
EYP-1901 in wet AMD�DAVIO 2 Phase 2 Clinical Trial�Topline Data December 4, 2023 ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Exhibit 99.2

Forward-Looking Statements ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, including but not limited to statements about the sufficiency of our existing cash resources into 2025; our expectations regarding the timing and clinical development of our product candidates, including EYP-1901 and EYP-2301; the potential for EYP-1901 as a novel sustained delivery treatment for serious eye diseases, including wet age-related macular degeneration, non-proliferative diabetic retinopathy and diabetic macular edema; and our longer term financial and business goals and expectations, are forward-looking statements. Some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements are risks and uncertainties inherent in our business including, without limitation: the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; our ability to access needed capital; termination or breach of current and future license agreements; our dependence on contract research organizations, co-promotion partners, and other outside vendors and service providers; effects of guidelines, recommendations and studies; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; product liability; industry consolidation; compliance with environmental laws; manufacturing risks; risks and costs of international business operations; volatility of our stock price; possible dilution; absence of dividends; the impact of instability in general business and economic conditions, including changes in inflation, interest rates and the labor market; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.
©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. IVT, intravitreal injection Pipeline represents potentially substantial opportunities using our bioerodible Durasert E™ IVT delivery technology EYP-1901 – vorolanib, a selective and patented TKI Positive topline Phase 2 data in wet AMD Phase 3 trials in wet AMD planned to initiate in 2H 2024 Topline Phase 2 data in NPDR anticipated in Q2 2024 Phase 2 trial in DME planned to commence in Q1 2024 EYP-2301 – razuprotafib, a patented TIE-2 agonist for serious retinal diseases Durasert® - proven, safe IVT drug delivery technology Routine in-office IVT injection Bioerodible and non-erodible formulations Safely administered to thousands of patient eyes across four FDA approved products with non-erodible formulations Strong Balance Sheet $136.0M of cash and investments on September 30, 2023 Cash runway into 2025 Committed to developing therapeutics to improve the lives of patients with serious retinal diseases

©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. EYP-1901 (vorolanib in Durasert E) a potential treatment for wet AMD featuring sustained delivery for 6-months or longer
©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. There is a Significant Need for More Durable Therapies in Wet AMD Many patients with wet AMD are chronically undertreated >80% of Retina Specialists say undertreatment is due to patient noncompliance, scheduling limitations or provider preference for less frequent dosing1 2 Current “treat and extend” protocol still places significant burden on physicians and patients Chronic disease treated with short acting anti-VEGF biologics A delay in care/missed visit can result in vision loss A delay in treatment of only 5.34 weeks resulted in vision loss2 3 1 4 An aging population means significantly more injections in a patient’s lifetime Current anti-VEGF treatments are dosed on average every two months in the United States3 1. 2022 PAT Survey; 2. American Academy of Ophthalmology, The Effect of Delay in Care Among Patients Requiring Intravitreal Injections, Welin Song, BS et al; 3. NIH Current and Upcoming Anti-VEGF Therapies and Dosing Strategies for the treatment of neovascular AMD: a comparative review, Saira Khanna et al, Dec. 2019
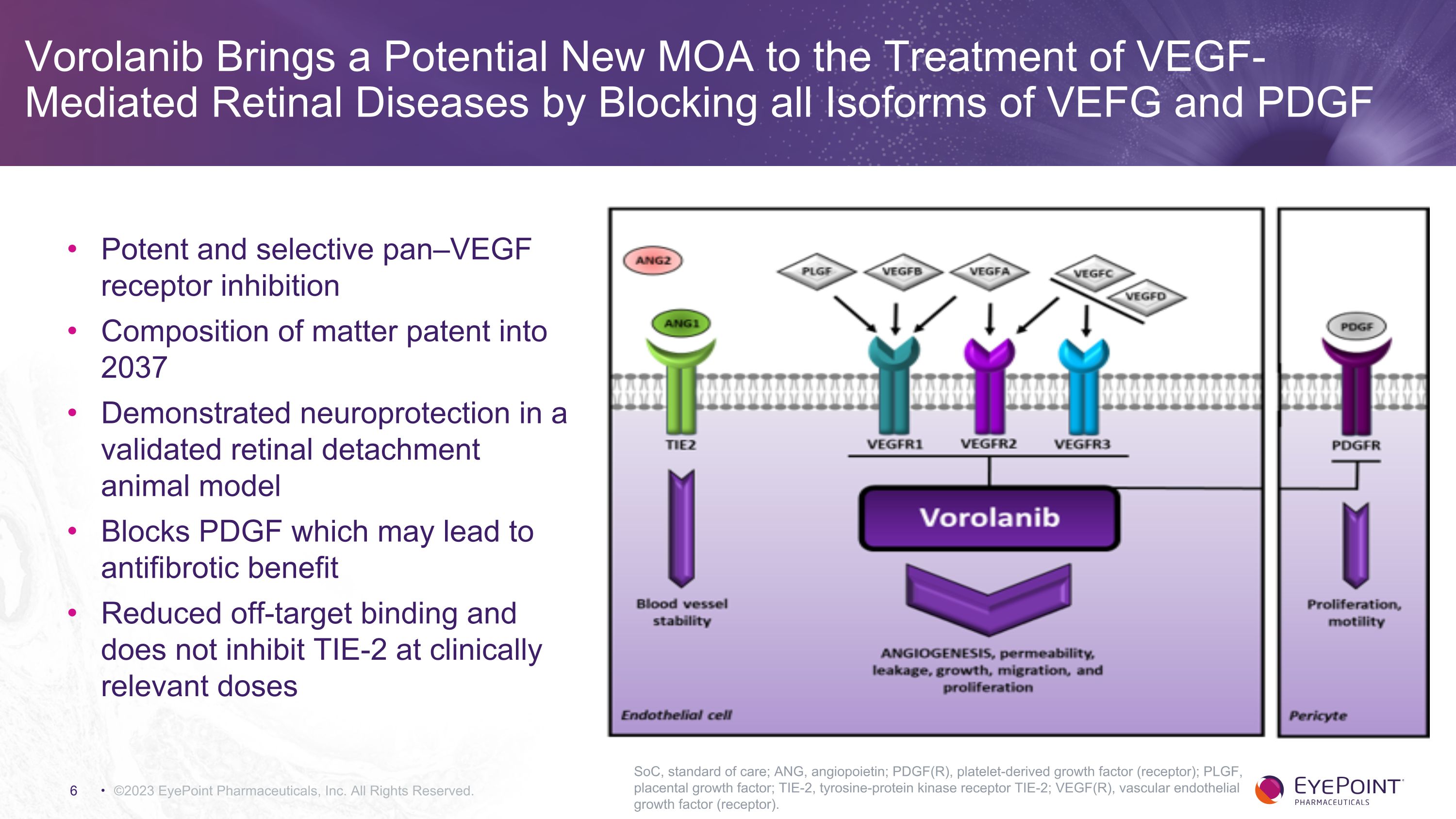
Vorolanib Brings a Potential New MOA to the Treatment of VEGF-Mediated Retinal Diseases by Blocking all Isoforms of VEFG and PDGF ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. SoC, standard of care; ANG, angiopoietin; PDGF(R), platelet-derived growth factor (receptor); PLGF, placental growth factor; TIE-2, tyrosine-protein kinase receptor TIE-2; VEGF(R), vascular endothelial growth factor (receptor). Potent and selective pan–VEGF receptor inhibition Composition of matter patent into 2037 Demonstrated neuroprotection in a validated retinal detachment animal model Blocks PDGF which may lead to antifibrotic benefit Reduced off-target binding and does not inhibit TIE-2 at clinically relevant doses

©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Safe Sustained IVT Drug Delivery Delivered by a single in-office IVT injection Continuous, stable release of drug Zero-order kinetics Durasert®: non-erodible Drug embedded within a bioerodible matrix coated with non-erodible polyimide shell: YUTIQ®1 ILUVIEN®1 RETISERT®2 VITRASERT®2 Durasert E™: bioerodible Insert consists of drug embedded within a bioerodible matrix Designed to deplete drug load before matrix fully erodes TECHNOLOGY�DURASERT® 1- licensed to Alimera; 2 – licensed to Bausch and Lomb
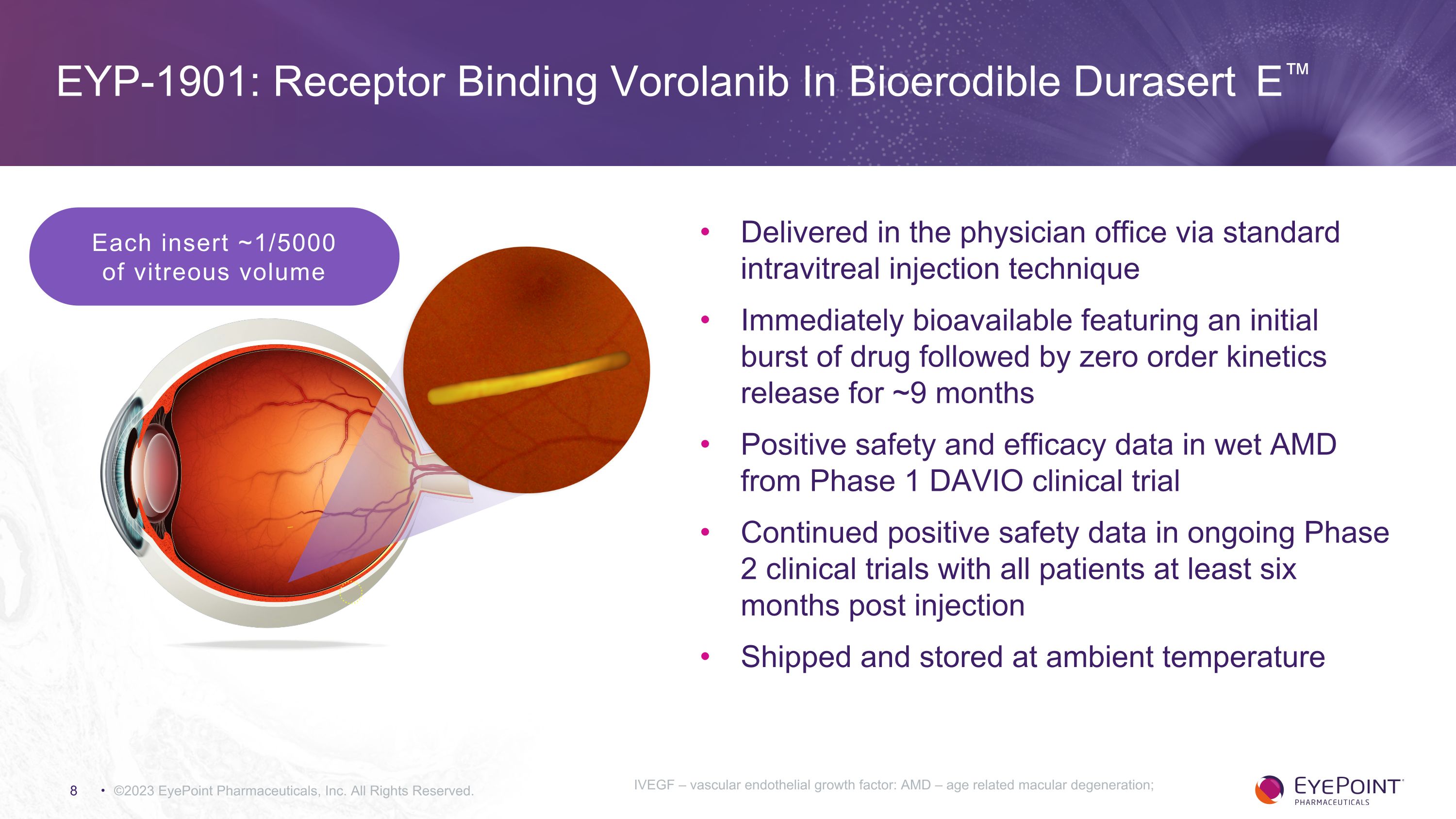
©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Each insert ~1/5000 �of vitreous volume Delivered in the physician office via standard intravitreal injection technique Immediately bioavailable featuring an initial burst of drug followed by zero order kinetics release for ~9 months Positive safety and efficacy data in wet AMD from Phase 1 DAVIO clinical trial Continued positive safety data in ongoing Phase 2 clinical trials with all patients at least six months post injection Shipped and stored at ambient temperature EYP-1901: Receptor Binding Vorolanib In Bioerodible Durasert E™ IVEGF – vascular endothelial growth factor: AMD – age related macular degeneration;
©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. The DAVIO 2 Clinical Trial – Background A non-inferiority trial evaluating two doses of EYP-1901 against an aflibercept control in wet AMD The DAVIO 2 clinical trial was designed to evaluate EYP-1901 in wet AMD and support Phase 3 clinical trials based on a Type C meeting with FDA Design: Multi-center, randomized, double-masked trial in patients with previously treated wet AMD Anti-VEGF supplement criteria: 5 letter loss with 75 microns of new fluid Other criteria 10 letter loss due to wet AMD 100 microns new fluid x 2 visits New retinal hemorrhage from wet AMD Investigator discretion Primary outcome: difference in mean change in BCVA from Day 1 to Week 28 and 32 (blended) Key secondary endpoints: safety, reduction in treatment burden, percent of eyes supplement-free up to six months and anatomical results
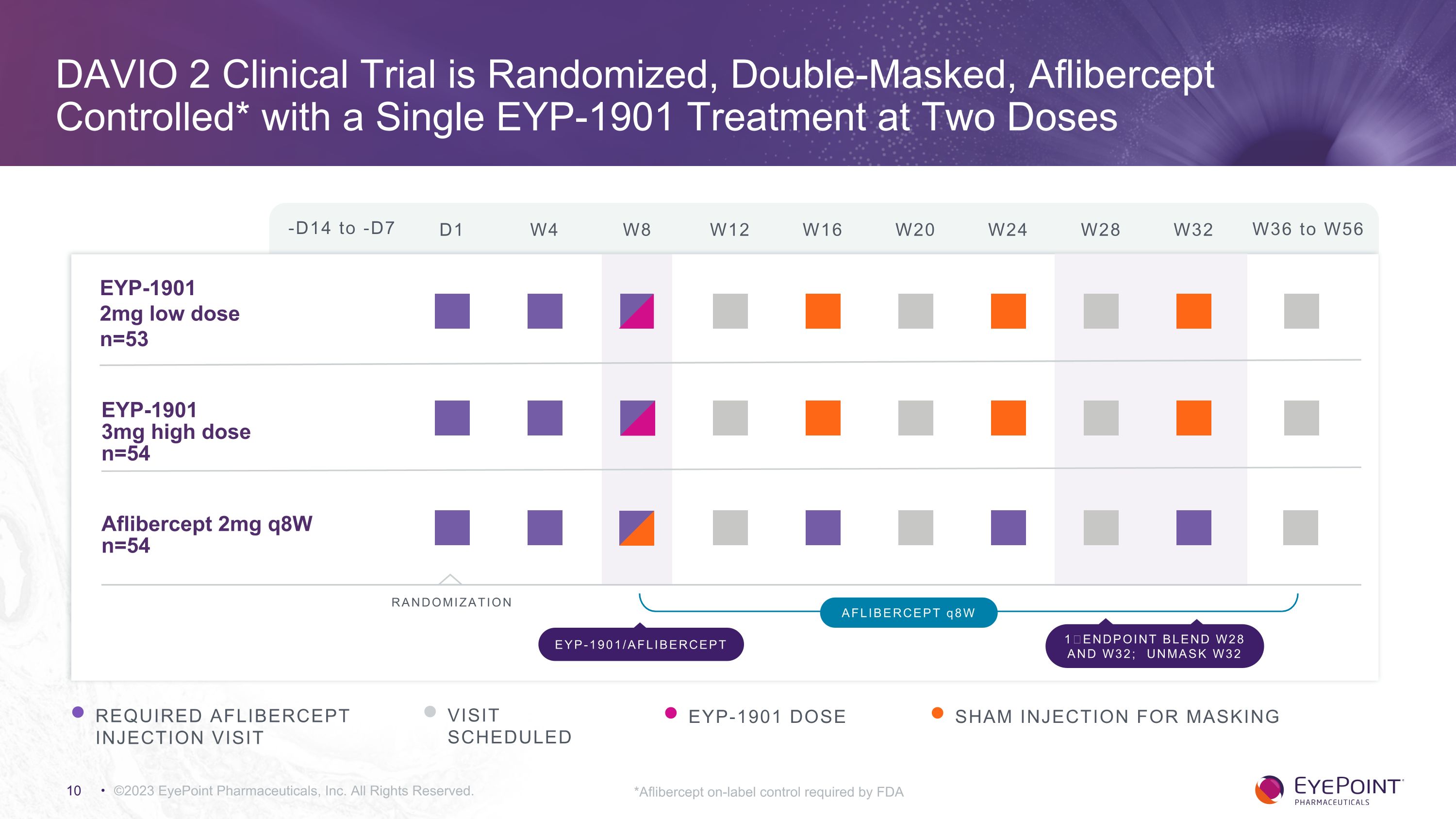
DAVIO 2 Clinical Trial is Randomized, Double-Masked, Aflibercept Controlled* with a Single EYP-1901 Treatment at Two Doses ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. -D14 to -D7 D1 W4 W8 W12 W16 W24 W32 W36 to W56 W20 W28 EYP-1901 �2mg low dose n=53 EYP-1901 �3mg high dose n=54 Aflibercept 2mg q8W n=54 RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED EYP-1901 DOSE AFLIBERCEPT q8W EYP-1901/AFLIBERCEPT 1⁰ ENDPOINT BLEND W28 AND W32; UNMASK W32 SHAM INJECTION FOR MASKING *Aflibercept on-label control required by FDA
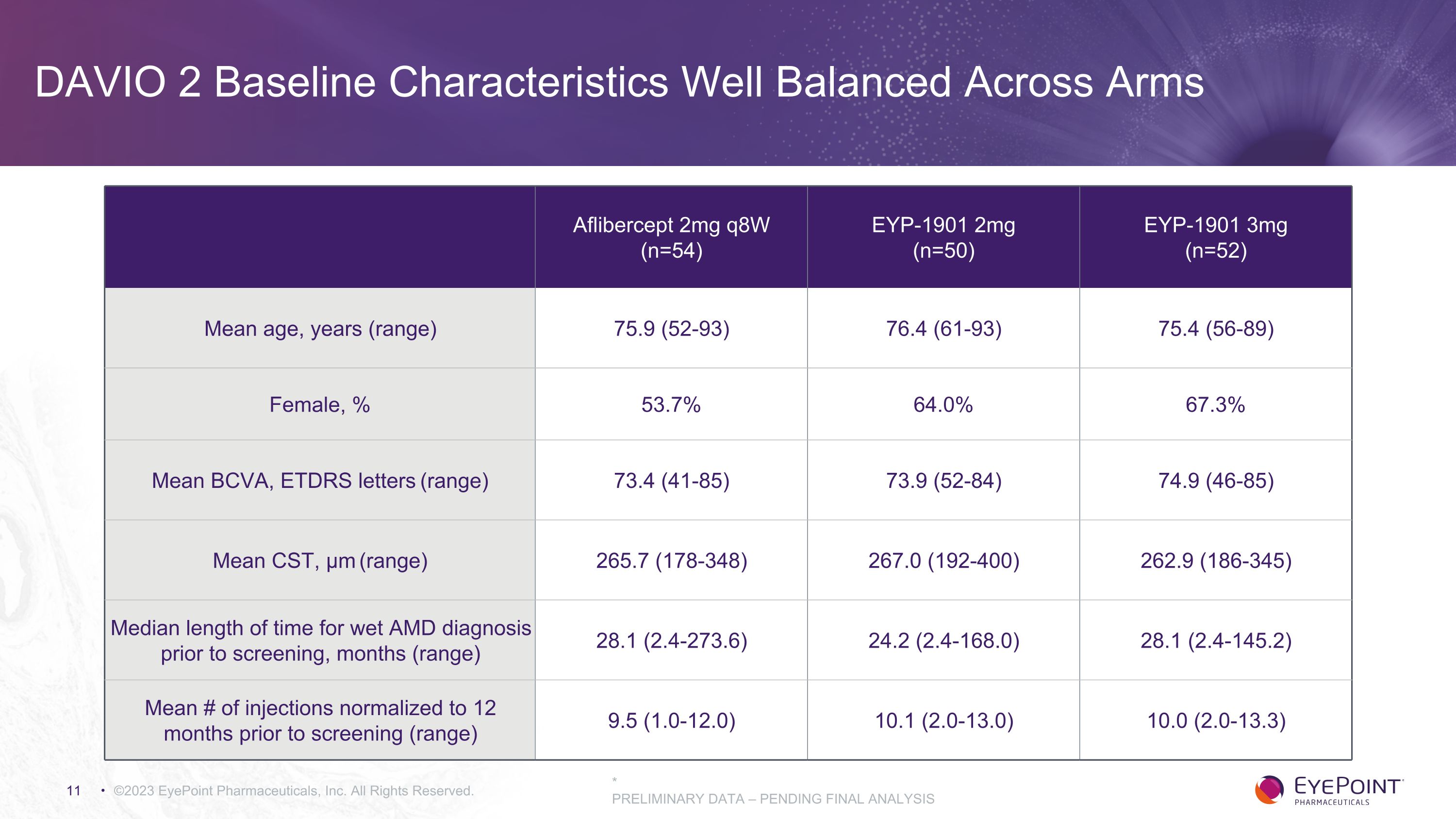
DAVIO 2 Baseline Characteristics Well Balanced Across Arms ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Aflibercept 2mg q8W (n=54) EYP-1901 2mg (n=50) EYP-1901 3mg (n=52) Mean age, years (range) 75.9 (52-93) 76.4 (61-93) 75.4 (56-89) Female, % 53.7% 64.0% 67.3% Mean BCVA, ETDRS letters (range) 73.4 (41-85) 73.9 (52-84) 74.9 (46-85) Mean CST, μm (range) 265.7 (178-348) 267.0 (192-400) 262.9 (186-345) Median length of time for wet AMD diagnosis prior to screening, months (range) 28.1 (2.4-273.6) 24.2 (2.4-168.0) 28.1 (2.4-145.2) Mean # of injections normalized to 12 months prior to screening (range) 9.5 (1.0-12.0) 10.1 (2.0-13.0) 10.0 (2.0-13.3) * PRELIMINARY DATA – PENDING FINAL ANALYSIS

©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Clinical Trial Topline Results A NON-INFERIORITY TRIAL VERSUS AN AFLIBERCEPT CONTROL
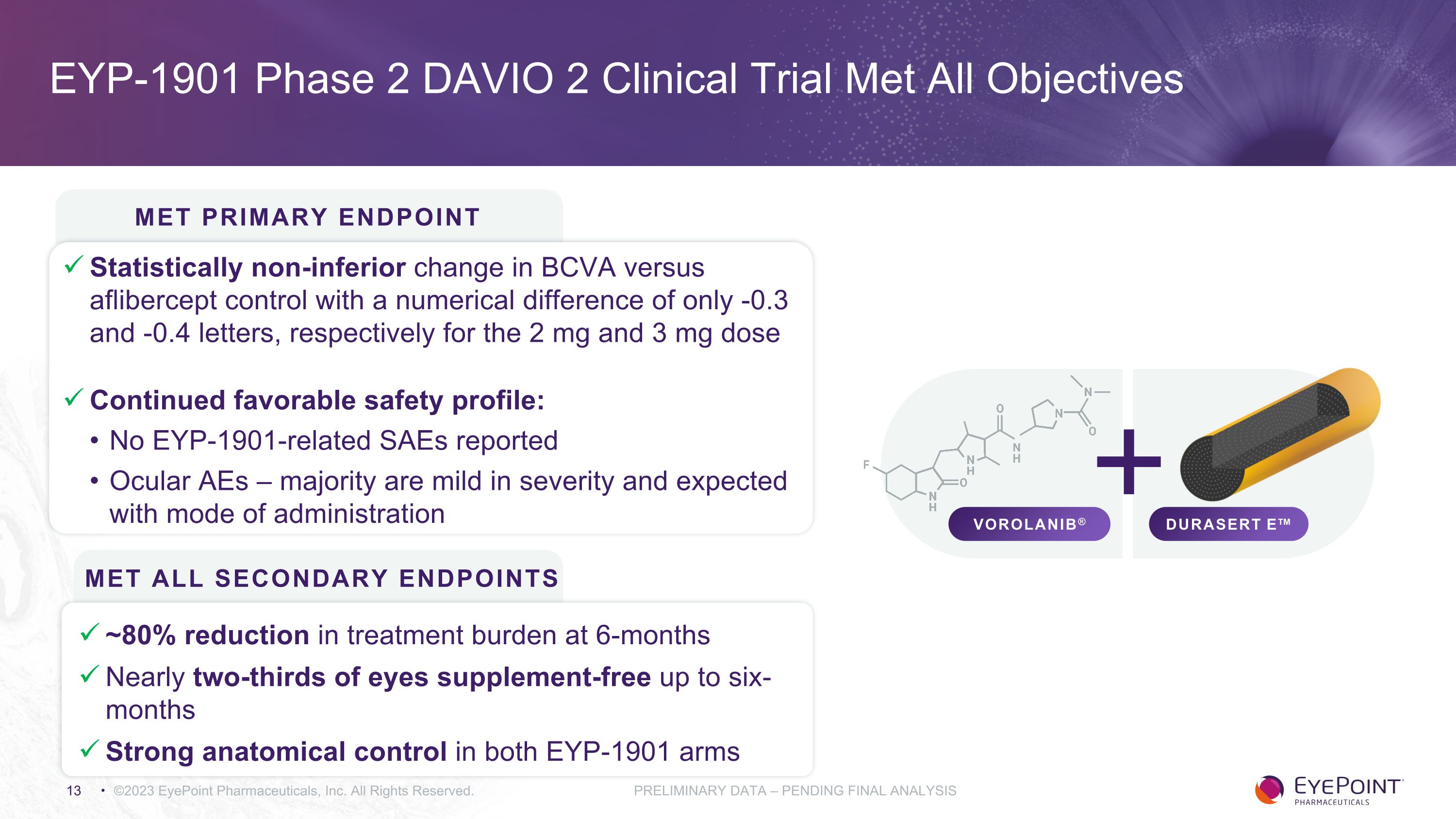
EYP-1901 Phase 2 DAVIO 2 Clinical Trial Met All Objectives ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS Met ALL Secondary Endpoints ~80% reduction in treatment burden at 6-months Nearly two-thirds of eyes supplement-free up to six-months Strong anatomical control in both EYP-1901 arms DURASERT E™ VOROLANIB® Statistically non-inferior change in BCVA versus aflibercept control with a numerical difference of only -0.3 and -0.4 letters, respectively for the 2 mg and 3 mg dose Continued favorable safety profile: No EYP-1901-related SAEs reported Ocular AEs – majority are mild in severity and expected with mode of administration Met Primary Endpoint
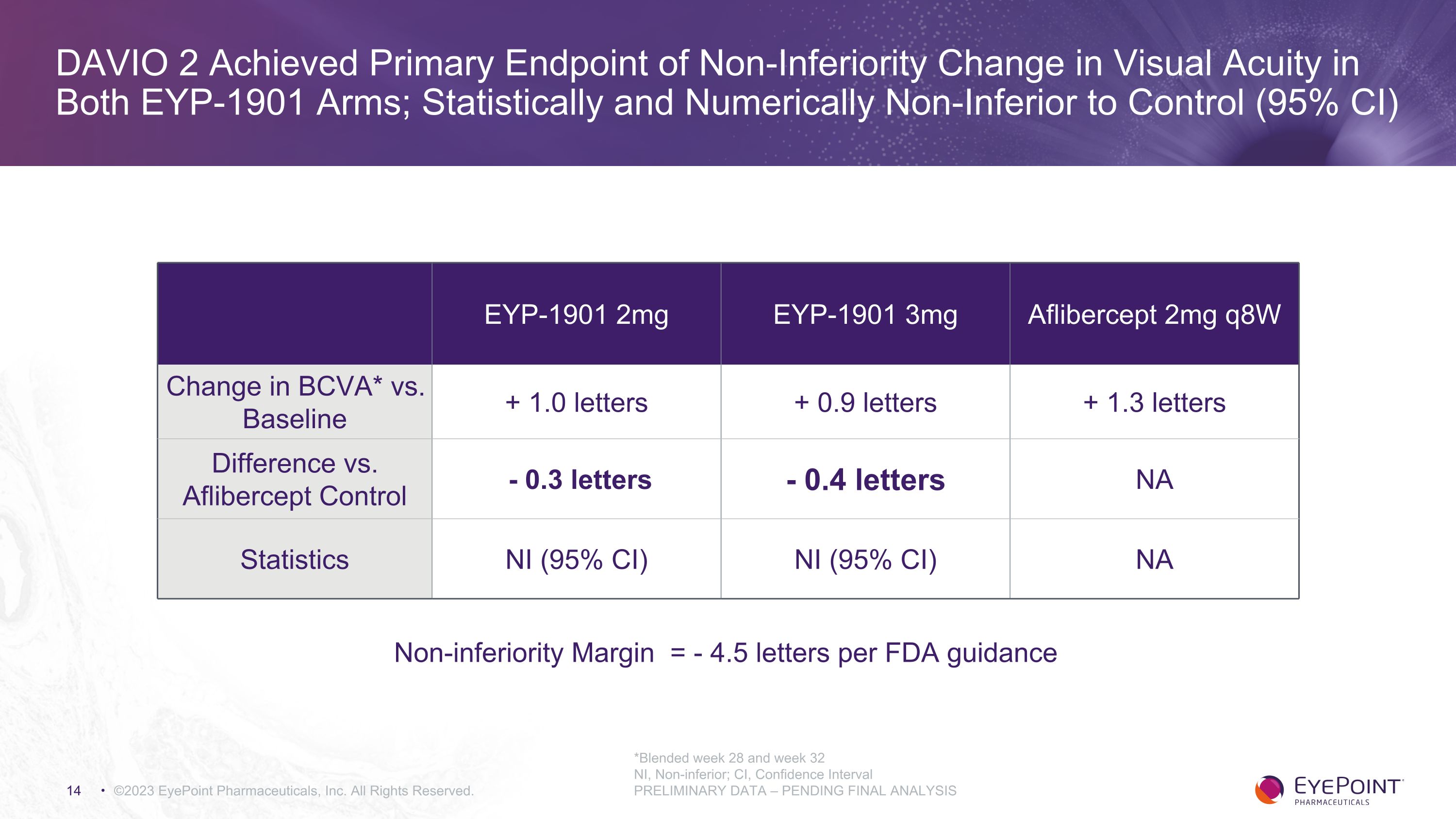
DAVIO 2 Achieved Primary Endpoint of Non-Inferiority Change in Visual Acuity in Both EYP-1901 Arms; Statistically and Numerically Non-Inferior to Control (95% CI) ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Blended week 28 and week 32 NI, Non-inferior; CI, Confidence Interval PRELIMINARY DATA – PENDING FINAL ANALYSIS EYP-1901 2mg EYP-1901 3mg Aflibercept 2mg q8W Change in BCVA* vs. Baseline + 1.0 letters + 0.9 letters + 1.3 letters Difference vs. Aflibercept Control - 0.3 letters - 0.4 letters NA Statistics NI (95% CI) NI (95% CI) NA Non-inferiority Margin = - 4.5 letters per FDA guidance
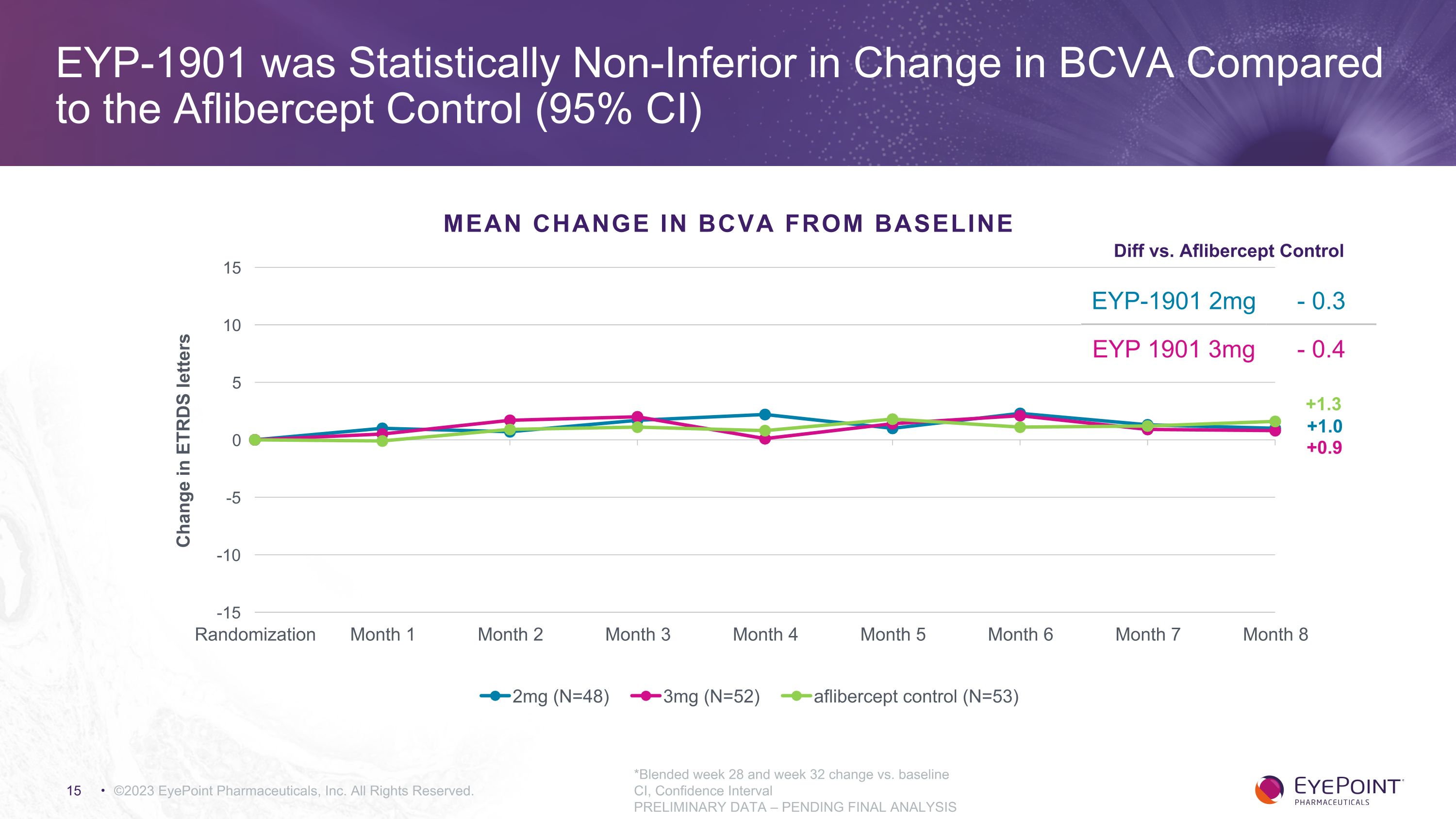
©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. +0.9 +1.0 +1.3 *Blended week 28 and week 32 change vs. baseline CI, Confidence Interval PRELIMINARY DATA – PENDING FINAL ANALYSIS EYP-1901 was Statistically Non-Inferior in Change in BCVA Compared to the Aflibercept Control (95% CI) MEAN CHANGE IN BCVA FROM BASELINE EYP-1901 2mg - 0.3 EYP 1901 3mg - 0.4 Diff vs. Aflibercept Control
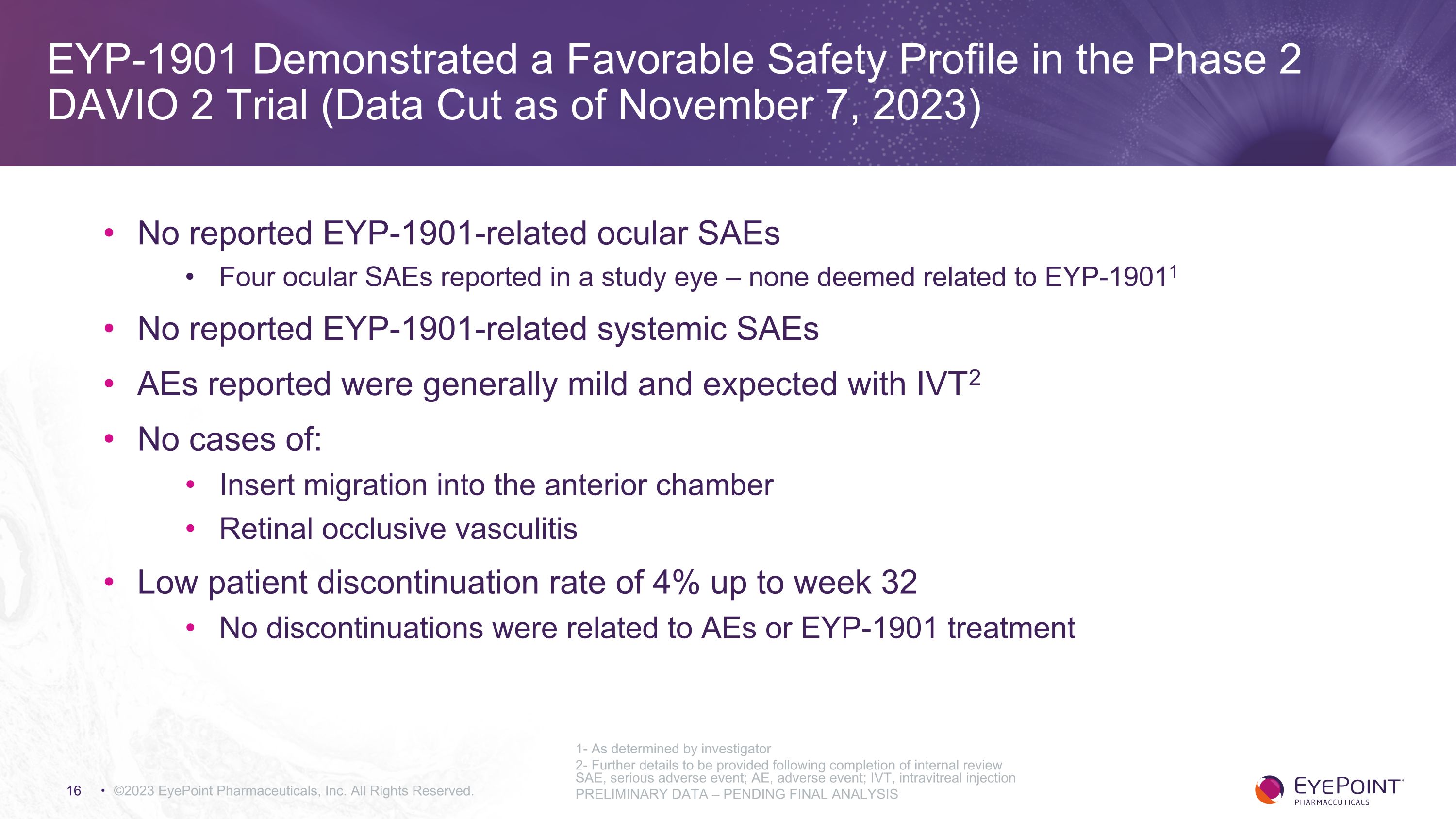
EYP-1901 Demonstrated a Favorable Safety Profile in the Phase 2 DAVIO 2 Trial (Data Cut as of November 7, 2023) ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. SAE, serious adverse event; AE, adverse event; IVT, intravitreal injection PRELIMINARY DATA – PENDING FINAL ANALYSIS 1- As determined by investigator 2- Further details to be provided following completion of internal review No reported EYP-1901-related ocular SAEs Four ocular SAEs reported in a study eye – none deemed related to EYP-19011 No reported EYP-1901-related systemic SAEs AEs reported were generally mild and expected with IVT2 No cases of: Insert migration into the anterior chamber Retinal occlusive vasculitis Low patient discontinuation rate of 4% up to week 32 No discontinuations were related to AEs or EYP-1901 treatment
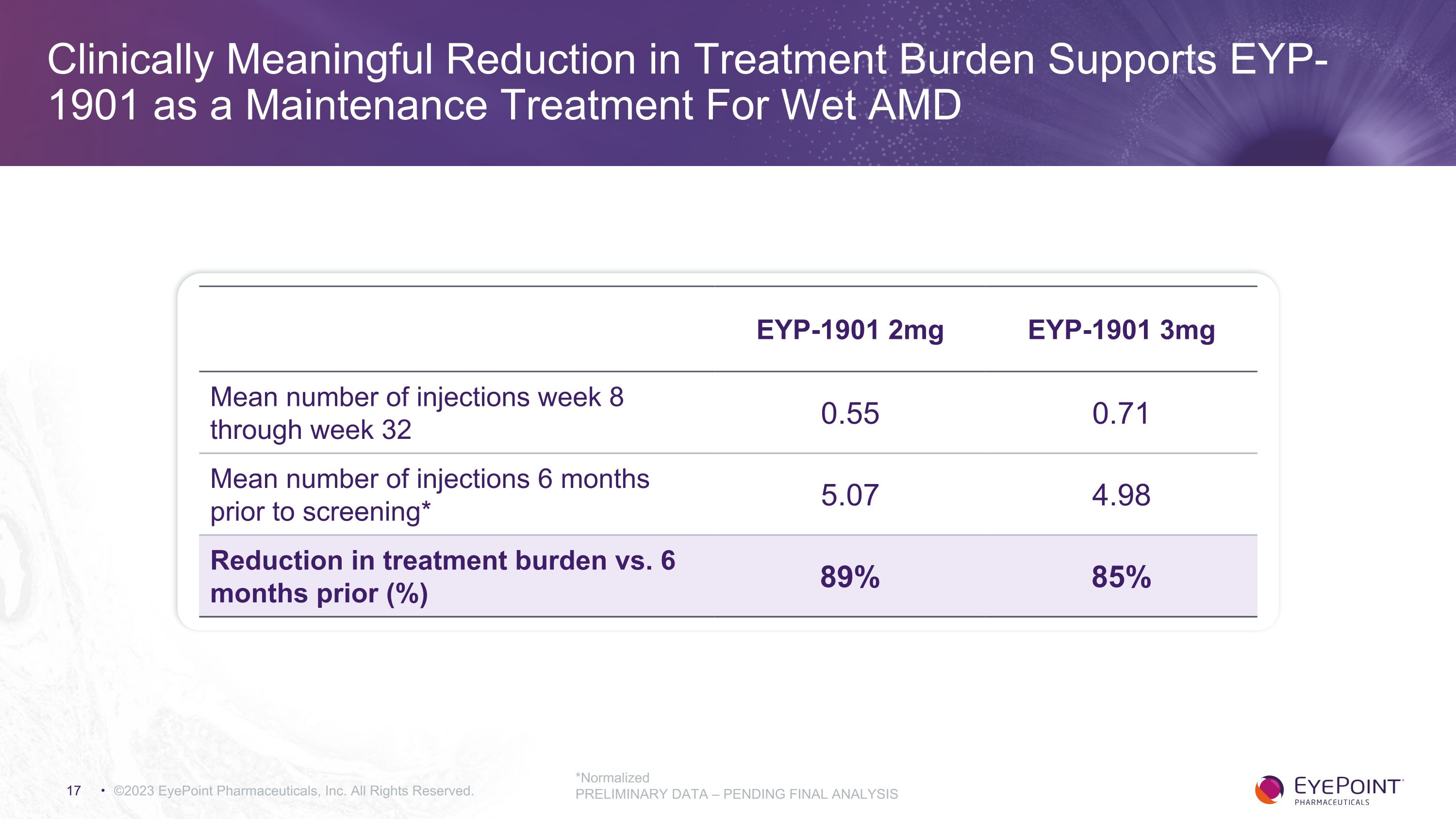
Clinically Meaningful Reduction in Treatment Burden Supports EYP-1901 as a Maintenance Treatment For Wet AMD ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Normalized PRELIMINARY DATA – PENDING FINAL ANALYSIS EYP-1901 2mg EYP-1901 3mg Mean number of injections week 8 through week 32 0.55 0.71 Mean number of injections 6 months prior to screening* 5.07 4.98 Reduction in treatment burden vs. 6 months prior (%) 89% 85%
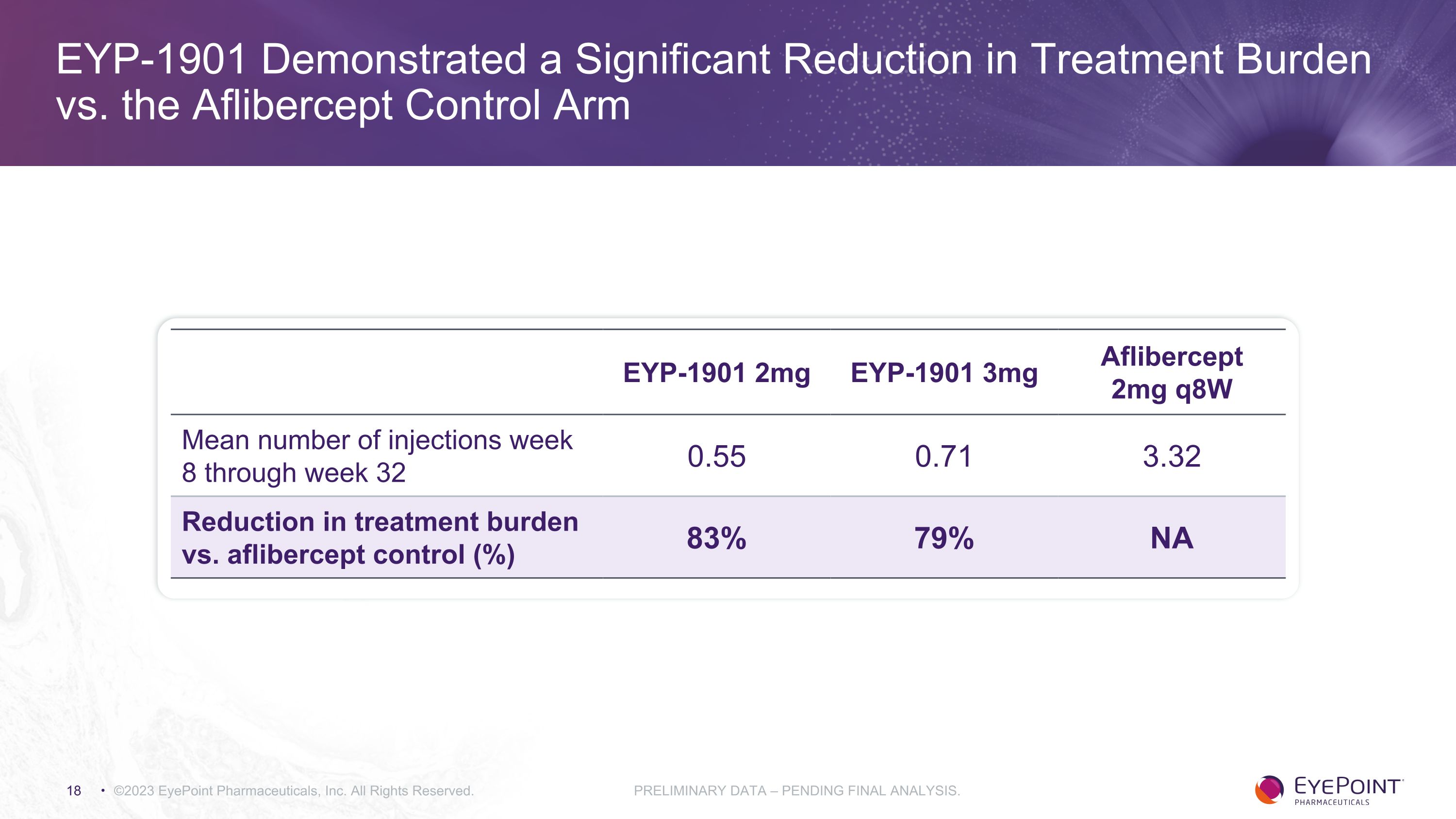
EYP-1901 Demonstrated a Significant Reduction in Treatment Burden vs. the Aflibercept Control Arm ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS. EYP-1901 2mg EYP-1901 3mg Aflibercept 2mg q8W Mean number of injections week 8 through week 32 0.55 0.71 3.32 Reduction in treatment burden vs. aflibercept control (%) 83% 79% NA
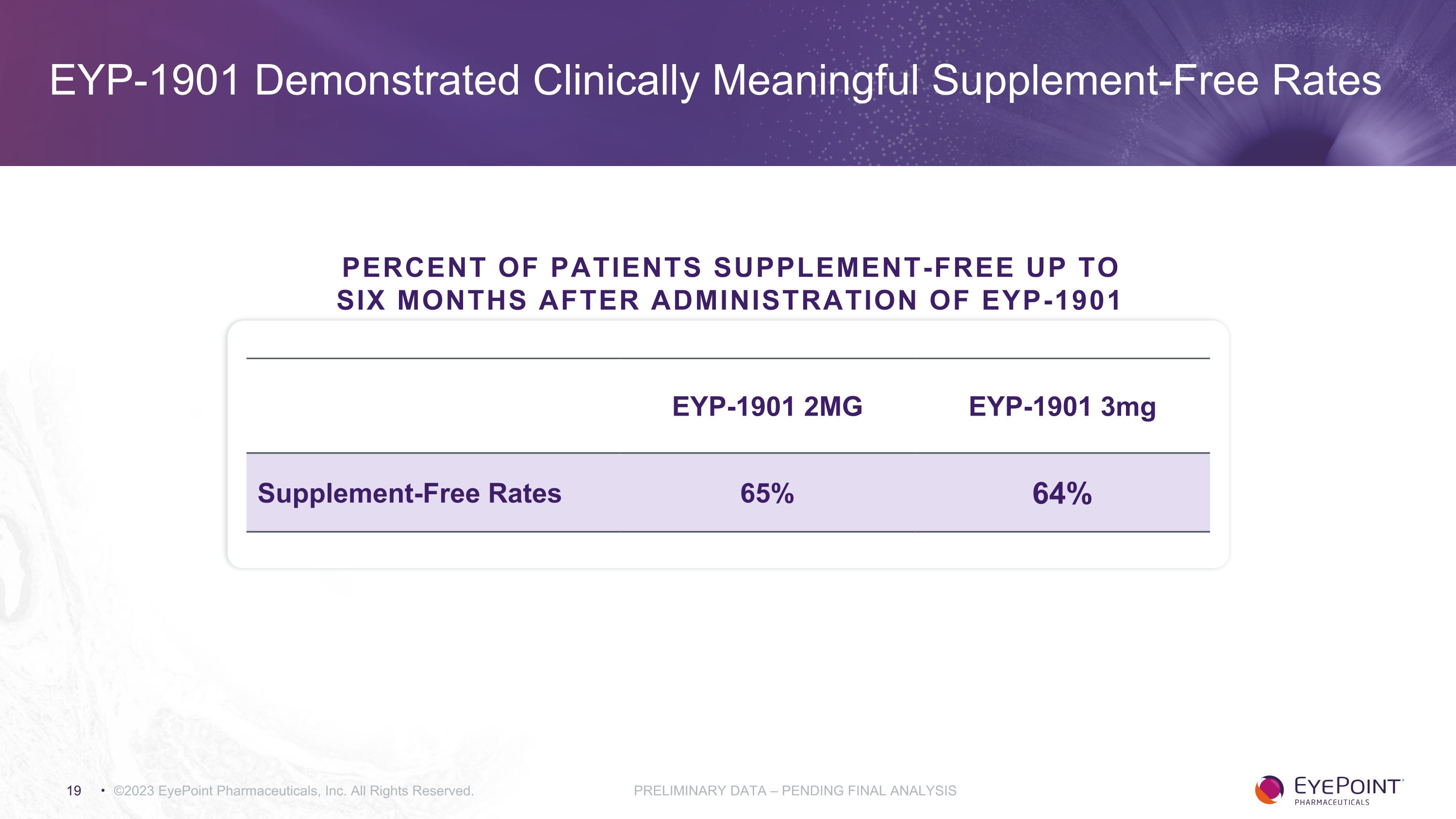
EYP-1901 Demonstrated Clinically Meaningful Supplement-Free Rates ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS EYP-1901 2MG EYP-1901 3mg Supplement-Free Rates 65% 64% Percent of Patients Supplement-Free up to Six Months After Administration of EYP-1901
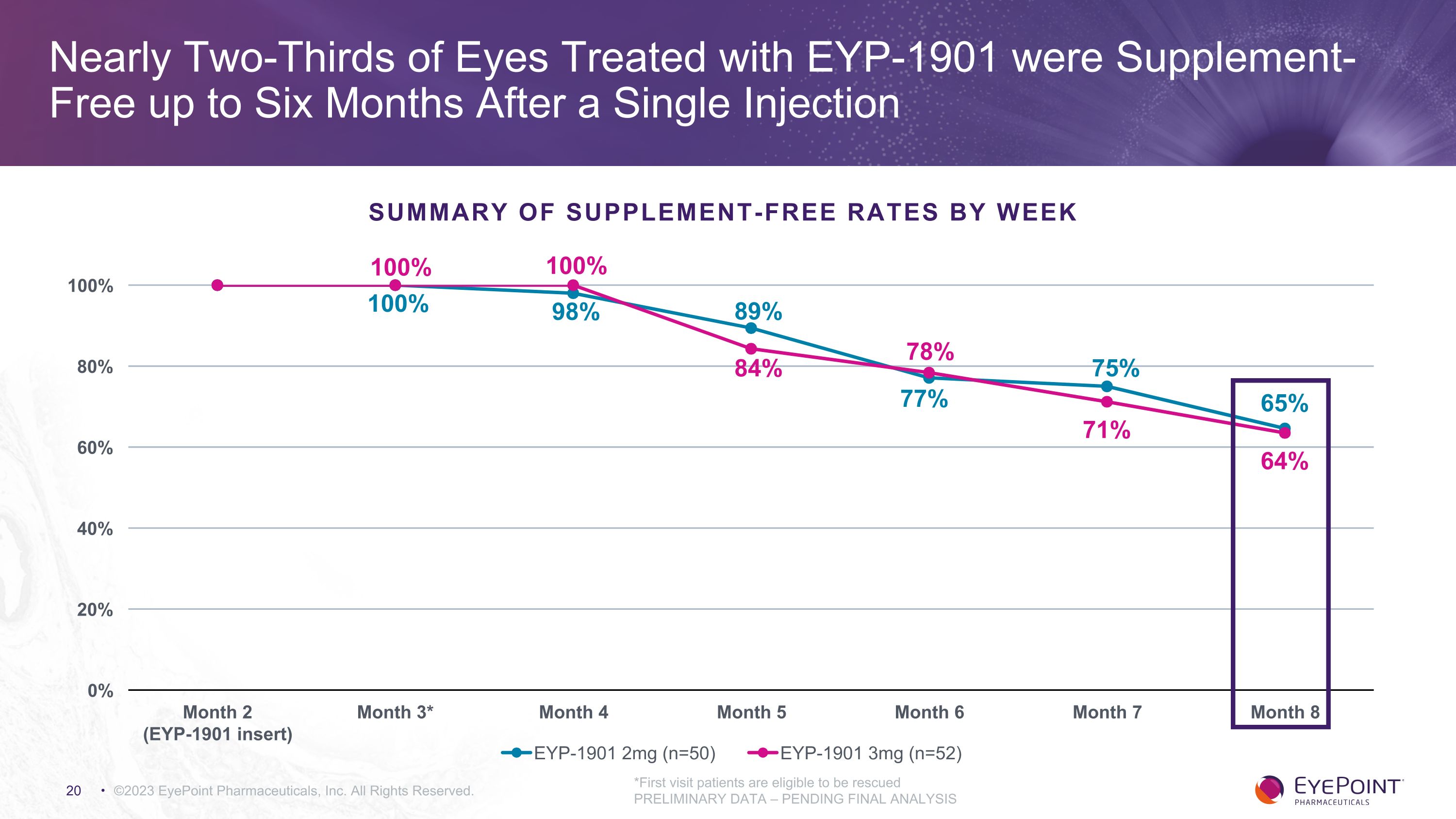
Nearly Two-Thirds of Eyes Treated with EYP-1901 were Supplement-Free up to Six Months After a Single Injection ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *First visit patients are eligible to be rescued PRELIMINARY DATA – PENDING FINAL ANALYSIS 100% Summary of Supplement-Free Rates by Week 100%
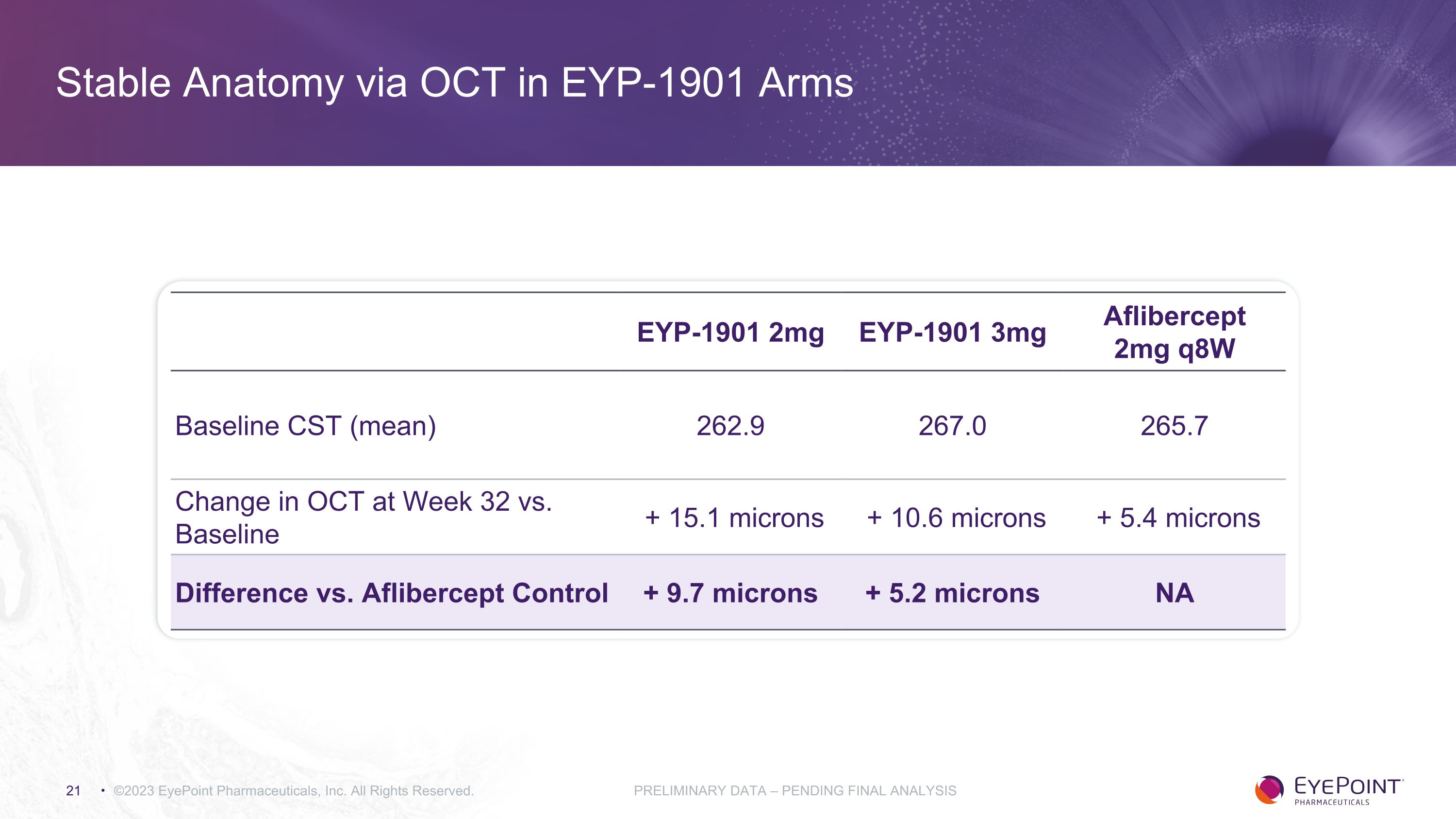
Stable Anatomy via OCT in EYP-1901 Arms EYP-1901 2mg EYP-1901 3mg Aflibercept 2mg q8W Baseline CST (mean) 262.9 267.0 265.7 Change in OCT at Week 32 vs. Baseline + 15.1 microns + 10.6 microns + 5.4 microns Difference vs. Aflibercept Control + 9.7 microns + 5.2 microns NA ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS
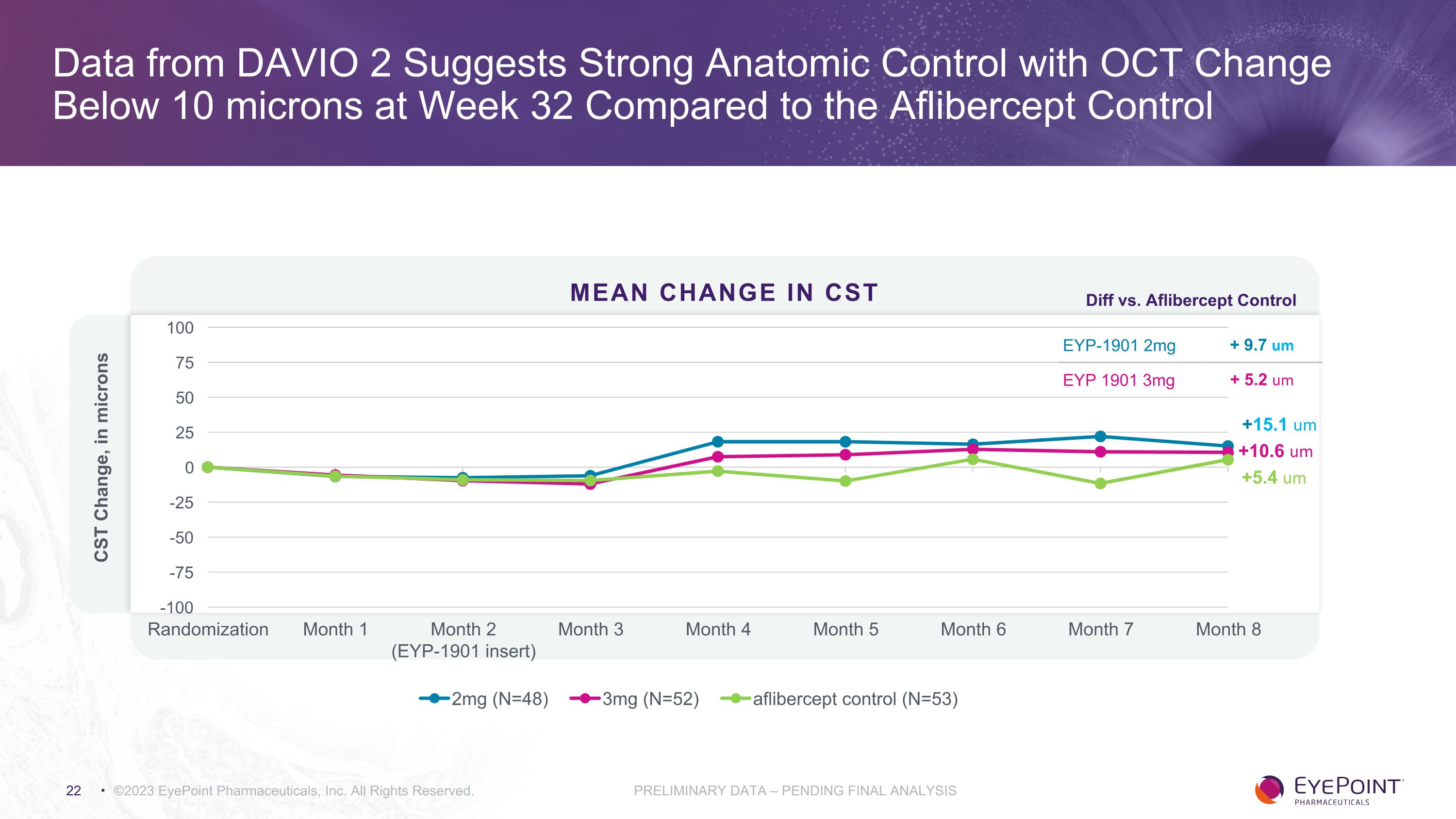
Data from DAVIO 2 Suggests Strong Anatomic Control with OCT Change Below 10 microns at Week 32 Compared to the Aflibercept Control ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS MEAN CHANGE in CST +10.6 um +15.1 um +5.4 um EYP-1901 2mg + 9.7 um EYP 1901 3mg + 5.2 um Diff vs. Aflibercept Control
EYP-1901 Phase 2 DAVIO 2 Clinical Trial Met All Objectives ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS Met ALL Secondary Endpoints ~80% reduction in treatment burden at 6-months Nearly two-thirds of eyes supplement-free up to six-months Strong anatomical control in both EYP-1901 arms DURASERT E™ VOROLANIB® Statistically non-inferior change in BCVA versus aflibercept control with a numerical difference of only -0.3 and -0.4 letters, respectively for the 2 mg and 3 mg dose Continued favorable safety profile: No EYP-1901-related SAEs reported Ocular AEs – majority are mild in severity and expected with mode of administration Met Primary Endpoint
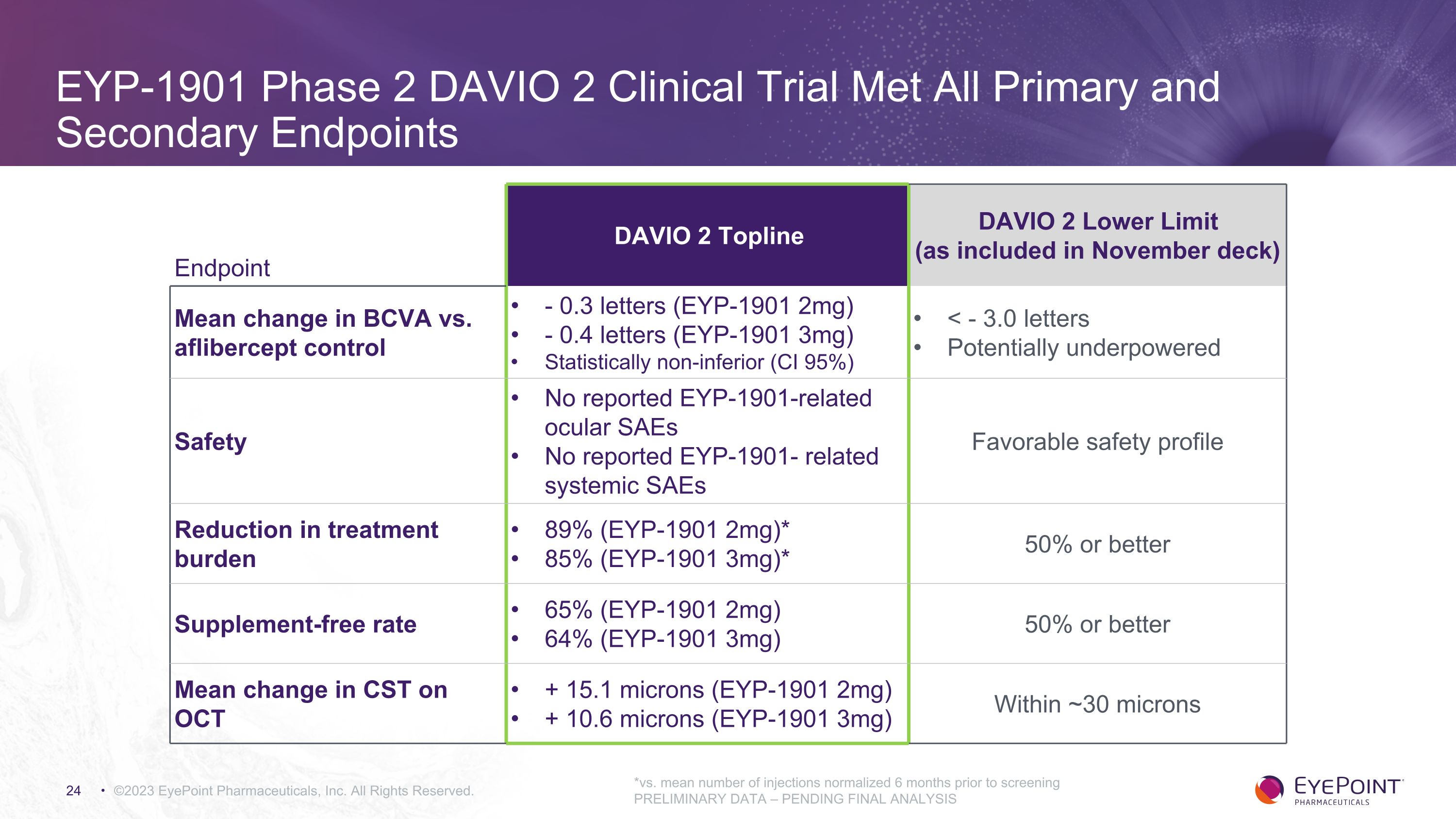
EYP-1901 Phase 2 DAVIO 2 Clinical Trial Met All Primary and Secondary Endpoints ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *vs. mean number of injections normalized 6 months prior to screening PRELIMINARY DATA – PENDING FINAL ANALYSIS Endpoint DAVIO 2 Topline DAVIO 2 Lower Limit (as included in November deck) Mean change in BCVA vs. aflibercept control - 0.3 letters (EYP-1901 2mg) - 0.4 letters (EYP-1901 3mg) Statistically non-inferior (CI 95%) < - 3.0 letters Potentially underpowered Safety No reported EYP-1901-related ocular SAEs No reported EYP-1901- related systemic SAEs Favorable safety profile Reduction in treatment burden 89% (EYP-1901 2mg)* 85% (EYP-1901 3mg)* 50% or better Supplement-free rate 65% (EYP-1901 2mg) 64% (EYP-1901 3mg) 50% or better Mean change in CST on OCT + 15.1 microns (EYP-1901 2mg) + 10.6 microns (EYP-1901 3mg) Within ~30 microns

EYP-1901 in wet AMD�DAVIO 2 Phase 2 Clinical Trial�Topline Data December 4, 2023 ©2023 EyePoint Pharmaceuticals, Inc. All Rights Reserved.
























