UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One) | | | | | | | | |
| | ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2022
OR
| | | | | | | | |
| | ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM ______ TO ______ |
Commission File Number: 001-32433
PRESTIGE CONSUMER HEALTHCARE INC.
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | |
| Delaware | | 20-1297589 |
(State or Other Jurisdiction of
Incorporation or Organization) | | (I.R.S. Employer Identification No.) |
660 White Plains Road
Tarrytown, New York 10591
(Address of Principal Executive Offices) (Zip Code)
(914) 524-6800 | | |
| (Registrant's Telephone Number, Including Area Code) |
|
| | | | | | | | |
| Securities registered pursuant to Section 12(b) of the Act: |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock, par value $0.01 per share | PBH | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | | | | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ | | | | Smaller reporting company | ☐ |
| | | | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the Registrant’s most recently completed second fiscal quarter ended September 30, 2021 was $2,798.6 million.
As of May 2, 2022, the registrant had 50,279,419 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Definitive Proxy Statement for the 2022 Annual Meeting of Stockholders (the “2022 Proxy Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent described herein.
TABLE OF CONTENTS | | | | | | | | |
| | | |
| | | Page |
| Part I | | |
| Item 1. | Business | |
| Item 1A. | Risk Factors | |
| Item 1B. | Unresolved Staff Comments | |
| Item 2. | Properties | |
| Item 3. | Legal Proceedings | |
| Item 4. | Mine Safety Disclosures | |
| | | |
| Part II | | |
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
| Item 6. | (Reserved) | |
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | |
| Item 8. | Financial Statements and Supplementary Data | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | |
| Item 9A. | Controls and Procedures | |
| Item 9B. | Other Information | |
| | | |
| Part III | | |
| Item 10. | Directors, Executive Officers and Corporate Governance | |
| Item 11. | Executive Compensation | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | |
| Item 14. | Principal Accounting Fees and Services | |
| | | |
| Part IV | | |
| Item 15. | Exhibits, Financial Statement Schedules | |
| Item 16. | Form 10-K Summary | |
| | | |
| | TRADEMARKS AND TRADENAMES | |
| | Trademarks and tradenames used in this Annual Report on Form 10-K are the property of Prestige Consumer Healthcare Inc. or its subsidiaries, as the case may be. We have italicized our trademarks or tradenames when they appear in this Annual Report on Form 10-K. | |
Part I.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”), including, without limitation, information within Management’s Discussion and Analysis of Financial Condition and Results of Operations. The following cautionary statements are being made pursuant to the provisions of the PSLRA and with the intention of obtaining the benefits of the “safe harbor” provisions of the PSLRA.
Forward-looking statements speak only as of the date of this Annual Report on Form 10-K. Except as required under federal securities laws and the rules and regulations of the SEC, we do not intend to update any forward-looking statements to reflect events or circumstances arising after the date of this Annual Report on Form 10-K, whether as a result of new information, future events or otherwise. As a result of the risks and uncertainties described below, readers are cautioned not to place undue reliance on forward-looking statements included in this Annual Report on Form 10-K or that may be made elsewhere from time to time by, or on behalf of, us. All forward-looking statements attributable to us are expressly qualified by these cautionary statements.
These forward-looking statements generally can be identified by the use of words or phrases such as "believe," "anticipate," "expect," "estimate," "plan," "project," "intend," "strategy," "goal," "objective," "future," "seek," "may," "might," "should," "would," "will," "will be," or other similar words and phrases. Forward-looking statements are based on current expectations and assumptions that are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated, including, without limitation:
•Price increases for raw materials, labor, energy and transportation costs, and for other input costs;
•Disruptions of supply of sourced goods or parts;
•The impact of the COVID-19 pandemic or other disease outbreaks on global economic conditions, consumer demand, retailer product availability, and business operations including manufacturing, supply chain and distribution;
•The high level of competition in our industry and markets;
•Our inability to increase organic growth via new product introductions, line extensions, increased spending on advertising and marketing support, and other new sales and marketing strategies;
•Our dependence on a limited number of customers for a large portion of our sales;
•Our inability to successfully identify, negotiate, complete and integrate suitable acquisition candidates and to obtain necessary financing;
•Changes by retailers in inventory management practices, delivery requirements, and demands for marketing and promotional spending in order to retain or increase shelf space or online share;
•Our inability to grow our international sales;
•General economic conditions and incidence levels affecting sales of our products and their respective markets;
•Volatility in or worsening conditions from geopolitical conflicts, public health issues, and other factors beyond our control;
•Financial factors, such as increases in interest rates and currency exchange rate fluctuations;
•Changing consumer trends, additional store brand or branded competition, accelerating shifts to online shopping or pricing pressures;
•Our dependence on third-party manufacturers to produce many of the products we sell and our ability to transfer production to our own facilities or other third-party suppliers;
•Our dependence on third-party logistics providers to distribute our products to customers;
•Disruptions in our distribution center or manufacturing facility;
•Potential changes in export/import and trade laws, regulations and policies including any increased trade restrictions or tariffs;
•Acquisitions, dispositions or other strategic transactions diverting managerial resources, and creating additional liabilities;
•Actions of government agencies in connection with our manufacturing plant, products, advertising or regulatory matters governing our industry;
•Product liability claims, product recalls and related negative publicity;
•Our inability to protect our intellectual property rights;
•Our dependence on third parties for intellectual property relating to some of the products we sell;
•Our inability to protect our information technology systems from threats or disruptions;
•Our dependence on third-party information technology service providers and their ability to protect against security threats and disruptions;
•Our assets being comprised virtually entirely of goodwill and intangibles and possible changes in their value based on adverse operating results and/or changes in the discount rate used to value our brands;
•Our dependence on key personnel;
•The costs associated with any claims in litigation or arbitration and any adverse judgments rendered in such litigation or arbitration;
•Our level of indebtedness and possible inability to service our debt or to obtain additional financing;
•The restrictions imposed by our financing agreements on our operations; and
•Changes in federal, state and other geographic tax laws.
For more information, see “Risk Factors” contained in Part I, Item 1A of this Annual Report on Form 10-K.
ITEM 1. BUSINESS
Overview
Unless otherwise indicated by the context, all references in this Annual Report on Form 10-K to “we,” “us,” “our,” the “Company” or “Prestige” refer to Prestige Consumer Healthcare Inc. and our subsidiaries. Prior to August 17, 2018, the Company's name was Prestige Brands Holdings, Inc. Reference to a year (e.g., “2022”) refers to our fiscal year ended March 31 of that year.
We formed as a Delaware corporation in 1996 and are engaged in the development, manufacturing, marketing, sales and distribution of well-recognized, brand name, over-the-counter (“OTC”) health and personal care products to mass merchandisers, drug, food, dollar, convenience, club and e-commerce stores in North America (the United States and Canada) and in Australia and certain other international markets. We use the strength of our brands, our established retail distribution network, a low-cost operating model and our experienced management team to our competitive advantage. Our ultimate success is dependent on several factors, including our ability to:
•Develop and execute effective sales, advertising and marketing programs to maintain or grow our share versus competitors over time;
•Establish and maintain our third-party manufacturing and distribution relationships to fulfill customer demands;
•Develop innovative new products;
•Continue to grow our presence in the United States and international markets through acquisitions and organic growth; and;
•Allocate capital effectively.
We have grown our product portfolio both organically and through acquisitions. We develop our existing brands by investing in new product lines, brand extensions and strong advertising support. Acquisitions of consumer health and personal care brands have also been an important part of our growth strategy. We pursue this growth following an acquisition through spending on advertising and marketing support, new sales and marketing strategies, improved packaging and formulations and innovative development of brand extensions.
We conduct our operations in two reportable segments: North American OTC Healthcare and International OTC Healthcare.
Our business, business model, competitive strengths and growth strategy face various risks that are described in "Risk Factors" in Part I, Item 1A of this Annual Report on Form 10-K.
The following summarizes the percent of our net revenues by segment:
| | | | | | | | | | | | | | | | | | | | | |
| | March 31, | |
| (In thousands) | | 2022 | | 2021 | | 2020 | |
| Segment: | | | | | | | |
| North American OTC Healthcare | | 89.1 | % | | 90.0 | % | | 89.2 | % | |
| International OTC Healthcare | | 10.9 | | | 10.0 | | | 10.8 | | |
| | | | | | | |
| Total | | 100.0 | % | | 100.0 | % | | 100.0 | % | |
For additional information concerning our business segments, please refer to Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Note 19 to the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
Major Brands and Market Position
Our major brands, set forth in the table below, have strong levels of consumer awareness and retail distribution across all major channels. These brands accounted for approximately 81.4%, 80.3%, and 80.5% of our total revenues for 2022, 2021, and 2020, respectively. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Major Brands | | Product Group | | Market Position (1) | | Market Segment (2) | | Brand Information | | | | |
North American OTC Healthcare: (3) | | | | | | | | | | |
| BC and Goody's | | Analgesics | | #1 | | Analgesic Powders | | Developed over 90 years ago, BC and Goody’s provide fast pain relief at the speed of powder | | | | |
| Boudreaux's Butt Paste | | Dermatologicals | | #3 | | Baby Ointments | | Products include various diaper rash treatments and skin protectants manufactured with high-quality ingredients | | | | |
| Chloraseptic | | Cough & Cold | | #1 | | Sore Throat Liquids and Lozenges (Medicated) | | Products include sprays and lozenges to relieve sore throats and mouth pain | | | | |
| Clear Eyes | | Eye & Ear Care | | #1 | | Redness Relief | | Effective line of eye care products that provide soothing comfort, including relief from redness and itchiness | | | | |
| Compound W | | Dermatologicals | | #1 | | Wart Removal | | Provides safe and effective at-home removal of common and plantar warts | | | | |
| Debrox | | Eye & Ear Care | | #1 | | Ear Wax Removal | | Provides a safe and gentle way to remove excess ear wax or water from ear canal | | | | |
| DenTek | | Oral Care | | #3 | | PEG Oral Care | | Products include dental guards, floss picks, interdental brushes, dental repair and kits, and tongue cleaners | | | | |
| Dramamine | | Gastrointestinal | | #1 | | Motion Sickness Relief | | Includes non-drowsy, kids', original and nausea-free formulas | | | | |
| Fleet | | Gastrointestinal | | #1 | | Adult Enemas and Suppositories | | Founded in 1869, products include enemas and other laxative products | | | | |
| Gaviscon | | Gastrointestinal | | #1 | | Upset Stomach Remedies | | Creates a protective foam barrier to help block stomach acid from splashing up into the esophagus | | | | |
| Luden's | | Cough & Cold | | #3 | | Cough Drops
(Non-Medicated) | | Cough drop brand that is over 130 years old and includes a variety of flavors | | | | |
| Monistat | | Women's Health | | #1 | | Vaginal Anti-Fungal | | Provides fast relief for yeast infections and is available in several different doses | | | | |
| Nix | | Dermatologicals | | #1 | | Lice and Parasite Treatments | | Effective and safe lice and super lice treatments | | | | |
| Summer's Eve | | Women's Health | | #1 | | Feminine Hygiene | | Offers a variety of feminine care products including washes, cloths, and sprays | | | | |
| TheraTears | | Eye & Ear Care | | #3 | | Dry Eye Relief | | Doctor created and recommended therapy for relief of dry eyes | | | | |
International OTC Healthcare: | | | | | | | | | | |
| Fess | | Cough & Cold | | #1 | | Nasal Saline Sprays and Washes | | Helps relieve nasal and sinus congestion due to allergy, hay fever, colds and flu
| | | | |
| Hydralyte | | Gastrointestinal | | #1 | | Oral Rehydration | | Relieves symptoms of dehydration and helps replace water and electrolytes lost due to vomiting, diarrhea, heavy sweating, vigorous exercise and occasional hangovers | | | | |
(1)We have prepared the information included in this Annual Report on Form 10-K with regard to the market position for our brands based in part on data generated by Information Resources, Inc. (“IRI”), for the 52-week period ended March 20, 2022. International information was derived from several sources. Fess and Hydralyte data are for the Australian market.
(2)“Market segment” is defined by us and is either a standard IRI category or a segment within a standard IRI category and is based on our product offerings and the categories in which we compete.
(3)Some brands in the North American OTC Healthcare segment are also sold in the International OTC Healthcare segment.
Our products are sold through multiple channels, including mass merchandisers, drug, food, dollar, convenience, club and e-commerce stores, which reduces our exposure to any single distribution channel.
Market Position
During 2022, approximately 67.5% of our total revenues were from major brands with a number one market position, compared with approximately 69.2% and 69.4% of total revenues during 2021 and 2020, respectively. In 2022, these brands included BC and Goody's, Chloraseptic, Clear Eyes, Compound W, Debrox, Dramamine, Fess, Fleet, Gaviscon, Hydralyte, Monistat, Nix, and Summer's Eve.
Competitive Strengths and Growth Strategy
We believe that our product portfolio is positioned for long-term growth based on the following factors:
Diversified Portfolio of Well-Recognized and Established Consumer Brands
We own and market a diverse portfolio of well-recognized consumer brands, some of which were established over 100 years ago. Our diverse portfolio of products provides us with multiple sources of growth and minimizes our reliance on any one product or category. We provide significant marketing support to our portfolio, which is designed to enhance our sales growth and our long-term profitability across our major brands and other significant brands, sometimes referred to as core brands.
Strong Competitor in Attractive Categories
We compete in product categories that address recurring consumer needs. We believe we are well positioned in these categories due to the long history and consumer awareness of our brands, our strong market positions, and our low-cost operating model. The markets in which we sell our products, however, are highly competitive and include numerous national and global manufacturers, distributors, marketers and retailers. As a result, any one or more of our brands could suffer a decline in market position or sales.
Proven Ability to Develop and Introduce New Products
We focus our marketing and product development efforts on the identification of under-served consumer needs, the design of products that directly address those needs, and the ability to extend our highly recognizable brand names to other products. One of our strategies is to broaden the categories in which we participate and increase our share within those categories through ongoing product innovation. As an example of this philosophy, in 2022 we launched a number of new products, including DenTek Fresh Protect Dental Guards, Dramamine Nausea Ginger Chews, BC Max Strength Lemonade-flavored powder, Summer’s Eve Spa Luxurious Wash and Clear Eyes Sensitive. In 2021, we launched Summer's Eve Active Chafe Gel and Sprays, Monistat Feminine Cleanser, and Compound W Gel Plus ConSeal Patches. While there is always a risk that sales of existing products may be reduced by new product introductions, our goal is to grow the overall sales of our brands.
Investments in Advertising and Marketing
We invest in advertising and marketing to drive the growth of our core brands. Our marketing strategy is focused primarily on consumer-oriented initiatives that target consumers via mass media, digital marketing, in-store programming and coupons. While the absolute level of marketing expenditures differs by brand and category, we have often increased the amount of investment in our brands after acquiring them.
Increasing Distribution Across Multiple Channels
Our broad distribution base attempts to ensure that our products are well positioned across all available channels and that we are able to participate in changing consumer retail trends. In an effort to ensure continued sales growth, we continue to focus on expanding our reliance on direct sales while reducing our reliance on brokers for our customers, other than our top 25 customers.
Pursuing Strategic Acquisitions
Acquisitions are a part of our overall strategy for growing revenue. We have a history of growth through acquisitions. In 2022, we acquired the consumer health business assets from Akorn Operating Company LLC. While we believe that there will continue to be a pipeline of acquisition candidates for us to investigate, strategic fit, availability of capital and relative cost are of the utmost importance in our decision to pursue such opportunities. We believe our business model allows us to integrate acquisitions in an efficient manner, while also providing opportunities to realize significant cost savings.
Growing Our International Business
International sales beyond the borders of North America represented 10.9%, 10.0% and 10.8% of total revenues in 2022, 2021, and 2020, respectively. We have designed and developed both products and packaging for specific international markets and expect that our international revenues as a proportion of our total revenues will continue to grow over the long-term.
A number of our brands have previously been sold internationally, and we seek to expand the number of brands sold through our existing international distribution network and continue to identify additional distribution partners for further expansion of our brands into other international markets.
Efficient Operating Model
To gain operating efficiencies, we oversee the production planning and quality control aspects of the manufacturing, warehousing and distribution of our products, while we primarily outsource the operating elements of these functions to well-established third-party providers. This approach allows us to benefit from their core competencies and maintain a highly variable cost structure with low overhead, limited working capital requirements, and minimal investment in capital expenditures.
Management Team with Proven Ability to Acquire, Integrate and Grow Brands
Our business has grown through acquisition and expansion of the many brands we have purchased as a result of the efforts of our experienced management team. Our management team has significant experience in consumer product marketing, sales, legal and regulatory compliance, product development and customer service. We rely on experienced personnel to bear the substantial responsibility of brand management and to effectuate our growth strategy.
Marketing and Sales
Our marketing strategy is based primarily on the acquisition and renovation of established consumer brands that possess what we believe to be significant brand value and unrealized potential and to grow categories with existing brands where we have leading market positions. Our marketing objective is to increase sales and market share by developing innovative new products and line extensions and executing creative and cost-effective advertising and marketing programs. This brand-building process involves the evaluation of the existing brand name, the development and introduction of innovative new products, and the execution of marketing support programs. Brand priorities will vary from year-to-year. Recognizing that financial resources are limited, we allocate our resources to focus on our core brands with the most impactful, consumer-relevant initiatives that we believe have the greatest opportunities for growth and financial success.
Customers
Our senior management team and dedicated sales force strive to maintain long-standing relationships with our top 25 domestic customers. We also contract with third-party sales management enterprises that interface directly with many of our remaining customers and report directly to members of our sales management team. In an effort to ensure continued sales growth, we continue to focus on expanding our reliance on direct sales while reducing our reliance on brokers for our non-top 25 customers.
We enjoy broad distribution across each of the major retail channels, including mass merchandisers, drug, food, dollar, convenience, club and e-commerce stores. The following table sets forth the percentage of gross sales for our domestic customers across our six major distribution channels during each of the past three years ended March 31:
| | | | | | | | | | | | | | | | | |
| | Percentage of Gross Sales(1) |
| Channel of Distribution | 2022 | | 2021 | | 2020 |
| Mass | 34.3 | | | 34.5 | | | 36.5 | |
| Drug | 25.4 | | | 22.4 | | | 25.6 | |
| Food | 13.9 | | | 15.0 | | | 15.4 | |
| Dollar | 6.3 | | | 7.7 | | | 6.6 | |
| Convenience | 3.5 | | | 3.2 | | | 3.9 | |
| Club | 1.6 | | | 1.8 | | | 1.4 | |
Other (2) | 15.0 | | | 15.4 | | | 10.6 | |
(1)Includes estimates for some of our wholesale customers that service more than one distribution channel.
(2)Includes e-commerce retailers such as Amazon.
Due to the diversity of our product lines, we believe that each of these channels is important to our business, and we continue to seek opportunities for growth in each channel.
We believe that our emphasis on strong customer relationships, speed and flexibility and leading sales technology capabilities, combined with consistent marketing support programs and ongoing product innovation, will continue to maximize our competitiveness in the increasingly complex retail environment.
During 2022, 2021, and 2020, Walmart accounted for approximately 20.5%, 21.6%, and 23.1%, respectively, of our gross revenues. We expect that for future periods, our top ten customers, including Walmart, will in the aggregate continue to account for a large portion of our sales.
Outsourcing and Manufacturing
In order to maximize our competitiveness and efficiently allocate our resources, third-party manufacturers fulfill most of our manufacturing needs. We have found that contract manufacturing often maximizes our flexibility and responsiveness to industry and consumer trends while minimizing the need for capital expenditures. We select contract manufacturers based on their core competencies and our perception of the best overall value, including factors such as (i) depth of services, (ii) professionalism and integrity of the management team, (iii) manufacturing agility, quality and capacity, (iv) regulatory compliance, and (v) competitive pricing. We require each of our suppliers, most of whom are based in the United States and Canada, to comply with our Supplier Code of Conduct, which sets forth the basic and minimal expectations that all suppliers must meet in order to do business with us. We also conduct thorough reviews of each potential manufacturer’s facilities, quality standards, capacity and financial stability. We generally purchase only finished products from our manufacturers.
Our primary contract manufacturers provide comprehensive services from product development through the manufacturing of finished goods. This approach results in minimal capital expenditures and maximizes our cash flow, which allows us to reinvest to support our marketing initiatives, fund brand acquisitions or repay outstanding indebtedness.
At March 31, 2022, we had relationships with 128 third-party manufacturers. Of those, we had long-term contracts with 23 manufacturers that produced items that accounted for approximately 69.0% of our gross sales for 2022, compared to 19 manufacturers with long-term contracts that accounted for approximately 70.5% of our gross sales in 2021. The fact that we do not have long-term contracts with certain manufacturers means that they could cease manufacturing our products at any time and for any reason or initiate arbitrary and costly price increases, which could have a material adverse effect on our business and results of operations. Although we are in the process of negotiating long-term contracts with certain key manufacturers, we may not be able to reach a timely agreement, which could have a material adverse effect on our business and results of operations.
Our long-term supply and manufacturing agreements explicitly outline the manufacturers’ obligations and product specifications with respect to the brand or brands being produced, including allocation of product liability risk. Pursuant to the terms of these agreements, the purchase price of products is subject to change due to fluctuations in input costs such as raw material, packaging components and labor costs.
Some of our other products are manufactured on a purchase order basis, which is generally based on batch sizes and results in no long-term obligations or commitments. To the extent we rely on purchase orders, rather than supply and manufacturing agreements, to govern our commercial relationships with suppliers, we typically rely on implied warranties with respect to the products manufactured, and we do not have specifically negotiated allocation of risk with these third-party manufacturers. With regard to our products both manufactured under long-term agreements and purchase orders, in periods of high inflation we have experienced and may continue to experience frequent increases in prices of products due to availability and fluctuations in input costs such as raw material, packaging components and labor costs.
In addition to relying on contract manufacturers, we operate a manufacturing facility in Lynchburg, Virginia, which manufactures products accounting for approximately 13% of our gross sales. We intend to move the manufacture of certain of our more highly regulated products to our facility in the future.
We believe that most of the raw materials and packaging components used to produce our products at our manufacturing facility in Virginia and at our third-party manufacturing facilities are generally available through multiple sources acquired on both a contract and purchase order basis but are also subject to inflationary pressure.
Warehousing and Distribution
We manage product distribution in the continental United States through one facility, which is owned and operated by GEODIS Logistics LLC ("GEODIS"), a third-party provider. We entered into an agreement with GEODIS in May 2019 and transitioned to this facility from our previous provider during fiscal 2020. GEODIS provides warehouse services including storage, handling and shipping, as well as transportation services, with respect to our full line of products, including (i) complete management services, (ii) carrier claims administration, (iii) proof of delivery, (iv) procurement, (v) report generation, and (vi) freight payment services.
Competition
The business of selling brand name consumer products in the OTC health and personal care market is highly competitive. This market includes numerous national and global manufacturers, distributors, marketers and retailers that actively compete for consumers’ business both in the United States and abroad. In addition, like most companies that market products in this category, we are experiencing continued competition from “private label” products introduced by major retail chains. While we believe that our branded products provide superior quality and benefits, we are unable to predict the extent to which consumers will purchase “private label” products as an alternative to branded products, although we expect that this could increase during an economic downturn or periods of high inflation.
Our principal competitors include AbbVie, Alcon, Bausch Health Companies Inc., Bayer, Combe, GlaxoSmithKline, Johnson & Johnson, Mondelez International, Reckitt Benckiser, Sanofi, Sunstar Group, The Honey Pot Company, and The Procter & Gamble Company.
We compete on the basis of numerous factors, including brand recognition, product quality, performance, value to customers, price, and product availability at the retail and e-commerce level. Advertising, marketing, merchandising and packaging, the timing of new product introductions, and line extensions also have a significant impact on customers’ buying decisions and, as a result, on our sales. The structure and quality of our sales force, as well as sell-through of our products, affect in-store and online positioning, wall display space and inventory levels for retail sale. Our markets are also highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market.
Many of the competitors noted above are larger and have substantially greater research and development and financial resources than we do, and may therefore have the ability to spend more aggressively and consistently on research and development, advertising and marketing, and to respond more effectively to changing business and economic conditions. See “Competitive Strengths” above for additional information regarding our competitive strengths and Part I, Item 1A “Risk Factors” below for additional information regarding competition in our industry.
Regulation
Product Regulation
The formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of our products are subject to extensive regulation by various U.S. federal agencies, including the U.S. Food and Drug Administration ("FDA"), the Federal Trade Commission ("FTC"), the Consumer Product Safety Commission (“CPSC”), and the Environmental Protection Agency (“EPA”), and various agencies of the states, localities and foreign countries in which our products are manufactured, marketed, distributed and sold. Our Regulatory and Quality team is guided by a senior member of management and staffed by individuals with appropriate quality, legal and regulatory experience. Our Regulatory, Quality and Operations teams work closely with our third-party manufacturers and our own manufacturing operation on quality-related matters. We monitor our own manufacturing operations and our third-party manufacturers' compliance with FDA and relevant foreign regulations and perform periodic audits to ensure compliance. This continual evaluation process is designed to ensure that our manufacturing processes and products are of high quality and in compliance with known regulatory and quality requirements. If the FDA or a foreign governmental authority chooses to audit a particular third-party manufacturing facility, we require the third-party manufacturer to notify us immediately and update us on the progress of the audit as it proceeds. If we or our manufacturers fail to comply with applicable regulations, we could become subject to significant claims or penalties or be required to discontinue the sale of the non-compliant products. In addition, the adoption of new regulations or changes in the interpretations of existing regulations may result in significant additional compliance costs or discontinuation of product sales.
Most of our U.S. OTC drug products are regulated pursuant to the FDA’s monograph system. The monographs set out the active ingredients and labeling indications that are permitted for certain broad categories of U.S. OTC drug products. When the FDA has finalized a particular monograph, it has concluded that a properly labeled product formulation is generally recognized as safe and effective and not misbranded. A tentative final monograph indicates that the FDA has not made a final determination about products in a category to establish safety and efficacy for a product and its uses. However, unless there is a serious safety or efficacy issue, the FDA typically will exercise enforcement discretion and permit companies to sell products conforming to a tentative final monograph until the final monograph is published. Products that comply with either final or tentative final monograph standards do not require pre-market approval from the FDA.
Certain of our U.S. OTC drug products are New Drug Application (“NDA”) or Abbreviated New Drug Application (“ANDA”) products and are manufactured and labeled in accordance with an FDA-approved submission. These products are subject to
reporting requirements as set forth in FDA regulations. Moving forward, OTC drug products will fall under the requirements of The Coronavirus Aid, Relief, and Economic Security Act ("CARES Act") signed into law on March 27, 2020, which includes the Over-the-Counter Monograph Safety, Innovation, and Reform Act. These new requirements are expected to be delineated by the FDA in 2023/2024.
Certain of our U.S. OTC Healthcare products are medical devices regulated by the FDA through a system that may involve pre-market clearance. During the review process, the FDA makes an affirmative determination as to the sufficiency of the label directions, cautions and warnings for the medical devices in question.
Certain of our products are considered cosmetics regulated by the FDA through the Federal Food, Drug, and Cosmetic Act ("FDC Act") and the Fair Packaging and Labeling Act. The FDA does not require pre-market clearance for cosmetics but manufacturers must ensure the products are not adulterated or misbranded.
In accordance with the FDC Act and FDA regulations, we and our third-party manufacturers of U.S. products must also comply with the FDA’s current Good Manufacturing Practices (“GMPs”). The FDA inspects our facilities and those of our third-party manufacturers periodically to determine that both we and our third-party manufacturers are complying with GMPs. We intend to move the manufacture of certain of our more regulated products to our own manufacturing facility, which will subject our facility to increased regulatory requirements and scrutiny with respect to both our existing and new operations there.
Our dietary supplement products are governed by the Dietary Supplement Health and Education Act of 1994 ("DSHEA"), which defines and regulates dietary supplements. Under DSHEA, supplements are also effectively regulated by the FDA for GMPs.
A number of our products are regulated by the CPSC under the Federal Hazardous Substances Act (“FHSA”), the Poison Prevention Packaging Act of 1970 (the “PPPA”) and the Consumer Products Safety Improvement Act of 2008 (“CPSIA”). In addition, a small number of our products are subject to regulation under the PPPA and can only be legally marketed if they are dispensed in child-resistant packaging or labeled for use in households where there are no children. The CPSIA requires us to make available to our customers certificates stating that we are in compliance with any applicable regulation administered by the CPSC.
Nix Lice Control Spray is considered a pesticide under the Federal Insecticide, Fungicide, and Rodenticide Act (“FIFRA”). Generally speaking, any substance intended for preventing, destroying, repelling, or mitigating any pest is considered to be a pesticide under FIFRA. Pesticides under FIFRA are required to be registered with the EPA and contain certain disclosures on the product labels. In addition, the contract manufacturers from which we source these products must be registered with the EPA. Our EPA registered products are also subject to state regulations and the rules and regulations of the various jurisdictions where these products are sold.
Our Canadian and international business is also subject to product regulations by local regulatory authorities in the various countries where these businesses operate, including regulations regarding manufacturing, labeling, marketing, distribution, sale and storage.
Sustainability and Environmental, Social and Governance (“ESG”) Regulations
We believe that sustainable operations are both financially and operationally beneficial to our business, and critical to the health of the communities in which we operate. Our operations are subject to federal, state, local and foreign laws, rules and regulations relating to environmental concerns, including air emissions, wastewater discharges, solid and hazardous waste management activities, and the safety of our employees. We endeavor to take actions necessary to comply with such regulations, including periodic environmental and health and safety audits of our facilities. The audits, conducted by independent firms with expertise in environmental, health and safety compliance, include site visits as well as a review of documentary information, to determine compliance with such federal, state, local and foreign laws, rules and regulations. We seek to ensure responsible sourcing of our products and to improve our suppliers’ environmental, labor, health and safety and ethical practices through our Supplier Code of Conduct. We seek to minimize our resource footprint at our locations with a focus on managing waste, water and energy consumption.
Other Regulations
We are also subject to a variety of other regulations in various foreign markets, including regulations pertaining to import/export, antitrust and pharmacovigilance issues. To the extent we decide to commence or expand operations in additional
countries, we may be required to obtain an approval, license or certification from the country’s ministry of health or comparable agency. We must also comply with product labeling and packaging regulations that may vary from country to country. Government regulations in both our domestic and international markets can delay or prevent the introduction, or require the reformulation or withdrawal, of some of our products. Our failure to comply with these regulations can also result in a product being removed from sale in a particular market, either temporarily or permanently. In addition, we are subject to FTC and state regulations, as well as foreign regulations, relating to our product claims and advertising. If we fail to comply with these regulations, we could be subject to enforcement actions and the imposition of penalties.
Impact of Regulations
Compliance with these various regulations has an impact on capital expenditures, earnings and our competitive position. Additional or shifting governmental regulation has and in the future could require reformulation of certain products to meet new standards, recalls or discontinuance of certain products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, expanded adverse event reporting or other new requirements. Those changes have and will continue to require capital investments in facilities and equipment to meet the requirements, as well as additional product development, material and production costs which may impact our earnings, financial condition and ability to compete.
Intellectual Property
We own a number of trademark registrations and applications in the United States, Canada and other foreign countries. The following are some of the most significant registered trademarks we own in the United States and/or Canada: BC, Boudreaux's Butt Paste, Chloraseptic, Clear Eyes, Compound W, Debrox, DenTek, Dramamine, Fleet, Gaviscon, Goody's, Hydralyte, Luden's, Monistat, Nix, Summer's Eve, Fess and TheraTears.
Our trademarks and tradenames are how we convey that the products we sell are “brand name” products. Our ownership of these trademarks and tradenames is very important to our business, as it allows us to compete based on the value and goodwill associated with these marks. Additionally, we own or license patents on innovative and proprietary technology. The patents evidence the unique nature of our products, provide us with exclusivity, and afford us protection from the encroachment of others. None of the patents that we own or license, however, is material to us on a consolidated basis. Enforcing our rights, or the rights of any of our licensors, represented by these trademarks, tradenames and patents is critical to our business and may require significant expense. If we are not able to effectively enforce our rights, others may be able to dilute our trademarks, tradenames and patents and diminish the value associated with our brands and technologies.
We do not own all of the intellectual property rights applicable to our products. In those cases where our third-party manufacturers own patents that protect our products, we are dependent on them as a source of supply for our products. In addition, we rely on our suppliers for their enforcement of their intellectual property rights against infringing products.
Seasonality
The first quarter of our fiscal year generally is the least profitable quarter due to the increased advertising and marketing spending to support those brands with a summer selling season, such as Clear Eyes products and Compound W, and generally the lowest level of sales attributable to multiple factors. The effectiveness of advertising and marketing campaigns in the third quarter influences sales of products such as Chloraseptic, Little Remedies, and Luden's during the fourth quarter cough and cold winter months. Additionally, the fourth quarter typically has the lowest level of advertising and marketing spending as a percent of revenue.
Human Capital Management
Our Culture & Diversity
Our mission is to deliver high-quality consumer health and personal care products that improve and enrich the lives of our consumers. Our Company culture is founded on the principles of Leadership, Trust, Change and Execution. Of those principles, Trust is among the most important. Trust in the safety and performance of our products, the integrity of our manufacturing and marketing processes, the character of our people, and the benefit to our consumers and society. We also reward employees who take ownership and embody our principle of Leadership with projects that positively impact our business, community and stakeholders.
We take pride in the wide range of backgrounds, races, nationalities, personalities, ideas, and talents that make up our organization. We continue to build on our long commitment of equal employment opportunity and anti-discrimination by consciously and proactively promoting inclusion and equity of people with unique cultural and ethnic heritage, color and backgrounds, gender identification and sexual orientation, and other traits. We endeavor to facilitate the acquisition of diverse attitudes, skills and talents particularly for future leadership roles through hiring, workplace practices and employee
development. We strive to create and sustain an environment where all employees are heard and inspired to achieve their full potential. We continually review our Company employee demographics to help us adhere to these principles.
We also believe in working productively with one another and with our stakeholders to ensure long-term success. Some of the ways we encourage this is by:
•Recruiting: With employees across the U.S. and the world, we understand the importance of hiring and advancement practices that ensure diversity and equality at all levels of the organization as well as talent development.
•Monitoring: We have a strict Code of Conduct and Ethics that fosters a work environment that is free from intimidation, harassment and violence. Our team employs a process to investigate and resolve any potential conduct or ethics concern that may violate our mission of diversity and inclusion. We use a third-party reporting avenue for employees to exercise any such concern with anonymity and confidentiality. Raising a concern honestly or participating in an investigation cannot be the basis for any adverse employment action, including termination, suspension, loss of benefits, threats, harassment or discrimination.
Our Employees
As of March 31, 2022, we had approximately 535 global employees. Approximately 90% of our workforce operates in the United States, 9% in Australia and Asia and 1% in Europe. 60% of our employees are salaried and 40% are paid hourly wages, mostly in production. We employ only a few part time employees. Our workforce is 52% female and 48% male. None of our employees are a party to a collective bargaining agreement. Management believes that our relations with employees are good.
Strategic Development and Empowerment
We encourage all employees to achieve their full potential by participating in our mentorship opportunities, career development programs and Company-provided learning tools. We provide meaningful responsibility and development opportunities to our employees worldwide. We employ a performance management process under which all employees receive reviews that not only assess performance, but identify specific developmental opportunities and learning goals for the individual. By empowering our employees to develop and enhance their skills through enterprise-wide tools, videos and coursework that focus on continuous learning and professional and personal development, we help all of our employees reach their full potential, which in turn helps our organization succeed.
Health and Safety
We are committed to providing a safe work environment for our employees and require employees to share this concern by abiding to rigorous safety measures. To enable this and assure that the message of health, safety and well-being are part of our work culture, we conduct regular training programs at our production facility. We seek to comply with all federal, state and/or local occupational safety and health standards and report our safety records in accordance with the Occupational Safety and Health Administration ("OSHA").
In March 2020, we implemented protocols in response to the COVID-19 pandemic across all locations, in an effort to ensure both the safety of our employees and compliance with federal and local requirements and guidelines. These protocols continued through fiscal 2022. During the pandemic, our dedicated production employees at our manufacturing facility in Lynchburg, VA continued to operate three shifts a day with enhanced safety and health measures in place to protect their well-being. Our office-based employees were initially transitioned to a remote workforce but later returned to the office with appropriate safeguards to protect their health and well-being. These measures have allowed employees the flexibility to attend to other unique personal and family needs brought on by the pandemic.
Our Community
We are a responsible corporate citizen and we resolve to live by our principles as we continue to grow our global business. We seek out opportunities to be active members of our communities to enhance the lives of our neighbors and consumers. We encourage employees to become involved in their respective communities, and we enable office locations the freedom to develop programs that are appropriate to their community needs. For example, our corporate headquarters office location traditionally has an annual “Day of Giving” where employees spend a day every December giving back to the nearby communities, while other office locations support their communities through various volunteerism events. With the pandemic and employees working remotely, our charitable efforts this year focused on feeding those in need in the communities where employees live and a Company contribution to Feeding Westchester, a nonprofit hunger relief network of food banks across the County of Westchester, NY.
Further information surrounding our Company’s human capital development and sustainability efforts are available on our Company’s website at https://www.prestigebrands.com/about-us/corporate-responsibility.
Economic Environment
In March 2020, the World Health Organization ("WHO") declared a global pandemic due to a new strain of coronavirus ("COVID-19"). The pandemic has caused significant volatility in the United States and global economies. In addition, the Russian invasion of Ukraine has led to further economic uncertainty. We expect economic conditions will continue to be highly volatile and uncertain and could affect demand for our products and put pressure on prices. We experienced a temporary but significant decline in consumer consumption of our brands in the first quarter of fiscal 2021, followed by more stable consumption and customer orders over the remainder of the year. Generally, throughout the pandemic, some categories were positively impacted (for instance, Women’s Health, Oral Care and Dermatological) and some categories negatively impacted (for instance, Cough & Cold and Gastrointestinal). The positively impacted categories benefited from the consumer shift to over-the-counter healthcare products, as consumers increased their focus on hygiene and self-care at home related to COVID-19. The declining categories were impacted by reduced incidence levels and usage rates due to shelter-at-home restrictions and limited travel-related activity. In fiscal 2022, we experienced solid consumer consumption and share gains across most of our brand portfolio. Our business also benefited from a significant increase in demand in travel-related categories and channels as well as the Cough & Cold category, previously impacted by the COVID-19 virus. We have continued to see changes in the purchasing patterns of our consumers, including the frequency of visits by consumers to retailers and a shift in many markets to purchasing our products online.
Both the COVID-19 pandemic and the geopolitical environment have also impacted the supply of labor and raw materials and exacerbated rising costs. Although we have not experienced a material disruption to our overall supply chain to date, we have and may continue to experience delays and backorders for certain ingredients and products, difficulty scheduling shipping for our products, as well as price increases from many of our suppliers for both shipping and product costs. In addition, labor shortages have impacted our manufacturing operations and may impact our ability to supply certain products to our customers. To date, the pandemic and other global conditions have not had a material negative impact on our operations, supply chain, overall costs or demand for most of our products or resulting aggregate sales and earnings, and, as such, it has also not negatively impacted our liquidity position. We continue to generate operating cash flows to meet our short-term liquidity needs. These circumstances could change, however, in this dynamic, unprecedented environment. If the COVID-19 outbreak worsens or geopolitical conditions cause further disruption in the global supply chain, the availability of labor or otherwise increase costs, it may materially affect our operations and those of third parties on which we rely, including causing disruptions in the supply and distribution of our products. The extent to which these conditions impact our results and liquidity will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity and duration of any further COVID-19 outbreak and recovery period and further global instability. These effects could have a material adverse impact on our business, liquidity, capital resources, and results of operations and those of the third parties on which we rely.
Available Information
Our Internet address is www.prestigeconsumerhealthcare.com. We make available free of charge on or through our Internet website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports, as well as the Proxy Statement for our annual stockholders’ meetings, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (the “SEC”). Information on our Internet website does not constitute a part of this Annual Report on Form 10-K and is not incorporated herein by reference, including any general statement incorporating by reference this Annual Report on Form 10-K into any filing under the Securities Act of 1933, as amended (the “Securities Act”), or under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
You may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
We have adopted a Code of Conduct and Ethics Policy, Code of Ethics for Senior Financial Employees, Policy and Procedures for Complaints Regarding Accounting, Internal Controls and Auditing Matters, Corporate Governance Guidelines, Audit Committee Pre-Approval Policy, and Charters for our Audit, Compensation and Nominating and Corporate Governance Committees, as well as a Related Persons Transaction Policy and Stock Ownership Guidelines. We will provide to any person without charge, upon request, a copy of the foregoing materials. Any requests for these documents from us should be made in writing to:
Prestige Consumer Healthcare Inc.
660 White Plains Road
Tarrytown, New York 10591
Attention: Secretary
We intend to disclose future amendments to these documents, policies and guidelines and any waivers of these documents, policies and guidelines, on our Internet website and/or through the filing of a Current Report on Form 8-K with the SEC, to the extent required under the Exchange Act.
ITEM 1A. RISK FACTORS
Risks Related to our Business and Industry
Price increases for raw materials, labor, energy, transportation costs and other manufacturer, logistics provider or distributor demands could continue to have an adverse impact on our margins.
The costs to manufacture and distribute our products are subject to fluctuation based on a variety of factors. Volatility and increases in commodity raw material (e.g. resins), packaging component prices, and labor, energy, and transportation costs and other input costs, including as a result of supply chain issues or shortages, could significantly affect our profit margin and could have a significant impact on our financial condition and results of operations if our raw material suppliers, third-party manufacturers, logistics providers or distributors pass along those costs to us. Certain product categories have been impacted by higher inflation due to, among other things, the continuing impacts of the COVID-19 pandemic, Russia’s invasion of Ukraine, civil unrest, and related labor shortages, global supply chain disruptions and the uncertain economic and geopolitical environment which has impacted our gross margin in 2022.
The manufacturers we use have and may continue to increase the cost of many of the products we purchase, which could adversely affect our margins in the event we are unable to pass along these increased costs to our customers or identify and qualify new manufacturers. If we are unable to increase the price for our products to our customers or continue to achieve cost savings in a rising cost environment, any such cost increases would likely reduce our gross margins and could have a material adverse effect on our financial condition and results of operations. If we increase the price of our products in order to maintain our current gross margins for our products, the increase may adversely affect demand for, and sales of, our products, which could have a material adverse effect on our business, financial condition and results of operations.
Volatility in or worsening of economic conditions from geopolitical conflicts, public health issues, and other factors beyond our control could reduce consumer spending, which could adversely impact demand for our products and our results of operations and financial condition.
Our financial performance depends on the stability of conditions that impact consumer spending. Adverse conditions in or volatility in financial markets or the economy could adversely impact consumer confidence, resulting in reduced consumer spending leading to reduced consumption of our products. The COVID-19 pandemic and the Russian invasion of Ukraine have both caused volatility in the global economy, including by creating supply chain issues and rising costs. Other factors beyond our control could also adversely impact demand for our products, including rising interest rates, inflation from rising costs, unemployment, and the availability of consumer financing. Any reduction in consumer demand for our products could have a material adverse impact on our results of operations and financial condition.
The high level of competition in our industry, much of which comes from competitors with greater resources, could adversely affect our business, financial condition and results of operations.
The business of selling brand name consumer products in the OTC health and personal care market is highly competitive. This market includes numerous manufacturers, distributors, marketers and retailers that actively compete for consumers’ business both in the United States and abroad. Many of these competitors are larger and have substantially greater resources than we do, and may therefore have the ability to spend more aggressively on research and development and advertising and marketing, and to respond more effectively to changing business and economic conditions, including in connection with inflation or recessionary conditions. If this were to occur, it could have a material adverse effect on our financial condition and results of operations.
Certain of our product lines that account for a large percentage of our sales have a smaller market share relative to our competitors. In some cases, we may have a number one market position but still have a relatively small share of the overall market. Alternatively, we may hold a number two market position but have a substantially smaller share of the market versus the number one competitor. See “Part I, Item 1. Business - Major Brands” of this Annual Report on Form 10-K for information regarding market share.
We compete for consumers’ attention based on a number of factors, including brand recognition, product quality, performance, value to consumers, price and product availability at the retail level. Advertising, marketing, merchandising and packaging and the timing of new product introductions and line extensions also have a significant impact on consumer buying decisions and, as a result, on our sales. Our markets are highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. New product innovations by our competitors, or our failure to develop new products, the failure of a new product launch by the Company, or the obsolescence of one or more of our products, could have a material adverse
effect on our business, financial condition and results of operations. If our advertising, marketing and promotional programs are not effective, our sales may decline.
The structure and quality of our sales force, as well as sell-through of our products, affect in-store and our e-commerce product position, wall display space and inventory levels for retail sale. If we are unable to maintain our current distribution network, product offerings for retail sale, inventory levels and in-store and online positioning of our products, our sales and operating results could be adversely affected.
In addition, competitors may attempt to gain market share by offering products at prices at or below those typically offered by us. The introduction or expansion of store brand products that compete with our products at a lower price point could in the future impact our sales and results of operations. This could be exacerbated by rising costs and other economic conditions that shift consumer demand to lower-priced products, as well as supply chain issues that result in reduced availability for our products. Competitive pricing may require us to reduce prices, which may result in lost revenue or a reduction of our profit margins. Future price adjustments by our competitors or our inability to react with price adjustments of our own could result in a loss of market share, which could have a material adverse effect on our financial condition and results of operations.
We depend on a limited number of customers with whom we have no long-term agreements for a large portion of our gross sales, and the loss of one or more of these customers or changes in their strategies and policies could reduce our gross sales and have a material adverse effect on our financial condition and results of operations.
During 2022, Walmart, which accounted for approximately 20.5% of our gross sales, was our only customer that accounted for more than 10% of our gross revenues. We expect that for future periods, our top ten customers, including Walmart, will, in the aggregate, continue to account for a large and potentially increasing portion of our sales. Many of our customers have sought to obtain lower pricing, logistics or other changes to the customer-supplier relationship. If we are unable to effectively respond to the demands of our customers, these customers could reduce their purchases of our products and increase their purchases of products from competitors. Reductions in inventory by our customers, the loss of one or more of our top customers, including as a result of consolidation in the retail industry, or any significant decrease in sales to these customers based on changes in their strategies or policies such as a reduction in the number of brands they carry, the amount of shelf space or positioning they dedicate to store brand products, inventory management, or a significant decrease in our retail display space or online positioning or in any of these customers’ stores, could reduce our sales and have a material adverse effect on our financial condition and results of operations.
In addition, our business is based primarily upon individual sales orders. We typically do not enter into long-term contracts with our customers. Accordingly, our customers could cease buying products or reduce the number of items they buy from us at any time and for any reason. The fact that we do not have long-term contracts with our customers means that we have no recourse in the event a customer no longer wants to purchase products from us or reduces the number of items purchased. If a significant number of our smaller customers, or any of our significant customers, elect not to purchase products from us, our financial condition and results of operations could be materially adversely affected.
We primarily depend on third-party manufacturers to produce the products we sell. If we are unable to maintain these manufacturing relationships or are unable to successfully transfer manufacturing to another third-party or our own manufacturing facility we may be unable to meet customer demand, and our business, sales and profitability could suffer as a result.
Many of our products are produced by a limited number of third-party manufacturers. Our ability to retain our current manufacturing relationships and engage in and successfully transition to new relationships or to our own manufacturing facility is critical to our ability to deliver quality products to our customers in a timely manner. Without adequate supplies of quality merchandise, our sales would decrease materially and our business would suffer. In the event that our primary third-party manufacturers are unable or unwilling to ship products to us in a timely manner, we would have to rely on secondary manufacturing relationships or, to the extent unavailable, identify and qualify new manufacturing relationships. Because of the unique manufacturing requirements of certain products, the Company may be unable to timely qualify new suppliers or at the quantities, quality and price levels needed. From time to time, certain of the Company's manufacturers have had difficulty meeting demand, which can and has caused shortages of our products. In such instances, we may not be able to identify or qualify secondary manufacturers for such products in a timely manner, and such manufacturers may not allocate sufficient capacity to allow us to meet our commitments to customers. In addition, identifying alternative manufacturers without adequate lead times may involve additional manufacturing expense, delay in production, or product disadvantage in the marketplace. In some instances, we may seek to transfer the manufacture of certain products to our own facilities, which may result in additional manufacturing expense, delay in production, additional regulatory requirements and other disruptions to our
business. In general, the consequences of not securing adequate, high quality and timely supplies of merchandise would negatively impact inventory levels, which could damage our reputation and result in lost customers and sales, and could have a material adverse effect on our business, financial condition and results of operations.
At March 31, 2022, we had relationships with 128 third-party manufacturers. Of those, we had long-term contracts with 23 manufacturers that produced items that accounted for approximately 69.0% of our gross sales for 2022, compared to 19 manufacturers with long-term contracts that produced approximately 70.5% of gross sales in 2021. The fact that we do not have long-term contracts with certain manufacturers means that they could cease manufacturing our products at any time and for any reason or initiate costly price increases, which could have a material adverse effect on our business and results of operations. Although we are in the process of negotiating long-term contracts with certain key manufacturers, we may not be able to reach a timely agreement, which could have a material adverse effect on our business and results of operations.
Disruption in our third-party distribution center or our Virginia manufacturing facility may prevent us from meeting customer demand, and our sales and profitability may suffer as a result.
Our product distribution in the United States is managed by a third-party through one primary distribution center in Clayton, Indiana, and we operate one manufacturing facility located in Lynchburg, Virginia, which manufactures many of the Summer's Eve and Fleet products, comprising approximately 13% of our gross revenues. A natural disaster, such as tornado, earthquake, flood, or fire, could damage our inventory and/or materially impair our ability to distribute our products to customers in a timely manner or at a reasonable cost. In addition, a serious disruption caused by performance or contractual issues with a third-party distribution manager, or labor shortages or contagious disease outbreaks or other public health emergencies at our distribution center or manufacturing facility could also materially impact our product distribution. Any disruption could result in increased costs, expense and/or shipping times, and could cause us to incur customer fees and penalties. We could also incur significantly higher costs and experience longer lead times should we be required to replace our distribution center, the third-party distribution managers or the manufacturing facility. As a result, any serious disruption could have a material adverse effect on our business, financial condition and results of operations.
The continuation of the COVID-19 pandemic, further outbreaks of COVID-19, or any future outbreak of other highly infectious diseases or public health emergencies could have a material adverse impact on our results of operations and financial condition.
Beginning in early 2020, the COVID-19 pandemic caused significant volatility in the global economy and resulted in materially reduced economic activity. Globally, numerous government orders and restrictions implemented to reduce the spread of COVID-19 required many businesses to temporarily close or limit operations and mandated that individuals substantially restrict daily activities, which adversely affected workforces, customers, and consumer sentiment, decreased consumer spending and increased unemployment.
Our operations are impacted by consumer spending levels, the availability of our products at retail stores or for online purchase, and our ability to manufacture and distribute products to our customers and consumers in an effective and efficient manner. Although the COVID-19 pandemic has not materially adversely impacted our business or our operations to date, we could experience a material adverse impact in future quarters if conditions worsen or if other pandemics occur. For example, sales have varied during the most restrictive phases of the COVID-19 pandemic, with some categories positively impacted (for instance, Women’s Health, Oral Care and Dermatological) and some categories negatively impacted (for instance, Cough & Cold, and Gastrointestinal). We have experienced adverse impacts from COVID-19, and we could experience adverse impacts from other pandemics, in a number of ways, including, but not limited to, the following:
•supply chain delays or disruptions due to closed supplier facilities or distribution centers, reduced workforces, scarcity of raw materials and scrutiny or embargoing of goods produced in infected areas;
•shutdown of our manufacturing facility due to illness or government order;
•reduced consumer demand for certain of our products as a result of the economic downturn or restrictions on in-person purchases;
•change in demand for or availability of our products as a result of retailers or distributors modifying their restocking, fulfillment, or shipping practices;
•decrease in our ability to develop innovative products due to reprioritization of suppliers and/or retailers;
•increase in working capital needs and/or an increase in trade accounts receivable write-offs as a result of increased financial pressures on our suppliers or customers;
•impairment in the carrying value of goodwill or intangible assets or a change in the useful life of definite-lived intangible assets from sustained changes in consumer purchasing behaviors, government restrictions, or financial results;
•increase in raw material and other input costs resulting from market volatility; and
•fluctuation in foreign currency exchange rates or interest rates resulting from market uncertainties.
While the COVID-19 pandemic has not negatively impacted our results of operations to date, the extent to which it, and any related global economic downturn, could affect our business, results of operations and financial condition in future quarters will depend on developments that are highly uncertain and cannot be predicted, including the severity and duration of any further outbreak and recovery period, the availability, acceptance and efficacy of vaccines, future actions taken by governmental authorities and other third parties in response to the pandemic, and the impact on our customers, employees and suppliers, distributors and other service providers. For example, we believe that government stimulus payments during the COVID-19 pandemic may have helped improve sales, and any termination or reduction of further stimulus funds may result in reduced consumer spending, which could adversely impact our results of operations. Accordingly, the ultimate impact on our financial condition and results of operations cannot be determined at this time. Nonetheless, we anticipate that it could adversely affect our results of operations and financial condition, including by negatively impacting demand for our products, restricting our operations and sales, marketing and distribution efforts, disrupting supply chain and manufacturing processes and other important business activities. Moreover, the effects of the COVID-19 pandemic could exacerbate the other risks described in this “Risk Factors” section of this Annual Report on Form 10-K.
Consumption trends for our products may not correlate to our results of operations.
We regularly review consumption levels for our brands to provide an indication of the strength of our expected results of operations. Total company consumption is based on domestic IRI multi-outlet + C-store retail sales for the relevant period, retail sales from other third parties for certain e-commerce sales in North America, Australia consumption based on IMS data, and other international net revenues as a proxy for consumption. Our calculation of consumption levels may not accurately reflect actual retail consumption given limitations of tracked data. In addition, many retailers have implemented inventory management strategies that include reductions in the amount of inventory they carry and related reductions in retail space and may continue such efforts in the future.
Product liability claims and product recalls and related negative publicity could adversely affect our sales and operating results.
We are dependent on consumers’ perception of the safety and quality of our products. Negative consumer perception may arise from product liability claims and product recalls, regardless of whether such claims or recalls involve us or our products. The mere publication of information asserting concerns about the safety of our products or the ingredients used in our products could have a material adverse effect on our business and results of operations. We believe our products are safe and effective when used in accordance with label directions. However, adverse publicity about ingredients used in our products may discourage consumers from buying our products containing those ingredients, which would have an adverse impact on our sales.
From time to time we are subject to various product liability claims. Claims could be based on allegations that, among other things, our products contain contaminants, include inadequate instructions or warnings regarding their use, or include inadequate warnings concerning side effects and interactions with other substances. Whether or not successful, product liability claims could result in negative publicity that could adversely affect the reputation of our brands and our business, sales and operating results. Additionally, we may be required to pay for losses or injuries purportedly caused by our products. In addition, we could be required for a variety of reasons to initiate product recalls, which we have done on several occasions. Any product recalls could have a material adverse effect on our business, financial condition and results of operations.
Although we have supply and manufacturing agreements with certain of our third-party manufacturers, which explicitly outline the allocation of product liability risk with respect to the products these manufacturers produce, some of our other products are manufactured on a purchase order basis. To the extent we rely on purchase orders to govern our commercial relationships with suppliers, we have not specifically negotiated the allocation of risk for product liability obligations. Instead, we typically rely on implied warranties from the suppliers with respect to these products. As a result, we may have difficulty enforcing these implied warranties, and we may bear all or a significant portion of any product liability obligations rather than transferring this risk to our third-party manufacturers.
In addition, although we maintain, and require our suppliers and third-party manufacturers to maintain, product liability insurance coverage, potential product liability claims may exceed the amount of insurance coverage or may be excluded under the terms of the policy, which could have a material adverse effect on our financial condition. In addition, in the future we may not be able to obtain adequate product liability insurance coverage or we may be required to pay higher premiums and accept higher deductibles in order to secure adequate product liability insurance coverage.
Risks Related to Acquisitions and Product Development
Our inability to successfully identify, negotiate, complete and integrate suitable acquisition candidates and to obtain necessary financing could have an adverse impact on our growth and our business, financial condition and results of operations.
Achievement of our strategic objectives includes the acquisition, or potentially the disposition, of certain brands or product lines, and these acquisitions and dispositions may not be successful.
The majority of our historical growth has been driven by acquiring other brands and companies. At any given time, we may be engaged in discussions with respect to possible acquisitions that are intended to enhance our product portfolio, enable us to realize cost savings, and further diversify our category, customer and channel focus. Our ability to successfully grow through acquisitions depends on our ability to identify, negotiate, complete and integrate suitable acquisition candidates and to obtain any necessary financing. However, we may not be able to identify and successfully negotiate suitable strategic acquisitions at attractive valuations, obtain financing for future acquisitions on satisfactory terms, or otherwise complete future acquisitions. All acquisitions entail various risks such that after completing an acquisition, we may also experience:
•Difficulties in integrating any acquired companies, suppliers, personnel and products into our existing business;
•Difficulties in realizing the benefits of the acquired company or products, including expected returns, margins, synergies and profitability;
•Higher costs of integration than we anticipated;
•Exposure to unexpected liabilities of the acquired business;
•Difficulties in retaining key employees of the acquired business who are necessary to operate the business;
•Difficulties in maintaining uniform standards, controls, procedures and policies throughout our acquired companies; or
•Adverse customer or stockholder reaction to the acquisition.
As a result, any acquisitions we pursue or complete could adversely impact our business, financial condition and results from operations. In addition, any acquisition could adversely affect our operating results as a result of higher interest costs from any acquisition-related debt and higher amortization expenses related to the acquired intangible assets.
In the event that we decide to divest of a brand or product line, we may encounter difficulty finding, or be unable to find, a buyer on acceptable terms in a timely manner.
Additionally, the pursuit of acquisitions and divestitures could also divert management's attention from our business operations and result in a delay in our efforts to achieve our strategic objectives.
If new products and product line extensions do not gain widespread customer acceptance or are otherwise discontinued, the Company's financial performance could be impacted.
The Company's future performance and growth depends on its ability to successfully develop and introduce new products and product line extensions. We cannot be certain that we will achieve our innovation goals. The successful development and introduction of new products involves substantial research, development, marketing and promotional expenditures, which the Company may be unable to recover if the new products do not gain widespread market acceptance. New product development and marketing efforts, including efforts to enter markets or product categories in which the Company has limited or no prior experience, have inherent risks. These risks include product development or launch delays, competitor actions, regulatory approval hurdles, and the failure of new products and line extensions to achieve anticipated levels of market acceptance. A negative outcome in any of these risks could adversely impact our results of operations.
Regulatory Risks
We face risks associated with doing business internationally.
Approximately 11% of our total revenues are attributable to our international business. We generally rely on brokers and distributors for the sale of our products in foreign countries. In addition, some of our third-party manufacturers are located outside the United States. Risks of doing business internationally include, but are not limited to:
•Political instability or declining economic conditions in the countries or regions where we operate or rely on third-party manufacturers or suppliers that adversely affect sales of our products or our ability to obtain adequate supply of our products;
•Currency controls that restrict or prohibit the payment of funds or the repatriation of earnings to the United States;
•Fluctuating foreign exchange rates that result in unfavorable increases in the price of our products or cause increases in the cost of certain products purchased from our foreign third-party manufacturers;
•Compliance with laws and regulations concerning ethical business practices;
•Trade restrictions and exchange controls;
•Difficulties in staffing and managing international operations;
•Difficulty protecting our intellectual property rights in these markets; and
•Increased costs of compliance with general business and tax regulations in these countries or regions.
Our operations are dependent on foreign distributors and sales agents for compliance and adherence to foreign laws and regulations that we may not be familiar with, and we cannot be certain that these distributors and sales agents will adhere to such laws and regulations or adhere to our business practices and policies. Any violation of laws and regulations by foreign distributors or sales agents or a failure of foreign distributors or sales agents to comply with applicable business practices and policies could result in legal or regulatory sanctions or potentially damage our reputation. If we fail to manage these risks effectively, we may not be able to continue our international operations, and our business and results of operations may be materially adversely affected.
Regulatory matters governing our industry could have a significant negative effect on our sales and operating costs.
In both the United States and in our foreign markets, our operations are affected by extensive laws, governmental regulations, administrative determinations, court decisions and similar constraints. Such laws, regulations and other constraints exist at the federal, state and local levels in the United States and at analogous levels of government in foreign jurisdictions.
The formulation, manufacturing, packaging, labeling, distribution, importation, marketing, sale and storage of our products are subject to extensive regulation by various U.S. federal agencies, including the FDA, FTC and CPSC, the EPA, and by various agencies of the states, localities and foreign countries in which our products are manufactured, distributed, stored and sold. The FDC Act and FDA regulations require that the manufacturing processes of our facilities and third-party manufacturers of U.S. products must also comply with the FDA’s GMPs. The FDA inspects our facilities and those of our third-party manufacturers periodically to determine if we and our third-party manufacturers are complying with GMPs. The health regulatory bodies of other countries have their own regulations and standards, which may or may not be consistent with the U.S. FDA GMPs. In addition, our and our suppliers’ operations are subject to the oversight of the Occupational Safety and Health Administration and some suppliers by the National Labor Relations Board. Our activities are also regulated by various agencies of the states, localities and foreign countries in which our products and their constituent materials and components are manufactured and sold. We intend to move the manufacture of certain of our more highly regulated products to our own manufacturing facility, which will subject our facility to increased regulatory requirements and scrutiny with respect to both our existing and new operations there.
If we or our third-party manufacturers or distributors fail to comply with applicable regulations, we could become subject to enforcement actions, significant penalties or claims, which could materially adversely affect our business, financial condition and results of operations. In addition, we could be required to:
•Suspend manufacturing operations;
•Modify product formulations or processes;
•Suspend the sale or require a recall of non-compliant products; or
•Change product labeling, packaging, distribution, storage, marketing, or advertising, or take other corrective action.
The adoption of new regulations or changes in the interpretation of existing regulations may result in significant compliance costs or the cessation of product sales and may adversely affect the marketing of our products, which could have a material adverse effect on our financial condition and results of operations.
In addition, our failure to comply with FDA, FTC, EPA or any other federal and state regulations, or with similar regulations in foreign markets, that cover our product registration, product claims and advertising, including direct claims and advertising by us, may result in enforcement actions and imposition of penalties, litigation by private parties, or otherwise materially adversely affect the distribution and sale of our products, which could have a material adverse effect on our business, financial condition and results of operations.
We are subject to increasing focus on Environmental, Social and Governance (“ESG”) issues, including those related to climate change.
As climate change, land use, water use, deforestation, recyclability or recoverability of packaging, plastic waste, ingredients and other ESG and sustainability concerns become more prevalent, federal, state and local governments, non-governmental organizations and our customers, consumers and investors are increasingly focused on these issues. This increased focus on sustainability may result in new laws, regulations and requirements that could cause disruptions in or increased costs associated with developing, manufacturing and distributing our products. We could also lose revenue if our consumers change brands, our customers refuse to buy our products, or investors choose not to invest in our debt or common stock if we do not meet their ESG and sustainability expectations. For example, since 2020, some of our major customers requested we respond to various questionnaires to evaluate our ESG efforts. Efforts to meet these standards could impact our costs, and failure to meet our customers’ expectations could impact our sales and business reputation. While we strive to minimize the environmental impact of our global operations, we may experience reduced demand for our products and loss of customers if we do not meet their ESG expectations, which could result in a material adverse effect on our financial condition and results of operations.
Risks Related to Intellectual Property and Data Privacy and Security
If we are unable to protect our intellectual property rights, our ability to compete effectively in the market for our products could be negatively impacted.
The market for our products depends to a significant extent upon the goodwill associated with our trademarks, tradenames and patents. Our trademarks and tradenames convey that the products we sell are “brand name” products. We believe consumers ascribe value to our brands, some of which are over 100 years old. We own or license the material trademarks, tradenames and patents used in connection with the packaging, marketing and sale of our products. These rights prevent our competitors or new entrants to the market from using our valuable brand names and technologies. Therefore, trademark, tradename and patent protection is critical to our business. Although most of our material intellectual property is registered in the United States and in applicable foreign countries, we may not be successful in asserting protection. If we were to lose the exclusive right to use one or more of our intellectual property rights, the loss of such exclusive right could have a material adverse effect on our financial condition and results of operations.
In addition, other parties may infringe on our intellectual property rights and may thereby dilute the value of our brands in the marketplace. Brand dilution could cause confusion in the marketplace and adversely affect the value that consumers associate with our brands, which could negatively impact our business and sales. In addition, third parties may assert claims against our intellectual property rights, and we may not be able to successfully resolve those claims, which would cause us to lose the right to use the intellectual property subject to those claims. Such loss could have a material adverse effect on our financial condition and results of operations. Furthermore, from time to time, we may be involved in litigation in which we are enforcing or defending our intellectual property rights, which could require us to incur substantial fees and expenses and have a material adverse effect on our financial condition and results of operations.
We depend on third parties for intellectual property relating to some of the products we sell, and our inability to maintain or enter into future license agreements may result in our failure to meet customer demand, which would adversely affect our business and operating results.
We have licenses or manufacturing agreements with third parties that own intellectual property (e.g., formulae, copyrights, trademarks, trade dress, patents and other technology) used in the manufacture and sale of certain of our products. In the event that any such license or manufacturing agreement expires or is otherwise terminated, we will lose the right to use the intellectual property covered by such license or agreement and will have to develop or obtain rights to use other intellectual property. Similarly, our rights could be reduced if the applicable licensor or third-party manufacturer fails to maintain or protect the licensed intellectual property because, in such event, our competitors could obtain the right to use the intellectual property without restriction. If this were to occur, we might not be able to develop or obtain replacement intellectual property in a timely or cost-effective manner. Additionally, any modified products may not be well-received by customers. The consequences of losing the right to use or having reduced rights to such intellectual property could negatively impact our business and sales due to our failure to meet consumer demand for the affected products or require us to incur costs for the development of new or different intellectual property, either of which could have a material adverse effect on our business, financial condition and results of operations. In addition, development of replacement products may be time-consuming and ultimately may not be feasible.
Virtually all of our assets consist of goodwill and intangible assets and are subject to impairment risk.
As our financial statements indicate, the majority of our assets consist of goodwill and intangible assets, principally the trademarks, tradenames and patents that we have acquired. On an annual basis, and otherwise when there is evidence that events or changes in circumstances indicate that the carrying value of intangible assets might not be recoverable, we assess the potential impairment of our goodwill and other intangible assets. If any of our brands sustain significant or prolonged declines in revenues or profitability or performance not in line with our expectations, the carrying value may no longer be recoverable, in which case a non-cash impairment charge may be recorded. For example, if the Company’s brand performance is weaker than projections used in valuation calculations, the value of such brands may become impaired. In the event that such analysis would result in the fair value being lower than the carrying value, we would be required to record an impairment charge. A significant charge in our financial statements would negatively impact our financial condition and results of operations. Although we experienced revenue declines in certain brands and we recorded non-cash impairment charges in recent years for certain assets, we continue to believe that the fair value of our brands exceed their carrying values as adjusted. However, sustained or significant future declines in revenue, profitability, lost distribution, other adverse changes in expected operating
results, and/or unfavorable changes in economic factors used to estimate fair value of certain brands including the discount rate could indicate that the fair value no longer exceeds the carrying value, in which case a non-cash impairment charge may be recorded in future periods. Should the value of those assets or other assets become further impaired or our financial condition be materially adversely affected in any way, our tangible assets that could be sold to repay our liabilities would be reduced. As a result, our creditors and investors may not be able to recoup the amount of the indebtedness that they have extended to us or the amount they have invested in us.
We rely significantly on information technology. Any inadequacy, interruption, theft or loss of data, malicious attack, integration failure, failure to maintain the security, confidentiality or privacy of sensitive data residing on our systems or other security failure of that technology could harm our ability to effectively operate our business and damage the reputation of our brands.
We rely extensively on our information technology systems, some of which are managed by third-party service providers, to manage the data, communications and business processes for all of our functions, including our marketing, sales (including e-commerce), manufacturing, logistics, customer service, accounting and administrative functions. These systems include programs and processes relating to internal communications and communications with other parties, ordering and managing materials from suppliers, converting materials to finished products, marketing and selling products to customers (including through e-commerce channels), customer order entry and order fulfillment, shipping product to customers, billing customers and receiving and applying payment, processing transactions, summarizing and reporting results of operations, complying with regulatory, legal or tax requirements, collecting and storing customer, consumer, employee, investor, and other stakeholder information and personal data, and other processes necessary to manage the Company's business.
We, and certain of our suppliers, have been, and likely will continue to be, subject to malware, computer viruses, computer hacking, attempted acts of data theft, phishing, other cyber-attacks and employee error or malfeasance related to our information technology systems. We do not believe that any of these attacks or events has had a material adverse impact on our business, but future attacks could result in a serious information security breach and have a material adverse impact on our business or results of operations.
Increased information technology security threats and more sophisticated computer crime, including advanced persistent threats, pose a potential risk to the security of the information technology systems, networks, and services of the Company, its customers and business partners, as well as the confidentiality, availability, and integrity of the data of the Company, its customers and business partners. As a result, the Company's information technology systems, networks or service providers could be damaged or cease to function properly or the Company could suffer a loss or disclosure of business, personal or stakeholder information, due to any number of causes, including system disruptions, catastrophic events, power outages, cyber-attacks and security breaches. To help guard against these possibilities, the Company provides employee security training and maintains a compliance program with updated security policies to help evaluate and address potential threats and attacks. The Company has also conducted regular security audits by an outside firm based on the National Institute of Standards and Technology ("NIST") standards to address any potential service interruptions or vulnerabilities. Further, the Company has implemented continuity and recovery plans in the event of a disruption. However, if these plans do not provide effective protection, the Company may suffer interruptions in its ability to manage or conduct its operations, including in all of the Company’s functions described above, which may adversely affect its business and results of operations. The Company maintains security risk insurance in the event of a cybersecurity breach or incident; however, the coverage may not be sufficient to cover all losses. The Company may need to expend additional resources in the future to continue to protect against, or to address problems caused by, any business interruptions or data security breaches.
Any breach of our data security, including the failure to maintain the security of confidential data and information or the misappropriation of such confidential data and information, could result in an unauthorized release or transfer of customer, consumer, user or employee information, or the loss of valuable business data or cause a disruption in our business. These events could give rise to unwanted media attention, damage our reputation, damage our customer, consumer or user relationships and result in lost sales, fines, lawsuits, remediation costs, or otherwise adversely impact the Company's results of operations and financial condition. We may also be required to expend significant capital and other resources to protect against or respond to or alleviate problems caused by a security breach.
As we conduct our operations, we move data across national borders, and consequently we are subject to a variety of continuously evolving and developing laws and regulations in the United States and abroad regarding privacy, data protection and data security. The scope of the laws that may be applicable to us is often uncertain and may be conflicting, particularly with respect to foreign laws. For example, the European Union’s General Data Protection Regulation (the “GDPR”), which greatly
increases the jurisdictional reach of European Union law and adds a broad array of requirements for handling personal data, including the public disclosure of significant data breaches, became effective in May 2018. In addition, several states have enacted data privacy laws applicable to entities serving or employing residents and other states are doing the same. We may not be able to comply with all of these evolving compliance and operational requirements and to do so may impose significant costs that are likely to increase over time.
Risks Related to our Financing
Our indebtedness could adversely affect our financial condition, and the significant amount of cash we need to service our debt would not be available to reinvest in our business.
At March 31, 2022, our total indebtedness, including current maturities, was approximately $1.5 billion.
Our indebtedness could:
•Increase our vulnerability to general adverse economic and industry conditions;
•Limit our ability to engage in strategic acquisitions;
•Require us to dedicate a substantial portion of our cash flow from operations toward repayment of our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, acquisitions and investments and other general corporate purposes;
•Limit our flexibility in planning for, or reacting to, changes in our business and the markets in which we operate;
•Place us at a competitive disadvantage compared to our competitors that have less debt; and
•Limit, among other things, our ability to borrow additional funds on favorable terms or at all.
The terms of the indentures governing our 3.750% senior notes due April 1, 2031 (the "2021 Senior Notes") and our 5.125% senior unsecured notes due January 15, 2028 (the "2019 Senior Notes"), and the credit agreement governing our term loan and revolving credit facility, allow us to issue and incur additional debt only upon satisfaction of the conditions set forth in those respective agreements. If new debt is added to current debt levels, the related risks described above could increase.
In July 2017, the United Kingdom Financial Conduct Authority announced its intention to transition away from LIBOR, with its full elimination to occur after 2021. The subsequent announcement in March 2021 indicated the last committed publication date for 1-month LIBOR will cease publishing immediately after June 30, 2023, and therefore may not continue to be available on the current basis (or at all) after this date. The Alternate Reference Rate Committee, convened by the Board of Governors of the Federal Reserve System and the New York Federal Reserve Bank, has endorsed the Secured Overnight Financing Rate ("SOFR") as its preferred replacement benchmark for U.S. dollar LIBOR. SOFR is calculated and published by the New York Federal Reserve Bank and reflects the combination of three overnight U.S. Treasury Repo Rates. The rate is different from LIBOR, in that it is a risk-free rate, is backward-looking instead of forward-looking, is a secured rate and currently is available primarily as an overnight rate rather than a 1-,3- or 6-month rate available for LIBOR. Our term loan and revolving credit facility currently use LIBOR as a benchmark for establishing the interest rate. If LIBOR ceases to exist and we do not want to use the alternative base rate under our term loan and/or cannot come to an agreement on a replacement rate under our revolving credit facility, we may need to renegotiate the terms of that indebtedness to replace LIBOR with SOFR or the new standard that is established. As a result, the potential effect of eliminating LIBOR could increase the cost of our variable rate indebtedness.
Any increase in LIBOR or other interest rates, which have been rising in fiscal 2022 and are expected to continue to rise in fiscal 2023, will increase our cost of servicing our variable rate debt and further limit our ability to fund working capital, capital expenditures, and acquisitions. At March 31, 2022, we had $123.3 million of borrowing capacity available under our revolving credit facility to support our operating activities.
Our capital structure and ability to engage in strategic transactions is limited in significant respects by the restrictive covenants in our senior credit facility and the indentures governing our senior notes.
Our senior credit facility and the indentures governing our senior notes impose restrictions that could impede our ability to enter into certain corporate transactions, as well as increase our vulnerability to adverse economic and industry conditions, by limiting our flexibility in planning for, and reacting to, changes in our business and industry. These restrictions limit our ability to, among other things:
•Borrow money or issue guarantees;
•Pay dividends, repurchase stock from, or make other restricted payments to, stockholders;
•Make investments or acquisitions;
•Use assets as security in other transactions;
•Sell assets or merge with or into other companies;
•Enter into transactions with affiliates;
•Sell stock in our subsidiaries; and
•Limits our subsidiaries' ability to pay dividends or make other payments to us.
Our ability to engage in these types of transactions is generally limited by the terms of the senior credit facility and the indentures governing the senior notes, even if we believe that a specific transaction would positively contribute to our future growth, operating results or profitability.
In addition, our senior credit facility requires us to maintain certain leverage, interest coverage and fixed charge ratios. Although we believe we can continue to meet and/or maintain the financial covenants contained in our credit agreement, our ability to do so may be affected by events outside our control. Covenants in our senior credit facility also require us to use 100% of the proceeds we receive from non-permitted debt issuances or certain issuances of refinancing debt to repay outstanding borrowings under our senior credit facility. Any failure by us to comply with the terms and conditions of the credit agreement and the indentures governing the senior notes could result in an event of default, which may allow our creditors to accelerate our debt and therefore have a material adverse effect on our financial condition.
The senior credit facility and the indentures governing the senior notes contain cross-default provisions that could result in the acceleration of all of our indebtedness.
The senior credit facility and the indentures governing the senior notes contain provisions that allow the respective creditors to declare all outstanding borrowings under one agreement to be immediately due and payable as a result of a default under another agreement. Consequently, failure to make a payment required by the indentures governing the senior notes, among other things, may lead to an event of default under the senior credit facility. Similarly, an event of default or failure to make a required payment at maturity under the senior credit facility, among other things, may lead to an event of default under the indentures governing the senior notes. If the debt under the senior credit facility and indentures governing the senior notes had both been accelerated, the aggregate amount immediately due and payable as of March 31, 2022 would have been approximately $1.5 billion. We presently do not have sufficient liquidity to repay these borrowings in the event they were to be accelerated, and we may not have sufficient liquidity in the future to do so. Additionally, we may not be able to borrow money from other lenders to enable us to refinance our indebtedness. At March 31, 2022, the book value of our current assets was $293.3 million. Although the book value of our total assets was $3,670.7 million, approximately $3,275.6 million was in the form of intangible assets, including goodwill of $579.0 million, a significant portion of which may not be available to satisfy our creditors in the event our debt is accelerated.
Any failure to comply with the restrictions of the senior credit facility, the indentures governing the senior notes or any other subsequent financing agreements may result in an event of default. Such default may allow the creditors to accelerate the related debt, as well as any other debt to which the cross-acceleration or cross-default provisions apply. In addition, the lenders may be able to terminate any commitments they had made to supply us with additional funding. As a result, any default by us under our credit agreement, indentures governing the senior notes or any other financing agreement could have a material adverse effect on our financial condition.
General Risk Factors
Litigation may adversely affect our business, financial condition and results of operations.
Our business is subject to the risk of, and from time to time in the ordinary course of business we are involved in, litigation by employees, customers, consumers, suppliers, competitors, regulators, stockholders or others through private actions, class actions, administrative proceedings, regulatory actions or other litigation. The outcome of litigation, particularly class action lawsuits and regulatory actions, is difficult to assess or quantify. Plaintiffs in these types of lawsuits may seek recovery of very large or indeterminate amounts, and the magnitude of the potential loss relating to such lawsuits may remain unknown for substantial periods of time. The cost to defend current and future litigation may be significant. There may also be adverse publicity associated with litigation that could decrease customer or consumer acceptance of our products, regardless of whether the allegations are valid or whether we are ultimately found liable. For example, although our marketing is evidence-based, consumers and competitors may challenge, and have challenged, certain of our marketing claims by alleging, among other things, false and misleading advertising with respect to advertising for certain of our products. Such challenges could result in our having to pay monetary damages or limit our ability to maintain current marketing claims. Conversely, we have, and may be required in the future to, initiate litigation against others to protect the value of our intellectual property and the related goodwill or enforce an agreement or contract that has been breached. These matters may be time consuming and expensive, but may be necessary to protect our assets and realize the benefits of the agreements and contracts that we have negotiated. As a result, litigation may adversely affect our business, financial condition and results of operations.
We depend on our key personnel, and the loss of the services provided by any of our executive officers or other key employees could harm our business and results of operations.
Our success depends to a significant degree upon the continued contributions of our senior management. These employees may voluntarily terminate their employment with us at any time. We may not be able to successfully retain existing personnel or identify, hire and integrate new personnel. While we believe we have developed depth and experience among our key personnel, our business may be adversely affected if one or more of these key individuals were to leave or were to experience serious illness, become disabled, or pass away. We do not maintain any key-man or similar insurance policies covering any of our senior management or key personnel.
Provisions in our amended and restated certificate of incorporation and Delaware law may discourage potential acquirers of our company, which could adversely affect the value of our securities.
Our amended and restated certificate of incorporation provides that our Board of Directors is authorized to issue from time to time, without further stockholder approval, up to five million shares of preferred stock in one or more series of preferred stock issuances. Our Board of Directors may establish the number of shares to be included in each series of preferred stock and determine, as applicable, the voting and other powers, designations, preferences, rights, qualifications, limitations and restrictions for such series of preferred stock. The shares of preferred stock could have preferences over our common stock with respect to dividends and liquidation rights. We may issue additional preferred stock in ways which may delay, defer or prevent a change in control of the Company without further action by our stockholders. The shares of preferred stock may be issued with voting rights that may adversely affect the voting power of the holders of our common stock by increasing the number of outstanding shares having voting rights, and by the creation of class or series voting rights.
Our amended and restated certificate of incorporation, as amended, contains additional provisions that may have the effect of making it more difficult for a third-party to acquire or attempt to acquire control of our company. In addition, we are subject to certain provisions of Delaware law that limit, in some cases, our ability to engage in certain business combinations with significant stockholders.
These provisions, either alone, or in combination with each other, give our current directors and executive officers the ability to significantly influence the outcome of a proposed acquisition of the Company. These provisions would apply even if an acquisition or other significant corporate transaction was considered beneficial by some of our stockholders. If a change in control or change in management is delayed or prevented by these provisions, the market price of our outstanding securities could be adversely impacted.
Changes in our provision for income taxes or adverse outcomes resulting from examination of our income tax returns could adversely affect our results.
Our provision for income taxes is subject to volatility and could be adversely affected by several factors, some of which are outside of our control, including:
•Changes in the income allocation methods for state taxes, and the determination of which states or countries have jurisdiction to tax our Company;
•An increase in non-deductible expenses for tax purposes, including certain stock-based compensation, executive compensation and impairment of goodwill;
•Transfer pricing adjustments;
•Tax assessments resulting from tax audits or any related tax interest or penalties that could significantly affect our income tax provision for the period in which the settlement takes place;
•Tax liabilities from acquired businesses;
•Changes in accounting principles; and
•Changes in tax laws or related interpretations, accounting standards, regulations, and interpretations in multiple tax jurisdictions in which we operate.
Significant judgment is required to determine the recognition and measurement of the attributes prescribed in Financial Accounting Standards Board (the "FASB") Accounting Standards Codification ("ASC") 740. As a multinational corporation, we conduct our business in several countries and are subject to taxation in many jurisdictions. The taxation of our business is subject to the application of multiple and sometimes conflicting tax laws and regulations as well as multinational tax conventions. Our effective tax rate is dependent upon the availability of tax credits and carryforwards. The application of tax laws and regulations is subject to legal and factual interpretation, judgment and uncertainty. Tax laws themselves are subject to change as a result of changes in fiscal policy, changes in legislation, and the evolution of regulations and court rulings. Consequently, taxing authorities may impose tax assessments or judgments against us that could materially impact our tax liability and/or our effective income tax rate.
In addition, we may be subject to examination of our income tax returns by the Internal Revenue Service and other tax authorities. If tax authorities challenge the relative mix of our U.S. and international income, or successfully assert the jurisdiction to tax our earnings, our future effective income tax rates could be adversely affected.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We lease our corporate headquarters located in Tarrytown, New York. Primary functions performed at the Tarrytown facility include marketing, sales, operations, quality control, regulatory affairs, finance, information technology and legal. The lease expires on December 31, 2027.
Our logistics provider, GEODIS, has leased a warehouse on our behalf located in Clayton, Indiana. This property serves as our primary warehouse. The lease expires on September 30, 2024.
We own an office and manufacturing facility in Lynchburg, Virginia.
ITEM 3. LEGAL PROCEEDINGS
We are involved from time to time in routine legal matters and other claims incidental to our business. We review outstanding claims and proceedings internally and with external counsel as necessary to assess probability and amount of potential loss. These assessments are re-evaluated at each reporting period and as new information becomes available to determine whether a reserve should be established or if any existing reserve should be adjusted. The actual cost of resolving a claim or proceeding ultimately may be substantially different than the amount of the recorded reserve. In addition, because it is not permissible under GAAP to establish a litigation reserve until the loss is both probable and estimable, in some cases there may be insufficient time to establish a reserve prior to the actual incurrence of the loss (upon verdict and judgment at trial, for example, or in the case of a quickly negotiated settlement). We believe the resolution of routine matters and other incidental claims, taking our reserves into account, will not have a material adverse effect on our business, financial condition or results of operations.
ITEM 4. MINE SAFETY DISCLOSURES
None.
Part II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is listed on The New York Stock Exchange (“NYSE”) under the symbol “PBH.”
Holders
As of May 5, 2022, there were 17 holders of record of our common stock. The number of record holders does not include beneficial owners whose shares are held in the names of banks, brokers, nominees or other fiduciaries.
Dividend Policy
Common Stock
We have not in the past paid, and do not expect to pay, cash dividends on our common stock. Instead, we anticipate that all of our earnings in the foreseeable future will be used in our operations, to facilitate strategic acquisitions, to repurchase our common stock, or to pay down our outstanding indebtedness. Any future determination to pay dividends will be at the discretion of our Board of Directors and will depend, among other factors, on our results of operations, financial condition, capital requirements and contractual restrictions limiting our ability to declare and pay cash dividends, including restrictions under our 2012 Term Loan and the indentures governing our senior notes, and any other considerations our Board of Directors deems relevant.
Part III, Item 12. "Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters" of this Annual Report on Form 10-K is incorporated herein by reference.
PERFORMANCE GRAPH
The following graph (“Performance Graph”) compares our cumulative total stockholder return since March 31, 2017, with the cumulative total stockholder return for the Russell 2000 Index, Standard & Poor's SmallCap 600 Index and our peer group index. The Company is included in each of the Standard & Poor's SmallCap 600 Index and the Russell 2000 Index. The Performance Graph assumes that the value of the investment in the Company’s common stock and each index was $100.00 on March 31, 2017. The Performance Graph was also prepared based on the assumption that all dividends paid, if any, were reinvested. The Peer Group Index is a self-constructed peer group consisting of companies in the consumer products industry with comparable revenues and market capitalization, from which the Company has been excluded. Two companies in the Old Peer Group, Akorn, Inc., and Amag Pharmaceuticals, Inc were replaced in the New Peer Group by Lannet Co. and Usana Health Sciences, Inc. as they ceased to be relevant peers due to bankruptcy and acquisition, respectively.
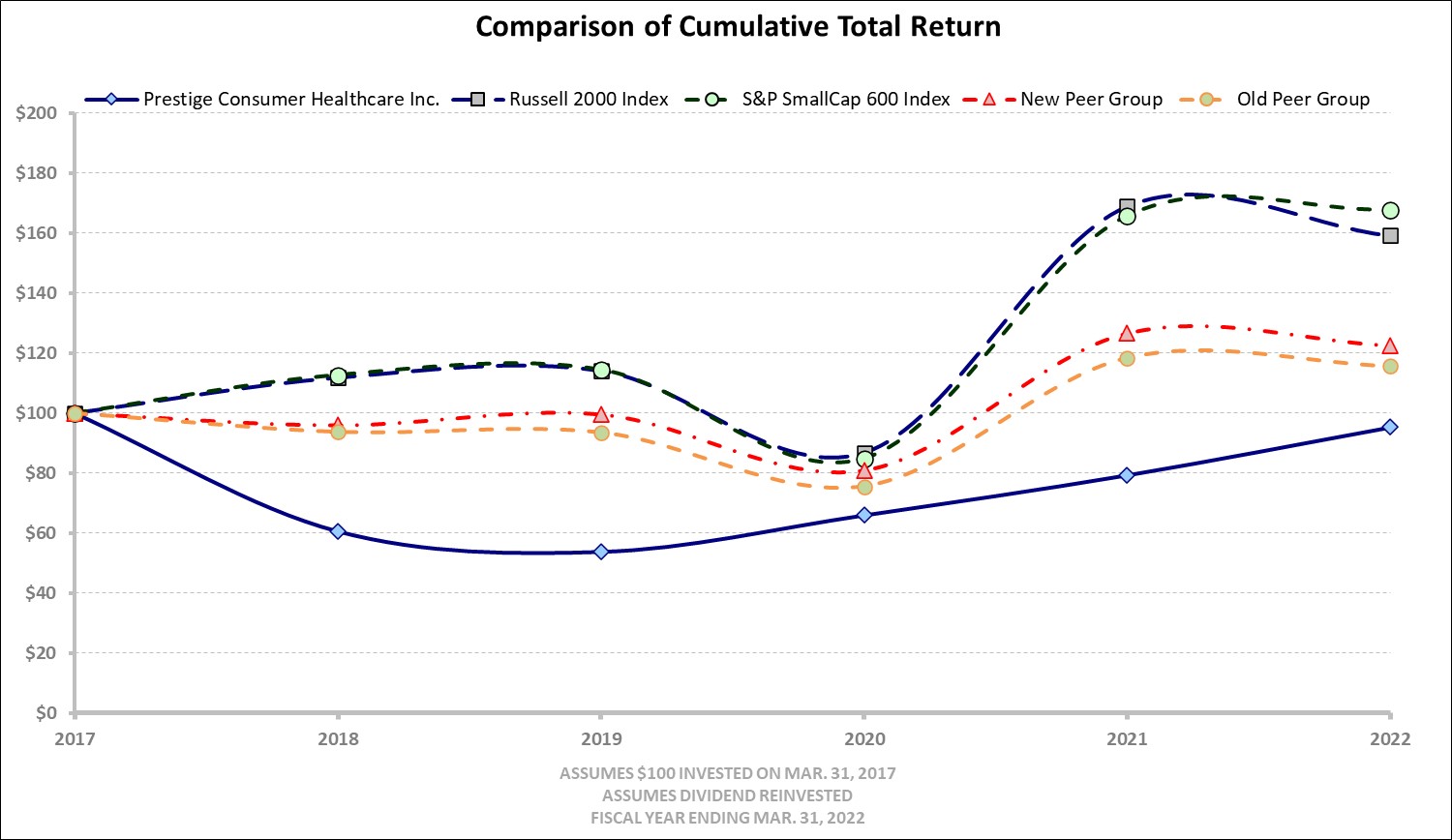
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| March 31, |
| Company/Market/Peer Group | 2017 | | 2018 | | 2019 | | 2020 | | 2021 | | 2022 |
| Prestige Consumer Healthcare Inc. | $ | 100.00 | | | $ | 60.69 | | | $ | 53.83 | | | $ | 66.02 | | | $ | 79.33 | | | $ | 95.28 | |
| Russell 2000 Index | 100.00 | | | 111.79 | | | 114.09 | | | 86.72 | | | 168.96 | | | 159.19 | |
| | | | | | | | | | | |
| S&P SmallCap 600 Index | 100.00 | | | 112.68 | | | 114.44 | | | 84.81 | | | 165.66 | | | 167.70 | |
New Peer Group Index (1) | 100.00 | | | 95.99 | | | 99.61 | | | 80.94 | | | 126.60 | | | 122.54 | |
Old Peer Group Index (2) | 100.00 | | | 93.71 | | | 93.55 | | | 75.58 | | 118.42 | | 115.64 |
(1) The New Peer Group index is comprised of: (i) B&G Food Holdings Corp., (ii) Hain Celestial Group, Inc., (iii) Church & Dwight Co., Inc., (iv) Helen of Troy, Ltd., (v) Vista Outdoors, Inc., (vi) Tupperware Brands Corporation, (vii) Revlon, Inc., (viii) Jazz Pharmaceuticals PLC, (ix) Edgewell Personal Care Company, (x) Energizer Holdings, Inc., (xi) Calavo Growers, Inc., (xii) Primo Water Corporation, (xiii) Lannet Co., and (xiv) Usana Health Sciences, Inc.
(2) The Old Peer Group index is comprised of: (i) B&G Food Holdings Corp., (ii) Hain Celestial Group, Inc., (iii) Church & Dwight Co., Inc., (iv) Helen of Troy, Ltd., (v) Vista Outdoors, Inc., (vi) Tupperware Brands Corporation, (vii) Revlon, Inc., (viii) Jazz Pharmaceuticals PLC, (ix) Edgewell Personal Care Company, (x) Energizer Holdings, Inc., (xi) Calavo Growers, Inc., (xii) Primo Water Corporation, (xiii) Akorn, Inc., and (xiv) Amag Pharmaceuticals, Inc.
The Performance Graph shall not be deemed incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any filing under the Securities Act or the Exchange Act, except to the extent that we specifically incorporate this information by reference, and shall not otherwise be deemed filed under such Acts.
ITEM 6. RESERVED
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read together with the Consolidated Financial Statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion and analysis may contain forward-looking statements that involve certain risks, assumptions and uncertainties that could cause actual results to differ materially from those implied or described by the forward-looking statements. Future results could differ materially from the discussion that follows for many reasons, including the factors described in Part I, Item 1A. “Risk Factors” in this Annual Report on Form 10-K, as well as those described in future reports filed with the SEC.
General
We are engaged in the development, manufacturing, marketing, sales and distribution of well-recognized, brand name OTC health and personal care products to mass merchandisers, drug, food, dollar, convenience and club stores, and e-commerce channels in North America (the United States and Canada) and in Australia and certain other international markets. We use the strength of our brands, our established retail distribution network, a low-cost operating model and our experienced management team to create our competitive advantage.
We have grown our product portfolio both organically and through acquisitions. We develop our existing brands by investing in new product lines, brand extensions and strong advertising support. Acquisitions of consumer health and personal care brands have also been an important part of our growth strategy. We have acquired well-recognized brands from consumer products and pharmaceutical companies and private equity firms. While certain of these brands have long histories of brand development and investment, we believe that, at the time we acquired them, most were considered “non-core” by their previous owners. As a result, these acquired brands did not benefit from adequate management focus and marketing support during the period prior to their acquisition, which created opportunities for us to reinvigorate these brands and improve their performance post-acquisition. After adding a core brand to our portfolio, we seek to increase its sales, market share and distribution in both existing and new channels through our established retail distribution network. We pursue this growth through increased spending on advertising and marketing support, new sales and marketing strategies, improved packaging and formulations and innovative development of brand extensions.
Acquisition
Acquisition of Akorn
On July 1, 2021, we completed the acquisition of the consumer health business assets from Akorn Operating Company LLC ("Akorn") pursuant to an Asset Purchase Agreement, dated May 27, 2021 (the "Purchase Agreement"), for a purchase price of $228.9 million in cash, subject to certain closing adjustments specified in the Purchase Agreement. As a result of the purchase, we acquired TheraTears and certain other over-the-counter consumer brands. The financial results from this acquisition are included in our North American and International OTC Healthcare segments. The purchase price was funded by a combination of available cash on hand, additional borrowings under the 2012 ABL Revolver and the net proceeds from the refinancing of our term loan originally entered into on January 31, 2012 (the "2012 Term Loan").
The acquisition was accounted for as a business combination. During 2022, we incurred acquisition-related costs of $5.1 million, which are included in General and administrative expense. In connection with the acquisition, we also entered into a supply arrangement with Akorn for a term of three years with optional renewals at prevailing market rates.
We prepared an analysis of the fair values of the assets acquired and liabilities assumed as of the date of acquisition. These purchase price allocations are preliminary as we are in the process of finalizing the valuation. The following table summarizes our preliminary allocation of the assets acquired and liabilities assumed as of the July 1, 2021 acquisition date.
| | | | | | |
| (In thousands) | | |
| July 1, 2021 | |
| | |
| | |
| Inventories | 6,455 | | |
| | |
| | |
| Goodwill | 1,648 | | |
| Intangible assets | 225,410 | | |
| | |
| Total assets acquired | 233,513 | | |
| | |
| Accounts payable | 478 | | |
| Reserves for sales allowances | 747 | | |
| Other accrued liabilities | 3,374 | | |
| | |
| | |
| Total liabilities assumed | 4,599 | | |
| Total purchase price | $ | 228,914 | | |
Based on this preliminary analysis, we allocated $195.9 million to non-amortizable intangible assets and $29.5 million to amortizable intangible assets. The non-amortizable intangible assets are classified as trademarks and, of the amortizable intangible assets, $20.4 million are classified as customer relationships and $9.1 million are classified as trademarks. We are amortizing the purchased amortizable intangible assets on a straight-line basis over an estimated weighted average useful life of 12.5 years.
We recorded goodwill of $1.6 million based on the amount by which the purchase price exceeded the preliminary estimate of the fair value of the net assets acquired. Goodwill is deductible and is being amortized for income tax purposes.
Economic Environment
In March 2020, the World Health Organization ("WHO") declared a global pandemic due to a new strain of coronavirus ("COVID-19"). The pandemic has caused significant volatility in the United States and global economies. In addition, the Russian invasion of Ukraine has led to further economic uncertainty. We expect economic conditions will continue to be highly volatile and uncertain and could affect demand for our products and put pressure on prices. We experienced a temporary but significant decline in consumer consumption of our brands in the first quarter of fiscal 2021, followed by more stable consumption and customer orders over the remainder of the year. Generally, throughout the pandemic, some categories were positively impacted (for instance, Women’s Health, Oral Care and Dermatological) and some categories negatively impacted (for instance, Cough & Cold and Gastrointestinal). The positively impacted categories benefited from the consumer shift to over-the-counter healthcare products, as consumers increased their focus on hygiene and self-care at home related to COVID-19. The declining categories were impacted by reduced incidence levels and usage rates due to shelter-at-home restrictions and limited travel-related activity. In fiscal 2022, we experienced solid consumer consumption and share gains across most of our brand portfolio. Our business also benefited from a significant increase in demand in travel-related categories and channels as well as the Cough & Cold category, previously impacted by the COVID-19 virus. We have continued to see changes in the purchasing patterns of our consumers, including the frequency of visits by consumers to retailers and a shift in many markets to purchasing our products online.
Both the COVID-19 pandemic and the geopolitical environment have also impacted the supply of labor and raw materials and exacerbated rising costs. Although we have not experienced a material disruption to our overall supply chain to date, we have and may continue to experience delays and backorders for certain ingredients and products, difficulty scheduling shipping for our products, as well as price increases from many of our suppliers for both shipping and product costs. In addition, labor shortages have impacted our manufacturing operations and may impact our ability to supply certain products to our customers. To date, the pandemic and other global conditions have not had a material negative impact on our operations, supply chain, overall costs or demand for most of our products or resulting aggregate sales and earnings, and, as such, it has also not negatively impacted our liquidity position. We continue to generate operating cash flows to meet our short-term liquidity needs. These circumstances could change, however, in this dynamic, unprecedented environment. If the COVID-19 outbreak worsens or geopolitical conditions cause further disruption in the global supply chain, the availability of labor or otherwise increase costs, it may materially affect our operations and those of third parties on which we rely, including causing disruptions in the supply and distribution of our products. The extent to which these conditions impact our results and liquidity will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity and duration of any further COVID-19 outbreak and recovery period and further global instability. These effects could have a material adverse impact on our business, liquidity, capital resources, and results of operations and those of the third parties on which we rely.
Tax Reform
On December 22, 2017, the Tax Cuts and Jobs Act (the "TCJA") was signed into law. The TCJA, among other things, reduced the U.S. federal corporate tax rate from 35% to 21% and imposed a new minimum tax on Global Intangible Low-Taxed Income ("GILTI") earned by foreign subsidiaries. In July 2020, final regulations were issued for GILTI, which include a high-tax exception for certain income earned by foreign subsidiaries if the foreign tax rate is in excess of 90% of the U.S. corporate tax rate of 21%.
Critical Accounting Estimates
Our significant accounting policies are described in the notes to the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. While all significant accounting policies are important to our Consolidated Financial Statements, certain of these policies may be viewed as being critical. Such policies are those that are both most important to the portrayal of our financial condition and results of operations and require our most difficult, subjective and complex estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses or the related disclosure of contingent assets and liabilities. These estimates are based on our historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ materially from these estimates. The following are our most critical accounting estimates:
Revenue Recognition, Customer Programs and Variable Consideration
Revenue is recognized when control of a promised good is transferred to a customer, in an amount that reflects the consideration that we expect to be entitled to receive in exchange for that good. This occurs either when finished goods are transferred to a common carrier for delivery to the customer or when product is picked up by the customer or the customer’s carrier.
Once a product has transferred to the common carrier or been picked up by the customer, the customer is able to direct the use of, and obtain substantially all of the remaining benefits from, the product. It is at this point that we have a right to payment and the customer has legal title.
Provisions for certain rebates, customer promotional programs, product returns, and discounts to customers are accounted for as variable consideration and recorded as a reduction in sales.
We record an estimate of future product returns, chargebacks and logistic deductions concurrent with recording sales, which is made using the most likely amount method that incorporates (i) historical return rates, (ii) current economic trends, (iii) changes in customer demand, (iv) product acceptance, (v) seasonality of our product offerings, and (vi) the impact of changes in product formulation, packaging and advertising.
We participate in the promotional programs of our customers to enhance the sale of our products. These promotional programs consist of direct-to-consumer incentives, such as coupons and temporary price reductions, as well as incentives to our customers, such as allowances for new distribution, including slotting fees, and cooperative advertising. The costs of such activities are recorded as a reduction to revenue when the related sale takes place. Estimates of the costs of these promotional programs are derived using the most likely amount method, which incorporates (i) historical sales experience, (ii) the current promotional offering, (iii) forecasted data, (iv) current market conditions, and (v) communication with customer purchasing/marketing personnel. At the completion of the promotional program, the estimated amounts are adjusted to actual results.
Pension Obligations and Expense
Certain employees of our Lynchburg manufacturing facility are covered by defined benefit pension plans. The Company’s policy is to contribute at least the minimum amount required under The Employee Retirement Income Security Act of 1974 ("ERISA"). The Company may elect to make additional contributions. Benefits are based on years of service and levels of compensation. On December 16, 2014, the decision was made to freeze the benefits under the Company's U.S. qualified defined benefit pension plan with an effective date of March 1, 2015. During the fourth quarter of 2021, we adopted a plan termination date of April 30, 2021 for the U.S. qualified defined benefit pension plan and began the plan termination process. The plan termination is expected to be completed in the first quarter of fiscal 2023.
Our discount rate assumption for our defined benefit plans changed to a range of 3.26% to 3.48% at March 31, 2022 from a range of 2.58% to 2.95% at March 31, 2021. This increase was due to market changes. While we do not currently anticipate a change in our fiscal 2023 assumptions, as a sensitivity measure, a 0.25% decline or increase in our qualified discount rate would increase or decrease our qualified pension expense by less than $0.1 million. Similarly, a 0.25% decrease or increase in
the expected return on our pension plan assets would increase or decrease our qualified pension expense by approximately $0.1 million.
The amounts that we recognize in our financial statements for pension benefit obligations are determined by actuarial valuations. Inherent in these valuations are certain assumptions, the more significant of which are: (i) the weighted average discount rate used for discounting the liability, (ii) the weighted average expected long-term rate of return on pension plan assets, (iii) the method used to determine the market-related value of pension plan assets, and (iv) the anticipated mortality rate tables. We believe the current assumptions used to estimate plan obligations and pension expense are appropriate in the current economic environment. However, as economic conditions change, we may change some of our assumptions, which could have a material impact on our financial condition and results of operations.
The funded status of our pension plans is dependent upon many factors, including returns on invested assets and the level of certain market interest rates. We review pension assumptions regularly, and we may from time to time make voluntary contributions to our pension plans that exceed the amounts required by statute. During fiscal 2022, we made total contributions to our pension plans of $0.4 million. We do not expect to make a contribution to our qualified defined benefit pension plan during fiscal 2023. Changes in interest rates and the market value of the securities held by the plans could materially change, positively or negatively, the funded status of the plans and affect the level of pension expense and required contributions.
Goodwill and Intangible Assets
Goodwill and intangible assets amounted to $3,275.6 million and $3,053.8 million at March 31, 2022 and 2021, respectively. At March 31, 2022 and 2021, goodwill and intangible assets were apportioned among similar product groups within our operating segments as follows:
| | | | | | | | | | | | | | | | | | | |
| March 31, 2022 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| | | | | | | |
| Goodwill | $ | 548,291 | | | $ | 30,685 | | | | | $ | 578,976 | |
| | | | | | | |
| Intangible assets | | | | | | | |
| Indefinite-lived | 2,391,517 | | | 85,042 | | | | | 2,476,559 | |
| Finite-lived | 198,353 | | | 21,723 | | | | | 220,076 | |
| Intangible assets, net | 2,589,870 | | | 106,765 | | | | | 2,696,635 | |
| Total | $ | 3,138,161 | | | $ | 137,450 | | | | | $ | 3,275,611 | |
| | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| March 31, 2021 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| | | | | | | |
| Goodwill | $ | 546,643 | | | $ | 31,436 | | | | | $ | 578,079 | |
| | | | | | | |
| Intangible assets | | | | | | | |
| Indefinite-lived | 2,195,617 | | | 86,371 | | | | | 2,281,988 | |
| Finite-lived | 190,462 | | | 3,279 | | | | | 193,741 | |
| Intangible assets, net | 2,386,079 | | | 89,650 | | | | | 2,475,729 | |
| Total | $ | 2,932,722 | | | $ | 121,086 | | | | | $ | 3,053,808 | |
| | | | | | | |
At March 31, 2022, the brands with the highest carrying value were Monistat, Summer's Eve, BC/Goody's, TheraTears and DenTek, comprising 60.6% of our total intangible assets value.
Goodwill and intangible assets comprise the majority of all of our assets. Goodwill represents the excess of the purchase price over the fair value of assets acquired and liabilities assumed in a business combination. Intangible assets generally represent our tradenames, brand names and patents. When we acquire a brand, we are required to make judgments regarding the value assigned to the associated intangible assets, as well as their respective useful lives. Management considers many factors both prior to and after the acquisition of an intangible asset in determining the value, as well as the useful life, assigned to each intangible asset that we acquire or continue to own and promote.
The most significant factors are:
•Brand History
A brand that has been in existence for a long period of time (e.g., 25, 50 or 100 years) generally warrants a higher valuation and longer life (sometimes indefinite) than a brand that has been in existence for a very short period of time. A brand that has been in existence for an extended period of time generally has been the subject of considerable investment by its previous owner(s) to support product innovation and advertising and marketing.
•Market Position
Consumer products that rank number one or two in their respective market generally have greater name recognition and are known as quality product offerings, which warrant a higher valuation and longer life than products that lag in the marketplace.
•Recent and Projected Sales Growth
Recent sales results present a snapshot as to how the brand has performed in the most recent time periods and represent another factor in the determination of brand value. In addition, projected sales growth provides information about the strength and potential longevity of the brand. A brand that has both strong current and projected sales generally warrants a higher valuation and a longer life than a brand that has weak or declining sales. Similarly, consideration is given to the potential investment, in the form of advertising and marketing, required to reinvigorate a brand that has fallen from favor.
•History of and Potential for Product Extensions
Consideration is given to the product innovation that has occurred during the brand’s history and the potential for continued product innovation that will determine the brand’s future. Brands that can be continually enhanced by new product offerings generally warrant a higher valuation and longer life than a brand that has always “followed the leader”.
After consideration of the factors described above, as well as current economic conditions and changing consumer behavior, management prepares a determination of an intangible asset’s value and useful life based on its analysis. Under accounting guidelines, goodwill is not amortized, but must be tested for impairment annually, or more frequently if an event occurs or circumstances change that would more likely than not reduce the fair value of the reporting unit below the carrying amount. In a similar manner, indefinite-lived assets are not amortized. They are also subject to an annual impairment test or more frequently if events or changes in circumstances indicate that the asset may be impaired. Additionally, at each reporting period an evaluation must be made to determine whether events and circumstances continue to support an indefinite useful life. Intangible assets with finite lives are amortized over their respective estimated useful lives and must also be tested for impairment whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable and exceeds its fair value.
On an annual basis, during the fourth fiscal quarter, concurrent with our annual strategic planning process, or more frequently if conditions indicate that the carrying value of the asset may not be recovered, management performs a review of both the values and, if applicable, useful lives assigned intangible assets and tests for impairment.
We report goodwill and indefinite-lived intangible assets in two reportable segments: North American OTC Healthcare and International OTC Healthcare. We identify our reporting units in accordance with the FASB ASC Subtopic 280. The carrying value and fair value for intangible assets and goodwill for a reporting unit are calculated based on key assumptions and valuation methodologies. As a result, any material changes to these assumptions could require us to record additional impairment in the future.
In the past, we have experienced declines in revenues and profitability of certain brands in the North American OTC Healthcare segment. Sustained or significant future declines in revenue, profitability, other adverse changes in expected operating results, and/or unfavorable changes in other economic factors used to estimate fair values of certain brands could indicate that fair value no longer exceeds carrying value, in which case additional non-cash impairment charges may be recorded in future periods.
Goodwill
Goodwill is tested for impairment annually and whenever events and circumstances indicate that impairment may have occurred. As of February 28, 2022 (our annual impairment review date), we had 14 reporting units with goodwill. As part of our annual test for impairment of goodwill, management estimates the discounted cash flows of each reporting unit to estimate their respective fair values. In performing this analysis, management considers current information and future events, such as competition, technological advances and changes in advertising support for our trademarks and tradenames, that could cause subsequent evaluations to utilize different assumptions. The discount rate utilized in the analysis, as well as future cash flows, may be influenced by such factors as changes in interest rates and rates of inflation. Additionally, should the related fair value of goodwill be adversely affected as a result of declining sales or margins caused by competition, changing consumer needs or preferences, technological advances or changes in advertising and marketing expenses, we may be required to record additional impairment charges in the future. In addition, we considered our market capitalization at February 28, 2022, as compared to the aggregate fair values of our reporting units, to assess the reasonableness of our estimates pursuant to the discounted cash flow methodology. An impairment charge is then recognized for the amount by which the carrying amount exceeds the reporting unit's fair value.
At February 28, 2021, in conjunction with the annual test for goodwill impairment, we recorded an impairment charge of $1.2 million to adjust the carrying amount of goodwill related to our Painstop brand in our International OTC Healthcare segment to its fair value.
At February 28, 2022, in conjunction with the annual test for goodwill impairment, we recorded an impairment charge of $0.3 million to write off the remaining goodwill related to our Painstop brand in our International OTC Healthcare segment.
As a result of our analysis at February 28, 2022, all other reporting units tested had a fair value that exceeded their carrying value by at least 10%. We performed a sensitivity analysis on our weighted average cost of capital and determined that a 50 basis point increase in the weighted average cost of capital would not have resulted in any of our other reporting units' implied fair value being less than their carrying value. Additionally, a 50 basis point decrease in the terminal growth rate used for each reporting unit would also not have resulted in any of our other reporting units’ implied fair value being less than their carrying value.
Indefinite-Lived Intangible Assets
Indefinite-lived intangibles are tested for impairment annually and whenever events and circumstances indicate that impairment may have occurred. We utilize the excess earnings method to estimate the fair value of our individual indefinite-lived intangible assets. The discount rate utilized in the analysis, as well as future cash flows, may be influenced by such factors as changes in interest rates and rates of inflation.
At each reporting period, management analyzes current events and circumstances to determine whether the indefinite life classification for a trademark or tradename continues to be valid. If circumstances warrant a change to a finite life, the carrying value of the intangible asset would then be amortized prospectively over the estimated remaining useful life.
Management tests the indefinite-lived intangible assets for impairment by comparing the carrying value of the intangible asset to its estimated fair value. Since quoted market prices are seldom available for trademarks and tradenames such as ours, we utilize present value techniques to estimate fair value. Accordingly, management’s projections are utilized to assimilate all of the facts, circumstances and expectations related to the trademark or tradename and estimate the cash flows over its useful life. In a manner similar to goodwill, future events, such as competition, technological advances and changes in advertising support for our trademarks and tradenames, could cause subsequent evaluations to utilize different assumptions. Once that analysis is completed, a discount rate is applied to the cash flows to estimate fair value. In connection with this analysis, management:
•Reviews period-to-period sales and profitability by brand;
•Analyzes industry trends and projects brand growth rates;
•Prepares annual sales forecasts;
•Evaluates advertising effectiveness;
•Analyzes gross margins;
•Reviews contractual benefits or limitations;
•Monitors competitors’ advertising spend and product innovation;
•Prepares projections to measure brand viability over the estimated useful life of the intangible asset; and
•Considers the regulatory environment, as well as industry litigation.
At February 28, 2022, in conjunction with the annual test for impairment of intangible assets, there were no indicators of impairment of indefinite-lived intangible assets under the analysis and accordingly, no impairment charge was taken.
As a result of our analysis at February 28, 2022, all indefinite-lived intangible assets tested had a fair value that exceeded their carrying value by at least 10%, with the exception of our TheraTears tradename which had a fair value exceeding its carrying value by 7%. We performed a sensitivity analysis of our weighted average cost of capital, and we determined that a 50 basis point increase in the weighted average cost of capital used to value the indefinite-lived intangible assets would not have resulted in any of our indefinite-lived intangible assets' fair value being less than their carrying value, with the exception of our TheraTears tradename. The TheraTears tradename was acquired on July 1, 2021 and, given the close proximity of the acquisition to the annual impairment test, we would expect the fair value to more closely approximate the carrying value. A 50 basis point increase in the weighted average cost of capital would result in an impairment charge of $4.3 million on our TheraTears tradename. Additionally, a 50 basis point decrease in the terminal growth rate used for each of our indefinite-lived intangible assets' would not have resulted in any of our indefinite-lived intangible assets' fair value being less than their carrying value.
Finite-Lived Intangible Assets
On an annual basis, or when events or changes in circumstances indicate the carrying value of the assets may not be recoverable, management performs a review similar to indefinite-lived intangible assets to ascertain the impact of events and circumstances on the estimated useful lives and carrying values of our trademarks and tradenames.
If the analysis warrants a change in the estimated useful life of the intangible asset, management will reduce the estimated useful life and amortize the carrying value prospectively over the shorter remaining useful life. Management’s projections are utilized to assimilate all of the facts, circumstances and expectations related to the trademark or tradename and estimate the cash flows over its useful life. Future events, such as competition, technological advances and changes in advertising support for our trademarks and tradenames, could cause subsequent evaluations to utilize different assumptions. In the event that the long-term projections indicate that the carrying value is in excess of the undiscounted cash flows expected to result from the use of the intangible assets, management is required to record an impairment charge. Once that analysis is completed, a discount rate is applied to the cash flows to estimate fair value. The impairment charge is measured as the excess of the carrying amount of the intangible asset over fair value, as calculated using the excess earnings method.
During the third quarter of 2021, we determined that the fair value of one of our finite-lived intangible assets in our International OTC Healthcare segment, Painstop, did not exceed its carrying amount. As such, we recorded an impairment charge of $1.2 million. The decline in the fair value of Painstop was primarily related to a decline in expected future sales due to a regulatory change that now requires Painstop to be prescribed by physicians rather than sold over-the-counter direct to consumers. At February 28, 2021, in conjunction with the annual test for impairment of intangible assets, there were no additional indicators of impairment of our finite-lived intangible assets and accordingly, no additional impairment charge was taken.
At February 28, 2022, in conjunction with the annual test for impairment of intangible assets, an impairment charge of $0.7 million was recorded. In connection with our long-term planning, two non-core brands in our North American OTC Healthcare segment were discontinued and therefore the related finite-lived intangible assets were written off.
Stock-Based Compensation
The Compensation and Equity topic of the FASB ASC 718 requires us to measure the cost of services to be rendered based on the grant-date fair value of the equity award. For most of our awards, compensation expense is to be recognized over the period during which an employee is required to provide service in exchange for the award, generally referred to as the requisite service period. We also grant performance stock units, which are contingent on the attainment of certain goals of the Company. Information utilized in the determination of fair value includes the following:
•Type of instrument (i.e., restricted shares, stock options, warrants or performance shares);
•Strike price of the instrument;
•Market price of our common stock on the date of grant;
•Discount rates;
•Duration of the instrument; and
•Volatility of our common stock in the public market.
Additionally, management must estimate the expected attrition rate of the recipients to enable it to estimate the amount of non-cash compensation expense to be recorded in our financial statements. While management prepares various analyses to
estimate the respective variables, a change in assumptions or market conditions, as well as changes in the anticipated attrition rates, could have a significant impact on the future amounts recorded as non-cash compensation expense.
Recent Accounting Pronouncements
A description of recently issued and adopted accounting pronouncements is included in the notes to the Consolidated Financial Statements in Item 8, Note 1 of this Annual Report.
Results of Operations
2022 compared to 2021
Total Segment Revenues
The following table represents total revenue by segment, including product groups, for each of the fiscal years ended March 31, 2022 and 2021.
| | | | | | | | | | | | | | | | | | | | |
| | | | | Increase (Decrease) |
| (In thousands) | 2022 | % | 2021 | % | Amount | % |
| North American OTC Healthcare | | | | | |
| Analgesics | $ | 117,868 | | 10.8 | | $ | 117,775 | | 12.5 | | $ | 93 | | 0.1 | |
| Cough & Cold | 86,855 | | 8.0 | | 56,158 | | 6.0 | | 30,697 | | 54.7 | |
| Women's Health | 249,136 | | 22.9 | | 252,535 | | 26.7 | | (3,399) | | (1.3) | |
| Gastrointestinal | 152,191 | | 14.0 | | 124,755 | | 13.2 | | 27,436 | | 22.0 | |
| Eye & Ear Care | 149,454 | | 13.9 | | 99,774 | | 10.6 | | 49,680 | | 49.8 | |
| Dermatologicals | 117,173 | | 10.8 | | 103,998 | | 11.0 | | 13,175 | | 12.7 | |
| Oral Care | 85,239 | | 7.8 | | 88,903 | | 9.4 | | (3,664) | | (4.1) | |
| Other OTC | 9,965 | | 0.9 | | 5,421 | | 0.6 | | 4,544 | | 83.8 | |
| Total North American OTC Healthcare | 967,881 | | 89.1 | | 849,319 | | 90.0 | | 118,562 | | 14.0 | |
| | | | | | |
| International OTC Healthcare | | | | | |
| Analgesics | 1,455 | | 0.1 | | 1,367 | | 0.1 | | 88 | | 6.4 | |
| Cough & Cold | 20,225 | | 1.9 | | 14,483 | | 1.5 | | 5,742 | | 39.6 | |
| Women's Health | 15,373 | | 1.4 | | 15,562 | | 1.7 | | (189) | | (1.2) | |
| Gastrointestinal | 52,368 | | 4.8 | | 36,381 | | 3.9 | | 15,987 | | 43.9 | |
| Eye & Ear Care | 13,995 | | 1.3 | | 10,635 | | 1.2 | | 3,360 | | 31.6 | |
| Dermatologicals | 3,213 | | 0.3 | | 3,085 | | 0.3 | | 128 | | 4.1 | |
| Oral Care | 12,282 | | 1.1 | | 12,528 | | 1.3 | | (246) | | (2.0) | |
| Other OTC | 20 | | — | 5 | | — | 15 | | 300.0 | |
| Total International OTC Healthcare | 118,931 | | 10.9 | | 94,046 | | 10.0 | | 24,885 | | 26.5 | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Total Consolidated | $ | 1,086,812 | | 100.0 | | $ | 943,365 | | 100.0 | | $ | 143,447 | | 15.2 | |
Total segment revenues for 2022 were $1,086.8 million, an increase of $143.4 million, or 15.2%, versus 2021. Both of our business segments contributed to the growth over the prior year.
North American OTC Healthcare Segment
Revenues for the North American OTC Healthcare segment increased $118.6 million, or 14.0%, during 2022 versus 2021. 2022 was primarily positively impacted by the Eye & Ear Care, Cough & Cold, and Gastrointestinal categories, as well as certain other categories. The positively impacted categories benefited from increased consumer travel, as well as improved demand, as a result of easing COVID-19 restrictions. The current period also benefited from the newly acquired TheraTears brand (included in the Eye & Ear Care category) as part of the Akorn asset acquisition, with revenues in North America of $42.2 million in 2022.
International OTC Healthcare Segment
Revenues for the International OTC Healthcare segment increased $24.9 million, or 26.5%, during 2022 versus 2021. The $24.9 million increase was mainly attributable to increased sales in our Australian subsidiary, primarily related to an increase in
sales of Hydralyte (included in the Gastrointestinal category) as a result of easing COVID-19 restrictions, as well as an increase in consumer illnesses.
Gross Profit
The following table represents our gross profit and gross profit as a percentage of total segment revenues, by segment for each of the fiscal years ended March 31, 2022 and 2021.
| | | | | | | | | | | | | | | | | | | | |
| |
| (In thousands) | | | | | Increase (Decrease) |
| Gross Profit | 2022 | % | 2021 | % | Amount | % |
| North American OTC Healthcare | $ | 548,719 | | 56.7 | | $ | 490,219 | | 57.7 | | $ | 58,500 | | 11.9 | |
| International OTC Healthcare | 71,927 | | 60.5 | | 57,253 | | 60.9 | | 14,674 | | 25.6 | |
| | | | | | |
| | $ | 620,646 | | 57.1 | | $ | 547,472 | | 58.0 | | $ | 73,174 | | 13.4 | |
Gross profit for 2022 increased $73.2 million, or 13.4%, versus 2021. As a percentage of total revenues, gross profit decreased to 57.1% in 2022 from 58.0% in 2021. The decrease in gross profit as a percentage of revenues was primarily due to increased supply chain costs and related to charges for the step up of the inventory valuation of the acquired Akorn brands in fiscal 2022 of $1.6 million.
North American OTC Healthcare Segment
Gross profit for the North American OTC Healthcare segment increased $58.5 million, or 11.9%, during 2022 versus 2021. As a percentage of North American OTC Healthcare revenues, gross profit decreased to 56.7% during 2022 from 57.7% during 2021, primarily due to increased supply chain costs and charges related to the inventory valuation of the acquired Akorn brands in fiscal 2022 of $1.6 million.
International OTC Healthcare Segment
Gross profit for the International OTC Healthcare segment increased $14.7 million, or 25.6%, during 2022 versus 2021. As a percentage of International OTC Healthcare revenues, gross profit decreased to 60.5% during 2022 from 60.9% during 2021, primarily due to increased supply chain costs.
Contribution Margin
Contribution margin is our segment measure of profitability. It is defined as gross profit less advertising and marketing expenses.
The following table represents our contribution margin and contribution margin as a percentage of total segment revenues, by segment for each of the fiscal years ended March 31, 2022 and 2021.
| | | | | | | | | | | | | | | | | | | | |
| |
| (In thousands) | | | | | Increase (Decrease) |
| Contribution Margin | 2022 | % | 2021 | % | Amount | % |
| North American OTC Healthcare | $ | 410,005 | | 42.4 | | $ | 367,362 | | 43.3 | | $ | 42,643 | | 11.6 | |
| International OTC Healthcare | 53,298 | | 44.8 | | 39,521 | | 42.0 | | 13,777 | | 34.9 | |
| | | | | | |
| | $ | 463,303 | | 42.6 | | $ | 406,883 | | 43.1 | | $ | 56,420 | | 13.9 | |
North American OTC Healthcare Segment
Contribution margin for the North American OTC Healthcare segment increased $42.6 million, or 11.6%, during 2022 versus 2021. As a percentage of North American OTC Healthcare revenues, contribution margin for the North American OTC Healthcare segment decreased to 42.4% during 2022 from 43.3% during 2021. The contribution margin decrease as a percentage of revenues was primarily due to an increase in advertising and marketing expenses as well as the decrease in gross profit margin noted above.
International OTC Healthcare Segment
Contribution margin for the International OTC Healthcare segment increased $13.8 million, or 34.9%, during 2022 versus 2021. As a percentage of International OTC Healthcare revenues, contribution margin for the International OTC Healthcare segment increased to 44.8% during 2022 from 42.0% during 2021. The contribution margin increase as a percentage of revenues was primarily due to decreased advertising and marketing expenses as a percentage of revenues in 2022.
General and Administrative
General and administrative expenses were $108.5 million for 2022 versus $85.5 million for 2021. The increase in general and administrative expenses was primarily due to increases in compensation costs and professional fees, as well as costs related to the acquisition of Akorn of $5.1 million.
Depreciation and Amortization
Depreciation and amortization expense was $24.9 million for 2022 versus $23.9 million for 2021. The increase in depreciation and amortization expenses was attributable to an increase in amortization expense due to the addition of certain brands purchased in conjunction with the Akorn acquisition, partly offset by certain assets being fully depreciated during 2022.
Interest Expense, Net
Interest expense, net was $64.3 million during 2022 versus $82.3 million during 2021. The average indebtedness remained at $1.6 billion during 2021 and 2022. The average cost of borrowing decreased to 4.1% for 2022 from 5.1% for 2021.
Loss on Extinguishment of Debt
During 2022, we recorded a loss on extinguishment of debt of $2.1 million, related to the amendment of our 2012 Term Loan on July 1, 2021. During 2021, we recorded a loss on extinguishment of debt of $12.3 million, representing the premium paid to redeem our 6.375% 2016 Senior Notes in March 2021 of $9.6 million and the related write off of debt costs of $2.7 million.
Income Taxes
The provision for income taxes during 2022 was $57.1 million versus $39.4 million in 2021. The effective tax rate on income before income taxes was 21.7% during 2022 versus 19.3% during 2021. The increase in the effective tax rate in 2022 compared to 2021 was primarily due to the final GILTI regulations issued in July 2020, which resulted in the release of the valuation allowance on foreign tax credit carryforwards of $5.4 million for 2021.
Results of Operations
2021 compared to 2020
For a discussion of fiscal 2021 compared to 2020, please refer to our 2021 Annual Report on Form 10-K Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations, filed with the SEC on May 7, 2021.
Liquidity and Capital Resources
Liquidity
Our primary source of cash comes from our cash flow from operations. In the past, we have supplemented this source of cash with various debt facilities, primarily in connection with acquisitions. We have financed our operations, and expect to continue to finance our operations over the next twelve months, with a combination of funds generated from operations and borrowings. Our principal uses of cash are for operating expenses, debt service, capital expenditures, share repurchases, and acquisitions. Based on our current levels of operations and anticipated growth, excluding acquisitions, we believe that our cash generated from operations and our existing credit facilities will be adequate to finance our working capital and capital expenditures through the next twelve months, although no assurance can be given in this regard. See "Economic Environment Since the Coronavirus Outbreak" above.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended March 31, | | $ Change |
| (In thousands) | 2022 | | 2021 | | 2020 | | 2022 vs. 2021 | 2021 vs. 2020 |
| Net cash provided by (used in): | | | | | | | | |
| Operating activities | $ | 259,922 | | | $ | 235,607 | | | $ | 217,124 | | | $ | 24,315 | | $ | 18,483 | |
| Investing activities | (256,511) | | | (22,243) | | | (16,570) | | | (234,268) | | (5,673) | |
| Financing activities | (7,569) | | | (279,419) | | | (131,431) | | | 271,850 | | (147,988) | |
| Effects of exchange rate changes on cash and cash equivalents | (959) | | | 3,597 | | | (1,893) | | | (4,556) | | 5,490 | |
| Net change in cash and cash equivalents | $ | (5,117) | | | $ | (62,458) | | | $ | 67,230 | | | $ | 57,341 | | $ | (129,688) | |
2022 compared to 2021
Operating Activities
Net cash provided by operating activities was $259.9 million for 2022 compared to $235.6 million for 2021. The $24.3 million increase in net cash provided by operating activities was due to an increase in net income after non-cash items, partly offset by increased working capital.
Investing Activities
Net cash used in investing activities was $256.5 million for 2022 compared $22.2 million for 2021. The increase was primarily due to acquisitions of $247.0 million in the current period, partly offset by a decrease in capital expenditures in the current period.
Financing Activities
Net cash used in financing activities was $7.6 million for 2022 compared to $279.4 million for 2021. In 2022, we had no net change in our long-term debt principal, as prepayments served to offset the borrowings to finance the Akorn acquisition and payments of debt costs of $6.1 million. In 2021, we reduced our outstanding long-term debt by $250.0 million, paid debt costs of $17.7 million and repurchased common stock of $11.9 million.
2021 compared to 2020
Operating Activities
Net cash provided by operating activities was $235.6 million for 2021 compared to $217.1 million for 2020. The $18.5 million increase in net cash provided by operating activities was due to an increase in net income after non-cash items, partly offset by increased working capital.
Investing Activities
Net cash used in investing activities was $22.2 million for 2021 compared $16.6 million for 2020. The increase was primarily due to an increase in capital expenditures in 2021.
Financing Activities
Net cash used in financing activities was $279.4 million for 2021 compared to $131.4 million for 2020. The increase was primarily due to increased repayments of debt of $97.0 million, decreased borrowings of $85.0 million and an increase in payment of debt costs of $11.1 million in 2021 compared to 2020, partly offset by a decrease in our repurchase of common stock of $44.8 million compared to the prior year.
Capital Resources
2012 Term Loan and 2012 ABL Revolver:
On January 31, 2012, Prestige Brands, Inc. ("the Borrower") entered into a senior secured credit facility, which consists of (i) a $660.0 million term loan (the "2012 Term Loan") with an original 7-year maturity and (ii) a $50.0 million asset-based revolving credit facility (the "2012 ABL Revolver") with an original 5-year maturity. In subsequent years, we have utilized portions of our accordion feature to increase the amount of our borrowing capacity under the 2012 ABL Revolver by $85.0 million to $135.0 million and reduced our borrowing rate on the 2012 ABL Revolver by 0.25% (discussed below). The 2012 Term Loan was issued with an original issue discount of 1.5% of the principal amount thereof, resulting in net proceeds to the Borrower of $650.1 million. The 2012 Term Loan is unconditionally guaranteed by Prestige Consumer Healthcare Inc. and certain of its domestic 100% owned subsidiaries, other than the Borrower. Each of these guarantees is joint and several. There are no significant restrictions on the ability of any of the guarantors to obtain funds from their subsidiaries or to make payments to the Borrower or the Company.
On February 21, 2013, we entered into Amendment No. 1 ("Term Loan Amendment No. 1") to the 2012 Term Loan. Term Loan Amendment No. 1 provided for the refinancing of all of the Borrower's existing Term B Loans with new Term B-1 Loans (the "Term B-1 Loans"). The interest rate on the Term B-1 Loans under Term Loan Amendment No. 1 was based, at our option, on a LIBOR rate plus a margin of 2.75% per annum, with a LIBOR floor of 1.00%, or an alternate base rate, with a floor of 2.00%, plus a margin. In addition, Term Loan Amendment No. 1 provided the Borrower with certain additional capacity to prepay subordinated debt, our then-outstanding senior notes and certain other unsecured indebtedness permitted to be incurred under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver.
On September 3, 2014, we entered into Amendment No. 2 ("Term Loan Amendment No. 2") to the 2012 Term Loan. Term Loan Amendment No. 2 provided for (i) the creation of a new class of Term B-2 Loans under the 2012 Term Loan (the "Term B-2 Loans") in an aggregate principal amount of $720.0 million, (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver, including additional investment, restricted payment and debt incurrence flexibility and financial maintenance covenant relief, and (iii) an interest rate on (x) the Term B-1 Loans that was based, at our option, on a LIBOR rate plus a margin of 3.125% per annum, with a LIBOR floor of 1.00%, or an alternate base rate, with a floor of 2.00%, plus a margin, and (y) the Term B-2 Loans that was based, at our option, on a LIBOR rate plus a margin of 3.50% per annum, with a LIBOR floor of 1.00%, or an alternate base rate, with a floor of 2.00%, plus a margin (with a margin step-down to 3.25% per annum, based upon achievement of a specified secured net leverage ratio).
Also on September 3, 2014, we entered into Amendment No. 3 ("ABL Amendment No. 3") to the 2012 ABL Revolver. ABL Amendment No. 3 provided for (i) a $40.0 million increase in revolving commitments under the 2012 ABL Revolver and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver, including additional investment, restricted payment and debt incurrence flexibility. Borrowings under the 2012 ABL Revolver, as amended, bore interest at a rate per annum equal to an applicable margin, plus, at our option, either (i) a base rate determined by reference to the highest of (a) the Federal Funds rate plus 0.50%, (b) the prime rate of Citibank, N.A., and (c) the LIBOR rate determined by reference to the cost of funds for U.S. dollar deposits for an interest period of one month, adjusted for certain additional costs, plus 1.00% or (ii) a LIBOR rate determined by reference to the costs of funds for U.S. dollar deposits for the interest period relevant to such borrowing, adjusted for certain additional costs. The applicable margin for borrowings under the 2012 ABL Revolver could be increased to 2.00% or 2.25% for LIBOR borrowings and 1.00% or 1.25% for base-rate borrowings, depending on average excess availability under the 2012 ABL Revolver during the prior fiscal quarter. In addition to paying interest on outstanding principal under the 2012 ABL Revolver, we were required to pay a commitment fee to the lenders under the 2012 ABL Revolver in respect of the unutilized commitments thereunder. The initial commitment fee rate was 0.50% per annum. The commitment fee rate will be reduced to 0.375% per annum at any time when the average daily unused commitments for the prior quarter is less than a percentage of total commitments by an amount set forth in the credit agreement covering the 2012 ABL Revolver. We may voluntarily repay outstanding loans under the 2012 ABL Revolver at any time without a premium or penalty.
On May 8, 2015, we entered into Amendment No. 3 ("Term Loan Amendment No. 3") to the 2012 Term Loan. Term Loan Amendment No. 3 provided for (i) the creation of a new class of Term B-3 Loans under the 2012 Term Loan (the "Term B-3 Loans") in an aggregate principal amount of $852.5 million, which combined the outstanding balances of the Term B-1 Loans of $207.5 million and the Term B-2 Loans of $645.0 million, and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility and financial maintenance covenant relief. The maturity date of the Term B-3 Loans remained the same as the Term B-2 Loans' original maturity date of September 3, 2021.
On June 9, 2015, we entered into Amendment No. 4 (“ABL Amendment No. 4”) to the 2012 ABL Revolver. ABL Amendment No. 4 provided for (i) a $35.0 million increase in the accordion feature under the 2012 ABL Revolver and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility and financial maintenance covenant relief and (iii) extended the maturity date of the 2012 ABL Revolver to June 9, 2020, which was five years from the effective date of ABL Amendment No. 4.
On February 5, 2016, we entered into Amendment No. 5 (“ABL Amendment No. 5”) to the 2012 ABL Revolver. ABL Amendment No. 5 temporarily suspended certain financial and related reporting covenants in the 2012 ABL Revolver until the earliest of (i) the date that was 60 calendar days following February 4, 2016, (ii) the date upon which certain of DenTek’s assets were included in the Company’s borrowing base under the 2012 ABL Revolver and (iii) the date upon which the Company received net proceeds from an offering of debt securities.
In connection with the Fleet acquisition, on January 26, 2017, we entered into Amendment No. 4 ("Term Loan Amendment No. 4") to the 2012 Term Loan. Term Loan Amendment No. 4 provided for (i) the refinancing of all of our outstanding term loans and the creation of a new class of Term B-4 Loans under the 2012 Term Loan (the "Term B-4 Loans") in an aggregate principal amount of $1,427.0 million and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility and financial maintenance covenant relief. Term Loan Amendment No.4 also extended the maturity date of the 2012 Term Loan to January 26, 2024. In addition, Citibank, N.A. was succeeded by Barclays Bank PLC as administrative agent under the 2012 Term Loan.
Also on January 26, 2017, we entered into Amendment No. 6 ("ABL Amendment No. 6") to the 2012 ABL Revolver. ABL Amendment No. 6 provided for (i) a $40.0 million increase in revolving commitments under the 2012 ABL Revolver, (ii) an extension of the maturity date of revolving commitments to January 26, 2022, and (iii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, including additional investment, restricted payment and debt incurrence flexibility consistent with Term Loan Amendment No. 4.
On March 21, 2018, we entered into Amendment No. 5 (“Term Loan Amendment No. 5”) to the 2012 Term Loan. Term Loan Amendment No. 5 provided for the creation of Term B-5 Loans (the "Term B-5 Loans") by repricing of the Term B-4 Loans under the credit agreement governing the 2012 Term Loan to an interest rate that is based, at our option, on a LIBOR rate plus a margin of 2.00% per annum, with a LIBOR floor of 0.00%, or an alternative base rate plus a margin of 1.00% per annum, with a floor of 1.00%.
On December 11, 2019, the Company and the Borrower entered into Amendment No. 7 ("ABL Amendment No. 7") to the 2012 ABL Revolver. ABL Amendment No. 7 provides for (i) an extension of the maturity date of the 2012 ABL Revolver to December 11, 2024, which is five years from the effective date of the ABL Amendment No. 7, (ii) increased flexibility under the 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility, (iii) an initial applicable margin for borrowings under the 2012 ABL Revolver that is 1.00% with respect to LIBOR borrowings and 0.0% with respect to base-rate borrowings (which may be increased to 1.25% or 1.50% for LIBOR borrowings and 0.25% or 0.50% for base-rate borrowings, depending on average excess availability under the facility during the prior fiscal quarter), and (iv) a commitment fee to the lenders under the 2012 ABL Revolver in respect of the unutilized commitments thereunder of 0.25% per annum.
On July 1, 2021, we entered into Amendment No. 6 ("Term Loan Amendment No. 6") to the 2012 Term Loan. Term Loan Amendment No. 6 provided for (i) the refinancing of our outstanding term loans and the creation of a new class of Term B-5 Loans under the credit agreement governing the 2012 Term Loan in an aggregate principal amount of $600.0 million, (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, and (iii) an interest rate on the Term B-5 Loans that is based, at the Borrower's option, on a LIBOR rate plus a margin of 2.00% per annum, with a LIBOR floor of 0.50%, or an alternative base rate plus a margin of 1.00% per annum. In addition, Term Loan Amendment No. 6 provided for an extension of the maturity date of the 2012 Term Loan to July 1, 2028. In connection with this refinancing, we recorded a loss on extinguishment of debt of $2.1 million to write off a portion of new and old debt costs relating to this refinancing. Under Term Loan Amendment No. 6, we are required to make quarterly payments each equal to 0.25% of the aggregate principal amount of the 2012 Term Loan.
For the year ended March 31, 2022, the average interest rate on the 2012 Term Loan was 3.6%. For the year ended March 31, 2022, the average interest rate on the amounts borrowed under the 2012 ABL Revolver was 1.2%.
2013 Senior Notes:
On December 17, 2013, the Borrower issued $400.0 million of senior unsecured notes, with an interest rate of 5.375% and a maturity date of December 15, 2021 (the "2013 Senior Notes"). These notes were redeemed on December 16, 2019 using the funds from the issuance of our 2019 Senior Notes described below. In conjunction with the redemption of our 2013 Senior Notes, we wrote off related debt costs of $2.2 million in the year ended March 31, 2020.
2016 Senior Notes:
On February 19, 2016, the Borrower completed the sale of $350.0 million aggregate principal amount of 6.375% senior notes due March 1, 2024 (the “Initial Notes”). On March 21, 2018, the Borrower completed the sale of $250.0 million additional aggregate principal amount of 6.375% senior notes due March 1, 2024 (the “Additional Notes”). Both the Initial Notes and the Additional Notes (the "2016 Senior Notes") were redeemed on March 1, 2021 using funds from the issuance of our 2021 Senior Notes described below. In conjunction with the redemption of the 2016 Senior Notes, we wrote off related debt costs of $2.7 million and paid a premium to redeem the 2016 Senior Notes of $9.6 million in the year ended March 31, 2021.
2019 Senior Notes:
On December 2, 2019, the Borrower issued $400.0 million aggregate principal amount of 5.125% senior notes due January 15, 2028 (the "2019 Senior Notes") pursuant to an indenture dated December 2, 2019, among the Borrower, the guarantors party thereto (including the Company) and U.S. Bank National Association, as trustee. We used the net proceeds from the 2019 Senior Notes, together with cash on hand, to redeem all $400.0 million of our outstanding 2013 Senior Notes, which were due in 2021, and to pay related fees and expenses.
2021 Senior Notes:
On March 1, 2021, we issued $600.0 million aggregate principal amount of 3.750% senior notes due April 1, 2031, (the "2021 Senior Notes") pursuant to an indenture dated March 1, 2021, among the Borrower, the guarantors party thereto (including the Company), and U.S. Bank National Association, as trustee. We used the net proceeds from the 2021 Senior Notes to redeem all $600.0 million of our outstanding 2016 Senior Notes, which were due in 2024, and to pay related fees and expenses.
Redemptions and Restrictions:
We have the option to redeem all or a portion of the 2019 Senior Notes at any time on or after January 15, 2023 at the redemption prices set forth in the indenture governing the 2019 Senior Notes, plus accrued and unpaid interest, if any. Subject to certain limitations, in the event of a change of control (as defined in the indenture governing the 2019 Senior Notes), the Borrower will be required to make an offer to purchase the 2019 Senior Notes at a price equal to 101% of the aggregate principal amount of the notes repurchased, plus accrued and unpaid interest, if any, to the date of repurchase.
We have the option to redeem all or a portion of the 2021 Senior Notes at any time on or after April 1, 2026 at the redemption prices set forth in the indenture governing the 2021 Senior Notes, plus accrued and unpaid interest, if any. Subject to certain limitations, in the event of a change of control (as defined in the indenture governing the 2021 Senior Notes), the Borrower will be required to make an offer to purchase the 2021 Senior Notes at a price equal to 101% of the aggregate principal amount of the notes repurchased, plus accrued and unpaid interest, if any, to the date of repurchase.
The credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes contain provisions that restrict us from undertaking specified corporate actions, such as asset dispositions, acquisitions, dividend payments, repurchases of common shares outstanding, changes of control, incurrences of indebtedness, issuance of equity, creation of liens, making of loans and transactions with affiliates. Additionally, the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes contain cross-default provisions, whereby a default pursuant to the terms and conditions of certain indebtedness will cause a default on the remaining indebtedness under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes. At March 31, 2022, we were in compliance with the covenants under our long-term indebtedness.
As of March 31, 2022, we had an aggregate of $1.5 billion of outstanding indebtedness, which consisted of the following:
•$400.0 million of 5.125% 2019 Senior Notes due January 15, 2028;
•$600.0 million of 3.750% 2021 Senior Notes due April 1, 2031; and
•$495.0 million of borrowings under the Term B-5 Loans due July 1, 2028.
As of March 31, 2022, we had no balance outstanding on the 2012 ABL Revolver and a borrowing capacity of $123.3 million.
Interest Rate Swaps
In January 2020, we entered into two interest rate swaps to hedge a total of $400.0 million of our variable interest debt. One swap settled on January 31, 2021 and the other settled on January 31, 2022.
Debt Covenants
Our debt facilities contain various financial covenants, including provisions that require us to maintain certain leverage, interest coverage and fixed charge ratios. Specifically, we must:
•Have a leverage ratio of less than 6.50 to 1.0 for the quarter ended March 31, 2022 and going forward (defined as, with certain adjustments, the ratio of our consolidated total net debt as of the last day of the fiscal quarter to our trailing twelve month consolidated net income before interest, taxes, depreciation, amortization, non-cash charges and certain other items (“EBITDA”));
•Have an interest coverage ratio of greater than 2.25 to 1.0 for the quarter ended March 31, 2022 and going forward (defined as, with certain adjustments, the ratio of our consolidated EBITDA to our trailing twelve month consolidated cash interest expense); and
•Have a fixed charge ratio of greater than 1.0 to 1.0 (defined as, with certain adjustments, the ratio of our consolidated EBITDA minus capital expenditures to our trailing twelve month consolidated interest paid, taxes paid and other specified payments). Our fixed charge requirement remains level throughout the term of the agreement.
At March 31, 2022, we were in compliance with the applicable financial and restrictive covenants under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes. Additionally, management anticipates that in the normal course of operations, we will be in compliance with the financial and restrictive covenants during fiscal 2023. During the year ended March 31, 2022, we made a required repayment
of $1.5 million as well as voluntary principal payments of $103.5 million against the outstanding balance under our 2012 Term Loan. Since we have made optional payments that exceed all of our required quarterly payments, we will not be required to make another payment on the 2012 Term Loan until maturity on July 1, 2028.
Commitments
As of March 31, 2022, we had ongoing commitments under various contractual and commercial obligations as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Payments Due by Period |
| (In millions) | | | Less than | | 1 to 3 | | 4 to 5 | | After 5 |
| Contractual Obligations | Total | | 1 Year | | Years | | Years | | Years |
| Long-term debt | $ | 1,495.0 | | | $ | — | | | $ | — | | | $ | — | | | $ | 1,495.0 | |
Interest on long-term debt (1) | 438.2 | | | 62.6 | | | 125.0 | | | 123.7 | | | 126.9 | |
| Purchase obligations: | | | | | | | | | |
Inventory costs (2) | 334.4 | | | 308.6 | | | 10.6 | | | 9.8 | | | 5.4 | |
Other costs (3) | 39.7 | | | 39.4 | | | 0.3 | | | — | | | — | |
| Operating leases | 22.4 | | | 6.4 | | | 10.7 | | | 3.8 | | | 1.5 | |
| Finance leases | 7.3 | | | 2.8 | | | 4.3 | | | 0.2 | | | — | |
| | | | | | | | | |
| | | | | | | | | |
Total contractual cash obligations (4) | $ | 2,337.0 | | | $ | 419.8 | | | $ | 150.9 | | | $ | 137.5 | | | $ | 1,628.8 | |
(1)Represents the estimated interest obligations on the outstanding balances at March 31, 2022 of the 2021 Senior Notes, 2019 Senior Notes, Term B-5 Loans, and 2012 ABL Revolver, assuming scheduled principal payments (based on the terms of the loan agreements). We estimate our future obligations for interest on our variable rate debt by assuming the weighted average interest rates in effect on each variable rate debt obligation at March 31, 2022 remain constant into the future. This is an estimate, as actual rates will vary over time. In addition, we assume that the average balance outstanding for the last month of fiscal 2022 remains the same for the remaining term of the agreement. The actual balance outstanding may fluctuate significantly in future periods, depending on the availability of cash flow from operations and future investing and financing considerations. Estimated interest obligations would be different under different assumptions regarding interest rates or timing of principal payments.
(2)Purchase obligations for inventory costs are legally binding commitments for projected inventory requirements to be utilized during the normal course of our operations.
(3)Purchase obligations for other costs are legally binding commitments for marketing, advertising and capital expenditures. Our capital expenditures primarily relate to manufacturing equipment. Activity costs for molds and equipment to be paid, based solely on a per unit basis without any deadlines for final payment, have been excluded from the table because we are unable to determine the time period over which such activity costs will be paid.
(4)We have excluded obligations related to uncertain tax positions because we cannot reasonably estimate when they will occur.
We do not have any off-balance sheet arrangements or financing activities with special-purpose entities.
Inflation
Inflationary factors such as increases in the costs of raw materials, packaging materials, purchased product, labor costs, transportation costs and overhead may adversely affect our operating results and financial condition. Although we do not believe that inflation has had a material impact on our financial condition or results of operations for the three most recent fiscal years, the COVID-19 pandemic, the Russian invasion of Ukraine and other supply and labor disruptions may have an inflationary impact on our costs and a high rate of inflation in the future could have a material adverse effect on our financial condition and results of operations. More volatility in crude oil prices may have an adverse impact on transportation costs, as well as certain petroleum based raw materials and packaging material. Although we make efforts to minimize the impact of inflationary factors, including raising prices to our customers, a high rate of pricing volatility associated with crude oil supplies or other raw materials used in our products may have an adverse effect on our operating results.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
We are exposed to interest rate risk because our 2012 Term Loan and 2012 ABL Revolver are variable rate debt. At March 31, 2022, approximately $495.0 million of our debt carries a variable rate of interest.
Holding other variables constant, including levels of indebtedness, a one percentage point increase in interest rates on our variable rate debt would have had an adverse impact on pre-tax loss and cash flows for the year ended March 31, 2022 of approximately $4.0 million.
Foreign Currency Exchange Rate Risk
During the years ended March 31, 2022 and 2021, approximately 13.2% and 12.0%, respectively, of our net revenues were denominated in currencies other than the U.S. Dollar. As such, we are exposed to transactions that are sensitive to foreign currency exchange rates, including insignificant foreign currency forward exchange agreements. These transactions are primarily with respect to the Canadian and Australian Dollar.
We performed a sensitivity analysis with respect to exchange rates for the year ended March 31, 2022 and 2021. Holding all other variables constant, and assuming a hypothetical 10.0% adverse change in foreign currency exchange rates, this analysis resulted in a 2.6% impact on pre-tax income of approximately $6.8 million for the year ended March 31, 2022 and a 2.2% impact on pre-tax loss of approximately $4.4 million for the year ended March 31, 2021.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The supplementary data required by this Item are described in Part IV, Item 15 of this Annual Report on Form 10-K and are presented beginning on page 89.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Prestige Consumer Healthcare Inc.
Audited Financial Statements
March 31, 2022
| | | | | |
Report of Independent Registered Public Accounting Firm
PricewaterhouseCoopers LLP | |
| Consolidated Statements of Income and Comprehensive Income for each of the three years in the period ended March 31, 2022 | |
| Consolidated Balance Sheets at March 31, 2022 and 2021 | |
| Consolidated Statements of Changes in Stockholders’ Equity for each of the three years in the period ended March 31, 2022 | |
| Consolidated Statements of Cash Flows for each of the three years in the period ended March 31, 2022 | |
| Notes to Consolidated Financial Statements | |
| Schedule II—Valuation and Qualifying Accounts for the years ended March 31, 2022, 2021 and 2020 | |
Management's Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act). Internal control over financial reporting is a process designed by, or under the supervision of the Chief Executive Officer and Chief Financial Officer and effected by the Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable, not absolute, assurance that the control objectives will be met. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate over time.
Management, with the participation of the Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company's internal control over financial reporting as of March 31, 2022. In making its evaluation, management has used the criteria established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control - Integrated Framework (2013 Framework).
Based on management's assessment utilizing the 2013 Framework, management concluded that the Company's internal control over financial reporting was effective as of March 31, 2022.
PricewaterhouseCoopers LLP, an independent registered public accounting firm, has issued a report on the effectiveness of our internal control over financial reporting as of March 31, 2022, which appears below.
Prestige Consumer Healthcare Inc.
May 6, 2022
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Prestige Consumer Healthcare Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Prestige Consumer Healthcare Inc. and its subsidiaries (the “Company”) as of March 31, 2022 and 2021, and the related consolidated statements of income and comprehensive income, of changes in stockholders’ equity and of cash flows for each of the three years in the period ended March 31, 2022, including the related notes and financial statement schedule listed in the accompanying index (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of March 31, 2022, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended March 31, 2022 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2022, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 8. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Goodwill and Indefinite-Lived Intangible Asset Impairment Assessments for Reporting Units and Tradenames of Certain Product Groups
As described in Notes 1, 5, and 6 to the consolidated financial statements, the Company’s consolidated goodwill and indefinite-lived intangible asset balances were $579 million and $2,697 million, respectively, as of March 31, 2022. Goodwill is classified as the excess of the purchase price over the fair market value of assets acquired and liabilities assumed in business combinations. Intangible assets generally represent tradenames, brand names and patents. Goodwill and indefinite-lived intangible assets are tested for impairment at least annually in the fourth fiscal quarter of each year, or more frequently if events or changes in circumstances indicate that the asset may be impaired. Goodwill is tested for impairment at the reporting unit level, which is one level below the operating segment level, and indefinite-lived intangible assets are tested at the individual asset level. An impairment loss is recognized if the carrying amount of the reporting unit or asset exceeds its fair value. Management utilized the discounted cash flow method to estimate the fair value of its reporting units and the excess earnings method to estimate the fair value of individual indefinite-lived intangible assets. Assumptions subject to significant uncertainties in developing the fair value measurement of the reporting units and indefinite-lived intangible assets include future sales, gross margins, advertising and marketing expenses, and the discount rates.
The principal considerations for our determination that performing procedures relating to goodwill and indefinite-lived intangible asset impairment assessments for reporting units and tradenames of certain product groups is a critical audit matter are (i) the significant judgment by management when developing the fair value measurements, (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to future sales, gross margins, advertising and marketing expenses, and the discount rates, and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s impairment assessments of goodwill and indefinite-lived intangible assets, including controls over the valuation of the Company’s reporting units and individual indefinite-lived intangible assets. These procedures also included, among others, (i) testing management’s process for developing the fair value measurements of the reporting units and tradenames for certain product groups, (ii) evaluating the appropriateness of methods used in developing the fair value measurements, (iii) testing the completeness and accuracy of underlying data used, and (iv) evaluating the reasonableness of significant assumptions used by management related to future sales, gross margins, advertising and marketing expenses, and the discount rates. Evaluating management’s assumptions related to future sales, gross margins, and advertising and marketing expenses involved evaluating whether the assumptions used by management were reasonable considering (i) current and historical performance, (ii) the consistency with external market and industry data, and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in the evaluation of the Company’s fair value methods and the discount rates.
/s/ PricewaterhouseCoopers LLP
Stamford, Connecticut
May 6, 2022
We have served as the Company’s auditor since at least 1999. We have not been able to determine the specific year we began serving as auditor of the Company.
Prestige Consumer Healthcare Inc.
Consolidated Statements of Income and Comprehensive Income
| | | | | | | | | | | | | | | | | |
| | Year Ended March 31, |
| (In thousands, except per share data) | 2022 | | 2021 | | 2020 |
| Revenues | | | | | |
| Net sales | $ | 1,086,770 | | | $ | 943,324 | | | $ | 962,936 | |
| Other revenues | 42 | | | 41 | | | 74 | |
| Total revenues | 1,086,812 | | | 943,365 | | | 963,010 | |
| | | | | |
| Cost of Sales | | | | | |
| Cost of sales excluding depreciation | 458,942 | | | 389,670 | | | 406,554 | |
| Cost of sales depreciation | 7,224 | | | 6,223 | | | 4,233 | |
| Cost of sales | 466,166 | | | 395,893 | | | 410,787 | |
| Gross profit | 620,646 | | | 547,472 | | | 552,223 | |
| | | | | |
| Operating Expenses | | | | | |
| Advertising and marketing | 157,343 | | | 140,589 | | | 147,194 | |
| General and administrative | 108,516 | | | 85,540 | | | 89,112 | |
| Depreciation and amortization | 24,868 | | | 23,941 | | | 24,762 | |
| | | | | |
| | | | | |
| Total operating expenses | 290,727 | | | 250,070 | | | 261,068 | |
| Operating income | 329,919 | | | 297,402 | | | 291,155 | |
| | | | | |
| Other expense (income) | | | | | |
| Interest expense, net | 64,287 | | | 82,328 | | | 96,224 | |
| Loss on extinguishment of debt | 2,122 | | | 12,327 | | | 2,155 | |
| Other expense (income), net | 1,052 | | | (1,366) | | | 1,625 | |
| Total other expense, net | 67,461 | | | 93,289 | | | 100,004 | |
| Income before income taxes | 262,458 | | | 204,113 | | | 191,151 | |
| Provision for income taxes | 57,077 | | | 39,431 | | | 48,870 | |
| Net income | $ | 205,381 | | | $ | 164,682 | | | $ | 142,281 | |
| | | | | |
| Earnings per share: | | | | | |
| Basic | $ | 4.09 | | | $ | 3.28 | | | $ | 2.81 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Diluted | $ | 4.04 | | | $ | 3.25 | | | $ | 2.78 | |
| | | | | |
| | | | | |
| | | | | |
| Weighted average shares outstanding: | | | | | |
| Basic | 50,259 | | | 50,210 | | | 50,723 | |
| Diluted | 50,842 | | | 50,605 | | | 51,140 | |
| | | | | |
| Comprehensive income, net of tax: | | | | | |
| Currency translation adjustments | (1,296) | | | 20,333 | | | (12,363) | |
| Unrealized gain (loss) on interest rate swaps | 1,819 | | | 3,045 | | | (4,864) | |
| Unrecognized net gain (loss) on pension plans | 246 | | | 1,172 | | | (1,187) | |
| Net gain on pension distribution reclassified to net income | — | | | (190) | | | — | |
| Total other comprehensive income (loss) | 769 | | | 24,360 | | | (18,414) | |
| Comprehensive income | $ | 206,150 | | | $ | 189,042 | | | $ | 123,867 | |
See accompanying notes.
Prestige Consumer Healthcare Inc.
Consolidated Balance Sheets | | | | | | | | | | | |
| (In thousands) | March 31, |
| Assets | 2022 | | 2021 |
| Current assets | | | |
| Cash and cash equivalents | $ | 27,185 | | | $ | 32,302 | |
| Accounts receivable, net of allowance of $19,720 and $16,457, respectively | 139,330 | | | 114,671 | |
| Inventories | 120,342 | | | 114,959 | |
| | | |
| Prepaid expenses and other current assets | 6,410 | | | 7,903 | |
| Total current assets | 293,267 | | | 269,835 | |
| | | |
| Property, plant and equipment, net | 71,300 | | | 70,059 | |
| Operating lease right-of-use assets | 20,372 | | | 23,722 | |
| Finance lease right-of-use assets, net | 6,858 | | | 8,986 | |
| Goodwill | 578,976 | | | 578,079 | |
| Intangible assets, net | 2,696,635 | | | 2,475,729 | |
| Other long-term assets | 3,273 | | | 2,863 | |
| Total Assets | $ | 3,670,681 | | | $ | 3,429,273 | |
| | | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities | | | |
| Accounts payable | $ | 55,760 | | | $ | 45,978 | |
| Accrued interest payable | 4,437 | | | 6,312 | |
| Operating lease liabilities, current portion | 6,360 | | | 5,858 | |
| Finance lease liabilities, current portion | 2,752 | | | 2,588 | |
| Other accrued liabilities | 74,113 | | | 61,402 | |
| Total current liabilities | 143,422 | | | 122,138 | |
| | | |
| | | |
| | | |
| Long-term debt, net | 1,476,658 | | | 1,479,653 | |
| Deferred income tax liabilities | 444,917 | | | 434,050 | |
| Long-term operating lease liabilities, net of current portion | 16,088 | | | 19,706 | |
| Long-term finance lease liabilities, net of current portion | 4,501 | | | 6,816 | |
| Other long-term liabilities | 7,484 | | | 8,612 | |
| Total Liabilities | 2,093,070 | | | 2,070,975 | |
| | | |
| Commitments and Contingencies – Note 17 | 0 | | 0 |
| | | |
| Stockholders’ Equity | | | |
| Preferred stock – $0.01 par value | | | |
| Authorized – 5,000 shares | | | |
| Issued and outstanding – None | — | | | — | |
| | | |
| Common stock – $0.01 par value | | | |
| Authorized – 250,000 shares | | | |
| Issued – 54,430 shares at March 31, 2022 and 53,999 shares at March 31, 2021 | 544 | | | 540 | |
| Additional paid-in capital | 515,583 | | | 499,508 | |
| Treasury stock, at cost – 4,151 shares at March 31, 2022 and 4,088 shares at March 31, 2021 | (133,648) | | | (130,732) | |
| Accumulated other comprehensive loss, net of tax | (19,032) | | | (19,801) | |
| Retained earnings | 1,214,164 | | | 1,008,783 | |
| Total Stockholders’ Equity | 1,577,611 | | | 1,358,298 | |
| Total Liabilities and Stockholders’ Equity | $ | 3,670,681 | | | $ | 3,429,273 | |
See accompanying notes.
Prestige Consumer Healthcare Inc.
Consolidated Statements of Changes in Stockholders’ Equity | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-in Capital | | Treasury Stock | | Accumulated
Other
Comprehensive Income (Loss) | | | | Retained
Earnings | | Total |
| (In thousands) | Shares | | Par
Value | | | Shares | | Amount | | | | |
| Balances at March 31, 2019 | 53,670 | | | $ | 536 | | | $ | 479,150 | | | 1,871 | | | $ | (59,928) | | | $ | (25,747) | | | | | $ | 701,820 | | | $ | 1,095,831 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 7,644 | | | — | | | — | | | — | | | | | — | | | 7,644 | |
| Exercise of stock options | 48 | | | 1 | | | 1,323 | | | — | | | — | | | — | | | | | — | | | 1,324 | |
| | | | | | | | | | | | | | | | | |
| Issuance of shares related to restricted stock | 87 | | | 1 | | | (1) | | | — | | | — | | | — | | | | | — | | | — | |
| Treasury share repurchases | — | | | — | | | — | | | 1,848 | | | (57,695) | | | — | | | | | — | | | (57,695) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | | | 142,281 | | | 142,281 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | (18,414) | | | | | — | | | (18,414) | |
| | | | | | | | | | | | | | | | | |
| Balances at March 31, 2020 | 53,805 | | | $ | 538 | | | $ | 488,116 | | | 3,719 | | | $ | (117,623) | | | $ | (44,161) | | | | | $ | 844,101 | | | $ | 1,170,971 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 8,543 | | | — | | | — | | | — | | | | | — | | | 8,543 | |
| Exercise of stock options | 120 | | | 1 | | | 2,850 | | | — | | | — | | | — | | | | | — | | | 2,851 | |
| | | | | | | | | | | | | | | | | |
| Issuance of shares related to restricted stock | 74 | | | 1 | | | (1) | | | — | | | — | | | — | | | | | — | | | — | |
| Treasury share repurchases | — | | | — | | | — | | | 369 | | | (13,109) | | | — | | | | | — | | | (13,109) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | | | 164,682 | | | 164,682 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Other comprehensive income | — | | | — | | | — | | | — | | | — | | | 24,360 | | | | | — | | | 24,360 | |
| | | | | | | | | | | | | | | | | |
| Balances at March 31, 2021 | 53,999 | | | $ | 540 | | | $ | 499,508 | | | 4,088 | | | $ | (130,732) | | | $ | (19,801) | | | | | $ | 1,008,783 | | | $ | 1,358,298 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 9,039 | | | — | | | — | | | — | | | | | — | | | 9,039 | |
| Exercise of stock options | 226 | | | 2 | | | 7,038 | | | — | | | — | | | — | | | | | — | | | 7,040 | |
| | | | | | | | | | | | | | | | | |
| Issuance of shares related to restricted stock | 205 | | | 2 | | | (2) | | | — | | | — | | | — | | | | | — | | | — | |
| Treasury share repurchases | — | | | — | | | — | | | 63 | | | (2,916) | | | — | | | | | — | | | (2,916) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | | | 205,381 | | | 205,381 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Other comprehensive | — | | | — | | | — | | | — | | | — | | | 769 | | | | | — | | | 769 | |
| | | | | | | | | | | | | | | | | |
| Balances at March 31, 2022 | 54,430 | | | $ | 544 | | | $ | 515,583 | | | 4,151 | | | $ | (133,648) | | | $ | (19,032) | | | | | $ | 1,214,164 | | | $ | 1,577,611 | |
See accompanying notes.
Prestige Consumer Healthcare Inc.
Consolidated Statements of Cash Flows | | | | | | | | | | | | | | | | | |
| | Year Ended March 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Operating Activities | | | | | |
| Net income | $ | 205,381 | | | $ | 164,682 | | | $ | 142,281 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | |
| Depreciation and amortization | 32,092 | | | 30,164 | | | 28,995 | |
| | | | | |
| Loss on sale or disposal of property and equipment | 271 | | | 220 | | | 713 | |
| | | | | |
| Deferred income taxes | 9,979 | | | 18,628 | | | 13,852 | |
| | | | | |
| Amortization of debt origination costs | 4,230 | | | 4,979 | | | 3,812 | |
| | | | | |
| Stock-based compensation costs | 9,039 | | | 8,543 | | | 7,644 | |
| | | | | |
| Loss on extinguishment of debt | 2,122 | | | 12,327 | | | 2,155 | |
| Non-cash operating lease cost | 6,706 | | | 7,082 | | | 8,786 | |
| | | | | |
| | | | | |
| | | | | |
| Impairment loss | 1,057 | | | 2,434 | | | — | |
| | | | | |
| Other | (9) | | | (7,854) | | | 84 | |
| Changes in operating assets and liabilities | | | | | |
| Accounts receivable | (24,654) | | | 36,872 | | | (2,849) | |
| Inventories | 663 | | | 2,972 | | | 2,930 | |
| Prepaid expenses and other current assets | 1,448 | | | (3,227) | | | 687 | |
| Accounts payable | 9,154 | | | (17,342) | | | 6,210 | |
| Accrued liabilities | 9,616 | | | (14,912) | | | 12,096 | |
| Operating lease liabilities | (6,448) | | | (6,718) | | | (8,824) | |
| | | | | |
| Other | (725) | | | (3,243) | | | (1,448) | |
| Net cash provided by operating activities | 259,922 | | | 235,607 | | | 217,124 | |
| | | | | |
| Investing Activities | | | | | |
| Purchases of property, plant and equipment | (9,642) | | | (22,243) | | | (14,560) | |
| | | | | |
| Escrow receipt | — | | | — | | | 750 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Acquisitions | (247,046) | | | — | | | (2,760) | |
| | | | | |
| | | | | |
| | | | | |
| Other | 177 | | | — | | | — | |
| Net cash used in investing activities | (256,511) | | | (22,243) | | | (16,570) | |
| | | | | |
| Financing Activities | | | | | |
| | | | | |
| | | | | |
| Proceeds from issuance of senior notes | — | | | 600,000 | | | 400,000 | |
| | | | | |
| | | | | |
| | | | | |
| Repayment of senior notes | — | | | (600,000) | | | (400,000) | |
| | | | | |
| | | | | |
| | | | | |
| Term Loan repayments | (600,000) | | | (195,000) | | | (48,000) | |
| Proceeds from refinancing of Term Loan | 597,000 | | | — | | | — | |
| Borrowings under revolving credit agreement | 85,000 | | | 15,000 | | | 100,000 | |
| Repayments under revolving credit agreement | (85,000) | | | (70,000) | | | (120,000) | |
| Payment of debt costs | (6,111) | | | (17,718) | | | (6,584) | |
| Payments of finance leases | (2,582) | | | (1,443) | | | (476) | |
| Proceeds from exercise of stock options | 7,040 | | | 2,851 | | | 1,324 | |
| | | | | |
| Fair value of shares surrendered as payment of tax withholding | (2,916) | | | (1,242) | | | (974) | |
| Repurchase of common stock | — | | | (11,867) | | | (56,721) | |
| Net cash used in financing activities | (7,569) | | | (279,419) | | | (131,431) | |
| | | | | |
| Effects of exchange rate changes on cash and cash equivalents | (959) | | | 3,597 | | | (1,893) | |
| (Decrease) increase in cash and cash equivalents | (5,117) | | | (62,458) | | | 67,230 | |
| Cash and cash equivalents - beginning of year | 32,302 | | | 94,760 | | | 27,530 | |
| Cash and cash equivalents - end of year | $ | 27,185 | | | $ | 32,302 | | | $ | 94,760 | |
| | | | | |
| Interest paid | $ | 61,364 | | | $ | 80,290 | | | $ | 92,166 | |
| Income taxes paid | $ | 46,568 | | | $ | 34,381 | | | $ | 30,602 | |
See accompanying notes.
Prestige Consumer Healthcare Inc.
Notes to Consolidated Financial Statements
1. Business and Basis of Presentation
Nature of Business
Prestige Consumer Healthcare Inc. (referred to herein as the “Company” or “we”, which reference shall, unless the context requires otherwise, be deemed to refer to Prestige Consumer Healthcare Inc. and all of its direct and indirect 100% owned subsidiaries on a consolidated basis) is engaged in the development, manufacturing, marketing, sales and distribution of over-the-counter (“OTC”) healthcare products to mass merchandisers, drug, food, dollar, convenience and club stores, and e-commerce channels in North America (the United States and Canada) and in Australia and certain other international markets. Prestige Consumer Healthcare Inc. is a holding company with no operations and is also the parent guarantor of the senior credit facility and the senior notes described in Note 9 to these Consolidated Financial Statements.
Economic Environment
In March 2020, the World Health Organization ("WHO") declared a global pandemic due to a new strain of coronavirus ("COVID-19"). The pandemic has caused significant volatility in the United States and global economies. In addition, the Russian invasion of Ukraine has led to further economic uncertainty. We expect economic conditions will continue to be highly volatile and uncertain and could affect demand for our products and put pressure on prices. We experienced a temporary but significant decline in consumer consumption of our brands in the first quarter of fiscal 2021, followed by more stable consumption and customer orders over the remainder of the year. Generally, throughout the pandemic, some categories were positively impacted (for instance, Women’s Health, Oral Care and Dermatological) and some categories negatively impacted (for instance, Cough & Cold and Gastrointestinal). The positively impacted categories benefited from the consumer shift to over-the-counter healthcare products, as consumers increased their focus on hygiene and self-care at home related to COVID-19. The declining categories were impacted by reduced incidence levels and usage rates due to shelter-at-home restrictions and limited travel-related activity. In fiscal 2022, we experienced solid consumer consumption and share gains across most of our brand portfolio. Our business also benefited from a significant increase in demand in travel-related categories and channels as well as the Cough & Cold category, previously impacted by the COVID-19 virus. We have continued to see changes in the purchasing patterns of our consumers, including the frequency of visits by consumers to retailers and a shift in many markets to purchasing our products online.
Both the COVID-19 pandemic and the geopolitical environment have also impacted the supply of labor and raw materials and exacerbated rising costs. Although we have not experienced a material disruption to our overall supply chain to date, we have and may continue to experience delays and backorders for certain ingredients and products, difficulty scheduling shipping for our products, as well as price increases from many of our suppliers for both shipping and product costs. In addition, labor shortages have impacted our manufacturing operations and may impact our ability to supply certain products to our customers. To date, the pandemic and other global conditions have not had a material negative impact on our operations, supply chain, overall costs or demand for most of our products or resulting aggregate sales and earnings, and, as such, it has also not negatively impacted our liquidity position. We continue to generate operating cash flows to meet our short-term liquidity needs. These circumstances could change, however, in this dynamic, unprecedented environment. If the COVID-19 outbreak worsens or geopolitical conditions cause further disruption in the global supply chain, the availability of labor or otherwise increase costs, it may materially affect our operations and those of third parties on which we rely, including causing disruptions in the supply and distribution of our products. The extent to which these conditions impact our results and liquidity will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity and duration of any further COVID-19 outbreak and recovery period and further global instability. These effects could have a material adverse impact on our business, liquidity, capital resources, and results of operations and those of the third parties on which we rely.
Basis of Presentation
Our Consolidated Financial Statements are prepared in accordance with accounting principles generally accepted in the United States of America ("GAAP"). All significant intercompany transactions and balances have been eliminated in consolidation. Our fiscal year ends on March 31st of each year. References in these Consolidated Financial Statements or notes to a year (e.g., “2022”) mean our fiscal year ended on March 31st of that year.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Although these estimates are
based on our knowledge of current events and actions that we may undertake in the future, actual results could differ from those estimates. As discussed below, our most significant estimates include those made in connection with the valuation of intangible assets, stock-based compensation, fair value of debt, sales returns and allowances, trade promotional allowances, inventory obsolescence, and accounting for income taxes and related uncertain tax positions.
Cash and Cash Equivalents
We consider all short-term deposits and investments with original maturities of three months or less to be cash equivalents. At March 31, 2022, approximately 44% of our cash is held by a bank in Australia and approximately 18% is held by a bank in Singapore. Substantially all of our remaining cash is held by a large U.S. domestic bank. We do not believe that, as a result of this concentration, we are subject to any unusual financial risk beyond the normal risk associated with commercial banking relationships. Substantially all of the Company's cash balances at March 31, 2022 are uninsured.
Accounts Receivable
We extend non-interest-bearing trade credit to our customers in the ordinary course of business. We maintain an allowance for doubtful accounts receivable based upon historical collection experience and expected collectability of the accounts receivable. In an effort to reduce credit risk, we (i) have established credit limits for all of our customer relationships, (ii) perform ongoing credit evaluations of customers’ financial condition, (iii) monitor the payment history and aging of customers’ receivables, and (iv) monitor open orders against an individual customer’s outstanding receivable balance.
Inventories
Inventories are stated at the lower of cost or net realizable value, where cost is determined by using the first-in, first-out method. We reduce inventories for the diminution of value resulting from product obsolescence, damage or other issues affecting marketability, equal to the difference between the cost of the inventory and its estimated net realizable value. Factors utilized in the determination of estimated net realizable value include (i) product expiration dates, (ii) current sales data and historical return rates, (iii) estimates of future demand, (iv) competitive pricing pressures, (v) new product introductions, and (vi) component and packaging obsolescence.
Property, Plant and Equipment
Property, plant and equipment are stated at cost and are depreciated using the straight-line method based on the following estimated useful lives:
| | | | | |
| | Years |
| Building | 5 to 40 |
| Machinery | 3 to 15 |
| Computer equipment and software | 3 to 5 |
| Furniture and fixtures | 7 to 10 |
| Leasehold improvements | * |
*Leasehold improvements are amortized over the lesser of the lease term or the estimated useful life of the related assets.
Expenditures for maintenance and repairs are charged to expense as incurred. When an asset is sold or otherwise disposed of, we remove the cost and associated accumulated depreciation from the respective accounts and recognize the resulting gain or loss in the Consolidated Statements of Income and Comprehensive Income.
Property, plant and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. An impairment loss is recognized if the carrying amount of the asset exceeds its fair value.
Goodwill
The excess of the purchase price over the fair market value of assets acquired and liabilities assumed in business combinations is classified as goodwill. Goodwill is not amortized, although the carrying value is tested for impairment at least annually in the fourth fiscal quarter of each year, or more frequently if events or changes in circumstances indicate that the asset may be impaired. Goodwill is tested for impairment at the reporting unit level, which is one level below the operating segment level. An impairment loss is recognized if the carrying amount of the reporting unit exceeds its fair value.
Intangible Assets
Intangible assets generally represent tradenames, brand names and patents and are stated at cost less accumulated amortization. For intangible assets with finite lives, amortization is computed using the straight-line method over estimated useful lives, typically ranging from 10 to 30 years.
Indefinite-lived intangible assets are tested for impairment at the individual asset level at least annually in the fourth fiscal quarter of each year, or more frequently if events or changes in circumstances indicate that the asset may be impaired. Intangible assets with finite lives are reviewed for impairment on an annual basis, or whenever events or changes in circumstances indicate that their carrying amount may exceed their fair values and may not be recoverable. An impairment loss is recognized if the carrying amount of the asset exceeds its fair value.
Debt Origination Costs
We have incurred debt origination costs in connection with the issuance of long-term debt. These costs are amortized over the term of the related debt, using the effective interest method for our senior notes and our term loan facility and the straight-line method for our revolving credit facility. Costs associated with our revolving credit facility are reported as a long-term asset and costs related to our senior notes and the term loan facility are recorded as a reduction of debt.
Revenue Recognition
Nature of Goods and Services
We recognize revenue from product sales. We primarily ship finished goods to our customers and operate in 2 segments: North American OTC Healthcare and International OTC Healthcare. The segments are based on differences in geographical area. The North America and International OTC Healthcare segments market a variety of personal care and over-the-counter products in the following product groups: Analgesics, Cough & Cold, Women's Health, Gastrointestinal, Eye & Ear Care, Dermatologicals, and Oral Care. Our products are distinct and separately identifiable on customer contracts or invoices, with each product sale representing a separate performance obligation.
We sell consumer products under a variety of brands through a broad distribution platform that includes mass merchandisers, drug, food, dollar, convenience and club stores, and e-commerce channels, all of which sell our products to consumers.
See Note 19 for disaggregated revenue information.
Satisfaction of Performance Obligations
Under ASC 606, revenue is recognized when control of a promised good is transferred to a customer, in an amount that reflects the consideration that we expect to be entitled to receive in exchange for that good. This occurs either when finished goods are transferred to a common carrier for delivery to the customer or when product is picked up by the customer or the customer’s carrier.
Once a product has transferred to the common carrier or been picked up by the customer, the customer is able to direct the use of, and obtain substantially all of the remaining benefits from, the product. It is at this point that we have a right to payment and the customer has legal title.
Variable Consideration
Provisions for certain rebates, customer promotional programs, product returns, and discounts to customers are accounted for as variable consideration and recorded as a reduction in sales.
We record an estimate of future product returns, chargebacks and logistic deductions concurrent with recording sales, which is made using the most likely amount method that incorporates (i) historical return rates, (ii) current economic trends, (iii) changes in customer demand, (iv) product acceptance, (v) seasonality of our product offerings, and (vi) the impact of changes in product formulation, packaging and advertising.
We participate in the promotional programs of our customers to enhance the sale of our products. These promotional programs consist of direct-to-consumer incentives, such as coupons and temporary price reductions, as well as incentives to our customers, such as allowances for new distribution, including slotting fees, and cooperative advertising. The costs of such activities are recorded as a reduction to revenue when the related sale takes place. Estimates of the costs of these promotional programs are derived using the most likely amount method, which incorporates (i) historical sales experience, (ii) the current promotional offering, (iii) forecasted data, (iv) current market conditions, and (v) communication with customer purchasing/marketing personnel. At the completion of the promotional program, the estimated amounts are adjusted to actual results.
Practical Expedients
Due to the nature (short duration) of our contracts with customers, we apply the practical expedient related to the disclosure of remaining performance obligations. Remaining performance obligations relate to contracts with a duration of less than one year, in which we have the right to invoice the customer at the time the performance obligation is satisfied for the amount of revenue recognized at that time. Accordingly, we have elected the practical expedient available under ASC 606 not to disclose
remaining performance obligations for our contracts. The period between when control of the promised products transfers to the customer and when the customer pays for the products is one year or less. As such, we do not adjust product consideration for the effects of a significant financing component. The amortization period of any asset resulting from incremental costs of obtaining a contract would be one year or less.
We expense incremental direct costs of obtaining a contract (broker commissions) when the related sale takes place.
We account for shipping and handling costs as fulfillment activities and therefore recognize them upon shipment of goods.
Cost of Sales
Cost of sales includes costs related to the manufacturing of our products, including raw materials, direct labor and indirect plant costs (including but not limited to depreciation), warehousing costs, inbound and outbound shipping costs, and handling and storage costs. Warehousing, shipping and handling and storage costs were $67.8 million for 2022, $52.1 million for 2021 and $61.9 million for 2020.
Advertising and Marketing Costs
Advertising and marketing costs are expensed as incurred. Allowances for distribution costs associated with products, including slotting fees, are recognized as a reduction of sales.
Stock-based Compensation
We recognize stock-based compensation expense by measuring the cost of services to be rendered based on the grant-date fair value of the equity award. Compensation expense is recognized over the period a grantee is required to provide service in exchange for the award, generally referred to as the requisite service period.
Pension Expense
Certain employees of our Lynchburg manufacturing facility are covered by defined benefit pension plans. The Company’s policy is to contribute at least the minimum amount required under The Employee Retirement Income Security Act of 1974 ("ERISA"). The Company may elect to make additional contributions. Benefits are based on years of service and levels of compensation. On December 16, 2014, the decision was made to freeze the benefits under the Company's U.S. qualified defined benefit pension plan with an effective date of March 1, 2015. During the third quarter of 2021, we offered participants of our qualified defined benefit plan the option to receive a lump sum payout of their benefits. The amount paid out of plan assets during the third quarter of 2021 to those who elected to take the lump sum payout was $7.0 million and we recognized a settlement gain of $0.2 million as a result of the payout. During the fourth quarter of 2021, we adopted a plan termination date of April 30, 2021 for the U.S. qualified defined benefit pension plan and began the plan termination process. The plan termination is expected to be completed in the first quarter of fiscal 2023.
The funded status of our pension plans is dependent upon many factors, including returns on invested assets and the level of certain market interest rates. We review pension assumptions regularly, and we may from time to time make voluntary contributions to our pension plans that exceed the amounts required by statute. Changes in interest rates and the market value of the securities held by the plans could materially change the funded status of the plans, positively or negatively, and affect the level of pension expense and required contributions in fiscal 2023 and beyond.
Income Taxes
On December 22, 2017, the Tax Cuts and Jobs Act (the "TCJA") was signed into law. The TCJA, among other things, reduced the U.S. federal corporate tax rate from 35% to 21% and imposed a new minimum tax on Global Intangible Low-Taxed Income ("GILTI") earned by foreign subsidiaries. In July 2020, final regulations were issued for GILTI, which include a high-tax exception for certain income earned by foreign subsidiaries if the foreign tax rate is in excess of 90% of the U.S. corporate tax rate of 21%.
Deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Income Taxes topic of the FASB ASC 740 prescribes a recognition threshold and measurement attributes for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The guidance only allows the recognition of those tax benefits that have a greater than 50% likelihood of being sustained upon examination by the various taxing authorities. As a result, we have applied such guidance in determining our tax uncertainties.
We are subject to taxation in the United States and various state and foreign jurisdictions.
We classify penalties and interest related to unrecognized tax benefits as income tax expense in the Consolidated Statements of Income and Comprehensive Income.
Earnings Per Share
Basic earnings per share is computed based on income available to common stockholders and the weighted average number of shares of common stock outstanding during the period. Diluted earnings per share is computed based on income available to common stockholders and the weighted average number of shares of common stock outstanding plus the effect of potentially dilutive common shares outstanding during the period using the treasury stock method, which includes stock options and restricted stock units ("RSUs"). Potential common shares, composed of the incremental common shares issuable upon the exercise of outstanding stock options and unvested RSUs, are included in the diluted earnings per share calculation to the extent that they are dilutive. In loss periods, the assumed exercise of in-the-money stock options and RSUs has an antidilutive effect, and therefore these instruments are excluded from the computation of diluted earnings per share. The following table sets forth the computation of basic and diluted earnings per share:
| | | | | | | | | | | | | | | | | |
| Year Ended March 31, |
| (In thousands, except per share data) | 2022 | | 2021 | | 2020 |
| Numerator | | | | | |
| Net income | $ | 205,381 | | | $ | 164,682 | | | $ | 142,281 | |
| | | | | |
| | | | | |
| | | | | |
| Denominator | | | | | |
| Denominator for basic earnings per share - weighted average shares outstanding | 50,259 | | | 50,210 | | | 50,723 | |
| Dilutive effect of unvested restricted stock units and options issued to employees and directors | 583 | | | 395 | | | 417 | |
| Denominator for diluted earnings per share | 50,842 | | | 50,605 | | | 51,140 | |
| | | | | |
| Earnings per Common Share: | | | | | |
| Basic net earnings per share | $ | 4.09 | | | $ | 3.28 | | | $ | 2.81 | |
| | | | | |
| | | | | |
| | | | | |
| Diluted net earnings per share | $ | 4.04 | | | $ | 3.25 | | | $ | 2.78 | |
| | | | | |
| | | | | |
For 2022, 2021, and 2020 there were 0.4 million, 0.5 million, and 0.3 million shares, respectively, attributable to outstanding stock-based awards that were excluded from the calculation of diluted earnings per share because their inclusion would have been anti-dilutive.
Leases
We lease real estate and equipment for use in our operations. These leases have lease terms of 1 to 10 years, some of which include options to terminate or extend leases for up to 1 to 5 years or on a month-to-month basis. The exercise of lease renewal options is at our sole discretion and our lease right-of-use ("ROU") assets and liabilities reflect only the options we are reasonably certain that we will exercise.
We determine if an arrangement is or contains a lease at inception by assessing whether the arrangement contains an identified asset and whether we have the right to control the identified asset. ROU assets represent our right to use an underlying asset for the lease term, and lease liabilities represent our obligation to make lease payments arising from the lease. Lease liabilities are recognized at the lease commencement date based on the present value of future lease payments over the lease term. ROU assets are based on the measurement of the lease liability and also include any lease payments made prior to or on lease commencement and exclude lease incentives and initial direct costs incurred, as applicable.
Variable lease payments, which do not vary based on an index or rate, are excluded from the ROU asset and lease liability determination. Variable lease payments are typically usage-based and are recorded in the period in which the obligation for those payments is incurred. Our lease agreements do not contain any material residual value guarantees or material restrictive covenants.
As the implicit rate in our leases is unknown, we used our incremental borrowing rate based on the information available at the date of adoption for existing leases and at the lease commencement date for new leases in determining the present value of future lease payments. We give consideration to our credit risk, term of the lease, total lease payments and adjust for the
impacts of collateral, as necessary, when calculating our incremental borrowing rates. Rent expense for our operating leases is recognized on a straight-line basis over the lease term.
For the measurement and classification of our lease agreements, we group lease and non-lease components into a single lease component for all underlying asset classes. We have also elected to exclude any leases within our existing classes of assets with a term of twelve months or less.
Recently Adopted Accounting Pronouncements
In December 2019, the FASB issued Accounting Standards Update ("ASU") 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. The amendments in this update eliminate the need for an organization to analyze whether certain exceptions apply for tax purposes. It also simplifies GAAP for certain taxes. The amendments in these updates are effective for us for fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. We adopted this standard effective April 1, 2021, and the adoption of this standard did not have a material impact on our Consolidated Financial Statements.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement. The amendments in this update modify the disclosure requirements in Topic 820, with a particular focus on Level 3 investments, by eliminating certain required disclosures and incorporating others. The amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. We adopted this standard effective April 1, 2020, and the adoption did not have a material impact on our Consolidated Financial Statements.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326) - Measurement of Credit Losses on Financial Instruments (with subsequent targeted amendments). The amendments in this update provide financial statement users with more useful information about expected credit losses on financial instruments and other commitments to extend credit held by a reporting entity at each reporting date. The guidance requires entities to utilize an expected credit loss model for certain financial instruments, including most trade receivables, which replaces the incurred credit loss model previously used. Under this new model, we are required to recognize estimated credit losses expected to occur over time using a broad range of information including historical information, current conditions and reasonable and supportable forecasts. The amendments in these updates were effective for us in the first quarter of our fiscal year 2021. We adopted this standard effective April 1, 2020 using the modified retrospective approach, and the adoption did not have a material impact on our Consolidated Financial Statements.
In August 2018, the FASB issued ASU 2018-14, Compensation - Retirement Benefits - Defined Benefit Plans - General (Topic 715-20): Disclosure Framework - Changes to the Disclosure Requirements for Defined Benefit Plans. The amendments in this update modify the disclosure requirements for employers that sponsor defined benefit pension or other postretirement plans by eliminating certain required disclosures and incorporating others. The amendments are effective for public companies for fiscal years ending after December 15, 2020. We adopted this standard effective April 1, 2021 and the adoption did not have a material impact on our Consolidated Financial Statements.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). This update amended a number of aspects of lease accounting, including requiring lessees to recognize all leases with a term greater than one year as a ROU asset and corresponding lease liability, measured at the present value of the lease payments. On April 1, 2019, we adopted Topic 842 using the modified retrospective approach. Results for the years ended March 31, 2022, 2021 and 2020 are presented under Topic 842.
Recently Issued Accounting Pronouncements
In March 2022, the FASB issued ASU 2022-02, Financial Instruments - Credit Losses (Topic 326): Troubled Debt Restructurings and Vintage Disclosures. This ASU responds to feedback received by the FASB during the post-implementation review of ASU 2016-13, Financial Instruments - Credit Losses (Topic 326) - Measurement of Credit Losses on Financial Instruments, which we adopted effective April 1, 2020 (see Recently Adopted Accounting Pronouncements above). The amendments in this update, among other things, eliminate the troubled debt restructuring recognition and measurement guidance and, instead, require the entity to evaluate whether the modification represents a new loan or a continuation of an existing loan. This ASU is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. The adoption of the standard is not expected to have a material effect on our Consolidated Financial Statements.
In March 2022, the FASB issued ASU 2022-01, Derivatives and Hedging (Topic 815): Fair Value Hedging - Portfolio Layer Method. The purpose of the ASU is to address questions raised on ASU 2017-12, Derivatives and Hedging (Topic 815): Targeted Improvements to Accounting for Hedging Activities. This ASU expands the currently used single-layer method of hedge accounting to allow multiple layers of a single closed portfolio under the method. This ASU is effective for fiscal years
beginning after December 15, 2022, and interim periods within those fiscal years. The impact of adoption of this new standard is not expected to have a material effect on our Consolidated Financial Statements.
In October 2021, the FASB issued ASU 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers. This ASU requires entities to apply Topic 606 to recognize and measure contract assets and liabilities in a business combination. This ASU is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. The impact of adoption of this new standard will depend on the magnitude of future acquisitions.
In March 2020, the FASB issued ASU 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting. This ASU provides optional expedient and exceptions for applying generally accepted accounting principles to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. In response to the concerns about structural risks of interbank offered rates (“IBORs”) and, particularly, the risk of cessation of the London Interbank Offered Rate (“LIBOR”), regulators in several jurisdictions around the world have undertaken reference rate reform initiatives to identify alternative reference rates that are more observable or transaction based and less susceptible to manipulation. The ASU provides companies with optional guidance to ease the potential accounting burden associated with transitioning away from reference rates that are expected to be discontinued. In January 2021, the FASB issued ASU 2021-01, which adds implementation guidance to clarify certain optional expedients in Topic 848. The ASUs can be adopted no later than December 31, 2022, with early adoption permitted. The adoption of the standard is not expected to have a material effect on our Consolidated Financial Statements.
2. Acquisition
Akorn
On July 1, 2021, we completed the acquisition of the consumer health business assets from Akorn Operating Company LLC ("Akorn"), pursuant to an Asset Purchase Agreement, dated May 27, 2021 (the "Purchase Agreement"), for a purchase price of $228.9 million in cash, subject to certain closing adjustments specified in the Purchase Agreement. As a result of the purchase, we acquired TheraTears and certain other over-the-counter consumer brands. The financial results from this acquisition are included in our North American and International OTC Healthcare segments. The purchase price was funded by a combination of available cash on hand, additional borrowings under our asset-based revolving credit facility (the "2012 ABL Revolver") and the net proceeds from the refinancing of our term loan entered into on January 31, 2012 (the "2012 Term Loan") (see Note 9).
The acquisition was accounted for as a business combination. During 2022, we incurred acquisition-related costs of $5.1 million, which are included in General and administrative expense. In connection with the acquisition, we also entered into a supply arrangement with Akorn for a term of three years with optional renewals at prevailing market rates.
We prepared an analysis of the fair values of the assets acquired and liabilities assumed as of the date of acquisition. These purchase price allocations are preliminary as we are in the process of finalizing the valuation. The following table summarizes our preliminary allocation of the assets acquired and liabilities assumed as of the July 1, 2021 acquisition date.
| | | | | | |
| (In thousands) | | |
| July 1, 2021 | |
| | |
| | |
| Inventories | $ | 6,455 | | |
| | |
| | |
| Goodwill | 1,648 | | |
| Intangible assets | 225,410 | | |
| | |
| Total assets acquired | 233,513 | | |
| | |
| Accounts payable | 478 | | |
| Reserves for sales allowances | 747 | | |
| Other accrued liabilities | 3,374 | | |
| | |
| | |
| Total liabilities assumed | 4,599 | | |
| Total purchase price | $ | 228,914 | | |
Based on this preliminary analysis, we allocated $195.9 million to non-amortizable intangible assets and $29.5 million to amortizable intangible assets. The non-amortizable intangible assets are classified as trademarks and, of the amortizable intangible assets, $20.4 million are classified as customer relationships and $9.1 million are classified as trademarks. We are amortizing the purchased amortizable intangible assets on a straight-line basis over an estimated weighted average useful life of 12.5 years (see Note 6).
We recorded goodwill of $1.6 million based on the amount by which the purchase price exceeded the preliminary estimate of the fair value of the net assets acquired (see Note 5). Goodwill is deductible and is being amortized for income tax purposes.
The financial impact of this acquisition was not material to our Consolidated Financial Statements, and, therefore, we have not presented pro forma results of operations for the acquisition.
3. Inventories
Inventories consist of the following: | | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Components of Inventories | | | |
| Packaging and raw materials | $ | 16,984 | | | $ | 8,463 | |
| Work in process | 338 | | | 326 | |
| Finished goods | 103,020 | | | 106,170 | |
| Inventories | $ | 120,342 | | | $ | 114,959 | |
Inventories are carried and depicted above at the lower of cost or net realizable value, which includes a reduction in inventory values of $4.9 million and $4.0 million at March 31, 2022 and 2021, respectively, related to obsolete and slow-moving inventory.
4. Property, Plant and Equipment
Property, plant and equipment, net consist of the following: | | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Components of Property, Plant and Equipment | | | |
| Land | $ | 550 | | | $ | 550 | |
| Building | 29,046 | | | 27,180 | |
| Machinery | 48,390 | | | 45,371 | |
| Computer equipment | 25,245 | | | 24,449 | |
| Furniture and fixtures | 3,238 | | | 3,256 | |
| Leasehold improvements | 9,080 | | | 9,071 | |
| Construction in progress | 16,466 | | | 14,537 | |
| | 132,015 | | | 124,414 | |
| Accumulated depreciation | (60,715) | | | (54,355) | |
| Property, plant and equipment, net | $ | 71,300 | | | $ | 70,059 | |
We recorded depreciation expense of $8.0 million, $8.5 million, and $8.8 million for 2022, 2021, and 2020, respectively.
5. Goodwill
The following table summarizes the changes in the carrying value of goodwill by operating segment for each of 2020, 2021, and 2022:
| | | | | | | | | | | | | | | | | | | |
| (In thousands) | North American OTC Healthcare | | International OTC Healthcare | | | | Consolidated |
| Balance – March 31, 2020 | | | | | | | |
| Goodwill | $ | 710,354 | | | $ | 28,536 | | | | | $ | 738,890 | |
| Accumulated impairment losses | (163,711) | | | — | | | | | (163,711) | |
| Balance - March 31, 2020 | 546,643 | | | 28,536 | | | | | 575,179 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Effects of foreign currency exchange rates | — | | | 4,147 | | | | | 4,147 | |
| Impairment loss | — | | | (1,247) | | | | | (1,247) | |
| | | | | | | |
| Balance – March 31, 2021 | | | | | | | |
| Goodwill | 710,354 | | | 32,683 | | | | | 743,037 | |
| Accumulated impairment losses | (163,711) | | | (1,247) | | | | | (164,958) | |
| Balance - March 31, 2021 | $ | 546,643 | | | $ | 31,436 | | | | | $ | 578,079 | |
| | | | | | | |
| Acquisitions | 1,648 | | | — | | | | | 1,648 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Effects of foreign currency exchange rates | — | | | (411) | | | | | (411) | |
| Impairment loss | — | | | (340) | | | | | (340) | |
| | | | | | | |
| Balance – March 31, 2022 | | | | | | | |
| Goodwill | 712,002 | | | 32,272 | | | | | 744,274 | |
| Accumulated impairment losses | (163,711) | | | (1,587) | | | | | (165,298) | |
| Balance - March 31, 2022 | $ | 548,291 | | | $ | 30,685 | | | | | $ | 578,976 | |
As discussed in Note 2, on July 1, 2021, we completed the acquisition of the consumer health business assets from Akorn. In connection with this acquisition, we recorded goodwill of $1.6 million based on the amount by which the purchase price exceeded the preliminary estimate of the fair value of the net assets acquired.
At February 28, 2021, in conjunction with the annual test for goodwill impairment, we recorded an impairment charge of $1.2 million to adjust the carrying amount of goodwill related to our International Analgesics reporting unit in our International OTC Healthcare segment to its fair value. The goodwill impairment was a result of the Painstop tradename impairment discussed in Note 5.
At February 28, 2022, in conjunction with the annual test for goodwill impairment, which coincides with our annual strategic planning process, we recorded an impairment charge of $0.3 million to write off the remaining goodwill related to our Painstop brand in our International OTC Healthcare segment.
We identify our reporting units in accordance with the FASB ASC Subtopic 280. The carrying value and fair value for intangible assets and goodwill for a reporting unit are calculated based on key assumptions and valuation methodologies. The discounted cash flow methodology is a widely-accepted valuation technique utilized by market participants in the transaction evaluation process and has been applied consistently. We also considered our market capitalization at February 28, 2022 and February 28, 2021, as compared to the aggregate fair values of our reporting units, to assess the reasonableness of our estimates pursuant to the discounted cash flow methodology. The estimates and assumptions made in assessing the fair value of our reporting units and the valuation of the underlying assets and liabilities are inherently subject to significant uncertainties related to future sales, gross margins, and advertising and marketing expenses, which can be impacted by increases in competition, changing consumer preferences, technical advances, increased product costs, supply chain/material cost inflation, or the potential impacts of COVID-19. The discount rate assumption may be influenced by such factors as changes in interest rates and rates of inflation, which can have an impact on the determination of fair value. If these assumptions are adversely affected, we may be required to record impairment charges in the future.
As a result of our analysis at February 28, 2022, all reporting units had a fair value that exceeded their carrying value by at least 10%. We performed a sensitivity analysis on our weighted average cost of capital and we determined that a 50 basis point increase in the weighted average cost of capital would not have resulted in any of our other reporting units' fair value being less than their carrying value. Additionally, a 50 basis point decrease in the terminal growth rate used for each reporting unit would also not have resulted in any of our other reporting units' fair value being less than their carrying value.
6. Intangible Assets
A reconciliation of the activity affecting intangible assets, net for each of 2022 and 2021 is as follows:
| | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2022 |
| (In thousands) | Indefinite-
Lived
Tradenames | | Finite-Lived
Tradenames and Customer Relationships | | Totals |
| Gross Carrying Amounts | | | | | |
| Balance – March 31, 2021 | $ | 2,281,988 | | | $ | 389,347 | | | $ | 2,671,335 | |
Additions(a) | 195,900 | | | 47,642 | | | 243,542 | |
| | | | | |
| | | | | |
| Tradename impairment | — | | | (1,700) | | | (1,700) | |
| Effects of foreign currency exchange rates | (1,329) | | | 885 | | | (444) | |
| Balance – March 31, 2022 | $ | 2,476,559 | | | $ | 436,174 | | | $ | 2,912,733 | |
| | | | | |
| Accumulated Amortization | | | | | |
| Balance – March 31, 2021 | $ | — | | | $ | 195,606 | | | $ | 195,606 | |
| Additions | — | | | 21,499 | | | 21,499 | |
| Tradename impairment | — | | | (983) | | | (983) | |
| Effects of foreign currency exchange rates | — | | | (24) | | | (24) | |
| Balance – March 31, 2022 | $ | — | | | $ | 216,098 | | | $ | 216,098 | |
| | | | | |
| Intangible assets, net – March 31, 2022 | $ | 2,476,559 | | | $ | 220,076 | | | $ | 2,696,635 | |
| | | | | |
| Intangible Assets, net by Reportable Segment: | | | | | |
| North American OTC Healthcare | $ | 2,391,517 | | | $ | 198,353 | | | $ | 2,589,870 | |
| International OTC Healthcare | 85,042 | | | 21,723 | | | 106,765 | |
| | | | | |
| Intangible assets, net – March 31, 2022 | $ | 2,476,559 | | | $ | 220,076 | | | $ | 2,696,635 | |
(a) On July 1, 2021, we completed the acquisition of certain assets from Akorn (see Note 2) and on December 15, 2021 our Australian subsidiary acquired the rights to the Zaditen brand in certain territories from Novartis Pharma AG. In connection with these acquisitions, we allocated $225.4 million to intangible assets based on our preliminary analysis for Akorn and $18.1 million for Zaditen.
| | | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2021 |
| (In thousands) | Indefinite-
Lived
Tradenames | | Finite-Lived
Tradenames and Customer Relationships | | | Totals |
| Gross Carrying Amounts | | | | | | |
| Balance – March 31, 2020 | $ | 2,265,331 | | | $ | 389,801 | | | | $ | 2,655,132 | |
| | | | | | |
| | | | | | |
| | | | | | |
| Tradename impairment | — | | | (1,186) | | | | (1,186) | |
| Effects of foreign currency exchange rates | 16,657 | | | 732 | | | | 17,389 | |
| Balance – March 31, 2021 | $ | 2,281,988 | | | $ | 389,347 | | | | $ | 2,671,335 | |
| | | | | | |
| Accumulated Amortization | | | | | | |
| Balance – March 31, 2020 | $ | — | | | $ | 175,741 | | | | $ | 175,741 | |
| Additions | | | 19,580 | | | | 19,580 | |
| | | | | | |
| Effects of foreign currency exchange rates | — | | | 285 | | | | 285 | |
| Balance – March 31, 2021 | $ | — | | | $ | 195,606 | | | | $ | 195,606 | |
| | | | | | |
| Intangible assets, net – March 31, 2021 | $ | 2,281,988 | | | $ | 193,741 | | | | $ | 2,475,729 | |
| | | | | | |
| Intangible Assets, net by Reportable Segment: | | | | | | |
| North American OTC Healthcare | $ | 2,195,617 | | | $ | 190,462 | | | | $ | 2,386,079 | |
| International OTC Healthcare | 86,371 | | | 3,279 | | | | 89,650 | |
| | | | | | |
| Intangible assets, net – March 31, 2021 | $ | 2,281,988 | | | $ | 193,741 | | | | $ | 2,475,729 | |
During the fourth quarter of each fiscal year, in conjunction with our strategic planning process, we perform our annual impairment analysis. We utilized the excess earnings method to estimate the fair value of our individual indefinite-lived intangible assets. The assumptions subject to significant uncertainties include the discount rate utilized in the analyses, as well as future sales, gross margins and advertising and marketing expenses. The discount rate assumption may be influenced by such factors as changes in interest rates and rates of inflation, which can have an impact on the determination of fair value. Additionally, should the related fair values of intangible assets be adversely affected as a result of declining sales or margins caused by competition, changing consumer needs or preferences, technological advances, increased product costs, changes in advertising and marketing expenses, or the potential impacts of COVID-19, we may be required to record impairment charges in the future.
During the third quarter of 2021, we determined that the fair value of one of our finite-lived intangible assets in our International OTC Healthcare segment, Painstop, did not exceed its carrying amount. As such, we recorded an impairment charge of $1.2 million. The decline in the fair value of Painstop was primarily related to a decline in expected future sales due to a regulatory change that now requires Painstop to be prescribed by physicians rather than sold over-the-counter direct to consumers.
At February 28, 2022, in conjunction with the annual test for impairment of intangible assets, we recorded an impairment charge of $0.7 million. In connection with our long-term planning, two non-core brands in our North American OTC Healthcare segment were discontinued and therefore the related finite-lived intangible assets were written off.
As a result of our analysis at February 28, 2022, all indefinite-lived intangible assets tested had a fair value that exceeded their carrying value by at least 10%, with the exception of our TheraTears tradename which had a fair value exceeding its carrying value by 7%. We performed a sensitivity analysis of our weighted average cost of capital, and we determined that a 50 basis point increase in the weighted average cost of capital used to value the indefinite-lived intangible assets would not have resulted in any of our indefinite-lived intangible assets' fair value being less than their carrying value, with the exception of our TheraTears tradename. The TheraTears tradename was acquired on July 1, 2021 and given the close proximity of the acquisition to the annual impairment test, we would expect the fair value to more closely approximate the carrying value. A 50 basis point increase in the weighted average cost of capital would result in an impairment charge of $4.3 million on our TheraTears tradename. Additionally, a 50 basis point decrease in the terminal growth rate used for each of our indefinite-lived
intangible assets' would not have resulted in any of our indefinite-lived intangible assets' fair value being less than their carrying value.
The weighted average remaining life for finite-lived intangible assets at March 31, 2022 was approximately 9.8 years, and the amortization expense for the year ended March 31, 2022 was $21.5 million. At March 31, 2022, finite-lived intangible assets are expected to be amortized over their estimated useful lives, which ranges from a period of 10 to 30 years, and the estimated amortization expense for each of the five succeeding years and periods thereafter is as follows (in thousands):
| | | | | |
| (In thousands) | |
| Year Ending March 31, | Amount |
| 2023 | $ | 22,576 | |
| 2024 | 22,533 | |
| 2025 | 20,479 | |
| 2026 | 18,232 | |
| 2027 | 16,591 | |
| Thereafter | 119,665 | |
| | $ | 220,076 | |
7. Leases
The components of lease expense for the years ended March 31, 2022 and 2021 were as follows:
| | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Finance lease cost: | | | |
| Amortization of right-of-use assets | $ | 2,579 | | | $ | 2,039 | |
| Interest on lease liabilities | 239 | | | 254 | |
| Operating lease cost | 6,670 | | | 6,754 | |
| Short term lease cost | 107 | | | 85 | |
| Variable lease cost | 42,868 | | | 46,771 | |
| Sublease income | — | | | (188) | |
| Total net lease cost | $ | 52,463 | | | $ | 55,715 | |
As of March 31, 2022, the maturities of lease liabilities were as follows:
| | | | | | | | | | | | | | | | | | | | |
| (In thousands) | | | | | | |
| Year Ending March 31, | | Operating Leases | | Financing Leases | | Total |
| 2023 | | $ | 6,931 | | | $ | 2,922 | | | $ | 9,853 | |
| 2024 | | 6,804 | | | 2,922 | | | 9,726 | |
| 2025 | | 4,624 | | | 1,509 | | | 6,133 | |
| 2026 | | 2,212 | | | 96 | | | 2,308 | |
| 2027 | | 1,889 | | | 80 | | | 1,969 | |
| Thereafter | | 1,705 | | | — | | | 1,705 | |
| Total undiscounted lease payments | | 24,165 | | | 7,529 | | | 31,694 | |
| Less amount of lease payments representing interest | | (1,717) | | | (276) | | | (1,993) | |
| Total present value of lease payments | | $ | 22,448 | | | $ | 7,253 | | | $ | 29,701 | |
The weighted average remaining lease term and weighted average discount rate were as follows: | | | | | | | | | | | |
| | | March 31, 2022 |
| Weighted average remaining lease term (years) | | |
| Operating leases | | 4.03 |
| Financing leases | | 2.64 |
| Weighted average discount rate | | |
| Operating leases | | 3.03 | % |
| Financing leases | | 2.96 | % |
Under our Master Services Agreement with GEODIS Logistics LLC ("GEODIS"), GEODIS purchased certain assets for our use that went into service during 2022, 2021 and 2020. The noncash lease liability arising from obtaining ROU assets was $0.5 million for the assets that went into service in 2022, $5.2 million for the assets that went into service in 2021 and $6.4 million for assets that went into service in 2020. These amounts represent noncash financing activities.
8. Other Accrued Liabilities
Other accrued liabilities consist of the following: | | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Accrued marketing costs | $ | 36,149 | | | $ | 29,955 | |
| Accrued compensation costs | 19,587 | | | 14,074 | |
| Accrued broker commissions | 1,179 | | | 1,023 | |
| Income taxes payable | 2,670 | | | 1,652 | |
| Accrued professional fees | 4,150 | | | 4,472 | |
| | | |
| | | |
| Accrued production costs | 3,686 | | | 2,882 | |
| | | |
| | | |
| Accrued sales tax | 5 | | | 2,368 | |
| Other accrued liabilities | 6,687 | | | 4,976 | |
| | $ | 74,113 | | | $ | 61,402 | |
9. Long-Term Debt
Long-term debt consists of the following, as of the dates indicated:
| | | | | | | | | | | | | | |
| (In thousands, except percentages) | | March 31,
2022 | | March 31,
2021 |
| | | | |
| 2021 Senior Notes bearing interest at 3.750%, with interest payable on April 1 and October 1 of each year. The 2021 Senior Notes mature on April 1, 2031. | | $ | 600,000 | | | $ | 600,000 | |
| | | | |
| | | | |
| 2019 Senior Notes bearing interest at 5.125%, with interest payable on January 15 and July 15 of each year. The 2019 Senior Notes mature on January 15, 2028. | | 400,000 | | | 400,000 | |
| 2012 Term B-5 Loans bearing interest at the Borrower's option at either LIBOR plus a margin of 2.00%, with a LIBOR floor of 0.00%, or an alternate base rate plus a margin of 1.00%, with a floor of 1.00%, due on January 26, 2024. | | — | | | 495,000 | |
| 2012 Term B-5 Loans bearing interest at the Borrower's option at either LIBOR plus a margin of 2.00%, with a LIBOR floor of 0.50%, or an alternate base rate plus a margin of 1.00% per annum, due on July 1, 2028. | | 495,000 | | | — | |
| | | | |
| | | | |
| | | | |
| | | | |
| Long-term debt | | 1,495,000 | | | 1,495,000 | |
| Less: unamortized debt costs | | (18,342) | | | (15,347) | |
| Long-term debt, net | | $ | 1,476,658 | | | $ | 1,479,653 | |
At March 31, 2022, we had no balance outstanding on the 2012 ABL Revolver and a borrowing capacity of $123.3 million.
2012 Term Loan and 2012 ABL Revolver:
On January 31, 2012, Prestige Brands, Inc. (the “Borrower") entered into a senior secured credit facility, which consists of (i) the $660.0 million 2012 Term Loan with an original 7-year maturity and (ii) the $50.0 million 2012 ABL Revolver with an original 5-year maturity. In subsequent years, we have utilized portions of our accordion feature to increase the amount of our borrowing capacity under the 2012 ABL Revolver by $85.0 million to $135.0 million and reduced our borrowing rate on the 2012 ABL Revolver by 0.25% (discussed below). The 2012 Term Loan was issued with an original issue discount of 1.5% of the principal amount thereof, resulting in net proceeds to us of $650.1 million. The 2012 Term Loan is unconditionally guaranteed by Prestige Consumer Healthcare Inc. and certain of its domestic 100% owned subsidiaries, other than the Borrower. Each of these guarantees is joint and several. There are no significant restrictions on the ability of any of the guarantors to obtain funds from their subsidiaries or to make payments to the Borrower or the Company.
On February 21, 2013, we entered into Amendment No. 1 ("Term Loan Amendment No. 1") to the 2012 Term Loan. Term Loan Amendment No. 1 provided for the refinancing of all of our existing Term B Loans with new Term B-1 Loans (the "Term B-1 Loans"). The interest rate on the Term B-1 Loans under Term Loan Amendment No. 1 was based, at our option, on a LIBOR rate plus a margin of 2.75% per annum, with a LIBOR floor of 1.00%, or an alternate base rate, with a floor of 2.00%, plus a margin. In addition, Term Loan Amendment No. 1 provided the Borrower with certain additional capacity to prepay subordinated debt, our then-outstanding senior notes and certain other unsecured indebtedness permitted to be incurred under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver.
On September 3, 2014, we entered into Amendment No. 2 ("Term Loan Amendment No. 2") to the 2012 Term Loan. Term Loan Amendment No. 2 provided for (i) the creation of a new class of Term B-2 Loans under the 2012 Term Loan (the "Term B-2 Loans") in an aggregate principal amount of $720.0 million, (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver, including additional investment, restricted payment and debt incurrence flexibility and financial maintenance covenant relief, and (iii) an interest rate on (x) the Term B-1 Loans that was based, at our option, on a LIBOR rate plus a margin of 3.125% per annum, with a LIBOR floor of 1.00%, or an alternate base rate, with a floor of 2.00%, plus a margin, and (y) the Term B-2 Loans that was based, at our option, on a LIBOR rate plus a margin of 3.50% per annum, with a LIBOR floor of 1.00%, or an alternate base rate, with a floor of 2.00%, plus a margin (with a margin step-down to 3.25% per annum, based upon achievement of a specified secured net leverage ratio).
Also, on September 3, 2014, we entered into Amendment No. 3 ("ABL Amendment No. 3") to the 2012 ABL Revolver. ABL Amendment No. 3 provided for (i) a $40.0 million increase in revolving commitments under the 2012 ABL Revolver and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver, including additional investment, restricted payment and debt incurrence flexibility. Borrowings under the 2012 ABL Revolver, as amended, bore interest at a rate per annum equal to an applicable margin, plus, at our option, either (i) a base rate determined by reference to the highest of (a) the Federal Funds rate plus 0.50%, (b) the prime rate of Citibank, N.A., and (c) the LIBOR rate determined by reference to the cost of funds for U.S. dollar deposits for an interest period of one month, adjusted for certain additional costs,
plus 1.00% or (ii) a LIBOR rate determined by reference to the costs of funds for U.S. dollar deposits for the interest period relevant to such borrowing, adjusted for certain additional costs. The applicable margin for borrowings under the 2012 ABL Revolver could be increased to 2.00% or 2.25% for LIBOR borrowings and 1.00% or 1.25% for base-rate borrowings, depending on average excess availability under the 2012 ABL Revolver during the prior fiscal quarter. In addition to paying interest on outstanding principal under the 2012 ABL Revolver, we were required to pay a commitment fee to the lenders under the 2012 ABL Revolver in respect of the unutilized commitments thereunder. The initial commitment fee rate was 0.50% per annum. The commitment fee rate will be reduced to 0.375% per annum at any time when the average daily unused commitments for the prior quarter is less than a percentage of total commitments by an amount set forth in the credit agreement covering the 2012 ABL Revolver. We may voluntarily repay outstanding loans under the 2012 ABL Revolver at any time without a premium or penalty.
On May 8, 2015, we entered into Amendment No. 3 ("Term Loan Amendment No. 3") to the 2012 Term Loan. Term Loan Amendment No. 3 provided for (i) the creation of a new class of Term B-3 Loans under the 2012 Term Loan (the "Term B-3 Loans") in an aggregate principal amount of $852.5 million, which combined the outstanding balances of the Term B-1 Loans of $207.5 million and the Term B-2 Loans of $645.0 million, and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility and financial maintenance covenant relief. The maturity date of the Term B-3 Loans remained the same as the Term B-2 Loans' original maturity date of September 3, 2021.
On June 9, 2015, we entered into Amendment No. 4 (“ABL Amendment No. 4”) to the 2012 ABL Revolver. ABL Amendment No. 4 provided for (i) a $35.0 million increase in the accordion feature under the 2012 ABL Revolver and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility and financial maintenance covenant relief and (iii) extended the maturity date of the 2012 ABL Revolver to June 9, 2020, which was five years from the effective date of ABL Amendment No. 4.
On February 5, 2016, we entered into Amendment No. 5 (“ABL Amendment No. 5”) to the 2012 ABL Revolver. ABL Amendment No. 5 temporarily suspended certain financial and related reporting covenants in the 2012 ABL Revolver until the earliest of (i) the date that was 60 calendar days following February 4, 2016, (ii) the date upon which certain of DenTek’s assets were included in the Company’s borrowing base under the 2012 ABL Revolver and (iii) the date upon which the Company received net proceeds from an offering of debt securities.
In connection with the Fleet acquisition, on January 26, 2017, we entered into Amendment No. 4 ("Term Loan Amendment No. 4") to the 2012 Term Loan. Term Loan Amendment No. 4 provided for (i) the refinancing of all of our outstanding term loans and the creation of a new class of Term B-4 Loans under the 2012 Term Loan (the "Term B-4 Loans") in an aggregate principal amount of $1,427.0 million and (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility and financial maintenance covenant relief. Term Loan Amendment No. 4 also extended the maturity date of the 2012 Term Loan to January 26, 2024. In addition, Citibank, N.A. was succeeded by Barclays Bank PLC as administrative agent under the 2012 Term Loan.
Also, on January 26, 2017, we entered into Amendment No. 6 ("ABL Amendment No. 6") to the 2012 ABL Revolver. ABL Amendment No. 6 provided for (i) a $40.0 million increase in revolving commitments under the 2012 ABL Revolver, (ii) an extension of the maturity date of revolving commitments to January 26, 2022, and (iii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, including additional investment, restricted payment and debt incurrence flexibility consistent with Term Loan Amendment No. 4.
On March 21, 2018, we entered into Amendment No. 5 (“Term Loan Amendment No. 5”) to the 2012 Term Loan. Term Loan Amendment No. 5 provided for the creation of Term B-5 Loans (the "Term B-5 Loans") by repricing of the Term B-4 Loans under the credit agreement governing the 2012 Term Loan to an interest rate that is based, at our option, on a LIBOR rate plus a margin of 2.00% per annum, with a LIBOR floor of 0.00%, or an alternative base rate plus a margin of 1.00% per annum, with a floor of 1.00%.
On December 11, 2019, we entered into Amendment No. 7 ("ABL Amendment No. 7") to the 2012 ABL Revolver. ABL Amendment No. 7 provides for (i) an extension of the maturity date of the 2012 ABL Revolver to December 11, 2024, which is five years from the effective date of ABL Amendment No. 7, (ii) increased flexibility under the 2012 ABL Revolver, including additional investment, restricted payment, and debt incurrence flexibility, (iii) an initial applicable margin for borrowings under the 2012 ABL Revolver that is 1.00% with respect to LIBOR borrowings and 0.0% with respect to base-rate borrowings (which may be increased to 1.25% or 1.50% for LIBOR borrowings and 0.25% or 0.50% for base-rate borrowings, depending on
average excess availability under the facility during the prior fiscal quarter), and (iv) a commitment fee to the lenders under the 2012 ABL Revolver in respect of the unutilized commitments thereunder of 0.25% per annum.
On July 1, 2021, we entered into Amendment No. 6 ("Term Loan Amendment No. 6") to the 2012 Term Loan. Term Loan Amendment No. 6 provided for (i) the refinancing of our outstanding term loans and the creation of a new class of Term B-5 Loans under the credit agreement governing the 2012 Term Loan in an aggregate principal amount of $600.0 million, (ii) increased flexibility under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver, and (iii) an interest rate on the Term B-5 Loans that is based, at the Borrower's option, on a LIBOR rate plus a margin of 2.00% per annum, with a LIBOR floor of 0.50%, or an alternative base rate plus a margin of 1.00% per annum. In addition, Term Loan Amendment No. 6 provided for an extension of the maturity date of the 2012 Term Loan to July 1, 2028. In connection with this refinancing, we recorded a loss on extinguishment of debt of $2.1 million to write off a portion of new and old debt costs relating to this refinancing. Under Term Loan Amendment No. 6, we are required to make quarterly payments each equal to 0.25% of the aggregate principal amount of the 2012 Term Loan.
The net proceeds from the new class of Term B-5 Loans were used to refinance our outstanding term loans, finance the acquisition of Akorn and pay fees and expenses incurred in connection with these transactions (see Note 2).
For the year ended March 31, 2022, the average interest rate on the 2012 Term Loan was 3.6% and the average interest rate on the amounts borrowed under the 2012 ABL Revolver was 1.2%. For the year ended March 31, 2021, the average interest rate on the 2012 Term Loan was 3.9% and the average interest rate on the amounts borrowed under the 2021 ABL Revolver was 1.7%. During the year ended March 31, 2022, we made required repayments of $1.5 million as well as voluntary principal payments of $103.5 million against the outstanding balance under our 2012 Term Loan. Since we have made optional payments that exceed all of our required quarterly payments, we will not be required to make another payment on the 2012 Term Loan until maturity on July 1, 2028.
2013 Senior Notes:
On December 17, 2013, the Borrower issued $400.0 million of senior unsecured notes, with an interest rate of 5.375% and a maturity date of December 15, 2021 (the "2013 Senior Notes"). These notes were redeemed on December 16, 2019 using the funds from the issuance of our 2019 Senior Notes described below. In conjunction with the redemption of our 2013 Senior Notes, we wrote off related debt costs of $2.2 million in the year ended March 31, 2020.
2016 Senior Notes:
On February 19, 2016, the Borrower completed the sale of $350.0 million aggregate principal amount of 6.375% senior notes due March 1, 2024 (the “Initial Notes”). On March 21, 2018, the Borrower completed the sale of $250.0 million additional aggregate principal amount of 6.375% senior notes due March 1, 2024 (the “Additional Notes”). Both the Initial Notes and the Additional Notes (the "2016 Senior Notes") were redeemed on March 1, 2021 using funds from the issuance of our 2021 Senior Notes described below. In conjunction with the redemption of the 2016 Senior Notes, we wrote off related debt costs of $2.7 million and paid a premium to redeem the 2016 Senior Notes of $9.6 million in the year ended March 31, 2021.
2019 Senior Notes:
On December 2, 2019, the Borrower issued $400.0 million aggregate principal amount of 5.125% senior notes due January 15, 2028 (the "2019 Senior Notes") pursuant to an indenture dated December 2, 2019, among the Borrower, the guarantors party thereto (including the Company) and U.S. Bank National Association, as trustee. We used the net proceeds from the 2019 Senior Notes, together with cash on hand, to redeem all $400.0 million of our outstanding 2013 Senior Notes, which were due in 2021, and to pay related fees and expenses.
2021 Senior Notes:
On March 1, 2021, the Borrower issued $600.0 million aggregate principal amount of 3.750% senior notes due April 1, 2031 (the "2021 Senior Notes") pursuant to an indenture dated March 1, 2021, among Prestige Brands, Inc., the guarantors party thereto (including the Company), and U.S. Bank National Association, as trustee. We used the net proceeds from the 2021 Senior Notes to redeem all $600.0 million of our outstanding 2016 Senior Notes, which were due in 2024, and to pay related fees and expenses.
Redemptions and Restrictions:
We have the option to redeem all or a portion of the 2019 Senior Notes at any time on or after January 15, 2023 at the redemption prices set forth in the indenture governing the 2019 Senior Notes, plus accrued and unpaid interest, if any. Subject to certain limitations, in the event of a change of control (as defined in the indenture governing the 2019 Senior Notes), the Borrower will be required to make an offer to purchase the 2019 Senior Notes at a price equal to 101% of the aggregate principal amount of the notes repurchased, plus accrued and unpaid interest, if any, to the date of repurchase.
We have the option to redeem all or a portion of the 2021 Senior Notes at any time on or after April 1, 2026 at the redemption prices set forth in the indenture governing the 2021 Senior Notes, plus accrued and unpaid interest, if any. Subject to certain limitations, in the event of a change of control (as defined in the indenture governing the 2021 Senior Notes), the Borrower will be required to make an offer to purchase the 2021 Senior Notes at a price equal to 101% of the aggregate principal amount of the notes repurchased, plus accrued and unpaid interest, if any, to the date of repurchase.
The credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes contain provisions that restrict us from undertaking specified corporate actions, such as asset dispositions, acquisitions, dividend payments, repurchases of common shares outstanding, changes of control, incurrences of indebtedness, issuance of equity, creation of liens, making of loans and transactions with affiliates. Additionally, the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes contain cross-default provisions, whereby a default pursuant to the terms and conditions of certain indebtedness will cause a default on the remaining indebtedness under the credit agreement governing the 2012 Term Loan and the 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes. At March 31, 2022, we were in compliance with the covenants under our long-term indebtedness.
Interest Rate Swaps:
In January 2020, we entered into two interest rate swaps to hedge a total of $400.0 million of our variable interest debt. One swap settled on January 31, 2021 and the other settled on January 31, 2022 (see Note 11 for further details).
As of March 31, 2022, aggregate future principal payments required in accordance with the terms of the 2012 Term Loan, 2012 ABL Revolver and the indentures governing the 2021 Senior Notes and the 2019 Senior Notes are as follows:
| | | | | | | | |
| (In thousands) | | |
| Year Ending March 31, | | Amount |
| 2023 | | $ | — | |
| 2024 | | — | |
| 2025 | | — | |
| 2026 | | — | |
| 2027 | | — | |
| Thereafter | 1,495,000 | |
| $ | 1,495,000 | |
10. Fair Value Measurements
For certain of our financial instruments, including cash, accounts receivable, accounts payable and other current liabilities, the carrying amounts approximate their respective fair values due to the relatively short maturity of these amounts.
The Fair Value Measurements and Disclosures topic of the FASB ASC 820 requires fair value to be determined based on the exchange price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market assuming an orderly transaction between market participants. The Fair Value Measurements and Disclosures topic established market (observable inputs) as the preferred source of fair value, to be followed by the Company's assumptions of fair value based on hypothetical transactions (unobservable inputs) in the absence of observable market inputs. Based upon the above, the following fair value hierarchy was created:
Level 1 - Quoted market prices for identical instruments in active markets;
Level 2 - Quoted prices for similar instruments in active markets, as well as quoted prices for identical or similar instruments in markets that are not considered active; and
Level 3 - Unobservable inputs developed by the Company using estimates and assumptions reflective of those that would be utilized by a market participant.
The market values have been determined based on market values for similar instruments adjusted for certain factors. As such, the 2021 Senior Notes, the 2019 Senior Notes, the Term B-5 Loans, the 2012 ABL Revolver and our interest rate swaps are measured in Level 2 of the above hierarchy (see summary below detailing the carrying amounts and estimated fair values of these instruments at March 31, 2022 and 2021).
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | March 31, 2022 | | March 31, 2021 |
| (In thousands) | | Carrying Value | | Fair Value | | Carrying Value | | Fair Value |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| 2019 Senior Notes | | $ | 400,000 | | | $ | 397,000 | | | $ | 400,000 | | | $ | 417,000 | |
| 2021 Senior Notes | | 600,000 | | | 534,000 | | | 600,000 | | | 570,000 | |
| 2012 Term B-5 Loans, No. 5 | | — | | | — | | | 495,000 | | | 493,763 | |
| 2012 Term B-5 Loans, No. 6 | | 495,000 | | | 493,144 | | | — | | | — | |
| 2012 ABL Revolver | | — | | | — | | | — | | | — | |
| Interest rate swaps | | — | | | — | | | 2,363 | | | 2,363 | |
At March 31, 2022 and 2021, we did not have any assets or liabilities measured in Level 1 or 3. During 2022, 2021 and 2020, there were no transfers of assets or liabilities between Levels 1, 2 and 3.
In accordance with ASU 2015-07, Fair Value Measurement (Topic 820): Disclosures for Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent), investments that are measured at fair value using net asset value ("NAV") per share as a practical expedient have not been classified in the fair value hierarchy.
11. Derivative Instruments
Changes in interest rates expose us to risks. To help us manage these risks, in January 2020, we entered into two interest rate swaps to hedge a total of $400.0 million of our variable interest debt. One swap settled on January 31, 2021 and other settled on January 31, 2022. The fair value of these interest rate swaps was reflected in the Consolidated Balance Sheets in other accrued liabilities. We do not use derivatives for trading purposes.
The following table summarizes the fair values of our derivative instruments as of the end of the periods shown:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | March 31, 2022 |
| (In thousands) | | Hedge Type | | Final Settlement Date | | Notional Amount | | Other Accrued Liabilities | | |
| | | | | | | | | | |
| Interest rate swap | | Cash flow | | 1/31/2022 | | $ | — | | | $ | — | | | |
| Total fair value | | | | | | | | $ | — | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | March 31, 2021 |
| (In thousands) | | Hedge Type | | Final Settlement Date | | Notional Amount | | Other Accrued Liabilities | | |
| | | | | | | | | | |
| Interest rate swap | | Cash flow | | 1/31/2022 | | $ | 200,000 | | | $ | (2,363) | | | |
| Total fair value | | | | | | | | $ | (2,363) | | | |
The following table summarizes our interest rate swaps, net of tax, for the periods shown:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands) | | Location | | 2022 | | 2021 | | 2020 |
| Gain (Loss) Recognized in Other Comprehensive (Loss) Income (effective portion) | Other comprehensive income (loss) | | $ | 1,819 | | | $ | 3,045 | | | $ | (4,864) | |
| Gain (Loss) Reclassified from Accumulated Other Comprehensive (Loss) Income into Income | Interest expense, net | | $ | (2,429) | | | $ | (4,760) | | | $ | 62 | |
Counterparty Credit Risk:
We manage our exposure to counterparty credit risk by only dealing with counterparties who are substantial international financial institutions with significant experience using such derivative instruments.
12. Stockholders' Equity
The Company is authorized to issue 250.0 million shares of common stock, $0.01 par value per share, and 5.0 million shares of preferred stock, $0.01 par value per share. The Board of Directors may direct the issuance of the undesignated preferred stock in one or more series and determine preferences, privileges and restrictions thereof.
Each share of common stock has the right to 1 vote on all matters submitted to a vote of stockholders. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to prior rights of holders of all classes of stock outstanding having priority rights as to dividends. No dividends have been declared or paid on the Company's common stock through March 31, 2022.
During the years ended March 31, 2022 and 2021, we repurchased shares of our common stock and recorded them as treasury stock. Our share repurchases consisted of the following:
| | | | | | | | | | | |
| Year Ended March 31, |
| 2022 | | 2021 |
| Shares repurchased pursuant to the provisions of the various employee restricted stock awards: | | | |
| Number of shares | 63,314 | | | 31,117 | |
| Average price per share | $46.04 | | $39.91 |
| Total amount repurchased | $2.9 million | | $1.2 million |
| | | |
| Shares repurchased in conjunction with our share repurchase program: | | | |
| | — | | | 337,117 | |
| Average price per share | — | | | $35.20 |
| Total amount repurchased | — | | | $11.9 million |
13. Share-Based Compensation
In connection with our initial public offering, the Board of Directors adopted the 2005 Long-Term Equity Incentive Plan (the “2005 Plan”), which provided for grants of up to a maximum of 5.0 million shares of restricted stock, stock options, RSUs and other equity-based awards. In June 2014, the Board of Directors approved, and in July 2014, our stockholders ratified, an increase of an additional 1.8 million shares of our common stock for issuance under the 2005 Plan, an increase of the maximum number of shares subject to stock options that could be awarded to any one participant under the 2005 Plan during any fiscal 12-month period from 1.0 million to 2.5 million shares, and an extension of the term of the 2005 Plan by ten years to February 2025. Directors, officers and other employees of the Company and its subsidiaries, as well as others performing services for the Company, were eligible for grants under the 2005 Plan.
On June 23, 2020, the Board of Directors adopted the Prestige Consumer Healthcare Inc. 2020 Long-Term Incentive Plan (the “2020 Plan”). The 2020 Plan became effective on August 4, 2020, upon the approval of the 2020 Plan by our stockholders. On June 23, 2020, a total of 2,827,210 shares were available for issuance under the 2020 Plan (comprised of 2,000,000 new shares plus 827,210 shares that were unissued under the 2005 Plan). All future equity awards will be made from the 2020 Plan, and the Company will not grant any additional awards under the 2005 Plan.
The following table provides information regarding our stock-based compensation:
| | | | | | | | | | | | | | | | | | | | |
| | March 31, |
| (In thousands) | | 2022 | | 2021 | | 2020 |
| Pre-tax share-based compensation costs charged against income | | $ | 9,039 | | | $ | 8,543 | | | $ | 7,644 | |
| Income tax benefit recognized on compensation costs | | $ | 633 | | | $ | 1,224 | | | $ | 1,207 | |
| Total fair value of options and RSUs vested during the period | | $ | 7,943 | | | $ | 6,796 | | | $ | 7,830 | |
| Cash received from the exercise of stock options | | $ | 7,040 | | | $ | 2,851 | | | $ | 1,324 | |
| Tax benefits realized from tax deductions resulting from RSU issuances and stock option exercises | | $ | 3,419 | | | $ | 1,153 | | | $ | 745 | |
At March 31, 2022, there were $2.8 million of unrecognized compensation costs related to unvested stock options under the 2005 Plan and the 2020 Plan, excluding an estimate for forfeitures which may occur. We expect to recognize such costs over a
weighted average period of 1.7 years. At March 31, 2022, there were $8.5 million of unrecognized compensation costs related to unvested RSUs and performance-based stock units ("PSUs") under the 2005 Plan and the 2020 Plan, excluding an estimate for forfeitures that may occur. We expect to recognize such costs over a weighted average period of 1.5 years.
At March 31, 2022, there were 2.5 million shares available for issuance under the 2020 Plan.
On May 3, 2021, the Compensation and Talent Management Committee (the "Committee") of our Board of Directors granted 77,345 performance units, 73,108 RSUs and stock options to acquire 222,660 shares of our common stock under the 2020 Plan to certain executive officers and employees. The stock options were granted at an exercise price of $44.33 per share, which was equal to the closing price for our common stock on the date of the grant.
A newly appointed independent member of the Board of Directors received a grant under the 2020 Plan of 1,636 RSUs on May 3, 2021.
Each of the independent members of the Board of Directors received a grant of 2,808 RSUs on August 3, 2021 under the 2020 Plan. The RSUs are fully vested upon receipt of the award and will be settled by delivery to each director of 1 share of our common stock for each vested RSU promptly following the earliest of (i) such director's death, (ii) such director's separation from service or (iii) a change in control of the Company.
Restricted Stock Units
RSUs granted to employees under the 2005 Plan and the 2020 Plan generally vest in three years, primarily upon the attainment of certain time vesting thresholds, and, in the case of performance share units, may also be contingent on the attainment of certain performance goals of the Company, including revenue and earnings before interest, income taxes, depreciation and amortization targets. The RSUs provide for accelerated vesting if there is a change of control, as defined in the 2005 Plan and the 2020 Plan. The RSUs granted to employees generally vest either ratably over three years or in their entirety on the three-year anniversary of the date of the grant. Upon vesting, the units will be settled in shares of our common stock. Termination of employment prior to vesting will result in forfeiture of the RSUs, unless otherwise accelerated by the Committee or, in the case of RSUs granted in May 2018 and later, subject to pro-rata vesting in the event of death, disability or retirement. The RSUs granted to directors prior to fiscal 2020 vest immediately upon grant, and will be settled by delivery to the director of 1 share of our common stock for each vested RSU promptly following the earliest of (i) the director's death, (ii) the director's disability or (iii) the six-month anniversary of the date on which the director's Board membership ceases for reasons other than death or disability. The RSUs granted to directors beginning in fiscal 2020 vest immediately upon grant, and will be settled by delivery to the director of 1 share of our common stock for each vested RSU promptly following the earliest of (i) the director's death, (ii) the director's separation from service or (iii) a change in control of the Company.
The fair value of the RSUs is determined using the closing price of our common stock on the date of the grant.
A summary of the Company’s RSUs granted under the 2005 Plan and 2020 Plan is presented below:
| | | | | | | | | | | | | | |
RSUs | | Shares
(in thousands) | | Weighted Average
Grant Date
Fair Value |
| | | | |
| Unvested at March 31, 2019 | | 299.9 | | | $ | 38.66 | |
| | | | |
| Granted | | 220.3 | | | 31.02 | |
| Vested | | (98.1) | | | 44.35 | |
| Forfeited | | (34.2) | | | 35.97 | |
| Unvested at March 31, 2020 | | 387.9 | | | 33.11 | |
| Vested at March 31, 2020 | | 124.2 | | | 30.54 | |
| | | | |
| Granted | | 179.7 | | | 40.22 | |
| Vested | | (100.2) | | | 42.94 | |
| Forfeited | | (10.4) | | | 43.37 | |
| Unvested at March 31, 2021 | | 457.0 | | | 33.52 | |
| Vested at March 31, 2021 | | 150.4 | | | 31.98 | |
| | | | |
| Granted | | 170.8 | | | 45.32 | |
| Vested | | (162.3) | | | 32.99 | |
| Forfeited | | (24.6) | | | 30.54 | |
| Unvested at March 31, 2022 | | 440.9 | | | 38.45 | |
| Vested at March 31, 2022 | | 106.8 | | | 36.42 | |
Options
The 2005 Plan and the 2020 Plan provide that the exercise price of options granted shall be no less than the fair market value of the Company's common stock on the date the options are granted. Options granted have a term of no greater than ten years from the date of grant and vest in accordance with a schedule determined at the time the option is granted, generally three years. The option awards provide for accelerated vesting in the event of a change in control, as defined in the 2005 Plan and the 2020 Plan. Except in the case of death, disability or retirement, termination of employment prior to vesting will result in forfeiture of the unvested stock options. Vested stock options will remain exercisable by the employee after termination of employment, subject to the terms in the 2005 Plan and the 2020 Plan.
The fair value of each option award is estimated on the date of grant using the Black-Scholes Option Pricing Model that uses the assumptions noted in the table below. Expected volatilities are based on the historical volatility of our common stock and other factors, including the historical volatilities of comparable companies. We use appropriate historical data, as well as current data, to estimate option exercise and employee termination behaviors. Employees that are expected to exhibit similar exercise or termination behaviors are grouped together for the purposes of valuation. The expected terms of the options granted are derived from our historical experience, management’s estimates, and consideration of information derived from the public filings of companies similar to us, and represent the period of time that options granted are expected to be outstanding. The risk-free rate represents the yield on U.S. Treasury bonds with a maturity equal to the expected term of the granted options.
| | | | | | | | | | | | | | | | | |
| | Year Ended March 31, |
| | 2022 | | 2021 | | 2020 |
| Expected volatility | 31.1% - 31.9% | | 32.1% - 32.2% | | 30.9% to 31.3% |
| Expected dividends | — | | — | | — |
| Expected term in years | 6.0 to 7.0 | | 6.0 to 7.0 | | 6.0 to 7.0 |
| Risk-free rate | 1.0% to 1.3% | | 0.5% | | 2.3% to 2.4% |
| Weighted average grant date fair value of options granted | $14.87 | | $12.91 | | $10.83 |
A summary of option activity under the 2005 Plan and 2020 Plan is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Shares
(in thousands) | | Weighted Average
Exercise
Price | | Weighted
Average
Remaining
Contractual Term | | Aggregate
Intrinsic
Value
(in thousands) |
| | | | | | | | |
| Outstanding at March 31, 2019 | | 944.6 | | | $ | 38.45 | | | | | |
| | | | | | | | |
| Granted | | 302.7 | | | 30.53 | | | | | |
| Exercised | | (47.9) | | | 27.60 | | | | | |
| Forfeited | | (79.2) | | | 32.62 | | | | | |
| Expired | | (100.0) | | | 50.31 | | | | | |
| Outstanding at March 31, 2020 | | 1,020.2 | | | 35.90 | | | | | |
| | | | | | | | |
| Granted | | 249.9 | | | 39.98 | | | | | |
| Exercised | | (119.6) | | | 23.83 | | | | | |
| Forfeited | | (21.7) | | | 34.65 | | | | | |
| Expired | | (13.9) | | | 52.86 | | | | | |
| Outstanding at March 31, 2021 | | 1,114.9 | | | 37.92 | | | | | |
| | | | | | | | |
| Granted | | 234.2 | | | 44.74 | | | | | |
| Exercised | | (226.0) | | | 31.15 | | | | | |
| Forfeited | | (13.7) | | | 37.83 | | | | | |
| Expired | | (8.5) | | | 56.63 | | | | | |
| Outstanding at March 31, 2022 | | 1,100.9 | | | 40.62 | | | 6.6 | | $ | 14,239 | |
| Exercisable at March 31, 2022 | | 621.3 | | | 40.79 | | | 5.2 | | $ | 8,226 | |
The aggregate intrinsic value of options exercised during 2022, 2021 and 2020 was $6.0 million, $2.2 million and $0.4 million, respectively.
14. Accumulated Other Comprehensive Loss
The table below presents accumulated other comprehensive income (loss) (“AOCI”), which affects equity and results from recognized transactions and other economic events, other than transactions with owners in their capacity as owners.
AOCI consisted of the following at March 31, 2022 and 2021:
| | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Components of Accumulated Other Comprehensive Loss | | | |
| Cumulative translation adjustment | $ | (20,204) | | | $ | (18,908) | |
| Unrealized loss on interest rate swaps, net of tax of $0 and $543, respectively | — | | | (1,819) | |
| Unrecognized net gain (loss) on pension plans, net of tax of $(350) and $(276), respectively | 1,172 | | | 926 | |
| | | |
| Accumulated other comprehensive loss, net of tax | $ | (19,032) | | | $ | (19,801) | |
15. Income Taxes
Income before income taxes consists of the following: | | | | | | | | | | | | | | | | | |
| Year Ended March 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| United States | $ | 236,381 | | | $ | 195,796 | | | $ | 167,508 | |
| Foreign | 26,077 | | | 8,317 | | | 23,643 | |
| | | | | |
| $ | 262,458 | | | $ | 204,113 | | | $ | 191,151 | |
The provision for income taxes consists of the following: | | | | | | | | | | | | | | | | | |
| Year Ended March 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Current | | | | | |
| Federal | $ | 34,446 | | | $ | 11,513 | | | $ | 24,051 | |
| State | 3,961 | | | 3,403 | | | 2,506 | |
| Foreign | 8,709 | | | 5,849 | | | 8,473 | |
| Deferred | | | | | |
| Federal | 12,055 | | | 14,430 | | | 14,119 | |
| State | (1,379) | | | 4,572 | | | (341) | |
| Foreign | (715) | | | (336) | | | 62 | |
| Total provision for income taxes | $ | 57,077 | | | $ | 39,431 | | | $ | 48,870 | |
The principal components of our deferred tax balances are as follows: | | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Deferred Tax Assets | | | |
| Allowance for doubtful accounts and sales returns | $ | 4,605 | | | $ | 4,267 | |
| Inventory capitalization | 2,201 | | | 1,124 | |
| Inventory reserves | 1,264 | | | 1,119 | |
| Net operating loss carryforwards | 115 | | | 115 | |
| State income taxes | 9,661 | | | 10,028 | |
| Accrued liabilities | 2,026 | | | 1,078 | |
| Accrued compensation | 4,597 | | | 3,736 | |
| Stock compensation | 4,548 | | | 4,369 | |
| Foreign tax credit | — | | | 699 | |
| | | |
| Lease liability | 6,808 | | | 8,132 | |
| Unrealized foreign exchange loss | 215 | | | 384 | |
| Other | 5,682 | | | 5,916 | |
| Total deferred tax assets | $ | 41,722 | | | $ | 40,967 | |
| | | |
| Deferred Tax Liabilities | | | |
| Property, plant and equipment | $ | (7,210) | | | $ | (7,245) | |
| Intangible assets | (471,327) | | | (458,713) | |
| Deferred cumulative catch-up adjustments - revenue recognition adjustments | — | | | (264) | |
| Right-of-use asset | (6,239) | | | (7,605) | |
| Total deferred tax liabilities | $ | (484,776) | | | $ | (473,827) | |
| | | |
| | | |
| | | |
| Net deferred tax liability | $ | (443,054) | | | $ | (432,860) | |
The net deferred tax liability shown above is net of $1.9 million of foreign deferred tax assets as of March 31, 2022 and $1.2 million of foreign deferred tax assets as of March 31, 2021.
We had no valuation allowance as of March 31, 2021 and March 31, 2022.
A reconciliation of the effective tax rate compared to the statutory U.S. Federal tax rate is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended March 31, |
| 2022 | | 2021 | | 2020 |
| (In thousands) | | | % | | | | % | | | | % |
| Income tax provision at statutory rate | $ | 55,116 | | | 21.0 | | | $ | 42,864 | | | 21.0 | | | $ | 40,142 | | | 21.0 | |
| Foreign tax provision | 2,876 | | | 1.1 | | | 3,972 | | | 1.9 | | | 2,498 | | | 1.3 | |
| State income taxes, net of federal income tax benefit | 1,737 | | | 0.7 | | | 7,284 | | | 3.6 | | | 1,606 | | | 0.8 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| R&D | (213) | | | (0.1) | | | (156) | | | (0.1) | | | (320) | | | (0.2) | |
| Compensation limitations | 1,563 | | | 0.6 | | | 735 | | | 0.4 | | | 562 | | | 0.3 | |
| Valuation allowance | — | | | — | | | (5,441) | | | (2.7) | | | 2,205 | | | 1.2 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Uncertain tax positions | (369) | | | (0.1) | | | (7,218) | | | (3.5) | | | — | | | — | |
| Other | (3,633) | | | (1.5) | | | (2,609) | | | (1.3) | | | 2,177 | | | 1.2 | |
| Total provision for income taxes | $ | 57,077 | | | 21.7 | | | $ | 39,431 | | | 19.3 | | | $ | 48,870 | | | 25.6 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Uncertain tax liability activity is as follows: | | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 | | 2020 |
| (In thousands) | | | | | |
| Balance – beginning of year | $ | 4,030 | | | $ | 10,369 | | | $ | 9,874 | |
| Additions based on tax positions related to the current year | — | | | — | | | 495 | |
| Reductions based on lapse of statute of limitations | (585) | | | (6,756) | | | — | |
| Payments and other movements | 117 | | | 417 | | | — | |
| Balance – end of year | $ | 3,562 | | | $ | 4,030 | | | $ | 10,369 | |
We recognize interest and penalties related to uncertain tax positions as a component of income tax (benefit) expense. We did not incur any material interest or penalties related to income taxes in 2022, 2021 or 2020. We reasonably anticipate that uncertain tax positions could decrease in the next year by approximately $0.5 million, principally due to the statute of limitation expirations if recognized and would impact the effective tax rate in a future period. We are subject to taxation in the United States and various state and foreign jurisdictions, and we are generally open to examination from the year ended March 31, 2018 forward.
16. Employee Retirement Plans
We have a defined contribution plan in which all U.S. full-time employees are eligible to participate. The participants may contribute from 1% to 70% of their compensation, as defined in the plan. We match 100% of the first 3%, plus 50% of the next 3%, of each participant's base compensation with full vesting immediately. We may also make additional contributions to the plan as determined by the Board of Directors. The total expense for the defined contribution plan was $1.7 million, $1.6 million and $1.5 million for 2022, 2021 and 2020, respectively.
Certain employees of our Lynchburg manufacturing facility are covered by defined benefit pension plans. The Company’s policy is to contribute at least the minimum amount required under ERISA. The Company may elect to make additional contributions. Benefits are based on years of service and levels of compensation. On December 16, 2014, the decision was made to freeze the benefits under the Company's U.S. qualified defined benefit pension plan (the "Plan") with an effective date of March 1, 2015.
During the fourth quarter of 2021, we adopted a plan termination date of April 30, 2021 for the Plan and began the Plan termination process. Pension obligations related to the Plan of $47.2 million are expected to be distributed through a combination of lump sum payments to eligible Plan participants who elect such payments and through the purchase of annuity contracts to the remaining participants. The benefit obligation for the Plan as of March 31, 2021 was therefore determined on a plan termination basis for which it is assumed that a portion of eligible active and deferred vested participants will elect lump sum payments. The Plan likely has sufficient assets to satisfy all transaction obligations and all payments related to the termination are expected to be paid out of Plan assets. No distributions have been made as of March 31, 2022. The plan termination is expected to be completed in the first quarter of fiscal 2023.
Benefit Obligations and Plan Assets
The following table summarizes the changes in the U.S. pension plan obligations and plan assets and includes a statement of the plans' funded status as of March 31, 2022 and 2021:
| | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Change in benefit obligation: | | | |
| Projected benefit obligation at beginning of period | $ | 56,818 | | | $ | 61,570 | |
| Interest cost | 1,107 | | | 1,972 | |
| Actuarial (gain) loss | (2,888) | | | 3,779 | |
| Benefits paid | (3,530) | | | (10,503) | |
| Projected benefit obligations at end of year | $ | 51,507 | | | $ | 56,818 | |
| | | |
| Change in plan assets: | | | |
| Fair value of plan assets at beginning of period | $ | 53,275 | | | $ | 52,760 | |
| Actual return on plan assets | (1,405) | | | 7,650 | |
| Employer contribution | 368 | | | 3,368 | |
| Benefits paid | (3,530) | | | (10,503) | |
| Fair value of plan assets at end of year | $ | 48,708 | | | $ | 53,275 | |
| | | |
| Funded status at end of year | $ | (2,799) | | | $ | (3,543) | |
Amounts recognized in the balance sheet at the end of the period consist of the following: | | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Noncurrent asset | $ | 1,534 | | | $ | 1,141 | |
| | | |
| Current liability | 365 | | | 357 | |
| Long-term liability | 3,968 | | | 4,327 | |
| Total liabilities | $ | 4,333 | | | $ | 4,684 | |
| | | |
| Total net liability | $ | (2,799) | | | $ | (3,543) | |
The primary components of Net Periodic Benefit Cost (Income) consist of the following: | | | | | | | | | | | | | | | | | |
| Year Ended March 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Interest cost | $ | 1,107 | | | $ | 1,972 | | | $ | 2,327 | |
| Expected return on assets | (1,163) | | | (2,336) | | | (2,886) | |
| Net periodic benefit cost (income) | $ | (56) | | | $ | (364) | | | $ | (559) | |
The following table provides information regarding our pension plans with an accumulated benefit obligation and a projected benefit obligation in excess of plan assets:
| | | | | | | | | | | |
| March 31, |
| (In thousands) | 2022 | | 2021 |
| Accumulated benefit obligation | $ | 51,507 | | | $ | 56,818 | |
| Fair value of plan assets | 48,708 | | | 53,275 | |
| | | |
| Projected benefit obligations | $ | 51,507 | | | $ | 56,818 | |
| Fair value of plan assets | 48,708 | | | 53,275 | |
The pension benefit amounts stated above include one pension plan that is an unfunded plan. The projected benefit obligation and accumulated benefit obligation for this unfunded plan were $4.3 million as of March 31, 2022 and $4.7 million as of March 31, 2021.
The following table includes amounts that are expected to be contributed to the plans by the Company. It reflects benefit payments that are made from the plans' assets as well as those made directly from the Company's assets. The amounts in the table are actuarially determined and reflect the Company's best estimate given its current knowledge; actual amounts could be materially different.
| | | | | |
| (In thousands) | Pension Benefits |
| Employer contributions: | |
| 2023 (expectation) to participant benefits | $ | 365 | |
| |
| Expected benefit payments year ending March 31, | |
| 2023 | $ | 3,452 | |
| 2024 | 3,519 | |
| 2025 | 3,481 | |
| 2026 | 3,415 | |
| 2027 | 3,385 | |
| 2028-2031 | 16,205 | |
We did not make any contributions to our qualified defined benefit plan during 2022, and we contributed $3.0 million and $1.0 million to our qualified defined benefit plan during 2021 and 2020, respectively.
During the third quarter of 2021, we offered participants of our qualified defined benefit plan the option to receive a lump sum payout of their benefits. The amount paid out of plan assets during the third quarter of 2021 to those who elected to take the lump sum payout was $7.0 million, and we recognized a settlement gain of $0.2 million as a result of the payout. The settlement credit was determined based on a remeasurement of the plan as of December 31, 2020, at which point a discount rate of 2.51% and an expected return assumption of 2.75% were selected. Those remeasurement assumptions were also used to determine the net periodic pension income for the plan for the fourth quarter of fiscal 2021.
The Company's primary investment objective for its qualified pension plan assets is to provide a source of retirement income for the Plan's participants and beneficiaries. The asset allocation for the Company's funded retirement plan as of March 31, 2022 and 2021, and the target allocation by asset category are as follows:
| | | | | | | | | | | | | | | | | |
| Percentage of Plan Assets |
| Asset Category | Target Allocation | | March 31, 2022 | | March 31, 2021 |
| | | | | |
| | | | | |
| | | | | |
| Real estate | — | % | | — | % | | 6 | % |
| Fixed income and cash | 100 | | | 100 | | | 94 | |
| Total | 100 | % | | 100 | % | | 100 | % |
The plan assets are invested in a portfolio consisting primarily of domestic fixed income held within collective investment trust funds as of March 31, 2022 due to the plan termination process which began during the fourth quarter of fiscal 2021 and the plan's positive funded status. The plan assets were invested in a portfolio consisting primarily of domestic fixed income as of March 31, 2022. These assets are measured at NAV as a practical expedient.
The following tables show the unrecognized actuarial loss (gain) included in accumulated other comprehensive income (loss) at March 31, 2022, 2021 and 2020:
| | | | | |
| (In thousands) | |
| Balances in accumulated other comprehensive loss as of March 31, 2020: | |
| Unrecognized actuarial loss | $ | 73 | |
| Unrecognized prior service credit | — | |
| |
| Balances in accumulated other comprehensive loss as of March 31, 2021: | |
| Unrecognized actuarial (gain) | $ | (1,202) | |
| Unrecognized prior service credit | — | |
| |
| Balances in accumulated other comprehensive loss as of March 31, 2022: | |
| Unrecognized actuarial (gain) | $ | (1,522) | |
| Unrecognized prior service credit | — | |
| |
| |
| |
| |
Assumptions used in determining the actuarial present value of the net periodic benefit cost (income) for the fiscal years ended March 31, 2022, 2021 and 2020 were as follows:
| | | | | | | | | | | | | | | | | |
| March 31, |
| 2022 | | 2021 * | | 2020 |
| Key assumptions: | | | | | |
| Discount rate | 2.58% to 2.95% | | 3.37% to 3.55% | | 3.80% to 3.99% |
| Expected return on plan assets, net of administrative fees | 2.25% | | 5.00% | | 5.75% |
| | | | | |
*The qualified plan was remeasured at December 31, 2020 for settlement accounting, at which point a discount rate of 2.51% and an expected return assumption of 2.75% were selected and used to determine the net periodic benefit cost (income) for the fourth quarter of fiscal 2021.
Assumptions used in determining the actuarial present value of the benefit obligation as of March 31, 2022 and 2021 were as follows: | | | | | | | | | | | |
| March 31, |
| 2022 | | 2021 |
| Key assumptions: | | | |
| Discount rate | 3.26% to 3.48% | | 2.58% to 2.95% |
| | | |
| | | |
The determination of the expected long-term rate of return was derived from an optimized portfolio using an asset allocation software program. The risk and return assumptions, along with the correlations between the asset classes, were entered into the program. Based on these assumptions and historical experience, the portfolio is expected to achieve a long-term rate of return of 2.75%. The investment managers engaged to manage the portfolio are expected to outperform their expected benchmarks on a relative basis over a full market cycle.
17. Commitments and Contingencies
We are involved from time to time in routine legal matters and other claims incidental to our business. We review outstanding claims and proceedings internally and with external counsel as necessary to assess probability and amount of potential loss. These assessments are re-evaluated at each reporting period and as new information becomes available to determine whether a reserve should be established or if any existing reserve should be adjusted. The actual cost of resolving a claim or proceeding ultimately may be substantially different than the amount of the recorded reserve. In addition, because it is not permissible under GAAP to establish a litigation reserve until the loss is both probable and estimable, in some cases there may be insufficient time to establish a reserve prior to the actual incurrence of the loss (upon verdict and judgment at trial, for example, or in the case of a quickly negotiated settlement). We believe the resolution of routine legal matters and other claims incidental to our business, taking our reserves into account, will not have a material adverse effect on our business, financial condition, or results of operations.
Lease Commitments
See Note 7 for a description of our operating and finance leases.
Purchase Commitments
We have supply agreements for the manufacture of some of our products. The following table shows the minimum amounts that we are committed to pay under these agreements:
| | | | | |
| (In thousands) | |
| Year Ending March 31, | Amount |
| 2023 | $ | 6,873 | |
| 2024 | 5,277 | |
| 2025 | 5,293 | |
| 2026 | 4,941 | |
| 2027 | 4,858 | |
| Thereafter | 5,367 | |
| | $ | 32,609 | |
18. Concentrations of Risk
Our revenues are concentrated in the areas of OTC Healthcare. We sell our products to mass merchandisers, drug, food, dollar, convenience and club stores and e-commerce channels. During 2022, 2021, and 2020, approximately 41.3%, 45.6%, and 42.6%, respectively, of our gross revenues were derived from our five top selling brands. One customer, Walmart, accounted for more than 10% of our gross revenues for each of the periods presented. During 2022, 2021, and 2020, Walmart accounted for approximately 20.5%, 21.6%, and 23.1%, respectively, of our gross revenues. At March 31, 2022, approximately 22.1% of our accounts receivable were owed by Walmart.
Our product distribution in the United States is managed by a third-party through one primary distribution center in Clayton, Indiana. In addition, we operate one manufacturing facility for certain of our products located in Lynchburg, Virginia, which manufacturers many of the Summer's Eve and Fleet products. A natural disaster, such as tornado, earthquake, flood, or fire, could damage our inventory and/or materially impair our ability to distribute our products to customers in a timely manner or at a reasonable cost. In addition, a serious disruption caused by performance or contractual issues with our third-party distribution manager or labor shortages or contagious disease outbreaks or other public health emergencies at our distribution center or manufacturing facility could materially impact our product distribution. Any disruption could result in increased costs, expense and/or shipping times, and could cause us to incur customer fees and penalties. We could also incur significantly higher costs and experience longer lead times if we need to replace our distribution center, the third-party distribution manager or the manufacturing facility. As a result, any serious disruption could have a material adverse effect on our business, financial condition and results of operations.
At March 31, 2022, we had relationships with 128 third-party manufacturers. Of those, we had long-term contracts with 23 manufacturers that produced items that accounted for approximately 69.0% of our gross sales for 2022, compared to 19 manufacturers with long-term contracts that accounted for approximately 70.5% of gross sales in 2021. The fact that we do not have long-term contracts with certain manufacturers means that they could cease manufacturing our products at any time and for any reason or initiate arbitrary and costly price increases, which could have a material adverse effect on our business and results from operations. Although we are continually in the process of negotiating long-term contracts with certain key manufacturers, we may not be able to reach a timely agreement, which could have a material adverse effect on our business and results of operations.
19. Business Segments
Segment information has been prepared in accordance with the Segment Reporting topic of FASB ASC 280. Our reportable segments consist of (i) North American OTC Healthcare and (ii) International OTC Healthcare. We evaluate the performance of our operating segments and allocate resources to these segments based primarily on contribution margin, which we define as gross profit less advertising and marketing expenses.
The tables below summarize information about our operating and reportable segments.
| | | | | | | | | | | | | | | | | | | |
| | Year Ended March 31, 2022 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Total segment revenues* | $ | 967,881 | | | $ | 118,931 | | | | | $ | 1,086,812 | |
| Cost of sales | 419,162 | | | 47,004 | | | | | 466,166 | |
| Gross profit | 548,719 | | | 71,927 | | | | | 620,646 | |
| Advertising and marketing | 138,714 | | | 18,629 | | | | | 157,343 | |
| Contribution margin | $ | 410,005 | | | $ | 53,298 | | | | | 463,303 | |
| Other operating expenses | | | | | | | 133,384 | |
| Operating income | | | | | | | $ | 329,919 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
*Intersegment revenues of $3.0 million were eliminated from the North American OTC Healthcare segment.
| | | | | | | | | | | | | | | | | | | |
| | Year Ended March 31, 2021 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Total segment revenues* | $ | 849,319 | | | $ | 94,046 | | | | | $ | 943,365 | |
| Cost of sales | 359,100 | | | 36,793 | | | | | 395,893 | |
| Gross profit | 490,219 | | | 57,253 | | | | | 547,472 | |
| Advertising and marketing | 122,857 | | | 17,732 | | | | | 140,589 | |
| Contribution margin | $ | 367,362 | | | $ | 39,521 | | | | | 406,883 | |
| Other operating expenses | | | | | | | 109,481 | |
| Operating income | | | | | | | $ | 297,402 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
* Intersegment revenues of $3.2 million were eliminated from the North American OTC Healthcare segment.
| | | | | | | | | | | | | | | | | | | |
| | Year Ended March 31, 2020 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Total segment revenues* | $ | 859,368 | | | $ | 103,642 | | | | | $ | 963,010 | |
| Cost of sales | 372,133 | | | 38,654 | | | | | 410,787 | |
| Gross profit | 487,235 | | | 64,988 | | | | | 552,223 | |
| Advertising and marketing | 127,972 | | | 19,222 | | | | | 147,194 | |
| Contribution margin | $ | 359,263 | | | $ | 45,766 | | | | | 405,029 | |
| Other operating expenses | | | | | | | 113,874 | |
| Operating income | | | | | | | $ | 291,155 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
*Intersegment revenues of $3.5 million were eliminated from the North America OTC Healthcare segment.
The tables below summarize information about our segment revenues from similar product groups.
| | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2022 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Analgesics | $ | 117,868 | | | $ | 1,455 | | | | | $ | 119,323 | |
| Cough & Cold | 86,855 | | | 20,225 | | | | | 107,080 | |
| Women's Health | 249,136 | | | 15,373 | | | | | 264,509 | |
| Gastrointestinal | 152,191 | | | 52,368 | | | | | 204,559 | |
| Eye & Ear Care | 149,454 | | | 13,995 | | | | | 163,449 | |
| Dermatologicals | 117,173 | | | 3,213 | | | | | 120,386 | |
| Oral Care | 85,239 | | | 12,282 | | | | | 97,521 | |
| Other OTC | 9,965 | | | 20 | | | | | 9,985 | |
| | | | | | | |
| Total segment revenues | $ | 967,881 | | | $ | 118,931 | | | | | $ | 1,086,812 | |
| | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2021 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Analgesics | $ | 117,775 | | | $ | 1,367 | | | | | $ | 119,142 | |
| Cough & Cold | 56,158 | | | 14,483 | | | | | 70,641 | |
| Women's Health | 252,535 | | | 15,562 | | | | | 268,097 | |
| Gastrointestinal | 124,755 | | | 36,381 | | | | | 161,136 | |
| Eye & Ear Care | 99,774 | | | 10,635 | | | | | 110,409 | |
| Dermatologicals | 103,998 | | | 3,085 | | | | | 107,083 | |
| Oral Care | 88,903 | | | 12,528 | | | | | 101,431 | |
| Other OTC | 5,421 | | | 5 | | | | | 5,426 | |
| | | | | | | |
| Total segment revenues | $ | 849,319 | | | $ | 94,046 | | | | | $ | 943,365 | |
| | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2020 |
| (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Analgesics | $ | 113,130 | | | $ | 877 | | | | | $ | 114,007 | |
| Cough & Cold | 87,601 | | | 23,505 | | | | | 111,106 | |
| Women's Health | 239,330 | | | 12,221 | | | | | 251,551 | |
| Gastrointestinal | 130,088 | | | 42,820 | | | | | 172,908 | |
| Eye & Ear Care | 100,245 | | | 11,911 | | | | | 112,156 | |
| Dermatologicals | 100,591 | | | 2,421 | | | | | 103,012 | |
| Oral Care | 83,323 | | | 9,882 | | | | | 93,205 | |
| Other OTC | 5,060 | | | 5 | | | | | 5,065 | |
| | | | | | | |
| Total segment revenues | $ | 859,368 | | | $ | 103,642 | | | | | $ | 963,010 | |
Our total segment revenues by geographic area are as follows: | | | | | | | | | | | | | | | | | |
| Year Ended March 31, |
| 2022 | | 2021 | | 2020 |
| United States | $ | 910,106 | | | $ | 799,038 | | | $ | 812,653 | |
| Rest of world | 176,706 | | | 144,327 | | | 150,357 | |
| Total | $ | 1,086,812 | | | $ | 943,365 | | | $ | 963,010 | |
Our consolidated goodwill and intangible assets have been allocated to the reportable segments as follows:
| | | | | | | | | | | | | | | | | | | |
March 31, 2022 (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Goodwill | $ | 548,291 | | | $ | 30,685 | | | | | $ | 578,976 | |
| | | | | | | |
| Intangible assets | | | | | | | |
| Indefinite-lived | 2,391,517 | | | 85,042 | | | | | 2,476,559 | |
| Finite-lived | 198,353 | | | 21,723 | | | | | 220,076 | |
| Intangible assets, net | 2,589,870 | | | 106,765 | | | | | 2,696,635 | |
| Total | $ | 3,138,161 | | | $ | 137,450 | | | | | $ | 3,275,611 | |
| | | | | | | | | | | | | | | | | | | |
March 31, 2021 (In thousands) | North American OTC
Healthcare | | International OTC
Healthcare | | | | Consolidated |
| Goodwill | $ | 546,643 | | | $ | 31,436 | | | | | $ | 578,079 | |
| | | | | | | |
| Intangible assets | | | | | | | |
| Indefinite-lived | 2,195,617 | | | 86,371 | | | | | 2,281,988 | |
| Finite-lived | 190,462 | | | 3,279 | | | | | 193,741 | |
| Intangible assets, net | 2,386,079 | | | 89,650 | | | | | 2,475,729 | |
| Total | $ | 2,932,722 | | | $ | 121,086 | | | | | $ | 3,053,808 | |
Our goodwill and intangible assets by geographic area are as follows:
| | | | | | | | | | | |
| Year Ended March 31, |
| 2022 | | 2021 |
| United States | $ | 3,138,161 | | | $ | 2,932,722 | |
| Rest of world | 137,450 | | | 121,086 | |
| Total | $ | 3,275,611 | | | $ | 3,053,808 | |
20. Subsequent Events
Share Repurchase Program
On May 3, 2022, the Company's Board of Directors authorized the repurchase of up to $50.0 million of the Company's issued and outstanding common stock. Under the authorization, the Company may purchase common stock through May, 2023 utilizing open market transactions, transactions structured through investment banking institutions, in privately-negotiated transactions, by direct purchases of common stock or a combination of the foregoing in compliance with the applicable rules and regulations of the Securities and Exchange Commission.
Share Based Compensation
On May 2, 2022, the Committee granted 67,959 performance units, 65,721 RSUs and stock options to acquire 195,526 shares of our common stock to certain executive officers and employees under the 2020 Plan. Performance units are earned based on achievement of the performance objectives set by the Committee and, if earned, vest in their entirety on the three-year anniversary of the date of grant. RSUs vest either 33.3% per year over three years or in their entirety on the three-year or five-year anniversary of the date of grant. Upon vesting, both performance units and RSUs will be settled in shares of our common stock. The stock options will vest 33.3% per year over three years and are exercisable for up to ten years from the date of grant. These stock options were granted at an exercise price of $54.47 per share, which is equal to the closing price for our common stock on the date of the grant. Except in cases of death, disability or retirement, termination of employment prior to vesting will result in forfeiture of the unvested performance units, RSUs and the stock options. Vested stock options will remain exercisable by the employee after termination, subject to the terms of the 2020 Plan.
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
(In thousands) | Balance at
Beginning of
Year | | Amounts
Charged to
Expense (Income) | | Deductions | | Other (a) | | Balance at
End of
Year |
| Year Ended March 31, 2022 | | | | | | | | | |
| Reserves for sales returns and allowance | $ | 12,163 | | | $ | 53,230 | | | $ | (51,579) | | | $ | 747 | | | $ | 14,561 | |
| Reserves for trade promotions | 18,422 | | | 89,547 | | | (84,270) | | | 945 | | | 24,644 | |
| Reserves for consumer coupon redemptions | 1,941 | | | 4,107 | | | (4,586) | | | 2,429 | | | 3,891 | |
| Allowance for doubtful accounts | 1,545 | | | 58 | | | (5) | | | — | | | 1,598 | |
| | | | | | | | | |
| | | | | | | | | |
| Year Ended March 31, 2021 | | | | | | | | |
| Reserves for sales returns and allowance | 15,809 | | | 46,195 | | | (49,841) | | | — | | | 12,163 | |
| Reserves for trade promotions | 18,389 | | | 83,479 | | | (83,446) | | | — | | | 18,422 | |
| Reserves for consumer coupon redemptions | 2,063 | | | 3,751 | | | (3,873) | | | — | | | 1,941 | |
| Allowance for doubtful accounts | 1,385 | | | 259 | | | (99) | | | — | | | 1,545 | |
| Deferred tax valuation allowance | 5,441 | | | (5,441) | | (b) | — | | | — | | | — | |
| | | | | | | | | |
| Year Ended March 31, 2020 | | | | | | | | |
| Reserves for sales returns and allowance | 8,973 | | | 57,505 | | | (50,669) | | | — | | | 15,809 | |
| Reserves for trade promotions | 15,491 | | | 88,502 | | | (85,604) | | | — | | | 18,389 | |
| Reserves for consumer coupon redemptions | 1,175 | | | 4,555 | | | (3,667) | | | — | | | 2,063 | |
| Allowance for doubtful accounts | 1,259 | | | 750 | | | (624) | | | — | | | 1,385 | |
| Deferred tax valuation allowance | 3,236 | | | 2,205 | | (c) | — | | | — | | | 5,441 | |
(a) Relates to opening balance sheet adjustments for our Akorn acquisition.
(b) Relates to the release of the valuation allowance on foreign tax credits.
(c) Relates to the unutilized foreign tax credit carryovers.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
The Company’s management, with the participation of its Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of the Company’s disclosure controls and procedures, as defined in Rule 13a–15(e) of the Exchange Act, as of March 31, 2022. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of March 31, 2022, the Company’s disclosure controls and procedures were effective to ensure that information required to be disclosed by the Company in the reports the Company files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
The report of management on our internal control over financial reporting as of March 31, 2022 and the attestation report of our independent registered public accounting firm on our internal control over financial reporting are set forth in Part II, Item 8. “Financial Statements and Supplementary Data" beginning on page 47 of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the quarter ended March 31, 2022 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
Part III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information required to be disclosed by this Item will be contained in the Company’s 2022 Proxy Statement under the headings “Election of Directors,” “Executive Compensation and Other Matters,” “Delinquent Section 16(a) Reports” and “Governance of the Company”, which information is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
Information required to be disclosed by this Item, including Items 402-(b) and 407-(e)(4) and (e)(5) of Regulation S-K, will be contained in the Company’s 2022 Proxy Statement under the headings “Executive Compensation and Other Matters”, “Governance of the Company”, "Compensation Discussion and Analysis", "Compensation Committee Report", and "Compensation Committee Interlocks and Insider Participation", which information is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information required to be disclosed by this Item will be contained in the Company’s 2022 Proxy Statement under the headings “Security Ownership of Certain Beneficial Owners and Management” and “Securities Authorized for Issuance Under Equity Compensation Plans”, which information is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information required to be disclosed by this Item will be contained in the Company’s 2022 Proxy Statement under the headings “Certain Relationships and Related Transactions”, “Election of Directors” and “Governance of the Company”, which information is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Information required to be disclosed by this Item will be contained in the Company’s 2022 Proxy Statement under the heading “Ratification of Appointment of the Independent Registered Public Accounting Firm”, which information is incorporated herein by reference.
Part IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1)Financial Statements
The financial statements and financial statement schedules listed below are set forth under Part II, Item 8 (pages 47 through 86) of this Annual Report on Form 10-K, which are incorporated herein to this Item as if copied verbatim.
| | |
| Prestige Consumer Healthcare Inc. |
Report of Independent Registered Public Accounting Firm, PricewaterhouseCoopers LLP, Auditor Firm ID 238 |
Consolidated Statements of Income and Comprehensive Income for each of the three years in
the period ended March 31, 2022 |
| Consolidated Balance Sheets at March 31, 2022 and 2021 |
| Consolidated Statements of Changes in Stockholders’ Equity for each of the three years in the period ended March 31, 2022 |
| Consolidated Statements of Cash Flows for each of the three years in the period ended March 31, 2022 |
| Notes to Consolidated Financial Statements |
| Schedule II—Valuation and Qualifying Accounts for the years ended March 31, 2022, 2021 and 2020 |
(a)(2)Financial Statement Schedules
Schedule II - Valuation and Qualifying Accounts listed in (a)(1) above is incorporated herein by reference as if copied verbatim. Schedules other than those listed in the preceding sentence have been omitted as they are either not required, not applicable, or the information has otherwise been shown in the Consolidated Financial Statements or notes thereto.
(b) Exhibit Index
| | | | | | | | |
| Exhibit No. | | Description |
| 2.1 | | |
| 2.2 | | |
| 2.3 | | |
| 3.1 | | |
| 3.1.1 | | |
| 3.2 | | |
| 3.3 | | |
| 4.1 | | |
| 4.2 | | Indenture, dated as of December 17, 2013, among Prestige Brands, Inc., as issuer, the Company and certain subsidiaries, as guarantors, and U.S. Bank National Association, as Trustee with respect to 5.375% Senior Notes due 2021 (filed as Exhibit 4.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on February 7, 2014).+ |
| 4.3 | | |
| | | | | | | | |
| 4.4 | | Third Supplemental Indenture, dated as of March 21, 2018, by and among Prestige Brands, Inc., as issuer, the Company and certain subsidiaries, as guarantors, and U.S. Bank National Association, as Trustee with respect to 6.375% Senior Notes due 2024 (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K filed with the SEC on March 21, 2018). + |
| 4.5 | | |
| 4.6 | | |
| 4.7 | | |
| 4.8 | | |
| 4.9 | | |
| 4.10 | | |
| 10.1 | | $660,000,000 Term Loan Credit Agreement, dated as of January 31, 2012, among Prestige Brands Inc., the Company, and certain subsidiaries of the Company as guarantors, Citibank, N.A., Citigroup Global Markets Inc., Morgan Stanley Senior Funding, Inc. and RBC Capital Markets (filed as Exhibit 10.3 to the Company's Annual Report on Form 10-K filed with the SEC on May 18, 2012). + |
| 10.2 | | Amendment No. 1, dated as of February 21, 2013, to the Term Loan Credit Agreement, dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other Guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A. as administrative agent (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the SEC on February 25, 2013). + |
| 10.3 | | Amendment No. 2, dated as of September 3, 2014, to the Term Loan Credit Agreement, dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other Guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A. as administrative agent (filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed with the SEC on February 5, 2015).+ |
| 10.4 | | Amendment No. 3, dated as of May 8, 2015, to the Term Loan Credit Agreement, dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other Guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A. as administrative agent (filed as Exhibit 10.6 to the Company's Annual Report on Form 10-K filed with the SEC on May 14, 2015).+ |
| 10.5 | | Amendment No. 4, dated as of January 26, 2017, to the Term Loan Credit Agreement, dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other guarantors from time to time party thereto, the lenders from time to time party thereto and Barclays Bank PLC (as successor in interest to Citibank, N.A.), as administrative agent (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 31, 2017). + |
| 10.6 | | Amendment No. 5, dated as of March 21, 2018, to the Term Loan Credit Agreement, dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other guarantors from time to time party thereto, the lenders from time to time party thereto and Barclays Bank PLC (as successor in interest to Citibank, N.A.), as administrative agent (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on March 21, 2018).+ |
| 10.7 | | Amendment No. 6 to the Term Loan Credit Agreement, dated as of July 1, 2021, among Prestige Consumer Healthcare Inc., Prestige Brands, Inc., the other guarantors from time to time party thereto, each lender from time to time party thereto and Barclays Bank PLC (as successor in interest to Citibank, N.A.), as administrative agent (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the SEC on July 1, 2021). + |
| 10.8 | | |
| 10.9 | | $50,000,000 ABL Credit Agreement, dated as of January 31, 2012, Among Prestige Brands, Inc., the Company, certain subsidiaries of the Company as guarantors, Citibank, N.A., Citigroup Global Markets Inc., Morgan Stanley Senior Funding, Inc. and RBC Capital Markets filed (filed as Exhibit 10.5 to the Company's Annual Report on Form 10-K filed with the SEC on May 18, 2012.).+ |
| 10.1 | | |
| | | | | | | | |
| 10.11 | | |
| 10.12 | | Amendment No. 3, dated as of September 3, 2014, to the ABL Credit Agreement (as amended by that certain Incremental Amendment, dated as of September 12, 2012, and that certain Incremental Amendment, dated as of June 11, 2013), dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other Guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A. as administrative agent, L/C issuer and swing line lender (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on September 3, 2014). + |
| 10.13 | | Amendment No. 4, dated as of June 9, 2015, to the ABL Credit Agreement (as amended by that certain Incremental Amendment, dated as of September 12, 2012, and that certain Incremental Amendment, dated as of June 11, 2013, and that certain Incremental Amendment dated as of September 3, 2014), dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other Guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A. as administrative agent, L/C issuer and swing line lender (filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed with the SEC on August 6, 2015).+ |
| 10.14 | | Amendment No. 5, dated as of February 4, 2016, to the ABL Credit Agreement, originally dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other Guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A. as administrative agent, L/C issuer and swing line lender (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K filed with the SEC on February 9, 2016). + |
| 10.15 | | Amendment No. 6, dated as of January 26, 2017, to the ABL Credit Agreement, originally dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A., as administrative agent, L/C issue and swing line lender (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on January 31, 2017). + |
| 10.16 | | Amendment No. 7, dated as of December 11, 2019, to the ABL Credit Agreement, originally dated as of January 31, 2012, among the Company, Prestige Brands, Inc., the other guarantors from time to time party thereto, the lenders from time to time party thereto and Citibank, N.A., as administrative agent, L/C issue and swing line lender (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 12, 2019). + |
| 10.17 | | |
| 10.18 | | |
| 10.19 | | |
| 10.2 | | |
| 10.21 | | |
| 10.22 | | |
| 10.23 | | |
| 10.24 | | |
| 10.25 | | |
| 10.26 | | |
| 10.27 | | |
| 10.28 | | |
| 10.29 | | |
| 10.30 | | |
| | | | | | | | |
| 10.31 | | |
| 10.32 | | |
| 10.33 | | |
| 10.34 | | Asset Purchase Agreement, dated July 2, 2018, by and among KIK International LLC, Prestige Brands International, Inc., The Spic and Span Company, Medtech Holdings, Inc. (as guarantor only) and Prestige Brands Holdings, Inc. (filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 2, 2018).+ |
| 10.35 | | AIA Document A141™-2014, Standard Form of Agreement, dated July 1, 2018, by and between C.B. Fleet Company, Incorporated, including its affiliates, subsidiaries, officers, directors, employees and agents and CRB Builders, LLC, including its affiliates, subsidiaries, officers, directors, employees and agents, as amended by Exhibit A Design-Build Amendment, dated March 16, 2020 (filed as Exhibit 10.34 to the Company’s 10-K filed with the SEC on May 8, 2020).*† |
| 10.36 | | |
| 21.1 | | |
| 23.1 | | |
| 31.1 | | |
| 31.2 | | |
| 32.1 | | |
| 32.2 | | |
| | | | | |
| * | Filed herewith. |
| | |
| † | Certain confidential portions have been omitted. |
| | |
| + | Incorporated herein by reference. |
| | |
| @ | Represents a management contract. |
| | |
| # | Represents a compensatory plan. |
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | Prestige Consumer Healthcare Inc. | |
| | | | |
| | By: | /s/ Christine Sacco | |
| | Name: | Christine Sacco | |
| | Title: | Chief Financial Officer | |
| | Date: | May 6, 2022 | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | | | | |
| /s/ RONALD M. LOMBARDI | | Director, President
and Chief Executive Officer | | May 6, 2022 |
| Ronald M. Lombardi | | (Principal Executive Officer) | | |
| | | | | |
| /s/ CHRISTINE SACCO | | Chief Financial Officer | | May 6, 2022 |
| Christine Sacco | | (Principal Financial Officer and | | |
| | | Principal Accounting Officer) | | |
| | | | | |
| /s/ JOHN E. BYOM | | Director | | May 6, 2022 |
| John E. Byom | | | | |
| | | | | |
| /s/ CELESTE A. CLARK | | Director | | May 6, 2022 |
| Celeste A. Clark | | | | |
| | | | |
| /s/ CHRISTOPHER J. COUGHLIN | | Director | | May 6, 2022 |
| Christopher J. Coughlin | | | | |
| | | | |
| /s/ SHEILA A. HOPKINS | | Director | | May 6, 2022 |
| Sheila A. Hopkins | | | | |
| | | | | |
| /s/ NATALE S. RICCIARDI | | Director | | May 6, 2022 |
| Natale S. Ricciardi | | | | |
| | | | | |
| /s/ DAWN M. ZIER | | Director | | May 6, 2022 |
| Dawn M. Zier | | | | |
| | | | |

