UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A INFORMATION
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
Filed by the Registrantx Filed by a Party other than the Registrant¨
Check the appropriate box:
| ¨ | Preliminary Proxy Statement |
| ¨ | Confidential, For Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| x | Definitive Proxy Statement |
| ¨ | Definitive Additional Materials |
| ¨ | Soliciting Material Pursuant to §240.14a-12 |
ACCENTIA BIOPHARMACEUTICALS, INC.
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ¨ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | (1) | Title of each class of securities to which transaction applies: |
| | (2) | Aggregate number of securities to which transaction applies: |
| | (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | (4) | Proposed maximum aggregate value of transaction: |
| ¨ | Fee paid previously with preliminary materials. |
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | (1) | Amount previously paid: |
| | (2) | Form, Schedule or Registration Statement No.: |
ACCENTIA BIOPHARMACEUTICALS, INC.
NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
TO BE HELD ON FEBRUARY 28, 2007
To the Shareholders of Accentia Biopharmaceuticals, Inc.:
You are cordially invited to attend the 2007 Annual Meeting of Shareholders of Accentia Biopharmaceuticals, Inc. (the “Company”), which will be held at the St. Louis Club, located at 7701 Forsyth Blvd., St. Louis, Missouri 63105, on February 28, 2007 at 11:00 a.m., local time, and any adjournments or postponements thereof for the following purposes:
| | 1. | To elect one director to hold office until the 2009 Annual Meeting of Shareholders and three directors to hold office until the 2010 Annual Meeting of Shareholders; |
| | 2. | To ratify the appointment of Aidman, Piser & Company as the Company’s independent registered public accounting firm for the fiscal year ended September 30, 2007; |
| | 3. | To approve the potential issuance of shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”), exceeding 19.99% of the number of shares outstanding on September 29, 2006 upon conversion of convertible debentures and exercise of warrants issued in a private placement financing for the purpose of complying with that certain Securities Purchase Agreement, dated September 29, 2006, among the Company and the investors and the rules governing the NASDAQ Global Market, formerly known as the NASDAQ National Market (the “NASDAQ”); and |
| | 4. | To consider and transact such other business as may properly come before the meeting or any adjournment thereof. |
Only shareholders of record at the close of business on January 16, 2007 will be entitled to vote at the annual meeting. Information relating to the matters to be considered and voted on at the annual meeting is set forth in the proxy statement accompanying this notice. A copy of our annual report for our 2006 fiscal year is also enclosed.
Please read the proxy statement and vote your shares as soon as possible. To ensure your representation at the annual meeting, please complete, date, sign, and return the enclosed proxy, even if you plan to attend the annual meeting. A proxy and a self-addressed stamped envelope are enclosed. If you attend the annual meeting, you may withdraw your proxy and vote in person.
|
| By Order of the Board of Directors, |
|
| /s/ Francis E. O’Donnell, Jr., M.D. |
| Francis E. O’Donnell, Jr., M.D. |
| Chairman of the Board and Chief Executive Officer |
January 26, 2007
ACCENTIA BIOPHARMACEUTICALS, INC.
324 South Hyde Park Ave., Suite 350
Tampa, Florida 33606
(813) 864-2554
PROXY STATEMENT
FOR
2007 ANNUAL MEETING OF SHAREHOLDERS
This Proxy Statement is furnished in connection with the solicitation of the accompanying proxies on behalf of the Board of Directors of Accentia Biopharmaceuticals, Inc. (the “Company”) for use at the Company’s 2007 Annual Meeting of Shareholders (the “Annual Meeting”) to be held on February 28, 2007 at 11:00 a.m., local time, at the St. Louis Club, 7701 Forsyth Blvd., St. Louis, Missouri 63105, and any adjournments or postponements of the Annual Meeting.
This Proxy Statement and the accompanying proxy forms, together with the Company’s 2006 Annual Report to Shareholders, are first being mailed to shareholders entitled to vote at the Annual Meeting on or about January 29, 2007.
ABOUT THE MEETING
Why am I receiving these materials?
At the Annual Meeting, shareholders will act upon matters described in the notice of meeting contained in this Proxy Statement, including the election of directors. We sent you this proxy statement and the enclosed proxy card because the Board of Directors of the Company is soliciting your proxy to vote at the Annual Meeting. You are invited to attend the Annual Meeting to vote on the proposals described in this proxy statement. However, you do not need to attend the meeting to vote your shares. Instead, you may simply complete, sign, and return the enclosed proxy card, or, if your shares are held by a broker, you may vote your shares by telephone or over the Internet, if authorized by your broker.
Who is entitled to vote?
Only holders of the Company’s common stock outstanding as of the close of business on January 16, 2007 (the “Record Date”) will be entitled to vote at the Annual Meeting. Each shareholder is entitled to one vote for each share of common stock he or she held on the Record Date.
Who can attend the Annual Meeting?
All shareholders, or individuals holding their duly appointed proxies, may attend the Annual Meeting. Appointing a proxy in response to this solicitation will not affect a shareholder’s right to attend the Annual Meeting and to vote in person. Please note that if you hold your shares in “street name” (in other words, through a broker, bank, or other nominee), you will need to bring a copy of a brokerage statement reflecting your stock ownership as of the Record Date to gain admittance to the Annual Meeting.
What constitutes a quorum?
A majority of the 32,143,011 shares of common stock outstanding on the Record Date must be represented, in person or by proxy, to provide a quorum at the Annual Meeting. If you vote, your shares will be part of the quorum. Shares represented by a proxy card either marked “ABSTAIN” or returned without voting instructions are counted as present for the purpose of determining whether the quorum requirement is satisfied. Also, in those instances where shares are held by brokers who have returned a proxy but are prohibited from exercising discretionary authority for beneficial owners who have not given voting instructions (“broker non-votes”), those shares will be counted as present for quorum purposes. However, broker non-votes will not be counted as votes for or against any proposal.
What is the effect of not voting?
It will depend on how your share ownership is registered. If you own shares as a registered holder and do not vote, your unvoted shares will not be represented at the meeting and will not count toward the quorum requirement. If a quorum is obtained, your unvoted shares will not affect whether a proposal is approved or rejected.
If you own shares in street name and do not vote, your broker may represent your shares at the meeting for purposes of obtaining a quorum. In the absence of your voting instructions, your broker may or may not vote your shares in its discretion depending on the proposals before the meeting. Your broker may vote your shares in its discretion on routine matters such as Proposal 1, the election of directors. With regard to non-routine matters (such as Proposal 3), broker non-votes will be counted towards a quorum, but will not be counted for any purpose in determining whether these matters have been approved. Abstentions will be counted toward the tabulation of votes and will have the same effect as negative notes. Once a share is represented at the Annual Meeting, it will be deemed present for quorum purposes throughout the Annual Meeting (including any adjournment or postponement of that meeting unless a new record date is or must be set for such adjournment or postponement).
How do I vote?
Shareholders who own shares registered directly with the Company’s transfer agent on the close of business on January 16, 2007 can appoint a proxy by mailing their signed proxy card in the enclosed envelope or by transmitting voting instructions over the Internet or by telephone. Any such shareholder who wishes to vote via the Internet can do so by accessingwww.proxyvote.com with his or her proxy card in hand, and any such shareholder who wishes to vote by telephone should call 1-800-690-6903 with his or her proxy card in hand. Street name holders may vote by telephone or Internet if their bank or broker makes those methods available, in which case the bank or broker will enclose the instructions with the Proxy Statement. The telephone and Internet voting procedures are designed to authenticate shareholders’ identities, to allow shareholders to give their voting instructions and to confirm that the shareholders’ instructions have been properly recorded.
Can I change my vote after I return my proxy card?
Yes. Even after you have submitted your proxy, you can change your vote at any time before the proxy is exercised by appointing a new proxy bearing a later date, by providing written notice to the Secretary of the Company that you are revoking your proxy, or by voting in person at the Annual Meeting. Presence at the Annual Meeting of a shareholder who has appointed a proxy does not in itself revoke a proxy. Unless so revoked, the shares represented by proxies received by the Board will be voted at the Annual Meeting. When a shareholder specifies a choice by means of the proxy, then the shares will be voted in accordance with such specifications. A written notice to the Company’s Secretary revoking your proxy must be sent to James A. McNulty, Secretary, Accentia Biopharmaceuticals, Inc., 324 South Hyde Park Ave., Suite 350, Tampa, Florida 33606.
What am I voting on?
You are voting on three proposals:
1. Election of four directors, with the following as the Board’s nominees:
| | • | | Edmund C. King (nominated as a Class I director with a term to end at the 2009 Annual Meeting of Shareholders); |
| | • | | John P. Dubinsky (nominated as a Class II director with a term to end at the 2010 Annual Meeting of Shareholders); |
| | • | | Francis E. O’Donnell, Jr., M.D. (nominated as a Class II director with a term to end at the 2010 Annual Meeting of Shareholders); and |
| | • | | Todd D. Thomason (nominated as a Class II director with a term to end at the 2010 Annual Meeting of Shareholders). |
2
2. Ratification of the appointment of Aidman, Piser & Company as the Company’s independent registered public accounting firm for the fiscal year ending September 30, 2007.
3. Approval of the potential issuance of shares of the Company’s Common Stock exceeding 19.99% of the Common Stock outstanding on September 29, 2006 pursuant to the terms of (a) certain 8% Secured Convertible Debentures due September 29, 2010 and Common Stock Purchase Warrants to purchase shares of the Company’s Common Stock issued by the Company in a private placement financing in September 2006 and (b) a Common Stock Purchase Warrant to purchase shares of the Company’s Common Stock issued to the Company’s placement agent in connection with such financing.
What are the Board’s recommendations?
The Board recommends a vote:
| | • | | For election of the nominated slate of directors. |
| | • | | For the ratification of the appointment of Aidman, Piser & Company as the Company’s independent registered public accounting firm for the fiscal year ending September 30, 2007. |
| | • | | For the approval of the potential issuance of shares of the Company’s Common Stock exceeding 19.99% of the Common Stock outstanding on September 29, 2006 pursuant to the terms of (a) certain 8% Secured Convertible Debentures due September 29, 2010 and Common Stock Purchase Warrants to purchase shares of the Company’s Common Stock issued by the Company in a private placement financing in September 2006 and (b) a Common Stock Purchase Warrant to purchase shares of the Company’s Common Stock issued to the Company’s placement agent in connection with such financing. |
If you sign and return your proxy card, unless you give other instructions on your proxy card, the persons named as proxy holders on the proxy card will vote in accordance with the recommendations of the Board.
How are votes counted?
Votes will be counted by the inspector of election appointed for the meeting, who will separately count “For” and “Withhold” votes, and with respect to proposals other than the election of directors, “Against” votes, abstentions, and broker non-votes. Abstentions will be counted towards the vote total for each proposal and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for any proposal.
If your shares are held by your broker as your nominee (that is, in “street name”), you will need to obtain a proxy form from the institution that holds your shares and follow the instructions included on that form regarding how to instruct your broker to vote your shares. If you do not give the broker or nominee specific instructions, your broker or nominee can vote your shares with respect to “discretionary” items, but not with respect to “non-discretionary” items. Discretionary items are proposals considered routine under the rules of the New York Stock Exchange on which your broker may vote shares held in street name in the absence of your voting instructions. On non-discretionary items for which you do not give your broker instructions, the shares will be treated as broker non-votes. Shares represented by such “broker non-votes” will, however, be counted in determining whether there is a quorum.
What vote is required to approve the proposals?
The four nominees for director receiving the greatest number of votes will be elected. With regard to the ratification of the appointment of Aidman, Piser & Company as the Company’s independent registered public accounting firm for fiscal 2007, the affirmative vote of a majority of the shares present or represented and entitled to vote at the Annual Meeting will constitute the ratification of the proposal. Provided that a quorum is present at the Annual Meeting, the affirmative vote of a majority of the shares present in person or represented by proxy and entitled to vote at the Annual Meeting will be required to approve the potential issuance of shares of
3
the Company’s Common Stock exceeding 19.99% of the Common Stock outstanding on September 29, 2006 pursuant to the terms of the Debentures and the Investor Warrants issued by the Company in the Financing and the Placement Agent Warrant issued in connection with the Financing.
Are there any other items that are to be discussed during the Annual Meeting?
No. The Company is not aware of any other matters that you will be asked to vote on at the Annual Meeting. If other matters are properly brought before the Annual Meeting, the Board or proxy holders will use their discretion on these matters as they may arise.
Who will count the vote?
ADP Investor Communication Services will count the vote and will serve as the inspector of the election at the Annual Meeting.
Who pays to prepare, mail, and solicit the proxies?
Proxies may be solicited by personal meeting, Internet, advertisement, telephone, and facsimile machine, as well as by use of the mails. Solicitations may be made by directors, officers, and other employees of the Company, as well as the Company’s investor relations firm, none of whom will receive additional compensation for such solicitations. The cost of soliciting proxies will be borne by the Company. It is anticipated that banks, brokerage houses, and other custodians, nominees, or fiduciaries will be requested to forward solicitation materials to their principals and to obtain authorization for the execution of proxies and that they will be reimbursed by the Company for their out-of-pocket expenses incurred in providing those services. All expenses of solicitation of proxies will be borne by the Company.
Delivery of Proxy Materials to Households
Pursuant to SEC rules, services that deliver the Company’s communications to shareholders that hold their stock through a bank, broker, or other holder of record may deliver to multiple shareholders sharing the same address a single copy of the Company’s annual report to shareholders and this proxy statement. Upon written or oral request, the Company will promptly deliver a separate copy of the annual report to shareholders and this proxy statement to any shareholder at a shared address to which a single copy of each document was delivered. Shareholders may notify the Company of their requests by calling the Company at (813) 864-2554 or by sending a written request addressed to the Company, Attention: Secretary, 324 South Hyde Park Ave., Suite 350, Tampa, Florida 33606.
How can I contact the members of the Board?
Shareholders may communicate with the full Board or individual directors by submitting such communications in writing to Accentia Biopharmaceuticals, Inc., Attn: Board of Directors (or the individual director(s)), 324 South Hyde Park Ave., Suite 350, Tampa, Florida 33606. Such communications will be delivered directly to the directors.
4
PROPOSAL 1—ELECTION OF DIRECTORS
The Board of Directors recommends the following nominees for election as directors and recommends that each shareholder vote “FOR” the nominees. Executed proxies in the accompanying form will be voted at the annual meeting in favor of the election as directors of the nominees named below, unless authority to do so is withheld.
Our Board of Directors presently consists of seven members and is divided into three classes (designated “Class I,” “Class II,” and “Class III”), with the number of directors in each class being as nearly equal in number as possible. Our Board of Directors was first divided into three classes in October 2005 at the time of our initial public offering. Our articles of incorporation and bylaws provide that the directors in each respective class will serve three-year terms expiring at the third annual meeting of shareholders after their elections or until their respective successors have been duly elected and qualified. The initial term of the Class I directors expired at our first annual shareholder meeting following our initial public offering (the 2006 Annual Meeting), the initial term of the Class II directors is scheduled to expire at this Annual Meeting, and the initial term of the Class III directors is scheduled to expire at our 2008 Annual Meeting. Accordingly, at the Annual Meeting, three Class II directors are required to be elected. Our Board of Directors, upon the recommendation of the Governance and Nominating Committee, has nominated John P. Dubinsky and Francis E. O’Donnell, Jr. to stand for re-election as Class II directors.
In addition, the Florida Business Corporation Act requires that any director elected by the Board of Directors to fill a vacancy on the Board must stand for re-election at the next annual meeting of the shareholders. Two directors, Edmund C. King and Todd D. Thomason, were elected as directors by the Board in October 2006 to fill vacancies on the Board. Therefore, in addition to the above-described nomination of Messrs. Dubinsky and O’Donnell as Class II directors, our Board of Directors, upon the recommendation of the Governance and Nominating Committee, has nominated Mr. King to stand for re-election to the Board at the Annual Meeting as a Class I director and Mr. Thomason to stand for re-election to the Board at the Annual Meeting as a Class II director.
The persons nominated for election have agreed to serve if elected, and the Board of Directors has no reason to believe that any of these nominees will be unavailable or will decline to serve. In the event, however, that any of the nominees are unable or decline to serve as a director at the time of the Annual Meeting, the persons designated as proxies will vote for any nominee who is designated by our current Board of Directors to fill the vacancy.
The four nominees for director named above currently are directors of the Company and are proposed to be elected at the Annual Meeting to serve until the 2010 annual meeting of shareholders (in the case of Class II directors) or until the 2009 annual meeting of shareholders (in the case of the Class I director). The remaining three directors will continue to serve as members of the Board for the terms described below. Directors are elected by a plurality of the votes cast (assuming a quorum is present at the Annual Meeting), meaning that the four nominees receiving the highest number of affirmative votes of the votes represented at the Annual Meeting will be elected as directors. Proxies solicited by the Board will be voted “FOR” the nominees named above unless a shareholder specifies otherwise.
The following is biographical information and the age (as of the Record Date) for each person nominated and each person whose term of office will continue after the Annual Meeting:
5
NOMINEES FOR DIRECTORS
Class I Directors (terms to expire at the 2009 annual meeting of shareholders)
Edmund C. King, age 71, was elected to the Board of Directors of the Company on October 27, 2006. Mr. King was a partner in Ernst & Young, an international accounting and consulting firm. While at Ernst & Young, Mr. King was that firm’s southern California senior healthcare partner and prior to that directed the southern California healthcare practice for Arthur Young & Company, one of the predecessor firms of Ernst & Young. During his 30 years with Ernst & Young, Mr. King counseled clients in structuring acquisitions and divestitures; advised on the development of strategic plans; directed the preparation of feasibility studies; assisted with operational and financial restructuring; directed and supervised audits of client financial statements; and provided expert witness testimony and technical SEC consultation. Commencing in 1999, Mr. King became a financial consultant to SmartGate, L.C. Mr. King has served as Chief Financial Officer and Director of Invisa, Inc. since November 2000 and Chief Financial Officer and Director of FlashPoint International, Inc. since October 2001. From January 1992, Mr. King has been a general partner of Trouver, an investment banking and financial consulting partnership. Mr. King is also a member of the Board of Directors of LTC Properties, Inc., NYSE listed real estate investment trust. Mr. King is a graduate of Brigham Young University having served on the National Advisory Council of that school’s Marriott School of Management, and has completed a Harvard University management course sponsored by Ernst & Young. Mr. King also has served as Chairman of the MPMA’s Long-Term Care Committee (Los Angeles Chapter) and is a past member of the National Association of Corporate Directors. He also holds a CPA certificate in the state of California.
Class II Directors (term to expire at the 2010 annual meeting of shareholders)
John P. Dubinsky, age 63, has served as a director of our company since October 2005. Mr. Dubinsky currently serves as President and Chief Executive Officer of Westmoreland Associates, LLC, a financial consulting firm that he founded in 1999. Prior to that, he served as the Chairman and Chief Executive Officer of Mercantile Bank, the lead bank of Mercantile Bancorporation, a publicly held banking corporation, from 1997 to September 1999, when Mercantile merged with Firstar Bank (now U.S. Bank). Mr. Dubinsky is currently President Emeritus-Midwest of U.S. Bank, one of the largest banks in the Midwestern U.S. He is also the CEO of Cortex, a not-for-profit partnership which is working to develop a biotechnology research district in St. Louis and whose sponsors include three major St. Louis research universities. From 1986 to 1997, Mr. Dubinsky was President and CEO of Mark Twain Bancshares, Inc., a publicly held banking corporation. Mr. Dubinsky currently serves as a director of Insituform Technologies, Inc., a publicly held provider of proprietary technologies and services for rehabilitating underground piping systems, and Stifel Financial Corporation, a publicly held financial services company, where he is a member of the audit and compensation committees. He also serves as director and trustee of various non-profit organizations, including serving as a trustee of Washington University in St. Louis, a director of BJC Health System, and a director of Barnes-Jewish Hospital. Mr. Dubinsky holds a Bachelor’s degree in political science and an MBA from Washington University.
Dr. Francis E. O’Donnell, Jr., M.D., age 57, has served as our Chairman of the Board since the Company’s founding in March 2002 and has served as our Chief Executive Officer since September 2003. Dr. O’Donnell also served as our President from September 2003 through November 2004. Since 1995, Dr. O’Donnell has served as manager of The Hopkins Capital Group, LLC, a biotechnology business development and investment company. Since May 2002, Dr. O’Donnell has also served as the Chairman of the Board of BioDelivery Sciences International, Inc., a publicly traded drug delivery technology company, and since June 2003, he has served as a director (and as Co Vice-Chairman since 2004) of Biovest International, Inc., our majority owned, publicly held subsidiary. He is co-founder and a director of RetinaPharma Technologies, Inc., a privately held biotechnology company developing novel pharmaceuticals and related products for the prevention, treatment, rescue, and recovery of ophthalmic and other neurodegenerative and neurovascular disease. He is the former Professor and Chairman, Department of Ophthalmology, St. Louis University School of Medicine. Dr. O’Donnell has published over 30 peer-reviewed scientific articles and has been awarded 34 U.S. patents. He is the recipient of
6
the 2000 Jules Stein Award from Retinitis Pigmentosa International and is a Trustee for St. Louis University and The Health Careers Foundation. Dr. O’Donnell is a graduate of the Johns Hopkins School of Medicine, where he received his specialty training at the Wilmer Ophthalmological Institute.
Todd D. Thomason, age 39, was elected to the Board of Directors of the Company on October 27, 2006. Since 2004, Mr. Thomason has been a Principal of Cornerstone Investment LLC and Owner of Thomason Developers which focus in real estate investment, development and property management in St. Louis and Kentucky-Barkley Lake. From 1994 to 2004, Mr. Thomason worked in Investment Banking for A.G. Edwards & Sons, Inc. where he was a Managing Director in the Healthcare Group with a focus in public and private capital raising and mergers and acquisitions. Prior to that Mr. Thomason was an Associate in Investment Banking for Chemical Securities, Inc. (now JPMorgan Chase) in the High Yield and Leasing Groups and a Financial Analyst in Investment Banking for Prudential-Bache Capital Funding in the Mergers and Acquisitions Group. Mr. Thomason holds a Bachelor’s degree in Economics and Philosophy from Columbia University and an MBA degree in Accounting and Finance from the University of Chicago.
7
DIRECTORS CONTINUING IN OFFICE
Class III Directors (terms to expire at the 2008 annual meeting of shareholders)
Steven R. Arikian, M.D., age 49, began serving as a director of our company in April 2002. Since November 2004, Dr. Arikian has served as President and Chief Operating Officer of Product Development and Market Services. In February 2005, his title was changed to President and Chief Operating Officer, Biopharmaceutical Products and Services. From January 2003 to November 2004, he was President of Pre-Market Services and Operations and from April 2002 to January 2003, he was President of Pre-Market Services. Since 1997, Dr. Arikian has served as the Chairman, Chief Executive Officer, and founder of our Analytica subsidiary, and September 2004, he has served and the Chairman and Chief Executive Officer of Biovest. Since 2003, Dr. Arikian has served as a director, and since 2004 has served as Chief Executive Officer, President, and Chairman, of Biovest International, Inc., our majority owned, publicly held subsidiary. Dr. Arikian began providing pharmaceutical clients with Clinical and Outcomes Research services in 1988. He served as President of The Center for Health Outcomes and Economics at Bristol Myers Squibb from May 1995 to July 1997, where he supervised a staff of over 50 professionals responsible for development of global health outcomes research. He has designed and implemented research projects in the United States, Canada, Latin America and Europe. Dr. Arikian holds a faculty appointment at the Columbia University Mailman School of Public Health. He has also held faculty appointments at the University of Toronto and the University of Kentucky. He is widely published in the peer-reviewed literature and has been a frequent speaker at industry and trade group sponsored meetings on topics including Formulary Management, Pharmaceutical Pricing, Multi-National Health Economic Studies, and Pharmacoepidemiology. Dr. Arikian is a graduate of Fordham University with a degree in Biology and is also a graduate of the University of Catania (Italy) Medical School.
Class I Directors (terms to expire at the 2009 annual meeting of shareholders)
David M. Schubert, age 40, has served as a director since October 2005. Mr. Schubert is currently the Chief Business Officer of Accelerator Corporation, a venture capital-backed biotechnology investment company located in Seattle, Washington. Prior to joining Accelerator in 2005, Mr. Schubert served as President and founder of Cellexsys, Inc., a privately held biotechnology company that he founded in January 2001 that was acquired by Chromos Molecular Systems in July 2004. Following the sale of Cellexsys, Mr. Schubert has worked as an independent consultant providing advisory services to biotechnology companies. Prior to founding Cellexsys, Mr. Schubert worked for Targeted Genetics Corporation, a publicly held developer of gene-based treatments, as Senior Director, Strategic Initiatives from April 2000 to December 2000 and as Senior Director, Communications and Strategic Relations from November 1997 through March 2000. Mr. Schubert’s prior work experience also includes serving as a Senior Market Manager- Immunotherapy for Baxter Healthcare Corporation. Mr. Schubert is a graduate of Eastern Nazarene College with Bachelor’s degrees in Biology and Psychology, Utah State University with a Master’s degree in Biology, and The Pennsylvania State University with an MBA.
Alan M. Pearce, age 57, has served as a director and our Chief Financial Officer since August 2004. Prior to serving as our Chief Financial Officer, Mr. Pearce served as Senior Vice President, Financial Services for McKesson Corporation, a large publicly traded healthcare company, from April 1999 to March 2004. Mr. Pearce also currently serves on the advisory boards of The Georgia Institute of Technology, or Georgia Tech, the Emory University BioEngineering Foundation, and The Hopkins Capital Group. He also previously served as a director and a member of the finance committee of XL Insurance. From September 2002 to September 2005, Mr. Pearce served as a director of BioDelivery Sciences International, Inc. Mr. Pearce is a graduate of Georgia Tech, where he earned a B.S. degree in Industrial Management, and the University of Texas, where he earned an MBA degree in finance.
8
PROPOSAL 2—RATIFICATION OF INDEPENDENT PUBLIC ACCOUNTANTS
The Board of Directors recommends the ratification of Aidman, Piser & Company as our independent registered public accounting firm for the fiscal year ending September 30, 2007 and recommends that each shareholder to vote “FOR” the ratification. Executed proxies in the accompanying form will be voted at the annual meeting in favor of the ratification of the independent registered public accounting firm, unless authority to do so is withheld.
At the Annual Meeting, the shareholders will be asked to ratify the appointment of Aidman, Piser & Company as our independent registered public accounting firm for the fiscal year ending September 30, 2007. The Audit Committee of our Board of Directors has recommended, and the Board of Directors has already selected, Aidman, Piser & Company as our independent registered public accounting firm for our fiscal year ending September 30, 2007. Aidman, Piser & Company also served as our independent registered public accounting firm during the fiscal year ended September 30, 2006. Unless a shareholder directs otherwise, proxies will be voted for the approval of the selection of Aidman, Piser & Company as our independent registered public accounting firm for the 2007 fiscal year. If the shareholders do not approve the appointment of Aidman, Piser & Company, the Board will consider the selection of other independent public accountants for the 2007 fiscal year, but will not be required to do so.
The affirmative vote of the majority of shares of common stock present or represented and entitled to vote at the Annual Meeting is required to ratify the selection of Aidman, Piser & Company. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the shareholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether this matter has been approved.
In the event the shareholders fail to ratify the appointment, the Board of Directors will reconsider its selection. Even if the selection is ratified, the Board of Directors, in its discretion, may direct the appointment of a different independent registered public accounting firm for such fiscal year if the Board of Directors feels that such a change would be in the best interests of our company and its shareholders.
Representatives of Aidman, Piser & Company will not be present at the Annual Meeting.
9
PROPOSAL 3—APPROVAL OF POTENTIAL ISSUANCE OF COMMON STOCK EXCEEDING 19.99% OF THE COMMON STOCK OUTSTANDING ON SEPTEMBER 29, 2006 UPON CONVERSION OF DEBENTURES AND EXERCISE OF WARRANTS
Introduction
In a September 2006 private placement financing transaction by the Company (the “Financing”), the Company entered into a Securities Purchase Agreement with certain institutional investors under which the Company issued to such investors 8% Secured Convertible Debentures due September 29, 2006 in the aggregate principal amount of $25,000,000 (the “Debentures”) and Common Stock Purchase Warrants to purchase shares of the Company’s Common Stock (the Investor Warrants”). The Company also issued a Common Stock Purchase Warrant to the placement agent for the financing (the “Placement Agent Warrant”). The Debentures are convertible into, and the Investor Warrants and Placement Agent Warrant are exercisable for, 9,615,385 shares, 3,136,201 shares, and 545,455 shares of Common Stock, respectively, subject to certain conversion and exercise restrictions and anti-dilution adjustments. The Financing closed on September 29, 2006.
Use of Proceeds from the Financing
The purpose of the Financing was to raise additional funding for working capital to continue the development of the Company’s proprietary intranasal anti-fungal therapy for chronic sinusitis, to support the commercialization of the Company’s specialty pharmaceutical products, and to repay certain short-term debt.
Terms of the Financing
The following descriptions summarize the material terms of the Financing; however, shareholders are urged to carefully read the forms of the Debentures and Warrants which are attached to the Form 8-K filed by the Company on October 2, 2006.
Debentures and Warrants
In the Financing, the Company issued Debentures in the aggregate principal amount of $25,000,000. The Debentures are convertible at any time at the option of the holder into shares of Common Stock at $2.60 per share, subject to adjustment for stock splits, stock dividends, and the like. In the event that the Company issues or grants in the future any rights to purchase any Common Stock, or other security convertible into Common Stock, for an effective per share price less than the conversion price then in effect, the conversion price of all unconverted Debentures will be decreased to equal such lower price. The Debentures are also exchangeable for shares of common stock of Biovest International, Inc. (“BVTI”) held by the Company at an exchange price of $1.00 per share, subject to adjustment for stock splits, stock dividends, and the like, at any time after the earlier to occur of (i) September 29, 2007 or (ii) such time as the closing price of BVTI’s common stock exceeds $2.25 for each of 20 consecutive trading days, subject to certain volume requirements and other conditions. BVTI is a majority-owned subsidiary of the Company. In the event that BVTI issues or grants in the future any rights to purchase any of BVTI’s common stock, or other security convertible into BVTI’s common stock, for a per share price less than the exchange price then in effect, the exchange price for all unconverted Debentures will be decreased to equal such lower price. The above-described adjustments to the conversion price or exchange price for future stock issuances by the Company or BVTI do not apply to certain exempt issuances, including stock issuances pursuant to employee stock option plans and strategic transactions.
Prior to maturity, the Debentures will bear interest at 8% per annum with interest payable quarterly in arrears in cash, or, at the Company’s option, in shares of Common Stock. The Company’s ability to pay interest with shares of Common Stock will be subject to specified conditions, including the existence of an effective registration statement covering the resale of the shares issued in payment of interest and certain minimum trading volumes in the stock to be issued. Shares delivered in payment of interest will be valued at 90% of the average of
10
the daily volume weighted average price of the shares for the 20 trading days prior to the interest payment date. From and after an event of default under the Debentures and for so long as the event of default is continuing, the Debentures will bear default interest at a rate of 18% per annum.
Beginning October 1, 2007, and on the 1st of each month thereafter, the Company will be required to redeem 1/37th of the face value of the Debentures in cash or, at the Company’s election, with shares of Common Stock, shares of BVTI common stock held by the Company, or a combination thereof. The Company’s ability to pay interest with shares of Company Common Stock or BVTI common stock is subject to specified conditions, including the existence of an effective registration statement covering the resale of the shares issued in payment of the redemption amount and certain minimum trading volumes in the stock to be issued. Any payment in common stock of either the Company or BVTI may not exceed 15% of the total dollar traded volume in the applicable stock for the 20 trading days prior to the amortization payment. Any common stock of the Company or BVTI delivered in satisfaction of an amortization payment will be valued at the lesser of (i) the conversion price or the exchange price, as the case may be, in effect at the time of the amortization payment or (ii) 90% of the average of the daily volume weighted average price of the applicable shares for the 20 trading days prior to the amortization payment. Any unconverted Debentures will become due on September 29, 2010.
In the event that the average of the daily volume weighted average price of the shares of Common Stock for any 20 consecutive trading days exceeds $6.50, the Company will have the right, but not the obligation, to require the holders of the Debentures to convert into Common Stock at the conversion price then in effect up to 50% of any outstanding Debentures (or 100% of any outstanding Debentures, in the event that the average of the daily volume weighted average price of the shares of the Common Stock for any 20 consecutive trading days exceeds 300% of the then-effective conversion price). Such a mandatory conversion is subject to specified conditions, including the existence of an effective registration statement covering the resale of the shares into which the Debentures are converted and certain minimum trading volumes in the stock to be issued.
At any time beginning on the first anniversary of the effectiveness of a registration statement covering the resale of the shares of Common Stock issuable upon conversion of the Debentures, the Company may redeem, subject to specified conditions and upon 20 trading days’ written notice, any or all of the outstanding Debentures for a redemption price of (i) cash of 120% of par plus accrued and unpaid interest on the Debentures to be redeemed and (ii) warrants to subscribe for a number of shares of Common Stock equal to the principal amount of the Debentures to be redeemed, divided by the conversion price then in effect. Such warrants will have an exercise price equal to the average of the daily volume weighted average price for the shares of Common Stock for the 20 trading day period immediately preceding the redemption and a term equal to the weighted average remaining term of the Debentures.
As a part of the Financing, the Company issued Investor Warrants to the purchasers of the Debentures giving them the right to purchase up to an aggregate of 3,136,201 shares of the Company’s common stock at an exercise price of $2.75 per share, provided that such Investor Warrants may be alternatively exercised for shares of BVTI common stock held by the Company at an exercise price of $1.10 per share. The Investor Warrant exercise prices are subject to adjustment for stock splits, stock dividends, and the like. The Investor Warrants may not be exercised for any shares of BVTI common stock until the earlier to occur of (i) September 29, 2007 or (ii) such time as the closing price of BVTI’s common stock exceeds $2.25 for each of 20 consecutive trading days, subject to certain volume requirements and adjustments. In the event that the Company in the future issues or grants any rights to purchase any Common Stock, or other security convertible into Common Stock, for a per share price less than the exercise price then in effect, the exercise price of the Investor Warrant with respect to shares of Common Stock will be reduced to equal such lower price and the number of shares of Common Stock for which the Investor Warrant may be exercised will be increased so that the total aggregate exercise price remains constant. In the event that BVTI in the future issues or grants any rights to purchase any of BVTI’s common stock, or other security convertible into BVTI’s common stock, for a per share price less than the exercise price then in effect, the exercise price of the Investor Warrant with respect to shares of BVTI’s common stock will be reduced to equal such lower price. The foregoing adjustments to the exercise price for both the
11
Common Stock and BVTI’s common stock for future stock issues will not apply to certain exempt issuances, including issuances pursuant to employee stock option plans and strategic transactions. In connection with the Financing, the Company also issued to the placement agent for the transaction a warrant to purchase an aggregate of 545,455 shares of Common Stock at an exercise price of $2.75 per share. All of the Warrants (including the warrants granted to the Placement Agent) will expire on September 29, 2011.
Unless and until shareholder approval of the Financing is obtained by the Company, the aggregate number of shares of Common Stock issuable upon the conversion of any of the Debentures and upon the exercise of any of the Investor Warrants is limited to 19.99% of the number of shares of Common Stock outstanding on the date of the closing of the Financing. In addition, the total number of shares of BVTI common stock held by the Company that may be transferred to the investors in the Financing pursuant to the Debentures or Investor Warrants may not exceed 18,000,000 shares in the aggregate. Pursuant to a Pledge Agreement among the Company and all of the purchasers of the Debentures, the Debentures are also secured by these 18,000,000 shares of BVTI common stock held by the Company.
Registration Rights
In connection with the Financing, the Company and the purchasers of the Debentures entered into a Registration Rights Agreement under which the Company was required, on or before November 1, 2006, to file a registration statement with the SEC covering the resale of the shares of Common Stock issuable pursuant to the Debentures and Investor Warrants and to use its best efforts to have the registration declared effective at the earliest date (but in no event later than 90 days after filing if there is no SEC review of the registration statement, or 120 days if there is an SEC review). This registration statement was filed by the Company on November 1, 2006 and declared effective by the SEC on November 17, 2006. BVTI and the purchasers of the Debentures entered into a similar registration rights agreement under which BVTI was required to file with the SEC and seek to have declared effective a registration statement covering the resale of the shares of BVTI common stock transferable by the Company pursuant to the Debentures and Investor Warrants. This registration statement was filed by BVTI on October 27, 2006 and was declared effective by the SEC on November 17, 2006.
Other Agreements
Shareholders holding more than 50% of the Common Stock have entered into voting agreements agreeing to vote their respective shares in favor of this proposal.
Nasdaq Shareholder Approval Requirements
The Common Stock is traded on the NASDAQ Global Market under the symbol “ABPI.” Consequently, the Company is subject to the Nasdaq Marketplace Rules (the “Marketplace Rules”). Although the issuance of the Debentures and Warrants did not require shareholder approval under Florida law, the Company’s articles of incorporation or bylaws, or the Marketplace Rules, the issuance of shares of Common Stock (upon exercise or conversion of the Debentures and Warrants) exceeding 19.99% of the Company’s Common Stock issued and outstanding on September 29, 2006 does require shareholder approval under Marketplace Rule 4350(i)(1)(D).
Marketplace Rule 4350(i)(1)(D) requires Nasdaq-listed issuers to obtain shareholder approval prior to any issuance or potential issuance of securities representing 20% or more of the outstanding common stock or voting power of the issuer (on an as-converted or as-exercised basis) before such issuance for a price less than the greater of the book or market value of the issuer’s common stock. For purposes of Rule 4350(i)(1)(D), the (i) outstanding common stock or voting power of the issuer is determined as of a date the issuer enters into a binding agreement with respect to such issuance or potential issuance and (ii) market value of the issuer’s common stock is deemed to be the closing bid price of the issuer’s common stock immediately prior to entering into such binding agreement.
12
Marketplace Rule 4350(i)(1)(D) is applicable to the Financing because, in the Financing, the Company became bound to issue the Debentures and Warrants on the date of closing. The closing bid price per share of the Common Stock immediately prior to the closing was $2.37, which price is greater than the book value of the Common Stock. Although the conversion price of the Debentures and the exercise price of the Investor Warrants and Placement Agent Warrants are initially above the market value of the Common Stock on the date of issuance, both the conversion price of the Debentures and the exercise price of the Investor Warrants and Placement Agent Warrants are subject to anti-dilution adjustment provisions, as described above, that could reduce the effective conversion price or exercise price to less than the market value of the underlying Common Stock on the date of issuance. Moreover, the Company could also be deemed to issue its Common Stock at less than market value in violation of Marketplace Rule 4350(i)(1)(D) if the Company issues Common Stock in the future in lieu of cash dividends or in redemption of principal under the Debentures as permitted by the Debentures. Furthermore, the aggregate number of shares of Common Stock potentially issuable upon conversion of the Debentures and exercise of the Warrants (without regard to any restrictions on such conversion or exercise), 13,297,041, exceeds 20% of the 31,716,279 shares of Common Stock outstanding on September 29, 2006, the date of the closing. In the Financing, the Company could potentially issue shares of Common Stock representing greater than 20% of the Company’s outstanding Common Stock and voting power for a price less than the greater of book or market value of the Common Stock. Accordingly, in order to comply with Marketplace Rule 4350(i)(1)(D), the Company must obtain shareholder approval before issuing shares of Common Stock upon conversion of the Debentures or exercise of the Warrants in excess of 19.99% of the Common Stock issued and outstanding on September 29, 2006.
To comply with Marketplace Rule 4350(i)(1)(D), we agreed in the Securities Purchase Agreement that we would hold a meeting of shareholders on or before February 28, 2007 for the purpose of obtaining shareholder approval authorizing the issuance of shares in excess of 19.99% of the Common Stock issued and outstanding on September 29, 2006. In addition, the Debentures provide that if the Company has not obtained shareholder approval authorizing the issuance of shares in excess of 19.99% of the Common Stock issued and outstanding on September 29, 2006, then “the Company may not issue, upon conversion of this Debenture, a number of shares of Common Stock which, when aggregated with any shares of Common Stock issued on or after the Original Issue Date and prior to such Conversion Date (A) in connection with any Debentures issued pursuant to the [Securities] Purchase Agreement, (B) in connection with any Warrants issued pursuant to the [Securities] Purchase Agreement and (C) in connection with any warrants issued to any registered broker-dealer as a fee in connection with the issuance of the Securities pursuant to the Securities Purchase Agreement, would exceed 6,340,084 shares of Common Stock (subject to adjustment for forward and reverse stock splits, recapitalizations and the like). . . .”
Effect of Shareholder Approval of Proposal 3
If the shareholders approve Proposal 3, then (i) the Company shall have obtained shareholder approval in satisfaction of Marketplace Rule 4350(i)(1)(D); (ii) the Company’s shareholders shall have authorized the issuance upon conversion of the Debentures and exercise of the Warrants of shares of Common Stock in excess of 19.99% of the shares of Common Stock issued and outstanding as of September 29, 2006; and (iii) the Company will be permitted to issue such shares of Common Stock upon conversion of the Debentures and exercise of the Warrants.
Conversion of the Debentures benefits the Company and its shareholders because, to the extent the outstanding principal amount of the Debentures is converted into shares of Common Stock, the Company is no longer obligated to pay such principal or interest otherwise due thereon, and the Company’s outstanding debt and interest expense will be reduced. Exercise of the Warrants benefits the Company and its shareholders because, upon such exercise, the Company will receive the exercise price per share of Common Stock issued. If the Warrants are exercised in full at the initial exercise price, then the exercise proceeds to the Company would be approximately $10.1 million. Even if the shareholders approve Proposal 3, the decision to convert the Debentures or to exercise the Warrants will remain with the holders thereof, and such holders may determine not to convert the Debentures or exercise the Warrants for any reason.
13
The 13,297,041 shares of Common Stock issuable upon conversion of the Debentures and exercise of the Warrants (without regard to additional shares which may become issuable due to anti-dilution adjustments) represent approximately 41.4% of the shares of Common Stock outstanding as of the Record Date and, assuming such shares of Common Stock are issued, represent a significant dilution of the voting interests of existing shareholders. The issuance of shares of Common Stock pursuant to the Debentures and the Warrants will also have a dilutive effect on earnings per share and may adversely affect the market price of the Common Stock.
The issuance of shares of Common Stock in connection with the Financing could have an anti-takeover effect because such issuance will make it more difficult for, or discourage an attempt by, a party to obtain control of the Company by tender offer or other means. The issuance of Common Stock upon conversion of the Debentures or exercise of the Warrants will increase the number of shares entitled to vote, increase the number of votes required to approve a change of control of the Company, and dilute the interest of a party attempting to obtain control of the Company. Proposal 3 is not part of a plan by the Board to adopt a series of anti-takeover measures. The Board does not have any knowledge of any effort by any person to accumulate the Company’s securities or obtain control of the Company by any means.
Effect of Failure to Obtain Shareholder Approval of Proposal 3
If the shareholders do not approve Proposal 3, then (i) the Debentures may not be converted into, and the Warrants may not be exercised for, shares of Common Stock in excess of 19.99% of the Common Stock issued and outstanding as of September 29, 2006, (ii) the Company will not enjoy the benefit of debt or interest expense reduction associated with the complete conversion of the Debentures, and (iii) the Company will not receive the exercise proceeds associated with the complete exercise of the Warrants. Furthermore, if the shareholders do not approve Proposal 3 at the Annual Meeting, then the Board must continue to seek shareholder approval thereof at the Company’s expense.
Recommendation of the Board of Directors
The Board unanimously recommends that shareholders vote FOR Proposal 3. Proxies solicited by the Board will be voted FOR Proposal 3 unless instructions to the contrary are given.
14
DIRECTORS AND EXECUTIVE OFFICERS
Board Meetings and Independence
During the fiscal year ended September 30, 2006 (“Fiscal 2006”), the Board of Directors of the Company held nine meetings, consisting of three in-person meetings and six telephonic meetings. In addition, the Board of Directors took action by written consent on eight occasions. Each director attended at least 75% of the aggregate of (1) the total number of meetings of the Board (held during the period for which he has been a director) and (2) the total number of meetings held by all committees of the Board on which he served (during the periods that he served). It is the Company’s current policy to strongly encourage directors to attend the Annual Meeting, but they are not required to attend. Two of the Board members attended the 2006 Annual Meeting.
Our Board of Directors presently has seven members, and biographical information regarding these directors (four of whom are director nominees) is set forth above under the caption “PROPOSAL 1—ELECTION OF DIRECTORS.” The Board has determined that four of its members are “independent directors” as defined under the rules of the Nasdaq Stock Market, Inc. and Rule 10A-3(b)(i) under the Securities Exchange Act of 1934. These four “independent directors” are David M. Schubert, John P. Dubinsky, Edmund C. King, and Todd D. Thomason.
Board Committees
The Board of Directors has established three standing committees: an Audit Committee, a Compensation Committee, and a Governance and Nominating Committee. Messrs. Schubert, Dubinsky, and King are members of each of these committees, and Mr. Thomason is a member of the Audit Committee. The following is a summary description of the respective responsibilities of the Board’s standing committees:
Audit Committee. The Audit Committee is responsible for overseeing the Company’s corporate accounting, financial reporting practices, audits of financial statements, and the quality and integrity of the Company’s financial statements and reports. In addition, the Audit Committee oversees the qualifications, independence and performance of the Company’s independent registered public accounting firm. Each member of the audit committee is able to read and understand fundamental financial statements, including the Company’s balance sheets, income statements, and cash flows statements. The Audit Committee held ten meetings during Fiscal 2006. Mr. Dubinsky serves as the chairman of the Audit Committee.
The Audit Committee performs the following functions, among others:
| | • | | appointing and replacing our independent accountants; |
| | • | | reviewing the results and scope of the independent accountants’ audit and the services provided by the independent accountants; |
| | • | | reviewing compliance with legal and regulatory requirements; |
| | • | | evaluating our audit and internal control functions; and |
| | • | | ensuring the integrity of our financial statements. |
The Board of Directors has determined that Mr. Dubinsky is an “audit committee financial expert” as that term is defined in Securities and Exchange Commission regulations. The Board of Directors has approved and adopted a written charter for the audit committee. A copy of this charter is posted on the Company’s website atwww.accentia.net in the “Investor Relations” section of the website.
Compensation Committee. The Compensation Committee is charged with overseeing the Company’s compensation policies, plans and programs. Mr. Schubert is the chairman of the Compensation Committee, although Steven Stogel, a former director of the Company, served as Chairman during Fiscal 2006. The
15
Compensation Committee held four meetings during Fiscal 2006. The Compensation Committee performs the following functions, among others, as set forth in its committee charter:
| | • | | recommending and approving salaries, incentive compensation, and equity-based plans for our executive officers and managers; |
| | • | | reviewing corporate goals and objectives relative to executive compensation; |
| | • | | evaluating our chief executive officer’s performance in light of corporate objectives; |
| | • | | setting our chief executive officer’s compensation based on the achievement of corporate objectives; |
| | • | | developing plans for chief executive officer succession; and |
| | • | | preparing and issuing reports required under the committee charter. |
A copy of the Compensation Committee’s charter is posted on the Company’s website atwww.accentia.net in the “Investor Relations” section of the website.
Governance and Nominating Committee. The Governance and Nominating Committee’s role is to oversee all aspects of the Company’s corporate governance functions on behalf of the Board and to evaluate candidates to serve as directors of the Company. Mr. Dubinsky is the chairman of the Governance and Nominating Committee. The Governance and Nominating Committee held four meetings during Fiscal 2006. The Governance and Nominating Committee performs the following functions, among others, as set forth in its committee charter:
| | • | | developing criteria for director selection; |
| | • | | identifying and recommending to the full Board of Directors the director-nominees to stand for election at annual meetings of the shareholders; |
| | • | | recommending members of the Board of Directors to serve on the various committees of the Board of Directors; |
| | • | | evaluating and ensuring the independence of each member of each committee of the Board of Directors; |
| | • | | recommending to the Board of Directors our corporate governance principles; and |
| | • | | recommending to the Board of Directors a code of conduct for the Company’s directors, officers and employees. |
Although the committee has not formulated any specific minimum qualifications that the committee believes must be met by a director-nominee that the committee recommends to the Board, the factors it will take into account will, as set forth in the committee charter, include judgment, skill, diversity, experiences with businesses and other organizations of comparable size, the interplay of the candidate’s experience with the experience of other directors, and the extent to which the candidate would be a desirable addition to the Board and any committees of the Board.
The Governance and Nominating Committee will consider recommendations for directorships submitted by shareholders. Any such director nominee recommendations should be sent together with appropriate biographical information concerning each proposed nominee. Recommendations by shareholders that are made in accordance with the procedures described in “Procedure for Submitting Shareholder Proposals” at the end of this document will receive the same consideration given to nominees of the Governance and Nominating Committee. A copy of the Governance and Nominating Committee’s charter is posted on the Company’s website atwww.accentia.net in the “Investor Relations” section of the website.
Director Compensation
Each non-employee director is entitled to receive an annual fee in the amount of $18,000 for each full year of service on our Board of Directors. In addition, such directors are entitled to receive automatic annual stock
16
option grants under our 2005 Equity Incentive Plan. Under the plan, each non-employee director will receive, on the day following the annual meeting of shareholders each year, a nonqualified stock option to purchase 20,000 shares of our common stock, as well as 5,000 additional shares for each committee on which the director serves on the grant date and 5,000 additional shares for each committee chair that the director holds on the grant date. Each non-employee director that first joined the Board in connection with our initial public offering in October 2005 received his first option grant on the date on which he first became a director, and such director’s second grant will not be made until the day following this 2007 Annual Meeting. Options granted to our non-employee directors under the 2005 Equity Incentive Plan have an exercise price equal to the fair market value of a share of our common stock on the option grant date, and the options will vest in three equal annual installments beginning on the first anniversary of the grant date.
Options to purchase 120,000 shares of our common stock were granted to non-employee directors under the 2005 Equity Incentive Plan during Fiscal 2006 at an exercise price of $8.00 per share. Subsequent to Fiscal 2006, in October 2006 a total of 35,000 options were granted to Edmund C. King and a total of 30,000 options were granted to Todd D. Thomason upon the commencement of their service as directors, and the exercise price of such options was $3.17 per share.
Members of the Board of Directors who are also officers or employees of the Company receive no separate compensation for serving as directors.
17
Report of Audit Committee
The Audit Committee is composed of four independent directors and operates under a written charter adopted by the Board of Directors. A copy of the Audit Committee Charter is posted on the Company’s website atwww.accentia.net in the “Investor Relations” section of the website. As described above, the Audit Committee is responsible for appointing and replacing our independent accountants; reviewing the results and scope of the independent accountants’ audit and the services provided by the independent accountants; reviewing compliance with legal and regulatory requirements; evaluating our audit and internal control functions; and ensuring the integrity of our financial statements. In fulfilling its oversight responsibilities, the Audit Committee reviewed the audited financial statements in the Annual Report with management, including a discussion of the quality, not just the acceptability, of the accounting principles, the reasonableness of significant judgments, and the clarity of disclosures in the financial statements.
The Audit Committee reviewed with the independent auditors, who are responsible for expressing an opinion on the conformity of those audited financial statements with generally accepted accounting principles, their judgments as to the quality, not just the acceptability, of our accounting principles, and such other matters as are required to be discussed with the Committee under generally accepted auditing standards. In addition, the Audit Committee has discussed with the independent auditors the auditors’ independence from management and the Company, including the matters in the written disclosures required by the Independence Standards Board and considered compatibility of nonaudit services with the auditors’ independence.
The Audit Committee discussed with our internal and independent auditors the overall scope and plans for their respective audits. The Committee meets with the internal and independent auditors, with and without management present, to discuss the results of their examinations, their evaluations of our internal controls, and the overall quality of our financial reporting. The Audit Committee held ten meetings during Fiscal 2006.
In reliance on the reviews and discussions referred to above, the Audit Committee recommended to our Board of Directors, and the Board approved, that the audited financial statements be included in our Annual Report on Form 10-K for the year ended September 30, 2006 for filing with the Securities and Exchange Commission. The Audit Committee and the Board of Directors have also recommended, subject to shareholder ratification, the selection of our independent auditors.
|
| THE AUDIT COMMITTEE |
|
|
| John P. Dubinsky |
| David M. Schubert |
| Edmund C. King |
| Todd D. Thomason |
January 9, 2007
18
Code of Business Conduct and Ethics
Our Board of Directors has adopted a Code of Business Conduct and Ethics that is applicable to all of the employees and directors of the Company and its subsidiaries. The text of the Code of Business Conduct and Ethics is posted on the website atwww.accentia.net in the “Investor Relations section of our website.
Communications with the Board of Directors
Shareholders may communicate with the full Board of Directors or individual directors by submitting such communications in writing to Accentia Biopharmaceuticals, Inc., Attention: Board of Directors (or the individual director(s)), 324 South Hyde Park Avenue, Suite 350, Tampa, Florida 33606. Such communications will be delivered directly to the directors.
Executive Officers
Set forth below is a table identifying our executive officers:
| | |
NAME | | POSITION |
Francis E. O’Donnell, Jr., M.D | | Chairman of the Board of Directors, Chief Executive Officer |
| |
Steven R. Arikian, M.D | | President and Chief Operating Officer, Biopharmaceutical Products and Services |
| |
Alan M. Pearce | | Chief Financial Officer |
| |
Samuel S. Duffey, Esq | | General Counsel |
Biographical information for each of these executive officers is set forth above under the caption “PROPOSAL 1—ELECTION OF DIRECTORS,” except for Samuel S. Duffey, whose biographical information and age (as of the record date) is as follows:
Samuel S. Duffey, age 61, has served as a director and our General Counsel since April 2003. Prior to that, Mr. Duffey practiced business law with Duffey and Dolan P.A. beginning in 1992. From February 2000 to September 2003, Mr. Duffey served as the non-executive chairman and as a member of the Board of Directors of Invisia, Inc., a small publicly held safety company, and from October 2001 to May 2004, Mr. Duffey also served as the non-executive chairman and as a member of the Board of Directors of FlashPoint International, Inc., a publicly held automotive parts company which is currently named Navitrak International Corporation. Mr. Duffey received his B.A. and J.D. degrees from Drake University.
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Under Section 16(a) of the Securities Exchange Act of 1934, as amended, an officer, director, or greater-than-10% shareholder of the Company must file a Form 4 reporting the acquisition or disposition of Company’s equity securities with the Securities and Exchange Commission no later than the end of the second business day after the day the transaction occurred unless certain exceptions apply. Transactions not reported on Form 4 must be reported on Form 5 within 45 days after the end of the Company’s fiscal year. Such persons must also file initial reports of ownership on Form 3 upon becoming an officer, director, or greater-than-10% shareholder. To the Company’s knowledge, based solely on a review of the copies of these reports furnished to it and written representations that no other reports were required, the officers, directors, and greater than 10% beneficial owners complied with all applicable Section 16(a) filing requirements during its fiscal year ended September 30, 2006, except that (1) each of Francis E. O’Donnell, Dennis Ryll, MOAB Investment, LP, and Timothy Ryll filed a late Form 4 on May 18, 2006 for a transaction that occurred on May 15, 2006; (2) each of John Dubinsky, Steven Stogel, Hopkins Capital Group, LLC, Dennis Ryll, Timothy Ryll, and Francis E. O’Donnell filed a late Form 4 on June 23, 2006 for a transaction that occurred on April 3, 2006 (MOAB Investments, LP filed a late Form 5 on January 26, 2007 to report this same transaction); (3) Alan Pearce and Samuel Duffey filed a late Form 4 and Form 5 on January 26, 2007 for option grants that occurred in February and December 2006; and (4) MOAB Investment, LP, Timothy Ryll, and Dennis Ryll filed a late Form 5 on January 26, 2007 to report gifts by MOAB Investment, LP that occurred during the 2006 fiscal year, and Steven Stogel filed a late Form 5 for a gift made by him in June 2006.
19
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the beneficial ownership of our common stock as of December 31, 2006 with respect to, (i) each of the Company’s directors and director nominees, (ii) each of the Company’s executive officers named in the Summary Compensation Table below, (iii) all directors and executive officers of the Company as a group, and (iv) each person known by the Company to own beneficially more than 5% of the Common Stock. The number and percentage of shares beneficially owned is determined under rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has sole or shared voting power or investment power and also any shares which the individual has the right to acquire beneficial ownership of within 60 days of December 31, 2006 through the exercise of any stock option or other right. Unless otherwise indicated in the footnotes, each person has sole voting and investment power with respect to the shares shown as beneficially owned. A total of 31,733,678 shares of the Company’s common stock were issued and outstanding as of December 31, 2006.
| | | | | |
Beneficial Owner | | Number of Shares of Common Stock
Beneficially Owned | | Percent | |
5% Stockholders | | | | | |
The Hopkins Capital Group, LLC(1) 709 The Hamptons Lane St. Louis, MO 63017 | | 4,385,992 | | 13.8 | % |
Timothy D. Ryll(2) 3608 N. Magnolia, #1N Chicago, IL 60613 | | 4,163,926 | | 13.1 | |
Pharmaceutical Product Development, Inc.(3) 3151 South 17th Street Wilmington, NC 28412 | | 4,270,323 | | 13.5 | |
Ronald E. Osman(4) 6530 Moake School Road Marion, IL 62959 | | 2,135,160 | | 6.7 | |
| | |
Named Executive Officers and Directors | | | | | |
Francis E. O’Donnell, Jr., M.D.(5) | | 4,801,136 | | 15.1 | |
Steven R. Arikian, M.D.(6) | | 1,045,556 | | 3.3 | |
Martin G. Baum(7) | | 883,490 | | 2.8 | |
Samuel S. Duffey, Esq.(8) | | 304,046 | | * | |
Alan M. Pearce(9) | | 889,498 | | 2.8 | % |
David M. Schubert(10) | | 11,666 | | * | |
John P. Dubinsky(11) | | 158,394 | | * | |
Todd D. Thomason(12) | | 10,000 | | * | |
Edmund King(13) | | 11,666 | | * | |
Executive Officers and Directors as Group
(9 persons) | | 8,115,452 | | 25.6 | % |
| (1) | Voting and investment power over the shares held by The Hopkins Capital Group, LLC (“Hopkins”) is exercised by its manager, Dr. Francis E. O’Donnell, Jr., our Chairman and Chief Executive Officer. |
| | (a) | 3,617,660 shares of common stock held by MOAB Investments, LP (“MOAB”) and 428,573 shares of our common stock held by MOAB-II Investments, LP (“MOAB-II” and, together with MOAB, the “MOAB Entities”) and |
| | (b) | 117,693 shares of common stock held by Timothy D. Ryll, as the Trustee of three different trusts (the “Timothy Ryll Trusts”). |
20
Mr. Timothy Ryll is the sole shareholder and sole director of MOAB Management Company, Inc., which is the sole general partner of each of the MOAB Entities. Mr. Timothy Ryll is the trustee of the Timothy Ryll Trusts. Mr. Timothy Ryll is the son of Dr. Dennis Ryll, one of our former directors. Dr. Dennis Ryll, a limited partner in each of the MOAB Entities, exercises no voting or investment power over any of our shares held by the MOAB Entities or the Timothy Ryll Trusts. Mr. Timothy Ryll exercises voting and investment power over the MOAB Entities and over the Timothy Ryll Trusts.
| (3) | These shares are held by Pharmaceutical Product Development International Holdings, Inc., or PPD International, a selling shareholder and a wholly owned subsidiary of Pharmaceutical Product Development, Inc. (“PPD”), a publicly held corporation. PPD exercises voting and investment control over PPD International. |
| | (a) | 405,681 shares of common stock held by MRB&B, LLC and |
| | (b) | 21,351 shares of common stock held by the Ronald E. Osman & Associates, Ltd. 401(k) Profit Sharing Plan. |
Mr. Osman is the manager of MRB&B, LLC and the trustee of the Ronald E. Osman & Associates, Ltd. 401(k) Profit Sharing Plan, exercising voting and investment power over both entities.
| | (a) | 4,385,992 shares of common stock held by Hopkins and 412,892 shares of common stock held by The Hopkins Capital Group II, LLC (“Hopkins II”) and |
| | (b) | 2,252 shares of common stock issuable pursuant to options held by Dr. O’Donnell that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
Dr. O’Donnell holds voting and investment power over shares held by each of Hopkins and Hopkins II as its manager.
| (6) | Includes 95,012 shares of common stock issuable pursuant to options that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
| | (a) | 336,804 shares of common stock issuable pursuant to options held by Mr. Baum that are currently exercisable or that are exercisable within 60 days of December 31, 2006; |
| | (b) | 91,863 shares of common stock held by the Martin and Doreen Baum Irrevocable Trust; |
| | (c) | 123,836 shares of common stock held by Mrs. Doreen Baum; and |
| | (d) | 1,588 shares of common stock issuable pursuant to options held by Mrs. Doreen Baum that are currently exercisable or that are exercisable within 60 days of December 31, 2006. Mrs. Doreen Baum is the wife of Mr. Baum. |
Mr. Baum has shared voting and investment power over the shares held by the Martin and Doreen Baum Irrevocable Trust. Mr. Baum ceased to be a director and executive officer of our company in October 2006.
| (8) | Consists of 304,046 shares of common stock issuable pursuant to options that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
| | (a) | 95,003 shares of common stock held by The Pearce Family Limited Partnership; |
| | (b) | 145,727 shares of common stock issuable pursuant to options held by Mr. Pearce that are currently exercisable or that are exercisable within 60 days of December 31, 2006; and |
| | (c) | 380,011 shares of common stock held jointly by Mr. Pearce and his wife. |
As a general partner of The Pearce Family Limited Partnership, Mr. Pearce exercises voting and investment power over The Pearce Family Limited Partnership.
| (10) | Consists of 11,666 shares of common stock issuable pursuant to options held by Mr. Schubert that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
| (11) | Includes 15,000 shares of common stock issuable pursuant to options held by Mr. Dubinsky that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
| (12) | Consists of 10,000 shares of common stock issuable pursuant to options held by Mr. Thomason that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
| (13) | Consists of 11,666 shares of common stock issuable pursuant to options held by Mr. King that are currently exercisable or that are exercisable within 60 days of December 31, 2006. |
21
COMPENSATION OF EXECUTIVE OFFICERS
The following summary compensation table sets forth information concerning compensation for services rendered to us in all capacities by our Chief Executive Officer and all other executive officers of our company whose salary and bonus exceeded $100,000, otherwise referred to as our named executive officers, for the year ended September 30, 2006.
Summary Compensation Table
| | | | | | | | | | |
| | | Annual Compensation | | Long-Term Compensation |
Name and Principal Position | | Year | | Salary | | Bonus | | Securities Underlying Options |
Francis E. O’Donnell, Jr., M.D. Chairman and Chief Executive Officer | | 2006
2005
2004 | | $
$
$ | —
—
— | | $
| —
—
— | | 60,000
20,000
20,000 |
| | | | |
Steven R. Arikian, M.D. President and Chief Operating Officer,
Biopharmaceutical Products and Services | | 2006
2005
2004 | |
| 448,166
416,041
361,262 | |
| —
52,571
85,250 | | 575,000
225,000
520,012 |
| | | | |
Martin G. Baum* President and Chief Operating Officer,
Specialty Pharmaceuticals | | 2006
2005
2004 | |
| 448,166
420,962
404,622 | |
| —
—
155,925 | | —
—
116,974 |
| | | | |
Samuel S. Duffey, Esq. General Counsel and Secretary | | 2006
2005
2004 | |
| 275,000
271,519
161,961 | |
| 30,000
—
— | | 541,168
—
618,765 |
| | | | |
Alan M. Pearce Chief Financial Officer | | 2006
2005
2004 | |
| 250,000
177,885
— | |
| —
—
— | | 37,425
—
— |
| * | Mr. Baum ceased to be a director and executive officer of the Company as of October 2006. |
22
Options Granted In the Last Fiscal Year
The following table sets forth information with respect to grants of stock options during Fiscal 2006 to our named executive officers. Potential realizable value represents hypothetical gains that could be achieved for the options if exercised at the end of the option term. The assumed 5% and 10% rates of stock price appreciation are provided in accordance with the rules of the SEC and do not represent our estimate or projection of our future common stock price.
| | | | | | | | | | | | | | | | | | | | |
| | | Individual Grants | | Potential Realizable Value At Assumed Annual Rates of Stock Prices Appreciation for option Term |
Name | | Options
Granted | | | Percent of Total Options/SARs
Granted to
Employees in Fiscal Year | | | Exercise
or Base Price ($/Share) | | Market Price
of Underlying Security on Date of Grant | | Expiration
Date | |
| | | | | | | 5% | | 10% |
Francis E. O’Donnell, Jr. M.D. | | 60,000 | (1) | | 2.4 | % | | $ | 1.13 | | $ | 1.03 | | 10/18/2016 | | $ | 32,866 | | $ | 92,493 |
Steven R. Arikian, M.D. | | 500,000 | (1) | | 19.6 | | | | 0.65 | | | 0.65 | | 2/10/2016 | | | 204,390 | | | 517,966 |
Samual S. Duffey, Esq. | | 500,000 | (1) | | 19.6 | | | | 0.65 | | | 0.65 | | 2/10/2016 | | | 204,390 | | | 517,966 |
| | 41,168 | | | 8.0 | | | | 7.59 | | | 6.90 | | 2/9/2016 | | | 150,237 | | | 424,311 |
Alan Pearce | | 37,425 | | | 7.3 | | | | 7.59 | | | 6.90 | | 2/9/2016 | | | 136,578 | | | 385,733 |
| (1) | Represents options to purchase shares of common stock of our Biovest subsidiary. Percentage based on an aggregate 2,550,341 shares of Biovest common stock subject to options granted by Biovest to its employees in the year ended September 30, 2006. |
Aggregate Option Exercises in Last Fiscal Year and Year-End Values
The following table sets forth information with respect to the aggregate stock option exercises by our named executive officers during Fiscal 2006 and the year-end value of unexercised options held by such executive officers at year end. With respect to such options, the values below are based on a value of $2.58 per share, which was the last reported sale price for our common stock on September 29, 2006. The following table also includes options granted to purchase shares of common stock of our majority-owned Biovest subsidiary, and with respect to such options, the values below are based on a value of $1.05 per share, which was the last reported sale price for such stock on the OTC Bulletin Board on September 29, 2006.
| | | | | | | | |
Name | | Shares
Acquired on
Exercise | | Value
Realized | | Number of Securities Underlying Unexercised Options/SARs at FY End (#) Exercisable/ Unexercisable | | Value of Unexercised In-the-money Options/SARs as FY End ($) Exercisable/
Unexercisable |
Francis E. O’Donnell, Jr. M.D. | | — | | — | | 42,252/— | | 22,822/— |
Steven R. Arikian, M.D. | | — | | — | | 819,979/425,033 | | 450,724/226,432 |
Martin G. Baum | | — | | — | | 373,490/3,314 | | 407,001/1,558 |
Samuel S. Duffey, Esq. | | — | | — | | 711,679/448,254 | | 374,704/231,115 |
Alan Pearce | | — | | — | | 12,478/24,947 | | —/— |
23
Employment Agreements with Executives
Francis E. O’Donnell, Jr., M.D.On January 1, 2005, we entered into an employment agreement with Dr. Francis E. O’Donnell, Jr., our Chairman, President and Chief Executive Officer. This agreement has an initial term of five years, and continues thereafter on an “at-will” basis, terminable during the “at-will” period by either party for any reason and at any time upon 30 days’ notice. Under the terms of the agreement, Dr. O’Donnell is entitled to a base salary of $1 per year. The agreement provides that, if we terminate Dr. O’Donnell’s employment without cause, because of disability, or for cause (except for dishonesty, misconduct, or unlawful acts that adversely affect us or pleading guilty or no contest to, or a conviction of, a felony or any crime involving moral turpitude, fraud, dishonesty, or misrepresentation), or he terminates his own employment for good reason, then he will be entitled to severance compensation in the amount of his base salary, health and welfare benefits, and all his options shall continue to vest for the 12-month period following the date of termination. Dr. O’Donnell will be deemed to have terminated his employment for good reason if he terminates because of a material breach of the agreement by us that is not cured within 30 days of written notice of the breach, the assignment by us without his consent to a position, responsibilities or duties of a materially lesser status or degree of responsibility, the relocation of our principal executive offices outside of Tampa, Florida, or we require him to be based anywhere other than our principal executive offices. The agreement provides that during the time of his employment and ending two years from the termination of the agreement, he may not solicit customers and will not engage in or own any business that is competitive with us.
Alan M. Pearce.On January 1, 2005, we entered into an employment agreement with Alan M. Pearce, our Chief Financial Officer. This agreement has an initial term of five years, and continues thereafter on an “at-will” basis, terminable during the “at-will” period by either party for any reason and at any time upon 30 days’ notice. Under the terms of the agreement, Mr. Pearce is entitled to an initial base salary of $250,000 per year, provided that our Compensation Committee is required to increase this salary following this offering to cause the salary to be commensurate with compensation paid to similarly-experienced chief financial officers in comparable companies. The agreement provides that Mr. Pearce is eligible to receive an annual performance bonus with a target of 50% of his annual base salary and a separate special bonus in connection with our initial public offering in the amount to be determined by the Compensation Committee. The agreement provides that, if we terminate Mr. Pearce’s employment without cause, because of disability, or for cause (except for dishonesty, misconduct, or unlawful acts that adversely affect us or pleading guilty or no contest to, or a conviction of, a felony or any crime involving moral turpitude, fraud, dishonesty, or misrepresentation), or Mr. Pearce terminates his own employment for good reason, then he will be entitled to severance compensation in the amount of his base salary, health and welfare benefits, and all his options shall continue to vest for the 12-month period following the date of termination. Mr. Pearce will be deemed to have terminated his employment for good reason if he terminates because of a material breach of the agreement by us that is not cured within 30 days of written notice of the breach, the assignment by us without his consent to a position, responsibilities or duties of a materially lesser status or degree of responsibility, the relocation of our principal executive offices outside of Tampa, Florida, or we require him to be based anywhere other than our principal executive offices. The agreement provides that during the time of his employment and ending two years from the termination of the agreement, he may not solicit customers and will not engage in or own any business that is competitive with us.
Steven R. Arikian, M.D.On October 19, 2004, we entered into an amended employment agreement with Dr. Steven R. Arikian, our President and Chief Operating Officer, Biopharmaceutical Products and Services. This agreement was further amended on February 10, 2005. The amended agreement expires in October 2009. Under the terms of the agreement, Dr. Arikian is entitled to a base salary of $426,825 per year, subject to a minimum 10% increase each year, and the agreement specifies that his salary shall not be less than the salary of our President and Chief Operating Officer, Specialty Pharmaceuticals. The agreement provides that, if we terminate Dr. Arikian’s employment without cause or if he terminates his own employment for good reason, then he will be entitled to severance compensation in the amount of his base salary and eligible for health insurance benefits for the two-year period following the date of termination. Dr. Arikian will be deemed to have terminated his employment for good reason if he terminates because of a reduction in base salary, a demotion or change in
24
duties or responsibilities, a breach of his employment agreement by the company that is not cured within 10 days of written notice, or a disposition of substantially all of the business or assets of our Analytica subsidiary. If we terminate Dr. Arikian’s employment for cause or he terminates his own employment without good reason, he will be entitled to receive his base salary for 6 months after termination. If we create an executive operating position senior to, or on par with, Dr. Arikian’s position (other than President and Chief Operating Officer, Specialty Pharmaceuticals), and if Dr. Arikian terminates his employment within 30 days of the creation of such position, then Dr. Arikian will be entitled to severance of 15 months base salary paid in 15 equal monthly installments. The agreement provides that, during Dr. Arikian’s employment, he may not solicit our customers and will not engage in or own any business that is competitive with the business of our Analytica subsidiary.
In connection with his employment, Dr. Arikian was granted on November 7, 2003 options to purchase up to 95,003 shares of our common stock at an exercise price of $2.11 per share. One-third of these options vest 12 months after the grant, with the remainder vesting daily over the following 24 months, and the options expire on November 7, 2013. Under the terms of the option agreement, in the event of a transfer of control (as defined in the option agreement) of the company, any unexercisable portion of the option will immediately vest as of a date prior to the transfer of control, which date will be determined by our Board of Directors in its sole discretion.
Samuel S. Duffey, Esq.On January 1, 2005, we entered into an employment agreement with Samuel S. Duffey, Esq., our General Counsel. This agreement extends for an initial term of five years, and continues thereafter on an “at-will” basis, terminable during the “at-will” period by either party for any reason and at any time upon 30 days’ notice. Under the terms of the agreement, Mr. Duffey is entitled to a base salary of $275,000 per year, and the agreement provides that Mr. Duffey is eligible to receive an annual performance bonus with a target of 50% of his annual base salary and a separate special bonus in connection with our initial public offering. The agreement provides that, if we terminate Mr. Duffey’s employment without cause, because of disability, or for cause (except for dishonesty, misconduct, or unlawful acts that adversely affect us or pleading guilty or no contest to, or a conviction of, a felony or any crime involving moral turpitude, fraud, dishonesty, or misrepresentation), or if Mr. Duffey terminates his own employment for good reason, then he will be entitled to severance compensation in the amount of his base salary, health and welfare benefits for 12 months after the termination, and all his options shall continue to vest for the 12-month period following the date of termination. Mr. Duffey will be deemed to have terminated his employment for good reason if he terminates because of a material breach of the agreement by us that is not cured within 30 days of written notice of the breach, the assignment by us without his consent to a position, responsibilities or duties of a materially lesser status or degree of responsibility, the relocation of our principal executive offices outside of Tampa, Florida, or we require him to be based anywhere other than our principal executive offices. The agreement provides that during the time of his employment and ending two years from the termination of the agreement, he may not solicit customers and will not engage in or own any business that is competitive with us.
In connection with his employment, Mr. Duffey was granted on November 7, 2003 options to purchase up to 118,754 shares of our common stock at an exercise price of $2.11 per share. One-third of these options vest 12 months after the grant, with the remainder vesting daily over the following 24 months, and the options expire on November 7, 2013. Under the terms of the option agreement, in the event of a transfer of control (as defined in the option agreement) of the company, any unexercisable portion of the option will immediately vest as of a date prior to the transfer of control, which date will be determined by our Board of Directors in its sole discretion.
25
EQUITY COMPENSATION PLANS
The following table summarizes securities authorized for issuance under equity compensation plans as of September 30, 2006 (our last completed fiscal year end):
| | | | | | | |
Plan Category | | Number of
securities to be issued upon exercise of outstanding options, warrants, and rights [a] | | Weighted-
average exercise price of outstanding options, warrants, and rights [b] | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column [a]) [c] |
Equity compensation plans approved by shareholders | | 2,186,992 | | $ | 3.35 | | 2,486,381 |
Equity compensation plans not approved by shareholders | | — | | | N/A | | — |
| | | | | | | |
Total | | 2,186,992 | | $ | 3.35 | | 2,486,381 |
| | | | | | | |
26
REPORT ON EXECUTIVE COMPENSATION BY COMPENSATION COMMITTEE
General
The Compensation Committee was established in 2005 and currently consists of David M. Schubert, John P. Dubinsky, and Edmund C. King. The Compensation Committee is comprised entirely of non-employee directors and is responsible for establishing the compensation of our senior management, including salaries, bonuses, termination arrangements, and other executive officer benefits. The Compensation Committee also administers our equity incentive plans.
Compensation Philosophy
The Compensation Committee’s goal is to establish and maintain compensation policies that will enable us to attract, motivate, and retain high-quality executives and to ensure that their individual interests are aligned with our long-term interests and our shareholders. The committee places heavy emphasis on paying for performance and believes that a significant portion of an executive’s total compensation opportunity should be at risk and tied to company performance. In addition, in the case of Dr. Francis E. O’Donnell, our Chairman and Chief Executive Officer, the committee has determined that compensation decisions with respect to Dr. O’Donnell should take into account the fact that Dr. O’Donnell is currently the largest beneficial owner of our common stock. Our compensation package generally consists of three principal components:
| | • | | Base salaries, as described in the employment agreements; |
| | • | | Annual incentive bonus eligibility; and |
| | • | | Stock option grants and other forms of equity-based compensation. |
Base Salary. With the exception of Dr. O’Donnell, base salary is the largest portion of the cash compensation package received by each of our executive officers. The base salary of each of our executive officers is governed by employment agreements that were entered into during 2004 and 2005, prior to our initial public offering. Subject to the terms of the employment agreements currently in effect, we intend to establish the base salary of each executive officer based, among other factors, on the Compensation Committee’s assessment of that executive officer’s position, responsibilities, experience, and performance. Our philosophy is to pay base salaries sufficient to attract and retain highly qualified executives. An executive officer’s level of responsibility is the primary factor used in determining base salary. Individual performance also is considered in determining any salary adjustment.
The Compensation Committee reviews and approves all executive officer salary adjustments as recommended by the Chief Executive Officer and determines whether to increase the base salary above the amount set forth in their employment agreements. The Compensation Committee reviews annually the performance of the Chief Executive Officer and the other officers and establishes their base salaries. As a result of Dr. O’Donnell’s substantial ownership interest in the Company, Dr. O’Donnell has agreed to accept a base salary of $1.00 per year for the 2005 and 2006 fiscal years. The Compensation Committee has determined that it will not increase the base salaries of the executive officers for the 2006 fiscal year.
Annual Bonus. Our executive officers are eligible for an annual cash bonuses under their respective employment agreements. No bonuses were paid to the executive officers for Fiscal 2006, except for a $30,000 performance-based bonus paid to Samuel S. Duffey, our General Counsel. However, the Compensation Committee plans to review the performance of the officers for Fiscal 2006 and may grant bonuses to specific officers as they deem appropriate.
Equity-Based Compensation. Our equity-based awards to our executive officers consist principally of stock options granted from time to time under our equity incentive plans. Stock option grants are based on various factors, including the executive officer’s position, responsibility and tenure, each executive officer’s
27
ability to contribute to our future success, and the other elements of such executive officer’s compensation. Generally, we use equity-based compensation to better align the interests of our executive officers with those of our shareholders. In addition, Dr. O’Donnell owns a significant amount of our common stock.
The Internal Revenue Code imposes a limitation on the deduction under Section 162(m) for certain executive officers’ compensation unless certain requirements are met. Our policy is to have all compensation fully deductible; however, we reserve the right to pay compensation that is not deductible if it is in our best interests.
|
| THE COMPENSATION COMMITTEE |
|
|
| John P. Dubinsky |
| David M. Schubert |
| Edmund C. King |
January 9, 2006
The report of the Compensation Committee shall not be deemed incorporated by reference by any general statement incorporating by reference this proxy statement into any filing under the Securities Act of 1933 or under the Securities Exchange Act of 1934, except to the extent that we specifically incorporate this information by reference, and shall not otherwise be deemed filed under such acts.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
The Compensation Committee consists of Messrs. Dubinsky, Schubert, and King. None of the members of the Compensation Committee has ever been an officer or employee of the Company or any of its subsidiaries. None of the Company’s executive officers currently serves, or in the past has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on the Board or Compensation Committee.
28
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
During our fiscal year ended September 30, 2006, we were a party to the following transactions with certain of our executive officers, directors, holders of more than 5% of our voting securities, and their respective affiliates. We believe that the terms of these transactions were no less favorable to us than the terms that could have been obtained from unaffiliated third parties.
Conversion of Accrued Dividends on Preferred Stock
During the fiscal year ended September 30, 2006, we issued a total of 17,091 shares of our common stock to the former holders of our Series E Convertible Preferred Stock in satisfaction of accrued but unpaid dividends owing to them with respect to such preferred stock. Our Series E Convertible Preferred Stock automatically converted into common stock upon the closing of our initial public offering in November 2005. The following directors, executive officers, and 5%-or-greater shareholders were among those who acquired shares of our common stock upon the conversion of the unpaid Series E dividends:
| | |
Name | | Number of Shares
Purchased |
The Hopkins Capital Group, LLC | | 4,333 |
MOAB Investments, LP | | 5,339 |
Dennis Ryll, M.D.* | | 252 |
Steven Stogel* | | 2,084 |
John Dubinsky | | 1,050 |
| * | Ceased to be a director as of October 2006 |
Loans Made to the Company by Affiliates and Related Parties
In August 2005, we entered into a credit facility with Hopkins Capital Group II, LLC, otherwise referred to as Hopkins II. Dr. Francis E. O’Donnell, Jr., our Chairman and Chief Executive Officer, is the sole manager of Hopkins II, and several irrevocable trusts established by Dr. O’Donnell collectively constitute the largest equity owners of Hopkins II. Additionally, Alan M. Pearce, our Chief Financial Officer and a director, and MOAB Investments, L.P. have both loaned funds to Hopkins II, and MOAB Investments, L.P. also holds an equity interest in Hopkins II. MOAB Investments, L.P. is a greater-than-5% shareholder of our company and an entity in which Dr. Dennis Ryll (a director of our company until October 2006) is a limited partner. In May 2006, we issued 412,892 shares of our common stock to Hopkins II as a result of the conversion of $3.3 million in principal and interest under this credit facility at a conversion rate of $8.00 per share, but no other payments have been by us under the credit facility during the fiscal year ended September 30, 2006. In the fourth quarter of the 2006 fiscal year, Hopkins II made additional advances of $0.08 million under the credit facility. As of September 30, 2006, $1.1 million in principal and $0.05 million in accrued but unpaid interest was outstanding under this credit facility. All principal and accrued but unpaid interest under this credit facility will become due on the earlier of August 16, 2007 or the completion by our company of a debt or equity financing that results in proceeds of more than $35.0 million (net of underwriting discounts, commissions, or placement agent fees). We may prepay the bridge loan at any time without penalty or premium.
Relationship with Biovest International, Inc.
In June 2003, we purchased 81% of the outstanding capital stock of Biovest International, Inc. for $20.0 million pursuant to an Investment Agreement with Biovest. During the fiscal years ended September 30, 2005 and 2006, we made advances to Biovest in excess of our required investment under the Investment Agreement, and these advances were made under a demand note bearing interest at prime rate. During the fiscal year ended September 30, 2006, we made total advances to Biovest under this demand note in the amount of $4.7 million after fulfilling our remaining payment obligation of $0.07 million under the Investment Agreement. As of September 30, 2006, the aggregate amount of principal and accrued but unpaid interest under this demand note was $4.7 million.
29
Dr. O’Donnell, our Chairman and Chief Executive Officer, is also Vice Chairman and a director of Biovest, and Dr. Arikian, one of our directors and our President and Chief Operating Officer, Biopharmaceutical Products and Services, is also President, Chairman, and Chief Executive Officer of Biovest.
Relationship with BioDelivery Sciences International, Inc.
We have entered into various transactions with BioDelivery Sciences International, Inc., a publicly traded drug delivery technology company. Dr. O’Donnell is a principal shareholder and Chairman of the Board of BioDelivery Sciences. Previously, Dr. O’Donnell also served as the President and Chief Executive Officer of BioDelivery Sciences. The Hopkins Capital Group, LLC and MOAB Investments, LP are principal shareholders of BioDelivery Sciences. Also, three employees are shared between BioDelivery Sciences and our company.
In April 2004, we entered into a license agreement with BioDelivery Sciences under which BioDelivery Sciences granted us an exclusive license to make, use, or sell its encochleated formulation of amphotericin B for topical treatments for CRS and asthma in the U.S. and European Union. In September 2004, we entered into an asset purchase agreement with BioDelivery Sciences under which we paid BioDelivery Sciences a fee of $2.5 million to expand the geographic scope of the license to make it worldwide and to reduce the royalty percentages to 7% on approved antifungal CRS therapies (but not asthma therapies) that utilize BioDelivery Sciences’ technology and 6% on any unapproved antifungal CRS therapies based on the Mayo Foundation license. Although this agreement remains in effect, we did not make any payments to BioDelivery Sciences under this agreement for royalties or otherwise during the fiscal year ended September 30, 2006.
In August 2004, BioDelivery Sciences acquired Arius Pharmaceuticals, Inc., a pharmaceutical development company that is our development partner for our Emezine product. In March 2004, prior to the acquisition of Arius by BioDelivery Sciences, we obtained exclusive U.S. distribution rights to Emezine under a distribution agreement that we entered into with Arius. Although this agreement remains in effect and Emezine is still under development, we did not make any payments to Arius under this agreement during the fiscal year ended September 30, 2006.
Relationship with Pharmaceutical Product Development, Inc.
In September 2004, Biovest entered into an expanded consulting agreement with PPD pursuant to which we have agreed to pay aggregate fees of approximately $4.6 million to PPD for vaccine-related services, including site development, patient enrollment, vendor management, and regulatory document collection. We paid PPD a total of $0.8 million in consulting fees during the fiscal year ended September 30, 2006.
Other Transactions
In April 2006, our Biovest subsidiary granted common stock purchase warrants to Steven Stogel and Dennis Ryll (formerly directors of our company until October 2006) and to Ronald Osman (a greater-than-5% beneficial owner of our common stock) in consideration of their personal guaranty of certain obligations of Biovest under a financing transaction. The warrants entitle Mr. Stogel, Dr. Ryll, and Mr. Osman to purchase 125,000 shares, 500,000 shares, and 250,000 shares of Biovest common stock, respectively, at an exercise price of $1.00 per share.
Dr. O’Donnell and the Francis E. O’Donnell Irrevocable Trust #1 (an irrevocable trust established by Dr. O’Donnell and in which he is the beneficiary) have pledged publicly traded securities to secure our company’s obligations under its credit facility with Laurus Master Fund, Ltd. The pledge remains in effect until the Laurus credit facility is paid in full.
Dr. O’Donnell holds a 50% partnership interest in a limited partnership that owns a private jet that we use for executive travel relating to company business. We reimburse the partnership for out-of-pocket costs for fuel, a per diem fee covering pilot costs (including overnight stays), and a flat hourly charge for flight time. During Fiscal 2006, we paid this partnership $0.3 million for the usage of the jet.
30
PERFORMANCE GRAPH
The material in this section is not “soliciting material,” is not deemed “filed” with the Securities and Exchange Commission, and is not to be incorporated by reference into any filing of Accentia Biopharmaceuticals, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended.
The following graph shows a comparison of the total cumulative returns of an investment of $100 in cash (i) on October 28, 2005, the first trading day following our initial public offering, in our common stock, (ii) on October 28, 2005 in the Nasdaq Composite Index, U.S. Companies and (iii) on October 28, 2005 in the Nasdaq Biotechnology Index, in each case through September 30, 2006. The comparisons in the graph are required by the SEC and are not intended to forecast or be indicative of the possible future performance of our common stock. The graph assumes that all dividends have been reinvested (to date, we have not declared any dividends).
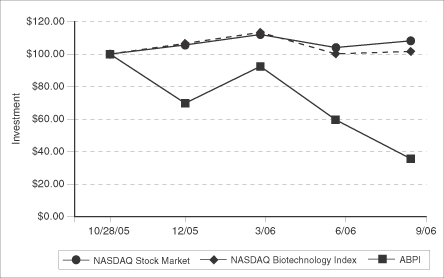
| | | | | | | | | | | | | | | |
| | | 10/28/05 | | 12/30/05 | | 3/31/06 | | 6/30/06 | | 9/29/06 |
Accentia Biopharmaceuticals, Inc. | | $ | 100.00 | | $ | 69.66 | | $ | 92.41 | | $ | 59.59 | | $ | 35.59 |
NASDAQ Biotechnology Index | | $ | 100.00 | | $ | 106.49 | | $ | 113.35 | | $ | 100.10 | | $ | 101.63 |
NASDAQ Composite Index, U.S. Companies | | $ | 100.00 | | $ | 105.53 | | $ | 111.96 | | $ | 103.93 | | $ | 108.07 |
31
PRINCIPAL ACCOUNTANT FEES AND SERVICES
The consolidated financial statements as of and for the years ended September 30, 2006, 2005, and 2004 have been audited by Aidman, Piser & Company, independent registered public accounting firm. We expect that representatives of Aidman, Piser & Company will be present at the Annual Meeting and will be available to respond to appropriate questions from shareholders.
Audit and Related Fees
During Fiscal 2006, the Company engaged Aidman, Piser & Company to perform the Fiscal 2006 audit and to prepare the Fiscal 2006 income tax returns.
Audit Fees. The aggregate audit fees billed by Aidman, Piser & Company for the fiscal years ended September 30, 2006 and 2005 were $283,887 and $371,000, respectively. Audit fees consist of fees billed for professional services rendered for the audit of the Company’s consolidated financial statements, for a restatement of the Company’s consolidated financial statements, and review of the interim condensed consolidated financial statements, as well as other professional services rendered in connection with the Company’s registration statement on Form S-3 filed in November 2006, the Company’s registration statements on Form S-1 filed in March 2006 and June 2006, and services that are normally provided by Aidman, Piser & Company in connection with statutory and regulatory filings or engagements, except those not required by statute or regulation.
Audit-Related Fees. The aggregate fees billed by Aidman, Piser & Company for assurance and related services related to the performance of the audit or review of the Company’s consolidated financial statements and not described above under “Audit Fees” were $188,440 in the fiscal year ended September 30, 2006 and $110,000 in the fiscal year ended September 30, 2005. During these two fiscal years, the Company’s audit-related services principally included audit services relating to the audit of Biovest International, Inc., a majority owned subsidiary of the Company, and services related to the audit of the Company’s 401(k) plan.
Tax Fees. During Fiscal 2006, Aidman, Piser & Company billed $48,217 to the Company for preparing the Company’s 2005 tax returns. The Company has engaged Aidman, Piser & Company to prepare its 2006 tax returns with expected fees for such services ranging from $55,000 to $60,000.
All Other Fees. There were no fees billed by Aidman, Piser & Company during Fiscal 2006 or Fiscal 2005 for professional services other than the services described under “Audit Fees,” “Audit-Related Fees” and “Tax Fees” above.
The Audit Committee does not believe the provision of non-audit services by the independent accountant impairs the ability of such accountant to maintain independence with regard to the Company.
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Auditors
The Audit Committee’s policy is to pre-approve all audit and permissible non-audit services provided by the Company’s independent auditors in order to assure that the provision of such services does not impair the auditor’s independence. These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to one year and any pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget. Management is required to periodically report to the Audit Committee regarding the extent of services provided by the independent auditors in accordance with this pre-approval, and the fees for the services performed to date. All such services and fees provided in Fiscal 2006 were pre-approved by the Audit Committee.
32
PROCEDURE FOR SUBMITTING SHAREHOLDER PROPOSALS
All notices of proposals by shareholders, whether or not be be included in the Company’s proxy materials, should be sent to the attention of the Secretary of the Company at 324 South Hyde Park Avenue, Suite 350, Tampa, Florida 33606. Any such notice to the Secretary shall set forth as to each matter the shareholder proposes to bring before the Annual Meeting (a) a brief description of the business desired to be brought before the Annual Meeting and the reasons for conducting such business at the Annual Meeting, (b) the name and address, as they appear on the books of the Company, of the shareholder proposing such business, and the name and address of the beneficial owner, if any, on whose behalf the proposal is made, (c) the class and number of shares of the Company which are owned beneficially and of record by such shareholder of record and by the beneficial owner, if any, on whose behalf the proposal is made, and (d) any material interest of such shareholder of record and the benefical owner, if any, on whose behalf the proposal is made in such business. The chairman of the meeting shall, if the facts warrant, determine and declare to the meeting that business was not properly brought before the meeting and in accordance with the procedures prescribed by the Bylaws, and if he should so determine, he shall so declare to the meeting and any such business not properly brought before the meeting shall not be transacted.
DEADLINE FOR RECEIPT OF SHAREHOLDER PROPOSALS
Shareholders may submit proposals on matters appropriate for shareholder action at annual meetings in accordance with the rules and regulations adopted by the Securities and Exchange Commission. Any proposal which an eligible shareholder desires to have included in the Company’s proxy statement and presented at the 2008 annual meeting of shareholders (which is expected to be held on or about February 28, 2008) will be included in the Company’s proxy statement and related proxy card if it is received by the Company no later than October 1, 2007 (120 calendar days prior to the anniversary of the mailing date of this Proxy Statement) and if it complies with Securities and Exchange Commission rules regarding inclusion of proposals in proxy statements.
Other deadlines apply to the submission of shareholder proposals for the 2008 annual meeting that are not required to be included in the Company’s proxy statement under Securities and Exchange Commission rules. With respect to these shareholder proposals for the 2008 annual meeting, the Company’s bylaws provide certain requirements for advance notification by shareholders of business to be conducted at annual meetings but not necessarily included in the Company’s proxy statement. In order to be timely, a shareholder notice must be delivered to or mailed and received in writing by the Company’s Secretary at the principal executive offices of the Company not less than 120 days prior to the date of the meeting. These requirements are separate from and in addition to requirements that a shareholder must meet in order to have a shareholder proposal included in the Company’s proxy statement.
OTHER MATTERS
The Board of Directors does not currently know of any other matters to be presented at the 2007 Annual Meeting. If any other matters properly come before the annual meeting, it is intended that the shares represented by Proxy will be voted with respect thereto in accordance with the judgement of the persons voting them.
|
By Order of the Board of Directors, |
|
| /s/ FRANCIS E. O’DONNELL, JR. |
| Francis E. O’Donnell, Jr., M.D. |
| Chairman of the Board and Chief Executive Officer |
January 26, 2007
33
| | |

324 SOUTH HYDE PARK AVENUE SUITE 350 TAMPA, FL 33606 | | VOTE BY INTERNET -www.proxyvote.com Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. ELECTRONIC DELIVERY OF FUTURE SHAREHOLDER COMMUNICATIONS If you would like to reduce the costs incurred by Accentia Biopharmaceuticals, Inc. in mailing proxy materials, you can consent to receiving all future proxy statements, proxy cards and annual reports electronically via e-mail or the Internet. To sign up for electronic delivery, please follow the instructions above to vote using the Internet and, when prompted, indicate that you agree to receive or access shareholder communications electronically in future years. VOTE BY PHONE - 1-800-690-6903 Use any touch-tone telephone to transmit your voting instructions up until 11:59 P.M. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you call and then follow the instructions. VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Accentia Biopharmaceuticals, Inc., c/o ADP, 51 Mercedes Way, Edgewood, NY 11717. |
| | | | |
TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: | | ACCBI1 | | KEEP THIS PORTION FOR YOUR RECORDS |
THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. | | DETACH AND RETURN THIS PORTION ONLY |
ACCENTIA BIOPHARMACEUTICALS, INC.
Vote on Directors
| | | | | | | | | | |
| 1. | | Election of four directors, with the following as the Board’s nominees: | | For
All | | Withhold
All | | For All
Except | | To withhold authority to vote for any individual nominee(s), mark “For All Except” and write the number(s) of the nominee(s) on the line below. |
| | | | | |
| | • (1) Edmund C. King (nominated as a Class I director with a term to end at the 2009 Annual Meeting of Shareholders); | | ¨ | | ¨ | | ¨ | | |
| | | | | |
| | • (2) John P. Dubinsky (nominated as a Class II director with a term to end at the 2010 Annual Meeting of Shareholders); | | | | | | | | |
| | | | | |
| | • (3) Francis E. O’Donnell, Jr., M.D. (nominated as a Class II director with a term to end at the 2010 Annual Meeting of Shareholders); and | | | | | | | | |
| | | | | |
| | • (4) Todd D. Thomason (nominated as a Class II director with a term to end at the 2010 Annual Meeting of Shareholders). | | | | | | | | |
| | | | | | | | |
| Vote on Proposals | | For | | Against | | Abstain |
| | | | |
| 2. | | Proposal to ratify the appointment of Aidman, Piser & Company as the Company’s independent registered public accounting firm for the fiscal year ending September 30, 2007. | | ¨ | | ¨ | | ¨ |
| | | | |
| 3. | | Proposal to approve the potential issuance of shares of the Company’s Common Stock exceeding 19.99% of the Common Stock outstanding on September 29, 2006 pursuant to the terms of (a) certain 8% Secured Convertible Debentures due September 29, 2010 and Common Stock Purchase Warrants to purchase shares of the Company’s Common Stock issued by the Company in a private placement financing in September 2006 (the “Financing”) and (b) a Common Stock Purchase Warrant to purchase shares of the Company’s Common Stock issued to the Company’s placement agent in connection with the Financing. | | ¨ | | ¨ | | ¨ |
| | |
| For address changes and/or comments, please check this box and write them on the back where indicated. | | ¨ |
| | | | |
| | Yes | | No |
| | |
| Please indicate if you plan to attend this meeting. | | ¨ | | ¨ |
| | |
| HOUSEHOLDING ELECTION - Please indicate if you consent to receive certain future investor communications in a single package per household. | | ¨ | | ¨ |
| | | | | | | | |
| | | | | | | | | |
| Signature [PLEASE SIGN WITHIN BOX] | | Date | | | | Signature (Joint Owners) | | Date |
ACCENTIA BIOPHARMACEUTICALS, INC.
Annual Meeting of Shareholders, February 28, 2007
THIS PROXY IS SOLICITED BY THE BOARD OF DIRECTORS
The undersigned shareholder of Accentia Biopharmaceuticals, Inc. hereby appoints Francis E. O’Donnell, Jr. and James A. McNulty, or either of them, as proxies, each with the power to appoint a substitute, and hereby authorizes them to represent and vote all shares of Accentia Biopharmaceuticals, Inc. that the undersigned is entitled to vote at the Annual Meeting of Shareholders of Accentia Biopharmaceuticals, Inc., and at any adjournments or postponements thereof, to be held on February 28, 2007, at 11:00 a.m. local time, at the St. Louis Club, 7701 Forsyth Blvd., St. Louis, Missouri 63105 , as indicated on the reverse side.
| | |
| Address Changes/Comments: | | |
| |
| |
| |
| |
(If you noted any Address Changes/Comments above, please mark corresponding box on the reverse side.)
(Continued and to be signed on the reverse side.)

