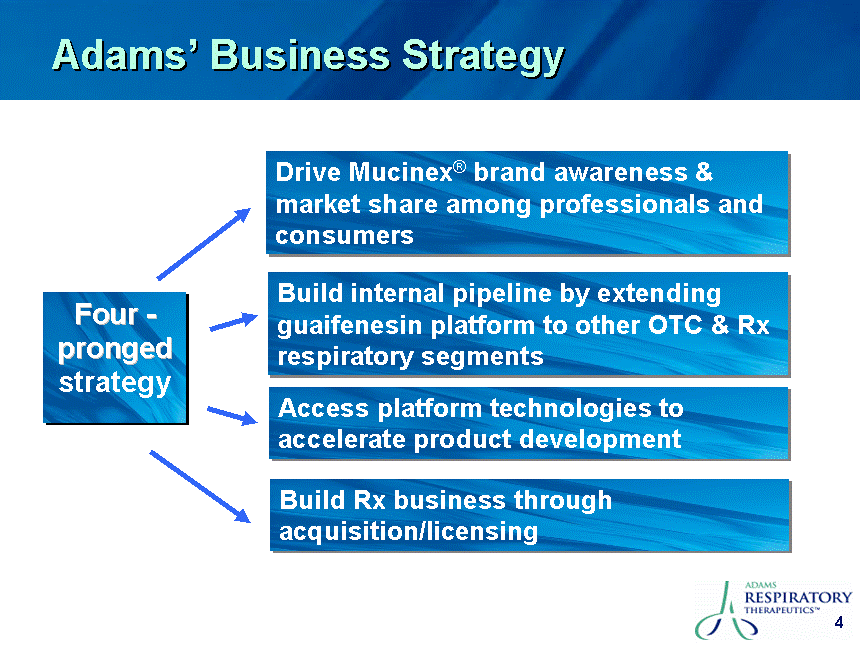
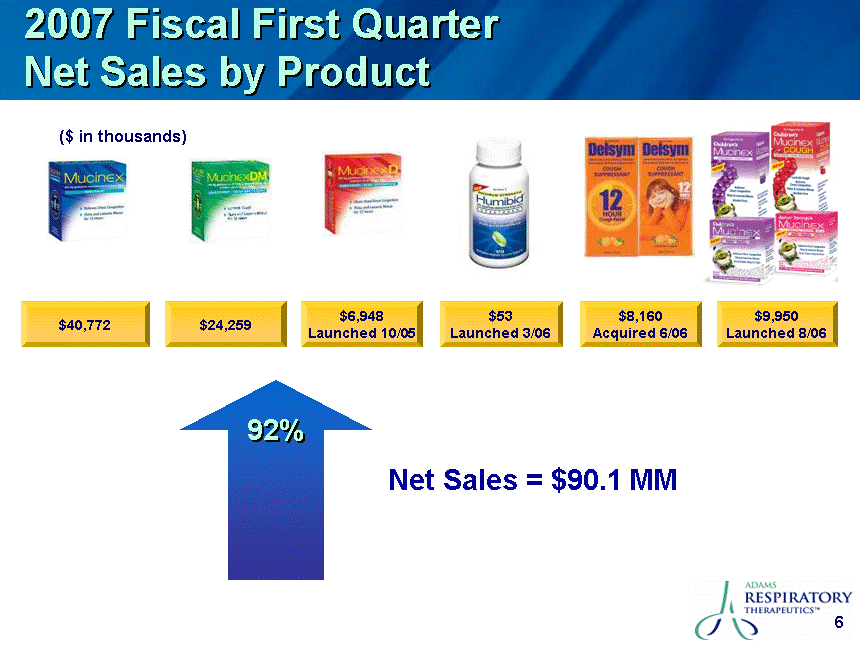
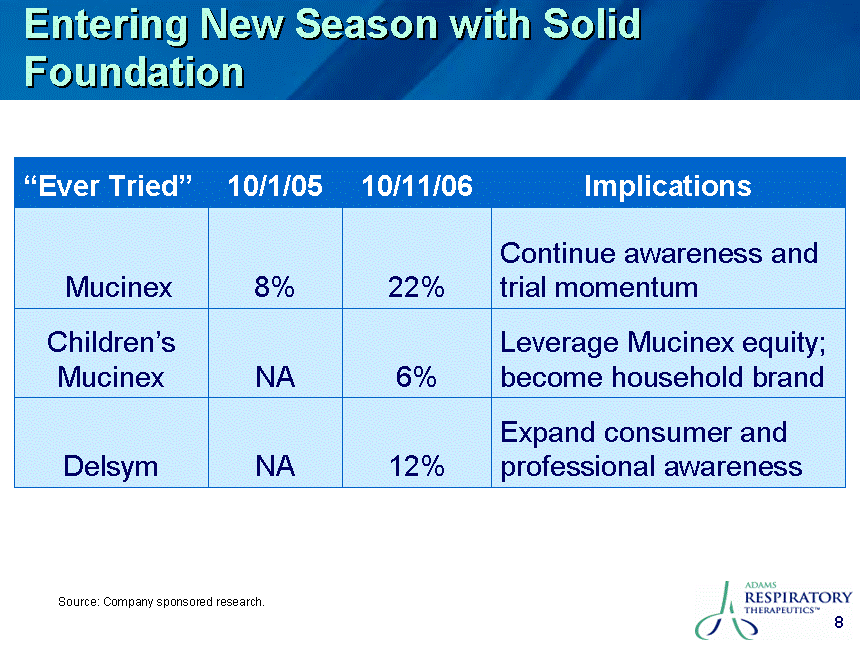
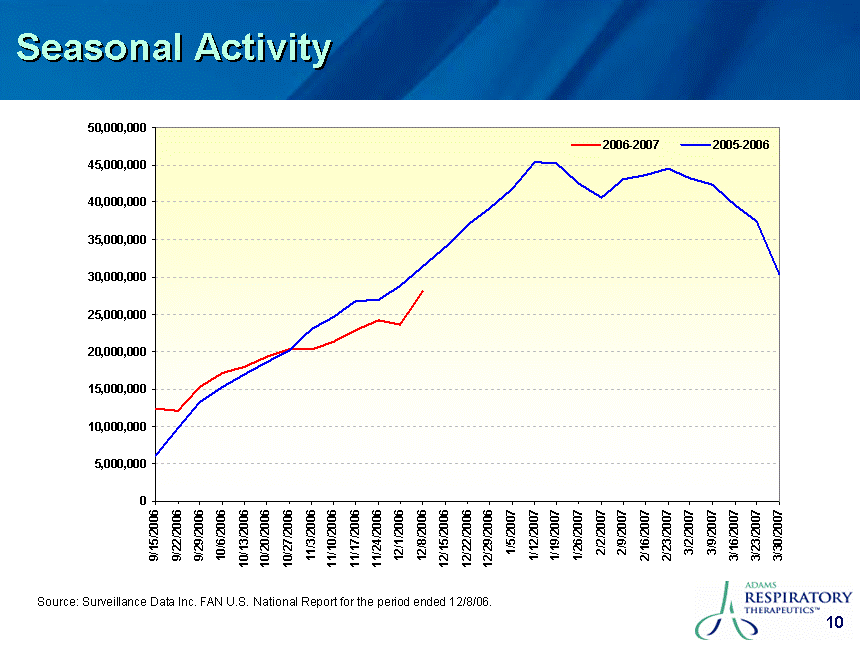
| Safe Harbor This presentation contains certain "forward-looking" statements. These statements are based on the current estimates and assumptions of the management of Adams Respiratory Therapeutics, Inc. (or "Adams") as of the date of this presentation and are naturally subject to uncertainty and changes in circumstances. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Actual results may vary materially from the expectations contained in this presentation. When used in this presentation, the words "may", "will", "should", "could", "would", "plan", "anticipate", "believe", "estimate", "intend", "project", "potential" and "expect" and similar expressions are intended to identify such forward-looking statements. Such forward-looking statements are subject to risks, uncertainties, assumptions and other factors that may cause the actual results of Adams to be materially different from those reflected in such forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others: Adams' ability to in-license or acquire new products and brands; Adams' ability to preserve its patent position in the reexamination process; Adams' ability to successfully extend the long-acting guaifenesin platform to new market segments and build the prescription drug market; the continued success of Mucinex SE, Mucinex DM and Mucinex D and the successful commercialization of Humibid, Delysm, Children's line of Mucinex products, and future products; Delsym's competitive position as the only OTC 12-hour liquid cough syrup; the FDA's removal from the market of products similar to Mucinex D, Mucinex DM and future products; Adams' ability to develop new products; Adams' ability to utilize technology in connection with product development; Adams' ability to complete the clinical trials of erdosteine and successfully commercialize it in the United States; leverage of the Mucinex brand name to increase market share and brand awareness; Adams' ability to successfully integrate the repurchase of the Ft. Worth plant from Cardinal Health; Adams' ability to comply with the FDA's manufacturing regulations, as well as the regulations of the DEA and other governmental agencies; Adams' ability to compete against other branded products, as well as against generic competition; Adams' ability to successfully defend its patent position and other risk factors set forth under Item 1A. Risk Factors in Adams' Annual Report on Form 10-K for the fiscal year ended June 30, 2006. Except to the extent required by applicable securities laws, Adams is not under any obligation to (and expressly disclaims any such obligation to) update its forward-looking statements, whether as a result of new information, future events, or otherwise. All statements contained in this presentation are made only as of the date of this presentation. |