
EHA 2017 June 23, 2017 NASDAQ: BLUE Exhibit 99.1

These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements we make regarding the initiation, timing, progress and results of our preclinical and clinical studies and our research and development programs, our ability to advance product candidates into, and successfully complete, clinical studies, and the timing or likelihood of regulatory filings and approvals are forward looking. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described in our most recent quarterly report on Form 10-Q, as well as our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Forward Looking Statements
Our Vision: Make Hope a Reality TRUE BLUE BLUE MOJO OUR PATIENTS OUR PEOPLE
Our Strategic Intent Severe Genetic Diseases Hematopoietic Stem Cells (HSCs) Immunotherapy T Cells Late Stage (LentiGlobin, Lenti-D) Big Near Term Opportunities (SCD, Multiple Myeloma) Best Team (350+) Great Partners Truly Integrated Platform Transformative Product Focus “Tetris” R&D (Anti-Pure Play) Lentiviral Gene Delivery Pure, Potent, Reproducible, Scalable Global Manufacturing Platform Virus and Drug Product Genome Editing Platform MegaTALs

T H E G E N E T H E R A P Y P R O D U C T C O M P A N Y Products on the Market Programs Nearing Commercialization Additional Programs in the Clinic 2+ 4+ 2+ Patient Impact ∞ World-class Gene Therapy Platform and Integrated Global Capabilities
How Do We Get There? Data, Execution and Development in 2017 LentiGlobin, TDT and SCD Update @ ASH LentiGlobin, TDT Update @ EHA CRB-401 Study Data (bb2121) @ ASCO DATA EXECUTION Initiate HGB-212 Study of LentiGlobin Confirm LentiD, CALD Clinical/ Regulatory Path Preparations for TDT EU MAA Filing File Next Generation BCMA IND (bb21217) Advance & Further Validate Gene Editing Platform DEVELOPMENT Lenti-D CALD Update @TBD
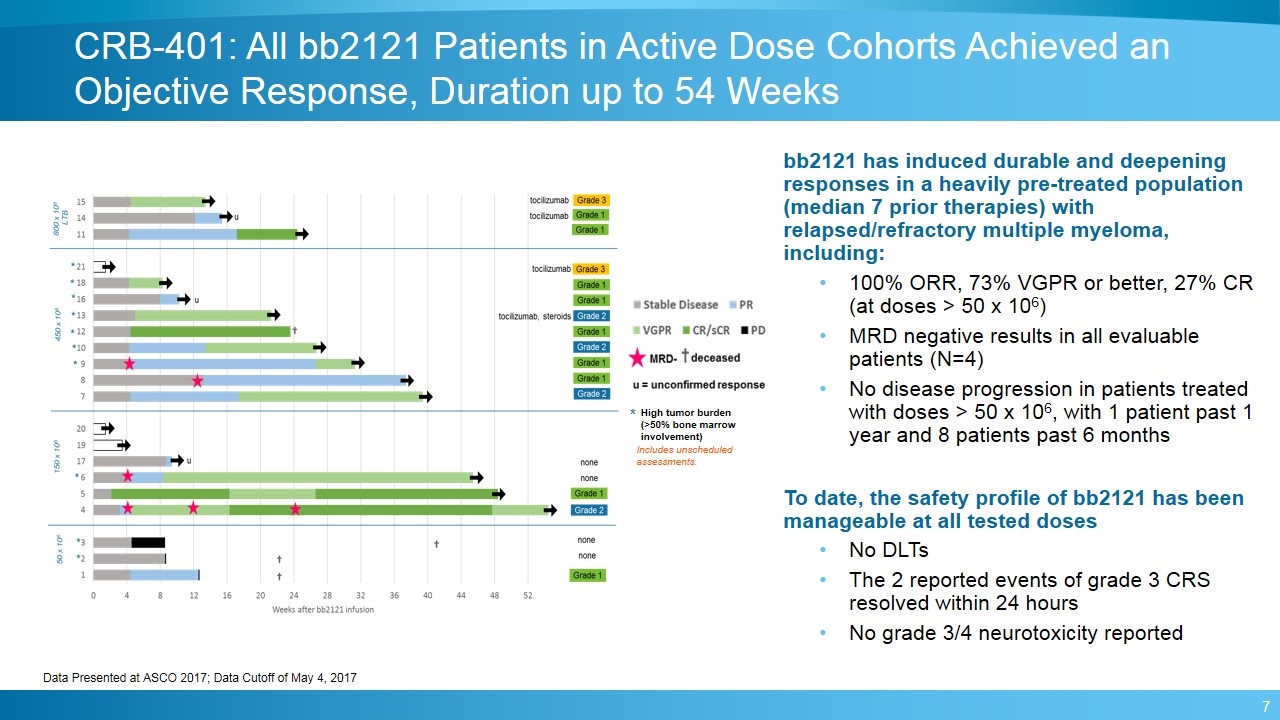
CRB-401: All bb2121 Patients in Active Dose Cohorts Achieved an Objective Response, Duration up to 54 Weeks * High tumor burden (>50% bone marrow involvement) Includes unscheduled assessments. bb2121 has induced durable and deepening responses in a heavily pre-treated population (median 7 prior therapies) with relapsed/refractory multiple myeloma, including: 100% ORR, 73% VGPR or better, 27% CR (at doses > 50 x 106) MRD negative results in all evaluable patients (N=4) No disease progression in patients treated with doses > 50 x 106, with 1 patient past 1 year and 8 patients past 6 months To date, the safety profile of bb2121 has been manageable at all tested doses No DLTs The 2 reported events of grade 3 CRS resolved within 24 hours No grade 3/4 neurotoxicity reported Data Presented at ASCO 2017; Data Cutoff of May 4, 2017

LentiGlobin Clinical Studies NORTHSTAR (HGB-204) Phase 1/2 single-center study; all genotypes NORTHSTAR-3 (HGB-212) Phase 1/2 multicenter study; all genotypes HGB-205 (TDT and SCD) All 18 patients treated, with ≥ 6 months follow-up 2 patients have completed 2-year analysis HGB-206 (Severe SCD) 4 TDT patients treated, with 11 – 33 months follow-up Phase 3, global, multicenter study; non- β 0 / β 0 genotypes First study to use new manufacturing process N=15 adults and adolescents, and N=8 pediatric patients Phase 3, multicenter, global study; β 0 / β 0 genotypes N=15 adults, adolescents and pediatric patients Initiation planned for 2017 NORTHSTAR-2 (HGB-207) Open label, multicenter, U.S. based study N=29 adults
Key Questions Transfusion-Dependent β-thalassemia (TDT): Northstar-2 and HGB-205 With our new manufacturing process in Northstar-2, are we able to consistently manufacture drug product (DP) with higher vector copy number (VCN) and proportion of transduced cells? How do the early results from Northstar-2… Compare to the results seen in non-β0/β0 patients in HGB-204? Read through to β0/β0 patients? Read through to SCD patients? What can we learn from the HGB-205 TDT patients? Severe Sickle Cell Disease (SCD) How do the data from the HGB-205 patients compare to ASH 2016 data? What does HGB-205 teach us regarding the potential impact of the protocol and manufacturing changes made in HGB-206?

A Phase 3 Study to Evaluate Safety and Efficacy of LentiGlobin Gene Therapy for Transfusion-Dependent β-thalassemia in Patients with non-β0/β0 Genotypes: The Northstar-2 (HGB-207) Trial Mark C. Walters, Alexis Thompson, Suradej Hongeng, Janet L. Kwiatkowski, Franco Locatelli, John Porter, Martin Sauer, Adrian Thrasher, Isabelle Thuret, Evangelia Yannaki, Alexandria Petrusich, Mohammed Asmal EHA 2017 Abstract S814
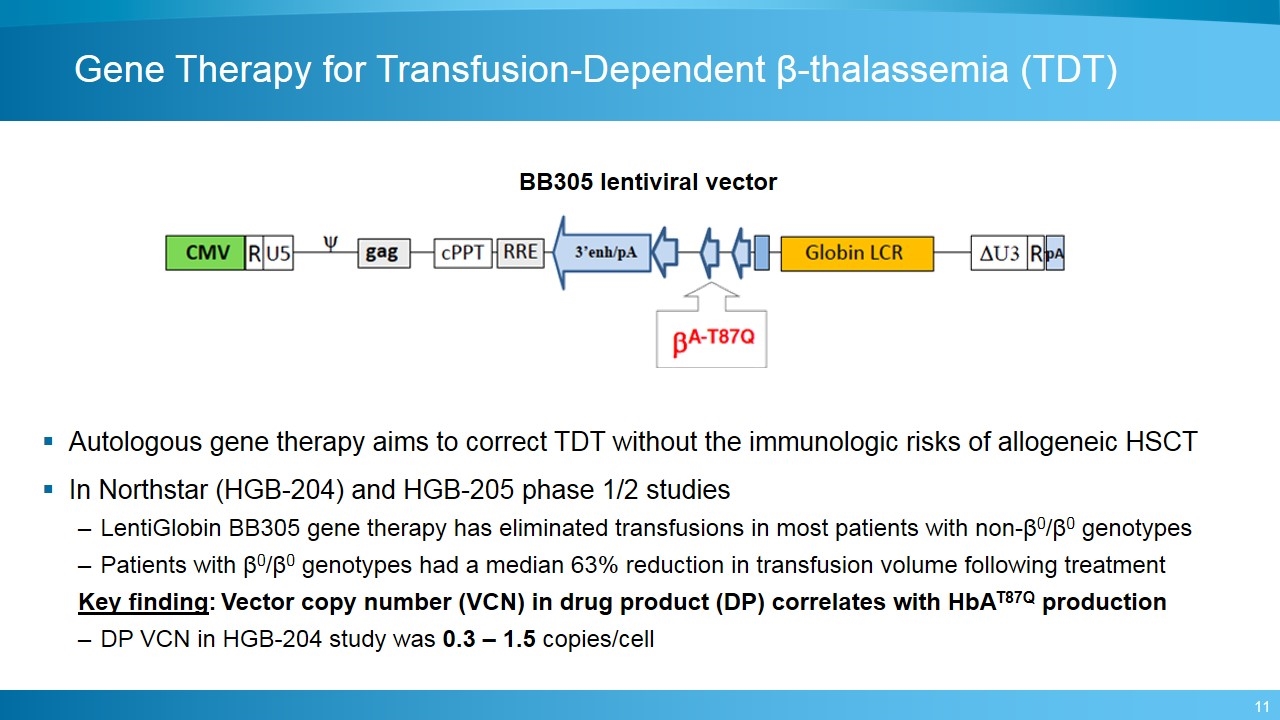
Gene Therapy for Transfusion-Dependent β-thalassemia (TDT) Autologous gene therapy aims to correct TDT without the immunologic risks of allogeneic HSCT In Northstar (HGB-204) and HGB-205 phase 1/2 studies LentiGlobin BB305 gene therapy has eliminated transfusions in most patients with non-β0/β0 genotypes Patients with β0/β0 genotypes had a median 63% reduction in transfusion volume following treatment Key finding: Vector copy number (VCN) in drug product (DP) correlates with HbAT87Q production DP VCN in HGB-204 study was 0.3 – 1.5 copies/cell BB305 lentiviral vector

Northstar-2 (HGB-207) Study of LentiGlobin BB305 Gene Therapy Investigating efficacy and safety of LentiGlobin BB305 in adolescents and adults with TDT and non-β0/β0 genotypes Uses refined manufacturing process to yield higher DP VCNs Primary endpoint: proportion of patients who achieve “transfusion independence” (TI) TI = maintain an average Hb≥ 9 g/dL without RBC transfusions for ≥12 months Data as of June 2, 2017
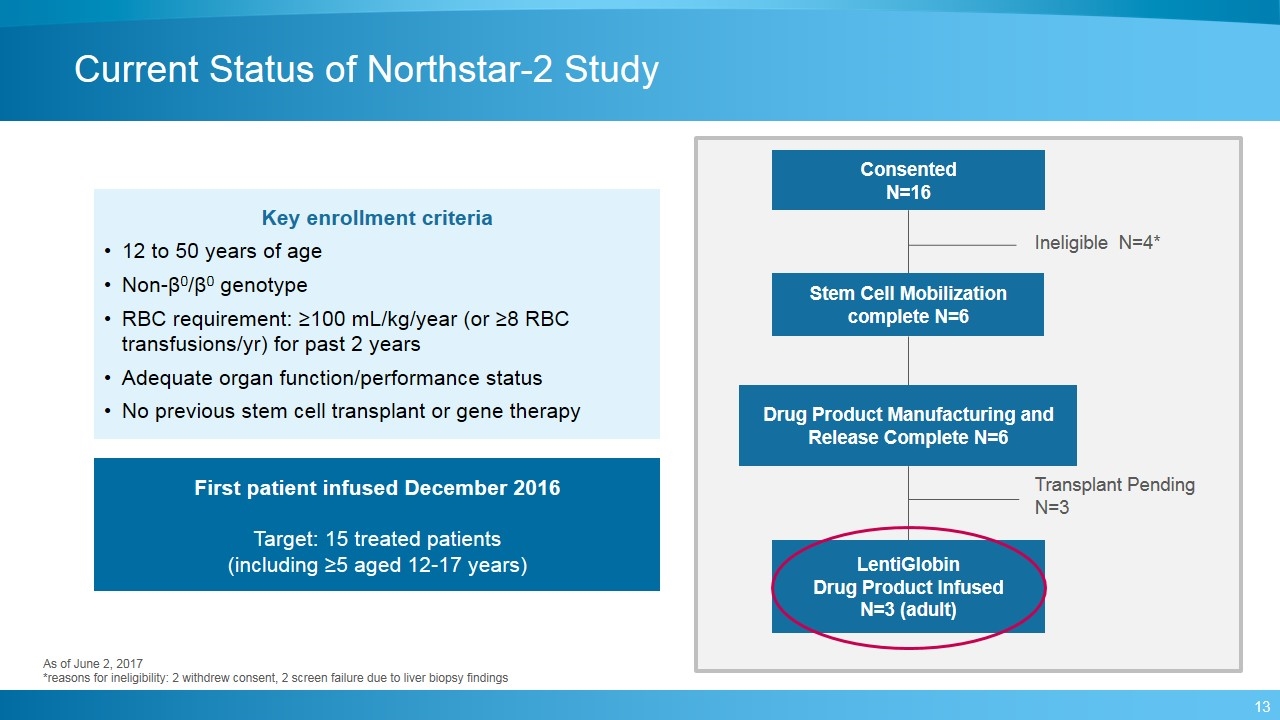
Current Status of Northstar-2 Study Consented N=16 Ineligible N=4* Stem Cell Mobilization complete N=6 Drug Product Manufacturing and Release Complete N=6 LentiGlobin Drug Product Infused N=3 (adult) First patient infused December 2016 Target: 15 treated patients (including ≥5 aged 12-17 years) Key enrollment criteria 12 to 50 years of age Non-β0/β0 genotype RBC requirement: ≥100 mL/kg/year (or ≥8 RBC transfusions/yr) for past 2 years Adequate organ function/performance status No previous stem cell transplant or gene therapy Transplant Pending N=3 As of June 2, 2017 *reasons for ineligibility: 2 withdrew consent, 2 screen failure due to liver biopsy findings
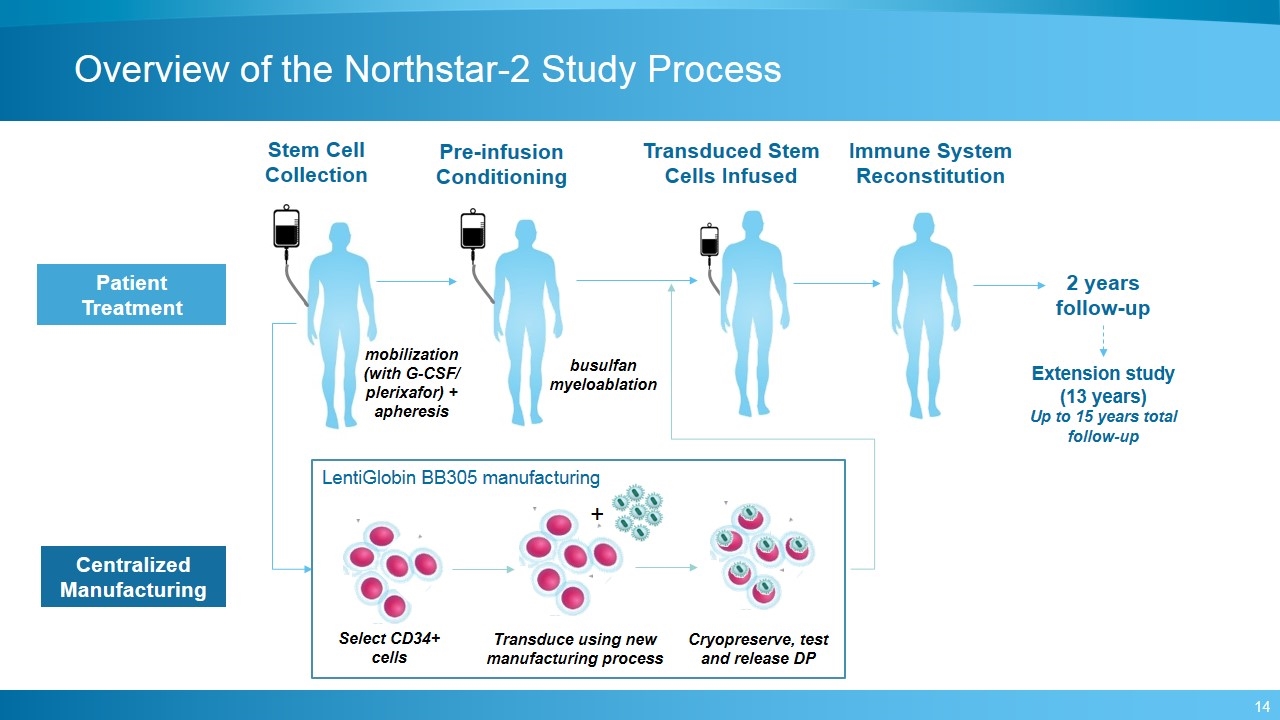
Overview of the Northstar-2 Study Process Patient Treatment Centralized Manufacturing Pre-infusion Conditioning Stem Cell Collection Transduced Stem Cells Infused Select CD34+ cells + Cryopreserve, test and release DP 2 years follow-up Extension study (13 years) Up to 15 years total follow-up Immune System Reconstitution Transduce using new manufacturing process LentiGlobin BB305 manufacturing busulfan myeloablation mobilization (with G-CSF/ plerixafor) + apheresis
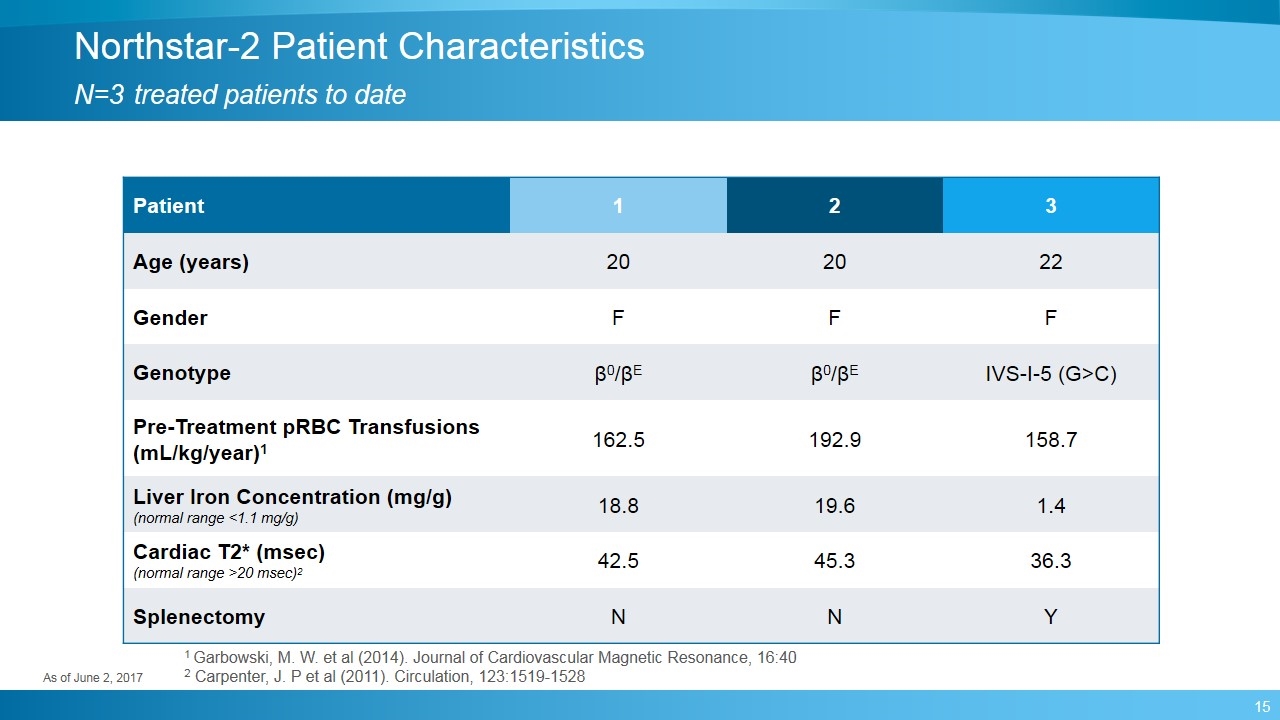
Northstar-2 Patient Characteristics N=3 treated patients to date Patient 1 2 3 Age (years) 20 20 22 Gender F F F Genotype β0/βE β0/βE IVS-I-5 (G>C) Pre-Treatment pRBC Transfusions (mL/kg/year)1 162.5 192.9 158.7 Liver Iron Concentration (mg/g) (normal range <1.1 mg/g) 18.8 19.6 1.4 Cardiac T2* (msec) (normal range >20 msec)2 42.5 45.3 36.3 Splenectomy N N Y As of June 2, 2017 1 Garbowski, M. W. et al (2014). Journal of Cardiovascular Magnetic Resonance, 16:40 2 Carpenter, J. P et al (2011). Circulation, 123:1519-1528
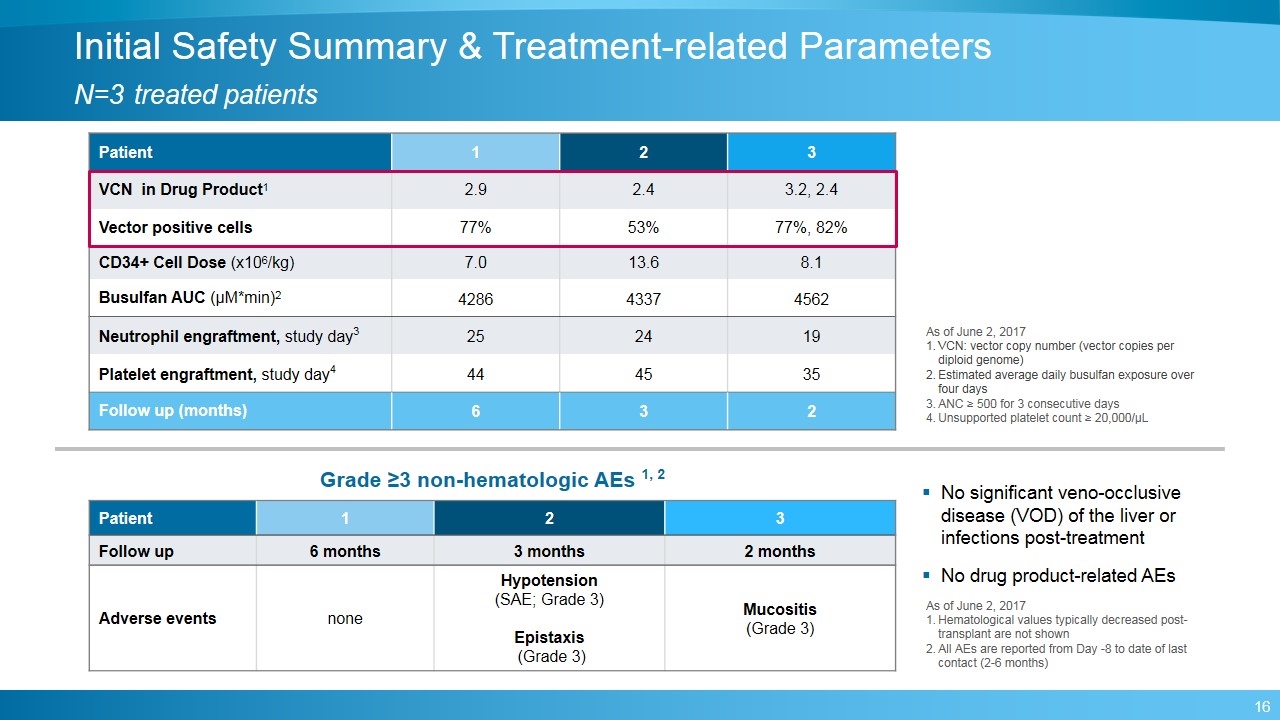
Initial Safety Summary & Treatment-related Parameters N=3 treated patients Patient 1 2 3 VCN in Drug Product1 2.9 2.4 3.2, 2.4 Vector positive cells 77% 53% 77%, 82% CD34+ Cell Dose (x106/kg) 7.0 13.6 8.1 Busulfan AUC (μM*min)2 4286 4337 4562 Neutrophil engraftment, study day3 25 24 19 Platelet engraftment, study day4 44 45 35 Follow up (months) 6 3 2 Patient 1 2 3 Follow up 6 months 3 months 2 months Adverse events none Hypotension (SAE; Grade 3) Epistaxis (Grade 3) Mucositis (Grade 3) Grade ≥3 non-hematologic AEs 1, 2 No significant veno-occlusive disease (VOD) of the liver or infections post-treatment No drug product-related AEs As of June 2, 2017 Hematological values typically decreased post-transplant are not shown All AEs are reported from Day -8 to date of last contact (2-6 months) As of June 2, 2017 VCN: vector copy number (vector copies per diploid genome) Estimated average daily busulfan exposure over four days ANC ≥ 500 for 3 consecutive days Unsupported platelet count ≥ 20,000/µL
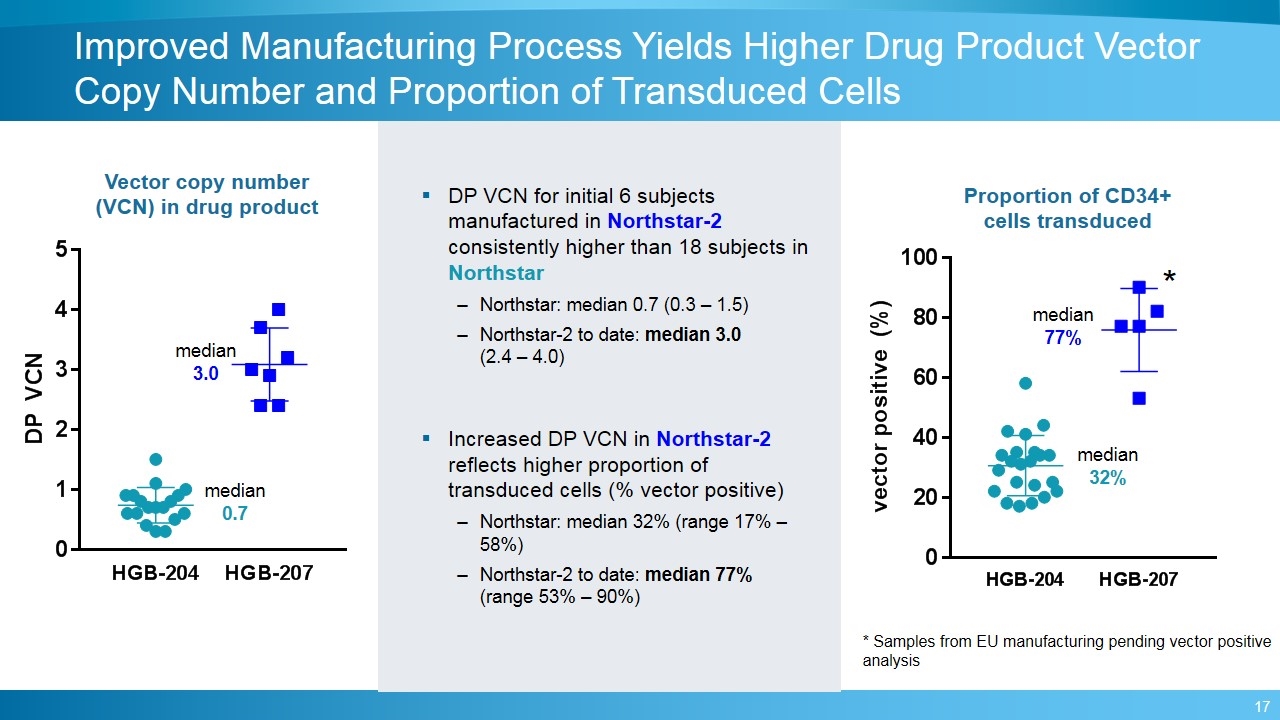
Improved Manufacturing Process Yields Higher Drug Product Vector Copy Number and Proportion of Transduced Cells Vector copy number (VCN) in drug product Proportion of CD34+ cells transduced median 0.7 median 3.0 median 32% median 77% DP VCN for initial 6 subjects manufactured in Northstar-2 consistently higher than 18 subjects in Northstar Northstar: median 0.7 (0.3 – 1.5) Northstar-2 to date: median 3.0 (2.4 – 4.0) Increased DP VCN in Northstar-2 reflects higher proportion of transduced cells (% vector positive) Northstar: median 32% (range 17% – 58%) Northstar-2 to date: median 77% (range 53% – 90%) * Samples from EU manufacturing pending vector positive analysis *
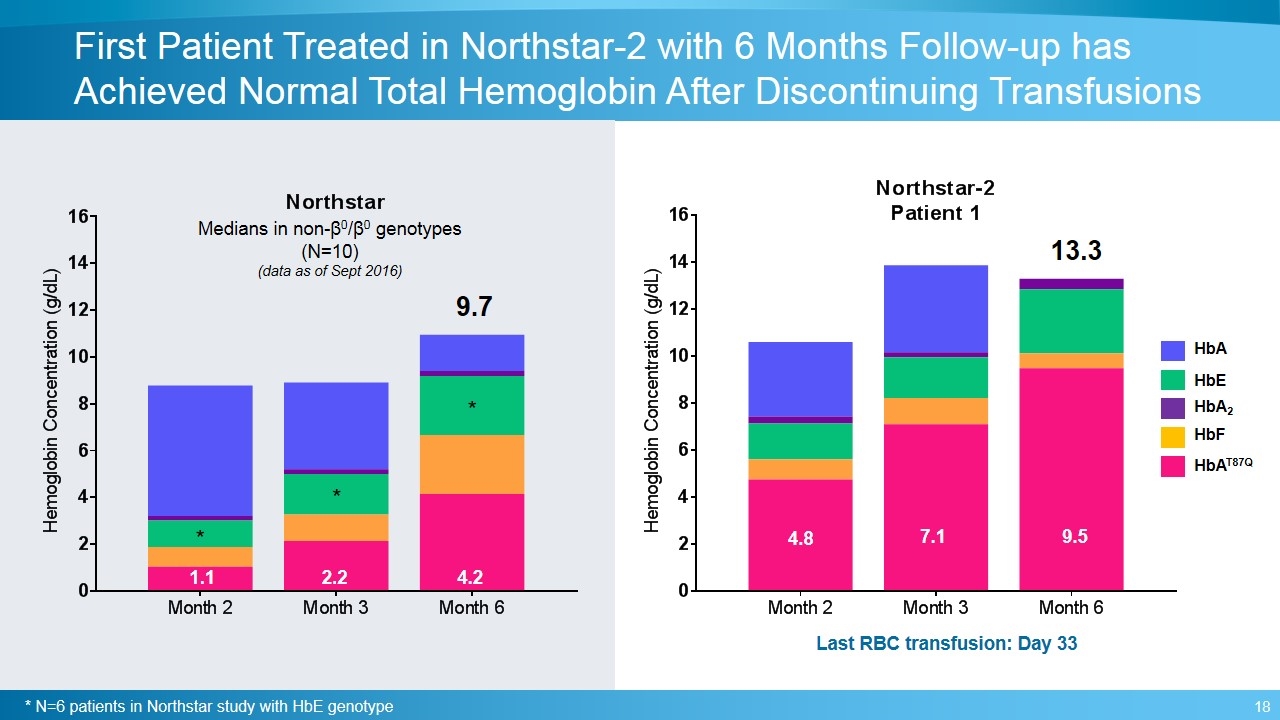
First Patient Treated in Northstar-2 with 6 Months Follow-up has Achieved Normal Total Hemoglobin After Discontinuing Transfusions 13.3 Last RBC transfusion: Day 33 4.8 7.1 9.5 HbAT87Q HbF HbA2 HbA HbE 4.2 2.2 1.1 Medians in non-β0/β0 genotypes (N=10) (data as of Sept 2016) 9.7 * N=6 patients in Northstar study with HbE genotype * * *
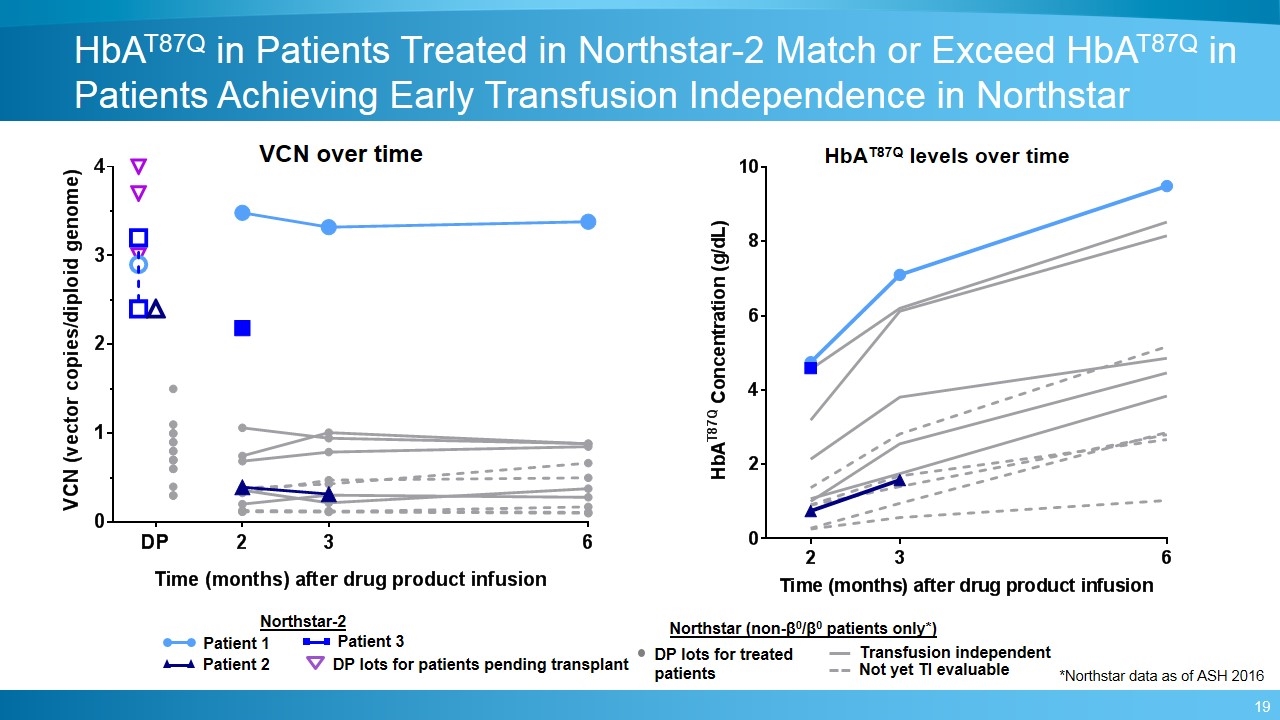
VCN over time Patient 1 Patient 2 Patient 3 Northstar (non-β0/β0 patients only*) HbAT87Q levels over time HbAT87Q in Patients Treated in Northstar-2 Match or Exceed HbAT87Q in Patients Achieving Early Transfusion Independence in Northstar DP lots for patients pending transplant Transfusion independent Not yet TI evaluable Northstar-2 *Northstar data as of ASH 2016 DP lots for treated patients
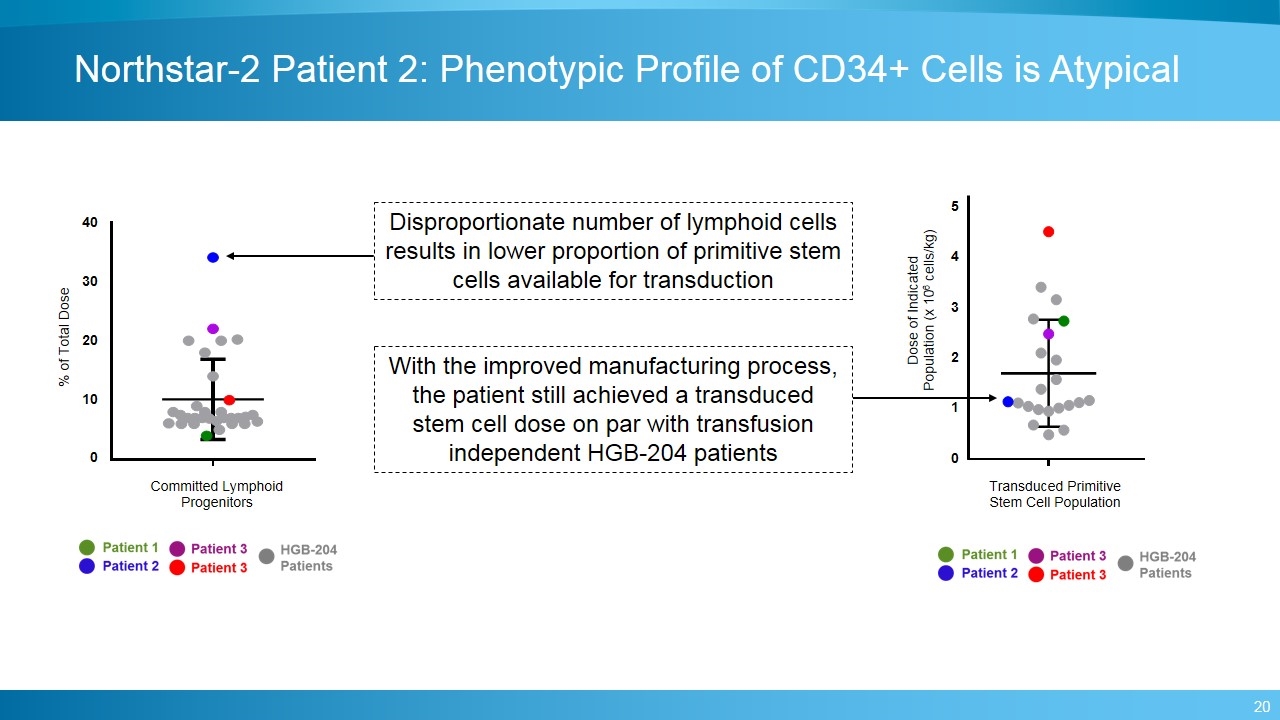
5 4 3 2 1 0 Dose of Indicated Population (x 106 cells/kg) Transduced Primitive Stem Cell Population Northstar-2 Patient 2: Phenotypic Profile of CD34+ Cells is Atypical Disproportionate number of lymphoid cells results in lower proportion of primitive stem cells available for transduction With the improved manufacturing process, the patient still achieved a transduced stem cell dose on par with transfusion independent HGB-204 patients 40 30 20 10 0 % of Total Dose Committed Lymphoid Progenitors
Summary and Next Steps The improved manufacturing process in Northstar-2 consistently yields higher DP VCNs and proportions of cells transduced Initial results show that the 3 patients treated to date have achieved in vivo VCN and HbAT87Q production as good or better than patients achieving transfusion independence in the first Northstar study No LentiGlobin-related AEs reported in Northstar-2 with 2 - 6 months follow-up AE profile of LentiGlobin continues to appear similar to autologous HCT with myeloablative busulfan Data on vector insertion pattern with higher DP VCN is pending HGB-212 β0/β0 genotypes Phase 3, multi-center, global study N=15 adults, adolescents and pediatric patients Initiation planned for 2017 Northstar-2 Summary Next Steps

HGB-205: Transfusion-Dependent Thalassemia (TDT)
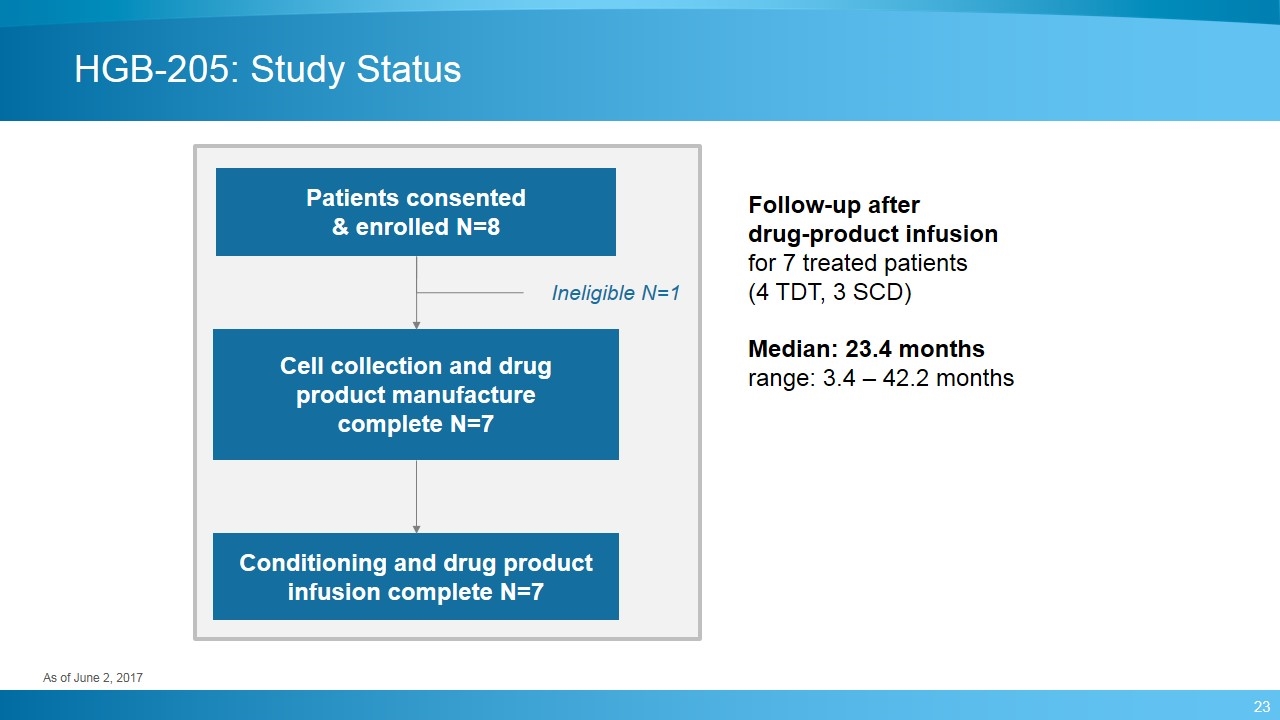
HGB-205: Study Status Patients consented & enrolled N=8 Cell collection and drug product manufacture complete N=7 Conditioning and drug product infusion complete N=7 Ineligible N=1 Follow-up after drug-product infusion for 7 treated patients (4 TDT, 3 SCD) Median: 23.4 months range: 3.4 – 42.2 months As of June 2, 2017
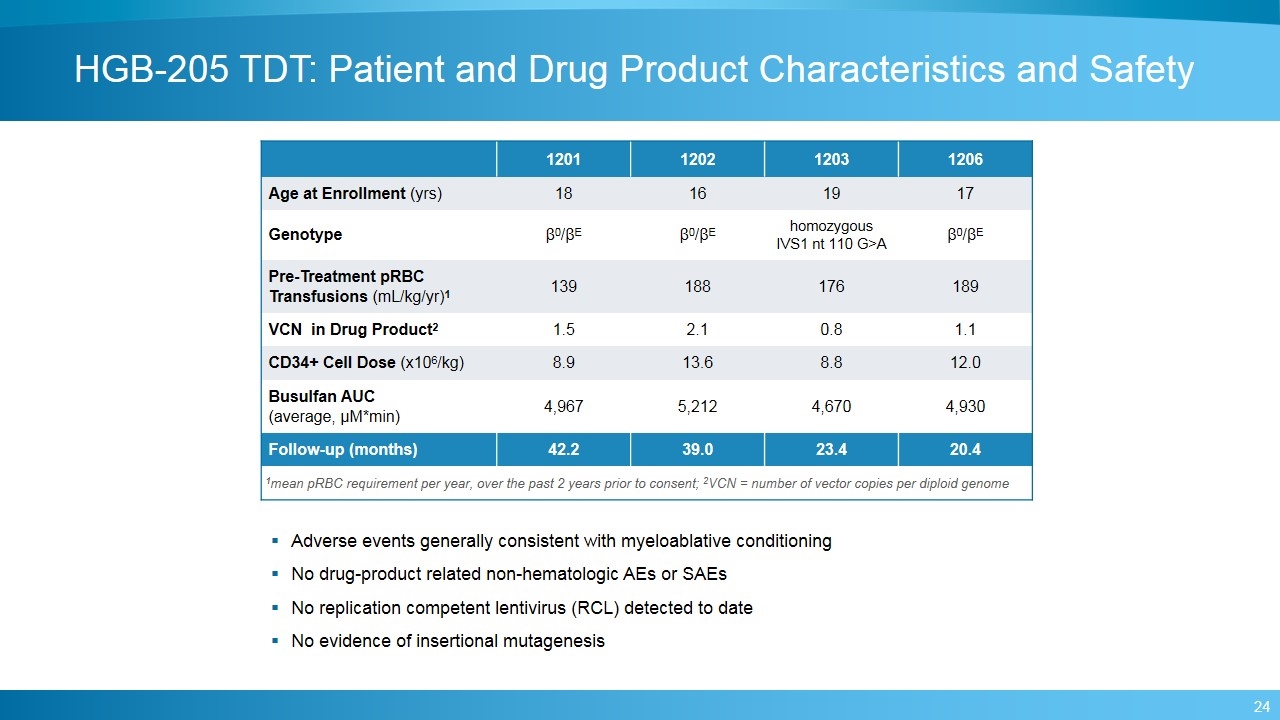
HGB-205 TDT: Patient and Drug Product Characteristics and Safety 1201 1202 1203 1206 Age at Enrollment (yrs) 18 16 19 17 Genotype β0/βE β0/βE homozygous IVS1 nt 110 G>A β0/βE Pre-Treatment pRBC Transfusions (mL/kg/yr)1 139 188 176 189 VCN in Drug Product2 1.5 2.1 0.8 1.1 CD34+ Cell Dose (x106/kg) 8.9 13.6 8.8 12.0 Busulfan AUC (average, μM*min) 4,967 5,212 4,670 4,930 Follow-up (months) 42.2 39.0 23.4 20.4 1mean pRBC requirement per year, over the past 2 years prior to consent; 2VCN = number of vector copies per diploid genome Adverse events generally consistent with myeloablative conditioning No drug-product related non-hematologic AEs or SAEs No replication competent lentivirus (RCL) detected to date No evidence of insertional mutagenesis
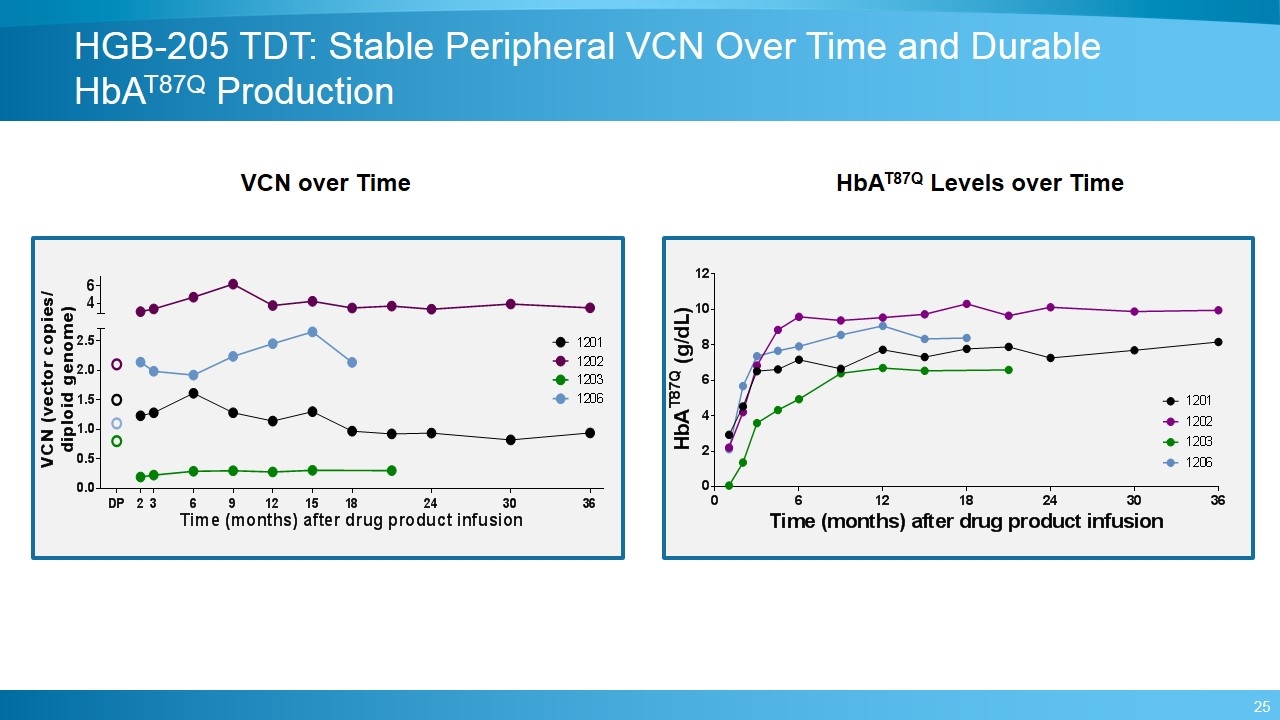
HGB-205 TDT: Stable Peripheral VCN Over Time and Durable HbAT87Q Production VCN over Time HbAT87Q Levels over Time
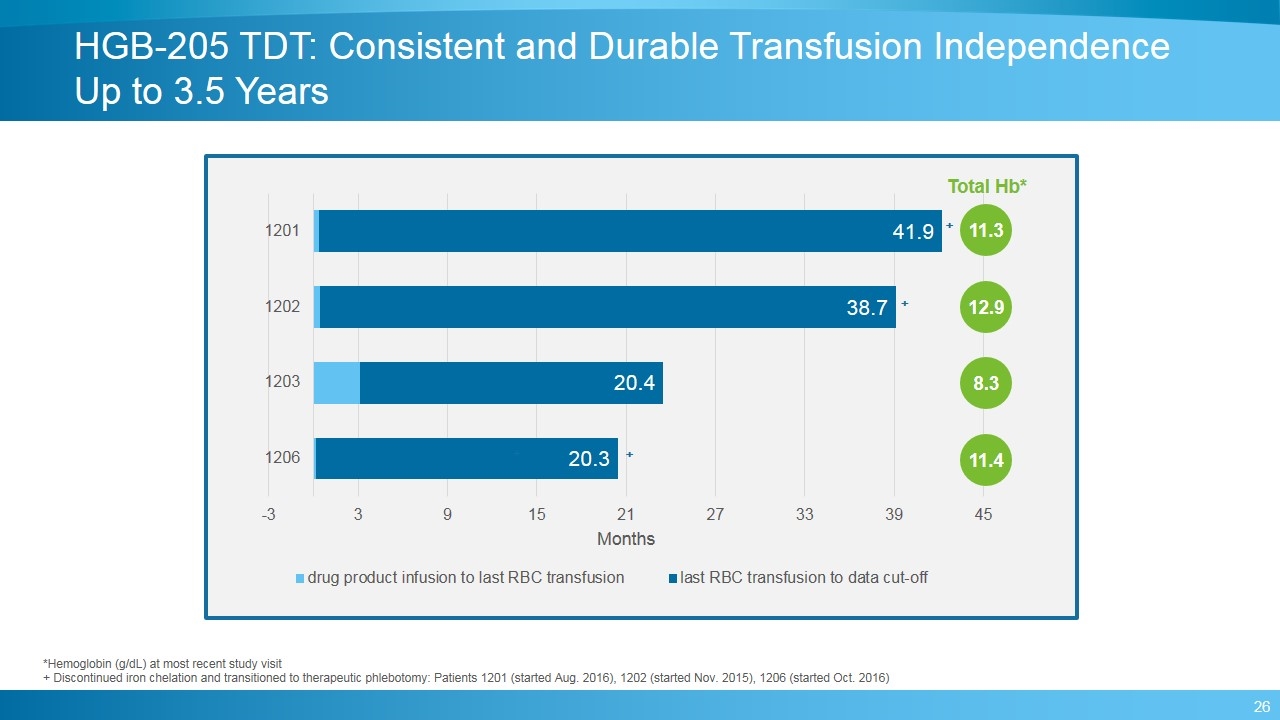
HGB-205 TDT: Consistent and Durable Transfusion Independence Up to 3.5 Years 11.3 8.3 12.9 11.4 Total Hb* *Hemoglobin (g/dL) at most recent study visit + Discontinued iron chelation and transitioned to therapeutic phlebotomy: Patients 1201 (started Aug. 2016), 1202 (started Nov. 2015), 1206 (started Oct. 2016)

HGB-205: Sickle Cell Disease (SCD)
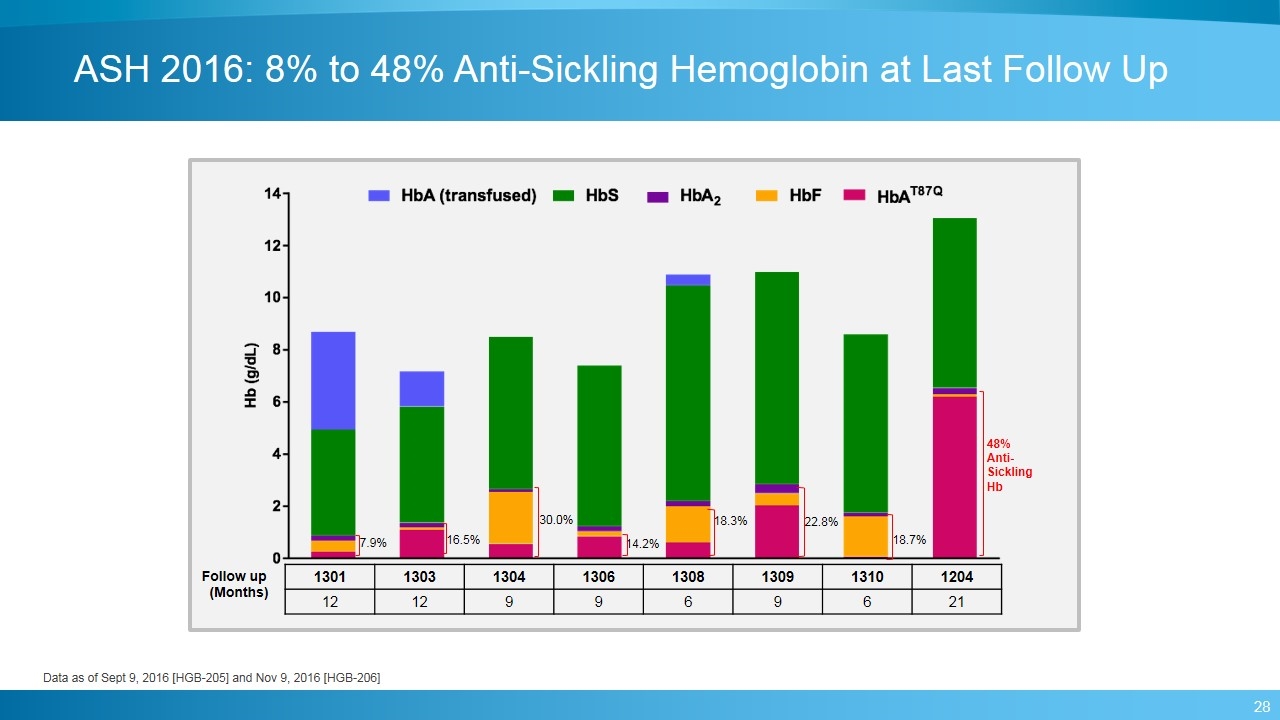
ASH 2016: 8% to 48% Anti-Sickling Hemoglobin at Last Follow Up 12 12 9 9 6 6 9 48% Anti-Sickling Hb 30.0% 14.2% 18.3% 22.8% 16.5% 7.9% 21 18.7% 1301 1303 1304 1306 1308 1309 1310 1204 12 12 9 9 6 9 6 21 Follow up (Months) Data as of Sept 9, 2016 [HGB-205] and Nov 9, 2016 [HGB-206]
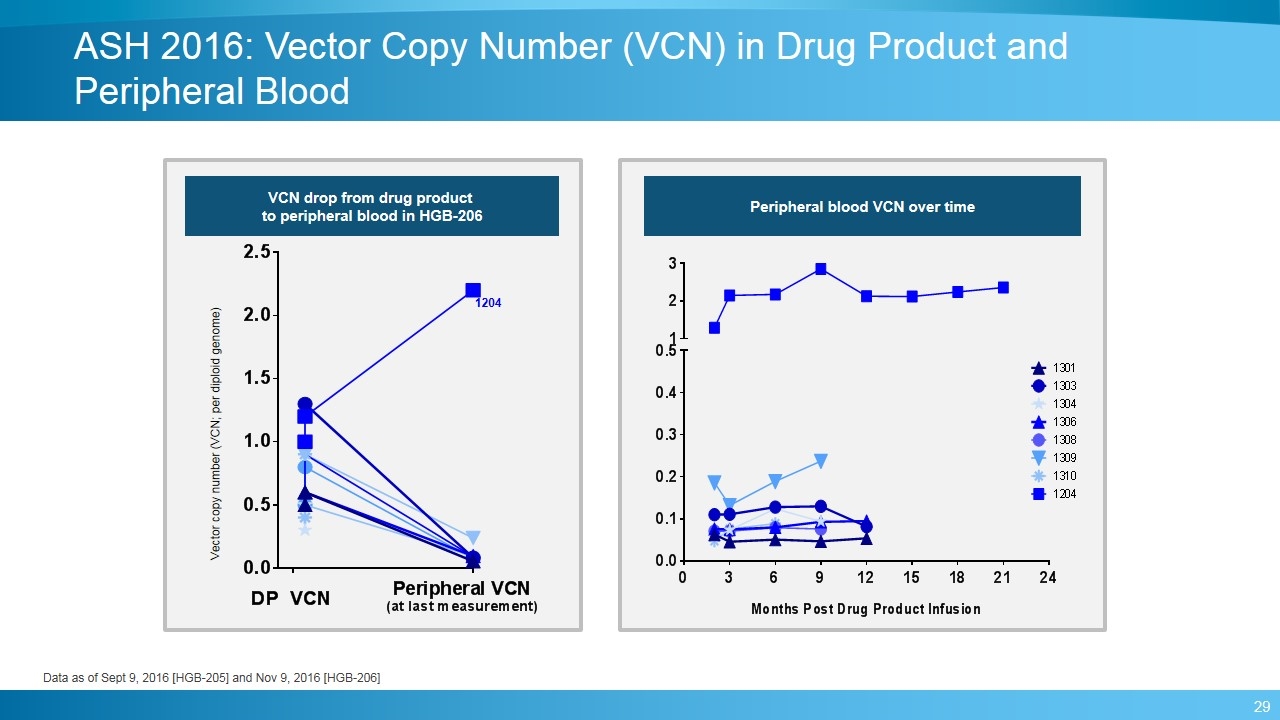
ASH 2016: Vector Copy Number (VCN) in Drug Product and Peripheral Blood Vector copy number (VCN; per diploid genome) VCN drop from drug product to peripheral blood in HGB-206 Peripheral blood VCN over time 1204 Data as of Sept 9, 2016 [HGB-205] and Nov 9, 2016 [HGB-206]
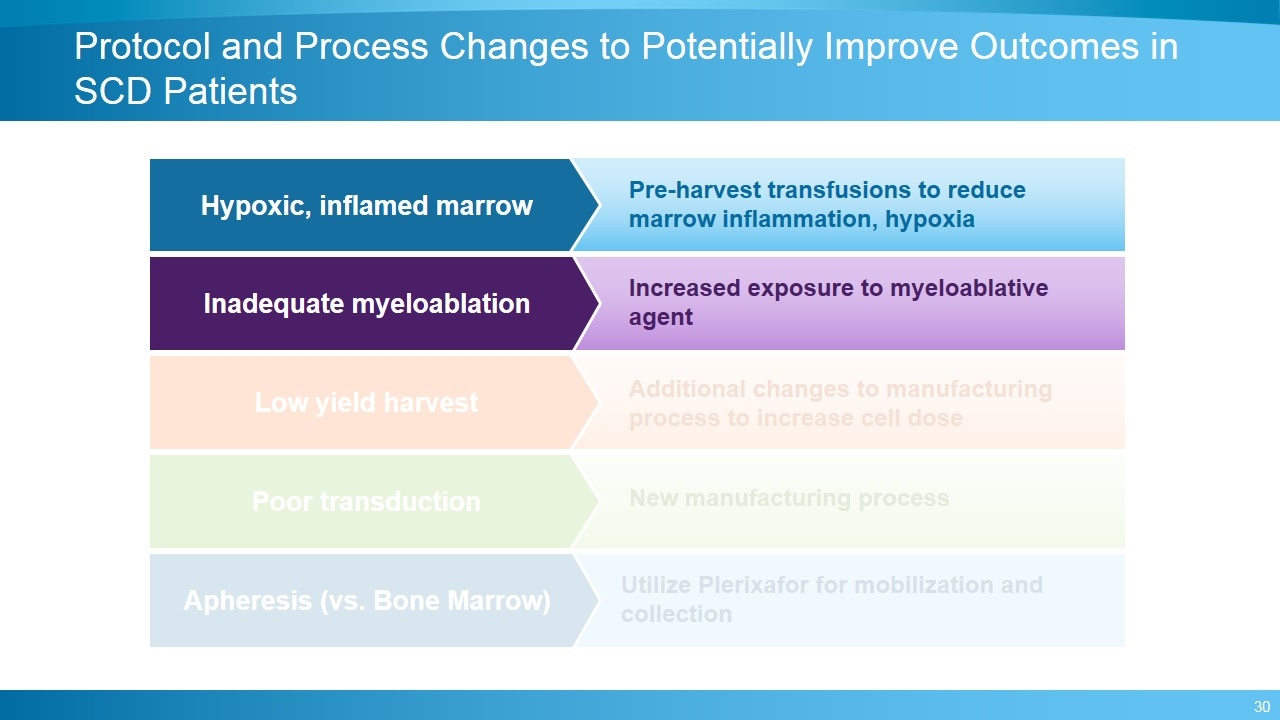
Protocol and Process Changes to Potentially Improve Outcomes in SCD Patients Hypoxic, inflamed marrow Pre-harvest transfusions to reduce marrow inflammation, hypoxia Low yield harvest Additional changes to manufacturing process to increase cell dose Poor transduction New manufacturing process Inadequate myeloablation Increased exposure to myeloablative agent Apheresis (vs. Bone Marrow) Utilize Plerixafor for mobilization and collection
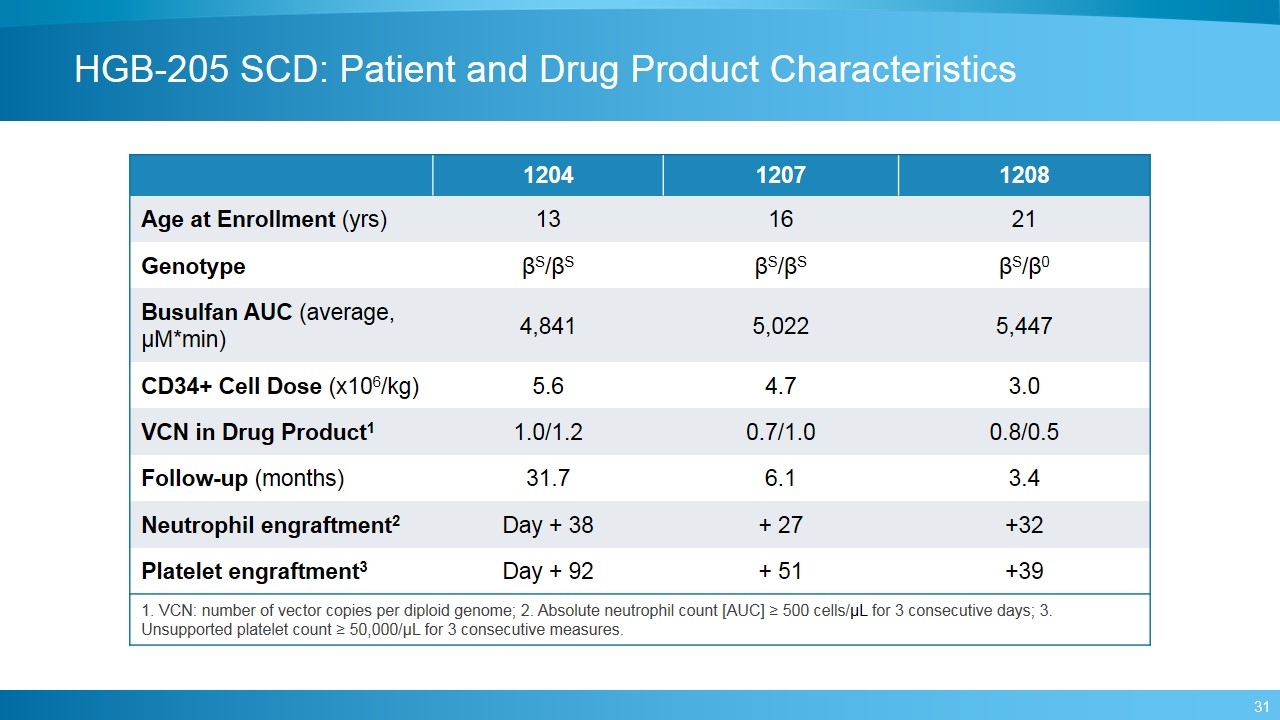
HGB-205 SCD: Patient and Drug Product Characteristics 1204 1207 1208 Age at Enrollment (yrs) 13 16 21 Genotype βS/βS βS/βS βS/β0 Busulfan AUC (average, μM*min) 4,841 5,022 5,447 CD34+ Cell Dose (x106/kg) 5.6 4.7 3.0 VCN in Drug Product1 1.0/1.2 0.7/1.0 0.8/0.5 Follow-up (months) 31.7 6.1 3.4 Neutrophil engraftment2 Day + 38 + 27 +32 Platelet engraftment3 Day + 92 + 51 +39 1. VCN: number of vector copies per diploid genome; 2. Absolute neutrophil count [AUC] ≥ 500 cells/μL for 3 consecutive days; 3. Unsupported platelet count ≥ 50,000/µL for 3 consecutive measures.
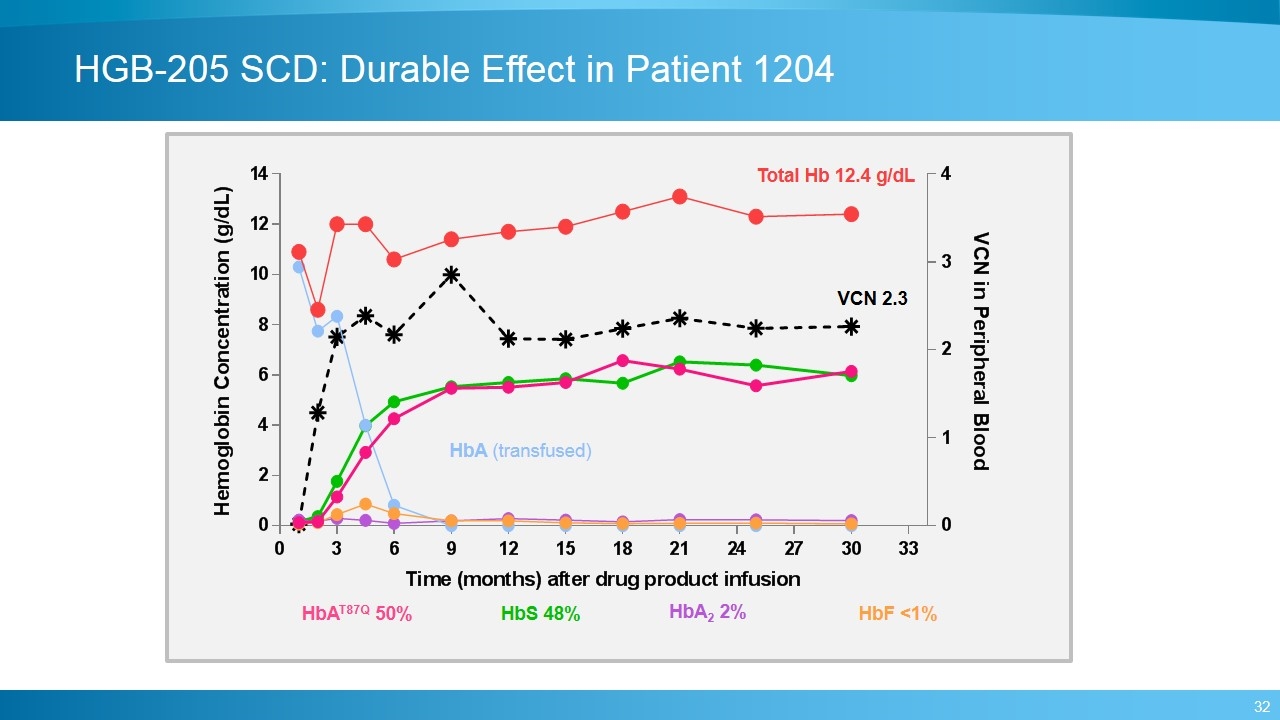
HGB-205 SCD: Durable Effect in Patient 1204 Total Hb 12.4 g/dL VCN 2.3 HbA (transfused) HbS 48% HbA2 2% HbF <1% HbAT87Q 50%
HGB-205 SCD: Safety and Clinical Status in Severe SCD Patients Adverse events generally consistent with myeloablative conditioning No drug-product related non-hematologic AEs or SAEs No replication competent lentivirus (RCL) detected to date No evidence of insertional mutagenesis Patient 1204 One episode of acute gastroenteritis at 30 months post-treatment, with vomiting and 2 days of fever up to 40°C, followed by a vaso-occlusive crisis (VOC) and was hospitalized HbAT87Q and peripheral blood VCN levels have remained stable (HbAT87Q 6.1 g/dL, VCN 2.3 at 30 months) Patient 1207 Pre-treatment history of frequent episodes of VOC and acute chest syndrome (ACS) despite hydroxyurea prior to beginning regular transfusions. Experienced an episode of ACS 6 months post-treatment and was hospitalized. HbAT87Q continues to increase with 1.8 g/dL at 6 months.
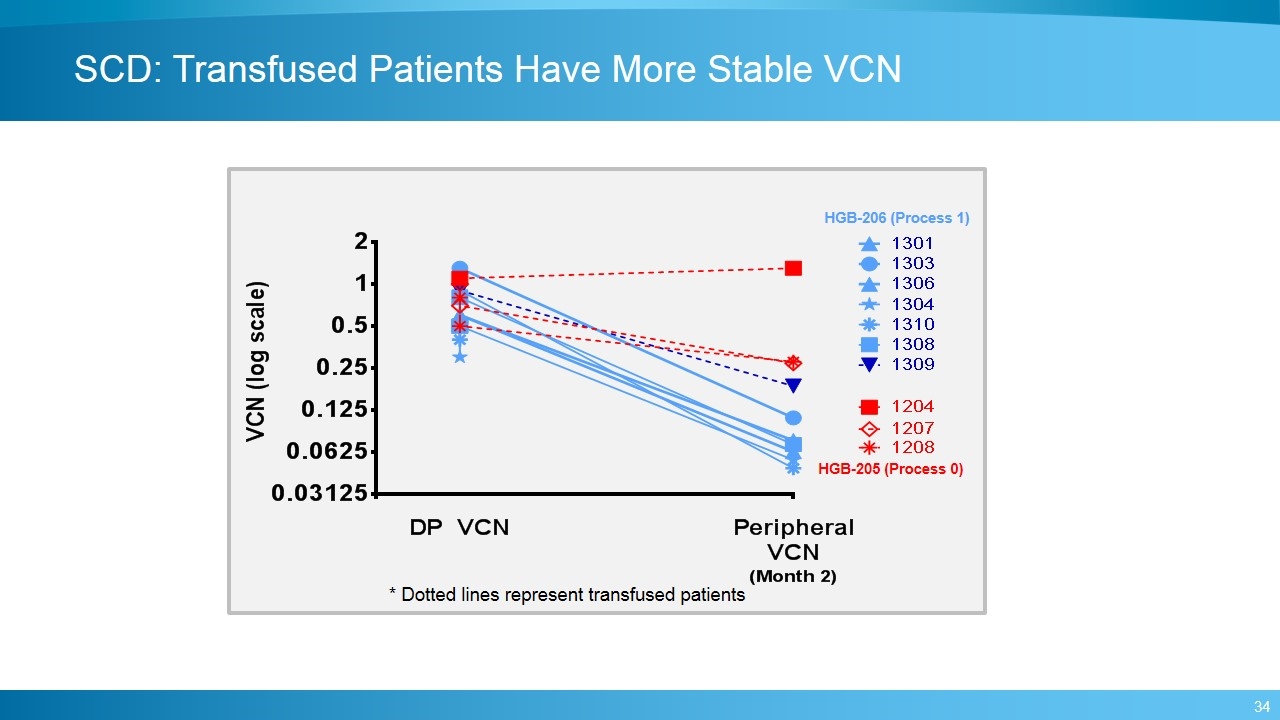
SCD: Transfused Patients Have More Stable VCN HGB-206 (Process 1) HGB-205 (Process 0) * Dotted lines represent transfused patients
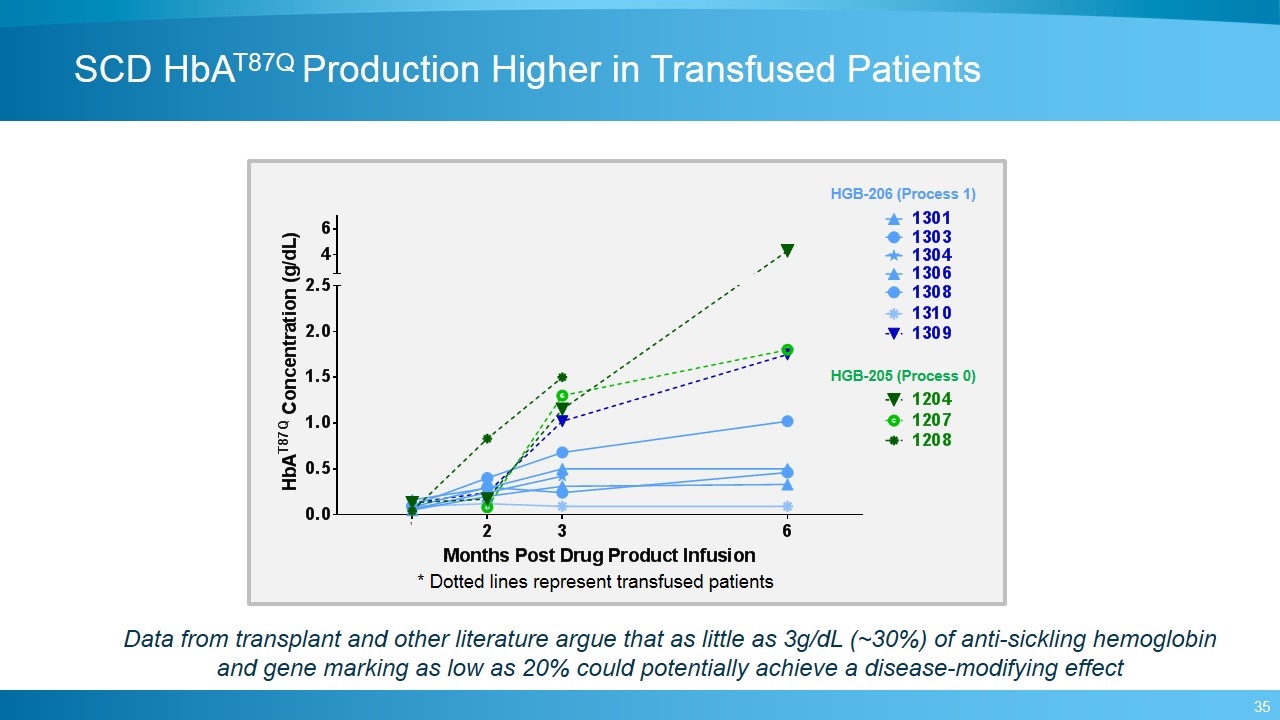
SCD HbAT87Q Production Higher in Transfused Patients HGB-206 (Process 1) HGB-205 (Process 0) Data from transplant and other literature argue that as little as 3g/dL (~30%) of anti-sickling hemoglobin and gene marking as low as 20% could potentially achieve a disease-modifying effect * Dotted lines represent transfused patients
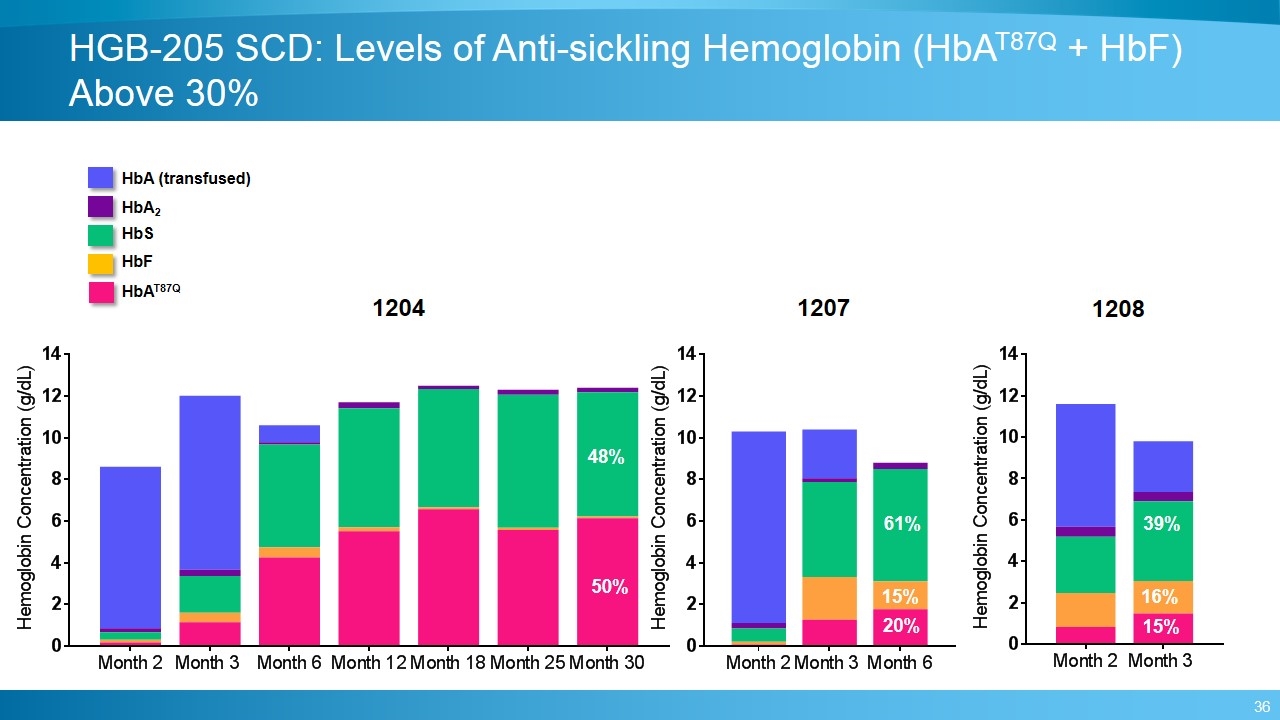
1204 1207 1208 50% 48% 20% 61% 15% 15% 39% 16% HbAT87Q HbF HbS HbA (transfused) HbA2 HGB-205 SCD: Levels of Anti-sickling Hemoglobin (HbAT87Q + HbF) Above 30%
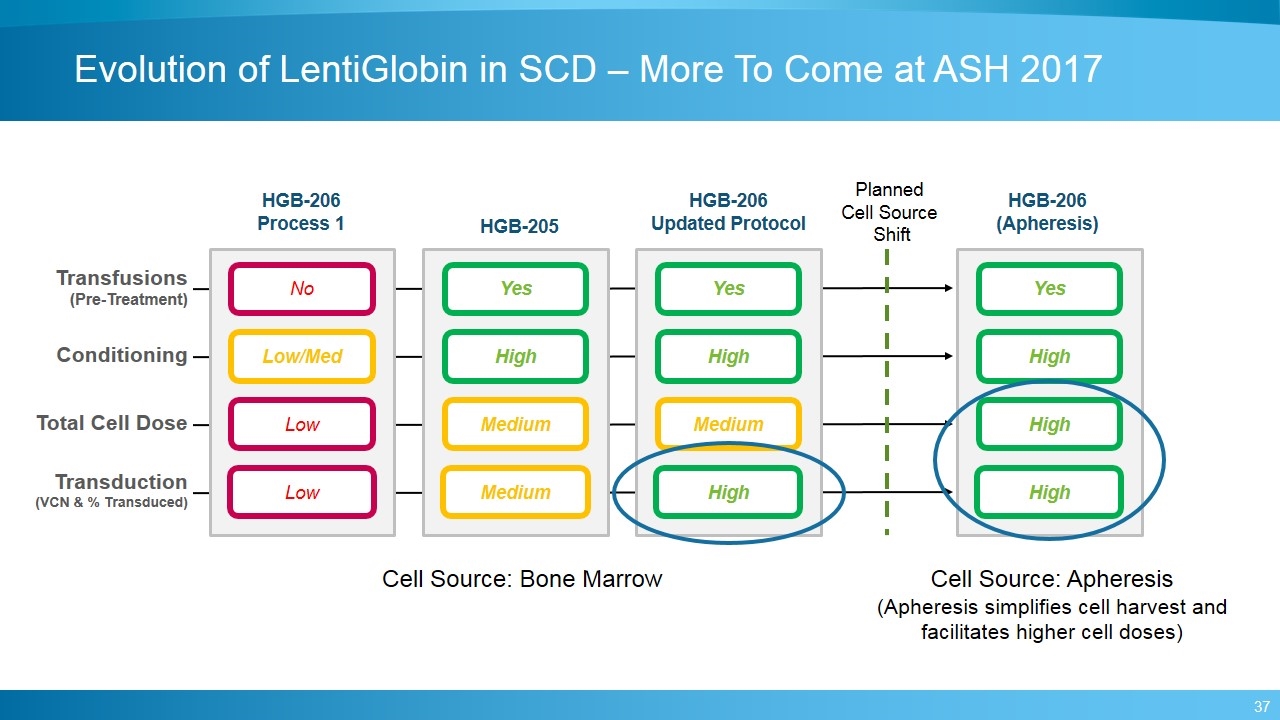
Evolution of LentiGlobin in SCD – More To Come at ASH 2017 HGB-206 Process 1 HGB-206 Updated Protocol HGB-206 (Apheresis) Low/Med Low Low No High Medium High Yes High High High Yes HGB-205 High Medium Medium Yes Transfusions (Pre-Treatment) Conditioning Total Cell Dose Transduction (VCN & % Transduced) Planned Cell Source Shift Cell Source: Apheresis (Apheresis simplifies cell harvest and facilitates higher cell doses) Cell Source: Bone Marrow

Closing
Key Questions Transfusion-Dependent β-thalassemia (TDT): Northstar-2 and HGB-205 With our new manufacturing process in Northstar-2, are we able to consistently manufacture drug product (DP) with higher vector copy number (VCN) and proportion of transduced cells? How do the early results from Northstar-2… Compare to the results seen in non- β0/ β0 patients in HGB-204? Read through to β0/ β0 patients? Read through to SCD patients? What can we learn from the HGB-205 TDT patients? Severe Sickle Cell Disease (SCD) How do the data from the HGB-205 patients compare to ASH 2016 data? What does HGB-205 teach us regarding the potential impact of the protocol and manufacturing changes made in HGB-206?

Bringing & Valuing Hope Go TRUE BLUE We Must Make Hope a Reality







































