Use these links to rapidly review the document
TABLE OF CONTENTS
INDEX TO FINANCIAL STATEMENTS
As filed with the Securities and Exchange Commission on July 9, 2014.
Registration No. 333-196875
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
IRADIMED CORPORATION
(Exact name of registrant as specified in its charter)
Delaware | 3841 | 73-1408526 | ||
(State or other jurisdiction of | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
1025 Willa Springs Dr.
Winter Springs, FL 32078
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Roger Susi
President
iRadimed Corporation
1025 Willa Springs Dr.
Winter Springs, FL 32078
(407) 677-8022
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to: | ||
Leib Orlanski, Esq. | Michael J. Kinkelaar, Esq. | |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ý
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company ý |
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee(4) | ||
|---|---|---|---|---|
Common Stock, par value $0.0001 per share | $12,075,000 | $1,555.26 | ||
Underwriters' Warrant to Purchase Common Stock(3) | $— | $— | ||
Common Stock Underlying Underwriters' Warrants, $0.0001 par value per share(3) | $1,365,000 | $175.81 | ||
Total Registration Fee | $1,731.07 | |||
| ||||
- (1)
- Includes the aggregate offering price of additional shares the underwriters have the option to purchase in this offering to cover over-allotments, if any.
- (2)
- Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
- (3)
- We have agreed to issue warrants exercisable within three years after the effective date of this registration statement representing 10% of the securities issued in the offering (the "Underwriter Warrant") to the underwriters. The initial issuance of the Underwriter Warrant and resales of shares of Common Stock issuable upon exercise of the Underwriter Warrant are registered hereby. See "Underwriting."
- (4)
- A total of $1,648.64 of the registration fee has been previously paid.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission acting pursuant to said section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JULY 9, 2014
![]()
1,750,000 Shares of Common Stock
This is the initial public offering of securities of iRadimed Corporation. We are offering to sell 1,750,000 shares of our common stock.
Prior to this offering, there has been no public market for our securities. The initial public offering price is expected to be between $5.00 and $6.00 per share. We intend to apply to list our shares of common stock on the NASDAQ Capital Market under the symbol "IRMD."
We are an "emerging growth company" and a "smaller reporting company" under federal securities laws and are subject to reduced public company reporting requirements. Investing in the common stock involves risks that are described in the "Risk Factors" section beginning on page 10 of this prospectus.
| | Per Share | Total | |||||
|---|---|---|---|---|---|---|---|
Public offering price | $ | $ | |||||
Underwriting discounts and commissions(1) | $ | $ | |||||
Proceeds, before expenses, to us | $ | $ | |||||
- (1)
- We have also granted warrants to the underwriters in connection with this offering. See "Underwriting" beginning on page 105 for a description of the compensation payable by us to the underwriters.
We have granted the underwriters a 45-day option to purchase a maximum of 262,500 additional shares from us at the public offering price, less the underwriting discounts and commissions, to cover over-allotments, if any.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Delivery of the shares will be made on or about , 2014.
Sole Book-Running Manager
Roth Capital Partners
Co-Manager
Monarch Capital Group
The date of this prospectus is , 2014
| | Page | |
|---|---|---|
Prospectus Summary | 1 | |
Offering Summary | 7 | |
Summary Financial and Other Data | 8 | |
Risk Factors | 10 | |
Special Note Regarding Forward-Looking Statements | 30 | |
Use of Proceeds | 32 | |
Dividend Policy | 33 | |
Capitalization | 34 | |
Dilution | 36 | |
Selected Financial and Other Data | 38 | |
Management's Discussion and Analysis of Financial Condition and Results of Operations | 41 | |
Business | 56 | |
Management | 81 | |
Executive and Director Compensation | 85 | |
Certain Relationships and Related Transactions | 91 | |
Beneficial Ownership of Common Stock | 92 | |
Description of Capital Stock | 94 | |
Shares Eligible for Future Sale | 99 | |
Material U.S. Federal Income Tax Considerations for Non-U.S. Holders | 101 | |
Underwriting | 105 | |
Legal Matters | 111 | |
Experts | 111 | |
Where You Can Find More Information | 111 | |
Index to Financial Statements | F-1 | |
Information Not Required in Prospectus | II-1 |
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. We take no responsibility for, and we provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our securities. Our business, financial condition, results of operations, and prospects may have changed since that date.
Through and including , 2014 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
For investors outside the U.S.: neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the U.S. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Our name, our logo, and other trademarks or service marks of ours appearing in this prospectus are the property of iRadimed Corporation. Trade names, trademarks, and service marks of other companies appearing in this prospectus are the property of their respective holders.
i
We use industry and market data throughout this prospectus, which we have obtained from internal market research, independent industry publications, or other publicly available information. Some data is also based on our good faith estimates, which are derived from our internal research, industry publications, and our management's knowledge and experience in the markets in which we operate. Although we believe that each such source and our internal data are reliable as of their respective dates, the information contained in such sources has not been independently verified. While we are not aware of any misstatements regarding any industry and market data or our internal data presented herein, such data are subject to change based on various factors, including those discussed under the heading "Risk Factors" in this prospectus. We have not commissioned, nor are we affiliated with, any of the independent industry sources we cite.
ii
This summary highlights information contained in other parts of this prospectus. Because it is a summary, it does not contain all of the information that you should consider before investing in the shares of common stock. You should read the entire prospectus carefully, including "Risk Factors," "Selected Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations," Business," and our financial statements and related notes before deciding to invest in our common stock. References in this prospectus to "iRadimed," "our company," "we," "our," and "us" refer to iRadimed Corporation, unless the context indicates otherwise.
Our Company
We are the leading provider of non-magnetic intravenous ("IV") infusion pump systems that are safe for use during magnetic resonance imaging ("MRI") procedures. Electromechanical medical devices and pumps contain magnetic and electronic parts which generate radio frequency ("RF") noise, create interference and are dangerous to operate in the presence of the powerful magnet which drives an MRI. Our mRidium (3850/3860+) IV pump systems have been designed with non-ferrous parts, ceramic ultrasonic motors, non-magnetic mobile stand and other special features in order to safely and predictably deliver anesthesia and other IV fluids during various MRI procedures. Many critically-ill patients cannot be removed from their vital medications, and children and infants must generally be sedated in order to remain immobile during an MRI scan. Our pump solution provides a seamless approach to providing IV fluids before, during and after an MRI scan. Given the rate of new MRI installations, expanding use of MRI procedures, growing attention to patient safety, and limited direct competition, we believe the market opportunity for our mRidium MRI compatible IV infusion pumps will grow over the next five years to over $500 million, with additional revenue generated from the sale of disposable IV sets used during every patient infusion.
In fiscal year 2012, we undertook a direct sales strategy in the United States. Today, our direct sales force consists of eight sales representatives, supplemented by two clinical support representatives, and our goal is to expand our U.S. sales force to 10 sales representatives and three clinical support representatives by the end of 2014. We have distribution agreements with 35 independent distributors selling our products internationally.
As of March 31, 2014 we estimate that we had approximately 1,917 IV infusion pump systems installed globally. Each system consists of an mRidium MRI compatible IV infusion pump, non-magnetic mobile stand, and proprietary disposable IV tubing sets and many systems contain additional optional upgrade accessories. We generate revenue from the one-time sale of pumps and accessories, ongoing service contracts and the sale of our proprietary disposable IV tubing sets, which are required to be used by our pump systems during each patient infusion. Our revenue growth has accelerated since initiating our direct sales effort. In fiscal year 2013, our revenue reached $11.3 million and our operating profit was $2.8 million representing an operating margin of 24.6%. This operating margin reflects the blended results of our IV infusion pumps, pump upgrades and disposable IV tubing sets.
Today, we believe we face limited direct competition for our MRI compatible IV infusion pump system. During 2013, our largest competitor announced its decision to commence removal of its pump systems from the U.S. market, and to discontinue support throughout the world by June 30, 2015 due to ongoing regulatory issues. Since our inception, we have initiated two voluntary recalls on some of our pump systems when we became aware of operating issues in field use. However, we are currently selling all of our pump systems, and we intend to aggressively market our mRidium 3860+ IV pump
1
system to the current users of our competitor's discontinued pump system as well as to new acute care facilities and trauma hospitals globally.
Our Products
The mRidium MRI compatible IV infusion pump system is based upon a patented non-magnetic ultrasonic motor and fluid control system and other uniquely-designed non-magnetic parts in order to provide accurate and dependable fluid delivery to patients undergoing a magnetic resonance ("MR") procedure. Our mRidium MRI compatible IV infusion pump system has been designed to offer numerous advantages to hospitals, clinicians and patients. mRidium's strengths include the following:
- •
- The only truly non-magnetic MRI compatible IV infusion pump system specifically designed and built for the MRI environment.
- •
- A mobile, rugged, easy-to-operate, and reliable system with a strong safety track record.
- •
- Able to operate virtually anywhere in the MRI scanner room; approved for use in the presence of 0.2T to 3T magnets and fully operational in up to a 10,000 gauss magnetic field.
- •
- The only non-magnetic MRI compatible IV infusion pump available with a Dose Error Reduction System ("DERS") to reduce the risk of medication errors and simplify clinician monitoring.
- •
- Available with a wireless remote display/control providing clinicians and technicians control and visibility from outside of the MRI scanner room.
- •
- Available with an add-on channel allowing for the easy addition of a second IV line for patients requiring multiple IV medications at a low incremental cost to the hospital.
- •
- Available with a built-in SpO2 monitor using Masimo SET® technology and a specially designed fiber optic SpO2 sensor allowing one device to monitor oxygen saturation levels while safely providing IV infusion during an MRI procedure.
Our MRI compatible IV infusion pump systems include the 3850, 3850R and 3860+ MRI compatible IV infusion pump, proprietary single-use IV tubing sets, a non-magnetic pole and a lithium battery. The newest model, 3860+, is currently being given the greatest marketing effort as we move to obsolete the 3850 type. In addition, we offer optional system upgrades including the 3865 Remote Display/Control, 3861 Side Car, DERS software feature, and an SpO2 sensor.
Market Opportunity
MRI provides physicians a noninvasive method to visualize vital organs and to identify blockages, growths and other difficult to detect diseases, conditions and other valuable diagnostic information. Hospitals and other medical facilities have been increasingly developing and using MRI for new procedures. These procedures include cardiac stress testing, intraoperative MRI and neurology MRI techniques. We believe that our mRidium products offer a unique and effective way to offer delivery of critical IV fluids safely and accurately in the expanding MRI field. While the benefits and utility of MRI systems and interventional MR are manifest and increasing, there are hazards intrinsic to the MR environment which must be respected. The MRI suite is a harsh place for medical devices, and safe and proper patient care requires specialty equipment that is specifically designed and built for the MR environment.
Intravenous fluids are needed during MRI procedures for many different reasons other than as contrast enhancement agents. Infusion pumps are often required in the MR scan room for patients who are not able to lie still without sedation or who require critical medications. For those medical facilities
2
that do not currently own an MRI compatible IV infusion pump, one of the most common methods is to attempt to use a conventional (magnetic) pump with long IV lines at a great distance from the patient and MR machine or from outside the MRI scanner room. However, overextended tubing can cause inaccurate drug delivery and false alarms or, more seriously, delayed alarms for equipment issues such as occlusion, especially when low-delivery rates are being used. Such makeshift extension sets can also affect the effectiveness of fluid delivery. A user's adjustment of dosage and other settings may take longer to reach the patient due to the over-extended tubing, with an attendant waste of oftentimes expensive drugs.
More seriously, there are risks in using a conventional IV pump that is mistakenly believed to be positioned at a safe distance from the MR scanner. The invisible powerful magnetic fields present during the MR procedure may result in metal objects in the MR environment being drawn with great force into the bore of the MR system, resulting in potentially deadly projectiles. Moreover, an MR scanner's radio frequency and time variant electro-magnetic field can induce currents in cables and other conductive materials near the MR system and cause the cables to heat. Hot cables may result in burns if they come into contact with a patient. Other problems in working in a MR environment include devices malfunctioning and low-quality images due to artifacts caused by RF interference.
During 2012, our only direct competitor in the MRI compatible IV infusion pump business, Bayer Radiology (formerly MEDRAD, Inc.), became the subject of an FDA recall with respect to its market-leading Continuum device. In late 2013, Bayer Radiology announced to its customers its decision to remove all Continuum pumps from the market. Bayer Radiology currently intends to complete its removal of its Continuum pumps no later than June 30, 2015, at which time Bayer Radiology will end its limited supply of its proprietary consumable IV sets that some current customers are receiving. As a result of Bayer Radiology's announced exit from the market, we anticipate that many Continuum customers will replace their MRI compatible pumps with our mRidium 3860+. Based on this exit from the market, plus the estimated size of the untapped portion of the market for MRI compatible IV infusion pumps, we believe our current market opportunity represents approximately 19,800 pump systems.
Our Business Strategy
Our goal is to provide access to MRI diagnostics to the patients who need assistance from IV pumps in the harsh magnetic MRI environment. We seek to grow our business by, among other things:
- •
- Driving market awareness of MRI compatible IV infusion pumps and the safety risks associated with using conventional IV pumps with long IV lines. We believe that the largest potential market for our MRI compatible IV infusion pumps is the segment of the market that is currently using workaround solutions. Such solutions include using conventional pumps outside the MRI scanner room and attaching multiple extension IV tubing sets through the wall or under the door into the magnet room to reach the patient. This practice of makeshift setups is fraught with risks to the patient and unnecessary costs and inefficiencies. We believe that increased market awareness and education will be required for these customers to appreciate the value for patients and the hospital of an efficient and patient-safe MRI environment which includes MRI compatible IV infusion pumps.
- •
- Expanding our MRI-focused sales force and customer service teams. We feel the single greatest impact of our commercialization strategy is the continued development and expansion of our direct sales force. Since there is currently no direct competitor for our MRI compatible IV infusion pump in the U.S., our focus is on expansion of the market through better education on advantages to patients, clinicians and hospitals of our pump systems and the shortcomings of current workaround solutions. Our challenge in the past
3
- •
- Continuing to innovate with MRI compatible patient care products. Our management team collectively has more than 100 years of experience with MRI compatible products. We have entrenched relationships with many of the industry's top thought leaders, and we have, and will continue to, closely collaborate with them to build upon mRidium's innovative MRI compatible technologies to create next generation pump systems and other MRI compatible products. We currently have under development a new MRI compatible resuscitation device which includes multi-parameter vital signs, and are researching the market for a patient thermal management unit.
- •
- Acquiring synergistic MRI patient care companies or products where we can leverage our experience and organization. We have an experienced team of engineering and operations managers committed to improving on existing MRI patient care designs through our internal development efforts and the acquisition of technologies and intellectual property of others. We have an effective and growing direct sales organization in the U.S. and a team of experienced international distributors that can effectively go to market with additional MRI patient care products. We intend to actively analyze opportunities to improve our product mix and profitability.
has been an understaffed sales team and our limited ability to educate new potential customers. We intend to devote a significant amount of time and resources to ensure that we provide our customers with a first-class clinical education to facilitate the adoption of our products. We believe that educated customers and potential customers, coupled with a positive user experience, will be critical to driving increased rates of utilization of our pump systems and their associated consumable IV sets.
Selected Risk Factors Associated with Our Business
An investment in our common stock involves substantial risks and uncertainties that may adversely affect our business, financial condition, results of operations, and cash flows. You should fully read and consider the information set forth under the "Risk Factors" section beginning on page 10 and all other information included in this prospectus before investing in our common stock. Some of the more significant risks relating to an investment in our company include the following:
- •
- Our financial performance is currently dependent on a single product which could be rendered obsolete or economically impractical by numerous factors, many of which are beyond our control;
- •
- We have single-source suppliers for multiple components of our products, and the disruption of any part of our supply chain could negatively impact our business;
- •
- If we or our suppliers fail to obtain, or experience delays in obtaining regulatory approval, our business could suffer;
- •
- We manufacture and store our products at a single facility;
- •
- We are highly dependent on our founder, Chief Executive Officer, President and controlling shareholder, Roger Susi, who will be able to exert significant control over matters subject to stockholder approval;
- •
- If we fail to maintain relationships with group purchasing organizations, sales of our products could decline;
- •
- Sales of our products generally require a lengthy sales cycle, which entails the passage of three to six months between initial discussions and the sale;
4
- •
- We rely on distributors to sell our products outside the U.S., and if they do not continue to purchase products from us our revenues could decline;
- •
- We may be unable to scale our operations successfully;
- •
- We may incur substantial product liability losses or become subject to other lawsuits relating to our products or business;
- •
- Our success depends on our ability to protect our intellectual property, and we cannot guarantee that the steps we have taken or will take in the future will be adequate;
- •
- The market for our stock is likely to be illiquid meaning that it may be difficult for you to sell your stock in the future;
- •
- If you purchase shares of common stock in this offering, you will incur immediate and substantial dilution; and
- •
- We may need to raise additional capital.
Corporate Information
Our company is incorporated under the name iRadimed Corporation in Delaware. We were originally incorporated in Oklahoma under the name IRI Development, Inc. in 1992, and we merged our Oklahoma corporation into the newly formed Delaware corporation in April 2014. Our principal executive offices are located at 1025 Willa Springs Dr., Winter Springs, FL 32078, and our telephone number is (407) 677-8022. Our internet address is www.iradimed.com. Information contained in, or accessed through, our website is not a part of this prospectus.
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act ("JOBS Act"), enacted in April 2012. An "emerging growth company" may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These reduced reporting requirements include:
- •
- not being required to comply with the audit or attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, as amended ("Sarbanes-Oxley Act");
- •
- reduced disclosure obligations regarding executive compensation in this prospectus and in our future periodic reports, proxy statements and registration statements; and
- •
- not being required to hold a nonbinding advisory vote on executive compensation or to seek stockholder approval of any golden parachute payments not previously approved.
We may take advantage of these reduced reporting obligations until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act of 1933, as amended ("Securities Act"). This fifth anniversary will occur in 2019. However, if certain events occur prior to the end of such five-year period, including if we become a "large accelerated filer," our annual gross revenue exceeds $1.0 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company.
5
The JOBS Act provides that an emerging growth company can utilize an extended transition period for complying with new or revised accounting standards. We are choosing to "opt out" of this transition period and, as a result, we will comply with new or revised accounting standards when they are required to be adopted by issuers. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
We are also currently considered a "smaller reporting company," which generally means that we have a public float of less than $75 million and annual revenues of less than $50 million during the most recently completed fiscal year. If we are still considered a "smaller reporting company" at such time as we cease to be an "emerging growth company," we will be subject to increased disclosure requirements. However, the disclosure requirements will still be less than they would be if we were not considered either an "emerging growth company" or a "smaller reporting company." Specifically, similar to "emerging growth companies", "smaller reporting companies" are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports.
6
| Issuer | iRadimed Corporation | |
| Common stock offered by us | 1,750,000 shares (2,012,500 shares if the underwriters' over-allotment is exercised in full). | |
| Underwriters' over-allotment option | We have granted the underwriters a 45-day option to purchase up to a maximum of 262,500 additional shares from us at the public offering price, less the underwriting discounts and commissions, to cover over-allotments, if any. | |
| Common stock outstanding after this offering | 10,150,000 shares (10,412,500 shares if the over-allotment option is exercised in full). | |
| Participation by Insiders | The Chairman of our Board of Directors has agreed to purchase up to $500,000 of our common stock in this offering. | |
| Use of proceeds | We intend to use approximately $1.5 to $2.0 million of the net proceeds from this offering to invest in our sales and marketing efforts, approximately $1.0 to $1.5 million to fund research and development of our products and product candidates, and approximately $1.0 to $1.5 million for working capital and other general corporate purposes. We also plan to use the remainder of the net proceeds for potential acquisitions of products or businesses; however, we currently do not have any agreements or commitments relating to any potential acquisitions. See "Use of Proceeds." | |
| Risk factors | You should read the section entitled "Risk Factors" beginning on page 10 of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. | |
| Proposed NASDAQ Capital Market Symbol | IRMD |
The number of shares of our common stock to be outstanding after this offering is based on a total of 7,000,000 shares of our common stock and 1,400,000 shares of our preferred stock, which will automatically convert into shares of common stock upon completion of this offering, outstanding as of March 31, 2014 and excludes:
- •
- 1,833,192 shares of common stock issuable upon exercise of options outstanding, with a weighted-average exercise price of $1.24 per share;
- •
- 175,000 shares of common stock issuable upon the exercise of the warrant to be issued to the underwriters as compensation in connection with this offering; and
- •
- 1,000,000 shares of common stock reserved for future grant under our 2014 Equity Incentive Plan.
Unless otherwise indicated, the information in this prospectus assumes:
- •
- the automatic conversion of all outstanding shares of preferred stock into 1,400,000 shares of common stock;
- •
- the 1.75 for 1 stock split ratio applied to all of our outstanding shares and stock options outstanding in connection with our reincorporation from Oklahoma to Delaware; and
- •
- no exercise of the underwriters' over-allotment option.
7
SUMMARY FINANCIAL AND OTHER DATA
The following tables summarize our financial and other data. You should read this summary financial and other data together with the section titled "Selected Financial and Other Data" included elsewhere in this prospectus and the section titled "Management's Discussion and Analysis of Financial Condition and Results of Operations" as well as our financial statements and related notes contained in this prospectus.
We have derived the statements of operations data for the years ended December 31, 2013 and 2012 from our audited financial statements and related notes contained in this prospectus. The summary financial data for the three months ended March 31, 2014 and 2013, and as of March 31, 2014, are derived from our unaudited financial statements and related notes contained in this prospectus and are not indicative of results to be expected for the full year. Moreover, our historical results are not necessarily indicative of the results that should be expected in the future.
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Statements of Operations Data: | |||||||||||||
Revenue | $ | 3,557,237 | $ | 2,628,269 | $ | 11,340,097 | $ | 7,685,061 | |||||
Cost of revenue(1) | 656,366 | 467,113 | 2,853,385 | 2,125,921 | |||||||||
| | | | | | | | | | | | | | |
Gross profit | 2,900,871 | 2,161,156 | 8,486,712 | 5,559,140 | |||||||||
| | | | | | | | | | | | | | |
Operating expenses: | |||||||||||||
General and administrative(1) | 1,092,695 | 503,189 | 2,392,305 | 1,550,034 | |||||||||
Sales and marketing(1) | 759,789 | 568,723 | 2,297,309 | 1,930,395 | |||||||||
Research and development(1) | 224,304 | 160,411 | 1,009,872 | 654,070 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses | 2,076,788 | 1,232,323 | 5,699,486 | 4,134,499 | |||||||||
| | | | | | | | | | | | | | |
Income from operations | 824,083 | 928,833 | 2,787,226 | 1,424,641 | |||||||||
Other income (expense), net | 3,452 | (3,340 | ) | (3,458 | ) | 7,424 | |||||||
| | | | | | | | | | | | | | |
Income before provision for income taxes | 827,535 | 925,493 | 2,783,768 | 1,432,065 | |||||||||
Provision for income taxes | 304,168 | 281,566 | 846,878 | 465,980 | |||||||||
| | | | | | | | | | | | | | |
Net income | $ | 523,367 | $ | 643,927 | $ | 1,936,890 | $ | 966,085 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share: | |||||||||||||
Basic | $ | 0.07 | $ | 0.09 | $ | 0.28 | $ | 0.14 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted | $ | 0.06 | $ | 0.08 | $ | 0.22 | $ | 0.11 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Weighted average shares outstanding:(2) | |||||||||||||
Basic | 7,000,000 | 7,000,000 | 7,000,000 | 7,000,000 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted | 8,859,015 | 8,492,475 | 8,624,314 | 8,462,240 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other Financial Data: | |||||||||||||
Non-GAAP income from operations(3) | $ | 986,892 | $ | 996,813 | $ | 3,059,145 | $ | 1,597,881 | |||||
Non-GAAP net income(4) | $ | 628,585 | $ | 685,011 | $ | 2,104,710 | $ | 1,062,606 | |||||
Free cash flow(5) | $ | 457,817 | $ | (72,319 | ) | $ | 1,311,222 | $ | 1,396,216 | ||||
- (1)
- Includes stock-based compensation expense as follows:
8
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Cost of revenue | $ | 959 | $ | 3 | $ | 13 | $ | 2,050 | |||||
General and administrative | 56,935 | 4,115 | 16,461 | 1,770 | |||||||||
Sales and marketing | 95,536 | 63,775 | 255,096 | 168,316 | |||||||||
Research and development | 9,379 | 87 | 349 | 1,104 | |||||||||
| | | | | | | | | | | | | | |
Total | $ | 162,809 | $ | 67,980 | $ | 271,919 | $ | 173,240 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (2)
- The basic and diluted net income per common share data in the statement of operations data for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited) and the years ended December 31, 2013 and 2012 take into account the 1.75:1 stock split effected in conjunction with our reincorporation from Oklahoma to Delaware. The number of diluted weighted average shares outstanding during each of the periods presented includes 1,400,000 shares of our outstanding convertible preferred stock, which will automatically convert into 1,400,000 shares of our common stock upon the closing of this offering.
- (3)
- Non-GAAP income from operations is a non-GAAP financial measure that we calculate as income from operations excluding stock-based compensation expense. For more information about non-GAAP income from operations and a reconciliation of non-GAAP income from operations to income from operations, the most directly comparable financial measure calculated and presented in accordance with U.S. Generally Accepted Accounting Principles ("GAAP"), see the section titled "Selected Financial and Other Data – Non-GAAP Financial Results."
- (4)
- Non-GAAP net income is a non-GAAP financial measure that we calculated as net income excluding stock-based compensation expense, net of tax. For more information about non-GAAP net income and a reconciliation of non-GAAP net income to net income, the most directly comparable financial measure calculated and presented in accordance with GAAP, see the section titled "Selected Financial and Other Data – Non-GAAP Financial Results."
- (5)
- Free cash flow is a non-GAAP financial measure that we calculate as net cash provided by operating activities less net cash used in investing activities for purchases of property and equipment. For more information about free cash flow and a reconciliation of free cash flow to net cash provided by operating activities, the most directly comparable financial measure calculated and presented in accordance with GAAP, see the section titled "Selected Financial and Other Data – Non-GAAP Financial Results."
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
Balance Sheets Data: | ||||||||||
Cash and cash equivalents | $ | 2,908,641 | $ | 2,461,559 | $ | 1,697,306 | ||||
Working capital | $ | 5,338,852 | $ | 4,931,949 | $ | 2,665,444 | ||||
Total assets | $ | 7,758,562 | $ | 6,986,871 | $ | 5,554,212 | ||||
Total stockholders' equity | $ | 6,111,136 | $ | 5,422,784 | $ | 3,220,200 | ||||
9
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information contained in this prospectus, including our financial statements and the related notes thereto, before making a decision to invest in our common stock. Our future operating results may vary substantially from anticipated results due to a number of risks and uncertainties, many of which are beyond our control. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. The following discussion highlights some of these risks and uncertainties and the possible impact of these risks on future results of operations. If any of the following risks occur, our business, financial condition or results of operations could be materially and adversely affected. In that case, the market value of our stock could decline substantially and you could lose part or all of your investment.
Risks Relating to Our Business and Financial Condition
Our financial performance is currently dependent on a single product.
Our current revenue and profitability is dependent on the sale of the mRidium 3860+ and 3850/R MRI compatible IV infusion pump system and the ongoing sale of disposable tubing sets related to them. Sales of the mRidium 3860+ and 3850/R MRI compatible IV infusion pump systems comprised approximately 85% or $3.0 million of our net revenue for the three months ended March 31, 2014 and approximately 80%, or $8.9 million, of our net revenue for the year ended December 31, 2013. Our near-term revenue and profitability will, accordingly, be dependent upon our ability to successfully market and sell this Class II medical device. Should our mRidium pump encounter technical problems in the field, unexpected competition, or regulatory issues with the FDA, our revenue could be materially adversely impacted.
The mRidium 3860+ or 3850/R MRI compatible IV infusion pumps could be rendered obsolete or economically impractical by numerous factors, many of which are beyond our control, including:
- •
- entrance of new competitors into our markets;
- •
- loss of key relationships with suppliers, group purchasing organizations, or end-user customers;
- •
- manufacturing or supply interruptions;
- •
- product liability claims;
- •
- our reputation and product market acceptance; and
- •
- product recalls or safety alerts.
Any major factor adversely affecting the sale of our mRidium 3860+ MRI compatible IV infusion pump would cause our revenues to decline and have a material adverse impact on our business, financial condition and our common stock.
Our continued success depends on the integrity of our supply chain, including multiple single-source suppliers, the disruption of which could negatively impact our business.
Many of the component parts of our mRidium MRI compatible IV infusion pumps are obtained through supply agreements with third parties. Some of these parts require our partners to engage in complex manufacturing processes. In light of our dependence on third-party suppliers, several of which are single-source suppliers, we are subject to inherent uncertainties and risks related to their ability to
10
produce parts on a timely basis, to comply with product safety and other regulatory requirements and to provide quality parts to us at a reasonable price.
For example, we are dependent upon a single vendor for the ultrasonic motor at the core of our mRidium MRI compatible IV infusion pump. If this vendor fails to meet our volume requirements, which we anticipate will increase over time, or if the vendor becomes unable or unwilling to continue supplying motors to us, this would impact our ability to supply our pumps to customers until a replacement source is secured. Our executed agreement with this vendor provides that the price at which we purchase products from the vendor is determined by mutual agreement from time to time or should material costs change. Although we have had a long history of stable pricing with this supplier, this provision may make it difficult for us to continue to receive motors from this vendor on favorable terms or at all if we do not agree on pricing in the future. In such event, it could materially and adversely affect our commercial activities, operating results and financial condition.
In the near term, we do not anticipate finding alternative sources for our primary suppliers, including single source suppliers. Therefore, if our primary suppliers become unable or unwilling to manufacture or deliver materials, we could experience protracted delays or interruptions in the supply of materials which would ultimately delay our manufacture of products for commercial sale, which could materially and adversely affect our development programs, commercial activities, operating results and financial condition.
Additionally, any failure by us to forecast demand for, or to maintain an adequate supply of, raw materials or finished products could result in an interruption in the supply of certain products and a decline in our sales.
The manufacture of our products requires strict adherence to regulatory requirements governing medical devices and if we or our suppliers encounter problems our business could suffer.
The manufacture of our pumps and products must comply with strict regulatory requirements governing Class II medical devices in the U.S. and other regulatory requirements in foreign locations. Problems may arise during manufacturing, quality control, storage or distribution of our products for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, manufacturing quality concerns, or problems with raw materials, electromechanical, software and other components, supplier issues, and natural disasters. If problems arise during production of our pump, the batch may have to be discarded. Manufacturing problems or delays could also lead to increased costs, lost sales, damage to customer relations, failure to supply penalties, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other batches of products. If problems are not discovered before the product is released to the market, voluntary recalls, corrective actions or product liability related costs may also be incurred. Should we encounter difficulties in the manufacture of our products or be subject to a product recall, our business could suffer materially.
We manufacture and store our products at a single facility in Florida.
Until recently, we manufactured and stored our products at a single facility in Winter Park, Florida. We are in the process of moving into a new larger facility being built in nearby Winter Springs by Susi, LLC, an entity controlled by our founder, Roger Susi, in order to expand our production and storage capabilities to accommodate our expected growth. If by reason of fire, hurricane or other natural disaster, or for any other reason, the facility is destroyed or seriously damaged, our ability to provide products to our customers would be seriously interrupted or impaired and our operating results and financial condition would be negatively affected.
11
More than 10% of our accounts receivables were held by one customer during the past two fiscal years, and our inability to collect on our accounts receivables held by significant customers may have an adverse effect on our business operations and financial condition.
We market our products to end users in the United States and to distributors internationally. Sales to end users in the United States are generally made on open credit terms. Management maintains an allowance for potential credit losses. Accounts receivables for one customer accounted for 10.8% of our gross accounts receivable as of December 31, 2013, while another single customer accounted for 15.4% of our gross accounts receivable as of December 31, 2012. As a result, we are exposed to a certain level of concentration of credit risk. If a major customer experiences financial difficulties, the effect on us could be material and have an adverse effect on our business, financial condition and results of operations.
If we fail to maintain relationships with GPOs, sales of our products could decline.
Our ability to sell our products to U.S. acute care facilities and outpatient imaging centers depends in part on our relationships with group purchasing organizations ("GPOs"). Many existing and potential customers for our products are members of GPOs. GPOs negotiate pricing arrangements and contracts, which are sometimes exclusive, with medical supply manufacturers and distributors, and these negotiated prices are made available to a GPO's affiliated hospitals and other members. We pay the GPOs an administrative fee in the form of a percentage of the volume of products sold to their affiliated hospitals and other members. If we are not an approved provider selected by a GPO, affiliated hospitals and other members may be less likely to purchase our products. Should a GPO negotiate a sole source or bundling contract covering a future competitor's products, we may be precluded from making sales to members of that GPO for the duration of the contractual arrangement. Our failure to renew contracts with GPOs may cause us to lose market share and could have a material adverse effect on our sales, financial condition and results of operations. We currently have GPO contracts with four major GPOs, and one of these contracts will expire in 2014 unless it is renewed. In the future, if another competitive supplier emerges, and we fail to keep our relationships and develop new relationships with GPOs, our competitive position would likely suffer.
Cost-containment efforts of our customers and purchasing groups could adversely affect our sales and profitability.
Our MRI compatible IV infusion pumps are considered capital equipment by many potential customers, and hence changes in the budgets of healthcare organizations and the timing of spending under these budgets and conflicting spending priorities can have a significant effect on the demand for our products and related services. Any decrease in expenditures by these healthcare facilities could decrease demand for our products and related services and reduce our revenue.
Any failure in our efforts to educate clinicians, anesthesiologists, radiologists, and hospital administrators regarding the advantages of our products could significantly limit our product sales.
Our future success will require us to educate a sufficient number of clinicians, anesthesiologists, radiologists, hospital administrators and other purchasing decision-makers about our products and the costs and benefits of MRI compatible IV infusion pump systems. If we fail to demonstrate the safety, reliability and economic benefits of our products to hospitals and acute medical facilities, our products may not be adopted and our sales will suffer.
The lengthy sales cycle for the mRidium 3860+ MRI compatible IV infusion pump could delay our sales.
The decision-making process of customers is often complex and time-consuming. Based on our experience, we believe the period between initial discussions concerning the mRidium 3860+ MRI compatible IV infusion pump and a purchase of a unit is three to six months. The process can be
12
delayed as a result of capital budgeting procedures. Moreover, even if one or two units are sold to a hospital, we believe that it will take additional time and experience with the mRidium 3860+ MRI compatible IV infusion pump before other medical professionals routinely use the mRidium 3860+ MRI compatible IV infusion pump for other procedures and in other departments of the hospital. Such time would delay potential sales of additional units and disposable tubing or additional optional accessories to that medical facility or hospital. These delays could have an adverse effect on our business, financial condition and results of operations.
Because we rely on distributors to sell our products outside of the U.S., our revenues could decline if our existing distributors do not continue to purchase products from us or if our relationship with any of these distributors is terminated.
We rely on distributors for all of our sales outside the U.S. and hence do not have direct control over foreign sales activities. These distributors also assist us with regulatory approvals and the education of physicians and government agencies. Our revenues outside the U.S. represented approximately 28.6% of our net revenues in fiscal year 2013, and we intend to continue our efforts to increase our sales in Europe, Japan and other countries. If our existing international distributors fail to sell our products or sell at lower levels than we anticipate, we could experience a decline in revenues or fail to meet our forecasts. We cannot be certain that we will be able to attract new international distributors that market our products effectively or provide timely and cost-effective customer support and service. None of our existing distributors are obligated to continue selling our products.
If we do not successfully develop and commercialize enhanced products or new products that remain competitive, we could lose revenue opportunities and customers, and our ability to achieve growth would be impaired.
The medical device industry is characterized by rapid product development and technological advances, which places our products at risk of obsolescence. Our long-term success depends upon the development and successful commercialization of new products, new or improved technologies and additional applications for non-magnetic infusion technology. The research and development process is time-consuming and costly and may not result in products or applications that we can successfully commercialize. If we do not successfully adapt our technology, products and applications, we could lose revenue opportunities and customers. In addition, we may not be able to improve our products or develop new products or technologies quickly enough to maintain a competitive position in our markets and continue to grow our business.
We are highly dependent on our founder, CEO, President, Director and controlling shareholder, Roger Susi.
Roger Susi developed our mRidium MRI compatible IV infusion pump system, and we believe that he will play a significant role in our continued success and in the development of new products including an MRI compatible device for patient resuscitation. Our current and future operations could be adversely impacted if we were to lose his services. We intend to carry key man life insurance on Roger Susi in the amount of $2,000,000. Accordingly, our success will be dependent on appropriately managing the risks related to executing a succession plan for Mr. Susi on a timely basis.
If we fail to attract and retain the talent required for our business, our business could be materially harmed.
Competition for highly skilled personnel is often intense in the medical device industry, and more specifically in the MRI compatible medical device industry. A number of our executives and employees are former employees of Invivo Corporation, where Mr. Susi developed the first MRI compatible patient monitoring system. If our current employees with experience in the MRI compatible device industry leave our company, we may have difficulty finding replacements with an equivalent amount of experience and skill, which could harm our operations. Our future success will also depend in part on our ability to identify, hire and retain additional personnel, including skilled engineers to develop new
13
products, and executives to oversee our marketing, sales, customer support and production staff. We may not be successful in attracting, integrating or retaining qualified personnel to meet our current growth plans or future needs. Our productivity may be adversely affected if we do not integrate and train our new employees quickly and effectively.
Also, to the extent we hire personnel from competitors, we may be subject to allegations that we have improperly solicited, or that they have divulged proprietary or other confidential information, or that their former employers own their inventions or work product.
We may be unable to scale our operations successfully.
Our plan is to grow rapidly. Our growth, if it occurs as planned, will place significant demands on our management and manufacturing capacity, as well as our financial, administrative and other resources. We cannot guarantee that any of the systems, procedures and controls we put in place will be adequate to support the manufacture and distribution of our products. Our operating results will depend substantially on the ability of our officers and key employees to manage changing business conditions and to implement and improve our financial, administrative and other resources. If we are unable to respond to and manage changing business conditions, or the scale of our products, services and operations, then the quality of our services, our ability to retain key personnel and our business could be harmed.
We engage in related party transactions, which result in a conflict of interest involving our management.
We have engaged in the past, and continue to engage, in related party transactions, particularly between our company and Roger Susi and his affiliates. One significant related party transaction is the lease agreement between our company and Susi, LLC, an affiliate of Roger Susi, with respect to our planned facility in Winter Springs, Florida. Additional detail regarding this lease is included in the section entitled "Properties" on page 79 of this prospectus. In addition, related party transactions present difficult conflicts of interest, could result in disadvantages to our company and may impair investor confidence, which could materially and adversely affect us. Related party transactions could also cause us to become materially dependent on related parties in the ongoing conduct of our business, and related parties may be motivated by personal interests to pursue courses of action that are not necessarily in the best interests of our company and our stockholders. Please refer to the section entitled "Certain Relationships and Related Transactions" on page 91 for further detail regarding related party transactions and our company's policies and procedures with respect to such transactions.
Risks Related to Our Industry
We are subject to substantial government regulation that is subject to change and could force us to make modifications to how we develop, manufacture and price our products.
The medical device industry is regulated extensively by governmental authorities, principally the FDA in the U.S. and corresponding state and foreign regulatory agencies. The majority of our manufacturing processes are required to comply with quality systems regulations, including current good manufacturing practice requirements that cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging and shipping of our products. Failure to comply with applicable medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspensions of production, refusal of the FDA or other regulatory agencies to grant pre-market clearances or approvals for our products, withdrawals or suspensions of future current clearances or approvals and criminal prosecution.
14
In addition, our products are subject to pre-approval requirements by the FDA and similar international agencies that govern a wide variety of product activities from design and development to labeling, manufacturing, promotion, sales and distribution. Compliance with these regulations may be time consuming, burdensome and expensive for us. The failure to obtain, or the loss or suspension of any such pre-approval, would negatively affect our ability to sell our products, and harm our anticipated revenues.
Foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we sell our products in foreign countries, we may be subject to rigorous regulation in the future. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated revenue.
If we fail to obtain, or experience significant delays in obtaining, FDA clearances or other necessary approvals to commercially distribute new products, our ability to grow will suffer.
Our current products are Class II medical devices and hence require regulatory pre-market approval by the FDA and other federal and state authorities prior to their sale in the U.S. Similar approvals are required by foreign governmental authorities for sale of our products outside of the U.S. We are responsible for obtaining the applicable regulatory approval for the commercial distribution of our products. As part of our growth strategy, we plan to seek approvals for new MRI compatible products. The process of obtaining approvals, particularly from the FDA, can be costly and time consuming, and there can be no assurance that we will obtain the required approvals on a timely basis, or at all. Failure to receive approvals for new products will hurt our ability to grow.
We are subject to risks associated with doing business outside of the U.S.
Sales to customers outside of the U.S. comprised approximately 28.6% of our revenue in fiscal 2013, and we expect that non-U.S. sales will contribute to future growth. A majority of our international sales originate from Europe and Japan, and we also make sales in Canada, Hong Kong, Australia, Mexico and certain parts of the Middle East. The risks associated with operations outside the United States include:
- •
- foreign regulatory and governmental requirements that could change and restrict our ability to manufacture and sell our products;
- •
- possible failure to comply with anti-bribery laws such as the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws in other jurisdictions;
- •
- foreign currency fluctuations that can impact our financial statements when foreign figures are translated into U.S. dollars;
- •
- different local product preferences and product requirements;
- •
- trade protection and restriction measures and import or export licensing requirements;
- •
- difficulty in establishing, staffing and managing non-U.S. operations;
- •
- failure to maintain relationships with distributors, especially those who have assisted with foreign regulatory or government clearances;
- •
- changes in labor, environmental, health and safety laws;
- •
- potentially negative consequences from changes in or interpretations of tax laws;
- •
- political instability and actual or anticipated military or political conflicts;
15
- •
- economic instability and inflation, recession or interest rate fluctuations;
- •
- uncertainties regarding judicial systems and procedures; and
- •
- minimal or diminished protection of intellectual property.
These risks, individually or in the aggregate, could have an adverse effect on our results of operations and financial condition.
We may incur product liability losses, or become subject to other lawsuits related to our products, business, and insurance coverage could be inadequate or unavailable to cover these losses.
Our business is subject to potential product liability risks that are inherent in the design, development, manufacture and marketing of our medical devices and consumable products. We carry third party product liability insurance coverage to protect against such risks, but there can be no assurance that our policy is adequate. In the ordinary course of business, we may become the subject of product liability claims and lawsuits alleging that our products have resulted or could result in an unsafe condition or injury to patients. Any product liability claim brought against us, with or without merit, could be costly to defend and could result in settlement payments and adjustments not covered by or in excess of our product liability insurance. We currently have third-party product liability insurance with maximum coverage of $3,000,000; however, such coverage requires a substantial deductible that we must pay before becoming eligible to receive any insurance proceeds. The deductible amount is currently equal to $25,000 per occurrence and $125,000 in the aggregate. We will have to pay for defending product liability or other claims that are not covered by our insurance. These payments could have a material adverse effect on our profitability and financial condition. Product liability claims and lawsuits, safety alerts, recalls or corrective actions, regardless of their ultimate outcome, could have a material adverse effect on our business, financial condition, reputation and on our ability to attract and retain customers. In addition, we may not be able to obtain insurance in the future on terms acceptable to us or at all.
Defects or failures associated with our products and/or our quality control systems could lead to the filing of adverse event reports, recalls or safety alerts and negative publicity and could subject us to regulatory actions.
Safety problems associated with our products could lead to a product recall or the issuance of a safety alert relating to such products and result in significant costs and negative publicity. An adverse event involving one of our products could require us to file an adverse event report with the FDA. Such disclosure could result in reduced market acceptance and demand for all of our products, and could harm our reputation and our ability to market our products in the future. In some circumstances, adverse events arising from or associated with the design, manufacture or marketing of our products could result in the suspension or delay of regulatory reviews of our applications for new product approvals or clearances.
We may also voluntarily undertake a recall of our products or temporarily shut down production lines based on internal safety, quality monitoring and testing data. For example, in August 2012, we initiated a voluntary recall of a particular lot of mRidium Series 1000 MR Infusion Sets, Type 1058 MR IV, an extension set used with our mRidium MRI compatible IV infusion pumps, due to an out-of-specification dimension of one section of the IV set. We retrieved and destroyed all unused infusion sets subject to the recall. In July 2013, the FDA notified us that it had concluded its audit and confirmed that the recall was considered terminated. In July 2013, we issued a voluntary recall of our MRI compatible IV infusion pump systems equipped with mRidium 1145 DERS Drug Library due to their potential risk in providing an incorrect recommended value for the infusion rate during the pump's initial infusion setup. To avoid future product recalls we have made and continue to invest in our quality systems, processes and procedures. We will continue to make improvements to our products and systems to further reduce issues related to patient safety. However, there can be no assurance our
16
systems will be sufficient. Future quality concerns, whether real or perceived, could adversely affect our operating results. For a more detailed description of recalls, see the section captioned "Governmental Regulation and Other Matters" in the "Business" section on page 73.
Our products or product types could be subject to negative publicity, which could have a material adverse effect on our financial position and results of operations and could cause the market value of our common stock to decline.
The market's perception of our products could be harmed if any of our products or similar products offered by others in our industry become the subject of negative publicity due to a product safety issue, withdrawal, recall, or are proven or are claimed to be harmful to patients. The harm to market perception may have a material adverse effect on our business, financial position and results of operations and could cause the market value of our common stock to decline.
Any acquisitions of technologies, products and businesses, may be difficult to integrate, could adversely affect our relationships with key customers, and/or could result in significant charges to earnings.
We plan to periodically review potential acquisitions of technologies, products and businesses that are complementary to our products and that could accelerate our growth. However, our company has never completed an acquisition and there can be no assurance that we will be successful in finding any acquisitions in the future. The process of identifying, executing and realizing attractive returns on acquisitions involves a high degree of uncertainty. Acquisitions typically entail many risks and could result in difficulties in integrating operations, personnel, technologies and products. If we are not able to successfully integrate our acquisitions, we may not obtain the advantages and synergies that the acquisitions were intended to create, which may have a material adverse effect on our business, results of operations, financial condition and cash flows, our ability to develop and introduce new products and the market price of our stock.
Recent U.S. healthcare policy changes, including the Affordable Care Act and PPACA, may have a material adverse effect on our financial condition and results of operations.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the "PPACA"), enacted in March 2010, implemented changes that are expected to significantly impact the medical device industry. Beginning on January 1, 2013, the Affordable Care Act imposed a 2.3% excise tax on sales of products defined as "medical devices" by the regulations of the FDA. We believe that all of our medical products are "medical devices" within the meaning of the FDA regulations. For the three months ended March 31, 2014 and the year ended December 31, 2013, we recorded $51,333 and $161,246, respectively, in medical device taxes, which is included as a component of general and administrative expense. If this tax rate is increased in future years, it would negatively impact our operating results.
Other significant measures contained in the PPACA include research on the comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. The PPACA also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. In addition, the PPACA established an Independent Payment Advisory Board ("IPAB"), to reduce the per capita rate of growth in Medicare spending. The IPAB has broad discretion to propose policies to reduce health care expenditures, which may have a negative impact on payment rates for services, including treatments and procedures which incorporate use of our products. The IPAB proposals may impact payments for treatments and procedures that use our technology beginning in 2016 and for hospital services beginning in 2020, and may indirectly reduce demand for our products.
17
The taxes imposed by the new federal legislation and the expansion in government's effect on the U.S. healthcare industry may result in decreased profits to us, lower reimbursements by payers for our products or reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations.
We are subject to healthcare fraud and abuse regulations that could result in significant liability, require us to change our business practices and restrict our operations in the future.
We and our customers are subject to various U.S. federal, state and local laws targeting fraud and abuse in the healthcare industry, including anti-kickback and false claims laws. Violations of these laws are punishable by criminal or civil sanctions, including substantial fines, imprisonment and exclusion from participation in healthcare programs such as Medicare and Medicaid, and Veterans' Administration health programs and health programs outside the U.S. These laws and regulations are broad in scope and are subject to evolving interpretations, which could require us to alter one or more of our sales or marketing practices. In addition, violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our sales, profitability and financial condition. Furthermore, since many of our customers rely on reimbursement from Medicare, Medicaid and other governmental programs to cover a substantial portion of their expenditures, if we or our customers are excluded from such programs as a result of a violation of these laws, it could have an adverse effect on our results of operations and financial condition. We have developed and implemented business practices and processes to train our personnel to perform their duties in compliance with healthcare fraud and abuse laws and conduct informal oversight to detect and prevent these types of fraud and abuse. However, we lack formal written policies and procedures at this time. If we are unable to formally document and implement the controls and procedures required in a timely manner or we are otherwise found to be in violation of such laws, we might suffer adverse regulatory consequences or face criminal sanctions, which could harm our operations, financial reporting or financial results.
The environment in which we operate makes it increasingly difficult to forecast our business performance.
Significant changes and volatility in the global financial markets, in the consumer and business environment, and our general competitive landscape may make it increasingly difficult for us to predict our revenues and earnings into the future. Our quarterly sales and profits depend substantially on the volume and timing of orders fulfilled during the quarter, and such orders are difficult to forecast. Product demand is dependent upon the capital spending budgets of our customers and prospects as well as government funding policies, and matters of public policy as well as product and economic cycles that can affect the spending decisions of these entities. As a result, any revenue or earnings guidance or outlook which we have given or might give may turn out to be inaccurate. Though we will endeavor to give reasonable estimates of future revenues and earnings at the time we give such guidance, based on then-current conditions, there is a significant risk that such guidance or outlook will turn out to be incorrect. Historically, companies that have overstated their operating guidance have suffered significant declines in their stock price when such results are announced to the public.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws.
The U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. We intend to adopt policies for compliance with these anti-bribery laws, which often carry substantial penalties. We cannot assure you that our internal control policies and procedures always will protect us from reckless or other inappropriate acts committed by our affiliates, employees or agents. Violations of these laws, or allegations of such violations, could have a material adverse effect on our business, financial position and results of operations and could cause the market value of our common stock to decline.
18
There are inherent uncertainties involved in estimates, judgments and assumptions used in the preparation of financial statements in accordance with United States GAAP. Furthermore, portions of GAAP require the use of fair value mathematical models which are variable in application and methodology from appraiser to appraiser. Any changes in estimates, judgments and assumptions used could have a material adverse effect on our business, financial position and operating results.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Such assumptions and estimates include those related to revenue recognition, accruals for product returns, valuation of inventory, impairment of intangibles and long-lived assets, accounting for income taxes and stock-based compensation and reserves for potential litigation. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, as discussed in greater detail in the section titled "Management's Discussion and Analysis of Financial Condition and Results of Operations." Our actual operating results may differ and fall below our assumptions and the financial forecasts of securities analysts and investors, resulting in a significant decline in our stock price.
We and our suppliers and customers are required to obtain regulatory approvals to comply with FDA regulations applicable to medical devices and infusion pumps, and these approvals could result in delays or increased costs in developing new products.
In 2010, the FDA issued a draft guidance document entitled "Total Product Life Cycle: Infusion Pump-Premarket Notification [510(k)] Submissions." Through this draft regulatory guidance, the FDA has established additional pre-market requirements for infusion pumps. At the same time, the FDA is also generally enhancing the pre-market requirements for medical devices. Although we cannot predict with certainty the future impact of these initiatives, it appears that the processes for obtaining regulatory approvals to market infusion pumps and related accessories are likely to become more costly and time consuming. In addition, the new requirements could result in longer delays for the approval of new products, or require modification or remediation of existing products in the market. Future delays in the receipt of, or failure to obtain, approvals could result in delayed or no realization of product revenues.
We and our suppliers and customers are required to maintain compliance with FDA regulations applicable to medical devices and infusion pumps, and it could be costly to comply with these regulations and to develop compliant products and processes. Failure to comply with these regulations could subject us to sanctions and could adversely affect our business.
Even if we are able to obtain approval for introducing new products to the market, we and our suppliers may not be able to remain in compliance with applicable FDA and other material regulatory requirements once clearance or approval has been obtained for a product. These requirements include, among other things, regulations regarding manufacturing practices, product labeling, off-label marketing, advertising and post-marketing reporting, adverse event reports and field alerts. Compliance with these FDA requirements is subject to continual review and is monitored through periodic inspections by the FDA. For example, the FDA conducted routine inspections of our facility in Winter Park, Florida in June 2010 and more recently between April 7 and April 16, 2014. The FDA issued a Form 483 on April 16, 2014 that identified eight observations. The majority of the observations related to procedural and documentation issues associated with the design, development, validation testing and documentation of software used in certain of our products. Other observations were related to the design validation of pump labeling, design analysis of tube stretching (which was an issue in the August 2012 voluntary recall that has been closed by the FDA), procedures for post-market design review, and control and procedures related to handling certain reported complaints. We submitted a response to the Form 483 in which we described our proposed corrective and preventative actions ("CAPA") to address each of the FDA's observations. If the FDA does not agree with our proposed CAPA plan, or
19
accepts them but finds that we have not implemented them adequately, or if we otherwise fail to comply with applicable regulatory requirements, the FDA could initiate an enforcement action, including issuing untitled letters, warning letters, fines, injunctions, consent decrees and/or civil penalties.
In addition, manufacturing flaws, component failures, design defects, off-label uses or inadequate disclosure of product related information could result in an unsafe condition or the injury or death of a patient. All of these events could harm our sales, margins and profitability in the affected periods and may have a material adverse effect on our business. Any adverse regulatory action or action taken by us to maintain appropriate regulatory compliance, with respect to these laws and regulations could disrupt our business and have a material adverse effect on our sales, profitability and financial condition. Furthermore, an adverse regulatory action with respect to any of our products or operating procedure or to our or our suppliers' manufacturing facility could materially harm our reputation in the marketplace. For a more detailed listing of the laws and regulations that significantly affect our business and operations, see the section captioned "Governmental Regulation and Other Matters" under the "Business" section on page 73.
Our operations are subject to environmental laws and regulations, with which compliance is costly and which exposes us to penalties for non-compliance.
Our business, products, and product candidates are subject to federal, state, and local laws and regulations relating to the protection of the environment, worker health and safety and the use, management, storage, and disposal of hazardous substances, waste, and other regulated materials. These environmental laws and regulations could require us to pay for environmental remediation and response costs at third-party locations where we dispose of or recycle hazardous substances. The costs of complying with these various environmental requirements, as they now exist or as may be altered in the future, could adversely affect our financial condition and results of operations.
Risks Relating to our Intellectual Property
Our success depends on our ability to protect our intellectual property.
We intend to rely on a combination of patents, trade secrets, know-how, license agreements and contractual provisions to establish and protect our proprietary rights to our technologies and products. We cannot guarantee that the steps we have taken or will take to protect our intellectual property rights will be adequate or that they will deter infringement, misappropriation or violation of our intellectual property. We may fail to secure patents that are important to our business, and we cannot guarantee that any pending U.S. patent application, if ultimately issued, will provide us some relative competitive advantage. Litigation may be necessary to enforce our intellectual property rights and to determine the validity and scope of our proprietary rights. Any litigation could result in substantial expenses and may not adequately protect our intellectual property rights. In addition, the laws of some of the countries in which our products may in the future be sold may not protect our products and intellectual property to the same extent as U.S. laws, or at all. We may be unable to protect our rights in trade secrets and unpatented proprietary technology in these countries. If our trade secrets become known, we may lose our competitive advantages.
Even if we are able to secure necessary patents in the U.S., we may not be able to secure necessary patents in foreign countries in which we sell our products or plan to sell our products. In March 2013, the U.S. transitioned to a "first inventor to file" system in which, assuming the other requirements for patentability are met, the first inventor to file a patent application is entitled to a patent. We may be subject to a third-party pre-issuance submission of prior art to the U.S. Patent and Trademark Office, or become involved in opposition, derivation, reexamination, inter parties review or interference proceedings challenging our patent rights or the patent rights of others. An adverse
20
determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third party patent rights.
The lack of registered trademarks and trade names could potentially harm the business.
We also rely on, or intend to rely on, our trademark, trade names, and brand names to distinguish our products from the products of our competitors and have registered or applied to register our own trademarks. We have filed one U.S. trademark application for "iRadimed," but there is no guarantee that our trademark application will be approved. We also have recently filed for trademark protection for the name of our U.S. product "mRidium". Third parties may also oppose any of our trademark applications or otherwise challenge our use of our claimed trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our product, which could result in loss of brand recognition and could require us to devote significant resources to advertising and marketing these new brands. Further, our competitors may infringe our trademarks or we may not have adequate resources to enforce our trademarks.
In October 2012, Radimed Gesellschaft für Kommunikationsdienstleistungen und Medizintechnik mbH ("Radimed") brought an action in Düsseldorf Regional Court against our German distributor alleging the name and sign "iRadimed" was confusingly similar to their German trademark "Radimed." A judgment was rendered against our German distributor preventing use of the name and sign "iRadimed" in Germany. We have however continued to sell products in Germany without any discernible effect by using the product name IRI Development. On July 31, 2013, Radimed filed a lawsuit against us and our founder, Roger Susi, in Düsseldorf Regional Court, alleging that we infringed their German and Community trademarks "Radimed" and seeking to prevent our use of the name, sign and domain name "iRadimed" in the European Union. In addition, Radimed is seeking unspecified damages. We will vigorously defend against the infringement claims. However, the ultimate outcome of the matter remains uncertain. If we receive an adverse judgment, we may be prevented from marketing our products in the European Union under the name and sign "iRadimed" and may be required to pay Radimed's attorneys' fees, the court fees and damages, which could materially adversely affect our business, operating results and financial condition.
Our unpatented trade secrets, know-how, confidential and proprietary information, and technology may be inadequately protected.
We rely on unpatented trade secrets, know-how and technology. This intellectual property is difficult to protect, especially in the medical device industry, where much of the information about a product must be submitted to regulatory authorities during the regulatory approval process. We seek to protect trade secrets, confidential information and proprietary information, in part, by entering into confidentiality and invention assignment agreements with employees, consultants, and others. These parties may breach or terminate these agreements, and we may not have adequate remedies for such breaches. Furthermore, these agreements may not provide meaningful protection for our trade secrets or other confidential or proprietary information or result in the effective assignment to us of intellectual property, and may not provide an adequate remedy in the event of unauthorized use or disclosure of confidential information or other breaches of the agreements. Despite our efforts to protect our trade secrets and our other confidential and proprietary information, we or our collaboration partners, board members, employees, consultants, contractors, or scientific and other advisors may unintentionally or willfully disclose our proprietary information to competitors.
There is a risk that our trade secrets and other confidential and proprietary information could have been, or could, in the future, be shared by any of our former employees with, and be used to the benefit of, any company that competes with us.
21
If we fail to maintain trade secret protection or fail to protect the confidentiality of our other confidential and proprietary information, our competitive position may be adversely affected. Competitors may also independently discover our trade secrets. Enforcement of claims that a third party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secret protections against them, which could have a material adverse effect on our business.
There can be no assurance of timely patent review and approval to minimize competition and generate sufficient revenues.
There can be no assurance that the Patent and Trademark Office will have sufficient resources to review our patent applications in a timely manner. Consequently, even if our patent applications are ultimately successful, our patent applications may be delayed, which would prevent intellectual property protection for our products. If we fail to successfully commercialize our products due to the lack of intellectual property protection, we may be unable to generate sufficient revenues to meet or grow our business according to our expected goals and this may have a materially adverse effect on our profitability, financial condition, and operations.
We may become involved in patent litigation or other intellectual property proceedings relating to our future product approvals, which could result in liability for damages or delay or stop our development and commercialization efforts.
The medical device industry has been characterized by significant litigation and other proceedings regarding patents, patent applications, and other intellectual property rights. The situations in which we may become parties to such litigation or proceedings may include any third parties (which may have substantially greater resources than we have) initiating litigation claiming that our products infringe their patent or other intellectual property rights; in such case, we will need to defend against such proceedings.
The large number of patents, the rapid rate of new patent applications and issuances, the complexities of the technologies involved and the uncertainty of litigation significantly increase the risks related to any patent litigation. Any potential intellectual property litigation also could force us to do one or more of the following:
- •
- stop selling, making, or using products that use the disputed intellectual property;
- •
- obtain a license from the intellectual property owner to continue selling, making, licensing, or using products, which license may require substantial royalty payments and may not be available on reasonable terms, or at all;
- •
- pay substantial damages or royalties to the party whose intellectual property rights we may be found to be infringing;
- •
- pay the attorney fees and costs of litigation to the party whose intellectual property rights we may be found to be infringing; or
- •
- redesign those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible.
If any of the foregoing events occur, we may have to withdraw existing products from the market or may be unable to commercialize one or more of our products, all of which could have a material adverse effect on our business, results of operations and financial condition. As the number of participants in our industry grows, the possibility of intellectual property infringement claims against us increases.
22
Furthermore, the costs of resolving any patent litigation or other intellectual property proceeding, even if resolved in our favor, could be substantial. Uncertainties resulting from the initiation and continuation of patent litigation or other intellectual property proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other intellectual property proceedings may also consume significant management time.
In the event that a competitor infringes upon our patent or other intellectual property rights, enforcing those rights may be costly, difficult, and time-consuming. Even if successful, litigation to enforce our intellectual property rights or to defend our patents against challenge could be expensive and time-consuming and could divert our management's attention. We may not have sufficient resources to enforce our intellectual property rights or to defend our patent or other intellectual property rights against a challenge. If we are unsuccessful in enforcing and protecting our intellectual property rights and protecting our products, it could materially harm our business. See "Legal and Regulatory Proceedings" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" for further details of our legal proceedings.
There may also be situations where we use our business judgment and decide to market and sell products, notwithstanding the fact that allegations of patent infringement(s) have not been finally resolved by the courts (i.e., an "at-risk launch"). The risk involved in doing so can be substantial because the remedies available to the owner of a patent for infringement may include, among other things, damages measured by the profits lost by the patent owner and not necessarily by the profits earned by the infringer. In the case of a willful infringement, the definition of which is subjective, such damages may be increased up to three times. An adverse decision could have a material adverse effect on our business, financial position and results of operations and could cause the market value of our common stock to decline.
We have been sued by Rydex Technologies LLC for alleged patent infringement, as discussed more fully under "Legal Proceedings." While this lawsuit was dismissed, the dismissal was without prejudice and we may therefore face this or one or more related lawsuits in the future.
In addition, we may indemnify our customers and distributors with respect to infringement by our products of the proprietary rights of third parties. Third parties may assert infringement claims against our customers or distributors. These claims may require us to initiate or defend protracted and costly litigation on behalf of our customers or distributors, regardless of the merits of these claims. If any of these claims succeed, we may be forced to pay damages on behalf of our customers or distributors or may be required to obtain licenses for the products they use. If we cannot obtain all necessary licenses on commercially reasonable terms, our customers may be forced to stop using our products.
We may be subject to claims that we, our board members, employees or consultants have used or disclosed alleged trade secrets or other proprietary information belonging to third parties and any such individuals who are currently affiliated with one of our competitors may disclose our proprietary technology or information.
As is commonplace in the medical device industry, some of our board members, employees and consultants are or have been associated with other medical device companies that compete with us. For example, Mr. Susi and a number of our other employees are former employees of Invivo Corporation. While associated with such other medical device companies, these individuals may have been exposed to research and technology similar to the areas of research and technology in which we are engaged. We may become subject to future claims that we, our employees, board members, or consultants have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of those companies. Litigation may be necessary to defend against such claims.
23
We have entered into confidentiality agreements with our executives and key consultants. However, we do not have, and are not planning to enter into, any confidentiality agreements with our non-executive directors because they have a fiduciary duty of confidentiality as directors. There is the possibility that any of our former board members, employees, or consultants who are currently employed at, or associated with, one of our competitors may unintentionally or willfully disclose our proprietary technology or information.
Risks Related to this Offering and Ownership of Our Common Stock
There is no established public market for our stock and a liquid public market for our common shares may never develop and therefore you may not be able to sell your shares.
Prior to this offering, there has been no public market for our common stock. The initial public offering price for the shares will be determined by negotiations between us and the underwriters and may not be indicative of prices that will prevail in the subsequent trading market. Given the relatively small size of our public offering there can be no assurance that an active trading market for our stock will ever develop. This means that you may be unable to sell your shares or you may be forced to sell your shares at a significant loss.
Our common stock price may be subject to significant fluctuations and volatility, and you may be unable to sell your shares at a fair price, or at all.
Our stock could be subject to wide fluctuations in price in response to various factors, including the following:
- •
- the commercial success or failure of our key products;
- •
- delayed or reduced orders from our customers;
- •
- manufacturing or supply interruptions;
- •
- changes or developments in laws or regulations applicable to our product candidates;
- •
- introduction of competitive products or technologies;
- •
- actual or anticipated variations in quarterly operating results;
- •
- failure to meet or exceed the estimates and projections of securities analysts or investors;
- •
- weakening economic and market conditions in the U.S.;
- •
- negative developments impacting the medical device industry in general and changes in the market valuations of companies deemed similar to us;
- •
- negative developments concerning our sources of manufacturing supply;
- •
- disputes or other developments relating to patents, trademarks or other proprietary rights;
- •
- litigation or investigations involving us, our industry, or both;
- •
- issuances of debt, equity or convertible securities at terms deemed unfavorable by the market;
- •
- major catastrophic events;
- •
- the expiration of contractual lock-up agreements;
- •
- sales of large blocks of our stock;
24
- •
- changes in our Board of Directors, management or key personnel; or
- •
- the other factors described in this "Risk Factors" section.
Any one of the factors above, or the cumulative effect of some of the factors referred to above, may result in significant fluctuations in our quarterly or annual operating results, fluctuations in our share price and investors' perception of our business. If we fail to meet or exceed such expectations, our business and stock price could be materially adversely affected.
If you purchase shares of common stock in this offering, you will incur immediate and substantial dilution.
If you purchase shares of common stock in this offering, you will incur immediate and substantial dilution in the amount of $4.13 per share, because the assumed initial public offering price of $5.50, which is the midpoint of the price range listed on the cover page of this prospectus, is substantially higher than the pro forma net tangible book value per share of our outstanding common stock. This dilution is due in large part to the fact that our earlier investors paid substantially less than the initial public offering price when they purchased their shares. Investors who purchase shares in this offering will contribute approximately 86.5% of the total amount of equity capital raised by us through the date of this offering, but will only own approximately 17.2% of our outstanding shares. In addition, you may also experience additional dilution upon future equity issuances or in the event the underwriters exercise their option to purchase additional shares. Additionally, you will experience additional dilution upon the exercise of stock options to purchase common stock granted to our employees and directors under our stock option and equity incentive plans. For additional information, see the "Dilution" section on page 36.
Future sales of our common stock may cause our stock price to decline.
If our existing stockholders sell, or indicate an intention to sell, our common stock in the public market after the contractual lock-up and other legal restrictions on resale lapse, the trading price of our common stock could decline. Our directors, officers and existing holders of all of our common stock, a total of 8,400,000 shares (taking into account the conversion of all of our preferred stock into common stock upon the closing of this offering), are subject to lock-up agreements that prevent them from selling any of their shares for a 180-day period. After the lock-up agreements expire, up to approximately 1.0 million shares will be eligible for immediate sale in the public market and approximately 7.4 million shares held by affiliates will become salable subject to volume limitations under Rule 144 under the Securities Act. Roth Capital Partners LLC may, in its sole discretion, permit shares subject to the lock-up to be sold prior to the 180-day expiration period. See the section titled "Shares Eligible For Future Sale" on page 99 for additional information.
Following the completion of this offering, we intend to file a registration statement under Form S-8 to register all of the shares issuable upon exercise of options outstanding or reserved for future issuance under our equity compensation plans. If these additional shares are sold, or if it is perceived that they will be sold, the trading price of our common stock could decline. See the section titled "Shares Eligible for Future Sale" on page 99 for additional information.
We may need to raise additional capital in the future, which could result in dilution to our stockholders and adversely affect stock price.
While we believe the proceeds from this offering will provide us with adequate capital to fund operations for at least the next 12 months, we may need to raise additional funds prior to that time. We may seek to sell additional equity or debt securities or to obtain an additional credit facility, which we may not be able to do on favorable terms, or at all. The sale of additional equity or convertible debt securities could result in additional dilution to our stockholders. If additional funds are raised through the issuance of debt securities or preferred stock, these securities could have rights that are
25
senior to holders of common stock and any debt securities could contain covenants that would restrict our operations. The sale of such securities could hurt demand for our common stock and lead our share price to decline.
Our management will have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management team, and in particular our founder and Chief Executive Officer, Roger Susi, will have broad discretion in the application of the net proceeds from this offering. Investors in the initial public offering will not have the opportunity as part of their investment decision to assess whether the net proceeds are being used appropriately. Many factors will determine how we spend the offering proceeds, and our ultimate choices may vary substantially from what is discussed in the prospectus. Our failure to apply these funds effectively could harm our business and financial condition, which could cause our stock price to decline. Pending their uses, we plan to invest the net proceeds of this offering in short- and medium-term, interest-bearing obligations; investment-grade instruments; certificates of deposit; direct or guaranteed obligations of the U.S. government and/or bank demand deposits. These investments may not yield a favorable return to our stockholders.
Roger Susi, who serves as a director and an executive officer, owns a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Prior to this offering, Roger Susi, our founder, who serves as one of our directors and Chief Executive Officer, and his affiliates beneficially own approximately 83% of our outstanding stock, taking into account preferred stock on an as-converted to common stock basis. Our directors, executive officers and each of our stockholders who own greater than 5% of our outstanding common stock and their affiliates, in the aggregate, will own approximately 74.0% of the outstanding shares of our common stock after this offering. These stockholders, if acting together, will be able to influence or control matters requiring approval by our stockholders, including the election of directors and the approval of mergers, acquisitions or other extraordinary transactions. They may also have interests that differ from yours and may vote in a way with which you disagree and which may be adverse to your interests. This concentration of ownership may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company and might ultimately affect the market price of our common stock.
Mr. Susi's majority ownership also qualifies our company as a "controlled company" and allows us to opt out of compliance with numerous corporate governance listing requirements.
In addition, we will qualify for the "controlled company" exemption under the corporate governance rules of the NASDAQ Stock Market until such a time as Mr. Susi does not control a majority of our outstanding common stock. As a "controlled company," we would be permitted to opt out of compliance with the requirements that a majority of our board of directors consist of independent directors, that our Board of Directors' compensation committee be comprised solely of independent directors, and that director nominees be selected or recommended to the Board of Directors for selection by independent directors. Upon the listing of our common stock on The NASDAQ Capital Market, a majority of our Board of Directors will be comprised of independent directors, our compensation committee will be comprised solely of independent directors, and our director nominees will be recommended for selection to our Board of Directors by a majority of our independent directors in a vote in which only independent directors may participate. Notwithstanding the availability of these exemptions, we have elected not to rely upon any of the exemptions afforded to a "controlled company" under NASDAQ rules. Our compliance is voluntary, however, and there can be no assurance that we will continue to comply with these standards in the future.
26
We do not intend to pay dividends for the foreseeable future.
The continued expansion of our business will require funding. Accordingly, we do not anticipate that we will pay any cash dividends on shares of our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our Board of Directors deems relevant. Investors seeking cash dividends should not purchase our common stock. Accordingly, if you purchase shares in this offering, realization of a gain on your investment will depend solely on the appreciation of the price of our common stock, which may never occur.
The requirements of being a public company may strain our resources, divert management's attention and affect our ability to attract and retain executive management and qualified board members.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended ("Exchange Act"), the Sarbanes-Oxley Act, the Dodd-Frank Act, the listing requirements of the NASDAQ Stock Market and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management's attention may be diverted from other business concerns, which could adversely affect our business and operating results. We may need to hire more employees in the future or engage outside consultants to monitor and advise us regarding compliance, which will increase our costs and expenses.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management's time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us, and our business may be adversely affected.
We also expect that being a public company and these new rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our Board of Directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
27
As a result of becoming a public company, we will be obligated to establish and maintain adequate internal controls. Failure to develop and maintain adequate internal controls or to implement new or improved controls could have a material adverse effect on our business, financial position and results of operations and could cause the market value of our common stock to decline.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort. Our internal controls over financial reporting are designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP. We are in the very early stages of the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act of 2002. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal controls over financial reporting, we will be unable to assert that our internal controls are effective.
We will be required to disclose changes made in our internal controls and procedures on a quarterly basis. However, our independent registered public accounting firm will not be required to report on the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until the later of the year following our first annual report required to be filed with the Securities and Exchange Commission ("SEC"), or the date we are no longer an "emerging growth company" as defined in the JOBS Act if we take advantage of the exemptions contained in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. Our remediation efforts may not enable us to avoid a material weakness in the future.
We believe that our business practices will become more visible following this offering, and this could impact our competitive environment and our risk of potential litigation.
As a result of disclosure of information in this prospectus and in filings required of a public company, our business and financial condition will become more visible potentially exposing us to new competition and threatened or actual litigation, including by competitors and other third parties. New competition could result in reduced sales of our products and adversely impact our profitability. If lawsuits prevail against us, our business and operating results could be adversely affected, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business and operating results.
We may become involved in securities class action litigation that could divert management's attention from our business and adversely affect our business and could subject us to significant liabilities.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices of small cap medical device companies. These broad market fluctuations as well a broad range of other factors, including the realization of any of the risks described in this "Risk Factors" section, may cause the market price of our common stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. We may become involved in this type of litigation in the future. Litigation is expensive and could divert management's attention and resources from our primary business, which could adversely affect our operating results. Any adverse determination in any such litigation or any amounts paid to settle any such actual or threatened litigation could require us to make significant payments. Such payment could have a material impact on how investors view our company and result in a decline in our stock price.
28
We are an "emerging growth company," and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an "emerging growth company," as defined in the JOBS Act, and intend to take advantage of certain exemptions from various reporting requirements. We cannot predict if investors will respond negatively to our reliance on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile.
As an "emerging growth company" we have also chosen to take advantage of certain provisions of the JOBS Act that allow us to provide you with less information in this prospectus than would otherwise be required. As a result it may be more difficult for you to evaluate an investment in our company.
If securities or industry analysts fail to initiate research coverage of our stock, or downgrade our stock, our trading volume might never develop and our stock price could decline.
The trading market for our common stock will depend, in part, on the research reports that securities or industry analysts publish about our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, trading market for our stock may never develop and the price of our stock could likely be negatively impacted. In the event securities or industry analysts initiate coverage, and later downgrade our stock, our stock price could decline.
Our charter documents and Delaware law have provisions that may discourage an acquisition of us by others and may prevent attempts by our stockholders to replace or remove our current management.
Upon completion of this offering, provisions in our charter documents, as well as provisions of the Delaware General Corporation Law ("DGCL"), could depress the trading price of our common stock by making it more difficult for a third party to acquire us at a price favorable to our shareholders. These provisions include:
- •
- authorizing the issuance of "blank check" preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval to defend against a takeover attempt; and
- •
- establishing advance notice requirements for nominations for election to our Board of Directors or for proposing matters that can be acted upon at stockholder meetings;
In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors. We are subject to Section 203 of the DGCL, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by our Board of Directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders, which could also affect the price that some investors are willing to pay for our common stock.
29
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains "forward-looking statements" that involve substantial risks and uncertainties. The forward-looking statements are contained principally in the sections entitled "Prospectus Summary," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business." In some cases, you can identify forward-looking statements by the following words: "may," "will," "could," "would," "should," "expect," "intend," "plan," "anticipate," "believe," "estimate," "predict," "project," "potential," "continue," "ongoing" or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements relate to future events or our future financial performance or condition and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements. These forward-looking statements include, but are not limited to, statements about:
- •
- our ability to retain the continued service of our key professional and to identify, hire and retain additional qualified professionals;
- •
- our expectations regarding the sales and marketing of our products and product candidates;
- •
- our expectations regarding the integrity of our supply chain for our products;
- •
- the timing and likelihood of FDA approvals and regulatory actions on our product candidates and product marketing activities;
- •
- the potential for adverse application of environmental, health and safety and other laws and regulations on our operations;
- •
- our expectations for market acceptance of our new products;
- •
- the potential for our marketed products to be withdrawn due to recalls, patient adverse events or deaths;
- •
- our ability to establish and maintain intellectual property on our products and our ability to successfully defend these in cases of infringement;
- •
- the implementation of our business strategies;
- •
- the potential for exposure to product liability claims;
- •
- our use of proceeds from this offering;
- •
- our financial performance expectations;
- •
- our ability to compete in the development and marketing of our products and product candidates with other competitors in the industry;
- •
- difficulties or delays in the development, production, manufacturing and marketing of new or existing products and services, including difficulties or delays associated with obtaining requisite regulatory approvals or clearances associated with those activities;
- •
- changes in laws and regulations or in the interpretation or application of laws or regulations, as well as possible failures to comply with applicable laws or regulations as a result of possible misinterpretations or misapplications;
- •
- cost-containment efforts of our customers, purchasing groups, third-party payers and governmental organizations;
30
- •
- costs associated with protecting our trade secrets and enforcing our patent, copyright and trademark rights, and successful challenges to the validity of our patents, copyrights or trademarks;
- •
- actions of regulatory bodies and other government authorities, including the FDA and foreign counterparts, that could delay, limit or suspend product development, manufacturing or sales or result in recalls, seizures, consent decrees, injunctions and monetary sanctions;
- •
- costs or claims resulting from potential errors or defects in our manufacturing that may injure persons or damage property or operations, including costs from remediation efforts or recalls;
- •
- the results, consequences, effects or timing of any commercial disputes, patent infringement claims or other legal proceedings or any government investigations;
- •
- interruption in our ability to manufacture our products or an inability to obtain key components or raw materials or increased costs in such key components or raw materials;
- •
- uncertainties in our industry due to government healthcare reform;
- •
- competitive pressures in the markets in which we operate;
- •
- the loss of, or default by, one or more key customers or suppliers; and
- •
- unfavorable changes to the terms of key customer or supplier relationships.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual results may differ materially from what we expect as expressed or implied by our forward-looking statements. In light of the significant risks and uncertainties to which our forward-looking statements are subject, you should not place undue reliance on or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. We discuss many of these risks and uncertainties in greater detail under the section entitled "Risk Factors" in this prospectus. These forward-looking statements represent our estimates and assumptions only as of the date of this prospectus regardless of the time of delivery of this prospectus or any sale of our common stock. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this prospectus.
31
We estimate that the net proceeds from our sale of 1,750,000 shares of common stock in this offering will be approximately $8.1 million (or $9.4 million if the underwriters exercise their overallotment option in full), based upon an assumed initial public offering price of $5.50 per share, the midpoint of the range on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses of $0.8 million payable by us.
A $1.00 increase or decrease in the assumed initial public offering price of $5.50 per share would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $1.6 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase or decrease of 100,000 in the number of shares we sell in the offering would increase or decrease the net proceeds to us by approximately $0.5 million, assuming that the assumed initial price to public remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We do not expect that a change in the initial price to the public or the number of shares by these amounts would have a material effect on the uses of proceeds from this offering, although it may accelerate the time at which we will need to seek additional capital.
We intend to use approximately $1.5 to $2.0 million of the net proceeds from this offering to invest in our sales and marketing efforts, approximately $1.0 to $1.5 million to fund research and development of our products and product candidates, and approximately $1.0 to $1.5 million for working capital and other general corporate purposes. We also plan to use the remainder of the funds for potential acquisitions of products or businesses that expand or complement our current business, as opportunities may arise, if at all. We currently do not have any agreements or commitments relating to any potential acquisitions for which we would use any of the net proceeds and we may not complete any such future acquisitions.
The amounts and timing of these expenditures will vary depending on a number of factors, including the amount of cash generated by our operations, competitive and technological developments, and the rate of growth, if any, of our business. We reserve the right to reallocate the proceeds of this offering in response to these and other contingencies. Accordingly, our management will have broad discretion in the application of the net proceeds, and investors will be relying on the judgment of our management regarding the application of the proceeds of this offering.
The other principal purposes of this offering are to:
- •
- increase our visibility in the markets we serve;
- •
- strengthen our balance sheet;
- •
- create a public market for our common stock;
- •
- facilitate our future access to the public capital markets;
- •
- provide liquidity for existing stockholders; and
- •
- improve the effectiveness of our equity compensation plans in attracting and retaining key employees.
We intend to invest the net proceeds in short and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or guaranteed obligations of the U.S. government and bank demand deposits, pending their use as described above.
32
In the past two fiscal years, and during the interim period, we have not paid cash dividends on our common stock or our preferred stock. We do not anticipate paying cash dividends on our common stock in the foreseeable future. Our preferred stock will be converted into common stock immediately prior to the offering and will no longer be outstanding. We currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determinations related to dividend policy will be made at the discretion of our Board of Directors. As a result, you will probably need to sell your shares to realize a return on your investment, and you may not be able to sell such securities at or above the price you paid for them.
33
The following table sets forth our cash and cash equivalents, investments and capitalization as of March 31, 2014:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to the conversion of our outstanding preferred stock into 1,400,000 shares of our common stock in connection with this offering; and
- •
- on a pro forma as adjusted basis to give further effect to the sale by us of 1,750,000 shares of our common stock in this offering at an assumed public offering price of $5.50 per share, the midpoint of the range on the cover of this prospectus, after deducting the underwriting discount and estimated offering expenses payable by us.
The pro forma and pro forma as adjusted information below is illustrative only, and our capitalization following the closing of this will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read the following table in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Description of Capital Stock," and our financial statements and related notes appearing elsewhere in this prospectus.
| | March 31, 2014 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma | Pro Forma As Adjusted(1) | |||||||
| | | | (unaudited) | |||||||
| | (in thousands, except share data) | |||||||||
Stockholders' equity: | ||||||||||
Preferred stock, par value $0.0001 per share; 10,000,000 shares authorized, 1,400,000 shares issued and outstanding, actual; no shares issued and outstanding, pro forma and pro forma as adjusted | $ | — | $ | — | $ | — | ||||
Common stock, par value $0.0001 per share; 90,000,000 shares authorized, 7,000,000 shares issued and outstanding, actual; 8,400,000 shares issued and outstanding, pro forma; 10,150,000 shares issued and outstanding, pro forma as adjusted | 1 | 1 | 1 | |||||||
Additional paid-in capital | 2,509 | 2,509 | 10,564 | |||||||
Retained earnings | 3,598 | 3,598 | 3,598 | |||||||
Accumulated other comprehensive income | 3 | 3 | 3 | |||||||
| | | | | | | | | | | |
Total stockholders' equity | 6,111 | 6,111 | 14,166 | |||||||
| | | | | | | | | | | |
Total capitalization | $ | 6,111 | $ | 6,111 | $ | 14,166 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- A $1.00 increase or decrease in the assumed initial public offering price of $5.50 per share, the midpoint of the price range reflected on the cover page of this prospectus, would increase or decrease, as applicable, our pro forma as adjusted cash and cash equivalents, and investments, additional paid-in capital, total stockholders' equity and total capitalization by approximately $1.6 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
34
The number of shares of our common stock to be outstanding after this offering is based on a total of 7,000,000 shares of our common stock and 1,400,000 shares of preferred stock, which will automatically convert into common stock upon the completion of this offering, outstanding as of March 31, 2014 and excludes:
- •
- 1,833,192 shares of common stock issuable upon exercise of options outstanding, with a weighted-average exercise price of $1.24 per share;
- •
- 175,000 shares of common stock issuable upon the exercise of the warrant to be issued to the underwriters as compensation in connection with this offering; and
- •
- 1,000,000 shares of common stock reserved for future grant under our 2014 Equity Incentive Plan.
Unless otherwise indicated, the information in this prospectus assumes:
- •
- the 1.75:1 conversion ratio applied to all of our outstanding shares and stock options outstanding in connection with our reincorporation from Oklahoma to Delaware; and
- •
- no exercise of the underwriters' over-allotment option.
35
If you invest in our common stock, your interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock upon completion of this offering, taking into account the automatic conversion of all outstanding shares of preferred stock into shares of common stock.
Investors participating in this offering will incur immediate and substantial dilution. Our pro forma net tangible book value as of March 31, 2014 was $5.9 million, or $0.70 per share of our common stock, taking into account the conversion of all of the preferred stock. Pro forma net tangible book value per share represents the amount of our total tangible assets (total assets less intangible assets) less total liabilities, divided by the number of shares of our common stock outstanding.
After giving effect to our sale in this offering of 1,750,000 shares of our common stock, at an assumed initial public offering price of $5.50 per share, the mid-point of the price range reflected on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of March 31, 2014 would have been $13.9 million, or $1.37 per share of our common stock. This represents an immediate increase in pro forma net tangible book value of $0.67 per share to our existing stockholders before this offering and an immediate dilution of $4.13 per share to new investors purchasing shares in this offering.
The following table illustrates this dilution:
Assumed initial public offering price per share | $ | 5.50 | |||||
Pro forma net tangible book value per common share as of March 31, 2014 | $ | 0.70 | |||||
Increase per share attributable to new investors | 0.67 | ||||||
| | | | | | | | |
Pro forma net tangible book value per share after this offering | 1.37 | ||||||
| | | | | | | | |
Dilution per share to new investors | $ | 4.13 | |||||
| | | | | | | | |
| | | | | | | | |
A $1.00 increase or decrease in the assumed initial public offering price of $5.50 per share, the mid-point of the price range reflected on the cover page of this prospectus, would increase or decrease, as applicable, our pro forma as adjusted net tangible book value as of March 31, 2014 by $0.16 per share and the dilution in pro forma as adjusted net tangible book value to investors in this offering by $0.84 per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The following table shows on an as pro forma as adjusted basis, as of March 31, 2014, after giving effect to this offering on an assumed initial public offering price of $5.50 per share, the mid-point of the price range reflected on the cover page of this prospectus, the difference between existing stockholders and new investors with respect to the total number of shares of common stock purchased from us, the total consideration paid to us for these shares, and the average price per share paid,
36
before deducting estimated underwriting discounts and commissions and estimated offering expenses (amounts in thousands, except per share amounts):
| | | | Total Consideration | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Shares Purchased | | ||||||||||||||
| | Average Price Per Share | |||||||||||||||
| | Number | Percent | Amount | Percent | ||||||||||||
Existing stockholders | 8,400 | 82.8 | % | $ | 1,500 | 13.5 | % | $ | 0.18 | |||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
New investors | 1,750 | 17.2 | 9,625 | 86.5 | $ | 5.50 | ||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Total | 10,150 | 100.0 | % | $ | 11,125 | 100.0 | % | $ | 1.10 | |||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing. Each $1.00 increase or decrease, as applicable, in the assumed initial public offering price of $5.50 per share, the midpoint of the price range reflected on the cover page of this prospectus, would increase or decrease, as applicable, total consideration paid by new investors by approximately $1.8 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
There will be further dilution to new investors with respect to the shares issued pursuant to stock options.
Except as otherwise indicated, the above discussion and tables assume no exercise of the underwriters' over-allotment option. If the underwriters exercise their over-allotment option in full, our existing stockholders would own 80.7% and our new investors would own 19.3% of the total number of shares of our common stock upon the completion of this offering, and the number of shares of common stock held by new investors participating in this offering will be increased to 2,012,500 shares.
The number of shares of our common stock to be outstanding after this offering is based on a total of 7,000,000 shares of our common stock and 1,400,000 shares of preferred stock, which will automatically convert into shares of common stock upon the completion of this offering, outstanding as of March 31, 2014 and excludes:
- •
- 1,833,192 shares of common stock issuable upon exercise of options outstanding, with a weighted-average exercise price of $1.24 per share;
- •
- 175,000 shares of common stock issuable upon the exercise of the warrant to be issued to the underwriters as compensation in connection with this offering; and
- •
- 1,000,000 shares of common stock reserved for future grant under our 2014 Equity Incentive Plan.
37
SELECTED FINANCIAL AND OTHER DATA
The following selected financial and other data should be read in conjunction with the section titled "Management's Discussion and Analysis of Financial Condition and Results of Operations" as well as our financial statements and related notes, each contained in this prospectus. We have derived the statements of operations data for the years ended December 31, 2013 and 2012 from our audited financial statements and related notes contained in this prospectus. The selected financial data for the three months ended March 31, 2014 and 2013, and as of March 31, 2014, are derived from our unaudited financial statements and related notes contained in this prospectus and are not indicative of results to be expected for the full year. Moreover, our historical results are not necessarily indicative of the results that should be expected in the future.
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Statements of Operations Data: | |||||||||||||
Revenue | $ | 3,557,237 | $ | 2,628,269 | $ | 11,340,097 | $ | 7,685,061 | |||||
Cost of revenue(1) | 656,366 | 467,113 | 2,853,385 | 2,125,921 | |||||||||
| | | | | | | | | | | | | | |
Gross profit | 2,900,871 | 2,161,156 | 8,486,712 | 5,559,140 | |||||||||
| | | | | | | | | | | | | | |
Operating expenses: | |||||||||||||
General and administrative(1) | 1,092,695 | 503,189 | 2,392,305 | 1,550,034 | |||||||||
Sales and marketing(1) | 759,789 | 568,723 | 2,297,309 | 1,930,395 | |||||||||
Research and development(1) | 224,304 | 160,411 | 1,009,872 | 654,070 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses | 2,076,788 | 1,232,323 | 5,699,486 | 4,134,499 | |||||||||
| | | | | | | | | | | | | | |
Income from operations | 824,083 | 928,833 | 2,787,226 | 1,424,641 | |||||||||
Other (expense) income, net | 3,452 | (3,340 | ) | (3,458 | ) | 7,424 | |||||||
| | | | | | | | | | | | | | |
Income before provision for income taxes | 827,535 | 925,493 | 2,783,768 | 1,432,065 | |||||||||
Provision for income taxes | 304,168 | 281,566 | 846,878 | 465,980 | |||||||||
| | | | | | | | | | | | | | |
Net income | $ | 523,367 | $ | 643,927 | $ | 1,936,890 | $ | 966,085 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share:(2) | |||||||||||||
Basic | $ | 0.07 | $ | 0.09 | $ | 0.28 | $ | 0.14 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted | $ | 0.06 | $ | 0.08 | $ | 0.22 | $ | 0.11 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Weighted average shares outstanding:(2) | |||||||||||||
Basic | 7,000,000 | 7,000,000 | 7,000,000 | 7,000,000 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted | 8,859,015 | 8,492,475 | 8,624,314 | 8,462,240 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other Financial Data: | |||||||||||||
Non-GAAP income from operations | $ | 986,892 | $ | 996,813 | $ | 3,059,145 | $ | 1,597,881 | |||||
Non-GAAP net income | $ | 628,585 | $ | 685,011 | $ | 2,104,710 | $ | 1,062,606 | |||||
Free cash flow | $ | 457,817 | $ | (72,319 | ) | $ | 1,311,222 | $ | 1,396,216 | ||||
- (1)
- Includes stock-based compensation expense as follows:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Cost of revenue | $ | 959 | $ | 3 | $ | 13 | $ | 2,050 | |||||
General and administrative | 56,935 | 4,115 | 16,461 | 1,770 | |||||||||
Sales and marketing | 95,536 | 63,775 | 255,096 | 168,316 | |||||||||
Research and development | 9,379 | 87 | 349 | 1,104 | |||||||||
| | | | | | | | | | | | | | |
Total | $ | 162,809 | $ | 67,980 | $ | 271,919 | $ | 173,240 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
38
- (2)
- The basic and diluted net income per common share data in the statement of operations data for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited) and for the years ended December 31, 2013 and 2012 take into account the 1.75:1 stock split effected in conjunction with our reincorporation from Oklahoma to Delaware. The number of diluted weighted average shares outstanding during each of the periods presented includes 1,400,000 shares of our outstanding convertible preferred stock, which will automatically convert into 1,400,000 shares of our common stock upon the closing of this offering.
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
Balance Sheets Data: | ||||||||||
Cash and cash equivalents | $ | 2,908,641 | $ | 2,461,559 | $ | 1,697,306 | ||||
Working capital | $ | 5,338,852 | $ | 4,931,949 | $ | 2,665,444 | ||||
Total assets | $ | 7,758,562 | $ | 6,986,871 | $ | 5,554,212 | ||||
Total stockholders' equity | $ | 6,111,136 | $ | 5,422,784 | $ | 3,220,200 | ||||
Non-GAAP Financial Results
We believe the use of non-GAAP income from operations, non-GAAP net income and free cash flow are helpful to our investors. These measures, which we refer to as our non-GAAP financial measures, are not prepared in accordance with GAAP. We calculate non-GAAP income from operations as income from operations excluding stock-based compensation expense. We calculate non-GAAP net income as net income excluding stock-based compensation expense, net of tax. Because of varying available valuation methodologies, subjective assumptions and the variety of equity instruments that can impact a company's non-cash expenses, we believe that providing non-GAAP financial measures that exclude stock-based compensation expense allow for meaningful comparisons between our operating results from period to period. We calculate free cash flow as net cash provided by operating activities less net cash used in investing activities for purchases of property and equipment. We consider free cash flow to be a liquidity measure that provides useful information to management and investors about the amount of cash generated by our business that can be used for strategic opportunities, including investing in our business, making strategic acquisitions and strengthening our balance sheet. All of our non-GAAP financial measures are important tools for financial and operational decision making and for evaluating our own operating results of different periods of time.
Our non-GAAP financial measures may not provide information that is directly comparable to that provided by other companies in our industry, as other companies in our industry may calculate non-GAAP financial results differently, particularly related to non-recurring, unusual items. In addition, there are limitations in using non-GAAP financial measures because the non-GAAP financial measures are not prepared in accordance with GAAP, may be different from non-GAAP financial measures used by other companies and exclude expenses that may have a material impact on our reported financial results. Further, stock-based compensation expense has been and will continue to be for the foreseeable future a significant recurring expense in our business and an important part of the compensation provided to our employees. The presentation of non-GAAP financial information is not meant to be considered in isolation or as a substitute for the directly comparable financial measures prepared in accordance with GAAP. We urge our investors to review the reconciliation of our non-GAAP financial measures to the comparable GAAP financial measures included below, and not to rely on any single financial measure to evaluate our business.
39
The following table reflects the reconciliation of income from operations to non-GAAP income from operations:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Income from operations | $ | 824,083 | $ | 928,833 | $ | 2,787,226 | $ | 1,424,641 | |||||
Excluding: | |||||||||||||
Stock-based compensation expense | 162,809 | 67,980 | 271,919 | 173,240 | |||||||||
| | | | | | | | | | | | | | |
Non-GAAP income from operations | $ | 986,892 | $ | 996,813 | $ | 3,059,145 | $ | 1,597,881 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The following table reflects the reconciliation of net income to non-GAAP net income:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Net income | $ | 523,367 | $ | 643,927 | $ | 1,936,890 | $ | 966,085 | |||||
Excluding: | |||||||||||||
Stock-based compensation expense, net of tax | 105,218 | 41,084 | 167,820 | 96,521 | |||||||||
| | | | | | | | | | | | | | |
Non-GAAP net income | $ | 628,585 | $ | 685,011 | $ | 2,104,710 | $ | 1,062,606 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The following table reflects the reconciliation of net cash provided by operating activities to free cash flow:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Net cash provided by (used in) operating activities | $ | 462,109 | $ | (51,074 | ) | $ | 1,474,397 | $ | 1,509,156 | ||||
Less: Purchases of property and equipment | 4,292 | 21,245 | 163,175 | 112,940 | |||||||||
| | | | | | | | | | | | | | |
Free cash flow | $ | 457,817 | $ | (72,319 | ) | $ | 1,311,222 | $ | 1,396,216 | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
40
MANAGEMENT'S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read this discussion and analysis together with our audited and unaudited financial statements, the notes to such statements and the other financial information included in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under the section entitled "Risk Factors" and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements. See "Special Note Regarding Forward-Looking Statements" for a discussion of the uncertainties, risks and assumptions associated with these statements.
Our Business
We are the leading provider of non-magnetic intravenous ("IV") infusion pump systems that are safe for use during magnetic resonance imaging ("MRI") procedures. Electromechanical medical devices and pumps contain magnetic and electronic parts which generate radio frequency noise, create interference and are dangerous to operate in the presence of the powerful magnet which drives an MRI. Our mRidium 3860+ MRI compatible IV infusion pump system has been designed with non-ferrous parts, ceramic ultrasonic motors, non-magnetic mobile stand and other special features in order to safely and predictably deliver anesthesia and other IV fluids during various MRI procedures. Many critically-ill patients cannot be removed from their vital medications, and children and infants must generally be sedated in order to remain immobile during an MRI scan.
As of March 31, 2014 we estimate that we had approximately 1,917 MRI compatible IV infusion pump systems installed globally. Each system consists of an mRidium MRI compatible IV infusion pump, mobile stand, and proprietary disposable IV tubing sets and many of these systems contain additional optional upgrade accessories. We primarily generate revenue from the one-time sale of pumps and accessories, in addition to revenue generated from ongoing service contracts and the sale of proprietary disposable tubing sets used during each patient infusion. The principal customers for our MRI compatible products include hospitals, acute care facilities and outpatient imaging centers.
We sell our MRI compatible products through our direct sales force in the U.S. and independent distributors internationally. In the U.S., we sell our products through our eight direct sales representatives and two clinical support representatives. We have distribution agreements for our products with 35 independent distributors selling our products internationally. Selling cycles for medical devices vary widely but are typically three to six months in duration. We also enter into agreements with healthcare supply contracting companies in the U.S., which enable us to sell and distribute our mRidium MRI compatible IV infusion pump systems to their member hospitals. Under these agreements, we are required to pay these group purchasing organizations ("GPOs") a fee of three percent of the sales of our products to their member hospitals. We currently have contracts with four major GPOs that effectively give us the ability to sell to more than 95% of all U.S. acute care facilities.
Financial Highlights and Outlook
Our total revenue increased $3.6 million, or 47.6%, to $11.3 million in 2013, from $7.7 million in 2012. This increase was primarily driven by sales of our MRI compatible IV infusion pump systems. Revenue increased approximately $0.9 million, or 35.3%, to $3.5 million for the three months ended March 31, 2014, compared to $2.6 million for the same period in 2013. As of March 31, 2014, our estimated installed base of IV infusion pumps increased to approximately 1,917 from 1,438 on March 31, 2013.
During 2013, our largest competitor announced its decision to commence removal of its pump systems from the U.S. market, and to discontinue support throughout the world by June 30, 2015 due
41
to ongoing regulatory issues. As a result, we believe that our mRidium MRI compatible IV infusion pump will be the only MRI compatible IV infusion pump available to customers beginning in the first half of 2015.
In 2014, we expect our revenues to increase as our expanded U.S. direct sales force continues to expand market awareness of the advantages of patient safety and operating efficiencies provided by our MRI compatible IV infusion pump. We intend to focus our efforts on converting users of our former competitor's pumps to our systems, in addition to targeting an increased number of hospitals and acute care facilities using MRI compatible IV infusion pumps. We expect operating expenses to increase in 2014 due to increased headcount, higher depreciation expense from additional capital expenditures, the costs associated with our new corporate headquarters and the costs of being a public company.
Application of Critical Accounting Policies
We prepare our financial statements in conformity with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and use assumptions that affect the reported amounts of assets, liabilities and related disclosures at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Our significant accounting policies are more fully described in Note 1 to the financial statements. However, we believe that the following critical accounting policies require the use of significant estimates, assumptions, and judgments. The use of different estimates, assumptions, and judgments could have a material effect on the reported amounts of assets, liabilities and related disclosures as of the date of the financial statements and revenue and expenses during the reporting period.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, the price is fixed or determinable, delivery has occurred and title and risk of loss has transferred and collection of the resulting receivable is reasonably assured. Terms of sale for most domestic sales are FOB destination, reflecting that title and risk of loss are assumed by the purchaser upon delivery. Terms of sales to international distributors are FOB shipping point, reflecting that title and risk of loss are assumed by the distributor at the shipping point.
Under the revenue recognition rules for tangible products, we allocate revenue from arrangements with multiple deliverables to each of the deliverables based upon their relative selling prices as determined by a selling-price hierarchy. A deliverable in an arrangement qualifies as a separate unit of accounting if 1) the delivered item has value to the customer on a stand-alone basis, and 2) the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered items is considered probable and substantially in control of the vendor. The principal deliverables in our multiple deliverable arrangements that qualify as separate units of accounting consist of (i) sales of medical devices and supplies, (ii) installation and training services, and (iii) extended warranty agreements.
We use a hierarchy to determine the selling price to be used for allocating revenue to deliverables as follows: (i) vendor-specific objective evidence of fair value ("VSOE"), (ii) third-party evidence of selling price ("TPE"), and (iii) best estimate of the selling price ("ESP"). VSOE of fair value is defined as the price charged when the same element is sold separately, or if the element has not yet been sold separately, the price for the element established by management having the relevant authority when it is probable that the price will not change before the introduction of the element into the marketplace. VSOE generally exists only when we sell the deliverable separately and is the price actually charged for that deliverable. For certain sales under GPO contracts, we have established VSOE
42
for all of the elements in our multiple element arrangements. This determination is based on the volume of sales to these customers in relation to our total sales and the discount tier in which those sales are made. For all other sales we rely on ESP, reflecting our best estimates of what the selling prices of elements would be if they were sold regularly on a stand-alone basis, to establish the amount of revenue to allocate to the undelivered elements. TPE generally does not exist for our products because of their uniqueness.
For products shipped under FOB shipping point terms, delivery is generally considered to have occurred when shipped. Undelivered elements in our sales arrangements, which are not considered to be essential to the functionality of a product, generally include installation and training services that are performed after the related products have been delivered and extended warranty agreements. Revenue related to undelivered installation and training services is deferred until such time as those services are complete, which is typically within 30 days of the related products being delivered to the customer's location. Revenue and direct acquisition costs related to undelivered extended warranty agreements are deferred and recognized ratably over the service period, which is between one and four years. Deferred revenue for extended warranty agreements is based on the price charged when the service is sold separately.
Shipping and handling charges billed to customers are included in revenue and shipping and handling related expenses are charged to cost of revenue. Advance payments from customers are recorded as deferred revenue and recognized as revenue as otherwise described above. Most of our sales are subject to 30 to 60 day customer-specified acceptance provisions. These provisions require us to estimate the amount of future returns and recognize revenue net of these potential returns.
In certain states we are required to collect sales taxes from our customers. These amounts are excluded from revenue and recorded as a liability.
GPOs negotiate volume purchase prices for hospitals, group practices and other clinics that are members of a GPO. Our agreements with GPOs typically include the following provisions:
- •
- Negotiated pricing for all group members;
- •
- Volume discounts and other preferential terms on their members' purchases from us;
- •
- Promotion of our products by the GPO to its members;
- •
- Payment of administrative fees by us to the GPO, based on purchases of our products by group members.
We do not sell to GPOs. Hospitals, group practices and other acute care facilities that are members of a GPO purchase products directly from us under the terms negotiated by the GPO. Negotiated pricing and discounts are recognized as a reduction of the selling price of products at the time of the sale. Revenue from sales to members of GPOs is otherwise consistent with revenue recognition policies described above.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable is recorded at the sales price of the related products and services. We assess the sufficiency of the allowance for estimated uncollectible accounts receivable. Estimates are based on historical collection experience and other customer-specific information, such as bankruptcy filings or liquidity problems of our customers. When it is determined that an account receivable is uncollectible, it is written off and relieved from the allowance. Any future determination that the allowance for estimated uncollectible accounts receivable is not properly stated could result in changes in operating expense and results of operations.
43
Inventory
Inventories are stated at the lower of standard cost, which approximates actual cost on a first-in, first-out basis, or market. We may be exposed to a number of factors that could result in portions of our inventory becoming either obsolete or in excess of anticipated usage. These factors include, but are not limited to, technological changes, competitive pressures in products and prices, and the introduction of new product lines. We regularly evaluate our ability to realize the value of inventory based on a combination of factors, including historical usage rates, forecasted sales, product life cycles, and market acceptance of new products. When inventory that is obsolete or in excess of anticipated usage is identified, it is written down to realizable salvage value or an inventory valuation allowance is established.
The estimates we use in projecting future product demand may prove to be incorrect. Any future determination that our inventory is overvalued could result in increases to our cost of sales and decreases to our operating margins and results of operations.
Stock-based compensation
We apply the fair value recognition provisions of Financial Accounting Standards Board Accounting Standards Codification Topic 718,Compensation – Stock Compensation ("ASC 718"). Determining the amount of stock-based compensation to be recorded requires us to develop estimates of the fair value of stock options as of their grant date. Stock-based compensation expense is recognized ratably over the requisite service period, which is the vesting period of the award. Calculating the fair value of stock-based awards requires that we make highly subjective assumptions. We use the Black-Scholes option pricing model to value our stock option awards. Use of this valuation methodology requires that we make assumptions as to the volatility of our common stock, the expected term of our stock options, the risk-free interest rate for a period that approximates the expected term of our stock options and our expected dividend yield. Because we are a privately-held company, we utilize the historical stock price volatility from a representative group of public companies to estimate expected stock price volatility. We selected companies from the medical device industry with market capitalizations that are similar to what we estimate our company would achieve upon a successful initial public offering. We intend to continue to utilize the historical volatility of the same or similar public companies to estimate expected volatility until a sufficient amount of historical information regarding the price of our publicly traded stock becomes available. We use the simplified method as prescribed by ASC 718 to calculate the expected term of stock options granted to employees as we do not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term of our stock option awards. The risk-free interest rate used for each grant is based on the U.S. Treasury yield curve in effect at the time of the grant for instruments with a similar expected life. We utilize a dividend yield of zero because we have no current intention to pay cash dividends. We estimated the fair value of options granted using a Black-Scholes option pricing model with the following assumptions:
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
Volatility | 112.8 | % | 113.0 | % | 116.8 | % | ||||
Expected term (years) | 7.0 | 7.0 | 7.0 | |||||||
Risk-free interest rate | 2.4 | % | 2.4 | % | 1.1 | % | ||||
Dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||||
Forfeiture rate | 10.0 | % | 10.0 | % | 0.0 | % | ||||
Stock-based compensation expense totaled $162,809 and $67,980 for the three months ended March 31, 2014 and 2013, respectively and $271,919 and $173,240 for the years ended December 31,
44
2013 and 2012, respectively. As of March 31, 2014 we had $2,148,989 of total unrecognized stock-based compensation expense, which is expected to be recognized over a weighted average period of 3.4 years. As of December 31, 2013 we had $2,117,081 of total unrecognized stock-based compensation expense, which is expected to be recognized over a weighted-average period of 3.6 years. We expect the future impact of stock-based compensation expense on our financial results to grow due to the potential increases in the value of our common stock, additional stock option grants and increased headcount.
Under ASC 718, we are required to estimate the level of forfeitures expected to occur and record stock-based compensation expense only for those awards that we ultimately expect will vest. We estimate our forfeiture rate based on historical experience and employee class.
The following table sets forth information with respect to stock options granted to employees and directors during the three months ended March 31, 2014 and during the year ended December 31, 2013:
Grant Date | Options Granted | Exercise Price | Estimated Fair Value of Common Stock per Share at Grant Date | Aggregate Grant Date Fair Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
January 1, 2013 | 17,500 | $ | 1.29 | $ | 1.59 | $ | 24,819 | ||||||
December 13, 2013 | 26,250 | $ | 1.29 | $ | 3.30 | $ | 80,259 | ||||||
December 31, 2013 | 523,509 | $ | 1.48 | $ | 3.30 | $ | 1,590,185 | ||||||
January 1, 2014 | 26,250 | $ | 1.48 | $ | 3.30 | $ | 71,820 | ||||||
January 1, 2014 | 12,250 | $ | 3.30 | $ | 3.30 | $ | 31,862 | ||||||
March 1, 2014 | 8,750 | $ | 3.30 | $ | 3.30 | $ | 22,759 | ||||||
March 25, 2014 | 26,250 | $ | 3.30 | $ | 3.30 | $ | 68,276 | ||||||
We used the estimated fair value per share of our common stock to determine stock-based compensation expense which is recorded in our financial statements. For each of the grant dates above, the stock options granted had exercise prices different than the estimated per share fair value of our common stock. In the past, some of the stock options we issued had exercise prices below the estimated fair value of our common stock on the grant date. This disqualified the treatment of those grants as incentive stock options. Going forward, our intention is to grant options to purchase shares of our common stock with an exercise price per share that is equal to the fair value per share of our common stock on the grant date.
As discussed above, one input of the Black-Scholes option pricing model is the fair value of our common stock, which is issued upon exercise of the option. Options to purchase shares of our common stock are intended to be granted with an exercise price per share that is no less than the fair value per share of our common stock on the date of grant, which is based on the information known to us on the date of grant. The fair value of our common stock was assessed on each grant date by our Board. For the periods presented, the historical valuations of our common stock were determined in accordance with the guidelines outlined in the applicable American Institute of Certified Public Accountants Practice Aid,Valuation of Privately-Held-Company Equity Securities Issued as Compensation ("AICPA Practice Aid"). In the absence of a public trading market, our Board considered all relevant facts and circumstances known at the time of valuation, made certain assumptions based on future expectations and exercised significant judgment to determine the fair value of our common stock. The factors considered by our Board in determining the fair value include, but are not limited to, the following:
- •
- Contemporaneous third-party valuation of our common stock as of December 31, 2013;
- •
- Retrospective third-party valuation of our common stock as of December 31, 2012;
45
- •
- Our historical financial results and estimated trends and projections of our future operating and financial performance;
- •
- The market performance of comparable, publicly traded companies; and
- •
- The overall economic and industry conditions and outlook.
December 31, 2013 Valuation
Certain members of our Board and management reviewed the contemporaneous third-party valuation of our common stock as of December 31, 2013, discussed the reasonableness of the assumptions, methodologies, analysis and conclusions in this report. After reviewing this report, we determined the fair market value of the Company's common stock. Our valuation of our common stock was conducted within the guidelines of the applicable AICPA Practice Aid. These guidelines prescribe certain valuation approaches for setting the value of an enterprise, such as the cost, market and income approaches, and various methodologies for allocating the value of an enterprise to common stock, such as the current value method, option pricing method, probability-weighted expected return method and the hybrid method.
Our valuation included the income approach and the market approach. Specifically, the Discounted Future Cash Flow ("DCF") method was used under the income approach. The Guideline Public Company ("GPC") method was used under the market approach to substantiate the conclusions derived from the income approach.
The method used to allocate enterprise value was the Probability-Weighted Expected Return Method ("PWERM"). Using the PWERM, the value of our common stock is estimated based upon an analysis of varying values for our common stock assuming the following possible future events for our company: (i) our initial public offering in 2014, (ii) our initial public offering in 2015, and (iii) we continue to operate as a private entity. For each of these possible events, a range of equity values is estimated based on a number of factors, which include income and market valuation approaches that factor in our estimates of future performance and performance of comparable public companies. For each possible future event, we determined the appropriate aggregate value to be allocated to holders of our shares of common stock based on the rights and preferences of each class of our stock at that time. Next, we estimated the timing of possible future event dates and applied a discount rate, based on our estimated weighted average cost of capital using the venture capital portfolio rates of return as referenced in the AICPA Practice Aid for companies in a similar stage of development as ours. We then multiplied the discounted value of our common stock under each scenario by an estimated probability for each of the possible events, resulting in a probability-weighted value per share of common stock. Finally, we applied a discount for lack of marketability to the weighted value per share to determine a value per common share.
Application of the above described approaches involved the use of estimates, judgments and assumptions, such as future cash flows, selection of comparable publicly traded companies and the selection of discount rates. Changes in our assumptions or the interrelationship of those assumptions could result in changes to our operating results.
December 31, 2012 Valuation
Certain members of our Board and management reviewed the retrospective third-party valuation of our common stock as of December 31, 2012, discussed the reasonableness of the assumptions, methodologies, analysis and conclusions in this report. After reviewing this report, we determined the fair market value of the Company's common stock. Our valuation of our common stock was conducted within the guidelines of the applicable AICPA Practice Aid. These guidelines prescribe certain valuation approaches for setting the value of an enterprise, such as the cost, market and income approaches, and
46
various methodologies for allocating the value of an enterprise to common stock, such as the current value method, option pricing method and the PWERM method.
Our valuation included the income approach and the market approach. Specifically, the DCF method was used under the income approach. The GPC method was used under the market approach to substantiate the conclusions derived from the income approach. The DCF method estimates enterprise value based on the estimated present value of future net cash flows the business is expected to generate over a forecasted period and an estimate of the present value of cash flows beyond that, which is referred to as terminal value. The estimated present value is calculated using a discount rate known as the weighted-average cost of capital, which accounts for the time value of money and the appropriate degree of risks inherent in the business. The GPC method considers multiples of various financial metrics of comparable publicly traded companies. These multiples were then applied to our financial metrics to derive a range of indicated values and compared to the DCF model to substantiate those results.
The enterprise value was then allocated to our common stock and preferred stock using the Option Pricing Model ("OPM"). The OPM treats common and preferred stock as call options on a company's enterprise value, with exercise prices based on the liquidation preference of our preferred stock. The common stock only has value if the funds available for distribution to shareholders exceed the value of the liquidation preference at the time of a liquidity event. Assuming these funds are available, the common stock is modeled as a call option that gives the shareholder the right, but not the obligation, to buy the underlying enterprise value at a predetermined exercise price. Thus, common stock is considered to be a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the preferred stock is liquidated. We assumed a five year holding period, which is consistent with the discrete periods used in the DCF analysis. The risk-free interest rates are based on the corresponding U.S. Treasury yield curve for periods that estimated the assumed holding period. Estimates of the volatility of our common stock used in the OPM were based on the volatility of common stock of comparable publicly traded companies used in the GPC method discussed above. We then applied a discount for lack of marketability to determine the fair value of our common stock and preferred stock.
Application of the above described approaches involved the use of estimates, judgments and assumptions, such as future cash flows, selection of comparable publicly traded companies and the selection of discount rates. Changes in our assumptions or the interrelationship of those assumptions could result in changes to our operating results.
Initial Public Offering
In consultation with the underwriters for this offering, we determined the estimated price range for this offering, as set forth on the cover page of this prospectus. The midpoint price range is $5.50 per share. In comparison, our estimate of the fair value of our common stock was $3.30 per share as of the December 31, 2013 valuation. We note that, as is typical in initial public offerings, the estimated price range for this offering was not derived using a formal determination of fair value, but was determined by negotiation between us and the underwriters. We believe the difference between the fair value of our common stock as of December 31, 2013, as determined by the contemporaneous third-party valuation discussed above, and the preliminary initial public offering price range, as recommended by the underwriters, is the result of the following factors:
- •
- The preliminary price range necessarily assumes the initial public offering will occur and a public market for our common stock has been created, and therefore reduces the discounts for marketability and illiquidity for our common stock, which were appropriately taken into consideration in the December 31, 2013 valuation;
47
- •
- Additional information obtained subsequent to December 31, 2013 regarding the activities of our primary competitor and its intention to remove its competing IV infusion pump from the market in 2014. Under the provisions of the AICPA Practice Aid, this information was appropriately excluded from the December 31, 2013 valuation;
- •
- The general condition of the securities markets and the recent market prices of, and the demand for, publicly traded common stock of generally comparable companies;
- •
- An assumption that there would be a receptive public trading market for medical device companies such as us; and
- •
- An assumption that there would be sufficient demand for our common stock to support an offering of the size contemplated in this prospectus.
Income taxes
We estimate certain components of our provision for income taxes. These estimates include, among other items, depreciation and amortization expense allowable for tax purposes, allowable tax credits, effective rates for state taxes and tax deductibility of certain other items. We adjust our annual effective income tax rate as additional information on outcomes or events becomes available.
We account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
We record net deferred tax assets to the extent we believe these assets will more likely than not be realized. In making such determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial operations. A valuation allowance is recorded to offset net deferred tax assets if, based upon the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
We recognize the tax benefit of uncertain tax positions in the financial statements based on the technical merits of the position. When the tax position is deemed more likely than not of being sustained, we recognize the largest amount of tax benefit that is greater than 50 percent likely of being ultimately realized upon settlement. We believe our tax positions are fully supportable
JOBS Act Accounting Election
We are an "emerging growth company" as defined in the Jumpstart Our Business Startups Act ("JOBS Act"), enacted in April 2012. An "emerging growth company" may take advantage of reduced reporting requirements that are otherwise applicable to public companies. For example, we will not have to provide an auditor's attestation report on our internal controls in future annual reports on Form 10-K as otherwise required by Section 404(b) of the Sarbanes-Oxley Act. The JOBS Act also permits us, as an "emerging growth company," to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to "opt out" of this provision and, as a result, we will comply with new or revised accounting standards when they are required to be adopted by issuers. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
48
Results of Operations
The following table sets forth for the periods indicated selected statements of operations data as a percentage of total revenue. Our historical operating results are not necessarily indicative of the results for any future period.
| | Percent of Revenue Three Months Ended March 31, | Percent of Revenue Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
Revenue | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | |||||
Cost of revenue | 18.5 | 17.8 | 25.2 | 27.7 | |||||||||
| | | | | | | | | | | | | | |
Gross profit | 81.5 | 82.2 | 74.8 | 72.3 | |||||||||
| | | | | | | | | | | | | | |
Operating expenses: | |||||||||||||
General and administrative | 30.7 | 19.1 | 21.1 | 20.2 | |||||||||
Sales and marketing | 21.4 | 21.6 | 20.3 | 25.1 | |||||||||
Research and development | 6.3 | 6.1 | 8.9 | 8.5 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses | 58.4 | 46.9 | 50.3 | 53.8 | |||||||||
| | | | | | | | | | | | | | |
Income from operations | 23.2 | 35.3 | 24.6 | 18.5 | |||||||||
Other (expense) income , net | 0.1 | (0.1 | ) | (0.0 | ) | 0.1 | |||||||
| | | | | | | | | | | | | | |
Income before provision for income taxes | 23.6 | 35.2 | 24.6 | 18.6 | |||||||||
Provision for income taxes | 8.6 | 10.7 | 7.5 | 6.1 | |||||||||
| | | | | | | | | | | | | | |
Net income | 14.7 | % | 24.5 | % | 17.1 | % | 12.5 | % | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Three Months Ended March 31, 2014 and 2013
Revenue
Revenue increased approximately $0.9 million, or 35.3%, to $3.5 million for the three months ended March 31, 2014, compared to $2.6 million for the same period in 2013. The increase was primarily attributable to an increase in sales of our MRI compatible IV infusion pump systems. As of March 31, 2014, our estimated installed base of IV infusion pumps increased to approximately 1,917 from 1,438 on March 31, 2013.
Revenue from sales in the U.S. increased approximately $0.4 million, or 20.5%, to $2.4 million for the three months ended March 31, 2014, from $2.0 million for the same period in 2013. Revenue from sales internationally increased approximately $0.5 million, or 86.1%, to $1.1 million for the three months ended March 31, 2014, from $0.6 million for the same period in 2013.
Revenue from devices was approximately $3.0 million, or 84.9% of total revenue for the three months ended March 31, 2014 compared to $2.2 million, or 83.7% of total revenue for the same period in 2013. During the three months ended March 31, 2014, we sold 146 MRI compatible IV infusion pumps compared to 91 for the same period in 2013. The average selling price of our MRI compatible IV infusion pump systems during the three months ended March 31, 2014 was approximately $20,700, compared to $24,800 for the same period in 2013. The decrease in our average selling price is the result of higher international sales during the three months ended March 31, 2014 compared to the same period in 2013.
Revenue from sales of our disposable IV sets and services was approximately $0.5 million, or 15.1% of total revenue for the three months ended March 31, 2014 compared to $0.4 million, or 16.3% of total revenue for the same period in 2013. We expect revenue from disposables and services to increase as the installed base of our MRI compatible IV infusion pumps and systems increases.
49
Cost of Revenue
Cost of revenue increased approximately $0.2 million, or 40.5%, to $0.7 million for the three months ended March 31, 2014, from $0.5 million for the same period in 2013. Gross profit increased approximately $0.7 million, or 34.2%, to $2.9 million for the three months ended March 31, 2014 from $2.2 million for the same period in 2013. Gross profit margin decreased to 81.5% for the three months ended March 31, 2014, from 82.2% for the same period in 2013 primarily due to higher international sales, partially offset by higher labor utilization and increased equipment utilization rates.
General and Administrative
General and administrative expense increased approximately $0.6 million, or 117.2%, to $1.1 million for the three months ended March 31, 2014, from $0.5 million for the same period in 2013. General and administrative expense as a percent of total revenue increased to 30.7% for the three months ended March 31, 2014, from 19.1% for the same period in 2013. This increase is primarily related to increased headcount and professional fees for the three months ended March 31, 2014 when compared to the same period in 2013. We expect general and administrative expenses to increase in 2014 as we continue to make investments in key personnel and back-office infrastructure to support our expected growth.
Sales and Marketing
Sales and marketing expense increased approximately $0.2 million, or 33.6%, to $0.8 million for the three months ended March 31, 2014, from $0.6 million for the same period in 2013. Sales and marketing expense as a percent of total revenue decreased to 21.4% for the three months ended March 31, 2014, from 21.6% for the same period in 2013. We expect continued growth in the size of our U.S. sales force in 2014 to support our expected increase in product sales.
Research and Development
Research and development expense increased approximately $0.1 million, or 39.8%, to $0.2 million for the three months ended March 31, 2014, from $0.1 million for the same period in 2013. Research and development expenses as a percent of total revenue increased to 6.3% for the three months ended March 31, 2014, from 6.1% for the same period in 2013. We believe that continued investment in research and development activities is essential to maintaining our position as the leading provider of MRI compatible IV infusion pumps and to our ability to develop innovative new product offerings, and therefore, we expect to continue making investments in these activities.
Other Income (Expense), Net
Other income (expense), net consists of dividend income, foreign currency losses, and other miscellaneous income. We reported other income of approximately $3,000 for the three months ended March 31, 2014, compared to other expense of approximately $3,000 for the same period in 2013. Dividend income was approximately $1,000 for the three months ended March 31, 2014 and 2013. We reported approximately $2,000 of foreign currency gains for the three months ended March 31, 2014 compared to losses of approximately $4,000 for the same period in 2013.
Income Taxes
We recorded income tax expense of $304,168 and $281,566 for the three months ended March 31, 2014 and 2013, respectively. The higher income tax expense for the first quarter of 2014 is due to an increase in our effective tax rate when compared to the same period in 2013. Our effective tax rate for the first three months of 2014 was 36.8% compared to 30.4% for the same period in 2013. The increase in our effective tax rate was the result of higher state tax expense and the expiration of the research and development tax credit at the end of 2013, partially offset by lower income before provision for income taxes.
50
Years Ended December 31, 2013 and 2012
Revenue
Revenue increased approximately $3.6 million, or 47.6%, to $11.3 million in 2013, from $7.7 million in 2012. The increase was primarily attributable to an increase in sales of our MRI compatible IV infusion pump systems. As of December 31, 2013, our estimated installed base of IV infusion pumps increased to approximately 1,771 from 1,347 at the end of 2012.
Revenue from sales in the U.S. increased approximately $2.3 million, or 39.2%, to $8.1 million in 2013, from $5.8 million in 2012. Revenue from sales internationally increased approximately $1.3 million, or 73.8%, to $3.2 million in 2013, from $1.9 million in 2012. During 2013, revenue from sales to one international customer represented 10.7% of total revenue.
Revenue from devices was approximately $9.3 million, or 82.3% of total revenue in 2013 compared to $6.0 million, or 78.2% of total revenue in 2012. During 2013, we sold 418 MRI compatible IV infusion pumps compared to 278 in 2012. Our sales during 2013 included two non-recurring orders from a customer in Saudi Arabia for 129 of our MRI compatible IV infusion pump systems. The average selling price of our MRI compatible IV infusion pump systems in 2013 was approximately $21,000, which was virtually the same price in 2012. This consistent pricing in 2013 and 2012 was primarily due to a shift in our product mix and the relatively higher contribution of international revenue as a percent of total revenue when compared to 2012 as our selling prices to international distributors are lower than those in the U.S.
Revenue from sales of our disposable IV sets and services was approximately $2.0 million, or 17.7% of total revenue in 2013 compared to $1.7 million, or 21.8% of total revenue in 2012. We expect revenue from disposables and services to increase as the installed base of our MRI compatible IV infusion pumps and systems increases.
Cost of Revenue
Cost of revenue increased approximately $0.8 million, or 34.2%, to $2.9 million in 2013, from $2.1 million in 2012 primarily as a result of higher revenue. Gross profit increased approximately $2.9 million, or 52.7%, to $8.5 million in 2013 from $5.6 million in 2012. Gross profit increased to 74.8% in 2013, from 72.3% in 2012 primarily due to higher labor utilization and increased equipment utilization rates.
General and Administrative
General and administrative expense increased approximately $0.8 million, or 54.3%, to $2.4 million in 2013, from $1.6 million in 2012. This increase is primarily due to higher employee benefit related costs and bonuses, the medical excise tax enacted under the Patient Protection and Affordable Care Act, various consulting services, administration fees paid to our GPO's, professional service costs and employee recruiting costs. General and administrative expense as a percent of total revenue increased to 21.1% in 2013, from 20.2% in 2012.
Sales and Marketing
Sales and marketing expense increased approximately $0.4 million, or 19.0%, to $2.3 million in 2013, from $1.9 million in 2012. This is primarily the result of higher salary and stock compensation costs as we increased the size of our sales team in 2013 when compared to 2012. Sales and marketing expense as a percent of total revenue decreased to 20.3% in 2013, from 25.1% in 2012 as a result of an increase in sales.
51
Research and Development
Research and development expense increased approximately $0.3 million, or 54.4%, to $1.0 million in 2013 from, $0.7 million in 2012. This is primarily the result of higher costs for outside engineering and technical writing services related to the continued research and development activities of new MRI compatible products and salary costs due to increased headcount. Research and development expenses as a percent of total revenue increased to 8.9% in 2013, from 8.5% in 2012.
Other Income (Expense), Net
We reported other expense of approximately $3,000 in 2013, compared to other income of approximately $7,000 in 2012. Dividend income was approximately $6,000 in 2013 and 2012. We reported approximately $23,000 of foreign currency losses in 2013 compared to losses of approximately $12,000 in 2012.
Income Taxes
We recorded income tax expense of approximately $0.8 million and $0.5 million in 2013 and 2012, respectively. The higher income tax expense in 2013 is due to higher income before provision for income taxes driven by increased sales. Our effective tax rate in 2013 was 30.4% compared to 32.5% in 2012. This decrease was primarily the result of a decrease in our state tax expense.
Liquidity and Capital Resources
Our principal sources of liquidity are our cash and cash equivalents balances, cash flow from operations and access to the financial markets. Our principal uses of cash are operating expenses, working capital requirements, capital expenditures and repayment of our officer note payable.
As of March 31, 2014, we had cash and cash equivalents of approximately $2.9 million, stockholders' equity of $6.1 million, and working capital of $5.3 million. As of December 31, 2013, we had cash and cash equivalents of $2.5 million, stockholders' equity of $5.4 million, and working capital of $4.9 million compared to cash and cash equivalents of $1.7 million, stockholders' equity of $3.2 million, and working capital of $2.7 million as of December 31, 2012.
In our early stages, our principal stockholder and Chief Executive Officer provided funding for operations in the form of an unsecured interest-free note payable with no specified due date. As of December 31, 2013 and 2012, approximately $6,000 and $520,000 remained outstanding. In March 2014, we repaid with cash the outstanding balance of the officer note payable. We do not expect to continue to borrow funds from this principal stockholder in the future.
For the three months ended March 31, 2014, cash provided by operations increased approximately $0.6 million to $0.5 million, compared to cash used in operations of $0.1 million for the same period in 2013. This increase was primarily the result of a net increase of approximately $0.2 million related to accrued income taxes resulting from the application of a tax overpayment made during 2013 and an increase of approximately $0.2 million in deferred revenue primarily resulting from the timing of product shipments and the sale of extended warranty contracts. The sum of our net income and certain non-cash expense items, such as stock compensation, depreciation and amortization was approximately $0.7 million for the three months ended March 31, 2014 and 2013.
For the years ended December 31, 2013 and 2012, cash provided by operations decreased by approximately $35,000 to $1.47 million in 2013, compared to $1.51 million in 2012. This decrease is the result of a net cash outflow of $218,000 in certain operating assets and liabilities. Accounts receivable increased by $0.4 million, or 25.0% in 2013, to $2.0 million, from $1.6 million in 2012. Inventory decreased by $46,000, or 3.3% in 2013 to $1.34 million, from $1.39 million in 2012. In aggregate, accounts payable and accrued expenses increased by $194,000, or 20.0% in 2013 to $1.16 million, from $970,000 in 2012. The sum of our net income and certain non-cash expense items, such as stock
52
compensation, depreciation and amortization was $2.3 million in 2013, compared to $1.2 million in 2012.
For the three months ended March 31, 2014 cash used in investing activities was approximately $9,000 compared to cash used in investing activities of approximately $25,000 for the same period in 2013. We used approximately $4,000 of cash to purchase property and equipment during the three months ended March 31, 2014 and approximately $21,000 for the same period in 2013. We expect our purchases of property and equipment to increase in 2014 compared to 2013 resulting from the relocation of our manufacturing and headquarters facility, which is scheduled to be completed during our third quarter of 2014.
For the year ended December 31, 2013, cash used in investing activities was approximately $197,000, compared to $166,000 in 2012. We used $163,000 of cash to purchase property and equipment in 2013 and $113,000 in 2012. In 2013, we capitalized $29,000 of internally developed software used in our products and costs associated with obtaining patents, compared to $49,000 in 2012.
For the year ended December 31, 2013, cash used in financing activities was approximately $513,000 compared to $239,000 in 2012, and consisted of payments applied to the officer note payable. As of December 31, 2013, we owed approximately $6,000 on this note, all of which was repaid in cash during March 2014.
Sales to end users in the United States are generally made on open credit terms. Management maintains an allowance for potential credit losses. At the end of 2013, one customer accounted for 10.8% of gross accounts receivable. At the end of 2012, one customer accounted for 15.4% of gross accounts receivable.
We have plans to relocate during 2014 and have entered into a lease, commencing July 1, 2014, for a new, nearby facility in Winter Springs, Florida that is approximately 27,000 square-feet. The facility will house our manufacturing operations and corporate headquarters. The new facility has been leased from Susi, LLC, an entity controlled by our President and CEO, Roger Susi. Pursuant to the terms of our lease for the Winter Springs property, the monthly base rent will be $32,583, adjusted annually for changes in the consumer price index.
We will have broad discretion over the use of the net proceeds in the offering. We intend to use the net proceeds from this offering primarily for research and development, sales and marketing, and for working capital and other general corporate purposes. We may also use a portion of the net proceeds for potential acquisitions of products or businesses; however, we currently do not have any agreements or commitments relating to any potential acquisitions.
We expect that our operating expenses will increase as a result of becoming a public company. Upon consummation of our initial public offering, we will become a public company, and we will need to comply with laws, regulations, and requirements that we did not need to comply with as a private company, including certain provisions of the Sarbanes-Oxley Act and related SEC regulations, and will need to comply with the requirements of NASDAQ if our securities are approved for listing. Compliance with the requirements of being a public company will require us to increase our operating expenses in order to pay our employees, legal counsel, and accountants to assist us in, among other things, external reporting, instituting, and monitoring a more comprehensive compliance and board governance function, establishing and maintaining internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act, and preparing and distributing periodic public reports in compliance with our obligations under the federal securities laws. In addition, being a public company will generally make it more expensive for us to obtain director and officer liability insurance.
53
We have an uncommitted revolving credit facility with Bank of America, National Association ("Bank of America") that provided for a maximum borrowing capacity of $100,000 throughout 2013. Advances on our uncommitted revolving credit facility can be in the form of variable rate advances, fixed rate advances, or term advances. Interest rates on variable interest rate advances would reset weekly at the then applicable LIBOR rate plus a spread, which is determined at the time of the advance. Finance charges on variable rate advances would accrue monthly and are due on the earlier of demand of the first business day of the succeeding month. Fixed rate advances would bear finance charges at a fixed rate of interest established at the time of the advance plus a spread for a period up to 12 months. Each fixed rate advance, together with all accrued finance charges, fees and other charges, would be due upon the earlier of demand or the last day of the fixed rate period. Term advances would bear finance charges at a fixed rate of interest established at the time of the advance plus a spread for a term greater than 12 months. Each term rate advance, together with all accrued finance charges, fees and other charges, would be due upon the earlier of demand or the last day of the term period. An early repayment penalty applies to fixed rate and term rate advances. The early repayment penalty is equal to the amount sufficient to compensate Bank of America for any loss, cost or expense incurred resulting from the early repayment, including any loss of anticipated profits and any loss or expense arising from the liquidation or reemployment of funds obtained by Bank of America for purposes of these advances. During the first three months of 2014 and the years ended December 31, 2013 and 2012, we did not request or obtain any advances from this revolving credit facility.
We believe our sources of liquidity, including cash flow from operations, existing cash and cash equivalents, and available financing sources will be sufficient to meet our projected cash requirements, including with respect to both the increased operating expenses we expect to incur in connection with being a public company, for at least the next 12 months. Any equity financing may be dilutive to stockholders and debt financing, if available, may involve restrictive covenants and increase our cost of capital. We will monitor our capital requirements to ensure our needs are in line with available capital resources. From time to time, we may explore additional financing sources to meet our working capital requirements, make continued investment in research and development, expand our business and acquire products or businesses that complement our current business. These actions would likely affect our future capital requirements and the adequacy of our available funds. Our future liquidity and capital requirements will depend on numerous factors, including the:
- •
- Amount and timing of revenue and expenses;
- •
- Extent to which our existing and new products gain market acceptance;
- •
- Extent to which we make acquisitions;
- •
- Cost and timing of product development efforts and the success of these development efforts;
- •
- Cost and timing of selling and marketing activities; and
- •
- Availability of borrowings under line of credit arrangements and the availability of other means of financing.
Quantitative and Qualitative Disclosures about Market Risk
Foreign Currency Exchange Risk
We have foreign currency risks related to our revenue and operating expenses denominated in currencies other than the U.S. dollar, principally the Japanese yen ("Yen). The volatility of the Yen depends on many factors that we cannot forecast with reliable accuracy. We have experienced and will continue to experience fluctuations in our net income as a result of transaction gains (losses) related to revaluing Yen denominated cash and accounts payable balances that are denominated in Yen. In the event our Yen denominated cash, accounts payable or expenses increase, our operating results may be
54
affected by fluctuations in the Yen exchange rate. If the U.S. Dollar uniformly increased or decreased in strength by 10% relative to the Yen, our net income would have correspondingly increased or decreased by an immaterial amount for the year ended December 31, 2013 and the three months ended March 31, 2014.
Interest Rate Risk
When able, we invest excess cash in bank money-market funds or discrete short-term investments. The fair value of our cash equivalents and short-term investments is sensitive to changes in the general level of interest rates in the U.S. If market interest rates were to change by 10% from levels at March 31, 2014, we expect the corresponding change in fair value of our investments would be immaterial. This is based on sensitivity analyses performed on our financial position as of March 31, 2014. Actual results may differ as our analysis of the effects of changes in interest rates does not account for, among other things, sales of securities prior to maturity and repurchase of replacement securities, the change in mix or quality of the investments in the portfolio, and changes in the relationship between short-term and long-term interest rates.
Off-Balance Sheet Arrangements
Under our amended and restated bylaws, we have agreed to indemnify our officers and directors for certain events or occurrences arising as a result of the officer or director serving in such capacity. We have a director and officer liability insurance policy that limits our exposure under these indemnifications and enables us to recover a portion of any future loss arising out of them. In addition, in the normal course of business, we enter into contracts that contain indemnification clauses whereby the Company indemnifies our customers against damages associated with product failures. We have obtained liability insurance providing coverage that limits our exposure for these indemnified matters. We have not incurred costs to defend lawsuits or settle claims related to these indemnities. We believe the estimated fair value of these indemnities is minimal and have not recorded a liability for these agreements as of March 31, 2014. We had no other off-balance sheet arrangements during the years ended December 31, 2013 or 2012 or the three months ended March 31, 2014 that had, or are reasonably likely to have, a material effect on our financial condition, results of operations, or liquidity.
Recent Accounting Pronouncements
SeeNote 1 – Organization and Significant Accounting Policies to the Financial Statements contained herein for a full description of recent accounting pronouncements including the respective expected dates of adoption and effects on results of our operations and financial condition.
55
Overview
We develop, manufacture, market and distribute MRI compatible products, and today, we are the global market leader in the sale of non-magnetic IV infusion pump systems. We were the first to develop an infusion delivery system that neutralizes the dangers and problems present during MRI procedures. Standard infusion pumps contain magnetic and electronic components which can create radio frequency interference and are dangerous to operate in the presence of the powerful magnet that drives an MR system. Our mRidium MRI compatible IV infusion pump system uses a patented non-magnetic ultrasonic motor and other uniquely-designed non-ferrous parts that enable accurate, safe and dependable fluid delivery to patients undergoing an MR procedure. With the expanding use of MRI procedures, both traditional procedures and new intraoperative and interventional procedures, safe and reliable infusion delivery in an MR environment is becoming increasingly important to hospitals and other medical providers. Our founder, President and Chief Executive Officer, Roger Susi, is a pioneer in the MRI compatible medical device industry, having invented the first MRI compatible patient monitoring system in 1986 and the first non-magnetic MRI safe infusion system in 2004. Since launching our first generation MRI compatible IV infusion pump system in 2005, we have continued to modify and improve our system, and we have leveraged our development strengths and unique market position to expand our customer base and profitability.
We sell our products primarily to acute care facilities and outpatient imaging centers, both in the United States and internationally. In fiscal year 2012, we undertook a direct sales strategy in the United States. Today, our direct sales force consists of eight sales representatives, supplemented by two clinical support representatives. Our goal is to expand our U.S. sales force to 10 by the end of 2014. We have distribution agreements with 35 independent distributors selling our products internationally.
As of March 31, 2014 we estimate that we had approximately 1,917 MRI compatible IV infusion pump systems installed globally. Each system consists of an mRidium MRI compatible IV infusion pump, mobile stand, and proprietary disposable IV tubing sets and many of these systems contain additional optional upgrade accessories. We generate revenue from the one-time sale of pumps and accessories, ongoing service contracts and the sale of disposable IV tubing used during each scan. Our revenue growth has accelerated since initiating our direct sales effort. In fiscal year 2013, our revenue reached $11.3 million and our operating profit was $2.8 million representing an operating margin of 24.6%. This operating margin reflects the blended results of our IV infusion pumps, pump upgrades and disposable IV tubing sets.
History and Development
Mr. Susi founded Invivo Research Inc. in 1979 where he developed the first MRI compatible patient monitoring system. Mr. Susi served as the President of Invivo Research Inc. from 1979 until 1998, and as its Chairman of the Board of Directors from 1998 until 2000. Under Mr. Susi's leadership, Invivo Research matured from a start-up medical device company into a leading producer of vital signs monitoring devices during MRI procedures. Invivo Research was acquired by Invivo Corporation in 1992, which began trading on the NASDAQ Stock Exchange in 1994. Mr. Susi served as a Director of Invivo Corporation from 1998 until 2000 and oversaw technical areas from 2000 to 2004. Invivo Corporation was acquired by Intermagnetics General Corporation in 2004 for $152 million, at which time Invivo Corporation had generated revenues of approximately $58.5 million for the prior 12 months. The Invivo system, currently owned by Koninklijke Philips NV (NYSE: PHG), continues to maintain its position as the market-leading MRI compatible vital signs monitor.
Mr. Susi began exploring the market for an MRI compatible IV infusion pump while at Invivo. Invivo subsequently disclaimed any interest in the infusion pump and acknowledged that Mr. Susi was
56
free to pursue the infusion pump development for his own account. Accordingly, after leaving Invivo in January 2004, Mr. Susi began the formal and detailed development of what subsequently has become our mRidium MRI compatible IV infusion pump system. During 2005, he assembled a team of individuals experienced in the medical device industry, many of whom were former employees of Invivo. This first generation MRI compatible IV infusion pump system and its associated proprietary IV tubing sets obtained FDA market clearance in March 2005 after which point we began our sales and marketing efforts.
We initially marketed the product ourselves in the U.S. with limited sales staff, and within one year, commenced international sales through a network of distributors. In 2006, we signed an exclusive distribution agreement with Mallinckrodt/Tyco Healthcare (now Covidien (NYSE: COV)) for domestic and Canadian distribution of our products including the mRidium 3850 MRI compatible IV infusion pump system. The exclusive arrangement ended in 2010, allowing us to implement a direct marketing strategy with our own sales force in the U.S. and Canada.
In 2009, we introduced our second generation MRI compatible IV infusion pump system, the mRidium 3860+ which improved upon the previous 3850 version in a number of areas, including the addition of SpO2, blood oxygen saturation monitoring, and remote wireless monitoring capability. An SpO2 monitor can signal when an insufficient level of oxygen is being supplied to the body. Our mRidium 3860+ is the leading MRI compatible IV infusion pump system on the market today. In 2011, we introduced the iMagox product line, a standalone SpO2 patient monitor which was based on our MRI compatible SpO2 monitoring system with a wireless remote control for international distribution only.
Need for MRI Compatible IV Infusion Pumps
MRI is a widely-used, non-invasive medical imaging technique to visualize vital organs, body function and to identify blockages, abnormalities and growths. MRI is generally considered safer than other scanning techniques that expose the body to radiation. This is particularly true for children. As such, hospitals and other medical facilities have been increasingly developing and using MRI for new procedures. These procedures include cardiac stress testing, intraoperative MRI and neurology MRI techniques. Our mRidium MRI compatible IV infusion pump offers a way to deliver critical IV fluids safely and accurately, thereby allowing the expanded use of MRI procedures.
While the benefits and utility of interventional MR are known, there are hazards intrinsic to the MR environment which must be respected. These hazards may be attributed to a powerful static magnetic field, pulsed gradient magnetic fields, and pulsed radio frequency fields. The MRI suite is a harsh place for medical devices, and safe and proper patient care requires specialty equipment that is specifically designed and built for the MR environment. Many of the dangers and problems present in the MR environment can be solved through use of non-magnetic equipment that have operational safeguards and that maintain performance standards within a harsh magnetic environment while maintaining patient safety. Designing an IV infusion pump system to operate safely and effectively in the MR environment requires overcoming significant technical hurdles.
Intravenous fluids are needed during MRI procedures for many different reasons. Infusion pumps provide sedation to patients who are not able to lie still during an MRI scan and a continuous flow of critical medications to seriously ill patients. Given the benefits to patient safety, radiology departments performing the scan, anesthesia departments delivering sedation and critical care specialists responsible for delivering critical medications during MRI procedures often initiate requests for an MRI compatible IV infusion pump.
57
Conventional Infusion Pumps and Other Inadequate Alternatives
For those medical facilities that do not currently own an MRI compatible IV infusion pump, there are five general methods that are used to deal with patients undergoing an MRI who require IV medications during their imaging procedure: (1) use conventional (magnetic) pumps with long IV lines that extend outside the MRI scanner room; (2) do not offer MRI treatment to patients requiring IV medications or sedation; (3) proceed and accept patients for an MRI procedure but stop the flow of IV fluids during the procedure; (4) allow the uncontrolled free drip of IV fluids; and (5) attempt to shield a conventional IV infusion pump. All of these approaches have severe drawbacks.
Use of multiple lengths of extension tubing can cause inaccurate readings and false alarms or, more seriously, delayed alarms for equipment issues such as occlusion, especially when low flow rates are being used. Such makeshift extension sets can also affect the effectiveness of fluid delivery. A clinician's adjustment of dosage and other settings may take longer to reach the patient due to the over-extended tubing.
Further, there are risks in using a conventional IV infusion pump that is mistakenly believed to be at a safe distance from the MR scanner. The powerful magnetic fields may cause metal objects in the MR environment to be drawn with great force into the bore of the MR system, resulting in potentially deadly projectiles. Moreover, an MR scanner's gradient magnetic field and RF fields can send currents in cables and other conductive materials that are near the MR system and cause the cables to heat. Hot cables may result in burns if they come into contact with a patient. Other problems include devices malfunctioning if they are not properly designed for use in the harsh MR environment and low-quality images due to artifacts caused by RF interference emitted from ancillary equipment.
To deal with the harsh environment of MR, some manufacturers have offered a "shielded box" solution for use with their conventional IV pumps. Outside of the U.S., B. Braun, Arcomed, and Fresenius Kabi recently introduced a proprietary RF shielded aluminum housing, known as a Faraday cage. The "shielded box" approach has also been offered internationally in the past by Mammendorfer Institut für Physik und Medizin, GmbH, but gained little traction. The major problem with this approach is that a highly magnetic conventional IV infusion pump is still being introduced into a hazardous MRI environment which can lead to projectile accidents. Moreover, a Faraday cage with the conventional IV infusion pump must be kept approximately 5 to 10 feet from the scanner. By contrast, our product can be safely placed anywhere in the scanner room including next to the scanner. We are not aware of any "shielded box" installations in use in the U.S. or any with a FDA 510(k) clearance and hence, we expect little current competition from this approach in the U.S.
We believe that our mRidium MRI compatible IV infusion pump system is the first and only product to provide an easy-to-operate, non-magnetic, safe and RF quiet solution and hence a truly MR compatible product.
Market Opportunities
Exit of Competitor from Market
During 2012, our only direct competitor in the MRI compatible IV infusion pump business, Bayer Radiology (formerly MEDRAD, Inc.), became the subject of an FDA recall with respect to its market-leading Continuum device. In mid-2012, Bayer Radiology announced that it was discontinuing, until further notice, all new sales of Continuum leaving us as the only global supplier of MRI compatible IV infusion pumps. During 2013, Bayer Radiology announced its decision to commence removal of its pump systems from the U.S. market, and to discontinue support throughout the world by June 30, 2015 due to ongoing regulatory issues, at which time Bayer Radiology will end its limited supply of its proprietary consumable IV sets that some current customers are receiving.
58
As a result of Bayer Radiology's announced exit from the market, we anticipate that many Continuum customers will replace their MRI compatible IV infusion pumps with our mRidium 3860+ system. We are continuing to expand our direct sales force in the U.S. and believe that our pump sales will be favorably impacted by the exodus of Bayer. We intend to market aggressively to this existing user base as well as to new potential users.
Expansion of Inter-Hospital Use of MRI Compatible IV Infusion Pumps
We currently market our MRI compatible infusion pumps primarily to the MRI departments of hospitals. We believe, however, that there is potential for expanded deployment of our MRI compatible IV infusion pumps within the Intensive Care Unit ("ICU"), Emergency Room ("ER"), and other locations within the hospital with a high probability that interventional radiology procedures will need to be performed on patients. Expanded use of our additional MRI compatible IV infusion pumps would serve as a type of transport pump and allow for consistent administration of IV fluids without interruption and easy transfer from the ICU or ER to the MRI scanner room.
It frequently becomes necessary for a patient in the ICU or ER who is connected to an IV infusion pump that is delivering critical medications to be quickly moved to the MRI facility for immediate imaging. The presence of multiple MRI compatible IV infusion pumps or pump channels, for each IV line, enables the orderly and rapid transfer between IV infusion pumps by allowing patients to be prepared for an MR procedure and setup on our mRidium MRI compatible IV infusion pump in the ICU or ER. Seriously ill patients are generally at higher risk when they are away from the resources of the ICU or ER, and efficient IV infusion pump transfer minimizes the time the patient spends away from the ICU or ER.
We believe there is a link between the number of infusions and infusion pumps and the onset of equipment-related adverse events during the intra-facility transport of critically ill patients. We therefore believe that placing our MRI compatible IV infusion pumps in ICU and ER facilities could reduce patient adverse events associated with the transport and pump exchange within the hospital.
Recently some hospitals have begun to use MRI during surgical procedures. Neurosurgical interventions have been at the forefront of this development in image-guided surgery, followed by otolaryngological procedures. As MR-guided intervention during surgery has been deployed, the degree of complexity in supplemental devices has increased markedly. Much of the effort required for successful implementation of intraoperative MRI has been in development and testing of anesthesia equipment, patient monitoring devices, infusion pumps and surgical instruments and accessories, all of which need to be MRI compatible if used near the MRI scanner. Intraoperative MRI is expanding demand for our MRI compatible IV infusion pump system from the MRI suite to the surgical suite of the hospital, again with multiple pump channels for multiple IV lines.
Market Summary
The following table summarizes the current potential worldwide market opportunity for MRI compatible IV infusion pumps systems. The worldwide base of installed MRI scanners is estimated at over 35,000, and we estimate that 15,500 of those scanners are located in acute care facilities with
59
favorable market conditions as defined by us, that would benefit directly from MRI compatible IV infusion pumps.
| | U.S. | ROW | |||||
|---|---|---|---|---|---|---|---|
Current Installed Base of MRI Scanners | 11,453 | 23,845 | |||||
Current Base of Acute Facilities with Pump favorable market conditions | 7,500 | 8,000 | |||||
Potential Pumps Based Upon Current Usage Practices (see below) | 12,000 | 9,600 | |||||
MEDRAD Replacement Pump Opportunity | 3,200 | 1,500 | |||||
U.S. Market
We believe that of the total 11,453 U.S. installed base of MRI scanners, approximately 7,500 are installed in acute care facilities of sufficient sophistication as to be considered supporting favorable market conditions for our MRI compatible IV infusion pump systems. Of the facilities currently using MRI compatible IV infusion pumps, many have elected to purchase more than one IV infusion pump per MRI scanner installed. Therefore, based upon our historical sales and customer pump purchases, we estimate that our current market opportunity in the U.S. represents 12,000 MRI compatible IV infusion pump systems to equip the 7,500 MRIs in acute care facilities. Near term, the U.S. market is also more advanced in the purchase of MRI compatible products and accessories and hence represents the greatest potential for our products.
Consider that today, we have approximately 1,180 mRidium MRI compatible IV infusion pumps sold in the U.S. in just over 560 facilities. Our current market opportunity is to equip the remaining MRIs located in acute care facilities, as well as to continue marketing to our existing customers and exploiting the operational merits and efficiencies of having multiple IV infusion pumps to support each MRI system. Included within the current 12,000 U.S. MRI compatible IV infusion pump opportunities are an estimated 3,200 MEDRAD systems which are being removed from the market no later than 2015. We intend to aggressively market our mRidium MRI compatible IV infusion pumps as replacement systems. Further, we estimate that new MRI scanner installations in the U.S. are growing at a rate of approximately 5% each year, 300 of which represent new installations at acute care facilities and hence, potential users of our mRidium MRI compatible IV infusion pump system.
International Markets
Market studies estimate the installed base of MRI scanners outside the U.S. to be 23,845 systems. We estimate that 8,000 of those systems are located in acute care facilities of sufficient sophistication as to be considered supporting favorable market conditions for MRI compatible IV infusion pumps. Based upon historical customer pump purchases and our sales history, many of our customers use more than one IV infusion pump system per MRI scanner. Outside the U.S., we estimate a potential opportunity to sell 9,600 MRI compatible IV infusion pump systems. Europe and Japan have represented the largest markets for our MRI compatible products in international markets, and we expect they shall continue to offer the greatest potential.
We have a total installed base in Europe of approximately 250 mRidium MRI compatible IV infusion pumps, and we estimate that competitors such as MEDRAD have placed significantly more than 250. We estimate that Europe has a current installed base of 3,700 MRI scanners located in acute care facilities with favorable market conditions offering us the potential to sell over 4,400 MRI compatible IV infusion pump systems. In terms of future growth, an estimated 250 new MRIs will be installed in these acute care facilities each year in Europe. Japan represents a market potential similar to that of Europe, but is likely to develop more gradually. We have a current installed base in Japan of approximately 75 mRidium MRI compatible IV infusion pumps, and we believe that Japan has
60
approximately 2,500 MRI scanners in acute care facilities with favorable market conditions creating the potential for us to sell 3,100 MRI compatible IV infusion pump systems.
The rest of the world outside of Europe and Japan ("ROW") represents a diverse area including developed markets such as Canada, Hong Kong, Australia, and certain parts of the Middle East. It also contains many less favorable markets that have lower quality of patient care, where patient safety and therefore MRI compatible IV infusion pumps are less of a priority. Though market studies show over 10,000 MRI scanners installed in these regions, we estimate that in the ROW 1,800 MRI scanners are located in acute care facilities with favorable market conditions and the potential for us to sell 2,100 MRI compatible IV infusion pump systems. We have 265 mRidium MRI compatible IV infusion pumps installed in the ROW, which includes two non-recurring orders from Saudi Arabia in 2013 totaling 129 mRidium MRI compatible IV infusion pump systems.
Our Products
The mRidium MRI compatible IV infusion pump system is based upon a patented non-magnetic ultrasonic motor and other uniquely-designed non-ferrous parts in order to provide accurate and dependable fluid delivery to patients undergoing an MRI procedure. Our mRidium MRI compatible IV infusion pump system has been designed to offer numerous advantages to hospitals, clinicians and patients. mRidium's strengths include the following:
- •
- The only truly non-magnetic MRI compatible IV infusion pump system specifically designed and built for the MRI environment.
- •
- A mobile, rugged, easy-to-operate, and reliable system with a strong safety record.
- •
- Able to operate virtually anywhere in the MRI scanner room; approved for use in the presence of 0.2T to 3T magnets and fully operational up to the 10,000 gauss-line.
- •
- The only non-magnetic MRI compatible IV infusion pump available with a Dose Error Reduction System ("DERS") to reduce the risk of medication errors and simplify clinician monitoring.
- •
- Available with a wireless remote display/control providing clinicians and technicians control and visibility from outside of the MRI scanner room.
- •
- Available with an add-on channel allowing for the easy addition of a second IV line for patients requiring multiple IV medications at a low incremental cost to the hospital.
- •
- Available with a built-in SpO2 monitor using Masimo SET® technology and a specially designed fiber optic SpO2 sensor allowing one device to monitor oxygen saturation levels while safely providing IV infusion during an MRI procedure.
The following diagram is an aerial view of an MRI scanner room with a top-of-the-line 3T magnet. The gauss-lines illustrate the distance from the magnet where various types of infusion pumps can safely operate. Our mRidium MRI compatible IV infusion pump is the only pump on the market
61
approved to operate safely and reliably near the patient (area shown in blue). All of the other pumps must either be placed at a distance from the MRI scanner or outside of the scanner room entirely.
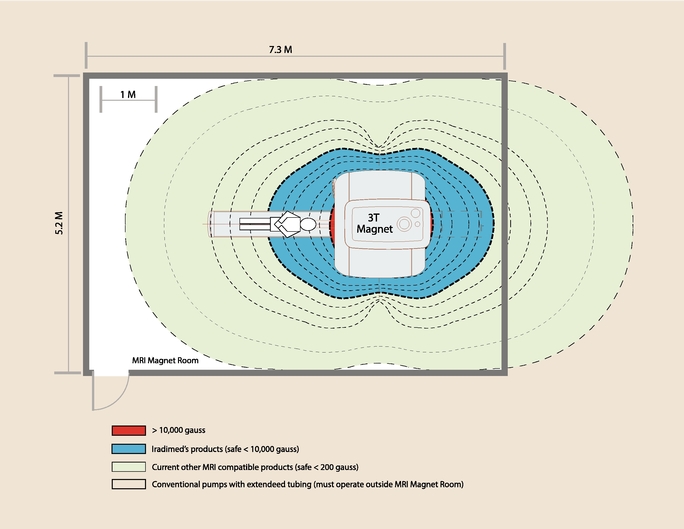
Our MRI compatible IV infusion pump system includes the 3860+ MRI compatible IV infusion pump, proprietary single-use IV tubing sets, a non-magnetic pole and a lithium battery. In addition, we offer optional upgrade systems including the 3865 Remote Display/Control, 3861 Side Car, DERS, and an SpO2 monitor as discussed below.
mRidium 3860+ MRI Compatible IV Infusion Pump
The mRidium 3860+ MRI compatible IV infusion pump system was introduced in 2009 and improved upon the strong performance and features of our first generation mRidium 3850 MRI compatible IV infusion pump system. Our pump systems can operate dependably in the presence of 0.2T to 3T magnets and are fully operational up to the 10,000 gauss-line. This means they are highly versatile and can operate virtually anywhere in the MRI scanner room, including close to the MRI scanner. The mRidium 3860+ MRI compatible IV infusion pump system has a 10-key numeric input keypad making our system easy to accurately program and operate. Our pumping range of 0.1 mL per hour to 1400 mL per hour provides a broad range of fluid flow control. Our broad range of infusion rates support differing patient needs including low levels for pediatric sedation, mid-levels for continued IV infusion of medications to critically-ill patients and high levels in the event of emergency situations. Our mRidium 3860+ MRI compatible IV infusion pump system offers a dose rate calculator, bolus dose programming, full alarm settings, and a rechargeable battery with a 12-hour battery life.
62
mRidium 3860+ IV Tubing Sets
The mRidium 3860+ MRI compatible IV infusion pump system utilizes proprietary fluid delivery tubing sets, each known as an "IV tubing set." Each use of our MRI compatible IV infusion pump requires a disposable IV tubing set. We offer a variety of IV tubing sets for varying MRI scenarios and these include our standard "spike" infusion set, syringe adapter infusion set, extension infusion set, and our unique bypass infusion set. All of our IV tubing sets are latex-free and DEHP-free.
- •
- mRidium 1056 Standard Infusion Set. Our standard "spike" infusion set features the ability to accurately deliver liquids from either a bottle or IV bag. Our standard infusion set contains two needle-free injection ports and is typically used when starting a new infusion from a bottle or bag.
- •
- mRidium 1057 Syringe Adapter Infusion Set. Our syringe adapter IV set enables users to provide accurate delivery of IV fluids directly from standard syringes. The vented syringe adapter set benefits from a low priming volume of 4 ml, which minimizes inefficient waste of medication. This product is most commonly used for cardiac medications, anesthesia, and pediatric drug delivery.
- •
- mRidium 1058 Extension Infusion Set. Our extension infusion set allows users to transfer a patient on a non-MRI infusion pump to our mRidium MRI compatible IV infusion pump. The user simply disconnects the existing IV tubing at the patient site and connects and primes the mRidium extension set to the existing IV tubing. Once removed from the conventional infusion pump and connected to our mRidium MRI compatible IV infusion pump, the user can program the pump and begin the infusion. The extension set includes one needle-free injection port and is typically used to provide uninterrupted critical medications to a severely ill patient during an MRI procedure.
- •
- mRidium 1055 MRI Infusion Bypass Set. Our bypass infusion set offers a cost effective solution to the difficult problem of how to change over from a non-MRI compatible IV infusion pump without breaking sterility. The bypass IV set includes integrated needle-free ports that can be activated only by a standard luer adapter, eliminating the need for new needles and reducing the risk of accidental transmission of blood borne pathogens.
MR IV Pole
We offer a fully-functional and weighted non-magnetic IV pole that is designed for mobility within the hospital and the MRI scanner room. The IV pole can support two mRidium MRI compatible IV infusion pumps, each with a side car. The IV pole is 66 inches (1.68 meters) high, stabilized with a wide pole radius and mobilized with five casters designed to roll easily during transport. The IV pole is equipped with four aluminum hooks for holding fluids.
Optional Features
Our 3860+ MRI compatible IV infusion pump system gives customers the ability to adapt their systems to meet their specific needs. In addition to our standard product features, we also offer system upgrades which include a wireless remote control/display, a modular add-on second IV channel through our "Side Car," DERS and an imbedded SpO2 monitor. We also offer rechargeable lithium polymer battery packs which have 12-hour battery life when not connected to an electrical outlet.
3865 mRidium Wireless Remote Display/Control
Our wireless remote control units allow for complete control and monitoring of the mRidium MRI compatible IV infusion pump system from the control room (outside of the MRI scan room). The 3865 mRidium Wireless Remote relays all commands and displays information bi-directionally between
63
the MRI compatible IV infusion pump and the remote control unit. Utilizing the same user interface and large bright display as the mRidium pump, our wireless remote control unit permits clinicians to adjust all pump parameters, including SpO2 monitoring parameters, rates, dose, volume, pump run/stop, alarms (adjust or reset), as well as real-time titration. Our remote control unit utilizes a proven MRI compatible 2.4 GHz FH (frequency hopping) spread spectrum radio technology for artifact-free operation that does not disturb the MRI imaging process. Clinicians may also use the remote control unit to adjust a second pump channel when used in combination with our Side Car unit discussed below. Our 3865 mRidium Wireless Remote also functions as a battery charger for our mRidium battery pack.
3861 Side Car Pump Module
Our Side Car Pump Module can be attached to our 3860+ mRidium MRI compatible IV infusion pump to provide a second channel for infusion delivery. This flexible option allows hospitals to convert their single-channel infusion pump into a dual-channel system designed to deliver both large and small volume fluids in the MRI scanner room. The side car is fully functional with our 3865 mRidium Wireless Remote, allowing clinicians the ability to control both channels with one remote control unit outside of the MR scanner room. The additional delivery line has all of the same features and benefits as the 3860+ mRidium MRI compatible IV infusion pump, as described above.
Dose Error Reduction System ("DERS")
Our DERS for use with our mRidium 3860+ MRI compatible IV infusion pump system incorporates the latest drug safety features for patients. The DERS system enables users to create a unique drug library and establish nominal values and limits for dose and concentration for specified infusion protocols. With DERS, patient safety and user convenience are supported by user-programmed infusion hard limits (maximum and minimum) and soft limits (high and low limits that require user confirmation to exceed). The dose applied via DERS is displayed and can be adjusted directly (titrated) on the running screen at any time during the infusion. The universal memory card port allows for easy archiving and updating of the drug library.
SpO2 Monitoring with Sensor and Accessories
Our mRidium 3860+ MRI compatible IV infusion pump system also offers state-of-the-art Masimo SET® SpO2TM capability providing a unique ability to have SpO2 monitoring and IV delivery combined in one unit. This feature offers users the ability to start sedations outside of the MRI scanner room, transport to the scanner, and then back to recovery without having to discontinue SpO2 monitoring on the patient. In addition, our fiber optic MRI-SpO2 sensor and accessories provide a safe connection between the patient and our MRI compatible IV infusion pumps. This fiber optic-based SpO2 sensor delivers outstanding performance while avoiding potentially hazardous heating or image artifact during MR scans. The method of patient attachment uses a medical-grade silicone rubber sensor grip that allows easy and convenient attachment to the patient's hand or foot, and accommodates pediatric, adult, and infant patients with various size grips.
We believe our mRidium 3860+ MRI compatible IV infusion pump system and its customizable features comprehensively and uniquely address the needs of MRI departments within hospitals and other medical facilities.
We also offer two products exclusively to non-U.S. customers. These products consist of the 2460 iMagox MRI SpO2 Monitor and the 2465 iMagox Remote Control.
64
iMagox 2460 MRI Pulse Oximeter – Approved for Use Outside of the U.S.
The iMagox 2460 MRI Pulse Oximeter System uses Masimo SET® Technology and is approved for use in the presence of 0.2 to 3T magnets and operational up to the 10,000 gauss-line. Our digital MRI pulse oximeter simultaneously measures and displays the functional oxygen saturation and pulse rate of adult, pediatric and infant patients. The large display provides digital and waveform data with SpO2 alarms and user messages, which can be easily seen within the MR scanner room. When fully charged, the battery supporting this system will provide up to 24 hours of continuous operation. The unique rear clamp mechanism swivels to allow mounting on either a non-magnetic IV pole, or for mounting to a bed side rail. This portability combined with the system's extended battery life gives clinicians at medical facilities the freedom to administer continuous patient monitoring before, during and after an MRI scan. Our iMagox system also provides an optional wireless remote and display described below.
iMagox 2465 MRI Oximeter Remote and Display – Approved for Use Outside of the U.S.
The iMagox 2465 Wireless Remote and Display allows for monitoring and control of the MRI Pulse Oximeter from outside the MR scanner room. Our remote allows users to adjust all oximeter parameters and reset alarms. The wireless remote, which is designed for plug-and-play use and requires no installation, is fitted with a large display and utilizes the same user interface as the 2460 MRI Pulse Oximeter. The remote also acts as a charger for a backup or spare battery pack for the iMagox 2460 MRI Pulse Oximeter. It utilizes a wireless link at 2.4 GHz for reliable communication with no image artifacts.
Strategy
Company Objective
Our objective is to become the leader in providing safe and effective care for all patients undergoing MRI procedures through the development and commercialization of a portfolio of MRI compatible products. By increasing the safety parameters of equipment operating within the harsh magnetic environment of the MRI scanner room, we hope to enable hospitals and other healthcare providers to offer the MRI diagnostic procedures patients desperately require. In particular, our goal is to increase the safety of MRI diagnostics for critically ill patients and children by minimizing potential complications with IV infusions and vital signs monitoring.
We seek to grow our business by, among other things:
Driving market awareness of MRI compatible IV infusion pumps and the safety risks associated with using conventional IV pumps with long IV lines.
We believe that the largest potential market for our MRI compatible IV infusion pumps is the segment of the market that is currently using workaround solutions. Such solutions include using conventional pumps outside the MRI scanner room and attaching multiple extension lines of IV tubing sets through the wall or under the door into the magnet room to reach the patient. This practice of makeshift setups is fraught with risks to the patient and unnecessary costs and inefficiencies. These risks and inefficiencies include:
- •
- Infection risk from running lengthy IV tubing sets through the wall or under the door;
- •
- Risk of inaccuracy from using a conventional IV infusion pump with multiple extension lines;
- •
- Potential medication occlusion and lengthy alarm notification delays due to multiple extension lines, posing a great risk to a patient on critical medications;
65
- •
- Excess medication costs due to the disposal of multiple extension IV tubing sets filled with unused medication at the end of the procedure; and
- •
- Lost productivity and MRI scanning time due to the lengthy set up time required for multiple extension lines.
We believe that increased market awareness and education will be required for potential customers to appreciate the value for patients and the hospital of an efficient and patient-safe MRI environment which includes MRI compatible IV infusion pumps.
Continuing to innovate with MRI compatible patient care products.
Our management team collectively has more than 100 years of experience with MRI compatible products. We have entrenched relationships with many of the industry's top thought leaders and we have, and will continue to, closely collaborate with them to build upon mRidium's innovative MRI compatible technologies to create next generation pump systems. We intend to leverage this experience and collaboration to innovate and commercialize other technologically-advanced MRI patient care products, such as a device for assisting resuscitation and a thermal management unit.
Developing a New Resuscitation/Monitoring System.
We currently have under development a new resuscitation device with multi-parameter vital signs that is MRI compatible and which we plan to launch in 2015. When providing anesthesia care in the MRI environment, the same requirements for safe resuscitation and monitoring that apply in a typical operating room must be satisfied. Our device is being developed using MRI compatible technology to safely deliver therapy and monitor all of the required basic vital signs parameters including electrocardiography, pulse oximetry, non-invasive blood pressure, capnography, and temperature. Our device will be designed to have a monitoring/remote station, with wireless communication capability outside of the scanner room (in the control room).
Acquiring synergistic MRI patient care companies, products or technology licenses to accelerate our product development and leverage our existing direct sales organization in the U.S.
We have an experienced team of engineering and operations managers committed to improving on existing MRI patient care designs through our internal development efforts and the acquisition of technologies and intellectual property of others. We have an effective and growing direct sales organization in the U.S. and a team of experienced international distributors that can effectively go to market with additional MRI patient care products. We intend to actively analyze opportunities to improve our product mix and profitability.
Commercial Strategy
We believe that the MRI compatible IV infusion pump market is still in its early stages of growth given the low rate of market penetration, and we aim to drive increased awareness and adoption of our products by:
Expanding our MRI-focused U.S. direct sales force and our international sales efforts.
We believe the most meaningful aspect of our commercialization strategy in the U.S. is the continued development and expansion of our direct sales force. Since there is no current direct competitor for an MRI compatible IV infusion pump, our focus is on expansion of the market through better education on advantages to patients, clinicians and hospitals of our products and the shortcomings of current workaround solutions. Our challenge in the past has been an understaffed direct sales team and a limited ability to educate our potential customers.
66
Since 2011, our U.S. sales team has grown from one sales representative to a team of eight direct sales representatives and two clinical support representatives. We intend to continue to grow our specialized, MRI product-focused sales team to 10 and to support it with clinical support representatives as the business dictates. We will market to current users of the Continuum system of our former competitor, Bayer Radiology, who will soon be without a viable solution. In addition, we believe that we can significantly increase sales of our mRidium MRI compatible IV infusion pump by also calling on anesthesia and critical care departments, to help influence hospitals' purchasing decisions. We believe that this strategy will likely expand the number of acute care facilities using our MRI compatible products and increase the average number of MRI compatible IV infusion pumps per acute MRI.
Internationally, our focus in 2014 and beyond is to work with our distributors in key target markets, such as Europe and Japan, to expand the business and augment our market penetration rates. In the future, we plan to expand our internal capacity to serve these high potential markets by adding dedicated regional sales managers located outside the U.S. to oversee our relationships at the local level.
Supporting commercial efforts with evidence-based information.
We currently have an initiative to develop a white paper that documents the risks and additional costs associated with using a workaround solution of running long lines from conventional IV pumps outside the MRI scanner room. We believe that this kind of evidence-based documentation will help us to provide widespread education to the clinicians that are driving clinical practice. We also believe that documented evidence will serve to inform the quality and risk management leaders in these organizations, which in turn may help drive the overall adoption of our mRidium MRI compatible IV infusion pump.
Providing best in class customer service and user experience.
We believe that the expectations of our customers for service and a superior user experience have risen with the advancement of technology. Once a customer purchases a mRidium MRI compatible IV infusion pump, it is imperative that they receive first-class clinical education and support to encourage adoption of our products. We have devoted a significant amount of time and training to ensure that this educational experience is a success. This training is performed most commonly by our sales staff and is augmented by our clinical support representatives; however, we intend to hire more clinical support specialists to improve our initial training experience and ongoing customer support. We believe that a positive user experience will be critical to driving increased rates of utilization of our products.
Intellectual Property
We protect our proprietary technology through a combination of trade secrets, confidentiality agreements and patents. During the development of our products, our founder, Roger Susi, obtained a number of patents regarding our MRI compatible IV infusion pump and related systems. Mr. Susi has irrevocably assigned these patents to us. We consider our patents important but do not believe our future success is dependent upon patents. We have nine issued U.S. patents and four issued foreign patents. We also have a number of U.S. patent applications pending. These patents and patent applications relate to several of our products, including our MRI compatible IV infusion pump system and its components. We intend to file patent applications with respect to future patentable developments and improvements when we believe that such protection is in our best interest.
67
We entered into a Purchasing and Licensing Agreement with Masimo Corporation (NASDAQ: MASI) ("Masimo") as of April 23, 2009. Under the agreement, Masimo granted us the nonexclusive worldwide license to incorporate Masimo's circuit boards into our MRI compatible IV infusion pumps, to make and sell our sensors for use with such pumps, and to distribute such pumps and sensors for use in MRI applications. The agreement provides for a five-year term which is automatically renewable for successive one-year periods, with either party having the right to terminate the agreement upon six months' notice before the end of any term. In March 2014, the agreement with Masimo was renewed for one year. There can be no assurance of our ability to renew this agreement in the future.
In April of 2013, Rydex Corporation ("Rydex") filed a Complaint against us in the U.S. District Court for the District of Delaware alleging infringement of U.S. Patent No. 5,913,180 entitled "Fluid Delivery Control Nozzle." On October 18, 2013, Rydex filed a Notice of Voluntary Dismissal stating that we had not answered such Complaint or filed a summary judgment motion. In November of 2013, Rydex filed an Amended Complaint against us in the same court alleging infringement of such patent. The claim was later voluntarily dismissed by Rydex and we believe the Rydex allegations were meritless, but there can be no assurance that these assertions, or any similar future assertions, will not result in liability or damages payable by us.
In October 2012, Radimed Gesellschaft für Kommunikationsdienstleistungen und Medizintechnik mbH ("Radimed") brought an action in Düsseldorf Regional Court against our German distributor alleging the name and sign "iRadimed" was confusingly similar to their German trademark "Radimed." A judgment was rendered against our German distributor preventing use of the name and sign "iRadimed" in Germany. We have however continued to sell products in Germany without any discernible effect by using the product name IRI Development. On July 31, 2013, Radimed filed a lawsuit against us and our founder, Roger Susi, in Düsseldorf Regional Court, alleging that we infringed their German and Community trademarks "Radimed" and seeking to prevent our use of the name, sign and domain name "iRadimed" in the European Union. In addition, Radimed is seeking unspecified damages. We will vigorously defend against the infringement claims. However, the ultimate outcome of the matter remains uncertain. If we receive an adverse judgment, we may be prevented from marketing our products in the European Union under the name and sign "iRadimed" and may be required to pay Radimed's attorneys' fees, the court fees and damages, which could materially adversely affect our business, operating results and financial condition.
We have applied to federally register the iRadimed trademark in the U.S.
We also rely on trade secret, copyright and other laws and on confidentiality agreements to protect our technology, but we believe that neither our patents nor other legal rights will necessarily prevent third parties from developing or using similar or related technology to compete against our products. Moreover, our technology may be viewed as improvements or adaptations of known MRI infusion technology, which might be duplicated or discovered through our patents, reverse engineering or both.
Sales and Marketing
We sell our MRI compatible products through our direct sales force in the U.S. and independent distributors internationally. In the U.S., we sell our products through our eight direct sales representatives and two clinical support representatives. We have distribution agreements for our products with 35 independent distributors selling our products internationally. We have developed an experienced team of distributors that have a strong MRI/radiology product portfolio and focus. Our international distributors are managed by our VP of International Sales, an industry veteran with over 20 years in the IV infusion pump business and eight years as our sales manager. Our distributors include Kyorin-Systemac and Saegeling Medizintechnik.
68
Selling cycles for medical devices vary widely but are typically three to six months in duration. To supplement the efforts of our sales and clinical support representatives, we produce and distribute videos that provide users of our mRidium products an easy means for learning clinical applications. These videos guide users through a detailed step-by-step process in using our products, including initial product set-up, selection of infusion sets, loading the infusion pump, programming the pump, managing alarms and alerts and prompts, SpO2 monitoring, and other advanced functions. Users also benefit from our detailed operator manuals and 24/7 technical support via telephone.
The principal customers for our MRI compatible products include hospitals and acute care facilities. A customer's decision to use our products is typically made by the radiologist and anesthesiologist, or department administrative director. We serve these customers through our sales and service specialists and believe that our specialists are well-positioned to build upon these customer relationships. We communicate with our customers on a regular basis in an attempt to understand potential issues or concerns as well as to improve our products and services in response to their needs. Product orders and inquiries are handled by trained service representatives who communicate with customers after equipment shipments, installations and service repair calls. All feedback and measures being taken to ensure satisfaction are reported regularly to our management. We have also implemented various other programs which enable us to assess our customers' needs. These programs include regular surveys and visits to product sites.
We enter into agreements with healthcare supply contracting companies, commonly referred to as GPOs in the U.S., which enable us to sell and distribute our mRidium MRI compatible IV infusion pump systems to their member hospitals. GPOs negotiate volume purchase prices for hospitals, group practices, and other clinics that are members of a GPO. Our agreements with GPOs typically include the following provisions:
- •
- Negotiated pricing for all group members;
- •
- Volume discounts and other preferential terms on their members' purchases from us;
- •
- Promotion of our products by the GPO to its members; and
- •
- Payment of administrative fees by us to the GPO, based on purchases of our products by group members.
Under these agreements, we are required to pay the GPOs a fee of three percent of the sales of our products to hospitals, group practices, and other acute care facilities that are members of the GPO. We currently have contracts with four major GPOs that effectively give us the ability to sell to more than 95% of all U.S. acute care facilities.
- •
- In September 2013 we entered into a nine-month agreement with MedAssets Supply Chain Systems. MedAssets has more than 4,200 participating hospitals and manages $50 billion in member supply expense.
- •
- In April 2013, we entered into a three-year agreement with HealthTrust Purchasing Group ("HealthTrust"), which is one of the largest healthcare supply contracting companies in the U.S. HealthTrust's members include 1,400 acute-care facilities, 800 ambulatory centers and members in more than 10,600 other locations including physician practices, long-term care and alternate care sites.
- •
- In September 2012, we entered into a three-year single-source supply contract with Novation Co., the largest healthcare supply contracting company in the U.S. Pursuant to this agreement, we are permitted to sell and distribute our mRidium MRI compatible IV
69
- •
- In January 2012, we entered into a three-year agreement with the Premier, Inc. healthcare alliance, which is one of the nation's largest performance improvement alliances of approximately 2,900 U.S. community hospitals and 100,000 alternate sites. Pursuant to this agreement, we joined Premier's ASCEND (Accelerated Supply Chain Endeavour) program that identifies and takes advantage of supply chain performance opportunities for its participating acute care facilities members.
infusion pump systems to all of Novation's current members which consist of 100,000 VHA, UHC, Children's Hospital Association and Provista member organizations.
During 2013, revenue from one international customer represented 10.7% of total revenue, which involved non-recurring orders from Saudi Arabia. In fiscal year 2013, 71.4% of our revenue was generated from U.S. sales and 28.6% was generated from international sales.
We generally do not have a significant backlog of firm orders, as our sales are made on purchase orders requiring just in time delivery.
Our mRidium MRI compatible IV infusion pump system received the Frost and Sullivan Technology award in 2005.
Manufacturing and Suppliers
We assemble our products in our facilities in Winter Springs, Florida, from components and sub-assemblies purchased from outside suppliers. We perform final assembly, testing and packaging to control quality and manufacturing efficiency. We purchase components and sub-assemblies from qualified suppliers that are subject to our stringent quality specifications and inspections by us. We conduct quality audits of our key suppliers, several of which are experienced in the supply of components to manufacturers of finished medical devices or disposables for use with these medical devices. Our historical track record of producing MRI compatible IV infusion pump systems has been good; however, there can be no assurance that this trend will continue or that we will be able to produce sufficient units to reach our expected revenue growth rates.
The non-magnetic ultra-sonic motor which drives our MRI compatible IV infusion pump is sole-sourced from a major multinational Japanese manufacturing company with whom we have an excellent long-term relationship. This company has exclusively provided us with these motors since 2005, and we recently renewed our exclusive supply agreement with this company for another five years through 2019. We have never encountered a significant supply interruption from this manufacturer and have received no indications that there might be disruptions of the supply of these motors in the future. We have routinely averaged no more than a two-month supply of these motors in our inventory. The supplier has committed to maintaining our supply and delivering on a timely basis. We have identified two additional suppliers who may be able to produce an ultra-sonic motor essentially identical to our current motor. However, we believe that adding or switching to an alternate supplier would require six months to a year.
Our former supplier of IV sets has ceased business operations. Though this has not had a material impact upon our operations, we now have two alternate suppliers of our IV sets. The first is our own in-house IV set manufacturing capability, and the second is an OEM located in central Florida. We have increased our current in-house clean room production capacity by adding staff and implementing a second production shift. We expect our in-house capabilities coupled with production capacity of the new OEM should be sufficient to meet expected customer demand for the foreseeable future.
70
Other sole or limited supply devices and components of our products include the following:
- •
- Force Sensor. This device is used to measure the fluid pressure within the IV tubing set and provide certain alarm functions. This part is supplied by a large multinational U.S.-based manufacturer. We have never experienced a delivery problem of this part, and we typically maintain three to six months inventory in supply. However, we have had an internal development effort underway to create and manufacture this device in-house. In the event of a failure of our current supplier to furnish sufficient force sensors, we believe we could assume the manufacture of this device in three to five months.
- •
- Bubble Detector. This device provides safety and alarm upon detection of air in the IV fluid line. We have never experienced delivery issues with this part, which is supplied by a major multinational U.S.-based company, and we generally maintain a three-month supply on hand. There is one alternate supplier whose part we have tested on a preliminary basis, and we believe we could switch to that source if needed, within approximately three or four months.
- •
- Lithium Polymer Battery Cells. This device is used to power our MRI compatible IV infusion pump. Though we keep several months' supply on hand and have alternate sources qualified, due to regulatory issues, changing to an alternate source could require three to four months.
- •
- LCD Displays. This component is currently a sole-sourced assembly from a large Chinese supplier who has a history of late delivery. We have an active ongoing effort to cultivate a second source, which we expect to be finalized by Q2 2014.
- •
- Various Molded and Cast Components. These custom tooled and molded/cast parts are subject to supply interruption should the tools become damaged. Replacement of these tools could require up to eight months. Our inventory of such custom molded and cast parts is typically on the order of six months.
We place significant emphasis on providing quality products and services to our customers. Quality management and oversight play an essential role in understanding and meeting customer requirements, effectively resolving quality issues and improving our products and services. We have a network of quality systems throughout our facilities that relate to the design, development, assembly, packaging, sterilization, handling, distribution and labeling of our products. To assess and facilitate compliance with applicable requirements, we periodically review our quality systems to determine their effectiveness and identify areas for improvement.
In January 2007, we received ISO 9001 and ISO 13485 certification and met the requirements under the European Medical Device Directive to use the CE Mark, thereby allowing us to continue to market our products in the European Community. These certificates were last renewed on January 17, 2013, and will need renewal again in January 2016.
Competition
We do not believe there is currently any direct competition for our MRI compatible IV infusion pump systems. Our only direct competitor in the MRI compatible IV infusion pump market, Bayer Radiology, formerly MEDRAD, Inc., announced during 2013 its decision to remove its competing Continuum pump system from the market, and to discontinue support throughout the world by June 30, 2015 due to ongoing regulatory issues. As a result, we believe that our mRidium 3860+ MRI compatible IV infusion pump is the only true MRI compatible IV infusion pump available today. Bayer Radiology's announcement provided that it planned to remove the actual product from customers in
71
the field, and that it would no longer offer the disposable proprietary IV tubing sets after June 30, 2015.
The medical device and IV infusion market is highly regulated and is typically one of the areas that the FDA scrutinizes closely for new market introductions. Because of this, the 510(k) FDA clearance process for new pumps is usually long and requires significant testing and documentation. This long development timeline coupled with the low market penetration to date may discourage new competitors from undertaking a complex project like building an MRI compatible IV infusion pump. However, the medical products industry is generally characterized by intense competition and extensive research and development. We believe, that the market for MRI compatible IV infusion pump products is in relatively early stages of development and may become highly competitive if, and when, the market develops further.
Outside of the U.S., we also compete with manufacturers of "shielded box" solutions that are intended to permit use of conventional IV pumps inside the MR scanner room. The providers of shielded boxes include B. Braun, Fresenius Kabi, MIPM Mammendorfer Institut für Physik und Medizin, and Arcomed. The market for medical products is subject to rapid change due to an increasingly competitive, cost-conscious environment and to government programs intended to reduce the cost of medical care. Many of these manufacturers and distributors of medical equipment are large, well-established companies whose resources, reputations and ability to leverage existing customer relationships might give them a competitive advantage over us. Our SpO2 products, which measure blood oxygen saturation, also compete indirectly with many other methods currently used to measure blood oxygen levels or the effects of low blood oxygen levels.
Another potential competitor may be CareFusion Corporation (NYSE: CFN), a major medical device manufacturer, given its dominant position in the conventional IV infusion pump market, in light of its $100 million minority investment in Caesarea Medical Electronics ("CME") in December 2013. CME manufactured Bayer Radiology's Continuum Pump System. In addition, B. Braun may seek to obtain FDA clearance for its SpaceStation MRI Trolley, currently only available outside the U.S., which allows traditional B. Braun IV infusion pumps to be used in the MR environment.
Many of our potential customers opt not to purchase our MRI compatible IV infusion pump systems and instead use makeshift workarounds, such as placing conventional IV infusion devices outside of the MR scanning room and utilizing extension tubing to reach the patient. To this extent, we are in competition with conventional IV infusion pump manufacturers and distributors.
There are many manufacturers of conventional IV infusion pump devices, and if any of these manufacturers, or other potential competitors, decide to enter into the MRI compatible IV infusion pump market, they may have competitive advantages over us. Many of these potential competitors have established reputations, customer relationships and marketing, distribution and service networks. In addition, they have substantially longer histories in the medical products industry, larger product lines and greater financial, technical, manufacturing, management, and research and development budgets. Many of these potential competitors may have long-term product supply relationships with our potential customers.
We believe that a company's reputation for producing accurate, reliable and technologically-advanced products, references from users, features (speed, safety, ease of use, patient convenience and range of applicability), product effectiveness and price are the principal competitive factors in the medical products industry.
72
Seasonality
Our business is seasonal. Our third quarter sales have typically been lower, compared to other fiscal quarters, principally because the fiscal quarter coincides with the summer vacation season, especially in the U.S., Europe, and Japan.
Research and Development
Our research and development efforts focus on developing innovative products by utilizing our established core competencies in MR compatible technologies and feedback from strategic relationships with hospitals, acute medical facilities and medical equipment manufacturers for new product ideas. Our research and development efforts are driven by the leadership of our founder, Roger Susi, assisted by five engineers and technical professionals with significant experience in product design.
Regulatory Matters
Governmental Regulation and Other Matters
Our medical device products are subject to extensive, complex and increasing oversight and regulation by the U.S. Food & Drug Administration ("FDA"), and other domestic and foreign governmental authorities. Our manufacturing and other facilities, and those of our suppliers, are subject to periodic inspections to verify compliance with current FDA and other governmental regulatory requirements. If it were determined that we were not in compliance with these laws and regulations, we could be subject to criminal or civil liability, or both, and other material adverse effects. We have compliance programs in place to support and monitor compliance with these laws and regulations. All of our products and facilities and those of our suppliers are subject to drug and medical device laws and regulations promulgated by the FDA and national and supranational regulatory authorities outside the U.S., including, for example, Health Canada's Health Products and Foods Branch, the U.K.'s Medicines and Healthcare Products Regulatory Agency, and Australia's Therapeutic Goods Agency. These authorities regulate a range of activities including, among other matters, manufacturing, post-marketing studies in humans, advertising and promotion, product labeling, post-marketing surveillance and adverse event reporting.
Quality Management Overview
We place significant emphasis on providing quality products to our customers. Quality management plays an essential role in understanding and meeting customer requirements, effectively resolving quality issues and improving our products. We have developed and implemented quality systems and compliance programs throughout our organization, and are involved in setting quality policies and managing internal and external quality performance. Our quality assurance department provides quality leadership and supervises our quality systems. We have a network of quality systems throughout our business departments and facilities that relate to the design, development, manufacturing, packaging, sterilization, handling, distribution and labeling of our products. To assess and facilitate compliance with applicable requirements, we regularly review our quality systems to determine their effectiveness and identify areas for improvement. We also conduct compliance training programs for our sales and marketing personnel and perform assessments of our suppliers of raw materials, components and finished goods. In addition, we conduct quality management reviews designed to inform management of key issues that may affect the quality of our products.
From time to time, we may determine that products manufactured or marketed by us do not meet our specifications, published standards or regulatory requirements. When a quality issue is identified, we investigate the issue and seek to take appropriate corrective action, such as withdrawal of the product from the market, correction of the product at the customer location, notice to the customer of revised labeling or a combination of these or other corrective actions.
73
Regulation of Medical Devices in the United States
The development, manufacture, sale and distribution of our medical device products are subject to comprehensive governmental regulation. Most notably, all of our medical devices sold in the United States are subject to the Food, Drug, and Cosmetic Act of 1938, as amended ("FDC Act"), as implemented and enforced by the FDA. The FDA, and in some cases other government agencies, such as the U.S. Federal Communications Commission ("FCC"), administer requirements covering the design, testing, safety, effectiveness, manufacturing, labeling, promotion and advertising, distribution and post-market surveillance of our products.
Unless an exemption applies, each medical device that we market must first receive either premarket notification (by making what is commonly called "a 510(k) submission") clearance or premarket approval (by filing a premarket approval application ("PMA") from the FDA pursuant to the FDC Act. In addition, certain modifications made to marketed devices also may require 510(k) clearance or approval of a PMA supplement. The FDA's 510(k) clearance process usually takes up to twelve months, but it can last longer. The process of obtaining PMA approval is much more costly, lengthy and uncertain. It generally takes from two to three years or even longer. All of our current products have been cleared through the 510(k) process. We cannot be sure that future products or modifications of current products, will qualify for the 510(k) pathway or whether 510(k) clearance or PMA approval will be obtained for any future product that we propose to market.
In 2010, the FDA issued a draft guidance document entitled "Total Product Life Cycle: Infusion Pump-Premarket Notification [510(k)] Submissions." Through this draft guidance, the FDA has established substantial additional pre-market requirements for new and modified infusion pumps. The FDA indicated more data demonstrating product safety would be required for future 510(k) submissions for infusion pumps, including more clinical and human factors data. Although the future impact of these initiatives is uncertain, it appears that the process for obtaining regulatory approvals to market infusion pumps is likely to become more costly and time consuming. It is possible that future new products or modifications of existing products will require PMAs rather than a 510(k). In addition, new requirements could result in longer delays for the approval of new products, modification of existing infusion pump products or remediation of existing products in the market. Future delays in the receipt of, or failure to obtain, approvals could result in delayed or no realization of product revenues.
After a device is placed on the market, numerous regulatory requirements continue to apply. Those regulatory requirements include the following: product listing and establishment registration; adherence to the Quality System Regulation ("QSR"), which requires stringent design, testing, control, documentation and other quality assurance procedures; labeling requirements and FDA prohibitions against the promotion of off-label uses or indications; adverse event reporting; post-approval restrictions or conditions, including post-approval study commitments; post-market surveillance requirements; the FDA's recall authority, whereby it can ask for, or require, the recall of products from the market; and requirements relating to voluntary corrections or removals.
All aspects of our manufacturing and distribution of regulated products and those of our suppliers are subject to substantial governmental oversight. Facilities used for the production, packaging, labeling, storage and distribution of medical devices must be registered with the FDA and other regulatory authorities. All manufacturing activities for these products must be conducted in compliance with current good manufacturing practices ("cGMPs"). Our manufacturing facilities and those of our suppliers are subject to periodic, routine and for-cause inspections to verify compliance with cGMPs. If, upon inspection, the FDA or another regulatory agency finds that a manufacturer has failed to comply with cGMPs, it could institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions, such as product recalls or seizures, monetary sanctions, consent decrees, injunctions to halt manufacturing and distributing products, civil or criminal sanctions, refusal
74
to grant clearances or approvals or delays in granting such clearances or approvals, import detentions of products made outside of the United States, restrictions on operations or withdrawal or suspension of existing approvals. The FDA also has the authority to request repair, replacement or refund of the cost of any medical device manufactured or distributed by us. These actions could result in, among other things, substantial modifications to our business practices and operations; a total or partial shutdown of production in one or more facilities while we or our suppliers remedy the alleged violation; the inability to obtain future pre-market clearances or approvals; and withdrawals or suspensions of current products from the market. Any of these events could disrupt our business and have a material adverse effect on our revenues, profitability and financial condition.
In June 2010, we received from the FDA an inspection report (commonly called an FDA Form 483) which contained four observations following a routine periodic inspection of our facility in Winter Park, Florida. The four observations in the FDA Form 483 related to our compliance with certain applicable regulations and standards. We submitted a written response to the FDA describing a corrective and preventative action plan, which included implementation of remediation programs and modification of our practices to address the FDA Form 483 observations. We did not receive a warning letter from the FDA or any other follow up to the June 2010 inspection.
More recently, the FDA conducted a routine inspection of our facility between April 7 and April 16, 2014. This was the first FDA inspection of our facility since the voluntary product recall in August 2012 of certain infusion sets and the voluntary recall in July 2013 of our DERS software. The FDA issued a Form 483 on April 16, 2014 that identified eight observations. In general, the observations involved issues related to the 2012 and 2013 product recalls (described in more detail under Product Recalls, below). The majority of the observations related to procedural and documentation issues associated with the design, development, validation testing and documentation of software used in certain of our products. Other observations were related to the design validation of pump labeling, design analysis of tube stretching, procedures for post-market design review, and control and procedures related to handling certain reported complaints. We submitted a response to the Form 483 in which we described our proposed corrective and preventative actions ("CAPA") to address each of the FDA's observations. If the FDA does not agree with our proposed CAPA plan, or accepts them but finds that we have not implemented them adequately, or if we otherwise fail to comply with applicable regulatory requirements, the FDA could initiate an enforcement action, including issuing untitled letters, warning letters, fines, injunctions, consent decrees and/or civil penalties.
Regulation of Medical Devices Outside of the United States
Medical device laws also are in effect in many of the non-U.S. markets in which we do business. These laws range from comprehensive device approval requirements for some or all of our products to requests for product data or certifications. Inspection of and controls over manufacturing, as well as monitoring of device-related adverse events, also are components of most of these regulatory systems. Most of our business is subject to varying degrees of governmental regulation in the countries in which we operate, and the general trend is toward increasingly stringent regulation balanced with a goal of optimizing international harmonization. For example, the European Union ("EU"), which currently relies on independent third parties, (called "Notified Bodies") rather than governmental authorities to review and certify medium and high risk medical devices, is moving to more governmental oversight of medical devices. Currently, the regulatory requirements for a broad spectrum of medical devices are covered in three European Medical Device Directives (adopted in the 1990's) with which manufacturers must comply in order to receive a CE Certificate of Conformity ("CE Mark") from a Notified Body. Only certified medical devices bearing a CE Mark can be sold in the EU and European Free Trade Association ("EFTA") countries and Turkey. EFTA includes Iceland, Norway, Principality of Liechtenstein and Switzerland.
75
In September 2012, the European Commission, ("EC"), proposed significant revisions to the regulatory framework for medical devices in the EU. The proposed changes include more oversight of Notified Bodies by governmental authorities, replacing the three European Medical Service Directives with two regulations and more stringent requirements for clinical evidence while also enhancing alignment with international guidelines to facilitate international trade. It is unknown how the proposed revisions will affect certification of future products or modifications of current products, but it is possible that more clinical data will be needed to support our applications, which would increase the costs and development time involved. We may lose our current quality system certification due to ISO Registrar difficulties as European authorities increase regulatory pressure or increased scrutiny resulting from the EU's Revised Medical Device Directive. The loss of the quality system certification may prevent product shipments to the EU and to other foreign markets, such as Canada. The EU has enacted legislation restricting the use of hazardous substances in electronic equipment (Directive 2011/65/EU, referred to as RoHS 2), such as our infusion pumps. The application of RoHS 2 to medical devices becomes effective as of July 22, 2014. If we are deemed not in compliance with RoHS 2 for key parts of our infusion pumps, such as the ultrasonic motor, the lithium battery cells or display, we will not be permitted to ship our products to EU markets. Certain of our component suppliers have not yet certified to us the compliance status of their parts. If our suppliers fail to supply us with RoHS 2 compliance certificates in a timely manner, we can conduct the compliance testing ourselves or replace the non-compliant supplier with a supplier with certified compliant parts. If, despite such efforts, we are unable to have RoHS 2 compliant products before the July 22, 2014 effective date, there will be an interruption of sales to the EU, which could significantly lower our revenues from foreign sales while we take further remedial measures.
Product Recalls
Infusion Set Recall. In August 2012, we initiated a voluntary recall of a particular lot of mRidium Series 1000 MR Infusion Sets, Type 1058 MR IV, an extension set intended for use with our mRidium MRI infusion pumps, when we became aware that one section of the IV set exceeded a specification dimension. We promptly retrieved and destroyed all unused extension infusion sets subject to the recall. We provided timely notice and cooperated with the FDA in its audit of the actions taken by us in connection with the recall. We submitted our final recall report on January 29, 2013 and requested FDA closure of the recall. On July 19, 2013, the FDA notified us that it had concluded its audit and confirmed that the recall was considered terminated.
Dose Error Reduction System ("DERS") Software Recall. Some of our mRidium 3860+ MRI compatible IV infusion pumps are equipped with a DERS. Due to a software issue observed on June 19, 2013, the drug dosage calculation indicated an incorrect recommended value for the flow rate when a specific key sequence was used during the infusion setup. As a result, a patient was infused with an incorrect flow rate. No harm to the patient was reported. On July 1, 2013, we issued an urgent medical device recall notice (the "DERS Recall") and promptly made available to our customers a software update to resolve the error. On July 2, 2013, the subject of the recall was discussed with the FDA by phone. On July 12, 2013, we provided written notification to the FDA of the DERS Recall and submitted a Medical Device Report (MDR) with the FDA describing the incident, the investigative and corrective actions taken, the reason for the DERS Recall and the recall strategy. On September 18, 2013, we notified the FDA that all of the pumps sold with the DERS kits had been successfully upgraded with the software correction and reported that the DERS Recall was completed as of September 16, 2013. We requested that the FDA officially close the DERS Recall. The FDA has not yet responded to our request of September 16, 2013 or more recent requests to close the DERS Recall. It is likely that the FDA wanted to conduct a follow-up inspection prior to closing the DERS Recall. The FDA completed an inspection of our facility on April 16, 2014. The majority of the observations cited on the Form 483 issued by the FDA were related to procedural and documentation issues associated with the design, development, validation testing and documentation of software used in
76
certain of our products. Other observations were related to the design validation of pump labeling, design analysis of tube stretching (which was an issue in the August 2012 voluntary recall that has been closed by the FDA), procedures for post-market design review, and control and procedures related to handling certain reported complaints. We submitted a response to the Form 483 in which we described our CAPA to address each of the FDA's observations. If the FDA does not agree with our proposed CAPA plan, or accepts them but finds that we have not implemented them adequately, or if we otherwise fail to comply with applicable regulatory requirements, the FDA could initiate an enforcement action, including issuing untitled letters, warning letters, fines, injunctions, consent decrees and/or civil penalties.
Overall Financial Impact from the Recalls. Management believes that the charges incurred related to these recalls individually or together were not significant or material to our operations or financial results. We expect to incur only minor additional aggregate charges over the next several months in connection with the final closeout activities related to the DERS Recall.
Corrective Actions from the Recalls. We take these recalls and related matters seriously and we have responded and will continue to respond fully, and in a timely manner, to the FDA and other governmental regulatory agencies. On April 16, 2014, we received a Form 483 from the FDA, which included observations related to issues that were generally related to both product recalls. We are in the process of formulating and submitting a response to the FDA, which will identify our proposed CAPA to address each of the FDA's observations in the Form 483. We cannot, however, give any assurances as to the expected date of resolution of the matters related to the DERS Recall or any of the observations in the Form 483.
We have made substantial investments in quality systems over the past two years. We will continue to make improvements to our products and systems to further reduce potential issues related to patient safety and avoid recalls in the future. Product quality plays a critical role in our success. While we believe that we have made significant improvements to our product quality and overall quality systems, further quality concerns, whether real or perceived, could adversely affect our results. Conversely, improving quality can be a competitive advantage and improve our results. For more information about risks related to these matters, see the section captioned "Defects or failures associated with our products and/or our quality system could lead to the filing of adverse event reports, recalls or safety alerts and negative publicity and could subject us to regulatory actions" in the "Risk Factors" section.
Healthcare Fraud and Abuse Laws
As a manufacturer and distributor of medical devices to hospitals and other healthcare providers, we and our customers are subject to laws which apply to Medicare, Medicaid, and other federal and state healthcare programs in the U.S. One such law, the Anti-kickback Statute, prohibits the solicitation, offer, payment or receipt of remuneration in return for referral or purchase, or in return for the recommending or arranging for the referral or purchase, of products covered by the programs. The Anti-kickback Statute provides a number of exceptions or "safe harbors" for particular types of transactions. While we generally do not file claims for reimbursement from government payers, the U.S. federal government has asserted theories of liability against manufacturers under the Federal False Claims Act, which prohibits the submission of false claims to Medicare, Medicaid, and other state and federal programs. Many states have similar fraud and abuse laws which may apply to us. Violations of these fraud and abuse-related laws are punishable by criminal or civil sanctions, including substantial fines, imprisonment and exclusion from participation in healthcare programs such as Medicare and Medicaid and health programs outside the United States. We have developed and implemented business practices and processes to train our personnel to perform their duties in compliance with healthcare fraud and abuse laws. While we conduct informal oversight to detect and prevent these types of fraud and abuse, we lack formal written policies and procedures at this time. If we were
77
unable to document and implement the controls and procedures required in a timely manner or otherwise violate such laws, we might suffer adverse regulatory consequences or face criminal sanctions, which could harm our operations, financial reporting or financial results.
Anti-Bribery Laws
Our global activities are subject to the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and other countries' anti-bribery laws that have been enacted in support of the Organization for Economic Cooperation and Development's Anti-bribery Convention. These laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. government officials with the intent to inappropriately gain a business advantage. They also require companies to maintain accurate books and records and internal financial controls. The U.K. Bribery Act also prohibits commercial bribery and makes it a crime for a company to fail to prevent bribery. Companies have the burden of proving that they have adequate procedures in place to prevent bribery. The enforcement of such laws in the U.S. and elsewhere has increased dramatically in the past few years, and authorities have indicated that the pharmaceutical and medical device industry is a significant focus for enforcement efforts.
Because of the predominance of government-sponsored healthcare systems around the world, many of our customer relationships outside of the United States are with governmental entities. Our policies mandate strict compliance with the anti-bribery laws. We operate in many parts of the world that have experienced governmental corruption to some degree, and in certain circumstances strict compliance with anti-bribery laws may conflict with local customs and practices.
We are in the process of evaluating and implementing a compliance program to ensure compliance with these laws by our employees and agents and to communicate our expectations of compliance to third parties, including our distributors and suppliers. The anti-bribery compliance program will include a risk assessment, policies and procedures that address significant aspects of anti-bribery legislation and enforcement priorities, training and communication to employees in commercial and other positions, and monitoring and auditing of our facilities, commercial operations and third parties with whom we do business.
Transparency Laws in the U.S. and Other Countries
There are numerous requirements imposed by states in the U.S. on the interaction of pharmaceutical and medical device companies with physicians. For example, several states and the District of Columbia either require the tracking and reporting of specific types of interactions with healthcare professionals or restrict such interactions. A similar requirement was imposed at the federal level under the "sunshine" provision of Patient Protection and Affordable Care Act, (the "Sunshine Provisions"), to track and report payments and "transfers of value" to U.S. physicians or teaching hospitals by manufacturers of medical products that are available for reimbursement by a federal insurer. It is expected that the Sunshine Provisions will preempt some but not all disclosure requirements under state laws. We are developing and implementing systems and processes to ensure compliance with both state and federal requirements as well as any applicable requirements in other countries, such as the U.K. and France, which have adopted similar reporting requirements.
Other Laws
We are also subject to California's Proposition 65, which sets forth a list of substances that are deemed by the State of California to pose a risk of carcinogenicity or birth defects. If any product sold in California contains such substances, the product must be accompanied by a prominent warning label alerting consumers that the product contains an ingredient linked to cancer or birth defect risk. Private
78
actions as well as California attorney general actions may be brought against non-compliant parties and can result in substantial costs and fines.
In the sale, delivery and servicing of our medical devices and software imbedded in some of our products outside of the United States, we must comply with various customs, export control, anti-boycott and trade embargo laws and regulations administered by U.S. and foreign government agencies, including the U.S. Customs and Border Protection, the Bureau of Industry and Security, the Department of Commerce and the Office of Foreign Assets Control Treasury Department, as well as others.
We are also subject to a variety of other laws, directives and regulations in and outside of the U.S., including those related to the following:
- •
- environmental laws and regulations;
- •
- the safety and health laws of the U.S. Occupational Safety and Health Act, which sets forth requirements for workplace conditions; and
- •
- the laws administered by the U.S. Department of Transportation and similar foreign agencies related to transporting materials defined as "hazardous" over land, sea, or through the air.
We use reasonable care to stay abreast of, and plan for, proposed legislation that could significantly affect our operations. Despite our training and compliance program, our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our employees or agents in violation of any of these laws.
Employees
As of May 22, 2014, we had 46 full-time employees, including six in research and development, 21 in manufacturing, 14 in sales and marketing and customer support services, and five in finance and administration. No employees are represented by a labor union. We have not experienced any work stoppages and consider our relations with our employees to be good.
Properties
Our principal offices are currently located in a leased facility of approximately 9,000 square feet located in Winter Park, Florida. The lease on our current facility expires in June 2014 and we anticipate month-to-month extensions until the new facility described below is ready for occupancy.
To accommodate our growing operations, we have plans to relocate during 2014 and have entered into a lease, commencing July 1, 2014, for a new, nearby facility in Winter Springs, Florida that is approximately 27,000 square-feet. The facility will house our manufacturing operations and corporate headquarters. The new facility has been leased from an entity controlled by our President and CEO, Roger Susi. Pursuant to the terms of our lease for the Winter Springs property, the monthly base rent will be $32,583, adjusted annually for changes in the consumer price index. The term of the lease expires on May 31, 2019. The lease will automatically renew for two successive terms of five years each beginning in 2019 and again in 2024, and thereafter, will be renewed for successive terms of one year each. The new manufacturing and office space is currently being constructed. We anticipate moving into the new space in July 2014 and having our operations fully functional in the new facility within 10 working-days. We do not believe a delay in moving would materially and adversely affect our ability to manufacture and ship products to our customers or otherwise affect our operations.
We do not own any real property that is materially important to our business.
79
Legal Proceedings
The technologies used in the medical device industry are protected by a wide array of patents and other intellectual property rights. As a result, third parties have in the past and may in the future assert infringement and misappropriation claims against us, our distribution partners or our content suppliers from time to time.
On April 16, 2013, Rydex Technologies LLC ("Rydex") filed a lawsuit against us, in Federal District Court in Delaware, asserting that we infringed a patent held by Rydex relating to "a fluid delivery nozzle for wireless communication to either an active or a passive device located on a vehicle." Rydex sought damages in an unspecified amount and recovery of costs and attorneys' fees. Although the claim was later voluntarily dismissed by Rydex and we believe the Rydex allegations were meritless, there can be no assurance that these assertions, or any similar future assertions, will not result in liability or damages payable by us.
In October 2012, Radimed Gesellschaft für Kommunikationsdienstleistungen und Medizintechnik mbH ("Radimed") brought an action in Düsseldorf Regional Court against our German distributor alleging the name and sign "iRadimed" was confusingly similar to their German trademark "Radimed." A judgment was rendered against our German distributor preventing use of the name and sign "iRadimed" in Germany. We have however continued to sell products in Germany without any discernible effect by using the name IRI Development. On July 31, 2013, Radimed filed a lawsuit against us and our founder, Roger Susi, in Düsseldorf Regional Court, alleging that we infringed their German and Community trademarks "Radimed" and seeking to prevent our use of the name, sign and domain name "iRadimed" in the European Union. In addition, Radimed is seeking unspecified damages. We will vigorously defend against the infringement claims. However, the ultimate outcome of the matter remains uncertain. If we receive an adverse judgment, we may be prevented from marketing our products in the European Union under the name and sign "iRadimed" and may be required to pay Radimed's attorneys' fees, the court fees and damages, which could materially adversely affect our business, operating results and financial condition.
The ultimate cost and outcome of any litigation or claim cannot be predicted with certainty. Whether or not an infringement or misappropriation claim is valid or successful, it could adversely affect our business by diverting management's attention or involving us in costly and time-consuming litigation. If we are not successful in defending any such claim, we may be required to pay past and future royalties to use technology or other intellectual property rights then in use, we may be required to enter into a license agreement and pay license fees or we may be required to stop using the technology or other intellectual property rights then in use. Any of these results could have a material adverse effect on our business, results of operations and financial condition.
80
Directors and Executive Officers
Our directors and executive officers and their ages and positions as of March 31, 2014 are as follows:
| Name | Age | Position(s) | |||
|---|---|---|---|---|---|
| Roger Susi. | 60 | Chief Executive Officer, President, and Director | |||
Chris Scott | 43 | Chief Financial Officer and Secretary | |||
Brent Johnson | 54 | Executive VP of Worldwide Sales and Marketing | |||
Fran Casey | 59 | Vice President of Regulatory and Quality Assurance | |||
Steve Nardi | 52 | Vice President of Manufacturing | |||
Louis Waldman | 59 | Controller | |||
James Hawkins(1)(2) | 58 | Chairman of the Board | |||
Monty Allen(1)(2) | 61 | Director | |||
Serge Novovich(1) | 81 | Director | |||
- (1)
- Member of the audit committee.
- (2)
- Member of the compensation committee.
Roger Susi. Mr. Susi is the founder of our Company and has served as our Chief Executive Officer and President and a director from inception. He has over 25 years of management experience in the medical device industry, including as a founder, Chairman and Chief Executive Officer of Invivo Research Inc., a medical device company and the predecessor to Invivo Corporation, which established MRI-safe patient monitoring. Mr. Susi served as a director of Invivo Corporation from 1998 through 2000 and as President of Invivo Research Inc. from 1979 through 1998. Mr. Susi is a biomedical engineer and received a B.S. in Biomedical Engineering from Case Western Reserve University in 1977. We believe Mr. Susi's extensive experience in the medical device industry and intimate knowledge of our Company as one of our founders qualify him to serve on our Board of Directors.
Chris Scott. Mr. Scott has served as our Chief Financial Officer since December 2013. Mr. Scott has extensive experience in finance and accounting. Mr. Scott held a management position at Darden Restaurants, Inc. from 2010 to 2013, where he provided accounting and reporting oversight. From 2002 to 2010, Mr. Scott served as an auditor and senior manager at KPMG LLP. Mr. Scott received a B.S. in Accounting from the University of Central Florida in 2002.
Brent Johnson. Mr. Johnson has served as our Executive Vice President of Worldwide Sales and Marketing since 2012. From 2009 to 2011, Mr. Johnson was the Vice President of Sales and Marketing at HyGreen Inc., which provides hygiene compliance systems for acute care facilities. Mr. Johnson was responsible for leading and executing HyGreen's commercialization strategy. From 1996 to 2007, Mr. Johnson was the Vice President of Worldwide Sales and Marketing at Invivo Corporation where he was responsible for leading Invivo's sales and marketing strategies. Mr. Johnson received a B.S. in Business Administration and Finance from San Diego State University in 1982.
Fran Casey. Mr. Casey has served as our Vice President of Quality Assurance and Regulatory Affairs since 2004. Mr. Casey is a biomedical engineer with more than 30 years of experience in the medical device field as a regulatory professional for large and medium sized companies. His experience
81
includes generating and/or obtaining FDA 510(k) clearances for over 50 Class II and III medical devices, including infusion products, and establishing ISO and FDA quality assurance systems. Mr. Casey brings to our Company more than 20 years of MRI compatible product experience. Mr. Casey received a B.S. in Biomedical Engineering from Temple University in 1978.
Steve Nardi. Mr. Nardi has served as our Vice President of Manufacturing since 2013. Mr. Nardi possesses over 25 years of engineering experience in the medical device industry, including senior management and principal engineer roles. Mr. Nardi received a B.S. in Engineering Technology from Northeastern University Boston in 2003 and an M.S. in Technology Commercialization from Northeastern University Boston in 2010.
Louis Waldman. Mr. Waldman has served as our Controller since 2005. Mr. Waldman brings more than 25 years of professional accounting experience in the medical device and oil refining industries. Mr. Waldman previously served as controller and VP of Finance at Invivo Research Inc. before joining us in 2005. Mr. Waldman received a B.S. in Accounting from Case Western Reserve University in 1977.
James Hawkins. Mr. Hawkins has served as Chairman of the Board of Directors since December 2013. Mr. Hawkins is the President and Chief Executive Officer of Natus Medical, Inc., a leading manufacturer of medical devices and software and a service provider for the newborn care, neurology, sleep, hearing and balance markets. Mr. Hawkins has held this position since 2004. In addition, he currently serves as a director of Natus Medical, the Digirad Corporation and IRIDEX Corporation. Mr. Hawkins sits on the audit and compensation committees of Digirad and on the audit and nominating and governance committees of IRIDEX. Prior to joining Natus, Mr. Hawkins was President, Chief Executive Officer, and a Director of Invivo Corporation, a provider of MRI-safe patient monitoring. Previously, Mr. Hawkins was the Chief Financial Officer of Sensor Control Corporation. He earned his undergraduate degree in Business Commerce from Santa Clara University and holds a Masters of Business Administration degree from San Francisco State University. In addition to his direct management experience in the medical device area, Mr. Hawkins has extensive investor contacts and experience with the public markets which we believe qualifies him to serve as Chairman of our Board of Directors.
Monty Allen. Mr. Allen has served as a member of our Board of Directors since January 2014. Mr. Allen served as Chief Financial Officer, Secretary and Treasurer of LENSAR, Inc., a medical device business from 2005 through 2011 and continues to serve LENSAR, Inc. as a financial advisor. Mr. Allen has nearly 40 years of accounting and finance experience including service as Chief Financial Officer of AgriDyne Technologies Inc., and Autonomous Technologies Corporation, which each completed an initial public offering during his tenure, in 1992 and 1996, respectively. Mr. Allen also served as Chief Financial Officer of LightPath Technologies Inc., a publicly-held manufacturer of optical components, from 2003 to 2005, and GlobeNet Capital Corporation, a privately-held developer of trading system software, from 1999 to 2001. Mr. Allen is a licensed CPA. Mr. Allen received a B.S. in Accounting and International Business from Florida State University in 1974 and an M.B.A. in General Management from Harvard University in 1978. We believe that Mr. Allen's past experience and expertise in the fields of accounting and finance qualify him to serve on our Board of Directors.
Serge Novovich, Esq. Mr. Novovich has served as a member of our Board of Directors since 2005. Mr. Novovich, an attorney and electrical engineer, served as corporate counsel for Memorex/Telex Corporation for more than 20 years. He is an experienced patent counsel and a former member of the Board of Directors of Invivo Research Inc. Mr. Novovich received a B.S.E.E. in Electrical Engineering from the University of Minnesota in 1957 and a J.D. from the University of Tulsa in 1971. We believe Mr. Novovich's experience in engineering and as an attorney qualifies him to serve on our Board of Directors.
82
Family Relationships
There are no family relationships among any of our officers or directors.
Board Composition and Independence
Our Board of Directors currently consists of four members. All directors hold office until their successors have been elected and qualified or until their earlier death, resignation, disqualification, or removal. Our Board of Directors has determined that Messrs. Hawkins, Novovich and Allen are independent within the meaning of NASDAQ listing standards.
Board Leadership Structure
In keeping with good corporate governance practices, we maintain a majority of independent directors and our committees are comprised solely of independent directors. Our current leadership structure separates the roles of Chairman and Chief Executive Officer. We believe that separating the position of Chief Executive Officer and Chairman of the Board of Directors allows our Chief Executive Officer to focus on our day-to-day business, while allowing the Chairman to lead the Board of Directors in its fundamental role of providing advice to, and independent oversight of, management. Our Board of Directors recognizes the time, effort and energy that the Chief Executive Officer must devote to his position in the current business environment. We also believe that this structure ensures a greater role for the independent directors in the oversight of our company and active participation of the independent directors in setting agendas and establishing priorities and procedures for the work of our Board of Directors. We expect and intend that the positions of Chairman of the Board of Directors and Chief Executive Officer will continue to be held by two individuals in the future.
Risk Oversight
One of the key functions of our Board of Directors is informed oversight of our risk management process. Our Board of Directors will not have a standing risk management committee, but rather intends to administer this oversight function directly through our Board of Directors as a whole, as well as through various Board of Directors standing committees that address risks inherent in their respective areas of oversight. In particular, our Board of Directors is responsible for monitoring and assessing strategic risk exposure, and our audit committee will have the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures. The audit committee also will, effective on the closing of our public offering, have the responsibility to issue guidelines and policies to govern the process by which risk assessment and management is undertaken, monitor compliance with legal and regulatory requirements, and oversee the performance of our internal audit function. Our compensation committee will assess and monitor whether any of our compensation policies and programs have the potential to encourage excessive risk-taking.
Board Committees
Our Board of Directors includes an audit committee and a compensation committee. Our audit and compensation committees are comprised solely of independent board members.
Audit committee. Our audit committee currently consists of Mr. Allen, who is the chair of the Committee, Mr. Hawkins and Mr. Novovich, each of whom has been determined by our Board of Directors to be independent in accordance with NASDAQ and SEC standards. Mr. Allen is an "audit committee financial expert" as the term is defined under the SEC regulations. The audit committee operates under a written charter. The functions of the audit committee include:
- •
- overseeing the engagement of our independent public accountants;
83
- •
- reviewing our audited financial statements and discussing them with the independent public accountants and our management;
- •
- meeting with the independent public accountants and our management to consider the adequacy of our internal controls; and
- •
- reviewing our financial plans, reporting recommendations to our full Board of Directors for approval and authorizing actions.
Both our independent registered accounting firm and internal financial personnel regularly meet with our audit committee and have unrestricted access to the audit committee.
Compensation committee. Our compensation committee currently consists of Mr. Hawkins, who is the chair of the committee, and Mr. Allen, each of whom has been determined by our Board of Directors to be independent in accordance with NASDAQ standards. Each member of our compensation committee is also a non-employee director, as defined pursuant to Rule 16b-3 promulgated under the Exchange Act, and an outside director, as defined pursuant to Section 162(m) of the Internal Revenue Code of 1986, as amended (the "Code"). The compensation committee operates under a written charter. The functions of the compensation committee include:
- •
- reviewing and, if deemed appropriate, recommending to our Board of Directors policies, practices, and procedures relating to the compensation of our directors, officers, and other managerial employees and the establishment and administration of our employee benefit plans;
- •
- determining or recommending to the Board of Directors the compensation of our executive officers; and
- •
- advising and consulting with our officers regarding managerial personnel and development.
Compensation Committee Interlocks and Insider Participation
None of the members of the compensation committee is or has ever been one of our officers or employees. None of our executive officers serves, or in the past has served, as a member of the compensation committee or on the Board of Directors of any entity that has one or more executive officers serving on our Board of Directors or compensation committee.
Code of Business Conduct and Ethics
In conjunction with this offering, our Board of Directors will adopt a code of business conduct and ethics that applies to our officers, directors, and employees. We intend to disclose any amendments to our code of business conduct and ethics or waivers of its requirements in filings under the Exchange Act.
84
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table sets forth total compensation earned by our named executive officers, who are comprised of our principal executive officer and our next two highest compensated executive officers other than the principal executive officer for the years ended December 31, 2013 and 2012.
Name and principal position | Year | Salary | Bonus | Option Awards(1) | All Other Compensation | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Roger Susi(3) | 2013 | $ | 117,212 | $ | 125,000 | — | $ | 9,688 | (2) | $ | 251,900 | ||||||||
Chief Executive Officer and President | 2012 | $ | 80,971 | $ | 100,000 | — | $ | 7,239 | (2) | $ | 188,210 | ||||||||
Chris Scott(4) | 2013 | — | — | $ | 263,340 | — | $ | 263,340 | |||||||||||
Chief Financial Officer | 2012 | — | — | — | — | — | |||||||||||||
Brent Johnson | 2013 | $ | 175,000 | $ | 58,418 | $ | 546,541 | $ | 16,537 | (5) | $ | 796,496 | |||||||
Executive VP of Worldwide Sales and Marketing | 2012 | $ | 168,260 | $ | 66,831 | $ | 402,498 | $ | 16,604 | (5) | $ | 654,193 | |||||||
- (1)
- This amount reflects the aggregate grant date fair value computed in accordance with ASC Topic 718. The assumptions that we used to calculate these amounts are discussed in "Management's Discussion and Analysis of Financial Condition and Results of Operations – Application of Critical Accounting Policies – Stock-based Compensation" and Note 6 to our financial statements appearing at the end of this prospectus.
- (2)
- This amount represents our matching contributions to the executive officer's contributions to their respective 401(k) retirement plans.
- (3)
- Mr. Susi also serves as a member of our Board of Directors but does not receive any compensation for his service as a director.
- (4)
- Mr. Scott was appointed as the Chief Financial Officer of our company in December 2013.
- (5)
- This amount includes $7,200 in an annual automobile allowance and the remaining amount represents our matching contributions to Mr. Johnson's 401(k) retirement plan.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth summary information regarding the outstanding equity awards for each of the named executive officers as of December 31, 2013.
| | Option Awards | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price | Option Expiration Date | |||||||||
Chris Scott, Chief Financial Officer | — | 96,250 | $ | 1.48 | 12/30/2023 | ||||||||
Brent Johnson, Executive VP of Worldwide Sales and Marketing | 216,388 | 216,388 | $ | 0.93 | 12/29/2021 | ||||||||
| 69,602 | 208,806 | $ | 0.93 | 10/31/2022 | |||||||||
| — | 199,759 | $ | 1.48 | 12/30/2023 | |||||||||
Employment Agreements
In April 2014, we entered into a written employment agreement with Roger Susi as our Chief Executive Officer and President. The agreement provides for a base annual salary of $225,000, eligibility for annual bonuses and eligibility for standard employee benefits. The agreement continues until terminated by us or by Mr. Susi in accordance with the terms of the agreement. If Mr. Susi is terminated by us without cause or he terminates his employment with us for good reason, each as defined under the agreement, we must pay him an amount equal to 12 months base salary. In the event
85
that we are involved in a change of control transaction, which generally means the transfer of ownership of more than 50% of our shares, and Mr. Susi terminates his employment with us for good reason, we must pay him an amount equal to three times his then current annual salary. The employment agreement also contains non-solicitation, non-compete, and confidentiality provisions and an employee innovation and proprietary information assignment to us by Mr. Susi of any of his inventions or innovations.
We entered into an employment agreement with Chris Scott as our Chief Financial Officer in December 2013, pursuant to which we agree to compensate Mr. Scott at a base annual salary of $145,000, with eligibility for annual bonuses and for standard employee benefits. The employment agreement also provides for a one-time grant of stock options exercisable to purchase 96,250 shares of our common stock. The agreement continues until terminated by us or by Mr. Scott in accordance with the terms of the agreement. If Mr. Scott is terminated by us without cause or he terminates his employment with us for good reason, each as defined under the agreement, we must pay him an amount equal to six months base salary. In the event that we are involved in a change of control transaction, which generally means the transfer of ownership of more than 50% of our shares, and Mr. Scott terminates his employment with us for good reason, we must pay him an amount equal to his then current annual salary but no less than $145,000. The agreement also contains non-solicitation, non-compete and confidentiality provisions.
We entered into a written employment agreement in December 2011 with Brent Johnson, our Executive Vice President of Worldwide Sales and Marketing. The employment agreement provides for a base annual salary of $175,000, eligibility for annual bonuses and for standard employee benefits. The agreement continues until terminated by us or by Mr. Johnson in accordance with the terms of the agreement. If Mr. Johnson is terminated by us without cause or he terminates his employment with us for good reason, we must pay him an amount equal to 12 months base salary. In the event that we are involved in a change of control transaction, which generally means the transfer of ownership of more than 50% of our shares, and Mr. Johnson terminates his employment with us for good reason, we must pay him an amount equal to his then-current annual salary. The employment agreement also contains non-solicitation, non-compete and confidentiality provisions.
Employee Benefit Plans
2005 Incentive Stock Plan
In connection with the merger agreement effecting the reincorporation of our Oklahoma entity into the newly formed Delaware corporation in April 2014, the Delaware corporation assumed all obligations associated with the Oklahoma corporation's 2005 Incentive Stock Plan (the "2005 Plan"). The 2005 Plan was terminated as of the date of the merger and no new awards will be granted thereunder. All outstanding stock options under the 2005 Plan were assumed and adopted, subject to the 1.75:1 conversion ratio of the merger, by the Company and continue to be outstanding and governed by the 2005 Plan. Upon expiration of any outstanding options under the 2005 Plan, the corresponding number of shares of common stock reserved will be cancelled and will not be made available for issuance under our new plan described below. A total of 1,862,000 shares of common stock are reserved for issuance under the 2005 Plan. As of March 31, 2014, options to purchase a total of 1,833,192 shares of common stock were issued and outstanding.
2014 Equity Incentive Plan
In April 2014, our Board of Directors adopted the iRadimed Corporation 2014 Equity Incentive Plan (the "2014 Plan"), which will be effective prior to the completion of this offering. Our shareholders also have approved the 2014 Plan. The following summary describes the material terms of the 2014 Plan.
86
Administration of the Plan. Our Board of Directors has such powers and authorities related to the administration of the 2014 Plan as are consistent with our corporate governance documents and applicable law. The Board of Directors may delegate to a committee administration of all or some parts of the 2014 Plan. Following the initial public offering and to the extent required by applicable law, the committee or sub-committee, as applicable, to which administrative responsibility will be delegated will be comprised of directors who (i) qualify as "outside directors" within the meaning of Section 162(m) of the Code, (ii) meet such other requirements as may be established from time to time by the SEC for plans intended to qualify for exemption under Rule 16b-3 (or its successor) under the Securities Exchange Act of 1934, as amended, and (iii) comply with the independence requirements of the stock exchange on which our common stock is listed.
Number of Authorized Shares. 1,000,000 shares of common stock have been reserved for issuance under the 2014 Plan. Any shares covered by an award that are forfeited, expired, cancelled, settled in cash, settled by issuance of fewer shares than the amount underlying the award, or otherwise terminated without delivery of shares to the grantee, will be available for future grants under the 2014 Plan. The number and class of shares available under the 2014 Plan and/or subject to outstanding awards may be equitably adjusted by our Board of Directors in the event of various changes in the capitalization of the Company.
Type of Awards. The following types of awards are available for grant under the 2014 Plan: ISOs, non-qualified stock options ("NSOs"), stock appreciation rights ("SARs"), restricted stock, restricted stock units, performance awards and other stock-based awards and cash awards.
Eligibility and Participation. Eligibility to participate in the 2014 Plan is limited to such employees, officers, directors, consultants and advisors of the Company, or of any affiliate, as our Board of Directors may determine and designate from time to time.
Grant of Options and SARs. Our Board of Directors may award ISOs, NSOs (together, "Options"), and SARs to grantees. Our Board of Directors is authorized to grant SARs either in tandem with or as a component of other awards or alone.
Exercise Price of Options and SARs. The exercise price per share of an Option will in no event be less than 100% of the fair market value per share of our stock underlying the award on the grant date. In no case will the exercise price of any Option be less than the par value of a share of our stock. A SAR will confer on the grantee a right to receive, upon exercise, a payment of the excess of (i) the fair market value of one share of our stock on the date of exercise over (ii) the grant price of the SAR as determined by our Board of Directors. The grant price will be fixed at the fair market value of a share of stock on the date of grant. SARs granted in tandem with an outstanding Option following the grant date of such Option may have a grant price that is equal to the Option's exercise price; provided, however, that the SAR's grant price may not be less than the fair market value of a share of stock on the grant date of the SAR. The exercise price of Options and SARs granted during our initial public offering will be the price per share of common stock of the Company for the offering, as established by the Board of Directors.
Vesting of Options and SARs. Our Board of Directors will determine when an Option or SAR will become exercisable and will include such information in the award agreement.
Special Limitations on ISOs. In the case of a grant of an Option intended to qualify as an ISO to a grantee that owns more than ten percent of the total combined voting power of all classes of our outstanding stock, its parent or any of its subsidiaries (a "Ten Percent Shareholder"), the exercise price of the Option will not be less than 110% of the fair market value of a share of our stock on the grant date. Additionally, an Option will constitute an ISO only (i) if the grantee is an employee of the Company or a subsidiary of the Company, (ii) to the extent specifically provided in the related award
87
agreement, and (iii) to the extent that the aggregate fair market value (determined at the time the option is granted) of the shares of stock with respect to which all ISOs held by such grantee become exercisable for the first time during any calendar year (under the 2014 Plan and all other plans of the grantee's employer and its affiliates) does not exceed $100,000.
Exercise of Options and SARs. An Option may be exercised by the delivery to us of written notice of exercise and payment in full of the exercise price (plus the amount of any taxes which we may be required to withhold). The minimum number of shares with respect to which an Option may be exercised, in whole or in part, at any time will be the lesser of (i) the number set forth in the applicable award agreement and (ii) the maximum number of shares available for purchase under the Option at the time of exercise. Our Board of Directors has the discretion to determine the method or methods by which a SAR may be exercised.
Expiration of Options and SARs. Options and SARs will expire at such time as our Board of Directors determines; provided, however that no Option may be exercised more than ten years from the date of grant, or in the case of an ISO held by a Ten Percent Shareholder, not more than five years from the date of grant.
Restricted Stock and Restricted Stock Units. At the time a grant of restricted stock or restricted stock units is made, our Board of Directors may, in its sole discretion, establish the applicable "restricted period" and prescribe restrictions in addition to or other than the expiration of the restricted period, including the satisfaction of corporate or individual performance objectives. Unless our Board of Directors otherwise provides in an award agreement, holders of restricted stock will have the right to vote such stock and the right to receive any dividends declared or paid with respect to such stock. Our Board of Directors may provide that any such dividends paid must be reinvested in shares of stock, which may or may not be subject to the same vesting conditions and restrictions applicable to such restricted stock. All distributions, if any, received by a grantee with respect to restricted stock as a result of any stock split, stock dividend, combination of shares, or other similar transaction will be subject to the restrictions applicable to the original grant. Holders of restricted stock units will have no rights as shareholders of the Company. Our Board of Directors may provide that the holder of restricted stock units will be entitled to receive dividend equivalent rights, which may be deemed reinvested in additional restricted stock units.
Performance Awards. The right of a grantee to exercise or receive a grant or settlement of any award, and the timing thereof, may be subject to such performance conditions as may be specified by our Board of Directors. Our Board of Directors may use such business criteria and other measures of performance as it may deem appropriate in establishing any performance conditions, and may, subject to certain limitations in the case of a performance award intended to qualify under Section 162(m) of the Code ("Section 162(m)"), exercise its discretion to reduce the amounts payable under any award subject to performance conditions. Following the completion of the Transition Period (as defined herein), if and to the extent required under Section 162(m), any power or authority relating to a performance award intended to qualify under Section 162(m), will be exercised by the committee and not our Board of Directors.
Other Stock-Based Awards. Our Board of Directors may, in its discretion, grant other stock-based awards, consisting of stock units or other awards, valued in whole or in part by reference to, or otherwise based upon, our common stock. The terms of such other stock-based awards will be set forth in the applicable award agreements.
Change in Control. Our Board of Directors may provide for actions that will be taken upon a change in control of the Company, including but not limited to, alternative vesting, termination or assumption of awards.
88
Nontransferability of Awards. Generally, during the lifetime of a grantee, only the grantee may exercise rights under the 2014 Plan and no award will be assignable or transferable other than by will or laws of descent and distribution. If authorized in the award agreement, a grantee may transfer, not for value, all or part of an award (other than an ISO) to certain family members (including trusts and foundations for the benefit thereof). Neither restricted stock nor restricted stock units may be sold, transferred, assigned, pledged or otherwise encumbered or disposed of during the restricted period or prior to the satisfaction of any other restrictions prescribed by our Board of Directors.
Separation from Service. Our Board of Directors may provide in the applicable award agreements for actions that will be taken upon a grantee's separation from service from the Company, including but not limited to, accelerated vesting or termination of awards.
Tax Withholding and Tax Offset Payments. We will have the right to deduct from payments of any kind otherwise due to a grantee any federal, state, or local taxes of any kind required by law to be withheld with respect to the vesting of or other lapse of restrictions applicable to an award or upon the issuance of any shares of stock upon the exercise of an Option or pursuant to an award.
Term of Plan. Unless earlier terminated by our Board of Directors, the authority to make grants under the 2014 Plan will terminate on the date that is ten years after it is adopted by our Board of Directors.
Amendment and Termination. Our Board of Directors may, at any time and from time to time, amend, suspend, or terminate the 2014 Plan as to any shares of stock as to which awards have not been made. An amendment will be contingent on approval of our shareholders to the extent stated by our Board of Directors, required by applicable law or required by applicable stock exchange listing requirements. No Awards will be made after termination of the 2014 Plan. No amendment, suspension, or termination of the 2014 Plan will, without the consent of the grantee, impair rights or obligations under any award theretofore awarded under the 2014 Plan.
New Plan Benefits. All grants of awards under the 2014 Plan will be discretionary. Therefore, in general, the benefits and amounts that will be received under the 2014 Plan are not determinable.
Federal Income Tax Consequences. The following is a summary of the general federal income tax consequences to the Company and to U.S. taxpayers of awards granted under the 2014 Plan. Tax consequences for any particular individual or under state or non-U.S. tax laws may be different. Special rules limit the deductibility of compensation paid to our CEO and to each of our four most highly compensated executive officers. Under Section 162(m), the annual compensation paid to each of these executives may not be deductible to the extent that it exceeds $1 million. However, we intend to rely on Treasury Regulation Section 1.162-27(f) which provides that the deduction limit of Section 162(m) does not apply to any remuneration paid pursuant to a compensation plan or agreement that existed during the period in which the company was not publicly held. Additionally, after the expiration of the grandfather period, we can preserve the deductibility of compensation over $1 million if certain conditions of Section 162(m) are met. These conditions include shareholder approval of the 2014 Plan, setting limits on the number of awards that any individual may receive and, for awards other than Options and SARs, establishing performance criteria that must be met before the award will actually be granted, be settled, vest or be paid. The 2014 Plan has been designed to permit the committee to grant awards that qualify as performance-based for purposes of satisfying the conditions of Section 162(m).
Registration of Shares. Following the completion of this offering we intend to file a registration statement on Form S-8 under the Securities Act to register 1,000,000 shares of common stock, which will be reserved for issuance under the 2014 Plan, as well as to register shares of common stock reserved for issuance pursuant to options issued under the 2005 Plan.
89
Limitation of Liability and Indemnification Matters
Our certificate of incorporation and amended and restated bylaws contain provisions indemnifying our directors and officers to the fullest extent permitted by law. In addition, as permitted by Delaware law, our amended and restated certificate of incorporation provides that no director will be liable to us or our stockholders for monetary damages due to breach of certain fiduciary duties as a director. The effect of this provision is to restrict our rights and the rights of our stockholders in derivative suits to recover monetary damages against a director due to breach of certain fiduciary duties as a director, except that a director will be personally liable for:
- •
- any breach of the director's duty of loyalty to us or our stockholders;
- •
- acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
- •
- the payment of dividends or the redemption or purchase of stock in violation of Delaware law; or
- •
- any transaction from which the director knowingly derived an improper personal benefit.
To the extent that our directors, officers, and controlling persons are indemnified under the provisions contained in our amended and restated certificate of incorporation, Delaware law, or contractual arrangements against liabilities arising under the Securities Act, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Director Compensation
We compensate non-employee members of the Board of Directors. Directors who are also employees do not receive cash or equity compensation for service on the Board of Directors in addition to compensation payable for their service as our employees. The non-employee members of our Board of Directors are reimbursed for travel, lodging, and other reasonable expenses incurred in attending Board of Directors or committee meetings.
The following table sets forth summary information concerning the compensation awarded to, paid to, or earned by the non-employee members of our Board of Directors for the fiscal year ended December 31, 2013.
Name | Fees Earned or Paid in Cash | Stock Awards | Option Awards(1) | All Other Compensation | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
James Hawkins | $ | — | $ | — | $ | 383,040 | $ | — | $ | 383,040 | ||||||
Serge Novovich | — | — | 71,820 | — | 71,820 | |||||||||||
Monty Allen(2) | — | — | — | — | — | |||||||||||
- (1)
- This amount reflects the aggregate grant date fair value computed in accordance with ASC Topic 718. The assumptions that we used to calculate these amounts are discussed in "Management's Discussion and Analysis of Financial Condition and Results of Operations – Application of Critical Accounting Policies – Stock-based Compensation" and Note 6 to our financial statements contained within the prospectus.
- (2)
- Mr. Allen was appointed as a member of the Board of Directors in January 2014. In connection with his appointment, he was granted options to purchase an aggregate of 26,250 shares of our common stock, which vest on a pro rata basis annually over four years from the date of grant.
After becoming a public company, our standard cash and equity components of our compensation policy for non-employee directors is expected to consist of a base annual fee of $15,000, an in-person board meeting fee of $2,500, a teleconference board meeting fee of $750, and an equity award grant of options to purchase 10,000 shares of our common stock. The compensation for directors may vary from time to time as determined by the compensation committee of our Board of Directors.
90
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
In addition to the compensation arrangements with directors and executive officers described above in "Management" and "Executive and Director Compensation," the following is a description of each transaction since January 1, 2012 and each currently proposed transaction in which (i) we have been or are to be a participant, (ii) the amount involved exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets at year end, and (iii) any of our directors, executive officers, holders of more than 5% of our capital stock, or any member of their immediate families or persons sharing their household had or will have a direct or indirect material interest.
Loan from CEO
From time to time, our founder and CEO, Roger Susi, loaned an aggregate of $1,393,498 to us, with no interest. As of December 31, 2013, the loan balance was $6,333. As of March 21, 2014, the loan was fully repaid. We do not expect to continue to borrow funds from this principal stockholder in the future.
Lease of Property from CEO
We entered into a lease, commencing July 1, 2014, for a new facility in Winter Springs, Florida owned by Susi, LLC, and entity controlled by our president and CEO, Roger Susi. The facility is approximately 27,000 square-feet and will house our manufacturing operations and corporate headquarters. Pursuant to the terms of our lease for this property, the monthly base rent will be $32,583, adjusted annually for changes in the consumer price index. The term of the lease expires on May 31, 2019. The lease will automatically renew for two successive terms of five years each beginning in 2019 and again in 2024, and thereafter, will be renewed for successive terms of one year each. The new manufacturing and office space is currently being constructed.
Purchase of Common Stock in this Offering by Director
At our request, the underwriters have reserved up to $500,000 of shares of our common stock offered by this prospectus for sale to James Hawkins, Chairman of our Board of Directors. The number of shares of common stock available for sale to the general public will be reduced by the amount of this purchase. All sales of reserved common stock to Mr. Hawkins will be made at the initial offering price set forth on the cover page of this prospectus.
Policies and Procedures for Related Party Transactions
In April 2014, we established an audit committee and adopted an audit committee charter. Our audit committee has the primary responsibility for reviewing and approving or disapproving "related party transactions," which are transactions between us and related persons in which the aggregate amount involved exceeds or may be expected to exceed $120,000 and in which a related person has or will have a direct or indirect material interest. For purposes of this policy, a related person is defined as a director, executive officer, nominee for director, or a greater than 5% beneficial owner of our common stock, in each case since the beginning of the most recently completed year, and their immediate family members. Our audit committee charter provides that the audit committee shall review and approve or disapprove any related party transactions.
91
BENEFICIAL OWNERSHIP OF COMMON STOCK
The following table sets forth information with respect to the beneficial ownership of our common stock as of the date of this prospectus, as adjusted to reflect the sale of common stock offered by us in this offering, for:
- •
- each person known by us to own beneficially more than 5% of our common stock;
- •
- each of our directors;
- •
- each of our named executive officers; and
- •
- all of our directors and executive officers as a group;
Applicable percentage ownership is based on 8,400,000 shares of common stock outstanding as of the date of this prospectus, which assumes the conversion of all outstanding shares of preferred stock into an aggregate of 1,400,000 shares of common stock. The percentage of beneficial ownership after this offering shown in the table is based on 10,150,000 shares of common stock outstanding after the closing of this offering, assuming no exercise of the underwriters' overallotment option.
Beneficial ownership is determined according to the rules of the SEC and generally means that a person possesses sole or shared voting or investment power of that security, including options that are currently exercisable or exercisable or deliverable within 60 days. Except as otherwise indicated, we believe that the beneficial owners of the common stock listed above, based on the information each of them has given to us, have sole investment and voting power with respect to their shares, except as otherwise noted or where community property laws may apply.
Except as otherwise noted, the address of each person or entity in the following table is c/o iRadimed Corporation, 1025 Willa Springs Dr., Winter Springs, FL 32078.
| | Beneficial Ownership Before Offering | Beneficial Ownership After the Offering | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name and Address of Beneficial Owner | Number of Shares | Percentage | Number of Shares | Percentage | |||||||||
5% Stockholders | |||||||||||||
Directors and Named Executive Officers | |||||||||||||
Roger Susi(2) | 7,000,000 | 83.3 | % | 7,000,000 | 69.0 | % | |||||||
James Hawkins(3) | 13,125 | * | 13,125 | * | |||||||||
Monty Allen | — | — | — | — | |||||||||
Louis Waldman(4) | 168,438 | 2.0 | % | 168,438 | 1.7 | ||||||||
Serge Novovich(5) | 144,375 | 1.7 | % | 144,375 | 1.4 | ||||||||
Chris Scott | — | — | — | — | |||||||||
Brent Johnson(6) | 285,989 | 3.3 | % | 285,989 | 2.7 | ||||||||
Fran Casey | 140,000 | 1.7 | % | 140,000 | 1.4 | ||||||||
Steve Nardi | — | — | — | — | |||||||||
All directors and named executive officers as a group (9 persons)(7) | 7,751,927 | 88.8 | % | 7,751,927 | 74.0 | % | |||||||
- *
- Represents beneficial ownership of less than 1% of the outstanding shares of common stock.
- (1)
- Unless otherwise indicated in the footnotes to this table and subject to applicable community property laws, we believe that the persons named in this table have sole voting and investment power with respect to all shares of common stock reflected in this table.
- (2)
- Includes 2,275,000 shares of common stock held by the Roger E. Susi Revocable Trust, 2,362,500 shares of common stock held by the Matthew Susi 2008 Dynasty Trust, and 2,362,500 shares of
92
- common stock held by the Phillip Susi 2008 Dynasty Trust. Roger Susi is the trustee for each of the foregoing trusts.
- (3)
- Includes 13,125 shares of common stock issuable upon exercise of share options that are currently exercisable or exercisable within 60 days. Beneficial ownership after the offering excludes up to $500,000 of shares of common stock purchased by Mr. Hawkins at a per share purchase price equal to the initial offering price set forth on the cover page of this prospectus.
- (4)
- Includes 28,438 shares of common stock issuable upon exercise of share options that are currently exercisable or exercisable within 60 days.
- (5)
- Includes 4,375 shares of common stock issuable upon exercise of share options that are currently exercisable or exercisable within 60 days and 140,000 shares of common stock held by Pacific Summit Capital LLC. Mr. Novovich is a manager of Pacific Summit Capital LLC and indirectly owns approximately 66.7% of Pacific Summit Capital LLC. Mr. Novovich disclaims beneficial ownership of the shares held by Pacific Summit Capital LLC except to the extent of his pecuniary interest.
- (6)
- Includes 285,989 shares of common stock issuable upon exercise of share options that are currently exercisable or exercisable within 60 days.
- (7)
- Includes 331,927 shares of common stock issuable upon exercise of share options that are currently exercisable or exercisable within 60 days. Includes 140,000 shares of common stock held by Pacific Summit Capital LLC, of which Mr. Novovich is a manager and partial owner (see footnote 5, above). Beneficial ownership after the offering excludes up to $500,000 of shares of common stock purchased by Mr. Hawkins at a per share purchase price equal to the initial offering price set forth on the cover page of this prospectus.
93
General
Under our certificate of incorporation, we are authorized to issue up to 100,000,000 shares, $.0001 par value per share, of which 90,000,000 shares may be common stock and 10,000,000 shares may be "blank check" preferred stock. The following description of our capital stock is subject to, and qualified in its entirety by, the provisions of our certificate of incorporation and amended and restated bylaws, which are included as exhibits to the registration statement of which this prospectus is a part, and by the provisions of applicable law.
Common Stock
As of March 31, 2014, there were 7,000,000 shares of our common stock outstanding that were held of record by three stockholders and 1,400,000 shares of Series A preferred stock held by eight stockholders that will automatically convert into common stock upon completion of this offering.
Each holder of our common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders. Common stockholders are not entitled to cumulative voting in the election of directors by our certificate of incorporation. This means that the holders of a majority of the shares voted will be able to elect all of the directors then standing for election. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the times and in the amounts that our Board of Directors may determine from time to time.
Upon our liquidation, dissolution or winding-up, the holders of our common stock will be entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any series of capital stock ranking senior to the common stock upon liquidation. Holders of common stock have no preemptive or conversion rights or other subscription rights. There will be no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and nonassessable, and the shares of common stock to be issued under this prospectus, when they are paid for, will be fully paid and nonassessable.
Preferred Stock
Upon the completion of this offering, all outstanding shares of Series A preferred stock will be automatically converted into an aggregate of 1,400,000 shares of our common stock. Under our certificate of incorporation, our Board of Directors has the authority, without further action by our stockholders, to authorize and issue shares of preferred stock in one or more series and to fix or alter the rights, preferences, privileges, qualifications and restrictions granted to or imposed upon any wholly unissued series of preferred stock, including dividend rights, conversion rights, voting rights, terms of redemption and any liquidation preferences, and to establish from time to time the number of shares constituting any such series or any of them. The issuance of preferred stock may result in one or more of the following:
- •
- decreasing the market price of our common stock;
- •
- restricting dividends on our common stock;
- •
- diluting the voting power of our common stock;
- •
- impairing the liquidation rights of our common stock; or
- •
- delaying or preventing a change in control of us without further action by our stockholders.
94
Underwriter Warrant
Please see "Underwriting-Commissions and Expenses" beginning on page 105 for a description of the warrant we have agreed to issue to the underwriters in this offering, subject to completion of this offering. We expect to enter into a warrant agreement in respect of the Underwriter Warrant prior to the closing of this offering.
Registration Rights
Upon the closing of this offering, all outstanding shares of preferred stock will automatically convert into 1,400,000 shares of common stock. The holders of these 1,400,000 shares of our common stock, in addition to the current holders of our 7,000,000 shares of common stock, will be entitled to certain rights with respect to the registration of such shares under the Securities Act. These holders have entered into the contractual "lock-up" agreements described in "Underwriting." As a result of these contractual restrictions, notwithstanding possible earlier eligibility for sale under the provisions of Rules 144 and 701, all outstanding shares will be available for sale only beginning 180 days after the effective date of this prospectus, subject in some cases to certain volume limitations.
The holders of these shares are entitled to certain piggyback registration rights. If we register any securities for public sale other than for our initial public offering, these holders will have the right to include their shares in the registration statement. In an underwritten offering, we have agreed to use our best efforts to cause the shares to be included in the underwriting on the same terms and conditions as the securities being sold through any such underwriters. We have agreed to indemnify the holders of this registration right against liabilities under the Securities Act, the Exchange Act, or other federal or state securities laws.
Dividend Rights
The holders of common stock shall be entitled to receive any dividends as may be declared from time to time by the Board of Directors, pro rata based on the number of shares of common stock held.
Delaware Law
We are subject to Section 203 of the Delaware General Corporation Law. In general, Section 203 defines an "interested stockholder" as any entity or person who beneficially owns, or an affiliate or associate of the corporation that at any time within three years prior to the date of determination of interested stockholder status did beneficially own, 15% or more of the outstanding voting stock of the corporation, and affiliates and associates of such person. Under this provision, we may not engage in any business combination with any interested stockholder for a period of three years following the date the stockholder became an interested stockholder, unless:
- •
- prior to that date our Board of Directors approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
- •
- upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock outstanding at the time the transaction began; or
- •
- on or following that date the business combination is approved by our Board of Directors and authorized at an annual or special meeting of stockholders, by the affirmative vote of at least two-thirds of the outstanding voting stock that is not owned by the interested stockholder.
95
Section 203 defines "business combination" to include:
- •
- any merger or consolidation involving the corporation and the interested stockholder;
- •
- any sale, lease, exchange, mortgage, transfer, pledge, or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
- •
- subject to some exceptions, any transaction that results in the issuance or transfer by the corporation or any of its direct or indirect subsidiaries of any stock of the corporation or of any such subsidiary to the interested stockholder;
- •
- any transaction involving the corporation or any of its direct or indirect subsidiaries that has the effect of increasing the proportionate share of the stock of any class or series of the corporation or of any such subsidiary beneficially owned by the interested stockholder; or
- •
- the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges, or other financial benefits provided by or through the corporation or any direct or indirect majority owned subsidiary.
Certificate of Incorporation and Amended and Restated Bylaws
Our certificate of incorporation and amended and restated bylaws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company. In particular, our certificate of incorporation and amended and restated bylaws, as applicable, among other things:
- •
- provide that special meetings of the stockholders may be called only by our Chairman of the Board, Chief Executive Officer, President, Chief Operating Officer, or the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors of our Board of Directors;
- •
- establish procedures with respect to stockholder proposals and stockholder nominations, including requiring that advance written notice of a stockholder proposal or director nomination generally must be received at our principal executive offices not less than 90 nor more than 120 days prior to the first anniversary of the previous year's annual meeting of stockholders;
- •
- do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in the Board of Directors and, as a result, may have the effect of deterring a hostile takeover or delaying or preventing changes in control or management of our company;
- •
- provide that vacancies on our Board of Directors may be filled by a majority of directors in office, although less than a quorum, and not by the stockholders; and
- •
- allow us to issue without stockholder approval up to 10,000,000 shares of undesignated preferred stock with rights senior to those of the common stock and that otherwise could adversely affect the rights and powers, including voting rights, of the holders of common stock. In some circumstances, this issuance could have the effect of decreasing the market price of the common stock as well as having the anti-takeover effect discussed above.
These provisions are intended to enhance the likelihood of continuity and stability in the composition of our Board of Directors and in the policies formulated by them and to discourage certain types of transactions that may involve an actual or threatened change of control of our
96
company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy fights. However, these provisions could have the effect of discouraging others from making tender offers for our shares that could result from actual or rumored takeover attempts. These provisions also may have the effect of preventing changes in our management.
Limitations of Director Liability and Indemnification of Directors, Officers, and Employees
As permitted by the Delaware General Corporation Law, provisions in our charter and amended and restated bylaws that will be in effect at the closing of this offering will limit or eliminate the personal liability of our directors. Consequently, directors will not be personally liable to us or our stockholders for monetary damages or breach of fiduciary duty as a director, except for liability for:
- •
- any breach of the director's duty of loyalty to us or our stockholders;
- •
- any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
- •
- any unlawful payments related to dividends or unlawful stock repurchases, redemptions or other distributions; or
- •
- any transaction from which the director derived an improper personal benefit.
These limitations of liability do not alter director liability under the federal securities laws and do not affect the availability of equitable remedies, such as an injunction or rescission.
In addition, our amended and restated bylaws provide that:
- •
- we will indemnify our directors and officers to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions, including an exception for indemnification in connection with a proceeding (or counterclaim) initiated by such persons; and
- •
- we will advance expenses, including attorneys' fees, to our directors and officers in connection with legal proceedings, subject to limited exceptions.
We intend to maintain general liability insurance that covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers, including liabilities under the Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or persons who control our company, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder's investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these provisions and the insurance are necessary to attract and retain talented and experienced directors and officers.
In a July 31, 2013, Radimed Gesellschaft für Kommunikationsdienstleistungen und Medizintechnik mbH ("Radimed") filed a lawsuit against us and our founder, Roger Susi, in Düsseldorf Regional Court, alleging that we infringed their German and Community trademarks "Radimed" and seeking to
97
prevent our use of the name, sign and domain name "iRadimed" in the European Union. In addition, Radimed is seeking unspecified damages. While we will vigorously defend against the infringement claims, the ultimate outcome of the matter remains uncertain. We are obligated to indemnify Roger Susi in his defense of this suit.
At present, there is no other pending litigation or proceeding involving any of our directors or officers where indemnification will be required or permitted. We are not aware of any other threatened litigation or proceedings that might result in a claim for such indemnification.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Corporate Stock Transfer, Inc.
The NASDAQ Capital Market
Before the date of this prospectus, there has been no public market for our common stock. We intend to apply to list our common stock on the NASDAQ Capital Market under the symbol "IRMD." Once our common stock is approved for trading, we anticipate that our common stock will be listed on the NASDAQ Capital Market.
98
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock. Future sales of substantial amounts of our common stock in the public market following this offering or the possibility of sales of this kind occurring could cause the prevailing market price of our common stock to fall and impede our ability to raise capital through an offering of equity securities.
Upon the completion of this offering, we will have a total of 10,150,000 shares of common stock outstanding based upon 8,400,000 shares outstanding as of March 31, 2014, assuming no exercise of the underwriters' option to purchase additional shares and no exercise of outstanding options prior to completion of this offering. The shares offered by this prospectus will be freely tradable unless they are purchased by our "affiliates," as defined in Rule 144 under the Securities Act. Shares purchased by affiliates may generally only be sold pursuant to an effective registration statement under the Securities Act or in compliance with the volume limitations of Rule 144.
The 8,400,000 shares of our common stock outstanding prior to our public offering (taking into account the automatic conversion of our preferred stock into common stock upon completion of this offering) are "restricted," which means they were originally sold in offerings that were not subject to a registration statement filed with the SEC. These restricted shares may generally be resold only through registration under the Securities Act or under an available exemption from registration, such as provided by Rule 144.
Lock-Up Agreements
Holders of all of our outstanding common stock, including holders of our preferred stock that will be automatically converted into common stock upon completion of this offering, have entered into the contractual "lock-up" agreements described in "Underwriting." As a result of these contractual restrictions, notwithstanding possible earlier eligibility for sale under the provisions of Rules 144 and 701, all outstanding shares will be available for sale only beginning 180 days after the date of this prospectus, subject in some cases to certain volume limitations.
Rule 144
In general, under Rule 144, as amended, a person (or persons whose shares are required to be aggregated) who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell those shares, subject only to the availability of current public information about us.
A person (or persons whose shares are aggregated) who is deemed to be an affiliate of ours and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
- •
- 1% of the number of common shares then outstanding, which will equal approximately 101,500 shares immediately after this offering (assuming no exercise of the underwriters' option to purchase additional shares and no outstanding options or warrants); or
- •
- the average weekly trading volume of our common shares on the NASDAQ Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale; provided, in each case, that we are subject to compliance with the Exchange Act periodic reporting requirements for at least 90 days before the sale, and subject to the lock-up agreements described above.
99
Sales under Rule 144 by affiliates or persons who have been affiliates within the previous 90 days are also subject to certain manner of sale provisions, notice requirements, and the availability of current public information about us.
Rule 701
Subject to certain limitations on the aggregate offering price of a transaction and other conditions, Rule 701 permits resales of shares issued prior to the date the issuer becomes subject to the reporting requirements of the Exchange Act, pursuant to certain compensatory benefit plans and contracts commencing 90 days after the issuer becomes subject to the reporting requirements of the Exchange Act, in reliance upon Rule 144 but without compliance with certain restrictions, including the holding period requirements. In addition, the SEC has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of these options, including exercises after the date the issuer becomes so subject. Securities issued in reliance on Rule 701 are restricted securities and subject to the contractual restrictions described above, beginning 90 days after the date of this prospectus, may be sold by persons other than "affiliates" subject only to the manner of sale provisions of Rule 144 and by "affiliates" under Rule 144 without compliance with its one-year minimum holding period requirement.
S-8 Registration Statement
We intend to file one or more registration statements on Form S-8 under the Securities Act covering the shares of common stock subject to outstanding options or reserved for issuance under our various stock option plans. Upon the effectiveness of the applicable registration statement, the shares covered by such registration statement will, subject to Rule 144 volume limitations applicable to affiliates, be available for sale in the open market, except to the extent that these shares are subject to vesting restrictions, lock-up agreements or the contractual restrictions described above.
Registration Rights
After the closing of this offering, the holders of approximately 8,400,000 shares of our common stock will be entitled to certain rights with respect to the registration of such shares under the Securities Act. These holders have the right to include their shares in the registration statements filed but have waived their registration rights in connection with this offering. These holders have also entered into the 180-day lock-up agreements.
100
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following is a summary of the material U.S. federal income tax consequences of the ownership and disposition of our common stock to non-U.S. holders, as defined below, but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, ("Code"), Treasury regulations promulgated thereunder, administrative rulings, and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income tax consequences different from those set forth below. We have not sought any ruling from the Internal Revenue Service, ("IRS"), with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance the IRS will agree with such statements and conclusions. This discussion assumes that the non-U.S. holder holds our common stock as a "capital asset" within the meaning of Section 1221 of the Code (generally, property held for investment).
This summary also does not address the tax considerations arising under the laws of any foreign, state or local jurisdiction or any U.S. federal non-income tax laws other than U.S. federal estate tax laws to the limited extent described below. In addition, this discussion does not address tax considerations applicable to an investor's particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
- •
- banks, insurance companies, or other financial institutions;
- •
- persons subject to the alternative minimum or net investment income tax;
- •
- tax-exempt organizations;
- •
- controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax;
- •
- dealers in securities or currencies;
- •
- traders in securities that elect to use a mark-to-market method of accounting for their securities holdings;
- •
- persons that own, or are deemed to own, more than five percent of our common stock (except to the extent specifically set forth below);
- •
- certain former citizens or long-term residents of the U.S.;
- •
- persons who hold our common stock as a position in a "hedging transaction," "straddle" "conversion transaction" or other risk reduction transaction;
- •
- persons who do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code; or
- •
- persons deemed to sell our common stock under the constructive sale provisions of the Code.
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
YOU ARE URGED TO CONSULT YOUR TAX ADVISOR WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO YOUR PARTICULAR SITUATION, AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION
101
OF OUR COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX RULES OR UNDER THE LAWS OF ANY STATE, LOCAL, FOREIGN, OR OTHER TAXING JURISDICTION OR UNDER ANY APPLICABLE TAX TREATY.
Non-U.S. Holder Defined
For purposes of this discussion, you are a non-U.S. holder if you are a holder other than a partnership or other entity classified as such for U.S. federal income tax purposes that, for U.S. federal income tax purposes, is not a U.S. person. For purposes of this discussion, you are a U.S. person if you are:
- •
- an individual citizen or resident of the U.S.;
- •
- a corporation or other entity taxable as a corporation created or organized in the U.S. or under the laws of the U.S. or any political subdivision thereof or otherwise treated as such for U.S. federal income tax purposes;
- •
- an estate whose income is subject to U.S. federal income tax regardless of its source; or
- •
- a trust (i) whose administration is subject to the primary supervision of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (ii) which has made an election to continue to be treated as a U.S. person.
Distributions
We do not plan to make any distributions on our common stock in the foreseeable future. If we do make future distributions on our common stock (other than certain pro rata distributions of our common stock), however, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of stock.
Any dividend paid to you generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, you must provide us with an IRS Form W-8BEN or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate.
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if an income tax treaty applies, that are attributable to a permanent establishment (or, if you are an individual, a fixed base) maintained by you in the U.S.) are exempt from such withholding tax. In order to obtain this exemption, you must provide us with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition, if you are a corporate non-U.S. holder, dividends you receive that are effectively connected with your conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty.
If you are eligible for a reduced rate of withholding tax pursuant to a tax treaty, you may generally obtain a refund of any excess amounts currently withheld if you timely file an appropriate claim for refund with the IRS. If you hold stock through a financial institution or other agent acting on your behalf, you will be required to provide appropriate documentation to the agent, which then will be
102
required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dispositions
You generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
- •
- the gain is effectively connected with your conduct of a U.S. trade or business (and, if an income tax treaty applies, the gain is attributable to a permanent establishment (or, if you are an individual, a fixed base) maintained by you in the U.S.);
- •
- you are an individual who is present in the U.S. for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met; or
- •
- our common stock constitutes a U.S. real property interest by reason of our status as a U.S. real property holding corporation ("U.S.RPHC"), for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the disposition or your holding period for our common stock.
We believe that we are not currently and will not become a U.S.RPHC. Because the determination of whether we are a U.S.RPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, however, there can be no assurance we will not become a U.S.RPHC if we acquire real property in the future. Even if we become a U.S.RPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests only if you actually or constructively hold more than five percent of such regularly traded common stock. Even if we become a U.S.RPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests only if you actually or constructively hold more than five percent of such regularly traded common stock.
If you are a non-U.S. holder described in the first bullet above, you will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and corporate non-U.S. holders described in the first bullet above may also be subject to the branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. If you are an individual non-U.S. holder described in the second bullet above, you will be required to pay a flat 30% tax on the gain derived from the sale, which tax may be offset by U.S. source capital losses (even though you are not considered a resident of the U.S.). You should consult any applicable income tax treaties that may provide for different rules.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address, and the amount of tax withheld, if any, regardless of whether withholding was required. A similar report is sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence. Payments of dividends or of proceeds on the disposition of stock made to you may be subject to information reporting and backup withholding unless you establish an exemption, for example by properly certifying your non-U.S. status on an IRS Form W-8BEN or another appropriate version of IRS Form W-8. Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that you are a U.S. person.
103
Backup withholding is not an additional tax; rather, the U.S. income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
U.S. Federal Estate Taxes
Common stock owned or treated as owned by an individual who is a non-U.S. person (as specifically defined for U.S. federal estate tax purposes) at the time of death will be included in the individual's gross estate for U.S. federal estate tax purposes and may be subject to U.S. federal estate tax, unless an applicable estate tax treaty provides otherwise.
Legislation Affecting Taxation of our Common Stock Held by or through Foreign Entities
Legislation enacted in 2010 generally will impose a U.S. federal withholding tax of 30% on dividends on and the gross proceeds of a disposition of our common stock, paid to a "foreign financial institution" (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. The legislation also generally will impose a U.S. federal withholding tax of 30% on dividends on and the gross proceeds of a disposition of our common stock paid to a non-financial foreign entity unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. An intergovernmental agreement between the U.S. and an applicable non-U.S. country may modify the requirements discussed above. This withholding obligation under this legislation with respect to dividends on our common stock will not begin until July 1, 2014 and with respect to the gross proceeds of a sale or other disposition of our common stock will not begin until January 1, 2017. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. Prospective investors are encouraged to consult with their own tax advisors regarding the possible implications of this legislation on their investment in our common stock.
Each prospective investor should consult its own tax advisor regarding the particular U.S. federal, state and local and foreign tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed change in applicable laws.
104
We have entered into an underwriting agreement with Roth Capital Partners, LLC, as representative of the several underwriters, with respect to the common stock being offered hereby. Subject to certain conditions, we have agreed to sell to the underwriters, and the underwriters have agreed to purchase from us, 1,750,000 shares of common stock.
Underwriters | Number of Shares | |||
|---|---|---|---|---|
Roth Capital Partners, LLC | ||||
Monarch Capital Group, LLC | ||||
| | | | | |
Total | 1,750,000 | |||
| | | | | |
The underwriters are offering the common stock subject to their acceptance of the common stock from us and subject to prior sale. The underwriting agreement provides that the obligation of the underwriters to pay for and accept delivery of the common stock offered by this prospectus is subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the common stock if any such shares are taken. However, the underwriters are not required to take or pay for the common stock covered by the underwriters' over-allotment option described below.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
At our request, the underwriters have reserved up to $500,000 of shares of our common stock offered by this prospectus for sale to James Hawkins, Chairman of our Board of Directors. The number of shares of common stock available for sale to the general public will be reduced by the amount of this purchase. All sales of reserved common stock to Mr. Hawkins will be made at the initial offering price set forth on the cover page of this prospectus.
Over-Allotment Option
The underwriters have an over-allotment option to purchase up to 15% of the number of shares sold in the offering. If the underwriters sell more shares than the above number, the underwriters have an option for 45 days from the date of this prospectus to buy up to an additional 262,500 shares of common stock from us at the public offering price, less the underwriting discounts and commissions, to cover these sales. The underwriters may exercise this option at any time, in whole or in part, within 45 days after the effective date of this prospectus.
Commissions and Expenses
The representative has advised us that the underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
105
The following table shows the public offering price, underwriting discount and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional shares.
| | Per Share | Without Over-Allotment Option | With Over-Allotment Option | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Public offering price | $ | $ | $ | |||||||
Underwriting discount | $ | $ | $ | |||||||
Proceeds, before expenses, to us | $ | $ | $ | |||||||
The expenses of the offering, not including the underwriting discount, are estimated at $ and are payable by us.
We have agreed to issue to the underwriters a warrant to purchase up to a total of 175,000 shares of common stock (10% of the shares of common stock sold in this offering) (the "Underwriter Warrant"), excluding shares issued pursuant to the underwriters' over-allotment option. The Underwriter Warrant will be exercisable in whole or in part commencing one year from the effective date of the offering through a period ending three years from the date of the offering. The Underwriter Warrant is exercisable at a per share price equal to $ per share, or 130% of the public offering price per share in the offering. The Underwriter Warrant has been deemed compensation by FINRA and is therefore subject to a lock-up of at least 180 days pursuant to Rule 5110(g)(1) of FINRA. The underwriters (or permitted assignees under Rule 5110(g)(1)) will not sell, transfer, assign, pledge, or hypothecate the Underwriter Warrant or the securities underlying the Underwriter Warrant, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Underwriter Warrant or the underlying securities for a period of 180 days from the effective date of the offering. The exercise price and number of shares issuable upon exercise of the Underwriter Warrant may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the exercise price of the Underwriter Warrant or underlying shares will not be adjusted for issuances of shares of common stock at a price below the Underwriter Warrant exercise price. We are registering the Underwriter Warrant and the Underwriter Warrant Shares issuable upon the exercise of the Underwriter Warrant under this Registration Statement on Form S-1.
We have also agreed to reimburse the underwriters for certain out-of-pocket expenses incurred by them, including fees and disbursements of their counsel, up to an aggregate of $75,000, with respect to this offering.
We estimate that the expenses paid by us in connection with the offering of our common stock, other than the underwriting discounts and commissions, will be approximately $800,000.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties contained in the underwriting agreement, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
Our executive officers, directors and our stockholders, who represent in the aggregate 100% of our currently outstanding shares of common stock, including holders of our preferred stock that will be automatically converted into common stock upon completion of this offering, have agreed to a 180-day
106
"lock-up" from the effective date of this prospectus of shares of common stock that they beneficially own.
NASDAQ Capital Market Listing
We intend to apply to list our common stock on the NASDAQ Capital Market under the symbol "IRMD."
Before this offering, there has been no public market for our common stock. The initial public offering price will be determined through negotiations among us and the underwriters. In addition to prevailing market conditions, the factors to be considered in determining the initial public offering price are
- •
- the valuation multiples of publicly traded companies that the underwriters believe to be comparable to us,
- •
- our financial information,
- •
- the history of, and the prospects for, our company and the industry in which we compete,
- •
- an assessment of our management, its past and present operations, and the prospects for, and timing of, our future revenues
- •
- the present state of our development; and
- •
- the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours.
An active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the public market at or above the initial public offering price.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares is completed, SEC rules may limit the underwriters from bidding for and purchasing our common stock. However, the underwriters may engage in transactions that stabilize the price of the common stock, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by an underwriter of a greater number of shares than it is required to purchase in the offering. "Covered" short sales are sales made in an amount not greater than the underwriters' option to purchase additional shares described above. An underwriter may close out any covered short position by either exercising its option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, an underwriter will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which it may purchase shares through the option granted to it. "Naked" short sales are sales in excess of such option. An underwriter must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
107
Similar to other purchase transactions, the underwriters' purchases to cover short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on the NASDAQ Capital Market, in the over-the-counter market or otherwise.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
In connection with the offering, the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
The underwriters and their affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They may in the future receive customary fees and commissions for these transactions.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area (each, a "Relevant Member State"), no offer of shares may be made to the public in that Relevant Member State other than:
- A.
- to any legal entity which is a qualified investor as defined in the Prospectus Directive;
- B.
- to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representative; or
- C.
- in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of shares shall require the Company or the representative to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person in a Relevant Member State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed that it is a "qualified investor" within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that term is
108
used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representative has been obtained to each such proposed offer or resale.
We, the representatives and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly any person making or intending to make an offer in that Relevant Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for us or the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither we nor the underwriters have authorized the making of any offer of shares in circumstances in which an obligation arises for us or the underwriters to publish a prospectus for such offer.
For the purpose of the above provisions, the expression "an offer to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member States) and includes any relevant implementing measure in the Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are "qualified investors" (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the "Order") and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "Relevant Persons"). This document must not be acted on or relied on in the United Kingdom by persons who are not Relevant Persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, Relevant Persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange ("SIX") or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
109
Neither this document nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes ("CISA"). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in Canada
The shares may be sold only to purchasers purchasing as principal that are both "accredited investors" as defined in National Instrument 45-106 Prospectus and Registration Exemptions and "permitted clients" as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from the prospectus requirements and in compliance with the registration requirements of applicable securities laws.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a "prospectus" as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the securities has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in China
The shares of common stock may not be offered or sold directly or indirectly to the public in the People's Republic of China ("China") and neither this prospectus, which has not been submitted to the Chinese Securities and Regulatory Commission, nor any offering material or information contained herein relating to the shares of common stock, may be supplied to the public in China or used in connection with any offer for the subscription or sale of shares of common stock to the public in China. The shares of common stock may only be offered or sold to China-related organizations which are authorized to engage in foreign exchange business and offshore investment from outside of China. Such China related investors may be subject to foreign exchange control approval and filing requirements under the relevant Chinese foreign exchange regulations.
110
The validity of the shares of common stock offered by this prospectus will be passed upon for us by K&L Gates LLP, Los Angeles, California. The underwriters are being represented by Procopio, Cory, Hargreaves & Savitch LLP, San Diego, California.
McGladrey LLP, an independent registered public accounting firm, has audited our financial statements as of December 31, 2013 and 2012, and for each of the years ended December 31, 2013 and 2012, as set forth in their report. We have included our financial statements in the prospectus and elsewhere in the registration statement in reliance on McGladrey LLP's report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC, in Washington, D.C., a registration statement on Form S-1 under the Securities Act with respect to the common stock offered in this prospectus. This prospectus, filed as part of the registration statement, does not contain all of the information set forth in the registration statement and its exhibits and schedules, portions of which have been omitted as permitted by the rules and regulations of the SEC. For further information about us and our common stock, we refer you to the registration statement and to its exhibits and schedules. Statements in this prospectus about the contents of any contract, agreement or other document are not necessarily complete and, in each instance, we refer you to the copy of such contract, agreement or document filed as an exhibit to the registration statement, with each such statement being qualified in all respects by reference to the document to which it refers. Anyone may inspect and copy the registration statement and its exhibits and schedules at the Public Reference Room the SEC maintains at 100 F Street, NE, Washington, D.C. 20549. You may obtain further information about the operation of the SEC's Public Reference Room by calling the SEC at 1-800-SEC-0330. You may also inspect the registration statement and its exhibits and schedules and other information without charge at the website maintained by the SEC. The address of this site is http://www.sec.gov.
We do not presently file periodic reports with the SEC, however, upon completion of this offering, we will become subject to the informational requirements of the Exchange Act and will be required to file reports, proxy statements and other information with the SEC. You will be able to inspect and copy these reports, proxy statements and other information at the Public Reference Room maintained by the SEC at the address noted above, and at the SEC's website http://www.sec.gov. We intend to furnish our stockholders with annual reports containing audited financial statements and make available quarterly reports containing unaudited financial statements. Our website address is www.iradimed.com. The contents of our website are not part of this prospectus and you should not consider the contents of our website in making an investment decision regarding our common stock.
111
IRADIMED CORPORATION FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
iRadimed Corporation
We have audited the accompanying balance sheets of iRadimed Corporation as of December 31, 2013 and 2012, and the related statements of operations and comprehensive income, stockholders' equity, and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of iRadimed Corporation as of December 31, 2013 and 2012, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
/s/ McGladrey LLP
Orlando, Florida
June 18, 2014
F-2
iRadimed Corporation
BALANCE SHEETS
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
ASSETS | ||||||||||
Current assets: | ||||||||||
Cash and cash equivalents | $ | 2,908,641 | $ | 2,461,559 | $ | 1,697,306 | ||||
Accounts receivable, net of allowance for doubtful accounts of $136,971 as of March 31, 2014 (unaudited) and $136,971 and $142,693 as of December 31, 2013 and 2012, respectively | 2,019,675 | 1,982,083 | 1,585,494 | |||||||
Investments | 250,787 | 246,203 | 250,794 | |||||||
Inventory | 1,426,157 | 1,340,331 | 1,385,986 | |||||||
Prepaid expenses and other current assets | 76,796 | 117,640 | 77,218 | |||||||
Prepaid income taxes | 153,524 | 170,496 | — | |||||||
Deferred income taxes | 66,436 | 65,961 | — | |||||||
| | | | | | | | | | | |
Total current assets | 6,902,016 | 6,384,273 | 4,996,798 | |||||||
Property and equipment, net | 320,421 | 327,343 | 265,617 | |||||||
Intangible assets, net | 260,635 | 267,024 | 276,029 | |||||||
Deferred income taxes | 6,515 | — | 12,528 | |||||||
Other assets | 268,975 | 8,231 | 3,240 | |||||||
| | | | | | | | | | | |
Total assets | $ | 7,758,562 | $ | 6,986,871 | $ | 5,554,212 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||||
Current liabilities: | ||||||||||
Accounts payable | $ | 812,009 | $ | 427,474 | $ | 408,424 | ||||
Accrued payroll and benefits | 436,265 | 655,362 | 541,253 | |||||||
Other accrued taxes | 33,591 | 80,787 | 20,321 | |||||||
Warranty reserve | 12,002 | 12,002 | 12,136 | |||||||
Deferred revenue | 163,882 | 207,395 | 389,398 | |||||||
Officer note payable | — | 6,333 | 519,730 | |||||||
Deferred income taxes | — | — | 52,803 | |||||||
Accrued income taxes | 105,415 | 62,971 | 387,289 | |||||||
| | | | | | | | | | | |
Total current liabilities | 1,563,164 | 1,452,324 | 2,331,354 | |||||||
Deferred revenue | 84,262 | 57,676 | 2,658 | |||||||
Deferred income taxes | — | 54,087 | — | |||||||
| | | | | | | | | | | |
Total liabilities | 1,647,426 | 1,564,087 | 2,334,012 | |||||||
| | | | | | | | | | | |
Stockholders' equity: | ||||||||||
Preferred stock, $0.0001 par value; 10,000,000 shares authorized; 1,400,000 issued and outstanding as of March 31, 2014 (unaudited), December 31, 2013 and 2012 | 140 | 140 | 140 | |||||||
Common stock; $0.0001 par value; 90,000,000 shares authorized; 7,000,000 shares issued and outstanding as of March 31, 2014 (unaudited), December 31, 2013 and 2012 | 700 | 700 | 700 | |||||||
Additional paid-in capital | 2,508,946 | 2,346,137 | 2,074,218 | |||||||
Retained earnings | 3,598,250 | 3,074,883 | 1,137,993 | |||||||
Accumulated other comprehensive income | 3,100 | 924 | 7,149 | |||||||
| | | | | | | | | | | |
Total stockholders' equity | 6,111,136 | 5,422,784 | 3,220,200 | |||||||
| | | | | | | | | | | |
Total liabilities and stockholders' equity | $ | 7,758,562 | $ | 6,986,871 | $ | 5,554,212 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to financial statements.
F-3
iRadimed Corporation
STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
| | For the Three Months Ended March 31, | For the Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Revenue | $ | 3,557,237 | $ | 2,628,269 | $ | 11,340,097 | $ | 7,685,061 | |||||
Cost of revenue | 656,366 | 467,113 | 2,853,385 | 2,125,921 | |||||||||
| | | | | | | | | | | | | | |
Gross profit | 2,900,871 | 2,161,156 | 8,486,712 | 5,559,140 | |||||||||
| | | | | | | | | | | | | | |
Operating expenses: | |||||||||||||
General and administrative | 1,092,695 | 503,189 | 2,392,305 | 1,550,034 | |||||||||
Sales and marketing | 759,789 | 568,723 | 2,297,309 | 1,930,395 | |||||||||
Research and development | 224,304 | 160,411 | 1,009,872 | 654,070 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses | 2,076,788 | 1,232,323 | 5,699,486 | 4,134,499 | |||||||||
| | | | | | | | | | | | | | |
Income from operations | 824,083 | 928,833 | 2,787,226 | 1,424,641 | |||||||||
Other income (expense), net | 3,452 | (3,340 | ) | (3,458 | ) | 7,424 | |||||||
| | | | | | | | | | | | | | |
Income before provision for income taxes | 827,535 | 925,493 | 2,783,768 | 1,432,065 | |||||||||
Provision for income taxes | 304,168 | 281,566 | 846,878 | 465,980 | |||||||||
| | | | | | | | | | | | | | |
Net income | $ | 523,367 | $ | 643,927 | $ | 1,936,890 | $ | 966,085 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other comprehensive (loss) income: | |||||||||||||
Change in fair value of available-for-sale securities, net of tax expense (benefit) of $1,171 and $77 for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), respectively, and $(3,352) and $1,661 for the years ended December 31, 2013 and 2012, respectively | 2,176 | 144 | (6,225 | ) | 3,084 | ||||||||
| | | | | | | | | | | | | | |
Comprehensive income | $ | 525,543 | $ | 644,071 | $ | 1,930,665 | $ | 969,169 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share: | |||||||||||||
Basic | $ | 0.07 | $ | 0.09 | $ | 0.28 | $ | 0.14 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted | $ | 0.06 | $ | 0.08 | $ | 0.22 | $ | 0.11 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Weighted average shares outstanding: | |||||||||||||
Basic | 7,000,000 | 7,000,000 | 7,000,000 | 7,000,000 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted | 8,859,015 | 8,492,475 | 8,624,314 | 8,462,240 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
See accompanying notes to financial statements.
F-4
iRadimed Corporation
STATEMENTS OF STOCKHOLDERS' EQUITY
| | Preferred Stock | Common Stock | Additional Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Income | Stockholders' Equity | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balances, December 31, 2011 | $ | 140 | $ | 700 | $ | 1,900,978 | $ | 171,908 | $ | 4,065 | $ | 2,077,791 | |||||||
Net income | — | — | — | 966,085 | — | 966,085 | |||||||||||||
Other comprehensive income | — | — | — | — | 3,084 | 3,084 | |||||||||||||
Stock-based compensation | — | — | 173,240 | — | — | 173,240 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2012 | $ | 140 | $ | 700 | $ | 2,074,218 | $ | 1,137,993 | $ | 7,149 | $ | 3,220,200 | |||||||
Net income | — | — | — | 1,936,890 | — | 1,936,890 | |||||||||||||
Other comprehensive loss | — | — | — | — | (6,225 | ) | (6,225 | ) | |||||||||||
Stock-based compensation | — | — | 271,919 | — | — | 271,919 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2013 | $ | 140 | $ | 700 | $ | 2,346,137 | $ | 3,074,883 | $ | 924 | $ | 5,422,784 | |||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes to financial statements.
F-5
iRadimed Corporation
STATEMENTS OF CASH FLOWS
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Operating activities: | |||||||||||||
Net income | $ | 523,367 | $ | 643,927 | $ | 1,936,890 | $ | 966,085 | |||||
Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||||
Depreciation and amortization | 20,768 | 31,996 | 139,040 | 106,281 | |||||||||
Non-cash disposal of property and equipment | — | — | — | (430 | ) | ||||||||
Stock-based compensation | 162,809 | 67,980 | 271,919 | 173,240 | |||||||||
Changes in operating assets and liabilities: | |||||||||||||
Accounts receivable | (37,592 | ) | (41,490 | ) | (396,589 | ) | (1,154,176 | ) | |||||
Inventory | (85,826 | ) | (125,149 | ) | 45,655 | (116,828 | ) | ||||||
Prepaid expenses and other current assets | 40,844 | (58,396 | ) | (40,422 | ) | (72,977 | ) | ||||||
Other assets | (1,688 | ) | (293 | ) | (4,991 | ) | (1,185 | ) | |||||
Deferred income taxes | (62,248 | ) | (13,115 | ) | (48,797 | ) | 78,839 | ||||||
Accounts payable | 125,479 | 57,843 | 19,050 | 337,317 | |||||||||
Accrued payroll and benefits | (219,097 | ) | (231,690 | ) | 114,109 | 404,952 | |||||||
Other accrued taxes | (47,196 | ) | 6,032 | 60,466 | 18,320 | ||||||||
Warranty reserve | — | (134 | ) | (134 | ) | 9,011 | |||||||
Deferred revenue | (16,927 | ) | (215,710 | ) | (126,985 | ) | 373,418 | ||||||
Accrued income taxes, net of prepaid income taxes | 59,416 | (172,875 | ) | (494,814 | ) | 387,289 | |||||||
| | | | | | | | | | | | | | |
Net cash provided by (used in) operating activities | 462,109 | (51,074 | ) | 1,474,397 | 1,509,156 | ||||||||
| | | | | | | | | | | | | | |
Investing activities: | |||||||||||||
Purchases of investments | (1,237 | ) | (1,037 | ) | (4,986 | ) | (4,707 | ) | |||||
Purchases of property and equipment | (4,292 | ) | (21,245 | ) | (163,175 | ) | (112,940 | ) | |||||
Patent and software costs | (3,165 | ) | (2,421 | ) | (28,586 | ) | (48,621 | ) | |||||
| | | | | | | | | | | | | | |
Net cash used in investing activities | (8,694 | ) | (24,703 | ) | (196,747 | ) | (166,268 | ) | |||||
| | | | | | | | | | | | | | |
Financing activities: | |||||||||||||
Repayment of officer note payable | (6,333 | ) | — | (513,397 | ) | (238,532 | ) | ||||||
| | | | | | | | | | | | | | |
Net cash used in financing activities | (6,333 | ) | — | (513,397 | ) | (238,532 | ) | ||||||
| | | | | | | | | | | | | | |
Net increase (decrease) in cash and equivalents | 447,082 | (75,777 | ) | 764,253 | 1,104,356 | ||||||||
Cash and cash equivalents, beginning of year | 2,461,559 | 1,697,306 | 1,697,306 | 592,950 | |||||||||
| | | | | | | | | | | | | | |
Cash and cash equivalents, end of year | $ | 2,908,641 | $ | 1,621,529 | $ | 2,461,559 | $ | 1,697,306 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Supplemental disclosure of cash flow information: | |||||||||||||
Cash paid for income taxes | $ | 307,000 | $ | 480,634 | $ | 1,390,049 | $ | — | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Deferred initial public offering costs included in Other assets | $ | 259,056 | $ | — | $ | — | $ | — | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
See accompanying notes to financial statements.
F-6
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
1 – Organization and Significant Accounting Policies
Organization
iRadimed Corporation ("iRadimed", the "Company", "we", "our") was incorporated in Oklahoma in July 1992 and reincorporated in Delaware in April 2014. We develop, manufacture, market and distribute Magnetic Resonance Imaging ("MRI") compatible products, and today, we are the sole provider of non-magnetic intravenous ("IV") infusion pump systems. We were the first to develop an infusion delivery system that neutralizes the dangers and problems present during MRI procedures. Our headquarters are in Winter Springs, Florida.
During the fourth quarter of 2013, the Company began taking steps to execute an initial public offering of its common stock.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities in the financial statements and the reported amount of revenue and expenses during the reporting period. Such estimates include allowances for potentially uncollectible accounts receivable, valuation of inventory, intangible assets, allocation of arrangement consideration, stock-based compensation, deferred income taxes, reserves for warranty obligations, and the provision for income taxes. Actual results could differ from those estimates.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, the price is fixed or determinable, delivery has occurred and title and risk of loss has transferred and collection of the resulting receivable is reasonably assured. Terms of sale for most domestic sales are FOB destination, reflecting that title and risk of loss are assumed by the purchaser upon delivery. Terms of sales to international distributors are FOB shipping point, reflecting that title and risk of loss are assumed by the distributor at the shipping point.
Under the revenue recognition rules for tangible products, we allocate revenue from arrangements with multiple deliverables to each of the deliverables based upon their relative selling prices as determined by a selling-price hierarchy. A deliverable in an arrangement qualifies as a separate unit of accounting if 1) the delivered item has value to the customer on a stand-alone basis, and 2) the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered items is considered probable and substantially in control of the vendor. The principal deliverables in our multiple deliverable arrangements that qualify as separate units of accounting consist of (i) sales of medical devices and supplies, (ii) installation and training services, and (iii) separately priced extended warranty agreements.
We use a hierarchy to determine the selling price to be used for allocating revenue to deliverables as follows: (i) vendor-specific objective evidence of fair value ("VSOE"), (ii) third-party evidence of selling price ("TPE"), and (iii) best estimate of the selling price ("ESP"). VSOE of fair value is defined as the price charged when the same element is sold separately, or if the element has not yet been sold separately, the price for the element established by management having the relevant authority when it is probable that the price will not change before the introduction of the element into the marketplace. VSOE generally exists only when we sell the deliverable separately and is the price
F-7
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
actually charged for that deliverable. For certain sales under group purchasing organization ("GPO") contracts, we have established VSOE for all of the elements in our multiple element arrangements. This determination is based on the volume of sales to these customers in relation to our total sales and the discount tier in which those sales are made. For all other sales we rely on ESP, reflecting our best estimates of what the selling prices of elements would be if they were sold regularly on a stand-alone basis, to establish the amount of revenue to allocate to the undelivered elements. TPE generally does not exist for our products because of their uniqueness.
For products shipped under FOB shipping point terms, delivery is considered to have occurred when shipped. Undelivered elements in our sales arrangements, which are not considered to be essential to the functionality of a product, generally include installation and training services that are performed after the related products have been delivered and extended warranty agreements. Revenue related to undelivered installation and training services is deferred until such time as those services are complete, which is typically within 30 days of the related products being delivered to the customer's location. Revenue and direct acquisition costs related to undelivered extended warranty agreements are deferred and recognized ratably over the service period, which is between one and four years. Deferred revenue for extended warranty agreements is based on the price charged when the service is sold separately.
Shipping and handling charges billed to customers are included in revenue and shipping and handling related expenses are charged to cost of revenue. Advance payments from customers are recorded as deferred revenue and recognized as revenue as otherwise described above. Most of our sales are subject to 30 to 60 day customer-specified acceptance provisions. These provisions require us to estimate the amount of future returns and recognize revenue net of these potential returns.
In certain States we are required to collect sales taxes from our customers. These amounts are excluded from revenue and recorded as a liability.
GPOs negotiate volume purchase prices for hospitals, group practices, and other clinics that are members of a GPO. Our agreements with GPOs typically include the following provisions:
- •
- Negotiated pricing for all group members;
- •
- Volume discounts and other preferential terms on their members' purchases from us;
- •
- Promotion of our products by the GPO to its members;
- •
- Payment of administrative fees by us to the GPO, based on purchases of our products by group members.
We do not sell to GPOs. Hospitals, group practices, and other acute care facilities that are members of a GPO purchase products directly from us under the terms negotiated by the GPO. Negotiated pricing and discounts are recognized as a reduction of the selling price of products at the time of the sale. Revenue from sales to members of GPOs is otherwise consistent with revenue recognition policies as previously described.
Cash Equivalents
All highly liquid instruments purchased with an original maturity of three months or less are classified as cash equivalents.
F-8
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable is recorded at the sales price of the related products and services. We assess the sufficiency of the allowance for estimated uncollectible accounts receivable. Estimates are based on historical collection experience and other customer-specific information, such as bankruptcy filings or liquidity problems of our customers. When it is determined that an account receivable is uncollectible, it is written off and relieved from the allowance. Any future determination that the allowance for estimated uncollectible accounts receivable is not properly stated could result in changes in operating expense and results of operations.
Investments
Our investments consist of two mutual funds and are considered available-for-sale. The specific identification method is used to determine the cost basis of investments sold. Our investments are recorded in our balance sheets at fair value. We classify our investments as current based on the nature of the investments and their availability for use in current operations. Unrealized gains and losses on our investments are included in accumulated other comprehensive income, net of tax. Realized gains or losses are recorded in sale of investments and impairment losses that are determined to be other-than-temporary are recorded in investment impairment losses in our statements of operations.
Fair Value Measurements
Fair value is the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants on the measurement date. A three-level valuation hierarchy requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability on the measurement date. The three levels of inputs are:
- •
- Level 1 – quoted prices (unadjusted) in active markets for an identical asset or liability.
- •
- Level 2 – quoted prices for a similar asset or liability in an active market or model-derived valuations in which all significant inputs are observable for substantially the full term of the asset or liability.
- •
- Level 3 – unobservable and significant to the fair value measurement of the asset or liability.
Financial instruments include cash and cash equivalents, investments, accounts receivable, accounts payable and accrued expenses. Cash and cash equivalents and investments are reported at their respective fair values on the balance sheet dates. The recorded carrying amount of accounts receivable, accounts payable and accrued expenses approximates their fair values due to their short-term maturities.
Inventory
Inventories are stated at the lower of standard cost, which approximates actual cost on a first-in, first-out basis, or market. We may be exposed to a number of factors that could result in portions of our inventory becoming either obsolete or in excess of anticipated usage. These factors include, but are not limited to, technological changes, competitive situations in products and prices, and the introduction of new product lines. We regularly evaluate our ability to realize the value of inventory
F-9
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
based on a combination of factors, including historical usage rates, forecasted sales, product life cycles, and market acceptance of new products. When inventory that is obsolete or in excess of anticipated usage is identified, it is written down to realizable salvage value or an inventory valuation allowance is established.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation expense is computed using the straight-line method over estimated useful lives of the respective assets, which are three to five years for computer software and hardware; five to seven years for furniture, fixtures, machinery and equipment. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the improvements.
Repair and maintenance costs that do not extend the useful life of our property and equipment are expensed as incurred.
Intangible Assets
Intangible assets include application and legal costs incurred to obtain patents. We capitalize these costs when we determine that probable future economic benefits exist. In making this determination, we consider the projected future operating results associated with the patents, industry and economic trends, and the entry of new products in the market. Costs incurred prior to this determination are expensed in the period they are incurred. We amortize capitalized patent costs using the straight-line method over their useful lives, which is typically 17 years. Periodic costs incurred to maintain existing patents are expensed as incurred.
Long-lived Assets
Long-lived assets are tested for impairment whenever changes in circumstances indicate the carrying value of these assets may be impaired. Impairment indicators include, but are not limited to, technological obsolescence, unfavorable court rulings, significant negative industry and economic trends, and significant underperformance relative to historical and projected future operating results. Impairment is considered to have occurred when the estimated undiscounted future cash flows related to the asset groups are less than its carrying value. Estimates of future cash flows involve consideration of many factors including the marketability of new products, product acceptance and lifecycle, competition, appropriate discount rates, and operating margins. An impairment is recognized as the amount by which the carrying value is less than the fair value of the asset or asset group.
Warranty
We provide for the estimated cost of product warranties at the time revenue is recognized. While we engage in product quality programs and processes, including actively monitoring and evaluating the quality of our suppliers, the estimated warranty obligation is affected by ongoing product failure rates, material usage costs and direct labor incurred in correcting a product failure. Actual product failure rates, material usage costs and the amount of labor required to repair products that differ from estimates result in revisions to the estimated liability. We warrant for a limited period of time that our products will be free from defects in materials and workmanship. We estimate warranty allowances based on historical warranty experience. Historically, warranty expenses have not been material to our financial statements.
F-10
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
Research & Development and Capitalized Software Development Costs
Research and development costs are expensed as incurred. Some of our products include embedded software which is essential to the product's functionality. Costs incurred in the research and development of new software components and enhancements to existing software components are expensed as incurred until technological feasibility has been established. We capitalize software development costs when the project reaches technological feasibility and cease capitalization when the project is ready for release. Capitalized software development costs are included in intangible assets and are amortized on a straight-line basis over the estimated useful life of the product. Amortization begins when the product is available for general release to the customer.
Advertising and Marketing
Advertising and marketing costs are expensed as they are incurred. For the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), advertising and marketing costs were $12,877 and $11,941, respectively. For the years ended December 31, 2013 and 2012, these costs were $78,882 and $84,930, respectively. Advertising and marketing costs are included in sales and marketing expense.
Medical Device Excise Taxes
Effective January 1, 2013, we became subject to the Medical Device Excise Tax applicable to sales of listed medical devices under the Patient Protection and Affordable Care Act ("ACA") enacted in 2010. The ACA requires us to pay 2.3% of the taxable sales value of devices sold. Qualifying sales are recorded on a gross basis. For the three months ended March 31, 2014 (unaudited), we recorded medical device excise taxes of $51,333. For the year ended December 31, 2013, we recorded medical device excise taxes of $161,246. Medical device excise taxes are included as a component of general and administrative expense.
Stock-Based Compensation
We recognize stock-based compensation expense associated with employee stock options on a straight-line basis over the requisite service period for the entire award, which is generally four years. The maximum contractual life of our stock options is ten years from the grant date. We utilize the Black-Scholes option pricing model to estimate the grant date fair value of those awards. The Black-Scholes option pricing model requires the input of certain assumptions including stock price, dividend yield, expected volatility, risk-free interest rate, and expected option life. Changes in these assumptions can materially affect the estimated fair value of our employee stock options.
The grant date stock price is based on third-party valuations that have been performed periodically and consideration of significant events impacting us since the date of the respective valuations; dividend yield is based on our expectation of dividend payments over the expected life of the option; expected volatility is based on a study of comparable, publicly traded companies with similar products and product life cycles; risk-free interest rate was the rate available on zero coupon U.S. government obligations with a term approximating the expected option life; the expected option life was calculated using the simplified method as there is no historical exercise data to rely upon.
Forfeitures of employee stock options are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from initial estimates. Stock-based compensation expense is recorded net of estimated forfeitures, such that expense is recorded only for those stock-based awards that are expected to vest.
F-11
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
The cash flow resulting from the tax benefits from tax deductions in excess of the compensation cost recognized for those options (excess tax benefits) is classified as a cash inflow from financing activities and a cash outflow from operating activities in our statements of cash flows. We treat tax deductions from certain stock option exercises as being realized when they reduce taxes payable in accordance with relevant tax law. Upon exercise, we issues new shares. To date, there have been no option exercises.
Income Taxes
We account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
We record net deferred tax assets to the extent we believe these assets will more likely than not be realized. In making such determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial operations. A valuation allowance is recorded to offset net deferred tax assets if, based upon the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
We recognize the tax benefit of uncertain tax positions in the financial statements based on the technical merits of the position. When the tax position is deemed more likely than not of being sustained, we recognize the largest amount of tax benefit that is greater than 50 percent likely of being ultimately realized upon settlement.
Foreign Currency
Gains and losses from transactions denominated in currencies other than our functional currency are included in other income and expense. For the three months ended March 31, 2014 (unaudited), net foreign currency transaction gain was $2,184. For the three months ended March 31, 2013 (unaudited), net foreign currency transaction loss was $4,389. For the years ended December 31, 2013 and 2012, net foreign currency transaction losses were $23,432 and $12,210, respectively. Foreign currency gains and losses result primarily from fluctuations in the exchange rate between the U.S. Dollar and the Japanese Yen.
Comprehensive Income
Comprehensive income includes net income and other comprehensive income items that are excluded from net income under U.S. generally accepted accounting principles. Comprehensive income includes unrealized gains and losses on our investments classified as available for sale.
Basic and Diluted Net Income per Share
Basic net income per share is based upon the weighted average number of common shares outstanding during the period. Diluted net income per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock. As discussed further in Note 5, the effect of our 1.75:1 stock split and recapitalization is reflected in the number of outstanding shares and per share information in the table below. Preferred stock and stock options granted by us represent the only dilutive effect reflected in diluted weighted-average shares outstanding.
F-12
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
The following table presents the computation of basic and diluted net income per share:
| | As of March 31, | As of December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Net income | $ | 523,367 | $ | 643,927 | $ | 1,936,890 | $ | 966,085 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Weighted-average common shares outstanding – Basic | 7,000,000 | 7,000,000 | 7,000,000 | 7,000,000 | |||||||||
Effect of dilutive securities: | |||||||||||||
Preferred stock | 1,400,000 | 1,400,000 | 1,400,000 | 1,400,000 | |||||||||
Stock options | 459,015 | 92,475 | 224,314 | 62,240 | |||||||||
| | | | | | | | | | | | | | |
Weighted-average common shares outstanding – Diluted | 8,859,015 | 8,492,475 | 8,624,314 | 8,462,240 | |||||||||
Basic net income per share | $ | 0.07 | $ | 0.09 | $ | 0.28 | $ | 0.14 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Diluted net income per share | $ | 0.06 | $ | 0.08 | $ | 0.22 | $ | 0.11 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Stock options to purchase shares of our common stock excluded from the calculation of diluted net income per share because the effect would have been anti-dilutive are as follows:
| | As of March 31, | As of December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Anti-dilutive stock options | 586,509 | 789,933 | 467,109 | 842,433 | |||||||||
Subsequent to December 31, 2013, we granted 73,500 options to employees for the purchase of our common stock under the terms of our incentive stock plan as described further in Note 6 below.
Certain Significant Risks and Uncertainties
We market our products to end users in the United States and to distributors internationally. Sales to end users in the United States are generally made on open credit terms. Management maintains an allowance for potential credit losses. At the end of 2013, one customer accounted for 10.8% of gross accounts receivable. At the end of 2012, one customer accounted for 15.4% of gross accounts receivable.
Revenue for 2013 included two non-recurring sales to a customer in Saudi Arabia for 129 of our MRI compatible IV infusion pumps. Revenue recorded in 2013 related to these orders represented 10.7% of total revenue for 2013.
We have deposited our cash and cash equivalents with various financial institutions. Our cash and cash equivalents balances exceed federally insured limits throughout the year. We have not incurred any losses related to these balances.
Our products require clearance from the Food and Drug Administration and international regulatory agencies prior to commercialized sales. The Company's future products may not receive required approvals. If the Company were denied such approvals, or if such approvals were delayed, it
F-13
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
would have a materially adverse impact on the Company's business, results of operations and financial condition.
Certain key components of our products essential to their functionality are sole-sourced. Any disruption in the availability of these components would have a materially adverse impact on our business, results of operations and financial condition.
Recent Accounting Pronouncements
Recently issued FASB guidance and Securities and Exchange Commission Staff Accounting Bulletins have either been implemented, with no significant effect, or are not applicable to us.
2 – Inventory
Inventory consists of:
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
Raw materials | $ | 1,179,936 | $ | 1,143,495 | $ | 1,165,356 | ||||
Work in process | 85,502 | 14,337 | 55,039 | |||||||
Finished goods | 160,719 | 182,499 | 165,591 | |||||||
| | | | | | | | | | | |
Total | $ | 1,426,157 | $ | 1,340,331 | $ | 1,385,986 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
3 – Property and Equipment
Property and equipment consist of:
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
Computer software and hardware | $ | 157,422 | $ | 154,709 | $ | 132,878 | ||||
Furniture and fixtures | 91,049 | 87,611 | 75,146 | |||||||
Leasehold improvements | 47,623 | 47,623 | 43,743 | |||||||
Machinery and equipment | 765,280 | 721,270 | 642,833 | |||||||
Tooling in-process | 693 | 46,562 | — | |||||||
| | | | | | | | | | | |
| 1,062,067 | 1,057,775 | 894,600 | ||||||||
Accumulated depreciation | (741,646 | ) | (730,432 | ) | (628,983 | ) | ||||
| | | | | | | | | | | |
Total | $ | 320,421 | $ | 327,343 | $ | 265,617 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Depreciation and amortization expense of property and equipment was $11,214 and $22,806 for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), respectively, and $101,449 and $72,455 in the years ended December 31, 2013 and 2012, respectively.
F-14
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
4 – Intangible Assets
The following table summarizes the components of intangible asset balances:
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
Patents – in use | $ | 228,520 | $ | 228,430 | $ | 215,389 | ||||
Patents – in process | 22,240 | 19,165 | 3,620 | |||||||
Internally developed software | 148,967 | 148,967 | 148,967 | |||||||
| | | | | | | | | | | |
| 399,727 | 396,562 | 367,976 | ||||||||
Accumulated amortization | (139,092 | ) | (129,538 | ) | (91,947 | ) | ||||
| | | | | | | | | | | |
Total | $ | 260,635 | $ | 267,024 | $ | 276,029 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Amortization expense of intangible assets was $9,554 and $9,190 for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), respectively, and $37,591 and $33,826 in the years ended December 31, 2013 and 2012, respectively.
Expected annual amortization expense for the next five years related to intangible assets is as follows:
2014 | $ | 38,218 | ||
2015 | 38,218 | |||
2016 | 20,915 | |||
2017 | 13,437 | |||
2018 | 13,437 |
5 – Capital Stock
Reincorporation
Effective April 14, 2014, we reincorporated as a Delaware corporation. As part of this reincorporation, we converted all previously outstanding shares of our Class A Common Stock and Class B Common Stock into a single class of common stock on a 1.75:1 conversion ratio and all previously outstanding shares of our Series A Preferred Stock were split on a 1.75:1 conversion ratio into new Series A Preferred Stock. Our Certificate of Incorporation provides that the Series A Preferred Stock will automatically be converted into shares of common stock immediately upon the earlier the closing on the first sale of shares of our common stock in in an initial firm commitment underwritten public offering pursuant to a registration statement under the Securities Act of 1933, as amended, where the aggregate public offering amount is not less than $10,000,000. In accordance with our Certificate of Incorporation, upon the sale of shares pursuant to this initial public offering, all of our Series A Preferred Stock will be automatically converted into common stock on a 1:1 conversion ratio. This recapitalization is accounted for as a stock split as the intent is to provide for wider
F-15
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
distribution of our common stock. The table below summarizes the effect of the stock split and conversion on our capital stock that was previously outstanding as of:
| December 31, 2013 and 2012 | ||||
|---|---|---|---|---|
Series A Preferred Stock outstanding – Pre recapitalization | 800,000 | |||
Stock split ratio | 1.75:1 | |||
Series A Preferred Stock outstanding – Post recapitalization | 1,400,000 | |||
| | | | | |
| | | | | |
Common stock outstanding – Pre recapitalization | ||||
Class A Common Stock | 400,000 | |||
Class B Common Stock | 3,600,000 | |||
| | | | | |
Total | 4,000,000 | |||
Stock split ratio | 1.75:1 | |||
Common stock outstanding – Post recapitalization | 7,000,000 | |||
| | | | | |
| | | | | |
As of the effective date of the reincorporation, we are now authorized to issue 90,000,000 shares of Common Stock with a par value of $0.0001 per share and 10,000,000 shares of Preferred Stock with a par value of $0.0001. Upon completion of the public offering, there will be 8,400,000 shares Common Stock outstanding and no shares of Series A Preferred Stock outstanding.
The effect of this stock split has been retroactively applied to per-share computations, share and option amounts for all periods presented within these financial statements and accompanying notes.
The rights and privileges of our Series A Preferred Stock and Common Stock are as follows:
Series A Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, of which 800,000 of these shares shall be designated as Series A Preferred Stock ("Preferred Stock") with a par value of $0.0001 per share.
Voting and Dividends. Prior to conversion, the holder of each share of Preferred Stock has the right to one vote for each share of Common Stock into which such Preferred Stock could then be converted. The holders of the Preferred Stock are entitled to receive dividends from legally available assets prior to any declaration or payment of dividends to Common Stock holders. Dividends on each share of Preferred Stock are initially at $0.06429 per year payable when and as declared by the Board and are non-cumulative. After payment of such dividends, any additional dividends or distributions are distributed among all holders of Common Stock and Preferred Stock in proportion to the number of shares of Common Stock that would be held by each holder if all shares of Preferred Stock were converted to Common Stock at the then effective conversion rate.
Liquidation. In the event of any liquidation, dissolution or winding up of our Company, either voluntary or involuntary, the holders of the Preferred Stock are entitled to receive, prior and in preference to any distribution of the proceeds resulting from such liquidation event to holders of the Common Stock, an amount equal to $1.07143 plus declared but unpaid dividends. If, upon occurrence of such liquidation event, the proceeds are insufficient to permit the payment of the aforementioned amount in full, then the entire proceeds shall be distributed ratably among all holders of the Preferred Stock in proportion to the full amount each holder would otherwise receive.
F-16
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
Conversion. Each share of Preferred Stock is convertible at any time, at the option of the holder, into such number of fully paid non-assessable shares of Common Stock as is determined by dividing the original issue price of each share of Preferred Stock by the applicable conversion price. The initial conversion price per share is $1.07143. Adjustments to the initial conversion price may result from a recapitalization event or changes in the number of common shares outstanding. Each share of Preferred Stock automatically converts into shares of fully paid non-assessable shares of Common Stock, at the then applicable conversion rate, upon (1) sale of our common stock through a qualified initial public offering, or (2) the date specified by written consent or agreement of the holders of a majority of the then outstanding shares of Preferred Stock, voting as a single class on an as-converted basis. As of March 31, 2014, December 31, 2013 and December 31, 2012, the original issue price and the applicable conversion price are both $1.07143, resulting in a 1:1 conversion ratio.
Redemption. Upon a majority vote of the then outstanding shares of Preferred Stock, we may, at our discretion, redeem or purchase shares of Preferred Stock. We also have a first right of refusal to repurchase shares of the Preferred Stock arising from a holder's proposal to sell such Preferred Stock.
Common Stock
We are authorized to issue 90,000,000 shares of Common Stock with a par value of $0.0001 per share.
Voting and Dividends. Each outstanding share of Common Stock shall entitle the holder thereof to one vote on each matter properly submitted to the stockholders of the Company for their vote except for matters related to potential amendments to our Certificate of Incorporation or matters that solely relate to the terms of one or more outstanding series of our Preferred Stock. Holders of our Common Stock are entitled to receive, when, as and if declared by the Board, dividends pro rata based on the number of shares of Common Stock held. These dividend rights are junior to those of the Preferred Stock holders' rights to dividends.
Liquidation. Liquidation preference of the Common Stock holders is junior to that of the Preferred Stock holders.
Redemption. The Common Stock is not redeemable.
6 – Stock-Based Compensation
During the years ended December 31, 2013 and 2012, we maintained one stock plan known as the iRadimed Corporation Incentive Stock Plan ("Plan"). Subsequent to the year ended December 31, 2013, our Board of Directors adopted and our shareholders approved the 2014 Equity Incentive Plan (see Note 15). The Plan is administered by our Board of Directors. The Plan authorizes the issuance of up to 1,862,000 common shares in connection with the granting of non-qualified stock options and incentive stock options. As of March 31, 2014 (unaudited) and December 31, 2013, and after the effect of our stock split as discussed in Note 5, there were 28,808 and 102,308, respectively, shares available for future awards.
F-17
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
Stock-based compensation was recognized as follows in the statement of operations:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Cost of revenue | $ | 959 | $ | 3 | $ | 13 | $ | 2,050 | |||||
General and administrative | 56,935 | 4,115 | 16,461 | 1,770 | |||||||||
Sales and marketing | 95,536 | 63,775 | 255,096 | 168,316 | |||||||||
Research and development | 9,379 | 87 | 349 | 1,104 | |||||||||
| | | | | | | | | | | | | | |
Total | $ | 162,809 | $ | 67,980 | $ | 271,919 | $ | 173,240 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
As of March 31, 2014 (unaudited) we had $2,148,989 of total unrecognized stock-based compensation expense, which is expected to be recognized over a weighted average period of 3.4 years. As of December 31, 2013, we had $2,117,081 of total unrecognized stock-based compensation expense, which is expected to be recognized over a weighted average period of 3.6 years. The total fair value of stock options that vested during the three months ended March 31, 2014 (unaudited) was $173,997. The total fair value of stock options that vested during the year ended December 31,2013 was $272,004.
Under the Plan, the strike price of non-qualified stock options awarded under the Plan may not be less than 85% of the fair value of our common stock on the date of grant. The strike price of incentive stock options awarded under the Plan may not be less than 100% of our common stock on the date of grant. Some of the options we issued in the past had exercise prices below the estimated fair value of our Common Stock on their respective grant date and hence do not qualify as incentive stock options. Going forward our intention is to issue options to our employees that qualify as incentive stock options. Options awarded under the Plan expire no more than ten years after the date of grant. Options are granted periodically throughout the year.
The weighted-average fair value of our options and the related assumptions used in the Black-Scholes model to record stock-based compensation are as follows:
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
Weighted-average fair value of common stock | $ | 3.30 | $ | 3.25 | $ | 1.59 | ||||
Dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||||
Expected volatility | 112.8 | % | 113.0 | % | 116.8 | % | ||||
Risk-free interest rate | 2.4 | % | 2.4 | % | 1.1 | % | ||||
Expected option life (years) | 7.0 | 7.0 | 7.0 | |||||||
Forfeiture rate | 10.0 | % | 10.0 | % | 0.0 | % | ||||
F-18
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
The following table presents a summary of our stock option activity as of and for the year ended December 31, 2013:
| | Options | Weighted-Average Exercise Price Per Share | Weighted-Average Remaining Contractual Life (Yrs) | Aggregate Intrinsic Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding beginning of period | 1,192,433 | $ | 1.04 | 6.6 | $ | 652,787 | |||||||
| | | | | | | | | | | | | | |
Options granted | 567,259 | 1.46 | |||||||||||
Options exercised | — | — | |||||||||||
Options canceled | — | — | |||||||||||
| | | | | | | | | | | | | | |
Outstanding end of period | 1,759,692 | $ | 1.18 | 7.7 | $ | 3,741,190 | |||||||
| | | | | | | | | | | | | | |
Exercisable | 721,302 | $ | 1.09 | 5.4 | $ | 1,593,550 | |||||||
| | | | | | | | | | | | | | |
The following table presents a summary of our stock option activity as of and for the three months ended March 31, 2014 (unaudited):
| | Options | Weighted-Average Exercise Price Per Share | Weighted-Average Remaining Contractual Life (Yrs) | Aggregate Intrinsic Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding beginning of period | 1,759,692 | $ | 1.18 | 7.7 | $ | 3,741,190 | |||||||
| | | | | | | | | | | | | | |
Options granted | 73,500 | 2.65 | |||||||||||
Options exercised | — | — | |||||||||||
Options canceled | — | — | |||||||||||
| | | | | | | | | | | | | | |
Outstanding end of period | 1,833,192 | $ | 1.24 | 7.5 | $ | 3,785,640 | |||||||
| | | | | | | | | | | | | | |
Exercisable | 721,302 | $ | 1.09 | 5.2 | $ | 1,593,550 | |||||||
| | | | | | | | | | | | | | |
The historical valuations of our common stock were determined in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid,Valuation of Privately-Held-Company Equity Securities Issued as Compensation. In the absence of a public trading market, we considered all relevant facts and circumstances known at the time of valuation, made certain assumptions based on future expectations and exercised significant judgment to determine the fair value of our common stock. The factors considered in determining the fair value include, but are not limited to, the following:
- •
- Retrospective third-party valuation of our common stock as of December 31, 2012;
- •
- Contemporaneous third-party valuation of our common stock as of December 31, 2013;
- •
- Our historical financial results and estimated trends and projections of our future operating and financial performance;
- •
- The market performance of comparable, publicly traded companies; and
- •
- The overall economic and industry conditions and outlook.
F-19
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
We granted the following stock options during the three months ended March 31, 2014 (unaudited) and for the years ended December 31, 2013 and 2012:
Grant Date | Options Granted | Exercise Price | Estimated Fair Value of Common Stock per Share at Grant Date | Aggregate Grant Date Fair Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
July 16, 2012 | 17,500 | $ | 1.29 | $ | 1.59 | $ | 25,224 | ||||||
November 1, 2012 | 278,408 | $ | 0.93 | $ | 1.59 | $ | 402,939 | ||||||
November 20, 2012 | 43,750 | $ | 1.48 | $ | 1.59 | $ | 61,503 | ||||||
January 1, 2013 | 17,500 | $ | 1.29 | $ | 1.59 | $ | 24,819 | ||||||
December 13, 2013 | 26,250 | $ | 1.29 | $ | 3.30 | $ | 80,259 | ||||||
December 31, 2013 | 523,509 | $ | 1.48 | $ | 3.30 | $ | 1,590,185 | ||||||
January 1, 2014 | 26,250 | $ | 1.48 | $ | 3.30 | $ | 71,820 | ||||||
January 1, 2014 | 12,250 | $ | 3.30 | $ | 3.30 | $ | 31,862 | ||||||
March 1, 2014 | 8,750 | $ | 3.30 | $ | 3.30 | $ | 22,759 | ||||||
March 25, 2014 | 26,250 | $ | 3.30 | $ | 3.30 | $ | 68,276 | ||||||
7 – Investments
Our available-for-sale securities consist of two mutual funds and are summarized in the following table:
| | Cost | Gross Unrealized Gains | Gross Unrealized Losses | Market Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
March 31, 2014 (unaudited) | $ | 246,018 | $ | 4,769 | $ | — | $ | 250,787 | |||||
December 31, 2013 | $ | 244,782 | $ | 1,421 | $ | — | $ | 246,203 | |||||
December 31, 2012 | $ | 239,796 | $ | 10,998 | $ | — | $ | 250,794 | |||||
8 – Fair Value Measurements
The fair value of our assets and liabilities subject to recurring fair value measurements are as follows:
| | Fair Value at March 31, 2014 (unaudited) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Fair Value | Quoted Prices in Active Market for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||
Mutual funds | $ | 250,787 | $ | 250,787 | $ | — | $ | — | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | Fair Value at December 31, 2013 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Fair Value | Quoted Prices in Active Market for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||
Mutual funds | $ | 246,203 | $ | 246,203 | $ | — | $ | — | |||||
| | | | | | | | | | �� | | | | |
| | | | | | | | | | | | | | |
F-20
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
| | Fair Value at December 31, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Fair Value | Quoted Prices in Active Market for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||
Mutual funds | $ | 250,794 | $ | 250,794 | $ | — | $ | — | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The fair values of our mutual funds are based upon quoted market prices and valuations provided by the third-party custodian of our mutual funds.
There were no transfers into or out of any Levels during the three months ended March 31, 2014 (unaudited) or for the years ended December 31, 2013 or 2012.
9 – Accumulated Other Comprehensive Income
The only component of accumulated other comprehensive income is as follows:
| | Unrealized Gains (Losses) on Available-For-Sale Securities | |||
|---|---|---|---|---|
Balances at December 31, 2011 | $ | 4,065 | ||
Gain (loss), net | 3,084 | |||
Reclassification realized in net earnings | — | |||
| | | | | |
Balances at December 31, 2012 | $ | 7,149 | ||
| | | | | |
Gain (loss), net | (6,225 | ) | ||
Reclassification realized in net earnings | — | |||
| | | | | |
Balances at December 31, 2013 | $ | 924 | ||
| | | | | |
| | | | | |
10 – Income Taxes
The components of the provision for income taxes are as follows:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Current taxes: | |||||||||||||
U.S. federal | $ | 316,971 | $ | 263,916 | $ | 764,452 | $ | 300,052 | |||||
State | 49,444 | 29,739 | 130,784 | 87,236 | |||||||||
| | | | | | | | | | | | | | |
Total current tax expense | 366,415 | 293,655 | 895,236 | 387,288 | |||||||||
Deferred taxes: | |||||||||||||
U.S. federal | (52,255 | ) | (3,569 | ) | (14,278 | ) | 91,242 | ||||||
State | (9,992 | ) | (8,520 | ) | (34,080 | ) | (12,550 | ) | |||||
| | | | | | | | | | | | | | |
Total deferred tax benefit | (62,247 | ) | (12,089 | ) | (48,358 | ) | 78,692 | ||||||
| | | | | | | | | | | | | | |
Income tax expense | $ | 304,168 | $ | 281,566 | $ | 846,878 | $ | 465,980 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-21
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred taxes are as follows:
| | As of March 31, | As of December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Deferred tax assets: | |||||||||||||
Current deferred tax assets: | |||||||||||||
Reserves and allowances | $ | 109,652 | $ | 104,801 | $ | 109,177 | $ | 103,341 | |||||
Other | — | 1,101 | — | 1,469 | |||||||||
| | | | | | | | | | | | | | |
Total current deferred tax assets | $ | 109,652 | $ | 105,902 | $ | 109,177 | $ | 104,810 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Noncurrent deferred tax assets: | |||||||||||||
Stock compensation | 214,555 | 80,028 | 152,906 | 55,736 | |||||||||
Research and development credits | — | 120,345 | — | 160,460 | |||||||||
| | | | | | | | | | | | | | |
Total noncurrent deferred tax assets | $ | 214,555 | $ | 200,373 | $ | 152,906 | $ | 216,196 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Deferred tax liabilities: | |||||||||||||
Current deferred tax liabilities: | |||||||||||||
Reserves and allowances | 42,674 | 125,734 | 42,674 | 153,421 | |||||||||
Other | 542 | 3,279 | 542 | 4,192 | |||||||||
| | | | | | | | | | | | | | |
Total current deferred tax liabilities | $ | 43,216 | $ | 129,013 | $ | 43,216 | $ | 157,613 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Noncurrent deferred tax liabilities | |||||||||||||
Depreciation and amortization | 208,040 | 204,499 | 206,993 | 203,668 | |||||||||
| | | | | | | | | | | | | | |
Total noncurrent deferred tax liabilities | $ | 208,040 | $ | 204,499 | $ | 206,993 | $ | 203,668 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
A reconciliation of the statutory U.S. federal tax rate to our effective rate is as follows:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
Statutory U.S. federal tax rate | 34.0 | % | 34.0 | % | 34.0 | % | 34.0 | % | |||||
Domestic production activities deduction | (2.4 | ) | (3.7 | ) | (3.7 | ) | (3.7 | ) | |||||
Research and development credits | — | (2.8 | ) | (2.8 | ) | (2.5 | ) | ||||||
State taxes, net of federal benefit | 4.8 | 2.3 | 2.3 | 3.4 | |||||||||
Permanent items | 0.4 | 0.6 | 0.6 | 1.3 | |||||||||
| | | | | | | | | | | | | | |
Total | 36.8 | % | 30.4 | % | 30.4 | % | 32.5 | % | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
As of March 31, 2014 (unaudited), December 31, 2013 and December 31, 2012, we have not identified or accrued for any uncertain tax positions. We are currently unaware of any uncertain tax positions that could result in significant payments, accruals or other material deviations in this estimate over the next 12 months.
We file tax returns in the United States Federal jurisdiction and many state jurisdictions. Our returns are not currently under examination by the Internal Revenue Service or other taxing
F-22
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
authorities. The Company is subject to income tax examinations for our United States federal and State income taxes for 2008 and subsequent years.
11 – Employee Benefit Plan
We sponsor a 401(k) tax-deferred savings plan under which eligible employees may elect to have a portion of their salary deferred and contributed to the plan. Employer matching contributions are determined by management and are discretionary. Employer matching contributions were approximately $43,154 and $24,651, respectively, for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), and $98,623 and $65,086, respectively, for the years ended December 31, 2013 and 2012. Employer contributions vest ratably over five years.
12 – Segment, Customer and Geographic Information
We operate in one reportable segment which is the development, manufacture and sale of MRI compatible products and IV infusion pump systems for use by hospitals and acute care facilities during MRI procedures.
In the U.S., we sell our products through our direct sales force and outside of the U.S. we sell our products through distributors who resell our products to end users.
Revenue information by geographic region is as follows:
| | Three Months Ended March 31, | Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2014 | 2013 | 2013 | 2012 | |||||||||
| | (unaudited) | | | ||||||||||
United States | $ | 2,449,109 | $ | 2,032,815 | $ | 8,100,907 | $ | 5,821,106 | |||||
International | 1,108,128 | 595,454 | 3,239,190 | 1,863,955 | |||||||||
| | | | | | | | | | | | | | |
| $ | 3,557,237 | $ | 2,628,269 | $ | 11,340,097 | $ | 7,685,061 | ||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Property and equipment, net information by geographic region is as follows:
| | | As of December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of March 31, 2014 | |||||||||
| | 2013 | 2012 | ||||||||
| | (unaudited) | | | |||||||
United States | $ | 245,430 | $ | 256,386 | $ | 207,290 | ||||
International | 74,991 | 70,957 | 58,327 | |||||||
| | | | | | | | | | | |
| $ | 320,421 | $ | 327,343 | $ | 265,617 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
During the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), respectively, revenue from devices was $3,020,389 and $2,200,715, while revenue from disposable IV sets and services were $536,848 and $427,554. During the years ended December 31, 2013 and 2012, respectively, revenue from devices was $9,335,753 and $6,009,640, while revenue from disposable IV sets and services were $2,004,344 and $1,675,421. Revenue for 2013 included two non-recurring sales to a customer in Saudi Arabia for 129 of our MRI compatible IV infusion pumps. Revenue recorded in 2013 related to these orders represented 10.7% of total revenue for 2013.
F-23
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
Long-lived assets held outside of the United States consist principally of tooling, which is a component of property and equipment, net.
13 – Commitments and Contingencies
Leases. We have entered into noncancelable operating leases for our facilities and office equipment. Future minimum lease payments under noncancelable operating leases as of December 31, 2013 are approximately $60,000. Minimum lease payments under noncancelable operating leases after 2014 are not material to our financial statements.
Rent expense, which is recorded on the straight-line method from commencement over the period of the lease, totaled $30,770 and $28,771 for the three months ended March 31, 2014 (unaudited) and 2013 (unaudited), respectively. Rent expense for the years ended December 31, 2013 and 2012 was $116,277 and $115,440, respectively. Minimum lease payments for each of our operating leases are even throughout their respective lease term.
In January 2014, we entered into a lease, commencing July 1, 2014, for a new facility in Winter Springs, Florida owned by Susi, LLC, and entity controlled by our president and CEO, Roger Susi. Pursuant to the terms of our lease for this property, the monthly base rent will be $32,583, adjusted annually for changes in the consumer price index. The term of the lease expires on May 31, 2019. The lease will automatically renew for two successive terms of five years each beginning in 2019 and again in 2024, and thereafter, will be renewed for successive terms of one year each.
Purchase commitments. We had various purchase orders for goods or services totaling approximately $1,529,710 at March 31, 2014 (unaudited) and $1,758,242 at December 31, 2013. No amounts related to these purchase orders have been recognized in our balance sheet.
Uncommitted Revolving Credit Facility. We have an uncommitted revolving credit facility with Bank of America, National Association ("Bank of America") that provided for a maximum borrowing capacity of $100,000 throughout 2013. Advances on this credit facility can be in the form of variable rate advances, fixed rate advances, or term advances. Interest rates on variable interest rate advances would reset weekly at the then applicable LIBOR rate plus a spread, which is determined at the time of the advance. Finance charges on variable rate advances would accrue monthly and are due on the earlier of demand of the first business day of the succeeding month. Fixed rate advances would bear finance charges at a fixed rate of interest established at the time of the advance plus a spread for a period up to 12 months. Each fixed rate advance, together with all accrued finance charges, fees and other charges, would be due upon the earlier of demand or the last day of the fixed rate period. Term advances would bear finance charges at a fixed rate of interest established at the time of the advance plus a spread for a term greater than 12 months. Each term rate advance, together with all accrued finance charges, fees and other charges, would be due upon the earlier of demand or the last day of the term period. An early repayment penalty applies to fixed rate and term rate advances. The early repayment penalty is equal to the amount sufficient to compensate Bank of America for any loss, cost or expense incurred resulting from the early repayment, including any loss of anticipated profits and any loss or expense arising from the liquidation or reemployment of funds obtained by Bank of America for purposes of these advances.
As security for any advances on this credit facility, we agreed to assign, transfer, pledge, grant and convey to Bank of America a continuing, first priority lien and security interest in all right, title and interest in the following property, whether owned now or hereafter acquired, in: (i) all of our securities;
F-24
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
(ii) monies, debts, claims, securities, securities entitlements, financial assets, investment property, instruments, certificates of deposit, general intangibles of any sort now or hereafter held or; (iii) proceeds from the sale of the foregoing. We are also required to maintain financial assets in our securities account that have a value at least equal to the amount required by Bank of America from time to time.
Events of default are typical for this type of instrument. Upon an event of default, Bank of America could perform any and all of the following: demand immediate payment of all amounts due; demand that we provide additional collateral; suspend or terminate our ability to obtain additional advances; liquidate securities held in our securities account; and increase the spread on any advance by 600 basis points.
During the years ended December 31, 2013 and 2012, we did not request or obtain any advances from this revolving credit facility.
Indemnifications. Under our amended and restated bylaws, we have agreed to indemnify our officers and directors for certain events or occurrences arising as a result of the officer or director serving in such capacity. We have a director and officer liability insurance policy that limits our exposure under these indemnifications and enables us to recover a portion of any future loss arising out of them.
In addition, in the normal course of business, we enter into contracts that contain indemnification clauses whereby the Company indemnifies our customers against damages associated with product failures. We have determined that these agreements fall within the scope of ASC 460,Guarantees. We have obtained liability insurance providing coverage that limits our exposure for these indemnified matters. We have not incurred costs to defend lawsuits or settle claims related to these indemnities. We believe the estimated fair value of these indemnities is minimal and have not recorded a liability for these agreements as of December 31, 2013.
Legal matters. We may from time to time become a party to various legal proceedings or claims that arise in the ordinary course of business. We do not believe that any current legal or administrative proceedings are likely to have a material effect on our business, financial condition, or results of operations.
In October 2012, Radimed Gesellschaft für Kommunikationsdienstleistungen und Medizintechnik mbH ("Radimed") brought an action in Düsseldorf Regional Court against our German distributor alleging the name and sign "iRadimed" was confusingly similar to their German trademark "Radimed." A judgment was rendered against our German distributor preventing use of the name and sign "iRadimed" in Germany. We have however continued to sell products in Germany without any discernible effect by using the name IRI Development. On July 31, 2013, Radimed filed a lawsuit against us and our founder, Roger Susi, in Düsseldorf Regional Court, alleging that we infringed their German and Community trademarks "Radimed" and seeking to prevent our use of the name, sign and domain name "iRadimed" in the European Union. In addition, Radimed is seeking unspecified damages. We have not accrued for any loss related to this matter as we believe that any such loss is not probable or estimable.
F-25
iRadimed Corporation
NOTES TO FINANCIAL STATEMENTS
14 – Officer Note Payable
In the early stages of the Company, our CEO provided funding for operations in the form of an unsecured interest-free note payable with no specified due date. As of December 31, 2013 and 2012, $6,333 and $519,730 remained outstanding. In March 2014, subsequent to our 2013 year end, we repaid with cash the outstanding balance of the note payable.
15 – Subsequent Events
Management has assessed subsequent events through June 18, 2014, which is the date these financial statements were available to be issued.
In April 2014, our Board of Directors adopted and our shareholders approved the 2014 Equity Incentive Plan ("2014 Plan"). The 2014 Plan reserves 1,000,000 shares of our common stock for awards of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards and other stock-based awards and cash awards.
F-26
![]()
1,750,000 Shares of Common Stock
P R O S P E C T U S
Sole Book-Running Manager
Roth Capital Partners
Co-Manager
Monarch Capital Group
, 2014
Through and including , 2014, (the 25th day after the date of this prospectus), all dealers effecting transactions in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this Registration Statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the SEC registration fee and the FINRA filing fee.
| | Amount | |||
|---|---|---|---|---|
SEC Filing Fee | $ | 1,649 | ||
FINRA Filing Fee | 2,195 | |||
The NASDAQ Capital Market Listing Fee | 50,000 | |||
Accounting Fees and Expenses | 150,000 | |||
Legal Fees and Expenses | 500,000 | |||
Transfer Agent and Registrar Fees and Expenses | 2,000 | |||
Printing Expenses | 60,000 | |||
Miscellaneous Expenses | 34,156 | |||
| | | | | |
| $ | 800,000 | |||
| | | | | |
| | | | | |
Item 14. Indemnification of Directors and Officers.
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except for breaches of the director's duty of loyalty to the corporation or its stockholders, acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of a law, authorizations of the payments of a dividend or approval of a stock repurchase or redemption in violation of Delaware corporate law or for any transactions from which the director derived an improper personal benefit. Our certificate of incorporation provides that no director will be liable to us or our stockholders for monetary damages for breach of fiduciary duties as a director, subject to the same exceptions as described above. We also expect to maintain standard insurance policies that provide coverage (1) to our directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments we may make to such officers and directors.
Section 145 of the General Corporation Law of the State of Delaware provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation and certain other persons serving at the request of the corporation in related capacities against expenses (including attorneys' fees), judgments, fines, and amounts paid in settlements actually and reasonably incurred by the person in connection with a threatened, pending, or completed action, suit or proceeding to which he or she is or is threatened to be made a party by reason of such position, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, indemnification is limited to expenses (including attorneys' fees) actually and reasonably incurred by the person in connection with defense or settlement of such action or suit and no indemnification shall be made with respect to any claim, issue, or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper. In addition, to the extent
II-1
that a present or former director or officer of a corporation has been successful on the merits or otherwise in defense of any action, suit, or proceeding described above (or claim, issue, or matter therein), such person shall be indemnified against expenses (including attorneys' fees) actually and reasonably incurred by such person in connection therewith. Expenses (including attorneys' fees) incurred by an officer or director in defending any civil, criminal, administrative, or investigative action, suit, or proceeding may be advanced by the corporation upon receipt of an undertaking by such person to repay such amount if it is ultimately determined that such person is not entitled to indemnification by the corporation under Section 145 of the General Corporation Law of the State of Delaware.
Our certificate of incorporation provides that we will, to the fullest extent permitted by law, indemnify any person made or threatened to be made a party to an action or proceeding by reason of the fact that he or she (or his or her testators or intestate) is or was our director or officer or serves or served at any other corporation, partnership, joint venture, trust or other enterprise in a similar capacity or as an employee or agent at our request, including service with respect to employee benefit plans maintained or sponsored by us, against expenses (including attorneys'), judgments, fines, penalties, and amounts paid in settlement incurred in connection with the investigation, preparation to defend, or defense of such action, suit, proceeding, or claim. However, we are not required to indemnify or advance expenses in connection with any action, suit, proceeding, claim, or counterclaim initiated by us or on behalf of us. Our amended and restated bylaws provide that we will indemnify and hold harmless each person who was or is a party or threatened to be made a party to any action, suit, or proceeding by reason of the fact that he or she is or was our director or officer, or is or was serving at our request in a similar capacity of another corporation, partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans (whether the basis of such action, suit, or proceeding is an action in an official capacity as a director or officer or in any other capacity while serving as a director of officer) to the fullest extent authorized by the Delaware General Corporation Law against all expense, liability and loss (including attorney's fees, judgments, fines, ERISA excise taxes, or penalties and amounts paid in settlement) reasonably incurred or suffered by such person in connection with such action, suit, or proceeding, and this indemnification continues after such person has ceased to be an officer or director and inures to the benefit of such person's heirs, executors, and administrators. The indemnification rights also include the right generally to be advanced expenses, subject to any undertaking required under Delaware General Corporation Law, and the right generally to recover expenses to enforce an indemnification claim or to defend specified suits with respect to advances of indemnification expenses.
Item 15. Recent Sales of Unregistered Securities.
From January 1, 2011 through March 31, 2014, the Registrant has granted stock options under the 2005 Incentive Stock Plan, as amended to directors, officers, and employees to purchase an aggregate of 1,421,942 shares of common stock, giving effect to the 1.75:1 conversion ratio effected in the reincorporation merger, with exercise prices ranging from $0.93 per share to $3.30 per share, with a weighted average exercise price of $1.25 per share. The issuances were made pursuant to the Registrant's 2005 Incentive Stock Plan in reliance on the exemption provided by Rule 701 promulgated under the Securities Act or in reliance on Section 4(a)(2) (formerly Section 4(2)) promulgated under the Securities Act as transactions by an issuer not involving a public offering.
II-2
Item 16. Exhibits and Financial Statement Schedules.
- (a)
- Exhibits
See Exhibit Index immediately following the signature page to this registration statement.
All other schedules are omitted because they are not required, are not applicable, or the information is included in the financial statements or the related notes to financial statements thereto.
- (a)
- The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(5) (ii) That, for the purpose of determining liability under the Securities Act to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-3
(6) For the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(f) The undersigned registrant hereby undertakes to provide to the representative of the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(i) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Winter Springs, State of Florida on this 9th day of July, 2014.
| IRADIMED CORPORATION | ||||
By: | /s/ ROGER SUSI Roger Susi Chief Executive Officer and President | |||
We, the undersigned directors and officers of iRadimed Corporation, hereby severally constitute and appoint Roger Susi and Chris Scott, and each of them, our true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement, and any subsequent registration statements pursuant to Rule 462 of the Securities Act of 1933, as amended and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as we might or could do in person, hereby ratifying and confirming all that each of said attorney-in-fact or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||||
|---|---|---|---|---|---|---|
| /s/ ROGER SUSI Roger Susi | Chief Executive Officer, President, and Director (principal executive officer) | July 9, 2014 | ||||
/s/ CHRIS SCOTT Chris Scott | Chief Financial Officer and Secretary (principal financial and accounting officer) | July 9, 2014 | ||||
* James Hawkins | Chairman of the Board | July 9, 2014 | ||||
II-5
Signature | Title | Date | ||||
|---|---|---|---|---|---|---|
* Serge Novovich | Director | July 9, 2014 | ||||
| * Monty Allen | Director | July 9, 2014 | ||||
*By: | /s/ ROGER SUSI Roger Susi Attorney-in-fact | |||||
II-6
| Exhibit No. | Description | ||
|---|---|---|---|
| 1.1 | * | Underwriting Agreement | |
| 3.1 | (1) | Certificate of Incorporation | |
| 3.2 | (1) | Amended and Restated Bylaws | |
| 4.1 | Specimen common stock certificate | ||
| 4.2 | * | Form of Underwriter Compensation Warrant Agreement | |
| 4.3 | (1) | Investors' Rights Agreement dated February 26, 2005 | |
| 5.1 | * | Opinion of K&L Gates LLP | |
| 10.1 | + | iRadimed Corporation 2005 Incentive Stock Plan adopted February 1, 2005 | |
| 10.2 | + | Form of Stock Option Agreement for iRadimed Corp. 2005 Incentive Stock Plan | |
| 10.3 | + | Iradimed Corporation 2014 Equity Incentive Plan | |
| 10.4 | + | Form of Stock Option Agreement for iRadimed Corporation 2014 Equity Incentive Plan | |
| 10.5 | (1) | Lease Agreement regarding 7457 Aloma Avenue dated April 12, 2011 between Roberts Supply Profit Sharing, LLC and the Registrant | |
| 10.6 | (1) | Amendment to Lease Agreement regarding 7457 Aloma Avenue dated September 16, 2013 between Roberts Supply Profit Sharing, LLC and the Registrant | |
| 10.7 | (1) | Lease Agreement regarding 1025 Willa Springs Dr. dated January 17, 2014 between Susi, LLC and the Registrant | |
| 10.8 | (1)+ | Employment Agreement between the Registrant and Christopher K. Scott dated December 16, 2013 | |
| 10.9 | (1)+ | Employment Agreement between the Registrant and Roger Susi dated April 14, 2014 | |
| 10.10 | (1)+ | Employment Agreement between the Registrant and Brent Johnson dated December 7, 2011 | |
| 10.11 | (1)† | Supply Agreement between the Registrant and Fukoku Co., Ltd. dated January 26, 2014 | |
| 10.12 | (1) | Patent Assignment Agreement by Roger E. Susi to the Registrant dated June 26, 2006 | |
| 10.13 | (1) | Patent Assignment Agreement by Roger E. Susi to the Registrant dated June 26, 2006 | |
| 10.14 | (1) | Patent Assignment Agreement by Roger E. Susi to the Registrant dated June 26, 2006 | |
| 10.15 | (1) | Patent Assignment Agreement by Roger E. Susi to the Registrant dated October 29, 2007 | |
| 10.16 | (1) | Patent Assignment Agreement by Roger E. Susi to the Registrant dated August 28, 2008 | |
| 10.17 | (1) | Patent Assignment Agreement by Roger E. Susi and David Hefele to the Registrant dated December 3, 2008 | |
| 10.18 | (1) | Patent Assignment Agreement by Roger E. Susi to the Registrant dated December 15, 2009 | |
| 23.1 | Consent of McGladrey LLP, Independent Registered Public Accounting Firm | ||
| 23.2 | * | Consent of K&L Gates, LLP (included in Exhibit 5.1) | |
II-7
| Exhibit No. | Description | ||
|---|---|---|---|
| 24.1 | (1) | Power of Attorney (included in the signature page to this registration statement) | |
- *
- To be filed by amendment.
- +
- Indicates a management contract or a compensatory plan.
- †
- Portions of this exhibit have been omitted pending a determination by the Securities and Exchange Commission as to whether these portions should be granted confidential treatment.
- (1)
- Previously filed.
II-8