Exhibit 99.1
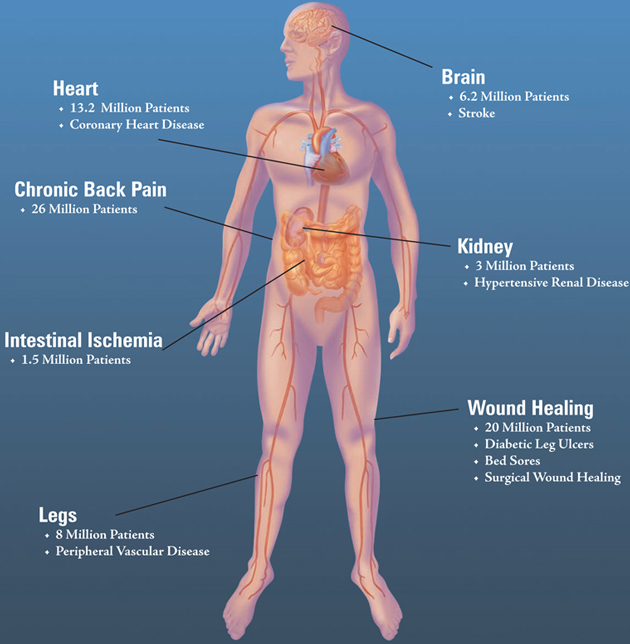
In the United States alone,77,900,000 people suffer
from some form of cardiovascular disease...
| | |
2006 Annual Review | |  |
...and nearly2,500 Americans die each day
from cardiovascular disease.
Cardiovascular disease claims more lives each year than the next four leading causes of death combined (cancer, chronic lower respiratory diseases, accidents and diabetes mellitus).
According to the American Heart Association’s Heart Disease and Stroke 2006 Statistics, in the United States:
| • | | 1 in 3 adult men and women has some form of cardiovascular disease |
| • | | 13.2 million suffer from coronary heart disease |
| • | | 7.2 million have had heart attacks |
| • | | 6.5 million have angina pectoris (chest pain) |
| • | | An estimated700,000 Americans will have a new heart attack and about500,000 will have a recurrent attack this year |
| • | | 5.5 million have had strokes |
| • | | Nearly2,500 die of cardiovascular disease (CVD) each day |
| • | | Every35 seconds someone will die from cardiovascular disease |
A Letter from our Co-Founder
Dear Friend,
| | |
Since it was founded in 1998, CardioVascular BioTherapeutics, Inc. (CVBT) has been dedicated to the development of regenerative medicine therapies. In comparison to “traditional” therapy or reparative medicine, regenerative medicine stimulates the growth of new cells and tissues defined as, angiogenesis, vasculogenesis, and neurogenesis. By developing these new therapies we are creating new hope for so many. The basic defect contributing to Coronary Heart Disease, Peripheral Vascular Disease and Stroke is atherosclerosis or the clogging of blood vessels. Atherosclerosis is also thought to be the cause of back pain due to lumbar ischemia (lack of blood flow to the back) and intestinal ischemia. Atherosclerosis is the “number one killer” in the Western World and Asia. This is also particularly true in the Indian Sub-Continent and the Middle East. Heart attack, heart failure, stroke, amputation, diabetic skin ulcers are all terms that induce fear in people. The morbidity and mortality of these diseases affect a large and growing number of people in the world. | |  |
CVBT has recently completed the treatment of all patients for its Phase I US trial for no-option heart patients, which followed two successful trials in Germany. A Phase II clinical trial is planned to commence in 2006. This trial will be the next step in bringing CVBT’s heart drug candidate formulated with Cardio Vascu-GrowTM closer to the market place.
Using the unique regenerative power of Cardio Vascu-GrowTM to stimulate cells and grow new vessels, CVBT will focus on developing therapies to treat Peripheral Vascular Disease and prevent wound healing problems including diabetic skin ulcers. New human clinical trials in this area will commence during 2006.
CVBT will continue its dedication to scientific research and development into regenerative medicine by expanding our research into new treatment options for other chronic diseases affecting our population such as chronic intestinal ischemia, and back pain caused by lumbar ischemia.
Diabetes mellitus (type 1 and type 2) is a very common disease affecting more and more people every year. At CVBT we are researching new treatments not only for the treatment of the complications of “Diabetes mellitus”, but potentially for the disease itself, through the stimulation of beta cell growth within the pancreas.
I am excited that we will also commence research into the stimulation of Cardiac Progenitor Cells, creating a potential treatment for heart failure, another common and disabling disease.
Organ ischemia whether it affects the heart, brain, skin or intestine causes significant morbidity and mortality. This disease affects many in the world today and will affect many more as the world’s population ages. Unfortunately, one of these diseases will affect you, a relative or a close friend. The good news is that CVBT has the potential to treat and prevent these illnesses.
Every life is a precious gift. The dynamic team at CVBT have made it their mission to develop therapies that will reduce suffering, improve the quality of life and increase the life expectancy of each individual affected by these devastating diseases.
Sincerely,

Thomas J. Stegmann, M.D.,
Director, Co-Founder, and Chief Clinical Officer
CardioVascular BioTherapeutics, Inc.
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 1 |
CardioVascular BioTheraputics, Inc. (CVBT) is a
biopharmaceutical company focused on
introducing new therapies for cardiovascular disease.
Cardio Vascu-GrowTM, the active ingredient in our drug candidates, is a protein, and is a member of the fibroblast growth factor family. Fibroblast growth factor-1 or, FGF-1, is a powerful stimulator of new blood vessel growth, a process referred to in the scientific community as “angiogenesis.” This is due to the fact that FGF-1 stimulates the growth and multiplication of the two main cell types of blood vessels, smooth muscle cells and endothelial cells. Extensive work by us and others has shown that, when FGF-1 is injected into the tissue and organs of animals with experimentally atherosclerotic disease, new blood vessels grow in the injected areas. Proteins such as Cardio Vascu-GrowTM represent a novel therapy for diseases characterized by inadequate blood flow to a tissue or organ. This growth factor is being tested in a wide variety of human diseases that could benefit from increased angiogenesis, including coronary artery disease, stroke, peripheral vascular disease of the legs and wound healing.
FDA-authorized clinical trials are being performed in the USA in patients with coronary artery disease with promising results. Additional trials are planned for the near future with our drug candidates for diabetics with wound healing deficiencies and vascular disease in their legs. Animal studies that mimic these human conditions have been performed and indicated that Cardio Vascu-GrowTM is an effective treatment in both animal models of wound healing and peripheral vascular disease. The large number of patients that suffer from these cardiovascular diseases make each of these medical indications a potential significant market.
Cardiovascular disease affects over77 millionpeople in the United States and CVBT has potential new therapies for a wide array of these cardiovascular diseases in its research and development portfolio.
2
The Muscle Tissue Group
As shown on the front page of this report, Cardio Vascu-GrowTM can be applied to multiple tissues in the body that become injured due to a decreased blood flow. This injury, or tissue ischemia, is most often caused by the blockage of arteries supplying blood to the tissue. The company’s current development efforts for a drug candidate containing Cardio Vascu-GrowTM as the active ingredient have been grouped according to the type of tissue that is proposed to be treated and includes:
The Muscle Tissue Group
Heart Applications
An Estimated 13.2 Million Americans Suffer From Coronary Heart Disease
The research to develop Cardio Vascu-GrowTM started in 1992 in Germany with Dr. Thomas Stegmann’s initial success in growing new blood vessels in animal hearts. This research led to the first human experiments in 1995/96 in 20 patients with no-option heart disease. The results were reported in the American Heart Association publication Circulation in February 1998. This first successful angiogenesis clinical trial in the human heart led to the founding of CardioVascular BioTherapeutics, Inc. to commercialize this medical breakthrough.
In 1998/99 Dr. Stegmann conducted a second clinical trial using the drug as the sole therapy in 20 no-option heart patients. The successful results of this trial were reported in the cardiology journal Cardiac and Vascular Regeneration in 2000. The cumulative results of the two clinical trials can be summarized as follows:
The potential of Cardio Vascu-GrowTM as a new treatment for coronary artery disease was convincingly demonstrated by Dr. Thomas Stegmann and his associates in two separate clinical trials performed in Germany.
A total of 40 patients with no viable surgical treatment options received injections into their hearts of human fibroblast growth factor, the active ingredient in Cardio Vascu-GrowTM. The clinical results from these studies, published in internationally-recognized medical journals, indicated that FGF-1 was a safe and effective treatment for severe coronary artery disease. The following results were observed:
| • | | 80% of patients showed a significant improvement in their stress exercise test. |
| • | | Hearts showed a five-fold increase in the stress SPECT perfusion score, indicating a significant improvement in blood flow to the heart muscle. |
| • | | No significant adverse safety effects of the therapy. |
| • | | Importantly, 90% of the patients had an improvement in their dominant clinical symptom, angina or chest pain. During the stress test, angina was either completely absent, or began at much higher levels of exertion. |
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 3 |
The Muscle Tissue Group
The Impact of Cardio Vascu-GrowTM in the Human Heart:
New Blood Vessel Growth in Treated Heart
| | |
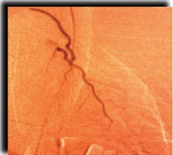 | | 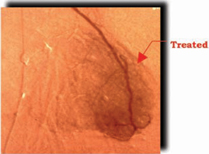 |
| without Cardio Vascu-GrowTM | | with 0.0 mg of Cardio Vascu-GrowTM |
Results shown above are from the first clinical trial performed with Cardio-Vascu-GrowTM in Germany. The blush of new vessel growth at 12 weeks is apparent in the figure at the right. (Source– Circulation,February 1998)
Cardio Vascu-GrowTM Increases Blood Flow
Into Heart Muscle
| | |
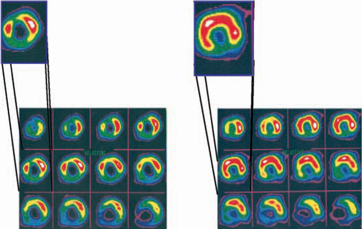 |
| Preoperatively | | 90 Days |
SPECT perfusion analysis from the second clinical trial with Cardio Vascu-GrowTM in Germany of a patient’s heart before and after receiving Cardio Vascu-GrowTM. This test measures the extent of blood perfusion in the heart and shows the dramatic increase in blood flow (depicted by red color in heart slices above) 90 days after injection of Cardio Vascu-GrowTM. (Source –Cardiac and Vascular Regeneration, 2000)
4
The Muscle Tissue Group
U.S. Clinical Trial in No-Option Heart Patients
CVBT is progressing along the pathway of commercializing one formulation of Cardio Vascu-GrowTM for use in the heart. The company has completed treatment of all patients in its first U.S. FDA-authorized clinical trials, which were closely patterned on the previous studies preformed by Dr. Stegmann in Germany. A total of 21 patients with 3 different dosage cohorts were treated in this U.S. trial.
Clinical endpoints measured in the Phase I Clinical Trial in no-option heart patients.
Study Endpoints
| | • | | Safety and tolerability of FGF1 (Adverse events) |
| | • | | Pharmacokinetics of FGF1 |
Efficacy
| | • | | Improved perfusion via SPECT Scan, Stress Echo, Angiography at 6 and/or 12 weeks |
| | • | | Change in CCS Anginal Classification |
| | • | | Change in Seattle Angina Questionnaire |
| | • | | Change in ophthalmologic exam |
| | • | | Change in laboratory values |
Although this was technically a Phase I trial, certain elements of a Phase II trial were incorporated into this first trial, including testing the drug in the target patient population and collecting efficacy data on the drug.
Six US medical centers were chosen to participate in this trial including:
| • | | University of Cincinnati Medical Center, Cincinnati, OH |
| • | | Penn State University Medical Center, Hershey, PA |
| • | | University of Alabama Medical Center, Birmingham, AL |
| • | | St. Joseph’s Hospital, Towson, MD |
| • | | St. Vincent’s Hospital, Bridgeport, CT |
| • | | JFK Hospital, West Palm Beach, FL |
Clinical trial design of our Phase I trial in no-option heart patients was as follows. Each boxed time point represents an office visit for the patient where safety and efficacy tests are performed.
Study Design

| • | | Stress and resting echocardiograms |
| • | | SPECT perfusion imaging scans |
| • | | Ophthalmologic Exams (6 exams throughout 52 weeks) |
This recently completed U.S. trial enrolled 21 no-option heart patients. No significant, unexpected adverse effects that have been attributed to the drug have been noted in any of the patients, and most patients see a significant decrease in their most common clinical symptom, angina or chest pain. The early results from this trial were so promising that one of the clinical trial sites, The University of Cincinnati Medical Center, was featured in several media segments including a report by the Cincinnati Enquirer, and a report on the national ABC Nightly News program.
As shown in the accompanying Table, which summarizes data on patients treated in the U.S. trials through 2006, there was a marked improvement in angina symptoms experienced by patients. At both 6 and 12 weeks there was a highly significant decrease in the severity of the patient’s chest pain with most patients falling from severe Class III and Class IV angina scores to more manageable Class I and II scores. These results parallel the results seen in the previous German clinical trials.
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 5 |
The Muscle Tissue Group
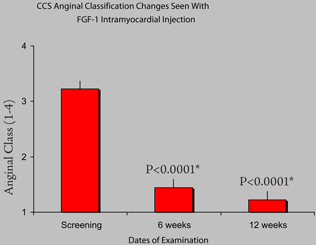
Reduction in chest pain and angina as measured by an angina questionnaire administered to no-option heart patients before surgery (screening) and at 6 and 12 weeks after receiving Cardio Vascu-GrowTM.(Source–Lynne Wagner, M.D., University of Cincinnati)
As enrollment and treatment of patients in this first clinical trial is completed, a Phase II trial is being designed and is planned to commence later this year in the same patient population.
The Phase II trial will focus more intensely on the efficacy of this formulation of Cardio Vascu-GrowTM in a larger patient population. In addition, the Phase II trial will have a control group of patients who do not receive our treatment but are given optimal medical care.
Adjunct Therapy in Bypass Patients
In addition to using this formulation of Cardio Vascu-GrowTM as the sole therapy to treat no-option heart patients, clinical protocols are being developed to test the drug candidate in patients who are receiving a bypass procedure. The goal in this clinical trial will be to examine the effect of treating areas of the heart with this formulation of Cardio Vascu-GrowTM where ischemia is due to the blockage of smaller vessels which cannot be surgically treated during the bypass procedure. Previous clinical studies carried out by academic researchers indicated that patients receiving both a bypass and an injection of a growth factor, related to Cardio Vascu-GrowTM, experience longer lasting relief from angina than patients only receiving a bypass.
CVBT’s drug candidate for no-option heart patients has been nationally recognized in media including:

6
The Muscle Tissue Group
Angina Pectoris
An estimated 6.5 million Americans experience severe angina due to diffuse coronary heart disease. This form of heart disease occurs when smaller coronary arteries throughout the heart become blocked, resulting in a diffuse form of coronary heart disease not amenable to bypass or stenting procedures. CVBT is developing clinical protocols to treat this class of patients and hopes to be able to deliver a new formulation of Cardio Vascu-GrowTM via a catheter, a much less invasive approach than surgery, which is currently being used. CVBT is working with a catheter company that has an injection catheter approved for clinical use.
This catheter is now scheduled to be tested in animals and, if successful, could greatly expand the potential use of this form of Cardio Vascu-GrowTM to patients with even mild angina, or perhaps, even as a preventative therapy for patients at risk for coronary artery disease. Additionally, if successful, a catheter delivery method could significantly lower the cost of delivering the drug to patients when compared to a surgical delivery.
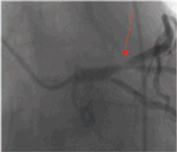
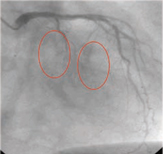
Diffuse Coronary Heart Disease
| | |
 | |  |
| |
Coronary Heart Disease caused by a blockage of a major artery | | Diffuse Coronary Heart Disease |
Arrow in photo on left indicates where a bypass procedure would be performed. Circled areas in photo on right would be where Cardio Vascu-GrowTM would be injected to induce new blood vessel growth in this area of the heart that can not be treated by bypass or stenting procedures, due to the small size of the coronary arteries. In diffuse coronary artery disease the vessels are too small to be bypassed. (Source – Thomas Stegmann, M.D.)
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 7 |
The Muscle Tissue Group
Vascular Disease of the Legs
An Estimated 8 Million Americans Suffer From Peripheral Vascular Disease
In the United States, an estimated 8 million Americans suffer from peripheral vascular disease (PVD). This occurs when blood vessels which feed the legs become blocked by atherosclerotic lesions, which results in pain while walking. This can evolve to more severe forms of the disease where circulation to the foot and leg become so reduced that amputation of the toes, foot, or leg is the only alternative. This disease increases in prevalence as people age or in patients with diabetes. CVBT’s objective for this medical indication is to attempt to grow new blood vessels with a different formulation of Cardio Vascu-GrowTM in the legs of these patients, with a drug candidate designed to re-establish blood flow to their ischemic leg muscles.
To achieve this objective, CVBT in the last year has successfully completed all of the pre-clinical testing of our Cardio Vascu-GrowTM drug candidate for PVD to initiate the first trials in humans with this disease. This has included extensive testing of the protein in an animal model of peripheral vascular disease. As shown in the accompanying figures, CVBT designed a study in an animal efficacy model of PVD, in which Cardio Vascu-GrowTM (FGF-1) was shown to stimulate new blood vessel growth in rabbit legs with restricted blood flow. This new vessel growth resulted in an increase capillary density and blood flow into the leg.
Design of an Animal Model of Peripheral Vascular Disease
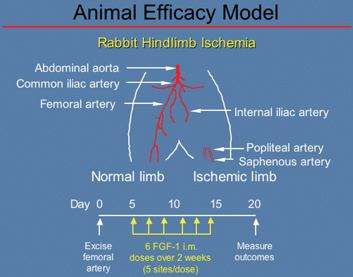
Animal model of peripheral vascular disease in which Cardio Vascu-GrowTM, formulated for use in the leg, was successfully evaluated. The femoral artery is removed to create ischemia in the affected leg of the animal and then the drug candidate is injected over a 2 week period. (Source–K. Thomas, Ph.D., CVBT)
8
The Muscle Tissue Group
Leg Angiograms
| | | | |
Baseline Control (Day 5) (25 + 0.4 vessels) | | 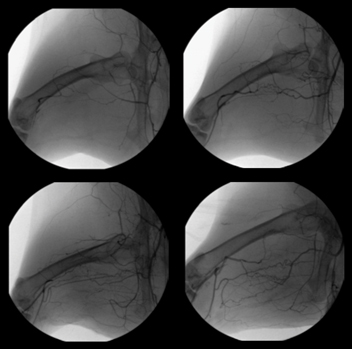 | | Vehicle Control (Day 20) (46 + 4 vessels) |
| | | |
1.0 mg/kg/dose (Day 20) (63 + 5 vessels*) | | | 10 mg/kg/dose (Day 20) (63 + 7 vessels*) |
* p<0.05 vs. vehicle control
FGF-1 increases number of angiographically visible vessels
Cardio Vascu-GrowTM for PVD stimulates new vessel growth in an animal model of peripheral vascular disease. 20 days following injection of Cardio Vascu-GrowTM, increased blood vessel growth is statistically significant over control animals at both doses of drug tested. (Source–American Cardiovascular Research Institute, CVBT)
FGF-1 Angiogenic Response
Adductor Muscle
| | | | |
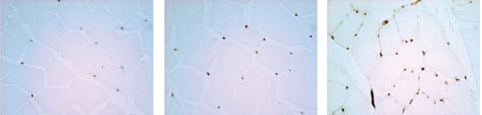 |
| | |
| | Gastrocnemius Muscle | | |
| | | | |
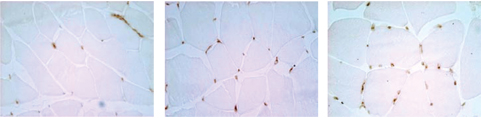 |
| | |
Vehicle Control (Day 20) | | 1.0 mg/kg/dose (Day 20) | | 10 mg/kg/dose (Day 20) |
FGF-1 increases capillary number and density
Cardio Vascu-GrowTM increases blood vessel density in ischemic leg muscles. A dose-dependent increase in both the number and density of new vessels is seen over control animals. (Source–American Cardiovascular Research Institute, CVBT)
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 9 |
The Muscle Tissue Group
Cardio Vascu-GrowTM for PVD Increases Vessel
Density and Blood Flow in an Animal Model of PVD
Ischemic Hindlimb
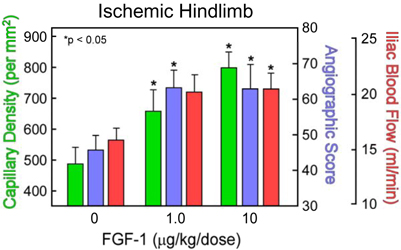
Summary of pre-clinical testing of Cardio Vascu-GrowTM in a rabbit model of PVD. The protein increases 3 measurements of efficacy in this model. (Source–American Cardiovascular Research Institute, CVBT)
After the successful testing of our drug candidate for PVD in these efficacy animal models of peripheral vascular disease, additional animal testing was performed in the last year to measure the relative toxicity and clearance of Cardio Vascu-GrowTM after administration to the leg muscles of animals. These tests were successfully completed and an application to the U.S. FDA to begin testing in U.S. patients this year is now being prepared. International experts who have participated in previous clinical trials in PVD at Duke University, Durham, N.C. have agreed to work with CVBT on our first clinical trial in this indication.
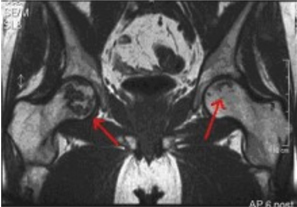
Patients with a symptom which is known as “claudication”, or pain upon walking, are the target group for our first clinical trial. As can be seen in the following figure these patients can have significant blockages in arteries either in their upper or lower leg (red arrows).
Once the location of the blocked arteries is visualized by angiograms or other forms of molecular imaging, injections of Cardio Vascu-GrowTM for PVD are administered to the affected muscles.
In collaboration with vascular surgeons, CVBT has designed a clinical protocol which will test Cardio Vascu-GrowTM for PVD in a patient population with leg pain or intermittent claudication. A key clinical readout in this first trial will be the length of time the patients can walk on a treadmill, both before and after treatment.
10
The Muscle Tissue Group
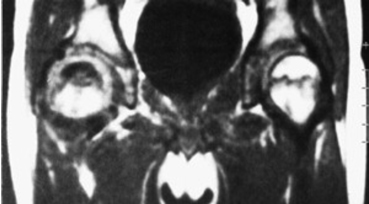
Peripheral Artery Lesions
Peripheral MR Angiograms
|
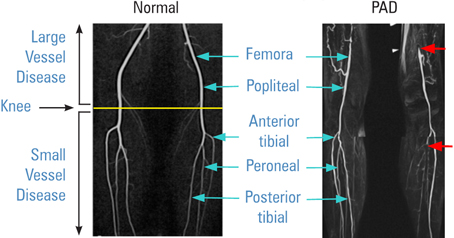 |
|
Leg muscle ischemia can be the result of blockage in larger above the knee and/or smaller below the knee arteries. (Source–CVBT) |
Chronic Back Pain and Lumbar Ischemia
An Estimated 26 Million Americans Suffer From Chronic Lower Back Pain
Chronic back pain, estimated in published reports to affect a staggering 26 million Americans, has recently been linked to blockage of blood vessels supplying the lower back, leading to a condition referred to as lumbar ischemia. European medical researchers have speculated that chronic back pain resulting from blockage of blood vessels in the lower back precedes the blockage of coronary arteries in the heart by ten years. CVBT is conducting a proof of concept clinical trial in Eastern Europe and Russia to test whether a drug candidate formulated with Cardio Vascu-GrowTM for lumbar muscle injections can successfully treat chronic back pain. This trial is being run by the contract clinical research organizatio bioRASI, a group with extensive clinical trial experience in this region of the world. If the results are positive, CVBT plans to then file an application to the U.S. FDA to allow a study to begin in chronic back pain patients in the U.S.
Lumbar Arteries
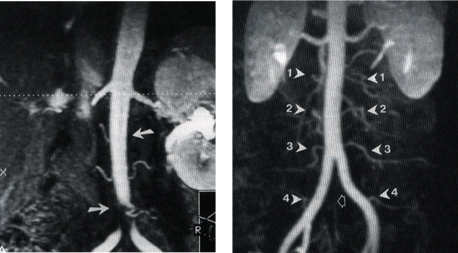
These angiograms illustrate the difference between normal and occluded lumbar arteries. On the left, as denoted by the arrowheads, all lumbar and middle sacral arteries are normal, whereas on the right several arteries are narrowed and the middle sacral artery is totally occluded. (Source– 2005 Conference on Regenerative Medicine, Maui, Hawaii)
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 11 |
Wound Healing Tissues
Wound Healing Tissues
An Estimated 20 Million Americans Suffer From Diabetic Leg Ulcers, Bed Sores and Surgical Wounds
Healing of FGF1-Treated Wounds in Diabetic Mice
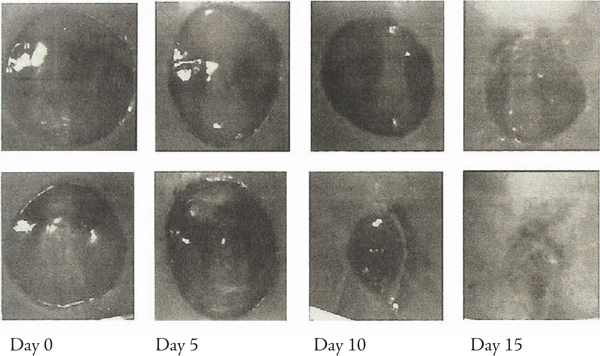
The top row shows untreated wounds in diabetic mice. The bottom row shows wounds treated with FGF-1. It is readily apparent at Days 10-15 that FGF-1 has accelerated the healing process. (Source –Journal of Investigative Dermatology104:850-855, 1995)
Wound healing requires angiogenesis for the healing process to occur. People with diminished healing abilities, including diabetics and the elderly, could greatly benefit from an agent that could stimulate the growth of new blood vessels in the wounded area.
This fact makes this indication an attractive target for treatment with a wound healing drug candidate containing Cardio Vascu-GrowTM. Earlier work has shown FGF-1 to be a potent stimulator of wound healing in animal models of wound healing performed in diabetic mice.
In the last year CVBT has completed its own pre-clinical testing of a viscous formulation of Cardio Vascu-GrowTM, especially developed for topical application, in animal models of wound healing. Using diabetic mice and following similar procedures as in previous investigations, CVBT has confirmed that Cardio Vascu-GrowTM potently stimulates dermal wound healing.
12
Wound Healing Tissues
Cardio Vascu-GrowTM is a Potent Stimulator of Wound Healing in Diabetic Mice
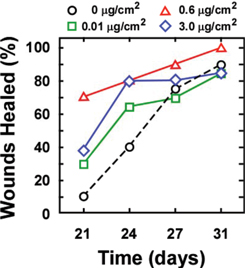
Twenty one days after treatment of wounds with a viscous formulation of Cardio Vascu-GrowTM almost 70% of the animals’ wounds have healed when treated with the 0.6 ug/cm2 dose of the growth factor, versus only 10% healing of the control animals’ wounds. (Source–CVBT)
Following the completion of efficacy testing in diabetic mice, CVBT then performed extensive animal toxicity testing in which high doses of Cardio Vascu-GrowTM, formulated for wound healing, were administered to experimental wounds. No adverse events were seen in these toxicity tests and the protein had no abnormal effect on the integrity or histology of the new dermal tissue that was generated after treatment with Cardio Vascu-GrowTM.Of clinical significance, when clearance studies were performed as part of these toxicity tests, no discernible quantity of Cardio Vascu-GrowTM could be detected in the blood circulation of these animals after relatively high doses of Cardio Vascu-GrowTM were placed on the wound surface.Because of this finding, CVBT will not have to monitor for potential adverse events in humans that may be anticipated if high amounts of Cardio Vascu-GrowTM were to enter the blood stream, including possible growth of new blood vessels in the eye.
An Investigational New Drug (IND) application was submitted to the FDA and CVBT was authorized by the FDA to test Cardio Vascu-GrowTM formulated for wound healing in patients with two types of impaired wound healing. This includes patients with diabetic foot ulcers and patients with venous stasis ulcers, open wounds that occur primarily on the calf region of the legs. An internationally recognized wound healing expert has agreed to be the Principal Investigator of CVBT’s first clinical trial, which will be conducted at the University of Pittsburgh Medical Center in Pittsburgh, PA.
Prior to initiation of this trial, clinical training with specialized digital photography to measure the area of the wounds will be provided by an industry leader in this area. Institutional Review Board (IRB) approval has been obtained in Pittsburgh to start the trial and patient recruitment is underway. As there are large numbers of potential patients, this clinical trial could progress rapidly and it is conceivable that this could be our first FDA approved drug for commercial sale. A summary of this first wound healing trial is given below.
Phase I Clinical Trial in Dermal Wound Healing
Title: A Phase I, Open Label, Single Dose, Dose Response, Pilot Study to Evaluate the Safety and Tolerability of Human Fibroblast Growth Factor-1 (FGF-1) in Patients with Diabetic or Venous Stasis Ulcers
Study Objectives:
| • | | Evaluate topical safety of Cardio Vascu-GrowTM |
| • | | Determine amount, if any, of the drug that enters the circulation |
| • | | A total of 4 diabetic foot ulcer and 4 venous stasis ulcer patients will be enrolled |
| • | | Digital photography will give an efficacy readout of effect of Cardio Vascu-GrowTM on wound healing |
In addition to this medical indication, CVBT is also actively exploring use of this formulation of Cardio Vascu-GrowTM in the areas of bed sores and healing of surgical wounds.
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 13 |
The Nerve Tissue Group
The Nerve Tissue Group
CVBT has focused primarily on developing new treatments that can stimulate new blood vessel growth into ischemic muscle tissues. However, blocked blood vessels can also severely injure other tissue types including nervous tissues. Stroke results from the same basic atherosclerotic process that causes coronary artery disease, but it is occurring in vessels that supply brain cells with blood.
Brain
An Estimated 6.2 Million Americans Suffer From Acute and Chronic Stroke
When blood flow is restricted or blocked in blood vessels supplying the brain or in vessels located within the brain, stroke results. Stroke is the number three cause of death in the United States, and an estimated 6.2 million people suffer from the often devastating long term effects of strokes in this country with 700,000 new strokes occuring every year. Cardio Vascu-GrowTM, in our preclinical animal studies, reduced the size of damaged brain tissue after an acute stroke and is currently being developed as a potential new therapy to treat this condition.
Patient with Cerebral Stroke

The outer red circle indicates ischemic brain tissue and is the target area for healing by a Cardio Vascu-GrowTM infusion. (Source– CVBT)
FGF Decreases Volume of Stroke in Rats
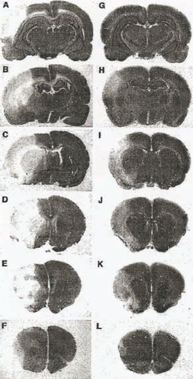
The untreated animal brain slices (A-F) show damaged areas of brain in white, indicating volume of strokes. The animal brain slices (G-L) were treated with FGF and show a significant decrease in volume of strokes (white areas). (Source – Brain Research 818 (1999) 140-146)
Numerous reports in the scientific literature indicate that FGF is a potent neuroprotective agent and can stimulate the growth of nerve cells. This is a new medical indication for Cardio Vascu-GrowTM. We are attempting to address the question of growing new nerve cells with our stroke drug candidate, instead of growing new blood vessels. This additional property of Cardio Vascu-GrowTM indicates that it may have utility in medical conditions where nerve loss has occurred such as neuropathy (nerve pain) and recovery from chronic stroke. In a recently completed stroke recovery animal study, we noted a significant decrease in the size of the stroke area in animals treated for 7 and 28 days with our stroke drug candidate containing Cardio Vascu-GrowTM, indicating a repopulating of the infarcted stroke area with new neurons. This new indication will be explored cautiously, but as this is an unmet medical need in this country, we believe a drug that could successfully treat this condition would be a significant medical advancement.
14
The Intestinal Tissue Group
Intestinal Tissue
An Estimated 1.5 Million Americans Suffer From Gastrointestinal Ischemia
Cardiovascular disease in the intestinal tract can cause severe pain in people, even after the simple act of eating a meal. A new potential application for Cardio Vascu-GrowTM is growing new blood vessels in the digestive tract to address the medical problem of intestinal ischemia and pancreatitis. Smaller blood vessels which feed the intestines or pancreas can become blocked by the same atherosclerotic process that affects the coronary arteries in the heart. This results in restricted blood flow to the digestive tract which can lead to pain, reduced absorption of nutrients, and diminished clearance of waste. It was reported at the 2005 Regenerative Medicine Conference in Maui, Hawaii, that this disease will affect, to some degree, almost everyone over the age of 70. Given the aging of populations throughout the western world, the number of people affected by this disease will only get larger. As little can be done currently for sufferers of this disease, Cardio Vascu-GrowTM has the potential to make a material difference for these patients. CVBT has just begun its research in this area. However, we believe this medical indication has the potential to be a significant marketplace for CVBT.
Intestinal Ischemia Due to Vessel Occlusion

A severe example of intestinal ischemia where large portions of the intestine (bright red areas) have been injured or destroyed. (Source– 2005 Conference on Regenerative Medicine, Maui, Hawaii)
Kidney Tissue
An Estimated 3 Million Americans Suffer From Hypertensive Renal Disease
This disease results from the blockage of arteries which supply the kidneys. In animal models of ischemic kidney disease, FGF-1 has been shown to stimulate new blood flow which results in an increased perfusion of the kidneys, and a decrease in the ischemic injury. CVBT will take a cautious approach to this disease as the kidney is a complex organ, however, there is currently no therapy that will reverse this disease.
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 15 |
Femoral Bone Ischemia
Femoral Bone Ischemia/Bone Repair
Close to 1 Million Fracture Repair Surgeries are Performed in the U.S. Each Year
Animal studies have shown that FGF stimulates bone repair. Mackenzie and coworkers reported (Plast Reconst Surgery, 2001) that recombinant human FGF-1 delivered in a fibrin carrier regenerates bone. In their rabbit model, FGF- 1 promoted a significant and unexpected increase in bone formation response. Their study is the first report on FGF-1 for bone formation in a critical-sized, long bone defect.
In addition, certain degenerative bone diseases result from an inadequate blood supply to the bone, as exemplified by ischemic bone necrosis of the hip. Research gels and collagen scaffolds can supply a slow and steady stream of growth factors to the damaged tissue. Could Cardio Vascu-GrowTM help increase bone formation and bone density? Would it be a valuable adjunct therapy to orthopedic surgeries, helping to speed recovery of the fracture? CVBT plans to begin testing of Cardio Vascu-GrowTM in animal models of bone regeneration and bone repair.
X-ray analysis of a patient suffering from necrosis of the head of the femur. The red arrows point to bone necrosis which is evident in the bone depicted in the lower panel. (Source–Thomas Stegmann, CVBT)
16
Islet Cell Regeneration in Diabetes
Islet Cell Regeneration in Diabetes
4.2 Million Diabetics are Dependent on Insulin
Recent articles in the medical literature indicate that selective growth factors have the potential to regenerate beta cells in the pancreas of diabetics. In particular, a recent article in Scientific American (December 2005) reported on researchers at the University of Alberta in Edmonton that are administering growth factors to stimulate the patients’ pancreas to grow its own beta cells. These researchers showed that these newly formed beta cells make appropriate concentrations of active insulin, and release the hormone in response to rising glucose levels.
The expansion of beta cell progenitors can also be driven through cell surface receptors that are potently activated by FGF-1 or Cardio Vascu-GrowTM. It is thus feasible to contemplate that FGF-1 could mimic or improve upon the success others have seen with other growth factors. Regenerating the body’s own insulin-producing cells has not only direct application to the 800,000 type I diabetics in the U.S. who suffer beta cell loss from an early age, but also to the 3.4 million (out of a total of 18 million) type II diabetics who become insulin dependent due to a phenomenon referred to as “pancreatic exhaustion.”
New Molecular Imaging Technologies to Diagnose and Manage Patients with Ischemia
It is clear that newer imaging modalities will be crucial to the proper diagnosis and management of the large class of patients who suffer from poor perfusion and ischemic disease. This was evident from a recent lecture given at the International Regenerative Medicine Foundation’s 3rd annual conference held in Kauai, Hawaii on February 20-22, 2006. In that lecture, Dr. David Rollo, Chief Medical Officer of Philips Medical Systems, a company which develops, manufactures and markets advanced imaging equipment and agents, spoke on the application of diagnostic imaging to assess the efficacy of FGF-1 for the diagnosis, treatment and management of ischemia. FGF-1 is the protein component of Cardio Vascu-GrowTM.
Dr. Rollo’s talk focused on the recent development by Philips of imaging agents which can specifically visualize, at high sensitivity, ischemic areas of the heart, brain and other tissues. As an example of a powerful new imaging agent, Dr. Rollo presented data on Tc-99m ECDG, which was shown to light up ischemic areas in the heart, as shown in the figure below. Ischemia in these tissues results from poor blood perfusion which is a consequence of blocked arteries. Dr. Rollo indicated that as FGF-1 is a potent inducer of new blood vessel growth, the new imaging agents could be utilized to first diagnose, and then assess the effect of FGF-1 given to patients with ischemic diseases. CVBT hopes to be able to utilize molecular imaging technologies to enhance the therapy it is targeting for the millions of patients with ischemic diseases.
| | | | |
| I-123 FAA ISCHEMIA STUDY | | 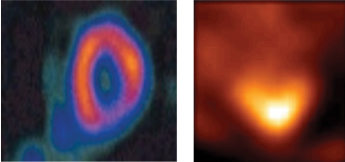 | | Tc-99m ECDG VIABILITY 27% Myocardial mass ischemic |
Two imaging agents visualizing ischemia in the heart. In particular, the agent Tc-99m ECDG (right panel) shows great promise in imaging cardiac ischemia. (Source–Dr. David Rollo, 3rd Annual Conference of the International Regenerative Medicine Foundation, Kauai, Hawaii, February 2006)
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 17 |
Chairman’s Message to Shareholders
At CardioVascular BioTherapeutics, Inc. (CVBT) we believe our proprietary drug candidates, in which Cardio Vascu-GrowTM is the active ingredient, represent a potential major medical breakthrough that will change the lives of tens of millions of people. We believe our drug candidates reduce pain and suffering and increase quality and length of life for millions of people. These people are our motivation and why we do not consider failure an option.
Stakeholders in CVBT’s Success
In all enterprises there are stakeholders, those who have a vested interest in the enterprise’s success. CVBT like all companies has traditional stakeholders, including shareholders, employees and suppliers. CVBT however, has special stakeholders; the millions of people suffering from diseases we believe we can treat.
Lack of blood flow to a tissue or organ is a disease that is responsible for over half of the deaths in the United States, Europe, Asia and the Middle East. Whether it is clogged arteries in the heart, brain, kidney, intestine, or complications from diabetes, each symptom is basically the same disease. We believe, based upon evidence, our drug grows new blood vessels that by-pass blockages. These new blood vessels re-establish the flow of blood, diminishing damage to the tissue or organ, and could save millions of people from pain, suffering and potential death from these diseases.
The team at CVBT is highly committed to achieving success. We have a duty and an obligation to all our stakeholders to succeed. Each of us has been touched by someone whose life has suffered due to these diseases. At CVBT, we have enjoyed meeting people who have been treated in the clinical trials. These are people who tell us how their lives have been saved and/or improved by Dr. Stegmann’s discovery. These people intensify our commitment to succeed.
Methods to achieve our objectives
To understand what we are trying to achieve, it is important to understand that we do not view the space CVBT fills from the traditional biotechnology perspective.
Why we are different
Why are we not following the traditional biotechnology formula? I have been told that 99 out of 100 biotechnology companies fail. As a businessman that sounds like poor odds to me. Why would I choose to follow an established formula that fails 99% of the time? As a rational businessman who has built successful businesses before, I would rather take normal business risk, which I believe we can succeed with, rather than follow blindly a business formula that has a 99% failure rate.
How are we different?
We are not following the traditional biotechnology business model espoused by most biotechnology experts. We are focused on making sound business decisions, not just taking the advice of the talking heads. We are not chasing a theory. Our science is tested and we now endeavor to prove it to the regulators. We have raised over $52 million dollars carefully from investors who believe in what we are doing. We have not turned over control of the company to venture capitalists who focus on short-term gain and are not focused on our stakeholders or their long term aspirations. Our company is owned and controlled by people who believe in our mission. We do not have the bureaucratic management that some big pharma companies deal with, so we are nimble and able to respond quickly to challenges. We are in this for the long run and do not plan to walk away from our life’s greatest purpose.
It is amazing to me how many people think we need to sell out or join up with others who have a track record of failure. If we believe in CVBT’s drug candidates and the medical benefits they bring to millions of people, why would we entrust its success to anyone else? We are going to advance our company and our drug as we believe is best for all our stakeholders. This will be based on rational business logic and financial discipline, not on a failed formula.
Progress of CVBT
In this Annual Review we have updated you on the significant scientific and medical progress we have made over the previous 12 months. Our progress advances continuously and I would advise visiting our website www.cvbt.com to obtain further updates.
Our progress is dependent upon many things outside of our control. However, regardless of how frustrating these issues and delays are, they are not important. What is important is that at each new barrier we adjust, modify, overcome and move forward. We deal with whatever problem comes our way and advance towards our objective.
18
Chairman’s Message to Shareholders
Clarity of CVBT Situation and Position
As Chairman of the Board, a major responsibility of mine is to guide the company towards our objectives. To do this, I meet with many people from biotechnology experts to regular individuals, all of whom have advice. What amazes me is the lack of vision and understanding of some of these experts. Many do not know the difference between a protein therapy and a gene therapy. Many believe all cleared FDA Phase I trials are the same. Many are unable to grasp the difference in marketplace opportunity and size. You need to understand all of the aforementioned factors to understand why CVBT is different and appreciate its current position.
In 1992, Dr. Stegmann started his lonely journey to test his idea; could a protein trigger angiogenesis in the human heart to by-pass blockage? There were no venture capitalist or big pharma supporting his view. Even the medical experts believed he was wrong. Dr Stegmann was alone in his belief. However his belief was vindicated when in February 1998 the American Heart Association published his medical research report in their publicationCirculation.
Dr. Wolfgang Priemer, Grant Gordon, Alexander Montano and I were convinced in March of 1998 that Dr. Stegmann’s medical discovery was real. We believed that Dr. Stegmann had discovered a major medical breakthrough and possibly the biggest biotechnology business opportunity yet. In early 1998, we founded CVBT with Dr. Stegmann. For 8 years, with the support of our investors, we have been working towards proving what we believe to be true.
Dr. Ralph Bradshaw, Dr. Jack Jacobs and Dr. Ken Thomas all scientists of molecular biology, studied Dr. Stegmann’s work. After their analysis they joined us in this quest to prove what we believe to be real. Mr. Mike Flaa joined us as our CFO to build a finance and administrative team of excellence.
Many others have completed their own investigation and become believers. Now we must journey through the regulatory authorities’ approval process. Our goal is regulatory approval of the different drug candidates containing Cardio Vascu-GrowTM for multiple medical indications and then its use by the medical public.
I believe within 36 months we will have the required proof for the regulatory authorities. Although three years seems a long time for many of us, it is the final quarter of the championship game and we are winning. I believe I can speak for most of our major shareholders and employees, that if our life’s legacy was saving millions of people’s lives and lessening their pain and suffering, it would be our ultimate business accolade.
CVBT has a passionate team of stakeholders. To us failure is not an option. We must succeed. It is the most important task in our lives.
I thank you for your faith in us, your trust in us, and your commitment. Our management team and major shareholders share with you their faith, their trust and most importantly their commitment to achieve our ultimate goal; to lessen the pain, suffering and death for the millions of people affected by these diseases by obtaining regulatory approval and ultimately the sale of our drug candidates.
Sincerely,
Daniel C. Montano
Chairman, President and CEO
April 24, 2006
| | | | |
(CVBT:OTCBB) | | CardioVascular BioTherapeutics, Inc. | | www.cvbt.com 19 |
Cardio Vascu-GrowTM
Stage of Development for Various Medical Indications
CVBT’s progress in commercializing our drug candidates containing Cardio Vascu-GrowTM.
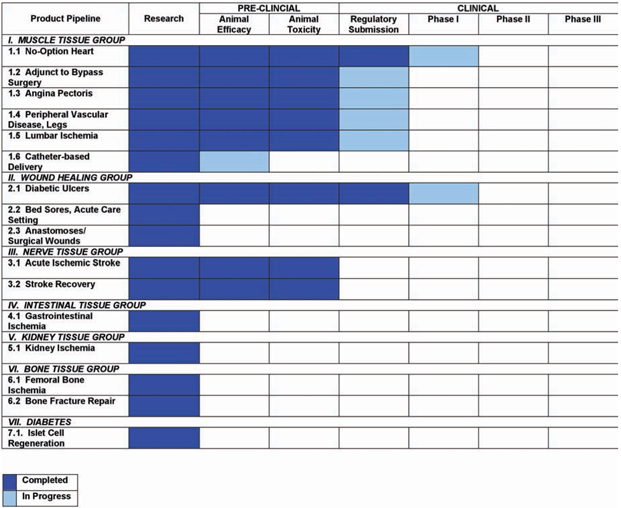
Stages of Drug Development
Research: Before animal testing, an extensive literature search of previous research studies is completed and evaluated.
Animal Efficacy: Before testing in humans, drugs are tested in animals to determine their efficacy or effectiveness.
Animal Toxicity: This animal testing stage evaluates the level of drug necessary for treatment without inducing harm.
Regulatory Submission: After successful animal studies, an “Investigational New Drug” (IND) application is submitted to the U.S. Food and Drug Administration (FDA) to conduct a clinical trial in humans.
Phase I Clinical Trial: A small group of people are given the experimental drug for the first time to evaluate its safety, determine a safe dosage range, and identify side effects.
Phase II Clinical Trial: The experimental drug is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III Clinical Trial: The experimental drug is given to large groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug to be used safely.
This Annual Report may contain forward-looking statements. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by the forward-looking statements. These risks and other factors include those listed under “Risk Factors”, on pages 15 through 25, and elsewhere in our Form 10-K filed with the SEC. We believe that the section entitled “Risk Factors” in our Form 10-K includes all material risks that could harm our business. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “intends,” “potential,” “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. Actual events or results may differ materially.
We believe that it is important to communicate our future expectations to our investors. However, there may be events in the future that we are not able to accurately predict or control and that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. Stockholders are cautioned that all forward-looking statements involve risks and uncertainties, and actual results may differ materially from those discussed as a result of various factors, including those factors described in the “Risk Factors” section of our Form 10-K. Stockholders and others should not place undue reliance on our forward-looking statements.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
For further information on CardioVascular BioTherapeutics, Inc. Investor Relations Contact:
Investor Relations Department
702.391.4999
investorrelations@cvbt.com
Selected Financial Information
As of and for the years ended December 31, 2003, 2004, and 2005
(US Dollars in thousands)
| | | | | | | | | | | | | | | | |
| | �� | 2003 | | | 2004 | | | 2005 | | | From Inception to
December 31, 2005 | |
| Balance Sheet Data | | | | | | | | | | | | | | | | |
Total Cash and Invested Assets | | $ | 1,634 | | | $ | 4,616 | | | $ | 8,812 | | | | | |
Total Assets | | $ | 4,918 | | | $ | 6,710 | | | $ | 9,488 | | | | | |
Convertible Debt | | $ | 8,261 | | | $ | 9,651 | | | $ | 0 | | | | | |
Shareholders’ (Deficit) Equity | | $ | (4,030 | ) | | $ | (5,383 | ) | | $ | 9,189 | | | | | |
| | | | |
| Operating Statement Data | | | | | | | | | | | | | | | | |
Research and Development | | $ | 750 | | | $ | 2,544 | | | $ | 3,091 | | | $ | 9,478 | |
General and Administrative | | $ | 920 | | | $ | 2,337 | | | $ | 7,979 | | | $ | 14,530 | |
Interest Expense | | $ | 672 | | | $ | 3,152 | | | $ | 1,593 | | | $ | 5,490 | |
Interest Income | | $ | 14 | | | $ | 34 | | | $ | 298 | | | $ | 360 | |
Net Loss | | $ | (2,329 | ) | | $ | (8,013 | ) | | $ | (12,366 | ) | | $ | (29,157 | ) |
| | | | |
| Cash flow Data | | | | | | | | | | | | | | | | |
Cash Flow from Operating Activity | | $ | (2,104 | ) | | $ | (4,127 | ) | | $ | (10,731 | ) | | $ | (22,914 | ) |
Cash Flow from Investing Activity | | $ | 4 | | | $ | (118 | ) | | $ | (232 | ) | | $ | (357 | ) |
Cash flow from financing activity | | $ | 3,715 | | | $ | 7,141 | | | $ | 15,108 | | | $ | 31,945 | |
Net Cash Flow | | $ | 1,615 | | | $ | 2,896 | | | $ | 4,145 | | | $ | 8,675 | |
We are a development-stage biopharmaceutical company in preclinical and clinical trials for our drug candidates in which Cardio Vascu-GrowTM is the active ingredient. We do not expect to generate revenues unless and until one of our drug candidates is approved by the FDA and we begin marketing it. We expect to continue to spend significant amounts on the development of our drug candidates. We expect to incur significant commercialization costs when we recruit a domestic sales force. We also plan to continue to invest in research and development for additional applications of Cardio Vascu-GrowTM and to develop new drug delivery technologies.
Most of our expenditures to date have been for research and development activities and general and administrative expenses. We outsource our clinical trials and our manufacturing and development activities to third parties to maximize efficiency and minimize our internal overhead. Manufacturing is outsourced to an affiliated entity. We expense our research and development costs as they are incurred. We began our Phase l FDA clinical trial with our first patient in December 2003. The trial has continued through 2005. We are uncertain as to what we expect to incur in future research and development costs for our clinical activities as these amounts are subject to the outcome of current preclinical activities, management’s continuing assessment of the economics of each individual research and development project and the internal competition for project funding.
We have raised $33,066,946 of net proceeds from inception through December 31, 2005, and we raised an additional $18,597,000 of net proceeds from the sale of Convertible Senior Secured Notes we closed in the first quarter of 2006.
For additional information on the Company’s financial condition and results of operations, see our form 10-K filed with the SEC.
| | | | |
CardioVascular BioTherapeutics, Inc. 1635 Village Center Circle, Suite 250 Las Vegas, NV 89134-0574 www.cvbt.com investorrelations@cvbt.com | |  | | |