Exhibit 99.2

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only ASSERT Topline Data Results ASSERT: Phase 3 study evaluating the safety and efficacy of Bylvay ® (odevixibat) in patients with Alagille syndrome October 2022 (Nasdaq: ALBO) For Media & Investors Only

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 2 Forward Looking Statements This presentation includes “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 19 95. Forward - looking statements include statements, other than statements of historical fact, regarding, among other things: Albireo’s expected cash runway; Albireo’s commercializatio n p lans; the plans for, or progress, scope, cost, initiation, duration, enrollment, results or timing for availability of results of, development of Bylvay ®, A3907, A2342 or any other Albireo product candidate or program; the target indication(s) for development or approval; the timing for anticipated regulatory filings; discussions with the FDA or EMA regarding our program s; potential regulatory approval and plans for potential commercialization of Bylvay in biliary atresia or ALGS or in additional countries; the impact of the Expanded Access Program; the potential benefits or c om petitive position of Bylvay or any other Albireo product candidate or program or the commercial opportunity in any target indication; or Albireo’s plans, ex pec tations or future operations, financial position, revenues, costs or expenses. Albireo often uses words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “int end s,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “planned,” “continue,” “guidance,” or the negative of these terms or other similar expressions to identify forwa rd - looking statements. Actual results, performance or experience may differ materially from those expressed or implied by any forward - looking statement as a result of various risks, uncertainti es and other factors, including, but not limited to: whether the regulatory filings to be made for Bylvay in patients with ALGS will be made on the timelines we expect and be approved by the FDA and EMA; whether the FDA and EMA wil l complete their respective reviews within target timelines, once determined; whether the FDA and EMA will require additional informatio n, whether we will be able to provide in a timely manner any additional information that the FDA and EMA request, and whether such additional information will be satisfactory to the FDA and EMA; there are no guarantees that Bylvay will be commercially successful; we may encounter issues, delays or other challenges in commercializing Bylvay ; whether Bylvay receives adequate reimbursement from third - party payors; the degree to which Bylvay receives acceptance from patients and physicians for its approved indication; challenges associated with execution of our sal es activities, which in each case could limit the potential of our product; challenges associated with supply and distribution activities, which in each case c oul d limit our sales and the availability of our product; results achieved in Bylvay in the treatment of patients with PFIC or other approved indications may be different than observed in clinical trials, and m ay vary among patients; potential negative impacts of the COVID - 19 pandemic, including on manufacturing, supply, conduct or initiation of clinical trials, or other aspects of our business; whether favorable findings from clinical trials of Bylvay to date, including findings in PFIC, ALGS and other indications, will be predictive of results from other clinical trials of Bylvay ; there is no guarantee that Bylvay will be approved in jurisdictions or for indications beyond the jurisdictions in which or indications for which Bylvay is currently approved; there is no guarantee that our other products candidates will be approved; estimates of the addressable patient population for target indications may prove to be incorrect; the outco me and interpretation by regulatory authorities of the ongoing third - party study pooling and analyzing of long - term PFIC patient data; the timing for initiation or completion of, or for avail ability of data from, clinical trials of Bylvay , including BOLD, and the Phase 2 clinical trial of A3907, and the outcomes of such trials; Albireo’s ability to obtain coverage, pricing or reimbu rse ment for approved products in the United States or Europe; delays or other challenges in the recruitment of patients for, or the conduct of, the Company’s clinical trials; any repurcha se by the Company of Sagard’s interest in the royalty interest payments under our royalty monetization agreement with Sagard could materially impact our financial condition; and the Company’s critical accounting policies. These and other risks and uncertainties that Albireo faces are described in greater detail under the heading “Risk Factors” in Albireo’s most recent An nua l Report on Form 10 - K or in subsequent filings that it makes with the Securities and Exchange Commission. As a result of risks and uncertainties that Albireo faces, the results or events in dicated by any forward - looking statement may not occur. Albireo cautions you not to place undue reliance on any forward - looking statement. In addition, any forward - looking statement in this presentation represents Albireo’s views only as of the date of this presentation and should not be relied upon as representing its views as of any subsequent date. Albireo disclaim s a ny obligation to update any forward - looking statement except as required by applicable law. All forward - looking statements speak only as of the date this presentation is made and should not be relied upon as representing our views as of any date after this presentation is made. We specifically disclaim any obligation to update any forward - looking statement, except as required by applicable law. “Albireo” is a trademark of Albireo AB. All other trademarks, service marks, service marks, trade names, logos and brand names identified in this presentation are the properties of their respecti ve owners.
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 3 On the Call Today Ron Cooper President and CEO Jan Mattsson, PhD Chief Scientific Officer, Head of R&D , & Co - Founder Nadia Ovchinsky, MD, MBA, FAALSD The Children’s Hospital at Montefiore Principal Investigator, ASSERT Study Simon Harford Chief Financial Officer Pamela Stephenson Chief Commercial Officer

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 4 POSITIVE TOPLINE DATA RESULTS Bylvay ® (odevixibat) in Alagille S yndrome Patients ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only
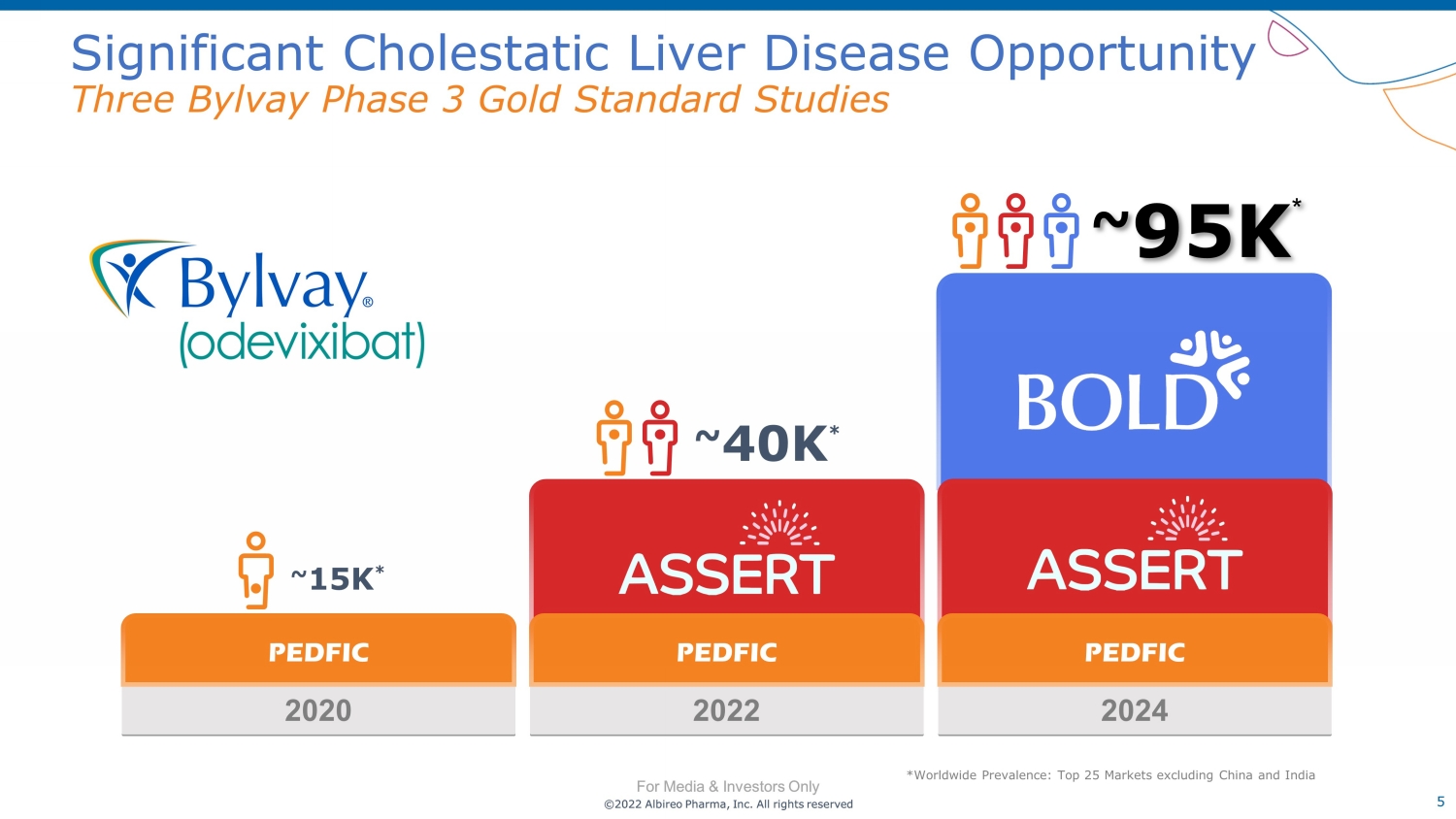
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 5 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Significant Cholestatic Liver Disease Opportunity Three Bylvay Phase 3 Gold Standard Studies *Worldwide Prevalence: Top 25 Markets excluding China and India 2020 PEDFIC ~ 15K * 2024 PEDFIC ~ 9 5K * 2022 PEDFIC ~ 40K *

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 6 Alagille Syndrome is a Devastating Disease May present in the first 3 months of life Cholestasis is the most common feature, typically presenting with unremitting pruritus Causes quality of life impairments including growth failure, sleep disturbances, disfiguring xanthomas Children with ALGS may need a liver transplant Alagille S yndrome (ALGS) A rare, life - threatening, genetic disorder, with multi - system involvement including paucity of liver bile ducts, leading to bile acid buildup.
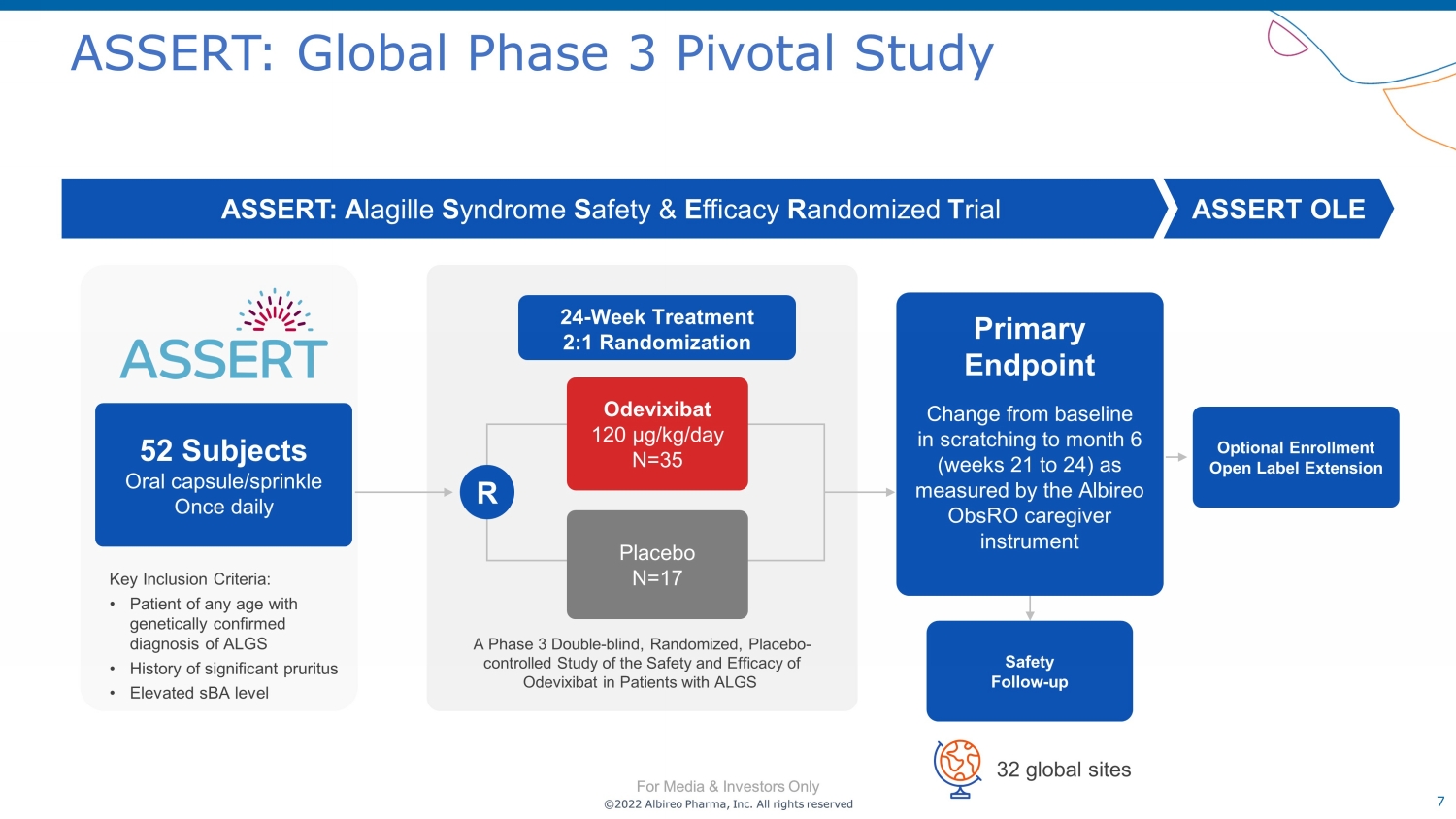
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 7 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only ASSERT: Global Phase 3 Pivotal Study Key Inclusion Criteria: • Patient of any age with genetically confirmed diagnosis of ALGS • History of significant pruritus • Elevated sBA level ASSERT: A lagille S yndrome S afety & E fficacy R andomized T rial ASSERT OLE A Phase 3 Double - blind, Randomized, Placebo - controlled Study of the Safety and Efficacy of Odevixibat in Patients with ALGS 24 - Week Treatment 2:1 Randomization R Placebo N=17 Odevixibat 120 µg/kg/day N=35 Safety Follow - up Optional Enrollment Open Label Extension 52 Subjects Oral capsule/sprinkle Once daily 32 global sites Primary Endpoint Change from baseline in scratching to month 6 (weeks 21 to 24) as measured by the Albireo ObsRO caregiver instrument
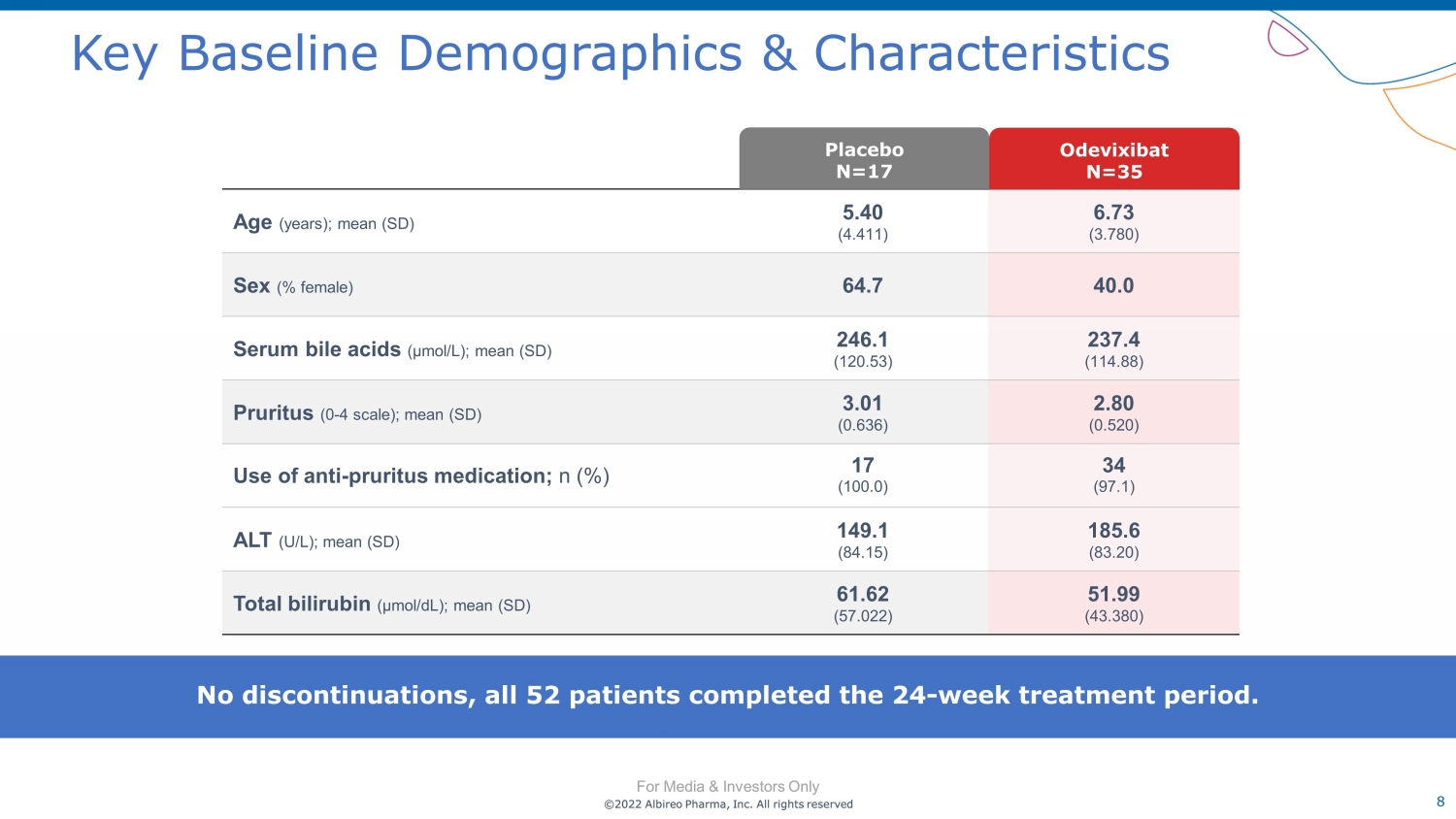
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 8 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Key Baseline Demographics & Characteristics Age (years); mean (SD) 5.40 (4.411) 6.73 (3.780) Sex (% female) 64.7 40.0 Serum bile acids (µmol/L); mean (SD) 246.1 (120.53) 237.4 (114.88) Pruritus (0 - 4 scale); mean (SD) 3.01 (0.636) 2.80 (0.520) Use of anti - pruritus medication; n (%) 17 (100.0) 34 (97.1) ALT (U/L); mean (SD) 149.1 (84.15) 185.6 (83.20) Total bilirubin (µmol/dL); mean (SD) 61.62 (57.022) 51.99 (43.380) Odevixibat N= 35 Placebo N= 17 No discontinuations, all 52 patients completed the 24 - week treatment period.

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 9 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Highly Statistically Significant Change in Pruritus and Serum Bile Acids 1. LS Mean at weeks 21 - 24 2. LS Mean at weeks 20 and 24 24 - week treatment with Bylvay led to highly statistically significant improvement in pruritus severity and reduction in serum bile acid levels compared to placebo Change from Baseline 1 (0 - 4) Primary: Pruritus Change from Baseline 2 (μmol/L) Key Secondary: Serum Bile Acids - 0.8 - 1.69 -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 Placebo (N=17) Odevixibat 120 µg/kg/day (N=35) 22.39 - 90.35 -100 -80 -60 -40 -20 0 20 40 Placebo (N=17) Odevixibat 120 µg/kg/day (N=35) p=0.002 p=0.001 Placebo (N=17) Odevixibat 120 µg/kg/day (N=35) Placebo (N=17) Odevixibat 120 µg/kg/day (N=35)
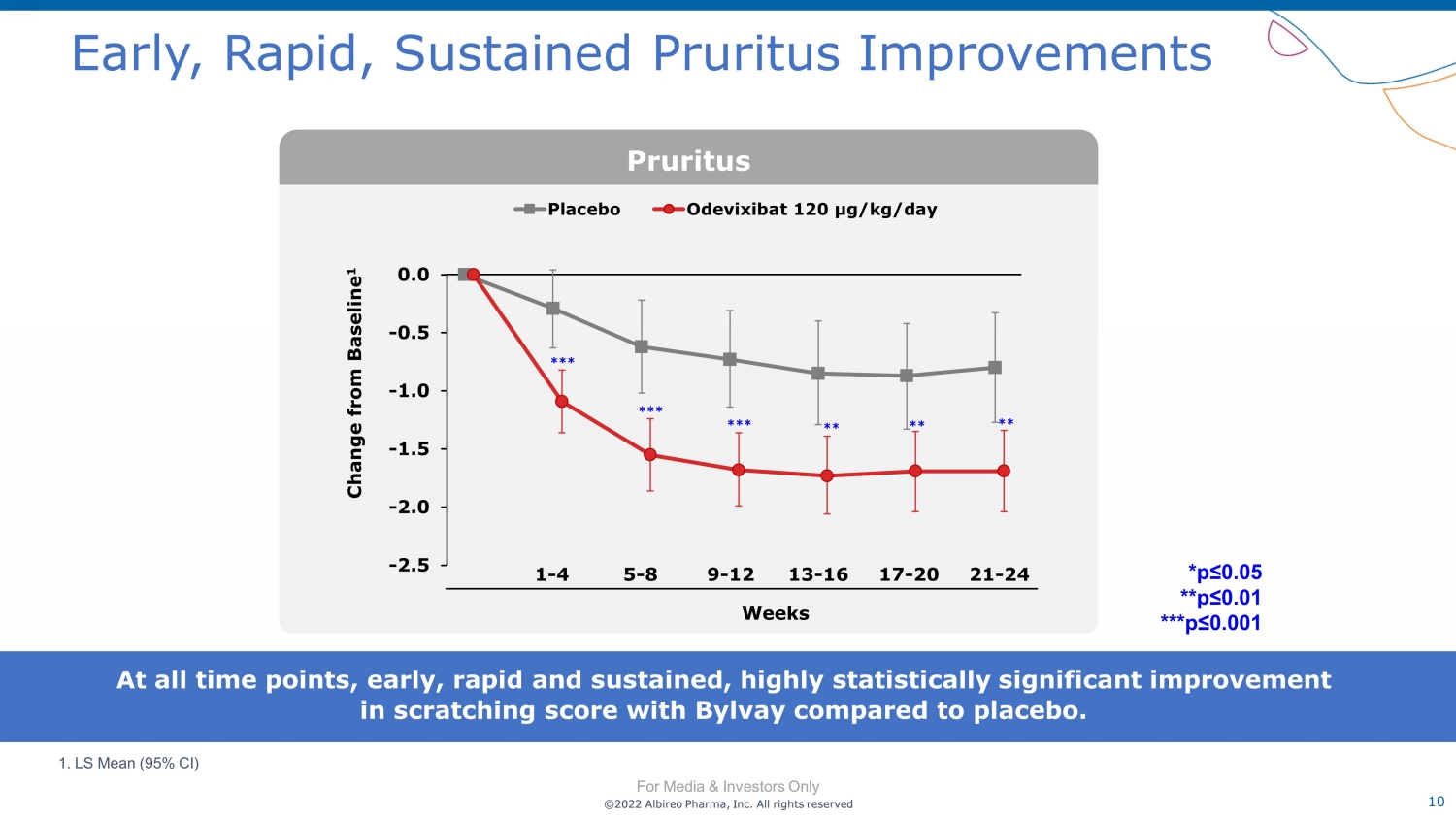
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 10 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Early, Rapid, Sustained Pruritus Improvements Change from Baseline 1 *** *** ** *** ** ** 1. LS Mean (95% CI) At all time points, early, rapid and sustained, highly statistically significant improvement in scratching score with Bylvay compared to placebo. Pruritus *p≤0.05 **p≤0.01 ***p≤0.001 Weeks 1 - 4 5 - 8 9 - 12 13 - 16 17 - 20 21 - 24 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 0.5 Placebo Odevixibat 120 µg/kg/day
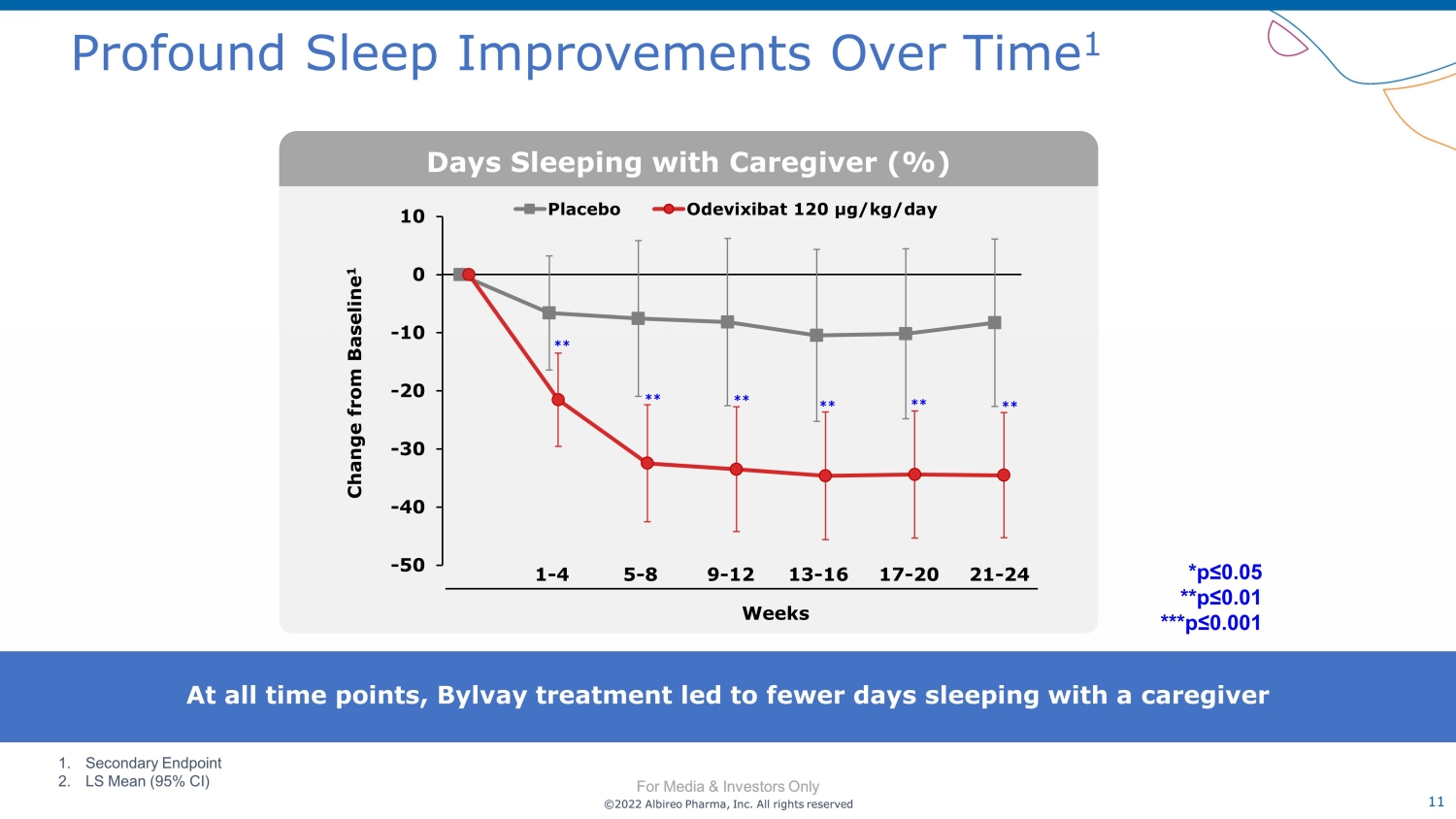
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 11 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Profound Sleep Improvements Over Time 1 At all time points, Bylvay treatment led to fewer days sleeping with a caregiver 1. Secondary Endpoint 2. LS Mean (95% CI) -50 -40 -30 -20 -10 0 10 Placebo Odevixibat 120 µg/kg/day Change from Baseline 1 ** ** ** ** ** ** Days Sleeping with Caregiver (%) *p≤0.05 **p≤0.01 ***p≤0.001 Weeks 1 - 4 5 - 8 9 - 12 13 - 16 17 - 20 21 - 24
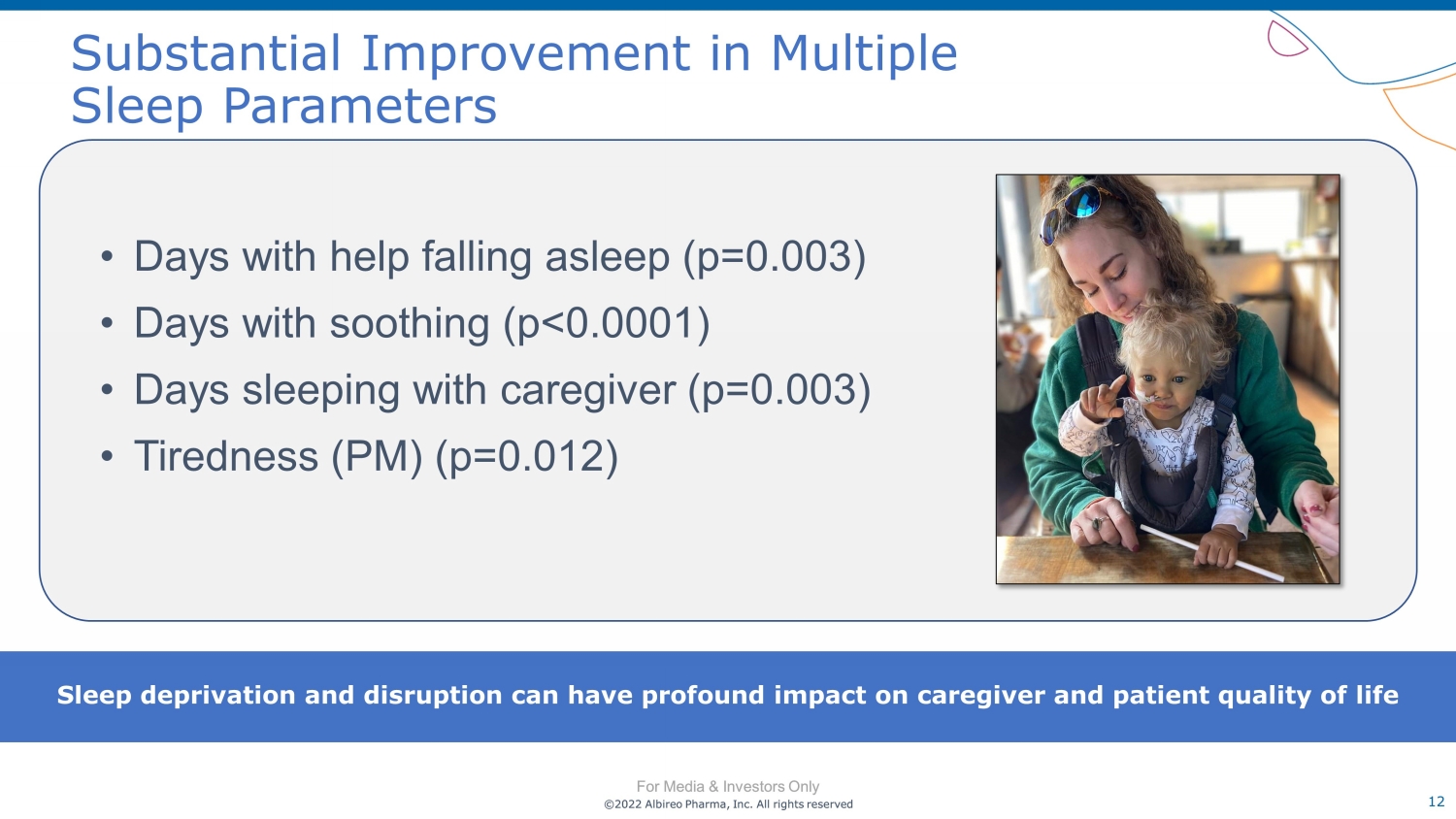
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 12 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Substantial Improvement in Multiple Sleep Parameters • Days with help falling asleep (p=0.003) • Days with soothing (p<0.0001) • Days sleeping with caregiver (p=0.003) • Tiredness (PM) (p=0.012) Sleep deprivation and disruption can have profound impact on caregiver and patient quality of life
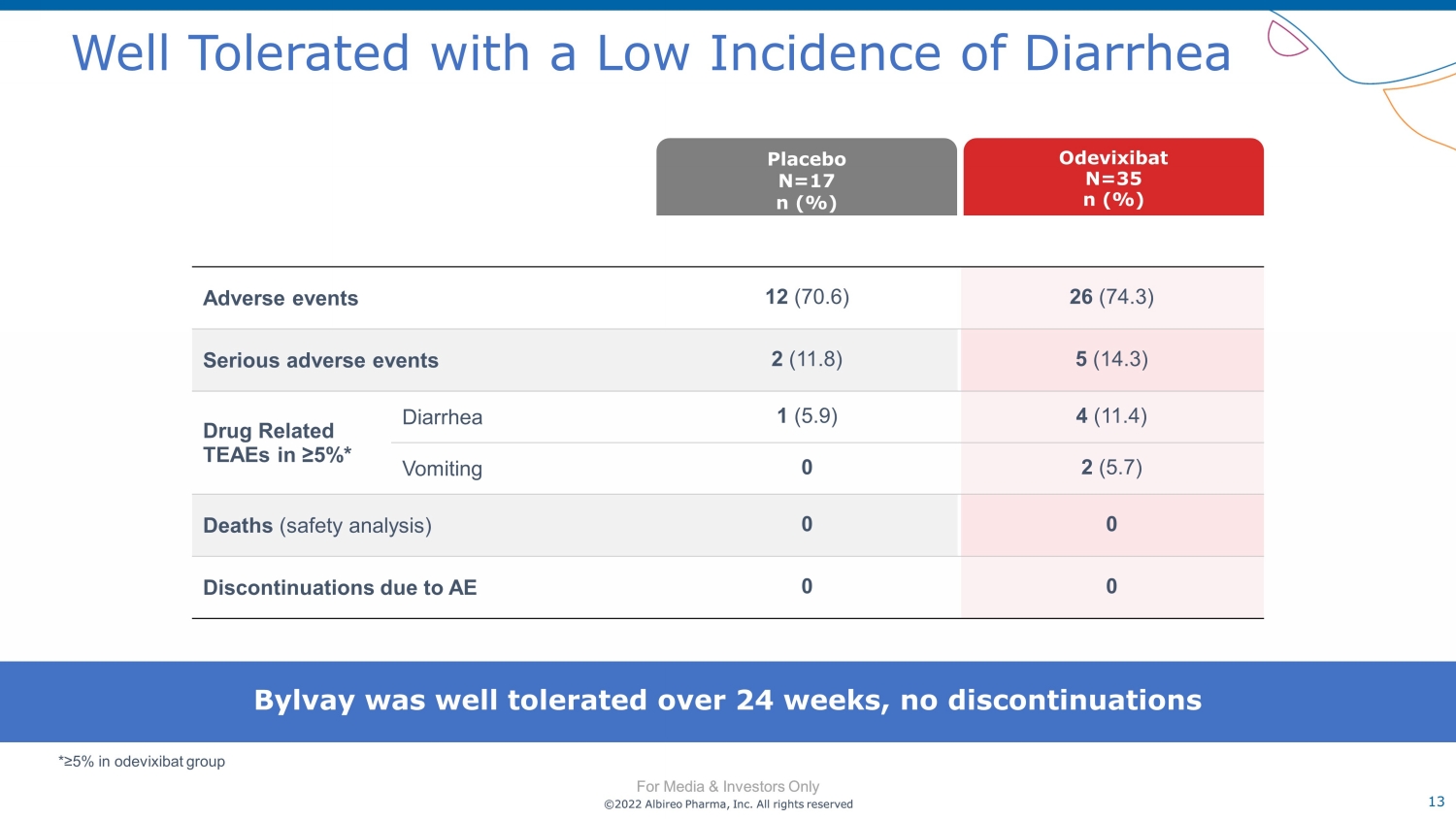
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 13 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Well Tolerated with a Low Incidence of Diarrhea Adverse events 12 (70.6) 26 (74.3) Serious adverse events 2 (11.8) 5 (14.3) Drug Related TEAEs in ≥5%* Diarrhea 1 (5.9) 4 (11.4) Vomiting 0 2 (5.7) Deaths (safety analysis) 0 0 Discontinuations due to AE 0 0 Odevixibat N= 35 n (%) Placebo N= 17 n (%) Bylvay was well tolerated over 24 weeks, no discontinuations *≥ 5% in odevixibat group
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 14 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Bylvay ASSERT Phase 3 Results Summary DIVERSE , GLOBAL STUDY POPULATION Trial enrolled patients from birth to young adults with both JAG1 and NOTCH2 mutations FIRST, ONLY & LARGEST PHASE 3 STUDY IN ALAGILLE SYNDROME R andomized , placebo - controlled WELL TOLERATED, NO DISCONTINUATIONS Low drug - related diarrhea rate, almost all patients rolled over to extension study Robust topline data to support regulatory filings – plans to complete in Q1 2023 ACHIEVED HIGHLY STATISTICAL SIGNIFICANCE Pruritus, serum bile acids, sleep efficacy endpoints

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 15 Nadia Ovchinsky , MD, MBA, FAALSD Director of Pediatric Hepatology Medical Director of Pediatric Liver Transplantation , Children's Hospital at Montefiore Professor of Pediatrics , Albert Einstein College of Medicine Nadia Ovchinsky , MD, MBA, FAALSD Principal Investigator, ASSERT Study
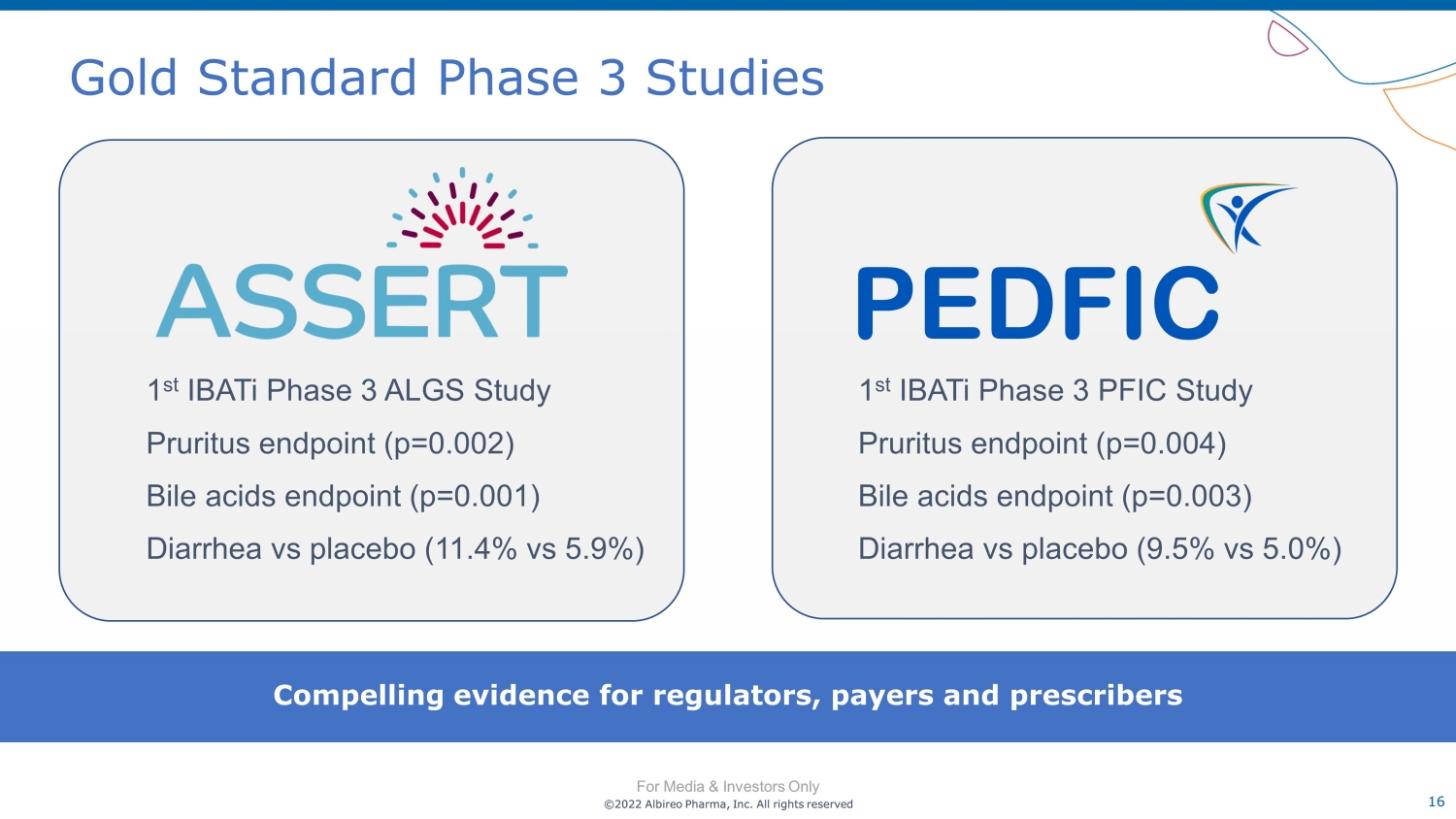
©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 16 ©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only Gold Standard Phase 3 Studies 1 st IBATi Phase 3 ALGS Study Pruritus endpoint (p=0.002) Bile acids endpoint (p=0.001) Diarrhea vs placebo (11.4% vs 5.9%) Compelling evidence for regulators, payers and prescribers 1 st IBATi Phase 3 PFIC Study Pruritus endpoint (p=0.004) Bile acids endpoint (p=0.003) Diarrhea vs placebo (9.5% vs 5.0%) PEDFIC

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only 17 Bylvay Plans and Priorities Strong financial foundation with over $ 270 million of cash US and Europe Regulatory Filings Q1 2023 Biliary Atresia Phase 3 Study Full Enrollment Q4 2022 Generate PFIC Sales Launch in Additional EU Countries

©2022 Albireo Pharma, Inc. All rights reserved For Media & Investors Only ASSERT Topline Data Results ASSERT: Phase 3 study evaluating the safety and efficacy of Bylvay ® (odevixibat) in patients with Alagille syndrome October 2022 (Nasdaq: ALBO) For Media & Investors Only

















