UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
For the fiscal year ended December 31, 2010
OR
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
For the transition period from ____ to____
Commission file number: 000-52018
VIRTUALSCOPICS, INC.
(Exact name of registrant as specified in its charter)
| DELAWARE | 04- 3007151 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| 500 Linden Oaks, Rochester, New York | 14625 |
| (Address of principal executive offices) | (Zip Code) |
(585) 249-6231
(Registrant's Telephone Number, Including Area Code)
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE EXCHANGE ACT:
Common Stock, $0.001 par value
NASDAQ Capital Market
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE EXCHANGE ACT:
TITLE OF EACH CLASS:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
¨ Yes x No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). * ¨
* Registrant is not yet required to submit Interactive Data Files pursuant to Rule 405 of Regulation S-T
Indicate by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting” in Rule 12b-2 of the Exchange Act.
Larger accelerated filer ¨ | Accelerated filer ¨ |
Non-accelerated filer ¨ (Do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o or No x
The aggregate market value of the issuer’s voting and non-voting common equity held by non-affiliates of the issuer as of December 31, 2010 was approximately $42,500,000 (calculated by excluding all shares held by executive officers, directors and holders known to the registrant of five percent or more of the voting power of the registrant's common stock, without conceding that such persons are “affiliates” of the registrant for purposes of the federal securities laws). This amount does not include any value for the issuer’s series A preferred stock or series B preferred stock, for which there is no established United States public trading market, or any value for the common stock issuable upon conversion of shares of such preferred stock.
As of February 28, 2011, there were outstanding 28,378,997 shares of the issuer’s common stock, $.001 par value.
Documents Incorporated By Reference: Portions of the Company's Proxy Statement to be delivered to the Company’s stockholders in connection with the Company’s 2010 Annual Meeting of Stockholders, which the Company plans to file with the Securities and Exchange Commission pursuant to Regulation 14A promulgated under the Securities Exchange Act of 1934, on or prior to April 30, 2011, are incorporated by reference in Part III (Items 10, 11, 12, 13 and 14) of this Form 10-K.
TABLE OF CONTENTS
| | | Page Numbers |
| PART I | | |
| | ITEM 1: Business | 2 |
| | ITEM 1A: Risk Factors | 14 |
| | ITEM 2: Properties | 18 |
| | ITEM 3: Legal Proceedings | 18 |
| PART II | | |
| | ITEM 5: Market For Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 19 |
| | ITEM 7: Management's Discussion and Analysis of Financial Condition and Results of Operations | 20 |
| | ITEM 8: Financial Statements and Supplementary Data | 25 |
| | ITEM 9: Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 25 |
| | ITEM 9A: Controls and Procedures | 25 |
| | ITEM 9B: Other Information | 26 |
| PART III | | |
| | ITEM 10: Directors, Executive Officers and Corporate Governance | 27 |
| | ITEM 11: Executive Compensation | 27 |
| | ITEM 12: Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 27 |
| | ITEM 13: Certain Relationships and Related Transactions, and Director Independence | 27 |
| | ITEM 14: Principal Accountant Fees and Services | 27 |
| PART IV | | |
| | ITEM 15: Exhibits | 27 |
PART I
FORWARD-LOOKING STATEMENTS
Some of the statements under the captions of this report on Form 10-K titled “Risk Factors,” “Management's Discussion and Analysis of Financial Condition and Results of Operations” or “Business,” contained or incorporated by reference elsewhere in this report, and in our other reports filed with the Securities Exchange Commission (“SEC”) constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which involve risks and uncertainties. These statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include statements that address activities, events or developments that we expect, believe or anticipate may occur in the future, including:
| | · | adverse economic conditions; |
| | · | loss of market share due to competing products and services; |
| | · | unexpected costs, lower than expected sales and revenues, and operating defects; |
| | · | adverse results of any legal proceedings; |
| | · | the volatility of our operating results and financial condition; |
| | · | inability to attract or retain qualified senior management and scientific personnel; |
| | · | inability to raise sufficient additional capital to operate our business, if necessary, and; |
| | · | other specific risks that may be referred to in this report. |
All statements, other than statements of historical facts, included in this report regarding our strategy, future operations, financial position, estimated revenue or losses, projected costs, prospects and plans and objectives of management are forward-looking statements. When used in this report, the words “may,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “project,” “plan,” “could,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. All forward-looking statements speak only as of the date of this report. We do not undertake any obligation to update any forward-looking statements or other information contained in this report. Existing stockholders and potential investors should not place undue reliance on these forward-looking statements. Although we believe that our plans, intentions and expectations reflected in or suggested by the forward-looking statements in this report are reasonable, we cannot assure our stockholders or potential investors that these plans, intentions or expectations will be achieved. We disclose important factors that could cause our actual results to differ materially from our expectations under “Risk Factors” and elsewhere in this report. These risk factors qualify all forward-looking statements attributable to us or persons acting on our behalf.
Information regarding market and industry statistics contained in this report is included based on information available to us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic analysis. We have not reviewed or included data from all sources, and we cannot assure our stockholders or potential investors of the accuracy or completeness of the data included in this report. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. We have no obligation to update forward-looking information to reflect actual results or changes in assumptions or other factors that could affect those statements. See “Risk Factors” for a more detailed discussion of uncertainties and risks that may have an impact on future results.
ITEM 1: Business
We are a provider of quantitative imaging for clinical trials serving the pharmaceutical, biotechnology and medical device industries. We have created a suite of image analysis software tools and applications which are used in detecting and measuring specific anatomical structures and metabolic activity using medical images. Our proprietary software and algorithms provide measurement capabilities designed to improve clinical research and development. We focus on applying our imaging technology to improve the efficiency and effectiveness of the pharmaceutical and medical device research and development processes. We believe our technology can also be used in improving the treatment planning for patients with cancer.
VirtualScopics, LLC was formed in 2000. In November 2005, VirtualScopics, LLC (“VS”), a New York limited liability company, entered into a securities exchange agreement with ConsultAmerica, Inc. (“CA”), a Delaware corporation. As a result of the exchange, the members of VS became the controlling stockholders of CA. CA did not have any meaningful operations prior to the merger. Immediately following the merger, CA changed its name to VirtualScopics, Inc. VirtualScopics, Inc. is a Delaware corporation and headquartered in Rochester, New York.
Business Overview
Our image-based measurement and visualization tools enable automated, accurate and reproducible measurement of minute changes that occur in anatomic structures in musculoskeletal, oncological, cardiological and neurological diseases. For pharmaceutical, biotechnology and medical device manufacturers, these tools can significantly alleviate or reduce clinical development bottlenecks by increasing the speed, accuracy and reliability of the demonstration of a new compound’s efficacy. Further, these measurements can be used to assess the viability of continuing a drug development project and eliminate as soon as possible a drug that is likely to fail. Early failure is critical to the pharmaceutical industry to prevent the expenditure of R&D funds on a drug that will not perform as expected. We believe that this is especially important today with the large number of compounds that are awaiting evaluation.
We have also begun pursuing the application of one of our technologies into the personalized medicine market. Specifically, we believe there could be further benefit of our blood flow and vascular permeability software tool in providing patients and oncologists information to determine whether an anti-angiogenic therapy is having the desired effect. We believe this application will better assist oncologists with treatment planning for patients undergoing anti-angiogenic cancer therapies. Over the past year we have received a significant amount of feedback relative to market acceptance from leading Oncologists, payers, pharmaceutical companies and equipment manufacturers. Based on this feedback, we have developed a strategy to begin the commercialization of the application. We will need to further validate the benefits of technology and will begin seeking opportunities to partner with an organization to assist with the process. We have also begun the regulatory process for obtaining 510k clearance from the FDA. We will continue to assess the best mechanism for channeling our application into the market as well as the process for obtaining reimbursement from payers; however, there can be no assurance that approval will be granted or we will experience significant demand for our application.
Benefits to Pharmaceutical, Biotech and Medical Device Companies
The benefits to pharmaceutical companies from using our image analysis tools can include shorter clinical development time, and earlier determination of the effectiveness or ineffectiveness of a new drug or compound. Our technology helps to curtail trials that are not likely to be beneficial and to avoid mistaken termination of compounds that are likely to prove efficacious, through:
| | · | improved precision in the measurement of existing biomarkers resulting in shorter observation periods, with beneficial cost savings within a clinical trial; |
| | · | new biomarkers, which are better correlated with disease states, again reducing trial length and therefore costs; and |
| | · | reduced processing time for image data analysis through automation. |
In addition, our technology reduces aggregate clinical development costs through:
| | · | improved precision of existing biomarkers, thus requiring smaller patient populations and lower administrative costs; and |
| | · | new biomarkers that serve as better correlates, leading to better early screening and elimination of weak drug candidates in pre-clinical trials. |
Benefits to Patients and Health Care Providers in Personalized Medicine
The specific opportunities that we are pursuing within personalized medicine are mostly related to the treatment monitoring of patients. Cancer is a leading cause of death throughout much of the developed world, and technologies for closely monitoring disease progression and response to treatment are currently lacking. We believe this presents us with a significant market opportunity.
In personalized medicine, our technology is designed to offer physicians and medical insurers better treatment planning of patients based on determination of patient response to compounds or other treatment options. For example, in oncology we have demonstrated the ability to determine whether patients are showing response to an anti-angiogenic drug after only 48 hours of treatment (Glenn Liu et al., “Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Pharmacodynamic Measure of Response After Acute Dosing of AG-013736, an Oral Angiogenesis Inhibitor, in Patients With Advanced Solid Tumors: Results From a Phase I Study,” Journal of Clinical Oncology, vol. 20, August 20, 2005).
We are the first company able to provide blood flow and volume measurements for cancer diagnosis and monitoring in a standardized and consistent way across multiple institutions (Jerry M. Collins, “Imaging and Other Biomarkers in Early Clinical Studies: One Step at a Time or Re-Engineering Drug Development?,” Journal of Clinical Oncology, vol. 20, August 20, 2005). These measurements are vital for assessing patient response to next-generation anti-angiogenic cancer drugs.
During 2010, we expanded our assessment and commercialization efforts within this market. We began a dialogue with the FDA to gather the Agency's feedback on our services and validate our assumptions regarding regulatory approval. We also gained insight into the level of supporting data that will be necessary to claim and demonstrate predictability. Based on this feedback we plan to do a staged approach with the regulatory approvals (i.e. not include the predictability claim in our first approval) in order to begin performing blood flow and vascular permeability measurements and collect any necessary data to demonstrate and claim predictability. Also, during 2010, we engaged consultants to assist in our efforts to commercialize this service. They gathered feedback from key opinion leaders within oncology, insurance companies and pharmaceutical companies on their assessment and utility of the service. Based on this feedback we believe the best strategy for the broadest market acceptance would be to partner with an organization to assist in the further validation of the application as well as the distribution. We are in the process of discussing the opportunity with those parties that expressed the most interest during the gathering of market research data. Based on the nature of the partnership and the amount of validation required to demonstrate strong predicative values we will make an assessment whether additional capital will be required.
Our Technology Solution
Oncology Applications
Automated Measurement of Tumor Structure in Oncology
Rapid determination of drug efficacy depends on precise measurement of tumor structure and function. Yet current practices - direct measurement from films and computer-aided tracing - can be time-consuming, inaccurate and highly variable. Manual approaches often lead to false conclusions when tumors take on abnormal shapes; where a two-dimensional analysis may indicate no change, a three dimensional analysis may show a significant change in tumor volume. The RECIST standard, still the primary imaging endpoint for assessing disease progression or response to treatment in many types of cancer, measures structural changes in tumors through a simple summation of longest diameters, limited to the axial imaging plane. Originally developed for x-ray imaging, it fails to take advantage of the far richer three dimensional information set available with today's imaging technologies.
Our semi-automated, statistically driven feature analysis provides greater precision, higher throughput and less dependence on a particular reader than manual tracing does. In retrospective analysis for a leading pharmaceutical company, our volumetric measurement showed that tumors found to be stable under RECIST actually were growing significantly. With our semi-automated analysis we could have discovered the failure sooner and avoided the expense of funding the next phase of clinical research. Conversely, volumetric measurement can greatly accelerate clinical research by preventing mistaken kills and identifying efficacious compounds sooner.
Innovation in Image-Based Biomarkers
With a multidisciplinary team of medical professionals (including staff radiologists), scientists and software developers, we deliver unparalleled innovation in the analysis of specific biomarkers. Measurements may include specific FDA-acknowledged (RECIST and tumor volume) biomarkers as well as secondary or exploratory endpoints such as cavitation/necrosis, or shape. By extracting substantially more information from existing imaging modalities such as CT or MRI, we believe we offer a more definite and efficient basis for determining the course of clinical trials.
Measurement of Blood Flow and Metabolic Activity
A growing number of anti-cancer drugs both on the market (e.g., Iressa and Avastin) and under development are designed to reduce the blood supply available to tumors, thereby depriving them of the ability to grow and spread. During development, these compounds require the ability to accurately measure blood flow and vascular permeability in vivo, in order to determine dose-response relationships and compound efficacy. In the clinic, this same capability is necessary in order to determine whether a particular patient is responding to treatment. We have developed a method, using dynamic contrast enhanced magnetic resonance imaging (DCE-MRI), to accomplish this. This technique involves repeated imaging, generally every five to ten seconds, for a period of several minutes before and after the injection of a gadolinium-based, FDA-approved, contrast agent. Tracer concentration changes over time can then be measured both in normal and cancerous tissues, and based on this information parameters such as blood flow, blood volume and vascular permeability can be derived. These parameters have been shown to relate directly to the activity of anti-angiogenesis and anti-vascular cancer drugs, and to allow the prediction of response or failure after only a few days of treatment.
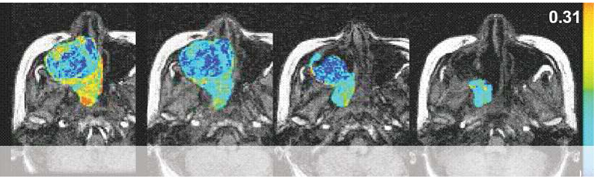
With dynamic contrast-enhanced series, changes in signal intensity can be related to tracer concentration in tissues. This information can be used to determine the blood flow to the tumor.
Musculoskeletal Applications
Our image analysis provides a degree of accuracy and reproducibility that cannot be duplicated by manual techniques. Standard endpoints, such as pain or functionality scoring are largely subjective and difficult to reproduce. Our quantitative imaging replaces subjective evaluation - knee pain ranked on a scale of 1 to 10 - with an objective quantification - volume of lost cartilage in cubic millimeters. Unlike manual assessment methods, our computer aided approach allows you to track the boundary location of each structure in a data set from one scan to another, even if the patient is not positioned in precisely the same way for each scan, or if there have been some anatomical changes between scans. For cartilage volumes and thickness measurements, the Coefficient of Variation (CV) typically falls between 2% and 4% - we can detect minute changes with statistical confidence, allowing our clients to reduce study populations or shorten study durations.
With our automated analysis, researchers can more confidently make the go/no go decision for a compound early in the evaluation process, allowing scarce resources to be allocated to the most promising candidates. In the evaluation of osteoarthritis, for example, MRI of the cartilage in the knee coupled with automated measurement of volume and composition shows disease changes in months; these changes would not be apparent for years using standard x-ray evaluation.
Reproducible medical image analysis is driven by computer image analysis algorithms that enable quantitative measurement of different structural parameters. Guided by the information present in the images, as well as embedded anatomical knowledge, the algorithms enable segmentation of different structures. From an MRI knee scan, for instance, it is possible to produce a three-dimensional reconstruction that graphically distinguishes cartilage from underlying bone, as well as from ligaments, fluid, degenerated menisci or inflamed synovium. This capability provides a valuable assessment tool for clinical research in osteoarthritis - a disease with multiple endpoints - because it allows sensitive and specific measurement of all the components of the knee joint and detects small changes in any of those components over time.
Medical Device and Biologics
New research continues to focus on the development of devices and/or biologics that will generate new and better cartilage for patients with osteoarthritis and knee injuries. Our technology uses a suite of tools to assist in the identification of cartilage lesions within the knee. These tools allow for the tracking of structural changes and the quality of new tissue being grown within those lesions. For example, we are currently working with leading biologic and medical device companies to determine the percent fill for lesions implanted with the device/biologic. This analysis serves as a useful tool in that it demonstrates the degree of success of the implant. It is presumed in the industry that the higher the percent fill the lower the degree of pain for the patient. We also provide quality of tissue assessments (i.e. T2 maps) to provide our customers with information on the composition of the repair tissue. It is also believed that the closer the repair tissue is to ‘normal’ tissue the longer the life span is of the repair tissue with the resulting benefit being the ultimate health and comfort of the patient.
Additionally, our motion tracking software capabilities allows us to more precisely measure changes in the structural and tissue quality measurements. It has been demonstrated that this technique can reduce the amount of variability inherent in these types of measurements, thereby, reducing the amount of patients necessary to demonstrate the effectiveness of the medical device and/or biologic.
Cardiovascular Applications
Cardiovascular disease is one of the leading causes of mortality within most developed countries. Early identification of the changes leading the disease can prompt early intervention which can result in longer and better quality of life as well as lower healthcare costs. Imaging provides a valuable tool for the assessment of early development of arterial plaques which can lead to arterial stenosis as well as stroke and myocardial infarction. The current primary imaging tool for screening cardiovascular patients is ultrasound but these carotid ultrasound scans produce a large amount of data which can be laborious and imprecise to analyze. We have developed a suite of patented semi-automated tools for the identification and measurement of carotid plaques which has proven to reduce analysis time to as little as 3 minutes per case compared to the current manual methods which can take over one hour. In addition, these tools have been tested against expert readers in the field and found to be highly precise and accurate and in many cases more sensitive to the appearance of small arterial plaques. This provides a valuable tool for screening of normal/healthy individuals as well as monitoring the use in patients enrolled in clinical trials.
More detailed information about plaque composition and progression can be obtained by using MRI. This modality has advantages over ultrasound in that it can precisely measure plaque volume as well as composition. This is important because it is widely recognized in the industry that certain plaques, in particular those with high lipid or necrotic cores, pose a much higher risk to the health of the patient, while those that are fibrous may pose a lower risk. Therefore, the ability to distinguish between benign and vulnerable plaques may enable treating physicians to better personalize the treatment for each patient. Additionally, certain drugs designed to reduce blood lipids may have greater effect on lipid rich plaques, making this a potentially beneficial screening tool for patients enrolled in clinical trials. Our patented semi-automated tools for the measurement of plaques in MRI and automated identification of lipids and calcification allows accurate and precise analysis of vulnerable plaques.
These proprietary ultrasound and MRI techniques for cardiovascular health are being used in large industry sponsored trials today.
Neurology Applications
Evaluating diseases such as multiple sclerosis (MS), epilepsy, and Alzheimer’s requires the identification and measurement of neurological structures and lesions. However, current methods for obtaining data points rely on subjective and error prone manual techniques. Manual tracing, especially of abnormal neurological structures, requires considerable expertise and time. Tracing introduces significant variability even when all measurements are made by one individual, an effect that is compounded with multiple operators. Intra- and inter-operator variability poses a major obstacle for researchers attempting to take advantage of the power of MRI analysis in the study of neurological disease. VirtualScopics eliminates these problems with automated, statistically driven feature analysis. Our algorithms employ the two types of knowledge that expert radiologists use to measure structures within the brain: differentiation of various tissue types and knowledge of structure, size, location, and shape. Our software incorporates an a priori model of neurological anatomy that enables the measurement of structures with indistinct boundaries such as the hippocampus. Knowledge of anatomical structures also improves reproducibility, allowing disease progression to be precisely monitored over time. To gain higher resolution and superior tissue separability, we reconstruct volumes by co-registering and fusing images from multiple imaging planes and pulse sequences. Moreover, its automatic reconstruction produces a smooth and continuous surface, much closer to actual shape than would result from manual segmentation.
Many neurological conditions can be detected and evaluated with quantitative measures of structures in MRI studies. While automated measurement tracks lesions in MS clinical trials, it also provides a critical tool in measuring hippocampal volume for diagnosing and monitoring both intractable temporal lobe epilepsy and Alzheimer’s disease. Validation studies prove that our automated approach provides greater speed, precision and accuracy in clinical trials than current manual methods do. In MS clinical trials, where current techniques to measure progress in drug development are largely manual, we provide an FDA-approved metric for quickly determining drug efficacy of MS compounds. A VirtualScopics validation study compared manual tracing using two VirtualScopics software algorithms for automated measurement: geometrically constrained region growth (GEORG) and directed clustering. Our Core Lab utilizes both algorithms to achieve an optimal system for quantification of MS lesions in multi-spectral MRI studies. In the MS validation study, mean processing time was 60 minutes for manual tracing, 10 minutes for GEORG, and 3 minutes for directed clustering. Intra- and inter-operator coefficients of variation were 5.1% and 16.5% for manual tracing, 1.4% and 2.3% for region growth and 1.5% and 5.2% for directed clustering. The study also compared our automated measurement and manual tracing from an expert radiologist against a phantom data set, obtained from the McConnell Brain Imaging Center. In all data sets, automated algorithms performed significantly better than manual tracing. Our automated measurements also proved more repeatable than manual methods, an important feature in multi-center clinical trials.
Sales and Marketing
Our sales and business development strategy is centered around the publication and presentation of our technology and services at targeted industry conferences along with an active marketing effort aimed at pharmaceutical, medical device, and biotechnology companies. To date, we have made significant inroads by having contracts with 12 of the 15 leading pharmaceutical, biotechnology and medical device companies. During 2010, we performed services for 121 projects representing 31 customers. We continue to grow our business by leveraging relationships with our current customers and through referrals. As a result, our current customers have been instrumental in introducing us to other therapeutic groups within their organization. Our marketing efforts are instrumental in broadening the awareness of VirtualScopics throughout the industry and educating current customers on the breadth of our services.
Complementing our sales and marketing effort, we actively participate in medical conferences to showcase our technology, services and results, as well as joint publications with sponsors which often results in highly visible, research. We have built a strong base of clinical collaborators across varied disease platforms.
We are continuing an active sales and marketing effort and are currently in discussions with a number of additional potential customers to form businesses and/or partnerships.
Industry Background and Market Trends
Market in Pharmaceutical and Medical Device Development
Industry Overview
The global Pharmaceutical market is expected to grow by 5-7% in 2011 with the industry forecasted to generate around $880 billion in revenues with an expected expansion to $975 billion by 2013. Although impacted by the economic downturn in 2009, the industry is insulated to a greater extent than other industries where consumer spending is far more discretionary. Other factors such as patent expirations, the introduction of cheaper generics, a slowdown in innovative product launches, and hurdles imposed by payers on market access and acceptance have contributed to record low sales growth this year. While the pharmaceutical market is expected to rebound as the global economy recovers, an unprecedented level of potential patent expirations in 2011 and 2012 will curb sales growth. In spite of these pressures, the demand for medicines and treatments is expected to rise due to 1) an aging world population with an increased need for medical care, 2) unhealthy lifestyles leading to increased frequency of chronic diseases, 3) high economic growth in emerging markets leading to an increased demand for better quality healthcare and 4) scientific advances that create the foundation for innovative treatments for previously untreatable diseases.
The global compound annual growth rate (CAGR) for pharmaceutical market growth is forecasted to be 4-7 percent through 2013.1The U.S. Pharmaceutical market is expected to expand by 3-5 percent in 2011. Emerging markets on the other hand are expected to grow collectively at a 15-17 percent rate in 2011 to $170-180 billion. Meanwhile, the five major European markets of Germany, France, Italy, Spain and the UK, along with Canada, will only grow at a 1-3% pace. Approximately 50 - 60 new chemical or biological products are expected to be launched over the next two years with at least 10 of them being potential blockbuster drugs.
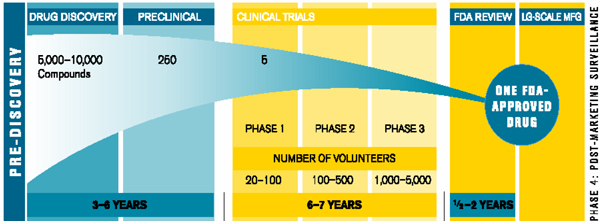
As the growth rate in the demand for prescription drugs decreases, it is getting harder for Pharmaceutical companies to maintain the same levels of R&D spending as in the past. Additionally, the cost and complexity of developing new drugs, in part due to the increased scrutiny over product safety and the pressure to demonstrate health outcomes earlier, has increased substantially relative to its eventual potential value. Due to the high attrition rates, with Phase 3 terminations alone doubling from 2007-2009 compared with 2004-2006, the total resources required to yield one successful product are rising. For every 5,000 -10,000 compounds that enter at the discovery stage, only one goes on to reach the market. The table below illustrates the complete R&D process from pre discovery to market.
1 imshealth.com - IMS Health Lowers 2009 Global Pharmaceutical Market Forecast to 2.5 – 3.5 Percent Growth
 2
2
Drug Development Process
Typically, most functions of the drug and medical device R&D process are managed by Clinical Research Organizations (CROs). Rising costs and falling productivity, among other trends are driving pharmaceutical companies to outsource an increasing range of functions to CROs in search of time and cost savings. This produced strong double-digit growth in the CRO sector between 2003 and 2008. The total CRO market size is estimated to reach $27bn by 2011 with a CAGR of 15%. The market is highly fragmented and the number of CROs worldwide has reached over 1,100 despite continued consolidation. The leading CRO’s are Quintiles (7.6%), Covance (4.7%) and PPD (4.3%)
Although the FDA has significantly reduced the average approval time for new drugs (1.1 years in 2005-2007 period), clinical development time has been increasing over the years, resulting in total development time being fairly flat in recent years (average of 8.6 years since 2002). Growing complexities in protocol design leading to longer clinical development times has been the major contributor to the rising costs that sponsors are facing. The table below illustrates the increasing trend in R&D costs over the years, while the number of new drug approvals continues to stagnate.
2 PHRMA Industry Report 2009

The current trend in drug development is for pharmaceutical companies to shift towards a niche market. The 'one size fits all' approach is being replaced by a more targeted, innovative approach to develop treatments for small patient groups with complicated diseases such as cancer, rheumatoid arthritis and immune disorders. Such 'niche buster drugs' are expected to exploit new technologies such as biomarkers and theranostics and will support the continued development of personalized medicine.
With the U.S administration trying to implement health care reform, increased regulatory oversight and pressure on drug companies to reduce prices, there is a need for R&D to become more efficient and reduce costs to prevent an innovation slowdown in the industry. Many leading pharmaceutical companies have restructured their R&D processes by establishing centers of R&D excellence and disease focused centers. Commercial success rate for new drugs is low, with only 2 out of 10 drugs matching or exceeding average R&D costs.
Because of these factors, we believe our quantitative imaging analysis offer a solution to these issues within drug development by providing more precise and reliable information in the assessment of compounds being developed. We believe our increased precision and reproducibility enable our customers to make more confident decisions on the efficacy of their compounds.
Quantitative Image Analysis Services
We have conducted research to determine the current size of the market for image analysis services in clinical trials supporting the pharmaceutical, biotech and medical device industries. Based on our research and discussion within the imaging CRO and medical device and drug development industries, we have found that the market is fragmented, with approximately $550 million in total annual revenues projected for 2010.
Prior to 2010, the industry underwent a growth phase as the use of imaging end-points is becoming more prevalent within the FDA. In 2010, we estimate that the market size was consistent with 2009 as companies experienced reductions in R&D spending. We currently estimate the annual growth rate for the market at 5% to 10% for the next five years. Our estimates are based on the amount of trials currently conducted within therapeutic areas that we work in. We also have performed a bottom-up calculation of the individual growth rates of the companies and academic centers within the industry. We believe that some of the largest players, which offer the broadest set of capabilities, are also growing. Specifically, BioClinica, Perceptive (division of Parexel), RadPharm and ICON.
Image Analysis Solutions in the Pharmaceutical and Medical Device Industries
It is well known that greater reproducibility of measurements can decrease the cost and time to market of compounds in development. The higher reproducibility of our automated analysis enables researchers to achieve statistically significant results with substantially smaller patient populations. Automated analysis greatly reduces the analyst variability and interaction time required to process clinical trial data. Published studies demonstrate that our automated analysis consistently yields a lower coefficient of variation than manual techniques. As measurement variation diminishes, so does the percentage change in a given structure necessary to determine whether a treatment is having the desired effect. In short, precise measurement allows companies to learn more from smaller populations earlier in the compound development process.
Drug discovery and development has been constrained by the lack of accurate image analysis tools and appropriate image-based biomarkers. In many musculoskeletal clinical studies, X-ray is the chosen modality for evaluating a compound’s efficacy. X-ray imaging in drug discovery has significant limitations, which include:
| | · | partial or complete inability to detect changes in a region of interest due to poor contrast or occlusion; |
| | · | the potential for inter/intra-observer variability - error in radiologist measurements can amount to upwards of 30% for small structures of interest; |
| | · | the need for a radiologist to perform manual tracings is not only subject to error, but is also time consuming; and |
| | · | reliance on a radiologist for biomarker measurements results in very limited throughput. |
The constraints mentioned above can add months and years to the drug discovery process.
The use of MRI and CT to determine drug efficacy is increasing, owing to its superior information content relative to X-ray. MRI and CT are more sensitive to pathology, provide higher contrast for soft tissue and are three-dimensional. These attributes improve the detection of disease and the ability to monitor disease progression over time. While MRI and CT are preferred modalities, they too suffer from the need to have a radiologist review the images, detect disease, monitor progression and, when necessary, perform manual calculations.
Intellectual Property
We consider our proprietary and patented technology and the technology for which we have applied for patent protection to be of importance to our business plan. We hold ten patents issued by the United States Patent and Trademark Office. These patents begin to expire in November 2018 through 2027. We have also applied for a number of other patents, both domestically and in foreign jurisdictions. To protect our proprietary technology, we rely primarily on a combination of confidentiality procedures, copyright, trademark and patent laws. Our policy is to require employees and consultants to execute confidentiality and invention assignment agreements upon the commencement of their relationship with us. These agreements provide that confidential information developed or made known during the course of a relationship with us must be kept confidential and not disclosed to third parties except in specific circumstances and for the assignment to us of intellectual property rights developed within the scope of the employment relationship.
Competition
Our main competitors are clinical research organizations (CROs) providing clinical trial services to pharmaceutical companies. As of the date of this report, we believe that none of the leading CROs have technology capabilities that are comparable to our technology. CROs typically provide manual and non-differentiated interpretation of medical images for the pharmaceutical industry. As a result, we believe that currently there is an opportunity for us to establish a technology advantage and a set of differentiated services in the advanced image-based biomarker market.
Our technology competition is largely comprised of a limited number of university research centers that are working on developing the next generation of image analysis tools. Aside from the university centers, there are a few commercial entities that have a desire to provide these advanced imaging services; however, we believe they are constrained by their lack of technical capabilities.
Competitors in Accelerating Pharmaceutical and Medical Device Development
The main CROs which participate in imaging trials are BioClinica, Core Lab Partners, Synarc, Perceptive and ICON. It is our understanding that these companies use predominately manual approaches that are unable to quantify minute structures in medical images. As a result, it may be difficult for them to offer differentiated services to achieve higher profit margins and at the same levels of reproducibility as ours. Additionally, some academic centers have worked on software that has applications for neurological diseases. These academic centers include Duke Image Analysis Laboratory, University of Pennsylvania, University of Montreal and University of California at San Francisco. However, we believe these organizations lack the required FDA compliance standards and ability to scale their operations to meet customer demand and we believe they offer inferior technology.
Government Regulation
Healthcare in the United States is heavily regulated by the federal government, and by state and local governments. The federal laws and regulations affecting healthcare change constantly, thereby increasing the uncertainty and risk associated with any healthcare-related company.
The federal government regulates healthcare through various agencies, including the following:
| | · | the Food and Drug Administration, or FDA, which administers the Food, Drug, and Cosmetic Act, or FD&C Act, as well as other relevant laws; |
| | · | Centers for Medicare & Medicaid Services, or CMS, which administers the Medicare and Medicaid programs; |
| | · | the Office of Inspector General, or OIG, which enforces various laws aimed at curtailing fraudulent or abusive practices, including by way of example, the Anti-Kickback Law, the Anti-Physician Referral Law, commonly referred to as Stark, the Anti-Inducement Law, the Civil Money Penalty Law, and the laws that authorize the OIG to exclude health care providers and others from participating in federal healthcare programs; and |
| | · | the Office of Civil Rights which administers the privacy aspects of the Health Insurance Portability and Accountability Act of 1996, or HIPAA. |
All of the aforementioned are agencies within the Department of Health and Human Services, or HHS. Healthcare is also provided or regulated, as the case may be, by the Department of Defense through its TriCare program, the Public Health Service within HHS under the Public Health Service Act, the Department of Justice through the Federal False Claims Act and various criminal statutes, and state governments under the Medicaid program and their internal laws regulating all healthcare activities.
FDA
The FDA regulates medical devices. A “medical device,” or device, is an article, including software and software associated with another medical device, which, among other things, is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease, in man or other animals. Computer software that complements a CT or MRI scan, such as VirtualScopics, we believe is considered a medical device and is therefore subject to FDA regulation. To date, our sales have been to the pharmaceutical and medical device industries to support their clinical trials. We would need to obtain FDA clearance or approval, as discussed below, before using our technology and services for diagnostic or treatment planning in a clinical setting. When we begin pursuing the application of our services in patient diagnostics, no assurance can be given that such clearance or approval would be granted or that it would be granted in a timely manner.
Devices are subject to varying levels of regulatory control, the most comprehensive of which requires that a clinical evaluation be conducted before a device receives approval for commercial distribution. In the United States, we generally are able to obtain permission to distribute a new device in two ways. The first applies to any new device that is substantially equivalent to a device first marketed prior to May 1976. In this case, to obtain FDA permission to distribute the device, we generally must submit a premarket notification application (a section 510(k) submission), and receive an FDA order finding substantial equivalence to a device (first marketed prior to May 1976) and permitting commercial distribution of that device for its intended use. A 510(k) submission must provide information supporting its claim of substantial equivalence to the predicate device.
If clinical data from human experience are required to support the 510(k) submission, these data must be gathered in compliance with investigational device exemption (IDE) regulations for investigations performed in the United States. The 510(k) process is normally used for software products of the type that we propose distributing. The FDA review process for premarket notifications submitted pursuant to section 510(k) takes on average about 90 days, but it can take substantially longer if the agency has concerns, and there is no guarantee that the agency will “clear” the device for marketing, in which case the device cannot be used for diagnosis and distributed in the United States. Nor is there any guarantee that the agency will deem the article subject to the 510(k) process, as opposed to the more time-consuming and resource intensive and problematic, premarket approval, or PMA, process described below.
The second, more comprehensive, approval process applies to a new device that is not substantially equivalent to a pre-1976 product. In this case, two steps of FDA approval generally are required before we can market the product in the United States. First, we must comply with IDE regulations in connection with any human clinical investigation of the device. Second, the FDA must review our PMA application, which contains, among other things, clinical information acquired under the IDE. The FDA will approve the PMA application if it finds there is reasonable assurance the device is safe and effective for its intended use.
Certain changes to existing devices that do not significantly affect safety or effectiveness can be made with in vitro testing under reduced regulatory procedures, generally without human clinical trials and by filing a PMA supplement to a prior PMA. Exported devices are subject to the regulatory requirements of each country to which the device is exported, as well as certain FDA export requirements.
After approval or clearance to market is given, the FDA and foreign regulatory agencies, upon the occurrence of certain events, have the power to withdraw the clearance or require changes to a device, its manufacturing process, or its labeling or additional proof that regulatory requirements have been met.
A device manufacturer is also required to register with the FDA. As a result, we may be subject to periodic inspection by the FDA for compliance with the FDA’s Quality System Regulation requirements and other regulations. In the European Community, we are required to maintain certain International Organization for Standardization (ISO) certifications in order to sell product and to undergo periodic inspections by notified bodies to obtain and maintain these certifications. These regulations require us to manufacture products and maintain documents in a prescribed manner with respect to design, manufacturing, testing and control activities. Further, we are required to comply with various FDA and other agency requirements for labeling and promotion. The Medical Device Reporting regulations require that we provide information to the FDA whenever there is evidence to reasonably suggest that a device may have caused or contributed to a death or serious injury or, if a malfunction were to occur, could cause or contribute to a death or serious injury. In addition, the FDA prohibits us from promoting a medical device for unapproved indications.
We currently meet the requirements of Good Clinical Practices: Consolidated Guidance, which governs the conduct of clinical trials, and our software complies with the FDA’s Regulation 21 CFR Part 11 (Electronic Records; Signatures) and 21 CFR Part 820.30, which outline the requirements for design controls in medical devices. As mentioned throughout this section, as we develop our approach into personalized medicine, FDA approval would most likely be required for the use of our software in that market.
Privacy Provisions of HIPAA
HIPAA, among other things, protects the privacy and security of individually identifiable health information by limiting its use and disclosure. HIPAA directly regulates “covered entities” (healthcare providers, insurers and clearinghouses) and indirectly regulates “business associates” with respect to the privacy of patients’ medical information. All entities that receive and process protected health information are required to adopt certain procedures to safeguard the security of that information. It is our policy to comply with HIPAA requirements.
Research and Development Costs
We incurred $1,130,744 and $1,058,676 in research and development costs for the years ended December 31, 2010 and 2009, respectively.
Customers
One customer accounted for 10% or more of our revenue during the year ended December 31, 2010, this same customer accounted for more than 10% of our revenue during the year ended December 31, 2009. The following table sets forth information as to revenue and percentage of revenue for these years for our three largest customers in 2010 and corresponding revenues for 2009:
| | | Years Ended December 31, | |
| Customer | | 2010 | | | 2009 | |
| | | | | | | | | | | | | |
| Novartis | | $ | 7,494,103 | | | | (56 | )% | | $ | 4,473,523 | | | | (43 | )% |
| Merck Serono | | $ | 721,069 | | | | (5 | )% | | $ | 851,385 | | | | (8 | )% |
| Johnson & Johnson | | $ | 642,616 | | | | (5 | )% | | $ | 470,825 | | | | (5 | )% |
Employees
As of December 31, 2010, we had 88 employees and seven contract radiologists. Of our employees, 81 are full-time.
ITEM 1A: Risk Factors
You should carefully consider the following risk factors before making an investment decision. If any of the following risks actually occur, our business, financial condition, or results of operations could be materially adversely affected. In such cases, the trading price of our common stock could decline, and you may lose all or part of your investment.
We have a history of operating losses and uncertain future profitability.
Prior to 2009, VirtualScopics incurred losses from operating activities since it began operations in 2001. As we continue to grow the business, we may face risks and difficulties in our business including uncertainties of further market penetration, competition, cost increases and delays in achieving business objectives. There can be no assurance that we will succeed in addressing any or all of these risks or that we will achieve future profitability and the failure to do so would have a material adverse effect on our business, financial condition and operating results.
If our products and services do not continue to attract interest from new and existing customers, we may not achieve future growth.
If we are unable to continue to attract interest in the industry for our services, we could fail to achieve future growth which would have a detrimental effect on our business. Our ability to generate revenues is highly dependent on building and maintaining relationships with leading pharmaceutical and biotechnology companies. No assurance can be given that a sufficient number of such companies will increase their demand for our services, thereby limiting the overall market for quantitative imaging services and not enable us to increase our revenue to the extent expected. In addition, the rate of the growth of MRI and CT image-based biomarkers is difficult to predict. Failure to attract and maintain a significant customer base would have a detrimental effect on our business, operating results and financial condition.
The majority of the contracts we have with customers are cancelable for any reason by giving 30 days advance notice.
Our customers typically engage us to perform services for them on a project-by-project basis and are required by us to enter into a written contractual agreement for the work, labor and services to be performed. Generally, our project contracts are terminable by the customer for any or no reason on 30 days’ advance notice to us. If a number of our customers were to exercise cancellation rights, our business and operating results would be materially and adversely affected.
If we are unable to manage and sustain our growth, our operating results would be adversely affected.
We have seen a growing demand for our image analysis services over the past several years. Although there can be no assurance that this will continue, if it does continue we may be unable to scale our capacity efficiently to meet this demand. If we are unable to do so, we may fail to maintain our operating margins or achieve expected operating margins. This may have a material and adverse effect on our operating results.
Our services may become obsolete if we do not effectively respond to rapid technological change on a timely basis.
Our services depend on the needs of our customers and their desire to utilize image-related services in drug and medical device development and clinical diagnosis and treatment. Since the image-based biomarker industry is characterized by evolving technologies, uncertain technology and limited availability of standards, we must respond to new research findings and technological changes affecting our customers. We may not be successful in developing and marketing, on a timely and cost-effective basis, new or modified products and services, which respond to technological changes, evolving customer requirements and competition. If we are unsuccessful in this regard, our business and operating results could be materially and adversely affected.
Although we believe that our products and services do not and will not infringe upon the patents or violate the proprietary rights of others, it is possible such infringement or violation has occurred or may occur which could have a material adverse effect on our business.
Portions of our business are reliant upon patented and patentable systems and methods used in our image analysis and related intellectual property. In the event that products and services we sell are deemed to infringe upon the patents or proprietary rights of others, we could be required to modify our products and services or obtain a license for the manufacture and/or sale of such products and services. In such event, there can be no assurance that we would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon our business. Moreover, there can be no assurance that we will have the financial or other resources necessary to enforce or defend a patent infringement or proprietary rights violation action. In addition, if our products and services or proposed products and services are deemed to infringe or likely to infringe upon the patents or proprietary rights of others, we could be subject to injunctive relief and, under certain circumstances, become liable for damages, which could also have a material adverse effect on our business.
We are subject to numerous pharmaceutical, medical device and healthcare industry regulations, which could adversely affect the nature and extent of the products and services we offer.
Many aspects of the pharmaceutical, medical device and healthcare industry are subject to regulation at the federal level. From time to time, the regulatory entities that have jurisdiction over the industry adopt new or modified regulations or take other actions as a result of their own regulatory processes or as directed by other governmental bodies. This changing regulatory environment could adversely affect the nature and extent of the services we are able to offer.
To date, our sales have been within the clinical trial industry. To enter the patient treatment or personalized medicine market we would need to obtain FDA clearance or approval before marketing our services in this area. There can be no assurance that such clearance or approval would be granted or that it would be granted in a timely manner. To effectively market our products to physicians as a treatment aid, we would also need to obtain appropriate coverage and favorable reimbursement from third-party payers, such as Medicare and insurance companies, in order to more fully benefit from the market opportunity. There can be no assurance that appropriate coverage would be granted or that reimbursement levels or conditions of coverage would be adequate to ensure acceptance among physicians. Additionally, the efforts of governments and third-party payers to contain or reduce the cost of health care, such as a number of legislative and regulatory proposals currently being discussed, could affect our ability to implement our plans in this area.
We may in the future experience competition from academic sites, imaging CROs, and other competing technologies.
Competition in the development of imaging solutions may become more widespread as with emerging technologies such as proteomics and genomics which can serve as predictive tools of drug efficacy. Competitors range from university-based research and development projects which would develop advanced tools to development stage companies and major domestic and international companies which would commercialize the tools. Some of these entities have greater financial, technical, marketing, sales, distribution and other resources than ours. There can be no assurance that we can continue to develop our technologies or that present or future competitors will not develop technologies that render our image-based biomarker industry obsolete or less marketable or that we will be able to introduce new products and product enhancements that are competitive with other products marketed by industry participants.
We have experienced significant demand from one customer, thereby increasing our dependence on the customer until we can further diversify our customer base.
While we continue to serve a broad range of customers, we’ve experienced strong demand from one of our customers, and our dependence on that customer to sustain our continued growth has increased. In 2010, this customer accounted for 56% of our revenue. We continue to see demand from other customers, however, not to the same significant pace. We continue to invest on our sales and marketing efforts to further diversify our customers and more broadly penetrate the market, in order to minimize reliance on any one customer. As with all of our contracts, this customer may terminate its contractual relationship with us for any or no reason on 30 days’ advance notice. A decision by the customer to cancel all of its studies with us could have an adverse impact on the growth of our business.
Consolidation within the pharmaceutical industry and changes within healthcare regulation may have an adverse impact on our business.
Over the past few years, there have been several mergers and acquisitions among pharmaceutical and biotechnology companies. Historically, these transactions have positively impacted our business due to a broader exposure within the merged entity, however, there can be no assurance that consolidation within the industry will continue to be beneficial to us. Additionally, with the recent political landscape and changes within the healthcare industry, there may be an adverse impact on our business if the cost of imaging significantly increases or no longer becomes standard of care for patients. Although, we don’t believe imaging will decline in its level of use, if it does we may need to reduce prices or invest in research to advance the education and science of medical imaging.
The trading price of our stock may be adversely affected if we are not able to continue to grow the business.
We intend to continue to use our cash on hand to broaden our market penetration of our services within the industry and pursue the application of one of our technologies in the personalized medicine market. If our plans or assumptions with respect to our business change or prove to be inaccurate, we may be required to use part or all of our cash to fund general operating expenses and/or reduce costs within the organization. This will depend on a number of factors, including, but not limited to:
| | · | the lack of significant cancellations of customer contracts |
| | · | the further market penetration of our products and services; and |
| | · | our ability to manage and sustain the growth of our business. |
We currently do not plan to raise additional capital, however, if we need to raise additional capital, it may not be available on acceptable terms, or at all. Our failure to obtain required capital, or the acquisition of capital on less favorable terms, would have a material adverse effect on our business. If we issue additional equity securities in the future, you could experience dilution or a reduction in priority of your securities.
The market price of our common stock may fluctuate significantly.
The market price of our common stock may fluctuate significantly in response to factors, some of which are beyond our control, such as the announcement of new products or product enhancements by us or our competitors; developments concerning intellectual property rights and regulatory approvals; quarterly variations in our competitors’ results of operations; changes in earnings estimates or recommendations by securities analysts; developments in our industry; product liability claims or other litigation; and general market conditions and other factors, including factors unrelated to our own operating performance.
Our common stock may be considered a “penny stock” and may be difficult to sell.
The SEC has adopted regulations which generally define “penny stock” to be an equity security that has a market or exercise price of less than $5.00 per share, subject to specific exemptions. The market price of our common stock is currently below $5.00 per share and therefore may be designated as a “penny stock” according to SEC rules. This designation requires any broker or dealer selling these securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase the securities. These rules may restrict the ability of brokers or dealers to sell our common stock and may affect the ability of our stockholders to sell their shares.
Our strategic alliance with PPD is an important aspect of our growth, and the market may not value our strategic alliance with PPD as we anticipate.
In 2010, we formed an alliance with PPD to provide a joint solution to provide clients with an integrated and customized clinical development and medical imaging solution for oncology clinical trials. If the market does not value this model as we anticipate, our ability to grow our business may be negatively impacted. Additionally, the agreement may be terminated by either party on 90 days notice. In the event PPD terminates the agreement, we may also experience a negative impact in our ability to experience the level of growth the company has historically achieved.
A significant number of the shares of our common stock are eligible for sale, and their sale could depress the market price of the our common stock.
Sales of a significant number of shares of our common stock in the public market or the possibility of such sales, could harm the market price of our common stock and impede our ability to raise capital through the issuance of equity securities. As of December 31, 2010, we had 27,414,620 shares of common stock outstanding. These shares are eligible for resale in the public market either immediately or subject to applicable volume, manner of sale, holding period and other limitations of Rule 144. In addition to these outstanding shares of common stock, the series B convertible preferred stock and the warrants to purchase common stock issued in our 2007 private placement are convertible into 2,774,494 shares of our common stock and registered for resale under a registration statement on Form S-3. Additionally, there are outstanding warrants issued prior to 2005 that are convertible into 444,888 shares of our common stock. There are also 2,647,191 shares of our common stock issuable upon conversion of our series A convertible preferred stock and warrants sold in our November 2005 private placement, eligible for resale under Rule 144. Additionally, we have filed registration statements on Form S-8 to register the sale of up to 6,900,000 shares issued or to be issued pursuant to our Amended and Restated 2006 Long-Term Incentive Plan. Finally, we have approximately 6,021,033 shares of common stock underlying options issued under our 2001, 2005, and 2006 Long-Term Incentive Plans that may be eligible for resale in the public market pursuant to an exemption under Rule 701 of the Securities Act. Sales of our common stock in the public market may have a depressive effect on the market for the shares of our common stock.
Our principal stockholders have significant voting power and may take actions that may not be in the best interests of other stockholders.
Our officers, directors, principal stockholders (greater than 10%) and their affiliates control approximately 30% of our outstanding voting securities. If these stockholders act together, they will be able to exert significant control over our management and affairs requiring stockholder approval, including approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of all our stockholders.
We do not anticipate paying dividends on our common stock in the foreseeable future, and the lack of dividends may have a negative effect on the stock price.
We currently intend to retain our future earnings to support operations and to finance expansion and meet dividend obligations on our series B convertible preferred stock. In addition, the terms of our series B preferred stock limit our ability to pay dividends to the holders of our common stock. Therefore, we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
ITEM 2: Properties
In July, 2007 we began leasing approximately 19,500 square feet of office space at our corporate headquarters in Rochester, New York. The base annual rent under the lease is $360,000, and increases three percent (3%) a year. During the first twenty months of the lease, the rent was paid in two portions: a cash portion of $156,000 annually, paid in equal monthly installments, increasing three percent (3%) annually; and, a stock portion of $204,000 annually, paid in equal monthly installments, increasing three percent (3%) annually. The stock portion was payable in shares of our common stock. In February 2009, the Landlord exercised their option to receive their remaining rental payments in all cash. During 2010 and 2009, we issued 0 and 88,132 shares, respectively, of the Company’s common stock ranging from $1.20 to $2.50 per share for a total value of $0 and $105,570, respectively. Management believes that the leased property is adequately covered by insurance.
ITEM 3: Legal Proceedings
None.
PART II
ITEM 5: Market For Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our shares of common stock are listed for trading on the NASDAQ Capital Market under the trading symbol “VSCP.” The following table sets forth the high and low closing sales prices for our common stock as reported on the NASDAQ Capital Market for the period from January 1, 2009 through December 31, 2010. These prices do not include retail markup, markdown or commission and may not necessarily represent actual transactions. Investors should not rely on historical stock price performance as an indication of future price performance.
Fiscal Year Ended December 31, 2009
| | | HIGH | | | LOW | |
| | | | | | | |
| First Quarter | | $ | 1.10 | | | $ | 0.43 | |
| Second Quarter | | | 1.26 | | | | 0.83 | |
| Third Quarter | | | 1.48 | | | | 1.01 | |
| Fourth Quarter | | | 1.22 | | | | 1.01 | |
Fiscal Year Ended December 31, 2010
| | | HIGH | | | LOW | |
| | | | | | | |
| First Quarter | | $ | 1.25 | | | $ | 0.82 | |
| Second Quarter | | | 1.35 | | | | 1.13 | |
| Third Quarter | | | 1.17 | | | | 0.90 | |
| Fourth Quarter | | | 2.50 | | | | 0.89 | |
As of February 28, 2011, we had approximately 28,378,997 registered holders of record of shares of our common stock.
Dividend Policy
We have never declared a cash dividend on our common stock. We intend to retain any earnings to fund future growth and the operation of our business and, therefore, we do not anticipate paying any cash dividends on our common stock in the foreseeable future. In addition, the terms of our series B preferred stock limit our ability to pay dividends to the holders of our common stock. Thereafter, dividends may be paid on our common stock only if and when declared by our board of directors and paid on an as-converted basis to the holders of our series A and series B convertible preferred stock.
Equity Compensation Plan Information The following table summarizes information, as of December 31, 2010, relating to our equity compensation plans:
| | | Number of Securities to be Issued Upon Exercise of Outstanding Options | | | Weighted-Average Exercise Price of Outstanding Options | | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a) | |
Plan Category | | (a) | | | (b) | | | (c) | |
| Equity compensation plans approved by security holders | | | 6,183,451 | (1) | | $ | 1.16 | | | | 2,228,190 | |
| Equity compensation plans not approved by security holders | | | 350,000 | (2) | | $ | 2.50 | | | | - | |
| Total | | | 6,533,451 | | | $ | 1.23 | | | | 2,228,190 | |
(1) This amount includes shares under the plans of VirtualScopics, LLC, in addition to 444,888 shares of our common stock to holders of warrants granted by VirtualScopics, LLC, in exchange for consideration in the form of goods and services. Also we agreed to issue 5,671,033 shares of common stock collectively, under our 2001, 2005 and 2006 Long Term Incentive Plans. Also included are 67,530 shares of common stock underlying warrants we issued to the placement agent in connection with our September 2007 private placement, which was approved by stockholders in November 2007.
(2) In November 2005, our Board of Directors granted to our Chairman and former CEO, Robert Klimasewski, an option to purchase 350,000 shares of our common stock at $2.50 per share.
Recent Sales of Unregistered Securities
During the quarter ended December 31, 2010, the Company issued 1,018,019 shares of common stock upon the conversion of 1,226 shares of series B convertible preferred stock. The Company did not receive any cash or other consideration in connection with the conversion. Additionally, no commission or other remuneration was paid by the Company in connection with the conversion. The issuance of common stock upon conversion of the series B convertible preferred stock was made in reliance on the exemption provided in Section 3(a)(9) of the Securities Act of 1933, as amended.
Issuer Repurchases of Equity Securities
None.
ITEM 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with VirtualScopics’ consolidated balance sheet, and related consolidated statements of operations, changes in stockholders’ equity and cash flows for the years ended December 31, 2010 and 2009, included elsewhere in this report. This discussion contains forward-looking statements, the accuracy of which involves risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements for many reasons including, but not limited to, those discussed in “Risk Factors” and elsewhere in this report. We disclaim any obligation to update information contained in any forward-looking statements.
Overview
VirtualScopics, Inc. is a leading provider of imaging solutions to accelerate drug and medical device development. We have developed a robust software platform for analysis and modeling of both structural and functional medical images. In combination with our industry-leading experience and expertise in advanced imaging biomarker measurement, this platform provides a uniquely clear window into the biological activity of drugs and devices in clinical trial patients, allowing our customers to make better decisions faster.
In July 2000, VirtualScopics was formed after being spun out of the University of Rochester. In June 2002, we purchased the underlying technology and patents created by VirtualScopics’ founders from the University of Rochester. In November 2005, we performed an exchange transaction with ConsultAmerica whereby prior to the exchange ConsultAmerica, Inc. had no meaningful operations and, therefore, the exchange was treated as recapitalization of VirtualScopics, LLC. Following the transaction, we succeeded to the business of VirtualScopics, Inc. as a provider of image-based biomarker solutions and have continued this business since that time. We own all rights to the patents underlying its technology.
Revenue over the past nine years has been derived primarily from image processing services in connection with pharmaceutical drug trials. For these services, we have been concentrating in the areas of oncology and osteoarthritis. We have also derived a small portion of revenue from consulting services, and pharmaceutical drug trials in the neurology and cardiovascular areas. We expect that the concentration of our revenue will continue in these services and in those areas in 2011. Revenues are recognized as the MRI and CT images that we process are quantified and delivered to our customers and/or the services are performed. Beginning in 2010, we began to pursue the personalized medicine market, however, we do not anticipate significant revenues from this market opportunity in 2011 as we are just beginning the process of executing on our regulatory, validation and commercialization strategy.
As of December 31, 2010, the amount remaining to be earned from active projects and awards was approximately $40 million. Once we enter into a new contract for participation in a drug trial, there are several factors that can effect whether we will realize the full benefits under the contract, and the time over which we will realize that revenue. Customers may not continue our services due to performance reasons with their compounds in development. Furthermore, the contracts may contemplate performance over multiple years. Therefore, revenue may not be realized in the fiscal year in which the contract is signed or the award is made. Recognition of revenue under the contract may also be affected by the timing of patient recruitment and image site identification and training. Additionally, the majority of contracts we have with customers are cancelable for any reason by giving 30 days advance notice.
Results of Operations
Results of Operations for Year Ended December 31, 2010 Compared to Year Ended December 31, 2009
Revenues
We had revenues of $13,393,000 for the year ended December 31, 2010 compared to $10,393,000 for the year ended December 31, 2009, representing a 29% increase. The increase in revenues is directly related to the growing demand for our services in the industry. We continue to see greater demand for our oncology analysis services as well as demand within the medical device industry. During 2010, we performed work for 31 customers, representing 121 different projects, in connection with their pharmaceutical drug trials primarily in the fields of oncology and musculoskeletal diseases (osteoarthritis and rheumatoid arthritis) along with various other projects. This compares to 28 customers representing 109 projects in 2009. At the end of 2010, our backlog (amounts remaining to be earned from active projects and awards) was $40 million, this compares to a backlog at the end of 2009 of $36 million, representing an 11% increase. As of the date of this report the backlog was $37 million, the reduction in the backlog from the end of 2010 is a result of recognized revenue for January and February 2011 as well as the cancellation of a few projects.
As of December 31, 2010, we had active projects with 12 of the leading 15 pharmaceutical and biotechnology companies in the world. In 2010, 73% of our revenues were generated from Phase II and Phase III studies compared to 64% in 2009. Additionally, for the year ended December 31, 2010, oncology, musculoskeletal and other projects represented 80%, 16%, and 4%, respectively, of our revenues. This compares to 76%, 17%, and 7%, respectively, for 2009.
Gross Profit
We had a gross profit of $6,824,000 for the year ended December 31, 2010 compared to $5,588,000 for the comparable period in 2009. Our gross profit improved year over year due to the increase in revenues as outlined above. Our gross margin is a reflection of the project mix that we experience during a given period. Start up activities associated with getting a project underway, including site initiation, qualification and training, yield a higher margin than image analyses. Therefore, depending on the project mix, we may experience fluctuations in our reported margins. We anticipate our gross margins (excluding reimbursements) will approximate 45-50% throughout 2011 based on the projects that are currently in our pipeline.
Research and Development
Research and development costs increased in 2010 by $72,000, or 7%, to $1,131,000, when compared to 2009. The increase was largely attributed to the allocation of resources within our research and development group during 2010 between internal and customer related projects. As of December 31, 2010, there were ten employees in our research and development group, this includes the algorithm and software development functions. Our research and development efforts are centered around improving the functionality of our existing algorithms and software platform as well as the refinement of our algorithms to new therapeutic areas. In 2011, we anticipate making investments within our software platform in order to generate more operating efficiencies and enhanced reporting tools as well as costs associated with our 510k filing with the FDA for our personalized medicine software application.
Sales and Marketing
Sales and marketing costs decreased in 2010 by $80,000, or 6%, to $1,184,000, when compared to 2009. The decrease was a result of the departure of our VP of Business Development during 2010. In February 2011, we hired an experienced individual in the position of VP and Business Development to drive greater penetration of the market. We anticipate additional investments in our sales and marketing efforts in 2011 as we execute on our revenue growth initiatives. We also plan to continue to develop our strategic alliance with PPD to expand and deepen new and existing customer relationships through an integrated joint solution to the industry. As of the date of this report, there are six individuals in our sales and marketing department.
General and Administrative
General and administrative expenses for the year ended December 31, 2010 were $3,125,000, representing a decrease of $51,000 or 2%, when compared to 2009. The slight decrease is mainly due to the reduction in stock compensation expense for vested stock options offset by higher consulting costs associated with our personalized medicine application. During 2011, the Company anticipates higher general and administrative costs as it continues its investment in its personalized medicine application.
Depreciation and Amortization
Depreciation and amortization charges increased for the year ended December 31, 2010 by $42,000 or 9%, to $512,000, when compared to 2009. This increase is attributed to the depreciation of newly purchased hardware and software to support the growth in revenues and employees. The amortization and depreciation costs are based on the timing and life of the patent costs and property and equipment. We continue to invest in our patent portfolio, however, do not anticipate significant expenditures to support our current business and future strategies. Our IT systems are the basis of our operating platform, therefore, we will continue to invest in our IT infrastructure to support our growth and ensure we have a robust and reliable operating system.
Other income(expense), net
Interest income for the year ended December 31, 2010 was $10,000, representing interest derived on the Company’s operating and savings accounts, compared to interest income of $7,000 in 2009. The increase in interest income was due to higher average account balances. Other expense for the years ended December 31, 2010 and 2009 was $25,000 and $22,000, respectively which relates to franchise taxes paid during the year. Additionally, we recognized a non-cash marked to market adjustment of $1,470,000 and $717,000 for the years ended December 31, 2010 and 2009, respectively, related to the loss in fair value of warrants that were issued in connection with our 2007 series B offering (see Financial Statement Note 5). Due to the price reset, or ratchet, provision within the warrant agreements, the accounting pronouncements require us to mark to market the change in fair value of the warrants during the reporting period. If our stock price appreciates during the reporting period, we report a loss, if our stock price declines, we will report a gain.
Net Loss
Our net loss for the year ended December 31, 2010 was $629,000 compared to a net loss of $1,011,000 for the year ended December 31, 2009. The decrease in our net loss over the prior period was related to higher revenues and controlled operating expenditures offset by the non-cash marked to market adjustment for the change in the fair value of certain outstanding warrants.
Liquidity and Capital Resources
Our working capital as of December 31, 2010 and 2009 was approximately $2,864,000 and $2,549,000, respectively. The increase in working capital was a result of the increase in operating income, cash and accounts receivable offset by increases in the liability associated with the valuation of warrants issued in our 2007 series B offering, in accordance with Accounting Standards Codification (“ASC”) 815-40.
Net cash provided by operating activities in 2010 was $679,000 compared to $1,850,000 in 2009. The decrease in the amount of cash provided by operating activities was primarily the result of the increase and timing of collection of our receivables due to the increased revenues. Also impacting our cash provided by operating activities was the reduction in the amount of unearned revenue at the end of 2010 compared to the end of 2009.
We invested $216,000 in the purchase of equipment and the acquisition of patents in 2010, compared to $362,000 for the investment in these items in 2009. This decrease represents a decrease in computer hardware and software purchased in 2010 compared to 2011 due to investments made in our storage and hardware equipment in 2010. We anticipate that our IT related costs will increase in 2011 as we support our growth which requires additional investments to be made in our operating system and infrastructure. During 2010, we incurred $12,000 in patent costs associated with filing costs for intellectual property, as compared to $45,000 in 2009. The reduction is due to the timing of office actions within our existing patent filings.
Net cash used in financing activities in 2010 was $215,000 compared to $304,000 in 2009. This decrease was due to lower required dividend payments on our series B preferred shares due to the conversion of the securities by the holders to common stock.
We currently expect that existing cash will be sufficient to fund our existing operations for the next 12 months and foreseeable future. If in the future our plans or assumptions change or prove to be inaccurate, we may be required to seek additional capital through public or private debt or equity financings. If we need to raise additional funds, we may not be able to do so on terms favorable to us, or at all. If we cannot raise sufficient funds on acceptable terms, we may have to curtail our level of expenditures and our rate of expansion.
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements, other than the consulting agreements and operating leases (as described in “Contractual Obligations” below) that have or are reasonably likely to have a current or future effect that is material to investors on our financial condition, revenues or expenses, results of operations, liquidity, capital resources or capital expenditures.
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2010 which we expect to have an effect on our liquidity and cash flow in future periods. (See Item 2: Description of Property for a full description of our lease obligations.)
| | | Payments Due by Period | |
| | | | | | Less than | | | | |
| | | Total | | | 1 Year | | | 1-2 Years | |
| | | | | | | | | | |
| Operating Leases | | $ | 518,181 | | | $ | 341,188 | | | $ | 176,993 | |
Recently Issued and Adopted Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2009-13, “Multiple-Deliverable Revenue Arrangements.” This ASU establishes the accounting and reporting guidance for arrangements including multiple revenue-generating activities. This ASU provides amendments to the criteria for separating deliverables, measuring and allocating arrangement consideration to one or more units of accounting. The amendments in this ASU also establish a selling price hierarchy for determining the selling price of a deliverable. Significantly enhanced disclosures are also required to provide information about a vendor’s multiple-deliverable revenue arrangements, including information about the nature and terms, significant deliverables, and its performance within arrangements. The amendments also require providing information about the significant judgments made and changes to those judgments and about how the application of the relative selling-price method affects the timing or amount of revenue recognition. The amendments in this ASU are effective prospectively for revenue arrangements entered into or materially modified in the fiscal years beginning on or after June 15, 2010. Early application is permitted. The Company is currently evaluating the impact the adoption of this standard will have on its consolidated financial position and results of operations.
In October 2009, the FASB issued ASU No. 2009-14, “Certain Revenue Arrangements That Include Software Elements.” This ASU changes the accounting model for revenue arrangements that include both tangible products and software elements that are “essential to the functionality,” and scopes these products out of current software revenue guidance. The new guidance will include factors to help companies determine what software elements are considered “essential to the functionality.” The amendments will now subject software-enabled products to other revenue guidance and disclosure requirements, such as guidance surrounding revenue arrangements with multiple-deliverables. The amendments in this ASU are effective prospectively for revenue arrangements entered into or materially modified in the fiscal years beginning on or after June 15, 2010. Early application is permitted. The Company is currently evaluating the impact the adoption of this standard will have on its consolidated financial position and results of operations.
In January 2010, the FASB issued ASU No. 2010-06, Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements. This ASU requires some new disclosures and clarifies some existing disclosure requirements about fair value measurement as set forth in Codification Subtopic 820-10. ASU 2010-06 amends Codification Subtopic 820-10 and now requires a reporting entity to use judgment in determining the appropriate classes of assets and liabilities and to provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. ASU 2010-06 is effective for interim and annual reporting periods beginning after December 15, 2009. As this standard relates specifically to disclosures, the adoption did not have an impact on the Company’s consolidated financial position and results of operations.
In February 2010, the FASB issued ASU 2010-09, "Subsequent Events (Topic 855) - Amendments to Certain Recognition and Disclosure Requirements." ASU 2010-09 requires an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement that an SEC filer disclose the date through which subsequent events have been evaluated. ASC 2010-09 was effective upon issuance. The adoption of this standard had no effect on our consolidated financial position or results of operations.
In March 2010, the FASB issued ASU No. 2010-17, Revenue Recognition— Milestone Method (Topic 605): Milestone Method of Revenue Recognition. This standard provides that the milestone method is a valid application of the proportional performance model for revenue recognition if the milestones are substantive and there is substantive uncertainty about whether the milestones will be achieved. Determining whether a milestone is substantive requires judgment that should be made at the inception of the arrangement. To meet the definition of a substantive milestone, the consideration earned by achieving the milestone (1) would have to be commensurate with either the level of effort required to achieve the milestone or the enhancement in the value of the item delivered, (2) would have to relate solely to past performance, and (3) should be reasonable relative to all deliverables and payment terms in the arrangement. No bifurcation of an individual milestone is allowed and there can be more than one milestone in an arrangement. The new standard is effective for interim and annual periods beginning on or after June 15, 2010. Early adoption is permitted. The Company is currently evaluating the impact the adoption of this standard will have on its consolidated financial position and results of operations.
ITEM 8: Financial Statements and Supplementary Data
The financial statements required hereby are located on pages F-1 through F-21 of this report.
ITEM 9: Changes In and Disagreements with Accountants on Accounting and Financial Disclosure
ITEM 9A: Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain a set of disclosure controls and procedures, as defined in Section 13a-15(e) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are designed to ensure that information required to be disclosed by us in the reports filed by us under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. We carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rule 13a-15(b) of the Exchange Act. Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report.
Notwithstanding the foregoing, there can be no assurance that the Company’s disclosure controls and procedures will detect or uncover all failures of persons within the Company to disclose material information otherwise required to be set forth in the Company’s periodic reports. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable, not absolute, assurance of achieving their control objectives.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of the financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only with proper authorizations; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management, under the supervision of and with the participation of the Chief Executive Officer and the Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2010 based on criteria for effective control over financial reporting described in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO. Based on this assessment, our management concluded that our internal control over financial reporting was effective as of December 31, 2010.
This annual report does not include an attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the Company's registered public accounting firm pursuant to Section 404(c) of the Sarbanes-Oxley Act of 2002, which permits the Company to provide only management's report in this annual report.
Changes in Internal Controls over Financial Reporting
An evaluation was performed under the supervision of the Company’s management, including the Chief Executive Officer and Chief Financial Officer, as required under Exchange Act Rules 13a-15(d) and 15d-15(d), of whether any change in the Company’s internal control over financial reporting occurred during the fiscal quarter ended December 31, 2010. Based on that evaluation, the Company’s management, including the Chief Executive Officer and Chief Financial Officer, concluded that no change in the Company’s internal controls over financial reporting occurred during the fiscal quarter ended December 31, 2010 that has materially affected or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B: Other Information
None.
PART III
ITEM 10: Directors, Executive Officers, Promoters and Control Persons; Compliance with Section 16(a) of the Exchange Act
The information required by this Item regarding our directors and executive officers is incorporated in this report by reference to our Proxy Statement for our 2011 Annual Meeting of Stockholders where such information appears under the heading “Directors and Executive Officers” in our Proxy Statement for our 2011 Annual Meeting of Stockholders.
Section 16(a) Beneficial Ownership Reporting Compliance
The discussion under the heading “Security Ownership of Certain Beneficial Owners and Management” in our definitive proxy statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference to such proxy statement.
Code of Ethics
The Company has adopted a Code of Ethics that is applicable to our principal executive officer and principal financial officer and can be viewed on our website www.virtualscopics.com.
ITEM 11. Executive Compensation
The information required by this Item is incorporated in this report by reference to our definitive Proxy Statement referred to in Item 10 above where such information appears under the heading “Executive Compensation and Other Matters.”
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item regarding security ownership of certain beneficial owners and management is incorporated in this report by reference to our definitive Proxy Statement referred to in Item 10 above where such information appears under the heading “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plans.”
ITEM 13. Certain Relationships and Related Transactions
The information required by this Item is incorporated in this report by reference to our definitive Proxy Statement referred to in Item 10 above where such information appears under the heading “Certain Relationships and Related Transactions.”
ITEM 14. Principal Accountant Fees and Services
The information required by this Item is incorporated in this report by reference to our definitive Proxy Statement referred to in Item 10 above where such information appears under the heading “Principal Accounting Fees and Services.”
ITEM 15: Exhibits
The list of exhibits required by this Item is incorporated in this Item by reference to the exhibit index attached after the signature page to this report.
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| March 28, 2011 | VirtualScopics, Inc. (Registrant) |
| | /s/ Molly Henderson |
| | Chief Business and Financial Officer, Sr. Vice President |
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jeffrey Markin and Molly Henderson, or either of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K and any documents related to this report and filed pursuant to the Securities and Exchange Act of 1934, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| DATE | SIGNATURE | | TITLE |
| | | | |
| March 28, 2011 | /s/ Jeffrey Markin | | President and Chief Executive Officer |
| | (Jeffrey Markin) | | (Principal Executive Officer) |
| | | | |
| /s/ Molly Henderson | | Chief Business and Financial Officer and |
| | (Molly Henderson) | | Sr. Vice President |
| | | | (Principal Financial and Accounting Officer) |
| | | | |
| /s/ Robert Klimasewski | | Chairman of the Board of Directors |
| | (Robert Klimasewski) | | |
| | | | |
| /s/ Norman Mintz | | Director |
| | (Norman Mintz) | | |
| | | | |
| /s/ Charles Phelps | | Director |
| | (Charles Phelps) | | |
| | | | |
| /s/ Sidney Knafel | | Director |
| | (Sidney Knafel) | | |
| | | | |
| /s/ Dan Kerpelman | | Director |
| | (Dan Kerpelman) | | |
| | | | |
| /s/ Terence Walts | | Director |
| | (Terence Walts) | | |
| | | | |
| /s/ Mostafa Analoui | | Director |
| | (Mostafa Analoui) | | |
| 2.1 | Securities Purchase Agreement dated September 12, 2007 by and among the VirtualScopics, Inc. and the Buyers listed on the Schedule of Buyers attached thereto (Incorporated herein by reference to Exhibit 10.1 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2007 (File No. 000-52018)). (Schedules and exhibits have been omitted pursuant to Regulation S-B Item 601(b)(2) and will be made available to the Commission upon request). |
| | |
| 3.1 | Certificate of Incorporation of VirtualScopics, Inc. dated April 21, 1988 (Incorporated herein by reference to Exhibit 3.1 of the VirtualScopics, Inc.’s Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on November 4, 2004 (File No. 333-120253)). |
| | |
| 3.2 | Certificate of Amendment of Certificate of Incorporation of VirtualScopics, Inc. dated February 2, 1989 (Incorporated herein by reference to Exhibit 3.1a of the VirtualScopics, Inc.’s Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on November 4, 2004 (File No. 333-120253)). |
| | �� |
| 3.3 | Certificate for Renewal and Revival of Certificate of Incorporation of VirtualScopics, Inc. dated February 23, 2004 (Incorporated herein by reference to Exhibit 3.1b of the VirtualScopics, Inc.’s Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on November 4, 2004 (File No. 333-120253)). |
| | |
| 3.4 | Certificate of Amendment of Certificate of Incorporation of VirtualScopics, Inc. dated August 20, 2004 (Incorporated herein by reference to Exhibit 3.1c of the VirtualScopics, Inc.’s Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on November 4, 2004 (File No. 333-120253)). |
| | |
| 3.5 | Certificate of Amendment of Certificate of Incorporation of VirtualScopics, Inc. dated October 7, 2005 (Incorporated by reference to Exhibit 3.5 the VirtualScopics, Inc.’s Annual Report on Form 10-KSB filed with the Securities and Exchange Commission on March 30, 2006 (File No. 333-120253)). |
| | |
| 3.6 | Certificate of Amendment to Certificate of Incorporation of VirtualScopics, Inc. dated November 4, 2005 (Incorporated herein by reference to Exhibit 3.1 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2005 (File No. 333-120253)). |
| | |
| 3.7 | Certificate of Designations, Powers, Preferences and Other Rights and Qualifications of Series A Convertible Preferred Stock of VirtualScopics, Inc. dated November 4, 2005 (Incorporated herein by reference to Exhibit 3.2 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2005 (File No. 333-120253)). |
| | |
| 3.8 | Certificate of Designation of Rights and Preferences of the Series B Preferred Stock of VirtualScopics, Inc. dated September 12, 2007 (Incorporated herein by reference to Exhibit 3.1 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2007 (File No. 000-52018)). |
| | |
| 3.9 | Certificate of Amendment to Certificate of Designation of Rights and Preferences of the Series B Preferred Stock of VirtualScopics, Inc. dated November 27, 2007 (Incorporated herein by reference to Exhibit 3.1 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 29, 2007 (File No. 000-52018)). |
| | |
| 3.10 | Amended and Restated Bylaws of VirtualScopics, Inc. dated August 28, 2007 (Incorporated herein by reference to Exhibit 3.9 to the VirtualScopics, Inc.’s Registration Statement of Form S-3 filed with the Securities Exchange Commission on October 11, 2007 (File No. 333-146635)). |
| 4.1 | Registration Rights Agreement Dated September 12, 2007 between the VirtualScopics, Inc. and the Buyers listed on the Schedule of Buyers thereto (Incorporated herein by reference to Exhibit 10.3 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2007 (File No. 000-52018)). |
| | |
| 4.2 | Form of Warrant to Purchase Common Stock of VirtualScopics (Incorporated herein by reference to Exhibit 10.2 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2007 (File No. 000-52018)). |
| | |
| 10.1 | VirtualScopics, Inc. 2005 Long Term Incentive Plan (Incorporated herein by reference to Exhibit 10.1 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2005 (File No. 333-120253)).* |
| | |
| 10.2 | Clinical Imaging Development and Services Agreement dated July 26, 2005 by and between VirtualScopics, LLC and Pfizer, Inc. (Incorporated herein by reference to Exhibit 10.6 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2005 (File No. 333-120253)). |
| | |
| 10.3 | Amendment to Clinical Imaging Development and Services Agreement dated October 5, 2006 by and between VirtualScopics, Inc. and Pfizer, Inc. (Incorporated herein by reference to Exhibit 10.1 to the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 28, 2006 (File No. 000-52018)). |
| | |
| 10.4 | Equipment Purchase Agreement dated December, 2003 by and between VirtualScopics, LLC and the University of Rochester Medical Center. (Incorporated herein by reference to Exhibit 10.9 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2005 (File No. 333-120253)). |
| | |
| 10.5 | Determination of Intellectual Property Rights Agreement dated July 1, 2002 by and between VirtualScopics, LLC and the University of Rochester (Incorporated herein by reference to Exhibit 10.10 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2005 (File No. 333-120253)). |
| | |
| 10.6 | Option Agreements with Robert Klimasewski dated November 5, 2005 (Incorporated herein by reference to Exhibit 10.18 to the VirtualScopics, Inc. Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on May 2, 2006 (File No. 333-133747)).* |
| | |
| 10.7 | Form of April 28, 2006 Indemnification Agreement by and among VirtualScopics, Inc. and the directors and officers of the VirtualScopics, Inc. (Incorporated herein by reference to Exhibit 10.19 to the VirtualScopics, Inc. Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on May 2, 2006 (File No. 333-133747)).* |
| | |
| 10.8 | VirtualScopics, Inc. Amended and Restated 2006 Long Term Incentive Plan (incorporated herein by reference to Appendix B to the Registrant’s Definitive Proxy Statement on Schedule 14A, filed with the Commission on April 10, 2009 (File No. 000-52018)) |
| | |
| 10.9 | 2007 Bonus Plan (Incorporated herein by reference to Exhibit 10.21 of the VirtualScopics, Inc. Annual Report on Form 10-KSB filed with the Securities and Exchange Commission on April 2, 2007 (File No. 000-52018)).* |
| | |
| 10.10 | Independent Director Compensation Plan (Incorporated herein by reference to Exhibit 10.22 of the VirtualScopics, Inc. Annual Report on Form 10-KSB filed with the Securities and Exchange Commission on April 2, 2007 (File No. 000-52018))* |
| | |
| 10.11 | Indemnification Agreement by and among VirtualScopics, Inc. and Norman Mintz, dated as of August 1, 2007. (Reference is made to the VirtualScopics, Inc. Form of Indemnification Agreement by and among VirtualScopics, Inc., and the directors and officers of the VirtualScopics, Inc. filed as Exhibit 10.19 to the VirtualScopics, Inc. Registration Statement on Form SB-2 filed with the Securities and Exchange Commission on May 2, 2006 (File No. 333-133747)).* |
| 10.12 | Standstill Agreement by and between VirtualScopics, Inc. Jeffrey Markin, dated September 12, 2007. (Incorporated herein by reference to Exhibit 10.4 of the VirtualScopics, Inc. Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2007 (File No. 000-52018)).* |
| | |
| 10.13 | Non-Employee Director Compensation Plan (Incorporated herein by reference to Exhibit 10.1 to the VirtualScopics, Inc. Report on Form 10-Q filed with the Securities and Exchange Commission on August 12, 2008 (File No. 000-52018)).* |
| | |
| 10.14 | 2009 Bonus Plan (Incorporated herein by reference to Exhibit 10.3 of the VirtualScopics, Inc. Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 15, 2009 (File No. 000-52018)).* |
| | |
| 10.15 | Employment Agreement dated February 24, 2009, by and between Jeffrey Markin and VirtualScopics, Inc.* (Incorporated herein by reference to Exhibit 10.17 to the VirtualScopics, Inc., Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 19, 2009 (File No. 000-52018)). |
| | |
| 10.16 | Employment Agreement dated February 24, 2009, by and between Molly Henderson and VirtualScopics, Inc.* (Incorporated herein by reference to Exhibit 10.18 to the VirtualScopics, Inc., 2008 Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 19, 2009 (File No. 000-52018)). |
| | |
| 10.17 | Strategic Alliance Agreement between VirtualScopics, Inc. and PPD Development, LP, dated October 20, 2010 (Incorporated herein by reference to Exhibit 10.1 to the VirtualScopics, Inc. Report on Form 10-Q filed with the Securities and Exchange Commission on November 12, 2010 (File No. 000-52018)). |
| | |
| 21 | Subsidiaries of VirtualScopics, Inc. |
| | |
| 23.1 | Consent of Marcum LLP |
| | |
| 24 | Power of Attorney (included on the signature page to this report) |
| | |
| 31.1 | Certification of Chief Executive Officer as required by Rule 13a-14 Or 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 Of The Sarbanes-Oxley Act of 2002 |
| | |
| 31.2 | Certification of Chief Financial Officer as required by Rule 13a-14 Or 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 Of The Sarbanes-Oxley Act of 2002 |
| | |
| 32.1 | Certification pursuant to 18 U.S.C. Section 1350 by the Chief Executive Officer, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
| 32.2 | Certification pursuant to 18 U.S.C. Section 1350 by the Chief Financial Officer, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
| * | Management contract or compensatory plan or arrangement. |
VirtualScopics, Inc. and Subsidiary
Index to Consolidated Financial Statements
| Report of Independent Registered Public Accounting Firm | F-2 |
| | |
| Consolidated Balance Sheets as of December 31, 2010 and 2009 | F-3 |
| | |
| Consolidated Statements of Operations for the Years Ended December 31, 2010 and 2009 | F-4 |
| | |
| Consolidated Statements of Changes in Stockholders’ Equity for the Years Ended December 31, 2010 and 2009 | F-5 |
| | |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2010 and 2009 | F-6 |
| | |
| Notes to Consolidated Financial Statements | F-7 - F-22 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of the
Board of Directors and Stockholders of
VirtualScopics, Inc. and Subsidiary
We have audited the accompanying consolidated balance sheets of VirtualScopics, Inc. and Subsidiary (the “Company”) as of December 31, 2010 and 2009, and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of VirtualScopics, Inc. and Subsidiary as of December 31, 2010 and 2009, and the results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ Marcum LLP
New York, New York
March 28, 2011
VirtualScopics, Inc. and Subsidiary
Consolidated Balance Sheets
| | | December 31, | |
| | | 2010 | | | 2009 | |
| Assets | | | |
| Current assets | | | |
| Cash | | $ | 4,576,060 | | | $ | 4,327,410 | |
| Accounts receivable, net of allowance for doubtful accounts of $30,000 and $20,786, respectively | | | 2,727,525 | | | | 1,481,381 | |
| Prepaid expenses and other current assets | | | 305,079 | | | | 387,247 | |
| Total current assets | | | 7,608,664 | | | | 6,196,038 | |
| | | | | | | | | |
| Patents, net | | | 1,711,501 | | | | 1,832,560 | |
| Property and equipment, net | | | 404,426 | | | | 456,169 | |
| Other assets | | | - | | | | 33,258 | |
| | | | | | | | | |
| Total assets | | $ | 9,724,591 | | | $ | 8,518,025 | |
| | | | | | | | | |
| Liabilities and Stockholders’ Equity | | | | | | | | |
| | | | | | | | | |
| Current liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 1,099,838 | | | $ | 658,430 | |
| Accrued payroll | | | 821,107 | | | | 837,177 | |
| Unearned revenue | | | 214,508 | | | | 1,011,498 | |
| Derivative liability | | | 2,609,708 | | | | 1,139,953 | |
| Total current liabilities | | | 4,745,161 | | | | 3,647,058 | |
| | | | | | | | | |
| Commitments and Contingencies (See Note 10) | | | | | | | | |
| | | | | | | | | |
| Stockholders’ Equity | | | | | | | | |
| Convertible preferred stock, $0.001 par value; 15,000,000 shares authorized; | | | | | | | | |
| Series A; 8,400 shares authorized; issued and outstanding, 3,188 and 3,438 at December 31, 2010 and 2009, respectively, liquidation preference $1,000 per share | | | 3 | | | | 3 | |
| | | | | | | | | |
| Series B; 6,000 shares authorized; issued and outstanding, 800 and 2,910 at December 31, 2010 and 2009, respectively, liquidation preference $1,000 per share | | | 1 | | | | 3 | |
| Common stock, $0.001 par value; 85,000,000 shares authorized; | | | | | | | | |
| issued and outstanding, 27,414,620 and 25,233,255 shares at December 31, 2010 and 2009, respectively | | | 27,415 | | | | 25,233 | |
| Additional paid-in capital | | | 15,090,254 | | | | 14,354,929 | |
| Accumulated deficit | | | (10,138,243 | ) | | | (9,509,201 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 4,979,430 | | | | 4,870,967 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 9,724,591 | | | $ | 8,518,025 | |
The accompanying notes are an integral part of these consolidated financial statements
VirtualScopics, Inc. and Subsidiary
Consolidated Statements of Operations
| | | For the Years Ended December 31, | |
| | | 2010 | | | 2009 | |
| | | | | | | |
| Revenues | | $ | 13,393,120 | | | $ | 10,392,825 | |
| Cost of services | | | 6,569,028 | | | | 4,804,987 | |
| Gross profit | | | 6,824,092 | | | | 5,587,838 | |
| Operating expenses | | | | | | | | |
| Research and development | | | 1,130,744 | | | | 1,058,676 | |
| Sales and marketing | | | 1,184,454 | | | | 1,264,801 | |
| General and administrative | | | 3,124,669 | | | | 3,074,047 | |
| Depreciation and amortization | | | 511,982 | | | | 469,585 | |
| Total operating expenses | | | 5,951,849 | | | | 5,867,109 | |
| Operating income (loss) | | | 872,243 | | | | (279,271 | ) |
| Other income (expense) | | | | | | | | |
| Interest income | | | 9,788 | | | | 7,116 | |
| Other expense | | | (25,318 | ) | | | (22,027 | ) |
| Loss on derivative financial instrument | | | (1,469,755 | ) | | | (717,045 | ) |
| Total other expense | | | (1,485,285 | ) | | | (731,956 | ) |
| Net loss before income taxes | | | (613,042 | ) | | | (1,011,227 | ) |
| | | | | | | | | |
| Provision for income taxes | | | (16,000 | ) | | | - | |
| | | | | | | | | |
| Net Loss | | | (629,042 | ) | | | (1,011,227 | ) |
| | | | | | | | | |
| Series B preferred stock cash dividend | | | 173,016 | | | | 304,318 | |
| Net loss attributable to common stockholders | | $ | (802,058 | ) | | $ | (1,315,545 | ) |
| | | | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.03 | ) | | $ | (0.05 | ) |
| | | | | | | | | |
| Weighted average number of common shares outstanding basic and diluted | | | 26,153,573 | | | | 24,102,126 | |
The accompanying notes are an integral part of these consolidated financial statements
VirtualScopics, Inc. and Subsidiary
Consolidated Statements of Changes in Stockholders’ Equity
For the Years Ended December 31, 2010 and 2009
| | | Series A | | | Series B | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Paid-In Capital | | | Deficit | | | Total | |
| Balances at December 31, 2008 | | | 3,976 | | | $ | 4 | | | | 4,226 | | | $ | 4 | | | | 23,502,352 | | | $ | 23,503 | | | $ | 16,546,550 | | | $ | (11,214,065 | ) | | $ | 5,355,996 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cumulative effect of change in accounting principle January 1, 2009 reclassification of equity-linked financial instruments to derivative liability | | | | | | | | | | | | | | | | | | | | | | | | | | | (3,138,999 | ) | | | 2,716,091 | | | | (422,908 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Conversion of Series A Preferred to Common Stock | | | (538 | ) | | | (1 | ) | | | | | | | | | | | 446,319 | | | | 446 | | | | (445 | ) | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Conversion of Series B Preferred to Common Stock | | | | | | | | | | | (1,316 | ) | | | (1 | ) | | | 1,092,752 | | | | 1,093 | | | | (1,092 | ) | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of stock option costs | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,027,561 | | | | | | | | 1,027,561 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock issued for rent | | | | | | | | | | | | | | | | | | | 88,132 | | | | 88 | | | | 105,482 | | | | | | | | 105,570 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Restricted stock units issuance | | | | | | | | | | | | | | | | | | | 103,700 | | | | 103 | | | | 120,190 | | | | | | | | 120,293 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Series B preferred stock cash dividends based on 8% annual rate | | | | | | | | | | | | | | | | | | | | | | | | | | | (304,318 | ) | | | | | | | (304,318 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,011,227 | ) | | | (1,011,227 | ) |
| Balances at December 31, 2009 | | | 3,438 | | | $ | 3 | | | | 2,910 | | | $ | 3 | | | | 25,233,255 | | | $ | 25,233 | | | $ | 14,354,929 | | | $ | (9,509,201 | ) | | $ | 4,870,967 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Conversion of Series A Preferred to Common Stock | | | (250 | ) | | | - | | | | | | | | | | | | 207,590 | | | | 208 | | | | (208 | ) | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Conversion of Series B Preferred to Common Stock | | | | | | | | | | | (2,110 | ) | | | (2 | ) | | | 1,752,057 | | | | 1,752 | | | | (1,750 | ) | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cashless Exercise of Warrants | | | | | | | | | | | | | | | | | | | 21,163 | | | | 21 | | | | (21 | ) | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of Employee Stock Options | | | | | | | | | | | | | | | | | | | 25,000 | | | | 25 | | | | 25,225 | | | | | | | | 25,250 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cashless exercise of employee stock options | | | | | | | | | | | | | | | | | | | 61,501 | | | | 62 | | | | (62 | ) | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Withholdings taxes paid on cashless exercise of employee stock options | | | | | | | | | | | | | | | | | | | | | | | | | | | (66,798 | ) | | | | | | | (66,798 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of stock option costs | | | | | | | | | | | | | | | | | | | | | | | | | | | 819,766 | | | | | | | | 819,766 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Restricted stock units issuance | | | | | | | | | | | | | | | | | | | 114,054 | | | | 114 | | | | 132,189 | | | | | | | | 132,303 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Series B preferred stock cash dividends based on 8% annual rate | | | | | | | | | | | | | | | | | | | | | | | | | | | (173,016 | ) | | | | | | | (173,016 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (629,042 | ) | | | (629,042 | ) |
| Balances at December 31, 2010 | | | 3,188 | | | $ | 3 | | | | 800 | | | $ | 1 | | | | 27,414,620 | | | $ | 27,415 | | | $ | 15,090,254 | | | $ | (10,138,243 | ) | | $ | 4,979,430 | |
The accompanying notes are an integral part of these consolidated financial statements
VirtualScopics, Inc. and Subsidiary
Consolidated Statements of Cash Flows
| | | For the Years Ended December 31, | |
| | | 2010 | | | 2009 | |
| | | | | | | |
| Cash flows from operating activities | | | | | | |
| | | | | | | | | |
| Net loss | | $ | (629,042 | ) | | $ | (1,011,227 | ) |
Adjustments to reconcile net loss to net cash provided by operating activities | | | | | | | | |
| Depreciation and amortization | | | 511,982 | | | | 469,585 | |
| Loss on disposition and write downs of property and equipment | | | - | | | | 2,904 | |
| Provision for doubtful accounts | | | 9,214 | | | | 20,786 | |
| Stock-based compensation | | | 863,522 | | | | 1,067,080 | |
| Derivative financial instrument | | | 1,469,755 | | | | 717,045 | |
| Changes in operating assets and liabilities | | | | | | | | |
| Accounts receivable | | | (1,255,358 | ) | | | (481,057 | ) |
| Prepaid expenses and other assets | | | (8,103 | ) | | | (123,950 | ) |
| Unearned revenue | | | (796,990 | ) | | | 719,904 | |
| Accounts payable and accrued expenses | | | 529,955 | | | | 185,765 | |
| Accrued payroll | | | (16,070 | ) | | | 282,752 | |
| Total adjustments | | | 1,307,907 | | | | 2,860,814 | |
| Net cash provided by operating activities | | | 678,865 | | | | 1,849,587 | |
| | | | | | | | | |
| Cash flows from investing activities | | | | | | | | |
| Purchase of equipment | | | (203,170 | ) | | | (317,067 | ) |
| Acquisition of patents | | | (12,481 | ) | | | (44,696 | ) |
| Net cash used in investing activities | | | (215,651 | ) | | | (361,763 | ) |
| | | | | | | | | |
| Cash flows from financing activities | | | | | | | | |
| Proceeds from the exercise of stock options | | | 25,250 | | | | - | |
| Withholding taxes paid on the cashless exercise of employee stock options | | | (66,798 | ) | | | - | |
| Cash dividends on series B preferred stock | | | (173,016 | ) | | | (304,318 | ) |
| Net cash used in financing activities | | | (214,564 | ) | | | (304,318 | ) |
| Net increase in cash | | | 248,650 | | | | 1,183,506 | |
| Cash | | | | | | | | |
| Beginning of year | | | 4,327,410 | | | | 3,143,904 | |
| End of year | | $ | 4,576,060 | | | $ | 4,327,410 | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information | | | | | | | | |
| Cash paid during the year for: | | | | | | | | |
| Taxes | | $ | 18,335 | | | $ | - | |
| | | | | | | | | |
| Non-cash financing activity: | | | | | | | | |
| Issuance of common stock for partial payment of rent in equity | | $ | - | | | $ | 105,570 | |
| Issuance of restricted stock units in settlement of accrued liability | | $ | 132,303 | | | $ | 120,293 | |
| Cashless exercise of employee stock options | | $ | 62 | | | $ | - | |
| Cashless exercise of warrants | | $ | 21 | | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
NOTE 1 - Organization and Basis of Presentation
Nature of Business
The Company’s headquarters are located in Rochester, New York. The Company has created a suite of image analysis software tools and applications which are used in detecting and analyzing specific structures in medical images. The Company’s developed software provides measurement and visualization capabilities designed to improve clinical research and development.
NOTE 2 - Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of VirtualScopics, Inc. and its wholly-owned subsidiary, VirtualScopics, LLC. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the year. Actual results could differ from those estimates. Estimates included in these consolidated financial statements relate to assessing the collectability of accounts receivable, the valuation of securities underlying share-based compensation and derivative instruments, realization of deferred tax assets, tax contingencies and any related valuation allowance, and the useful lives and potential impairment of the Company’s property and equipment and intangible assets. Estimates and assumptions are reviewed periodically and the effects of revisions are reflected in the period that they are determined to be necessary.
Reclassifications
Certain prior period amounts have been reclassified to conform to the current year presentation.
Cash and Cash Equivalents
The Company considers all highly liquid investments when purchased with a maturity of three months or less to be cash equivalents. At December 31, 2010 and 2009, the Company had no cash equivalents.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist of cash. At times, our cash may be uninsured or in deposit accounts that exceed the Federal Deposit Insurance Corporation (“FDIC”) insurance limits.
Accounts Receivable
Accounts receivable are stated at estimated net realizable value. Accounts receivable are comprised of balances due from customers net of estimated allowances for uncollectible accounts, if any. In determining collectability, historical trends are evaluated and specific customer issues are reviewed to arrive at appropriate allowances, if any. The allowance for doubtful accounts at December 31, 2010 and 2009 was $30,000 and $20,768, respectively.
Right to Use Equipment
In April 2004, the Company obtained the right to use a Magnetic Resonance Imaging (“MRI”) machine owned by the University of Rochester for a period of seven years (Note 11). The Company has recorded the value of the right in other assets in the accompanying consolidated balance sheets and is amortizing the asset based on usage over the life of the agreement.
For the years ended December 31, 2010 and 2009, the total amount charged to amortization on this asset which is included in the accompanying consolidated statements of operations was $123,529 and $125,530, respectively. As of December 31, 2010 and 2009, the unamortized balance of this asset is $33,258 and $156,787, respectively, of which $33,258 and $123,529, respectively is classified within prepaid expenses and other current assets for 2010 and 2009, and $33,258 is classified within other assets as of December 31, 2009.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
Patents
Costs incurred to acquire and file for patents, including legal costs, are capitalized as long-lived assets and amortized on a straight-line basis over the lower of the estimated useful life or legal life of the patent, which is 20 years.
Property and Equipment
Property and equipment are carried at cost less accumulated depreciation. When retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and any resulting gain or loss is recognized and included in the consolidated statement of operations.
Expenditures for maintenance and repairs, which do not generally extend the useful life of the assets, are charged to expense as incurred. Gains or losses on disposal of property and equipment are reflected in general and administrative expense in the consolidated statement of operations in the period of disposal.
Depreciation is computed using the straight-line method over the following useful lives:
| | | Years | |
| Office/computer equipment | | 3-5 | |
| Furniture and fixtures | | 5-7 | |
| Software | | 3 | |
Leasehold improvements, which are included in property and equipment, are recorded at cost less accumulated depreciation. Depreciation on leasehold improvements is computed using the straight-line method over the shorter of their estimated useful lives or the lease term, whichever is shorter.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including intangible assets other than goodwill, for impairment whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable. In connection with this review, the Company also reevaluates the periods of depreciation and amortization for these assets. The Company assesses recoverability by determining whether the net book value of the related asset will be recovered through the projected undiscounted future cash flows of the asset. If the Company determines that the carrying value of the asset may not be recoverable, it measures any impairment based on the projected future discounted cash flows as compared to the asset’s carrying value. Through December 31, 2010, the Company has not recorded any impairment charges on its long-lived assets.
Derivative Financial Instruments
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. The Company evaluates all of its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. Due to the price reset, or ratchet, provision associated with the warrants issued with the Company’s series B preferred stock, and to the placement agent in the series B financings, the Company is required to recognize a liability for the current fair value of these warrants at each reporting period. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. For stock-based derivative financial instruments, the Company uses the Black-Scholes option valuation model to value the derivative instruments at inception and on subsequent valuation dates. Although the Company determined the warrants include an implied downside protection feature, it performed a Monte-Carlo simulation and concluded that the value of the feature is de minimus and the use of the Black-Scholes valuation model is considered to be a reasonable method to value the warrants. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within 12 months of the balance sheet date (Note 5).
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
Revenue Recognition
The Company recognizes revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when an agreement exists, services and products are provided to the customer, prices are fixed or determinable, and collectability is reasonably assured. Revenues are reduced for estimated discounts and other allowances, if any.
The Company provides advanced medical image analysis on a per analysis basis, and recognizes revenue when the image analysis is completed and delivered to the customer. Revenue related to project, data, and site management services is recognized as the services are rendered and in accordance with the terms of the contract. Consulting revenue is recognized once the services are rendered and typically charged as an hourly rate.
Occasionally, the Company provides software development services to its customers, which may require significant development, modification, and customization. Software development revenue is billed on a fixed price basis and recognized upon delivery of the software and acceptance by the customer on a completed contract basis. The Company does not sell software, software licenses, upgrades or enhancements, or post-contract customer services.
Reimbursements received by the Company from its customers for out-of-pocket expenses incurred are reported as revenue in the financial statements.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income and the reversal of deferred tax liabilities during the period in which related temporary differences become deductible. The benefit of tax positions taken or expected to be taken in the Company’s income tax returns are recognized in the consolidated financial statements if such positions are more likely than not of being sustained.
Research and Development
Research and development expense relates to the development of new products and processes including significant improvements to existing products. These costs are expensed as incurred. Research and development costs for the year ended December 31, 2010 and 2009 were $1,130,744 and $1,058,676, respectively.
Fair Value of Financial Instruments
Fair value of financial instruments is defined as an exit price, which is the price that would be received upon sale of an asset or paid upon transfer of a liability in an orderly transaction between market participants at the measurement date. The degree of judgment utilized in measuring the fair value of assets and liabilities generally correlates to the level of pricing observability. Financial assets and liabilities with readily available, actively quoted prices or for which fair value can be measured from actively quoted prices in active markets generally have more pricing observability and require less judgment in measuring fair value. Conversely, financial assets and liabilities that are rarely traded or not quoted have less price observability and are generally measured at fair value using valuation models that require more judgment. These valuation techniques involve some level of management estimation and judgment, the degree of which is dependent on the price transparency of the asset, liability or market and the nature of the asset or liability. The Company has categorized its financial assets and liabilities measured at fair value into a three-level hierarchy. See Note 5 – Derivative Liability for a further discussion regarding the Company’s measurement of financial assets and liabilities at fair value.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
Stock Based Compensation
For the year ended December 31, 2010 and 2009, the Company’s consolidated statements of operations reflect stock-based compensation expense for stock options granted under its long-term stock incentive plans amounting to $819,766 and $1,027,561, respectively.
Stock options issued under the Company’s long-term incentive plans are granted with an exercise price equal to no less than the market price of the Company’s stock at the date of grant and expire up to ten years from the date of grant. These options generally vest over a three- or four-year period.
The fair value of stock options granted was determined on the grant date using assumptions for risk free interest rate, the expected term, expected volatility, and expected dividend yield. The risk free interest rate is based on U.S. Treasury zero-coupon yield curve over the expected term of the option. The expected term assumption is determined using the weighted average midpoint between vest and expiration for all individuals within the grant. Since the Company has limited historical volatility information, it bases its expected volatility on the historical volatility of similar entities whose share prices are publicly available averaged with the Company’s historical volatility excluding the first ten months due to the discreet and non-recurring nature of the trading. In making its determination as to similarity, the Company considered the industry, stage of life cycle, size, and financial leverage of such other entities. The Company’s model includes a zero dividend yield assumption, as the Company has not historically paid nor does it anticipate paying dividends on its common stock. The Company’s model does not include a discount for post-vesting restrictions, as the Company has not issued awards with such restrictions. The periodic expense is then determined based on the valuation of the options, and at that time an estimated forfeiture rate is used to reduce the expense recorded. The Company’s estimate of pre-vesting forfeitures is primarily based on the Company’s historical experience and is adjusted to reflect actual forfeitures as the options vest.
The following assumptions were used to estimate the fair value of options granted for the year ended December 31, 2010 and 2009 using the Black-Scholes option-pricing model:
| | | December 31, | |
| | | 2010 | | | 2009 | |
| Risk free interest rate | | | 2.76 | % | | | 2.81 | % |
| Expected term (years) | | | 6.19 | | | | 8.10 | |
| Expected volatility | | | 57.69 | % | | | 73.6 | % |
| Expected dividend yield | | | - | | | | - | |
Loss Per Share
Basic loss per share is computed by dividing the net loss applicable to common shares by the weighted average number of common shares outstanding during the period. Diluted loss attributable to common shares adjusts basic loss per share for the effects of convertible securities, warrants, stock options and other potentially dilutive financial instruments only in the periods in which such effect is dilutive. The shares issuable upon the conversion of preferred stock, the exercise of stock options and warrants are excluded from the calculation of net loss per share as their effect would be antidilutive.
Securities that could potentially dilute earnings per share in the future that were not included in the computation of diluted loss per share consist of the following as of December 31:
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
| | | 2010 | | | 2009 | |
| Series A convertible preferred stock | | | 2,647,191 | | | | 2,854,780 | |
| Series B convertible preferred stock | | | 664,286 | | | | 2,416,341 | |
| Warrants to purchase common stock | | | 2,555,096 | | | | 2,679,652 | |
| Options to purchase common stock | | | 6,021,033 | | | | 4,907,492 | |
| | | | | | | | | |
| Total | | | 11,887,606 | | | | 12,858,265 | |
Recently Adopted Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2009-13, “Multiple-Deliverable Revenue Arrangements.” This ASU establishes the accounting and reporting guidance for arrangements including multiple revenue-generating activities. This ASU provides amendments to the criteria for separating deliverables, measuring and allocating arrangement consideration to one or more units of accounting. The amendments in this ASU also establish a selling price hierarchy for determining the selling price of a deliverable. Significantly enhanced disclosures are also required to provide information about a vendor’s multiple-deliverable revenue arrangements, including information about the nature and terms, significant deliverables, and its performance within arrangements. The amendments also require providing information about the significant judgments made and changes to those judgments and about how the application of the relative selling-price method affects the timing or amount of revenue recognition. The amendments in this ASU are effective prospectively for revenue arrangements entered into or materially modified in the fiscal years beginning on or after June 15, 2010. Early application is permitted. The Company is currently evaluating the impact the adoption of this standard will have on its consolidated financial position and results of operations.
In October 2009, the FASB issued ASU No. 2009-14, “Certain Revenue Arrangements That Include Software Elements.” This ASU changes the accounting model for revenue arrangements that include both tangible products and software elements that are “essential to the functionality,” and scopes these products out of current software revenue guidance. The new guidance will include factors to help companies determine what software elements are considered “essential to the functionality.” The amendments will now subject software-enabled products to other revenue guidance and disclosure requirements, such as guidance surrounding revenue arrangements with multiple-deliverables. The amendments in this ASU are effective prospectively for revenue arrangements entered into or materially modified in the fiscal years beginning on or after June 15, 2010. Early application is permitted. The Company is currently evaluating the impact the adoption of this standard will have on its consolidated financial position and results of operations.
In January 2010, the FASB issued ASU No. 2010-06, Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements. This ASU requires some new disclosures and clarifies some existing disclosure requirements about fair value measurement as set forth in Codification Subtopic 820-10. ASU 2010-06 amends Codification Subtopic 820-10 and now requires a reporting entity to use judgment in determining the appropriate classes of assets and liabilities and to provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. ASU 2010-06 is effective for interim and annual reporting periods beginning after December 15, 2009. As this standard relates specifically to disclosures, the adoption did not have an impact on the Company’s consolidated financial position and results of operations.
In February 2010, the FASB issued ASU 2010-09, "Subsequent Events (Topic 855) - Amendments to Certain Recognition and Disclosure Requirements." ASU 2010-09 requires an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement that an SEC filer disclose the date through which subsequent events have been evaluated. ASC 2010-09 was effective upon issuance. The adoption of this standard had no effect on our consolidated financial position or results of operations.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
In March 2010, the FASB issued ASU No. 2010-17, Revenue Recognition— Milestone Method (Topic 605): Milestone Method of Revenue Recognition. This standard provides that the milestone method is a valid application of the proportional performance model for revenue recognition if the milestones are substantive and there is substantive uncertainty about whether the milestones will be achieved. Determining whether a milestone is substantive requires judgment that should be made at the inception of the arrangement. To meet the definition of a substantive milestone, the consideration earned by achieving the milestone (1) would have to be commensurate with either the level of effort required to achieve the milestone or the enhancement in the value of the item delivered, (2) would have to relate solely to past performance, and (3) should be reasonable relative to all deliverables and payment terms in the arrangement. No bifurcation of an individual milestone is allowed and there can be more than one milestone in an arrangement. The new standard is effective for interim and annual periods beginning on or after June 15, 2010. Early adoption is permitted. The Company is currently evaluating the impact the adoption of this standard will have on its consolidated financial position and results of operations.
NOTE 3 - Property and Equipment
Property and equipment consisted of the following as of December 31:
| | | 2010 | | | 2009 | |
| Office/computer equipment | | $ | 954,614 | | | $ | 882,776 | |
| Furniture and fixtures | | | 216,454 | | | | 165,286 | |
| Software | | | 299,403 | | | | 229,101 | |
| Leasehold improvements | | | 107,388 | | | | 99,375 | |
| | | | 1,577,859 | | | | 1,376,538 | |
| Less: accumulated depreciation | | | (1,173,433 | ) | | | (920,369 | ) |
| | | $ | 404,426 | | | $ | 456,169 | |
Depreciation expense amounted to $254,913 and $213,473 for the years ended December 31, 2010 and 2009, respectively.
The Company disposed of and wrote-off property and equipment with $1,849 of cost and $1,849 of accumulated depreciation for the year ended December 31, 2010 as compared to $163,917 and 161,013 for the year ended December 31, 2009.
NOTE 4 - Patents
On May 24, 2002, the Company purchased from the University of Rochester, a related party, certain patents developed by the Company’s founders and previously licensed by the Company under an Exclusive Right Agreement. The Company paid $1,500,000 and issued warrants to acquire 357,075 shares of common stock to the University of Rochester for the full right and title to the patents. The warrants were recorded at fair value which totaled $157,000. Since May 24, 2002, the Company has invested an additional $1,026,029 in connection with improving and expanding its patent portfolio. These costs consist predominately of legal and filing fees and historically have been capitalized as long-lived assets. For the years ended December 31, 2010 and 2009, the Company capitalized $12,481 and $44,696, respectively, of legal expenses and filing fees associated with its patents.
Accumulated amortization on the patents amounted to $971,527 as of December 31, 2010. Amortization expense for the years ended December 31, 2010 and 2009 amounted to $133,540 and $132,582, respectively. The estimated amortization expense on the patents for the next five years and thereafter is as follows:
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
| For the Years Ending | | | |
| December 31, | | Amount | |
| 2011 | | $ | 135,618 | |
| 2012 | | | 135,618 | |
| 2013 | | | 135,618 | |
| 2014 | | | 135,618 | |
| 2015 | | | 135,618 | |
| Thereafter | | | 1,033,412 | |
| Total | | $ | 1,711,502 | |
NOTE 5 - Derivative Liability
Effective January 1, 2009, the Company adopted a new accounting policy requiring it to change the manner in which it accounts for certain equity instruments. The warrants issued with the Company’s series B preferred stock, and to the placement agent in the series B financing, do not have fixed settlement provisions because their exercise prices may be lowered if the Company issues securities at lower prices in the future. The Company was required to include the reset provisions in order to protect the warrant holders from the potential dilution associated with future financings. Accordingly, the warrants were recognized as a derivative instrument and have been re-characterized as derivative liabilities. The fair value of these liabilities are re-measured at the end of every reporting period with the change in value reported in the statement of operations.
The derivative liabilities were valued using the Black-Scholes option valuation model and the following assumptions on the following dates:
| | | December 31, 2010 | | | December 31, 2009 | | | January 1, 2009 | | | November 5, 2005 | |
| Series A warrants: | | | | | | | | | | | | |
| Risk-free interest rate | | | - | | | | - | | | | 2.24 | % | | | 4.66 | % |
| Expected volatility | | | - | | | | - | | | | 72.9 | % | | | 59.6 | % |
| Expected life (in years) | | | - | | | | - | | | | .92 | | | | 4 | |
| Expected dividend yield | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | |
| Number of warrants | | | - | | | | - | | | | 1,400,000 | | | | 1,400,000 | |
| | | | | | | | | | | | | | | | | |
| Fair value | | $ | - | | | $ | - | | | $ | 14,840 | | | $ | 1,299,900 | |
| | | December 31, 2010 | | | December 31, 2009 | | | January 1, 2009 | | | September 17, 2007 | |
| Series B warrants: | | | | | | | | | | | | |
| Risk-free interest rate | | | 1.39 | % | | | 2.62 | % | | | 2.24 | % | | | 4.37 | % |
| Expected volatility | | | 57.6 | % | | | 67.5 | % | | | 57.8 | % | | | 70.0 | % |
| Expected life (in years) | | | 3.69 | | | | 4.67 | | | | 5.67 | | | | 7 | |
| Expected dividend yield | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | |
| Number of warrants | | | 2,110,208 | | | | 2,234,764 | | | | 2,234,764 | | | | 2,234,764 | |
| | | | | | | | | | | | | | | | | |
| Fair value | | $ | 2,609,708 | | | $ | 1,139,953 | | | $ | 408,068 | | | $ | 1,839,099 | |
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
The risk-free interest rate was based on rates established by the Federal Reserve. The Company’s expected volatility was based on the Company’s historical volatility and of similar entities whose share prices are publicly available. The expected life of the warrants was determined by the expiration date of the warrants. The expected dividend yield was based upon the fact that the Company has not historically paid dividends on its common stock, and does not expect to pay dividends on its common stock in the future.
The change in accounting for warrants was implemented in the first quarter of 2009 and was reported as a cumulative effect of a change in accounting principle. As a result, the cumulative effect on the accounting for the warrants was as follows:
Derivative Instrument | | Decrease in Additional Paid- In-Capital | | | Decrease in Accumulated Deficit | | | Increase in Derivative Liability | |
| Warrants | | $ | 3,138,999 | | | $ | 2,716,091 | | | $ | 422,908 | |
The warrants were originally recorded at their relative fair value as an increase in additional paid-in-capital. Changes in accumulated deficit include $2,716,091 in gains resulting from the decrease in the fair value of the derivative liabilities through December 31, 2008. The derivative liability amount reflects the fair value of each derivative instrument as of the January 1, 2009 date of implementation. The $1,469,755 and $717,045 change in fair value during 2010 and 2009, respectively, is reported in our consolidated statement of operations as a loss on derivative financial instruments. The fair value of these liabilities will be re-measured at the end of every reporting period and the change in fair value will be reported in our consolidated statement of operations as a gain or loss on derivative financial instruments.
Fair Value Measurement
Valuation Hierarchy
ASC 820, “Fair Value Measurements and Disclosures,” establishes a valuation hierarchy for disclosure of the inputs to valuation used to measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full term of the financial instrument. Level 3 inputs are unobservable inputs based on the Company’s own assumptions used to measure assets and liabilities at fair value. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
The following table provides the assets and liabilities carried at fair value measured on a recurring basis as of December 31, 2010 and 2009:
| | | | | | Fair Value Measurements at December 31, 2010 | |
| | | Total Carrying Value at December 31, 2010 | | | Quoted prices in active markets (Level 1) | | | Significant other observable inputs (Level 2) | | | Significant unobservable inputs (Level 3) | |
| Derivative liabilities | | $ | 2,609,708 | | | $ | - | | | $ | - | | | $ | 2,609,708 | |
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
| | | | | | Fair Value Measurements at December 31, 2009 | |
| | | Total Carrying Value at December 31, 2009 | | | Quoted prices in active markets (Level 1) | | | Significant other observable inputs (Level 2) | | | Significant unobservable inputs (Level 3) | |
| Derivative liabilities | | $ | 1,139,953 | | | $ | - | | | $ | - | | | $ | 1,139,953 | |
The carrying amounts of cash, accounts receivable, accounts payable, and accrued liabilities approximate their fair value due to their short maturities. The derivative liabilities are measured at fair value using quoted market prices and estimated volatility factors, and are classified within Level 3 of the valuation hierarchy. There were no changes in the valuation techniques during the year ended December 31, 2010.
The following table sets forth a summary of the changes in the fair value of our Level 3 financial liabilities that are measured at fair value on a recurring basis:
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | |
| Beginning balance | | $ | (1,139,953 | ) | | $ | (422,908 | ) |
| Net unrealized loss on derivative financial instruments | | | (1,469,755 | ) | | | (717,045 | ) |
| Ending balance | | $ | (2,609,708 | ) | | $ | (1,139,953 | ) |
NOTE 6 – Stockholders’ Equity
Common Stock
The Company has authorized 85,000,000 shares of common stock, par value $0.001. As of December 31, 2010, the Company had reserved 2,327,937 shares of common stock for issuance under its 2001 and 2005 long-term incentive plans, another 350,000 shares of common stock issued to a previous CEO outside of one of its long-term incentive plans, and 6,900,000 shares for its 2006 Long-term Incentive Plan.
Preferred Stock
The Company has authorized 15,000,000 shares of preferred stock, par value $0.001 per share, of which 8,400 are designated as Series A Convertible Preferred Stock (“Series A Preferred”) and 6,000 are designated as Series B Convertible Preferred Stock (“Series B Preferred”) as specified in the Certificate of Designation (the “Certificate”).
During November and December 2005, the Company completed a private placement totaling 7,000 units at a purchase price of $1,000 per unit. Each unit consisted of one share of Series A Preferred, convertible into 400 shares of common stock, and a detachable warrant to purchase 200 shares of common stock at an exercise price of $4.00 per share. Gross proceeds from the private placement amounted to $7,000,000 and net proceeds amounted to approximately $6,000,000. As a result of the private placement, in September 2007 (see below), Series A Preferred is now convertible into 830.36 shares of the Company’s common stock. All Series A warrants have expired.
On September 17, 2007, the Company completed a private placement of 4,350 shares of Series B convertible preferred stock, par value $0.001 per share, and warrants to purchase the Company’s common stock, par value $0.001 per share, for an aggregate purchase price of $4,350,000. Each share of the Series B convertible preferred stock is initially convertible, at the holder’s election, into approximately 830.36 shares of common stock and has a liquidation preference that is pari passu with the Company’s Series A convertible preferred stock and senior to the Company’s common stock. Cumulative dividends on the Series B convertible preferred stock accrue on the initial stated value of $1,000 per share at an annual rate of 8%, payable monthly in cash and/or shares of the Company’s common stock, at the option of the Company. As of December 31, 2010, cash dividend payments amounted to $173,016 and there were no accrued but unpaid dividends to Series B convertible preferred stockholders. During the years ended December 31, 2010 and 2009, cash dividends paid aggregated to $173,016 and $304,318, respectively.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
The warrants have a seven-year term and are initially exercisable into 2,167,232 shares of common stock. The warrants are exercisable, at the holder’s election, for shares of the Company’s common stock in either a cash or cashless exercise. Fifty percent of the warrants have an initial exercise price equal to $1.2043 per share and the other fifty percent have an initial exercise price of $1.3849 per share. The Company also issued warrants to the financial advisor in the transaction, Canaccord Adams, Inc., to purchase 67,530 shares of common stock, which was recorded at fair value of approximately $57,000 and was recognized as additional paid in capital. The value of the warrants was computed using the Black-Scholes option-pricing model with the following assumptions: risk free rate of 2.62%, contractual term of seven years, expected volatility of 67.5%, 0% expected dividend yield, stock price of $1.01 per share, and exercise prices of $1.2043 and $1.3849 per share. During 2010, there was a cashless exercise of 124,554 warrants that resulted in the issuance of 21,163 shares of the Company’s common stock. There were no exercises prior to 2010.
The conversion feature of the Company’s warrants do not have fixed settlement provisions because their exercise prices may be lowered if the Company issues securities at lower prices in the future. The Company included the reset provisions in order to protect the warrant holders from the potential dilution associated with future financings. Accordingly the warrants have been classified as derivative liabilities as discussed above.
During the year ended December 31, 2010, 250 shares of the Company’s series A convertible preferred stock were converted into 207,590 shares of the Company’s common stock and 2,110 shares of series B convertible preferred stock were converted in 1,752,057 shares of the Company’s common stock.
NOTE 7 – Share-Based Compensation
Stock Options
As of the end of 2010, the Company’s 2001 Long-Term Incentive Plan, 2005 Long-Term Incentive Plan and 2006 Long-Term Incentive Plan have a total of 6,021,033 in stock option grants and 114,054 in restricted stock issued to directors, officers and employees of the Company for a total of 6,135,087. In May 2007, the stockholders of the Company approved the adoption of the Company’s 2006 Stock Plan (the “Plan”), which amended the Company’s 2005 and 2001 Stock Plan. The Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to employees and for the grant of non-statutory stock options, restricted stock, and stock appreciation rights to employees, directors, and consultants. The Compensation Committee of the Company’s board of directors administers the Plan and has the authority to make awards under the Plan and establish vesting and other terms, but cannot grant stock options at less than the fair value of the Company’s common stock on the date of grant or re-price stock options previously granted. The employee stock options granted under the Plan generally vest ratably over three to four years of service and expire seven to ten years from the date of grant (or ninety days after the termination of employment). As of December 31, 2010, 2,228,190 stock options remained eligible for grant under the 2006 Long-Term Incentive Plan. The 2001 and 2005 Long-Term Incentive Plans have been closed for additional grants.
On November 4, 2009, the Company executed a stock option exchange program whereby eligible employees and directors could elect to receive one stock option at the closing market price on November 4, 2009 for two previously granted stock options. Pursuant to the exchange offer, 1,366,250 eligible options (representing 40% of the total stock options eligible for exchange) were exchanged for new stock options with similar terms (including an additional year vesting period) to purchase 683,125 shares of common stock. The exercise price of the new options is $1.20 per share, which was the closing price of the Company’s common stock on November 4, 2009 as reported by the Nasdaq Capital Market. The exchange did not result in incremental fair value or an additional charge to the Company’s consolidated statement of operations for the year ended December 31, 2009.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
During the years ended December 31, 2010 and 2009, the Company granted options to purchase 1,574,328 and 1,468,184 shares of common stock, respectively, to employees at exercise prices of $0.94, $1.06, $1.12, $1.18, $1.22 and $1.28, which approximated the fair value on the respective grant dates. Shares granted after November 4, 2005 (the exchange transaction date) were made under the 2006 VirtualScopics, Inc., Long Term Incentive Plan. These options generally vest ratably during the first four years following their issuance and have a ten-year life. There were a total of 250,000 options exercised as of December 31, 2010, resulting in the issuance of 86,501 shares of the Company’s common stock and cash proceeds of $25,250.
A summary of the option activity for the year ended December 31, 2010 and 2009 are as follows:
| | | Number of Shares | | | Weighted Average Exercise Price | | | Weighted- Average Remaining Contractual Term | | | Aggregate Intrinsic Value | |
| | | | | | | | | | | | | |
| Options outstanding at January 1, 2009 | | | 4,676,868 | | | $ | 1.64 | | | | | | | |
| Granted | | | 1,468,184 | | | | 1.06 | | | | | | | |
| Cancelled | | | (1,379,250 | ) | | | (3.02 | ) | | | | | | |
| Options outstanding at December 31, 2009 | | | 4,765,802 | | | | 1.28 | | | | 6.10 | | | | 326,412 | |
| Granted | | | 1,574,328 | | | | 1.07 | | | | | | | | | |
| Exercised | | | (250,000 | ) | | | (0.77 | ) | | | | | | | | |
| Cancelled | | | (143,282 | ) | | | (1.66 | ) | | | | | | | | |
| Options outstanding at December 31, 2010 | | | 5,946,848 | | | | 1.24 | | | | 6.30 | | | | 5,640,172 | |
| Options exercisable at December 31, 2010 | | | 2,981,354 | | | | 1.43 | | | | 4.37 | | | | 2,367,595 | |
Additional information with respect to the outstanding options as of December 31, 2010 is as follows:
| | | Options Outstanding | | | Options Exercisable | |
Exercise Prices | | Number Outstanding at December 31, 2010 | | | Weighted Average Remaining Life (Years) | | | Weighted Average Exercise Price | | | Number Exercisable at December 31,
2010 | | | Weighted Average Exercise Price | |
| | | | | | | | | | | | | | | | |
| $ 0.43 - 1.19 | | | 3,449,025 | | | | 7.10 | | | | 0.93 | | | | 1,231,314 | | | | 0.87 | |
| $ 1.20 - 1.45 | | | 1,558,119 | | | | 6.11 | | | | 1.23 | | | | 812,086 | | | | 1.23 | |
| $ 1.88 - 1.90 | | | 249,330 | | | | 1.56 | | | | 1.90 | | | | 247,580 | | | | 1.90 | |
| $ 2.36 - 2.50 | | | 606,874 | | | | 4.30 | | | | 2.44 | | | | 606,874 | | | | 2.44 | |
| $ 3.03 – 4.15 | | | 83,500 | | | | 5.61 | | | | 3.04 | | | | 83,500 | | | | 3.04 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 5,946,848 | | | | 6.30 | | | | 1.24 | | | | 2,981,354 | | | | 1.43 | |
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
The weighted-average grant-date fair value of options granted during the year ended December 31, 2010 and 2009 was $951,092 and $1,552,150, respectively.
For the years ended December 31, 2010 and 2009, the Company recorded a total of $819,766 and $1,027,561, respectively, in stock-based compensation expense, which is allocated as follows:
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | |
| | | | | | | |
| Cost of service revenues | | $ | 31,144 | | | $ | 27,516 | |
| Research and development | | | 102,653 | | | | 83,365 | |
| Sales and marketing | | | 19,094 | | | | 24,492 | |
| General and administrative | | | 666,875 | | | | 892,188 | |
| Total stock-based compensation | | $ | 819,766 | | | $ | 1,027,561 | |
A summary of the status of the non-vested shares as of December 31, 2010 and changes during the year ended December 31, 2010, is presented below:
| | | | | | | |
| Non-vested Shares | | Shares | | | Weighted-Average Grant-Date Fair Value Per Share | |
| Non-vested at January 1, 2009 | | | 1,688,002 | | | $ | 1.65 | |
| Granted | | | 1,468,184 | | | | 1.06 | |
| Vested | | | 598,517 | | | | 3.90 | |
| Cancelled Grants | | | (1,379,250 | ) | | | (3.02 | ) |
| Non-vested at December 31, 2009 | | | 2,375,453 | | | $ | 1.51 | |
| Granted | | | 1,574,328 | | | | 0.60 | |
| Vested | | | (938,330 | ) | | | (0.54 | ) |
| Cancelled Grants | | | (45,957 | ) | | | (0.79 | ) |
| Non-vested at December 31, 2010 | | | 2,965,494 | | | $ | 0.71 | |
As of December 31, 2010, there was $910,429 of total unrecognized compensation cost related to non-vested share-based compensation arrangements. This cost is expected to be recognized over a weighted-average period of 2.31 years. The total fair value of shares vested during the year ended December 31, 2010 amounted to $510,689.
Prior to 2008, the Company issued options under the 2006 Long-Term Incentive Plan to non-employee consultants for radiological services performed. These options to non-employees vest immediately, have exercise prices ranging from $1.12 to $6.85 and a term of seven or six years from the date of grant. The value of the options was based on the fair value of the services performed and were included in the Company’s statements of operations. During 2010, a total of 67,505 of the non-employee options expired.
The total amount of stock options outstanding as of December 31, 2010 is:
| Stock options granted to employees | | | 5,946,848 | |
| Stock options granted to consultants | | | 74,185 | |
| Total outstanding | | | 6,021,033 | |
During 2010, and through the date of this report, a total of 1,574,328 stock options were granted, of that amount 1,020,795 were granted to executive officers of the Company.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
Restricted Stock Awards
A restricted stock award entitles the recipient to receive shares of unrestricted common stock upon vesting of the award. The fair value of each restricted stock award is determined upon granting of the shares and the related compensation expense is recognized ratably over the vesting period and charged to the income statements as non-cash compensation expense. Restricted stock awards granted but unvested shares are forfeited upon termination of employment, unless otherwise agreed. The fair value of restricted stock issued under the Plan is determined based on the closing price of the Company’s common stock on the grant date. Under the provisions of the 2006 Long Term Incentive Plan, the Company may grant restricted stock to its employees, Board members and consultants. During 2006, the Board of Directors Compensation Committee approved an equity based compensation structure for non-employee Board members. As of December 31, 2010 and 2009, respectively, there were 265,806 and 229,682 shares of common stock that have been issued or are reserved for issuance as restricted stock units under the Plan. As of December 31, 2010, the Company has a liability of $60,313 (equating to 48,052 restricted stock units) related to awards that will be given to certain Board members, on pre-determined dates, in lieu of cash for their services as Board members during 2010. As the shares are issued by the transfer agent, the liability is reduced. In 2010 and 2009, 114,054 and 103,700, respectively, restricted stock awards were issued to members of the Board for their services on the Board under the 2006 Long Term Incentive Plan. The restricted stock awards are fully vested and non-forfeitable and are therefore included in the outstanding common stock of the Company as of December 31, 2010. The weighted average grant date fair value of restricted stock issued by the Company during 2010 and 2009 were $132,303 and $120,293 respectively.
The Company incurred $43,756 and $39,519 in compensation expense in 2010 and 2009 related to the restricted stock awards for services by Board members for those respective periods.
NOTE 8 - Benefit Plan
The Company has a defined contribution plan which covers all of its full-time employees. The employees’ annual contributions are limited to the maximum allowed under the Internal Revenue Code. During 2009, the Company began a matching contribution to participants 401k plans equal to 50% of the participants’ contributions up to a maximum 3% of annual wages. The Company paid a total of $105,678 and $28,023 in 2010 and 2009 to participants representing the employer contribution amount.
NOTE 9 - Income Taxes
The Company has identified its federal tax return and its state tax return in New York as “major” tax jurisdictions, as defined. Based on the Company’s evaluation, it has been concluded that there are no significant uncertain tax positions requiring recognition in the Company’s consolidated financial statements. The Company’s evaluation was performed for the tax years ended 2007 through 2010, the only periods subject to examination. The Company believes that its income tax positions and deductions will be sustained on audit and does not anticipate any adjustments that will result in a material change to its financial position. The Company does not expect its unrecognized tax benefit to change during the next 12 months. As of December 31, 2010, all of the Company’s deferred tax assets were fully reserved by a valuation allowance equal to 100% of the net deferred tax assets. During 2011, the Company will assess the likelihood of recognizing a portion of its deferred tax assets and will make an assessment of whether it should reduce the valuation allowance.
The Company has significant net operating loss and business credit carryovers which are subject to a valuation allowance due to the uncertain nature of the realization of the losses. Section 382 of the Internal Revenue Code imposes certain limitations on the utilization of net operating loss carryovers and other tax attributes after a change in control. If the Company has a change in ownership, such change could significantly limit the possible utilization of such carryovers. Since its formation, the Company has raised capital through the issuance of capital stock which, combined with purchasing shareholders' subsequent disposition of these shares, has resulted in an ownership change as defined by Section 382, and also could result in an ownership change in the future upon subsequent disposition.
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
The Company will recognize interest and penalties accrued related to unrecognized tax benefits as components of its income tax provision. The Company does not have any interest and penalties accrued related to unrecognized tax benefits.
The Company accounts for income taxes in accordance with ASC 740, Income Taxes. Under ASC 740, deferred income taxes and liabilities are recognized based on temporary differences between the financial statement and tax basis of assets and liabilities using the enacted tax rates in effect in the years in which the differences are expected to reverse. ASC 740 requires recognition of net deferred tax assets to the extent it is more likely than not that such net assets will be realized. To the extent that the Company believes that its net deferred tax assets will not be realized, a valuation allowance must be recorded against those assets.
The income tax provision consists of the following:
| | | 2010 | | | 2009 | |
| Current | | | | | | |
| Federal | | $ | 16,000 | | | $ | - | |
| State | | | - | | | | - | |
| | | | 16,000 | | | | - | |
| Deferred | | | | | | | | |
| Federal | | | - | | | | - | |
| State | | | - | | | | - | |
| | | | - | | | | - | |
| | | | | | | | | |
| Total income tax provision | | $ | 16,000 | | | $ | - | |
During the year ending December 31, 2010, the company utilized approximately 1,187,000 of its net operating loss carryforward. The utilization of this net operating loss carryforward resulted in a reduction of the deferred tax asset for the net operating loss of 416,000. The company also had a decrease in the valuation allowance related to other deferred tax assets of 79,000. The company had a total decrease in the valuation allowance of 495,000.
The Company has net operating loss carryforwards (“NOLs”) of approximately $7,773,000 as of December 31, 2010 that will be available to offset future taxable income. Approximately $158,000 of the NOL carryforward, if realized, will result in a benefit to be recorded in APIC. The NOLs are due to expire in 2025 through 2028. The Company has concluded that a full valuation allowance was appropriate for the NOLs as they are more likely than not to be utilized prior to their expiration.
The total net deferred tax asset and liabilities as of December 31, 2010 and 2009 consists of the following:
| | | 2010 | | | 2009 | |
| Net operating loss carryforwards | | $ | 2,945,983 | | | $ | 3,362,588 | |
| Intangible assets | | | 1,024,124 | | | | 1,140,944 | |
| Accrued expenses | | | 124,001 | | | | 115,944 | |
| Credit carryforwards | | | 110,783 | | | | - | |
| Stock-based compensation | | | 909,241 | | | | 948,222 | |
| Total deferred tax asset | | | 5,114,132 | | | | 5,567,698 | |
| | | | | | | | | |
| Deferred tax liability: | | | | | | | | |
| Property and equipment | | | (81,385 | ) | | | (39,825 | ) |
| Subtotal | | | 5,032,747 | | | | 5,527,873 | |
| Less: valuation allowance | | | 5,032,747 | | | | (5,527,873 | ) |
| Total net deferred tax asset | | $ | - | | | $ | - | |
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
The difference between the federal statutory and effective income tax rates for the years ended December 31, 2010 and 2009 is as follows:
| | | 2010 | | | 2009 | |
| Federal statutory tax rate | | | (34.00 | )% | | | (34.00 | )% |
| State and local income taxes, net of federal benefit | | | (4.69 | )% | | | (4.69 | )% |
| Stock-based compensation | | | 43.51 | % | | | 66.20 | % |
| Loss from derivative financial instrument | | | 90.40 | % | | | 27.43 | % |
| Research and development credit | | | (15.07 | )% | | | - | |
| Other | | | 1.10 | % | | | 2.92 | % |
| | | | 81.25 | % | | | 57.86 | % |
| | | | | | | | | |
| Less: valuation allowance | | | (78.71 | )% | | | (57.86 | )% |
| Provision for income taxes | | | 2.54 | % | | | 0.00 | % |
NOTE 10 - Commitments and Contingencies
Operating Leases
In July, 2007 we began leasing approximately 19,500 square feet of office space at our corporate headquarters in Rochester, New York. The base annual rent under the lease is $360,000, and increases three percent (3%) a year. During the first twenty months of the lease, the rent was paid in two portions: a cash portion of $156,000 annually, paid in equal monthly installments, increasing three percent (3%) annually; and, a stock portion of $204,000 annually, paid in equal monthly installments, increasing three percent (3%) annually. The stock portion was payable in shares of our common stock. In February 2009, the Landlord exercised their option to receive their remaining rental payments in all cash. During 2010 and 2009, we issued 0 and 88,132 shares, respectively, of the Company’s common stock ranging from $1.20 to $2.50 per share for a total value of $0 and $105,570, respectively. Management believes that the leased property is adequately covered by insurance.
In April 2010, the Company entered into a lease agreement for certain equipment. The lease is for 36 months and will expire in March 2013.
Total rent expense for the years ended December 31, 2010 and 2009 was $325,148 and $331,065, respectively.
Future minimum rental commitments under non-cancelable operating leases are as follows:
| For the Years Ending | | | |
| December 31, | | Amount | |
| 2011 | | $ | 341,188 | |
| 2012 | | | 175,687 | |
| 2013 | | | 1,306 | |
| Total | | $ | 518,181 | |
VirtualScopics, Inc. and Subsidiary
Notes to Consolidated Financial Statements
NOTE 11 - Related Parties
On June 26, 2002, the Company entered into a multi-year strategic relationship with Pfizer Inc. (“Pfizer”) to accelerate the discovery, validation and application of image-based biomarkers for clinical research. Under the terms of the agreement, Pfizer invested $2,500,000 in the Company, for a 10% ownership interest. The Company used $1,500,000 of the investment to purchase the intellectual property that it was licensing from the University of Rochester. In August 2005, the agreement was extended for another two years and provided for termination by Pfizer by giving a 30-day advance written notice. Additionally, in November 2006, the agreement was further extended until July 2008, with automatic renewals unless either party provides written notice 60 days prior to the next anniversary. Revenues generated from this customer were $90,473 and $100,969 for the years ended December 31, 2010 and 2009, respectively. As of December 31, 2010 and 2009, the accounts receivable balance from Pfizer was $22,464 and $8,383, respectively.
In December 2002, the Company received an investment of $2,450,000 from GE Medical Systems. Upon receipt of the proceeds of this investment, the Company purchased an MRI machine from this investor for $2,300,000. During 2003, the equipment was sold to the University of Rochester, a related party, for $2,300,000 of which $1,250,000 was received in cash proceeds and the balance of $1,050,000 was applied as an advance payment for use of the equipment and is recorded in other assets on the balance sheet as of December 31, 2010 and is being amortized based on usage over the life of the agreement. The Company’s retained right to use the machine exclusively one day a week expires in March, 2011. The equipment is being used by the Company for research and to broaden its ability to service its customers. Revenues generated from the University of Rochester were $22,847 and $39,547 for the years ended December 31, 2010 and 2009, respectively. As of December 31, 2010 and 2009, the accounts receivable balance from the University of Rochester was $0 and $8,655, respectively.
NOTE 12 - Major Customers
The Company’s top customer accounted for approximately 56% of total revenue for the year ended December 31, 2010 and 59% of accounts receivable. For the year ended December 31, 2009, the largest customer accounted for 43% of total revenue and 45% of accounts receivable.
NOTE 13 – Subsequent Event
Subsequent to December 31, 2010, there were 1,208,170 series B warrants exercised that resulted in the issuance of 618,694 shares of the Company’s common stock and cash proceeds of $105,001. Of the warrants exercised, 1,120,982 were through cashless exchanges.

 2
2