
NASDAQ: EYPT Investor Presentation April 2019 Exhibit 99.1

Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: Various statements made in this release are forward-looking, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. This presentation is intended for communication for investors only. Nothing in this presentation should be construed as promoting the use of DEXYCU™, YUTIQ™ or other product candidates. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, including but not limited to statements about our commercialization of YUTIQ and DEXYCU, the potential for our products to alter the treatment landscape for ocular diseases, the expected use of proceeds from our refinancing transactions and our belief that the amounts available from the CRG credit facility together with our current cash and cash equivalent position are sufficient to fund our operations and debt service obligations through the remainder of 2019, are forward-looking statements. Some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements include uncertainties with respect to: our ability to achieve profitable operations and access to needed capital; fluctuations in our operating results; our ability to successfully produce commercial supply of YUTIQ and DEXYCU and successfully commercialize YUTIQ and DEXYCU in the U.S.; our ability to successfully build a commercial infrastructure and enter into and maintain commercial agreements for the launch of YUTIQ and DEXYCU; the successful release of our YUTIQ line extension shorter-acting treatment for uveitis; potential off-label sales of ILUVIEN for non-infectious posterior segment uveitis (“NIPU”); consequences of fluocinolone acetonide side effects for YUTIQ; consequences of dexamethasone side effects for DEXYCU; successful commercialization of, and receipt of revenues from Alimera Life Sciences, Inc. (“Alimera”) from its sales of ILUVIEN for diabetic macular edema (“DME”); Alimera’s ability to obtain additional marketing approvals and the effect of pricing and reimbursement decisions on sales of ILUVIEN for DME; Alimera’s ability to obtain marketing approval for ILUVIEN in its licensed territories for NIPU; potential declines in Retisert royalties; our ability to market and sell products; the success of current and future license agreements; termination or breach of current license agreements; our dependence on contract research organizations, contract sales organizations, vendors and investigators; effects of competition and other developments affecting sales of products; market acceptance of products; effects of guidelines, recommendations and studies; protection of intellectual property and avoiding intellectual property infringement; retention of key personnel; product liability; industry consolidation; compliance with environmental laws; manufacturing risks; risks and costs of international business operations; legislative or regulatory changes; volatility of stock price; possible dilution; absence of dividends; and other factors described in our filings with the Securities and Exchange Commission. You should read and interpret any forward-looking statements in light of these risks. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.

Acquired Icon Bioscience to transform business and accelerate growth Launched YUTIQTM (Feb 4, 2019) and DEXYCUTM (Mar 12, 2019) (J-Code reimbursement in place for both products) Established a strong leadership team with seasoned executives to lead our commercial strategy and manage our sales infrastructure Executing on strategy to commercialize our own products, expand our ophthalmology portfolio and utilize our existing technology platforms Obtained $80M+ from equity and debt partners in 2018 Chronic non-infectious uveitis affecting the posterior segment of the eye Postoperative inflammation following ocular surgery EyePoint Highlights: Transformational Opportunity in Ophthalmology

Management with Proven Commercial Track Record & Highly Experienced Board of Directors Dr. Göran Ando Chairman of the Board Dr. David J Mazzo Director Nancy Lurker President and CEO Dr. John Landis Director Doug Godshall Director Dr. Jay Duker Director Dr. David Guyer Director Ron Eastman Director Board of Directors Nancy Lurker President and CEO David Price Chief Financial Officer Dario Paggiarino, M.D. Chief Medical Officer Jack Weet, Ph.D. SVP, Regulatory Affairs & Quality Kristine Peterson Director Michael W Rogers Director
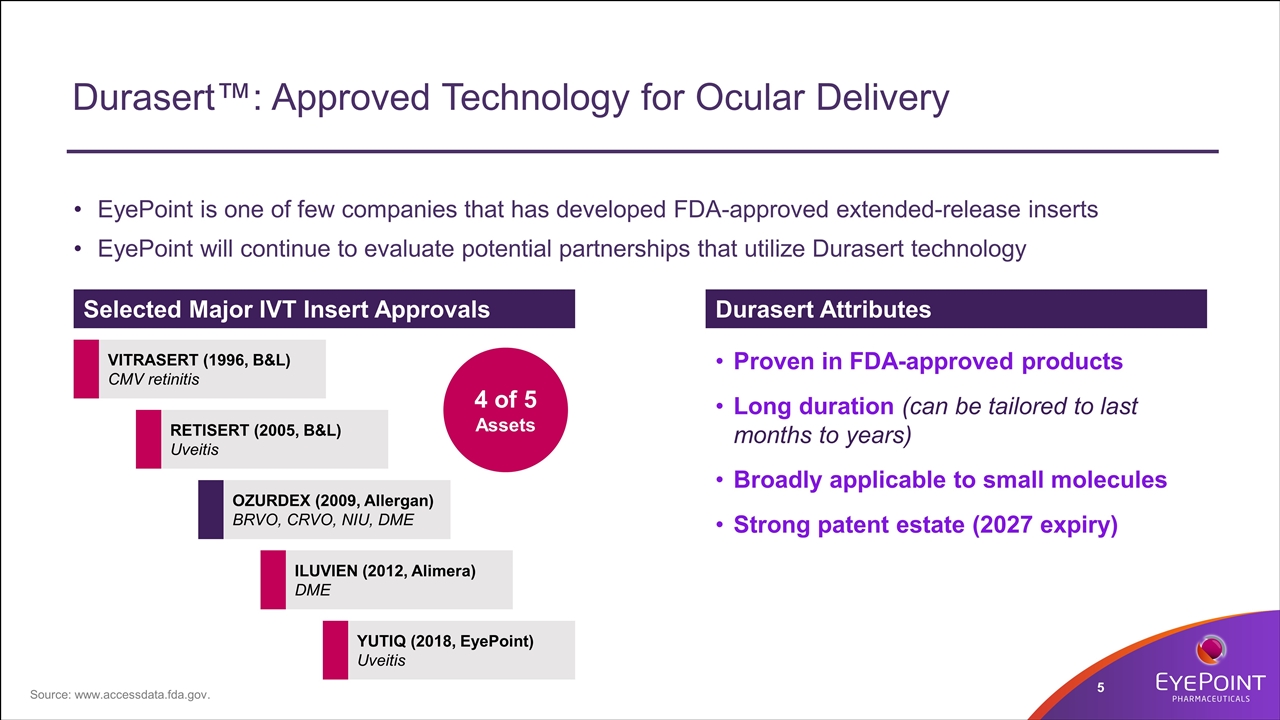
Durasert™: Approved Technology for Ocular Delivery EyePoint is one of few companies that has developed FDA-approved extended-release inserts EyePoint will continue to evaluate potential partnerships that utilize Durasert technology OZURDEX (2009, Allergan) BRVO, CRVO, NIU, DME RETISERT (2005, B&L) Uveitis VITRASERT (1996, B&L) CMV retinitis ILUVIEN (2012, Alimera) DME YUTIQ (2018, EyePoint) Uveitis Selected Major IVT Insert Approvals 4 of 5 Assets Durasert Attributes Proven in FDA-approved products Long duration (can be tailored to last months to years) Broadly applicable to small molecules Strong patent estate (2027 expiry) Source: www.accessdata.fda.gov.
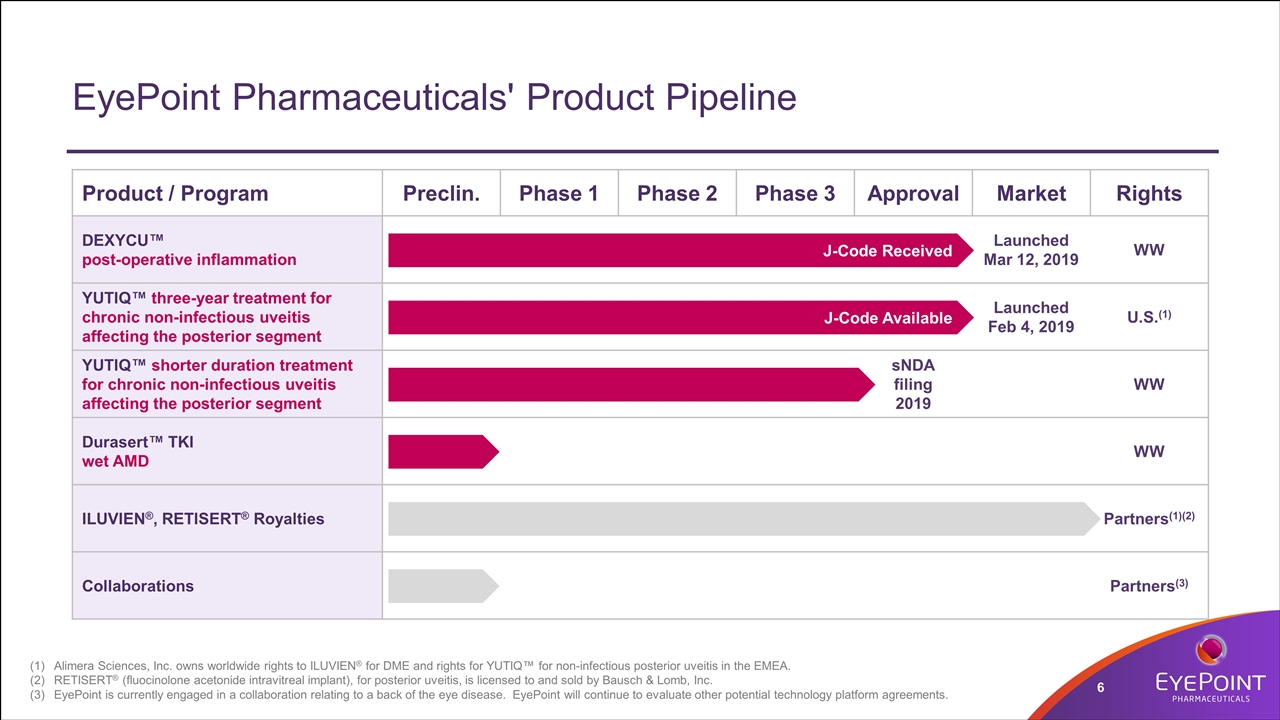
EyePoint Pharmaceuticals' Product Pipeline Product / Program Preclin. Phase 1 Phase 2 Phase 3 Approval Market Rights DEXYCU™ post-operative inflammation Launched Mar 12, 2019 WW YUTIQ™ three-year treatment for chronic non-infectious uveitis affecting the posterior segment Launched Feb 4, 2019 U.S.(1) YUTIQ™ shorter duration treatment for chronic non-infectious uveitis affecting the posterior segment sNDA filing 2019 WW Durasert™ TKI wet AMD WW ILUVIEN®, RETISERT® Royalties Partners(1)(2) Collaborations Partners(3) J-Code Received J-Code Available Alimera Sciences, Inc. owns worldwide rights to ILUVIEN® for DME and rights for YUTIQ™ for non-infectious posterior uveitis in the EMEA. RETISERT® (fluocinolone acetonide intravitreal implant), for posterior uveitis, is licensed to and sold by Bausch & Lomb, Inc. EyePoint is currently engaged in a collaboration relating to a back of the eye disease. EyePoint will continue to evaluate other potential technology platform agreements.

DEXYCUTM & YUTIQTM Commercialization Roadmap Medical Education Plan Rolled Out Multiple KOL Advisory Boards & significant presence at key Congresses Robust Publication plan and key papers published with continued data flow MSL team complete Contract Sales Organization in Place (44 reps in total) Dedicated sales team has been interviewed and chosen by EyePoint Management 34 Key Account Managers (KAMs) focused exclusively on DEXYCUTM 10 KAMs focused exclusively on YUTIQTM KAMs and back office support managed by CSO National Sales Director and DMs managed by EyePoint Payor and Reimbursement Team in Place Dedicated team in place Reimbursement support services will be provided J-Code (J1095) received for DEXYCUTM J-Code available for YUTIQTM Third party logistics (3PL) in place EyePoint Assist launched

Postoperative inflammation following ocular surgery
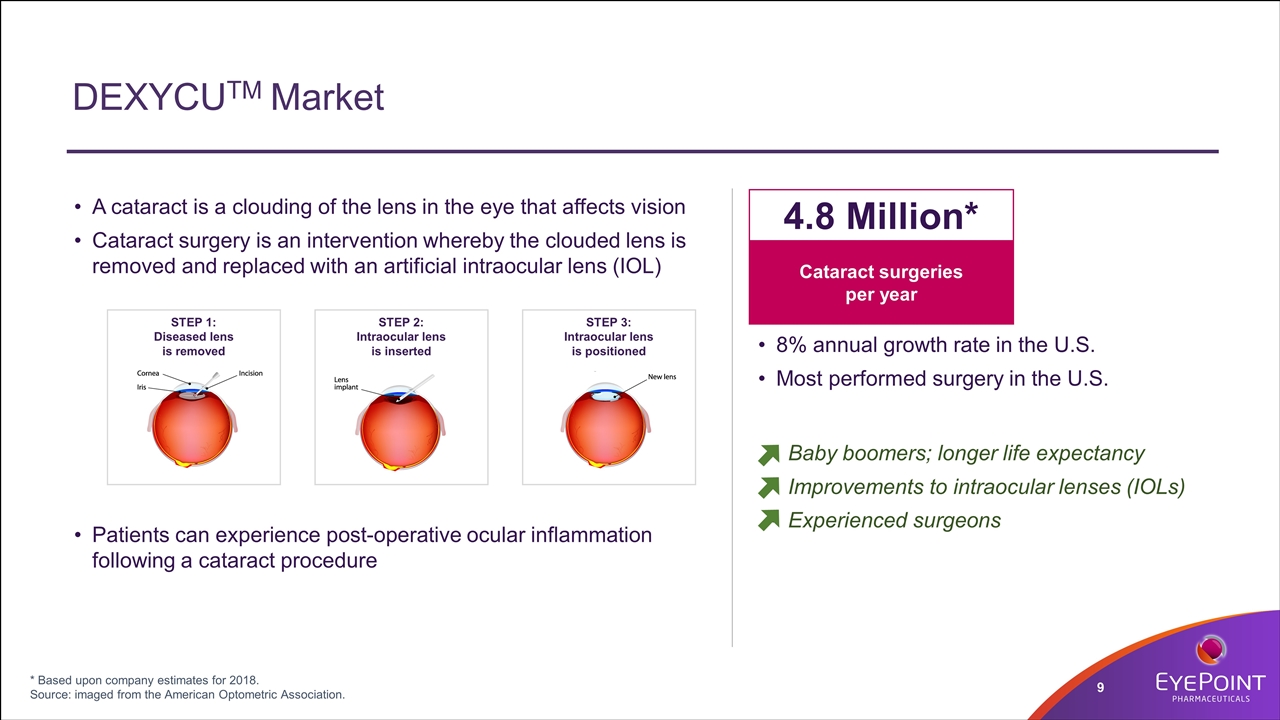
DEXYCUTM Market 8% annual growth rate in the U.S. Most performed surgery in the U.S. Cataract surgeries per year 4.8 Million* Baby boomers; longer life expectancy Improvements to intraocular lenses (IOLs) Experienced surgeons * Based upon company estimates for 2018. Source: imaged from the American Optometric Association. A cataract is a clouding of the lens in the eye that affects vision Cataract surgery is an intervention whereby the clouded lens is removed and replaced with an artificial intraocular lens (IOL) Patients can experience post-operative ocular inflammation following a cataract procedure STEP 2: Intraocular lens is inserted STEP 3: Intraocular lens is positioned STEP 1: Diseased lens is removed
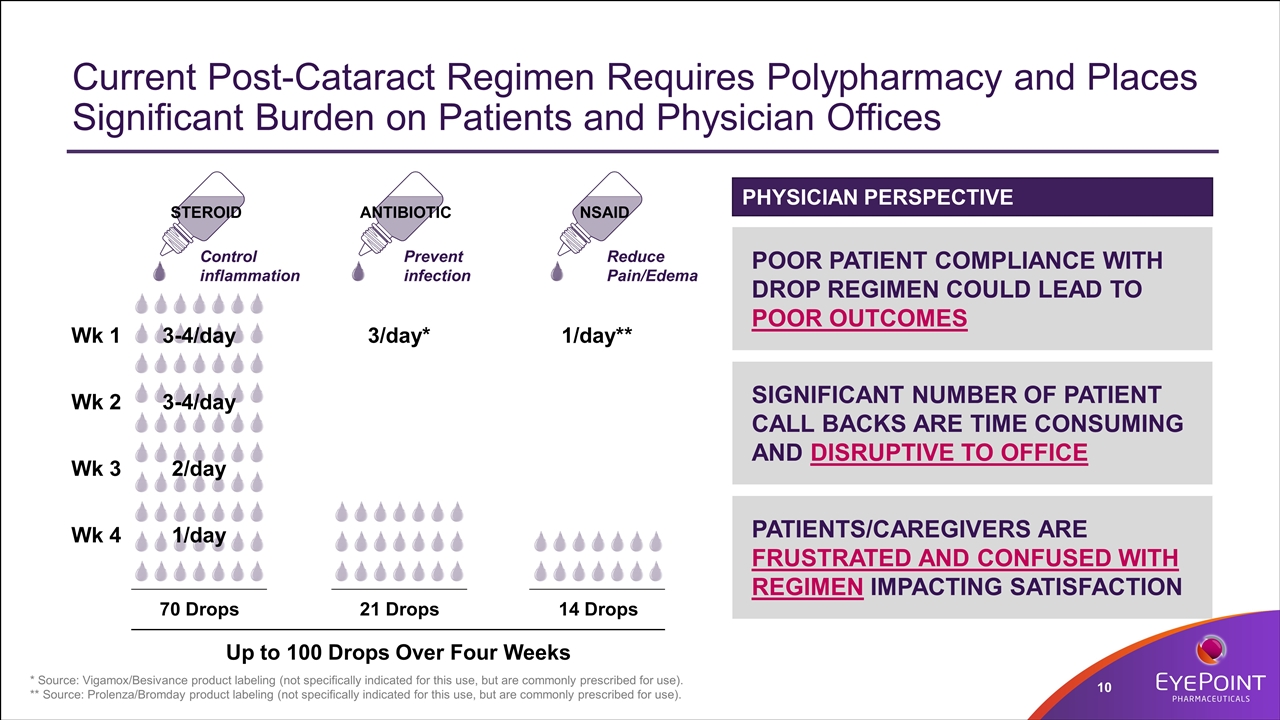
Current Post-Cataract Regimen Requires Polypharmacy and Places Significant Burden on Patients and Physician Offices * Source: Vigamox/Besivance product labeling (not specifically indicated for this use, but are commonly prescribed for use). ** Source: Prolenza/Bromday product labeling (not specifically indicated for this use, but are commonly prescribed for use). Wk 1 Wk 2 Wk 3 Wk 4 21 Drops 3/day* Up to 100 Drops Over Four Weeks ANTIBIOTIC Prevent infection 14 Drops 1/day** NSAID Reduce Pain/Edema 70 Drops 3-4/day 3-4/day 2/day 1/day STEROID Control inflammation POOR PATIENT COMPLIANCE WITH DROP REGIMEN COULD LEAD TO POOR OUTCOMES SIGNIFICANT NUMBER OF PATIENT CALL BACKS ARE TIME CONSUMING AND DISRUPTIVE TO OFFICE PATIENTS/CAREGIVERS ARE FRUSTRATED AND CONFUSED WITH REGIMEN IMPACTING SATISFACTION PHYSICIAN PERSPECTIVE
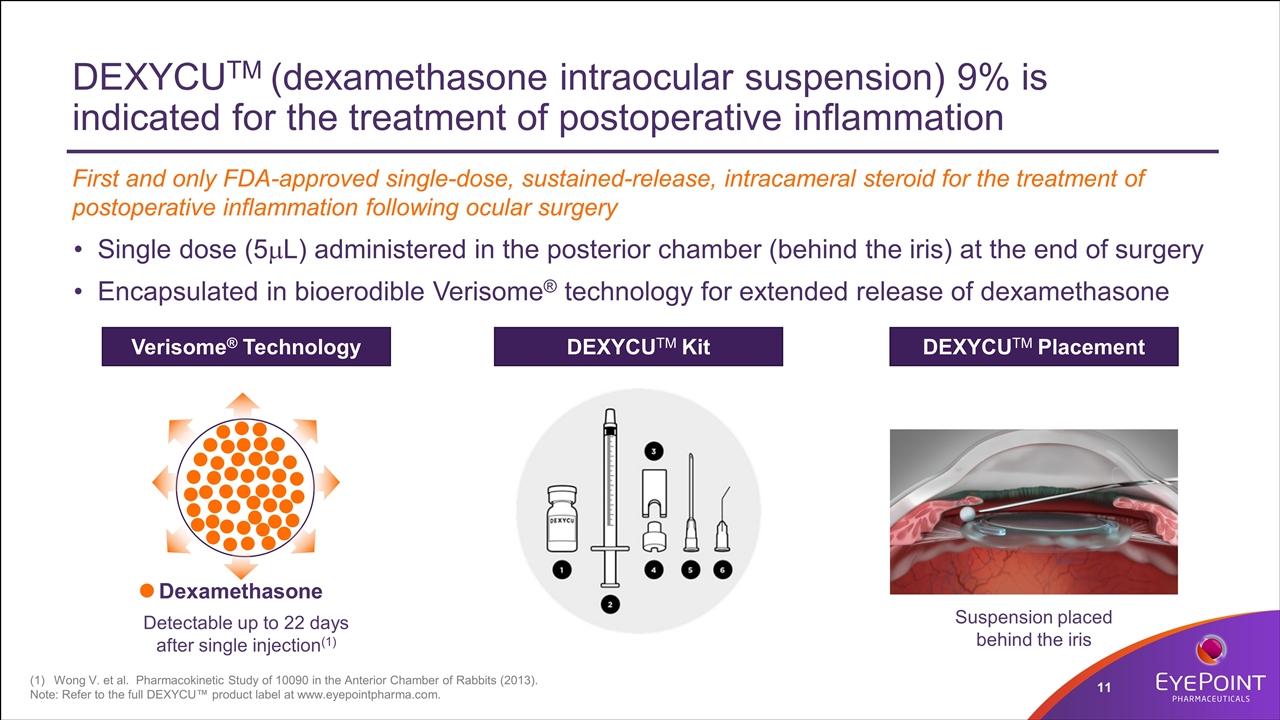
DEXYCUTM (dexamethasone intraocular suspension) 9% is indicated for the treatment of postoperative inflammation Single dose (5mL) administered in the posterior chamber (behind the iris) at the end of surgery Encapsulated in bioerodible Verisome® technology for extended release of dexamethasone Wong V. et al. Pharmacokinetic Study of 10090 in the Anterior Chamber of Rabbits (2013). Note: Refer to the full DEXYCU™ product label at www.eyepointpharma.com. Dexamethasone Verisome® Technology DEXYCUTM Kit DEXYCUTM Placement Detectable up to 22 days after single injection(1) Suspension placed behind the iris First and only FDA-approved single-dose, sustained-release, intracameral steroid for the treatment of postoperative inflammation following ocular surgery
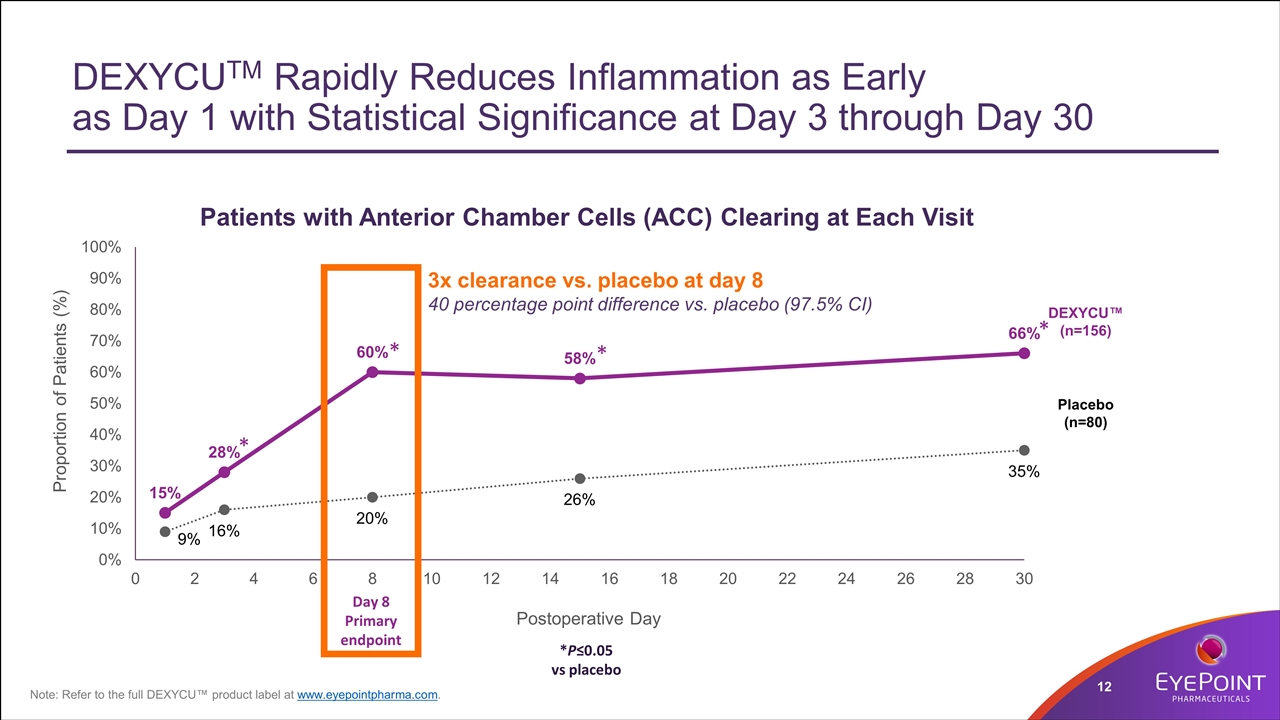
DEXYCUTM Rapidly Reduces Inflammation as Early as Day 1 with Statistical Significance at Day 3 through Day 30 * *P≤0.05 vs placebo Day 8 Primary endpoint Placebo (n=80) DEXYCU™ (n=156) * * * Note: Refer to the full DEXYCU™ product label at www.eyepointpharma.com. 3x clearance vs. placebo at day 8 40 percentage point difference vs. placebo (97.5% CI)
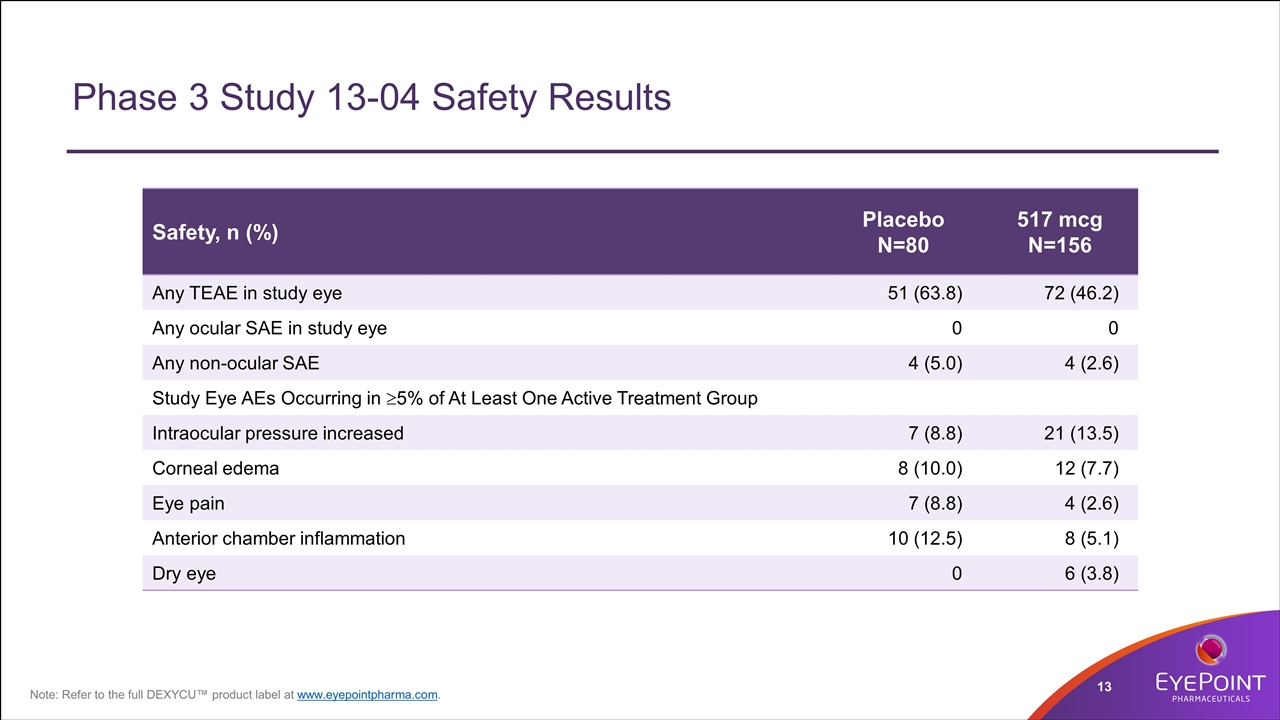
Phase 3 Study 13-04 Safety Results Safety, n (%) Placebo N=80 517 mcg N=156 Any TEAE in study eye 51 (63.8) 72 (46.2) Any ocular SAE in study eye 0 0 Any non-ocular SAE 4 (5.0) 4 (2.6) Study Eye AEs Occurring in ³5% of At Least One Active Treatment Group Intraocular pressure increased 7 (8.8) 21 (13.5) Corneal edema 8 (10.0) 12 (7.7) Eye pain 7 (8.8) 4 (2.6) Anterior chamber inflammation 10 (12.5) 8 (5.1) Dry eye 0 6 (3.8) Note: Refer to the full DEXYCU™ product label at www.eyepointpharma.com.
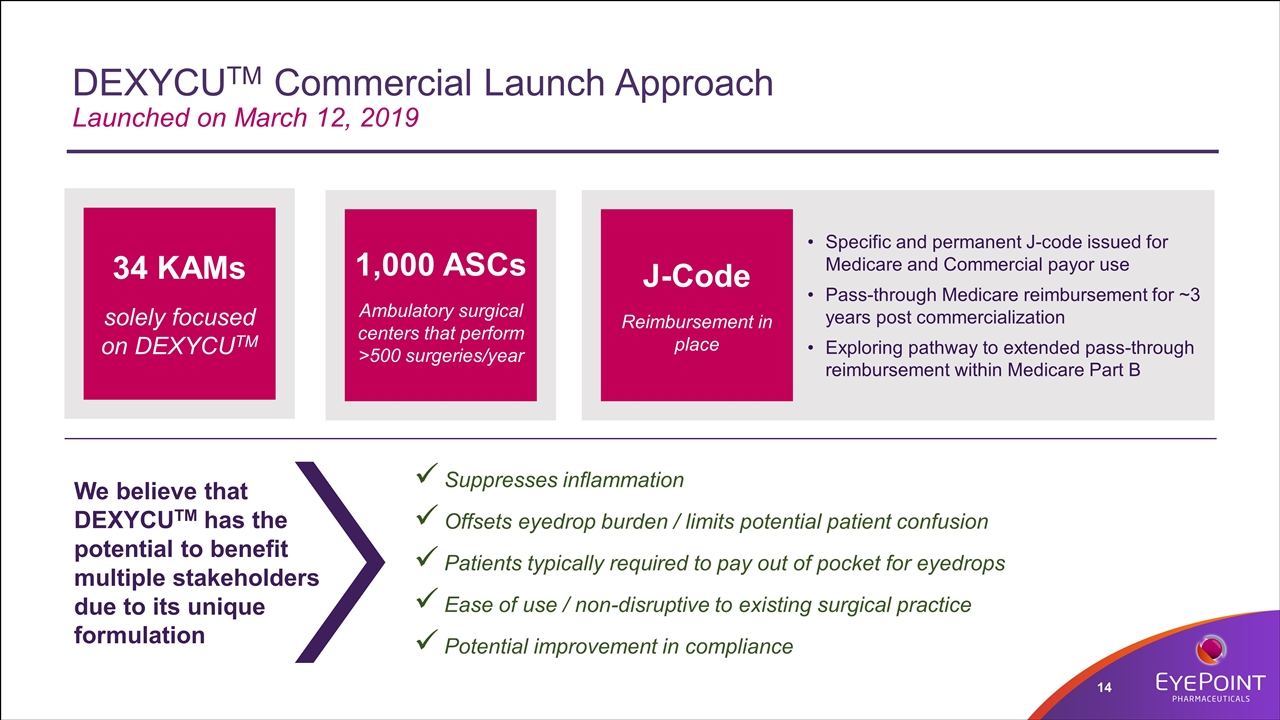
DEXYCUTM Commercial Launch Approach Launched on March 12, 2019 Suppresses inflammation Offsets eyedrop burden / limits potential patient confusion Patients typically required to pay out of pocket for eyedrops Ease of use / non-disruptive to existing surgical practice Potential improvement in compliance 34 KAMs solely focused on DEXYCUTM 1,000 ASCs Ambulatory surgical centers that perform >500 surgeries/year J-Code Reimbursement in place Specific and permanent J-code issued for Medicare and Commercial payor use Pass-through Medicare reimbursement for ~3 years post commercialization Exploring pathway to extended pass-through reimbursement within Medicare Part B We believe that DEXYCUTM has the potential to benefit multiple stakeholders due to its unique formulation
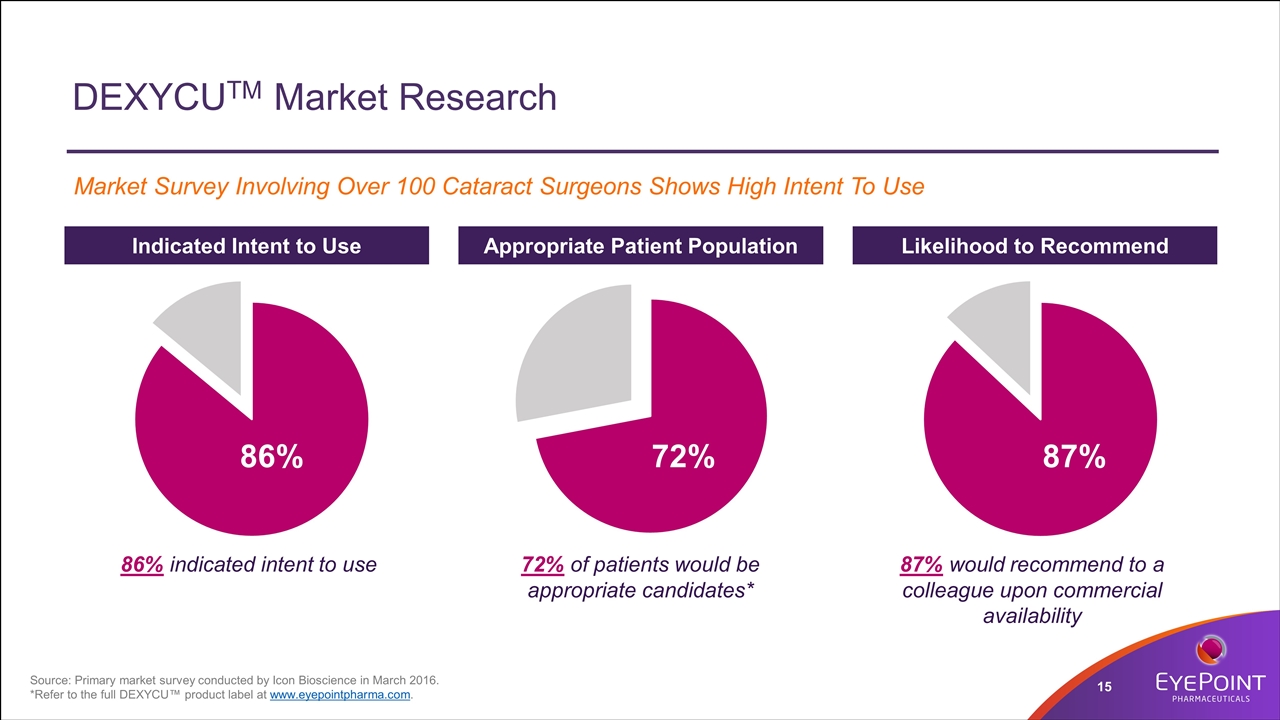
DEXYCUTM Market Research Market Survey Involving Over 100 Cataract Surgeons Shows High Intent To Use Indicated Intent to Use Likelihood to Recommend Appropriate Patient Population 86% indicated intent to use 72% of patients would be appropriate candidates* 87% would recommend to a colleague upon commercial availability 86% 72% 87% Source: Primary market survey conducted by Icon Bioscience in March 2016. *Refer to the full DEXYCU™ product label at www.eyepointpharma.com.

Chronic Non-Infectious Uveitis Affecting the Posterior Segment of the Eye
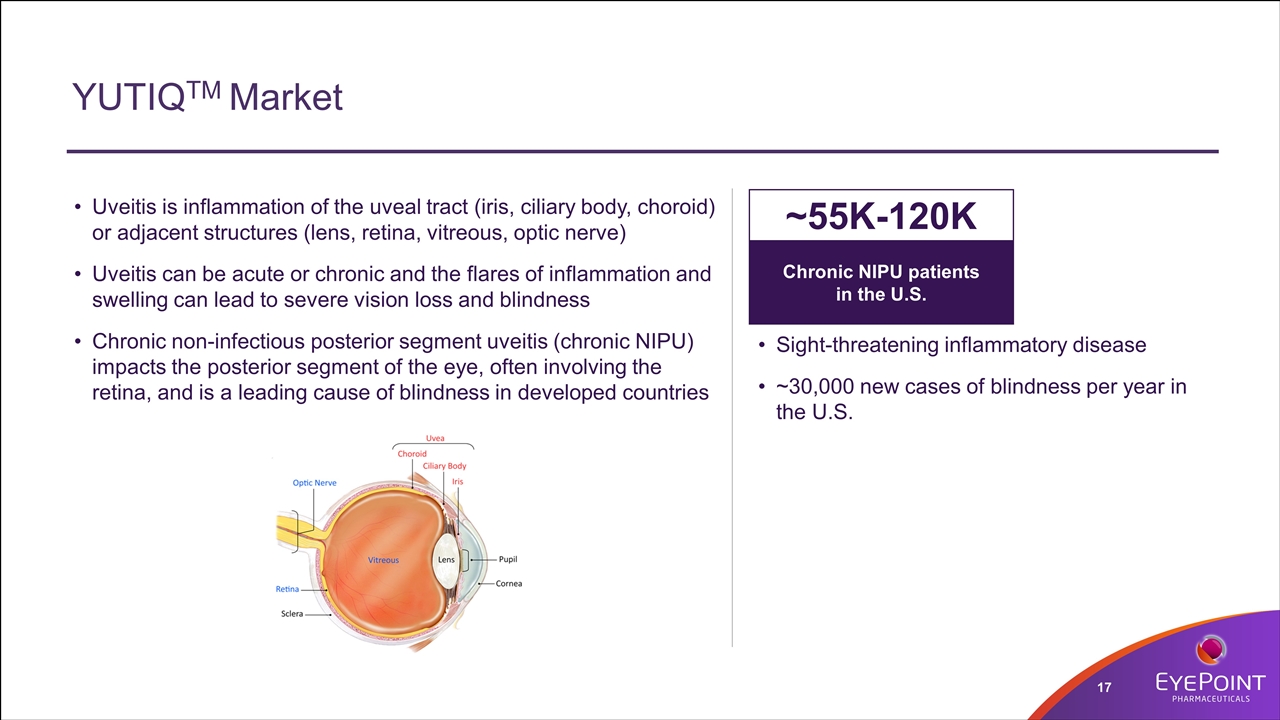
YUTIQTM Market Sight-threatening inflammatory disease ~30,000 new cases of blindness per year in the U.S. Uveitis is inflammation of the uveal tract (iris, ciliary body, choroid) or adjacent structures (lens, retina, vitreous, optic nerve) Uveitis can be acute or chronic and the flares of inflammation and swelling can lead to severe vision loss and blindness Chronic non-infectious posterior segment uveitis (chronic NIPU) impacts the posterior segment of the eye, often involving the retina, and is a leading cause of blindness in developed countries Chronic NIPU patients in the U.S. ~55K-120K
YUTIQ™ (fluocinolone acetonide intravitreal implant) 0.18 mg for chronic non-infectious uveitis affecting the posterior segment of the eye YUTIQ provides consistent micro dosing of corticosteroid up to three years without drug peaks and valleys and has been shown to significantly decrease the recurrence of flares primary goal of therapy in uveitis Chronic NIPU is treated both aggressively and frequently by physicians in order to minimize the disease flares Periocular and intravitreal steroid injections, and systemic delivery of corticosteroids are routinely used to treat chronic NIPU The current standard of care treatment provides sustained release of steroids over a period of 3 to 4 months
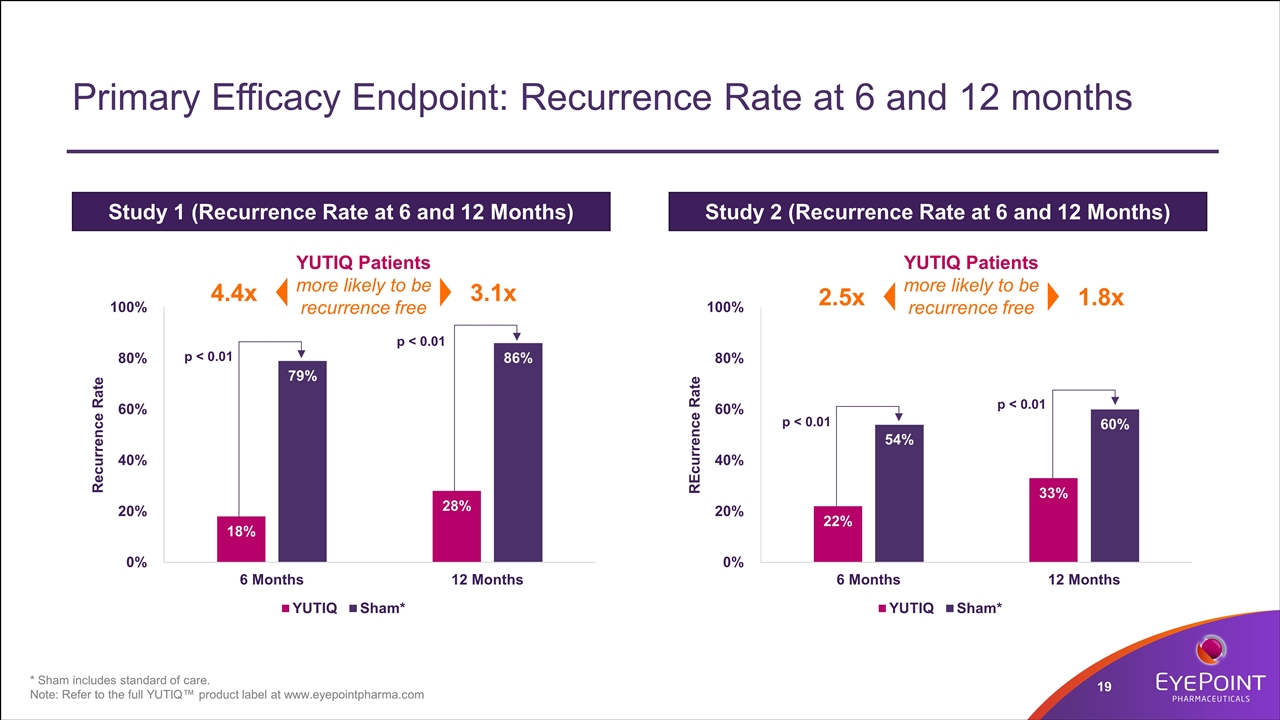
Primary Efficacy Endpoint: Recurrence Rate at 6 and 12 months Study 1 (Recurrence Rate at 6 and 12 Months) Study 2 (Recurrence Rate at 6 and 12 Months) 4.4x 3.1x p < 0.01 p < 0.01 p < 0.01 p < 0.01 YUTIQ Patients more likely to be recurrence free * Sham includes standard of care. Note: Refer to the full YUTIQ™ product label at www.eyepointpharma.com 2.5x 1.8x YUTIQ Patients more likely to be recurrence free
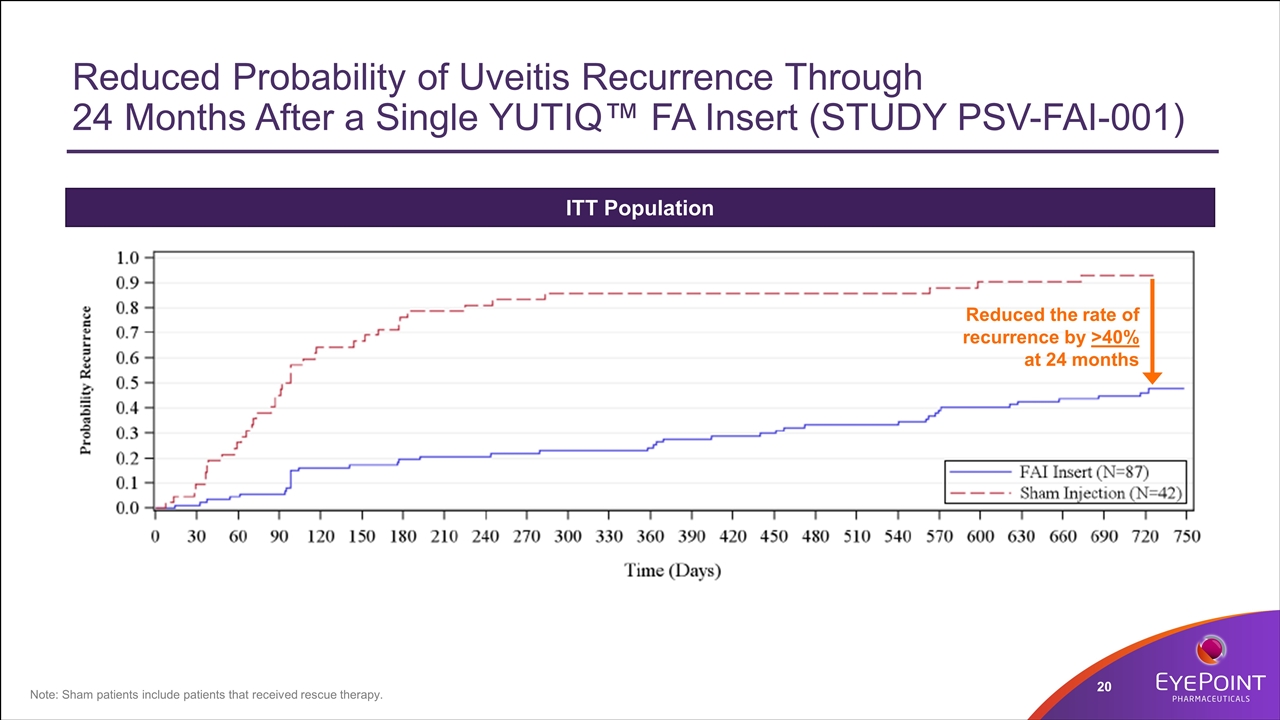
Reduced Probability of Uveitis Recurrence Through 24 Months After a Single YUTIQ™ FA Insert (STUDY PSV-FAI-001) ITT Population Note: Sham patients include patients that received rescue therapy. Reduced the rate of recurrence by >40% at 24 months
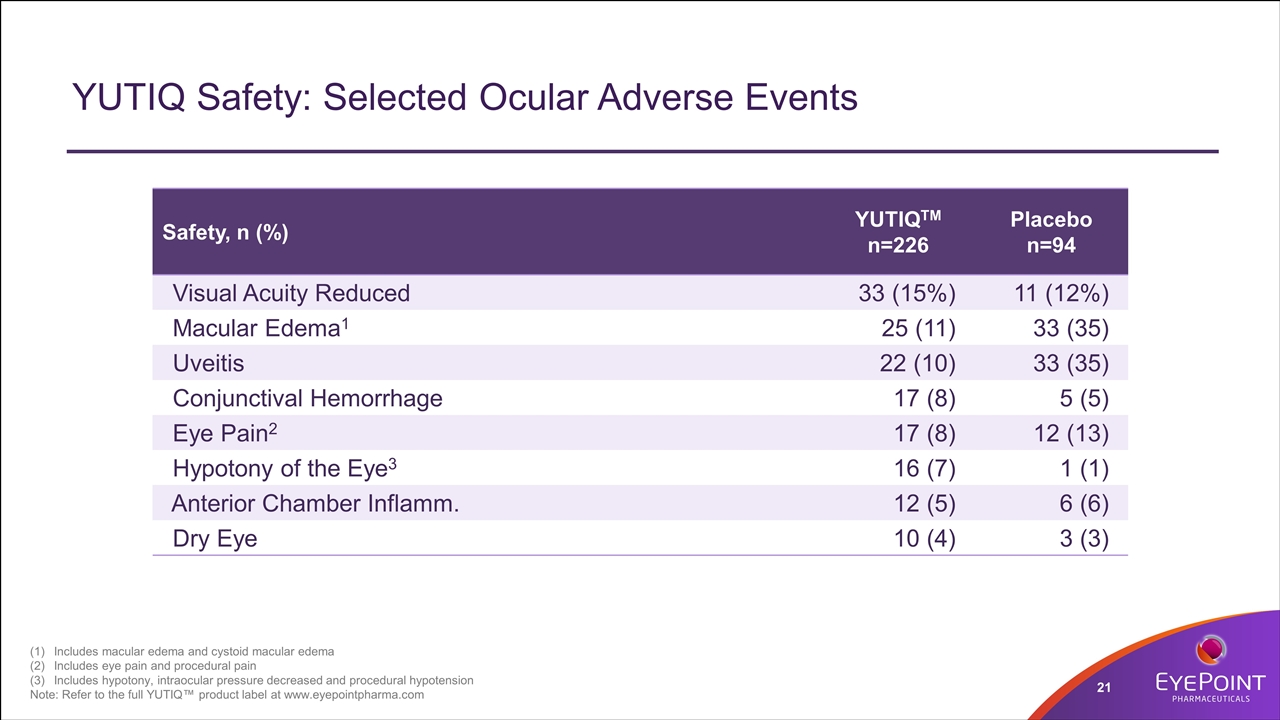
YUTIQ Safety: Selected Ocular Adverse Events Safety, n (%) YUTIQTM n=226 Placebo n=94 Visual Acuity Reduced 33 (15%) 11 (12%) Macular Edema1 25 (11) 33 (35) Uveitis 22 (10) 33 (35) Conjunctival Hemorrhage 17 (8) 5 (5) Eye Pain2 17 (8) 12 (13) Hypotony of the Eye3 16 (7) 1 (1) Anterior Chamber Inflamm. 12 (5) 6 (6) Dry Eye 10 (4) 3 (3) Includes macular edema and cystoid macular edema Includes eye pain and procedural pain Includes hypotony, intraocular pressure decreased and procedural hypotension Note: Refer to the full YUTIQ™ product label at www.eyepointpharma.com
YUTIQTM Commercial Launch Approach Launched on February 4, 2019 Longest duration product indicated for chronic non-infectious uveitis Non-disruptive / corticosteroids remain current standard of care Goal of treatment is to prevent flares that can lead to blindness 10 KAMs solely focused on YUTIQTM J-Code Available Reimbursement will be obtained initially from an existing, not miscellaneous, J-Code Application has been made for a permanent and specific YUTIQTM J-Code We believe that YUTIQTM fits naturally into the current treatment paradigm for chronic NIPU and provides physicians with a differentiated alternative to existing therapies Consistent micro-dosing of corticosteroid over time without drug peaks and valleys
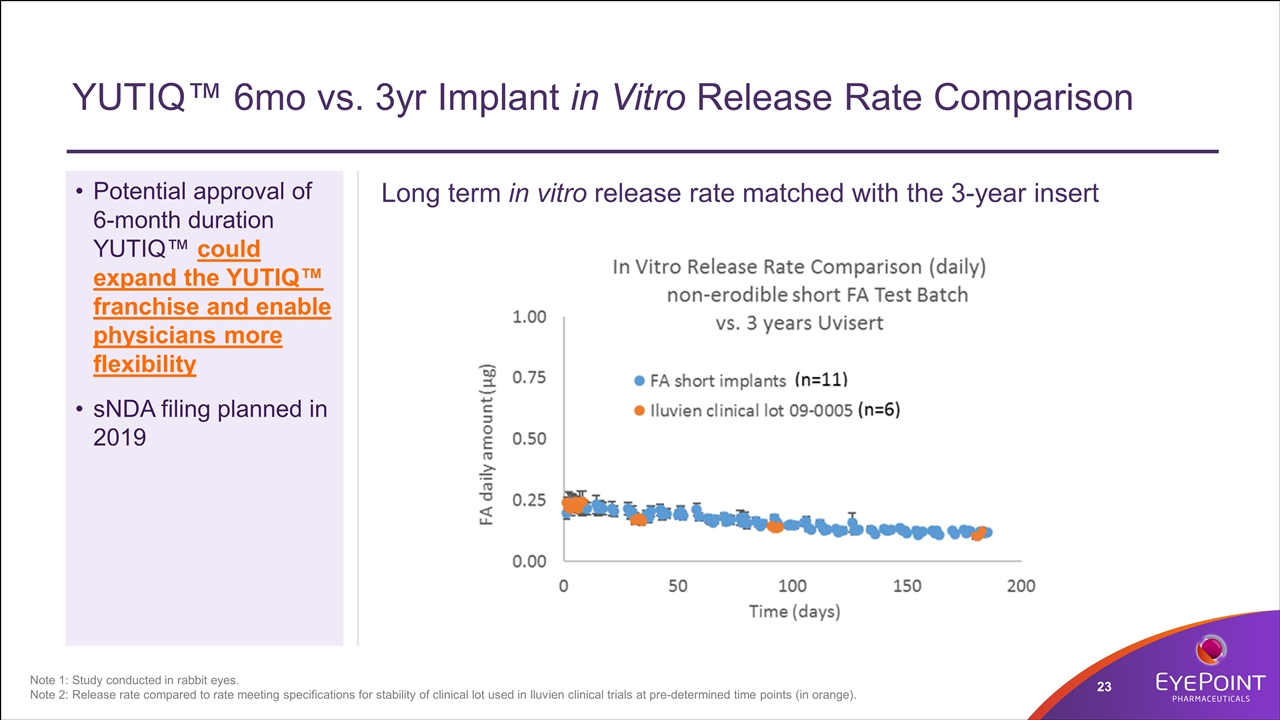
YUTIQ™ 6mo vs. 3yr Implant in Vitro Release Rate Comparison Long term in vitro release rate matched with the 3-year insert Note 1: Study conducted in rabbit eyes. Note 2: Release rate compared to rate meeting specifications for stability of clinical lot used in Iluvien clinical trials at pre-determined time points (in orange). Potential approval of 6-month duration YUTIQ™ could expand the YUTIQ™ franchise and enable physicians more flexibility sNDA filing planned in 2019

Company Milestones & Strategy DEXYCUTM launched on March 12, 2019 YUTIQTM launched February 4, 2019 YUTIQTM 6-month formulation sNDA submission in 2019 Continued development and progression Durasert™ TKI Potential partnerships surrounding Durasert™ and Verisome® technologies Evaluating in-licensing and M&A opportunities Exploring pathway to extended reimbursement outside of cataract bundle within Medicare Part B
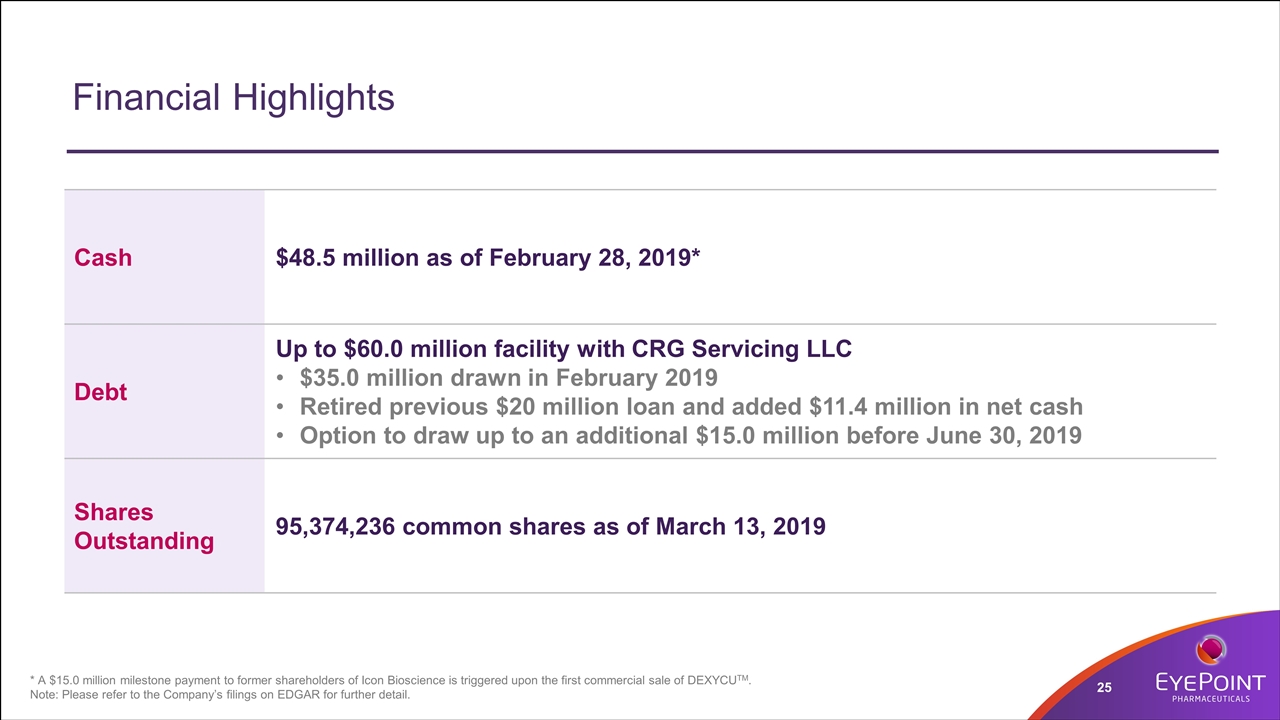
Financial Highlights * A $15.0 million milestone payment to former shareholders of Icon Bioscience is triggered upon the first commercial sale of DEXYCUTM. Note: Please refer to the Company’s filings on EDGAR for further detail. Cash $48.5 million as of February 28, 2019* Debt Up to $60.0 million facility with CRG Servicing LLC $35.0 million drawn in February 2019 Retired previous $20 million loan and added $11.4 million in net cash Option to draw up to an additional $15.0 million before June 30, 2019 Shares Outstanding 95,374,236 common shares as of March 13, 2019

Evaluate potential partnerships surrounding Durasert™ and Verisome® technologies Launched YUTIQTM (Feb 4, 2019) and DEXYCUTM (Mar 12, 2019) (J-Code reimbursement in place for both products) Utilize our platform to progress assets that address unmet medical needs (YUTIQTM 6-month formulation, DurasertTM TKI) Execute on strategy to commercialize our own products and seek extended reimbursement on DEXYCUTM Evaluate potential in-licensing and M&A opportunities Chronic non-infectious uveitis affecting the posterior segment of the eye Postoperative inflammation following ocular surgery EyePoint Highlights: Transformational Opportunity in Ophthalmology

























