
Corporate Presentation Nasdaq: AKAO JP Morgan Healthcare Conference Blake Wise CEO January 11, 2018

Forward Looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding future operational and financial results and positions, business strategy, prospective products, potential market, commercial opportunity and market share, availability and potential sources of funding, clinical trial results, product approvals and regulatory pathways, research and development costs, timing (including but limited to timing of clinical development and approval), strategies for completion and likelihood of success for our business activities, our regulatory timelines, and plans for future operations, are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Such risks and uncertainties include, among others, those inherent in the preclinical and clinical development process; the uncertainties of having an NDA accepted by the FDA, the risks and uncertainties of the regulatory approval process; commercialization and gaining market acceptance; conditions when bacteria will evolve resistance to plazomicin, C-Scape or other antibiotics; third party claims alleging infringement of patents and proprietary rights or seeking to invalidate Achaogen's patents or proprietary rights; and the risk that Achaogen's proprietary rights may be insufficient to protect its product candidates. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 filed with the Securities and Exchange Commission on March 14, 2017 and our Form 10-Q for the quarter ended September 30, 2017, filed with the Securities and Exchange Commission and our future periodic Form 10-Ks or Form 10-Qs. Except as required by applicable law, we assume no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. This presentation concerns plazomicin, C-Scape and other product candidates, none of which have been approved for commercialization. They are currently limited to investigational use, and (except as specifically described herein) no representation is made to their safety or effectiveness for the purposes for which they are being investigated. © Achaogen, Inc. All rights reserved.
Achaogen: Innovative Products for Serious Infections Large and growing patient populations Strong physician interest Value-based pricing potential Significant Commercial Opportunity Committed to addressing the urgent threat posed by multi-drug resistant (MDR) bacterial infections Pipeline of innovative product candidates Proven R&D and Commercial teams Unique Antibacterial Company Unique and differentiated therapy for CRE infections Phase 3 results positive: reduced mortality in CRE Breakthrough Therapy designation and priority review; FDA action date of June 25, 2018 Best-in-Class NDA Candidate: Plazomicin Much needed oral therapy for ESBL+ cUTI infections Combination of previously approved compounds (ceftibuten + clavulanate); streamlined development path Phase 1 results positive; plan to initiate Phase 3 in 2018 Innovative Phase 3 Candidate: C-Scape

Multi-Drug Resistance Poses an Urgent and Growing Threat Resistance rising rapidly2 High morbidity and mortality (up to 50% in BSI due to CRE)1,3 Limited treatment options4 Significant economic burden to healthcare system & society5 © Achaogen, Inc. All rights reserved. CDC Urgent1 CDC Serious1 Priority Pathogens 1 CDC Antibiotic Resistance Threats in the United States, 2013 http://www.cdc.gov/drugresistance/threat-report-2013/ 2 The Surveillance Network database: CDDEP.org Resistance Map; Braykov et. al., 2013 3 Ben-David et al. Clin Microbiol Infect 2012;18(1):54–60. 4 Daikos et al., Clin Microbiol Rev. 2012 Oct;25(4):682-707. 5 Bartsch et al., Clin Microbiol Infect. 2016 Sep 15. pii: S1198-743X(16)30389-5. WHO published Feb. 27, 2017
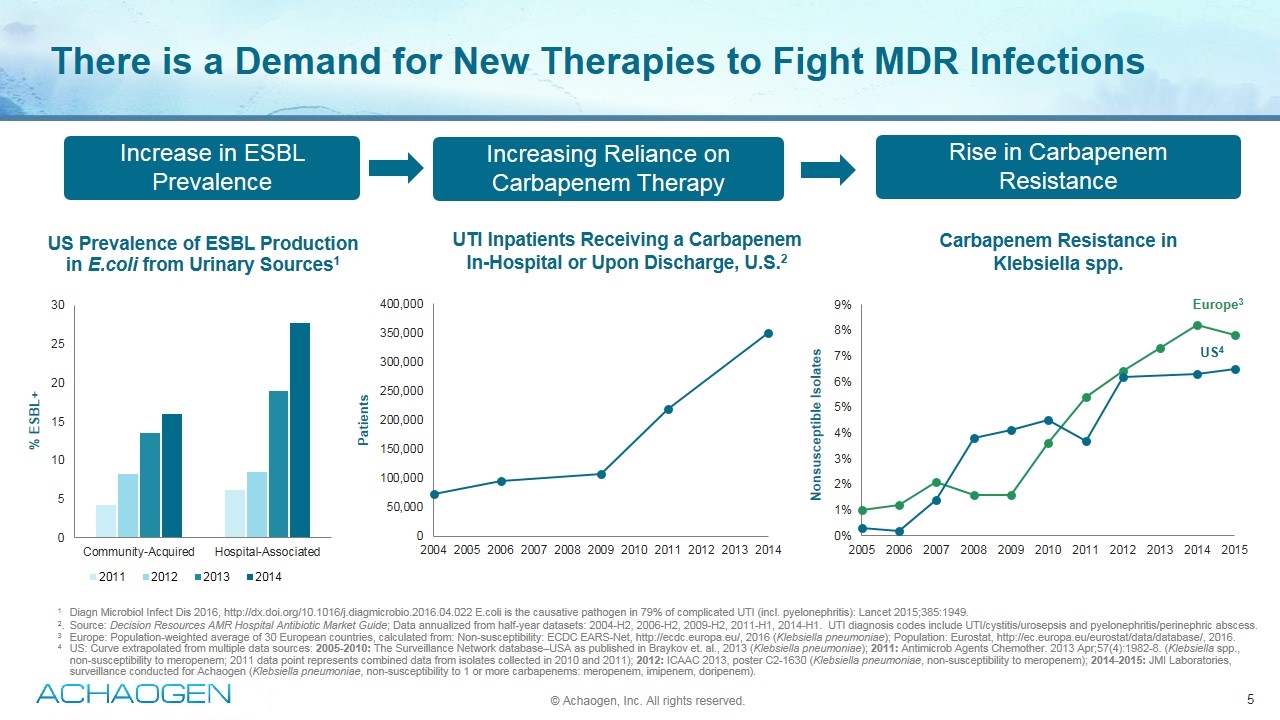
There is a Demand for New Therapies to Fight MDR Infections © Achaogen, Inc. All rights reserved. 1Diagn Microbiol Infect Dis 2016, http://dx.doi.org/10.1016/j.diagmicrobio.2016.04.022 E.coli is the causative pathogen in 79% of complicated UTI (incl. pyelonephritis): Lancet 2015;385:1949. 2.Source: Decision Resources AMR Hospital Antibiotic Market Guide; Data annualized from half-year datasets: 2004-H2, 2006-H2, 2009-H2, 2011-H1, 2014-H1. UTI diagnosis codes include UTI/cystitis/urosepsis and pyelonephritis/perinephric abscess. 3 Europe: Population-weighted average of 30 European countries, calculated from: Non-susceptibility: ECDC EARS-Net, http://ecdc.europa.eu/, 2016 (Klebsiella pneumoniae); Population: Eurostat, http://ec.europa.eu/eurostat/data/database/, 2016. 4 US: Curve extrapolated from multiple data sources: 2005-2010: The Surveillance Network database–USA as published in Braykov et. al., 2013 (Klebsiella pneumoniae); 2011: Antimicrob Agents Chemother. 2013 Apr;57(4):1982-8. (Klebsiella spp., non-susceptibility to meropenem; 2011 data point represents combined data from isolates collected in 2010 and 2011); 2012: ICAAC 2013, poster C2-1630 (Klebsiella pneumoniae, non-susceptibility to meropenem); 2014-2015: JMI Laboratories, surveillance conducted for Achaogen (Klebsiella pneumoniae, non-susceptibility to 1 or more carbapenems: meropenem, imipenem, doripenem). Carbapenem Resistance in Klebsiella spp. Europe3 US4 UTI Inpatients Receiving a Carbapenem In-Hospital or Upon Discharge, U.S.2 Increase in ESBL Prevalence Increasing Reliance on Carbapenem Therapy Rise in Carbapenem Resistance
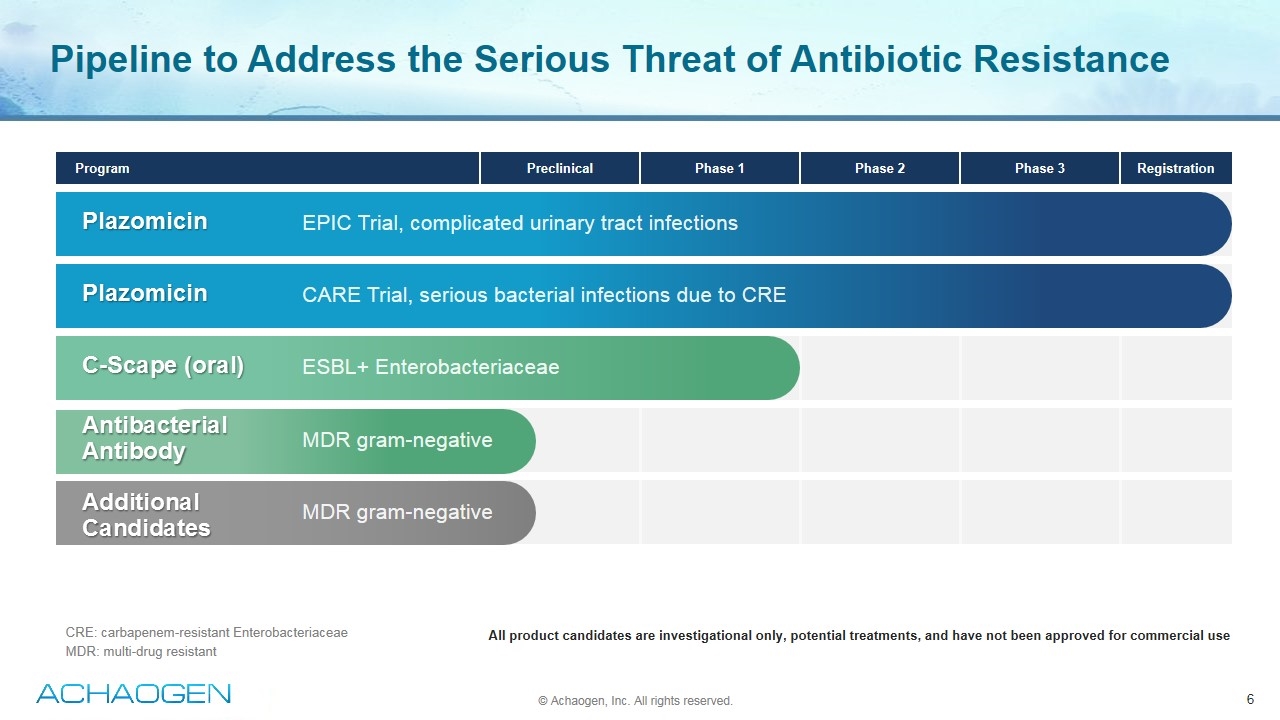
CRE: carbapenem-resistant Enterobacteriaceae MDR: multi-drug resistant Pipeline to Address the Serious Threat of Antibiotic Resistance Program Preclinical Phase 1 Phase 2 Phase 3 Registration All product candidates are investigational only, potential treatments, and have not been approved for commercial use Plazomicin Plazomicin C-Scape (oral) Antibacterial Antibody Additional Candidates EPIC Trial, complicated urinary tract infections CARE Trial, serious bacterial infections due to CRE ESBL+ Enterobacteriaceae MDR gram-negative MDR gram-negative © Achaogen, Inc. All rights reserved.
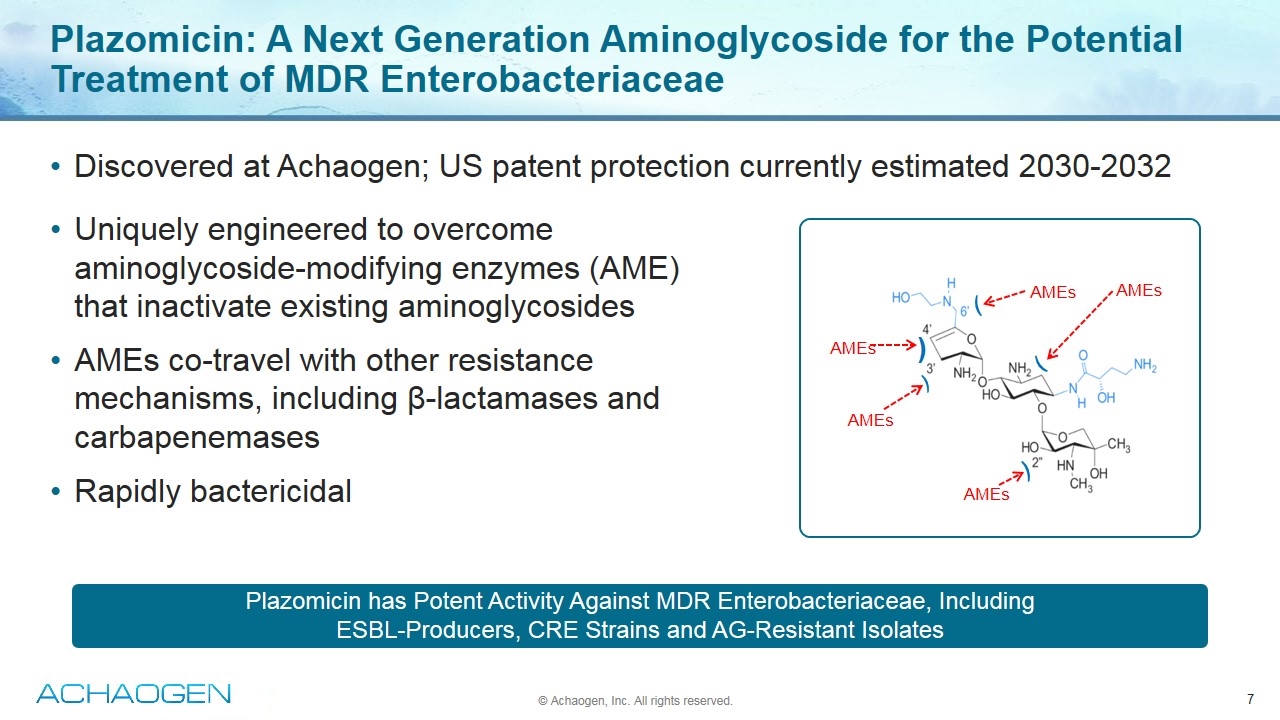
Plazomicin has Potent Activity Against MDR Enterobacteriaceae, Including ESBL-Producers, CRE Strains and AG-Resistant Isolates Plazomicin: A Next Generation Aminoglycoside for the Potential Treatment of MDR Enterobacteriaceae Discovered at Achaogen; US patent protection currently estimated 2030-2032 AMEs AMEs AMEs AMEs AMEs ) Uniquely engineered to overcome aminoglycoside-modifying enzymes (AME) that inactivate existing aminoglycosides AMEs co-travel with other resistance mechanisms, including β-lactamases and carbapenemases Rapidly bactericidal © Achaogen, Inc. All rights reserved.
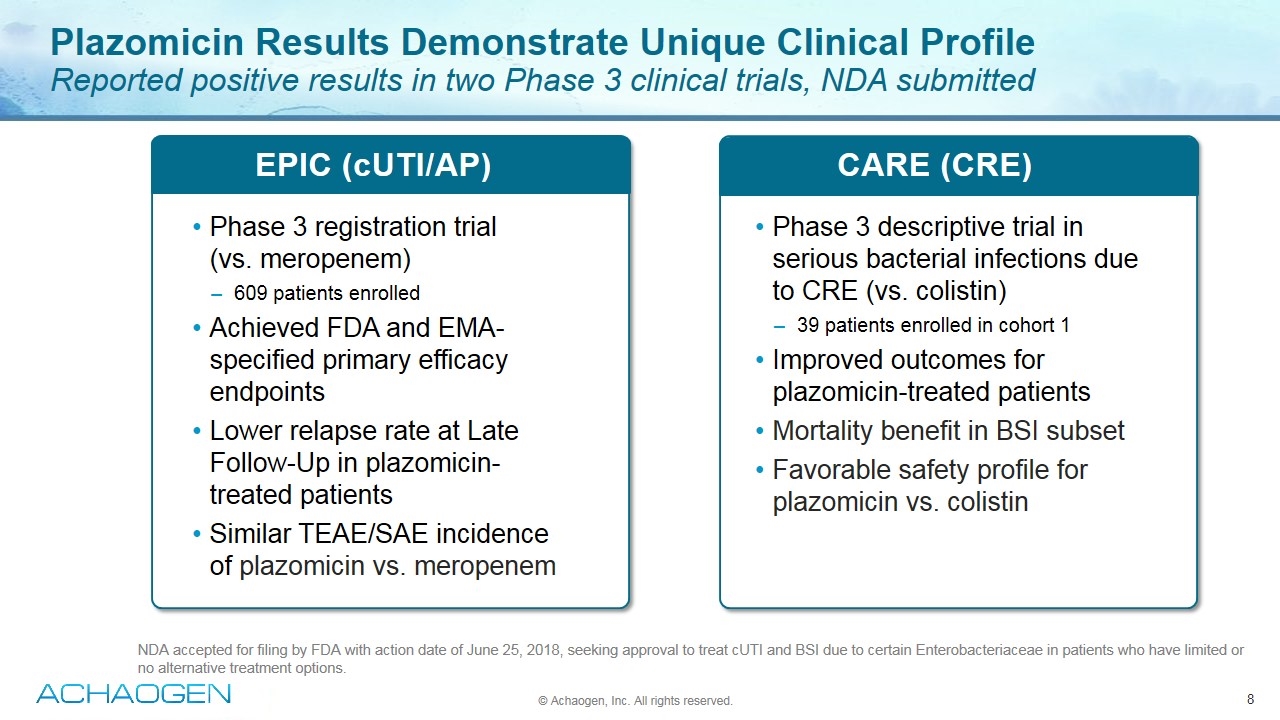
Plazomicin Results Demonstrate Unique Clinical Profile Reported positive results in two Phase 3 clinical trials, NDA submitted Phase 3 registration trial (vs. meropenem) 609 patients enrolled Achieved FDA and EMA-specified primary efficacy endpoints Lower relapse rate at Late Follow-Up in plazomicin-treated patients Similar TEAE/SAE incidence of plazomicin vs. meropenem EPIC (cUTI/AP) CARE (CRE) © Achaogen, Inc. All rights reserved. Phase 3 descriptive trial in serious bacterial infections due to CRE (vs. colistin) 39 patients enrolled in cohort 1 Improved outcomes for plazomicin-treated patients Mortality benefit in BSI subset Favorable safety profile for plazomicin vs. colistin NDA accepted for filing by FDA with action date of June 25, 2018, seeking approval to treat cUTI and BSI due to certain Enterobacteriaceae in patients who have limited or no alternative treatment options.
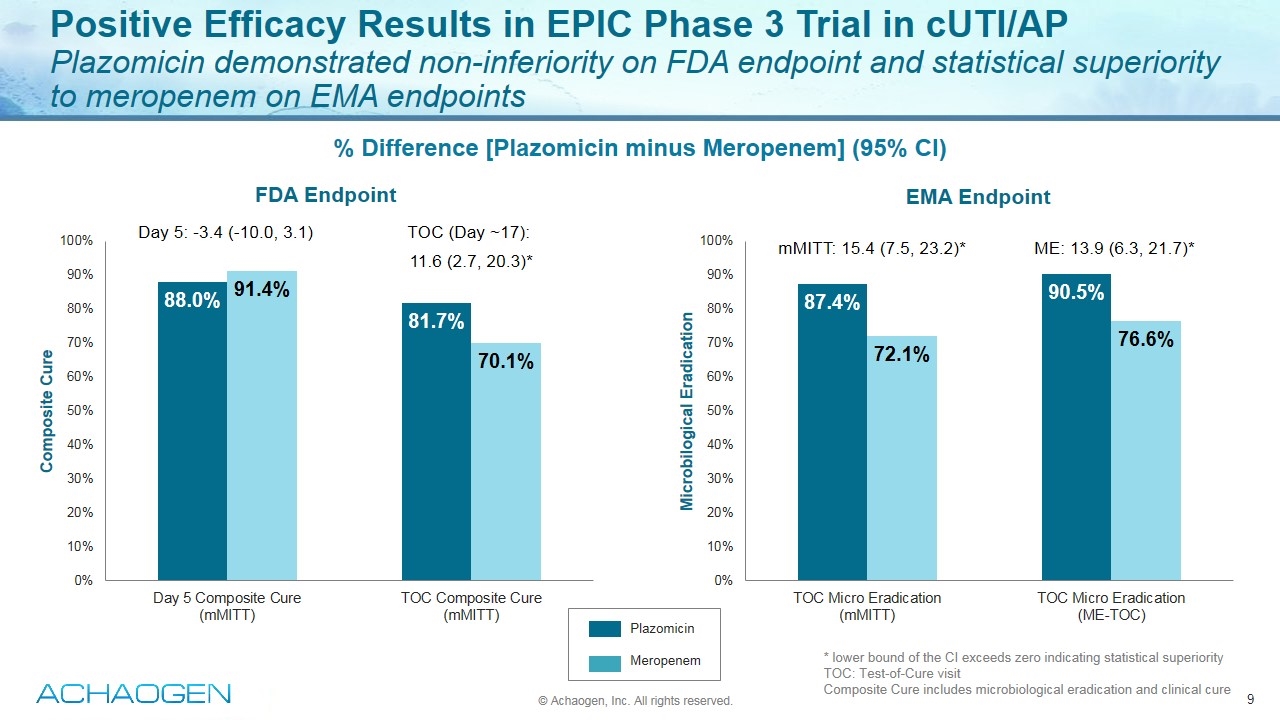
Positive Efficacy Results in EPIC Phase 3 Trial in cUTI/AP Plazomicin demonstrated non-inferiority on FDA endpoint and statistical superiority to meropenem on EMA endpoints * lower bound of the CI exceeds zero indicating statistical superiority TOC: Test-of-Cure visit Composite Cure includes microbiological eradication and clinical cure % Difference [Plazomicin minus Meropenem] (95% CI) Day 5: -3.4 (-10.0, 3.1) TOC (Day ~17): 11.6 (2.7, 20.3)* mMITT: 15.4 (7.5, 23.2)* ME: 13.9 (6.3, 21.7)* Plazomicin Meropenem FDA Endpoint EMA Endpoint © Achaogen, Inc. All rights reserved.
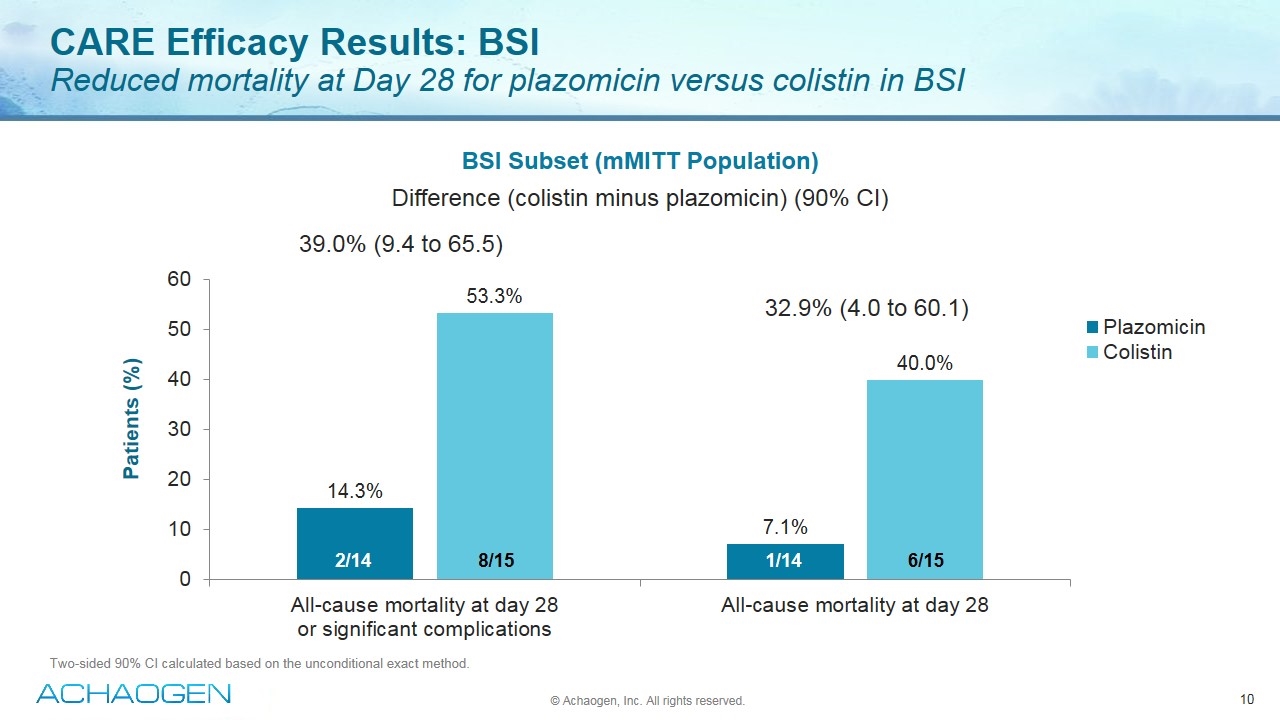
CARE Efficacy Results: BSI Reduced mortality at Day 28 for plazomicin versus colistin in BSI Two-sided 90% CI calculated based on the unconditional exact method. Plazomicin Colistin 2/14 8/15 1/14 6/15 39.0% (9.4 to 65.5) 32.9% (4.0 to 60.1) BSI Subset (mMITT Population) Difference (colistin minus plazomicin) (90% CI) © Achaogen, Inc. All rights reserved. Patients (%)
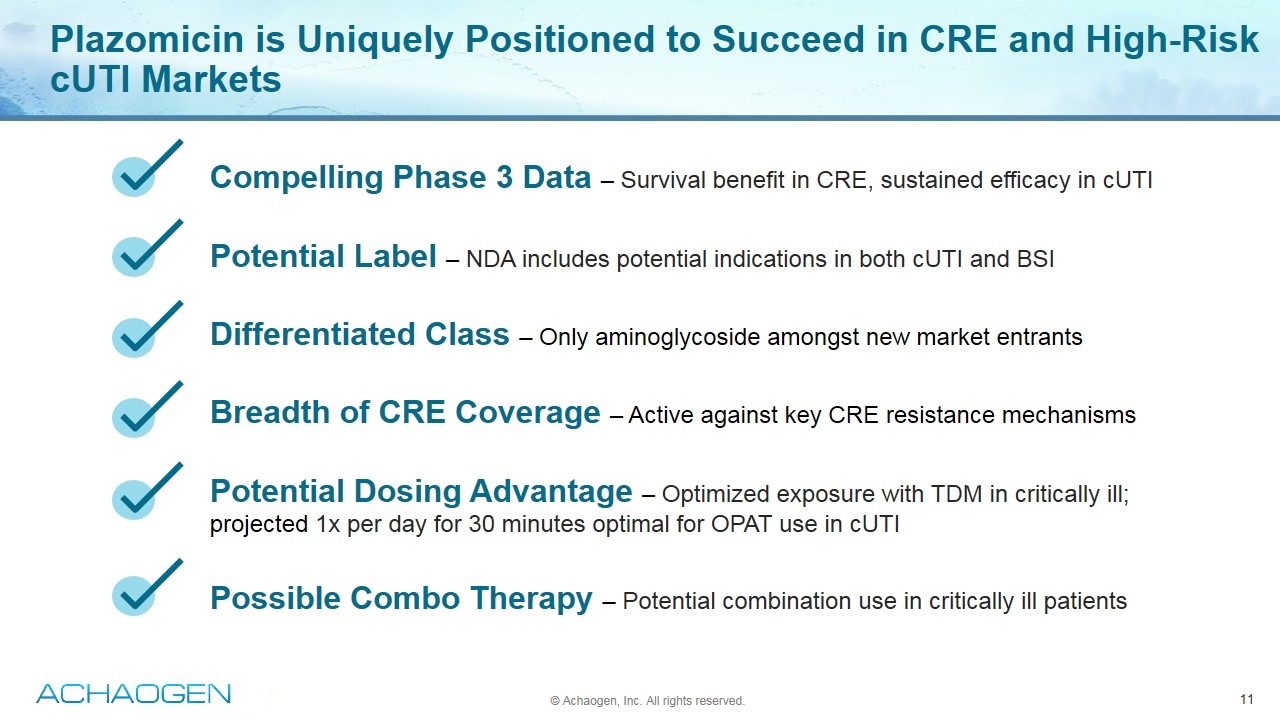
Plazomicin is Uniquely Positioned to Succeed in CRE and High-Risk cUTI Markets Compelling Phase 3 Data – Survival benefit in CRE, sustained efficacy in cUTI Potential Label – NDA includes potential indications in both cUTI and BSI Differentiated Class – Only aminoglycoside amongst new market entrants Breadth of CRE Coverage – Active against key CRE resistance mechanisms Potential Dosing Advantage – Optimized exposure with TDM in critically ill; projected 1x per day for 30 minutes optimal for OPAT use in cUTI Possible Combo Therapy – Potential combination use in critically ill patients © Achaogen, Inc. All rights reserved.
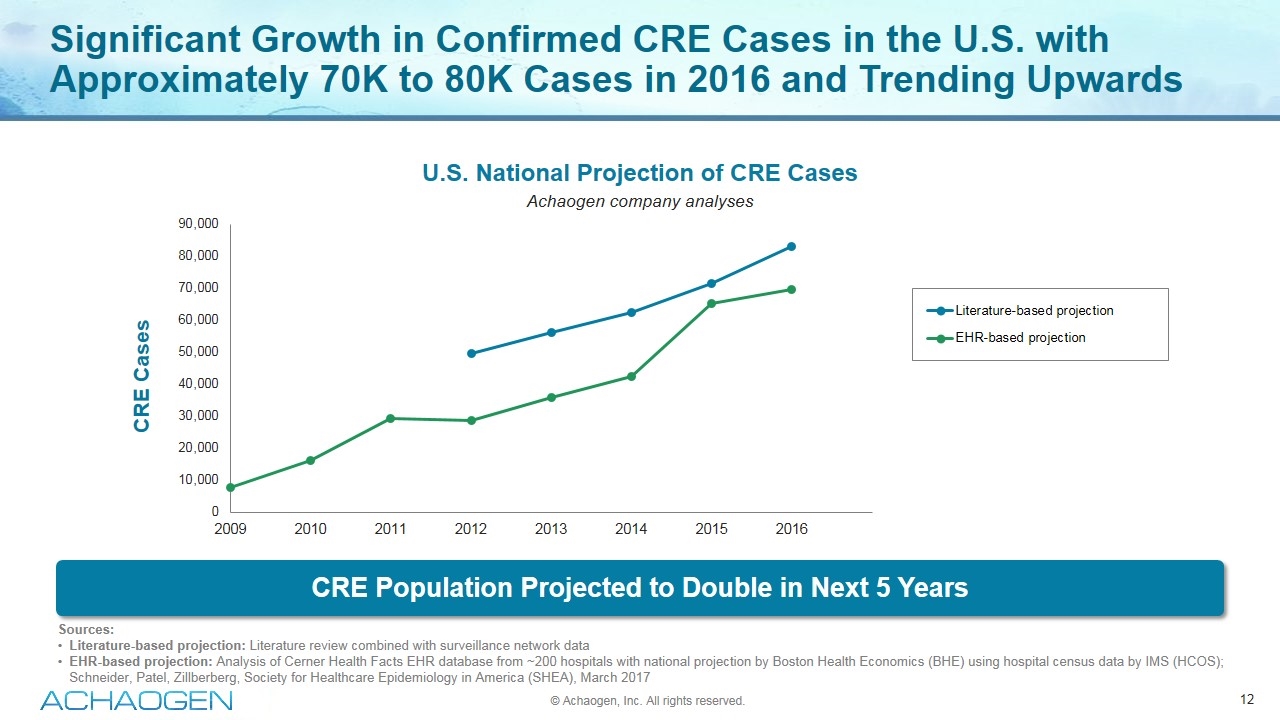
Significant Growth in Confirmed CRE Cases in the U.S. with Approximately 70K to 80K Cases in 2016 and Trending Upwards Sources: Literature-based projection: Literature review combined with surveillance network data EHR-based projection: Analysis of Cerner Health Facts EHR database from ~200 hospitals with national projection by Boston Health Economics (BHE) using hospital census data by IMS (HCOS); Schneider, Patel, Zillberberg, Society for Healthcare Epidemiology in America (SHEA), March 2017 CRE Population Projected to Double in Next 5 Years U.S. National Projection of CRE Cases Achaogen company analyses © Achaogen, Inc. All rights reserved.
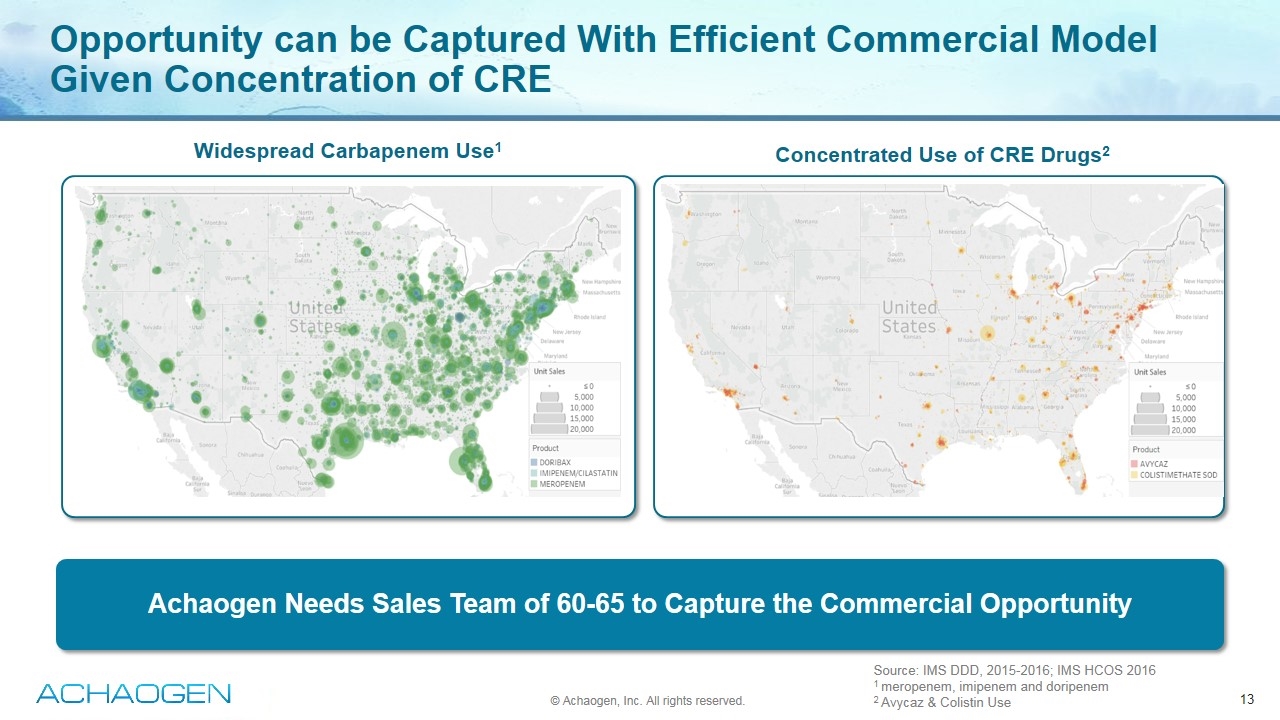
Opportunity can be Captured With Efficient Commercial Model Given Concentration of CRE Source: IMS DDD, 2015-2016; IMS HCOS 2016 1 meropenem, imipenem and doripenem 2 Avycaz & Colistin Use Widespread Carbapenem Use1 Concentrated Use of CRE Drugs2 © Achaogen, Inc. All rights reserved. Achaogen Needs Sales Team of 60-65 to Capture the Commercial Opportunity
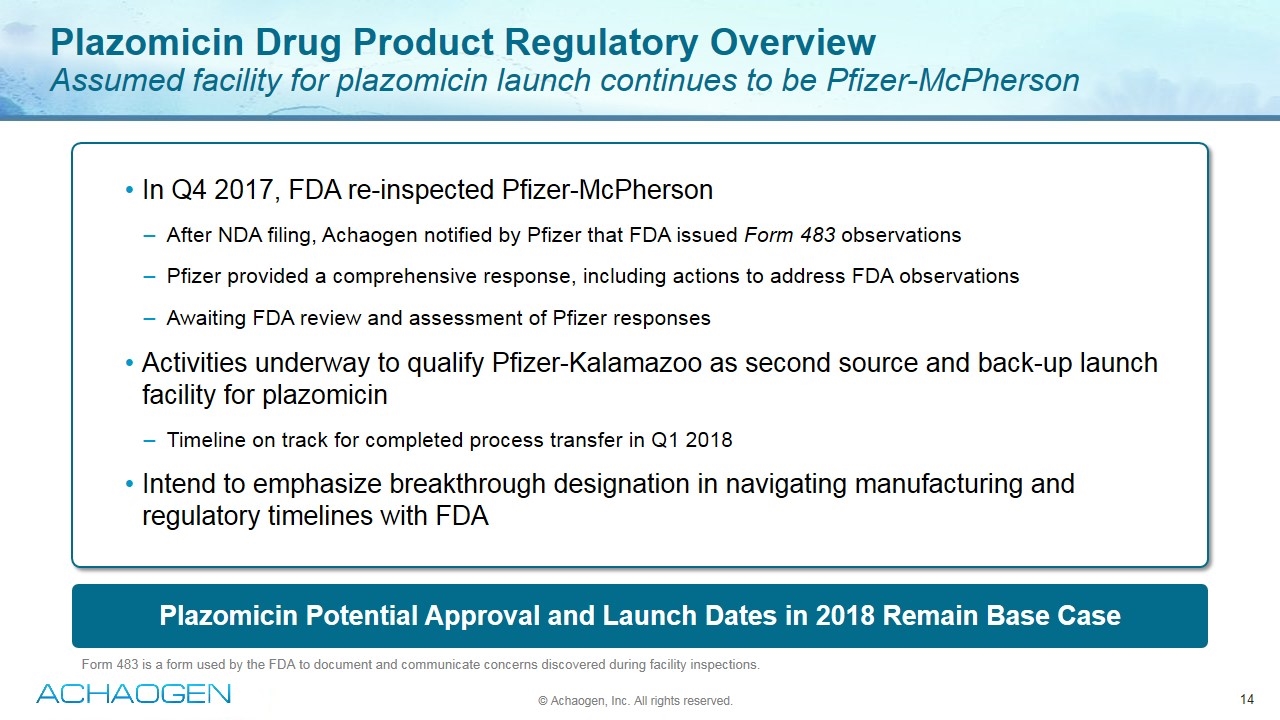
Plazomicin Potential Approval and Launch Dates in 2018 Remain Base Case Plazomicin Drug Product Regulatory Overview Assumed facility for plazomicin launch continues to be Pfizer-McPherson © Achaogen, Inc. All rights reserved. Form 483 is a form used by the FDA to document and communicate concerns discovered during facility inspections. In Q4 2017, FDA re-inspected Pfizer-McPherson After NDA filing, Achaogen notified by Pfizer that FDA issued Form 483 observations Pfizer provided a comprehensive response, including actions to address FDA observations Awaiting FDA review and assessment of Pfizer responses Activities underway to qualify Pfizer-Kalamazoo as second source and back-up launch facility for plazomicin Timeline on track for completed process transfer in Q1 2018 Intend to emphasize breakthrough designation in navigating manufacturing and regulatory timelines with FDA

C-Scape: Oral Antibiotic Candidate for cUTI © Achaogen, Inc. All rights reserved.
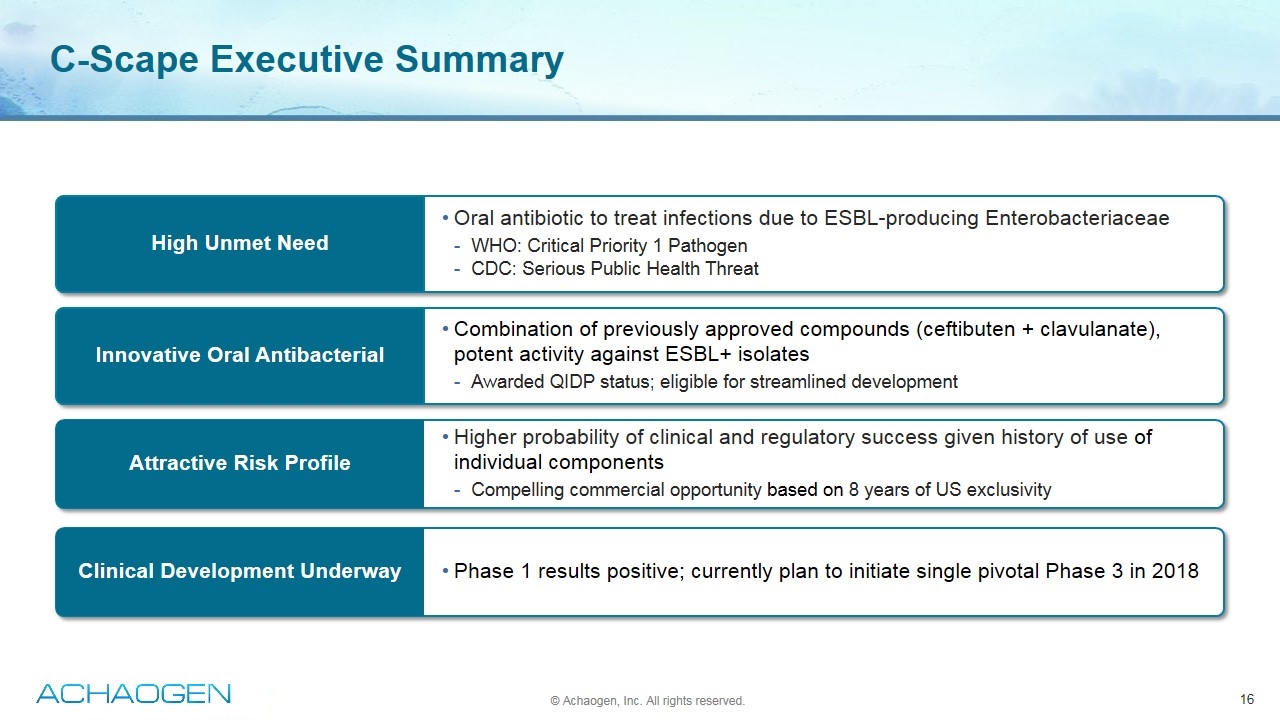
C-Scape Executive Summary Oral antibiotic to treat infections due to ESBL-producing Enterobacteriaceae WHO: Critical Priority 1 Pathogen CDC: Serious Public Health Threat Higher probability of clinical and regulatory success given history of use of individual components Compelling commercial opportunity based on 8 years of US exclusivity Phase 1 results positive; currently plan to initiate single pivotal Phase 3 in 2018 Combination of previously approved compounds (ceftibuten + clavulanate), potent activity against ESBL+ isolates Awarded QIDP status; eligible for streamlined development High Unmet Need Innovative Oral Antibacterial Attractive Risk Profile Clinical Development Underway © Achaogen, Inc. All rights reserved.
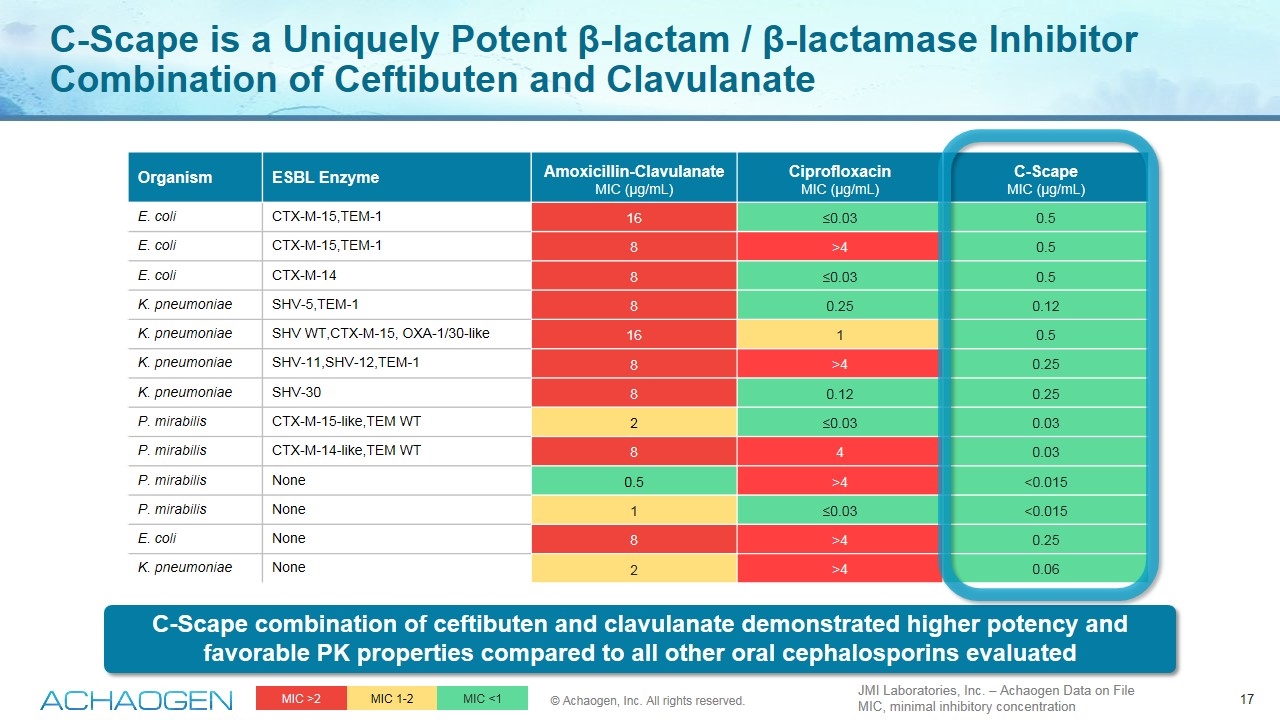
Organism ESBL Enzyme Amoxicillin-Clavulanate MIC (µg/mL) Ciprofloxacin MIC (µg/mL) C-Scape MIC (µg/mL) E. coli CTX-M-15,TEM-1 16 ≤0.03 0.5 E. coli CTX-M-15,TEM-1 8 >4 0.5 E. coli CTX-M-14 8 ≤0.03 0.5 K. pneumoniae SHV-5,TEM-1 8 0.25 0.12 K. pneumoniae SHV WT,CTX-M-15, OXA-1/30-like 16 1 0.5 K. pneumoniae SHV-11,SHV-12,TEM-1 8 >4 0.25 K. pneumoniae SHV-30 8 0.12 0.25 P. mirabilis CTX-M-15-like,TEM WT 2 ≤0.03 0.03 P. mirabilis CTX-M-14-like,TEM WT 8 4 0.03 P. mirabilis None 0.5 >4 <0.015 P. mirabilis None 1 ≤0.03 <0.015 E. coli None 8 >4 0.25 K. pneumoniae None 2 >4 0.06 MIC >2 MIC 1-2 MIC <1 JMI Laboratories, Inc. – Achaogen Data on File MIC, minimal inhibitory concentration C-Scape is a Uniquely Potent β-lactam / β-lactamase Inhibitor Combination of Ceftibuten and Clavulanate © Achaogen, Inc. All rights reserved. C-Scape combination of ceftibuten and clavulanate demonstrated higher potency and favorable PK properties compared to all other oral cephalosporins evaluated
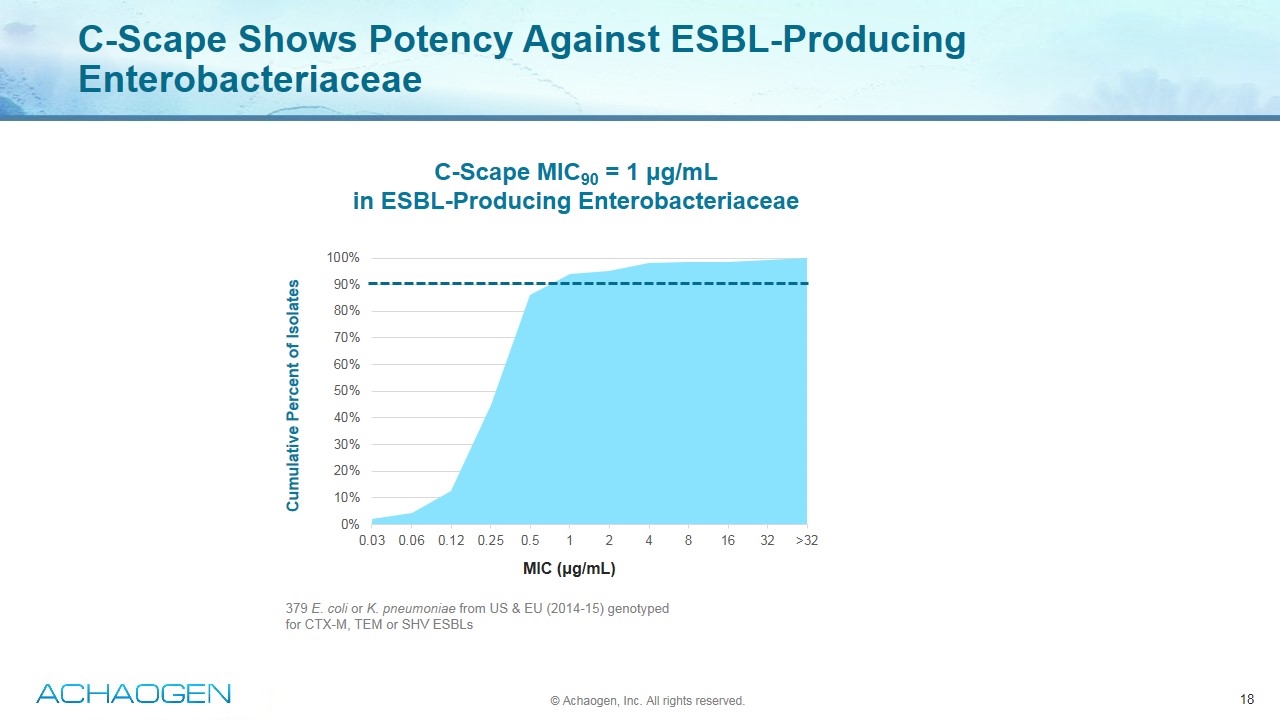
C-Scape Shows Potency Against ESBL-Producing Enterobacteriaceae Cumulative Percent of Isolates MIC (μg/mL) C-Scape MIC90 = 1 μg/mL in ESBL-Producing Enterobacteriaceae 379 E. coli or K. pneumoniae from US & EU (2014-15) genotyped for CTX-M, TEM or SHV ESBLs © Achaogen, Inc. All rights reserved.
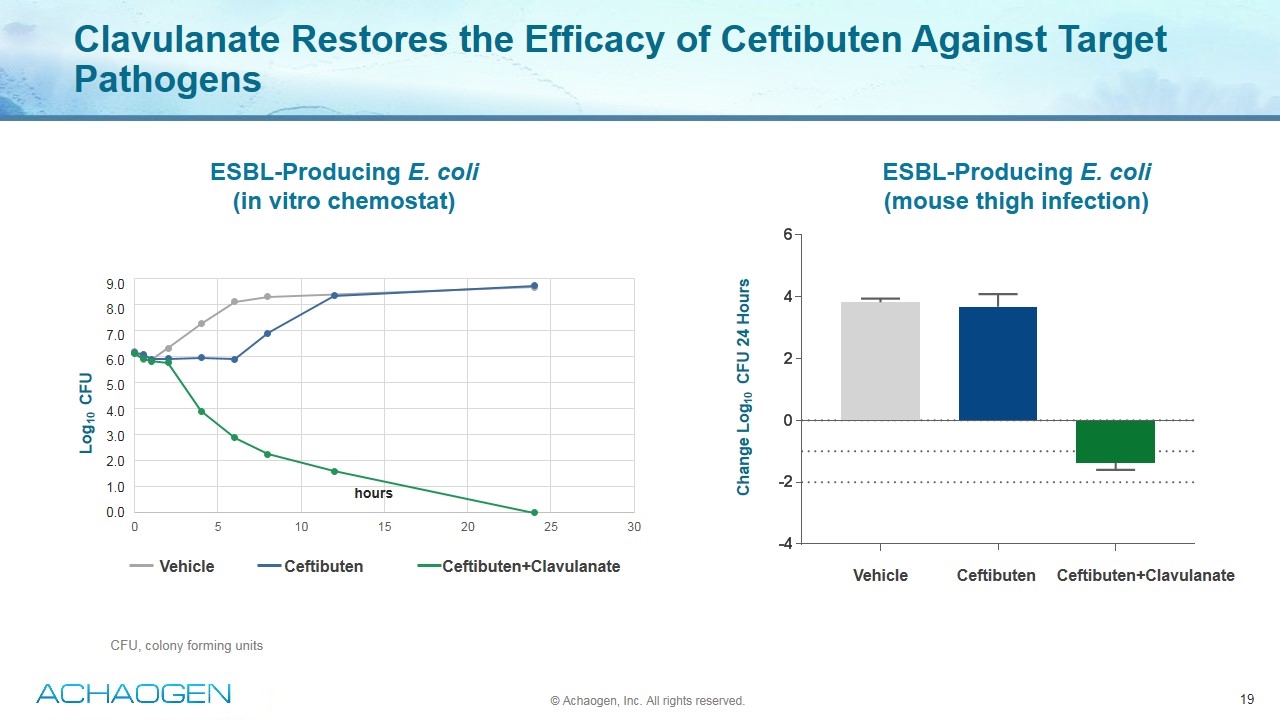
Clavulanate Restores the Efficacy of Ceftibuten Against Target Pathogens © Achaogen, Inc. All rights reserved. hours 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 Vehicle Ceftibuten Ceftibuten+Clavulanate Ceftibuten Ceftibuten+Clavulanate Vehicle Log10 CFU Change Log10 CFU 24 Hours ESBL-Producing E. coli (in vitro chemostat) ESBL-Producing E. coli (mouse thigh infection) CFU, colony forming units
C-Scape Phase 1 Top-Line Results The combination of ceftibuten/clavulanate was well tolerated when administered for 14 days across all dosing regimens tested No serious adverse events (SAEs), grade 3 or 4 adverse events, or adverse events leading to discontinuation of study drug were observed Safety profile was consistent with expectations for ceftibuten/clavulanate when administered based on existing product labels The most commonly reported adverse events included vascular access site bruising, headache, diarrhea, gastroenteritis and nausea. The incidence of each of the most common adverse events was comparable between the active treatment groups and placebo. Preliminary pharmacokinetics following administration of ceftibuten/clavulanate in combination were similar to those following administration of each compound alone, indicating no drug-drug interaction between ceftibuten/clavulanic acid © Achaogen, Inc. All rights reserved.
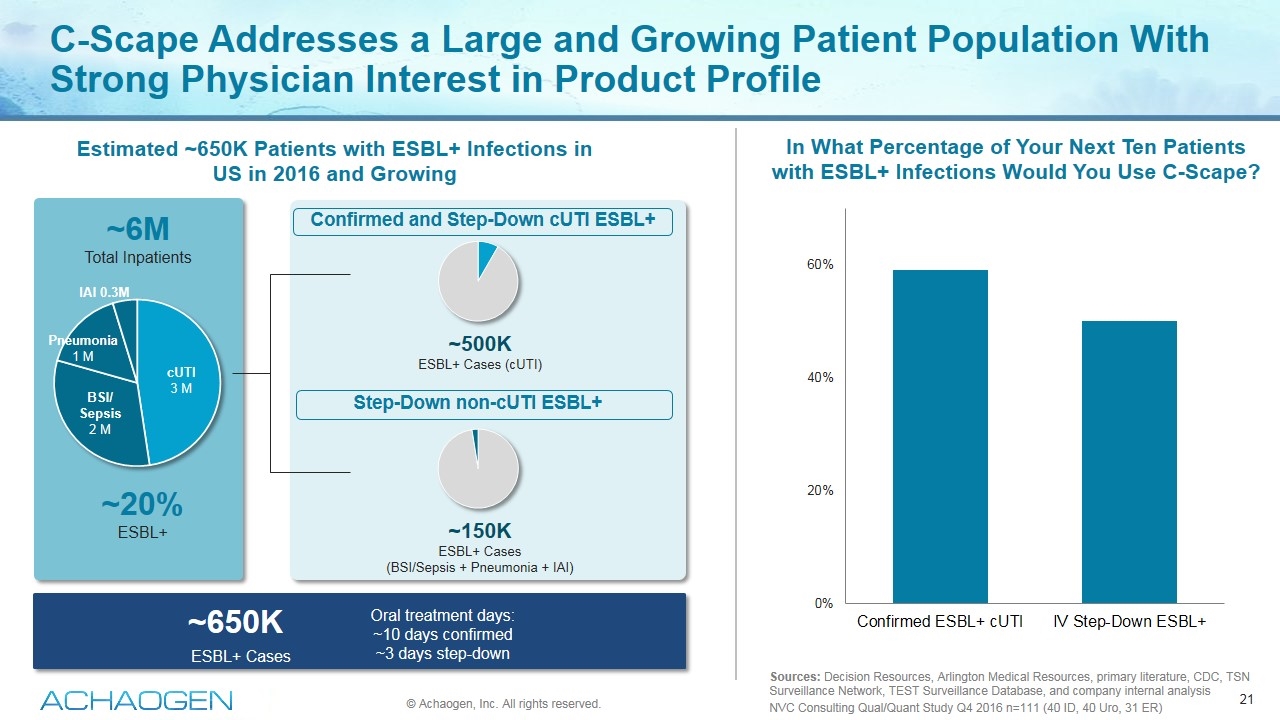
C-Scape Addresses a Large and Growing Patient Population With Strong Physician Interest in Product Profile Sources: Decision Resources, Arlington Medical Resources, primary literature, CDC, TSN Surveillance Network, TEST Surveillance Database, and company internal analysis NVC Consulting Qual/Quant Study Q4 2016 n=111 (40 ID, 40 Uro, 31 ER) ~6M Total Inpatients ~650K ESBL+ Cases Confirmed and Step-Down cUTI ESBL+ Step-Down non-cUTI ESBL+ ~500K ESBL+ Cases (cUTI) ~150K ESBL+ Cases (BSI/Sepsis + Pneumonia + IAI) cUTI 3 M BSI/ Sepsis 2 M Pneumonia 1 M IAI 0.3M Oral treatment days: ~10 days confirmed ~3 days step-down © Achaogen, Inc. All rights reserved. ~20% ESBL+ In What Percentage of Your Next Ten Patients with ESBL+ Infections Would You Use C-Scape? Estimated ~650K Patients with ESBL+ Infections in US in 2016 and Growing

Financial Snapshot $199.4 million in cash, cash equivalents, short-term investments at 9/30/17 Additional restricted cash of $10.7 million associated with Gates Foundation partnership at 9/30/17 Funding partnerships BARDA non-dilutive funding of $124.3 million for plazomicin development BARDA non-dilutive funding of up to $18 million for C-Scape development Gates Foundation grant and equity funding up to $20.5 million for antibody development $25 million loan agreement with Solar Capital Research coverage Chris Shibutani (Cowen); Kevin Kedra (Gabelli); Adnan Butt (Guggenheim); Ed Arce (HC Wainwright); Paul Matteis (Leerink); Difei Yang (Mizuho); Alan Carr (Needham); Stephen D. Willey (Stifel); Edward H. Nash (Suntrust); Robert Driscoll (Wedbush); Y. Katherine Xu (William Blair) © Achaogen, Inc. All rights reserved.
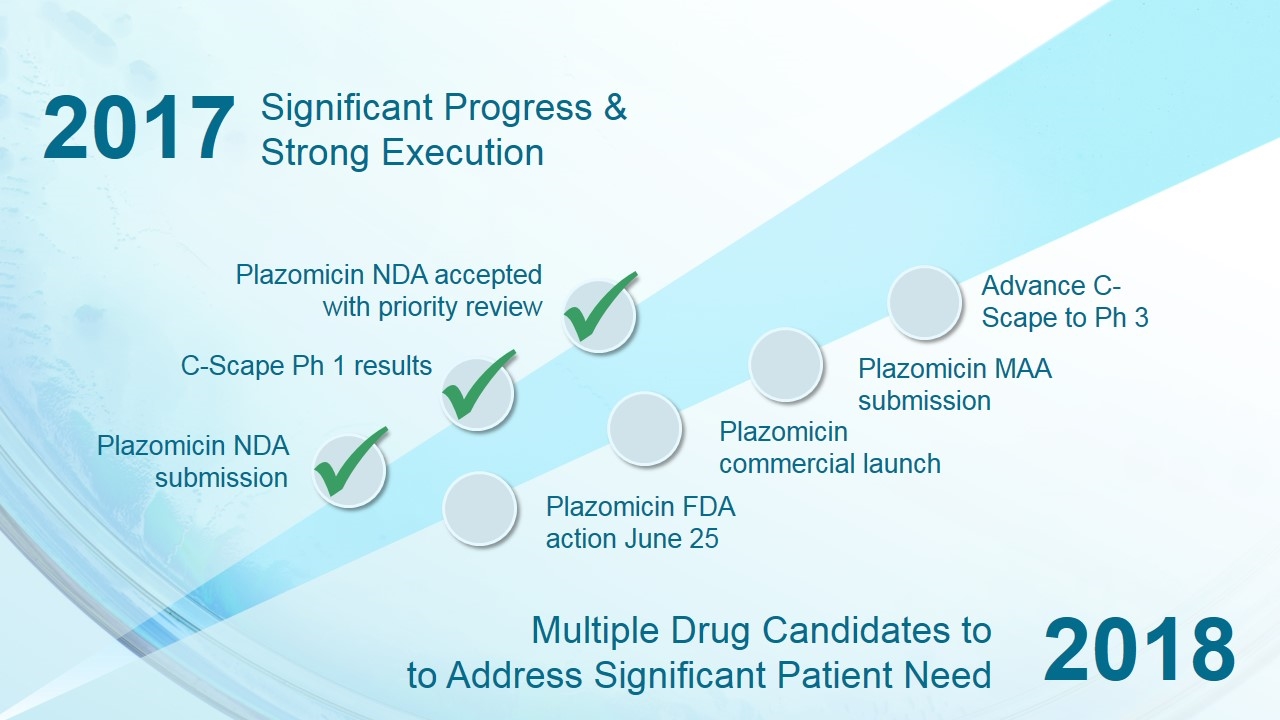
2017 2018 ü Significant Progress & Strong Execution Multiple Drug Candidates to to Address Significant Patient Need ü Plazomicin NDA submission C-Scape Ph 1 results Advance C-Scape to Ph 3 Plazomicin commercial launch Plazomicin FDA action June 25 Plazomicin MAA submission ü Plazomicin NDA accepted with priority review






















