Filed pursuant to Rule 424(b)(3)
Registration Nos. 333-138131
333-138131-01
333-138131-02
333-138131-03
333-138131-04
333-138131-05
$390,000,000

Warner Chilcott Corporation
8 3/4 Senior Subordinated Notes due 2015
The 8 3/4% Senior Subordinated Notes due 2015 (the “notes”) were issued on January 13, 2005 and will mature on February 1, 2015. Interest on the notes is paid on February 1 and August 1 of each year.
The notes are our unsecured senior subordinated obligations and rank junior to all our existing and future senior indebtedness, including indebtedness under our senior secured credit facility. The notes are guaranteed on a senior subordinated basis by Warner Chilcott Limited, Warner Chilcott Holdings Company III, Limited (“Holdings”), and Warner Chilcott Intermediate (Luxembourg) S.à r.l. (“Luxco”), our indirect and direct parent companies, respectively, and by Holdings’ U.S. and Puerto Rican subsidiaries.
We may redeem some or all of the notes at any time prior to February 1, 2010 at a redemption price equal to the make-whole amount set forth in this prospectus. We may also redeem some or all of the notes at any time on or after February 1, 2010 at the redemption prices set forth in this prospectus. There is no sinking fund for the notes.
You should consider carefully the “Risk Factors” beginning on page 16 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
This prospectus is to be used by Credit Suisse Securities (USA) LLC and J.P. Morgan Securities Inc. in connection with offers and sales related to market making transactions in the notes. Credit Suisse Securities (USA) LLC and J.P. Morgan Securities Inc. may act as principal or agent in such transactions. Such sales will be made at prices related to prevailing market prices at the time of sale.
July 6, 2007
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus.
INDUSTRY AND MARKET DATA
Industry and market data used throughout this prospectus were obtained through reports of IMS Health Incorporated (“IMS”). While we believe that the reports of IMS are reliable and appropriate, we have not independently verified such data.
TRADEMARKS
We have proprietary rights to a number of trademarks used in this prospectus which are important to our business, including Doryx®, Duricef®, Estrace®, Estrostep®, femhrt®, Femcon™, Femring®, Femtrace®, Loestrin®, Moisturel®, Ovcon®, Sarafem® and Warner Chilcott®. Dovonex®, Taclonex®, Dovobet® and Daivobet® are registered trademarks of LEO Pharma A/S (“LEO Pharma”). We have omitted the “®” and “™” trademark designation for such trademarks in this prospectus. Nevertheless, all rights to such trademarks named in this prospectus are reserved.
i
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere, or incorporated by reference, in this prospectus. It may not contain all the information that is important to you. You should read this entire prospectus, including the information incorporated by reference herein, carefully, including “Risk Factors,” the consolidated financial statements and the related notes thereto, before making an investment decision. Unless otherwise noted or the context otherwise requires, references in this prospectus to “Warner Chilcott,” “the Company,” “our company,” “we,” “us” or “our” for periods after January 5, 2005 refer to Warner Chilcott Limited, and its direct and indirect subsidiaries (the “Successor”), following the consummation of the acquisition of Warner Chilcott PLC by Warner Chilcott Acquisition Limited on January 5, 2005 (the “Acquisition Date”) and the other transactions described in this summary under “The Transactions” and for periods prior to the Acquisition Date refer to the historical operations of Warner Chilcott PLC, the public company that was acquired in connection with the Transactions, together with its subsidiaries (the “Predecessor”). The Predecessor operated and reported using a fiscal year ending on September 30. After the acquisition of the Predecessor, we changed our fiscal year to a calendar year. Therefore, all references in this prospectus to “fiscal year 2002,” “fiscal year 2003” or “fiscal year 2004” are to the twelve months ended September 30 of the year referenced and all references in this prospectus to “year ended 2005” or “ year ended 2006” are to the twelve months ending December 31, 2005 and 2006, respectively.
Our Business
We are a leading specialty pharmaceutical company focused on segments of the U.S. pharmaceutical market, currently women’s healthcare and dermatology. We are a fully-integrated company with internal resources dedicated to product development, manufacturing and the promotion of our products. We have established strong franchises in these two areas through our precision marketing techniques and specialty sales forces which now comprise approximately 491 representatives. We believe that our proven product development capabilities, coupled with our ability to execute acquisitions and in-licensing transactions and develop partnerships, such as our relationship with LEO Pharma, will enable us to sustain and grow these franchises. We operate two manufacturing facilities located in Fajardo, Puerto Rico and Larne, Northern Ireland (UK).
Our franchises are comprised of complementary portfolios of established branded, development-stage and new products, including our recently introduced products, Loestrin 24 Fe, Taclonex and Femcon Fe (formerly Ovcon 35 Fe). Our women’s healthcare franchise is anchored by our strong presence in the hormonal contraceptive and hormone therapy (“HT”) categories and our dermatology franchise is built on our established positions in the markets for psoriasis and acne therapies. In April 2006, we launched Loestrin 24 Fe, an oral contraceptive with a novel patented 24-day dosing regimen, with the goal of growing the market share position we have achieved with our Ovcon and Estrostep products in the hormonal contraceptive market. In November 2006, we introduced Femcon Fe which is the first and only chewable oral contraceptive approved by the United States Food and Drug Administration (“FDA”). We also have a significant presence in the HT market, primarily through our products femhrt and Estrace Cream. In dermatology, our psoriasis product Dovonex enjoys the leading position in the United States for the non-steroidal topical treatment of psoriasis. We strengthened and extended our position in the market for psoriasis therapies with the April 2006 launch of Taclonex, the first once-a-day topical psoriasis treatment that combines betamethasone dipropionate, a corticosteroid, with calcipotriene, the active ingredient in Dovonex. Our product Doryx is the leading branded oral tetracycline in the United States for the treatment of acne. In 2005, we launched Doryx delayed-release tablets.
1
Our strategy is to grow our specialty pharmaceutical products business by focusing on therapeutic areas dominated by specialist physicians. We believe that we will continue to drive organic growth by employing our precision marketing techniques. Furthermore, we intend to supplement our growth and broaden our market position in our existing franchises through ongoing product development. Our internal product development is focused on new products, proprietary product improvements and new and enhanced dosage forms. We selectively review potential product in-licensing, acquisition and partnership opportunities within our franchises, such as our relationship with LEO Pharma, or in market segments that have characteristics similar to our current markets. We believe that our streamlined corporate organization and resulting ability to react rapidly to changing market dynamics enhances our ability to execute on our strategy.
In our development of new products and product enhancements, we have a track record of successful and cost-efficient internal product development with six new drug application (“NDA”) approvals from the FDA since March 2003. Our research and development team has significant experience and proven capabilities in specialty chemistry, pharmaceutical development and clinical development. We focus our research and development efforts primarily on developing new products that target therapeutic areas with established regulatory guidance, making proprietary improvements to our existing products and developing new and enhanced dosage forms. Through this focused approach to research and development, we seek to enhance the value of our franchises by investing in relatively low-risk projects.
The U.S. pharmaceutical market generated sales of approximately $286 billion in 2006 and has grown at a compound annual growth rate of approximately 7.6% since 2001, according to IMS. Large pharmaceutical companies have been consolidating and are focusing on developing and marketing “blockbuster” drugs that have the potential of generating more than $1 billion in annual revenues. The focus by large pharmaceutical companies on blockbuster products creates opportunities for specialty pharmaceutical companies like us to compete effectively in smaller but lucrative therapeutic markets.
Dovonex and Taclonex Transactions
In April 2003, our Predecessor, Warner Chilcott PLC, entered into a major strategic alliance in dermatology with LEO Pharma, the developer and owner of Dovonex and Taclonex (and owner of the patents covering these products), and Bristol-Myers Squibb Company (“Bristol-Myers”), the then-exclusive licensee of Dovonex in the United States.
Dovonex is the leading non-steroidal topical treatment for psoriasis. For each quarter over the last four years, Dovonex has had the number one share of both revenues and prescriptions in the non-steroidal topical treatment segment. Often prescribed as a combination therapy with a topical corticosteroid, we believe that Dovonex enjoys wide brand recognition and acceptance among dermatologists as a leading treatment for mild to moderate psoriasis. From April 2003 through December 2005, we promoted Dovonex in the United States under a co-promotion agreement with Bristol-Myers. Under the agreement, we promoted Dovonex and were compensated based upon levels of sales of Dovonex achieved by Bristol-Myers. On January 1, 2006, we acquired the exclusive U.S. sales and marketing rights to Dovonex from Bristol-Myers for a purchase price of $205.2 million (including inventory on hand) plus a 5% royalty on net sales of Dovonex through 2007. We funded the payment of the purchase price by borrowing $200.0 million in delayed-draw term loans under our senior secured credit facility. On January 1, 2006, our license and supply agreement with LEO Pharma for Dovonex became effective and our co-promotion agreement with Bristol-Myers was terminated. Our acquisition of the U.S. sales and marketing rights to Dovonex did not require any significant launch costs or an increase in the size of our sales forces as we had been promoting Dovonex since 2003.
2
Under the LEO Pharma license and supply agreement, we are required to pay LEO Pharma a supply fee for Dovonex equal to 20% of net sales and a royalty equal to 10% of net sales (each as calculated under the terms of the agreement). The royalty will be reduced to 5% if a generic equivalent is introduced.
Taclonex is a once-a-day topical psoriasis treatment that combines calcipotriene, the active ingredient in Dovonex, with the corticosteroid, betamethasone dipropionate, in a single treatment. In April 2003, we entered into agreements with LEO Pharma relating to the development and U.S. commercialization of Taclonex. Taclonex (also known in some countries as Dovobet or Daivobet is currently marketed by or on behalf of LEO Pharma in over 70 countries, including the United Kingdom, Canada and France. LEO Pharma’s New Drug Application (“NDA”) for Taclonex was approved by the FDA on January 9, 2006. Under our agreements with LEO Pharma for Dovonex and Taclonex, we paid LEO Pharma $2.0 million in December 2001, an additional $10.0 million in April 2003 and a final milestone payment of $40.0 million on February 6, 2006 that was triggered by FDA approval of Taclonex. As of September 14, 2005, we became the exclusive licensee of Taclonex in the United States, subject to the terms of our agreement with LEO Pharma. Under the agreement we are required to pay LEO Pharma a supply fee for Taclonex ranging from 20% to 25% of net sales and royalties ranging from 10% to 15% of net sales (each as calculated under the terms of the agreement).
Expansion of LEO Pharma Relationship
LEO Pharma has agreed to expand the scope of our Dovonex and Taclonex licenses to include exclusive U.S. sales and marketing rights to all of LEO Pharma’s product improvements, new and enhanced dosage forms and new products that contain calcipotriene or a combination of calcipotriene and a steroid until 2020. LEO Pharma also granted us a right of first refusal and last offer for the U.S. sales and marketing rights to all products developed by LEO Pharma principally for the treatment or prevention of dermatological diseases through 2010. In connection with these expanded agreements, we paid LEO Pharma an aggregate of $37.0 million during the year ended December 31, 2005. We may make additional payments under these agreements upon the achievement of various development milestones relating to certain identified products and product improvements. These payments could aggregate up to $150.0 million. In addition, we have agreed to pay a supply fee and royalties to LEO Pharma on the net sales of those products. We may also agree to make additional payments for products that have not been identified or that are covered under the right of first refusal and last offer.
3
Franchise Focus and Principal Marketed Products
We market a diversified portfolio of branded products in our women’s healthcare and dermatology franchises. Within these franchises, we compete primarily in four therapeutic categories: hormonal contraception and hormone therapy in women’s healthcare and psoriasis and acne in dermatology. The following chart presents our franchises and their respective therapeutic categories with revenues generated in 2006.

| (1) | Includes Sarafem, our product to treat premenstrual dysphoric disorder. |
4
| | | | | | | | |
Our Principal Products
|
| | | Product (Active Ingredient)
| | Indication
| | Patent
Expiry(1)
| | 2006 Revenue
($mm)
|
| | | Hormonal Contraception | | | | | | |
| | | Loestrin 24 FE (Norethindrone acetate and ethinyl estradiol) | | Prevention of pregnancy | | July 2014 | | $44.2(2) |
| | Ovcon 35 and Ovcon 50 (Norethindrone and ethinyl estradiol) | | Prevention of pregnancy | | Patent expired
prior to 2000 | | $73.8 |
| | | | |
| | | Femcon Fe (Norethindrone and ethinyl estradiol) | | Prevention of pregnancy | | June 2019 | | $7.5(3) |
| | | | |
| | | Estrostep Fe (Norethindrone acetate and ethinyl estradiol) | | Prevention of pregnancy and treatment of moderate acne in women who desire oral contraception | | April 2008(4) | | $103.0 |
| | | | |
| | | Hormone Therapy | | | | | | |
| | | femhrt 1/5 and .5/2.5 (Norethindrone acetate and ethinyl estradiol) | | Oral treatment of moderate to severe vasomotor symptoms and urogenital symptoms associated with menopause | | May 2010(4) | | $58.7 |
| | | | |
| | | Estrace Cream (17-beta estradiol) | | Vaginal cream for treatment of vaginal and vulvar atrophy | | Patent expired
March 2001 | | $65.8 |
| | | | |
| | | Psoriasis | | | | | | |
| | | Taclonex(5) (Calcipotriene and betamethasone dipropionate) | | Topical treatment of psoriasis | | January 2020 | | $60.1(2) |
| | | Dovonex (Calcipotriene) | | Topical treatment of psoriasis | | Ointment-December 2007 Cream and topical solution-June 2015(6) | | $146.9 |
| | | | |
| | | Acne | | | | | | |
| | | Doryx (Doxycycline hyclate) | | Oral adjunctive therapy for severe acne | | December 2022 | | $102.4 |
| | (1) | See “Risk Factors—Risks Relating to Our Business—If generic products that compete with any of our branded pharmaceutical products are approved, sales of our products may be adversely affected.” | |
| | (2) | We launched Loestrin 24 Fe and Taclonex in April 2006. | |
| | (3) | We introduced Femcon Fe in November 2006 (formerly Ovcon 35 Fe). | |
| | (4) | Pursuant to an agreement to settle patent litigation against Barr Laboratories, Inc. (“Barr”), we granted Barr a non-exclusive license to launch generic versions of the product six months prior to expiration of our patents. | |
| | (5) | Taclonex was previously referred to as “Dovobet.” The name was changed during the review by the FDA of the NDA for the product. | |
| | (6) | LEO Pharma has received notices of Paragraph IV certifications in respect of its patent on Dovonex solution which may result in a generic equivalent entering the market as early as January 2008. See Note 11 to the Notes to the Condensed Consolidated Financial Statements for the quarter ended March 31, 2007, incorporated by reference herein. | |
5
New Product Launches
In April 2006, we launched Loestrin 24 Fe, an oral contraceptive with a novel patented 24-day dosing regimen. The majority of oral contraceptive products currently used in the United States are based on a regimen of 21 days of active hormonal pills followed by seven days of placebo. By contrast, with Loestrin 24 Fe women take the active pills for 24 consecutive days followed by four days of placebo. The clinical data show that at the end of the sixth cycle, women who took Loestrin 24 Fe had periods that averaged 2.7 days, compared to 3.9 days with the traditional 21-day regimen. In a national survey of women aged 18 - 49 conducted by Harris Interactive® (funded by us), 85% of women who currently use or have ever used a birth control pill felt that having a shorter period would make a positive difference.
In April 2006, we launched Taclonex, the first and only dual-action therapy of its kind. Taclonex is a once-a-day topical psoriasis treatment that combines betamethasone dipropionate with calcipotriene, the active ingredient in Dovonex. In clinical trials, 81% of patients using Taclonex achieved a significant reduction in disease severity after only 4 weeks of use as compared to 49% of patients using Dovonex alone and 64% of patients using betamethasone dipropionate alone. Many patients with psoriasis have adopted complicated dosing regimens including daily and intermittent use of multiple products. The once-daily dosing of Taclonex provides an opportunity to enhance patient compliance.
In November 2006, we introduced Femcon Fe, the first and only chewable oral contraceptive to receive FDA approval. Femcon Fe’s chewable design provides a convenient, new option for women “on-the-go” taking oral contraceptives.
Our Competitive Strengths
We believe that we possess the following competitive strengths:
| | Ÿ | | Strong franchises in women’s healthcare and dermatology. We are well positioned to grow our franchises through our portfolio of established brands and the growth opportunities from our new products, particularly Loestrin 24 Fe and Taclonex. These franchises are supported by significant intellectual property. |
| | Ÿ | | Effective and efficient sales and marketing approach. We market products that are promotionally sensitive. Using our precision marketing techniques, we deploy our sales representatives and other marketing resources to consistently target physicians with the highest potential to prescribe our products. This approach enables us to optimize our promotional efforts. |
| | Ÿ | | Substantial development capabilities and pipeline. We use our proven internal product development expertise to maintain and grow our franchises. Our internal development efforts have yielded six NDA approvals since 2003. Our exclusive product development relationship with LEO Pharma also provides us with access to additional pipeline opportunities for dermatology products. |
| | Ÿ | | Integrated manufacturing capabilities. The manufacturing and packaging capabilities of our Puerto Rico operations provide us with a reliable source of supply for a number of our brands, including our hormonal products. The integration of our development efforts with our ability to scale up our manufacturing from pilot to production quantities allows us to more efficiently develop products. |
| | Ÿ | | Strong free cash flow generation. Our business generates strong free cash flow as a result of our high sales force productivity and efficient investment in product development activities, coupled with an attractive corporate tax rate and modest capital expenditure requirements. |
6
| | Ÿ | | Experienced management team. The members of our senior management team have an average of over 21 years of experience in the pharmaceutical industry. Through this experience we have gained a deep base of industry knowledge, and built a proven track record of growing market share for new and established products. |
Our Strategy
We intend to continue to develop our specialty pharmaceutical products business by focusing on smaller therapeutic markets, driving organic growth by employing our precision marketing techniques, developing and marketing new products, proprietary product improvements and new and enhanced dosage forms and selectively reviewing potential product in-licensing, acquisition and partnership opportunities within our franchises, such as our relationship with LEO Pharma, or in market segments that have characteristics similar to our current markets.
We believe that the following are risks we face in executing our business strategies:
| | Ÿ | | Generic competition with our branded products. Our branded pharmaceutical products are or may become subject to competition from generic equivalents because there is no proprietary protection for some of the branded pharmaceutical products we sell or our patents are not sufficiently broad or because we lose proprietary protection due to the expiration of a patent. |
| | Ÿ | | Infringement on our intellectual property. If we are unable to protect our trademarks, patents and other intellectual property from infringement, our business prospects may be harmed. We may not have adequate remedies for any infringement, and the expense of bringing lawsuits against infringers could cause us not to continue these suits and abandon the affected products. |
| | Ÿ | | Delays in production of our products. We may face delays in qualifying our manufacturing facility in Puerto Rico for the manufacture of new products or for our other products that are currently manufactured for us by third parties. In addition, we or our contract manufacturers may not be able to manufacture our products without interruption, and our contract manufacturers may not comply with their obligations under our various supply arrangements. |
| | Ÿ | | Downward pricing pressures. Pricing pressures from third-party payors, including managed care organizations, government sponsored health systems and regulations relating to Medicare and Medicaid, healthcare reform, pharmaceutical reimbursement and pricing in general could decrease our revenues. In addition, sales of our products may be adversely affected by the consolidation among wholesale drug distributors and the growth of large retail drug store chains. |
| | Ÿ | | Changes in laws and regulations. Our future operating results could be adversely affected by changes in laws and regulations, including changes in the FDA approval processes that may cause delays in, or prevent the approval of, new products, new laws, regulations and judicial decisions affecting product marketing, promotion or the healthcare field generally, and new laws or judicial decisions affecting intellectual property rights. |
| | Ÿ | | Product liability claims and/or product recalls. Unforeseen side-effects caused by, or manufacturing defects inherent in, the products sold by us could result in injury or death. The occurrence of such an event could result in product liability claims and/or recall of one or more of our products. |
For a discussion of these and other risks associated with our business, including risks related to our strategy and risks related to this offering, see “Risk Factors” beginning on page 16 of this prospectus.
7
Our Sponsors
Our sponsors, Bain Capital, LLC, DLJ Merchant Banking, J.P. Morgan Partners, LLC and Thomas H. Lee Partners are each leading global private equity firms with established track records of successful investments and extensive experience managing investments in the healthcare industry.
The Transactions
In November 2004, affiliates of Bain Capital, LLC, DLJ Merchant Banking, J.P. Morgan Partners, LLC and Thomas H. Lee Partners, who we refer to collectively in this prospectus as the “Sponsors,” reached an agreement to acquire Warner Chilcott PLC. The acquisition became effective on January 5, 2005, and thereafter, following a series of transactions, we acquired 100% of the share capital of Warner Chilcott PLC. We refer to this transaction in this prospectus as the “Acquisition.” To complete the Acquisition, the Sponsors, certain of their limited partners and certain members of our management, indirectly funded equity contributions to us and certain of our subsidiaries, the proceeds of which were used to purchase 100% of Warner Chilcott PLC’s share capital. On January 18, 2005, certain of our subsidiaries borrowed an aggregate of $2,020 million, consisting of an initial drawdown of $1,420 million under a $1,790 million senior secured credit facility and the issuance of the notes by Warner Chilcott Corporation. The proceeds from the acquisition financings, together with cash on hand at Warner Chilcott PLC, were used to pay the selling stockholders $3,014 million, to retire all of Warner Chilcott PLC’s outstanding share options for $70 million, to retire all of Warner Chilcott PLC’s previously outstanding funded indebtedness totaling $195 million and to pay related fees and expenses. In this prospectus, we refer to the Acquisition, together with the related financings, as the “Transactions.”
8
The following chart reflects our corporate structure following the Transactions, the completion of a series of internal restructuring transactions after the completion of the Acquisition and the initial public offering of Warner Chilcott Limited on September 20, 2006. Guarantor references designate guarantors of the notes. Not all guarantors of our senior secured credit facility are shown:
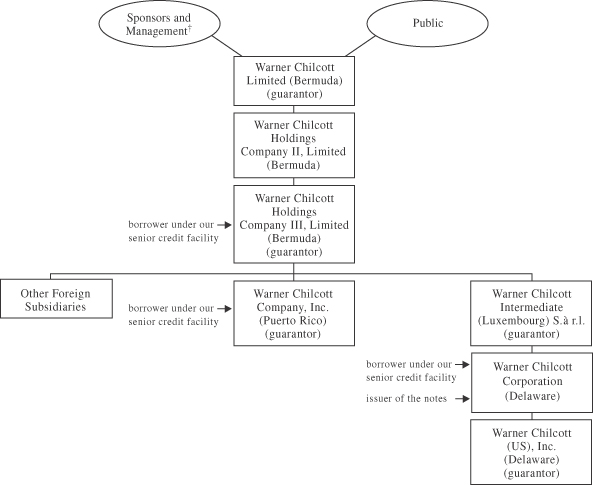
| † | The investment by the Sponsors, certain of their limited partners and certain members of our management was in equity interests in each of Warner Chilcott Limited and Warner Chilcott Holdings Company II, Limited. A portion of the equity interests in Warner Chilcott Holdings Company II, Limited were redeemed and the remainder converted into Class A common shares of Warner Chilcott Limited in connection with Warner Chilcott Limited’s initial public offering. |
The issuer of the notes is Warner Chilcott Corporation. At the time of the funding of the Transactions, Warner Chilcott Holdings Company III, Limited (which we refer to in this prospectus as “Holdings”) was capitalized with approximately $1,283 million of equity that was contributed to it, indirectly, by the Sponsors, certain of their limited partners and certain members of our management. Of that amount, $400.0 million was further contributed to the issuer of the notes. Following the completion of a series of internal restructuring transactions, Warner Chilcott Corporation became the direct 100% shareholder of Warner Chilcott (US), Inc. (our U.S. operating subsidiary which was previously a wholly-owned indirect subsidiary of Warner Chilcott PLC). The notes are guaranteed by Warner Chilcott Limited, Holdings, Warner Chilcott Intermediate (Luxembourg) S.à r.l., Warner Chilcott Company, Inc. and Warner Chilcott (US), Inc.
9
Corporate Information
Our registered office is located at Canon’s Court, 22 Victoria Street, Hamilton HM12, Bermuda. Our telephone number is (441) 295-2244. Our website is accessible throughwww.warnerchilcott.com. Information on, or accessible through, this website is not a part of, and is not incorporated into, this prospectus.
Summary of Terms of the Notes
The summary below describes the principal terms of the notes. Some of the terms and conditions described below are subject to important limitations and exceptions. The “Description of the Notes” section of this prospectus contains a more detailed description of the terms and conditions of the notes.
Issuer | Warner Chilcott Corporation |
Securities | $390,000,000 aggregate principal amount of 8 3/4% Senior Subordinated Notes due 2015 registered under the Securities Act. The notes were issued under an indenture dated January 18, 2005. |
Maturity | February 1, 2015. |
Interest Payment Dates | February 1 and August 1 of each year. |
Guarantees | The notes are guaranteed, jointly and severally, on a senior subordinated basis, by Warner Chilcott Limited, Warner Chilcott Holdings Company III, Limited and Warner Chilcott Intermediate (Luxembourg) S.à r.l., Warner Chilcott Corporation’s indirect and direct parent companies, respectively, and by Holdings’ U.S. and Puerto Rican subsidiaries. See “Description of the Notes—Guarantees.” |
Ranking | The notes and the guarantees are our and our guarantors’ unsecured senior subordinated obligations. The notes rank: |
| | • | | junior to all of our and our guarantors’ existing and future senior indebtedness, including borrowings under our senior secured credit facility; |
| | • | | equally in right of payment with all of our and our guarantors’ future unsecured senior subordinated indebtedness; |
| | • | | senior in right of payment to any of our or our guarantors’ future indebtedness that is expressly subordinated in right of payment to the notes; and |
| | • | | effectively junior to all of the existing and future indebtedness, including trade payables, of our subsidiaries that have not guaranteed the notes. |
10
As of March 31, 2007, the notes and the guarantees ranked junior to:
| | • | | $1,097.8 million of our senior indebtedness, all of which consisted of borrowings and guarantees under our senior secured credit facility; and |
| | • | | $15.5 million of total liabilities of our non-guarantor subsidiaries, including trade payables and accrual for taxes but excluding intercompany obligations and guarantees by them of borrowings under our senior secured credit facility. |
At December 31, 2006 and March 31, 2007, the subsidiaries of Holdings that are not guarantors accounted for approximately $322.4 million, or 10.2%, and $313.0 million, or 10.0%, respectively, of our total assets and a nominal amount of revenues (after intercompany eliminations).
Optional Redemption | We may redeem some or all of the notes at any time prior to February 1, 2010 at a redemption price equal to the make-whole amount set forth under “Description of the Notes—Optional Redemption.” We may also redeem some or all of the notes at any time on or after February 1, 2010 at the redemption price listed under “Description of the Notes—Optional Redemption.” |
Change of Control | If we experience a change of control (as defined in the indenture), we will be required to make an offer to repurchase the notes at a price equal to 101% of the principal amount thereof, plus accrued interest, if any, to the date of purchase. See “Description of the Notes—Repurchase at the Option of Holders—Change of Control.” |
Restrictive Covenants | The indenture governing the notes contains certain covenants that, among other things, limit the ability of Holdings and its restricted subsidiaries to: |
| | • | | incur or guarantee additional indebtedness or issue preferred stock; |
| | • | | pay dividends or make distributions to our stockholders; |
| | • | | repurchase or redeem capital stock or subordinated indebtedness; |
| | • | | incur restrictions on the ability of our subsidiaries to pay dividends or to make other payments to us; |
| | • | | enter into transactions with our affiliates; |
| | • | | enter into other lines of business; and |
| | • | | merge or consolidate with other companies or transfer all or substantially all of our assets. |
These limitations are subject to a number of exceptions and qualifications. See “Description of the Notes—Certain Covenants.”
11
Summary Historical Consolidated Financial Data
The following table sets forth our summary historical consolidated financial data. Except for the data relating to the years ended December 31, 2006 and 2005 and the three months ended March 31, 2007 and 2006, all data below reflects the consolidated financial data of the Predecessor. The year ended December 31, 2005 was our first fiscal year following the Acquisition. The financial statements relating to this period reflect the Acquisition as if the closing took place on January 1, 2005 and the operating results for the period January 1 through January 4, 2005 were ours. The period included only two business days and the impact on the results of operations during the period was not material. Our fiscal year ends on December 31 versus the Predecessor’s year-end of September 30.
The summary consolidated financial data as of and for the years ended December 31, 2006 and 2005, as of and for the quarter ended December 31, 2004, and as of and for the fiscal year ended September 30, 2004 presented in this table have been derived from our audited consolidated financial statements and related notes incorporated by reference in this prospectus. The summary consolidated financial data as of and for the quarter ended December 31, 2003 presented in this table have been derived from our unaudited consolidated financial statements and related notes, which are not included or incorporated by reference in this prospectus. The summary consolidated financial data as of and for the fiscal years ended September 30, 2003 and 2002 presented in this table are derived from our audited consolidated financial statements and related notes which are not included or incorporated by reference in this prospectus. Summary historical consolidated financial data for the three months ended March 31, 2007 and 2006 and the condensed consolidated balance sheet data as of March 31, 2007 and 2006, has been derived from our unaudited consolidated financial statements and related notes, which have been incorporated by reference in this prospectus.
The summary historical data included below and elsewhere in this prospectus are not necessarily indicative of future performance. This information is only a summary and should be read in conjunction with “Capitalization,” “Selected Historical Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and related notes included, or incorporated by reference, in this prospectus.
12
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Fiscal Year Ended
September 30,
| | | Transition Period
Quarter Ended
December 31,
| | | Year Ended
December 31,
| | | Three Months
Ended March 31,
| |
| | | Predecessor
| | | Predecessor
| | | Successor
| | | Successor
| |
| | | 2002
| | | 2003
| | | 2004
| | | 2003
| | | 2004
| | | 2005
| | | 2006
| | | 2006
| | | 2007
| |
| | | | | | | | | | | | (Unaudited) | | | | | | | | | | | | (Unaudited) | | | (Unaudited) | |
(dollars and share amounts in thousands, except per share amounts) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenue(1)(2) | | $ | 172,231 | | | $ | 365,164 | | | $ | 490,248 | | | $ | 124,789 | | | $ | 136,893 | | | $ | 515,253 | | | $ | 754,457 | | | $ | 166,461 | | | $ | 218,421 | |
Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of sales (excluding amortization and impairments)(3) | | | 19,366 | | | | 42,042 | | | | 53,488 | | | | 11,408 | | | | 34,529 | | | | 95,224 | | | | 151,750 | | | | 31,807 | | | | 50,597 | |
Selling, general and administrative(3)(4) | | | 47,174 | | | | 124,786 | | | | 146,205 | | | | 37,745 | | | | 41,463 | | | | 162,670 | | | | 253,937 | | | | 38,286 | | | | 77,898 | |
Impairment of intangible assets | | | — | | | | — | | | | — | | | | — | | | | — | | | | 38,876 | | | | — | | | | — | | | | — | |
Research and development | | | 16,000 | | | | 24,874 | | | | 26,558 | | | | 6,692 | | | | 4,608 | | | | 58,636 | | | | 26,818 | | | | 9,571 | | | | 7,432 | |
Amortization of intangible assets(3)(5) | | | 18,252 | | | | 38,106 | | | | 52,374 | | | | 13,185 | | | | 21,636 | | | | 233,473 | | | | 253,425 | | | | 58,826 | | | | 57,553 | |
Acquired in-process research and development(3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 280,700 | | | | — | | | | — | | | | — | |
Transaction costs(3) | | | — | | | | — | | | | — | | | | — | | | | 50,973 | | | | 35,975 | | | | — | | | | — | | | | — | |
Net interest expense(3) | | | 18,916 | | | | 7,686 | | | | 9,256 | | | | 3,152 | | | | 1,214 | | | | 147,934 | | | | 206,994 | | | | 45,092 | | | | 30,944 | |
Accretion on preferred stock of subsidiary(6) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 31,533 | | | | 26,190 | | | | 8,701 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) before taxes | | | 52,523 | | | | 127,670 | | | | 202,367 | | | | 52,607 | | | | (17,530 | ) | | | (569,768 | ) | | | (164,657 | ) | | | (25,822 | ) | | | (6,003 | ) |
Provision (benefit) for income taxes | | | 18,858 | | | | 41,380 | | | | 59,390 | | | | 12,312 | | | | 11,558 | | | | (13,122 | ) | | | (11,147 | ) | | | 1,434 | | | | (1,501 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) from continuing operations | | | 33,665 | | | | 86,290 | | | | 142,977 | | | | 40,295 | | | | (29,088 | ) | | | (556,646 | ) | | | (153,510 | ) | | | (27,256 | ) | | | (4,502 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Discontinued operations, net of tax(7) | | | 111,511 | | | | 9,865 | | | | 8,711 | | | | 2,405 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | 145,176 | | | $ | 96,155 | | | $ | 151,688 | | | $ | 42,700 | | | $ | (29,088 | ) | | $ | (556,646 | ) | | $ | (153,510 | ) | | $ | (27,256 | ) | | $ | (4,502 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Other Data | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Ratio of earnings to fixed charges(12) | | | 2.7 | x | | | 11.8 | x | | | 18.5 | x | | | 14.7 | x | | | (12 | ) | | | (12 | ) | | | 0.1 | x | | | 0.4 | x | | | 0.8 | x |
Per Share Data(8): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Earnings (loss) per share—basic | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | (7.19 | ) | | $ | (1.63 | ) | | $ | (0.55 | ) | | $ | (0.02 | ) |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | 7.35 | | | $ | 6.33 | | | $ | 2.05 | | | $ | n.m. | |
Earnings (loss) per share—diluted | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | (7.19 | ) | | $ | (1.63 | ) | | $ | (0.55 | ) | | $ | (0.02 | ) |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | 7.34 | | | $ | 6.33 | | | $ | 2.05 | | | $ | n.m. | |
Weighted average shares outstanding—basic | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 88,311 | | | | 133,897 | | | | 89,269 | | | | 248,619 | |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 10,642 | | | | 10,280 | | | | 10,671 | | | | n.m. | |
Weighted average shares outstanding—diluted | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 88,311 | | | | 133,897 | | | | 89,269 | | | | 248,619 | |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 10,668 | | | | 10,282 | | | | 10,672 | | | | n.m. | |
Balance Sheet Data (at period end): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 315,571 | | | $ | 88,571 | | | $ | 186,251 | | | $ | 167,500 | | | $ | 229,565 | | | $ | 11,502 | | | $ | 84,464 | | | $ | 20,809 | | | $ | 68,502 | |
Total assets(3)(9)(10) | | | 1,072,231 | | | | 1,456,419 | | | | 1,419,295 | | | | 1,614,403 | | | | 1,454,243 | | | | 3,041,877 | | | | 3,162,545 | | | | 3,246,964 | | | | 3,119,593 | |
Total long-term debt(3)(9)(10)(11) | | | 49,158 | | | | 341,078 | | | | 191,701 | | | | 341,582 | | | | 192,199 | | | | 1,989,500 | | | | 1,550,750 | | | | 2,226,000 | | | | 1,487,802 | |
Preferred stock in subsidiary(6) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 435,925 | | | | — | | | | 444,689 | | | | — | |
Shareholders’ equity(3) | | | 909,007 | | | | 997,125 | | | | 1,126,640 | | | | 1,051,701 | | | | 1,104,087 | | | | 332,510 | | | | 1,328,232 | | | | 307,945 | | | | 1,323,128 | |
| (1) | The increase in product revenues from fiscal year 2002 to fiscal year 2003 and from fiscal year 2003 to fiscal year 2004 was in part attributable to product acquisitions. |
13
| (2) | In March 2004, we sold the U.S. and Canadian rights to our then-marketed Loestrin products to a unit of Barr. Following this sale, we continued to earn revenue from supplying Loestrin under a supply agreement. Our total revenue for fiscal years 2004 and 2003, excluding revenue attributable to Loestrin product sales, were $464.0 million and $326.6 million, respectively. |
| (3) | Completing the Acquisition affected our financial condition, results of operations and cash flows in the following ways: |
| | a. | In the quarter ended December 31, 2004 we incurred $51.0 million of transaction costs and $3.7 million of incremental selling, general and administrative costs directly related to the closing of the Acquisition; and |
| | b. | During the year ended December 31, 2005 we completed the Acquisition for total consideration of $3,152.1 million, which was funded by approximately $1,282.8 million of equity contributions, $1,420.0 million of senior secured debt (including $20.0 million borrowed under the revolving credit facility at the time of acquisition), $600.0 million of the notes and cash on hand. We recorded adjustments to the fair value of our assets and liabilities as of the date of the Acquisition. During 2005, the following items were included in our operating results: |
| | • | a charge of $22.4 million in cost of sales representing the write-off of the purchase price allocated to the fair value of our opening inventory, |
| | • | $7.8 million of incremental selling, general and administrative costs directly related to the closing of the Acquisition, |
| | • | $4.9 million in selling, general and administrative expense for the management fee to our Sponsors, |
| | • | increased amortization expense resulting from the write-up of our identified intangible assets, |
| | • | a $280.7 million write-off of acquired in-process research and development, |
| | • | $36.0 million of transaction costs, and |
| | • | increased interest expense from the indebtedness we incurred to complete the Acquisition. |
| (4) | In 2006, SG&A costs include $42.1 million of expenses directly related to our IPO. |
| (5) | In January 2005 (at the time of the Acquisition), intangible assets were re-valued at fair market value increasing the amounts to be amortized from that of the Predecessor. |
| (6) | Our wholly-owned subsidiary, Warner Chilcott Holdings Company II, Limited, issued 404,439 Preferred Shares in connection with the Transactions. The Preferred Shares are entitled to cumulative preferential dividends at an accretion rate of 8% per annum, compounded quarterly. All of the Preferred Shares were either converted to Class A common shares or redeemed for cash at the time of the IPO in September 2006. |
| (7) | Discontinued operations represented our Pharmaceutical services businesses, which were Interactive Clinical Technologies, Inc. (sold in August 2002), our Clinical Trial Services business (sold in May 2002) and our Chemical Synthesis Services business (sold in December 2001). In addition, in December 2003, we sold our Pharmaceutical Development and Manufacturing Services business, in April 2004 we sold our U.K. Pharmaceutical Sales and Marketing business and in May 2004 we sold our U.K.-based sterile solutions business. We received $343.0 million of proceeds net of costs for the sale of these businesses. The high level of discontinued operations in 2002 is due to the gain on disposal of our above-mentioned Pharmaceutical services business of $101.1 million, net of taxes of $3.9 million. No business was divested in fiscal year 2003. The discontinued operations for fiscal year 2004 included a gain on disposal of $5.4 million, net of a tax charge of $11.8 million on the sale of our Pharmaceutical Development and Manufacturing Services business, our U.K. Pharmaceutical Sales and Marketing business and our U.K.-based sterile solutions business. |
| (8) | The Company purchased all outstanding shares of the Predecessor as part of the Acquisition, making the Predecessor’s earnings per share not comparable to the Successor’s earnings per share. The Successor was in a net loss position in both 2005 and 2006. The effect from the exercise of outstanding stock options and the vesting of restricted shares during the periods would have been anti-dilutive. Accordingly, the shares issuable upon exercise of such stock options and the restricted shares have not been included in the calculation of diluted earnings per share. The December 31, 2006 earnings per share of the Class L common shares is calculated through September 30, 2006 as there were no Class L common shares outstanding during the fourth quarter of 2006 or the first quarter of 2007. |
| (9) | During the year ended December, 2006 we completed the acquisition of the U.S. sales and marketing rights to Dovonex for $205.2 million and paid the final milestone payment for Taclonex of $40.0 million. We borrowed $240.0 million in connection with these transactions and recorded $238.5 million in intangible assets. |
| (10) | In 2002, we repurchased 2 million ordinary shares (0.5 million ADS equivalents). In 2004, we repurchased 2.8 million ordinary shares (0.7 million ADS equivalents). |
| (11) | Reflects the issuance of $350.0 million of long-term debt to finance a portion of the acquisition of products for approximately $650.0 million in 2003. |
| (12) | For purposes of these computations, earnings consist of income from continuing operations before income taxes plus fixed charges, plus the amortization of capitalized interest, less interest capitalized. Fixed charges include interest on indebtedness from both continuing and discontinued operations, amortization of debt issuance costs, capitalized interest and the portion of lease rental expense representative of interest. In the opinion of management, we estimate the interest component of lease rental expense to be one third of lease rental expense. For the quarter ended December 31, 2004 and the year ended December 31, 2005, our earnings were insufficient by $17.5 million and $569.8 million, respectively, to cover our fixed charges for such periods. |
14
RECENT DEVELOPMENTS
Legal Proceedings
Certain legal proceedings in which we are involved are discussed in Note 11 to the Condensed Consolidated Financial Statements included in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007. The following discussion is limited to recent developments concerning our legal proceedings with respect to the Federal Trade Commission lawsuits regarding the exercise of an option for a five-year exclusive license to Barr’s abbreviated new drug application (“ANDA”) referencing Ovcon 35. This section should be read in conjunction with our Quarterly Report and unless otherwise indicated, all proceedings discussed in our Quarterly Report remain outstanding.
Federal Trade Commission Lawsuits Regarding Exercise of Option for a Five-Year Exclusive License to ANDA Referencing Ovcon 35
In connection with the eight direct purchaser lawsuits filed against us and Barr in the U.S. District Court for the District of Columbia, on March 12, 2007, the direct purchaser class action plaintiffs filed a motion for class certification. Defendants filed a response in opposition on April 12, 2007. The direct purchaser class action plaintiffs filed a reply on May 21, 2007. The motion is fully briefed and is pending before the U.S. District Court.
With respect to the actions brought by the third-party payor plaintiffs and the consumer plaintiffs, we agreed to settlements in principle on April 9, 2007. We signed a settlement agreement with the third-party payor plaintiffs on May 15, 2007, and with the consumer plaintiffs on May 17, 2007. On April 16, 2007, we agreed to a settlement in principle with the state plaintiffs. Counsel for the consumer plaintiffs filed a motion for preliminary approval of the settlement on June 6, 2007, and counsel for the third-party payor plaintiffs filed a motion for preliminary approval of the settlement on June 12, 2007. We filed a joint motion with the state plaintiffs on June 12, 2007, asking the Court to approve our proposed final order. All three settlements remain subject to necessary approvals by the parties and the U.S. District Court.
15
RISK FACTORS
You should carefully consider the risk factors set forth below as well as the other information contained in this prospectus before making an investment decision. Additional risks and uncertainties not currently known to us or those we currently deem to be immaterial may also materially and adversely affect our business operations. Any of the following risks could materially adversely affect our business, financial condition, results of operations or cash flows. In such cases, you may lose all or part of your original investment in our common stock.
Risks Related to the Notes
We have a substantial amount of indebtedness, which may adversely affect our cash flow and our ability to operate our business, remain in compliance with debt covenants and make payments on our indebtedness, including the notes.
We have a significant amount of indebtedness. As of March 31, 2007, we had total indebtedness of $1,487.8 million.
Our substantial level of indebtedness increases the possibility that we may be unable to generate cash sufficient to pay, when due, the principal of, interest on or other amounts due in respect of our indebtedness. Our substantial indebtedness, combined with our lease and other financial obligations and contractual commitments could have other important consequences to you as a holder of the notes. For example, it could:
| | Ÿ | | make it more difficult for us to satisfy our obligations with respect to our indebtedness, including the notes, and any failure to comply with the obligations of any of our debt instruments, including financial and other restrictive covenants, could result in an event of default under the indenture governing the notes and the agreements governing such other indebtedness; |
| | Ÿ | | make us more vulnerable to adverse changes in general economic, industry and competitive conditions and adverse changes in government regulation; |
| | Ÿ | | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flows to fund working capital, capital expenditures, acquisitions and other general corporate purposes; |
| | Ÿ | | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| | Ÿ | | place us at a competitive disadvantage compared to our competitors that have less debt; and |
| | Ÿ | | limit our ability to borrow additional amounts for working capital, capital expenditures, acquisitions, debt service requirements, execution of our business strategy or other purposes. |
Any of the above listed factors could materially adversely affect our business, financial condition and results of operations. Furthermore, our interest expense could increase if interest rates increase because debt under our senior secured credit facility bears interest at our option at either adjusted LIBOR plus an applicable margin or the alternate base rate plus an applicable margin. If we do not have sufficient earnings to service our debt, we may be required to refinance all or part of our existing debt, sell assets, borrow more money or sell securities, none of which we can guarantee we will be able to do.
In addition, the senior secured credit facility and the indenture governing the notes contain financial and other restrictive covenants that limit our ability to engage in activities that may be in our long-term best interests. Our failure to comply with those covenants could result in an event of default which, if not cured or waived, could result in the acceleration of all our debt.
16
Despite current indebtedness levels, we and our subsidiaries may still be able to incur substantially more debt. This could further exacerbate the risks associated with our substantial leverage.
We and our subsidiaries may be able to incur substantial additional indebtedness in the future. Although the indenture governing the notes and the senior secured credit facility contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of significant qualifications and exceptions, and the indebtedness incurred in compliance with these restrictions could be substantial. Our senior secured credit facility and the indenture governing the notes permit the incurrence of additional borrowings of up to $150.0 million under our revolving credit facility, subject, in the case of our senior secured credit facility, to compliance with the covenants and conditions to borrowings under our senior secured credit facility. All of those borrowings would rank senior to the notes and the guarantees thereof. If new debt is added to our and our subsidiaries’ current debt levels, the related risks that we and they face would be increased. In addition, the indenture does not prevent us from incurring obligations that do not constitute indebtedness. Any additional borrowings could be senior to the notes and the related guarantees. If we incur additional debt above the levels that were in effect at the time of the closing of the Transactions, the risks associated with our substantial leverage will increase.
To service our indebtedness, we will require a significant amount of cash. Our ability to generate cash depends on many factors beyond our control, and any failure to meet our debt service obligations could harm our business, financial condition and results of operations.
Our ability to pay interest on and principal of the notes and to satisfy our other debt obligations principally will depend upon our future operating performance. As a result, prevailing economic conditions and financial, business and other factors, many of which are beyond our control, will affect our ability to make these payments.
If we do not generate sufficient cash flow from operations to satisfy our debt service obligations, including payments on the notes, we may have to undertake alternative financing plans, such as refinancing or restructuring our indebtedness, selling assets, reducing or delaying capital investments or seeking to raise additional capital. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. In addition, the terms of existing or future debt instruments, including the senior secured credit facility, and the indenture governing the notes offered hereby, may restrict us from adopting some of these alternatives. Our inability to generate sufficient cash flow to satisfy our debt service obligations, or to refinance our obligations at all or on commercially reasonable terms, would have an adverse effect, which could be material, on our business, financial condition and results of operations, as well as on our ability to satisfy our obligations in respect of the notes.
Repayment of our debt, including the notes, is dependent on cash flow generated by our subsidiaries.
All of Warner Chilcott Limited, Holdings, Luxco and Warner Chilcott Corporation are holding companies, and since immediately following the Transactions, all of their operating assets have been owned by their subsidiaries. Repayment of our indebtedness, including the notes, is dependent on the generation of cash flow by our subsidiaries and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Unless they are guarantors of the notes, our subsidiaries do not have any obligation to pay amounts due on the notes or to make funds available for that purpose. Our subsidiaries may not be able to, or be permitted to, make distributions to enable us to make
17
payments in respect of our indebtedness, including the notes. Each of our subsidiaries is a distinct legal entity and, under certain circumstances, legal and contractual restrictions may limit our ability to obtain cash from our subsidiaries. While the indenture governing the notes limits the ability of our subsidiaries to incur consensual restrictions on their ability to pay dividends or make other intercompany payments to us, these limitations are subject to certain qualifications and exceptions. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness, including the notes.
Since each of Warner Chilcott Limited, Holdings and Luxco is a holding company with no significant operations, the guarantees by Warner Chilcott Limited, Holdings and Luxco provide little, if any, additional credit support for the notes and investors should not rely on these guarantees in evaluating an investment in the notes.
Only certain of the subsidiaries of Holdings guarantee the notes, and the assets of the non-guarantor subsidiaries may not be available to make payments on the notes.
Certain foreign subsidiaries of Holdings, other than Luxco and Warner Chilcott Company, Inc., do not guarantee the notes. As of March 31, 2007, the non-guarantor subsidiaries accounted for approximately $313.0 million, or 10.0%, of our total assets and a nominal amount of revenues (after intercompany eliminations). Certain of these non-guarantor subsidiaries are guarantors of the borrowers’ obligations under our senior secured credit facility. In addition, the indenture permits these subsidiaries to incur additional amounts of indebtedness in the future. In the event that any non-guarantor subsidiary becomes insolvent, liquidates, reorganizes, dissolves or otherwise winds up, holders of its indebtedness and its trade creditors generally will be entitled to payment on their claims from the assets of that subsidiary before any of those assets are made available to us. Consequently, your claims in respect of the notes will be effectively subordinated to all of the liabilities of the non-guarantor subsidiaries, including their obligations under or in respect of our senior secured credit facility, trade payables, and the claims, if any, of third party holders of preferred equity interests in the non-guarantor subsidiaries.
The terms of our senior secured credit facility and the indenture governing the notes restrict our current and future operations, particularly our ability to respond to changes in our business or to take certain actions.
Our senior secured credit facility and the indenture governing the notes contain, and any future indebtedness of ours would likely contain, a number of restrictive covenants that impose significant operating and financial restrictions on Warner Chilcott Corporation, Holdings and the other restricted subsidiaries of Holdings, including restrictions on our ability to engage in acts that may be in our best long-term interests. Our senior secured credit facility includes financial covenants, including requirements that we:
| | Ÿ | | maintain minimum interest coverage ratios; and |
| | Ÿ | | not exceed maximum total leverage ratios. |
Our senior secured credit facility limits the ability of Warner Chilcott Corporation, Holdings and their restricted subsidiaries to make capital expenditures and requires that they use a portion of excess cash flow and proceeds of certain asset sales that are not reinvested in their business and other dispositions to repay indebtedness under the senior secured credit facility.
Our senior secured credit facility also includes covenants restricting, among other things, the ability of Warner Chilcott Corporation, Holdings and its restricted subsidiaries to:
| | Ÿ | | incur or assume additional debt or guarantees or issue preferred stock; |
18
| | Ÿ | | pay dividends, or make redemptions and repurchases, with respect to capital stock; |
| | Ÿ | | prepay, or make redemptions and repurchases of, subordinated debt; |
| | Ÿ | | make loans and investments; |
| | Ÿ | | make capital expenditures; |
| | Ÿ | | engage in mergers, acquisitions, asset sales, sale/leaseback transactions and transactions with affiliates; |
| | Ÿ | | change the business conducted by us or our subsidiaries; and |
| | Ÿ | | amend the terms of subordinated debt. |
The indenture relating to the notes also contains numerous covenants including, among other things, restrictions on Warner Chilcott Corporation’s, Holdings’ and the restricted subsidiaries of Holdings’ ability to:
| | Ÿ | | incur or guarantee additional indebtedness or issue disqualified or preferred stock; |
| | Ÿ | | pay dividends or make other equity distributions; |
| | Ÿ | | repurchase or redeem capital stock; |
| | Ÿ | | make investments or other restricted payments; |
| | Ÿ | | sell assets or consolidate or merge with or into other companies; |
| | Ÿ | | create limitations on the ability of our restricted subsidiaries to make dividends or distributions to us; and |
| | Ÿ | | engage in transactions with affiliates. |
The operating and financial restrictions and covenants in these debt agreements and any future financing agreements may adversely affect our ability to finance future operations or capital needs or to engage in other business activities. A breach of any of the restrictive covenants in the senior secured credit facility would result in a default under the senior secured credit facility. If any such default occurs, the lenders under the senior secured credit facility may elect to declare all outstanding borrowings, together with accrued interest and other fees, to be immediately due and payable, or enforce their security interest, any of which would result in an event of default under the notes. The lenders will also have the right in these circumstances to terminate any commitments they have to provide further borrowings.
Your right to receive payment on the notes and the guarantees are junior to the rights of the lenders under our senior secured credit facility and to all of our and the guarantors’ other senior indebtedness, including any of our or the guarantors’ future senior debt.
The notes and the guarantees rank in right of payment behind all of our and the guarantors’ existing senior indebtedness, including borrowings under our senior secured credit facility, and rank in right of payment behind all of our and the guarantors’ future borrowings, except any future indebtedness that expressly provides that it ranks equally or is junior in right of payment to the notes and the guarantees. See “Description of the Notes—Ranking.” As of March 31, 2007 the notes and the guarantees were contractually subordinated to approximately $1,097.8 million of senior debt. In addition, our senior secured credit facility and the indenture governing the notes permit us to incur up to $150.0 million in additional borrowings under our revolving credit facility (of which $0 was outstanding as of December 31, 2006 and March 31, 2007), subject, in the case of our senior secured credit facility, to compliance with the covenants and conditions to borrowings under the senior secured
19
credit facility, which borrowings would be senior to the notes and the guarantees. We are also permitted to incur substantial additional indebtedness, including senior indebtedness, in the future.
We and the guarantors may not pay principal, premium, if any, interest or other amounts on account of the notes or the guarantees in the event of a payment default or certain other defaults in respect of certain of our senior indebtedness, including debt under the senior secured credit facility, unless the senior indebtedness has been paid in full or the default has been cured or waived. In addition, in the event of certain other defaults with respect to the senior indebtedness, we or the guarantors may not be permitted to pay any amount on account of the notes or the guarantees for a designated period of time. See “Description of the Notes—Ranking—Payment of Notes.” Because of the subordination provisions in the notes and the guarantees, in the event of a bankruptcy, liquidation, reorganization or similar proceeding relating to us or a guarantor, our or the guarantor’s assets will not be available to pay obligations under the notes or the applicable guarantee until we have, or the guarantor has, made all payments in cash on its senior indebtedness. Sufficient assets may not remain after all these payments have been made to make any payments on the notes or the applicable guarantee, including payments of principal or interest when due.
Pursuant to the subordination provisions of the indenture governing the notes, all payments on the notes and the guarantees will be blocked in the event of a payment default on designated senior debt and may be blocked for up to 179 consecutive days in the event of certain non-payment defaults on designated senior debt.
In addition, in the event of a bankruptcy, liquidation or reorganization or similar proceeding relating to us or the guarantors, holders of the notes will participate with trade creditors and all other holders of our and the guarantors’ senior subordinated indebtedness, as the case may be, in the assets, if any, remaining after we and the guarantors have paid all of the senior indebtedness. However, because the indenture requires that amounts otherwise payable to holders of the notes in a bankruptcy or similar proceeding be paid to holders of senior indebtedness instead, holders of the notes may receive less, ratably, than holders of trade payables or other unsecured, unsubordinated creditors in any such proceeding. In any of these cases, we and the guarantors may not have sufficient funds to pay all of our creditors, and holders of the notes may receive less, ratably, than the holders of senior indebtedness. See “Description of the Notes—Ranking.”
The notes are not secured by our assets and the lenders under our senior secured credit facility are entitled to remedies available to a secured lender, which gives them priority over you to collect amounts due to them.
In addition to being subordinated to all our and the guarantors’ existing and future senior debt, the notes and the guarantees are not secured by any of our or their assets. Loans under our senior secured credit facility are secured by a first priority security interest in substantially all of our, Holdings’, Warner Chilcott Company, Inc.’s and the subsidiary guarantors’ assets and in all of the capital stock held by us, Holdings, Warner Chilcott Company, Inc. and the subsidiary guarantors (but, other than in the case of Warner Chilcott Company, Inc., limited to 65% of the voting stock in the case of direct foreign subsidiaries and 0% in the case of indirectly held foreign subsidiaries). Loans under our senior secured credit facility are also secured by a non-recourse pledge of the capital stock of Holdings by its direct parent company, Warner Chilcott Holdings Company II, Limited. In addition, loans made to Holdings and Warner Chilcott Company, Inc. under the senior secured credit facility are also secured by a first priority security interest in substantially all the assets of our material foreign subsidiaries who guarantee loans under our senior secured credit facility. As of March 31, 2007, we had $1,097.8 million of senior secured indebtedness. If we become insolvent or are liquidated, or if payment under the senior secured credit facility or in respect of any other secured indebtedness is accelerated, the lenders under our senior secured credit facility or holders of other secured indebtedness will be entitled
20
to exercise the remedies available to a secured lender under applicable law (in addition to any remedies that may be available under documents pertaining to our senior secured credit facility or other senior debt). Upon the occurrence of any default under our senior secured credit facility (and even without accelerating the indebtedness under our senior secured credit facility), the lenders may be able to prohibit the payment of the notes and the guarantees either by limiting our ability to access our cash flow or under the subordination provisions contained in the indenture governing the notes.
We may not be able to repurchase the notes upon a change of control.
Upon the occurrence of certain change of control events, we will be required to offer to repurchase all notes that are outstanding at 101% of the principal amount thereof, plus any accrued and unpaid interest, and additional interest, if any. The senior secured credit facility provides that certain change of control events (including a Change of Control as defined in the indenture relating to the notes) constitute a default. Any future credit agreement or other agreements relating to senior indebtedness to which we become a party would likely contain similar provisions. If we experience a change of control that triggers a default under our senior secured credit facility, we could seek a waiver of such default or seek to refinance our senior secured credit facility. In the event we do not obtain such a waiver or refinance the senior secured credit facility, such default could result in amounts outstanding under our senior secured credit facility being declared due and payable. In the event we experience a change of control that results in our having to repurchase your notes, we may not have sufficient financial resources to satisfy all of our obligations under our senior secured credit facility and/or the notes. A failure to make the applicable change of control offer or to pay the applicable change of control purchase price when due would result in a default under the indenture.
In addition, the change of control covenant in the indenture governing the notes does not cover all corporate reorganizations, mergers or similar transactions and may not provide you with protection in a highly leveraged transaction. Furthermore, the definition of “Permitted Holders” under the indenture governing the notes includes a “group” in which the Sponsors, their affiliates and officers of our company collectively have beneficial ownership, directly or indirectly, of more than 50% of the total voting power of the voting stock of Holdings or any of its direct or indirect parent entities owned by such group. As a result, the Sponsors may be able to transfer a significant portion of their holdings in our company without triggering a change of control under the indenture. See “Description of the Notes—Certain Covenants,” “Description of the Notes—Repurchase at the Option of Holders—Change of Control” and “Description of the Notes—Certain Definitions—Permitted Holders.” The purchase offer requirements could also delay or make it more difficult for others to effect a change of control.
Federal and state statutes allow courts, under specific circumstances, to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees and require note holders to return payments received from us or the guarantors.
A portion of the proceeds from the sale of the notes was applied, together with the equity investment made by the Sponsors, certain of their limited partners and certain members of our management and borrowings under our senior secured credit facility, to make payments to holders of capital stock and options of Warner Chilcott PLC in connection with the Transactions. Luxco, Warner Chilcott Limited, Holdings, Holdings’ U.S. subsidiaries and Warner Chilcott Company, Inc. have guaranteed our obligations under the notes. Our issuance of the notes and the issuance of the guarantees by the guarantors may be subject to review under state and federal laws if a bankruptcy, liquidation or reorganization case or a lawsuit, including in circumstances in which bankruptcy is not involved, were commenced at some future date by, or on behalf of, our unpaid creditors or the unpaid creditors of a guarantor. Under the federal bankruptcy laws and comparable provisions of state fraudulent transfer laws, a court may void or otherwise decline to enforce the notes or a guarantor’s guaranty, or subordinate the notes or such guaranty to our or the applicable guarantor’s existing and future indebtedness. While the relevant laws may vary from state to state, a court might do so if it found that when we issued the notes or the applicable guarantor entered into its guaranty or, in some
21
states, when payments became due under the notes or such guaranty, we or the applicable guarantor received less than reasonably equivalent value or fair consideration and either:
| | Ÿ | | we or the applicable guarantor were or was insolvent, or rendered insolvent, by reason of such incurrence; |
| | Ÿ | | we or the applicable guarantor were or was engaged in a business or transaction for which our or the applicable guarantor’s remaining assets constituted unreasonably small capital; or |
| | Ÿ | | we or the applicable guarantor intended to incur, or believed that we or the applicable guarantor would incur, debts beyond our or such guarantor’s ability to pay such debts as they mature. |
The court might also void the notes or a guaranty, without regard to the above factors, if the court found that we issued the notes or the applicable guarantor entered into its guaranty with actual intent to hinder, delay or defraud our or its creditors. In addition, any payment by us under the notes or by a guarantor pursuant to its guarantee could be voided and required to be returned to us, such guarantor or to a fund for the benefit of our or such guarantor’s creditors. In the event of a finding that a fraudulent conveyance occurred, you may not receive any payment on the notes.
A court would likely find that we or a guarantor did not receive reasonably equivalent value or fair consideration for the notes or such guaranty if we or such guarantor did not substantially benefit directly or indirectly from the issuance of the notes or the applicable guarantee. Our use of proceeds from the Transactions, which included the distribution of a substantial portion of the proceeds from the offering of the notes to Warner Chilcott PLC’s shareholders, could increase the risk of such a finding. If a court were to void the notes or a guaranty, you would no longer have a claim against us or the applicable guarantor, as the case may be. Sufficient funds to repay the notes may not be available from other sources, including the remaining guarantors, if any. In addition, the court might direct you to repay any amounts that you already received from us or any guarantor, as the case may be.
The measures of insolvency for purposes of these fraudulent transfer laws vary depending upon the law applied in any proceeding to determine whether a fraudulent transfer has occurred. Generally, however, an entity would be considered insolvent if:
| | Ÿ | | the sum of its debts, including contingent liabilities, was greater than the fair saleable value of its assets; or |
| | Ÿ | | if the present fair saleable value of its assets was less than the amount that would be required to pay the probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or |
| | Ÿ | | it cannot pay its debts as they become due. |
To the extent a court voids the notes or, any of the guarantees as fraudulent transfers or holds the notes or any of the guarantees unenforceable for any other reason, holders of notes would cease to have any direct claim against us or the applicable guarantor. If a court were to take this action, our or the applicable guarantor’s assets would be applied first to satisfy our or the applicable guarantor’s liabilities, if any, before any portion of its assets could be applied to the payment of the notes.
Each guaranty (other than a guaranty by a company that is a direct or indirect parent of the issuer of the notes) will contain a provision intended to limit that guarantor’s liability to the maximum amount that it could incur without causing the incurrence of obligations under its guaranty to be a fraudulent transfer. This provision may not be effective to protect those guarantees from being voided under fraudulent transfer law, or may reduce that guarantor’s obligation to an amount that effectively makes its guaranty worthless.
22
Risks Relating to Our Business
If generic products that compete with any of our branded pharmaceutical products are approved, sales of our products may be adversely affected.
Our branded pharmaceutical products are or may become subject to competition from generic equivalents because there is no proprietary protection for some of the branded pharmaceutical products we sell or because our patent protection expires or is not sufficiently broad. Ovcon 50, Ovcon 35, Estrace Tablets and Estrace Cream are currently not protected by patents. Generic equivalents are currently available for Ovcon 35 and Estrace Tablets.
Patents covering the following products will expire within the next five years:
| | |
Product
| | Patent Expires
|
Dovonex Ointment | | December 2007 |
Estrostep | | April 2008 |
Sarafem | | May 2008 |
femhrt | | May 2010 |
Although part of our strategy includes the ongoing development of proprietary product improvements to our existing products and new and enhanced dosage forms, other companies may attempt to compete with our original products losing patent protection, we may not be successful in obtaining FDA approval of our new and enhanced dosage products and doctors may not prescribe these products.
Generic equivalents for some of our branded pharmaceutical products are sold by other pharmaceutical companies at a lower cost. After the introduction of a generic competitor, a significant percentage of the prescriptions written for the branded product may be filled with the generic version at the pharmacy, resulting in a commensurate loss in sales of the branded product. In addition, legislation enacted in the United States allows or, in a few instances, in the absence of specific instructions from the prescribing physician mandates, the use of generic products rather than branded products where a generic equivalent is available. Competition from generic equivalents could have a material adverse impact on our revenues, financial condition, results of operations and cash flows.
On September 25, 2006, we terminated the exclusivity provision of our license agreement with Barr relating to Ovcon 35. On October 26, 2006, Barr launched a generic version of Ovcon 35. Sales of Ovcon 35 have predictably eroded as a result of the launch of the generic.
Potential generic competitors may also challenge our patents. For example, Watson Pharmaceuticals, Inc. submitted an ANDA in April, 2006 seeking approval to market a generic version of Loestrin 24 Fe prior to the expiration of our patent. We have filed an infringement lawsuit against Watson in response to this submission. In addition, under an agreement to settle patent claims against Barr relating to our Estrostep oral contraceptive and our femhrt HT product, we granted Barr a non-exclusive license to launch generic versions of Estrostep and femhrt six months prior to expiration of our patents in 2008 and 2010, respectively. We cannot predict what effect, if any, such matters will have on our financial condition, results of operations and cash flows.
Our trademarks, patents and other intellectual property are valuable assets and if we are unable to protect them from infringement or challenges, our business prospects may be harmed.
Due to our focus on branded products, we consider our trademarks to be valuable assets. Therefore, we actively manage our trademark portfolio, maintain long-standing trademarks and obtain trademark registrations for new brands. We also police our trademark portfolio against infringement.
23
Our efforts to defend our trademarks may be unsuccessful against competitors or other violating entities and we may not have adequate remedies for any breach because, for example, a violating company may be insolvent.
We also rely on patents, trade secrets and proprietary knowledge to protect our products. We take steps to protect our proprietary rights by filing applications for patents on certain inventions, by entering into confidentiality, non-disclosure and assignment of invention agreements with our employees, consultants, licensees and other companies and enforcing our legal rights against third parties that we believe may infringe our intellectual property rights. We do not ultimately control whether we will be successful in enforcing our legal rights against third party infringers, whether our patent applications will result in issued patents, whether our patents will be subjected to inter parte reexamination by the United States Patent and Trademark Office, whether our confidentiality, non-disclosure and assignment of invention agreements will not be breached and whether we will have adequate remedies for any such breach, or that our trade secrets will not otherwise become known by competitors.
There has been substantial litigation in the pharmaceutical industry with respect to the manufacture, use and sale of new products that are the subject of conflicting patent rights, and in the past, as noted above, we have been involved in this type of litigation. These lawsuits relate to the validity and infringement of patents. The expense of bringing lawsuits against infringers or defending lawsuits brought against us could cause us not to continue these suits and abandon the affected products. The ultimate outcome of this type of litigation, if brought, may not be favorable and could adversely impact our business, financial condition, results of operations and cash flows.
Delays in production could have a material adverse impact on our business.
Our principal pharmaceutical manufacturing facility located in Fajardo, Puerto Rico currently manufactures most of our women’s healthcare oral dose products, including Loestrin 24 Fe, Femcon Fe, Estrostep Fe and Ovcon 50 oral contraceptives and packages delayed-release Doryx tablets, Femhrt, Ovcon 35 and Dovonex and Taclonex samples. Because the manufacture of pharmaceutical products requires precise and reliable controls, and due to significant compliance obligations imposed by laws and regulations, we may face delays in qualifying the Fajardo facility for the manufacture of new products or for our other products that are currently manufactured for us by third parties.
In addition, certain of our pharmaceutical products are currently manufactured for us under contracts with third parties. Our contract manufacturers may not be able to manufacture our products without interruption, may not comply with their obligations under our various supply arrangements, and we may not have adequate remedies for any breach. From time to time our contract manufacturers have been unable to meet all of our orders, which has led to the depletion of our safety stock and temporary shortages of trade supply and promotional samples.
Failure by our own manufacturing facility or any third party manufacturer (each a “Product Supplier”) to comply with regulatory requirements could adversely affect their ability to supply products to us. All facilities and manufacturing techniques used for the manufacture of pharmaceutical products must be operated in conformity with current Good Manufacturing Practices (“cGMPs”). In complying with cGMP requirements, Product Suppliers must continually expend time, money and effort in production, record-keeping and quality assurance and control to ensure that the product meets applicable specifications and other requirements for product safety, efficacy and quality. Manufacturing facilities are subject to periodic unannounced inspections by the FDA and other regulatory authorities. Failure to comply with applicable legal requirements including, in the case of our manufacturing facility located in Fajardo, certain obligations that we assumed in our purchase of the facility arising out of a consent decree entered into by the previous owner subjects the Product Suppliers to possible legal or
24
regulatory action, including shutdown, which may adversely affect their ability to supply us with product. In addition, adverse experiences with the use of products must be reported to the FDA and could result in the imposition of market restrictions through labeling changes or product removal.
The FDA must approve suppliers of certain active and inactive pharmaceutical ingredients and certain packaging materials used in our products as well as suppliers of finished products. The development and regulatory approval of our products are dependent upon our ability to procure these ingredients, packaging materials and finished products from FDA-approved sources. FDA approval of a new supplier would be required if, for example, active ingredients, packaging materials or finished products were no longer available from the initially approved supplier or if that supplier had its approval from the FDA withdrawn. The qualification of a new Product Supplier could potentially delay the manufacture of the drug involved. Furthermore, we may not be able to obtain active ingredients, packaging materials or finished products from a new supplier on terms that are as favorable to us as those agreed with the initially approved supplier or at reasonable prices.
A delay in supplying, or failure to supply, products by any Product Supplier could result in our inability to meet the demand for our products and adversely affect our revenues, financial condition, results of operations and cash flows.
Pricing pressures from third-party payors, including managed care organizations, government sponsored health systems and regulations relating to Medicare and Medicaid, healthcare reform, pharmaceutical reimbursement and pricing in general could decrease our revenues.
Our commercial success in producing, marketing and selling products depends, in part, on the availability of adequate reimbursement from third-party healthcare payors, such as managed care organizations, and government bodies and agencies for the cost of the products and related treatment. The market for our products may be limited by actions of third-party payors.
Managed care organizations and other third-party payors try to negotiate the pricing of medical services and products to control their costs, including by developing formularies to encourage plan beneficiaries to utilize preferred products for which the plans have negotiated favorable terms. Exclusion of a product from a formulary, or placement of a product on a disfavored formulary tier, can lead to sharply reduced usage in the managed care organization patient population. If our products are not included within an adequate number of formularies or if adequate reimbursement levels are not provided, or if reimbursement policies increasingly favor generic products, our market share and business could be negatively affected.
Recent reforms in Medicare added an out-patient prescription drug reimbursement beginning in 2006 for all Medicare beneficiaries. The federal government and private plans contracting with the government to deliver the benefit, through their purchasing power under these programs, are demanding discounts from pharmaceutical companies that may implicitly create price controls on prescription drugs. These reforms may decrease our future revenues from products such as Dovonex and Taclonex that are covered by the Medicare drug benefit. Further, a number of other legislative and regulatory proposals aimed at changing the healthcare system have been proposed. While we cannot predict whether any such proposals will be adopted or the effect such proposals may have on our business, the existence of such proposals, as well as the adoption of any proposal, may increase industry-wide pricing pressures, thereby adversely affecting our results of operations and cash flows.
Changes in laws and regulations could affect our results of operations, financial position or cash flows.
Our future operating results, financial position or cash flows could be adversely affected by changes in laws and regulations such as (i) changes in the FDA approval processes that may cause
25
delays in, or prevent the approval of, new products, (ii) new laws, regulations and judicial decisions affecting product marketing, promotion or the healthcare field generally, (iii) new laws or judicial decisions affecting intellectual property rights and (iv) changes in the application of tax principles, including tax rates, new tax laws, or revised interpretations of existing tax laws and precedents, which result in a shift of taxable earnings between tax jurisdictions.
Taxing authorities could reallocate our taxable income among our subsidiaries, which could increase our consolidated tax liability.
We conduct operations world-wide through subsidiaries in various tax jurisdictions. Certain aspects of the transactions between our subsidiaries, including our transfer pricing (which is the pricing we use in the transfer of products and services among our subsidiaries) and our intercompany financing arrangements could be challenged by applicable taxing authorities. While we believe both our transfer pricing and our intercompany financing arrangements are reasonable, either or both could be challenged by the applicable taxing authorities and, following such challenge, our taxable income could be reallocated among our subsidiaries. Such reallocation could both increase our consolidated tax liability and adversely affect our financial condition, results of operations and cash flows.
Changes in market conditions, including lower than expected cash flows or revenues for our branded pharmaceutical products, may result in our inability to realize the value of these products, in which case we may have to record an impairment charge.
The pharmaceutical industry is characterized by rapid product development and technological change, and as a result, our pharmaceutical products could be rendered obsolete or their value may be significantly decreased by the development of new technology or new pharmaceutical products to treat the conditions currently addressed by our products, technological advances that reduce the cost of production or marketing or pricing actions by one or more of our competitors. Some of the companies we compete against have significantly greater resources than we do, and therefore may be able to adapt more quickly to new or emerging technologies and changes in customer requirements, or devote greater resources to the promotion and sale of their products than we can. Our inability to compete successfully with respect to these or other factors may materially and adversely affect our cash flows or revenues, may result in our inability to realize the value of our branded pharmaceutical products, including products acquired from third parties, and may require us to record an impairment charge.
The loss of the services of members of our senior management team or scientific staff or the inability to attract and retain other highly qualified employees could impede our ability to meet our strategic objectives and adversely affect our business.
Our success is dependent on attracting and retaining highly qualified scientific, sales and management staff, including our Chief Executive Officer, Roger Boissonneault. We face intense competition for personnel from other companies, academic institutions, government entities and other organizations. The loss of key personnel, or our failure to attract and retain other highly qualified employees, may impede our ability to meet our strategic objectives.
Pursuant to our business strategy, we intend to develop proprietary product improvements as well as new products. This strategy may require us to hire additional employees with expertise in areas that relate to product development. We cannot fully anticipate or predict the time and extent to which we will need to hire this type of specialized personnel. As a result, we may not be successful in attracting and retaining the personnel necessary to pursue our business strategy fully. In addition, if competition continues to intensify, then our cost of attracting and retaining employees may escalate.
26
Product liability claims and product recalls could harm our business.
The development, manufacture, testing, marketing and sale of pharmaceutical products entail significant risk of product liability claims or recalls. Our products are, in the substantial majority of cases, designed to affect important bodily functions and processes. Unforeseen side-effects caused by, or manufacturing defects inherent in, the products sold by us could result in exacerbation of a patient’s condition, further deterioration of the patient’s condition or even death. The occurrence of such an event could result in product liability claims and/or recall of one or more of our products. Claims may be brought by individuals seeking relief for themselves or, in certain jurisdictions, by groups seeking to represent a class. For example, approximately 556 product liability suits have been filed against us in connection with the HT products, femhrt and Estrace. The lawsuits were likely triggered by the July 2002 and March 2004 announcement by the National Institutes of Health (“NIH”) of the early terminations of two large-scale randomized controlled clinical trials, which were part of the Women’s Health Initiative (“WHI”), examining the long-term effect of HT on the prevention of coronary heart disease and osteoporotic fractures, and any associated risk for breast cancer in postmenopausal women. In the case of the trial terminated in 2002, which examined combined estrogen and progestogen therapy (the “E&P Arm of the WHI Study”), the safety monitoring board determined that the risks exceeded the benefits, when comparing estrogen and progestogen therapy to a placebo. WHI investigators found that combined estrogen and progestogen therapy did not prevent heart disease in the study subjects and despite a decrease in the incidence of hip fracture and colorectal cancer, there was an increased risk of invasive breast cancer, coronary heart disease, stroke, blood clots and dementia. In the trial terminated in 2004, which examined estrogen therapy, the trial was ended one year early because the NIH did not believe that the results were likely to change in the time remaining in the trial and that the increased risk of stroke could not be justified for the additional data that could be collected in the remaining time. As in the E&P Arm of the WHI study, WHI investigators again found that estrogen only therapy did not prevent heart disease and although study subjects experienced fewer hip fractures and no increase in the incidence of breast cancer compared to subjects randomized to placebo, there was an increased incidence of stroke and blood clots in the legs. The estrogen used in the WHI Study was conjugated equine estrogen and the progestin was medroxyprogesterone acetate, the compounds found in Premarin® and Prempro™, products marketed by Wyeth. See Note 11 of the Notes to the Condensed Consolidated Financial Statements for the quarter ended March 31, 2007 incorporated by reference in this prospectus.
Product liability insurance coverage is expensive, can be difficult to obtain and may not be available in the future on acceptable terms, if at all. Partly as a result of product liability lawsuits related to pharmaceuticals, product liability and other types of insurance have become more difficult and costly to obtain. Our product liability insurance may not cover all the future liabilities we might incur in connection with the development, manufacture or sale of our products. In addition, we may not continue to be able to obtain insurance on satisfactory terms or in adequate amounts.
A successful claim or claims brought against us in excess of available insurance coverage could subject us to significant liabilities and have a material adverse effect on our business, financial condition, results of operations and cash flows. Such claims could also harm our reputation and the reputation of our products, thereby adversely affecting our ability to market our products successfully. In addition, irrespective of the outcome of product liability claims, defending a lawsuit with respect to such claims could be costly and significantly divert management’s attention from operating our business. Furthermore, we could be rendered insolvent if we do not have sufficient financial resources to satisfy any liability resulting from such a claim or to fund the legal defense of such a claim.
Product recalls may be issued at our discretion or at the discretion of certain of our suppliers, the FDA, other government agencies and other entities that have regulatory authority for pharmaceutical sales. From time to time, we have recalled some of our products; however, to date none of these
27
recalls have been significant. Any recall of a significant product could materially adversely affect our business by rendering us unable to sell that product for some time.
Sales of our products may be adversely affected by the consolidation among wholesale drug distributors and the growth of large retail drug store chains.
The network through which we sell our products has undergone significant consolidation marked by mergers and acquisitions among wholesale distributors and the growth of large retail drugstore chains. As a result, a small number of large wholesale distributors control a significant share of the market, and the number of independent drug stores and small drugstore chains has decreased. In the year ended December 31, 2006 and the three months ended March 31, 2007, three of these large distributors and a large retail drug store chain accounted for an aggregate of 87% and 89% of our net revenues, respectively. In addition, excess inventory levels held by large distributors may lead to periodic and unanticipated future reductions in revenues and cash flows. Consolidation of drug wholesalers and retailers, as well as any increased pricing pressure that those entities face from their customers, including the U.S. government, may increase pricing pressure and place other competitive pressures on drug manufacturers, including us.
If we fail to comply with government regulations we could be subject to fines, sanctions and penalties that could adversely affect our ability to operate our business.
We are subject to regulation by regional, national, state and local agencies, including the FDA, the Drug Enforcement Administration, the Department of Justice, the Federal Trade Commission (the “FTC”), the Office of Inspector General of the U.S. Department of Health and Human Services and other regulatory bodies, as well as governmental authorities in those foreign countries in which we manufacture or distribute some of our products. The Federal Food, Drug, and Cosmetic Act (“FDCA”), the Public Health Service Act and other federal and state statutes and regulations govern to varying degrees the research, development, manufacturing and commercial activities relating to prescription pharmaceutical products, including pre-clinical and clinical testing, approval, production, labeling, sale, distribution, import, export, post-market surveillance, advertising, dissemination of information and promotion.
Our sales, marketing, research and other scientific/educational programs must also comply with the anti-kickback and fraud and abuse provisions of the Social Security Act, the False Claims Act, the privacy provisions of the Health Insurance Portability and Accountability Act, and similar state laws. Pricing and rebate programs must comply with the Medicaid drug rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
Non-compliance with government regulations and other legal requirements can result in civil and criminal fines, the recall of products, the total or partial suspension of manufacture and/or distribution, seizure of products, injunctions, whistleblower lawsuits, failure to obtain approval of pending product applications, withdrawal of existing product approvals, exclusion from participation in government healthcare programs and other sanctions. Any threatened or actual government enforcement action can also generate adverse publicity and require that we devote substantial resources that could be used productively on other aspects of our business. Any of these enforcement actions could affect our ability to commercially distribute our products and could materially and adversely affect our business, financial condition, results of operations and cash flows.
28
New legal and regulatory requirements could make it more difficult for us to obtain new or expanded approvals for our products, and could limit or make more burdensome our ability to commercialize our approved products.
Numerous proposals have been made in recent months and years to impose new requirements on drug approvals, expand post-approval requirements, and restrict sales and promotional activities. For example, federal legislation has been proposed that would require all new drug applicants to submit risk evaluation and minimization plans to monitor and address potential safety issues for products upon approval, grant FDA the authority to impose risk management measures for marketed products and to mandate labeling changes in certain circumstances, and establish new requirements for disclosing the results of clinical trials. Additional measures have also been proposed to address perceived shortcomings in FDA’s handling of drug safety issues, and to limit pharmaceutical company sales and promotional practices that some see as excessive or improper. If these or other legal or regulatory changes are enacted, it may become more difficult or burdensome for us to obtain new or expanded approvals for our products, any approvals we receive may be more restrictive or come with onerous post-approval requirements, our ability to commercialize approved products successfully may be hindered, and our business may be harmed as a result.
Delays and uncertainties in clinical trials or the government approval process for new products could result in lost market opportunities and hamper our ability to recoup costs associated with product development.
FDA approval is generally required before a prescription drug can be marketed. For innovative, or non-generic, new drugs, an FDA-approved NDA is required before the drugs may be marketed in the United States. The NDA must contain data to demonstrate that the drug is safe and effective for its intended uses, and that it will be manufactured to appropriate quality standards. The clinical trials required to obtain regulatory approvals can be complex and expensive, and their outcomes are uncertain. Positive results from preclinical studies and early clinical trials do not ensure positive results in later clinical trials that form the basis of an application for regulatory approval. Even where clinical trials are completed successfully, FDA may determine that a product does not present an acceptable risk benefit profile, and may not approve an NDA or may only approve an NDA with significant restrictions or conditions. The drug development and approval process can be time-consuming and expensive without assurance that the data will be adequate to justify approval of proposed new products. If we are unable to obtain governmental approval for our NDAs, we will not be able to commercialize our products and recoup our research and development costs. Furthermore, even if we obtain regulatory approvals, the terms of any product approval, including labeling, may be more restrictive than desired and could affect the marketability of our products, and the approvals may be contingent upon burdensome post-approval study commitments. If we are unable to obtain timely product approvals on commercially viable terms, our profitability and business could suffer.
We may not be able to successfully identify, develop, acquire, license or market new products as part of growing our business.
In order to grow and achieve success in our business, we must continually identify, develop, acquire and license new products that we can ultimately market. Any future growth through new product acquisitions will be dependent upon the continued availability of suitable acquisition candidates at favorable prices and upon advantageous terms and conditions. Even if such opportunities are present, we may not be able to successfully identify products as candidates for potential acquisition, licensing, development or collaborative arrangements. Moreover, other companies, many of which may have substantially greater financial, marketing and sales resources, are competing with us for the right to acquire such products.
If an acquisition candidate is identified, the third parties with which we seek to cooperate may not select us as a potential partner or we may not be able to enter into arrangements on commercially
29
reasonable terms or at all. Furthermore, we do not know if we will be able to finance the acquisition or integrate an acquired product into our existing operations. The negotiation and completion of potential acquisitions could cause significant diversion of management’s time and resources and potential disruption of our ongoing business. Future product acquisitions would likely result in the incurrence of debt and contingent liabilities and an increase in interest expense and amortization expenses as well as significant charges relating to integration costs.
At each stage between developing or sourcing new products and marketing these products, there are a number of risks and uncertainties, and failure at any stage could have a material adverse effect on our ability to achieve commercial success with a product or to maintain or increase revenues, profits and cash flow. In addition, if we are unable to manage the challenges surrounding product development, acquisitions or the successful integration of acquisitions, it could have materially adverse effects on our business, financial condition, results of operations and cash flows.
Prescription drug importation from Canada and other countries could increase pricing pressure on certain of our products and could decrease our revenues and profit margins.
Under current U.S. law, U.S. individuals may import prescription drugs that are unavailable in the United States from Canada and other countries for their personal use under specified circumstances. Other imports, although illegal under U.S. law, also enter the country as a result of the resource constraints and enforcement priorities of the FDA and the U.S. Customs Service. In addition, in December 2003, the United States enacted the import provisions of the Medicare Prescription Drug, Improvement, and Modernization Act, which would permit pharmacists and wholesalers to import prescription drugs into the United States from Canada under specified circumstances. These additional import provisions will not take effect until the Secretary of Health and Human Services makes a required certification regarding the safety and cost savings of imported drugs and the FDA has promulgated regulations setting forth parameters for importation. These conditions have not been met to date, and the law has thus not taken effect. However, legislative proposals have been introduced to remove these conditions and implement the changes to the current import laws, or to create other changes that would allow foreign versions of our products priced at lower levels than in the United States to be imported or reimported to the United States from Canada, Europe and other countries. If these provisions take effect, the volume of prescription drug imports from Canada and elsewhere could increase significantly, and our products would face competition from lower priced imports.
Even if these provisions do not take effect and alter current law, the volume of prescription drug imports from Canada and elsewhere could increase due to a variety of factors, including the further spread of Internet pharmacies and actions by certain state and local governments to facilitate Canadian and other imports. These imports may harm our business.
We currently sell femhrt in Canada. In addition, Estrace Tablets, Dovonex and Taclonex (sold as “Dovobet” in Canada) are sold in Canada by third parties. For the year ended December 31, 2006 and the three months ended March 31, 2007, Dovonex, Taclonex, femhrt and Estrace Tablets accounted for 36% and 40% of our total revenues, respectively. Due to government price regulation in Canada, these products are generally sold in Canada for lower prices than in the United States. As a result, if these drugs are imported into the United States from Canada, we may experience reduced revenue or profit margins.
The perceived health effects of estrogen and combined estrogen-progestogen hormone therapy products may affect the acceptability and commercial success of our HT products.
Estrace Tablets, Estrace Cream, Femring and Femtrace are estrogen therapy products, and femhrt is a combined estrogen-progestogen therapy product. These HT products are used by women
30
to alleviate symptoms associated with menopause. Recent studies have analyzed the health effects of estrogen therapy and estrogen-progestogen therapy products and the American College of Obstetricians and Gynecologists has recommended that consumers use these products in the lowest possible dose for the shortest possible duration. We believe the publicity surrounding some of these studies resulted in a significant industry-wide decrease in the number of prescriptions being written for estrogen therapy and estrogen-progestogen therapy products, including femhrt. The ultimate outcome of these studies and any further changes in labeling for our products may further affect the acceptability of our products by patients, the willingness of physicians to prescribe our products for their patients or the duration of their therapy. In any such event, our overall rate of growth may be lower.
Our exercise of an option to acquire a five-year license to Barr’s ANDA, which references our Ovcon 35 oral contraceptive, is the subject of suits by 34 states, the District of Columbia and numerous private plaintiffs.
In March 2004, for $1.0 million, Barr granted us an option to acquire a five-year exclusive license under an ANDA owned by Barr for which our Ovcon 35 oral contraceptive is the reference drug. In May 2004, we exercised this option for an additional payment of $19.0 million. At that time, we entered into a finished product supply agreement under which Barr agreed to provide us with our requirements for finished Ovcon products throughout the term of the license.
On November 7, 2005, the Federal Trade Commission (the “FTC”) filed suit against us and Barr in the U.S. District Court for the District of Columbia. The FTC suit alleged that our agreements with Barr relating to Ovcon 35 (the “Ovcon Agreements”) constituted unfair competition under Section 5 of the FTC Act and sought an injunction to remove the Ovcon Agreements’ exclusivity provisions and other equitable relief. On October 23, 2006 the Court approved a settlement and entered a final order in this case.
On November 7, 2005, 21 states plus the District of Columbia filed suit against Barr and us in the U.S. District Court for the District of Columbia. An additional 13 states subsequently joined the suit. The suit by the state plaintiffs alleged that the Ovcon Agreements violate Section 1 of the Sherman Act and various state antitrust and consumer protection statutes. The state plaintiffs sought civil penalties, injunctive and equitable relief, and attorneys’ fees.
Eight direct purchaser lawsuits were filed against us and Barr in the U.S. District Court for the District of Columbia. The direct purchaser plaintiffs allege that the Ovcon Agreements violate Section 1 of the Sherman Act. All of the direct purchaser plaintiffs seek treble damages, injunctive relief, and costs including attorneys’ fees. Six of the lawsuits are class actions. The remaining two suits are brought on behalf of individual direct purchasers. On April 14, 2006 the six direct purchaser class action plaintiffs jointly filed an amended consolidated class action complaint and dismissed their complaints in the remaining five cases. On November 27, 2006, the Court appointed Magistrate Judge Alan Kay as a mediator for settlement of the direct purchaser cases at the parties’ request. On March 12, 2007, the direct purchaser class action plaintiffs filed a motion for class certification. Defendants filed a response in opposition on April 12, 2007. The direct purchaser class action plaintiffs filed a reply on May 21, 2007. The motion is fully briefed and is pending before the U.S. District Court.
One third-party-payor class action lawsuit filed suit against us and Barr in the U.S. District Court for the District of Columbia. The third-party-payor plaintiffs allege that the Ovcon Agreements violate Section 1 of the Sherman Act, the antitrust laws and consumer protection laws of various states and the District of Columbia, and constitute a cause of action for unjust enrichment in unspecified jurisdictions. The third-party-payor plaintiffs sought an injunction, treble damages, the amounts by which defendants have been unjustly enriched, restitution, disgorgement, a constructive trust, and costs including attorneys’ fees.
31
On March 6, 2006, a personal use consumer plaintiff filed a class action lawsuit against us and Barr in the U.S. District Court for the District of Columbia. The plaintiffs alleged that the Ovcon Agreements violate Sections 1 and 2 of the Sherman Act, the antitrust and/or consumer protection laws of various states and the unjust enrichment laws of fifty states. The consumer plaintiffs sought treble damages, injunctive relief, restitution, disgorgement, and costs, including attorney’s fees.
In August 2006, we completed manufacturing validation for a chewable version of Ovcon 35, Ovcon 35 Fe (now Femcon Fe). In September of 2006, we launched Ovcon 35 Fe and stopped shipping Ovcon 35 to consumers as we began the process of transitioning from Ovcon 35 to Ovcon 35 Fe. As a result of the launch of Ovcon 35 Fe, we had expected to engage in discussions with Barr about the effect of the launch on the parties’ agreements. However, our review of our agreements with Barr and the resulting decision to terminate the exclusivity provisions in these agreements relating to Ovcon 35 was accelerated when, on September 25, 2006, the FTC unexpectedly filed a motion for preliminary injunctive relief that would have required us to preserve inventories of Ovcon 35 in its possession and to continue certain marketing and sales activities related to Ovcon 35, which, if granted, would have had a materially adverse effect on our plans for the sale and marketing of Ovcon 35 Fe. Accordingly, on September 25, 2006 we unilaterally signed a waiver which terminated the exclusivity provisions contained in the agreements with Barr. The remaining provisions of the Barr agreements remained unchanged. On September 26, 2006, Barr announced that it would launch a generic version of Ovcon 35 in October 2006. In October 2006, Barr launched a generic version of Ovcon 35. Sales of Ovcon have predictably eroded as a result of the launch of the generic. See “Risk Factors—Risks Relating to Our Business—If generic products that compete with any of our branded pharmaceutical products are approved, sales of our products may be adversely affected.” On October 5, 2006, we filed a motion to dismiss the FTC case for lack of subject matter jurisdiction on the ground that the relief sought by the FTC had been mooted by our waiver of the exclusivity provisions included in the Ovcon Agreements and Barr’s announcement of its plans to launch a generic version of Ovcon 35.
On October 10, 2006, we agreed to a settlement in principle with the FTC. As a result, the FTC withdrew its motion for a preliminary injunction, and we withdrew our motion to dismiss for lack of subject matter jurisdiction. The Court approved the settlement and entered the agreed final order on October 23, 2006. The settlement is a final resolution of all claims filed by the FTC against us. Under the terms of the settlement, with respect to any product for which we have a new drug application filed with the FDA, we cannot enter into an agreement with a filer of an ANDA for the product (an “ANDA Filer”), that has all of the following terms: (i) we provide the ANDA Filer with anything of value, (ii) the ANDA Filer agrees to limit its research, development, manufacturing, distribution or sale of a generic version of the product and (iii) the agreement unreasonably restrains competition. In addition, under the settlement we cannot enter into any product supply agreement with an ANDA Filer that limits the filer’s development, manufacturing, distribution or sale of the product. The FTC settlement agreement specifies that we are subject to these restrictions for 10 years. We are not required to pay any monetary damages. We also agreed under the settlement to continue to fill orders for Ovcon 35 until January 23, 2007. In November 2006, we changed the trade name of Ovcon 35 Fe to Femcon Fe in order to reduce potential market confusion with Ovcon 35.
On April 9, 2007, we agreed to settlements in principle in the actions brought by the third-party payor plaintiffs and the consumer plaintiffs. On April 16, 2007, we agreed to a settlement in principle in the action brought by the 34 states and the District of Columbia. Under the proposed settlements, all claims in these actions will be dismissed and the litigations will be terminated in exchange for cash payments and/or donations of product samples amounting to approximately $7.5 million in the aggregate (or approximately $12.0 million in the aggregate if the product samples are valued at the fair market value of commercial trade product). The $7.5 million was recorded as an expense in our condensed consolidated statement of operations for the quarter ended March 31, 2007.
32
Additionally, under the terms of the settlement with the state plaintiffs, with respect to any product for which we have a new drug application filed with the FDA, we cannot enter into an agreement with an ANDA Filer, that has all of the following terms: (i) we provide the ANDA Filer with anything of value, (ii) the ANDA Filer agrees to limit its research, development, manufacturing, distribution or sale of a generic version of the product and (iii) the agreement unreasonably restrains competition. In addition, under the settlement we cannot enter into any product supply agreement with an ANDA Filer that limits the filer’s development, manufacturing, distribution or sale of the product. The settlement agreement in the state action specifies that we are subject to these restrictions for 10 years.
We signed a settlement agreement with the third-party payor plaintiffs on May 15, 2007, and with the consumer plaintiffs on May 17, 2007. Counsel for the consumer plaintiffs filed a motion for preliminary approval of the settlement on June 6, 2007, and counsel for the third-party payor plaintiffs filed a motion for preliminary approval of the settlement on June 12, 2007. We filed a joint motion with the state plaintiffs on June 12, 2007, asking the Court to approve our proposed final order. All three settlements remain subject to necessary approvals by the parties and the U.S. District Court.
We do not believe that the terms of any of these settlements will have an adverse effect on our financial position, results of operations or ongoing business activities. These settlements do not affect the related pending actions brought by the direct purchasers.
We continue to vigorously defend these lawsuits. Although it is impossible to predict with certainty the impact that the above described settlements will have on the continuing action, or the outcome of any litigation, we are confident in the merits of our defenses and do not anticipate an unfavorable outcome. An estimate of the range of potential loss to us, if any, relating to the direct purchaser proceedings is not possible at this time. If the plaintiffs in these private lawsuits are ultimately successful, we may be required to pay damages which could have an adverse impact on our financial condition, results of operations and cash flows.
We have recorded a significant amount of intangible assets, which may never generate the returns we expect.
The Acquisition has resulted in significant increases in identifiable intangible assets and goodwill. Identifiable intangible assets, which include trademarks and trade names, license agreements and patents acquired in acquisitions, were $1,483.4 million at March 31, 2007, representing approximately 48% of our total assets. Goodwill, which relates to the excess of cost over the fair value of the net assets of the businesses acquired, was $1,244.2 million at March 31, 2007, representing approximately 40% of our total assets. The majority of our intangible assets are owned by our Puerto Rican subsidiary.
Goodwill and identifiable intangible assets are recorded at fair value on the date of acquisition. Under Financial Accounting Standards Board Statement No. 142, goodwill is reviewed at least annually for impairment and definite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that their carrying value may not be recoverable. Future impairment may result from, among other things, deterioration in the performance of the acquired business or product line, adverse market conditions and changes in the competitive landscape, adverse changes in applicable laws or regulations, including changes that restrict the activities of the acquired business or product line, and a variety of other circumstances. The amount of any impairment is recorded as a charge to the statement of operations. We may never realize the full value of our intangible assets. Any future determination requiring the write-off of a significant portion of intangible assets would have an adverse effect on our financial condition and results of operations. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included in our Form 10-K and Quarterly Report on Form 10-Q incorporated by reference in this prospectus.
33
We have not completed our evaluation of our internal controls over financial reporting with respect to compliance with Section 404 of the Sarbanes-Oxley Act of 2002.
We are required to comply with the internal control evaluation and certification requirements of Section 404 of the Sarbanes-Oxley Act of 2002 by no later than the end of our 2007 fiscal year. We have begun the process of determining whether our existing internal controls over financial reporting systems are compliant with Section 404. If it is determined that we are not in compliance with Section 404, we may be required to implement new internal control procedures. We may experience higher than anticipated operating expenses as well as outside auditor fees during the implementation of these changes and thereafter. Further, we may need to hire additional qualified personnel in order for us to be compliant with Section 404.
If we are unable to conclude that internal controls over financial reporting are effective and to obtain an unqualified report on internal controls from our independent auditors as required under Section 404 of the Sarbanes-Oxley Act there could be an adverse impact on trading prices for our securities and an adverse affect on our ability to access the capital markets.
If we fail to comply with our reporting and payment obligations under the Medicaid rebate program or other governmental pricing programs, we could be subject to additional reimbursements, penalties, sanctions and fines which could have a material adverse effect on our business.
We participate in the federal Medicaid rebate program established by the Omnibus Budget Reconciliation Act of 1990, as well as several state supplemental rebate programs. Under the Medicaid rebate program, we pay a rebate to each state Medicaid program for our products that are reimbursed by those programs. The minimum amount of the rebate for each unit of product is set by law as 15.1% of the average manufacturer price (“AMP”) of that product, or if it is greater, the difference between AMP and the best price available from us to any customer. The rebate amount also includes an inflation adjustment, if necessary.
As a manufacturer currently of single source, innovator multiple source and non-innovator multiple source products, rebate calculations vary among products and programs. The calculations are complex and, in certain respects, subject to interpretation by us, governmental or regulatory agencies and the courts. The Medicaid rebate amount is computed each quarter based on our submission to Centers for Medicare and Medicaid Services at the U.S. Department of Health and Human Services of our current AMP and best price for each of our products. The terms of our participation in the program impose an obligation to correct the prices reported in previous quarters, as may be necessary. Any such corrections could result in an overage or underage in our rebate liability for past quarters, depending on the direction of the correction. In addition to retroactive rebates (and interest, if any), if we are found to have knowingly submitted false information to the government, the statute provides for civil monetary penalties in the amount of $100,000 per item of false information. Governmental agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid.
Federal law requires that any company that participates in the Medicaid rebate program extend comparable discounts to qualified purchasers under the Public Health Services (“PHS”) pharmaceutical pricing program. The PHS pricing program extends discounts comparable to the Medicaid rebates to a variety of community health clinics and other entities that receive health services grants from the PHS, as well as hospitals that serve a disproportionate share of economically disadvantaged patients.
34
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains certain forward-looking statements, including, without limitation, statements concerning the conditions in our industry, expected cost savings, our operations, our economic performance and financial condition, including, in particular, statements relating to our business and growth strategy and product development efforts. The words “may,” “might,” “will,” “should,” “estimate,” “project,” “plan,” “anticipate,” “expect,” “intend,” “outlook,” “believe” and other similar expressions are intended to identify forward-looking statements and information. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of their dates. These forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain and subject to a number of risks and uncertainties. These risks and uncertainties include, without limitation, those identified under “Risk Factors” and elsewhere, or incorporated by reference, in this prospectus.
The following list represents some, but not necessarily all, of the factors that could cause actual results to differ from historical results or those anticipated or predicted by our forward-looking statements:
| | Ÿ | | competitive factors in the industry in which we operate; |
| | Ÿ | | our ability to protect our intellectual property; |
| | Ÿ | | a delay in qualifying our manufacturing facility to produce our products or production or regulatory problems with either third party manufacturers upon whom we may rely for some of our products or our own manufacturing facility; |
| | Ÿ | | pricing pressures from reimbursement policies of private managed care organizations and other third party payors, government sponsored health systems, the continued consolidation of the distribution network through which we sell our products, including wholesale drug distributors and the growth of large retail drug store chains; |
| | Ÿ | | government regulation affecting the development, manufacture, marketing and sale of pharmaceutical products, including our ability and the ability of companies with whom we do business to obtain necessary regulatory approvals; |
| | Ÿ | | the loss of key senior management or scientific staff; |
| | Ÿ | | an increase in litigation, including product liability claims and patent litigation; |
| | Ÿ | | our ability to manage the growth of our business by successfully identifying, developing, acquiring or licensing and marketing new products, obtain regulatory approval and customer acceptance of those products, and continued customer acceptance of our existing products; and |
| | Ÿ | | the other factors that are described under “Risk Factors.” |
We caution you that the foregoing list of important factors is not exclusive. In addition, in light of these risks and uncertainties, the matters referred to in the forward-looking statements contained, or incorporated by reference, in this prospectus may not in fact occur. We undertake no obligation to publicly update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
35
USE OF PROCEEDS
We will not receive any cash proceeds from the market making in the notes undertaken by our affiliates.
The net proceeds from the initial offering of the notes, after deducting the initial purchasers’ discounts and commissions and offering expenses payable by us, were approximately $582 million. We used the net proceeds from the offering of the notes, together with the proceeds from borrowings under the senior secured credit facility, cash on hand and equity contributions from the Sponsors, certain of their limited partners and certain members of our management, to fund the Transactions, repay substantially all of Warner Chilcott PLC’s and its subsidiaries’ existing debt and pay the fees and expenses related thereto.
DIVIDEND POLICY
We have never paid cash dividends on our common stock and we intend to retain our future earnings, if any, to fund the growth of our business. We therefore do not anticipate paying any cash dividends on our common stock in the foreseeable future. Our future decisions concerning the payment of dividends on our common stock will depend upon our results of operations, financial condition and capital expenditure plans, as well as any other factors that the board of directors, in its sole discretion, may consider relevant.
36
CAPITALIZATION
The following table sets forth our capitalization as of March 31, 2007.
This table should be read in conjunction with “Selected Historical Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and notes thereto included, or incorporated by reference, in this prospectus.
| | | |
| | | March 31,
2007
|
| | | Actual
|
| (in millions), except share and per share data | | �� |
Cash and cash equivalents | | $ | 68.5 |
| | |
|
|
Long-term indebtedness: | | | |
Senior secured revolving credit facility drawdowns(1) | | $ | — |
Secured term loan(2) | | | 1,097.8 |
Senior subordinated notes | | | 390.0 |
| | |
|
|
Total long-term indebtedness | | | 1,487.8 |
Total shareholders’ equity | | | 1,323.1 |
| | |
|
|
Total capitalization | | $ | 2,810.9 |
| | |
|
|
| (1) | Our senior secured credit facility includes a revolving credit facility providing for loans of up to $150.0 million. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity and Capital Resources—Senior secured credit facility” and Note 8 of the Notes to the Condensed Consolidated Financial Statements for the three months ended March 31, 2007, incorporated by reference in this prospectus. |
| (2) | Our senior secured credit facility provides for term loans in an aggregate principal amount of $1,640.0 million, including a senior secured term loan in the amount of up to $1,400.0 million, all of which was drawn immediately prior to closing the Acquisition, and a delayed-draw senior secured term loan in the amount of up to $240.0 million, which was drawn in early 2006 for the purpose of acquiring the exclusive U.S. sales and marketing rights to Dovonex and making a $40.0 million milestone payment to LEO Pharma following FDA approval of Taclonex. Our senior secured credit facility also provides for an uncommitted incremental term loan facility in an aggregate principal amount of up to $250.0 million. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity and Capital Resources—Senior Secured Credit Facility” and Note 8 of the Notes to the Condensed Consolidated Financial Statements for the three months ended March 31, 2007, incorporated by reference in this prospectus. |
37
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected historical consolidated financial data. Except for the data relating to the years ended December 31, 2006 and 2005 and the three months ended March 31, 2007 and 2006, all data below reflects the consolidated financial data of the Predecessor. The year ended December 31, 2005 was our first fiscal year following the Acquisition. The financial statements relating to this period reflect the Acquisition as if the closing took place on January 1, 2005 and the operating results for the period January 1 through January 4, 2005 were ours. The period included only two business days and the impact on the results of operations during the period was not material. Our fiscal year ends on December 31 versus the Predecessor’s year-end of September 30.
The selected consolidated financial data as of and for the years ended December 31, 2006 and 2005, as of and for the quarter ended December 31, 2004, and as of and for the fiscal year ended September 30, 2004 presented in this table have been derived from our audited consolidated financial statements and related notes incorporated by reference in this prospectus. The selected consolidated financial data as of and for the quarter ended December 31, 2003 presented in this table have been derived from our unaudited consolidated financial statements and related notes, which are not included or incorporated by reference in this prospectus. The selected consolidated financial data as of and for the fiscal years ended September 30, 2003 and 2002 presented in this table are derived from our audited consolidated financial statements and related notes which are not included or incorporated by reference in this prospectus. Selected historical consolidated financial data for the three months ended March 31, 2007 and 2006 and the condensed consolidated balance sheet data as of March 31, 2007 and 2006 has been derived from our unaudited consolidated financial statements and related notes, which have been incorporated by reference in this prospectus.
The selected historical data included below and elsewhere in this prospectus are not necessarily indicative of future performance. This information is only a summary and should be read in conjunction with “Capitalization,” “Selected Historical Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and related notes included, or incorporated by reference, in this prospectus.
38
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Fiscal Year Ended
September 30,
| | | Transition Period
Quarter Ended
December 31,
| | | Year Ended
December 31,
| | | Three Months
Ended March 31,
| |
| | | Predecessor
| | | Predecessor
| | | Successor
| | | Successor
| |
| | | 2002
| | | 2003
| | | 2004
| | | 2003
| | | 2004
| | | 2005
| | | 2006
| | | 2006
| | | 2007
| |
| | | | | | | | | | | | (Unaudited) | | | | | | | | | | | | (Unaudited) | | | (Unaudited) | |
(dollars and share amounts in
thousands, except per share
amounts) | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenue(1)(2) | | $ | 172,231 | | | $ | 365,164 | | | $ | 490,248 | | | $ | 124,789 | | | $ | 136,893 | | | $ | 515,253 | | | $ | 754,457 | | | $ | 166,461 | | | $ | 218,421 | |
Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of sales (excluding amortization and impairments)(3) | | | 19,366 | | | | 42,042 | | | | 53,488 | | | | 11,408 | | | | 34,529 | | | | 95,224 | | | | 151,750 | | | | 31,807 | | | | 50,597 | |
Selling, general and administrative(3)(4) | | | 47,174 | | | | 124,786 | | | | 146,205 | | | | 37,745 | | | | 41,463 | | | | 162,670 | | | | 253,937 | | | | 38,286 | | | | 77,898 | |
Impairment of intangible assets | | | — | | | | — | | | | — | | | | — | | | | — | | | | 38,876 | | | | — | | | | — | | | | — | |
Research and development | | | 16,000 | | | | 24,874 | | | | 26,558 | | | | 6,692 | | | | 4,608 | | | | 58,636 | | | | 26,818 | | | | 9,571 | | | | 7,432 | |
Amortization of intangible assets(3)(5) | | | 18,252 | | | | 38,106 | | | | 52,374 | | | | 13,185 | | | | 21,636 | | | | 233,473 | | | | 253,425 | | | | 58,826 | | | | 57,553 | |
Acquired in-process research and development(3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 280,700 | | | | — | | | | — | | | | — | |
Transaction costs(3) | | | — | | | | — | | | | — | | | | — | | | | 50,973 | | | | 35,975 | | | | — | | | | — | | | | — | |
Net interest expense(3) | | | 18,916 | | | | 7,686 | | | | 9,256 | | | | 3,152 | | | | 1,214 | | | | 147,934 | | | | 206,994 | | | | 45,092 | | | | 30,944 | |
Accretion on preferred stock of subsidiary(6) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 31,533 | | | | 26,190 | | | | 8,701 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) before taxes | | | 52,523 | | | | 127,670 | | | | 202,367 | | | | 52,607 | | | | (17,530 | ) | | | (569,768 | ) | | | (164,657 | ) | | | (25,822 | ) | | | (6,003 | ) |
Provision (benefit) for income taxes | | | 18,858 | | | | 41,380 | | | | 59,390 | | | | 12,312 | | | | 11,558 | | | | (13,122 | ) | | | (11,147 | ) | | | 1,434 | | | | (1,501 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) from continuing operations | | | 33,665 | | | | 86,290 | | | | 142,977 | | | | 40,295 | | | | (29,088 | ) | | | (556,646 | ) | | | (153,510 | ) | | | (27,256 | ) | | | (4,502 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Discontinued operations, net of tax(7) | | | 111,511 | | | | 9,865 | | | | 8,711 | | | | 2,405 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | 145,176 | | | $ | 96,155 | | | $ | 151,688 | | | $ | 42,700 | | | $ | (29,088 | ) | | $ | (556,646 | ) | | $ | (153,510 | ) | | $ | (27,256 | ) | | $ | (4,502 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Other Data | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Ratio of earnings to fixed charges(12) | | | 2.7 | x | | | 11.8 | x | | | 18.5 | x | | | 14.7 | x | | | (12 | ) | | | (12 | ) | | | 0.1 | x | | | 0.4 | x | | | 0.8 | x |
Per Share Data(8): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Earnings (loss) per share—basic | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | (7.19 | ) | | $ | (1.63 | ) | | $ | (0.55 | ) | | $ | (0.02 | ) |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | 7.35 | | | $ | 6.33 | | | $ | 2.05 | | | $ | n.m. | |
Earnings (loss) per share—diluted | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | $ | (7.19 | ) | | $ | (1.63 | ) | | $ | (0.55 | ) | | $ | (0.02 | ) |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | �� | | | n.m. | | | $ | 7.34 | | | $ | 6.33 | | | $ | 2.05 | | | $ | n.m. | |
Weighted average shares outstanding—basic | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 88,311 | | | | 133,897 | | | | 89,269 | | | | 248,619 | |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 10,642 | | | | 10,280 | | | | 10,671 | | | | n.m. | |
Weighted average shares outstanding—diluted | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Class A | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 88,311 | | | | 133,897 | | | | 89,269 | | | | 248,619 | |
Class L | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | n.m. | | | | 10,668 | | | | 10,282 | | | | 10,672 | | | | n.m. | |
Balance Sheet Data (at period end): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 315,571 | | | $ | 88,571 | | | $ | 186,251 | | | $ | 167,500 | | | $ | 229,565 | | | $ | 11,502 | | | $ | 84,464 | | | $ | 20,809 | | | $ | 68,502 | |
Total assets(3)(9)(10) | | | 1,072,231 | | | | 1,456,419 | | | | 1,419,295 | | | | 1,614,403 | | | | 1,454,243 | | | | 3,041,877 | | | | 3,162,545 | | | | 3,246,964 | | | | 3,119,593 | |
Total long-term debt(3)(9)(10)(11) | | | 49,158 | | | | 341,078 | | | | 191,701 | | | | 341,582 | | | | 192,199 | | | | 1,989,500 | | | | 1,550,750 | | | | 2,226,000 | | | | 1,487,802 | |
Preferred stock in subsidiary(6) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 435,925 | | | | — | | | | 444,689 | | | | — | |
Shareholders’ equity(3) | | | 909,007 | | | | 997,125 | | | | 1,126,640 | | | | 1,051,701 | | | | 1,104,087 | | | | 332,510 | | | | 1,328,232 | | | | 307,945 | | | | 1,323,128 | |
| (1) | The increase in product revenues from fiscal year 2002 to fiscal year 2003 and from fiscal year 2003 to fiscal year 2004 was in part attributable to product acquisitions. |
| (2) | In March 2004, we sold the U.S. and Canadian rights to our then-marketed Loestrin products to a unit of Barr. Following this sale, we continued to earn revenue from supplying Loestrin under a supply agreement. Our total revenue for fiscal years 2004 and 2003, excluding revenue attributable to Loestrin product sales, were $464.0 million and $326.6 million, respectively. |
| (3) | Completing the Acquisition affected our financial condition, results of operations and cash flows in the following ways: |
| | a. | In the quarter ended December 31, 2004 we incurred $51.0 million of transaction costs and $3.7 million of incremental selling, general and administrative costs directly related to the closing of the Acquisition; and |
39
| | b. | During the year ended December 31, 2005 we completed the Acquisition for total consideration of $3,152.1 million, which was funded by approximately $1,282.8 million of equity contributions, $1,420.0 million of senior secured debt (including $20.0 million borrowed under the revolving credit facility at the time of acquisition), $600.0 million of the notes and cash on hand. We recorded adjustments to the fair value of our assets and liabilities as of the date of the Acquisition. During 2005, the following items were included in our operating results: |
| | • | | a charge of $22.4 million in cost of sales representing the write-off of the purchase price allocated to the fair value of our opening inventory, |
| | • | | $7.8 million of incremental selling, general and administrative costs directly related to the closing of the Acquisition, |
| | • | | $4.9 million in selling, general and administrative expense for the management fee to our Sponsors, |
| | • | | increased amortization expense resulting from the write-up of our identified intangible assets, |
| | • | | a $280.7 million write-off of acquired in-process research and development, |
| | • | | $36.0 million of transaction costs, and |
| | • | | increased interest expense from the indebtedness we incurred to complete the Acquisition. |
| (4) | In 2006, SG&A costs include $42.1 million of expenses directly related to our IPO. |
| (5) | In January 2005 (at the time of the Acquisition), intangible assets were re-valued at fair market value increasing the amounts to be amortized from that of the Predecessor. |
| (6) | Our wholly-owned subsidiary, Warner Chilcott Holdings Company II, Limited, issued 404,439 Preferred Shares in connection with the Transactions. The Preferred Shares are entitled to cumulative preferential dividends at an accretion rate of 8% per annum, compounded quarterly. All of the Preferred Shares were either converted to Class A common shares or redeemed for cash at the time of the IPO in September 2006. |
| (7) | Discontinued operations represented our Pharmaceutical services businesses, which were Interactive Clinical Technologies, Inc. (sold in August 2002), our Clinical Trial Services business (sold in May 2002) and our Chemical Synthesis Services business (sold in December 2001). In addition, in December 2003, we sold our Pharmaceutical Development and Manufacturing Services business, in April 2004 we sold our U.K. Pharmaceutical Sales and Marketing business and in May 2004 we sold our U.K.-based sterile solutions business. We received $343.0 million of proceeds net of costs for the sale of these businesses. The high level of discontinued operations in 2002 is due to the gain on disposal of our above-mentioned Pharmaceutical services business of $101.1 million, net of taxes of $3.9 million. No business was divested in fiscal year 2003. The discontinued operations for fiscal year 2004 included a gain on disposal of $5.4 million, net of a tax charge of $11.8 million on the sale of our Pharmaceutical Development and Manufacturing Services business, our U.K. Pharmaceutical Sales and Marketing business and our U.K.-based sterile solutions business. |
| (8) | The Company purchased all outstanding shares of the Predecessor as part of the Acquisition, making the Predecessor’s earnings per share not comparable to the Successor’s earnings per share. The Successor was in a net loss position in both 2005 and 2006. The effect from the exercise of outstanding stock options and the vesting of restricted shares during the periods would have been anti-dilutive. Accordingly, the shares issuable upon exercise of such stock options and the restricted shares have not been included in the calculation of diluted earnings per share. The December 31, 2006 earnings per share of the Class L common shares is calculated through September 30, 2006 as there were no Class L common shares outstanding during the fourth quarter of 2006 or the first quarter 2007. |
| (9) | During the year ended December, 2006 we completed the acquisition of the U.S. sales and marketing rights to Dovonex for $205.2 million and paid the final milestone payment for Taclonex of $40.0 million. We borrowed $240.0 million in connection with these transactions and recorded $238.5 million in intangible assets. |
| (10) | In 2002, we repurchased 2 million ordinary shares (0.5 million ADS equivalents). In 2004, we repurchased 2.8 million ordinary shares (0.7 million ADS equivalents). |
| (11) | Reflects the issuance of $350.0 million of long-term debt to finance a portion of the acquisition of products for approximately $650.0 million in 2003. |
| (12) | For purposes of these computations, earnings consist of income from continuing operations before income taxes plus fixed charges, plus the amortization of capitalized interest, less interest capitalized. Fixed charges include interest on indebtedness from both continuing and discontinued operations, amortization of debt issuance costs, capitalized interest and the portion of lease rental expense representative of interest. In the opinion of management, we estimate the interest component of lease rental expense to be one third of lease rental expense. For the quarter ended December 31, 2004 and the year ended December 31, 2005, our earnings were insufficient by $17.5 million and $569.8 million, respectively, to cover our fixed charges for such periods. |
40
DESCRIPTION OF THE NOTES
You can find the definitions of certain terms used in this description under the subheading “—Certain Definitions.” In this description, the term “Company” refers only to Warner Chilcott Corporation and not to any of its Subsidiaries and the term “Holdings” refers only to Warner Chilcott Holdings Company III, Limited and not to any of its Subsidiaries.
The Company issued the notes under an indenture (the “Indenture”) among itself, the Guarantors and Wells Fargo Bank, National Association, as trustee (the “Trustee”). The terms of the notes include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act of 1939, as amended (the “Trust Indenture Act”).
The following description is a summary of the material provisions of the Indenture; all material information regarding the notes and the rights of the holders of the notes are summarized herein. The following description does not restate the Indenture in its entirety. We urge you to read the Indenture because it, and not this description, defines your rights as holders of the notes. Copies of the Indenture may be obtained from the Company upon request. Certain defined terms used in this description but not defined below under “—Certain Definitions” have the meanings assigned to them in the Indenture.
The registered holder of any note will be treated as the owner of it for all purposes. Only registered holders will have rights under the Indenture.
Brief Description of the Notes and the Guarantees
The notes:
| | Ÿ | | are general unsecured obligations of the Company; |
| | Ÿ | | are subordinated in right of payment to all existing and future Senior Debt; and |
| | Ÿ | | arepari passu in right of payment with any future Senior Subordinated Indebtedness of the Company. |
The Guarantees:
| | Ÿ | | are general unsecured obligations of each Guarantor; |
| | Ÿ | | are subordinated in right of payment to all existing and future Guarantor Senior Debt of such Guarantor; and |
| | Ÿ | | arepari passu in right of payment with any future Senior Subordinated Indebtedness of such Guarantor. |
As of the date of this prospectus, all of the Subsidiaries of Holdings, including the Company, are “Restricted Subsidiaries.” Under the circumstances described under the caption “—Certain Covenants—Restricted Payments” and the definition of “Unrestricted Subsidiary,” Holdings is permitted to designate certain of its Subsidiaries as “Unrestricted Subsidiaries.” Unrestricted Subsidiaries are not subject to the restrictive covenants of the Indenture. Unrestricted Subsidiaries do not guarantee the notes.
Principal, Maturity and Interest
The Company issued notes in an aggregate principal amount of $600.0 million. On October 31, 2006, we redeemed $210.0 million in aggregate principal amount of the notes. See “—Optional Redemption.” The Indenture governing the notes provides for the issuance of additional notes having
41
identical terms and conditions to the notes (the “Additional Notes”), subject to compliance with the covenants contained in the Indenture. Any Additional Notes will be part of the same issue as the notes and will vote on all matters with the notes. Such Additional Notes will be identical in all material respects to the notes, except that notes offered in the future will have different issuance dates and may have different issuance prices. The notes will mature on February 1, 2015.
The notes were issued in denominations of $1,000 and integral multiples of $1,000. Interest on the notes accrues at the rate of 8 3/4% per annum and is payable semi-annually in arrears on February 1 and August 1. The Company makes each interest payment to the holders of record of the notes on the immediately preceding January 15 in the case of the February 1 payment and July 15 in the case of the August 1 payment.
Interest on the notes accrues from the date of original issuance or, if interest has already been paid, from the date it was most recently paid. Interest is computed on the basis of a 360-day year comprised of twelve 30-day months.
Methods of Receiving Payments on the Notes
If a holder has given wire transfer instructions to the Company at least three Business Days prior to the applicable payment date, the Company, through the paying agent or otherwise, will pay all principal, interest and premium on that holder’s notes in accordance with those instructions. All other payments on the notes will be made at the office or agency of the paying agent and registrar within the City and State of New York, unless the Company elects to make interest payments by check mailed to the holders at their address set forth in the register of holders.
Paying Agent and Registrar for the Notes
The Company will maintain one or more paying agents (each, a “paying agent”) for the notes.
The Company will also maintain one or more registrars (each, a “registrar”) and a transfer agent. The Trustee will serve as initial registrar and transfer agent at its corporate trust office. The registrar and the transfer agent in New York will maintain a register reflecting ownership of notes outstanding from time to time and will make payments on and facilitate transfer of notes on behalf of the Company.
The Company may change the paying agents, the registrars or the transfer agents without prior notice to the holders. The Company or any of its Restricted Subsidiaries may act as a paying agent or registrar.
Transfer and Exchange
A holder may transfer or exchange notes in accordance with the Indenture. The registrar and the Trustee may require a holder to furnish appropriate endorsements and transfer documents in connection with a transfer of notes. Holders will be required to pay all taxes due on transfer. The Company is not required to transfer or exchange any note selected for redemption. Also, the Company is not required to transfer or exchange any note for a period of 15 days before a selection of notes to be redeemed.
Ranking
Senior Debt versus Notes
The payment of principal, interest, premium, if any, and Additional Interest, if any, on, and other obligations with respect to the notes and the payment of any Guarantee will be subordinated in right of
42
payment to the prior payment in full of all Senior Debt, or the Guarantor Senior Debt of the relevant Guarantor, as the case may be, including, without limitation, the obligations of the Company and such Guarantor under the Credit Agreement and under any Senior Debt or any Guarantor Senior Debt incurred after the Issue Date.
As of March 31, 2007:
(1) Senior Debt was $1,097.8 million, which consisted of secured indebtedness under the Credit Agreement, excluding potential additional borrowings of $150 million under the revolving credit facility and $250 million under the uncommitted incremental term loan facility, in each case constituting part of the Credit Agreement, any of which would constitute Senior Debt if and when borrowed; and
(2) Guarantor Senior Debt was $888.8 million, all of which represents the Guarantors’ Guarantee of indebtedness under the Credit Agreement, excluding potential additional borrowings under the revolving credit facility or the uncommitted incremental term loan facility, in each case constituting part of the Credit Agreement, any of which Guarantees would constitute Guarantor Senior Debt if and when granted.
Although the Indenture contains limitations on the amount of additional Indebtedness that the Company, and the Guarantors (other than Warner Chilcott Limited which is not so limited) may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Debt or Guarantor Senior Debt, as applicable. See “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock.”
Liabilities of Subsidiaries versus Notes
All of the operations of Holdings, Luxco and the Company are conducted through their respective Subsidiaries. Some of the Subsidiaries of Holdings are not guaranteeing the notes, and, as described below under “—Guarantees,” Guarantees of Subsidiaries may be released under certain circumstances. In addition, future Subsidiaries of Holdings may not be required to Guarantee the notes. Claims of creditors of any Subsidiaries that are not Guarantors, including trade creditors and creditors holding indebtedness or guarantees issued by such Subsidiaries, and claims of preferred stockholders of such Subsidiaries generally will have priority with respect to the assets and earnings of such Subsidiaries over the claims of creditors of the Company, including holders of the notes, even if such claims do not constitute senior debt of such Subsidiaries. Accordingly, the notes and each Guarantee will be effectively subordinated to creditors (including trade creditors) and preferred stockholders, if any, of such Subsidiaries that are not Guarantors.
Certain Subsidiaries of Holdings that are not Guarantors guarantee the obligations of Holdings and Warner Chilcott Company, Inc., a Puerto Rican corporation and a Guarantor, under the Credit Agreement. The Subsidiaries of Holdings that are not Guarantors accounted for a nominal amount of total revenues of Holdings for the twelve months ended December 31, 2006 and the three months ended March 31, 2007, after elimination of intercompany amounts. Although the Indenture limits the incurrence of Indebtedness and preferred stock by Holdings and certain of its Subsidiaries, such limitation is subject to a number of significant qualifications. Moreover, the Indenture does not impose any limitation on the incurrence by such Subsidiaries of liabilities that are not considered Indebtedness under the Indenture. See “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock.”
Other Senior Subordinated Indebtedness versus Notes
Only Indebtedness of the Company or any Guarantor that is Senior Debt or Guarantor Senior Debt, as applicable, ranks senior to the notes and the relevant Guarantee in accordance with the
43
provisions of the Indenture. The notes and each Guarantee in all respects rankpari passu with all other Senior Subordinated Indebtedness of the Company and the relevant Guarantor, respectively.
The Company and the Guarantors (other than Warner Chilcott Limited) have agreed in the Indenture that it and they will not incur any Indebtedness that is subordinate or junior in right of payment to Senior Debt or Guarantor Senior Debt, unless such Indebtedness is Senior Subordinated Indebtedness of the Company or the Guarantors (other than Warner Chilcott Limited), as applicable, or is expressly subordinated in right of payment to Senior Subordinated Indebtedness of the Company or the Guarantors, as applicable. The Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to secured Indebtedness merely because it is unsecured or (2) Senior Debt or Guarantor Senior Debt as subordinated or junior to any other Senior Debt or Guarantor Senior Debt merely because it has a junior priority with respect to the same collateral.
Payment of Notes
The holders of Senior Debt will be entitled to receive payment in full of all Obligations due in respect of such Senior Debt (including interest accruing after the commencement of any bankruptcy proceeding at the rate provided for in the documentation with respect to the applicable Senior Debt, whether or not such interest is an allowed or allowable claim under applicable law) before the holders of notes will be entitled to receive any payment with respect to the notes, in the event of any distribution to creditors of the Company:
(1) in a liquidation or dissolution of the Company;
(2) in a bankruptcy, reorganization, insolvency, receivership or similar proceeding relating to the Company or its property;
(3) in an assignment for the benefit of creditors; or
(4) in any marshaling of the Company’s assets and liabilities.
The Company also may not make any payment in respect of the notes (except that holders may receive and retain Permitted Junior Securities or payments pursuant to the provisions described under the sections entitled “—Legal Defeasance and Covenant Defeasance” or “—Satisfaction and Discharge,” if the subordination provisions described in this section were not violated at the time the applicable amounts were deposited in trust or with the Trustee, as applicable, pursuant to such sections) if:
(1) a payment default on Designated Senior Debt occurs and is continuing beyond any applicable grace period; or
(2) any other default occurs and is continuing on any series of Designated Senior Debt that permits holders of that series of Designated Senior Debt to accelerate its maturity without further notice (except such notice as may be required to effect such acceleration) and the Trustee receives a notice of such default (a “Payment Blockage Notice”) from the Representative of that series of Designated Senior Debt.
Payments on the notes may and will be resumed:
(1) in the case of a payment default, upon the date on which such default is cured or waived; and
(2) in the case of a nonpayment default, upon the earliest of (x) the date on which such nonpayment default is cured or waived, (y) 179 days after the date on which the applicable Payment Blockage Notice is received and (z) the date the Trustee receives notice from the Representative for such Designated Senior Debt rescinding the Payment Blockage Notice, unless the maturity of any Designated Senior Debt has been accelerated.
44
No new Payment Blockage Notice may be delivered unless and until 360 days have elapsed since the delivery of the immediately prior Payment Blockage Notice.
No nonpayment default that existed or was continuing on the date of delivery of any Payment Blockage Notice to the Trustee will be, or be made, the basis for a subsequent Payment Blockage Notice unless such default has been cured or waived for a period of not less than 90 days.
If the Trustee or any holder of the notes receives a payment or distribution in respect of the notes (except that holders may receive and retain Permitted Junior Securities or payments pursuant to the provisions described under the sections entitled “—Legal Defeasance and Covenant Defeasance” or “—Satisfaction and Discharge,” if the subordination provisions described in this section were not violated at the time the applicable amounts were deposited in trust or with the Trustee, as applicable, pursuant to such sections) when the payment or distribution is prohibited by the subordination provisions of the Indenture, then the Trustee or the holder, as the case may be, will hold the payment in trust for the benefit of the holders of Senior Debt. Upon the proper written request of the holders of Senior Debt, the Trustee or the holder, as the case may be, will deliver the amounts in trust to the holders of Senior Debt or their proper Representative.
The Company must promptly notify holders of Senior Debt if payment of the notes is accelerated because of an Event of Default.
A Guarantor’s obligations under its Guarantee are senior subordinated obligations. As such, the rights of holders of the notes to receive payment by a Guarantor pursuant to its Guarantee will be subordinated in right of payment to the rights of holders of Guarantor Senior Debt of such Guarantor. The terms of the subordination and payment blockage provisions described above with respect to the Company’s obligations under the notes apply equally to a Guarantor and the obligations of such Guarantor under its Guarantee.
As a result of the subordination provisions described above, in the event of a bankruptcy, liquidation or reorganization of the Company, holders of notes may recover less ratably than creditors of the Company who are holders of Senior Debt. See “Risk Factors—Risks Related to the Notes—Your right to receive payment on the notes and the guarantees are junior to the rights of the lenders under our senior secured credit facility and to all of our and the guarantors’ other senior indebtedness, including any of our or the guarantors’ future senior debt.”
Optional Redemption
At any time prior to February 1, 2008, the Company may on one or more occasions redeem in the aggregate up to 35% of the aggregate principal amount of the notes issued under the Indenture (calculated after giving effect to any issuance of additional notes), with the net cash proceeds of one or more Equity Offerings, at a redemption price of 108.750% of the principal amount of the notes, plus accrued and unpaid interest and Additional Interest, if any, to the redemption date (provided that if the Equity Offering is an offering by Holdings or any of its direct or indirect parent corporations, a portion of the net cash proceeds thereof equal to the amount required to redeem any such notes is contributed to the equity capital of the Company); provided, however, that:
(1) at least 65% of the aggregate principal amount of the notes (calculated after giving effect to any issuance of additional notes) must remain outstanding immediately after the occurrence of each such redemption (excluding in such calculation notes held by Holdings and its Subsidiaries); and
(2) the redemption occurs within 90 days of the date of the closing of such Equity Offering.
On October 31, 2006, following the initial public offering of Warner Chilcott Limited, we redeemed $210.0 million in aggregate principal amount of the notes.
45
The notes may be redeemed, in whole or in part, at any time prior to February 1, 2010, at the option of the Company upon not less than 30 nor more than 60 days’ prior notice mailed by first-class mail to each holder’s registered address, at a redemption price equal to 100% of the principal amount of the notes redeemed plus the Applicable Premium as of, and accrued and unpaid interest and Additional Interest, if any, to, the applicable redemption date (subject to the right of holders of record on the relevant record date to receive interest due on the relevant interest payment date).
On or after February 1, 2010, the Company may redeem all or a part of the notes at its option, upon not less than 30 nor more than 60 days’ notice, at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest and Additional Interest, if any, on the notes to be redeemed to the applicable redemption date (subject to the right of holders of record on the relevant record date to receive interest due on the relevant interest payment date), if redeemed during the twelve-month period beginning on February 1 of the years indicated below:
| | | |
Year
| | Percentage
| |
2010 | | 104.375 | % |
2011 | | 102.917 | % |
2012 | | 101.458 | % |
2013 and thereafter | | 100.000 | % |
The Company may acquire notes by means other than a redemption, whether by tender offer, open market purchases, negotiated transactions or otherwise, in accordance with applicable securities laws, so long as such acquisition does not otherwise violate the terms of the Indenture.
Guarantees
The Guarantors jointly and severally guarantee the Company’s obligations under the Indenture and the notes on a senior subordinated, full and unconditional basis. Each Guarantee is subordinated to the applicable Guarantor Senior Debt on the same basis as the notes are subordinated to Senior Debt. The obligations of each Guarantor (other than a company that is a direct or indirect parent of the Company) under its Guarantee are limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance or fraudulent transfer under applicable law. In addition, since each of Warner Chilcott Limited, Holdings and Luxco is a holding company with no significant operations, the Guarantees by Warner Chilcott Limited, Holdings and Luxco provide little, if any, additional credit support for the notes and investors should not rely on these Guarantees in evaluating an investment in the notes.
Each Guarantor may consolidate with or merge into or sell its assets to the Company or another Guarantor without limitation, or with, into or to any other Persons upon the terms and conditions set forth in the Indenture. See “—Certain Covenants—Merger, Consolidation or Sale of Assets.” The Guarantee of a Guarantor will be released in the event that:
(a) (x) the sale, disposition or other transfer (including through merger or consolidation) of all of the Capital Stock (or any sale, disposition or other transfer of Capital Stock following which the applicable Guarantor is no longer a Restricted Subsidiary), or all or substantially all the assets, of the applicable Guarantor, in each case other than Holdings or a Subsidiary of Holdings, if such sale, disposition or other transfer is made in compliance with the provisions of the Indenture described under “—Repurchase at the Option of Holders—Asset Sales” and (y) such Guarantor is released from its guarantee, if any, of, and all pledges and security, if any, granted in connection with the Credit Agreement and any other Indebtedness of Holdings or any Restricted Subsidiary,
46
(b) Holdings designates any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in accordance with the provisions of the Indenture described under “—Certain Covenants—Restricted Payments” and the definition of “Unrestricted Subsidiary,”
(c) in the case of any Restricted Subsidiary which after the Issue Date is required to guarantee the notes pursuant to the covenant described under “—Certain Covenants—Additional Subsidiary Guarantees,” the release or discharge of the guarantee by such Restricted Subsidiary of Indebtedness of Holdings or any Restricted Subsidiary or the repayment of the Indebtedness or Disqualified Stock, in each case, which resulted in the obligation to guarantee the notes;
(d) if the Company exercises its legal defeasance option or its covenant defeasance option as described under “—Legal Defeasance and Covenant Defeasance” or if its obligations under the Indenture are discharged in accordance with the terms of the Indenture; or
(e) such Guarantor is also a guarantor or borrower under the Credit Agreement as in effect on the Issue Date and, at the time of release of its Guarantee, (x) has been released from its guarantee of, and all pledges and security, if any, granted in connection with the Credit Agreement, (y) is not an obligor under any Indebtedness (other than Indebtedness permitted to be incurred pursuant to clause (7), (9), (10) or (15) of the second paragraph of the covenant described under “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock”) and (z) does not guarantee any Indebtedness of the Company or any of the other Guarantors; or
(f) in the case of Warner Chilcott Limited, if it requests such release at any time.
Subject to certain exceptions, if a Guarantor that is a Foreign Subsidiary or is a Puerto Rican corporation is required by applicable law or by the interpretation or administration thereof to withhold or deduct any amount for or on account of any present or future tax, duty, levy, impost, assessment or other governmental charge (including penalties, interest and other liabilities related thereto) (“Taxes”) imposed or levied by or on behalf of the government of the jurisdiction of organization or incorporation of such Guarantor or any political subdivision or any authority or agency therein or thereof having power to tax, or within any other jurisdiction in which such Guarantor is resident for tax purposes or any jurisdiction from or through which payment under its Guarantee is made, such Guarantor will be required to pay under its Guarantee such additional amounts (“Additional Amounts”) as may be necessary so that the net amount received by holders of the notes (including Additional Amounts) after such withholding or deduction will not be less than the amount that holders of the notes would have received if such Taxes had not been withheld or deducted.
Mandatory Redemption
The Company is not required to make mandatory redemption or sinking fund payments with respect to the notes. However, under certain circumstances, the Company may be required to offer to purchase notes as described under the captions “—Repurchase at the Option of Holders—Change of Control” and “—Repurchase at the Option of Holders—Asset Sales.” The Company may at any time and from time to time purchase notes in the open market or otherwise.
Repurchase at the Option of Holders
Change of Control
If a Change of Control occurs, unless the Company at such time has given notice of redemption under the second or third paragraph under the caption “—Optional Redemption” with respect to all outstanding notes, each holder of notes will have the right to require the Company to repurchase all or any part (equal to $1,000 or an integral multiple of $1,000) of that holder’s notes pursuant to a Change of Control Offer on the terms set forth in the Indenture. In the Change of Control Offer, the Company
47
will offer a Change of Control Payment in cash equal to 101% of the aggregate principal amount of notes repurchased plus accrued and unpaid interest and Additional Interest, if any, on the notes repurchased, to the date of purchase. Within 30 days following any Change of Control, unless the Company at such time has given notice of redemption under the second or third paragraph under the caption “—Optional Redemption” with respect to all outstanding notes, the Company will mail a notice to each holder describing the transaction or transactions that constitute the Change of Control and offering to repurchase notes on the Change of Control Payment Date specified in the notice, which date will be no earlier than 30 days and no later than 60 days from the date such notice is mailed, pursuant to the procedures required by the Indenture and described in such notice. The Company will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with the repurchase of the notes as a result of a Change of Control. To the extent that the provisions of any securities laws or regulations conflict with the Change of Control provisions of the Indenture, the Company will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations under the Change of Control provisions of the Indenture by virtue of such conflict.
On the Change of Control Payment Date, the Company will, to the extent lawful:
(1) accept for payment all notes or portions of notes properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the Change of Control Payment in respect of all notes or portions of notes properly tendered; and
(3) deliver or cause to be delivered to the Trustee the notes properly accepted together with an officers’ certificate stating the aggregate principal amount of notes or portions of notes being purchased by the Company.
The paying agent will promptly mail to each holder of notes properly tendered the Change of Control Payment for such notes, and the Trustee will promptly authenticate and mail (or cause to be transferred by book entry) to each holder a new note equal in principal amount to any unpurchased portion of the notes surrendered, if any;provided that each new note will be in a principal amount of $1,000 or an integral multiple of $1,000. The Company will publicly announce the results of the Change of Control Offer on or as soon as practicable after the Change of Control Payment Date.
The Company will not be required to make a Change of Control Offer upon a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by the Company and purchases all notes properly tendered and not withdrawn under the Change of Control Offer. A Change of Control Offer may be made in advance of a Change of Control or conditional upon the occurrence of a Change of Control, if a definitive agreement is in place for the Change of Control at the time the Change of Control Offer is made.
The Credit Agreement prohibits the Company from purchasing notes, and will also provide that the occurrence of certain change of control events with respect to Holdings would constitute a default thereunder. Prior to complying with any of the provisions of this “Change of Control” covenant under the Indenture governing the notes, but in any event within 90 days following a Change of Control, to the extent required to permit the Company to comply with this covenant, the Company will either repay all outstanding Senior Debt or obtain the requisite consents, if any, under all agreements governing outstanding Senior Debt. If the Company does not repay such Senior Debt or obtain such consents, the Company will remain prohibited from purchasing notes in a Change of Control, which after appropriate notice and lapse of time would result in an Event of Default under the Indenture, which would in turn constitute a default under the Credit Agreement. In such circumstance, the subordination provisions of the Indenture would likely restrict payment to the holders of notes.
48
Future indebtedness that Warner Chilcott Limited, or Holdings or its Subsidiaries may incur may contain prohibitions on the occurrence of certain events that would constitute a Change of Control or require the repurchase of such indebtedness upon a Change of Control. Moreover, the exercise by the holders of their right to require the Company to repurchase their notes could cause a default under such indebtedness, even if the Change of Control itself does not, due to the financial effect of such repurchase on Holdings. Finally, the Company’s ability to pay cash to the holders of notes following the occurrence of a Change of Control may be limited by its then existing financial resources. There can be no assurance that sufficient funds will be available when necessary to make any required repurchases.
The provisions described above that require the Company to make a Change of Control Offer following a Change of Control will be applicable whether or not any other provisions of the Indenture are applicable. Except as described above with respect to a Change of Control, the Indenture does not contain provisions that permit the holders of the notes to require that the Company repurchase or redeem the notes in the event of a takeover, recapitalization or similar transaction.
The Change of Control purchase feature of the notes may in certain circumstances make more difficult or discourage a sale or takeover of Holdings or its Subsidiaries and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between Holdings, the Company and the initial purchasers of the notes. Holdings or the Company have no present intention to engage in a transaction involving a Change of Control, although it is possible that they could decide to do so in the future. Subject to the limitations discussed below, Holdings or its Subsidiaries could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Indenture, but that could increase the amount of indebtedness outstanding at such time or otherwise affect the capital structure of Holdings or its credit ratings. Restrictions on the ability of Holdings and its Subsidiaries to incur additional Indebtedness are contained in the covenants described under “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock”. Such restrictions can only be waived with the consent of the holders of a majority in principal amount of the notes then outstanding. Except for the limitations contained in such covenants, however, the Indenture does not contain any covenants or provisions that may afford holders of the notes protection in the event of a highly leveraged transaction.
The definition of Change of Control includes a phrase relating to the direct or indirect sale, lease, transfer, conveyance or other disposition of “all or substantially all” of the properties or assets of Holdings and its Subsidiaries taken as a whole. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, the ability of a holder of notes to require the Company to repurchase its notes as a result of a sale, lease, transfer, conveyance or other disposition of less than all of the assets of Holdings and its Subsidiaries taken as a whole to another Person or group may be uncertain.
Asset Sales
Holdings will not, and will not permit any of its Restricted Subsidiaries to, consummate an Asset Sale unless:
(1) Holdings (or such Restricted Subsidiary, as the case may be) receives consideration at the time of the Asset Sale at least equal to the fair market value of the assets or Equity Interests issued or sold or otherwise disposed of;
(2) in the case of Asset Sales involving consideration in excess of $10.0 million, the fair market value is determined in good faith by Holdings’ Board of Directors; and
(3) except for any Permitted Asset Swap, at least 75% of the consideration received in the Asset Sale by Holdings or such Restricted Subsidiary is in the form of cash or Cash Equivalents.
49
For purposes of clause (3) above, the amount of (i) any liabilities (as shown on Holdings’ or the applicable Restricted Subsidiary’s most recent balance sheet or in the notes thereto) of Holdings or any Restricted Subsidiary (other than liabilities that are by their terms subordinated to the notes or the Guarantees) that are assumed by the transferee of any such assets and from which Holdings and all Restricted Subsidiaries have been validly released by all creditors in writing, (ii) any securities received by Holdings or such Restricted Subsidiary from such transferee that are converted by Holdings or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale and (iii) any Designated Noncash Consideration received by Holdings or any of its Restricted Subsidiaries in such Asset Sale having an aggregate fair market value (as determined in good faith by the Board of Directors of Holdings), taken together with all other Designated Noncash Consideration received pursuant to this clause (iii) that is at that time outstanding, not to exceed the greater of (x) $75.0 million and (y) $75.0 million plus or minus, as applicable, an amount equal to 6% of Consolidated Net Income of Holdings for the period (taken as one accounting period) from January 1, 2005 to the end of Holding’s most recently ended fiscal quarter prior to the date on which such Designated Noncash Consideration is received (with the fair market value of each item of Designated Noncash Consideration being measured at the time received without giving effect to subsequent changes in value), shall be deemed to be cash for purposes of this paragraph and for no other purpose.
Within 365 days after the receipt of any Net Proceeds from an Asset Sale, Holdings may apply those Net Proceeds at its option:
(1) to permanently reduce Obligations under Senior Debt (and to correspondingly reduce commitments with respect thereto) or Indebtedness that rankspari passu with the notes (provided that if Holdings shall so reduce Obligations under Indebtedness that rankspari passu with the notes, it will equally and ratably reduce Obligations under the notes by causing the Company to make an offer (in accordance with the procedures set forth below for an Asset Sale Offer (as defined below)) to all holders of notes to purchase at a purchase price equal to 100% of the principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, on thepro rata principal amount of notes) or Indebtedness of a Restricted Subsidiary that (x) is a Subsidiary of the Company and (y) is not a Guarantor, in each case other than Indebtedness owed to Holdings or an Affiliate of Holdings;
(2) to an investment in (A) any one or more businesses; provided that such investment in any business is in the form of the acquisition of Capital Stock and results in Holdings or a Restricted Subsidiary owning an amount of the Capital Stock of such business such that such business constitutes a Restricted Subsidiary, (B) capital expenditures or (C) other assets, in each of (A), (B) and (C), used or useful in a Permitted Business; and/or
(3) to an investment in (A) any one or more businesses; provided that such investment in any business is in the form of the acquisition of Capital Stock and it results in Holdings or a Restricted Subsidiary owning an amount of the Capital Stock of such business such that such business constitutes a Restricted Subsidiary, (B) properties or (C) assets that, in each of (A), (B) and (C), replace the businesses, properties and assets that are the subject of such Asset Sale.
Any Net Proceeds from an Asset Sale not applied or invested in accordance with the preceding paragraph within 365 days from the date of the receipt of such Net Proceeds shall constitute “Excess Proceeds,” provided that if during such 365-day period Holdings or a Restricted Subsidiary enters into a definitive binding agreement committing it to apply such Net Proceeds in accordance with the requirements of clause (2) or (3) of the immediately preceding paragraph after such 365th day, such 365-day period will be extended with respect to the amount of Net Proceeds so committed for a period not to exceed 180 days until such Net Proceeds are required to be applied in accordance with such agreement (or, if earlier, until termination of such agreement).
50
When the aggregate amount of Excess Proceeds exceeds $20.0 million, Holdings, or the applicable Restricted Subsidiary (including the Company), will make an offer (an “Asset Sale Offer”) to all holders of notes and Indebtedness that rankspari passu with the notes and contains provisions similar to those set forth in the Indenture with respect to offers to purchase with the proceeds of sales of assets to purchase, on apro rata basis, the maximum principal amount of notes and such otherpari passu Indebtedness that may be purchased out of the Excess Proceeds. The offer price in any Asset Sale Offer will be equal to 100% of principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase, and will be payable in cash.
Pending the final application of any Net Proceeds, Holdings, or the applicable Restricted Subsidiary (including the Company), may temporarily reduce revolving credit borrowings or otherwise invest the Net Proceeds in any manner that is not prohibited by the Indenture.
If any Excess Proceeds remain after consummation of an Asset Sale Offer, Holdings, or the applicable Restricted Subsidiary (including the Company), may use those Excess Proceeds for any purpose not otherwise prohibited by the Indenture. If the aggregate principal amount of notes tendered into such Asset Sale Offer exceeds the amount of Excess Proceeds, the Trustee will select the notes to be purchased on apro rata basis. Upon completion of each Asset Sale Offer, the amount of Excess Proceeds will be reset at zero.
Holdings, or the applicable Restricted Subsidiary (including the Company), will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with each repurchase of notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the Asset Sale provisions of the Indenture, Holdings, or the applicable Restricted Subsidiary (including the Company), will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations under the Asset Sale provisions of the Indenture by virtue of such conflict.
Selection and Notice
If less than all of the notes are to be redeemed at any time, the Trustee will select notes for redemption as follows:
(1) if the notes are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange on which the notes are listed; or
(2) if the notes are not listed on any national securities exchange, on a pro rata basis to the extent practicable.
No notes of $1,000 or less can be redeemed in part. Notices of redemption will be mailed by first-class mail at least 30 but not more than 60 days before the redemption date to each holder of notes to be redeemed at its registered address, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the notes or a satisfaction and discharge of the Indenture. Notices of redemption may not be conditional.
If any note is to be redeemed in part only, the notice of redemption that relates to that note will state the portion of the principal amount of that note that is to be redeemed. A new note in principal amount equal to the unredeemed portion of the original note will be issued in the name of the holder of that note upon cancellation of the original note. Notes called for redemption become due on the date fixed for redemption. On and after the redemption date, interest ceases to accrue on notes or portions of them called for redemption.
51
Book-Entry
The notes were initially issued in the form of one or more Global Securities registered in the name of The Depository Trust Company (“DTC”) or its nominee. DTC or its nominee has credited the accounts of Persons holding through it with the respective principal amounts of the notes represented by such Global Security purchased by such Persons in the original issuance. Ownership of beneficial interests in a Global Security will be limited to Persons that have accounts with DTC (“participants”) or Persons that may hold interests through participants. Any Person who acquired an interest in a Global Security through an offshore transaction in reliance on Regulation S of the Securities Act may hold such interest through Clearstream Banking, S.A. or Euroclear Bank S.A./N.V., as operator of the Euroclear System. Ownership of beneficial interests in a Global Security is shown on, and the transfer of that ownership interest will be effected only through, records maintained by DTC (with respect to participants’ interests) and such participants (with respect to the owners of beneficial interests in such Global Security other than participants). The laws of some jurisdictions require that certain purchasers of securities take physical delivery of such securities in definitive form. Such limits and such laws may impair the ability to transfer beneficial interests in a Global Security.
Payment of principal of and interest on notes represented by a Global Security will be made in immediately available funds to DTC or its nominee, as the case may be, as the sole registered owner and the sole Holder represented thereby for all purposes under the Indenture. The Company has been advised by DTC that upon receipt of any payment of principal of or interest on any Global Security, DTC will immediately credit, on its book entry registration and transfer system, the accounts of participants with payments in amounts proportionate to their respective beneficial interests in the principal or face amount of such Global Security as shown on the records of DTC. Payments by participants to owners of beneficial interests in a Global Security held through such participants will be governed by standing instructions and customary practices as is now the case with securities held for customer accounts registered in “street name” and will be the sole responsibility of such participants.
A Global Security may not be transferred except as a whole by DTC or a nominee of DTC to a nominee of DTC or to DTC. A Global Security is exchangeable for certificated notes only if:
(1) DTC notifies the Company that it is unwilling or unable to continue as a depositary for such Global Security or if at any time DTC ceases to be a clearing agency registered under the Exchange Act;
(2) the Company in its discretion at any time determines not to have all the notes represented by such Global Security; or
(3) there shall have occurred and be continuing a Default or an Event of Default with respect to the notes represented by such Global Security.
Any Global Security that is exchangeable for certificated notes pursuant to the preceding sentence will be exchanged for certificated notes in authorized denominations and registered in such names as DTC or any successor depositary holding such Global Security may direct. Subject to the foregoing, a Global Security is not exchangeable, except for a Global Security of like denomination to be registered in the name of DTC or any successor depositary or its nominee. In the event that a Global Security becomes exchangeable for certificated notes,
(1) certificated notes will be issued only in fully registered form in denominations of $1,000 or integral multiples thereof;
(2) payment of principal of, and premium, if any, and interest on, the certificated notes will be payable, and the transfer of the certificated notes will be registrable, at the office or agency of the Company maintained for such purposes; and
52
(3) no service charge will be made for any registration of transfer or exchange of the certificated notes, although the Company may require payment of a sum sufficient to cover any tax or governmental charge imposed in connection therewith.
So long as DTC or any successor depositary for a Global Security, or any nominee, is the registered owner of such Global Security, DTC or such successor depositary or nominee, as the case may be, will be considered the sole owner or Holder represented by such Global Security for all purposes under the Indenture and the notes. Except as set forth above, owners of beneficial interests in a Global Security will not be entitled to have the notes represented by such Global Security registered in their names, will not receive or be entitled to receive physical delivery of certificated notes in definitive form and will not be considered to be the owners or holders of any notes under such Global Security. Accordingly, each Person owning a beneficial interest in a Global Security must rely on the procedures of DTC or any successor depositary, and, if such Person is not a participant, on the procedures of the participant through which such Person owns its interest, to exercise any rights of a holder under the Indenture. The Company understands that under existing industry practices, in the event that requests any action of holders are requested or that an owner of a beneficial interest in a Global Security desires to give or take any action which a holder is entitled to give or take under the Indenture, DTC or any successor depositary would authorize the participants holding the relevant beneficial interest to give or take such action and such participants would authorize beneficial owners owning through such participants to give or take such action or would otherwise act upon the instructions of beneficial owners owning through them.
DTC has advised the Company that DTC is a limited-purpose trust company organized under the Banking Law of the State of New York, a member of the Federal Reserve System, a “clearing corporation” within the meaning of the New York Uniform Commercial Code and a “clearing agency” registered under the Exchange Act. DTC was created to hold the securities of its participants and to facilitate the clearance and settlement of securities transactions among its participants in such securities through electronic book-entry changes in accounts of the participants, thereby eliminating the need for physical movement of securities certificates. DTC’s participants include securities brokers and dealers, banks, trust companies, clearing corporations and certain other organizations some of whom (or their representatives) own DTC. Access to DTC’s book-entry system is also available to others, such as banks, brokers, dealers and trust companies, that clear through or maintain a custodial relationship with a participant, either directly or indirectly.
Although DTC has agreed to the foregoing procedures in order to facilitate transfers of interests in Global Securities among participants of DTC, it is under no obligation to perform or continue to perform such procedures, and such procedures may be discontinued at any time. Neither the Company nor the Trustee has any responsibility for the performance by DTC or its participants or indirect participants of their respective obligations under the rules and procedures governing their operations.
Certain Covenants
Restricted Payments
Holdings will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(a) declare or pay any dividend or make any other distribution on account of Holdings’ or any of its Restricted Subsidiaries’ Equity Interests, including any dividend or distribution payable in connection with any merger or consolidation (other than (A) dividends or distributions by Holdings payable in Equity Interests (other than Disqualified Stock) of Holdings or in options, warrants or other rights to purchase such Equity Interests (other than Disqualified Stock), (B) dividends or distributions by a Restricted Subsidiary payable solely to Holdings or any other
53
Restricted Subsidiary or (C), in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly Owned Subsidiary, pro rata dividends or distributions to minority stockholders of such Restricted Subsidiary (or owners of an equivalent interest in the case of a Subsidiary that is an entity other than a corporation) provided that Holdings or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities);
(b) purchase, redeem or otherwise acquire or retire for value any Equity Interests of Holdings or any direct or indirect parent entity of Holdings held by any Person (other than by a Restricted Subsidiary), including in connection with any merger or consolidation;
(c) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value, in each case prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness (other than (x) Indebtedness permitted under clauses (7) and (8) of the definition of “Permitted Debt” or (y) the purchase, repurchase or other acquisition or retirement of Indebtedness subordinated or junior in right of payment to the notes purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase, acquisition or retirement); or
(d) make any Restricted Investment (all such payments and other actions set forth in these clauses (a) through (d) being collectively referred to as “Restricted Payments”),
unless, at the time of and after giving effect to such Restricted Payment:
(1) no Default or Event of Default has occurred and is continuing or would occur as a consequence of such Restricted Payment;
(2) Holdings would, at the time of such Restricted Payment and after givingpro forma effect thereto as if such Restricted Payment had been made at the beginning of the applicable four-quarter period, have been permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described below under the caption “—Incurrence of Indebtedness and Issuance of Preferred Stock”; and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by Holdings and the Restricted Subsidiaries after the Issue Date (excluding Restricted Payments permitted by clauses (2), (3), (4), (5), (6), (8), (10), (11), (12), (13), (16), (17) and (18) of the next succeeding paragraph), is less than the sum, without duplication, of
(a) 50% of the Consolidated Net Income of Holdings for the period (taken as one accounting period) from January 1, 2005 to the end of Holdings’ most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment (or, in the case such Consolidated Net Income for such period is a deficit, minus 100% of such deficit), plus
(b) 100% of the aggregate net cash proceeds and the fair market value, as determined in good faith by the Board of Directors of Holdings, of property and marketable securities received by Holdings after the Issue Date from the issue or sale of (x) Equity Interests of Holdings (including Retired Capital Stock (as defined below) but excluding (i) cash proceeds received from the sale of Equity Interests of Holdings and, to the extent actually contributed to Holdings, Equity Interests of Holdings’ direct or indirect parent corporations to members of management, directors or consultants of Holdings, any direct or indirect parent corporation of Holdings and the Subsidiaries of Holdings after the Issue Date to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph, (ii) cash proceeds received from the sale of Refunding Capital Stock (as defined below) to the extent such amounts have been applied
54
to Restricted Payments made in accordance with clause (2) of the next succeeding paragraph, (iii) Designated Preferred Stock, (iv) the Cash Contribution Amount and (v) Disqualified Stock) or (y) debt securities of Holdings that have been converted into such Equity Interests of Holdings (other than Refunding Capital Stock or Equity Interests or convertible debt securities of Holdings sold to a Restricted Subsidiary or Holdings, as the case may be, and other than Disqualified Stock or Designated Preferred Stock or debt securities that have been converted into Disqualified Stock or Designated Preferred Stock), plus
(c) 100% of the aggregate amount of cash and the fair market value, as determined in good faith by the Board of Directors of Holdings, of property and marketable securities contributed to the capital of Holdings after the Issue Date (other than (i) by a Restricted Subsidiary, (ii) any Excluded Contributions, (iii) any Disqualified Stock, (iv) any Refunding Capital Stock, (v) any Designated Preferred Stock, (vi) the Cash Contribution Amount and (vii) cash proceeds applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph), plus
(d) without duplication of any amounts included in clause (4) of the paragraph below and to the extent not already included in Consolidated Net Income, 100% of the aggregate amount received in cash and the fair market value, as determined in good faith by the Board of Directors of Holdings, of property and marketable securities received after the Issue Date by means of (A) the sale or other disposition (other than to Holdings or a Restricted Subsidiary) of Restricted Investments made by Holdings or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from Holdings or its Restricted Subsidiaries and repayments of loans or advances which constitute Restricted Investments of Holdings or its Restricted Subsidiaries or (B) the sale (other than to Holdings or a Restricted Subsidiary) of the Capital Stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by a Restricted Subsidiary pursuant to clause (10) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary, plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary or the merger or consolidation of an Unrestricted Subsidiary into Holdings or a Restricted Subsidiary or the transfer of assets of an Unrestricted Subsidiary to Holdings or a Restricted Subsidiary, the fair market value of the Investment in such Unrestricted Subsidiary, as determined by the Board of Directors of Holdings in good faith at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary or at the time of such merger, consolidation or transfer of assets (other than an Unrestricted Subsidiary to the extent the Investment in such Unrestricted Subsidiary was made by a Restricted Subsidiary pursuant to clause (10) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment), plus
(f) $25.0 million.
The preceding provisions will not prohibit:
(1) the payment of any dividend within 60 days after the date of declaration thereof, if at the date of declaration such payment would have complied with the provisions of the Indenture;
(2) (A) the redemption, repurchase, retirement or other acquisition of any Equity Interests of Holdings or any direct or indirect parent corporation of Holdings (“Retired Capital Stock”) or Indebtedness subordinated to the notes in exchange for or out of the net cash proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary or Holdings) of Equity Interests of Holdings or contributions to the equity capital of Holdings (in each case, other than
55
Disqualified Stock and the Cash Contribution Amount) (“Refunding Capital Stock”) and (B) the declaration and payment of dividends on the Retired Capital Stock out of the net cash proceeds of the substantially concurrent sale (other than to a Subsidiary of Holdings or to an employee stock ownership plan or any trust established by Holdings or any of its Subsidiaries) of Refunding Capital Stock;
(3) the redemption, repurchase or other acquisition or retirement of Indebtedness subordinated to the notes made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the borrower thereof which is incurred in compliance with the covenant “—Incurrence of Indebtedness and Issuance of Preferred Stock” so long as (A) the principal amount of such new Indebtedness does not exceed the principal amount of the Indebtedness subordinated to the notes being so redeemed, repurchased, acquired or retired for value plus related fees and expenses and the amount of any reasonable premium required to be paid under the terms of the instrument governing the Indebtedness subordinated to the notes being so redeemed, repurchased, acquired or retired, (B) such new Indebtedness is subordinated to the notes and any Guarantees thereof at least to the same extent as such Indebtedness subordinated to such notes so redeemed, repurchased, acquired or retired, (C) such new Indebtedness has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Indebtedness subordinated to such notes being so redeemed, repurchased, acquired or retired and (D) such new Indebtedness has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Indebtedness subordinated to such notes being so redeemed, repurchased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of common Equity Interests of Holdings or any of its direct or indirect parent corporations held by any future, present or former employee, director or consultant of Holdings, any of its Subsidiaries or any of its direct or indirect parent corporations (or their permitted transferees, assigns, estates or heirs) pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, provided, however, that the aggregate amount of Restricted Payments made under this clause (4) does not exceed in any calendar year $7.5 million (with unused amounts in any calendar year being carried over to the two immediately succeeding calendar years); and provided, further, that such amount in any calendar year may be increased by an amount not to exceed (A) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of Holdings and, to the extent contributed to Holdings, Equity Interests of any of its direct or indirect parent corporations, in each case to members of management, directors or consultants of Holdings, any of its Subsidiaries or any of its direct or indirect parent corporations that occurs after the Issue Date plus (B) the amount of any cash bonuses otherwise payable to members of management, directors or consultants of Holdings or any of its Subsidiaries or any of its direct or indirect parent corporations in connection with the Transactions that are foregone in return for the receipt of Equity Interests of Holdings or any of its direct or indirect parent corporations pursuant to a deferred compensation plan of such corporation plus (C) the cash proceeds of “key man” life insurance policies received by Holdings or its Restricted Subsidiaries after the Issue Date (provided that Holdings may elect to apply all or any portion of the aggregate increase contemplated by clauses (A), (B) and (C) above in any calendar year) less (D) the amount of any Restricted Payments previously made pursuant to clauses (A), (B) and (C) of this clause (4);
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of Holdings or any Restricted Subsidiary issued or incurred in accordance with this covenant to the extent such dividends are included in the definition of Fixed Charges for such entity;
(6) the declaration and payment of dividends or distributions to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued after the Issue Date
56
and the declaration and payment of dividends to any direct or indirect parent corporation of Holdings the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) of any direct or indirect parent corporation of Holdings issued after the Issue Date; provided, however, that (A) for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock, after giving effect to such issuance (and the payment of dividends or distributions thereon) on apro forma basis, Holdings would have had a Fixed Charge Coverage Ratio of at least 2.0 to 1 and (B) the aggregate amount of dividends declared and paid pursuant to this clause (6) does not exceed the net cash proceeds actually received by Holdings from any such sale of Designated Preferred Stock (other than Disqualified Stock) issued after the Issue Date;
(7) Intentionally omitted;
(8) repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the payment of dividends on Holdings’ common stock following the first public offering of Holdings’ common stock or the common stock of any of its direct or indirect parent corporations after the Issue Date, of up to 6.0% per annum of the net cash proceeds received by or contributed to Holdings after the Issue Date in any such public offering, other than public offerings with respect to Holdings’ common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Investments that are made with Excluded Contributions;
(11) other Restricted Payments in an aggregate amount not to exceed $50.0 million;
(12) cash dividends or other distributions on Holdings’ or any Restricted Subsidiary’s Capital Stock used to, or the making of loans, the proceeds of which will be used to, fund the payment of fees and expenses incurred in connection with the Transactions or the IPO, in each case to the extent permitted (to the extent applicable) by the covenant described under “—Transactions with Affiliates;”
(13) distributions or payments of Securitization Fees and purchases of Securitization Assets pursuant to a Securitization Repurchase Obligation in connection with a Qualified Securitization Financing;
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to provisions similar to those described under the captions “—Repurchase at the Option of Holders—Change of Control” and “—Repurchase at the Option of Holders—Asset Sales;” provided that a Change of Control Offer or Asset Sale Offer, as applicable, has been made and all notes tendered by holders of the notes in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed or acquired for value;
(15) Intentionally omitted;
(16) the payment of the consideration to shareholders and holders of options and warrants of Warner Chilcott PLC in connection with the scheme of arrangement constituting part of the Transactions;
(17) the declaration and payment of dividends to, or the making of loans to, a direct or indirect parent corporation of Holdings in amounts required for such Person to pay, without duplication:
(A) franchise taxes and other fees, taxes and expenses required to maintain its corporate existence;
57
(B) income taxes to the extent such income taxes are attributable to the income of Holdings and the Restricted Subsidiaries and, to the extent of the amount actually received from the Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of the Unrestricted Subsidiaries, provided, however, that in each case the amount of such payments in any fiscal year does not exceed the amount of income taxes that Holdings and the Restricted Subsidiaries would be required to pay for such fiscal year were Holdings and the Restricted Subsidiaries to pay such taxes as a stand-alone taxpayer;
(C) customary salary, bonus, severance and other benefits payable to officers and employees of such direct or indirect parent corporation of Holdings to the extent such salaries, bonuses, severance and other benefits are attributable to the ownership or operation of Holdings and its Restricted Subsidiaries;
(D) general corporate overhead expenses for such direct or indirect parent corporation of Holdings to the extent such expenses are attributable to the ownership or operation of Holdings and its Restricted Subsidiaries; and
(E) reasonable fees and expenses incurred in connection with any unsuccessful debt or equity offering by such direct or indirect parent corporation of Holdings;
(18) cash payments in lieu of the issuance of fractional shares in connection with the exercise of warrants, options or other securities convertible into or exchangeable for Capital Stock of Holdings; provided, however, that any such cash payment shall not be for the purpose of evading the limitation of the covenant described under this subheading (as determined in good faith by the Board of Directors of Holdings); or
(19) the payment of dividends and other distributions to any direct or indirect parent corporation of Holdings in an amount equal to any reduction in taxes actually realized by Holdings and its Restricted Subsidiaries in the form of refunds or from deductions when applied to offset income or gain as a direct result of (i) transaction fees, (ii) commitment and other financing fees or (iii) severance, change in control and other compensation expense incurred in connection with the repurchase or rollover of stock options or transaction bonuses, in each case in connection with the Transactions;
provided, however, that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (2), (5), (6), (9), (11), (13), (14) and (19) above, no Default or Event of Default shall have occurred and be continuing or would occur as a consequence thereof.
The amount of all Restricted Payments (other than cash) will be the fair market value on the date of the Restricted Payment of the asset(s) or securities proposed to be transferred or issued by Holdings or such Subsidiary, as the case may be, pursuant to the Restricted Payment. The fair market value of any assets or securities that are required to be valued by this covenant will be determined in good faith by the Board of Directors of Holdings. Such determination must be based upon an opinion or appraisal issued by an Independent Financial Advisor if the fair market value exceeds $30.0 million.
As of the Issue Date, all of Holdings’ Subsidiaries will be Restricted Subsidiaries. Holdings will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the second to last sentence of the definition of Unrestricted Subsidiary. For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding investments by Holdings and the Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the second paragraph of the definition of Investments. Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time under this covenant or the definition of Permitted Investments and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries will not be subject to any of the restrictive covenants described in this summary.
58
Incurrence of Indebtedness and Issuance of Preferred Stock
Holdings will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise, with respect to (collectively “incur”) any Indebtedness (including Acquired Debt) and will not permit any of its Restricted Subsidiaries (other than the Company) to issue any shares of Preferred Stock; provided, however, that the Company, Holdings and any Restricted Subsidiary that is a Guarantor may incur Indebtedness (including Acquired Debt) and any Restricted Subsidiary that is a Guarantor may issue Preferred Stock if the Fixed Charge Coverage Ratio of Holdings for its most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Preferred Stock is issued would have been at least 2.0 to 1 determined on apro forma basis (including apro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred or the Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The first paragraph of this covenant will not prohibit the incurrence of any of the following (collectively, “Permitted Debt”):
(1) the incurrence by the Company or a Guarantor of Indebtedness under the Credit Agreement together with the incurrence by Holdings or any Restricted Subsidiary of the guarantees thereunder and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), up to an aggregate principal amount, of $1,790 million outstanding at any one time, less the amount of all mandatory principal payments (with respect to revolving borrowings and letters of credit, only to the extent revolving commitments are correspondingly reduced) actually made by the borrower thereunder in respect of Indebtedness thereunder with Net Proceeds from Asset Sales;
(2) the incurrence by the Company and the Guarantors of Indebtedness represented by the notes (including any Guarantee thereof) issued on the Issue Date and the incurrence by the Company and the Guarantors of Indebtedness represented by the Exchange notes issued in exchange for the notes issued on the Issue Date (including any Guarantee thereof);
(3) Existing Indebtedness (other than Indebtedness described in clauses (1) or (2));
(4) Indebtedness (including Capitalized Lease Obligations) incurred by Holdings or any Restricted Subsidiary to finance the purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Permitted Business (whether through the direct purchase of assets or the Capital Stock of any Person owning such assets) in an aggregate principal amount that, when aggregated with the principal amount of all other Indebtedness then outstanding and incurred pursuant to this clause (4), does not exceed the greater of (x) $25.0 million and (y) $25.0 million plus or minus, as applicable, an amount equal to 2% of Consolidated Net Income of Holdings for the period (taken as one accounting period) from January 1, 2005 to the end of Holding’s fiscal quarter most recently ended prior to the date on which such Indebtedness is incurred;
(5) Indebtedness incurred by Holdings or any Restricted Subsidiary constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including without limitation letters of credit in respect of workers’ compensation claims, health, disability or other employee benefits or property, casualty or liability insurance or self-insurance or other Indebtedness with respect to reimbursement-type obligations regarding workers’ compensation claims; provided, however, that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
59
(6) Indebtedness arising from agreements of Holdings or a Restricted Subsidiary providing for indemnification, adjustment of purchase price or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided, however, that (A) such Indebtedness is not reflected on the balance sheet of Holdings or any Restricted Subsidiary (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (A)) and (B) the maximum assumable liability in respect of all such Indebtedness shall at no time exceed the gross proceeds including noncash proceeds (the fair market value of such noncash proceeds being measured at the time received and without giving effect to any subsequent changes in value) actually received by Holdings and any Restricted Subsidiaries in connection with such disposition;
(7) Indebtedness of Holdings owed to and held by any Restricted Subsidiary or Indebtedness of a Restricted Subsidiary owed to and held by Holdings or any other Restricted Subsidiary; provided, however, that (A) any subsequent issuance or transfer of any Capital Stock or any other event that results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any subsequent transfer of any such Indebtedness (except to Holdings or a Restricted Subsidiary) shall be deemed, in each case, to constitute the incurrence of such Indebtedness by the issuer thereof and (B) if the Company or a Guarantor is the obligor on such Indebtedness, such Indebtedness is expressly subordinated in right of payment to all obligations of the Company or such Guarantor with respect to the notes;
(8) shares of Preferred Stock of a Restricted Subsidiary issued to Holdings or a Restricted Subsidiary; provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to Holdings or a Restricted Subsidiary) shall be deemed in each case to be an issuance of such shares of Preferred Stock;
(9) Hedging Obligations of Holdings or any Restricted Subsidiary (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting (A) interest rate risk with respect to any Indebtedness that is permitted by the terms of the Indenture to be outstanding or (B) exchange rate risk with respect to any currency exchange;
(10) obligations in respect of performance and surety bonds and performance and completion guarantees provided by Holdings or any Restricted Subsidiary or obligations in respect of letters of credit related thereto, in each case in the ordinary course of business or consistent with past practice;
(11) Indebtedness of the Company or any Guarantor or Preferred Stock of any Restricted Subsidiary that is a Guarantor not otherwise permitted hereunder in an aggregate principal amount or liquidation preference which, when aggregated with the principal amount and liquidation preference of all other Indebtedness and Preferred Stock then outstanding and incurred pursuant to this clause (11), does not at any one time outstanding exceed $125.0 million;
(12) (x) any guarantee by the Company or a Guarantor of Indebtedness or other obligations of any Restricted Subsidiary (other than the Company) so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Indenture;provided that if such Indebtedness is by its express terms subordinated in right of payment to the notes or the Guarantee of such Restricted Subsidiary or Holdings, as applicable, any such guarantee of such Guarantor with respect to such Indebtedness shall be subordinated in right of payment to such Guarantor’s Guarantee with respect to the notes substantially to the same extent as such Indebtedness is subordinated to the notes or the Guarantee of such
60
Restricted Subsidiary or Holdings, as applicable, (y) any guarantee by a Restricted Subsidiary that is not a Guarantor of Indebtedness of another Restricted Subsidiary that is not a Guarantor incurred in accordance with the terms of the Indenture, and (z) any guarantee by a Guarantor of Indebtedness of the Company incurred in accordance with the terms of the Indenture;
(13) the incurrence by Holdings or any Restricted Subsidiary of Indebtedness or Preferred Stock that serves to refund or refinance any Indebtedness incurred as permitted under the first paragraph of this covenant and clauses (2), (3) and (4) above, this clause (13) and clauses (14) and (21) below or any Indebtedness issued to so refund or refinance such Indebtedness including additional Indebtedness incurred to pay premiums and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided, however, that such Refinancing Indebtedness (A) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of the Indebtedness being refunded or refinanced, (B) to the extent such Refinancing Indebtedness refinances Indebtedness subordinated orpari passu to the notes or the Guarantees, such Refinancing Indebtedness is subordinated orpari passu to the notes or the Guarantees at least to the same extent as the Indebtedness being refinanced or refunded, (C) shall not include (x) Indebtedness or Preferred Stock of a Subsidiary that is not a Guarantor that refinances Indebtedness or Preferred Stock of the Company or a Guarantor or (y) Indebtedness or Preferred Stock of Holdings or a Restricted Subsidiary that refinances Indebtedness or Preferred Stock of an Unrestricted Subsidiary, (D) shall not be in a principal amount in excess of the principal amount of, premium, if any, accrued interest on, and related fees and expenses of, the Indebtedness being refunded or refinanced and (E) shall not have a stated maturity date prior to the Stated Maturity of the Indebtedness being refunded or refinanced; and provided further, that subclauses (A), (B) and (E) of this clause (13) will not apply to any refunding or refinancing of any Senior Debt;
(14) Indebtedness or Preferred Stock of a Person incurred and outstanding on or prior to the date on which such Person was acquired by Holdings or any Restricted Subsidiary or merged into Holdings or a Restricted Subsidiary in accordance with the terms of the Indenture; provided that such Indebtedness or Preferred Stock is not incurred in connection with or in contemplation of, or to provide all or any portion of the funds or credit support utilized to consummate, such acquisition or merger; and provided further, that after giving effect to such incurrence of Indebtedness either (A) Holdings would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of this covenant or (B) such Fixed Charge Coverage Ratio would be greater than immediately prior to such acquisition;
(15) Indebtedness arising from the honoring by a bank or financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business; provided that such Indebtedness is extinguished within five business days of its incurrence;
(16) Indebtedness of Holdings or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Agreement in a principal amount not in excess of the stated amount of such letter of credit;
(17) Indebtedness incurred by a Securitization Subsidiary in a Qualified Securitization Financing that is not recourse to Holdings or any of its Restricted Subsidiaries, other than a Securitization Subsidiary (except for Standard Securitization Undertakings);
(18) Indebtedness incurred by a Foreign Subsidiary, provided, however, that the aggregate principal amount of Indebtedness incurred under this clause (18) which, when aggregated with the principal amount of all other Indebtedness then outstanding and incurred pursuant to this clause (18), does not exceed the greater of (x) $20.0 million and (y) $20.0 million plus or minus, as applicable, an amount equal to 2% of Consolidated Net Income of the Foreign Subsidiaries of
61
Holdings for the period (taken as one accounting period) from January 1, 2005 to the end of Holdings’ fiscal quarter most recently ended prior to the date on which such Indebtedness is incurred;
(19) Indebtedness consisting of promissory notes issued by the Company or any Guarantor to current or former officers, directors and employees, their respective estates, spouses or former spouses to finance the purchase or redemption of Equity Interests of Holdings or any of its direct or indirect parent corporations permitted by the covenant described under the caption “—Restricted Payments;”
(20) Contribution Indebtedness;
(21) Indebtedness of the Company or a Guarantor incurred in connection with or in contemplation of, or to provide all or any portion of the funds or credit support utilized to consummate, the acquisition by the Company or such Guarantor of property used or useful in a Permitted Business (including a Product) (whether through the direct purchase of assets or the purchase of Capital Stock of, or merger or consolidation with, any Person owning such assets); provided that the Fixed Charge Coverage Ratio of Holdings for its most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date on which such Indebtedness is incurred, determined on apro forma basis as if such Indebtedness had been incurred and the application of proceeds therefrom had occurred at the beginning of such four-quarter period, (A) would have been at least 1.75 to 1 and (B) would have been greater than such Fixed Charge Coverage Ratio immediately prior to such acquisition or merger; and
(22) Non-Recourse Product Financing Indebtedness; provided, however that the aggregate principal amount of any such Indebtedness, when taken together with all other Indebtedness of Holdings or any Restricted Subsidiary incurred pursuant to this clause (22) and then outstanding, does not exceed $100.0 million.
For purposes of determining compliance with this “—Incurrence of Indebtedness and Issuance of Preferred Stock” covenant, in the event that an item of proposed Indebtedness meets the criteria of more than one of the categories of Permitted Debt described in clauses (1) through (22) above, or is entitled to be incurred pursuant to the first paragraph of this covenant, Holdings will be permitted to classify and later reclassify such item of Indebtedness in any manner that complies with this covenant, and such item of Indebtedness will be treated as having been incurred pursuant to only one of such categories. Accrual of interest, the accretion of accreted value and the payment of interest in the form of additional Indebtedness will not be deemed to be an incurrence of Indebtedness for purposes of this covenant. Notwithstanding the foregoing, Indebtedness under the Credit Agreement outstanding on the Issue Date will be deemed to have been incurred on such date in reliance on the exception provided by clause (1) of the definition of Permitted Debt and Holdings shall not be permitted to reclassify all or any portion of such Indebtedness.
For purposes of determining compliance with any U.S. dollar restriction on the incurrence of Indebtedness where the Indebtedness incurred is denominated in a different currency, the amount of such Indebtedness will be the U.S. Dollar Equivalent determined on the date of the incurrence of such Indebtedness; provided, however, that if any such Indebtedness denominated in a different currency is subject to a currency agreement with respect to U.S. dollars covering all principal, premium, if any, and interest payable on such Indebtedness, the amount of such Indebtedness expressed in U.S. dollars will be as provided in such currency agreement. The principal amount of any refinancing Indebtedness incurred in the same currency as the Indebtedness being refinanced will be the U.S. Dollar Equivalent of the Indebtedness being refinanced, except to the extent that (1) such U.S. Dollar Equivalent was determined based on a currency agreement, in which case the refinancing Indebtedness will be determined in accordance with the preceding sentence, and (2) the principal amount of the refinancing
62
Indebtedness exceeds the principal amount of the Indebtedness being refinanced, in which case the U.S. Dollar Equivalent of such excess will be determined on the date such refinancing Indebtedness is incurred. The maximum amount of Indebtedness that Holdings and its Restricted Subsidiaries may incur pursuant to this covenant shall not be deemed to be exceeded, with respect to any outstanding Indebtedness, solely as a result of fluctuations in the exchange rate of currencies.
Limitation on Layering
The Indenture governing the notes provides that Holdings will not, and will not permit the Company or any Restricted Subsidiary that is a Guarantor to, directly or indirectly, incur any Indebtedness that is or purports to be by its terms (or by the terms of any agreement governing such Indebtedness) contractually subordinated or junior in right of payment to any Senior Debt (including Acquired Debt) or Guarantor Senior Debt (including Acquired Debt) of Holdings or such other Guarantor, as the case may be, unless such Indebtedness is either:
(1) Senior Subordinated Indebtedness; or
(2) subordinate in right of payment to the notes or the Guarantees, as the case may be.
Liens
Holdings will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, assume or suffer to exist any Lien that secures obligations under any Indebtedness rankingpari passu with or subordinated to the notes or a related Guarantee on any asset or property of Holdings or any Restricted Subsidiary, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Indebtedness subordinated to the notes or the Guarantees, the notes and any related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; or
(2) in all other cases, the notes and any related Guarantees are equally and ratably secured,
except that the foregoing shall not apply to:
(i) Liens existing on the Issue Date to the extent and in the manner such Liens are in effect on the Issue Date;
(ii) Liens securing the notes and the related Guarantees and the Exchange Notes (including Exchange Notes issued in exchange for Additional Notes issued and secured by a Lien in each case in accordance with the terms of the Indenture) and the related Guarantees;
(iii) Liens securing Senior Debt or Guarantor Senior Debt and the related guarantees of such Senior Debt or Guarantor Senior Debt; and
(iv) Permitted Liens.
Dividend and Other Payment Restrictions Affecting Subsidiaries
Holdings will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create or permit to exist or become effective any consensual encumbrance or restriction on the ability of any such Restricted Subsidiary to:
(1) pay dividends or make any other distributions on its Capital Stock to Holdings or any of its Restricted Subsidiaries, or with respect to any other interest or participation in, or measured by, its profits, or pay any Indebtedness owed to Holdings or any of its Restricted Subsidiaries;
63
(2) make loans or advances to Holdings or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to Holdings or any of its Restricted Subsidiaries.
However, the preceding restrictions will not apply to encumbrances or restrictions existing under or by reason of:
(1) contractual encumbrances or restrictions in effect (x) pursuant to the Credit Agreement or related documents as in effect on the Issue Date or (y) on the Issue Date, including, without limitation, pursuant to Existing Indebtedness and related documentation;
(2) the Indenture, the notes and the Guarantees (including any Exchange Notes with respect to the notes and related Guarantees);
(3) purchase money obligations or other obligations described in clause (4) of the second paragraph of “—Incurrence of Indebtedness and Issuance of Preferred Stock” for property acquired in the ordinary course of business that in each case impose restrictions of the nature discussed in clause (3) above in the first paragraph of this covenant on the property so acquired;
(4) applicable law or any applicable rule, regulation or order;
(5) any agreement or other instrument of a Person acquired by Holdings or any Restricted Subsidiary in existence at the time of such acquisition (but not created in connection therewith or in contemplation thereof or to provide all or a portion of the funds or credit support utilized to consummate such acquisition), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person, or the property or assets of the Person, so acquired;
(6) contracts for the sale of assets, including without limitation, customary restrictions with respect to a Subsidiary pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(7) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under the captions “—Incurrence of Indebtedness and Issuance of Preferred Stock” and “—Liens” that limits the right of the debtor to dispose of the assets securing such Indebtedness;
(8) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(9) other Indebtedness or Preferred Stock (i) of Holdings, the Company or any Restricted Subsidiary that is a Guarantor, in each case that is incurred subsequent to the Issue Date pursuant to the covenant described under “—Incurrence of Indebtedness and Issuance of Preferred Stock” or (ii) that is incurred by a Foreign Subsidiary of Holdings subsequent to the Issue Date pursuant to clauses (1), (4) or (16) of the second paragraph of the covenant described under “—Incurrence of Indebtedness and Issuance of Preferred Stock”;
(10) customary provisions in joint venture agreements and other similar agreements entered into in the ordinary course of business;
(11) customary provisions contained in leases, subleases, licenses or asset sale agreements and other agreements; and
(12) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) of the first paragraph above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (1) through (11) above; provided that the encumbrances or restrictions imposed by such amendments, modifications, restatements, renewals, increases,
64
supplements, refundings, replacements or refinancings are, in the good faith judgment of Holdings’ Board of Directors, not materially less favorable to the holders of the notes than encumbrances and restrictions contained in such predecessor agreements and do not affect the Company’s and Guarantor’s ability, taken as a whole, to make payments of interest and scheduled payments of principal in respect of the notes, in each case as and when due; provided further, however, that with respect to agreements existing on the Issue Date, any refinancings or amendments thereof contain such encumbrances or restrictions that are not materially less favorable to the holders of the notes than the encumbrances or restrictions contained in such agreements as in effect on the Issue Date.
Merger, Consolidation or Sale of Assets
The Company or Holdings may not, directly or indirectly: (1) consolidate or merge with or into another Person (whether or not the Company or Holdings, as applicable, is the surviving corporation); or (2) sell, assign, transfer, convey, lease or otherwise dispose of all or substantially all of the properties or assets of the Company and its Subsidiaries or Holdings and its Subsidiaries, as applicable, taken as a whole, in one or more related transactions, to another Person; unless:
(1) either: (a) the Company or Holdings, as applicable, is the surviving corporation; or (b) the Person formed by or surviving any such consolidation or merger (if other than the Company or Holdings, as applicable) or to which such sale, assignment, transfer, conveyance, lease or other disposition has been made is, in the case of the Company, a corporation organized or existing under the laws of the United States, any state of the United States or the District of Columbia or, in the case of Holdings, a corporation organized or existing under the laws of the United States, any state of the United States or the District of Columbia or Bermuda or the Grand Duchy of Luxembourg (the Company, Holdings or such Person, including the Person to which such sale, assignment, transfer, conveyance, lease or other disposition has been made, as the case may be, being herein called the “Successor Company”);
(2) the Successor Company (if other than the Company or Holdings, as applicable) assumes all the obligations of the Company or Holdings, as applicable, under the notes, the Indenture and the Registration Rights Agreement pursuant to agreements reasonably satisfactory to the Trustee;
(3) immediately after such transaction, no Default or Event of Default exists; and
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if the same had occurred at the beginning of the applicable four-quarter period, either (a) the Successor Company would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described under “—Incurrence of Indebtedness and Issuance of Preferred Stock” or (b) the Fixed Charge Coverage Ratio for the Successor Company and its Restricted Subsidiaries would be greater than such ratio for Holdings and its Restricted Subsidiaries immediately prior to such transaction.
The Indenture also provides for similar provisions relating to any consolidation, merger or sale, assignment, transfer, conveyance, lease or other disposition of all or substantially all of the properties or assets of a Guarantor (other than Warner Chilcott Limited and Holdings), excluding clause (4) above. See “—Guarantees.”
For purposes of this covenant, the sale, lease, conveyance, assignment, transfer or other disposition of all or substantially all of the properties and assets of one or more Restricted Subsidiaries of Holdings, including the Company, which properties and assets, if held by Holdings instead of such Restricted Subsidiaries, would constitute all or substantially all of the properties and assets of Holdings
65
on a consolidated basis, shall be deemed to be the sale, lease, conveyance, assignment, transfer or other disposition of all or substantially all of the properties and assets of Holdings.
The Predecessor will be released from its obligations under the Indenture and the Successor Company will succeed to, and be substituted for, and may exercise every right and power of, the Company or Holdings, as the case may be, under the Indenture, but, in the case of a lease of all or substantially all its assets, the Predecessor will not be released from the obligation to pay the principal of and interest on the notes.
Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve “all or substantially all” of the property or assets of a Person.
This “—Merger, Consolidation or Sale of Assets” covenant will not apply to a sale, assignment, transfer, conveyance, lease or other disposition of assets between or among Holdings and its Restricted Subsidiaries. Notwithstanding the foregoing, clauses (3) and (4) will not be applicable to (a) any Restricted Subsidiary consolidating with, merging into or selling, assigning, transferring, conveying, leasing or otherwise disposing of all or part of its properties and assets to Holdings or to another Restricted Subsidiary and (b) Holdings or the Company merging with an Affiliate solely for the purpose and with the sole effect of reincorporating the Company or Holdings, as the case may be, in another jurisdiction so long as the amount of Indebtedness of Holdings and its Restricted Subsidiaries is not increased thereby.
Transactions with Affiliates
Holdings will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, assign, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate (each, an “Affiliate Transaction”) involving aggregate consideration in excess of $5.0 million, unless:
(1) the Affiliate Transaction is on terms that are no less favorable to Holdings or the relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by Holdings or such Restricted Subsidiary with an unrelated Person; and
(2) (a) with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate consideration in excess of $15.0 million, a majority of the disinterested members of the Board of Directors of Holdings have determined in good faith that the criteria set forth in the immediately preceding clause (1) are satisfied and have approved the relevant Affiliate Transaction as evidenced by a resolution of the Board of Directors of Holdings; and
(b) with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate consideration in excess of $75.0 million, the Board of Directors of Holdings shall also have received a written opinion as to the fairness to Holdings and its Restricted Subsidiaries of such Affiliate Transaction from a financial point of view issued by an Independent Financial Advisor.
The following items will not be deemed to be Affiliate Transactions and, therefore, will not be subject to the provisions of the prior paragraph:
(1) any transaction with Holdings, a Restricted Subsidiary or joint venture or similar entity which would constitute an Affiliate Transaction solely because Holdings or a Restricted Subsidiary owns an equity interest in or otherwise controls such Restricted Subsidiary, joint venture or similar entity;
66
(2) Restricted Payments and Permitted Investments (other than pursuant to clauses (3) and (10) of the definition thereof) permitted by the Indenture;
(3) the payment to the Sponsors, any of their Affiliates, and officers of Holdings or any of its Restricted Subsidiaries, of management, consulting, monitoring and advisory fees, termination payments and related reasonable expenses pursuant to (A) the Advisory Services and Monitoring Agreement or any amendment thereto (so long as any such amendment is not less advantageous to the holders of the notes in any material respect than the Advisory Services and Monitoring Agreement) or (B) other agreements as in effect on the Issue Date that are (x) entered into in connection with the Transactions and (y) as described in this prospectus or any amendment thereto (so long as any such amendment is not less advantageous to the holders of the notes in any material respect than the original agreement as in effect on the Issue Date);
(4) the payment of reasonable and customary compensation and fees to, and indemnities provided on behalf of (and entering into related agreements with) officers, directors, employees or consultants of Holdings, any of its direct or indirect parent corporations, or any Restricted Subsidiary, as determined in good faith by the Board of Directors of Holdings or senior management thereof;
(5) payments made by Holdings or any Restricted Subsidiary to the Sponsors and any of their Affiliates for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures, which payments are approved by a majority of the disinterested members of the Board of Directors of Holdings in good faith;
(6) transactions in which Holdings or any Restricted Subsidiary delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to Holdings or such Restricted Subsidiary from a financial point of view;
(7) payments or loans (or cancellations of loans) to employees or consultants of Holdings or any of its direct or indirect parent corporations or any Restricted Subsidiary which are approved by the Board of Directors of Holdings and which are otherwise permitted under the Indenture, but in any event not to exceed $10.0 million in the aggregate outstanding at any one time;
(8) payments made or performance under any agreement as in effect on the Issue Date or described in the prospectus (other than the Advisory Services and Monitoring Agreement and Shareholders Agreement, but including, without limitation, each of the other agreements entered into in connection with the Transactions);
(9) the existence of, or the performance by Holdings or any of its Restricted Subsidiaries of its obligations under the terms of, the Shareholders Agreement (including any registration rights agreement or purchase agreements related thereto to which it is a party on the Issue Date and any similar agreement that it may enter into thereafter); provided, however, that the existence of, or the performance by Holdings or any of its Restricted Subsidiaries of its obligations under, any future amendment to the Shareholders Agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (9) to the extent that the terms of any such existing agreement together with all amendments thereto, taken as a whole, or new agreement are not otherwise more disadvantageous to holders of the notes in any material respect than the original agreement as in effect on the Issue Date;
(10) the Transactions and the payment of all transaction, underwriting, commitment and other fees and expenses incurred in connection with the Transactions;
(11) transactions with customers, clients, suppliers, or purchasers or sellers of goods or services, in each case in the ordinary course of business and otherwise in compliance with the terms of the Indenture that are fair to Holdings or its Restricted Subsidiaries, in the reasonable determination of the members of the Board of Directors of Holdings or the senior management
67
thereof, or are on terms at least as favorable as would reasonably have been entered into at such time with an unaffiliated party; and
(12) if otherwise permitted hereunder, the issuance of Equity Interests (other than Disqualified Stock) of Holdings to any Permitted Holder, any director, officer, employee or consultant of Holdings or its Subsidiaries or any other Affiliates of Holdings (other than a Subsidiary).
Business Activities
Holdings will not, and will not permit any Restricted Subsidiary to, engage in any business other than Permitted Businesses, except to such extent as would not be material to Holdings and its Subsidiaries taken as a whole.
Payments for Consent
Holdings will not, and will not permit any of its Subsidiaries to, directly or indirectly, pay or cause to be paid any consideration to or for the benefit of any holder of notes for or as an inducement to any consent, waiver or amendment of any of the terms or provisions of the Indenture or the notes unless such consideration is offered to be paid and is paid to all holders of the notes that so consent, waive or agree to amend in the time frame set forth in the solicitation documents relating to such consent, waiver or agreement.
Additional Guarantees
After the Issue Date, Holdings will cause (i) each of its Domestic Subsidiaries (other than the Company or any Unrestricted Subsidiary) that incurs any Indebtedness (other than Indebtedness permitted to be incurred pursuant to clause (7), (9), (10) or (15) of the second paragraph of the covenant described under “—Incurrence of Indebtedness and Issuance of Preferred Stock”) and (ii) each Restricted Subsidiary (other than the Company) that guarantees any Indebtedness of the Company or any of the Guarantors (other than a Foreign Subsidiary that guarantees Indebtedness of another Foreign Subsidiary, Holdings or any Restricted Subsidiary that is incorporated in Puerto Rico), in each case, at the same time, to execute and deliver to the Trustee a Guarantee pursuant to which such Restricted Subsidiary will unconditionally Guarantee, on a joint and several basis, the full and prompt payment of the principal of, premium, if any and interest on the notes and all other obligations under the Indenture on the same terms and conditions as those set forth in the Indenture.
Each Guarantee will be limited to an amount not to exceed the maximum amount that can be guaranteed by that Restricted Subsidiary without rendering the Guarantee, as it relates to such Restricted Subsidiary, voidable under applicable law relating to fraudulent conveyance or fraudulent transfer or similar laws affecting the rights of creditors generally.
Each Guarantee shall be released in accordance with the provisions of the Indenture described under “—Guarantees.”
Reports
Whether or not required by the Commission, so long as any notes are outstanding, if not filed electronically with the Commission through the Commission’s Electronic Data Gathering, Analysis, and Retrieval System (or any successor system), Holdings will furnish to the holders of notes, within the time periods specified in the Commission’s rules and regulations:
(1) all quarterly and annual financial information that would be required to be contained in a filing with the Commission on Forms 10-Q and 10-K (or Form 20-F if Holdings is a “foreign private
68
issuer” as such term is defined under the rules and regulations of the Commission), other than the Quarterly Report on Form 10-Q for the fiscal quarter ended December 31, 2004, if Holdings were required to file such Forms, including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and, with respect to the annual information only, a report on the annual financial statements by Holdings’ certified independent accountants; and
(2) all current reports that would be required to be filed with the Commission on Form 8-K if Holdings were required to file such reports.
In addition, whether or not required by the Commission, after the consummation of the Exchange Offer or the effectiveness of the Shelf Registration Statement, Holdings will file a copy of all of the information and reports referred to in clauses (1) and (2) above with the Commission for public availability within the time periods specified in the Commission’s rules and regulations (unless the Commission will not accept such a filing) and make such information available to securities analysts and prospective investors upon request. In addition, Holdings has agreed that, for so long as any notes remain outstanding, it will furnish to the holders of the notes and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
Notwithstanding the foregoing, Holdings’ obligations under “—Reports” shall be deemed satisfied (i) with respect to the Form 10-K or 20-F, as applicable, for fiscal year 2004 (or, if the Issuer changes its fiscal year end to December 31, for the period ended December 31, 2004) prior to the commencement of the Registered Exchange Offer or the effectiveness of the Shelf Registration Statement by the filing with the Commission of the Exchange Offer Registration Statement or Shelf Registration Statement, and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act with respect to fiscal year 2004 (or, if the Issuer changes its fiscal year end to December 31, for the period ended December 31, 2004) within the time periods and in accordance with the provisions of the Registration Rights Agreement or (ii) for so long as Warner Chilcott Limited is a Guarantor, has no material assets, liabilities or operations other than its ownership of Holdings and has a reporting obligation under Section 13 or Section 15(d) of the Exchange Act, if Warner Chilcott Limited files with the Commission the reports set forth above and includes in a note to its financial statements included in such reports the condensed consolidating financial information specified by Rule 3-10 of Regulation S-X.
Events of Default and Remedies
Under the Indenture, an Event of Default is defined as any of the following:
(1) the Company defaults in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the notes, whether or not such payment is prohibited by the subordination provisions of the Indenture;
(2) the Company defaults in the payment when due of interest or Additional Interest, if any, on or with respect to the notes and such default continues for a period of 30 days, whether or not such payment is prohibited by the subordination provisions of the Indenture;
(3) Holdings or the Company defaults in the performance of, or breaches any covenant, warranty or other agreement contained in, the Indenture (other than a default in the performance or breach of a covenant, warranty or agreement which is specifically dealt with in clauses (1) or (2) above) and such default or breach continues for a period of 60 days after the notice specified below;
(4) a default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by Holdings or any Restricted Subsidiary or the payment of which is guaranteed by Holdings or any Restricted
69
Subsidiary (other than Indebtedness owed to Holdings or a Restricted Subsidiary), whether such Indebtedness or guarantee now exists or is created after the Issue Date, if (A) such default either (1) results from the failure to pay any such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or (2) relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity and (B) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $40.0 million (or its foreign currency equivalent) or more at any one time outstanding;
(5) certain events of bankruptcy affecting the Company, Holdings or any Significant Subsidiary;
(6) the failure by the Company, Holdings or any Significant Subsidiary to pay final judgments aggregating in excess of $40.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after the applicable judgment becomes final, and, with respect to any such judgments covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed; or
(7) the Guarantee of a Significant Subsidiary or any group of Subsidiaries that, taken together as of the date of the most recent audited financial statements of Holdings, would constitute a Significant Subsidiary ceases to be in full force and effect (except as contemplated by the terms hereof) or any Guarantor denies or disaffirms its obligations under the Indenture or any Guarantee, other than by reason of the release of the Guarantee in accordance with the terms of the Indenture, and such Default continues for 10 days.
If an Event of Default (other than an Event of Default specified in clause (5) above with respect to the Company) shall occur and be continuing, the Trustee or the holders of at least 25% in principal amount of outstanding notes under the Indenture may declare the principal of and accrued interest on such notes to be due and payable by notice in writing to the Company and the Trustee specifying the respective Event of Default and that it is a “notice of acceleration” (the “Acceleration Notice”), and the same shall become immediately due and payable upon the first to occur of an acceleration under the Credit Agreement and five business days after receipt by the Company and the Representative under the Credit Agreement of such Acceleration Notice but only if such Event of Default is then continuing.
If an Event of Default specified in clause (5) above with respect to the Company occurs and is continuing, then all unpaid principal of, and premium, if any, and accrued and unpaid interest on all of the outstanding notes shallipso facto become and be immediately due and payable without any declaration or other act on the part of each Trustee or any holder of the notes.
The Indenture provides that, at any time after a declaration of acceleration with respect to the notes as described in the two preceding paragraphs, the holders of a majority in principal amount of the notes may rescind and cancel such declaration and its consequences:
(1) if the rescission would not conflict with any judgment or decree;
(2) if all existing Events of Default have been cured or waived except nonpayment of principal or interest that has become due solely because of the acceleration;
(3) to the extent the payment of such interest is lawful, interest on overdue installments of interest and overdue principal, which has become due otherwise than by such declaration of acceleration, has been paid;
(4) if the Company has paid the Trustee its reasonable compensation and reimbursed the Trustee for its expenses, disbursements and advances; and
70
(5) in the event of the cure or waiver of an Event of Default of the type described in clause (5) of the description above of Events of Default, the Trustee shall have received an Officers’ Certificate and an opinion of counsel that such Event of Default has been cured or waived.
No such rescission shall affect any subsequent Default or impair any right consequent thereto.
The holders of a majority in principal amount of the notes issued and then outstanding under the Indenture may waive any existing Default or Event of Default under such Indenture, and its consequences, except a default in the payment of the principal of or interest on such notes.
In the event of any Event of Default specified in clause (4) of the first paragraph above, such Event of Default and all consequences thereof (excluding, however, any resulting payment default) will be annulled, waived and rescinded, automatically and without any action by the Trustee or the holders of the notes, if within 20 days after such Event of Default arose the Company delivers an Officers’ Certificate to the Trustee stating that (x) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged or (y) the holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default or (z) the default that is the basis for such Event of Default has been cured, it being understood that in no event shall an acceleration of the principal amount of the notes as described above be annulled, waived or rescinded upon the happening of any such events.
Holders of the notes may not enforce the Indenture or the notes except as provided in the Indenture and under the Trust Indenture Act of 1939, as amended. Subject to the provisions of the Indenture relating to the duties of the Trustee, the Trustee is under no obligation to exercise any of its rights or powers under the Indenture at the request, order or direction of any of the holders of the notes, unless such holders have offered to the Trustee reasonable indemnity. Subject to all provisions of the Indenture and applicable law, the holders of a majority in aggregate principal amount of the then outstanding notes issued under such Indenture have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or exercising any trust or power conferred on the Trustee.
Holdings is required to deliver to the Trustee annually a statement regarding compliance with the Indenture. Upon becoming aware of any Default or Event of Default, Holdings is required to deliver to the Trustee a statement specifying such Default or Event of Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of Holdings, any of its Subsidiaries or any of its direct or indirect parent corporations, as such, will have any liability for any obligations of the Company or any Guarantor under the notes, the Indenture, the Guarantees, or for any claim based on, in respect of, or by reason of, such obligations or their creation. Each holder of notes by accepting a Note waives and releases all such liability. The waiver and release are part of the consideration for issuance of the notes. The waiver may not be effective to waive liabilities under the federal securities laws, and it is the view of the Commission that such waiver is against public policy.
Governing Law
The Indenture and the notes are governed by, and construed in accordance with, the laws of the State of New York.
71
Legal Defeasance and Covenant Defeasance
The Company may, at its option and at any time, elect to have all of its obligations and the obligations of the Guarantors discharged with respect to the outstanding notes issued under the Indenture (“Legal Defeasance”) except for:
(1) the rights of holders of outstanding notes issued thereunder to receive payments in respect of the principal of, or interest or premium and Additional Interest, if any, on such notes when such payments are due from the trust referred to below;
(2) the Company’s obligations with respect to the notes issued thereunder concerning issuing temporary notes, registration of notes, mutilated, destroyed, lost or stolen notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Company’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Indenture.
In addition, the Company may, at its option and at any time, elect to have its obligations and the obligations of the Guarantors released with respect to certain covenants that are described in the Indenture (“Covenant Defeasance”) and thereafter any omission to comply with those covenants will not constitute a Default or Event of Default with respect to the notes issued thereunder. In the event that a Covenant Defeasance occurs, certain events (not including nonpayment, bankruptcy, receivership, rehabilitation and insolvency events of the Company but including such events with respect to Holdings or any Significant Subsidiary other than the Company) described under “—Events of Default and Remedies” will no longer constitute an Event of Default with respect to the notes issued under the Indenture.
In order to exercise either Legal Defeasance or Covenant Defeasance under the Indenture:
(1) the Company must irrevocably deposit with the Trustee, in trust, for the benefit of the holders of the notes issued thereunder, cash in U.S. dollars, non-callable U.S. Government Securities, or a combination of cash in U.S. dollars and non-callable U.S. Government Securities, in amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, or interest and premium and Additional Interest, if any, on the outstanding notes issued thereunder on the stated maturity or on the applicable redemption date, as the case may be, and the Company must specify whether the notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Company has delivered to the Trustee an opinion of counsel reasonably acceptable to the Trustee confirming that (a) the Company has received from, or there has been published by, the Internal Revenue Service a ruling or (b) since the date of the Indenture, there has been a change in the applicable federal income tax law, in either case to the effect that, and based thereon such opinion of counsel will confirm that, the holders of the outstanding notes will not recognize income, gain or loss for federal income tax purposes as a result of such Legal Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Company has delivered to the Trustee an opinion of counsel reasonably acceptable to the Trustee confirming that the holders of the outstanding notes will not recognize income, gain or loss for federal income tax purposes as a result of such Covenant Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
72
(4) no Default or Event of Default has occurred and is continuing on the date of such deposit (other than a Default or Event of Default resulting from the borrowing of funds to be applied to such deposit and the grant of any Lien securing such borrowings) or insofar as Events of Default resulting from the borrowing of funds or insolvency events are concerned, at any time in the period ending on the 123rd day after the date of deposit;
(5) such Legal Defeasance or Covenant Defeasance will not result in a breach or violation of, or constitute a default under any material agreement or instrument (other than the Indenture) to which the Company or any Guarantor is a party or by which the Company or any Guarantor is bound;
(6) the Company must deliver to the Trustee an opinion of counsel to the effect that: (a) the trust funds will not be subject to any rights of holders of Senior Debt or Guarantor Senior Debt, including, without limitation, those arising under the Indenture; and (b) the trust funds will not be subject to the effect of the preference provisions of Section 547 of the United States Federal Bankruptcy Code;
(7) the Company must deliver to the Trustee an Officers’ Certificate stating that the deposit was not made by the Company with the intent of preferring the holders of notes over the other creditors of the Company or any Guarantor or with the intent of defeating, hindering, delaying or defrauding creditors of the Company or any Guarantor or others; and
(8) the Company must deliver to the Trustee an Officers’ Certificate and an opinion of counsel, each stating that all conditions precedent relating to the Legal Defeasance or the Covenant Defeasance have been complied with.
Amendment, Supplement and Waiver
Except as provided in the next two succeeding paragraphs, the Indenture or the notes issued thereunder may be amended or supplemented with the consent of the holders of at least a majority in principal amount of the notes then outstanding issued thereunder (including, without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, notes), and any existing default or compliance with any provision of the Indenture or the notes issued thereunder may be waived (except a default in respect of the payment of principal or interest on the notes) with the consent of the holders of a majority in principal amount of the then outstanding notes issued thereunder (including, without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, notes).
Without the consent of each holder affected, an amendment or waiver of the Indenture may not (with respect to any notes held by a non-consenting holder):
(1) reduce the principal amount of notes issued thereunder whose holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed maturity of any note or alter the provisions with respect to the redemption of the notes issued thereunder (other than provisions relating to the covenants described above under the caption “—Repurchase at the Option of Holders” except as set forth in item (10) below);
(3) reduce the rate of or change the time for payment of interest on any note issued thereunder;
(4) waive a Default or Event of Default in the payment of principal of, or interest or premium, or Additional Interest, if any, on the notes issued thereunder (except a rescission of acceleration of the notes issued thereunder by the holders of at least a majority in aggregate principal amount of the notes issued thereunder with respect to a nonpayment default and a waiver of the payment default that resulted from such acceleration);
73
(5) make any note payable in money other than that stated in the notes;
(6) make any change in the provisions of the Indenture relating to waivers of past Defaults or the rights of holders of notes to receive payments of principal of, or interest or premium or Additional Interest, if any, on the notes issued thereunder or impair the right of any holder of notes to institute suit for the enforcement of any payment on or with respect to such holder’s notes;
(7) waive a redemption payment with respect to any note issued thereunder (other than a payment required by one of the covenants described above under the caption “—Repurchase at the Option of Holders” except as set forth in item (10) below);
(8) make any change in the ranking or priority of any note that would adversely affect the noteholders;
(9) modify the Guarantees in any manner adverse to the holders of the notes;
(10) amend, change or modify in any material respect the obligation of the Company or Holdings, as applicable, to make and consummate a Change of Control Offer in respect of a Change of Control that has occurred or make and consummate an Asset Sale Offer in respect of an Asset Sale that has been consummated after a requirement to make an Asset Sale Offer has arisen; or
(11) make any change in the preceding amendment and waiver provisions.
Notwithstanding the preceding, without the consent of any holder of notes, the Company, the Guarantors and the Trustee may amend or supplement the Indenture or the notes issued thereunder:
(1) to cure any ambiguity, mistake, defect or inconsistency;
(2) to provide for uncertificated notes in addition to or in place of certificated notes;
(3) to provide for the assumption by a Successor Company or a successor company of a Guarantor, as applicable, of the Company’s or such Guarantor’s obligations under the Indenture;
(4) to make any change that would provide any additional rights or benefits to the holders of notes or that does not adversely affect the legal rights under the Indenture of any such holder;
(5) to secure the notes;
(6) to comply with requirements of the Commission in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act of 1939, as amended;
(7) to add a Guarantee of the notes;
(8) to release a Guarantor upon its sale or designation as an Unrestricted Subsidiary or other permitted release from its Guarantee; provided that such sale, designation or release is in accordance with the applicable provisions of the Indenture.
Satisfaction and Discharge
The Indenture will be discharged and will cease to be of further effect as to all notes issued thereunder, when:
(1) either:
(a) all notes that have been authenticated, except lost, stolen or destroyed notes that have been replaced or paid and notes for whose payment money has been deposited in trust and thereafter repaid to the Company, have been delivered to the Trustee for cancellation; or
74
(b) all notes that have not been delivered to the Trustee for cancellation have become due and payable by reason of the mailing of a notice of redemption or otherwise or will become due and payable by reason of the mailing of a notice of redemption or otherwise within one year and the Company has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the holders of the notes, cash in U.S. dollars, non-callable U.S. Government Securities, or a combination thereof, in amounts as will be sufficient without consideration of any reinvestment of interest, to pay and discharge the entire Indebtedness on the notes not delivered to the Trustee for cancellation for principal, premium and Additional Interest, if any, and accrued interest to the date of maturity or redemption;
(2) no Default or Event of Default has occurred and is continuing on the date of the deposit or will occur as a result of the deposit (other than a Default resulting from borrowing of funds to be applied to such deposit and the grant of any Lien securing such borrowing) and the deposit will not result in a breach or violation of, or constitute a default under, any other material instrument to which the Company is a party or by which the Company is bound;
(3) the Company has paid or caused to be paid all sums payable by it under the Indenture; and
(4) the Company has delivered irrevocable instructions to the Trustee under the Indenture to apply the deposited money toward the payment of the notes issued thereunder at maturity or the redemption date, as the case may be.
In addition, the Company must deliver an Officers’ Certificate and an opinion of counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Notices
All notices to the holders will be valid if published in a leading English language daily newspaper published in New York City or such other English language daily newspaper with general circulation in the United States. Any notice will be deemed to have been given on the date of publication or, if so published more than once on different dates, on the date of first publication. It is expected that publication will normally be made inThe Wall Street Journal. If publication as provided above is not practicable, notice will be given in such other manner, and shall be deemed to have been given on such date, as the Trustee may approve.
Concerning the Trustee
If the Trustee becomes a creditor of the Company, the Indenture limits its right to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee will be permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the Commission for permission to continue to serve as trustee or resign.
The holders of a majority in principal amount of the then outstanding notes issued under the Indenture have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default occurs and is continuing, the Trustee will be required, in the exercise of its power, to use the degree of care of a prudent man in the conduct of his own affairs. Subject to such provisions, the Trustee will be under no obligation to exercise any of its rights or powers under the Indenture at the request of any holder of notes, unless such holder has offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
75
Certain Definitions
Set forth below are certain defined terms used in the Indenture. Reference is made to the Indenture for a more detailed presentation of all such terms, as well as any other capitalized terms used herein for which no definition is provided.
“Acquired Debt” means, with respect to any specified Person:
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, or to provide all or any portion of the funds or credit support utilized in connection with, such other Person merging with or into, or becoming a Restricted Subsidiary of, such specified Person; and
(2) Indebtedness secured by an existing Lien encumbering any asset acquired by such specified Person.
“Advisory Services and Monitoring Agreement” means the Advisory Services and Monitoring Agreement to be dated as of January 18, 2005, by and among Warner Chilcott Holdings Company III, Limited, Warner Chilcott Company, Inc., the Company and Affiliates of each of the Sponsors, as in effect on the Issue Date.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“ApplicablePremium” means, with respect to any Note on any applicable redemption date, the greater of:
(1) 1.0% of the then outstanding principal amount of the Note; and
(2) the excess of:
(a) the present value at such redemption date of (i) the redemption price of the Note at February 1, 2010 (such redemption price being set forth in the table appearing above under the caption “—Optional Redemption”) plus (ii) all required interest payments due on the Note, through February 1, 2010 (excluding accrued but unpaid interest), computed using a discount rate equal to the Treasury Rate as of such redemption date plus 50 basis points; over
(b) the then outstanding principal amount of the Note.
“Asset Sale” means (i) the sale, conveyance, transfer, lease or other disposition (whether in a single transaction or a series of related transactions) of property or assets (including by way of a sale and leaseback) of Holdings or any Restricted Subsidiary (each referred to in this definition as a “disposition”) or (ii) the issuance or sale of Equity Interests of any Restricted Subsidiary (whether in a single transaction or a series of related transactions), in each case, other than:
(1) a disposition of Cash Equivalents or obsolete or worn out property or equipment in the ordinary course of business or inventory (or other assets) held for sale in the ordinary course of business and dispositions of property no longer used or useful in the conduct of the business of Holdings and its Restricted Subsidiaries;
(2) the disposition of all or substantially all of the assets of Holdings in a manner permitted pursuant to the covenant contained under the caption “Certain Covenants—Merger, Consolidation or Sale of Assets” or any disposition that constitutes a Change of Control pursuant to the Indenture;
76
(3) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, pursuant to the covenant contained under the caption “Certain Covenants—Restricted Payments” or the granting of a Lien permitted by the covenant contained under the caption “Certain Covenants—Liens”;
(4) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of transactions with an aggregate fair market value of less than $10.0 million;
(5) any disposition of property or assets or issuance of securities by a Restricted Subsidiary to Holdings or by Holdings or a Restricted Subsidiary to another Restricted Subsidiary;
(6) the lease, assignment, sublease, license or sublicense of any real or personal property in the ordinary course of business;
(7) any sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary (with the exception of Investments in Unrestricted Subsidiaries made pursuant to clause (10) of the definition of “Permitted Investments”);
(8) foreclosures on assets;
(9) disposition of an account receivable in connection with the collection or compromise thereof;
(10) sales of Securitization Assets and related assets of the type specified in the definition of “Securitization Financing” to a Securitization Subsidiary in connection with any Qualified Securitization Financing; and
(11) a transfer of Securitization Assets and related assets of the type specified in the definition of “Securitization Financing” (or a fractional undivided interest therein) by a Securitization Subsidiary in a Qualified Securitization Financing.
“Bank Indebtedness” means all Obligations pursuant to the Credit Agreement.
“Beneficial Owner” has the meaning assigned to such term in Rule 13d-3 and Rule 13d-5 under the Exchange Act, except that in calculating the beneficial ownership of any particular “person” (as that term is used in Section 13(d)(3) of the Exchange Act), such “person” will be deemed to have beneficial ownership of all securities that such “person” has the right to acquire by conversion or exercise of other securities, whether such right is currently exercisable or is exercisable only upon the occurrence of a subsequent condition. The terms “Beneficially Owns”, “Beneficially Owned” and “Beneficial Ownership” have a corresponding meaning.
“Board of Directors” means:
(1) with respect to a corporation, the board of directors of the corporation;
(2) with respect to a partnership, the board of directors of the general partner of the partnership; and
(3) with respect to any other Person, the board or committee of such Person serving a similar function.
“Capital Stock” means:
(1) in the case of a corporation, capital stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of capital stock;
77
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) in accordance with GAAP.
“Cash Contribution Amount” means the aggregate amount of cash contributions made to the capital of the Company, Holdings or any other Guarantor described in the definition of “Contribution Indebtedness.”
“Cash Equivalents” means:
(1) U.S. dollars or, in the case of Holdings or any Foreign Subsidiary, such local currencies held by it from time to time in the ordinary course of business;
(2) securities issued or directly and fully and unconditionally guaranteed or insured by the government or any agency or instrumentality of the United States, the United Kingdom or any member state of the European Union whose legal tender is the euro having maturities of not more than 12 months from the date of acquisition;
(3) certificates of deposit, time deposits and eurodollar time deposits with maturities of 12 months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding 12 months and overnight bank deposits, in each case, with any lender party to the Credit Agreement or with any commercial bank having capital and surplus in excess of $250 million;
(4) repurchase obligations for underlying securities of the types described in clauses (2) and (3) above entered into with any financial institution meeting the qualifications specified in clause (3) above;
(5) commercial paper maturing within 12 months after the date of acquisition and having a rating of at least A-1 from Moody’s or P-1 from S&P;
(6) readily marketable direct obligations issued by any state of the United States or any political subdivision thereof having one of the two highest rating categories obtainable from either Moody’s or S&P with maturities of 12 months or less from the date of acquisition;
(7) instruments equivalent to those referred to in clauses (1) to (6) above denominated in euro or pounds sterling or any other foreign currency comparable in credit quality and tenor to those referred to above and customarily used by corporations for cash management purposes in any jurisdiction outside the United States to the extent reasonably required in connection with any business conducted by any Restricted Subsidiary organized in such jurisdiction; and
(8) investment in funds which invest substantially all of their assets in Cash Equivalents of the kinds described in clauses (1) through (7) of this definition.
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease, transfer or other conveyance, in one or a series of related transactions, of all or substantially all of the assets of the Company and its Subsidiaries, taken as a whole, or Holdings and its Subsidiaries, taken as a whole, to any Person other than to a Permitted Holder;
(2) Holdings becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by any
78
Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than the Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of Beneficial Ownership, directly or indirectly, of 50% or more of the total voting power of the Voting Stock of Holdings or any of its direct or indirect parent entities;
(3) the first day on which the majority of the Board of Directors of either of Holdings or the Company then in office shall cease to consist of individuals who (i) were members of such Board of Directors on the Issue Date or (ii) were either (x) nominated for election by such Board of Directors, a majority of whom were directors on the Issue Date or whose election or nomination for election was previously approved by a majority of such directors or who were designated or appointed pursuant to clause (y) below, or (y) designated or appointed by a Permitted Holder (each of the directors selected pursuant to clauses (i) and (ii),“Continuing Directors”); or
(4) the failure at any time by Holdings to Beneficially Own, directly or indirectly, 100% of the Voting Stock of the Company.
“Code” means the United States Internal Revenue Code of 1986, as amended from time to time, and the regulations promulgated and rulings issued thereunder. Section references to the Code are to the Code, as in effect on the Issue Date, and any subsequent provisions of the Code, amendatory thereof, supplemental thereto or substituted therefor.
“Commission” means the U.S. Securities and Exchange Commission.
“Consolidated Depreciation and Amortization Expense” means, with respect to any Person for any period, the total amount of depreciation and amortization expense, including the amortization of deferred financing fees, and other noncash charges (excluding any noncash item that represents an accrual or reserve for a cash expenditure for a future period) of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, the sum, without duplication, of: (a) consolidated interest expense of such Person and its Restricted Subsidiaries for such period (including amortization of original issue discount, interest payable with respect to Non-Recourse Product Financing Indebtedness, noncash interest payments (other than imputed interest as a result of purchase accounting), commissions, discounts and other fees and charges owed with respect to letters of credit and bankers’ acceptance financing, the interest component of Capitalized Lease Obligations, net payments (if any) pursuant to interest rate Hedging Obligations, but excluding amortization of deferred financing fees or expensing of any bridge or other financing fees, in each case, relating to the Specified Financings) and (b) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued, less (c) interest income actually received in cash for such period; provided, however, that Securitization Fees shall not be deemed to constitute Consolidated Interest Expense.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided, however, that
(1) any net after-tax extraordinary, unusual or nonrecurring gains or losses (including, without limitation, severance, relocation, transition and other restructuring costs and litigation settlements or losses) shall be excluded;
(2) the Net Income for such period shall not include the cumulative effect of a change in accounting principle(s) during such period;
79
(3) any net after-tax gains or losses attributable to asset dispositions other than in the ordinary course of business (as determined in good faith by the Board of Directors of Holdings) and any gain (or loss) realized upon the sale or other disposition of any Capital Stock of any Person shall be excluded;
(4) the Net Income for such period of any Person that is not a Subsidiary of such Person, or that is an Unrestricted Subsidiary, or that is accounted for by the equity method of accounting, shall be excluded; provided that, to the extent not already included, Consolidated Net Income of such Person shall be (A) increased by the amount of dividends or other distributions that are actually paid in cash (or to the extent converted into cash) to the referent Person or a Restricted Subsidiary thereof in respect of such period (subject in the case of dividends paid or distributions made to a Restricted Subsidiary (other than a Guarantor) to the limitations contained in clause (5) below) and (B) decreased by the amount of any equity of Holdings in a net loss of any such Person for such period to the extent Holdings has funded such net loss;
(5) solely for the purpose of determining the amount available for Restricted Payments under clause (3) of the first paragraph of “Certain Covenants—Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than a Guarantor) shall be excluded if the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not permitted at the date of determination without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived; provided that the Consolidated Net Income of such Person shall be, subject to the exclusion contained in clause (3) above, increased by the amount of dividends or similar distributions that are actually paid in cash (or to the extent converted into cash) to such Person or a Restricted Subsidiary thereof (subject to the provisions of this clause (5)) in respect of such period, to the extent not already included therein;
(6) non-cash compensation charges, including any such charges arising from stock options, restricted stock grants or other equity-incentive programs shall be excluded;
(7) any net after-tax gains or losses (less all fees and expenses or charges relating thereto) attributable to the early extinguishment of Indebtedness shall be excluded; and
(8) the effect of any non-cash items resulting from any amortization, write-up, write-down or write-off of assets (including intangible assets, goodwill and deferred financing costs but excluding inventory) in connection with the Transactions or any future acquisition, merger, consolidation or similar transaction (excluding any such non-cash item to the extent that it represents an accrual of or reserve for cash expenditures in any future period except to the extent such item is subsequently reversed) shall be excluded.
Notwithstanding the foregoing, for the purpose of the covenant contained under the caption “—Certain Covenants—Restricted Payments” only, there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by Holdings and the Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments made by Holdings and the Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments made by Holdings and any Restricted Subsidiary, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under clause (3)(d) of the first paragraph of the covenant contained under the caption “—Certain Covenants—Restricted Payments.”
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary
80
obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent, (i) to purchase any such primary obligation or any property constituting direct or indirect security therefor, (ii) to advance or supply funds (A) for the purchase or payment of any such primary obligation or (B) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor, or (iii) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Contribution Indebtedness” means Indebtedness of the Company or any Guarantor in an aggregate principal amount not greater than twice the aggregate amount of cash contributions (other than Excluded Contributions) made to the capital of the Company or such Guarantor after the Issue Date; provided that such Contribution Indebtedness:
(1) if the aggregate principal amount of such Contribution Indebtedness is greater than one times such cash contributions to the capital of the Company or such Guarantor, as applicable, the amount of such excess shall be (A)(x) Subordinated Indebtedness (other than Secured Indebtedness) or (y) Senior Subordinated Indebtedness (other than Secured Indebtedness) and (B) Indebtedness with a Stated Maturity later than the Stated Maturity of the notes, and
(2) (a) is incurred within 180 days after the making of such cash contributions and (b) is so designated as Contribution Indebtedness pursuant to an Officers’ Certificate on the date of the incurrence thereof.
“Credit Agreement” means that certain credit agreement, dated as of January 18, 2005, among Holdings, the Company, Warner Chilcott Company, Inc., Credit Suisse, as Administrative Agent, and the lenders party thereto, including any related notes, guarantees, collateral documents, instruments and agreements executed in connection therewith, and in each case as amended, restated, supplemented, modified, renewed, refunded, replaced or refinanced from time to time in one or more agreements or indentures (in each case with the same or new lenders or institutional investors), including any agreement extending the maturity thereof or otherwise restructuring all or any portion of the Indebtedness thereunder or increasing the amount loaned or issued thereunder or altering the maturity thereof (provided that such increase in borrowings is permitted under the covenant entitled “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock”).
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Deferred Product Payments” means the deferred product payments in respect of the Dovonex and/or Dovobet acquisitions.
“Designated Noncash Consideration” means the fair market value of noncash consideration received by Holdings or any of its Restricted Subsidiaries in connection with an Asset Sale that is so designated as Designated Noncash Consideration pursuant to an Officers’ Certificate setting forth the basis of such valuation, less the amount of cash or Cash Equivalents received in connection with a subsequent sale of such Designated Noncash Consideration.
“Designated Preferred Stock” means Preferred Stock of Holdings or any direct or indirect parent corporation of Holdings (other than Disqualified Stock), that is issued for cash (other than to Holdings or any of its Subsidiaries or an employee stock ownership plan or trust established by Holdings or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officers’ Certificate, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of the covenant described under “—Certain Covenants—Restricted Payments.”
81
“Designated Senior Debt” means:
(1) any Bank Indebtedness that constitutes Senior Debt; and
(2) any other Senior Debt permitted under the Indenture the principal amount of which is $25.0 million or more and that has been designated by Holdings in the instrument evidencing that Senior Debt as “Designated Senior Debt.”
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms (or by the terms of any security into which it is convertible or for which it is putable or exchangeable), or upon the happening of any event, matures or is mandatorily redeemable (other than as a result of a change of control or asset sale), pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the final maturity date of the notes or the date the notes are no longer outstanding; provided, however, that if such Capital Stock is issued to any plan for the benefit of employees of Holdings or any of its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by Holdings or any of its Subsidiaries in order to satisfy applicable statutory or regulatory obligations.
“Domestic Restricted Subsidiary” means any direct or indirect Restricted Subsidiary of Holdings that was formed under the laws of the United States, any state of the United States or the District of Columbia and Warner Chilcott Company, Inc., a Puerto Rican corporation.
“Domestic Subsidiary” means any direct or indirect Subsidiary of Holdings that was formed under the laws of the United States, any state of the United States or the District of Columbia and Warner Chilcott Company, Inc., a Puerto Rican corporation.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period plus, without duplication,
(1) the provision for taxes based on income or profits, plus franchise or similar taxes, of such Person for such period deducted in computing Consolidated Net Income,plus
(2) Consolidated Interest Expense of such Person for such period to the extent the same was deducted in calculating such Consolidated Net Income,plus
(3) Consolidated Depreciation and Amortization Expense of such Person for such period to the extent such depreciation and amortization were deducted in computing Consolidated Net Income,plus
(4) any reasonable expenses or charges incurred in connection with any Equity Offering, Permitted Investment, acquisition, recapitalization or Indebtedness permitted to be incurred under the Indenture (in each case whether or not consummated) or the Transactions (including, without limitation, the fees payable to the Sponsors pursuant to the Advisory Services and Monitoring Agreement in connection with the Transactions) and, in each case, deducted in such period in computing Consolidated Net Income,plus
(5) the amount of any restructuring charges or reserves (which, for the avoidance of doubt, shall include retention, severance, systems establishment cost, excess pension charges, contract termination costs, including future lease commitments, and costs to consolidate facilities and relocate employees) deducted in such period in computing Consolidated Net Income,plus
(6) any other noncash charges (including any impairment charges and the impact of purchase accounting, including, but not limited to, the amortization of inventory step-up) reducing Consolidated Net Income for such period (excluding any such charge that represents an accrual or reserve for a cash expenditure for a future period),plus
82
(7) any net gain or loss resulting from Hedging Obligations relating to currency exchange risk,plus
(8) the amount of management, monitoring, consulting, advisory fees, termination payments and related expenses paid to the Sponsors (or any accruals relating to such fees and related expenses) during such period pursuant to the Advisory Services and Monitoring Agreement,plus
(9) Securitization Fees to the extent deducted in calculating Consolidated Net Income for such period,plus
(10) any net after-tax income or loss from discontinued operations and any net after-tax gains or losses on disposal of discontinued operations, less
(11) noncash items increasing Consolidated Net Income of such Person for such period (excluding any items which represent the reversal of any accrual of, or cash reserve for, anticipated cash charges made in any prior period).
Notwithstanding the foregoing, the provision for taxes based on the income or profits of, and the depreciation and amortization and non-cash charges of, a Restricted Subsidiary (other than a Guarantor) shall be added to Consolidated Net Income to compute EBITDA only to the extent (and in the same proportion, including by reason of minority interests) that the net income or loss of such Restricted Subsidiary was included in calculating Consolidated Net Income and only if a corresponding amount would be permitted at the date of determination to be dividended to Holdings by such Restricted Subsidiary without any prior governmental approval (which has not been obtained) or would not be restricted from being so dividended, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or in similar distributions has been legally waived.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock (but excluding any debt security that is convertible into, or exchangeable for, Capital Stock).
“Equity Offering” means any public or private sale of common stock or Preferred Stock of Holdings or any of its direct or indirect parent corporations (excluding Disqualified Stock of Holdings), other than (i) public offerings with respect to common stock of Holdings or of any of its direct or indirect parent corporations registered on Form S-4 or Form S-8, (ii) any such public or private sale that constitutes an Excluded Contribution or (iii) an issuance to any Subsidiary of Holdings.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the Commission promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds, in each case received by Holdings and its Restricted Subsidiaries from:
(1) contributions to its common equity capital; and
(2) the sale (other than to a Subsidiary or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of Holdings or any Subsidiary) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock),
in each case designated as Excluded Contributions pursuant to an Officers’ Certificate on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph of the covenant contained under the caption “—Certain Covenants—Restricted Payments.”
83
“Existing Indebtedness” means Indebtedness of Holdings and its Subsidiaries (other than Indebtedness under the Credit Agreement) in existence on the date of the Indenture.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period consisting of such Person and its Restricted Subsidiaries’ most recently ended four fiscal quarters for which internal financial statements are available, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that Holdings or any Restricted Subsidiary incurs, assumes, guarantees or repays any Indebtedness or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated givingpro forma effect to such incurrence, assumption, guarantee or repayment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period and as if Holdings or such Restricted Subsidiary had not earned the interest income actually earned during such period in respect of such cash used to repay, repurchase, defease or otherwise discharge such Indebtedness.
If Investments, acquisitions, dispositions, mergers or consolidations (as determined in accordance with GAAP) have been made by Holdings or any Restricted Subsidiary during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Calculation Date, then the Fixed Charge Coverage Ratio shall be calculated on apro forma basis assuming that all such Investments, acquisitions, dispositions, mergers or consolidations (and the change in any associated Fixed Charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period.
If since the beginning of such period any Person (that subsequently became a Restricted Subsidiary or was merged with or into Holdings or any Restricted Subsidiary since the beginning of such period) shall have made any Investment, acquisition, disposition, merger or consolidation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated givingpro forma effect thereto for such period as if such Investment, acquisition, disposition, merger or consolidation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, wheneverpro forma effect is to be given to an Investment, acquisition, disposition, merger or consolidation (including, without limitation, the Transactions) and the amount of income or earnings relating thereto, thepro forma calculations shall be determined in good faith by a responsible financial or accounting Officer of Holdings and shall comply with the requirements of Rule 11-02 of Regulation S-X promulgated by the Commission, except that suchpro forma calculations may include operating expense reductions for such period resulting from the transaction which is being givenpro forma effect that (A) have been realized or (B) for which the steps necessary for realization have been taken (or are taken concurrently with such transaction) or (C) for which the steps necessary for realization are reasonably expected to be taken within the six month period following such transaction and, in each case, including, but not limited to, (a) reduction in personnel expenses, (b) reduction of costs related to administrative functions, (c) reduction of costs related to leased or owned properties and (d) reductions from the consolidation of operations and streamlining of corporate overhead, provided that, in each case, such adjustments are set forth in an Officers’ Certificate signed by Holdings’ chief financial officer and another Officer which states (i) the amount of such adjustment or adjustments, (ii) in the case of items (B) or (C) above, that such adjustment or adjustments are based on the reasonable good faith beliefs of the Officers executing such Officers’ Certificate at the time of such execution and (iii) that any related incurrence of Indebtedness is permitted pursuant to the Indenture. If any Indebtedness bears a floating rate of interest and is being givenpro forma effect, the interest on such Indebtedness shall be calculated as if
84
the rate in effect on the Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness if the related hedge has a remaining term in excess of twelve months).
Interest on a Capitalized Lease Obligation shall be deemed to accrue at the interest rate reasonably determined by a responsible financial or accounting officer of Holdings to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on apro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as Holdings may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of, without duplication, (a) Consolidated Interest Expense (excluding all noncash interest expense and amortization/accretion of original issue discount, in each case, in connection with the Specified Financings (including any original issue discount created by fair value adjustments to Existing Indebtedness as a result of purchase accounting)) of such Person for such period, (b) all cash dividends paid, accrued and/or scheduled to be paid or accrued during such period (excluding items eliminated in consolidation) on any series of Preferred Stock of such Person and its Subsidiaries and (c) all cash dividends paid, accrued and/or scheduled to be paid or accrued during such period (excluding items eliminated in consolidation) on any series of Disqualified Stock of such Person and its Subsidiaries.
“Foreign Subsidiary” means any Subsidiary of Holdings that is not a Domestic Subsidiary.
“GAAP” means generally accepted accounting principles in the United States in effect on the date of the Indenture. For purposes of this description of the notes, the term “consolidated” with respect to any Person means such Person consolidated with its Restricted Subsidiaries and does not include any Unrestricted Subsidiary.
“guarantee” means a guarantee other than by endorsement of negotiable instruments for collection in the ordinary course of business, direct or indirect, in any manner including, without limitation, through letters of credit or reimbursement agreements in respect thereof, of all or any part of any Indebtedness or other obligations. When used as a verb, “guarantee” shall have a corresponding meaning.
“Guarantee” means any guarantee of the obligations of the Company under the Indenture and the notes by a Guarantor in accordance with the provisions of the Indenture. When used as a verb, “Guarantee” shall have a corresponding meaning.
“Guarantor” means any Person that incurs a Guarantee of the notes; provided that upon the release and discharge of such Person from its Guarantee in accordance with the Indenture, such Person shall cease to be a Guarantor. On the Issue Date the Guarantors were Holdings, Luxco, Warner Chilcott Company, Inc., and Warner Chilcott (US), Inc. In addition, in connection with filing the registration statement of which this prospectus forms a part, Warner Chilcott Limited became an additional Guarantor of the notes.
“Guarantor Senior Debt” means, with respect to any Guarantor, the principal of, premium, if any, and interest (including any interest accruing subsequent to the filing of a petition of bankruptcy at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed
85
or allowable claim under applicable law) on any Indebtedness and any Securitization Repurchase Obligation of such Guarantor, whether outstanding on the Issue Date or thereafter created, incurred or assumed, unless, in the case of any particular obligation, the instrument creating or evidencing the same or pursuant to which the same is outstanding expressly provides that such obligation shall be subordinate orpari passu in right of payment to the Guarantee of such Guarantor. Without limiting the generality of the foregoing, “Guarantor Senior Debt” shall also include the principal of, premium, if any, interest (including any interest accruing subsequent to the filing of a petition of bankruptcy at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed or allowable claim under applicable law) on, and all other amounts owing in respect of (including guarantees of the foregoing obligations):
(1) all monetary obligations of every nature of such Guarantor under, or with respect to, the Credit Agreement, including, without limitation, obligations to pay principal, premium and interest, reimbursement obligations under letters of credit, fees, expenses and indemnities (and guarantees thereof); and
(2) all Hedging Obligations (and guarantees thereof),
in each case whether outstanding on the Issue Date or thereafter incurred.
Notwithstanding the foregoing, “Guarantor Senior Debt” shall not include:
(1) any Indebtedness of such Guarantor to a Subsidiary of such Guarantor (other than any Securitization Repurchase Obligation);
(2) Indebtedness to, or guaranteed on behalf of, any shareholder, director, officer or employee of such Guarantor or any Subsidiary of such Guarantor (including, without limitation, amounts owed for compensation), other than Indebtedness under the Credit Agreement;
(3) Indebtedness to trade creditors and other amounts incurred in connection with obtaining goods, materials or services (including guarantees thereof or instruments evidencing such liabilities);
(4) Indebtedness represented by Capital Stock;
(5) any liability for federal, foreign, state, local or other taxes owed or owing by such Guarantor;
(6) that portion of any Indebtedness incurred in violation of any of the covenants contained under the captions “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock” or “—Certain Covenants—Limitations on Layering”;
(7) Indebtedness which, when incurred and without respect to any election under Section 1111(b) of Title 11, United States Code, is without recourse to such Guarantor; and
(8) any Indebtedness which is, by its express terms, subordinated in right of payment to any other Indebtedness of such Guarantor.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under:
(1) currency exchange, interest rate or commodity swap agreements, currency exchange, interest rate or commodity cap agreements and currency exchange, interest rate or commodity collar agreements; and
(2) other agreements or arrangements designed to protect such Person against fluctuations in currency exchange, interest rates or commodity prices.
“Holdings” means Warner Chilcott Holdings Company III, Limited.
86
“Implementation Agreement” means the Implementation Agreement, dated October 27, 2004, as amended and restated on November 9, 2004 and November 16, 2004, among Bain Capital Partners LLC, DLJMB Overseas Partners III, C.V., J.P. Morgan Partners (BHCA), L.P., Thomas H. Lee (Alternative) Fund V, L.P., Waren Acquisition Limited and Warner Chilcott PLC.
“Indebtedness” means, with respect to any Person,
(a) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(i) in respect of borrowed money,
(ii) evidenced by bonds, notes, debentures or similar instruments or letters of credit (or, without duplication, reimbursement agreements in respect thereof),
(iii) representing the deferred and unpaid balance of the purchase price of any property (including Capitalized Lease Obligations), except any such balance that constitutes a trade payable or similar obligation to a trade creditor in each case accrued in the ordinary course of business or
(iv) representing any Hedging Obligations,
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP,
(b) Disqualified Stock of such Person,
(c) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the Indebtedness of another Person (other than by endorsement of negotiable instruments for collection in the ordinary course of business), and
(d) to the extent not otherwise included, Indebtedness of another Person secured by a Lien on any asset owned by such Person (whether or not such Indebtedness is assumed by such Person);
provided, however, that Contingent Obligations incurred in the ordinary course of business and not in respect of borrowed money shall be deemed not to constitute Indebtedness.
“Independent Financial Advisor” means an accounting, appraisal or investment banking firm or consultant to Persons engaged in a Permitted Business of nationally recognized standing that is, in the good faith judgment of the Board of Directors of Holdings, qualified to perform the task for which it has been engaged.
“Investments” means, with respect to any Person, all direct or indirect investments by such Person in other Persons (including Affiliates) in the forms of loans (including guarantees or other obligations), advances or capital contributions (including by means of any transfer of cash or other property to others or any payment for property or services for the account or use of others, but excluding accounts receivable, trade credit, advances to customers, commission, travel and similar advances to officers and employees, in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of such Person in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. If Holdings or any Subsidiary of Holdings sells or otherwise disposes of any Equity Interests of any direct or indirect Subsidiary of Holdings such that, after giving effect to any such sale or disposition, such
87
Person is no longer a Subsidiary of Holdings, Holdings will be deemed to have made an Investment on the date of any such sale or disposition equal to the fair market value of the Equity Interests of such Subsidiary not sold or disposed of in an amount determined as provided in the last paragraph of the covenant described above under the caption “—Certain Covenants—Restricted Payments.”
For purposes of the definition of “Unrestricted Subsidiary” and the covenant described above under the caption “—Certain Covenants—Restricted Payments,” (i) “Investments” shall include the portion (proportionate to Holdings’ equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of Holdings at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided, however, that upon a redesignation of such Subsidiary as a Restricted Subsidiary, Holdings shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to (x) Holdings’ “Investment” in such Subsidiary at the time of such redesignation less (y) the portion (proportionate to Holdings’ equity interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; (ii) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer, in each case as determined in good faith by the Board of Directors of Holdings and (iii) any transfer of Capital Stock that results in an entity which became a Restricted Subsidiary after the Issue Date ceasing to be a Restricted Subsidiary shall be deemed to be an Investment in an amount equal to the fair market value (as determined by the Board of Directors of Holdings in good faith as of the date of initial acquisition) of the Capital Stock of such entity owned by Holdings and the Restricted Subsidiaries immediately after such transfer.
“Issue Date” means January 18, 2005.
“Lien” means, with respect to any asset, any mortgage, lien, pledge, charge, security interest or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Luxco” means Warner Chilcott Intermediate (Luxembourg) S.à r.l., a company organized under the laws of the Grand Duchy of Luxembourg.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends or accretion of any Preferred Stock.
“Net Indebtedness to EBITDA Ratio” means, with respect to Holdings, the ratio of: (a) the Indebtedness (which, for purposes of any calculations of the Net Indebtedness to EBITDA Ratio shall include, without duplication, any Qualified Securitization Financing) of Holdings and its Restricted Subsidiaries, as of the end of the most recently ended fiscal quarter,plus the amount of any Indebtedness incurred subsequent to the end of such fiscal quarter,less the amount of cash and Cash Equivalents that would be stated on the balance sheet of Holdings and held by Holdings as of such date of determination, as determined in accordance with GAAP to (b) Holdings’ EBITDA for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date on which the event for which such calculation is being made shall occur (the “Measurement Period”); provided, however, that: (i) in making such computation, Indebtedness shall include the greater of (x) the average daily balance outstanding under any revolving credit facility
88
during the most recently ended fiscal quarter and (y) the actual amount of Indebtedness outstanding under any revolving credit facility as of the date for which such calculation is being made; and (ii) if Holdings or any of its Restricted Subsidiaries consummates a material acquisition or an Asset Sale or other disposition of assets subsequent to the commencement of the Measurement Period but prior to the event for which the calculation of the Net Indebtedness to EBITDA Ratio is made, then the Net Indebtedness to EBITDA Ratio shall be calculated givingpro forma effect to such material acquisition or Asset Sale or other disposition of assets, as if the same had occurred at the beginning of the applicable period. Anypro forma calculations necessary pursuant to this “Net Indebtedness to EBITDA Ratio” shall be made in accordance with the provisions relating topro forma adjustments set forth in the definition of “Fixed Charge Coverage Ratio.”
“Net Proceeds” means the aggregate cash proceeds received by Holdings or any Restricted Subsidiary in respect of any Asset Sale, in each case net of legal, accounting and investment banking fees, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), repayment of Indebtedness that is secured by the property or assets that are the subject of such Asset Sale and any deduction of appropriate amounts to be provided by Holdings as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by Holdings after such sale or other disposition thereof, including, without limitation, pension and other post employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Non-Guarantor Restricted Subsidiary” means any Restricted Subsidiary that is not a Guarantor.
“Non-Recourse Product Financing Indebtedness” means any Indebtedness incurred by Holdings or any Restricted Subsidiary solely for the purpose of financing (whether directly or through a partially- owned joint venture) the production, acquisition or development of Products produced, acquired or developed after the Issue Date (including any Indebtedness assumed in connection with the production, acquisition or development of any such Product or secured by a Lien on any such Product prior to the production, acquisition or development thereof) where the recourse of the creditor in respect of that Indebtedness is limited to Product revenues generated by such Product or any rights pertaining thereto and where the Indebtedness is unsecured, except for Liens over such Product or revenues and such rights, and any extension, renewal, replacement or refinancing of such Indebtedness. “Non-Recourse Product Financing Indebtedness” excludes, for the avoidance of doubt, any Indebtedness raised or secured against Products where the proceeds are used for any other purposes.
“Obligations” means any principal, interest, penalties, fees, indemnifications, reimbursements (including, without limitation, reimbursement obligations with respect to letters of credit), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the Board, the Chief Executive Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Company, or Holdings, as applicable.
“Officers’ Certificate” means a certificate signed on behalf of the Company, or Holdings, as applicable, by two Officers of the Company, or Holdings, as applicable, one of whom is the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of the Company, or Holdings, as applicable, that meets the requirements set forth in the Indenture.
89
“Permitted Asset Swap” means any transfer of property or assets by Holdings or any of its Restricted Subsidiaries in which at least 90% of the consideration received by the transferor consists of properties or assets (other than cash) that will be used in a Permitted Business; provided that the aggregate fair market value of the property or assets being transferred by Holdings or such Restricted Subsidiary is not greater than the aggregate fair market value of the property or assets received by Holdings or such Restricted Subsidiary in such exchange (provided, however, that in the event such aggregate fair market value of the property or assets being transferred or received by Holdings or such Restricted Subsidiary is (x) less than $30.0 million, such determination shall be made in good faith by the Board of Directors of Holdings and (y) greater than or equal to $30.0 million, such determination shall be made by an Independent Financial Advisor).
“Permitted Business” means the business and any services, activities or businesses incidental, or directly related or similar to, any line of business engaged in by Holdings and its Subsidiaries as of the Issue Date or any business activity that is a reasonable extension, development or expansion thereof or ancillary thereto.
“Permitted Debt”is defined under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock.”
“Permitted Holders” means (i) each of the Sponsors and their respective Affiliates, but not including, however, any portfolio companies of any of the Sponsors, (ii) Officers, provided that if such Officers beneficially own more shares of Voting Stock of Holdings or any of its direct or indirect parent entities than the number of such shares beneficially owned by all the Officers as of the Issue Date or acquired by Officers within 90 days immediately following the Issue Date, such excess shall be deemed not to be beneficially owned by Permitted Holders, and (iii) any “group” (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members, provided that in the case of such “group” and without giving effect to the existence of such “group” or any other “group,” such Sponsors, Affiliates and Officers (subject, in the case of Officers, to the foregoing limitation), collectively, have beneficial ownership, directly or indirectly, of more than 50% of the total voting power of the Voting Stock of Holdings or any of its direct or indirect parent entities held by such “group”.
“Permitted Investments” means
(1) any Investment by Holdings in any Restricted Subsidiary or by a Restricted Subsidiary in another Restricted Subsidiary;
(2) any Investment in cash and Cash Equivalents;
(3) any Investment by Holdings or any Restricted Subsidiary in a Person that is engaged in a Permitted Business if as a result of such Investment (A) such Person becomes a Restricted Subsidiary or (B) such Person, in one transaction or a series of related transactions, is merged, consolidated or amalgamated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, Holdings or a Restricted Subsidiary;
(4) any Investment in securities or other assets not constituting cash or Cash Equivalents and received in connection with an Asset Sale made pursuant to the provisions described above under the caption “—Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date and any modification, replacement, renewal or extension thereof; provided that the amount of any such Investment may be increased (x) as required by the terms of such Investment as in existence on the Issue Date or (y) as otherwise permitted under the Indenture;
90
(6) loans and advances to employees and any guarantees made in the ordinary course of business, but in any event not in excess of $10.0 million in the aggregate outstanding at any one time;
(7) any Investment acquired by Holdings or any Restricted Subsidiary (A) in exchange for any other Investment or accounts receivable held by Holdings or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable or (B) as a result of a foreclosure by Holdings or such Restricted Subsidiary with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(8) Hedging Obligations permitted under clause (9) of the definition of “Permitted Debt;”
(9) loans and advances to officers, directors and employees for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business;
(10) any Investment by Holdings or a Restricted Subsidiary in a Permitted Business having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (10) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash and/or marketable securities), not to exceed the greater of (x) $100.0 million and (y) $100.0 million plus or minus, as applicable, an amount equal to 7% of Consolidated Net Income of Holdings for the period (taken as one accounting period) from January 1, 2005 to the end of Holdings’ fiscal quarter most recently ended prior to the date on which such Investment is made (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(11) Investments the payment for which consists of Equity Interests of Holdings or any of its direct or indirect parent corporations (exclusive of Disqualified Stock);
(12) guarantees (including Guarantees) of Indebtedness permitted under the covenant contained under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock” and performance guarantees consistent with past practice;
(13) Investments consisting of licensing of intellectual property pursuant to joint marketing arrangements with other Persons; and
(14) any Investment in a Securitization Subsidiary or any Investment by a Securitization Subsidiary in any other Person in connection with a Qualified Securitization Financing, including, without limitation, Investments of funds held in accounts permitted or required by the arrangements governing such Qualified Securitization Financing or any related Indebtedness; provided, however, that any Investment in a Securitization Subsidiary is in the form of a Purchase Money Note, contribution of additional Securitization Assets or an equity interest.
“Permitted Junior Securities” means
(1) Equity Interests in the Company, Holdings, any other Guarantor or any direct or indirect parent of Holdings issued pursuant to a plan of reorganization or readjustment; or
(2) unsecured debt securities of the Company or Holdings issued pursuant to a plan of reorganization or readjustment that are subordinated to all Senior Debt or, as applicable, Guarantor Senior Debt of Holdings (and any debt securities issued in exchange for Senior Debt or such Guarantor Senior Debt) to substantially the same extent as, or to a greater extent than, the notes are subordinated to Senior Debt under the Indenture;
provided that to the extent that any Senior Debt or Guarantor Senior Debt, as the case may be, outstanding on the date of consummation of any such plan of reorganization or readjustment is not
91
paid in full in cash on such date, the holders of any such Senior Debt or Guarantor Senior Debt not so paid in full in cash have consented to the terms of such plan of reorganization or readjustment.
“Permitted Liens” means the following types of Liens:
(1) deposits of cash or government bonds made in the ordinary course of business to secure surety or appeal bonds to which such Person is a party;
(2) Liens in favor of issuers of performance, surety, bid, indemnity, warranty, release, appeal or similar bonds or with respect to other regulatory requirements or letters of credit or bankers’ acceptance issued, and completion guarantees provided for, in each case pursuant to the request of and for the account of such Person in the ordinary course of its business or consistent with past practice;
(3) Liens on property or shares of stock of a Person at the time such Person becomes a Subsidiary; provided, however, that such Liens are not created or incurred in connection with, or in contemplation of, or to provide all or any portion of the funds or credit support utilized in connection with, such other Person becoming such a Subsidiary; provided, further, however, that such Liens may not extend to any other property owned by Holdings or any Restricted Subsidiary;
(4) Liens on property at the time Holdings or a Restricted Subsidiary acquired the property, including any acquisition by means of a merger or consolidation with or into Holdings or any Restricted Subsidiary; provided, however, that such Liens are not created or incurred in connection with, or in contemplation of, or to provide all or any portion of the funds or credit support utilized for, such acquisition; provided, further, however, that such Liens may not extend to any other property owned by Holdings or any Restricted Subsidiary;
(5) Liens securing Hedging Obligations so long as the related Indebtedness is permitted to be incurred under the Indenture and is secured by a Lien on the same property securing such Hedging Obligation;
(6) Liens on specific items of inventory or other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances or letters of credit issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(7) Liens in favor of Holdings or any Restricted Subsidiary;
(8) Liens to secure any Indebtedness that is incurred to refinance any Indebtedness that has been secured by a Lien existing on the Issue Date or referred to in clauses (3), (4) and (19)(B) of this definition; provided, however, that such Liens (x) are no less favorable to the holders of the notes, taken as a whole, and are not more favorable to the lienholders with respect to such Liens than the Liens in respect of the Indebtedness being refinanced; and (y) do not extend to or cover any property or assets of Holdings or any of its Restricted Subsidiaries not securing the Indebtedness so refinanced;
(9) Liens on Securitization Assets and related assets of the type specified in the definition of “Securitization Financing” incurred in connection with any Qualified Securitization Financing;
(10) Liens for taxes, assessments or other governmental charges or levies not yet delinquent, or which are being contested in good faith by appropriate proceedings promptly instituted and diligently conducted or for property taxes on property that Holdings or one of its Subsidiaries has determined to abandon if the sole recourse for such tax, assessment, charge, levy or claim is to such property;
(11) judgment liens in respect of judgments that do not constitute an Event of Default so long as such Liens are adequately bonded and any appropriate legal proceedings that may have
92
been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(12) pledges, deposits or security under workmen’s compensation, unemployment insurance and other social security laws or regulations, or deposits to secure the performance of tenders, contracts (other than for the payment of Indebtedness) or leases, or deposits to secure public or statutory obligations, or deposits as security for contested taxes or import or customs duties or for the payment of rent, or deposits or other security securing liabilities to insurance carriers under insurance or self-insurance arrangements, in each case incurred in the ordinary course of business or consistent with past practice;
(13) Liens imposed by law, including carriers’, warehousemen’s, materialmen’s, repairmen’s and mechanics’ Liens, in each case for sums not overdue by more than 30 days or if more than 30 days overdue, are unfiled and no other action has been taken to enforce such Lien or which are being contested in good faith by appropriate proceedings promptly instituted and diligently conducted;
(14) encumbrances, ground leases, easements or reservations of, or rights of others for, licenses, rights of way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning, building codes or other restrictions (including, without limitation, minor defects or irregularities in title and similar encumbrances) as to the use of real properties or Liens incidental to the conduct of business or to the ownership of properties that do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business;
(15) leases, licenses, subleases or sublicenses granted to others in the ordinary course of business that do not (x) interfere in any material respect with the business of Holdings or any of its material Restricted Subsidiaries (including the Company) or (y) secure any Indebtedness;
(16) the rights reserved or vested in any Person by the terms of any lease, license, franchise, grant or permit held by Holdings or any of its Restricted Subsidiaries or by a statutory provision, to terminate any such lease, license, franchise, grant or permit, or to require annual or periodic payments as a condition to the continuance thereof;
(17) banker’s Liens, rights of set-off or similar rights and remedies as to deposit accounts or other funds maintained with a depositary institution, provided that (a) such deposit account is not a dedicated cash collateral account and is not subject to restrictions against access by Holdings or any of its Subsidiaries in excess of those set forth by regulations promulgated by the Federal Reserve Board or other applicable law and (b) such deposit account is not intended by Holdings or any Restricted Subsidiary to provide collateral to the depositary institution;
(18) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases or consignments entered into by Holdings and its Restricted Subsidiaries in the ordinary course of business;
(19) (A) other Liens securing Indebtedness for borrowed money with respect to property or assets with an aggregate fair market value (valued at the time of creation thereof) of not more than $10.0 million at any time and (B) Liens securing Indebtedness incurred to finance the construction, purchase or lease of, or repairs, improvements or additions to, property of such Person; provided, however, that (x) the Lien may not extend to any other property (except for accessions to such property) owned by such Person or any of its Restricted Subsidiaries at the time the Lien is incurred, (y) such Liens attach concurrently with or within 270 days after the acquisition, repair, replacement, construction or improvement (as applicable) of the property subject to such Liens and (z) with respect to Capitalized Lease Obligations, such Liens do not at any time extend to or cover any assets (except for accessions to such assets) other than the assets subject to such Capitalized Lease Obligations; provided that individual financings of equipment provided by one lender may be cross collateralized to other financings of equipment provided by such lender;
93
(20) Liens (i) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection, (ii) attaching to commodity trading accounts or other commodities brokerage accounts incurred in the ordinary course of business; and (iii) in favor of a banking institution arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(21) Liens encumbering reasonable customary initial deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(22) Liens that are contractual rights of set-off (i) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (ii) relating to pooled deposit or sweep accounts of Holdings or any Restricted Subsidiary to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of Holdings and its Restricted Subsidiaries or (iii) relating to purchase orders and other agreements entered into with customers of Holdings or any Restricted Subsidiary in the ordinary course of business;
(23) Liens solely on any cash earnest money deposits made by Holdings or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted under the Indenture;
(24) Liens with respect to the assets of a Restricted Subsidiary that is not a Guarantor securing Indebtedness of such Restricted Subsidiary incurred in accordance with the covenant contained under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock;” and
(25) Liens to secure Non-Recourse Product Financing Indebtedness permitted to be incurred pursuant to clause (22) of Permitted Debt, which Liens may not secure Indebtedness other than Non-Recourse Product Financing Indebtedness and which Liens may not attach to assets other than the items of Product produced, acquired or developed with the proceeds of such Indebtedness.
“Person” means any individual, corporation, partnership, joint venture, association, joint stock company, trust, unincorporated organization, limited liability company or government or other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends upon liquidation, dissolution or winding up.
“Product” means any product developed, acquired, produced, marketed or promoted by Holdings or any of its Subsidiaries in connection with the conduct of a Permitted Business.
“Purchase Money Note” means a promissory note of a Securitization Subsidiary evidencing a line of credit, which may be irrevocable, issued by Holdings or any Subsidiary of Holdings to such Securitization Subsidiary in connection with a Qualified Securitization Financing, which note is intended to finance that portion of the purchase price that is not paid in cash or a contribution of equity and which (a) shall be repaid from cash available to the Securitization Subsidiary, other than (i) amounts required to be established as reserves, (ii) amounts paid to investors in respect of interest, (iii) principal and other amounts owing to such investors and (iv) amounts paid in connection with the purchase of newly generated receivables and (b) may be subordinated to the payments described in clause (a).
“Qualified Proceeds” means assets that are used or useful in, or Capital Stock of any Person engaged in, a Permitted Business;provided that the fair market value of any such assets or Capital Stock shall be determined by the Board of Directors of Holdings in good faith, except that in the event the value of any such assets or Capital Stock exceeds $25.0 million, the fair market value thereof shall be determined by an Independent Financial Advisor.
94
“Qualified Securitization Financing” means any Securitization Financing of a Securitization Subsidiary that meets the following conditions: (i) the Board of Directors of Holdings shall have determined in good faith that such Qualified Securitization Financing (including financing terms, covenants, termination events and other provisions) is in the aggregate economically fair and reasonable to Holdings and the Securitization Subsidiary, (ii) all sales of Securitization Assets and related assets to the Securitization Subsidiary are made at fair market value (as determined in good faith by Holdings) and (iii) the financing terms, covenants, termination events and other provisions thereof shall be market terms (as determined in good faith by Holdings) and may include Standard Securitization Undertakings. The grant of a security interest in any Securitization Assets of Holdings or any of its Restricted Subsidiaries (other than a Securitization Subsidiary) to secure Indebtedness under the Credit Agreement and any Refinancing Indebtedness with respect thereto shall not be deemed a Qualified Securitization Financing.
“Representative” means the trustee, agent or representative (if any) for an issue of Senior Debt; provided that if, and for so long as, any Designated Senior Debt lacks such a representative, then the Representative for such Designated Senior Debt shall at all times constitute the holders of a majority in outstanding principal amount of such Designated Senior Debt.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of Holdings (including the Company and any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided, however, that upon the occurrence of an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of Restricted Subsidiary.
“S&P” means Standard and Poor’s Ratings Services, a division of The McGraw-Hill Companies, Inc., and any successor to its rating business.
“Secured Indebtedness” means any Indebtedness secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the Commission promulgated thereunder.
“Securitization Assets” means any accounts receivable or other revenue streams from Products subject to a Qualified Securitization Financing.
“Securitization Fees” means reasonable distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Securitization Subsidiary in connection with any Qualified Securitization Financing.
“Securitization Financing” means any transaction or series of transactions that may be entered into by Holdings or any of its Subsidiaries pursuant to which Holdings or any of its Subsidiaries may sell, convey or otherwise transfer to (a) a Securitization Subsidiary (in the case of a transfer by Holdings or any of its Subsidiaries) and (b) any other Person (in the case of a transfer by a Securitization Subsidiary), or may grant a security interest in, any Securitization Assets (whether now existing or arising in the future) of Holdings or any of its Subsidiaries, and any assets related thereto including, without limitation, all collateral securing such Securitization Assets, all contracts and all guarantees or other obligations in respect of such Securitization Assets, proceeds of such Securitization Assets and other assets which are customarily transferred or in respect of which security interests are customarily granted in connection with asset securitization transactions involving Securitization Assets and any Hedging Obligations entered into by Holdings or any such Subsidiary in connection with such Securitization Assets.
95
“Securitization Repurchase Obligation” means any obligation of a seller of Securitization Assets in a Qualified Securitization Financing to repurchase Securitization Assets arising as a result of a breach of a representation, warranty or covenant or otherwise, including, without limitation, as a result of a receivable or portion thereof becoming subject to any asserted defense, dispute, off set or counterclaim of any kind as a result of any action taken by, any failure to take action by or any other event relating to the seller.
“Securitization Subsidiary” means a Wholly Owned Subsidiary of Holdings (or another Person formed for the purposes of engaging in a Qualified Securitization Financing in which Holdings or any Subsidiary of Holdings makes an Investment and to which Holdings or any Subsidiary of Holdings transfers Securitization Assets and related assets) which engages in no activities other than in connection with the financing of Securitization Assets of Holdings or its Subsidiaries, all proceeds thereof and all rights (contingent and other), collateral and other assets relating thereto, and any business or activities incidental or related to such business, and which is designated by the Board of Directors of Holdings or such other Person (as provided below) as a Securitization Subsidiary and (a) no portion of the Indebtedness or any other obligations (contingent or otherwise) of which (i) is guaranteed by Holdings or any other Subsidiary of Holdings (excluding guarantees of obligations (other than the principal of, and interest on, Indebtedness) pursuant to Standard Securitization Undertakings), (ii) is recourse to or obligates Holdings or any other Subsidiary of Holdings in any way other than pursuant to Standard Securitization Undertakings or (iii) subjects any property or asset of Holdings or any other Subsidiary of Holdings, directly or indirectly, contingently or otherwise, to the satisfaction thereof, other than pursuant to Standard Securitization Undertakings, (b) with which neither Holdings nor any other Subsidiary of Holdings has any material contract, agreement, arrangement or understanding other than on terms which Holdings reasonably believes to be no less favorable to Holdings or such Subsidiary than those that might be obtained at the time from Persons that are not Affiliates of Holdings and (e) to which neither Holdings nor any other Subsidiary of Holdings has any obligation to maintain or preserve such entity’s financial condition or cause such entity to achieve certain levels of operating results. Any such designation by the Board of Directors of Holdings or such other Person shall be evidenced to the Trustee by filing with the Trustee a certified copy of the resolution of the Board of Directors of Holdings or such other Person giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing conditions.
“Senior Debt” means the principal of, premium, if any, and interest (including any interest accruing after the commencement of any bankruptcy proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed or allowable claim under applicable law) on any Indebtedness and any Securitization Repurchase Obligation of the Company, whether outstanding on the Issue Date or thereafter created, incurred or assumed, unless, in the case of any particular obligation, the instrument creating or evidencing the same or pursuant to which the same is outstanding expressly provides that such obligation shall be subordinate orpari passu in right of payment to the notes. Without limiting the generality of the foregoing, “Senior Debt” shall also include the principal of, premium, if any, interest (including any interest accruing after the commencement of any bankruptcy proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed or allowable claim under applicable law) on, and all other amounts owing in respect of (including guarantees of the foregoing obligations):
(1) all monetary obligations of every nature of the Company under, or with respect to, the Credit Agreement, including, without limitation, obligations to pay principal, premium and interest, reimbursement obligations under letters of credit, fees, expenses and indemnities (and guarantees thereof); and
(2) all Hedging Obligations (and guarantees thereof),
in each case whether outstanding on the Issue Date or thereafter incurred.
96
Notwithstanding the foregoing, “Senior Debt” shall not include:
(1) any Indebtedness of the Company to a Subsidiary of the Company (other than any Securitization Repurchase Obligation);
(2) Indebtedness to, or guaranteed on behalf of, any shareholder, director, officer or employee of the Company or any Subsidiary of the Company (including, without limitation, amounts owed for compensation), other than Indebtedness under the Credit Agreement;
(3) Indebtedness to trade creditors and other amounts incurred in connection with obtaining goods, materials or services (including guarantees thereof or instruments evidencing such liabilities);
(4) Indebtedness represented by Capital Stock;
(5) any liability for federal, foreign, state, local or other taxes owed or owing by the Company;
(6) that portion of any Indebtedness incurred in violation of the covenant contained under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock,” or “—Certain Covenants—Limitations on Layering;”
(7) Indebtedness which, when incurred and without respect to any election under Section 1111(b) of Title 11, United States Code, is without recourse to the Company; and
(8) any Indebtedness which is, by its express terms, subordinated in right of payment to any other Indebtedness of the Company.
“Senior Subordinated Indebtedness” means the notes (in the case of the Company), a Guarantee (in the case of a Guarantor) and any other Indebtedness of the Company or a Guarantor that specifically provides that such Indebtedness is to rankpari passu with the notes or such Guarantee, as the case may be, in right of payment and is not subordinated by its terms in right of payment to any Indebtedness or other obligation of the Company or such Guarantor which is not Senior Debt (in the case of the Company) or Guarantor Senior Debt (in the case of a Guarantor).
“Shareholders Agreement” means the Shareholders Agreement to be dated as of January 18, 2005, by and among Warner Chilcott Holdings Company, Limited, Warner Chilcott Holdings Company II, Limited, Warner Chilcott Holdings Company III, Limited and the investment funds affiliated with the Sponsors and certain of their limited partners that are signatories thereto.
“Significant Subsidiary” means any Restricted Subsidiary that would be “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such Regulation is in effect on the date hereof.
“Specified Financings” means the financings included in the Transactions and offering of the notes.
“Sponsors” means Bain Capital Partners LLC, DLJ Merchant Banking III, Inc., J.P. Morgan Partners LLC, and Thomas H. Lee Partners, L.P.
“Standard Securitization Undertakings” means representations, warranties, covenants and indemnities entered into by Holdings or any Subsidiary of Holdings which Holdings has determined in good faith to be customary in a Securitization Financing, including, without limitation, those relating to the servicing of the assets of a Securitization Subsidiary, it being understood that any Securitization Repurchase Obligation shall be deemed to be a Standard Securitization Undertaking.
“Stated Maturity” means, with respect to any installment of interest or principal on any series of Indebtedness, the date on which the payment of interest or principal was scheduled to be paid in the
97
original documentation governing such Indebtedness, and will not include any contingent obligations to repay, redeem or repurchase any such interest or principal prior to the date originally scheduled for the payment thereof.
“Subordinated Indebtedness” means (a) with respect to the Company, any Indebtedness of the Company that is by its terms subordinated in right of payment to the notes and (b) with respect to any Guarantor of the notes, any Indebtedness of such Guarantor that is by its terms subordinated in right of payment to its Guarantee of the notes.
“Subsidiary” means, with respect to any specified Person:
(1) any corporation, association or other business entity, of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or controlled, directly or indirectly, by that Person or one or more of the other Subsidiaries of that Person (or a combination thereof); and
(2) any partnership, joint venture, limited liability company or similar entity of which (x) more than 50% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise and (y) such Person or any Wholly Owned Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
“Transactions” means the transactions contemplated by (i) the Implementation Agreement, (ii) the Credit Agreement and (iii) this offering of the notes and any bridge facility entered into in lieu of such offering prior to the Issue Date.
“Treasury Rate” means, as of the applicable redemption date, the yield to maturity as of such redemption date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two business days prior to such redemption date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from such redemption date to February 1, 2010; provided, however, that if the period from such redemption date to February 1, 2010 is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Unrestricted Subsidiary” means (i) any Subsidiary of Holdings (other than the Company) that at the time of determination is an Unrestricted Subsidiary (as designated by the Board of Directors of Holdings, as provided below) and (ii) any Subsidiary of an Unrestricted Subsidiary. The Board of Directors of Holdings may designate any Subsidiary of Holdings (including any existing Subsidiary and any newly acquired or newly formed Subsidiary, but excluding the Company) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, Holdings or any Subsidiary of Holdings (other than any Subsidiary of the Subsidiary to be so designated); provided that (a) any Unrestricted Subsidiary must be an entity of which shares of the Capital Stock or other equity interests (including partnership interests) entitled to cast at least a majority of the votes that may be cast by all shares or equity interests having ordinary voting power for the election of directors or other governing body are owned, directly or indirectly, by Holdings, (b) such designation complies with the covenant contained under the caption “—Certain Covenants—Restricted Payments” and (c) each of (I) the Subsidiary to be so designated and (II) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any
98
Indebtedness pursuant to which the lender has recourse to any of the assets of Holdings or any Restricted Subsidiary. The Board of Directors of Holdings may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default or Event of Default shall have occurred and Holdings could incur $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test described under the first paragraph of “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock.” Any such designation by the Board of Directors of Holdings shall be notified by Holdings to the Trustee by promptly filing with the Trustee a copy of the board resolution giving effect to such designation and an Officers’ Certificate certifying that such designation complied with the foregoing provisions.
“U.S. Dollar Equivalent” means with respect to any monetary amount in a currency other than U.S. dollars, at any time for determination thereof, the amount of U.S. dollars obtained by converting such foreign currency involved in such computation into U.S. dollars at the spot rate for the purchase of U.S. dollars with the applicable foreign currency as published inThe Wall Street Journal in the “Exchange Rates” column under the heading “Currency Trading” on the date two business days prior to such determination.
Except as described under “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock”, whenever it is necessary to determine whether Holdings has complied with any covenant in the Indenture or a Default has occurred and an amount is expressed in a currency other than U.S. dollars, such amount will be treated as the U.S. Dollar Equivalent determined as of the date such amount is initially determined in such currency.
“U.S. Government Securities” means securities that are
(a) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged or
(b) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such U.S. Government Securities or a specific payment of principal of or interest on any such U.S. Government Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the U.S. Government Securities or the specific payment of principal of or interest on the U.S. Government Securities evidenced by such depository receipt.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the Board of Directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness at any date, the number of years obtained by dividing:
(1) the sum of the products obtained by multiplying (a) the amount of each then remaining installment, sinking fund, serial maturity or other required payments of principal, including payment at final maturity, in respect of the Indebtedness, by (b) the number of years (calculated to the nearest one-twelfth) that will elapse between such date and the making of such payment; by
(2) the then outstanding principal amount of such Indebtedness.
99
“Wholly Owned Restricted Subsidiary” is any Wholly Owned Subsidiary that is a Restricted Subsidiary.
“Wholly Owned Subsidiary” of any Person means a Subsidiary of such Person, 100% of the outstanding Capital Stock or other ownership interests of which (other than directors’ qualifying shares and shares issued to foreign nationals under applicable law) shall at the time be owned by such Person or by one or more Wholly Owned Subsidiaries of such Person or by such Person and one or more Wholly Owned Subsidiaries of such Person.
100
UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of the material U.S. federal income tax consequences of ownership and disposition of notes, but it does not purport to be a comprehensive description of all of the tax considerations that may be relevant to a particular person’s decision to acquire notes. This discussion applies only to holders who hold notes as capital assets for U.S. federal income tax purposes. This discussion does not describe all of the U.S. federal income tax consequences that may be relevant to a holder in light of the holder’s particular circumstances or to holders subject to special rules, such as:
| | Ÿ | | certain financial institutions; |
| | Ÿ | | dealers in securities or foreign currencies; |
| | Ÿ | | persons holding notes as part of a hedge, straddle or other integrated transaction; |
| | Ÿ | | persons whose functional currency is not the U.S. dollar; |
| | Ÿ | | partnerships or other entities classified as partnerships for U.S. federal income tax purposes; |
| | Ÿ | | persons subject to the alternative minimum tax; or |
| | Ÿ | | tax exempt organizations. |
This discussion is based on the Internal Revenue Code of 1986, as amended, administrative pronouncements, judicial decisions and final, temporary and proposed Treasury regulations, all as of the date hereof, all of which are subject to change, possibly on a retroactive basis. Persons considering the purchase of notes are urged to consult their tax advisors with regard to the application of the U.S. federal income tax laws to their particular situations as well as any tax consequences arising under the laws of any state, local or non-U.S. taxing jurisdiction.
U.S. Holders
As used herein, the term “U.S. Holder” means a beneficial owner of a note that is for U.S. federal income tax purposes:
| | Ÿ | | a citizen or resident of the United States; |
| | Ÿ | | a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States or of any political subdivision thereof; or |
| | Ÿ | | an estate or trust the income of which is subject to United States federal income taxation regardless of its source. |
The term U.S. Holder also includes certain former citizens and residents of the United States.
Payments of Interest
Interest paid on a note will be taxable to a U.S. Holder as ordinary interest income at the time it accrues or is received in accordance with the holder’s method of accounting for U.S. federal income tax purposes.
Regardless of a U.S. Holder’s method of accounting for U.S. federal income tax purposes, a U.S. Holder may elect to include in gross income all interest that accrues on the note (including stated interest and any market discount or de minimis market discount, as discussed below under “—Market
101
Discount,” as adjusted by any amortizable bond premium, as discussed below under “—Amortizable Bond Premium”) in accordance with a constant yield method based on the compounding of interest (a “constant yield election”). Such election must be made on the holders U.S. federal income tax return in the year the notes are acquired and may be revoked only with the permission of the Internal Revenue Service (the “IRS”). U.S. Holders should consult their own tax advisors regarding the rules for the constant yield election.
Market Discount
If a U.S. Holder purchases a note for an amount that is less than its principal amount, the difference will be treated as market discount for U.S. federal income tax purposes, unless this difference is less than a specified de minimis amount.
A U.S. Holder will be required to treat any gain on the sale, exchange or other disposition of a note as ordinary income to the extent of any market discount accrued on the note at the time of such sale, exchange or other disposition unless the market discount has been previously included in income by the holder pursuant to (i) an election by the holder to include market discount in income as it accrues, or (ii) a constant yield election.
If the note is disposed of in certain nontaxable transactions, a U.S. Holder generally must include any accrued market discount as ordinary income as if such holder had sold the note in a taxable transaction at its then fair market value. In addition, the holder may be required to defer, until the maturity of the note or its earlier disposition (including certain nontaxable transactions), the deduction of all or a portion of the interest expense on any indebtedness incurred or maintained to purchase or carry such note.
Amortizable Bond Premium
If a U.S. Holder purchases a note for an amount that is greater than its principal amount, the difference will be treated as amortizable bond premium for U.S. federal income tax purposes. In general, the holder may elect to amortize this premium, using a constant yield method, over the remaining term of the note. A U.S. Holder generally may use the amortizable bond premium allocable to an accrual period to offset interest required to be included in such holder’s income with respect to the note in that accrual period. A U.S. Holder who elects to amortize bond premium must reduce his tax basis in the note by the amount of the premium amortized in any year. An election to amortize bond premium applies to all taxable debt obligations then owned and thereafter acquired by the holder and may be revoked only with the consent of the IRS.
If a U.S. Holder makes a constant yield election described above under “—Payments of Interest” for a note with amortizable bond premium, such election will result in a deemed election to amortize bond premium for all of the holder’s debt instruments with amortizable bond premium and may be revoked only with the permission of the IRS with respect to debt instruments acquired after revocation.
Sale, Exchange or Other Disposition of the Notes
Upon the sale, exchange or other disposition of a note, a U.S. Holder will recognize taxable gain or loss equal to the difference between the amount realized on the sale, exchange or other disposition and the holder’s adjusted tax basis in the note. For these purposes, the amount realized does not include any amount attributable to accrued interest. Amounts attributable to accrued interest are treated as interest as described under “—Payments of Interest” above.
Except as described above under “—Market Discount,” gain or loss realized on the sale, exchange or other disposition of a note will generally be capital gain or loss and will be long-term
102
capital gain or loss if at the time of sale, exchange or other disposition the note has been held for more than one year. In the case of certain non-corporate U.S. Holders, long-term capital gains are currently subject to a maximum U.S. federal tax rate of 15%. The availability of capital losses by U.S. Holders is subject to limitations.
Information Reporting and Backup Withholding
Information returns will be filed with the Internal Revenue Service (the “IRS”) in connection with payments on the notes and the proceeds from a sale or other disposition of the notes. A U.S. Holder will be subject to backup withholding on these payments if the U.S. Holder fails to provide its taxpayer identification number to the paying agent and comply with certain certification procedures or otherwise establish an exemption from backup withholding. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle the holder to a refund, provided that the required information is furnished to the IRS.
Non-U.S. Holders
As used herein, the term “Non-U.S. Holder” means a beneficial owner of a note that is, for U.S. federal income tax purposes:
| | Ÿ | | an individual who is classified as a nonresident for U.S. federal income tax purposes; |
| | Ÿ | | a foreign corporation; or |
| | Ÿ | | a foreign estate or trust. |
“Non-U.S. Holder” does not include a holder who is an individual present in the United States for 183 days or more in the taxable year of disposition and who is not otherwise a resident of the United States for U.S. federal income tax purposes. Such a holder is urged to consult his or her own tax advisor regarding the U.S. federal income tax consequences of the sale, exchange or other disposition of a note.
Subject to the discussion below concerning backup withholding:
| | Ÿ | | Payments of principal, interest and premium on the notes by the Company or any paying agent to any Non-U.S. Holder will not be subject to U.S. federal withholding tax, provided that, in the case of interest, |
| | • | | the holder does not own, actually or constructively, 10% or more of the total combined voting power of all classes of stock of the Company entitled to vote and is not a controlled foreign corporation related, directly or indirectly, to the Company through stock ownership; and |
| | • | | the certification requirement described below has been fulfilled with respect to the beneficial owner. |
| | Ÿ | | A Non-U.S. Holder of a note will not be subject to U.S. federal income tax on gain realized on the sale, exchange or other disposition of such note, unless the gain is effectively connected with the conduct by the holder of a trade or business in the United States, subject to an applicable income tax treaty providing otherwise. |
Certification Requirement
Interest will not be exempt from withholding tax unless the beneficial owner of that note certifies on IRS Form W-8BEN, under penalties of perjury, that it is not a United States person.
103
If a Non-U.S. Holder of a note is engaged in a trade or business in the United States, and if interest on the note is effectively connected with the conduct of this trade or business, the Non-U.S. Holder, although exempt from the withholding tax discussed in the preceding paragraph, will generally be taxed in the same manner as a U.S. Holder (see “—U.S. Holders” above), subject to an applicable income tax treaty providing otherwise, except that the holder will be required to provide to the Company a properly executed IRS Form W-8ECI in order to claim an exemption from withholding tax. These holders should consult their own tax advisors with respect to other U.S. tax consequences of the ownership and disposition of notes, including the possible imposition of a 30% branch profits tax.
U.S. Federal Estate Tax
Individual Non-U.S. Holders and entities the property of which is potentially includible in such an individual’s gross estate for U.S. federal estate tax purposes (for example, a trust funded by such an individual and with respect to which the individual has retained certain interests or powers), should note that, absent an applicable treaty benefit, a note or coupon will be treated as U.S. situs property subject to U.S. federal estate tax if payments on the note, if received by the decedent at the time of death, would have been:
| | Ÿ | | subject to U.S. federal withholding tax (even if the W-8BEN certification requirement described above were satisfied); or |
| | Ÿ | | effectively connected to the conduct by the holder of a trade or business in the United States. |
Information Reporting and Backup Withholding
Information returns will be filed with the IRS in connection with payments on the notes. Unless the Non-U.S. Holder complies with certification procedures to establish that it is not a United States person, information returns may be filed with the IRS in connection with the proceeds from a sale or other disposition and the Non-U.S. Holder may be subject to backup withholding on payments on the notes or on the proceeds from a sale or other disposition of the notes. The certification procedures required to claim the exemption from withholding tax on interest described above will satisfy the certification requirements necessary to avoid backup withholding as well. The amount of any backup withholding from a payment to a Non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle the holder to a refund, provided that the required information is furnished to the IRS.
104
PLAN OF DISTRIBUTION
This prospectus has been prepared for use by J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC in connection with offers and sales of the notes in market making transactions effected from time to time. J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC may act as principal or agent in these transactions. These sales will be made at prevailing market prices at the time of sale. We will not receive any of the proceeds of these sales. We have agreed to indemnify J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC against certain liabilities, including liabilities under the Securities Act, and to contribute payments which J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC might be required to make in respect thereof.
As of March 31, 2007, affiliated funds of J.P. Morgan Partners, LLC, an affiliate of J.P. Morgan Securities Inc., owned approximately 15.2% of the outstanding share capital of Warner Chilcott Limited and certain of our directors are employed by an affiliate of J.P. Morgan Securities Inc. As of March 31, 2007, affiliated funds of DLJ Merchant Banking III, Inc., an affiliate of Credit Suisse Securities (USA) LLC, owned approximately 15.2% of the outstanding share capital of Warner Chilcott Limited and certain of our directors are employed by an affiliate of Credit Suisse Securities (USA) LLC.
105
LEGAL MATTERS
Davis Polk & Wardwell, New York, New York has passed upon the validity of the notes on our behalf.
EXPERTS
The financial statements of Warner Chilcott Limited as of and for the years ended December 31, 2006 and 2005 and of Warner Chilcott PLC for the three months ended December 31, 2004 and for the year ended September 30, 2004 incorporated in this prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2006, have been so incorporated by reference in reliance on the reports of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
INFORMATION INCORPORATED BY REFERENCE
The following documents previously filed with the SEC are hereby incorporated by reference in this prospectus (other than filings or portions of filings that are furnished under the applicable SEC rules rather than filed):
| | Ÿ | | Our Annual Report on Form 10-K for the year ended December 31, 2006, filed with the SEC on March 26, 2007; |
| | Ÿ | | Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007, filed with the SEC on May 11, 2007; |
| | Ÿ | | Our Current Report on Form 8-K filed with the SEC on May 11, 2007; |
| | Ÿ | | Our Proxy Statement on Schedule 14A for our 2007 Annual Meeting of Shareholders, filed with the SEC on April 25, 2007. |
We hereby undertake to provide without charge to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, upon written or oral request of any such person, a copy of any and all of the information that has been or maybe incorporated by reference in this prospectus, other than exhibits to such documents unless such exhibits have been specifically incorporated by reference thereto. Requests for such copies should be directed to our Investor Relations department, at the following address:
Warner Chilcott Corporation
Rochelle Fuhrmann
rfuhrmann@wcrx.com
100 Enterprise Drive
Rockaway, New Jersey 07866
(973) 442-3200
We have filed with the SEC, Washington, D.C. 20549, a registration statement on Form S-1 under the Securities Act with respect to the notes offered hereby. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules thereto. For further information with respect to Warner Chilcott Corporation, Warner Chilcott Limited and the other guarantors of the notes and the notes, reference is made to the registration statement and the exhibits and any schedules filed therewith. Statements contained in this prospectus as to the contents of any contract or other document referred to are not necessarily complete and in each instance, if such
106
contract or document is filed as an exhibit, reference is made to the copy of such contract or other document filed as an exhibit to the registration statement, each statement being qualified in all respects by such reference. A copy of the registration statement, including the exhibits and schedules thereto, may be read and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that site is http://www.sec.gov.
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, and file periodic reports and other information with the SEC. We also furnish our shareholders with annual reports containing consolidated financial statements certified by an independent public accounting firm. We also maintain an Internet site at www.warnerchilcott.com. Our website and the information contained therein or connected thereto shall not be deemed to be incorporated into this prospectus or the registration statement of which it forms a part.
107




