Exhibit 99.1

Exhibit 99.1
2007 ANNUAL REVIEW
CardioVascular
BioTherapeutics, Inc.
Treating Cardiovascular Disease with Neo-Angiogenesis
CORONARY HEART DISEASE · PERIPHERAL ARTERIAL DISEASE · STROKE
WOUND HEALING DISORDERS · CHRONIC BACK PAIN

Cover Image: SPECT (Single Photon Emission Computed Tomography) perfusion images taken from the hearts of CHD patients pre-treatment (left) and 90 days after FGF-1141 sole treatment (right). Significant post-treatment improvement of myocardial perfusion was demonstrated by red coloured areas (red: indicating normalized perfusion & blood supply of the heart — in contrast to green & blue: indicating restricted myocardial perfusion).

TABLE OF CONTENTS
President’s Message 2
Company Background 4
Development Program 6
Marketing & Commercialization 13
Appendix 17

PRESIDENT’S MESSAGE
Since its inception in 1998, CardioVascular Bio Therapeutics, Inc. (CVBT) has been dedicated to finding a solution to patients suffering — and even dying — from cardiovascular diseases. As a result and consequence of my personal experience as a cardiovascular surgeon with these patients — forming the majority of all hospitalized patients in the Western world — I strongly felt obligated to develop new and better treatment modalities for patients entrusted to me. The basic reason for this overriding ambition is my experience with the limitations of existing treatment techniques, i.e. balloon angioplasty (PTCA) and bypass surgery (CABG), particularly in patients with advanced coronary heart disease. Quite often in the operating theatre it became apparent that the given conventional treatment for many patients was not efficient or sophisticated enough, to provide them with sufficient long term improvement of their coronary circulation and myocardial perfusion. The induction of neo-angiogenesis by the human (protein) growth factor FGF-1141 became a very real solution — and a new hope for many patients with advanced and diffuse coronary heart disease. Thus, angiogenic treatment of these patients was introduced into the scientific community, and simultaneously into the clinic. Since then, CardioVascular Bio Therapeutics, Inc., has made continuous and significant progress — and furthermore, the Company has added new medical indications for its angiogenic therapy, including wound healing disorders (both diabetic and venous stasis ulcers), peripheral arterial disease (PAD), chronic back pain, and stroke — with all being diseases having in common a lack of adequate arterial perfusion in tissues and organs.
I am very pleased to report substantial progress and advancement for CardioVascular Bio Therapeutics, Inc., in particular for this year — 2007, and look forward to a very promising 2008 and beyond, especially relating to new developments in our company’s Research & Development efforts, as well as new initiatives in our clinical trials. As I mentioned in my letter one year ago — when the last patient of our Coronary Heart Disease trial had received their angiogenic treatment — the Phase I Heart trial was completely enrolled, and this year the final one year post-treatment visits were completed. The independent DSMC (Data and Safety Monitoring Committee)

did not find any significant or unexpected safety concerns, and the activity of the treatment with the Company’s active pharmaceutical ingredient (API), FGF-1141, confirmed the preceding clinical trials in Germany. With these excellent results in Phase I, CardioVascular Bio-Therapeutics, Inc., can now move forward to start the international Phase II Heart trial in patients with severe Coronary Heart Disease (CVBT-141H).
During the last year, the final details of the Phase I Peripheral Arterial Disease (PAD) trial were discussed and outlined, and the Company received authorization from the FDA to start the trial (CVBT-141C), scheduled to commence shortly after the final approvals are obtained from the Institutional Review Boards of our clinical trial sites. Furthermore, the Phase I Wound Healing trial achieved substantial progress with close to 90% of patients now enrolled and treated with no safety concerns noted (CVBT-141B). Finally, the proof-of-concept trial using our drug product for the treatment of chronic back pain related to impaired arterial perfusion (CVBT-141E) This actively under way in Eastern Europe.
In addition, the Company is continuously performing basic scientific research through its specialized and renowned scientists and consultants. This research includes such areas as diabetes mellitus, heart failure, osteoporosis, and — in particular — stroke. I am very pleased that our pre-clinical research into stroke is producing very encouraging results, in both acute and chronic animal models of stroke. As is common knowledge, despite many medical efforts, the treatment options for patients suffering an acute stroke, or presenting in a chronic stroke setting, have been very limited for many years, with no causative treatment
available. With FGF-1141, CardioVascular Bio Therapeutics, Inc., offers a possible new dimension into an effective treatment for stroke victims, which will offer them a possible new hope.
In surprising contrast to the substantial progress made in the field of clinical trials and scientific research, CardioVascular Bio Therapeutics, Inc., met with a decrease of its share price during the course of the year 2006. There may be different reasons for this effect, however one explanation may be that the fundamental difference between
“protein therapy” (as with FGF-1141) and “gene therapy” is still not well understood by investment bankers and institutional investors (and their analysts, too!). CardioVascular Bio Therapeutics, Inc., therefore, has increased its efforts to “educate” the public regarding this important distinction — by reminding them of the proven potential and efficacy of its protein therapy, FGF-1141. We have included on the Company’s web-site (www.cvbt.com), a number of published papers describing that difference between protein and gene therapy.
Being traded only on the bulletin board (OTC-BB, symbol: CVBT.OB) also may have been part of the rather disappointing market valuation and market acceptance. Enforced and supported by the given fact that many of CVBT’s shareholder are based in Europe, the management decided to list CVBT’s shares at the London Stock Exchange. This goal is set to become a reality in 2007. CardioVascular Bio Therapeutics, Inc., being Sarbanes-Oxley compliant, also was forced to increase its administrational efforts to stay current with such compliance.
I would like to cordially thank you — for your continuous trust in the management of CardioVascular Bio- Therapeutics, Inc., and in the committed CVBT staff, and for your ongoing support. I thank all members of CVBT’s scientific and administrative staff— for their passionate and steadfast daily work.
We all know that CVBT is owned by its shareholders — you are CVBT. And we all agree that we have to fulfill our mission by obtaining regulatory approval for our drug products – thus, giving a new chance of life for critically ill patients suffering from cardiovascular diseases.
Thomas J. Stegmann, M.D., FETCS
Co-Founder, Co-President, Chief Medical Officer
CardioVascular Bio Therapeutics, Inc.
COMPANY BACKGROUND
CardioVascular Bio Therapeutics is a biopharmaceutical company focused on developing new drugs for the treatment of cardiovascular diseases where the growth of new blood vessels can improve the outcome for patients with these diseases.
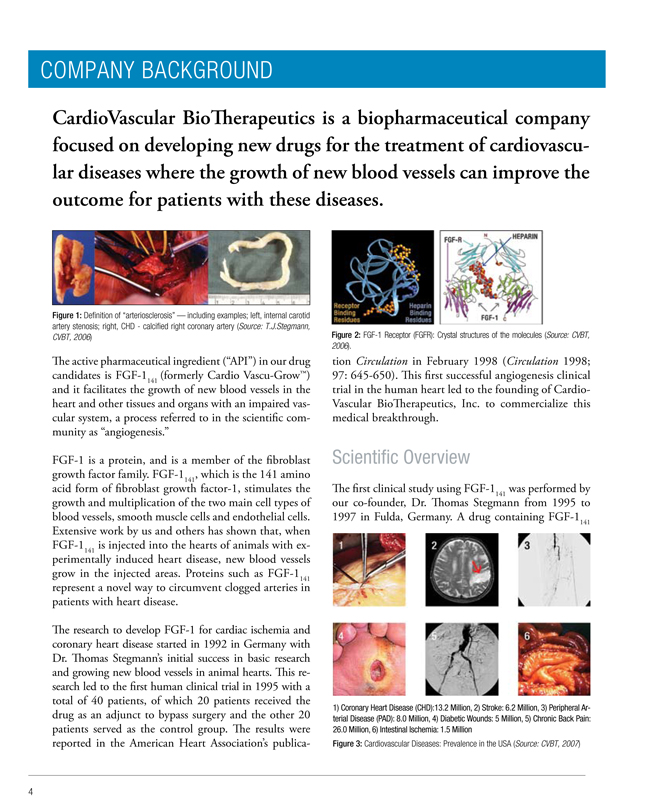
Figure 1: Definition of “arteriosclerosis” — including examples; left, internal carotid artery stenosis; right, CHD—calcified right coronary artery (Source: T.J.Stegmann, CVBT, 2006)
The active pharmaceutical ingredient (“API”) in our drug candidates is FGF-1141 (formerly Cardio Vascu-Grow™) and it facilitates the growth of new blood vessels in the heart and other tissues and organs with an impaired vascular system, a process referred to in the scientific community as “angiogenesis.”
FGF-1 is a protein, and is a member of the fibroblast growth factor family. FGF-1141, which is the 141 amino acid form of fibroblast growth factor-1, stimulates the growth and multiplication of the two main cell types of blood vessels, smooth muscle cells and endothelial cells. Extensive work by us and others has shown that, when FGF-1141 is injected into the hearts of animals with experimentally induced heart disease, new blood vessels grow in the injected areas. Proteins such as FGF-1141 represent a novel way to circumvent clogged arteries in patients with heart disease.
The research to develop FGF-1 for cardiac ischemia and coronary heart disease started in 1992 in Germany with Dr. Thomas Stegmann’s initial success in basic research and growing new blood vessels in animal hearts. This research led to the first human clinical trial in 1995 with a total of 40 patients, of which 20 patients received the drug as an adjunct to bypass surgery and the other 20 patients served as the control group. The results were reported in the American Heart Association’s publication
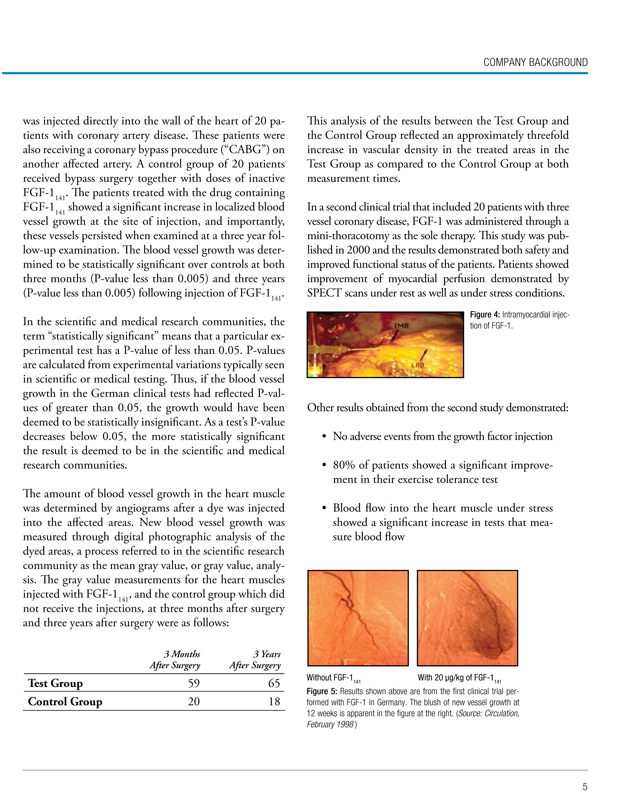
Figure 2: FGF-1 Receptor (FGFR): Crystal structures of the molecules (Source: CVBT, 2006).
Circulation in February 1998 (Circulation 1998; 97: 645-650). This first successful angiogenesis clinical trial in the human heart led to the founding of CardioVascular Bio Therapeutics, Inc. to commercialize this medical breakthrough.
Scientific Overview
The first clinical study using FGF-1141 was performed by our co-founder, Dr. Thomas Stegmann from 1995 to 1997 in Fulda, Germany. A drug containing FGF-1141
1) Coronary Heart Disease (CHD):13.2 Million, 2) Stroke: 6.2 Million, 3) Peripheral Arterial Disease (PAD): 8.0 Million, 4) Diabetic Wounds: 5 Million, 5) Chronic Back Pain: 26.0 Million, 6) Intestinal Ischemia: 1.5 Million
Figure 3: Cardiovascular Diseases: Prevalence in the USA (Source: CVBT, 2007)
COMPANY BACKGROUND
was injected directly into the wall of the heart of 20 patients with coronary artery disease. These patients were also receiving a coronary bypass procedure (“CABG”) on another affected artery. A control group of 20 patients received bypass surgery together with doses of inactive FGF-1141. The patients treated with the drug containing FGF-1141 showed a significant increase in localized blood vessel growth at the site of injection, and importantly, these vessels persisted when examined at a three year follow-up examination. The blood vessel growth was determined to be statistically significant over controls at both three months (P-value less than 0.005) and three years (P-value less than 0.005) following injection of FGF-1141.
In the scientific and medical research communities, the term “statistically significant” means that a particular experimental test has a P-value of less than 0.05. P-values are calculated from experimental variations typically seen in scientific or medical testing. Thus, if the blood vessel growth in the German clinical tests had reflected P-values of greater than 0.05, the growth would have been deemed to be statistically insignificant. As a test’s P-value decreases below 0.05, the more statistically significant the result is deemed to be in the scientific and medical research communities.
The amount of blood vessel growth in the heart muscle was determined by angiograms after a dye was injected into the affected areas. New blood vessel growth was measured through digital photographic analysis of the dyed areas, a process referred to in the scientific research community as the mean gray value, or gray value, analysis. The gray value measurements for the heart muscles
injected with FGF-1141, and the control group which did not receive the injections, at three months after surgery and three years after surgery were as follows:
3 Months After Surgery
3 Years After Surgery
Test Group 59 65
Control Group 20 18
This analysis of the results between the Test Group and the Control Group reflected an approximately threefold increase in vascular density in the treated areas in the Test Group as compared to the Control Group at both measurement times.
In a second clinical trial that included 20 patients with three vessel coronary disease, FGF-1 was administered through a mini-thoracotomy as the sole therapy. This study was published in 2000 and the results demonstrated both safety and improved functional status of the patients. Patients showed improvement of myocardial perfusion demonstrated by SPECT scans under rest as well as under stress conditions.
Figure 4: Intramyocardial injection of FGF-1.
Other results obtained from the second study demonstrated:
• No adverse events from the growth factor injection
• 80% of patients showed a significant improvement in their exercise tolerance test
• Blood flow into the heart muscle under stress showed a significant increase in tests that measure blood flow
Without FGF-1141 With 20 µg/kg of FGF-1141
Figure 5: Results shown above are from the first clinical trial performed with FGF-1 in Germany. The blush of new vessel growth at 12 weeks is apparent in the figure at the right. (Source: Circulation, February 1998 )
DEVELOPMENT PROGRAM
Severe Coronary Heart Disease: Surgical Delivery of FGF-1
We have completed the enrollment and treatment of all patients specified in our Phase I clinical trial protocol which was closely patterned on the previous studies performed by Dr. Stegmann in Germany. A total of 21 patients were treated at six participating U.S. medical centers. No significant, unexpected adverse effects were attributable to the drug, and most patients saw a significant decrease in their symptoms of angina or chest pain. The early results from this trial were of such promise, that one of the clinical trial sites, The University of Cincinnati Medical Center, was featured in several media segments including a report by the Cincinnati Enquirer, and a report on the national ABC Nightly News program. A full study report for this trial will be submitted to the FDA.
As shown in Figure 6, which summarizes the data of patients treated in the U.S. trial through 2006, there was a marked improvement (i.e. decrease) in angina symptoms. At both 6 and 12 weeks there was a highly significant decrease in the severity of the patient’s chest pain with most patients falling from severe Class III and Class IV angina scores to more manageable Class I and II scores. These results parallel the results seen in the previous German clinical trials.
We are currently collecting the final safety data at 12 month follow-up visits on our patients who were treated in our Phase I clinical trial. We filed a report with the FDA listing all adverse events or other safety issues observed in this trial up to and including the 12 week follow-up visit of all patients who participated in this trial.
CSS ANGINAL CLASSIFICATION CHANGES SEEN WITH FGF-1 INTRAMYOCARDIAL INJECTION
ANGINAL CLASS (1-4)
4 3 2 1
P<0.0001*
P<0.0001*
DATES OF EXAMINATION
Figure 6: Reduction in chest pain as measured by an angina questionnaire administered to no-option heart patients before surgery (screening) and at 6 and 12 weeks after receiving FGF-1. (Source: Lynne Wagner, M.D., University of Cincinnati, 2005)
We have finalized the development of a clinical protocol for our Phase II study, where we will test our drug candidate in an expanded patient population in both U.S. and foreign clinical trial sites.
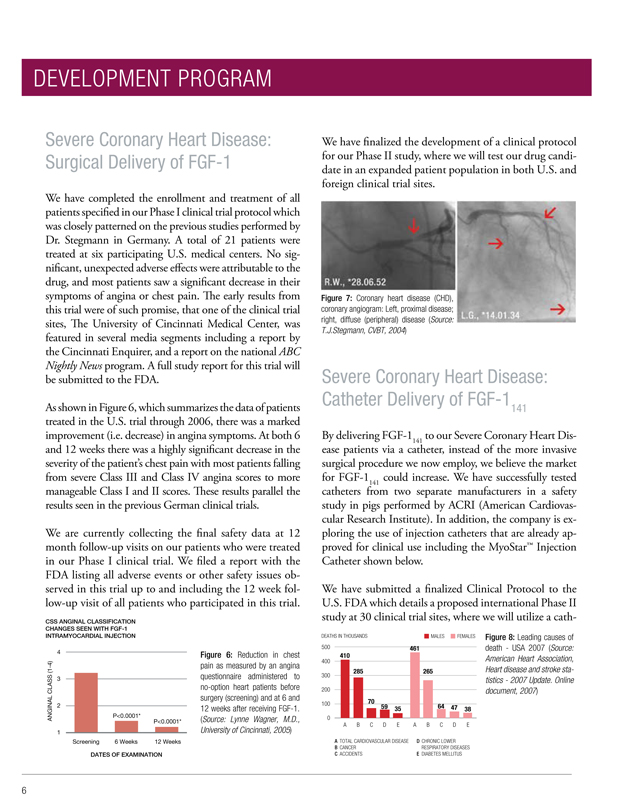
Figure 7: Coronary heart disease (CHD), coronary angiogram: Left, proximal disease; right, diffuse (peripheral) disease (Source: T.J.Stegmann, CVBT, 2004)
Severe Coronary Heart Disease: Catheter Delivery of FGF-1141
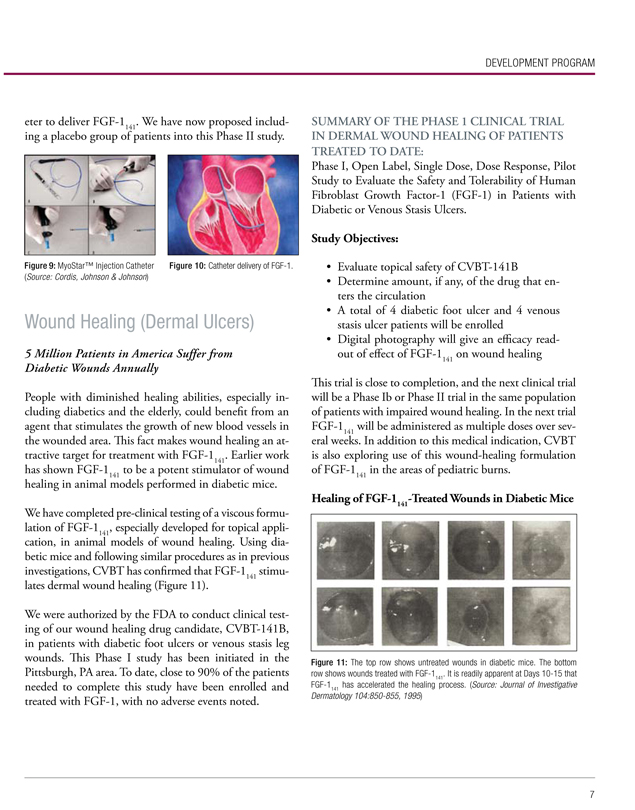
By delivering FGF-1141 to our Severe Coronary Heart Disease patients via a catheter, instead of the more invasive surgical procedure we now employ, we believe the market for FGF-1141 could increase. We have successfully tested catheters from two separate manufacturers in a safety study in pigs performed by ACRI (American Cardiovascular Research Institute). In addition, the company is exploring the use of injection catheters that are already approved for clinical use including the MyoStar™ Injection Catheter shown below.
We have submitted a finalized Clinical Protocol to the U.S. FDA which details a proposed international Phase II study at 30 clinical trial sites, where we will utilize a catheter
DEATHS IN THOUSANDS
MALES FEMALES
500 400 300 200 100 0
461 410 285 265 70 59 35 64 47 38
A B C D E A B C D E
A TOTAL CARDIOVASCULAR DISEASE
B CANCER
C ACCIDENTS
D CHRONIC LOWER RESPIRATORY DISEASES
E DIABETES MELLITUS
Figure 8: Leading causes of death—USA 2007 (Source: American Heart Association, Heart disease and stroke statistics—2007 Update. Online document, 2007)
to deliver FGF-1141. We have now proposed including a placebo group of patients into this Phase II study.
Figure 9: MyoStar™ Injection Catheter (Source: Cordis, Johnson & Johnson)
Figure 10: Catheter delivery of FGF-1.
Wound Healing (Dermal Ulcers)
5 Million Patients in America Suffer from Diabetic Wounds Annually
People with diminished healing abilities, especially including diabetics and the elderly, could benefit from an agent that stimulates the growth of new blood vessels in the wounded area. This fact makes wound healing an attractive target for treatment with FGF-1141. Earlier work has shown FGF-1141 to be a potent stimulator of wound healing in animal models performed in diabetic mice.
We have completed pre-clinical testing of a viscous formulation of FGF-1141, especially developed for topical application, in animal models of wound healing. Using diabetic mice and following similar procedures as in previous investigations, CVBT has confirmed that FGF-1141 stimulates dermal wound healing (Figure 11).
We were authorized by the FDA to conduct clinical testing of our wound healing drug candidate, CVBT-141B, in patients with diabetic foot ulcers or venous stasis leg wounds. This Phase I study has been initiated in the Pittsburgh, PA area. To date, close to 90% of the patients needed to complete this study have been enrolled and treated with FGF-1, with no adverse events noted.
SUMMARY OF THE PHASE 1 CLINICAL TRIAL IN DERMAL WOUND HEALING OF PATIENTS TREATED TO DATE:
Phase I, Open Label, Single Dose, Dose Response, Pilot Study to Evaluate the Safety and Tolerability of Human Fibroblast Growth Factor-1 (FGF-1) in Patients with Diabetic or Venous Stasis Ulcers.
Study Objectives:
• Evaluate topical safety of CVBT-141B
• Determine amount, if any, of the drug that enters the circulation
• A total of 4 diabetic foot ulcer and 4 venous stasis ulcer patients will be enrolled
• Digital photography will give an efficacy read out of effect of FGF-1141 wound healing
This trial is close to completion, and the next clinical trial will be a Phase Ib or Phase II trial in the same population of patients with impaired wound healing. In the next trial FGF-1141 will be administered as multiple doses over several weeks. In addition to this medical indication, CVBT is also exploring use of this wound-healing formulation of FGF-1141 in the areas of pediatric burns.
Healing of FGF-1141-Treated Wounds in Diabetic Mice
Figure 11: The top row shows untreated wounds in diabetic mice. The bottom row shows wounds treated with FGF-1141. It is readily apparent at Days 10-15 that FGF-1141 has accelerated the healing process. (Source: Journal of Investigative Dermatology 104:850-855, 1995)
CVBT 2007 ANNUAL REVIEW
Peripheral Arterial Disease (PAD)
An Estimated 8 Million Americans Suffer from Peripheral Arterial Disease.
Lower extremity Peripheral Arterial Disease (PAD) occurs in the blood vessels of the legs and feet. PAD is a progressive disease that involves the hardening and narrowing of the arteries due to a gradual buildup of plaque. PAD of the lower extremities is a major cause of diminished ability to walk, and advanced cases can lead to leg amputation. An area of research we are exploring is growing new blood vessels with a PAD drug candidate, CVBT-141C that may improve the blood supply to the legs and feet to decrease pain and prevent tissue loss. We collaborated with a research institute, American Cardiovascular Research Institute (ARCI) that conducted an animal efficacy study for PAD in rabbits. Injections of our PAD drug candidate were given 3 times a week for a 2-week period into the ischemic leg. Results from these studies demonstrated that the drug candidate caused increased iliac artery blood flow and collateral vessel density and micro-vascular development in the legs of the treated animals. The Company has received FDA authorization to initiate its Phase I clinical trial in PAD patients suffering from intermittent claudication.
FGF-1 Stimulates Arterial Growth in Ischemic Rabbit Leg
Figure 12: FGF-1 for PAD stimulates new vessel growth in an animal model of peripheral arterial disease. 20 days following injection of FGF-1, increased blood vessel growth is statistically significant over control animals at both doses of drug tested. (Source: CardioVascular BioTherapeutics, Inc.)
A. Control, B. 1µg/kg, C. 3µg/ kg, D. 30µg/kg
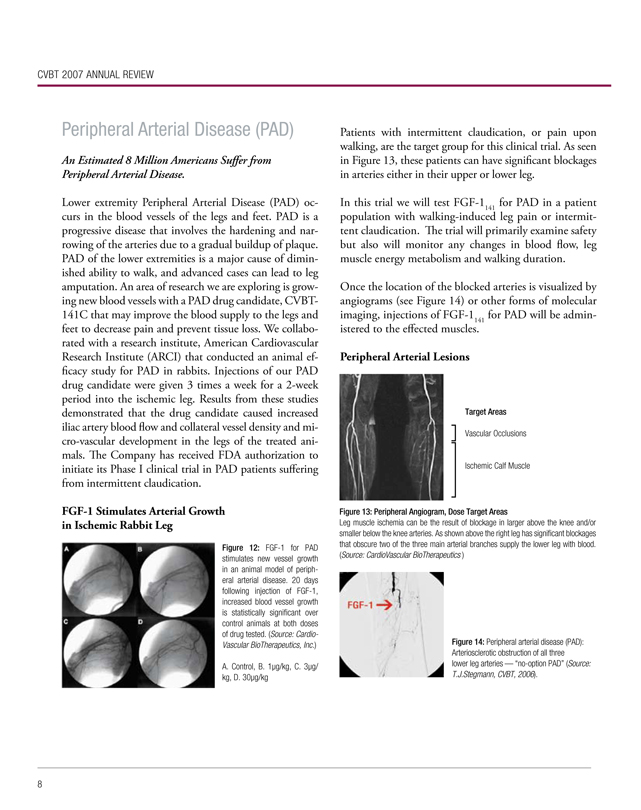
Patients with intermittent claudication, or pain upon walking, are the target group for this clinical trial. As seen in Figure 13, these patients can have significant blockages in arteries either in their upper or lower leg.
In this trial we will test FGF-1141 for PAD in a patient population with walking-induced leg pain or intermittent claudication. The trial will primarily examine safety but also will monitor any changes in blood flow, leg muscle energy metabolism and walking duration.
Once the location of the blocked arteries is visualized by angiograms (see Figure 14) or other forms of molecular imaging, injections of FGF-1141 for PAD will be administered to the effected muscles.
Peripheral Arterial Lesions
Target Areas
Vascular Occlusions
Ischemic Calf Muscle
Figure 13: Peripheral Angiogram, Dose Target Areas
Leg muscle ischemia can be the result of blockage in larger above the knee and/or smaller below the knee arteries. As shown above the right leg has significant blockages that obscure two of the three main arterial branches supply the lower leg with blood. (Source: CardioVascular BioTherapeutics )
Figure 14: Peripheral arterial disease (PAD): Arteriosclerotic obstruction of all three lower leg arteries — “no-option PAD” (Source: T.J.Stegmann, CVBT, 2006).

DEVELOPMENT PROGRAM
Disc Ischemia
According to a study reported in the Journal of General Internal Medicine, direct healthcare expenditures have been estimated at 100 billion dollars annually. Over half of these costs were utilized for treating chronic low back which has no known cause or cure. Degenerative disc disease, felt to be a major contributor of chronic low back pain, can be surgically treated by removing the disc and fusing the vertebrae together or replacing its function with an artificial implant. However, the reason why discs degenerate (or break down) is still largely unknown. In the 1990’s, a Finnish researcher demonstrated that the lumbar and spinal arteries become abnormally narrow (or diseased due to atherosclerosis) about the same time that blood vessels narrow in other areas of the body, such as the heart. In addition, these narrowed (or: stenotic) arteries were seen next to discs that were degenerating. It was concluded that discs degenerate because of poor access to blood borne nutrients. The lack of a safe and objective method for detecting spinal blood flow and nutrient transport into the disc has been the obstacle to confirming this theory. Recently however, powerful Magnetic Resonance Imaging scanners (MRI) have been developed that can provide this information. Because of this, the Orthopaedic Education and Research Institute of Southern California is performing a CVBT funded MRI study on 50 patients with chronic low back pain. The study’s investigators, from the University of California, Irvine Department of Radiology, are utilizing a powerful MRI research scanner and innovative software to look for patterns of abnormal blood flow associated with degenerating discs. Depending upon the results of the study, these innovative MRI techniques may become commonplace in the diagnosis of chronic low back pain. In addition, the possibility that ischemia may play a significant role in this condition could revolutionize its treatment, providing hope for sufferers worldwide.
Lumbar Ischemia
An Estimated 26 Million Americans Suffer from Chronic Lower Back Pain
Chronic back pain, estimated in published reports to affect a staggering 26 million Americans, has recently been linked to blockage of blood vessels supplying the lower back, leading to a condition referred to as lumbar ischemia. European medical researchers have speculated that chronic back pain resulting from blockage of blood vessels in the lower back precedes the blockage of coronary arteries in the heart by ten years. CVBT is conducting a proof of concept clinical trial in Eastern Europe and Russia to test whether a drug candidate formulated with FGF-1 for lumbar muscle injections can successfully treat chronic back pain. To date, 3 patients have been treated with a low dose of the drug, with no adverse events reported. If the results are positive in the proof of concept trial, CVBT plans to then file an application to the U.S. FDA to allow a study to begin in chronic back pain patients in the U.S.
Lumbar Arteries
Figure 15: This angiogram illustrates the difference between normal and occluded lumbar arteries. On the left, as denoted by the arrowheads, all lumbar and middle sacral arteries are normal, whereas on the right several arteries are narrowed and the middle sacral artery is totally occluded. (Source: L. Kaupilla et al., Spine 2004; 29: 2147ff.)
CVBT 2007 ANNUAL REVIEW
Pre-Clinical Research & Development
Our pre-clinical research and development is focused on developing new drug candidates in which is FGF-1141 is the active pharmaceutical ingredient (API), for the treatment of cardiovascular diseases where the growth of new blood vessels can improve the outcome for patients with these diseases. We conduct research to identify and evaluate medical indications that may benefit from our drug candidates. When, in our opinion, the evidence and results of our research warrant, a potential new drug candidate is graduated from research to development. Each drug candidate follows a similar development pathway. The first step is animal studies. We look for the correct biological response of drug candidates using FGF-1141 in animal models of cardiovascular disease. We also perform toxicity studies where we will look for any expected or unexpected side effects of our drug candidates compared to similar products on the market, if such products exist. Then we may initiate a clinical development program, which will commence with the submission of an IND application to the U.S. FDA. Human studies may begin once the FDA allows the IND to proceed. The Company works with the FDA on a case-by-case basis to determine the extent of clinical testing with each protein product in development.
STROKE
CVBT has focused primarily on developing new treatments that can stimulate new blood vessel growth into ischemic muscle tissues. However, blocked blood vessels can also severely injure other tissue types including nervous tissues. Stroke results either from the same basic atherosclerotic process that causes coronary artery disease, but it is occurring in vessels that supply brain cells with blood, or from a hemorrhagic cerebral event (13% of all strokes).
Stroke is the number three cause of death in the United States, and an estimated 6.2 million people suffer from the often devastating long term effects of strokes in this country with 500,000 new strokes, and about 200,000 recurrent strokes occurring every year. On average, every 45 seconds someone in the US has a stroke.
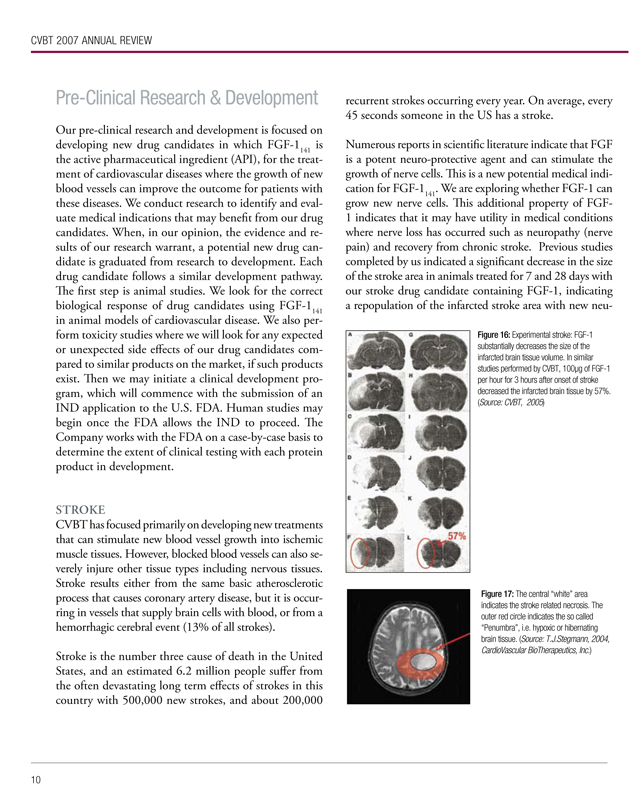
Numerous reports in scientific literature indicate that FGF is a potent neuro-protective agent and can stimulate the growth of nerve cells. This is a new potential medical indication for FGF-1141. We are exploring whether FGF-1 can grow new nerve cells. This additional property of FGF-1 indicates that it may have utility in medical conditions where nerve loss has occurred such as neuropathy (nerve pain) and recovery from chronic stroke. Previous studies completed by us indicated a significant decrease in the size of the stroke area in animals treated for 7 and 28 days with our stroke drug candidate containing FGF-1, indicating a repopulation of the infarcted stroke area with new neu-
Figure 16: Experimental stroke: FGF-1 substantially decreases the size of the infarcted brain tissue volume. In similar studies performed by CVBT, 100µg of FGF-1 per hour for 3 hours after onset of stroke decreased the infarcted brain tissue by 57%. (Source: CVBT, 2005)
Figure 17: The central “white” area indicates the stroke related necrosis. The outer red circle indicates the so called “Penumbra”, i.e. hypoxic or hibernating brain tissue. (Source: T.J.Stegmann, 2004, CardioVascular BioTherapeutics, Inc.)
DEVELOPMENT PROGRAM
rons. This new indication will be explored cautiously, but as this is an unmet medical need in this country, we believe a drug that could successfully treat this condition would be a significant medical advancement.
BONE DISEASE
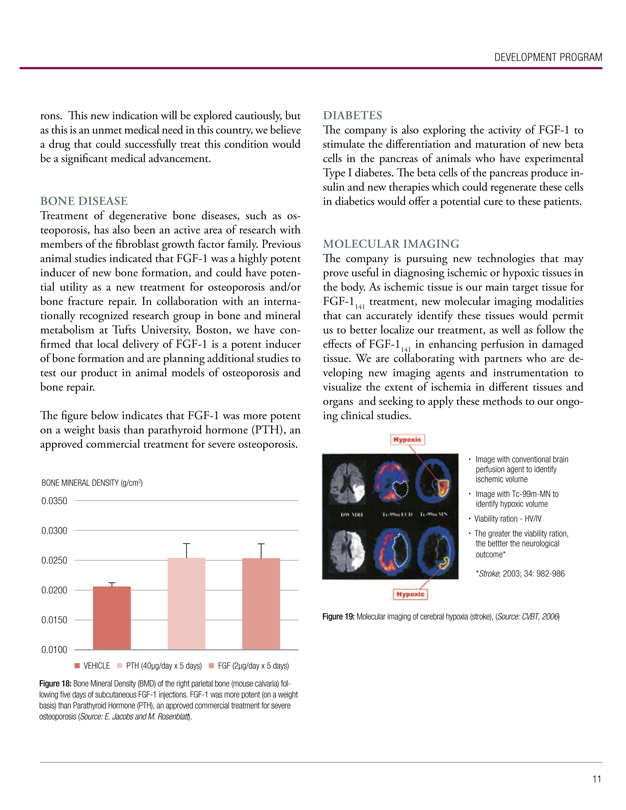
Treatment of degenerative bone diseases, such as osteoporosis, has also been an active area of research with members of the fibroblast growth factor family. Previous animal studies indicated that FGF-1 was a highly potent inducer of new bone formation, and could have potential utility as a new treatment for osteoporosis and/or bone fracture repair. In collaboration with an internationally recognized research group in bone and mineral metabolism at Tufts University, Boston, we have confirmed that local delivery of FGF-1 is a potent inducer of bone formation and are planning additional studies to test our product in animal models of osteoporosis and bone repair.
The figure below indicates that FGF-1 was more potent on a weight basis than parathyroid hormone (PTH), an approved commercial treatment for severe osteoporosis.
BONE MINERAL DENSITY (g/cm2)
0.0350 0.0300 0.0250 0.0200 0.0150 0.0100
VEHICLE PTH (40µg/day x 5 days) FGF (2µg/day x 5 days)
Figure 18: Bone Mineral Density (BMD) of the right parietal bone (mouse calvaria) following five days of subcutaneous FGF-1 injections. FGF-1 was more potent (on a weight basis) than Parathyroid Hormone (PTH), an approved commercial treatment for severe osteoporosis (Source: E. Jacobs and M. Rosenblatt).
DIABETES
The company is also exploring the activity of FGF-1 to stimulate the differentiation and maturation of new beta cells in the pancreas of animals who have experimental Type I diabetes. The beta cells of the pancreas produce insulin and new therapies which could regenerate these cells in diabetics would offer a potential cure to these patients.
MOLECULAR IMAGING
The company is pursuing new technologies that may prove useful in diagnosing ischemic or hypoxic tissues in the body. As ischemic tissue is our main target tissue for FGF-1141 treatment, new molecular imaging modalities that can accurately identify these tissues would permit us to better localize our treatment, as well as follow the effects of FGF-1141 in enhancing perfusion in damaged tissue. We are collaborating with partners who are developing new imaging agents and instrumentation to visualize the extent of ischemia in different tissues and organs and seeking to apply these methods to our ongoing clinical studies.
Image with conventional brain perfusion agent to identify ischemic volume
Image with Tc-99m-MN to identify hypoxic volume
Viability ration - HV/IV
The greater the viability ration, the bettter the neurological outcome*
*Stroke; 2003; 34: 982-986
Figure 19: Molecular imaging of cerebral hypoxia (stroke), (Source: CVBT, 2006)
CVBT 2007 ANNUAL REVIEW
DEVELOPMENT PIPELINE
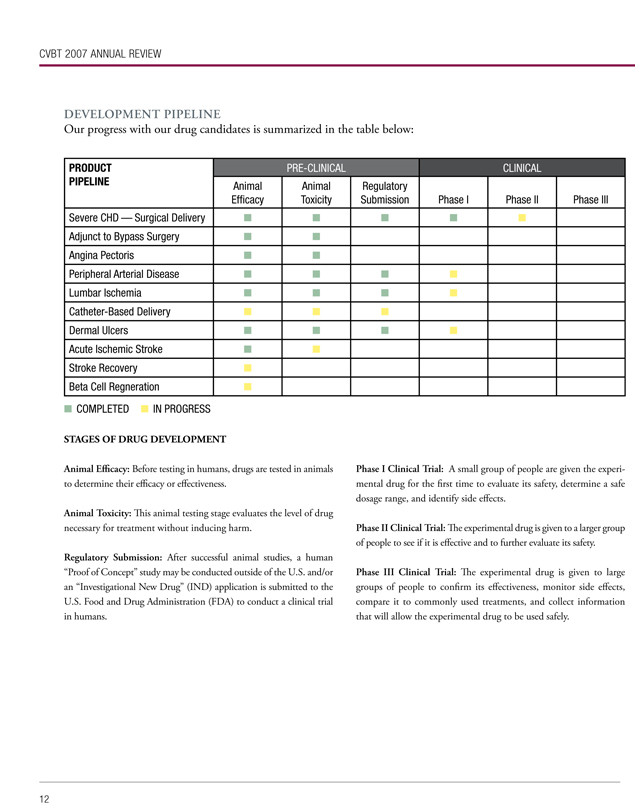
Our progress with our drug candidates is summarized in the table below:
PRODUCT PIPELINE
Severe CHD — Surgical Delivery
Adjunct to Bypass Surgery
Angina Pectoris
Peripheral Arterial Disease
Lumbar Ischemia
Catheter-Based Delivery
Dermal Ulcers
Acute Ischemic Stroke
Stroke Recovery
Beta Cell Regneration
PRE-CLINICAL
CLINICAL
Animal Efficacy
Animal Toxicity
Regulatory Submission
Phase I
Phase II
Phase III
COMPLETED IN PROGRESS
STAGES OF DRUG DEVELOPMENT
Animal Efficacy: Before testing in humans, drugs are tested in animals to determine their efficacy or effectiveness.
Animal Toxicity: This animal testing stage evaluates the level of drug necessary for treatment without inducing harm.
Regulatory Submission: After successful animal studies, a human “Proof of Concept” study may be conducted outside of the U.S. and/or an “Investigational New Drug” (IND) application is submitted to the U.S. Food and Drug Administration (FDA) to conduct a clinical trial in humans.
Phase I Clinical Trial: A small group of people are given the experimental drug for the first time to evaluate its safety, determine a safe dosage range, and identify side effects.
Phase II Clinical Trial: The experimental drug is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III Clinical Trial: The experimental drug is given to large groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug to be used safely.

MARKETING & COMMERCIALIZATION
Establish Marketing, Sales and Distribution
We believe that strategic investments in pre-launch marketing may benefit our shareholders. We are developing our pre-launch marketing investment priorities for each of our future drugs by defining:
Where we should target — Geographic priorities based upon market opportunity and potential
Whom we should target — High priority relevant customer segments that are particularly influential prior to launch
When we should be communicating — when is the right time to reach high priority customer segments
What we should be saying — defining messaging that is targeted at our high priority customer segments
How we should be communicating — defining and leveraging marketing channels that are the most effective and efficient in reaching our targets
Our geographic investment strategy is risk adjusted to account for the legal environment, economic policies, accounting standards, and the regulatory environment. This risk adjusted geographic analysis is the basis for our business development efforts and early stage marketing investments. As such, we are evaluating licensing, marketing, sales and distribution opportunities in the United States, Europe, and Japan.
In defining our marketing, sales and distribution opportunities, we weigh market opportunity against sales and distribution complexity, and thus risk and cost. This is the basis for defining our approach to either outsourcing or insourcing these functions through licensing, partnership agreements, or developing internal capabilities.
OUR STAKEHOLDER SEGMENTS
We believe that people and organizations that are important to our company can be grouped into three segments: advocates, approvers, and adopters. Advocates are influential segments and opinion leaders that can accelerate awareness of our products, solutions, and company. Approvers are large institutions that control the approval, sale, pricing, and licensing of our drugs, procedures, and intellectual property. Adopters are people
Maximizing Optimizing CONTRIBUTION Profitability and
Peak Sales
Extending Brand Life
Accelerating Uptake
Maximizing Laying a Pre-Launch Marketing
Commercial Foundation
Postpone Marketing
Potential for Success
0 Until After Launch
Discovery and Market
Pre-launch Launch Maturity Development Penetration and Growth
CLINICAL TRIALS
Figure 20: Marketing Objectives by Stage and Product Lifecycle (Source: Understanding Pharma: A Primer on How Pharmaceutical Companies Really Work; John J. Campbell, 2005, p. 126)
CVBT 2007 ANNUAL REVIEW
and organizations that are gate keepers to the adoption of our drugs and the use of our drugs, diagnostics, and procedures with patients.
Across these three segments, Healthcare Providers (physicians, medical professionals, and hospitals/clinics), Patients, Care Givers (family members and friends of patients), Investors, Suppliers, Regulators, and Payers engage in different activities that influence our progress through clinical trials and pave the way for our market success. Understanding their needs and how to meet them is a key focus on ensuring that we have advocates, approval, and adopters for our drugs.
Commercialization
HEALTH ECONOMICS OF OUR LEADING DRUG CANDIDATE
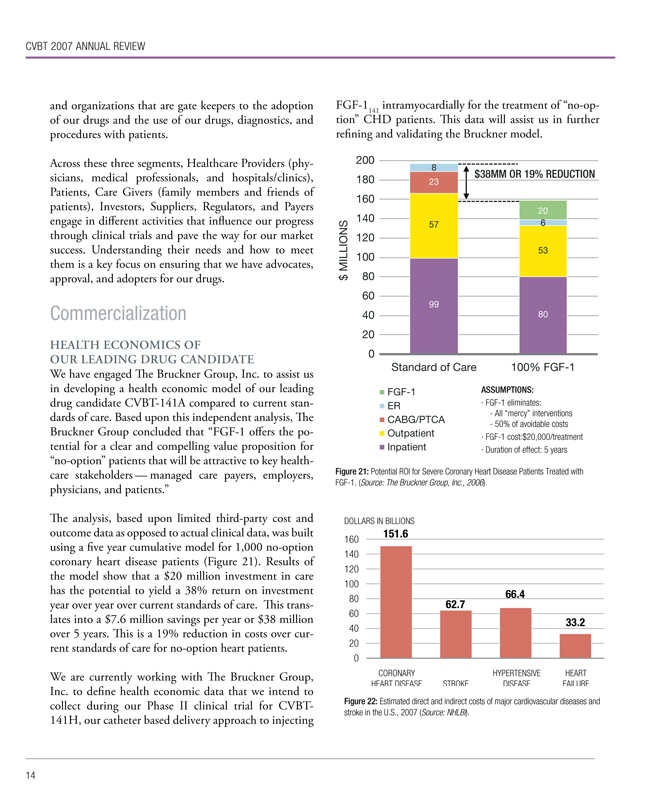
We have engaged The Bruckner Group, Inc. to assist us in developing a health economic model of our leading drug candidate CVBT-141A compared to current standards of care. Based upon this independent analysis, The Bruckner Group concluded that “FGF-1 offers the potential for a clear and compelling value proposition for “no-option” patients that will be attractive to key health-care stakeholders — managed care payers, employers, physicians, and patients.”
The analysis, based upon limited third-party cost and outcome data as opposed to actual clinical data, was built using a five year cumulative model for 1,000 no-option coronary heart disease patients (Figure 21). Results of the model show that a $20 million investment in care has the potential to yield a 38% return on investment year over year over current standards of care. This translates into a $7.6 million savings per year or $38 million over 5 years. This is a 19% reduction in costs over current standards of care for no-option heart patients.
We are currently working with The Bruckner Group, Inc. to define health economic data that we intend to collect during our Phase II clinical trial for CVBT-141H, our catheter based delivery approach to injecting FGF-1141 intramyocardially for the treatment of “no-option” CHD patients. This data will assist us in further refining and validating the Bruckner model.
200 180 160 140 120 100 80 60 40 20 0
8 23 $38MM OR 19% REDUCTION
57 6 5320
99 80
$MILLIONS
Standard of Care 100% FGF-1
FGF-1
ER
CABG/PTCA
Outpatient
Inpatient
ASSUMPTIONS:
FGF-1 eliminates:
- All “mercy” interventions
- 50% of avoidable costs
FGF-1 cost:$20,000/treatment
Duration of effect: 5 years
Figure 21: Potential ROI for Severe Coronary Heart Disease Patients Treated with FGF-1. (Source: The Bruckner Group, Inc., 2006)
DOLLARS IN BILLIONS
160 140 120 100 80 60 40 20 0
151.6 62.7 66.4 33.2
CORONARY HEART DISEASE STROKE HYPERTENSIVE DISEASE HEART FAILURE
Figure 22: Estimated direct and indirect costs of major cardiovascular diseases and stroke in the U.S., 2007 (Source: NHLBI).

MARKETING & COMMERICIALIZATION
Key Media Placements
Original Articles
Red Herring, “Hearts Tough to Mend,” October 9, 2006
“We found statistically increased working capacity for these patients three months after the treatments,” Dr. Stegmann reports. “ The angina symptoms in all patients were at least reduced and in many of them the symptoms are vanishing. Some of the patients who were not able to do anything before the treatments have returned to work…
… The patient just lives his or her life while the heart muscle creates the new vessels.”
BioExecutive International, “Patents, Partnerships and R&D Take Aim at Heart Disease,” April 18, 2007
“CardioVascular Bio Therapeutics (CVBT) announced in January that the U.S. Patent and Trademark Office granted its patent entitled, ‘Method of Producing Biologically Active Human Acidic Fibroblast Growth Factor and its Use in Promoting Angiogenesis.’…
… A growing body of evidence indicates that for angiogenesis therapy in the heart to work optimally, the angiogenic agent must be a protein, and that this protein should be injected directly into the heart muscle, two critical points covered in our patent.”
Genetic Engineering News, “CVBT Obtains Patent for Injection of Angiogenic Protein into the Heart”, January 9, 2007
United Press International, “CVBT Gets Patent on Direct-Inject Heart Rx”, January 9, 2007
Byline Articles
Drug Discovery Today, “Combating Cardiovascular Disease With Protein-based Angiogenic Therapy”, December 6, 2006
Drug Discovery News, “Treating Disease: Protein Therapy vs. Gene Therapy”, December 13, 2006
Las Vegas Review-Journal, “The R-J Goes To a Party: Heart Group Honors Siegfried and Roy, UMC Trauma Center”, March 1, 2007
Buffalo News, “Protein Therapy Advances”, March 20, 2007
Good Clinical Practice Journal, “Protein Promise in Heart Disease”, March 2007
Drug & Market Development, “Protein Promise in Heart Disease”, April 2007

DISCLOSURE
This Annual Review may contain forward-looking statements. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by the forward-looking statements. These risks and other factors include those listed under “Risk Factors”, on pages 5 through 25, and elsewhere in our Form 10-K filed with the SEC. We believe that the section entitled “Risk Factors” in our Form 10-K includes all material risks that could harm our business. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “intends,” “potential,” “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. Actual events or results may differ materially.
We believe that it is important to communicate our future expectations to our investors. However, there may be events in the future that we are not able to accurately predict or control and that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. Stockholders are cautioned that all forward-looking statements involve risks and uncertainties, and actual results may differ materially from those discussed as a result of various factors, including those factors described in the “Risk Factors” section of our Form 10-K. Stockholders and others should not place undue reliance on our forward-looking statements.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
For further information on CardioVascular Bio Therapeutics, Inc. Investor Relations Contact:
Investor Relations Department 702.391.4999 investorrelations@cvbt.com

APPENDIX

Cardiovascular indications
Protein promise in heart disease
Protein therapies are usually associated with anticancer regimens, however some initial clinical trials indicate that they could play a significant role in treating cardiovascular disease, based on their angiogenic properties. Dr Thomas J Stegmann explains
KEYWORDS: Cardiovascular disease (CVD); Protein therapy; Angiogenesis
Cardiovascular disease is the number one killer in the US, claiming around 2,500 lives a day, or one death every 35 seconds, according to the American Heart Association. This is more than the next four leading causes of death combined – cancer, chronic lower respiratory diseases, accidents and diabetes mellitus. And although there are several treatment options across the clinical spectrum of cardiovascular disease, a significant proportion of patients are unsuitable for many of the regimens on offer (see Box below).1–3 To combat this unmet need, protein therapy is being explored as a potential treatment. Although most of the 866 ongoing US FDA-authorised studies investigating protein therapy are cancer trials, 29 studies are focusing on protein therapy for cardiovascular disease (CVD). So far, the results look promising, and this article considers why this approach could be so valuable.
Magic molecules
Proteins are the most important molecules inside every living cell, tissue and organ, and perform all functions necessary for life: they regulate all our physiological reactions, they metabolise carbohydrates and fats, they defend our bodies against bacteria and viruses, they work as enzymes, exquisitely potent hormones, antibodies, cytokines and signalling peptides that transmit information into cells – they do everything.
Most human diseases are related to the malfunctioning of particular proteins, either systemically or locally. Today, a variety of human proteins can be produced with relative ease by means of genetic engineering and recombinant DNA technology. Large quantities of the desired protein can be made in mammalian cells, yeast or bacteria. Novel protein expression systems, including the newly introduced phage technology process, allow biologically active, properly folded, engineered
Heart disease: Prevalence, socio-economic impact and treatment
The clinical spectrum of cardiovascular disease comprises: coronary heart disease (CHD), congenital cardiovascular defects, heart failure, stroke, high blood pressure and peripheral arterial disease. Of the 71.3 million American adults with one or more types of CVD, 27.4 million are estimated to be aged 65 or older. Preliminary mortality data show that CVD was the underlying cause of death in 37.3% of all deaths, or one of every 2.7, in the US in 2003. CVD as an underlying or contributing cause of death (1.4 million deaths) made up 58% of deaths in 2002. The estimated direct and indirect cost of CVD for 2006 is US$403.1 billion.1
In Europe, the numbers are similar: CVD causes nearly half of all deaths in Europe (49%) and in the EU (42%).2 Each year, CVD causes more than 4.35 million deaths in Europe and over 1.9 million deaths in the EU. CVD is the main cause of death in women in all countries of Europe and is the main cause of death in men in all countries except France and San Marino. CHD by itself is the single most common cause of death in Europe, accounting for 1.95 million deaths a year. Overall, CVD is estimated to cost the EU economy US$169 billion a year.2 Methods of treating patients with severe medically refractory CHD include various catheter-based techniques (percutaneous coronary intervention or PCI) and such modifications as laser angioplasty, rotablator techniques and stent implantation; also surgical procedures, particularly coronary artery bypass grafting (CABG).
An estimated 664,000 PCI procedures on 652,000 patients and 467,000 CABG operations on 268,000 patients were performed in 2003 in the US.1 A significant number (estimated at 150,000 a year) of these patients, however, present with diffuse coronary artery disease, proximal stenosis and additional peripheral stenoses/ occlusions of the coronary artery branches, small vessels, or severe general co-morbidities, which makes them unsuitable both for PCI and for CABG (‘no-option’ heart patients). Owing to hereditary factors, Asian Indians in particular show this unfavourable pattern of CHD.
GCPj © Informa UK Ltd March 2007 www.GCPj.com

Cardiovascular indications
proteins to be manufactured and purified in high yield at a very reasonable cost.
Therapeutic proteins can be used as highly effective medical treatments – protein therapy – for a wide array of diseases in which the protein is either lacking or deficient (growth hormone and insulin), or the therapeutic protein is used to inhibit a biological process (antibodies that block blood supply to tumours).
A range of proteins and protein-based therapies are accepted treatments and have been introduced into the market: examples are Interleukin-2 for the treatment of HIV infection and advanced tumour stages; Interferon (IFN) alfa-2b and IFN alfa-2a (today most commonly used as pegylated form) for the treatment of hepatitis B and C; granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-GSF) for the treatment of HIV infection, haematological diseases and cancer; mycophenol for the treatment of chronic organ rejection and immunosuppression, or tumour necrosis factor (TNF) inhibitors for the treatment of rheumatoid arthritis. However, as yet no angio-genic protein-based therapy has been approved by the US FDA for the treatment of CVD.
Angiogenesis and CVD
To understand the implications of protein therapy in CVD, it is worth looking at the process of angiogenesis (the formation of new blood vessels) and its role in both disease and potential treatment. Research into angiogenesis started nearly 30 years ago and focused mainly on the inhibition of angiogenesis to limit tumour growth. In the 1980s, the isolation, characterisation and purification of the first angiogenic growth factors were reported; subsequently, inhibitors of angiogenesis were developed. Examples include angiostatin, endostatin, and tumstatin. In 2004, the US FDA approved the first anti-angiogenic protein therapy for cancer, Avastin (bevacizumab, rhuMAb-VEGF) to treat metastatic colorectal cancer in combination with established chemotherapy.
Research into angiogenesis and heart disease established that in blood vessels of mature
organisms, the endothelial cells remain in a quiescent, non-proliferate state until they are stimulated by conditions, such as wounding, inflammation, hypoxia or ischaemia. The development of new vessels can be considered as the result of several processes:
• Dissolution of the matrix underlying the endothelial cell layer
• Migration, adhesion and proliferation of endothelial cells
• Formation of a new three-dimensional tube, which then lengthens from its tip as circulation is re-established
• In larger vessels, vascular smooth muscle cells also migrate and adhere to the newly deposited matrix of the nascent vessel.
In the blood vessels of mature organisms, the edothelial cells remain in a quiescent state until stimulated by conditions such as wounding, inflammation, hypoxia or ischaemia
FGF -1 FGF -1/FGF receptor
FGFR Heparin
Receptor Heparin binding binding FGF -1 residues residues
Figure 1: Three-dimensional structures of fibroblast growth factor 1 (FGF-1) and FGF-1 receptor (FGFR). The receptor binding sites are indicated in yellow, the heparin binding sites in blue.
Angiogenic growth factors (proteins) induce, promote and/or interfere with all these steps of angiogenesis.
A large variety of very potent proteins and polypeptides, termed ‘growth factors’, exists in the human body. Growth factors are naturally occurring proteins capable of stimulating cellular proliferation, maturation, and differentiation; they typically act as signalling molecules between cells and bind to specific receptors on the surface of their target cells. The best-known growth factors with proven angiogenic potency are fibroblast growth factor-1 (FGF-1) and vascular endothelial growth factor-1 or
-A (VEGF-1). FGF-1 stimulates the proliferation and differentiation of all cell types necessary for building an arterial vessel – endothelial cells, smooth muscle cells, and fibroblasts (adventitial cells) – whereas VEGF-1 mainly stimulates endothelial cells, and increases the permeability of the vessel wall.
Developing protein therapies
In contrast to the still very active research and development of anti-angiogenic proteins for treatment of malignant tumours, research into the use of angiogenic proteins in promoting angiogenesis as a therapeutic option for CVD is still at
www.GCPj.com GCPj © Informa UK Ltd March 2007
Control Myocardium Myocardium
IMA (control) (+FGF-1) 160 *p<0.005 140 120
gray scale 100* * Angiographic 80
FGF-1
60 40 20 0
3 months 3 years Full contrast
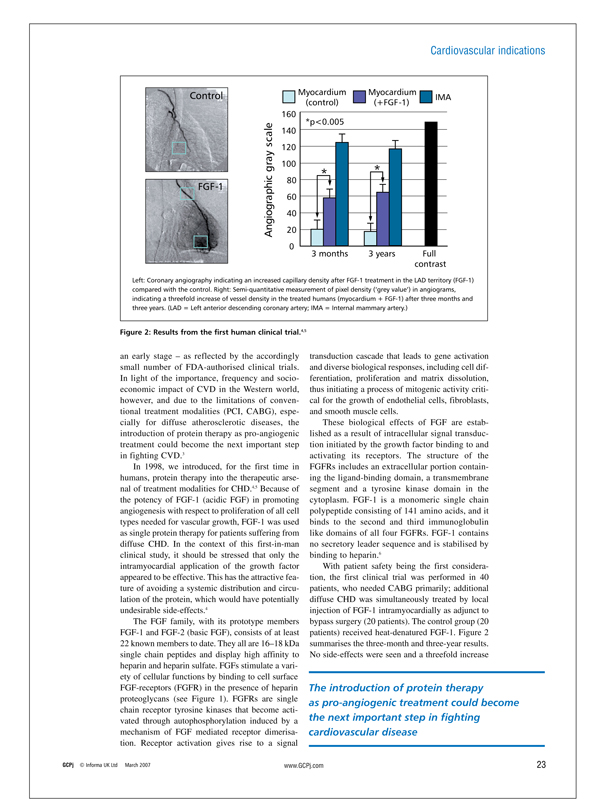
Left: Coronary angiography indicating an increased capillary density after FGF-1 treatment in the LAD territory (FGF-1) compared with the control. Right: Semi-quantitative measurement of pixel density (‘grey value’) in angiograms, indicating a threefold increase of vessel density in the treated humans (myocardium + FGF-1) after three months and three years. (LAD = Left anterior descending coronary artery; IMA = Internal mammary artery.)
Figure 2: Results from the first human clinical trial.4,5
an early stage – as reflected by the accordingly small number of FDA-authorised clinical trials. In light of the importance, frequency and socioeconomic impact of CVD in the Western world, however, and due to the limitations of conventional treatment modalities (PCI, CABG), especially for diffuse atherosclerotic diseases, the introduction of protein therapy as pro-angiogenic treatment could become the next important step in fighting CVD.3 In 1998, we introduced, for the first time in humans, protein therapy into the therapeutic arsenal of treatment modalities for CHD.4,5 Because of the potency of FGF-1 (acidic FGF) in promoting angiogenesis with respect to proliferation of all cell types needed for vascular growth, FGF-1 was used as single protein therapy for patients suffering from diffuse CHD. In the context of this first-in-man clinical study, it should be stressed that only the intramyocardial application of the growth factor appeared to be effective. This has the attractive feature of avoiding a systemic distribution and circulation of the protein, which would have potentially undesirable side-effects.4 The FGF family, with its prototype members FGF-1 and FGF-2 (basic FGF), consists of at least 22 known members to date. They all are 16–18 kDa single chain peptides and display high affinity to heparin and heparin sulfate. FGFs stimulate a variety of cellular functions by binding to cell surface FGF-receptors (FGFR) in the presence of heparin proteoglycans (see Figure 1). FGFRs are single chain receptor tyrosine kinases that become activated through autophosphorylation induced by a mechanism of FGF mediated receptor dimerisation. Receptor activation gives rise to a signal
transduction cascade that leads to gene activation and diverse biological responses, including cell differentiation, proliferation and matrix dissolution, thus initiating a process of mitogenic activity critical for the growth of endothelial cells, fibroblasts, and smooth muscle cells.
These biological effects of FGF are established as a result of intracellular signal transduction initiated by the growth factor binding to and activating its receptors. The structure of the FGFRs includes an extracellular portion containing the ligand-binding domain, a transmembrane segment and a tyrosine kinase domain in the cytoplasm. FGF-1 is a monomeric single chain polypeptide consisting of 141 amino acids, and it binds to the second and third immunoglobulin like domains of all four FGFRs. FGF-1 contains no secretory leader sequence and is stabilised by binding to heparin.6 With patient safety being the first consideration, the first clinical trial was performed in 40 patients, who needed CABG primarily; additional diffuse CHD was simultaneously treated by local injection of FGF-1 intramyocardially as adjunct to bypass surgery (20 patients). The control group (20 patients) received heat-denatured FGF-1. Figure 2 summarises the three-month and three-year results. No side-effects were seen and a threefold increase
The introduction of protein therapy as pro-angiogenic treatment could become the next important step in fighting cardiovascular disease
GCPj © Informa UK Ltd March 2007 www.GCPj.com
Cardiovascular indications
Ischaemic territory Stress SPECT
Preop 90 days(0 = no perfusion, 16 = full perfusion)
14 12 10 8 6 4 2 0
*p < 0.001
Rest Stress
18/20 patients improved
SPECT perfusion summed score
Preop 45 days 90 days
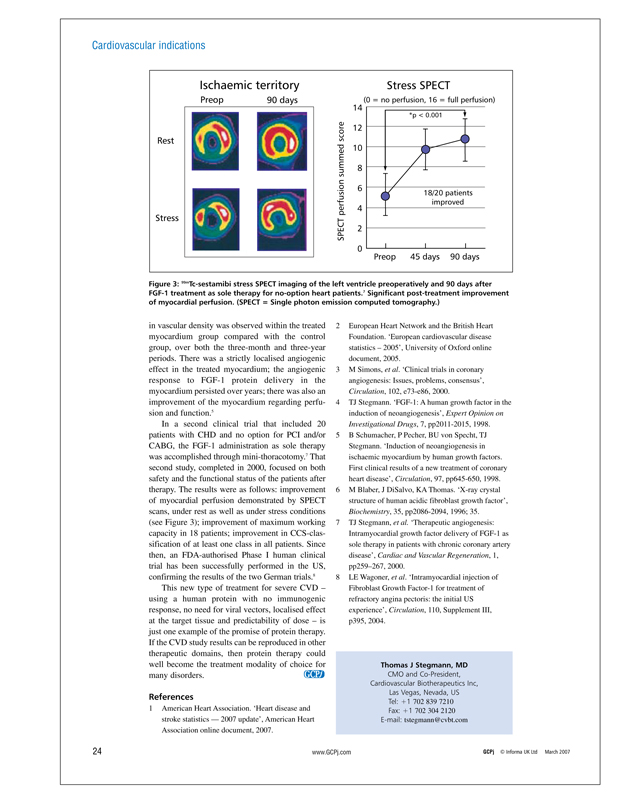
Figure 3: 99mTc-sestamibi stress SPECT imaging of the left ventricle preoperatively and 90 days after FGF-1 treatment as sole therapy for no-option heart patients.7 Significant post-treatment improvement of myocardial perfusion. (SPECT = Single photon emission computed tomography.)
in vascular density was observed within the treated myocardium group compared with the control group, over both the three-month and three-year periods. There was a strictly localised angiogenic effect in the treated myocardium; the angiogenic response to FGF-1 protein delivery in the myocardium persisted over years; there was also an improvement of the myocardium regarding perfusion and function.5
In a second clinical trial that included 20 patients with CHD and no option for PCI and/or CABG, the FGF-1 administration as sole therapy was accomplished through mini-thoracotomy.7 That second study, completed in 2000, focused on both safety and the functional status of the patients after therapy. The results were as follows: improvement of myocardial perfusion demonstrated by SPECT scans, under rest as well as under stress conditions (see Figure 3); improvement of maximum working capacity in 18 patients; improvement in CCS-classification of at least one class in all patients. Since then, an FDA-authorised Phase I human clinical trial has been successfully performed in the US, confirming the results of the two German trials.8
This new type of treatment for severe CVD –using a human protein with no immunogenic response, no need for viral vectors, localised effect at the target tissue and predictability of dose – is just one example of the promise of protein therapy. If the CVD study results can be reproduced in other therapeutic domains, then protein therapy could well become the treatment modality of choice for many disorders.
References
1 American Heart Association. ‘Heart disease and stroke statistics — 2007 update’, American Heart Association online document, 2007.
2 European Heart Network and the British Heart Foundation. ‘European cardiovascular disease statistics – 2005’, University of Oxford online document, 2005.
3 M Simons, et al. ‘Clinical trials in coronary angiogenesis: Issues, problems, consensus’, Circulation, 102, e73-e86, 2000.
4 TJ Stegmann. ‘FGF-1: A human growth factor in the induction of neoangiogenesis’, Expert Opinion on Investigational Drugs, 7, pp2011-2015, 1998.
5 B Schumacher, P Pecher, BU von Specht, TJ Stegmann. ‘Induction of neoangiogenesis in ischaemic myocardium by human growth factors. First clinical results of a new treatment of coronary heart disease’, Circulation, 97, pp645-650, 1998.
6 M Blaber, J DiSalvo, KA Thomas. ‘X-ray crystal structure of human acidic fibroblast growth factor’, Biochemistry, 35, pp2086-2094, 1996; 35.
7 TJ Stegmann, et al. ‘Therapeutic angiogenesis: Intramyocardial growth factor delivery of FGF-1 as sole therapy in patients with chronic coronary artery disease’, Cardiac and Vascular Regeneration, 1, pp259–267, 2000.
8 LE Wagoner, et al. ‘Intramyocardial injection of Fibroblast Growth Factor-1 for treatment of refractory angina pectoris: the initial US experience’, Circulation, 110, Supplement III, p395, 2004.
Thomas J Stegmann, MD
CMO and Co-President, Cardiovascular Biotherapeutics Inc, Las Vegas, Nevada, US
Tel: +1 702 839 7210 Fax: +1 702 304 2120 E-mail: tstegmann@cvbt.com
www.GCPj.com GCPj © Informa UK Ltd March 2007

CardioVascular BioTherapeutics, Inc.
1635 VILLAGE CENTER CIRCLE, SUITE 250 LAS VEGAS, NV 89134 (702) 839-7200 www.cvbt.com























