
J.P. Morgan Healthcare Conference Presentation January 14, 2025 Jay Duker, M.D. President and CEO ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Exhibit 99.1

Legal Disclaimers ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, are forward-looking statements , including but not limited to statements regarding: our expectations regarding the timing and clinical development of DURAVYU™ in Wet AMD and DME, our expectations regarding the enrollment, dosing and data readouts for the LUGANO trial and the LUCIA trial; our optimism that that DURAVYU has the potential to change the current treatment paradigm and revolutionize real-world outcomes for patients suffering from serious retinal diseases; our belief that DURAVYU has the potential to maintain a majority of patients with active disease with no supplemental anti-VEGF therapy for six months or longer; and our expectations regarding the timing and clinical development of our other product candidates, including EYP-2301. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, these risks and uncertainties include the timing, progress and results of the company’s clinical development activities; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the risk that results of clinical trials may not be predictive of future results, and interim and preliminary data are subject to further analysis and may change as more data becomes available; unexpected safety or efficacy data observed during clinical trials; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for approval of the company’s product candidates; changes in the regulatory environment; changes in expected or existing competition; the success of current and future license agreements; our dependence on contract research organizations, and other outside vendors and service providers; product liability; the impact of general business and economic conditions; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; delays, interruptions or failures in the manufacture and supply of our product candidates; the availability of and the need for additional financing; our ability to obtain additional funding to support our clinical development programs; uncertainties regarding the timing and results of the August 2022 subpoena from the U.S. Attorney’s Office for the District of Massachusetts; uncertainties regarding the FDA warning letter pertaining to our Watertown, MA manufacturing facility; and other factors described in our filings with the Securities and Exchange Commission (SEC). More detailed information on these and additional factors that could affect our actual results are described in our filings with the SEC, including our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, as revised or supplemented by its Quarterly Reports on Form 10-Q and other documents filed with the SEC. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. EyePoint undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
The Leader in Sustained Release Drug Delivery for Retinal Disease ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Most robust dataset in wet AMD among all sustained delivery programs Two ongoing global Phase 3 non-inferiority pivotal trials in wet AMD Strong balance sheet with ~$370M in cash and equivalents1; cash runway into 2027 DURAVYU™: patent protected vorolanib featuring new MOA delivered via best-in-class technology, Durasert E™ Only sustained release TKI with active program in DME bolstered by highly positive interim Phase 2 clinical data 1. As of December 31, 2024. Unaudited estimate, inclusive of net proceeds from October 2024 equity financing. MOA, mechanism of action; wet AMD, wet age-related macular degeneration; DME, diabetic macular edema 1 2 3 4 5

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. wet AMD, wet age-related macular degeneration; DME, diabetic macular edema; PK, pharmacokinetic; GA, geographic atrophy Pipeline Leveraging Durasert E™ Drug Delivery Technology trial underway non-clinical Durasert E™ Programs Indication Discovery Pre-Clin Phase 1 Phase 2 Phase 3 Anticipated Next Milestone DURAVYU™ – (vorolanib intravitreal insert) (f/k/a EYP-1901) Wet AMD DME EYP-2301 – razuprotafib (TIE-2 agonist) serious retinal diseases Enrollment completion in 2H 2025 Full data Q1 2025 FDA meeting expected Q2 2025 Pre-clin tox and PK data PIVOTAL TRIALS UNDERWAY Positive interim BCVA and CST data Evaluating additional pipeline opportunities

IVT, intravitreal DURASERT E™ TECHNOLOGY Sustained-Release Drug Delivery Standard in-office IVT injection Continuous dosing Zero-order kinetics drug release Bioerodible Durasert E™: Solid injectable insert Drug formulated within a bioerodible matrix Designed to release drug load before matrix fully erodes Favorable safety profile across multiple indications ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Insert is 1/5000 of Vitreous Volume
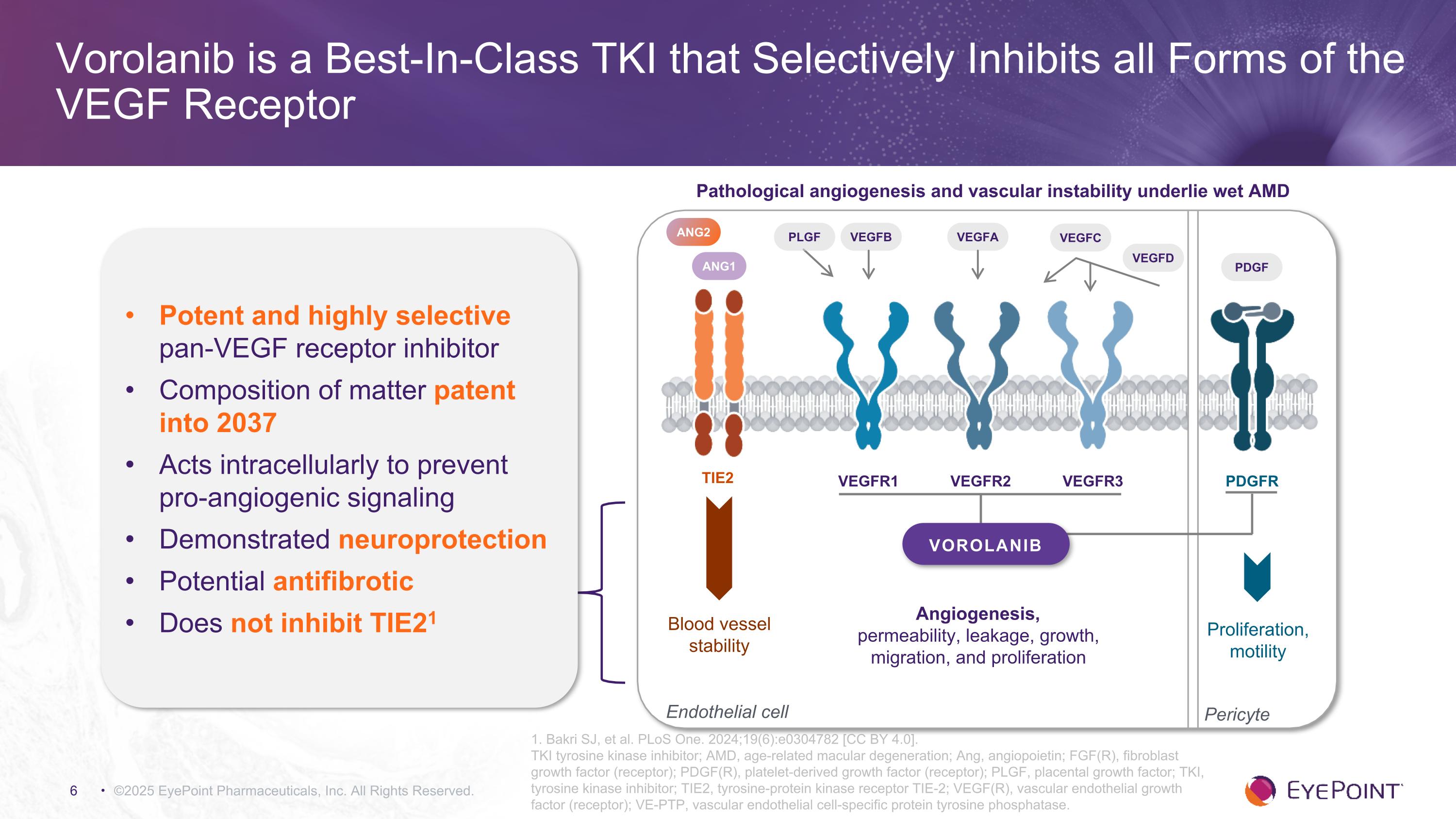
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. Bakri SJ, et al. PLoS One. 2024;19(6):e0304782 [CC BY 4.0]. TKI tyrosine kinase inhibitor; AMD, age-related macular degeneration; Ang, angiopoietin; FGF(R), fibroblast growth factor (receptor); PDGF(R), platelet-derived growth factor (receptor); PLGF, placental growth factor; TKI, tyrosine kinase inhibitor; TIE2, tyrosine-protein kinase receptor TIE-2; VEGF(R), vascular endothelial growth factor (receptor); VE-PTP, vascular endothelial cell-specific protein tyrosine phosphatase. Vorolanib is a Best-In-Class TKI that Selectively Inhibits all Forms of the VEGF Receptor Potent and highly selective pan-VEGF receptor inhibitor Composition of matter patent into 2037 Acts intracellularly to prevent�pro-angiogenic signaling Demonstrated neuroprotection Potential antifibrotic Does not inhibit TIE21 Endothelial cell VEGFB VEGFA VEGFC VEGFD VEGFR1 VEGFR2 VEGFR3 TIE2 PDGFR ANG1 ANG2 PDGF Angiogenesis, �permeability, leakage, growth, migration, and proliferation Blood vessel stability Proliferation, motility PLGF Pericyte VOROLANIB Pathological angiogenesis and vascular instability underlie wet AMD
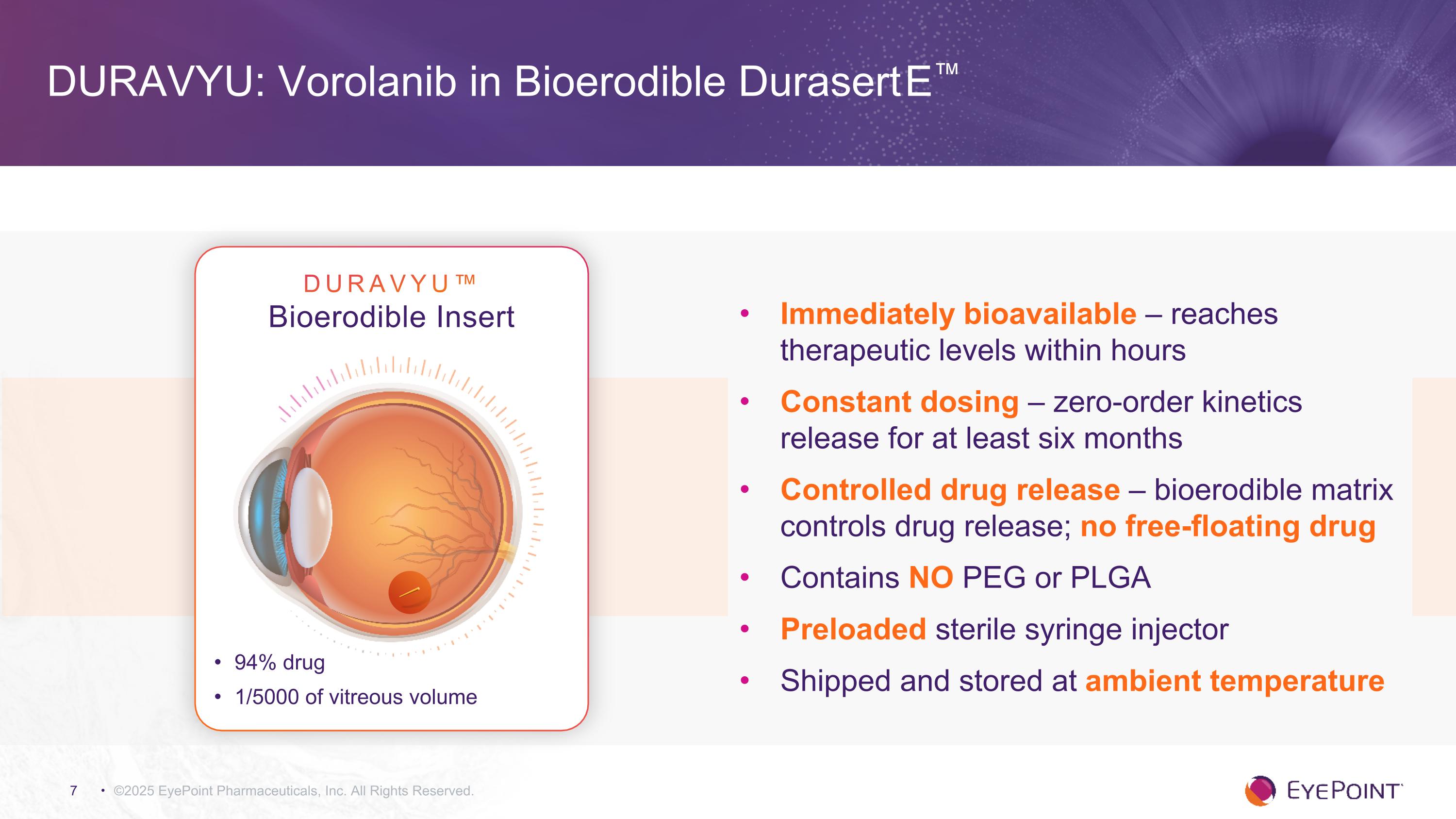
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Immediately bioavailable – reaches therapeutic levels within hours Constant dosing – zero-order kinetics release for at least six months Controlled drug release – bioerodible matrix controls drug release; no free-floating drug Contains NO PEG or PLGA Preloaded sterile syringe injector Shipped and stored at ambient temperature DURAVYU: Vorolanib in Bioerodible Durasert E™ DURAVYU™ Bioerodible Insert 94% drug 1/5000 of vitreous volume
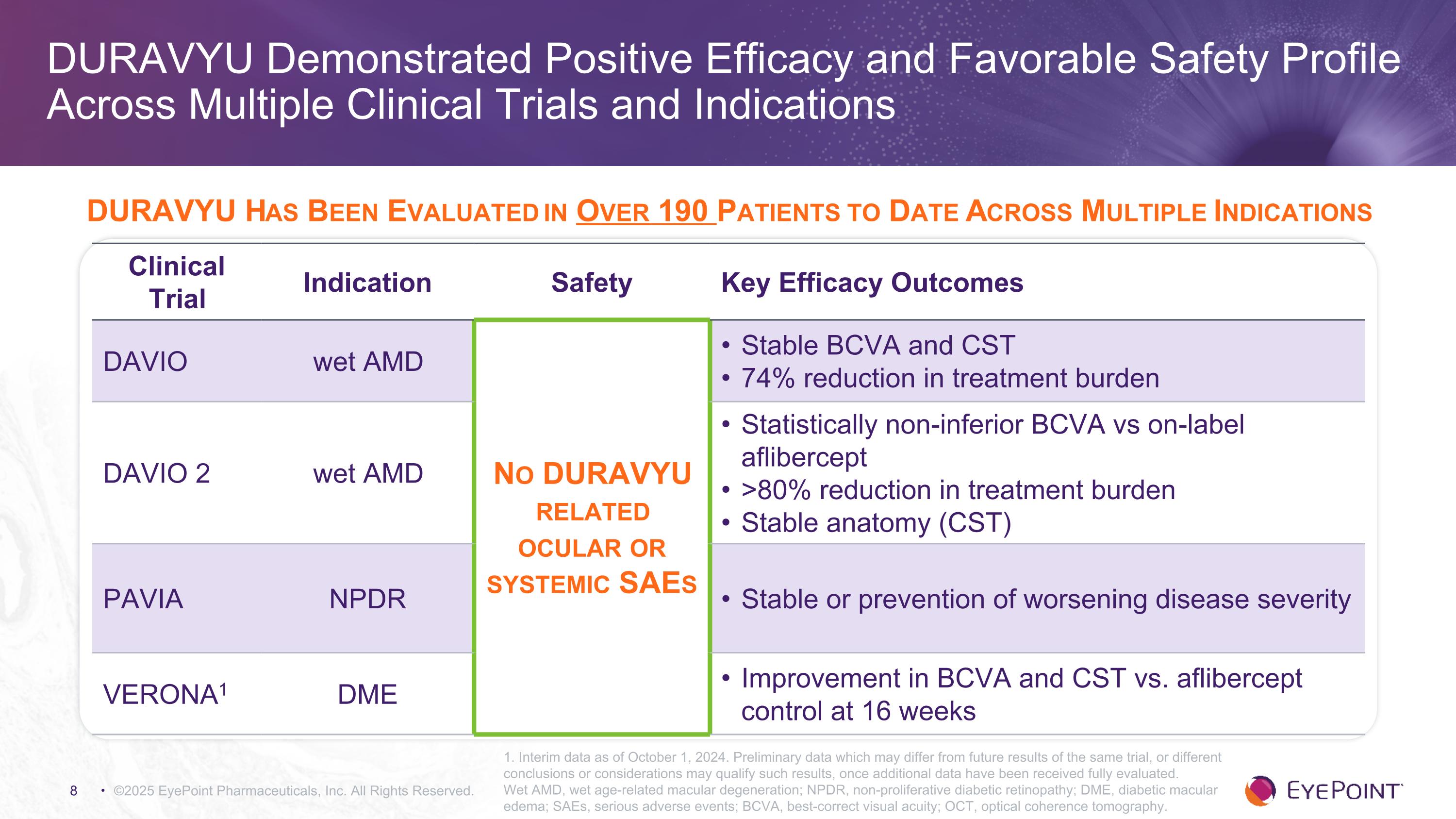
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Demonstrated Positive Efficacy and Favorable Safety Profile Across Multiple Clinical Trials and Indications 1. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated. Wet AMD, wet age-related macular degeneration; NPDR, non-proliferative diabetic retinopathy; DME, diabetic macular edema; SAEs, serious adverse events; BCVA, best-correct visual acuity; OCT, optical coherence tomography. Clinical Trial Indication Safety Key Efficacy Outcomes DAVIO wet AMD No DURAVYU related ocular or systemic SAEs Stable BCVA and CST 74% reduction in treatment burden DAVIO 2 wet AMD Statistically non-inferior BCVA vs on-label aflibercept >80% reduction in treatment burden Stable anatomy (CST) PAVIA NPDR Stable or prevention of worsening disease severity VERONA1 DME Improvement in BCVA and CST vs. aflibercept control at 16 weeks DURAVYU Has Been Evaluated in Over 190 Patients to Date Across Multiple Indications

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Positive Results in�wet AMD as a 6-Month Maintenance Therapy A NON-INFERIORITY TRIAL VERSUS AN AFLIBERCEPT CONTROL
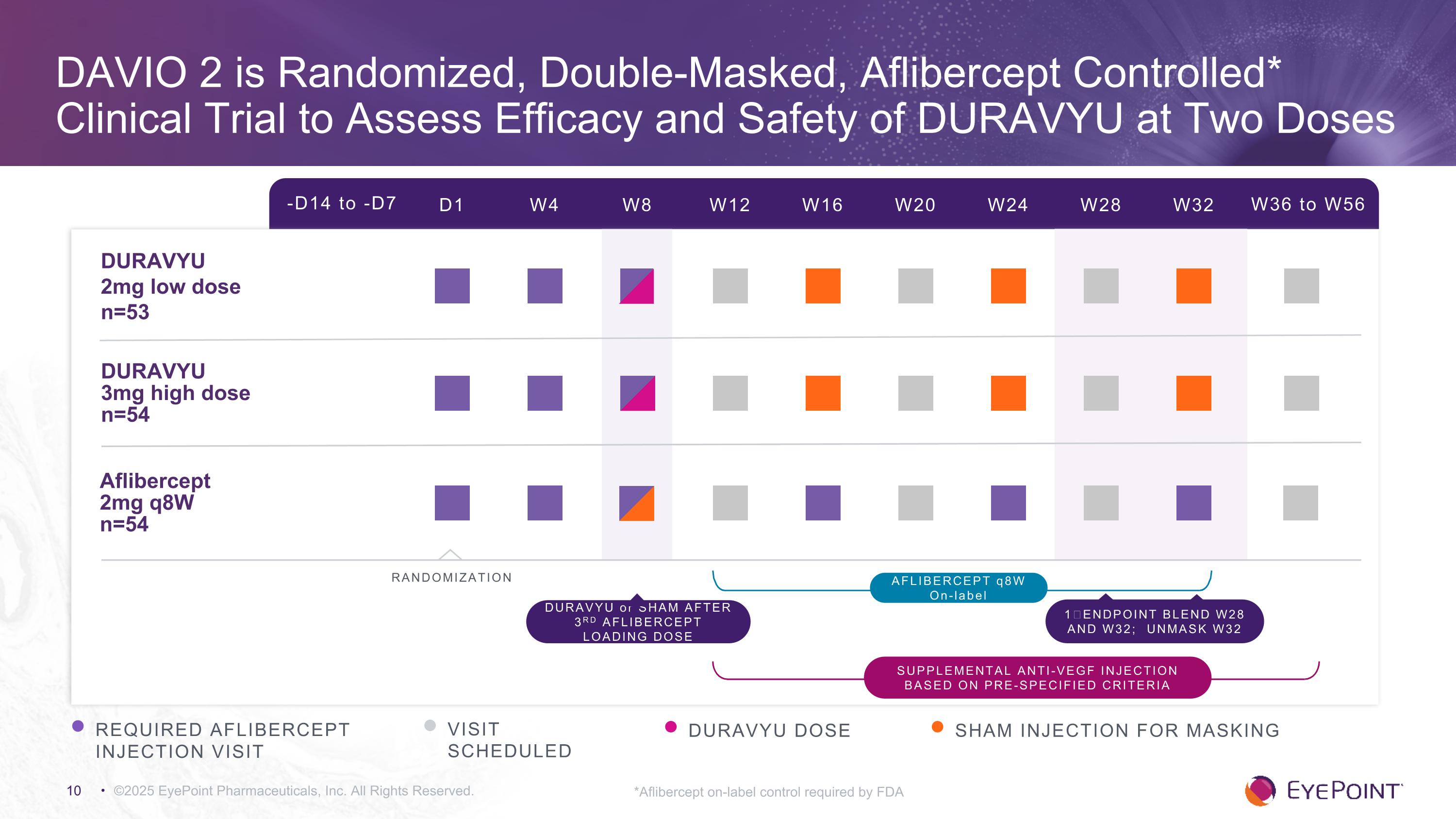
DAVIO 2 is Randomized, Double-Masked, Aflibercept Controlled* Clinical Trial to Assess Efficacy and Safety of DURAVYU at Two Doses ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Aflibercept on-label control required by FDA -D14 to -D7 D1 W4 W8 W12 W16 W24 W32 W36 to W56 W20 W28 DURAVYU �2mg low dose n=53 DURAVYU �3mg high dose n=54 Aflibercept 2mg q8W n=54 RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED DURAVYU DOSE AFLIBERCEPT q8W On-label DURAVYU or SHAM AFTER 3RD AFLIBERCEPT LOADING DOSE 1⁰ ENDPOINT BLEND W28 AND W32; UNMASK W32 SHAM INJECTION FOR MASKING Supplemental anti-VEGF injection based on pre-specified criteria

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Phase 2 DAVIO 2 Clinical Trial Met All Endpoints DAVIO 2 Endpoint 2mg dose 3mg dose R Primary: Non-inferior change in BCVA vs. aflibercept - 0.3 letters - 0.4 letters R Secondary: Favorable safety profile1 No DURAVYU-related SAEs R Secondary: Reduction in treatment burden vs. 6 mos. prior 89% 85% R Secondary: Reduction in treatment burden vs. aflibercept 82% 76% R Secondary: Supplement-free up to 6 months 63% 88% of eyes had 0 or 1 supplemental injections 63% 83% of eyes had 0 or 1 supplemental injections R Secondary: Anatomical control vs. aflibercept +12.4um +5.2um

+1.0* +1.3* +0.9* -0.5 -0.1 -0.5 ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU was Statistically Non-Inferior in Change in BCVA Compared to the Aflibercept Control *Blended week 28 and week 32 change vs. baseline **Month 8 represents 6 months after DURAVYU injection 1 – AAO 2022 presentation, Paolo Lanzetta, on behalf of the PULSAR study investigators MEAN CHANGE IN BCVA FROM BASELINE In the Pulsar trial, HD Eylea (16-week 8mg arm) change in BCVA vs. 2mg Eylea was -1.4 letters1 Aflibercept Month 9 Month 10 Month 11 Month 12 Aflibercept Aflibercept Aflibercept Aflibercept Mean Change in BCVA vs Aflibercept at Six Months DURAVYU 2mg -0.3* DURAVYU 3mg -0.4* Primary Endpoint:
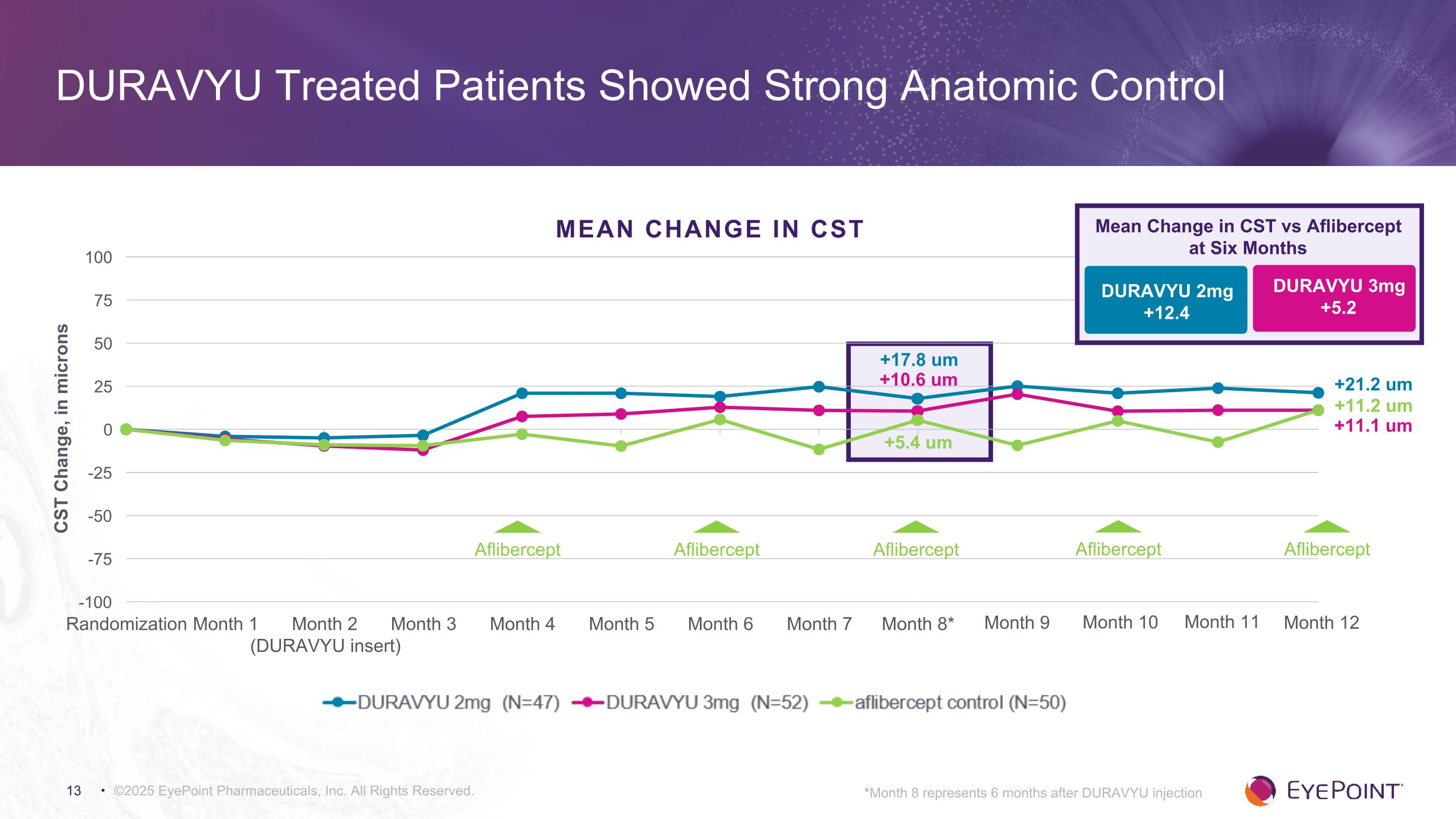
+10.6 um +17.8 um +5.4 um +11.2 um +21.2 um +11.1 um ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Treated Patients Showed Strong Anatomic Control *Month 8 represents 6 months after DURAVYU injection MEAN CHANGE IN CST Aflibercept Month 9 Month 10 Month 11 Month 12 Aflibercept Aflibercept Aflibercept Aflibercept Mean Change in CST vs Aflibercept at Six Months DURAVYU 2mg +12.4 DURAVYU 3mg +5.2

Meaningful Supplement-Free Rates in Eyes Treated with DURAVYU Support DURAVYU as a Potential 6-Month Treatment for Wet AMD ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *First visit patients are eligible to be supplemented **Month 8 represents 6 months post DURAVYU injection Summary of Supplement-Free Rates by Month 100% 100% Aflibercept Aflibercept Aflibercept 96% Month 9 Month 10 Month 11 Month 12 Month 2 (DURAVYU insert) Month 3* Month 4 Month 5 Month 6 Month 7 Month 8** Aflibercept Aflibercept 100% 100% 94%

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU�LUGANO/LUCIA�Phase 3 Pivotal Trials in wet AMD NON-INFERIORITY VERSUS AN AFLIBERCEPT CONTROL

Phase 3 Trials are Designed to Enable Global Regulatory Approvals for DURAVYU ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Demonstrate DURAVYU, when administered every six months, achieves similar visual outcomes to on-label aflibercept while reducing treatment burden ~400 patients per trial Two arms 2.7mg DURAVYU aflibercept on-label control DURAVYU dosing every 6-months One-year efficacy and safety endpoint for NDA submission Primary Endpoint: difference in mean change in BCVA from Day 1 to Week 52 and 56 (blended) versus aflibercept control Secondary endpoints: safety, reduction in treatment burden, percent of eyes supplement-free, anatomical stability LUGANO and LUCIA Trials: global, randomized, double-masked, aflibercept controlled Objective Trial Design Endpoints

DURAVYU™ in Wet AMD Phase 3 Pivotal Trial Design ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Screening D1 W4 W8 W12 W16 W24 W32 W84-W92 W20 W28 DURAVYU™ �2.7mg Aflibercept 2mg q8W RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED DURAVYU™ DOSE AFLIBERCEPT q8W 1⁰ ENDPOINT BLEND W52 AND W56; UNMASK W56 SHAM INJECTION FOR MASKING W36 W40 W44 W48 W52 W56 W60-W76 W80 W96 EOS AFLIBERCEPT Q8W Sham injection For Masking Supplemental anti-VEGF injection based on strict criteria AFLIBERCEPT load DURAVYU DURAVYU DURAVYU DURAVYU LUGANO/LUCIA First Patient Dosed Topline Data in 2026

Exceptional Enrollment to Date Driven by Significant Investigator and Patient Enthusiasm ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. June 2024 Final Phase 3 protocols October 2024 First patient in LUGANO trial dosed December 2024 First patient in LUCIA trial dosed 2H 2025 Expected full enrollment of both Phase 3 pivotal trials Top line data for both Phase 3 pivotal trials anticipated in 2026 January 2025 LUGANO trial ~1/3 enrolled; LUCIA exceeding expectations 2026 Topline data for pivotal program

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 VERONA Clinical Trial in DME – 16-Week Interim Results all patients Have completed the week 16 visit DME, diabetic macular edema Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated.

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DME, diabetic macular edema; VEGF, vascular endothelial growth factor; BCVA, best corrected visual acuity; OCT, optical coherence tomography; CST, central subfield thickness DURAVYU dosing Visit Scheduled aflibercept injection Sham injection DURAVYU 1.3mg (n=10) DURAVYU 2.7mg (n=11) Aflibercept 2mg single injection (n=6) Supplemental Anti-VEGF injection based on prespecified criteria Objectives: Evaluate the safety and efficacy of DURAVYU in DME Collect dose-ranging data to inform Phase 3 clinical trials Primary endpoint: time to supplemental anti-VEGF injection up to week 24 Key Secondary endpoints: safety, change in BCVA vs. aflibercept control and anatomical control (CST) Primary endpoint -D28 to -D7 D1 W4 W8 W12 W16 W20 W24 Positive interim data; full topline data anticipated in Q1 2025 Phase 2 VERONA Clinical Trial is a Randomized, Open-Label, Aflibercept Controlled Trial as a Potential Treatment for DME
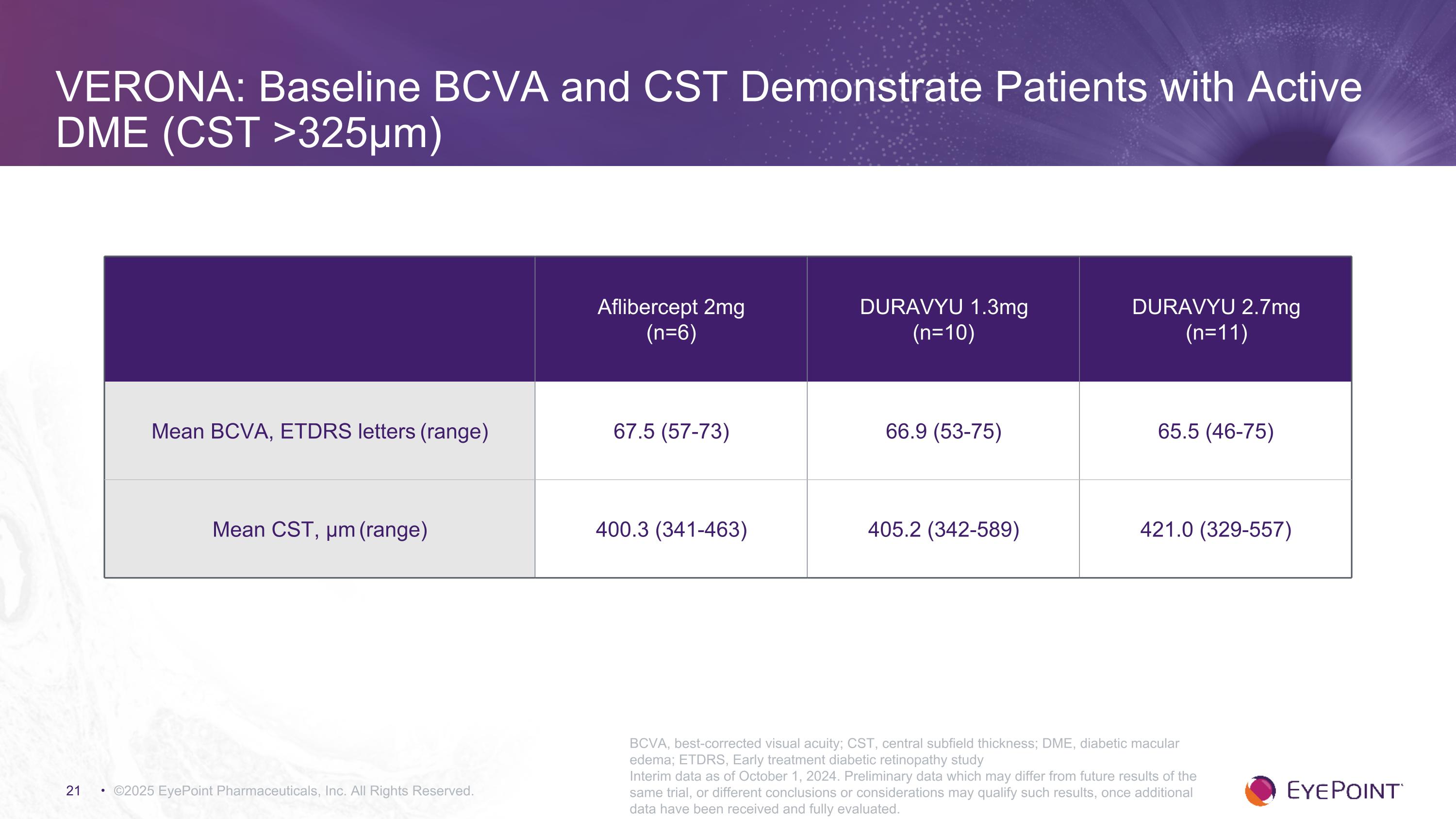
VERONA: Baseline BCVA and CST Demonstrate Patients with Active DME (CST >325μm) ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Aflibercept 2mg (n=6) DURAVYU 1.3mg (n=10) DURAVYU 2.7mg (n=11) Mean BCVA, ETDRS letters (range) 67.5 (57-73) 66.9 (53-75) 65.5 (46-75) Mean CST, μm (range) 400.3 (341-463) 405.2 (342-589) 421.0 (329-557) BCVA, best-corrected visual acuity; CST, central subfield thickness; DME, diabetic macular edema; ETDRS, Early treatment diabetic retinopathy study Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated.

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. VERONA: DURAVYU 2.7mg Demonstrated Clinically Meaningful Improvement in BCVA at 16 Weeks ~Six Letters Better vs. Aflibercept Control DURAVYU 2.7mg +5.7 Mean Change in BCVA vs Aflibercept DURAVYU 1.3mg +0.6 +3.8 +8.9 +3.2 MEAN CHANGE IN BCVA FROM BASELINE BCVA, best-corrected visual acuity Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated.
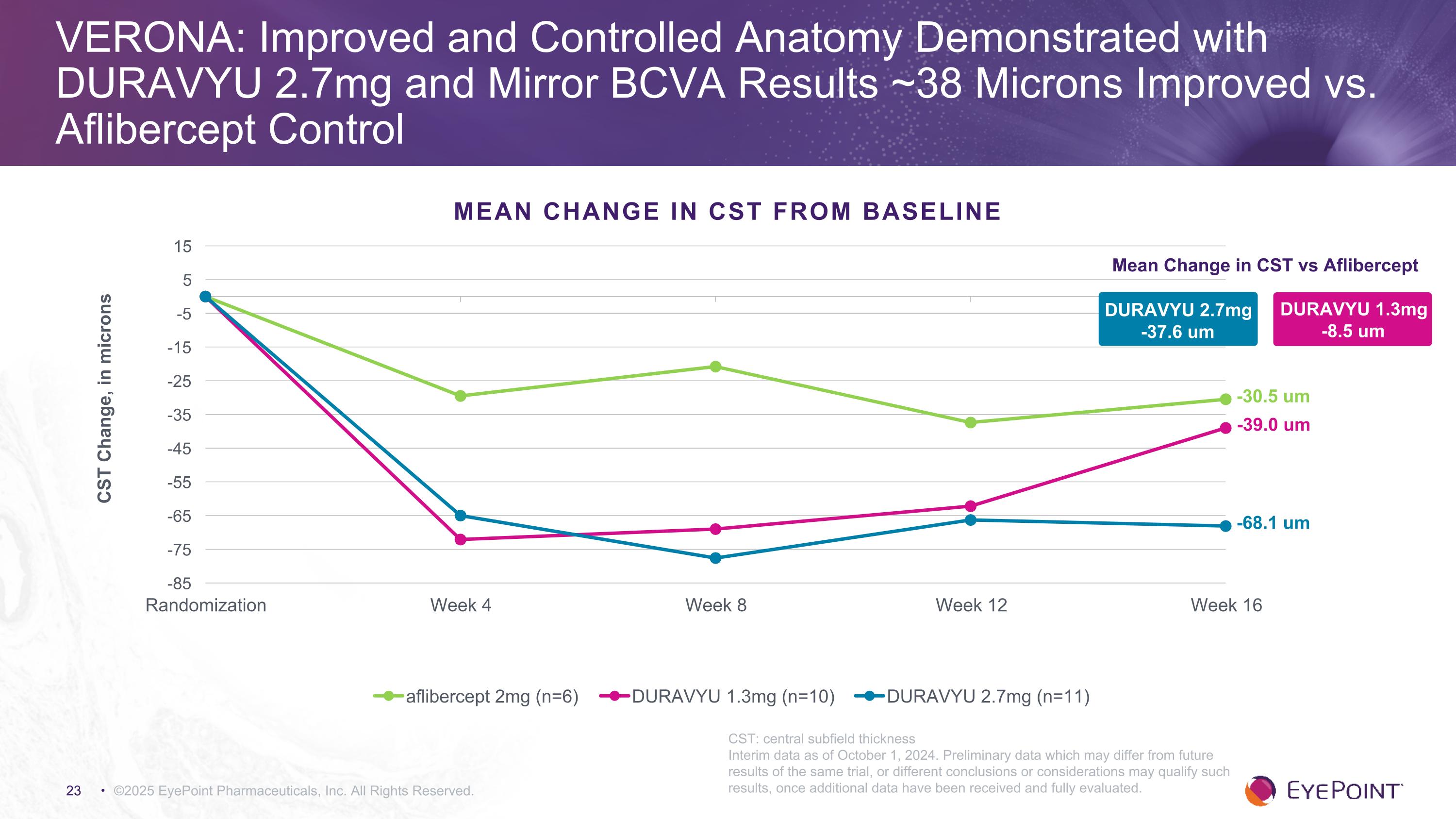
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. VERONA: Improved and Controlled Anatomy Demonstrated with DURAVYU 2.7mg and Mirror BCVA Results ~38 Microns Improved vs. Aflibercept Control Mean Change in CST vs Aflibercept DURAVYU 1.3mg -8.5 um -39.0 um -68.1 um -30.5 um MEAN CHANGE IN CST FROM BASELINE CST: central subfield thickness Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. DURAVYU 2.7mg -37.6 um
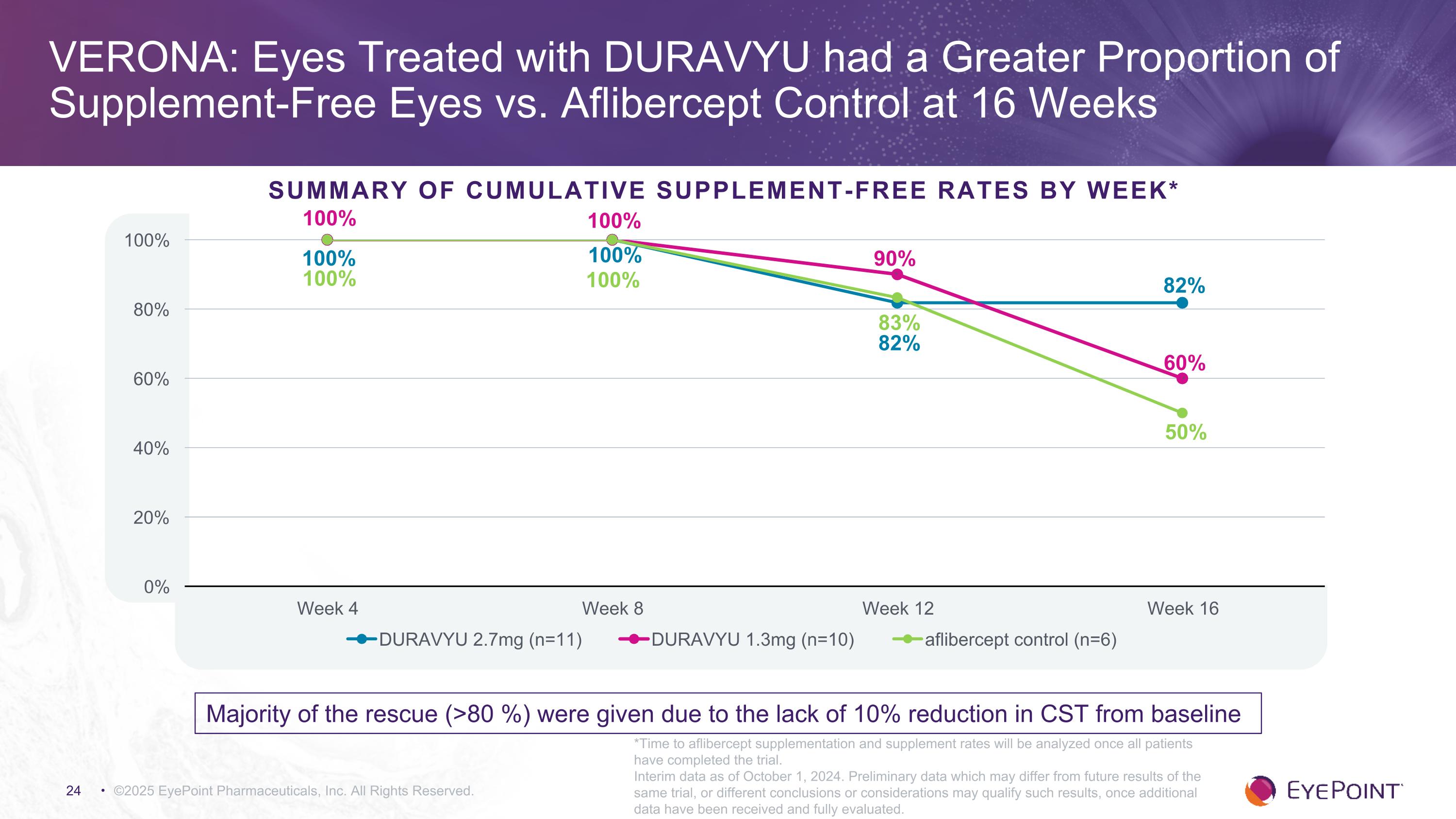
VERONA: Eyes Treated with DURAVYU had a Greater Proportion of �Supplement-Free Eyes vs. Aflibercept Control at 16 Weeks ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Summary of Cumulative Supplement-Free Rates by Week* 100% 100% 100% *Time to aflibercept supplementation and supplement rates will be analyzed once all patients have completed the trial. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Majority of the rescue (>80 %) were given due to the lack of 10% reduction in CST from baseline

VERONA:�Positive Interim Data Supports DURAVYU as a Potential Treatment for DME��Data support potential for vision improvement in DME as well as superior dosing intervals ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2.7MG EFFICACY 16-Week Results: Early and sustained BCVA improvement Early and sustained CST improvement Greater proportion of supplement-free eyes vs. aflibercept control1 Improvements in BCVA and CST appear to be driven by treatment with DURAVYU and not supplemental injections DURAVYU OVERALL SAFETY Results: No ocular or systemic DURAVYU-related SAEs No cases of: Endophthalmitis Retinal vasculitis (occlusive or non-occlusive) Intraocular inflammation (IOI) Insert migration into the anterior chamber 1. Time to aflibercept supplementation and supplement rates will be analyzed once all patients have completed the trial. DME, diabetic macular edema; BCVA, best-corrected visual acuity; CST, central subfield thickness; SAEs, serious adverse events. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated.

Commercial Manufacturing Facility Opened in October 2024 ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. FDA, Federal Drug Administration; EMA, European Medicines Agency; cGMP, current good manufacturing practice Located in Northbridge, MA Built to US FDA and EU EMA standards 40,000sf cGMP manufacturing facility Built to EYPT specifications by landlord preserving upfront cash investment New manufacturing site for commercial production of DURAVYU

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. EYP-2301: razuprotafib in Durasert E™ A sustained delivery tie-2 agonist for severe retinal diseases

EYP-2301: Razuprotafib in Durasert E™ is a Patented TIE-2 Agonist as a Potential New MOA for Treating Serious Retinal Diseases ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. Heier et al. Retina, 2021;41:1-19. and Joussen et al. Eye 2021; 35:1305-1316.; 2. Hammes, et. Al – Diabetes.2011 Jan 1; 3. Shen et al. JCI, 2014; 124:4564; 3. Campochiaro et al. Ophthalmology, 2016; 123:1722-1730; 4. Phase 2 TIME 2a clinical trial conducted by Aerpio. 5.Campochiaro et al. PubMed 2016 123(8):1722-1730. DOI: 10.1016/j.ophtha.2016.04.025 EYP-2301 targets vascular endothelial protein tyrosine phosphatase (VE-PTP) activating TIE-2 and downregulating ANG2 to maintain vascular stability in the retina P P EYP-2301 Blood vessel lumen Intracellular space VE-PTP ANG2 Tie-2 activation combined with VEGF inhibition has the potential to enhance efficacy and extend durability1 of treatment Razuprotafib (f/k/a AKB-9778) delivered subcutaneously demonstrated preclinical and clinical proof of concept in posterior segment disease 2,3 In a Phase 2 clinical trial, razuprotafib combined with ranibizumab, was more effective than ranibizumab alone at reducing macular edema with a favorable safety and tolerability profile4,5

The Leader in Sustained Release Drug Delivery for Retinal Disease ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Most robust dataset in wet AMD among all sustained delivery programs Two ongoing global Phase 3 non-inferiority pivotal trials in wet AMD Strong balance sheet with ~$370M in cash and equivalents1; cash runway into 2027 DURAVYU™: patent protected vorolanib with new MOA delivered via best-in-class technology, Durasert E™ Only sustained release TKI with active program in DME bolstered by highly positive interim Phase 2 clinical data 1. As of December 31, 2024. Unaudited estimate, inclusive of net proceeds from October 2024 equity financing. MOA, mechanism of action; wet AMD, wet age-related macular degeneration; DME, diabetic macular edema 1 2 3 4 5

J.P. Morgan Healthcare Conference Presentation January 14, 2025 Jay Duker, M.D. President and CEO ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved.





























