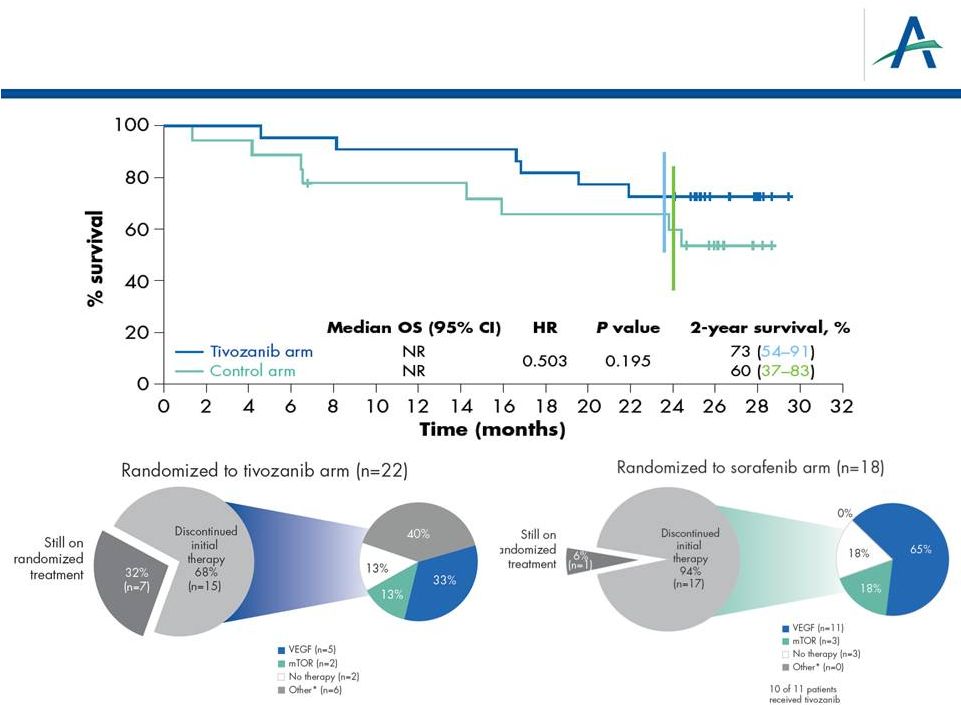
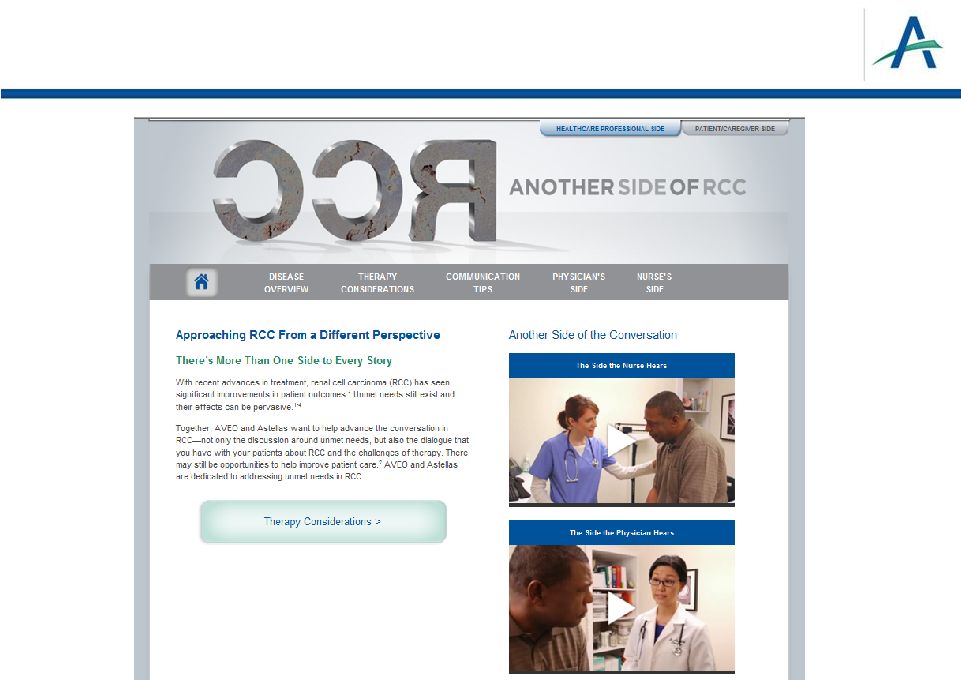
Forward-Looking Statements 2 This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: the planned development, commercialization and manufacturing plans, timelines and strategies for tivozanib; the potential therapeutic advantages and benefits of tivozanib; the timing and results of our ongoing and planned preclinical studies and clinical trials; the potential benefits of our strategic partnership agreements, including our agreement with Astellas; our plans to leverage our Human Response Platform™ to inform clinical development; our intellectual property position and strategies; the expected RCC market and potential of tivozanib to obtain regulatory approval and enter this market; our anticipated plans for success in the oncology markets; the anticipated dates for the FDA’s ODAC meeting to review our NDA for tivozanib and the completion of the FDA’s review of the NDA; and AVEO having sufficient capital to fund its operations into the second quarter of 2014 and our estimates for 2013 expected year end cash balance. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make due to a number of important factors, including risks and uncertainties inherent in pharmaceutical research and development, such as those related to: our ability to successfully develop, test and gain approval of our product candidates, including regulatory approval of tivozanib to treat advanced renal cell cancer; potential delays in the initiation of other clinical trials of tivozanib; our ability to obtain, maintain and enforce intellectual property rights; competition; our dependence on our alliance partners and other third parties; our ability to obtain necessary financing; adverse economic conditions; and other risk factors discussed in the “Risk Factors” and elsewhere in our Current Report on Form 8-K that was filed with the Securities and Exchange Commission (“SEC”) on January 16, 2013, and other periodic filings we make with the SEC. All forward-looking statements contained in this presentation speak only as of the date hereof, and we undertake no obligation to update any of these statements, except as required by law. |