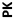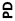BIOD-961 STUDY 6-101 RESULTS
Biodel’s Glucagon Formulation for Use in a Proprietary Auto-Reconstitution Device for the Treatment of
Severe Hypoglycemia Meets Primary Efficacy Endpoint in Phase 1 Clinical Trial
BIOD-961 Administration Results in Glucagon Exposure and Glucose Response that Meets Standard Bioequivalence
Criteria When Compared to Marketed Glucagon
Conference Call and Webcast Will be Held on Monday, March 16, 2015 at 8:30 a.m. Eastern Daylight Time
DANBURY, Conn., _____- Biodel Inc. (Nasdaq: BIOD) announced positive preliminary results from Study 6-101, a Phase 1 clinical trial comparing Biodel’s lyophilized glucagon formulation BIOD-961, designed for use in a proprietary Glucagon Emergency Management (GEM) auto-reconstitution device, to Eli Lilly’s Glucagon Emergency Rescue Kit and Novo Nordisk’s GlucaGen® HypoKitTM, which are marketed for the treatment of severe hypoglycemia. Study 6-101 was a randomized, single-center, double blind, six-period cross over study in 15 healthy volunteers who received each glucagon administered subcutaneously (SC) and intramuscularly (IM) in a randomized treatment sequence. The objectives of the study were to compare the pharmacokinetic (PK) profiles, the pharmacodynamic (PD, glucose) responses, and the PK/PD relationships of IM and SC dosing, as well as to assess safety profiles of the three test glucagons.
Data Highlights
●Overall, PK and PD (glucose response) profiles among all three glucagon formulations were statistically indistinguishable.
●Although this study was designed as an exploratory comparison of PK and PD profiles, standard regulatory criteria for PK and PD bioequivalence were satisfied when comparing BIOD-961 to Eli Lilly’s Glucagon Emergency Rescue Kit and Novo Nordisk’s GlucaGen® HypoKitTM.
●As expected, nausea was the most common adverse event for all glucagon formulations. The incidence of adverse events was similar among treatments.
Biodel formulated BIOD-961 for use in a user-friendly injection device, referred to as the Glucagon Emergency Management (GEM) system. The GEM device is a customized version of an auto-reconstitution syringe developed by Unilife Corporation. The device is specifically designed to address and expand an underserved market currently wrestling with cumbersome glucagon kits that are especially difficult to use and operate during an emergency for the treatment of severe hypoglycemia.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
Dr. Alan Krasner, chief medical officer of Biodel, stated “We are very pleased that in this exploratory trial BIOD-961 shows the ability to meet pharmacokinetic and pharmacodynamic criteria for bioequivalence to a marketed glucagon formulation. The FDA requires PK and PD bioequivalence as co-primary objectives of our pivotal trial for approval of this product. We look forward to sharing these data with the FDA and discussing the components of the remaining development program, including the design of the pivotal bioequivalence trial.”
Dr. Errol De Souza, president and chief executive officer of Biodel, stated: “The results of this study exceeded expectations. This is the first glucagon formulation in development for the treatment of severe hypoglycemia which has demonstrated comparable pharmacokinetic and pharmacodynamic profiles to marketed glucagon formulations. In addition to the pivotal clinical trial, a human factors study is planned for the first half 2015 to compare the usability of the GEM device to the existing manually reconstituted commercial glucagon kits in the hands of representative caregivers, with the final pivotal human factors study expected to be completed by the end of 2015. We plan to request an end-of-Phase 2 meeting with the FDA in the second calendar quarter of 2015 and submit the NDA in mid-2016”.
Study Design
Study 6-101 was a Phase 1 randomized, single-center, double blind, six-period cross over study designed to evaluate the PK and PD profiles of BIOD-961 compared to marketed glucagon formulations manufactured by Eli Lilly and Novo Nordisk. BIOD-961 is a lyophilized glucagon formulation designed for use in the GEM auto-reconstitution device. On six separate dosing days, each subject received 1 mg of one of the test glucagons delivered either SC or IM. Fifteen normal, healthy subjects were randomized into the study and ten completed all dosings. The objectives of the study were to compare the PK profiles, the PD (glucose) responses, and the PK/PD relationships of IM and SC dosing, as well as to assess safety profiles of the three test glucagons. These data will facilitate selection of an appropriate marketed glucagon to use as a comparator in the planned pivotal study, in which the primary efficacy endpoint for approval is to demonstrate PK and PD bioequivalence of BIOD-961 to one of the marketed comparators.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
About Glucagon Emergency Management (GEM) Device for the Treatment of Severe Hypoglycemia
Biodel's Glucagon Emergency Management (GEM) system is a lyophilized glucagon formulation provided in a proprietary auto-reconstitution device intended for the rescue treatment of patients with severe hypoglycemia. GEM is expected to be available in separate presentations for the delivery of pediatric (0.5 mg) and adult (1.0 mg) dosages of glucagon. The GEM device provides automatic needle retraction after dose delivery.
There is currently an unmet medical need for a more user-friendly glucagon emergency injection device since the complexity of existing kits increases caregiver training requirements, the likelihood of dosing errors (especially dosing only with diluent) and the time required to deliver the rescue therapy. It is believed that these factors lead to relatively low prescription, fill, refill and usage rates of existing glucagon emergency kits. The annual U.S. glucagon market for the treatment of severe hypoglycemia is approximately $160 million.
The GEM device has been designed to require little to no training for use and is intended to be a more user-friendly glucagon presentation compared to emergency kits currently marketed by Eli Lilly and Novo Nordisk. The automatic reconstitution of the glucagon formulation eliminates the possibility of dosing only with diluent. The automatic needle retraction after dose delivery reduces the risk of accidental needle stick injuries. Dating and storage of the GEM device is anticipated to be comparable to existing glucagon kits.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
Pharmacokinetic (PK) and Pharmacodynamic (PD) Parameters: Biodel’s Glucagon Formulation (BIOD-961) vs.Eli Lilly’s Glucagon Emergency Rescue Kit (Lilly) vs. Novo Nordisk’s GlucaGen®HypoKitTM(Novo) |
Parameter | Treatment Groups |
Subcutaneous Injection (1 mg Glucagon) | | Intramuscular Injection (1 mg Glucagon) |
BIOD-961
(n=12) | Lilly
(n=11) | Novo
(n=11) | | BIOD-961
(n=10) | Lilly
(n=11) | Novo
(n=12) |
| | | | | | | | |
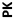 | Cmax
(µg/L) | 9.01 ± 0.75 | 7.87 ± 0.79 | 9.16 ± 0.59 | | 6.80 ± 0.52 | 6.75 ± 0.31 | 7.22 ± 0.44 |
AUC0-240 min
(µg*min/L) | 559.5 ± 28.7 | 520.8 ± 29.8 | 570.5 ± 26.8 | | 409.5 ± 39.2 | 402.6 ± 27.8 | 441.4 ± 42.7 |
Tmax
(min) | 26.7 ± 3.76 | 23.2 ± 1.82 | 20.8 ± 1.83 | | 15.5 ± 2.07 | 17.7 ± 4.39 | 14.6 ± 2.64 |
| | | | | | | | |
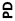 | BGmax
(mg/dL) | 178.2 ± 7.3 | 170.9 ± 5.3 | 175.3 ± 5.9 | | 182.9 ± 6.5 | 171.1 ± 6.7 | 174.8 ± 5.5 |
BGAUC 0-240 min
(mg*min/dL) | 26118 ± 630 | 25915 ± 685 | 25240 ± 424 | | 26184 ± 607 | 24960 ± 568 | 25274 ± 485 |
Time to
BGmax(min) | 41.7 ± 4.1 | 43.6 ± 4.2 | 34.6 ± 3.2 | | 40.5 ± 2.5 | 40.5 ± 3.4 | 44.6 ± 3.8 |
Data represent the Mean ± Standard Error of the Mean
There were no significant differences between BIOD-961 and Lilly or Novo glucagon on any of the PK or PD parameters.
Cmax= Maximal glucagon concentration
AUC0-240 min= Area under the glucagon concentration vs. time curve in the 240 minutes after injection
BGmax= Maximal glucose concentration
BGAUC 0-240 min= Area under the blood glucose concentration vs. time curve in the 240 minutes after injection |
Conference Call and Webcast Information
Biodel's senior management will host a conference call on Monday, March 16, 2015 at 8:30 a.m. Eastern Daylight Time to discuss these results. Live audio of the conference call will be available to investors, members of the news media and the general public by dialing +1 (877) 407-7181 (Toll-Free US & Canada) or +1 (201) 689-8047 (International). To access the call by live audio webcast, please log on to the investor section of the company's website athttp://www.biodel.com. An archived version of the audio webcast will be available on Biodel's website until January 14, 2015 or by dialing +1 (877) 660-6853 (Toll Free US & Canada) or +1 (201) 612-7415 (International) and entering conference ID number 13588552.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
About Biodel Inc.
Biodel Inc. is a specialty biopharmaceutical company focused on the development and commercialization of innovative treatments for diabetes that may be safer, more effective and more convenient for patients. Biodel's product candidates are developed by applying proprietary technologies to existing drugs in order to improve their therapeutic profiles. More information about Biodel is available atwww.biodel.com.
Safe-Harbor Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements about future activities related to the clinical development plans for Biodel's product candidates, potential timing, design and outcomes of clinical trials and Biodel's ability to develop and commercialize its product candidates. Forward-looking statements represent Biodel's management's judgment regarding future events. All statements, other than statements of historical facts, including statements regarding Biodel's strategy, future operations, future clinical trial results, future financial position, future revenues, projected costs, prospects, plans and objectives of management are forward-looking statements. The words "anticipates," "believes," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Biodel's forward-looking statements are subject to a number of known and unknown risks and uncertainties that could cause actual results, performance or achievements to differ materially from those described or implied in the forward-looking statements, including, but not limited to, the progress, timing or success of Biodel's research and development and clinical programs for Biodel's product candidates; Biodel's ability to conduct the development work necessary to finalize the formulation and design of Biodel's auto-reconstitution glucagon rescue product candidate, as well as the preclinical studies, clinical trials and manufacturing activities necessary to support the filing of a new drug application, or NDA, to the U.S. Food and Drug Administration, or FDA, for that product candidate; Biodel's ability to engage a strategic partner in the further development of Biodel's prandial ultra-rapid-acting insulin formulations, including BIOD-531, which uses regular human insulin, or RHI, as the active pharmaceutical ingredient, and Biodel's insulin analog-based formulations; the success of Biodel's formulation development work to improve the stability of Biodel's newer ultra-rapid-acting insulin analog-based formulations while maintaining the pharmacokinetic and injection site toleration characteristics associated with earlier formulations; the results of Biodel's real-time stability programs for Biodel's RHI-, insulin analog- and glucagon-based product candidates, including the reproducibility of earlier, smaller scale, stability studies and Biodel's ability to accurately project long term stability on the basis of accelerated testing; Biodel's ability to accurately anticipate technical challenges that the company may face in the development of Biodel's ultra-rapid-acting RHI- and insulin analog-based product candidates or Biodel's glucagon rescue product candidates; Biodel's ability to secure approval by the FDA for Biodel's product candidates under Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act; Biodel's ability to enter into collaboration arrangements for the commercialization of Biodel's product candidates and the success or failure of any such collaborations into which the company enters, or Biodel's ability to commercialize its product candidates on its own; Biodel's ability to enforce Biodel's patents for Biodel's product candidates and Biodel's ability to secure additional patents for Biodel's product candidates; and other factors identified in our most recent annual report on Form 10-K for the fiscal year ended September 30, 2014. The company disclaims any obligation to update any forward-looking statements as a result of events occurring after the date of this press release.
BIOD-G
CONTACT: John Graziano (The Trout Group), +1-646-378-2942
SOURCE Biodel Inc.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release