
ATHENEX Corporate Presentation September 2019 CONFIDENTIAL Nasdaq: ATNX Exhibit 99.1

2 Except for historical information, all of the statements, expectations, and assumptions contained in this presentation constitute forward-looking statements. These statements include descriptions regarding the intent, belief or current expectations of Athenex, Inc. (the “Company”), its officers or its management with respect to the consolidated results of operations and financial condition of the Company. These statements can be recognized by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “foresee,” “guidance,” “intend,” “likely,” “may,” “plan,” “potential,” “predict,” “preliminary,” “prepare,” “probable,” “project,” “promising,” “seek,” “should,” “will,” or words of similar expressions. Such forward-looking statements are not guarantees of future performance and involve risks and uncertainties. Actual results might differ materially from those explicit or implicit in the forward-looking statements. Important factors that could cause actual results to differ materially include: the development stage of the Company’s primary clinical candidates and related risks involved in drug development, clinical trials, regulation, manufacturing and commercialization; the Company’s reliance on third parties for success in certain areas of its business; the Company’s history of operating losses and need to raise additional capital to continue as a going concern; competition; intellectual property risks; risks relating to doing business in China; the uncertainty of when, if at all, we will be able to resume producing API in our Chongqing plant; and the other risk factors set forth from time to time in the Company’s public filings with the SEC, copies of which are available for free in the Investor Relations section of the Company’s website at http://ir.athenex.com/financial-information/sec-filings?c=254495&p=irol-sec or upon request from the Company’s Investor Relations Department. Information about the Company and any forward-looking statements contained in this presentation are provided and made only as of 9 September 2019 and should not be relied upon as predictions of future events. The Company assumes no obligation and does not undertake to revise or update forward-looking statements to reflect future events or circumstances, except as required by law. DISCLAIMER This presentation does not constitute or form part of any offer for sale or subscription of or solicitation or invitation of any offer to buy or subscribe for any securities. Neither this presentation nor any part of it shall form the basis of or be relied upon in connection with any contract or commitment whatsoever. Specifically, this presentation does not constitute a “prospectus” within the meaning of the Securities Act of 1933, as amended. Our historical results are not necessarily indicative of results to be expected for any future period. The financial data contained in this presentation for the periods and as of the dates indicated are qualified by reference to and should be read in conjunction with our financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in our public filings with the U.S. Securities and Exchange Commission (the “SEC”). Forward Looking Statements
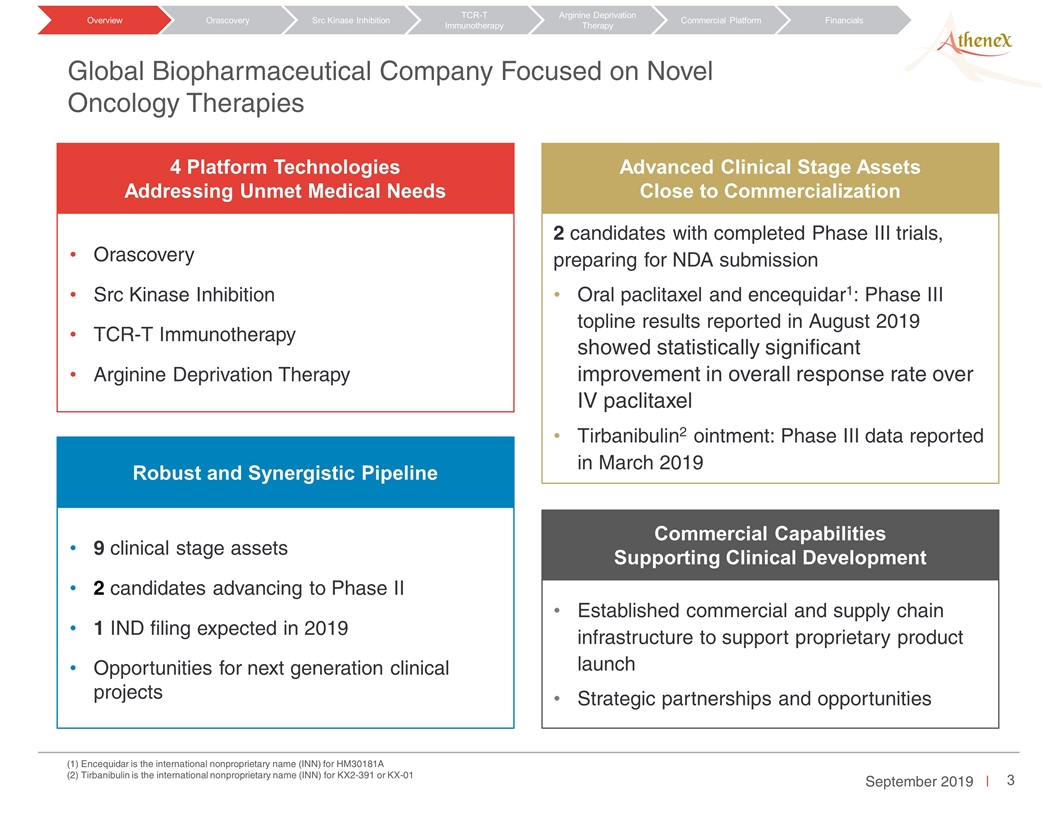
2 candidates with completed Phase III trials, preparing for NDA submission Oral paclitaxel and encequidar1: Phase III topline results reported in August 2019 showed statistically significant improvement in overall response rate over IV paclitaxel Tirbanibulin2 ointment: Phase III data reported in March 2019 9 clinical stage assets 2 candidates advancing to Phase II 1 IND filing expected in 2019 Opportunities for next generation clinical projects Established commercial and supply chain infrastructure to support proprietary product launch Strategic partnerships and opportunities Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy 4 Platform Technologies Addressing Unmet Medical Needs Robust and Synergistic Pipeline Commercial Capabilities Supporting Clinical Development Advanced Clinical Stage Assets Close to Commercialization (1) Encequidar is the international nonproprietary name (INN) for HM30181A (2) Tirbanibulin is the international nonproprietary name (INN) for KX2-391 or KX-01 Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Global Biopharmaceutical Company Focused on Novel Oncology Therapies
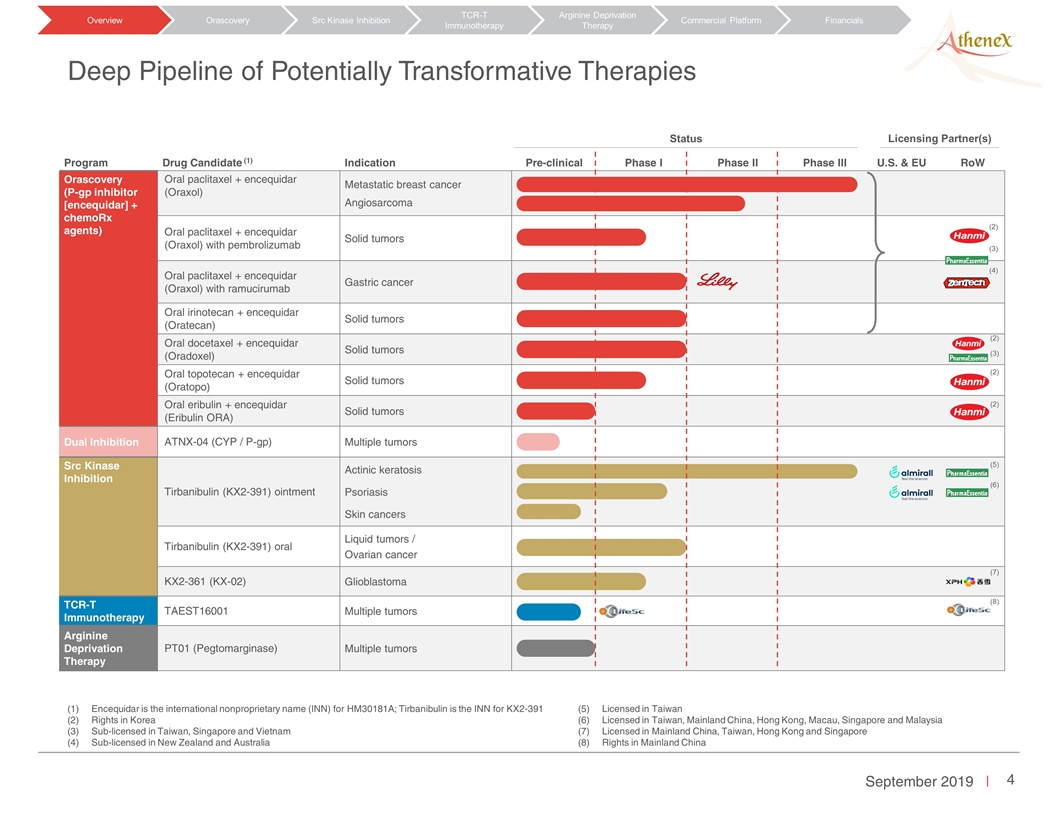
Program Drug Candidate (1) Indication Pre-clinical Phase I Phase II Phase III U.S. & EU RoW Orascovery (P-gp inhibitor [encequidar] + chemoRx agents) Oral paclitaxel + encequidar (Oraxol) Metastatic breast cancer Angiosarcoma Oral paclitaxel + encequidar (Oraxol) with pembrolizumab Solid tumors Oral paclitaxel + encequidar (Oraxol) with ramucirumab Gastric cancer Oral irinotecan + encequidar (Oratecan) Solid tumors Oral docetaxel + encequidar (Oradoxel) Solid tumors Oral topotecan + encequidar (Oratopo) Solid tumors Oral eribulin + encequidar (Eribulin ORA) Solid tumors Dual Inhibition ATNX-04 (CYP / P-gp) Multiple tumors Src Kinase Inhibition Tirbanibulin (KX2-391) ointment Actinic keratosis Psoriasis Skin cancers Tirbanibulin (KX2-391) oral Liquid tumors / Ovarian cancer KX2-361 (KX-02) Glioblastoma TCR-T Immunotherapy TAEST16001 Multiple tumors Arginine Deprivation Therapy PT01 (Pegtomarginase) Multiple tumors Deep Pipeline of Potentially Transformative Therapies Encequidar is the international nonproprietary name (INN) for HM30181A; Tirbanibulin is the INN for KX2-391 Rights in Korea Sub-licensed in Taiwan, Singapore and Vietnam Sub-licensed in New Zealand and Australia Licensed in Taiwan Licensed in Taiwan, Mainland China, Hong Kong, Macau, Singapore and Malaysia Licensed in Mainland China, Taiwan, Hong Kong and Singapore Rights in Mainland China Licensing Partner(s) Status (2) (3) (4) (2) (2) (3) (2) (5) (6) (7) (8) Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials
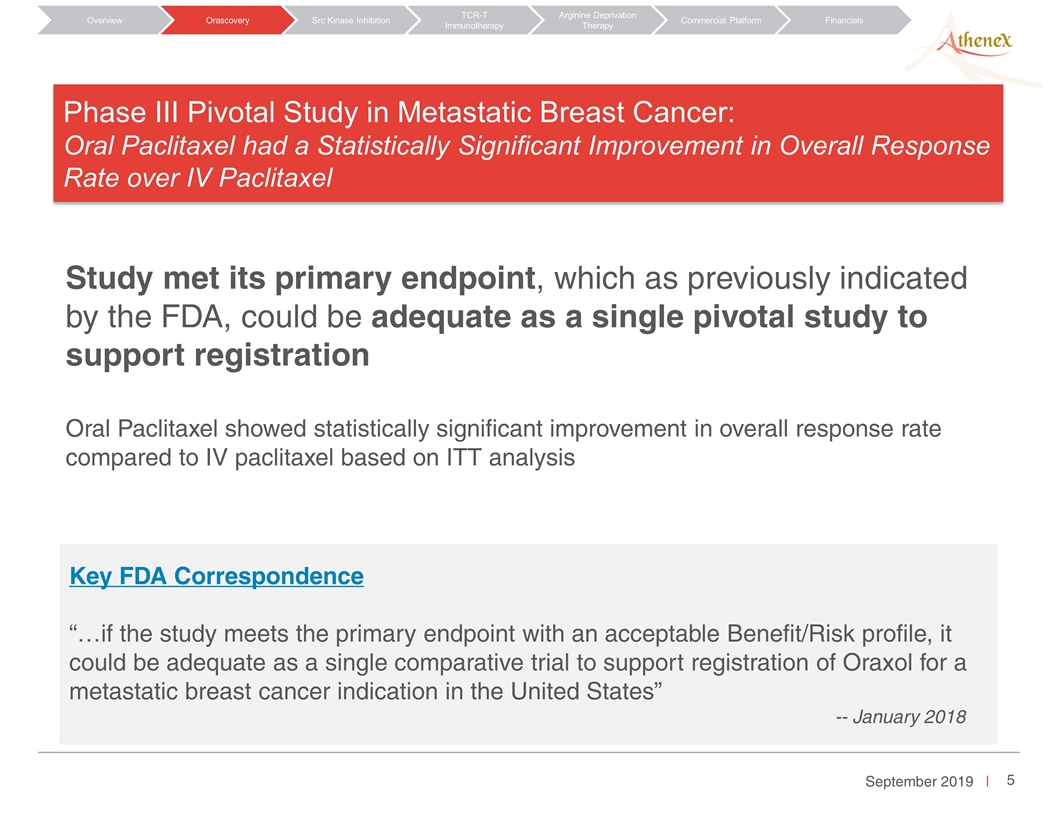
Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Phase III Pivotal Study in Metastatic Breast Cancer: Oral Paclitaxel had a Statistically Significant Improvement in Overall Response Rate over IV Paclitaxel Study met its primary endpoint, which as previously indicated by the FDA, could be adequate as a single pivotal study to support registration Oral Paclitaxel showed statistically significant improvement in overall response rate compared to IV paclitaxel based on ITT analysis Key FDA Correspondence “…if the study meets the primary endpoint with an acceptable Benefit/Risk profile, it could be adequate as a single comparative trial to support registration of Oraxol for a metastatic breast cancer indication in the United States” -- January 2018
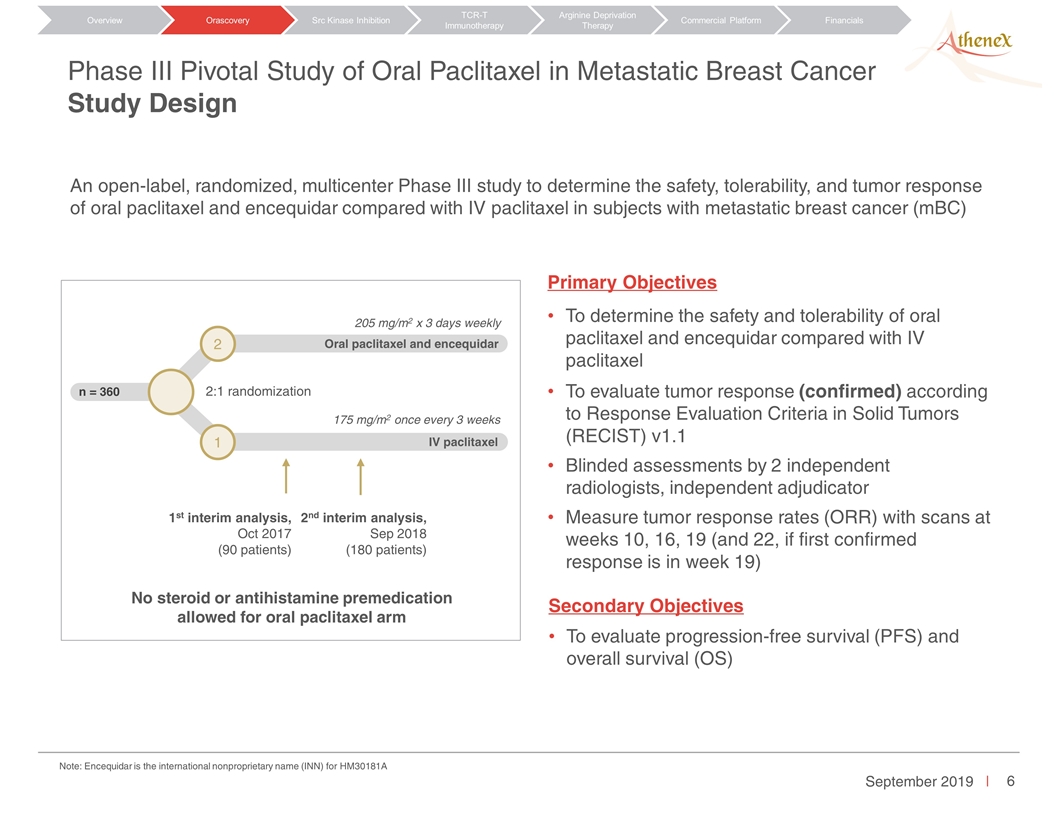
An open-label, randomized, multicenter Phase III study to determine the safety, tolerability, and tumor response of oral paclitaxel and encequidar compared with IV paclitaxel in subjects with metastatic breast cancer (mBC) Primary Objectives To determine the safety and tolerability of oral paclitaxel and encequidar compared with IV paclitaxel To evaluate tumor response (confirmed) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 Blinded assessments by 2 independent radiologists, independent adjudicator Measure tumor response rates (ORR) with scans at weeks 10, 16, 19 (and 22, if first confirmed response is in week 19) Secondary Objectives To evaluate progression-free survival (PFS) and overall survival (OS) Phase III Pivotal Study of Oral Paclitaxel in Metastatic Breast Cancer Study Design Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials n = 360 Oral paclitaxel and encequidar 2:1 randomization 1st interim analysis, Oct 2017 (90 patients) 2nd interim analysis, Sep 2018 (180 patients) No steroid or antihistamine premedication allowed for oral paclitaxel arm 175 mg/m2 once every 3 weeks 205 mg/m2 x 3 days weekly IV paclitaxel 2 1 Note: Encequidar is the international nonproprietary name (INN) for HM30181A
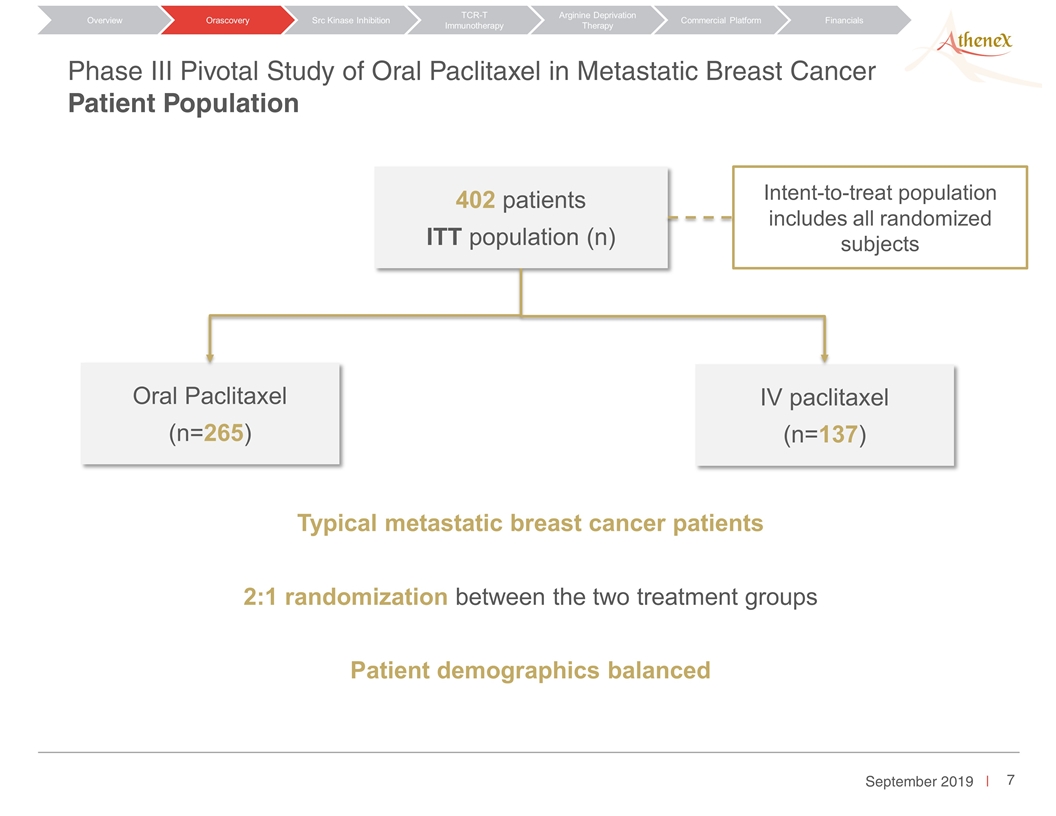
Phase III Pivotal Study of Oral Paclitaxel in Metastatic Breast Cancer Patient Population Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials 402 patients ITT population (n) Typical metastatic breast cancer patients 2:1 randomization between the two treatment groups Patient demographics balanced Oral Paclitaxel (n=265) IV paclitaxel (n=137) Intent-to-treat population includes all randomized subjects
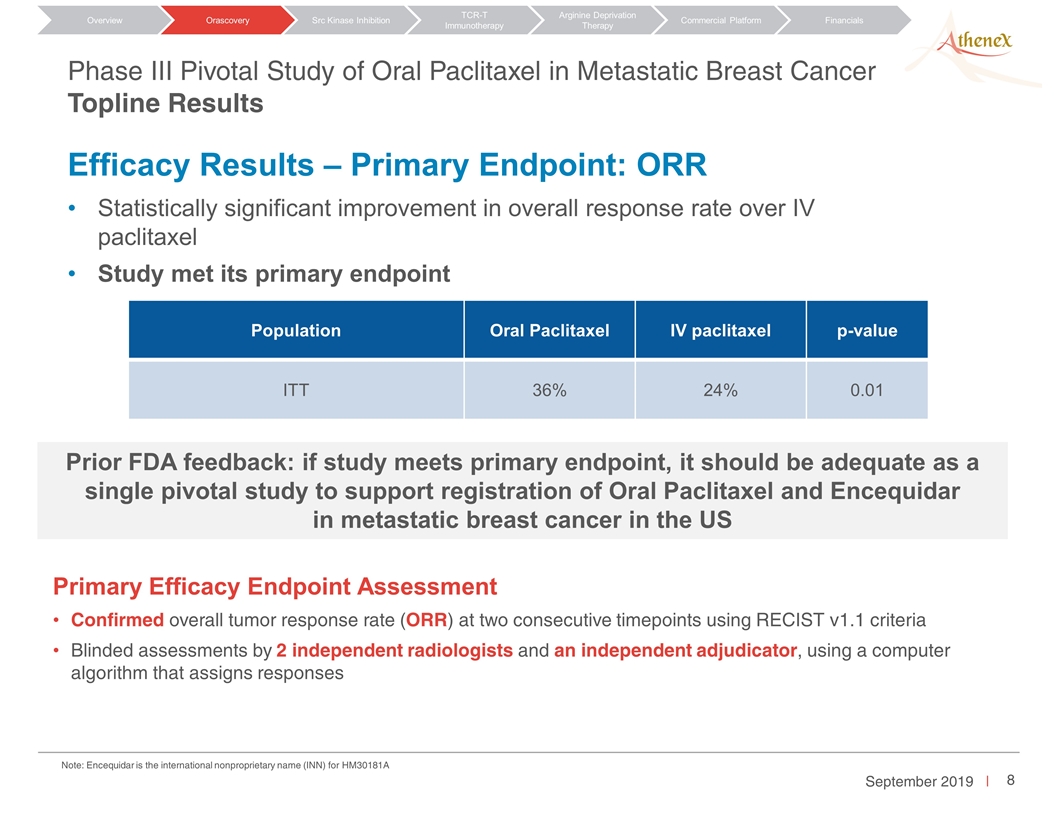
Phase III Pivotal Study of Oral Paclitaxel in Metastatic Breast Cancer Topline Results Population Oral Paclitaxel IV paclitaxel p-value ITT 36% 24% 0.01 Efficacy Results – Primary Endpoint: ORR Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Primary Efficacy Endpoint Assessment Confirmed overall tumor response rate (ORR) at two consecutive timepoints using RECIST v1.1 criteria Blinded assessments by 2 independent radiologists and an independent adjudicator, using a computer algorithm that assigns responses Statistically significant improvement in overall response rate over IV paclitaxel Study met its primary endpoint Prior FDA feedback: if study meets primary endpoint, it should be adequate as a single pivotal study to support registration of Oral Paclitaxel and Encequidar in metastatic breast cancer in the US Note: Encequidar is the international nonproprietary name (INN) for HM30181A
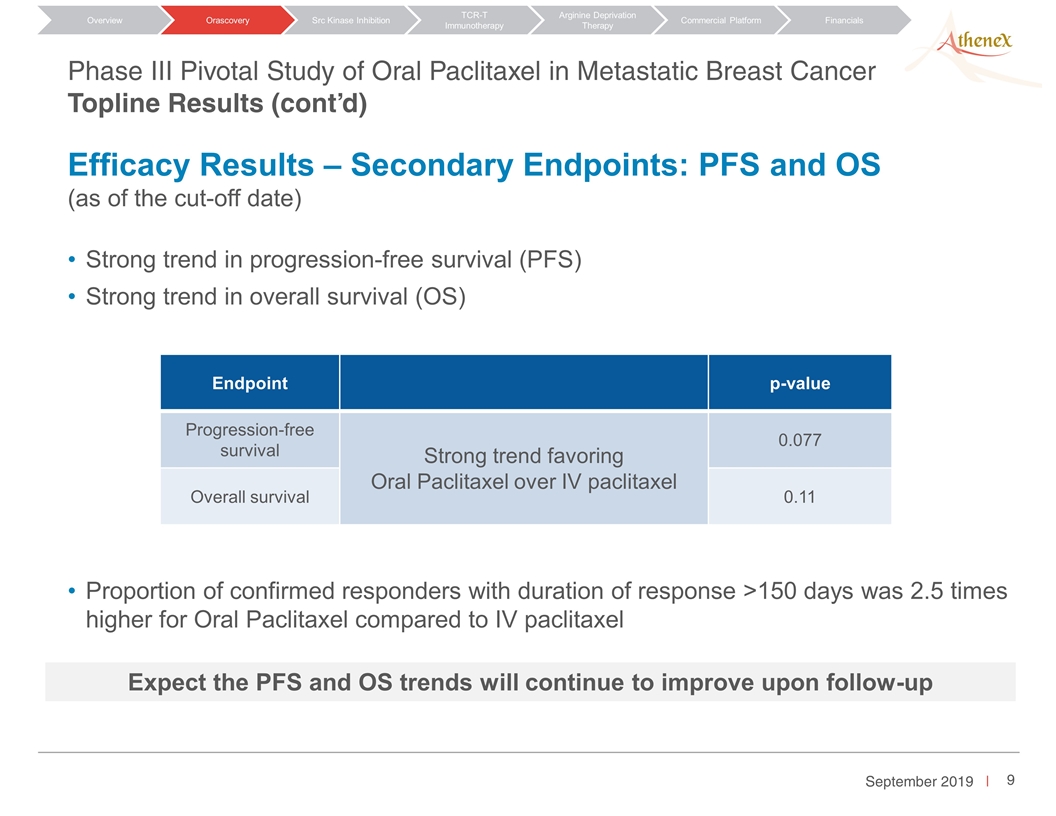
Strong trend in progression-free survival (PFS) Strong trend in overall survival (OS) Proportion of confirmed responders with duration of response >150 days was 2.5 times higher for Oral Paclitaxel compared to IV paclitaxel Endpoint p-value Progression-free survival Strong trend favoring Oral Paclitaxel over IV paclitaxel 0.077 Overall survival 0.11 Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Phase III Pivotal Study of Oral Paclitaxel in Metastatic Breast Cancer Topline Results (cont’d) Efficacy Results – Secondary Endpoints: PFS and OS (as of the cut-off date) Expect the PFS and OS trends will continue to improve upon follow-up
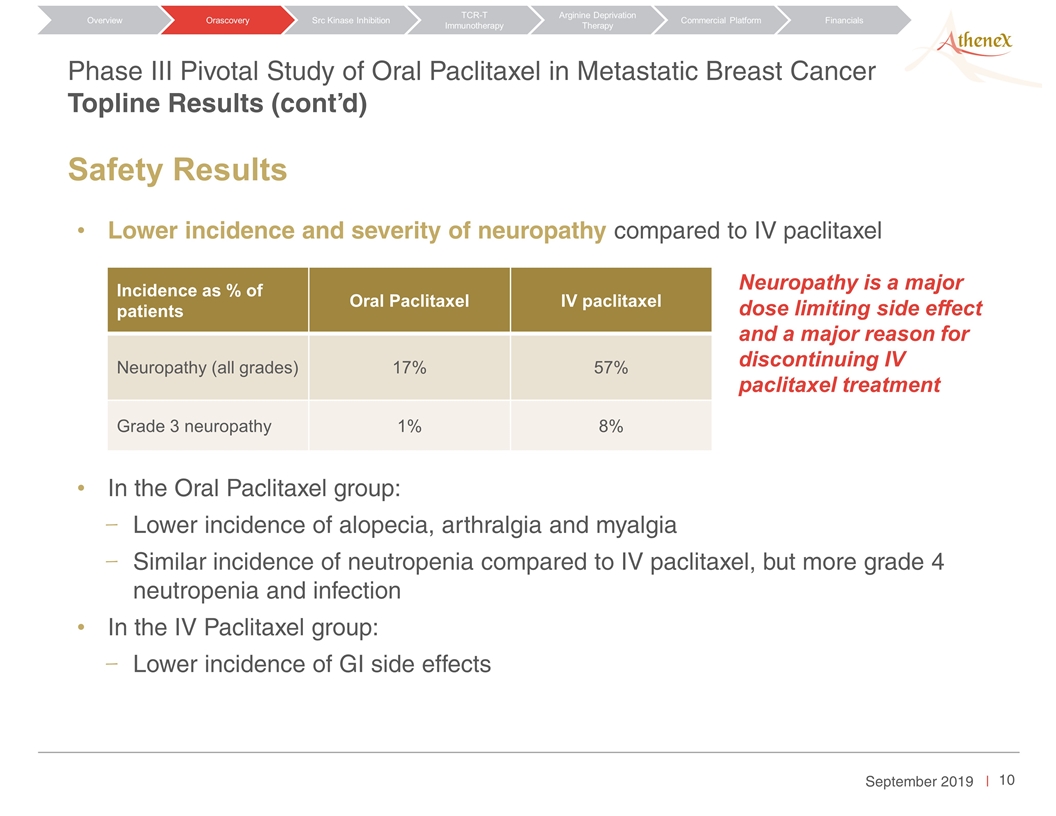
Phase III Pivotal Study of Oral Paclitaxel in Metastatic Breast Cancer Topline Results (cont’d) Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Lower incidence and severity of neuropathy compared to IV paclitaxel In the Oral Paclitaxel group: Lower incidence of alopecia, arthralgia and myalgia Similar incidence of neutropenia compared to IV paclitaxel, but more grade 4 neutropenia and infection In the IV Paclitaxel group: Lower incidence of GI side effects Incidence as % of patients Oral Paclitaxel IV paclitaxel Neuropathy (all grades) 17% 57% Grade 3 neuropathy 1% 8% Neuropathy is a major dose limiting side effect and a major reason for discontinuing IV paclitaxel treatment Safety Results

Study met primary endpoint showing statistically significant improvement in overall response rate for Oral Paclitaxel compared to IV paclitaxel based on intent-to-treat (ITT) analysis Prior FDA feedback that this could be adequate to support an NDA Strong trend in progression-free survival (PFS) and overall survival (OS) of Oral Paclitaxel compared to IV paclitaxel Proportion of confirmed responders with duration of response >150 days was 2.5 times higher for Oral Paclitaxel than IV paclitaxel Neuropathy was observed in less patients treated with Oral Paclitaxel compared to IV paclitaxel Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Phase III Pivotal Study of Oral Paclitaxel in Metastatic Breast Cancer Summary of Topline Results
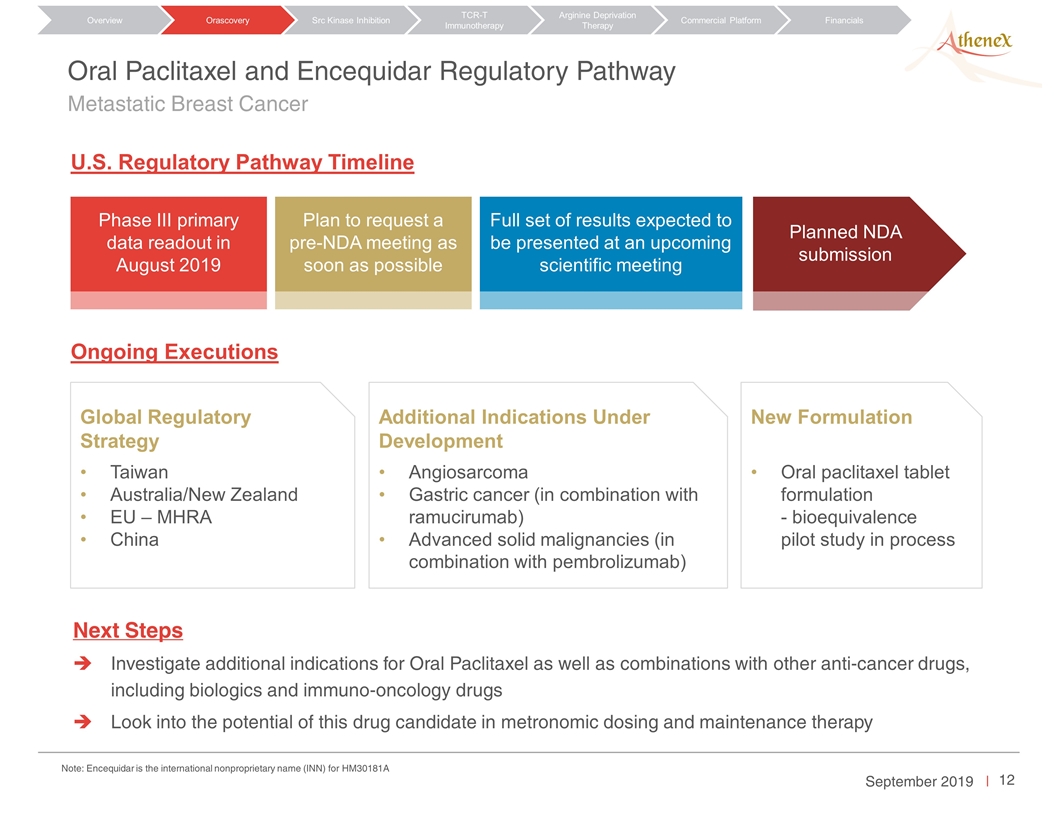
Plan to request a pre-NDA meeting as soon as possible Planned NDA submission Next Steps Investigate additional indications for Oral Paclitaxel as well as combinations with other anti-cancer drugs, including biologics and immuno-oncology drugs Look into the potential of this drug candidate in metronomic dosing and maintenance therapy Oral Paclitaxel and Encequidar Regulatory Pathway Metastatic Breast Cancer Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials To be confirmed by Johnson and Rudolf U.S. Regulatory Pathway Timeline Phase III primary data readout in August 2019 Full set of results expected to be presented at an upcoming scientific meeting Ongoing Executions Global Regulatory Strategy Taiwan Australia/New Zealand EU – MHRA China Additional Indications Under Development Angiosarcoma Gastric cancer (in combination with ramucirumab) Advanced solid malignancies (in combination with pembrolizumab) New Formulation Oral paclitaxel tablet formulation - bioequivalence pilot study in process Note: Encequidar is the international nonproprietary name (INN) for HM30181A
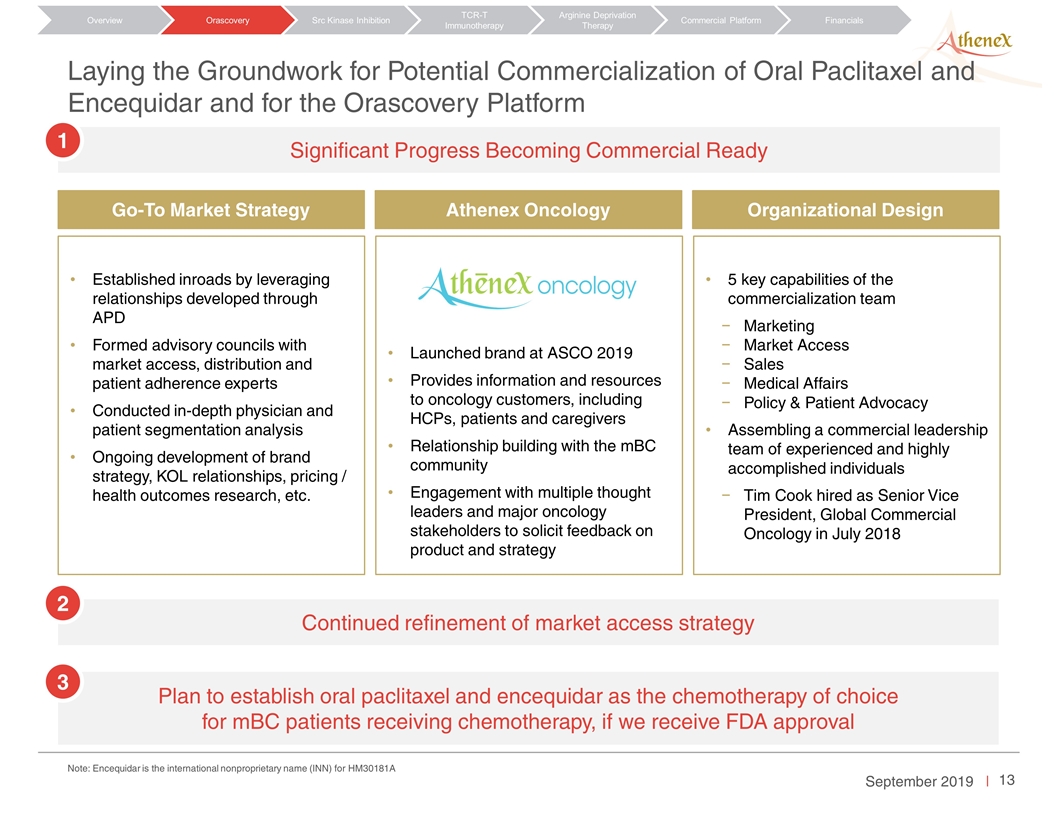
Laying the Groundwork for Potential Commercialization of Oral Paclitaxel and Encequidar and for the Orascovery Platform Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Significant Progress Becoming Commercial Ready Continued refinement of market access strategy Plan to establish oral paclitaxel and encequidar as the chemotherapy of choice for mBC patients receiving chemotherapy, if we receive FDA approval Established inroads by leveraging relationships developed through APD Formed advisory councils with market access, distribution and patient adherence experts Conducted in-depth physician and patient segmentation analysis Ongoing development of brand strategy, KOL relationships, pricing / health outcomes research, etc. Launched brand at ASCO 2019 Provides information and resources to oncology customers, including HCPs, patients and caregivers Relationship building with the mBC community Engagement with multiple thought leaders and major oncology stakeholders to solicit feedback on product and strategy 5 key capabilities of the commercialization team Marketing Market Access Sales Medical Affairs Policy & Patient Advocacy Assembling a commercial leadership team of experienced and highly accomplished individuals Tim Cook hired as Senior Vice President, Global Commercial Oncology in July 2018 Go-To Market Strategy Athenex Oncology Organizational Design 1 2 3 Note: Encequidar is the international nonproprietary name (INN) for HM30181A
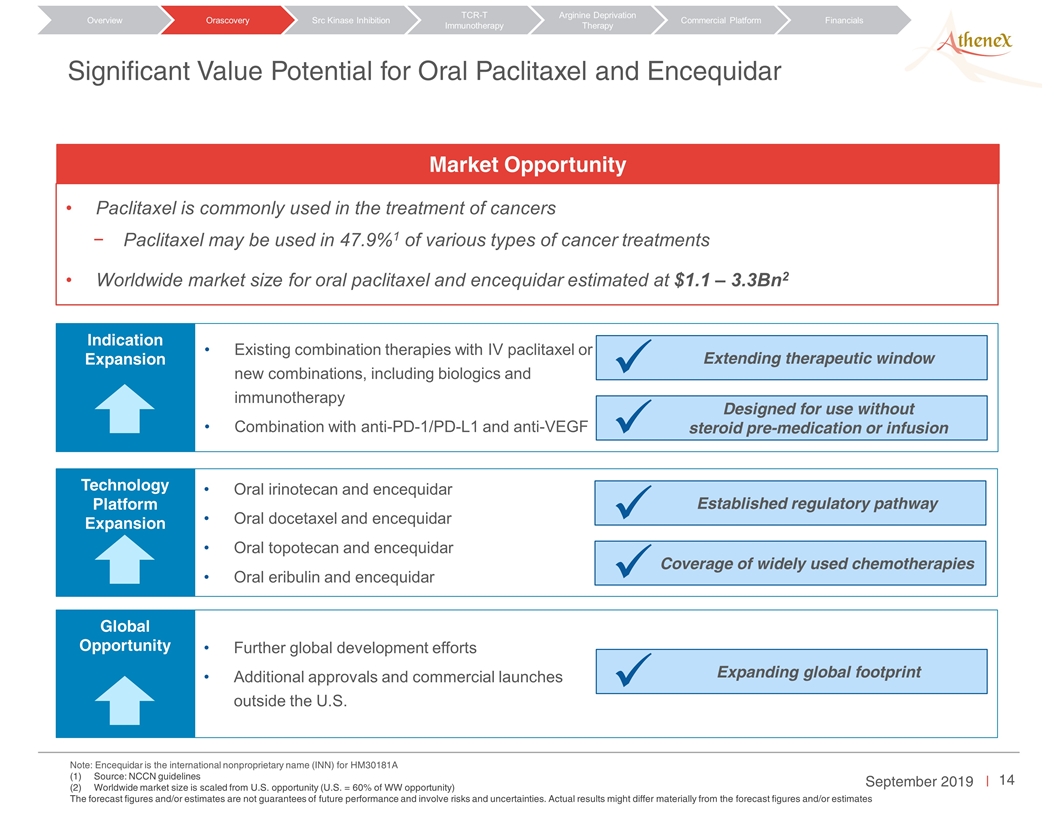
Significant Value Potential for Oral Paclitaxel and Encequidar Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Paclitaxel is commonly used in the treatment of cancers Paclitaxel may be used in 47.9%1 of various types of cancer treatments Worldwide market size for oral paclitaxel and encequidar estimated at $1.1 – 3.3Bn2 Market Opportunity Note: Encequidar is the international nonproprietary name (INN) for HM30181A Source: NCCN guidelines Worldwide market size is scaled from U.S. opportunity (U.S. = 60% of WW opportunity) The forecast figures and/or estimates are not guarantees of future performance and involve risks and uncertainties. Actual results might differ materially from the forecast figures and/or estimates Existing combination therapies with IV paclitaxel or new combinations, including biologics and immunotherapy Combination with anti-PD-1/PD-L1 and anti-VEGF Indication Expansion Extending therapeutic window Designed for use without steroid pre-medication or infusion ü ü Oral irinotecan and encequidar Oral docetaxel and encequidar Oral topotecan and encequidar Oral eribulin and encequidar Technology Platform Expansion Established regulatory pathway Coverage of widely used chemotherapies ü ü Further global development efforts Additional approvals and commercial launches outside the U.S. Global Opportunity Expanding global footprint ü
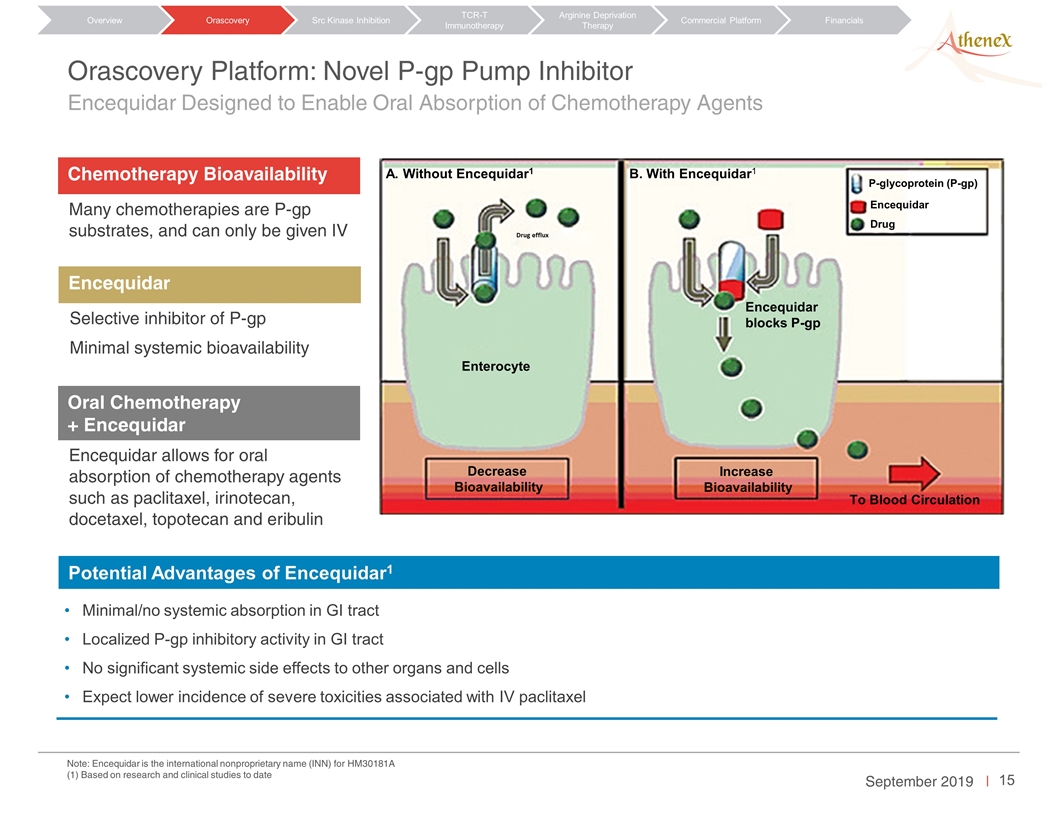
Note: Encequidar is the international nonproprietary name (INN) for HM30181A (1) Based on research and clinical studies to date Orascovery Platform: Novel P-gp Pump Inhibitor Encequidar Designed to Enable Oral Absorption of Chemotherapy Agents Chemotherapy Bioavailability Many chemotherapies are P-gp substrates, and can only be given IV Encequidar Selective inhibitor of P-gp Minimal systemic bioavailability Oral Chemotherapy + Encequidar Encequidar allows for oral absorption of chemotherapy agents such as paclitaxel, irinotecan, docetaxel, topotecan and eribulin Minimal/no systemic absorption in GI tract Localized P-gp inhibitory activity in GI tract No significant systemic side effects to other organs and cells Expect lower incidence of severe toxicities associated with IV paclitaxel Potential Advantages of Encequidar1 A. Without Encequidar1 B. With Encequidar1 Drug efflux Enterocyte Decrease Bioavailability P-glycoprotein (P-gp) To Blood Circulation Encequidar blocks P-gp Encequidar Drug Increase Bioavailability Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials
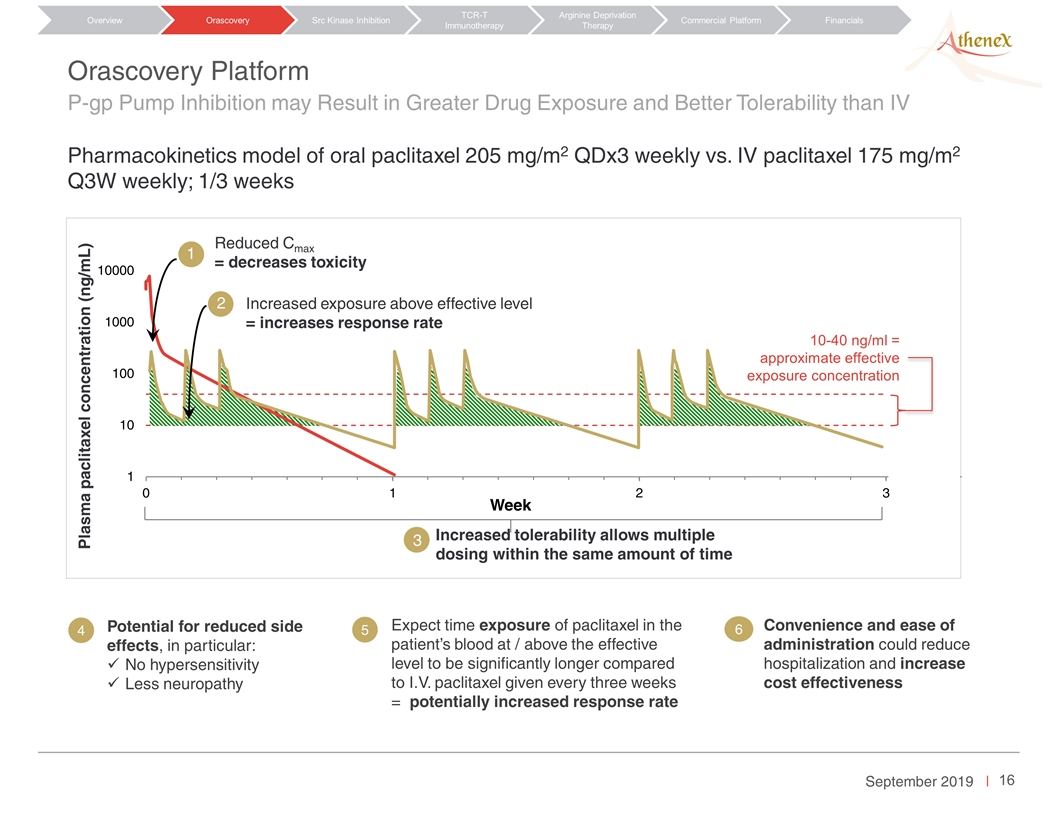
Orascovery Platform P-gp Pump Inhibition may Result in Greater Drug Exposure and Better Tolerability than IV Pharmacokinetics model of oral paclitaxel 205 mg/m2 QDx3 weekly vs. IV paclitaxel 175 mg/m2 Q3W weekly; 1/3 weeks Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials 4 5 Week 3 Plasma paclitaxel concentration (ng/mL) 1 2 Increased tolerability allows multiple dosing within the same amount of time Increased exposure above effective level = increases response rate Reduced Cmax = decreases toxicity 2 Potential for reduced side effects, in particular: No hypersensitivity Less neuropathy Expect time exposure of paclitaxel in the patient’s blood at / above the effective level to be significantly longer compared to I.V. paclitaxel given every three weeks = potentially increased response rate Convenience and ease of administration could reduce hospitalization and increase cost effectiveness 4 5 6 10-40 ng/ml = approximate effective exposure concentration
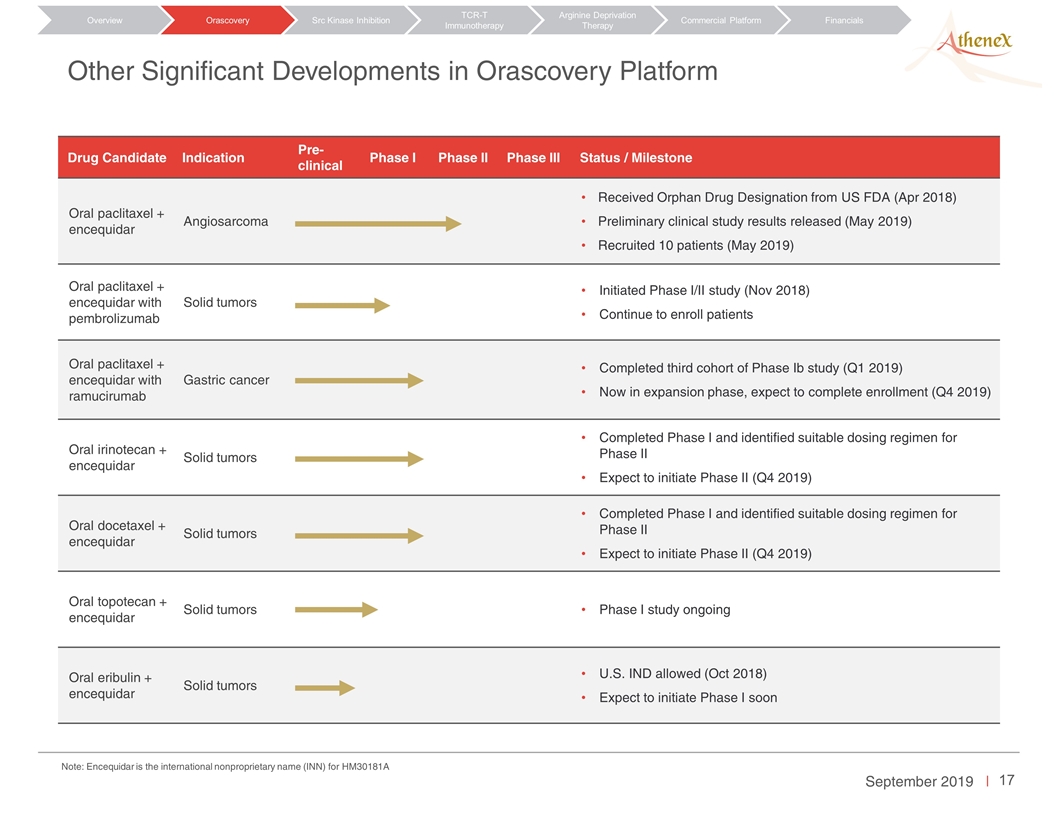
Other Significant Developments in Orascovery Platform Drug Candidate Indication Pre-clinical Phase I Phase II Phase III Status / Milestone Oral paclitaxel + encequidar Angiosarcoma Received Orphan Drug Designation from US FDA (Apr 2018) Preliminary clinical study results released (May 2019) Recruited 10 patients (May 2019) Oral paclitaxel + encequidar with pembrolizumab Solid tumors Initiated Phase I/II study (Nov 2018) Continue to enroll patients Oral paclitaxel + encequidar with ramucirumab Gastric cancer Completed third cohort of Phase Ib study (Q1 2019) Now in expansion phase, expect to complete enrollment (Q4 2019) Oral irinotecan + encequidar Solid tumors Completed Phase I and identified suitable dosing regimen for Phase II Expect to initiate Phase II (Q4 2019) Oral docetaxel + encequidar Solid tumors Completed Phase I and identified suitable dosing regimen for Phase II Expect to initiate Phase II (Q4 2019) Oral topotecan + encequidar Solid tumors Phase I study ongoing Oral eribulin + encequidar Solid tumors U.S. IND allowed (Oct 2018) Expect to initiate Phase I soon Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Note: Encequidar is the international nonproprietary name (INN) for HM30181A
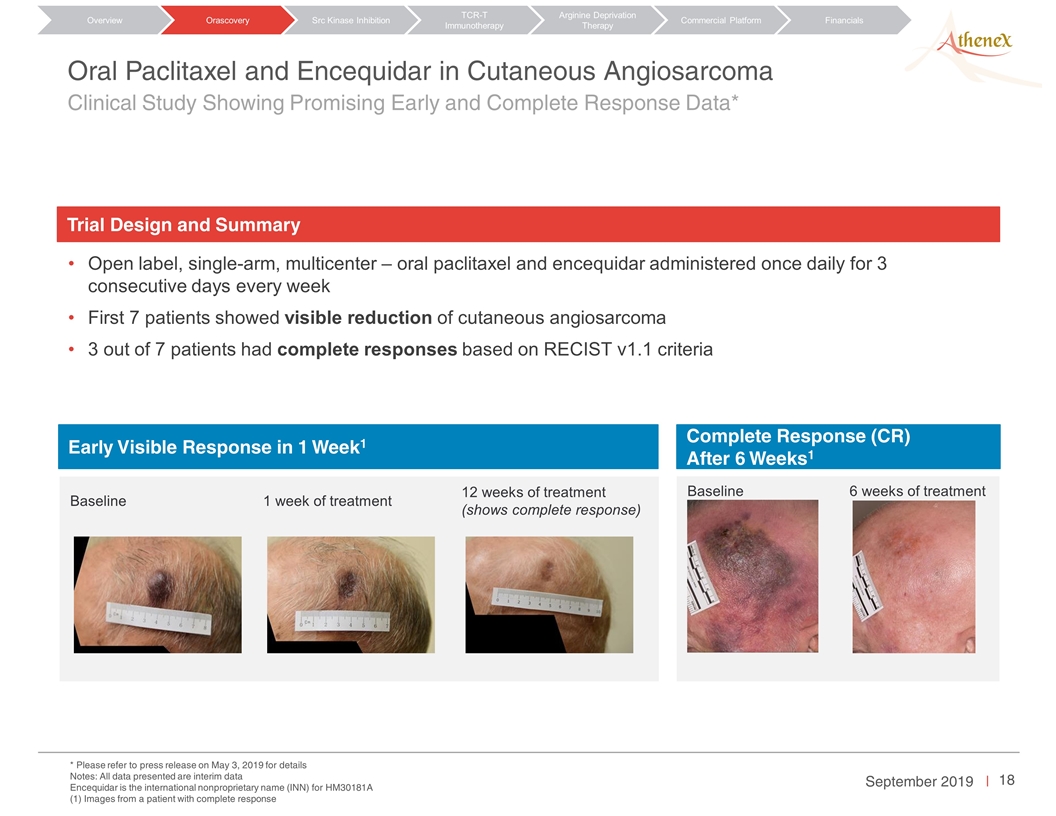
Baseline 1 week of treatment 12 weeks of treatment (shows complete response) Baseline 6 weeks of treatment Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Oral Paclitaxel and Encequidar in Cutaneous Angiosarcoma Clinical Study Showing Promising Early and Complete Response Data* Open label, single-arm, multicenter – oral paclitaxel and encequidar administered once daily for 3 consecutive days every week First 7 patients showed visible reduction of cutaneous angiosarcoma 3 out of 7 patients had complete responses based on RECIST v1.1 criteria * Please refer to press release on May 3, 2019 for details Notes: All data presented are interim data Encequidar is the international nonproprietary name (INN) for HM30181A (1) Images from a patient with complete response Early Visible Response in 1 Week1 Trial Design and Summary Complete Response (CR) After 6 Weeks1
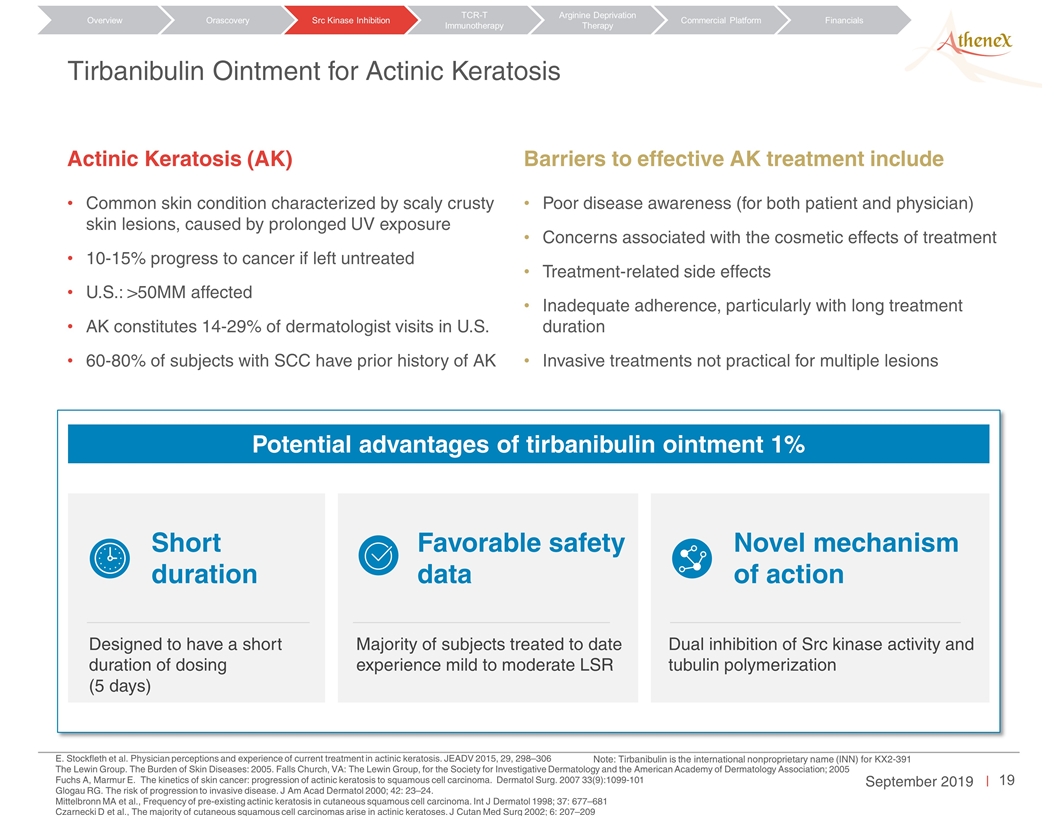
Tirbanibulin Ointment for Actinic Keratosis Potential advantages of tirbanibulin ointment 1% Designed to have a short duration of dosing (5 days) Short duration Favorable safety data Majority of subjects treated to date experience mild to moderate LSR Novel mechanism of action Dual inhibition of Src kinase activity and tubulin polymerization Actinic Keratosis (AK) Common skin condition characterized by scaly crusty skin lesions, caused by prolonged UV exposure 10-15% progress to cancer if left untreated U.S.: >50MM affected AK constitutes 14-29% of dermatologist visits in U.S. 60-80% of subjects with SCC have prior history of AK E. Stockfleth et al. Physician perceptions and experience of current treatment in actinic keratosis. JEADV 2015, 29, 298–306 The Lewin Group. The Burden of Skin Diseases: 2005. Falls Church, VA: The Lewin Group, for the Society for Investigative Dermatology and the American Academy of Dermatology Association; 2005 Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007 33(9):1099-101 Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol 2000; 42: 23–24. Mittelbronn MA et al., Frequency of pre-existing actinic keratosis in cutaneous squamous cell carcinoma. Int J Dermatol 1998; 37: 677–681 Czarnecki D et al., The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg 2002; 6: 207–209 Note: Tirbanibulin is the international nonproprietary name (INN) for KX2-391 Efficacy Results of KX2-391 Ointment in the Field Treatment of Actinic Keratosis Study KX01-AK-003 KX01-AK-004 % of Subjects in the Intent-To-Treat Population KX2-391 Vehicle p-value KX2-391 Vehicle p-value (Number of Subjects) N=175 N=176 N=178 N=173 100% AK Clearance on Day 57 44% (N=77) 5% (N=8) <0.0001a 54% (N=97) 13% (N=22) <0.0001a Face 50% 6% <0.0001 61% 14% <0.0001 Scalp 30% 2% <0.0001 41% 11% 0.0003 ≥75% AK Clearance on Day 57 68% 16% <0.0001a 76% 20% <0.0001a Note: a= p-value calculated based on Cochran-Mantel-Haenszel (CMH) Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Barriers to effective AK treatment include Poor disease awareness (for both patient and physician) Concerns associated with the cosmetic effects of treatment Treatment-related side effects Inadequate adherence, particularly with long treatment duration Invasive treatments not practical for multiple lesions
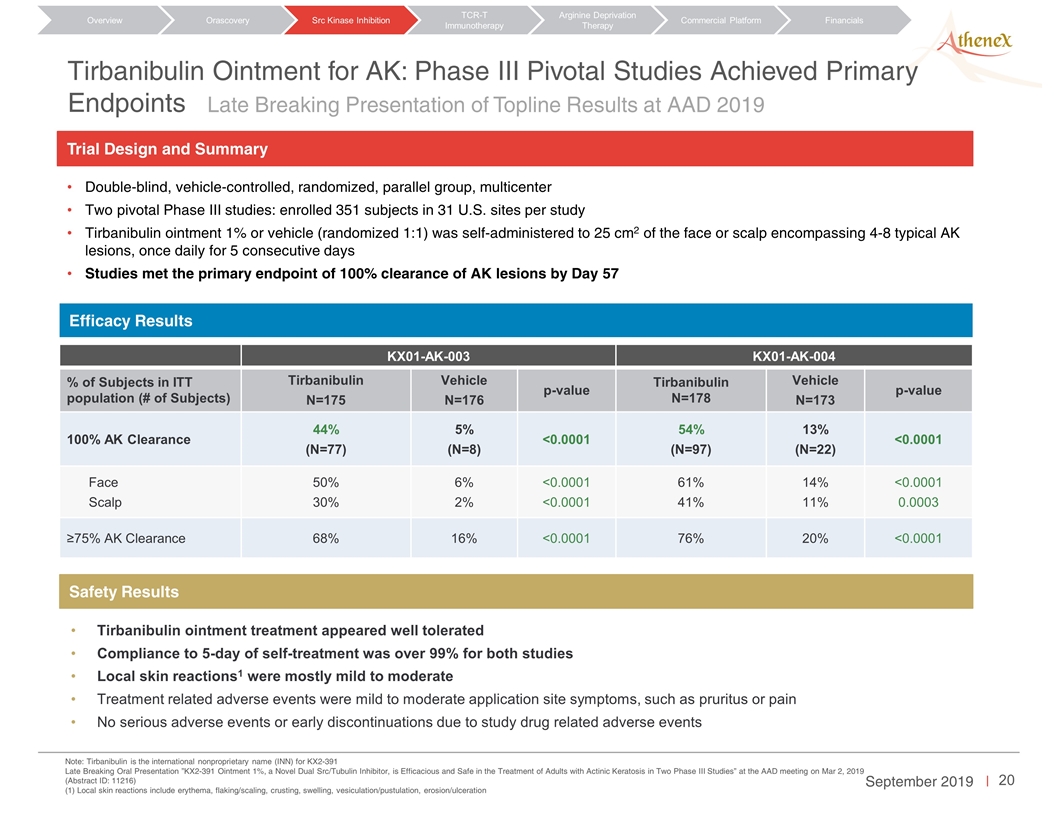
Tirbanibulin Ointment for AK: Phase III Pivotal Studies Achieved Primary Endpoints Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Late Breaking Presentation of Topline Results at AAD 2019 KX01-AK-003 KX01-AK-004 % of Subjects in ITT population (# of Subjects) Tirbanibulin N=175 Vehicle N=176 p-value Tirbanibulin N=178 Vehicle N=173 p-value 100% AK Clearance 44% (N=77) 5% (N=8) <0.0001 54% (N=97) 13% (N=22) <0.0001 Face Scalp 50% 30% 6% 2% <0.0001 <0.0001 61% 41% 14% 11% <0.0001 0.0003 ≥75% AK Clearance 68% 16% <0.0001 76% 20% <0.0001 Tirbanibulin ointment treatment appeared well tolerated Compliance to 5-day of self-treatment was over 99% for both studies Local skin reactions1 were mostly mild to moderate Treatment related adverse events were mild to moderate application site symptoms, such as pruritus or pain No serious adverse events or early discontinuations due to study drug related adverse events Note: Tirbanibulin is the international nonproprietary name (INN) for KX2-391 Late Breaking Oral Presentation ”KX2-391 Ointment 1%, a Novel Dual Src/Tubulin Inhibitor, is Efficacious and Safe in the Treatment of Adults with Actinic Keratosis in Two Phase III Studies” at the AAD meeting on Mar 2, 2019 (Abstract ID: 11216) (1) Local skin reactions include erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, erosion/ulceration Efficacy Results Safety Results Double-blind, vehicle-controlled, randomized, parallel group, multicenter Two pivotal Phase III studies: enrolled 351 subjects in 31 U.S. sites per study Tirbanibulin ointment 1% or vehicle (randomized 1:1) was self-administered to 25 cm2 of the face or scalp encompassing 4-8 typical AK lesions, once daily for 5 consecutive days Studies met the primary endpoint of 100% clearance of AK lesions by Day 57 Trial Design and Summary
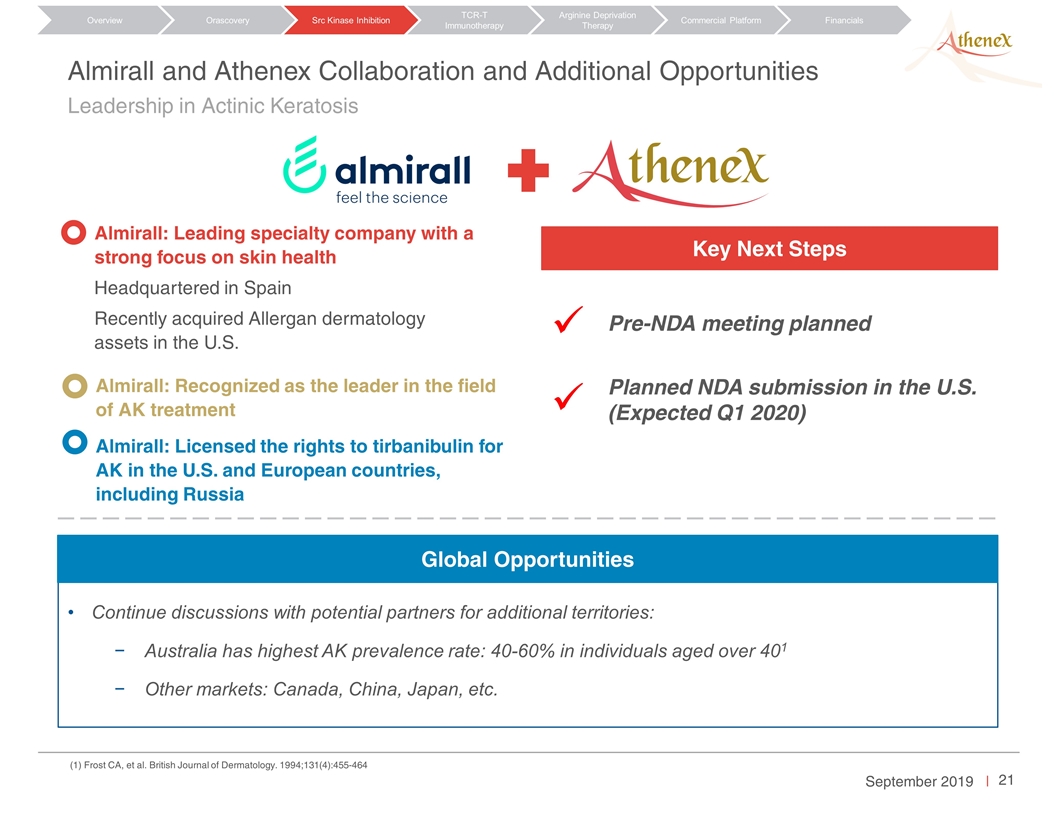
Leadership in Actinic Keratosis Almirall and Athenex Collaboration and Additional Opportunities Almirall: Leading specialty company with a strong focus on skin health Headquartered in Spain Recently acquired Allergan dermatology assets in the U.S. Almirall: Recognized as the leader in the field of AK treatment (1) Frost CA, et al. British Journal of Dermatology. 1994;131(4):455-464 Almirall: Licensed the rights to tirbanibulin for AK in the U.S. and European countries, including Russia Key Next Steps Phase III follow-up data NDA submission in the U.S. [Pre-NDA meeting planned?] Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Key Next Steps Planned NDA submission in the U.S. (Expected Q1 2020) Pre-NDA meeting planned ü ü Continue discussions with potential partners for additional territories: Australia has highest AK prevalence rate: 40-60% in individuals aged over 401 Other markets: Canada, China, Japan, etc. Global Opportunities
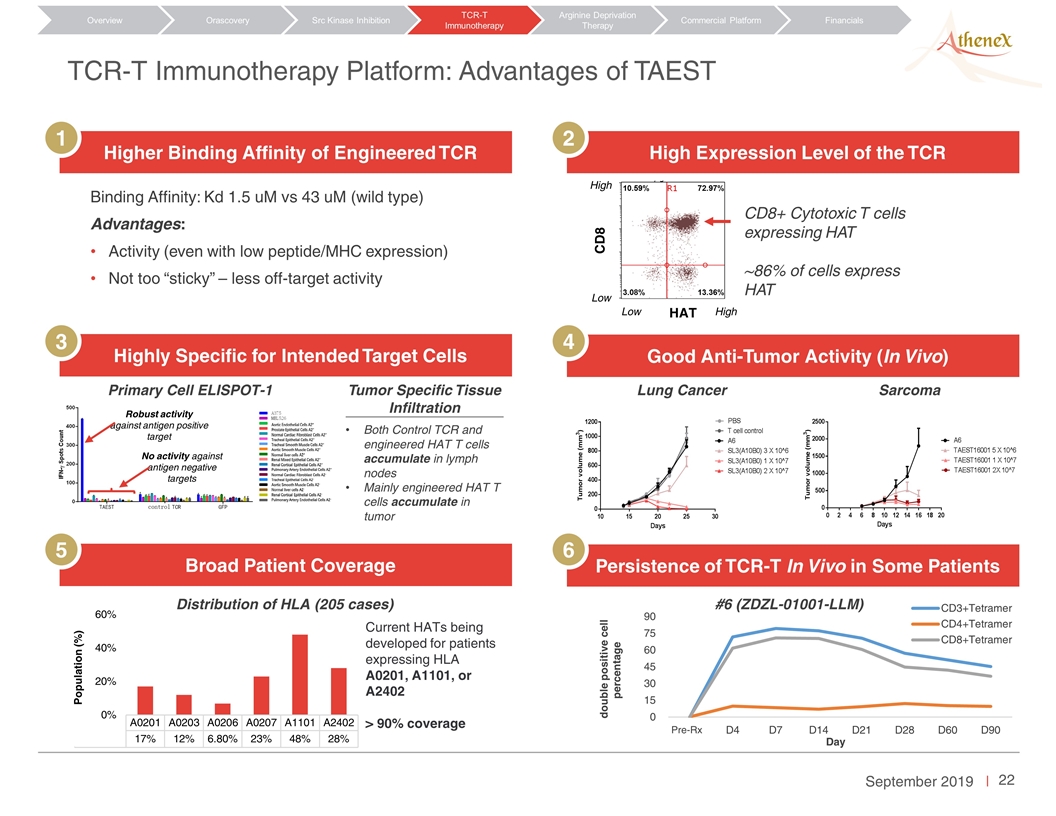
TCR-T Immunotherapy Platform: Advantages of TAEST Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Higher Binding Affinity of Engineered TCR High Expression Level of the TCR Highly Specific for Intended Target Cells Good Anti-Tumor Activity (In Vivo) Broad Patient Coverage Persistence of TCR-T In Vivo in Some Patients 1 2 3 4 5 6 Binding Affinity: Kd 1.5 uM vs 43 uM (wild type) Advantages: Activity (even with low peptide/MHC expression) Not too “sticky” – less off-target activity CD8 Low Low High HAT High CD8+ Cytotoxic T cells expressing HAT ~86% of cells express HAT No activity against antigen negative targets Robust activity against antigen positive target Tumor Specific Tissue Infiltration Both Control TCR and engineered HAT T cells accumulate in lymph nodes Mainly engineered HAT T cells accumulate in tumor Lung Cancer Sarcoma Current HATs being developed for patients expressing HLA A0201, A1101, or A2402 > 90% coverage Primary Cell ELISPOT-1 #6 (ZDZL-01001-LLM) Distribution of HLA (205 cases)
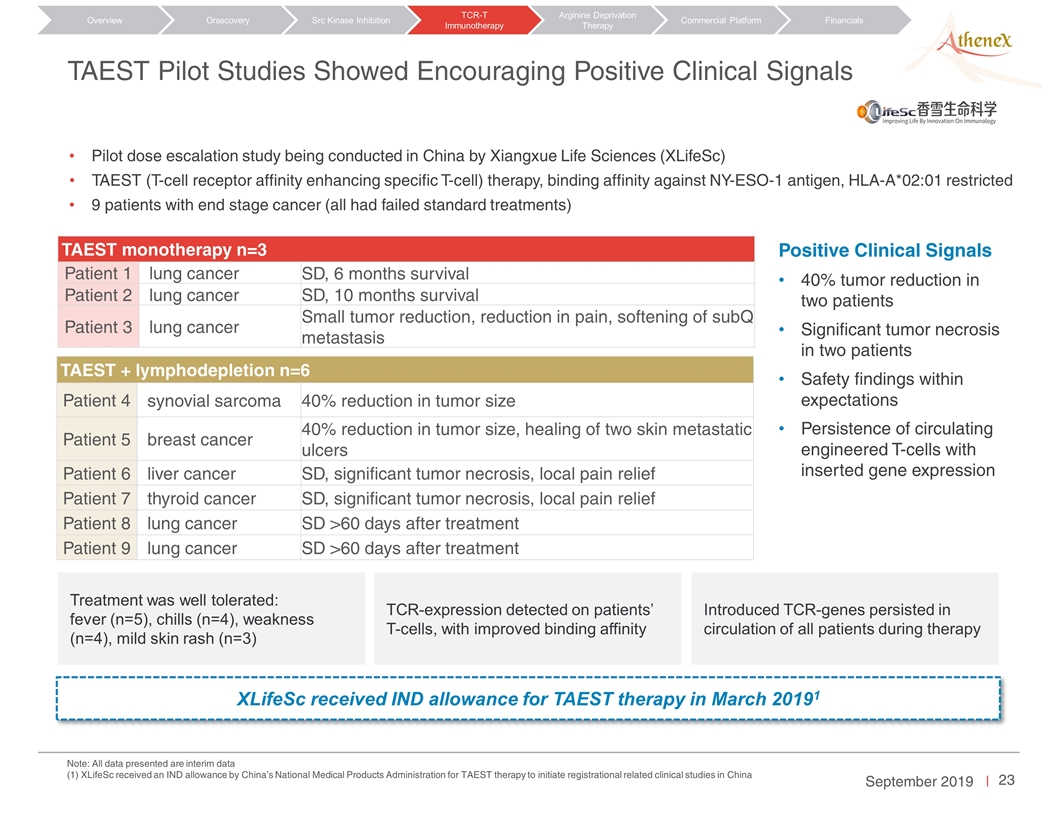
TAEST Pilot Studies Showed Encouraging Positive Clinical Signals TAEST monotherapy n=3 Patient 1 lung cancer SD, 6 months survival Patient 2 lung cancer SD, 10 months survival Patient 3 lung cancer Small tumor reduction, reduction in pain, softening of subQ metastasis Pilot dose escalation study being conducted in China by Xiangxue Life Sciences (XLifeSc) TAEST (T-cell receptor affinity enhancing specific T-cell) therapy, binding affinity against NY-ESO-1 antigen, HLA-A*02:01 restricted 9 patients with end stage cancer (all had failed standard treatments) TAEST + lymphodepletion n=6 Patient 4 synovial sarcoma 40% reduction in tumor size Patient 5 breast cancer 40% reduction in tumor size, healing of two skin metastatic ulcers Patient 6 liver cancer SD, significant tumor necrosis, local pain relief Patient 7 thyroid cancer SD, significant tumor necrosis, local pain relief Patient 8 lung cancer SD >60 days after treatment Patient 9 lung cancer SD >60 days after treatment Treatment was well tolerated: fever (n=5), chills (n=4), weakness (n=4), mild skin rash (n=3) Introduced TCR-genes persisted in circulation of all patients during therapy TCR-expression detected on patients’ T-cells, with improved binding affinity Positive Clinical Signals 40% tumor reduction in two patients Significant tumor necrosis in two patients Safety findings within expectations Persistence of circulating engineered T-cells with inserted gene expression XLifeSc received IND allowance for TAEST therapy in March 20191 Note: All data presented are interim data (1) XLifeSc received an IND allowance by China’s National Medical Products Administration for TAEST therapy to initiate registrational related clinical studies in China Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials
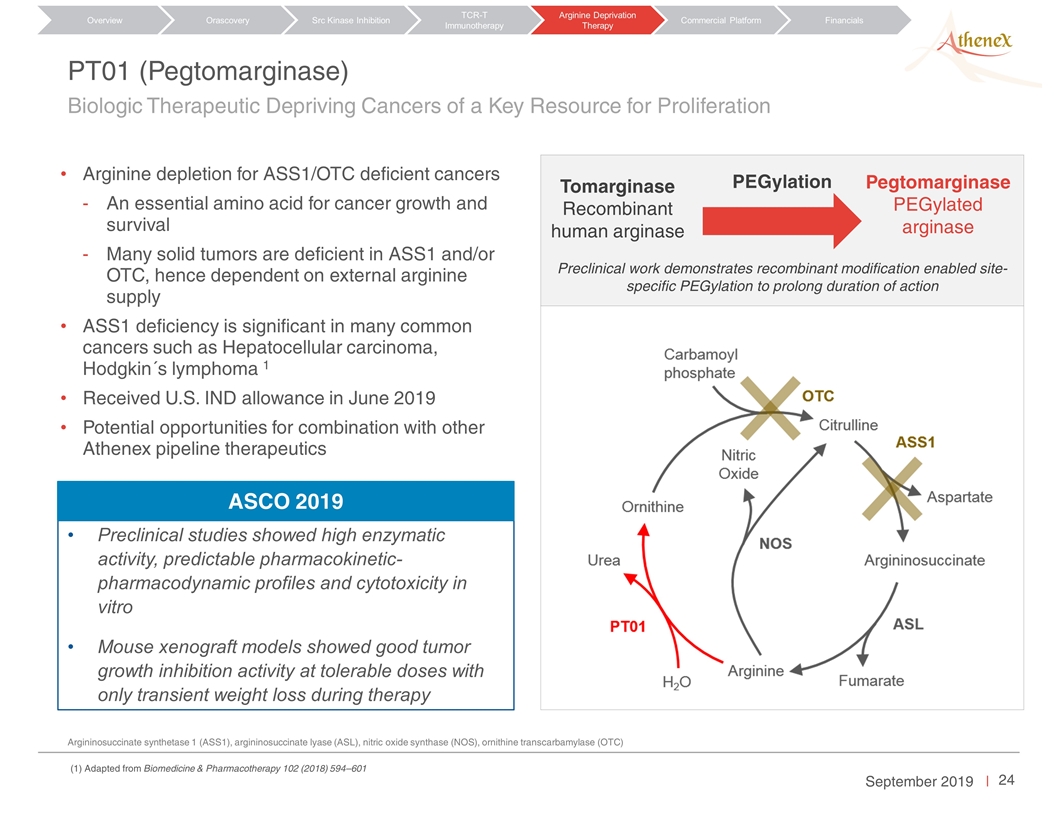
PT01 (Pegtomarginase) Arginine depletion for ASS1/OTC deficient cancers An essential amino acid for cancer growth and survival Many solid tumors are deficient in ASS1 and/or OTC, hence dependent on external arginine supply ASS1 deficiency is significant in many common cancers such as Hepatocellular carcinoma, Hodgkin´s lymphoma 1 Received U.S. IND allowance in June 2019 Potential opportunities for combination with other Athenex pipeline therapeutics Argininosuccinate synthetase 1 (ASS1), argininosuccinate lyase (ASL), nitric oxide synthase (NOS), ornithine transcarbamylase (OTC) Preclinical work demonstrates recombinant modification enabled site-specific PEGylation to prolong duration of action PEGylation Tomarginase Recombinant human arginase Pegtomarginase PEGylated arginase Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Biologic Therapeutic Depriving Cancers of a Key Resource for Proliferation Preclinical studies showed high enzymatic activity, predictable pharmacokinetic-pharmacodynamic profiles and cytotoxicity in vitro Mouse xenograft models showed good tumor growth inhibition activity at tolerable doses with only transient weight loss during therapy ASCO 2019 (1) Adapted from Biomedicine & Pharmacotherapy 102 (2018) 594–601
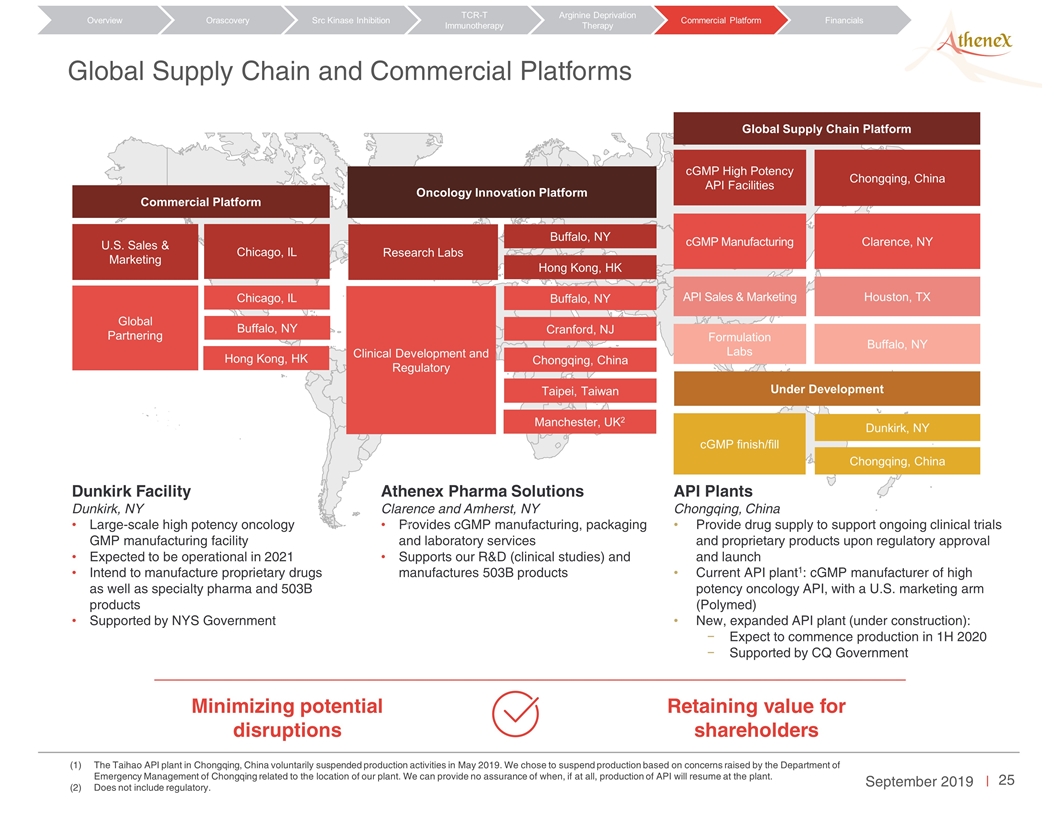
The Taihao API plant in Chongqing, China voluntarily suspended production activities in May 2019. We chose to suspend production based on concerns raised by the Department of Emergency Management of Chongqing related to the location of our plant. We can provide no assurance of when, if at all, production of API will resume at the plant. Does not include regulatory. Global Supply Chain and Commercial Platforms Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Minimizing potential disruptions Retaining value for shareholders Dunkirk Facility Dunkirk, NY Large-scale high potency oncology GMP manufacturing facility Expected to be operational in 2021 Intend to manufacture proprietary drugs as well as specialty pharma and 503B products Supported by NYS Government Athenex Pharma Solutions Clarence and Amherst, NY Provides cGMP manufacturing, packaging and laboratory services Supports our R&D (clinical studies) and manufactures 503B products API Plants Chongqing, China Provide drug supply to support ongoing clinical trials and proprietary products upon regulatory approval and launch Current API plant1: cGMP manufacturer of high potency oncology API, with a U.S. marketing arm (Polymed) New, expanded API plant (under construction): Expect to commence production in 1H 2020 Supported by CQ Government Oncology Innovation Platform Research Labs Buffalo, NY Clinical Development and Regulatory Hong Kong, HK Buffalo, NY Cranford, NJ Chongqing, China Taipei, Taiwan Manchester, UK2 Commercial Platform U.S. Sales & Marketing Chicago, IL Global Partnering Chicago, IL Buffalo, NY Hong Kong, HK Global Supply Chain Platform Under Development cGMP High Potency API Facilities Chongqing, China cGMP Manufacturing Clarence, NY Formulation Labs Buffalo, NY cGMP finish/fill Dunkirk, NY Chongqing, China API Sales & Marketing Houston, TX
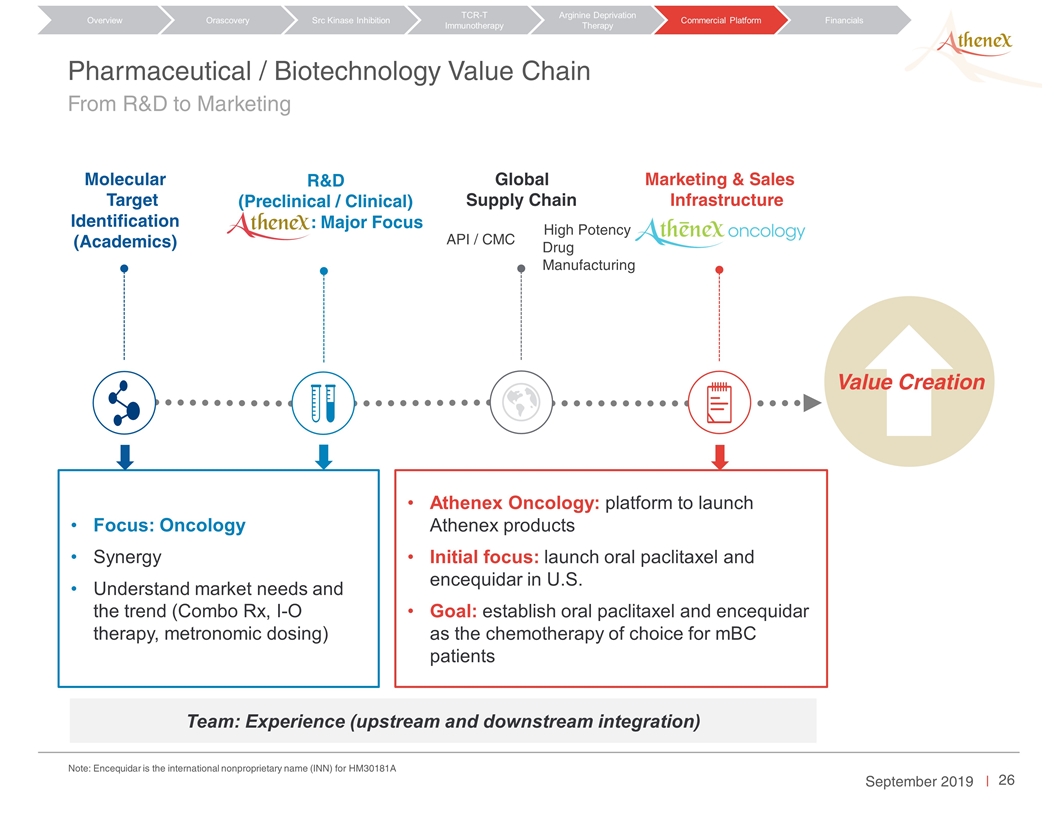
Pharmaceutical / Biotechnology Value Chain From R&D to Marketing Team: Experience (upstream and downstream integration) Molecular Target Identification (Academics) Marketing & Sales Infrastructure API / CMC R&D (Preclinical / Clinical) Athenex: Major Focus Global Supply Chain High Potency Drug Manufacturing Value Creation Focus: Oncology Synergy Understand market needs and the trend (Combo Rx, I-O therapy, metronomic dosing) Athenex Oncology: platform to launch Athenex products Initial focus: launch oral paclitaxel and encequidar in U.S. Goal: establish oral paclitaxel and encequidar as the chemotherapy of choice for mBC patients Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Note: Encequidar is the international nonproprietary name (INN) for HM30181A
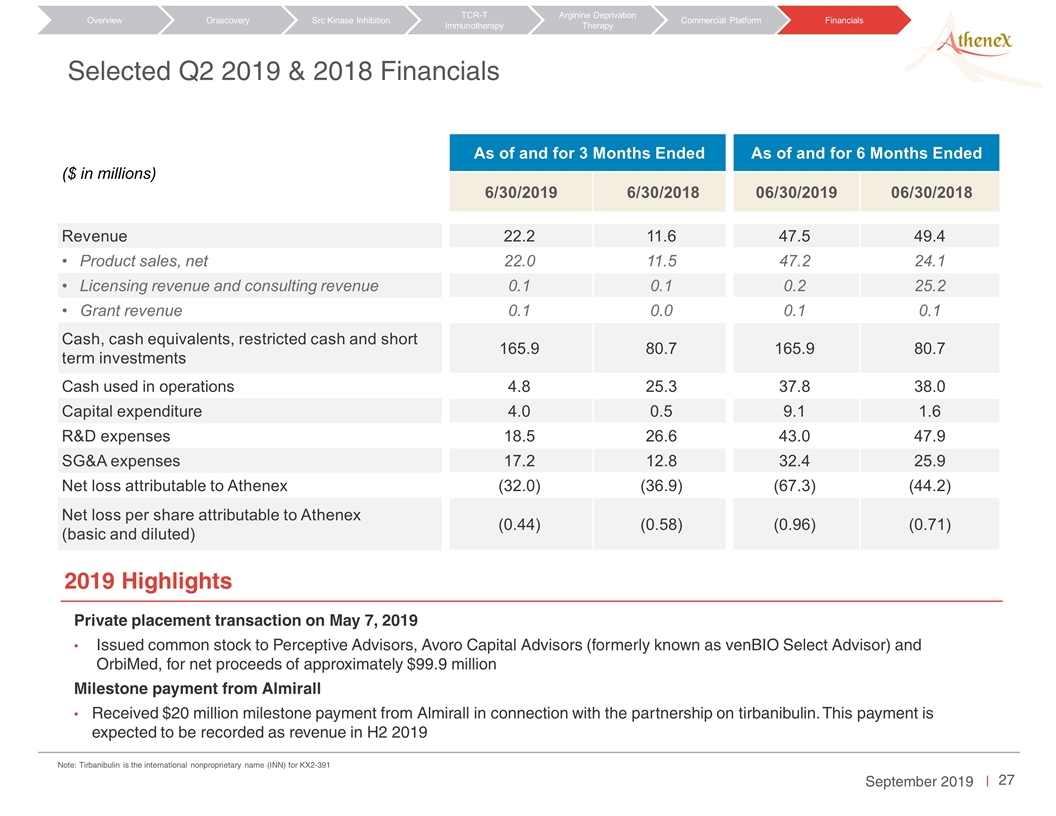
Selected Q2 2019 & 2018 Financials ($ in millions) As of and for 3 Months Ended As of and for 6 Months Ended 6/30/2019 6/30/2018 06/30/2019 06/30/2018 Revenue 22.2 11.6 47.5 49.4 Product sales, net 22.0 11.5 47.2 24.1 Licensing revenue and consulting revenue 0.1 0.1 0.2 25.2 Grant revenue 0.1 0.0 0.1 0.1 Cash, cash equivalents, restricted cash and short term investments 165.9 80.7 165.9 80.7 Cash used in operations 4.8 25.3 37.8 38.0 Capital expenditure 4.0 0.5 9.1 1.6 R&D expenses 18.5 26.6 43.0 47.9 SG&A expenses 17.2 12.8 32.4 25.9 Net loss attributable to Athenex (32.0) (36.9) (67.3) (44.2) Net loss per share attributable to Athenex (basic and diluted) (0.44) (0.58) (0.96) (0.71) Private placement transaction on May 7, 2019 Issued common stock to Perceptive Advisors, Avoro Capital Advisors (formerly known as venBIO Select Advisor) and OrbiMed, for net proceeds of approximately $99.9 million Milestone payment from Almirall Received $20 million milestone payment from Almirall in connection with the partnership on tirbanibulin. This payment is expected to be recorded as revenue in H2 2019 2019 Highlights Orascovery Src Kinase Inhibition TCR-T Immunotherapy Arginine Deprivation Therapy Overview Commercial Platform Financials Note: Tirbanibulin is the international nonproprietary name (INN) for KX2-391
Management Team with Deep Pharma & Biotech Experience Led the Development of Many Global Drugs Prior Experience Selected Drugs Developed Johnson Lau MBBS, MD, FRCP Chief Executive Officer, Chairman 25+ years experience Jeff Yordon Chief Operating Officer 45+ years experience Rudolf Kwan MBBS, MRCP Chief Medical Officer 30+ years experience Simon Pedder PhD Chief Business & Strategy Officer 25+ years experience Randoll Sze Chief Financial Officer 10+ years experience Timothy Cook Senior Vice President, Global Oncology 25+ years experience Daniel Lang MD President, Axis Therapeutics Limited 25+ years experience Wing Kai Chan MBBS, MD, FRACP Head of Clinical Operations, APAC 35+ years experience William Zuo PhD President, China 20+ years experience

Q&A




























