Exhibit 99.1
| NASDAQ: ADLS |
| 2 Safe Harbor Statement This presentation contains forward-looking statements about Advanced Life Sciences Holdings, Inc., including statements regarding the clinical trials and regulatory pathway of cethromycin. Forward-looking statements represent our management’s judgment regarding future events. The Company does not undertake any obligations to update any forward-looking statements whether as a result of new information, future events or otherwise. Our actual results could differ materially from those discussed herein due to several factors including the success and timing of our clinical trials and our ability to obtain and maintain regulatory approval and labeling of our product candidates; our plans to develop and commercialize our product candidates; the loss of key scientific or management personnel; the size and growth of potential markets for our product candidates and our ability to serve those markets; regulatory developments in the U.S. and foreign countries; the rate and degree of market acceptance of any future products; the accuracy of our estimates regarding expenses, future revenues and capital requirements; our ability to obtain and maintain intellectual property protection for our product candidates; the successful development of our sales and marketing capabilities; the success of competing drugs that become available; and the performance of third party collaborators and manufacturers. These and additional risks and uncertainties are detailed in the Company’s filings with the Securities and Exchange Commission. |
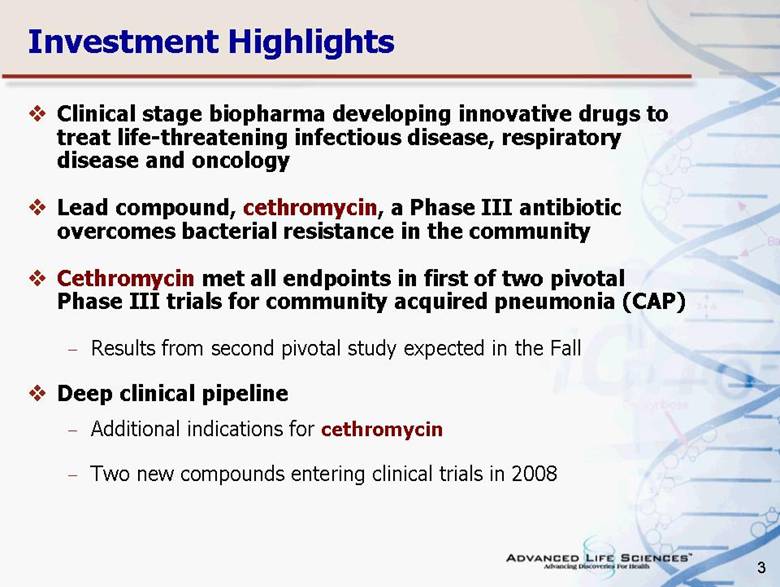
| 3 Investment Highlights Clinical stage biopharma developing innovative drugs to treat life-threatening infectious disease, respiratory disease and oncology Lead compound, cethromycin, a Phase III antibiotic overcomes bacterial resistance in the community Cethromycin met all endpoints in first of two pivotal Phase III trials for community acquired pneumonia (CAP) Results from second pivotal study expected in the Fall Deep clinical pipeline Additional indications for cethromycin Two new compounds entering clinical trials in 2008 |
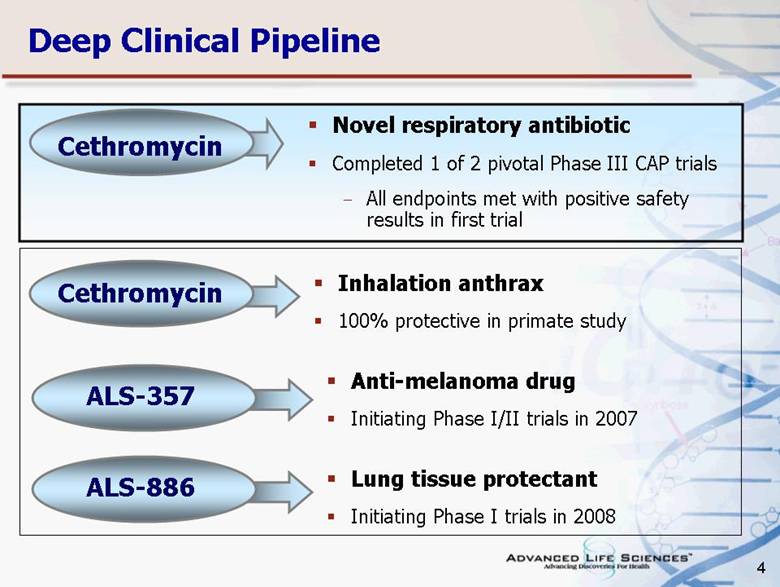
| 4 Lung tissue protectant Initiating Phase I trials in 2008 Anti-melanoma drug Initiating Phase I/II trials in 2007 Inhalation anthrax 100% protective in primate study Novel respiratory antibiotic Completed 1 of 2 pivotal Phase III CAP trials All endpoints met with positive safety results in first trial Deep Clinical Pipeline ALS-357 ALS-886 Cethromycin Cethromycin |
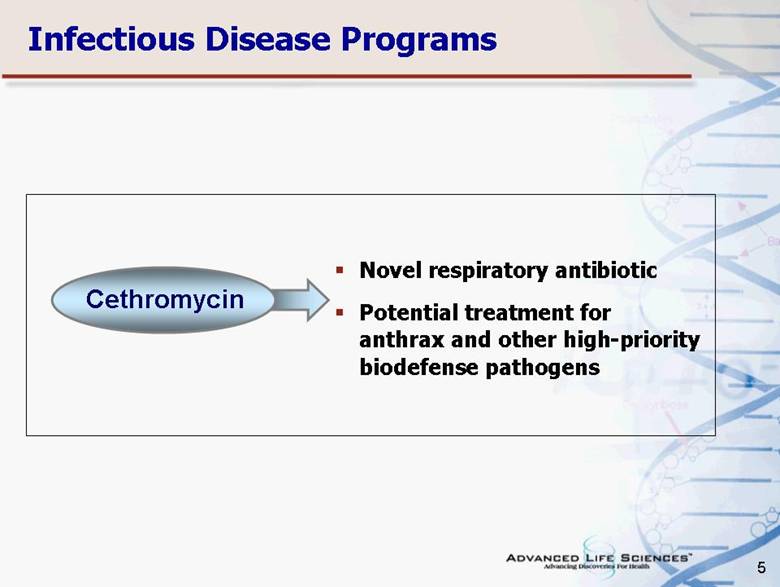
| 5 Novel respiratory antibiotic Potential treatment for anthrax and other high-priority biodefense pathogens Cethromycin Infectious Disease Programs |
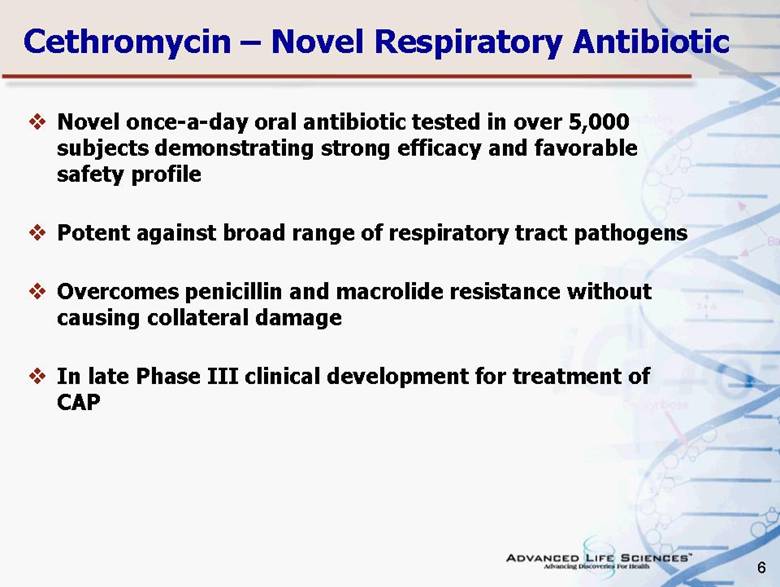
| 6 Cethromycin – Novel Respiratory Antibiotic Novel once-a-day oral antibiotic tested in over 5,000 subjects demonstrating strong efficacy and favorable safety profile Potent against broad range of respiratory tract pathogens Overcomes penicillin and macrolide resistance without causing collateral damage In late Phase III clinical development for treatment of CAP |
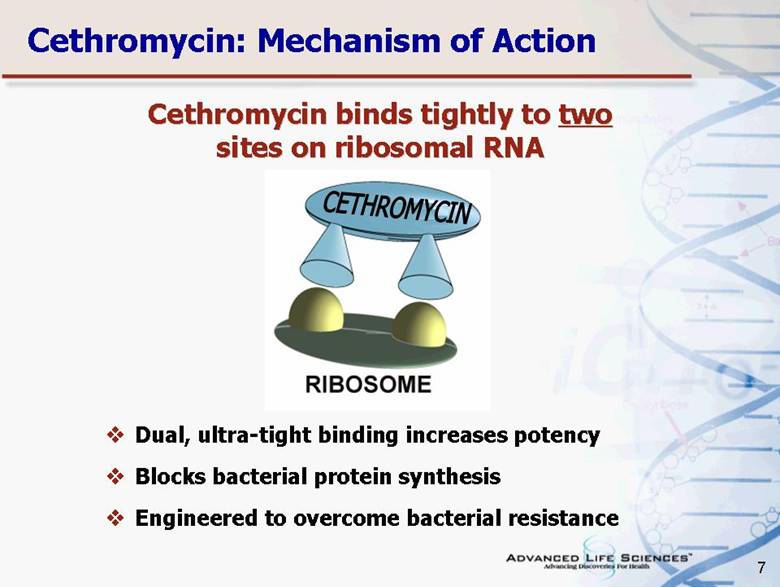
| 7 Dual, ultra-tight binding increases potency Blocks bacterial protein synthesis Engineered to overcome bacterial resistance Cethromycin: Mechanism of Action Cethromycin binds tightly to two sites on ribosomal RNA |
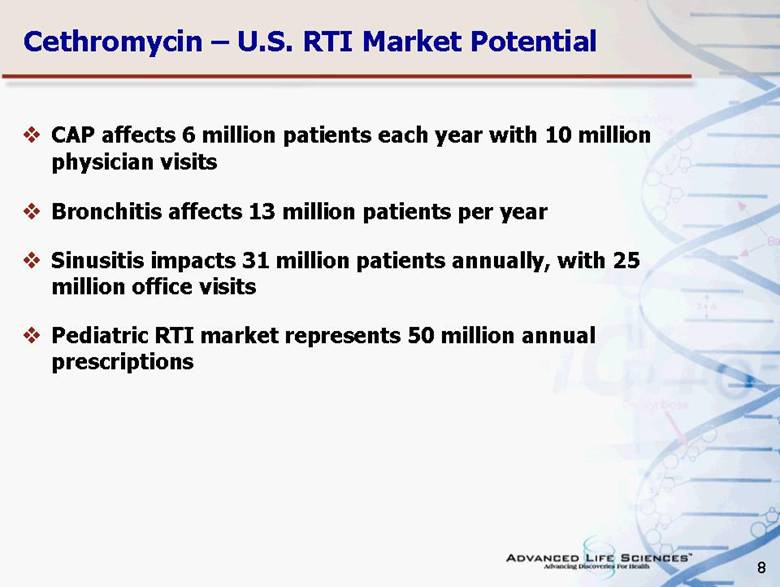
| 8 Cethromycin – U.S. RTI Market Potential CAP affects 6 million patients each year with 10 million physician visits Bronchitis affects 13 million patients per year Sinusitis impacts 31 million patients annually, with 25 million office visits Pediatric RTI market represents 50 million annual prescriptions |
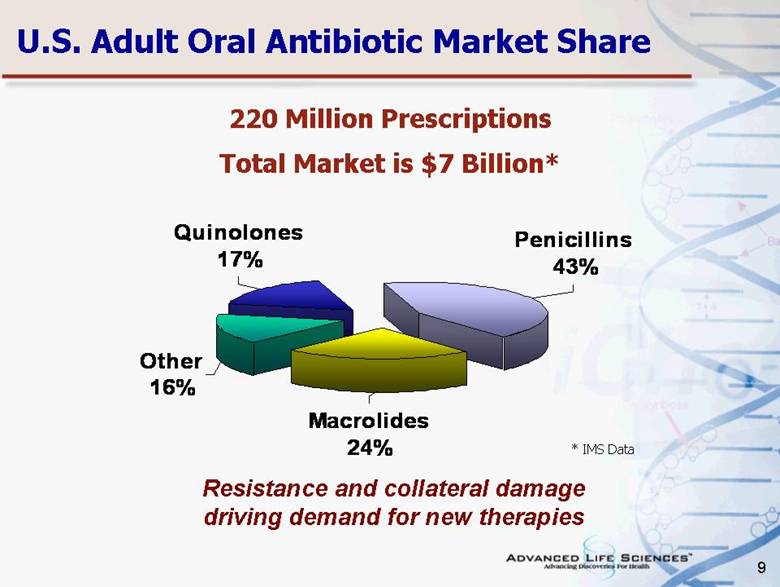
| 9 U.S. Adult Oral Antibiotic Market Share 220 Million Prescriptions Total Market is $7 Billion* * IMS Data Resistance and collateral damage driving demand for new therapies Macrolides 24% Other 16% Quinolones 17% Penicillins 43% |
| 10 Cethromycin Initial Focus: Potential Treatment for CAP |
| 11 Cethromycin – Potential Treatment for CAP CAP is gold standard for RTI and the 6th leading cause of death in the U.S. 6 million cases of CAP annually in the U.S. with annual spend of $2 billion for antibiotics Clear regulatory pathway to approval in CAP Potent activity against Streptococcus pneumoniae – most prevalent pathogen in CAP Increased lung concentration and long post-antibiotic effect boosts CAP potency Most rapid, cost effective path to successful market launch |
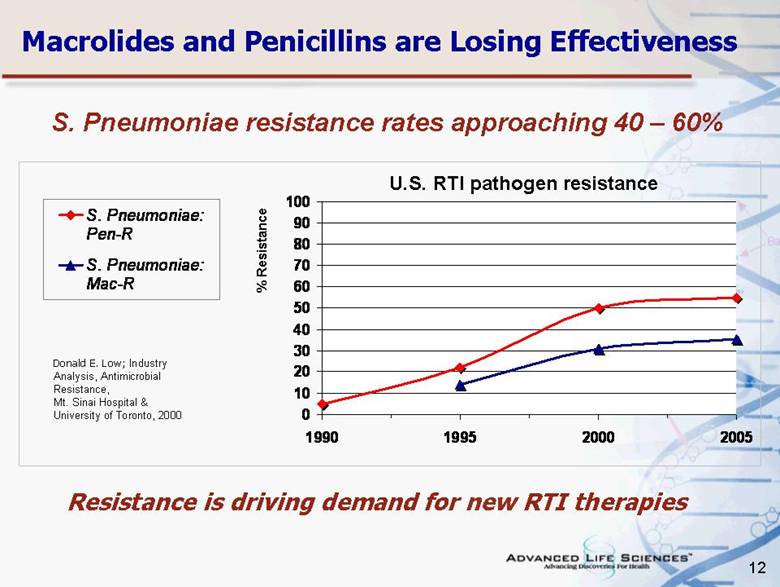
| 12 Macrolides and Penicillins are Losing Effectiveness Resistance is driving demand for new RTI therapies Donald E. Low; Industry Analysis, Antimicrobial Resistance, Mt. Sinai Hospital & University of Toronto, 2000 % Resistance S. Pneumoniae resistance rates approaching 40 – 60% U.S. RTI pathogen resistance 0 10 20 30 40 50 60 70 80 90 100 1990 1995 2000 2005 S. Pneumoniae: Pen-R S. Pneumoniae: Mac-R |
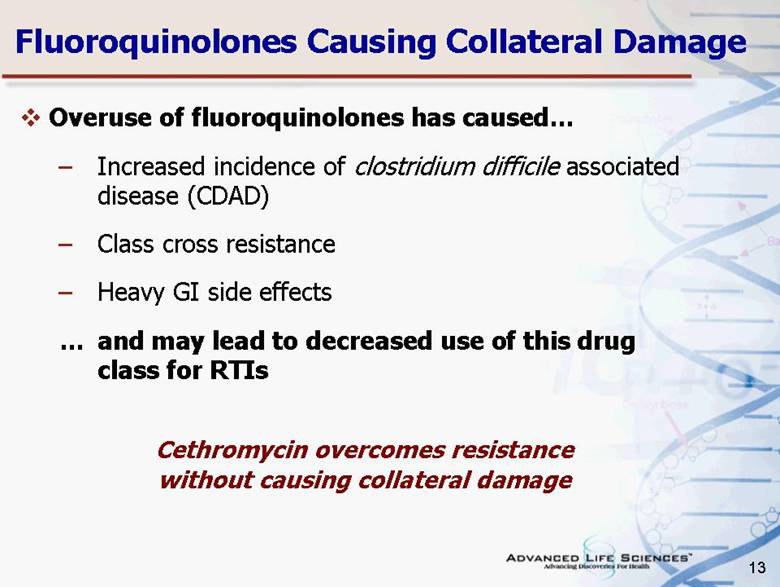
| 13 Fluoroquinolones Causing Collateral Damage Overuse of fluoroquinolones has caused Increased incidence of clostridium difficile associated disease (CDAD) Class cross resistance Heavy GI side effects and may lead to decreased use of this drug class for RTIs Cethromycin overcomes resistance without causing collateral damage |
| 14 Growing Demand for Antibiotics that Overcome Resistance without Collateral Damage Resistance in RTIs is a $5 billion economic burden in the U.S.1 Cost of hospitalized CAP patient failing penicillins or macrolides is $15,000 vs. $200 office visit with prescription2 Economic burden for CDAD is over $1 billion and increasing3 MCOs seek new antibiotics not associated with resistance or CDAD risk to avoid cost 1 “CARTI: New Opportunities for Effective Management”, Iannini, Paul, MD, Paladino, Joseph, PharmD, Kressel, Amy, MD, CPE Forum Vol.5 No. 1, February 2006 2” “The Effect of Macrolide Resistance on the treatment of CARTI”; Low, Donald MD, Moellering, Robert, MD, File, Thomas, MD 45th ICAAC, December 2005 3 “Healthcare costs and nosocomial diarrhea due to Clostridium difficile”; Kyne,L., Hamel, M.B., Polavaram, R., Kelly, C.P. Clin. Infect. Dis. 34:346-353 |
| 15 Cethromycin – Phase III Clinical Trial Design Powered to demonstrate clinical cure rate non-inferiority using 95% confidence interval FDA requires demonstration of non-inferiority to current standard of care for CAP trials Cethromycin was dosed 300 mg QD vs. Biaxin® dosed at 250 mg BID for 7 days Two Phase III, double-blind, randomized trials with over 200 clinical sites worldwide and over 1,000 total patients Trial CL-05 in US, Canada and South Africa Enrollment completed, results due in Fall Trial CL-06 in South America, Europe and Israel Trial completed, met all endpoints |
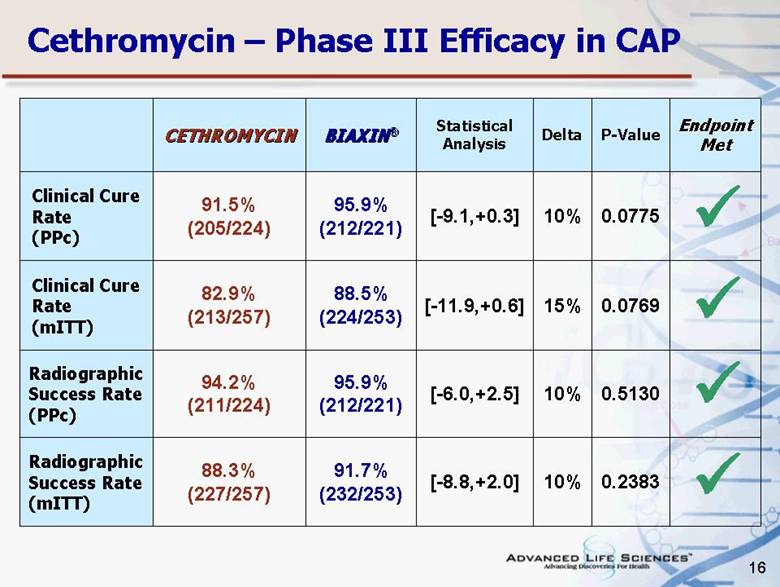
| 16 Cethromycin – Phase III Efficacy in CAP CETHROMYCIN BIAXIN® Statistical Analysis Delta P-Value Endpoint Met Clinical Cure Rate (PPc) 91.5% (205/224) 95.9% (212/221) [-9.1,+0.3] 10% 0.0775 Clinical Cure Rate (mITT) 82.9% (213/257) 88.5% (224/253) [-11.9,+0.6] 15% 0.0769 Radiographic Success Rate (PPc) 94.2% (211/224) 95.9% (212/221) [-6.0,+2.5] 10% 0.5130 Radiographic Success Rate (mITT) 88.3% (227/257) 91.7% (232/253) [-8.8,+2.0] 10% 0.2383 |
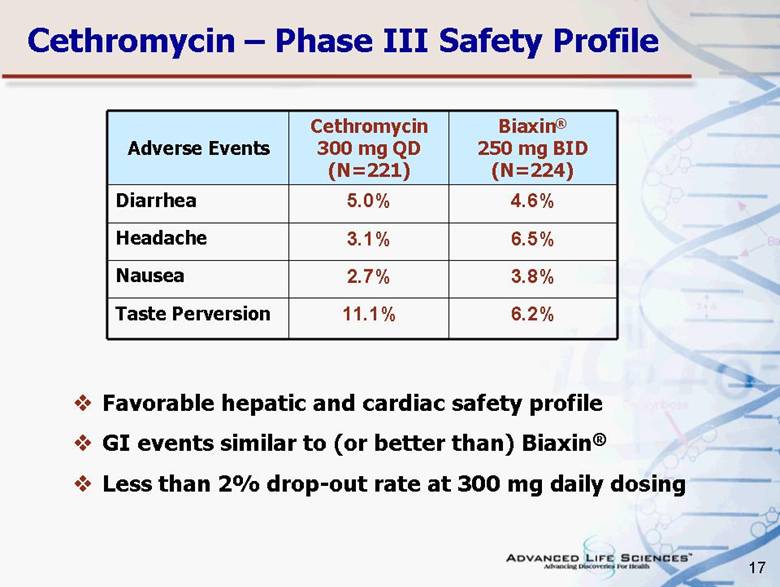
| 17 Cethromycin – Phase III Safety Profile Adverse Events Cethromycin 300 mg QD (N=221) Biaxin® 250 mg BID (N=224) Diarrhea 5.0% 4.6% Headache 3.1% 6.5% Nausea 2.7% 3.8% Taste Perversion 11.1% 6.2% Favorable hepatic and cardiac safety profile GI events similar to (or better than) Biaxin® Less than 2% drop-out rate at 300 mg daily dosing |
| 18 Global Commercialization Strategy Assuming FDA approval, launch in CAP with commercial partner with PCP sales force With commercial partner, co-develop cethromycin for other indications; option to co-promote Expect milestone and royalty based deal structure Engage commercial partner in Europe and ROW U.S. Market Launch Ex-U.S. Market Launch Key patents expire in 2016, with opportunity for extension |
| 19 Cethromycin Potential Treatment for Anthrax and Other Biodefense Pathogens |
| 20 Cethromycin – Biodefense Applications Need novel agents with new mechanisms for bioterrorism protection Studies in collaboration with USAMRIID and NIAID Superior potency in vitro vs. approved drugs against more than 30 anthrax strains and other high-priority pathogens Shown to be 100% protective for prophylactic treatment of inhalation anthrax in primates Outperformed Cipro®, current standard of care FDA has granted cethromycin Orphan Drug status for treatment of anthrax Anthrax indication will augment NDA submission for CAP |
| 21 Cethromycin Milestones: Past and Future |
| 22 Milestones Achieved To Date Reported positive data for cethromycin as in 1st of 2 pivotal trials in CAP Established manufacturing partnerships for API and drug product Formed National Advisory Board of physician experts to support NDA and commercialization processes Reported positive data from anthrax primate study Collaboration with USAMRIID and NIAID for other pathogens Granted Orphan Drug status for cethromycin as treatment for inhalation anthrax |
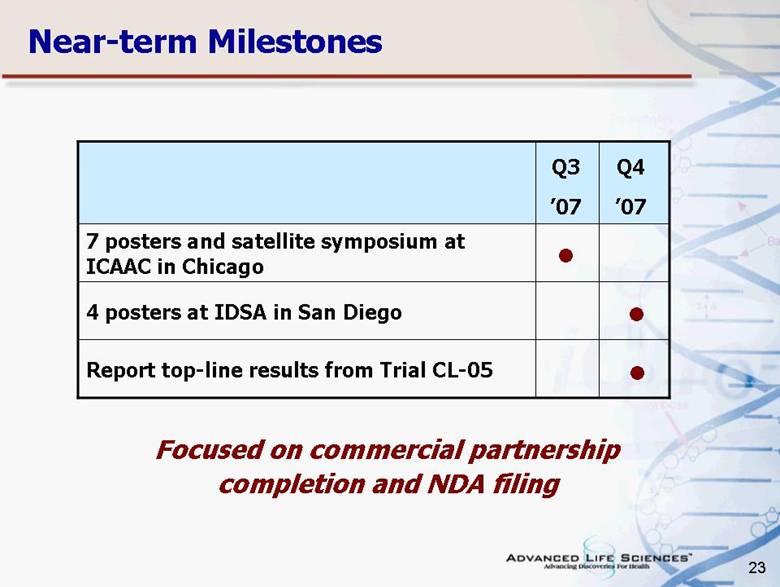
| 23 Near-term Milestones 7 posters and satellite symposium at ICAAC in Chicago Report top-line results from Trial CL-05 4 posters at IDSA in San Diego Q3 ’07 Q4 ’07 • • • Focused on commercial partnership completion and NDA filing |
| 24 Oncology Programs Anti-melanoma drug Initiating Phase I/II trials in early 2008 ALS-357 |
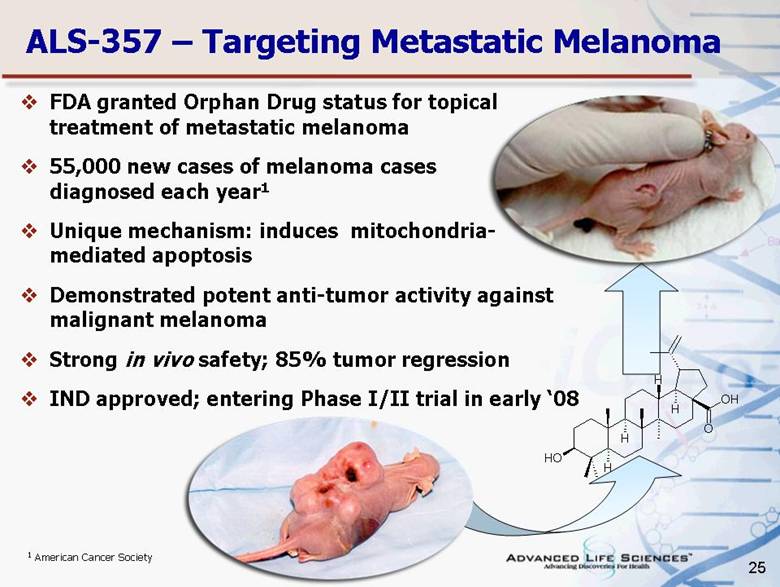
| 25 ALS-357 – Targeting Metastatic Melanoma FDA granted Orphan Drug status for topical treatment of metastatic melanoma 55,000 new cases of melanoma cases diagnosed each year1 Unique mechanism: induces mitochondria- mediated apoptosis Demonstrated potent anti-tumor activity against malignant melanoma Strong in vivo safety; 85% tumor regression IND approved; entering Phase I/II trial in early ‘08 1 American Cancer Society O O H O H H H H H |
| 26 Lung tissue protectant IND approved; expected to enter Phase I trials in 2008 ALS-886 Respiratory Programs |
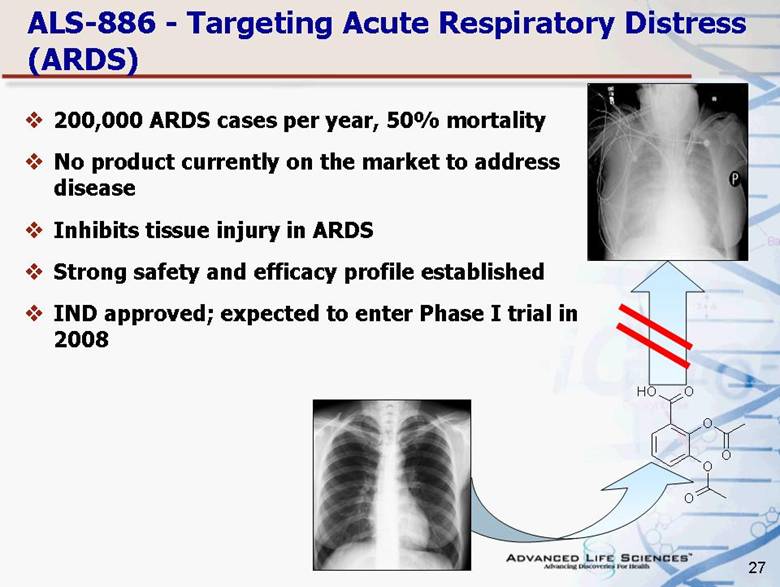
| 27 ALS-886 - Targeting Acute Respiratory Distress (ARDS) 200,000 ARDS cases per year, 50% mortality No product currently on the market to address disease Inhibits tissue injury in ARDS Strong safety and efficacy profile established IND approved; expected to enter Phase I trial in 2008 O O O O H O O |
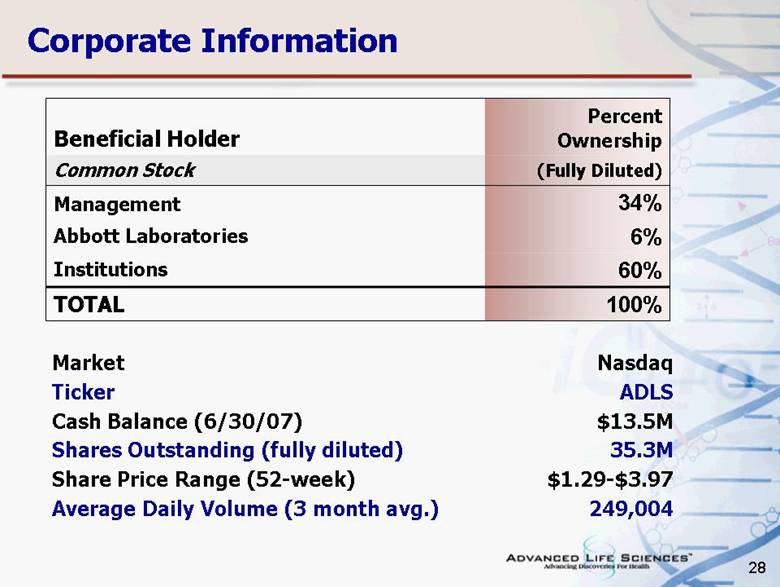
| 28 Corporate Information Beneficial Holder Percent Ownership Common Stock (Fully Diluted) Management 34% Abbott Laboratories 6% Institutions 60% TOTAL 100% Market Nasdaq Ticker ADLS Cash Balance (6/30/07) $13.5M Shares Outstanding (fully diluted) 35.3M Share Price Range (52-week) $1.29-$3.97 Average Daily Volume (3 month avg.) 249,004 |
| 29 Investment Highlights Developing innovative drugs to treat life-threatening infectious disease, respiratory disease and oncology Lead compound, cethromycin, a Phase III antibiotic overcomes bacterial resistance in the community Cethromycin met all endpoints in first of two pivotal Phase III trials for community acquired pneumonia (CAP) Results from second pivotal study expected in the Fall Deep clinical pipeline Additional indications for cethromycin Two new compounds entering clinical trials in 2008 |