- HGENQ Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Humanigen (HGENQ) 8-KRegulation FD Disclosure
Filed: 12 Apr 22, 9:00am
Exhibit 99.1

Executive Overview April 2022 Humanigen, Inc.

2 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this presentation are forward - looking statements . Forward - looking statements reflect management's current knowledge, assumptions, judgment, and expectations regarding future performance or events . Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and you should be aware that actual events or results may differ materially from those contained in the forward - looking statements . Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward - looking statements, including, without limitation, statements regarding : Humanigen’s beliefs as to the potential benefits of lenzilumab as a treatment for hospitalized COVID - 19 patients ; its beliefs as to the potential of lenzilumab to improve patient survival when used before ICU admission and progression of respiratory failure ; statements regarding the therapeutic potential of targeting a single upstream cytokine earlier in the COVID - 19 disease process ; its efforts to request and receive Emergency Use Authorization or Conditional Marketing Authorization for lenzilumab in COVID - 19 in the US and UK and other territories, as applicable its beliefs and projections regarding the need for lenzilumab as a therapeutic if authorized or approved ; the company’s projections for anticipated supply of lenzilumab through the end of 2022 ; the effectiveness of its preparations to commercialize lenzilumab in the UK and other markets, if CMA or other marketing approval were granted ; its efforts to mitigate its manufacturing expenses pending receipt of a marketing authorization or approval from a regulatory agency such as MHRA, EMA or FDA ; its ability to resolve payment disputes with certain of its CMOs and other service providers on favorable terms ; and its other plans to initiate or participate in planned clinical trials and otherwise explore the effectiveness of lenzilumab and other candidates in its development portfolio as therapies for other inflammation and immune - oncology indications . Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in the company’s lack of profitability and need for additional capital to conduct its business ; its dependence on partners to further the development of its product candidates ; the uncertainties inherent in the development, attainment of the requisite regulatory authorizations and approvals and launch of any new pharmaceutical product ; challenges associated with manufacturing and commercializing a biologic such as lenzilumab ; the outcome of pending or future litigation ; and the various risks and uncertainties described in the "Risk Factors" sections and elsewhere in Humanigen's periodic and other filings with the Securities and Exchange Commission . All forward - looking statements are expressly qualified in their entirety by this cautionary notice . You should not rely upon any forward - looking statements as predictions of future events . The Company undertakes no obligation to revise or update any forward - looking statements made in this presentation to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law .
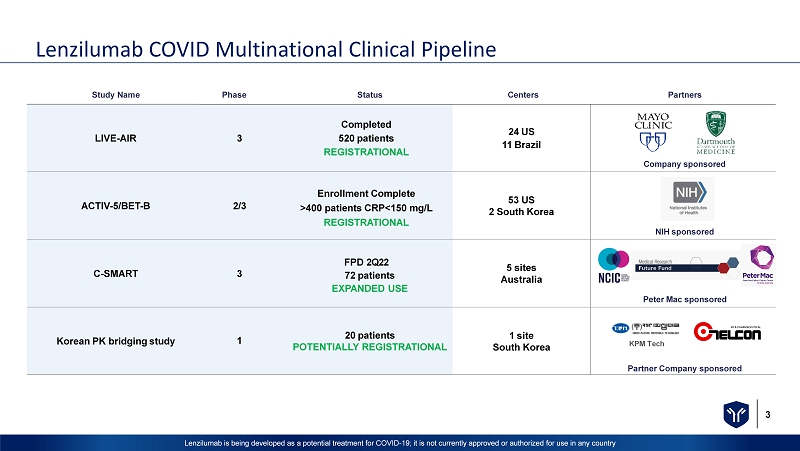
3 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Lenzilumab COVID Multinational Clinical Pipeline S t ud y N a m e Phase Status Centers Partners LIVE - AIR 3 Completed 520 patients REGISTRATIONAL 24 US 11 Brazil C o m p a n y s pon s o re d ACTIV - 5/BET - B 2/3 Enrollment Complete >400 patients CRP<150 mg/L REGISTRATIONAL 53 US 2 South Korea NIH sponsored C - SMART 3 FPD 2Q22 72 patients EXPANDED USE 5 sites Australia Peter Mac sponsored Korean PK bridging study 1 20 patients POTENTIALLY REGISTRATIONAL 1 site South Korea Partner Company sponsored K PM T ec h
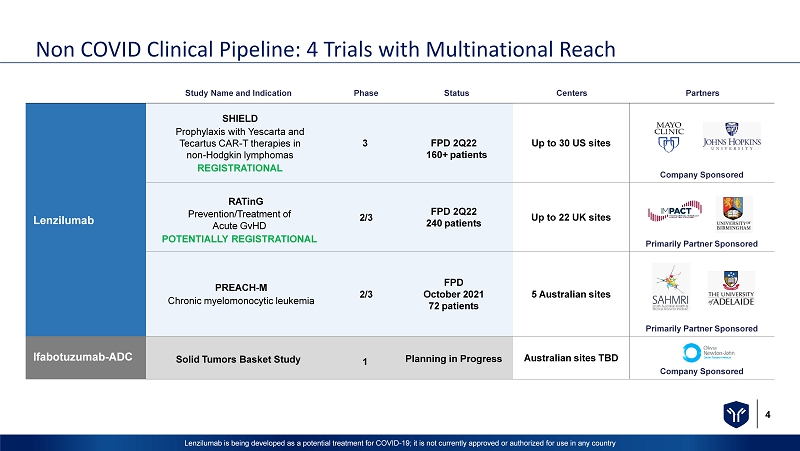
4 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Non COVID Clinical Pipeline: 4 Trials with Multinational Reach Study Name a n d I nd i ca ti o n Phase Status Centers Partners Lenzilumab SHIELD P r o p h y l a xis w i t h Yescarta and Tecartus CAR - T therapies in non - Hodgkin lymphoma s REGISTRATIONAL 3 FPD 2Q22 16 0 + patients Up to 30 US sites Company Sponsored RATinG Prevention/Treatment of Acute GvHD POTENTIALLY REGISTRATIONAL 2/3 F PD 2Q22 240 patients Up to 22 UK sites Primarily Partner Sponsored PREACH - M Chronic myelomonocytic leukemia 2 /3 F PD O c t o b e r 202 1 72 patients 5 Australian sites Primarily Partner Sponsored Ifabotuzumab - ADC Solid Tumors Basket Study 1 Planning in Progress Australian sites TBD Company Sponsored

5 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Key COVID - 19 U.S. Statistics Delta Omicron Alpha 1 https://covid.cdc.gov/covid - data - tracker/#new - hospital - admissions (Accessed April 7, 2022 ) 2 https://covid.cdc.gov/covid - data - tracker/#trends_dailydeaths (Accessed April 7, 2022 ) 3 https://covid.cdc.gov/covid - data - tracker/#vaccinations_vacc - total - admin - rate - total (Accessed April 7, 2022) BA.1 Patients Admitted 1 Patients in Hospital 1 9,980 Latest available Daily Deaths 2 674 Latest available 1,298 Latest daily 896,004 YTD - Total Vaccination Rate 3 ~77% One Dose ~45% Booster ~66% Full July 3, 2021 Delta Dominant Variant BA.2 March 26, 2022 Ba.2 Dominant Variant December 18, 2021 Ba.1 Dominant Variant Delta Emerges Omicron Emerges

6 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Flu Provides Example of Endemic Market COVID - 19 has many similarities to other viruses that have been around for decades, but also several key differences (e.g., COVID mutates 2X−10X faster than flu 2 ) that increase the likelihood that COVID - 19 will sustain for years, despite vaccines and individual immunity Despite vaccines and antivirals, there is still a sustainable need for effective therapeutics Burden of Influenza 1 The Nature of a Virus • Vaccines are not 100% effective • Vaccine efficacy proven to wane • Immune escape variant may emerge from vaccinated population experiencing breakthrough infections *CDC estimates from the 2019 - 2020 season are preliminary and may change as data are finalized 1. CDC: Disease Burden of Influenza. Hospitalization and death numbers are estimates (Accessed April 6, 2022). https://www.cdc.g ov/ flu/about/burden/index.html 2. Trevor Bedford, Fred Hutchinson Cancer Research Center, presentation to FDA Vaccines and Related Biological Products Advisory Co mmittee on April 6, 2022 Estimated Range of Flu Seasons In U.S. 2010 - 11 through 2019 - 20 Season Hospitalizations Deaths 2010 - 2011 290,000 37,000 2011 - 2012 140,000 12,000 2012 - 2013 570,000 43,000 2013 - 2014 350,000 38,000 2014 - 2015 590,000 51,000 2015 - 2016 280,000 23,000 2016 - 2017 500,000 38,000 2017 - 2018 710,000 52,000 2018 - 2019 380,000 28,000 2019 - 2020* 380,000* 20,000* Average 419,000 34,200
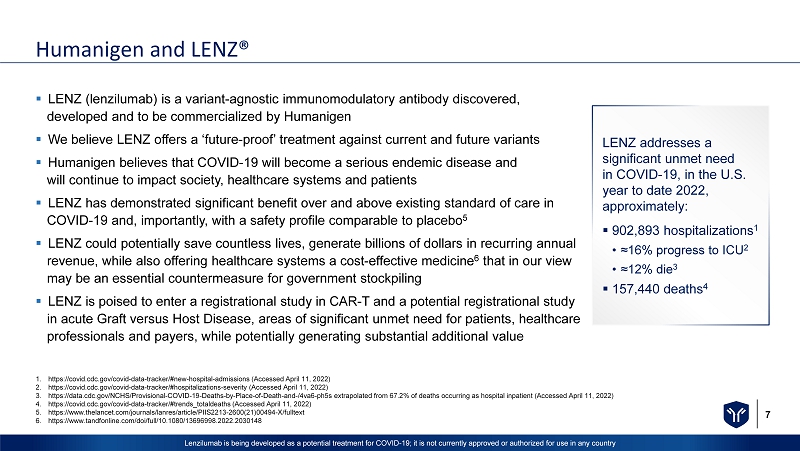
7 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Humanigen and LENZ® ▪ LENZ (lenzilumab) is a variant - agnostic immunomodulatory antibody discovered, developed and to be commercialized by Humanigen ▪ We believe LENZ offers a ‘future - proof’ treatment against current and future variants ▪ Humanigen believes that COVID - 19 will become a serious endemic disease and will continue to impact society, healthcare systems and patients ▪ LENZ has demonstrated significant benefit over and above existing standard of care in COVID - 19 and, importantly, with a safety profile comparable to placebo 5 ▪ LENZ could potentially save countless lives, generate billions of dollars in recurring annual revenue, while also offering healthcare systems a cost - effective medicine 6 that in our view may be an essential countermeasure for government stockpiling ▪ LENZ is poised to enter a registrational study in CAR - T and a potential registrational study in acute Graft versus Host Disease, areas of significant unmet need for patients, healthcare professionals and payers, while potentially generating substantial additional value 1. https://covid.cdc.gov/covid - data - tracker/#new - hospital - admissions (Accessed April 11, 2022) 2. https://covid.cdc.gov/covid - data - tracker/#hospitalizations - severity (Accessed April 11, 2022) 3. https://data.cdc.gov/NCHS/Provisional - COVID - 19 - Deaths - by - Place - of - Death - and - /4va6 - ph5s extrapolated from 67.2% of deaths occurri ng as hospital inpatient (Accessed April 11, 2022) 4. https://covid.cdc.gov/covid - data - tracker/#trends_totaldeaths (Accessed April 11, 2022) 5. https://www.thelancet.com/journals/lanres/article/PIIS2213 - 2600(21)00494 - X/fulltext 6. https://www.tandfonline.com/doi/full/10.1080/13696998.2022.2030148 LENZ addresses a significant unmet need in COVID - 19, in the U.S. year to date 2022, approximately: ▪ 902,893 hospitalizations 1 • ≈16% progress to ICU 2 • ≈12% die 3 ▪ 157,440 deaths 4
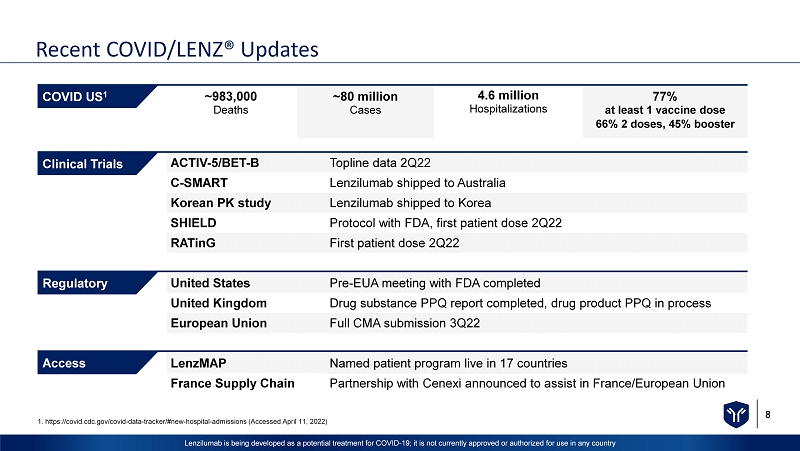
8 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry ACTIV - 5/BET - B Topline data 2Q22 C - SMART Lenzilumab shipped to Australia Korean PK study Lenzilumab shipped to Korea SHIELD Protocol with FDA, first patient dose 2Q22 RATinG First patient dose 2Q22 United States Pre - EUA meeting with FDA completed United Kingdom Drug substance PPQ report completed, drug product PPQ in process European Union Full CMA submission 3Q22 LenzMAP Named patient program live in 17 countries France Supply Chain Partnership with Cenexi announced to assist in France/European Union ~983,000 Deaths Clinical Trials Regulatory Access COVID US 1 ~80 million Cases 4.6 million Hospitalizations 77% at least 1 vaccine dose 66% 2 doses, 45% booster Recent COVID/LENZ® Updates 1. https://covid.cdc.gov/covid - data - tracker/#new - hospital - admissions (Accessed April 11, 2022)
9 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry The Lancet Respiratory Medicine: Peer - Reviewed LIVE - AIR Phase 3 Publication LIVE - AIR showed that lenzilumab treatment of hospitalised patients with COVID - 19 can improve the likelihood of survival without the need for mechanical ventilation, with a safety profile similar to that of placebo 60% of LIVE - AIR patients were on room air or low - flow oxygen support. … (Raising) the possibility that lenzilumab might be positioned for use before ICU admission and progression of respiratory failure requiring high - flow oxygen and non - invasive or invasive ventilation Findings might indicate the therapeutic potential of targeting a single upstream cytokine earlier in the disease process, guided by baseline CRP. ... The study contributes to the emerging body of evidence about how CRP concentrations relate to the pathogenesis of COVID - 19 and to patient and treatment selection Temesgen Z., et al. Lancet Respir Med 2021. Published Online December 1, 2021. https://doi.org/10.1016/S2213 - 2600(21)00494 - X View Article
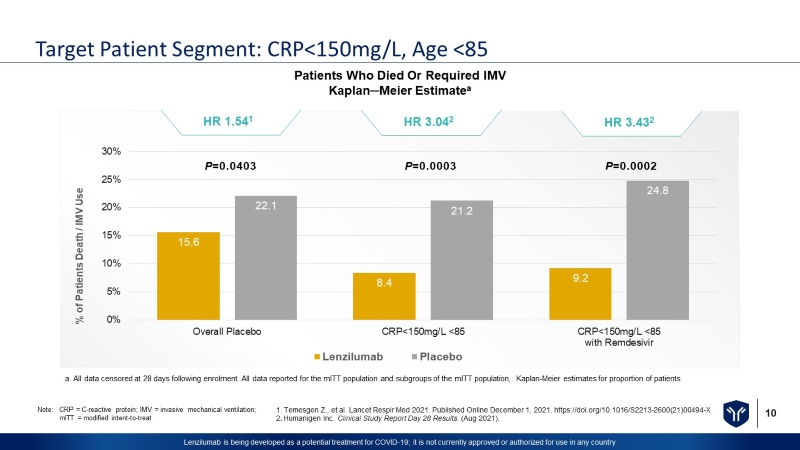
10 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Target Patient Segment: CRP<150mg/L, Age <85 Patients Who Died Or Required IMV Kaplan─Meier Estimate a 15.6 8.4 9.2 22.1 21.2 24.8 0% 5% 10% 15% 20% 25% 30% Overall Placebo CRP<150mg/L <85 CRP<150mg/L <85 with Remdesivir % of Patients Death / IMV Use Lenzilumab Placebo P =0.0403 P =0.0003 P =0.0002 HR 1.54 1 HR 3.04 2 HR 3.43 2 1. Temesgen Z., et al. Lancet Respir Med 2021. Published Online December 1, 2021. https://doi.org/10.1016/S2213 - 2600(21)00494 - X 2 . Humanigen Inc. Clinical Study Report Day 28 Results . (Aug 2021). Note: CRP = C - reactive protein; IMV = invasive mechanical ventilation; mITT = modified intent - to - treat a. All data censored at 28 days following enrolment. All data reported for the mITT population and subgroups of the mITT population, Kaplan - Meier estimates for proportion of patients
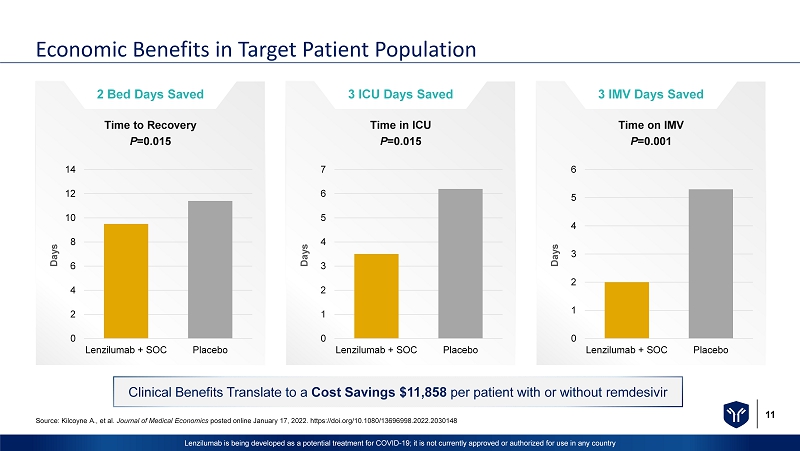
11 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Economic Benefits in Target Patient Population Clinical Benefits Translate to a Cost Savings $11,858 per patient with or without remdesivir 2 Bed Days Saved 0 2 4 6 8 10 12 14 Lenzilumab + SOC Placebo Days Time to Recovery P =0.015 3 ICU Days Saved 0 1 2 3 4 5 6 7 Lenzilumab + SOC Placebo Days Time in ICU P =0.015 3 IMV Days Saved 0 1 2 3 4 5 6 Lenzilumab + SOC Placebo Days Time on IMV P =0.001 Source: Kilcoyne A., et al. Journal of Medical Economics posted online January 17, 2022. https://doi.org/10.1080/13696998.2022.2030148
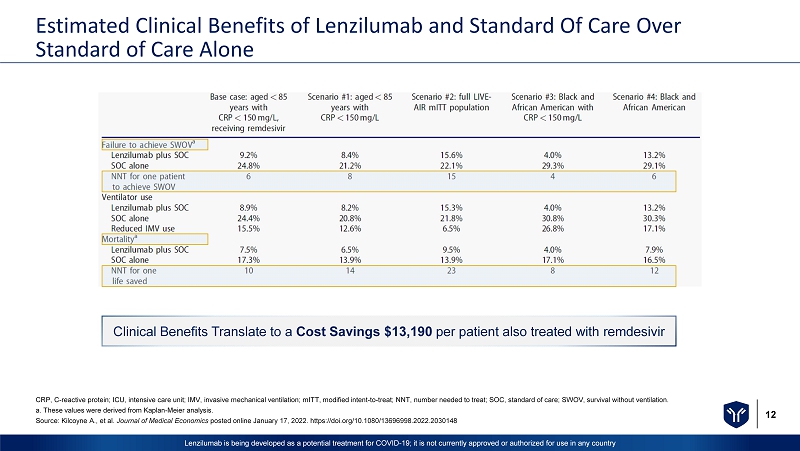
12 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Estimated Clinical Benefits of Lenzilumab and Standard Of Care Over Standard of Care Alone CRP, C - reactive protein; ICU, intensive care unit; IMV, invasive mechanical ventilation; mITT , modified intent - to - treat; NNT, number needed to treat; SOC, standard of care; SWOV, survival without ventilation. a. These values were derived from Kaplan - Meier analysis. Source: Kilcoyne A., et al. Journal of Medical Economics posted online January 17, 2022. https://doi.org/10.1080/13696998.2022.2030148 Clinical Benefits Translate to a Cost Savings $13,190 per patient also treated with remdesivir
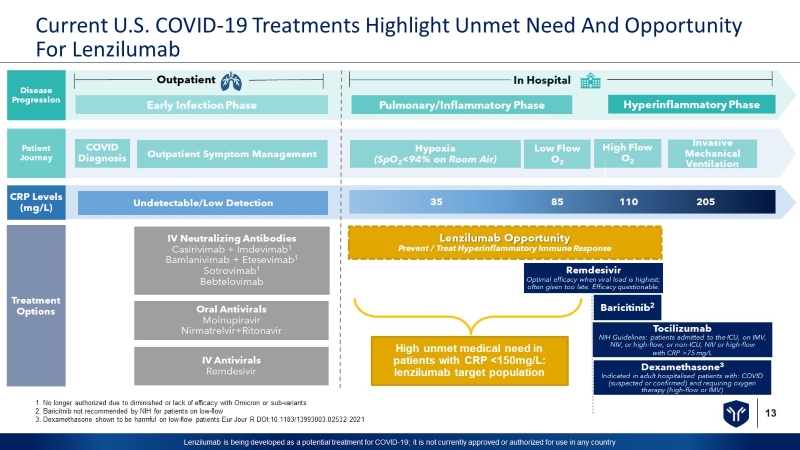
13 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Current U.S. COVID - 19 Treatments Highlight Unmet Need And Opportunity For Lenzilumab In Hospital Hyperinflammatory Phase Pulmonary/Inflammatory Phase Early Infection Phase Dexamethasone 3 Indicated in adult hospitalised patients with: COVID (suspected or confirmed) and requiring oxygen therapy (high - flow or IMV) Lenzilumab Opportunity Prevent / Treat Hyperinflammatory Immune Response Tocilizumab NIH Guidelines: patients admitted to the ICU, on IMV, NIV, or high - flow, or non - ICU, NIV or high - flow with CRP >75 mg/L Remdesivir Optimal efficacy when viral load is highest; often given too late. Efficacy questionable. COVID Diagnosis Outpatient Invasive Mechanical Ventilation Hypoxia (SpO 2 <94% on Room Air) Baricitinib 2 Low Flow O 2 High Flow O 2 Disease Progression Patient Journey CRP Levels (mg/L) Treatment Options Outpatient Symptom Management Undetectable/Low Detection 35 85 110 20 5 IV Neutralizing Antibodies Casirivimab + Imdevimab 1 Bamlanivimab + Etesevimab 1 Sotrovimab 1 Bebtelovimab Oral Antivirals Molnupiravir Nirmatrelvir+Ritonavir IV Antivirals Remdesivir High unmet medical need in patients with CRP <150mg/L: lenzilumab target population 1. No longer authorized due to diminished or lack of efficacy with Omicron or sub - variants 2. Baricitnib not recommended by NIH for patients on low - flow 3. Dexamethasone shown to be harmful on low - flow patients Eur Jour R DOI:10.1183/13993003.02532 - 2021
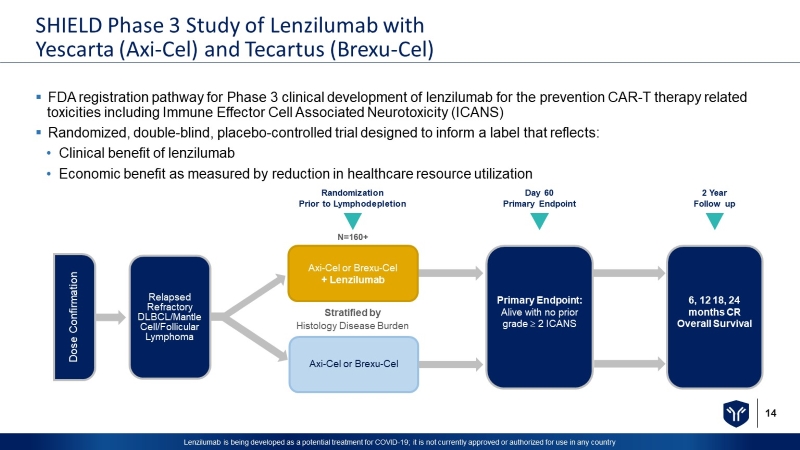
14 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry SHIELD Phase 3 Study of Lenzilumab with Yescarta (Axi - Cel) and Tecartus (Brexu - Cel) ▪ FDA registration pathway for Phase 3 clinical development of lenzilumab for the prevention CAR - T therapy related toxicities including Immune Effector Cell Associated Neurotoxicity (ICANS) ▪ Randomized, double - blind, placebo - controlled trial designed to inform a label that reflects: • Clinical benefit of lenzilumab • Economic benefit as measured by reduction in healthcare resource utilization Axi - Cel or Brexu - Cel + Lenzilumab Axi - Cel or Brexu - Cel Stratified by Histology Disease Burden Primary Endpoint: Alive with no prior grade 2 ICANS 6, 12 18, 24 months CR Overall Survival Dose Confirmation Relapsed Refractory DLBCL/Mantle Cell/Follicular Lymphoma Randomization Prior to Lymphodepletion Day 60 Primary Endpoint 2 Year Follow up N=160+

15 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry ▪ 22 sites selected ▪ UK regulatory submission completed ▪ First patient to be dosed 2Q22 RATinG Study: Addressing High Unmet Need in aGvHD To be conducted in collaboration with the IMPACT Group in UK SOC Lenzilumab + SOC vs Placebo + SOC Intermediate/ High Risk Low Risk Allogeneic Stem Cell Transplant aGvHD Diagnosis Outcome prediction using “MAGIC” algorithm
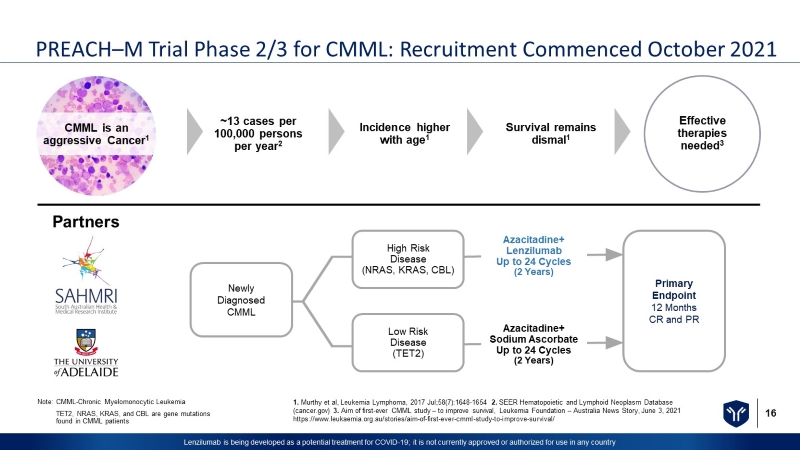
16 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry PREACH – M Trial Phase 2/3 for CMML: Recruitment Commenced October 2021 1. Murthy et al, Leukemia Lymphoma, 2017 Jul;58(7):1648 - 1654 2. SEER Hematopoietic and Lymphoid Neoplasm Database (cancer.gov) 3. Aim of first - ever CMML study – to improve survival, Leukemia Foundation – Australia News Story, June 3, 2021 https://www.leukaemia.org.au/stories/aim - of - first - ever - cmml - study - to - improve - survival/ Note: CMML - Chronic Myelomonocytic Leukemia TET2, NRAS, KRAS, and CBL are gene mutations found in CMML patients Partners Newly Diagnosed CMML Primary Endpoint 12 Months CR and PR Azacitadine+ Sodium Ascorbate Up to 24 Cycles (2 Years) Azacitadine+ Lenzilumab Up to 24 Cycles (2 Years) High Risk Disease (NRAS, KRAS, CBL) Low Risk Disease (TET2) ~13 cases per 100,000 persons per year 2 Incidence higher with age 1 Survival remains dismal 1 Effective therapies needed 3 CMML is an aggressive Cancer 1
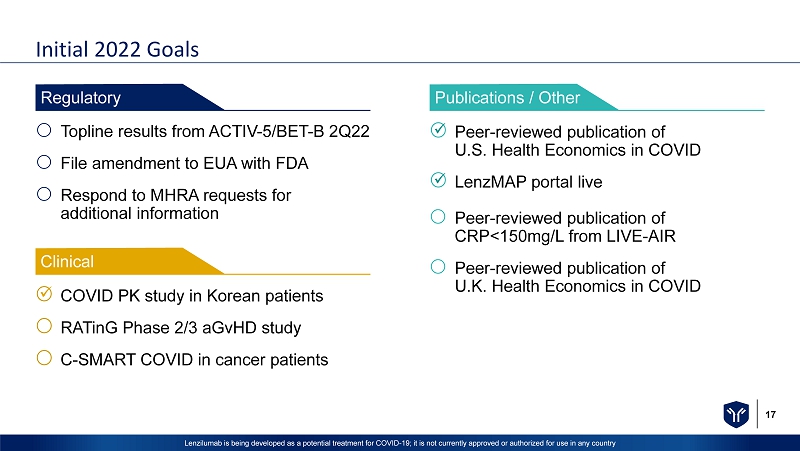
17 Lenzilumab is being developed as a potential treatment for COVID - 19; it is not currently approved or authorized for use in any c ountry Initial 2022 Goals COVID PK study in Korean patients RATinG Phase 2/3 aGvHD study C - SMART COVID in cancer patients Clinical Topline results from ACTIV - 5/BET - B 2Q22 File amendment to EUA with FDA Respond to MHRA requests for additional information Regulatory Peer - reviewed publication of U.S. Health Economics in COVID LenzMAP portal live Peer - reviewed publication of CRP<150mg/L from LIVE - AIR Peer - reviewed publication of U.K. Health Economics in COVID Publications / Other x

Thank You For Your Attentio n VISIT OUR SIT E