UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14D-9
(Rule 14d-101)
SOLICITATION/RECOMMENDATION STATEMENT UNDER
SECTION 14(d)(4) OF THE SECURITIES EXCHANGE ACT OF 1934
SECTION 14(d)(4) OF THE SECURITIES EXCHANGE ACT OF 1934
ev3 Inc.
(Name of Subject Company)
ev3 Inc.
(Names of Person(s) Filing Statement)
Common Stock, par value $0.01 per share
(Title of Class of Securities)
(Title of Class of Securities)
26928A200
(CUSIP Number of Class of Securities)
(CUSIP Number of Class of Securities)
Kevin M. Klemz
Senior Vice President, Secretary and Chief Legal Officer
ev3 Inc.
3033 Campus Drive
Plymouth, Minnesota 55441
(763) 398-7000
Senior Vice President, Secretary and Chief Legal Officer
ev3 Inc.
3033 Campus Drive
Plymouth, Minnesota 55441
(763) 398-7000
(Name, Address and Telephone Number of Person Authorized to Receive
Notice and Communications on Behalf of the Person(s) Filing Statement)
Notice and Communications on Behalf of the Person(s) Filing Statement)
Copies To:
| Bruce A. Machmeier, Esq. | Steven J. Gartner, Esq. | |
| Amy E. Culbert, Esq. | Adam M. Turteltaub, Esq. | |
| Patrick J. Pazderka, Esq. | Willkie Farr & Gallagher LLP | |
| Oppenheimer Wolff & Donnelly LLP | 787 Seventh Street | |
| 45 South Seventh Street, Suite 3300 | New York, New York 10019 | |
| Minneapolis, Minnesota 55402-1509 | (212) 728-8000 | |
| (612) 607-7000 |
| þ | Check the box if the filing relates solely to preliminary communications made before the commencement of a tender offer. |

| ev3 and Covidien Proposed Acquisition June 1, 2010 |

| 2 2 Forward-Looking Statements This presentation contains forward-looking statements that are not historical facts. Covidien and ev3 have identified some of these forward-looking statements with words like "believe," "may," "could," "would," "might," "possible," "will," "should," "expect," "intend," "plan," "anticipate," or "continue", the negative of these words, other terms of similar meaning or the use of future dates. Forward-looking statements in this presentation include without limitation statements regarding the expected timing of the completion of the transaction and statements regarding the effect of the transaction on Covidien's business and its future financial performance and financial condition. Investors and security holders are cautioned not to place undue reliance on these forward-looking statements. Actual results could differ materially from those currently anticipated due to a number of risks and uncertainties. Risks and uncertainties that could cause results to differ from expectations include: uncertainties as to the timing of the transaction; uncertainties as to how many of ev3's stockholders will tender their shares in the offer; the risk that competing offers will be made; the possibility that various closing conditions for the transaction may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the transaction; the effects of disruption from the transaction making it more difficult to maintain relationships with employees, customers, vendors and other business partners; the risk that stockholder litigation in connection with the transaction may result in significant costs of defense, indemnification and liability; other business effects, including the effects of industry, economic or political conditions outside of Covidien's and ev3's control; transaction costs; actual or contingent liabilities; and other risks and uncertainties discussed in Covidien's and ev3's filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" sections of Covidien's and ev3's most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q, as well as the tender offer documents to be filed by Covidien and the Solicitation/Recommendation Statement to be filed by ev3. Neither Covidien nor ev3 undertakes any obligation to update any forward-looking statements as a result of new information, future developments or otherwise, except as expressly required by law. All forward-looking statements in this presentation are qualified in their entirety by this cautionary statement. |

| Important Information About the Tender Offer This presentation is neither an offer to purchase nor a solicitation of an offer to sell any securities of ev3 Inc. COV Delaware Corporation ("Purchaser"), an indirect, wholly owned subsidiary of Covidien, has not yet commenced the tender offer for the shares of ev3 common stock. Upon commencement of the tender offer, Purchaser will file with the SEC a tender offer statement on Schedule TO and related exhibits, including the offer to purchase, letter of transmittal, and other related documents. Following commencement of the tender offer, ev3 will file with the SEC a tender offer solicitation/recommendation statement on Schedule 14D-9. These documents will contain important information about Covidien, ev3, the transaction and other related matters. Investors and security holders are urged to read each of these documents carefully when they are available. Investors and security holders will be able to obtain free copies of the tender offer statement, the tender offer solicitation/recommendation statement and other documents filed with the SEC by Purchaser and ev3 through the web site maintained by the SEC at www.sec.gov. In addition, investors and security holders will be able to obtain free copies of these documents by contacting Covidien Investor Relations at 508-452-4650 or investor.relations@covidien.com or ev3 Inc., Julie Tracy, Sr. Vice President , Chief Communications Officer at 949-680-1375 or jtracy@ev3.net. |
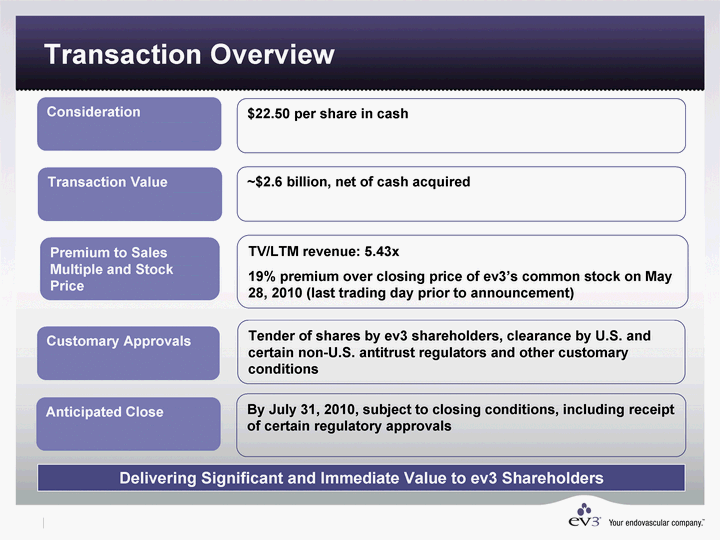
| Transaction Overview Anticipated Close Consideration Customary Approvals By July 31, 2010, subject to closing conditions, including receipt of certain regulatory approvals $22.50 per share in cash TV/LTM revenue: 5.43x 19% premium over closing price of ev3's common stock on May 28, 2010 (last trading day prior to announcement) Tender of shares by ev3 shareholders, clearance by U.S. and certain non-U.S. antitrust regulators and other customary conditions ~$2.6 billion, net of cash acquired Delivering Significant and Immediate Value to ev3 Shareholders Transaction Value Premium to Sales Multiple and Stock Price |

| 5 ev3 at a Glance ev3 is: A leading global medical device company focused on breakthrough endovascular treatments for peripheral vascular and neurovascular disease Two business segments serve estimated $2.6+ billion worldwide market Broadest and most innovative product portfolio - over 100 products, with 15 new products launched in last 12 months Market leader in peripheral plaque excision and #2 worldwide revenue share in neurovascular Products sold in over 65 countries |

| Covidien at a Glance Covidien is: A leading manufacturer of medical devices and supplies, diagnostic imaging agents and pharmaceuticals, with 2009 revenue of $10.7 billion and industry-leading profit margins 42,000 employees worldwide in more than 60 countries, with products sold in over 140 countries Committed to creating innovative medical solutions for better patient outcomes and delivering value through clinical leadership and excellence |

| Creating a Global Leader in the Peripheral Vascular and Neurovascular Markets Advances our broad platform of peripheral vascular and neurovascular technologies with leading global healthcare products company Leverages ev3's world-class sales organization to better serve patients and clinicians Leverages both organizations' expertise in developing and marketing innovative products Together, committed to creating innovative medical solutions for better patient outcomes and delivering value through clinical leadership and excellence Combination meaningfully increases ability to ensure we meet needs of clinician customers around the world and patients they serve |

| Roadmap To Completion Event Expected Timeline Execute definitive agreement Solicit tender of shares from ev3 shareholders Receive regulatory clearances Close transaction Completed Tender offer documents to be filed by mid-June - offer period to remain open for at least 20 business days By July 31, 2010 By July 31, 2010 |

| Conclusion Offers immediate return on investment for shareholders Advances our broad platform of peripheral vascular and neurovascular technologies with leading global healthcare products company Leverages combined organization's world-class sales forces and expertise in developing and marketing innovative products Provides opportunity for further innovation to support endovascular market growth and procedure penetration worldwide Anticipated to close by July 31, 2010, subject to customary closing conditions, including receipt of certain regulatory approvals |

| NASDAQ: EVVV www.ev3.net Contact Info: Julie Tracy - Sr. VP, Chief Communications Officer (949) 680-1375 jtracy@ev3.net |