UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K/A
Amendment No. 1
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year ended December 31, 2006
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 000-51169
ASPREVA PHARMACEUTICALS CORPORATION
(Exact name of registrant as specified in its charter)
| | | |
British Columbia, Canada | | 98-0435540 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
1203 – 4464 Markham Street, Victoria, B.C., Canada | | V8Z 7X8 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (250) 744-2488
Securities registered pursuant to Section 12(b) of the Act:
| | | |
| Title of Each Class | | Name of Each Exchange on Which Registered |
| Common Stock, without par value | | The NASDAQ Stock Market LLC
(NASDAQ Global Select Market) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by checkmark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o
Indicate by checkmark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by checkmark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15 (d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by checkmark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. Yes þ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act.
Large accelerated filer þ Accelerated filer o Non-accelerated filer o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes o No þ
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant on June 30, 2006, the last business day of the registrant’s most recently completed second fiscal quarter, was $731,282,930 (based on the closing sales price of the registrant’s common stock on that date). Shares of the registrant’s common stock held by each officer and director and each person who owns 5% or more of the outstanding common stock of the registrant have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of January 31, 2007, 35,161,888 shares of the registrant’s common stock were issued and outstanding.
EXPLANATORY NOTE
This Amendment No. 1 on Form 10-K/A (“Amendment No. 1”) amends Aspreva Pharmaceuticals Corporation’s Annual Report on Form 10-K, as filed on February 26, 2007 (the “Original Filing”), and is being filed solely to replace Part II, Item 5, Part III, Item 10 through Item 14 and to update Item 15. Except as otherwise stated herein, no other information contained in the Original Filing has been updated by this Amendment No. 1. None of the information in this Amendment No. 1 modifies or updates disclosures in the Original Filing (including the exhibits to the Original Filing, except for the updated Exhibits 31.1 and 31.2) other than as set forth herein.
This Amendment No. 1 should be read in conjunction with our periodic filings made with the Securities and Exchange Commission, or the SEC, subsequent to the date of the Original Filing, including any amendments to those filings, as well as any Current Reports filed on Form 8-K subsequent to the date of the Original Filing. In addition, in accordance with applicable rules and regulations promulgated by the SEC, this Form 10-K/A includes updated certifications from our Chief Executive Officer and Chief Financial Officer as Exhibits 31.1 and 31.2.
CURRENCY
In this Amendment No. 1, unless otherwise specified, all monetary amounts are in United States dollars. To the extent that such monetary amounts are derived from Aspreva’s consolidated financial statements contained in this Annual Report on Form 10-K, as amended, they have been translated into United States dollars in accordance with our accounting policies as described therein. All other monetary amounts have been translated into United States dollars at the April 10, 2007 noon buying rate published by the Federal Reserve Bank of New York, being US$1.00 = C$1.1479. The noon buying rate published by the Federal Reserve Bank of New York as at December 31, 2006 was US$1.00 = C$1.1652, as at December 31, 2005 was US$1.00 = C$1.1656 and as at December 31, 2004 was US$1.00 = C$1.2034. The annual average noon buying rate published by the Federal Reserve Bank of New York for 2006 was US$1.00 = C$1.1340, for 2005, US$1.00 = C$1.2115, and for 2004, US$1.00 = C$1.3273.
ASPREVA PHARMACEUTICALS CORPORATION
TABLE OF CONTENTS
| | | | |
| | | PART II |
| Item 5. | | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 1 |
| | | PART III |
| Item 10. | | Directors, Executive Officers and Corporate Governance | 5 |
| Item 11. | | Executive Compensation | 9 |
| Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 27 |
| Item 13. | | Certain Relationships and Related Transactions, and Director Independence | 31 |
| Item 14. | | Principal Accountant Fees and Services | 34 |
| | | PART IV |
| Item 15. | | Exhibits and Financial Statement Schedules | 35 |
| SIGNATURES | 36 |
| EXHIBIT INDEX | 37 |
PART II
| Item 5. | | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
NASDAQ Global Select Market and Toronto Stock Exchange
Our common shares are quoted on the NASDAQ Global Select Market under the symbol “ASPV”, and on the Toronto Stock Exchange under the symbol “ASV”. The following table sets out, for the periods indicated, the high and low closing sales prices and trading volumes of our common shares, as reported by the NASDAQ Global Select Market and Toronto Stock Exchange for the years ended December 31, 2006 and 2005.
| | | | | | | | | | | | | | | | | |
| | | NASDAQ Global Select Market | | | The Toronto Stock Exchange | |
| | | High | | | Low | | | High | | | Low | |
| 2006 | | (U.S.$) | | | (U.S.$) | | | (CAD$) | | | (CAD$) | |
| First Quarter | | $ | 28.87 | | | $ | 15.62 | | | $ | 33.38 | | | $ | 18.01 | |
| Second Quarter | | $ | 34.00 | | | $ | 22.52 | | | $ | 38.07 | | | $ | 26.20 | |
| Third Quarter | | $ | 27.72 | | | $ | 20.05 | | | $ | 31.00 | | | $ | 22.00 | |
| Fourth Quarter | | $ | 26.35 | | | $ | 17.47 | | | $ | 29.68 | | | $ | 19.92 | |
| | | | | | | | | | | | | | | | | |
| | | NASDAQ Global Select Market | | | The Toronto Stock Exchange | |
| | | High | | | Low | | | High | | | Low | |
| 2005 | | (U.S.$) | | | (U.S.$) | | | (CAD$) | | | (CAD$) | |
| First Quarter (1) | | $ | 16.30 | | | $ | 11.70 | | | $ | 20.00 | | | $ | 13.68 | |
| Second Quarter | | $ | 16.41 | | | $ | 12.80 | | | $ | 20.20 | | | $ | 15.50 | |
| Third Quarter | | $ | 17.25 | | | $ | 13.32 | | | $ | 20.79 | | | $ | 16.20 | |
| Fourth Quarter | | $ | 16.25 | | | $ | 11.18 | | | $ | 18.99 | | | $ | 13.55 | |
| | |
| (1) | | On March 4, 2005 we completed our initial public offering. |
As of December 31, 2006, there were approximately 69 shareholders of record of our common shares, one of which was Cede & Co., a nominee for Depository Trust Company, or DTC, and one of which was The Canadian Depository for Securities Limited, or CDS. All of our common shares held by brokerage firms, banks and other financial institutions in the U.S. and Canada as nominees for beneficial owners are considered to be held of record by Cede & Co. in respect of brokerage firms, banks and other financial institutions located in the U.S., and by CDS in respect of brokerage firms, banks and other financial institutions located in Canada. Cede & Co. and CDS are each considered to be one shareholder of record.
Dividend Policy
Aspreva has not declared or paid any dividends on its common shares since inception. We anticipate that we will retain any future earnings to finance the expansion of our business and we do not anticipate paying dividends in the foreseeable future.
Equity Compensation Plan Information
Information regarding our equity compensation plans is included under Part III, Item 12 the caption “Securities Authorized for Issuance Under Equity Compensation Plans” and is incorporated herein by reference.
Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities during the three months ended December 31, 2006.
1
Recent Sales of Unregistered Securities
We did not sell or issue any unregistered securities during the three months ended December 31, 2006.
Exchange Controls
Limitations on the ability to acquire and hold our common shares may be imposed by the Competition Act (Canada). This legislation permits the Commissioner of Competition of Canada, or Commissioner, to review any acquisition of a significant interest in us. This legislation grants the Commissioner jurisdiction, for up to three years, to challenge this type of acquisition before the Canadian Competition Tribunal if the Commissioner believes that it would, or would be likely to, substantially reduce or prevent competition in any market in Canada.
This legislation also requires any person who intends to acquire our common shares to file a notification with the Canadian Competition Bureau if certain financial thresholds are exceeded, and that person would hold more than 20% of our common shares. If a person already owns 20% or more of our common shares, a notification must be filed when the acquisition would bring that person’s holdings to over 50%. Where a notification is required, the legislation prohibits completion of the acquisition until the expiration of a statutory waiting period, unless the Commissioner provides written notice that he or she does not intend to challenge the acquisition.
There is no law, governmental decree or regulation in Canada that restricts the export or import of capital, or which would affect the remittance of dividends or other payments by us to non-resident holders of our common shares, other than withholding tax requirements.
There is no limitation imposed by Canadian law or our notice of articles or articles on the right of non-residents to hold or vote our common shares, other than those imposed by the Investment Canada Act (Canada), or Investment Act.
The Investment Act requires each individual, government or agency thereof, corporation, partnership, trust or joint venture that is not a “Canadian” as defined in the Investment Act, referred to in this discussion as a “non-Canadian” who commences a new business activity in Canada or acquires control of an existing Canadian business, where the establishment or acquisition of control is not a reviewable transaction, to file a notification with Industry Canada. The Investment Act generally prohibits the implementation of a reviewable transaction by a non-Canadian unless after review the minister responsible for the Investment Act is satisfied that the investment is likely to be of net benefit to Canada. An investment in our common shares by a non-Canadian would be reviewable under the Investment Act if it were an investment to acquire control of us and the value of our assets were C$5.0 million or more. The Investment Act provides for special review thresholds for World Trade Organization, or WTO, member country investors, including United States investors. Under the Investment Act, an investment in our common shares by a non-Canadian who is a “WTO investor” (as defined in the Investment Act) would be reviewable only if it were an investment to acquire control of us and the value of our assets was equal to or greater than a specified amount, which increases in stages. The specified amount is C$265.0 million in 2006. The threshold amount is subject to an annual adjustment on the basis of a prescribed formula in the Investment Act to reflect inflation and real growth within Canada.
The acquisition of a majority of the voting interests of an entity or of a majority of the undivided ownership interests in the voting shares of an entity that is a corporation is deemed to be acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquiror through the ownership of voting shares. The acquisition of less than one-third of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is deemed not to be acquisition of control of that corporation. Certain transactions in relation to our common shares would be exempt from review from the Investment Act, including:
| • | | acquisition of our common shares by a person in the ordinary course of that person’s business as a trader or dealer in securities; |
| |
| • | | acquisition or control of us in connection with the realization of security granted for a loan or other financial |
2
| | | assistance and not for any purpose related to the provisions of the Investment Act; and |
| |
| • | | acquisition or control of us by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of us, through the ownership of voting interests, remains unchanged. |
Material United States and Canadian Income Tax Considerations
The following is a summary of certain Canadian and U.S. federal income tax considerations applicable to holders of common shares of Aspreva. These tax considerations are stated in brief and general terms and are based on Canadian and U.S. law currently in effect. There are other potentially significant Canadian and U.S. federal income tax considerations and provincial, state and local income tax considerations with respect to ownership and disposition of the common shares which are not discussed herein. The tax considerations relative to ownership and disposition of the common shares may vary from shareholder to shareholder depending on the shareholder’s particular status. Accordingly, shareholders and prospective shareholders are encouraged to consult with their tax advisors regarding tax considerations, which may apply to the particular situation.
Canadian Federal Tax Information
Dividends paid on the common shares held by non-residents of Canada will generally be subject to Canadian withholding tax at the rate of 25%. The Canada-U.S. Income Tax Convention (1980) (the “Convention”) provides that the withholding rate on dividends paid to U.S. residents on the common shares is generally 15%.
Gains on sales or other dispositions of the common shares of Aspreva by a U.S. resident generally are not subject to Canadian income tax, unless the shareholder realizes the gains in connection with a business carried on in Canada. A gain realized upon the disposition of the common shares by a U.S. resident that is otherwise subject to Canadian tax may be exempt from Canadian tax under the Convention.
Where the common shares are disposed of by way of an acquisition of such common shares by Aspreva, other than a purchase in the open market in the manner in which common shares normally would be purchased by any member of the public in the open market, the amount paid by Aspreva in excess of the paid-up capital of such common shares will be treated as a dividend and will be subject to non-resident withholding tax as described above.
U.S. Federal Tax Information
Distributions with respect to our common shares generally will be taxable as dividends to the extent of Aspreva’s earnings and profits, determined under U.S. tax principles, subject to the same preferential rate that applies to long-term capital gain (currently, 15%). Under current law, for taxable years beginning after December 31, 2010, distributions will be taxed at ordinary rates without the benefit of such preferential rates.
Corporate U.S. Holders generally will not be allowed a deduction for dividends received in respect of distributions on our common shares. Dividends will be treated as income from sources outside the U.S., but generally will be “passive income,” or in the case of a financial services entity, “financial services income” (and, for taxable years beginning after December 31, 2005, as “general category income”) for U.S. foreign tax credit purposes.
Special rules apply to U.S. Holders that hold stock in a “passive foreign investment company” (“PFIC”). A foreign corporation generally will be a PFIC for any taxable year in which either (i) 75% or more of its gross income is passive income or (ii) 50% or more of the average value of its assets consist of assets that produce, or that are held for the production of, passive income. For this purpose, passive income generally includes, among other things, interest, dividends, rents, royalties and gains from certain commodities transactions.
We believe that we were not a PFIC in 2005 or 2006 and anticipate that we will not be a PFIC with respect to any subsequent taxable year. However, we have been a PFIC in the past and there can be no assurance that we will not be considered a PFIC in a future taxable year, because status under the PFIC rules is determined annually and is based in part on factors not entirely within our control (such as market capitalization).
3
A U.S. Holder whose common shares were held at any time during a taxable year in which we were a PFIC may be subject to increased tax liability upon the sale, exchange or other disposition of those shares of our common shares or upon the receipt of certain distributions. These adverse tax consequences will not apply, however, if a U.S. Holder timely filed and maintained (and in certain cases, continue to maintain) a qualified electing fund (“QEF”) election to be taxed annually on the holder’s pro rata portion of our ordinary earnings and net capital gains.
We intend to comply with all record-keeping, reporting and other requirements so that U.S. Holders, who must make or continue to maintain a QEF election may do so. However, if meeting those record-keeping and reporting requirements becomes onerous, we may decide, in our sole discretion, that such compliance is impractical and will so notify U.S. Holders. UNTIL SUCH TIME, U.S. HOLDERS THAT DESIRE TO MAKE OR MAINTAIN A QEF ELECTION MAY CONTACT OUR INVESTMENT RELATIONS GROUP FOR THE PFIC ANNUAL INFORMATION STATEMENT, WHICH MAY BE USED TO COMPLETE THEIR ANNUAL QEF ELECTION FILINGS. THIS STATEMENT IS ALSO AVAILABLE ON OUR WEBSITE AT: WWW.ASPREVA.COM.
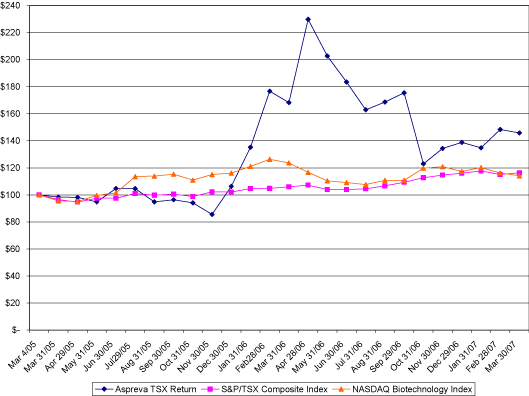
Share Price Performance Graph
The graph below compares cumulative total shareholder return on our common shares since our initial public offering on March 4, 2005 with the total cumulative return of the S&P/TSX Composite Index and the NASDAQ Biotechnology Index over the same period.
The graph above assumes $100 invested on March 4, 2005 in our common shares and in each index. The share price shown above for our common shares is historical and not indicative of future price performance.
This section is not “soliciting material,” is not deemed “filed” with the SEC, and is not to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language contained in such filing.
4
PART III
| Item 10. | | Directors, Executive Officers and Corporate Governance. |
Executive Officers
Information concerning our Executive Officers is set forth under “Executive Officers of the Registrant” in Part I of the Annual Report on Form 10-K, and is incorporated herein by reference.
Directors
The following table sets forth as of March 31, 2007, the name, age and position of each person who serves as an Aspreva director.
| | | | | | | | | |
| Name of Director and | | | | | | | |
| Municipality of Residence | | Age | | | Current Position | | Director Since |
Richard M. Glickman
| | | 48 | | | Chairman and Chief Executive Officer | | January 2002 |
| Sidney, British Columbia | | | | | | | | |
| | | | | | | | | |
Noel F. Hall
| | | 45 | | | President and Director | | January 2002 |
| Victoria, British Columbia | | | | | | | | |
| | | | | | | | | |
Kirk K. Calhoun, C.P.A.(1)
| | | 62 | | | Director | | September 2004 |
| Santa Monica, California | | | | | | | | |
| | | | | | | | | |
Ronald M. Hunt(2),(3)
| | | 42 | | | Director | | March 2004 |
| Westport, Connecticut | | | | | | | | |
| | | | | | | | | |
William L. Hunter, M.D., Msc.
| | | 44 | | | Director | | June 2006 |
| Vancouver, British Columbia | | | | | | | | |
| | | | | | | | | |
Julia G. Levy, Ph.D.(1)
| | | 72 | | | Director | | December 2004 |
| Vancouver, British Columbia | | | | | | | | |
| | | | | | | | | |
R. Hector MacKay-Dunn, Q.C.(3)
| | | 56 | | | Director and Corporate Secretary | | December 2004 |
| Vancouver, British Columbia | | | | | | | | |
| | | | | | | | | |
George M. Milne, Ph.D.(2)(3)(4)
| | | 63 | | | Director | | September 2004 |
| Boca Grande, Florida | | | | | | | | |
| | | | | | | | | |
Arnold L. Oronsky, Ph.D.(1) (2)
| | | 67 | | | Director | | March 2004 |
| Los Altos Hills, California | | | | | | | | |
Notes:
| | |
| (1) | | Member of our Audit Committee. |
| |
| (2) | | Member of our Compensation Committee. |
| |
| (3) | | Member of our Nominating and Corporate Governance Committee. |
| |
| (4) | | Lead Director. |
Richard M. Glickmanis a co-founder of Aspreva and has been our Chairman of the Board and Chief Executive Officer since January 2002. In 1990, Mr. Glickman co-founded Stressgen Biotechnologies Corporation, a biotechnology company, and served as its Chief Executive Officer until 2000. From 2000 to 2006, Mr. Glickman served as a director of Vigil Health Solutions Inc., a healthcare services company, and served as the Chairman of the Board from 2000 to 2005. Mr. Glickman currently serves as a director of Cardiome Pharma Corp. and Medgenesis Therapeutix Inc. Mr. Glickman holds a B.Sc. in Microbiology and Immunology from McGill University.
Noel F. Hallis a co-founder of Aspreva and has been our President and a member of our Board of Directors since January 2002. In 1995, Mr. Hall co-founded the life sciences practice of Hill and Knowlton, a consulting firm, and until 2002 served as head of global strategic planning for the firm’s worldwide pharmaceutical consulting practice.
5
From 1992 to 1995, Mr. Hall was Director of Corporate Affairs for the United Kingdom and Northern Europe for The Wellcome Foundation Ltd., now part of GlaxoSmithKline PLC, a pharmaceutical company. From 1985 to 1990, Mr. Hall worked in market development with Abbott Laboratories Ltd., a pharmaceutical company. From 1983 to 1985, Mr. Hall was a regional sales manager with Leo Laboratories Ltd., a pharmaceutical company. Mr. Hall holds an M.L.S.O. from Paddington Technical College, University of Westminster.
Kirk K. Calhoun, C.P.A.has been a member of our Board of Directors since September 2004. Mr. Calhoun joined Ernst & Young LLP, an independent registered public accounting firm, in 1965 and served as a partner of the firm from 1975 until his retirement in 2002. Mr. Calhoun is a Certified Public Accountant. Mr. Calhoun serves on the Board of Directors of Adams Respiratory Therapeutics, Inc., a specialty pharmaceutical company, Abraxis BioScience, Inc., a pharmaceutical company, and Replidyne, Inc., a biopharmaceutical company. Mr. Calhoun holds a B.S. in Accounting from the University of Southern California.
Ronald M. Hunthas been a member of our Board of Directors since March 2004. Since 2005, Mr. Hunt has been a Managing Director of New Leaf Venture Partners, L.L.C., a spin-off of the Sprout Group, a venture capital affiliate of Credit Suisse First Boston. New Leaf Venture Partners, L.L.C. was formed in 2005 and manages the healthcare portfolio of the Sprout Group. From 1998 to 2005, Mr. Hunt held various positions, including partner and director, with the Sprout Group. From 1996 to 1998, Mr. Hunt served as a strategy and operations consultant with The Healthcare Group, a consulting firm. From 1994 to 1996, Mr. Hunt served as a strategy and operations consultant with Coopers and Lybrand Consulting, a consulting firm. From 1992 to 1994, Mr. Hunt served in a marketing role with Johnson & Johnson, a pharmaceutical company. From 1986 to 1992, Mr. Hunt served in various marketing, strategic planning and sales positions with SmithKline Beecham, now GlaxoSmithKline PLC, a pharmaceutical company. Mr. Hunt holds a B.S. from Cornell University and an M.B.A. from The Wharton School at the University of Pennsylvania.
William L. Hunter, MD, MSc.Dr. Hunter became a director of Aspreva on June 8, 2006. Dr. Hunter is a founder of Angiotech Pharmaceuticals Inc., and has been Chief Executive Officer and President since 1997 and has been a member of Angiotech’s scientific and management teams since its inception. He serves as a director of Angiotech, NeuroMed Technologies, Inc. and the Michael Smith Foundation for Health Research and served as a director of AnorMED Inc. prior to its acquisition by Genzyme Corp. in 2006. Dr. Hunter is also an advisory board member for the Biotechnology MBA Program at the University of Western Ontario’s Ivey School of Business and an active member of the Government of British Columbia Premier’s Technology Council. Dr. Hunter has been honored with many awards including, most recently, the 2005 BC Innovative Council’s Cecil Green Award for Science and Technology Entrepreneurship and the 2006 Canadian Venture Capital Association’s Entrepreneur of the Year. He received his Bachelor of Science Degree from McGill University, Montreal in 1985 and his Masters of Science and Doctor of Medicine Degree from the University of British Columbia in 1989 and 1992, respectively.
Julia G. Levy, Ph.D.has been a member of our Board of Directors since December 2004. In 1981, Dr. Levy co-founded QLT Inc., a biopharmaceutical company, and has been a member of its Board of Directors since 1983. Since 2002, Dr. Levy has been Executive Chairman of the Scientific Advisory Board of QLT. From 1996 to 2002, Dr. Levy was the President and Chief Executive Officer of QLT. From 1986 to 1996, Dr. Levy held positions with QLT as a Vice President, Senior Vice President and Acting President and Chief Executive Officer. From 1973 to 1999, Dr. Levy was a Professor of Microbiology at the University of British Columbia. Dr. Levy is a Fellow of the Royal Society of Canada. Dr. Levy holds a B.A. from the University of British Columbia and a Ph.D. in Experimental Pathology from the University of London.
R. Hector MacKay-Dunn, Q.C.has been a member of our Board of Directors since December 2004, and prior to that was a member of our Board of Directors from June 2003 to September 2004. Mr. MacKay-Dunn has been our Corporate Secretary since September 2004. Mr. MacKay-Dunn is a senior partner with Farris, Vaughan, Wills & Murphy LLP, a Vancouver based law firm, and has been with the firm since 1978. Mr. MacKay-Dunn is the Chair of the British Columbia Innovation Council, a Provincial Crown Agency, a member of the University of British Columbia Industry Liaison (UILO) Advisory Council and a member of the University of British Columbia Faculty of Science Dean’s Advisory Council. Mr. MacKay-Dunn holds a B.A. and an LL.B. from the University of British Columbia and was appointed Queen’s Counsel by the British Columbia Cabinet in 2003.
6
George M. Milne, Ph.D.has been a member of our Board of Directors since September 2004. Since 2003, Dr. Milne has been a venture partner at Radius Ventures, a venture capital firm focused on the life science industry. From 2000 to 2003, Dr. Milne was Executive Vice President of Global Research and Development at Pfizer, Inc., a pharmaceutical company. While at Pfizer, Dr. Milne also held a number of senior management positions including President of Pfizer’s Central Research with global responsibility for Human and Veterinary Research and Development from 1993 to 2000. Dr. Milne is a member of the Board of Directors of Charles River Laboratories, Inc., a drug development service company, MedImmune, Inc., a biopharmaceutical company, and Mettler Toledo International, Inc., a precision instruments company. Dr. Milne holds a B.S. in Chemistry from Yale University and a Ph.D. in Organic Chemistry from the Massachusetts Institute of Technology.
Arnold L. Oronsky, Ph.D.has been a member of our Board of Directors since March 2004. Dr. Oronsky is a general partner at InterWest Partners, a venture capital firm focusing on investments in life sciences and information technology. From 1989 to 1994, Dr. Oronsky was a special limited partner at InterWest. Dr. Oronsky is a senior lecturer in the Department of Medicine at Johns Hopkins Medical School. From 1980 to 1993, Dr. Oronsky was the Vice President for Discovery Research at the Lederle Laboratories division of American Cyanamid Company, a pharmaceutical company. From 1970 to 1972, Dr. Oronsky was assistant professor at Harvard Medical School, where he also served as a research fellow from 1968 to 1970. From 1973 to 1976, Dr. Oronsky was the head of the Inflammation, Allergy, and Immunology Research program for Ciba-Geigy Pharmaceutical Company. Dr. Oronsky was a director of BioTransplant Incorporated, a biopharmaceutical company, which filed for bankruptcy in February 2003 and was ordered into bankruptcy in April 2004. Dr. Oronsky is a director of the following biopharmaceutical companies: Anesiva Inc., Dynavax Technologies Corporation and Metabasis Therapeutics, Inc. Dr. Oronsky holds a Ph.D. in Immunology from Columbia University and a Ph.D. from Columbia University’s College of Physicians & Surgeons.
There are no family relationships between any of our executive officers and/or directors.
Section 16(A) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires a registrant’s directors and executive officers, and persons who own more than 10% of a registered class of a registrants’ securities, to file with the SEC initial reports of ownership and reports of changes in ownership of common shares and other equity securities of the registrant. As we are a “foreign private issuer” pursuant to Rule 3a12-3 of the Securities Exchange Act of 1934, Aspreva and the persons referred to above are exempt from the reporting and liability provisions of Section 16(a). However, under Canadian provincial securities laws, the persons referred to above are required to file reports in electronic format through the System for Electronic Disclosure by Insiders, or SEDI, disclosing changes in beneficial ownership of, or control or direction over, our common shares and other securities. Our shareholders can access such reports atwww.sedi.ca.
Code of Conduct
We have adopted a code of conduct that applies to all or our officers, directors and employees. Our code of conduct is available on our website atwww.aspreva.com. Shareholders may request a copy of our code of conduct without charge from our Vice President, Investor Relations and Corporate Relations at (250) 744-2488.
Our Board of Directors and management review and discuss from time to time the effectiveness of our code of conduct and any areas or systems that may be further improved. We have not filed a material change report that pertains to any conduct of any of our directors or executive officers that constitutes a departure from our code of conduct. If we make any substantive amendments to our code of conduct, or grant any waiver from a provision of our code of conduct to any of our executive officers or directors, we will promptly disclose the nature of the amendment or waiver on our website.
Aspreva complies with the relevant provisions under the Business Corporations Act (British Columbia) that deal with conflict of interest in the approval of agreements or transactions and our code of conduct sets out additional guidelines in relation to conflict of interest situations. Aspreva, through directors’ and officers’ questionnaires and other systems, also gathers and monitors relevant information in relation to potential conflicts of interest that one of our directors or officers may have.
Aspreva was founded on, and the business continues to be successful largely as a result of, a commitment to ethical conduct. Employees are regularly reminded about their obligations in this regard and senior management demonstrates a culture of integrity and monitors employees compliance with our code of conduct to the extent possible.
7
Identification of Audit Committee and Financial Expert
We maintain an Audit Committee consisting of directors, Mr. Calhoun, Dr. Levy and Dr. Oronsky, each of whom is a non-employee member of our Board of Directors. Mr. Calhoun chairs the committee and is our Audit Committee financial expert (as is currently defined under the applicable SEC rules). Our Board of Directors has determined that each member of our Audit Committee is an independent member of our Board of Directors under the current requirements of the NASDAQ Market and the rules and regulations of the SEC and Canadian provincial securities regulatory authorities.
Board of Directors Nomination Procedures
Our Board of Directors is responsible for approving nominees for election as directors. However, our Nominating and Corporate Governance Committee is responsible for reviewing, soliciting and recommending nominees to our Board of Directors.
In evaluating prospective nominees, our Nominating and Corporate Governance Committee looks for the following minimum qualifications: strong business acumen, extensive previous experience as an executive or director with successful companies, the highest standards of integrity and ethics, and a willingness and ability to make the necessary time commitment to diligently perform the duties of a director. Nominees are selected with a view to our best interests as a whole, rather than as representative of any particular stakeholder or category of stakeholders. Our Nominating and Corporate Governance Committee will also consider the skill sets of the incumbent directors when recruiting replacements to fill vacancies in our Board of Directors. Our Board of Directors prefers a mix of experience among its members to maintain a diversity of viewpoints and ensure that our Board of Directors can achieve its objectives. When a vacancy on our Board of Directors occurs, in searching for a new director, the Nominating and Corporate Governance Committee will identify particular areas of specialization which it considers beneficial, in addition to the general qualifications, having regard to the skill sets of the other members of our Board of Directors. Potential nominees and their respective references are interviewed extensively in person by the Nominating and Corporate Governance Committee before any nomination is endorsed by that committee. All nominations proposed by the Nominating and Corporate Governance Committee must receive the approval of our Board of Directors.
Our Board of Directors will also consider any director nominees proposed by our shareholders. Since our initial public offering in March 2005, our Board of Directors has not received any such shareholder nominations and, as a result, has not considered it necessary to develop separate formal procedures for the submission and review of nominations by shareholders. Shareholders may submit nominations to our Board of Directors by addressing a communication to the chair of the Nominating and Corporate Governance Committee and providing sufficient information to the committee to permit it to conduct an assessment of the qualifications of the proposed nominee, including biographical information about the candidate and his or her professional experience, confirmation of the candidate’s willingness to serve as a director, and complete contact information for the candidate and the nominating shareholder. As a matter of policy, our Nominating and Corporate Governance Committee is committed to giving due and fair consideration to proposed nominations submitted by our shareholders using the same criteria and processes as other nominations which come before the committee. To date, our Board of Directors has received no shareholder nominations for directors.
Shareholder Communications With Our Board of Directors
Shareholders may communicate with our Board of Directors or any of its directors by sending written communications addressed to Aspreva Pharmaceuticals Corporation at #1203-4464 Markham Street, Victoria, British Columbia, Canada, V8Z 7X8, Attention Vice President, Investor Relations and Corporate Communications. Any communication sent must state the number of our common shares owned by the shareholder making the communication. Our Vice President, Investor Relations and Corporate Communications will review each communication and will forward such communication to our Board of Directors, or to any individual director to whom the communication is addressed, unless the communication is unduly hostile, threatening or similarly inappropriate, in which case, our Vice President, Investor Relations and Corporate Communications shall discard the communication. All communications that relate to questionable accounting or auditing matters involving Aspreva should be addressed directly to the chair of our Audit Committee at the address noted above.
8
| Item 11. | | Executive Compensation. |
REPORT OF THE COMPENSATION COMMITTEE OF THE
BOARD OF DIRECTORS ON EXECUTIVE COMPENSATION(1)
The Compensation Committee of our Board of Directors is responsible for establishing the policies and programs that govern all of the compensation of our executive officers. The Compensation Committee is comprised of independent Directors, who are appointed by the Board. The Compensation Committee Charter, approved by the Board, clearly states the duties and responsibilities of the Compensation Committee. The Board evaluates and must approve all recommendations of the Compensation Committee relating to the compensation of our executive officers, in order for those recommendations to be implemented.
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis with management and based on its review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Amendment No. 1 due to its content accurately reflecting the philosophy of our compensation program and the compensation program in total as designed by the Compensation Committee.
Compensation Committee of the Board of Directors
Ronald M. Hunt
George M. Milne
Arnold L. Oronsky
| | |
| (1) | | The material in this report is not “soliciting material,” is not deemed filed with the SEC, and is not to be incorporated by reference into any Aspreva filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language contained in such filing. |
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
During 2006, Mr. Hunt , Dr. Milne and Dr. Oronsky served on our Compensation Committee. None of our executive officers serve as members of the Compensation Committee or Board of Directors of any entity that has an executive officer serving as a member of our Compensation Committee or Board of Directors.
COMPENSATION DISCUSSION AND ANALYSIS
The Compensation Committee of our Board of Directors, composed entirely of independent directors, administers our executive compensation program. The Compensation Committee oversees our compensation and benefit plans and policies, administers our stock plans and reviews and recommends to the Board of Directors all compensation decisions relating to our executive officers. Our Chief Executive Officer, Chief Financial Officer and our other three most highly compensated executive officers who earn at least $100,000 of total compensation in a particular year are referred to in this Amendment No. 1 as our named executive officers.
Compensation Philosophy
Aspreva’s general compensation philosophy with respect to executive officers is to provide compensation that:
| | • | | attracts and retains high caliber executive officers by offering competitive base salaries and benefits programs; |
| |
| | • | | motivates executive officers to meet high performance standards through annual and long term incentive programs; and |
| |
| | • | | aligns the interests of our executive officers with our intermediate and long-term objectives, and the long-term interests of our shareholders, through stock option grants. |
Setting Executive Compensation
The Compensation Committee oversees a compensation arrangement for our executive officers designed to support our business strategy and to attract and retain the key talent necessary to achieve our business objectives. The Compensation Committee reviews the linkage between corporate and individual performance and recommends salary adjustments, annual cash bonuses and stock option grants pursuant to the Aspreva 2002 Incentive Stock Option Plan.
The Compensation Committee endeavours to position executive compensation between the 60th and the 75th percentile of the range of compensation levels for comparable specialty pharmaceutical, biopharmaceutical and biotechnology companies; subject in all cases to the performance of each individual executive officer. The Compensation Committee also utilizes data from independent external compensation surveys which are used to provide comparative benchmark compensation data. Such comparative companies have historically been other North American companies at a similar stage of development.
During 2006, management used the Radford Biotechnology — Executive Survey, a widely-used independent published survey, to benchmark executive officer compensation. Management also engaged Towers Perrin, a compensation consultant, to research and assess our overall employee as well as executive compensation against their proprietary database of companies. Towers Perrin was engaged to provide consultative services on our compensation mix, long term incentive plans, dilution management and possible modifications to our benefit plans. The Compensation Committee did not separately engage any compensation consultant. This research and the continuing use of independent survey data will continue to guide the committee’s determination of appropriate compensation as Aspreva continues to evolve.
For 2006 and 2007, the Radford survey data was used to provide focused data on salary, bonus and option levels. The Radford data is drawn from a largely U.S.-based group of over 200 companies, operating in the biotechnology, pharmaceutical and specialty pharmaceutical sectors. The data is subdivided into groups based on number of employees. Management and the Compensation Committee determined the appropriate subgroup based on employee numbers and revenue. This subgroup contains over 50 companies, including companies such as Pharmion Corporation and QLT Inc. This peer group data will be reviewed annually to ensure it remains appropriate to the size, revenue, employee base, stage of evolution and complexity of Aspreva’s business model and performance. As Aspreva’s executive team is primarily based in Canada, the data from the Radford survey and Towers Perrin is used on a nominal basis to provide guidance towards compensation levels for such executives.
9
The compensation targets for each executive officer is the 60th to 75th percentile in salary and annual bonus, and the 60th percentile for stock option awards. Individual performance is considered when determining actual changes to salary, the proposed cash bonus and any stock option awarded. The Compensation Committee also receives documentary support on individual executive officer performance. The Chief Executive Officer conducts reviews of the executive team, evaluates the performance of executive officers on an annual basis and makes recommendations to the Compensation Committee with respect to annual salary adjustments, bonuses and stock option grants. The Compensation Committee collectively considers the performance of Aspreva, the individual performance of each executive officer, the current compensation of the executive officer and the relevant benchmark data to ensure appropriate market alignment. The Compensation Committee exercises its own discretion in determining salary adjustments and discretionary cash and equity-based awards for all executive officers. Mr. Glickman, our Chairman of the Board and Chief Executive Officer is recused from the meeting prior to any discussion or approval of his individual compensation.
The Compensation Committee considers each executive officer separately and its determinations reflect a number of considerations, including general participation in the leadership, operation and oversight of Aspreva as a whole; the work conducted to deliver on specific objectives (both personal and corporate) and the success of those objectives in addressing the overall goals set with the Board of Directors annually. Where an individual executive officer has taken on additional or changed responsibilities, these are reflected in their position descriptions and if significant, their title and relevant benchmark. The Compensation Committee has not considered actual share ownership in its compensation decisions as no executive officer beneficially owns greater than 5% of our common shares.
Elements of Annual Compensation
Executive officer compensation consists of three main components: base salary, annual cash bonuses and long-term incentive compensation in the form of stock options. The Compensation Committee does not have any formal policies or guidelines for allocating compensation between these components. The Compensation Committee has used market driven benchmark data to assist it in setting the balance and the weighting varies year to year. The actual balance is driven by the external benchmark data, which in turn reflects the changing nature of the market for the personnel we seek to attract and retain. For 2006, the relative emphasis of these three main components for the named executive officers was approximately 40% base salary, 10% annual cash incentive and 50% stock options, calculated using salary, bonus and stock option expensing values as shown in the “Summary Compensation Table” on a full year basis. Our goal is to provide a competitive compensation package that provides a balance between base salary, bonuses based on work towards and delivery of specified short term objectives and longer term incentives that support the on-going growth and evolution of Aspreva.
During 2006, the balance between salary, bonus and stock options changed slightly from 2005, in part due to new-hire stock option grants to new executive officers, which are larger than annual grants and bonuses for 2006 being smaller in scale than 2005 bonuses, due to the partial completion of some corporate goals and changing benchmark information. For 2006, the Compensation Committee determined that although a number of targeted corporate objectives were met, some were not fully achieved. As a result, cash bonuses were reduced. Other than this adjustment, comparative benchmarks and personal performance had the most significant impact on executive compensation in 2006.
Determinants of Executive Compensation
Base Salary
In determining the base salary for each of our executive officers the Compensation Committee considers the officer’s experience, position within Aspreva and performance in the past year. The Compensation Committee also reviewed independently published survey data from Radford and Towers Perrin of base salaries for executive officers with comparative companies. The Compensation Committee does not apply specific formulas to determine annual increases.
Most of our executive officers are paid in Canadian dollars and for the purposes hereof such amounts have been translated into United States dollars. Please see the section entitled “Currency”. The base salaries of each of our named executive officers for 2006 are presented in the “Summary Compensation Table” below. For 2006:
10
| • | | Mr. Glickman our Chief Executive Officer received a salary increase of 3.5% based on market and individual performance factors; |
| |
| • | | Mr. Hall our President received a salary increase of 2.2% based on market and individual performance factors; |
| |
| • | | Mr. Cousins our Executive Vice-President and Chief Finance Officer received a salary increase of 15% based on his appointment as Executive Vice President as well as market and individual performance factors; |
| |
| • | | Dr. Jones received a salary increase of 8.2% based on his change of role into the clinical and regulatory function, his promotion to Chief Scientific Officer as well as market and individual performance factors; and |
| |
| • | | Mr. Goulburn received a salary increase of 8.2% based on his promotion to Executive Vice President of Pharmaceutical Operations and market and individual performance factors. |
In 2007, our named executive officers’ base salaries were increased from 2006 levels in the range of 2.2% to 15%, with a weighted average of 6.6%. The increase in base salaries in 2006 and 2007 reflected the continuing maturation of Aspreva with the focus remaining on the attraction of new personnel and retention of outstanding executive officers through evolving roles. We believe the adjusted base salaries are consistent with those provided by comparable specialty pharmaceutical, biopharmaceutical and biotechnology companies of similar size and stage of development and operations.
Annual Cash Bonus
2006
During 2006, the employment agreements with each of our executive officers provided for annual cash bonuses ranging between 30% to 50% of the respective executive officer’s annual base salary, as determined by our Board of Directors. Please see the section below entitled “Employment Agreements” for a description of such agreements. The Compensation Committee reviews annually: (i) the annual corporate objectives of Aspreva, which in general include operating, business development and clinical and product development goals; and (ii) the general personal objectives of each executive officer as proposed by our Chief Executive Officer. The Compensation Committee undertakes a review to establish the extent to which such objectives have been met for the purpose of determining each executive officer’s annual cash bonus to recommended to the Board of Directors for approval. In reviewing the performance of each named executive officer, the Compensation Committee also considers each executive officer’s level of leadership, teamwork and general participation in the development of individuals responsible to the executive officer. The potential annual cash bonus that may be granted to our executive officers was weighted 40% on the achievement of corporate objectives and 60% on the achievement of individual objectives.
For 2006, our primary corporate objectives included:
| | • | | complete one asset acquisition transaction consistent with our corporate strategy relating to indication partnering; |
| |
| | • | | advance clinical trial programs to milestones and within budget; |
| |
| | • | | successfully deliver global medical affairs programs to plan and within budget; |
| |
| | • | | build-out innovative commercial capability, proactively supporting business development initiatives; |
| |
| | • | | instill and maintain Sarbanes-Oxley Act of 2002 compliancy throughout Aspreva; and |
| |
| | • | | strengthen human capital programs and services. |
For 2006, individual objectives generally included, but were not limited to:
11
| | • | | completing a transaction which supports our business model and aligns to Aspreva’s overall business strategy; |
| |
| | • | | delivery of core functional initiatives and programs; |
| |
| | • | | further enhancing and evolving our capabilities to assess, develop and commercialize new assets; and |
| |
| | • | | managing Aspreva to meet and exceed any compliance expectations, to deliver results, build shareholder value and within budgets. |
All of the corporate objectives set forth above were viewed by the executive team and the Board of Directors as challenging and only deliverable if Aspreva was extremely focused. These corporate objectives were viewed as providing the basis for further corporate growth, while establishing the appropriate infrastructure to allow Aspreva to secure and effectively develop new product candidates for development.
For 2006, each executive officer provided a review of their achievements to the Chief Executive Officer who presented the data and his evaluation of each executive officer to the Compensation Committee. The Compensation Committee recommended, and the Board of Directors approved, cash bonuses to our named executive officers in the range of 50% to 84% of their maximum allowable bonus, with an average of 64% of their maximum allowable bonus. With respect to the achievement of corporate objectives the Compensation Committee determined that such goals had been achieved at the 60% level. In reaching this determination the Compensation Committee considered that although a corporate transaction consistent with our corporate strategy was not achieved during 2006, the executive officers had actively evaluated and diligently pursued potential transactions and had acted in the best interests of Aspreva and our stockholders in such evaluations. In addition, the Compensation Committee determined that a number of other corporate objectives had also been significantly advanced, thereby warranting a partial award. The overall bonus levels were as follows: Mr. Glickman — 60%; Mr. Hall — 50%; Mr. Cousins — 84%; Mr. Goulburn — 80%; and Dr. Jones - 64%. These bonuses were paid in February 2007 and are reflected in the “Summary Compensation Table” below.
2007
For 2007, we have determined that the payment of any bonus will have a “double trigger”: (i) the completion of a transaction consistent with our corporate strategy, as well as then (ii) performance against their other key objectives for 2007. The latter will drive the actual amount of any individual bonus. The objectives for 2007 are consistent with and expand upon the primary objectives for 2006. In essence, they focus on securing additional product candidates for development and commercialization, functioning in a compliant and ethical manner; the execution of our financial transactions, conducting clinical trials and regulatory submissions (and ultimately our commercial activities when appropriate) in a professional, effective and efficient manner. Our corporate objectives for 2007 are aligned functionally to allow all employees to understand how they contribute to Aspreva’s progress and success.
All of our corporate objectives for 2007 have been designed with three key tenets:
| | • | | exceed strategic partner expectations; |
| |
| | • | | create long term shareholder value through sustainability and profitability; and |
| |
| | • | | operate in a fashion consistent with our values of being “beyond compliance.” |
Equity Awards
A portion of our executive compensation is designed to align the interests of our executive officers with our intermediate and long-term objectives, and the long-term interests of our shareholders. Stock options are an integral part of achieving these objectives as they provide our executive officers with the opportunity to participate in the growth and development of Aspreva. Stock options are awarded to our executive officers at the commencement of
12
their employment and annually based on their performance and the achievement of corporate and personal objectives. Stock options are also awarded from time to time if deemed necessary based on our regular assessments of the comparative benchmark compensation data for executive officers. The stock option grants to each of our named executive officers in 2006 are presented in the table below entitled “Grants of Plan-Based Awards in 2006.” The Compensation Committee determined that such awards in 2006 were consistent with comparable benchmark data and presented their recommendations relating to all the executive officers to the Board of Directors for approval.
The Board of Directors has not delegated the authority to grant stock options to any executive officer or a committee comprised of executive officers. The Board of Directors has delegated the authority to the Compensation Committee to grant stock options to employees other than executive officers. The Board of Directors approves all grants to executive officers upon recommendation from the Compensation Committee. Each stock option is granted with an exercise price in Canadian dollars equal to the closing price of our common shares for the trading session ending immediately prior to the time of grant, as reported on the Toronto Stock Exchange and for the purposes hereof has been translated into United States dollars. Our current practice is not to grant stock option awards to executive officers and annual awards to our employees at times where Aspreva is in possession of material non-disclosed information.
The Compensation Committee continues to monitor the competitive landscape and recognizes that alternative equity vehicles are becoming more widely used. Consequently, the Compensation Committee has approved the amendment of our 2002 Incentive Stock Option Plan to allow for the issuance of tandem stock appreciation rights, restricted stock units and deferred stock units.
Severance and Change in Control Arrangements
We have entered into agreements with our executive officers providing severance and change in control benefits, the terms of which are described below under “Severance and Change in Control Agreements.” We believe these severance and change in control benefits are an essential element of our overall executive compensation package and assist us in recruiting and retaining talented individuals and aligning the executive officer’s interests with the interests of our stockholders.
Change in Control Arrangements
With respect to change of control benefits, Aspreva provides severance compensation if an executive officer is terminated in connection with a change of control transaction. These change of control benefits that are structured on a “double-trigger” basis, meaning that before an executive officer can receive severance compensation: (i) a change of control must occur and (ii) within 12 months of such change of control, the executive officer’s employment must be terminated for good reason or without cause. These provisions were included to motivate our executive officers to act in the best interests of our stockholders by removing the distraction of post-change of control uncertainties faced by the executive officers with regard to their continued employment and compensation. We believe that double-trigger change of control severance compensation is attractive to maintain continuity and retention of key management personnel and is consistent with our compensation philosophy.
Severance Arrangements
We also believe that the other severance benefits are appropriate, particularly with respect to a termination by Aspreva without cause since in that scenario, both Aspreva and the executive officer have a mutually-agreed-upon severance package that is in place prior to any termination event which we believe provides Aspreva with greater flexibility to make a change in executive management if such a change is in the stockholders’ best interests.
13
Benefit Programs
We provide customary employment benefits to all our employees, including our executive officers. Benefits are provided at Aspreva’s expense and include:
| | • | | medical and extended health care; |
| |
| | • | | dental care; |
| |
| | • | | vision care; |
| |
| | • | | employee life insurance; |
| |
| | • | | accidental death and dismemberment insurance; and |
| |
| | • | | short and long term disability insurance. |
Additional life and health insurance is provided to our Chief Executive Officer. We do not have a corporate sponsored pension plan.
For more recent executive officer new hires, sign-on bonuses and relocation support have been provided, as appropriate. In these cases, if the individual is terminated for cause or leaves without good reason, such amounts may be repaid based upon provisions that allow for repayment on a sliding scale over three years from date of employment.
Indemnification Agreements; D&O Liability Insurance
We have entered and expect to continue to enter into agreements to indemnify our directors and executive officers as determined by the Board of Directors. These agreements provide for indemnification for related expenses including attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers. We also maintain directors’ and officers’ liability insurance for the benefit of such persons.
Share Ownership Guidelines
While we believe equity ownership directly aligns the risk profile of our executive officers with that of the stockholders we have not at this time adopted any share ownership guidelines for our executive officers. Each of our Chief Executive Officer, President and Chief Financial Officer hold common shares that were purchased or awarded prior to our initial public offering in March 2005.
Accounting and Tax Consideration
Aspreva early adopted Financial Accounting Standards Board statement No. 123, “Share-Based Payment,” or SFAS 123 and we adopted the fair value provisions of Financial Accounting Standards Board statement No. 123R (revised 2004), “Share-Based Payment,” or SFAS 123(R) effective January 1, 2006. Under SFAS 123(R), Aspreva estimates and records an expense for each award for equity compensation (including stock options) over the vesting period of the award. The Compensation Committee has determined to retain the stock option plan for the foreseeable future and therefore to record this expense on an on-going basis according to SFAS 123(R). The Compensation Committee has approved the amendment of our 2002 Incentive Stock Option Plan to allow for the issuance of tandem stock appreciation rights, restricted stock units and deferred stock units, and will ensure the appropriate compliance to any and all accounting and tax rules that may apply.
The Compensation Committee has not yet established any policies for determining which if any forms of incentive compensation awarded to Aspreva’s U.S.-based executive officers shall be designed to qualify as “performance-based compensation” with respect to Section 162 (m) of the Internal Revenue Code of 1986. To maintain flexibility
14
in compensating our executive officers in a manner designed to promote Aspreva’s long term strategic goals, the Compensation Committee retains the right to evaluate the effects of compensation limits of Section 162 (m) on any compensation it proposes to grant in the future.
EXECUTIVE COMPENSATION
The following table shows for 2004, 2005 and 2006, compensation awarded or paid to, or earned by, our Chief Executive Officer, Chief Financial Officer and our other three most highly compensated executive officers in 2006 who received at least $100,000 of total compensation. We refer to such persons elsewhere in this Amendment No. 1 as our named executive officers.
Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Stock | | Option | | All Other | | |
| Name and Principal | | | | | | Salary | | Bonus | | Awards | | Awards | | Compensation | | |
| Position | | Year | | ($) | | ($) | | ($)(2) | | ($)(3) | | ($) | | Total ($) |
Richard M.
| | | 2006 | | | $ | 463,000 | (1) | | $ | 139,000 | (1) | | | — | | | $ | 351,000 | | | $ | 24,000 | (4) | | $ | 977,000 | |
Glickman, Chief
| | | 2005 | | | | 373,000 | (1) | | | 136,000 | (1) | | | — | | | | 139,000 | | | | 16,000 | | | | 664,000 | |
Executive Officer
| | | 2004 | | | | 279,000 | (1) | | | 295,000 | (1) | | | — | | | | — | | | | 7,000 | | | | 581,000 | |
| and Chairman | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Noel F. Hall,
| | | 2006 | | | | 362,000 | (1) | | | 90,000 | (1) | | | — | | | | 265,000 | | | | 4,000 | | | | 721,000 | |
| President | | | 2005 | | | | 334,000 | (1) | | | 85,000 | (1) | | | — | | | | 116,000 | | | | 3,000 | | | | 538,000 | |
| | | | 2004 | | | | 272,000 | (1) | | | 277,000 | (1) | | | — | | | | — | | | | 3,000 | | | | 552,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Bruce G.
| | | 2006 | | | | 274,000 | (1) | | | 80,000 | (1) | | $ | 113,000 | | | | 378,000 | | | | 4,000 | | | | 849,000 | |
Cousins, C.A.,
| | | 2005 | | | | 223,000 | (1) | | | 81,000 | (1) | | | 113,000 | | | | 266,000 | | | | 4,000 | | | | 687,000 | |
Executive Vice
| | | 2004 | | | | 121,000 | (1) | | | 98,000 | (1) | | | 94,000 | | | | 304,000 | | | | 2,000 | | | | 619,000 | |
President and Chief
Financial Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Charles F.
| | | 2006 | | | | 268,000 | | | | 75,000 | | | | — | | | | 635,000 | | | | 24,000 | (7) | | | 1,002,000 | |
Goulburn,
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Executive Vice
| | | 2004 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
President, Global
Pharmaceutical
Operations(5) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Richard Jones,
| | | 2006 | | | | 218,000 | (1) | | | 43,000 | (1) | | | — | | | | 525,000 | | | | 59,000 | (8) | | | 845,000 | |
D.M., MRCP,
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Chief Scientific
| | | 2004 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Officer(6) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Compensation is paid in Canadian dollars and, for the purposes hereof, has been translated into United States dollars in accordance with the accounting policies described in our audited consolidated financial statements. Such translation uses the annual average noon buying rate published by the Federal Reserve Bank of New York for 2006, being US$1.00 = C$1.1340, for 2005, being US$1.00 = C$1.2115, and for 2004, being US$1.00 = C$1.3273. |
| |
| (2) | | Valuation based on the dollar amount of stock awards recognized for financial statements reporting purposes pursuant to SFAS 123(R) with respect to the applicable year. The assumptions made in the valuation of the stock awards are discussed in footnote 9, “Common Shares” to Aspreva’s consolidated financial statement contained in Aspreva’s Annual Report on Form 10-K for the year ended December 31, 2006 and includes amounts from stock awards granted in and prior to the applicable year. |
15
| | |
| (3) | | Valuation based on the dollar amount of option grants recognized for financial statement reporting purposes pursuant to SFAS 123(R) with respect to 2006 and thus includes amount from awards granted in and prior to the applicable year. The assumptions made in the valuation of the options are discussed in footnote 9, “Common Shares” to Aspreva’s consolidated financial statements contained in Aspreva’s Annual Report on Form 10-K for the year ended December 31, 2006. |
| |
| (4) | | This amount includes $14,000 for personal travel. |
| |
| (5) | | Mr. Goulburn was appointed an executive officer in November 2006. |
| |
| (6) | | Dr. Jones joined Aspreva in March 2006. |
| |
| (7) | | This amount includes $20,000 for medical benefits. |
| |
| (8) | | This amount includes $43,000 for relocation expenses and $13,000 for a signing bonus. |
16
Grants of Plan-Based Awards
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Grant Date |
| | | | | | | All Other Option | | Exercise or | | Fair Value of |
| | | | | | | Awards: Number of | | Base Price of | | Stock and |
| | | | | | | Securities Underlying | | Option | | Option |
| | | | | | | Options | | Awards | | Awards |
| Name | | Grant Date | | (#) | | ($/Sh)(1) | | $(2) |
| Richard M. Glickman | | | 2/15/06 | | | | 35,000 | (3) | | $ | 24.02 | | | $ | 508,000 | |
| | | | | | | | | | | | | | | | | |
| Noel F. Hall | | | 2/15/06 | | | | 25,000 | (3) | | | 24.02 | | | | 363,000 | |
| | | | | | | | | | | | | | | | | |
| Bruce G. Cousins, C.A. | | | 2/15/06 | | | | 15,000 | (3) | | | 24.02 | | | | 218,000 | |
| | | | | | | | | | | | | | | | | |
| Charles F. Goulburn | | | 2/15/06 | | | | 15,000 | (3) | | | 24.02 | | | | 218,000 | |
| | | | | | | | | | | | | | | | | |
| Richard Jones, D.M., MRCP | | | 3/24/06 | | | | 100,000 | (4) | | | 27.01 | | | | 1,616,000 | |
| | |
| (1) | | Each stock option was granted with an exercise price in Canadian dollars equal to the closing price of our common shares on the date of grant, as reported on the Toronto Stock Exchange and for the purposes hereof has been translated into United States dollars. See “Currency”. |
| |
| (2) | | The dollar value shown for a stock option is based on the fair value as of the grant date. The assumptions made in the valuation of the option are discussed in footnote 9, “Common Shares” to Aspreva’s consolidated financial statements contained in Aspreva’s Annual Report on Form 10-K for the year ended December 31, 2006. |
| |
| (3) | | Option granted under the Aspreva 2002 Incentive Stock Option Plan. Options vest in equal monthly amounts over a 4-year period. |
| |
| (4) | | Option granted under the Aspreva 2002 Incentive Stock Option Plan. Options vest in equal monthly amounts over a 3-year period, commencing on the 1 year anniversary of the date of grant. |
17
Outstanding Equity Awards at Fiscal Year End
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Option Awards | | Stock Awards |
| | | Number | | | | | | | | | | | | | | |
| | | of | | Number of | | | | | | | | | | | | |
| | | Securities | | Securities | | | | | | | | | | Number of | | Market Value |
| | | Underlying | | Underlying | | | | | | | | | | Shares or | | of Shares or |
| | | Unexercised | | Unexercised | | Option | | | | | | Units of Stock | | Units of Stock |
| | | Options | | Options | | Exercise | | Option | | That Have | | That Have |
| | | (#) | | (#) | | Price | | Expiration | | Not Vested | | Not Vested |
| Name | | Exercisable | | Unexercisable | | ($)(1) | | Date | | (#) | | ($)(5) |
| |
| Richard M. Glickman | | | 8,021 | | | | 26,979 | (2) | | $ | 24.02 | | | | 2/14/16 | | | | — | | | | — | |
| | | | 20,854 | | | | 14,896 | (3) | | | 14.98 | | | | 4/19/15 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Noel F. Hall | | | 5,729 | | | | 19,271 | (2) | | | 24.02 | | | | 2/14/16 | | | | — | | | | — | |
| | | | 17,500 | | | | 12,500 | (3) | | | 14.98 | | | | 4/19/15 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Bruce G. Cousins, C.A. | | | 3,161 | | | | 11,563 | (2) | | | 24.02 | | | | 2/14/16 | | | | 1,427 | | | $ | 29,296 | |
| | | | 11,496 | | | | 33,333 | (2) | | | 15.20 | | | | 9/19/15 | | | | — | | | | — | |
| | | | 3,972 | | | | 5,417 | (3) | | | 14.98 | | | | 4/19/15 | | | | — | | | | — | |
| | | | 18,233 | | | | — | (3) | | | 4.88 | | | | 1/23/14 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Charles F. Goulburn | | | 3,437 | | | | 11,563 | (2) | | | 24.02 | | | | 2/14/16 | | | | — | | | | — | |
| | | | 14,275 | | | | 82,028 | (4) | | | 4.88 | | | | 12/9/14 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Richard Jones, D.M., MRCP | | | — | | | | 100,000 | (4) | | | 27.01 | | | | 3/23/16 | | | | — | | | | — | |
| | |
| (1) | | Each stock option was granted with an exercise price in Canadian dollars equal to the closing price of our common shares on the date of grant, as reported on the Toronto Stock Exchange. Stock options granted prior to Aspreva’s initial public offering in March 2005 have an exercise price equal to the fair market value of our common shares as determined by the Compensation Committee. For the purposes hereof the exercise price has been translated into United States dollars. See “Currency”. |
| |
| (2) | | Option granted under the Aspreva 2002 Incentive Stock Option Plan. Options vest in equal monthly amounts over a 4-year period. |
| |
| (3) | | Option granted under the Aspreva 2002 Incentive Stock Option Plan. Options vest in equal monthly amounts over a 3-year period. |
| |
| (4) | | Option granted under the Aspreva 2002 Incentive Stock Option Plan. Options vest in equal monthly amounts over a 3-year period, commencing on the 1 year anniversary of the date of grant. |
| |
| (5) | | Calculated by multiplying the closing market price of Aspreva’s common shares on December 31, 2006 ($20.53) as reported on the Toronto Stock Exchange by the number of shares of restricted common shares that have not vested. |
18
Option Exercises and Stock Vested
| | | | | | | | | | | | | | | | | |
| | | Option Awards | | Stock Awards |
| | | Number of | | | | | | Number of | | |
| | | Shares | | | | | | Shares | | Value |
| | | Acquired | | Value Realized | | Acquired on | | Realized |
| | | on Exercise | | on Exercise | | Vesting | | on Vesting |
| Name | | (#) | | ($)(1)(2) | | (#) | | ($)(2)(3) |
| |
| Richard M. Glickman | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Noel F. Hall | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Bruce G. Cousins, C.A. | | | 79,225 | | | $ | 1,558,000 | | | | 8,560 | | | $ | 210,000 | |
| | | | | | | | | | | | | | | | | |
| Charles F. Goulburn | | | 32,097 | | | | 630,000 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Richard Jones, D.M., MRCP | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | Calculated by determining the difference between the closing price of our common shares on the date of exercise as reported on the Toronto Stock Exchange and the exercise price of the options. |
| |
| (2) | | Options are exercised in Canadian dollars and, for the purposes hereof, values have been translated into United States dollars in accordance with the accounting policies described in our audited consolidated financial statements. See “Currency”. |
| |
| (3) | | Calculated by multiplying, in each vesting period, the number of shares vested by the closing price of our common shares on the vesting date, as reported on the Toronto Stock Exchange. |
19
Pension Benefits
Our named executive officers did not participate in, or otherwise receive any benefits under, any pension or retirement plan sponsored by us during the year ended December 31, 2006.
Nonqualified Deferred Compensation
Our named executive officers did not earn any nonqualified compensation benefits from us during the year ended December 31, 2006.
COMPENSATION OF DIRECTORS
In 2006, each of our non-employee directors was eligible to receive the following compensation:
| | • | | Lead Director receives an annual retainer of $30,000; |
| |
| | • | | each of the other non-employee director receives an annual retainer of $20,000; |
| |
| | • | | each non-employee director receives $1,500 for each board meeting attended in person ($750 for meetings attended by video or telephone conference); |
| |
| | • | | the chair of our Audit Committee receives an additional annual retainer of $10,000, and each of the chairs of our Compensation Committee and Nominating and Corporate Governance Committee receives an additional annual retainer of $5,000; |
| |
| | • | | each member of such committees receives $1,000 per committee meeting attended in person ($500 for meetings attended by video or telephone conference); |
| |
| | • | | each non-employee director, upon their election to our board, receives an initial stock option grant to purchase 30,000 of our common shares pursuant to the Plan, as well as annual stock option grants to purchase 10,000 common shares pursuant to the Plan. These stock options vest in equal monthly amounts for 24 months and 12 months, respectively, from the date of grant. |
All of our directors are reimbursed for out-of-pocket expenses incurred in attending board and committee meetings in accordance with company policy.
For services rendered to us in 2006, our non-employee directors earned aggregate cash total compensation of $233,000 and each received stock options to purchase 90,000 common shares. The following table outlines the compensation paid to our non-employee directors for services rendered to us in 2006.
20
Director Compensation
| | | | | | | | | | | | | |
| | | Fees Earned or | | Option | | |
| | | Paid in | | Awards | | |
| | | Cash | | ($) | | Total |
| Name | | ($)(1) | | (2)(3)(4) | | ($) |
| |
| Kirk K. Calhoun, C.P.A. | | $ | 38,000 | | | $ | 236,000 | | | $ | 274,000 | |
| | | | | | | | | | | | | |
| Ronald M. Hunt | | | 37,000 | | | | 191,000 | | | | 228,000 | |
| | | | | | | | | | | | | |
| Julia G. Levy, Ph.D. | | | 26,000 | | | | 315,000 | | | | 341,000 | |
| | | | | | | | | | | | | |
| R. Hector MacKay-Dunn, Q.C. | | | 40,000 | | | | 206,000 | | | | 246,000 | |
| | | | | | | | | | | | | |
| George M. Milne, Ph.D. | | | 39,000 | | | | 235,000 | | | | 274,000 | |
| | | | | | | | | | | | | |
| Arnold L. Oronsky, Ph.D. | | | 31,000 | | | | 191,000 | | | | 222,000 | |
| | | | | | | | | | | | | |
| William Hunter, M.D. | | | 15,000 | | | | 197,000 | | | | 212,000 | |
| (1) | | Reflects retainer fees, meeting fees and committee chair fees. |
| |
| (2) | | Valuation based on the dollar amount of stock options recognized for financial statement reporting purposes pursuant to SFAS 123(R) during 2006. The assumptions made in the valuation of the options are discussed in footnote 9, “Common Shares” to Aspreva’s consolidated financial statements contained in Aspreva’s Annual Report on Form 10-K for the year ended December 31, 2006. Each stock option was granted with an exercise price in Canadian dollars equal to the closing price of our common shares on the date of grant, as reported on the Toronto Stock Exchange, and for the purposes hereof has been translated into United States dollars. See “Currency”. |
| |
| (3) | | At December 31, 2006 Aspreva’s non-employee directors each held an aggregate number of stock options as follows: Mr. Calhoun, 54,353 shares; Mr. Hunt, 21,666 shares; Dr. Levy, 31,883 shares; Mr. MacKay-Dunn, 24,166 shares; Dr. Milne, 54,353 shares; Dr. Oronsky, 21,666 shares; and Dr. Hunter, 30,000 shares. |
| |
| (4) | | The grant date fair value of each stock option granted to each director in fiscal 2006 is $181,000, with the exception of Dr. Hunter, whose grant date value fair value is $482,000. The assumptions made in the valuation of the options are discussed in footnote 9 — “Common Shares” to Aspreva’s consolidated financial statements contained in Aspreva’s Annual Report on Form 10-K for the year ended December 31, 2006. |
EMPLOYMENT AGREEMENTS
The following provides information regarding employment contracts with our named executive officers.
Richard M. Glickman
In January 2002, we entered into an employment agreement with Mr. Glickman, our Chairman and Chief Executive Officer. Mr. Glickman currently receives an annual base salary of $479,000, subject to increases at the discretion of our Board of Directors. In 2007, Mr. Glickman is also eligible for a discretionary performance bonus of up to 50% of his annual base salary as determined by our Board of Directors.
Noel F. Hall
In January 2002, we entered into an employment agreement with Mr. Hall, our President. Mr. Hall currently receives an annual base salary of $370,000, subject to increases at the discretion of our Board of Directors. In 2007,
21
Mr. Hall is also eligible for a discretionary performance bonus of up to 50% of his annual base salary as determined by our Board of Directors.
Bruce G. Cousins
In January 2004, we entered into an employment agreement with Mr. Cousins, our Executive Vice President and Chief Financial Officer. The agreement is effective as of March 2004, continues for three years, and is renewable thereafter by mutual written agreement by the parties for successive one year terms. Mr. Cousins currently receives an annual base salary of $314,000, subject to increases at the discretion of our Board of Directors. In 2007, Mr. Cousins is also eligible for a discretionary performance bonus of up to 40% of his annual base salary as determined by our Board of Directors.
Richard Jones
In November 2005, we entered into an employment agreement with Dr. Jones, as a Senior Vice President. The employment agreement continues for four years, and is renewable thereafter by mutual written agreement of the parties for successive one year terms. Pursuant to the employment agreement, Dr. Jones received a signing bonus of $13,000 and was reimbursed $43,000 for relocation expenses. Dr. Jones currently receives an annual base salary of $277,000, subject to increases at the discretion of our Board of Directors. In 2007, Dr. Jones is also eligible for a discretionary performance bonus of up to 35% of his annual base salary as determined by our Board of Directors. Under the agreement, Dr. Jones was granted a stock option to purchase 100,000 common shares at an exercise price of $27.01 per share. As of March 1, 2007, Dr Jones was appointed as our Chief Scientific Officer.
Charles F. Goulburn
In September 2004, we entered into an employment agreement with Mr. Goulburn effective as of October 18, 2004. Mr. Goulburn currently receives an annual base salary of $290,000, subject to increases at the discretion of our Board of Directors. In 2007, Mr. Goulburn is also eligible for a discretionary performance bonus of up to 40% of his annual base salary as determined by our Board of Directors.
SEVERANCE AND CHANGE OF CONTROL AGREEMENTS
The following provides information regarding termination of employment and change of control arrangements with our named executive officers.
Richard M. Glickman
Under Mr. Glickman’s employment agreement, either we or Mr. Glickman may terminate his employment at any time. If we terminate Mr. Glickman’s employment without cause; prior to January 2008, we are obligated to pay to him, depending upon the year of employment in which he is terminated, a lump sum of up to 12 months of his then current base salary. After January 2008, we are obligated to pay to him, depending upon the year of employment in which he is terminated, a lump sum of up to 18 months of his then current base salary, plus such other sums owed for arrears of salary, vacation pay and any performance bonus. We are also obligated to pay for the maintenance of Mr. Glickman’s benefits, prior to January 2008 for a period of up to 12 months, and after January 2008 for a period of up to 18 months. If Mr. Glickman obtains a new source of remuneration for personal services, the payment of benefits will cease six months from the date of termination of his employment, excluding the notice period.
In January 2002, we entered into a change of control agreement with Mr. Glickman. If within 12 months following a change of control of Aspreva, Mr. Glickman terminates his employment for good reason, or we terminate his employment other than for cause, we are obligated to pay to Mr. Glickman a lump sum equal to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. In such case, we are also obligated to maintain Mr. Glickman’s benefits for the 12 month period and his unvested stock options will immediately vest.
22
The following table describes the potential payments to Mr. Glickman upon his termination without good cause, or resignation for good reason, if applicable, both in connection with a change of control and not in connection with a change of control, as if such termination or resignation had occurred on December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Change of Control | | No Change of Control |
| | | | | | | Equity | | | | | | | | | | Equity | | |
| Name | | Salary(1) | | Acceleration (2) | | Benefits(3) | | Salary(1) | | Acceleration | | Benefits(4) |
| Richard M. Glickman | | $ | 457,000 | | | $ | 213,000 | | | $ | 9,000 | | | $ | 457,000 | | | $ | 0 | | | $ | 9,000 | |
| | |
| (1) | | Represents 12 months of continued salary. |
| |
| (2) | | Calculated based on a change of control taking place as of December 31, 2006 and assuming a price per share of $20.93, which is the closing price of our common shares as reported on the Toronto Stock Exchange on December 31, 2006. This represents the full acceleration of unvested stock options held by Mr. Glickman. |
| |
| (3) | | Represents 12 months of health benefits. |
| |
| (4) | | Represents 12 months of health benefits. |
Noel F. Hall
Under Mr. Hall’s employment agreement, either we or Mr. Hall may terminate his employment at any time. If we terminate Mr. Hall’s employment without cause; prior to January 2008, we are obligated to pay to him, depending upon the year of employment in which he is terminated, a lump sum of up to 12 months of his then current base salary, after January 2008, we are obligated to pay to him, depending upon the year of employment in which he is terminated, a lump sum of up to 18 months of his then current base salary, plus such other sums owed for arrears of salary, vacation pay and any performance bonus. We are also obligated to pay for the maintenance of Mr. Hall’s benefits, prior to January 2008 for a period of up to 12 months, and after January 2008 for a period of up to 18 months. If Mr. Hall obtains a new source of remuneration for personal services, the payment of benefits will cease six months from the date of termination of his employment, excluding the notice period.
In January 2002, we entered into a change of control agreement with Mr. Hall. If within 12 months following a change of control of Aspreva, Mr. Hall terminates his employment for good reason, or we terminate his employment other than for cause, we are obligated to pay to Mr. Hall a lump sum equal to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. In such case, we are also obligated to maintain Mr. Hall’s benefits for the 12 month period and his unvested stock options will immediately vest.
The following table describes the potential payments to Mr. Hall upon his termination without good cause, or resignation for good reason, if applicable, both in connection with a change of control and not in connection with a change of control, as if such termination or resignation had occurred on December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Change of Control | | No Change of Control |
| | | | | | | Equity | | | | | | | | | | Equity | | |
| Name | | Salary(1) | | Acceleration (2) | | Benefits(3) | | Salary(1) | | Acceleration | | Benefits(4) |
| Noel F. Hall | | $ | 357,000 | | | $ | 178,000 | | | $ | 5,000 | | | $ | 357,000 | | | $ | 0 | | | $ | 5,000 | |
| | |
| (1) | | Represents 12 months of continued salary. |
23
| | |
| (2) | | Calculated based on a change of control taking place as of December 31, 2006 and assuming a price per share of $20.93, which is the closing price of our common shares as reported on the Toronto Stock Exchange on December 31, 2006. This represents the full acceleration of unvested stock options held by Mr. Hall. |
| |
| (3) | | Represents 12 months of health benefits. |
| |
| (4) | | Represents 12 months of health benefits. |
Bruce G. Cousins
Under Mr. Cousins’ employment agreement, either we or Mr. Cousins may terminate his employment at any time. If we terminate Mr. Cousins’ employment without cause; prior to March 2010, we are obligated to pay to him, depending upon the year of employment in which he is terminated, a lump sum of up to 12 months of his then current base salary, after March 2010, we are obligated to pay to him, depending upon the year of employment in which he is terminated, a lump sum of up to 18 months of his then current base salary, plus such other sums owed for arrears of salary, vacation pay and any performance bonus. We are also obligated to pay for the maintenance of Mr. Cousin’s benefits, for a period of 6 months following termination.
In January 2004, we entered into a change of control agreement with Mr. Cousins. If within 12 months following a change of control of Aspreva, Mr. Cousins terminates his employment for good reason, or we terminate his employment other than for cause, we are obligated to pay to Mr. Cousins a lump sum equal to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. In such case, we are also obligated to maintain Mr. Cousins’ benefits for the 12 month period and his unvested stock options will immediately vest.
The following table describes the potential payments to Mr. Cousins upon his termination without good cause, or resignation for good reason, if applicable, both in connection with a change of control and not in connection with a change of control, as if such termination or resignation had occurred on December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Change of Control | | No Change of Control |
| | | | | | | Equity | | | | | | | | | | Equity | | |
| Name | | Salary(1) | | Acceleration (2) | | Benefits(3) | | Salary(1) | | Acceleration | | Benefits(4) |
| Bruce G. Cousins | | $ | 270,000 | | | $ | 637,000 | | | $ | 5,000 | | | $ | 270,000 | | | $ | 0 | | | $ | 2,500 | |
| | |
| (1) | | Represents 12 months of continued salary. |
| |
| (2) | | Calculated based on a change of control taking place as of December 31, 2006 and assuming a price per share of $20.93, which is the closing price of our common shares as reported on the Toronto Stock Exchange on December 31, 2006. This represents the full acceleration of unvested stock options held by Mr. Cousins. |
| |
| (3) | | Represents 12 months of health benefits. |
| |
| (4) | | Represents 6 months of health benefits. |
24
Richard Jones
Under Dr. Jones’ employment agreement, either we or Dr. Jones may terminate his employment at any time. If we terminate Dr. Jones’ employment without cause, we are obligated to pay to Dr. Jones, depending on the year of employment in which he is terminated, a lump sum of up to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. We are also obligated to maintain Dr. Jones’ benefits for a period of six months following termination.
In November 2005, we entered into a change of control agreement with Dr. Jones, effective as of March 6, 2006. If within 12 months following a change of control of Aspreva, Dr. Jones terminates his employment for good reason, or we terminate his employment other than for cause, we are obligated to pay to Dr. Jones a lump sum equal to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. In such case, we are also obligated to maintain Dr. Jones’ benefits for the 12 month period and his unvested stock options will immediately vest.
The following table describes the potential payments to Dr. Jones upon his termination without good cause, or resignation for good reason, if applicable, both in connection with a change of control and not in connection with a change of control, as if such termination or resignation had occurred on December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Change of Control | | No Change of Control |
| | | | | | | Equity | | | | | | | | | | Equity | | |
| Name | | Salary(1) | | Acceleration (2) | | Benefits(3) | | Salary(4) | | Acceleration | | Benefits(5) |
| Richard Jones | | $ | 270,000 | | | $ | 0 | | | $ | 5,000 | | | $ | 135,000 | | | $ | 0 | | | $ | 2,000 | |
| | |
| (1) | | Represents 12 months of continued salary. |
| |
| (2) | | Calculated based on a change of control taking place as of December 31, 2006 and assuming a price per share of $20.93, which is the closing price of our common shares as reported on the Toronto Stock Exchange on December 31, 2006. This represents the full acceleration of unvested stock options held by Dr. Jones. |
| |
| (3) | | Represents 12 months of health benefits. |
| |
| (4) | | Represents 6 months of continued salary. |
| |
| (5) | | Represents 6 months of health benefits. |
Charles F. Goulburn
Under Mr. Goulburn’s employment agreement, either we or Mr. Goulburn may terminate his employment at any time. If we terminate Mr. Goulburn’s employment without cause, we are obligated to pay to Mr. Goulburn, depending on the year of employment in which he is terminated, a lump sum of up to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. We are also obligated to maintain Mr. Goulburn’s benefits for a period of six months following termination.
In October 2006, we entered into a change of control agreement with Mr. Goulburn, effective as of October 24, 2006. If within 12 months following a change of control of Aspreva, Mr. Goulburn terminates his employment for good reason, or we terminate his employment other than for cause, we are obligated to pay to Mr. Goulburn a lump sum equal to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. In such case, we are also obligated to maintain Mr. Goulburn’s benefits for the 12 month period and his unvested stock options will immediately vest.
25
The following table describes the potential payments to Mr. Goulburn upon his termination without good cause, or resignation for good reason, if applicable, both in connection with a change of control and not in connection with a change of control, as if such termination or resignation had occurred on December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Change of Control | | No Change of Control |
| | | | | | | Equity | | | | | | | | | | Equity | | |
| Name | | Salary(1) | | Acceleration (2) | | Benefits(3) | | Salary(4) | | Acceleration | | Benefits(5) |
| Charles Goulburn | | $ | 277,000 | | | $ | 1,546,000 | | | $ | 24,000 | | | $ | 185,000 | | | $ | 0 | | | $ | 12,000 | |
| | |
| (1) | | Represents 12 months of continued salary. |
| |
| (2) | | Calculated based on a change of control taking place as of December 31, 2006 and assuming a price per share of $20.93, which is the closing price of our common shares as reported on the Toronto Stock Exchange on December 31, 2006. This represents the full acceleration of unvested stock options held by Mr. Goulburn. |
| |
| (3) | | Represents 12 months of health benefits. |
| |
| (4) | | Represents 8 months of continued salary. |
| |
| (5) | | Represents 6 months of health benefits. |
26
| Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The following table sets forth information regarding beneficial ownership of our common shares as of March 31, 2007 by:
| | • | | each of our directors; |
| |
| | • | | each of our executive officers named in the Summary Compensation Table below; |
| |
| | • | | all of our directors and current executive officers as a group; and |
| |
| | • | | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common shares. |
Unless otherwise indicated, to our knowledge, each shareholder possesses sole voting and investment power over the shares listed, except for shares owned jointly with that person’s spouse.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Except as noted by footnote, and subject to community property laws where applicable, the persons named in the table below have sole voting and investment power with respect to all common shares shown as beneficially owned by them.
The table below lists the applicable percentage ownership based on 35,172,657 common shares outstanding as of March 31, 2007. Options to purchase our common shares that are exercisable within 60 days of March 31, 2007, are deemed to be beneficially owned by the persons holding these options for the purpose of computing percentage ownership of that person, but are not treated as outstanding for the purpose of computing any other person’s ownership percentage.
27
| | | | | | | | | |
| | | Beneficial Ownership | |
| | | Number of | | | | |
| Name and Address of Beneficial Owner | | Common Shares | | | Percent of Total | |
5% Shareholders | | | | | | | | |
| | | | | | | | | |
Hbm Bioventures (Cayman) Ltd.(1) | | | 3,199,154 | | | | 9.1 | % |
c/o HBM Bioventures Ltd.
Grabenstrasse 25
CH-6340 Baar, Switzerland | | | | | | | | |
| | | | | | | | | |
Entities And Persons Affiliated With The Sprout Group(2) | | | 2,937,049 | | | | 8.4 | |
11 Madison, Ave. 26th Floor
New York, New York 10010 | | | | | | | | |
| | | | | | | | | |
Entities Affiliated with InterWest Partners(3) | | | 2,000,000 | | | | 5.7 | |
2710 Sand Hill Road, Second Floor
Menlo Park, California 94025 | | | | | | | | |
| | | | | | | | | |
Executive Officers and Directors | | | | | | | | |
| | | | | | | | | |
Richard M. Glickman(4) | | | 1,250,936 | | | | 3.6 | |
Noel F. Hall(5) | | | 1,214,838 | | | | 3.5 | |
| | | | | | | | | |
Bruce G. Cousins, C.A.(6) | | | 48,003 | | | | * | |
| | | | | | | | | |
| Richard Jones, D.M. (Oxon), MRCP (UK) | | | — | | | | * | |
| | | | | | | | | |
Charles F. Goulburn(7) | | | 26,096 | | | | * | |
| | | | | | | | | |
Kirk K. Calhoun, C.P.A.(7) | | | 54,353 | | | | * | |
| | | | | | | | | |
Ronald M. Hunt(8) | | | 2,958,715 | | | | 8.4 | |
| | | | | | | | | |
William L. Hunter, M.D., Msc.(7) | | | 12,500 | | | | * | |
| | | | | | | | | |
Julia G. Levy, Ph.D.(7) | | | 31,883 | | | | * | |
| | | | | | | | | |
R. Hector MacKay-Dunn, Q.C.(7) | | | 24,166 | | | | * | |
| | | | | | | | | |
George M. Milne, Ph.D.(9) | | | 61,353 | | | | * | |
| | | | | | | | | |
Arnold L. Oronsky, Ph.D.(10) | | | 2,023,086 | | | | 5.8 | |
| | | | | | | | | |
| All Current executive officers and directors as a group (12 persons) | | | 7,705,929 | | | | 21.9 | |
Notes:
| | |
| * | | Represents beneficial ownership of less than one percent of our common shares. |
| |
| (1) | | Includes 535,228 shares held by HBM BioVentures (Barbados) Ltd. HBM BioVentures (Cayman) Ltd. is the indirect beneficial shareholder of HBM BioVentures (Barbados) Ltd. |
| |
| (2) | | Represents 2,880,215 shares held by Sprout Capital IX, L.P., 14,976 shares held by Sprout Entrepreneurs Funds, L.P., and 41,858 shares held by James Neidel. Mr. Neidel is a managing director of New Leaf Venture Partners, L.L.C., or New Leaf. New Leaf has entered into an agreement with an affiliate of Credit Suisse First Boston whereby New Leaf provides investment advisory services to the Sprout Group, a venture capital affiliate of Credit Suisse First Boston. The Sprout Group includes Sprout Capital IX, L.P. and Sprout Entrepreneurs Fund, L.P. collectively, the Sprout Funds. Mr. Neidel is a limited partner of a general partner of Sprout Capital |
28
| | |
| | | IX, L.P. The Sprout Funds disclaim beneficial ownership of the shares beneficially owned by Mr. Neidel and Ronald M. Hunt, as described in footnote 8. |
| |
| (3) | | Represents 1,336,025 shares held by InterWest Partners VII, L.P., 578,780 shares held by InterWest Partners VIII, L.P., 64,048 shares held by InterWest Investors VII, L.P., 16,537 shares held by InterWest Investors Q VIII, L.P. and 4,610 shares held by InterWest Investors VIII, L.P., collectively, the InterWest Funds. The InterWest Funds disclaim beneficial ownership of the shares beneficially owned by Arnold L. Oronsky, as described in footnote 10. |
| |
| (4) | | Represents 123,050 shares held by Richard M. Glickman, 73,831 shares held by the Glickman Family Trust, of which Terra Louise Carlson, the sister-in-law of Mr. Glickman, is the trustee, 1,018,292 shares held by Glickman Properties Ltd., a British Columbia company, and 35,763 shares issuable upon exercise of stock options within 60 days of March 31, 2007. Mr. Glickman is the sole shareholder of Glickman Properties Ltd. |
| |
| (5) | | Represents 7,419 held by Noel F. Hall, 7,419 held by Sandra MacPherson, 600,000 held by the Noel Francis Hall Spousal Trust, 600,000 held by the Sandra MacPherson Spousal Trust, and 28,645 shares issuable upon exercise of stock options within 60 days of March 31, 2007. |
| |
| (6) | | Represents 43,723 shares issuable upon exercise of stock options within 60 days of March 31, 2007 and 4,280 shares held by Mr. Cousins’ spouse, Carolyn Cousins. |
| |
| (7) | | Represents shares issuable upon exercise of stock options within 60 days of March 31, 2007. |
| |
| (8) | | Includes the shares listed in footnote 2. Also includes 21,666 shares issuable upon exercise of stock options within 60 days of March 31, 2007. Ronald M. Hunt is a managing director of New Leaf and a limited partner of a general partner of Sprout Capital IX, L.P. Mr. Hunt has shared voting and investment powers over the shares beneficially owned by the Sprout Funds. Mr. Hunt disclaims beneficial ownership of the shares beneficially owned by the Sprout Funds and James Neidel, except to the extent of his pecuniary interest therein. |
| |
| (9) | | Includes 7,000 shares held by George M. Milne and 54,353 shares issuable upon exercise of stock options within 60 days of March 31, 2007. |
| |
| (10) | | Includes the shares described in footnote 3. Also includes 1,420 shares held by Arnold L. Oronsky and 21,666 shares issuable upon exercise of stock options within 60 days of March 31, 2007. Arnold L. Oronsky is a managing director of InterWest Management Partners VII, L.L.C., the general partner of InterWest Partners VII, L.P. and InterWest Investors VII, L.P. Dr. Oronsky is also a managing director of InterWest Management Partners VIII, L.L.C., the general partner of InterWest Investors VIII, L.P., InterWest Investors Q VIII, L.P. and InterWest Partners VIII, L.P. Dr. Oronsky has shared voting and investment powers over the shares beneficially owned by the InterWest Funds. Dr. Oronsky disclaims beneficial ownership of the shares beneficially owned by the InterWest Funds, except to the extent of his pecuniary interest therein. |
29
Securities Authorized For Issuance Under Equity Compensation Plans
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2006:
| | | | | | | | | | | | | |
| | | | | | | | | | | Number of | |
| | | | | | | | | | | securities remaining | |
| | | | | | | | | | | available for future | |
| | | Number of | | | Weighted- | | | issuance under | |
| | | securities issuable | | | average exercise | | | equity compensation | |
| | | upon exercise of | | | price of | | | plans (excluding | |
| | | outstanding | | | outstanding | | | securities reflected | |
| | | options | | | options | | | in column a) | |
| Plan Category | | (a) | | | (b) | | | (c) | |
Equity compensation plans approved by securityholders(1) | | | 2,011,371 | | | $ | 15.61 | | | | 1,101,569 | |
| | | | | | | | | | | | | |
Equity compensation plans not approved by securityholders(2) | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Total | | | 2,011,371 | | | $ | 15.61 | | | | 1,101,569 | |
| | | | | | | | | | |
Notes:
| | |
| (1) | | Includes only the 2002 Aspreva Incentive Stock Option Plan. |
| |
| (2) | | No shares are currently available for issuance under the 2002 Aspreva Incentive Stock Purchase Plan Trust. To the extent that shares are returned, we may reissue such shares. |
2002 Aspreva Incentive Stock Purchase Plan Trust
Our 2002 Aspreva Incentive Stock Purchase Plan Trust, or the Trust, was created pursuant to an agreement, as amended and restated as of January 28, 2002, between us and Richard M. Glickman, our Chairman and Chief Executive Officer. The Trust has not been approved by our shareholders. The Trust was established for the benefit of our directors, officers, employees and consultants and those of our affiliated corporations for the purpose of distributing common shares held by the Trust to such persons. Mr. Glickman has been appointed trustee of the Trust. The agreement sets forth the terms and conditions under which the trustee will hold, manage, invest, reinvest, administer and distribute the assets of the Trust. In 2002, the Trust subscribed for 1,284,000 of our common shares, for total proceeds of $62.00. The trustee may, at any time and from time to time pay, transfer or distribute any capital or income of the Trust to or for the benefit of any one or more of the participants in such proportions and manner and as the trustee in his sole and unfettered discretion may determine, including without limitation in accordance with a request or direction delivered to the trustee from Aspreva from time to time. As of April 10, 2007, the trustee had distributed all 1,284,000 common shares to various participants.
In the event that the trustee resigns or is removed or a vacancy in the trusteeship arises for any reason, the trustee shall assign all assets of the Trust to its successor who shall be appointed by Aspreva. The trustee may elect to wind-up the Trust at any time, provided that the trustee shall wind-up the Trust no later than January 2082. The agreement may be amended or terminated at any time by an instrument in writing executed by us and the trustee, provided that no amendments shall authorize or permit any part of the Trust to be used for or diverted to purposes other than those provided for under the terms of the agreement. Upon such termination, the trustee will wind-up the Trust.
30
| |
| Item 13. | | Certain Relationships and Related Transactions, and Director Independence. |
Policies and Procedures For Related Party Transactions
Related Party Transactions are governed by our Code of Conduct policy and Aspreva’s management monitors adherence to this policy. Additionally, each director and executive officer annually delivers to Aspreva a questionnaire that includes, among other things, a request for information relating to any transactions in which both the director, executive officer, or their respective family members, and Aspreva participates, and in which the director, executive officer, or such family member, has a material interest. Our Audit Committee reviews all such transactions listed in the questionnaires, or that are brought to its attention by management or otherwise. After review, the Audit Committee approves, ratifies or disapproves such transactions.
In 2006, we engaged in the following transactions with our directors, officers and holders of more than 5% of our common shares, and affiliates thereof.
Employment, Severance and Change of Control Agreements
We have entered into employment agreements and change of control agreements with our executive officers. For more information regarding these agreements, see“Executive Compensation — Employment Agreements” and“Executive Compensation — Severance and Change of Control Arrangements”.
Consulting Agreement With Dr. Michael R. Hayden and Genworks Inc.
In January 2002, we entered into a consulting agreement with Dr. Michael R. Hayden, our co-founder and Chief Medical Advisor, and Genworks Inc., a Nova Scotia company. Dr. Hayden is the sole shareholder of Genworks Inc. The agreement has an effective date of March 1, 2002. Under the agreement, we retain Genworks Inc. to provide certain services related to scientific and clinical matters or other mutually agreed upon medical or human genetic matters, and Dr. Hayden performs such services on behalf of Genworks Inc. Dr. Hayden also chairs our Medical Advisory Board pursuant to the agreement. In 2006, we paid Dr. Hayden $156,000. Furthermore, we paid to the Center of Molecular Medicine and Therapeutics at the University of British Columbia $10,000 for providing administrative assistance to Genworks Inc.
The agreement terminates on March 1, 2008, unless earlier terminated in accordance with its terms. However, the term of the agreement will be extended for successive one year terms unless the parties otherwise agree or the agreement is terminated in accordance with its terms.
During 2006, Dr. Hayden beneficially owned more than 5% of our outstanding common shares. As of December 31, 2006 Dr. Hayden beneficially owned 2.5% of our outstanding common shares.
Agreement With Richard M. Glickman
In December 2004, we entered into an agreement with Richard M. Glickman, our Chairman and Chief Executive Officer, whereby he agreed to act as one of the administrators of our subsidiary, Aspreva Pharmaceuticals SA. The agreement provides that Mr. Glickman is not entitled to any remuneration for acting in this capacity.
Legal Fees
We retain Farris, Vaughan, Wills & Murphy LLP, a law firm where R. Hector MacKay-Dunn, Q.C., a member of our Board of Directors and our Corporate Secretary, is a senior partner. In 2006, we incurred legal fees payable to Farris, Vaughan, Wills & Murphy LLP of $554,000.
Director and Officer Indemnification
Under the British Columbia Business Corporations Act, or BCBCA, we may indemnify an individual who:
| | • | | is or was our director or officer; |
| |
| | • | | is or was a director or officer of another corporation: (a) at the time when such corporation is or was an affiliate of ours; or, (b) at our request; or |
| |
| | • | | at our request, is or was, acting in a similar capacity of another entity, |
31
against a judgment, penalty or fine awarded or imposed in, or an amount paid in settlement of any legal proceeding or investigative action, whether current, threatened, pending or completed, in which such eligible party is involved because of that association with us or other entity.
However, indemnification is prohibited under the BCBCA if:
| | • | | such eligible party did not act honestly and in good faith with a view to our best interests (or the other entity, as the case may be); and |
| |
| | • | | in the case of a proceeding other than a civil proceeding, such eligible party did not have reasonable grounds for believing that such person’s conduct was lawful. |
We may not indemnify or pay the expenses of an eligible party in respect of an action brought against an eligible party by or on behalf of us.
The BCBCA allows us to pay, as they are incurred in advance of a final disposition of a proceeding, the expenses actually and reasonably incurred by the eligible party, provided that, we receive from such eligible party an undertaking to repay the amounts advanced if it is ultimately determined that such payment is prohibited.
Despite the foregoing, on application by us or an eligible party, a court may:
| | • | | order us to indemnify an eligible party in respect of an eligible proceeding; |
| |
| | • | | order us to pay some or all of the expenses incurred by an eligible party in an eligible proceeding; |
| |
| | • | | order enforcement of or any payment under an indemnification agreement; |
| |
| | • | | order us to pay some or all of the expenses actually and reasonably incurred by a person in obtaining the order of the court; and |
| |
| | • | | make any other order the court considers appropriate. |
The BCBCA provides that we may purchase and maintain insurance for the benefit of an eligible party (or their heirs and personal or other legal representatives of the eligible party) against any liability that may be incurred by reason of the eligible party being or having been a director or officer, or in an equivalent position of ours or that of an associated corporation.
Our articles provide that, subject to the BCBCA, we must indemnify our directors, former directors or alternate directors and his or her heirs and legal personal representatives against all judgments, penalties or fines awarded or imposed in, or an amount paid in settlement of, all legal proceedings, investigative actions or other eligible proceedings (whether current, threatened, pending or completed) to which such person is or may be liable, and we must, after the final disposition of a legal proceeding, investigative action or other eligible proceeding, pay the expenses (which includes costs, charges and expenses, including legal and other fees but does not include judgments, penalties, fines or amounts paid in settlement of a proceeding) actually and reasonably incurred by such person in respect of that proceeding.
We maintain liability insurance which insures our directors and officers against certain losses and which insures us against our obligations to indemnify our directors and officers.
In February 2005, we also entered into indemnity agreements with our directors and executive officers which provide, among other things, that we will indemnify him or her for expenses actually and reasonably incurred by such person in respect of a proceeding in which such person is or may be joined as a party or is or may be liable for or in respect of penalty by reason of such person being or having been a director or officer; provided that, we shall not indemnify such person if, among other things, he or she did not act honestly and in good faith with a view to our best interests and, in the case of a proceeding other than a civil proceeding, the person did not have reasonable grounds for believing that his or her conduct in respect of which the proceeding was brought was lawful.
32
At present, we are not aware of any pending or threatened litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification would be required or permitted.
Director Independence
Our Board of Directors assumes responsibility for stewardship of Aspreva. The mandate of our Board of Directors is to supervise the management of the business and affairs of Aspreva. Our Board of Directors delegate day-to-day managerial responsibilities to management, and any responsibility not delegated to senior management or to a committee of the board remains with the full Board of Directors.
Our Board of Directors is currently composed of nine directors as noted above. Our Board of Directors has determined that seven of the nine members of the board are independent (78%) under the current requirements of the NASDAQ Market and the rules and regulations of the Canadian provincial securities regulatory authorities. Our independent directors are as follows: Kirk K. Calhoun, Ronald M. Hunt, William L. Hunter Julia G. Levy, R. Hector MacKay-Dunn, Q.C., George M. Milne and Arnold L. Oronsky. Richard M. Glickman, our Chairman and Chief Executive Officer, and Noel F. Hall, our President, are not independent as a result of being officers of Aspreva.
As our Chairman and Chief Executive Officer, Richard M. Glickman, is not independent, our Board of Directors in December 2005 appointed George M. Milne as our Lead Director.
Our Board of Directors currently has three committees: the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. Our Board of Directors has determined that each member of our Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee is an independent member of our Board of Directors under the current requirements of the NASDAQ Market and the rules and regulations of the SEC, if applicable, and Canadian provincial securities regulatory authorities.
33
| |
| Item 14. | | Principal Accountant Fees and Services. |
Fees of Ernst & Young LLP
The aggregate fees billed for professional services rendered to us by Ernst & Young LLP in 2005 and 2006 are as follows:
| | | | | | | | | | | |
| | | | | Fees ($) | |
| Type of Service | | Description of Services | | 2006 | | | 2005 | |
| Audit Fees | | Audit; Annual Report on Form 10-K; Quarterly | | $ | 747,000 | | | $ | 380,000 | |
| | | Reports on Form 10-Q; Assessment of internal | | | | | | | | |
| | | controls under Sarbanes-Oxley Act Regulation 404; other | | | | | | | | |
| | | regulatory filings | | | | | | | | |
| | | | | | | | | | | |
| Audit-Related Fees | | Revenue Model Assessment | | — | | | 15,000 | |
| | | | | | | | | | | |
Total Audit and Audit-Related Fees | | | | $ | 747,000 | | | $ | 395,000 | |
| | | | | | | | | |
| | | | | | | | | | | |
| Tax Fees | | Compliance, Transfer Pricing | | $ | 160,000 | | | $ | 195,000 | |
| | | | | | | | | | | |
| All Other Fees | | | | | — | | | | — | |
| | | | | | | | | |
| | | | | | | | | | | |
Total Fees | | | | $ | 907,000 | | | $ | 590,000 | |
| | | | | | | | | |
Our Audit Committee considered and concluded that the provision by Ernst & Young LLP of such audit and tax related services, and other services as were provided to us in 2005 and 2006, is compatible with maintaining the independence of Ernst & Young LLP.
Our Audit Committee’s policy is to pre-approve all audit and permissible non-audit services rendered by Ernst & Young LLP. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services and tax services up to specified amounts. Pre-approval may also be given as part of our Audit Committee’s approval of the scope of the engagement of Ernst & Young LLP, or on an individual case-by-case basis before Ernst & Young LLP is engaged to provide each service.
Because our initial public offering was completed on March 4, 2005, our Audit Committee was not required to, and did not pre-approve, all of the fees described above that were rendered prior to our initial public offering.
34
PART IV
| Item 15. | | Exhibits and Financial Statement Schedules. |
| | (a) | | The following documents are included as part of this Annual Report on Form 10-K/A: |
The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as a part of this Amendment No. 1 to the Annual Report.
35
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| | ASPREVA PHARMACEUTICALS CORPORATION
| |
| Date: April 30, 2007 | By: | /s/ Richard M. Glickman | |
| | | Richard M. Glickman | |
| | | Chairman and Chief Executive Officer
(Principal Executive Officer) | |
| |
POWER OF ATTORNEY
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| | | | | | | | | | | |
| By: | | /s/ Richard M. Glickman | | Date: April 30, 2007 | | By: | | /s/ Bruce G. Cousins | | Date: April 30, 2007 |
| | | Richard M. Glickman | | | | | | Bruce G. Cousins | | |
| | | Chief Executive Officer and | | | | | | Chief Financial Officer and Executive | | |
| | | Chairman | | | | | | Vice President | | |
| | | (Principal Executive Officer) | | | | | | (Principal Financial and Accounting Officer) | | |
| | | | | | | | | | | |
| By: | | * | | Date: April 30, 2007 | | By: | | * | | Date: April 30, 2007 |
| | | Kirk K. Calhoun | | | | | | Julia G. Levy | | |
| | | Director | | | | | | Director | | |
| | | | | | | | | | | |
| By: | | * | | Date: April 30, 2007 | | By: | | * | | Date: April 30, 2007 |
| | | Noel F. Hall | | | | | | Arnold L. Oronsky | | |
| | | Director | | | | | | Director | | |
| | | | | | | | | | | |
| By: | | * | | Date: April 30, 2007 | | By: | | * | | Date: April 30, 2007 |
| | | R. Hector MacKay-Dunn | | | | | | Ronald M. Hunt | | |
| | | Director | | | | | | Director | | |
| | | | | | | | | | | |
| By: | | * | | Date: April 30, 2007 | | By: | | * | | Date: April 30, 2007 |
| | | George M. Milne | | | | | | William Hunter | | |
| | | Director | | | | | | Director | | |
| | | | | |
| | | |
| * By: | /s/ Richard M. Glickman | | |
| | Richard M. Glickman | | |
| | Attorney-in-Fact | | |
36
EXHIBIT INDEX
| | | |
| Exhibit No. | | Description |
| 31.1 | | Certification of the Chief Executive Officer, as required by Rule 13a-14(a) of the Securities and Exchange Act of 1934, as amended. |
| 31.2 | | Certification of the Chief Financial Officer, as required by Rule 13a-14(a) of the Securities and Exchange Act of 1934, as amended. |
37