
Jay S. Duker, M.D. Chief Operating Officer EyePoint Pharma 8-month Results of a Tyrosine Kinase Inhibitor (Vorolanib) in a Bio-erodible Durasert® Implant for Previously Treated Wet AMD: The DAVIO Trial Exhibit 99.1

Employee - EyePoint Pharma Board of Directors - Sesen Bio - Hubble Tx Consultant - Aura Bio Financial Interest Disclosure – Jay S. Duker, M.D.

Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, including but not limited to statements about our expectations regarding the potential benefits of our partnerships and strategic alliances with other companies, as well as the timing and clinical development of our product candidates, including EYP-1901; the potential for EYP-1901 as a vital, novel twice-yearly treatment for wet age-related macular degeneration, diabetic retinopathy and retinal vein occlusion; and our longer term financial and business goals and expectations, are forward-looking statements. Some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements are risks and uncertainties inherent in our business including, without limitation: the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the extent to which COVID-19 impacts our business; our ability to achieve profitable operations and access to needed capital; fluctuations in our operating results; our ability to successfully produce sufficient commercial quantities of YUTIQ® and DEXYCU® and to successfully commercialize YUTIQ and DEXYCU in the U.S.; our ability to sustain and enhance an effective commercial infrastructure and enter into and maintain commercial agreements for YUTIQ and DEXYCU; the development of our YUTIQ line extension shorter-duration treatment for non-infectious uveitis affecting the posterior segment of the eye; the success of current and future license agreements, including our agreements with Ocumension Therapeutics and Equinox Science; termination or breach of current license agreements, including our agreements with Ocumension Therapeutics and Equinox Science; our dependence on contract research organizations, co-promotion partners, and other outside vendors and service providers; effects of competition and other developments affecting sales of products; market acceptance of products; effects of guidelines, recommendations and studies; protection of intellectual property and avoiding intellectual property infringement; retention of key personnel; product liability; industry consolidation; compliance with environmental laws; manufacturing risks; risks and costs of international business operations; volatility of our stock price; possible dilution; absence of dividends; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized. Forward looking statements

All objectives successfully met: Proof of Concept for Vorolanib in wet AMD Positive Safety Data No ocular SAEs reported No drug-related systemic SAEs reported Ocular AEs - majority mild and to be expected EFFICACY and DURABILITY Positive Efficacy Data Stable VA and OCT Median time to supplemental anti-VEGF: 6 months 76 % rescue-free up to 4 months 53 % rescue-free up to 6 months 41 % rescue-free up to 9 months Clinically significant reduction in treatment burden by 79 % at six mo – 75 % at 8 mo SAFETY DAVIO Take Home Messages: EYP-1901 Phase 1 Clinical Trial Met All Objectives
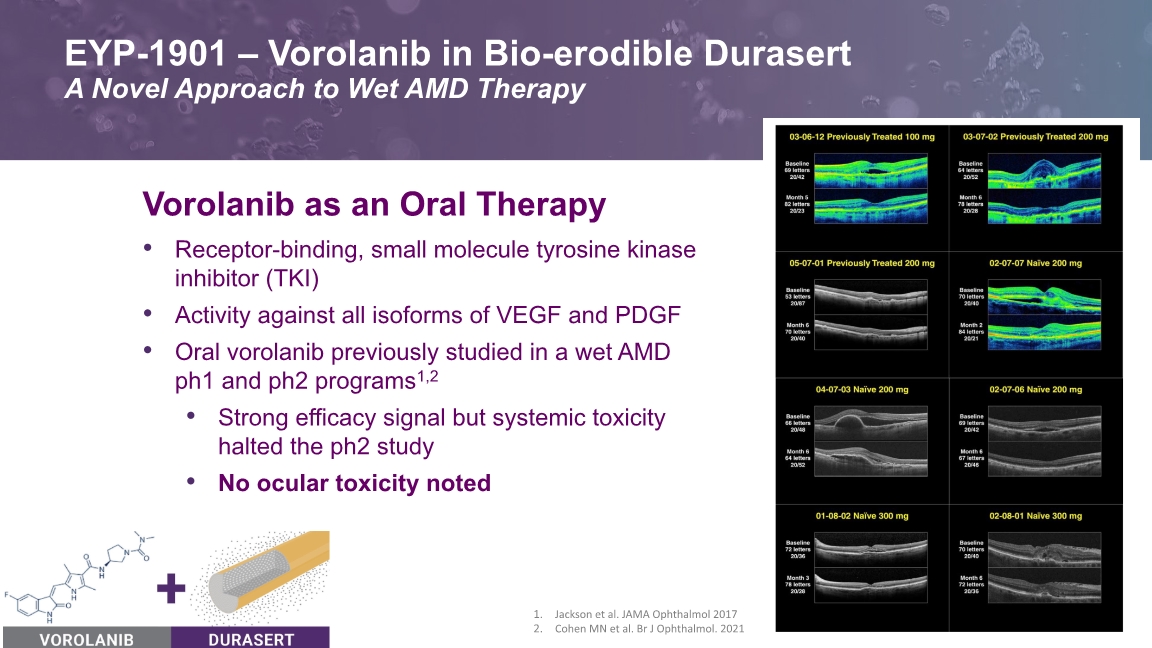
EYP-1901 – Vorolanib in Bio-erodible Durasert A Novel Approach to Wet AMD Therapy 5 Vorolanib as an Oral Therapy Receptor-binding, small molecule tyrosine kinase inhibitor (TKI) Activity against all isoforms of VEGF and PDGF Oral vorolanib previously studied in a wet AMD ph1 and ph2 programs1,2 Strong efficacy signal but systemic toxicity halted the ph2 study No ocular toxicity noted Jackson et al. JAMA Ophthalmol 2017 Cohen MN et al. Br J Ophthalmol. 2021
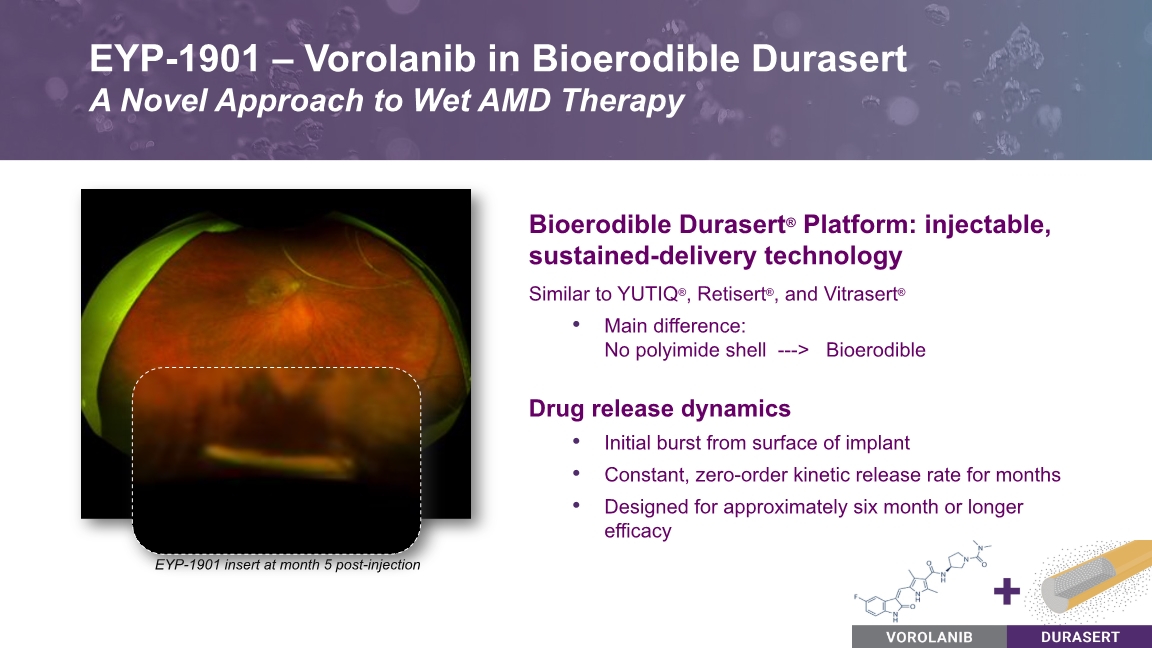
Bioerodible Durasert® Platform: injectable, sustained-delivery technology Similar to YUTIQ®, Retisert®, and Vitrasert® Main difference: No polyimide shell ---> Bioerodible Drug release dynamics Initial burst from surface of implant Constant, zero-order kinetic release rate for months Designed for approximately six month or longer efficacy EYP-1901 – Vorolanib in Bioerodible Durasert A Novel Approach to Wet AMD Therapy
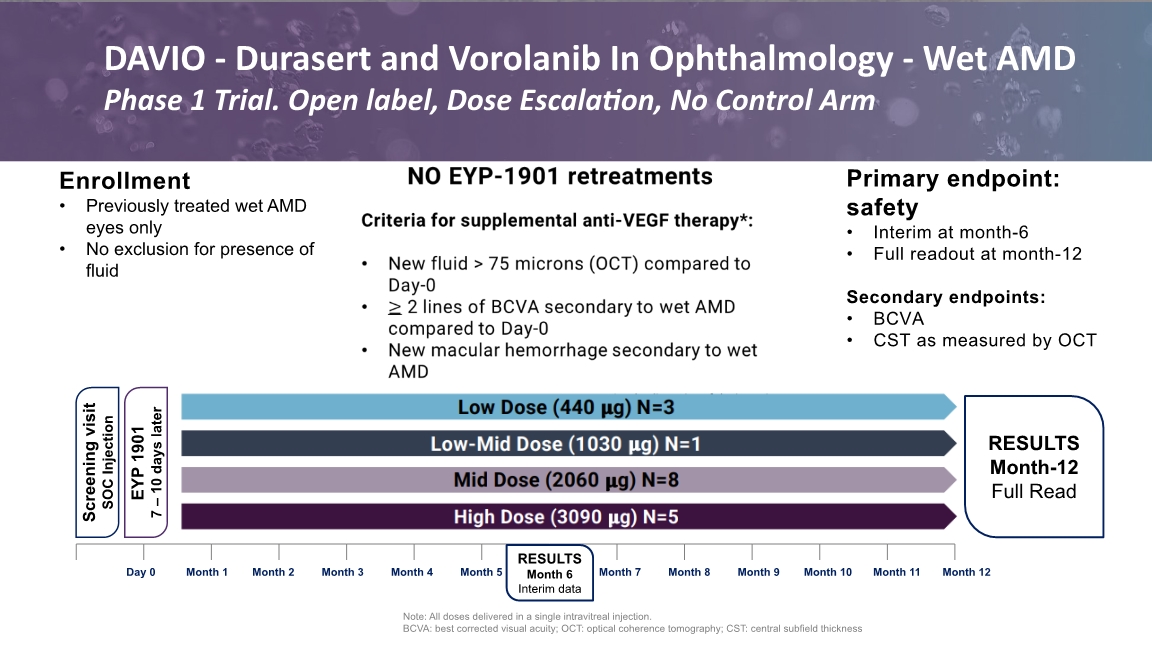
NO EYP-1901 retreatments Criteria for supplemental anti-VEGF therapy*: New fluid > 75 microns (OCT) compared to Day-0 2 lines of BCVA secondary to wet AMD compared to Day-0 New macular hemorrhage secondary to wet AMD Enrollment Previously treated wet AMD eyes only No exclusion for presence of fluid Primary endpoint: safety Interim at month-6 Full readout at month-12 Secondary endpoints: BCVA CST as measured by OCT Note: All doses delivered in a single intravitreal injection. BCVA: best corrected visual acuity; OCT: optical coherence tomography; CST: central subfield thickness Screening visit SOC Injection RESULTS Month-12 Full Read RESULTS Month 6 Interim data EYP 1901 7 – 10 days later DAVIO - Durasert and Vorolanib In Ophthalmology - Wet AMD Phase 1 Trial. Open label, Dose Escalation, No Control Arm
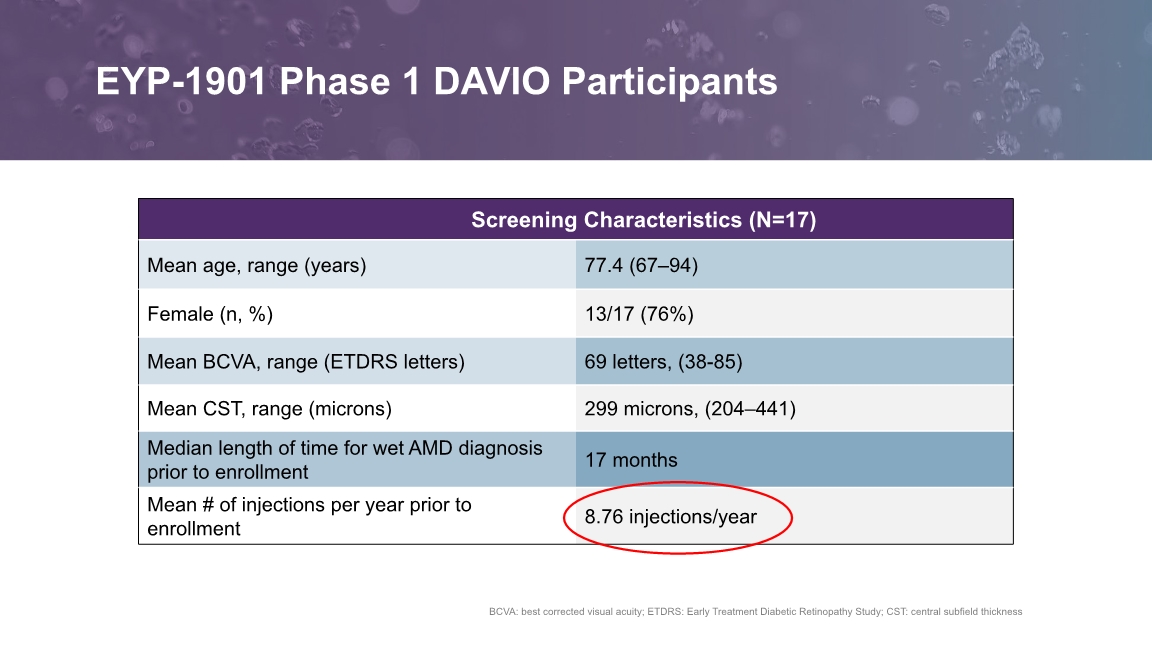
BCVA: best corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study; CST: central subfield thickness EYP-1901 Phase 1 DAVIO Participants
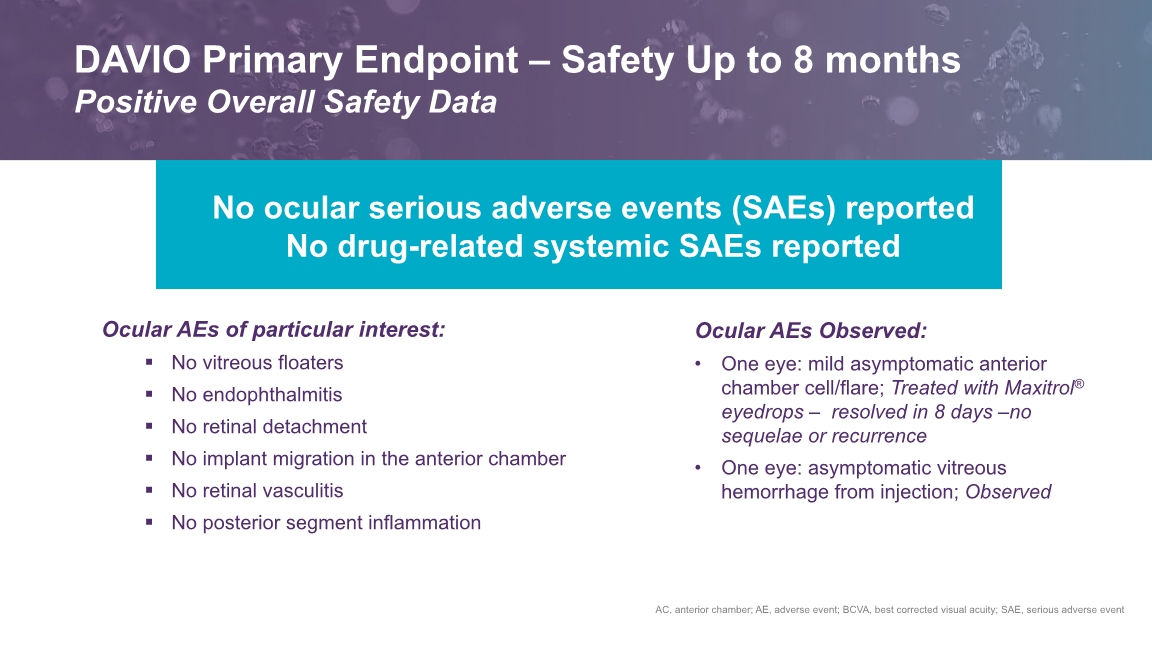
Ocular AEs of particular interest: No vitreous floaters No endophthalmitis No retinal detachment No implant migration in the anterior chamber No retinal vasculitis No posterior segment inflammation AC, anterior chamber; AE, adverse event; BCVA, best corrected visual acuity; SAE, serious adverse event No ocular serious adverse events (SAEs) reported No drug-related systemic SAEs reported Ocular AEs Observed: One eye: mild asymptomatic anterior chamber cell/flare; Treated with Maxitrol® eyedrops – resolved in 8 days –no sequelae or recurrence One eye: asymptomatic vitreous hemorrhage from injection; Observed DAVIO Primary Endpoint – Safety Up to 8 months Positive Overall Safety Data
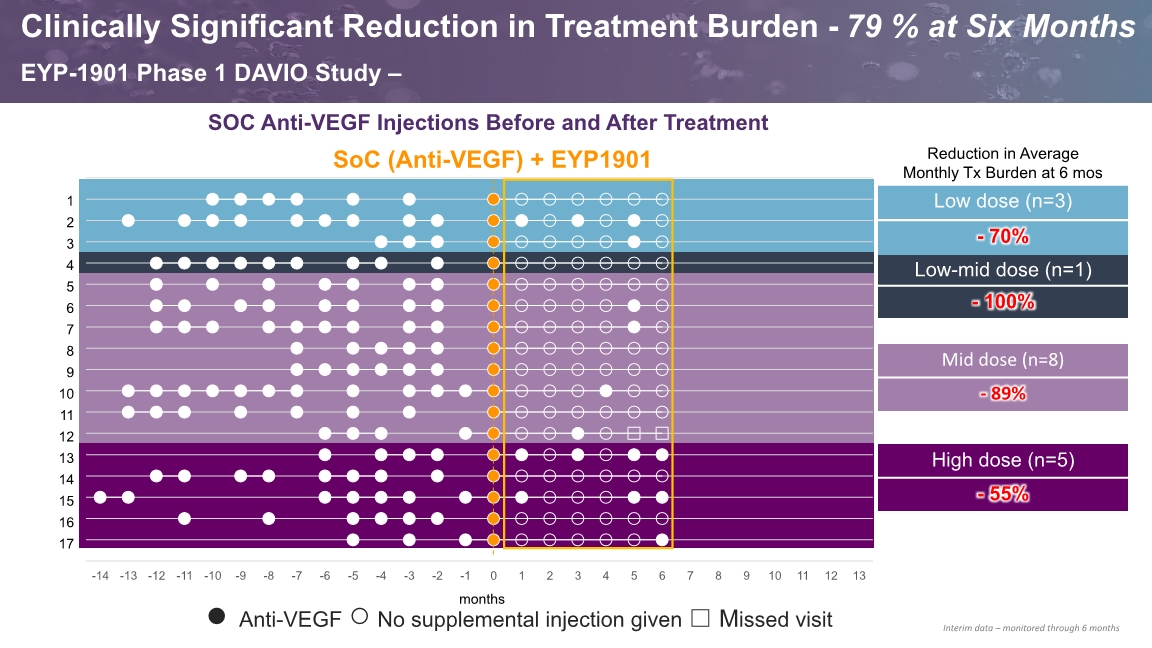
SOC Anti-VEGF Injections Before and After Treatment SoC (Anti-VEGF) + EYP1901 ● Anti-VEGF ○ No supplemental injection given ☐ Missed visit months Clinically Significant Reduction in Treatment Burden - 79 % at Six Months EYP-1901 Phase 1 DAVIO Study – Interim data – monitored through 6 months
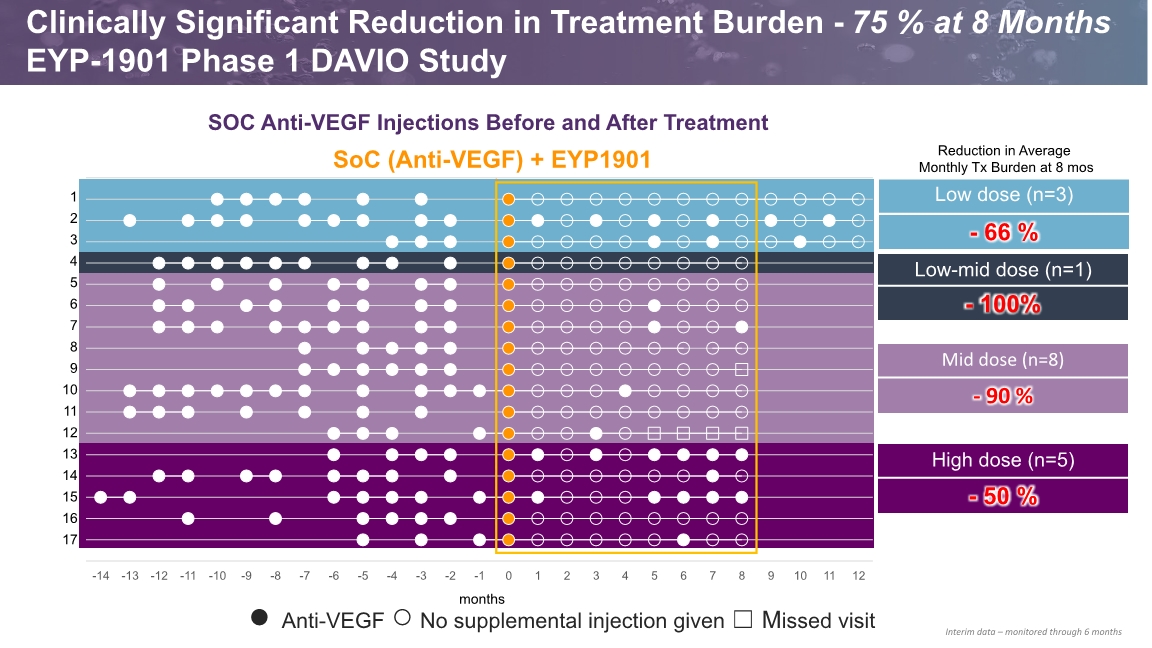
SOC Anti-VEGF Injections Before and After Treatment SoC (Anti-VEGF) + EYP1901 ● Anti-VEGF ○ No supplemental injection given ☐ Missed visit months Clinically Significant Reduction in Treatment Burden - 75 % at 8 Months EYP-1901 Phase 1 DAVIO Study Interim data – monitored through 6 months
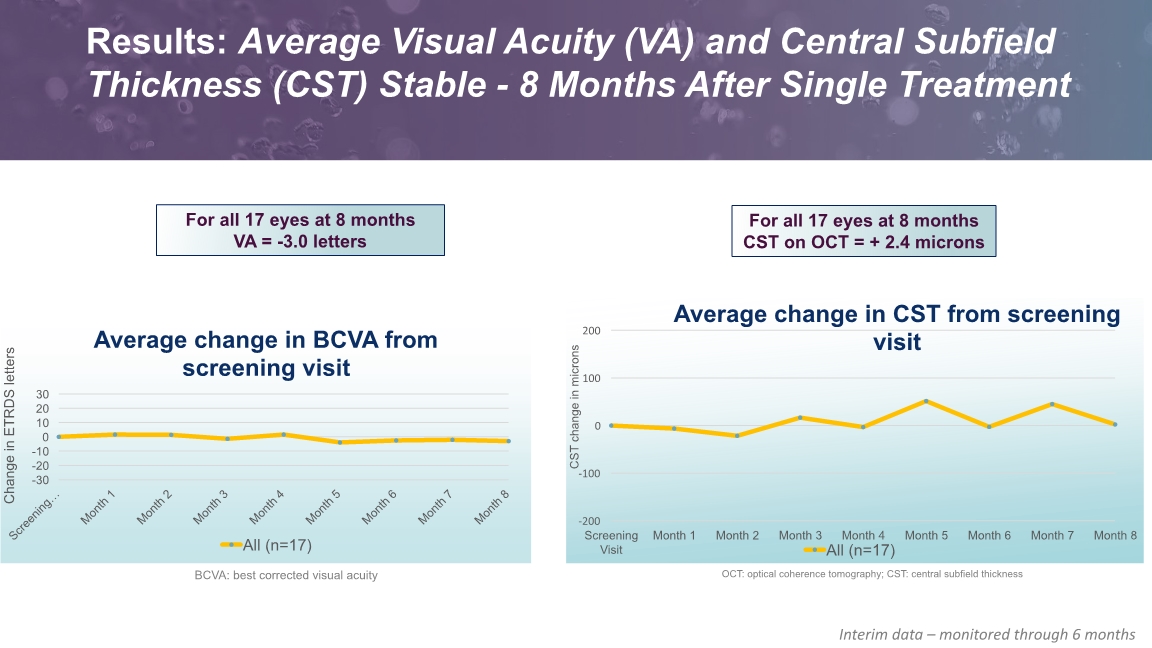
Results: Average Visual Acuity (VA) and Central Subfield Thickness (CST) Stable - 8 Months After Single Treatment BCVA: best corrected visual acuity Interim data – monitored through 6 months For all 17 eyes at 8 months VA = -3.0 letters OCT: optical coherence tomography; CST: central subfield thickness For all 17 eyes at 8 months CST on OCT = + 2.4 microns
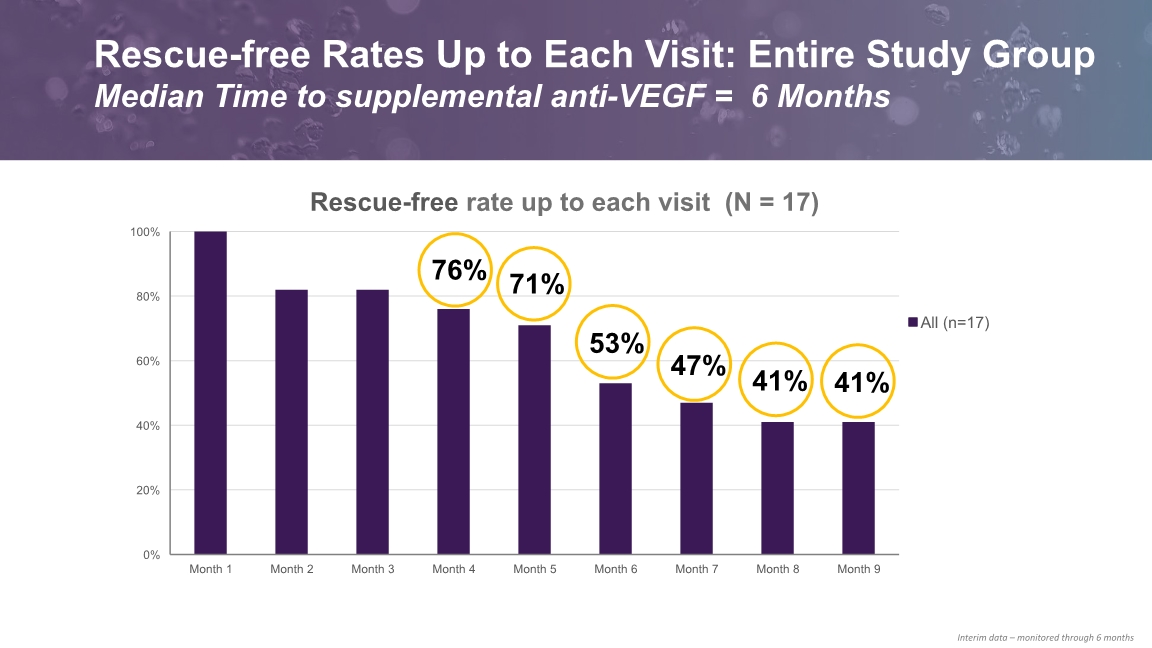
Interim data – monitored through 6 months Rescue-free Rates Up to Each Visit: Entire Study Group Median Time to supplemental anti-VEGF = 6 Months
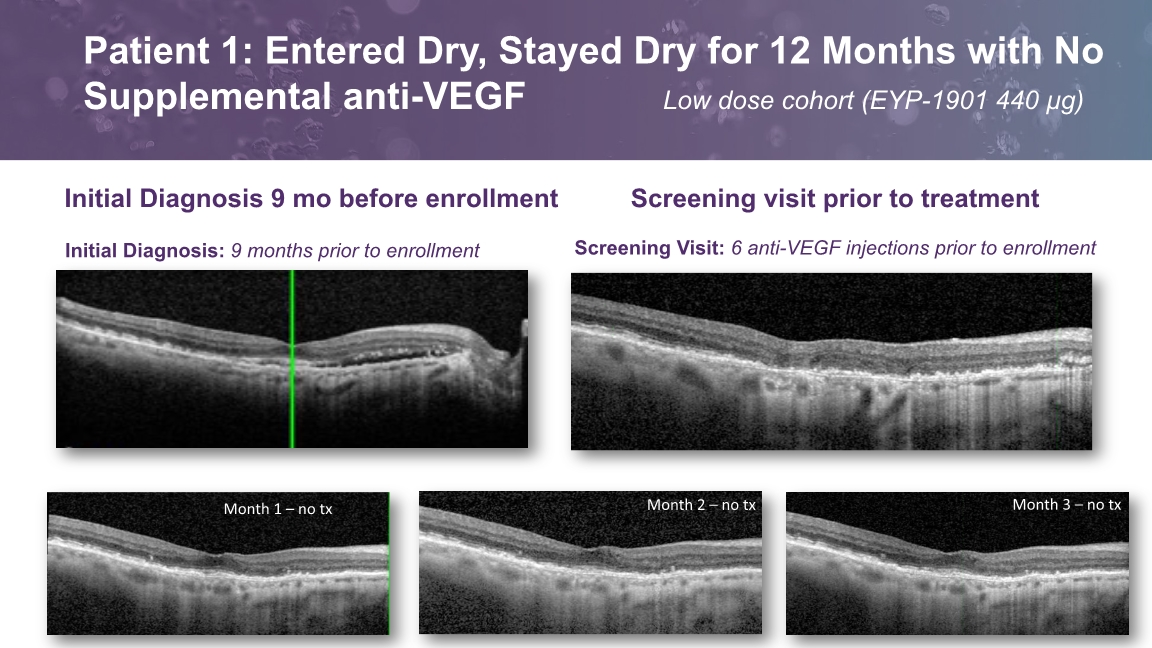
Screening Visit: 6 anti-VEGF injections prior to enrollment Initial Diagnosis: 9 months prior to enrollment Initial Diagnosis 9 mo before enrollment Screening visit prior to treatment Patient 1: Entered Dry, Stayed Dry for 12 Months with No Supplemental anti-VEGF Low dose cohort (EYP-1901 440 µg)
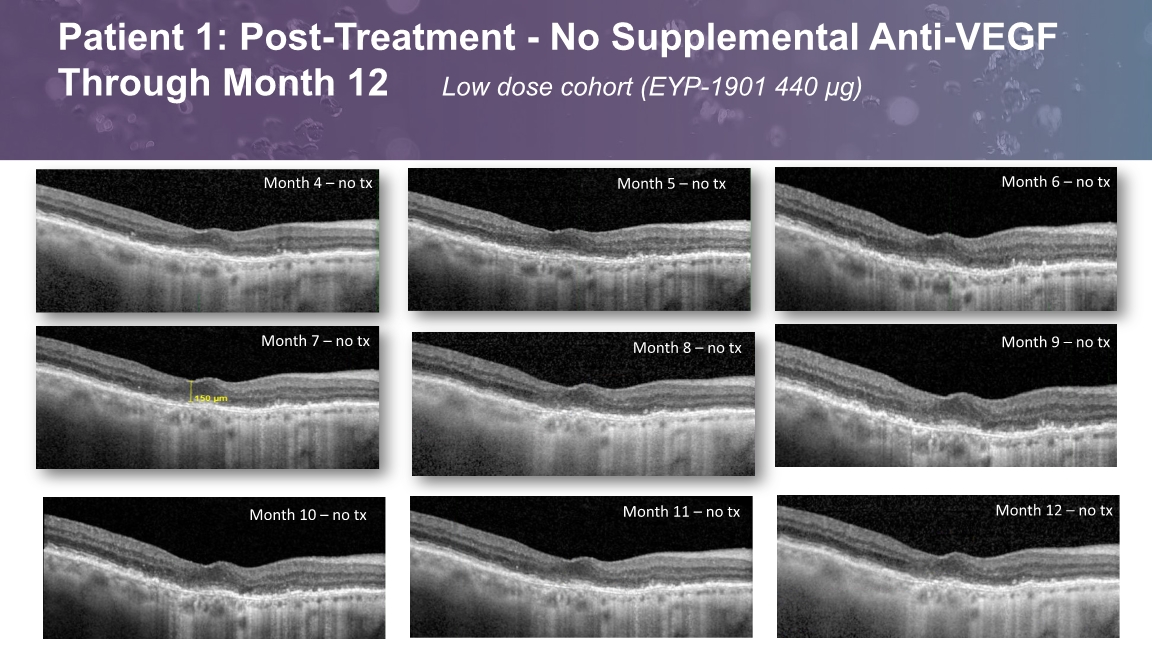
Month 5 – no tx Patient 1: Post-Treatment - No Supplemental Anti-VEGF Through Month 12 Low dose cohort (EYP-1901 440 µg)
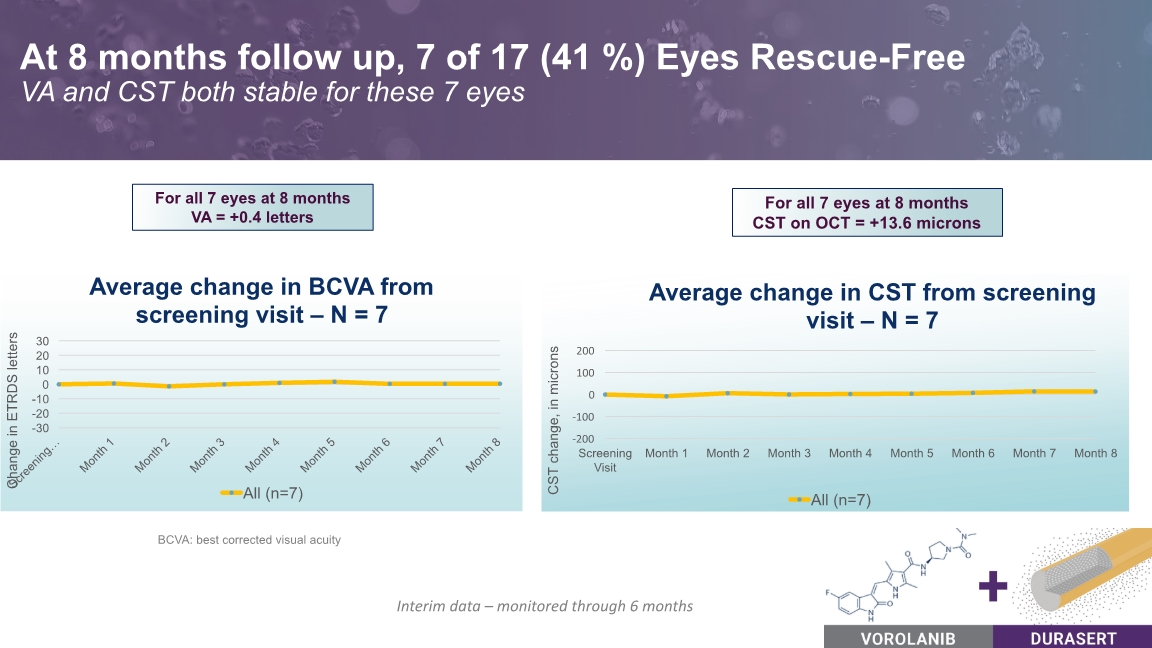
At 8 months follow up, 7 of 17 (41 %) Eyes Rescue-Free VA and CST both stable for these 7 eyes For all 7 eyes at 8 months VA = +0.4 letters BCVA: best corrected visual acuity Interim data – monitored through 6 months For all 7 eyes at 8 months CST on OCT = +13.6 microns
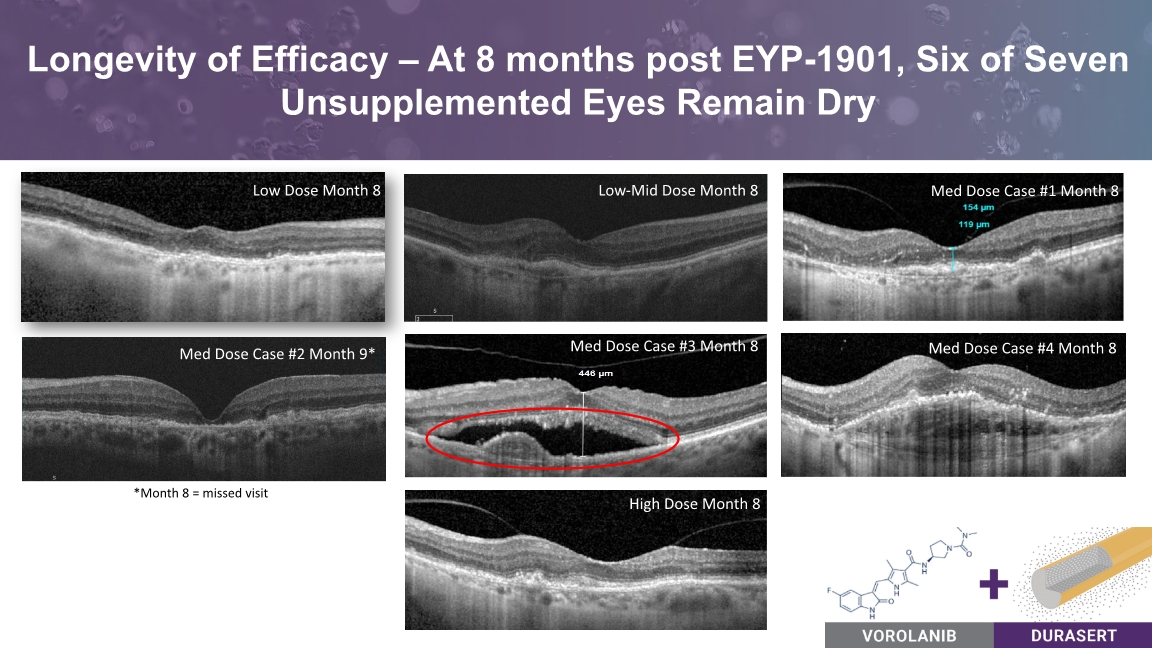
Med Dose Case #3 Month 8 Longevity of Efficacy – At 8 months post EYP-1901, Six of Seven Unsupplemented Eyes Remain Dry Med Dose Case #4 Month 8 Low-Mid Dose Month 8 Low Dose Month 8 Med Dose Case #1 Month 8 High Dose Month 8 Med Dose Case #2 Month 9* *Month 8 = missed visit
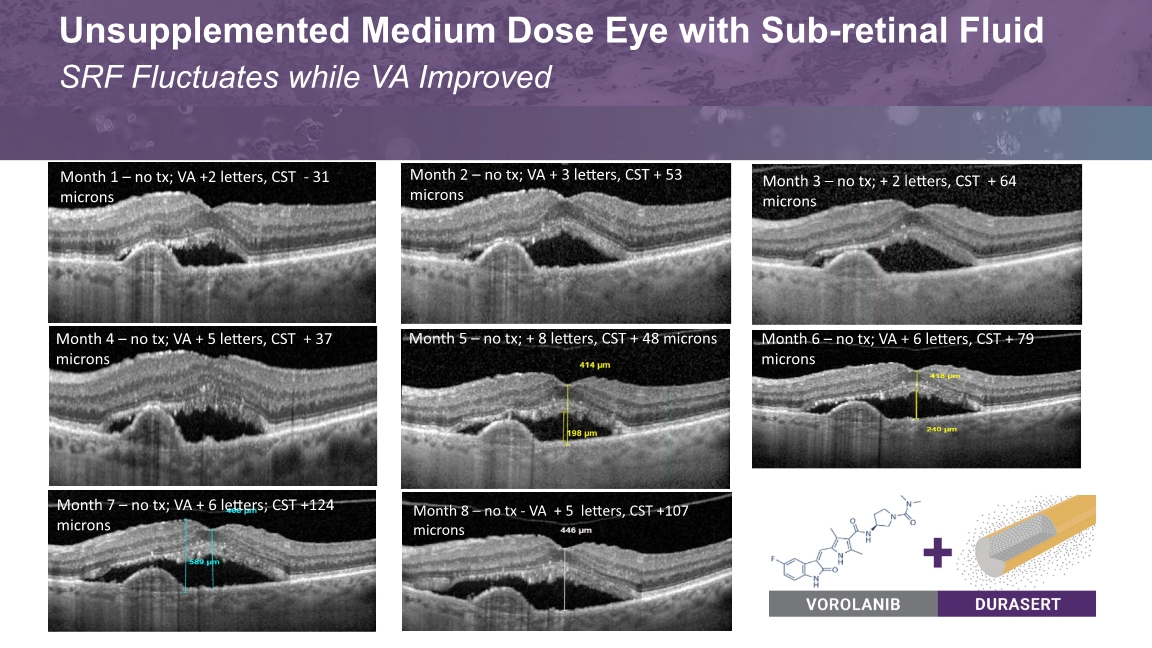
Month 5 – no tx Med Dose Case #1 Month 10 Med Dose Case #4 Month 8 Low-Mid Dose Month 9 18 | EYEPOINT PHARMACEUTICALS
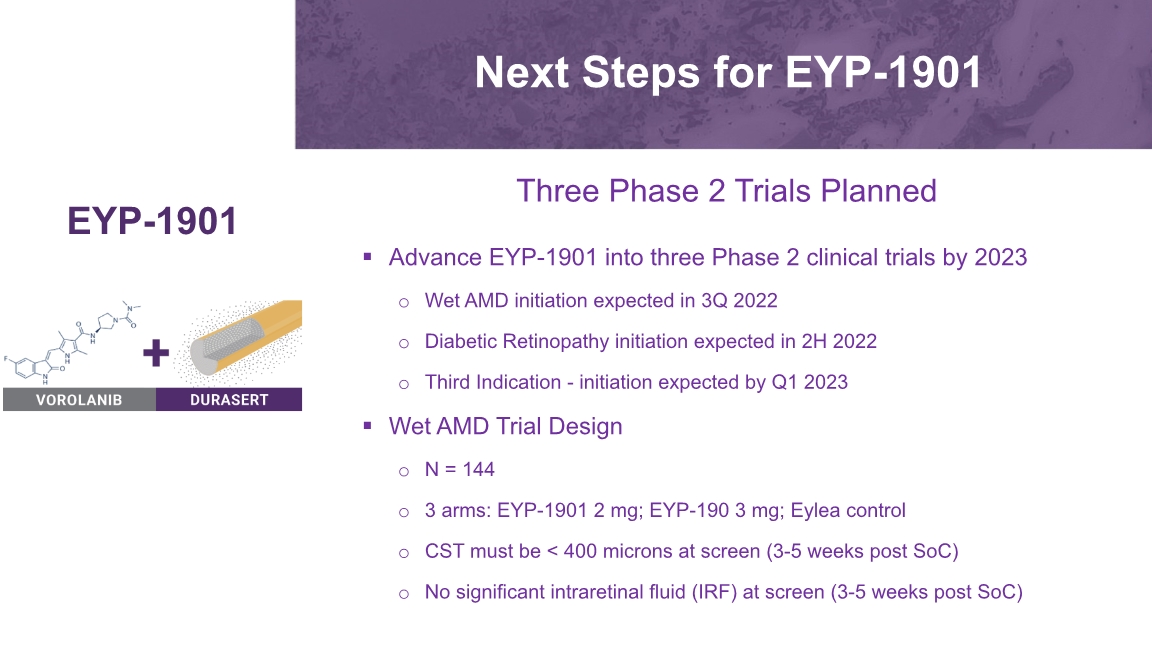
Next Steps for EYP-1901 Advance EYP-1901 into three Phase 2 clinical trials by 2023 Wet AMD initiation expected in 3Q 2022 Diabetic Retinopathy initiation expected in 2H 2022 Third Indication - initiation expected by Q1 2023 Wet AMD Trial Design N = 144 3 arms: EYP-1901 2 mg; EYP-190 3 mg; Eylea control CST must be < 400 microns at screen (3-5 weeks post SoC) No significant intraretinal fluid (IRF) at screen (3-5 weeks post SoC) EYP-1901 Three Phase 2 Trials Planned
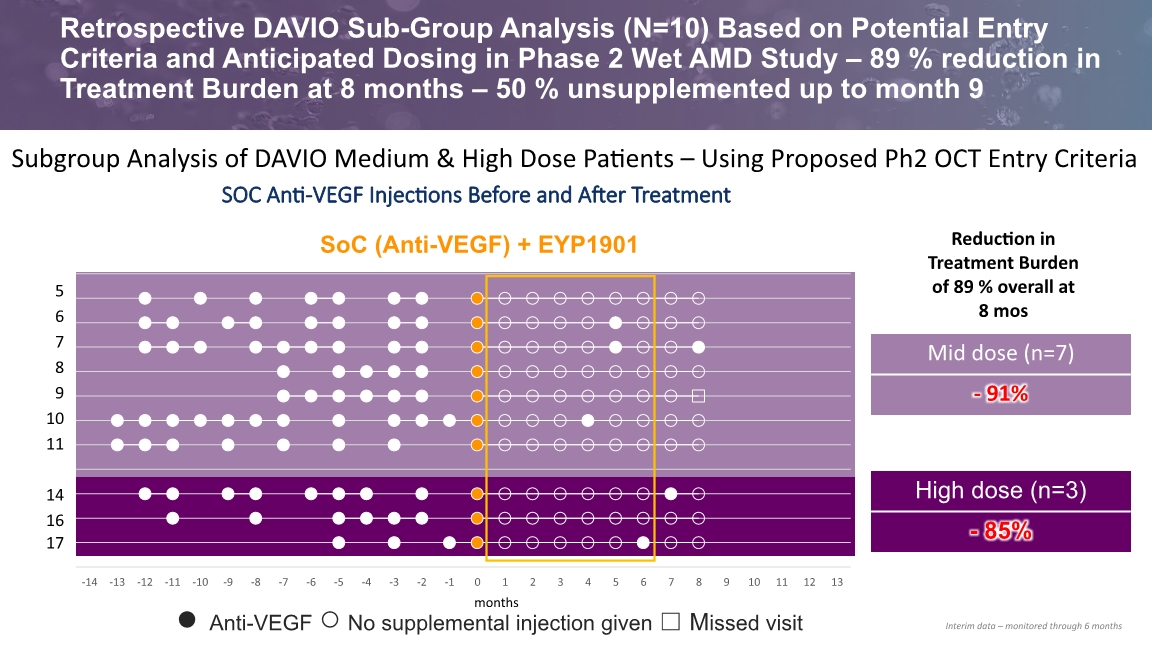
Retrospective DAVIO Sub-Group Analysis (N=10) Based on Potential Entry Criteria and Anticipated Dosing in Phase 2 Wet AMD Study – 89 % reduction in Treatment Burden at 8 months – 50 % unsupplemented up to month 9 SoC (Anti-VEGF) + EYP1901 ● Anti-VEGF ○ No supplemental injection given ☐ Missed visit months Subgroup Analysis of DAVIO Medium & High Dose Patients – Using Proposed Ph2 OCT Entry Criteria SOC Anti-VEGF Injections Before and After Treatment Reduction in Treatment Burden of 89 % overall at 8 mos Interim data – monitored through 6 months

All objectives successfully met: Proof of Concept for Vorolanib in wet AMD Positive Safety Data No ocular SAEs reported No drug-related systemic SAEs reported Ocular AEs - majority mild and to be expected EFFICACY and DURABILITY Positive Efficacy Data Stable VA and OCT Median time to supplemental anti-VEGF: 6 months 76 % rescue-free up to 4 months 53 % rescue-free up to 6 months 41 % rescue-free up to 9 months Clinically significant reduction in treatment burden by 79 % at six mo – 75 % at 8 mo SAFETY DAVIO Take Home Messages: EYP-1901 Phase 1 Clinical Trial Met All Objectives




















