QuickLinks -- Click here to rapidly navigate through this documentAs filed with the Securities and Exchange Commission on May 23, 2007
Registration No. 333-141655
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
NovaCardia, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | | 2834 | | 33-0989994 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification Number) |
12750 High Bluff Drive, Suite 300
San Diego, CA 92130
(858) 509-0455
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant's Principal Executive Offices)
Randall E. Woods
President and Chief Executive Officer
NovaCardia, Inc.
12750 High Bluff Drive, Suite 300
San Diego, CA 92130
(858) 509-0455
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copies to:
Barbara L. Borden, Esq.
J. Patrick Loofbourrow, Esq.
Cooley Godward Kronish LLP
4401 Eastgate Mall
San Diego, CA 92121
(858) 550-6000 | | Charles K. Ruck, Esq.
Cheston J. Larson, Esq.
Latham & Watkins LLP
12636 High Bluff Drive, Suite 400
San Diego, CA 92130
(858) 523-5400
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the "Securities Act"), check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell or accept an offer to buy the securities under this preliminary prospectus until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and we are not soliciting an offer to buy these securities in any state where such offer or sale is not permitted.
Subject to completion, dated May 23, 2007
Prospectus
shares

Common stock
This is an initial public offering of shares of common stock by NovaCardia, Inc. NovaCardia is selling shares of common stock. The estimated initial public offering price is between $ and $ per share.
We have applied for listing of our common stock on the NASDAQ Global Market under the symbol NCAR.
|
| | Per share
| | Total
|
|---|
|
| Initial public offering price | | $ | | | $ | |
Underwriting discounts and commissions |
|
$ |
|
|
$ |
|
Proceeds to NovaCardia, before expenses |
|
$ |
|
|
$ |
|
|
|
|
|
|
|
|
|
NovaCardia has granted to the underwriters an option for a period of 30 days to purchase up to additional shares of common stock.
Investing in the common stock involves a high degree of risk. See "Risk factors" beginning on page 8.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| JPMorgan | | Credit Suisse |
Pacific Growth Equities, LLC |
|
First Albany Capital
|
, 2007
Table of contents
| | Page
|
|---|
| Prospectus summary | | 1 |
| Risk factors | | 8 |
| Forward-looking statements | | 40 |
| Use of proceeds | | 42 |
| Dividend policy | | 43 |
| Capitalization | | 44 |
| Dilution | | 47 |
| Selected financial data | | 50 |
| Management's discussion and analysis of financial condition and results of operations | | 52 |
| Business | | 67 |
| Management | | 91 |
| Executive compensation | | 99 |
| Transactions with related persons | | 119 |
| Principal stockholders | | 126 |
| Description of capital stock | | 129 |
| Shares eligible for future sale | | 135 |
| Material U.S. federal income tax consequences to non-U.S. holders | | 138 |
| Underwriting | | 141 |
| Legal matters | | 145 |
| Experts | | 145 |
| Where you can find more information | | 145 |
| Index to financial statements | | F-1 |
You should rely only on information contained or incorporated by reference in this prospectus. We have not authorized anyone to provide you with information different from or in addition to that contained in this prospectus. We are offering to sell, and seeking offers to buy, common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of shares of our common stock.
No action is being taken in any jurisdiction outside the United States to permit a public offering of the shares of our common stock or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to that jurisdiction.
Prospectus summary
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially "Risk factors" and our financial statements and notes to these financial statements, before deciding to invest in shares of our common stock.
NovaCardia, Inc.
We are a clinical-stage pharmaceutical company focused on developing drugs to treat major cardiovascular diseases that are underserved by existing therapies. We have two compounds in clinical development, KW-3902 for congestive heart failure, or CHF, and K201 (JTV-519) for atrial fibrillation, or A-Fib, an irregular heartbeat. We believe each compound has potential for use in both acute and chronic settings.
We are currently testing the intravenous, or IV, formulation of KW-3902 in Phase 3 clinical trials for the treatment of the acute form of CHF in patients with renal impairment and fluid overload. We expect to have the results from our pivotal Phase 3 trials in mid-2008. We also plan to initiate a Phase 2 clinical trial of the oral formulation of KW-3902 for the treatment of chronic CHF in the second half of 2007. In addition, we intend to initiate a Phase 2 trial of the IV formulation of K201 (JTV-519) for the acute treatment of A-Fib in the second half of 2007.
We hold commercial rights to our product candidates in major markets, including the United States and Europe, but excluding Asia, and plan to establish a hospital-based sales force to market the IV formulations of our product candidates in the United States. We plan to partner with third parties for the commercialization of our IV product candidates outside the United States and for the further development and commercialization of our oral product candidates in all regions where we have commercial rights. To expand our product pipeline, we intend to in-license additional product candidates with the potential to treat cardiovascular diseases.
KW-3902
There are nearly five million people in the United States with CHF, according to the American Heart Association, or AHA. Many CHF patients experience acute CHF, a rapid deterioration of their condition, requiring urgent treatment in the hospital. Renal function is an important determinant of the management and outcome of both acute and chronic CHF, and we estimate that more than half of CHF patients have some degree of renal impairment, based on a study published inCirculation in 2004. Worsening renal function during hospital stay is a strong predictor of negative outcomes, including length of hospital stay and mortality. Fluid overload is one of the major causes of hospitalization for CHF patients, and approximately 80% of CHF patients are on chronic diuretic therapy to manage their fluid overload at the time of their hospitalization, according to the ADHERE Registry, a registry of acute heart failure patient cases in the United States. However, the use of diuretic therapy, such as Lasix, to manage fluid overload in CHF patients may lead to further deterioration of renal function. As a result, we believe there is a significant need for a new therapy that protects renal function while further reducing fluid overload in both acute and chronic CHF patients.
1
KW-3902 is an adenosine A1 receptor antagonist that selectively blocks the effect of adenosine on the adenosine A1 receptor. In the kidneys, adenosine acts through the adenosine A1 receptor to restrict blood flow to the kidneys, which may result in worsening renal function and may also increase reabsorption of salt and water, thereby contributing to fluid overload. By blocking the adenosine A1 receptor, KW-3902 increases blood flow to the kidneys and reduces reabsorption of salt and water. We believe that these effects have the potential to improve the treatment of CHF patients by preserving renal function, improving the symptoms of fluid overload and enabling the more effective use of other CHF therapies. Therefore, KW-3902 may improve short- and long-term outcomes in CHF patients. We are developing IV and oral formulations of KW-3902 to be co-administered with diuretics for the treatment of acute and chronic CHF, respectively.
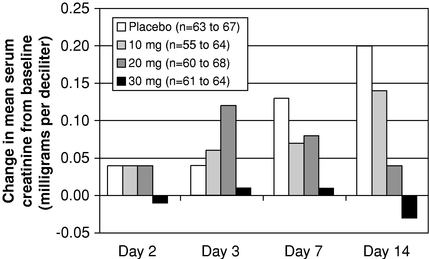
We have completed three multi-center, placebo-controlled Phase 2 clinical trials of the IV formulation of KW-3902 in which we demonstrated that KW-3902 preserves renal function and improves diuresis in acute CHF patients. In addition, in March 2007, we completed a preliminary analysis of data obtained from 276 patients in our 304-patient pilot Phase 3 clinical trial of KW-3902. In this analysis, we observed an improvement in patients' shortness of breath, a symptom for which CHF patients are frequently hospitalized, and a persistent maintenance of renal function. In addition, we confirmed the optimal dosing regimen for our Phase 3 trials and we did not observe an adverse safety profile for KW-3902.
We initiated two pivotal Phase 3 clinical trials of the IV formulation of KW-3902 in May 2007 and expect to have results from these trials in mid-2008. We have designed these pivotal trials to examine the effect of KW-3902 on dyspnea, or shortness of breath, renal function and worsening heart failure. We plan to initiate two additional Phase 3 trials of the IV formulation of KW-3902 and expect to commence one of these trials in mid-2007 and the other trial in the third quarter of 2007. We plan to commence a Phase 2 clinical trial of an oral formulation of KW-3902 in outpatients with chronic CHF in the second half of 2007.
K201 (JTV-519)
We are developing K201 (JTV-519) to treat A-Fib, a condition that afflicts over 2.2 million people in the United States and leads to over 440,000 hospitalizations per year, according to the most recent data from the AHA. A-Fib is an independent risk factor for stroke and increases the severity of symptoms experienced by CHF patients.
We are developing IV and oral formulations of K201 (JTV-519) to address acute and chronic A-Fib, respectively. We believe K201 (JTV-519) has the potential to treat A-Fib without the side effects, such as life-threatening ventricular arrhythmias, associated with current therapies. We plan to commence a Phase 2 clinical trial of the IV formulation of K201 (JTV-519) for the acute treatment of A-Fib in the second half of 2007.
2
Strategy
Our strategy is to in-license, develop and commercialize drugs to treat major cardiovascular diseases that are underserved by existing therapies. We intend to:
- •
- rapidly develop and commercialize our product candidates;
- •
- partner with third parties for the commercialization of our IV product candidates outside the United States and for the further development and commercialization of our oral product candidates in all regions where we hold commercial rights;
- •
- establish a hospital-based sales force to sell our products in the acute care setting in the United States; and
- •
- in-license additional cardiovascular product candidates.
Risks relating to our business
Our business and our ability to execute on our business strategy are subject to a number of risks of which you should be aware before you decide to buy our common stock. In particular, you should consider the following risks, which are discussed more fully in "Risk factors" beginning on page 8:
- •
- We are highly dependent on the success of our two product candidates, KW-3902 and K201 (JTV-519), and we cannot give any assurance that either product candidate will receive regulatory approval or be successfully commercialized.
- •
- KW-3902 and K201 (JTV-519) may not demonstrate adequate efficacy, may cause undesirable side effects or may have other properties that could delay or prevent their regulatory approval or commercialization or limit their commercial potential.
- •
- The timing of clinical development activities is hard to predict, and delays in the clinical trials for either of our product candidates could result in increased costs and delay or limit our ability to obtain regulatory approval.
- •
- We rely on third parties to conduct our clinical trials and manufacture our product candidates, and we cannot be sure that they will successfully carry out their contractual duties or meet expected deadlines.
- •
- If we fail to enter into collaborations with respect to the oral formulations of our product candidates, we may not be able to execute this portion of our business strategy.
- •
- Even if our product candidates are approved by regulatory authorities, they may still face regulatory and commercialization difficulties and will face intense competition.
- •
- Our two product candidates, KW-3902 and K201 (JTV-519), are both in-licensed from third parties, and any dispute with these parties may adversely affect our ability to develop and commercialize these product candidates.
- •
- We have had a history of losses since our inception, and we expect that losses will increase in future periods. Our net loss was $17.9 million in 2006 and $8.5 million during the first quarter of 2007 and, as of March 31, 2007, we had an accumulated deficit of $51.5 million.
- •
- We may not have adequate intellectual property protection for our product candidates.
3
Corporate information
We were incorporated in Delaware in November 2001. Our principal executive offices are located at 12750 High Bluff Drive, Suite 300, San Diego, California 92130 and our telephone number is (858) 509-0455. Our corporate website address iswww.novacardia.com. We do not incorporate the information contained on, or accessible through, our website into this prospectus, and you should not consider it part of this prospectus. Unless the context indicates otherwise, as used in this prospectus, the terms "NovaCardia," "we," "us" and "our" refer to NovaCardia, Inc., a Delaware corporation.
We have applied for registration of the trademark "NovaCardia" and our NovaCardia logo with the United States Patent and Trademark Office. This prospectus also contains trademarks and tradenames of other companies, including Lasix® and ADENTRI™, and those trademarks and tradenames are the property of their respective owners.
4
The offering
| Common stock offered by NovaCardia | | shares |
| Common stock to be outstanding after this offering | | shares |
| Over-allotment option | | We have granted the underwriters an option for a period of 30 days to purchase up to additional shares of common stock. |
| Use of proceeds | | We intend to use the net proceeds from this offering to fund clinical development of KW-3902 and K201 (JTV-519) and for working capital and general corporate purposes. See "Use of proceeds" beginning on page 42. |
| Proposed NASDAQ Global Market symbol | | NCAR |
The number of shares of common stock that will be outstanding after this offering is based on 88,543,718 shares outstanding as of March 31, 2007, after giving pro forma effect to the assumed cash exercise of warrants to purchase 548,800 shares of our Series B preferred stock outstanding as of March 31, 2007 that will terminate if not exercised prior to the effectiveness of this offering, and excludes:
- •
- 7,609,832 shares of common stock subject to outstanding options under our 2003 equity incentive plan, with a weighted average exercise price of $0.21 per share;
- •
- 1,233,949 shares of common stock reserved for future issuance under our 2003 equity incentive plan;
- •
- 18,000,000 shares of common stock reserved for future issuance under our 2007 equity incentive plan, 2007 non-employee directors' stock option plan and 2007 employee stock purchase plan, each of which will become effective upon the completion of this offering;
- •
- 663,809 shares of common stock subject to outstanding warrants, with a weighted average exercise price of $0.97 per share; and
- •
- up to 933,333 shares of common stock subject to an outstanding warrant issued in April 2007, with an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts under our credit facility (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price).
Unless otherwise stated, all information contained in this prospectus assumes:
- •
- a 1-for- reverse stock split of our common stock to be effected immediately prior to the completion of this offering;
- •
- the conversion of all outstanding shares of our preferred stock, including 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering, into an aggregate of 85,817,499 shares of common stock upon the completion of this offering;
- •
- the filing of our amended and restated certificate of incorporation and adoption of our amended and restated bylaws, which will occur upon the completion of this offering; and
- •
- no exercise of the underwriters' over-allotment option.
5
Summary financial data
The following tables present our summary financial data and should be read together with our financial statements and notes and "Management's discussion and analysis of financial condition and results of operations" appearing elsewhere in this prospectus. The summary statement of operations data for the years ended December 31, 2004, 2005 and 2006, and the summary balance sheet data as of December 31, 2006, are derived from our audited financial statements, which are included elsewhere in this prospectus. The summary statement of operations data for the period from November 30, 2001 (inception) through March 31, 2007 and for the three months ended March 31, 2006 and 2007, and the summary balance sheet data as of March 31, 2007, are derived from our unaudited financial statements, which are included elsewhere in this prospectus. Our historical results are not necessarily indicative of our future results.
| |
| | Years ended December 31,
| | Three months
ended March 31,
| |
| |
|---|
| | Period from
November 30, 2001
(inception) through
March 31, 2007
| |
|---|
(in thousands, except per share data)
| |
|---|
| | 2004
| | 2005
| | 2006
| | 2006
| | 2007
| |
|---|
| |
| Statement of operations data: | | | | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | |
| | Development | | $ | 6,343 | | $ | 9,023 | | $ | 14,184 | | $ | 1,776 | | $ | 7,576 | | $ | 41,699 | |
| | Selling, general and administrative | | | 1,782 | | | 1,973 | | | 2,124 | | | 402 | | | 1,219 | | | 8,573 | |
| | |
| |
| Total operating expenses | | | 8,125 | | | 10,996 | | | 16,308 | | | 2,178 | | | 8,795 | | | 50,272 | |
| | |
| |
| Loss from operations | | | (8,125 | ) | | (10,996 | ) | | (16,308 | ) | | (2,178 | ) | | (8,795 | ) | | (50,272 | ) |
| Other income (expense): | | | | | | | | | | | | | | | | | | | |
| | Interest income | | | 173 | | | 139 | | | 560 | | | 20 | | | 375 | | | 1,322 | |
| | Interest expense | | | — | | | (203 | ) | | (2,161 | ) | | (246 | ) | | (88 | ) | | (2,509 | ) |
| | |
| |
| Total other income (expense) | | | 173 | | | (64 | ) | | (1,601 | ) | | (226 | ) | | 287 | | | (1,187 | ) |
| | |
| |
| Net loss | | | (7,952 | ) | | (11,060 | ) | | (17,909 | ) | | (2,404 | ) | | (8,508 | ) | | (51,459 | ) |
| Deemed dividend related to beneficial conversion feature on Series B preferred stock | | | — | | | — | | | — | | | — | | | (8,080 | ) | | (8,080 | ) |
| | |
| |
| Net loss attributable to common stockholders | | $ | (7,952 | ) | $ | (11,060 | ) | $ | (17,909 | ) | $ | (2,404 | ) | $ | (16,588 | ) | $ | (59,539 | ) |
| | |
| |
| Basic and diluted net loss per share(1): | | | | | | | | | | | | | | | | | | | |
| | Historical | | $ | (6.09 | ) | $ | (5.96 | ) | $ | (8.37 | ) | $ | (1.21 | ) | $ | (7.13 | ) | | | |
| | |
| | | | |
| | Pro forma | | | | | | | | $ | (0.45 | ) | | | | $ | (0.23 | ) | | | |
| | | | | | | | |
| | | | |
| | | | |
| Shares used to compute basic and diluted net loss per share(1): | | | | | | | | | | | | | | | | | | | |
| | Historical | | | 1,305 | | | 1,855 | | | 2,139 | | | 1,984 | | | 2,326 | | | | |
| | |
| | | | |
| | Pro forma | | | | | | | | | 40,179 | | | | | | 73,148 | | | | |
| | | | | | | | |
| | | | |
| | | | |
| | | | | | | | | | | | | | | | | | | | |
| |
- (1)
- Please see Note 1 to our financial statements for an explanation of the method used to calculate the historical and pro forma net loss per share and the number of shares used in the computation of the per share amounts.
6
|
March 31, 2007
(in thousands)
| | Actual
| | Pro forma(1)
| | Pro forma
as adjusted(2)
|
|---|
|
|---|
| |
| | (unaudited)
|
|---|
| Balance sheet data: | | | | | | | | | |
| Cash, cash equivalents and short-term investments | | $ | 42,509 | | $ | 47,509 | | $ | |
| Working capital | | | 34,301 | | | 40,423 | | | |
| Total assets | | | 48,640 | | | 53,640 | | | |
| Long-term debt (including current portion) | | | 2,631 | | | 7,631 | | | |
| Deficit accumulated during the development stage | | | (51,459 | ) | | (51,459 | ) | | |
| Total stockholders' equity | | | 38,850 | | | 38,850 | | | |
|
- (1)
- The pro forma balance sheet data give effect to $5.0 million borrowed in April 2007 and the restructuring of our outstanding indebtedness in April 2007 in connection with an amendment to our loan and security agreement with Lighthouse Capital Partners V, L.P.
- (2)
- The pro forma as adjusted balance sheet data also give effect to the assumed cash exercise of warrants to purchase 548,800 shares of our Series B preferred stock outstanding as of March 31, 2007 that will terminate if not exercised prior to the effectiveness of this offering at an exercise price of $1.125 per share, resulting in aggregate proceeds of $617,400, the sale of shares of our common stock in this offering at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us and the automatic conversion of all preferred stock, including the 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering. A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering and our cash, cash equivalents and short-term investments, working capital, total assets and total stockholders' equity by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us. The pro forma as adjusted information is illustrative only and, following the completion of this offering, will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
7
Risk factors
An investment in shares of our common stock involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information appearing elsewhere in this prospectus, before deciding to invest in our common stock. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects. In these circumstances, the market price of our common stock could decline, and you may lose all or part of the money you paid to buy our common stock.
We are highly dependent on the success of our two product candidates, KW-3902 and K201 (JTV-519), and we cannot give any assurance that either product candidate will receive regulatory approval or be successfully commercialized.
We are a clinical-stage pharmaceutical company, and we do not currently have any commercial products that generate revenues or any other sources of revenue. We may never be able to successfully develop marketable products. We are dependent on the success of our two product candidates, KW-3902 and K201 (JTV-519). In particular, we are highly dependent on the success of our most advanced product candidate, the intravenous, or IV, formulation of KW-3902, which we in-licensed from Kyowa Hakko Kogyo Co., Ltd., or Kyowa Hakko, in August 2003. We initiated our pivotal Phase 3 clinical trials of the IV formulation of KW-3902 in May 2007 for the treatment of the acute form of congestive heart failure, or CHF, in patients with renal impairment and fluid overload. We plan to commence a Phase 2 clinical trial of an oral formulation of KW-3902 in outpatients with chronic CHF in the second half of 2007.
KW-3902 is an adenosine A1 receptor antagonist, and there are currently no adenosine A1 receptor antagonists that have received regulatory approval from the United States Food and Drug Administration, or FDA, for the treatment of CHF. Although we received favorable results in our Phase 2 clinical trials and pilot Phase 3 clinical trial of the IV formulation of KW-3902, we cannot give any assurance that our pivotal Phase 3 trials for the treatment of acute CHF in patients with renal impairment and fluid overload will achieve the clinical endpoints or serve as the basis for pursuing regulatory approval from the FDA. In particular, based on our discussions with the FDA, we believe that the FDA may require that we define treatment failure in our pivotal trials in a different manner from our pilot trial. This change could alter our ability to achieve favorable results. Although we plan to commence a Phase 2 clinical trial of an oral formulation of KW-3902, we are still optimizing the oral formulation of KW-3902. As such, we have not yet clinically tested any oral formulations. We may not be able to develop a suitable oral formulation of KW-3902.
Our second product candidate, K201 (JTV-519), was in-licensed from Aetas Pharma Co., Ltd., or Aetas, in September 2006. We expect to commence a Phase 2 clinical trial of the IV formulation of K201 (JTV-519) for the acute treatment of atrial fibrillation, or A-Fib, an irregular heartbeat, in the second half of 2007. We have not yet filed an Investigational New Drug Application, or IND, or its foreign equivalent for K201 (JTV-519), and there is no guarantee that we will be able to successfully file an IND or commence Phase 2 trials of K201 (JTV-519) for the treatment of A-Fib on our projected timeline, or at all.
If we are unable to develop, receive approval for or successfully commercialize KW-3902 or K201 (JTV-519), we will not be able to generate product revenues.
8
The clinical trial protocol and design for our planned Phase 3 clinical trials of KW-3902 may not be sufficient to allow us to submit a New Drug Application, or NDA, or demonstrate safety or efficacy at the level required by the FDA for product approval.
The clinical trial protocols and design of our Phase 3 clinical trials of the IV formulation of KW-3902 for the treatment of acute CHF in patients with renal impairment and fluid overload may prove to be insufficient for product approval. We are conducting our Phase 3 clinical trials of the IV formulation of KW-3902 to examine whether KW-3902, when co-administered with the IV diuretic furosemide, or Lasix, to patients with acute CHF will result in:
- •
- an improvement in patient-reported shortness of breath, also known as dyspnea; and
- •
- the absence of the occurrence of any of the following events within a certain time period after the first dosing with KW-3902 or placebo:
- •
- death or readmission to the hospital;
- •
- the worsening of heart failure requiring the use of rescue therapy; or
- •
- the worsening of renal function.
In particular, the measure of a patient's dyspnea and our other clinical endpoints have some subjective elements that are not as clear as other clinical endpoints that are based on specific objective measures. It is possible that the FDA could determine that there is inadequate evidence of dyspnea improvement.
Even if we achieve positive results on the endpoints for a trial, the results may not be sufficient to demonstrate efficacy at the level required by the FDA for product approval. It is possible that we may make modifications to the clinical trial protocols or designs of one or more of our Phase 3 clinical trials of the IV formulation of KW-3902 that delay the enrollment in, or the completion of, our clinical trials or delay our pursuit of regulatory approval of KW-3902.
In March 2006, we met with the FDA to discuss the clinical trial requirements for submission of an NDA for the IV formulation of KW-3902. We have not sought FDA agreement on our current primary endpoint or the statistical plans under which the results of our pivotal clinical trials will be analyzed. We have not submitted and do not plan to submit a Special Protocol Assessment, or SPA, which drug development companies sometimes use to obtain an agreement with the FDA concerning the design and size of a clinical trial intended to form the primary basis of an effectiveness claim. Without the concurrence of the FDA on an SPA or otherwise, we cannot be certain that the design, conduct and data analysis approach for our Phase 3 trials will be sufficient to allow us to submit or receive approval of an NDA for the IV formulation of KW-3902. If the FDA requires us, or we otherwise determine, to amend our protocols, change our clinical trial designs, increase enrollment targets or conduct additional clinical trials, our ability to obtain regulatory approval on our projected timeline would be jeopardized and we could be required to make significant additional expenditures related to clinical development. Any failure to obtain approval of KW-3902 on the timeline that we currently anticipate, or at all, would have a material and adverse impact on our business.
9
Because the results of early clinical trials are not necessarily predictive of future results, KW-3902, K201 (JTV-519) or any other product candidate we advance into clinical trials may not have favorable results in later clinical trials, if any, or receive regulatory approval.
Our product candidates are prone to the risks of failure inherent in drug development. We will be required to demonstrate through well-controlled clinical trials of KW-3902 and K201 (JTV-519) that our product candidates are safe and effective for use in a diverse population for their target indications before we can seek regulatory approvals for their commercial sale. Success in early clinical trials does not mean that later clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety or efficacy despite having progressed through initial clinical testing. All of our Phase 2 clinical trials, as well as our pilot Phase 3 clinical trial, involved relatively small patient populations. Because these patient populations were relatively small, our data from these trials may contain statistical anomalies and may not be predictive of later- stage trials. Moreover, based on our discussions with the FDA, we believe that the FDA may require that we change the definition of treatment failure to be used for the primary endpoint in our pivotal trials from the definition that we used in our pilot trial.
Companies frequently suffer significant setbacks in advanced clinical trials, even after earlier clinical trials have shown promising results. Any of our planned Phase 3 clinical trials of the IV formulation of KW-3902, Phase 2 clinical trial of the oral formulation of KW-3902 or Phase 2 trial of the IV formulation of K201 (JTV-519) may not be successful for a variety of reasons, including the clinical trial designs, the failure to enroll a sufficient number of patients, undesirable side effects and other safety concerns and the inability to demonstrate sufficient efficacy.
We modified the primary endpoint for our two PROTECT trials from that studied in the pilot Phase 3 clinical trial. This change may affect the results we observe in our pivotal Phase 3 trials as compared to the preliminary data from our pilot Phase 3 trial. Further, Phase 3 trials of the IV formulation of KW-3902 will enroll significantly more patients than we have enrolled in clinical trials of KW-3902 to date. The FDA has indicated that a minimum of 1,500 patients must have been administered the IV formulation of KW-3902 at doses that are equal to or above the dose for which we seek regulatory approval. We expect to enroll 2,359 patients in our Phase 3 trials. The data collected from clinical trials with larger patient populations may not demonstrate sufficient safety and efficacy to support regulatory approval of KW-3902 or any other product candidate we advance into clinical trials. If KW-3902, K201 (JTV-519) or any other product candidate we advance into clinical trials fails to demonstrate sufficient safety or efficacy, we would experience potentially significant delays in, or be required to abandon, development of that product candidate.
KW-3902, K201 (JTV-519) and any other product candidates we may advance into clinical development are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays or prevent the receipt of the required approvals to commercialize our product candidates.
The clinical development, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution of KW-3902, K201 (JTV-519) or any future product candidates are subject to extensive regulation by the FDA in the United States and by comparable governmental authorities in foreign markets. In the United States, we are
10
not permitted to market our product candidates until we receive approval of an NDA from the FDA. The process of obtaining NDA approval is expensive, often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. Approval policies or regulations may change and the FDA has substantial discretion in the drug approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product candidates, regulatory approval is never guaranteed. We intend to seek product approvals in countries outside the United States where we have commercial rights, including Europe. As a result, we will be subject to regulation by the European Medicines Agency, or EMEA, as well as the other regulatory agencies in many of these countries. The FDA, EMEA and any of the applicable European and other regulatory bodies can delay, limit or deny approval of a product candidate for many reasons, including:
- •
- a product candidate may not be safe and effective;
- •
- regulatory agencies may not find the data from preclinical testing and clinical trials to be sufficient;
- •
- the FDA may not accept clinical data from trials which are conducted in countries where the standard of care is potentially different from the United States;
- •
- the protocol, design or implementation of our clinical trials may not be consistent with the requirements of each applicable regulatory agency;
- •
- regulatory agencies may not approve of our third-party manufacturers' processes or facilities; or
- •
- regulatory agencies may change their approval policies or adopt new regulations.
In addition, recent events raising questions about the safety of certain marketed drugs may result in increased cautiousness by the FDA and other regulatory authorities in reviewing new drugs based on safety, efficacy or other regulatory considerations and may result in significant delays in obtaining regulatory approvals. Any delay in obtaining, or inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates.
KW-3902, as an adenosine A1 receptor antagonist, increases the risk of seizure, which may delay or prevent its regulatory approval. We also expect that any approved label for KW-3902 will include a contra-indication for some patients with heightened seizure risk and will require other patients with heightened seizure risk to be co-administered one or more anti-seizure medications, which may limit the commercial potential of KW-3902.
KW-3902, like other adenosine A1 receptor antagonists, lowers patient seizure threshold and may contribute to seizures in CHF patients who are already at risk for seizure. In our Phase 2 clinical trials, two patients with risk factors for seizure had seizures that were determined to be drug-related. One of the patients subsequently died. As a result of the increased risk of seizure associated with the administration of KW-3902, we designed our Phase 3 clinical trials to exclude certain patients at risk for seizure, including patients with certain known medical histories, including, but not limited to, brain tumors, history of seizure, brain surgery within two years, alcohol withdrawal, stroke within two years, advanced multiple sclerosis, penetrating head trauma and late-stage Alzheimer's disease, from participating in the trials. In
11
addition, we require patients believed to have an elevated risk of seizure who are not excluded from our Phase 3 trials by the above criteria to take a prophylactic anti-seizure medication that raises the seizure threshold. We also extended the infusion time of KW-3902 to four hours. Although no seizures were observed in the 304 patients enrolled in our pilot Phase 3 clinical trial of the IV formulation of KW-3902, of which 225 received one or more doses of KW-3902, we believe that, given the baseline rate of seizures in heart failure patients and the size of the patient populations expected to be enrolled in these pivotal Phase 3 trials, some patients will likely experience seizures. KW-3902 could fail to receive regulatory approval for safety reasons if the FDA determines that the number of patients who experience seizures during our pivotal Phase 3 trials is unacceptable.
Even if we are able to obtain regulatory approval of the IV formulation of KW-3902 in acute CHF patients with renal impairment and fluid overload, we expect that the label will require that certain patients with heightened seizure risk will not be prescribed KW-3902 and that other patients with a heightened seizure risk be co-administered a benzodiazepine or another type of anti-seizure medication. Physicians may decide not to administer KW-3902 due to concerns over the potential side effects or other impact of administering an anti-seizure medication to an acute CHF patient. This or other concerns may diminish the usage of KW-3902 and limit its commercial success.
KW-3902, K201 (JTV-519) or any other product candidate we advance into clinical trials may cause undesirable side effects or have other properties that may delay or prevent their regulatory approval or commercialization or limit their commercial potential.
Undesirable side effects caused by KW-3902, K201 (JTV-519) or any other product candidate we advance into clinical trials could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications. This, in turn, could prevent us from commercializing the affected product candidate and generating revenues from its sale. In addition to seizure, other side effects were observed in our clinical trials of KW-3902 to date. The most common side effects observed in patients who received either KW-3902 or the IV placebo were worsening CHF, abnormal heart rhythms, kidney and urinary problems, hypokalemia (a low amount of potassium in the blood), aches and cramps, headaches, low blood pressure, nausea, constipation, hypoglycemia, insomnia, dizziness, anxiety and anemia.
We have not yet tested K201 (JTV-519) for the treatment of patients with A-Fib and we currently do not know what side effects, if any, will be observed in patients with A-Fib who receive K201 (JTV-519). Although K201 (JTV-519) has been administered to date in 141 people, including both healthy volunteers and patients with acute coronary syndrome, it has not been administered to treat A-Fib in humans.
In addition, if KW-3902, K201 (JTV-519) or any other product candidate we develop receives marketing approval and we or others later identify undesirable side effects caused by the product, a number of significant negative consequences could result, including:
- •
- regulatory authorities may withdraw their approval of the affected product;
- •
- regulatory authorities may require a larger clinical benefit for approval to offset the risk;
12
- •
- regulatory authorities may require the addition of labeling statements that could diminish the usage of the product or otherwise limit the commercial success of the affected product;
- •
- we may be required to change the way the affected product is administered, conduct additional clinical trials or change the labeling of the product;
- •
- we may choose to discontinue sale of the affected product;
- •
- we could be sued and held liable for harm caused to patients;
- •
- we may not be able to enter into collaboration agreements on acceptable terms and execute on our business model; and
- •
- our reputation may suffer.
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing the affected product, which in turn could delay or prevent us from generating any revenues from the sale of the affected product.
We may experience delays in the commencement of our clinical trials, which could result in increased costs to us and delay our ability to pursue regulatory approval.
Delays in the commencement of clinical testing could significantly impact our product development costs. We do not know whether all of our planned Phase 3 clinical trials of the IV formulation of KW-3902, Phase 2 clinical trial of the oral formulation of KW-3902 or Phase 2 clinical trial of the IV formulation of K201 (JTV-519) will begin on time, or at all. The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
- •
- obtaining regulatory clearance to commence a clinical trial;
- •
- identifying, recruiting and training suitable clinical investigators;
- •
- reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation, may be subject to modification from time to time and may vary significantly among different CROs and trial sites;
- •
- obtaining sufficient quantities of a product candidate for use in clinical trials;
- •
- obtaining institutional review board, or IRB, approval to conduct a clinical trial at a prospective site;
- •
- identifying, recruiting and enrolling patients to participate in a clinical trial; and
- •
- retaining patients who have initiated a clinical trial but may withdraw due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or personal issues.
Any delays in the commencement of our clinical trials will delay our ability to pursue regulatory approval for our product candidates. In addition, many of the factors that cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate.
13
Delays in the completion of clinical testing could result in increased costs to us and delay our ability to generate product revenues.
Once a clinical trial has begun, patient recruitment and enrollment may be slower than we anticipate. Our Phase 3 clinical trials of the IV formulation of KW-3902 will enroll significantly more patients than we have enrolled in clinical trials of KW-3902 to date. We expect to enroll 2,359 patients in our Phase 3 clinical trials. Because the patients we intend to enroll in our REACH UP Phase 3 trial are a smaller subset of the CHF patient population, we expect patient enrollment for this trial to take longer than the patient enrollment in our other Phase 3 trials.
In our pivotal Phase 3 clinical trials, we are enrolling patients who meet a higher BNP threshold than the patients we enrolled in our pilot Phase 3 trial. BNP is an indicator of heart failure and raising the BNP threshold will decrease the possibility of including patients without CHF in our Phase 3 trials. Approximately 75% of the patients enrolled in our pilot Phase 3 trial met the higher BNP threshold. As a result, we may experience slower enrollment in our pivotal Phase 3 trials than in our pilot Phase 3 trial.
We anticipate that some of our clinical trial sites may also be enrolling acute CHF patients in clinical trials for other sponsors, including potential competitors, and as a result we may face competition to enroll acute CHF patients in our Phase 3 clinical trials. Delays in enrollment in our Phase 3 trials of the IV formulation of KW-3902 would result in delays in our ability to pursue regulatory approval of KW-3902.
Clinical trials may also be delayed as a result of ambiguous or negative interim results. Further, a clinical trial may be suspended or terminated by us, the IRB or the Data Monitoring Committee overseeing the clinical trial, any of our clinical trial sites with respect to that site or the FDA or other regulatory authorities due to a number of factors, including:
- •
- failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
- •
- inspection of the clinical trial operations or clinical trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold;
- •
- unforeseen safety issues or any determination that the clinical trial presents unacceptable health risks; and
- •
- lack of adequate funding to continue the clinical trial.
Changes in regulatory requirements and guidance also may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to IRBs for re-examination, which may impact the costs, timing and successful completion of a clinical trial. If we experience delays in the completion of, or if we must terminate, any clinical trial of KW-3902, K201 (JTV-519) or any other product candidate we advance into clinical trials, our ability to obtain regulatory approval for that product candidate will be delayed and the commercial prospects, if any, for the product candidate may be harmed. In addition, many of these factors may also ultimately lead to the denial of regulatory approval of a product candidate. Even if we ultimately commercialize KW-3902 or K201 (JTV-519), other therapies for the same indications may have been introduced to the market during the period we have been delayed and such therapies may have established a competitive advantage over our product candidates.
14
We are relying on Hesperion Ltd., or Hesperion, to conduct and oversee our Phase 3 clinical trials of the IV formulation of KW-3902 and will rely upon third parties to conduct our other planned clinical trials. If Hesperion or other third parties do not meet our deadlines or otherwise conduct the trials as required, we may not be able to obtain regulatory approval for or commercialize our product candidates when expected or at all.
We have contracted with Hesperion to serve as our CRO for the Phase 3 clinical trials of the IV formulation of KW-3902. We have contracted with Duke Clinical Research Institute, or DCRI, to provide safety surveillance and conduct pharmacoeconomic sub-studies for these Phase 3 trials. We also rely upon medical institutions, clinical investigators and contract laboratories to conduct our trials in accordance with our clinical protocols. Our CROs, investigators and other third parties play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical trials.
There is no guarantee that Hesperion, DCRI, other CROs, investigators and other third parties will devote adequate time and resources to our clinical trials or perform as contractually required. If Hesperion, DCRI or any other third parties upon which we rely for administration and conduct of our clinical trials fail to meet expected deadlines, fail to adhere to our clinical protocols or otherwise perform in a substandard manner, our clinical trials may be extended, delayed or terminated, and we may not be able to commercialize our product candidates.
If any of our clinical trial sites terminate for any reason, we may experience the loss of follow-up information on patients enrolled in our ongoing clinical trials unless we are able to transfer the care of those patients to another qualified clinical trial site. In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be jeopardized.
If we fail to enter into a collaboration with respect to the advanced clinical development and commercialization of the oral formulations of our product candidates, we may not be able to execute this portion of our business strategy.
Currently, our strategy is to enter into collaborations with pharmaceutical and biotechnology companies for the commercialization of our oral product candidates in all regions where we hold commercial rights and for commercialization of our IV product candidates outside the United States. We may also pursue collaborations in which our partners provide funding for our future clinical development. It may be difficult for us to find suitable third parties that are willing to enter into collaborations on economic terms that are acceptable to us, or at all.
If we are not able to enter into collaborations for the oral formulations of our product candidates, we could be required to undertake and fund product development, clinical trials, manufacturing and marketing activities solely at our expense. Failure to enter into collaborations for the oral formulations of our product candidates would substantially increase our requirements for capital, which may not be available to us.
15
Even if KW-3902 or K201 (JTV-519) receives regulatory approval, our product candidates may still face future commercial and regulatory difficulties.
We may fail to commercialize successfully any of our product candidates that ultimately receive regulatory approval. For example, the availability of alternative treatments, cost effectiveness, the cost of manufacturing the product on a commercial scale, labeling restrictions and the effect of competition with other drugs may adversely impact the commercial success of a drug. If we fail to commercialize our product candidates as expected, we may be unable to generate sufficient revenue to attain or maintain profitability.
In addition, the FDA may impose significant restrictions on the indicated uses or marketing of a product or impose ongoing requirements for potentially costly post-approval clinical trials or studies. Our product candidates will also be subject to ongoing FDA requirements governing the labeling, packaging, storage, advertising, promotion, recordkeeping and submission of safety and other post-market information.
Even if our product candidates receive regulatory approval in the United States, we may never obtain regulatory approval or successfully commercialize our products outside of the United States.
In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks described above regarding FDA approval in the United States. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. To date, we have not initiated any discussions with the EMEA or other foreign regulatory authorities with respect to seeking regulatory approval of KW-3902 for any indication in Europe or in any other country outside the United States. As in the United States, the regulatory approval process in Europe and in other countries is a lengthy and challenging process. Failure to obtain regulatory approval in other countries or any delay or setback in obtaining such approval could have the same adverse effects detailed above regarding FDA approval in the United States. Such effects include the risk that our product candidates may not be approved for all indications requested, which could limit the uses of our product candidates and adversely impact potential product sales and/or collaboration opportunities, and that such approval may be subject to limitations on the indicated uses for which the product may be marketed or require costly, post-marketing follow-up studies.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
16
Because we in-licensed KW-3902 from Kyowa Hakko and K201 (JTV-519) from Aetas, any dispute with these licensors may adversely affect our ability to develop and commercialize the applicable product candidates.
Both of our current product candidates were in-licensed from third parties. In August 2003, we licensed exclusive rights from Kyowa Hakko to the IV and oral formulations of our product candidate KW-3902 for the treatment of CHF and other indications in major markets, including the United States and Europe, but excluding Asia. Pursuant to the terms of our license agreement with Kyowa Hakko, Kyowa Hakko has the right to terminate the agreement in the event of an uncured material breach of the license agreement by us.
We entered into a license agreement with Aetas in September 2006 and supplemented this license agreement in January 2007 for the exclusive rights to the IV and oral formulations of our product candidate, K201 (JTV-519), for the treatment of cardiac arrhythmia and other cardiovascular indications in major markets, including the United States and Europe, but excluding Asia. Under the terms of our license agreement, Aetas has the right to terminate the agreement in the event of a material breach by us. Aetas also has the right to terminate the agreement in the event we fail to commence dosing, without reasonable reasons, in specified clinical trials of the IV formulation of K201 (JTV-519) by predetermined dates or otherwise fail to use commercially reasonable efforts to develop the product candidate, in each case as determined by Aetas in its sole discretion.
As a result, if there is any conflict, dispute or disagreement between us and Kyowa Hakko or Aetas regarding our rights or obligations under the license agreements, our ability to develop and commercialize the affected product candidate and our ability to enter into collaboration or marketing agreements for the affected product candidate may be adversely affected. Any loss of our rights under our license agreements could delay or completely terminate our product development efforts for the affected product candidate.
In addition, our agreements with Kyowa Hakko and Aetas permit our licensors to assign their rights under the respective license agreements to affiliates and to any third party, including our current or potential future competitors, in connection with the sale or transfer of the business or assets associated with the licensed product candidate. In addition, under the license agreement with Aetas, Aetas may assign its rights with our consent, which is not to be unreasonably withheld. If our licensors were to assign their rights to a third party, such parties may have interests that are adverse to us and may take actions that adversely affect our ability to commercialize our product candidates.
Any adverse developments that occur during any clinical trials conducted by Kyowa Hakko or Aetas, or their respective licensees, may affect our ability to obtain regulatory approval or commercialize our product candidates.
Kyowa Hakko retains the rights to develop and commercialize KW-3902 and Aetas retains the rights to develop and commercialize K201 (JTV-519) in Japan, China, India, Indonesia, Korea, Singapore, Taiwan, Thailand and other specified Asian countries. If either Kyowa Hakko or Aetas or any of their future licensees decides to conduct clinical trials with respect to these respective product candidates and serious adverse events occur during these trials, the FDA and other regulatory authorities may delay, limit or deny approval of our drug candidates or require us to conduct additional clinical trials as a condition to marketing approval, which would increase our costs. If we receive marketing approval for KW-3902 or K201 (JTV-519) and
17
a new and serious safety issue is identified in connection with clinical trials conducted by one of our licensors or their other licensees, the FDA and other regulatory authorities may withdraw their approval of the product or otherwise restrict our ability to market and sell our products. In addition, treating physicians may be less willing to administer our product candidates due to concerns over such adverse events which would limit our ability to commercialize the affected product candidate.
If our competitors develop treatments for CHF patients with renal impairment and fluid overload, treatments for chronic CHF patients or treatments for patients with A-Fib that are approved more quickly, marketed more effectively or demonstrated to be more effective than our product candidates, our commercial opportunity will be reduced or eliminated.
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition from many different sources, including pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions, many of which have significantly greater financial and other resources than we.
The cardiovascular indications for which we are developing products have a number of established therapies and products under development with which our product candidates will compete. Although there are currently no adenosine A1 receptor antagonists that have received regulatory approval from the FDA for the treatment of CHF, we believe that Biogen Idec Inc.'s product candidate, ADENTRI, and Solvay S.A.'s, or Solvay, product candidate, SLV-320, are both in Phase 2 clinical trials.
Several companies are engaged in the clinical development of vasopression antagonists for the treatment of CHF, including Otsuka Pharmaceuticals Group, which has tolvaptan in Phase 3 clinical trials, and Cardiokine Inc., which has Lixivaptan in Phase 2 clinical trials. PDL BioPharma, Inc. has the vasodilator, ularitide, a synthetic form of urodilatin, in Phase 2 development for acute CHF. Two other companies have inotropes, which are agents that increase or decrease the force or energy of muscular contractions, in clinical trials for the treatment of CHF, including Abbott Laboratories, which has levosimendan in Phase 3 trials, and Cytokinetics Inc., which has CK-1827452 in Phase 1 clinical trials. While these products will not compete directly with KW-3902, physicians may choose to use one therapy over another.
Current drugs in development for the acute treatment of A-Fib include vernakalant, which is being developed by Cardiome Pharma Corp., or Cardiome, in partnership with Astellas Pharma Inc., for which an NDA has been submitted. Currently in development for the long-term maintenance of A-Fib are Sanofi-Aventis S.A.'s dronedarone, which is in Phase 3 clinical trials, and the oral formulation of Cardiome's vernakalant, which is in Phase 2 clinical trials. Solvay's Tedisamil is also in clinical development for the treatment of arrthymias, including A-Fib.
New developments, including the development of other drug technologies and methods of preventing the incidence of disease, occur in the pharmaceutical and life sciences industries at a rapid pace. These developments may render our product candidates obsolete or noncompetitive. Compared to us, many of our potential competitors have substantially greater:
- •
- research and development resources, including personnel and technology;
- •
- regulatory experience;
18
- •
- drug development, clinical trial and drug commercialization experience;
- •
- experience and expertise in exploitation of intellectual property rights; and
- •
- capital resources.
As a result of these factors, our competitors may obtain regulatory approval of their products more rapidly than we or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop products for the treatment of CHF, A-Fib or other cardiovascular indications we pursue that are more effective, better tolerated, more useful and less costly than ours and may also be more successful in manufacturing and marketing their products. Our competitors may succeed in obtaining approvals from the FDA and foreign regulatory authorities for their product candidates sooner than we do for ours. In addition, if we receive regulatory approvals for our products, manufacturing efficiency is likely to be a significant competitive factor. We currently have no commercial manufacturing infrastructure. There can be no assurance that we can develop or contract for these capabilities on acceptable economic terms, or at all.
In addition, Kyowa Hakko retains the rights to develop and commercialize KW-3902 and Aetas retains the rights to develop and commercialize K201 (JTV-519) for fields of use outside the fields of use for which we have a license in the United States, Europe and other countries where we have commercial rights. In the event that our licensors, or their respective future licensees, successfully develop and commercialize these product candidates for use in fields outside of the fields of use licensed to us in countries where we hold commercial rights to these product candidates, we may face significant competition from our licensors or their future licensees as physicians and other healthcare providers may prescribe, and patients may use, the products of our licensors or their licensees for off-label use. We will receive no revenues from the sale of such products by our licensors or their future licensees.
We will also face competition from these third parties in recruiting and retaining qualified personnel, establishing clinical trial sites and patient registration for clinical trials and in identifying and in-licensing new product candidates.
We do not have internal manufacturing capabilities, and if we fail to develop and maintain supply relationships with manufacturers, we may be unable to develop or commercialize our product candidates.
All of our manufacturing is outsourced to third parties with oversight by our internal managers, and we do not plan to build manufacturing capabilities. The production and manufacture of KW-3902 and K201 (JTV-519) employ small molecule organic chemistry procedures that require a high level of expertise. We currently rely on DSM Pharma Chemicals, Inc. for the production of the drug substance of KW-3902 and Patheon Italia S.p.A., or Patheon, for the production of finished IV and oral KW-3902 product candidates. Patheon is in the process of moving our KW-3902 production from a plant in Puerto Rico to a plant in Italy. Although we have sufficient inventory of finished KW-3902 for our clinical trials, we could experience temporary supply interruptions in connection with the facility move. In addition, although KW-3902 is manufactured from readily available raw materials using standard pharmaceutical methods and equipment and we can obtain the raw materials from multiple suppliers, the replacement of any one of our manufacturers would lead to significant delays
19
and increase our costs. We currently do not have long-term supply arrangements with any of these suppliers.
Our ability to develop and commercialize our product candidates depends, in part, on our ability to outsource the manufacturing at a competitive cost, in accordance with regulatory requirements and in sufficient quantities for clinical testing and eventual commercialization. Our manufacturers have not manufactured commercial batches of KW-3902 or clinical supplies of K201 (JTV-519). These manufacturers may encounter difficulties with the small- and large-scale formulation and manufacturing processes required to manufacture clinical and commercial quantities of our product candidates. Such difficulties could result in delays in our clinical trials and regulatory submissions, in the commercialization of KW-3902, K201 (JTV-519) or another product candidate or, if KW-3902, K201 (JTV-519) or any other product candidate is approved, in the recall or withdrawal of the product from the market. Further, development of large-scale manufacturing processes may require additional validation studies, which the FDA must review and approve. If our manufacturers fail to deliver the required commercial quantities on a timely basis and at commercially reasonable prices, we may be unable to meet demand for our products and could lose potential revenue.
Our manufacturers must comply with current good manufacturing practices, or cGMP, enforced by the FDA through its facilities inspection program and review of submitted technical information. We have little control over manufacturers' compliance with these regulations and standards and they may not comply. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any quantities supplied by third parties is compromised due to their failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize the affected product candidate, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay of clinical trials, regulatory submissions, approvals or commercialization of our product candidates, entail higher costs or result in our being unable to effectively commercialize our products.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell any products we may develop, we may not be able to effectively market and sell our products and generate product revenue.
We do not currently have the infrastructure for the sales, marketing and distribution of our pharmaceutical products, and we must build this infrastructure or make arrangements with third parties to perform these functions in order to commercialize our products. We intend to establish (either internally or through a contract sales force) a hospital-based sales force to sell the IV formulations of KW-3902 and K201 (JTV-519), if approved, in the United States. We plan to partner with third parties to commercialize our IV product candidates outside the United States. We also plan to enter into collaborations to develop further, promote and sell the oral formulations of our product candidates within and outside of the United States.
The establishment and development of our own sales force in the United States or the establishment of a contract sales force to market any products we may develop will be expensive and time-consuming and could delay any product launch, and we cannot be certain that we would be able to successfully develop this capability. If we are unable to establish our sales and marketing capability or any other non-technical capabilities necessary to
20
commercialize any products we may develop, we will need to contract with third parties to market and sell such products in the United States. We currently possess limited resources and may not be successful in establishing our own internal sales force or in establishing arrangements with third-parties on acceptable terms, if at all.
If KW-3902, K201 (JTV-519) or any other product candidate that we may successfully develop does not achieve broad market acceptance among physicians, patients, healthcare payors and the medical community, the revenues that we generate from their sales will be limited.
Even if our product candidates receive regulatory approval, they may not gain market acceptance among physicians, patients, healthcare payors and the medical community. Coverage and reimbursement of our product candidates by third-party payors, including government payors, generally is also necessary for commercial success. The degree of market acceptance of any of our approved products will depend on a number of factors, including:
- •
- our ability to provide acceptable evidence of safety and efficacy;
- •
- the relative convenience and ease of administration;
- •
- the prevalence and severity of any adverse side effects;
- •
- warnings or limitations contained in a product's labeling;
- •
- the availability of alternative treatments;
- •
- pricing and cost effectiveness;
- •
- the effectiveness of our or any future collaborators' sales and marketing strategies;
- •
- our ability to obtain sufficient third-party coverage or reimbursement; and
- •
- the willingness of patients to pay out of pocket in the absence of third-party coverage.
Currently, acute CHF patients are prescribed primarily inexpensive generic drugs. Hospitals are generally reimbursed at a fixed dollar amount per hospital stay for acute CHF patients. The amount of reimbursement that hospitals receive is generally less than the average amount spent by the hospital on the treatment of acute CHF patients. Because reimbursement amounts generally do not increase to cover the cost of non-generic drugs in the event they are prescribed, physicians may resist prescribing KW-3902 or other non-generic drugs to acute CHF patients, even if there is valid pharmacoeconomic justification (such as a shorter hospital stay or reduced rate of hospital readmission) to offset the cost. Although we believe that we will be able to demonstrate adequate pharmacoeconomic justification, there is no guarantee that physicians will adopt the use of KW-3902 because of its cost or for other factors. If KW-3902, K201 (JTV-519) or any other product candidate we may develop is approved but does not achieve an adequate level of acceptance by physicians, hospitals, healthcare payors and patients, we may not generate sufficient revenue from these products and we may not become or remain profitable.
We may incur substantial liabilities from any product liability claims if our insurance coverage for those claims is inadequate.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials, and will face an even greater risk if we sell our product candidates commercially. An individual may bring a liability claim against us if one of our
21
product candidates causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
- •
- decreased demand for our product candidates;
- •
- impairment to our business reputation;
- •
- withdrawal of clinical trial participants;
- •
- costs of related litigation;
- •
- distraction of management's attention from our primary business;
- •
- substantial monetary awards to patients or other claimants;
- •
- the inability to commercialize our product candidates; and
- •
- loss of revenues.
The estate of the patient who suffered a seizure in a Phase 2 clinical trial of the IV formulation of KW-3902 and subsequently died has filed a malpractice claim against the clinical investigator. Although we are not named as a defendant in the pending lawsuit, it is possible that the complaint may be amended or we may be joined as a defendant in the case.
We have obtained product liability insurance coverage for our clinical trials with a $5.0 million annual aggregate limit. Our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for any of our product candidates. However, we may be unable to obtain this product liability insurance on commercially reasonable terms and with insurance coverage that will be adequate to satisfy any liability that may arise. On occasion, large judgments have been awarded in class action or individual lawsuits relating to marketed drugs. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
We are subject to uncertainty relating to healthcare reform measures and reimbursement policies which, if not favorable, could hinder or prevent our product candidates' commercial success.
Our ability to commercialize our product candidates successfully will depend in part on the extent to which governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our product candidates and related treatments. As a threshold for coverage and reimbursement, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors also are increasingly challenging the effectiveness of, and prices charged for, medical products and services and influence the purchase of healthcare services and products. Legislative proposals to reform healthcare or reduce government insurance programs may result in lower prices for our product candidates or exclusion of our product candidates from coverage and reimbursement programs. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could significantly reduce our revenues from the sale of any approved product. We cannot provide any assurances that
22
we will be able to obtain third-party coverage or reimbursement for our product candidates in whole or in part.
In the United States, there have been a number of legislative and regulatory changes to the healthcare system in ways that could affect our future revenues and profitability and the future revenues and profitability of our potential customers. For example, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 established a new Part D prescription drug benefit, which became effective January 1, 2006. It remains difficult to predict the impact that the prescription drug program will have on us and our industry. Under the prescription drug benefit, Medicare beneficiaries can obtain prescription drug coverage from private sector plans that are permitted to limit the number of prescription drugs that are covered in each therapeutic category and class on their formularies.
There also have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare costs to contain or reduce costs of healthcare may adversely affect one or more of the following:
- •
- our ability to set a price we believe is fair for our products;
- •
- our ability to generate revenues and achieve profitability;
- •
- the future revenues and profitability of our potential customers, suppliers and collaborators; and
- •
- the availability of capital.
In certain foreign markets, the pricing of prescription drugs is subject to government control and reimbursement may, in some cases, be unavailable. In the United States, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and the reform of the Medicare and Medicaid systems. While we cannot predict the full outcome of the implementation of this legislation, it is possible that this new Medicare prescription drug benefit, which will be managed by private health insurers and other managed care organizations, will result in decreased reimbursement for prescription drugs, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to market our products and generate revenues. It is also possible that other proposals having a similar effect will be adopted.
We will need to increase the size of our organization and the scope of our outside vendor relationships, and we may experience difficulties in managing growth.
As of April 30, 2007, we had 11 full-time employees. In addition, we have engaged part-time individual consultants to assist us with establishing accounting systems and internal systems of control over financial reporting. We will need to expand our managerial, operational, financial and other resources in order to manage and fund our operations and clinical trials, continue our research and development activities, and commercialize our product candidates. Our management and scientific personnel, systems and facilities currently in place may not be
23
adequate to support our future growth. Our need to effectively manage our operations, growth and various projects requires that we:
- •
- manage our clinical trials effectively, including our planned pivotal Phase 3 clinical trials of KW-3902 and our proposed Phase 2 clinical trial of K201 (JTV-519);
- •
- manage our internal development efforts effectively while carrying out our contractual obligations to licensors, contractors and other third parties;
- •
- continue to improve our operational, financial and management controls and reporting systems and procedures; and
- •
- attract and retain sufficient numbers of talented employees.
To date, we have utilized the services of outside vendors or consultants to perform tasks including clinical trial management, statistics and analysis, regulatory affairs, formulation development and other drug development functions. We depend on the services of Hesperion as our CRO for the conduct of our Phase 3 clinical trials of the IV formulation of KW-3902. We are also utilizing the consulting services of Momentum Research Inc. to assist with our internal oversight of Hesperion and other third parties involved in these Phase 3 trials. Our growth strategy may also entail expanding our group of contractors or consultants to implement these tasks going forward. Because we rely on a substantial number of consultants, effectively outsourcing many key functions of our business, we will need to be able to effectively manage these consultants to ensure that they successfully carry out their contractual obligations and meet expected deadlines. However, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for our product candidates or otherwise advance our business. There can be no assurance that we will be able to manage our existing consultants or find other competent outside contractors and consultants on economically reasonable terms, or at all. If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may be unable to successfully implement the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
If we fail to attract and keep key management and clinical development personnel, we may be unable to successfully develop or commercialize our product candidates.
We will need to expand and effectively manage our managerial, operational, financial and other resources in order to successfully pursue our clinical development and commercialization efforts for KW-3902, K201 (JTV-519) and our future product candidates. Our success depends on our continued ability to attract, retain and motivate highly qualified management, clinical development, regulatory and sales and marketing personnel. We are highly dependent on the development, regulatory, commercial and financial expertise of our senior management. The loss of the services of any of our senior management, particularly Randall E. Woods, our president and chief executive officer, or Howard C. Dittrich, M.D., our chief medical officer, could delay or prevent the commercialization of our product candidates. If we lose any members of our senior management team, we may not be able to find suitable replacements, and our business may be harmed as a result. We do not maintain "key man" insurance policies on the lives of these individuals or the lives of any of our other employees. Although we have
24
employment agreements with each of our executive officers, we employ these individuals on an at-will basis and their employment can be terminated by us or them at any time, for any reason and with or without notice. We will need to hire additional personnel as we continue to expand our manufacturing, research and development activities.
We may not be able to attract or retain qualified management and scientific personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses, particularly in the San Diego, California area, where our offices are located. Our industry has experienced a high rate of turnover of management personnel in recent years. In addition to the competition for personnel, the San Diego area in particular is characterized by a high cost of living. As such, we could have difficulty attracting experienced personnel to our company and may be required to expend significant financial resources in our employee recruitment and retention efforts. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will impede significantly the achievement of our development objectives, our ability to raise additional capital and our ability to implement our business strategy. In particular, if we lose any members of our senior management team, we may not be able to find suitable replacements and our business may be harmed as a result.
We have scientific and clinical advisors who assist us in formulating our development and clinical strategies. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, our advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours.
We do not have any internal drug discovery or research capabilities, and if we fail to successfully identify and acquire or in-license additional product candidates, we may have limited growth opportunities.
An important part of our business strategy is to develop a broad pipeline of product candidates through in-licensing of drug candidates that have already undergone pre-clinical studies and Phase 1 clinical trials. We do not have any internal drug discovery or research capabilities. As a result, the success of our in-licensing strategy depends upon our ability to identify, select and acquire or in-license promising pharmaceutical product candidates. Currently, we are not actively in-licensing any particular additional drug candidates.
The process of proposing, negotiating and implementing an in-license of a product candidate is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license. We have limited resources to identify and execute the acquisition or in-licensing transaction and integrate the acquired or licensed assets and resources into our current infrastructure. Moreover, we may devote resources to potential in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. We may not be able to acquire the rights to additional product candidates on terms that we find acceptable, or at all.
Future in-licensing transactions may entail numerous operational and financial risks including:
- •
- exposure to unknown liabilities;
- •
- disruption of our business and diversion of our management's time and attention;
25
- •
- incurrence of substantial debt or dilutive issuances of securities to pay for acquisitions or in-licensing transactions; and
- •
- high acquisition and integration costs.
Product candidates that we in-license will likely require additional development efforts prior to commercial sale, including product development, extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe or effective for approval by regulatory authorities. In addition, we cannot provide assurance that any product candidates that we acquire or in-license for further development will be manufactured profitably or achieve market acceptance.
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
In addition to FDA restrictions on the marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical and medical device industries in recent years. These laws include anti-kickback statutes and false claims statutes.
The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or, in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally-financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not, in all cases, meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the company's marketing of the product for unapproved, and thus non-reimbursable, uses. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer's products from reimbursement under government programs, criminal fines and imprisonment.
26
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge could have a material adverse effect on our business, financial condition and results of operations.
In addition, as part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. This practice is regulated by the FDA and other governmental authorities, including, in particular, requirements concerning record keeping and control procedures. Any failure to comply with the regulations may result in significant criminal and civil penalties as well as damage to our credibility in the marketplace.
Risks relating to our intellectual property
Our success depends upon our ability to protect our intellectual property and our proprietary technologies, and the intellectual property protection for KW-3902 and K201 (JTV-519) depends in part on third parties.
Our commercial success depends on obtaining and maintaining patent protection and trade secret protection for our product candidates and their formulations and uses and our other proprietary technologies, as well as successfully defending these patents against third-party challenges. There can be no assurance that our patent applications or the patent applications we have in-licensed will result in additional patents being issued or that issued patents will afford protection against competitors with similar technology, nor can there be any assurance that the patents issued will not be infringed, designed around or invalidated by third parties. Even issued patents may later be found unenforceable, or be modified or revoked in proceedings instituted by third parties before various patent offices or in courts.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents.
The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
- •
- we might not have been the first to file patent applications for these or similar inventions;
- •
- we might not have been the first to make the inventions covered by each of our pending patent applications and issued patents;
- •
- others may independently develop similar or alternative technologies or duplicate any of our technologies;
- •
- the patents of others may have an adverse effect on our business;
- •
- it is possible that none of our or our licensors' pending patent applications will result in issued patents;
27
- •
- any patents we obtain or our licensors' issued patents may not encompass commercially viable products, may not provide us with any competitive advantages, or may be challenged by third parties;
- •
- any patents we obtain or our in-licensed issued patents may not be valid or enforceable; or
- •
- we may not develop additional proprietary technologies that are patentable.
If we or our licensors fail to appropriately prosecute and maintain patent protection for KW-3902, K201 (JTV-519) and any other product candidate that we may develop, our ability to develop and commercialize KW-3902, K201 (JTV-519) or any other product candidate we develop may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products. This failure to properly protect the intellectual property rights relating to KW-3902, K201 (JTV-519) or any other product candidate we develop could have a material adverse effect on our financial condition and results of operation.
Proprietary trade secrets and unpatented know-how are also very important to our business. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with employees, consultants and advisors, third parties may still obtain this information or we may be unable to protect our rights. Enforcing a claim that a third party illegally obtained and is using our trade secrets or unpatented know-how is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secret information. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how, and we would not be able to prevent their use.
The long-term intellectual property protection for KW-3902 is primarily focused on methods of use and manufacturing and formulations.
Various patent applications and patents are directed to KW-3902 and its methods of use and manufacturing, along with KW-3902 formulations. We use the term "composition of matter" in referring to a patent or application that claims a chemical entity; "method of use" to refer to a patent or application that claims the treatment of an individual or patient; "formulation" to refer to a patent or application that claims a combination of an active ingredient with excipients and/or other ingredients; and "manufacturing" to refer to a patent or application that claims a method of making a formulation or composition of matter. The U.S. composition of matter patent for KW-3902 is due to expire in 2011. We cannot guarantee that lack of composition of matter protection after 2011 will not adversely impact the strength of our protection for KW-3902 or that any of these patents will be found valid and enforceable, or that third parties will be found to infringe any of our issued patent claims. There can be no assurance that any of the patent applications will issue in any jurisdiction. Moreover, we cannot predict the breadth of claims that may be allowed in our or our licensors' patent applications or the actual enforceable scope of the KW-3902 method of manufacturing and use and formulation patents. Our strategy for preventing generic competition relies on our ability to obtain and maintain regulatory exclusivity and effective patent rights. Once any regulatory exclusivity expires, if certain of our pending patent applications do not issue, or if certain of our patents or our licensor's patents have expired or are determined to be invalid, not
28
infringed or unenforceable, we might not be able to prevent others from marketing and selling generic versions of KW-3902.
Since our U.S. composition of matter patent for KW-3902 is due to expire in 2011, absent patent protection, we expect to rely primarily on the Hatch-Waxman Act and similar foreign statutes to prevent third parties from marketing and selling generic versions of KW-3902.
The U.S. composition of matter patent for KW-3902 is due to expire in 2011. Although we have various patent applications and patents that are directed to the methods of manufacturing and use of KW-3902, together with KW-3902 formulations, these patents and patent applications may not adequately prevent others from marketing and selling generic versions of KW-3902. We may therefore have no direct means to prevent third parties from making, selling, using or importing KW-3902 in major markets, including the United States and Europe. Instead, we expect to rely upon the United States Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, and applicable foreign legislation, to achieve market exclusivity for KW-3902. For NDAs for new chemical entities not previously approved, the Hatch-Waxman Act provides for marketing exclusivity to the first applicant to gain approval for a particular drug by prohibiting acceptance or approval of an abbreviated new drug application, or ANDA, from a generic competitor for up to five years after approval of the original NDA. This exclusivity only applies to submissions of an ANDA and would not prevent a third party from conducting pivotal clinical trials and thereafter filing a complete regulatory submission for KW-3902. Our competitors will be free during any period of statutory exclusivity to develop the data necessary either to file an ANDA at the end of the exclusivity period or to conduct studies in support of a complete NDA filing during the period of market exclusivity. Although statutory market exclusivity in the United States and Europe may apply even when the composition of matter patent has already expired, it is possible that KW-3902 will not qualify for such exclusivity, or alternatively, the terms of the Hatch-Waxman Act, or similar foreign statutes, could be amended to our disadvantage. If we do not qualify for marketing exclusivity for KW-3902, the competition we face would increase, reducing our potential revenues.
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in that litigation would have a material adverse effect on our business.
Our commercial success also depends upon our ability and the ability of our future collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. Numerous United States and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing products. Because patent applications can take many years to issue, there may be currently pending applications, unknown to us, which may later result in issued patents that our product candidates or proprietary technologies may infringe. Similarly, there may be issued patents relevant to our product candidates of which we are not aware.
We may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our product candidates and/or proprietary technologies infringe their intellectual property rights. If one of these patents was found to cover our product candidates, proprietary technologies or their uses, we or our future
29
collaborators could be required to pay damages and could be unable to commercialize our product candidates or use our proprietary technologies unless we or they obtained a license to the patent. A license may not be available to us or our future collaborators on acceptable terms, if at all. In addition, during litigation, the patent holder could obtain a preliminary injunction or other equitable right which could prohibit us from making, using or selling our products, technologies or methods.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally. If a third party claims that we or our collaborators infringe its intellectual property rights, we may face a number of issues, including, but not limited to:
- •
- infringement and other intellectual property claims which, with or without merit, may be expensive and time-consuming to litigate and may divert our management's attention from our core business;
- •
- substantial damages for infringement, including treble damages and attorneys' fee, which we may have to pay if a court decides that the product or proprietary technology at issue infringes on or violates the third party's rights;
- •
- a court prohibiting us from selling or licensing the product or using the proprietary technology unless the third party licenses its technology to us, which it is not required to do;
- •
- if a license is available from the third party, we may have to pay substantial royalties, fees and/or grant cross licenses to our technology; and
- •
- redesigning our products or processes so they do not infringe, which may not be possible or may require substantial funds and time.
We have not conducted an extensive search of patents issued to third parties, and no assurance can be given that such patents do not exist, have not been filed, or could not be filed or issued, which contain claims covering our products, technology or methods. Because of the number of patents issued and patent applications filed in our technical areas or fields, we believe there is a significant risk that third parties may allege they have patent rights encompassing our products, technology or methods.
Other product candidates that we may in-license or acquire could be subject to similar risks and uncertainties.
We may be subject to claims that our consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, we engage the services of consultants to assist us in the development of our product candidates. Many of these consultants were previously employed at or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or their former or current customers. Litigation may be necessary to defend against these claims. Even
30
if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks relating to our finances and capital requirements
We expect our net operating losses to continue for at least several years, and we are unable to predict the extent of future losses or when we will become profitable, if ever.
We are a development stage company with a limited operating history. We have incurred substantial net operating losses since our inception. We have financed our operations through private placements of preferred stock and debt and have incurred losses in each year since our inception in November 2001. For the year ended December 31, 2006, we had a net loss of $17.9 million, and for the three months ended March 31, 2007, we had a net loss of $8.5 million. As of March 31, 2007, we had an accumulated deficit of approximately $51.5 million. These losses, combined with expected future losses, have had and will continue to have a material and adverse effect on our stockholders' equity and working capital. We expect to continue to incur operating losses for the next several years as we pursue the clinical development and our potential market launch of our two product candidates and expand our infrastructure to support our development and pre-market launch activities and increase selling, general and administrative expenses to comply with public company reporting requirements. Because of the numerous risks and uncertainties associated with our development efforts and other factors, we are unable to predict the extent of any future losses or when we will become profitable, if ever. In addition, if we obtain regulatory approval for any of our product candidates, our losses may increase, at least initially, because we may incur significant sales, marketing and outsourced manufacturing expenses as well as continued development expenses. We will need to obtain regulatory approval and successfully commercialize KW-3902, K201 (JTV-519) and/or other future product candidates before we can generate revenues which would have the potential to lead to profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
We have not generated any revenue from our product candidates and may never become profitable.
Our ability to become profitable depends upon our ability to generate significant continuing revenues. To obtain significant continuing revenues, we must succeed, either alone or with others, in developing, obtaining regulatory approval for and manufacturing and marketing, KW-3902, K201 (JTV-519) or any other product candidates with significant market potential. To date, our product candidates have not generated any revenues, and we do not know when, or if, we will generate any revenue. Our ability to generate revenue depends on a number of factors, including, but not limited to:
- •
- successful completion of clinical trials of the IV formulations of KW-3902 and K201 (JTV-519) and any other product candidates we advance into clinical trials;
- •
- achievement of regulatory approval for the IV formulations of KW-3902 and K201 (JTV-519) and any other product candidates we advance into clinical trials;
- •
- manufacturing commercial quantities of our products at acceptable cost levels if regulatory approvals are obtained;
31
- •
- successful sales, distribution and marketing of our future products, if any; and
- •
- our entry into collaborative arrangements or co-promotion agreements to market and sell our products outside the United States and in certain markets in the United States.
If we are unable to generate significant continuing revenues, we will not become profitable and we may be unable to continue our operations without continued funding.
We will need substantial additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our research and development programs or commercialization efforts.
Our operations have consumed substantial amounts of cash since inception. To date, our sources of cash have been primarily limited to private placements of preferred stock and debt. We expect to continue to spend substantial amounts on development, including significant amounts on conducting clinical trials for our two product candidates, manufacturing clinical supplies and expanding our drug development programs. Our cash flow used for operating activities in 2006 was an average of approximately $1.5 million per month, compared to an average of approximately $0.9 million per month in 2005. Our cash flow used for operating activities during the first quarter of 2007 was an average of approximately $2.4 million per month. We expect that our monthly cash used by operations will continue to increase for the next several years.
We believe that the net proceeds from this offering, together with interest thereon, and our existing cash, cash equivalents and short-term investments and existing borrowing availability under our credit facility, will be sufficient to fund our operations through at least the end of 2008, and to allow us to complete our pivotal Phase 3 clinical trials of the IV formulation of KW-3902, our planned Phase 2 clinical trial of the oral formulation of KW-3902 and our planned Phase 2 trial of the IV formulation of K201 (JTV-519). We have based these estimates, however, on assumptions that may prove to be wrong, and we could spend our available financial resources much faster than we currently expect. Further, we will need to raise additional capital following this offering to fund our operations and continue to conduct clinical trials to support potential regulatory approval of marketing applications.
The amount and timing of our future funding requirements will depend on many factors, including, but not limited to, the following:
- •
- the progress of our clinical trials, including expenses to support the trials and milestone payments that may become payable under our license agreements;
- •
- the costs and timing of regulatory approvals;
- •
- the costs and timing of clinical and commercial manufacturing supply arrangements for each product candidate;
- •
- the costs of establishing sales or distribution capabilities;
- •
- the success of the commercialization of our products;
- •
- our ability to establish and maintain strategic collaborations, including licensing and other arrangements;
32
- •
- the costs involved in enforcing or defending patent claims or other intellectual property rights; and
- •
- the extent to which we in-license or invest in other indications, product candidates or businesses.
We will be required to raise additional capital to complete the development and commercialization of our current product candidates. Future capital requirements will also depend on the extent to which we acquire or in-license additional product candidates. We currently have no commitments or agreements relating to any of these types of transactions.
Until we can generate a sufficient amount of product revenue and achieve profitability, we expect to finance future cash needs through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements, as well as through interest income earned on cash balances. We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue one or more of our drug development programs. We also may be required to relinquish, license or otherwise dispose of rights to product candidates or products that we would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available.
Our short operating history makes it difficult to evaluate our business and prospects.
We were incorporated in November 2001 and did not commence our drug development activities until August 2003. Our operations to date have been limited to organizing and staffing our company and conducting product development activities for our two product candidates. We have not yet demonstrated an ability to obtain regulatory approval for or commercialize a product candidate. Consequently, any predictions about our future performance may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
Raising additional funds by issuing securities or through licensing or lending arrangements may cause dilution to you, restrict our operations or require us to relinquish proprietary rights.
We may raise additional funds through public or private equity offerings, debt financings or licensing arrangements. To the extent that we raise additional capital by issuing equity securities, your ownership will be diluted. Our loan and security agreement with Lighthouse Capital Partners V, L.P., or Lighthouse Capital Partners, is secured by a pledge of all of our assets other than intellectual property, and contains a negative pledge on our intellectual property and a variety of operational covenants, including limitations on our ability to incur liens or additional debt, pay dividends, redeem our stock, make certain investments and engage in certain merger, consolidation or asset sale transactions, among other restrictions. Any future debt financing we enter into may involve similar or more onerous covenants that restrict our operations. Any borrowings under the loan and security agreement with Lighthouse Capital Partners or any future debt financing will need to be repaid, which creates additional financial risk for us, particularly if our business or prevailing financial market conditions are not conducive to paying-off or refinancing our outstanding debt obligations.
33
In addition, if we raise additional funds through licensing arrangements, it may be necessary to relinquish potentially valuable rights to our product candidates, or grant licenses on terms that are not favorable to us. If adequate funds are not available, our ability to achieve profitability or to respond to competitive pressures would be significantly limited and we may be required to delay, significantly curtail or eliminate the development of one or more of our product candidates.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our operating results will be affected by numerous factors, including:
- •
- the development status of our product candidates, including results of our clinical trials and whether any of our product candidates receive regulatory approval;
- •
- variations in the level of expenses related to our product candidates or clinical development programs, including relating to the timing of invoices from and other billing practices of, our CROs and clinical trial sites;
- •
- our execution of collaborative, co-promotion, licensing or other arrangements, and the timing of payments we may make or receive under these arrangements;
- •
- our in-license of additional drug compounds for clinical development or termination of existing clinical development programs;
- •
- any litigation in which we may become involved; and
- •
- changes in accounting principles.
Quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the Securities and Exchange Commission, or SEC, and the Nasdaq Stock Market, or Nasdaq, have imposed various new requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these new compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. As a public company, it will be more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure. In particular, commencing in fiscal year 2008, we
34
must perform system and process evaluation and testing of our internal controls over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies or material weaknesses in our internal controls over financial reporting. We expect to incur significant expense and devote substantial management effort toward ensuring compliance with Section 404. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identify deficiencies in our internal controls that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities, which would entail expenditure of additional financial and management resources.
Currency fluctuations may negatively affect our financial condition.
We incur significant expenses, including for clinical trials, outside the United States based on contractual obligations denominated in currencies other than the U.S. dollar. Some of our clinical trial expenses outside the United States are paid in Euros and Swiss Francs. We pay our CRO Hesperion for services outside the United States in U.S. dollars based upon fees accrued in Swiss Francs and converted into U.S. dollars at the average currency exchange rate for U.S. dollars into Swiss Francs for the prior month. Our other clinical trial expenses incurred in Euros or Swiss Francs are expected to increase as a result of the commencement of our pivotal Phase 3 clinical trials of the IV formulation of KW-3902. We generally pay pass through expenses in the currency in which the expenses are incurred. As a result, our business is affected by fluctuations in exchange rates between the U.S. dollar and foreign currencies. Our reporting currency is the U.S. dollar and, therefore, financial positions are translated into U.S. dollars at the applicable foreign exchange rates. Exchange rate fluctuations may adversely affect our revenues, results of operations, financial position and cash flows. Based on our expenses paid in Euros and Swiss Francs during the three months ended March 31, 2007, a movement of 10% in U.S. dollar to Euro or U.S. dollar to Swiss Franc exchange rates would have resulted in an increase or decrease in our aggregate expenses during the three months ended March 31, 2007 of approximately $185,000 or $265,000, respectively. If we are successful in establishing international sales, these sales may also be denominated in foreign currencies and hence subject to volatility due to changes in foreign exchange rates.
Risks relating to the securities markets and investment in our common stock
There may not be a viable public market for our common stock.
Prior to this offering, there has been no public market for our common stock, and there can be no assurance that a regular trading market will develop and continue after this offering or that the market price of our common stock will not decline below the initial public offering price. The initial public offering price will be determined through negotiations between us and the representatives of the underwriters and may not be indicative of the market price of our common stock following this offering. Among the factors considered in such negotiations are prevailing market conditions, certain of our financial information, market valuations of other companies that we and the representatives of the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant. See "Underwriting" for additional information.
35
Market volatility may affect our stock price and the value of your investment.
Following this offering, the market price for our common stock is likely to be volatile, in part because our shares have not been traded publicly. In addition, the market price of our common stock may fluctuate significantly in response to a number of factors, most of which we cannot control, including:
- •
- changes in the development status of or clinical trial results for our product candidates, including the results for our Phase 3 clinical trials of the IV formulation of KW-3902;
- •
- the resources available to us to fund our clinical trial programs on our desired schedule;
- •
- the establishment or termination of any potential collaborations with third parties;
- •
- announcements of new products or technologies or other events by us or our competitors;
- •
- clinical and regulatory developments with respect to other companies developing cardiovascular drugs;
- •
- variations in our quarterly operating results;
- •
- changes in securities analysts' estimates of our financial performance;
- •
- regulatory developments in the United States and foreign countries;
- •
- fluctuations in stock market prices and trading volumes of similar companies or in the broader markets generally;
- •
- sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders;
- •
- additions or departures of key personnel;
- •
- discussion of us or our stock price by the financial and scientific press and in online investor communities; and
- •
- changes in accounting principles generally accepted in the United States.
An active public market for our common stock may not develop or be sustained after this offering. We will negotiate and determine the initial public offering price with representatives of the underwriters and this price may not be indicative of prices that will prevail in the trading market after the offering. As a result, you may not be able to sell your shares of common stock at or above the offering price. In addition, class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Any such litigation brought against us could result in substantial costs and a diversion of management's attention and resources, which could hurt our business, operating results and financial condition.
36
Our management team may allocate the net proceeds from this offering in ways that you and other stockholders may not approve.
Our management will have considerable discretion over the use of proceeds from this offering. We intend to use the net proceeds from this offering to:
- •
- fund clinical development of KW-3902 and K201 (JTV-519), including our proposed Phase 3 clinical trials of the IV formulation of KW-3902 for the treatment of acute CHF in patients with renal impairment and fluid overload; and
- •
- fund our working capital and general corporate purposes.
We may use a portion of the net proceeds from this offering for potential in-licensing activities. We have no present understandings, commitments or agreements with respect to any in-licenses and no portion of the net proceeds from this offering has been allocated for any specific transaction. You will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. Until the net proceeds are used, they may be placed in investments that do not produce significant income or that may lose value.
Investors purchasing common stock in this offering will incur substantial dilution as a result of this offering and future equity issuances, and, as a result, our stock price could decline.
The initial public offering price for this offering is substantially higher than the pro forma, net tangible book value per share of our outstanding common stock. As a result, investors purchasing common stock in this offering will pay a price that substantially exceeds the value of our tangible assets after subtracting liabilities and will incur immediate dilution of $ per share, based on an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus. This dilution is due in large part to earlier investors having paid substantially less than the initial public offering price when they purchased certain of their shares. Investors purchasing shares of common stock in this offering will contribute approximately % of the total amount we have raised since our inception, and will own approximately % of our total common stock immediately following the completion of this offering, in each case based on an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus.
To the extent outstanding stock options and warrants are exercised, there will be further dilution to new investors.
We believe that the net proceeds from this offering, together with interest thereon, and our existing cash, cash equivalents and short-term investments and existing borrowing availability under our credit facility, will be sufficient to fund our operations through at least the end of 2008. Because we will need to raise additional capital to fund our clinical development and research programs, among other things, we may conduct substantial additional equity offerings. These future equity issuances, together with the exercise of outstanding options and warrants and any additional shares issued in connection with acquisitions, will result in further dilution to investors.
37
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and bylaws, both of which will become effective upon the completion of this offering, may delay or prevent an acquisition of us or a change in our management. These provisions include a classified board of directors, a prohibition on actions by written consent of our stockholders and the ability of our board of directors to issue preferred stock without stockholder approval. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which, subject to certain exceptions, prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Although we believe these provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
If our executive officers, directors and largest stockholders choose to act together, they may be able to control our operations and act in a manner that advances their best interests and not necessarily those of other stockholders.
After this offering, our executive officers, directors and holders of 5% or more of our outstanding common stock will beneficially own approximately % of our common stock. As a result, these stockholders, acting together, will be able to control all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of this group of stockholders may not always coincide with our interests or the interests of other stockholders, and they may act in a manner that advances their best interests and not necessarily those of other stockholders.
Future sales of our common stock may depress our stock price.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding shares of common stock based on the number of shares outstanding as of March 31, 2007. This includes the shares that we are selling in this offering, which may be resold in the public market immediately unless held by an affiliate of ours, and the conversion of all of the shares of our preferred stock, including the 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants outstanding as of March 31, 2007 that will terminate if not exercised prior to the effectiveness of this offering. Of the remaining shares, shares are currently restricted as a result of securities laws or lock-up agreements but will be available for resale in the public market as described in the "Shares eligible for future sale" section of this prospectus. As a result of the lock-up agreements between our underwriters and our security holders and the provisions of Rule 144, Rule 144(k) and Rule 701 under the Securities Act of 1933, as amended,
38
or Securities Act, the shares of our common stock (excluding the shares sold in this offering) that will be available for sale in the public market are as follows:
- •
- shares will be eligible for sale under Rule 144(k) or Rule 701 upon the expiration of the lock-up agreements, beginning 180 days after the date of this prospectus;
- •
- shares will be eligible for sale under Rule 144 upon the expiration of the lock-up agreements, subject to volume limitations, manner of sale requirements and other restrictions, beginning 180 days after the date of this prospectus; and
- •
- shares will be eligible for sale, upon the exercise of vested options, upon the expiration of the lock-up agreements, beginning 180 days after the date of this prospectus.
Moreover, after this offering, holders of up to approximately 87,481,308 shares of common stock (including shares of our common stock issuable upon the exercise of warrants) will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. These rights will continue following this offering and will terminate seven years following the completion of this offering, or for any particular holder with registration rights who holds less than one percent of our outstanding capital stock, at such time following this offering when all securities held by that stockholder that are subject to registration rights may be sold pursuant to Rule 144 under the Securities Act within a single 90 day period. We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to the lock-up agreements described in the "Underwriting" section of this prospectus.
We have never paid dividends on our capital stock, and because we do not anticipate paying any cash dividends in the foreseeable future, capital appreciation, if any, of our common stock will be your sole source of gain on an investment in our stock.
We have paid no cash dividends on any of our classes of capital stock to date and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Furthermore, our loan and security agreement with Lighthouse Capital Partners restricts our ability to pay dividends by prohibiting us from paying any dividends other than dividends payable solely in our capital stock without the prior written consent of Lighthouse Capital Partners. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
We may become involved in securities class action litigation that could divert management's attention and harm our business.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of pharmaceutical companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant stock price volatility in recent years. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management's attention and resources, which could adversely affect our business.
39
Forward-looking statements
This prospectus contains forward-looking statements, including statements regarding the progress and timing of our clinical trials, the goals of our research and development activities, the safety and efficacy of our product candidates, the potential success of our existing licensing arrangements and future collaborations and commercial agreements, our expected future revenues, operations and expenditures, and our projected cash needs, that are based on our management's beliefs and assumptions and on information currently available to our management. The forward-looking statements are contained principally in "Prospectus summary," "Risk factors," "Management's discussion and analysis of financial condition and results of operations" and "Business." Forward-looking statements relate to future events or our future financial performance and include information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, the effects of future regulation and competition. Forward-looking statements include all statements that are not historical facts and generally can be identified by terms such as "anticipates," "believes," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" or the negative of those terms, and similar expressions.
Forward-looking statements include, but are not limited to, statements about:
- •
- our ability to demonstrate the safety and efficacy of KW-3902 and K201 (JTV-519) in clinical trials and successfully complete clinical development of KW-3902 and K201 (JTV-519);
- •
- the trial design and the expected timing for the commencement or completion of our Phase 3 clinical trials of the IV formulation of KW-3902;
- •
- our planned Phase 2 clinical trial of the IV formulation of K201 (JTV-519) and our planned Phase 2 trial of the oral formulation of KW-3902 and the expected availability of results;
- •
- our ability to enroll sufficient numbers of patients in our Phase 3 trials of the IV formulation of KW-3902, our planned Phase 2 trial of the oral formulation of KW-3902 and our planned Phase 2 trial of the IV formulation of K201 (JTV-519);
- •
- our ability to execute successfully our plan to in-license additional cardiovascular drug candidates;
- •
- our ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of any of our product candidates that may be approved for sale;
- •
- the content and timing of submissions to, and decisions made by, the FDA and other regulatory agencies;
- •
- our ability to obtain and maintain regulatory approval of KW-3902 and K201 (JTV-519);
- •
- regulatory developments in the United States and foreign countries;
- •
- the rate and degree of market acceptance of any future products we commercialize;
- •
- our ability to secure the manufacture of sufficient amounts of our product candidates for clinical trials and, if approved, products for commercialization activities;
40
- •
- our reliance on third parties to conduct our clinical trials;
- •
- our ability to establish a sufficient hospital-based sales and marketing force or enter into collaborative arrangements or co-promotion agreements with third parties to sell and market any of our product candidates that may be approved for sale;
- •
- the accuracy of our estimates regarding expenses, future revenues and capital requirements;
- •
- the success of competing drugs that are or become available; and
- •
- our ability to raise additional funds in the capital markets, through arrangements with collaborators or from other sources.
Forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We discuss these risks in greater detail in "Risk factors." Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements represent our management's beliefs and assumptions only as of the date of this prospectus. You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. The forward-looking statements contained in this prospectus are excluded from the safe harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended.
41
Use of proceeds
We estimate that the net proceeds from the sale of the shares of common stock we are offering will be approximately $ million, based upon an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us. Each $1.00 increase or decrease in the assumed public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase or decrease, respectively, the net proceeds to us from this offering by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us. If the underwriters fully exercise the over-allotment option, we estimate that our net proceeds from this offering will be approximately $ million.
We intend to use the majority of the net proceeds from this offering to fund the clinical development, including related regulatory developments and manufacturing activities, of KW-3902 and K201 (JTV-519). In particular, we anticipate that:
- •
- approximately $40.7 million of the net proceeds will be used to fund our proposed Phase 3 clinical trials of and other activities relating to the IV formulation of KW-3902 for treatment of acute CHF in patients with renal impairment and fluid overload;
- •
- approximately $1.6 million of the net proceeds will be used to fund our proposed Phase 2 clinical trial of and other activities relating to the oral formulation of KW-3902 for the treatment of chronic CHF in outpatients; and
- •
- approximately $2.9 million of the net proceeds will be used to fund our proposed Phase 2 clinical trial of and other activities relating to the IV formulation of K201 (JTV-519) for the acute treatment of A-Fib.
Due to the risks inherent in the clinical trial process, we are unable to estimate with any certainty the total costs or when we will incur these costs in the continued development of our product candidates for potential commercialization.
We anticipate using the remaining balance of the net proceeds from this offering for working capital and general corporate purposes.
We may use a portion of the net proceeds from this offering for potential in-licensing activities. Although we are not actively in-licensing any particular additional drug candidates, we expect to expand our product pipeline, as resources allow, by in-licensing additional cardiovascular product candidates. To the degree that we pursue any of these transactions, the amount of proceeds that we have available for working capital and general corporate purposes may decrease.
Pending the use of the net proceeds from this offering, we intend to invest these funds in short-term, interest-bearing investment-grade securities.
The amounts and timing of our actual expenditures will depend on numerous factors, including the progress in, and costs of, our clinical trials and other product development programs. We
42
therefore cannot estimate the amount of net proceeds to be used for all of the purposes described above. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceeds.
We believe that the net proceeds from this offering, together with interest thereon, our existing cash, cash equivalents and short-term investments and existing borrowing availability under our credit facility, will be sufficient to fund our operations through at least the end of 2008.
Dividend policy
We have never declared or paid any cash dividends on our common stock and do not expect to pay cash dividends in the foreseeable future. The terms of our loan and security agreement with Lighthouse Capital Partners V, L.P. prohibit us from paying any dividends other than dividends payable solely in our capital stock without its prior written consent. Any future determination related to dividend policy will be made at the discretion of our board of directors.
43
Capitalization
The following table sets forth our capitalization as of March 31, 2007:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to $5.0 million borrowed in April 2007 and the restructuring of our outstanding indebtedness in April 2007 in connection with an amendment to our loan and security agreement with Lighthouse Capital Partners V, L.P.; and
- •
- on a pro forma as adjusted basis to give effect to the pro forma adjustments described above and:
- •
- the filing of an amended and restated certificate of incorporation to authorize 100,000,000 shares of common stock and 5,000,000 shares of undesignated preferred stock;
- •
- the assumed cash exercise of warrants to purchase 548,800 shares of our Series B preferred stock outstanding as of March 31, 2007 that will terminate if not exercised prior to the effectiveness of this offering at an exercise price of $1.125 per share, resulting in aggregate proceeds of $617,400;
- •
- the conversion of all our outstanding shares of preferred stock, including the 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering, into an aggregate of 85,817,499 shares of common stock upon the completion of this offering; and
- •
- the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us.
44
You should read the information in this table together with our financial statements and accompanying notes and "Management's discussion and analysis of financial condition and results of operations" appearing elsewhere in this prospectus.
|
March 31, 2007
(in thousands, except per share data)
| | Actual
| | Pro forma
| | Pro forma
as adjusted(1)
|
|---|
|
| |
| | (unaudited)
|
|---|
| Long-term debt, less current portion | | $ | 953 | | $ | 7,074 | | $ | |
| Stockholders' equity: | | | | | | | | | |
| | Convertible preferred stock, $0.001 par value: 88,547,619 shares authorized and 85,268,699 shares issued and outstanding, actual; 88,547,619 shares authorized and 85,268,699 shares issued and outstanding, pro forma; 5,000,000 shares authorized and no shares issued and outstanding, pro forma as adjusted | | | 85 | | | 85 | | | |
| | Common stock, $0.001 par value: 105,000,000 shares authorized and 2,726,219 shares issued and outstanding, actual and pro forma; 100,000,000 shares authorized and shares issued and outstanding, pro forma as adjusted | | | 3 | | | 3 | | | |
| | Additional paid-in capital(2) | | | 90,218 | | | 90,218 | | | |
| | Accumulated other comprehensive income | | | 3 | | | 3 | | | |
| | Deficit accumulated during the development stage | | | (51,459 | ) | | (51,459 | ) | | |
| | |
|
| | | Total stockholders' equity(2) | | | 38,850 | | | 38,850 | | | |
| | |
|
| | | | Total capitalization(2) | | $ | 39,803 | | $ | 45,924 | | $ | |
| | |
|
|
- (1)
- The pro forma as adjusted information is illustrative only and following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
- (2)
- A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) each of pro forma as adjusted additional paid-in capital, total stockholders' equity and total capitalization by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us.
45
The number of shares of our common stock that will be outstanding after the completion of this offering is based on 88,543,718 shares outstanding as of March 31, 2007, after giving pro forma effect to the assumed cash exercise of warrants to purchase 548,800 shares of our Series B preferred stock outstanding as of March 31, 2007 that will terminate if not exercised prior to the effectiveness of this offering, and excludes the following:
- •
- 7,609,832 shares of common stock subject to outstanding options under our 2003 equity incentive plan, with a weighted average exercise price of $0.21 per share;
- •
- 1,233,949 shares of common stock reserved for future issuance under our 2003 equity incentive plan;
- •
- 18,000,000 shares of common stock reserved for future issuance under our 2007 equity incentive plan, 2007 non-employee directors' stock option plan and 2007 employee stock purchase plan, each of which will become effective upon the completion of this offering; and
- •
- 663,809 shares of common stock subject to outstanding warrants, with a weighted average exercise price of $0.97 per share, which do not expire if unexercised at or prior to the closing of this offering.
In April 2007, we issued an additional warrant to our lender, Lighthouse Capital Partners V, L.P., to purchase up to 933,333 shares of Series B preferred stock at an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price).
46
Dilution
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock after this offering. The historical net tangible book value of our common stock as of March 31, 2007 was approximately $38.9 million, or approximately $0.44 per share of common stock, based on the number of shares of common stock outstanding as of March 31, 2007. Historical net tangible book value per share is determined by dividing the number of outstanding shares of our common stock, after giving effect to the conversion of all outstanding shares of our preferred stock into 85,268,699 shares of our common stock, into our total tangible assets (total assets less intangible assets) less total liabilities.
Investors participating in this offering will incur immediate, substantial dilution. After giving effect to the sale of common stock offered in this offering at an assumed initial public offering price of $ per share, the mid-point range of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us, and after giving effect to the conversion of all outstanding shares of our preferred stock as of March 31, 2007, including the 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering, into an aggregate of 88,543,718 shares of common stock upon completion of this offering, our pro forma as adjusted net tangible book value as of March 31, 2007 would have been approximately $ million, or approximately $ per share of common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to existing common stockholders, and an immediate dilution of $ per share to investors participating in this offering. The following table illustrates this per share dilution:
|
| Assumed initial public offering price per share, the mid-point of the price range set forth on the cover page of this prospectus | | | | | $ | |
| | Historical net tangible book value per share as of March 31, 2007, including conversion of outstanding preferred stock as of March 31, 2007 | | $ | 0.44 | | | |
| | Pro forma increase in net tangible book value per share attributable to investors participating in this offering and the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering | | $ | | | | |
| | |
| | | |
| Pro forma as adjusted net tangible book value per share after this offering | | | | | $ | |
| | | | | |
|
| Pro forma dilution per share to investors participating in this offering | | | | | $ | |
| | | | | |
|
|
47
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted net tangible book value by $ , the pro forma as adjusted net tangible book value per share after this offering by $ per share and the dilution in pro forma as adjusted net tangible book value per share to investors in this offering by $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us.
If the underwriters exercise their over-allotment option in full to purchase additional shares of common stock in this offering, the pro forma as adjusted net tangible book value per share after the offering would be $ per share, the increase in the pro forma net tangible book value per share to existing stockholders would be $ per share and the dilution to new investors purchasing common stock in this offering would be $ per share.
The following table summarizes, on a pro forma as adjusted basis as of December 31, 2006, the differences between the number of shares of common stock purchased from us, the total consideration, which includes the assumed cash exercise for aggregate proceeds of $617,400 of warrants to purchase an aggregate of 548,800 shares of Series B preferred stock outstanding as of March 31, 2007 that will terminate if not exercised prior to the closing of this offering and the net proceeds received from the issuance of common stock and preferred stock, and the average price per share paid by existing stockholders and by investors participating in this offering, after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us, at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus.
|
| | Shares purchased
| | Total consideration
| |
|
|---|
| | Average
price
per share
|
|---|
| | Number
| | Percent
| | Amount
| | Percent
|
|---|
|
| Existing stockholders before this offering | | 88,543,718 | | % | | $ | 89,220,092 | | % | | $ | 1.01 |
| Investors participating in this offering | | | | | | | | | | | | |
| | |
|
| | Total | | | | 100.0% | | | | | 100.0% | | $ | |
| | |
| |
| |
|
|
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by new investors by $ million, or increase (decrease) the percentage of total consideration paid by new investors by %, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us.
The discussion and tables above assume no exercise of the underwriters' over-allotment option or any outstanding options or warrants. If the underwriters' over-allotment option is exercised in full, the number of shares of common stock held by existing stockholders will be reduced to % of the total number of shares of common stock to be outstanding after this offering,
48
and the number of shares of common stock held by investors participating in this offering will be increased to shares or % of the total number of shares of common stock to be outstanding after this offering.
As of March 31, 2007, there were:
- •
- 7,609,832 shares of common stock subject to outstanding options under our 2003 equity incentive plan, with a weighted average exercise price of $0.21 per share;
- •
- 1,233,949 shares of common stock reserved for future issuance under our 2003 equity incentive plan;
- •
- 548,800 shares of common stock subject to outstanding warrants, having a weighted average exercise price of $1.125 per share, which will terminate if not exercised prior to the closing of this offering; and
- •
- 663,809 shares of common stock subject to outstanding warrants, with a weighted average exercise price of $0.97 per share, which do not expire if unexercised at or prior to the closing of this offering.
In April 2007, we issued an additional warrant to our lender, Lighthouse Capital Partners V, L.P., to purchase up to 933,333 shares of Series B preferred stock at an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price).
Effective upon the completion of this offering, an aggregate of 9,000,000, 3,000,000 and 6,000,000 shares of our common stock will be reserved for issuance under our 2007 equity incentive plan, our 2007 non-employee directors' stock option plan and our 2007 employee stock purchase plan, respectively, and these share reserves will also be subject to automatic annual increases in accordance with the terms of the plans. Furthermore, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any of these options or warrants are exercised, new options are issued under our equity incentive plans or we issue additional shares of common stock or other equity securities in the future, there will be further dilution to investors participating in this offering.
49
Selected financial data
The following selected financial data should be read together with our financial statements and accompanying notes and "Management's discussion and analysis of financial condition and results of operations" appearing elsewhere in this prospectus. Our historical results are not necessarily indicative of our future results. The selected statement of operations data for the years ended December 31, 2004, 2005 and 2006, and the selected balance sheet data as of December 31, 2005 and 2006, are derived from our audited financial statements, which are included elsewhere in this prospectus. The summary statement of operations data for the period from November 30, 2001 (inception) through March 31, 2007 and for the three months ended March 31, 2006 and 2007, and the summary balance sheet data as of March 31, 2007, are derived from our unaudited financial statements, which are included elsewhere in this prospectus. The selected statement of operations data for the years ended December 31, 2003, and the selected balance sheet data as of December 31, 2003 and 2004, are derived from our audited financial statements, which are not included in this prospectus. The selected statement of operations data for the year ended December 31, 2002 and the selected balance sheet data as of December 31, 2002 are derived from our unaudited financial statements, which are not included in this prospectus. Our historical results are not necessarily indicative of our future results.
| |
| |
| |
| |
| |
| |
| |
| |
| | Period from
November 30,
2001
(inception)
through
March 31,
2007
| |
|---|
| | Years ended December 31,
| | Three months
ended March 31,
| |
|---|
(in thousands,
except per share data)
| |
|---|
| | 2002
| | 2003
| | 2004
| | 2005
| | 2006
| | 2006
| | 2007
| |
|---|
| |
| Statement of operations data: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Development | | $ | 192 | | $ | 4,370 | | $ | 6,343 | | $ | 9,023 | | $ | 14,184 | | $ | 1,776 | | $ | 7,576 | | $ | 41,699 | |
| | Selling, general and administrative | | | 100 | | | 1,324 | | | 1,782 | | | 1,973 | | | 2,124 | | | 402 | | | 1,219 | | | 8,573 | |
| | |
| |
| Total operating expenses | | | 292 | | | 5,694 | | | 8,125 | | | 10,996 | | | 16,308 | | | 2,178 | | | 8,795 | | | 50,272 | |
| | |
| |
| Loss from operations | | | (292 | ) | | (5,694 | ) | | (8,125 | ) | | (10,996 | ) | | (16,308 | ) | | (2,178 | ) | | (8,795 | ) | | (50,272 | ) |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Interest income | | | — | | | 75 | | | 173 | | | 139 | | | 560 | | | 20 | | | 375 | | | 1,322 | |
| | Interest expense | | | (14 | ) | | (42 | ) | | — | | | (203 | ) | | (2,161 | ) | | (246 | ) | | (88 | ) | | (2,509 | ) |
| | |
| |
| Total other income (expense) | | | (14 | ) | | 33 | | | 173 | | | (64 | ) | | (1,601 | ) | | (226 | ) | | 287 | | | (1,187 | ) |
| | |
| |
| Net loss | | | (306 | ) | | (5,661 | ) | | (7,952 | ) | | (11,060 | ) | | (17,909 | ) | | (2,404 | ) | | (8,508 | ) | | (51,459 | ) |
| Deemed dividend related to beneficial conversion feature on Series B preferred stock | | | — | | | — | | | — | | | — | | | — | | | — | | | (8,080 | ) | | (8,080 | ) |
| | |
| |
| Net loss attributable to common stockholders | | $ | (306 | ) | $ | (5,661 | ) | $ | (7,952 | ) | $ | (11,060 | ) | $ | (17,909 | ) | $ | (2,404 | ) | $ | (16,588 | ) | $ | (59,539 | ) |
| | |
| |
| Basic and diluted net loss per share(1): | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Historical | | $ | (0.31 | ) | $ | (6.58 | ) | $ | (6.09 | ) | $ | (5.96 | ) | $ | (8.37 | ) | $ | (1.21 | ) | $ | (7.13 | ) | | | |
| | |
| | | | |
| | Pro forma | | | | | | | | | | | | | | $ | (0.45 | ) | | | | $ | (0.23 | ) | | | |
| | | | | | | | | | | | | | |
| | | | |
| | | | |
| Shares used to compute basic and diluted net loss per share(1): | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Historical | | | 1,000 | | | 860 | | | 1,305 | | | 1,855 | | | 2,139 | | | 1,984 | | | 2,326 | | | | |
| | |
| | | | |
| | Pro forma | | | | | | | | | | | | | | | 40,179 | | | | | | 73,148 | | | | |
| | | | | | | | | | | | | | |
| | | | |
| | | | |
| |
- (1)
- Please see Note 1 to our financial statements for an explanation of the method used to calculate the historical and pro forma net loss per share and the number of shares used in the computation of the per share amounts.
50
| |
| | December 31,
| | March 31,
| |
|---|
(in thousands)
| | 2002
| | 2003
| | 2004
| | 2005
| | 2006
| | 2007
| |
|---|
| |
| Balance sheet data: | | | | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and short-term investments | | $ | 23 | | $ | 15,763 | | $ | 8,458 | | $ | 2,458 | | $ | 31,524 | | $ | 42,509 | |
| Working capital (deficit) | | | (367 | ) | | 15,783 | | | 7,790 | | | (144 | ) | | 26,299 | | | 34,301 | |
| Total assets | | | 23 | | | 16,105 | | | 8,745 | | | 2,917 | | | 35,901 | | | 48,640 | |
| Notes payable to stockholders | | | 200 | | | — | | | — | | | — | | | — | | | — | |
| Long-term debt (including current portion) | | | — | | | — | | | — | | | 4,499 | | | 3,024 | | | 2,631 | |
| Deficit accumulated during the development stage | | | (368 | ) | | (6,029 | ) | | (13,981 | ) | | (25,041 | ) | | (42,950 | ) | | (51,459 | ) |
| Total stockholders' equity (deficit) | | | (367 | ) | | 15,905 | | | 7,927 | | | (2,910 | ) | | 28,735 | | | 38,850 | |
| |
51
Management's discussion and analysis of
financial condition and results of operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with "Selected financial data" and our financial statements and related notes appearing elsewhere in this prospectus. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited to, those set forth under "Risk factors" and elsewhere in this prospectus.
Overview
Background
We are a clinical-stage pharmaceutical company focused on developing drugs to treat major cardiovascular diseases that are underserved by existing therapies. We have two compounds in clinical development, KW-3902 and K201 (JTV-519), each with potential for use in both acute and chronic settings. We are currently testing the intravenous, or IV, formulation of KW-3902 in Phase 3 clinical trials for the treatment of the acute form of congestive heart failure, or CHF, in patients with renal impairment and fluid overload. We are also developing an oral formulation of KW-3902 for the treatment of chronic CHF. In addition, we are developing K201 (JTV-519) for the treatment of atrial fibrillation, or A-Fib, an irregular heartbeat.
We have incurred significant net losses since our inception. As of March 31, 2007, we had an accumulated deficit of approximately $51.5 million. To date, we have not had any revenue and our losses have resulted from costs incurred in connection with our development activities, including costs of clinical trial activities associated with our current product candidates, licensing fees, patent costs and selling, general and administrative expenses. We expect to continue to incur operating losses for the next several years as we pursue the clinical development and potential market launch of our product candidates, expand our infrastructure to support our development and pre-market launch activities, increase selling, general and administrative expenses to comply with public company reporting requirements and potentially in-license additional cardiovascular product candidates.
Revenues
We have not generated any revenues to date and we have not entered into any collaboration or other agreements under which we could generate revenue in the future.
Development expenses
The majority of our operating expenses to date have been incurred in development activities. Our development expenses consist primarily of costs associated with clinical trials managed by contract research organizations, or CROs, manufacturing costs and license fees. Salaries and related employee benefits for certain personnel and regulatory expenses are also included in development expenses. Our most significant historical development expenses relate primarily to the clinical trials of the IV formulation of KW-3902. We charge all development expenses to operations as incurred because the underlying technology associated with these expenditures relates to our development efforts and has no alternative future use and because we have not established that our product candidates are technologically feasible.
52
At this time, due to the risks inherent in the clinical trial process and given the early stage of our product development programs, we are unable to estimate with any certainty the costs we will incur in the continued development of our product candidates for potential commercialization. Clinical development timelines, the probability of success and development costs vary widely. While we are currently focused on advancing each of our product development programs, our future development expenses will depend on the clinical success of each product candidate, as well as ongoing assessments as to each product candidate's commercial potential. We may seek collaboration partners to further develop the oral formulations of our two product candidates. In addition, we cannot forecast with any degree of certainty which product candidates will be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
We use external service providers and vendors to conduct our clinical trials, to manufacture our product candidates to be used in clinical trials and to provide various other development related products and services. Commencing January 1, 2007, we began tracking these external costs on a project basis.
We use our internal development resources across several projects and many resources are not attributable to specific projects. Accordingly, we have included these costs in the "unallocated" category in the table below.
The following summarizes our development expenses for the periods indicated:
|
| | Years ended December 31,
| | Three months
ended March 31,
|
|---|
Product candidate (in millions)
| | 2004
| | 2005
| | 2006
| | 2006
| | 2007
|
|---|
|
| KW-3902 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | 6.0 |
| K201 (JTV-519) | | | — | | | — | | | — | | | — | | | 0.6 |
| Unallocated | | | 6.3 | | | 9.0 | | | 14.2 | | | 1.8 | | | 1.0 |
| | |
|
| | | $ | 6.3 | | $ | 9.0 | | $ | 14.2 | | $ | 1.8 | | $ | 7.6 |
| | |
|
|
We have included the costs incurred prior to January 1, 2007 in the "unallocated" category in the table above because such costs were not tracked on a project basis, even though most of these costs were associated with the development of KW-3902. We in-licensed K201 (JTV-519) from Aetas in September 2006 and incurred only diligence and business development related expenses, including professional fees, prior to that time. Prior to January 1, 2007, external services were used primarily to conduct our Phase 2 clinical trial of KW-3902 and manufacture our KW-3902 product candidate.
53
The following table details our unallocated development expenses for the periods indicated:
|
| | Years ended December 31,
|
|---|
Unallocated development expenses (in millions)
| | 2004
| | 2005
| | 2006
|
|---|
|
| | External clinical trial and regulatory | | $ | 2.8 | | $ | 5.5 | | $ | 7.5 |
| | Manufacturing | | | 1.5 | | | 1.3 | | | 2.6 |
| | License and related fees | | | 0.1 | | | 0.2 | | | 1.2 |
| | Salary, benefits and related | | | 1.0 | | | 1.1 | | | 1.2 |
| | Professional services and consulting | | | 0.6 | | | 0.4 | | | 1.0 |
| | Other | | | 0.3 | | | 0.5 | | | 0.7 |
| | |
|
| Total unallocated | | $ | 6.3 | | $ | 9.0 | | $ | 14.2 |
| | |
|
|
We expect our development expenses to be substantial over the next few years as we continue the advancement of our product development programs. We do not expect material net cash inflows from our development programs for at least several years and do not expect to have product revenue until 2010. We completed an analysis of the preliminary data from our PROTECT pilot Phase 3 clinical trial of the IV formulation of KW-3902 in March 2007. We initiated two pivotal Phase 3 trials of the IV formulation of KW-3902 in May 2007, and intend to initiate our proposed REACH UP Phase 3 trial and PULSE RENAL Phase 3 trial in the third quarter of 2007 and the Phase 2 clinical trial of an oral formulation of KW-3902 in the second half of 2007. In addition, we plan to commence a Phase 2 trial of the IV formulation of K201 (JTV-519) for the treatment of A-Fib in the second half of 2007. The lengthy process of undertaking clinical trials and seeking regulatory approval for our product candidates requires the expenditure of substantial resources. Any delay in completing clinical trials or in obtaining regulatory approvals could cause development expense to increase and, in turn, have a material adverse effect on our results of operations.
Selling, general and administrative expenses
Our selling, general and administrative expenses consist primarily of salaries and benefits for personnel in executive, finance, accounting, marketing and business development and internal systems support functions. Our administrative expenses include facility costs, professional fees for legal, consulting and accounting services and insurance costs. We anticipate increases in selling, general and administrative expenses as we add personnel, comply with the reporting obligations applicable to publicly-held companies, incur the expense of our new, larger facility and continue to build our corporate infrastructure in support of our continued development activities for the IV formulations of KW-3902 and K201 (JTV-519).
Interest income
Our interest income consists primarily of interest earned on our cash, cash equivalents and short-term investments.
Interest expense
Our interest expense to date has consisted primarily of interest expense on our outstanding loan balance under our loan and security agreement with Lighthouse Capital Partners V, L.P., or Lighthouse Capital Partners, the amortization of warrant valuations related to our various debt issuances and the beneficial conversion feature embedded in loans from our stockholders in February and June of 2006. We expect our interest expense, including the amortization of
54
warrant valuations, to increase as a result of our amendment to increase the borrowing availability under our credit facility with Lighthouse Capital Partners, and the issuance of additional warrants to purchase shares of our Series B preferred stock, as described under "—Liquidity and capital resources" below.
Income taxes
As of December 31, 2006, we had federal and state net operating loss carryforwards of approximately $36.4 million and $37.7 million, respectively. If not used, the net operating loss carryforwards will begin expiring in 2021 for federal income tax purposes and in 2013 for state income tax purposes. As of December 31, 2006, we had federal research credit carryforwards of $1.6 million, which will begin expiring in 2021. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, substantial changes in our ownership may limit the amount of net operating loss carryforwards that could be used annually in the future to offset taxable income. Any such annual limitation may significantly reduce our ability to use net operating losses before they expire. In each period since our inception, we have recorded a valuation allowance for the full amount of our deferred tax asset, as the realization of the deferred tax asset is uncertain. As a result, we have not recorded any federal or state income tax benefit in our statement of operations.
Critical accounting policies and estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in conformity with generally accepted accounting principles in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and related disclosures. Actual results could differ from those estimates.
We believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements.
Development expenses
A substantial portion of our on-going development activities are performed under agreements we enter into with external service providers, including CROs, who conduct many of our development activities. We accrue for costs incurred under these contracts based on factors such as estimates of work performed, milestones achieved, patient enrollment and experience with similar contracts. As actual costs become known, we adjust our accruals. We anticipate our estimated accruals will increase as we expand the level of development activity performed by external service providers in connection with our Phase 3 clinical trials of the IV formulation of KW-3902 and the commencement of our Phase 2 clinical trial of the IV formulations of K201 (JTV-519) and the Phase 2 trial of the oral formulation of KW-3902. As a public company, we will be required to make these estimates within a shorter time period based on less actual data, which may cause our estimates to be less accurate and result in a material change in our accruals, which could also materially affect our results of operations within any fiscal period. To date, there have been no material changes in our development expense estimates.
Stock-based compensation
Effective January 1, 2006, we adopted Statement of Financial Accounting Standards, or SFAS, No. 123(R),Share-Based Payment, which revises SFAS No. 123,Accounting for Stock-Based Compensation, and supersedes Accounting Principles Board, or APB, Opinion No. 25,
55
Accounting for Stock Issued to Employees. We adopted SFAS No. 123(R) effective January 1, 2006, prospectively for new equity awards issued subsequent to December 31, 2005. SFAS No. 123(R) requires that share-based payment transactions with employees be recognized in the financial statements based on their fair value and recognized as compensation expense over the vesting period in the related expense categories of the statement of operations. Prior to SFAS No. 123(R), we disclosed the pro forma effects of applying SFAS No. 123 under the minimum value method. Our results of operations for the year ended December 31, 2006 and the three months ended March 31, 2007 included stock-based compensation expense of approximately $62,000 and $253,000, respectively.
We estimate the fair value of options granted using the Black-Scholes option-pricing model and the assumptions described below. The weighted average expected life of options for 2006 reflects the application of the simplified method set out in Securities and Exchange Commission Staff Accounting Bulletin, or SAB, No. 107. The simplified method defines the life as the average of the contractual term of the options and the weighted average vesting period for all option tranches. Estimated volatility for fiscal 2006 also reflects the application of SAB No. 107 interpretive guidance and, accordingly, incorporates historical volatility of similar public entities. We base the risk-free interest rate on the implied yield in effect at the time of an option grant on United States Treasury zero-coupon issues with equivalent remaining terms. We have never paid any cash dividends on our common stock and do not anticipate paying any cash dividends for the foreseeable future. The assumptions used in the Black-Scholes model were 6.08 years for the expected term, 70.0% for the expected volatility, 4.54% for the risk free rate and 0% for dividend yield for the year ended December 31, 2006. The assumptions used in the Black-Scholes model were 6.07 years for the expected term, 68% for the expected volatility, 4.56% for the risk free rate and 0% for dividend yield for the three months ended March 31, 2007. We may elect to use different assumptions under the Black-Scholes option-pricing model in the future. Future expense amounts for any particular quarterly or annual period could be affected by changes in our assumptions. In addition, under the provisions of SFAS No. 123(R), we include an estimate of the number of option awards that will be forfeited in future periods. Based on the fact that the 2006 and 2007 option grants are substantially in-the-money, the likelihood of turnover was assumed to be very low. As such, we assumed a forfeiture rate of zero. We reassess the rate of forfeiture on a quarterly basis and the compensation expense may be adjusted based upon the changing rate of forfeiture.
As of March 31, 2007, we had approximately $6.2 million of unrecognized stock-based compensation costs related to unvested equity awards. As of March 31, 2007, we had outstanding vested options to purchase 2,337,200 shares of our common stock and unvested options to purchase 5,272,632 shares of our common stock with an intrinsic value of $ million and $ million, respectively, based on an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus.
Prior to January 1, 2006, we applied the intrinsic-value-based method of accounting prescribed by APB Opinion No. 25 and related interpretations. Under this method, if the exercise price of the award equaled or exceeded the fair value of the underlying stock on the measurement date, no compensation expense was recognized. The measurement date was the date on which the final number of shares and exercise price were known and was generally the grant date for awards to employees and directors. If the exercise price of the award was below the fair value of the underlying stock on the measurement date, then compensation cost was recorded,
56
using the intrinsic-value method, and was generally recognized in the statements of operations over the vesting period of the award.
The fair value of our common stock has historically been established contemporaneously by our board of directors, the members of which are knowledgeable in valuing the securities of development stage enterprises. In addition, starting in 2005, our board of directors also considered certain information prepared by applying valuation principles in determining fair market value for stock option pricing purposes. At the time of issuances of equity awards, we believed our estimates of the fair value of our common stock were reasonable and consistent with our understanding of how similarly situated companies in our industry were valued. We have considered the guidance in the American Institute of Certified Public Accountants, or AICPA, Audit and Accounting Practice Aid Series,Valuation of Privately-Held-Company Equity Securities Issued as Compensation, to determine the fair value of our common stock for purposes of setting the exercise prices of stock options granted to employees and others. This guidance emphasizes the importance of operational development in determining the value of the enterprise. Our key operational and financing milestones are as follows:
- •
- entry into the license agreement with Kyowa Hakko for KW-3902 in August 2003;
- •
- issuance of 26,190,447 shares of Series A preferred stock to certain new investors and certain related parties at $0.84 per share in August 2003;
- •
- convening of our end-of-Phase 2 meeting with the United States Food and Drug Administration, or FDA, for the IV formulation of KW-3902 in March 2006;
- •
- entry into the license agreement with Aetas for K201 (JTV-519) in September 2006;
- •
- initiation of our pilot Phase 3 clinical trial of the IV formulation of KW-3902 in September 2006;
- •
- issuance of 42,822,222 shares of Series B preferred stock to certain new investors and certain related parties at $1.125 per share in September 2006;
- •
- expansion of our license agreement with Aetas in January 2007 to include the oral formulation of K201 (JTV-519);
- •
- hiring of an experienced chief financial officer in January 2007;
- •
- completion of a preliminary analysis of data from our PROTECT pilot Phase 3 clinical trial on February 27, 2007;
- •
- issuance of 16,000,000 shares of Series B preferred stock to certain related parties at $1.125 per share in March 2007; and
- •
- entry into an amendment to our loan and security agreement with Lighthouse Capital Partners in April 2007 to increase our credit facility by an additional $15.0 million.
As a development stage company, we have been focused on product development activities and formation of corporate infrastructure with an unproven business model. Prior to 2006, we had in-licensed a single product candidate and completed one round of preferred stock financing. We had spent substantially all of the proceeds we had received from our equity and debt financing as of the end of 2005 and had current liabilities in excess of current assets. Based on these factors, we were considered to be in the early stage of development, pursuant to the AICPA guidance, where the preferences of the preferred stockholders, in particular the
57
liquidation preferences, are very significant when compared to our enterprise value. For these reasons, our board of directors estimated the fair value of our common stock at $0.14 per share as of December 31, 2005. In December 2006, given the absence of an active market for our common stock, our board of directors determined the estimated fair value of our common stock on the date of grant based on several factors, including the price of $1.125 per share at which Series B preferred stock was issued to investors in September 2006, and the rights, preferences and privileges of the preferred stock relative to the common stock, important developments relating to the results of our clinical trials, our stage of development and business strategy, financial position and the likelihood of achieving a liquidity event for our outstanding shares of stock. The rights, preferences and privileges of each series of preferred stock include a liquidation preference, dividend provisions, antidilution protective provisions and voting preferences, among other rights, while the common stock has none of these features. On the date of issuance, these preferences were considered significant and our board of directors concluded at that time that the common stock had a nominal fair value compared to the preferred stock, primarily because the likelihood of achieving a liquidity event could not be determined at that time. Based on these and other factors, in December 2006, our board of directors increased our common stock valuation to $0.16 per share. Based on the same factors considered above for the period ended December 31, 2006 and also considering the expansion of our license agreement with Aetas in January 2007 and completion of a preliminary analysis of data from our PROTECT pilot Phase 3 clinical trial near the end of February 2007, our board of directors increased our common stock valuation to $0.38 per share during the quarter ended March 31, 2007.
In the first quarter of 2007, in connection with the preparation of our financial statements necessary for this offering, we performed a market-based valuation of our common stock in order to reassess retrospectively the estimated fair value of our common stock for financial reporting purposes. There are significant judgments and estimates inherent in the determination of the reassessed fair values. These judgments and estimates include determinations of the appropriate valuation methods, including selection and weighting among asset-based, income-based and market-based approaches. Our use of a market-based approach requires selection and weighting of appropriate market comparables. For these and other reasons, the reassessed fair values used to compute stock-based compensation expense for financial reporting purposes may not reflect the fair values that would result from the application of other valuation methods, including accepted valuation methods for tax purposes.
Our market-based evaluation of our enterprise value was determined by considering the pre-offering valuations of three recently public biopharmaceutical companies that we selected, based in part on information and materials provided by investment bankers, taking into account such factors as the stage of development and type of indication being pursued. Using this market-based approach, we determined a range of enterprise values and then determined a price per common share of $2.04, which was calculated using our fully diluted capitalization prior to this offering at the low end of the enterprise valuation range.
Considering our market-based determination of our enterprise value noted above, we then reassessed the fair value of our common stock during prior periods. For the period from March 2006 through September 2006, which is the period during which we initiated and completed the sale of our Series B preferred stock, we determined that the reassessed fair value was $1.125 per share. This represents the price at which we completed the sale of our Series B preferred stock in September 2006. Using the market-based approach, we determined
58
that the Series B preferred stock per share price represented the reassessed fair value because the financing was done at arm's length, with approximately 44% of the total amount raised coming from new investors. This financing became feasible following the presentation to the FDA of our Phase 2 data in March 2006 and the decision to proceed with further development of KW-3902. For periods prior to March 2006, we believe our common stock valuations as originally determined by our board of directors were appropriate because of our early stage of development, our weak cash position and financial condition at that time and the liquidation preferences of our outstanding preferred stock.
During the fourth quarter of 2006, we increased our reassessed fair value of our common stock to $1.33 per share based upon a 35% discount to our market-based valuation of $2.04 per share. We concluded that a 35% discount reflected the attainment during the quarter of the various operational and financing milestones noted above and represented a reasonable assessment of the risk of not completing this offering, given that we were at least six months prior to completing the offering.
In the first quarter of 2007, we increased the reassessed fair value of our common stock to $1.63 per share based upon a 20% discount to our market-based valuation of $2.04 per share. We concluded that a 20% discount reflected the attainment during the quarter of the various operational and financing milestones noted above and represented a reasonable assessment of the risk of not completing this offering, given that we were at least three months prior to completing the offering.
Based on our reassessed fair values of our common stock, we concluded that the options to purchase 1,872,900 shares of our common stock granted in December 2006 at $0.16 per share were at an exercise price below the reassessed value of $1.33 per share and that the options to purchase 3,185,000 shares of our common stock granted at a weighted average exercise price of $0.35 per share during the first quarter of 2007 were below the weighted average reassessed fair value of $1.60 per share. We computed the reassessed fair value of the options granted in December 2006 and the first quarter of 2007 to be $1.23 per share and $1.39 per share, respectively, in accordance with SFAS No. 123(R), which will be amortized over a vesting period of four years.
We record equity instruments issued to non-employees as expense at their fair value over the related service period as determined in accordance with SFAS No. 123(R) and Emerging Issues Task Force, or EITF, 96-18,Accounting for Equity Instruments That are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling Goods and Services, and we periodically revalue them as the equity instruments vest.
Results of operations
Comparison of three months ended March 31, 2007 and 2006
Development expenses. Development expenses increased to $7.6 million for the three months ended March 31, 2007, from $1.8 million for the comparable period during 2006. This increase of $5.8 million primarily was due to a $2.8 million increase in clinical trial and regulatory costs related to the Phase 3 clinical trials of the IV formulation of KW-3902, a $2.3 million increase in manufacturing costs related to KW-3902 and a $0.5 million increase related to a payment for the amendment of the K201 (JTV-519) license agreement.
59
Selling, general and administrative expenses. Selling, general and administrative expenses increased to $1.2 million for the three months ended March 31, 2007 from $0.4 million for the comparable period during 2006. This increase of $0.8 million primarily was due to a $0.3 million increase in personnel and related costs due to the expansion of our finance personnel to prepare for this offering, a $0.2 million increase in consulting and professional services and $0.3 million increase in other selling, general and administrative costs.
Interest income. Interest income increased to $0.4 million for the three months ended March 31, 2007 from $20,000 for the comparable period during 2006. This increase of $0.4 million was due to the increase in our average cash and investment balances, primarily as a result of the net proceeds from preferred stock sales.
Interest expense. Interest expense decreased to $0.1 million for the three months ended March 31, 2007 from $0.2 million for the comparable period during 2006. Interest expense in 2007 primarily consisted of interest related to the outstanding borrowings under our credit facility with Lighthouse Capital Partners. This decrease of $0.1 million was primarily due to interest expense of $0.1 million related to an embedded beneficial conversion feature on stockholder bridge loans that were issued in early 2006 and converted to equity in September 2006.
Comparison of the years ended December 31, 2006 and 2005
Development expenses. Development expenses increased to $14.2 million for the year ended December 31, 2006, from $9.0 million for the comparable period during 2005. This increase of $5.2 million primarily was due to a $2.0 million increase in external clinical trial and regulatory costs related to KW-3902, a $1.3 million increase in manufacturing costs related to KW-3902, a $1.0 million increase related to the in-licensing of K201 (JTV-519), a $0.6 million increase in professional services and consulting costs and $0.3 million of other development costs.
Selling, general and administrative expenses. Selling, general and administrative expenses increased to $2.1 million for the year ended December 31, 2006 from $2.0 million for the comparable period during 2005. This increase of $0.1 million primarily was due to increased personnel and related costs.
Interest income. Interest income increased to $0.6 million for the year ended December 31, 2006 from $0.1 million for the comparable period during 2005. This increase of $0.5 million was due to the increase in our average cash and investment balances and higher market interest rates in 2006.
Interest expense. Interest expense increased to $2.2 million for the year ended December 31, 2006 from $0.2 million for the comparable period during 2005. This increase of $2.0 million was primarily due to interest expense of $0.9 million related to the warrants issued in connection with the original issuance and amendment of a credit facility with Lighthouse Capital Partners and certain stockholder loans used as bridge financing between February 2006 and September 2006. Interest expense also increased $0.5 million related to the outstanding balances on the Lighthouse Capital Partners credit facility and stockholder bridge loans and $0.6 million related to an embedded beneficial conversion feature on the stockholder bridge loans.
60
Comparison of years ended December 31, 2005 and 2004
Development expenses. Development expenses increased to $9.0 million for the year ended December 31, 2005, from $6.3 million for the comparable period during 2004. This increase of $2.7 million primarily was due to the clinical development of KW-3902, for which we commenced Phase 2 clinical trials in August 2004.
Selling, general and administrative expenses. Selling, general and administrative expenses increased to $2.0 million for the year ended December 31, 2005 from $1.8 million for the comparable period during 2004. This increase of $0.2 million primarily was due to increased personnel and related costs.
Interest income. Interest income decreased to $0.1 million for the year ended December 31, 2005 from $0.2 million for the comparable period during 2004. This decrease of $0.1 million primarily was due to the decrease in average cash and investment balances in 2005.
Interest expense. Interest expense increased to $0.2 million for the year ended December 31, 2005 from zero for the comparable period during 2004. This increase of $0.2 million was primarily due to interest paid in connection with September 2005 borrowings under our credit facility with Lighthouse Capital Partners.
Liquidity and capital resources
Since inception, our operations have been financed primarily through the private placement of equity securities and both long-term and short-term debt financings. Through March 31, 2007, we received net proceeds of approximately $88.3 million (including the conversion of stockholder loans) from the sale of shares of our preferred and common stock as follows:
- •
- from December 2001 to March 2007, we issued and sold a total of 2,726,219 shares of common stock for aggregate net proceeds of approximately $140,000;
- •
- in August 2003, we issued and sold a total of 26,190,477 shares of Series A preferred stock for aggregate net proceeds of approximately $21.9 million;
- •
- in September 2006, we issued and sold 42,822,222 shares of Series B preferred stock for aggregate net proceeds of approximately $48.0 million, including the conversion of $5.2 million in convertible bridge notes and related interest;
- •
- in March 2007, we issued and sold 256,000 shares of Series B preferred stock upon the exercise of outstanding warrants for aggregate net proceeds of $288,000; and
- •
- also in March 2007, we issued and sold 16,000,000 shares of Series B preferred stock for aggregate net proceeds of approximately $18.0 million.
In March 2005, we entered into a loan and security agreement with Lighthouse Capital Partners for $5.0 million, which was fully drawn on September 1, 2005. After giving effect to the restructuring of this loan in April 2007, the then outstanding principal balance is due under the same terms as the $5 million borrowed in April 2007. As of December 31, 2006, $3.0 million in principal remained outstanding under the loan and security agreement. In connection with the loan and security agreement, we issued a warrant to Lighthouse Capital Partners to purchase up to 357,142 shares of Series A preferred stock at an exercise price of $0.84 per share.
61
In July 2006, we amended our loan and security agreement with Lighthouse Capital Partners to provide for additional financing of up to $3.0 million and immediately borrowed the $3.0 million. Upon the close of our Series B preferred stock financing in September 2006, we repaid the additional $3.0 million. In connection with the amendment to the loan and security agreement, we issued a warrant to Lighthouse Capital Partners to purchase up to 306,667 shares of Series B preferred stock at an exercise price of $1.125 per share. Under the terms of our existing loan and security agreement with Lighthouse Capital Partners, we are precluded from entering into certain financing and other transactions, including disposing of certain assets and paying dividends, and are subject to various non-financial covenants. Our loan and security agreement with Lighthouse Capital Partners restricts our ability to pay dividends by prohibiting us from paying any dividends other than dividends payable solely in our capital stock without the prior written consent of Lighthouse Capital Partners.
In April 2007, we amended our loan and security agreement with Lighthouse Capital Partners to increase our credit facility by an additional $15.0 million. $5.0 million was funded in connection with the execution of the amendment and the remaining $10.0 million is available through September 1, 2007. Interest accrues at the prime rate plus 1.5% per year, with interest-only payments through December 2007. The interest rate floats during the interest-only period based upon any changes in the prime rate and will become fixed at January 1, 2008. Principal and interest payments will commence on January 1, 2008 and are required to be made in 36 monthly installments. In connection with the increased credit facility, we issued Lighthouse Capital Partners a warrant to purchase up to 933,333 shares of our Series B preferred stock at an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts under our credit facility (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price). Our loan and security agreement with Lighthouse Capital Partners is secured by all of our assets other than intellectual property and there is a negative pledge on our intellectual property and a variety of operational covenants. In connection with the increased credit facility, the outstanding indebtedness under our existing loan and security agreement with Lighthouse Capital Partners was restructured.
During March 2007, we completed the sale of 16,000,000 shares of Series B preferred stock for aggregate net proceeds of approximately $18.0 million. The Series B preferred stock was sold at a price per share consistent with our September 2006 Series B preferred stock financing, which is below the anticipated initial public offering price. Accordingly, pursuant to EITF Issue No. 98-5,Accounting for Convertible Securities with Beneficial Conversion Features and EITF No. 00-27,Application of Issue 98-5 to Certain Convertible Instruments, we have recorded a deemed dividend on the Series B preferred stock equal to the number of shares of Series B preferred stock sold multiplied by the difference between the $1.63 per share estimated fair value for financial reporting purposes of the underlying common stock and the $1.125 Series B conversion price per share. The deemed dividend of $8,080,000 had no net impact on stockholders' equity but increased the net loss attributable to common stockholders in the calculation of basic and diluted net loss per common share during the first quarter of 2007.
As of December 31, 2006 and March 31, 2007 we had $12.3 million and $28.6 million, respectively, in cash and cash equivalents, primarily consisting of money market funds, and $19.2 million and $13.9 million, respectively, of short-term investments, all of which were corporate debt securities.
62
Net cash used in operating activities was $6.7 million, $10.4 million, $17.4 million and $7.1 million for the years ended December 31, 2004, 2005 and 2006 and the three months ended March 31, 2007, respectively. Net cash used in each of these periods was primarily a result of external development expenses, clinical trial costs, personnel-related costs, third-party supplier expenses and professional fees.
Net cash provided by investing activities was $9.2 million, $3.6 million and $5.5 million for the years ended December 31, 2004 and 2005 and the three months ended March 31, 2007, respectively. Net cash used in investing activities was $19.2 million in 2006. The activity in each period was primarily due to net purchases or maturities of investment securities.
Net cash provided by financing activities was $4.5 million, $46.4 million and $17.9 million for the years ended December 31, 2005 and 2006 and the three months ended March 31, 2007, respectively. In 2005, financing activities consisted primarily of $5.0 million of proceeds from a loan and security agreement offset by $0.5 million of repayments on the related debt. In 2006, we received $42.8 million related to sales of equity securities and received $8.0 million related to investor and non-investor bridge loans offset by $4.5 million of related debt repayments. In the three months ended March 31, 2007, we received $18.3 million related to sales of equity securities and repaid $0.4 million of our outstanding indebtedness.
We cannot be certain if, when or to what extent we will receive cash inflows from the commercialization of our product candidates. We expect our development expenses to be substantial and to increase over the next few years as we continue the advancement of our product development programs.
As a development stage pharmaceutical company focused on in-licensing and developing proprietary pharmaceutical product candidates, we have entered into license agreements to acquire the rights to develop and commercialize our two product candidates, KW-3902 and K201 (JTV-519). Pursuant to these agreements, we obtained exclusive licenses to the patent rights and know-how for both IV and oral formulations of KW-3902 and K201 (JTV-519) for selected indications and in selected territories. Under the KW-3902 license agreement, we paid $2.0 million in up-front fees and may be required to make future milestone payments totaling up to $19.5 million upon the achievement of various milestones related to regulatory or commercial events for the first two indications, including $1.5 million that we expect to pay to Kyowa Hakko in June 2007 as a result of having initiated our Phase 3 clinical trials of the IV formulation of KW-3902. We are obligated to negotiate and agree to additional milestone payments before submitting an NDA for a third indication. Under the K201 (JTV-519) license agreement, we paid $1.5 million in up-front fees and may be required to make future milestone payments totaling up to $18.0 million upon the achievement of various milestones related to regulatory or commercial events. Under both agreements, we are also obligated to pay royalties on any net sales of the licensed products.
Our future capital uses and requirements depend on numerous forward-looking factors. These factors include but are not limited to the following:
- •
- the progress of our clinical trials, including expenses to support the trials and milestone payments that may become payable under our license agreements;
- •
- the costs and timing of regulatory approvals;
63
- •
- the costs and timing of clinical and commercial manufacturing supply arrangements for each product candidate;
- •
- the costs of establishing sales or distribution capabilities;
- •
- the success of the commercialization of our products;
- •
- our ability to establish and maintain strategic collaborations, including licensing and other arrangements;
- •
- the costs involved in enforcing or defending patent claims or other intellectual property rights; and
- •
- the extent to which we in-license or invest in other indications, products or businesses.
We expect that our monthly cash used by operations will continue to increase for the next several years. We believe that the net proceeds from this offering, together with interest thereon, and our existing cash, cash equivalents and short-term investments and existing borrowing availability under our credit facility, will be sufficient to fund our operations through at least the end of 2008.
Until we can generate significant cash from our operations, we expect to continue to fund our operations with existing cash resources generated from the proceeds of offerings of our equity securities and from our existing and from available additional borrowings under our loan and security agreement. In addition, we may finance future cash needs through the sale of additional equity securities, strategic collaboration agreements and other debt financing. We may not be successful in obtaining strategic collaboration agreements at all or on favorable terms or in receiving milestone or royalty payments under those strategic collaboration agreements. In addition, we cannot be sure that our existing cash and investment resources will be adequate, that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or our stockholders. Having insufficient funds may require us to delay, scale back or eliminate some or all of our clinical development programs, relinquish some or even all rights to product candidates at an earlier stage of development or renegotiate less favorable terms than we would otherwise choose. Failure to obtain adequate financing also may adversely affect our ability to operate as a going concern. If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders would likely result. If we raise additional funds by incurring additional debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
64
Contractual obligations and commitments
The following table describes our long-term contractual obligations and commitments as of December 31, 2006:
|
| | Payments due by period
|
|---|
(in thousands)
| | Total
| | 2007
| | 2008
| | 2009
| | 2010
| | 2011
| | Beyond
|
|---|
|
| Principal and interest payable under credit facility(1) | | $ | 3,323 | | $ | 1,874 | | $ | 1,449 | | $ | — | | $ | — | | $ | — | | $ | — |
| Operating lease obligations(2) | | | 94 | | | 94 | | | — | | | — | | | — | | | — | | | — |
| License obligations(3) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
| Contractual obligations to CROs and manufacturers(4) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
| | |
|
| Total | | $ | 3,417 | | $ | 1,968 | | $ | 1,449 | | $ | — | | $ | — | | $ | — | | $ | — |
| | |
|
|
- (1)
- Amounts do not include the $5.0 million borrowed in April 2007 in connection with an amendment to our loan and security agreement with Lighthouse Capital Partners and do not reflect the restructuring in April 2007 of our existing indebtedness to Lighthouse Capital Partners. As a result of the restructuring in April 2007, we will make principal and interest payments to Lighthouse Capital Partners (with respect to the existing indebtedness outstanding as of April 2007 and excluding the additional $5.0 million borrowed in April 2007) of $902,676 in 2008, $902,676 in 2009 and $1,026,511 in 2010.
- (2)
- Amounts do not include payments due in connection with our sublease for our new facility. Our operating lease obligations under the sublease are as follows: an aggregate of $38,500 in 2007, $289,071 in 2008, $299,189 in 2009 and $179,164 in 2010, for a total obligation of $805,924.
- (3)
- License obligations do not include a $1.5 million payment we expect to make to Kyowa Hakko in June 2007 as a result of having initiated our Phase 3 clinical trials of the IV formulation of KW-3902, a $500,000 payment to Aetas in February 2007 in connection with the amendment of the K201 (JTV-519) license agreement and payments of up to $36.0 million due upon the occurrence of certain future milestones related to regulatory or commercial events. We may also be required to pay royalties on any net sales of the licensed products. License payments may be increased based on the timing of various milestones and the extent to which the licensed technologies are pursued for other indications. These milestone payments and royalty payments under our license agreements are not included in the table above because we cannot, at this time, determine when or if the related milestones will be achieved or the events triggering the commencement of payment obligations will occur.
- (4)
- Excludes total budgeted payments of approximately $26.8 million as of December 31, 2006 for ongoing clinical trials pursuant to task orders under our clinical research agreements and amounts under outstanding purchase orders or contracts for manufacturing of supplies as of December 31, 2006. Under our CRO agreement for the Phase 3 clinical trials of KW-3902, we have a right to terminate a specific trial for good cause, such as identification of medical risk to patients, a showing that the
drug is ineffective or evidence of regulatory action by the FDA terminating or suspending a trial, in which case we would not have to pay for budgeted expenses that had not yet been incurred.
We also enter into other agreements or purchase orders with third parties to manufacture our product candidates, conduct our clinical trials and perform data collection and analysis. Our payment obligations under certain of these agreements depend upon the progress of our development programs. Therefore, we are unable at this time to estimate with certainty the future costs we will incur under these agreements or purchase orders.
Related person transactions
For a description of our related person transactions, see "Transactions with related persons" beginning on page 119.
Recent accounting pronouncements
In July 2006, FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109, or FIN No. 48, which clarifies the recognition threshold and measurement attribute for the financial statement recognition and
65
measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 also provides guidance on recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. We have adopted FIN No. 48 as of January 1, 2007 and determined that the adoption of FIN No. 48 did not have a material effect on our financial statements.
Off-balance sheet arrangements
We have not engaged in any off-balance sheet activities.
Quantitative and qualitative disclosures about market risk
Our cash, cash equivalents and investments as of March 31, 2007 consisted primarily of cash, money market funds and corporate debt securities with maturities through September 2007. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of United States interest rates, particularly because the majority of our investments are in short-term corporate debt securities. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. Some of the securities that we invest in may be subject to market risk. This means that a change in prevailing interest rates may cause the value of the investment to fluctuate. For example, if we purchase a security that was issued with a fixed interest rate and the prevailing interest rate later rises, the value of our investment will probably decline. To minimize this risk, we intend to continue to maintain our portfolio of cash equivalents and short-term investments in a variety of securities including money market funds and corporate debt securities, all with various maturities. In general, money market funds are not subject to market risk because the interest paid on such funds fluctuates with the prevailing interest rate.
We incur significant expenses, including for clinical trials, outside the United States based on contractual obligations denominated in currencies other than the U.S. dollar. Some of our clinical trial expenses outside the United States are paid in Euros and Swiss Francs. We pay our CRO Hesperion for services outside the United States in U.S. dollars based upon fees accrued in Swiss Francs and converted into U.S. dollars at the average currency exchange rate for U.S. dollars into Swiss Francs for the prior month. Our other clinical trial expenses incurred in Euros or Swiss Francs are expected to increase as a result of the commencement of our pivotal Phase 3 clinical trials of the IV formulation of KW-3902. At the end of each reporting period, these liabilities are converted to U.S. dollars at the then applicable foreign exchange rate. We generally pay pass through expenses in the currency in which the expenses are incurred. As a result, our business is affected by fluctuations in exchange rates between the U.S. dollar and foreign currencies. We do not enter into foreign currency hedging transactions to mitigate our exposure to foreign currency exchange risks. Exchange rate fluctuations may adversely affect our expenses, results of operations, financial position and cash flows. However, to date, these fluctuations have not been significant. Based on our expenses paid in Euros and Swiss Francs during the three months ended March 31, 2007, a movement of 10% in U.S. dollar to Euro or U.S. dollar to Swiss Franc exchange rates would have resulted in an increase or decrease in our aggregate expenses during the three months ended March 31, 2007 of approximately $185,000 or $265,000, respectively.
66
Business
Overview
We are a clinical-stage pharmaceutical company focused on developing drugs to treat major cardiovascular diseases that are underserved by existing therapies. We have two compounds in clinical development, KW-3902 and K201 (JTV-519), each with potential for use in both acute and chronic settings. We are currently testing the intravenous, or IV, formulation of KW-3902 in Phase 3 clinical trials for the treatment of the acute form of congestive heart failure, or CHF, in patients with renal impairment and fluid overload. We are also developing an oral formulation of KW-3902 for the treatment of chronic CHF. In addition, we are developing K201 (JTV-519) for the treatment of atrial fibrillation, or A-Fib, an irregular heartbeat.
There are nearly five million people in the United States with CHF, according to the American Heart Association, or AHA. Many CHF patients experience acute CHF, a rapid deterioration of their condition, requiring urgent treatment in the hospital. Renal function is an important determinant of the management and outcome of CHF, and worsening renal function during hospital stay is a strong predictor of negative outcomes, including length of hospital stay and mortality. We estimate that more than half of CHF patients have some degree of renal impairment based on a study published inCirculation in 2004. Fluid overload is one of the major causes of hospitalization for CHF patients, and approximately 80% of patients are on chronic diuretic therapy to manage their fluid overload at the time of their hospitalization, according to the ADHERE Registry, a registry of acute heart failure patient cases in the United States. However, the use of diuretic therapy such as furosemide, or Lasix, to manage fluid overload in CHF patients may lead to further deterioration of renal function.
We are developing KW-3902, a selective adenosine A1 receptor antagonist, to improve the treatment of CHF patients by preserving renal function, improving the symptoms of fluid overload and enabling the optimal use of other CHF therapies. We are developing both IV and oral formulations of KW-3902 to be co-administered with diuretics for the treatment of acute and chronic CHF, respectively. We have completed three multi-center, placebo-controlled Phase 2 clinical trials and have obtained preliminary data from 276 patients out of a 304-patient pilot Phase 3 clinical trial of the IV formulation of KW-3902. We initiated two pivotal Phase 3 trials of the IV formulation of KW-3902 for the treatment of acute CHF in May 2007 and expect to have results from these trials in mid-2008. We intend to initiate two additional Phase 3 trials of the IV formulation of KW-3902 and expect to commence one of these trials in mid-2007 and the other trial in the third quarter of 2007. In addition, we plan to commence a Phase 2 trial of an oral formulation of KW-3902 in outpatients with chronic CHF in the second half of 2007.
We are developing K201 (JTV-519) for the treatment of A-Fib. A-Fib afflicts over 2.2 million people in the United States and leads to over 440,000 hospitalizations per year, according to the most recent data from the AHA. A-Fib is an independent risk factor for stroke, and patients with CHF frequently also require treatment for A-Fib. We believe that K201 (JTV-519) has the potential to treat A-Fib without the side effects, such as life-threatening ventricular arrhythmias, associated with current therapies. We are developing IV and oral formulations of K201 (JTV-519) to address acute and chronic A-Fib, respectively. We plan to commence a Phase 2 clinical trial of the IV formulation for the acute treatment of K201 (JTV-519) in the second half of 2007.
We hold commercial rights to KW-3902 and K201 (JTV-519) in major markets, including the United States and Europe, but excluding Asia. We intend to market the IV formulations of our
67
product candidates in the United States by establishing a hospital-based sales force. We plan to enter into commercialization arrangements with third parties to access the acute care markets outside the United States and the chronic care markets in territories where we hold the rights to our product candidates. We in-licensed KW-3902 from Kyowa Hakko Kogyo Co., Ltd., or Kyowa Hakko, and K201 (JTV-519) from Aetas Pharma Co., Ltd., or Aetas, and we plan to in-license additional product candidates for the treatment of cardiovascular diseases.
CHF background
Overview
CHF is a widespread and debilitating disease most often caused by a weakening or stiffening of the heart muscle, which leads to a progressive loss in the heart's ability to pump blood effectively throughout the body. It can be caused by a number of disease states that damage heart muscle, including coronary artery disease, heart attack and high blood pressure. As the heart loses its ability to pump effectively, blood backs up into the blood vessels of the lungs, causing increases in pulmonary vascular pressure. As this pressure increases, fluid moves from the pulmonary blood vessels into the air spaces in the lung, causing pulmonary congestion and shortness of breath, a CHF symptom known as dyspnea. Fluid accumulation in other parts of the body, known as fluid overload, leads to swelling of the abdomen, legs and feet. As the disease progresses, these symptoms can severely impact the patient's quality of life, limiting his or her ability to perform simple activities such as walking across the room.
There are nearly five million people in the United States with CHF, according to the AHA. With the aging population and more patients surviving the early stages of cardiovascular diseases, the prevalence of CHF is increasing. Approximately 550,000 new cases of CHF are reported in the United States each year, according to the AHA. In addition, one in five patients with CHF will die within one year of diagnosis, and 80% of male patients and 70% of female patients under 65 suffering from CHF will die within eight years of diagnosis.
Many CHF patients experience a rapid deterioration of their condition requiring urgent treatment in the hospital. This acute form of CHF accounts for over one million hospital admissions with CHF as the primary diagnosis, and up to two million hospital admissions with CHF as the secondary diagnosis, according to the Cleveland Clinic, and is the leading cause of hospitalization in patients over 65, according to the Centers for Disease Control.
Current therapies for CHF
Patients with chronic CHF are typically treated with a combination of oral medications, including angiotensin converting enzyme inhibitors, or ACE inhibitors, angiotensin receptor blockers, or ARBs, beta-blockers, vasodilators, diuretics and digoxin. These drugs act to decrease the work of the heart, decrease the resistance against which the heart must pump, reduce fluid overload or strengthen the contraction of the heart.
Upon admission to the hospital, acute CHF patients are typically treated with IV diuretics, generally IV furosemide, or Lasix, and sometimes with positive inotropes and vasodilators. Diuretics, the most commonly used therapy, increase sodium and water excretion, which in turn reduces fluid overload associated with acute CHF. Positive inotropes and vasodilators serve to increase the efficiency of the circulatory system and provide relief of the symptoms associated with acute CHF. As a patient's disease progresses, the frequency of hospitalizations increases, along with the need for higher doses of diuretics for treatment of acute CHF.
68
The current treatments for acute CHF all have limitations. Positive inotropes have been shown to cause increased mortality rates. Systemic vasodilators can cause a dangerous decline in blood pressure, which may lead to deterioration of organ function, including renal function. Diuretics are valued for their quick onset and overall effectiveness. However, diuretics generally lose their effectiveness over time and higher doses are often associated with worsening renal function.
Renal function in CHF
Physicians are becoming increasingly aware that an important relationship exists between the heart and the kidney in CHF. We believe this complex interaction, known as the cardio-renal syndrome, increasingly influences how doctors manage the CHF patient as the disease progresses. Multiple studies have demonstrated that renal dysfunction is a strong independent predictor of worse short- and long-term outcomes in patients with CHF. For example, during hospital stays, a greater than 0.3 milligram per deciliter increase in serum creatinine, a commonly used marker of worsening renal function, predicts longer hospital stays and higher mortality rates. Worsening renal function makes effective treatment with diuretics more difficult, may increase workload of the heart, may trigger arrhythmias and may result in the production of chemical mediators that may adversely affect the cardiovascular system. In addition, in the presence of renal impairment, we believe physicians often decrease use of outcome-improving drugs such as ACE inhibitors for fear that these drugs will further worsen renal function.
Adenosine and renal function
Adenosine has been found to play an important role in regulating renal function. Adenosine is a naturally occurring molecule that acts via several kinds of adenosine receptors throughout the body. The kidney is the only organ in which adenosine acts to constrict the blood vessels, most notably the artery that supplies the glomerulus, the filtering unit of the kidney.
In a healthy kidney, the complex action of adenosine fine-tunes blood flow, renal function and the handling of salt and water. On the other hand, in patients with CHF, adenosine's action may be more prominent and counter-therapeutic. By acting specifically through the adenosine A1 receptor, adenosine may decrease renal function by restricting renal blood flow and cause increased reabsorption of salt and water through the proximal tubule of the kidney. As renal function deteriorates over time, higher doses of diuretics are required in order to achieve the same effects. As a result, adenosine may be further activated and the cycle continues, leading to further worsening of renal function.
As a result, we believe that a therapy that blocks the adenosine A1 receptor in the kidney will preserve or improve renal function while enhancing diuresis, resulting in reduced fluid overload and better short- and long-term outcomes in patients with CHF.
KW-3902: New approach to CHF
KW-3902 is an adenosine A1 receptor antagonist that selectively blocks the effect of adenosine on the adenosine A1 receptor. We believe that administration of KW-3902 will result in blockade of adenosine-mediated vasoconstriction in the kidney and inhibition of salt and water reabsorption in the proximal tubule of the kidney, thereby maintaining renal function and facilitating diuresis. We believe other compounds intended to improve renal function via vasodilator effects have been ineffective because they are not selective to the kidney, and non-selective vasodilation results in the retention of salt and water. To date, no other
69
vasodilator has demonstrated the selective renal vasodilation attribute of KW-3902 that is critical for preserving renal function.
We believe that these effects may:
- •
- preserve renal function through increased blood flow to the kidney;
- •
- facilitate diuresis to reduce fluid overload;
- •
- reduce the need for higher doses of diuretics or other acute CHF therapies that are associated with worsening renal function and other side effects; and
- •
- enable the optimal use of outcome-improving drugs such as ACE inhibitors and ARBs that may be underutilized in patients with worsening renal function.
As a result, KW-3902 may provide the following benefits for patients:
- •
- improved symptoms and quality of life;
- •
- shorter and less frequent hospital stays; and
- •
- increased survival.
KW-3902 IV formulation: Clinical development overview
To date, we have completed three multi-center Phase 2 clinical trials and a pilot Phase 3 clinical trial of the IV formulation of KW-3902 in patients with acute CHF. We initiated two pivotal Phase 3 trials of the IV formulation of KW-3902 in May 2007. We intend to initiate two additional Phase 3 trials of the IV formulation of KW-3902 and expect to commence one of these trials in mid-2007 and the other trial in the third quarter of 2007. We plan to commence a Phase 2 trial of the oral formulation of KW-3902 in outpatients with chronic CHF in the second half of 2007. To date, KW-3902 has been administered to more than 400 healthy volunteers and patients and was generally well tolerated in these individuals.
Prior to our licensing of KW-3902 from Kyowa Hakko in September 2003, Kyowa Hakko and Hoechst Marion Roussel, Inc., or HMR, conducted Phase 1 clinical trials of the IV formulation of KW-3902 in approximately 36 healthy volunteers. KW-3902 was generally well tolerated in these trials. An additional 106 patients were enrolled in Phase 1 trials of an oral formulation of KW-3902. Based on the results of these Phase 1 trials conducted by Kyowa Hakko and HMR, we filed an Investigational New Drug Application, or IND, for the IV formulation of KW-3902 in August 2004 and commenced our first Phase 2 clinical trial of KW-3902 in August 2004.
Phase 2 clinical trials
CKI-201 clinical trial in acute CHF patients with mild to moderate renal impairment
In August 2005, we completed a multi-center, randomized, double-blind, placebo-controlled, dose-ranging trial evaluating the effects of KW-3902, both as monotherapy and in co-administration with IV Lasix, on diuresis and renal function. We conducted the trial in hospitalized patients with acute CHF and renal impairment, which we defined for purposes of this trial as creatinine clearance of 20 to 80 milliliters per minute, who suffered from fluid overload and required treatment with IV diuretics. Creatinine clearance is a common measurement of renal function and is determined by comparing the concentration of creatinine excreted in a volume of urine to the concentration of creatinine in the blood. Higher creatinine clearance indicates greater renal function.
70
The primary objective of this trial was to determine the optimal IV dose range of KW-3902 by comparing the diuretic effect of a range of single IV doses of KW-3902 versus placebo over a six-hour period. A secondary objective was to evaluate the effect on renal function of KW-3902 when co-administered with IV Lasix. In this trial, 146 patients were randomly assigned to placebo or 2.5, 15, 30 or 60 milligram doses of the IV formulation of KW-3902 infused over two hours daily for up to three days. IV Lasix could be co-administered at any time after six hours on day one.
In this trial, we made the following observations:
- •
- Enhanced diuresis—a dose response and a statistically significant improvement in urine volume at the 30 milligram dose versus placebo.
- •
- Reduced necessary doses of diuretics—when co-administered with standard diuretic therapy, KW-3902 allowed for the use of lower doses of diuretics and enabled earlier discontinuation of IV diuretic therapy.
- •
- Preserved renal function—when co-administered with standard diuretic therapy, KW-3902 preserved renal function relative to placebo in the 2.5, 15 and 30 milligram doses.
Based on the results described above, we concluded that 30 milligrams was the optimal dose and that KW-3902, when added to standard diuretic therapy, may prove beneficial in preserving renal function and facilitating diuresis.
CKI-202 clinical trial in acute CHF patients refractory to diuretic therapy
In December 2005, we completed a multi-center, randomized, double-blind, placebo-controlled, dose-escalation clinical trial evaluating the effects of KW-3902 on renal function and diuresis. We conducted the trial in patients with acute CHF who were not adequately responding to high-dose diuretic therapy. The mean creatinine clearance of these patients was 35 milliliters per minute.
The primary objective of this trial was to determine if a single administration of KW-3902, at each of the four trial doses of 10, 30, 60 and 100 milligrams, given over a two-hour period, could increase urine output to a clinically significant degree from the baseline compared to placebo. Secondary objectives included evaluation of the effect of KW-3902 on renal function as measured by changes in creatinine clearance. In this trial, 34 patients who were non-responsive to diuretics were randomly assigned to placebo or one of the three trial doses of KW-3902. Twenty-three patients were treated with KW-3902. This trial was not designed to assess the statistical significance of effects.
In this trial, we made the following observations:
- •
- Diuretic effect in patients who are non-responsive to diuretics—an increase in urine volume relative to placebo with the best effect observed in patients who received the 30 milligram dose.
- •
- Preserved renal function—improved or maintained creatinine clearance for the 30 milligram dose compared to a worsening in creatinine clearance in patients treated with placebo.
Based on these results, we believe KW-3902 may prove beneficial in facilitating diuresis while simultaneously preserving renal function in patients who are not responsive to conventional therapy.
71
CKI-203 clinical trial in CHF outpatients with mild renal impairment
In November 2005, we completed a multi-center, randomized, double-blind, placebo-controlled, two-way crossover Phase 2 clinical trial evaluating the effects of the IV formulation of KW-3902, when co-administered with IV Lasix, on 32 treated outpatients with stable heart failure and renal impairment, which we defined for purposes of this trial as creatinine clearance of 30 to 80 milliliters per minute.
The primary objective of this trial was to compare the effect on renal function of a single 30 milligram dose of the IV formulation of KW-3902 administered over two hours, when co-administered with 80 milligrams of IV Lasix, to placebo when co-administered with IV Lasix. We measured renal function based on glomerular filtration rate during the three-hour period before the administration of KW-3902 and IV Lasix, as well as for the eight-hour period following treatment. The secondary endpoint for this trial was to compare the effect of KW-3902 and IV Lasix to placebo and IV Lasix on renal blood flow during the same time period following treatment.
Glomerular filtration rate, or GFR, is the standard measure of how well a person's kidneys are filtering wastes from their blood. GFR is determined by comparing the concentration of an intravenously administered compound, with specific properties, in a volume of urine and comparing it to the concentration of that compound in the blood.
In this trial, we made the following observations:
- •
- Improved renal function—when co-administered with IV Lasix, KW-3902 showed a statistically significant increase in GFR of 32% compared to 8% for placebo over the eight-hour time interval.
- •
- Increased renal blood flow—when co-administered with IV Lasix, KW-3902 increased renal blood flow by 56% compared to 18% for placebo at the four- to eight-hour time interval.
- •
- Persistent effect on renal function—patients receiving KW-3902 on the first visit in this study demonstrated a persistent increase in GFR when returning for their follow-up visit after a median of six days compared to those who were first treated with placebo.
Based on these results, we believe KW-3902 is a selective renal vasodilator that improves renal function in CHF patients with renal impairment and may have a delayed or persistent effect beyond the first eight hours.
Phase 2 clinical trials safety data
KW-3902 was generally well tolerated in patients in our Phase 2 clinical trials. The most common side effects observed in patients who received either KW-3902 or placebo were worsening CHF, abnormal heart rhythms, kidney and urinary problems, hypokalemia (a low amount of potassium in the blood), aches and cramps, headaches, low blood pressure, nausea, constipation, hypoglycemia, insomnia, dizziness, anxiety and anemia.
In our Phase 2 clinical trials, two patients with risk factors for seizure had seizures that were determined to be drug-related. One patient who received KW-3902 at a dose of 60 milligrams experienced a seizure during the day two infusion. Before the second day of treatment with KW-3902, this diabetic patient had experienced wide swings in glucose concentrations, a potential seizure trigger, and had been given a drug for nasal decongestion with known pro-convulsant activity. Another patient with an undiagnosed brain tumor, a significant risk
72
factor for seizures, received a single 30 milligram dose of the IV formulation of KW-3902 and developed a seizure shortly after completing the infusion of KW-3902.
Although seizures can be seen more frequently in hospitalized CHF patients, we believe that KW-3902, as an adenosine A1 receptor antagonist, may be associated with the lowering of seizure thresholds and may precipitate seizures in individuals already at risk for seizure. As a result, we have designed our Phase 3 clinical trials in consideration of the potential seizure risk.
Phase 3 clinical trials
In March 2006, we met with the FDA to discuss the clinical trial requirements for submission of a new drug application, or NDA, for the IV formulation of KW-3902. The FDA has indicated that a minimum of 1,500 patients must have been administered the IV formulation of KW-3902 at doses that are equal to or above the dose for which we seek regulatory approval. We expect to enroll 2,359 patients in our Phase 3 clinical trials. Our PROTECT 1 and PROTECT 2 trials, described below, are our pivotal trials and we believe that these trials, if successful, will be adequate to support regulatory approval for KW-3902. The FDA has also indicated that if our REACH UP trial is successful and one or both of our PROTECT clinical trials are successful, our REACH UP trial could serve as a separate pivotal trial of KW-3902. Our Phase 3 clinical program will encompass the following trials:
|
KW-3902 Phase 3 clinical trial program
|
|---|
Trial name
| | Objectives
| | Patient population
| | Trial size
| | Status
|
|---|
|
| PROTECT Pilot | | Confirm safety, evaluate primary endpoints and select a dose for planned pivotal trials | | Acute CHF patients with mild to severe renal impairment who required treatment with IV diuretics to reduce fluid overload | | 304 | | Preliminary analysis completed |
PROTECT 1 |
|
Evaluate effect on dyspnea, renal function and incidence of worsening heart failure in acute CHF patients with renal impairment undergoing IV diuresis |
|
Acute CHF patients with mild to severe renal impairment who require treatment with IV diuretics to reduce fluid overload |
|
600 |
|
Ongoing; data anticipated in mid-2008 |
PROTECT 2 |
|
Identical to PROTECT 1 |
|
Identical to PROTECT 1 |
|
600 |
|
Identical to PROTECT 1 |
REACH UP |
|
Evaluate effect on renal function and heart failure severity |
|
Hospitalized CHF patients with moderate to severe renal impairment being treated for fluid overload and who have experienced a documented worsening of renal function |
|
480 |
|
To begin in mid-2007 |
PULSE RENAL |
|
Evaluate serial infusion in outpatients |
|
Outpatients with heart failure |
|
375 |
|
To begin in the third quarter of 2007 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
73
PROTECT Pilot trial results
In March 2007, we completed a preliminary analysis of the 14-day data from the PROTECT pilot Phase 3 clinical trial. We analyzed data from 276 patients out of a total 304 patients for which data were available as of February 1, 2007. This trial was designed to evaluate the safety of the IV formulation of KW-3902, to optimize the dose regimen and confirm the Phase 3 clinical trial sample size. The pilot trial was conducted at approximately 80 sites in the United States, Europe, Israel and Russia, and included 304 patients who were randomized to placebo or 10, 20 or 30 milligram doses of the IV formulation of KW-3902 infused daily for up to three days and co-administered with IV Lasix. In the trial, 225 patients received KW-3902 and 79 patients received placebo. We enrolled hospitalized acute CHF patients with renal impairment, which we defined for purposes of this trial as creatinine clearance of 20 to 80 milliliters per minute, who required treatment with IV diuretics to treat fluid overload. This trial was not designed to assess the statistical significance of effects.
As a result of the increased risk of seizure associated with the administration of KW-3902 and the occurrence of seizures in two patients in our Phase 2 clinical trials, we excluded patients who were at risk for seizure, including patients with certain known medical histories, including, but not limited to, brain tumors, history of seizure, brain surgery within two years, alcohol withdrawal, stroke within two years, advanced multiple sclerosis, penetrating head trauma and late-stage Alzheimer's disease, from participating in the trial. Patients believed to have an elevated risk of seizure but not excluded from the trial by the above criteria were prophylactically administered a benzodiazepine, an anti-seizure medicine that raises the seizure threshold. We also extended the infusion time of KW-3902 to four hours.
The primary endpoint for the PROTECT pilot Phase 3 trial was the proportion of patients in the categories of treatment failure, success or no change.
Treatment success was defined as:
- •
- improvement in patient-reported dyspnea within 48 hours;
- •
- physician determination that the patient had improved such that IV diuretics could be converted to oral; and
- •
- the absence of treatment failure.
Treatment failure was defined as:
- •
- death or readmission to the hospital within seven days of the first dose of KW-3902 or placebo;
- •
- the worsening of heart failure requiring the use of rescue therapy more than 24 hours following the first dose of KW-3902 or placebo to the earlier of discharge or day seven; or
- •
- the worsening of renal function defined by an increase of greater than a 0.3 milligrams per deciliter in serum creatinine from the first dose of KW-3902 or placebo to the earlier of discharge or day seven.
A preliminary analysis of the 14-day data received from 276 patients out of a total of 304 patients showed a strong trend toward efficacy for the 30 milligram dose. We did not observe an adverse safety profile of KW-3902. Specifically, there were no seizures reported.
74
As shown in the chart below, based on an analysis of the preliminary data from the pilot Phase 3 clinical trial, the 30 mg dose had a higher rate of treatment success and a lower rate of treatment failure when compared to placebo.
As shown in the chart below, the 30 milligram dose showed a positive effect on renal function over time compared to placebo. In addition, although KW-3902 was administered for only the first three days of the trial, we observed an apparent dose response in mean serum creatinine at day 14. The measurement of serum creatinine level is used to evaluate renal function. These data are consistent with our prior findings and indicate that KW-3902 appears to have a persistent effect on renal function.
While we believe the results of our pilot trial are promising, based on our discussions with the FDA, we believe that the FDA may require us to define treatment failure in a different manner in our pivotal trials. This could alter our ability to achieve favorable results. We intend to meet with the FDA to further clarify this definition.
In our PROTECT pilot Phase 3 clinical trial, we enrolled patients whose entry BNP or NT-pro-BNP, both indicators of heart failure, was at least 250 or 1,000 picograms per milliliter,
75
respectively. As part of the design of our PROTECT pilot Phase 3 trial, we analyzed patients with BNP higher than 500 picograms per milliliter. While a BNP threshold of 250 picograms per milliliter has been shown to be a sensitive threshold when making the diagnosis of heart failure, increasing this BNP threshold to 500 picograms per milliliter decreases the possibility of including patients without CHF in the trial. When we examined the patients who met the higher BNP threshold, the difference between KW-3902 and placebo-treated patients was even greater. Approximately 75% of the patients enrolled in this trial met this higher BNP threshold. Our pivotal Phase 3 trials will include this higher BNP threshold.
The protocol in our PROTECT pilot Phase 3 clinical trial stated that "success" was to be determined at 48 hours by the presence of both moderate or marked improvement of patient-reported dyspnea and investigator determination that the patient was sufficiently improved such that the patient could be converted from IV to oral Lasix. As part of our analytical plan, we examined the predictive value of patient-reported dyspnea with and without the physician assessment.
We have modified the definition of success in the primary endpoint of our PROTECT trials to include only the patient-reported improvement in dyspnea at both 24 and 48 hours. In addition, worsening renal function will be based on the measurement of serum creatinine at day seven rather than the earlier of discharge or day seven. The latter change was made to ensure consistency in day of measure for all patients.
The chart below demonstrates the preliminary results of our PROTECT pilot Phase 3 clinical trial using the increased BNP threshold and the modified "success" and "failure" definitions. The results demonstrated a substantially higher rate of treatment success and a lower rate of treatment failure in patients who received KW-3902 when compared to placebo. We based the clinical and statistical design of our pivotal Phase 3 trials on the belief that we will achieve similar differences in those studies.
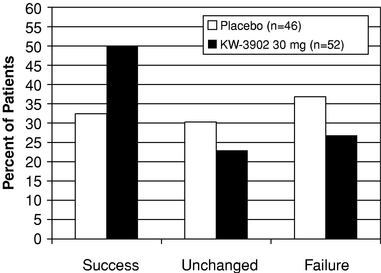
Based upon the preliminary results of the PROTECT pilot Phase 3 clinical trial, we have selected 30 milligrams infused daily over a period of four hours for up to three days to be the dosing regimen used in our pivotal PROTECT Phase 3 trials. While these modified results account for the change in the BNP threshold and the change in the definition of success, they do not
76
account for any changes in the definition of treatment failure that we may need to make after our meeting with the FDA.
PROTECT 1 and PROTECT 2 Phase 3 clinical trials
In May 2007, we initiated our pivotal PROTECT Phase 3 clinical trials of the IV formulation of KW-3902 at approximately 160 trial sites in the United States, Canada, Europe, Israel and Russia and potentially other countries, and we plan to enroll 600 patients in each trial.
The clinical trial design for the PROTECT trials is identical to the design of the pilot trial but will include a revised BNP threshold and modified definitions of "success" and "failure."
Treatment success will be defined as:
- •
- improvement in patient-reported dyspnea at 24 and 48 hours; and
- •
- the absence of treatment failure.
Treatment failure will be defined as:
- •
- death or readmission to the hospital within seven days following the first dose of KW-3902 or placebo;
- •
- the worsening of heart failure requiring the use of rescue therapy more than 24 hours following the first dose of KW-3902 or placebo to the earlier of discharge or day seven; or
- •
- the worsening of renal function defined by an increase of greater than a 0.3 milligrams per deciliter in serum creatinine following the first dose of KW-3902 or placebo on day seven.
Based on our discussions with the FDA, we believe that the FDA may require us to change the definition of treatment failure to extend the measurements of serum creatinine following first dose beyond day seven and to change the objective measure of worsening of heart failure. We intend to meet with the FDA to further clarify this definition.
REACH UP Phase 3 clinical trial
Our REACH UP Phase 3 clinical trial will study KW-3902 in CHF patients with renal impairment, which we defined for the purposes of this trial as creatinine clearance of 20 to 60 milliliters per minute, who are hospitalized for treatment of fluid overload and who have experienced a documented worsening of renal function, as measured by an increase in serum creatinine of at least 25% and at least 0.3 milligrams per deciliter, following the patient's admission to the hospital, or of at least 40% and at least 0.3 milligrams per deciliter within the past 30 days, in the case of outpatients with chronic CHF. The dosing regimen, criteria for exclusion related to seizure risk and co-administration of a benzodiazepine for this trial will be the same as those for our PROTECT trials.
Beginning in mid-2007, we intend to enroll approximately 480 patients, including 80 patients in a pilot phase designed to determine the appropriateness of the primary endpoint and to assess the adequacy of the trial size for this patient population. The primary endpoint for the REACH UP trial will be the proportion of treatment failures within 30 days of randomization. Treatment failure for this trial will be essentially the same as defined in the PROTECT trials, except that failure may occur out to 30 days after randomization.
77
PULSE RENAL Phase 3 clinical trial
Our PULSE RENAL Phase 3 clinical trial is designed to study the effects of intermittent infusions of KW-3902 on renal function and heart failure signs and symptoms in outpatients with chronic CHF. Beginning in the third quarter of 2007, we plan to enroll approximately 375 patients in this trial. We expect the trial to include one group of approximately 75 patients who will receive placebo once a week for four weeks, one group of approximately 150 patients who will receive 30 milligrams of KW-3902 once a week for four weeks and one group of approximately 150 patients who will alternatively receive 30 milligrams of KW-3902 and placebo on a weekly basis for four weeks. We intend to examine the change in renal function, the degree of shortness of breath, ability to walk over six minutes and a panel of safety measures over time.
KW-3902: Oral formulation
Prior to our in-licensing of KW-3902, an oral formulation of KW-3902 was tested in single and repeated dose studies in healthy volunteers and patients with fluid overload due to cardiac failure, kidney disease or other causes. KW-3902 has demonstrated oral bioavailability and a pharmacodynamic response, as measured by increased urine volume.
We are optimizing our oral formulation of KW-3902 and, assuming success, intend to begin in the second half of 2007 a Phase 2 clinical trial of KW-3902 in chronic CHF patients with renal impairment who are on diuretic therapy. At an appropriate time, we intend to enter into a partnership for the development and commercialization of the oral formulation of KW-3902.
K201 (JTV-519)
We are developing K201 (JTV-519) for the treatment of A-Fib. We in-licensed K201 (JTV-519) from Aetas in September 2006. Based on preclinical studies conducted by others, we believe K201 (JTV-519) has the potential to treat A-Fib effectively without the side effects associated with current therapies, such as life-threatening ventricular arrhythmias. K201 (JTV-519) acts by modifying ion current through potassium, sodium and calcium channels. We plan to commence a Phase 2 clinical trial of the IV formulation of K201 (JTV-519) for the acute treatment of A-Fib in the second half of 2007.
Atrial fibrillation therapeutic opportunity
A-Fib afflicts over 2.2 million people in the United States and leads to over 440,000 hospitalizations per year, according to the most recent data from the AHA. A-Fib, which can be acute or chronic, is a condition characterized by chaotic electrical activity in the upper chambers of the heart, also known as the atria. A-Fib may result in a rapid, irregular heartbeat that causes the patient to have symptoms of shortness of breath, chest pain or dizziness. This erratic heartbeat affects the heart's ability to pump properly, which may also result in pooling of blood in the atria that may ultimately form into a blood clot. This blood clot may subsequently dislodge and travel to the brain, resulting in a stroke. According to the AHA, approximately 15% to 20% of strokes occur in people with A-Fib.
A-Fib is seen frequently in patients with other cardiovascular conditions. For example, approximately 31% of hospitalized CHF patients also have A-Fib, according to the ADHERE Registry. A-Fib increases the severity of symptoms experienced by CHF patients.
78
Current therapies for A-Fib include IV and oral drugs to control heart rate, as well as drugs and procedures to control the rhythm of the heart. Current drug therapies for A-Fib have limitations, including organ toxicity and increased risk of life-threatening ventricular arrhythmias. When A-Fib patients fail to respond to drug therapies, physicians may use electrical cardioversion, which involves shocking the patient to reset the heart, or an ablation procedure, which involves ablating, or destroying, the area of the heart tissue that is causing the abnormal heart rhythms, as second line therapies. However, use of these procedures is limited by patient discomfort, the need for anesthesia and limited eligibility. As a result, we believe there remains a significant need for drugs that can control heart rhythm without the side effects of current therapies.
Development of K201 (JTV-519)
Prior to our in-licensing of K201(JTV-519), the safety of the IV formulation of K201 (JTV-519) was evaluated in 141 individuals, including healthy volunteers and patients with acute coronary syndrome. K201 (JTV-519) was generally well tolerated at a range of dose levels and demonstrated no significant adverse events. We believe that these studies and the preclinical studies that preceded them will support the filing of an investigational new drug, or IND, application to initiate a Phase 2 clinical trial during the second half of 2007. Our Phase 2 trial will be designed to determine the safety, efficacy and appropriate dose of the IV formulation of K201 (JTV-519) for the treatment of acute A-Fib. We are also developing an oral formulation of K201 (JTV-519) to be used for the management of chronic A-Fib.
Strategy
Our goal is to become a leading cardiovascular pharmaceutical company. In the near term, we will continue to pursue the development and regulatory approval of our product candidates. Specifically, we intend to:
- •
- complete our Phase 3 clinical trials and obtain regulatory approval for the IV formulation of KW-3902;
- •
- initiate our Phase 2 clinical trials of the oral formulation of KW-3902 in the second half of 2007; and
- •
- file an IND application or its foreign equivalent for the IV formulation of K201 (JTV-519) and commence one or more Phase 2 trials in the second half of 2007.
In the longer term, we plan to expand and commercialize our portfolio of cardiovascular products. Specifically, we intend to:
- •
- enter into commercialization arrangements with third parties to sell our IV product candidates outside of the United States and to further develop and commercialize our oral product candidates in all regions where we hold commercial rights;
- •
- establish a hospital-based sales force to sell our products in the acute care setting in the United States;
- •
- maximize the market potential of our product candidates through available intellectual property protection and regulatory exclusivity;
- •
- in-license additional cardiovascular product candidates; and
79
- •
- pursue additional indications, administration regimens, formulations and commercial opportunities for our product candidates.
Sales and marketing
We do not currently have an organization for the sales, marketing and distribution of our of our pharmaceutical products. We intend to commercialize our product candidates by establishing (either internally or through a contract sales force) a hospital-based sales force to sell the IV formulations of KW-3902 and K201 (JTV-519), if approved, in the United States. We plan to partner with third parties to commercialize our IV product candidates outside the United States. We also plan to enter into collaborations to develop further, promote and sell the oral formulations of our product candidates within and outside of the United States.
Specifically, we intend to:
- •
- establish a hospital-based sales force in the United States consisting of approximately 150 to 200 experienced cardiovascular sales representatives;
- •
- build a marketing organization;
- •
- establish commercialization alliances with larger or more specialized pharmaceutical and sales organization; and
- •
- generate and use pharmacoeconomic data to support the cost savings and therapeutic benefits of our product candidates.
Intellectual property
We rely upon a combination of in-licensed patent rights and our own patent applications, trade secrets and know-how to protect KW-3902 and K201 (JTV-519). We have exclusive rights to one issued patent directed to composition of matter and two issued patents directed to formulations relating to KW-3902 in the United States and we own 13 United States patent applications that are directed at methods of use, manufacturing and analogs of KW-3902. We use the term "composition of matter" in referring to a patent or application that claims a chemical entity; "method of use" to refer to a patent or application that claims the treatment of an individual or patient; "formulation" to refer to a patent or application that claims a combination of an active ingredient with excipients and/or other ingredients; and "manufacturing" to refer to a patent or application that claims a method of making a formulation or composition of matter. In addition, we have exclusive rights to two issued patents and one pending application in the United States with respect to composition of matter and methods of use relating to K201 (JTV-519). We have exclusive rights to six issued patents and we own 34 patent applications in various foreign countries with respect to KW-3902 that corresponds to some of the issued and pending United States patents and patent applications relating to KW-3902. We also have exclusive rights to four issued patents and three pending patent applications in various foreign countries with respect to K201 (JTV-519) that correspond to some of the issued and pending United States patents and patent applications relating to K201 (JTV-519). We also seek to protect our trade secrets and our know-how relating to our products and our business, including the manufacturing process. These intellectual property rights are in addition to any regulatory exclusivity we may be able to obtain.
80
KW-3902 is currently protected in the United States under United States Patent No. 5,290,782, which provides composition of matter coverage for the KW-3902 compound, and United States Patent Nos. 6,210,687 and 6,254,889, which cover certain IV and oral formulations of KW-3902, all of which we have in-licensed on an exclusive basis from Kyowa Hakko under a license agreement described in more detail below. In addition, we own 11 United States patent applications that are directed at methods of use of KW-3902 in combinations with diuretics or other therapeutics, one United States patent application relating to KW-3902 analogs and one United States patent application relating to a manufacturing method for a KW-3902 product. Kyowa Hakko is responsible for the prosecution and maintenance of the patents licensed from Kyowa Hakko and we are responsible for the related costs. The KW-3902 patents and patents that may issue under pending applications that we own and have in-licensed from Kyowa Hakko are expected to expire on dates ranging from 2011 to 2027 in the absence of any patent term extension. Our strategy for preventing generic competition relies on our ability to obtain and maintain regulatory exclusivity and effective patent rights. Once any regulatory exclusivity expires, if certain of our pending patent applications do not issue, or if certain of our patents or our licensor's patents have expired or are determined to be invalid, not infringed or unenforceable, we might not be able to prevent others from marketing and selling generic versions of KW-3902.
K201 (JTV-519) is currently protected in the United States under United States Patent No. 5,416,066, which covers the K201 (JTV-519) compound, and United States Patent No. 6,506,745, which is directed at the method of use of K201 (JTV-519) for the treatment of A-Fib, both of which have been in-licensed on an exclusive basis from Aetas under a license agreement described in more detail below. In addition, we have exclusive rights to any patents that may issue under a pending United States patent application that is directed at methods of use of K201 (JTV-519) for the treatment of heart failure. Aetas is responsible for the prosecution and maintenance of the patents in-licensed from Aetas and the related costs. The K201 (JTV-519) patents and patents that may issue under pending applications that we own and have in-licensed from Aetas are expected to expire on dates ranging from 2012 through 2025 in the absence of any patent term extension.
Licensing and clinical trial agreements
Kyowa Hakko
In August 2003, we entered into a license agreement with Kyowa Hakko, a Japanese pharmaceutical company, under which we received an exclusive license to develop and commercialize KW-3902 for the treatment of CHF and other specified indications in major markets, including the United States and Europe, but excluding Asia. In consideration for the license, we paid Kyowa Hakko approximately $2.4 million in up-front fees, and may be required to make future milestone payments totaling up to $19.5 million upon the achievement of various milestones related to regulatory or commercial events for the first two indications, including $1.5 million that we expect to pay to Kyowa Hakko in June 2007 as a result of having initiated our Phase 3 clinical trials of the IV formulation of KW-3902. In addition, we have an obligation to negotiate and agree to additional milestone payments before submitting an NDA for a third indication. We also have an obligation to pay royalties on any net sales of the licensed products. The agreement was supplemented in March 2005. Under the terms of the agreement, we must use commercially reasonable efforts to develop and commercialize KW-3902.
81
In consideration for the license, we granted Kyowa Hakko a non-exclusive and fully-paid license to any improvements that we make to the patents that are in-licensed to us under the agreement for use in geographic areas in which we do not have the right to develop and commercialize KW-3902 and use in fields not licensed to us in all geographic areas, subject to certain limitations. Under the terms of the license agreement, we are allowed to grant sublicenses to third parties with the prior approval of Kyowa Hakko, provided that Kyowa Hakko may not unreasonably withhold its approval. Any of our potential sublicensees will not be allowed to further sublicense any rights that we grant to them.
The agreement terminates upon the later of the expiration of the last licensed patent right or 15 years from the date of first commercial sale of KW-3902 for any indication in specified major markets, unless the agreement is earlier terminated. Either party may terminate the agreement in the event of material breach by the other party, subject to prior notice and the opportunity to cure, or the other party enters into bankruptcy or is dissolved for any reasons other than in connection with a merger or acquisition. In addition, we may terminate the agreement by providing written notice to Kyowa Hakko at least 90 days prior to any contemplated termination.
Aetas
We entered into a license agreement with Aetas, a Japanese pharmaceutical company, in September 2006 and supplemented the license agreement in January 2007. Under the terms of our license agreement with Aetas, as supplemented, we received an exclusive license to develop and commercialize the IV formulation of K201 (JTV-519) for the treatment of cardiac arrhythmia and another potential indication and the oral formulation of K201 (JTV-519) in all cardiovascular indications in major markets, including the United States and Europe, but excluding Asia. In consideration for the license, we paid Aetas $1.5 million in up-front fees and may be required to make future milestone payments totaling up to $18.0 million upon the achievement of various milestones related to regulatory or commercial events. We also have an obligation to pay royalties on any net sales of the licensed products. Under the terms of the agreement, we must use commercially reasonable efforts to develop and commercialize K201 (JTV-519) and commence dosing in specified clinical trials by predetermined dates.
In consideration for the license, we granted Aetas an exclusive, royalty-free and fully-paid license to any improvements that we make to the patents that are in-licensed to us under the agreement for use in geographic areas in which we do not have the right to develop and commercialize K201 (JTV-519). Under the terms of the license agreement, we are allowed to grant sublicenses to third parties with the prior approval of Aetas, provided that Aetas may not unreasonably withhold its approval.
The agreement terminates upon the later of the expiration of the last licensed patent right or 10 years from the date of first commercial sale of K201 (JTV-519) on a country-by-country basis, unless the agreement is earlier terminated. Either party may terminate the agreement in the event of material breach by the other party, subject to prior notice and the opportunity to cure, or the other party enters into bankruptcy or is dissolved for any reasons other than in connection with a merger or acquisition. In addition, we may terminate the agreement by providing specified written notice to Aetas related to safety, efficacy, intellectual property or regulatory concerns. Aetas also has the right to terminate the agreement by providing written notice to us in the event we fail to commence dosing, without reasonable reasons, in specified clinical trials of the IV formulation of K201 (JTV-519) by predetermined dates or otherwise fail to use commercially reasonable efforts to develop the product candidate, in each case as determined by Aetas in its sole discretion. In the event the agreement is terminated and Aetas successfully commercializes K201 (JTV-519) after such termination, Aetas will be obligated to pay us royalties on net sales of K201 (JTV-519).
82
Hesperion Ltd.
In May 2007, we entered into a master clinical services agreement with Hesperion Ltd., or Hesperion, to serve as our CRO for the Phase 3 clinical trials of the IV formulation of KW-3902. Hesperion was previously subcontracted by Duke Clinical Research Institute, or DCRI, as our CRO for the pilot Phase 3 trials outside the United States. Under the terms of the agreement, Hesperion will conduct our Phase 3 trials of the IV formulation of KW-3902, and will be responsible for identifying and contracting with all clinical trial sites and enrolling patients for any such trials. DCRI will continue to provide safety surveillance and conduct pharmacoeconomic sub-studies for these Phase 3 trials.
We may terminate a specific trial that Hesperion is engaged in immediately upon showing of good cause, such as indentification of a medical risk to patients, departure from Hesperion or removal from a specific trial of key individuals utilized by Hesperion for the trials, a showing that a study drug is ineffective or receipt of notice of regulatory action by the FDA terminating or suspending a trial. In addition, we may terminate the agreement or a specific trial for any reason upon advance notice to Hesperion. Either party may also terminate the agreement for an uncured breach of the other party. If not terminated earlier, the agreement expires after five years unless a trial is underway at such time, in which case the agreement expires following the completion of such trial.
Competition
Most cardiovascular indications for which we are developing products have a number of established therapies with which our product candidates will compete. Most major pharmaceutical companies and many biotechnology companies are aggressively pursuing new cardiovascular drug development programs, including both therapies with traditional as well as novel mechanisms of action. Most of these companies are more established than us and have significantly greater financial and other resources than we do. We are aware of many competitive products currently marketed or under development that are used to treat some of the cardiovascular indications we have targeted.
Although there are currently no adenosine A1 receptor antagonists that have received regulatory approval from the FDA for the treatment of CHF, we believe that Biogen Idec Inc.'s product candidate, ADENTRI, and Solvay S.A.'s, or Solvay, product candidate, SLV-320, are both in Phase 2 clinical trials. Several companies are engaged in the clinical development of vasopression antagonists for the treatment of CHF, including Otsuka Pharmaceuticals Group, which has tolvaptan in Phase 3 clinical trials, and Cardiokine Inc., which has Lixivaptan in Phase 2 clinical trials. PDL BioPharma, Inc. has the vasodilator, ularitide, a synthetic form of urodilatin, in Phase 2 development for acute CHF. Two other companies have inotropes, which are agents that increase or decrease the force or energy of muscular contractions, in clinical trials for the treatment of CHF, including Abbott Laboratories, which has levosimendan in Phase 3 trials, and Cytokinetics Inc., which has CK-1827452 in Phase 1 clinical trials. While these products will not compete directly with KW-3902, physicians may choose to use one therapy over another one.
Current drugs in development for the acute treatment of A-Fib include vernakalant, which is being developed by Cardiome Pharma Corp., or Cardiome, in partnership with Astellas Pharma Inc., for which an NDA has been submitted. Currently in development for the long-term maintenance of A-Fib are Sanofi-Aventis S.A.'s dronedarone, which is in Phase 3 clinical trials, and the oral formulation of Cardiome's vernakalant, which is in Phase 2 clinical
83
trials. Solvay's Tedisamil is in clinical development for the treatment of arrthymias, including A-Fib.
Manufacturing
We neither currently possess nor do we plan to develop our own manufacturing capabilities. All of our manufacturing is outsourced to third parties with oversight by our internal managers. The production and manufacture of KW-3902 and K201 (JTV-519) employ small molecule organic chemistry procedures that require a high level of expertise. We currently rely on DSM Pharma Chemicals, Inc. for the production of the drug substance of KW-3902 and Patheon Italia S.p.A., or Patheon, for the production of, finished IV and oral KW-3902 product candidates. Although KW-3902 is manufactured from readily available raw materials using standard pharmaceutical methods and equipment and although we can obtain the raw materials from multiple suppliers, the replacement of any one of our manufacturers will lead to significant delays and increase our costs. We currently do not have long-term supply arrangements with any of these suppliers. We intend to continue this practice of outsourcing our manufacturing services to third parties for any future clinical trials and large-scale commercialization of KW-3902, and for any other product candidate we may acquire for further development.
Government regulation
FDA approval process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, labeling, promotion and marketing, and distribution of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and criminal prosecution.
Pharmaceutical product development in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of a notice of claimed investigational exemption or an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes several years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry and formulation, as well as animal trials to assess the potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in
84
compliance with federal regulations, the FDA's good clinical practice requirements, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients must be submitted to the FDA as part of the IND.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not commented on or questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics and pharmacological actions and safety, including side effects associated with increasing doses. Phase 2 usually involves trials in a limited patient population, to determine dosage tolerance and optimum dosage, identify possible adverse effects and safety risks, and provide preliminary support for the efficacy of the drug in the indication being studied. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to further evaluate clinical efficacy and to further test for safety within an expanded patient population, typically at geographically dispersed clinical trial sites.
After successful completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product's pharmacology, chemistry, manufacture, and controls. The cost of preparing and submitting an NDA is substantial. Under federal law, the submission of NDAs is additionally subject to substantial application user fees, currently exceeding $890,000, and the manufacturer and/or sponsor under an approved NDA are also subject to annual product and establishment user fees, currently exceeding $49,000 per product and $313,000 per establishment. These fees are typically increased annually.
The FDA has 60 days from its receipt of a NDA to determine whether the application will be accepted for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for non-priority drug products are reviewed within ten months. The review process may be extended by the FDA for three additional months to consider certain information or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the
85
application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will inspect the facility or the facilities at which the drug is manufactured, and will not approve the product unless compliance with current good manufacturing practices is satisfactory or if the NDA does not contain sufficient information to conclude that the drug is safe or that the drug is effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues an approval letter, an approvable letter or a not-approvable letter. Both approvable and not-approvable letters generally outline the deficiencies in the submission and may require additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA's satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require post-approval testing and surveillance to monitor the drug's safety or efficacy and may impose other conditions, including labeling restrictions which can materially impact the potential market and profitability of the drug. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
The Hatch-Waxman Act
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent with claims that cover the applicant's product. Upon approval of a drug, each of the patents listed in the application for the drug are then published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential competitors in support of approval of an abbreviated new drug application, or ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. ANDA applicants are not required to conduct or submit results of pre-clinical or clinical tests to prove the safety or effectiveness of their drug product, other than the requirement for bioequivalence testing. Drugs approved in this way are commonly referred to as "generic equivalents" to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA's Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. A certification that the new product will not infringe the already approved product's listed patents or that such patents are invalid is called a paragraph iv certification. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a paragraph iv certification to the FDA, the applicant must also send notice of the paragraph iv certification to the NDA and patent holders once the NDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a legal challenge to the paragraph iv certification. The filing of a patent infringement lawsuit within
86
45 days of the receipt of a paragraph iv certification automatically prevents the FDA from approving the A NDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. Federal law provides a period of five years following approval of a drug containing no previously approved active ingredients, during which ANDAs for generic versions of those drugs cannot be submitted unless the submission accompanies a challenge to a listed patent, in which case the submission may be made four years following the original product approval. Federal law provides for a period of three years of exclusivity following approval of a listed drug that contains previously approved active ingredients but is approved in a new dosage form, route of administration or combination, or for a new use, the approval of which was required to be supported by new clinical trials conducted by or for the sponsor, during which the FDA cannot grant effective approval of an ANDA based on that listed drug.
Other U.S. regulatory requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. A company can make only those claims relating to safety and efficacy that are approved by the FDA.
Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk minimization action plans, and surveillance to monitor the effects of approved products or place conditions on any approvals that could restrict the commercial applications of these products. In addition, quality control as well as drug manufacture, packaging, and labeling procedures must continue to conform to current good manufacturing practices, or cGMPs, after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to access compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
87
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
International regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our future product candidates. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, marketing authorizations may be submitted either under a centralized or mutual recognition procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The mutual recognition procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval.
88
In Canada, applications for marketing authorizations are submitted to Health Canada, which is a centralized regulatory body overseeing prescription drug approvals for all of Canada. At present, Health Canada targets 355 days for application review and approvals. Once approved, the sponsor has the right to sell the drug in Canada; however, placement on the reimbursement formularies in the various Canadian provinces may take an unspecified amount of time.
In addition to regulations in Europe and the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial distribution of our future product candidates.
Reimbursement
Our ability to commercialize our product candidates successfully will depend in part on the extent to which the United States and foreign governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our product candidates and related treatments. As a threshold for coverage and reimbursement, in the United States, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors also are increasingly challenging the effectiveness of and prices charged for medical products and services. Also, the trend toward managed healthcare in the United States, which could significantly influence the purchase of healthcare services and products, as well as legislative proposals to reform healthcare or reduce government insurance programs, may result in lower prices for our product candidates or exclusion of our product candidates from coverage and reimbursement programs. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could significantly reduce our revenues from the sale of any approved product. We cannot provide any assurances that we will be able to obtain third-party coverage or reimbursement for our product candidates in whole or in part.
Currently, acute CHF patients are prescribed primarily inexpensive generic drugs. Hospitals are reimbursed by Medicare a fixed dollar amount per hospital stay for acute CHF patients, which amount is generally less than the average amount spent on the treatment of acute CHF patients. Because Medicare reimbursement amounts do not increase to cover the cost of non-generic drugs, hospitals and physicians may resist prescribing KW-3902 to acute CF patients or other non-generic drugs even if there is valid pharmacoeconomic justification (such as a shorter hospital stay or reduced hospital readmission) in order to avoid increasing the costs of treatment. Although we believe that we will be able to demonstrate adequate pharmacoeconomic justification, there is no guarantee that physicians will adopt the use of KW-3902 because of its cost or for other factors.
In the United States, there have been a number of legislative and regulatory changes to the healthcare system in ways that could affect our future revenues and profitability and the future revenues and profitability of our potential customers. For example, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, revised the payment methodologies for many drugs, which resulted in reduced reimbursement to providers. Additionally, the MMA created an outpatient prescription drug benefit which became effective on January 1, 2006. This new benefit is administered by private pharmacy benefit managers and other managed care organizations and is putting increased pressure on the pharmaceutical industry to reduce prices.
89
There also have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare costs to contain or reduce costs of healthcare may adversely affect one or more of the following:
- •
- our ability to set a price we believe is fair for our products;
- •
- our ability to generate revenues and achieve profitability;
- •
- the future revenues and profitability of our potential customers, suppliers and collaborators; and
- •
- the availability of capital.
In certain foreign markets, the pricing of prescription drugs is subject to government control and reimbursement may in some cases be unavailable. In the United States, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and the reform of the Medicare and Medicaid systems. While we cannot predict the full outcome of the implementation of this legislation, it is possible that this new Medicare prescription drug benefit, which will be managed by private health insurers and other managed care organizations, will result in decreased reimbursement for prescription drugs, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to market our products and generate revenues. It is also possible that other proposals having a similar effect will be adopted.
Legal proceedings
We are not currently involved in any legal proceedings.
Facilities
In March 2007, we entered into a sublease agreement for a new facility with approximately 8,500 square feet of office space in San Diego, California, which will expire in July 2010. We began to occupy this space in early May 2007. We also currently lease approximately 3,900 square feet of office space in San Diego, California, under a lease agreement that expires in September 2007. We have no laboratory, research or manufacturing facilities. We believe that our current and planned facilities are adequate for our needs for the immediate future and that, should it be needed, suitable additional space will be available to accommodate expansion of our operations on commercially reasonable terms.
Insurance
We maintain liability insurance for our clinical trials, and intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for any of our product candidates. Insurance coverage is becoming increasingly expensive, and we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against product liabilities.
Employees
As of April 30, 2007, we employed 11 full-time employees who are engaged in clinical development, regulatory affairs, manufacturing and product development, business development and marketing, finance and administration. None of our employees is subject to a collective bargaining agreement. We consider our relationship with our employees to be good.
90
Management
Executive officers, directors and key employees
The following table sets forth information regarding our executive officers, directors and key employees as of April 30, 2007:
|
Name
| | Age
| | Position
|
|---|
|
| Executive officers and directors | | | | |
Randall E. Woods |
|
55 |
|
President, Chief Executive Officer and Director |
Howard C. Dittrich, M.D. |
|
54 |
|
Chief Medical Officer |
John E. Crawford |
|
52 |
|
Chief Financial Officer |
Eckard Weber, M.D. |
|
57 |
|
Director and Chairman |
Stuart J. M. Collinson, Ph.D.(1)(2) |
|
47 |
|
Director |
Nina Kjellson(1)(3) |
|
32 |
|
Director |
David G. Lowe, Ph.D.(2)(3) |
|
50 |
|
Director |
Camille D. Samuels(2)(3) |
|
35 |
|
Director |
Joseph L. Turner(1)(3) |
|
55 |
|
Director |
Key employees |
|
|
|
|
Paul Chamberlin, M.D. |
|
42 |
|
Vice President, Medical |
Brian K. Farmer |
|
34 |
|
Senior Director of Corporate Development |
Mark Mugerditchian |
|
49 |
|
Senior Vice President, Manufacturing and Product Development |
|
|
|
|
|
|
- (1)
- Member of the audit committee.
- (2)
- Member of the compensation committee.
- (3)
- Member of the corporate governance and nominating committee.
Executive officers and directors
Randall E. Woods has served as our president and chief executive officer and as a member of our board of directors since May 2004. Prior to joining us, from May 1996 until July 2003, Mr. Woods served as president and chief executive officer of Corvas International, Inc., a biopharmaceutical company that focused on cancer and cardiovascular disease, which was acquired by Dendreon Corporation in July 2003. Prior to his employment at Corvas, Mr. Woods served as the president of the United States Operations of Boehringer Mannheim Pharmaceuticals Corporation, a pharmaceutical company, from March 1994 to March 1996, and was vice president of marketing and sales at Boehringer Mannheim from December 1993 to February 1994. Prior to that, he served in various capacities at Eli Lilly and Company, a pharmaceutical company, from 1973 to December 1993. Mr. Woods currently serves as the chairman for the advisory board of the University of California, San Diego's Sulpizio Family Cardiovascular Center and serves on the board of directors of BIOCOM, a life science industry
91
association in Southern California. Mr. Woods holds a B.S. degree in biology from Ball State University and an M.B.A. from Western Michigan University.
Howard C. Dittrich, M.D., is one of our co-founders and has served as our chief medical officer since August 2006. From August 2003 to August 2006, Dr. Dittrich served as our senior vice president of clinical and regulatory affairs. Since July 1996, Dr. Dittrich has held an appointment as clinical professor of medicine at the University of California, San Diego. Prior to joining us, from January 2001 to July 2002, Dr. Dittrich was senior vice president and chief medical officer for Alliance Pharmaceutical Corp., a pharmaceutical research and development company. From December 1998 to December 2000, Dr. Dittrich served as executive vice president of Molecular Biosystems, Inc., a developer of medical products. Prior to that, he served as Molecular Biosystems' vice president of research/medical & regulatory affairs from November 1996 to December 1998 and as executive director of medical affairs from May 1996 to November 1996. Dr. Dittrich served as a consultant to Molecular Biosystems from 1989 to 1996. Dr. Dittrich was a full-time faculty member of the University of California, San Diego, Department of Medicine from 1984 to May 1996. Dr. Dittrich holds a B.S. degree from University of Iowa and an M.D. from the University of Iowa College of Medicine.
John E. Crawford has served as our chief financial officer since January 2007. From May 2006 to December 2006, Mr. Crawford served as chief financial and administrative officer of Cabrellis Pharmaceuticals Corporation, a specialty biopharmaceutical company focused on the treatment of cancer, which was acquired by Pharmion Corporation in November 2006. Prior to that, from January 2006 to May 2006, Mr. Crawford served as chief financial officer of Conforma Therapeutics Corporation, a biotechnology company, which was acquired by Biogen Idec, Inc. From 1997 to December 2005, Mr. Crawford was a private consultant to life sciences and technology firms. During this time, Mr. Crawford served on the board of directors of several private companies and was a consultant to Archimedes Technology Group, LLC, a plasma physics and engineering firm. Mr. Crawford was the founding president of Corvas and held various positions within the company from 1987 to 1999, including that of chief financial officer. Mr. Crawford currently serves on the board of directors of Lipid Sciences, Inc., a biotechnology company that focuses on the treatment of cardiovascular disease, HIV and other viral infections. Mr. Crawford holds a B.S. degree in mathematical sciences from Stanford University and an M.B.A. from the University of Chicago Graduate School of Business.
Eckard Weber, M.D. is one of our co-founders and has served as chairman of our board of directors since our inception in November 2001. Dr. Weber also served as our president from our inception in November 2001 to May 2004 and our acting chief executive officer from our inception to March 2003 and between March 2004 and May 2004. From March 2003 to December 2004, Dr. Weber also served as a consultant to us. Dr. Weber is also a partner at Domain Associates, L.L.C., a private venture capital management firm focused on life sciences, a position he has held since 2001. Dr. Weber has been a founder, founding chief executive officer and board member of multiple biopharmaceutical companies in the Domain portfolio including Cytovia, Inc., Acea Pharmaceuticals, Inc., Novalar Pharmaceuticals, Inc., Novacea, Inc., Domain AntiBacterial Acquisition Corporation, Ascenta Therapeutics, Inc., Konova, Inc., Renovia, Inc. (now Ocera Therapeutics, Inc.), Tragara Pharmaceuticals, Syndax Pharmaceuticals and Tobira Therapeutics, Inc. Dr. Weber is currently chairman of the board of Orexigen Therapeutics, Inc., a publicly held biopharmaceutical company, and a member of the board of directors of Novacea, Inc., a publicly held biopharmaceutical company, and he currently serves as interim
92
chief executive officer of Tramoxia Therapeutics, Inc., an early-stage biopharmaceutical company. He is also a current board member of Biovascular, Inc., Ocera Therapeutics, Inc. and Diobex, Inc. Dr. Weber holds a B.S. degree from Kolping College in Germany and an M.D. from the University of Ulm Medical School in Germany.
Stuart J. M. Collinson, Ph.D. has served as a member of our board of directors since August 2003. He has been a member of Forward Ventures, a venture capital fund, since 2002. He served as chairman, chief executive officer and president of Aurora Biosciences Corporation, a developer of drug discovery systems, from 1999 until the acquisition of Aurora Biosciences in 2001 by Vertex Pharmaceuticals, Inc., a publicly held biotechnology company. Before joining Aurora Biosciences, Dr. Collinson served as a consultant to Aurora Biosciences from 1998 to 1999 and as chief executive officer of Andaris, Ltd., a privately held microcapsule technology company, from June 1998 to November 1998. Prior to joining Andaris, Dr. Collinson held senior management positions with Glaxo Wellcome, a pharmaceutical company, from December 1994 through June 1998, most recently serving as co-chairman of the hospital and critical care therapy management team and director of hospital and critical care. Dr. Collinson is currently a member of the board of directors of Vertex. Dr. Collinson received an M.B.A. from Harvard Business School and a Ph.D. in physical chemistry from the University of Oxford.
Nina Kjellson has served as a member of our board of directors since September 2006. Ms. Kjellson became a Partner in the Life Sciences Group of InterWest Partners, a venture capital firm, in 2002. Ms. Kjellson currently represents InterWest on the boards of directors of Alvine Pharmaceuticals, Inc., Nereus Pharmaceuticals, Inc. and Trius Therapeutics, Inc. Ms. Kjellson is an observer on the boards of AkaRx, Inc., Devax, Inc., Kanisa Pharmaceuticals and MacuSight, Inc. Prior to joining InterWest, Ms. Kjellson was an investment manager at Bay City Capital, a life sciences merchant bank, from 2000 to 2002, and a research associate researching pharmaceutical and biotechnology stocks at Oracle Partners, a healthcare-focused hedge fund from 1999 to 2000. She began her career conducting health policy and survey research with the Kaiser Family Foundation, a philanthropic health policy organization from 1997 to 1999. Ms. Kjellson holds a B.S. degree in human biology from Stanford University.
David G. Lowe, Ph.D. has served as a member of our board of directors since September 2006. Dr. Lowe joined Skyline Ventures, a venture capital firm, in 2002 and became a partner at Skyline in 2003. Prior to joining Skyline, Dr. Lowe was employed at Genentech, Inc., a biotherapeutics company, from 1985 to 2001, where he led drug discovery activities in biologics and small molecule therapeutics. Dr. Lowe held various positions while at Genentech, Inc., including as director of cardiovascular research. Dr. Lowe currently serves on the boards of directors of NimbleGen Systems, Inc., Applied Genetics Technologies Corporation and Proteon Therapeutics. Dr. Lowe holds B.S. and Ph.D. degrees in biochemistry from the University of Toronto, Canada.
Camille D. Samuels has served as a member of our board of directors since August 2003. She has served as a managing director of Versant Ventures, a venture capital firm, since November 2001, where she specializes in biotechnology investments. From 1998 to 2000, Ms. Samuels served as director of business development at Tularik Inc., a human therapeutics company that was acquired by Amgen Inc. in August 2004. She also directed Tularik's Technology Acquisition Group. From 1993 to 1996, Ms. Samuels served as an associate consultant with LEK Consulting, a business consulting firm. Ms. Samuels currently serves on the board of directors of Novacea, Inc., a publicly held biopharmaceutical company, Achaogen, Inc.
93
and Kythera Biopharmaceuticals, Inc. Ms. Samuels holds a B.S. degree in biology from Duke University and an M.B.A. from Harvard Business School.
Joseph L. Turner has served as a member of our board of directors since February 2007. From December 1999 to November 2006, Mr. Turner served in various capacities at Myogen, Inc., a publicly held biopharmaceutical company, which was acquired by Gilead Sciences, Inc., or Gilead, in November 2006. From November 2006, to January 2007, Mr. Turner served in a transition capacity at Myogen following Myogen's acquisition by Gilead. Prior to Myogen's acquisition by Gilead, from December 1999 to November 2006, Mr. Turner served initially as Myogen's acting chief financial officer in a part-time capacity, and, from September 2000, as the chief financial officer of Myogen. Mr. Turner also served as senior vice president of finance and administration of Myogen from December 2003 to November 2006, and as vice president of finance and administration from September 2000 to November 2003. From July 1999 to May 2000, Mr. Turner was an independent strategic consultant to emerging companies. From November 1997 to June 1999, Mr. Turner worked at Centaur Pharmaceuticals, a biopharmaceutical company, where he served in several positions, including vice president of finance and chief financial officer. From March 1992 to October 1997, Mr. Turner served as vice president, finance and chief financial officer of Cortech, Inc., a biopharmaceutical company. Previously, Mr. Turner spent 12 years with Eli Lilly and Company, where he held a variety of financial management positions both within the United States and abroad. Mr. Turner holds an M.A. in molecular, cellular and developmental biology from the University of Colorado and an M.B.A. from the University of North Carolina at Chapel Hill and a B.A. in chemistry from Swarthmore College.
Key employees
Paul Chamberlin, M.D., our vice president of medical, joined us in March 2007. From September 2000 to March 2007, Dr. Chamberlin served in various positions at EPIX Pharmaceuticals, Inc., a pharmaceutical company, including as executive medical director, clinical research from August 2006 to March 2007, as executive medical director from July 2005 to August 2006, as associate medical director from March 2003 to July 2005, as senior director, clinical development and drug safety officer from December 2001 to March 2003 and as senior manager, business and clinical development from September 2000 to December 2001. Prior to EPIX, from July 1999 to June 2000, Dr. Chamberlin was a resident at Mount Auburn Hospital (Harvard Medical School). Dr. Chamberlin holds a B.A. degree in biology from the University of Pennsylvania and an M.D. degree from Boston University School of Medicine.
Brian K. Farmer, our senior director of corporate development, joined us initially as the senior manager of business development in July 2004. Prior to joining us, from April 2004 to July 2004, Mr. Farmer was a consultant to us. From March 2003 to April 2004, Mr. Farmer was an independent consultant. From May 2000 to March 2003, Mr. Farmer held marketing positions at Eli Lilly and Company. Prior to Eli Lilly, from September 1996 to August 1999, Mr. Farmer held marketing positions at Prudential HealthCare, which was acquired by Aetna, and public health management positions with the San Antonio Metropolitan Health District from July 1994 to September 1996. Mr. Farmer holds a B.S. degree in political science from Trinity University and an M.B.A. from the University of Texas.
Mark Mugerditchian, our senior vice president of manufacturing and product development since January 2006, joined us initially as the vice president of manufacturing and process
94
development in August 2003. From June 2002 to August 2003, Mr. Mugerditchian was a consultant to various biopharmaceutical companies, including us. From May 2001 to June 2002, Mr. Mugerditchian served as executive director of contract manufacturing and project management at Gensia Sicor Pharmaceuticals, Inc., a pharmaceutical company, where he was responsible for directing new drug application product development and contract manufacturing activities. From March 1994 to May 2001, Mr. Mugerditchian served in various management roles at Dura Pharmaceuticals, Inc., a specialty respiratory pharmaceutical and pulmonary drug delivery company. Prior to March 1994, Mr. Mugerditchian held various manufacturing management positions at Abbott Laboratories, Inc., Key Pharmaceuticals, Inc. and Fujisawa USA. Mr. Mugerditchian holds a B.S. degree in chemical engineering from the University of Illinois.
Board composition
Our business and affairs are organized under the direction of our board of directors, which currently consists of seven members. The primary responsibilities of our board of directors are to provide oversight, strategic guidance, counseling and direction to our management. Our board of directors meets on a regular basis and additionally as required. Written board materials are distributed in advance of meetings as a general rule, and our board of directors schedules meetings with and receives presentations from members of our senior management on a regular basis and as required.
Our board of directors has determined that five of our seven directors, Dr. Collinson, Ms. Kjellson, Dr. Lowe, Ms. Samuels and Mr. Turner, are independent directors, as defined by Rule 4200(a)(15) of the National Association of Securities Dealers.
Effective upon the completion of this offering, we will divide our board of directors into three classes, as follows:
- •
- Class I, which will consist of Dr. Weber and Dr. Collinson, whose terms will expire at our annual meeting of stockholders to be held in 2008;
- •
- Class II, which will consist of Mr. Turner, Ms. Samuels and Ms. Kjellson, whose terms will expire at our annual meeting of stockholders to be held in 2009; and
- •
- Class III, which will consist of Dr. Lowe and Mr. Woods, whose terms will expire at our annual meeting of stockholders to be held in 2010.
At each annual meeting of stockholders to be held after the initial classification, the successors to directors whose terms then expire will serve until the third annual meeting following their election and until their successors are duly elected and qualified. The authorized size of our board is currently eight members. The authorized number of directors may be changed only by resolution of the board of directors, subject to the rights of any holders of preferred stock. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in our control or management. Our directors may be removed for cause by the affirmative vote of the holders of at least 662/3% of our voting stock.
95
Board committees
Our board of directors has established an audit committee, a compensation committee and a corporate governance and nominating committee.
Audit committee
Our audit committee consists of Dr. Collinson, Ms. Kjellson and Mr. Turner. Our board of directors has determined that each of the members of our audit committee satisfies the Nasdaq Stock Market, LLC, or Nasdaq, and SEC independence requirements. Mr. Turner serves as the chair of our audit committee. The functions of this committee include, among other things:
- •
- evaluating the performance, independence and qualifications of our independent auditors and determining whether to retain our existing independent auditors or engage new independent auditors;
- •
- reviewing and approving the engagement of our independent auditors to perform audit services and any permissible non-audit services;
- •
- monitoring the rotation of partners of our independent auditors on our engagement team as required by law;
- •
- reviewing our annual and quarterly financial statements and reports and discussing the statements and reports with our independent auditors and management;
- •
- reviewing with our independent auditors and management significant issues that arise regarding accounting principles and financial statement presentation, and matters concerning the scope, adequacy and effectiveness of our financial controls;
- •
- reviewing with management and our auditors any earnings announcements and other public announcements regarding corporate progress;
- •
- establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters and other matters;
- •
- preparing the report that the SEC requires in our annual proxy statement;
- •
- reviewing and approving any related party transactions and reviewing and monitoring compliance with our code of conduct and ethics;
- •
- reviewing at least annually our investment policy; and
- •
- reviewing and evaluating, at least annually, the performance of the audit committee and its members, including compliance of the audit committee with its charter.
Our board of directors has determined Mr. Turner qualifies as an audit committee financial expert within the meaning of SEC regulations and the Nasdaq Marketplace Rules. In making this determination, our board has considered the formal education and nature and scope of experience that Mr. Turner has previously had with reporting companies. Both our independent registered public accounting firm and management periodically meet privately with our audit committee.
96
Compensation committee
Our compensation committee consists of Dr. Collinson, Dr. Lowe and Ms. Samuels. Dr. Lowe serves as the chair of our compensation committee. The functions of this committee include, among other things:
- •
- reviewing and approving the compensation and other terms of employment of our executive officers;
- •
- reviewing and approving performance goals and objectives relevant to the compensation of our executive officers and assessing their performance against these goals and objectives;
- •
- evaluating and approving the equity incentive plans, compensation plans and similar programs advisable for us, as well as modification or termination of existing plans and programs;
- •
- evaluating and recommending to our board of directors the type and amount of compensation to be paid or awarded to board members;
- •
- administering our equity incentive plans;
- •
- establishing policies with respect to equity compensation arrangements;
- •
- reviewing the competitiveness of our executive compensation programs and evaluating the effectiveness of our compensation policy and strategy in achieving expected benefits to us;
- •
- reviewing and approving the terms of any employment agreements, severance arrangements, change in control protections and any other compensatory arrangements for our executive officers;
- •
- reviewing with management and approving our disclosures under the caption "Compensation discussion and analysis" in our periodic reports to be filed with the SEC;
- •
- preparing the report that the SEC requires in our annual proxy statement; and
- •
- reviewing and evaluating, at least annually, the performance of the compensation committee.
Each member of our compensation committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Securities Exchange Act of 1934, as amended, is an outside director, as defined pursuant to Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, and satisfies the Nasdaq independence requirements.
Corporate governance and nominating committee
Our corporate governance and nominating committee consists of Dr. Lowe, Ms. Kjellson, Ms. Samuels and Mr. Turner. Our board of directors has determined that each of the members of this committee satisfies the Nasdaq independence requirements. Ms. Samuels serves as the chair of our corporate governance and nominating committee. The functions of this committee include, among other things:
- •
- identifying, reviewing and evaluating candidates to serve on our board of directors consistent with criteria approved by our board of directors;
97
- •
- determining the minimum qualifications for service on our board of directors;
- •
- evaluating director performance on the board and applicable committees of the board and determining whether continued service on our board is appropriate;
- •
- interviewing, evaluating, nominating and recommending individuals for membership on our board of directors;
- •
- assisting the members of our compensation committee in reviewing and recommending to our board of directors the compensation arrangements for our non-employee directors;
- •
- evaluating nominations by stockholders of candidates for election to our board of directors;
- •
- considering and assessing the independence of members of our board of directors;
- •
- developing a set of corporate governance policies and principles, including a code of ethics and reviewing and recommending to our board of directors any changes to such policies and principles;
- •
- considering questions of possible conflicts of interest of directors as such questions arise;
- •
- developing a set of corporate governance principles for us and periodically reviewing and assessing these principles and their application and recommending to our board of directors any amendments to our corporate governance principles; and
- •
- evaluating at least annually, the performance of the nominating and corporate governance committee.
98
Executive compensation
Compensation discussion and analysis
Overview
The compensation committee of our board of directors, composed entirely of independent directors, administers our executive compensation program. The role of the compensation committee is to oversee our compensation and benefit plans and policies, to administer our stock plans and to review and approve annually all compensation decisions relating to all executive officers.
Compensation philosophy
Our compensation programs are designed to:
- •
- attract, motivate and retain executives of outstanding ability and potential;
- •
- reward the achievement of key strategic and financial performance measures; and
- •
- ensure that executive compensation is meaningfully related to the creation of stockholder value.
Our compensation committee believes that our compensation programs should include short-and long-term components, including cash and equity-based compensation, and should reward performance as measured against established goals. The compensation committee evaluates both performance and compensation to make sure that the compensation provided to executives remains competitive relative to compensation paid by companies of similar size and stage of development operating in the life sciences industry, taking into account our relative performance and our own strategic goals.
Setting executive compensation
The compensation committee reviews and determines on an annual basis the compensation to be paid to our chief executive officer, executive officers and key employees. As part of this process, we conduct an annual benchmark review of the aggregate level of our executive compensation, as well as the mix of elements used to compensate our executive officers. As a private company, we have based this review on surveys of executive compensation paid by private life sciences companies conducted by third-party providers such as the Biotechnology Employment Development Coalition (which is a survey of approximately 93 public and private life sciences companies in San Diego, California), and the J. Robert Scott Compensation and Entrepreneurship Report in Life Sciences (which is a survey of approximately 170 private life sciences and medical device companies in the United States). In addition, our compensation committee has historically taken into account input from other independent members of our board of directors and publicly available data relating to the compensation practices and policies of other companies within and outside our industry.
The compensation committee retained compensation consultant, Strategic Apex Group, in February 2007 to assist in the review of the chief executive officer's compensation. Strategic Apex Group assisted the compensation committee in creating a peer group of life sciences companies with a similar profile who have become public companies since the beginning of 2006, reviewing elements of compensation and providing compensation alternatives for
99
consideration. The peer group consisted of 19 public companies with a median market capitalization of $230 million. The peer group companies were:
|
Peer Group
|
|---|
|
Achillion Pharmaceuticals
Acorda Pharmaceuticals
Alexza Pharmaceuticals
Altus Pharmaceuticals
BioMimetic Therapeutics
Cadence Pharmaceuticals
Cleveland BioLabs |
|
Emergent BioSolutions
Iomai
Northstar Neuroscience
Novacea
Oculus Innovative Sciences
Omrix Biopharmaceuticals
Osiris Therapeutics |
|
Replidyne
SGX Pharmaceuticals
Targacept
Trubion Pharmaceuticals
Valera Pharmaceuticals |
|
The compensation committee generally benchmarks executive compensation against the median compensation paid by peer companies. While benchmarking may not always be appropriate as a stand-alone tool for setting compensation due to the aspects of our business and objectives that may be unique to us, the compensation committee generally believes that gathering this information is an important part of our compensation-related decision-making process.
Our compensation committee intends to retain the services of third-party executive compensation specialists from time to time, as it sees fit, in connection with the establishment of cash and equity compensation and related policies.
Role of chief executive officer in compensation decisions
The chief executive officer evaluates the performance of other executive officers and employees on an annual basis and makes recommendations to the compensation committee with respect to annual salary adjustments, bonuses and annual stock option grants. The compensation committee exercises its own discretion in determining salary adjustments and discretionary cash and equity-based awards for all executive officers and senior employees.
Elements of executive compensation
The compensation program for our executive officers consists principally of base salary, annual cash incentive compensation, long-term compensation in the form of stock options and severance/termination protection. As a private company, our compensation program has been weighted toward long-term compensation as opposed to short-term or cash-based compensation. If we achieve our corporate goals, we expect the equity awards held by our executives to be the major component of overall compensation. As discussed in more detail below, base compensation is based primarily on market factors and our annual executive bonus plan generally targets cash bonus opportunities at a percentage of base salary. The amount of cash compensation generally does not influence the amount of equity awards granted to our executives.
Base salary
Base salaries for our executives are established based on the scope of their responsibilities and individual experience, taking into account competitive market compensation paid by other companies for similar positions within the life sciences industry. Base salaries are reviewed annually, and adjusted from time to time to realign salaries with market levels after taking into
100
account individual responsibilities, performance and experience. The compensation committee does not apply specific formulas to determine increases.
For 2007, our chief executive officer's base salary increased 4.5% based on market and performance factors and our chief medical officer's base salary increased 16.7% due to his promotion and based upon market and performance factors. Our compensation committee believes that the base salary levels of our executives are commensurate with the general salary levels for similar positions in life sciences companies of similar size and stage of development and operations.
Annual executive bonus plan
In addition to base salaries, we believe that performance-based cash bonuses play an important role in providing incentives to our executives to achieve defined annual corporate goals. Near the beginning of each year, the compensation committee reviews a detailed set of overall corporate performance goals and value-creating milestones for the current year prepared by management that are intended to apply to the executives' bonus awards and, with some distinctions, to the bonus awards for all of our other employees. Our compensation committee works with management to develop final corporate performance goals that are set at a level that the compensation committee believes management can reasonably achieve with hard work over the next year.
At the end of each year, our compensation committee, determines the level of achievement for each corporate goal and value-creating milestone. Final determinations as to discretionary bonus levels are then based in part on the achievement of these corporate goals or milestones, as well as the compensation committee's assessment as to the overall success of our company and the development of our business and the performance of individual employees. The compensation committee has designed the bonus plan to be flexible and discretionary and has not pre-assigned weights to any particular goals or developed a formula for awarding bonuses based upon achievement of corporate or individual goals. The corporate goals and milestones, may vary, from time to time, depending on our overall strategic objectives, but relate generally to factors such as achievement of clinical and regulatory milestones for product candidates, identifying and in-licensing new product candidates, establishment of collaborative arrangements and to financial factors such as raising or preserving capital. Typically, the compensation committee does not set individual performance goals. The compensation committee reserves the discretion to award bonuses based upon its assessment of corporate and individual performance, and bonuses may be above or below target levels.
In December 2006, our compensation committee awarded discretionary bonuses of $82,500 to Mr. Woods and $40,000 to Dr. Dittrich based upon its assessment of our performance against our corporate goals during 2006, including our achievement of regulatory and clinical development milestones relating to our lead product candidates, the closing of our Series B preferred stock financing and our in-licensing of K201 (JTV 519) and based upon competitive bonus levels.
Our compensation committee has established target bonus awards of up to 25% of base salary for executives for 2007. The amount of actual bonuses paid to our executives will be determined by our compensation committee and our board of directors and paid at the end of 2007, or in early 2008, and may be above or below target bonus levels.
101
Long-term incentive program
We believe that by providing our executives the opportunity to increase their ownership of our stock, the best interests of stockholders and executives will be more aligned and we will encourage long-term performance. The stock awards enable our executive officers to participate in the appreciation of stockholder value, while personally participating in the risks of business setbacks. We have not adopted stock ownership guidelines and our equity benefit plans have provided our executive officers the only means to acquire equity or equity-linked interests in our company.
Prior to this offering, we granted equity awards primarily through our 2003 equity incentive plan, which was adopted by our board of directors and stockholders to permit the grant of stock options, stock appreciation rights, restricted stock and other stock-based awards to our officers, directors, scientific advisory board members, employees and consultants. The material terms of our 2003 equity incentive plan are further described under "—Equity benefit plans" below.
In 2006, certain named executive officers were awarded stock options under our 2003 equity incentive plan in the amounts indicated in the section below entitled "Grants of plan-based awards." In connection with raising additional equity capital in March 2007, the compensation committee, with the assistance of its compensation consultant, reviewed the equity ownership levels of chief executive officers at the same peer group of public companies used to review overall compensation. Based upon its review of the benchmark information, its assessment of individual performance and its assessment of the employee retention value of equity awards subject to vesting requirements, in March 2007, the compensation committee made additional options awards to our chief executive officer, certain of our other named executive officers and other key employees. These stock option awards are further described under "—Outstanding equity awards at December 31, 2006." In making annual equity awards to existing employees, the compensation committee takes into account its assessment of individual performance contributions of employees, competitive equity awards based upon position and level of responsibility and the recommendations of our chief executive officer.
In the absence of a public trading market for our common stock, our board of directors has determined the fair market value of our common stock in good faith based upon consideration of a number of relevant factors, including the status of our regulatory and clinical development efforts, financial status, market conditions and valuations obtained from an independent valuation firm in November 2005, December 2006, February 2007 and March 2007.
All equity awards to our employees and directors were granted at no less than the fair market value of our common stock on the date of each award. We do not have any program, plan or obligation that requires us to grant equity compensation on specified dates and, because we have not been a public company, we have not made equity grants in connection with the release or withholding of material non-public information. Authority to make equity grants to executive officers rests with our compensation committee, although our compensation committee does consider the recommendations of our chief executive officer for grants to officers other than himself.
In connection with this offering, our board of directors has adopted new equity benefit plans described under "—Equity benefit plans" below. The 2007 equity incentive plan will replace our existing 2003 equity incentive plan immediately following this offering and, as described below,
102
will afford our compensation committee much greater flexibility in making a wide variety of equity awards. The option to participate in our 2007 employee stock purchase plan, which we have adopted and that will become effective after this offering, will also be available to all executive officers following this offering on the same basis as our other employees.
Stock appreciation rights
Our 2007 equity incentive plan authorizes us to grant stock appreciation rights, or SARs, which are more fully described below under "—Equity benefit plans." To date, no SARs have been awarded to any of our executive officers. However, our compensation committee, in its discretion, may in the future elect to make such grants to our executive officers if it deems it advisable.
Restricted stock grants or awards
Our 2003 equity incentive plan and our 2007 equity incentive plan authorize us to grant restricted stock or restricted stock awards. Our compensation committee did not authorize the grant of restricted stock or restricted stock awards pursuant to our equity benefit plans to any of our executive officers in the year ended December 31, 2006. However, our compensation committee, in its discretion, may in the future elect to make such grants to our executive officers if it deems it advisable.
Severance and change in control benefits
Our named executive officers, who are designated below under "—Summary compensation table," are entitled to certain severance and change in control benefits, the terms of which are described below under "—Post employment compensation." We believe these severance and change in control benefits are an essential element of our overall executive compensation package and assist us in recruiting and retaining talented individuals and aligning the executive's interests with the best interests of the stockholders.
Other compensation
Our executive officers who were parties to employment agreements prior to this offering will continue, following this offering, to be parties to such employment agreements in their current form until such time as our compensation committee determines in its discretion that revisions to such employment agreements are advisable. In addition, consistent with our compensation philosophy, we intend to continue to maintain the current benefits for our executive officers, which are also available to all of our other employees; however, our compensation committee, in its discretion, may in the future revise, amend or add to the benefits of any executive officer if it deems it advisable. The material terms of our employment agreements with our named executive officers are described below under "—Post employment compensation—Potential payment under employment arrangements."
103
Summary compensation table
The following table summarizes the compensation that we paid to our chief executive officer, a former chief medical officer and our current chief medical officer, who are our only current and former executive officers whose combined salary and bonus exceeded $100,000 during the year ended December 31, 2006. We refer to these current and former officers in this prospectus as our named executive officers. No other executive officer received salary and bonus compensation from us in excess of $100,000 in the year ended December 31, 2006.
|
Name and principal position
| | Year
| | Salary
| | Bonus
| | Option
awards(1)
| | Total
|
|---|
|
Randall E. Woods
President and Chief Executive Officer | | 2006 | | $ | 330,000 | | $ | 82,500 | | $ | 25,395 | | $ | 437,895 |
Howard C. Dittrich, M.D.
Chief Medical Officer | | 2006 | | | 270,000 | | | 40,000 | | | 13,240 | | | 323,240 |
Michael J. B. Tansey, M.D., Ph.D.(2)
Former Chief Medical Officer | | 2006 | | | 100,419 | | | — | | | 614 | | | 101,033 |
|
- (1)
- Calculated in accordance with SFAS No. 123(R) using the modified prospective transition method without consideration of forfeitures.
- (2)
- Dr. Tansey resigned as our chief medical officer in May 2006.
Post-employment compensation
The amount of compensation payable to each named executive officer upon voluntary termination, involuntary termination without cause, termination following a change in control or termination in the event of disability or death of the executive is shown below.
Payments made upon termination
Regardless of the manner in which a named executive officer's employment terminates, the named executive officer is entitled to receive amounts earned during his term of employment, including salary and unused vacation pay.
Potential payment under employment arrangements
Under our employment offer letter to Mr. Woods, if we terminate his employment at any time with or without cause, as defined in the letter agreement, he is entitled to receive any of his unpaid prorated base salary along with all benefits and expense reimbursements to which he is entitled by virtue of his past employment with us. In addition, if Mr. Woods is terminated without cause, he is also entitled to be compensated at his then annual base salary for six months from his date of termination. If Mr. Woods is terminated without cause or resigns for good reason within 13 months following a change in control, as defined in the terms of his stock option agreement, the vesting of all of Mr. Woods' outstanding stock options will be accelerated in full.
Under our employment offer letter to Dr. Dittrich, if we terminate his employment at any time with or without cause, he is entitled to receive any unpaid prorated base salary along with all benefits and expense reimbursements to which he is entitled by virtue of his past employment with us. In addition, if Dr. Dittrich is terminated without cause, he is also entitled to be compensated at his then annual base salary for three months from his date of termination. If
104
Dr. Dittrich is terminated without cause or resigns for good reason within 13 months following a change in control, as defined in his stock option agreement, the vesting of all of Dr. Dittrich's outstanding stock options will be accelerated in full.
Under our employment agreement with Mr. Crawford, if we terminate Mr. Crawford's employment without cause, he is entitled to be compensated at his then annual base salary for nine months from his date of termination. In the event that Mr. Crawford is terminated without cause or resigns for good reason within 13 months following a change in control, as defined in his stock option agreement, the vesting of all of Mr. Crawford's outstanding stock options will be accelerated in full.
The following table and summary set forth potential payments payable to our current executive officers upon termination of employment or a change in control. Our compensation committee may in its discretion revise, amend or add to the benefits if it deems advisable. The table below reflects amounts payable to our executive officers assuming their employment was terminated on December 31, 2006:
|
| | Upon termination without cause
| | Upon termination without cause or resignation for good reason following a change in control
|
|---|
Name
| | Salary
| | Bonus
| | Benefits
| | Equity
awards
| | Total
| | Salary
| | Bonus
| | Benefits
| | Equity
awards(3)
| | Total
|
|---|
|
| Randall E. Woods | | $ | 165,000 | (1) | — | | — | | — | | $ | 165,000 | | $ | 165,000 | (1) | — | | — | | $ | 971,019 | | $ | 1,136,019 |
| Howard C. Dittrich, M.D. | | | 67,500 | (2) | — | | — | | — | | | 67,500 | | | 67,500 | (2) | — | | — | | | 542,793 | | | 610,293 |
|
- (1)
- Represents six months of continued salary based upon his 2006 base salary.
- (2)
- Represents three months of continued salary based upon his 2006 base salary.
- (3)
- Calculated in accordance with SFAS No. 123(R) using the modified prospective transition method without consideration of forfeitures.
Dr. Tansey resigned as our chief medical officer in May 2006. He did not receive any severance payments or benefits and all of his stock options had terminated as of December 31, 2006.
Grants of plan-based awards
All stock options granted to our named executive officers are incentive stock options, to the extent permissible under the Internal Revenue Code of 1986, as amended, or the Code. The exercise price per share of each stock option granted to our named executive officers was equal to the fair market value of our common stock as determined in good faith by our board of directors on the date of the grant. All stock options were granted under our 2003 plan.
105
We omitted columns related to non-equity and equity incentive plan awards as none of our named executive officers earned any such awards during 2006. The following table sets forth certain information regarding grants of plan-based awards to our named executive officers for 2006.
|
Name
| | Grant date
| | Approval date
| | All option awards: number of shares of stock or units (#)
| | Exercise or base price of option awards
($/share)(1)
| | Grant date fair value of option awards
($)(2)
|
|---|
|
| Randall E. Woods(3) | | 12/12/06 | | 12/12/06 | | 800,000 | | 0.16 | | 984,000 |
| Howard C. Dittrich, M.D.(4). | | 12/12/06 | | 12/12/06 | | 450,000 | | 0.16 | | 553,500 |
| Michael J. B. Tansey, M.D., Ph.D. | | — | | — | | — | | — | | — |
|
- (1)
- Represents the per share fair market value of our common stock, as determined in good faith by our board of directors on the grant date.
- (2)
- Calculated in accordance with SFAS No. 123(R) using the modified prospective transition method without consideration of forfeitures.
- (3)
- 25% of the total number of shares subject to each of Mr. Woods' options vest on the 12 month anniversary of the applicable grant date with the remainder vesting over the following 36 months. In connection with certain events following a change in control, 100% of the unvested shares under each of the stock options granted to Mr. Woods will vest in full.
- (4)
- 25% of the total number of shares subject to Dr. Dittrich's options vest on the 12 month anniversary of the applicable grant date with the remainder vesting over the following 36 months. In connection with certain events following a change in control, 100% of the unvested shares under each of the stock options granted to Dr. Dittrich will vest in full.
Outstanding equity awards at December 31, 2006
The following table sets forth certain information regarding outstanding equity awards granted to our named executive officers that remain outstanding as of December 31, 2006.
| |
| | Option awards
| |
|---|
Name
| | Number of
securities
underlying
unexercised
options
(#)
exercisable
| | Number of
securities
underlying
unexercised
options
(#)
unexercisable
| | Option
exercise
price
($)
| | Option
expiration
date
| |
|---|
| |
| Randall E. Woods | | 800,000
160,000
1,638,024 | | —
—
— | | 0.16
0.14
0.10 | | 12/11/16(1
12/18/15(1
5/10/14(1 | )
)
) |
| Howard C. Dittrich, M.D. | | 450,000
55,000
573,308 | | —
—
— | | 0.16
0.14
0.10 | | 12/11/16(1
12/20/15(1
9/4/13(2 | )
)
) |
| Michael J. B. Tansey, M.D., Ph.D. | | — | | — | | — | | — | |
| |
- (1)
- One quarter of the common stock underlying each option vests on the one year anniversary of the date of grant with the remainder vesting in equal installments on a monthly basis over the next three years.
- (2)
- One quarter of the common stock underlying the option vested on December 1, 2003 with the remainder vesting in equal installments on a monthly basis over the next three years.
106
In March 2007, our board of directors granted options exercisable for an additional 1,000,000 shares of our common stock to Mr. Woods and an option exercisable for an additional 600,000 shares of our common stock to Dr. Dittrich at an exercise price of $0.38 per share subject to our customary vesting schedule.
Option exercises and stock vested
Our named executive officers did not exercise any stock option awards that are subject to vesting during the year ended December 31, 2006.
Option repricings
We have not engaged in any option repricings or other modifications to any of our outstanding equity awards during the year ended December 31, 2006.
Pension benefits
None of our named executive officers participates in or have account balances in qualified or non-qualified defined benefit plans sponsored by us. Our compensation committee may elect to adopt qualified or non-qualified benefit plans in the future if it determines that doing so is in our best interests.
Nonqualified deferred compensation
None of our named executive officers participates in or have account balances in nonqualified defined contribution plans or other nonqualified deferred compensation plans maintained by us. Our compensation committee may elect to provide our officers and other employees with non-qualified defined contribution or other nonqualified deferred compensation benefits in the future if it determines that doing so is in our best interests.
Equity benefit plans
2003 equity incentive plan
Our board of directors adopted and our stockholders approved the 2003 equity incentive plan, or 2003 plan, in August 2003. As of April 30, 2007, 656,219 shares of common stock have been issued upon the exercise of options granted, options to purchase 7,609,832 shares of common stock were outstanding and 1,233,949 shares of common stock remained available for future grant under the 2003 plan. Upon the effective date of this offering, the 2003 plan will be terminated and no further option grants will be made under the 2003 plan and any shares then remaining available for future grant, plus any shares underlying outstanding options that expire or are forfeited, will be allocated to our 2007 equity incentive plan.
Administration. Our board of directors administers the 2003 plan. Our board of directors, however, may delegate this authority to a committee of one or more board members. Our board has delegated such authority nonexclusively to our compensation committee. The board of directors or a committee of the board has the authority to construe, interpret, amend and modify the 2003 plan as well as to determine the terms of an option. Our board of directors may amend or modify the 2003 plan at any time. However, no amendment or modification
107
shall adversely affect the rights and obligations with respect to outstanding options unless the holder consents to that amendment or modification.
Eligibility. The 2003 plan permits us to grant stock awards, including options, restricted stock, stock appreciation rights and stock bonuses to our employees, directors and consultants. Our board of directors has granted only stock options under the 2003 plan. A stock option may be an incentive stock option within the meaning of Section 422 of the Code or a nonstatutory stock option.
Stock option provisions generally. In general, the duration of a stock option granted under the 2003 plan cannot exceed ten years. The exercise price of an incentive stock option cannot be less than 100% of the fair market value of the common stock on the date of grant. The exercise price of a nonstatutory stock option cannot be less than 85% of the fair market value of the common stock on the date of grant. An incentive stock option may be transferred only on death, but a nonstatutory stock option may be transferred as permitted in an individual stock option agreement. Stock option agreements may provide that the stock options may be early exercised subject to our right of repurchase of unvested shares. In addition, our board of directors may reprice any outstanding option or, with the permission of the optionholder, may cancel any outstanding option and grant a substitute option.
Incentive stock options may be granted only to our employees. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to which incentive stock options are exercisable for the first time by an optionholder during any calendar year under all of our stock plans may not exceed $100,000. An incentive stock option granted to a person who at the time of grant owns or is deemed to own more than 10% of the total combined voting power of all classes of our outstanding stock or any of our affiliates must have a term of no more than five years and an exercise price that is at least 110% of fair market value at the time of grant.
Effect on stock options of certain corporate transactions. If we dissolve or liquidate, then outstanding stock options under the 2003 plan will terminate immediately prior to such dissolution or liquidation. However, we treat outstanding stock options differently in the following situations:
- •
- a sale of all or substantially all of our assets;
- •
- a sale or other disposition of at least 90% of our outstanding securities;
- •
- a merger or consolidation in which we are not the surviving corporation; or
- •
- a reverse merger in which we are the surviving corporation but the shares of our common stock outstanding immediately preceding the merger are converted by virtue of the merger into other property.
In the event any of the above situations occurs, if the surviving entity determines not to assume or substitute for these stock options, the vesting of stock options held by persons whose service with us or our affiliates has not already terminated will accelerate in full prior to such corporate transaction.
108
Other provisions. If there is a transaction or event which changes our stock that does not involve our receipt of consideration, such as a merger, consolidation, reorganization, stock dividend or stock split, our board of directors will appropriately adjust the class and the maximum number of shares subject to the 2003 plan.
2007 equity incentive plan
Our board of directors adopted the 2007 equity incentive plan, or 2007 plan, in March 2007 and we expect our stockholders will approve the 2007 plan prior to the closing of this offering. The 2007 plan will become effective immediately upon the signing of the underwriting agreement for this offering. The 2007 plan will terminate in March 2017, unless sooner terminated by our board of directors.
Stock awards. The 2007 plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance-based stock awards, and other forms of equity compensation, or collectively, stock awards. In addition, the 2007 plan provides for the grant of performance cash awards. Incentive stock options may be granted only to employees. All other awards may be granted to employees, including officers, non-employee directors and consultants.
Share reserve. Following this offering, the aggregate number of shares of our common stock that may be issued initially pursuant to stock awards under the 2007 plan is 9,000,000 shares. In addition, the number of shares of our common stock reserved for issuance will automatically increase (i) on January 1st, from January 1, 2008 through January 1, 2017, by the least of (a) four percent of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year, (b) 6,000,000 shares or (c) a number determined by our board of directors that is less than (a) or (b). The reserve will also include any shares reserved under the 2003 plan that are not subject to outstanding options at the effective date of the 2007 plan (1,233,949 shares as of April 30, 2007) plus any shares that are issuable pursuant to options under the 2003 plan that are forfeited or expire from time to time. The maximum number of shares that may be issued pursuant to the exercise of incentive stock options under the 2007 plan is equal to 18,000,000 shares, as increased from time to time pursuant to annual increases.
No person may be granted stock awards covering more than 5,000,000 shares of our common stock under the 2007 plan during any calendar year pursuant to stock options or stock appreciation rights. In addition, no person may be granted a performance stock award covering more than 1,000,000 shares or a performance cash award covering $2,000,000 in any calendar year. Such limitations are designed to help assure that any deductions to which we would otherwise be entitled with respect to such stock awards will not be subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code.
If a stock award granted under the 2007 plan expires or otherwise terminates without being exercised in full, or is settled in cash, the shares of our common stock not acquired pursuant to the stock award again become available for subsequent issuance under the 2007 plan. In addition, the following types of shares under the 2007 plan may become available for the grant of new stock awards under the 2007 plan: (a) shares that are forfeited to or repurchased by us prior to becoming fully vested; (b) shares withheld to satisfy income or employment
109
withholding taxes; (c) shares used to pay the exercise price of an option in a net exercise arrangement; and (d) shares tendered to us to pay the exercise price of an option. Shares issued under the 2007 plan may be previously unissued shares or reacquired shares bought on the open market. As of the date hereof, no shares of our common stock have been issued under the 2007 plan.
Administration. Our board of directors has delegated its authority to administer the 2007 plan to our compensation committee. Subject to the terms of the 2007 plan, our board of directors or an authorized committee, referred to as the plan administrator, determines recipients, dates of grant, the numbers and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability and vesting. Subject to the limitations set forth below, the plan administrator will also determine the exercise price of options granted, the consideration to be paid for restricted stock awards and the strike price of stock appreciation rights.
The plan administrator has the authority to reprice any outstanding stock award under the 2007 plan without the approval of our stockholders.
Stock options. Incentive and nonstatutory stock options are granted pursuant to incentive and nonstatutory stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for a stock option, within the terms and conditions of the 2007 plan, provided that the exercise price of a stock option cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2007 plan vest at the rate specified by the plan administrator.
The plan administrator determines the term of stock options granted under the 2007 plan, up to a maximum of ten years, except in the case of certain incentive stock options, as described below. Unless the terms of an optionholder's stock option agreement provide otherwise, if an optionholder's relationship with us, or any of our affiliates, ceases for any reason other than for cause, disability or death, the optionholder may exercise any vested options for a period of three months following the cessation of service. If an optionholder's service relationship with us is terminated for cause, then the option terminates immediately. If an optionholder's service relationship with us, or any of our affiliates, ceases due to disability or death, or an optionholder dies within a certain period following cessation of service, the optionholder or a beneficiary may exercise any vested options for a period of 12 months in the event of disability and 18 months in the event of death. The option term may be extended in the event that exercise of the option following termination of service is prohibited by applicable securities laws. In no event, however, may an option be exercised beyond the expiration of its term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the plan administrator and may include (a) cash, check, bank draft or money order, (b) a broker-assisted cashless exercise, (c) the tender of common stock previously owned by the optionholder, (d) a net exercise of the option and (e) other legal consideration approved by the plan administrator.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An
110
optionholder may designate a beneficiary, however, who may exercise the option following the optionholder's death.
Tax limitations on incentive stock options. Incentive stock options may be granted only to our employees. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive stock options that are exercisable for the first time by an optionholder during any calendar year under all of our stock plans may not exceed $100,000. No incentive stock option may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates unless (a) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and (b) the term of the incentive stock option does not exceed five years from the date of grant.
Restricted stock awards. Restricted stock awards are granted pursuant to restricted stock award agreements adopted by the plan administrator. Restricted stock awards may be granted in consideration for (a) cash, check, bank draft or money order, (b) past or future services rendered to us or our affiliates, or (c) any other form of legal consideration. Shares of common stock acquired under a restricted stock award may, but need not, be subject to a share repurchase option in our favor in accordance with a vesting schedule to be determined by the plan administrator. Rights to acquire shares under a restricted stock award may be transferred only upon such terms and conditions as set by the plan administrator.
Restricted stock unit awards. Restricted stock unit awards are granted pursuant to restricted stock unit award agreements adopted by the plan administrator. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award may be settled by cash, delivery of stock, a combination of cash and stock as deemed appropriate by the plan administrator, or in any other form of consideration set forth in the restricted stock unit award agreement. Additionally, dividend equivalents may be credited in respect of shares covered by a restricted stock unit award. Except as otherwise provided in the applicable award agreement, restricted stock units that have not vested will be forfeited upon the participant's cessation of continuous service for any reason.
Stock appreciation rights. Stock appreciation rights are granted pursuant to stock appreciation rights agreements adopted by the plan administrator. The plan administrator determines the strike price for a stock appreciation right which cannot be less than 100% of the fair market value of our common stock on the date of grant. Upon the exercise of a stock appreciation right, we will pay the participant an amount equal to the product of (a) the excess of the per share fair market value of our common stock on the date of exercise over the strike price, multiplied by (b) the number of shares of common stock with respect to which the stock appreciation right is exercised. A stock appreciation right granted under the 2007 plan vests at the rate specified in the stock appreciation right agreement as determined by the plan administrator.
The plan administrator determines the term of stock appreciation rights granted under the 2007 plan, up to a maximum of ten years. If a participant's service relationship with us, or any of our affiliates, ceases, then the participant, or the participant's beneficiary, may exercise any vested stock appreciation right for three months (or such longer or shorter period specified in
111
the stock appreciation right agreement) after the date such service relationship ends. In no event, however, may a stock appreciation right be exercised beyond the expiration of its term.
Performance awards. The 2007 plan permits the grant of performance stock awards and performance cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code. To assure that the compensation attributable to performance-based awards will so qualify, our compensation committee can structure such awards so that stock will be issued or paid pursuant to such award only upon the achievement of certain pre-established performance goals during a designated performance period. The maximum benefit number of shares that may be granted to a participant in any calendar year attributable to performance stock awards may not exceed 1,000,000 shares of common stock and the maximum value that may be granted to a participant in any calendar year attributable to performance cash awards may not exceed $2,000,000.
Other stock awards. The plan administrator may grant other awards based in whole or in part by reference to our common stock. The plan administrator will set the number of shares under the award and all other terms and conditions of such awards.
Changes to capital structure. In the event that there is a specified type of change in our capital structure, such as a stock split, appropriate adjustments will be made to (a) the number of shares reserved under the 2007 plan, (b) the maximum number of shares by which the share reserve may increase automatically each year, (c) the maximum number of options, stock appreciation rights and performance stock awards and performance cash awards that can be granted in a calendar year, (d) the number of shares for which options are subsequently to be made as initial and annual grants to new and continuing non-employee directors and (e) the number of shares and exercise price or strike price, if applicable, of all outstanding stock awards.
Corporate transactions. In the event of certain significant corporate transactions, awards under the 2007 plan may be assumed, continued or substituted for by any surviving or acquiring entity or its parent company. If the surviving or acquiring entity or its parent company elects not to assume, continue or substitute for such stock awards, then (a) with respect to any such stock awards that are held by individuals whose service with us or our affiliates has not terminated prior to the effective date of the corporate transaction, the vesting and exercisability provisions of such stock awards will be accelerated in full and such awards will be terminated if not exercised prior to the effective date of the corporate transaction, and (b) all other outstanding stock awards will terminate if not exercised prior to the effective date of the corporate transaction. Our board of directors has the discretion to:
- •
- arrange for the assumption, continuation, or substitution of a stock award by a surviving or acquiring entity or parent company;
- •
- accelerate the vesting of a stock award and provide for its termination prior to the effective time of the corporate transaction; or
- •
- provide for the surrender of a stock award in exchange for a payment equal to the excess of (a) the value of the property that the optionholder would have received upon the exercise of
112
Changes in control. Our board of directors has the discretion to provide that a stock award under the 2007 plan will immediately vest as to all or any portion of the shares subject to the stock award (a) immediately upon the occurrence of certain specified change in control transactions, whether or not such stock award is assumed, continued or substituted by a surviving or acquiring entity in the transaction or (b) in the event a participant's service with us or a successor entity is terminated actually or constructively within a designated period following the occurrence of certain specified change in control transactions. Stock awards held by participants under the 2007 plan will not vest automatically on such an accelerated basis unless specifically provided by the participant's applicable award agreement.
2007 non-employee directors' stock option plan
Our board of directors adopted the 2007 non-employee directors' stock option plan, or directors' plan, in March 2007 and we expect our stockholders will approve our directors' plan prior to the closing of this offering. The directors' plan will become effective immediately upon the signing of the underwriting agreement for this offering. The directors' plan will terminate at the discretion of our board of directors. The directors' plan provides for the automatic grant of nonstatutory stock options to purchase shares of our common stock to our non-employee directors.
Share reserve. An aggregate of 3,000,000 shares of our common stock are reserved for issuance under the directors' plan. This amount will be increased annually on the first day of our fiscal year, from 2008 until 2017, by the aggregate number of shares of our common stock subject to options granted as initial grants and annual grants under the directors' plan during the immediately preceding year. However, our board of directors will have the authority to designate a smaller number of shares by which the authorized number of shares of our common stock will be increased.
Shares of our common stock subject to stock options that have expired or otherwise terminated under the directors' plan without having been exercised in full shall again become available for grant under the directors' plan. Shares of our common stock issued under the directors' plan may be previously unissued shares or reacquired shares bought on the market or otherwise. If the exercise of any stock option granted under the directors' plan is satisfied by tendering shares of our common stock held by the participant, then the number of shares tendered shall again become available for the grant of awards under the directors' plan.
Administration. The directors' plan will be administered by our board of directors, which in turn may delegate authority to administer the plan to a committee.
Stock options. Stock options will be granted pursuant to stock option agreements. The exercise price of the options granted under the directors' plan will be equal to 100% of the fair market value of our common stock on the date of grant. Initial grants vest in equal monthly installments over three years after the date of grant and annual grants vest in equal monthly installments over 12 months after the date of grant.
113
In general, the term of stock options granted under the directors' plan may not exceed ten years. If an optionholder's service relationship with us, or any affiliate of ours, ceases, then the optionholder or his or her beneficiary may exercise any vested options for such period as provided under the terms of the stock option agreement.
Acceptable consideration for the purchase of our common stock issued under the directors' plan may include cash, a "net" exercise, common stock previously owned by the optionholder or a program developed under Regulation T as promulgated by the Federal Reserve Board.
Generally, an optionholder may not transfer a stock option other than by will or the laws of descent and distribution. However, an optionholder may transfer an option under certain circumstances with our written consent if a Form S-8 registration statement is available for the exercise of the option and the subsequent resale of the shares. In addition, an optionholder may designate a beneficiary who may exercise the option following the optionholder's death.
Automatic grants
- •
- Initial grant. Any person who becomes a non-employee director after the completion of this offering will automatically receive an initial grant of an option to purchase 60,000 shares of our common stock upon his or her election, subject to adjustment by our board of directors from time to time. These options will vest in equal monthly installments over three years.
- •
- Annual grant. In addition, any person who is a non-employee director on the date of each annual meeting of our stockholders automatically will be granted, on the annual meeting date, beginning with our 2008 annual meeting, an option to purchase 50,000 shares of our common stock, or the annual grant, subject to adjustment by our board of directors from time to time. However, the size of an annual grant made to a non-employee director who is elected after the completion of this offering and who has served for less than 12 months at the time of the annual meeting will be reduced by 25% for each full quarter prior to the date of grant during which such person did not serve as a non-employee director. These options will vest in equal monthly installments over 12 months.
Changes to capital structure. In the event there is a specified type of change in our capital structure not involving the receipt of consideration by us, such as a stock split or stock dividend, the number of shares reserved under the directors' plan and the number of shares and exercise price of all outstanding stock options will be appropriately adjusted.
Corporate transactions. In the event of certain corporate transactions, including change in control transactions, the vesting options held by non-employee directors whose service has not been terminated as of, or immediately prior to, the effective time of the corporate transaction, generally will be accelerated in full and all options outstanding under the directors' plan will be terminated if not exercised prior to the effective date of the corporate transaction.
Plan amendments. Our board of directors will have the authority to amend or terminate the directors' plan. However, no amendment or termination of the directors' plan will adversely affect any rights under awards already granted to a participant unless agreed to by the affected participant. We will obtain stockholder approval of any amendment to the directors' plan as required by applicable law.
114
2007 employee stock purchase plan
Our board of directors adopted our 2007 employee stock purchase plan, or 2007 purchase plan, in March 2007 and we expect our stockholders will approve the 2007 purchase plan prior to the closing of this offering. The 2007 purchase plan will become effective immediately upon the signing of the underwriting agreement for this offering.
Share reserve. Following this offering, the 2007 purchase plan authorizes the issuance of 6,000,000 shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. The number of shares of our common stock reserved for issuance will automatically increase on January 1st, from January 1, 2008 through January 1, 2017, by the least of (a) one percent of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year, (b) 1,500,000 shares, or (c) a number determined by our board of directors that is less than (a) or (b). The 2007 purchase plan is intended to qualify as an "employee stock purchase plan" within the meaning of Section 423 of the Code. As of the date hereof, no shares of our common stock have been purchased under the 2007 purchase plan.
Administration. Our board of directors has delegated its authority to administer the 2007 purchase plan to our compensation committee. The 2007 purchase plan is implemented through a series of offerings of purchase rights to eligible employees. Under the 2007 purchase plan, we may specify offerings with a duration of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. An offering may be terminated under certain circumstances.
Payroll deductions. Generally, all regular employees, including executive officers, employed by us or by any of our designated affiliates, may participate in the 2007 purchase plan and may contribute, normally through payroll deductions, up to 15% of their earnings for the purchase of our common stock under the 2007 purchase plan. Unless otherwise determined by our board of directors, common stock will be purchased for accounts of employees participating in the 2007 purchase plan at a price per share equal to the lower of (a) 85% of the fair market value of a share of our common stock on the first date of an offering or (b) 85% of the fair market value of a share of our common stock on the date of purchase.
Limitations. Employees may have to satisfy one or more of the following service requirements before participating in the 2007 purchase plan, as determined by our board of directors: (a) customarily employed for more than 20 hours per week, (b) customarily employed for more than five months per calendar year or (c) continuous employment with us or one of our affiliates for a period of time not to exceed two years. No employee may purchase shares under the 2007 purchase plan at a rate in excess of $25,000 worth of our common stock based on the fair market value per share of our common stock at the beginning of an offering for each year such a purchase right is outstanding. Finally, no employee will be eligible for the grant of any purchase rights under the 2007 purchase plan if immediately after such rights are granted, such employee has voting power over 5% or more of our outstanding capital stock measured by vote or value.
Changes to capital structure. In the event that there is a specified type of change in our capital structure, such as a stock split, appropriate adjustments will be made to (a) the number
115
of shares reserved under the 2007 purchase plan, (b) the maximum number of shares by which the share reserve may increase automatically each year and (c) the number of shares and purchase price of all outstanding purchase rights.
Corporate transactions. In the event of certain significant corporate transactions, any then-outstanding rights to purchase our stock under the 2007 purchase plan will be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase rights, then the participants' accumulated payroll contributions will be used to purchase shares of our common stock within ten business days prior to such corporate transaction, and such purchase rights will terminate immediately.
401(k) Plan
We maintain a defined contribution employee retirement plan, or 401(k) plan, for our employees. Our executive officers are also eligible to participate in the 401(k) plan on the same basis as our other employees. The 401(k) plan is intended to qualify as a tax-qualified plan under Section 401(k) of the Code. The plan provides that each participant may contribute up to the lesser of 100% of his or her pre-tax compensation or the statutory limit, which is $15,500 for calendar year 2007. Participants that are 50 years or older can also make "catch-up" contributions, which in calendar year 2007 may be up to an additional $5,000 above the statutory limit. Under the 401(k) plan, each participant is fully vested in his or her deferred salary contributions when contributed. Participant contributions are held and invested by the plan's trustee.
Non-employee director compensation
Prior to February 1, 2007, we did not provide cash compensation to directors for their services as directors or members of committees of the board of directors. We have reimbursed and will continue to reimburse our non-employee directors for their travel, lodging and other reasonable expenses incurred in attending meetings of our board of directors and committees of the board of directors.
In March 2007, our board of directors adopted a compensation program for our non-employee directors, or the Non-Employee Director Compensation Policy. The Non-Employee Director Compensation Policy is effective for our non-venture capital board member as of February 1, 2007 and will be effective for all of our other non-employee directors on the effective date of this offering. Pursuant to the Non-Employee Director Compensation Policy, each member of our board of directors who is not our employee will receive the following cash compensation for board services, as applicable:
- •
- $20,000 per year for service as a board member;
- •
- $15,000 per year for service as chairperson of the audit committee and $8,500 per year each for service as chairperson of the compensation committee or the nominating and corporate governance committee;
- •
- $1,500 for each in-person board meeting and $750 for each telephonic board meeting;
116
- •
- $1,000 for each in-person committee meeting; and
- •
- $500 for each telephonic committee meeting.
In addition, our non-employee directors will receive initial and annual, automatic, non-discretionary grants of nonqualified stock options under the terms and provisions of our directors' plan, which will become effective as of the effective date of this offering.
Each non-employee director joining our board of directors after the closing of this offering will automatically be granted a non-statutory stock option to purchase 60,000 shares of common stock with an exercise price equal to the then fair market value of our common stock under our directors' plan. On the date of each annual meeting of our stockholders beginning in 2008, each non-employee director will also automatically be granted a non-statutory stock option to purchase 50,000 shares of our common stock on that date with an exercise price equal to the then fair market value of our common stock under our 2007 directors' plan. Initial grants will vest monthly over three years and annual grants will vest in twelve equal monthly installments. All stock options granted under our directors' plan will have a term of ten years and vesting will automatically accelerate upon the closing of a change in control transaction.
For a more detailed description of our directors' plan, see "—Equity benefit plans" above.
No compensation was paid to non-employee directors during 2006.
Compensation committee interlocks and insider participation
No member of our compensation committee has ever been an executive officer or employee of ours. None of our executive officers currently serves, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our board of directors or compensation committee. We have had a compensation committee for three years. Prior to establishing the compensation committee, our full board of directors made decisions relating to compensation of our executive officers.
Limitation of liability and indemnification
Our amended and restated certificate of incorporation, which will become effective upon the completion of this offering, limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
- •
- breach of their duty of loyalty to the corporation or its stockholders;
- •
- act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
- •
- unlawful payment of dividends or redemption of shares; or
- •
- transaction from which the directors derived an improper personal benefit.
117
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
Our amended and restated bylaws, which will become effective upon the completion of this offering, provide that we will indemnify our directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law. Our amended and restated bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our amended and restated bylaws permit such indemnification. We have obtained a policy of directors' and officers' liability insurance.
We have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amended and restated bylaws. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
At present, there is no pending litigation or proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or Securities Act, may be permitted to directors, executive officers or persons controlling us, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
118
Transactions with related persons
The following includes a summary of transactions since January 1, 2004 and certain transactions prior to that date to which we have been a party, in which the amount involved in the transaction exceeded $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than five percent of our capital stock had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under "Executive compensation." We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm's-length transactions.
Policies and procedures for transactions with related persons
We have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and approval or ratification of "related-persons transactions." For purposes of our policy only, a "related-person transaction" is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any "related person" are participants involving an amount that exceeds $50,000. Transactions involving compensation for services provided to us as an employee, director, consultant or similar capacity by a related person are not covered by this policy. A related person is any executive officer, director or a holder of more than five percent of our common stock, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, where a transaction has been identified as a related-person transaction, management must present information regarding the proposed related-person transaction to our audit committee (or, where approval by our audit committee would be inappropriate, to another independent body of our board of directors) for consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the direct and indirect interests of the related persons, the benefits of the transaction to us and whether any alternative transactions are available. To identify related-person transactions in advance, we rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related-person transactions, our audit committee takes into account the relevant available facts and circumstances including, but not limited to:
- •
- the risks, costs and benefits to us;
- •
- the impact on a director's independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated;
- •
- the terms of the transaction;
- •
- the availability of other sources for comparable services or products; and
- •
- the terms available to or from, as the case may be, unrelated third parties or to or from our employees generally.
119
In the event a director has an interest in the proposed transaction, the director must recuse himself or herself form the deliberations and approval. Our policy requires that, in determining whether to approve, ratify or reject a related-person transaction, our audit committee must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, the best interests of us and our stockholders, as our audit committee determines in the good faith exercise of its discretion. We did not previously have a formal policy concerning transactions with related persons.
Sales of securities
In December 2001, we issued an aggregate of 1,000,000 shares of common stock for aggregate consideration of $1,000 to a director and certain of our affiliated stockholders. In August 2003, we issued 1,070,000 shares of common stock for aggregate consideration of $1,070 to a former executive officer and other parties. In August 2003, we issued in a private placement shares of our Series A preferred stock at a per share price of $0.84 to a five percent stockholder at the time of the issuance. In September 2006, we issued to certain of our five percent stockholders in a private placement shares of our Series B preferred stock at a per share price of $1.125. In March 2007, we issued in a private placement to certain of our five percent stockholders and a director shares of our Series B preferred stock at a per share price of $1.125.
The following table sets forth the aggregate number of shares of our common stock and preferred stock acquired between December 2001 and March 2007 by the listed directors, executive officers, certain former executive officers or holders of more than five percent of our common stock, or their affiliates.
|
Purchaser
| | Common
stock
| | Series A preferred stock(1)
| | Series B preferred stock(2)
|
|---|
|
| 5% stockholders | | | | | | |
| | Domain Partners and its affiliates(3) | | 200,000 | | 8,333,333 | | 13,217,302 |
| | Forward Ventures V, L.P.(4) | | — | | 5,952,381 | | 7,703,152 |
| | InterWest Partners IX, L.P.(5) | | — | | — | | 9,194,360 |
| | Montreux Equity Partners and its affiliates(6) | | — | | 4,761,908 | | 6,161,833 |
| | Skyline Ventures and its affiliates(7) | | — | | — | | 13,521,117 |
| | Versant Ventures and its affiliates(8) | | — | | 5,952,379 | | 5,939,391 |
| Executive officers and directors | | | | | | |
| | Eckard Weber, M.D. | | 800,000 | | — | | 2,666,668 |
| Former executive officers, directors and founders | | | | | | |
| | Kenneth J. Widder, M.D. | | 560,000 | | — | | — |
| | Lauren Otsuki | | 440,000 | | — | | — |
|
- (1)
- Includes shares issued upon conversion of approximately $1.2 million in aggregate principal amount under outstanding promissory notes that were issued to certain purchasers in connection with bridge financings in 2001 and 2003. See "—Bridge Financings—Pre-Series A financing" for additional information.
- (2)
- Includes shares issued upon conversion of approximately $4.9 million in aggregate principal amount and unpaid interest accrued under outstanding convertible promissory notes that were issued to certain purchasers in connection with a February 2006 bridge financing. See "—Bridge financings—2006 bridge financing" for additional information.
- (3)
- Includes 195,384 shares of common stock, 8,141,020 shares of Series A preferred stock and 12,911,561 shares of Series B preferred stock held by Domain Partners V, L.P., and 4,616 shares of common stock, 192,313 shares of Series A preferred stock and 305,471 shares of Series B preferred stock held by DP V Associates, L.P. The voting and investment power over these shares are held by the managing members of One Palmer Square Associates V, L.L.C., the general partner of Domain
120
Partners V, L.P. and DP V Associates, L.P. Dr. Weber, the chairman of our board of directors, is an employee of Domain Associates, L.L.C., the manager of Domain Partners V, L.P. and DP V Associates, L.P. Dr. Weber has no ownership interest, or voting or investment power with respect to the shares held by Domain Partners V, L.P. and DP V Associates, L.P.
- (4)
- Dr. Collinson, a member of our board of directors, is the managing member of Forward Ventures V, L.P. and has voting and investment power over the shares held by Forward Ventures V, L.P. Dr. Collinson disclaims beneficial ownership of these shares except to the extent of his pecuniary interest therein.
- (5)
- The voting and disposition of the shares held by InterWest Partners IX, L.P. is determined by InterWest Management Partners IX, LLC, the general partner of InterWest Partners IX, L.P. Ms. Kjellson, a member of our board of directors, is a member of InterWest Management Partners IX, LLC and has voting and investment power over the shares held by InterWest Partners IX, L.P. Ms. Kjellson disclaims beneficial ownership of these shares except to the extent of her pecuniary interest therein.
- (6)
- Includes 2,714,287 shares of Series A preferred stock and 2,324,960 shares of Series B preferred stock held by Montreux Equity Partners II SBIC, L.P., and 2,047,621 shares of Series A preferred stock and 3,836,873 shares of Series B preferred stock held by Montreux Equity Partners III SBIC, L.P. Daniel K. Turner III, a former member of our board of directors, and Howard D. Palefsky have voting and investment power with respect to the shares held by these entities and they each disclaim beneficial ownership except to the extent of their respective pecuniary interests therein.
- (7)
- Includes 10,816,893 shares of Series B preferred stock held by Skyline Venture Partners Qualified Purchaser Fund IV, L.P., 2,638,530 shares of Series B preferred stock held by Skyline Venture Partners Qualified Purchaser Fund III, L.P., and 65,694 shares of Series B preferred stock held by Skyline Venture Partners III, L.P. The voting and investment power over these shares are held by the general partners of each fund as follows: Skyline Venture Management IV, LLC is the general partner of Skyline Venture Partners Qualified Purchaser Fund IV, L.P. and Skyline Venture Management III, LLC is the general partner of both Skyline Venture Partners III, L.P. and Skyline Venture Partners Qualified Purchaser Fund III, L.P. As the managing directors of Skyline Venture Management IV, LLC and Skyline Venture Management III, LLC, John G. Freund and Yasunori Kaneko may be deemed to beneficially own these shares. Dr. Lowe, a member of our board of directors, is a partner of Skyline Ventures and, although he has influence over these shares, he has no voting or investment power of the shares held by these entities. Dr. Lowe, Mr. Freund and Mr. Kaneko each disclaims beneficial ownership of these shares except to the extent of his pecuniary interest therein.
- (8)
- Includes 5,790,732 shares of Series A preferred stock and 5,778,106 shares of Series B preferred stock held by Versant Venture Capital II, L.P., 109,892 shares of Series A preferred stock and 109,642 shares of Series B preferred stock held by Versant Affiliates Fund II-A, L.P., and 51,755 shares of Series A preferred stock and 51,643 shares of Series B preferred stock held by Versant Side Fund II, L.P. Ms. Samuels, a member of our board of directors, is the managing director of Versant Ventures II, L.L.C., the general partner of these entities, and has voting and investment power over the shares held by these entities. Ms. Samuels disclaims beneficial ownership of these shares except to the extent of her pecuniary interest therein.
In connection with our Series A preferred stock financing and Series B preferred stock financing, we entered into various stockholder agreements with the holders of our preferred stock relating to voting rights, information rights and registration rights, among other things. These stockholder agreements will terminate upon the completion of this offering, except for the registration rights granted under our amended and restated investor rights agreement, as more fully described in "Description of capital stock—Registration rights."
Bridge financings
Pre-Series A bridge financing
In December 2001, we issued non-negotiable promissory notes in an aggregate principal amount of $200,000 to certain holders of more than five percent of our capital stock and their affiliates. The notes accrued interest at the rate of 7.0% per year and, upon the request of the lenders, could be secured against our intellectual property. In January 2003 and April 2003, we issued additional non-negotiable promissory notes to the same investors. The January 2003 and April 2003 notes accrued interest at the rate of 6.75% per year.
121
The following table sets forth the names of the holders of more than five percent of our capital stock who participated in our December 2001, January 2003 and April 2003 financings and the principal amount of each loan:
|
| | Principal loan amount
|
|---|
Purchaser
| | December 2001 notes
| | January 2003 notes
| | April 2003
notes
|
|---|
|
| Domain Partners and its affiliates | | $200,000(1) | | $500,000(2) | | $500,000(3) |
|
- (1)
- Includes one note in the principal amount of $195,384 issued to Domain Partners V, L.P. and one note in the principal amount of $4,616 issued to DP V Associates, L.P.
- (2)
- Includes one note in the principal amount of $488,461 issued to Domain Partners V, L.P. and one note in the principal amount of $11,539 issued to DP V Associates, L.P.
- (3)
- Includes one note in the principal amount of $488,461 issued to Domain Partners V, L.P. and one note in the principal amount of $11,539 issued to DP V Associates, L.P.
All of the non-negotiable promissory notes and unpaid interest accrued under such notes were converted into shares of our Series A preferred stock in connection with our August 2003 Series A preferred stock financing. See "—Sales of securities" above for additional information.
2006 bridge financing
In February and June 2006, as part of a bridge financing, we issued unsecured convertible promissory notes in an aggregate principal amount of approximately $5.0 million to certain investors in two tranches. The notes accrued interest at the rate of 7.0% per year and were automatically convertible into shares of our preferred stock in the event we completed a preferred stock financing of at least $30.0 million, in the event of our sale or in certain other circumstances. All principal and unpaid interest accrued under the notes were subsequently converted into shares of our Series B preferred stock in connection with our Series B preferred stock financing in September 2006.
In connection with our February and June 2006 bridge financing, in addition to the notes, we also issued, in two tranches, to the purchasers of our notes, warrants that were exercisable for shares of preferred stock to be issued in our next round of preferred stock financing determined based on the conversion price at which the notes were to be converted. We received aggregate consideration of $11,976 for these warrants. In September 2006, these warrants were adjusted pursuant to their terms to become exercisable for shares of our Series B preferred stock at an exercise price of $1.125 per share. Warrants exercisable for up to an aggregate of 536,533 shares of our Series B preferred stock that were issued in the first tranche will terminate upon the earlier of February 2013 or completion of an initial public offering and warrants exercisable for up to an aggregate of 268,267 shares of our Series B preferred stock that were issued in the second tranche will terminate upon the earlier of June 2013 or completion of an initial public offering. All of the warrants issued in connection with our February and June 2006 bridge financing, which are exercisable for an aggregate 804,800 shares of our Series B preferred stock, will terminate if not exercised prior to the effectiveness of this offering. In March 2007, two of our warrant purchasers, Domain Partners V, L.P. and DP V Associates, L.P., exercised their warrants and purchased 256,000 shares of our Series B preferred stock for an aggregate exercise price of $288,000. The remaining warrants, which are exercisable for an aggregate 548,880 shares of our Series B preferred stock, will terminate if not exercised prior to the effectiveness of this offering.
122
The following table sets forth the names of the holders of more than five percent of our capital stock who participated in our bridge financings and the principal amount of each loan:
|
Purchaser
| | Principal loan
amount
|
|---|
|
| Domain Partners and its affiliates(1) | | $ | 1,600,000 |
| Forward Ventures V, L.P. | | | 1,150,000 |
| Montreux Equity Partners and its affiliates(2) | | | 900,000 |
| Versant Ventures and its affiliates(3) | | | 1,130,000 |
|
- (1)
- Includes one note in the principal amount of $1,550,500 issued to Domain Partners V, L.P. and one note in the principal amount of $50,000 issued to DP V Associates, L.P.
- (2)
- Includes one note in the principal amount of $500,000 issued to Montreux Equity Partners II SBIC, L.P. and one note in the principal amount of $400,000 issued to Montreux Equity Partners III SBIC, L.P.
- (3)
- Includes one note in the principal amount of $1,100,000 issued to Versant Venture Capital II, L.P., one note in the principal amount of $10,000 issued to Versant Side Fund II, L.P. and one note in the principal amount of $20,000 issued to Versant Affiliates Fund II-A, L.P.
All of the unsecured convertible promissory notes were converted into shares of our Series B preferred stock in connection with our September 2006 Series B preferred stock financing. See "—Sales of securities" above for additional information.
Intellectual property assignment
In May 2003, we entered into a letter agreement with Windamere II, LLC and Windamere III, LLC, or collectively, Windamere, whereby we agreed to purchase certain intellectual property relating to adenosine A1 receptor antagonists from Windamere in exchange for 892,857 shares of our Series A preferred stock. The purchase of the intellectual property closed concurrently with the closing of our Series A preferred stock financing in August 2003. In addition, Windamere purchased an additional 297,619 shares of our Series A preferred stock at $0.84 per share at the closing of our Series A preferred stock financing. Kenneth J. Widder, M.D., our former acting chief executive officer, was a general partner of Windamere.
Investor rights agreement
We have entered into an agreement with the purchasers of our outstanding preferred stock and the holders of warrants to acquire our preferred stock, including entities with which certain of our directors are affiliated, that provides for certain rights relating to the registration of their shares of common stock issuable upon conversion of their preferred stock. These rights will continue following this offering and will terminate seven years following the completion of this offering, or for any particular holder with registration rights, at such time following this offering when all securities held by that stockholder subject to registration rights may be sold pursuant to Rule 144 under the Securities Act. All holders of our preferred stock are party to this agreement. See "Description of capital stock—Registration rights" for additional information.
Right of first refusal and co-sale agreement
We have entered into a right of first refusal and co-sale agreement with the purchasers of our outstanding preferred stock, including entities with which certain of our directors are affiliated, and certain key holders of our common stock. Under this agreement, certain key holders of our
123
common stock are subject to contractual restrictions relating to their proposed transfer of our common stock. In addition, in the event such key holders or the purchasers of our preferred stock desire to transfer their shares of our common stock to a third party and we do not exercise our right of first refusal under our bylaws to repurchase such shares, in whole or in part, then the holders of our preferred stock have a right, on a pro-rata basis, to purchase such shares of common stock proposed to be transferred. The holders of our preferred stock also have a right of co-sale under this agreement to sell their own stock, on a pro-rata basis, in the event of a proposed transfer of stock by such key holders. This agreement will terminate upon the closing of this offering.
Voting agreement
The election of the members of our board of directors is governed by a voting agreement that we entered into with certain holders of our common stock and holders of our preferred stock and related provisions of our amended and restated certificate of incorporation. The holders of a majority of our Series A preferred stock have designated Dr. Weber, Dr. Collinson and Ms. Samuels for election to our board of directors. The holders of a majority of our Series B preferred stock have designated Ms. Kjellson and Dr. Lowe for election to our board of directors. The holders of a majority of our common stock and preferred stock, voting together as a single class, have designated Mr. Woods and Mr. Turner for election to our board of directors. Upon the closing of this offering, the voting agreement will terminate in its entirety and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors.
Employment agreements
We have entered into employment agreements with our executive officers, as more fully described in "Executive compensation—Employment agreements."
Consulting arrangements
Dr. Weber, the chairman of our board of directors, served as our acting chief executive officer from our inception in November 2001 to March 2003. From March 2003 to December 2004, Dr. Weber provided consulting services to us with respect to the development of our product candidates and the potential in-licensing of additional product candidates and we paid Dr. Weber $10,000 per month as compensation for his services. In August 2003, we formalized Dr. Weber's consulting arrangement with us by entering into a consulting agreement with Dr. Weber. We paid Dr. Weber an aggregate of $195,210 under the terms of our consulting arrangements with him.
In May 2003, we entered into a consulting agreement with Kenneth J. Widder, M.D., our acting chief executive officer from March 2003 to March 2004, under which Dr. Widder provided consulting services to us in connection with serving as our acting chief executive officer. We paid Dr. Widder an aggregate of $331,625 as compensation for his services under the agreement. We terminated the agreement in March 2004.
In May 2003 and August 2003, we entered into consulting agreements with a consulting company controlled by Lauren Otsuki, one of our founding stockholders, under which the consulting company provided consulting services to us with respect to the development of our
124
product candidates. We paid the consulting company an aggregate of $454,142 for services provided under the agreements. The May 2003 agreement terminated upon the consummation of our Series A preferred stock financing in August 2003 and the August 2003 agreement terminated in August 2005.
Severance and change in control arrangements
Some of our executive officers are entitled to certain severance and change in control benefits, as more fully described in "Executive compensation—Post-employment compensation."
Stock options granted to executive officers and directors
We have granted stock options to our executive officers and directors, as more fully described in "Executive compensation."
In addition, in February 2007, our board of directors granted options exercisable for 800,000 shares of our common stock to John E. Crawford, our chief financial officer, at an exercise price of $0.28 per share subject to our customary vesting schedule.
Indemnification agreements
We have entered into indemnification agreements with each of our directors and executive officers, as described in "Executive compensation—Limitation of liability and indemnification."
125
Principal stockholders
The following table sets forth information regarding beneficial ownership of our capital stock by:
- •
- each person, or group of affiliated persons, known by us to beneficially own more than five percent of our common stock;
- •
- each of our directors;
- •
- each of our named executive officers; and
- •
- all of our directors and executive officers as a group.
The percentage ownership information shown in the table is based upon 88,543,718 shares of common stock outstanding as of April 30, 2007, which assumes the conversion of all outstanding shares of preferred stock into common stock, including 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering, and the issuance of shares of common stock in this offering. The percentage ownership information assumes no exercise of the underwriters' over-allotment option.
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than five percent of our common stock. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable on or before June 29, 2007, which is 60 days after April 30, 2007. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
126
Except as otherwise noted below, the address for each person or entity listed in the table is c/o NovaCardia, Inc., 12651 High Bluff Drive, Suite 200, San Diego, CA 92130.
|
| |
| | Percentage of shares beneficially owned
|
|---|
| | Number of
shares
beneficially
owned
|
|---|
Name and address of beneficial owner
| | Before
offering
| | After
offering
|
|---|
|
| 5% or greater stockholders | | | | | | |
Domain Partners V, L.P. and its affiliates(1)
One Palmer Square, Suite 515
Princeton, New Jersey 08542 | | 22,006,365 | | 24.9 | % | % |
Forward Ventures V, L.P.(2).
9393 Town Center Drive, Suite 200
San Diego, CA 92121 | | 13,839,533 | | 15.6 | | |
InterWest Partners IX, LP(3)
2710 Sand Hill Road, Second Floor
Menlo Park, CA 94025 | | 9,194,360 | | 10.4 | | |
Montreux Equity Partners II SBIC, L.P. and its affiliates(4)
3000 Sand Hill Road, Suite 260
Menlo Park, CA 94025 | | 11,067,741 | | 12.5 | | |
Skyline Venture Partners III, L.P. and its affiliates(5)
525 University Ave., Suite 520
Palo, Alto, CA 94301 | | 13,521,117 | | 15.3 | | |
Versant Venture Capital II, L.P. and its affiliates(6)
3000 Sand Hill Road
Building 4, Suite 210
Menlo Park, CA 94025 | | 12,072,570 | | 13.6 | | |
| Directors and named executive officers | | | | | | |
| Randall E. Woods(7) | | 3,598,024 | | 3.9 | | |
| Howard C. Dittrich, M.D.(8) | | 1,678,308 | | 1.9 | | |
| Michael J. B. Tansey, M.D., Ph.D.(9) | | 250,000 | | * | | |
| Eckard Weber, M.D.(10) | | 3,466,668 | | 3.9 | | |
| Stuart J. M. Collinson, Ph.D.(2) | | 13,839,533 | | 15.6 | | |
| Nina Kjellson(3) | | 9,194,360 | | 10.4 | | |
| David G. Lowe, Ph.D.(11) | | — | | * | | |
| Camille D. Samuels(6) | | 12,072,570 | | 13.6 | | |
| Joseph T. Turner(12) | | 60,000 | | * | | |
| All executive officers and directors as a group (9 persons)(13) | | 44,709,463 | | 47.4 | | |
|
- *
- Represents beneficial ownership of less than one percent.
- (1)
- Includes 21,495,965 shares of common stock held by Domain Partners V, L.P. and 510,400 shares of common stock held by DP V Associates, L.P. The voting and investment power over these shares are held by the managing members of One Palmer Square Associates V, L.L.C., the general partner of Domain Partners V, L.P. and DP V Associates, L.P. Dr. Weber, the chairman of our board of directors, is an employee of Domain Associates, L.L.C., the manager of Domain Partners V, L.P. and DP V Associates, L.P. Dr. Weber has no ownership interest, or voting or investment power with respect to the shares held by Domain Partners V, L.P. and DP V Associates, L.P.
- (2)
- Assumes the cash exercise of warrants to purchase 184,000 shares of common stock which are exercisable within 60 days of April 30, 2007. Dr. Collinson, a member of our board of directors, is the managing member of Forward Ventures V, L.P. and has voting and investment power over the shares held by Forward Ventures V, L.P. Dr. Collinson disclaims beneficial ownership of these shares except to the extent of his pecuniary interest therein.
127
- (3)
- The voting and disposition of the shares held by InterWest Partners IX, L.P. is determined by InterWest Management Partners IX, LLC, the general partner of InterWest Partners IX, L.P. Ms. Kjellson, a member of our board of directors, is a member of InterWest Management Partners IX, LLC and has voting and investment power over the shares held by InterWest Partners IX, L.P. Ms. Kjellson disclaims beneficial ownership of these shares except to the extent of her pecuniary interest therein.
- (4)
- Includes 5,039,247 shares of common stock held by Montreux Equity Partners II SBIC, L.P. and 5,884,494 shares of common stock held by Montreux Equity Partners III SBIC, L.P. Also assumes the cash exercise of warrants to purchase 80,000 shares of common stock held by Montreux Equity Partners II SBIC, L.P. and warrants to purchase 64,000 shares of common stock held by Montreux Equity Partners III SBIC, L.P., in each case, that are exercisable within 60 days of April 30, 2007. Daniel K. Turner III, a former member of our board of directors, and Howard D. Palefsky have voting and investment power with respect to the shares held by these entities and they each disclaim beneficial ownership except to the extent of their respective pecuniary interests therein.
- (5)
- Includes 10,816,893 shares of common stock held by Skyline Venture Partners Qualified Purchaser Fund III, L.P., 2,638,530 shares of common stock held by Skyline Venture Partners Qualified Purchaser Fund IV, L.P., and 65,694 shares of common stock held by Skyline Venture Partners III, L.P. The voting and investment power over these shares are held by the general partners of each fund as follows: Skyline Venture Management IV, LLC is the general partner of Skyline Venture Partners Qualified Purchaser Fund IV, L.P. and Skyline Venture Management III, LLC is the general partner of both Skyline Venture Partners III, L.P. and Skyline Venture Partners Qualified Purchaser Fund III, L.P. As the managing directors of Skyline Venture Management IV, LLC and Skyline Venture Management III, LLC, John G. Freund and Yasunori Kaneko may be deemed to beneficially own these shares. Dr. Lowe, a member of our board of directors, is a partner of Skyline Ventures and, although he has influence over these shares, he has no voting or investment power of the shares held by these entities. Dr. Lowe, Mr. Freund and Mr. Kaneko each disclaims beneficial ownership of these shares except to the extent of his pecuniary interest therein.
- (6)
- Includes 11,568,838 shares of common stock held by Versant Venture Capital II, L.P., 219,534 shares of common stock held by Versant Affiliates Fund II-A, L.P. and 103,398 shares of common stock held by Versant Side Fund II, L.P. Also assumes the cash exercise of warrants to purchase 176,000 shares of common stock held by Versant Venture Capital II, L.P., warrants to purchase 3,200 shares of common stock held by Versant Affiliates Fund II-A, L.P. and warrants to purchase 1,600 shares of common stock held by Versant Side Fund II, L.P., in each case, that are exercisable within 60 days of April 30, 2007. The voting and investment power over these shares are held by Versant Ventures Management, L.L.C., which is the general partner of these entities. Ms. Samuels, a member of our board of directors, is the managing director of Versant Ventures II, L.L.C., the general partner of these entities, and has voting and investment power over the shares held by these entities. Ms. Samuels disclaims beneficial ownership of these shares except to the extent of her pecuniary interest therein.
- (7)
- Mr. Woods has the right to acquire these shares pursuant to outstanding options which are immediately exercisable within 60 days of April 30, 2007, of which 2,241,256 shares would be subject to repurchase by us within 60 days of April 30, 2007.
- (8)
- Dr. Dittrich has the right to acquire these shares pursuant to outstanding options which are immediately exercisable within 60 days of April 30, 2007, of which 1,084,375 shares are subject to repurchase by us within 60 days of April 30, 2007.
- (9)
- Dr. Tansey resigned as our chief medical officer in May 2006.
- (10)
- Dr. Weber has sole voting and investment power over these shares. Does not include any shares beneficially owned by Domain Partners V, L.P. and its affiliates over which Dr. Weber has no voting or investment power.
- (11)
- Dr. Lowe is a partner of Skyline Ventures and, although he has influence over the shares included in footnote (5) above, he has no voting or investment power of the shares held by the entities listed in footnote (5) above.
- (12)
- Mr. Turner has the right to acquire these shares pursuant to an outstanding option which is immediately exercisable within 60 days of April 30, 2007, of which 53,334 shares are subject to repurchase by us within 60 days of April 30, 2007.
- (13)
- Includes the shares referred to in footnotes (2), (3), (6), (7), (8), (10), (11) and (12) above. In addition, includes 400,000 shares held by John E. Crawford, our chief financial officer, all of which would be subject to repurchase by us within 60 days of April 30, 2007, and 400,000 shares subject to outstanding options held by Mr. Crawford which are immediately exercisable within 60 days of April 30, 2007, all of which would be subject to repurchase by us within 60 days of April 30, 2007.
128
Description of capital stock
Upon completion of this offering and the filing of our amended and restated certificate of incorporation, our authorized capital stock will consist of 100,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share.
The following is a summary of the rights of our common stock and preferred stock. This summary is not complete. For more detailed information, please see our amended and restated certificate of incorporation and amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is a part.
Common stock
Outstanding shares. On April 30, 2007, there were 2,726,219 shares of common stock outstanding, held of record by 10 stockholders. This amount excludes our outstanding shares of preferred stock as of April 30, 2007, which will convert into 85,268,699 shares of common stock upon completion of this offering. Based on shares of common stock outstanding as of April 30, 2007, which assumes the conversion of all outstanding shares of our preferred stock, including the shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering, and the issuance of shares of common stock in this offering, there will be shares of common stock outstanding upon the closing of this offering.
As of April 30, 2007, there were 7,609,832 shares of common stock subject to outstanding options, and up to 1,330,475 shares of common stock subject to outstanding warrants (based on the shares issuable upon exercise of these warrants as of April 30, 2007), after excluding the warrants that will terminate if not exercised prior to the effectiveness of this offering.
Voting rights. Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of the stockholders, including the election of directors. Our amended and restated certificate of incorporation and amended and restated bylaws do not provide for cumulative voting rights. Because of this, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose.
Dividends. Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of our outstanding shares of common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation. In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Rights and preferences. Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of common stock are
129
subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Fully paid and nonassessable. All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Preferred stock
On April 30, 2007, there were 85,268,699 shares of preferred stock outstanding, held of record by 18 stockholders. Upon the closing of this offering, all outstanding shares of preferred stock will have been converted into shares of common stock. See Note 9 to our financial statements for a description of the currently outstanding preferred stock. Following this offering, our certificate of incorporation will be amended and restated to delete all references to such shares of preferred stock. Under the amended and restated certificate of incorporation, our board of directors will have the authority, without further action by the stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. We have no current plans to issue any shares of preferred stock.
Warrants
As of April 30, 2007, there were outstanding warrants to purchase the following shares of our capital stock:
|
Description
| | Number of shares of common
stock after this offering
| | Weighted average exercise
price after this offering
|
|---|
|
| Series A Preferred Stock | | 357,142 | | $ | 0.84 |
| Series B Preferred Stock(1) | | 548,800 | | | 1.125 |
| Series B Preferred Stock(2) | | 973,333 | | | 1.125 |
|
- (1)
- These warrants will terminate if not exercised prior to the completion of this offering.
- (2)
- Includes a warrant held by Lighthouse Capital Partners V, L.P. that is exercisable, as of April 30, 2007, for an aggregate of 666,666 shares of Series B preferred stock. Up to an additional 266,667 shares of Series B preferred stock may become exercisable under this warrant if we borrow additional amounts under our credit facility with this lender (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price). These additional shares are not included in the chart above.
In March 2005, in connection with a loan and security agreement with Lighthouse Capital Partners V, L.P., or Lighthouse Capital Partners, we issued one warrant to this lender to purchase approximately 238,095 shares of our Series A preferred stock, at an initial exercise price of $0.84 per share. In September 2005, the number of shares of our Series A preferred
130
stock that is subject to this warrant automatically increased to the number that is equal to 2% of the aggregate advances then outstanding under the loan and security agreement divided by $0.84. This warrant is exercisable through March 2012. In March 2007, we amended this warrant to clarify that the automatic increase is capped at a maximum of 119,047 shares of our Series A preferred stock. This warrant will become exercisable for up to an aggregate of 357,142 shares of our common stock, at an exercise price equal to $0.84 per share, upon completion of this offering.
In February and June 2006, as part of a bridge financing, we issued in two tranches to certain investors unsecured convertible promissory notes in an aggregate principal amount of approximately $5.0 million and warrants that were exercisable for a number of shares of preferred stock to be issued in our next round of preferred stock financing determined based on the conversion price at which the notes were to be converted. We received aggregate consideration of $11,975 for these warrants. In September 2006, these warrants were adjusted pursuant to their terms to become exercisable for shares of our Series B preferred stock at an exercise price of $1.125 per share. Warrants exercisable for up to an aggregate of 536,533 shares of our Series B preferred stock that were issued in the first tranche will terminate upon the earlier of February 2013 or completion of an initial public offering and warrants exercisable for up to an aggregate of 268,267 shares of our Series B preferred stock that were issued in the second tranche will terminate upon the earlier of June 2013 or completion of an initial public offering. All of the warrants issued in connection with our February and June 2006 bridge financing, which are exercisable for an aggregate 804,800 shares of our Series B preferred stock, will terminate if not exercised prior to the effectiveness of this offering. In March 2007, two of our warrant purchasers, Domain Partners V, L.P. and DP V Associates, L.P., exercised their warrants and purchased 256,000 shares of our Series B preferred stock for an aggregate exercise price of $288,000. The remaining warrants, which are exercisable for an aggregate 548,800 shares of our Series B preferred stock, will terminate if not exercised prior to the effectiveness of this offering.
In July 2006, we issued one warrant to Lighthouse Capital Partners in connection with our existing loan and security agreement. This warrant was amended in October 2006. Under the terms of this warrant, as amended, the warrant is exercisable for 306,667 shares of our Series B preferred stock at an initial exercise price of $1.125 per share. This warrant is exercisable through July 2013 if not exercised prior to the consummation of this offering. This warrant will become exercisable for an aggregate of 306,667 shares of our common stock, at an exercise price equal to $1.125 per share, upon completion of this offering.
In April 2007, in connection with an amendment to our loan and security agreement to increase our credit facility by an additional $15.0 million, we issued to Lighthouse Capital Partners an additional warrant to purchase up to 933,333 shares of Series B preferred stock at an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts under our credit facility (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price).
Each of these warrants has a net exercise provision under which its holder may, in lieu of payment of the exercise price in cash, surrender the warrant and receive a net amount of shares based on the fair market value of our common stock at the time of exercise of the
131
warrant after deduction of the aggregate exercise price. Each of these warrants also contains provisions for the adjustment of the exercise price and the aggregate number of shares issuable upon the exercise of the warrant in the event of stock dividends, stock splits, reorganizations and reclassifications and consolidations.
The holders of certain of these warrants are entitled to registration rights under our amended and restated investor rights agreement, as described in "—Registration rights" below.
Registration rights
Under our amended and restated investor rights agreement, 180 days after the effective date of this offering, the holders of 85,268,699 shares of common stock, warrants to purchase up to 548,800 shares of common stock that will terminate if not exercised prior to the effectiveness of this offering and warrants to purchase up to 1,330,475 shares of common stock (based on the shares issuable upon exercise of these warrants as of April 30, 2007) that do not expire if unexercised at or prior to the closing of this offering, or their transferees, have the right to require us to register their shares with the SEC so that those shares may be publicly resold, or to include their shares in any registration statement we file.
Demand registration rights. At any time beginning 180 days after the effective date of this offering, the holders of at least a majority of the shares having registration rights have the right to demand that we file up to two registration statements. These registration rights are subject to specified conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration under certain circumstances.
Form S-3 registration rights. If we are eligible to file a registration statement on Form S-3, each holder of shares having registration rights has the right to demand that we file up to two registration statements for the holders on Form S-3 within a year of such request so long as the aggregate offering price, net of any underwriters' discounts or commissions, of securities to be sold under the registration statement on Form S-3 is at least $1,000,000, subject to specified exceptions, conditions and limitations.
"Piggyback" registration rights. If we register any securities for public sale, stockholders with registration rights will have the right to include their shares in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement, but not below 25% of the total number of shares included in the registration statement, except for this offering in which the underwriters have excluded any shares held by existing investors.
Expenses of registration. We will pay all expenses, other than underwriting discounts and commissions, relating to all demand registrations, Form S-3 registrations and piggyback registrations.
Expiration of registration rights. The registration rights described above will terminate upon the earlier of either seven years following the closing of this offering or, as to a given holder of registrable securities, when such holder of registrable securities can sell all of such holder's registrable securities pursuant to Rule 144 promulgated under the Securities Act in a single transaction without registration or any other restrictions.
132
Delaware anti-takeover law and provisions of our amended and restated certificate of incorporation and amended and restated bylaws
Delaware anti-takeover law. We are subject to Section 203 of the Delaware General Corporation Law. Section 203 generally prohibits a public Delaware corporation from engaging in a "business combination" with an "interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
- •
- prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
- •
- the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
- •
- on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock which is not owned by the interested stockholder.
Section 203 defines a business combination to include:
- •
- any merger or consolidation involving the corporation and the interested stockholder;
- •
- any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation;
- •
- subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; and
- •
- the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation.
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Amended and restated certificate of incorporation and amended and restated bylaws. Provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon the completion of this offering, may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock.
133
Among other things, our amended and restated certificate of incorporation and amended and restated bylaws:
- •
- permit our board of directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change in our control);
- •
- provide that the authorized number of directors may be changed only by resolution of the board of directors;
- •
- provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
- •
- divide our board of directors into three classes;
- •
- require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent;
- •
- provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder's notice;
- •
- do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); and
- •
- provide that special meetings of our stockholders may be called only by the chairman of the board, our chief executive officer or by the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors.
The amendment of any of these provisions would require approval by the holders of at least 662/3% of our then outstanding common stock.
NASDAQ Global Market listing
We are applying to have our common stock included for listing on the NASDAQ Global Market under the symbol NCAR.
Transfer agent and registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar's address is c/o Computershare Stockholder Services Inc., 250 Royall Street, Canton, MA 02021.
134
Shares eligible for future sale
Immediately prior to this offering, there has been no public market for our common stock. Future sales of substantial amounts of common stock in the public market could adversely affect prevailing market prices. Furthermore, since only a limited number of shares will be available for sale shortly after this offering because of contractual and legal restrictions on resale described below, sales of substantial amounts of common stock in the public market after the restrictions lapse could adversely affect the prevailing market price for our common stock as well as our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of April 30, 2007, and after giving pro forma effect to the automatic conversion of all of our outstanding shares of preferred stock, including the 548,800 shares of Series B preferred stock issued upon the assumed cash exercise of warrants that will terminate if not exercised prior to the effectiveness of this offering, upon the closing of this offering, shares of our common stock will be outstanding, assuming no exercise of the underwriters' over-allotment option and no exercise of outstanding options or outstanding warrants. All of the shares sold in this offering will be freely tradable unless held by one of our affiliates. Except as set forth below, the remaining shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements. These remaining shares will generally become available for sale in the public market as follows:
- •
- shares of common stock will be eligible for immediate sale upon the closing of this offering;
- •
- shares of common stock will be eligible for sale under Rule 144 or Rule 701 upon expiration of lock-up agreements 180 days after the date of this prospectus, unless the lock-up is otherwise extended; and
- •
- shares of common stock will be eligible for sale from time to time thereafter upon expiration of their respective one-year holding periods, but could be sold earlier if the holders exercise any available registration rights.
Rule 144
In general, under Rule 144 under the Securities Act, as in effect on the date of this prospectus, a person who has beneficially owned shares of our common stock for at least one year would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
- •
- one percent of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or
- •
- the average weekly trading volume of our common stock on the NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
135
Rule 144(k)
Pursuant to Rule 144(k) promulgated under the Securities Act as in effect on the date of this prospectus, a person who is not deemed to have been one of our affiliates at any time during the 90 days preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years, including the holding period of any prior owner other than an affiliate, is entitled to sell the shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144. shares of our common stock will qualify for resale under Rule 144(k) within 180 days of the date of this prospectus.
Rule 701
Rule 701 under the Securities Act, as in effect on the date of this prospectus, permits resales of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers, directors or consultants who purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and under "Underwriting" and will become eligible for sale at the expiration of those agreements.
Lock-up agreements
We, along with our directors, executive officers and substantially all of our other stockholders, optionholders and warrantholders, have agreed with the underwriters that for a period of 180 days after the date of this prospectus, we or they will not, without the prior written consent of J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC and subject to other limited exceptions, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase an option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or securities convertible into or exchangeable or exercisable for shares of common stock, enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction is to be settled by delivery of shares of common stock or such other securities, in cash or otherwise, or publicly disclose the intention to make any such offer, pledge, sale, contract, grant, transfer or disposition, or enter into any such swap or other agreement. J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC, may, in their joint discretion, at any time without prior notice, release for sale in the public market all or any portion of the shares subject to the lock-up agreements, subject to certain limitations.
The 180-day restricted period described in the preceding paragraph will be extended if:
- •
- during the last 17 days of the 180-day lock-up period, we issue a earnings release or material news or a material event relating to us occurs; or
- •
- prior to the expiration of the 180-day lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day lock-up period, in which case the lock-up period will be extended until 18 days following the date of
136
the release of the earnings results or the occurrence of the material news or material event, unless J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC waive such extension in writing.
Upon expiration of the 180-day restricted period, certain of our directors, warrantholders and holders of more than 5% of our capital stock have the right to require us to register their shares under the Securities Act. See "—Registration rights" below.
Registration rights
Upon the closing of this offering, the holders of 85,268,699 shares of common stock, warrants to purchase up to 548,800 shares of common stock that will terminate if not exercised prior to the effectiveness of this offering and warrants to purchase up to 1,330,475 shares of common stock (based on the shares issuable upon exercise of these warrants as of April 30, 2007) that do not expire if unexercised at or prior to the closing of this offering will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the 180-day lock-up arrangement described above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates, immediately upon the effectiveness of this registration. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock. See "Description of capital stock—Registration rights" for additional information.
Equity incentive plans
We intend to file with the SEC a registration statement under the Securities Act covering the shares of common stock reserved for issuance under our 2003 equity incentive plan, our 2007 equity incentive plan, 2007 non-employee directors' stock option plan and our 2007 employee stock purchase plan. The registration statement is expected to be filed and become effective as soon as practicable after the completion of this offering. Accordingly, shares registered under the registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the 180-day lock-up arrangement described above, if applicable.
137
Material U.S. federal income tax
consequences to non-U.S. holders
The following is a general discussion of the material U.S. federal income tax consequences of the ownership and disposition of our common stock to a non-U.S. holder. For the purpose of this discussion, a non-U.S. holder is any holder that for U.S. federal income tax purposes is not a U.S. person. For purposes of this discussion, the term U.S. person means:
- •
- an individual citizen or resident of the U.S.;
- •
- a corporation or other entity taxable as a corporation or a partnership or entity taxable as a partnership created or organized in the U.S. or under the laws of the U.S. or any political subdivision thereof;
- •
- an estate whose income is subject to U.S. federal income tax regardless of its source; or
- •
- a trust (x) whose administration is subject to the primary supervision of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (y) which has made an election to be treated a U.S. person.
If a partnership holds common stock, the tax treatment of a partner will generally depend on the status of the partner and upon the activities of the partnership. Accordingly, we urge partnerships that hold our common stock and partners in such partnerships to consult their tax advisors.
This discussion assumes that a non-U.S. holder will hold our common stock issued pursuant to the offering as a capital asset (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant in light of a non-U.S. holder's special tax status or special tax situations. U.S. expatriates, life insurance companies, tax-exempt organizations, dealers in securities or currency, banks or other financial institutions, investors whose functional currency is other than the U.S. dollar, and investors that hold common stock as part of a hedge, straddle or conversion transaction are among those categories of potential investors that are subject to special rules not covered in this discussion. This discussion does not address any tax consequences arising under the laws of any state, local or non-U.S. taxing jurisdiction. Furthermore, the following discussion is based on current provisions of the Internal Revenue Code and Treasury Regulations and administrative and judicial interpretations thereof, all as in effect on the date hereof, and all of which are subject to change, possibly with retroactive effect. Accordingly, we urge each non-U.S. holder to consult a tax advisor regarding the U.S. federal, state, local and non-U.S. income and other tax consequences of acquiring, holding and disposing of shares of our common stock.
Dividends
We have not paid any dividends on our common stock and we do not plan to pay any dividends for the foreseeable future. However, if we do pay dividends on our common stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those dividends exceed our current and accumulated earnings and
138
profits, the dividends will constitute a return of capital and will first reduce a holder's basis, but not below zero, and then will be treated as gain from the sale of stock.
Any dividend (out of earnings and profits) paid to a non-U.S. holder of common stock generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable tax treaty. To receive a reduced treaty rate, a non-U.S. holder must provide us with an IRS Form W-8BEN or other appropriate version of Form W-8 certifying qualification for the reduced rate.
Dividends received by a non-U.S. holder that are effectively connected with a U.S. trade or business conducted by the non-U.S. holder are exempt from such withholding tax. To obtain this exemption, a non-U.S. holder must provide us with an IRS Form W-8ECI properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits, subject to any applicable tax treaty providing otherwise. In addition to the graduated tax described above, dividends received by corporate non-U.S. holders that are effectively connected with a U.S. trade or business of the corporate non-U.S. holder may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable tax treaty.
A non-U.S. holder of common stock that is eligible for a reduced rate of withholding tax pursuant to a tax treaty may obtain a refund of any excess amounts currently withheld if an appropriate claim for refund is filed with the IRS.
Gain on disposition of common stock
A non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
- •
- the gain is effectively connected with a U.S. trade or business of the non-U.S. holder (which gain, in the case of a corporate non-U.S. holder, must also be taken into account for branch profits tax purposes), subject to any applicable tax treaty providing otherwise;
- •
- the non-U.S. holder is an individual who is present in the U.S. for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met; or
- •
- our common stock constitutes a U.S. real property interest by reason of our status as a "U.S. real property holding corporation" for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the disposition or the holder's holding period for our common stock. We believe that we are not currently, and that we will not become, a "U.S. real property holding corporation" for U.S. federal income tax purposes.
Backup withholding and information reporting
Generally, we must report annually to the IRS the amount of dividends paid, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder. Pursuant to tax treaties or other agreements, the IRS may make its reports available to tax authorities in the recipient's country of residence.
139
Payments of dividends or of proceeds on the disposition of stock made to a non-U.S. holder may be subject to backup withholding (currently at a rate of 28%) unless the non-U.S. holder establishes an exemption, for example, by properly certifying its non-U.S. status on a Form W-8BEN or another appropriate version of Form W-8. Notwithstanding the foregoing, backup withholding may apply if either we or our paying agent has actual knowledge, or reason to know, that the holder is a U.S. person.
Backup withholding is not an additional tax. Rather, the U.S. income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund may be obtained, provided that the required information is furnished to the IRS.
140
Underwriting
J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC are the representatives of the underwriters. Subject to the terms and conditions of the underwriting agreement, the underwriters named below, through their representatives, have severally agreed to purchase from us the following respective numbers of shares of our common stock:
|
Underwriter
| | Number of shares
|
|---|
|
| J.P. Morgan Securities Inc. | | |
| Credit Suisse Securities (USA) LLC | | |
| Pacific Growth Equities, LLC | | |
| First Albany Capital Inc. | | |
| | |
|
| | Total | | |
|
The underwriting agreement provides that the underwriters are obligated to purchase all of the shares of our common stock in this offering if any are purchased, other than those shares covered by the over-allotment option described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated.
The underwriters will offer shares of our common stock in a public offering in the United States and to institutional investors elsewhere. Each underwriter may offer and sell shares of our common stock anywhere in the world where it is legally permitted to do so. There are no minimum or maximum limits on how many shares of common stock may be offered or sold in any particular country or region. Some of the underwriters are expected to make offers and sales both inside and outside the United States through their respective selling agents. Any offers or sales in the United States will be conducted by broker-dealers registered with the SEC.
We have granted to the underwriters a 30-day option to purchase on a pro rata basis up to additional shares at the initial public offering price listed on the cover page of this prospectus, less the underwriting discounts and commissions. The option may be exercised only to cover any over-allotments of common stock.
The total underwriting discounts and commissions paid to the underwriters will be % of the total offering price of the common stock. The following table summarizes the compensation and estimated expenses we will pay:
|
| | Without
over-allotment exercise
| | With
over-allotment exercise
|
|---|
|
| Per share | | $ | | | $ | |
| Total | | | | | | |
|
The underwriters propose to offer shares of common stock initially at the public offering price on the cover page of this prospectus. Any shares of common stock sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. Any such securities dealers may resell any shares of common stock purchased from the underwriters to other brokers or dealers at a discount of up to $ per share from
141
the initial public offering price. If all of the shares of common stock are not sold at the initial offering price, the representatives may change the offering price and the other selling terms.
See "Shares eligible for future sale" for a discussion of certain transfer restrictions.
We have applied for listing of our common stock on the NASDAQ Global Market under the symbol NCAR.
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by a negotiation between us and the underwriters and will not necessarily reflect the market price of the common stock following the offering. The principal factors that will be considered in determining the public offering price will include:
- •
- the valuation multiples of publicly traded companies that we and the representatives believe to be comparable to us;
- •
- the information in this prospectus and otherwise available to the underwriters;
- •
- market conditions for initial public offerings;
- •
- the history and the prospects for the industry in which we compete;
- •
- an assessment of our management, our past and present operations and the prospects for, and timing of, our future sales;
- •
- the present state of our development and our current financial condition;
- •
- the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours; and
- •
- the general condition of the securities markets at the time of this offering.
Neither we nor the underwriters can assure you that the initial public offering price will correspond to the price at which the common stock will trade in the public market subsequent to the offering or that an active trading market for the common stock will develop and continue after the offering.
In connection with the offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Securities Exchange Act of 1934, as amended, or the Securities Exchange Act, as follows:
- •
- Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum.
- •
- Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option and/or purchasing shares in the open market.
142
- •
- Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering.
- •
- Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
These stabilizing transactions and syndicate covering transactions may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. If these activities are commenced, they are required to be conducted in accordance with applicable laws and regulations, and they may be discontinued at any time. These transactions may be effected on the NASDAQ Global Market or otherwise and, if commenced, may be discontinued at any time.
The underwriters do not expect sales to discretionary accounts to exceed 5% of the total number of shares offered.
We have agreed that, subject to certain limited exceptions, for a period of 180 days after the date of this prospectus, we will not (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, our ordinary shares or any securities convertible into or exercisable or exchangeable for our ordinary shares or (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of our ordinary shares, whether any such transaction described in (i) or (ii) above is to be settled by delivery of our ordinary shares or such other securities, in cash or otherwise, without the prior written consent of the J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC, other than the ordinary shares to be sold in this initial public offering and any of our ordinary shares issued upon the exercise of options granted under our existing employee share option plans. However, in the event that either, (i) during the last 17 days of the lock-up period, we issue an earnings release or material news or a material event relating to us occurs or (ii) prior to the expiration of the lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the lock-up period, then the expiration of the lock-up period will be extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC waive such extension in writing.
Our executive officers, directors and stockholders have entered into lock-up agreements pursuant to which they have agreed that they will not, for a period of 180 days after the date of this prospectus without the prior written consent of J.P. Morgan Securities Inc. and Credit
143
Suisse Securities (USA) LLC and subject to other limited exceptions, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase an option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or securities convertible into or exchangeable or exercisable for shares of common stock, enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction is to be settled by delivery of shares of common stock or such other securities, in cash or otherwise, or publicly disclose the intention to make any such offer, pledge, sale, contract, grant, transfer or disposition, or enter into any such swap or other agreement. The lock-up agreements permit transfers of our common stock subject to certain restrictions. J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC may, in their joint discretion, at any time and without notice, release for sale in the public market all or any portion of the shares subject to the lock-up agreements subject to certain limitations. After the 180-day lock-up period, these shares may be sold, subject to applicable securities laws. However, in the event that either (i) during the last 17 days of the lock-up period, we issue an earnings release or material news or a material event relating to us occurs; or (ii) prior to the expiration of the lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the lock-up period, the lock-up period will be extended until 18 days following the date of the release of the earnings results or the occurrence of the material news or material event, unless J.P. Morgan Securities Inc. and Credit Suisse Securities (USA) LLC waive such extension in writing.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in that respect.
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering, and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling group members for sale to their on-line brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make Internet distributions on the same basis as other allocations.
144
Legal matters
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Cooley Godward Kronish LLP, San Diego, California. The underwriters are being represented by Latham & Watkins LLP, San Diego, California. As of the date of this prospectus, GC&H Investments, LLC, an entity comprised of partners and associates of Cooley Godward Kronish LLP, beneficially owns an aggregate of 54,084 shares of our preferred stock, which will convert into an aggregate of 54,084 shares of our common stock upon the completion of this offering. Latham & Watkins LLP and certain attorneys and investment funds affiliated with the firm collectively own an aggregate of 27,042 shares of our preferred stock, which will convert into an aggregate of 27,042 shares of our common stock upon the completion of this offering.
Experts
Ernst & Young LLP, independent registered public accounting firm, have audited our financial statements at December 31, 2005 and 2006, and for each of the three years in the period ended December 31, 2006, as set forth in their report. We have included our financial statements in this prospectus and elsewhere in the registration statement in reliance upon Ernst & Young LLP's report, given on their authority as experts in accounting and auditing.
Where you can find more information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933, as amended, with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC's website at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
Upon the closing of this offering, we will be subject to the information reporting requirements of the Securities Exchange Act of 1934, as amended, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and web site of the SEC referred to above. We also maintain a website atwww.novacardia.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
145
NovaCardia, Inc.
Index to financial statements
| Report of independent registered public accounting firm | | F-2 |
Balance sheets |
|
F-3 |
Statements of operations |
|
F-4 |
Statements of stockholders' equity (deficit) |
|
F-5 |
Statements of cash flows |
|
F-8 |
Notes to financial statements |
|
F-9 |
F-1
Report of independent registered public accounting firm
The Board of Directors and Stockholders
NovaCardia, Inc.
We have audited the accompanying balance sheets of NovaCardia, Inc. (a development stage company) as of December 31, 2005 and 2006 and the related statements of operations, stockholders' equity (deficit), and cash flows for each of the three years in the period ended December 31, 2006. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of NovaCardia, Inc. (a development stage company) at December 31, 2005 and 2006, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2006, in conformity with generally accepted accounting principles in the United States.
As discussed in Note 1 to the financial statements, on January 1, 2006, NovaCardia, Inc. changed its method of accounting for share-based payments as required by Statement of Financial Accounting Standards No. 123 (revised in 2004),Share-Based Payment.
San Diego, California
March 26, 2007
F-2
NovaCardia, Inc.
(a development stage company)
Balance sheets
| |
| | December 31,
| |
| |
| |
|---|
| |
| | Pro forma
stockholders'
equity at
March 31, 2007
| |
|---|
| | March 31,
2007
| |
|---|
| | 2005
| | 2006
| |
|---|
| |
| |
| |
| | (unaudited)
| | (unaudited)
| |
|---|
| Assets | | | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | | | |
| | Cash and cash equivalents | | $ | 2,458,219 | | $ | 12,277,584 | | $ | 28,601,177 | | | | |
| | Short-term investments | | | — | | | 19,246,306 | | | 13,908,097 | | | | |
| | Prepaid and other current assets | | | 89,314 | | | 552,738 | | | 629,736 | | | | |
| | Restricted cash | | | 105,437 | | | — | | | — | | | | |
| | |
| | | | |
| Total current assets | | | 2,652,970 | | | 32,076,628 | | | 43,139,010 | | | | |
Property and equipment, net |
|
|
86,875 |
|
|
219,717 |
|
|
224,407 |
|
|
|
|
| Other assets | | | 177,582 | | | 3,604,592 | | | 5,276,854 | | | | |
| | |
| | | | |
| Total assets | | $ | 2,917,427 | | $ | 35,900,937 | | $ | 48,640,271 | | | | |
| | |
| | | | |
Liabilities and stockholders' equity (deficit) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities: | | | | | | | | | | | | | |
| | Accounts payable | | $ | 1,081,399 | | $ | 2,967,843 | | $ | 5,379,163 | | | | |
| | Accrued liabilities | | | 241,364 | | | 1,174,138 | | | 1,779,817 | | | | |
| | Current portion of long term-debt | | | 1,474,309 | | | 1,635,594 | | | 1,678,601 | | | | |
| | |
| | | | |
| Total current liabilities | | | 2,797,072 | | | 5,777,575 | | | 8,837,581 | | | | |
| Deferred rent | | | 6,346 | | | — | | | — | | | | |
| Long-term debt, less current portion | | | 3,024,230 | | | 1,388,635 | | | 952,509 | | | | |
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity (deficit): |
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Convertible preferred stock, $0.001 par value: | | | | | | | | | | | | | |
| | | Series A convertible preferred stock, 26,547,619 shares authorized; 26,190,477 shares issued and outstanding at December 31, 2005 and 2006 and March 31, 2007 (unaudited); aggregate liquidation preference of $22,000,000; no shares issued and outstanding pro forma (unaudited) | | | 26,190 | | | 26,190 | | | 26,190 | | $ | — | |
| | | Series B convertible preferred stock, 62,000,000 shares authorized; no shares, 42,822,222 shares and 59,078,222 shares issued and outstanding at December 31, 2006 and March 31, 2007 (unaudited), respectively; aggregate liquidation preference of $66,463,000; no shares issued and outstanding pro forma (unaudited) | | | — | | | 42,822 | | | 59,078 | | | — | |
| | Common stock, $0.001 par value; 105,000,000 shares authorized; 2,076,219 shares, 2,326,219 shares and 2,726,219 shares issued and outstanding at December 31, 2005 and 2006 and March 31, 2007 (unaudited), respectively; 87,994,918 shares issued and outstanding pro forma (unaudited) | | | 2,076 | | | 2,326 | | | 2,726 | | | 87,995 | |
| | Additional paid-in capital | | | 22,102,427 | | | 71,605,516 | | | 90,218,148 | | | 90,218,147 | |
| | Accumulated other comprehensive income | | | — | | | 8,190 | | | 2,804 | | | 2,804 | |
| | Deficit accumulated during the development stage | | | (25,040,914 | ) | | (42,950,317 | ) | | (51,458,765 | ) | | (51,458,765 | ) |
| | |
| |
| Total stockholders' equity (deficit) | | | (2,910,221 | ) | | 28,734,727 | | | 38,850,181 | | $ | 38,850,181 | |
| | |
| |
| |
| Total liabilities and stockholders' equity (deficit) | | $ | 2,917,427 | | $ | 35,900,937 | | $ | 48,640,271 | | | | |
| | |
| | | | |
| |
See accompanying notes.
F-3
NovaCardia, Inc.
(a development stage company)
Statements of operations
| |
| |
| |
| |
| |
| |
| | Period from
November 30,
2001 (inception)
through
March 31,
2007
| |
|---|
| | Years ended December 31,
| | Three months ended March 31,
| |
|---|
| | 2004
| | 2005
| | 2006
| | 2006
| | 2007
| |
|---|
| |
| |
| |
| |
| | (unaudited)
| | (unaudited)
| | (unaudited)
| |
|---|
| Operating expenses: | | | | | | | | | | | | | | | | | | | |
| | Development | | $ | 6,343,032 | | $ | 9,023,431 | | $ | 14,184,309 | | $ | 1,776,186 | | $ | 7,576,344 | | $ | 41,699,167 | |
| | Selling, general and administrative | | | 1,781,757 | | | 1,972,987 | | | 2,123,896 | | | 402,004 | | | 1,219,305 | | | 8,572,739 | |
| | |
| |
| Total operating expenses | | | 8,124,789 | | �� | 10,996,418 | | | 16,308,205 | | | 2,178,190 | | | 8,795,649 | | | 50,271,906 | |
| | |
| |
| Loss from operations | | | (8,124,789 | ) | | (10,996,418 | ) | | (16,308,205 | ) | | (2,178,190 | ) | | (8,795,649 | ) | | (50,271,906 | ) |
| Other income (expense): | | | | | | | | | | | | | | | | | | | |
| | Interest income | | | 173,012 | | | 139,374 | | | 560,342 | | | 19,795 | | | 374,910 | | | 1,322,120 | |
| | Interest expense | | | — | | | (203,354 | ) | | (2,161,540 | ) | | (245,968 | ) | | (87,709 | ) | | (2,508,979 | ) |
| | |
| |
| Total other income (expense) | | | 173,012 | | | (63,980 | ) | | (1,601,198 | ) | | (226,173 | ) | | 287,201 | | | (1,186,859 | ) |
| | |
| |
| Net loss | | | (7,951,777 | ) | | (11,060,398 | ) | | (17,909,403 | ) | | (2,404,363 | ) | | (8,508,448 | ) | | (51,458,765 | ) |
| Deemed dividend related to beneficial conversion feature on Series B preferred stock | | | — | | | — | | | — | | | — | | | (8,080,000 | ) | | (8,080,000 | ) |
| | |
| |
| Net loss attributable to common stockholders | | $ | (7,951,777 | ) | $ | (11,060,398 | ) | $ | (17,909,403 | ) | $ | (2,404,363 | ) | $ | (16,588,448 | ) | $ | (59,538,765 | ) |
| | |
| |
| Basic and diluted net loss per share attributable to common stockholders | | $ | (6.09 | ) | $ | (5.96 | ) | $ | (8.37 | ) | $ | (1.21 | ) | $ | (7.13 | ) | | | |
| | |
| | | | |
| Shares used to compute basic and diluted net loss per share attributable to common stockholders | | | 1,305,243 | | | 1,854,896 | | | 2,138,967 | | | 1,984,007 | | | 2,326,219 | | | | |
| | |
| | | | |
| Pro forma basic and diluted net loss per share attributable to common stockholders | | | | | | | | $ | (0.45 | ) | | | | $ | (0.23 | ) | | | |
| | | | | | | | |
| | | | |
| | | | |
| Shares used to compute pro forma basic and diluted net loss per share attributable to common stockholders | | | | | | | | | 40,178,881 | | | | | | 73,147,985 | | | | |
| | | | | | | | |
| | | | |
| | | | |
| |
See accompanying notes.
F-4
NovaCardia, Inc.
(a development stage company)
Statements of stockholders' equity (deficit)
Period from November 30, 2001 (inception) through March 31, 2007
| |
| | Series A
convertible
preferred stock
| | Series B
convertible
preferred stock
| |
| |
| |
| |
| |
| |
| |
|---|
| | Common stock
| |
| | Accumulated
other
comprehensive
income
(loss)
| | Deficit
accumulated
during the
development
stage
| |
| |
|---|
| | Additional
paid-in
capital
| | Total
stockholders'
equity
(deficit)
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Shares
| | Amount
| |
|---|
| |
| | Issuance of common stock to founders for cash at $0.001 per share in December 2001 | | — | | $ | — | | — | | $ | — | | 1,000,000 | | $ | 1,000 | | $ | — | | $ | — | | $ | — | | $ | 1,000 | |
| | Net loss and comprehensive loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (61,807 | ) | | (61,807 | ) |
| | |
| |
| Balance at December 31, 2001 | | — | | | — | | — | | | — | | 1,000,000 | | | 1,000 | | | — | | | — | | | (61,807 | ) | | (60,807 | ) |
| | Net loss and comprehensive loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (306,235 | ) | | (306,235 | ) |
| | |
| |
| Balance at December 31, 2002 | | — | | | — | | — | | | — | | 1,000,000 | | | 1,000 | | | — | | | — | | | (368,042 | ) | | (367,042 | ) |
| | Issuance of common stock to founders for cash at $0.001 per share in August 2003 | | — | | | — | | — | | | — | | 1,070,000 | | | 1,070 | | | — | | | — | | | — | | | 1,070 | |
| | Issuance of Series A preferred stock for cash, conversion of debt and intellectual property at $0.84 per share, net of $80,332 of offering costs, in August 2003 | | 26,190,477 | | | 26,190 | | — | | | — | | — | | | — | | | 21,893,478 | | | — | | | — | | | 21,919,668 | |
| | Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Unrealized gain on investment securities | | — | | | — | | — | | | — | | — | | | — | | | — | | | 12,390 | | | — | | | 12,390 | |
| | | Net loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (5,660,697 | ) | | (5,660,697 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (5,648,307 | ) |
| | |
| |
| Balance at December 31, 2003 | | 26,190,477 | | | 26,190 | | — | | | — | | 2,070,000 | | | 2,070 | | | 21,893,478 | | | 12,390 | | | (6,028,739 | ) | | 15,905,389 | |
| | Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Unrealized loss on investment securities | | — | | | — | | — | | | — | | — | | | — | | | — | | | (26,520 | ) | | — | | | (26,520 | ) |
| | | Net loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (7,951,777 | ) | | (7,951,777 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (7,978,297 | ) |
| | |
| |
| Balance at December 31, 2004 | | 26,190,477 | | | 26,190 | | — | | | — | | 2,070,000 | | | 2,070 | | | 21,893,478 | | | (14,130 | ) | | (13,980,516 | ) | | 7,927,092 | |
See accompanying notes.
F-5
NovaCardia, Inc.
(a development stage company)
Statements of stockholders' equity (deficit) (continued)
Period from November 30, 2001 (inception) through March 31, 2007
| |
| | Series A
convertible
preferred stock
| | Series B
convertible
preferred stock
| |
| |
| |
| |
| |
| |
| |
|---|
| | Common stock
| |
| | Accumulated
other
comprehensive
income
(loss)
| | Deficit
accumulated
during the
development
stage
| |
| |
|---|
| | Additional
paid-in
capital
| | Total
stockholders'
equity
(deficit)
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Shares
| | Amount
| |
|---|
| |
| Balance at December 31, 2004 | | 26,190,477 | | 26,190 | | — | | — | | 2,070,000 | | 2,070 | | 21,893,478 | | (14,130 | ) | (13,980,516 | ) | 7,927,092 | |
| | Exercise of common stock options for cash at $0.10 per share in November 2005 | | — | | — | | — | | — | | 6,219 | | 6 | | 616 | | — | | — | | 622 | |
| | Issuance of warrants in connection with debt financing | | — | | — | | — | | — | | — | | — | | 208,333 | | — | | — | | 208,333 | |
| | Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | |
| | | Unrealized gain on investment securities | | — | | — | | — | | — | | — | | — | | — | | 14,130 | | — | | 14,130 | |
| | | Net loss | | — | | — | | — | | — | | — | | — | | — | | — | | (11,060,398 | ) | (11,060,398 | ) |
| | | | | | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive loss | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (11,046,268 | ) |
| | |
| |
| Balance at December 31, 2005 | | 26,190,477 | | 26,190 | | — | | — | | 2,076,219 | | 2,076 | | 22,102,427 | | — | | (25,040,914 | ) | (2,910,221 | ) |
| | Issuance of Series B preferred stock for cash and conversion of debt at $1.125 per share, net of $205,248 of offering costs, in September 2006 | | — | | — | | 42,822,222 | | 42,822 | | — | | — | | 47,926,930 | | — | | — | | 47,969,752 | |
| | Stock-based compensation | | — | | — | | — | | — | | — | | — | | 62,086 | | — | | — | | 62,086 | |
| | Exercise of common stock options for cash at $0.10 per share in August 2006 | | — | | — | | — | | — | | 250,000 | | 250 | | 24,750 | | — | | — | | 25,000 | |
| | Issuance of warrants in connection with debt financing | | — | | — | | — | | — | | — | | — | | 861,579 | | — | | — | | 861,579 | |
| | Beneficial conversion feature in connection with convertible debt financing | | — | | — | | — | | — | | — | | — | | 627,744 | | — | | — | | 627,744 | |
| | Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | |
| | | Unrealized gain on investment securities | | — | | — | | — | | — | | — | | — | | — | | 8,190 | | — | | 8,190 | |
| | | Net loss | | — | | — | | — | | — | | — | | — | | — | | — | | (17,909,403 | ) | (17,909,403 | ) |
| | | | | | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive loss | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (17,901,213 | ) |
| | |
| |
| Balance at December 31, 2006 | | 26,190,477 | | 26,190 | | 42,822,222 | | 42,822 | | 2,326,219 | | 2,326 | | 71,605,516 | | 8,190 | | (42,950,317 | ) | 28,734,727 | |
See accompanying notes.
F-6
NovaCardia, Inc.
(a development stage company)
Statements of stockholders' equity (deficit) (continued)
Period from November 30, 2001 (inception) through March 31, 2007
| |
| | Series A
convertible
preferred stock
| | Series B
convertible
preferred stock
| |
| |
| |
| |
| |
| |
| |
|---|
| | Common stock
| |
| | Accumulated
other
comprehensive
income
(loss)
| | Deficit
accumulated
during the
development
stage
| |
| |
|---|
| | Additional
paid-in
capital
| | Total
stockholders'
equity
(deficit)
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Shares
| | Amount
| |
|---|
| |
| Balance at December 31, 2006 | | 26,190,477 | | | 26,190 | | 42,822,222 | | | 42,822 | | 2,326,219 | | | 2,326 | | | 71,605,516 | | | 8,190 | | | (42,950,317 | ) | | 28,734,727 | |
| | Issuance of Series B preferred stock for cash at $1.125 per share, net of $23,416 of offering costs, in March 2007 (unaudited) | | — | | | — | | 16,000,000 | | | 16,000 | | — | | | — | | | 17,960,584 | | | — | | | — | | | 17,976,584 | |
| | Stock-based compensation (unaudited) | | — | | | — | | — | | | — | | — | | | — | | | 252,704 | | | — | | | — | | | 252,704 | |
| | Exercise of common stock options for cash at $0.28 per share in February 2007 (unaudited) | | — | | | — | | — | | | — | | 400,000 | | | 400 | | | 111,600 | | | — | | | — | | | 112,000 | |
| | Exercise of preferred stock warrants for cash at $1.125 per share in March 2007 (unaudited) | | — | | | — | | 256,000 | | | 256 | | — | | | — | | | 287,744 | | | — | | | — | | | 288,000 | |
| | Comprehensive loss (unaudited): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Unrealized loss of investment securities (unaudited) | | — | | | — | | — | | | — | | — | | | — | | | — | | | (5,386 | ) | | — | | | (5,386 | ) |
| | | Net loss (unaudited) | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (8,508,448 | ) | | (8,508,448 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| | | Total comprehensive loss (unaudited) | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (8,513,834 | ) |
| | |
| |
| Balance at March 31, 2007 (unaudited) | | 26,190,477 | | $ | 26,190 | | 59,078,222 | | $ | 59,078 | | 2,726,219 | | $ | 2,726 | | $ | 90,218,148 | | $ | 2,804 | | $ | (51,458,765 | ) | $ | 38,850,181 | |
| | |
| |
See accompanying notes.
F-7
NovaCardia, Inc.
(a development stage company)
Statements of cash flows
| |
| | Years ended December 31,
| | Three months
ended March 31,
| |
| |
|---|
| | Period from
November 30, 2001
(inception) through
March 31, 2007
| |
|---|
| | 2004
| | 2005
| | 2006
| | 2006
| | 2007
| |
|---|
| |
| | | | | | | | | | | | | (unaudited) | | | (unaudited) | | | (unaudited) | |
| Operating activities | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (7,951,777 | ) | $ | (11,060,398 | ) | $ | (17,909,403 | ) | $ | (2,404,363 | ) | $ | (8,508,448 | ) | $ | (51,458,765 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | | | | | |
| | Depreciation | | | 51,045 | | | 59,485 | | | 53,978 | | | 15,059 | | | 11,232 | | | 187,556 | |
| | Stock-based compensation | | | — | | | — | | | 62,086 | | | — | | | 252,704 | | | 314,790 | |
| | Amortization of premium (discount) on investment securities | | | 425,048 | | | 83,211 | | | (158,159 | ) | | — | | | (232,254 | ) | | 309,275 | |
| | Non-cash interest expense | | | — | | | 40,986 | | | 1,540,002 | | | 134,846 | | | 15,689 | | | 1,596,677 | |
| | Issuance of preferred stock in exchange for intellectual property | | | — | | | — | | | — | | | — | | | — | | | 750,000 | |
| | Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | | | | |
| | | Prepaid and other current assets | | | 113,702 | | | 5,267 | | | (3,953,189 | ) | | (222,421 | ) | | (783,107 | ) | | (4,835,845 | ) |
| | | Accounts payable | | | 468,366 | | | 445,944 | | | 1,886,444 | | | (344,007 | ) | | 1,884,569 | | | 4,852,412 | |
| | | Accrued expenses and deferred rent | | | 150,427 | | | 65,235 | | | 1,088,297 | | | 58,631 | | | 252,666 | | | 1,588,673 | |
| | |
| |
| Net cash used in operating activities | | | (6,743,189 | ) | | (10,360,270 | ) | | (17,389,944 | ) | | (2,762,255 | ) | | (7,106,949 | ) | | (46,695,227 | ) |
| Investing activities | | | | | | | | | | | | | | | | | | | |
| Purchases of property and equipment | | | (65,749 | ) | | (9,054 | ) | | (186,820 | ) | | (501 | ) | | (15,922 | ) | | (411,963 | ) |
| Purchases of investment securities | | | (2,310,244 | ) | | (1,260,095 | ) | | (22,829,957 | ) | | — | | | (2,434,923 | ) | | (43,578,568 | ) |
| Maturities of investment securities | | | 11,650,000 | | | 4,964,000 | | | 3,750,000 | | | — | | | 8,000,000 | | | 29,364,000 | |
| Restricted cash | | | (45,000 | ) | | (60,437 | ) | | 105,437 | | | 45,000 | | | — | | | — | |
| | |
| |
| Net cash provided by (used in) investing activities | | | 9,229,007 | | | 3,634,414 | | | (19,161,340 | ) | | 44,499 | | | 5,549,155 | | | (14,626,531 | ) |
| Financing activities | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock, net | | | — | | | 622 | | | 25,000 | | | — | | | 112,000 | | | 139,692 | |
| Costs related to initial public offering | | | — | | | — | | | — | | | — | | | (102,078 | ) | | (102,078 | ) |
| Issuance of preferred stock, net | | | — | | | — | | | 42,777,883 | | | — | | | 17,976,584 | | | 80,724,135 | |
| Issuance of warrants | | | — | | | — | | | 12,076 | | | 7,186 | | | 288,000 | | | 300,076 | |
| Proceeds from notes payable | | | — | | | 5,000,000 | | | 3,000,000 | | | — | | | — | | | 8,000,000 | |
| Repayments of notes payable | | | — | | | (501,461 | ) | | (4,474,310 | ) | | (354,355 | ) | | (393,119 | ) | | (5,368,890 | ) |
| Proceeds from related party stockholder loans | | | — | | | — | | | 5,030,000 | | | 3,018,000 | | | — | | | 6,230,000 | |
| | |
| |
| Net cash provided by financing activities | | | — | | | 4,499,161 | | | 46,370,649 | | | 2,670,831 | | | 17,881,387 | | | 89,922,935 | |
| | |
| |
| Net increase (decrease) in cash and cash equivalents | | | 2,485,818 | | | (2,226,695 | ) | | 9,819,365 | | | (46,925 | ) | | 16,323,593 | | | 28,601,177 | |
| Cash and cash equivalents at beginning of period | | | 2,199,096 | | | 4,684,914 | | | 2,458,219 | | | 2,458,219 | | | 12,277,584 | | | — | |
| | |
| |
| Cash and cash equivalents at end of period | | $ | 4,684,914 | | $ | 2,458,219 | | $ | 12,277,584 | | $ | 2,411,294 | | $ | 28,601,177 | | $ | 28,601,177 | |
| | |
| |
| Supplemental schedule of non-cash investing and financing activities | | | | | | | | | | | | | | | | | | | |
| Conversion of related party stockholder loans and accrued interest for preferred stock | | $ | — | | $ | — | | $ | 5,191,869 | | $ | — | | $ | — | | $ | 6,391,869 | |
| | |
| |
| Unrealized gain (loss) on investment securities | | $ | (26,520 | ) | $ | 14,130 | | $ | 8,190 | | $ | — | | $ | (5,386 | ) | $ | 2,804 | |
| | |
| |
| |
See accompanying notes.
F-8
NovaCardia, Inc.
(a development stage company)
Notes to financial statements
(Information as of March 31, 2007 and thereafter and for the three months ended March 31, 2006 and 2007 and the period from November 30, 2001 (inception) through March 31, 2007 is unaudited)
1. Organization and summary of significant accounting policies
Organization and business
We were incorporated in Delaware in November 2001. We are a clinical-stage pharmaceutical company focused on developing drugs to treat major cardiovascular diseases that are underserved by existing therapies. We have two compounds in clinical development, KW-3902 and K201 (JTV-519), each with potential for use in both acute and chronic settings. We are currently testing the intravenous, or IV, formulation of KW-3902 in Phase 3 clinical trials for the treatment of the acute form of congestive heart failure, or CHF, in patients with renal impairment and fluid overload. We are also developing an oral formulation of KW-3902 for the treatment of chronic CHF. In addition, we are developing K201 (JTV-519) for the treatment of atrial fibrillation, or A-Fib, an irregular heartbeat.
We have incurred significant net losses since our inception. As of March 31, 2007, we had an accumulated deficit of approximately $51.5 million. To date, we have not had any revenue and our losses have resulted from costs incurred in connection with our development activities, including costs of clinical trial activities associated with our current product candidates, licensing fees, patent costs and selling, general and administrative expenses. Accordingly, we are considered to be in the development stage.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Unaudited interim financial statements
The accompanying unaudited interim balance sheet as of March 31, 2007, the statements of operations and cash flows for the three months ended March 31, 2006 and 2007 and the period from November 30, 2001 (inception) through March 31, 2007 and the statement of stockholders' equity for the three months ended March 31, 2007 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments necessary to present fairly our financial position as of March 31, 2007 and results of operations and cash flows for the three months ended March 31, 2006 and 2007. The results of operations for the three months ended March 31, 2007 are not necessarily
F-9
indicative of the results to be expected for the year ending December 31, 2007 or for any other interim period or for any other future year.
Unaudited pro forma stockholders' equity
The unaudited pro forma stockholders' equity information in the accompanying balance sheet assumes the automatic conversion of the outstanding shares of convertible preferred stock at March 31, 2007 into 85,268,699 shares of common stock as though the completion of the initial public offering contemplated by this prospectus had occurred on March 31, 2007. We have excluded new common stock in such initial public offering and any related net proceeds are excluded from the pro forma information.
Cash and cash equivalents
Cash and cash equivalents consist of cash and other highly liquid investments with original maturities of three months or less from the date of purchase.
Investments
In accordance with Statement of Financial Accounting Standards, or SFAS, No. 115, Accounting for Certain Investments in Debt and Equity Securities, we classify all securities as available-for-sale, as the sale of such securities may be required prior to maturity to implement our strategies. We carry these securities at fair value, with the unrealized gains and losses reported as a component of accumulated other comprehensive income (loss) until realized. We realize gains and losses from the sale of available-for-sale securities, if any, on a specific identification basis.
Fair value of financial instruments
The carrying amounts of all financial instruments, accounts payable and accrued expenses are reasonable estimates of their fair value because of the short maturity of these items. Based on the borrowing rates currently available to us for loans with similar terms, we believe the fair value of the long-term debt approximates its carrying value.
Concentration of credit risk
Financial instruments, which potentially subject us to significant concentration of credit risk, consist primarily of cash and cash equivalents, investment securities and restricted cash. We maintain deposits in federally insured financial institutions in excess of federally insured limits.
F-10
We believe the financial positions of the depository institutions holding our deposits significantly reduce our exposure to credit risk. Additionally, we have established guidelines regarding diversification of our investments and their maturities, which are designed to maintain safety and liquidity.
Property and equipment
We state property and equipment, including leasehold improvements, at cost. We calculate depreciation expense using the straight-line method over the estimated useful lives of the assets, generally three to five years.
Impairment of long-lived assets
In accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, we review long-lived assets, such as property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. We measure recoverability of assets to be held and used by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, we recognize an impairment charge for the amount by which the carrying value of the asset exceeds the fair value of the asset. Assets to be disposed of would be separately presented in the balance sheet and reported at the lower of the carrying amount or the fair value less costs to sell, and are no longer depreciated. We would present separately the assets and liabilities of a disposed group classified as held for sale in the appropriate asset and liability sections of the balance sheet. Although we have accumulated losses since inception, we believe the future cash flows to be received from the long-lived assets will exceed the assets' carrying value and, accordingly, we have not recognized any impairment losses through March 31, 2007.
Development expenses
We account for development costs in accordance with SFAS No. 2,Accounting for Research and Development Costs. SFAS No. 2 specifies that development costs should be charged to expense until technological feasibility has been established for the product. Once we establish technological feasibility, we capitalize all product costs until the product is available for general release to customers. We have determined that we will reach technological feasibility for our product candidates when regulatory authorities grant approval to sell the product. Our development expenses consist primarily of license fees, salaries and related employee benefits,
F-11
costs associated with clinical trials managed by our contract research organizations, or CROs, and costs associated with non-clinical activities, such as regulatory expenses. We use external service providers and vendors to conduct clinical trials, to manufacture product candidates to be used in clinical trials and to provide various other development-related products and services. Through March 31, 2007, development expenses relate predominantly to the in-licensing and clinical trials of KW-3902, our product candidate for the treatment of CHF.
Patent costs
We expense costs related to filing and pursuing patent applications as incurred since recoverability of such expenditures is uncertain.
Income taxes
We account for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. We measure tax assets and liabilities using the enacted tax rates expected to apply to taxable income in the years in which we expect to recover or settle those temporary differences. We recognize the effect of a change in tax rates on deferred tax assets and liabilities in income in the period that includes the enactment date. We provide a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized.
In July 2006, the Financial Accounting Standards Board, or FASB, issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109, or FIN No. 48, which clarifies the recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 also provides guidance on recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. We have adopted FIN No. 48 as of January 1, 2007 and determined that the adoption of FIN No. 48 did not have a material impact on our financial position and results of operations. We have not recognized interest or penalties related to income tax for any period through March 31, 2007. We have not currently completed a study to assess whether a change in control has occurred or whether there have been multiple changes of control since our formation due to the significant complexity and cost associated with such study and the possibility that there could be
F-12
additional changes in the future. If we experienced a greater than 50 percentage point change or shift in ownership over a three year time frame since formation, utilization of our net operating loss or research and development credit carryforwards would be subject to an annual limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code. The annual limitation generally is determined by multiplying the value of our stock at the time of the ownership change (subject to certain adjustments) by the applicable long-term tax-exempt rate. Any limitation may result in expiration of a portion of the net operating loss or research and development credit carryforwards before utilization. Further, until a study is completed and any limitation known, no amounts are being presented as a uncertain tax position under FIN No. 48. Tax returns for all years are subject to future examination by tax authorities.
Stock-based compensation
Effective January 1, 2006, we adopted the provisions of SFAS No. 123(R),Share-Based Payment, using the prospective transition method and therefore we will not restate prior period results. SFAS No. 123(R) supersedes Accounting Principles Board, or APB, Opinion No. 25,Accounting for Stock Issued to Employees, and related interpretations, and revises guidance in SFAS No. 123,Accounting for Stock-Based Compensation. Under this transition method, we recognize compensation cost related to all equity instruments granted prior to, but not yet vested as of, the adoption date based on the grant-date fair value estimated in accordance with the original provisions of SFAS No. 123; however, we exclude those options issued prior to but unvested on January 1, 2006 and valued using the minimum value method from the options subject to SFAS No. 123(R). We recognize compensation costs related to all equity instruments granted after January 1, 2006 at the grant-date fair value of the awards in accordance with the provisions of SFAS No. 123(R). Additionally, under the provisions of SFAS No. 123(R), we include an estimate of the number of the awards that will be forfeited in calculating compensation costs, and recognize these over the requisite service period of the awards on a straight-line basis.
During the year ended December 31, 2006, we recorded $62,086, or $0.03 per share, of stock-based compensation expense as a result of the adoption of SFAS No. 123(R). Of this amount, we allocated $38,383 to development expenses and $23,703 to selling, general and administrative expenses based on the department to which the associated employee reports. No related tax benefits of the stock-based compensation costs have been recognized since our inception.
F-13
The following table shows the assumptions used to compute the stock-based compensation costs for the stock options granted using the Black-Scholes option pricing model.
|
| | Year ended December 31, 2006
| | Three months
ended
March 31,
2007
|
|---|
|
| Employee stock options | | | | |
| Risk-free interest rate | | 4.54% | | 4.56% |
| Dividend yield | | 0.00% | | 0.00% |
| Expected life of options (years) | | 6.08 | | 6.07 |
| Volatility | | 70.00% | | 68.00% |
|
We derived the risk-free interest rate assumption from the United States Treasury's rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued. We based the assumed dividend yield on our expectation of not paying dividends in the foreseeable future. We calculated the weighted average expected life of options using the simplified method as prescribed by Securities and Exchange Commission Staff Accounting Bulletin, or SAB, No. 107,Share-Based Payment. This decision was based on the lack of relevant historical data due to our limited historical experience. In addition, due to our limited historical data, the estimated volatility also reflects the application of SAB No. 107, incorporating the historical volatility of comparable companies with publicly-available share prices. Based on the fact that the 2006 and 2007 option grants are substantially in-the-money, the likelihood of turnover was assumed to be very low. As such, we assumed a forfeiture rate of zero and will adjust our forfeiture rate in the period in which actual forfeitures occur.
The weighted average grant-date fair values of stock options granted during the year ended December 31, 2006 and the three months ended March 31, 2007 was $1.23 per share and $1.39 per share, respectively.
As of December 31, 2006, we had approximately $2,200,000 of unrecognized stock-based compensation costs related to the non-vested balance of the 1,872,900 stock options granted during the year ended December 31, 2006 and expect to recognize such compensation over a weighted average period of 3.89 years. As of March 31, 2007, we had approximately $6,200,000 of unrecognized stock-based compensation costs related to non-vested stock options and expect to recognize such compensation over a weighted average period of 3.79 years.
F-14
Prior to January 1, 2006, we applied the intrinsic-value-based method of accounting prescribed by APB Opinion No. 25, and related interpretations including FASB Interpretation No. 44,Accounting for Certain Transactions involving Stock Compensation—an interpretation of APB Opinion No. 25, to account for equity-based awards to employees and directors. Under this method, if the exercise price of the award equaled or exceeded the fair value of the underlying stock on the measurement date, we recognized no compensation expense. The measurement date was the date on which the final number of shares and exercise prices were known (generally the grant date for awards to employees and directors). If the exercise price of the award was below the fair value of the underlying stock on the measurement date, then we recorded compensation cost, using the intrinsic-value method, and we generally recognized this in the statements of operations over the vesting period of the award.
The effect on net loss as if the fair-value-based method had been applied to all outstanding and unvested awards in each period would have been less than a $25,000 increase in the net loss for each period in the period from November 30, 2001 (inception) through December 31, 2005. For purposes of disclosures required by SFAS No. 123, we amortized the estimated fair value of the options on a straight-line basis over the vesting period. We estimated the fair value of these awards using the minimum value pricing model, with these weighted-average assumptions for 2004 and 2005: risk-free interest rate of 3.00%; dividend yield of 0%; expected volatility of 0%; and an expected life of five years.
We record equity instruments issued to non-employees as expense at their fair value over the related service period as determined in accordance with SFAS No. 123(R) and Emerging Issues Task Force, or EITF, 96-18,Accounting for Equity Instruments That are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling Goods and Services, and we periodically revalue them as the equity instruments vest. Stock-based compensation related to the 13,435 non-employee common stock options that vested during the three months ended March 31, 2007 was $17,583.
Comprehensive income (loss)
SFAS No. 130,Reporting Comprehensive Income, requires that we report all components of comprehensive loss, including net loss, in the financial statements in the period in which we recognize them. The definition of comprehensive loss includes all changes in equity during a period from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on investments.
F-15
Net loss per share
We calculate basic net loss per share attributable to common stockholders by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding for the period, without consideration for common stock equivalents. We calculate diluted net loss per share attributable to common stockholders by dividing the net loss attributable to common stockholders by the weighted average number of common stock equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, we consider convertible preferred stock, stock options and warrants to be common stock equivalents, but include them in the calculation of diluted net loss per share attributable to common stockholders only when their effect is dilutive.
We calculate the unaudited pro forma basic and diluted net loss per share attributable to common stockholders by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding for the period plus the weighted average number of common shares resulting from the assumed conversion of the outstanding shares of convertible preferred stock. We calculate the assumed conversion using the
F-16
as-if-converted method, as if such conversion had occurred as of the beginning of each period presented or as of the original issuance date, if later.
| |
| | Years ended December 31,
| | Three months
ended March 31,
| |
|---|
| | 2004
| | 2005
| | 2006
| | 2006
| | 2007
| |
|---|
| |
| Historical | | | | | | | | | | | | | | | | |
| Numerator: | | | | | | | | | | | | | | | | |
| Net loss | | $ | (7,951,777 | ) | $ | (11,060,398 | ) | $ | (17,909,403 | ) | $ | (2,404,363 | ) | $ | (8,508,448 | ) |
| Deemed dividend related to beneficial conversion feature on Series B preferred stock | | | — | | | — | | | — | | | — | | | (8,080,000 | ) |
| | |
| |
| Net loss attributable to common stockholders | | $ | (7,951,777 | ) | $ | (11,060,398 | ) | $ | (17,909,403 | ) | $ | (2,404,363 | ) | $ | (16,588,448 | ) |
| | |
| |
| Denominator: | | | | | | | | | | | | | | | | |
| | Weighted average common shares outstanding | | | 2,070,000 | | | 2,070,733 | | | 2,176,904 | | | 2,076,219 | | | 2,486,219 | |
| | Weighted average unvested common shares subject to repurchase | | | (764,757 | ) | | (215,837 | ) | | (37,937 | ) | | (92,212 | ) | | (160,000 | ) |
| | |
| |
| | Denominator for basic and diluted net loss per share attributable to common stockholders | | | 1,305,243 | | | 1,854,896 | | | 2,138,967 | | | 1,984,007 | | | 2,326,219 | |
| | |
| |
| Basic and diluted net loss per share attributable to common stockholders | | $ | (6.09 | ) | $ | (5.96 | ) | $ | (8.37 | ) | $ | (1.21 | ) | $ | (7.13 | ) |
| | |
| |
| Pro forma | | | | | | | | | | | | | | | | |
| Net loss attributable to common stockholders | | | | | | | | $ | (17,909,403 | ) | | | | $ | (16,588,448 | ) |
| | | | | | | | |
| | | | |
| |
| Pro forma basic and diluted net loss per share attributable to common stockholders | | | | | | | | $ | (0.45 | ) | | | | $ | (0.23 | ) |
| | | | | | | | |
| | | | |
| |
| Shares used above | | | | | | | | | 2,138,967 | | | | | | 2,326,219 | |
| Pro forma adjustments to reflect assumed conversion of preferred stock | | | | | | | | | 38,039,914 | | | | | | 70,821,766 | |
| | | | | | | | |
| | | | |
| |
| Pro forma shares used to compute basic and diluted net loss per share attributable to common stockholders | | | | | | | | | 40,178,881 | | | | | | 73,147,985 | |
| | | | | | | | |
| | | | |
| |
| |
Historical outstanding anti-dilutive securities not included in diluted net loss per share attributable to common stockholders calculation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock (as converted) | | | 26,190,477 | | | 26,190,477 | | | 69,012,699 | | | 26,190,477 | | | 85,268,699 | |
| Preferred stock warrants (as converted) | | | — | | | 322,095 | | | 1,433,562 | | | 456,228 | | | 1,212,609 | |
| Common stock options | | | 3,149,839 | | | 3,909,043 | | | 5,014,832 | | | 3,909,043 | | | 7,609,832 | |
| Common stock subject to repurchase | | | 316,661 | | | 116,657 | | | — | | | 66,656 | | | 400,000 | |
| | |
| |
| | | | 29,656,977 | | | 30,538,272 | | | 75,461,093 | | | 30,622,404 | | | 94,491,140 | |
| | |
| |
| |
F-17
NovaCardia, Inc.
(a development stage company)
Notes to financial statements (continued)
(Information as of March 31, 2007 and thereafter and for the three months ended March 31, 2006 and 2007 and the period from November 30, 2001 (inception) through March 31, 2007 is unaudited)
2. Investment securities
Investment securities consist entirely of corporate debt securities. All investments held as of December 31, 2006 and March 31, 2007 have contractual maturities of less than six months. Based on an evaluation of the credit standing of each issuer, we believe we will be able to collect all amounts due according to the contractual terms. We had no realized losses on sales of investment securities for the years ended December 31, 2004, 2005 and 2006 and the three months ended March 31, 2007.
3. Property and equipment
Property and equipment consist of:
| |
| |
| | December 31,
| |
| |
|---|
| | Useful
Lives
| | March 31,
2007
| |
|---|
| | 2005
| | 2006
| |
|---|
| |
| Furniture and fixtures | | 5 years | | $ | 72,038 | | $ | 75,111 | | $ | 75,111 | |
| Computer and office equipment | | 3 years | | | 137,183 | | | 165,931 | | | 181,853 | |
| Manufacturing equipment | | 5 years | | | — | | | 47,650 | | | 47,650 | |
| Construction in process | | — | | | — | | | 107,349 | | | 107,349 | |
| | | | |
| |
| | | | | | 209,221 | | | 396,041 | | | 411,963 | |
| Less accumulated depreciation and amortization | | | | | (122,346 | ) | | (176,324 | ) | | (187,556 | ) |
| | | | |
| |
| | | | | $ | 86,875 | | $ | 219,717 | | $ | 224,407 | |
| | | | |
| |
| |
4. Other assets
Other assets consist of:
|
| | December 31,
| |
|
|---|
| | March 31,
2007
|
|---|
| | 2005
| | 2006
|
|---|
|
| Clinical trial deposits | | $ | — | | $ | 3,500,000 | | $ | 4,170,756 |
| Deferred initial public offering costs | | | — | | | — | | | 981,842 |
| Debt issuance costs | | | 167,347 | | | 104,592 | | | 124,256 |
| Deposits | �� | | 10,235 | | | — | | | — |
| | |
|
| | | $ | 177,582 | | $ | 3,604,592 | | $ | 5,276,854 |
| | |
|
|
F-18
Included in other assets at March 31, 2007 are capitalized offering costs of $981,842, which are incremental costs directly attributable to our proposed initial public offering. Upon consummation of our initial public offering, such costs will be applied to the offering proceeds; however, in the event the offering is not consummated, such costs will be expensed. Also included in other assets at March 31, 2007 are clinical trial deposits of $4,170,756, which are refundable deposits that we are obligated to maintain with our CRO until estimated remaining costs under the related contracts fall below our balance on deposit.
5. Accrued liabilities
Accrued liabilities consist of:
|
| | December 31,
| |
|
|---|
| | March 31,
2007
|
|---|
| | 2005
| | 2006
|
|---|
|
| Clinical trial and related costs | | $ | — | | $ | 962,437 | | | 1,053,843 |
| Accrued payroll and related costs | | | 168,299 | | | 82,252 | | | 185,621 |
| Accrued interest | | | 39,088 | | | 26,277 | | | 22,862 |
| Deferred rent | | | 5,962 | | | 6,346 | | | 4,314 |
| Other accrued liabilities | | | 28,015 | | | 96,826 | | | 513,177 |
| | |
|
| | | $ | 241,364 | | $ | 1,174,138 | | $ | 1,779,817 |
| | |
|
|
6. Long-term debt
In March 2005, we entered into a loan and security agreement, or the Loan Agreement, for $5,000,000, which was fully drawn on September 1, 2005. The note is due August 1, 2008 and bears interest at 8.25% annually and is payable in equal monthly installments of $156,185. In addition, the note requires a final interest payment of $200,000 in August 2008.
In connection with the Loan Agreement, we became obligated to issue warrants to purchase up to an aggregate of 357,142 shares of Series A preferred stock, and, as of December 31, 2006, we had issued warrants to purchase up to 322,095 shares of Series A preferred stock at an exercise price of $0.84 per share. The warrants are fully exercisable upon issuance and will expire on March 21, 2012. We determined the $208,333 fair value of the warrants using the Black-Scholes valuation model, recorded as debt issuance costs, and amortized to interest expense over the 36 month term of the related note. We used the following assumptions to
F-19
value the warrants: risk-free interest rate of 4.08% to 4.33%; dividend yield of 0%; expected volatility of 70%; and contractual term of 6.56 to 7.00 years.
In July 2006, we amended the Loan Agreement, or the Amended Loan Agreement, to provide for additional financing of up to $3,000,000. In July 2006, we borrowed the entire $3,000,000 of available credit under the Amended Loan Agreement and repaid such borrowings in September 2006.
In connection with the Amended Loan Agreement, we issued a warrant to purchase 306,667 shares of Series B preferred stock at an exercise price of $1.125 per share. We received $100 for these warrants. The warrants are fully exercisable on December 31, 2006 and will expire on July 14, 2013. We determined the $239,100 fair value of the warrants using the Black-Scholes valuation model, originally recorded as debt issuance costs, and fully amortized to interest expense upon the repayment of the related promissory note in September 2006. We used the following assumptions to value the warrants: risk-free interest rate of 4.68%; dividend yield of 0%; expected volatility of 70%; and contractual term of 6.81 years.
For the years ended December 31, 2004, 2005, 2006 and the period from November 30, 2001 (inception) through December 31, 2006, we included $0, $40,986, $301,854 and $342,840, respectively, in interest expense related to the amortization of the fair value of warrants granted under the Loan Agreement and Amended Loan Agreement.
Future minimum payments under the Amended Loan Agreement as of December 31, 2006 are as follows:
| |
| 2007 | | $ | 1,874,224 | |
| 2008 | | | 1,449,483 | |
| | |
| |
| Total | | | 3,323,707 | |
| Less interest | | | (299,478 | ) |
| | |
| |
| Principal due | | | 3,024,229 | |
| Less current portion of long-term debt | | | (1,635,594 | ) |
| | |
| |
| Long-term debt, less current portion | | $ | 1,388,635 | |
| | |
| |
| |
Interest expense (excluding the non-cash interest charges related to convertible debt and warrants) for the years ended December 31, 2005 and 2006, and for the period November 30, 2001 (inception) through December 31, 2006 was $162,368, $387,105 and $549,473, respectively.
F-20
7. Commitments
Operating leases
In August 2004, we leased our corporate headquarters under a non-cancelable operating lease agreement that expires in September 2007. Future minimum lease payments under the operating lease are $94,341 for the nine months ending September 30, 2007. Rent expense, including common area charges, for the years ended December 31, 2004, 2005 and 2006, and for the period from November 30, 2001 (inception) through December 31, 2006 was $134,049, $120,143, $118,779 and $439,142, respectively.
In March 2007, we entered into a non-cancelable operating lease for our new corporate headquarters that expires in July 2010. Future minimum lease payments under the operating lease for the years ending December 31, 2007, 2008, 2009 and 2010 are $38,500, $289,071, $299,189 and $179,164, respectively.
8. License agreements
Kyowa Hakko Kogyo Co., Ltd.
In August 2003, we entered into a license agreement with Kyowa Hakko Kogyo Co., Ltd., or Kyowa Hakko, under which we received an exclusive license to develop and commercialize KW-3902 for the treatment of CHF and other specified indications in major markets, including the United States and Europe, but excluding Asia. In consideration for the license, we paid Kyowa Hakko $2,350,000 in up-front fees (of which we paid $2,050,000 in 2003, $100,000 in 2004 and $200,000 in 2005), and may be required to make future milestone payments totaling up to $19,500,000 upon the achievement of various milestones related to regulatory or commercial events for the first two indications, including $1,500,000 that we expect to pay to Kyowa Hakko in June 2007 as a result of having initiated our Phase 3 clinical trials of the IV formulation of KW-3902. We have an obligation to negotiate and agree to additional milestone payments before submitting an NDA for a third indication. We also have an obligation to pay royalties on any net sales of the licensed products.
The agreement is sublicensable with the prior approval of Kyowa Hakko, provided that Kyowa Hakko may not unreasonably withhold its approval. Any of our potential sublicensees will not be allowed to further sublicense any rights that we grant to them. The agreement terminates upon the later of the expiration of all of the in-licensed patents under the agreement or 15 years from the date of first commercial sale of KW-3902 for any indication in a major market, unless the parties terminate the agreement earlier. Either party may terminate the agreement in the event of material breach by the other party, subject to prior notice and the
F-21
opportunity to cure, or the other party enters into bankruptcy or is dissolved for any reasons other than in connection with a merger or acquisition. In addition, we may terminate the agreement by providing written notice to Kyowa Hakko at least 90 days prior to any contemplated termination.
Aetas Pharma Co., Ltd.
In September 2006, we entered into a license agreement with Aetas Pharma Co., Ltd., or Aetas, and supplemented the license agreement in January 2007. Under the terms of the license agreement with Aetas, as supplemented, we received an exclusive license to develop and commercialize the IV formulation of K201 (JTV-519) for the treatment of cardiac arrhythmia and another potential indication and the oral formulation of K201 (JTV-519) in all cardiovascular indications in major markets, including the United States and Europe, but excluding Asia. In consideration for the license, we paid Aetas $1,500,000 in up-front fees (of which we paid $1,000,000 in 2006 and $500,000 in 2007) and may be required to make future milestone payments totaling up to $18,000,000 upon the achievement of various milestones related to regulatory or commercial events. We are also obligated to pay royalties on any net sales of the licensed products.
The agreement terminates upon the later of the expiration of the last licensed patent right or 10 years from the date of first commercial sale of K201 (JTV-519) on a country-by-country basis, unless the agreement is earlier terminated. Either party may terminate the agreement in the event of material breach by the other party, subject to prior notice and the opportunity to cure, or the other party enters into bankruptcy or is dissolved for any reasons other than in connection with a merger or acquisition. In addition, we may terminate the agreement by providing specified written notice to Aetas related to safety, efficacy, intellectual property or regulatory concerns. Aetas also has the right to terminate the agreement by providing written notice to us in the event we fail to commence dosing, without reasonable reasons, in specified clinical trials of the IV formulation of K201 (JTV-519) by predetermined dates or otherwise fail to use commercially reasonable efforts to develop the product candidate, in each case as determined by Aetas in its sole discretion. In the event the agreement is terminated and Aetas successfully commercializes K201 (JTV-519) after such termination, Aetas will be obligated to pay us royalties on net sales of K201 (JTV-519).
Since both of our product candidates are still under development, we have recorded and will continue to record all payments to Kyowa Hakko and Aetas as development expenses until the product candidates reach technological feasibility.
F-22
9. Stockholders' equity
Convertible preferred stock
In August 2003, we sold 26,190,477 shares of Series A convertible preferred stock to certain new investors and certain related parties, at $0.84 per share, resulting in gross proceeds of $22,000,000. The gross proceeds consisted of $20,050,000 of cash, $1,200,000 from the conversion of debt (see Note 12) and $750,000 of intellectual property (see Note 12). We incurred offering costs of $80,332, resulting in net proceeds of $21,919,668, of which $19,969,668 was cash.
In September 2006, we sold 42,822,222 shares of Series B convertible preferred stock to certain new investors and certain related parties, at $1.125 per share, resulting in gross proceeds of $48,175,000. The gross proceeds consisted of $42,983,131 of cash and $5,191,869 from the conversion of debt and accrued interest (see Note 12). We incurred offering costs of $205,248, resulting in net proceeds of $47,969,752, of which $42,777,883 was cash.
In March 2007, we issued 16,000,000 shares of Series B convertible preferred stock to certain related parties at $1.125 per share for cash of $18,000,000. We incurred offering costs of $23,416, resulting in net cash proceeds of $17,976,584. We sold these shares of Series B preferred stock at a price per share below the anticipated initial public offering price. Accordingly, pursuant to EITF No. 98-5,Accounting for Convertible Securities with Beneficial Conversion Features, and EITF No. 00-27,Application of Issue No. 98-5 to Certain Convertible Instruments, we have recorded a deemed dividend on the Series B preferred stock in an amount equal to the number of shares of Series B preferred stock sold multiplied by the difference between the $1.63 per share estimated fair value for financial reporting purposes of the underlying common stock and the Series B preferred stock conversion price of $1.125 per share. The $8,080,000 deemed dividend had no net impact on stockholders' equity and increased the net loss attributable to common stockholders in the calculation of basic and diluted net loss per common share during 2007.
Each holder of Series A and Series B preferred stock has the right, at the option of the holder at any time, to convert shares of preferred stock into shares of common stock at a conversion ratio of one-to-one, subject to further adjustment for stock splits, certain capital reorganizations and dilutive stock issuances. Each share of preferred stock will automatically convert into shares of common stock, at the then effective applicable conversion rate upon the earlier of: (i) the day preceding the closing of the sale of our common stock in connection with a firmly underwritten public offering in which we receive gross proceeds of at least $30,000,000 at a price of at least $1.6875 per share (as adjusted from time to time) or (ii) the
F-23
consent of at least 662/3% of the then outstanding shares of Series A preferred stock, as a single class, and Series B preferred stock, as a single class.
The holders of Series A and Series B preferred stock have rights to receive, when, as and if declared by our board of directors out of legally available funds, non-cumulative dividends payable to holders of the preferred stock in an amount equal to $0.0672 and $0.09 per share, respectively, in preference and priority to the payment of any dividends on common stock. As of December 31, 2006, no dividends have been declared by the board of directors.
In the event of our liquidation, dissolution or winding up, the holders of Series A and Series B preferred stock have rights to receive in preference to the holders of common stock, the amount of $0.84 and $1.125 per share, respectively, plus declared and unpaid dividends, if any. If the assets and funds available to be distributed among the holders of the preferred stock shall be insufficient to permit the payment to such holders of the full preferences, then the entire assets and funds legally available for distribution to such holders shall be distributed ratably based on the total due each such holder. Any of our remaining assets will be distributed ratably among the holders of the common stock.
The holders of the Series A and Series B preferred stock have the right to one vote for each share of common stock into which such Series A and Series B preferred stock could then be converted; and with respect to such vote, such holder shall have full voting rights and powers equal to the voting rights and powers of the holders of common stock.
Founders shares
In December 2001, we issued 195,384 shares of common stock to Domain Partners V, L.P., 4,616 shares of common stock to DP V Associates, L.P., and 800,000 shares of common stock to Eckard Weber, M.D., for $0.001 per share, resulting in aggregate proceeds to us of $1,000.
The common stock sold to Dr. Weber was subject to a stock restriction agreement whereby we had the right to repurchase the common stock in the event Dr. Weber was no longer providing certain services to us. The right to repurchase the common stock lapsed by 25% in July 2003 and thereafter lapsed on a monthly basis until it fully lapsed in July 2006.
In August 2003, we issued 440,000 shares of common stock to Lauren Otsuki, the president of our consultant, Crescent Consulting Ltd., for $0.001 per share, resulting in proceeds to us of $440. The shares were subsequently transferred to Hermosa Family Limited Partnership, an affiliate of Crescent Consulting Ltd. The common stock was subject to a stock restriction
F-24
agreement whereby we had the right to repurchase the common stock in the event the consultant was no longer providing certain services to us. The right to repurchase the common stock lapsed by 25% in October 2003 and fully lapsed in May 2004. In August 2003, we issued 560,000 shares of common stock to our then acting chief executive officer, Kenneth J. Widder, M.D., for $0.001 per share, resulting in proceeds to us of $560. The common stock was subject to a stock restriction agreement whereby we had the right to repurchase the common stock in the event Dr. Widder was no longer providing certain services to us. The right to repurchase the common stock lapsed by 25% in October 2003 and fully lapsed in May 2004.
Stock options
In August 2003, we adopted the 2003 Equity Incentive Plan, or the 2003 Plan, under which shares of common stock are reserved for issuance to our employees, non-employee directors and consultants. The 2003 Plan provides for the grant of incentive stock options, non-statutory stock options, phantom stock and rights to purchase restricted stock to eligible recipients. Recipients of incentive stock options shall be eligible to purchase shares of our common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The maximum term of options granted under the Plan is ten years. The options generally vest 25% on the first anniversary of the original vesting date, with the balance vesting monthly over the remaining three years.
F-25
NovaCardia, Inc.
(a development stage company)
Notes to financial statements (continued)
(Information as of March 31, 2007 and thereafter and for the three months ended March 31, 2006 and 2007 and the period from November 30, 2001 (inception) through March 31, 2007 is unaudited)
9. Stockholders' equity (continued)
A summary of our stock option activity under the 2003 Plan and related information are as follows:
|
| |
| | Options outstanding
|
|---|
| | Shares
available
for grant
| | Number
of shares
| | Weighted
average
exercise
price
| | Weighted
average
contractual
term (years)
| | Aggregate
intrinsic
value
|
|---|
|
| | Initial shares reserved | | 4,500,000 | | — | | | | | | | | |
| | Options granted | | (1,439,715 | ) | 1,439,715 | | $ | 0.10 | | | | | |
| | |
| | | | | | | | |
| Balance at December 31, 2003 | | 3,060,285 | | 1,439,715 | | $ | 0.10 | | | | | |
| | Options granted | | (1,720,124 | ) | 1,720,124 | | $ | 0.10 | | | | | |
| | Options cancelled | | 10,000 | | (10,000 | ) | $ | 0.10 | | | | | |
| | |
| | | | | | | | |
| Balance at December 31, 2004 | | 1,350,161 | | 3,149,839 | | $ | 0.10 | | | | | |
| | Options granted | | (794,204 | ) | 794,204 | | $ | 0.12 | | | | | |
| | Options cancelled | | 28,781 | | (28,781 | ) | $ | 0.10 | | | | | |
| | Options exercised | | — | | (6,219 | ) | $ | 0.10 | | | | | |
| | |
| | | | | | | | |
| Balance at December 31, 2005 | | 584,738 | | 3,909,043 | | $ | 0.10 | | | | | |
| | Additional shares reserved | | 2,500,000 | | — | | | | | | | | |
| | Options granted | | (1,872,900 | ) | 1,872,900 | | $ | 0.16 | | | | | |
| | Options cancelled | | 517,111 | | (517,111 | ) | $ | 0.10 | | | | | |
| | Options exercised | | — | | (250,000 | ) | $ | 0.10 | | | | | |
| | |
| | | | | | | | |
| Balance at December 31, 2006 | | 1,728,949 | | 5,014,832 | | $ | 0.13 | | 8.37 | | $ | 6,042,229 |
| | | | | | | | | |
|
| | Additional shares reserved | | 2,500,000 | | — | | | | | | | | |
| | Options granted | | (3,195,000 | ) | 3,195,000 | | $ | 0.35 | | | | | |
| | Options cancelled | | 200,000 | | (200,000 | ) | $ | 0.16 | | | | | |
| | Options exercised | | — | | (400,000 | ) | $ | 0.28 | | | | | |
| | |
| | | | | | | | |
| Balance at March 31, 2007 | | 1,233,949 | | 7,609,832 | | $ | 0.21 | | 8.75 | | $ | 10,805,229 |
| | |
|
| Exercisable at March 31, 2007 | | | | 7,609,832 | | $ | 0.21 | | 8.75 | | $ | 10,805,229 |
| | | | |
|
|
In connection with the preparation of a registration statement for us to sell shares of our common stock in an initial public offering, management, all of whom are related parties, reassessed the estimated fair value for financial reporting purposes of our common stock in
F-26
light of the expected completion of the offering. At the time of the issuances of stock options, we believed our estimates of the fair value for financial reporting purposes of our common stock were reasonable and consistent with our understanding of how similarly situated companies in our industry were valued.
Information on stock options granted from November 30, 2001 (inception) through March 31, 2007 is summarized as follows:
Grant date
| | Number of
options
granted
| | Exercise
price
| | Fair value
per share
| | Intrinsic
value per
share
|
|---|
| September 5, 2003 | | 1,429,715 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| December 2, 2003 | | 10,000 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| May 11, 2004 | | 1,648,024 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| November 18, 2004 | | 72,100 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| July 12, 2005 | | 175,000 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| July 27, 2005 | | 2,500 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| September 28, 2005 | | 245,704 | | $ | 0.10 | | $ | 0.10 | | $ | — |
| December 21, 2005 | | 371,000 | | $ | 0.14 | | $ | 0.14 | | $ | — |
| December 12, 2006 | | 1,872,900 | | $ | 0.16 | | $ | 1.33 | | $ | 1.17 |
| February 23, 2007 | | 988,000 | | $ | 0.28 | | $ | 1.53 | | $ | 1.25 |
| March 20, 2007 | | 2,207,000 | | $ | 0.38 | | $ | 1.63 | | $ | 1.25 |
Based on the reassessed fair values of our common stock, we concluded that the 1,872,900 common stock options granted at $0.16 per share to employees on December 12, 2006 were at exercise prices below the reassessed value of $1.33 per share and the aggregate 3,195,000 common stock options granted at a weighted average of $0.35 per share to employees and consultants on February 23, 2007 and March 20, 2007 were at exercise prices below the weighted average reassessed value of $1.60 per share.
The aggregate exercise date intrinsic value of options exercised during the year ended December 31, 2006 was approximately $256,000. The weighted average grant-date fair value of the 2,174,997 vested options at December 31, 2006 was $0.01 per share and the weighted average grant-date fair value of the 797,253 shares which vested during the year ended December 31, 2006 was $0.01 per share. The weighted average grant-date fair value of the options cancelled during the year ended December 31, 2006 was $0.01 per share.
F-27
Shares reserved for future issuance
Common stock reserved for future issuance consists of:
|
| | December 31,
2006
| | March 31,
2007
|
|---|
|
| Conversion of preferred stock | | 69,012,699 | | 85,268,699 |
| Stock options issued and outstanding | | 5,014,832 | | 7,609,832 |
| Shares available for future option grants | | 1,728,949 | | 1,233,949 |
| Preferred stock warrants | | 1,433,562 | | 1,212,609 |
| | |
|
| | | 77,190,042 | | 95,325,089 |
| | |
|
|
10. Income taxes
Deferred tax assets at December 31, 2005 and 2006 consist of:
| |
| | 2005
| | 2006
| |
|---|
| |
| Deferred tax assets: | | | | | | | |
| Net operating loss carryforwards | | $ | 8,888,000 | | $ | 14,924,000 | |
| Research and development credits | | | 1,111,000 | | | 1,759,000 | |
| Intangibles | | | 938,000 | | | 1,336,000 | |
| Other | | | 13,000 | | | 28,000 | |
| | |
| |
| Total deferred tax assets | | | 10,950,000 | | | 18,047,000 | |
| Less valuation allowance | | | (10,950,000 | ) | | (18,047,000 | ) |
| | |
| |
| | | $ | — | | $ | — | |
| | |
| |
| |
At December 31, 2006, we have federal and state net operating loss carryforwards of approximately $36,447,000 and $37,691,000, respectively. The federal and state loss carryforwards begin to expire in 2021 and 2013, respectively, unless previously utilized. We also have federal and state research credit carryforwards of approximately $1,567,000 and $296,000, respectively. The federal research credit carryforwards will begin expiring in 2021 unless previously utilized.
Pursuant to Section 382 of the Code, use of our net operating loss carryforwards will be limited if a cumulative change in ownership of more than 50% has occurred within a three-year period.
F-28
We have determined that it is more likely than not we will not realize the net deferred tax asset under SFAS No. 109. Accordingly, we have established a valuation allowance in the amount of $18,047,000 at December 31, 2006.
11. Employee benefits
We have a defined contribution 401(k) plan for employees who are at least 21 years of age. Employees are eligible to participate in the plan beginning on the first day of the month following date of hire. Under the terms of the plan, employees may make voluntary contributions as a percentage of compensation. We had not made any matching contributions as of March 31, 2007.
12. Related party transactions
Consulting arrangements
From March 2003 to December 2004, Dr. Eckard Weber, the chairman of our board of directors and our acting chief executive officer from our inception in November 2001 to March 2003, provided consulting services to us with respect to the development and commercialization of our product candidates and the potential in-licensing of additional product candidates. In August 2003, we formalized Dr. Weber's consulting arrangement with us by entering into a consulting agreement with Dr. Weber. As compensation for his services, Dr. Weber was paid $71,694, $123,516 and $195,210, respectively, for the years ended December 31, 2003 and 2004 and the period from November 30, 2001 (inception) to December 31, 2006. Dr. Weber provided no consulting services in 2005 or 2006.
In May 2003, we entered into a consulting agreement with Dr. Widder, our acting chief executive officer from March 2003 to March 2004, under which Dr. Widder provided consulting services to us in connection with serving as our chief executive officer. As compensation for his services, Dr. Widder was paid $92,484 and $331,625, respectively, for the year ended December 31, 2004 and the period from November 30, 2001 (inception) to December 31, 2006. Dr. Widder's consulting agreement terminated in March 2004 and he provided no consulting services in 2005 and 2006.
In May 2003 and August 2003, we entered into a consulting agreement with Crescent Consulting, Ltd., an entity controlled by a founding stockholder, under which the consultant provided consulting services to us with respect to the development of our product candidates. As compensation for services, we paid the consultant $236,218, $70,232 and $454,142,
F-29
respectively, for the years ended December 31, 2004 and 2005 and the period from November 30, 2001 (inception) to December 31, 2006. The May 2003 consulting agreement terminated upon the consummation of our Series A preferred stock financing in August 2003 and the August 2003 agreement terminated in August 2005 and the consultant did not provide any consulting services in 2006.
Notes payable to stockholders
In December 2001, we received $200,000 from stockholders upon the issuance of 7.0% convertible promissory notes. In January 2003, we received $500,000 from stockholders upon the issuance of 6.75% convertible promissory notes. In April 2003, we received $500,000 from stockholders upon the issuance of 6.75% convertible promissory notes. The $1,200,000 of outstanding promissory notes converted to Series A preferred stock at a price of $0.84 per share in August 2003.
We recognized interest expense of $729, $14,000, $41,647 and $56,376 for the years ended December 31, 2001, 2002 and 2003 and the period from November 30, 2001 (inception) through December 31, 2006, respectively. After the August 2003 conversion of outstanding note balances and payment of the $56,376 balance of accrued interest expense, we recognized no additional interest expense.
Purchase of intellectual property
In May 2003, we entered into a letter agreement with Windamere II, LLC and Windamere III, LLC, or Windamere, whereby we agreed to purchase certain intellectual property relating to adenosine A1 receptor antagonists from Windamere in exchange for 892,857 shares of Series A preferred stock. The $750,000 valuation of the shares issued was recorded as development expense in 2003. The purchase of the intellectual property closed concurrently with the closing of our Series A preferred stock financing on August 18, 2003. In addition, Windamere purchased an additional 297,619 shares of our Series A preferred stock at $0.84 per share at the closing of our Series A preferred stock financing. In August 2003, we sold 70,000 shares of common stock at $0.001 per share to two individuals affiliated with Windamere, including to Dr. Widder, our former acting chief executive officer. Dr. Widder was a general partner of Windamere.
F-30
Investor bridge financing
In February and June 2006, as part of a bridge financing, we issued unsecured convertible promissory notes in an aggregate principal amount of $5,030,000 to certain investors in two tranches. The notes accrued interest at the rate of 7.0% per year. The outstanding principal balance of $5,030,000 and accrued interest balance of $161,870 converted into an aggregate of 4,614,995 shares of Series B preferred stock in connection with our Series B preferred stock financing in September 2006.
In connection with the bridge financing, we issued warrants to purchase an aggregate of 804,800 shares of Series B preferred stock at an exercise price of $1.125 per share. We received aggregate consideration of $11,976 for these warrants. The warrants became exercisable on September 21, 2006 and will expire at the earlier of September 21, 2013 or the completion of an initial public offering. We used the Black-Scholes valuation model to determine the $622,379 fair value of the warrants, originally recorded as debt issuance costs, and fully amortized to interest expense upon the conversion of the related promissory notes on September 21, 2006. We used the following assumptions to value the warrants: risk-free interest rate of 4.68%; dividend yield of 0%; expected volatility of 70%; and contractual term of 6.44 to 6.70 years. Additionally, after allocating the proceeds, we determined there was a beneficial conversion feature for the unsecured convertible promissory notes of $627,744 for the year ended December 31, 2006, which we included in interest expense in the accompanying statements of operations.
13. Subsequent events
In April 2007, we amended our loan and security agreement with Lighthouse Capital Partners V, L.P., or Lighthouse Capital Partners, to increase our credit facility by an additional $15.0 million. $5.0 million was funded in connection with executing the amendment and the remaining $10.0 million is available through September 1, 2007. Interest accrues at the prime rate plus 1.5% per year, with interest-only payments through December 2007. The interest rate floats during the interest-only period based upon any changes in the prime rate and will become fixed at January 1, 2008. Principal and interest payments will commence on January 1, 2008 and are required to be made in 36 monthly installments. In connection with the increased credit facility, we issued Lighthouse Capital Partners a warrant to purchase up to 933,333 shares of our Series B preferred stock at an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts under our credit facility (with the actual number of shares
F-31
becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price). Our loan and security agreement with Lighthouse Capital Partners is secured by all of our assets other than intellectual property and there is a negative pledge on our intellectual property. We determined the approximately $1,200,000 fair value of the currently exercisable portion of the warrant using the Black-Scholes valuation model. Such amount will be recorded as a debt issuance cost and amortized to interest expense over the 45-month term of the related note. In connection with the increased credit facility, the outstanding indebtedness under our existing loan and security agreement with Lighthouse Capital Partners was restructured.
F-32
shares

Common stock
Prospectus
| JPMorgan | | Credit Suisse |
Pacific Growth Equities, LLC |
|
First Albany Capital
|
, 2007
Until , 2007, all dealers that buy, sell or trade in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
Information not required in prospectus
Item 13. Other expenses of issuance and distribution.
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale of the common stock being registered. All amounts shown are estimates except for the SEC registration fee, the NASD filing fee and the NASDAQ Global Market filing fee.
|
| | Amount to be paid
|
|---|
|
| SEC registration fee | | $ | 2,648 |
| NASD filing fee | | | 9,125 |
| NASDAQ Global Market filing fee | | | 100,000 |
| Blue sky qualification fees and expenses | | | * |
| Printing and engraving expenses | | | * |
| Legal fees and expenses | | | * |
| Accounting fees and expenses | | | * |
| Transfer agent and registrar fees and expenses | | | * |
| Miscellaneous expenses | | | * |
| | |
|
| | Total | | $ | * |
|
- *
- To be provided by amendment.
Item 14. Indemnification of directors and officers.
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer, director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation's best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys' fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation's best interests except that no indemnification is permitted without
II-1
judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses which such officer or director has actually and reasonably incurred. Our amended and restated certificate of incorporation and amended and restated bylaws, each of which will become effective upon the closing of this offering, provide for the indemnification of our directors and officers to the fullest extent permitted under the Delaware General Corporation Law.
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
- •
- transaction from which the director derives an improper personal benefit;
- •
- act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
- •
- unlawful payment of dividends or redemption of shares; or
- •
- reach of a director's duty of loyalty to the corporation or its stockholders.
Our amended and restated certificate of incorporation and amended and restated bylaws include such a provision. Expenses incurred by any officer or director in defending any such action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director or officer is not entitled to be indemnified by us.
Section 174 of the Delaware General Corporation Law provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption, may be held liable for such actions. A director who was either absent when the unlawful actions were approved, or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered in the books containing minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful acts.
As permitted by the Delaware General Corporation Law, we have entered into indemnity agreements with each of our directors and executive officers, that require us to indemnify such persons against any and all expenses (including attorneys' fees), witness fees, damages, judgments, fines, settlements and other amounts incurred (including expenses of a derivative action) in connection with any action, suit or proceeding, whether actual or threatened, to which any such person may be made a party by reason of the fact that such person is or was a director, an officer or an employee of NovaCardia or any of its affiliated enterprises, provided that such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to our best interests and, with respect to any criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification thereunder.
II-2
At present, there is no pending litigation or proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
We have an insurance policy covering our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, or Securities Act, or otherwise.
We plan to enter into an underwriting agreement which provides that the underwriters are obligated, under some circumstances, to indemnify our directors, officers and controlling persons against specified liabilities, including liabilities under the Securities Act.
Reference is made to the following documents filed as exhibits to this registration statement regarding relevant indemnification provisions described above and elsewhere herein:
|
Exhibit document
| | Number
|
|---|
|
| Form of Underwriting Agreement | | 1.1 |
| Form of Amended and Restated Certificate of Incorporation to be effective upon completion of this offering | | 3.2 |
| Form of Amended and Restated Bylaws to be effective upon completion of this offering | | 3.4 |
| Amended and Restated Investor Rights Agreement dated September 31, 2006 between the Registrant and certain of its stockholders | | 4.7 |
| Amendment No. 1 to Amended And Restated Investors' Rights Agreement, dated March 20, 2007, by and among the Registrant and certain of its stockholders | | 4.8 |
| Form of Indemnity Agreement | | 10.1 |
|
Item 15. Recent sales of unregistered securities.
The following sets forth information regarding all unregistered securities sold since our inception through April 30, 2007:
- (1)
- Since inception through April 30, 2007, we have granted stock options to purchase up to 8,786,943 shares of our common stock to employees, consultants and directors under to our 2003 equity incentive plan at exercise prices ranging from $0.10 to $0.38 per share. Of these options, options exercisable for up to 755,892 shares of common stock have been cancelled without being exercised, options to purchase 656,219 shares of common stock have been exercised of which no shares have been repurchased and options exercisable for up to 7,609,832 shares of common stock remain outstanding.
- (2)
- In December 2001, we issued 1,000,000 shares of our common stock to three accredited investors for an aggregate purchase price of $1,000.
- (3)
- In December 2001, we issued non-negotiable promissory notes in an aggregate principal amount of $200,000 to two of our existing investors. The notes accrued interest at the rate of 7.0% per year. All principal under the notes was subsequently converted into shares of our Series A preferred stock in connection with our Series A preferred stock financing in August 2003.
II-3
- (4)
- In January 2003, we issued non-negotiable promissory notes in an aggregate principal amount of $500,000 to two of our existing investors. The notes accrued interest at the rate of 6.75% per year. All principal under the notes was subsequently converted into shares of our Series A preferred stock in connection with our Series A preferred stock financing in August 2003.
- (5)
- In April 2003, we issued non-negotiable promissory notes in an aggregate principal amount of $500,000 to two of our existing investors. The notes accrued interest at the rate of 6.75% per year. All principal under the notes was subsequently converted into shares of our Series A preferred stock in connection with our Series A preferred stock financing in August 2003.
- (6)
- In August 2003, we issued 1,070,000 shares of our common stock to four accredited investors for an aggregate purchase price of $1,070.
- (7)
- In August 2003, in connection with our Series A preferred stock financing, we issued 26,190,447 shares of our Series A preferred stock to 10 accredited investors, at $0.84 per share, for an aggregate purchase price of approximately $22.0 million. Approximately $1.2 million of the purchase price was paid for by the surrender and cancellation of the non-negotiable promissory notes issued in December 2001, January 2003 and April 2003, and $750,000 of the purchase price was paid in consideration for the assignment to us of certain intellectual property rights.
- (8)
- In March 2005, in connection with a loan and security agreement, we issued one warrant to one lender to purchase approximately 238,095 shares of our Series A preferred stock, at an initial exercise price of $0.84 per share. In September 2005, the number of shares of our Series A preferred stock that is subject to this warrant automatically increased to the number that is equal to 2% of the aggregate advances then outstanding under the loan and security agreement divided by $0.84. This warrant is exercisable through March 2012. In March 2007, we amended this warrant to clarify that the automatic increase is capped at a maximum of 119,047 shares of our Series A preferred stock. This warrant will become exercisable for an aggregate of 357,142 shares of our common stock, at an exercise price equal to $0.84 per share, upon completion of this offering.
- (9)
- In February and June 2006, as part of a bridge financing, we issued unsecured convertible promissory notes in an aggregate principal amount of approximately $5.0 million to certain existing investors in two tranches. The notes accrued interest at the rate of 7.0% per year and were automatically convertible into shares of our preferred stock in the event we completed a preferred stock financing of at least $30.0 million, in the event of our sale or in certain other circumstances. All principal and unpaid interest accrued under the notes were subsequently converted into shares of our Series B preferred stock in connection with our Series B preferred stock financing in September 2006.
- (10)
- In connection with our February and June 2006 bridge financing, in addition to the notes, we also issued, in two tranches, to the purchasers of our notes, warrants that were exercisable for a number of shares of preferred stock to be issued in our next round of preferred stock financing determined based on the conversion price at which the notes were to be converted. We received aggregate consideration of $11,975 for these warrants. In September 2006, these warrants were adjusted pursuant to their terms to become exercisable for shares of our Series B preferred stock at an exercise price of $1.125 per share. Warrants exercisable for up to
II-4
an aggregate of 536,533 shares of our Series B preferred stock that were issued in the first tranche will terminate upon the earlier of February 2013 or completion of an initial public offering and warrants exercisable for up to an aggregate of 268,267 shares of our Series B preferred stock that were issued in the second tranche will terminate upon the earlier of June 2013 or completion of an initial public offering. All of the warrants issued in connection with our February and June 2006 bridge financing, which are exercisable for an aggregate 804,800 shares of our Series B preferred stock, will terminate if not exercised prior to the effectiveness of this offering. These warrants, which are exercisable for an aggregate 804,800 shares of our Series B preferred stock, will terminate if not exercised prior to the effectiveness of this offering.
- (11)
- In July 2006, in connection with a loan and security agreement, we issued one warrant to one lender. This warrant was amended in October 2006. Under the terms of this warrant, as amended, the warrant is exercisable for 306,667 shares of our Series B preferred stock at an initial exercise price of $1.125 per share. This warrant is exercisable through July 2013. This warrant will become exercisable for an aggregate of 306,667 shares of our common stock, at an exercise price equal to $1.125 per share, upon completion of this offering.
- (12)
- In September 2006, in connection with our Series B preferred stock financing, we issued an aggregate of 42,822,222 shares of our Series B preferred stock to 17 accredited investors, at $1.125 per share, for an aggregate purchase price of approximately $48.2 million.
- (13)
- In March 2007, we sold an additional 16,000,000 shares of our Series B preferred stock in a private placement to 16 of our existing accredited investors, at $1.125 per share, for an aggregate purchase price of $18.0 million.
- (14)
- In March 2007, two investors who purchased warrants in connection with our February and June 2006 bridge financing exercised their warrants for an aggregate of 256,000 shares of our Series B preferred stock at an exercise price of $1.125 per share, for an aggregate purchase price of $288,000.
- (15)
- In April 2007, in connection with an amendment to our existing loan and security agreement, we issued a warrant to our existing lender to purchase up to 933,333 shares of Series B preferred stock at an exercise price of $1.125 per share, 666,666 shares of which are immediately exercisable and the remaining 266,667 shares of which will become exercisable if we borrow additional amounts under our credit facility (with the actual number of shares becoming exercisable equaling 3.0% of the amount actually drawn down under the credit facility, divided by the exercise price). This warrant will become exercisable for up to an aggregate of 933,333 shares of our common stock, at an exercise price equal to $1.125 per share, upon completion of this offering and will remain exercisable through April 2014.
The offers, sales and issuances of the securities described in paragraph (1) were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such securities were our employees, directors or bona fide consultants and received the securities under our 2003 equity incentive plan. Appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about us.
II-5
The offers, sales, and issuances of the securities described in paragraphs (2) through (15) were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act and/or Rule 506 promulgated under Regulation D promulgated thereunder as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor within the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships, to information about us.
Item 16. Exhibits and financial statement schedules.
(a) Exhibits.
|
Exhibit
number
| | Description of document
|
|---|
|
1.1† |
|
Form of Underwriting Agreement. |
| 3.1(1) | | Registrant's Amended and Restated Certificate of Incorporation, as currently in effect. |
| 3.2(1) | | Form of the Registrant's Amended and Restated Certificate of Incorporation to become effective upon completion of this offering. |
| 3.3(1) | | Registrant's Bylaws, as currently in effect. |
| 3.4(1) | | Form of the Registrant's Amended and Restated Bylaws to become effective upon completion of this offering. |
| 4.1(1) | | Reference is made to exhibits 3.1 through 3.4. |
| 4.2† | | Form of Common Stock Certificate of the Registrant. |
| 4.3(1) | | Preferred Stock Purchase Warrant issued by the Registrant on March 21, 2005 to Lighthouse Capital Partners V, L.P. |
| 4.4(1) | | Amendment No. 1 to Preferred Stock Purchase Warrant, dated March 20, 2007, by and between the Registrant and Lighthouse Capital Partners V, L.P. |
| 4.5(1) | | Preferred Stock Purchase Warrant issued by the Registrant on July 14, 2006 to Lighthouse Capital Partners V, L.P. |
| 4.6(1) | | Amendment No. 1 to the Stock Purchase Warrant, dated as of October 2, 2006, by and between the Registrant and Lighthouse Capital Partners V, L.P. |
| 4.7(1) | | Amended and Restated Investors' Rights Agreement, dated September 21, 2006, by and among the Registrant and certain of its stockholders. |
| 4.8 | | Amendment No. 1 to Amended and Restated Investors' Rights Agreement, dated March 21, 2007, by and among the Registrant and certain of its stockholders. |
| 4.9(2) | | Preferred Stock Purchase Warrant, dated April 11, 2007, by and between the Registrant and Lighthouse Capital Partners V, L.P. |
| 5.1† | | Opinion of Cooley Godward Kronish LLP. |
| 10.1+ | | Form of Indemnity Agreement by and between the Registrant and its directors and executive officers. |
| | | |
II-6
| 10.2+(1) | | 2003 Equity Incentive Plan and Form of Option Agreement, Form of Stock Option Grant Notice, Exercise Notice and Restricted Stock Purchase Agreement and Notice of Exercise thereunder. |
| 10.3+(1) | | 2007 Equity Incentive Plan and Form of Stock Option Agreement, Form of Stock Option Grant Notice and Notice of Exercise thereunder. |
| 10.4+(1) | | 2007 Non-Employee Directors' Stock Option Plan and Form of Stock Option Agreement, Form of Initial and Annual Stock Option Grant Notice and Notice of Exercise thereunder. |
| 10.5+(1) | | 2007 Employee Stock Purchase Plan and Form of Offering Document thereunder. |
| 10.6+(1) | | Offer Letter Agreement, dated April 5, 2004, between the Registrant and Randall E. Woods. |
| 10.7+(1) | | Offer Letter Agreement, dated August 1, 2003, between the Registrant and Howard C. Dittrich, M.D. |
| 10.8+(1) | | Employment Agreement, dated January 2, 2007, between the Registrant and John E. Crawford. |
| 10.9(1) | | Office Lease, dated July 29, 2004, by and between the Registrant and R.B. Income Properties. |
| 10.10* | | Master Clinical Services Agreement, dated May 7, 2007, by and between the Registrant and Hesperion Ltd. |
| 10.11(2) | | Sublease Agreement, dated March 28, 2007, by and between the Registrant and Jones Day. |
| 10.12* | | License Agreement, dated August 14, 2003, between the Registrant and Kyowa Hakko Kogyo Co., Ltd. |
| 10.13*(1) | | Supplement to License Agreement, dated March 30, 2005, between the Registrant and Kyowa Hakko Kogyo Co., Ltd. |
| 10.14*(1) | | Settlement Agreement, dated March 31, 2005, between the Registrant and Kyowa Hakko Kogyo Co., Ltd. |
| 10.15* | | License Agreement, dated September 1, 2006, between the Registrant and Aetas Pharma Co., Ltd. |
| 10.16* | | Supplementary Agreement to License Agreement, dated January 5, 2007, between the Registrant and Aetas Pharma Co., Ltd. |
| 10.17(1) | | Loan and Security Agreement No. 4611, dated March 21, 2005, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 10.18(1) | | Amendment No. 01 to Loan and Security Agreement No. 4611, dated July 14, 2006, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 10.19* | | Development, Technology Transfer and Pre-Commercial Manufacturing Agreement, dated June 5, 2006, between the Registrant and DSM Pharma Chemicals, Inc. |
| 10.20*(1) | | Product Development Service Proposal, dated February 8, 2007, between the Registrant and Patheon Italia S.p.A. |
| 10.21*(1) | | Transfer & Commercial Manufacture Proposal, dated February 16, 2007, between the Registrant and Patheon Italia S.p.A. |
| 10.22+(1) | | Description of Annual Executive Bonus Plan. |
| | | |
II-7
| 10.23(2) | | Amendment No. 02 to Loan and Security Agreement, dated September 11, 2006, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 10.24(2) | | Amendment No. 03 to Loan and Security Agreement, dated April 11, 2007, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 23.1 | | Consent of Independent Registered Public Accounting Firm. |
| 23.2† | | Consent of Cooley Godward Kronish LLP. Reference is made to Exhibit 5.1. |
| 24.1(1) | | Power of Attorney. |
|
- †
- To be filed by amendment.
- +
- Indicates management contract or compensatory plan.
- *
- Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
- (1)
- Filed with the Registrant's Registration Statement on Form S-1 on March 29, 2007.
- (2)
- Filed with Amendment No. 1 to the Registrant's Registration Statement on Form S-1 on May 4, 2007.
(b) Financial statement schedules.
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
Item 17. Undertakings.
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The Registrant hereby undertakes that:
- (a)
- For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
- (b)
- For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-8
Signatures
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Amendment No. 2 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on the 23rd day of May, 2007.
| | | NOVACARDIA, INC. |
| | | | | |
| | | | | |
| | | By: | | |
| | | | | /s/ Randall E. Woods
Randall E. Woods
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 to the Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
| | Title
| | Date
|
|---|
|
|
|
|
|
|
/s/ Randall E. Woods
Randall E. Woods |
|
President, Chief Executive Officer and Member of the Board of Directors
(Principal Executive Officer) |
|
May 23, 2007 |
/s/ John E. Crawford
John E. Crawford |
|
Chief Financial Officer
(Principal Financial and Accounting Officer) |
|
May 23, 2007 |
*
Eckard Weber, M.D. |
|
Chairman of the Board of Directors |
|
May 23, 2007 |
*
Stuart J. M. Collinson, Ph.D. |
|
Member of the Board of Directors |
|
May 23, 2007 |
*
Nina Kjellson |
|
Member of the Board of Directors |
|
May 23, 2007 |
| | | | | |
II-9
*
David G. Lowe, Ph.D. |
|
Member of the Board of Directors |
|
May 23, 2007 |
*
Camille D. Samuels |
|
Member of the Board of Directors |
|
May 23, 2007 |
*
Joseph L. Turner |
|
Member of the Board of Directors |
|
May 23, 2007 |
*By: |
/s/ JOHN E. CRAWFORD
John E. Crawford
Attorney-in-Fact |
|
|
|
May 23, 2007 |
|
II-10
Exhibit index
|
Exhibit
number
| | Description of document
|
|---|
|
1.1† |
|
Form of Underwriting Agreement. |
| 3.1(1) | | Registrant's Amended and Restated Certificate of Incorporation, as currently in effect. |
| 3.2(1) | | Form of the Registrant's Amended and Restated Certificate of Incorporation to become effective upon completion of this offering. |
| 3.3(1) | | Registrant's Bylaws, as currently in effect. |
| 3.4(1) | | Form of the Registrant's Amended and Restated Bylaws to become effective upon completion of this offering. |
| 4.1(1) | | Reference is made to exhibits 3.1 through 3.4. |
| 4.2† | | Form of Common Stock Certificate of the Registrant. |
| 4.3(1) | | Preferred Stock Purchase Warrant issued by the Registrant on March 21, 2005 to Lighthouse Capital Partners V, L.P. |
| 4.4(1) | | Amendment No. 1 to Preferred Stock Purchase Warrant, dated March 20, 2007, by and between the Registrant and Lighthouse Capital Partners V, L.P. |
| 4.5(1) | | Preferred Stock Purchase Warrant issued by the Registrant on July 14, 2006 to Lighthouse Capital Partners V, L.P. |
| 4.6(1) | | Amendment No. 1 to the Stock Purchase Warrant, dated as of October 2, 2006, by and between the Registrant and Lighthouse Capital Partners V, L.P. |
| 4.7(1) | | Amended and Restated Investors' Rights Agreement, dated September 21, 2006, by and among the Registrant and certain of its stockholders. |
| 4.8 | | Amendment No. 1 to Amended and Restated Investors' Rights Agreement, dated March 21, 2007, by and among the Registrant and certain of its stockholders. |
| 4.9(2) | | Preferred Stock Purchase Warrant, dated April 11, 2007, by and between the Registrant and Lighthouse Capital Partners V, L.P. |
| 5.1† | | Opinion of Cooley Godward Kronish LLP. |
| 10.1+ | | Form of Indemnity Agreement by and between the Registrant and its directors and executive officers. |
| 10.2+(1) | | 2003 Equity Incentive Plan and Form of Option Agreement, Form of Stock Option Grant Notice, Exercise Notice and Restricted Stock Purchase Agreement and Notice of Exercise thereunder. |
| 10.3+(1) | | 2007 Equity Incentive Plan and Form of Stock Option Agreement, Form of Stock Option Grant Notice and Notice of Exercise thereunder. |
| 10.4+(1) | | 2007 Non-Employee Directors' Stock Option Plan and Form of Stock Option Agreement, Form of Initial and Annual Stock Option Grant Notice and Notice of Exercise thereunder. |
| 10.5+(1) | | 2007 Employee Stock Purchase Plan and Form of Offering Document thereunder. |
| 10.6+(1) | | Offer Letter Agreement, dated April 5, 2004, between the Registrant and Randall E. Woods. |
| 10.7+(1) | | Offer Letter Agreement, dated August 1, 2003, between the Registrant and Howard C. Dittrich, M.D. |
| 10.8+(1) | | Employment Agreement, dated January 2, 2007, between the Registrant and John E. Crawford. |
| | | |
| 10.9(1) | | Office Lease, dated July 29, 2004, by and between the Registrant and R.B. Income Properties. |
| 10.10* | | Master Clinical Services Agreement, dated May 7, 2007, by and between the Registrant and Hesperion Ltd. |
| 10.11(2) | | Sublease Agreement, dated March 28, 2007, by and between the Registrant and Jones Day. |
| 10.12* | | License Agreement, dated August 14, 2003, between the Registrant and Kyowa Hakko Kogyo Co., Ltd. |
| 10.13*(1) | | Supplement to License Agreement, dated March 30, 2005, between the Registrant and Kyowa Hakko Kogyo Co., Ltd. |
| 10.14*(1) | | Settlement Agreement, dated March 31, 2005, between the Registrant and Kyowa Hakko Kogyo Co., Ltd. |
| 10.15* | | License Agreement, dated September 1, 2006, between the Registrant and Aetas Pharma Co., Ltd. |
| 10.16* | | Supplementary Agreement to License Agreement, dated January 5, 2007, between the Registrant and Aetas Pharma Co., Ltd. |
| 10.17(1) | | Loan and Security Agreement No. 4611, dated March 21, 2005, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 10.18(1) | | Amendment No. 01 to Loan and Security Agreement No. 4611, dated July 14, 2006, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 10.19* | | Development, Technology Transfer and Pre-Commercial Manufacturing Agreement, dated June 5, 2006, between the Registrant and DSM Pharma Chemicals, Inc. |
| 10.20*(1) | | Product Development Service Proposal, dated February 8, 2007, between the Registrant and Patheon Italia S.p.A. |
| 10.21*(1) | | Transfer & Commercial Manufacture Proposal, dated February 16, 2007, between the Registrant and Patheon Italia S.p.A. |
| 10.22+(1) | | Description of Annual Executive Bonus Plan. |
| 10.23(2) | | Amendment No. 02 to Loan and Security Agreement, dated September 11, 2006, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 10.24(2) | | Amendment No. 03 to Loan and Security Agreement, dated April 11, 2007, between the Registrant and Lighthouse Capital Partners V, L.P. |
| 23.1 | | Consent of Independent Registered Public Accounting Firm. |
| 23.2† | | Consent of Cooley Godward Kronish LLP. Reference is made to Exhibit 5.1. |
| 24.1(1) | | Power of Attorney. |
|
- †
- To be filed by amendment.
- +
- Indicates management contract or compensatory plan.
- *
- Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
- (1)
- Filed with the Registrant's Registration Statement on Form S-1 on March 29, 2007.
- (2)
- Filed with Amendment No. 1 to the Registrant's Registration Statement on Form S-1 on May 4, 2007.
QuickLinks
Table of contentsProspectus summaryThe offeringSummary financial dataRisk factorsForward-looking statementsUse of proceedsDividend policyCapitalizationDilutionSelected financial dataManagement's discussion and analysis of financial condition and results of operationsBusinessManagementExecutive compensationTransactions with related personsPrincipal stockholdersDescription of capital stockShares eligible for future saleMaterial U.S. federal income tax consequences to non-U.S. holdersUnderwritingLegal mattersExpertsWhere you can find more informationNovaCardia, Inc. Index to financial statementsReport of independent registered public accounting firmNovaCardia, Inc. (a development stage company) Balance sheetsNovaCardia, Inc. (a development stage company) Statements of operationsNovaCardia, Inc. (a development stage company) Statements of cash flowsNovaCardia, Inc. (a development stage company) Notes to financial statements (Information as of March 31, 2007 and thereafter and for the three months ended March 31, 2006 and 2007 and the period from November 30, 2001 (inception) through March 31, 2007 is unaudited)PART II Information not required in prospectusSignaturesExhibit index