UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
S Quarterly Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the quarterly period endedMarch 31, 2008
£ Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the transition period from _____ to _____
Commission File Number: 000-52238
CELLCYTE GENETICS CORPORATION
(Exact name of registrant as specified in its charter)
Nevada | | 86-1127046 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
| | | |
1725 200th Street S.E., Suite 103
Bothell, Washington | |
98021
|
(Address of principal executive offices) | | (Zip Code) |
| | | |
| | | |
(425) 483-6101 | | |
(Registrant's telephone number, including are code) | | |
| | | |
Indicate by checkmark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YesS No£
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer£ | Accelerated filer£ |
Non-accelerated filer£ (Do not check if a smaller reporting company) | Smaller reporting companyS |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes£ NoS
Indicate the number of shares outstanding of each of the issuer's classes of common equity, as of the latest practicable date.60,873,848 shares of common stock outstanding as of May 13, 2008.
CELLCYTE GENETICS CORPORATION
Quarterly Report On Form 10-Q
For The Quarterly Period Ended March 31, 2008
FORWARD-LOOKING STATEMENTS
This Form 10-Q for the quarterly period ended March 31, 2008 contains forward-looking statements that involve risks and uncertainties. Forward-looking statements in this document include, among others, statements regarding our capital needs, business plans and expectations. Such forward-looking statements involve assumptions, risks and uncertainties regarding, among others, the success of our business plan, availability of funds, government regulations, operating costs, our ability to achieve significant revenues, our business model and other factors. Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may", "will", "should", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict", "potential" or "continue", the negative of such terms or other comparable terminology. In evaluating these statements, you shou ld consider various factors, including the assumptions, risks and uncertainties set forth in our Annual Report on Form 10-KSB for the year ended December 31, 2007 and other reports and documents we have filed with or furnished to the Securities and Exchange Commission. These factors or any of them may cause our actual results to differ materially from any forward-looking statement made in this document. While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding future events, our actual results will likely vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein. The forward-looking statements in this document are made as of the date of this document and we do not intend or undertake to update any of the forward-looking statements to conform these statements to actual results, except as required by applicable law, including the securities l aws of the United States.
__________
- 2 -
PART I- FINANCIAL INFORMATION
Item 1. Financial Statements
The following unaudited interim consolidated financial statements of CellCyte Genetics Corporation are included in this Quarterly Report on Form 10-Q:
Description | Page |
Consolidated Balance Sheets as at March 31, 2008 and December 31, 2007: | 4 |
Interim Consolidated Statements of Operations for the Three Months ended March 31, 2008 and 2007 and for the period from January 14, 2005 (Date of Inception) to March 31, 2008: | 5 |
Interim Consolidated Statements of Cash Flows for the Three Months ended March 31, 2008 and 2007 and for the period from January 14, 2005 (Date of Inception) to March 31, 2008: | 6 |
Notes to Interim Consolidated Financial Statements: | 7 |
__________
- 3 -
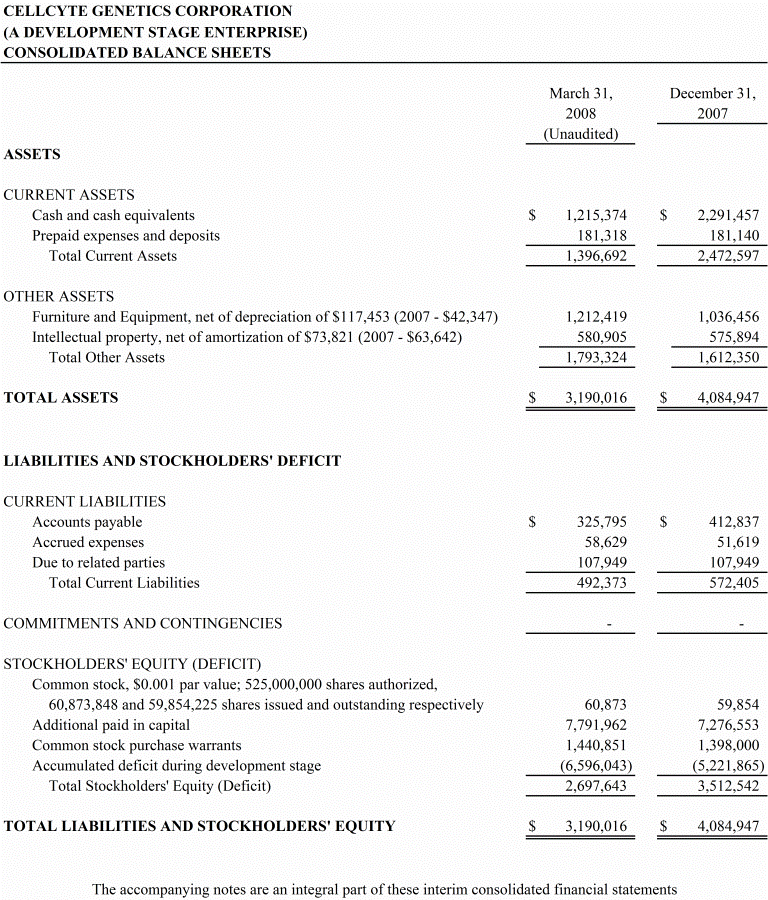
- 4 -
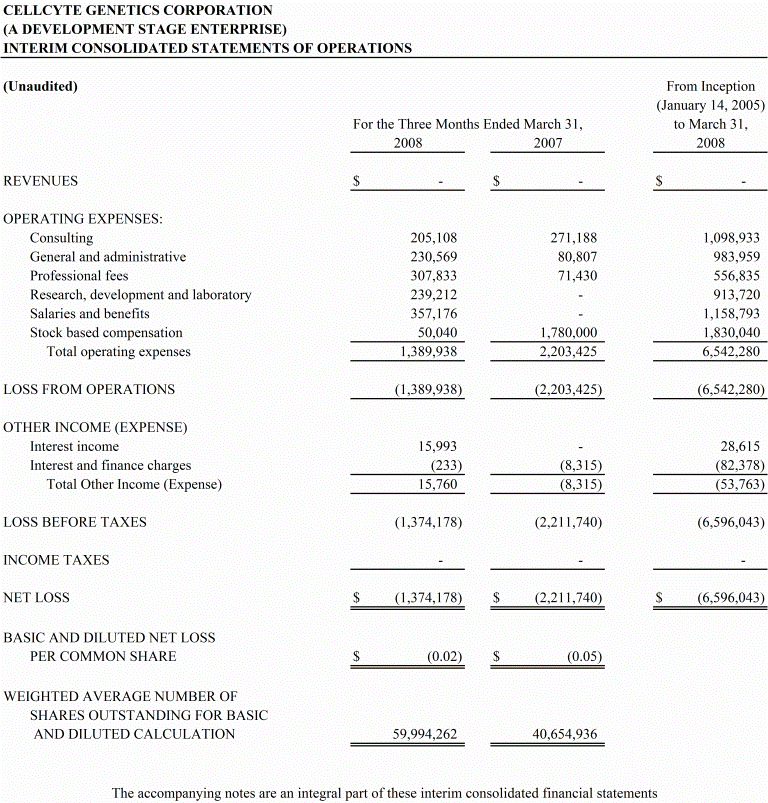
- 5 -

- 6 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
NOTE 1 - DESCRIPTION OF BUSINESS AND BASIS OF PRESENTATION
The Company was incorporated as Shepard Inc. March 9, 2004 in the state of Nevada and changed its name to CellCyte Genetics Corporation ("CGN") effective February 16, 2007.
Effective January 19, 2007, CGN entered into an Agreement in Principle to acquire 100% of the issued and outstanding shares of CellCyte Genetics Corporation (a development stage enterprise) ("CGW"), a private Washington State corporation incorporated January 4, 2005, in exchange for 16,000,000 restricted shares of CGN's common stock, and the transfer of a further 18,750,000 shares of CGN's restricted common stock and 2,500,000 shares of CGN's non-restricted common stock from certain shareholders of CGN to the shareholders of CGW. Effective March 14, 2007, the acquisition of CGW was completed by the execution of a Share Exchange Agreement (the "Agreement"), which, in addition to the share issuances and transfers described above, finalized certain other terms of the acquisition.
As a result of the completion of this acquisition and the related transactions, the former shareholders of CGW own approximately 62% of the outstanding shares of common stock of CGN representing 37,250,000 of the then 59,854,224 total issued and outstanding shares of common stock of CGN.
This acquisition is in essence a recapitalization of the Company and has been accounted for in accordance with the principles applicable to accounting for reverse acquisitions with CGW, the legal subsidiary, being treated as the accounting parent and CGN, the legal parent, being treated as the accounting subsidiary. Accordingly, the consolidated results of operations and cash flows of the Company include those of CGW for all periods presented and those of CGN subsequent to the date of the reverse acquisition. The comparative Balance Sheet as at December 31, 2007 is that of CGW.
CGW is an emerging biotechnology company engaged in the discovery and development of stem cell therapeutic products. The Company is developing clinical-stage therapeutic agents and treatments for oncology, diabetes, heart, liver, lung and kidney diseases as well as for stem cell bone marrow and organ transplants. The Company's discoveries involve stem cell regimens without using embryonic stem cells. As the Company is in the development stage, it has not realized any revenues from its planned operations.
As shown in the accompanying financial statements, the Company has incurred significant losses since inception and has not generated any revenues to date. The future of the Company is dependent upon its ability to obtain sufficient financing and upon achieving future profitable operations. These factors, among others, raise substantial doubt about the Company's ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The accompanying unaudited interim consolidated financial statements have been prepared in accordance with generally accepted principals for interim financial information and with the instruction to Form 10-Q of Regulation S-K. They may not include all information and footnotes required by United States generally accepted accounting principals for complete financial statements. However, except as disclosed herein, there has been no material changes in the information disclosed in the notes to the financial statements for the year ended December 31, 2007 included in the Company's Annual Report on Form 10-KSB filed with the Securities and Exchange Commission. The interim unaudited financial statements should be read in conjunction with those financial statements included in the Form 10-KSB. In the opinion of Management, all adjustments considered necessary for a fair presentation, which unless otherwise disclosed herein, consisting primarily of normal recurring adjustments, have been mad e. Operating results for the three months ended March 31, 2008 are not necessarily indicative of the results that may be expected for the year ending December 31, 2008.
- 7 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies is presented to assist in understanding the Company's financial statements. The financial statements and notes are representations of the Company's management, which is responsible for their integrity and objectivity. These accounting policies conform to accounting principles generally accepted in the United States of America and have been consistently applied in the preparation of the financial statements.
Basis of Consolidation
These consolidated financial statements include the accounts of CGW since its incorporation on January 4, 2005 and CGN since the reverse acquisition on March 14, 2007. All inter-company balances and transactions have been eliminated.
Cash and Cash Equivalents
The Company considers all highly liquid investments and short-term debt instruments with maturities of three months or less from date of purchase to be cash equivalents. The Company had cash equivalents equal to $1,078,490 on deposit with Merrill Lynch at March 31, 2008.
Concentration of Risks
The Company maintains its cash and cash equivalents in commercial accounts at a major financial institution. Although the financial institution is considered creditworthy and has not experienced any losses on its deposits as of March 31, 2008, the Company's cash balances exceeded Federal Deposit Insurance Corporation (FDIC) limits at March 31, 2008 by $1,115,374. A balance of up to $100,000 is FDIC insured with Bank of America. No FDIC insurance is available on the balance on account with Merrill Lynch Institutional Money Market Fund.
Earnings per Share
The Company computes net income (loss) per common share in accordance with Statement of Financial Accounting Standards No. 128, "Earnings per Share". Net income (loss) per share is based upon the weighted average number of outstanding common shares and the dilutive effect of common share equivalents, such as options and warrants to purchase common stock, convertible preferred stock and convertible notes, if applicable, that are outstanding each year. Basic and diluted earnings per share were the same at the reporting dates of the accompanying financial statements, as including common stock equivalents in the calculation of diluted earnings per share would have been anti-dilutive. For all periods prior to the reverse acquisition, the number of shares outstanding is deemed to be the number of shares issued and transferred to the shareholders of CBW to effect the reverse acquisition, being 37,250,000.
- 8 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
Fair Value of Financial Instruments
The Company's financial instruments may consist of cash and cash equivalents and other current liabilities. All instruments are accounted for on an historical cost basis, which, due to the short maturity of these financial instruments, approximates the fair value at the reporting dates of these financial statements.
SFAS No. 157,Fair Value Measurements ("SFAS157"), defines fair value, establishes a framework for measuring fair value in accordance with t generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS157 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value as follows:
Level 1. Observable inputs such as quoted prices in active markets
Level 2. Inputs, other than quoted prices in active markets, that are observable either directly or indirectly and
Level 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The Company does not have any assets or liabilities measured at fair value on a recurring basis at March 31, 2008. The Company did not have any fair value adjustments for assets and liabilities measured at fair value on a nonrecurring basis during the three months ended March 31, 2008.
Intangible Assets
The Company's intangible assets primarily consist of patents and intellectual property, which are carried at the purchase price and/or the legal cost to obtain them less accumulated amortization. Patents and licenses are being amortized over their estimated useful lives, which range from seven to seventeen years.
Going Concern
As shown in the accompanying financial statements, the Company had working capital of approximately $906,074 an accumulated deficit of $6,596,043 incurred through March 31, 2008. The Company has no revenues. Management forecasts operations during the coming year will need approximately $7,200,000 in additional working capital, which is expected to come from issuance of convertible notes, equipment financing and private placements of stock. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
Income Taxes
The Company follows the provisions of Statement of Financial Accounting Standards No. 109, "Accounting for Income Taxes" (hereinafter "SFAS No. 109"), under which deferred tax assets and liabilities are determined based on differences between financial reporting and tax basis of assets and liabilities. Deferred tax assets and liabilities are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The provisions of SFAS No. 109 also require the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that the realization of such benefits is more likely than not. To the extent that it is more likely than not that such benefits will not be received, the Company records a valuation allowance against the related deferred tax asset.
- 9 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
Long-Lived Assets and Intangibles
In accordance with Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets", the Company examines on a periodic basis the carrying value of its tangible and intangible assets to determine whether there are any impairment losses. This standard establishes a single accounting model for long-lived assets to be disposed of by sale, including discontinued operations, and requires that these long-lived assets be measured at the lower of carrying amount or fair value less cost to sell, whether reported in continuing operations or discontinued operations. The determination of any impairment would include a comparison of estimated future cash flows anticipated to be generated during the remaining life of the assets to the net carrying value of the assets. To March 31, 2008, no asset impairments have been identified or recorded.
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Expenditures for repairs and maintenance are expensed as incurred.
Research and Development Costs
Research and development costs are expensed as incurred. The cost of intellectual property purchased from others that is immediately marketable or that has an alternative future use is capitalized and amortized as intangible assets. Capitalized costs are amortized using the straight-line method over the estimated economic life of the related asset. The Company periodically reviews its capitalized intangible assets to assess recoverability based on the projected undiscounted cash flows from operations, and impairments are recognized in operating results when a permanent diminution in value occurs.
Compensated Absences
Employees of the Company are entitled to paid vacation, and sick days, depending on job classification, length of service, and other factors. The Company accrued vacation pay in the amounts of $ 46,555 at March 31, 2008.
Recent Accounting Pronouncements
On March 19, 2008, the Financial Accounting Standards Board (FASB) announced the issuance of Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities, (FAS 161). FAS 161 amends FASB Statement No. 133, Accounting for Derivative Instruments and Hedging Activities, (FAS 133) and was issued in response to concerns and criticisms about the lack of adequate disclosure of derivative instruments and hedging activities. FAS 161 is focused on requiring enhanced disclosure on 1) how and why an entity uses derivative instruments and hedging activities; 2) how derivative instruments and related hedging activities are accounted for under FAS 133; and 3) how derivative instruments and related hedging activities affect an entity's cash flows, financial position and performance.
- 10 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
To accomplish the three objectives listed above, FAS 161 requires: 1) qualitative disclosures regarding the objectives and strategies for using derivative instruments and engaging in hedging activities in the context of an entity's overall risk exposure; 2) quantitative disclosures in tabular format of the fair values of derivative instruments and their gains and losses; and 3) disclosures about credit-risk related contingent features in derivative instruments.
FAS 161 is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008. Management has not determined the effect that adopting this statement would have on the Company's financial position or results of operations.
Stock-Based Compensation
The Company accounts for its stock option plan under the recognition and measurement principles of the revised statement to Statement of Financial Accounting Standards No. 123, "Share-Based Payments" (hereinafter "SFAS No. 123 (R)"). Accordingly, compensation cost has been recognized using the fair value method and expected term accrual requirements as prescribed in SFAS No. 123 (R). The Company has recorded $1,830,040 in stock-based compensation expense through March 31, 2008 in connection with the acquisition of CGW and other stock options granted as described further in Note 8 below.
Use of Estimates
The accompanying financial statements are prepared in conformity with accounting principles generally accepted in the United States of America and include certain estimates and assumptions which affect the reported amounts of assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Accordingly, actual results may differ from those estimates.
NOTE 3 - INTELLECTUAL PROPERTY AND PROPERTY AND EQUIPMENT
In 2006 and 2007, the Company paid a total of $25,000 and $90,000 on the execution of an intellectual property licensing agreement with U.S. Department of Veterans Affairs (VA). The license agreement relates to certain patents pending and contains royalty payment arrangements as well as funding guarantees. Because of certain internal VA intellectual property timeline problems regarding technology transfer to CGW, the VA has not required CGW to meet any funding requirements as of December 31, 2006. Because the VA was unable to transfer research notebooks, it precluded CGW from beginning technology development and securing related funding and/or grants. With the completion of the recent financing transaction CGW has now met the funding guarantee requirements included in the VA license.
As of December 31, 2007, the Company had $70,002 of capitalized patents and $454,534 of capitalized patents pending. Through March 31, 2008 the Company incurred an additional $15,190 of capitalized patents pending. These amounts consist of the legal fees expended to pursue and prosecute a range of patent claims related to stem cell delivery and purification.
- 11 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
The Company amortizes patents (and licenses for the use of patents) over the remaining life of the patents, which ranges from 7 to 17 years.
| | | March 31, | | December 31, |
| | | 2008 | | 2007 |
Licenses | | $ | 115,000 | | $ | 115,000 |
Patents | | | 70,002 | | | 70,002 |
Patents pending | | | 469,724 | | | 454,534 |
Accumulated amortization | | | (73,821) | | | (63,642) |
Licenses and patents, net | | $ | 580,905 | | $ | 575,894 |
As of December 31, 2007, the Company had capitalized a total of $1,078,803 in computer equipment, laboratory equipment and tenant improvements. Through March 31, 2008 the Company incurred additional $92,762 of computer equipment, $54,055 of laboratory equipment and $104,251 of tenant improvements. Property and equipment are stated at cost. The Company depreciates computer and office equipment is over a three year useful life, laboratory equipment over a five year useful life and tenant improvements over a five year useful life
| | March 31, | | December 31, |
| | | 2008 | | 2007 |
Laboratory equipment | | $ | 559,623 | | $ | 505,568 |
Computer and office equipment | | | 393,378 | | | 300,616 |
Tenant improvements | | | 376,871 | | | 272,620 |
Accumulated depreciation | | | (117,453) | | | (42,347) |
Property and equipment, net | | $ | 1,212,419 | | $ | 1,036,456 |
NOTE 4 - COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company has operating leases for its office and storage space, all of which are month to month. Rent expense relating to the operating leases was approximately $70,497 for the period ended March 31, 2008.
On April 1, 2007 the Company entered into a 63 month lease in a new facility in Bothell Washington. Total commitment in lease cost for the term of the lease is approximately $1,817,858
Year | | Lease Expense |
2008 | $ | 344,766 |
2009 | | 370,644 |
2010 | | 383,736 |
2011 | | 396,864 |
2012 | | 307,521 |
2013 | | 14,237 |
Total | $ | 1,817,858 |
- 12 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
Executive Employment Contracts
The Company entered into employment agreements with each of Gary A. Reys, Chief Executive Officer, and Dr. Ronald Berninger, Executive Vice President and Chief Scientific Officer on June 1, 2007. The Company entered into an employment agreement with Nathan A. McDonald, Vice President of Finance on January 1, 2008. The Company has also entered into a CEO Award Agreement with Mr. Reys. The CEO agreement term is for a period of five years; with a daily three year "evergreen" feature such that the remaining term of his employment will always be at least three years. In consideration for his services, the Company has agreed to pay Mr. Reys an annual salary of $350,000, provided, however, that effective upon reaching an aggregate funding of $15,000,000, Mr. Reys' base salary will be increased to $425,000 per year. The CEO Award Agreement target is presently $750,000 in cash and the actual award will be calculated by multiplying the target award by the percentage score achieved by the CEO bas ed on assessment of performance under its business plan. The CSO agreement term is for a period of five years; with a daily three year "evergreen" feature such that the remaining term of his employment will always be at least three years. In consideration for his services, the Company has agreed to pay Dr. Berninger an annual salary of $205,000, provided, however, that effective upon successfully completing Phase I clinical trials, Dr. Berninger's base salary will be increased to $225,000 per year. The VP of Finance agreement is for a term ending upon the termination of the agreement as described below. In consideration for his services, the Company has agreed to pay Mr. McDonald an annual salary of $165,000. The agreement may be terminated (i) if Mr. McDonald is incapacitated from illness, accident or other disability and unable to perform his duties for a cumulative period of four months in any 12 consecutive month period, upon the Company or Mr. McDonald giving the other not less than 30 days prior writt en notice, (ii) by either party without Cause (as defined in the agreement) upon 14 days prior written notice, (iii) by the Company for Cause, or (iv) upon cessation of the Company's business.
NOTE 5 - CAPITAL STOCK
Common Stock
The Company has authorized 525,000,000 shares of its common stock, $0.001 par value. The Company had issued and outstanding 60,873,848 and 59,854,225 shares of its common stock at March 31, 2008 and December 31, 2007, respectively.
During the period ended March 31, 2008, the Company issued common shares as follows:
- 67,146 shares of common stock were issued on the exercise of common stock purchase warrants at a price of $3.00 per warrant for total proceeds of $201,438.
- 112,477 shares of common stock were issued in exchange for services with an estimated fair value of $97,801.
- 840,000 units were issued at a price of $0.25 per unit for total proceeds of $210,000. Each unit consists of one common share of CGN and one warrant to acquire an additional common share of CGN at a price of $0.75 per share, exercisable for a period ending 18 months from the date of issuance of the Units.
- 13 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
The estimated fair value of the warrants issued in connection with the financing of $63,000 has been recorded as a separate component of stockholders' equity and was determined on a relative fair value basis using the Black-Scholes option pricing model using the following weighted average assumptions: expected option life of 1.50 years, risk-free interest rate of 1.60%, dividend yield of 0% and expected volatility of 135%.
The Company's common stock purchase warrant activity for the period ended March 31, 2008 is as follows:
| | Warrants
Outstanding | | Weighted Average
Exercise Price | | Weighted Average
Remaining Life |
| | | | | |
Balance, December 31, 2006 | - | $ | - | | - |
Warrants issued | 4,659,225 | | 3.00 | | |
Warrants exercised | - | | - | | |
Warrants expired | - | | - | | |
Balance, December 31, 2007 | 4,659,225 | | 3.00 | | 0.75 years |
Warrants issued | 840,000 | | 0.75 | | |
Warrants exercised | (67,146) | | 3.00 | | |
Warrants expired | - | | - | | |
Balance, March 31, 2008 | 5,432,079 | $ | 2.65 | | 0.65 years |
NOTE 6 - COMMON STOCK OPTIONS
During the period ended March 31, 2008, the Company granted a total of 715,000 stock options to certain employees, officers, directors and consultants of the Company to acquire shares of the Company's common stock at a price of $0.33 per share for a period of 10 years. The fair value of the stock options granted during the period was estimated to be $185,900 and was determined using the Black-Scholes option pricing model using the following weighted average assumptions: expected option life of 5 years; risk-free interest rate of 2.48%; dividend yield of 0%; and expected volatility of 110%. These options vest at a rate of 25% on grant and 25% at the end of each of year one through year three from the grant date. As of March 31, 2008, the Company had recorded stock based compensation of $50,040 in connection with the vesting of the options leaving $135,860 to be expensed on future vesting.
- 14 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
The Company's stock option activity for the period ended March 31, 2008 is as follows:
| | Options
Outstanding | | Weighted Average
Exercise Price | | Weighted Average
Remaining Life |
| | | | | |
Balance, December 31, 2006 | - | $ | - | | - |
Options granted | 2,000,000 | | 1.50 | | |
Options exercised | - | | - | | |
Options cancelled and expired | - | | - | | |
Balance, December 31, 2007 | 2,000,000 | | 1.50 | | 7.31 years |
Options granted | 715,000 | | 0.33 | | |
Options exercised | - | | - | | |
Options cancelled and expired | - | | - | | |
Balance, March 31, 2008 | 2,715,000 | $ | 1.19 | | 7.06 years |
NOTE 7 - RELATED PARTY TRANSACTIONS
Two officers, Gary Reys and Dr, Ronald Berninger, who are also members of the Company's board of directors served as employees to the Company on various business, strategic, and technical issues. The Company paid and expensed a total of $516,250 for these services in 2007 and $129,500 during the three month period ended March 31, 2008. In addition to the fees, reimbursable expenses were also paid under these contracts. The total amount paid for these expenses was $113,517 in 2007 and $15,040 during the three month period ended March 31, 2008. At period end December 31, 2007, the Company also carried a payable to Sheryl Reys in the amount of $852 and at period end March 31, 2008, $20,401 was paid as reimbursable expenses to Sheryl Reys for supplies and furniture purchased for the new facility.
Shareholder Advances
During the twelve month period ended December 31, 2007 a shareholder of the Company paid various outstanding payables on behalf of the Company. As of March 31, 2008 the Company owed this shareholder $107,949. These advances are unsecured, non-interest bearing with no set terms of repayment.
- 15 -
CELLCYTE GENETICS CORPORATION
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2008
(unaudited)
NOTE 8 - LEGAL DISPUTES
During the reporting period, three shareholder lawsuits were filed against us, all of which were filed in the United States federal court for the Western District of Washington:Armbruster v. Cellcyte Genetics Corporation, et. al,No. C08-0047,Tolerico v. Cellcyte Genetics Corporation, et. al., No. C08-0163 andPruitt v. Cellcyte Genetics Corporation, et. al., No. C08-0178. Gary Reys and Ronald Berninger are also named in all three lawsuits, and John Fluke is named in theTolericolawsuit. The three lawsuits are virtually identical and allege,inter alia, that the Company, and its officers and directors filed misleading statements with the Securities and Exchange Commission regarding the Company's products, and that the Company posted misleading information regarding an officer on its website. The lawsuits claim that investors purchased our stock based on the alleged misleading statements and the plaintiffs are seeking monet ary relief. The three lawsuits have been consolidated and an amended complaint will be filed. None of the lawsuits has been certified for class action status as of the date of this report. We are disputing the basis for all three lawsuits and intend to vigorously defend against them. While we do not believe any of the lawsuits has merit, an adverse outcome could have a material adverse effect on our financial position and results.
We are also currently engaged in a dispute with a former employee, who voluntarily resigned in 2007. The employee is asserting claims for monetary damages based upon a constructive discharge theory and other claims. The employee has been ordered to arbitrate her claims pursuant to the terms of a contract between the employee and CellCyte. We do not believe that the employee's claims have merit, nor do we believe that an adverse outcome will have a material adverse effect on our financial position. The employee has opened her own biotech company and is on notice regarding that which CellCyte deems proprietary information. At this time, we do not believe that CellCyte's intellectual property is imminently threatened. Should we determine that the employee is trying to operate her new endeavor with intellectual property that belongs to CellCyte, we will take appropriate action.
Although management currently believes that resolving claims against us, individually or in aggregate, will not have a material adverse impact on our financial position, our results of operations, or our cash flows, these matters are subject to inherent uncertainties and management's view of these matters may change in the future.
In January 2008, the Securities and Exchange Commission ("SEC") notified the Company that it was commencing an informal non-public inquiry relating to certain matters related to the Company's filings. The SEC requested that the Company voluntarily produce documents relating to its investigation. On May 12, 2008, the Company learned that the informal inquiry commenced by the SEC had become a formal investigation. The Company believes that the focus of the formal investigation is substantially the same as the informal inquiry and intends to continue to fully cooperate with the SEC regarding this matter. The company is committed to resolving the issues raised in connection with the investigation.
- 16 -
Item 2. Management's Discussion and Analysis
As used in this Quarterly Report: (i) the terms the "Company", "our company", "we", "us" and "our" refer to CellCyte Genetics Corporation, a Nevada corporation, and its subsidiaries, unless the context requires otherwise; (ii) references to "CellCyte" mean CellCyte Genetics Corporation, a Washington corporation; and (iii) all dollar amounts refer to United States dollars unless otherwise indicated.
The following discussion of our plan of operations, results of operations and financial condition as at and for the three months ended March 31, 2008 should be read in conjunction with (i) our unaudited interim consolidated financial statements and related notes for the three months ended March 31, 2008 included in this Quarterly Report, and (ii) our Annual Report on Form 10-KSB for the year ended December 31, 2007, including our annual audited financial statements set forth therein.
Overview
We were an exploration stage company engaged in the acquisition and exploration of mineral properties with a view to exploiting any mineral deposits we discovered. Prior to our year ended December 31, 2006, we owned a 100% beneficial interest in one mineral claim known as the CB-1 claim.
During the 2006 fiscal year, we retained an independent geologist to conduct exploration on the CB-1 claim and to provide us with recommendations for additional exploration on the property. All rock samples that were taken from the property during exploration did not contain any significant quantities of gold. In addition, we were not able to locate any other zones of altered rock on the CB-1 claim which could possibly host economic mineralization. Accordingly, we have not conducted any further exploration of the CB-1 claim and allowed the claim to expire on November 10, 2006.
On March 30, 2007, we completed the acquisition of all of the issued and outstanding shares of CellCyte pursuant to a Share Exchange Agreement among CellCyte, the shareholders of CellCyte and the Company dated March 14, 2007. For more information regarding the terms of the acquisition and related private placement of our common stock, see our Annual Report on Form 10-KSB for the year ended December 31, 2007 filed with the SEC on April 14, 2008.
As a consequence of our Company's acquisition of CellCyte, our business is now focused on the discovery and development of stem cell enabling therapeutic products. See our Annual Report on Form 10-KSB for the year ended December 31, 2007 for more information.
The CellCyte acquisition constitutes a recapitalization of the Company and has been accounted for in accordance with principles applicable to accounting for reverse acquisitions with CellCyte, the legal subsidiary, being treated as the accounting parent and the Company, the legal parent, being treated as the accounting subsidiary. Accordingly, the consolidated results of operations and cash flows of the Company set forth in this Quarterly Report include those of CellCyte for all periods presented and those of the Company subsequent to the date of the CellCyte acquisition. The comparative balance sheet as at December 31, 2007 is that of CellCyte.
Plan of Operations
Our plan of operations for the next 12 months is to:
(a) conduct pre-clinical lab research at our new state-of-the-art biotechnology research and development facility in Bothell, WA.;
- 17 -
(b) complete negotiations with our manufacturing partner to supply our glycoprotein compound for our internal research, academic collaborations, toxicity study and clinical trials;
(c) complete negotiations in preparation for our academic collaborations, toxicity study and file an IND with the FDA for approval to begin a Phase I trial, and plan for Phase II clinical studies; and
(d) complete the design and sourcing of components to begin small scale manufacturing of our cell expansion device technology for use in collaborative research with academic and institutional research organization.
We anticipate that we will incur an aggregate of $7,200,000 in expenses for the next 12 months as follows:
(a) $1,122,000 for facility and equipment related expenses;
(b) $2,725,000 for pre-clinical work, quality assurance, manufacturing of biologic material, toxicity study, academic collaborations, engage contract research organization for our Phase I clinical trial and further research collaborations;
(c) $105,000 for design and device manufacturing;
(d) $610,000 for legal expenses related to intellectual property prosecution and accounting expenses related to quarterly reviews and annual audits;
(e) $2,115,000 for salaries and consulting; and
(f) $523,000 for general and administrative, travel, conferences and public relations.
During the next 12 months we anticipate that we will not generate any revenue. We had cash of $1,215,374 and working capital of $906,074 at March 31, 2008. We presently do not have sufficient cash to fund our operations for more than the next three months. Accordingly, we anticipate that we will require additional financing to enable us to pay our planned expenses for the next 12 months and pursue our plan of operations.
Subsequent to the 12 month period following the date of this Quarterly Report, we will be required to obtain additional financing in order to continue to pursue our business plan. We believe that debt financing will not be an alternative for funding as we do not have tangible assets to secure any debt financing. We anticipate that additional funding will be in the form of equity financing from the sale of our common stock. We cannot provide investors with any assurance that we will be able to raise sufficient funding from the sale of our common stock to fund our business plan going forward. In the absence of such financing, our business plan will fail. Even if we are successful in obtaining equity financing to fund our business plan, there is no assurance that we will obtain the funding necessary to pursue our plan over the long-term.
Results of Operations
Three Months Ended March 31, 2008 Compared to Three Months Ended March 31, 2007
The following table sets out our consolidated loss for the periods indicated:
- 18 -
| | Three Months Ended
March 31, 2008 | Three Months Ended
March 31, 2007 |
| | (Unaudited) | (Unaudited) |
Revenues | $ -- | | $ -- | |
Operating Expenses | | | | |
Consulting | 205,108 | | 271,188 | |
General and administrative | 230,569 | | 80,807 | |
Professional fees | 307,833 | | 71,430 | |
Research, development and
laboratory | 239,212 | | -- | |
Salaries and benefits | 357,176 | | -- | |
Stock based compensation | 50,040 | | 1,780,000 | |
Other Income (Expense) | 15,760 | | (8,315) | |
Net Loss | (1,374,178) | | (2,211,740) | |
Revenues
We have had no operating revenues since our inception on January 14, 2005 to March 31, 2008. We anticipate that we will not generate any revenues for so long as we are a development stage company.
Consulting Fees
In the three months ended March 31, 2008, consulting fees decreased to $205,108, compared to $271,188 in the prior period of 2007, primarily due to the growth in our internal operations.
General and Administrative Expenses
Our general and administrative expenses in the three months ended March 31, 2008 increased to $230,569 from $80,807 in the prior period of 2007, primarily as a result of increased operations due to the acquisition of additional intellectual property, rent of additional research facilities and overhead costs associated with our growing organization.
Professional Fees
In the three months ended March 31, 2008, we incurred professional fees of $307,833, compared to $71,430 in the prior period of 2007, primarily related to the increase in costs relating to completing our year-end audit and litigation matters.
Research, Development and Laboratory Expenses
In the three months ended March 31, 2008, we incurred research, development and laboratory expenses of $239,212, compared to $Nil in the prior period of 2007, due to the change in our plan of operations and commencement of research and development of our products.
- 19 -
Salaries and Benefits
In the three months ended March 31, 2008, we paid salaries and benefits of $357,176 to our directors, officers and employees, compared to $Nil in the prior period of 2007, due to the hiring of 14 employees.
Stock-based Compensation
In the three months ended March 31, 2008, we incurred stock based compensation expenses of $50,040 in connection with the compensation of our officers and employees. In the three months ended March 31, 2007, we incurred stock based compensation expenses of $1,780,000 related to the issuance of options to acquire up to 2,000,000 of our shares of common stock, in connection with the acquisition of CellCyte.
Net Loss
As a result of the above, our net loss for the three months ended March 31, 2008 was $1,374,178, compared to $2,211,740 in the prior period of 2007.
Liquidity and Capital Resources
As of March 31, 2008, we had cash in the amount of approximately $1,215,374 and working capital of $906,074. Our planned expenditures over the next 12 months are expected to amount to approximately $7,200,000 and will exceed our cash reserves and working capital. We presently do not have sufficient cash to fund our operations for more than the next three months. We anticipate that we will require additional financing in order to pursue our plan of operations for the next 12 months. There can be no assurance that we will obtain any additional financing in the amounts required or on terms favorable to us. If we are unable to obtain additional financing, we may have to abandon our business activities and plan of operations.
We believe that debt financing will not be an alternative for funding as we do not have tangible assets to secure any debt financing. We anticipate that additional funding will be in the form of equity financing from the sale of our common stock. However, we cannot provide investors with any assurance that we will be able to raise sufficient funding from the sale of our common stock to fund our plan of operations going forward. In the absence of such financing, our business plan will fail. Even if we are successful in obtaining equity financing, there is no assurance that we will obtain the funding necessary to pursue our business plan. If we do not continue to obtain additional financing going forward, we will be forced to abandon our plan of operations.
Cash Used in Operating Activities
Cash used in operating activities in the three months ended March 31, 2008 was $1,221,263, compared to $408,225 in the prior period of 2007. Operating activities used cash primarily relating to the commencement of our new plan of operations subsequent to the acquisition of CellCyte. We anticipate that cash used in operating activities will increase in 2008 as we pursue our plan of operations. We have funded our operations primarily from the issuance of our common stock.
Cash Used In Investing Activities
In the three months ended March 31, 2008, investing activities used cash of $266,258, compared to $100,198 in the prior period of 2007, primarily to fund the acquisition of intellectual property and the purchase of furniture and lab and office equipment for our facility.
- 20 -
Cash Provided By Financing Activities
We have funded our business to date primarily from sales of our common stock. In the three months ended March 31, 2008, we raised proceeds of $411,438, compared to $5,971,751 in the prior period of 2007, from the sale of our common stock.
Going Concern
As shown in the accompanying financial statements, we have incurred significant losses since inception and have not generated any revenues to date. The future of our Company is dependent upon our ability to obtain sufficient financing and upon achieving future profitable operations. These factors, among others, raise substantial doubt about our Company's ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Future Financings
We anticipate continuing to rely on equity sales of our common shares in order to continue to fund our business operations. Issuances of additional shares will result in dilution to our existing shareholders. There is no assurance that we will achieve any additional sales of our equity securities or arrange for debt or other financing to fund our business plan.
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to stockholders.
Critical Accounting Policies
Our financial statements are impacted by the accounting policies used and the estimates and assumptions made by management during their preparation. A complete summary of these policies is included in Note 2 of the notes to our historical financial statements. We have identified below the accounting policies that are of particular importance in the presentation of our financial position, results of operations and cash flows and which require the application of significant judgment by management.
Use of Estimates
The accompanying financial statements are prepared in conformity with accounting principles generally accepted in the United States of America and include certain estimates and assumptions which affect the reported amounts of assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Accordingly, actual results may differ from those estimates.
Income Taxes
The Company follows the provisions of Statement of Financial Accounting Standards No. 109, "Accounting for Income Taxes" (hereinafter "SFAS No. 109"), under which deferred tax assets and liabilities are determined based on differences between financial reporting and tax basis of assets and liabilities. Deferred tax assets and liabilities are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The provisions of SFAS No. 109 also require the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that the realization of such benefits is more likely than not. To the extent that it is more likely than not that such benefits will not be received, the Company records a valuation allowance against the related deferred tax asset.
- 21 -
Intangible Assets
The Company's intangible assets primarily consist of patents and intellectual property, which are carried at the purchase price and/or the legal cost to obtain them less accumulated amortization. Patents and licenses are being amortized over their estimated useful lives, which range from seven to seventeen years.
Research and Development Costs
Research and development costs are expensed as incurred. The cost of intellectual property purchased from others that is immediately marketable or that has an alternative future use is capitalized and amortized as intangible assets. Capitalized costs are amortized using the straight-line method over the estimated economic life of the related asset. The Company periodically reviews its capitalized intangible assets to assess recoverability based on the projected undiscounted cash flows from operations, and impairments are recognized in operating results when a permanent diminution in value occurs.
Stock-Based Compensation
The Company accounts for its stock option plan under the recognition and measurement principles of the revised statement to Statement of Financial Accounting Standards No. 123, "Share-Based Payments" (hereinafter "SFAS No. 123 (R)"). Accordingly, compensation cost has been recognized using the fair value method and expected term accrual requirements as prescribed in SFAS No. 123 (R). The Company has recorded $1,830,040 in stock-based compensation expense through March 31, 2008 in connection with the acquisition of CGW and other stock options granted as described further in Note 8 of the financial statements included herein.
Recent Accounting Pronouncements
On March 19, 2008, the Financial Accounting Standards Board (FASB) announced the issuance of Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities, (FAS 161). FAS 161 amends FASB Statement No. 133, Accounting for Derivative Instruments and Hedging Activities, (FAS 133) and was issued in response to concerns and criticisms about the lack of adequate disclosure of derivative instruments and hedging activities. FAS 161 is focused on requiring enhanced disclosure on 1) how and why an entity uses derivative instruments and hedging activities; 2) how derivative instruments and related hedging activities are accounted for under FAS 133; and 3) how derivative instruments and related hedging activities affect an entity's cash flows, financial position and performance.
To accomplish the three objectives listed above, FAS 161 requires: 1) qualitative disclosures regarding the objectives and strategies for using derivative instruments and engaging in hedging activities in the context of an entity's overall risk exposure; 2) quantitative disclosures in tabular format of the fair values of derivative instruments and their gains and losses; and 3) disclosures about credit-risk related contingent features in derivative instruments.
FAS 161 is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008. Management has not determined the effect that adopting this statement would have on the Company's financial position or results of operations.
- 22 -
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not Applicable.
Item 4T. Controls And Procedures
Evaluation of Disclosure Controls and Procedures
In connection with the preparation of this Quarterly Report on Form 10-Q, an evaluation was carried out by our management, with the participation of the Chief Executive Officer and Principal Accounting Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 ("Exchange Act")) as of March 31, 2008. Disclosure controls and procedures are designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms and that such information is accumulated and communicated to management, including the Chief Executive Officer and the Principal Accounting Officer, to allow timely decisions regarding required disclosures. Based on its evaluation, our management concluded, as of the end of the period covered by this report, that our disclosure c ontrols and procedures were effective.
There has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) during the quarter ended March 31, 2008 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
__________
- 23 -
PART II- OTHER INFORMATION
Item 1. Legal Proceedings
During the reporting period, three shareholder lawsuits were filed against us, all of which were filed in the United States federal court for the Western District of Washington:Armbruster v. Cellcyte Genetics Corporation, et. al,No. C08-0047,Tolerico v. Cellcyte Genetics Corporation, et. al., No. C08-0163 andPruitt v. Cellcyte Genetics Corporation, et. al., No. C08-0178. Gary Reys and Ronald Berninger are also named in all three lawsuits, and John Fluke is named in theTolericolawsuit. The three lawsuits are virtually identical and allege,inter alia, that the Company, and its officers and directors filed misleading statements with the Securities and Exchange Commission regarding the Company's products, and that the Company posted misleading information regarding an officer on its website. The lawsuits claim that investors purchased our stock based on the alleged misleading statements and the plaintiffs are seeking monet ary relief. The three lawsuits have been consolidated and an amended complaint will be filed. None of the lawsuits has been certified for class action status as of the date of this report. We are disputing the basis for all three lawsuits and intend to vigorously defend against them. While we do not believe any of the lawsuits has merit, an adverse outcome could have a material adverse effect on our financial position and results.
We are also currently engaged in a dispute with a former employee, who voluntarily resigned in 2007. The employee is asserting claims for monetary damages based upon a constructive discharge theory and other claims. The employee has been ordered to arbitrate her claims pursuant to the terms of a contract between the employee and CellCyte. We do not believe that the employee's claims have merit, nor do we believe that an adverse outcome will have a material adverse effect on our financial position. The employee has opened her own biotech company and is on notice regarding that which CellCyte deems proprietary information. At this time, we do not believe that CellCyte's intellectual property is imminently threatened. Should we determine that the employee is trying to operate her new endeavor with intellectual property that belongs to CellCyte, we will take appropriate action.
Although management currently believes that resolving claims against us, individually or in aggregate, will not have a material adverse impact on our financial position, our results of operations, or our cash flows, these matters are subject to inherent uncertainties and management's view of these matters may change in the future.
In January 2008, the Securities and Exchange Commission ("SEC") notified the Company that it was commencing an informal non-public inquiry relating to certain matters related to the Company's filings. The SEC requested that the Company voluntarily produce documents relating to its investigation. On May 12, 2008, the Company learned that the informal inquiry commenced by the SEC had become a formal investigation. The Company believes that the focus of the formal investigation is substantially the same as the informal inquiry and intends to continue to fully cooperate with the SEC regarding this matter. The company is committed to resolving the issues raised in connection with the investigation.
Item 1A. Risk Factors
Not Applicable.
Item 2. Unregistered Sales of Equity Securities
During the period ended March 31, 2008, the Company issued securities as follows:
- 24 -
- 67,146 shares of common stock were issued on the exercise of common stock purchase warrants at a price of $3.00 per warrant for total proceeds of $201,438 pursuant to Rule 506 to accredited investors.
- 112,477 shares of common stock were issued in exchange for services with an estimated fair value of $97,801.
- 840,000 units were issued at a price of $0.25 per unit for total proceeds of $210,000 pursuant to Rule 506 to accredited investors.Each unit consists of one common share of CGN and one warrant to acquire an additional common share of CGN at a price of $0.75 per share, exercisable for a period ending 18 months from the date of issuance of the Units.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Submission of Matters to a Vote of Security Holders
None.
Item 5. Other Information
None.
Item 6. Exhibits
The following exhibits are included with this Quarterly Report on Form 10-Q:
Exhibit
Number | Description of Exhibit |
3.1 | Articles of Incorporation, as amended. Incorporated by reference to our Registration Statement on Form SB-2 filed on May 4, 2005. |
3.2 | Bylaws. Incorporated by reference to our Current Report on Form 8-K filed May 14, 2004. |
10.1 | Agreement in Principle between Shepard Inc. and CellCyte Genetics Corporation and the Principal Vendor of CellCyte Genetics Corporation dated January 26, 2007. Incorporated by reference to our Current Report on Form 8-K filed February 2, 2007. |
10.2 | Share Exchange Agreement among CellCyte Genetics Corporation (Nevada), CellCyte Genetics Corporation (Washington) and the Vendors of CellCyte Genetics Corporation (Washington) dated March 14, 2007. Incorporated by reference to our Current Report on Form 8-K filed March 15, 2007. |
10.3 | Employment Agreement between the Company and Gary A. Reys. Incorporated by reference to our Current Report on Form 8-K filed June 5, 2007. |
10.4 | Employment Agreement between the Company and Dr. Ronald W. Berninger. Incorporated by reference to our Current Report on Form 8-K filed June 5, 2007. |
- 25 -
Exhibit
Number | Description of Exhibit |
10.5 | Consulting Agreement between the Company and Jack Wynn & Co., Inc. Incorporated by reference to our Registration Statement on Form SB-2 filed June 29, 2007. |
10.6 | CEO Award Agreement between the Company and Gary A. Reys. Incorporated by reference to our Current Report on Form 8-K filed June 5, 2007. |
10.7 | Sublease Agreement between the Company and Icogenex Corporation. Incorporated by reference to our Registration Statement on Form SB-2 filed June 29, 2007. |
10.8 | Lease Agreement between the Company and Teachers Insurance and Annuity Association of America, Inc. Incorporated by reference to our Registration Statement on Form SB-2 filed June 29, 2007. |
10.9 | 2007 Stock Option/Restricted Stock Plan. Incorporated by reference to our Registration Statement on Form SB-2 filed June 29, 2007. |
31.1 | Rule 13a-14(a)/15(d)-14(a) Certification of Chief Executive Officer. |
31.2 | Rule 13a-14(a)/15(d)-14(a) Certification of Principal Accounting Officer. |
32.1 | 18 U.S.C. Section 1350 Certification of Chief Executive Officer. |
32.2 | 18 U.S.C. Section 1350 Certification of Principal Accounting Officer. |
__________
- 26 -
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | CELLCYTE GENETICS CORPORATION |
Dated: May 15, 2008. Per: |
/s/ "Gary A. Reys"
Gary A Reys
President, Chief Executive Officer and a director
|
__________


