UNITED STATES SECURITIES AND EXCHANGECOMMISSION
Washington, D.C., 20549
FORM SB-2
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
CELLCYTE GENETICS CORPORATION |
(Exact name of registrant as specified in charter) |
Nevada
(State or jurisdiction of incorporation or organization) | 2836
(Primary Standard Industrial Classification Code Number) | 86-1127046
(I.R.S. Employer Identification No.) |
Carillon Point, 5400 Carillon Point, Kirkland, Washington, U.S.A., 98033
Telephone: (425) 576-4106 |
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices) |
GARY A. REYS
President, Chief Executive Officer and a director
Carillon Point, 5400 Carillon Point, Kirkland, Washington, U.S.A., 98033
Telephone: (425) 576-4106 |
(Name, address, including zip code, and telephone number, including area code, of agent for service) |
With a copy to:
Thomas J. Deutsch, Esq.
LANG MICHENER LLP
1500 Royal Centre, 1055 West Georgia Street, Vancouver, British Columbia, Canada, V6E 4N7
Telephone: (604) 689-9111 and Facsimile: (604) 685-7084 |
Approximate date of commencement of proposed sale to the public: From time to time after this Registration Statement is declared effective.
If any securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.[X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registrations statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. [ ]
CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities
to be Registered(1) | Amount to be Registered(2) | Proposed Maximum Offering Price Per Share(3) | Proposed Maximum Aggregate Offering Price(3) | Amount of
Registration Fee(4) |
Shares of common stock, par value $0.001 per share | 678,060(5) | $5.40 | $3,661,524 | $112.41 |
Shares of common stock, par value $0.001 per share, underlying common stock purchase warrants | 678,060(6) | $5.40 | $3,661,524 | $112.41 |
Shares of common stock, par value $0.001 per share | 205,000(7) | $5.40 | $1,107,000 | $33.98 |
Shares of common stock, par value $0.001 per share | 3,981,164(8) | $5.40 | $21,498,285 | $660.00 |
Shares of common stock, par value, $0.001 per share, underlying common stock purchase warrants | 3,981,164(9) | $5.40 | $21,498,285 | $660.00 |
Totals: | 9,523,448 | | $51,426,619 | $1,578.80 |
__________
(1) In the event of a stock split, stock dividend or similar transaction involving the common shares of the Registrant, in order to prevent dilution, the number of shares of common stock registered shall be automatically increased to cover additional shares in accordance with Rule 416(a) under the United StatesSecurities Act of 1933, as amended (the "Securities Act").
(2) Includes shares of our common stock, par value $0.001 per share, currently owned by the selling shareholders (each a "Selling Shareholder") and shares of common stock which are issuable upon the exercise of warrants held by certain of the Selling Shareholders, all of which may be offered pursuant to this registration statement. In addition to the shares set forth in the table, the amount to be registered includes an indeterminate number of shares issuable upon the exercise of the warrants as such number may be adjusted as a result of stock splits, stock dividends and similar transactions in accordance with Rule 416 of the Securities Act.
(3) The Proposed Maximum Offering Price per Share is calculated in accordance with Rule 457(c) and Rule 457(g) of the Securities Act, based upon the last reported bid and ask price for our common stock on the OTC Bulletin Board on June 21, 2007, the date that our shares last traded. The Proposed Aggregate Maximum Aggregate Offering Price is based on the Proposed Maximum Offering Price Per Share times the total number of shares of common stock to be registered. These amounts are estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) and Rule 457(g) of the Securities Act.
(4) The fee is calculated in accordance with Rule 457(c) and Rule 457(g) of the Securities Act. We have based the fee calculation on the average of the last reported bid and ask price for our common stock on the OTC Bulletin Board on June 21, 2007, the date that our shares last traded.
(5) On March 30, 2007, we issued an aggregate of 678,060 units (the "Settlement Units") upon the settlement of $1,017,088 in principal and interest owing to certain creditors of a subsidiary at a deemed settlement price of $1.50 per Settlement Unit, with each Settlement Unit being comprised of one share of our common stock and one share purchase warrant (each a "Settlement Warrant"). These 678,060 shares of common stock represent the shares of our common stock issued in connection with the issuance of the Settlement Units.
(6) These 678,060 shares of common stock represent the 678,060 shares of our common stock issuable upon exercise of the Settlement Warrants. Each Settlement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share. The Settlement Warrants are exercisable commencing on the date of issuance and ending on the earlier of 18 months from the date of issuance and twelve months from the effective date of this registration statement.
(7) On March 30, 2007, we issued an aggregate of 205,000 shares of our common stock in connection with the cancellation of outstanding warrants to acquire shares in the capital of a subsidiary at a deemed price of $1.50 per share.
(8) On March 30, 2007, we issued an aggregate of 3,981,164 units (each a "Private Placement Unit") at a price of $1.50 per unit, with each Private Placement Unit consisting of one share of our common stock and one common stock purchase warrant (each a "Private Placement Warrant"). These 3,981,164 shares of common stock represent the shares of our common stock issued in connection with the issuance of the Private Placement Units.
(9) These 3,981,164 shares of common stock represent the 3,981,164 shares of our common stock issuable upon exercise of the Private Placement Warrants. Each Private Placement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share. The Private Placement Warrants are exercisable on the date of issuance for a period ending on the earlier of 18 months from the date of issuance and 12 months from the effective date of this registration statement.
__________
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
ii
SUBJECT TO COMPLETION, DATED JUNE 29, 2007
PROSPECTUS
CELLCYTE GENETICS CORPORATION
OFFERING OF 9,523,448 SHARES OF COMMON STOCK
This prospectus relates to the offering of 4,864,224 shares of our common stock by the selling shareholders (each a "Selling Shareholder") named in this prospectus under the heading "Selling Shareholders". In addition, this prospectus relates to the offering of 4,659,224 shares issuable upon the exercise of certain outstanding warrants to acquire shares of our common stock by certain of the Selling Shareholders. These shares include the following shares, all as described in this prospectus under "Selling Shareholders":
- the resale by certain Selling Shareholders, and their transferees, donees or successors, of an aggregate of 678,060 shares of our common stock issued on March 30, 2007 pursuant to the settlement of certain outstanding indebtedness of a subsidiary;
- the resale by certain Selling Shareholders, and their transferees, donees or successors, of an aggregate of 205,000 shares of our common stock issued on March 30, 2007 upon the cancellation of certain outstanding warrants of a subsidiary;
- the resale by certain Selling Shareholders, and their transferees, donees or successors, of an aggregate of 3,981,164 shares of our common stock issued on March 30, 2007 pursuant to a private placement;
- the resale by certain Selling Shareholders, and their transferees, donees or successors, of an aggregate of 678,060 shares of our common stock issuable upon the exercise of 678,060 common stock purchase warrants issued pursuant to the settlement of certain outstanding indebtedness of a subsidiary; and
- the resale by certain Selling Shareholders, and their transferees, donees or successors, of an aggregate of 3,981,164 shares of our common stock issuable upon the exercise of 3,981,164 common stock purchase warrants issued pursuant to the private placement.
We will not receive any proceeds from the sale of shares by the Selling Shareholders. However, we will receive proceeds upon the exercise of any common stock purchase warrants that may be exercised by certain of the Selling Shareholders. If all of the warrants are exercised we will receive proceeds in an amount of $13,977,672.
Our common stock is registered under Section 12(g) of the United StatesSecurities Exchange Act of 1934, as amended (the "Exchange Act"), and is quoted on the NASD Over-the-Counter Bulletin Board (the "OTCBB") under the symbol "CCYG.OB". The last reported sales price per share of our common stock as reported by the OTCBB on June 21, 2007, the date that our shares last traded, was $5.40.
Our principal offices are located at Carillon Point Centre, 5400 Carillon Point, Kirkland, Washington, U.S.A., 98033, and our telephone number is (425) 576-4106.
The purchase of the securities offered through this prospectus involves a high degree of risk. You should carefully read and consider the section of this prospectus titled "Risk Factors" beginning on page 7 before buying any of our shares of common stock.
The information in this prospectus is not complete and may be changed. The Selling Shareholders may not sell or offer these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offence.
The date of this prospectus is ____________, 2007.
__________
The following table of contents has been designed to help you find important information contained in this prospectus. We encourage you to read the entire prospectus.
TABLE OF CONTENTS |
Item | Page No. |
ABOUT THIS PROSPECTUS | 3 |
REFERENCES | 3 |
PROSPECTUS SUMMARY | 4 |
RISK FACTORS | 7 |
FORWARD-LOOKING STATEMENTS | 14 |
USE OF PROCEEDS | 15 |
SELLING SHAREHOLDERS | 15 |
PLAN OF DISTRIBUTION | 19 |
LEGAL PROCEEDINGS | 21 |
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS | 21 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 24 |
DESCRIPTION OF SECURITIES | 25 |
EXPERTS | 26 |
INTERESTS OF NAMED EXPERTS AND COUNSEL | 26 |
DISCLOSURE OF SEC POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES | 27 |
ORGANIZATION SINCE INCORPORATION | 27 |
DESCRIPTION OF BUSINESS | 27 |
DESCRIPTION OF PROPERTY | 40 |
MANAGEMENT'S DISCUSSION AND ANALYSIS OR PLAN OF OPERATIONS | 41 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 47 |
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER INFORMATION | 48 |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS | 50 |
INDEMNIFICATION OF DIRECTORS AND OFFICERS | 51 |
EXECUTIVE COMPENSATION | 52 |
WHERE YOU CAN FIND MORE INFORMATION | 53 |
FINANCIAL STATEMENTS | 55 |
__________
2
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission. The registration statement containing this prospectus, including the exhibits to the registration statement, also contains additional information about CellCyte Genetics Corporation and the securities offered under this prospectus. That registration statement can be read at the Securities and Exchange Commission's website (located atwww.sec.gov) or at the Securities and Exchange Commission's Public Reference Room mentioned under the heading "Where You Can Find More Information" of this prospectus.
You should rely only on the information contained in this document or to which we have referred you. We have not authorized anyone to provide you with information that is different. This document may only be used where it is legal to sell these securities. The information in this document may only be accurate on the date of this document. Our business, financial condition or results of operations may have changed since that date.
REFERENCES
As used in this prospectus: (i) the terms "we", "us", "our" and the "Company" mean CellCyte Genetics Corporation, a Nevada Corporation, and its subsidiaries, unless the context otherwise requires; (ii) references to "CellCyte" are to our wholly-owned subsidiary, CellCyte Genetics Corporation, a Washington corporation, acquired on March 30, 2007; (iii) "SEC" refers to the United States Securities and Exchange Commission; (iv) "Securities Act" refers to the United StatesSecurities Act of 1933, as amended; (v) "Exchange Act" refers to the United StatesSecurities Exchange Act of 1934, as amended; and (vi) all dollar amounts refer to United States dollars unless otherwise indicated.
__________
3
PROSPECTUS SUMMARY
The following summary highlights selected information contained in this prospectus. This summary does not contain all the information you should consider before investing in the securities. Before making an investment decision, you should read the entire prospectus carefully, including the "Risk Factors" section, the financial statements and the notes to the financial statements.
The Company
We were incorporated under the laws of the State of Nevada on March 9, 2004 under the name "Shepard Inc." On December 22, 2006, we effected a forward split of our shares of common stock on the basis of seven new shares of common stock for each one share of common stock outstanding on that date and increased our authorized share capital from 75,000,000 shares of common stock to 525,000,000 shares of common stock. On February 16, 2007, Shepard Inc. was merged with a wholly-owned subsidiary, CellCyte Genetics Corporation, a Nevada corporation, in contemplation of the acquisition of all of the issued and outstanding shares of CellCyte and the name of our company was changed to CellCyte Genetics Corporation.
We were an exploration stage company engaged in the acquisition and exploration of mineral properties with a view to exploiting any mineral deposits we discovered. Prior to the our Company's most recent year ended December 31, 2006, we owned a 100% beneficial interest in one mineral claim known as the CB-1 claim.
During the last fiscal year we retained an independent geologist to conduct exploration on the CB-1 claim and to provide us with recommendations for additional exploration on the property. All rock samples that were taken from the property during exploration did not contain any significant quantities of gold. In addition, we were not able to locate any other zones of altered rock on the CB-1 claim which could possibly host economic mineralization. Accordingly, we do not plan to conduct any further exploration of the CB-1 claim and we plan to allow the claim to expire.
On March 30, 2007, we acquired all of the issued and outstanding shares of CellCyte and our business is now focused on the discovery and development of stem cell enabling therapeutic products.
Our executive offices are located at Carillon Point Centre, 5400 Carillon Point, Kirkland, Washington, U.S.A., 98033, and our telephone number is (425) 576-4106.
The Offering
The Issuer: | | CellCyte Genetics Corporation |
The Selling Shareholders: | | The selling shareholders (each a "Selling Shareholder") are comprised of our existing shareholders who: - on March 30, 2007, were issued an aggregate of 678,060 units (each a "Settlement Unit") of the Company upon settlement of $1,017,088 in principal and interest owing to certain creditors of CellCyte at a deemed price of $1.50 per Settlement Unit, with each Settlement Unit consisting of one share of our common stock and one common stock purchase warrant (each a "Settlement Warrant");
- on March 30, 2007, purchased an aggregate of 3,981,164 units (each a "Private Placement Unit") of the Company at a price of $1.50 per Private Placement Unit, with each Private Placement Unit consisting of one share of our common stock and one common stock purchase warrant (each a "Private Placement Warrant"); and
- on March 30, 2007, were issued an aggregate of 205,000 shares of common stock of the Company at a deemed price of $1.50 per share upon the cancellation of certain outstanding share purchase warrants of CellCyte.
The Selling Shareholders are named in this prospectus under "Selling Shareholders". |
4
Shares Offered by the Selling Shareholders: | | The Selling Shareholders are offering up to an aggregate of 9,523,448 shares of our common stock as described below. See "Selling Shareholders" for more information. |
Offering Price: | | The Selling Shareholders may, from time to time, sell any or all of their shares of common stock offered by this prospectus on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed prices, at prevailing market prices, at prices related to prevailing market prices, at varying prices or at negotiated prices. The shares may be sold directly or through agents or broker-dealers acting as agents on behalf of the Selling Shareholders. We will pay substantially all the expenses relating to the registration of the shares offered by this prospectus, except for sales commissions and other fees applicable to sales of the shares. See "Plan of Distribution" for a description of the methods of distribution. |
Use of Proceeds: | | We will not receive any of the proceeds from the sale of shares by the Selling Shareholders. However, we will receive proceeds upon the exercise of any common stock purchase warrants that may be exercised by the Selling Shareholders. If all of the warrants are exercised we will receive proceeds in an amount of $13,977,672. The proceeds, if any, would be used for general corporate purposes, including for the purchase of lab and research equipment, research and development and working capital. |
Market for our Common Stock: | | Our common stock is quoted on the OTC Bulletin Board under the symbol "CCYG.OB". The last reported sale price for our shares on the OTC Bulletin Board on June 21, 2007, the date that our shares last traded, was $5.40 per share. |
Outstanding Shares of Common Stock: | | There were 59,854,224 shares of our common stock issued and outstanding as at June 28, 2007. If all warrants are exercised, then there would be 64,513,448 shares of our common stock issued and outstanding. |
Risk Factors: | | See "Risk Factors" and the other information in this prospectus for a discussion of the factors you should consider before deciding to invest in our securities. |
This prospectus relates to the offer of 4,864,224 shares of our common stock by the Selling Shareholders named in this prospectus under the heading "Selling Shareholders". We will not receive any proceeds from the sale of the currently outstanding common stock by the Selling Shareholders. In addition, this prospectus relates to the offer of 4,659,224 shares of our common stock issuable upon the exercise of certain outstanding warrants to acquire shares of our common stock by certain of the Selling Shareholders. These shares include the following shares, all as described in this prospectus under "Selling Shareholders":
On March 30, 2007, we issued an aggregate of 678,060 Settlement Units, at a deemed price of $1.50 per Settlement Unit, with each Settlement Unit consisting of one share of our common stock and one Settlement Warrant, to certain of the Selling Shareholders named herein, in connection with the settlement of an aggregate of $1,017,088 in principal and interest owing by CellCyte to certain of its creditors. Each Settlement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share commencing on the date of issuance for a period ending on the earlier of 18 months from the date of issuance of the Settlement Units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
5
On March 30, 2007, we issued an aggregate of 3,981,164 Private Placement Units at a price of $1.50 per Private Placement Unit, with each Private Placement Unit consisting of one share of our common stock and one Private Placement Warrant, to certain of the Selling Shareholders named herein. Each Private Placement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share commencing on the date of issuance for a period ending on the earlier of 18 months from the date of issuance of the Private Placement Units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
On March 30, 2007, we issued an aggregate of 205,000 shares of our common stock to certain of the Selling Shareholders named herein upon the cancellation of outstanding warrants to acquire shares in the capital of CellCyte at a deemed price of $1.50 per share.
Summary of Financial Data
The following selected financial data has been derived from and should be read in conjunction with: (i) our audited financial statements for the years ended December 31, 2006 and 2005, together with the notes to these financial statements; (ii) the audited financial statements of CellCyte for the years ended December 31, 2006 and 2005, together with the notes to those financial statements; (iii) the pro forma financial statements of the Company as at March 31, 2007; (iv) our interim financial statements for the three months ended March 31, 2007; and (v) the section of this prospectus entitled "Management's Discussion and Analysis or Plan of Operations", included elsewhere herein.
We were an exploration stage company engaged in the acquisition and exploration of mineral properties with a view to exploiting any mineral deposits we discovered. Prior to the our Company's most recent year ended December 31, 2006, we owned a 100% beneficial interest in one mineral claim known as the CB-1 claim.
During the last fiscal year we retained an independent geologist to conduct exploration on the CB-1 claim and to provide us with recommendations for additional exploration on the property. All rock samples that were taken from the property during exploration did not contain any significant quantities of gold. In addition, we were not able to locate any other zones of altered rock on the CB-1 claim which could possibly host economic mineralization. Accordingly, we do not plan to conduct any further exploration of the CB-1 claim and we plan to allow the claim to expire.
On March 30, 2007, we acquired all of the issued and outstanding shares of CellCyte and our business is now focused on the discovery and development of stem cell enabling therapeutic products.
The closing of the CellCyte acquisition represented a change in control of our Company. For accounting purposes, this change in control constitutes a re-capitalization of the Company, and the acquisition has been accounted for as a reverse merger whereby we, as the legal acquirer, are treated as the acquired entity, and CellCyte, as the legal subsidiary, is treated as the acquiring company with the continuing operations. The results of operations and cash flows of the Company are considered to be a continuation of the results of CellCyte.
As the Company did not have any material operations prior to the acquisition of CellCyte and the acquisition of CellCyte has been accounted for as a reverse merger, the financial information presented below for the periods prior to the three months ended March 31, 2007 is that of CellCyte.
Balance Sheet Data
| | CellCyte | |
| | As at
December 31, 2006 | As at
December 31, 2005 | As at
March 31, 2007 |
| | (Audited) | (Audited) | (Unaudited) |
Cash and cash equivalents | $287,750 | $52,444 | $779,327 |
Working capital (deficiency) | (535,135) | (542,055) | 5,357,180 |
Total assets | 658,616 | 289,869 | 6,221,841 |
6
| | CellCyte | |
| | As at
December 31, 2006 | As at
December 31, 2005 | As at
March 31, 2007 |
| | (Audited) | (Audited) | (Unaudited) |
Current liabilities | 824,085 | 604,699 | 399,844 |
Long-term liabilities | 535,000 | --- | --- |
Total stockholders' equity (deficiency) | (700,469) | (314,830) | 5,821,997 |
Statement of Operations Data
| | CellCyte | |
| | Fiscal Year Ended December 31, 2006 | Year Ended
December 31, 2005 | Three Months Ended
March 31, 2007 |
| | (Audited) | (Audited) | (Unaudited) |
Operating expenses | $(334,901) | $(291,689) | $(2,203,425) |
Net loss | (385,639) | (315,030) | (2,211,740) |
Net loss per share | (0.019) | (0.016) | (0.05) |
RISK FACTORS
An investment in our common stock involves a number of very significant risks. You should carefully consider the following risks and uncertainties in addition to other information in this prospectus in evaluating our company and its business before purchasing shares of our common stock. Our business, operating results and financial condition could be seriously harmed due to any of the following risks. The risks described below may not be all of the risks facing our company. Additional risks not presently known to us or that we currently consider immaterial may also impair our business operations. You could lose all or part of your investment due to any of these risks.
Risks Related to our Business
Any adverse development in the initial clinical trial for stem cell technology could substantially depress our stock price and prevent us from raising the capital we will need to further develop our stem cell technology.
To an unusual extent, our ability to progress as a company is significantly dependent on a single early stage clinical trial. Any clinical, regulatory or other development that prevents or delays us from conducting our initial clinical trial for our products, or any safety issue or adverse side effect to any patient that occurs during the trial, or the failure of this initial trial to enroll patients and proceed to completion as anticipated or to show the results expected by investors, would likely significantly depress our stock price and could prevent us from raising the substantial additional capital we will require to further develop stem cell technologies.
Our financial situation is precarious and, based on currently estimated operating expenses, our existing capital resources may not be sufficient to fund our operations beyond the next 10 months.
We have incurred significant operating losses and negative cash flows from operations since inception. We have not achieved profitability and may not be able to realize sufficient revenues to achieve or sustain profitability in the future. We expect to incur additional and increasing operating losses. We have limited liquidity and capital resources and must obtain significant additional capital resources in order to sustain our product development efforts and for acquisition of technologies and intellectual property rights, preclinical and clinical testing of our anticipated products, pursuit of regulatory approvals, acquisition of capital equipment, laboratory and office facilities, establishment of production capabilities, maintaining and enforcing our intellectual property portfolio, general and administrative expenses and other working capital requirements. We rely on cash reserves and proceeds from equity offerings to fund our operations. If we exhaust our cash reserves a nd are unable to realize adequate financing, we may be unable to meet operating obligations and be required to initiate bankruptcy proceedings. Our existing capital resources may not be sufficient to fund our operations beyond the next 10 months. We intend to pursue opportunities to obtain additional financing in the future through equity and debt financings, corporate alliances, grants and collaborative research arrangements. The source, timing and availability of any future financing will depend principally upon market conditions, interest rates and, more specifically, on our progress in our exploratory, preclinical and future clinical development programs. Funding may not be available when needed -- at all or on terms acceptable to us. Lack of necessary funds may require us to delay, scale back or eliminate some or all of our research and product development programs and/or our capital expenditures or to license our potential products or technologies to third parties.
7
Our product development programs are based on novel technologies and are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of our therapeutic products creates significant challenges in regards to product development and optimization, manufacturing, government regulation, third party reimbursement and market acceptance. For example, the FDA has relatively little experience with stem cell-based therapeutics, and the pathway to regulatory approval for our product candidates may accordingly be more complex and lengthy than the pathway for new conventional drugs. These challenges may prevent us from developing and commercializing products on a timely or profitable basis or at all.
Our technology is at an early stage of development, and we may fail to develop any commercially acceptable or profitable products.
Before we may market any product, we must obtain regulatory approval from the FDA and equivalent foreign agencies after conducting extensive preclinical studies and clinical trials that demonstrate that our product candidates are safe and effective for each disease for which we seek approval. We have limited experience in conducting clinical trials. We expect that none of our stem cell therapy product candidates will be commercially available for three years, if at all.
Our programs are still at the preclinical phase for our stem cell candidates and are in the development phase. While the FDA has permitted us to go forward with our proposed Phase I clinical trial of our proprietary stem cell therapy products, that trial has not yet enrolled or treated any patients and there can be no assurance that the clinical investigators will be able to identify suitable candidates for the trial or of a successful outcome of the trial if candidates are enrolled. We may fail to discover the stem cells we are seeking, to develop any products, to obtain regulatory approvals, to enter clinical trials, or to commercialize any products. We may elect to delay or discontinue preclinical studies or clinical trials based on unfavorable results. Any product using stem cell technology may fail to:
- survive and persist in the desired location;
- provide the intended therapeutic benefits;
- properly integrate into existing tissue in the desired manner; or
- achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing.
In addition, our products may cause undesirable side effects. Results of preclinical research may not be indicative of the results that will be obtained in later stages of preclinical or clinical research. If regulatory authorities do not approve our products or if we fail to maintain regulatory compliance, we would be unable to commercialize our products, and our business and results of operations would be harmed. Furthermore, because stem cells are a new form of therapy, the marketplace may not accept any products we may develop. If we do succeed in developing products, we will face many potential obstacles such as the need to obtain regulatory approvals and to develop or obtain manufacturing, marketing and distribution capabilities. In addition, we will face substantial additional risks such as product liability claims.
8
Moreover, because our cell therapy treatments may be derived from tissue of individuals other than the patient (that is, they will be "non-self" or "allogeneic" transplant products), patients may require the use of immunosuppressive drugs such as cyclosporine, FK506, or others to prevent rejection of the cells. While immunosuppression is now standard in connection with allogeneic transplants of various kinds, long-term maintenance on immunosuppressive drugs can produce complications that include infection, cancer, cardiovascular disease, renal dysfunction and other side effects depending upon which immunosuppressive regimen is employed. Immunosuppression has not been tested with our therapies since we have not yet conducted any clinical trials.
We may need but fail to obtain partners to support our stem cell development efforts and to commercialize our technology.
Equity and debt financings alone may not be sufficient to fund the cost of developing our stem cell technologies, and we may need to rely on our ability to reach partnering arrangements to provide financial support for our stem cell discovery and development efforts. In addition, in order to successfully develop and commercialize our technology, we may need to enter into a wide variety of arrangements with corporate sponsors, pharmaceutical companies, universities, research groups and others. While we have engaged, and expect to continue to engage in discussions regarding such arrangements, we have not reached any agreement, and we may fail to obtain any such agreement on terms acceptable to us. Even if we enter into these arrangements, we may not be able to satisfy our obligations under them or renew or replace them after their original terms expire. Furthermore, these arrangements may require us to grant certain rights to third parties, including exclusive marketing rights to one or more products, may require us to issue securities to our collaborators or may contain other terms that are burdensome to us. If any of our collaborators terminates our relationship with us or fails to perform our obligations in a timely manner, the development or commercialization of our technology and potential products may be adversely affected.
We have a history of operating losses, and we may fail to obtain revenues or become profitable.
We expect to continue to incur substantial operating losses in the future in order to conduct our research and development activities, and, if those activities are successful, to fund clinical trials and other expenses. These expenses include the cost of acquiring technology, product testing, acquiring regulatory approvals, establishing production, marketing, sales and distribution programs and administrative expenses. We have not earned any revenues from sales of any product. We expect that any revenues we may obtain in the foreseeable future will be derived from, cooperative agreements, research grants, investments and interest on invested capital. We currently have no cooperative agreements, we have no current research grant for our stem cell technology, and we may not obtain any such agreements or additional grants in the future or receive any revenues from them.
If we are unable to protect our patents and proprietary rights, our business, financial condition and results of operations will be harmed.
We own or license a number of patents and pending patent applications related to various stem cells and methods of deriving and using them, including human neural stem cell cultures. Patent protection for products such as those we propose to develop is highly uncertain and involves complex and continually evolving factual and legal questions. The governmental authorities that consider patent applications can deny or significantly reduce the patent coverage requested in an application before or after issuing the patent. Consequently, we do not know whether any of our pending applications will result in the issuance of patents, if any existing or future patents will provide sufficient protection or significant commercial advantage or if others will circumvent these patents. We cannot be certain that we were the first to discover the inventions covered by each of our pending patent applications or that we were the first to file patent applications for such inventions because paten t applications are secret until they are published, and because publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Patents may not issue from our pending or future patent applications or, if issued, may not be of commercial benefit to us. In addition, our patents may not afford us adequate protection from competing products. Third parties may challenge our patents or governmental authorities may declare them invalid or reduce their scope. In the event that a third party has also filed a patent application relating to inventions claimed in our patent applications, we may have to participate in proceedings to determine priority of invention. Even if a patent issues, a court could decide that the patent was issued invalidly. Because patents issue for a limited term, our patents may expire before we utilize them profitably. Procedures of the European Patent Office, third parties may oppose our issued European patents during the relevant opposition peri od. These proceedings and oppositions could result in substantial uncertainties and cost for us, even if the eventual outcome is favorable to us, and the outcome might not be favorable to us.
9
If we learn of third parties who infringe our patent rights, we may need to initiate legal proceedings to enforce our patent rights. These proceedings may entail significant costs, and these third parties may have significantly greater financial resources than us. We may not prevail in these proceedings.
Proprietary trade secrets and unpatented know-how are also important to our research and development activities. We cannot be certain that others will not independently develop the same or similar technologies on their own or gain access to our trade secrets or disclose such technology or that we will be able to meaningfully protect our trade secrets and unpatented know-how. We require our employees, consultants, and significant scientific collaborators and sponsored researchers to execute confidentiality agreements upon the commencement of an employment or consulting relationship with us. These agreements may, however, fail to provide meaningful protection or adequate remedies for us in the event of unauthorized use, transfer or disclosure of such information or technology.
If others are first to discover and patent the stem cells we are seeking to discover, we could be blocked from further work on those stem cells.
Because the first person or entity to discover and obtain a valid patent to a particular stem cell may effectively block all others, it will be important for us or our collaborators to be the first to discover any stem cell and methods that we are seeking to discover. Failure to be the first could prevent us from commercializing all of our research and development affected by that patent.
If we are unable to obtain necessary licenses to third-party patents and other rights, we may not be able to commercially develop our expected products.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have received patents relating to cell therapy, stem cells and other technologies potentially relevant to or necessary for our expected products. We cannot predict which, if any, of the applications will issue as patents, and there may be existing patents of which we are currently unaware which the commercialization of our product candidates would infringe. If third party patents or patent applications contain valid claims that our technology infringes upon their technology, we may be prevented from commercializing that technology unless the third party is willing to grant a license to us. We may be unable to obtain licenses to the relevant patents at a reasonable cost, if at all, and may also be unable to develop or obtain alternative non-infringing technology. If we are unable to obtain such licenses or develop non-infringing technology at a reasonable cost, our business could be significantly harmed. Also, any infringement lawsuits commenced against us may result in significant costs, divert our management's attention and result in an award against us for substantial damages.
We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, some aspects of our stem cell product candidates involve the use of growth factors, antibodies and other reagents that may, in certain cases, be the subject of third party rights. Before we commercialize any product using these growth factors, antibodies or reagents, we may need to obtain license rights from third parties or use alternative growth factors, antibodies and reagents that are not then the subject of third party patent rights. We currently believe that the commercialization of our products as currently planned will not infringe these third party rights, or, alternatively, that we will be able to obtain necessary licenses or otherwise use alternate non-infringing technology. However, third parties may nonetheless bring suit against us claiming infringement. If we are unable to prove that our technology does not infringe their patents, or if we are unable to obtain necessary licenses or otherwise use alternative non-infringing technology, we may not be able to commercialize any products. Also, if we use alternative non-infringing technology, we may need to demonstrate comparability in subsequent clinical trials.
We have obtained rights to technologies, processes and compounds that we believe may be important to the development of our products. These licensors, however, may cancel our licenses or convert them to non-exclusive licenses if we fail to use the relevant technology or otherwise breach these agreements. Loss of these licenses could expose us to the risk that our technology infringes the rights of third parties. We can give no assurance that any of these licenses will provide effective protection against our competitors.
10
We compete with companies that have significant advantages over us.
It is expected that our therapeutic products will have to compete with a variety of therapeutic products and procedures. Major pharmaceutical companies currently offer a number of pharmaceutical products to treat lysosomal storage disorders, neurodegenerative and liver diseases, diabetes and other diseases for which our technologies may be applicable. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. The market for therapeutic products is large, and competition is intense. We expect competition to increase. We believe that o ur most significant competitors will be fully integrated pharmaceutical companies and more established biotechnology companies. Smaller companies may also be significant competitors, particularly through collaborative arrangements with large pharmaceutical or biotechnology companies. Many of these competitors have significant products approved or in development that could be competitive with our products.
Competition for any stem cell products that we may develop may be in the form of existing and new drugs, other forms of cell transplantation, ablative and simulative procedures, and gene therapy. Some competitors are also trying to develop stem and progenitor cell-based technologies. These products may compete with our therapeutic products based on efficacy, safety, cost and intellectual property positions.
We may also face competition from companies that have filed patent applications relating to the use of genetically modified cells to treat disease, disorder or injury. In the event our therapies should require the use of such genetically modified cells, it may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted or may be available only on unfavorable terms.
If we develop products that receive regulatory approval, we would then have to compete for market acceptance and market share. For certain of our products, an important success factor will be the timing of market introduction of competitive products. This is a function of the relative speed with which we and our competitors can develop products, complete the clinical testing and approval processes, and supply commercial quantities of a product to market. These competitive products may also impact the timing of clinical testing and approval processes by limiting the number of clinical investigators and patients available to test our products.
While we believe that the primary competitive factors will be product efficacy, safety, and the timing and scope of regulatory approvals, other factors include, in certain instances, obtaining marketing exclusivity under the Orphan Drug Act, availability of supply, marketing and sales capability, reimbursement coverage, price, and patent and technology position.
Development of our technology is subject to and restricted by extensive government regulation, which could impede our business.
Our research and development efforts, as well as any future clinical trials, and the manufacturing and marketing of any products we may develop, will be subject to and restricted by extensive regulation by governmental authorities in the United States and other countries. The process of obtaining FDA and other necessary regulatory approvals is lengthy, expensive and uncertain. We or our collaborators may fail to obtain the necessary approvals to commence or continue clinical testing or to manufacture or market our potential products in reasonable time frames, if at all. In addition, the United States Congress and other legislative bodies may enact regulatory reforms or restrictions on the development of new therapies that could adversely affect the regulatory environment in which we operate or the development of any products we may develop.
11
We base our research and development on the use of human stem cells obtained from fetal tissue. The federal and state governments and other jurisdictions impose restrictions on the use of fetal tissue, including those incorporated in the recent federal current Good Tissue Practice, or cGTP, regulations. These regulatory and other constraints could prevent us from obtaining cells and other components of our products in the quantity or quality needed for their development or commercialization. These restrictions change from time to time and may become more onerous. Additionally, we may not be able to identify or develop reliable sources for the cells necessary for our potential products -- that is, sources that follow all state and federal guidelines for cell procurement. Certain components used to manufacture our stem cell product candidates may need to be manufactured in compliance with the FDA's Good Manufacturing Practices, or cGMP. Accordingly, we may need to enter into supply agr eements with companies that manufacture these components to cGMP standards.
Government regulation and threatened regulation of embryonic tissue may lead top researchers to leave the field of stem cell research, or the country, in order to assure that their careers will not be impeded by restrictions on their work. Similarly, these factors may induce the best graduate students to choose other fields less vulnerable to changes in regulatory oversight, thus exacerbating the risk, discussed below, that we may not be able to attract and retain the scientific personnel we need in face of the competition among pharmaceutical, biotechnology and health care companies, universities and research institutions for what may become a shrinking class of qualified individuals. In addition, we cannot assure you that constraints on the use of embryonic stem cells will not be extended to use of fetal stem cells. Moreover, it is possible that concerns regarding research using embryonic stem cells will negatively impact our stock price and our ability to attract collaborators and in vestors.
We may apply for status under the Orphan Drug Act for some of our therapies to gain a seven-year period of marketing exclusivity for those therapies. The United States Congress in the past has considered, and in the future again may consider, legislation that would restrict the extent and duration of the market exclusivity of an orphan drug. If enacted, such legislation could prevent us from obtaining some or all of the benefits of the existing statute even if we were to apply for and obtain orphan drug status with respect to a potential product.
We are dependent on the services of key personnel.
We are highly dependent on the principal members of our management and scientific staff and some of our outside consultants, including our chief executive officer and our vice presidents. Although we have entered into employment agreements with some of these individuals, they may terminate their agreements at any time. In addition, our operations are dependent upon our ability to attract and retain additional qualified scientific and management personnel. We may not be able to attract and retain the personnel we need on acceptable terms given the competition for experienced personnel among pharmaceutical, biotechnology and health care companies, universities and research institutions.
Our activities involve hazardous materials and experimental animal testing; improper handling of these animals and materials by our employees or agents could expose us to significant legal and financial penalties.
Our research and development activities involve the controlled use of hazardous chemicals and potentially hazardous biological materials such as human tissue and animals. Their use subjects us to environmental and safety laws and regulations such as those governing laboratory procedures, exposure to blood-borne pathogens, use of animals and the handling of biohazardous materials. Compliance with current or future laws and regulations may be expensive and the cost of compliance could adversely affect us.
Although we believe that our safety procedures for using, handling, storing and disposing of hazardous and potentially hazardous materials comply with the standards prescribed by California and federal regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of such an accident or of any violation of these or future laws and regulations, state or federal authorities could curtail our use of these materials; we could be liable for any civil damages that result, the cost of which could be substantial; and we could be subjected to substantial fines or penalties. In addition, any failure by us to control the use, disposal, removal or storage, or to adequately restrict the discharge, or to assist in the cleanup of hazardous chemicals or hazardous, infectious or toxic substances could subject us to significant liability. Any such liability could exceed our resources and could have a material adverse effect on our business, financial c ondition and results of operations. Moreover, an accident could damage our research and manufacturing facilities and operations and result in serious adverse effects on our business.
12
The manufacture, development and commercialization of stem cell products expose us to product liability claims, which could lead to substantial liability.
By developing and, ultimately, commercializing medical products, we are exposed to the risk of product liability claims. Product liability claims against us could entail substantial litigation costs and damage awards against us. We are in the process of obtaining liability insurance that covers our clinical trials, and we will need to increase our insurance coverage if and when we begin commercializing products. We may not be able to obtain insurance on acceptable terms, if at all, and the policy limits on our insurance policies may be insufficient to cover our liability.
Since health care insurers and other organizations may not pay for our products or may impose limits on reimbursements, our ability to become profitable could be reduced.
In both domestic and foreign markets, sales of potential products are likely to depend in part upon the availability and amounts of reimbursement from third party health care payor organizations, including government agencies, private health care insurers and other health care payors, such as health maintenance organizations and self-insured employee plans. There is considerable pressure to reduce the cost of therapeutic products, and government and other third party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products and by refusing, in some cases, to provide any coverage for uses of approved products for disease indications for which the FDA has not granted marketing approval. Significant uncertainty exists as to the reimbursement status of newly approved health care products or novel therapies such as ours. Even if we obtain regulatory approval to market our products, we can give no assurance that reimbursement will be provided by such payors at all or without substantial delay or, if such reimbursement is provided, that the approved reimbursement amounts will be sufficient to enable us to sell products we develop on a profitable basis. Changes in reimbursement policies could also adversely affect the willingness of pharmaceutical companies to collaborate with us on the development of our stem cell technology. We also expect that there will continue to be a number of federal and state proposals to implement government control over health care costs. Efforts at health care reform are likely to continue in future legislative sessions. We do not know what legislative proposals federal or state governments will adopt or what actions federal, state or private payers for health care goods and services may take in response to health care reform proposals or legislation. We cannot predict the effect government control and other health care reforms may have on our business.
We have limited liquidity and capital resources and may not obtain the significant capital resources we will need to sustain our research and development efforts.
We have limited liquidity and capital resources and must obtain substantial additional capital to support our research and development programs, for acquisition of technology and intellectual property rights and, to the extent we decide to undertake these activities ourselves, for preclinical and clinical testing of our anticipated products, pursuit of regulatory approvals, establishment of production capabilities, maintaining and enforcing our intellectually property portfolio, establishment of marketing and sales capabilities and distribution channels, and general administrative expenses. If we do not obtain the necessary capital resources, we may have to delay, reduce or eliminate some or all of our research and development programs or license our technology or any potential products to third parties rather than commercialize them ourselves. We intend to pursue our needed capital resources through equity and debt financings, corporate alliances, grants and collaborative resear ch arrangements. We may fail to obtain the necessary capital resources from any such sources when needed or on terms acceptable to us. Our ability to complete successfully any such arrangements will depend upon market conditions and, more specifically, on continued progress in our research and development efforts.
Ethical and other concerns surrounding the use of stem cell therapy may negatively affect regulatory approval or public perception of our product candidates, which could reduce demand for our products.
The use of stem cells for research and therapy has been the subject of debate regarding related ethical, legal and social issues. Although these concerns have mainly been directed to the use of embryonic stem cells, which we do not use, the distinction between embryonic and non-embryonic stem cells is frequently overlooked; moreover, our use of human stem cells from fetal sources might raise these or similar concerns. Negative public attitudes toward stem cell therapy could result in greater governmental regulation of stem cell therapies, which could harm our business. For example, concerns regarding such possible regulation could impact our ability to attract collaborators and investors. Also, existing regulatory constraints on the use of embryonic stem cells may in the future be extended to use of fetal stem cells, and these constraints might prohibit or restrict us from conducting research or commercializing products. Government regulation and threatened regulation of embry onic tissue could also harm our ability to attract and retain qualified scientific personnel by causing top researchers to leave the country or the field of stem cell research altogether; and by encouraging the best graduate students to choose other fields that are less vulnerable to changes in regulatory oversight.
13
Risks Related to the Securities Market
Our stock price will likely be highly volatile, which may negatively affect our ability to obtain additional financing in the future.
The market price of our stock is likely to be highly volatile due to the risks and uncertainties described in this risk factors section, as well as other factors, including:
- our ability to develop and test our technology;
- our ability to patent or obtain licenses to necessary technology;
- conditions and publicity regarding the industry in which we operate, as well as the specific areas our product candidates seek to address;
- competition in our industry;
- price and volume fluctuations in the stock market at large that are unrelated to our operating performance; and
- comments by securities analysts, or our failure to meet market expectations.
As a result of this volatility, an investment in our stock is subject to substantial risk. Furthermore, the volatility of our stock price could negatively impact our ability to raise capital in the future.
We are contractually obligated to issue shares in the future, diluting the interest of current shareholders.
As of March 31, 2007, there were outstanding warrants to purchase 4,659,000 shares of our common stock and options to purchase 2,000,000 shares of our common stock. Moreover, we expect to issue additional options to purchase shares of our common stock to compensate employees, consultants and directors, and may issue additional shares to raise capital, to acquire other companies or technologies, to pay for services or for other corporate purposes. Any such issuances will have the effect of diluting the interest of current shareholders.
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties. Forward-looking statements in this prospectus include, among others, statements regarding our capital needs, business plans and expectations. Such forward-looking statements involve assumptions, risks and uncertainties regarding, among others, the success of our business plan, availability of funds, government regulations, operating costs, our ability to achieve significant revenues, our business model and products and other factors. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may", "will", "should", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict", "potential" or "continue", the negative of such terms or other comparable terminology. In evaluating these statements, you should consider various factors, includin g the assumptions, risks and uncertainties outlined in this prospectus under "Risk Factors". These factors or any of them may cause our actual results to differ materially from any forward-looking statement made in this prospectus. While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding future events, our actual results will likely vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein. The forward-looking statements in this prospectus are made as of the date of this prospectus and we do not intend or undertake to update any of the forward-looking statements to conform these statements to actual results, except as required by applicable law, including the securities laws of the United States.
14
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of the shares of common stock offered by the Selling Shareholders under this prospectus. We would receive gross proceeds in the approximate amount of $13,977,672 assuming the exercise of all warrants of which the underlying common stock are being offered hereby. The proceeds, if any, would be used for general corporate purposes, including for the purchase of lab and research equipment, research and development and working capital.
All proceeds from the sale of the shares will be for the account of the Selling Shareholders, and they will pay any and all expenses incurred by them for brokerage, accounting or tax services or any other expenses incurred by them in disposing of their shares. We will however incur substantially all of the costs associated with the filing of this prospectus and the registration statement of which it forms a part.
SELLING SHAREHOLDERS
The Selling Shareholders named in this prospectus are offering all of the 9,523,448 shares of common stock offered through this prospectus. The Selling Shareholders are comprised of our existing shareholders who:
- on March 30, 2007, were issued an aggregate of 678,060 Settlement Units upon settlement of $1,017,088 in principal and interest owing to certain creditors of CellCyte at a deemed price of $1.50 per Settlement Unit, with each Settlement Unit consisting of one share of our common stock and one Settlement Warrant;
- on March 30, 2007, purchased an aggregate of 3,981,164 Private Placement Units at a price of $1.50 per Private Placement Unit, with each Private Placement Unit consisting of one share of our common stock and one Private Placement Warrant; and
- on March 30, 2007, were issued an aggregate of 205,000 shares of our common stock at a deemed price of $1.50 upon the cancellation of certain outstanding share purchase warrants of CellCyte.
This prospectus relates to the offer of 4,864,224 shares of our common stock by the Selling Shareholders named in this prospectus under the heading "Selling Shareholders". We will not receive any proceeds from the sale of the currently outstanding common stock by the Selling Shareholders. In addition, this prospectus relates to the offer of 4,659,224 shares of our common stock issuable upon the exercise of certain outstanding warrants to acquire shares of our common stock by certain of the Selling Shareholders. These shares include the following shares:
On March 30, 2007, we issued an aggregate of 678,060 Settlement Units at a deemed price of $1.50 per Settlement Unit, with each Settlement Unit consisting of one share of our common stock and one Settlement Warrant, to certain of the Selling Shareholders named herein, in connection with the settlement of an aggregate of $1,017,088 in principal and interest owing by CellCyte to certain of its creditors. Each Settlement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share commencing on the date of issuance for a period ending on the earlier of 18 months from the date of issuance of the Settlement Units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
15
On March 30, 2007, we issued an aggregate of 3,981,164 Private Placement Units at a price of $1.50 per Private Placement Unit, with each Private Placement Unit consisting of one share of our common stock and one Private Placement Warrant, to certain of the Selling Shareholders named herein. Each Private Placement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share commencing on the date of issuance for a period ending on the earlier of 18 months from the date of issuance of the Private Placement Units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
On March 30, 2007, we issued an aggregate of 205,000 shares of our common stock upon the cancellation of outstanding warrants to acquire shares in the capital of CellCyte at a deemed price of $1.50 per share.
The private placement transactions were completed in reliance of Rule 506 of Regulation D of the Securities Act, with respect to investors in the United States, and in reliance of Rule 903 of Regulation S of the Securities Act, with respect to those investors who were not "U.S. Persons", within the meaning of Regulation S, and who were otherwise outside of the United States. Sales to United States investors pursuant to Rule 506 of Regulation D were limited to investors who qualified as "accredited investors" within the meaning of Rule 501(a) of Regulation D.
The following table sets forth information as of June 28, 2007, regarding the ownership of the shares of common stock to be sold by the Selling Shareholders. The number of shares indicated in respect of the transactions relating to the issuance of the Settlement Units and Private Placement Units includes both the shares issued in respect of these units and the shares issuable to the Selling Shareholders upon exercise of the Loan Warrants, Settlement Warrants and Private Placement Warrants.
Information with respect to ownership is based upon information obtained from the Selling Shareholders. Information with respect to "Shares Owned Prior to this Offering" includes the shares issuable upon exercise of the warrants held by the Selling Shareholders. The "Number of Shares Being Offered" includes the shares acquired by the Selling Shareholders in the transactions described above and the shares that are issuable upon exercise of the warrants acquired by the Selling Shareholders. Information with respect to "Shares Owned After this Offering" assumes the sale of all of the shares offered by this prospectus and no other purchases or sales of our common stock by the Selling Shareholders. Except as described below and to our knowledge, the named Selling Shareholders own and have sole voting and investment power over all shares or rights to these shares. Except for their ownership of common stock described below, none of the Selling Shareholders had or have any material relationship with us. On information and belief based upon query by the Company to the Selling Shareholders, we do not believe that any Selling Shareholders are registered broker-dealers or affiliates of registered broker-dealers.
| | Shares Owned
Prior To This Offering | Number of Shares Being Offered | Shares Owned
AfterThis Offering |
Name of Selling Shareholder | Number(1) | Percentage(2) | Number | Number | Percentage(2) |
Settlement Units | | | | | |
Brian Carver | 15,584 | * | 15,584 | Nil | Nil |
GBR Investments, LLC(3) | 495,132 | * | 495,132 | Nil | Nil |
Mark and Tracey Hilliard | 15,584 | * | 15,584 | Nil | Nil |
Steven A. and Karen L. Huber | 15,002 | * | 15,002 | Nil | Nil |
Faye Lock and Ming Lin | 77,960 | * | 77,960 | Nil | Nil |
Jack K. Lowry | 15,440 | * | 15,440 | Nil | Nil |
Samuel D. Lowry and Terry Lowry | 15,592 | * | 15,592 | Nil | Nil |
Brett Nesland | 14,832 | * | 14,832 | Nil | Nil |
Pacific Blue Capital LLC(4) | 414,390 | * | 414,390 | Nil | Nil |
Gary Pearce | 31,144 | * | 31,144 | Nil | Nil |
16
| | Shares Owned
Prior To This Offering | Number of Shares Being Offered | Shares Owned
AfterThis Offering |
Name of Selling Shareholder | Number(1) | Percentage(2) | Number | Number | Percentage(2) |
Ernest and Kim Perry | 15,592 | * | 15,592 | Nil | Nil |
Karen Perry | 31,162 | * | 31,162 | Nil | Nil |
Sherry Presgrove | 15,580 | * | 15,580 | Nil | Nil |
William V. Roll | 37,542 | * | 37,542 | Nil | Nil |
Richard I. Rynes | 68,726 | * | 68,726 | Nil | Nil |
James A. Wold and Ann E. Wold | 15,028 | * | 15,028 | Nil | Nil |
Robert Wold | 62,370 | * | 62,370 | Nil | Nil |
Shares for Warrants | | | | | |
Brian Carver | 3,000 | * | 3,000 | Nil | Nil |
GBR Investments, LLC(3) | 70,000 | * | 70,000 | Nil | Nil |
Mark and Tracey Hilliard | 3,000 | * | 3,000 | Nil | Nil |
Steven A. and Karen L. Huber | 2,000 | * | 2,000 | Nil | Nil |
Faye Lock and Ming Lin | 15,000 | * | 15,000 | Nil | Nil |
Jack K. Lowry | 2,000 | * | 2,000 | Nil | Nil |
Samuel D. Lowry and Terry Lowry | 3,000 | * | 3,000 | Nil | Nil |
Brett Nesland | 2,000 | * | 2,000 | Nil | Nil |
Pacific Blue Capital LLC(4) | 58,000 | * | 58,000 | Nil | Nil |
Gary Pearce | 6,000 | * | 6,000 | Nil | Nil |
Ernest and Kim Perry | 3,000 | * | 3,000 | Nil | Nil |
Karen Perry | 6,000 | * | 6,000 | Nil | Nil |
Sherry Presgrove | 3,000 | * | 3,000 | Nil | Nil |
William V. Roll | 5,000 | * | 5,000 | Nil | Nil |
Richard I. Rynes | 10,000 | * | 10,000 | Nil | Nil |
James A. Wold and Ann E. Wold | 2,000 | * | 2,000 | Nil | Nil |
Robert Wold | 12,000 | * | 12,000 | Nil | Nil |
Private Placement Units | | | | | |
Avasha Group Ltd.(5) | 666,666 | 1.1% | 666,666 | Nil | Nil |
Cynthia Lynn Carruthers | 20,000 | * | 20,000 | Nil | Nil |
Tim & Dana Chace | 66,666 | * | 66,666 | Nil | Nil |
Eunice M. Stevens Trust, UTD September 23, 1999(6) | 100,000 | * | 100,000 | Nil | Nil |
Golden West Investments(7) | 2,000,000 | 3.3% | 2,000,000 | Nil | Nil |
Mark A. Hilliard | 66,666 | * | 66,666 | Nil | Nil |
Steven and Karen Huber | 66,666 | * | 66,666 | Nil | Nil |
Faye C. Lock | 89,666 | * | 89,666 | Nil | Nil |
17
| | Shares Owned
Prior To This Offering | Number of Shares Being Offered | Shares Owned
AfterThis Offering |
Name of Selling Shareholder | Number(1) | Percentage(2) | Number | Number | Percentage(2) |
Brett Nesland | 120,000 | * | 120,000 | Nil | Nil |
Newport Capital Corp.(8) | 1,666,666 | 2.7% | 1,666,666 | Nil | Nil |
Pacific Blue Capital LLC(4) | 253,334 | * | 253,334 | Nil | Nil |
Ernest & Kimberly Perry | 80,000 | * | 80,000 | Nil | Nil |
Phoenix Asset Corp.(9) | 2,000,000 | 3.3% | 2,000,000 | Nil | Nil |
Dana Pierce | 333,332 | * | 333,332 | Nil | Nil |
Sherry Presgrove | 22,666 | * | 22,666 | Nil | Nil |
Kenneth & Barbara Regua | 133,334 | * | 133,334 | Nil | Nil |
Niles R.W. Robbins | 666,666 | 1.1% | 666,666 | Nil | Nil |
Michael Terrane Shutt | 10,000 | * | 10,000 | Nil | Nil |
Thomas R. Ulie | 133,334 | * | 133,334 | Nil | Nil |
James A. Wold | 66,666 | * | 66,666 | Nil | Nil |
Total: | 9,523,448 | | 9,523,448 | Nil | Nil |
* Less than one percent.
- Includes shares of common stock currently held by the Selling Shareholder and shares issuable upon exercise of all warrants owned by certain of the Selling Shareholder.
- The applicable percentage of ownership is based on 59,854,224 shares of common stock outstanding as of June 28, 2007, plus the number of shares of common stock that would be outstanding if all of the warrants held by such Selling Shareholder were exercised.
- Rick Rathman, as managing member of GBR Investments, LLC, has discretionary authority to purchase, vote and dispose of the securities on behalf of GBR Investments, LLC. Mr. Rathman disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
- Pacific Blue Capital LLC owns an aggregate of 725,724 shares of our common stock and warrants to acquire up to 667,724 shares of our common stock, representing 2.3% of our outstanding shares of commons stock. Tom Grossi, as managing member of Pacific Blue Capital LLC, has discretionary authority to purchase, vote and dispose of the securities on behalf of Pacific Blue Capital LLC. Mr. Grossi disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
- Dr. Gerald Hoop, as managing director of Avasha Group Ltd., has discretionary authority to purchase, vote and dispose of the securities on behalf of Avasha Group Ltd. Dr. Hoop disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
- Eunice H. Stevens, as trustee of Eunice H. Stevens Trust, UTD September 23, 1999, has discretionary authority to purchase, vote and dispose of the securities on behalf of Eunice H. Stevens Trust, UTD September 23, 1999. Eunice H. Stevens disclaims beneficial ownership as to such securities except to the extent of her pecuniary interests therein.
- Trustell Ltd. is the sole director of Golden West Investments. Barry Dempsey is the sole director of Trustell Ltd. Mr. Dempsey has discretionary authority to purchase, vote and dispose of the securities on behalf of Golden West Investments. Mr. Dempsey disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
- Brent Pierce is an officer of Newport Capital Corp. and has discretionary authority to purchase, vote and dispose of the securities on behalf of Newport Capital Corp. Mr. Pierce disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
- Fitzroy Holdings, Ltd. is the sole director of Phoenix Asset Corp. Barry Dempsey is a director of Fitzroy Holdings. Mr. Dempsey has discretionary authority to purchase, vote and dispose of the securities on behalf of Phoenix Asset Corp. Mr. Dempsey disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
Because a Selling Shareholder may offer by this prospectus all or some part of the common shares which it holds, no estimate can be given as of the date hereof as to the number of common shares actually to be offered for sale by a Selling Shareholder or as to the number of common shares that will be held by a Selling Shareholder upon the termination of such offering.
18
PLAN OF DISTRIBUTION
Timing of Sales
The Selling Shareholders may offer and sell the shares covered by this prospectus at various times. The Selling Shareholders will act independently of us in making decisions with respect to the timing, manner and size of each sale.
No Known Agreements to Resell the Shares
To our knowledge, no Selling Shareholder has any agreement or understanding, directly or indirectly, with any person to resell the shares covered by this prospectus.
Offering Price
The Selling Shareholders will sell their shares to the public at:
- the market price prevailing at the time of sale;
- a price related to such prevailing market price; or
- such other price as the selling shareholders determine from time to time.
The sales price to the public will vary according to the selling decisions of each Selling Shareholder and the market for our stock at the time of resale.
Manner of Sale
The shares may be sold by means of one or more of the following methods:
- a block trade in which the broker-dealer so engaged will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
- purchases by a broker-dealer as principal and resale by that broker-dealer for its account pursuant to this prospectus;
- ordinary brokerage transactions in which the broker solicits purchasers;
- through options, swaps or derivative;
- in transactions to cover short sales;
- privately negotiated transactions; or
- in a combination of any of the above methods.
The Selling Shareholders may sell their shares directly to purchasers or may use brokers, dealers, underwriters or agents to sell their shares. Brokers or dealers engaged by the selling shareholders may arrange for other brokers or dealers to participate. Brokers or dealers may receive commissions, discounts or concessions from the Selling Shareholders, or, if any such broker-dealer acts as agent for the purchaser of shares, from the purchaser in amounts to be negotiated immediately prior to the sale. The compensation received by brokers or dealers may, but is not expected to, exceed that which is customary for the types of transactions involved. Broker-dealers may agree with a Selling Shareholder to sell a specified number of shares at a stipulated price per share, and, to the extent the broker-dealer is unable to do so acting as agent for a Selling Shareholder, to purchase as principal any unsold shares at the price required to fulfill the broker-dealer commitment to the Selling Sharehol der. Broker-dealers who acquire shares as principal may thereafter resell the shares from time to time in transactions, which may involve block transactions and sales to and through other broker-dealers, including transactions of the nature described above, in the over-the-counter market or otherwise at prices and on terms then prevailing at the time of sale, at prices then related to the then-current market price or in negotiated transactions. In connection with resales of the shares, broker-dealers may pay to or receive from the purchasers of shares commissions as described above.
19
We agreed to file a registration statement with the SEC in accordance with the requirements of the Securities Act in order to register the resale by the investors of the shares issued and the shares issuable upon exercise of the warrants relating to the Settlement Units and Private Placement Units and shares issued upon cancellation of the CellCyte warrants, completed pursuant to Rule 506 of Regulation D and Rule 903 of Regulation S under the Securities Act. We agreed to have the registration statement declared effective within six months from the date of the issuance of the units relating to the settlement of indebtedness and Private Placement and shares issued upon cancellation of the CellCyte warrants.
We will keep the registration statement effective for a period of not less than twelve months from the effective date of the registration statement, including the filing of such amendments and supplements to the registration statement and this prospectus as may be necessary to comply with the provisions of the Securities Act.
If our Selling Shareholders enter into arrangements with brokers or dealers, as described above, we are obligated to file a post-effective amendment to this registration statement disclosing such arrangements, including the names of any broker dealers acting as underwriters.
Sales Pursuant to Rule 144
Any shares of common stock covered by this prospectus which qualify for sale pursuant to Rule 144 under the Securities Act may be sold under Rule 144 rather than pursuant to this prospectus.
Regulation M
We will advise the Selling Shareholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the Selling Shareholders and their affiliates. Regulation M under the Exchange Act prohibits, with certain exceptions, participants in a distribution from bidding for, or purchasing for an account in which the participant has a beneficial interest, any of the securities that are the subject of the distribution. Accordingly, the Selling Shareholders are not permitted to cover short sales by purchasing shares while the distribution is taking place. Regulation M also governs bids and purchases made in order to stabilize the price of a security in connection with a distribution of the security. In addition, we will make copies of this prospectus available to the Selling Shareholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act.
State Securities Laws
Under the securities laws of some states, the common shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the common shares may not be sold unless the shares have been registered or qualified for sale in the state or an exemption from registration or qualification is available and is complied with.
Expenses of Registration
We are bearing all costs relating to the registration of the common stock, including the Selling Shareholders' expenses relating to the registration. These expenses are estimated to be $42,578.80, including, but not limited to, legal, accounting, printing and mailing fees. The Selling Shareholders, however, will pay any commissions or other fees payable to brokers or dealers in connection with any sale of the common stock.
20
LEGAL PROCEEDINGS
Management is not aware of any legal proceedings contemplated by any governmental authority or any other party involving us or our properties. As of the date of this registration statement, no director, officer or affiliate is (i) a party adverse to us in any legal proceeding, or (ii) has an adverse interest to us in any legal proceedings. Management is not aware of any other legal proceedings pending or that have been threatened against us or our properties.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Our directors and executive officers and their respective ages as of June 28, 2007 are as follows:
Name and Municipality ofResidence |
Age
| Current Office with
CellCyte Genetics Corporation | Director Since |
Gary A. Reys
Freeland, Washington, U.S.A. | 62 | Chairman, President, Chief Executive Officer, Chief Financial Officer, Principal Executive Officer, Principal Accounting Officer and a director | March 30, 2007 |
Dr. Ronald W. Berninger, Ph.D.
Mukilteo, Washington, U.S.A. | 62 | Secretary, Executive Vice President, Chief Scientific Officer and a director | March 30, 2007 |
John M. Fluke, Jr.
Bellevue, Washington, U.S.A. | 64 | Director | April 16, 2007 |
The following is a description of the business background of the directors and executive officers of our Company:
Gary A. Reys - Chairman, President, Chief Executive Officer, Chief Financial Officer, Principal Executive Officer, Principal Financial Officer and a director
Mr. Reys brings over 30 years of experience with both international Fortune 100 and 500 publicly traded companies and emerging-growth companies in the pharmaceutical, biotechnology and medical device sectors. He held executive positions with Pfizer and with Rhone Poulenc Rorer (now Aventis), North America. Mr. Reys was one of the pioneers in the generic pharmaceutical industry as part of a five member founding executive team forming Schein Pharmaceutical, taking the company through an IPO and the acquisition by Bayer AG. Mr. Reys served in various capacities for Goldline Laboratories, positioning the company for the acquisition by IVAX, an international pharmaceutical holding company (now Teva Pharmaceutical), retiring from IVAX as Vice President of Business Development in 1993. Mr. Reys served as Executive Vice President and Chief Financial Officer of IBV Technologies, a division of the McKesson Corporation from May 1994 to December 1997. Mr. Reys served as Chief Executive Office r and President of Genespan, a cell expansion and DNA biotechnology company from January 1998 to February 2000. Mr. Reys served as Chief Executive Officer, President and Chief Financial Officer for Clear Medical from February 2000 to March 2001, where he positioned the company as the first FDA approved high-level disinfectant re-processor of medical devices. Mr. Reys co-founded and served as the President and Chief Executive Officer of Cennapharm, a biopharmaceutical company from April 2001 to May 2003. Mr. Reys co-founded CellCyte in June 2003. Mr. Reys attended the University of Washington where he majored in finance, later receiving a CPA designate. Mr. Reys is a past member of the Washington Society of Certified Public Accountants. He also serves as a member of the University of Washington's Graduate School, M.B.A. Mentor Advisory Board.
Dr. Ronald W. Berninger, Ph.D. - Secretary, Executive Vice-President, Chief Scientific Officer and a director
Dr. Berninger has over 20 years experience in building research teams and moving products from R&D to manufacturing and into the marketplace. Dr. Berninger co-founded CellCyte in June 2003. Dr. Berninger served as Vice President and Director of R&D and QA/Regulatory for Genespan Corporation from September 1996 to February 2002 and also for Cennapharm Corporation from May 2002 to May 2003. In both positions he was responsible for formulation of drugs and building the infrastructure for R&D, Quality, Regulatory, and Documentation. Prior to that, Dr. Berninger served as Senior Scientist and Head of Biochemistry at CellPro, a stem cell company. As a member of the award winning Ceprate.. SC Concentrator development team, he was instrumental in establishing the first FDA approved process for purification, biotinylation and formulation of a CD34 IgM monoclonal antibody for purification of stem cells. Dr. Berninger also played a vital role in defini ng the chemistry for the affinity gel employed in the Ceprate.. product used for cancer and gene therapy applications. Dr. Berninger served as Senior Scientist for NeoRx Corporation where he used his expertise in chemistry to play a pivotal role in purifying and chemically modifying monoclonal antibodies to target tumor cells in diagnostic and clinical applications.
21
Dr. Berninger served as Assistant Professor at the Tufts University School of Medicine from 1980 to 1986. Prior to that appointment, Dr. Berninger was a Fellow, Instructor and Assistant Professor at the John Hopkins University School of Medicine from 1975 to 1980. He received his B.S. degree in Chemistry from Drexel University in 1968 and in 1972 was awarded a Ph.D. in Organic Chemistry from the University of Pittsburgh. Dr. Berninger has received numerous awards, patents and honors for his scientific discoveries and has over 70 scientific publications.
John M. Fluke, Jr. - Director
Mr. Fluke is the Chairman of Fluke Capital Management, LP; the Fluke family's investment and trust management company. He founded the company in 1976. Mr. Fluke graduated from the University of Washington in 1964, with a Bachelor of Science Degree in Electrical Engineering, and received his Master's Degree in Electrical Engineering from Stanford University in 1966.
Mr. Fluke joined his father's firm, John Fluke Mfg. Co., in 1966 as a design engineer and held various engineering and general management positions. He was elected to the board of that company in 1976, appointed Chief Executive Officer in 1983 and Chairman from 1984. He served as CEO through 1987, Chairman through 1990 and a director of the Fluke Corp. through July 1998 when the company was acquired by Danaher Corporation.
Mr. Fluke currently serves on the Boards of three emerging - or re-emerging - companies: Abacast, Inc. (third generation peer-to-peer streaming software); MaestroSoft, Inc. (charitable event management software); and TeraCloud, Inc. (maker of high performance mainframe computer storage management software). Over the last three decades many of Mr. Fluke's governance positions with smaller companies have required direct and frequent interaction with management to balance the achievement (or maintenance) of cash flow positive performance without deviating from best governance practices.
Mr. Fluke is a director of PACCAR Inc. (since 1984), where he also serves as audit committee financial expert and chairman of the compensation committee. He also currently serves as a director of Tully's Coffee Company (since 2005) and chairs its audit committee. Mr. Fluke is a former director and chairman of the audit committee for PRIMUS International (2003 to 2006) and American Seafoods Group (2003 to 2006) where he served on the audit committee. He has also served on the boards and audit committees of Peoples National Bank (1984 to 1993) and US Bank of Washington (1993 to 1997).
Mr. Fluke is a past Chairman of the Greater Seattle Chamber of Commerce (1993) and continues to serve on its board and is a past Chairman of the Washington State China Relations Council (1985 to 1987). He concurrently served as a national board member of the American Electronics Association and the National Association of Manufacturers as well as the Executive Committees of both trade organizations (1985 to 1990).
Mr. Fluke continues to serve as a member of the University of Washington's Engineering Visiting Committee (since 1982), the University of Washington Business Advisory Board (since 1987), the University of Washington Applied Physics Laboratory Advisory Board (since 1992) and the Pacific Museum of Flight (since 1988). He also currently serves as the Chairman of the board of Junior Achievement of Washington and recently was elected to the board of the Washington Policy Center. Mr. Fluke previously served on the Stanford University Engineering Advisory Council (1985 to 1989). He is a trustee-emeritus of the University of Puget Sound (1984-2002), a past trustee of St. Thomas School of Medina, WA (1988 to 1995) and served on the Washington State Higher Education Coordinating Board (1987 to 2000) and the Washington Business Roundtable and its Education Committee (1984 to 2001).
22
Mr. Fluke is a past President of PONCHO (1993), Seattle's oldest fund-raising auction for the arts, a past President of the Seattle Council of the Boy Scouts of America (1984 to 1985) and a former member of the boards of the Seattle Symphony Orchestra (1981 to 1990), the 5th Avenue Theater Association (1985 to 1990) and the Nature Conservancy (1997 to 2001).
Term of Office
Our directors are appointed for a one-year term to hold office until the next annual general meeting of our shareholders or until removed from office in accordance with our Bylaws. Our officers are appointed by our Board of Directors and hold office until removed by the board.
Family Relationships
None of the directors or officers of our Company are related to one another.
Significant Employees
In addition to our executive officers, we have two significant employees: Dr. Theresa A. Deisher, Ph.D., who is our head of research and development, and Dr. H. Andreas Kalmes, Ph.D., who heads our nuclear imaging and pharmacology. Following is a description of the business background of these persons.
Dr. Theresa A. Deisher, Ph.D. - Vice President, Research and Development
Dr. Deisher is an internationally renowned cardiovascular scientist with extensive expertise in the biotechnology industry. Her research programs have encompassed all areas of cardiovascular physiology and disease, including heart failure, myocardial infarction, thrombosis, stroke, diabetes, obesity, hematopoiesis, immune modulation, cardiotoxicity, and regenerative medicine. Dr. Deisher was the first scientist to discover and characterize adult cardiac stem cells, for which she holds the patent. Dr. Deisher's most recent appointment prior to CellCyte was as Principal Scientist at Amgen, Inc. from July 2002 to September 2006, where she led an interdepartmental team developing antibody therapeutics for the treatment of chronic heart failure, acute myocardial infarction and chemotherapeutic cardiotoxicity. During her tenure at Amgen, Dr. Deisher's research group pioneered the use of rodent non-invasive imaging techniques. From October 2000 to August 2006Dr. Deisher served as Senior Staff Scientist, Vascular Biology at Immunex Corp. in Seattle, WA leading projects in Anti-Thrombotics and Inflammation and Myocardial Repair which she successfully transferred over to Amgen with the Immunex acquisition. Dr. Deisher's research has resulted in numerous patent awards, out-licensing deals, and clinical programs. Dr. Deisher received her Ph.D. from Stanford University's Department of Molecular and Cellular Physiology, and her B.A. degree from Stanford University's Department of Human Biology, graduating with honors and distinction. Dr. Deisher has authored numerous manuscripts, has 17 issued or pending patent applications, and is a frequent lecturer on stem cell research, inflammation and cardiology.
Dr. H. Andreas Kalmes, Ph.D. - Vice President, Pharmacology
Dr. Kalmes has an extensive background in biomedical research with specific expertise in vascular biology, inflammation, oncogenic growth factor signalling pathways and general cell biology. Dr. Kalmes is an expert in preclinical cardiovascular disease models. Prior to joining CellCyte, Dr. Kalmes was a Senior Scientist at Amgen, Inc. from July 2002 to August 2006, where he and his team investigated the involvement of inflammation and the role of specific inflammatory cytokines in the events leading to atherosclerotic plaque rupture, heart attack and stroke. Dr. Kalmes was responsible for establishing models of atherosclerosis that were used to validate drug targets and measure efficacy of therapeutic candidates. He also established novel non-invasive optical imaging techniques that can be used to measure macrophage-mediated inflammation of atherosclerosis plaque. Prior to Amgen, Dr. Kalmes was with Immunex Corp. from June 2 001 to July 2002, where he contributed to forming the Vascular Biology Department. Before venturing into the biotechnology industry, Dr. Kalmes was at the University of Washington School of Medicine in Seattle, WA, first as a Senior Fellow and then as Acting Instructor in the Department of Vascular Surgery. His research during this time led to the discovery of a novel pathway for the injury-induced activation of arterial smooth muscle via the epidermal growth factor receptor. Prior to that, Dr. Kalmes was a post-doctoral fellow at the University of Würzburg, Germany, where he worked on intracellular signalling pathways that are involved in tumor formation, and where he identified novel regulators for the Raf family of oncogenic proteins. Dr. Kalmes received his Ph.D. from the University of Mainz for his research on a tumor suppressor protein - work that he had conducted at the German Cancer Research Center in Heidelberg, Germany - graduating Magna Cum Laude. Dr. Kalmes also holds an M.S. degree that he received from the University of Mainz. Dr. Kalmes has authored numerous manuscripts in peer-reviewed journals.
23
We have no significant employees other than the officers and directors described above.
Conflicts of Interest
We do not have any procedures in place to address conflicts of interest that may arise between our business and our directors' other business activities. We are not aware of any existing conflicts between us and our directors' other business activities.
Audit Committee
The sole member of our current Audit Committee is Mr. Fluke.
Our Board of Directors has determined that Mr. Fluke qualifies as a "financial expert" and that he is independent according to the standards for Audit Committee member independence prescribed by the American Stock Exchange.
Involvement in Certain Legal Proceedings
None of our directors, executive officers or control persons have been involved in any of the following events during the past five years:
- any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
- any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
- being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or
- being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information with respect to the beneficial ownership of our common stock by each stockholder known by us to be the beneficial owner of more than 5% of our common stock and by each of our current directors and executive officers. Each person has sole voting and investment power with respect to the shares of common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated. As of June 28, 2007, there are 59,854,224 shares of common stock issued and outstanding.
24
| | As of March 30, 2007 |
Name and Address of Beneficial Owner | Shares(1) | Percent |
Named Executive Officers and Directors(2) | | |
Gary A. Reys
Chairman, President, Chief Executive Officer, Chief Financial Officer, Principal Executive Officer, Principal Accounting Officer and a director | 18,625,000 | 31.1% |
Dr. Ronald W. Berninger, Ph.D.
Secretary, Executive Vice President, Chief Scientific Officer and a director | 18,625,000 | 31.1% |
John M. Fluke, Jr.
Director | 150,000(3) | * |
Directors and Executive Officers as a Group (3 Persons) | 37,400,000 | 62.5% |
Beneficial Owners of in Excess of 5% (other than Executive Officers and Directors) | | |
Not applicable | Nil | Nil |
* Less than one percent.
- Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. A s a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person's actual ownership or voting power with respect to the number of shares of common stock actually outstanding on June 28, 2007. As of June 28, 2007, there were 59,854,224 shares issued and outstanding.
- The address of the executive officers and directors is c/o Carillon Point Centre, 5400 Carillon Point, Kirkland, Washington, U.S.A., 98033.
- Represents options to acquire up to 150,000 shares of our common stock at an exercise price of $1.50 per share for a period of five years.
Changes in Control
We are unaware of any contract, or other arrangement or provision, the operation of which may at a subsequent date result in a change of control of our company.
DESCRIPTION OF SECURITIES
General
Our authorized capital stock consists of 525,000,000 shares of common stock at a par value of $0.001 per share.
Common Stock
As of June 28, 2007, there were 59,854,224 shares of our common stock issued and outstanding held by approximately 100 stockholders of record. Holders of our common stock are entitled to one vote for each share on all matters submitted to a stockholder vote. Holders of common stock do not have cumulative voting rights. Therefore, holders of a majority of the shares of common stock voting for the election of directors can elect all of the directors. Two persons present and being, or representing by proxy, shareholders are necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our articles of incorporation.
Holders of common stock are entitled to share in all dividends that the board of directors, in its discretion, declares from legally available funds. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over the common stock.
25
Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock.
Preferred Stock
We do not have any authorized class of preferred.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we do not anticipate paying any cash dividends in the foreseeable future.
Share Purchase Warrants
We issued and have outstanding warrants to purchase an aggregate of 4,659,225 shares of our common stock which were issued on March 30, 2007, and are exercisable at a price of $3.00 per share for a period ending on the earlier of 18 months from the date of issuance of the warrants and 12 months from the effective date of the registration statement of which this prospectus form a part.
Options
We have issued and have outstanding options to purchase an aggregate of 2,000,000 shares of our common stock exercisable at a price of $1.50 per share for a period of ten years from the date of issuance on March 30, 2007.
Convertible Securities
We have not issued and do not have outstanding any securities convertible into shares of our common stock or any rights convertible or exchangeable into shares of our common stock, other than the warrants and options described above.
EXPERTS
The audited consolidated financial statements of the Company included in this prospectus have been audited by Morgan & Company, Chartered Accountants, an independent registered public accounting firm, to the extent and for the periods set forth in their report appearing elsewhere in this prospectus. These financial statements are included in reliance upon the authority of said firm as an expert in auditing and accounting.
The audited consolidated financial statements of CellCyte included in this prospectus have been audited by Williams & Webster, P.S., Certified Public Accountants, an independent registered public accounting firm, to the extent and for the periods set forth in their report appearing elsewhere in this prospectus. These financial statements are included in reliance upon the authority of said firm as an expert in auditing and accounting.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock offered hereby was employed on a contingency basis, or had, or is to receive, in connection with such offering, a substantial interest, direct or indirect, in the Company, nor was any such person connected with the Company as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
26
DISCLOSURE OF SEC POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our directors and officers are indemnified as provided by theNevada Revised Statutes, our Articles of Incorporation and our Bylaws.
We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling precedent, submit the question of whether such indemnification is against public policy to a court of appropriate jurisdiction. We will then be governed by the court's decision.
ORGANIZATION SINCE INCORPORATION
We were incorporated under the laws of the State of Nevada on March 9, 2004 under the name "Shepard Inc." On December 22, 2006, we effected a forward split of our shares of common stock on the basis of seven new shares of common stock for each one share of common stock outstanding on that date and increased our authorized share capital from 75,000,000 shares of common stock to 525,000,000 shares of common stock. On February 16, 2007, Shepard Inc. was merged with a wholly-owned subsidiary, CellCyte Genetics Corporation, the Nevada corporation, in contemplation of the acquisition of all of the issued and outstanding shares of CellCyte and the name of our company was changed to CellCyte Genetics Corporation.
John M. Fluke, Jr. is an "independent" director of the Company as that term is defined in Rule 121 of the American Stock Exchange listing standards.
DESCRIPTION OF BUSINESS
Overview
General
We were incorporated under the laws of the State of Nevada on March 9, 2004 under the name "Shepard Inc.". On December 22, 2006, we effected a forward split of our shares of common stock on the basis of seven new shares of common stock for each one share of common stock outstanding on that date and increased our authorized share capital from 75,000,000 shares of common stock to 525,000,000 shares of common stock. On February 16, 2007, Shepard Inc. was merged with a wholly-owned subsidiary, CellCyte Genetics Corporation, the Nevada corporation, in contemplation of the acquisition of all of the issued and outstanding shares of CellCyte and the name of our Company was changed to CellCyte Genetics Corporation.
Our Prior Business
We were an exploration stage company engaged in the acquisition and exploration of mineral properties with a view to exploiting any mineral deposits we discovered. Prior to our most recent year ended December 31, 2006, we owned a 100% beneficial interest in one mineral claim known as the CB-1 claim.
During the last fiscal year, we retained an independent geologist to conduct exploration on the CB-1 claim and to provide us with recommendations for additional exploration on the property. All rock samples that were taken from the property during exploration did not contain any significant quantities of gold. In addition, we were not able to locate any other zones of altered rock on the CB-1 claim which could possibly host economic mineralization. Accordingly, we do not plan to conduct any further exploration of the CB-1 claim and we plan to allow the claim to expire.
27
The CellCyte Acquisition
On March 30, 2007, we completed the acquisition of all of the issued and outstanding shares (the "Purchased Shares") of CellCyte (the "Closing") pursuant to a Share Exchange Agreement among CellCyte, the shareholders of CellCyte (the "Vendors") and the Company dated as fully executed on March 14, 2007 (the "Share Exchange Agreement"). The Share Exchange Agreement replaced, in its entirety, the terms and conditions of our previous Agreement In Principle, dated for reference January 17, 2006 as fully executed on January 26, 2007 (the "Agreement In Principle"), as previously entered into among the Company, CellCyte and a principal Vendor of CellCyte; all as envisioned by the terms and conditions of the Agreement In Principle (and, collectively, the "CellCyte Acquisition" herein).
In accordance with the terms and conditions of the Share Exchange Agreement the total purchase price for the Purchased Shares consisted of the issuance from treasury by the Company to the order and direction of the Vendors at Closing of an aggregate of 16,000,000 restricted common shares in the capital of the Company at a deemed issuance price of $1.50 per common share. In addition, and in accordance with the terms of the Share Exchange Agreement and at the Closing of the CellCyte Acquisition: (i) Robert Harris, a former director of our Company, transferred an aggregate of 18,750,000 restricted common shares in the capital of the Company held by him to the order and direction of the Vendors at a deemed transfer price of $0.001 per common share; and (ii) certain other shareholders of the Company transferred at closing an aggregate of 2,500,000 common shares in the capital of the Company to the order and direction of the Vendors at the same deemed transfer price.
Pursuant to the terms of each of the original Agreement In Principle and the resulting Share Exchange Agreement, the Company, on its behalf and on behalf of certain investors (each a "Lender"), had, on or about January 26, 2007, loaned to CellCyte (the "Loan") the aggregate principal sum of $1,000,000 from proceeds of the private placement of units described below. Interest on such Loan accrued at a rate of 5% per annum, compounded semi-annually and not in advance, and the Loan was to mature 120 calendar days from the date of the advancement of the principal sum. The principal sum and interest on the Loan was secured by way of a senior, subordinated (subordinated only to our existing bank indebtedness), fixed and floating charge on all of the assets of CellCyte. Under the terms of the Share Exchange Agreement, each of the Lenders had the right and option, exercisable until the close of business on the date of maturity of the Loan, to convert any principal sum, interest or any other sum owing under the Loan to such Lender into units of the Company ("Loan Units") at a deemed conversion price of $1.50 per Loan Unit, with each Loan Unit being comprised of one common share and one non-transferable share purchase warrant of the Company exercisable for a period ending on the earlier of 18 months from the date of issuance of the Loan Units by the Company and 12 months from the effective date of the Company's registration statement of which this prospectus forms a part, at an exercise price of $3.00 per common share. The Lenders converted the Loan into Loan Units on March 30, 2007.
Under the terms of the Share Exchange Agreement, at the Closing of the CellCyte Acquisition an aggregate of $1,017,088 in principal and interest owing by CellCyte to certain of its creditors was settled through the issuance of an aggregate of 678,060 units of the Company at a deemed settlement price of $1.50 per Settlement Unit, with each such Settlement Unit having the same terms and conditions as set forth for the Loan Units of the Company as described above.
In addition, and at the Closing of the CellCyte Acquisition, all outstanding options to acquire shares in the capital of CellCyte were exchanged for options to acquire an aggregate of 2,000,000 common shares in the capital of the Company at an exercise price of $1.50 per share for a period of five years, and all options to acquire shares in the capital of CellCyte were cancelled. In addition, and again at Closing, all outstanding warrants to acquire shares in the capital of CellCyte were exchanged for 205,000 shares in the capital of the Company, and all warrants to acquire shares in the capital of CellCyte were also cancelled.
28
In accordance with the terms of the Share Exchange Agreement, the Company raised, by way of a private placement of units of the Company, a total of $5,971,751 at a subscription price of $1.50 per unit with each Private Placement Unit being comprised of one common share and one non-transferable share purchase warrant of the Company with each warrant being exercisable for one additional common share of the Company on the same terms and conditions for exercise as set forth for the Loan Warrants. Prior to Closing, the Company, on its behalf and on behalf of certain investors, transferred an aggregate of $1,000,000 to CellCyte in connection with the Loan from proceeds of the Private Placement. An aggregate of $4,971,751 from the Private Placement was subsequently advanced by the Company to CellCyte at Closing, and at Closing and each of the Lenders, the Company and CellCyte agreed that the original Loan of $1,000,000 would be converted for Loan Units in the manner as set forth above.
As a consequence of our Company's acquisition of CellCyte, our business is now focused on the discovery and development of stem cell enabling therapeutic products.
Accounting Treatment
The closing of the CellCyte acquisition represented a change in control of our Company. For accounting purposes, this change in control constitutes a re-capitalization of the Company, and the acquisition has been accounted for as a reverse merger whereby we, as the legal acquirer, are treated as the acquired entity, and CellCyte, as the legal subsidiary, is treated as the acquiring company with the continuing operations. The results of operations and cash flows of the Company are considered to be a continuation of the results of CellCyte.
Our Business
Overview
CellCyte was organized under the laws of the State of Washington, U.S.A., on January 14, 2005.
As a result of the acquisition of CellCyte, we are a development stage biotechnology company involved in the discovery and development of stem cell enabling therapeutic products.
Stem cells are unprogrammed cells in the human body that could be described as 'shape shifters'. These cells have the ability to change into other types of cells. Stem cells are at the center of the new field of regenerative medicine. Because stem cells can become bone, muscle, cartilage and other specialized types of cells, they have the potential to treat many diseases, including coronary disease, parkinson's, alzheimer's, diabetes and cancer. They are also being used to regenerate organs, and could potentially reduce the need for organ transplants and related surgeries.
Stem cells can typically be broken into five types:
- Embryonic Stem Cells - stem cells taken from human embryos;
- Fetal Stem Cells - stem cells taken from aborted fetal tissue;
- Umbilical Stem Cells - stem cells take from umbilical cords;
- Adult Stem Cells - stem cells taken from adult tissue; and
- Amniotic Stem Cells - stem cells taken from human amniotic fluid.
Embryonic, amniotic and fetal stem cells have the potential to morph into a greater variety of cells than adult stem cells.
In April 2001, researchers at UCLA and the University of Pittsburgh found stem cells in fat drawn out of liposuction patients. More recently, in January 2007, researchers at Wake Forest University School of Medicine and the Harvard University School of Medicine Unit discovered that stem cells from the amniotic fluid of pregnant women could be retrieved and cultivated. Previously, stem cells were found only in bone marrow, brain tissue and fetal tissue which are sources that have raised both logistical and ethical issues. Stem cells from fat have the ability to mature into other types of specific cells, including muscle, bone and cartilage, but how many other types is still unknown.
29
Prior to being transplanted into a person's tissue to begin regeneration of that tissue, stem cells have to go through differentiation. Differentiation is the process by which scientists pre-specialize the stem cells, almost like pre-programming the stem cells to become specific cells. These cells are then injected into the area of the body being targeted for tissue regeneration. When stem cells come into contact with growth chemicals in the body, the chemicals program the stem cells to grow into the tissue surrounding it.
Stem cells are already being used to treat leukemia and some joint repairs. For example, a bone-marrow transplant is accomplished by injecting stem cells from a donor into the bloodstream of the patient. Stem cells from bone marrow also have the ability to repair the liver. Researchers are studying stem cells to find out if they could correct brain damage resulting from parkinson's disease.
Our Products
We have discovered and are developing certain stem cell enabling therapeutic products. These therapeutic products make it possible for the first delivery of stem cells through a patient's own circulatory vascular system directly targeting those cells to a specific diseased or damaged organ.
These discoveries are the first stem cell enabling drugs to enter Investigational New Drug ("IND") supported by the United States Food and Drug Administration ("FDA") clinical trials and they offer applicability across a number of diseases. These products are expected to be supplied in a single use vial form with the first product launch scheduled for 2010.
Fundamentally, our discoveries are the operating (or delivery) systems and platforms that stem cells require to become viable therapeutic applications. Our management has strategically combined the company's therapeutic discoveries with platforms of proven and synergistic device technologies that have repeatedly been demonstrated to enhance stem cell interventions and other cellular therapy approaches. This has provided us with both a therapeutic product pipeline and a proven device division with opportunities in drug development, research and therapeutic interventions.
We have an extensive and broad intellectual property framework and product pipeline consisting of United States patents issued, United States patents pending and eleven foreign patents pending covering numerous therapeutic discoveries relating to stem cell organ delivery and repair compounds. In addition, the company has patents pending for an adenocarcinoma tumor diagnostic and patents pending for the discovery of a novel technology that can deliver any cell type to the liver.
Therapeutic Pipeline
Stem Cell Organ Delivery, Retention and Repair
Two unmet significant challenges and hindrances to stem cell organ repair, regeneration and curative treatments have been the lack of a non-invasive, non-damaging technology to deliver adequate quantities of stem cells to a specific diseased, damaged or dying organ, and the lack of a method or mechanism to keep the stem cells in the organ. These have been the essential barriers considered necessary to be overcome before any foreseeable and reasonable organ repair or organ curative regeneration therapeutic procedure can take place.
Targeting delivery to and retention of stem cells in specific organs are our lead therapeutic technologies. These discoveries offer treatments across a broad number of diseases and are significant advancements in the pursuit of a medically viable procedure to deliver stem cells to damaged, diseased or dying organs and to repair those organs.
Our discoveries permit the first delivery and retention of stem cells through the organ's circulatory vascular system, where the cells are nourished and encouraged to differentiate under optimal conditions into muscle and tissue. These discoveries have been proven in extensive late-stage animal studies to allow non-invasive and non-destructive delivery of both haematopoietic (blood forming) CD34+ and mesenchymal (MSC) stem cells without damage to the organ. We believe that these discoveries will enable delivery and organ cell retention of stem cells derived from any source.
30
These compounds' products are proprietary, organ specific and precisely target and deliver stem cells to the heart, CCG-TH3O, liver, CCG-TL35, or the lung, CCG-TL4O. The company is also in late-stage development and analyzing data on an additional formulation, CCG-TK45, which has shown to target, deliver and retain stem cells in the kidney for repair. In the future the company plans to explore the delivery of stem cells to any region of the body including the brain, where they can take up residency and repair damaged areas.
In the discovery and development of these compounds, years of the most extensive human stem cell nuclear imaging studies to date in animals were conducted. Combining histological staining of the human stem cells, these studies confirmed that the compositions precisely delivered the stem cells. Furthermore, it showed that the compositions contained a signalling mechanism that also kept the stem cells in the desired organ. Additionally, the industry's most extensive stem cell biodistribution studies were completed. These studies further verified that the stem cells remained in the specific organ under optimal conditions and rapidly began differentiating into vital tissue and muscle cells needed for organ repair and regeneration.
To date, other stem cell organ research and clinical trials have focused stem cell interventions, mostly on the heart. Organs, such as the liver, lung and kidney present more significant delivery challenges due to their structure and high susceptibility to damage, leaving the present invasive techniques being used in heart studies as a less viable option. The ability of our compounds to deliver stem cells through the circulatory/vascular system and retain the cells in a specific organ open therapeutic opportunities for repair and regeneration of organs, and for us to become the gatekeeper for all stem cell organ delivery methodologies.
The first organ we have scheduled to enter human clinical trials is the heart. Until now, all research and methods for administering stem cells to damaged heart muscle and tissue has been by injection into and around the damaged tissue areas of the heart via catheter, needle or open-heart surgery. The most recent clinical trials where published in September 2007, in the New England Journal of Medicine demonstrating that the majority of the administered stem cells leak out of the heart injection site (only 1-3% remains) and the invasive nature of the procedure resulted in further damage to the already impaired heart. Our preclinical studies have shown that one administration through a standard IV into the circulatory/vascular system with our proprietary composition prior to stem cell infusion resulted in over 77% of the stem cells delivered to the heart and remaining in the heart where they differentiated into heart cells.
Our suite of proprietary stem cell delivery compounds are obtained from existing FDA approved processes currently used in the blood industry. The purified compounds are proprietarily modified before they are formulated and placed into single use vials. The company is advancing compound CCG-TH3O into human trials for repair of the heart with an IND submission scheduled for the second half of 2007, and phase I clinical trials are expected to begin shortly thereafter. Due to the technology's similarities, we anticipate that an IND for the liver compound, CCG-TL35 and lung compound CCGTL4O will follow within months of compound CCG-TH3O for the heart. Because of the importance of these discoveries and their lack of toxicity and safety concerns, we plan to seek FDA fast track designation once the appropriate IND is filed.
We are also pursuing an application to allow patients to use their own stem cells to repair their damaged organs. This procedure was not a reality until discovery of our stem cell delivery and organ targeting compounds. The approach will be to administer granulocyte-colony stimulating factor (G-CSF), the current standard of medicine used to mobilize stem cells from the patients own bone marrow into their bloodstream. Once the cells are in the bloodstream, the patient's damaged organ will be treated non-invasively, via IV delivery with compound, CCG-TH3O and the patients own stem cells are expected to migrate directly to the damaged organ, remain there and initiate repair. In a patient suffering from a heart attack, no time would be lost waiting for matching donor cells, as this intervention could be performed within a few hours after the heart attack. Being able to intervene quickly lessens further damage to the heart, so there is less need for organ regeneration. We believe that thi s technology could become the first-line intervention after the patient is stabilized.
This technology is also being developed to play a role as a standard procedure in organ transplants. This is the first technology believed to have several applications in organ transplant, e.g., maintaining a patient's organ waiting for a transplant, providing and extending the life of the donor organ and reducing or perhaps eliminating organ rejection after transplant. The therapy could also provide treatment to patients who are not eligible for transplantation, such as infants too small to accept organs or patients with diseases that render them unable to withstand long and complex organ transplant surgery.
31
We are working closely with Swedish Medical Center in Seattle and their internationally recognized organ transplant group, with the Hope Heart Program at the Benaroya Research Institute in Seattle, as well as with other international transplant physicians to further this intervention. Between heart and liver alone, the International Society of Organ Transplants estimates there are over 8,000 transplants each year in the United States totalling estimated direct and non-direct costs over $6 billion. It is further estimated that 20% of all organ donations are rendered useless as the organs die or become compromised before they can be transplanted.
Cell Targeting to the Liver
There are a large number of diseases that affect the liver such as haemophilia, cirrhosis, hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis (NASH), and diabetes. Another of our discoveries is the technology that can direct various cells to the liver and keep the cell in the organ. This technology is product based, to be supplied in a single use vial, is easily administered and could be a significant step toward a cure for many liver diseases.
Specifically, in the case of diabetics, the beta cell portion of the islet cell is destroyed in the pancreas. Islets are now being implanted into the liver where they produce insulin. The procedure is long, invasive and the cells do not stay longer than 12 to 24 months, therefore requiring repeat procedures. Administering the islet cells using our technology could allow for non-invasive implantation of the islet cells, and longer duration of insulin production would be expected as our technology may enhance organ specific cell delivery by up to 25 fold. Due to the product/process synergies with compound CCG-TL35, an IND for this compound could follow closely after the initiation of clinical studies in the heart.
Furthermore, using our 3-dimensional cell expansion technology, expanded islet cells from a patient could be banked in the event more islet cells are needed. This would open vast new liver therapeutic treatments.
Trafficking Stem Cells to the Bone Marrow
Stem cell trafficking for use in bone marrow transplants is a compound we are developing. The compound is to be supplied in a single use vial form and administered through an IV before the stem cell bone marrow transplant procedure. This compound is believed to direct the stem cells to stay in the blood circulation rather than the cells accumulating in the lungs and liver as is now the case.
Through our research, it was discovered that the liver and lungs contain unknown stem cell receptor sites that physiologically attract and gather stem cells. We are developing a novel compound, CCG-T50, to block these receptor sites. In doing so, the stem cells migrated directly to the bone marrow, therefore increasing the effective dose of stem cells available for engraftment and restoration of the body's immune system. With the increased amount of stem cells available for engraftment the current lengthy time for reconstitution of a patient's blood and immune system has been shown to be greatly reduced. With a more rapid and higher quality engraftment, fewer complications are expected, including marked decreases in immune responses, reductions in the risk of graft versus host disease (GVHD), and fewer patient deaths. An infection or a simple cold, which cannot be controlled with drugs while the patient's immune system is compromised while awaiting stem cell engraftment, can be termin al. In addition, this product is believed to have anti-inflammatory properties, which are seen to further lessen the possibility of GVHD.
Clinical trials to demonstrate efficacy for this product will average two weeks per patient and the FDA will not require patients to be followed for several years as is the case with many other product candidates. The company is hopeful of FDA approval in a relatively short timeframe. Phase I clinical trials on this product are expected to begin in 2007.
32
Oncology - Adenocarcinoma Tumor Detection
Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. According to the National Cancer Institute, cancer is a major cause of death in the United States, second only to cardiovascular disease. Despite significant progress in researchers understanding of ovarian, breast, prostate and colon cancers (adenocarcinoma tumors) early detection still remains largely unsatisfactory.
One of our more advanced licensed technologies is the only known adenocarcinoma tumor detection that can spot tumors at their very earliest stage. This technology has the potential to set new standards for early cancer detection and overcome the limitations of current cancer diagnostics by identifying a specific tumor biomarker indicating the presence of a tumor before symptoms begin, and when the opportunity for a cure is highest. Beyond a diagnostic, this technology could provide a therapeutic option for adenocarcinoma tumors as well. This discovery is a highly specific intervention for ovarian, breast, prostate and colon cancers.
This technology has already entered an FDA approved Phase I clinical trial on women with metastatic breast cancer and on women with ovarian cancer. The patient's white blood cells were isolated from the patient's blood, cultured with our proprietary peptide, CCG-T55 to a specific marker inherent in adenocarcinoma cancer tumor cells. This resulted in production of Cytotoxic T lymphocyte (CTLLJs) that are specific to adenocarcinoma cancer tumor cells. This novel technology detected tumors the size of a grain of rice (-P2 mm) containing only a few cancer cells. This is compared to current tumor diagnostics that are only capable of finding much larger tumors (5-10 mm) the size of a pea, containing hundreds or thousands of cancer cells.
A finding in preclinical research was discovered when mice implanted with human breast cancer cells (adenocarcinomas) lived more than twice as long as those not treated with this technology. This finding provides an opportunity for this technology to be become an additional therapeutic intervention for physicians to target tumors at their very earliest stage, before they proliferate to an advanced life threatening phase. This technology is expected to be developed in collaboration with a major pharma or biotechnology company.
Our Device Division
Cell Expansion
We purchased cell expansion technology as a complementary addition to our therapeutic portfolio. The technology has patents issued and is in the manufacturing stage, with the device slated for market entry within 12 months. This is a three-dimensional bioreactor cell expansion and incubation technology. The single use bioreactor chamber can emulate human body tissues and has already proven successful in growing, expanding and extending the life of 14 different cell lines, including embryonic stem cells. Stem cells, islet cells and other cells are significantly influenced by oxygen, and this technology has demonstrated superior oxygenation of any cell compared to all other methods for growing and expanding cells. One of our three-dimensional bioreactor chambers, for example, can grow 10 billion cells because of its oxygenation ability. We are continuing to expand and plan to leverage this technology.
As an example, stem cells collected from one individual's cord blood can only treat a child or a small adult one time, although therapy requires more. Once a person reaches a certain weight (approximately 150 pounds) there are not enough stem cells from a single individual's cord blood for a therapeutic intervention. Our technology is believed to make possible the treatment of many patients from one cord blood collection. Achieving this objective would reduce the number of necessary cord blood collections and importantly, once a match is found, permit the expansion of the matching stem cells for multiple therapeutic interventions.
A significant potential application of this technology is in the treatment of multiple myeloma. These treatments have not been possible to date, because the stem cells required for these interventions must be immunologically identical and supplied in significant numbers. We believe our technology will now enable sufficient quantities of patient matched stem cells to be supplied in a vial form for this therapeutic treatment and may enable other new stem cell interventions.
33
Still another application which we are working in conjunction with the Pacific Northwest Research Institute and the University of British Columbia in Vancouver, Canada (which has North America's largest islet cell transplant centre) is using our cell expansion device to both extend the life of islet cells and for the first expansion of islet cells for use in diabetic patients. Either of these applications would be a significant therapeutic advancement to treat diabetes, one of the worlds most pervasive, debilitating and growing diseases.
As stated previously, our cell expansion device emulates human body tissues, and for that reason physicians in diabetes research institutions are exploring the possibility for its use as an artificial pancreas for islet cells to reside and produce insulin. It is envisioned that through a relatively simple procedure, a miniaturized version of the devise could be implanted in the abdominal area of the patient just under the skin, mush like a pacemaker.
An additional application for this technology is the expansion of stem cells and other cells for third world countries and minorities, as stem cell banks for these populations are very limited or do not currently exist. Further adding to our revenues, our cell expansion technology has already provided collaboration opportunities with researchers across a wide range of disciplines.
Stem Cell Purification
Our stem cell purification platform is a second-generation stem cell purification technology based on a proven immunoaffinity capture for stem cells. Our co-founder, Dr. Ronald W. Berninger, successfully developed the first generation of this technology. This technology's first generation has undergone successful clinical trials involving 3,500 patients in 250 clinics in 28 countries and 60 cancer centers in the United States saving many lives, and has received FDA approval, as well as European regulatory approvals.
The second generation system has been designed to be significantly more robust with an emphasis on supporting our stem cell organ targeting and delivery technologies. Additional revenue applications are in traditional stem cell bone marrow transplants, where purified stem cells are required for self-donor transplants to assure cancer free blood regeneration. Highly purified stem cells from bone marrow eliminate T cells which reduces GVHD immune response, a serious complication in bone marrow transplant. We will use our purification platform for applications in cancer, genetic blood disorders, and autoimmune disease.
Our plan of operations includes critical mass in the research, clinical, drug development and therapeutic markets by offering the most commercially viable purification technology. The FDA is driving these markets and have indicated they will require companies to show biodistribution in clinical trials, i.e., where stem cells go and to be able to account for them. Such trials will require purified stem cells in the form of a pharmaceutical grade therapeutic agent.
Our Business Model
Our business model focuses on the parallel development of products having multiple and complementary applications to treat a variety of diseases in large markets. We plan to focus on industry-leading technologies far along the research and development pathway offering significant breakthroughs, carrying both strong defendable and supportive clinical data with technologically dominant intellectual property positions.
We believe this model offers the shortest timeline to FDA approval for our therapeutic products and a pathway to begin generating revenues. We believe our technologies and the following strategies will make this possible:
Focus on Synergistic Technologies
We plan on only developing and commercializing breakthrough discoveries with large market demand that have undergone extensive research with defendable and positive outcomes. Our broad product platform was chosen because it offers a wide range of possible commercial and therapeutic applications, either individually or in combination with our other product candidates or those of other companies. We believe this approach will afford us the opportunity to quickly advance our research and broaden our intellectual property portfolio while mitigating many of the risks and costs of early stage clinical development.
34
In that regard, the CTLs required for our adenocarcinoma tumors detection I therapeutic technology will be grown and produced using our cell expansion technology. We also intend to move research forward for expansion of islet cells and as an implantable device. We believe these synergistic technologies further advance and leverage our plan for early revenues beyond those that may also be obtained via collaborative or development agreements with other companies.
FDA Approval Process
A significant part of our plan of operations is to work proactively with the FDA and to be a leading force in moving its compounds forward in the regulatory environment. We have attracted top regulatory experts to our team and in early IND enabling discussions have received constructive direction that will support timely IND filings for initiation of phase I studies for its products.
Our plan is to strategically select product candidates that through minor modifications could have multiple disease applications and which may afford us the opportunity to further support the IND enabling preclinical research. The potential benefits to these so-called piggy-back technologies (medications/regimens altered for more than one indication) are that subsequent compounds may have an accelerated path into the clinic and full studies are most often not required, as FDA approval is more likely to be granted on an indication extension basis rather than as a new drug. We believe that many of our product candidates could qualify for the FDAs Fast Track status and intends to seek those designations once the INDs are filed.
Licensing and Acquisitions
We have licensed and acquired product candidates and plan to continue to do so in the future. This strategy is expected to continue to lessen its development costs, expand its intellectual property pipeline, shorten time to commercialization, and offer broad marketing and early revenue opportunities.
For example, we have finalized exclusive worldwide licenses from the Department of Veteran Affairs for six lead therapeutic product candidates, as well as two additional technologies. We have acquired intellectual property assets of a biotechnology company including two additional issued patents.
Collaboration
We intend to continue to seek collaborations with biotechnology, pharmaceutical and research organizations that would enhance the development and speed to market of our product candidates. We have had advanced discussions with two major pharmaceutical companies that could lead to collaborations designed to enable it to retain a significant portion of the potential economic benefit while offsetting a substantial portion of the financial burden of final development and marketing of its therapeutic candidates. We have also identified potential product candidates of other companies that have not yet been shown to be viable therapeutics, but which we believe may do so in combination with one or more of its technologies.
We have not generated any revenues to date and have incurred operating losses since inception.
As at March 31, 2007, we had cash in the amount of approximately $779,327, restricted cash in the amount of $4,971,751 and working capital of $5,357,180. Our planned expenditures over the next twelve months are expected to amount to approximately $6,700,000 and will exceed our cash reserves and working capital. We presently do not have sufficient cash to fund our operations for more than the next 10 months. We anticipate that we will require additional financing in order to pursue our plan of operations for the next twelve months. There can be no assurance that we will obtain any additional financing in the amounts required or on terms favorable to us. If we are unable to obtain additional financing, we may have to abandon our business activities and plan of operations.
Beyond the next 12 months, we will be required to obtain additional financing in order to continue to pursue our plan of operations. It is anticipated that additional funding will be in the form of equity financing from the sale of our common stock. However, we cannot provide investors with any assurance that we will be able to raise sufficient funding from the sale of our common stock to fund our plan of operations going forward. If we do not obtain additional financing, we may be forced to abandon our business activities and plan of operations.
35
Patents, Proprietary Rights and Licenses
We believe that proprietary protection of our therapeutic products is critical to our business. We vigorously seek out intellectual property that we believe might be useful in connection with our products, and have an aggressive program of protecting our intellectual property. We believe that our know-how will also provide a significant competitive advantage, and intend to continue to develop and protect our proprietary know-how. We may also from time to time seek to acquire licenses to important externally developed technologies.
We have exclusive or non-exclusive rights to a portfolio of patents and patent applications related to our products and methods of deriving and using them. These patents and patent applications relate to compositions of matter, methods of obtaining such cells, and methods for preparing, transplanting and utilizing such cells. Currently, our United States patent portfolio includes two issued United States patents. More than three additional patent applications are pending. In addition, we have foreign counterparts to many of the United States applications and patents; counterparts to 11 United States patents or applications have issued in various countries. We also rely upon trade-secret protection for our confidential and proprietary information and takes active measures to control access to that information.
Our policy is to require our employees, consultants and significant scientific collaborators and sponsored researchers to execute confidentiality agreements upon the commencement of an employment or consulting relationship. These agreements generally provide that all confidential information developed or made known to the individual by us during the course of the individual's relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees and consultants, the agreements generally provide that all inventions conceived by the individual in the course of rendering services to us shall be our exclusive property.
We have obtained exclusive license rights from the United States Department of Veteran Affairs to technologies, processes and compounds that we believe may be important to the development of our products. These agreements typically require us to pay license fees, meet certain diligence obligations and, upon commercial introduction of certain products, pay royalties. These licenses may be cancelled or converted to non-exclusive licenses if we fail to use the relevant technology or breaches the agreements. Loss of such licenses could expose us to the risks of third party patents and/or technology. There can be no assurance that any of these licenses will provide effective protection against competitors.
The patent positions of biotechnology companies, including our company, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced before or after the patent is issued. Consequently, we do not know whether any pending applications will result in the issuance of patents, or if any existing or future patents will provide significant protection or commercial advantage or will be circumvented by others. Since patent applications are secret until the applications are published (usually eighteen months after the earliest effective filing date), and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make the inventions covered by each pending patent applications or that we were the first to file patent applications for such inventions. There can be no assurance that patents will issue from pending or future patent applications or, if issued, that such patents will be of commercial benefit to us, afford us adequate protection from competing products, or not be challenged or declared invalid.
In the event that a third party has also filed a patent application relating to inventions claimed in our patent applications, we may have to participate in interference proceedings declared by the United States Patent and Trademark Office to determine priority of invention, which could result in substantial uncertainties and cost, even if the eventual outcome is favorable to us . There can be no assurance that our patents, if issued, would be held valid by a court of competent jurisdiction.
36
A number of biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapy, stem cells and other technologies potentially relevant to or required by our expected products. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed.
If third party patents or patent applications contain claims infringed by our technology and such claims or claims in issued patents are ultimately determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative non-infringing technology. If we are unable to obtain such licenses or develop or obtain alternative non-infringing technology at a reasonable cost, we may not be able to develop certain products commercially. There can be no assurance that we will not be obliged to defend our company in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require us to seek licenses from third parties, or require us to cease using such technology.
Research and Development
We spent approximately $230,500 and $166,000 in 2005 and 2006, respectively, on research and development, comprised primarily of licensing of patents and expenses relating to pursuing and prosecuting a range of patent claims related to stem cell delivery and purification.
Competition
It is expected that our therapeutic products will have to compete with a variety of therapeutic products and procedures. Major pharmaceutical companies currently offer a number of pharmaceutical products to treat lysosomal storage disorders, neurodegenerative and liver diseases, diabetes and other diseases for which our technologies may be applicable. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. The market for therapeutic products is large, and competition is intense. We expect competition to increase. We believe that our m ost significant competitors will be fully integrated pharmaceutical companies and more established biotechnology companies. Smaller companies may also be significant competitors, particularly through collaborative arrangements with large pharmaceutical or biotechnology companies. Many of these competitors have significant products approved or in development that could be competitive with our products.
Competition for any stem cell products that we may develop may be in the form of existing and new drugs, other forms of cell transplantation, ablative and simulative procedures, and gene therapy. Some competitors are also trying to develop stem and progenitor cell-based technologies. These products may compete with our therapeutic products based on efficacy, safety, cost and intellectual property positions.
We may also face competition from companies that have filed patent applications relating to the use of genetically modified cells to treat disease, disorder or injury. In the event our therapies should require the use of such genetically modified cells, we may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted or may be available only on unfavorable terms.
If we develop products that receive regulatory approval, we would then have to compete for market acceptance and market share. For certain of our products, an important success factor will be the timing of market introduction of competitive products. This is a function of the relative speed with which our and our competitors can develop products, complete the clinical testing and approval processes, and supply commercial quantities of a product to market. These competitive products may also impact the timing of clinical testing and approval processes by limiting the number of clinical investigators and patients available to test our products.
37
While we believe that the primary competitive factors will be product efficacy, safety, and the timing and scope of regulatory approvals, other factors include, in certain instances, obtaining marketing exclusivity under the Orphan Drug Act, availability of supply, marketing and sales capability, reimbursement coverage, price, and patent and technology position.
Government Regulation
Our research and development activities and the future manufacturing and marketing of our therapeutic products are, and will continue to be, subject to regulation for safety and efficacy by numerous governmental authorities in the United States and other countries.
In the United States, pharmaceuticals, biologicals and medical devices are subject to rigorous FDA regulation. The Federal Food, Drug and Cosmetic Act, as amended, and the Public Health Service Act, as amended, the regulations promulgated thereunder, and other Federal and state statutes and regulations govern, among other things, the testing, manufacture, safety, efficacy, labelling, storage, export, record keeping, approval, marketing, advertising and promotion of our potential products. Product development and approval within this regulatory framework takes a number of years and involves significant uncertainty combined with the expenditure of substantial resources. In addition, the federal, state, and other jurisdictions have restrictions on the use of fetal tissue.
The steps required before therapeutic products may be marketed in the United States include:
| | Steps | Considerations |
1. | Preclinical laboratory and animal tests | Preclinical tests include laboratory evaluation of the cells and the formulation intended for use in humans for quality and consistency.In vivo studies are performed in normal animals and specific disease models to assess the potential safety and efficacy of the cell therapy product. |
2. | Submission to the FDA of an Investigational New Drug application (IND), which must become effective before United States human clinical trials may commence | The IND is submitted to the FDA with the preclinical and manufacturing data, a proposed development plan and a proposed protocol for a study in humans. The IND becomes effective 30 days following receipt by the FDA, provided there are no questions, requests for delay or objections from the FDA. If the FDA has questions or concerns, it notifies the sponsor, and the IND will then be on clinical hold until the sponsor responds satisfactorily |
3. | Adequate and well-controlled human clinical trials to establish the safety and efficacy of the product | Clinical trials involve the evaluation of a potential product under the supervision of a qualified physician, in accordance with a protocol that details the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Each protocol is submitted to the FDA as part of the IND. The protocol for each clinical study must be approved by an independent Institutional Review Board ("IRB") of the institution at which the study is conducted and the informed consent of all participants must be obtained. The IRB reviews the existing information on the product, considers ethical factors, the safety of human subjects, the potential benefits of the therapy and the possible liability of the institution. The IRB is responsible for ongoing safety assessment of the subjects during the clinical investigation. |
| | | Clinical development is traditionally conducted in three sequential phases, Phase 1, 2 and 3. |
38
| | Steps | Considerations |
| | | Phase 1 studies for a cell therapy product are designed to evaluate safety in a small number subjects in a selected patient population by assessing adverse effects, and may include multiple dose levels. This study may also gather preliminary evidence of a beneficial effect on the disease. |
| | | Phase 2 may involve studies in a limited patient population to determine biological and clinical effects of the product and to identify possible adverse effects and safety risks of the product in the selected patient population. |
| | | Phase 3 trials would be undertaken to conclusively demonstrate clinical benefit or effect and to test further for safety within a broader patient population, generally at multiple study sites. |
| | | The FDA continually reviews the clinical trial plans and results and may suggest changes or may require discontinuance of the trials at any time if significant safety issues arise. |
4. | Submission to the FDA of marketing authorization applications | The results of the preclinical studies and clinical studies are submitted to the FDA in the form of marketing approval authorization applications. |
5. | FDA approval of the application(s) prior to any commercial sale or shipment of the drug. Biologic product manufacturing establishments located in certain states also may be subject to separate regulatory and licensing requirement | The testing and approval process will require substantial time, effort and expense. The time for approval is affected by a number of factors, including relative risks and benefits demonstrated in clinical trials, the availability of alternative treatments and the severity of the disease. Additional animal studies or clinical trials may be requested during the FDA review period, which might add to that time. |
After FDA approval for the product, the manufacturing and the initial indications, further clinical trials may be required to gain approval for the use of the product for additional indications. The FDA may also require unusual or restrictive post-marketing testing and surveillance to monitor for adverse effects, which could involve significant expense, or may elect to grant only conditional approvals.
FDA Manufacturing Requirements
Among the conditions for product licensure is the requirement that the prospective manufacturer's quality control and manufacturing procedures conform to the FDA's current good manufacturing practice (cGMP) requirements. Even after product licensure approval, the manufacturer must comply with cGMP on a continuing basis, and what constitutes cGMP may change as the state of the art of manufacturing changes. Domestic manufacturing facilities are subject to regular FDA inspections for cGMP compliance, which are normally held at least every two years. Foreign manufacturing facilities are subject to periodic FDA inspections or inspections by the foreign regulatory authorities with reciprocal inspection agreements with the FDA. Domestic manufacturing facilities may also be subject to inspection by foreign authorities.
Orphan Drug Act
The Orphan Drug Act provides incentives to drug manufacturers to develop and manufacture drugs for the treatment of diseases or conditions that affect fewer than 200,000 individuals in the United States. Orphan drug status can also be sought for treatments for diseases or conditions that affect more than 200,000 individuals in the United States if the sponsor does not realistically anticipate our product becoming profitable from sales in the United States. We may apply for orphan drug status for certain of our therapies. Under the Orphan Drug Act, a manufacturer of a designated orphan product can seek tax benefits, and the holder of the first FDA approval of a designated orphan product will be granted a seven-year period of marketing exclusivity in the United States for that product for the orphan indication. While the marketing exclusivity of an orphan drug would prevent other sponsors from obtaining approval of the same compound for the same indication, it would not prevent o ther compounds or products from being approved for the same use including, in some cases, slight variations on the originally designated orphan product.
39
FDA Human Cell and Tissue Regulations
Our research and development is based on the use of human stem cells. The FDA has initiated a risk-based approach to regulating Human Cell, Tissue and Cellular and Tissue-based products and has published current Good Tissue Practice (cGTP) regulations. As part of this approach, the FDA has published final rules for registration of establishments that engage in the recovery, screening, testing, processing, storage or distribution of human cells, tissues, and cellular and tissue-based products, and for the listing of such products. In addition, the FDA has published rules for making suitability and eligibility determinations for donors of cells and tissue and for current good tissue practice for manufacturers using them, which came into effect in May 2005. We cannot yet determine the full effects of this regulatory initiative, including precisely how it may affect the extent of regulatory obligations associated with multipotent stem cell research, and the manufacture and marketin g of stem cell products.
Other Regulations
In addition to safety regulations enforced by the FDA, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act and other present and potential future foreign, Federal, state and local regulations.
Outside the United States, we will be subject to regulations that govern the import of drug products from the United States or other manufacturing sites and foreign regulatory requirements governing human clinical trials and marketing approval for our products. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursements vary widely from country to country. In particular, the European Union, or EU, is revising our regulatory approach to biotechnology products, and representatives from the United States, Japan and the EU are in the process of harmonizing and making more uniform the regulations for the registration of pharmaceutical products in these three markets.
Employees
We currently employ 12 people not including our executive officers.
Description of Property
We and CellCyte maintain an executive and business office located at Carillon Point Centre, 5400 Carillon Point, Kirkland, Washington, U.S.A., 98033.
We have entered into a 63 month lease for a new facility in Bothell, Washington, to be used for general office, administration, lab, research and development use associated with the biomedical life sciences field. The premises area is comprised of 24,829 square feet and the facility also has a project area comprised of 361,439 square feet.
We also lease space in a research facility located at 454 North 34th Street, Seattle, Washington, the term of which ends on August 31, 2007.
See "Management's Discussion and Analysis - Material Commitments" for more information.
40
MANAGEMENT'S DISCUSSION AND ANALYSIS OR PLAN OF OPERATIONS
The following selected financial data has been derived from and should be read in conjunction with: (i) our audited financial statements for the years ended December 31, 2006 and 2005, together with the notes to these financial statements; (ii) the audited financial statements of CellCyte for the years ended December 31, 2006 and 2005, together with the notes to those financial statements; (iii) the pro forma financial statements of the Company as at March 31, 2007; (iv) our interim financial statements for the three months ended March 31, 2007; and (v) the section of this prospectus entitled "Description of Business", included elsewhere herein.
We were an exploration stage company engaged in the acquisition and exploration of mineral properties with a view to exploiting any mineral deposits we discovered. Prior to the our Company's most recent year ended December 31, 2006, we owned a 100% beneficial interest in one mineral claim known as the CB-1 claim.
During the last fiscal year we retained an independent geologist to conduct exploration on the CB-1 claim and to provide us with recommendations for additional exploration on the property. All rock samples that were taken from the property during exploration did not contain any significant quantities of gold. In addition, we were not able to locate any other zones of altered rock on the CB-1 claim which could possibly host economic mineralization. Accordingly, we do not plan to conduct any further exploration of the CB-1 claim and we plan to allow the claim to expire.
On March 30, 2007, we acquired all of the issued and outstanding shares of CellCyte and our business is now focused on the discovery and development of stem cell enabling therapeutic products.
The closing of the CellCyte acquisition represented a change in control of our Company. For accounting purposes, this change in control constitutes a re-capitalization of the Company, and the acquisition has been accounted for as a reverse merger whereby we, as the legal acquirer, are treated as the acquired entity, and CellCyte, as the legal subsidiary, is treated as the acquiring company with the continuing operations. The results of operations and cash flows of the Company are considered to be a continuation of the results of CellCyte.
As the Company did not have any material operations prior to the acquisition of CellCyte and the acquisition of CellCyte has been accounted for as a reverse merger, the financial information presented below for the periods prior to the three months ended March 31, 2007 is that of CellCyte.
The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below and elsewhere in this prospectus, particularly in the section entitled "Risk Factors".
Plan of Operations
Our plan of operations for the next 12 months is to:
(a) complete tenant improvements, purchase and install necessary lab and research equipment and move into our new state-of-the-art biotechnology research and development facility in Bothell, WA.;
(b) complete pre-clinical lab research at our interim facility in preparation for our Phase I trail, complete our Phase I study and begin Phase II collaborative research studies; and
(c) complete the design and sourcing of components to begin small scale manufacturing of our cell expansion device technology.
We anticipate that we will incur an aggregate of $6,700,000 in expenses for the next 12 months as follows:
41
(a) $1,770,000 for facility and equipment related expenses;
(b) $830,000 for pre-clinical work, quality assurance, manufacturing of biologic material, Phase I clinical trial and further research collaborations;
(c) $175,000 for design and device manufacturing;
(d) $850,000 for legal expenses related to intellectual property prosecution and accounting expenses related to quarterly reviews and annual audits;
(e) $2,950,000 for salaries and consulting; and
(f) $125,000 for general and administrative, travel, conferences and public relations.
During the next 12 months we anticipate that we will not generate any revenue. We had cash and cash equivalents of $779,327 and working capital of $5,357,180 at March 31, 2007, including $4,971,751 of restricted cash (held in trust as a result of a private placement that was released in April 2007). Accordingly, we anticipate that we will require additional financing to enable us to pay our planned expenses for the next 12 months and pursue our plan of operations.
Subsequent to the 12 month period following the date of this prospectus, we will be required to obtain additional financing in order to continue to pursue our business plan. We believe that debt financing will not be an alternative for funding as we do not have tangible assets to secure any debt financing. We anticipate that additional funding will be in the form of equity financing from the sale of our common stock. We cannot provide investors with any assurance that we will be able to raise sufficient funding from the sale of our common stock to fund our business plan going forward. In the absence of such financing, our business plan will fail. Even if we are successful in obtaining equity financing to fund our business plan, there is no assurance that we will obtain the funding necessary to pursue our plan over the long-term.
Results of Operations
The following table sets forth selected financial information relating to our company for the periods indicated:
| | CellCyte | Three Months Ended
March 31, 2007 |
Year Ended
December 31, 2006 | Year Ended
December 31, 2005 |
Revenues | $Nil | | $Nil | | $Nil | |
Operating Expenses | | | | | | |
General and administrative | (112,636) | | (67,052) | | (351,995) | |
Professional Fees | (222,265) | | (224,637) | | (71,430) | |
Stock-based compensation | Nil | | Nil | | (1,780,000) | |
Net Loss | (385,639) | | (315,030) | | (2,211,740) | |
We have been funding our initial operations by way of private placements. We expect we will require additional capital to meet our long term operating requirements. We expect to raise additional capital through, among other things, the sale of equity or debt securities.
Three Months Ended March 31, 2007 Compared to the Three Months Ended March 31, 2006
Revenues
We have had no operating revenues since our inception on January 14, 2005 to March 31, 2007. We anticipate that we will not generate any revenues for so long as we are a development stage company.
42
General and Administrative Expenses
Our general and administrative expenses in the three months ended March 31, 2007 increased to $351,995 from $27,559 in the prior period of 2006, primarily as a result of increased operations due to the acquisition of additional intellectual property.
Professional Fees
In the three months ended March 31, 2007, we incurred professional fees of $71,430, compared to $47,143 in the prior period of 2006, primarily related to the acquisition of additional intellectual property.
Stock-based Compensation
In the three months ended March 31, 2007, we incurred stock based compensation expense of $1,780,000, compared to $nil in the prior period of 2006, directly related to the issuance of options to acquire up to 2,000,000 of our shares of common stock, in connection with the acquisition of CellCyte.
Net Loss
As a result of the above, our net loss for the three months ended March 31, 2007 was $2,211,740, compared to $82,465 in the prior period of 2006.
Year Ended December 31, 2006 Compared to Year Ended December 31, 2005
Revenues
We have had no operating revenues since our inception on January 14, 2005 to December 31, 2006. We anticipate that we will not generate any revenues for so long as we are a development stage company.
General and Administrative Expenses
Our general and administrative expenses in the year ended December 31, 2006 increased to $112,636 from $67,052 in the year ended December 31, 2005, primarily as a result of increased operations due to the acquisition of additional intellectual property.
Professional Fees
In the year ended December 31, 2006, we incurred professional fees of $222,265, compared to $224,637 in the year ended December 31, 2005, primarily related to the acquisition of additional intellectual property.
Net Loss
As a result of the above, our net loss for the year ended December 31, 2006 was $385,639, compared to $315,030 in the year ended December 31, 2005.
Liquidity and Capital Resources
As at March 31, 2007, we had cash in the amount of approximately $779,327 and working capital of $5,357,180. Our planned expenditures over the next twelve months are expected to amount to approximately $6,700,000 and will exceed our cash reserves and working capital. We presently do not have sufficient cash to fund our operations for more than the next 10 months. We anticipate that we will require additional financing in order to pursue our plan of operations for the next twelve months. There can be no assurance that we will obtain any additional financing in the amounts required or on terms favorable to us. If we are unable to obtain additional financing, we may have to abandon our business activities and plan of operations.
43
We believe that debt financing will not be an alternative for funding as we do not have tangible assets to secure any debt financing. We anticipate that additional funding will be in the form of equity financing from the sale of our common stock. However, we cannot provide investors with any assurance that we will be able to raise sufficient funding from the sale of our common stock to fund our plan of operations going forward. In the absence of such financing, our business plan will fail. Even if we are successful in obtaining equity financing, there is no assurance that we will obtain the funding necessary to pursue our business plan. If we do not continue to obtain additional financing going forward, we will be forced to abandon our plan of operations.
Cash Used in Operating Activities
Cash used in operating activities in the three months ended March 31, 2007 was $403,479, which reflected our net loss, compared to operating activities providing cash of $58,146 in the prior period of 2006. An increase in accounts payable provided cash of $58,719, partially offset by a decrease in accrued expenses which used cash of $45,000. We anticipate that cash used in operating activities will increase in 2007 as we pursue our plan of operations.
Cash used in operating activities in the year ended December 31, 2006 was $165,946, which reflected our net loss, compared to $106,080 in the year ended December 31, 2005. An increase in accounts payable, accrued interest and accrued expenses provided cash of $137,065, $50,678 and $21,023, respectively in the year ended December 31, 2006, compared to $167,439, $23,343 and $23,917, respectively in the year ended December 31, 2005.
Cash Used In Investing Activities
In the three months ended March 31, 2007, investing activities used cash of $104,944, compared to $108,829 in the prior period of 2006, primarily as a result of the acquisition of intellectual property.
In the year ended December 31, 2006, investing activities used cash of $165,946, compared to $231,676 in the December 31, 2005, relating primarily to the acquisition of intellectual property.
Cash Provided By Financing Activities
We have funded our business to date primarily from sales of our common stock. In the three months ended March 31, 2007, we raised proceeds of $5,971,751, compared to $10,000 in the prior period of 2006, from the sale of our common stock.
In the year ended December 31, 2006, financing activities provided cash of $545,000, compared to $390,200 in the year ended December 31, 2005, primarily from the issuance of convertible notes.
Going Concern
As shown in the accompanying financial statements, we have incurred significant losses since inception and have not generated any revenues to date. The future of our Company is dependent upon our ability to obtain sufficient financing and upon achieving future profitable operations. These factors, among others, raise substantial doubt about our Company's ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Future Financings
We anticipate continuing to rely on equity sales of our common shares in order to continue to fund our business operations. Issuances of additional shares will result in dilution to our existing shareholders. There is no assurance that we will achieve any additional sales of our equity securities or arrange for debt or other financing to fund our business plan.
44
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Material Commitments
The Company has operating leases for its office and storage space, all of which are month to month. Rent expense relating to the operating leases was approximately $31,593 for the period ended March 31, 2007.
Subsequent to the period end March 31, 2007, the Company entered into a 63-month lease in a new facility in Bothell, Washington beginning April 1, 2007. Total commitment in leases cost for the term of the lease is approximately $1,590,123.
Purchase of Significant Equipment
The Company intends to spend approximately $1.5 million in 2007 to purchase equipment for its new laboratory and office space, including approximately $500,000 to purchase a state-of-the-art NanoSPECT/CT In-ViVo Animal Imager, Dual Detector with CT.
Critical Accounting Policies
Our financial statements are impacted by the accounting policies used and the estimates and assumptions made by management during their preparation. A complete summary of these policies is included in Note 2 of the notes to our historical financial statements. We have identified below the accounting policies that are of particular importance in the presentation of our financial position, results of operations and cash flows and which require the application of significant judgment by management.
Use of Estimates
Our financial statements and accompanying notes have been prepared in accordance with United States generally accepted accounting principles applied on a consistent basis and include certain estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results may differ from those estimates. Significant estimates include the estimate of useful lives of intellectual property for amortization purposes, and the estimate of fair market value of stock-based compensation.
Income Taxes
The Company follows the provisions of Statement of Financial Accounting Standards No. 109, "Accounting for Income Taxes" (hereinafter "SFAS No. 109"), under which deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities. Deferred tax assets and liabilities are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The provisions of SFAS No. 109 also require the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that the realization of such benefits is more likely than not. To the extent that it is more likely than not those benefits will not be received, the Company records a valuation allowance against the related deferred tax asset.
Intangible Assets
The Company's intangible assets primarily consist of patents and intellectual property, which are carried at the purchase price and/or the legal cost to obtain them. Patents and licenses are being amortized over their estimated useful lives, which range from seven to seventeen years.
45
Research and Development Costs
Research and development costs are expensed as incurred. The cost of intellectual property purchased from others that is immediately marketable or that has an alternative future use is capitalized and amortized as intangible assets. Capitalized costs are amortized using the straight-line method over the estimated economic life of the related asset. The Company periodically reviews its capitalized intangible assets to assess recoverability based on the projected undiscounted cash flows from operations, and impairments are recognized in operating results when a permanent diminution in value occurs.
Stock-Based Compensation
The Company accounts for its stock option plan under the recognition and measurement principles of the revised statement to Statement of Financial Accounting Standards No. 123, "Share-Based Payments" (hereinafter "SFAS No. 123 (R)"). Accordingly, compensation cost has been recognized using the fair value method and expected term accrual requirements as prescribed in SFAS No. 123 (R). The Company had no stock-based compensation expense for the years ended December 31, 2006 and 2005 and $1,780,000 in stock-based compensation expense for the three months ended March 31, 2007.
Recent Accounting Pronouncements
In February 2007, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 159, "The Fair Value Option for Financial Assets and Financial Liabilities - Including an amendment of FASB Statement No. 115" (hereinafter "SFAS No. 159"). This statement permits entities to choose to measure many financial instruments and certain other items at fair value. The objective is to improve financial reporting by providing entities with the opportunity to mitigate volatility in reported earnings caused by measuring related assets and liabilities differently without having to apply complex hedge accounting provisions. This Statement is expected to expand the use of fair value measurement, which is consistent with the Board's long-term measurement objectives for accounting for financial instruments. This statement is effective as of the beginning of an entity's first fiscal year that begins after November 15, 2007, although earlier adoption is permitted. Management has not determined the effect that adopting this statement would have on the Company's financial condition or results of operation.
In September 2006, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 157, "Fair Value Measurements" (hereinafter "SFAS No. 157) which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles (GAAP), and expands disclosures about fair value measurements. Where applicable, SFAS No. 157 simplifies and codifies related guidance within GAAP and does not require any new fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. Earlier adoption is encouraged. The Company does not expect the adoption of SFAS No. 157 to have a significant immediate effect on its financial position or results of operations.
In June 2006, the Financial Accounting Standards Board issued FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109" (hereinafter "FIN 48"), which prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006. The Company does not expect the adoption of FIN 48 to have a material immediate impact on its financial reporting. The Company is currently evaluating the impact, if any, the adoption of FIN 48 will have on its disclosure requirements.
46
In March 2006, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 156, "Accounting for Servicing of Financial Assets--an amendment of FASB Statement No. 140." This statement requires an entity to recognize a servicing asset or servicing liability each time it undertakes an obligation to service a financial asset by entering into a servicing contract in any of the following situations: a transfer of the servicer's financial assets that meets the requirements for sale accounting; a transfer of the servicer's financial assets to a qualifying special-purpose entity in a guaranteed mortgage securitization in which the transferor retains all of the resulting securities and classifies them as either available-for-sale securities or trading securities; or an acquisition or assumption of an obligation to service a financial asset that does not relate to financial assets of the servicer or its consolidated affiliates. The statement also requires all sep arately recognized servicing assets and servicing liabilities to be initially measured at fair value, if practicable and permits an entity to choose either the amortization or fair value method for subsequent measurement of each class of servicing assets and liabilities. The statement further permits, at its initial adoption, a one-time reclassification of available for sale securities to trading securities by entities with recognized servicing rights, without calling into question the treatment of other available for sale securities under Statement 115, provided that the available for sale securities are identified in some manner as offsetting the entity's exposure to changes in fair value of servicing assets or servicing liabilities that a servicer elects to subsequently measure at fair value and requires separate presentation of servicing assets and servicing liabilities subsequently measured at fair value in the statement of financial position and additional disclosures for all separately recognized se rvicing assets and servicing liabilities. This statement is effective for fiscal years beginning after September 15, 2006, with early adoption permitted as of the beginning of an entity's fiscal year. Management believes the adoption of this statement will have no immediate impact on the Company's financial condition or results of operations.
In February 2006, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 155, "Accounting for Certain Hybrid Financial Instruments, an Amendment of FASB Standards No. 133 and 140" (hereinafter "SFAS No. 155"). This statement established the accounting for certain derivatives embedded in other instruments. It simplifies accounting for certain hybrid financial instruments by permitting fair value remeasurement for any hybrid instrument that contains an embedded derivative that otherwise would require bifurcation under SFAS No. 133 as well as eliminating a restriction on the passive derivative instruments that a qualifying special-purpose entity ("SPE") may hold under SFAS No. 140. This statement allows a public entity to irrevocably elect to initially and subsequently measure a hybrid instrument that would be required to be separated into a host contract and derivative in its entirety at fair value (with changes in fair value recognized in earnings) so long as that instrument is not designated as a hedging instrument pursuant to the statement. SFAS No. 140 previously prohibited a qualifying special-purpose entity from holding a derivative financial instrument that pertains to a beneficial interest other than another derivative financial instrument. This statement is effective for fiscal years beginning after September 15, 2006, with early adoption permitted as of the beginning of an entity's fiscal year. Management believes the adoption of this statement will have no immediate impact on the Company's financial condition or results of operations.
In May 2005, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 154, "Accounting Changes and Error Corrections," (hereinafter "SFAS No. 154") which replaces Accounting Principles Board Opinion No. 20, "Accounting Changes", and SFAS No. 3, "Reporting Accounting Changes in Interim Financial Statements - An Amendment of APB Opinion No. 28". SFAS No. 154 provides guidance on accounting for and reporting changes in accounting principle and error corrections. SFAS No. 154 requires that changes in accounting principle be applied retrospectively to prior period financial statements and is effective for fiscal years beginning after December 15, 2005. The Company does not expect SFAS No. 154 to have a material impact on its consolidated financial position, results of operations, or cash flows.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
Except as disclosed below, there are no transactions, since the beginning of our Company's last fiscal year, or any currently proposed transactions, in which our Company was or is to be a participant where the amount involved exceeds the lesser of $120,000 or one percent of the average of our Company's total assets at year-end for each of the fiscal years since the date of our incorporation, and in which any "related person" had or will have a direct or indirect material interest. "Related party" includes
47
(a) any of our directors or officers;
(b) any person proposed as a nominee for election as a director;
(c) any person who beneficially owns, directly or indirectly, shares carrying more than 10% of the voting rights attached to our outstanding shares of common stock; or
(d) any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law of any of the foregoing persons who has the same house as any of such person.
As a consequence of the completion of the Share Exchange Agreement:
(a) we issued 8,000,000 restricted common shares to each of Gary A. Reys and Dr. Ronald W. Berninger;
(b) an aggregate of 10,625,000 shares were transferred to each of Mr. Reys and Dr. Berninger by certain of our shareholders; and
(c) Mr. Reys and Dr. Berninger were appointed as directors and executive officers of our Company.
Mr. Reys and Dr. Berninger, who are also members of CellCyte's Board of Directors, served as consultants to CellCyte on various business, strategic, and technical issues. CellCyte paid and expensed a total of $143,000 for these services in 2005 ($107,000 to Mr. Reys and $36,000 to Dr. Berninger) and $144,000 for these services in 2006 ($94,000 to Mr. Reys and $50,000 to Dr. Berninger) and $100,000 through March 31, 2007 ($75,000 to Mr. Reys and $25,000 to Dr. Berninger). In addition to the fees, reimbursable expenses were also paid under this contract. The total amount paid for these expenses was $43,187 in 2005 and $41,040 in 2006 and $31,634 through March 31, 2007. The use of officers as contractors is considered an interim arrangement while CellCyte was in startup operations.
Robert Harris, a former director and officer, and Gary A. Reys and Dr. Ronald W. Berninger, current directors and officers, may be considered promoters of the Company. None of Mr. Harris, Mr. Reys or Dr. Berninger has received anything of value from us in their capacities as promoters of our Company.
Material Contracts
We have entered into employment agreements with Gary A. Reys, our Chairman, President and Chief Executive Officer, and Dr. Ronald W. Berninger, our Secretary, Executive Vice President and Chief Scientific Officer, which are described under "Executive Compensation - Employment Agreements".
We have entered into a consulting agreement with Jack Wynn & Co., Inc. to organize and implement a news media relations and news coverage/media placement program, as well as provide information on government actions as they relate to our products and technologies. The agreement is for a six month term ending October 31, 2007. Under the agreement, we are required to pay to Jack Wynn & Co., Inc. an aggregate of $48,000.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER INFORMATION
Market for our Common Stock
Our common shares are listed for trading on the OTC Bulletin Board under the trading symbol "CCYG". The first trade in the Company's common stock occurred on or about March 23, 2007.
Holders of Our Common Stock
As of the date of this prospectus we have approximately 100 registered shareholders.
48
Rule 144 Shares
A total of up to approximately 2,250,000 shares of our common stock (inclusive of those held by our affiliates) are available for resale to the public currently and a total of up to approximately a further 41,909,225 shares of our common stock issued pursuant to the Share Exchange Agreement, and the related Settlement Units and the Private Placement Units offered and sold under the contemporaneous settlement of CellCyte indebtedness and Private Placement under the Share Exchange Agreement (inclusive of those now held by our affiliates) are available for resale to the public after March 30, 2008 in accordance with the volume and trading limitations of Rule 144 of the Securities Act.
In general, under Rule 144, as promulgated under the Securities Act, and as currently in effect, a person who has beneficially owned shares of a company's common stock for at least one year is entitled to sell within any three month period a number of shares that does not exceed the greater of:
(a) 1% of the number of shares of the company's common stock then outstanding; or
(b) the average weekly trading volume of the company's common stock during the four calendar weeks preceding the filing of a notice on form 144 with respect to the sale.
Sales under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about the company.
Under Rule 144(k), a person who is not one of the Company's affiliates at any time during the three months preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years, is entitled to sell shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144.
As of the date of this prospectus, persons who are our affiliates hold up to approximately 39,500,000 of the shares that will be eligible for Rule 144 sales. These persons would, however, be subject to the volume limitation discussed above and would not become eligible to use Rule 144(k) until at least three months after resigning as an officer and director, and then only if they retained less than ten percent of the aggregate amount of common shares then outstanding.
Registration Rights
We have granted registration rights with respect to the securities underlying the Settlement Units and the Private Placement Units offered and sold under the contemporaneous settlement of CellCyte indebtedness and Private Placement effected contemporaneously with the Closing of the CellCyte Acquisition. At any time that the Company prepares to register any of its securities for public sale, holders of these securities will have the right to also have their securities registered for public resale under the same registration statement.
Dividends
There are no restrictions in our Articles of Incorporation or Bylaws that restrict us from declaring dividends. The Nevada Revised Statutes, however, do prohibit us from declaring dividends where, after giving effect to the distribution of the dividend:
(a) we would not be able to pay our debts as they become due in the usual course of business; or
(b) our total assets would be less than the sum of our total liabilities, plus the amount that would be needed to satisfy the rights of shareholders who have preferential rights superior to those receiving the distribution.
We have not declared any dividends. We do not plan to declare any dividends in the foreseeable future.
49
Equity Compensation Plans
The table set forth below presents information relating to our equity compensation plans as of the date of this prospectus:
Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights
(a) | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights
(b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding column (a)) |
Equity Compensation Plans Approved by Security Holders(1) | 2,000,000 | 1.50 | Nil |
Equity Compensation Plans Not Approved by Security Holders(2) | 4,659,000 | Nil | Nil |
(1) On March 30, 2007, we issued options to acquire up to 2,000,000 shares of our common stock at an exercise price of $1.50 per share exercisable for a period of five years pursuant to the Plan.
(2) On March 30, 2007, we issued warrants to acquire up to 4,659,225 common shares of the Company that are exercisable at a price of $3.00 per share for a period ending on the earlier of 18 months from the date of issuance of the warrants and 12 months from the effective date of the Company's registration statement of which this prospectus forms a part.
Recent Sales of Unregistered Securities
In connection with the acquisition of CellCyte, the (i) 16,000,000 restricted common shares issued by the Company to the Vendors, (ii) the Loan Units that have now been issued upon the conversion of the Loan, (iii) the Settlement Units issued in respect of the settlement of principal and interest owing by CellCyte to certain creditors, (iv) the options of the Company issued in place of options of CellCyte, (v) the shares of the Company issued in place of the warrants of CellCyte and (vi) the Private Placement Units issued under the Private Placement, were issued or are issuable to accredited investors (as defined in Rule 501 under Regulation D under the Securities Act) in reliance on Rule 506 under Regulation D under the Securities Act, or were issued or are issuable in offshore transactions (as defined in Rule 902 under Regulation S under the Securities Act) in reliance on Regulation S under the Securities Act, based upon representations made by the purchasers. The 18,750,0 00 restricted common shares and 2,500,000 common shares in the capital of the Company transferred to the order and direction of Vendors under the Share Exchange Agreement were transferred pursuant to an unregistered private transaction. Such securities have not been registered under the Securities Act or under any state securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
We engaged Williams & Webster, P.S., Certified Public Accountants, as our principal independent registered public accounting firm effective April 27, 2007. Concurrent with this appointment, Morgan & Company, Chartered Accountants, have resigned as our principal independent registered public accounting firm effective April 27, 2007. The decision to change our principal independent registered public accounting firm has been approved by our board of directors.
The report of Morgan & Company on our financial statements for the past two years did not contain an adverse opinion or disclaimer of opinion, nor was it modified as to uncertainty, audit scope, or accounting principles, other than to state that there is substantial doubt as to our ability to continue as a going concern.
During the two most recent fiscal years and the subsequent period through to the date of their resignation, there were no disagreements between us and Morgan & Company on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreements, if not resolved to the satisfaction of Morgan & Company, would have caused them to make reference thereto in their reports on our audited financial statements.
50
We provided Morgan & Company with a copy of our Current Report on Form 8-K filed April 30, 2007 in respect of the change of our auditor and requested that Morgan & Company furnish us with a letter addressed to the Securities and Exchange Commission stating whether or not they agree with the statements made in that Current Report on Form 8-K and, if not, stating the aspects with which they do not agree. We received the requested letter from Morgan & Company wherein they have confirmed their agreement to our disclosures in such Current Report on Form 8-K. A copy of Morgan & Company's letter has been filed as an exhibit to such Current Report on Form 8-K.
Williams & Webster, P.S. have not been consulted on any matter relating to the application of accounting principles to a specific transaction, either completed or contemplated, or the type of audit opinion that might be rendered on the Company's financial statements.
Indemnification of Directors and Officers
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons under Nevada law or otherwise, we have been advised that the opinion of the Securities and Exchange Commission is that such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Our officers and directors are indemnified as provided by the Nevada Revised Statutes (the "NRS") and our Bylaws.
Under the NRS, director immunity from liability to a company or its shareholders for monetary liabilities applies automatically unless it is specifically limited by a company's articles of incorporation that is not the case with our articles of incorporation. Excepted from that immunity are:
- a wilful failure to deal fairly with the company or its shareholders in connection with a matter in which the director has a material conflict of interest;
- a violation of criminal law (unless the director had reasonable cause to believe that his or her conduct was lawful or no reasonable cause to believe that his or her conduct was unlawful);
- a transaction from which the director derived an improper personal profit; and
- wilful misconduct.
Our Bylaws provide that we will indemnify our directors and officers to the fullest extent not prohibited by Nevada law; provided, however, that we may modify the extent of such indemnification by individual contracts with our directors and officers; and, provided, further, that we shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless:
- such indemnification is expressly required to be made bylaw;
- the proceeding was authorized by our Board of Directors;
- such indemnification is provided by us, in our sole discretion, pursuant to the powers vested us under Nevada law; or
- such indemnification is required to be made pursuant to the bylaws.
Our Bylaws provide that we will advance all expenses incurred to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was our director or officer, or is or was serving at our request as a director or executive officer of another company, partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request. This advance of expenses is to be made upon receipt of an undertaking by or on behalf of such person to repay said amounts should it be ultimately determined that the person was not entitled to be indemnified under our bylaws or otherwise.
51
Our Bylaws also provide that no advance shall be made by us to any officer in any action, suit or proceeding, whether civil, criminal, administrative or investigative, if a determination is reasonably and promptly made: (a) by the Board of Directors by a majority vote of a quorum consisting of directors who were not parties to the proceeding; or (b) if such quorum is not obtainable, or, even if obtainable, a quorum of disinterested directors so directs, by independent legal counsel in a written opinion, that the facts known to the decision- making party at the time such determination is made demonstrate clearly and convincingly that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to our best interests.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons against liability under the Securities Act, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
EXECUTIVE COMPENSATION
The following table sets forth the compensation paid to our Chief Executive Officer and those executive officers that earned more than $100,000 in 2006 (the "Named Executive Officers"):
Summary Compensation Table
Name and Principal Position | Year | Salary
($) | Bonus
($) | Stock Awards
($) | Option Awards ($) | Non-Equity Incentive Plan Compensation
($) | Non-qualified Deferred Compen-sation Earnings
($) | All Other Compen-sation
($) | Total
($) |
Michael Eyre,
Prior President and CEO(*) | 2006 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Glen Macdonald,
Prior Secretary, Treasurer and CFO(*) | 2006 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
(*) Both Named Executive Officers resigned from their respective positions with the Company upon the Closing of the CellCyte Acquisition.
None of our directors have received monetary compensation since our incorporation to the date of this prospectus. We currently do not pay any compensation to our directors.
Stock Option Grants
We had not granted any stock options to our executive officers prior to the Closing of the CellCyte Acquisition.
Stock Option Plan
In connection with the Closing of the CellCyte Acquisition, on March 30, 2007, our Board of Directors adopted the CellCyte's original 2007 Stock Option/Restricted Stock Plan (the "Plan"). The following summary of the Plan is not complete and is qualified in its entirety by reference to the Plan; a copy of which Plan has been filed with the SEC.
The purpose of the Plan is to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to employees and consultants and to promote the success of the Company's business. The number of shares available for issuance under the Plan is 2,000,000shares. If an award under the Plan should expire or become unexercisable for any reason and without having been exercised in full, or surrendered pursuant to an option exchange program, then the shares that were subject to such expiry or unexercised award shall again become available for future grant under the Plan. The Plan is administered by the Company's Board or a committee appointed by the Board. The Plan administrator has the authority, in its discretion, to determine all matters relating to awards granted under the Plan, including the selection of individuals to be granted awards, the type of award to be granted, the number of shares to be covered by each award and al l terms, conditions, restrictions and limitations of an award granted under the Plan. The term of the Plan is ten years from the date of its adoption by the Board.
52
We have issued options to acquire up to 2,000,000 of our shares of common stock at an exercise price of $1.50 per share exercisable for five years.
Employment Agreements
We have entered into employment agreements with each of Gary A. Reys, our Chairman, President and Chief Executive Officer, and Dr. Ronald W. Berninger, our Secretary, Executive Vice President and Chief Scientific Officer. We have also entered into a CEO Award Agreement with Mr. Reys. The following summary of each such agreement is not complete and is qualified in its entirety by reference to each such agreement; a copy of each such agreement having been filed as an exhibit to our Current Report on Form 8-K filed with the SEC on June 5, 2007.
We have entered into an Employment Agreement with Gary A. Reys dated June 1, 2007, pursuant to which Mr. Reys has agreed to serve as our Chairman, Chief Executive Officer and President for a period of five years, provided, however, that starting with the second anniversary of the date of the agreement, the agreement shall be extended under a daily three year "evergreen" feature such that the remaining term of his employment will always be at least three years. In consideration for his services, we have agreed to pay Mr. Reys an annual salary of $350,000, provided, however, that effective upon our reaching an aggregate funding of $15,000,000, Mr. Reys' base salary will be increased to $425,000 per year. In addition, Mr. Reys is entitled to reasonable housing and living expenses if he is required to maintain a resident apart from his permanent residence and we have also agreed to lease an automobile for Mr. Reys. Mr. Reys is also entitled to participate in em ployee benefit plans maintained by us, including group medical, dental, vision, disability, life insurance and flexible spending account plans. If Mr. Reys' employment with us terminates other than voluntarily or for Cause (as defined in the agreement) and he signs and does not revoke a standard release of claims with us, then Mr. Reys shall have the option of receiving continuing payments of his current salary plus any payments equal to his most recent annual performance bonus for a period of 36 months from the date of such termination (or a lump sum representing such salary continuation and bonus) and all of his unvested stock options shall become fully vested at the time of the termination and shall have an exercise period of 24 months from the date of termination. If Mr. Reys' employment with us terminates voluntarily or for Cause, then all unexercised and vested options terminate immediately and all payments of compensation by us to him will terminate immediately (except as to amounts a lready earned) and Mr. Reys will only be eligible for severance benefits in accordance with our established policies as in effect at that time. If, within 12 months following a Change of Control (as defined in the agreement), Mr. Reys' employment with us is subject to voluntarily or Involuntarily Termination (as defined in the agreement) or we terminate his employment other than for Cause, then he shall receive severance as if he were terminated other than voluntarily or for Cause.
We have entered into an Employment Agreement with Dr. Ronald W. Berninger dated June 1, 2007, pursuant to which Dr. Berninger has agreed to serve as Executive Vice President and Chief Scientific Officer for a period of five years, provided, however, that starting with the second anniversary of the date of the agreement, the agreement shall be extended under a daily three year "evergreen" feature such that the remaining term of his employment will always be at least three years. In consideration for his services, we have agreed to pay Dr. Berninger an annual salary of $205,000, provided, however, that effective upon our successfully completing Phase I clinical trials, Dr. Berninger's base salary will be increased to $225,000 per year. In addition, Dr. Berninger is entitled to reasonable housing and living expenses if he is required to maintain a resident apart from his permanent residence. Dr. Berninger is also entitled to participate in employee benefi t plans maintained by us, including group medical, dental, vision, disability, life insurance and flexible spending account plans. If Dr. Berninger's employment with us terminates other than voluntarily or for Cause (as defined in the agreement) and he signs and does not revoke a standard release of claims with us, then Dr. Berninger shall have the option of receiving continuing payments of his current salary plus any payments equal to his most recent annual performance bonus for a period of 36 months from the date of such termination (or a lump sum representing such salary continuation and bonus) and all of his unvested stock options shall become fully vested at the time of the termination and shall have an exercise period of 24 months from the date of termination. If Dr. Berninger's employment with us terminates voluntarily or for Cause, then all unexercised and vested options will terminate immediately and all payments of compensation by us to him will terminate immediately (except as to amounts already earned) and Dr. Berninger will only be eligible for severance benefits in accordance with our established policies as in effect at that time. If, within 12 months following a Change of Control (as defined in the agreement), Dr. Berninger's employment with us is subject to voluntarily or Involuntarily Termination (as defined in the agreement) or we terminate his employment other than for Cause, then he shall receive severance as if he were terminated other than voluntarily or for Cause.
53
On June 1, 2007, we also entered into a CEO Award Agreement with Mr. Reys. The purpose of the agreement is to specify how incentive compensation payments shall be calculated in connection with the satisfactory implementation of our business plan moving forward by Mr. Reys. Under the agreement it has presently been determined by our Board of Directors that the following items shall be in place in sufficient form and magnitude so as to allow our company and Mr. Reys to satisfactorily perform all of the activities required by our present and proposed business plan: (i) staffing, (ii) facilities, (iii) equipment, (iv) financing and (v) schedules. Our Board of Directors will make an independent assessment of Mr. Reys' performance relative to these categories of our business plan; and the Board will assign a score between 0 and 30 reflecting the degree to which each category has been implemented by Mr. Reys. This score will be used to determine the actual award to be paid to Mr. Reys. The t arget award is presently $750,000 in cash and the actual award will be calculated by multiplying the target award by the percentage score achieved by Mr. Reys. The award is to be paid as soon as practicable following the completion of each stage of our business plan, and at present the Board is considering its first assessment of Mr. Reys' performance under its business plan in the fall of this year.
WHERE YOU CAN FIND MORE INFORMATION
We are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy any document we file at the SEC's Public Reference Room at One Station Place, 100 F Street, N.E., Washington, D.C. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. You can also obtain copies of our SEC filings by going to the SEC's website athttp://www.sec.gov.
We have filed with the SEC a registration statement on Form SB-2 under the Securities Act with respect to the securities offered under this prospectus. This prospectus, which forms a part of that registration statement, does not contain all information included in the registration statement. Certain information is omitted and you should refer to the registration statement and its exhibits. With respect to references made in this prospectus to any contract or other document of the Company, the references are not necessarily complete and you should refer to the exhibits attached to the registration statement for copies of the actual contract or document.
No agent of the Company or other person has been authorized to give any information or to make any representation in connection with this offering other than those contained in this prospectus and, if given or made, such information or representation must not be relied upon as having been authorized by us. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any of the securities offered hereby by anyone in any jurisdiction in which such offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so or to any person to whom it is unlawful to make such offer or solicitation. Neither the delivery of this prospectus nor any sale made hereunder shall, under any circumstances, create any implication that the information contained herein is correct as of any time subsequent to the date of this prospectus.
54
FINANCIAL STATEMENTS
(a) Financial statements.
The following financial statements of CellCyte are included in this prospectus:
- Report of Independent Registered Independent Accounting Firm, dated February 28, 2007;
- Balance Sheets as at December 31, 2005 and 2006;
- Statements of Operations for the period from January 14, 2005 (date of inception) to December 31, 2006 and the years ended December 31, 2005 and 2006;
- Statement of Shareholders' Deficit for the period from January 14, 2005 (date of inception) to December 31, 2006;
- Statements of Cash Flows for the period from January 14, 2005 (date of inception) to December 31, 2006 and the years ended December 31, 2005 and 2006; and
- Notes to the Financial Statements.
The following financial statements of the Company are included in this prospectus:
Annual Financial Statements
- Report of Independent Registered Independent Accounting Firm, dated February 28, 2007;
- Balance Sheets as at December 31, 2005 and 2006;
- Statements of Operations for the period from March 9, 2004 (date of inception) to December 31, 2006 and the years ended December 31, 2005 and 2006;
- Statement of Shareholders' Deficit for the period from March 9, 2004 (date of inception) to December 31, 2006;
- Statements of Cash Flows for the period from March 9, 2004 (date of inception) to December 31, 2006 and the years ended December 31, 2005 and 2006; and
- Notes to the Financial Statements.
Interim Financial Statements (Unaudited)
- Balance Sheets as at March 31, 2007 and December 31, 2006;
- Interim Consolidated Statements of Operations for the Three Months Ended March 31, 2007 and 2006 and for the period from January 14, 2005 (date of inception) to March 31, 2007;
- Interim Consolidated Statements of Cash Flows for the Three Months Ended March 31, 2007 and 2006 and for the period from January 14, 2005 (date of inception) to March 31, 2007; and
- Notes to Interim Consolidated Financial Statements.
55
(b) Pro Forma Consolidated Financial Statements (Unaudited).
The following pro forma financial information reflecting the acquisition of CellCyte is included in this prospectus:
- Pro Forma Consolidated Balance Sheet as at December 31, 2006; and
- Notes to Pro forma Consolidated Statements.
56
CELLCYTE GENETICS CORPORATION
FINANCIAL STATEMENTS
March 31, 2007
57
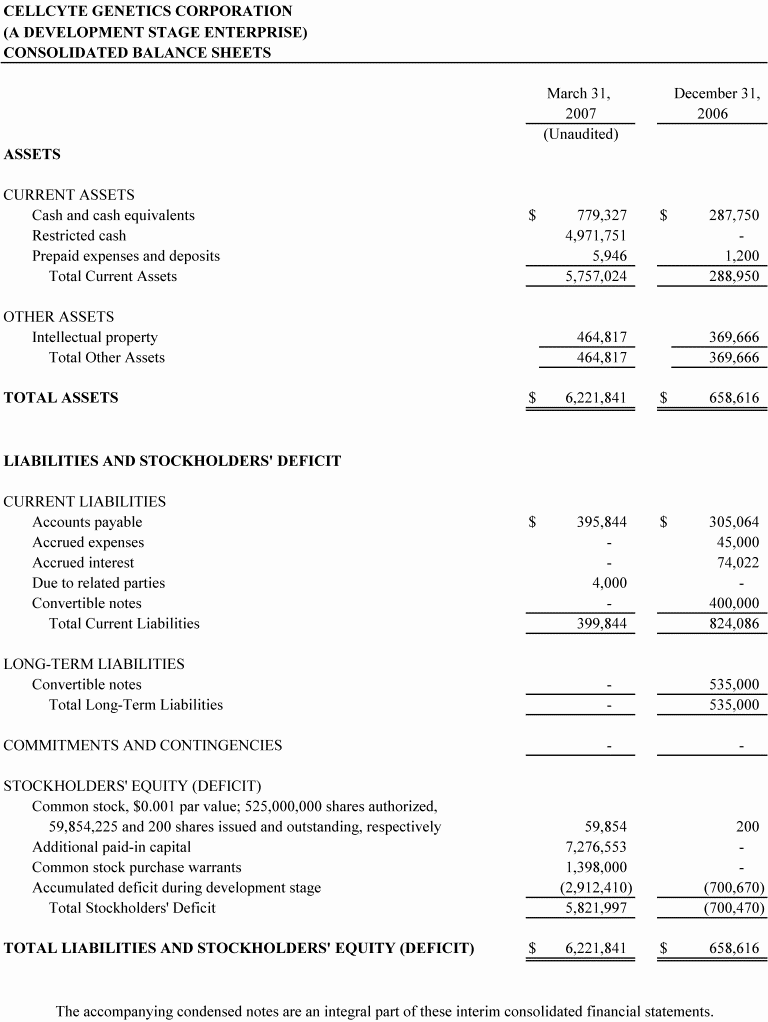
58
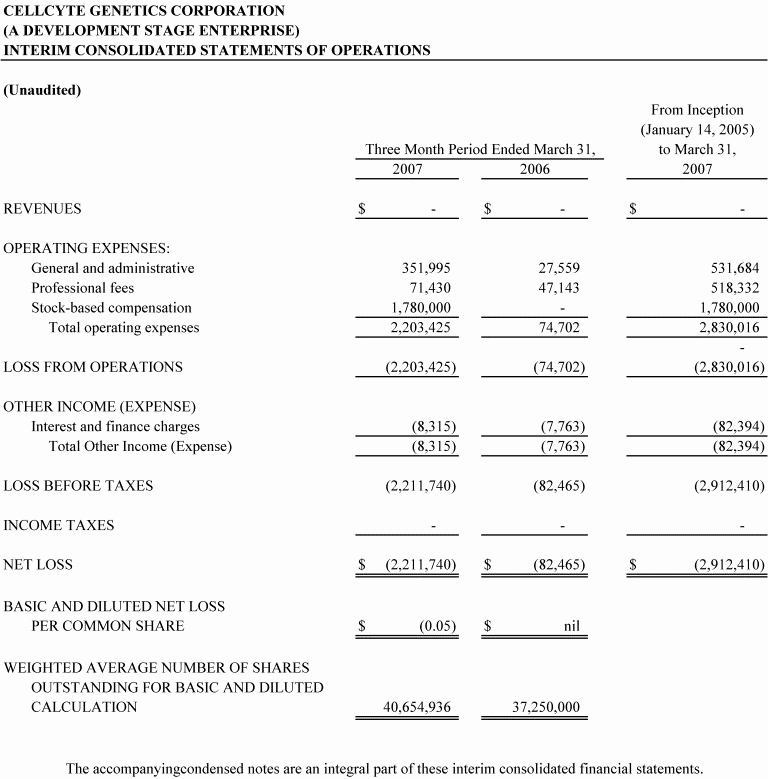
59
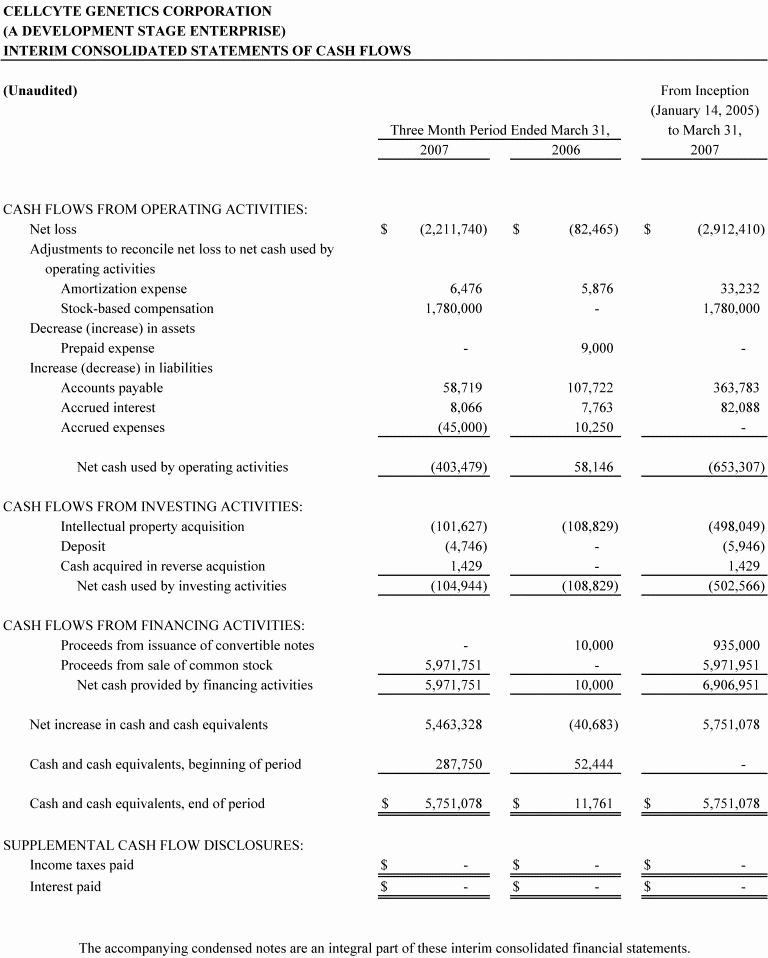
60
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
NOTE 1 - DESCRIPTION OF BUSINESS
The Company was incorporated as Shepard Inc. March 9, 2004 in the State of Nevada and changed its name to CellCyte Genetics Corporation ("CGN") effective February 16, 2007.
Effective January 19, 2007, CGN entered into an agreement in principle to acquire 100% of the issued and outstanding shares of CellCyte Genetics Corporation (a development stage enterprise) ("CGW"), a private Washington State corporation incorporated January 14, 2005, in exchange for 16,000,000 restricted shares of CGN's common stock, and the transfer of a further 18,750,000 shares of CGN's restricted common stock and 2,500,000 shares of CGN's non-restricted common stock from certain shareholders of CGN to the shareholders of CGW. Effective March 14, 2007, the acquisition of CGW was completed by the execution of a share exchange agreement (the "Agreement"), which, in addition to the share issuances and transfers described above, finalized certain other terms of the acquisition.
As a result of the completion of this acquisition and the related transactions as described in Note 3, the former shareholders of CGW own approximately 62% of the outstanding shares of common stock of CGN representing 37,250,000 of the 59,854,224 total issued and outstanding shares of common stock of CGN.
This acquisition is in essence a recapitalization of the Company and has been accounted for in accordance with the principles applicable to accounting for reverse acquisitions with CGW, the legal subsidiary, being treated as the accounting parent and CGN, the legal parent, being treated as the accounting subsidiary. Accordingly, the consolidated results of operations and cash flows of the Company include those of CGW for all periods presented and those of CGN subsequent to the date of the reverse acquisition. The comparative balance sheet as at December 31, 2006 is that of CGW.
CGW is an emerging biotechnology company engaged in the discovery and development of stem cell therapeutic products. The Company is developing clinical-stage therapeutic agents and treatments for oncology, diabetes, heart, liver, lung and kidney diseases as well as for stem cell bone marrow and organ transplants. The Company's discoveries involve stem cell regimens without using embryonic stem cells. As the Company is in the development stage, it has not realized any revenues from its planned operations.
As shown in the accompanying financial statements, the Company has incurred significant losses since inception and has not generated any revenues to date. The future of the Company is dependent upon its ability to obtain sufficient financing and upon achieving future profitable operations. These factors, among others, raise substantial doubt about the Company's ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Unaudited Financial Statements
The accompanying unaudited interim consolidated financial statements have been prepared in accordance with generally accepted principals for interim financial information and with the instruction to Form 10-QSB of Regulation S-B. They do not include all information and footnotes required by United States generally accepted accounting principals for complete financial statements. However, except as disclosed herein, there has been no material changes in the information disclosed in the notes to the financial statements for the year ended December 31, 2006 included in the Company's Annual Report on Form 10-KSB filed with the Securities and Exchange Commission. The interim unaudited financial statements should be read in conjunction with those financial statements included in the Form 10-KSB and the Form 8-K dated March 30, 2007. In the opinion of Management, all adjustments considered necessary for a fair presentation, consisting solely of normal recurring adjustments, have been made . Operating results for the three months ended March 31, 2007 are not necessarily indicative of the results that may be expected for the year ending December 31, 2007.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies is presented to assist in understanding the Company's financial statements. The financial statements and notes are representations of the Company's management, which is responsible for their integrity and objectivity. These accounting policies conform to accounting principles generally accepted in the United States of America and have been consistently applied in the preparation of the financial statements.
61
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
Basis of Consolidation
These consolidated financial statements include the accounts of CGW since its incorporation on January 14, 2005 and CGN. Since the reverse acquisition on March 14, 2007 (refer to Note 3), all inter-company balances and transactions have been eliminated.
Accounts Receivable
The Company carries its accounts receivable at net realizable value. On a periodic basis, the Company evaluates its accounts receivable and considers the need for an allowance for doubtful accounts, based on Company past and expected collections, and current credit conditions. The Company had no receivables or bad debts at its annual reporting dates in the accompanying financial statements.
Cash and Cash Equivalents
The Company considers restricted cash, all highly liquid investments and short-term debt instruments with maturities of three months or less from date of purchase to be cash equivalents. The Company had no cash equivalents at March 31, 2007.
Concentration of Risks
The Company maintains its cash and cash equivalents in commercial accounts at a major financial institution. Although the financial institution is considered creditworthy and has not experienced any losses on its deposits as of March 31, 2007, the Company's cash balances exceeded Federal Deposit Insurance Corporation limits at March 31, 2007 by approximately $679,327.
Earnings per Share
The Company computes net income (loss) per common share in accordance with Statement of Financial Accounting Standards No. 128, "Earnings per Share". Net income (loss) per share is based upon the weighted average number of outstanding common shares and the dilutive effect of common share equivalents, such as options and warrants to purchase common stock, convertible preferred stock and convertible notes, if applicable, that are outstanding each year. Basic and diluted earnings per share were the same at the reporting dates of the accompanying financial statements, as including common stock equivalents in the calculation of diluted earnings per share would have been anti-dilutive. For all periods prior to the reverse acquisition, the number of shares outstanding is deemed to be the number of shares issued and transferred to the shareholders of CBW to effect the reverse acquisition, being 37,250,000.
Fair Value of Financial Instruments
The Company's financial instruments may consist of cash and cash equivalents, prepaid expenses and other deferred charges, accounts payable, accrued expenses and other current liabilities. All instruments are accounted for on an historical cost basis, which, due to the short maturity of these financial instruments, approximates the fair value at the reporting dates of these financial statements.
Income Taxes
The Company follows the provisions of Statement of Financial Accounting Standards No. 109, "Accounting for Income Taxes" (hereinafter "SFAS No. 109"), under which deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities. Deferred tax assets and liabilities are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The provisions of SFAS No. 109 also require the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that the realization of such benefits is more likely than not. To the extent that it is more likely than not those benefits will not be received, the Company records a valuation allowance against the related deferred tax asset.
Intangible Assets
The Company's intangible assets primarily consist of patents and intellectual property, which are carried at the purchase price and/or the legal cost to obtain them. Patents and licenses are being amortized over their estimated useful lives, which range from seven to seventeen years.
Long-Lived Assets and Intangibles
In accordance with Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets," the Company examines on a periodic basis the carrying value of its tangible and intangible assets to determine whether there are any impairment losses. This standard establishes a single accounting model for long-lived assets to be disposed of by sale, including discontinued operations, and requires that these long-lived assets be measured at the lower of carrying amount or fair value less cost to sell, whether reported in continuing operations or discontinued operations. The determination of any impairment would include a comparison of estimated future cash flows anticipated to be generated during the remaining life of the assets to the net carrying value of the assets. As of March 31, 2007, no asset impairments have been identified or recorded.
62
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. The Company's had no property and equipment assets at March 31, 2007. Expenditures for repairs and maintenance are expensed as incurred
Research and Development Costs
Research and development costs are expensed as incurred. The cost of intellectual property purchased from others that is immediately marketable or that has an alternative future use is capitalized and amortized as intangible assets. Capitalized costs are amortized using the straight-line method over the estimated economic life of the related asset. The Company periodically reviews its capitalized intangible assets to assess recoverability based on the projected undiscounted cash flows from operations, and impairments are recognized in operating results when a permanent diminution in value occurs.
Revenue Recognition
The Company recognizes revenue when persuasive evidence of an arrangement exists and delivery has occurred, provided the fee is fixed or determinable and collection is probable. The Company assesses whether the fee is fixed and determinable based on the payment terms associated with the transaction. If a fee is based upon a variable such as acceptance by the customer, the Company accounts for the fee as not being fixed and determinable. In these cases, the Company defers revenue and recognizes it when it becomes due and payable. The Company assesses the probability of collection based on a number of factors, including past transaction history with the customer and the current financial condition of the customer. If the Company determines that collection of a fee is not reasonably assured, revenue is deferred until the time collection becomes reasonably assured.
Revenue from support agreements is recognized on a straight-line basis over the life of the contract, although the fee is due and payable at the time the agreement is signed or upon annual renewal.
Stock-Based Compensation
The Company accounts for its stock option plan under the recognition and measurement principles of the revised statement to Statement of Financial Accounting Standards No. 123, "Share-Based Payments" (hereinafter "SFAS No. 123 (R)"). Accordingly, compensation cost has been recognized using the fair value method and expected term accrual requirements as prescribed in SFAS No. 123 (R). The Company has recorded $1,780,000 in stock-based compensation expense through March 31, 2007 in connection with the acquisition of CGW as described further in Note 8 below.
Use of Estimates
The accompanying financial statements are prepared in conformity with accounting principles generally accepted in the United States of America and include certain estimates and assumptions which affect the reported amounts of assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Accordingly, actual results may differ from those estimates.
NOTE 3 - ACQUISITION OF CELLCYTE GENETICS CORPORATION
Effective January 19, 2007, CGN entered into an agreement in principle to acquire 100% of the issued and outstanding shares of CGW in exchange for 16,000,000 restricted shares of CGN's common stock, and the transfer of a further 18,750,000 shares of CGN's restricted common stock and 2,500,000 shares of CGN's non-restricted common stock from certain shareholders of CGN to the shareholders of CGW. Effective March 14, 2007, the acquisition of CGW was completed by the execution of a share exchange agreement (the "Agreement"), which, in addition to the share issuances and transfers described above, finalized the following terms of the acquisition: (i) the completion of a private placement of 3,981,164 units of CGN at a price of $1.50 per unit with each unit consisting of one common share of CGN and one warrant to acquire an additional common share of CGN at a price of $3.00 per share; (ii) the settlement of a total of $1,017,088 in debts of CGW through the issuance of units of CGN at a price o f $1.50 per unit with each unit being consistent with the private placement units above; (iii) the replacement of 2,000,000 stock options previously outstanding in CGW with 2,000,000 stock options in CGN at a price of $1.50 per share; and (iv) the issuance of 205,000 restricted shares of CGN's common stock in exchange for 205,000 share purchase warrants previously outstanding in CGW.
63
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
At the completion of this acquisition and related transactions, the former shareholders of CGW owned approximately 62% of the outstanding shares of common stock of CGN representing 37,250,000 of the anticipated 59,854,224 total issued and outstanding shares of common stock of CGN.
Accordingly, the acquisition has been accounted for as a recapitalization using accounting principles applicable to reverse acquisitions whereby the financial statements subsequent to the date of the transaction are presented as a continuation of CGW. Under reverse acquisition accounting CGW (the legal subsidiary) has been treated as the accounting parent (acquirer) and CGN (the legal parent) has been treated as the accounting subsidiary (acquiree). The value assigned to the common stock of consolidated CGN on acquisition of CGW is equal to the book value of the common stock of CGW plus the book value of the net assets of CGN as at the date of the acquisition as adjusted for certain concurrent transactions.
In accordance with the provisions for accounting for business combinations, the fair value of the options issued by CGN to replace options previously outstanding in CGW and the shares issued by CGN to replace warrants previously outstanding in CGW are considered part of the business combination purchase price. However, as these items are charged to CGN's share capital and deficit, they have no impact on the net book value of the net assets of CGN.
As at the date of the acquisition, the book value of the net assets of CGN was as follows:
Cash | $ | 1,429 |
Accounts payable | | (32,061) |
Due to related party | | (4,000) |
Preliminary net assets (liabilities) | | (34,632) |
Concurrent share issuance on settlement of debt (see above) | | 1,017,088 |
Adjusted CGN net assets | $ | 982,456 |
The consolidated capital stock of the Company immediately following the acquisition is determined as follows:
CGW - book value of capital stock | $ | 200 |
CGN - adjusted book value of net assets | | 982,456 |
Consolidated capital stock accounts post recapitalization | $ | 982,656 |
| | |
Allocated as follows: | | |
Common Stock - 55,873,060 common shares, $0.001 par value | $ | 55,873 |
Additional paid-in capital | | 723,783 |
Common stock purchase warrants | | 203,000 |
| $ | 982,656 |
NOTE 4 - CONVERTIBLE DEBT
As of December 31, 2006, the CGW had issued a total of $935,000 of convertible promissory notes to certain private accredited investors. Accrued interest on the notes equalled approximately $74,022 at year end 2006.
The outstanding principal and interest on the promissory notes is payable by CellCyte in 2007 and 2008. The outstanding principal amount of the promissory notes and all unpaid interest is automatically convertible into CellCyte securities issued in a qualified equity or equity based financing or combination of equity financings with gross proceeds totalling at least $1,000,000. For purposes of determining the number of equity securities to be received by the holders of the 8% convertible promissory notes upon such exchange, such holders will be deemed to have tendered 100% of the outstanding principal amount of the promissory notes and all accrued but unpaid interest as payment of the purchase price in such qualified financing.
64
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
In the event CellCyte does not complete a qualified financing, CellCyte is obligated to repay the entire principal balance in 2007 and 2008. Interest on the outstanding principal balance of the notes shall be computed on the basis of the actual number of days elapsed and a year of three hundred and sixty (360) days and shall be payable on the maturity date by the Company in cash or, at the option of the Company, in shares of the Company's equity securities.
While the original debenture agreements called for the prospective issuance of warrants to the note holders, these warrants were never issued. In subsequent agreements, the holders of convertible notes agreed to relinquish any rights to warrants in view of the prospective conversion of their notes into shares of the Company's common stock. The Company's calculation of the warrants' fair value at the time of the debentures' issuance resulted in no value attributable to the warrants.
In connection with the reverse acquisition as described in Note 3, the convertible promissory notes and accrued interest totalling $1,017,088 were converted into units of CGN at a price of $1.50 per unit, resulting in the issuance of 678,060 units as described in detail in Note 3.
In addition, as a condition of the recapitalization, CGW issued 205,000 shares of common stock on replacement and settlement of the never issued common stock purchase warrants previously outstanding in CGW.
NOTE 5 - INTELLECTUAL PROPERTY
In 2006 and 2007, the Company paid a total of $25,000 and $90,000, respectively, in the execution of an intellectual property licensing agreement with U.S. Department of Veterans Affairs ("VA"). The license agreement relates to certain patents pending and contains royalty payment arrangements as well as funding guarantees. Because of certain internal VA intellectual property timeline problems regarding technology transfer to CellCyte, the VA has not required CellCyte to meet any funding requirements as of December 31, 2006. Because the VA was unable to transfer research notebooks, it precluded CellCyte from beginning technology development and securing related funding and/or grants. With the completion of the recent financing transaction CellCyte has now met the funding guarantee requirements included in the VA license.
At March 31, 2007 and year end 2006 and 2005, the Company had $70,002 of capitalized patents and $313,047, $301,420 and $160,474, respectively, of capitalized patents pending. These amounts consist of the legal fees expended to pursue and prosecute a range of patent claims related to stem cell delivery and purification.
The Company amortizes patents (and licenses for the use of patents) over their remaining estimated lives, which ranges from 7 to 17 years.
| | March 31, | | December 31, |
| | 2007 | | 2006 |
Licenses | | $ | 115,000 | | | $ | 25,000 | |
Patents | | | 70,002 | | | | 70,002 | |
Patents pending | | | 313,047 | | | | 301,420 | |
Accumulated amortization | | | (33,232) | | | | (26,756) | |
Licenses and patents, net | | $ | 464,817 | | | $ | 369,666 | |
NOTE 6 - COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company has operating leases for its office and storage space, all of which are month to month. Rent expense relating to the operating leases was approximately $31,593 for the period ended March 31, 2007.
Subsequent to the period end March 31, 2007, the Company entered into a 63-month lease in a new facility in Bothell, Washington beginning April 1, 2007. Total commitment in leases cost for the term of the lease is approximately $1,590,123.
65
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
Executive Employment Contracts
In 2005, the Company entered into employment contracts with two key Company executives. As of March 31, 2007, these employment contracts have not been initiated because the executives agreed to forfeit the rights to any salaried compensation until such time as the Company's liquidity improves. Accordingly, there are no accrued expenses or liabilities associated with the contracts.
NOTE 7 - CAPITAL STOCK
Common Stock
The Company has authorized 525,000,000 shares of its common stock, $0.001 par value. The Company had issued and outstanding 38,990,000 shares of its common stock at December 31, 2005 and 2006, respectively.
During the period ended March 31, 2007, the issued common shares as follows:
- 16,000,000 shares of common stock were issued on acquisition of CGW as described in Note 3.
- 3,981,164 units were issued at a price of $1.50 per unit for total proceeds of $5,971,751. Each unit consists of one common share of CGN and one warrant to acquire an additional common share of CGN at a price of $3.00 per share, exercisable for a period ending earlier of 18 months from date of issuance of the units or 12 months from the effective date of the proposed registration statement.
- 678,060 units were issued on settlement of convertible promissory notes and accrued interest totalling $1,017,088 at a price of $1.50 per unit with each unit being consistent with the private placement units above.
- 205,000 shares of common stock with an estimated fair value of $246,000 were issued on replacement and settlement of the never issued common stock purchase warrants previously outstanding in CGW as described in Note 4.
The estimated fair value of the warrants issued in connection with the financing and debt settlement of $1,398,000 has been recorded as a separate component of stockholders' equity and was determined using the Black-Scholes option pricing model using the following weighted average assumptions: expected option life of 1.50 years; risk-free interest rate of 4.90%; dividend yield of 0%; and expected volatility of 100%.
NOTE 8 - COMMON STOCK OPTIONS
CGW common stock options
In 2005, CCW adopted the 2005 Stock Option/Restricted Stock (hereinafter "the Plan") under which 5,450,000 shares of common stock were reserved for issuance under qualified options, nonqualified options, stock appreciation rights and other awards as set forth in the plan.
During 2005 and 2006, CCW agreed to issue a total of 575,000 common stock options at an exercise price of $0.10 per share to consultants to CCW. The options fair value was determined to be $0 based upon CCW calculation using the Black-Scholes option pricing calculation formula and CCW subsequently rescinded its agreement to issue options.
CGN common stock options
In connection with the recapitalization as described in Note 3, CGN granted a total of 2,000,000 options to replace the 2,000,000 options previously outstanding in CGW. In accordance with the provisions for accounting for business combinations, the fair value of the options of $1,780,000 is considered part of the business combination purchase price. The Company determined the fair value of these stock options using the Black-Scholes option pricing model using the following weighted average assumptions: expected option life of 10 years; risk-free interest rate of 4.70%; dividend yield of 0%; and expected volatility of 100%.
66
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2007
(unaudited)
NOTE 9 - RELATED PARTY TRANSACTIONS
Two officers who are also members of the Company's board of directors served as consultants to the Company on various business, strategic, and technical issues. The Company paid and expensed a total of $143,000 for these services in 2005, $144,000 for these services in 2006 and $100,000 through March 31, 2007. In addition to the fees, reimbursable expenses were also paid under this contract. The total amount paid for these expenses was $43,187 in 2005, $41,040 in 2006 and $31,634 through March 31, 2007. The use of officers as contractors is considered an interim arrangement while the Company was in start-up operations.
__________
67
CELLCYTE GENETICS CORPORATION (WASHINGTON)
Financial Statements
December 31, 2006
CELLCYTE GENETICS CORPORATION
Table of Contents

REPORT OF THE INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM |
F-1
|
FINANCIAL STATEMENTS | |
| | Balance Sheets | F-2 |
| | Statements of Operations | F-3 |
| | Statement of Stockholders' Equity | F-4 |
| | Statements of Cash Flows | F-5 |
NOTES TO FINANCIAL STATEMENTS | F-6 |
CellCyte Genetics Corporation
Kirkland, WA
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We have audited the accompanying balance sheets of CellCyte Genetics Corporation (a development stage enterprise) as of December 31, 2006 and 2005, and the related statements of operations, stockholders' deficit and cash flows for the year s then endedand from inception (January 14, 2005) through December 31, 2006. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of CellCyte Genetics Corporation as of December 31, 2006 and 2005 and the results of its operations, stockholders' deficit and cash flows for the years then ended and from inception (January 14, 2005) through December 31, 2006 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company's operating losses, negative working capital, and delinquent notes payable raise substantial doubt about its ability to continue as a going concern. Management's plans regarding the resolution of this issue are also discussed in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Williams & Webster, P.S.
Certified Public Accountants
Spokane, Washington
February 28, 2007
F-1
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
BALANCE SHEETS | |
|
|
| | | | Year Ended December 31, |
| | | | 2006 | | 2005 |
ASSETS | | | | | | |
CURRENT ASSETS | | | | | |
| Cash and cash equivalents | $ | 287,750 | | $ | 52,444 |
| Prepaid expenses | | - | | | 9,000 |
| Deposits | | 1,200 | | | 1,200 |
| | Total Current Assets | | 288,950 | | | 62,644 |
OTHER ASSETS | | | | | |
| Intellectual property | | 369,666 | | | 227,225 |
| | Total Other Assets | | 369,666 | | | 227,225 |
| | | | | | | | |
TOTAL ASSETS | $ | 658,616 | | $ | 289,869 |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS' DEFICIT | | | | | |
CURRENT LIABILITIES | | | | | |
| Accounts payable | $ | 305,064 | | $ | 167,439 |
| Accrued expenses | | 45,000 | | | 23,917 |
| Accrued interest | | 74,021 | | | 23,343 |
| Convertible notes | | 400,000 | | | 390,000 |
| | Total Current Liabilities | | 824,085 | | | 604,699 |
LONG TERM LIABILITIES | | | | | |
| Convertible notes | | 535,000 | | | - |
| | Total Long Term Liabilities | | 535,000 | | | - |
COMMITMENTS AND CONTINGENCIES | | - | | | - |
| | | | | | | | |
STOCKHOLDERS' DEFICIT | | | | | |
| Preferred stock, no par value; 10,000,000 shares authorized, no shares issued and outstanding | | - | | | |
| Common stock, $0.00001 par value; 40,000,000 shares authorized, 20,000,000 shares issued and outstanding | | 200 | | | 200 |
| Accumulated deficit during development stage | | (700,669) | | | (315,030) |
| | Total Stockholders' Deficit | | (700,469) | | | (314,830) |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 658,616 | | $ | 289,869 |
The accompanying notes are an integral part of these financial statements.
F-2
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
STATEMENTS OF OPERATIONS | |
|
|
| | | | | | | | | From Inception |
| | | | | | | | | (January 14, 2005) |
| | | Year Ended December 31, | to December 31, |
| | | 2006 | | 2005 | | 2006 |
| | | | | | | | | | |
REVENUES | $ | - | | $ | - | | $ | - |
| | | | | | | | | | |
OPERATING EXPENSES: | | | | | | | | |
| General and administrative | | 112,636 | | | 67,052 | | | 179,688 |
| Professional fees | | 222,265 | | | 224,637 | | | 446,902 |
| | Total operating expenses | | 334,901 | | | 291,689 | | | 626,590 |
| | | | | | | | | | - |
LOSS FROM OPERATIONS | | (334,901) | | | (291,689) | | | (626,590) |
| | | | | | | | | | |
OTHER INCOME (EXPENSE) | | | | | | | | |
| Interest expense | | (50,678) | | | (23,341) | | | (74,019) |
| Finance charges | | (60) | | | - | | | (60) |
| | Total Other Income(Expense) | | (50,738) | | | (23,341) | | | (74,079) |
| | | | | | | | | | |
LOSS BEFORE TAXES | | (385,639) | | | (315,030) | | | (700,669) |
| | | | | | | | | | |
INCOME TAXES | | - | | | - | | | - |
| | | | | | | | | | |
NET LOSS | $ | (385,639) | | $ | (315,030) | | $ | (700,669) |
| | | | | | | | | | |
BASIC AND DILUTED NET LOSS | | | | | | | | |
| PER COMMON SHARE | $ | (0.019) | | $ | (0.016) | | | |
| | | | | | | | | | |
WEIGHTED AVERAGE NUMBER OF SHARES |
| OUTSTANDING FOR BASIC AND DILUTED CALCULATION | | 20,000,000 | | | 20,000,000 | | | |
The accompanying notes are an integral part of these financial statements.
F-3
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
STATEMENTS OF STOCKHOLDERS' DEFICIT | |
|
|
| | | | | | | Accumulated | |
| | | | | | | Deficit during | Total |
| Preferred Stock | Common Stock | Development | Stockholders' |
| Shares | Amount | Shares | Amount | Stage | Deficit |
| | | | | | | | | | |
Initial Contribution by Founders | - | $ | - | 20,000,000 | $ | 200 | $ | - | $ | 200 |
| | | | | | | | | | |
Net loss for year ended December 31, 2005 | - | | - | - | | - | | (315,030) | | (315,030) |
| | | | | | | | | | |
| | | | | | | | | | |
Balance,
December 31, 2005 | - | | - | 20,000,000 | | 200 | | (315,030) | | (314,830) |
| | | | | | | | | | |
Net loss for year ended December 31, 2006 | - | | - | - | | - | | (385,639) | | (385,639) |
| | | | | | | | | | |
| | | | | | | | | | |
Balance,
December 31, 2006 | - | $ | - | 20,000,000 | $ | 200 | $ | (700,669) | $ | (700,469) |
| | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-4
CELLCYTE GENETICS CORPORATION | | | | | | | | | |
(A DEVELOPMENT STAGE ENTERPRISE) | | | | | | | | | |
STATEMENTS OF CASH FLOWS | | | | | | | | | |
| | | | | | | | | | | | From Inception |
| | | | | | | | | | | | (January 14, 2005) |
| | | | | | Year Ended December 31, | | to December 31, |
| | | | | | 2006 | | 2005 | | 2006 |
| | | | | | | | | | | | | |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | |
| Net loss | | $ | (385,639) | | $ | (315,030) | | $ | (700,669) |
| Adjustments to reconcile net loss to net cash used by | | | | | | |
| | operating activities | | | | | | | | | |
| | | Amortization expense | | | 23,505 | | | 3,251 | | | 26,756 |
| Decrease (increase) in assets | | | | | | | | | |
| | | Prepaid expense | | | 9,000 | | | (9,000) | | | - |
| Increase (decrease) in liabilities | | | | | | | | | |
| | | Accounts payable | | | 137,625 | | | 167,439 | | | 305,064 |
| | | Accrued interest | | | 50,678 | | | 23,343 | | | 74,021 |
| | | Accrued expenses | | | 21,083 | | | 23,917 | | | 45,000 |
| | | | | | | | | | | | | |
| | | | Net cash used by operating activities | | | (143,748) | | | (106,080) | | | (249,828) |
| | | | | | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | |
| | | Intellectual property acquisition | | | (165,946) | | | (230,476) | | | (396,422) |
| | | Deposit | | | - | | | (1,200) | | | (1,200) |
| | | | Net cash used by investing activities | | | (165,946) | | | (231,676) | | | (397,622) |
| | | | | | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | |
| | | Proceeds from issuance of convertible notes | | 545,000 | | | 390,000 | | | 935,000 |
| | | Proceeds from sale of common stock | | | - | | | 200 | | | 200 |
| | | | Net cash provided by financing activities | | 545,000 | | | 390,200 | | | 935,200 |
| | | | | | | | | | | | | |
| Net increase in cash and cash equivalents | | | 235,306 | | | 52,444 | | | 287,750 |
| | | | | | | | | | | | | |
| Cash and cash equivalents, beginning of period | | | 52,444 | | | - | | | - |
| | | | | | | | | | | | | |
| Cash and cash equivalents, end of period | | $ | 287,750 | | $ | 52,444 | | $ | 287,750 |
| | | | | | | | | | | | | |
SUPPLEMENTAL CASH FLOW DISCLOSURES: | | | | | | | | | |
| Income taxes paid | | $ | - | | $ | - | | $ | - |
| Interest paid | | $ | - | | $ | - | | $ | - |
The accompanying notes are an integral part of these financial statements.
F-5
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
NOTE 1 - DESCRIPTION OF BUSINESS
CellCyte Genetics Corporation was incorporated on January 14, 2005 in the State of Washington and maintains offices in Kirkland, Washington.
CellCyte Genetics Corporation (hereinafter "CellCyte" or the "Company") is an emerging biotechnology company engaged in the discovery and development of stem cell therapeutic products. The Company is developing clinical-stage therapeutic agents and treatments for oncology, diabetes, heart, liver, lung and kidney diseases as well as for stem cell bone marrow and organ transplants. The Company's discoveries involve stem cell regimens without using embryonic stem cells. As the Company is in the development stage, it has not realized any revenues from its planned operations.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies of CellCyte Genetics Corporation is presented to assist in understanding the Company's financial statements. The financial statements and notes are representations of the Company's management, which is responsible for their integrity and objectivity. These accounting policies conform to accounting principles generally accepted in the United States of America and have been consistently applied in the preparation of the financial statements.
Accounts Receivable
The Company carries its accounts receivable at net realizable value. On a periodic basis, the Company evaluates its accounts receivable and considers the need for an allowance for doubtful accounts, based on Company past and expected collections, and current credit conditions. The Company had no receivables or bad debts at its annual reporting dates in the accompanying financial statements.
Cash and Cash Equivalents
The Company considers all highly liquid investments and short-term debt instruments with maturities of three months or less from date of purchase to be cash equivalents. The Company had no cash equivalents at December 31, 2005 or December 31, 2006.
Concentration of Risks
The Company maintains its cash and cash equivalents in commercial accounts at a major financial institution. Although the financial institution is considered creditworthy and has not experienced any losses on its deposits as of December 31, 2006, the Company's cash balances exceeded Federal Deposit Insurance Corporation limits at December 31, 2006 by approximately $187,750.
Earnings per Share
The Company computes net income (loss) per common share in accordance with Statement of Financial Accounting Standards No. 128, "Earnings per Share." Net income (loss) per share is based upon the weighted average number of outstanding common shares and the dilutive effect of common share equivalents, such as options and warrants to purchase common stock, convertible preferred stock and convertible notes, if applicable, that are outstanding each year. Basic and diluted earnings per share were the same at the reporting dates of the accompanying financial statements, as including common stock equivalents in the calculation of diluted earnings per share would have been anti-dilutive.
Fair Value of Financial Instruments
The Company's financial instruments may consist of cash and cash equivalents, prepaid expenses and other deferred charges, accounts payable, accrued expenses and other current liabilities. All instruments are accounted for on an historical cost basis, which, due to the short maturity of these financial instruments, approximates the fair value at the reporting dates of these financial statements.
F-6
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
Going Concern
As shown in the accompanying financial statements, the Company had an accumulated deficit of $682,669 incurred through December 31, 2006. The Company has no revenues, and negative working capital. Management forecasts operations during the coming year will need approximately $3,250,000 in additional working capital, which is expected to come from issuance of convertible notes, equipment financing and private placements of stock.
Income Taxes
The Company follows the provisions of Statement of Financial Accounting Standards No. 109, "Accounting for Income Taxes" (hereinafter "SFAS No. 109"), under which deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities. Deferred tax assets and liabilities are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The provisions of SFAS No. 109 also require the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that the realization of such benefits is more likely than not. To the extent that it is more likely than not that such benefits will not be received, the Company records a valuation allowance against the related deferred tax asset.
Intangible Assets
The Company's intangible assets primarily consist of patents and intellectual property, which are carried at the purchase price and/or the legal cost to obtain them. Patents and licenses are being amortized over their estimated useful lives, which range from seven to seventeen years.
Long-Lived Assets and Intangibles
In accordance with Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets," the Company examines on a periodic basis the carrying value of its tangible and intangible assets to determine whether there are any impairment losses. This standard establishes a single accounting model for long-lived assets to be disposed of by sale, including discontinued operations, and requires that these long-lived assets be measured at the lower of carrying amount or fair value less cost to sell, whether reported in continuing operations or discontinued operations. The determination of any impairment would include a comparison of estimated future cash flows anticipated to be generated during the remaining life of the assets to the net carrying value of the assets. For the years ended December 31, 2005 and 2006, no asset impairments were identified or recorded.
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. The Company's had no property and equipment assets at December 31, 2005 or December 31, 2006. Expenditures for repairs and maintenance are expensed as incurred.
Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 157, "Fair Value Measurements" (hereinafter "SFAS No. 157) which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles (GAAP), and expands disclosures about fair value measurements. Where applicable, SFAS No. 157 simplifies and codifies related guidance within GAAP and does not require any new fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. Earlier adoption is encouraged. The Company does not expect the adoption of SFAS No. 157 to have a significant immediate effect on its financial position or results of operations.
F-7
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
In June 2006, the Financial Accounting Standards Board issued FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109" (hereinafter "FIN 48"), which prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006. The Company does not expect the adoption of FIN 48 to have a material immediate impact on its financial reporting. The Company is currently evaluating the impact, if any, the adoption of FIN 48 will have on its disclosure requirements.
In March 2006, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 156, "Accounting for Servicing of Financial Assets--an amendment of FASB Statement No. 140." This statement requires an entity to recognize a servicing asset or servicing liability each time it undertakes an obligation to service a financial asset by entering into a servicing contract in any of the following situations: a transfer of the servicer's financial assets that meets the requirements for sale accounting; a transfer of the servicer's financial assets to a qualifying special-purpose entity in a guaranteed mortgage securitization in which the transferor retains all of the resulting securities and classifies them as either available-for-sale securities or trading securities; or an acquisition or assumption of an obligation to service a financial asset that does not relate to financial assets of the servicer or its consolidated affiliates. The statement also requires all separately recognized servicing assets and servicing liabilities to be initially measured at fair value, if practicable and permits an entity to choose either the amortization or fair value method for subsequent measurement of each class of servicing assets and liabilities. The statement further permits, at its initial adoption, a one-time reclassification of available for sale securities to trading securities by entities with recognized servicing rights, without calling into question the treatment of other available for sale securities under Statement 115, provided that the available for sale securities are identified in some manner as offsetting the entity's exposure to changes in fair value of servicing assets or servicing liabilities that a servicer elects to subsequently measure at fair value and requires separate presentation of servicing assets and servicing liabilities subsequently measured at fair value in the statement of financial position and additional disclosures for all separately recognize d servicing assets and servicing liabilities. This statement is effective for fiscal years beginning after September 15, 2006, with early adoption permitted as of the beginning of an entity's fiscal year. Management believes the adoption of this statement will have no impact on the Company's financial condition or results of operations.
In February 2006, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 155, "Accounting for Certain Hybrid Financial Instruments, an Amendment of FASB Standards No. 133 and 140" (hereinafter "SFAS No. 155"). This statement established the accounting for certain derivatives embedded in other instruments. It simplifies accounting for certain hybrid financial instruments by permitting fair value remeasurement for any hybrid instrument that contains an embedded derivative that otherwise would require bifurcation under SFAS No. 133 as well as eliminating a restriction on the passive derivative instruments that a qualifying special-purpose entity ("SPE") may hold under SFAS No. 140. This statement allows a public entity to irrevocably elect to initially and subsequently measure a hybrid instrument that would be required to be separated into a host contract and derivative in its entirety at fair value (with changes in fair value recognized in earnings) so long as that instrument is not designated as a hedging instrument pursuant to the statement. SFAS No. 140 previously prohibited a qualifying special-purpose entity from holding a derivative financial instrument that pertains to a beneficial interest other than another derivative financial instrument. This statement is effective for fiscal years beginning after September 15, 2006, with early adoption permitted as of the beginning of an entity's fiscal year. Management believes the adoption of this statement will have no impact on the Company's financial condition or results of operations.
F-8
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
In May 2005, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards No. 154, "Accounting Changes and Error Corrections," (hereinafter "SFAS No. 154") which replaces Accounting Principles Board Opinion No. 20, "Accounting Changes", and SFAS No. 3, "Reporting Accounting Changes in Interim Financial Statements - An Amendment of APB Opinion No. 28". SFAS No. 154 provides guidance on accounting for and reporting changes in accounting principle and error corrections. SFAS No. 154 requires that changes in accounting principle be applied retrospectively to prior period financial statements and is effective for fiscal years beginning after December 15, 2005. The Company does not expect SFAS No. 154 to have a material impact on its consolidated financial position, results of operations, or cash flows.
Research and Development Costs
Research and development costs are expensed as incurred. The cost of intellectual property purchased from others that is immediately marketable or that has an alternative future use is capitalized and amortized as intangible assets. Capitalized costs are amortized using the straight-line method over the estimated economic life of the related asset. The Company periodically reviews its capitalized intangible assets to assess recoverability based on the projected undiscounted cash flows from operations, and impairments are recognized in operating results when a permanent diminution in value occurs.
Revenue Recognition
The Company recognizes revenue when persuasive evidence of an arrangement exists and delivery has occurred, provided the fee is fixed or determinable and collection is probable. The Company assesses whether the fee is fixed and determinable based on the payment terms associated with the transaction. If a fee is based upon a variable such as acceptance by the customer, the Company accounts for the fee as not being fixed and determinable. In these cases, the Company defers revenue and recognizes it when it becomes due and payable. The Company assesses the probability of collection based on a number of factors, including past transaction history with the customer and the current financial condition of the customer. If the Company determines that collection of a fee is not reasonably assured, revenue is deferred until the time collection becomes reasonably assured.
Revenue from support agreements is recognized on a straight-line basis over the life of the contract, although the fee is due and payable at the time the agreement is signed or upon annual renewal.
Stock-Based Compensation
The Company accounts for its stock option plan under the recognition and measurement principles of the revised statement to Statement of Financial Accounting Standards No. 123, "Share-Based Payments" (hereinafter "SFAS No. 123 (R)"). Accordingly, compensation cost has been recognized using the fair value method and expected term accrual requirements as prescribed in SFAS No. 123 (R) The Company had no stock-based compensation expense for the years ended December 31, 2006 and 2005.
Use of Estimates
The accompanying financial statements are prepared in conformity with accounting principles generally accepted in the United States of America and include certain estimates and assumptions which affect the reported amounts of assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Accordingly, actual results may differ from those estimates.
F-9
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
NOTE 3 - CONVERTIBLE DEBT
As of December 31, 2005, the Company had issued 8% convertible promissory notes in the aggregate principal amount of $390,000 to certain private accredited investors. Accrued interest on the notes equalled approximately $23,343 at year end 2005. As of December 31, 2006, these one year promissory notes were in default.
As of December 31, 2006, the Company had issued a total of $930,000 of convertible promissory notes to certain private accredited investors. Accrued interest on the notes equalled approximately $74,022 at year end 2006.
The following chart provides maturity and interest rate information on the Company's convertible debt:
| | December 31, |
| | 2006 | | 2005 |
Convertible debt with an interest rate of 8%, | | | | | | |
uncollateralized, due in 2006, delinquent | | $ | 390,000 | | $ | 390,000 |
Convertible debt with an interest rate of 8%, | | | | | | |
uncollateralized, due in 2007 | | | 10,000 | | | - |
Convertible debt with an interest rate of 8%, | | | | | | |
uncollateralized, due in 2008 | | | 400,000 | | | - |
Convertible debt with an interest rate of 0%, | | | | | | |
uncollateralized, due in 2008 | | | 135,000 | | | - |
| | | | | | |
Less current portion | | | ( 400,000) | | | ( 390,000) |
| | | | | | |
Long-term portion | | $ | 535,000 | | $ | - |
Future maturities of long-term debt are as follows:
Year ending December 31, | | | |
2006 (delinquent) | | $ | 390,000 |
2007 | | | 10,000 |
2008 | | | 535,000 |
2009 | | | - |
| | | | $ | 935,000 |
The outstanding principal and interest on the promissory notes issued in 2006 is payable by CellCyte in 2007 and 2008. The outstanding principal amount of the promissory notes and all unpaid interest is automatically convertible into CellCyte securities issued in a qualified equity or equity based financing or combination of equity financings with gross proceeds totaling at least $1,000,000. For purposes of determining the number of equity securities to be received by the holders of the 8% convertible promissory notes upon such exchange, such holders will be deemed to have tendered 100% of the outstanding principal amount of the promissory notes and all accrued but unpaid interest as payment of the purchase price in such qualified financing.
F-10
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
In the event CellCyte does not complete a qualified financing, CellCyte is obligated to repay the entire principal balance in 2007 and 2008. Interest on the outstanding principal balance of the notes shall be computed on the basis of the actual number of days elapsed and a year of three hundred and sixty (360) days and shall be payable on the maturity date by the Company in cash or, at the option of the Company, in shares of the Company's equity securities.
While the original debenture agreements called for the prospective issuance of warrants to the note holders, these warrants were never issued. In subsequent agreements, the holders of convertible notes agreed to relinquish any rights to warrants in view of the prospective conversion of their notes into shares of the Company's common stock. The Company's calculation of the warrants' fair value at the time of the debentures' issuance resulted in no value attributable to the warrants.
NOTE 4 - INTELLECTUAL PROPERTY
In 2006, the Company paid a total of $25,000 in the execution an intellectual property licensing agreement with U.S. Department of Veterans Affairs (VA). The license agreement relates to certain patents pending and contains royalty payment arrangements as well as funding guarantees. Because of certain internal VA intellectual property timeline problems regarding technology transfer to CellCyte, the VA has not required CellCyte to meet any funding requirements as of December 31, 2006. Because the VA was unable to transfer research notebooks, it precluded CellCyte from beginning technology development and securing related funding and/or grants.
At year end 2006 and 2005, the Company had $70,002 of capitalized patents and $301,420 and $160,474, respectively, of capitalized patents pending. These amounts consist of the legal fees expended to pursue and prosecute a range of patent claims related to stem cell delivery and purification.
The Company amortizes patents (and licenses for the use of patents) over the remaining life of the patents, which ranges from 7 to 17 years.
| | December 31, |
| | 2006 | | 2005 |
Licenses | | $ | 25,000 | | $ | - |
Patents | | | 70,002 | | | 70,002 |
Patents pending | | | 301,420 | | | 160,474 |
Accumulated amortization | | | (26,756) | | | (3,251) |
Licenses and patents, net | | $ | 369,666 | | $ | 227,225 |
NOTE 5 - COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company has operating leases for its office and storage space, all of which are month to month. Rent expense relating to the operating leases was approximately $36,843 and $8,401 for the years ended December 31, 2006 and 2005, respectively.
F-11
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
Executive Employment Contracts
In 2005, the Company entered into employment contracts with two key Company executives. As of December 31, 2006, these employment contracts have not been initiated because the executives agreed to forfeit the rights to any salaried compensation until such time as the Company's liquidity improves. Accordingly, there are no accrued expenses or liabilities associated with the contracts.
NOTE 6 - INCOME TAXES
At December 31, 2006, the Company had deferred tax assets calculated at an expected blended rate of 34% of approximately $235,264, principally arising from net operating loss carryforwards (NOL) for income tax purposes. As management of the Company cannot determine that it is more likely than not that the Company will realize the benefit of its deferred tax assets, a valuation allowance equal to the deferred tax assets has been established at December 31, 2006. The significant components of the deferred tax asset are as follows:
| | December 31, |
| | 2006 | | 2005 |
NOL - Beginning of year | | $ | 310,888 | | $ | - |
Expiration of NOL | | | - | | | - |
Loss for year | | | 381,066 | | | 310,888 |
NOL - End of year | | $ | 691,954 | | $ | 310,888 |
| | | | | | |
Deferred tax asset | | $ | 235,264 | | $ | 105,702 |
Deferred tax asset valuation allowance | | | (235,264) | | | (105,702) |
Net deferred tax assets | | $ | - | | $ | - |
At December 31, 2006, the Company has net operating tax loss carryforwards of approximately $691,954, which expire in the years 2025 through 2026. The change in the allowance account was an increase of $129,562 for the year ending December 31, 2006.
NOTE 7 - CAPITAL STOCK
Preferred Stock
The Company has authorized 10,000,000 shares of no par preferred stock. No preferred shares have been designated or issued by the Company.
Common Stock
The Company has authorized 40,000,000 shares of its common stock, $0.001 par value. The Company had issued and outstanding 20,000,000 shares of its common stock at December 31, 2005 and 2006, respectively.
NOTE 8 - COMMON STOCK OPTIONS
In 2005, the Company adopted the 2005 Stock Option/Restricted Stock, (hereinafter "the Plan") under which 5,450,000 shares of common stock are reserved for issuance under qualified options, nonqualified options, stock appreciation rights and other awards as set forth in the Plan.
F-12
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006
Under the Plan, qualified options are available for issuance to employees of the Company and nonqualified options are available for issuance to consultants and advisors. The Plan provides that the exercise price of a qualified option cannot be less than the fair market value on the date of grant and the exercise price of a nonqualified option must be determined on the date of grant. Options granted under the Plan generally vest three to five years from the date of grant and generally expire ten years from the date of grant.
During 2005 and 2006, the Company agreed to issue a total of 575,000 common stock options at an exercise price of $0.10 per share to consultants to the Company. The options fair value was determined to be $0 based upon the Company's calculation using the Black-Scholes option pricing calculation formula and the Company rescinded its agreement to issue options. Accordingly, no expense was recorded.
NOTE 9 - RELATED PARTY TRANSACTIONS
Two officers who are also members of the Company's board of directors served as consultants to the Company on various business, strategic, and technical issues. The Company paid and expensed a total of $143,000 for these services in 2005 and $144,000 for these services in 2006. In addition to the fees, reimbursable expenses were also paid under this contract. The total amount paid for these expenses was $43,187 in 2005 and $41,040 in 2006. The use of officers as contractors is considered an interim arrangement while the Company was in startup operations.
__________
F-13
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
FINANCIAL STATEMENTS
DECEMBER 31, 2006
(Stated in U.S. Dollars)
F-14

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM To the Directors and Stockholders of
CellCyte Genetics Corporation
(formerly Shepard Inc)
(A Development Stage Company) We have audited the accompanying balance sheets of CellCyte Genetics Corporation. (formerly Shepard Inc.) (a development stage company) as at December 31, 2006 and 2005, and the related statements of operations, cash flows, and stockholders' equity (deficiency) for each of the two years in the period ended December 31, 2006, and for the cumulative period from March 9, 2004 (date of inception) to December 31, 2006. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion. In our opinion, these financial statements present fairly, in all material respects, the financial position of the Company as at December 31, 2006 and 2005, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2006 and 2005, and for the cumulative period from March 9, 2004 (date of inception) to December 31, 2006, in conformity with United States generally accepted accounting principles. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered net losses and negative cash flows from operations since its inception that raise substantial doubt about its ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. |
Vancouver, Canada February 28, 2007 | /s/ Morgan & Company Chartered Accountants |

|
F-15
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
BALANCE SHEETS
(Stated in U.S. Dollars)
| | DECEMBER 31 |
| | 2006 | 2005 |
| | | | |
ASSETS | | | | |
| | | | | |
Current | | | | |
Cash and cash equivalents | $ | 181 | $ | 18,669 |
| | | | | |
LIABILITIES | | | | |
| | | | | |
Current | | | | |
Accounts payable and accrued liabilities | $ | 21,774 | $ | 7,205 |
Due to related party (Note 4) | | 3,300 | | - |
| | | 25,074 | | 7,205 |
| | | | | |
STOCKHOLDERS' EQUITY (DEFICIENCY) | | | | |
| | | | | |
Share Capital | | | | |
Authorized: | | | | |
525,000,000 common shares with a par value of $0.001 | | | | |
| | | | | |
Issued: | | | | |
38,990,000 common shares (2005-38,990,000 common shares)
| | 38,990 | | 38,990 |
| | | | | |
Additional paid-in capital | | (3,990) | | (3,990) |
| | | | | |
Deficit Accumulated During The Exploration Stage | | (59,893) | | (23,536) |
| | | (24,893) | | 11,464 |
| | | | | |
| | $ | 181 | $ | 18,669 |
See accompanying notes
F-16
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
STATEMENTS OF OPERATIONS
(Stated in U.S. Dollars)
| YEAR
ENDED
DECEMBER 31 | YEAR
ENDED
DECEMBER 31 | CUMULATIVE
PERIOD FROM
INCEPTION
MARCH 9
2004 TO
DECEMBER 31 |
|
|
|
|
|
| 2006 | 2005 | 2006 |
| | | | | |
Revenue | $ | - | $ | - | $ | - |
| | | | | | |
Expenses | | | | | | |
Mineral interest acquisition costs | | - | | - | | 2,053 |
Exploration expenses | | - | | 5,000 | | 5,000 |
Office and sundry | | 1,574 | | 1,354 | | 3,354 |
Professional fees | | 20,926 | | 10,543 | | 33,684 |
Regulatory fees | | 315 | | 1,445 | | 1,760 |
Transfer agent fees | | 13,542 | | 500 | | 14,042 |
| | | | | | |
Net Loss | $ | (36,357) | $ | (18,842) | $ | (59,893) |
| | | | | | |
| | | | | | |
Net Loss Per Share | $ | (0.00) | $ | (0.00) | | |
| | | | | | |
| | | | | | |
Weighted Average Number Of Shares Outstanding | |
38,990,000
| |
38,990,000
| | |
See accompanying notes
F-17
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
STATEMENTS OF CASH FLOWS
(Stated in U.S. Dollars)
| | | CUMULATIVE |
| | | PERIOD FROM |
| | | INCEPTION |
| YEAR | YEAR | MARCH 9 |
| ENDED | ENDED | 2004 TO |
| DECEMBER 31 | DECEMBER 31 | DECEMBER 31 |
| 2006 | 2005 | 2006 |
| | | | | | |
Cash Flows From Operating Activities | | | | | | |
Net Loss | $ | (36,357) | $ | (18,842) | $ | (59,893) |
| | | | | | |
Items to reconcile net loss to net cash used in operating
activities | | | | | | |
Mineral interest acquisition costs | | - | | - | | 2,053 |
Accounts payable and accrued liabilities | |
14,569
| |
2,572
| |
21,774
|
Due to related party | | 3,300 | | | | 3,300 |
| | (18,488) | | (16,270) | | (32,766) |
| | | | | | |
Cash Flows From Financing Activity | | | | | | |
Issue of share capital | | - | | - | | 35,000 |
| | | | | | |
Cash Flows From Investing Activity | | | | | | |
Mineral interest acquisition costs | | - | | - | | (2,053) |
| | | | | | |
Increase (Decrease) In Cash And Cash Equivalents During The Year | |
(18,488)
| |
(16,270)
| |
181
|
| | | | | | |
Cash And Cash Equivalents, Beginning Of Year | |
18,669
| |
34,939
| |
-
|
| | | | | | |
Cash And Cash Equivalents, End Of Year | $ | 181 | $ | 18,669 | $ | 181 |
| | | | | | |
| | | | | | |
Supplementary Disclosure Of Cash Flow Information | | | | | | |
Interest paid | $ | - | $ | - | $ | - |
Income taxes paid | $ | - | $ | - | $ | - |
See accompanying notes
F-18
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS' EQUITY
PERIOD FROM DATE OF INCEPTION, MARCH 9, 2004,
TO DECEMBER 31, 2006
| | | | | DEFICIT | |
| | | | | ACCUMULATED | |
| | COMMON SHARES | ADDITIONAL | DURING THE | |
| | | PAR | PAID-IN | EXPLORATION | |
| | NUMBER | VALUE | CAPITAL | STAGE | TOTAL |
| | | | | | | | | | |
Balance, March 9, 2004 (Date of inception) | - | $ | - | $ | - | $ | - | $ | - |
| | | | | | | | | | |
Capital stock issued for cash: | | | | | | | | | |
May 2004 at $0.001 | 3,000,000 | | 3,000 | | - | | - | | 3,000 |
September 2004 at $0.01 | 2,500,000 | | 2,500 | | 22,500 | | - | | 25,000 |
September 2004 at $0.10 | 70,000 | | 70 | | 6,930 | | - | | 7,000 |
Net Loss | - | | - | | - | | (4,694) | | (4,694) |
| | | | | | | | | | |
Balance, December 31, 2004 | 5,570,000 | | 5,570 | | 29,430 | | (4,694) | | 30,306 |
| | | | | | | | | | |
Net Loss | - | | - | | - | | (18,842) | | (18,842) |
| | | | | | | | | | |
Balance, December 31, 2005 | 5,570,000 | | 5,570 | | 29,430 | | (23,536) | | 11,464 |
| | | | | | | | | | |
December 22, 2006 - Stock split adjustment | 33,420,000 | | | | | | | | |
| | | | | | | | | | |
Net Loss | - | | - | | - | | (36,357) | | (36,357) |
| | | | | | | | | | |
Balance, December 31, 2006 | 38,990,000 | $ | 5,570 | $ | 29,430 | $ | (59,893) | $ | (24,893) |
All share amounts have been retroactively adjusted to reflect the 7 for 1 forward common stock split on December 22, 2006.
See accompanying notes
F-19
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
1. NATURE OF BUSINESS
Organization
The Company was incorporated in the State of Nevada, U.S.A., on March 9, 2004.
Exploration Stage Activities
The Company has been in the exploration stage since its formation and has not yet realized any revenues from its planned operations. The Company commenced its exploration stage activities by acquiring an exploration prospect in the mining sector.
Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern.
As shown in the accompanying financial statements, the Company has incurred a net loss of $59,893 for the period from March 9, 2004 (inception) to December 31, 2006, and has no revenue. The future of the Company is dependent upon its ability to obtain financing and upon future profitable operations from the development of its mineral properties. Management has plans to seek additional capital through a private placement of its common stock. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts of and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
a) Use of Estimates and Assumptions
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
b) Cash and Cash Equivalents
Cash and cash equivalents consist of all cash balances and all highly liquid investments purchased with a maturity of three months or less.
F-20
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
c) Exploration Costs
The Company expenses all exploration costs as incurred.
d) Mineral Property Interests
The Company capitalizes acquisition and option costs of mineral property rights. The amount capitalized represents fair value at the time the mineral rights were acquired. The accumulated costs of acquisition for properties that are developed to the stage of commercial production will be amortized using the unit-of-production method.
e) Asset Impairment
Long-lived assets are tested for recoverability whenever events or changes in circumstances indicate the carrying amount may not be recoverable, pursuant to guidance established in Statement of Financial Accounting Standards No. 144 ("SFAS 144"), "Accounting for the Impairment or Disposal of Long-lived Assets". The Company determines impairment by comparing the undiscounted future cash flows estimated to be generated by its assets to their respective carrying amounts. If impairment is deemed to exist, the assets will be written down to fair value.
f) Foreign Currency Translation
The Company's functional currency is the U.S. dollar. Transactions in foreign currency are translated into U.S. dollars as follows:
i) monetary items at the rate prevailing at the balance sheet date;
ii) non-monetary items at the historical exchange rate;
iii) revenue and expense at the average rate in effect during the applicable accounting period.
Gain or loss on foreign currency transactions are included in the statement of operations.
F-21
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
g) Fair Value of Financial Instruments
The carrying value of cash and cash equivalents, accounts payable and accrued liabilities, and amounts due to related party approximates their fair value because of the short maturity of these instruments. The Company's operations are in Canada and virtually all of its assets and liabilities are giving rise to significant exposure to market risks from changes in foreign currency rates. The Company's financial risk is the risk that arises from fluctuations in foreign exchange rates and the degree of volatility of these rates. Currently, the Company does not use derivative instruments to reduce its exposure to foreign currency risk.
h) Income Taxes
Deferred taxes are provided on an asset and liability method whereby deferred tax assets are recognized for taxable temporary differences, and operating loss, tax credit carryforwards and deferred tax liabilities are recognized for deductible temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases.
i) Basic and Diluted Net Loss Per Share
The Company computes net loss per share in accordance with SFAS No. 128, "Earnings per Share". SFAS No. 128 requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net loss available to common shareholders by the weighted average number of shares outstanding during the period. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock. The Company has no potentially issuable common stock at December 31, 2006.
j) Adoption of New Accounting Policy
Stock - Based Compensation
The Company has a stock-based compensation plan, whereby stock options are granted in accordance with the policies of regulatory authorities.
F-22
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
j) Adoption of New Accounting Policy (Continued)
Prior to January 1, 2006, the Company accounted for stock-based awards under the recognition and measurement provisions of Accounting Principles Board Opinion ("APB") No. 25, "Accounting for Stock Issued to Employees" using the intrinsic value method of accounting, under which compensation expense was only recognized if the exercise price of the Company's employee stock options was less than the market price of the underlying common stock on the date of grant. Effective January 1, 2006, the Company adopted the fair value recognition provisions of SFAS No. 123R "Share Based Payments", using the modified prospective transition method. Under that transition method, compensation cost is recognized for all share-based payments granted prior to, but not yet vested as of January 1, 2006, based on the grant date fair value estimated in accordance with the original provisions of SFAS No. 123, and compensation cost for all share-based payments granted subsequent to January 1, 2006, based on the gran t-date fair value estimated in accordance with the provisions of SFAS 123R. Results for prior periods have not been restated. There was no change to the Company income from operations for the period ended December 31, 2006, than if it had continued to account for share-based compensation under APB No. 25.
All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued.
k) Comprehensive Loss
SFAS No. 130, "Reporting Comprehensive Income," establishes standards for the reporting and display of comprehensive loss and its components in the financial statements. As at December 31, 2006, the Company has no items that represent a comprehensive loss and, therefore, has not included a schedule of comprehensive loss in the financial statements.
F-23
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
3. RECENT ACCOUNTING PRONOUNCEMENT
In February 2006, the FASB issued SFAS No. 155, "Accounting for Certain Hybrid Financial Instruments-an amendment of FASB Statements No. 133 and 140", to simplify and make more consistent the accounting for certain financial instruments. SFAS No. 155 amends SFAS No. 133, "Accounting for Derivative Instruments and Hedging Activities", to permit fair value re-measurement for any hybrid financial instrument with an embedded derivative that otherwise would require bifurcation, provided that the whole instrument is accounted for on a fair value basis. SFAS No. 155 amends SFAS No. 140, "Accounting for the Impairment or Disposal of Long-Lived Assets", to allow a qualifying special-purpose entity to hold a derivative financial instrument that pertains to a beneficial interest other than another derivative financial instrument. SFAS No. 155 applies to all financial instruments acquired or issued after the beginning of an entity's first fiscal year that begins after September 15, 2006, with earl ier application allowed. The adoption of this statement is not expected to have a material effect on the Company's future reported financial position or results of operations.
In March 2006, the FASB issued SFAS No. 156, "Accounting for Servicing of Financial Assets, an amendment of FASB Statement No. 140, Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities". This statement requires all separately recognized servicing assets and servicing liabilities be initially measured at fair value, if practicable, and permits for subsequent measurement using either fair value measurement with changes in fair value reflected in earnings or the amortization and impairment requirements of Statement No. 140. The subsequent measurement of separately recognized servicing assets and servicing liabilities at fair value eliminates the necessity for entities that manage the risks inherent in servicing assets and servicing liabilities with derivatives to qualify for hedge accounting treatment and eliminates the characterization of declines in fair value as impairments or direct write-downs. SFAS No. 156 is effective for an entity's first fisca l year beginning after September 15, 2006. The adoption of this statement is not expected to have a material effect on the Company's future reported financial position or results of operations.
In September 2006, the FASB issued SFAS No. 157, "Fair Value Measurements". The objective of SFAS 157 is to increase consistency and comparability in fair value measurements and to expand disclosures about fair value measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 applies under other accounting pronouncements that require or permit fair value measurements and does not require any new fair value measurements. The provisions of SFAS No. 157 are effective for fair value measurements made in fiscal years beginning after November 15, 2007. The adoption of this statement is not expected to have a material effect on the Company's future reported financial position or results of operations.
F-24
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
3. RECENT ACCOUNTING PRONOUNCEMENT (Continued)
In September 2006, the FASB issued SFAS No. 158, "Employers' Accounting for Defined Benefit Pension and Other Postretirement Plans - an amendment of FASB Statements No. 87, 88, 106, and 132(R)". This statement requires employers to recognize the over-funded or under-funded status of a defined benefit postretirement plan (other than a multiemployer plan) as an asset or liability in its statement of financial position and to recognize changes in that funded status in the year in which the changes occur through comprehensive income of a business entity or changes in unrestricted net assets of a not-for-profit organization. This statement also requires an employer to measure the funded status of a plan as of the date of its year-end statement of financial position, with limited exceptions. The provisions of SFAS No. 158 are effective for employers with publicly traded equity securities as of the end of the fiscal year ending after December 15, 2006. The adoption of this statement is not expect ed to have a material effect on the Company's future reported financial position or results of operations.
4. DUE TO RELATED PARTY
Amounts due to a director are non-interest bearing, unsecured and repayable on demand. The loan is unsecured and repayable on demand.
5. MINERAL PROSPECT
Pursuant to an agreement dated June 9, 2004, the Company has acquired a 100% interest, subject to a 2.5% production royalty, in a mineral prospect located in the Northwest Territories, Canada, for cash consideration of $2,053 and conducted a work program of $5,000. As at December 31, 2006, the Company abandoned its mineral claim prospect.
6. COMMON STOCK
During the period from March 9, 2004 (inception) to December 31, 2004, the Company issued a total of 38,990,000 (post-split) common shares for total cash proceeds of $35,000.
On December 22, 2006, the Company effected a forward stock split on the basis of the issue of seven new common shares for the cancellation of 1 old common share. All share amounts have been retroactively adjusted for all periods presented.
At December 31, 2006, there were no outstanding stock options or warrants.
F-25
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2006 AND 2005
(Stated in U.S. Dollars)
7. SUBSEQUENT EVENTS
a) Proposed acquisition
On January 26, 2007, the Company entered into an agreement in principle with CellCyte Genetics, ("CellCyte"), a Washington corporation, and a shareholder of CellCyte (with the other shareholders of CellCyte to also be parties to a formal agreement to replace the agreement in principle) in connection with the proposed acquisition by the Company from the shareholders of CellCyte of all of the issued and outstanding shares in the capital of CellCyte.
The total purchase price for the shares of CellCyte consists of 16,000,000 restricted common shares in the capital of the Company. In addition, an officer and director of the Company is required to transfer an aggregate of 18,750,000 restricted common shares in the capital of the Company held by him to the shareholders of CellCyte and certain other shareholders of the Company will be required to transfer an aggregate of 2,500,000 common shares in the capital of the Company to the shareholders of CellCyte.
The Company, on its behalf and on behalf of certain investors, has agreed to use its commercially reasonable efforts to advance, by way of a loan to CellCyte, an aggregate principal sum of up to $1,000,000 bearing interest at a rate of 5% per annum, compounded semi-annually and not in advance, and maturing 120 calendar days from the date of the advancement of the principal sum. The principal sum and interest on the loan will be secured by way of a senior, subordinated (subordinated only to CellCyte's existing bank indebtedness), fixed and floating charge on all of the assets of CellCyte. Each of the investors will have the right and option, exercisable until the close of business on the date of maturity of the loan, to convert any principal sum, interest or any other sum owing under the loan to them into units of the Company at a price of $1.50 per unit, with each unit being comprised of one common share and one non-transferable share purchase warrant of the Company exercisable for a per iod ending on the earlier of 18 months from the date of issuance of the units and 12 months from the effective date of a registration statement to be filed to register the common shares underlying the warrants, if any.
Furthermore, an aggregate of $1,017,088 in principal and interest currently owing by CellCyte to certain of its creditors is expected to be settled through the issuance of units of the Company at a price of $1.50 per unit, with the terms and conditions of these units being the same as the units to be issued in respect of the loan described above.
All outstanding options to acquire shares in the capital of CellCyte will be exchanged for options to acquire an equivalent number of shares in the capital of the Company at an exercise price of $1.00 per share, and such options to acquire shares in the capital of CellCyte will be cancelled. All outstanding warrants to acquire shares in the capital of CellCyte will also be exchanged for warrants to acquire shares in the capital of the Company at a cashless exercise price of $1.50 per share, and the warrants to acquire shares in the capital of CellCyte will be cancelled.
F-26
The Company has agreed to raise, by way of a private placement of common shares or units of the Company, a minimum of $5,000,000 and a maximum of $6,500,000 at a subscription price of not less than $1.50 per common share or unit, with each unit being comprised of not greater than one common share and one non-transferable share purchase warrant of the Company, with each warrant being exercisable for not greater than one additional common share of the Company for a period ending at the earlier of 18 months from the date of issuance of the units and 12 months from the effective date of a proposed registration statement to register the common shares underlying the warrants, if any, at an exercise price of $3.00 per share. Not less than $4,000,000 from the private placement is to be advanced by the Company to CellCyte at the closing of the proposed purchase of the shares of CellCyte and up to $1,000,000 from the private placement may take the form of the loan described above.
b) Name change
On February 16, 2007, the Company changed its name to CellCyte Genetics Corporation in anticipation of the proposed acquisition of CellCyte Genetics Corporation, a Washington State company.
__________
F-27
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
PRO FORMA CONSOLIDATED BALANCE SHEET
DECEMBER 31, 2006
(Unaudited)
INTRODUCTION |
| |
PRO FORMA CONSOLIDATED BALANCE SHEET |
| |
NOTES TO PRO FORMA CONSOLIDATED BALANCE SHEET |
| |
F-28
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
PRO FORMA CONSOLIDATED BALANCE SHEET
(Unaudited)
INTRODUCTION
Effective January 19, 2007, CellCyte Genetics Corporation (formerly Shepard Inc.), (a development stage company), ("CGN" or the "Company"), a Nevada corporation, entered into an Agreement in Principle to acquire 100% of the issued and outstanding shares of CellCyte Genetics Corporation (a development stage enterprise) ("CGW"), a private Washington State corporation, in exchange for 16,000,000 restricted shares of CGN's common stock, and the transfer of a further 18,750,000 shares of CGN's restricted common stock and 2,500,000 shares of CGN's non-restricted common stock from certain shareholders of CGN to the shareholders of CGW. Effective March 14, 2007, the acquisition of CGW was completed by the execution of a Share Exchange Agreement (the "Agreement"), which, in addition to the share issuances and transfers described above, finalized the following terms of the acquisition: (i) the completion of a private placement of 3,981,165 units of CGN at a price of $1.50 per unit with each unit c onsisting of one common share of CGN and one warrant to acquire an additional common share of CGN at a price of $3.00 per share; (ii) the settlement of a total of $1,017,088 in debts of CGW through the issuance of units of CGN at a price of $1.50 per unit with each unit being consistent with the private placement units above; (iii) the replacement of 2,000,000 stock options previously outstanding in CGW with 2,000,000 stock options in CGN at a price of $1.50 per share; and (iv) the issuance of 205,000 restricted shares of CGN's common stock in exchange for 205,000 share purchase warrants previously outstanding in CGW.
As a result of the completion of this transaction and the other assumptions contained herein, the former shareholders of CGW will own approximately 62% of the outstanding shares of common stock of CGN representing 37,250,000 of the anticipated 59,854,255 total issued and outstanding shares of common stock of CGN.
Accordingly, the acquisition will be accounted for as a recapitalization using accounting principles applicable to reverse acquisitions whereby the financial statements subsequent to the date of the transaction will be presented as a continuation of CGW. Under reverse acquisition accounting CGW (the legal subsidiary) will be treated as the accounting parent (acquirer) and CGN (the legal parent) will be treated as the accounting subsidiary (acquiree). The value assigned to the common stock of consolidated CGN on acquisition of CGW will be equal to the book value of the common stock of CGW plus the book value of the net assets of CGN as at the date of the acquisition, less costs of the transaction.
The pro forma consolidated balance sheet has been prepared to reflect the consolidated balance sheet of CGN as at December 31, 2006 assuming the acquisition of CGW had occurred effective December 31, 2006. As the results of operations of consolidated CGN are considered to be a continuation of the results of CGW, and as the audited financial statements of CGW as at December 31, 2006, and for the period from January 14, 2005 (inception) to December 31, 2006, have been included in the Company's filing on Form 8-K/A, no pro forma statements of operations have been presented.
The pro forma consolidated balance is based on the following financial statements:
CGN - audited balance sheet as at December 31, 2006.
CGW - audited balance sheet as at December 31, 2006.
This pro forma consolidated balance sheet should be read in conjunction with CGN's December 31, 2006 audited financial statements as filed on Form 10-KSB and CGW's audited financial statements as at December 31, 2006 included in CGN's filing on Form 8-K/A dated April 5, 2007.
F-29
CELLCYTE GENETICS CORPORATION | | | | | | | | | | |
(A DEVELOPMENT STAGE COMPANY) | | | | | | | | | | |
PRO FORMA CONSOLIDATED BALANCE SHEET | | | | | | | | | | |
DECEMBER 31, 2006 | | | | | | | | | | | |
(unaudited) | | | | | | | | | | (expressed in United States dollars) |
| | | | | | | | | | | | | | |
| | | | | CellCyte | CellCyte | | | | | | | | Pro forma |
| | | | | (Nevada) | (Wash) | Consolidated | Consolidated |
| | | | | CGN | CGW | Pro forma Adjustments | CGN |
| | | | 31-Dec-06 | 31-Dec-06 | (a) | (b) | (c) | (d) | (e) | (f) | (g) | 31-Dec-06 |
| | | | | | | | | | | | | | |
ASSETS | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
CURRENT ASSETS | | | | | | | | | | | |
| | Cash and cash equivalents | $ 181 | $ 287,750 | $ - | $ - | $ - | $5,975,751 | $ - | $ - | $ - | $ 6,263,682 |
| Deposits | | - | 1,200 | - | - | - | - | - | - | - | 1,200 |
| | | | | | | | | | | | | | |
| Total Current Assets | 181 | 288,950 | - | - | - | 5,975,751 | - | - | - | 6,264,882 |
| | | | | | | | | | | | | | |
OTHER ASSETS | | | | | | | | | | | | |
| Intellectual property | - | 369,666 | - | - | - | - | - | - | - | 369,666 |
| | | | | | | | | | | | | | |
| Total Other Assets | - | 369,666 | - | - | - | - | - | - | - | 369,666 |
| | | | | | | | | | | | | | |
TOTAL ASSETS | | | $ 181 | $ 658,616 | $ - | $ - | $ - | $5,975,751 | $ - | $ - | $ - | $ 6,634,548 |
| | | | | | | | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | | | |
| | | | | | | | | | | | | | |
CURRENT LIABILITIES | | | | | | | | | | | |
| | Accounts payable and accrued liabilities | $ 21,774 | $ 350,064 | $ - | $ - | $ - | $- | $ - | $ - | $ - | $ 371,838 |
| | Accrued interest | | - | 74,021 | (74,021) | - | - | - | - | - | - | - |
| | Convertible notes | | - | 400,000 | (400,000) | - | - | - | - | - | - | - |
| Due to related parties | 3,300 | - | - | - | - | - | - | - | - | 3,300 |
| | | | | | | | | | | | | | |
| Total Current Liabilities | 25,074 | 824,085 | (474,021) | - | - | - | - | - | - | 375,138 |
| | | | | | | | | | | | | | |
LONG TERM LIABILITES | | | | | | | | | | | |
| Convertible notes | | - | 535,000 | (535,000) | - | - | - | - | - | - | - |
| | | | | | | | | | | | | | |
| Total long term liabilities | - | 535,000 | (535,000) | - | - | - | - | - | - | - |
| | | | | | | | | | | | | | |
STOCKHOLDERS' EQUITY (DEFICIENCY) | | | | | | | | | | |
| | Common stock | | 5,570 | 200 | - | - | - | - | (5,570) | - | 59,654 | 59,854 |
| | Additional paid in capital | 29,430 | - | 1,017,088 | 1,780,000 | 246,000 | 5,975,751 | (3,072,518) | 992,195 | (1,457,654) | 5,510,292 |
| | Common stock purchase warrants | - | - | - | - | - | - | - | - | 1,398,000 | 1,398,000 |
| Deficit | | | (59,893) | (700,669) | (8,067) | (1,780,000) | (246,000) | - | 2,085,893 | - | - | (708,736) |
| | | | | | | | | | | | | | |
| Total Stockholders' Equity (deficiency) | (24,893) | (700,469) | 1,009,021 | - | - | 5,975,751 | (992,195) | 992,195 | - | 6,259,410 |
| | | | | | | | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | $ 181 | $ 658,616 | $ - | $ - | $ - | $5,975,751 | $(992,195) | $ 992,195 | $ - | $ 6,634,548 |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of this pro forma consolidated balance sheet |
F-30
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
PRO FORMA CONSOLIDATED BALANCE SHEET
(Unaudited)
NOTE 1 - ACQUISITION
By way of an Agreement in Principle effective January 19, 2007, the terms of which were finalized by Share Exchange Agreement completed Marcy 14, 2007, CGN acquired 100% of the issued and outstanding common shares of CGW. The completion of the terms of the Agreement will result in the former shareholders of CGW owning approximately 62% of the issued and outstanding shares of CGN's common. Under the terms of the Agreement, CGN has agreed to:
- issue 16,000,000 restricted shares of common stock to the shareholders of CGW;
- arrange for the transfer of 18,750,000 restricted shares of CGN's common stock from a Director of CGN to the shareholders of CGW;
- arrange for the transfer of 2,500,000 unrestricted shares of CGN's common stock from certain shareholders of CGN to the shareholders of CGW;
- complete a private placement for proceeds of between $5,000,000 and $6,500,000 through the issuance of units of CGN at a price of $1.50 per unit with each unit consisting of one share of CGN's common stock and one share purchase warrant entitling the holder to acquire an additional share of CGN's common stock at a price of $3.00 per share. The share purchase warrants are exercisable for a period ending the later of (i) 18 months from the date of issuance and (ii) 12 months following the effective date of registration statement, if any, pursuant to which the warrants are proposed for registration;
- settle a total of $1,017,088 in debts of CGW through the issuance of units of CGN at a price of $1.50 per unit with each unit being consistent with the private placement units described above;
- grant 2,000,000 options to acquire shares of CGN's common stock at a price of $1.50 per share to replace stock options outstanding in CGW; and
- issue 205,000 restricted shares of CGN's common stock in exchange for the cancellation of a total of 205,000 share purchase warrants outstanding in CGW.
This pro forma consolidated balance sheet has been prepared assuming the completion of the proposed acquisition and all related conditions having been satisfied effective December 31, 2006.
For purposes of this pro forma consolidated balance sheet the acquisition has been accounted for as a recapitalization using accounting principles applicable to reverse acquisitions with CGW (the legal subsidiary) being treated as the accounting parent (acquirer) and CGN (the legal parent) being treated as the accounting subsidiary (acquiree). Under reverse acquisition accounting the value assigned to the common stock of consolidated CGN on Acquisition of CGW will be equal to the book value of the common stock of CGW plus the book value of the net assets of CGN as at the date of the acquisition, less costs of the transaction.
The pro forma book value of CGN's capital stock as at December 31, 2006 is calculated as follows:
| | |
CGW capital stock | $ 200 |
CGN adjusted net assets - from 2(e) below | 992,195 |
| | |
| | 992,395 |
Concurrent private placement financing - from 2(d) below | 5,975,751 |
| | |
CGN pro forma capital stock, December 31, 2006 | $ 6,968,146 |
F-31
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
PRO FORMA CONSOLIDATED BALANCE SHEET
(Unaudited)
NOTE 1 - ACQUISITION (continued)
CGN's pro forma capital stock as at December 31, 2006 is made up as follows: | |
| | |
Capital stock | $ 59,854 |
Additional paid-in capital | 5,510,292 |
Common stock purchase warrants | 1,398,000 |
| | |
| | $ 6,968,146 |
NOTE 2 - PRO FORMA ADJUSTMENTS
(a) Record shares issued by CGN on settlement of Debts of CGW
As a condition of the Share Exchange Agreement, CGN agreed to settle a total of $1,017,088 of debts of CGW through the issuance of 678,060 units at a price of $1.50 per unit with each unit consisting of one share of CGN's common stock and one share purchase warrant entitling the holder to acquire an additional share of CGN's common stock at a price of $3.00 per share. The share purchase warrants are exercisable for a period ending the later of (i) 18 months from the date of issuance and (ii) 12 months following the effective date of registration statement, if any, pursuant to which the warrants are proposed for registration. As at December 31, 2006, the CGW financial statements reflect a total of $1,099,021 of Convertible notes and accrued interest in connection with the amounts to be settled. A pro forma adjustment has been recorded to reflect (i) additional interest accrued to the date of settlement of $8,067 and (ii) settlement of the debts effective December 31, 2006. The estim ated fair value of the warrants issued of $203,000 has been recorded as a separate component of stockholders' equity on a pro forma basis in Note 2(g). The fair value of the warrants was estimated using the Black-Scholes option pricing model with the following assumptions: risk free interest rate of 4.90%; dividend yield of 0%; expected volatility of 100%; and an expected life of the warrants of 1.5 years.
(b) Record fair value of stock options
In connection with the acquisition, CGN has agreed to grant 2,000,000 stock options as replacements of and in exchange for the cancellation of all existing stock options outstanding in CGW. Accounting principles applicable to business combinations require that the fair value of stock options granted in connection with a business combination, to the extent that the options relate to prior service, be recorded as part of the business combination purchase price at fair value. A pro forma adjustment has been made to record the fair value of 2,000,000 stock options granted at an exercise price of $1.50 per share. The options were valued in accordance with the provisions of SFAS No. 123R by applying the fair value method using the Black-Scholes option-pricing model.
The fair value of the stock options was estimated to be $1,780,000 by using the Black-Scholes option pricing model with the following assumptions: risk free interest rate of 4.70%; dividend yield of 0%; expected volatility of 100%; and an expected life of the options of 5 years. As these options relate to prior service and are fully vested when granted, the entire amount of the fair value has been recorded as part of the purchase price.
The value assigned to the shares issued in connection with this reverse acquisition is calculated as described in Note 1 and, as a result, amounts that would otherwise form part of the purchase price for standard business combinations are charged to the deficit of the legal parent for reverse acquisitions. A pro forma adjustment has been made to charge the fair value of the stock options $1,780,000 to the deficit of CGN which is subsequently eliminated in pro forma adjustment 2(e).
F-32
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
PRO FORMA CONSOLIDATED BALANCE SHEET
(Unaudited)
NOTE 2 - PRO FORMA ADJUSTMENTS (continued)
(c) Record shares issued by CGN for share purchase warrants of CGW
In connection with the acquisition, CGN has agreed to issue 205,000 restricted shares of common stock to certain holders of warrants to acquire shares of the common stock of CGW in exchange for the cancellation of the CNW share purchase warrants. The fair value of the 205,000 restricted shares of common stock was estimated to be $246,000. Accounting principles applicable to business combinations require that the fair value of these shares issued in exchange for the cancellation of the CGW share purchase warrants be recorded as part of the business combination purchase price.
The value assigned to the shares issued in connection with this reverse acquisition is calculated as described in Note 1 and, as a result, amounts that would otherwise form part of the purchase price for standard business combinations are charged to the deficit of the legal parent for reverse acquisitions. A pro forma adjustment has been made to charge the fair value of these shares of common stock of $246,000 to the deficit of CGN which is subsequently eliminated in pro forma adjustment 2(e).
(d) Record CGN concurrent private placement financing
In connection with the acquisition, CGN agreed to conduct a private placement for proceeds of between $5,000,000 and $6,500,000 through the issuance of units of CGN at a price of $1.50 per unit with each unit consisting of one share of CGN's common stock and one share purchase warrant entitling the holder to acquire an additional share of CGN's common stock at a price of $3.00 per share. The share purchase warrants are exercisable for a period ending the later of (i) 18 months from the date of issuance and (ii) 12 months following the effective date of registration statement, if any, pursuant to which the warrants are proposed for registration. A pro forma adjustment has been recorded to reflect the actual private placement proceeds raised of $5,975,751 on the issuance of 3,981,165 units assumed to be effective December 31, 2006. The estimated fair value of the warrants issued of $1,195,000 has been recorded as a separate component of stockholders' equity on a pro forma basis in Not e 2(g). The fair value of the warrants was estimated using the Black-Scholes option pricing model with the following assumptions: risk free interest rate of 4.90%; dividend yield of 0%; expected volatility of 100%; and an expected life of the warrants of 1.5 years.
(e) Elimination of CGN's stockholder's equity
In accordance with reverse acquisition accounting the financial statements subsequent to the date of the acquisition will be presented as a continuation of CGW and, as a result, the stockholders' equity of CGN, which is equal to the book value of net assets, has been eliminated as follows:
| |
Capital Stock
| Additional Paid-in Capital |
Deficit
| Total Stockholder's Equity |
| | | | | |
CGN - balance, December 31, 2006 | $ 5,570 | $ 29,430 | $ (59,893) | $ (24,893) |
Pro forma adjustment (a) | - | 1,017,088 | - | 1,017,088 |
Pro forma adjustment (b) | - | 1,780,000 | (1,780,000) | - |
Pro forma adjustment (c) | - | 246,000 | (246,000) | - |
| | | | | |
CGN - balance before elimination | 5,570 | 3,072,518 | (2,085,893) | 992,195 |
| | | | | |
Pro forma adjustment (e), to eliminate | (5,570) | (3,072,518) | 2,085,893 | (992,195) |
| | | | | |
| | $ - | $ - | $ - | $ - |
F-33
CELLCYTE GENETICS CORPORATION
(Formerly Shepard Inc.)
(A Development Stage Company)
PRO FORMA CONSOLIDATED BALANCE SHEET
(Unaudited)
NOTE 2 - PRO FORMA ADJUSTMENTS (continued)
(f) Record value assigned to CGN under reverse acquisition accounting
As at the date of the acquisition, CGN did not have any significant operations or assets and control of CGN was passed to the former shareholders of CGW. As a result, the transaction will be accounted for as a recapitalization using accounting principles applicable to reverse acquisitions and, accordingly, no goodwill is recorded and the value assigned to CGN is equal to the book value of the net assets of CGN as at the date of the acquisition. As at December 31, 2006, the adjusted book value of the net assets of CGN (as calculated above) is $992,195.
(g) Restatement of share capital under reverse acquisition accounting
In accounting for this reverse acquisition the legal share capital is that of CGN (the legal parent) and the value of share capital is calculated as described in Note 1. Upon completion of the proposed Acquisition, CGN will have 59,854,255 of its $US 0.0001 par value common shares issued and outstanding. In addition, CGN will have 4,659,225 common stock purchase warrants outstanding with a fair value as described and calculated in (a) and (d) above. A pro forma adjustment is required to reconcile the pro forma consolidated share capital as follows:
| |
Capital Stock
|
Additional Paid-inCapital
| Common Stock PurchaseWarrants |
Total ShareCapital
|
| | | | | |
CGN as at December 31, 2006 | $ 5,570 | $ 29,430 | $ - | $ 35,000 |
CGW as at December 31, 2006 | 200 | - | - | 200 |
Pro forma adjustment (a) | - | 1,017,088 | - | 1,017,088 |
Pro forma adjustment (b) | - | 1,780,000 | - | 1,780,000 |
Pro forma adjustment (c) | - | 246,000 | - | 246,000 |
Pro forma adjustment (d) | - | 5,975,751 | - | 5,975,751 |
Pro forma adjustment (e) | (5,570) | (3,072,518) | - | (3,078,088) |
Pro forma adjustment (f) | - | 992,195 | - | 992,195 |
Pro forma adjustment (g), to reconcile | 59,654 | (1,457,654) | 1,398,000 | - |
| | | | | |
Pro forma balance,
December 31, 2006 (unaudited) |
$ 59,854
|
$ 5,510,292
|
$ 1,398,000
|
$ 6,968,146
|
__________
F-34
Dealer Prospectus Delivery Obligation
Until ___________, 2007, all dealers that effect transactions in these securities, whether or not participating in the offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 24. INDEMNIFICATION OF OFFICERS AND DIRECTORS
Our officers and directors are indemnified as provided by theNevada Revised Statutes ("NRS"), our Articles of Incorporation and our Bylaws.
NRS Section 78.7502 provides that:
(i) a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys' fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful;
(ii) a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys' fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if he acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jur isdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper; and
(iii) to the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding, or in defense of any claim, issue or matter therein, the corporation shall indemnify him against expenses, including attorneys' fees, actually and reasonably incurred by him in connection with the defense.
NRS Section 78.751 provides that we may make any discretionary indemnification only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances. The determination must be made:
(a) by our stockholders;
(b) by our board of directors by majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding;
(c) if a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent legal counsel in a written opinion;
(d) if a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal counsel in a written opinion; or
(e) by court order.
II-1
Our Certificate of Incorporation and Articles provide that no director or officer shall be personally liable to our company, any of our stockholders or any other for damages for breach of fiduciary duty as a director or officer involving any act or omission of such director or officer unless such acts or omissions involve intentional misconduct, fraud or a knowing violation of law, or the payment of dividends in violation of the General Corporate Law of Nevada.
Further, our Bylaws provide that we shall, to the fullest and broadest extent permitted by law, indemnify all persons whom we may indemnify pursuant thereto. We may, but shall not be obligated to, maintain insurance, at our expense, to protect ourselves and any other person against any liability, cost or expense. We shall not indemnify persons seeking indemnity in connection with any threatened, pending or completed action, suit or proceeding voluntarily brought or threatened by such person unless such action, suit or proceeding has been authorized by a majority of the entire Board of Directors.
Insofar as indemnification for liabilities arising under the Securities Act might be permitted to directors, officers or persons controlling our company under the provisions described above, we have been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ITEM 25. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following is a list of the expenses to be incurred by us in connection with the preparation and filing of this registration statement. All amounts shown are estimates except for the SEC registration fee:
SEC registration fee | $1,578.80 |
Accounting fees and expenses | $5,000.00 |
Legal fees and expenses | $30,000.00 |
Transfer agent and registrar fees | $2,500.00 |
Fees and expenses for qualification under state securities laws | $1,500.00 |
Miscellaneous (including Edgar filing fees) | $2,000.00 |
Total: | $42,578.80 |
We are paying all expenses of the offering listed above. No portion of these expenses will be borne by the selling shareholders. The selling shareholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage or underwriting discounts or commissions paid by the selling shareholders to broker-dealers in connection with the sale of their shares.
ITEM 26. RECENT SALES OF UNREGISTERED SECURITIES
During the past three years, we have sold unregistered securities in private placement offerings as follows:
On March 30, 2007, we issued an aggregate of 678,060 Settlement Units at a deemed price of $1.50 per Settlement Unit, with each Settlement Unit consisting of one share of our common stock and one Settlement Warrant, to certain of the Selling Shareholders named herein, in connection with the settlement of an aggregate of $1,017,088 in principal and interest owing by CellCyte to certain of its creditors. Each Settlement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share commencing on the date of issuance for a period ending on the earlier of 18 months from the date of issuance of the Settlement Units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
II-2
On March 30, 2007, we issued an aggregate of 3,981,164 Private Placement Units at a price of $1.50 per Private Placement Unit, with each Private Placement Unit consisting of one share of our common stock and one Private Placement Warrant, to certain of the Selling Shareholders named herein. Each Private Placement Warrant entitles the holder to purchase one share of our common stock at an exercise price of $3.00 per share commencing on the date of issuance for a period ending on the earlier of 18 months from the date of issuance of the Private Placement Units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
On March 30, 2007, we issued an aggregate of 205,000 shares of our common stock upon the cancellation of outstanding warrants to acquire shares in the capital of CellCyte at a deemed rate of $1.50.
On March 30, 2007, we issued options to acquire 2,000,000 shares of our common stock at a price of $1.50 per share in place of options to acquire shares of CellCyte.
The private placement transactions were completed in reliance of Rule 506 of Regulation D of the Securities Act, with respect to investors in the United States, and in reliance of Rule 903 of Regulation S of the Securities Act, with respect to those investors who were not "U.S. Persons", within the meaning of Regulation S, and who were otherwise outside of the United States. Sales to United States investors pursuant to Rule 506 of Regulation D were limited to investors who qualified as "accredited investors" within the meaning of Rule 501(a) of Regulation D.
In addition, we issued 16,000,000 restricted common shares to the Vendors of CellCyte, and 18,750,000 restricted common shares and 2,500,000 common shares in the capital of the Company were transferred to the Vendors, in connection with the acquisition of CellCyte. These transactions were completed pursuant to an unregistered private transaction. Such securities have not been registered under the Securities Act or under any states securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements.
ITEM 27. EXHIBITS
The following exhibits are filed with this registration statement on Form SB-2:
Exhibit Number | Description of Exhibit |
3.1 | Articles of Incorporation, as amended(1) |
3.2 | Bylaws(2) |
4.1 | Share Exchange Agreement among the Company, CellCyte Genetics Corporation, the Washington Company and the Shareholders of CellCyte Genetics Corporation, the Washington Company(3) |
4.2 | Employment Agreement between the Company and Gary A. Reys(4) |
4.3 | Employment Agreement between the Company and Dr. Ronald W. Berninger(4) |
4.4 | Consulting Agreement between the Company and Jack Wynn & Co., Inc. |
4.5 | CEO Award Agreement between the Company and Gary A. Reys.(4) |
4.6 | Sublease Agreement between the Company and Icogenex Corporation. |
4.7 | Lease Agreement between the Company and Teachers Insurance and Annuity Association of America, Inc. |
5.1 | Opinion of Lang Michener LLP, with consent to use, regarding the legality of the securities being registered. |
10.1 | 2007 Stock Option / Restricted Stock Plan. |
23.1 | Consent of Independent Auditors, Morgan & Company. |
23.2 | Consent of Independent Auditors, Williams & Webster, P.S. |
(1) Incorporated by reference to our Registration Statement on Form SB-2 filed with the SEC on May 4, 2005.
(2) Incorporated by reference to our Current Report on Form 8-K filed with the SEC on May 14, 2004.
(3) Incorporated by reference to our Current Report on Form 8-K filed with the SEC on April 6, 2007.
(4) Incorporated by reference to our Current Report on Form 8-K filed with the SEC on June 5, 2007.
II-3
ITEM 28. UNDERTAKINGS
The undersigned registrant hereby undertakes:
1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information set forth in this registration statement; provided that any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in the volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement; and
(iii) To include any additional or changed material information with respect to the plan of distribution.
2. That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
3. To remove from registration by means of a post-effective amendment any of the securities being registered hereby which remain unsold at the termination of the offering;
4. That, for the purpose of determining liability of the undersigned small business issuer under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned small business issuer undertakes that in a primary offering of securities of the undersigned small business issuer pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned small business issuer will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned small business issuer relating to the offering required to be filed pursuant to Rule 424 of Regulation C of the Securities Act;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned small business issuer or used or referred to by the undersigned small business issuer;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned small business issuer or its securities provided by or on behalf of the undersigned small business issuer; and
(iv) Any other communication that is an offer in the offering made by the undersigned small business issuer to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the provisions above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, and is, therefore, unenforceable.
II-4
In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by one of our directors, officers, or controlling persons in the successful defense of any action, suit or proceeding, is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification is against public policy as expressed in the Securities Act of 1933, and we will be governed by the final adjudication of such issue.
Each prospectus filed pursuant to Rule 424(b) of the Securities Act of 1933 as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
__________
II-5
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form SB-2 and authorized this registration statement to be signed on its behalf by the undersigned in the City of Seattle, Washington, on June 29, 2007.
| | | CELLCYTE GENETICS CORPORATION
By: /s/ Gary A. Reys
_________________________________
Gary A. Reys
Chairman, President, Chief Executive Officer, Chief Financial Officer, Principal Executive Officer, Principal Accounting Officer and a director |
POWER OF ATTORNEY
Know all persons by these presents that that each individual whose signature appears below constitutes and appoints Gary A. Reys as a true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing under Rule 462 promulgated under the Securities Act of 1933, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all inten ts and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any one of them, or his or their substitutes, may lawfully do or cause to be done by virtue hereof.
In accordance with the requirements of the Securities Act of 1933, this amendment to the registration statement was signed by the following persons in the capacities and on the dates stated.
Signature | Title | Date |
/s/ Gary A. Reys
Gary A. Reys
|
Chairman, President, Chief Executive Officer, Chief Financial Officer, Principal Executive Officer, Principal Accounting Officer and a director
| June 29, 2007 |
/s/ Dr. Ronald W. Berninger
Dr. Ronald W. Berninger
|
Secretary, Executive Vice President, Chief Scientific Officer and a director
| June 29, 2007 |
/s/ John M. Fluke, Jr.
John M. Fluke, Jr.
|
Director
| June 29, 2007 |
__________



