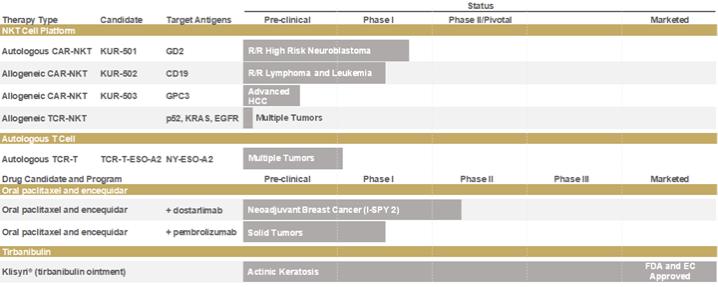
Overview of Safety Observations in Oral Paclitaxel Studies
Studies to date have indicated that Oral Paclitaxel does not result in hypersensitivity reactions when given without premedication for hypersensitivity type reactions, in contrast to the premedication requirement for IV paclitaxel, namely steroids and antihistamines. No new toxicity, apart from those typically observed with paclitaxel, was observed. Infusion related reactions, including hypersensitivity type reactions, have not been observed in patients administered Oral Paclitaxel. Additionally, severe toxicities associated with IV administration of paclitaxel, including neuropathy and alopecia, are at a lower incidence and severity for Oral Paclitaxel.
As of December 31, 2021, approximately 16% of patients treated in clinical studies with Oral Paclitaxel experienced SAEs that were considered to be related to the study treatment. The most common were neutropenia in approximately 3% of patients and febrile neutropenia in approximately 4.7% of patients; pneumonia in approximately 2.7% of patients; septic shock in approximately 1.7% of patients; sepsis in 1.5% of patients; dehydration in approximately 1.2% of patients; gastroenteritis, anemia, diarrhea, mucositis, gastrointestinal bleeding, vomiting, nausea and hypokalaemia, pneumonitis, colitis, tachycardia and atrial fibrillation, each in less than one percent of patients. Other serious treatment-related infections were reported in approximately 1.7% of patients. Approximately 3.3% of patients had other treatment-related SAEs (one patient each including rectal bleeding, altered state of consciousness, cardiac arrest, cardiogenic shock, hypotension, pancytopenia, multiorgan failure, renal failure, pleural effusion, supraventricular tachycardia, respiratory failure, malnutrition, dyspnoea, cardiac failure, upper abdominal pain, fatigue, decreased appetite, pulmonary tuberculosis and peripheral sensory neuropathy).
Oral Irinotecan and Encequidar
Current and Planned Clinical Development
A Phase 1 MTD study has been clinically completed. This study is to determine the MTD of Oral Irinotecan, when given once every three weeks, in subjects with advanced malignancies. We have identified a dosing regimen suitable for Phase 2. However, further development of Oral Irinotecan has been suspended at this time.
Overview of Safety Observations in Oral Irinotecan Studies
In our Oral Irinotecan clinical studies to date, the SAEs observed that were deemed to be at least possibly related to Oral Irinotecan include diarrhea, rash, gastrointestinal hemorrhage, anorexia, vomiting, nausea, enteritis, asthenia, neutropenia, increased alanine aminotransferase, increased aspartate aminotransferase, and C diff infection. As the clinical development program is still in its early stages, we do not yet have meaningful statistics on safety, including adverse events, to report.
Oral Docetaxel and Encequidar
Current and Planned Clinical Studies
A Phase 1 dose escalation U.S. based trial for Oral Docetaxel in patients with various solid tumors given once every three weeks is ongoing. Another Phase 1 study to identify the absolute bioavailability of Oral Docetaxel in prostate cancer patients has been completed. Based on Phase 1 results so far, we believe that we can achieve similar exposure to IV docetaxel with one to three days of dosing every three weeks. Alternative schedules such as weekly or 2 of 3 weeks are also being explored. Further development of Oral Docetaxel has been suspended at this time.
Overview of Safety Observations in Oral Docetaxel Studies
We expect that the overall safety profile of Oral Docetaxel will be similar to that of IV docetaxel, with differences related to the route of administration. As with Oral Paclitaxel, premedication has not been required and hypersensitivity type reactions have not been observed. As of December 31, 2021, in our Oral Docetaxel clinical studies, the SAEs observed that were deemed to at least possibly related to Oral Docetaxel include gastrointestinal toxicity; vomiting, nausea and diarrhea in 1 subject.
Oral Topotecan and Encequidar
A Phase 1 clinical trial in advanced malignancies for Oral Topotecan has completed enrollment. Further development of Oral Topotecan has been suspended at this time. As the clinical development program has enrolled few patients, we do not have meaningful statistics on safety, including adverse events, to report.
11