Exhibit 99.1
Biodel Inc and Albireo Limited Share Exchange Agreement
NASDAQ: BIOD ? October 11, 2016
Cautionary Note Regarding Forward-Looking Statements
Certain statements contained in this presentation regarding matters that are not historical facts are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, known as the PSLRA. These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Albireo undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,”
“intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to those described in the documents Biodel has filed with the SEC as well as the possibility that Biodel may be unable to obtain stockholder approval required for the proposed transaction with us, the expected timing and likelihood of completion of the proposed transaction, the inability to successfully integrate the businesses or the risk that such integration may be more difficult, time-consuming or costly than expected, the occurrence of any event, change or other circumstances that could give rise to the termination of the share exchange agreement, the inability of the parties to meet expectations regarding the accounting and tax treatments of the proposed transaction, the potential for the proposed transaction to involve unexpected costs, the risk that the parties may not be able to satisfy the conditions to the proposed transaction in a timely manner or at all, risks related to disruption of management time from ongoing business operations due to the proposed transaction, the risk that the expected benefits of the proposed combination are not realized, and the risk that any announcements relating to the proposed transaction could have adverse effects on the market price of Biodel’s common stock. Risks and uncertainties related to
Albireo that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to: whether the preliminary data from the ongoing Phase 2 trial of A4250 in children with chronic cholestasis will be confirmed following database lock; whether the ongoing Phase 2 trial of A4250 in children with chronic cholestasis will be sufficient to support advancement into a pivotal trial in Progressive Familial Intrahepatic Cholestasis (PFIC); the timing and outcome of the planned meeting with the FDA regarding the anticipated pivotal program for A4250 in PFIC; the designs, endpoints, numbers of patients and treatment periods for trials that will be required to support approval of A4250 to treat PFIC or any other orphan pediatric liver disease; whether
the cash resources of Biodel and proceeds from the planned concurrent financing will be sufficient to advance A4250 through completion of a planned pivotal trial in PFIC; the timing for initiation or completion of, or availability of data from, ongoing or future trials of A4250, including a planned pivotal trial in PFIC, or elobixibat and the outcomes of such trials; whether results of the first cohort of patients in the ongoing Phase 2 trial of A4250 in primary biliary cholangitis will be predictive of similar or better results in the trial’s second cohort; delays or other challenges in the recruitment of patients for current or future trials of any Albireo product candidate; the medical benefit that may be derived from A4250, elobixibat, A3384 or any other Albireo product candidate; the ability to establish a strategic alliance, collaboration or licensing or other comparable arrangement on favorable terms for elobixibat in the United States and Europe; whether or when
EA Pharma filed a new drug application for elobixibat in Japan; the extent to which Albireo’s agreement with EA Pharma for elobixibat generates nondilutive income to Albireo; whether the FDA will consider a single additional trial sufficient to establish the efficacy of elobixibat to support approval for the treatment of chronic idiopathic constipation in the
United States; Albireo’s ability to protect its intellectual property; the competitive environment and commercial opportunity for a potential treatment for PFIC and other orphan pediatric cholestatic liver diseases; whether findings from nonclinical studies and clinical trials of IBAT inhibitors will be predictive of future clinical success for an Albireo IBAT inhibitor in the treatment of nonalcoholic steatohepatitis (NASH); and the timing and success of submission, acceptance and approval of regulatory filings. In addition, market and industry statistics contained in this presentation are based on information available to us that we believe to be reliable but have not independently verified.
All forward-looking statements speak only as of the date this presentation is made and should not be relied upon as representing our views as of any date after this presentation is made. We specifically disclaim any obligation to update any forward-looking statement, except as required by applicable law.
“Albireo” is a trademark of Albireo AB. All other trademarks, service marks, service marks, trade names, logos and brand names identified in this presentation are the properties of their respective owners.
©2016 Albireo Limited. All rights reserved.
Additional Information and Where You Can Find It
This presentation may be deemed to be solicitation material in respect of the proposed transaction between Biodel Inc. (Biodel), Albireo Limited (Albireo) and Albireo stockholders and noteholders. In connection with the proposed transaction, Biodel filed with the Securities and Exchange Commission (SEC) on September 19, 2016 a definitive proxy statement in connection with the solicitation of proxies for its 2016 Annual Meeting and has mailed the definitive proxy statement to Biodel stockholders. BIODEL URGES INVESTORS AND STOCKHOLDERS TO READ THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION, AS WELL AS OTHER DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THESE DOCUMENTS, BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. This presentation is not a substitute for the definitive proxy statement or any other documents that Biodel may file with the SEC or send to Biodel stockholders in connection with the proposed transaction. Before making any voting decision, investors and security holders are urged to read the proxy statement and all other relevant documents filed or that will be filed with the SEC in connection with the proposed transaction as they become available because they will contain important information about the proposed transaction and related matters. You may obtain free copies of the proxy statement and all other documents filed or that will be filed with the SEC regarding the proposed transaction at the website maintained by the SEC www.sec.gov. Copies of the proxy statement are available free of charge on Biodel’s website at www.biodel.com or by contacting Biodel’s Corporate Secretary at 203-796-5000 or by mail at Investor Relations, Biodel Inc., 100 Saw Mill Road, Danbury, Connecticut 06810.
Participants in Solicitation – Biodel, Albireo and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from the holders of Biodel common stock in connection with the proposed transaction. Information about these participants, and a description of their direct and indirect interests, by security holdings or otherwise, may be found in the definitive proxy statement that Biodel filed with the SEC on September 19, 2016 relating to its 2016 Annual Meeting of Stockholders. Other information regarding participants in the proxy solicitation may be contained in other relevant materials filed by Biodel with the SEC. You may obtain free copies of these documents as described in the preceding paragraph.
©2016 Albireo Limited. All rights reserved.
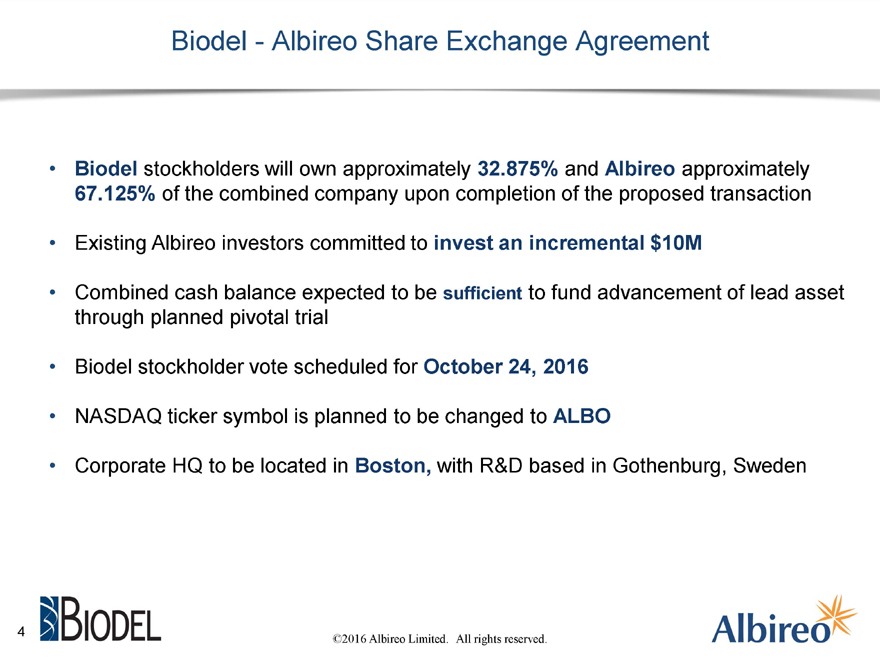
Biodel—Albireo Share Exchange Agreement
Biodel stockholders will own approximately 32.875% and Albireo approximately 67.125% of the combined company upon completion of the proposed transaction
Existing Albireo investors committed to invest an incremental $10M
Combined cash balance expected to be sufficient to fund advancement of lead asset through planned pivotal trial
Biodel stockholder vote scheduled for October 24, 2016
NASDAQ ticker symbol is planned to be changed to ALBO
Corporate HQ to be located in Boston, with R&D based in Gothenburg, Sweden
©2016 Albireo Limited. All rights reserved.
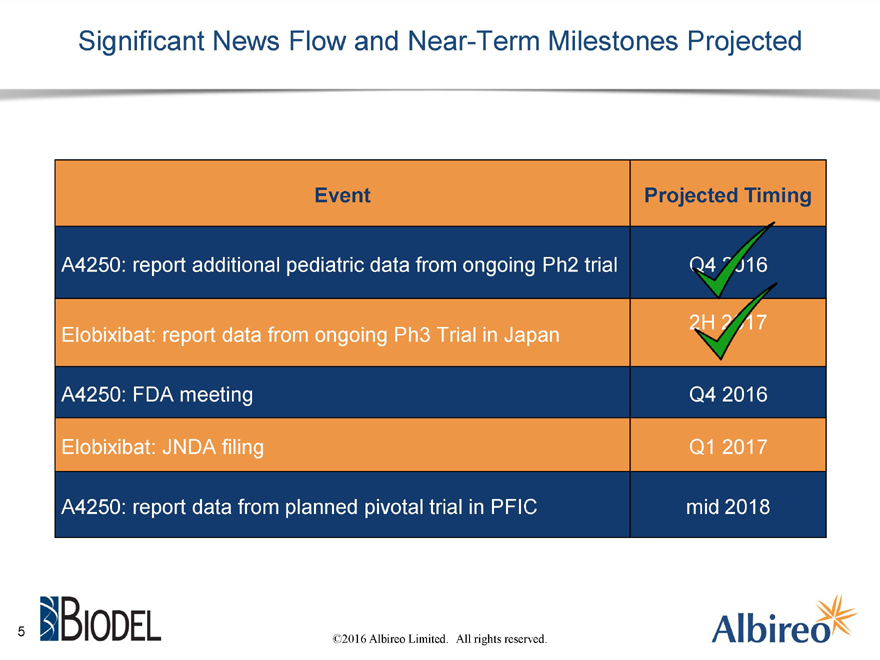
Significant News Flow and Near-Term Milestones Projected
Event Projected Timing
A4250: report additional pediatric data from ongoing Ph2 trial
Elobixibat: report data from ongoing Ph3 Trial in Japan
A4250: FDA meeting Q4 2016
Elobixibat: JNDA filing Q1 2017
A4250: report data from planned pivotal trial in PFIC mid 2018
©2016 Albireo Limited. All rights reserved.
Hope for Children with Orphan Liver Diseases Through Bile Acid Modulation
Albireo
Innovative Science + Deep Pipeline + Experienced Management
Leader in the development of bile acid modulators to treat liver and GI diseases
Three clinical assets, including lead Phase 2 orphan product candidate and Phase 3 constipation product candidate
Multiple near-term inflection opportunities
Strong patent portfolio, with orphan drug designation and future potential for valuable Pediatric Priority Review Voucher for lead product candidate (A4250)
AstraZeneca spinout backed by syndicate of leading biotech investors — TPG, TVM Capital, Phase4 Partners
Highly experienced management team with track record of developing and commercializing blockbuster drugs
©2016 Albireo Limited. All rights reserved.
Deep Biotech and Pharma Experienced Management Team
Ron Cooper
President and CEO
BMS
(President of Europe)
Paresh Soni Jan Mattsson, PhD
MBChB, FCP, PhD Chief Operating Officer
Chief Medical Officer (Co-founder)
Alexion, Amarin, Pfizer AstraZeneca
Per-Göran Gillberg, PhD Tom Shea
VP, Development Chief Financial Officer
(Co-founder)
Cubist, Euthymics, Neurovance,
AstraZeneca, Pharmacia, Kabi EBI, Tolerx, Epirus
Kristina Torfgård, PhD Pete Zorn
VP, Clinical SVP, Corporate Development and and Regulatory Affairs General Counsel
AstraZeneca Santaris, Targacept
©2016 Albireo Limited. All rights reserved.
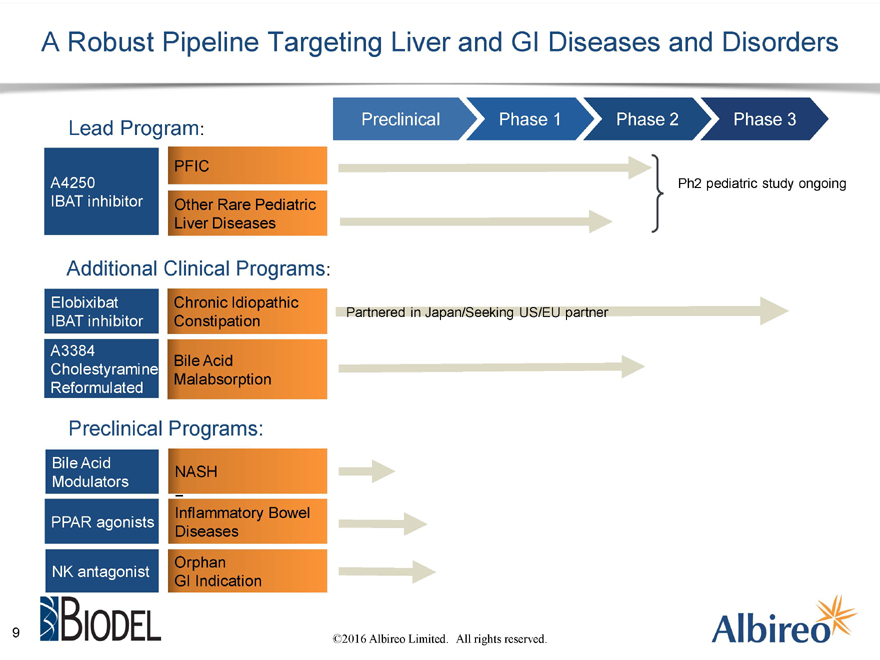
A Robust Pipeline Targeting Liver and GI Diseases and Disorders
Lead Program: Preclinical Phase 1 Phase 2 Phase 3
PFIC
A4250 Ph2 pediatric study ongoing
IBAT inhibitor Other Rare Pediatric
Liver Diseases
Additional Clinical Programs:
Elobixibat Chronic Idiopathic
Partnered in Japan/Seeking US/EU partner
IBAT inhibitor Constipation A3384
Bile Acid
Cholestyramine
Malabsorption Reformulated
Preclinical Programs:
Bile Acid
NASH
Modulators
Inflammatory Bowel PPAR agonists Diseases
Orphan NK antagonist
GI Indication
©2016 Albireo Limited. All rights reserved.
9
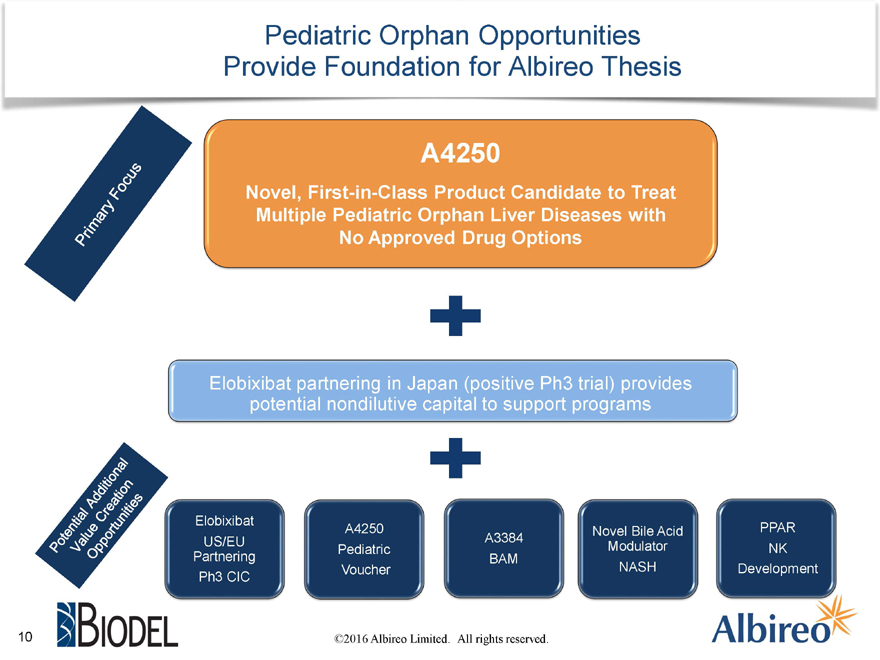
Pediatric Orphan Opportunities Provide Foundation for Albireo Thesis
A4250
Novel, First-in-Class Product Candidate to Treat Multiple Pediatric Orphan Liver Diseases with No Approved Drug Options
Elobixibat partnering in Japan (positive Ph3 trial) provides potential nondilutive capital to support programs
Elobixibat
A4250 Novel Bile Acid PPAR US/EU A3384 Pediatric Modulator NK
Partnering BAM
Voucher NASH Development Ph3 CIC
©2016 Albireo Limited. All rights reserved.
10
A4250 for Orphan Pediatric
Liver Diseases
Positioned to Become First Drug Approved for Progressive Familial Intrahepatic Cholestasis
A4250
The market opportunity…
Multiple pediatric orphan liver diseases and disorders with no approved drugs; lifetime treatment
The science…
Elevated serum bile acids linked to both progressive liver disease and debilitating pruritus
Positive effects on serum bile acid levels and pruritus in preclinical and early clinical development
The exclusivity period…
Orphan designation in PFIC provides 7 years in US + 10 years in EU
Composition of matter to 2025*
Method of use for liver disease to 2031 (EU allowed, US pending)
*includes estimated H-W PTE
©2016 Albireo Limited. All rights reserved.
12
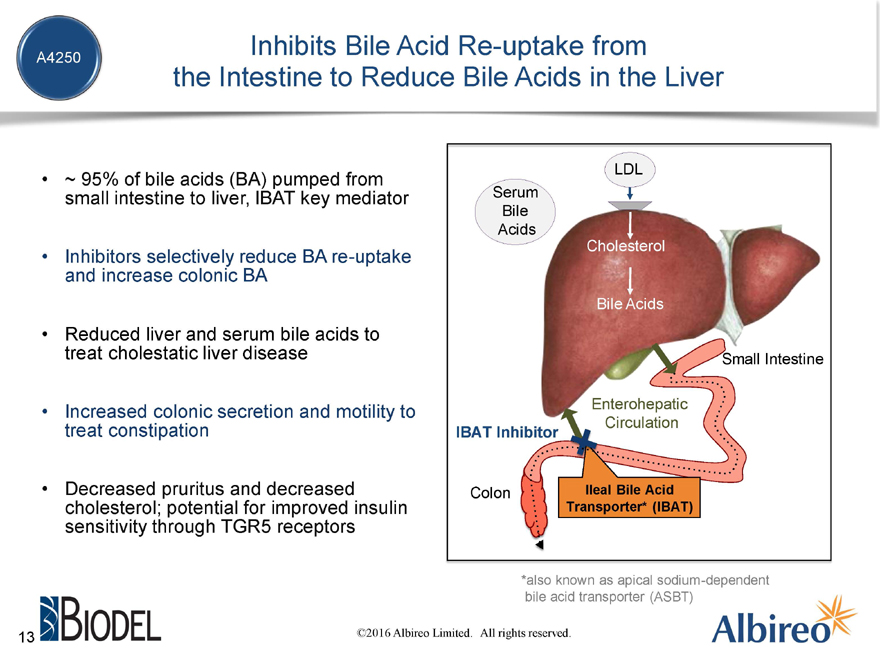
~ 95% of bile acids (BA) pumped from small intestine to liver, IBAT key mediator
Inhibitors selectively reduce BA re-uptake and increase colonic BA
Reduced liver and serum bile acids to treat cholestatic liver disease
Increased colonic secretion and motility to treat constipation
Decreased pruritus and decreased cholesterol; potential for improved insulin sensitivity through TGR5 receptors
Serum Bile Acids
*also known as apical sodium-dependent bile acid transporter (ASBT)
©2016 Albireo Limited. All rights reserved.
13
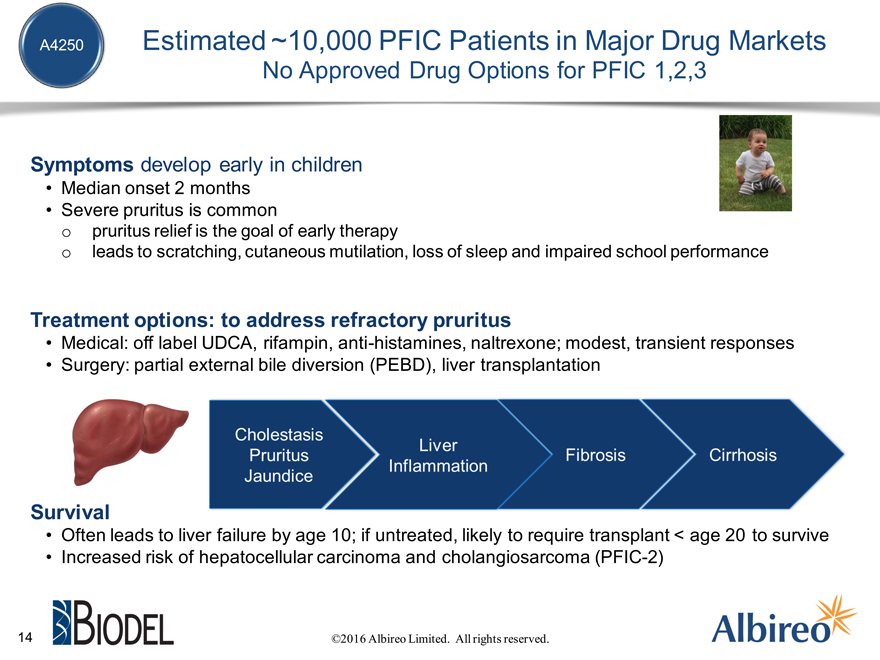
A4250 Estimated ~10,000 PFIC Patients in Major Drug Markets
No Approved Drug Options for PFIC
Symptoms develop early in children
Median onset 2 months Severe pruritus is common
pruritus relief is the goal of early therapy leads to scratching, cutaneous mutilation, loss of sleep and impaired school performance
Treatment options: to address refractory pruritus
Medical: off label UDCA, rifampin, anti-histamines, naltrexone; modest, transient responses Surgery: partial external bile diversion (PEBD), liver transplantation
Survival
Often leads to liver failure by age 10; if untreated, likely to require transplant < age 20 to survive Increased risk of hepatocellular carcinoma and cholangiosarcoma (PFIC-2)
©2016 Albireo Limited. All rights reserved.
14
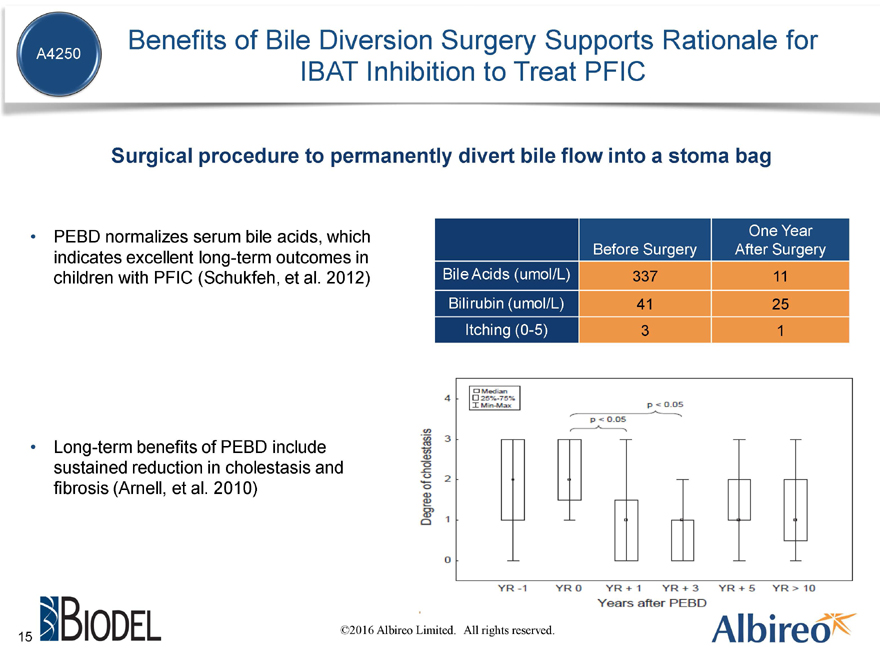
Benefits of Bile Diversion Surgery Supports Rationale for IBAT Inhibition to Treat PFIC
Surgical procedure to permanently divert bile flow into a stoma bag
PEBD normalizes serum bile acids, which indicates excellent long-term outcomes in children with PFIC (Schukfeh, et al. 2012)
Long-term benefits of PEBD include sustained reduction in cholestasis and fibrosis (Arnell, et al. 2010)
One Year Before Surgery After Surgery Bile Acids (umol/L) 337 11 Bilirubin (umol/L) 41 25 Itching (0-5) 3 1
©2016 Albireo Limited. All rights reserved.
15
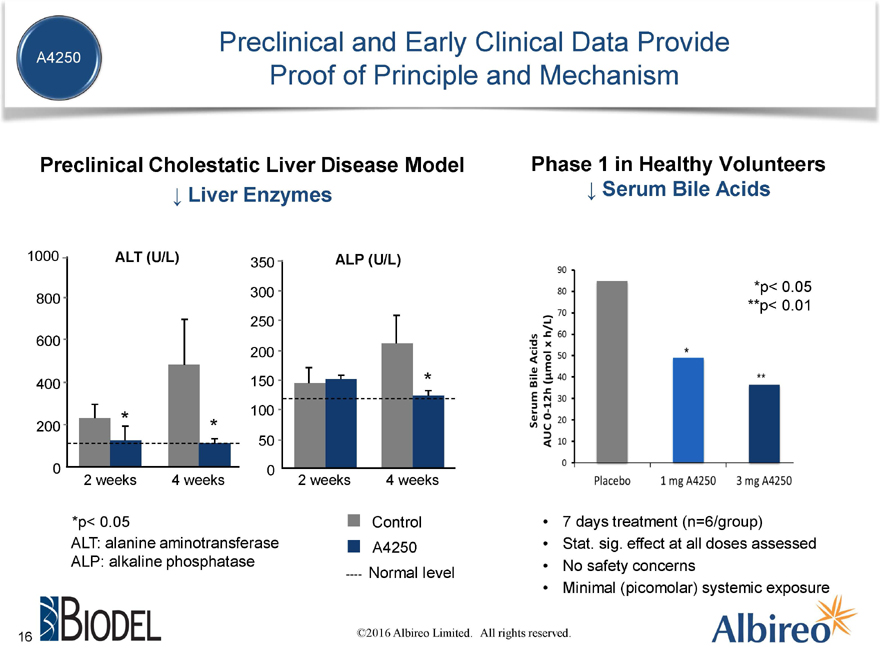
Preclinical and Early Clinical Data Provide
A4250
Proof of Principle and Mechanism
Preclinical Cholestatic Liver Disease Model Phase 1 in Healthy Volunteers
? Liver Enzymes ? Serum Bile Acids
1000 ALT (U/L) ALP (U/L)
350
300 *p< 0.05
800 **p< 0.01
250 600 200
400 150 *
0 0
2 | | weeks 4 weeks 2 weeks 4 weeks |
*p< 0.05 Control 7 days treatment (n=6/group)
ALT: alanine aminotransferase Stat. sig. effect at all doses assessed
A4250
ALP: alkaline phosphatase No safety concerns
—— Normal level
Minimal (picomolar) systemic exposure
©2016 Albireo Limited. All rights reserved.
16
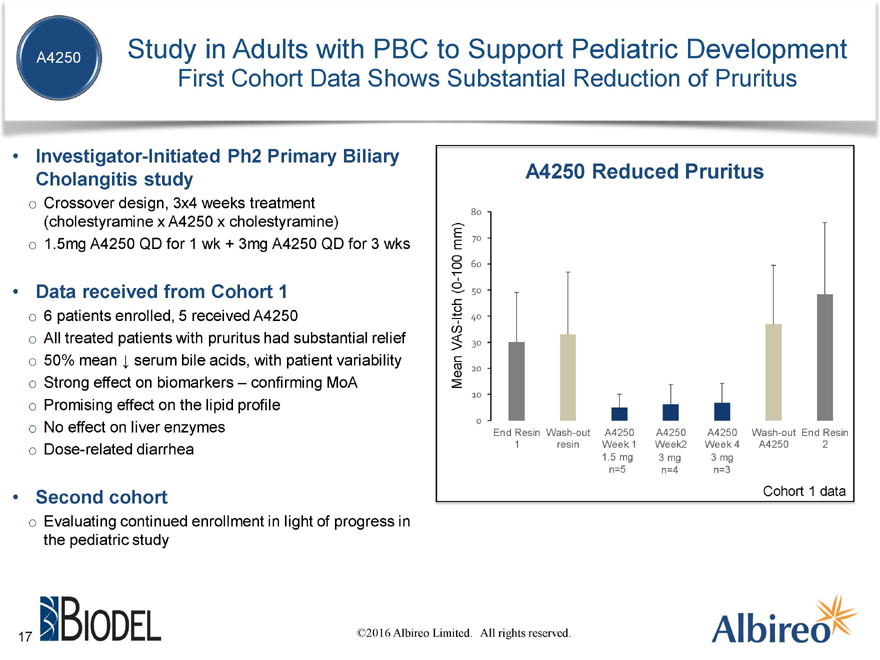
Study in Adults with PBC to Support Pediatric Development
First Cohort Data Shows Substantial Reduction of Pruritus
Investigator-Initiated Ph2 Primary Biliary
Cholangitis study
Crossover design, 3x4 weeks treatment
(cholestyramine x A4250 x cholestyramine)
1.5mg | | A4250 QD for 1 wk + 3mg A4250 QD for 3 wks |
Data received from Cohort 1
6 | | patients enrolled, 5 received A4250 |
All treated patients with pruritus had substantial relief
50% mean ? serum bile acids, with patient variability
Strong effect on biomarkers – confirming MoA
Promising effect on the lipid profile
No effect on liver enzymes
Dose-related diarrhea
Second cohort
Evaluating continued enrollment in light of progress in
the pediatric study
1.5 mg 3 mg 3 mg n=5 n=4 n=3
©2016 Albireo Limited. All rights reserved.
17
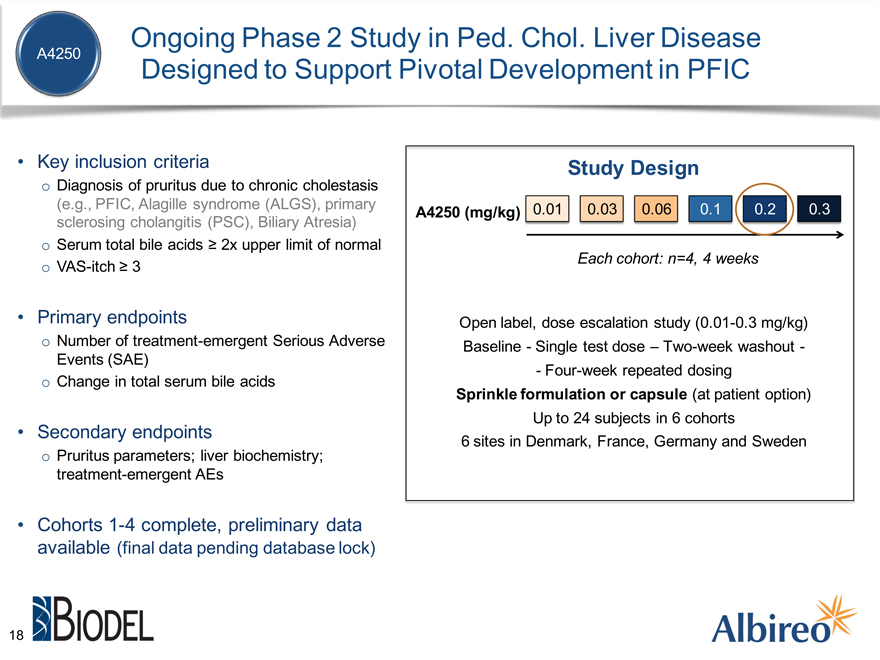
Ongoing Phase 2 Study in Ped. Chol. Liver Disease
A4250
Designed to Support Pivotal Development in PFIC
Key inclusion criteria
Diagnosis of pruritus due to chronic cholestasis
(e.g., PFIC, Alagille syndrome (ALGS), primary sclerosing cholangitis (PSC), Biliary Atresia) o Serum total bile acids ? 2x upper limit of normal o VAS-itch ? 3
Primary endpoints
Number of treatment-emergent Serious Adverse
Events (SAE) o Change in total serum bile acids
Secondary endpoints
Pruritus parameters; liver biochemistry; treatment-emergent AEs
Cohorts 1-4 complete with preliminary data available (pending database lock)
Open label, dose escalation study (0.01-0.3 mg/kg) Baseline—Single test dose – Two-week washout—
- Four-week repeated dosing
Sprinkle formulation or capsule (at patient option)
Up to 24 subjects in 6 cohorts
6 | | sites in Denmark, France, Germany and Sweden |
18
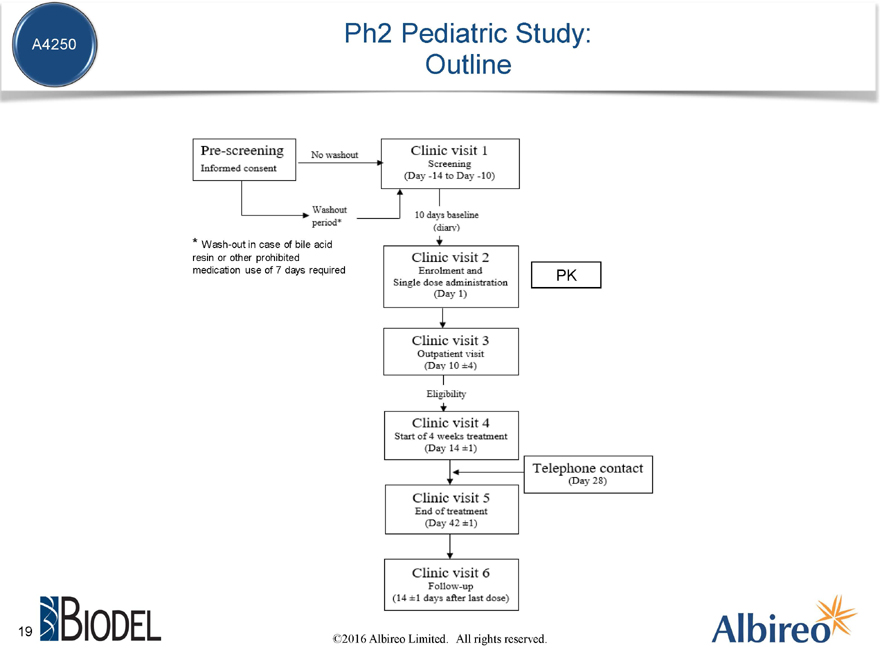
Ph2 Pediatric Study: Outline
* | | Wash-out in case of bile acid resin or other prohibited medication use of 7 days required PK |
©2016 Albireo Limited. All rights reserved.
19
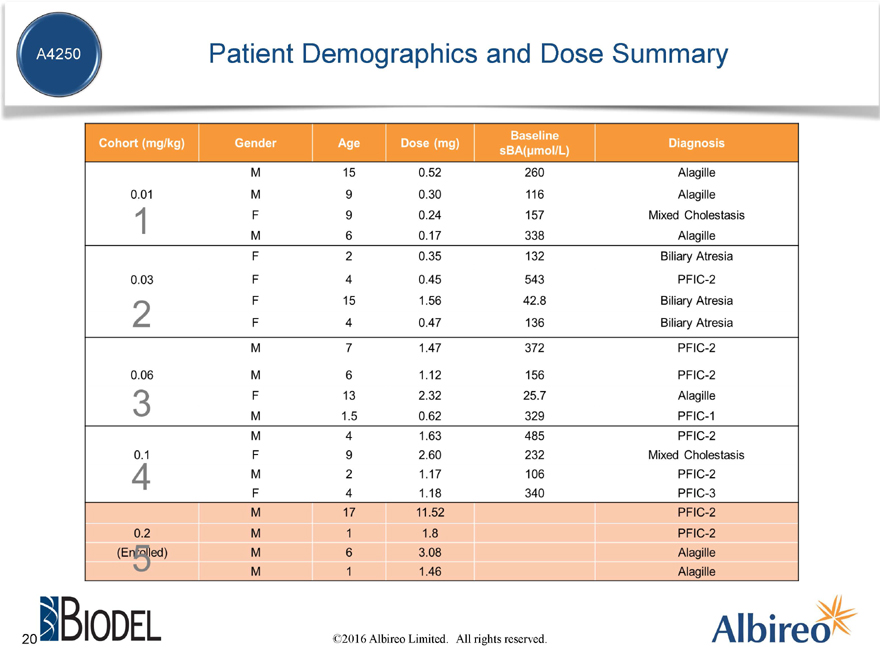
Patient Demographics and Dose Summary
©2016 Albireo Limited. All rights reserved.
20
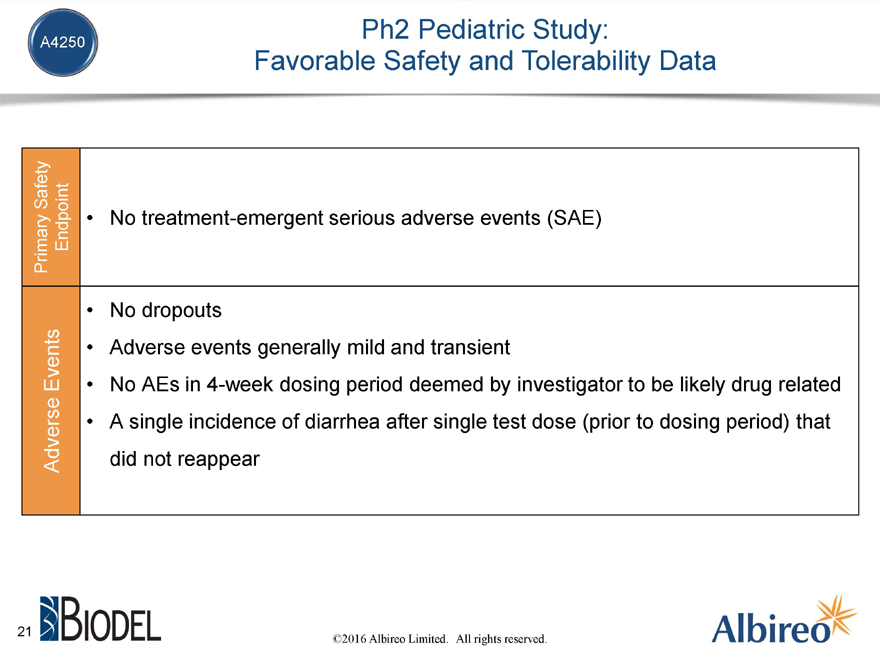
Ph2 Pediatric Study:
Favorable Safety and Tolerability Data
Primary Safety
Adverse Events
Endpoint
No treatment-emergent serious adverse events (SAE)
No dropouts
Adverse events generally mild and transient
No AEs in 4-week dosing period deemed by investigator to be likely drug related A single incidence of diarrhea after single test dose (prior to dosing period) that did not reappear
©2016 Albireo Limited. All rights reserved.
21
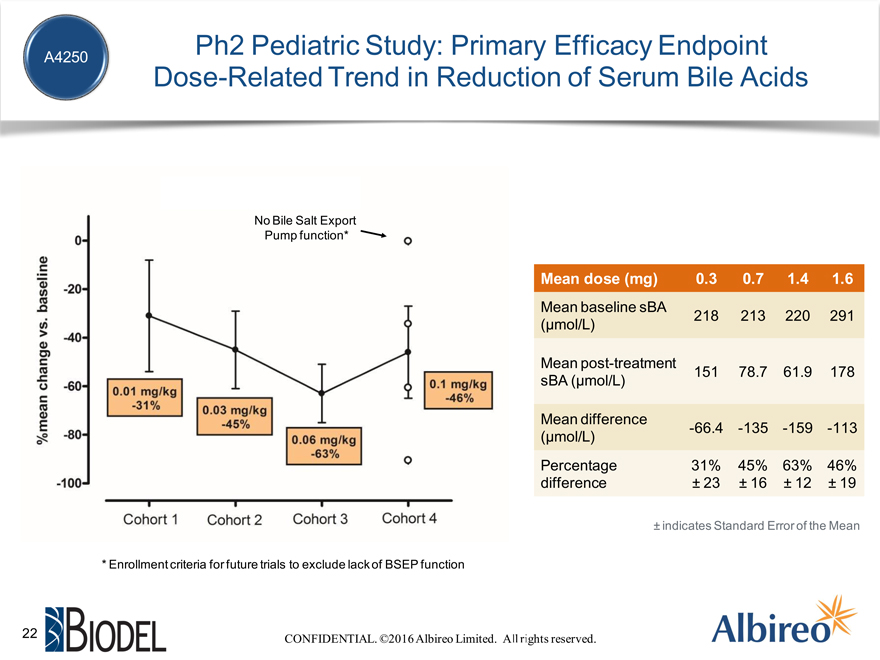
Ph2 Pediatric Study: Primary Efficacy Endpoint Dose-Related Trend in Reduction of Serum Bile Acids
No Bile Salt Export Pump function*
Mean dose (mg) 0.3 0.7 1.4 1.6
Mean baseline sBA
218 213 220 291 (µmol/L)
Mean post-treatment
151 78.7 61.9 178 sBA (µmol/L)
Mean difference
-66.4 -135 -159 -113 (µmol/L)
Percentage 31% 45% 63% 46% difference ± 23 ± 16 ± 12 ± 19
* Enrollment criteria for future trials to exclude lack of BSEP function
± indicates Standard Error of the Mean
CONFIDENTIAL. ©2016 Albireo Limited. All rights reserved.
22
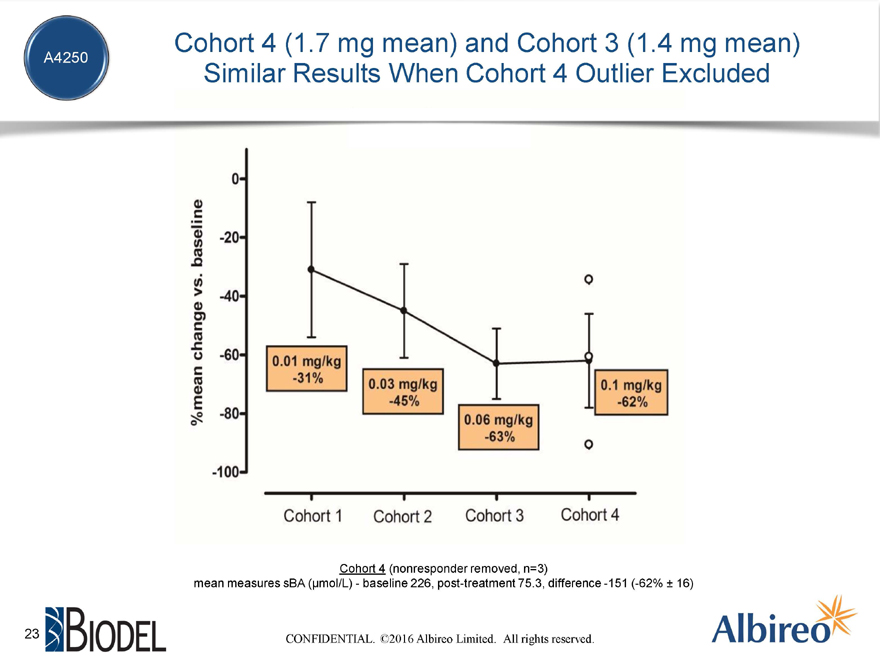
Cohort 4 (1.7 mg mean) and Cohort 3 (1.4 mg mean)
Similar Results When Cohort 4 Outlier Excluded
Cohort 4 (nonresponder removed, n=3)
mean measures sBA (µmol/L)—baseline 226, post-treatment 75.3, difference -151 (-62% ± 16)
CONFIDENTIAL. ©2016 Albireo Limited. All rights reserved.
23
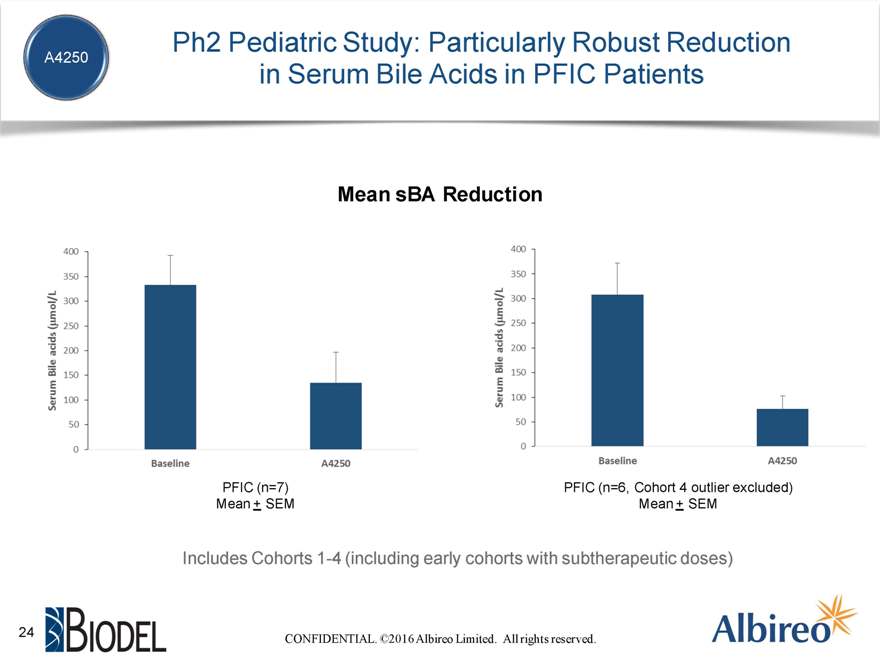
Ph2 Pediatric Study: Particularly Robust Reduction in Serum Bile Acids in PFIC Patients Mean sBA Reduction PFIC (n=7) PFIC (n=6, Cohort 4 outlier excluded) Mean + SEM Mean + SEM Includes Cohorts 1-4 (including early cohorts with subtherapeutic doses) CONFIDENTIAL. ©2016 Albireo Limited. All rights reserved.
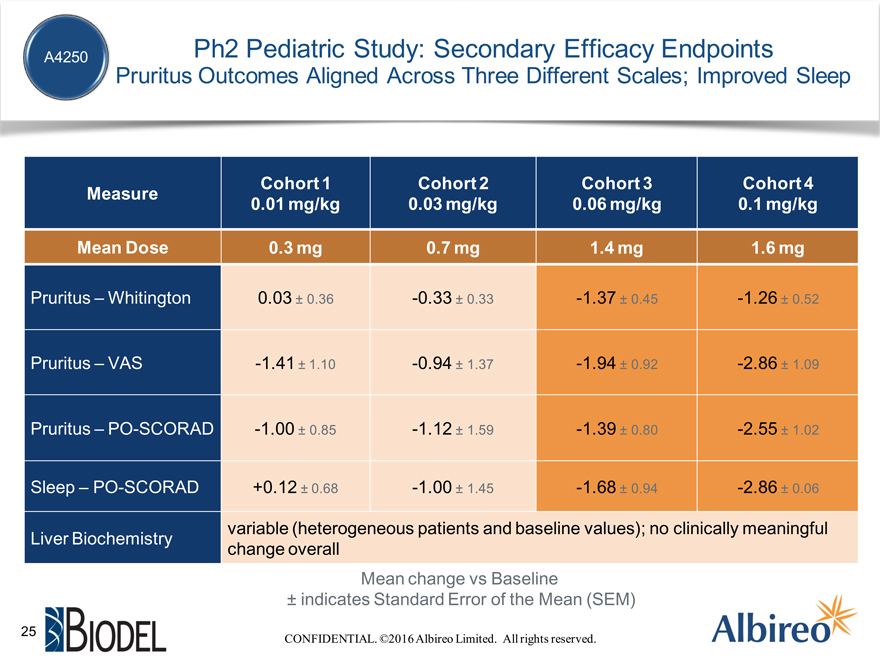
A4250 Ph2 Pediatric Study: Secondary Efficacy Endpoints
Pruritus Outcomes Aligned Across Three Different Scales; Improved Sleep
Cohort 1 Cohort 2 Cohort 3 Cohort 4
Measure
0.01 mg/kg 0.03 mg/kg 0.06 mg/kg 0.1 mg/kg
Mean Dose 0.3 mg 0.7 mg 1.4 mg 1.6 mg
Pruritus – Whitington 0.03 ± 0.36 -0.33 ± 0.33 -1.37 ± 0.45 -1.26 ± 0.52
Pruritus – VAS -1.41 ± 1.10 -0.94 ± 1.37 -1.94 ± 0.92 -2.86 ± 1.09
Pruritus – PO-SCORAD -1.00 ± 0.85 -1.12 ± 1.59 -1.39 ± 0.80 -2.55 ± 1.02
Sleep – PO-SCORAD +0.12 ± 0.68 -1.00 ± 1.45 -1.68 ± 0.94 -2.86 ± 0.06
Liver Biochemistry variable (heterogeneous patients and baseline values); no clinically meaningful change overall
Mean change vs Baseline
± indicates Standard Error of the Mean (SEM)
CONFIDENTIAL. ©2016 Albireo Limited. All rights reserved.
25
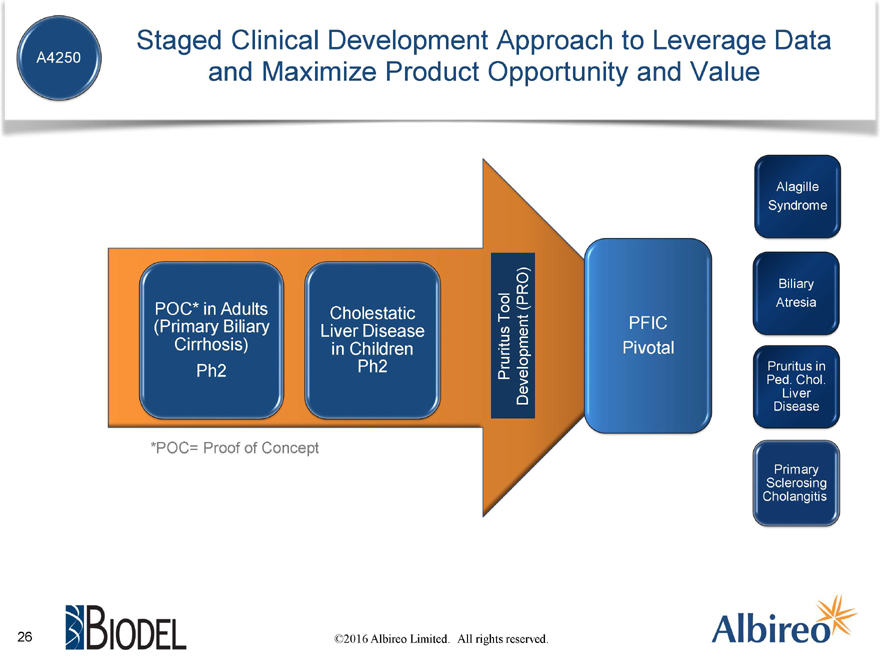
A4250 Staged Clinical Development Approach to Leverage Data and Maximize Product Opportunity and Value
Alagille
Syndrome
Biliary (PRO) Atresia
POC* in Adults Cholestatic Tool
(Primary Biliary Liver Disease PFIC
Cirrhosis) in Children Pivotal
Ph2 Ph2 Pruritus Pruritus in
Ped. Chol.
Development Liver
Disease
*POC= Proof of Concept
Primary
Sclerosing
Cholangitis
©2016 Albireo Limited. All rights reserved.
26
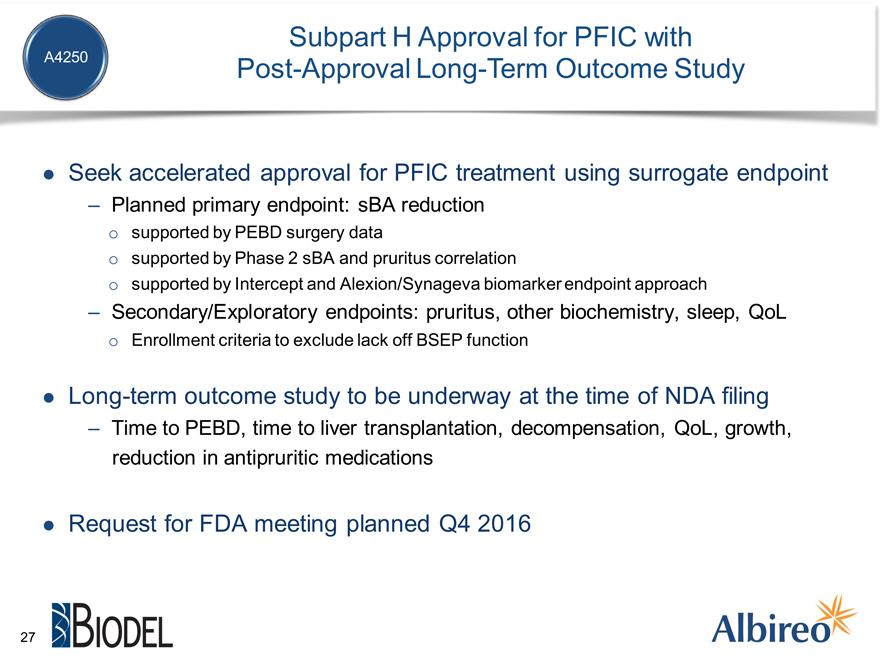
Subpart H Approval for PFIC with
A4250 Post-Approval Long-Term Outcome Study
Seek accelerated approval for PFIC treatment using surrogate endpoint
Planned primary endpoint: sBA reduction
supported by PEBD surgery data supported by Phase 2 sBA and pruritus correlation supported by Intercept and Alexion/Synageva biomarker endpoint approach
Secondary/Exploratory endpoints: pruritus, other biochemistry, sleep, QoL
Enrollment criteria to exclude lack off BSEP function
Long-term outcome study to be underway at the time of NDA filing
Time to PEBD, time to liver transplantation, decompensation, QoL, growth, reduction in antipruritic medications
Request for FDA meeting planned Q4 2016
27
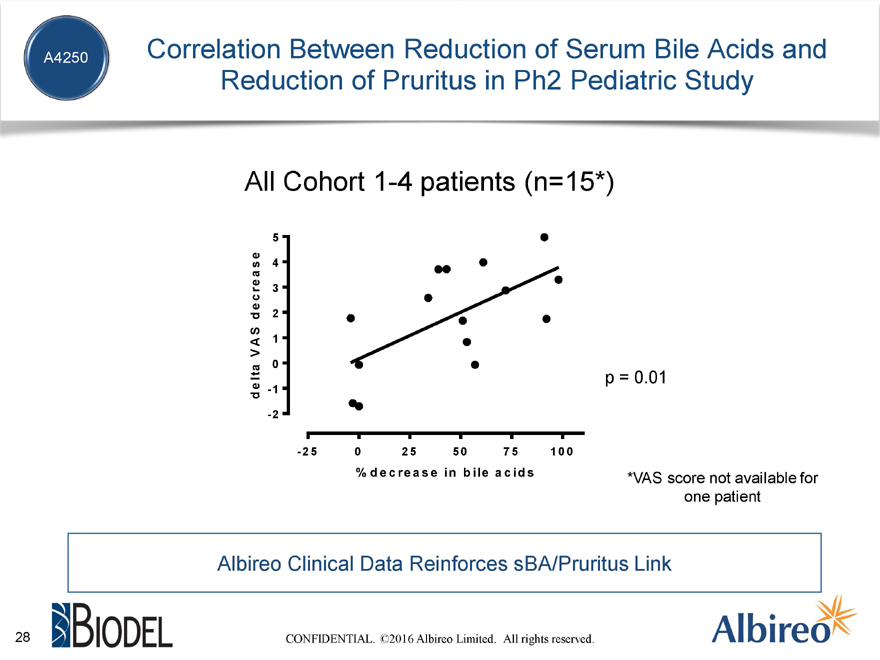
Correlation Between Reduction of Serum Bile Acids and Reduction of Pruritus in Ph2 Pediatric Study
All Cohort 1-4 patients (n=15*)
5 | | e s 4 a re 3 c e d 2 S A 1 V |
0
lta p = 0.01
e—1 d
- 2
- 2 5 0 2 5 5 0 7 5 1 0 0
% d e c re a s e in b ile a c id s *VAS score not available for one patient
Albireo Clinical Data Reinforces sBA/Pruritus Link
CONFIDENTIAL. ©2016 Albireo Limited. All rights reserved.
28
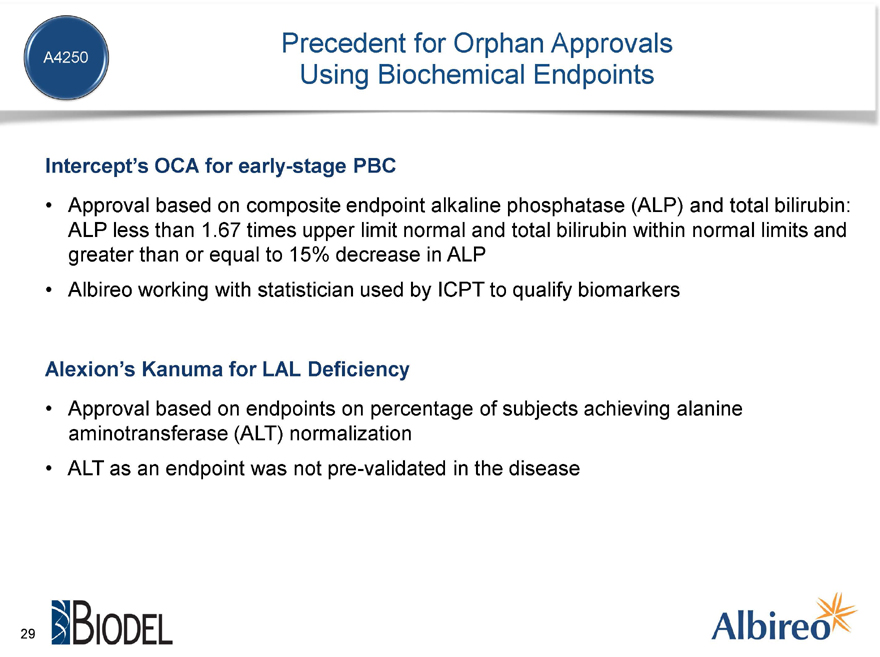
Precedent for Orphan Approvals Using Biochemical Endpoints
Intercept’s OCA for early-stage PBC
Approval based on composite endpoint alkaline phosphatase (ALP) and total bilirubin: ALP less than 1.67 times upper limit normal and total bilirubin within normal limits and greater than or equal to 15% decrease in ALP
Albireo working with statistician used by ICPT to qualify biomarkers
Alexion’s Kanuma for LAL Deficiency
Approval based on endpoints on percentage of subjects achieving alanine aminotransferase (ALT) normalization ALT as an endpoint was not pre-validated in the disease
29
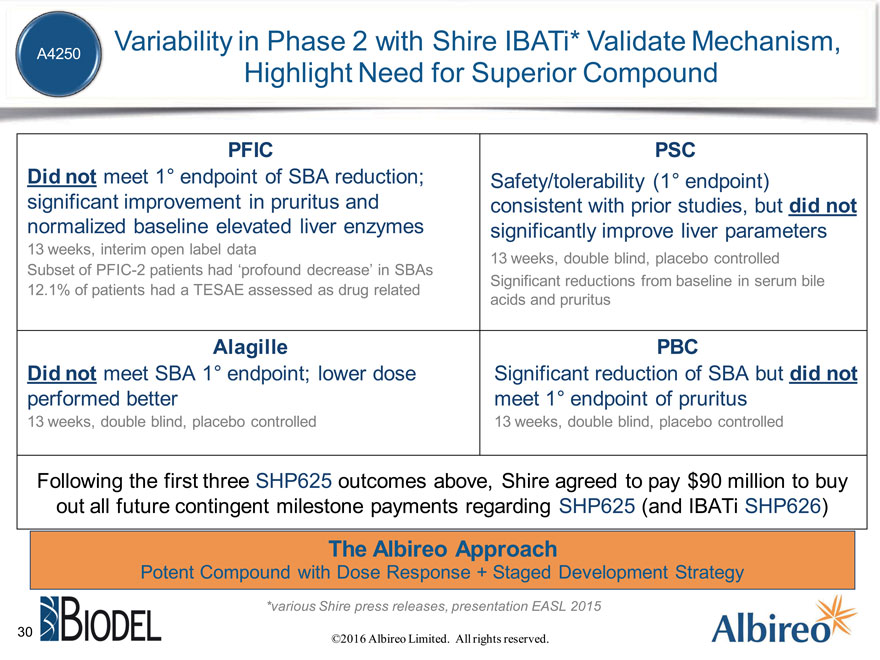
Variability in Phase 2 with Shire IBATi* Validate Mechanism,
A4250
Highlight Need for Superior Compound
PFIC PSC
Did not meet 1° endpoint of SBA reduction; Safety/tolerability (1° endpoint) significant improvement in pruritus and consistent with prior studies, but did not normalized baseline elevated liver enzymes significantly improve liver parameters
13 weeks, interim open label data
13 weeks, double blind, placebo controlled
Subset of PFIC-2 patients had ‘profound decrease’ in SBAs
Significant reductions from baseline in serum bile
12.1% of patients had a TESAE assessed as drug related acids and pruritus
Alagille PBC
Did not meet SBA 1° endpoint; lower dose Significant reduction of SBA but did not performed better meet 1° endpoint of pruritus
13 weeks, double blind, placebo controlled 13 weeks, double blind, placebo controlled
Following the first three SHP625 outcomes above, Shire agreed to pay $90 million to buy out all future contingent milestone payments regarding SHP625 (and IBATi SHP626)
The Albireo Approach
Potent Compound with Dose Response + Staged Development Strategy
*various Shire press releases, presentation EASL 2015
©2016 Albireo Limited. All rights reserved.
30
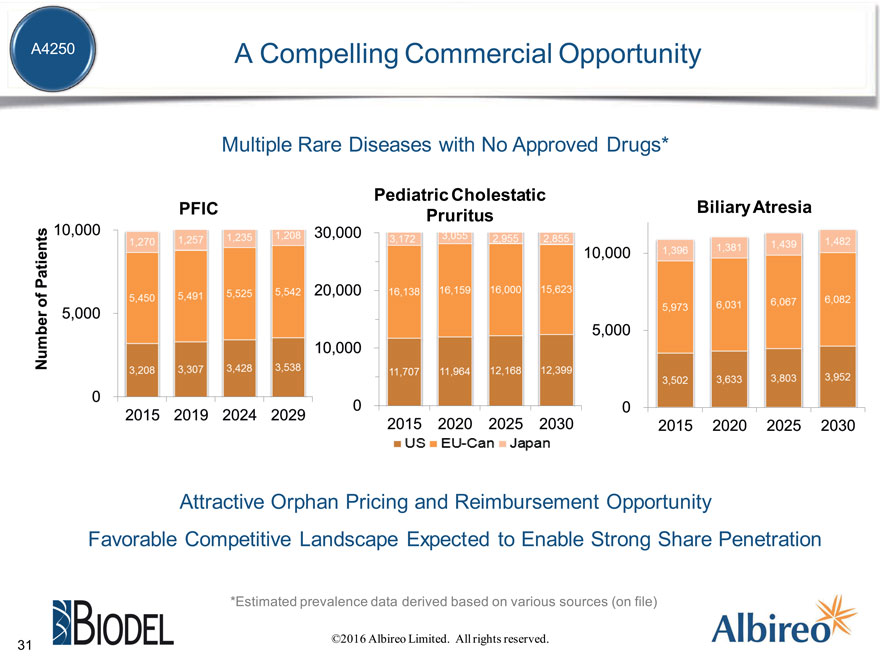
A4250 A Compelling Commercial Opportunity
Multiple Rare Diseases with No Approved Drugs*
Pediatric Cholestatic
Biliary Atresia Pruritus
Attractive Orphan Pricing and Reimbursement Opportunity
Favorable Competitive Landscape Expected to Enable Strong Share Penetration
*Estimated prevalence data derived based on various sources (on file)
©2016 Albireo Limited. All rights reserved.
31
Pipeline Programs
Elobixibat CIC Ph3 A3384 BAM Ph2 NASH Preclinical
First and Only Large Bowel-Acting Agent for CIC that Increases Both Secretion AND Motility
Elobixibat
The market opportunity…
o 156% dollar Rx market growth since 2011 (IMS Monthly NPA December 2014)
The data…
o Strong US Ph2b efficacy data with favorable AE profile o Positive Pivotal Phase 3 trial in Japan, with JNDA filing expected Q1 2017 o Favorable trends in data from abbreviated US Ph3
The commercial potential…
Blockbuster sales potential Differentiated by mechanism’s site of action and ability to stimulate both secretion and motility Further investment gated against US/EU partnering
The exclusivity period…
Composition of matter to 2027*, method of use for CIC/IBS to 2024 Polymorph patent with term to 2034 + pending patent with potential term to 2035 Downstream Rx to OTC under evaluation
*includes estimated H-W PTE
©2016 Albireo Limited. All rights reserved.
33
Primary Endpoint Met with High Statistical Significance
Elobixibat
in Ph3 Trial In Japan
Licensed to EA Pharma (Eisai/Ajinomoto) in 2012 for Japan*
License follows positive Phase 2b trial in the United States
Approximately $31.1 million in upfront and milestone payments received to date
Positive Phase 3 Pivotal Trial in chronic constipation in Japan
Primary endpoint (regulatory endpoint in Japan) met with high statistical significance
Change in weekly SBMs from baseline to the first treatment week compared with placebo
All secondary efficacy endpoints assessed statistically also met with high statistical significance
Change in complete SBM frequency, time to first SBM, constipation severity and stool consistency
No SAEs; substantially all AEs characterized as mild in severity
EA Pharma expected to file a JNDA in Q1 2017
US/EU
Ph2b (US): primary endpoint met with high statistical significance
Ph3 (US/EU ex-licensee): effects shown despite only 40% of planned enrollment (n=690)
*plus five other Asian markets (not China)
CONFIDENTIAL. ©2016 Albireo Limited. All rights reserved.
34
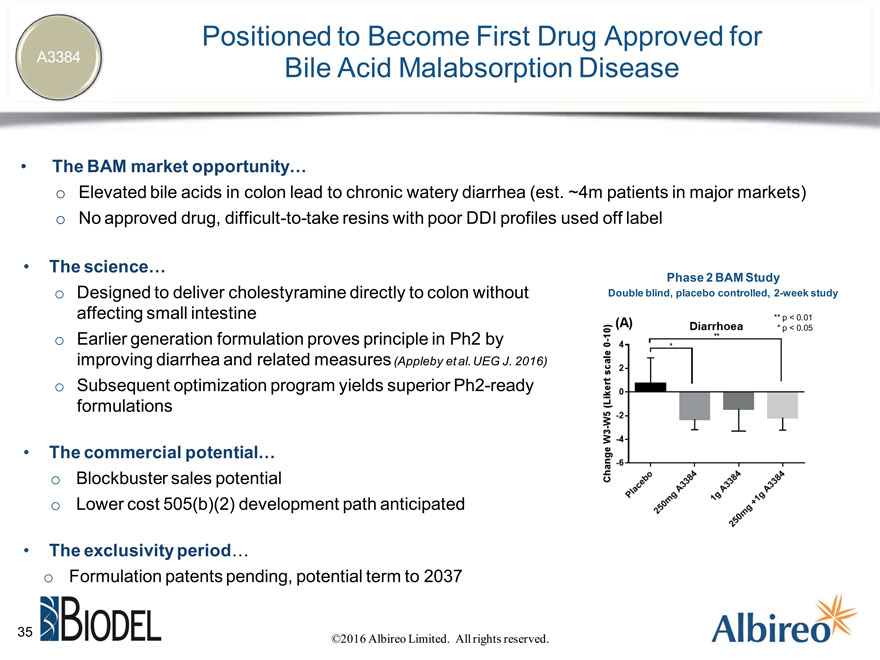
Positioned to Become First Drug Approved for A3384 Bile Acid Malabsorption Disease
The BAM market opportunity… o Elevated bile acids in colon lead to chronic watery diarrhea (est. ~4m patients in major markets) o No approved drug, difficult-to-take resins with poor DDI profiles used off label
The science…
Phase 2 BAM Study
Designed to deliver cholestyramine directly to colon without Double blind, placebo controlled, 2-week study affecting small intestine ** p < 0.01
Earlier generation formulation proves principle in Ph2 by improving diarrhea and related measures (Appleby et al. UEG J. 2016) Subsequent optimization program yields superior Ph2-ready formulations
The commercial potential… Blockbuster sales potential Lower cost 505(b)(2) development path anticipated
The exclusivity period… Formulation patents pending, potential term to 2037
©2016 Albireo Limited. All rights reserved.
35
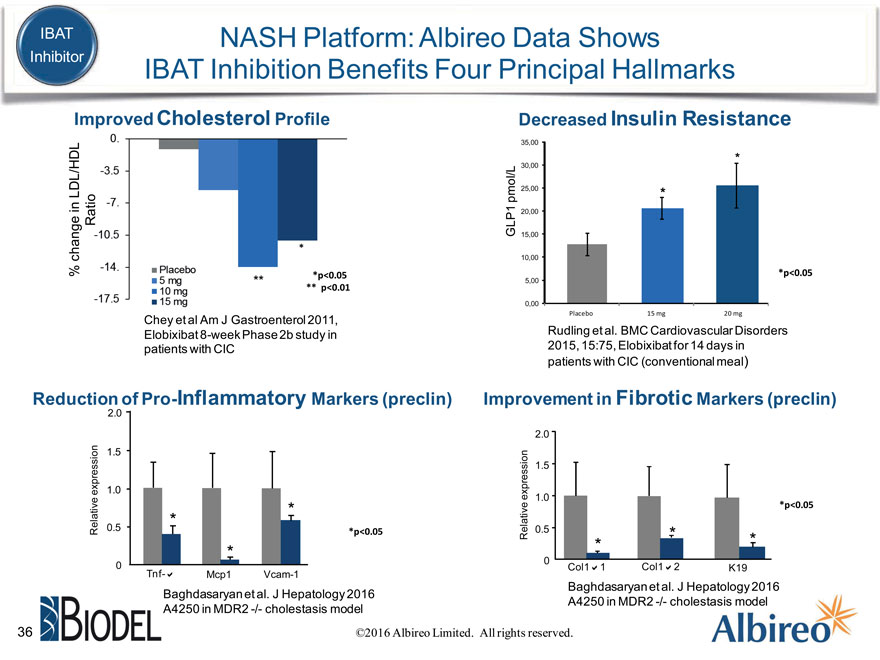
IBAT NASH Platform: Albireo Data Shows Inhibitor IBAT Inhibition Benefits Four Principal Hallmarks
Improved Cholesterol Profile Decreased Insulin Resistance
35,00
30,00 * 25,00
LDL/HDL * pmol/L
in 20,00
Ratio GLP1
15,00
change 10,00
% *p<0.05 *p<0.05
** 5,00
** p<0.01
0,00
Placebo 15 mg 20 mg
Chey et al Am J Gastroenterol 2011, Rudling et al. BMC Cardiovascular Disorders Elobixibat 8-week Phase 2b study in 2015, 15:75, Elobixibat for 14 days in patients with CIC patients with CIC (conventional meal)
Reduction of Pro-Inflammatory Markers (preclin) Improvement in Fibrotic Markers (preclin)
2.0
2.0 1.5 1.5 expression 1.0 expression 1.0
Relative 0.5 0.5
*p<0.05 Relative * *
0
0 Tnf-a Mcp1 Vcam-1 Col1a1 Col1a2 K19
Baghdasaryan et al. J Hepatology 2016 Baghdasaryan et al. J Hepatology 2016 A4250 in MDR2 -/- cholestasis model A4250 in MDR2 -/- cholestasis model
©2016 Albireo Limited. All rights reserved.
36
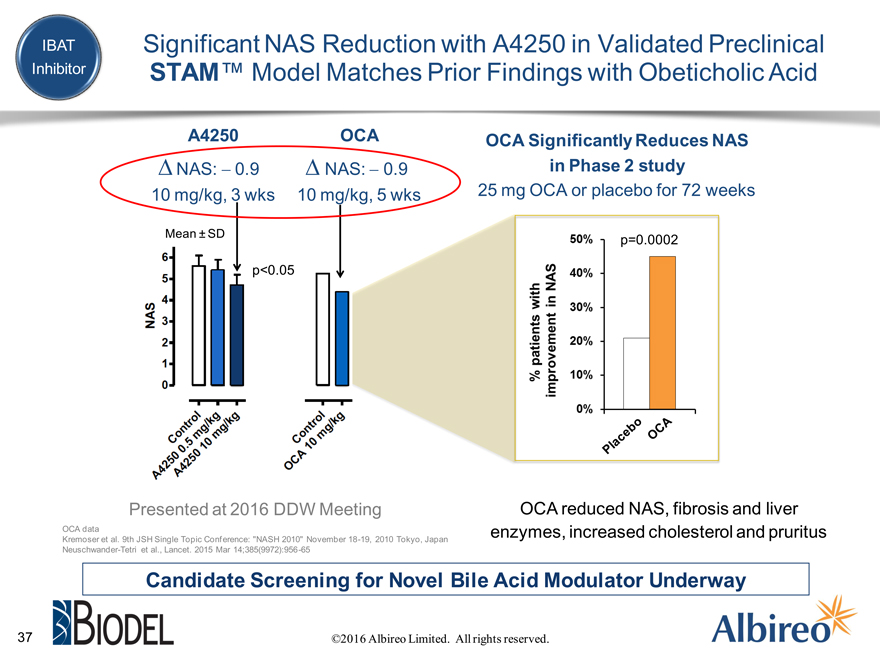
IBAT Significant NAS Reduction with A4250 in Validated Preclinical
Inhibitor STAM™ Model Matches Prior Findings with Obeticholic Acid
A4250 OCA OCA Significantly Reduces NAS D?NAS: -?0.9 D?NAS: -?0.9 in Phase 2 study
10 mg/kg, 3 wks 10 mg/kg, 5 wks 25 mg OCA or placebo for 72 weeks
Mean ± SD
p<0.05
Presented at 2016 DDW Meeting OCA reduced NAS, fibrosis and liver
OCA data enzymes, increased cholesterol and pruritus
Kremoser et al. 9th JSH Single Topic Conference: “NASH 2010” November 18-19, 2010 Tokyo, Japan Neuschwander-Tetri et al., Lancet. 2015 Mar 14;385(9972):956-65
Candidate Screening for Novel Bile Acid Modulator Underway
©2016 Albireo Limited. All rights reserved.
37
Summary
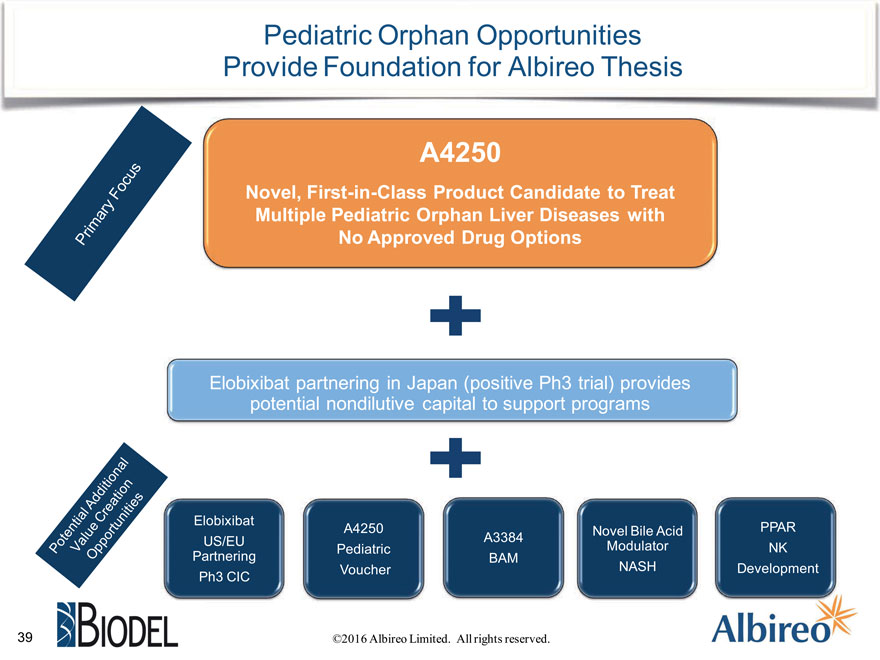
Pediatric Orphan Opportunities Provide Foundation for Albireo Thesis
A4250
Novel, First-in-Class Product Candidate to Treat Multiple Pediatric Orphan Liver Diseases with No Approved Drug Options
Elobixibat partnering in Japan (positive Ph3 trial) provides potential nondilutive capital to support programs
Elobixibat
A4250 Novel Bile Acid PPAR US/EU A3384 Pediatric Modulator NK
Partnering BAM
Voucher NASH Development Ph3 CIC
©2016 Albireo Limited. All rights reserved.
39
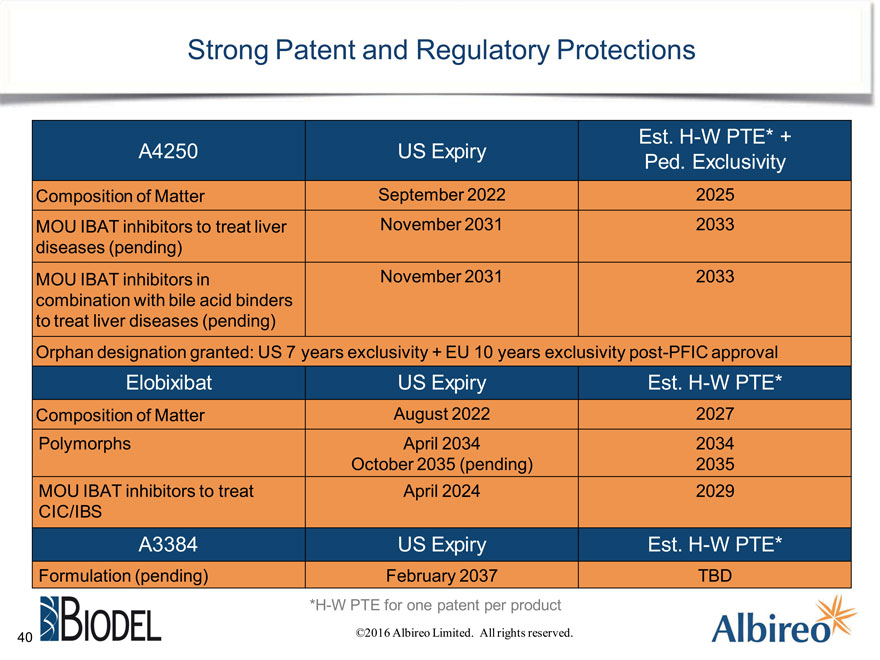
Strong Patent and Regulatory Protections
Est. H-W PTE* + A4250 US Expiry Ped. Exclusivity
Composition of Matter September 2022 2025 MOU IBAT inhibitors to treat liver November 2031 2033 diseases (pending) MOU IBAT inhibitors in November 2031 2033 combination with bile acid binders to treat liver diseases (pending) Orphan designation granted: US 7 years exclusivity + EU 10 years exclusivity post-PFIC approval
Elobixibat US Expiry Est. H-W PTE*
Composition of Matter August 2022 2027 Polymorphs April 2034 2034 October 2035 (pending) 2035
MOU IBAT inhibitors to treat April 2024 2029
CIC/IBS
A3384 US Expiry Est. H-W PTE*
Formulation (pending) February 2037 TBD
*H-W PTE for one patent per product
©2016 Albireo Limited. All rights reserved.
40
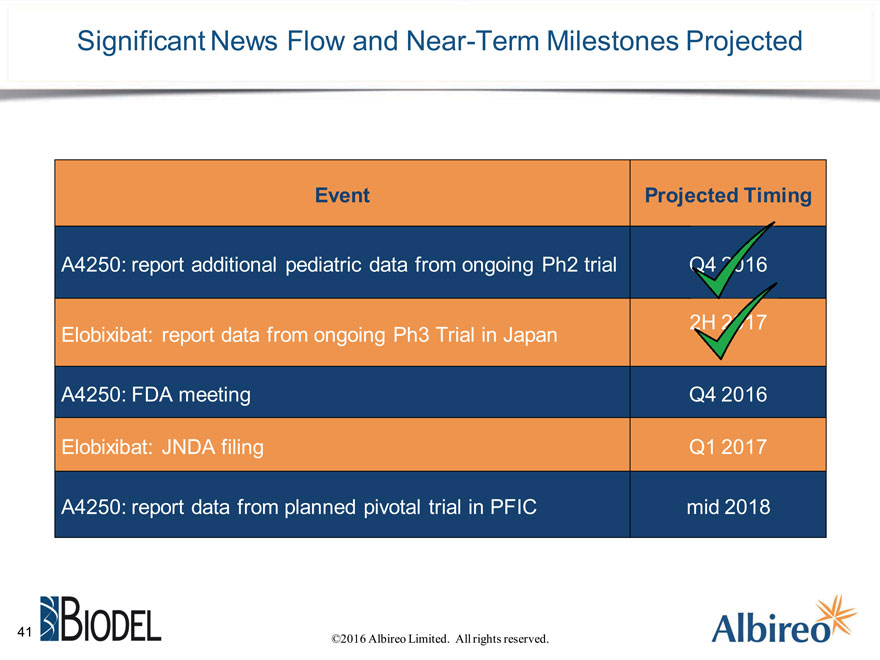
Significant News Flow and Near-Term Milestones Projected
Event Projected Timing
A4250: report additional pediatric data from ongoing Ph2 trial
Elobixibat: report data from ongoing Ph3 Trial in Japan
A4250: FDA meeting Q4 2016
Elobixibat: JNDA filing Q1 2017
A4250: report data from planned pivotal trial in PFIC mid 2018
©2016 Albireo Limited. All rights reserved.
41
Albireo
Innovative Science + Deep Pipeline + Experienced Management
Leader in the development of bile acid modulators to treat liver and GI diseases
Three clinical assets (lead Ph2 orphan and Ph3 constipation product candidates)
Multiple near-term inflection opportunities
Funding expected to be sufficient to advance A4250 through planned pivotal PFIC trial
Highly experienced management team with strong track record
©2016 Albireo Limited. All rights reserved.
42
Thank you
NASDAQ: BIOD ? October 11, 2016