UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended - MARCH 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-50601
SYNUTRA INTERNATIONAL, INC.
| DELAWARE | | 13-4306188 |
(State or Other jurisdiction of Incorporation or Organization) | | I. R. S. Employer Identification No. |
2275 RESEARCH BLVD., SUITE 500
ROCKVILLE, MARYLAND 20850
301-840-3888
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
2275 RESEARCH BLVD., SUITE 500
ROCKVILLE, MARYLAND 20850
301-840-3888
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | | Name of Each Exchange on Which Registered |
| Common Stock $0.0001 Par Value | | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by referenced in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨ Smaller reporting company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant based on the closing sales price of the registrant’s common stock on September 30, 2008 (the last business day of the registrant’s most recently completed second fiscal quarter), as reported on the Nasdaq Global Select Market, was $362.3 million. For purposes of this disclosure, shares of Common Stock held by persons who hold more than 10% of the outstanding shares of Common Stock and shares held by officers and directors of the registrant have been excluded in that such persons may be deemed to be affiliates. This determination is not necessarily conclusive for other purposes.
As of June 12, 2009, there were 54,000,713 shares of the registrant’s Common Stock outstanding.
TABLE OF CONTENTS
| | | PAGE |
| PART I | |
| | | |
| ITEM 1. | BUSINESS | 4 |
| ITEM 1A. | RISK FACTORS | 16 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS | 38 |
| ITEM 2. | PROPERTIES | 39 |
| ITEM 3. | LEGAL PROCEEDINGS | 40 |
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS | 41 |
| | | |
| PART II | |
| | | |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDERS MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 42 |
| ITEM 6. | SELECTED FINANCIAL DATA | 44 |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 46 |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 68 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 70 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 106 |
| ITEM 9A. | CONTROLS AND PROCEDURES | 107 |
| ITEM 9B. | OTHER INFORMATION | 109 |
| | | |
| PART III | |
| | | |
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 110 |
| ITEM 11. | EXECUTIVE COMPENSATION | 114 |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 119 |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 122 |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES | 124 |
| | | |
| PART IV | |
| | | |
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES | 125 |
| | | |
| SIGNATURES | | |
CONVENTIONS THAT APPLY TO THIS ANNUAL REPORT ON FORM 10-K
Except where the context otherwise requires and for purposes of this Annual Report on Form 10-K only:
| | • | “we,” “us,” “our company,” “our,” and “Synutra” refer to Synutra International, Inc., and its consolidated subsidiaries; |
| | • | “China” or “PRC” refers to the People’s Republic of China, excluding Taiwan and the Special Administrative Regions of Hong Kong and Macau; |
| | • | all references to “ton” or “tons” are to “tonne” or “metric ton;” |
| | • | all references to “Renminbi” or “RMB” are to the legal currency of China; and |
| | • | all references to “U.S. dollars,” “dollars,” or “$” are to the legal currency of the United States. |
Amounts may not always add to the totals due to rounding.
Unless otherwise noted, all translations from Renminbi to U.S. dollars were made at the middle rate published by the People’s Bank of China, or the middle rate, as of March 31, 2009, which was RMB6.8359 to $1.00. We make no representation that the Renminbi amounts referred to in this Annual Report on Form 10-K could have been or could be converted into U.S. dollars at any particular rate or at all. On June 12, 2009, the middle rate was RMB6.8325 to $1.00.
PART I
This Annual Report on Form 10-K, or Form 10-K, contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, that are based on our current expectations, assumptions, estimates and projections about us and our industry. All statements other than statements of historical fact in this Form 10-K are forward-looking statements. In some cases, these forward-looking statements can be identified by words or phrases such as “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “is/are likely to,” “may,” “plan,” “should,” “will,” “aim,” “potential,” “continue,” or other similar expressions. The forward-looking statements included in this Form 10-K relate to, among others:
| | • | our goals and strategies; |
| | • | our future business development, financial condition and results of operations; |
| | • | the expected growth of the nutritional products and infant formula markets in China; |
| | • | market acceptance of our products; |
| | • | our expectations regarding demand for our products; |
| | • | our ability to stay abreast of market trends and technological advances; |
| | • | competition in the infant formula industry in China; |
| | • | PRC governmental policies and regulations relating to the nutritional products and infant formula industries; and |
| | • | general economic and business conditions in China. |
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may turn out to be incorrect. Our actual results could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in the “Item 1 . Business,” “Item 1A. Risk Factors,” “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and other sections in this Form 10-K.
The forward-looking statements are made as of the date of this Form 10-K. We undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date on which the statements are made or to reflect the occurrence of unanticipated events.
ITEM 1. BUSINESS
GENERAL DEVELOPMENT AND NARRATIVE DESCRIPTION OF BUSINESS
We are a leading infant formula company in China. We principally produce, market, and sell our products under the “Shengyuan,” or “Synutra,” name, together with other complementary brands. We focus on selling premium infant formula products, which are supplemented by more affordable infant formulas targeting the mass market as well as other nutritional products and ingredients. We sell our products through an extensive nationwide sales and distribution network covering 29 provinces and provincial-level municipalities in China. As of March 31, 2009, this network comprised over 480 distributors and over 800 sub-distributors who sell our products in over 65,000 retail outlets.
We entered into the prepared baby food business in October 2008 by acquiring the business from Beijing Huilian Food Co., Ltd. We also began our nutritional ingredients and supplements business, which include the production of Chondroitin sulfate, and processing of microencapsulated Docosahexanoic Acid (“DHA”) and Arachidonic Acid (“ARA”), at Meitek Technology Co., Ltd., one of our PRC subsidiaries.
In September 2008, our business was severely interrupted by the melamine contamination incident in which the products of 22 Chinese formula producers, including ours, were found to be contaminated by melamine, a substance not approved for use in food and linked to approximately 300,000 kidney illnesses among infants and children in China. We believe that the melamine contamination was resulted from tainted milk supplies provided by third-party suppliers in the Hebei and Inner Mongolia regions of China. We did not add the melamine to the affected products and our normal testing procedures, which are carried out in accordance with Chinese government health regulations and requirements and internationally-accepted quality control procedures, were not equipped to and did not detect the melamine contamination. On September 16, 2008, we announced a compulsory recall on certain lots of U-Smart products and a voluntary recall of other products that were contaminated or suspected to be contaminated by melamine. The melamine contamination incident severely damaged our reputation and our business suffered significant losses from product recall, inventory write down/write-off and subsequent loss of sales, see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In addition, there can be no assurance that additional issues will not be identified in the future and this may have an adverse effect on our results of operations. See Part I - Item 1A. Risk Factors - We are highly dependent upon consumers’ perception of the safety and quality of our products. Any ill effects, product liability claims, recalls, adverse publicity or negative public perception regarding particular ingredients or products or our industry in general, could harm our reputation and damage our brand, and adversely affect our results of operations.
Due to the significant reduction of sales caused by the melamine contamination incident and replacement of recalled products, our net sales for the fiscal year ended March 31, 2009 decreased by 13.7% to $312.5 million from $362.1 million for the prior fiscal year. Our gross profit for the fiscal year ended March 31, 2009 decreased by 71.4% to $53.4 million from $186.5 million for the prior fiscal year. Our net loss for the fiscal year ended March 31, 2009 was $100.5 million, as compared to net income of $45.7 million for the prior fiscal year. The net loss for the fiscal year ended March 31, 2009 compared to the prior fiscal year was attributable primarily to the significant cost of product recall and decreased sales from the second half of September 2008 to March 2009.
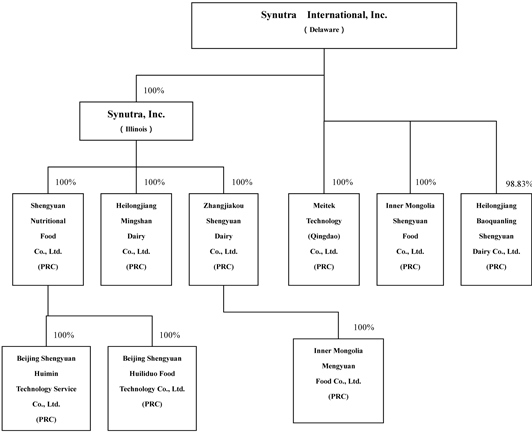
CORPORATE STRUCTURE AND HISTORY
We are a Delaware holding company and conduct substantially all of our business through our operating subsidiaries in China. We own all or majority of the equity interests in our operating subsidiaries, directly or indirectly, through Synutra, Inc., or Synutra Illinois, an intermediate holding company. Synutra Illinois was incorporated in Illinois in 2000 and has no other significant assets and operations of its own. Our corporate structure reflects common practice for companies with operations in the PRC where separate legal entities are often required or advisable for tax or administrative reasons.
We began our business operations in China in 1998. In a series of related transactions between 2003 and 2005, Synutra Illinois acquired all the interest in our operating subsidiaries in China.
On July 15, 2005, Synutra Illinois completed a reverse acquisition transaction with Vorsatech Ventures, Inc., or Vorsatech. Upon the consummation of this share exchange transaction, Vorsatech’s total issued and outstanding common stock equaled 50,000,713 shares, including 48,879,500 shares issued pursuant to the reverse acquisition transaction and 1,121,213 shares owned by Vorsatech’s existing stockholders. Thereafter, Synutra Illinois became Vorsatech’s wholly owned subsidiary and Vorsatech became the reporting entity for our business. We subsequently changed the name of the reporting entity to Synutra International, Inc.
In July 2005, we changed our fiscal year end from December 31 to March 31 beginning with the fiscal year ended March 31, 2005. This change was intended to simplify communication with stockholders and enables the reporting of our financial results in a timeframe consistent with Vorsatech.
On May 24, 2007, we entered into a Common Stock Purchase Agreement with Warburg Pincus Private Equity IX, L.P., or Warburg, pursuant to which we sold to Warburg 4,000,000 shares of common stock for an aggregate purchase price of $66 million. The closing of the transaction took place on June 15, 2007.
The following is a brief description of our major operating subsidiaries in China.
| | • | Shengyuan Nutritional Food Co., Ltd., or Shengyuan Nutrition, formerly known as Qingdao St. George Dairy Co., Ltd., located in Qingdao, Shandong, China, was established by Synutra Illinois in September 2001 and is engaged in the dry-blending, packaging, shipping and distribution of all of our powdered formula products. |
| | • | Heilongjiang Mingshan Dairy Co., Ltd., or Mingshan, formerly known as Luobei Shengyuan Dairy Co., Ltd., located in Luobei, Heilongjiang, China, was established in April 2001 and is engaged in raw milk processing and the production of powdered formula. Synutra Illinois acquired 67% and 33% of the ownership interest in Mingshan from Sheng Zhi Da and Xiuqing Meng, the wife of Liang Zhang, our chairman and chief executive officer, respectively, in January 2005. |
| | • | Zhangjiakou Shengyuan Dairy Co., Ltd., or Zhangjiakou, located in Zhangjiakou, Hebei, China, was established in March 2004 with Synutra Illinois and Sheng Zhi Da holding 40% and 60%, respectively, of its equity interests and is engaged in raw milk processing and the production of powdered formula. Synutra Illinois acquired the remaining 60% ownership interest in Zhangjiakou from Sheng Zhi Da in April 2005. |
| | • | Inner Mongolia Shengyuan Food Co., Ltd., or Inner Mongolia Shengyuan, located in Zhenglanqi, Inner Mongolia, China, was established in September 2006 and has been constructing its production facilities since its establishment. Inner Mongolia Shengyuan is expected to commence production of nutritional food products in late 2009. |
| | • | Inner Mongolia Mengyuan Food Co., Ltd., or Mengyuan, located in Fengzhen, Inner Mongolia, China, commenced operations in July 2007 and is engaged in raw milk processing. Mengyuan was acquired by Zhangjiakou from its then shareholders in November 2006. |
| | • | Meitek Technology (Qingdao) Co., Ltd., or Meitek, formerly known as Mei Tai Technology (Qingdao) Co, Ltd. located in Qingdao, Shandong, China, was established in November 2006 to produce certain nutritional supplements and ingredients. Meitek began operations in October 2008. |
| | • | Heilongjiang Baoquanling Shengyuan Dairy Co., Ltd., or Baoquanling, located in Junchuan, Heilongjiang, China, is engaged in raw milk processing and the production of powdered formula. Liang Zhang previously owned an 80% interest in Baoquanling. We acquired Zhang Liang’s equity interest in Baoquanling for $1.4 million in May 2007. Immediately after the acquisition of this equity interest, we made an aggregate capital injection of $29.7 million into Baoquanling and, as a result, our ownership interest in Baoquanling increased to 99%. |
| | • | Beijing Shengyuan Huiliduo Food Technology Co., Ltd., or Huiliduo, located in Beijing, China, was established in July 2008 to produce prepared baby food. |
| | • | Beijing Shengyuan Huimin Technology Service Co., Ltd., or Huimin, a variable interest entity which was incorporated on July 10, 2008 and is engaged in diagnostic services for pregnant women. As of March 31, 2009, Huimin had not commenced its operations. |
The following chart which includes major subsidiaries reflects our organizational structure as of the date of this Form 10-K.
OUR BRANDS
We primarily market our products under the Synutra, or Shengyuan, name which has been associated with infant formula products in China for more than 10 years. In addition to the Synutra, or Shengyuan name, our products are marketed in China under brands that we have developed through our national sales and marketing efforts.
Synutra Family of Brands
The Synutra family of brands includes several of China’s leading infant formula and children’s nutrition brands, including Super and U-Smart. We have positioned the Synutra family of brands as high quality brands, which provide unique, clinically supported health and developmental benefits. The Synutra family of brands features products that include DHA and ARA, which support brain, visual and nervous system development for infants. Building upon the strength of our brand equity, we have extended the Synutra family of brands into the fast growing children’s nutrition market, such as prepared baby foods.
Complementary Brands
In addition to the Synutra family of brands, we market several other brands targeted at various consumer segments and designed to meet the nutritional needs of broad consumer populations in China. These brands include the Mingshan (powdered formula), Helanruniu or Holsteina (adult formula), Meitek (nutritional supplements), and Huiliduo (prepared baby foods).
OUR PRODUCTS
Our nutritional products are grouped by category of production process and usage as well as internal resources allocation: (1) powdered formula, (2) baby foods and (3) nutritional ingredients and supplements. Sales of powdered formula, baby foods and nutritional ingredients and supplements comprised approximately 91.1%, 0.1% and 8.8% of our net sales for the fiscal year ended March 31, 2009.
Powdered Formula Products
Powdered formula segment covers the sale of powdered infant and adult formula products. It includes the brands of Super, U-Smart, Mingshan which was launched in October 2008 and Helanruniu, or Holsteina, which was launched in December 2008. Infant formula is our primary product line in the powdered formula segment, accounting for 85.9%, 84.8% and 72.3% of our total net sales for the fiscal years ended March 31, 2009, 2008, and 2007, respectively.
Each of our Super, U-Smart and Mingshan product lines has multiple formulations designed to meet nutritional requirements and help promote a baby or child’s healthy growth at each developmental stage. We endeavor to bring our infant formula products closer to breast milk. We have devoted resources to extensively adjust our product portfolio, upgrade our product lines, and add new products or line extensions to respond to market needs and target a wider group of consumers. To meet consumer expectations, we also periodically upgrade our product concepts, packaging, and pricing of our products.
We supplement our powdered infant formula products with other nutritional products for both adults and children. Our products are targeted at, and come in formulations that are developed to address specific types of consumer profiles, such as middle-aged and elderly consumers with cardiologic health issues, diabetic conditions, and calcium deficiency. Furthermore, we have developed a product specially designed for young adults to address their calcium and other nutrient fortification needs. Our products for women and young adults have also undergone product extensions and upgrades to further clarify the health and nutritional message and product image we intend to convey.
We continue to improve our rice cereal products as supplemental and functional foods to our powdered infant and children formula products. These improvements included upgrades to packaging as well as product extensions with new functionalities, new tastes and flavors, and new protein sources such as fish and chicken.
Baby Food Products
Baby food segment covers the sale of prepared baby food and nutritional snacks for babies and children. It includes the brand of Huiliduo which was launched in the fiscal quarter ended March 31, 2009 and the nutritional snacks component which we expect to launch in fiscal year 2010. These products are designed to be part of a child’s appropriate diet with enhanced nutrition value at different stages of development.
Nutritional Ingredients and Supplements
Nutritional ingredients and supplements segment covers the production and sale of nutritional ingredients and supplements such as chondroitin sulfate, microencapsulated DHA and ARA. In the past, we had sourced and exported chondroitin sulfate, a nutrient for joint health, to U.S. industrial customers through our exclusive third-party agent. With the completion of our Meitek facilities in October 2008, we will be able to produce chondroitin sulfate ourselves. In addition, our Meitek facilities can produce microencapsulated DHA and ARA powders and other nutritional ingredients and supplements for our own use and also for external industrial customers.
PRODUCTION
Powdered Formula Processing
Processing of our powdered formula begins with the collection and preparation of raw milk from dairy farmers. Local dairy farmers bring their dairy cattle to collection stations owned by us or third parties where raw milk is automatically received using fully enclosed, stainless-steel vacuum milking machines. These collection stations collect and transport the raw milk to our production facilities which are located within 100 kilometers of these milk collection stations, except for our Qingdao facility. Although raw milk can remain fresh for up to 72 hours, we normally process it within 24 hours. Once received, the raw milk will no longer have any contact with air and is immediately processed with refrigeration equipment that cools the raw milk within four seconds to approximately four degrees Celsius. The raw milk is then stored in air-tight tanks in preparation for advanced processes, which include milk fat separation, sterilization and spray-drying. Spray-dried milk powder is transported to our Qingdao facility for further processing or stored locally for commercial resale.
At the Qingdao facility, dried milk powder is mixed in large automated mechanical mixers with whey protein powder and other additives in a method know as dry-blending. Our dry-blending equipment can automatically adjust the level of ingredients to achieve the complex formulations required by our premium products. The resulting milk powder is then checked to ensure proper granule size before packaging and distribution.
Since the melamine contamination incident, we have been using imported milk powder from Europe and New Zealand for our Super, U-Smart and Holsteina brands. Currently, milk powder produced at our own facilities is mainly used for commercial resale. Only the Mingshan series of products continues to use locally produced raw milk. At our Mingshan facilities, sterilized raw milk is mixed with whey protein powder and other nutrients to the specifications of product formula through wet mixing method. The resulting mixture is then spray dried into milk powder and transported to our Qingdao facility for final packaging.
Packaging
The bulk of our powdered formula and other nutritional products come in three types of retail packaging: tin canisters, standup/display pouches, or sealed packages in a box. All packaging labels carry product information, nutritional profile, user instructions, product tracing data and shelf life date, product certification status, quality control and assurance remarks, manufacturer contact information, as well as customer service information that comply with PRC labeling requirements. Selected products are also retail-packaged in single-use sizes. Before any product leaves our packaging facility to distributors, we generally engage in an extensive testing and inspection of the final product.
Production and Packaging Facilities
Our processing and packaging facilities are located in various locations in China, including Beijing, Qingdao, Luobei, Zhangjiakou, Fengzhen, Zhenglanqi and Junchuan. These facilities encompass approximately 114,000 square meters of office, plant, and warehouse space. Our packaging and distribution headquarters located in Qingdao includes over 2,330 square meters of owned office space. All of our production facilities are built based on the GMP standard, with equipment imported from Europe and all of our facilities that have commenced operation have ISO9000 and HACCP series qualifications with some also being ISO14000 certified. We also have leased office space in Beijing covering approximately 6,000 square meters. Synutra Illinois leases an executive office in Rockville, Maryland, United States.
We currently own and operate five processing facilities and one packaging facility for our powdered formula production. As of March 31, 2009, we had raw milk processing capacity of 40,000 tons per year, packaging capacity of 82,000 tons per year and dry-blending processing capacity of 73,000 tons per year.
Our Qingdao facility serves as our packaging plant. Various ingredients, such as milk powder milk, whey protein powder and nutritional additives arrive at our Qingdao facility from our production facilities and our suppliers, and the Qingdao facility repackages ingredients into retail-size tin canisters or stand up/display pouches or sealed packages in boxes. This packaging facility also provides inventory control and logistics management, product quality monitoring and product development assistance. As of March 31, 2009, our packaging facility had an installed capacity of 82,000 tons per year.
Our production facility for prepared baby foods is located in Beijing. As of March 31, 2009, this facility had a processing capacity of 3.6 million jars per year.
Our production facility for nutritional snacks is located in Zhenglanqi, Inner Mongolia. This facility, upon completion in the later half of 2009, is expected to have processing capacity of 80,000 tons per year.
Our production facility for nutritional ingredients and supplements is located in Qingdao. As of March 31, 2009, this facility had a processing capacity of 700 tons per year for chondroitin sulfate, 1,000 tons per year for collagen protein, and 700 tons for microencapsulated DHA and ARA powders and other nutritional ingredients.
For information with respect to the installed capacity, location and function of our processing and packaging facilities, see “Item 2. Properties”.
RAW MATERIALS AND SUPPLIERS
Raw Materials
Our business depends on maintaining a regular and adequate supply of high-quality raw materials. A key ingredient for our powdered formulas is high-quality raw milk. We pay market prices, or premium prices in certain regions for our raw milk. Our milk suppliers are primarily dairy farmers located throughout Heilongjiang and Hebei provinces and Inner Mongolia.
In the aftermath of the melamine contamination incident, we decided to use imported milk powder for the production of our higher end powdered formula products. We currently source the majority of milk powder from Europe and New Zealand.
Whey protein powder is the other key ingredient used in the production of our powdered infant formula products and our other dairy-based products. Like all powdered milk producers, we use whey protein powder as the active ingredient to help reconstituted dairy-based formula to mimic the consistency of breast milk, which can constitute as much as 40.0% of the final powdered infant formula product by weight. Whey protein powder is a byproduct of cheese-making processes, and is difficult and costly to produce as a stand-alone product. Since China is not a large consumer or producer of cheese and cheese products, we and other domestic producers typically obtain whey protein powder in volume from overseas sources, such as France.
Based on our experience, prices of milk powder and whey protein powder can fluctuate over relatively short periods of time depending on market conditions. Our sourcing team carefully monitors price movements and makes major purchases at times when prices are low, subject to projected customer order flow and other factors.
Some of our powdered milk products, including our powdered infant formulas, also include additives such as DHA and ARA fatty acids and other nutritional additives. DHA and ARA fatty acids are long-chain poly-unsaturated fatty acids found in breast milk that are believed to aid in the development of an infant’s brain, eyes and nervous system. Studies have suggested that DHA and ARA fortification can replicate some of the nutritional benefits of breast milk in infant formulas. Currently we are producing microencapsulated DHA and ARA powders at our Meitek facility which began operations in October 2008 for both internal use and external sales.
We use vegetable oils in our dry-spraying powder infant formula production processes as a binder for the dry ingredients, helping diminish the occurrence of “lumpiness” or uneven texture when reconstituting powdered infant formula.
We purchase animal cartilage from third-party suppliers, including overseas slaughtering houses, for the production of chondroitin sulfate, a substance that provides nutrients for joints, tendon, ligaments and bones.
Suppliers and Supplier Arrangements
Prior to September 2008, we were able to meet our milk powder production needs by purchasing raw milk on the open market in established dairy regions in northern and northeastern China. We generally negotiate the purchase price of raw milk with many dairy farmers and cooperatives.
Since the melamine contamination incident, we have been purchasing milk powder from Europe and New Zealand. We generally negotiate the prices for each separate purchase on spot and do not sign long term contracts with our suppliers.
Prior to June 2007, we obtained substantially all of our supply of whey protein powder from Honnete, a large volume importer of processed dairy products in China. Honnete, a company controlled by Liang Zhang, our chairman and chief executive officer, is a major supplier of China’s whey protein powder. Beginning in June 2007, we began sourcing substantially all of our whey protein powder directly from Eurosérum, Honnete’s supplier in France.
SALES AND DISTRIBUTION
Sales
We generally sell our products directly to distributors and in limited circumstances directly to retailers. Our recent marketing efforts for our nutritional products have focused on extending retail coverage in terms of geography and market sectors. Our sales and marketing approach combines advertising, brand-building and store-level promotions. Our sales team of more than 300 individuals use our customer relations management, or CRM, database in order to acquire, process, and manage targeted customer information.
We have built a sales network that currently covers 29 provinces and provincial-level municipalities. Our sales group is divided into multiple sub-sales regions. Each sub-sales region covers between eight to twenty urban sales areas which act as an independent operating unit, while each urban sales area covers three to twenty county sales areas. As of March 31, 2009, we had a sales and marketing force of 3,400 employees, complemented by more than 16,000 commissioned field nutrition consultants or retail site promoters employed by our distributors and sub-distributors to promote and sell our products.
Although we sell primarily to our distributors and a few resellers, our sales teams work directly with each outlet to manage the sales process and to collect customer and purchasing related data. We use multiple criteria to select our distributors, including reviewing each potential distributor’s financial condition. We intend to expand our sales organization into additional cities and municipalities that we do not currently serve. Our city managers have their own monthly budgets and budgetary responsibility, empowering them to plan and execute their sales and marketing plans without further budgetary approval of the provincial managers. City managers are also rotated periodically between various cities. We have recently set up a sales budget management team to manage our sales expenses and to supervise the execution of our budgeting plan. This team reports directly to the president of marketing and sales.
We compensate our sales personnel through a combination of fixed salaries and bonuses determined based on sales growth. Our targeted sales incentive programs compensate our sales personnel on a product-specific level, thereby enabling us to incentivize our sales personnel to focus their sales and promotion efforts on certain product lines, such as our premium product lines or larger product packages.
Distribution
We primarily work directly with over 480 distributors, who in turn work with over 800 sub-distributors, and more than 65,000 retail outlets. Our packaging subsidiary, Shengyuan Nutritional Food, also serves as our national distribution center for our distributors in China. We generally require our distributors to pay full purchase price for our products in cash prior to delivery. Starting from the beginning of 2007 and prior to the melamine contamination incident, we have offered no more than 60 days of credit for our products to selected distributors. In light of the financial difficulties experienced by our distributors as a result of the melamine contamination incident, we have extended credit periods to no more than 90 days to more distributors. In addition to our credit policies, we ask our distributors to provide monthly inventory reports for us to monitor their inventory levels. Our sales personnel also regularly inspect distributors’ inventories to identify and control any potential inventory buildup by our distributors. We employ trucking companies locally and nationally to distribute retail packaged products to various regional and provincial distributors.
Distributors normally have exclusive distribution rights in their respective regions and cities to distribute our products, and are also responsible for developing the sub-distributors in their own region and cities. We typically enter into a contract with each of our distributors that establishes the range of sales obligations and their respective pricing ranges. However, our obligation to sell and the distributor’s obligation to purchase arise only at the time a purchase order is accepted. We seek to carefully manage our distributors through an evaluation system that monitors and grades each distributor with respect to performance criteria such as monthly sales and investment in promotional activities. We seek to incentivize well-performing distributors by providing discounts, larger sales territory and other incentives. While we do not directly manage our sub-distributors, we do track sub-distributor performance through coordinated efforts between our own sales personnel in the field and distributors. Our distributors generally have the right to return products due to package damage.
We currently distribute our nutritional products across China. Our logistics center in our Qingdao facilities occupies an area of 39,000 square meters. This logistics center can currently dispatch 4,800 tons of our products for shipment to our distributors per month. Our Qingdao facility also has the capability to respond to urgent requests for product shipments within an average of five days.
We currently work with approximately 27 transportation companies to transport our goods directly from our Qingdao facilities to distributors in a timely and efficient manner.
We have an enterprise resource planning system, or ERP system that monitors inventory management. This ERP system is linked to our financial information system.
MARKETING, ADVERTISING AND PROMOTION
Advertising
We advertise through various media, including television, print media and the Internet. Additionally, we conduct promotional activities with supermarket chains and entertainment companies in order to reach our target market.
We started nationwide television advertising coverage in September 2006. In certain cases, we supplement our nationwide television coverage with local television coverage. We also pursue advertising over the Internet. Our advertising spending was $72.8 million, $30.3 million and $16.4 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively. This increase enabled us to secure prime-time placements with China Central Television and other premium regional or satellite television stations. In the aftermath of the melamine contamination incident, we also intensified our efforts to improve our corporate image and brand name and to recover our lost market share.
Marketing and Promotion
As part of our sales and marketing approach, our sales force works with more than 7,400 healthcare facilities across China to provide maternity, infant nutrition and health education programs. We have also established a national customer service call center providing live and toll-free information support to consumers in prenatal, nursing, baby care education, product information, and complaint and dispute resolution.
We provide displays, posters and other promotional print to retail outlets and sales consultants employed by our distributors at each point of sale. We also pay entry fees to various retail outlets to place our products within such outlets. We collect customer information through surveys voluntarily provided by each customer via the point of sale or via mailed forms provided to our customers in each product package. We also have promotional activities with supermarket chains and entertainment companies in order to reach our target market.
QUALITY CONTROL
We place primary importance on quality. We have established quality control and food safety management systems for the purchase of raw materials, raw milk checks, raw milk processing, packaging, storage and transportation. We use commercial strength 25KG poly kraft bags for packaging before shipping the formula products to our retail packaging and distribution facilities. Additionally, we maintain cold storage areas at each of our four processing facilities to store fluid milk. All of our processing facilities are equipped with in-house laboratories for quality assurance and quality control purposes. Our laboratory in Qingdao facilities has been qualified as a National Standard Laboratory by the China National Accreditation Service for Conformity Assessment.
In order to ensure the quality and safety of our ingredients and products, we have also installed testing equipment and have implemented control procedures at each stage of production, including at the initial raw material purchase stage. There are over 1,100 quality control points throughout the entire production process, including 24 quality control points at the milk collection stations. During every step of production, transportation and storage, we employ strict internal regulations and monitoring by highly trained employees. Additionally, we have been increasing our investment in quality control equipment and training. We also maintain our own quality control personnel at each of our third-party producers’ facilities and we rigorously test all products shipped to us from our third-party producers.
Highlights of our quality control procedures are summarized below, organized by the main stages of production:
Purchase of Raw Milk:
| | • | Raw milk procurement manager conducts pre-purchase assessment of dairy farmers and requests issuance of clean bill of health for dairy cows; |
| | • | Procurement staff conducts on-site inspection in strict compliance with our quality standards and rejects nonconforming supply; |
| | • | Inspection of specimen—sampling in the process of raw milk collection for inspection at our facilities pursuant to national standards; and |
| | • | Sterilization of all equipment for raw milk collection. |
Milk Powder Production:
| | • | Strict compliance with production process control procedure, HACCP Plan implemented at all plants; |
| | • | All raw materials are subject to prior inspection; |
| | • | Detailed process designed for all parts of the production process including pretreatment, vaporization, drying, powder receiving, cooling and packaging; |
| | • | Maintain hygiene standards for staff, equipment, environment and any other object; and |
| | • | Inspection conducted throughout the production process. |
Packaging, Storage and Transport:
| | • | Establishment and practice of total process management with respect to product identification and traceability; |
| | • | Strict inspection before warehousing of products; |
| | • | Maintain hygiene standards in the course of transport and storage; and |
| | • | Products must be positioned strictly according to their category during transport and storage. |
Since the melamine contamination incident, we have been importing a large quantity of milk powder from Europe and New Zealand. All of our milk powder imports are inspected by China’s import-export inspection and quarantine authorities at landing. In addition, our Qingdao laboratory tests each batch of imported milk powder using the strictest standards for quality assurance.
RESEARCH AND DEVELOPMENT
Our research and development activities focus on new product formulation, new ingredient development, creation of new methods to incorporate certain nutrients in our products, and improvement in product tastes and ingredient shelf stabilities. We engage in regular product refinement and new product development for our dairy-based formula products, as well as other forms of foods and nutritional supplements.
We utilize our research and development facilities to engage in the development of trial products that improve our technical capabilities and serve to promote our brand image. We also engage third-party research institutions to research and develop such trial products for us.
We seek to leverage our research and development resources in order to extend our new product pipeline. We believe we can accomplish this goal with new formulations and product concepts in dairy-based formula products as well as other nutritional food products and supplements.
In addition to new formulations and products, we have also developed a variety of delivery systems such as orally delivered supplements in a pill format and single use packages which can provide the formula to the end-user in convenient single packets instead of bulky large canisters.
We also plan to open a new research and development facility in Beijing, which we expect to be operational by late 2010.
During the fiscal years ended March 31, 2009, 2008 and 2007, we spent approximately 0.3% of net sales per year on research and development.
COMPETITION
The infant formula industry in China is highly competitive. We generally compete with both multinational and domestic Chinese infant formula producers. Competitive factors include brand recognition, perceived quality, advertising, formulation, packaging and price. Many of our competitors have significant market share in the markets we compete in. Our principal competitors can be classified generally into the following two groups:
Multinational Producers
| | • | Abbot Laboratories’ Ross Products Division, a U.S. producer and distributor of infant formulas marketed under the brand names of Similac and Enfalac family of formulas; |
| | • | Mead Johnson Nutrition Co., or Mead Johnson, formerly a Bristol-Myers Squibb Company Division, a U.S. producer and distributor of the Enfamil family of formulas; |
| | • | Groupe Danone SA’s Numico division, or Numico, a Dutch producer of baby foods, which sells and markets infant formula products in China under the Dumex brand; |
| | • | Nestlé Suisse SA, or Nestlé, a Swiss producer and distributor of starter and follow-up formulas, milk, cereals, oral supplements and performance foods marketed under Nestlé brands such as Carnation; and |
| | • | Wyeth, a U.S. producer and distributor of infant formula sold under private label brands. |
Domestic Producers
| | • | Inner Mongolia Yili Industrial Group Co., Ltd., or Yili, a PRC producer and distributor of liquid and powdered milk under their Yili brand; |
| | • | Beingmate Group Company Limited, or Beingmate, a PRC producer and distributor of infant formula products under their Beingmate brand; |
| | • | Guangdong Yashili Group Co., Ltd., or Yashili, a PRC consumer brand marketer which sells a line of infant formula products under their Yashili brand; and |
| | • | American Dairy, Inc., a PRC producer and distributor of milk formula products under their Feihe brand. |
According to data collected by the PRC National Commercial Information Center, or CIC, an entity affiliated with the PRC General Chamber of Commerce responsible for collecting retail sales data, the top ten brands accounted for 78.4% of total infant formulas sold in China in 2008. Among the top ten participants in the infant formula market, we believe top domestic companies such as us are competing increasingly more effectively with multinational producers.
INTELLECTUAL PROPERTY
All of our product formulations have been developed in-house and are proprietary. We have not registered or applied for protections in China for most of our intellectual property or proprietary technologies relating to the formulations of our powdered infant formula. See “Item 1A. Risk factors—Risks Related to Our Business—Failure to adequately protect our intellectual property rights may undermine our competitive position, and litigation to protect our intellectual property rights may be costly.” Although we believe that, as of today, patents and copyrights have not been essential to maintaining our competitive market position, we intend to assess appropriate occasions in the future for seeking patent and copyright protections for those aspects of our business that provide significant competitive advantages.
We have 69 registered trademarks in China, one registered trademark in Hong Kong, and one registered trademark in the United States. Additionally, we have 143 trademark applications pending approval in China.
We rely on trade secret protection and confidentiality agreements to protect our proprietary information and know-how. Our management and each of our research and development personnel have entered into a standard annual employment contract, which includes a confidentiality clause and a clause acknowledging that all inventions, designs, trade secrets, works of authorship, developments and other processes generated by them on our behalf are our property, and assigning to us any ownership rights that they may claim in those works. Each of our third-party producers enters into a standard form confidentiality agreement. Despite our precautions, it may be possible for third parties also to obtain and use, without our consent, intellectual property that we own or are licensed to use. Unauthorized use of our intellectual property by third parties, and the expenses incurred in protecting our intellectual property rights, may adversely affect our business. See “Item 1A. Risk factors—Risks Related to Our Business—Failure to adequately protect our intellectual property rights may undermine our competitive position, and litigation to protect our intellectual property rights may be costly.”
ENVIRONMENTAL MATTERS
Our manufacturing facilities are subject to various pollution control regulations with respect to noise, water and air pollution and the disposal of waste and hazardous materials. We are also subject to periodic inspections by local environmental protection authorities. Our operating subsidiaries have received certifications from the relevant PRC government agencies in charge of environmental protection indicating that their business operations are in material compliance with the relevant PRC environmental laws and regulations. We are not currently subject to any pending actions alleging any violations of applicable PRC environmental laws.
OUR EMPLOYEES
As of March 31, 2009, we employed approximately 6,200 employees in all of our facilities, with approximately 600 management staff and research and development employees, approximately 2,200 production employees, approximately 3,400 sales and marketing employees, including approximately 300 database sales and marketing specialists, approximately 700 employees working on educational programs in healthcare facilities, and approximately 50 customer relations employees. Our employees are not represented by a labor organization or covered by a collective bargaining agreement. We have not experienced any work stoppages.
We offer our employees both a base salary and a profit sharing program composed of performance bonuses and rewards for exceptional performance. As required by PRC regulations, we participate in various employee benefit plans that are organized by municipal and provincial governments, including pension, work-related injury benefits, maternity insurance, medical and unemployment benefit plans. We are required under PRC law to make contributions to the employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time. Members of the retirement plan are entitled to a pension equal to a fixed proportion of the salary prevailing at the member’s retirement date.
REGULATION
The food industry, of which nutritional and infant formula products form a part, is subject to extensive regulations in China. This section summarizes the most significant PRC regulations governing our business in China.
Food Hygiene and Safety Laws and Regulations
As a producer of nutritional products, and particularly dairy-based infant formula products, in China, we are subject to a number of PRC laws and regulations governing the manufacturing (including composition of ingredients), labeling, packaging, safety and hygiene of food products:
| • | the PRC Product Quality Law; |
| • | the PRC Food Hygiene Law; |
| • | the Access Conditions for Dairy Products Processing Industry; |
| • | the Implementation Rules on the Administration and Supervision of Quality and Safety in Food Producing and Processing Enterprises; |
| • | the Regulation on the Administration of Production Licenses for Industrial Products; |
| • | the General Standards for the Labeling of Prepackaged Foods; |
| • | the Implementation Measures on Examination of Dairy Product Production Permits; |
| • | the Standardization Law; |
| • | the Raw Milk Collection Standard; |
| • | the Whole Milk Powder, Skimmed Milk Powder, Sweetened Whole Milk Powder and Flavored Milk Powder Standards; and |
| • | the General Technical Requirements for Infant Formula Powder and Supplementary Cereal for Infants and Children. |
These laws and regulations set out safety and hygiene standards and requirements for various aspects of food production, such as the use of additives, production, packaging, handling, labeling and storage, as well as facilities and equipment. Failure to comply with these laws and regulations may result in confiscation of our products and proceeds from the sales of non-compliant products, destruction of our products and inventory, fines, suspension of production and operation, product recalls, revocation of licenses, and, in extreme cases, criminal liability.
As a result of the melamine contamination incident, the PRC government authorities have conducted several dairy industry inspections. In addition to the initial 22 companies implicated in the incident, these subsequent government inspections have identified other companies with unacceptable contamination in their products. On October 7, 2008, the State General Administration of Quality Supervision, Inspection and Quarantine (“AQSIQ”) issued a national standard on the detection of melamine in raw milk and dairy based products. On October 9, 2008, the State Council promulgated with immediate effect a Regulation for the Quality and Safety Supervision of Dairy Based Products, which, among other things, imposes more stringent requirements for inspection, production, packaging, labeling and product recall on dairy product producers. This regulation also established a “Black-List” system to ensure that illegal business operators in the dairy production chain are timely disclosed and severely punished.
Environmental Regulations
We are subject to various governmental regulations related to environmental protection. The major environmental regulations applicable to us include:
| • | the Environmental Protection Law of the PRC; |
| • | the Law of PRC on the Prevention and Control of Water Pollution; |
| • | Implementation Rules of the Law of PRC on the Prevention and Control of Water Pollution; |
| • | the Law of PRC on the Prevention and Control of Air Pollution; |
| • | Implementation Rules of the Law of PRC on the Prevention and Control of Air Pollution; |
| • | the Law of PRC on the Prevention and Control of Solid Waste Pollution; and |
| • | the Law of PRC on the Prevention and Control of Noise Pollution. |
We are periodically inspected by local environmental protection authorities. Our operating subsidiaries have received certifications from the relevant PRC government agencies in charge of environmental protection indicating that their business operations are in compliance with the relevant PRC environmental laws and regulations.
Dairy Industry Access Conditions and Policies
In March 2008, the PRC National Development and Reform Commission, or the NDRC, promulgated the Access Conditions for Dairy Products Processing Industry, or the Access Conditions. The Access Conditions set forth the conditions an entity must satisfy in order to engage, or continue to engage, in the dairy products processing business, including technique and equipment, product quality, energy and water consumption, sanitation and environmental protection, as well as production safety. Any new or continuing dairy products processing projects or enterprises will be required to meet all the conditions and requirements set forth in the Access Conditions. For projects or enterprises that already commenced operations before the promulgation of the Access Conditions, improvements or rectification actions may need to be taken in order to have such projects or enterprises meet the conditions within two years of the effective date of the Access Conditions on April 1, 2010.
The Access Conditions also set forth some requirements relating to the location, processing capacity and raw milk source for any new or continuing dairy products processing project or enterprise. Any new or continuing dairy products processing projects or enterprises that fail to meet the requirements will not be able to procure land, license, permits, loan facility and electricity necessary for the processing of dairy products, and those projects or enterprises already in operation before the promulgation of the Access Conditions will be deregistered and ordered to shut down if they fail to meet the conditions within a two-year rectification period. We believe that all of our existing entities and facilities meet the requirements under the Access Conditions. See “Item 1A. Risk Factor—Risks Associated with Doing Business in China—Changes in the regulatory environment for dairy and infant nutrition products in China could negatively impact our business.”
In May 2008, the NDRC issued the Dairy Industry Policies, or the Policies. According to the PRC government, the Policies are the first set of comprehensive government policies on the PRC dairy industry, covering a broad range of matters such as industry planning, closure of inefficient capacity, milk supply, quality control and product safety, environment protection and promotion of milk consumption. Moreover, the Policies provide conditions that new entrants to the dairy industry must meet in addition to the conditions set forth in the Access Conditions.
FINANCIAL INFORMATION ABOUT SEGMENTS AND GEOGRAPHIC AREAS
Historically we reported our results as a single reporting segment in the financial statements since operating segments such as the nutritional ingredients and supplements segment was under construction with minor operating expenses and had not met the quantitative threshold as described under SFAS 131 "Disclosure about Segments of an Enterprise and Related Information". In this fiscal year, we expanded the number of reportable segments from one to three segments, which are powdered formula, baby food and nutritional ingredients and supplements, in order to better reflect the manner in which management analyzes the Company’s performance. Please refer to Note 17 to the Consolidated Financial Statements for further discussion about segments and geographic areas.
AVAILABLE INFORMATION
Our Internet website address is www.synutra.com. We make available at this address, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the United States Securities and Exchange Commission, or SEC. Information available on our website is not incorporated by reference in and is not deemed a part of this Form 10-K.
Because of the following factors, as well as other factors affecting our financial condition and operating results, past financial performance should not be considered to be a reliable indicator of future performance, and investors should not use historical trends to anticipate results or trends in future periods.
You should carefully consider the following risks and other information in this Form 10-K before making an investment decision with respect to our common stock. The following risks and uncertainties could materially adversely affect our business, results of operations and financial condition. The risks described below are not the only ones we face. Additional risks that we are not presently aware of or that we currently believe are immaterial may also impair our business operations.
RISKS RELATED TO OUR BUSINESS
We are highly dependent upon consumers’ perception of the safety and quality of our products. Any ill effects, product liability claims, recalls, adverse publicity or negative public perception regarding particular ingredients or products or our industry in general, could harm our reputation and damage our brand, and adversely affect our results of operations.
We sell products for human consumption, which involves risks such as product contamination, spoilage and tampering. We may be subject to liability if the consumption of any of our products causes injury, illness or death. Adverse publicity or negative public perception regarding particular ingredients, our products, our actions relating to our products, or our industry in general could result in a substantial drop in demand for our products. This negative public perception may include publicity regarding the safety or quality of particular ingredients or products in general, of other companies or of our products or ingredients specifically. Negative public perception may also arise from regulatory investigations or product liability claims, regardless of whether those investigations involve us or whether any product liability claim is successful against us.
On September 16, 2008, China’s Administration of Quality Supervision, Inspection and Quarantine, or China AQSIQ, announced its finding that the formula products of 22 Chinese formula producers, including certain lots of our U-Smart products, were contaminated by melamine, a substance not approved for use in food and linked to the recent illness and deaths of infants and children in China. To date, there have been six reported deaths and approximately 300,000 children have suffered kidney-related illnesses due to the contaminated infant formula of one of our competitors. This contamination incident has resulted in significant negative publicity for the entire domestic dairy and formula industries in China and demand for domestically-produced dairy and formula products, including our products, has declined significantly. We have recalled our affected U-Smart products as well as all other products produced at the same facilities in the Hebei and Inner Mongolia regions of China, where we believe the contaminated milk supplies originated. We also suspended production at our facilities in Qingdao, Hebei and Inner Mongolia for two weeks pending government and internal investigations. The initial estimated cost of this action was $101.5 million which was recognized as a charge to cost of sales, selling and distribution expenses and general and administrative expenses in our consolidated statement of income for the fiscal year ended March 31, 2009. These costs are still subject to finalization, and we cannot assure you that this will be the total cost for the recall or that the total cost will not significantly exceed our estimates.
Although we have not confirmed any cases of kidney-related or other illnesses caused by our products, we cannot assure you that such cases will not surface in the future. The Chinese government has provided medical screening, treatment, and care for consumers affected by melamine contamination in infant formula products. We have contributed a net amount of $2.3 million to a compensation fund set up by China Dairy Industry Association to settle existing and potential claims arising in China from families of infants affected by melamine contamination. We cannot assure you that the Chinese government will not seek further reimbursement from dairy and formula product manufacturers, including us.
We believe the contamination incident has already negatively impacted our brand and reputation in China. It has also affected investor confidence in us as reflected by the significant decrease in our stock price since September 16, 2008. We cannot predict the long term effect this recall and the negative publicity associated with the contamination incident will have on our reputation among our customers, consumers and investors. Our results of operations and financial position would be severely impacted if we have failed to accurately estimate the costs of this product recall or if our customers and consumers cease to purchase our products as a result of the contamination incident.
In the past, there have also been occurrences of counterfeiting and imitation of products in China that have been widely publicized. We cannot guarantee that contamination or counterfeiting or imitation of our or similar products will not occur in the future or that we will be able to detect it and deal with it effectively. Any occurrence of contamination or counterfeiting or imitation could negatively impact our corporate and brand image or consumers’ perception of our products or similar nutritional products generally, particularly if the counterfeit or imitation products cause injury or death to consumers. For example, in April 2004, sales of counterfeit and substandard infant formula in Anhui, China caused the deaths of 13 infants as well as harming many others. Although this incident did not involve the counterfeiting of our products, it caused significant negative publicity for the entire infant formula industry in China. The mere publication of information asserting that infant formula ingredients or products may be harmful could have a material adverse effect on us, regardless of whether these reports are scientifically supported or concern our products or the raw materials used in our products.
In addition, we believe that the recent melamine incident and any other adverse news related to formula products in China will also result in increased regulatory scrutiny of our industry, which may result in increased costs and reduce our margins and profitability. The government has enhanced its regulations on the industry aimed to ensure the safety and quality of dairy products, including but not limited to compulsory batch by batch inspection. This is likely to increase our operating costs and capital expenditure.
If we fail to obtain raw materials in the quantity and the quality we need, and at commercially acceptable prices, our results of operations, financial condition and business prospects would be materially and adversely affected.
Our business requires certain key raw materials, such as raw milk, milk powder and whey protein powder. We may experience a shortage in the supply of certain raw materials in the future, which could materially and adversely affect our production and results of operations. We do not have guaranteed supply contracts with any of our raw material suppliers, and some of our suppliers may, without notice or penalty, terminate their relationship with us at any time. We also rely on a small number of suppliers for some of our raw materials, such as whey protein powder and imported milk powder. After the melamine contamination incident, we have started to import milk powder from Europe and New Zealand for our U-Smart, Super and Helanruniu, or Holsteina products as consumers have less confidence in domestically-produced milk powder. If any supplier is unwilling or unable to provide us with high quality raw materials in required quantities and at acceptable prices, we may be unable to find alternative sources or at commercially acceptable prices, on satisfactory terms, in a timely manner, or at all. Our inability to find or develop alternative sources could result in delays or reductions in production, product shipments or a reduction in our profit margins. Moreover, these suppliers may delay material shipments or supply us with inferior quality raw materials that may adversely impact the timely delivery or the quality of our products. If any of these events were to occur, our product quality, competitive position, reputation and business could suffer.
In addition, some of the raw materials used in our business are imported, such as whey protein powder and milk powder. Our imported raw materials are subject to various PRC governmental permit requirements, approval procedures and import duties, and may also, from time to time, be subject to export controls and other legal restrictions imposed by foreign countries. Should the PRC government refuse to issue the necessary permits or approvals to us or our suppliers, or take any administrative actions to limit imports of certain raw materials, or if we or our suppliers fail to pay any required import duties, or if governmental agencies or laws of foreign countries prevent the timely export of certain raw materials we require to China, our ability to produce and sell our products in China could be materially and adversely affected. In addition, import duties increase the cost of our products and may make them less competitive.
Finally, certain suppliers of raw materials within our supply chain may intentionally or inadvertently contaminate our raw material supplies or provide us with substandard raw material supplies that adversely impact the quality of our products exposing our customers to health risks and damaging our reputation, brand and financial condition. For a more detailed description of this risk, and in particular the impact of the recent melamine contamination incident in China, see Part 1 - Item 1A. Risk Factors — We are highly dependent upon consumers’ perception of the safety and quality of our products. Any ill effects, product liability claims, recalls, adverse publicity or negative public perception regarding particular ingredients or products or our industry in general, could harm our reputation and damage our brand, and adversely affect our results of operations.
We depend on raw milk to produce our dairy-based nutritional products. Any interruption in our supply of raw milk could materially and adversely affect our results of operations, financial condition and business prospects.
We purchase most of our raw milk from individual dairy farmers and cooperatives without long-term contractual arrangements. Our raw milk supply is limited by the ability of the individual dairy farmers and cooperatives to provide raw milk in the amount and quality to meet our requirements. Raw milk production is, in turn, influenced by numerous factors beyond our control such as: (1) seasonal factors, with dairy cows generally producing more milk in temperate weather as opposed to cold or hot weather and extended unseasonably cold or hot weather potentially leading to lower than expected production; (2) environmental factors, with the volume and quality of milk produced by dairy cows closely linked to the quality of the nourishment provided by the environment around them; and (3) impact of governmental agricultural and environmental policy, with government grants, subsidies, provision of land, technical assistance and other agricultural and environmental policies having a direct effect on the viability of individual dairy farmers and dairy cooperatives, and the numbers of dairy cows and quantities of milk they are able to produce. Individual dairy farmers bring their cattle to collection stations owned by us or third parties. In order to meet our projected needs, we expect that we will need to continue to increase the number of milk collection centers from which we source our raw milk. We cannot assure you that we will be able to establish relationships with additional milk collection centers or that there will be sufficient supplies of raw milk from individual dairy farmers and cooperatives to be provided to any milk collection centers. Any interruption in our supply of raw milk could materially and adversely affect our results of operations, financial condition and business prospects.
Our results of operations may be affected by fluctuations in availability and price of raw materials.
The raw materials we use are subject to price fluctuations due to various factors beyond our control, including increasing market demand, inflation, severe climatic and environmental conditions, commodity price fluctuations, currency fluctuations, changes in governmental and agricultural regulations and programs and other factors. We also expect that our raw material prices will continue to fluctuate and be affected by inflation in the future. Changes to our raw materials prices may result in increases in production and packaging costs, and we may be unable to raise the prices of our products to offset these increased costs in the short-term or at all. As a result, our results of operations may be materially and adversely affected.
We might face inventory write-down if milk powder inventory continues to increase and milk powder prices continue to decline.
Since the melamine contamination incident, we have been using imported milk powder for the production of our Super, U-Smart, and Helanruniu series of products. In the meantime, our domestic production facilities continued to purchase raw milk locally to produce milk powder for commercial resale and for our Mingshan series of products. Due to the decline in the consumption of dairy based products in the PRC as a result of the melamine contamination incident and the significant increase in milk powder imports, there has been a nationwide inventory build up of domestically produced milk powder in the PRC. According to the Dairy Industry Association of China, as of March 31, 2009, surplus milk powder inventory in the PRC was estimated at 300,000 tons and is expected to continue to rise. Such inventory build up has caused a significant decline in milk powder prices. If milk powder inventory continues to rise and the milk powder prices continue to fall, we might face significant inventory write-down which will adversely affect our financial results.
Any major outbreak of illness or disease relating to cows in China and in the regions in which we import milk powder could lead to significant shortfalls in the supply of our raw milk and milk powder, and could result in consumers avoiding dairy products, which could result in substantial declines in our sales and possibly substantial losses.
A major outbreak of any illness or disease in cows in China and globally could lead to a serious loss of consumer confidence in, and demand for, dairy products. A major outbreak of mad cow disease (bovine spongiform encephalopathy), bovine tuberculosis, or bovine TB, or other serious disease in the principal regions supplying our raw milk and milk powder could lead to significant shortfalls in the supply of our raw milk and milk powder. Limited cases of bovine TB have occurred in several parts of China in the past. Furthermore, adverse publicity about these types of concerns, whether or not valid, may discourage consumers from buying dairy products or cause production and delivery disruptions. If consumers generally were to avoid our products, our sales would decline substantially and we could suffer substantial losses.
We may experience problems with product quality or product performance, or the perception of such problems, which could adversely affect our reputation or result in a decrease in customers and revenue, unexpected expenses and loss of market share.
Our operating results depend, in part, on our ability to deliver high quality products on a timely and cost-effective manner. Our quality control and food safety management systems are complex. For example, there are over 1,100 quality control points throughout the whole production process. If the quality of any of our products deteriorated, it could result in delays in shipments, cancellations of orders or customer returns and complaints, loss of goodwill, and harm to our brand and reputation. In addition, in the aftermath of the melamine incident, we purchase a significant portion of our milk powder from overseas suppliers, mainly in Europe and New Zealand for our U-Smart, Super and Helanruniu, or Holsteina products. We may be unable to exercise the same degree of quality control over these overseas suppliers as we can over our own facilities. Any quality problems associated with the milk powder produced by these suppliers would also affect our products’ quality and lead to negative publicity against us, adversely affecting our reputation and brand, and causing a decrease in sales of our products and a loss of market share. For example, the recent melamine contamination incident in China has resulted in certain of our products being contaminated, impacting our brand and reputation.
Product liability claims against us could result in adverse publicity and potentially significant monetary damages.
As with other infant formula producers, we are also exposed to risks associated with product liability claims if the consumption of infant formula products we sell results in injury or death. We cannot predict what impact such product liability claims or resulting negative publicity would have on our business or on our brand image. The successful assertion of product liability claims against us could result in potentially significant monetary damages, diversion of management resources and require us to make significant payments and incur substantial legal expenses. We do not have product liability insurance and have not made provisions for potential product liability claims. Therefore, we may not have adequate resources to satisfy a judgment if a successful claim is brought against us. Even if a product liability claim is not successfully pursued to judgment by a claimant, we may still incur substantial legal expenses defending against such a claim and our brand image and reputation would suffer. Finally, serious product quality concerns could result in governmental action against us, which, among other things, could result in the suspension of production or distribution of our products, loss of certain licenses, or other governmental penalties.
For example, the recent melamine contamination incident in China has resulted in certain of our products being contaminated. As a result, lawsuits have been filed against us in both China and the U.S. by Chinese families alleged to have been affected by melamine contamination, seeking compensatory and punitive damages. See “Item 3 - Legal Proceedings.” We may incur significant legal expenses and be subject to significant monetary damages in connection with such claims, which may adversely affect our results of operations and further harm our reputation and damage our brand. Further, we cannot assure you that we will not become subject to future product liability claims in connection with the melamine contamination incident. See “Risk Factors — We are highly dependent upon consumers’ perception of the safety and quality of our products. Any ill effects, product liability claims, recalls, adverse publicity or negative public perception regarding particular ingredients or products or our industry in general could harm our reputation and damage our brand and adversely affect our results of operations.”
Our sales, results of operations, brand image and reputation could be materially and adversely affected if we fail to efficiently manage our operations without interruption, or fail to ensure that our products are delivered on time.
Our business requires successful coordination of several sequential and complex processes, the disruption of any of which could interrupt our operations and materially and adversely affect our relationships with our distributors, sub-distributors and end-customers, our brand name and reputation, and our financial performance. Our operations involve the coordination of raw material sourcing from third parties, internal production processes and external distribution processes. We may face difficulties in coordinating the various aspects of our production processes, resulting in downtime and delays.
In addition, we may encounter interruptions in our production processes due to a catastrophic loss or events beyond our control, such as fires, explosions, labor disturbances, earthquakes or other natural disasters. If there is a stoppage in production at any of our facilities, even if only temporary, or delays in deliveries to our customers, our business and reputation could be severely affected. Along with many other producers of dairy and consumer products in China, we generally rely on third-party transportation operators and distributors for the delivery of our products. Delivery may be disrupted for various reasons, many of which are beyond our control, including natural disasters, weather conditions or social unrest and strikes, which could lead to delayed or lost deliveries. In addition, transportation and related infrastructure conditions are often generally under-developed in some of the regions where we sell our products. We currently do not have business interruption insurance to offset these potential losses, delays and risks, so a material interruption of our business operations could severely damage our business.
We rely primarily on third-party distributors and cannot assure you that their marketing and distribution of our products will be effective or will not harm our brand and reputation. Moreover, if we fail to timely identify and appoint additional or replacement distributors as needed, or are unable to successfully manage our distribution network, our operating results could suffer.
We do not sell our products directly to our end customers. Instead, we primarily rely on third-party distributors and sub-distributors for the sale and distribution of our products. We sell our products through an extensive nationwide sales and distribution network covering 29 provinces and provincial-level municipalities in China. As of March 31, 2009, this network comprised over 480 distributors and over 800 sub-distributors who sell our products in over 65,000 retail outlets. Our distributors normally have exclusive distribution rights in their respective regions and cities, and are also responsible for developing the sub-distributors located in their own regions and cities. In addition, our distributors are not required to exclusively distribute our products. We typically do not enter into long-term agreements with distributors and have no control over their everyday business activities. Consequently, our distributors may engage in activities that are prohibited under our arrangements with them, that violate PRC laws and regulations governing the dairy industry or other PRC laws and regulations generally, or that are otherwise harmful to our business or our reputation. Due to our dependence on distributors for the sale and distribution of our products to retail outlets, any one of the following events could cause material fluctuations or declines in our revenue and have a material adverse effect on our financial condition and results of operations:
| • | reduction, delay or cancellation of orders from one or more of our distributors; |
| • | selection or increased sales by our distributors of our competitors’ products; and |
| • | our failure to timely identify and appoint additional or replacement distributors upon the loss of one or more of our distributors. |
The competition for distributors is intense in our industry in China and many of our competitors are expanding their distribution networks in China. We may not be able to compete successfully against the larger and better-funded sales and marketing operations of some of our current or future competitors, especially if these competitors provide more favorable arrangements for distributors. As a result, we may lose some of our distributors to our competitors, which may cause us to lose some or all of our favorable arrangements with such distributors and may even result in the termination of our relationships with some of our distributors. While we do not believe we are substantially dependent upon any individual distributor, finding replacement distributors could be time-consuming and any resulting delay may be disruptive and costly to our business. In addition, we may not be able to successfully manage our distributors and the cost of any consolidation or further expansion of our distribution network may exceed the revenue generated from these efforts. The occurrence of any of these factors could result in a significant decrease in the sales volume of our products and therefore materially harm our financial condition and results of operations.
We maintain inventories of raw materials and finished products, and our inventories may spoil.
Most of our finished products have an average shelf life of 18 to 24 months. Our raw materials, excluding raw milk, have an average shelf life of 12 months. Our inventory levels are based, in part, on our expectations regarding future sales. While we do not currently maintain large inventory levels for long periods, we may in future periods experience inventory buildup if our sales slow for any reason. Any significant shortfall in sales may result in higher inventory levels of raw materials and finished products than we require, thereby increasing our risk of inventory spoilage and corresponding inventory write-downs and write-offs, which may materially and adversely affect our results of operations.
Our results of operations and business prospects may be impaired by changing consumer preferences if we do not develop and offer products to meet changing preferences.
Consumer preferences evolve over time and the success of our products depends on our ability to identify the tastes and nutritional needs of our customers and to offer products that appeal to their preferences. We introduce new products and improved products from time to time and incur significant development and marketing costs. If our products fail to meet consumer preferences, then our strategy to grow sales and profits with new products will be less successful.
More mothers may breastfeed their babies rather than use our products, resulting in reduced demand for our products and adversely affecting our revenues.
Our results of operations are affected by the number of mothers who choose to use our products rather than breastfeeding their babies. Much publicly available data suggests that breastfeeding has many health benefits for the baby that cannot be replicated by dairy-based infant formula products. Additionally, popular literature, cultural pressure, government policies and medical advice in China generally promote the benefits of breastfeeding. For example, on August 1, 2007, China’s Ministry of Health issued an Infant Feeding Strategy which promoted breastfeeding and requested all local relevant departments to publicize the benefits of breastfeeding through radio broadcasting, television and newspapers during World Breastfeeding Week, which took place in early August 2007. Thus, to the extent that private, public and government sources increasingly promote the benefits of breastfeeding, there could be a reduced demand for our products and our revenues could be adversely affected.
In addition, we believe the recent melamine contamination incident has deteriorated customer confidence in the safety and quality of infant formula products and the number of mothers (and future mothers) who choose to breastfeed their babies may significantly increase. The overall market demand for infant formula products may slow or decline and any reduced demand for our products will negatively impact our revenues and growth prospects.
Failure to execute our expansion plan could adversely affect our financial condition and results of operations.
We have plans to increase our annual production capacity to meet an expected increase in demand for our products. Our decision to increase our production capacity is based in part on our projections of increases in our sales volume and growth in the size of the infant formula product market in China. If actual customer demand does not meet our projections, we will likely suffer overcapacity problems and have idle capacity, which may materially and adversely affect our financial condition and results of operations. Our future success depends on our ability to expand our business to address expected growth in demand for our current and future products. Our ability to add production capacity and increase output is subject to significant risks and uncertainties, including:
| • | the availability of additional funding to expand our production capacity, build new processing and packaging facilities, make additional investments in our subsidiaries, acquire additional businesses or production facilities, purchase additional fixed assets and purchase raw materials on favorable terms or at all; |
| • | delays and cost overruns as a result of a number of factors, many of which may be beyond our control, such as problems with equipment vendors and suppliers of raw materials; |
| • | failure to maintain high quality control standards; |
| • | global or local shortage of raw materials, such as raw milk or whey protein powder; |
| • | our inability to obtain, or delays in obtaining, required approvals by relevant government authorities; |
| • | diversion of significant management attention and other resources; and |
| • | failure to execute our expansion plan effectively. |
As our business grows, we will need to implement a variety of new and upgraded operational and financial systems, procedures and controls, including improvements to our accounting and other internal management systems by dedicating additional resources to our reporting and accounting functions, and improvements to our record keeping and contract tracking system. We will need to respond to competitive market conditions and continue to enhance existing products and develop new products, and retain existing customers and attract new customers. We will also need to recruit more personnel and train and manage our growing employee base. Furthermore, we will need to maintain and expand our relationships with our current and future customers, suppliers, distributors and other third parties, and there is no guarantee that we will succeed.
If we encounter any of the risks described above, or are otherwise unable to establish or successfully operate additional production capacity or to increase production output, we may be unable to grow our business and revenues, reduce our operating costs, maintain our competitiveness or improve our profitability, and our business, financial condition, results of operations and prospects may be adversely affected.
Our planned expansion into many of China’s major urban centers may be costly, time-consuming and difficult. If we do not successfully expand into such markets, our results of operations and prospects would be materially and adversely affected.
Our future success significantly depends upon our ability to successfully expand into many of China’s major urban centers. To further promote our brand and generate demand for our products in such markets, we intend to significantly increase spending on marketing and promotion. We may be unable to attract a sufficient number of distributors with the experience and ability to penetrate these markets, and our selected distributors may not be suitable for selling our products in these markets for other reasons. We may also fail to attract new customers in such markets who may have less familiarity with our brand and products. Furthermore, we may fail to anticipate and address competitive conditions in these new markets that are different from those in our existing primary markets. These competitive conditions may make it difficult or impossible for us to effectively operate in these markets. If our expansion efforts in existing and new markets are unsuccessful, our results of operations and prospects would be materially and adversely affected.
Part of our strategy involves the development of new products and new business segments, and if we fail to timely develop new products or we incorrectly gauge the potential market for new products, our financial results would be adversely affected.
We plan to utilize our in-house research and development capabilities to develop new products that could become new sources of revenue for us in the future and help us to diversify our revenue base. We have also entered into new business segments such as the prepared baby food and nutritional snacks segment, and the nutritional ingredients and supplements segment. Our future research and development efforts will focus on expanding our product offerings beyond dairy-based nutritional products and into new products segments such as nutritional snacks and, prepared baby food. If we fail to timely develop these and other new products or if we incorrectly gauge market demand for such new products, we may not be able to grow our sales revenue at expected growth rates and may incur expenses and capital expenditure costs relating to the development of new products that are not offset by the sales they generate.
We operate in a competitive environment, which may lead to declining revenue growth or other circumstances that would negatively affect our results of operations.
The market for pediatric nutritional products is competitive, and we believe that competition in this market will continue to intensify. We believe that the principal competitive factors in our markets are brand recognition, perceived quality, advertising, formulation, packaging, and price. We face significant competition from a number of competitors, including multinational companies, such as Abbot Laboratories’ Ross Products Division, Mead Johnson, Nestle, Numico and Wyeth, and domestic companies, such as Beingmate, Yashili, Feihe and Yili. See “Item 1. Business—Competition”. Many of our competitors have longer operating histories, greater name recognition, significantly larger market shares, access to larger customer bases and significantly greater economies of scale in financial, sales and marketing, production, distribution, technical and other resources than we do. Some of these competitors have used, and we expect will continue to use, more aggressive pricing strategies, greater amounts of incentives and subsidies for distributors, retailers and customers and more advanced processes and technologies. Furthermore, consolidation among industry participants in China may potentially result in stronger domestic competitors that are better able to compete as end-to-end suppliers as well as competitors who are more specialized in particular areas and geographic markets. This could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, as a result of the recent melamine contamination incident, customers have lost confidence in infant formula produced by domestic companies for the time being, which gives multinational infant formula companies an advantage over us.
In order to compete successfully in our markets, we will need to restore customer confidence in our brand and products, develop new products and enhance our product offerings while maintaining price competitiveness. Even if we successfully restore customer confidence, if and to the extent we fail to develop new products that differentiate us from our competitors, we may need to compete largely on price, which may cause our operating margins to decline. Our inability to compete successfully against competitors and pricing pressures could result in lost customers, loss of market share and reduced operating margins, which would adversely impact our results of operations.
If we grant employee stock options and other stock-based compensation in the future, we will be required to recognize stock-based compensation expense, which would adversely affect our results of operations.
As of the date of this Form 10-K, we have not granted any stock-based compensation and thus have not been required to reflect any such expenses in our results of operations. We adopted a stock -based compensation plan in June 2008 and intend to grant certain employees and directors stock-based compensation, which we believe will be important to attract and retain key personnel. We will be required to account for compensation costs for all stock options, including stock options granted to our directors and employees, using the fair value method and recognize the expense in our consolidated statement of operations in accordance with Statement of Financial Accounting Standard No. 123-R, "Share-Based Payment" which may have a material adverse effect on our results of operations.
We have previously experienced certain material weaknesses and deficiencies with our internal control over financial reporting. Among other things, our previous material weaknesses and deficiencies have made it necessary for us to restate certain of our financial statements in the recent past. Any further failure to accurately report our financial results, including any resulting restatement, could result in harm to our business, loss of investor confidence in our financial reporting and a lower trading price of our common stock, or possibly lead to the delisting of our common stock by the Nasdaq Global Select Market.
Due in part to material weaknesses and deficiencies in our internal control over financial reporting, we were required to restate our financial statements for the fiscal years ended March 31, 2006 and 2007, for the year ended December 31, 2004, for the transition period for the three months ended March 31, 2005 and for the fiscal quarters ended June 30, 2006, September 30, 2006 and December 31, 2006. In connection with the review of our financial statements for the fiscal year ended March 31, 2008, our independent registered public accounting firm and our management identified certain material weaknesses and significant deficiencies. A material weakness is defined by the Public Company Accounting Oversight Board, or PCAOB, as a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. A significant deficiency is defined by the PCAOB as a deficiency, or combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of our financial reporting.
Due to the material weaknesses and significant deficiencies in our internal control over financial reporting—as evidenced by the significant number and magnitude of out-of-period adjustments identified during the year-end and period-end closing process and the resulting restatements—we previously concluded in our prior SEC filings that our disclosure controls and procedures and our internal control over financial reporting were not effective at the reasonable assurance level, and, prior to the restatement of certain prior periods, that investors should not rely on our prior financial statements. We have taken steps to remediate our material weaknesses and deficiencies in our internal control over financial reporting, and we believe that the material weakness identified as of March 31, 2008 has been fully remediated, see “Part II. Item 9A. Controls and Procedures.” As a result, our management has concluded that our internal control over financial reporting was effective as of March 31, 2009, and our independent registered public accounting firm has issued an attestation report, which has concluded that our internal control over financial reporting is effective in all material respects. However, there remains one significant deficiency and four deficiencies in our internal control over financial reporting which we are in the process of remediating but which have not been fully remediated, see “Part II, Item 9A. Controls and Procedures.” There can be no assurance that these deficiencies will not contribute to, or cause, possible material weaknesses in the future or, that we will be able to implement effectively new or improved controls or that our management or our independent registered public accounting firm will determine that our disclosure controls and procedures or our internal control over financial reporting will be effective in the future. Moreover, a lack of effective internal control over financial reporting in the future could cause us to fail to provide accurate financial statements or fail to meet our reporting obligations, either of which could cause investors to lose confidence in our reported financial information, and have a negative effect on the trading price of our common stock. Our failure to meeting our reporting obligations in a timely fashion may also lead to the delisting of our common stock by the Nasdaq Global Select Market.
Our success depends to a substantial degree upon our senior management and key personnel, and our business operations may be significantly and negatively affected if we fail to attract and retain highly competent senior management and key personnel.
Our future success depends substantially on the continued services of our key personnel including, particularly, Liang Zhang, our chairman and chief executive officer. We rely substantially on their experience in the dairy and nutritional products industry, similar business operations and sales and marketing and on their relationships and ability to work with our suppliers and distributors and other customers. If we lose the services of one or more of these key personnel, we may not be able to replace them readily, if at all, with suitable or qualified candidates, and may incur additional expenses to recruit and retain new officers and key personnel, which could severely disrupt our business and growth. We do not maintain key-man life insurance for any of our key personnel.
In addition, if any of these key personnel joins a competitor or forms a competing company, we may lose some of our distributors. We have entered into employment agreements with each of these key personnel, which contain confidentiality and non-competition provisions. Moreover, if any disputes were to arise between these key personnel and us, it is not clear, in light of uncertainties associated with the PRC legal system, what the court decisions would be and the extent to which these court decisions could be enforced in China, where most of these key personnel reside and hold some of their assets. See “Item 1A. Risks Associated with Doing Business in China—Uncertainties with respect to the PRC legal system could limit the legal protections available to you and us.” Furthermore, as we expect to continue to expand our operations and develop new products, we will need to continue attracting and retaining experienced management and key research and development personnel.
Competition for experienced management and research and development personnel in China is intense, and the availability of experienced, suitable and qualified candidates is limited. Competition for these individuals may require us to pay higher compensation and other benefits in order to attract and retain them, which could have a material adverse effect on our financial condition and results of operations. We may also be unable to attract or retain the personnel necessary to achieve our business objectives, and any failure in this regard could severely disrupt our business and growth.
Liang Zhang’s association with other businesses could impede his ability to devote sufficient time to our business and could present potential conflicts of interest.
Liang Zhang, our founder, chairman, chief executive officer and principal beneficial stockholder, controls several other companies, including Honnete, a supplier of whey protein powder in China. Liang Zhang devotes most of his time to our affairs and the remainder of his time to the affairs of the other companies. Liang Zhang’s decision-making responsibilities for these other companies are similar in the areas of public relations, risk management and strategic planning. As a result, conflicts of interest may arise from time to time and we cannot assure you that they will be resolved in our favor. Additionally, even though Liang Zhang devotes most of his time to our affairs and is further accountable to us and our stockholders as a fiduciary, which requires that he exercise good faith and due care in handling our affairs, we cannot assure you this will always be the case and his existing responsibilities to other businesses and entities may limit the amount of time he can spend on our affairs.
Our chairman and chief executive officer, Liang Zhang, beneficially owns a substantial amount of our common stock and will have significant influence over our corporate affairs.
Our founder, chairman and chief executive officer, Liang Zhang, beneficially owns approximately 66.67% of our outstanding common stock through a company owned by his wife as of March 31, 2009. Accordingly, Liang Zhang will be able to direct the outcome of matters requiring approval of the stockholders, including the election of our directors and other corporate actions such as:
| • | our merger with or into another company; |
| • | a sale of substantially all of our assets; and |
| • | amendments to our certificate of incorporation. |
The decisions of Liang Zhang may conflict with our interests or the interests of our other stockholders.
We may not succeed in identifying suitable acquisition targets, which could adversely affect our ability to expand our operations and service offerings and enhance our competitiveness.
We have pursued and may in the future pursue strategic acquisition opportunities to increase our scale and geographic presence and expand the number of our product offerings. However, we may not be able to identify suitable acquisition or investment candidates, or, even if we do identify suitable candidates, we may not be able to complete those transactions on terms commercially favorable to us or at all, which could adversely affect our competitiveness and our growth prospects.
If we acquire other companies in the future, we could face the following risks:
| • | difficulty in assimilating the target company’s personnel, operations, products, services and technology into our operations; |
| • | the presence of unforeseen or unrecorded liabilities; |
| • | entry into unfamiliar markets; |
| • | inability to generate sufficient revenues to offset acquisition costs; and |
| • | tax and accounting issues. |
Many recently joined employees may decide not to work with us or to leave shortly after joining our company. These difficulties could disrupt our ongoing business, distract our management and current employees and increase our expenses, including write-offs or impairment charges. Acquired companies also may not perform to our expectations for various reasons, including the loss of key personnel, key distributors, key suppliers or key customers, and our strategic focus may change. As a result, we may not realize the benefits we anticipated. If we fail to integrate acquired businesses or realize the expected benefits, we may lose the return on the investment in these acquisitions or incur additional transaction costs and our operations may be negatively impacted as a result. Further, any acquisition or investment that we attempt, whether or not completed, or any media reports or rumors with respect to any such transactions, may adversely affect our competitiveness, our growth prospects, and the value of our common stock.
The recent disruptions in the overall economy and the financial markets may adversely impact our business and results of operations and may limit our access to additional financing.
As widely reported, financial markets in the United States, Europe and Asia have been experiencing extreme disruptions in recent months, including, among other things, extreme volatility in security prices, the failure and near failure of a number of large financial services companies, severely diminished liquidity and credit availability, rating downgrades of certain investments and declining valuations of others.
The infant formula industry can be affected by macro economic factors, including changes in national, regional, and local economic conditions, employment levels and consumer spending patterns. Though the recent disruptions in the overall economy and financial markets is less severe in China than in the U.S., it could reduce consumer confidence in the economy and negatively affect consumers’ spending, which could be harmful to our financial position and results of operations.
In addition, if the capital and credit markets continue to experience volatility and the availability of funds remains limited, we will incur increased costs associated with equity and/or debt financing. It is possible that our ability to access the capital and credit markets may be limited by these or other factors at a time when we would like, or need, to do so, which could have an impact on our ability to refinance maturing debt and/or react to changing economic and business conditions.
As a result of losses associated with the recent melamine contamination incident in China, we may not be able to meet certain financial covenants under our credit facility and may incur additional expenses in connection with the change of loan arrangements.
As a result of the recent melamine contamination incident, we have incurred substantial losses for the quarters ended September 30, 2008, December 31, 2008 and March 31, 2009 from product recall and inventory write-off. Though we expect this to be a short-term financial challenge for us, we have not been able to meet the financial covenants set forth in our credit facility for the quarters ended September 30, 2008, December 31, 2008 and March 31, 2009, and possibly continue to experience difficulties meeting the financial covenants for the next several quarters. We are requesting the lenders to grant us a waiver of financial covenants and amend loan agreements with us. There can be no assurance that we will be able to obtain such waiver on terms and conditions acceptable to us. We expect that in consideration for the waiver, we will be subject to increased interest rates and additional fees as a result of downgrading of our credit rating by the lenders due to the melamine contamination incident as well as the current market disruption. If we are unable to obtain the waiver, we will be in default and may be required to prepay the outstanding loans as a result of the default. We may not be able to refinance our debt on terms acceptable to us, or at all, and there can be no assurance that additional lines-of-credit or financing instruments will be available in amounts or on terms acceptable to us, if at all, under the current market condition.
Our business is capital intensive and our growth strategy may require additional capital that may not be available on favorable terms or at all.
We have, in the past, obtained loans and sold our common stock to raise additional capital. Our business requires significant capital and although we believe that our current cash, and cash flow from operations will be sufficient to meet our present and reasonably anticipated cash needs, we may, in the future, require additional cash resources due to changed business conditions, implementation of our strategy to expand our production capacity or other investments or acquisitions we may decide to pursue. If our own financial resources are insufficient to satisfy our capital requirements, we may seek to sell additional equity or debt securities or obtain additional credit facilities. For example, the recent melamine contamination incident has significantly impacted our liquidity due to our product recall and required us to obtain additional funding through short-term loans for working capital purposes after the incident. In addition to these short-term loans, we may need to obtain additional private or public financing including debt or equity financing and there can be no assurance that such financing will be available as needed or, if available, on terms favorable to us. Due to the current stock market conditions, we have terminated our proposed public offering of common stock in November 2008. Any additional equity financing may be dilutive to stockholders and such additional equity securities may have rights, preferences or privileges that are senior to those of our existing common stock. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations. If we are unable to generate sufficient cash flow from operating activities or obtain funds for required payments of interest and principal on such additional indebtedness, or if we fail to comply with our debt covenants, we will be in default. In addition, changes in our debt rating due to the recent melamine contamination incident could have a material adverse effect on our interest costs and financing sources. Financing may not be available in amounts or on terms acceptable to us, if at all. Any failure by us to raise additional funds on terms favorable to us, or at all, could limit our ability to expand our business operations and could harm our overall business prospects.
We have recently incurred operating losses and our future profitability is uncertain; this may have a harmful effect on our business and the value of our common stock.
As a result of the recent melamine contamination incident, we have incurred substantial losses for the quarters ended September 30, 2008, December 31, 2008 and March 31, 2009 from product recall and inventory write-down and write-off. Though we expect this to be a short-term financial challenge for us, we believe the contamination incident has negatively impacted our brand and reputation in China and may impact our future revenues. We have incurred expenses relating to the melamine contamination incident and we may incur more expenses in the future relating to expanding our production capacity or other investments or acquisitions we may decide to pursue. If our revenue does not increase or if we fail to maintain our expenses at an amount less than our projected revenues, we will not be able to achieve or sustain operating profitability on a consistent basis. Given our planned operating and capital expenditures as well as our overall business plan, for the foreseeable future we expect our results of operations to fluctuate, and during this period we may continue to incur losses. Our lack of profitability may have an adverse effect on the market value of our common stock and on our cash flow and liquidity.
We may face liquidity challenges to meet our debt obligations and may require additional funding in the future.
We had positive cash flow from operations of $38.2 million for the fiscal year ended March 31, 2008, but we had negative cash flow from operations of $109.5 million for the fiscal year ended March 31, 2009. At March 31, 2009, we had short-term debt of $224.6 million, consisting of short-term loans from banks in the amount of $182.6 million, an additional $7.5 million in short-terms loans from related parties, and $34.5 million under the New ABN Loan which has been reclassified as a current liability since we did not meet our financial covenants under the loan as of March 31, 2009. The majority of our short-term debt will become due by December 31, 2009. Our ability to meet our debt obligations will depend on our future performance, which will be affected by financial, business, domestic and foreign economic conditions and other factors, many of which we are unable to control. We believe the contamination incident has negatively impacted our brand and reputation in China and may impact our future revenues. As a result, we cannot be assured that our revenues will provide cash flow in excess of our cash needs, and we therefore may have negative cash flow in the future. If our cash flow is not sufficient to service our debt, we may be required to obtain additional financing in the future, and such additional financing may not be available at times, in amounts or on terms acceptable to us or at all, which would have a material adverse effect on our business.
The report of our Independent Registered Public Accounting Firm contains a reference raising substantial doubt about our ability to continue as a “going concern.”
Because of our limited capital resources and our non-compliance with certain covenants under our New ABN loan agreement, coupled with our significant operating losses and negative cash flows from operations for the fiscal year ended March 31, 2009, our independent registered public accounting firm has included a reference in its report on our financial statements for the fiscal year ended March 31, 2009 with a statement raising substantial doubt regarding our ability to continue as a “going concern.” As of March 31, 2009, we had a working capital deficit of approximately $80.4 million. While we are attempting to renegotiate the terms and covenants of our New ABN loan agreement and are currently in the process of evaluating funding alternatives including seeking refinancing of certain of our short-term loans from PRC banks, we may continue to have a deficit in our working capital.
Substantial doubt about our ability to continue as a going concern could affect our relationships with suppliers or customers. In accordance with generally accepted accounting principles in the U.S., Synutra’s balance sheet generally states the book value of Synutra’s assets, which does not necessarily represent the value that could be realized from the assets if Synutra could not continue as a going concern.
We also import milk powder and any disruption in this source of milk powder could materially and adversely affect our business and results of operations.
Since the melamine contamination incident, we have been using imported milk powder from suppliers in Europe and New Zealand for our major product series, such as Super, U-Smart, and Helanruniu, or Holsteina. While these arrangements help us to regain customer confidence and manage operating costs, they also reduce our direct control over a portion of our production. As our overseas suppliers are not legally obligated to supply us with milk powder, we cannot assure you that they will always meet our demand. They may encounter capacity constraints or may be unable or unwilling to provide us milk powder in required quantities or at acceptable costs. Our inability to find or develop alternate suppliers, if required, could result in delays or reductions in production and product shipments. Moreover, these overseas suppliers may delay milk powder shipments or supply us with inferior quality milk powder that may adversely impact the timely delivery or the quality of our products. For a more detailed description of risks associated with product quality, see “Risk Factors — We may experience problems with product quality or product performance, or the perception of such problems, which could adversely affect our reputation or result in a decrease in customers and revenue, unexpected expenses and loss of market share.”
We have limited insurance coverage and do not carry any business interruption insurance, third-party liability insurance for our production facilities or insurance that covers the risk of loss of our products in shipment.
Operation of our facilities involves many risks, including equipment failures, natural disasters, industrial accidents, power outages, labor disturbances and other business interruptions. Furthermore, if any of our products are faulty or contaminated, then we may become subject to product liability claims or we may have to engage in a product recall. For details about our recent recall of contaminated products in connection with the melamine contamination incident, please see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Overview.”
We do not carry any business interruption insurance, product recall or third-party liability insurance for our production facilities or with respect to our products to cover claims pertaining to personal injury or property or environmental damage arising from defects in our products, product recalls, accidents on our property or damage relating to our operations. Therefore, our existing insurance coverage may not be sufficient to cover all risks associated with our business. As a result, we may be required to pay for financial and other losses, damages and liabilities, including those caused by natural disasters and other events beyond our control, out of our own funds, which could have a material adverse effect on our business, financial condition and results of operations. For examples, all of the costs we have incurred to date and may incur in the future that are related to our recent product recall in connection with the melamine contamination incident may not be covered by our existing insurance policies.
Failure to adequately protect our intellectual property rights may undermine our competitive position, and litigation to protect our intellectual property rights may be costly.
We rely primarily on trade secrets and other contractual restrictions to protect our intellectual property. We have not registered or applied for protections in China for most of our intellectual property or proprietary technologies relating to the formulations of powdered infant formula that we produce. In order to protect our proprietary technology and processes, we also rely in part on nondisclosure agreements with our employees, licensing partners, third-party producers, consultants, agents and other organizations to which we disclose our proprietary information. The actions we have taken to protect our intellectual property rights may not be adequate to provide us with meaningful protection or commercial advantage. As a result, third parties may use the intellectual property or proprietary technologies that we have developed and compete with us, which could have a material adverse effect on our business, financial condition and operating results.
PRC intellectual property-related laws and their implementation are still under development. Accordingly, intellectual property rights and confidentiality protections in China may not be as effective as in the United States or many other countries. In addition, policing unauthorized use of proprietary technology can be difficult and expensive. Litigation may be necessary to enforce our intellectual property rights and the outcome of any such litigation may not be in our favor. Given the relative unpredictability of China’s legal system and potential difficulties enforcing a court judgment in China, there is no guarantee that we would be able to halt the unauthorized use of our intellectual property through litigation in a timely manner or at all. Furthermore, any such litigation may be costly and may divert management attention away from our business and cause us to expend significant resources. An adverse determination in any such litigation will impair our intellectual property rights and may harm our business, prospects and reputation. In addition, we have no insurance coverage against litigation costs and would have to bear all costs arising from such litigation to the extent we are unable to recover them from other parties. The occurrence of any of the foregoing could have a material adverse impact on our business, financial condition and results of operations.
We may be exposed to infringement or misappropriation claims by third parties, which, if determined adversely against us, could disrupt our business and subject us to significant liability to third parties.
Our success largely depends on our ability to use and develop our know-how and product formulations without infringing upon the intellectual property rights of third parties. We may be subject to litigation involving claims of infringement or violation of other intellectual property rights of third parties. The holders of patents and other intellectual property rights potentially relevant to our product offerings may be unknown to us or may otherwise make it difficult for us to acquire a license on commercially acceptable terms.
There may also be technologies licensed to and relied on by us that are subject to infringement or other corresponding allegations or claims by others which could damage our ability to rely on such technologies. In addition, although we endeavor to ensure that companies that work with us possess appropriate intellectual property rights or licenses, we cannot fully avoid the risks of intellectual property rights infringement created by suppliers of raw materials used in our products, our third-party producers, or by companies with which we work in cooperative research and development activities. Our current or potential competitors, many of which have substantial resources and have made substantial investments in competing technologies, may have obtained or may obtain patents that will prevent, limit or interfere with our ability to make, use or sell our products in China or other countries. The defense of intellectual property claims, including patent infringement suits, and related legal and administrative proceedings can be both costly and time-consuming, and may significantly divert the efforts and resources of our technical and management personnel. Furthermore, an adverse determination in any such litigation or proceeding to which we may become a party could cause us to:
| • | seek licenses from third parties, which may not be available on reasonable terms or at all; |
| • | pay additional ongoing royalties, which could decrease our profit margins; |
| • | redesign our products, which may be costly, if possible at all; or |
| • | be restricted by injunctions. |
These factors could effectively prevent us from pursuing some or all of our business and result in our customers or potential customers deferring, canceling or limiting their purchase or use of our products, which could have a material adverse effect on our financial condition and results of operations.
Our products may not achieve market acceptance.
We are currently selling our products principally in northern, central, and eastern China. Achieving market acceptance for our products, particularly in new markets, will require substantial marketing efforts and the expenditure of significant funds. There is substantial risk that any new markets may not accept or be as receptive to our products. In addition, we intend to market our products as premium and super-premium products and to adopt a corresponding pricing model, which may not be accepted in new or existing markets. Market acceptance of our current and proposed products will depend, in large part, upon our ability to inform potential customers that the distinctive characteristics of our products make them superior to competitive products and justify their pricing. Our current and proposed products may not be accepted by consumers or able to compete effectively against other premium or non-premium dairy products. Lack of market acceptance would limit our revenues and profitability.
RISKS ASSOCIATED WITH DOING BUSINESS IN CHINA
Adverse changes in political and economic policies of the PRC government could impede the overall economic growth of China, which could reduce the demand for our products and damage our business.
We conduct substantially all of our operations and generate most of our revenues in China. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in China. The PRC economy differs from the economies of most developed countries in many respects, including:
| • | the higher level of government involvement and regulation; |
| • | the early stage of development of the market-oriented sector of the economy; |
| • | the higher rate of inflation; |
| • | the higher level of control over foreign exchange; and |
| • | government control over the allocation of many resources. |
As the PRC economy has been transitioning from a planned economy to a more market-oriented economy, the PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. While these measures may benefit the overall PRC economy, they may also have a negative effect on us.
Although the PRC government has in recent years implemented measures emphasizing the utilization of market forces for economic reform, the PRC government continues to exercise significant control over economic growth in China through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and imposing policies that impact particular industries or companies in different ways.
Any adverse change in the economic conditions or government policies in China could have a material adverse effect on the overall economic growth and the level of consumer spending in China, which in turn could lead to a reduction in demand for our products and consequently have a material adverse effect on our business and prospects.
PRC food hygiene and safety laws may become more onerous, which may adversely affect our operations and financial performance and lead to an increase in our costs which we may be unable to pass on to our customers.
Operators within the PRC dairy industry and infant formula sector are subject to compliance with PRC food hygiene and safety laws and regulations. Such laws and regulations require all enterprises engaged in the production of dairy and infant formula products to obtain a hygiene license. They also set out hygiene standards with respect to food and food additives, packaging and containers, and labeling on packaging as well as hygiene requirements for food production and sites, facilities and equipment used for the transportation and the sale of food. Failure to comply with PRC food hygiene and safety laws may result in fines, suspension of operations, loss of hygiene license and, in certain cases, criminal proceedings against an enterprise and its management. Although we are in compliance with current PRC food hygiene and safety laws and regulations, in the event that such laws and regulations become more stringent or widen in scope, we may fail to comply with such laws, or if we comply, our production and distribution costs may increase, and we may be unable to pass these additional costs on to our customers. For example, in response to the recent melamine contamination incident, the PRC State Council abolished the regulations on inspection exemptions for food on September 18, 2008 so that our products were to be subject to batch by batch inspection going forward. In addition, the PRC State Council promulgated with immediate effect the Regulation on Supervision and Administration of Quality and Safety of Dairy Products on October 9, 2008 which, among other things, imposes more stringent requirements for inspection, production, packaging, labeling and product recall on dairy product producers. All these new measures are likely to increase our costs which we may be unable to pass on to our customers.
Changes in the regulatory environment for dairy and infant nutrition products in China could negatively impact our business.
The dairy and infant nutrition product industries in China are regulated and regulatory changes may affect both our customers and us. Any changes in regulations that impose additional requirements for construction of new production lines and facilities or expansion of existing facilities will require us to secure additional government approvals for our current production expansion projects. Similarly, additional safety and quality control regulations could impact our costs of production. For example, on March 18, 2008, the PRC National Development and Reform Commission, or NDRC, promulgated the Access Conditions for Dairy Products Processing Industry, or the Access Conditions, which became effective on April 1, 2008. The Access Conditions impose new conditions that an entity must satisfy in order to engage, or continue to engage, in the dairy products processing business. For a more detailed description of these requirements, see “Item 1. Business—Regulation”. Although we believe our existing entities and facilities meet the Access Condition requirements, it is possible that our future expansion plans or the establishment of new entities may fail to meet one or more of the requirements under the Access Conditions in the future. In addition, in response to the recent melamine contamination incident, the relevant PRC government authorities may impose more stringent conditions on entering or remaining in the dairy products industry. Failure to comply with these or any other changes in regulations affecting our business could have a material adverse effect on our business and our results of operations. In addition, the indirect impact of any such changes could adversely affect our business even if the specific regulations do not directly apply to our products or us.
Uncertainties with respect to the PRC legal system could limit the legal protections available to you and us.
We conduct substantially all of our business through our operating subsidiaries in China. Our operating subsidiaries are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to foreign-invested enterprises. The PRC legal system is based on written statutes, and prior court decisions may be cited for reference, but have limited precedential value. Over the past several decades, a series of new PRC laws and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, since the PRC legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involve uncertainties, which may limit legal protections available to you and us. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
If we are found to have failed to comply with applicable laws, we may incur additional expenditures or be subject to significant fines and penalties.
Our operations are subject to PRC laws and regulations applicable to us. However, many PRC laws and regulations are uncertain in their scope, and the implementation of such laws and regulations in different localities could have significant differences. In certain instances, local implementation rules and/or the actual implementation are not necessarily consistent with the regulations at the national level. Although we strive to comply with all the applicable PRC laws and regulations, we cannot assure you that the relevant PRC government authorities will not later determine that we have not been in compliance with certain laws or regulations. For example, there is a PRC regulation requiring all employees to make contributions to a housing provident fund based on their salary level and their employers to match such contributions. However, many employees are reluctant to make such contributions as they do not perceive such contribution will benefit them, and this regulation has generally not been rigorously enforced. We have allowed our employees to make contributions on a voluntary basis and then match their contributions. However, we cannot assure you that in the future the PRC government will not start to enforce this regulation more rigorously. Although there are no material penalties stipulated in this regulation, if we are found to not be in compliance, we may be required to bring current any past deficiencies, which could adversely affect our financial condition and results of operations.
In addition, our facilities and products are subject to many laws and regulations administered by the PRC State Administration for Industry and Commerce, the PRC State Administration of Taxation, the PRC Ministry of Health and Hygiene Permitting Office, the PRC General Administration of Quality Supervision, Inspection and Quarantine, and the PRC State Food and Drug Administration Bureau relating to the processing, packaging, storage, distribution, advertising, labeling, quality, and safety of food products. Our failure to comply with these and other applicable laws and regulations in China could subject us to administrative penalties and injunctive relief, as well as civil remedies, including fines, injunctions and recalls of our products. It is possible that changes to such laws, more rigorous enforcement of such laws or with respect to our current or past practices, could have a material adverse effect on our business, operating results and financial condition. Further, additional environmental, health or safety issues relating to matters that are not currently known to management may result in unanticipated liabilities and expenditures.
The PRC government exerts substantial influence over the manner in which we conduct our business activities.
The PRC government has exercised and continues to exercise substantial control over virtually every sector of the PRC economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in PRC laws and regulations, including those relating to how we conduct our business, taxation, import and export tariffs, environmental regulations, land use rights, property and other matters. We believe that our operations in China are in material compliance with all applicable legal and regulatory requirements. However, the central or local governments of the jurisdictions in which we operate may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations.
Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof and could require us to divest ourselves of any interest we then hold in PRC properties or joint ventures.
Restrictions on currency exchange may limit our ability to receive and use our sales revenue effectively.
Most of our sales revenue and expenses are denominated in Renminbi. Under PRC law, the Renminbi is currently convertible under the “current account,” which includes dividends and trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans. Currently, our PRC operating subsidiaries may purchase foreign currencies for settlement of current account transactions, including payments of dividends to us, without the approval of the PRC State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. However, the relevant PRC government authorities may limit or eliminate our ability to purchase foreign currencies in the future.
Foreign exchange transactions by PRC operating subsidiaries under the capital account continue to be subject to significant foreign exchange controls and require the approval of or registration with PRC government authorities, including SAFE. In particular, if our PRC operating subsidiaries borrow foreign currency through loans from us or other foreign lenders, these loans must be registered with SAFE, and if we finance the subsidiaries by means of additional capital contributions, these capital contributions must be approved by certain government authorities, including the PRC Ministry of Commerce, or their respective local branches. These limitations could affect our PRC operating subsidiaries’ ability to obtain foreign exchange through debt or equity financing.
Recent PRC regulations relating to investment activities by, and holdings in entities outside of China of, PRC residents and citizens, may subject our PRC resident and citizen stockholders to personal liability and limit our ability to acquire PRC companies or to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute profits to us or otherwise materially and adversely affect us.
In October 2005, SAFE issued a public notice, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice, which requires PRC residents and citizens, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of China, referred to as an “offshore special purpose company,” for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any PRC resident that is the stockholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in China. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any PRC stockholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the PRC subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
Many of the terms and provisions in the SAFE notice, and its implementing regulations, remain unclear and implementation by central SAFE and local SAFE branches of the SAFE notice have been inconsistent since its adoption. Based on the advice of our PRC counsel, De Heng Law Offices, and after consultation with relevant SAFE officials, we believe our PRC resident stockholders were required to complete their respective SAFE registrations pursuant to the SAFE notice. The local SAFE branch may not accept their applications for SAFE registration until more detailed rules or announcements concerning the penalties for those who failed to make their SAFE registrations are implemented. However, we believe the likely penalties for failure to complete the SAFE registration will be nominal and there should be no other legal obstacles for our PRC resident stockholders to complete or amend their respective SAFE registrations. Moreover, because of uncertainty over how the SAFE notice will be interpreted and implemented, and how or whether SAFE will apply it to us, we cannot predict how it will affect our business operations or future strategies. For example, our present and prospective PRC subsidiaries’ ability to conduct foreign exchange activities, such as the remittance of dividends and foreign currency-denominated borrowings, may be subject to compliance with the SAFE notice by our PRC resident beneficial holders. In addition, such PRC residents may not always be able to complete the necessary registration procedures required by the SAFE notice. We also have little control over either our present or prospective direct or indirect stockholders or the outcome of such registration procedures. A failure by our PRC resident beneficial holders or future PRC resident stockholders to comply with the SAFE notice, if SAFE requires it, could subject us to fines or legal sanctions, restrict our overseas or cross-border investment activities, limit our subsidiary’s ability to make distributions or pay dividends or affect our ownership structure, which could adversely affect our business and prospects.
Fluctuations in exchange rates could adversely affect our business and the value of our common stock.
The value of our common stock will be indirectly affected by the foreign exchange rate between the U.S. dollar and the Renminbi and between those currencies and other currencies in which our sales may be denominated. Because substantially all of our earnings and cash assets are denominated in Renminbi, appreciation or depreciation in the value of the Renminbi relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of any dividend we issue that will be exchanged into U.S. dollars and earnings from, and the value of, any U.S. dollar-denominated investments we make in the future.
Since July 2005, the Renminbi has no longer been pegged to the U.S. dollar. Although the People’s Bank of China, or PBOC, regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the Renminbi may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long-term. Moreover, it is possible that in the future the PRC authorities may lift restrictions on fluctuations in the Renminbi exchange rate and lessen intervention in the foreign exchange market.
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may enter into hedging transactions in the future, the availability and effectiveness of these transactions may be limited, and we may not be able to successfully hedge our exposure at all. In addition, our foreign currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert Renminbi into foreign currencies.
Currently, some of our raw materials and major equipment are imported. In the event that the U.S. dollar appreciated against the Renminbi, our costs may increase. If we cannot pass the resulting cost increases on to our customers, our profitability and operating results will suffer. In addition, to the extent we enter markets outside China in the future, we may be increasingly subject to the risk of foreign currency fluctuations.
Restrictions under PRC law on our PRC subsidiaries’ ability to make dividends and other distributions could materially and adversely affect our ability to grow, make investments or acquisitions that could benefit our business, pay dividends to you, and otherwise fund and conduct our businesses.
Substantially all of our revenues are earned by our PRC subsidiaries. However, PRC regulations restrict the ability of our PRC subsidiaries to make dividends and other payments to their offshore parent company. PRC legal restrictions permit payments of dividend by our PRC subsidiaries only out of their accumulated after-tax profits, if any, determined in accordance with PRC accounting standards and regulations. Each of our PRC subsidiaries is also required under PRC laws and regulations to allocate at least 10% of our annual after-tax profits determined in accordance with PRC GAAP to a statutory general reserve fund until the amounts in such fund reaches 50% of our registered capital. Allocations to these statutory reserve funds can only be used for specific purposes and are not transferable to us in the form of loans, advances or cash dividends. As of March 31, 2009, the amount of our restricted net assets was $76.9 million. Any limitations on the ability of our PRC subsidiaries to transfer funds to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
Any future outbreak of severe acute respiratory syndrome or avian influenza in China, or similar adverse public health developments, may disrupt our business and operations.
Our business and operations could be materially and adversely affected by the outbreak of swine flu, avian influenza, severe acute respiratory syndrome, or SARS, or other similar adverse public health development. Recently, there have been reports on the occurrences of swine flu in various countries. Any prolonged recurrence of an adverse public health development may result in the temporary closure of businesses in China by the PRC government in order to avoid congregation in closed spaces to help prevent disease transmission. Such occurrences would disrupt our business operations and adversely affect our results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of avian influenza, SARS or any other epidemic.
Under China’s New EIT Law, we may be classified as a “resident enterprise” of China. This classification could result in unfavorable tax consequences to us and our non-PRC shareholders.
The New EIT Law provides that enterprises established outside of China whose “de facto management bodies” are located in China are considered “resident enterprises” and are generally subject to the uniform 25% enterprise income tax rate on their worldwide income. In addition, a recent circular issued by the State Administration of Taxation regarding the standards used to classify certain Chinese-invested enterprises established outside of China as “resident enterprises” clarified that dividends and other income paid by such “resident enterprises” will be considered to be PRC source income, subject to PRC withholding tax, currently at a rate of 10%, when recognized by non-PRC shareholders. This recent circular also subjects such “resident enterprises” to various reporting requirements with the PRC tax authorities. Under the implementation regulations to the enterprise income tax, a “de facto management body” is defined as a body that has material and overall management and control over the manufacturing and business operations, personnel and human resources, finances and treasury, and acquisition and disposition of properties and other assets of an enterprise. In addition, the recent circular mentioned above details that certain Chinese-invested enterprises will be classified as “resident enterprises” if the following are located or resident in China: senior management personnel and departments that are responsible for daily production, operation and management; financial and personnel decision making bodies; key properties, accounting books, company seal, and minutes of board meetings and shareholders’ meetings; and half or more of the directors or senior management having voting rights. If the PRC tax authorities determine that Synutra, Inc. or Synutra International, Inc a “resident enterprise,” we may be subject to enterprise income tax at the rate of 25% on our worldwide income and dividends paid by us to our non-PRC shareholders and, while less clear, capital gains recognized by them with respect to the sale of our stock, may be subject to a PRC withholding tax. This will have an impact on our effective tax rate, a material adverse effect on our net income and results of operations, and may require us to withhold tax on our non-PRC shareholders. We are actively monitoring the “resident enterprise” classification rules and are evaluating appropriate organizational changes to avoid this treatment, to the extent possible.
If we were treated as a “resident enterprise” by PRC tax authorities, we would be subject to tax in both the U.S. and China, and our PRC tax may not be creditable against our U.S. tax.
The M&A Rule establishes more complex procedures for some acquisitions of Chinese companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in China.
On August 8, 2006, six PRC regulatory agencies, including the China Securities Regulatory Commission, or CSRC, promulgated the Provisions Regarding Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rule, which became effective on September 8, 2006. The M&A Rule establishes additional procedures and requirements that could make some acquisitions of Chinese companies by foreign investors more time-consuming and complex, including requirements in some instances that the PRC Ministry of Commerce be notified in advance of any change-of-control transaction and in some situations, require approval of the PRC Ministry of Commerce when a foreign investor takes control of a Chinese domestic enterprise. In the future, we may grow our business in part by acquiring complementary businesses, although we do not have any plans to do so at this time. The M&A Rule also requires PRC Ministry of Commerce anti-trust review of any change-of-control transactions involving certain types of foreign acquirers. Complying with the requirements of the M&A Rule to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the PRC Ministry of Commerce, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
You may have difficulty enforcing judgments against us.
We are a Delaware holding company and most of our assets are located outside of the United States. Most of our current operations are conducted in the PRC. In addition, most of our directors and officers are nationals and residents of countries other than the United States. A substantial portion of the assets of these persons is located outside the United States. As a result, it may be difficult for you to effect service of process within the United States upon these persons. It may also be difficult for you to enforce in U.S. courts judgments on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors, most of whom are not residents in the United States and the substantial majority of whose assets are located outside of the United States. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts. DeHeng Law Offices, our counsel as to PRC law, has advised us that the recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. Courts in China may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based on treaties between China and the country where the judgment is made or on reciprocity between jurisdictions. China does not have any treaties or other arrangements that provide for the reciprocal recognition and enforcement of foreign judgments with the United States. In addition, according to the PRC Civil Procedures Law, courts in the PRC will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates basic principles of PRC law or national sovereignty, security or public interest. So it is uncertain whether a PRC court would enforce a judgment rendered by a court in the United States.
RISKS RELATED TO THE MARKET FOR OUR STOCK GENERALLY
The market price of our common stock is volatile, and its value may be depressed at a time when you want to sell your holdings.
The market price of our common stock is volatile, and this volatility may continue. For instance, between July 1, 2008 and March 31, 2009, the price of our common stock, as reported on Nasdaq on which our common stock has traded, ranged between $4.60 and $52.24. Though we believe the recent fluctuation resulted mainly from the melamine contamination incident and the current worldwide market disruption, numerous factors, many of which are beyond our control, may cause the market price of our common stock to fluctuate significantly.
In addition to market and industry factors, the price and trading volume for our common stock may be highly volatile for specific business reasons. Factors such as variations in our revenues, earnings and cash flow, announcements of new investments, cooperation arrangements or acquisitions, and fluctuations in market prices for our products could cause the market price for our shares to change substantially.
Securities class action litigation is often instituted against companies following periods of volatility in their stock price. This type of litigation could result in substantial costs to us and divert our management’s attention and resources.
Moreover, the trading market for our common stock will be influenced by research or reports that industry or securities analysts publish about us or our business. If one or more analysts who cover us downgrade our common stock, the market price for our common stock would likely decline. If one or more of these analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which, in turn, could cause the market price for our common stock or trading volume to decline.
Furthermore, securities markets may from time to time experience significant price and volume fluctuations for reasons unrelated to operating performance of particular companies. These market fluctuations may adversely affect the price of our common stock and other interests in our company at a time when you want to sell your interest in us.
Although publicly traded, the trading market in our common stock has been substantially less liquid than the average stock quoted on the Nasdaq Global Select Market, and this low trading volume may adversely affect the price of our common stock.
Although our common stock is traded on the Nasdaq Global Select Market, the trading volume of our common stock has generally been very low. Reported average daily trading volume in our common stock for the three month period ended June 12, 2009 was approximately 36,700 shares. Limited trading volume will subject our shares of common stock to greater price volatility and may make it difficult for you to sell your shares of common stock at a price that is attractive to you.
Provisions in our charter documents and under Delaware law could discourage a change-of-control that our stockholders may consider favorable.
Provisions in our amended and restated certificate of incorporation and bylaws may have the effect of delaying or preventing a change of control or changes in our management. These provisions include the following:
| • | our board of directors is divided into three classes, with approximately one-third of our directors elected each year; |
| • | our board of directors has the right to elect directors to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
| • | our stockholders must provide timely notice for any stockholder proposals and director nominations; |
| • | we have adopted provisions that eliminate the personal liability of directors for monetary damages for actions taken as a director, with certain exceptions; and |
| • | our board of directors may issue, without stockholder approval, shares of undesignated preferred stock, which makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us. |
As a Delaware corporation, we are also subject to certain Delaware anti-takeover provisions. Under Delaware law, a corporation may not engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Our board of directors could rely on Delaware law to prevent or delay an acquisition of us.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our headquarters are located at Beijing with around 6,000 square meters of leased office space. Synutra Illinois leases an executive office in Rockville, Maryland, USA. Our processing and packaging facilities are located in various locations in China, including Beijing, Qingdao, Luobei, Zhangjiakou, Fengzhen, Zhenglanqi and Junchuan. These facilities encompass approximately 114,000 square meters of office, plant, and warehouse space. Our packaging and distribution headquarters located in Qingdao includes over 2,330 square meters of owned office space. All of our production facilities are built based on the GMP standard, with equipment imported from Europe and all of our facilities that have commenced operation have ISO9000 and HACCP series qualifications with some also being ISO14000 certified.
We currently own and operate five processing facilities and one packaging facility for our powder formula products. As of March 31, 2009, we had raw milk processing capacity of 40,000 tons per year, packaging capacity of 82,000 tons per year and dry-blending processing capacity of 73,000 tons per year.
The following table sets forth certain information with respect to our production, processing and packaging facilities.
| | Province/ Region | | Installed Capacity as of March 31, 2009 | | Description | | Property Right |
| | | | | (tons per year) | | | | |
| Zhangjiakou facility | | Hebei | | | 22,000 | | Raw milk processing | | Land Use Right |
| Luobei facility | | Heilongjiang | | | 4,000 | | Raw milk processing | | Land Use Right * |
| Fengzhen facility | | Inner Mongolia | | | 7,000 | | Raw milk processing | | Land Use Right |
| Junchuan facility | | Heilongjiang | | | 7,000 | | Raw milk processing | | Land Use Right |
| Qingdao facility | | Shandong | | | 73,000 | | Dry-mixing of all of our powdered formula products | | Land Use Right |
| | | | | | 82,000 | | Packaging of all of our products | | Land Use Right |
| Beijing facility | | Beijing | | | 400 | | Production of prepared baby foods | | Land Use Right |
| Zhenglanqi facility | | Inner Mongolia | | | 80,000 | | Production and processing of nutritional snacks and other non-core products | | Land Use Right |
| Qingdao Meitek facility | | Shandong | | | 2,400 | | Production and processing of nutritional ingredients and supplement and other non-core products | | Land Use Right |
* Expires in August 2010.
There is no private land ownership in China. Individuals and companies are permitted to acquire land use rights for specific purposes and for limited periods. Each period may be renewed at the expiration of the initial and any subsequent terms. Granted land use rights are transferable and may be used as security for borrowings and other obligations.
We believe that our facilities are adequate for our current operations and any increase in production in the near term will not require additional space.
ITEM 3. LEGAL PROCEEDINGS
As of March 31, 2009, the end of the period covered by this report, the Company was subject to various legal proceedings and claims discussed below, as well as certain other legal proceedings and claims that have not been fully resolved and that have arisen in the ordinary course of business. Other than as discussed below, in the opinion of management, the Company does not have a potential liability related to any current legal proceedings and claims that would individually or in the aggregate have a material adverse effect on its financial condition or operating results. However, the results of legal proceedings cannot be predicted with certainty. The Company intends to contest each lawsuit vigorously but should the Company fail to prevail in any of these legal matters or should several of these legal matters be resolved against the Company in the same reporting period, the operating results of a particular reporting period could be materially adversely affected, see “Item 1A. Risk Factors - Risks Related to Our Business - Product liability claims against us could result in adverse publicity and potentially significant monetary damages.” Management continues to evaluate the lawsuits discussed below and based on the stage of these proceedings, management is unable to reasonably estimate the likelihood of any loss or the amount or range of any potential loss that could result from the litigation. Therefore, no accrual has been established for any potential loss in connection with these lawsuits.”
On January 15, 2009 , a lawsuit was filed in the U.S. on behalf of 54 Chinese families alleged to be affected by melamine contamination, against Synutra International, Inc. and Synutra Inc. in the U.S. District Court for the District of Maryland, alleging negligent or intentional infliction of personal injury, negligent or intentional infliction of emotional distress, battery, breach of warranty, fraudulent or negligent misrepresentation, seeking compensation for punitive damages in the amount of US$500 million, together with any compensatory damages. We filed a motion in April 2009 to dismiss the case on grounds of "Forum Non-Convenience,” failure to state a claim, and failure to join an indispensable party, and the opposition filed a Memorandum of Opposition to Motion to Dismiss on May 20, 2009. We have been given time to respond to the Opposition, and we will do so on or before June 15 2009 with an additional filing. After June 15, 2009, the presiding Judge of the Court will decide, with no time limit, if the Court will take the case or render its dismissal. At this stage, the management is unable to predict the outcome of such lawsuit.
On March 2, 2009 , a lawsuit was filed in China on behalf of 54 Chinese families alleged to be affected by melamine contamination against our subsidiary, Shengyuan Nutritional Food Co., Ltd. at Qingdao Intermediate People’s Court, seeking compensation for damages, including medical and other expenses, emotional harm and punitive damages, for an aggregate amount over US$1.0 million (RMB6.9 million). Further, on April 14, 2009 , a lawsuit was filed on behalf of 73 families (including the original 54 families) against our subsidiary, Shengyuan Nutritional Food Co., Ltd. at Shandong Higher People’s Court on April 14, 2009, seeking compensation for damages of over US$1.7 million (RMB11.3 million). To date, we have not been notified by either court of acceptance of any case against us in China.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
Our stockholders held an annual meeting at 10:00 A.M. local time on March 12, 2009, at our office located at 106 Dong Lu Yuan, Tongzhou District, Beijing, China. At the annual meeting, our stockholders considered and voted to (i) elect two Class I directors to our Board of Directors, and (ii) ratify the appointment of Deloitte Touche Tohmatsu CPA Ltd. as our independent registered public accountant for the fiscal year ended March 31, 2009.
All directors nominated by the Company were re-elected. The following is a separate tabulation with respect to the vote for each nominee:
| Name | | Total Votes For | | Total Votes Withheld |
| Liang Zhang | | | 40,701,302 | | 39,467 |
| William W. Wu | | | 40,703,434 | | 37,335 |
The appointment of Deloitte Touche Tohmatsu CPA Ltd. as our independent registered public accountant was ratified. The following is a breakdown of the vote on such matter:
| | | | | | | | | | |
| Total Votes For | | Total Votes Against | | | Abstain | | | Broker Non-Votes | |
| 40,740,284 | | | 485 | | | | 0 | | | | 13,259,944 | |
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDERS MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
PRICE RANGE OF OUR COMMON STOCK
Our common stock has been trading on the Nasdaq Global Select Market under the symbol “SYUT” since November 8, 2007. Our common stock was previously quoted on the Over-The-Counter Bulletin Board, or OTCBB, under the trading symbol “SYUT.OB” until April 11, 2007. On April 12, 2007, our common stock was listed on the Nasdaq Global Market and subsequently approved for listing on the Nasdaq Global Select Market on November 8, 2007.
The following table sets forth the high and low bid prices for our common stock on the OTCBB, prior to April 12, 2007 and our common stock on the Nasdaq Global or Global Select Market since April 12, 2007 for the periods indicated. The high and low bid prices reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
| | The OTCBB Bid Price per Share(1) | | The Nasdaq Global/Global Select Market Price per Share(2) | |
| | High | | Low | | High | | Low | |
| | | | | | | | | | | | | |
| Fiscal Year Ending March 31, 2010 | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| First Quarter (through June 12, 2009) | | $ | N/A | | | $ | N/A | | | $ | 13.37 | | | $ | 6.87 | |
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended March 31, 2009 | | | | | | | | | | | | | | | | |
| | | | | | | | | |
| Fourth Quarter | | $ | N/A | | | $ | N/A | | | $ | 11.97 | | | $ | 4.33 | |
| | | | | | | | | | | | | | | | | |
| Third Quarter | | | N/A | | | | N/A | | | | 23.58 | | | | 7.30 | |
| | | | | | | | | | | | | | | | | |
| Second Quarter | | | N/A | | | | N/A | | | | 52.24 | | | | 11.83 | |
| | | | | | | | | | | | | | | | | |
| First Quarter | | | N/A | | | | N/A | | | | 34.00 | | | | 29.30 | |
| | | | | | | | | |
| Fiscal Year Ended March 31, 2008 | | | | | | | | | | | | | | | | |
| | | | | | | | | |
| Fourth Quarter | | $ | N/A | | | $ | N/A | | | $ | 34.01 | | | $ | 22.41 | |
| | | | | | | | | |
| Third Quarter | | | N/A | | | | N/A | | | | 41.31 | | | | 24.38 | |
| | | | | | | | | |
| Second Quarter | | | N/A | | | | N/A | | | | 31.25 | | | | 19.81 | |
| | | | | | | | | |
| First Quarter | | | 12.00 | | | | 11.68 | | | | 26.50 | | | | 11.50 | |
| | | | | | | | | |
(1) Through April 11, 2007.
(2) From April 12, 2007 forward.
As of June 12, 2009, we had approximately 39 registered stockholders of our common stock on record. This number does not include shares held by brokerage clearing houses, depositories or otherwise in unregistered form or shares held by a custodian for the benefit of our employees.
DIVIDEND POLICY
We have never declared or paid any dividends on shares of our common stock. We intend to retain any future earnings to fund the development and growth of our business, and we do not anticipate paying any dividends in the foreseeable future.
Our loan agreement with ABN AMRO Bank N.V., Hong Kong Branch, or ABN, restricts our ability to pay any dividends that, when taken in the aggregate with other payments by us within a 12-month period, exceed 30.0% of our consolidated net income for such period. For a description of the ABN loan, see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Outstanding Indebtedness.” We may enter into agreements in the future that restrict our ability to make distributions to stockholders.
Substantially all of our revenues are earned by our PRC subsidiaries. However, PRC regulations restrict the ability of our PRC subsidiaries to make dividends and other payments to their offshore parent company. PRC legal restrictions permit payments of dividend by our PRC subsidiaries only out of their accumulated after-tax profits, if any, determined in accordance with PRC accounting standards and regulations. Each of our PRC subsidiaries is also required under PRC laws and regulations to allocate at least 10% of our annual after-tax profits determined in accordance with PRC GAAP to a statutory general reserve fund until the amounts in such fund reaches 50% of our registered capital. Allocations to these statutory reserve funds can only be used for specific purposes and are not transferable to us in the form of loans, advances or cash dividends. In addition, there are restrictions on the distribution of share capital from the Company’s PRC subsidiaries. As of March 31, 2009, the amount of our restricted net assets was $76.9 million.
RECENT SALES OF UNREGISTERED SECURITIES
None.
| ITEM 6. | SELECTED FINANCIAL DATA |
In July 2005, we changed our fiscal year end from December 31 to March 31 beginning with the fiscal year ended March 31, 2005. The following selected consolidated financial data for the fiscal years ended March 31, 2009, 2008 and 2007 are derived from our audited consolidated financial statements included elsewhere in this Form 10-K. The financial data for the years ended March 31, 2006 and December 31, 2004 and three months ended March 31, 2005 are derived from audited consolidated financial statements which are not included in this Form 10-K. The financial data for the three months ended March 31, 2004 are derived from our unaudited consolidated financial statements, which is not included in this Form 10-K. The unaudited financial statements reflect, in the opinion of management, all adjustments necessary for the fair presentation of the financial condition and the results of operations for such periods. The results of operations for past accounting periods are not necessarily indicative of the results to be expected for any future accounting period.
The financial data set forth below should be read in conjunction with, and are qualified in their entirety by reference to, “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our consolidated financial statements and related notes included elsewhere in this Form 10-K.
| | | Fiscal Year Ended March 31, | | Three Months Ended March 31, | | Year Ended December 31, | |
| | | 2009 | | 2008 | | 2007 | | 2006 | | 2005 | | 2004 | | 2004 | |
| | | (in thousands except earnings per share data) | |
| Selected Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | |
| Net sales | | $ | 312,528 | | $ | 362,090 | | $ | 216,605 | | $ | 132,289 | | $ | 18,692 | | $ | 12,782 | | $ | 57,542 | |
| Cost of sales | | | 259,086 | | | 175,568 | | | 109,900 | | | 76,653 | | | 9,124 | | | 7,556 | | | 33,605 | |
| Gross profit | | | 53,442 | | | 186,522 | | | 106,705 | | | 55,636 | | | 9,568 | | | 5,226 | | | 23,937 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Selling and distribution expenses | | | 44,178 | | | 34,449 | | | 25,561 | | | 15,494 | | | 4,563 | | | 2,722 | | | 9,428 | |
| Advertising and promotion expenses | | | 115,478 | | | 76,388 | | | 52,322 | | | 20,908 | | | 1,071 | | | 373 | | | 8,479 | |
| General and administrative expenses | | | 25,455 | | | 16,013 | | | 7,031 | | | 5,896 | | | 665 | | | 555 | | | 3,218 | |
| Other operating income, net | | | 5,790 | | | 1,492 | | | 1,109 | | | 734 | | | - | | | - | | | 271 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Income (loss) from operations | | | (125,879 | ) | | 61,164 | | | 22,900 | | | 14,072 | | | 3,269 | | | 1,576 | | | 3,083 | |
| Interest expense | | | 4,857 | | | 6,354 | | | 1,896 | | | 1,784 | | | 183 | | | 145 | | | 638 | |
| Interest income | | | 341 | | | 1,801 | | | 356 | | | 238 | | | 75 | | | 18 | | | 88 | |
| Other income (expense), net | | | (580 | ) | | (3,084 | ) | | 110 | | | (44 | ) | | (58 | ) | | (8 | ) | | (239 | ) |
| Income (loss) before provision for income tax | | | (130,975 | ) | | 53,527 | | | 21,470 | | | 12,482 | | | 3,103 | | | 1,441 | | | 2,294 | |
| Provision for income tax | | | (30,386 | ) | | 7,855 | | | 1,596 | | | 1,446 | | | 32 | | | 19 | | | 76 | |
| Net income before minority interests | | | (100,589 | ) | | 45,672 | | | 19,874 | | | 11,036 | | | 3,071 | | | 1,422 | | | 2,218 | |
| Minority interests | | | (40 | ) | | 11 | | | - | | | 1 | | | (1,365 | ) | | 488 | | | 2,598 | |
| Net income attributable to stockholders | | $ | (100,549 | ) | $ | 45,661 | | $ | 19,874 | | $ | 11,035 | | $ | 4,436 | | $ | 934 | | $ | (380 | ) |
| Earnings per share-basic | | $ | (1.86 | ) | $ | 0.86 | | $ | 0.40 | | $ | 0.23 | | $ | 0.10 | | $ | 0.02 | | $ | (0.01 | ) |
| Earnings per share-diluted | | $ | (1.86 | ) | $ | 0.85 | | $ | 0.40 | | $ | 0.23 | | $ | 0.10 | | $ | 0.02 | | $ | (0.01 | ) |
| | | March 31, | | | December 31, | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2004 | |
| | | (in thousands) | |
| Selected Balance Sheet Data: | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 37,736 | | | $ | 97,425 | | | $ | 20,836 | | | $ | 5,677 | | | $ | 5,812 | | | $ | 5,893 | |
| Working capital (deficit) | | | (80,432 | ) | | | 111,230 | | | | (8,281 | ) | | | (14,270 | ) | | | (15,920 | ) | | | (17,837 | ) |
| Total assets | | | 472,571 | | | | 294,318 | | | | 127,271 | | | | 83,009 | | | | 64,192 | | | | 59,849 | |
| Total long-term liabilities | | | 20,468 | | | | 39,993 | | | | 4,138 | | | | — | | | | 4,833 | | | | 4,833 | |
| Total stockholders’ equity | | $ | 76,859 | | | $ | 171,259 | | | $ | 42,701 | | | $ | 20,951 | | | $ | 7,622 | | | $ | 2,990 | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes that appear elsewhere in this Form 10-K. In addition to historical consolidated financial information, the following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Form 10-K, particularly in “Item 1A. Risk Factors.”
OVERVIEW
We are a leading infant formula company in China. We principally produce, market and sell our products under the “Shengyuan,” or “Synutra,” name, together with other complementary brands. We focus on selling premium infant formula products, which are supplemented by more affordable infant formulas targeting the mass market as well as other nutritional products and ingredients. We sell our products through an extensive nationwide sales and distribution network covering 29 provinces and provincial-level municipalities in China. As of March 31, 2009, this network comprised over 480 distributors and over 800 sub-distributors who sell our products in over 65,000 retail outlets.
Historically we reported our results as a single reporting segment in the financial statements since operating segments such as the nutritional ingredients and supplements segment was under construction with minor operating expenses and had not met the quantitative threshold as described under SFAS 131 "Disclosure about Segments of an Enterprise and Related Information". In this fiscal year, we expanded the number of reportable segments from one to three in order to better reflect the manner in which management analyzes our performance. The three segments are:
| | Powdered formula segment: Powdered formula segment covers the sale of powdered infant and adult formula products. It includes the brands of Super, U-Smart, Mingshan which was launched in October 2008 and Helanruniu which was launched in December 2008; |
| · | Baby food segment: Baby food segment covers the sale of prepared baby food and nutritional snacks for babies and children. It includes the brand of Huiliduo which was launched in March 2009 and the nutritional snacks component which we expect to launch in late 2009; |
| · | Nutritional ingredients and supplements segment: Nutritional ingredients and supplements segment covers the production and sale of nutritional ingredients and supplements such as chondroitin sulfate, and microencapsulated DHA and ARA. |
On September 16, 2008, we announced a compulsory recall on certain lots of U-Smart products and a voluntary recall of other products that were contaminated or suspected to be contaminated by melamine, a substance not approved for use in food and linked to recent illnesses among infants and children in China. The cost of this action during the year ended March 31, 2009 was $101.5 million, including the cost of product replacement of $48.1 million in cost of sales, the write-down and write-off of affected inventory of $48.5 million in cost of sales, the net amount of $2.3 million to a compensation fund set up by China Dairy Industry Association to settle existing and potential claims arising in China from families of infants affected by melamine contamination in general and administrative expenses, and freight charges of $2.6 million in selling and distribution expenses, of which $4.5 million was recorded as a product recall provision in the consolidated balance sheet as of March 31, 2009. These costs represent our estimate of probable costs based on available data and take into account factors such as expected return rates for the affected units, unit replacement costs, logistical expenses and expenses relating to the hiring of temporary contractors to assist with our recall efforts.
Our product recall does not involve significant cash pay-out to our distributors or customers. Rather, both distributors and customers receive new products of the same value in exchange for recalled products. Since the product recall took place during our second, third and fourth fiscal quarters, our financial results for these quarters were severely impacted and we recognized considerably less revenue compared to the same periods in the prior year.
There have been certain legal proceedings brought against us in connection with the melamine contamination incident, which may have an adverse effect on our results of operations, see Part I - Item 3. Legal Proceedings and Part I - Item 1A. Risk Factors - Product liability claims against us could result in adverse publicity and potential significant monetary damages. Although management is not aware of any additional significant issues associated with the melamine contamination incident, there can be no assurance that additional issues will not be identified in the future and this may have an adverse effect on our results of operations. See Part I - Item 1A. Risk Factors - We are highly dependent upon consumers’ perception of the safety and quality of our products. Any ill effects, product liability claims, recalls, adverse publicity or negative public perception regarding particular ingredients or products or our industry in general could harm our reputation and damage our brand and adversely affect our results of operations.
Since the beginning of October 2008, all of our manufacturing facilities have been returned to service, after passing government inspections and obtaining permission from governing authorities. We continue to comply with random and unscheduled government testing and to conduct enhanced and systematic testing in-house.
In order to regain customer confidence and to ensure products of the highest quality, we are now using imported milk powder from Europe and New Zealand in our U-Smart, Super and Helanruniu, or Holsteina series products. Through our network of distributors, we have restocked substantially all of our contracted shelf space nationwide with new U-Smart series products and new Super series products produced after October 1, 2008. All such products have been found free of melamine and compliant with various government tests.
In October 2008, we acquired a prepared baby food business from Huilian, the “Huiliduo” brand series, and set up a subsidiary, Beijing Shengyuan Huiliduo Food Technology Co., Ltd. (“Huiliduo”), to receive this business. Huiliduo began operations in March 2009.
In late October 2008, we launched an entirely new series of infant formula products, the “Mingshan” series, aimed at the expansive lower and mid-end Chinese markets. We began selling the Mingshan series of products in December 2008.
In December 2008, we launched an entirely new series of powdered adult formula products, the “Helanruniu” or “Holsteina” series, aimed at the higher-end powdered adult formula markets. We began selling the Helanruniu series of products in December 2008.
Due to the significant reduction of sales caused by the melamine contamination incident and replacement of recalled products, our net sales for the fiscal year ended March 31, 2009 decreased by 13.7% to $312.5 million from $362.1 million for the prior fiscal year. Our gross profit for the fiscal year ended March 31, 2009 decreased by 71.4% to $53.4 million from $186.5 million for the prior fiscal year. Our net loss for the fiscal year ended March 31, 2009 was $100.5 million, as compared to net income of $45.7 million for the prior fiscal year.
The net loss for the fiscal year ended March 31, 2009 compared to the prior fiscal year was attributable primarily to the significant cost of product recall and decreased sales from the second half of September 2008 to March 2009.
The Company’s main operations are located in mainland China. Though the recent disruptions in the overall economy and financial markets is less severe in China than in the U.S., it could reduce consumer confidence in the economy and negatively affect consumers’ spending, which could be harmful to our financial position and results of operations. See Part I - Item 1A. Risk Factors - The recent disruptions in the overall economy and the financial markets may adversely impact our business and results of operations and may limit our access to additional financing.
FACTORS AFFECTING OUR RESULTS OF OPERATIONS
Our operating results are primarily affected by the following factors:
Perceptions of Product Quality and Safety
Rising consumer wealth in China has contributed to a greater acceptance by consumers in China of and desire for higher-priced products with perceived quality advantages associated with such products. Thus, we believe that infant formula producers with a reputation for quality and safety should be able to command higher average selling prices and thereby generate higher gross margins than competitors who do not possess the same perceived reputation for quality and safety. Conversely, any decrease in consumer perceptions of quality and safety could adversely impact such producers’ sales and gross margins. Moreover, a decrease in the quality and safety of any particular product could trigger wider negative perception of the decrease in the quality and safety of all producers, thereby affecting the industry generally. For example, the recent melamine contamination incident had resulted in a significant reduction in the sales of a number of major dairy product companies in China, including us. If a future market crisis involving any of our products should occur, especially if management failed to respond to such crisis in a timely and effective manner, our brand recognition and reputation could be severely damaged, which could adversely affect our results of operations. See Part I - Item 1A. Risk Factors—Risks Related to Our Business—We are highly dependent upon consumers’ perception of the safety and quality of our products. Any ill effects, product liability claims, recalls, adverse publicity or negative public perception regarding particular ingredients or products or our industry in general could harm our reputation and adversely affect our results of operations.
Brand Recognition and Customer Loyalty
In recent years, there has been growing demand in China for premium infant formula products due to increasing consumer awareness of brand image and nutritional value of the products offered by leading producers. Although the market is still highly competitive, we believe that companies with strong national brands and customer loyalty will increasingly capture market share from regional brands with less brand recognition. Moreover, we believe brand recognition and customer loyalty are predominantly influenced by customer perceptions of the quality and safety of branded products. We believe the recent melamine contamination incident involving 22 infant formula producers have increased the importance of consumer perceptions of quality and safety and the need to maintain and increase brand recognition and customer loyalty.
Competition and Market Position
While China’s infant formula market is expected to grow significantly, competition is intense. The market has become highly fragmented in recent years as an increasing number of infant formula producers have entered the market. Based on CIC data, in 2008, there were over 30 companies selling infant formula in China. We face significant competition from domestic and multinational producers. A small number of multinational players enjoy significant market share in China, particularly in the more affluent major urban areas, based on greater brand name recognition among Chinese consumers. In addition, competition from domestic producers has become more intense in recent years, especially from large national milk companies, such as Yili, Yashili, Beingmate and Feihe, that have entered the infant formula market.
We focus on developing and marketing premium products for the infant formula market in China. By leveraging our focused marketing strategy, our brand name and our sales and marketing infrastructure, we have been able to sell infant formula products to consumers in China’s small to mid-size cities and rural areas and are perceived to deliver premium quality that justifies our premium prices. This strategy has allowed us to maintain and improve our market share in our primary markets.
Product Offering and Pricing
Infant formula has been, and is expected to remain, our primary product. Due to rising economic affluence in China, infant formula products have become more affordable, resulting in the rapid growth of the overall market for infant formula in China. Despite the recent rapid growth, we believe much of the market is still underserved with respect to infant formula. We believe this growth in demand will help drive sales for many PRC infant formula producers, but companies with strong brand loyalty and extensive distribution networks in China will have greater ability to capitalize on such growth as well as to increase prices and pass on higher raw material costs to customers. This can be accomplished through launching higher-priced new infant formula product lines (such as our Super infant formula products) or re-launching older product lines with higher prices and improved product features (such as our U-Smart infant formula products).
Raw Material Supply and Prices
The per unit costs of producing our infant formula are subject to the supply and price volatility of raw milk and other raw materials, which are affected by the PRC and global markets. For example, raw milk prices are affected by factors such as geographic location, fluctuations in production and competition. Historically, we have been able to meet our raw milk supply needs by building our processing facilities close to our milk suppliers and by maintaining long-term business relationships with milk collection stations. Since the melamine contamination incident, we have been using imported milk powder for our Super, U-Smart and Helanruniu or Holsteina series products. This has led to a significant reduction in our raw milk procurement.
Although we have not used as much raw milk in the aftermath of the melamine incident, increases in the price of raw milk, milk powder and whey protein powder would negatively impact our gross margins if we are not able to offset such price increases through increases in our selling price or change in product mix. However, a significant drop in milk powder prices may also adversely affect our business and cause us to face inventory write-down as we currently continue to produce large amount of milk powder domestically for commercial resale. See Part I - Item 1A. Risk Factors—Risks Related to Our Business—We might face inventory write-down if milk power inventory continues to increase and milk powder prices continue to decline.
Advertising and Sales Promotion Costs
We have historically relied on our extensive distribution network, our consumer education programs and customer relation services to market and sell our products. We substantially increased our television advertising expenditures during the fiscal year ended March 31, 2009 as part of our strategy of improving brand recognition on a national level and to promote our premium products. We intend to continue to spend significant amounts on national advertising and promotion efforts. In the aftermath of the melamine contamination incident, we also intensified our efforts to improve our corporate image and brand name and to recover our lost market share.
TAXATION
We file separate tax returns in the United States and China. Income taxes of our subsidiaries are calculated in accordance with taxation principles currently effective in the PRC. For Synutra Illinois and Synutra Delaware, applicable U.S. tax laws are followed.
On March 16, 2007, the National People’s Congress of the PRC approved and promulgated a new tax law, which took effect beginning January 1, 2008. The Company’s PRC subsidiaries then measure and pay enterprise income tax pursuant to the new tax law. Under the new tax law, foreign investment enterprise and domestic companies are subject to a uniform tax rate of 25%. The new tax law provides a five-year transition period from its effective date for those enterprises which were established before the promulgation date of the new tax law and which were entitled to a preferential lower tax rate under the then effective tax laws or regulations.
We operate under tax holidays in PRC, which are effective through December 2012. The impact of these tax holidays decreased PRC taxes by $3.2 million, $14.0 million and $5.2 million for fiscal year 2009, 2008 and 2007, respectively. The benefit of the tax holidays on net income per share was $0.06, $0.26 and $0.10 for fiscal year 2009, 2008 and 2007, respectively.
Some of the Company's PRC subsidiaries are eligible under the transition rules to continue enjoying tax holidays or reduced tax rate until expiration. The following table illustrates the applicable tax rate and tax holidays of major PRC subsidiaries under the new EIT Law:
| | | Statutory Tax Rate | | |
| Name of Subsidiaries | | Beginning January 1, 2008 | | Tax Holiday (based on calendar year) |
| Shengyuan Nutritional Food Co., Ltd. | | | 25 | % | 2 years tax free (2004, 2005); 12% (2006, 2007); 12.5% (2008) |
| Qingdao Shengyuan Dairy Co., Ltd. | | | 25 | % | No tax holiday |
| Heilongjiang Mingshan Dairy Co., Ltd. | | | 25 | % | 2 years tax free (2006, 2007); 3 years tax at 12.5% (2008-10) |
| Zhangjiakou Shengyuan Dairy Co., Ltd. | | | 25 | % | 2 years tax free (2006, 2007); 3 years tax at 12.5% (2008-10) |
| Inner Mongolia Shengyuan Food Co., Ltd. | | | 25 | % | 2 years tax free (2008, 2009); 3 years tax at 12.5% (2010-12) |
| Inner Mongolia Mengyuan Food Co., Ltd. | | | 25 | % | No tax holiday |
| Meitek Technology (Qingdao) Co., Ltd. | | | 25 | % | 2 years tax free (2008, 2009); 3 years tax at 12.5% (2010-12) |
| Heilongjiang Baoquanling Shengyuan Dairy Co., Ltd. | | | 25 | % | No tax holiday |
| Beijing Shengyuan Huiliduo Food Technology Co., Ltd. | | | 25 | % | No tax holiday |
Substantially all of our income may be derived from dividends we receive from our PRC operating subsidiaries described above. The New EIT Law and its implementing rules generally provide that a 10% withholding tax applies to China-sourced income derived by non-resident enterprises for PRC enterprise income tax purposes. We expect that such 10% withholding tax will apply to dividends paid to us by our PRC subsidiaries but this treatment will depend on our status as a non-resident enterprise. For detailed discussion of PRC tax issues related to resident enterprise status, see Part-I - Item 1A. Risk Factors — Risks Associated with Doing Business in China — Under China's New EIT Law, we may be classified as a ‘resident enterprise’ of China. This classification could result in unfavorable tax consequences to us and our non-PRC shareholders.
Each of our PRC subsidiaries files stand-alone tax returns and we do not file a consolidated tax return.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
We prepare our financial statements in accordance with US GAAP. The preparation of these financial statements requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Management periodically evaluates the estimates and judgments made. Management bases its estimates and judgments on historical experience and on various factors that are believed to be reasonable under the circumstances. Actual results may differ from these estimates as a result of different assumptions or conditions.
The following critical accounting policies affect the more significant judgments and estimates used in the preparation of our financial statements.
Revenue
We recognize revenue when title and risk and rewards for the products are transferred to the customer, price is fixed and determinable and collectability is reasonably assured. At the time of the sale, we also record estimates for a variety of sales deductions, including value added taxes, rebates, discounts and incentives, trade promotions and product returns. Sales deductions are reported as a reduction of revenue. Most of our nutritional product sales are made through distributors. Our revenue arrangements with most of our distributors require distributor advance payment prior to any shipment and delivery of goods by us to such distributors. Under this distributor arrangement, evidenced by purchase order together with advance payment, sales revenue is realized and earned upon acceptance of delivery of products by the distributors. We apply this revenue recognition policy uniformly to all nutritional products, including all dairy-based pediatric and adult nutritional products.
A small fraction of our nutritional product sales are through supermarket retailers directly. Our revenue arrangement with some of these retailers includes right of return clause. Our price to the supermarkets is fixed. The supermarkets’ obligation to us would not be changed in the event of theft or physical destruction or damage of the product. We recognize revenue when the supermarkets have paid us, or the supermarkets are obligated to pay us and the obligation is not contingent on resale of the product. The amount of future returns are estimated and recognized in the current period.
Our gross sales are subject to various deductions, primarily comprised of rebates and discounts to distributors and retailers. These deductions represent estimates of the related obligations, requiring the use of judgment when estimating the impact of these sales deductions on gross sales for a reporting period. We report these adjustments as a reduction of gross sales to arrive at net sales.
| • | We offer rebates to distributors and supermarket retailers to sustain and increase our product market share. These rebate programs provide that distributors and supermarket retailers receive a rebate after attaining certain performance parameters relating to product purchases, formulary status and/or pre-established market share milestones relative to competitors. Since rebates are contractually agreed upon, we estimate rebates based on the specific terms in each agreement, historical experience, anticipated reimbursement channel mix and product growth rates. We consider the sales performance of products subject to rebates and other contract discounts and adjust the provision periodically to reflect actual experience. Actual amount may differ if the level of redemption rates and performance vary from estimates. The Company records rebates as a reduction of revenues in the year in which these programs are offered. |
| • | We record a provision for estimated sales returns due to package damage, merchandise slow moving at certain retail customer shelf, and termination of distributorships, which we estimate through a comparison of historical return data to related sales. We use historical rates of return and adjust for known or expected changes in the marketplace when appropriate. The sales return amount represents management’s best estimates based on the available information at the time of estimate is made. |
| • | For product sales and promotions at supermarkets and shopping malls, certain expenses in relation to shelf display, end-cap placement, bar-coding, banner advertising, etc. are paid to supermarkets and shopping mall operators. These expenses are deducted from revenues in accordance with EITF 01-9 “Accounting for Consideration Given by a Vendor to a Customer (Including a Reseller of the Vendor’s Product).” |
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts primarily based on the age of receivables and factors surrounding the credit risk of specific customers. We regularly review the recoverability and age of our trade receivables. If there is a deterioration of customers' creditworthiness or actual defaults are higher than our historical experience, we will provide additional allowances.
Inventories
Our inventories are stated at the lower of cost or net realizable value. The valuation of inventory requires us to estimate excess and slow moving inventory. The determination of the value of excess and slow moving inventory is based upon assumptions of future demands and market conditions. If actual market conditions are less favorable than those projected by management, inventory write-downs may be required. We routinely evaluate quantities and value of our inventories in light of current market conditions and market trends, and record write-down against the cost of inventories for a decline in net realizable value. Inventory write-down charges establish a new cost basis for inventory. In estimating obsolescence, we utilize our backlog information and project future demand. Market conditions are subject to change and actual consumption of inventories could differ from forecasted demand. Furthermore, the price of raw milk, our primary raw material, is subject to fluctuations based on global supply and demand. If actual market conditions are less favorable or other factors arise that are significantly different than those anticipated by management, additional inventory write-downs or increases in obsolescence reserves may be required. Our management continually monitors the changes in the purchase price paid for raw milk. Our products have a limited life cycle and obsolescence has not historically been a significant factor in the valuation of inventories given our inventory turnover
Accounting for Warrants
In April 2007, we issued warrants to purchase 400,000 shares of common stock to ABN as part of certain financing arrangements with ABN. The fair value of the warrants was approximately $2.7 million at the date of grant, estimated using the Black-Scholes-Merton option pricing model. Determining the fair value of the warrant charge requires us to make highly subjective assumptions, including expected contractual life of the warrants and the price volatility of the underlying shares. We estimate stock price volatility based on our historical volatility of stock. The assumptions used in calculating the fair value of the warrants represent our management’s best estimates, but these estimates involve inherent uncertainties and the application of management’s judgment.
Income Taxes
The provision for income taxes has been determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. The provision for income taxes represents income taxes paid or payable for the current year plus the change in deferred taxes during the year. Deferred taxes result from differences between the financial and tax bases of our assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. The assessment of whether or not a valuation allowance is required often requires significant judgment including the long-range forecast of future taxable income and the evaluation of tax planning initiatives. Adjustments to the deferred tax valuation allowances are made to earnings in the period when such assessments are made.
Our tax rate is based on expected income, statutory tax rates and tax planning opportunities available in the various jurisdictions in which we operate. For interim financial reporting, we estimate the annual tax rate based on projected taxable income for the full year and record a quarterly income tax provision in accordance with the anticipated annual rate. As the year progresses, we refine the estimates of the year’s taxable income as new information becomes available, including year-to-date financial results. This continual estimation process often results in a change to our expected effective tax rate for the year. When this occurs, we adjust the income tax provision during the quarter in which the change in estimate occurs so that the year-to-date provision reflects the expected annual tax rate. Significant judgment is required in determining our effective tax rate and in evaluating its tax positions.
In accordance with SFAS No. 109, “Accounting for Income Taxes,” we recognize deferred tax assets and liabilities based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities. Deferred tax assets represent items to be used as a tax deduction or credit in future tax returns for which we have already properly recorded the tax benefit in the income statement. At least quarterly, we assess the likelihood that the deferred tax asset balance will be recovered from future taxable income. We take into account such factors as prior earnings history, expected future earnings, carry-back and carry-forward periods, and tax strategies that could potentially enhance the likelihood of a realization of a deferred tax asset. To the extent recovery is unlikely, a valuation allowance is established against the deferred tax asset and increasing our income tax expense in the year such determination is made.
APB Opinion No. 23, “Accounting for Income Taxes, Special Areas,” does not require U.S. income taxes to be provided on foreign earnings when such earnings are indefinitely reinvested offshore. We periodically evaluate our investment strategies with respect to each foreign tax jurisdiction in which we operate to determine whether foreign earnings will be indefinitely reinvested offshore and, accordingly, whether U.S. income taxes should be provided when such earnings are recorded. As of March 31, 2009, we believed all earnings generated in China would be permanently reinvested and as a result, we did not record any income taxes on such earnings.
We adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes, an Interpretation of FASB Statement No. 109” (FIN 48) effective April 1, 2007. In accordance with FIN 48, we recognize a tax benefit associated with an uncertain tax position when, in our judgment, it is more likely than not that the position will be sustained upon examination by a taxing authority. For a tax position that meets the more-likely-than-not recognition threshold, we initially and subsequently measure the tax benefit as the largest amount that we judge to have a greater than 50% likelihood of being realized upon ultimate settlement with a taxing authority. Our liability associated with unrecognized tax benefits is adjusted periodically due to changing circumstances, such as the progress of tax audits, case law developments and new or emerging legislation. Such adjustments are recognized entirely in the period in which they are identified. Our effective tax rate includes the net impact of changes in the liability for unrecognized tax benefits and subsequent adjustments as considered appropriate by management. As of March 31, 2009, we had recorded FIN 48 liabilities of $964,000 for our PRC subsidiaries.
We classify interest and penalties recognized on the liability for unrecognized tax benefits as income tax expense. For the fiscal year ended March 31, 2009, the unrecognized tax benefit did not change significantly and the amount of interest and penalties related to uncertain tax position is immaterial.
Product Recall
We establish a reserve for product recall on a product-specific basis when circumstances giving rise to the recall become known. Facts and circumstances related to the recall, including where the product affected by the recall or withdrawal is located (e.g., with consumers, in customers’ inventory, or in the Company’s inventory), the expected product return rates by our distributor and end-customers, cost estimates for shipping and handling for returns and estimated replacement costs are considered when establishing a product recall reserve. These factors are updated and reevaluated each period and the related reserves are adjusted when these factors indicate that the recall reserve is either not sufficient to cover or exceeds the estimated product recall expenses.
Impairment of Goodwill and Indefinite Lived Intangible Assets
We account for goodwill and intangible assets with indefinite lives in accordance with SFAS No. 142, “Goodwill and Other Intangible Assets,” or SFAS 142. SFAS No. 142 states that goodwill and intangible assets with indefinite lives are not amortized, but are instead reviewed for impairment annually (or more frequently if impairment indicators arise). We conduct our annual impairment testing on March 31 to determine if we will be able to recover all or a portion of the carrying value of goodwill and intangible assets with indefinite lives.
The application of the impairment test requires judgment, including the identification of reporting units, assignments of assets and liabilities to reporting units and the determination of the fair value of each reporting unit. Further, the impairment test involves the use of accounting estimates and assumptions related to future operating results. Consistent with the requirements of SFAS No. 142, the fair values of our reporting units are generally based on discounted cash flow projections that are believed to be reasonable under current and forecasted circumstances, the results of which form the basis for making judgments about carrying values of the reported net assets of our reporting units.
We will continue to closely monitor the 2010 results and projections for our reporting units and the economic conditions of the product end-markets. Any significant change in market conditions and estimates or judgments could give rise to impairment in the period that the change becomes known.
Prior to performing the goodwill impairment testing process for a reporting unit under SFAS 142, if there is reason to believe that other non-goodwill related intangible assets may be impaired, these other intangible assets must first be tested for impairment under SFAS No. 142 or SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets,” or SFAS No. 144. Assets governed by SFAS No. 144 require a recoverability test for impairment whereby the gross undiscounted cash flows are determined specific to the asset. For non-goodwill related intangible assets with indefinite lives, a fair value determination is made. If the carrying value of the asset exceeds the fair value, then impairment occurs. The carrying values of these assets are impaired as necessary to provide the appropriate carrying value for the goodwill impairment calculation.
These impairment tests also involve the use of accounting estimates and assumptions believed to be reasonable, the results of which form the basis for our conclusions. Significant changes to these estimates and assumptions could adversely impact our conclusion to these impairment tests.
No goodwill or indefinite lived intangible assets have been impaired during any of the periods presented.
RESULTS OF OPERATIONS
The following table sets forth, for the periods indicated, our consolidated statements of operations and certain other information, each expressed as a percentage of net sales.
| | | Fiscal Years Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | Amount | | | % of Net Sales | | | Amount | | | % of Net Sales | | | Amount | | | % of Net Sales | |
| | | (in thousands) | |
| Net sales | | $ | 312,528 | | | | 100.0 | % | | $ | 362,090 | | | | 100.0 | % | | $ | 216,605 | | | | 100.0 | % |
| Cost of sales | | | 259,086 | | | | 82.9 | % | | | 175,568 | | | | 48.5 | % | | | 109,900 | | | | 50.7 | % |
| Gross profit | | | 53,442 | | | | 17.1 | % | | | 186,522 | | | | 51.5 | % | | | 106,705 | | | | 49.3 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Selling and distribution expenses | | | 44,178 | | | | 14.1 | % | | | 34,449 | | | | 9.5 | % | | | 25,561 | | | | 11.8 | % |
| Advertising and promotion expenses | | | 115,478 | | | | 36.9 | % | | | 76,388 | | | | 21.1 | % | | | 52,322 | | | | 24.2 | % |
| General and administrative expenses | | | 25,455 | | | | 8.1 | % | | | 16,013 | | | | 4.4 | % | | | 7,031 | | | | 3.2 | % |
| Other operating income, net | | | 5,790 | | | | 1.9 | % | | | 1,492 | | | | 0.4 | % | | | 1,109 | | | | 0.5 | % |
| Income (loss) from operations | | | (125,879 | ) | | | -40.3 | % | | | 61,164 | | | | 16.9 | % | | | 22,900 | | | | 10.6 | % |
| Interest expense | | | 4,857 | | | | 1.6 | % | | | 6,354 | | | | 1.8 | % | | | 1,896 | | | | 0.9 | % |
| Interest income | | | 341 | | | | 0.1 | % | | | 1,801 | | | | 0.5 | % | | | 356 | | | | 0.2 | % |
| Other income (expense), net | | | (580 | ) | | | -0.2 | % | | | (3,084 | ) | | | -0.9 | % | | | 110 | | | | 0.1 | % |
| Income (loss) before provision (benefit) for income tax | | | (130,975 | ) | | | -41.9 | % | | | 53,527 | | | | 14.8 | % | | | 21,470 | | | | 9.9 | % |
| Provision (benefit) for income tax | | | (30,386 | ) | | | -9.7 | % | | | 7,855 | | | | 2.2 | % | | | 1,596 | | | | 0.7 | % |
| Net income (loss) before minority interests | | | (100,589 | ) | | | -32.2 | % | | | 45,672 | | | | 12.6 | % | | | 19,874 | | | | 9.2 | % |
| Minority interests | | | (40 | ) | | | -0.0 | % | | | 11 | | | | 0.0 | | | | — | | | | — | % |
| Net income (loss) attributable to shareholders | | $ | (100,549 | ) | | | -32.2 | % | | $ | 45,661 | | | | 12.6 | % | | $ | 19,874 | | | | 9.2 | % |
FISCAL YEAR ENDED MARCH 31, 2009 COMPARED TO FISCAL YEAR ENDED MARCH 31, 2008
Net Sales
Net sales for the fiscal year ended March 31, 2009 decreased by 13.7% to $312.5 million from $362.1 million for the prior fiscal year. This decrease in net sales was mainly due to the significant product recall and decrease in sales which resulted from the melamine contamination incident, partially offset by the increases in volume and average selling price before the melamine contamination incident.
Powdered formula segment
Net sales of our powdered formula products, including infant milk formula and other powdered formula products for children and adults under our Super, U-Smart, Mingshan and Helanruniu brand names, accounted for 91.1% of our total sales for the fiscal year ended March 31, 2009. Net sales of our powdered formula products for the fiscal year ended March 31, 2009 decreased by 11.3% to $284.8 million from $321.1 million for the prior fiscal year, primarily as a result of the following factors:
| ● | Sales volume of powdered formula products decreased by 26.5% to 30,383 tons for the fiscal year ended March 31, 2009 from 41,359 tons for the prior fiscal year, due primarily to a slow-down in sales activities in the second half of September to March 2009 following the melamine contamination incident, partially offset by a significant business growth experienced in the months from April to August, 2008. |
| ● | The average selling price of our powdered formula products for the fiscal year ended March 31, 2009 increased by 20.7% to $9,374 per ton from $7,764 per ton for the prior fiscal year. This increase in average selling price was a combined result of a greater proportion of higher-priced products in our product mix and an increase of our sales price in August 2008. |
Baby food segment
We acquired the prepared baby food business from Huilian in October 2008. The business began to generate sales for us in March 2009, and we are currently in the process of providing shipments of prepared baby food to be placed in stores. Our nutritional snack business was still in its pre-operations stage and hence did not have sales for the fiscal year ended March 31, 2009. We plan to begin its operations in late 2009.
Nutritional ingredients and supplements segment
Net sales of nutritional ingredients and supplements segment for the fiscal year ended March 31, 2009 was $3.4 million, representing basically all inter-segment sales of nutritional ingredients, such as microencapsulated DHA and ARA, which were used in the production of powdered infant formula products. We did not have any net sales in the fiscal year ended March 31, 2008 as the nutritional ingredients and supplements segment had not begun its operations by March 31, 2008.
Cost of Sales
Cost of sales for the fiscal year ended March 31, 2009 increased by 47.6% to $259.1 million from $175.6 million for the prior fiscal year. The increase in the cost of sales is due primarily to the increase of product recall related cost, partially offset by a decrease in the sales volume of our powdered formula products.
Powdered formula segment
Cost of sales for the powdered formula products for the fiscal year ended March 31, 2009 increased by 64.7% to $234.5million from $142.4 million for the prior fiscal year. The increase in the cost of sales is due primarily to increase of product recall related cost, partially offset by a decrease in the sales volume of our powdered formula products. The portion of the product recall cost, which has been recognized in cost of sales for the fiscal year ended March 31, 2009, was $96.6 million, reflecting the cost of recalled products of $48.1 million and the write-down and write-off of affected inventory of $48.5 million. The sales volume of powdered formula products sold for the fiscal year ended March 31, 2009 decreased by 10,976 tons as compared to the prior fiscal year.
Baby food segment
We acquired the prepared baby food business from Huilian in October 2008. The business began to generate sales for us in March 2009 and we are currently in the process of providing shipments of prepared baby food to be placed in stores. Our nutritional snack business was still in its pre-operations stage and hence did not have cost of sales for the fiscal year ended March 31, 2009. We plan to begin its operations in late 2009.
Nutritional ingredients and supplements segment
Cost of sales of nutritional ingredients and supplements segment for the fiscal year ended March 31, 2009 was $3.2 million, representing basically all cost of inter-segment sales of nutritional ingredients such as microencapsulated DHA and ARA which are used in the production of infant powdered formula products. We did not have any cost of sales in the fiscal year ended March 31, 2008 as the nutritional ingredients and supplements segment had not begun its operations by March 31, 2008.
Gross Profit and Gross Margin
As a result of the foregoing, gross profit for the fiscal year ended March 31, 2009 decreased by 71.4% to $53.4 million from $186.5 million for the prior fiscal year. Gross profit for our powdered formula products for the fiscal year ended March 31, 2009 decreased by 71.9% to $50.3 million from $178.7 million for the prior fiscal year due primarily to the significant costs in relation to product recall including the cost of recalled products and the write-down and write-off of affected inventory, and due also to decreased sales in the last three fiscal quarters following the melamine contamination incident. Gross profit for our baby food segment and nutritional ingredients and supplements segment for the fiscal year ended March 31, 2009 were $0.2 million and nil, respectively.
Our overall gross margin decreased to 17.1% for the fiscal year ended March 31, 2009 from 51.5% for the prior fiscal year. Our gross margin for powdered formula products was 17.7% for the fiscal year ended March 31, 2009, as compared to 55.7% for the prior fiscal year. The decrease in our gross margin for powdered formula products was primarily due to the significant cost of the product recall and increased free products offered to our customers in the aftermath of the melamine contamination incident, partially offset by an increase in the proportion of sales of our higher margin infant formula products. Our gross margin for baby food segment and nutritional ingredients and supplements segment for the fiscal year ended March 31, 2009 were 5.8% and nil, respectively.
Selling and Distribution Expenses
Selling and distribution expenses for the fiscal year ended March 31, 2009 increased by 28.5% to $44.2 million from $34.4 million for the prior fiscal year. This increase was primarily due to an increase in compensation expenses for our sales force, an increase in travel and communication expenses and the freight charges relating to product recall and replacement. Total compensation for our sales force for the fiscal year ended March 31, 2009 increased by 49.7% to $21.7 million from $14.5 million for the prior fiscal year. This increase was primarily due to the compensation paid to the field promoters (nutrition consultants), who were previously paid by our distributors on commission basis, during the months of October to December 2008 following the melamine contamination incident. We took up such expenses in order to retain the services of these field promoters as their commission based salaries decreased significantly during this period. In addition, the increase in the number of sales staff to 2,997 as of March 31, 2009 from 2,780 as of March 31, 2008 also contributed to the increase in compensation expense relating to our sales force. Travel and communication expenses for the fiscal year ended March 31, 2009 increased by 69.0% to $7.1 million from $4.2 million for the prior fiscal year, due mainly to the expansion of our business. The freight charges of $2.6 million relating to product recall and replacement also contributed to the increase in selling and distribution expenses.
Advertising and Promotion Expenses
Advertising and promotion expenses for the fiscal year ended March 31, 2009 increased by 51.2% to $115.5 million from $76.4 million for the prior fiscal year. Advertising expenses for the fiscal year ended March 31, 2009, which accounted for 63.0% of total advertising and promotion expenses, increased by 140.3% to $72.8 million from $30.3 million for the prior fiscal year, due primarily to our intensified efforts to improve our corporate image and brand name and to recover our lost market share following the melamine contamination incident. Promotion expenses for the fiscal year ended March 31, 2009, which accounted for 37.0% of total advertising and promotion expenses, decreased by 7.4% to $42.7 million from $46.1 million for the prior fiscal year.
General and Administrative Expenses
General and administrative expenses for the fiscal year ended March 31, 2009 increased by 59.4% to $25.5 million from $16.0 million for the prior fiscal year. The increase in general and administrative expenses was primarily due to a net contribution of $2.3 million made to the compensation fund set up by China Dairy Industry Association for the settlement of existing and potential claims in China from families of infants affected by melamine contamination, an increase of $2.6 million in salary and social insurance as result of the increased headcount, an increase of $1.7 million in rental expenses for new office space, partially offset by a decrease of $1.0 million in legal and professional expenses associated with our cancelled public offering.
Other operating income, net
Other operating income for the fiscal year ended March 31, 2009 increased by 286.7% to $5.8 million from $1.5 million for the prior fiscal year. The increase in other operating income was primarily due to a grant received from local government in recognition of our significant contribution to the local economy.
Interest Income
Interest income for the fiscal year ended March 31, 2009 decreased to $0.3 million from $1.8 million for the prior fiscal year due to significant decrease in our cash and cash equivalent balances.
Interest Expense
Interest expense for the fiscal year ended March 31, 2009 decreased to $4.9 million from $6.4 million for the prior fiscal year, due primarily to the amortization of debt discount associated with the issuance of warrants to ABN which amounted to $2.6 million in the fiscal year ended March 31, 2008, which was offset by the increased interest expenses due to significant borrowing after the melamine contamination incident in the fiscal year ended March 31, 2009.
Provision (benefit) for Income Tax
As a result of the net loss arising primarily from the cost of the product recall, we recorded an income tax benefit of $30.4 million for the fiscal year ended March 31, 2009, as compared to an income tax expense of $7.9 million for the fiscal year ended March 31, 2008. Our effective tax rate increased to 23.2% for the fiscal year ended March 31, 2009 from 14.7% for the prior fiscal year. This increase in our effective income tax rate was primarily due to the expirations of tax holidays enjoyed by certain subsidiaries in China.
Net Income (Loss) Attributable to Shareholders
As a result of the foregoing, net loss attributable to shareholders for the fiscal year ended March 31, 2009 was $100.5 million, as compared to net income of $45.7 million for the prior fiscal year.
FISCAL YEAR ENDED MARCH 31, 2008 COMPARED TO FISCAL YEAR ENDED MARCH 31, 2007
Net Sales
Net sales for the fiscal year ended March 31, 2008 increased by 67.2% to $362.1 million from $216.6 million for the prior fiscal year. This increase in net sales was a result of an increase in volume of products sold and an increase in average selling price. The increase in the volume of products sold was partly driven by the growth of the infant formula market in China and increased market awareness for our products as a result of our marketing initiatives. These marketing initiatives were built around a TV advertising campaign that started in September 2006, which was enhanced by continued targeted sales and promotional activities at the store level.
Powdered formula segment
Net sales of our powdered formula products, including infant formula and other nutritional products for children and adults under our Super, U-Smart, U-Strong, and National Standards sub-brands, accounted for 88.7% of our total sales for the fiscal year ended March 31, 2008. Net sales of our powdered formula products for the fiscal year ended March 31, 2008 increased by 91.7% to $321.1 million from $167.5 million for the prior fiscal year, primarily as a result of the following factors:
| • | Sales volume of powdered formula products increased by 48.0% to 41,359 tons for the fiscal year ended March 31, 2008 from 27,942 tons for the prior fiscal year. |
| • | The average selling price of our powdered formula products for the fiscal year ended March 31, 2008 increased by 29.5% to $7,764 per ton from $5,995 per ton for the prior fiscal year. This increase in average selling price was primarily due to an increase in sales of Super infant formula products, which resulted in a greater proportion of higher-priced products in our product mix, and a substantial increase in the average selling price for our U-Smart infant formula products resulted primarily from our introduction of U-Smart Gold Package. |
Baby food segment and Nutritional ingredients and supplements segment
Baby food segment and nutritional ingredients and supplements segment had not began their operations by March 31, 2008.
Cost of Sales
Cost of sales for the fiscal year ended March 31, 2008, including purchases from third-party producers, increased by 59.8% to $175.6 million from $109.9 million for the prior fiscal year. The increase in cost of sales was due to an increase in the cost of sales for our powdered formula products.
Powdered formula segment
Cost of sales for the powdered formula products for the fiscal year ended March 31, 2008 increased by 118.4% to $142.4 million from $65.2 million for the prior fiscal year. The increase of 13,417 tons of powdered formula products sold for the fiscal year ended March 31, 2008 as compared to the prior fiscal year was the primary reason for the increase in cost of sales. The increase in unit prices of raw materials (principally raw milk and whey protein powder) for the fiscal year ended March 31, 2008 as compared to the prior fiscal year also contributed to the increase in our cost of sales. To a lesser extent, our increase in cost of sales for the fiscal year ended March 31, 2008 was also due to the increase in the percentage of premium products sold.
Baby food segment and Nutritional ingredients and supplements segment
Baby food segment and nutritional ingredients and supplements segment had not began their operation by March 31, 2008.
Gross Profit and Gross Margin
As a result of the foregoing, gross profit for the fiscal year ended March 31, 2008 increased by 74.8% to $186.5 million from $106.7 million for the prior fiscal year. Gross profit for our powdered formula products for the fiscal year ended March 31, 2008 increased by 74.7% to $178.7 million from $102.3 million for the prior fiscal year.
Our gross margin increased to 51.5% for the fiscal year ended March 31, 2008 from 49.3% for the prior fiscal year. Our gross margin for powdered formula products was 55.7% for the fiscal year ended March 31, 2008, as compared to 61.1% for the prior fiscal year. Our overall gross margin increased primarily due to an increase in the proportion of sales of our powdered formula products, which had a higher margin than our other products. The decrease in the gross margin of our powdered formula products was primarily due to rising raw material costs, which was partially offset by a significant increase in the proportion of sales of our higher margin infant formula products. The gross margin of our powdered formula products was also negatively impacted by the increased supermarket/shopping mall related expenses, such as end-cap placement costs and admission fees, which are recoded as reduction of revenue, due to our rapid expansion into supermarkets/shopping malls.
Selling and Distribution Expenses
Selling and distribution expenses for the fiscal year ended March 31, 2008 increased by 34.8% to $34.4 million from $25.6 million for the prior fiscal year. This increase was primarily a result of increased compensation expenses for our sales force, and an increase in shipping and handling expenses as well as travel expenses, partially offset by a decrease in entertainment, event and communication expenses. Total compensation for our sales force for the fiscal year ended March 31, 2008 increased by 58.7% to $14.5 million from $9.2 million for the prior fiscal year. This increase was primarily due to the continuation of our targeted sales incentive programs. In addition, the increase in the number of sales staff to 2,780 as of March 31, 2008 from 2,050 as of March 31, 2007 also contributed to the increase in compensation expense relating to our sales force. As a result of increases in sales and business activities, shipping and handling expenses for the fiscal year ended March 31, 2008 increased by 67.5% to $6.7 million from $4.0 million for the prior fiscal year, generally in proportion to the increase in sales. Travel expenses for the fiscal year ended March 31, 2008 increased by 48.0% to $2.9 million from $2.0 million for the prior fiscal year.
Advertising and Promotion Expenses
Advertising and promotion expenses for the fiscal year ended March 31, 2008 increased by 46.0% to $76.4 million from $52.3 million for the prior fiscal year, due to increased nationwide TV advertising and promotional activities at the store level, such as offering promotional products and organizing promotional events. Advertising expenses for the fiscal year ended March 31, 2008 , which accounted for 39.7% of total advertising and promotion expenses, increased by 84.8% to $30.3 million from $16.4 million for the prior fiscal year. Promotion expenses for the fiscal year ended March 31, 2008, which accounted for 60.3% of total advertising and promotion expenses, increased by 28.1% to $46.1 million from $36.0 million for the prior fiscal year.
General and Administrative Expenses
General and administrative expenses for the fiscal year ended March 31, 2008 increased by 127.7% to $16.0 million from $7.0 million for the prior fiscal year, primarily as a result of increased legal and professional fees and costs for staff salaries and office expenses. Legal and professional fees for the fiscal year ended March 31, 2008 increased by 435.6% to $4.8 million from $0.9 million for the prior fiscal year due to increased corporate transactions. Staff salaries and social insurance for the fiscal year ended March 31, 2008 increased by 93.1% to $4.6 million from $2.4 million for the prior fiscal year, primarily due to increased corporate headcount and average salary levels. Office expenses for the fiscal year ended March 31, 2008 increased by 210.0% to $1.5 million from $0.5 million for the prior fiscal year, primarily due to the addition of subsidiaries and expansion of operations.
Other operating income, net
Other operating income for the fiscal year ended March 31, 2008 increased by 36.4% to $1.5 million from $1.1 million for the prior fiscal year.
Interest Income
Interest income for the fiscal year ended March 31, 2008 increased to $1.8 million from $0.4 million for the prior fiscal year due to significant increases in our cash and cash equivalent balances.
Interest Expense
Interest expense for the fiscal year ended March 31, 2008 increased to $6.4 million from $1.9 million for the prior fiscal year, due primarily to borrowings from ABN, and the amortization of debt discount associated with the issuance of warrants to ABN.
Provision for Income Tax
The provision for income taxes, which is computed on an individual legal entity basis, was $7.9 million and $1.6 million for the fiscal years ended March 31, 2008 and 2007, respectively. Our effective tax rate increased to 14.7% for the fiscal year ended March 31, 2008 from 7.4% for the prior fiscal year. This increase in our effective income tax rate was due to the expiration of a tax holiday enjoyed by Zhangjiakou Shengyuan, and the provision for unrealizable deferred tax assets for our operations.
Net Income Attributable to Shareholders
As a result of the foregoing, net income attributable to shareholders for the fiscal year ended March 31, 2008 increased by 129.8% to $45.7 million from $19.9 million for the prior fiscal year.
LIQUIDITY AND CAPITAL RESOURCES
The accompanying consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates the realization of assets and the liquidation of liabilities in the normal course of business. As a result of the melamine incident and the related product recall, we have experienced significant operating losses and negative cash flows from operations for the fiscal year ended March 31, 2009. As of March 31, 2009, we had a working capital deficit of approximately $80.4 million. In addition, we have not been in compliance with certain covenants in our New ABN loan agreement as of March 31, 2009. We are attempting to renegotiate the terms and covenants of the New ABN loan agreement. As a result of the occurrence of these recent economic events, the ensuing operating losses and negative cash flows and our failure to meet our debt covenants, substantial doubts have been raised about our ability to continue as a going concern. We are currently in the process of evaluating funding alternatives including seeking refinancing of certain short-term loans from PRC banks. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We intend to seek to overcome any substantial doubt concerning our ability to continue as going concern by continuing to pursue our strategic operating goals for enhanced profitability and by obtaining new debt and/or equity financing. Any substantial doubt about our ability to continue as a going concern could also affect our relationship with our trade suppliers and their willingness to continue to conduct business with us on terms consistent with historical practice. These suppliers might respond to an apparent weakening of our liquidity position and to address their own liquidity needs may request faster payment of invoices, new or increased deposits or other assurances. If this were to happen, our need for cash would be intensified and we might be unable to make payments to our suppliers as they become due.
The recent melamine contamination incident has significantly impacted our liquidity. Since the damage to our reputation caused by the melamine contamination incident will take time to recover, the net sales of our powdered formula products were negatively impacted in the fiscal year ended March 31, 2009. In the meantime, we incurred substantial cash outflow for the purchase of raw materials and for operating expenses.
Accordingly, we have had discussions with local banks to obtain short term financing to support our operational needs. As of March 31, 2009, we had short-term borrowings from local banks of $182.6 million with a weighted average interest rate of 4.1%. The loans were secured by the pledge of certain fixed assets held by the Company’s subsidiaries, pledge of land use right in Qingdao, China and pledge of cash deposits which was recorded as restricted cash. The maturity dates of the short term loans from local banks outstanding at March 31, 2009 range from April 2009 to March 2010. As of the date of the filing of this 10-K, all outstanding short-term loans that have become due have been repaid. As of March 31, 2009, we have unsecured long-term borrowing from local banks of $8.8 million maturing in March 2011 with an interest rate of 5.4%. In addition to the loans from local banks, we also borrowed from related parties short term loans amounting to $7.5 million with a weighted average interest rate of 7.8% to finance our acquisition of the Helanruniu trademarks and to support our normal operating needs. The maturity dates of the short term loans outstanding from related parties at March 31, 2009 are in October 2010. As of March 31, 2009, we are not able to meet the financial covenants of the ABN loan and hence it was reclassified to current liabilities since we considered this debt callable by the bank. As of the date of the filing of this 10-K, the waiver has not been granted. Prior to the melamine contamination incident, cash generated from our operating activities were sufficient for normal operating needs, and financing from banks was normally related to investing activities such as expansion of our manufacturing plant.
In order to maintain sufficient funds for our operations, we have postponed the payment of certain accounts payable. The payment terms of accounts payable were usually three months. We negotiated with some suppliers and extended the payment terms.
We do not expect significant cash outflow in relation to the product recall and subsequent replacement for future fiscal quarters other than the cash outflow for the accrued freight charge of $519,000 at March 31, 2009. However, we cannot provide assurance in this regard.
Our cash and cash equivalent balance decreased by $59.7 million to $37.7 million at March 31, 2009, as compared to $97.4 million at March 31, 2008.
The following table sets forth, for the periods indicated, certain information relating to our cash flows:
| | | Fiscal Years Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (in thousands) | |
| Net cash provided by (used in) operating activities | | $ | (109,488 | ) | | $ | 38,229 | | | $ | 14,766 | |
| | | | | | | | | | | | | |
| Net cash used in investing activities | | | (127,287 | ) | | | (33,096 | ) | | | (17,908 | ) |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 174,871 | | | | 64,731 | | | | 16,425 | |
| | | | | | | | | | | | | |
| Effect of foreign currency translation on cash and cash equivalents | | | 2,215 | | | | 6,725 | | | | 1,876 | |
| | | | | | | | | | | | | |
| Net cash flow | | $ | (59,689 | ) | | $ | 76,589 | | | $ | 15,159 | |
Cash Flows from Operating Activities
Net cash used in operating activities was $109.5 million for the fiscal year ended March 31, 2009, as compared to net cash provided by operating activities of $38.2 million for the prior fiscal year. Net cash used in operating activities for the fiscal year ended March 31, 2009 was mainly due to net loss of $100.5 million, non-cash items not affecting 2009 cash flows of $20.0 million, and an $11.1 million increase in working capital. The changes in working capital for the fiscal year ended March 31, 2009, were primarily related to a $50.9 million increase in inventories due to the increase in goods-in-transit for imported milk powder, a $77.0 million increase in accounts payable due to delayed payment to our suppliers resulting from our tightened liquidity position and payable for goods-in-transit for imported milk powder, a $13.4 million increase in accounts receivable due to our extended credit term to certain distributors, a $5.6 million increase in income tax receivable due to overpaid income tax before the melamine incident, and a $4.5 million increase in product recall provision. In the fiscal year ended March 31, 2009, we spent $238.8 million in purchasing raw materials and other production materials, $33.0 million in staff compensation and social welfare, $33.8 million in other taxes, $197.1 million in selling and distribution, advertising and promotion, and general and administrative expenses, and received $403.8 million from our customers.
Net cash provided by operating activities was $38.2 million for the fiscal year ended March 31, 2008, as compared to net cash provided by operating activities of $14.8 million for the prior fiscal year. Net cash provided by operating activities for the fiscal year ended March 31, 2008 was mainly due to net income of $45.7 million, non-cash items not affecting 2008 cash flows of $5.6 million, partially offset by $13.0 million of negative changes in working capital. The changes in working capital for the year ended March 31, 2008, were primarily related to a $39.1 million increase in inventory, a $5.7 million increase in prepaid expense and other current assets, and a $4.1 million increase in accounts receivable, partially offset by a $9.4 million decrease in due from related parties, a $6.8 million increase in accounts payable, and a $8.1 million increase in other liabilities. The increase in inventory was due to several factors: our increased production and sales, the acquisition of Baoquanling and its inventory, and direct whey protein purchase from Eurosérum in France. Due to the distance of Eurosérum and the longer delivery time, we kept more whey protein inventory in our warehouse and had more whey protein in-transit as our inventory. There were no material policy changes in our credit terms to customers and payment terms to suppliers for the fiscal year ended March 31, 2008. In the fiscal year ended March 31, 2008, we spent $198.4 million in purchasing raw materials and other production materials, $21.7 million in staff compensation and social welfare, $19.7 million in other taxes, $143.5 million in selling and distribution, advertising and promotion, and general and administrative expenses, and received $431.5 million from our customers.
Net cash provided by operating activities was $14.8 million for the fiscal year ended March 31, 2007, mainly due to net income of $19.9 million, non-cash items not affecting 2007 cash flows of $1.9 million, partially offset by $7.1 million of negative changes in working capital. The changes in working capital for the fiscal year ended March 31, 2007 were primarily related to a $3.3 million increase in advances from customers, a $4.1 million increase in deferred revenue, partially offset by a $4.6 million increase in inventory, a $4.0 million increase in accounts receivable, and a $3.1 million increase in amounts due from related parties. The increases in inventory, accounts receivable, and advances from customers primarily resulted from the increase in our sales. The increase in amounts due from related parties primarily resulted from increased prepayment to Honnete for the purchase of whey protein.
Cash Flows from Investing Activities
Net cash used in investing activities was $127.3 million for the fiscal year ended March 31, 2009, as compared to $33.1 million for the prior fiscal year. Cash invested in purchases of property and equipment was $44.9 million and $36.4 million for the fiscal year ended March 31, 2009 and 2008, respectively. This increase in net cash used in investing activities is primarily due to our plant expansion to increase our production capacity prior to the melamine contamination incident, partially offset by the suspension of major investing projects in the aftermath of such event. We expect the suspension will slow down our expansion into the nutritional snack business which we expect to launch in late 2009, but it will not materially affect our production capacity to fulfill our customers’ order requirements. Restricted cash increased by $73.9 million for the fiscal year ended March 31, 2009, as compared to a decrease of $4.2 million for the prior fiscal year due to a significant increase in our bank borrowings. Restricted cash represents cash deposited with banks as security against the issuance of letters of credit for the import of machinery and raw materials and as pledges for certain short-term borrowings.
Net cash used in investing activities was $33.1 million for the fiscal year ended March 31, 2008, as compared to $17.9 million for the prior fiscal year. This was due primarily to our plant expansion to increase our production capacities. Cash invested in purchases of property and equipment was $36.5 million and $21.6 million for the fiscal years ended March 31, 2008, and 2007, respectively. Restricted cash decreased by $4.2 million for the fiscal year ended March 31, 2008, as compared to a decrease of $3.5 million for the prior fiscal year. Restricted cash represents cash deposited with banks as security against the issuance of letters of credit.
Cash Flows from Financing Activities
Net cash provided by financing activities was $174.9 million for the fiscal year ended March 31, 2009. Cash provided by financing activities during the fiscal year ended March 31, 2009 was primarily related to $238.2 million short-term loans from PRC banks in China, $7.5 million loan from related parties, $8.8 million long-term loans from domestic banks in China, offset by $77.3 million repayment of short-term loans from PRC banks and $1.9 million repayment of long-term loans from PRC banks.
Net cash provided by financing activities was $64.7 million for the fiscal year ended March 31, 2008. Cash provided by financing activities during the fiscal year ended March 31, 2008 was primarily related to $65.8 million in the proceeds from the issuance of common stock to Warburg, $60.2 million short-term loans from domestic banks in China and ABN, and a $35.0 million long-term loan from ABN, offset by the repayment of $96.2 million in short-term borrowings from domestic banks in China and ABN.
Net cash provided by financing activities for the fiscal year ended March 31, 2007 was $16.4 million, primarily related to the proceeds from bank loans of $32.6 million, partially offset by the repayment of short-term borrowings of $16.2 million.
Outstanding Indebtedness
On April 19, 2007, the Company entered into a bridge loan agreement with ABN AMRO Bank N.V., Hong Kong branch (“ABN”), in the amount of $35.0 million (the “Bridge Loan Agreement”). The Loan bore interest at the one-month London interbank offered rate for deposits in US dollars plus 2.5% with interest payable on the last day of each month. The Company was required to pay to ABN a commitment fee of 1% on the daily amount of the unused Commitment Amount. The Loans were secured by a pledge of 25,000,000 shares of the Company’s Common Stock owned by Beams Power Investment Limited, a British Virgin Islands company, the capital stock of which is beneficially owned by Liang Zhang, the Chief Executive Officer of the Company. Pursuant to the Loan Agreement, Liang Zhang and Xiuqing Meng, Mr. Zhang’s wife, guaranteed the Company’s obligations thereunder. The principal amount and unpaid accrued interest under the Bridge Loan Agreement were repaid on October 18, 2007.
In addition, pursuant to a USD facility side letter and warrant agreement dated April 19, 2007 between the Company and ABN, the Company was obligated to issue warrants to ABN to purchase up to 400,000 shares of the Company’s common stock (the “Warrant Agreement”). On April 19, 2007 and October 11, 2007, respectively, the Company issued warrants to purchase 200,000 shares of its common stock at $8.84 per share and the remaining 200,000 shares at the same price. All of the warrants may be exercised up to the third anniversary of the completion of a “Qualified Public Offering”, as defined in the Warrant Agreement.
On October 11, 2007, ABN and another lender provided a three year term loan, or the New ABN Loan, to the Company in the aggregate amount of $35 million. The principal amount, and any unpaid accrued interest on the New ABN Loan, will be due on October 11, 2010 and may be prepaid without penalty. The proceeds of the New ABN Loan were used to pay the outstanding principal and accrued interest of a six-month term loan provided to the Company by ABN on April 19, 2007, or the Original ABN Loan. The New ABN Loan bears interest at LIBOR for deposits in U.S. dollars plus 3.5% with interest payable on the last day of each three-month period. The loan is secured by a pledge of 10,000 shares of common stock, or 100% of the outstanding common stock of Synutra Illinois.
The loan agreement for the New ABN Loan provides for mandatory prepayment upon the occurrence of certain events, and contains customary covenants for financings of this type, including restrictions on the incurrence of liens, payment of dividends, and disposition of properties. The loan agreement for the New ABN Loan also contains certain financial covenants, including a requirement to maintain specified leverage and interest coverage ratio, tangible net worth, and indebtedness to tangible net worth ratio. The Company has performed an analysis of the relevant ratios and found that due to the net loss which resulted from the significant costs of the product recall, including inventory write-down and write-off, the Company was not able to meet any of the financial covenant requirements as of March 31, 2009. Further, the Company anticipates that it may have difficulty meeting these financial covenant requirements for the next several fiscal quarters. The Company has requested the lenders to waive these financial covenants for the next several fiscal quarters. The lenders have proposed waiver fee which equals to 0.75% of the aggregate principal amount outstanding and an increase in the interest rate of 200 basis points to LIBOR for deposits in U.S. dollars plus 5.5%. The lenders have also proposed a revised repayment schedule of quarterly amortizations commencing in October 2009 with $5.0 million in October 2009, $5.0 million in January 2010, $10 .0 million in April 2010, $10.0 million in July 2010, and $5.0 million on maturity. Any non-payment would constitute a default, for which the banks have rights to enforce on the existing share pledge. As of the date of the filing of this 10-K, the waiver has not been granted. The Company is currently in discussions with the lenders on the waiver and is unable to predict when, or if the waiver will be granted. Accordingly, the Company has reclassified the outstanding balance of $34.5 million under the loan to current liabilities since the Company considers this debt callable by the bank. The Company may also incur additional expenses in connection with the modification of loan arrangements.
In addition to the New ABN Loan, as of March 31, 2009 and March 31, 2008, the Company had short-term loans from PRC banks in the amount of $182.6 million and $21.2 million, respectively. The maturity dates of the short term loans outstanding from PRC banks at March 31, 2009 range from April 2009 to March 2010. As of the date of the filing of this 10-K, all outstanding short-term loans that have become due have been repaid. The weighted average interest rate on short-term loans from PRC banks outstanding at March 31, 2009 and March 31, 2008 was 4.1% and 7.08%, respectively. The loans at March 31, 2009 were secured by the pledge of certain fixed assets held by the Company and its subsidiaries, a pledge of the Company’s land use right and pledge of cash deposits. The value of fixed assets pledged was $34.2 million and $18.1 million as of March 31, 2009 and March 31, 2008, respectively. The value of land use right pledged was $3.4 million and nil as of March 31, 2009 and March 31, 2008, respectively. The value of cash pledged was $66.1million which was recorded in restricted cash and nil as of March 31, 2009 and March 31, 2008, respectively.
As of March 31, 2009 and 2008, the Company had long-term loans which are unsecured debt, from PRC banks in the amount of $8.8 million and $1.9 million, respectively. The maturity date of the loan term loans outstanding from PRC banks at March 31, 2009 is in March 2011. The weighted average interest rate on outstanding long-term loans at March 31, 2009 and 2008 was 5.4% and 3.2%, respectively.
Apart from the short-term loans and long-term loans from banks, the Company also had short term loans from related parties in the amount of $7.5 million and nil as of March 31, 2009 and March 31, 2008 respectively. The maturity dates of the short term loans outstanding from related parties at March 31, 2009 are in October 2010. The balance as of March 31, 2009 included a US dollar loan of $3.9 million and a RMB loan of $3.6 million, and the interest rates were 10.0% and 5.5% respectively. The interest rate of the US dollar loan is benched mark to that of US dollar borrowing from third parties. The outstanding amount under these loans from related parties was $6.6 million as of June 12, 2009.
Tabular Disclosure of Contractual Obligations
Our cash flows from operations are dependent on a number of factors, including fluctuations in our operating results, accounts receivable collections, inventory management, and the timing and amount of tax and other payments. As a result, the impact of contractual obligations on our liquidity and capital resources in future periods should be analyzed in conjunction with such factors. In addition, we plan for and measure our liquidity and capital resources through an annual budgeting process.
Below is a table setting forth our contractual obligations as of March 31, 2009:
| | | Total | | | Less than 1 year | | | 1-3 years | | | 3-5 years | | | More than 5 years | |
| | | (in thousands) | |
| Long-term debt and related interest payment obligations | | $ | 9,719 | | | $ | 474 | | | $ | 9,245 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Capital lease obligations | | | 18,038 | | | | — | | | | 857 | | | | 946 | | | | 16,235 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating lease obligations | | | 66,880 | | | | 650 | | | | 3,368 | | | | 3,606 | | | | 59,256 | |
| | | | | | | | | | | | | | | | | | | | | |
| Advertising and purchase of raw materials commitments | | | 19,211 | | | | 19,211 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Capital expenditure commitments | | | 6,004 | | | | 6,004 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 119,852 | | | $ | 26,339 | | | $ | 13,470 | | | $ | 4,552 | | | $ | 75,491 | |
We computed the long-term debt-related interest based on the interest rate as of March 31, 2009.
As of March 31, 2009, our FIN 48 liability was $964,000. We are unable to reasonably estimate the timing of the effective settlement of this tax position.
Capital Expenditures
Our capital expenditures were $44.8 million, $36.4 million and $21.6 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively. Our capital expenditures were mainly used for the expansion of our production plants. In order to meet our immediate working capital needs in the aftermath of the melamine contamination incident, we decided to temporarily postpone projects that were not urgently needed for our operations, such as our baby beverage project and the furnishing of our head office building. Our capital expenditure increased for the fiscal year ended March 31, 2009 due to projects we were undertaking to expand our production capacity prior to the melamine contamination incident. We do not anticipate significant capital expenditure in the next several fiscal quarters. All future capital expenditure plans will be dependent upon our cash position and operating status.
Off-Balance Sheet Arrangements
We have not entered into any guarantee contract or commitments to guarantee the payment obligations of any third parties except for the guarantee issued to the Zhangbei Branch of the Agricultural Bank of China related to various bank loans of $1.1 million to 104 dairy farmers in the Zhangbei area of Hebei Province in China, which was recorded as contingent liability of $0.4 million. Such loans matured on December 15, 2007 but as of March 31, 2009, none of these loans have been repaid. We do not have any retained or contingent interest in assets transferred to an unconsolidated entity or similar arrangement that serves as credit, liquidity or market risk support to such entity for such assets. We do not have any obligation, including a contingent obligation, under a contract that would be accounted for as a derivative instrument, except that it is both indexed to the registrant's own stock and classified in stockholders' equity in the registrant's statement of financial position, and therefore excluded from the scope of FASB Statement of Financial Accounting Standards No. 133, Accounting for Derivative Instruments and Hedging Activities. We do not have any obligation, including a contingent obligation, arising out of a variable interest in an unconsolidated entity that is held by and material to us where such entity provides financing, liquidity, market risk or credit risk support to, or engages in leasing, hedging or research and development services with us.
Recent Accounting Pronouncements
In December 2007, the FASB issued SFAS No. 141 (R), “Business Combinations” (“SFAS No. 141(R)”). SFAS No. 141(R) requires an entity to recognize the assets acquired, liabilities assumed, contractual contingencies, and contingent consideration at their fair value on the acquisition date. Subsequent changes to the estimated fair value of contingent consideration will be reflected in earnings until the contingency is settled. SFAS No. 141(R) also requires acquisition-related costs and restructuring costs to be expensed as incurred rather than treated as part of the purchase price. The adoption of SFAS No. 141(R) will change our accounting treatment for business combinations for which the acquisition date is on or after April 1, 2009. SFAS 141R amends SFAS 109, “Accounting for Income Taxes,” such that adjustments made to valuation allowances on deferred taxes and acquired tax contingencies associated with acquisitions that closed prior to the effective date of SFAS 141R would also apply the provisions of SFAS 141R.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements” to improve the relevance, comparability, and transparency of financial information provided to investors by requiring all entities to report noncontrolling (minority) interests in subsidiaries in the same way as required in the consolidated financial statements. Moreover, SFAS No. 160 eliminates the diversity that currently exists in accounting for transactions between an entity and noncontrolling interests by requiring they be treated as equity transactions. SFAS No. 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. We are currently evaluating whether the adoption of SFAS No. 160 will have a significant effect on its consolidated financial position, results of operations or cash flows.
In March 2008, the FASB issued SFAS No. 161, “Disclosures about Derivative Instruments and Hedging Activities - an Amendment of FASB Statement 133.” SFAS No. 161 provides new disclosure requirements for an entity’s derivative and hedging activities. SFAS No. 161 is effective for periods beginning after November 15, 2008. We have not yet determined the impact on its consolidated financial statements of adopting SFAS No. 161.
In April 2008, the FASB issued Staff Position No. FAS 142-3, “Determination of the Useful Life of Intangible Assets” (FSP FAS 142-3). This position amends the factors an entity should consider when developing renewal or extension assumptions used in determining the useful life over which to amortize the cost of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets.” FSP FAS 142-3 requires an entity to consider its own historical experience in renewing or extending similar arrangements in determining the amortizable useful life. Additionally, this position requires expanded disclosures related to the determination of intangible asset useful lives. FSP FAS 142-3 is effective for fiscal years beginning after December 15, 2008, and may impact any intangible assets the Company acquires in future transactions. The guidance for determining the useful life of a recognized intangible asset must be applied prospectively to intangible assets acquired after the effective date. The disclosure requirements, though, shall be applied prospectively to all intangible assets recognized as of the effective date. Early adoption is prohibited. We expect to adopt FSP FAS 142-3 as of April 1, 2009.
In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles" (FAS 162). FAS 162 identifies the sources of accounting principles and the framework for selecting the principles used in the preparation of financial statements. FAS 162 is effective 60 days following SEC approval.
In June 2008, the FASB ratified EITF Issue 07-5, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock” (“EITF 07-5”). This Issue addresses the determination of whether an instrument (or an embedded feature) is indexed to an entity’s own stock. The EITF will be effective for years beginning after December 15, 2008. We are currently evaluating whether the adoption of EITF Issue 07-5 will have a significant effect on its consolidated financial position, results of operations or cash flows.
At the November 24, 2008 meeting, the FASB ratified the consensus reached by the Task Force in Issue No. 08-7: Accounting for Defensive Intangible Assets (EITF 08-7). EITF 08-7 requires entities that will acquire a defensive intangible asset after the effective date of Statement 141(R), to account for the acquired intangible asset as a separate unit of accounting and amortize the acquired intangible asset over the period during which the asset would diminish in value. EITF 08-7 is effective for defensive intangible assets acquired in fiscal years beginning on or after December 15, 2008. We are currently evaluating the impact of this statement on our consolidated financial statements.
In May 2009, the FASB issued Statement of Financial Accounting Standards No. 165, "Subsequent Events" (SFAS No.165). SFAS 165 establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. Specifically, SFAS 165 provides (i) the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements; (ii) the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements; and (iii) the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. SFAS 165 is effective for interim or annual financial periods ending after June 15, 2009 and shall be applied prospectively.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
The objective of our policies is to mitigate potential income statement, cash flow and fair value exposures resulting from possible future adverse fluctuations in rates. We evaluate our exposure to market risk by assessing the anticipated near-term and long-term fluctuations in interest rates and foreign exchange rates. This evaluation includes the review of leading market indicators, discussions with financial analysts and investment bankers regarding current and future economic conditions and the review of market projections as to expected future rates.
Foreign Exchange Risk
The value of the Renminbi against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions. Since July 2005, the Renminbi has no longer been pegged to the U.S. dollar. Although the PBOC regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the Renminbi may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future, PRC authorities may lift restrictions on fluctuations in the Renminbi exchange rate and lessen intervention in the foreign exchange market.
Because substantially all of our earnings and cash assets are denominated in Renminbi, appreciation or depreciation in the value of the Renminbi relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. As a result, we face exposure to adverse movements in currency exchange rates as the financial results of our Chinese operations are translated from local currency into U.S. dollars upon consolidation. If the U.S. dollar weakens against the Renminbi, the translation of our foreign-currency-denominated balances will result in increased net assets, net revenues, operating expenses, and net income or loss. Similarly, our net assets, net revenues, operating expenses, and net income or loss will decrease if the U.S. dollar strengthens against the Renminbi. Additionally, foreign exchange rate fluctuations on transactions denominated in Renminbi other than the functional currency result in gains and losses that are reflected in our Consolidated Statement of Income. Our operations are subject to risks typical of international business, including, but not limited to, differing economic conditions, changes in political climate, differing tax structures, other regulations and restrictions, and foreign exchange rate volatility.
Considering the RMB balance of our cash and cash equivalents as of March 31, 2009, which amounted to $37.7 million, a 1.0% change in the exchange rates between the Renminbi and the U.S. dollar would result in an increase or decrease of approximately $0.4 million of the balance.
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may enter into hedging transactions in the future, the availability and effectiveness of these transactions may be limited, and we may not be able to successfully hedge our exposure at all. In addition, our foreign currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert Renminbi into foreign currencies.
Inflation
Inflationary factors, such as increases in the cost of our products and overhead costs, could impair our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of gross margin and selling, general and administrative expenses as a percentage of sales revenue if the selling prices of our products do not increase with these increased costs.
Interest Rate Risk
We did not experience any material changes in interest rate exposures during the fiscal year ended March 31, 2009. Based upon economic conditions and leading market indicators at March 31, 2009, we do not foresee a significant adverse change in interest rates in the near future and do not use interest rate derivatives to manage exposure to interest rate changes.
Currently, we are exposed to interest rate risk primarily associated with: the New ABN Loan, a variable-rate debt obligation based on LIBOR, with the carrying value of $35.0 million which approximates its fair value at March 31, 2009.
Concentration of Credit Risk
We are subject to concentrations of credit risk consisting primarily of accounts receivable. We perform ongoing credit evaluations with respect to the financial condition of our debtors, but do not require collateral. In order to determine the value of our accounts receivable, we record a provision for doubtful accounts to cover probable credit losses. Our management reviews and adjusts this allowance periodically based on historical experience and its evaluation of the collectibility of outstanding accounts receivable.
Commodities Risk
Raw milk, imported milk powder and whey protein are the principal raw materials in the Company’s business, accounting for over 70% of the Company’s cost of sales. Domestically produced milk powder and other dairy based ancillary products are also the Company’s products for commercial sales. As such, the Company’s is exposed to the fluctuations in the price of raw milk, milk powder and whey protein. During the past two years, the rise and fall in the prices of these commodities have significantly affected the Company’s business operations and profitability. A significant rise in the prices of these raw materials will adversely affect the gross margin of the Company’s powdered formula products. In the meantime, a significant decrease in the price of milk powder may also adversely affect the gross margin of domestically produced milk powder and other dairy based ancillary products for commercial sales.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | | |
| Reports of Independent Registered Public Accounting Firms | | 71 |
| | |
| Consolidated Balance Sheets as of March 31, 2009 and 2008 | | 73 |
| | |
| Consolidated Statements of Income for the fiscal years ended March 31, 2009, 2008 and 2007 | | 74 |
| | |
| Consolidated Statements of Shareholders’ Equity for the fiscal years ended March 31, 2009, 2008 and 2007 | | 75 |
| | |
| Consolidated Statements of Cash Flows for the fiscal years ended March 31, 2009, 2008 and 2007 | | 76 |
| | |
| Notes to Consolidated Financial Statements | | 77 |
| | |
| Financial Statement Schedules | | 125 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Synutra International, Inc.
Rockville, Maryland
We have audited the accompanying consolidated balance sheets of Synutra International, Inc. and subsidiaries (the “Company”) as of March 31, 2009 and 2008, and the related consolidated statements of income and comprehensive income (loss), shareholders’ equity, and cash flows for each of the two years in the period ended March 31, 2009 and related financial statement schedules. These financial statements and financial statement schedules are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedules based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Synutra International, Inc. and subsidiaries at March 31, 2009 and 2008, and the results of their operations and their cash flows for each of the two years in the period ended March 31, 2009, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly, in all material respect, the information set forth therein.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2(A) to the consolidated financial statements, the Company's losses from operations, negative working capital and non-compliance with certain debt covenants raise substantial doubt about its ability to continue as a going concern. Management's plans concerning these matters are also discussed in Note 11 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in Note 14 to the consolidated financial statements, effective April 1, 2007, the Company adopted Financial Accounting Standards Board Interpretation No. 48 “Accounting for Uncertainty in Income Taxes – an Interpretation of FASB Statement No. 109”.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of March 31, 2009, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated June 15, 2009 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ Deloitte Touche Tohmatsu CPA Ltd.
Shanghai, China
June 15, 2009
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
and Stockholders
Synutra International, Inc. and Subsidiaries
Rockville, Maryland
We have audited the accompanying consolidated statements of income, changes in shareholders' equity, cash flows, and related financial statement schedules included in Schedule I and II of Synutra International, Inc. and Subsidiaries for the year ended March 31, 2007. These consolidated financial statements and related financial statement schedules are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the results of its operations and its cash flows of Synutra International, Inc. and Subsidiaries for the year ended March 31, 2007, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the related financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly in all material respects, the information set forth therein.
As described more fully in Note 17, the segment information disclosure for the year ended March 31, 2007 has been restated to reflect three reporting segments.
/s/ Rotenberg & Co., LLP
Rotenberg & Co., LLP
Rochester, New York
June 26, 2007 (June 11, 2009 as to the segment information restatement discussed in Note 17)
SYNUTRA INTERNATIONAL, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share par value)
| | | March 31, | | | March 31, | |
| | | 2009 | | | 2008 | |
| ASSETS | | | | | | |
| Current Assets: | | | | | | |
| Cash and cash equivalents | | $ | 37,736 | | | $ | 97,425 | |
| Restricted cash | | | 84,338 | | | | 9,744 | |
| Accounts receivable, net of allowance of $1,452 and $185, respectively | | | 23,826 | | | | 11,325 | |
| Inventories | | | 114,724 | | | | 61,853 | |
| Due from related parties | | | 2,463 | | | | — | |
| Deferred tax assets | | | 16,276 | | | | 1,448 | |
| Income tax receivable | | | 1,476 | | | | — | |
| Prepaid expenses and other current assets | | | 13,436 | | | | 10,800 | |
| Assets held for sale | | | — | | | | 1,316 | |
| | | | | | | | | |
| Total current assets | | | 294,275 | | | | 193,911 | |
| | | | | |
| Property, plant and equipment, net | | | 144,481 | | | | 85,719 | |
| Land use rights, net | | | 6,374 | | | | 4,496 | |
| Intangible assets, net | | | 3,136 | | | | — | |
| Goodwill | | | 1,435 | | | | — | |
| Deferred tax assets | | | 18,464 | | | | 2,077 | |
| Other assets | | | 4,406 | | | | 8,115 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 472,571 | | | $ | 294,318 | |
| | | | | | | | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Short-term loans | | $ | 224,647 | | | $ | 21,228 | |
| Long-term debt-current portion | | | — | | | | 1,923 | |
| Accounts payable | | | 112,968 | | | | 26,438 | |
| Due to related parties | | | 5,172 | | | | 2,970 | |
| Advances from customers | | | 5,448 | | | | 9,465 | |
| Income tax payable | | | — | | | | 4,168 | |
| Product recall provision | | | 4,547 | | | | — | |
| Other current liabilities | | | 21,925 | | | | 16,489 | |
| | | | | | | | | |
| Total current liabilities | | | 374,707 | | | | 82,681 | |
| Long-term debt | | | 8,777 | | | | 34,184 | |
| Deferred revenue | | | 4,681 | | | | 4,559 | |
| Capital lease obligations | | | 5,254 | | | | — | |
| Other long term liabilities | | | 1,756 | | | | 1,250 | |
| | | | | | | | | |
| Total liabilities | | | 395,175 | | | | 122,674 | |
| | | | | |
| Minority interest | | | 537 | | | | 385 | |
| | | | | |
| Shareholders’ equity: | | | | | | | | |
| Common stock, $.0001 par value: 250,000 authorized; 54,001 and 54,001 issued and outstanding at March 31, 2009 and 2008, respectively | | | 5 | | | | 5 | |
| Additional paid-in capital | | | 76,607 | | | | 76,607 | |
| Retained earnings (Accumulated deficit) | | | (23,674 | ) | | | 76,875 | |
| Accumulated other comprehensive income | | | 23,921 | | | | 17,772 | |
| | | | | | | | | |
| Total shareholders’ equity | | | 76,859 | | | | 171,259 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 472,571 | | | $ | 294,318 | |
The accompanying notes are an integral part of the consolidated financial statements.
SYNUTRA INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF INCOME
AND COMPREHENSIVE INCOME
(in thousands except earnings per share data)
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Net sales | | $ | 312,528 | | | $ | 362,090 | | | $ | 216,605 | |
| Cost of sales | | | 259,086 | | | | 175,568 | | | | 109,900 | |
| | | | | | | | | | | | | |
| Gross profit | | | 53,442 | | | | 186,522 | | | | 106,705 | |
| | | | | | |
| Selling and distribution expenses | | | 44,178 | | | | 34,449 | | | | 25,561 | |
| Advertising and promotion expenses | | | 115,478 | | | | 76,388 | | | | 52,322 | |
| General and administrative expenses | | | 25,455 | | | | 16,013 | | | | 7,031 | |
| Other operating income, net | | | 5,790 | | | | 1,492 | | | | 1,109 | |
| | | | | | | | | | | | | |
| Income (loss) from operations | | | (125,879 | ) | | | 61,164 | | | | 22,900 | |
| | | | | | |
| Interest expense | | | 4,857 | | | | 6,354 | | | | 1,896 | |
| Interest income | | | 341 | | | | 1,801 | | | | 356 | |
| Other income (expense), net | | | (580 | ) | | | (3,084 | ) | | | 110 | |
| | | | | | | | | | | | | |
| Income (loss) before provision for income tax | | (130,975 | ) | | | 53,527 | | | | 21,470 | |
| Provision (benefit) for income tax | | | (30,386 | ) | | | 7,855 | | | | 1,596 | |
| | | | | | | | | | | | | |
| Net income (loss) before minority interests | | | (100,589 | ) | | | 45,672 | | | | 19,874 | |
| | | | | | |
| Minority interests | | | (40 | ) | | | 11 | | | | — | |
| | | | | | | | | | | | | |
| Net income (loss) attributable to shareholders | | $ | (100,549 | ) | | $ | 45,661 | | | $ | 19,874 | |
| | | | | | | | | | | | | |
| Other comprehensive income | | | 6,149 | | | | 14,840 | | | | 1,876 | |
| | | | | | | | | | | | | |
| Total comprehensive income (loss) | | $ | (94,400 | ) | | $ | 60,501 | | | $ | 21,750 | |
| | | | | | | | | | | | | |
| Earnings (loss) per share - basic | | $ | (1.86 | ) | | $ | 0.86 | | | $ | 0.40 | |
| | | | | | | | | | | | | |
| Earnings (loss) per share - diluted | | $ | (1.86 | ) | | $ | 0.85 | | | $ | 0.40 | |
| | | | | | | | | | | | | |
| Weighted average common share outstanding - basic | | | 54,001 | | | | 53,170 | | | | 50,001 | |
| Weighted average common share outstanding - diluted | | | 54,001 | | | | 53,476 | | | | 50,001 | |
The accompanying notes are an integral part of the consolidated financial statement.
SYNUTRA INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in thousands)
| | | Common Stock | | | Additional paid-in capital | | | Retained earnings (Accumulated deficit) | | | Accumulated other comprehensive income | | | Total shareholders’ equity | |
| | | Shares | | | Amount | |
| Balance, March 31, 2006 | | | 50,001 | | | $ | 5 | | | $ | 8,226 | | | $ | 11,664 | | | $ | 1,056 | | | $ | 20,951 | |
| Net income | | | — | | | | — | | | | — | | | | 19,874 | | | | — | | | | 19,874 | |
| Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 1,876 | | | | 1,876 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, March 31,2007 | | | 50,001 | | | $ | 5 | | | $ | 8,226 | | | $ | 31,538 | | | $ | 2,932 | | | $ | 42,701 | |
| Issuance of common stock, net of issuance costs of $206 | | | 4,000 | | | | — | | | | 65,794 | | | | — | | | | — | | | | 65,794 | |
| Issuance of warrants | | | — | | | | — | | | | 2,587 | | | | — | | | | — | | | | 2,587 | |
| Adoption of FIN 48 | | | — | | | | — | | | | — | | | | (375 | ) | | | — | | | | (375 | ) |
| Acquisition of a subsidiary | | | — | | | | — | | | | — | | | | 51 | | | | — | | | | 51 | |
| Net income | | | — | | | | — | | | | — | | | | 45,661 | | | | — | | | | 45,661 | |
| Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 14,840 | | | | 14,840 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, March 31,2008 | | | 54,001 | | | $ | 5 | | | $ | 76,607 | | | $ | 76,875 | | | $ | 17,772 | | | $ | 171,259 | |
| Net loss | | | — | | | | — | | | | — | | | | (100,549 | ) | | | — | | | | (100,549 | ) |
| Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 6,149 | | | | 6,149 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, March 31,2009 | | | 54,001 | | | $ | 5 | | | $ | 76,607 | | | $ | (23,674 | ) | | $ | 23,921 | | | $ | 76,859 | |
The accompanying notes are an integral part of the consolidated financial statements.
SYNUTRA INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Cash flow from operating activities: | | | | | | | | | | | | |
| Net income (loss) | | $ | (100,549 | ) | | $ | 45,661 | | | $ | 19,874 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
| Amortization of debt discount | | | — | | | | 2,733 | | | | — | |
| Amortization of debt issuance costs | | | 321 | | | | 539 | | | | — | |
| Depreciation and amortization | | | 7,649 | | | | 4,646 | | | | 2,057 | |
| Write down of intangible asset | | | 880 | | | | — | | | | — | |
| Bad debt expense | | | 2,138 | | | | (47 | ) | | | 397 | |
| (Gain)/loss on short term investment | | | — | | | | — | | | | (77 | ) |
| (Gain)/ Loss on disposal of property, plant and equipment | | | (20 | ) | | | 249 | | | | — | |
| Impairment of property, plant and equipment | | | — | | | | 175 | | | | — | |
| Deferred income tax | | | (31,124 | ) | | | (2,695 | ) | | | (432 | ) |
| Minority interest | | | 152 | | | | 11 | | | | — | |
| | | | | | | | | | | | | |
| Changes in assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable | | | (13,399 | ) | | | (4,149 | ) | | | (4,044 | ) |
| Inventories | | | (50,940 | ) | | | (39,066 | ) | | | (4,616 | ) |
| Due from related parties | | | (3,557 | ) | | | 9,400 | | | | (3,139 | ) |
| Prepaid expenses and other current assets | | | (2,691 | ) | | | (5,676 | ) | | | (1,341 | ) |
| Accounts payable | | | 76,975 | | | | 6,831 | | | | (994 | ) |
| Due to related parties | | | 4,880 | | | | 1,909 | | | | (1,366 | ) |
| Advances from customers | | | (4,248 | ) | | | 4,606 | | | | 3,330 | |
| Income tax receivable | | | (5,636 | ) | | | — | | | | — | |
| Income tax payable | | | — | | | | 5,037 | | | | (668 | ) |
| Deferred revenue | | | 986 | | | | — | | | | 4,138 | |
| Product recall provision | | | 4,547 | | | | — | | | | — | |
| Other liabilities | | | 4,148 | | | | 8,065 | | | | 1,647 | |
| | | | | | | | | | | | | |
| Net cash provided by (used in) operating activities | | | (109,488 | ) | | | 38,229 | | | | 14,766 | |
| | | | | | | | | | | | | |
| Cash flow from investing activities: | | | | | | | | | | | | |
| Acquisition of property, plant and equipment | | | (44,827 | ) | | | (36,453 | ) | | | (21,584 | ) |
| Acquisition of intangible assets | | | (2,474 | ) | | | — | | | | — | |
| Disposal of investment in a subsidiary | | | — | | | | (1,046 | ) | | | — | |
| Proceeds from sales of short term investment | | | — | | | | — | | | | 120 | |
| Change in restricted cash | | | (73,934 | ) | | | 4,213 | | | | 3,527 | |
| Business acquisitions | | | (6,052 | ) | | | 190 | | | | — | |
| Proceeds from disposal of property, plant and equipment | | | — | | | | — | | | | 29 | |
| Advance to related companies | | | — | | | | (3,324 | ) | | | — | |
| Repayment of advance to related companies | | | — | | | | 3,324 | | | | — | |
| | | | | | | | | | | | | |
| Net cash used in investing activities | | | (127,287 | ) | | | (33,096 | ) | | | (17,908 | ) |
| | | | | | | | | | | | | |
| Cash flow from financing activities: | | | | | | | | | | | | |
| Proceeds from short-term loans | | | 245,710 | | | | 60,179 | | | | 32,625 | |
| Repayment of short-term loans | | | (77,314 | ) | | | (96,242 | ) | | | (16,200 | ) |
| Proceeds from long-term loans | | | 8,777 | | | | 35,000 | | | | — | |
| Repayment of long-term loans | | | (1,923 | ) | | | — | | | | — | |
| Payments on capital lease obligations | | | (379 | ) | | | — | | | | — | |
| Proceeds from issuance of common stock, net of issuance costs | | | — | | | | 65,794 | | | | — | |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 174,871 | | | | 64,731 | | | | 16,425 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash and cash equivalents | | | 2,215 | | | | 6,725 | | | | 1,876 | |
| | | | | | | | | | | | | |
| Net change in cash and cash equivalents | | | (59,689 | ) | | | 76,589 | | | | 15,159 | |
| Cash and cash equivalents, beginning of year | | | 97,425 | | | | 20,836 | | | | 5,677 | |
| | | | | | | | | | | | | |
| Cash and cash equivalents, end of year | | $ | 37,736 | | | $ | 97,425 | | | $ | 20,836 | |
| | | | | | | | | | | | | |
| Supplemental cash flow information: | | | | | | | | | | | | |
| Interest paid | | $ | 5,718 | | | $ | 3,769 | | | $ | 2,119 | |
| Income tax paid | | $ | 6,443 | | | $ | 6,486 | | | $ | 2,714 | |
| | | | |
| Non-cash investing and financing activities: | | | | | | | | | | | | |
| Purchase of property, plant and equipment by accounts payable | | $ | 7,501 | | | $ | 4,149 | | | $ | — | |
| Issuance of warrant in relation to the long-term debt | | $ | — | | | $ | 2,733 | | | $ | — | |
| Assets acquired under capital lease | | $ | 5,336 | | | $ | — | | | $ | — | |
| Business acquisitions by accounts payable | | $ | 1,521 | | | $ | — | | | $ | — | |
| Disposal of property by due from related parties | | $ | 1,726 | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of the consolidated financial statements
SYNUTRA INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FISCAL YEARS ENDED MARCH 31, 2009 AND 2008
| 1. | ORGANIZATION AND PRINCIPAL ACTIVITIES |
Directly or through its wholly owned subsidiary, Synutra Inc., an Illinois corporation (“Synutra Illinois”), Synutra International, Inc. (collectively with its subsidiaries, the “Company” or “Synutra”) owns all or majority of the equity interests of the entities in the People’s Republic of China (“China” or “PRC”) that are principally engaged in the production, marketing and distribution of dairy based nutritional products under the Company’s own brands in China. The Company is a leader in sales of infant formula products in China.
The Company produces, markets and sells nutritional products under “Shengyuan”, or “Synutra”, name, together with other complementary brands. The Company focuses on selling premium infant formula products, which are supplemented by more affordable infant formula products targeting the mass market as well as other nutritional products, such as adult powdered formula and prepared baby food, and certain nutritional ingredients and supplements.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
The consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. Under that assumption, it is expected that assets will be realized and liabilities will be satisfied in the normal course of business. Following the government announcement in mid September 2008 that formula products of the Company and 21 other manufacturers had been contaminated with melamine, the Company conducted a compulsory recall of certain lots of U-Smart products and a voluntary recall of all other products produced before September 16, 2008 at the same facilities, where the Company believed the contaminated milk supplies originated. As a direct result of the incident, the Company has experienced significant operating losses and negative cash flows from operations for the fiscal year ended March 31, 2009 and, as of March 31, 2009, has a working capital deficit of approximately $80.4 million caused primarily by the product recall costs including cost of replacing products, shipping charges and inventory write down/ write off, and subsequent loss of sales. As discussed in Note 11 to the financial statements, at March 31, 2009, the Company was not in compliance with certain covenants of its loan agreement due to the losses suffered as a result of the melamine issue. The occurrence of these recent economic events, the ensuing operating losses and negative cash flows and the failure of the Company to meet its debt covenants raise substantial doubt as to the Company's ability to continue as a going concern. Management is attempting to renegotiate the terms and covenants of the loan agreement and is in the process of evaluating funding alternatives including seeking refinance of certain short-term loans from PRC banks. The accompanying consolidated financial statements do not reflect any adjustments relating to the recoverability and reclassification of assets and liabilities as that might be necessary if the Company is unable to continue as a going concern.
The consolidated financial statements include the financial statements of Synutra International, Inc. and its subsidiaries, its variable interest entity and its subsidiaries. All significant inter-company accounts and transactions have been eliminated in consolidation. The variable interest entity and its subsidiaries have not commenced their planned operations as of March 31, 2009.
The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results may differ from those estimates.
| D. | Cash and cash equivalents |
Cash and cash equivalents consist of cash on hand and highly liquid investments which are unrestricted as to withdrawal or use, and which have original maturities of three months or less when purchased.
Restricted cash are bank demand deposits used as security against letter of credits and short-term borrowings. This is used by the Company as a short term instrument to reduce financing cost.
Trade receivables are recognized and carried at the original invoice amount less allowance for any uncollectible amounts. An estimate for doubtful accounts is made when collection of the full amount is no longer probable. Bad debts are written off as incurred.
Inventories are stated at the lower of cost or market. Cost is calculated on the moving-average basis and includes all costs to acquire and other costs incurred in bringing the inventories to their present location and condition. The Company evaluates market of its inventories on a regular basis and records a provision for loss to reduce the computed weighted-average cost if it exceeds market. Potential losses from obsolete and slow-moving inventories are recorded when identified. Market is current replacement cost, which does not exceed the net realizable value, that is, estimated selling price in the ordinary course of business less reasonable predictable cost of completion and disposal, and is not less than net realizable value reduced by an allowance for normal profit margin.
There is no private land ownership in China. Companies or individuals are authorized to possess and use the land only through land use rights granted by the PRC government. Land use rights are amortized using the straight-line method over the lease term of 20 to 50 years.
| I. | Property, plant and equipment, net |
Property, plant and equipment are carried at cost. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized.
When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition.
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets or, when applicable, the life of the lease, whichever is shorter. The useful lives for property, plant and equipment are as follows:
| Buildings | 20 - 40 years |
| Plant and machinery | 5 - 10 years |
| Office equipment and furnishings | 3 - 5 years |
| Motor vehicles | 5 years |
| Others | 5 years |
Leases are classified as capital or operating leases. A lease that transfers to the lessee substantially all the benefits and risks incidental to ownership is classified as a capital lease. At inception, a capital lease is recorded at present value of minimum lease payments or the fair value of the asset, whichever is less. Assets under capital leases are amortized on a basis consistent with that of similar fixed assets or the lease term, whichever is less. Operating lease costs are recognized on a straight-line basis over the lease term.
| K. | Construction in progress |
Construction in progress represents direct costs of construction or acquisition and design fees incurred. Capitalization of these costs ceases and the construction in progress is transferred to plant and equipment when substantially all the activities necessary to prepare the assets for their intended use are completed. No depreciation is provided until it is completed and ready for intended use.
The capitalized interest recognized up to March 31, 2009, 2008 and 2007 was $1.4 million, $848,000 and $726,000 respectively, associated with construction in progress.
| L. | Impairment of long-lived assets |
The Company evaluates its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When these events occur, the Company measures impairment by comparing the carrying amount of the assets to future undiscounted net cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Company would recognize an impairment loss based on the fair value of the assets.
| M. | Impairment of goodwill and indefinite lived intangible assets |
SFAS No. 142 requires the Company to complete a two-step goodwill impairment test. The first step compares the fair value of each reporting unit to its carrying amount, including goodwill and indefinite lived intangible assets. If the fair value of each reporting unit exceeds its carrying amount, goodwill is not considered to be impaired and the second step will not be required. If the carrying amount of a reporting unit exceeds its fair value, the second step compares the implied fair value of goodwill and indefinite lived intangible assets to the carrying value of a reporting unit’s goodwill. The implied fair value of goodwill is determined in a manner similar to accounting for a business combination with the allocation of the assessed fair value determined in the first step to the assets and liabilities of the reporting unit. The excess of the fair value of the reporting unit over the amounts assigned to the assets and liabilities is the implied fair value of goodwill. This allocation process is only performed for purposes of evaluating goodwill impairment and does not result in an entry to adjust the value of any assets or liabilities. An impairment loss is recognized for any excess in the carrying value of goodwill over the implied fair value of goodwill.
Intangible assets with an indefinite life are tested for impairment annually or more frequently if events or changes in circumstances indicate that the asset might be impaired. The impairment test consists of a comparison of the fair value of the intangible asset to its carrying amount. If the carrying amount exceeds the fair value, an impairment loss is recognized equal in amount to that excess.
Management performs its annual goodwill impairment test on March 31. No goodwill or indefinite lived intangible assets have been impaired during any of the periods presented.
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. Parties are also considered to be related if they are subject to common control or common significant influence. Related parties may be individuals or corporate entities.
The following entities are considered to be related parties to the Company because they are affiliates of the Company under the common control of the Company’s major shareholder. These related parties act only as the Company’s suppliers or distributors and there are no other relationships wherein the Company has the ability to exercise significant influence over the operating and financial policies of these parties. The Company is not obligated to provide any type of financial support to these related parties. The related parties were excluded from consolidation due to equity structure and other business reasons. The economic substance of each entity apart from its relationship with the Company is discussed in the table below:
| | Economic Substance |
| Sheng Zhi Da Dairy Group Corporation (“Sheng Zhi Da”) | | Engages in buying and selling packaging materials, vitamin and mineral pre-mixes, and other food ingredients. |
| | |
| Beijing Kelqin Dairy Co. Ltd. (“Kelqin”) | | Produces and distributes retail-packaged yogurt products in Beijing. |
| | |
| St. Angel (Beijing) Business Service Co., Ltd. (“St. Angel”) | | Publishes catalogues and engages in advertising and direct marketing of consumer products featured in catalogues. |
| | |
| Beijing Honnete Dairy Co., Ltd. (“Honnete”) | | Engages in importing and distributing whey protein products to commercial customers. |
| | |
| Beijing Ao Naier Feed Stuff LLC (“Ao Naier”) | | Engages in buying and selling whey protein and other protein substitutes. Ao Naier became an unrelated party since January 2008. |
| | |
| Beijing Luding Xueyuan Trading Co., Ltd. (“Luding Xueyuan”) | | Operates retail shelf spaces in supermarkets in Beijing. Luding Xueyuan became an unrelated party since January 2008. |
| | | |
| Qingdao Luyin Waste Disposal Investment Management Co., Ltd. (“Luyin”) | | Engages in waste disposal and sewage treatment activities. |
The Company accounts for income taxes in accordance with SFAS No. 109, “Accounting for Income Taxes,” which requires recognition of deferred tax liabilities and assets for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax liabilities and assets are determined based on the temporary difference between the financial statement and tax basis of assets and liabilities using presently enacted tax rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
In July 2006, the Financial Accounting Standard Board (“FASB”) issued Financial Interpretation (“FIN”) No. 48, “Accounting for Uncertainty in Income Taxes,” which clarifies the accounting for uncertainty in income taxes recognized in the financial statements in accordance with SFAS No. 109, “Accounting for Income Taxes.” FIN No. 48 provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. Income tax positions must meet a more-likely-than-not recognition threshold at the effective date to be recognized upon the adoption of FIN 48 and in subsequent periods. This interpretation also provides guidance on measurement, derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006.
The Company adopted the provisions of FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes, on April 1, 2007. As a result of the implementation of Interpretation 48, the Company recognized approximately a $375,000 increase in the liability for unrecognized tax benefits, which was accounted for as a reduction to the April 1, 2007 balance of retained earnings. Note 14 further describes the impact of the adoption of FIN No. 48 - an interpretation of FASB Statement No. 109.
The Company recognizes interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying consolidated statement of operations. Accrued interest and penalties are included within the related tax liability line in the consolidated balance sheet.
| P. | Foreign currency translation |
The functional currency and reporting currency of Synutra International Inc. and Synutra Illinois are United States Dollar (“US Dollar”). Monetary assets and liabilities denominated in currencies other than the US dollar are translated into US dollar at the rates of exchange ruling at the balance sheet date. Transactions in currencies other than the US dollar during the year are converted into the US dollar at the applicable rates of exchange prevailing on the day transactions occurred. Transaction gains and losses are recognized in the statements of operations.
The financial records of the Company’s PRC subsidiaries are maintained in Renminbi (“RMB”) which are their functional currency. Assets and liabilities are translated at the exchange rates at the balance sheet date, equity accounts are translated at historical exchange rates and revenues, expenses, gains and losses are translated using the average rate for the year. Translation adjustments are reported as cumulative translation adjustments and are shown as a separate component of accumulated other comprehensive income in the statement of shareholders’ equity.
In July 2005, the PRC began to value the RMB against a basket of currencies of its major trading partners, including the U.S. This measure has allowed the RMB to fluctuate within a narrow band vis a vis the U.S. dollar. Since the adoption of this managed flexible exchange rate policy, the RMB has been under pressure to appreciate against the U.S. dollar. This has affected the changes in the foreign currency translation gain (loss) as reflected in other income (expense), net of $(252,000), $(1,110,000) and $38,000 for the fiscal years ended March 31, 2009, 2008 and 2007, respectively.
The Company recognizes revenue when title and risk and rewards for the products are transferred to the customer, price is fixed or determinable, and collectibility is reasonably assured. At the time of sale, the Company also records estimates for a variety of sales deductions, including value added taxes, rebates, discounts and incentives, trade promotions and product returns. Sales deductions are reported as a reduction of revenue. Most of the Company’s nutritional product sales are made through distributors. The Company’s revenue arrangement with most of its distributors requires distributor advance payment prior to any shipment and delivery of goods by the Company to such distributors. Under this distributor arrangement, evidenced by purchase order together with advance payment, sales revenue is realized and earned upon acceptance of delivery of products by the distributors. The Company applies this revenue recognition policy uniformly to all nutritional products, including all dairy-based pediatric and adult nutritional products.
A small fraction of the Company’s nutritional product sales are through supermarket retailers directly. The Company’s revenue arrangement with some of these retailers includes right of return clause. The Company’s price to the supermarkets is fixed. The supermarkets’ obligation to the Company would not be changed in the event of theft or physical destruction or damage of the product. The Company recognizes revenue when the supermarkets have paid the Company, or the supermarkets are obligated to pay the Company and the obligation is not contingent on resale of the product. The amount of future returns are estimated and recognized in the current period.
The Company’s gross sales are subject to various deductions, primarily comprised of rebates and discounts to distributors and retailers. These deductions represent estimates of the related obligations, requiring the use of judgment when estimating the impact of these sales deductions on gross sales for a reporting period. The Company reports these adjustments as a reduction of gross sales to arrive at net sales.
| • | The Company offers rebates to distributors and supermarket retailers to sustain and increase the Company’s product market share. These rebate programs provide that distributors and supermarket retailers receive a rebate after attaining certain performance parameters relating to product purchases, formulary status and/or pre-established market share milestones relative to competitors. Since rebates are contractually agreed upon, the Company estimate rebates based on the specific terms in each agreement, historical experience, anticipated reimbursement channel mix and product growth rates. The Company considers the sales performance of products subject to rebates and other contract discounts and adjusts the provision periodically to reflect actual experience. Actual amount may differ if the level of redemption rates and performance vary from estimates. The Company records rebates as a reduction of revenues in the year in which these programs are offered. |
| • | The Company records a provision for estimated sales returns due to package damage, merchandise slow moving at certain retail customer shelf, and termination of distributorships, which the Company estimates through a comparison of historical return data to related sales. The Company uses historical rates of return and adjusts for known or expected changes in the marketplace when appropriate. The sales return amount represents management’s best estimates based on the available information at the time of estimate is made. |
| • | For product sales and promotions at supermarkets and shopping malls, certain expenses in relation to shelf display, end-cap placement, bar-coding, banner advertising, etc. are paid to supermarkets and shopping mall operators. These expenses are deducted from revenues in accordance with EITF 01-9 “Accounting for Consideration Given by a Vendor to a Customer (Including a Reseller of the Vendor’s Product)”. |
| R. | Shipping and handling costs |
All shipping and handling costs are expensed as incurred and outbound freight is not billed to customers. Shipping and handling costs are included in selling and distribution expenses. The expenses were $7.4 million, $6.7 million and $4.0 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively.
| S. | Advertising and promotion expenses |
Advertising and promotion expenses include advertising expenses, promotion staff salaries and promotion product expenses. Advertising expenses were $72.8 million, $30.3 million and $16.4 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively.
Government grants for revenue and/or expenses should be recognized in income when the related revenue and/or expense are recorded. Government grants related to property, plant, and equipment should be netted against the depreciation expense of the related assets over the useful lives of these assets. Government subsidies relating to specific borrowings are recorded as an offset to the interest expense over the term of these borrowings. Unrestricted government subsidies from local governmental agencies allowing the Company full discretion in the fund utilization were $5.8 million, $1.5 million and $1.1 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively, which was recorded in other operating income in the consolidated statements of income. In addition, the Company received $4.6 million (RMB 32.0 million) of government subsidy in fiscal year 2007 which was directly related to the manufacturing facility construction. Such subsidy was recorded in deferred revenue and will be amortized to offset the depreciation expenses associated with the underlining property.
Mandatory contributions are made to the Government’s health, retirement benefit and unemployment schemes at the statutory rates in force during the period, based on gross salary payments. The cost of these payments is charged to the statement of income in the same period as the related salary cost.
| V. | Comprehensive income (loss) |
The Company has adopted the provisions of Statement of Financial Accounting Standards No. 130, “Reporting Comprehensive Income” (“SFAS No. 130”). SFAS No. 130 establishes standards for the reporting and display of comprehensive income, its components and accumulated balances in a full set of general-purpose financial statements. SFAS No. 130 defines comprehensive income to include all changes in equity except those resulting from investments by owners and distributions to owners, including adjustments to accumulated foreign currency translation.
| W. | Fair value of financial instruments |
The carrying value of financial instruments including cash, receivables, accounts payable, accrued expenses and debt, approximates their fair value at March 31, 2009 and 2008 due to the relatively short-term nature of these instruments. The carrying value of long-term debt approximates its fair value as it bears variable interest rate which reflects the current market yield level for comparable loans. The carrying value of long term receivable approximates its fair value as it represents the present value of future payments to be received.
On April 1, 2008, the Company adopted SFAS No. 157, Fair Value Measurements (“SFAS 157”) that was not delayed by FASB Staff Position FAS 157-2 (“FSP FAS 157-2”). FSP FAS 157-2 delays the effective date of SFAS 157 as it applies to non-financial assets and liabilities that are not required to be measured at fair value on a recurring (at least annual) basis. As a result of the delay, SFAS 157 will be applied to the Company’s non-financial assets and liabilities effective on April 1, 2009. SFAS 157 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (also referred to as an exit price). SFAS 157 also establishes a three-level fair value hierarchy for classifying financial instruments that is based on whether the inputs to the valuation techniques used to measure fair value are observable or unobservable. The three levels of the SFAS 157 fair value hierarchy are described below:
| Level 1: | Quoted prices (unadjusted) in active markets for identical assets or liabilities. |
| Level 2: | Observable market-based inputs, other than quoted prices in active markets for identical assetsor liabilities. |
| Level 3: | Unobservable inputs. |
As of March 31, 2009, the Company did not have any financial assets or liabilities that were measured at fair value on a recurring basis subsequent to initial recognition.
| X. | Concentration of credit risk |
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of trade accounts receivable. The Company performs ongoing credit evaluations with respect to the financial condition of its creditors, but does not require collateral. In order to determine the value of the Company’s accounts receivable, the Company records a provision for doubtful accounts to cover probable credit losses. Management reviews and adjusts this allowance periodically based on historical experience and its evaluation of the collectibility of outstanding accounts receivable.
The Company expanded the number of reportable segments from one to three in order to better reflect the manner in which management analyzes the Company’s performance including powdered formula, baby food, and nutritional ingredients and supplements. For more information, see Note 17 to these Consolidated Financial Statements.
| Z. | Recently issued accounting pronouncements |
In December 2007, the FASB issued SFAS No. 141, “Business Combinations: (Revised 2007)” (“SFAS 141R”). SFAS 141R is relevant to all transactions or events in which one entity obtains control over one or more other businesses. SFAS 141R requires an acquirer to recognize any assets and noncontrolling interest acquired and liabilities assumed to be measured at fair value as of the acquisition date. Liabilities related to contingent consideration are recognized and measured at fair value on the date of acquisition rather than at a later date when the amount of the consideration may be resolved beyond a reasonable doubt. This revised approach replaces SFAS 141, “Business Combinations” (“SFAS 141”) cost allocation process in which the cost of an acquisition was allocated to the individual assets acquired and liabilities assumed based on their respective fair value. SFAS 141R requires any acquisition-related costs and restructuring costs to be expensed as incurred as opposed to allocating such costs to the assets acquired and liabilities assumed as previously required by SFAS 141. Under SFAS 141R, an acquirer recognizes liabilities for a restructuring plan in purchase accounting only if the requirements of SFAS No. 146, “Accounting for Costs Associated with Exit or Disposal Activities”, are met. SFAS 141R allows for the recognition of pre-acquisition contingencies at fair value only if these contingencies are likely to materialize. If this criterion is not met at the acquisition date, then the acquirer accounts for the non-contractual contingency in accordance with recognition criteria set forth under SFAS No. 5, “Accounting for Contingencies”, in which case no amount should be recognized in purchase accounting. SFAS 141R is effective as of the beginning of an entity’s first fiscal year that begins after December 15, 2008 with the exception of the accounting for valuation allowances on deferred taxes and acquired tax contingencies. SFAS 141R amends SFAS 109, “Accounting for Income Taxes,” such that adjustments made to valuation allowances on deferred taxes and acquired tax contingencies associated with acquisitions that closed prior to the effective date of SFAS 141R would also apply the provisions of SFAS 141R. The Company is currently evaluating the impact, if any, of this statement on its consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements” to improve the relevance, comparability, and transparency of financial information provided to investors by requiring all entities to report noncontrolling (minority) interests in subsidiaries in the same way as required in the consolidated financial statements. Moreover, SFAS No. 160 eliminates the diversity that currently exists in accounting for transactions between an entity and noncontrolling interests by requiring they be treated as equity transactions. SFAS No. 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. The Company is currently evaluating whether the adoption of SFAS No. 160 will have a significant effect on its consolidated financial position, results of operations or cash flows.
In March 2008, the FASB issued SFAS No. 161, “Disclosures about Derivative Instruments and Hedging Activities - an Amendment of FASB Statement 133.” SFAS No. 161 provides new disclosure requirements for an entity’s derivative and hedging activities. SFAS No. 161 is effective for periods beginning after November 15, 2008. The Company has not yet determined the impact on its consolidated financial statements of adopting SFAS No. 161.
In April 2008, the FASB issued Staff Position No. FAS 142-3, “Determination of the Useful Life of Intangible Assets” (FSP FAS 142-3). This position amends the factors an entity should consider when developing renewal or extension assumptions used in determining the useful life over which to amortize the cost of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets.” FSP FAS 142-3 requires an entity to consider its own historical experience in renewing or extending similar arrangements in determining the amortizable useful life. Additionally, this position requires expanded disclosures related to the determination of intangible asset useful lives. FSP FAS 142-3 is effective for fiscal years beginning after December 15, 2008, and may impact any intangible assets the Company acquires in future transactions. The guidance for determining the useful life of a recognized intangible asset must be applied prospectively to intangible assets acquired after the effective date. The disclosure requirements, though, shall be applied prospectively to all intangible assets recognized as of the effective date. Early adoption is prohibited. The Company expects to adopt FSP FAS 142-3 as of April 1, 2009.
In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles" (FAS 162). FAS 162 identifies the sources of accounting principles and the framework for selecting the principles used in the preparation of financial statements. FAS 162 is effective 60 days following SEC approval.
In June 2008, the FASB ratified EITF Issue 07-5, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock” (“EITF 07-5”). This Issue addresses the determination of whether an instrument (or an embedded feature) is indexed to an entity’s own stock. The EITF will be effective for years beginning after December 15, 2008. The Company is currently evaluating whether the adoption of EITF Issue 07-5 will have a significant effect on its consolidated financial position, results of operations or cash flows.
At the November 24, 2008 meeting, the FASB ratified the reached in EITF Issue No. 08-7, “Accounting for Defensive Intangible Assets” (“EITF 08-7”). EITF 08-7 requires entities that will acquire a defensive intangible asset after the effective date of Statement 141(R), to account for the acquired intangible asset as a separate unit of accounting and amortize the acquired intangible asset over the period during which the asset would diminish in value. EITF 08-7 is effective for defensive intangible assets acquired in fiscal years beginning on or after December 15, 2008. The Company is currently evaluating the impact of this statement on its consolidated financial statements.
On April 1, 2009, the FASB issued FASB Staff Position ("FSP") No. 141(R)-1, Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies (“FSP 141(R)-1”), which amends the guidance in SFAS 141R to establish a model for pre-acquisition contingencies that is similar to the one entities used under SFAS 141. Under the FSP, an acquirer is required to recognize at fair value an asset acquired or a liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, then the acquirer follows the recognition criteria in SFAS 5 Accounting for Contingencies and FIN 14 Reasonable Estimation of the Amount of a Loss—an interpretation of FASB Statement No. 5 to determine whether the contingency should be recognized as of the acquisition date or after it. The FSP is effective for business combinations whose acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The adoption of FSP 141(R)-1 will change the Company’s accounting treatment for business combinations on a prospective basis beginning on April 1, 2009.
On April 9, 2009, the FASB issued FSP FAS 157-4, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly ("FSP 157-4"). FSP 157-4 provides additional guidance for estimating fair value in accordance with FASB 157 when the volume and level of activity for the asset or liability have significantly decreased. This FSP also includes guidance on identifying circumstances that indicate a transaction is not orderly. The FSP will be effective for the Company for interim and annual periods beginning July 1, 2009. The Company does not expect the adoption of FSP 157-4 to have a material impact on the Company's consolidated financial statements or the fair values of its financial assets and liabilities.
On April 9, 2009, the FASB issued FASB Staff Position No. FAS 107-1 and APB 28-1, Interim Disclosures about Fair Value of Financial Instruments (“FSP FAS 107-1 and APB 28-1”), to require disclosures about fair value of financial instruments for interim reporting periods of publicly traded companies as well as in annual financial statements. This FSP also amends APB Opinion No. 28, Interim Financial Reporting, to require those disclosures in summarized financial information at interim reporting periods. The FSP will be effective for the Company for interim and annual periods beginning July 1, 2009.
In May 2009, the FASB issued Statement of Financial Accounting Standards No. 165, "Subsequent Events" (SFAS No.165). SFAS 165 establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. Specifically, SFAS 165 provides (i) the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements; (ii) the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements; and (iii) the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. SFAS 165 is effective for interim or annual financial periods ending after June 15, 2009 and shall be applied prospectively.
| AA. | Changes in Presentation |
Certain prior year amounts have been reclassified to conform to the current year presentation.
On October 8, 2008, the Company acquired certain assets from Beijing Huilian Food Co., Ltd. (“Huilian”) which is a provider of prepared baby food in mainland China, with total consideration of RMB 45 million (approximately US$ 6.6 million). As a result of the acquisition, the Company has expanded into the production of prepared baby food beginning with the fiscal quarter ended March 31, 2009. For accounting purposes, the acquisition has been accounted for as a business combination in accordance with SFAS No. 141. Under the purchase method of accounting, the assets acquired and liabilities assumed from Huilian are recorded at the date of acquisition at their respective fair values. Financial statements and reported results of operations of the company have reflected these values, with the results of Huilian included from October 8, 2008, in the consolidated statements of income.
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition.
| | | (In thousands) | |
| | | | |
| Property, plant and equipment | | $ | 1,870 | |
| Land use right | | | 1,697 | |
| Intangible assets | | | 1,582 | |
| Goodwill | | | 1,435 | |
| Total | | $ | 6,584 | |
Of the $1.6 million of intangible assets acquired, $0.3 million was assigned to registered trademarks that are not subject to amortization; $0.4 million was assigned to know-how that have a useful life of approximately 5 years; $0.9 million was assigned to a customer relationship that was written off at the date of acquisition as the Company did not intend to utilize such asset and the write-off was included in selling and distribution expense. Acquired identifiable intangible assets have been allocated to the Baby Food reporting segment.
Goodwill of $1.4 million arising from this acquisition was assigned to the Baby Food segment. The impairment test of goodwill will be performed at the last day of each fiscal year or upon the occurrence of certain events and change in circumstances as required by SFAS 142.
On September 16, 2008, the Company announced a compulsory recall on certain lots of U-Smart products and a voluntary recall of other products that were contaminated or suspected to be contaminated by melamine, a substance not approved for use in food and linked to recent illnesses among infants and children in China. The cost of this action during the year ended March 31, 2009 was $101.5 million, including the cost of product replacement of $48.1 million in cost of sales, the write-down and write-off of affected inventory of $48.5 million in cost of sales, the net amount of $2.3 million to a compensation fund set up by China Dairy Industry Association to settle existing and potential claims arising in China from families of infants affected by melamine contamination in general and administrative expenses, and freight charges of $2.6 million in selling and distribution expenses, of which $4.5 million was recorded as a product recall provision in the consolidated balance sheet as of March 31, 2009. These costs represent the Company’s estimate of probable costs based on available data and take into account factors such as expected return rates for the affected units, unit replacement costs, logistical expenses and expenses relating to the hiring of temporary contractors to assist with the Company’s recall efforts.
Should actual product recall costs differ from the estimated costs, the Company would have to reassess the impact of the product recall on the Company’s financial results and revise the estimated product recall accrual accordingly. Following is a summary of the liabilities related to the product recall that were recorded during the fiscal year ended March 31, 2009:
| | | (In thousands) | |
| Balance at April 1, 2008 | | $ | — | |
| Product recall expenses | | | 53,038 | |
| Replacement of products | | | (44,002 | ) |
| Payment of cash, net | | | (4,395 | ) |
| Foreign currency translation | | | (94 | ) |
| Balance at March 31, 2009 | | $ | 4,547 | |
The Company’s inventories at March 31, 2009 and 2008 are summarized as follows:
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Raw materials | | $ | 96,361 | | | $ | 30,550 | |
| Work-in-progress | | | 11,739 | | | | 16,786 | |
| Finished goods | | | 6,624 | | | | 14,517 | |
| Total Inventories | | $ | 114,724 | | | $ | 61,853 | |
| 6. | DUE FROM (TO) RELATED PARTIES AND RELATED PARTY TRANSACTIONS |
| A. | Classification of related party balances by name |
| a. | Due from related parties |
| | March 31, 2009 | | March 31, 2008 | |
| | (In thousands) | |
| Sheng Zhi Da Dairy Group Corporation | | $ | 1,726 | | | $ | — | |
| Beijing Honnete Dairy Co., Ltd. | | | 737 | | | | — | |
| Total Due from Related Companies | | $ | 2,463 | | | $ | — | |
In June 2008, the Company sold two commercial buildings, one of which was classified as assets held for sale as of March 31, 2008, to Sheng Zhi Da Dairy Group Corporation, an entity 100% controlled by the Company’s CEO, at the carrying value of $1.7 million. The amount represents the balance due from Sheng Zhi Da Dairy Group Corporation as of March 31, 2009.
| b. | Due to related parties |
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Sheng Zhi Da Dairy Group Corporation | | | 2,113 | | | | 2,404 | |
| St. Angel (Beijing) Business Service Co., Ltd. | | | 2 | | | | 4 | |
| Beijing Honnete Dairy Co., Ltd. | | | 3,057 | | | | 562 | |
| Total Due to Related Companies | | $ | 5,172 | | | $ | 2,970 | |
The Company had certain related party borrowings. See Note 11. Except for the related party borrowings, the amount due to and due from related parties were unsecured and interest free.
| B. | Sales to related parties |
In the fiscal years ended March 31, 2009, 2008 and 2007, the Company’s sales to the related parties mainly included industrial milk powder to Kelqin, Luding Xueyuan and Baoquanling; whey protein to Honnete; milk fat and non-fat dry milk to Kelqin and Honnete; formulation ingredients to Ao Naier, BQL and Luding Xueyuan.
The following tables categorize sales to related companies as main product sales and ancillary product sales as presented in the income statements:
| a. | Main product sales to related parties |
| | Year Ended March 31, | |
| | 2009 | | 2008 | | 2007 | |
| | (In thousands) | |
| Beijing Kelqin Dairy Co., Ltd. | | $ | — | | | $ | — | | | $ | 5,230 | |
| Beijing Luding Xueyuan Trading Co., Ltd. | | | — | | | | 1,497 | | | | 686 | |
| Heilongjiang Baoquanling Shengyuan Dairy Co., Ltd. | | | — | | | | — | | | | 637 | |
| Total | | $ | — | | | $ | 1,497 | | | $ | 6,553 | |
| b. | Ancillary product sales to related parties |
| | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (In thousands) | |
| Beijing Honnete Dairy Co., Ltd. | | $ | 2,255 | | | $ | — | | | $ | 6,128 | |
| Beijing Kelqin Dairy Co., Ltd. | | | — | | | | 143 | | | | 311 | |
| Heilongjiang Baoquanling Shengyuan Dairy Co., Ltd. | | | — | | | | — | | | | 3,401 | |
| Beijing Ao Naier Feed Stuff LLC | | | — | | | | — | | | | 130 | |
| Beijing Luding Xueyuan Trading Co., Ltd. | | | — | | | | — | | | | 260 | |
| St.Angel (Beijing) Business Service Co., Ltd. | | | — | | | | — | | | | 32 | |
| Total | | $ | 2,255 | | | $ | 143 | | | $ | 10,262 | |
| | | | | | | | | | | | | |
| Total of main product and ancillary product sales to related companies | | $ | 2,255 | | | $ | 1,640 | | | $ | 16,815 | |
| C. | Purchases from related parties |
In the fiscal year ended March 31, 2009, 2008 and 2007, the Company’s purchases from related parties included whey protein powders from Kelqin and Honnete, spray-dried milk powder from BQL, and catalogues, brochures, and marketing materials from St. Angel.
| | Year Ended March 31, | |
| | 2009 | | 2008 | | 2007 | |
| | (In thousands) | |
| Beijing Honnete Dairy Co., Ltd. | | $ | 10,376 | | | $ | 17,862 | | | $ | 11,460 | |
| St. Angel (Beijing) Business Service Co., Ltd. | | | 1,639 | | | | 988 | | | | 19 | |
| Beijing Kelqin Dairy Co., Ltd. | | | 154 | | | | 3,286 | | | | 3,033 | |
| Heilongjiang Baoquanling Shengyuan Dairy Co., Ltd. | | | — | | | | — | | | | 15,139 | |
| Total | | $ | 12,169 | | | $ | 22,136 | | | $ | 29,651 | |
| 7. | PREPAID EXPENSES AND OTHER CURRENT ASSETS |
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Staff advance | | $ | — | | | $ | 96 | |
| Advance to suppliers | | | 584 | | | | 2,338 | |
| Prepaid advertising expense | | | 535 | | | | 5,656 | |
| Receivable due to disposal of a subsidiary | | | 870 | | | | 972 | |
| Other tax receivable | | | 3,148 | | | | — | |
| Subsidy receivable | | | 5,060 | | | | — | |
| Others | | | 3,239 | | | | 1,738 | |
| | | | | | | | | |
| Total | | $ | 13,436 | | | $ | 10,800 | |
| 8. | PROPERTY, PLANT AND EQUIPMENT, NET |
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Property, plant and equipment, cost: | | | | | | |
| Buildings | | $ | 51,693 | | | $ | 27,004 | |
| Plant and machinery | | | 65,020 | | | | 38,430 | |
| Office equipment and furnishings | | | 3,076 | | | | 2,534 | |
| Motor vehicles | | | 2,963 | | | | 1,340 | |
| Others | | | 543 | | | | 348 | |
| | | | | | | | | |
| Total cost | | $ | 123,295 | | | $ | 69,656 | |
| | | | | | | | | |
| Less: Accumulated depreciation: | | | | | | | | |
| Buildings | | | 4,472 | | | | 2,831 | |
| Plant and machinery | | | 11,804 | | | | 6,933 | |
| Office equipment and furnishings | | | 1,183 | | | | 705 | |
| Motor vehicles | | | 885 | | | | 545 | |
| Others | | | 273 | | | | 197 | |
| | | | | | | | | |
| Total accumulated depreciation | | | 18,617 | | | | 11,211 | |
| | | | | | | | | |
| Construction in progress | | | 39,803 | | | | 27,274 | |
| | | | | | | | | |
| Property, plant and equipment, net | | $ | 144,481 | | | $ | 85,719 | |
Construction in progress primarily represents the construction of manufacturing facilities and related equipment, and administrative buildings.
The Company recorded depreciation expense of $7.5 million, $4.6 million and $1.8 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively.
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Land use rights, cost | | $ | 6,757 | | | $ | 4,744 | |
| Less: Accumulated amortization | | | 383 | | | | 248 | |
| Land use rights, net | | $ | 6,374 | | | $ | 4,496 | |
The Company recorded amortization expense of $128,000, $80,000 and $40,000 for the fiscal years ended March 31, 2009, 2008 and 2007, respectively.
| 10. | INTANGIBLE ASSETS, NET |
| | | March 31, 2009* | |
| | | Gross Carrying Amount | | | Accumulated Amortization | | | Write-off | | | Net Carrying Amount | |
| | | (In thousands) | |
| Know-how (5-year useful life) | | $ | 398 | | | $ | 40 | | | $ | — | | | $ | 358 | |
| Customer relationship | | | 880 | | | | — | | | | 880 | | | | — | |
| Registered trademark (indefinite life) | | | 2,778 | | | | — | | | | — | | | | 2,778 | |
| | | $ | 4,056 | | | $ | 40 | | | $ | 880 | | | $ | 3,136 | |
* Nil figures for the comparable periods in fiscal year 2008.
The intangible assets acquired in the fiscal year ended March 31, 2009 included the know-how, customer relationship and registered trademarks arising from the acquisition of Huilian’s prepared baby food business discussed in Note 3, and the registered trademarks of Helanruniu, or Holsteina, that are being used for the Company’s powdered adult formula products.
Amortization expense was $40,000 for the fiscal year ended March 31, 2009.
On April 19, 2007, the Company entered into a bridge loan agreement with ABN AMRO Bank N.V., Hong Kong branch (“ABN”), in the amount of $35.0 million (the “Bridge Loan Agreement”). The Loan bore interest at the one-month London interbank offered rate for deposits in US dollars plus 2.5% with interest payable on the last day of each month. The Company was required to pay to ABN a commitment fee of 1% on the daily amount of the unused Commitment Amount. The Loans were secured by a pledge of 25,000,000 shares of the Company’s Common Stock owned by Beams Power Investment Limited, a British Virgin Islands company, the capital stock of which is beneficially owned by Liang Zhang, the Chief Executive Officer of the Company. Pursuant to the Loan Agreement, Liang Zhang and Xiuqing Meng, Mr. Zhang’s wife, guaranteed the Company’s obligations thereunder. The principal amount and unpaid accrued interest under the Bridge Loan Agreement were repaid on October 18, 2007.
In addition, pursuant to a USD facility side letter and warrant agreement dated April 19, 2007 between the Company and ABN, the Company was obligated to issue warrants to ABN to purchase up to 400,000 shares of the Company’s common stock (the “Warrant Agreement”). On April 19, 2007 and October 11, 2007, respectively, the Company issued warrants to purchase 200,000 shares of its common stock at $8.84 per share and the remaining 200,000 shares at the same price. All of the warrants may be exercised up to the third anniversary of the completion of a “Qualified Public Offering”, as defined in the warrant agreement.
The fair value of the warrants was $2.7 million at the grant date, estimated on the basis of the Black-Scholes-Merton option-pricing formula with the following assumptions:
| | | | | |
| Expected volatility | | | 43.86 | % |
| Risk-free interest rate | | | 4.66 | % |
| Expected dividend yield | | | 0.00 | % |
| Contractual life of the warrant (years) | | | 4.2 | |
The proceeds of the 2007 loan were allocated to the debt and the warrants based on their relative values, resulting in $32.4 million and $2.6 million being allocated to the debt and warrants, respectively. This amount has been recorded as an increase to additional paid in capital with a corresponding debt discount, which was fully amortized as of March 31, 2008.
On October 11, 2007, ABN and another lender provided a three year term loan, or the New ABN Loan, to the Company in the aggregate amount of $35 million. The principal amount, and any unpaid accrued interest on the New ABN Loan, will be due on October 11, 2010 and may be prepaid without penalty. The proceeds of the New ABN Loan were used to pay the outstanding principal and accrued interest of a six-month term loan provided to the Company by ABN on April 19, 2007, or the Original ABN Loan. The New ABN Loan bears interest at LIBOR for deposits in U.S. dollars plus 3.5% with interest payable on the last day of each three-month period. The loan is secured by a pledge of 10,000 shares of common stock, or 100% of the outstanding common stock of Synutra Illinois.
The loan agreement for the New ABN Loan provides for mandatory prepayment upon the occurrence of certain events, and contains customary covenants for financings of this type, including restrictions on the incurrence of liens, payment of dividends, and disposition of properties. The loan agreement for the New ABN Loan also contains certain financial covenants, including a requirement to maintain specified leverage and interest coverage ratio, tangible net worth, and indebtedness to tangible net worth ratio. The Company has performed an analysis of the relevant ratios and found that due to the net loss which resulted from the significant costs of the product recall, including inventory write-down and write-off, the Company was not able to meet any of the financial covenant requirements as of March 31, 2009. Further, the Company anticipates that it may have difficulty meeting these financial covenant requirements for the next several fiscal quarters. The Company has requested the lenders to waive these financial covenants for the next several fiscal quarters. The lenders have proposed waiver fee which equals to 0.75% of the aggregate principal amount outstanding and an increase in the interest rate of 200 basis points to LIBOR for deposits in U.S. dollars plus 5.5%. The lenders have also proposed a revised repayment schedule of quarterly amortizations commencing in October 2009 with $5.0 million in October 2009, $5.0 million in January 2010, $10.0 million in April 2010, $10.0 million in July 2010, and $5.0 million on maturity. Any non-payment would constitute a default, for which the banks have rights to enforce on the existing share pledge. As of the issuance date of consolidated financial statement, the waiver has not been granted. The Company is currently in discussions with the lenders on the waiver and is unable to predict when, or if the waiver will be granted. Accordingly, the Company has reclassified the outstanding balance of $34.5 million under the loan to current liabilities since the Company considers this debt callable by the bank. The Company may also incur additional expenses in connection with the modification of loan arrangements.
In addition to the New ABN Loan, as of March 31, 2009 and March 31, 2008, the Company had short-term loans from PRC banks in the amount of $182.6 million and $21.2 million, respectively. The maturity dates of the short term loans outstanding from PRC banks at March 31, 2009 range from April 2009 to March 2010. The weighted average interest rate on short-term loans from PRC banks outstanding at March 31, 2009 and March 31, 2008 was 4.1% and 7.08%, respectively. The loans at March 31, 2009 were secured by the pledge of certain fixed assets held by the Company and its subsidiaries, a pledge of the Company’s land use right and pledge of cash deposits. The value of fixed assets pledged was $34.2 million and $18.1 million as of March 31, 2009 and March 31, 2008, respectively. The value of land use right pledged was $3.4 million and nil as of March 31, 2009 and March 31, 2008, respectively. The value of cash pledged was $66.1 million which was recorded in restricted cash and nil as of March 31, 2009 and March 31, 2008, respectively.
As of March 31, 2009 and 2008, the Company had long-term loans which are unsecured debt, from PRC banks in the amount of $8.8 million and $1.9 million, respectively. The maturity date of the loan term loans outstanding from PRC banks at March 31, 2009 is in March 2011. The weighted average interest rate of outstanding long-term loans at March 31, 2009 and March 31, 2008 was 5.4% and 3.2%, respectively.
Apart from the short-term loans and long-term loans from banks, the Company also had short term loans from related parties in the amount of $7.5 million and nil as of March 31, 2009 and March 31, 2008 respectively. The maturity dates of the short term loans outstanding from related parties at March 31, 2009 are in October 2010. The balance as of March 31, 2009 included a US dollar loan of $3.9 million and a RMB loan of $3.6 million, and the interest rates were 10.0% and 5.5% respectively. The interest rate of the US dollar loan is benched mark to that of US dollar borrowing from third parties.
| 12. | OTHER CURRENT LIABILITIES |
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Other tax payables | | | — | | | | 2,661 | |
| Accrued freight charges | | $ | 434 | | | $ | 837 | |
| Accrued rebate and slotting fee | | | 844 | | | | 371 | |
| Deferred government subsidies | | | 991 | | | | — | |
| Accrued professional service fees | | | 1,046 | | | | 1,240 | |
| Payroll and bonus payables | | | 3,615 | | | | 1,852 | |
| Accrued selling and marketing expenses | | | 4,118 | | | | 3,627 | |
| Accrued advertising and promotion expenses | | | 5,984 | | | | 3,140 | |
| Others | | | 4,893 | | | | 2,761 | |
| Total | | $ | 21,925 | | | $ | 16,489 | |
| 13. | OBLIGATIONS UNDER CAPITAL LEASES |
Future minimum capital lease payments at March 31, 2009 are as follows:
| | | Year Ending | |
| | | March 31, | |
| | | (In thousands) | |
| 2010 | | $ | — | |
| 2011 | | | 384 | |
| 2012 | | | 473 | |
| 2013 | | | 473 | |
| 2014 | | | 473 | |
| 2015 and thereafter | | | 16,235 | |
| | | | | |
| Total minimum lease payments | | $ | 18,038 | |
| Less: Amount representing interest | | | 12,784 | |
| | | | | |
| Present value of minimum lease payments | | | 5,254 | |
| | | | | |
| Current | | | — | |
| Long-term | | | 5,254 | |
| Total | | | 5,254 | |
The interest rate associated with the capital leases is 7.8% per annum.
| A. | Tax law of each tax jurisdictions |
United States
Under the federal and state income tax laws of United States, the Company is subject to tax on its income or capital gains. As at March 31, 2009, the Company’s subsidiary in United States does not have any assessable profit and accordingly, no provision for federal and state income taxes have been provided thereon.
China
On March 16, 2007, the National People’s Congress of the PRC approved and promulgated the new Enterprise Income Tax Law ("new EIT Law"), which took effect beginning January 1, 2008. Under the new EIT law, foreign investment enterprises and domestic companies are subject to a uniform tax rate of 25%. The new tax law provides a five-year transition period from its effective date for certain qualifying enterprises which were established before the promulgation date of the new tax law and which were entitled to a preferential lower tax rate or tax holiday under the then effective tax laws or regulations.
Some of the Company's PRC subsidiaries are eligible under the transition rules to continue enjoying tax holidays or reduced tax rate until expiration. The following table illustrates the applicable tax rate and tax holidays of major PRC subsidiaries under the new EIT Law:
| | | | Statutory Tax Rate | | | |
| | | Beginning January 1, 2008 | | | Tax Holiday (based on calendar year) |
| Shengyuan Nutritional Food Co., Ltd. | | | 25 | % | | 2 years tax free (2004, 2005); 12% (2006, 2007); 12.5% (2008) |
| Qingdao Shengyuan Dairy Co., Ltd. | | | 25 | % | | No tax holiday |
| Heilongjiang Mingshan Dairy Co., Ltd. | | | 25 | % | | 2 years tax free (2006, 2007); 3 years tax at 12.5% (2008-10) |
| Zhangjiakou Shengyuan Dairy Co., Ltd. | | | 25 | % | | 2 years tax free (2006, 2007); 3 years tax at 12.5% (2008-10) |
| Inner Mongolia Shengyuan Food Co., Ltd. | | | 25 | % | | 2 years tax free (2008, 2009); 3 years tax at 12.5% (2010-12) |
| Inner Mongolia Mengyuan Food Co., Ltd. | | | 25 | % | | No tax holiday |
| Meitek Technology (Qingdao) Co., Ltd. | | | 25 | % | | 2 years tax free (2008, 2009); 3 years tax at 12.5% (2010-12) |
| Heilongjiang Baoquanling Shengyuan Dairy Co., Ltd. | | | 25 | % | | No tax holiday |
| Beijing Shengyuan Huiliduo Food Technology Co., Ltd. | | | 25 | % | | No tax holiday |
| B. | Components of income (loss) before income taxes |
For financial reporting purposes, income (loss) before income taxes includes the following components:
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (In thousands) | |
| United States | | $ | (3,320 | ) | | $ | (7,235 | ) | | $ | (126 | ) |
| PRC | | | (127,655 | ) | | | 60,762 | | | | 21,596 | |
| | | $ | (130,975 | ) | | $ | 53,527 | | | $ | 21,470 | |
| C. | Reconciliation from income tax at statutory rate to reported amount of income tax expense |
The income tax expense reconciled to the tax expense computed at the US statutory rate was approximately as follows for the years ended March 31, 2009, 2008 and 2007:
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (In thousands) | |
| Income tax provision at US federal statutory rate of 34% | | $ | (44,531 | ) | | $ | 18,199 | | | $ | 7,300 | |
| Foreign tax rate differential | | | 11,489 | | | | (2,393 | ) | | | (215 | ) |
| PRC tax holiday effect | | | (3,228 | ) | | | (13,978 | ) | | | (5,231 | ) |
| Change in valuation allowance | | | 3,072 | | | | 2,135 | | | | — | |
| FIN 48 unrecognized tax benefits | | | 81 | | | | 508 | | | | — | |
| Nondeductible (nontaxable) items | | | 2,731 | | | | 3,384 | | | | (258 | ) |
| Total income tax expenses | | $ | (30,386 | ) | | $ | 7,855 | | | $ | 1,596 | |
Certain Company’s PRC subsidiaries are eligible for tax holidays. The impact of these tax holidays decreased/(increased)PRC taxes by $(3.2) million, $14.0 million and $5.2 million for the fiscal year ended March 31, 2009, 2008 and 2007, respectively. The benefit/(expense) of the tax holidays on earnings per share was $0.06, $0.26 and $0.10 for the fiscal year ended March 31, 2009, 2008 and 2007, respectively.
| D. | Deferred tax assets by type of temporary difference, credits and change in valuation allowance |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows:
| | | March 31, 2009 | | March 31, 2008 | |
| | | (In thousands) | |
| Deferred tax assets - current | | | | | | | |
| Inventory write-down | | | $ | 8,005 | | | $ | — | |
| Promotional expenses carried forward | | | | 7,367 | | | | — | |
| Net operating loss | | | | 151 | | | | 287 | |
| Accrued expenses | | | | 88 | | | | 1,210 | |
| Others | | | | 909 | | | | 227 | |
| Subtotal | | | | 16,520 | | | | 1,724 | |
| Less: valuation allowance | | | | (244 | ) | | | (276 | ) |
| Total current deferred tax assets | | | $ | 16,276 | | | $ | 1,448 | |
| | | | | | | | | | |
| Deferred tax assets – non current | | | | | | | | | |
| Net operating loss | | | $ | 20,988 | | | $ | 1,462 | |
| Tax credit carryforward for PRC equipment purchasing | | | | 2,083 | | | | 2,028 | |
| Fixed assets | | | | 278 | | | | 320 | |
| Others | | | | 78 | | | | 126 | |
| Subtotal | | | | 23,427 | | | | 3,936 | |
| Less: valuation allowance | | | | (4,963 | ) | | | (1,859 | ) |
| Total non-current deferred tax assets | | | $ | 18,464 | | | $ | 2,077 | |
The net change during the year in the total valuation allowance is as follows:
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Balance at beginning of year | | $ | 2,135 | | | $ | — | |
| Additions during the year | | | 3,072 | | | | 2,135 | |
| Balance at end of year | | $ | 5,207 | | | $ | 2,135 | |
At March 31, 2009, net operating loss carryforward of Synutra Illinois and Synutra International, Inc., who file consolidated tax return, is approximately $13.8 million, which expires through 2029. Subject to the federal income tax rate of 34%, the tax benefit is $4.7 million as of March 31, 2009. Synutra Illinois and Synutra International, Inc. do not have enough profit in the foreseeable future to realize the tax benefit. The Company believes that it is more likely than not that the benefit from the net operating loss carryforward will not be realized. In recognizing this risk, the Company has fully provided a valuation allowance on the deferred tax assets relating to these operating loss carryforward. If or when recognized, the tax benefits relating to any reversal of the valuation allowance on deferred tax assets at March 31, 2009 will be recognized as a reduction of income tax expense. At March 31, 2009, deductible net operating loss carryforwards of PRC subsidiaries were $69.2 million, which expires through 2014. Subject to the PRC income tax rate of 25% and applicable tax holidays, the tax benefit is $16.5 million as of March 31, 2009.. The Company believes that it is more likely than not that the benefit from the net operating loss carryforward relating to its PRC subsidiaries will be realized through future profits. The amount of the deferred tax assets considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carry forward periods are reduced.
| E. | Significant components of income tax expense |
Income taxes for the PRC subsidiaries are calculated on a separate entity basis. Each of the Company’s PRC subsidiaries files stand-alone tax returns. The provisions for the Company’s income taxes for the fiscal years ended March 31, 2009, 2008 and 2007, respectively, are summarized as follows:
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (In thousands) | |
| Current tax | | | | | | | | | |
| US | | $ | — | | | $ | — | | | $ | — | |
| PRC | | | 738 | | | | 10,550 | | | | 2,028 | |
| | | | | | | | | | | | | |
| | | | 738 | | | | 10,550 | | | | 2,028 | |
| | | | | | | | | | | | | |
| Deferred tax | | | | | | | | | | | | |
| Deferred tax-benefit-US | | | — | | | | 218 | | | | (218 | ) |
| Deferred tax-benefit-PRC | | | (31,124 | ) | | | (2,913 | ) | | | (214 | ) |
| | | | | | | | | | | | | |
| | | | (31,124 | ) | | | (2,695 | ) | | | (432 | ) |
| | | | | | | | | | | | | |
| Total | | $ | (30,386 | ) | | $ | 7,855 | | | $ | 1,596 | |
| F. | Uncertainty in income tax |
Effective April 1, 2007, the Company adopted FIN 48, which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken in the tax return. This interpretation also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods and income tax disclosures.
The adoption of FIN 48 has reduced the retained earnings as of April 1, 2007, by $375,000, including interest and penalties amounting to $55,000, with a corresponding increase in the liability for uncertain tax positions. The above mentioned liability is recorded in other long term liabilities in the consolidated balance sheet. In accordance with the Company’s policies, it accrued and classified interest and penalties related to unrecognized tax benefits as a component of income tax provision. For the year ended March 31, 2009, the Company assessed its FIN 48 provisions and has recognized an additional net liability (including adjustments of interest) of $81,000. The Company does not anticipate any significant increases or decreases to its liability for unrecognized tax benefits within the next 12 months.
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits for the year:
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| Balance at beginning of year | | $ | 883 | | | $ | 375 | |
| Additions based on tax positions related to the current year | | | 343 | | | | 508 | |
| Reductions for tax positions of prior years | | | (262 | ) | | | — | |
| Balance at end of year | | $ | 964 | | | $ | 883 | |
The balance of unrecognized tax benefits at March 31, 2009, if recognized, would affect the effective tax rate.
According to PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of taxes is due to computational errors made by the taxpayer or withholding agent. The statute of limitations will be extended to five years under special circumstances, which are not clearly defined (but an underpayment of tax liability exceeding RMB100,000 is specifically listed as a special circumstance). In the case of a related party transaction, the statute of limitations is 10 years. There is no statute of limitations in the case of tax evasion. The Company is subject to taxation in the US and the PRC. The statute of limitations in the US is three years.
As of March 31, 2008, the Company's foreign subsidiaries had approximately $74.1million of undistributed earnings which were considered to be indefinitely reinvested, and accordingly, no provision for income taxes has been provided thereon. Upon distribution of those earnings in the form of dividends or otherwise, the Company would be subject to withholding taxes payable to various foreign countries. For the fiscal year ended March 31, 2009, the Company's foreign subsidiaries did not generate any distributable earnings.
| 15. | EARNINGS PER SHARE (EPS) |
For purposes of calculating basic and diluted earnings per share, the Company used the following weighted average common shares outstanding:
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (In thousands except for per share data) | |
| Net income (loss) attributable to common shareholders | | $ | (100,549 | ) | | $ | 45,661 | | | $ | 19,874 | |
| | | | | | | | | | | | | |
| Basic weighted average common shares outstanding | | | 54,001 | | | | 53,170 | | | | 50,001 | |
| Dilutive potential common shares from warrants | | | — | | | | 306 | | | | — | |
| | | | | | | | | | | | | |
| Diluted weighted average shares outstanding | | | 54,001 | | | | 53,476 | | | | 50,001 | |
| | | | | | | | | | | | | |
| Earnings (loss) per share-basic | | $ | (1.86 | ) | | $ | 0.86 | | | $ | 0.40 | |
| | | | | | | | | | | | | |
| Earnings (loss) per share-diluted | | $ | (1.86 | ) | | $ | 0.85 | | | $ | 0.40 | |
The warrants to purchase 400,000 shares of common stock granted to ABN in connection with the Original ABN Loan were excluded from the computation of diluted earnings per share for the fiscal year ended March 31, 2009 as they would be anti-dilutive. There were no anti-dilutive warrants excluded from the computation of diluted earnings per share for the fiscal years ended March 31, 2008 and 2007.
| 16. | COMMITMENTS AND CONTINGENCIES |
As of March 31, 2009, the Company had outstanding commitments in the amount of $19.2million for advertising and raw materials purchase within the next twelve months.
As of March 31, 2009, the Company’s capital commitments amounted to $6.0 million in relation to asset improvement and plant expansion within the next twelve months.
| C. | Operating lease commitments |
The Company leases certain office facilities and certain warehouses under non-cancellable operating leases. The operating lease commitments of the Company at March 31, 2009 are as follows:
| | | Year Ending | |
| | | March 31, | |
| | | (In thousands) | |
| 2010 | | $ | 650 | |
| 2011 | | | 1,532 | |
| 2012 | | | 1,836 | |
| 2013 | | | 1,836 | |
| 2014 | | | 1,770 | |
| 2015 and thereafter | | | 59,256 | |
| Total | | $ | 66,880 | |
As of March 31, 2009, the end of the period covered by this report, the Company was subject to various legal proceedings and claims discussed below, as well as certain other legal proceedings and claims that have not been fully resolved and that have arisen in the ordinary course of business. Other than as discussed below, in the opinion of management, the Company does not have a potential liability related to any current legal proceedings and claims that would individually or in the aggregate have a material adverse effect on its financial condition or operating results. However, the results of legal proceedings cannot be predicted with certainty. The Company intends to contest each lawsuit vigorously but should the Company fail to prevail in any of these legal matters or should several of these legal matters be resolved against the Company in the same reporting period, the operating results of a particular reporting period could be materially adversely affected. Management continues to evaluate the lawsuits discussed below and based on the stage of these proceedings, management is unable to reasonably estimate the likelihood of any loss or the amount or range of any potential loss that could result from the litigation. Therefore, no accrual has been established for any potential loss in connection with these lawsuits.
On January 15, 2009, a lawsuit was filed in the U.S. on behalf of 54 Chinese families alleged to be affected by melamine contamination, against Synutra International, Inc. and Synutra Inc. in the U.S. District Court for the District of Maryland, alleging negligent or intentional infliction of personal injury, negligent or intentional infliction of emotional distress, battery, breach of warranty, fraudulent or negligent misrepresentation, seeking compensation for punitive damages in the amount of US$500 million, together with any compensatory damages. The Company filed a motion in April to dismiss the case on grounds of "Forum Non-Convenience” and the opposition filed a Memorandum of Opposition to Motion to Dismiss on May 20, 2009. The Company had been given time to respond to the Opposition, and we will do so on or before June 15, 2009 with an additional filing. After June 15, 2009, the presiding Judge of the Court will decide, with no time limit, if the Court will take the case or render its dismissal. At this stage, the management is unable to predict the outcome of such lawsuit.
On March 2, 2009, a lawsuit was filed in China on behalf of 54 Chinese families alleged to be affected by melamine contamination against a subsidiary, Shengyuan Nutritional Food Co., Ltd. at Qingdao Intermediate People’s Court, seeking compensation for damages, including medical and other expenses, emotional harm and punitive damages, for an aggregate amount over US$1.0 million (RMB6.9 million). Further, on April 14, 2009, a lawsuit was filed on behalf of 73 families (including the original 54 families) against a subsidiary, Shengyuan Nutritional Food Co., Ltd. at Shandong Higher People’s Court on April 14, 2009, seeking compensation for damages of over US$1.7 million (RMB11.3 million). To date, the Company has not been notified by either court of acceptance of any case against the Company in China.
As at March 31, 2009, the Company had an outstanding guarantee issued to the Zhangbei Branch of the Agricultural Bank of China related to various bank loans totaling $1.1 million to 104 dairy farmers in the Zhangbei Area (the “Guarantee”). Such loans matured on December 25, 2007, but as of March 31, 2009, none of the loans had been repaid and the total outstanding loan amount remained $1.1 million. The Company has prepared its best estimate of possible losses related to these loans based on a weighed average range of likely probabilities and potential payments that would be required under the Guarantee, and estimated a potential loss of $367,000 related to the Guarantee.
| 17. | SEGMENT REPORTING AND GEOGRAPHIC INFORMATION |
During the fiscal year ended March 31, 2009, Meitek, a PRC subsidiary of the Company which is engaged in the production of nutritional ingredients and supplements such as chondroitin sulphate, microencapsulated Docosahexanoic Acid (“DHA”) and Arachidonic Acid (“ARA”), completed its construction process and began its operations. In the same fiscal year, the Company acquired the prepared baby food business from Huilian which had begun operation by March 31, 2009. Historically the Company reported results as a single reporting segment in the financial statements since operating segments such as nutritional ingredients and supplements were under construction with minor operating expenses and had not met the quantitative threshold as described under SFAS 131 "Disclosure about Segments of an Enterprise and Related Information". The chief operating decision maker manages the business, including making operating decisions (such as allocating resources) and evaluating operating performance, on a gross profit (loss) basis. The Company expanded the number of reportable segments from one to three in order to better reflect the manner in which management analyzes the Company’s performance including powdered formula, baby food, and nutritional ingredients and supplements. All prior period segment information has been recast to reflect the current period presentation. The Company accounts for inter-segment sales and transfers as if the sales or transfers were to third parties, that is, at current market prices. The activities of each segment are as follows:
Powdered Formula - Sales of powdered infant and adult formula products.
Baby Food - Sales of prepared baby food and nutritional snacks for babies and children.
Nutritional Ingredients and Supplements - Sales of nutritional ingredients and supplements such as chondroitin sulphate, and microencapsulated DHA and ARA.
"All Other" includes non-core businesses such as toll packaging, toll drying service and sales of ingredients and materials to industrial customers.
The Company’s underlying accounting records are maintained on a legal entity basis for government and public reporting requirements. Segment disclosures are on a performance basis consistent with internal management reporting. The intangible assets which were recognized upon the acquisition of the prepared baby food business from Huilian in the fiscal year ended March 31, 2009 and related amortization are allocated to the baby food segment. The following segment information has been prepared in accordance with the internal accounting policies of the Company, as described above.
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (In thousands) | |
| NET SALES | | | | | | | | | |
| Powdered formula | | $ | 284,818 | | | $ | 321,130 | | | $ | 167,499 | |
| Baby food | | | 271 | | | | — | | | | — | |
| Nutritional ingredients and supplements | | | 3,367 | | | | — | | | | — | |
| All other | | | 27,439 | | | | 40,960 | | | | 49,106 | |
| Intersegment sales elimination | | | (3,367 | ) | | | — | | | | — | |
| | | | | | | | | | | | | |
| Net sales | | $ | 312,528 | | | $ | 362,090 | | | $ | 216,605 | |
| | | | | | | | | | | | | |
| GROSS PROFIT | | | | | | | | | | | | |
| Powdered formula | | $ | 50,336 | | | $ | 178,728 | | | $ | 102,282 | |
| Baby food | | | 150 | | | | — | | | | — | |
| Nutritional ingredients and supplements | | | — | | | | — | | | | — | |
| All other | | | 2,956 | | | | 7,794 | | | | 4,423 | |
| Intersegment profit elimination | | | — | | | | | | | | | |
| | | | | | | | | | | | | |
| Gross profit | | $ | 53,442 | | | $ | 186,522 | | | $ | 106,705 | |
| | | | | | | | | | | | | |
| Selling and distribution expenses | | | 44,178 | | | | 34,449 | | | | 25,561 | |
| Advertising and promotion expenses | | | 115,478 | | | | 76,388 | | | | 52,322 | |
| General and administrative expenses | | | 25,455 | | | | 16,013 | | | | 7,031 | |
| Other operating income, net | | | 5,790 | | | | 1,492 | | | | 1,109 | |
| | | | | | | | | | | | | |
| Income (loss) from operations | | | (125,879 | ) | | | 61,164 | | | | 22,900 | |
| Interest expense | | | 4,857 | | | | 6,354 | | | | 1,896 | |
| Interest income | | | 341 | | | | 1,801 | | | | 356 | |
| Other income (expense), net | | | (580 | ) | | | (3,084 | ) | | | 110 | |
| | | | | | | | | | | | | |
| Income (loss) before provision (benefit) for income tax and minority interests | | $ | (130,975 | ) | | $ | 53,527 | | | $ | 21,470 | |
| | | | | | | | | | | | | |
| CAPITAL EXPENDITURE | | | | | | | | | | | | |
| Powdered formula | | $ | 45,492 | | | $ | 24,567 | | | $ | 14,462 | |
| Baby food | | | 4,389 | | | | 6,088 | | | | 6,349 | |
| Nutritional ingredients and supplements | | | 8,929 | | | | 10,853 | | | | 772 | |
| All other | | | 3,231 | | | | 1 | | | | 1 | |
| | | | | | | | | | | | | |
| Total | | $ | 62,041 | | | $ | 41,509 | | | $ | 21,584 | |
| | | | | | | | | | | | | |
| DEPRECIATION AND AMORTIZATION | | | | | | | | | | | | |
| Powdered formula | | $ | 6,686 | | | $ | 4,558 | | | $ | 2,056 | |
| Baby food | | | 351 | | | | 61 | | | | — | |
| Nutritional ingredients and supplements | | | 608 | | | | 26 | | | | — | |
| All other | | | 4 | | | | 1 | | | | 1 | |
| | | | | | | | | | | | | |
| Total | | $ | 7,649 | | | $ | 4,646 | | | $ | 2,057 | |
| | | March 31, 2009 | | | March 31, 2008 | |
| | | (In thousands) | |
| TOTAL ASSETS | | | | | | |
| Powdered formula | | $ | 443,946 | | | $ | 271,114 | |
| Baby food | | | 30,291 | | | | 19,200 | |
| Nutritional ingredients and supplements | | | 49,611 | | | | 11,835 | |
| All other | | | 115,923 | | | | 119,580 | |
| Intersegment elimination | | | (167,200 | ) | | | (127,411 | ) |
| | | | | | | | | |
| Total | | $ | 472,571 | | | $ | 294,318 | |
| | | | | | | | | |
| LONG LIVED ASSETS | | | | | | | | |
| Powdered formula | | $ | 106,726 | | | $ | 68,932 | |
| Baby food | | | 21,223 | | | | 12,441 | |
| Nutritional ingredients and supplements | | | 22,630 | | | | 8,839 | |
| All other | | | 276 | | | | 3 | |
| | | | | | | | | |
| Total | | $ | 150,855 | | | $ | 90,215 | |
Consolidated revenue is generated from sales in the following areas:
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Sales in Mainland China | | | 98.9 | % | | | 94.8 | % | | | 92.4 | % |
| Sales to United States | | | 1.1 | % | | | 5.2 | % | | | 7.6 | % |
Only one customer in the U.S. accounted for more than 5% of the Company’s total sales in fiscal year 2008 and 2007
All of the Company’s long-lived assets are located in China.
| 18. | MAINLAND CHINA CONTRIBUTION PLAN AND RESTRICTED NET ASSETS |
| A. | China Contribution Plan |
Full time employees of the Company in the PRC participate in a government-mandated multi-employer defined contribution plan pursuant to which certain pension benefits, medical care, unemployment insurance and other welfare benefits are provided to employees. PRC labor regulations require the Company to accrue for these benefits based on certain percentage of the employees’ salaries. The total contribution for such employee benefits were $4.9 million, $2.7 million and $1.2 million for the fiscal years ended March 31, 2009, 2008 and 2007, respectively.
Relevant PRC statutory laws and regulations permit payments of dividends by the Company’s PRC subsidiaries only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. PRC laws and regulations require that annual appropriations of 10% of after-tax income should be set aside prior to payment of dividends as a general reserve fund. In addition, there are restrictions on the distribution of share capital from the Company’s PRC subsidiaries. As a result of these PRC laws and regulations, the Company’s PRC subsidiaries and PRC affiliates are restricted in their ability to transfer a portion of their net assets to the Company in the form of dividends, loans or advances. Such restricted portions amounted to approximately $76.9 million and $128.7 million as of March 31, 2009 and 2008, respectively.
| 19. | QUARTERLY FINANCIAL DATA (UNAUDITED) |
Summarized quarterly financial information in fiscal years ended March 31, 2009 and 2008 is as follows:
| | | Fiscal Year 2009 | | | Fiscal Year 2008 | |
| | | First | | | Second | | | Third | | | Fourth | | | First | | | Second | | | Third | | | Fourth | |
| | | (In thousands except per share data) | |
| Revenue | | $ | 127,380 | | | $ | 94,791 | | | $ | 17,658 | | | $ | 72,699 | | | $ | 67,492 | | | $ | 86,202 | | | $ | 100,205 | | | $ | 108,191 | |
| Gross Profit (loss) | | | 66,895 | | | | (26,723 | ) | | | (9,760 | ) | | | 23,030 | | | | 37,062 | | | | 46,626 | | | | 50,578 | | | | 52,256 | |
| Net Income (loss) | | | 15,644 | | | | (49,680 | ) | | | (49,342 | ) | | | (17,171 | ) | | | 5,340 | | | | 9,772 | | | | 11,203 | | | | 19,346 | |
| Weighted average common share outstanding, basic | | | 54,001 | | | | 54,001 | | | | 54,001 | | | | 54,001 | | | | 50,667 | | | | 54,001 | | | | 54,001 | | | | 54,001 | |
| Weighted average common share outstanding, diluted | | | 54,291 | | | | 54,001 | | | | 54,001 | | | | 54,001 | | | | 50,786 | | | | 54,132 | | | | 54,304 | | | | 54,682 | |
| Earnings per share, basic | | $ | 0.29 | | | $ | (0.92 | ) | | $ | (0.91 | ) | | $ | (0.32 | ) | | $ | 0.11 | | | $ | 0.18 | | | $ | 0.21 | | | $ | 0.36 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Earnings per share, diluted | | $ | 0.29 | | | $ | (0.92 | ) | | $ | (0.91 | ) | | $ | (0.32 | ) | | $ | 0.11 | | | $ | 0.18 | | | $ | 0.21 | | | $ | 0.35 | |
SYNUTRA INTERNATIONAL, INC.
ADDITIONAL INFORMATION - FINANCIAL STATEMENT SCHEDULE I
Financial information of parent company
BALANCE SHEETS
(in thousands, except share par value)
| | | March 31, 2009 | | March 31, 2008 | |
| ASSETS | | | | | | | |
| Current Assets: | | | | | | | |
| Cash and cash equivalents | | | $ | 441 | | | $ | 2,174 | |
| Prepaid expenses and other current assets | | | | 1 | | | | 171 | |
| | | | | | | | | | |
| Total current assets | | | | 442 | | | | 2,345 | |
| Due from subsidiaries | | | | 39,540 | | | | 40,318 | |
| | | | | | |
| Investments in subsidiaries | | | | 73,098 | | | | 164,569 | |
| Other assets | | | | 4,153 | | | | 788 | |
| | | | | | | | | | |
| TOTAL ASSETS | | | $ | 117,233 | | | $ | 208,020 | |
| | | | | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | | |
| | | | | | |
| Current Liabilities: | | | | | | | | | |
| Short term loan | | | | 38,364 | | | | — | |
| Other current liabilities | | | | 1,166 | | | | 1,615 | |
| | | | | | | | | | |
| Total current liabilities | | | | 39,530 | | | | 1,615 | |
| Due to subsidiaries | | | | 307 | | | | 577 | |
| | | | | | |
| Long term loans | | | | — | | | | 34,184 | |
| | | | | | | | | | |
| Total liabilities | | | | 39,837 | | | | 36,376 | |
| | | | | | |
| Minority interest | | | | 537 | | | | 385 | |
| | | | | | |
| Shareholders’ equity: | | | | | | | | | |
| Common stock, $.0001 par value: 250,000 authorized; 54,001 and 54,001 issued and outstanding, respectively | | | | 5 | | | | 5 | |
| Additional paid-in capital | | | | 76,607 | | | | 76,607 | |
| Retained earnings (Accumulated deficit) | | | | (23,674 | ) | | | 76,875 | |
| Accumulated other comprehensive income | | | | 23,921 | | | | 17,772 | |
| | | | | | | | | | |
| Total shareholders’ equity | | | | 76,859 | | | | 171,259 | |
| | | | | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | | $ | 117,233 | | | $ | 208,020 | |
Financial Information of parent company
STATEMENTS OF INCOME
(in thousands except earnings per share data)
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Operating expenses: | | | | | | | | | |
| General and administrative expenses | | $ | 2,605 | | | $ | 3,073 | | | $ | — | |
| | | | | | | | | | | | | |
| Loss from operations | | | 2,605 | | | | 3,073 | | | | — | |
| Interest expense | | | 1,342 | | | | 4,466 | | | | — | |
| Interest income | | | (13 | ) | | | (1,064 | ) | | | (4 | ) |
| Other income, net | | | (1,000 | ) | | | — | | | | — | |
| Equity in earnings (losses) of subsidiaries | | | (97,615 | ) | | | 52,136 | | | | 19,870 | |
| | | | | | | | | | | | | |
| Net income (loss) attributable to shareholders | | $ | (100,549 | ) | | $ | 45,661 | | | $ | 19,874 | |
| | | | | | | | | | | | | |
| Earnings (loss) per share-basic | | $ | (1.86 | ) | | $ | 0.86 | | | $ | 0.40 | |
| | | | | | | | | | | | | |
| Earnings (loss) per share-diluted | | $ | (1.86 | ) | | $ | 0.85 | | | $ | 0.40 | |
| | | | | | | | | | | | | |
| Weighted average common share outstanding-basic | | | 54,001 | | | | 53,170 | | | | 50,001 | |
| Weighted average common share outstanding-diluted | | | 54,001 | | | | 53,476 | | | | 50,001 | |
Financial Information of parent company
STATEMENTS OF CASH FLOWS
(in thousands)
| | | Year Ended March 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Cash flow from operating activities: | | | | | | | | | | | | |
| Net income | | $ | (100,549 | ) | | $ | 45,661 | | | $ | 19,874 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
| Equity in (earnings) losses of subsidiaries | | | 97,615 | | | | (52,136 | ) | | | (19,870 | ) |
| Amortization of debt discount | | | — | | | | 2,733 | | | | — | |
| Amortization of debt issuance costs | | | 321 | | | | 539 | | | | — | |
| Changes in assets and liabilities: | | | | | | | | | | | | |
| Due from subsidiaries | | | 778 | | | | (40,318) | | | | — | |
| Prepaid expenses and other current assets | | | (1,855 | ) | | | (710) | | | | — | |
| Due to subsidiaries | | | 110 | | | | 172 | | | | 405 | |
| Other current liabilities | | | 3,730 | | | | 799 | | | | — | |
| Other assets | | | (1,658 | ) | | | (788) | | | | — | |
| | | | | | | | | | | | | |
| Net cash provided by (used in) operating activities | | | (1,508 | ) | | | (44,048) | | | | 409 | |
| | | | | | | | | | | | | |
| Cash flow from investing activities: | | | | | | | | | | | | |
| Capital injection at subsidiaries | | | (225 | ) | | | (54,981) | | | | — | |
| | | | | | | | | | | | | |
| Net cash used in investing activities | | | (225 | ) | | | (54,981) | | | | — | |
| | | | | | | | | | | | | |
| Cash flow from financing activities: | | | | | | | | | | | | |
| Proceeds from bridge loan | | | — | | | | 35,000 | | | | — | |
| Repayment of bridge loan | | | — | | | | (35,000) | | | | — | |
| Proceeds from long-term loan | | | — | | | | 35,000 | | | | — | |
| Proceeds from issuance of common stock, net of issuance costs | | | — | | | | 65,794 | | | | — | |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | — | | | | 100,794 | | | | — | |
| | | | | | | | | | | | | |
| Net change in cash and cash equivalents | | | (1,733 | ) | | | 1,765 | | | | 409 | |
| Cash and cash equivalents, beginning of year | | | 2,174 | | | | 409 | | | | — | |
| | | | | | | | | | | | | |
| Cash and cash equivalents, end of year | | $ | 441 | | | $ | 2,174 | | | $ | 409 | |
Note to Schedule I
The parent company financial statements have been prepared using the same accounting principles and policies described in the notes to the consolidated financial statements, with the only exception being that the company accounts for its subsidiaries using the equity method. Please refer to the notes to the consolidated financial statements presented above for additional information and disclosures with respect to these financial statements.
Schedule I has been provided pursuant to the requirements of Rule 12-04(a) and 5-04(c) of Regulation S-X, which require condensed financial information as to the financial position, changes in financial position and results of operations of a parent company as of the same dates and for the same periods for which audited consolidated financial statements have been presented when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year.
SYNUTRA INTERNATIONAL, INC.
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
(In thousands)
| | Balance at Beginning of Year | | | Charged to Costs and Expenses | | | Charged to Other Accounts | | | Balance at End of Year | |
| Allowance for accounts receivable | | | | | | | | | | | | |
| - 2009 | | $ | 185 | | | $ | 1,267 | | | $ | — | | | $ | 1,452 | |
| - 2008 | | $ | 241 | | | $ | (56 | ) | | $ | — | | | $ | 185 | |
| - 2007 | | $ | 417 | | | $ | — | | | $ | (176 | ) | | $ | 241 | |
| | | | | | | | | | | |
| Allowance for other receivables | | | | | | | | | | | | | | | | |
| - 2009 | | $ | 585 | | | $ | 887 | | | $ | — | | | $ | 1,472 | |
| - 2008 | | $ | 576 | | | $ | 9 | | | $ | | | | $ | 585 | |
| - 2007 | | $ | 3 | | | $ | 573 | | | $ | — | | | $ | 576 | |
| | | | | | | | | | | |
| Allowance for deferred tax assets | | | | | | | | | | | | | | | | |
| - 2009 | | $ | 2,135 | | | $ | 3,072 | | | $ | — | | | $ | 5,207 | |
| - 2008 | | $ | — | | | $ | 2,135 | | | $ | — | | | $ | 2,135 | |
| - 2007 | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
On July 27, 2007, we dismissed Rotenberg Co., or Rotenberg, our then independent registered public accounting firm, who was engaged to audit our financial statements. We dismissed Rotenberg because the growth and size of our operations required us to select an accountant with greater resources and a more global scale. The dismissal was effective immediately and was approved by the audit committee of our board of directors.
Rotenberg’s audit reports on our consolidated financial statements for the year ended March 31, 2007 did not contain any adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles.
In connection with the audits of our financial statements for the fiscal year ended March 31, 2007, there were no disagreements between Rotenberg and us on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure which, if not resolved to Rotenberg’s satisfaction, would have caused Rotenberg to make reference to the subject matter in their report on our consolidated financial statements for such years; and there were no reportable events, as listed in Item 304(a)(1)(v) of Regulation S-K.
On July 27, 2007, we engaged Deloitte Touche Tohmatsu CPA Ltd., or Deloitte, as our new independent registered public accounting firm. The engagement was approved by the audit committee of our board of directors. No relationship existed in any manner between Deloitte and us prior to the date we engaged Deloitte.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Management, including our Chief Executive Officer and Chief Financial Officer, does not expect that its disclosure controls and procedures or its internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Conclusion Regarding Effectiveness of Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act) as of the end of the period covered by this report. Based on such evaluation, the Company’s Chief Executive Officer and Chief Financial Officer have concluded that, as of March 31, 2009, the Company’s disclosure controls and procedures were effective to ensure that the information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is (a) recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and (b) accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). The Company’s internal control system was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of its consolidated financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of March 31, 2009. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control — Integrated Framework. The Company’s management concluded that, as of March 31, 2009, the Company’s internal control over financial reporting was effective based on these criteria.
Our independent registered public accounting firm, Deloitte Touche Tohmatsu CPA Ltd., has audited the effectiveness of our internal control over financial reporting as of March 31, 2009, as stated in its report, which appears on page 109 of this annual report on Form 10-K.
Remediation and Changes in Internal Control over Financial Reporting
As previously reported in the Company’s Annual Report on Form 10-K for the year ended March 31, 2008, management had concluded that as of March 31, 2008, the Company’s internal control over financial reporting was not effective. Management’s conclusion that the Company’s internal control over financial reporting was not effective was based on management’s identification of one material weakness and four deficiencies in the Company’s internal control over financial reporting, as defined in Auditing Standard No. 5, “An Audit of Internal Control Over Financial Reporting That Is Integrated with An Audit of Financial Statements.”
Our material weakness related to:
| | • | | (i) an insufficient complement of personnel in our corporate accounting and financial reporting function with an appropriate level of technical accounting knowledge, experience, and training in the application of generally accepted accounting principles commensurate with the Company’s complex financial accounting and reporting requirements and materiality thresholds; and (ii) an inadequacy of competent staff in our internal audit function to effectively monitor the Company’s operational, financial and compliance-oriented controls, including controls over the financial closing and reporting process. |
Our deficiencies related to:
| | • | | inadequate training of employees, especially new hires, on our antifraud programs and code of ethics; |
| | • | | inadequate segregation of duties related to our stock count process and lack of controls to regularly monitor, document and determine the value of certain inventories on a timely basis; |
| | • | | inadequate controls related to managing staff cash advances; and |
| | • | | failure to maintain an appropriate level of security within our computer operating systems and applications. |
The Company discussed the material weakness and deficiencies in its internal control over financial reporting with the Audit Committee of the Board of Directors. The following specific remedial actions were initiated and completed during the Company’s most recent fiscal year to address the material weakness in our internal control over financial reporting:
| | • | | Reorganize and restructure the Company’s corporate accounting staff (“Corporate Accounting”) by (1) revising the reporting structure and establishing clear roles, responsibilities, and accountability, (2) hiring additional technical accounting personnel to address the Company’s complex accounting and financial reporting requirements; (3) assessing the technical accounting capabilities at our subsidiaries to ensure the right complement of knowledge, skills, and training; (4) hiring an experienced internal audit manager to lead the Company’s independent internal audit function; (5) conducting formal training sessions for accounting personnel to improve their familiarity with U.S. generally accepted accounting principles including any recent accounting pronouncements; (6) restaffing the internal audit personnel to improve the efficiency of the internal audit function; and (7) having our internal audit play a more active role in our internal control by conducting more audit planning, tests and implementing remediation steps at our subsidiaries. |
| | • | | Improve period-end closing procedures by (1) ensuring that account reconciliations and analyses for significant financial statement accounts are reviewed for completeness and accuracy by qualified accounting personnel, (2) implementing a process that ensures the timely review and approval of complex accounting estimates by qualified accounting personnel and subject matter experts, where appropriate, and (3) developing better monitoring controls at Corporate Accounting and at our subsidiaries and (4) documenting and implementing antifraud programs and controls as well as comprehensive risk assessment of procedures, programs and controls. |
As a result of the remediation steps above, which were completed in the fourth quarter of the fiscal year ended March 31, 2009, management believes that the material weakness has been remediated.
The Company has also taken the following specific remedial actions to address the deficiencies in our internal control over financial reporting:
| | • | | Providing training programs for new hires and continuing training programs for current employees on our antifraud programs, corporate governance guidelines and code of ethics, including enhancing policies and procedures for detecting and preventing fraud; |
| | • | | Reorganizing the stock count process by including staff from different departments to ensure adequate segregation of duties; |
| | • | | Implementing procedures for monitoring staff cash advances including introducing tighter controls which include regular checks and alerts to manage staff cash advances; and |
| | • | | Increasing the level of security within our computer operating system and applications through keeping a record of audit logs and performing regular review of such audit logs and implementing adequate password controls. |
As a result of these remedial actions, management believes that our deficiencies identified above have been remediated. However, as of March 31, 2009, management concluded that we still have one significant deficiency and four deficiencies as set forth below:
Our significant deficiency related to:
| | · | an inadequate control over cutoff of certain marketing expenses, primarily in the category of slotting fees, which could result in a material impact on our financial statements. |
Our deficiencies related to:
| | · | an incomplete stock count of inventory at certain warehouses; |
| | · | a malfunction of our anti-fraud hotline which may lead to ineffective monitoring over fraud risk; |
| | · | recording of investments outside of the months in which they occurred; and |
| | · | an inappropriate delegation of authority in the financial reporting process of certain subsidiaries. |
The Company has discussed the significant deficiency and deficiencies identified above with the Audit Committee, and is in the process of developing and implementing remediation plans to address these deficiencies. These plans which are currently being implemented include improving the cutoff procedures for marketing expenses despite our largely dispersed sales network, implementing stricter controls to ensure complete coverage of the inventory stock-taking process, clarifying the instructions on how to use our anti-fraud hotline, implementing controls to ensure that all investments are accurately calculated and recorded in the appropriate months, and revisiting certain job allocations to ensure proper segregation of duties.
Other than as described above, management does not believe that there have been any other changes in the Company’s internal control over financial reporting during the Company’s most recent fiscal quarter, which have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Synutra International Inc.
Rockville, Maryland
We have audited Synutra International Inc. and subsidiaries (the “Company’s”) internal control over financial reporting as of March 31, 2009, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on that risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2009, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements and financial statement schedules as of and for the year ended March 31, 2009, of the Company and our report dated June 15, 2009 expressed an unqualified opinion on those financial statements and financial statement schedules and included explanatory paragraphs regarding the Company’s going concern and the adoption of FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes – an Interpretation of FASB Statement No. 109”, effective April 1, 2007.
/s/ Deloitte Touche Tohmatsu CPA Ltd.
Shanghai, China
June 15, 2009
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
EXECUTIVE OFFICERS, DIRECTORS AND SIGNIFICANT EMPLOYEES
The following sets forth information about our directors and executive officers as of March 31, 2009:
| Name | | Age | | Position |
| Directors and Executive Officers | | | | |
| Liang Zhang | | 49 | | Chairman of the Board of Directors and Chief Executive Officer |
| Jinrong Chen | | 49 | | Director(1)(2)(3) |
| Yiu-Chun Chan | | 56 | | Director(1)(2)(3) |
| Lei Lin | | 41 | | Director(1)(2)(3) |
| William W. Wu | | 47 | | Director and President—Marketing and Sales |
| Weiguo Zhang | | 51 | | President and Chief Operating Officer |
| Lawrence Lee | | 44 | | Chief Financial Officer |
| Xisen Mu | | 51 | | President—Production |
| (1) | Member of Audit Committee. |
| (2) | Member of Compensation Committee. |
| (3) | Member of Nominating Committee. |
Liang Zhang. Liang Zhang is our founder and has served as the chairman of our board of directors and chief executive officer since we became a public company in 2005. Mr. Zhang has worked in the food ingredients industry since the 1980s and founded his first entrepreneurial venture, Honnete, in the early 1990s. Honnete has since become the dominant supplier of whey protein products in China. Mr. Zhang has been recognized as a business leader in the dairy industry in China, serving as vice-chairman of the China Dairy Industry Association. Mr. Zhang received a bachelor’s degree in French language and literature from Nanjing International Relations Institute of China.
Jinrong Chen. Jinrong Chen has served as a director of our board of directors since June 27, 2006. Ms. Chen has served as associate professor at the School of Economics and Management of Tsinghua University in Beijing since 2001, specializing in corporate finance management, securities analysis, financial operations, corporate governance and controls. In addition to her academic career with top business schools in China, Ms. Chen also advises public companies and private businesses in China. Ms. Chen received her bachelor’s degree in accounting from Beijing Institute of Electronics & Information and her MBA degree from Renmin University of China.
Yiu-Chun Chan. Yiu-Chun Chan has served as a director of our board of directors since December 3, 2006. Mr. Chan has over 30 years of experience in marketing agricultural and food products in the Greater China area, and is the chief executive officer and founder of P R Consultants Limited. Previously, he served as an Executive Director of Lintas Hong Kong Limited and a partner of Times Direct Marketing Asia—the largest privately held direct marketing company in South East Asia. Mr. Chan began his career marketing agricultural products at Sunkist Growers, where he was responsible for the advertising and promotion activities in the Hong Kong market. Mr. Chan received his Diploma in communications from Hong Kong Baptist University.
Lei Lin. Lei Lin has served as a director of our board of directors since October 1, 2007. Mr. Lin is president and co-chief executive officer of Sinotrust, a leading consulting company in China which Mr. Lin founded in 1992. Mr. Lin received his bachelor’s degree in applied economic mathematics from Renmin University of China.
Weiguo Zhang. Weiguo Zhang has been our chief operating officer and president since 2005 and is primarily responsible for our financial market operations, including investor relations, corporate development, and international strategic development. Mr. Zhang first joined us as president of Synutra Illinois in 2001 to oversee our U.S. operations, including information support in research and technologies and business development. Prior to joining us, Mr. Zhang was the managing director of Bambridge International, Ltd., which he founded in 1995. Mr. Zhang received a bachelor’s degree in English language and literature from the Nanjing International Relations Institute and a master’s degree in international economics and American foreign policy from the School of Advanced International Studies from John Hopkins University.
Lawrence Lee. Lawrence Lee has served as our chief financial officer since October 1, 2007. Mr. Lee also served as a director of our board of directors from December 3, 2006 to September 30, 2007. From August 1, 2004 to September 30, 2007, Mr. Lee was vice president and chief financial officer of Kasen International Holdings Limited, a public company listed on the Hong Kong Stock Exchange. Prior to that, Mr. Lee served as chief financial officer at Eagle Brand Holdings Limited, a company listed on the Singapore Stock Exchange. Mr. Lee’s experience also includes serving as a financial controller at the Korean division of Exel Plc, and serving as a senior auditor at Waste Management Inc.’s international department in London. Mr. Lee is an associate member of the Association of Chartered Certified Accountants. Mr. Lee received a bachelor’s degree in management and engineering from Beijing Institute of Technology, a master’s degree in economics from Renmin University of China, and a master’s degree in accounting and finance from the London School of Economics.
William W. Wu. William W. Wu has served as our president of marketing and sales since July 2007 and is currently a member of our board of directors. Mr. Wu served as our vice president of marketing and sales since December 2005. Before joining us, Mr. Wu worked as a member of the senior management on sales, marketing and market research with Bristol-Myers Squibb (China) since 2001. Prior to that, Mr. Wu also worked in the marketing departments of Bristol Myers Squibb, Merck-Medco, a pharmacy benefit manager affiliated with Merck, later spun off from Merck as an independent company, and other sales and marketing organizations in the United States. Mr. Wu received a master’s degree in American studies from Beijing Foreign Studies University and his Ph.D. in sociology from the University of North Carolina at Chapel Hill.
Xisen Mu. Xisen Mu has served as our president of production since January 2007. Before joining us, Mr. Mu worked as general manager of Heilongjiang Dairy Group since 2001. Prior to that, Mr. Mu held senior positions with other major dairy companies in Heilongjiang province. He has more than 20 years of experience in the dairy industry in China. Mr. Mu received a diploma in management from Qiqihar Institute of Light Industry.
Our directors have a term of office expiring at the next annual general meeting, unless re-elected or earlier vacated in accordance with our bylaws. Our officers are appointed by and serve at the discretion of the board of directors. All officers have a term of office lasting until their removal or replacement by our board of directors. There are no family relationships among our directors or officers.
Board of Directors
Our board of directors currently has five directors, consisting of three independent directors, all of whom are independent as defined by the applicable listing requirements of the NASDAQ Global Select Market. All directors are elected to hold office until our next annual meeting of stockholders and until their successors have been elected or until their earlier resignation or removal.
On June 11, 2008, our board of directors and stockholders approved our amended and restated certificate of incorporation, which became effective on October 17, 2008. Our amended and restated certificate of incorporation provides that our board of directors shall be divided into three classes of directors. The three classes, which are required to be as nearly equal in number as possible, will be designated class I, class II and class III, and serve staggered three-year terms and hold office until their term of office expires or until such time as they are removed from office by resolution of our stockholders. To effect such staggered terms, our amended and restated bylaws provides that the directors first elected to class I shall serve for a term ending on the annual meeting date following the end of 2008, the directors first elected to class II shall serve for a term ending on the second annual meeting date following the end of 2008, and the directors first elected to class III shall serve for a term ending on the third annual meeting date following the end of 2008. Our board of directors has designated Liang Zhang and William Wu as class I directors, Jinrong Chen and Yiu-Chun Chan as class II directors and Lei Lin as a class III director. At the stockholder’s annual meeting on March 12, 2009, Liang Zhang and William Wu were re-elected as class I directors, as discussed in “Part II - Item 4. Submission of Matters to a Vote of Security Holders”.
Board Committees
Our board has established the committees described below and may establish others from time to time.
Audit Committee
Our audit committee consists of Jinrong Chen, Lei Lin and Yiu-Chun Chan, all of whom are independent as defined by the applicable listing requirements of the NASDAQ Global Select Market. Ms. Chen is the chairperson of our audit committee and serves as the financial expert of the committee. The audit committee oversees our accounting and financial reporting processes and the audits of our financial statements. The audit committee is responsible for, among other things:
| | • | | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures; |
| | • | | coordinating the oversight and reviewing the adequacy of our internal control over financial reporting with both management and the independent registered public accounting firm; |
| | • | | establishing policies and procedures for the receipt and retention of accounting related complaints and concerns, including a confidential, anonymous mechanism for the submission of concerns by employees; |
| | • | | periodically reviewing legal compliance matters, including securities trading policies, periodically reviewing significant accounting and other risks or exposures to our company, reviewing and, if appropriate, approving all transactions between our company or its subsidiaries and any related party (as described in Item 404 of Regulation S-K) and periodically reviewing business expenses of our chief executive officer; and |
| | • | | establishing policies for the hiring of employees and former employees of the independent registered public accounting firm. |
Compensation Committee
Our compensation committee consists of Jinrong Chen, Yiu-Chun Chan, and Lei Lin. Ms. Chen is the chairperson of our compensation committee. The purpose of our compensation committee is to discharge the responsibilities of our board of directors relating to compensation of our executive officers. Specific responsibilities of our compensation committee include:
| | • | | reviewing and recommending approval of compensation of our executive officers; |
| | • | | administering our stock incentive and employee stock purchase plans; and |
| | • | | reviewing and making recommendations to our board with respect to incentive compensation and equity plans. |
Nominating Committee
Our nominating committee consists of Yiu-Chun Chan, Jinrong Chen, and Lei Lin. Mr. Chan is the chairman of our nominating committee. The purpose of our nominating committee is to be primarily responsible for identifying individuals qualified to serve as members of the board of directors and recommending to the board the persons to be nominated by the board as nominees for director at each annual meeting of our stockholders. Specific responsibilities of our nominating committee include:
| | • | | developing and recommending to the board of directors criteria for board of directors and committee membership; |
| | • | | identifying individuals qualified to become board of directors members; |
| | • | | recommending to the board of directors the persons to be nominated for election as directors and to each of the board of directors’ committees; |
| | • | | developing and recommending to the board of directors a code of ethical conduct and a set of corporate governance policies and practices; and |
| | • | | monitoring and evaluating the performance of the board of directors and leading the board in an annual self-assessment of its practices and effectiveness. |
Section 16(a) Beneficial Ownership Reporting Requirements
Section 16(a) of the Securities Exchange Act of 1934 requires our executive officers, directors and persons who own beneficially more than ten percent of a registered class of our equity securities to file reports of ownership and changes in ownership with the Securities and Exchange Commission and the NASDAQ Global Select Market. The Securities and Exchange Commission has established specific due dates for these reports, and we must disclose in this 10-K any late filings during the fiscal year ended March 31, 2009. These persons are required by regulation of the SEC to furnish copies of all Section 16(a) forms to the Company. Based solely upon our review of the copies of such forms received by the Company, we believe that during the year ended March 31, 2009 our officers, directors and greater than ten percent beneficial owners complied with the Section 16(a) filing requirements.
Code of Ethics
The Company has a code of ethics that applies to all of the Company’s employees, including its principal executive officer, principal financial officer and principal accounting officer, and the Board of Directors. A copy of this code is available on the Company's website at www.synutra.com. The Company intends to disclose any changes in or waivers from its code of ethics by posting such information on its website or by filing a Form 8-K.
| ITEM 11. | EXECUTIVE COMPENSATION |
COMPENSATION DISCUSSION AND ANALYSIS
This section describes the material elements of compensation earned by our executive officers during the fiscal year ended March 31, 2009. Our executive compensation programs are determined and approved by the compensation committee of our board of directors.
Liang Zhang and Lawrence Lee, who serve as our chief executive officer and our chief financial officer, respectively, and William W. Wu were our “named executive officers” for the fiscal year ended March 31, 2009, and the compensation for these officers is reported in the “Summary Compensation Table” below. No other executive officer received compensation in excess of $100,000 for the fiscal year ended March 31, 2009. Unless otherwise noted, the amounts reported in this Form 10-K have been converted from Renminbi to U.S. dollars based on the conversion rate as of March 31, 2009 of RMB6.8359 to $1.00.
Overview of Executive Compensation Program
The compensation committee has responsibility for establishing, implementing and monitoring our executive compensation program philosophy and practices. The compensation committee seeks to ensure that the total compensation paid to our executive officers is fair, reasonable and competitive.
Compensation Philosophy and Objectives
The compensation committee believes that an effective executive compensation program should provide base annual compensation that is reasonable in relation to the individual executive’s job responsibilities and reward the achievement of both annual and long-term strategic goals of our company. Because of the size of our company, the small number of executive officers in our company, and our company’s financial priorities, the compensation committee has decided not to implement or offer any retirement plans, deferred compensation plans or other similar plans for our executive officers. Accordingly, for the fiscal year ended March 31, 2009, the components of our executive compensation program consisted of cash salary only. On June 11, 2008, the Company adopted the 2008 Stock Incentive Plan (the “Plan”). The Plan provides the Company with the ability to grant stock-based awards to all employees, officers and directors. Although the compensation committee currently has not granted any stock-based awards, the compensation committee will consider using equity incentive grants in the future.
The compensation committee also takes into account in making its decisions the executive compensation programs of other manufacturing and marketing companies operating in China. The compensation committee may in future years reassess the levels of equity and cash compensation offered to our executives in light of our profitability and other performance factors.
Role of Executive Officers in Compensation Decisions
Our compensation committee and chief executive officer annually review the performance of each executive officer (other than the chief executive officer, whose performance is reviewed only by the compensation committee). The compensation committee makes all decisions with respect to compensation for the chief executive officer and will approve grants of equity awards to all of our executive officers. Decisions regarding the non-equity compensation of our executive officers other than our chief executive officer are made by our chief executive officer.
Setting Executive Compensation
In making its compensation decisions, our compensation committee does not retain outside compensation consultants. Instead, the compensation committee reviews compensation data for executives of other listed companies located in China. The compensation committee utilizes this data to set compensation for our executive officers at levels targeted at or around the average of the compensation amounts provided to similarly situated executives at comparable local companies considering, for each executive, their individual experience level and the responsibilities of their position with us. There is no pre-established policy or target for the allocation between cash and non-cash incentive compensation.
Employment Agreements
We have entered into standard employment agreements with each of our executive officers, including the named executive officers. The terms and conditions of these employment agreements are determined via negotiations between the employee parties and us, and the framework and structure of the agreements are intended to comply with applicable PRC labor and employment laws.
Liang Zhang’s employment agreement was automatically extended and has an indefinite term and provides for an annual base salary of approximately $175,544. William W. Wu’s employment agreement has a two-year term that ends on June 30, 2009, which will be automatically extended for another two years upon expiration, and provides for an annual base salary of approximately $122,881. Lawrence Lee’s employment agreement was automatically extended for another two years for a term that ends on December 31, 2010 and provides for an annual base salary of approximately $110,593.
2009 Executive Compensation Components
For the fiscal year ended March 31, 2009, the principal component of compensation for our executive officers was their base salary. We provide executive officers with a base salary to compensate them for services rendered during the fiscal year. Base salary ranges for the executive officers are determined for each executive based on his or her position and responsibility.
During its review of base salaries for executives, the compensation committee primarily considers:
| | • | | the negotiated terms of each executive’s employment agreement; |
| | • | | internal review of the executive’s compensation, both individually and relative to other executive officers; and |
| | • | | individual performance of the executive. |
Salary levels are typically considered annually as part of our performance review process, as well as upon a change in job responsibility. Merit-based increases to salaries are based on the compensation committee’s assessment of the individual’s performance.
Our executive officers did not receive any annual bonus or equity-based compensation for the fiscal year ended March 31, 2009.
Summary Compensation Table — Fiscal Years Ended March 31, 2009, 2008 and 2007
The following table presents information regarding compensation of our named executive officers for services rendered during the fiscal years ended March 31, 2009, 2008 and 2007. The amounts reported in this table have been converted from Renminbi to U.S. dollars based on the March 31, 2009 conversion rate of RMB 6.8359 to $1.00.
Name and Principal Position | | Year ended March, 31 | | Salary ($) | | Bonus ($) | | Stock Awards ($) | | Option Awards ($) | | Non-Equity Incentive Plan Compensation ($) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ($) | | Total ($) | |
| (a) | | (b) | | (c) | | (d) | | (e) | | (f) | | (g) | | (h) | | (i) | | (j) | |
Liang Zhang(1) Chairman of the Board and Chief Executive Officer | | | 2009 2008 2007 | | 175,544 170,964 32,334 | | | — — — | | — — — | | | — — — | | — — — | | | — — — | | — — — | | | 175,544 170,964 32,334 | |
Lawrence Lee(2) Chief Financial Officer | | | 2009 2008 2007 | | 110,593 56,988 — | | | — — — | | — — — | | | — — — | | — — — | | | — — — | | — 10,407 — | | | 110,593 67,395 — | |
William W. Wu President — Marketing and Sales | | | 2009 2008 2007 | | 122,881 119,675 119,675 | | | — — — | | — — — | | | — — — | | — — — | | | — — — | | — — — | | | 122,881 119,675 119,675 | |
| (1) | Because of his significant equity interest in us, Liang Zhang elected not to receive any form of compensation, monetary or otherwise, prior to January 2007. The amount for 2007 reflects the base salary paid to Liang Zhang for the last three months of the fiscal year ended March 31, 2007. |
| (2) | As noted above, Lawrence Lee was appointed our chief financial officer effective October 1, 2007. The amounts reported in column (i) reflect retainer fees for his service on our board of directors prior to his appointment as our chief financial officer. |
Compensation Committee Report
The Compensation Committee has reviewed and discussed with management the “Compensation Discussion and Analysis” required by Item 402(b) of Regulation S-K and, based on such review and discussions, has recommended to our board of directors that the foregoing “Compensation Discussion and Analysis” be included in this Annual Report.
Compensation Committee of the Board of Directors
Jinrong Chen (Chairperson)
Yiu-Chun Chan
Lei Lin
Plan-Based Awards — Fiscal Year Ended March 31, 2009
None of our named executive officers received any grants of options or other stock-based awards during the fiscal year ended March 31, 2009. Additionally, none of our named executive officers held any outstanding options or other stock-based awards as of the last day of the fiscal year ended March 31, 2009, nor did any of our named executive officers exercise any options or hold any other stock awards that vested during the fiscal year ended March 31, 2009.
Potential Payments upon Termination of Employment or Change of Control
We have not entered into any arrangements with our executive officers to provide severance or change of control benefits. Upon a termination of employment by us, an employee is generally entitled under PRC labor law to one month’s severance pay for each full year he or she has been employed with us (with a minimum of one month’s severance pay and a maximum of 12 months’ severance pay). We have not made such severance provisions for our executive officers as the amount is not material.
Stock Incentive Plan
We have adopted the 2008 Stock Incentive Plan to provide an additional means to attract, motivate, retain and reward selected employees and other eligible persons. Employees, officers, directors, and consultants that provide services to us or one of our subsidiaries may be selected to receive awards under the Plan.
Our board of directors, or a committee of directors appointed by the board, will have the authority to administer the Plan. The administrator of the plan will have broad authority to:
| | • | | select participants and determine the types of awards that they are to receive; |
| | • | | determine the number of shares that are to be subject to awards and the terms and conditions of awards, including the price (if any) to be paid for the shares or the award; |
| | • | | cancel, modify or waive our rights with respect to, or modify, discontinue, suspend or terminate any or all outstanding awards, subject to any required consents; |
| | • | | construe and interpret the terms of the Plan; |
| | • | | accelerate or extend the vesting or exercisability or extend the term of any or all outstanding awards; |
| | • | | subject to the other provisions of the Plan, make certain adjustments to an outstanding award and authorize the conversion, succession or substitution of an award; and |
| | • | | allow the purchase price of an award or shares of our common stock to be paid in the form of cash, check or electronic funds transfer, by the delivery of already-owned shares of our common stock or by a reduction of the number of shares deliverable pursuant to the award, by services rendered by the recipient of the award, by notice and third-party payment or cashless exercise on such terms as the administrator may authorize, or any other form permitted by law. |
A total of 12,500,000 shares of our common stock have been authorized for issuance with respect to awards granted under the Plan. Any shares subject to awards that are not paid or exercised before they expire or are terminated, as well as shares used to pay the purchase or exercise price of awards or related tax withholding obligations, will become available for other award grants under the Plan. As of the date of this filing, no awards have been granted under the Plan, and the full number of shares authorized under the Plan will be available for award purposes.
Awards under the Plan may be in the form of incentive or nonqualified stock options, stock appreciation rights, restricted stock, stock bonuses and other forms of awards granted or denominated in our common stock or units of our common stock. Awards under the plan generally will not be transferable other than by will or the laws of descent and distribution, except that the plan administrator may authorize certain transfers for tax or estate planning purposes.
Nonqualified stock options and other awards may be granted at prices below the fair market value of the common stock on the date of grant. Restricted stock awards can be issued for nominal or the minimum lawful consideration. Incentive stock options must have an exercise price that is at least equal to the fair market value of our common stock, or 110% of fair market value of our common stock for incentive stock option grants to any 10% owner of our common stock, on the date of grant. These and other awards may also be issued solely or in part for services. Awards are generally paid in cash or shares of our common stock. Subject to applicable tax law, the plan administrator may provide for the deferred payment of awards and may determine the terms applicable to deferrals.
As is customary in incentive plans of this nature, the number and kind of shares that will be available under the Plan and any outstanding awards, as well as the exercise or purchase prices of awards, will be subject to adjustment in the event of certain reorganizations, mergers, combinations, recapitalizations, stock splits, stock dividends or other similar events that change the number or kind of shares outstanding, and extraordinary dividends or distributions of property to the stockholders. In no case (except due to an adjustment referred to above or any repricing that may be approved by our stockholders) will any adjustment be made to a stock option or stock appreciation right award under the Plan (by amendment, cancellation and regrant, exchange or other means) that would constitute a repricing of the per-share exercise or base price of the award.
Upon the occurrence of any of the following:
| | • | | any merger, combination, consolidation, or other reorganization; |
| | • | | any exchange of our common stock or other securities; |
| | • | | a sale of all or substantially all of our business, stock or assets; |
| | • | | any other event in which we do not survive (or do not survive as a public company in respect of our common stock); |
Outstanding awards under the Plan will generally become fully vested and will terminate upon the transaction, unless the awards are assumed by the successor entity or otherwise continue in the circumstances.
Our board of directors may amend or terminate the Plan at any time, but no such action will affect any outstanding award in any manner materially adverse to a participant without the consent of the participant. The Plan and any Plan amendments will be submitted to stockholders for their approval as required by applicable law or any applicable listing agency. The Plan is not exclusive — our board of directors and compensation committee may grant stock and performance incentives or other compensation, in stock or cash, under other plans or authority.
The plan will terminate on June 10, 2018. However, the plan administrator will retain its authority until all outstanding awards are exercised or terminated. The maximum term of options, stock appreciation rights and other rights to acquire common stock under the plan is ten years after the initial date of the award.
Director Compensation
The following table presents information regarding the compensation for the fiscal year ended March 31, 2009 to members of our board of directors who were not also employed by us (referred to as our “non-employee directors”) during the fiscal year. The compensation paid to Liang Zhang, Lawrence Lee and William W. Wu, each of whom is employed by us, is presented below in the table titled “Summary Compensation Table—Fiscal Years Ended March 31, 2009, 2008 and 2007” and the related explanatory notes.
| Name | | Fees Earned or Paid in Cash ($) | | | Stock Awards ($)(1) | | | Option Awards ($)(1) | | | Non-Equity Incentive Plan Compensation ($) | | | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | | | All Other Compensation ($) | | | Total ($) | |
| Jinrong Chen | | | 40,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 40,000 | |
| Yiu-Chun Chan | | | 40,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 40,000 | |
| Lei Lin | | | 40,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 40,000 | |
| (1) | None of our non-employee directors held any outstanding options or other stock-based awards as of the last day of the fiscal year ended March 31, 2009. |
Non-Employee Director Compensation
Currently, each of our non-employee directors is entitled to receive an annual cash retainer of $40,000 for his or her services as a director. In addition, our non-employee directors are reimbursed for travel, lodging and other reasonable out-of-pocket expenses incurred in attending meetings of the board and board committees. Our non-employee directors do not receive any equity-based awards or other compensation for their service as directors.
Compensation Committee Interlocks and Insider Participation
Jinrong Chen, Yiu-Chun Chan and Lei Lin each served on the compensation committee during the fiscal year ended March 31, 2009. None of these directors is or was an executive officer of our company or had any relationships requiring disclosure by us under the SEC’s rules requiring disclosure of certain relationships and related-party transactions. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, the executive officers of which served as a director or member of our compensation committee during the fiscal year ended March 31, 2009.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Security Ownership of Certain Beneficial Owners
The following table sets forth information, as of June 12, 2009, regarding the beneficial ownership of our common stock by:
| • | each person known by us to be a beneficial owner of more than five percent of our outstanding common stock; |
| • | each of our directors and director nominees; |
| • | each of our named executive officers; and |
| • | all directors and executive officers as a group. |
The amounts and percentage of common stock beneficially owned are reported on the basis of regulations of the SEC governing the determination of beneficial ownership of securities. Under the rules of the SEC, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or “investment power,” which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days after June 12, 2009. Under these rules, more than one person may be deemed a beneficial owner of the same securities and a person may be deemed a beneficial owner of securities as to which he has no economic interest. Except as indicated by footnote, the persons named in the table below have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them.
Percentage of class is based on 54,000,713 shares of common stock outstanding as of June 12, 2009. Unless otherwise noted below, the address of the persons listed on the table is 2275 Research Blvd., Suite 500, Rockville, Maryland 20850.
| | | Shares Beneficially Owned Prior to This Offering | |
| Name | | Number | | | Percent | |
| OFFICERS AND DIRECTORS | | | | | | |
Liang Zhang, Director and Chief Executive Officer(1) | | | 36,000,000 | | | | 66.67 | % |
| Lawrence Lee, Chief Financial Officer | | | — | | | | — | |
| Weiguo Zhang, President and Chief Operating Officer | | | 2,000 | | | | * | |
| William W. Wu, Director and President of Marketing and Sales | | | — | | | | — | |
| Jinrong Chen, Director | | | — | | | | — | |
| Yiu—Chun Chan, Director | | | — | | | | — | |
| Lei Lin, Director | | | — | | | | — | |
| Xisen Mu, President of Production | | | — | | | | — | |
| All Officers and Directors as a Group | | | 36,002,000 | | | | 66.67 | % |
| | | | | | | | | |
| PRINCIPAL STOCKHOLDER | | | | | | | | |
Warburg Pincus Private Equity IX, L.P.(2) | | | 5,000,000 | | | | 9.26 | % |
| (1) | This amount includes 36,000,000 shares owned by Beams Power Investment Limited, or Beams, a British Virgin Islands company. In addition Beams had issued a senior convertible note dated April 23, 2008, to the Warburg Pincus Entities, as defined below, convertible into up to 1,000,000 shares of common stock held by Beams. Liang Zhang has dispositive and voting power over investments by Beams. Liang Zhang’s wife, Xiuqing Meng, is the sole shareholder and director of Beams. Of the shares held by Beams, 5,967,000 shares were pledged to Warburg (as defined below) pursuant to a share pledge agreement, dated April 23, 2008 and an additional 8,000,000 shares were pledged to Warburg on December 4, 2008, see “Item 13. Certain Relationships and Related Transactions, and Director Independence.” |
| (2) | According to a Schedule 13D/A filed by Warburg Pincus Private Equity IX, L.P., or Warburg, with the SEC on September 1, 2008, Warburg Pincus IX LLC, or WP IX LLC, is the sole general partner of Warburg. Warburg Pincus Partners LLC, or WP Partners, is the sole managing member of WP IX LLC, and Warburg Pincus & Co., or WP, is the sole managing member of WP Partners. Charles R. Kaye and Joseph P. Landy are general partners of WP and managing members and co-presidents of Warburg Pincus LLC, or WP LLC, which manages Warburg. WP, WP Partners, WP IX LLC, Warburg and WP LLC are collectively referred to as the “Warburg Pincus Entities.” Messrs. Kaye and Landy may be deemed to indirectly beneficially own the shares held by Warburg because of their affiliation with the Warburg Pincus Entities. Messrs. Kaye and Landy disclaim beneficial ownership of the shares held by Warburg except to the extent of their pecuniary interest therein. The address of the Warburg Pincus Entities is 466 Lexington Avenue, New York, New York, 10017. This amount includes 4,000,000 shares of common stock held directly by the Warburg Pincus Entities and up to 1,000,000 shares of common stock issuable to the Warburg Pincus Entities pursuant to the Senior Exchangeable Note, dated April 23, 2008, issued by Beams Power Investment Limited. |
Securities Authorized for Issuance Under Equity Compensation Plans
We currently have a 2008 Stock Incentive Plan which has been approved by our shareholders. The plan will terminate on June 10, 2018. To date, we have not issued any awards under the Plan.
The following table sets forth the number of common shares remaining available for future issuance under the plan as of March 31, 2009.
| | | | | | | | | Number of Common Shares | |
| | | | | | | | | Remaining Available for | |
| | | | | | | | | Future Issuance Under | |
| | | Number of Common | | | | | | Equity | |
| | | Shares to be Issued | | | Weighted-Average | | | Compensation Plans | |
| | | Upon Exercise of | | | Exercise Price of | | | (Excluding Shares | |
| | | Outstanding Options, | | | Outstanding Options, | | | Reflected in | |
| Plan Category | | Warrants and Rights | | | Warrants and Rights | | | the First Column) | |
| | | | | | | | | | |
| Equity compensation plans approved by shareholders | | | — | | | | — | | | | 12,500,000 | |
| Equity compensation plans not approved by shareholders | | | — | | | | — | | | | — | |
| Total | | | — | | | | — | | | | 12,500,000 | |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Transactions with Affiliate Companies controlled by Liang Zhang
Our chairman, chief executive officer and principal stockholder, Liang Zhang, controls several other companies in China. Among these companies, we sell butter and skimmed milk powder to Beijing Kelqin Dairy Co., Ltd. and Honnete, formulation ingredients to Beijing Ao Naier Feed Stuff LLC, our name brand products to St. Angel (Beijing Business Service) and engage the services of Beijing Luding Xueyuan for direct sales, catalogue sales, and regional retail outlets distribution. We also purchased whey protein powder from Honnete and Beijing Kelqin Dairy Co., Ltd., and catalogues, brochures, and marketing materials from Beijing Sheng Long Media Co., Ltd. In January 2008, Liang Zhang sold all his interest in Beijing Ao Naier Feed Stuff LLC, and Beijing Luding Xueyuan.
Honnete imports whey protein from Eurosérum. We purchased substantially all of our whey protein powder from Honnete until December 2007, when we started to purchase substantially all of our whey protein powder directly from Eurosérum. For the fiscal year ended March 31, 2009, we purchased $10.4 million of whey protein powder from Honnete.
The following table sets forth the value of our sales to our affiliated companies during the period indicated.
| | | Year Ended March 31, 2009 | |
| | | (in thousands) | |
| Beijing Honnete Dairy Co., Ltd. | | $ | 2,255 | |
| Total | | $ | 2,255 | |
The following table sets forth the value of our purchases from our affiliated companies during the period indicated.
| | | Year ended March 31, 2009 | |
| | | (in thousands) | |
| Beijing Honnete Dairy Co., Ltd. | | $ | 10,376 | |
| St. Angel (Beijing Business Service) | | | 1,639 | |
| Beijing Sheng Long Media Co., Ltd. | | | 154 | |
| Total | | $ | 12,169 | |
In June 2008, we sold two commercial buildings, one of which was classified as assets held for sale as of March 31, 2008, to Sheng Zhi Da Dairy Group Corporation, an entity 100% controlled by our CEO, at the carrying value of $1.7 million. The amount represents the balance due from Sheng Zhi Da Dairy Group Corporation as of March 31, 2009.
We also had short term loans from Honnete and Luyin, which were controlled by our CEO, in the amount of $7.5 million as of March 31, 2009, and the amount has decreased to $6.6 million as of June 12, 2009. The maturity dates of the short term loans outstanding from related parties at March 31, 2009 are in October 2010. The balance as of March 31, 2009 included a US dollar loan of $3.9 million and a RMB loan of $3.6 million, and the interest rates were 10.0% and 5.5% respectively. In the fiscal year ended March 31, 2009, we did not make any interest payment to Honnete and Luyin. The accrued interest expenses associated with the loans from our related parties was $238,200 as of March 31, 2009.
Registration Rights Agreement with Warburg
On June 15, 2007, we issued 4,000,000 shares of common stock to Warburg Pincus Private Equity IX, L.P., or Warburg, for $66.0 million and entered into a registration rights agreement with Warburg. Pursuant to this registration rights agreement, we granted Warburg certain customary registration rights, including demand, piggyback and Form S-3 registration rights. Subject to the provisions of the registration rights agreement and the restrictions of its lock-up agreement, Warburg will be entitled to require us to register the resale of its shares under the Securities Act.
On April 23, 2008, Beams Power Investment Limited, or Beams, a British Virgin Islands limited liability company and our majority stockholder, and Warburg entered into a note purchase agreement. Pursuant to this note purchase agreement, Beams issued Warburg a senior convertible note in an aggregate principal amount of $30 million, which is convertible into up to 1,000,000 shares of our common stock held by Beams. In addition, Beams and Warburg entered into a share pledge agreement, dated April 23, 2008, pursuant to which Beams agreed to pledge an aggregate of 5,967,000 of shares of our common stock held by Beams as initial collateral for the loan covered by the note. On December 4, 2008, Beams agreed to pledge an additional 8,000,000 shares of shares of our common stock held by Beams as collateral for the loan covered by the note.
In connection with the issuance of the note and the share pledge agreement, we entered into a registration rights agreement, dated April 23, 2008, with Beams and Warburg, pursuant to which we granted Warburg certain customary registration rights, including demand, piggyback and Form S-3 registration rights, with respect to the shares of our common stock Warburg acquires or may acquire pursuant to the note purchase agreement, the note and the share pledge agreement.
Review, Approval or Ratification of Transactions with Related Parties
All transactions involving the Company and its officers, directors, principal shareholders and their affiliates will be and have been approved by our audit committee.
Director Independence
The Board has determined all Board members, excluding Liang Zhang and William W. Wu, are independent under the applicable NASDAQ rules. The Board has also determined the members of each committee of the Board are independent under the listing standards of the NASDAQ Global Select Market. In making these determinations, the Board considered, among other things, the types and amounts of the commercial dealings between the Company and the companies and organizations with which the directors are affiliated.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
On July 27, 2007, we engaged Deloitte Touche Tohmatsu CPA Ltd. (“DTTC”) as our new independent auditors. The engagement was approved by the Audit Committee of our Board of Directors. No relationship existed in any manner between DTTC and us prior to the date we engaged DTTC.
AUDIT FEES
DTTC was paid aggregate fees of approximately $1,202,520 and $1,234,190 for the fiscal year ended March 31, 2009 and 2008, respectively, for professional services rendered for the audit of our annual financial statements and for the reviews of the financial statements included in our quarterly reports on Form 10-Q.
AUDIT-RELATED FEES
DTTC was paid aggregate fees of approximately nil and $467,735 for the fiscal year ended March 31, 2009 and 2008, respectively, for assurance and related services reasonably related to the performance of the audit or review of the Registrant’s financial statements.
TAX FEES
DTTC was paid aggregate fees of approximately $13,000 and nil for the fiscal year ended March 31, 2009 and 2008, respectively, for professional services rendered for tax compliance, tax advice and tax planning.
ALL OTHER FEES
DTTC was paid no other fees for any other services rendered to the Company for the fiscal year ended March 31, 2009 and 2008.
AUDIT COMMITTEE PRE-APPROVAL POLICIES AND PROCEDURES
Our Audit Committee’s policy is to pre-approve all audit and permissible non-audit services provided by our independent auditors. These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to one year and any pre-approval is detailed as to the particular service or category of services. The independent auditor and management are required to periodically report to the Audit Committee regarding the extent of services provided by the independent auditor in accordance with this pre-approval.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (a) | List of Documents Filed as a Part of this Annual Report on Form 10-K: |
1. See Index to Consolidated Financial Statements in Item 8 of this Report.
2. See Index to Consolidated Financial Statements in Item 8 of this Report, and the Consolidated Financial Schedules listed on such index filed as part of this Report are filed as part of this Report.
3. The exhibits listed on the exhibit index filed as part of this Report are filed or incorporated by reference as part of this Report
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, on this 15th day of June, 2009.
| SYNUTRA INTERNATIONAL, INC. |
| | |
| By: | |
| | Liang Zhang |
| | Chief Executive Officer and Chairman |
Each person whose signature appears below appoints each of Liang Zhang and Lawrence Lee, severally and not jointly, to be his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any amendments to our Annual Report on Form 10-K for the fiscal year ended March 31, 2009; granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully for all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, shall lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities.
| Name | | Position | | Date |
| | | |
| | | | |
| Liang Zhang | | Chief Executive Officer, and Chairman (Principal Executive Officer) | | June 15, 2009 |
| | | |
| | | | |
| Lawrence Lee | | Chief Financial Officer (Principal Financial and Accounting Officer) | | June 15, 2009 |
| | | |
| | | | |
| Jinrong Chen | | Director | | June 15, 2009 |
| | | |
| | | | |
| Yiu-Chun Chan | | Director | | June 15, 2009 |
| | | |
| | | | |
| Lei Lin | | Director | | June 15, 2009 |
| | | |
| | | | |
| William W. Wu | | Director | | June 15, 2009 |
EXHIBITS INDEX
| | Description |
| 3.1 | | Amended and Restated Certificate of Incorporation of Synutra International, Inc. (incorporated by reference to Exhibit 3.1 to the Registrant’s Quarterly Report on Form 10-Q filed on November 11, 2008) |
| | |
| 3.2 | | Amended and Restated Bylaws of Synutra International, Inc. (incorporated by reference to Exhibit 3.2 to the Registrant’s Quarterly Report on Form 10-Q filed on November 11, 2008) |
| | |
| 4.1 | | Specimen common stock certificate (incorporated by reference to Exhibit 4.1 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | |
| 10.1 | | Employment Agreement between Qingdao Shengyuan Dairy Co., Ltd. and Liang Zhang, dated December 20, 2006 (incorporated by reference to Exhibit 10.1 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | |
| 10.2 | | Employment Agreement between Qingdao Shengyuan Dairy Co., Ltd. and Lawrence Lee, dated September 1, 2007 (incorporated by reference to Exhibit 10.2 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | |
| 10.3 | | Employment Agreement between the Beijing Representative Office of Qingdao Shengyuan Dairy Co., Ltd. and William W. Wu, dated July 1, 2007 (incorporated by reference to Exhibit 10.3 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | |
| 10.4 | | Employment Agreement between the Beijing Representative Office of Qingdao Shengyuan Dairy Co., Ltd. and Weiguo Zhang, dated January 1, 2007 (incorporated by reference to Exhibit 10.4 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | |
| 10.5 | | Employment Agreement between Shengyuan Nutritional Food Co., Ltd. and Xisen Mu, dated July 1, 2007 (incorporated by reference to Exhibit 10.5 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | |
| 10.6 | | Warrant Agreement among Beams Power Investment Limited, Synutra International, Inc., the Bank of New York as Warrant Agent, and ABN AMRO Bank N.V., Hong Kong Branch, dated April 19, 2007 (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on April 24, 2007) |
| | |
| 10.7 | | Registration Rights Agreement between Synutra International, Inc. and ABN AMRO Bank N.V., Hong Kong Branch, dated April 19, 2007 (incorporated by reference to Exhibit 4.3 to the Registrant’s Current Report on Form 8-K filed on April 24, 2007) |
| 10.8 | | Common Stock Purchase Agreement between Synutra International, Inc. and Warburg Pincus Private Equity IX, L.P. , dated May 24, 2007 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K/A filed on June 1, 2007) |
| | |
| 10.9 | | Registration Rights Agreement between Synutra International, Inc. and Warburg Pincus Private Equity IX, L.P., dated June 15, 2007 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K/A filed on June 1, 2007) |
| | |
| 10.10 | | Voting and Co-Sale Agreement among Synutra International, Inc., Beams Power Investment Limited, and Warburg Pincus Private Equity IX, L.P., dated June 15, 2007 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K/A filed on June 1, 2007) |
| | |
| 10.11 | | Registration Rights Agreement among Synutra International, Inc., Beams Power Investment Limited and Warburg Pincus Private Equity IX, L.P., dated April 23, 2008, (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on April 24, 2008) |
| | |
| 10.12 | | Loan Agreement among Synutra International, Inc., ABN AMRO Bank N.V. and certain lenders party thereto, dated October 11, 2007 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on October 17, 2007) |
| | | |
| 10.13 | | Collateral Agreement among Synutra International, Inc., ABN AMRO Bank N.V. and Synutra, Inc., dated October 11, 2007 (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on October 17, 2007). |
| | | |
| 10.14 | | US Dollar Facility Fee Letter Agreement between Synutra International, Inc. and ABN AMRO Bank N.V., dated October 11, 2007 (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on October 17, 2007). |
| | | |
| 10.15 | | Synutra International, Inc. 2008 Stock Incentive Plan (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on June 17, 2008) |
| | | |
| 10.16A | | Form of Incentive Stock Option Agreement under Synutra International, Inc. 2008 Stock Incentive Plan |
| | | |
| 10.16B | | Form of Nonqualified Stock Option Agreement under Synutra International, Inc., 2008 Stock Incentive Plan |
| | | |
| 10.17 | | Asset Purchase Agreement, dated July 14, 2008, between Sheng Yuan Nutritional Food Co., Ltd. and Beijing Huilian Food Co, Ltd. (incorporated by reference to Exhibit 2.1 from the Registrant’s Current Report on Form 8-K filed on July 18, 2008) |
| 14.1 | | Code of Ethics for Chief Executive Officer and Senior Financial Officers (incorporated by reference to Exhibit 14. 1 to the Registrant’s Annual Report on Form 10-K filed on June 16, 2008) |
| | | |
| 16.1 | | Letter from Rotenberg & Co to the Securities and Exchange Commission dated July 24, 2007 (incorporated by reference to Exhibit 16.1 to the Registrant’s Current Report on Form 8-K filed on July 27, 2007) |
| | |
| 21.1 | | List of Subsidiaries |
| | |
| 24.1 | | Power of Attorney (included on the Signature Page of this Annual Report on Form 10-K) |
| | |
| 31.1 | | Certification of Principal Executive Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended |
| | |
| 31.2 | | Certification of Principal Financial Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended |
| | |
| 32.1 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Chief Executive Officer) |
| | |
| 32.2 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Chief Financial Officer) |