Exhibit 99.1
MyMD Pharmaceuticals’ Lead Compound MYMD-1 Demonstrates Superior Anti-inflammatory Effects over Top-Selling Therapies in a Pivotal Preclinical Model of Rheumatoid Arthritis
MyMD seeks to disrupt the $31 billion global market for rheumatoid arthritis (RA) drug therapies with MYMD-1, which could potentially prove safer, more effective and better tolerated than current options
Submission of Investigational New Drug (IND) application and protocols for a Phase 2 RA study planned for second half of 2022
BALTIMORE, MD. – April 12, 2022 – MyMD Pharmaceuticals, Inc. (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage pharmaceutical company committed to extending healthy lifespan, today announced positive new preclinical study data demonstrating the potential effectiveness of MYMD-1 for the treatment of rheumatoid arthritis (RA). MYMD-1 demonstrated a significantly greater anti-inflammatory effect than current TNF-alpha inhibitors on the market, the top three of which represent an estimated $31 billion market (based on 2020 revenues)1.
The comparative study of arthritis, using the CAIA model2, was conducted and analyzed by Charles River Laboratories International, a full-service contract research organization for drug discovery and development. Study results showed that MYMD-1 inhibited inflammation more effectively in the RA model by 30% and 70% of the top two marketed drugs, respectively.
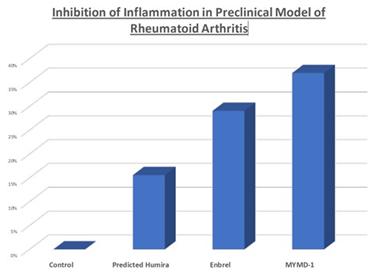
3
1 https://www.yahoo.com/now/abbvies-humira-still-number-1-224014673.html
2 Animal models of arthritis mimic many of the features of arthritis in humans and have been used successfully in establishing proof of concept for new treatment compounds. Of the animal models available, the murine Collagen Antibody-Induced Arthritis (CAIA) model is modeled after RA, and is widely used because of the similar putative etiology to human disease. Moreover, microscopic changes associated with rheumatoid arthritis (e.g., inflammation, bone erosion, and bone degeneration) are observed in CAIA control animals.
3 Predicted Humira response was calculated based on previous comparisons of Enbrel vs. Humira in the same CAIA model during a previous experiment by Charles River.
“We are excited about the impressive new data we are announcing today that shows a highly favorable comparison of our lead drug candidate MYMD-1 to the gold standard rheumatoid arthritis drug therapies currently on the market today. A small fraction of this enormous $31 billion market represents a highly attractive opportunity for MyMD,” said Chris Chapman, M.D., President, Director and Chief Medical Officer of MyMD.
“Utilizing the strong proof-of-concept and efficacy data from our pre-clinical model, we are preparing to submit to the FDA an Investigational New Drug (IND) application for MYMD-1 as treatment for RA, along with protocols for a Phase 2 study, in the second half of this year.”
In addition to the greater efficacy shown in the preclinical model of RA, we believe that MYMD-1 holds significant advantages over current anti-TNF-alpha therapies:
| ● | Smaller molecular size with easy access throughout the body, including the brain; | |
| ● | Selective inhibition of TNF-alpha production by lymphocytes involved in autoimmune disease, but not macrophages involved in front line immune protection against bacteria and viral infections; | |
| ● | Not shown to cause serious side effects in preclinical studies that is common with traditional therapies that treat inflammation; | |
| ● | Simultaneous inhibition of pro-inflammatory cytokines TNF-alpha, IL-6 and IL-17; and | |
| ● | Ease of oral dosing rather than by needle injection. Drug candidate MYMD-1 is the only TNF-alpha inhibitor that is dosed orally. |
MyMD is currently conducting a Phase 2 clinical trial of MYMD-1 as a therapy for delaying aging and expanding healthy lifespan. The primary endpoint for the Phase 2 double-blind, placebo-controlled clinical trial is to achieve a reduction in circulating levels of TNF-alpha within 28 days of therapy.
In addition to aging and rheumatoid arthritis, MYMD-1’s distinct action in regulating the immune system and treating chronic inflammation is being developed for the treatment of multiple sclerosis (MS), diabetes, and inflammatory bowel disease.
About Rheumatoid Arthritis
Rheumatoid arthritis is a chronic autoimmune disease and an inflammatory form of arthritis. The disorder preferentially attacks the joints and is characterized by inflammation and bone erosion. In the progression of RA, joints are infiltrated by white blood cells that produce the pro-inflammatory cytokines TNF-alpha, IL-6 and IL-17. Rheumatoid arthritis is the most common form of autoimmune arthritis, affecting more than 1.3 million Americans4 and occuring at any age. Studies have determined that the number of people suffering from rheumatoid arthritis may rise to over 78 million by 20405.
About MYMD-1
Originally developed for autoimmune diseases, MYMD-1’s primary purpose is to slow the aging process, prevent sarcopenia and frailty, and extend healthy lifespan. Because it can cross the blood-brain barrier and gain access to the central nervous system (CNS), MYMD-1 is also positioned to be a possible treatment for brain-related disorders. Its mechanism of action and efficacy in diseases including multiple sclerosis (MS) and thyroiditis have been studied through collaborations with several academic institutions.
MYMD-1 has shown effectiveness in pre-clinical and clinical studies in regulating the immune system by performing as a selective inhibitor of tumor necrosis factor-alpha (TNF-α), a driver of chronic inflammation. Unlike other therapies, MYMD-1 has been shown in these studies to selectively block TNF-α when it becomes overactivated in autoimmune diseases and cytokine storms, but not block it from doing its normal job of being a first responder to any routine type of moderate infection. MYMD-1’s ease of oral dosing is another differentiator compared to currently available TNF-α blockers, all of which require delivery by injection or infusion. No approved TNF inhibitor has ever been dosed orally. In addition, the drug is not immunosuppressive and has not been shown to cause the serious side effects common with traditional therapies that treat inflammation.
4 https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis
5 Data published by Research and Markets, March 16, 2018
About MyMD Pharmaceuticals, Inc.
MyMD Pharmaceuticals, Inc. (Nasdaq: MYMD), a clinical stage pharmaceutical company committed to extending healthy lifespan, is focused on developing two novel therapeutic platforms that treat the causes of disease rather than only addressing the symptoms. MYMD-1 is a drug platform based on a clinical stage small molecule that regulates the immune system to control TNF-α, which drives chronic inflammation, and other pro-inflammatory cell signaling cytokines. MYMD-1 is being developed to delay aging, increase longevity, and treat autoimmune diseases and COVID-19- associated depression. The Company’s second drug platform, Supera-CBD, is being developed to treat chronic pain, addiction and epilepsy. Supera-CBD is a novel synthetic derivative of cannabidiol (CBD) and is being developed to address and improve upon the rapidly growing CBD market, which includes both FDA approved drugs and CBD products not currently regulated as drugs. For more information, visit www.mymd.com.
Cautionary Statement Regarding Forward-Looking Statements
This press release may contain forward-looking statements. These forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any expected future results, performance, or achievements. Forward-looking statements speak only as of the date they are made and none of MyMD nor its affiliates assume any duty to update forward-looking statements. Words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “may,” “plan,” “will,” “would’’ and other similar expressions are intended to identify these forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, without limitation: the timing of, and MyMD’s ability to, obtain and maintain regulatory approvals for clinical trials of MyMD’s pharmaceutical candidates; the timing and results of MyMD’s planned clinical trials for its pharmaceutical candidates; the amount of funds MyMD requires for its pharmaceutical candidates; increased levels of competition; changes in political, economic or regulatory conditions generally and in the markets in which MyMD operates; MyMD’s ability to retain and attract senior management and other key employees; MyMD’s ability to quickly and effectively respond to new technological developments; MyMD’s ability to protect its trade secrets or other proprietary rights, operate without infringing upon the proprietary rights of others and prevent others from infringing on MyMD’s proprietary rights; and the impact of the ongoing COVID-19 pandemic on MyMD’s results of operations, business plan and the global economy. A discussion of these and other factors with respect to MyMD is set forth in the Company’s Annual Report on Form 10-K for the year ended December 31, 2021, filed by MyMD on March 31, 2022. Forward-looking statements speak only as of the date they are made and MyMD disclaims any intention or obligation to revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Investor Contact:
Robert Schatz
(646) 421-9523
rschatz@mymd.com
www.mymd.com
Media Contact:
media@mymd.com