EXHIBIT 99.1
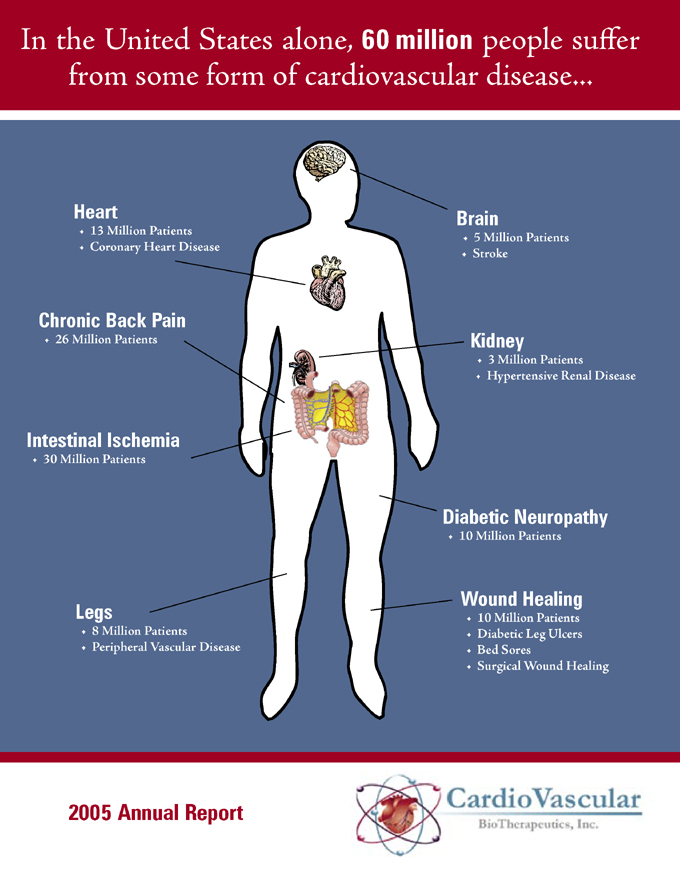
In the United States alone, 60 million people suffer from some form of cardiovascular disease
Heart
13 Million Patients
Coronary Heart Disease
Brain
5 Million Patients
Stroke
Chronic Back Pain
26 Million Patients
Kidney
3 Million Patients
Hypertensive Renal Disease
Intestinal Ischemia
30 Million Patients
Diabetic Neuropathy
10 Million Patients
Legs
8 Million Patients
Peripheral Vascular Disease
Wound Healing
10 Million Patients
Diabetic Leg Ulcers
Bed Sores
Surgical Wound Healing
2005 Annual Report
CardioVascular
BioTherapeutics, Inc..

…and nearly 2,600 Americans die each day from cardiovascular disease.
Cardiovascular disease claims more lives each year than the next five leading causes of death combined (cancer, chronic lower respiratory diseases, accidents, diabetes mellitus, and influenza/pneumonia).
According to the American Heart Association’s 2004 statistics in the United States:
Nearly 2,600 die of cardiovascular disease (CVD) each day
An estimated 700,000 people will develop coronary artery disease and angina every year
20 million people have some form of heart disease
13.2 million suffer from coronary artery disease
7.8 million per year experience heart attacks
4.8 million adults have had strokes
About 500,000 will have recurrent heart attacks
Every 36 seconds, someone will suffer a heart attack or stroke
2 CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com

CardioVascular BioTheraputics, Inc. (CVBT) is a biopharmaceutical company solely focused on introducing new therapies for cardiovascular disease.
CardioVascular BioTheraputics, Inc. is headquartered in Henderson, Nevada with contract R&D/manufacturing facilities and contract administrative facilities adjacent to the University of California, Irvine. CVBT’s drug candidate is Cardio Vascu-GrowTM, a potent protein growth factor which stimulates the growth of new blood vessels, a biological process termed “angiogenesis.” This growth factor is being tested in a wide variety of human diseases that would benefit from increased angiogenesis, including coronary artery disease, stroke, peripheral vascular disease of the legs and wound healing.
FDA clinical studies are being performed in the USA in patients with coronary artery disease with promising results. Additional testing is planned for the near future with the drug candidate in diabetics with wound healing deficiencies and vascular disease in their legs. Animal studies that mimic these human conditions have been performed and indicated that Cardio Vascu-GrowTM is an effective wound healing treatment. The large number of patients that suffer from these cardiovascular diseases make each of these medical indications a potential significant market opportunity for Cardio Vascu-GrowTM.
Cardiovascular disease affects over 60 million people in the United States and CVBT has new therapies for a wide array of these cardiovascular diseases in its research and development portfolio.
View of the University of California at Irvine Research Campus from the CVBT contract R&D/manufacturing facility. (Source- CVBT)
CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 3

As shown on the front page of this report, Cardio Vascu-GrowTM can be applied to multiple tissues in the body that become injured due to a decreased blood flow. This injury, or tissue ischemia, is most often caused by the blockage of arteries supplying blood to the tissue. The company’s current development efforts for Cardio Vascu-GrowTM have been grouped according to the type of tissue that is proposed to be treated and includes:
1. The Muscle Tissue Group
A. Heart Applications
13 Million Patients with Coronary Artery Disease
The research to develop Cardio Vascu-GrowTM started in 1992 in Germany with Dr. Thomas Stegmann’s initial success in growing new blood vessels in animal hearts. This research led to the first human experiments in 1995/96 in 20 patients with no-option heart disease. The results were reported in the American Heart Association’s publication, Circulation, in February 1998. This first successful angiogenesis clinical trial in the human heart led to the founding of Cardio Vascular BioTherapeutics, Inc. to commercialize this medical breakthrough.
In 1998/99 Dr. Stegmann conducted a second clinical trial using the drug as the sole therapy in 20 no-option heart patients. The successful results of this trial were reported in the cardiology journal, Cardiac and Vascular Regeneration in 2000. The cumulative results of the two clinical trials can be summarized as follows:
The potential of Cardio Vascu-GrowTM as a new treatment for coronary artery disease was convincingly demonstrated by Dr. Thomas Stegmann and his associates in two separate clinical trials performed in Germany. A total of 40 patients faced with no viable surgical treatment options received injections of Cardio Vascu-GrowTM into their hearts. The clinical results from these studies, published in internationally-recognized medical journals, indicated that Cardio Vascu-GrowTM was a safe and effective treatment for severe coronary artery disease. The following results were observed:
80% of patients showed a significant improvement in their stress exercise test.
Hearts showed a five-fold increase in the stress SPECT perfusion score, indicating a significant improvement in blood flow to the heart muscle.
No significant adverse safety effects of the therapy.
Importantly, 90% of the patients had an improvement in their dominant clinical symptom, angina or chest pain. During the stress exercise test, angina was either completely absent, or began at much higher levels of exertion.
4 CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com
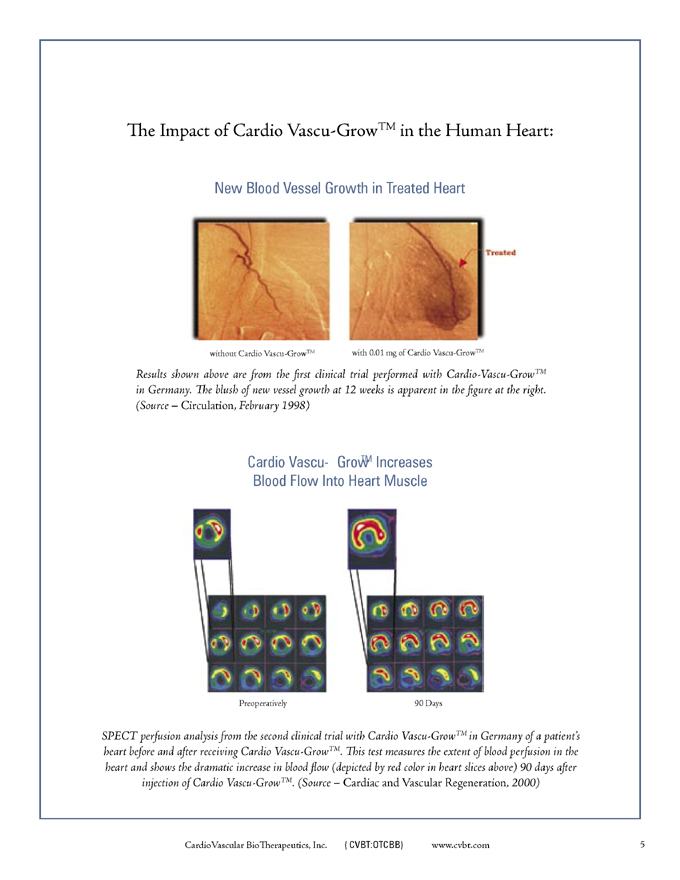
The Impact of Cardio Vascu-GrowTM in the Human Heart:
New Blood Vessel Growth in Treated Heart
without Cardio Vascu-GrowTM with 0.01 mg of Cardio Vascu-GrowTM
Results shown above are from the first clinical trial performed with Cardio -Vascu-GrowTM in Germany. The blush of new vessel growth at 12 weeks is apparent in the figure at the right. (Source – Circulation, February 1998)
Cardio Vascu-GrowTM Increases Blood Flow Into Heart Muscle
Preoperatively 90 Days
SPECT perfusion analysis from the second clinical trial with Cardio Vascu-GrowTM in Germany of a patient’s heart before and after receiving Cardio Vascu-GrowTM. This test measures the extent of blood perfusion in the heart and shows the dramatic increase in blood flow (depicted by red color in heart slices above) 90 days after injection of Cardio Vascu-GrowTM. (Source—Cardiac and Vascular Regeneration, 2000)
CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 5

1. The Muscle Tissue Group
U.S. Clinical Trial in No-Option
Heart Patients
In 2005, CVBT is well along the pathway of commercializing Cardio Vascu-GrowTM for use in the heart. In 2003, CVBT started U.S. FDA-authorized clinical trials which are now ongoing at six US medical centers including:
University of Cincinnati Medical Center, Cincinnati, OH
Penn State University Medical Center, Hershey, PA
University of Alabama Medical Center, Birmingham, AL
St. Joseph’s Hospital, Towson, MD
St. Vincent’s Medical Center, Bridgeport, CT
JFK Hospital, West Palm Beach, FL
The first results from this trial, which were highly promising, were reported by the Cincinnati Enquirer, and on ABC Nightly News on May 12, 2004. In addition, medical researchers from the University of Cincinnati Hospital were invited to give a Plenary Session presentation on their clinical results at the 2004 American Heart Association’s meeting in New Orleans. The current studies are examining different doses of Cardio Vascu-GrowTM and the drug’s effect to stimulate new blood vessel growth in the hearts of no-option patients.
Adjunct Treatment in By-Pass Patients
In addition to using Cardio Vascu-GrowTM as the sole therapy to treat no-option heart patients, clinical protocols are also being developed to test the application of Cardio Vascu-GrowTM in patients who are receiving a bypass procedure. The idea in this clinical trial will be to examine the effect of treating areas of the heart with Cardio Vascu-GrowTM where ischemia is due to the blockage of smaller vessels, which cannot be surgically treated during the bypass procedure. Previous clinical studies with Cardio Vascu-GrowTM indicated that patients receiving both a bypass and injection of the growth factor experience longer lasting relief from angina than patients only receiving a bypass.
Angina Pectoris
An estimated 6 million Americans experience severe angina due to diffuse coronary artery disease. This form of heart disease occurs when smaller coronary arteries throughout the heart become blocked resulting in a diffuse form of coronary artery disease not amenable to bypass or stenting procedure.
Cardio Vascu-GrowTM has been nationally recognized in media including:
THE WALL STREET JOURNAL.
(February, 1998)
American Heart Association(R)
(November, 2004)
(May 12, 2004)
The NEW ENGLAND
JOURNAL of MEDIClNE
(1998)
CENTRAL PENN BUSINESS JOURNAL.
(February 27, March 19, July 2, 2004)
THE ENQITRER
(March 24, 2004)
6 CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com

The Muscle Tissue Group
Diffuse Coronary Heart Disease
Coronary Heart Disease caused by a blockage of a major artery
Diffuse Coronary Heart Disease
Arrow in photo on left indicates where bypass procedure would be performed. Circled areas in photo on right would be where Cardio Vascu-GrowTM would be injected to induce new blood vessel growth in the area. In diffuse coronary artery disease the vessels are too small to be bypassed. (Source – Thomas Stegmann, M.D.)
Delivery of Cardio Vascu-GrowTM via an Injection Catheter
CardioVascular BioTherapeutics, Inc. Injection Catheter
A prototype injection catheter to introduce Cardio Vascu-GrowTM by a less invasive procedure is scheduled for animal testing. (Source – CVBT)
CVBT is developing an injection catheter, which has been designed and prototypes manufactured, that should permit the application of Cardio Vascu-GrowTM by a much less invasive catheter procedure. This catheter is now scheduled to be tested in animals and its introduction could greatly expand the potential use of Cardio Vascu-GrowTM to patients with even mild angina, or perhaps, even as a preventative therapy for patients at risk for coronary artery disease. Additionally, if successful, the catheter could significantly lower the cost of delivering the drug to patients when compared to a surgical delivery.
CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 7
1. The Muscle Tissue Group
B. Vascular Disease of the Legs
An estimated 8 million Americans suffer from peripheral vascular disease (PVD). This occurs when blood vessels which feed the legs become blocked by atherosclerotic lesions, which results in pain while walking. This can evolve to more severe forms of the disease where circulation to the foot and leg become so reduced that amputation of the toes, foot, or legs is the only alternative. This disease increases in prevalence as people age or in patients with diabetes.
The objective is to attempt to grow new blood vessels in the legs around the blockage, and thereby re-establish blood flow to the ischemic leg muscles in these patients.
CVBT has started testing Cardio Vascu-GrowTM in animal models of peripheral vascular disease and this work may support an application to the U.S. FDA to begin testing in U.S. patients by the end of this year.
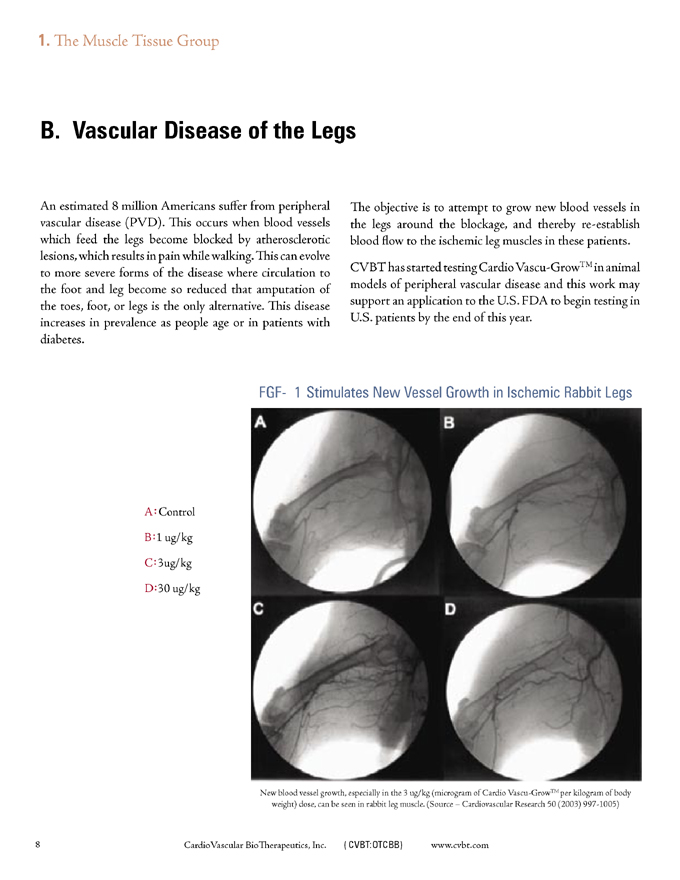
FGF-1 Stimulates New Vessel Growth in Ischemic Rabbit Legs
A: Control B:1ug/kg C:3ug/kg D:30 ug/kg
New blood vessel growth, especially in the 3 ug/kg (microgram of Cardio Vascu-GrowTM per kilogram of body weight) dose, can be seen in rabbit leg muscle. (Source – Cardiovascular Research 50 (2003) 997-1005)
8 Cardio Vascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com
1. The Muscle Tissue Group
C. Chronic Back Pain and Lumbar Ischemia
Chronic back pain, which we estimate, based on published reports, affects a staggering 26 million Americans, has recently been linked to blockage of blood vessels supplying the lower back, leading to a condition referred to as lumbar ischemia. European medical researchers have speculated that chronic back pain resulting from blockage of blood vessels in the lower back precedes the blockage of coronary arteries in the heart by ten years. CVBT is negotiating to participate in a proof of concept clinical trial in Europe in the second half of 2005 to test whether Cardio Vascu-GrowTM can successfully treat chronic back pain. If the results are positive, CVBT plans to then file an application to the U.S. FDA to allow a study to begin in chronic back pain patients in the U.S.
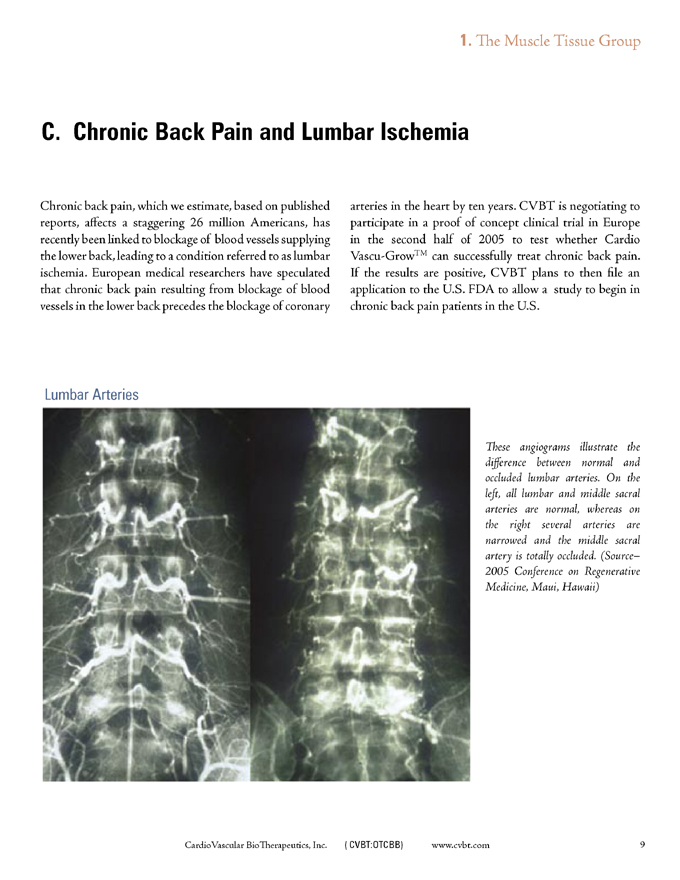
Lumbar Arteries
These angiograms illustrate the difference between normal and occluded lumbar arteries. On the left, all lumbar and middle sacral arteries are normal, whereas on the right several arteries are narrowed and the middle sacral artery is totally occluded. (Source-2005 Conference on Regenerative Medicine, Maui, Hawaii)
CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 9
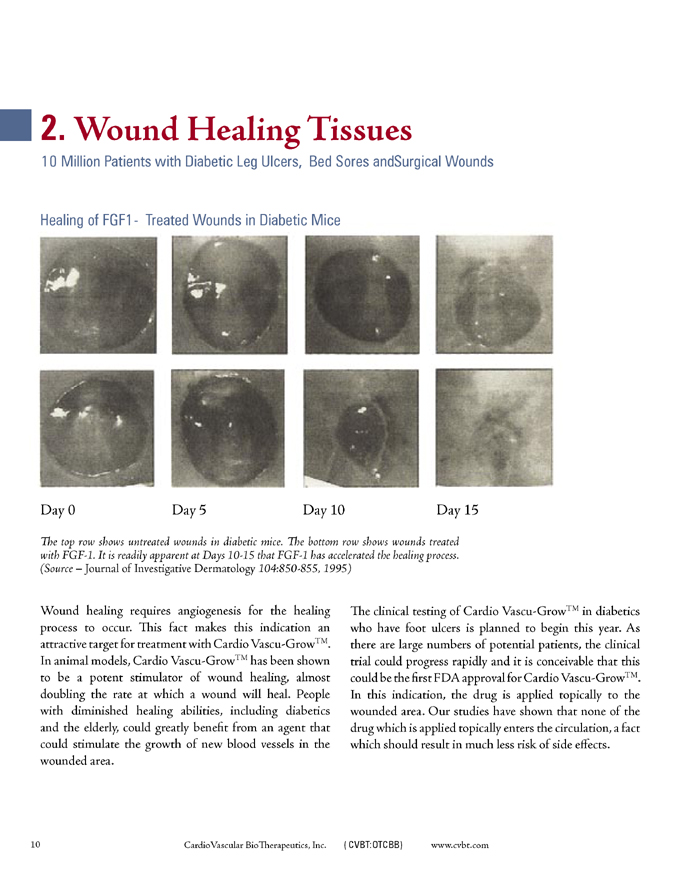
2. Wound Healing Tissues
10 Million Patients with Diabetic Leg Ulcers, Bed Sores and Surgical Wounds
Healing of FGF1-Treated Wounds in Diabetic Mice
Day 0 Day 5 Day 10 Day 15
The top row shows untreated wounds in diabetic mice. The bottom row shows wounds treated with FGF-1. It is readily apparent at Days 10-15 that FGF-1 has accelerated the healing process. (Source – Journal of Investigative Dermatology 104:850-855,1995)
Wound healing requires angiogenesis for the healing process to occur. This fact makes this indication an attractive target for treatment with Cardio Vascu-GrowTM. In animal models, Cardio Vascu-GrowTM has been shown to be a potent stimulator of wound healing, almost doubling the rate at which a wound will heal. People with diminished healing abilities, including diabetics and the elderly, could greatly benefit from an agent that could stimulate the growth of new blood vessels in the wounded area.
The clinical testing of Cardio Vascu-GrowTM in diabetics who have foot ulcers is planned to begin this year. As there are large numbers of potential patients, the clinical trial could progress rapidly and it is conceivable that this could be the first FDA approval for Cardio Vascu-GrowTM. In this indication, the drug is applied topically to the wounded area. Our studies have shown that none of the drug which is applied topically enters the circulation, a fact which should result in much less risk of side effects.
10 CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com
3. The Nerve Tissue Group
CVBT has focused most intensely on developing new treatments that can stimulate new blood vessel growth into ischemic muscle tissues. However, blocked blood vessels can also severely injure other tissue types including nervous tissues. Stroke results from the same basic atherosclerotic process that causes coronary artery disease, but now this process is occurring in vessels that supply brain cells with blood.
Brain 5 Million Stroke Patients
When blood flow is restricted or blocked in blood vessels supplying the brain or in vessels located within the brain, stroke results. Stroke is the number three cause of death in the United States, and an estimated 5 million people suffer from the often devastating long term effects of strokes in this country. Cardio Vascu-GrowTM, in our pre-clinical animal studies, reduced the size of damaged brain tissue after a stroke and is currently being developed as a potential new therapy to treat this condition.
Patient with Cerebral Stroke
The outer red circle indicates ischemic brain tissue and is the target area for healing by a Cardio Vascu-GrowTM infusion. (Source- CVBT)
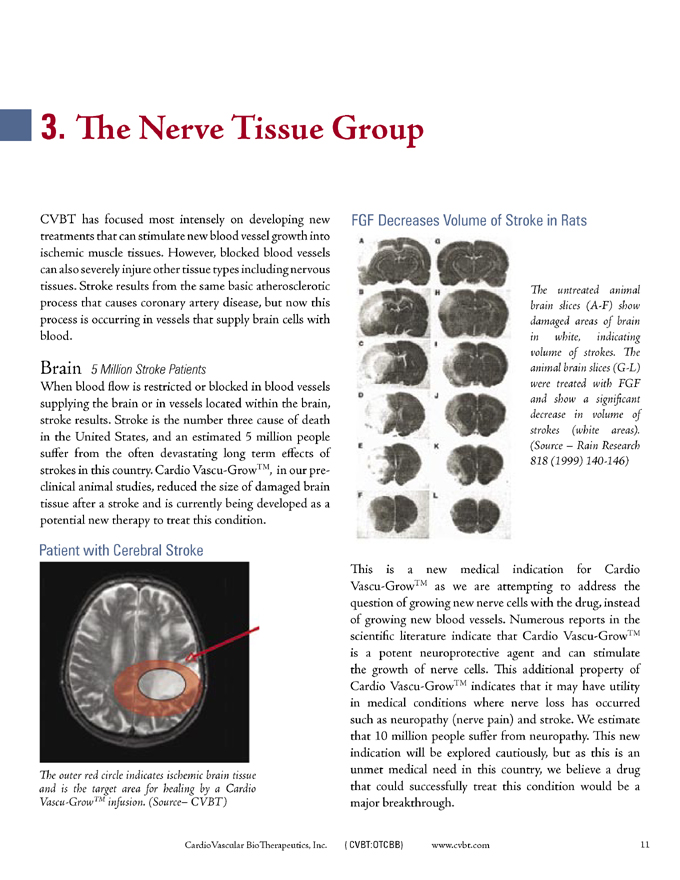
FGF Decreases Volume of Stroke in Rats
The untreated animal brain slices (A-F) show damaged areas of brain in white, indicating volume of strokes. The animal brain slices (G-L) were treated with FGF and show a significant decrease in volume of strokes (white areas). (Source – Rain Research 818 (1999) 140-146)
This is a new medical indication for Cardio Vascu-GrowTM as we are attempting to address the question of growing new nerve cells with the drug, instead of growing new blood vessels. Numerous reports in the scientific literature indicate that Cardio Vascu-GrowTM is a potent neuroprotective agent and can stimulate the growth of nerve cells. This additional property of Cardio Vascu-GrowTM indicates that it may have utility in medical conditions where nerve loss has occurred such as neuropathy (nerve pain) and stroke. We estimate that 10 million people suffer from neuropathy. This new indication will be explored cautiously, but as this is an unmet medical need in this country, we believe a drug that could successfully treat this condition would be a major breakthrough.
CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 11
4. Intestinal Tissue
We estimate, based on physicicans’ estimates of prevalence, that 30 million Americans suffer with Intestinal Ischemia.
CVBT has just started its work in to this area which affects a large number of mainly elderly Americans and has the potential to be a significant marketplace for Cardio Vascu-GrowTM.
Cardiovascular disease in the intestinal tract can cause severe pain in people, even after the simple act of eating a meal. A new potential application for Cardio Vascu-GrowTM is in the growing of new blood vessels in the digestive tract to address the medical problem of intestinal ischemia and pancreatitis. Smaller blood vessels which feed the intestines or pancreas can become blocked by the same atherosclerotic process that affects the coronary arteries in the heart. This results in restricted blood flow to the digestive tract which can lead to pain, reduced absorption of nutrients, and diminished clearance of waste. It was reported at the 2005 Regenerative Medicine Conference, Maui, Hawaii, that this disease will affect, to some degree, almost everyone over the age of 70. Given the aging of populations throughout the western world, the number of people affected by this disease will only get larger. As little can be done currently for sufferers of this disease, Cardio Vascu-GrowTM has the potential to make a material difference for these patients.
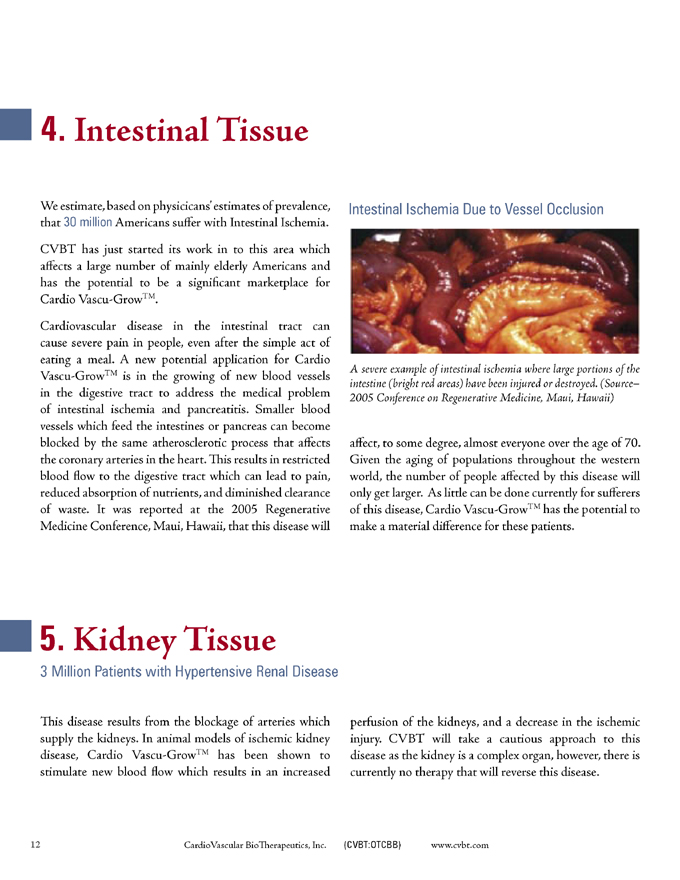
Intestinal Ischemia Due to Vessel Occlusion
A severe example of intestinal ischemia where large portions of the intestine (bright red areas) have been injured or destroyed. (Source – 2005 Conference on Regenerative Medicine, Maui, Hawaii)
5. Kidney Tissue
3 Million Patients with Hypertensive Renal Disease
This disease results from the blockage of arteries which supply the kidneys. In animal models of ischemic kidney disease, Cardio Vascu-GrowTM has been shown to stimulate new blood flow which results in an increased perfusion of the kidneys, and a decrease in the ischemic injury. CVBT will take a cautious approach to this disease as the kidney is a complex organ, however, there is currently no therapy that will reverse this disease.
12 CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com
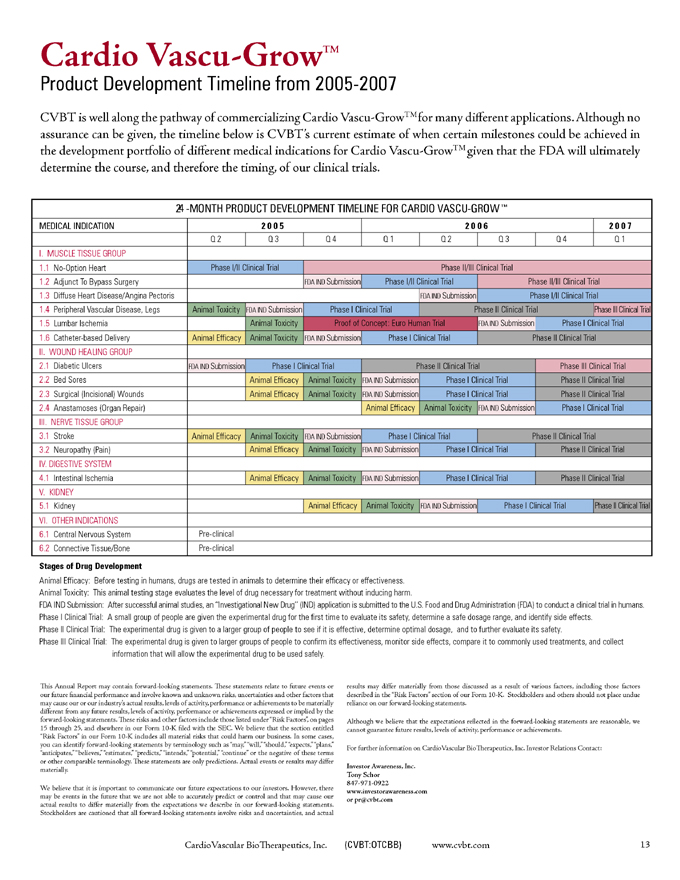
Cardio Vascu-GrowTM
Product Development Timeline from 2005-2007
CVBT is well along the pathway of commercializing Cardio Vascu-GrowTM for many different applications. Although no assurance can be given, the timeline below is CVBT’s current estimate of when certain milestones could be achieved in the development portfolio of different medical indications for Cardio Vascu-GrowTM given that the FDA will ultimately determine the course, and therefore the timing, of our clinical trials.
24-MONTH PRODUCT DEVELOPMENT TIMELINE FOR CARDIO VASCU-GROWTM
MEDICAL INDICATION 2005 2006 2007
Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1
1. MUSCLE TISSUE GROUP
1.1 No-Option Heart Phase I/II Clinical Trial Phase II/III Clinical Trial
1.2 Adjunct To Bypass Surgery FDA IND Submission Phase I/II Clinical Trial Phase II/III Clinical Tr al
1.3 Diffuse Heart Disease/Angina Pectoris FDA IND Submission Phase I/II Clinical Trial
1.4 Peripheral Vascular Disease, Legs Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial Phase III Clinical Trial
1.5 Lumbar Ischemia Animal Toxicity Proof of Concept: Euro Human Trial FDA IND Submission Phase I Clinical Trial
1.6 Catheter-based Delivery Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
II. WOUND HEALING GROUP
2.1 Diabetic Ulcers FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial Phase III Clinical Trial
2.2 Bed Sores Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
2.3 Surgical (Incisional) Wounds Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
2.4 Anastamoses (Organ Repair) Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial
III. NERVE TISSUE GROUP
3.1 Stroke Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
3.2 Neuropathy (Pain) Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
IV. DIGESTIVE SYSTEM
4.1 Intestinal Ischemia Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
V. KIDNEY
5.1 Kidney Animal Efficacy Animal Toxicity FDA IND Submission Phase I Clinical Trial Phase II Clinical Trial
VI. OTHER INDICATIONS
6.1 Central Nervous System Pre-clinical
6.2 Connective Tissue/Bone Pre-clinical
Stages of Drug Development
Animal Efficacy: Before testing in humans, drugs are tested in animals to determine their efficacy or effectiveness.
Animal Toxicity: This animal testing stage evaluates the level of drug necessary for treatment without inducing harm.
FDA IND Submission: After successful animal studies, an “Investigational New Drug” (IND) application is submitted to the U.S. Food and Drug Administration (FDA) to conduct a clinical trial in humans.
Phase I Clinical Trial: A small group of people are given the experimental drug for the first time to evaluate its safety, determine a safe dosage range, and identify side effects.
Phase II Clinical Trial: The experimental drug is given to a larger group of people to see if it is effective, determine optimal dosage, and to further evaluate its safety.
Phase III Clinical Trial: The experimental drug is given to larger groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug to be used safely.
This Annual Report may contain forward-looking statements. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by the forward-looking statements. These risks and other factors include those listed under “Risk Factors”, on pages 15 through 25, and elsewhere in our Form 10-K filed with the SEC. We believe that the section entitled “Risk Factors” in our Form 10-K includes all material risks that could harm our business. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “intends,” “potential,” “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. Actual events or results may differ materially.
We believe that it is important to communicate our future expectations to our investors. However, there may be events in the future that we are not able to accurately predict or control and that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. Stockholders are cautioned that all forward-looking statements involve risks and uncertainties, and actual results may differ materially from those discussed as a result of various factors, including those factors described in the “Risk Factors” section of our Form 10-K. Stockholders and others should not place undue reliance on our forward-looking statements.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
For further information on Cardio Vascular BioTherapeutics, Inc. Investor Relations Contact:
Investor Awareness, Inc. Tony Schor 847-971-0922 www.investorawareness.com or pr@cvbt.com
Cardio Vascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 13

Chairman’s Message to Shareholders
April of 2005 marks the seven year anniversary of the founding of CardioVascular BioTherapuetics, Inc. (CVBT or Company).
From a modest start in March of 1998, we have advanced our company toward our goals. We have raised over $33 million of investment capital, and we have advanced the clinical trials on our drug candidate Cardio Vascu-GrowTM. Our investigation into new possible applications for our protein drug candidate which grows new blood vessels in the human body has identified many new exciting opportunities.
The first thing to be addressed in this Annual Report is to thank all of the people who have helped us advance this mission. That mission is the treatment of cardiovascular circulatory diseases. I thank you, the Board of Directors thanks you, and all of our potential patients thank you. This thank you is extended to all of our investors, employees, consultants, advisors, clinical personnel, patients, friends, and many more. We are thankful to all of you for what you have helped us accomplish.
Our Company, CardioVascular BioTherapeutics, Inc. (CVBT), has several major goals to accomplish. The first goal is to become a commercially viable business. The second goal is to conduct our actions in a manner that allows everyone to trust in our integrity to do the right thing. The third goal is to continue to identify and develop revolutionary new medical treatments for the number one cause of death in the developed world. We must achieve all of these goals in order to advance the Company and succeed in our mission to provide treatment for cardiovascular circulatory diseases.
Dr. Stegmann, in 1992, started this adventure of treating circulatory disease by growing new blood vessels. I joined him in 1998 after he had worked on this project for six years. In 1998, Dr. Thomas Stegmann, Dr. Wolfgang Priemer, Alex Montano, Grant Gordon and I started down a long path to build this Company, CVBT. An easy journey, it has not been.
Dr. Stegmann’s initial work focused on growing new blood vessels in the heart, the number one cause of death in the developed world. CVBT initially placed its primary focus on treating heart disease. However, during the last seven years, we have identified additional potential applications for our drug candidate, Cardio Vascu-GrowTM. Examples of these potential medical applications are to treat Peripheral Vascular Disease in the legs, to treat Lumbar Ischemia in the back, to address stroke issues in the brain, to improve wound healing, and perhaps to address intestinal ischemia and pancreatitis, ischemia in the kidneys, Neuropathy, diseases of the Central Nervous system, uses towards connective tissues, eyes, hips, and more. Our goals and our opportunities have multiplied beyond what could have been imagined only three years ago.
The last seven years have been a great adventure. I believe the next seven years will be even more exciting for CVBT and our people.
It seems to me the next seven years will be even more interesting and rewarding for you the CVBT shareholders.
Looking into CVBT’s future, the next 24 to 36 months will provide clarity as to the viability of CVBT as a commercial business, evidence of our ability to conduct ourselves in a manner which makes our shareholders proud to be associated with us, and validate our ability to identify and develop revolutionary new medical treatments for cardiovascular circulatory diseases. Said another way, in the next 24 to 36 months we will know if we are succeeding in our mission to treat the number one cause of death and suffering in the developed world, cardiovascular circulatory diseases.
We, the CVBT family, appreciate and thank you for your faith and support. So much has been accomplished over the past seven years, and while we are still at the beginning of succeeding in our three primary goals, we shall do everything in our power to succeed in our mission.
Sincerely,
Daniel C. Montano Chairman, President, and CEO
April 26, 2005
14 CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com
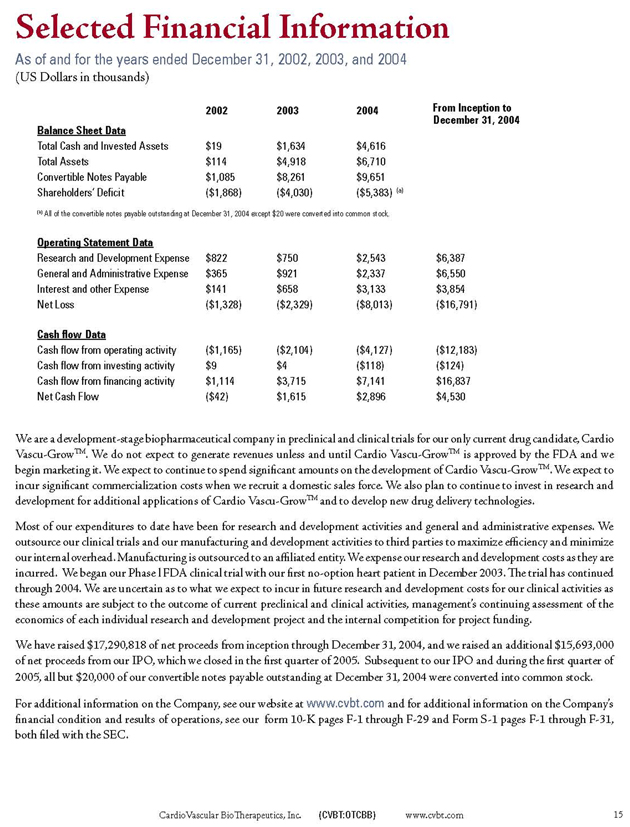
Selected Financial Information
As of and for the years ended December 31, 2002, 2003, and 2004
(US Dollars in thousands)
2002
2003
2004
From Inception to December 31, 2004
Balance Sheet Data
Total Cash and Invested Assets $19 $1,634 $4,616
Total Assets $114 $4,918 $6,710
Convertible Notes Payable $1,085 $8,261 $9,651
Shareholders’ Deficit ($1,868) ($4,030) ($5,383) (a)
(a) All of the convertible notes payable outstanding at December 31, 2004 except $20 were converted into common stock.
Operating Statement Data
Research and Development Expense $822 $750 $2,543 $6,387
General and Administrative Expense $365 $921 $2,337 $6,550
Interest and other Expense $141 $658 $3,133 $3,854
Net Loss ($1,328) ($2,329) ($8,013) ($16,791)
Cash flow Data
Cash flow from operating activity ($1,165) ($2,104) ($4,127) ($12,183)
Cash flow from investing activity $9 $4 ($118) ($124)
Cash flow from financing activity $1,114 $3,715 $7,141 $16,837
Net Cash Flow ($42) $1,615 $2,896 $4,530
We are a development-stage biopharmaceutical company in preclinical and clinical trials for our only current drug candidate, Cardio Vascu-Grow™. We do not expect to generate revenues unless and until Cardio Vascu-Grow™ is approved by the FDA and we begin marketing it. We expect to continue to spend significant amounts on the development of Cardio Vascu-Grow™. We expect to incur significant commercialization costs when we recruit a domestic sales force. We also plan to continue to invest in research and development for additional applications of Cardio Vascu-Grow™ and to develop new drug delivery technologies.
Most of our expenditures to date have been for research and development activities and general and administrative expenses. We outsource our clinical trials and our manufacturing and development activities to third parties to maximize efficiency and minimize our internal overhead. Manufacturing is outsourced to an affiliated entity. We expense our research and development costs as they are incurred. We began our Phase l FDA clinical trial with our first no-option heart patient in December 2003. The trial has continued through 2004. We are uncertain as to what we expect to incur in future research and development costs for our clinical activities as these amounts are subject to the outcome of current preclinical and clinical activities, management’s continuing assessment of the economics of each individual research and development project and the internal competition for project funding.
We have raised $17,290,818 of net proceeds from inception through December 31, 2004, and we raised an additional $15,693,000 of net proceeds from our IPO, which we closed in the first quarter of 2005. Subsequent to our IPO and during the first quarter of 2005, all but $20,000 of our convertible notes payable outstanding at December 31, 2004 were converted into common stock.
For additional information on the Company, see our website at www.cvbt.com and for additional information on the Company’s financial condition and results of operations, see our form 10-K pages F-1 through F-29 and Form S-1 pages F-1 through F-31, both filed with the SEC.
CardioVascular BioTherapeutics, Inc. (CVBT:OTCBB) www.cvbt.com 15

CardioVascular BioTherapeutics, Inc.
17 00 West Horizon Ridge Pkwy, Ste 100
Henderson, NV 89012 www.cvbt.com pr@cvbt.com
CardioVascular
BioTherapeutics, Inc.
Tony Schor (Investor Relations)
(847) 97 1- 0922 Investor Awareness, Inc. www.investorawareness.com















