
January 2016 Corporate Overview Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: AVEO’s plans to leverage biomarkers and pursue strategic partnerships for certain of its assets; AVEO’s goals and business strategy; the timing, design and results of preclinical and clinical trials; the timing and outcome of meetings with and applications to regulatory authorities by AVEO and its partners; the competitive landscape for AVEO’s therapeutic candidates; AVEO’s ability to demonstrate tivozanib’s safety and efficacy as a combination therapy and AVEO’s estimates for its cash runway. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements AVEO makes due to a number of important factors, including substantial risks and uncertainties relating to: AVEO’s ability to successfully implement its strategic plans; AVEO’s ability to successfully develop, test and gain regulatory approval of its product candidates, including its companion diagnostics; developments, expenses and outcomes related to AVEO’s ongoing shareholder litigation and SEC investigation, including the risk that the Company may be required to incur substantial costs to settle such matters; AVEO’s ability to obtain necessary financing required to perform its clinical trials and achieve its other goals; AVEO’s ability to establish and maintain strategic partnerships; AVEO’s ability to obtain and maintain intellectual property rights; competition; AVEO’s dependence on its strategic partners and other third parties; adverse general economic and industry conditions; and those risk factors discussed in the “Risk Factors” and elsewhere in AVEO’s Annual Report on Form 10-K for the year ended December 31, 2014, and other periodic filings AVEO makes with the SEC. All forward-looking statements contained in this presentation speak only as of the date of this presentation, and AVEO undertakes no obligation to update any of these statements, except as required by law.

AVEO Oncology Highlights Lead Asset: Tivozanib Core focus: retain North American tivozanib rights >$1Bn U.S. RCC market Partner Ex-N.A. (EUSA, Pharmstandard) and for ocular (Ophthotech) in 2014/15 Phase 3 TIVO-1 study demonstrated improved PFS in advanced RCC Study may support registration in Europe/Russia OS confounded by crossover; FDA requested additional study Phase 3 refractory RCC study may support 1st and 3rd line indications in U.S. Exploring immunotherapy combination in RCC and CRC biomarker Pipeline to be funded through partnerships Novartis with AV-380; Biodesix with ficlatuzumab; seeking partner for AV-203 Estimated $34M in cash and securities at year end 2015 Excludes $3.5M due from Novartis Potential for >$40M in additional partnership payments in next 18 months Total of >$850M in potential partnership payments Lean organization, experienced management team and Board
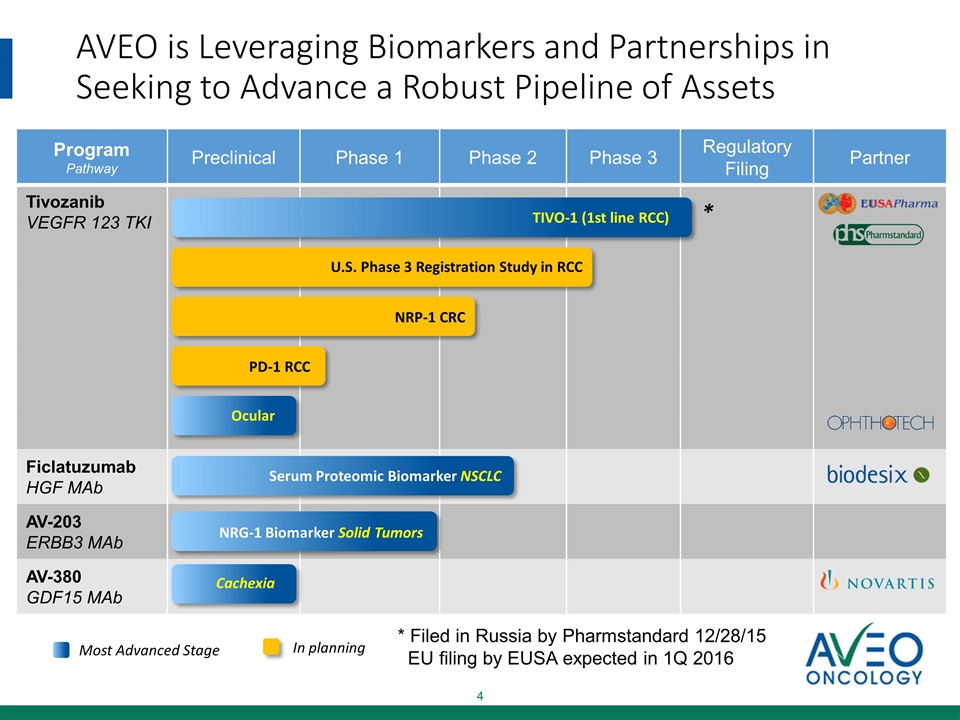
AVEO is Leveraging Biomarkers and Partnerships in Seeking to Advance a Robust Pipeline of Assets Program Pathway Preclinical Phase 1 Phase 2 Phase 3 Regulatory Filing Partner Tivozanib VEGFR 123 TKI Ficlatuzumab HGF MAb AV-203 ERBB3 MAb AV-380 GDF15 MAb Most Advanced Stage Cachexia NRG-1 Biomarker Solid Tumors Serum Proteomic Biomarker NSCLC Ocular In planning NRP-1 CRC PD-1 RCC TIVO-1 (1st line RCC) U.S. Phase 3 Registration Study in RCC * Filed in Russia by Pharmstandard 12/28/15 EU filing by EUSA expected in 1Q 2016 *
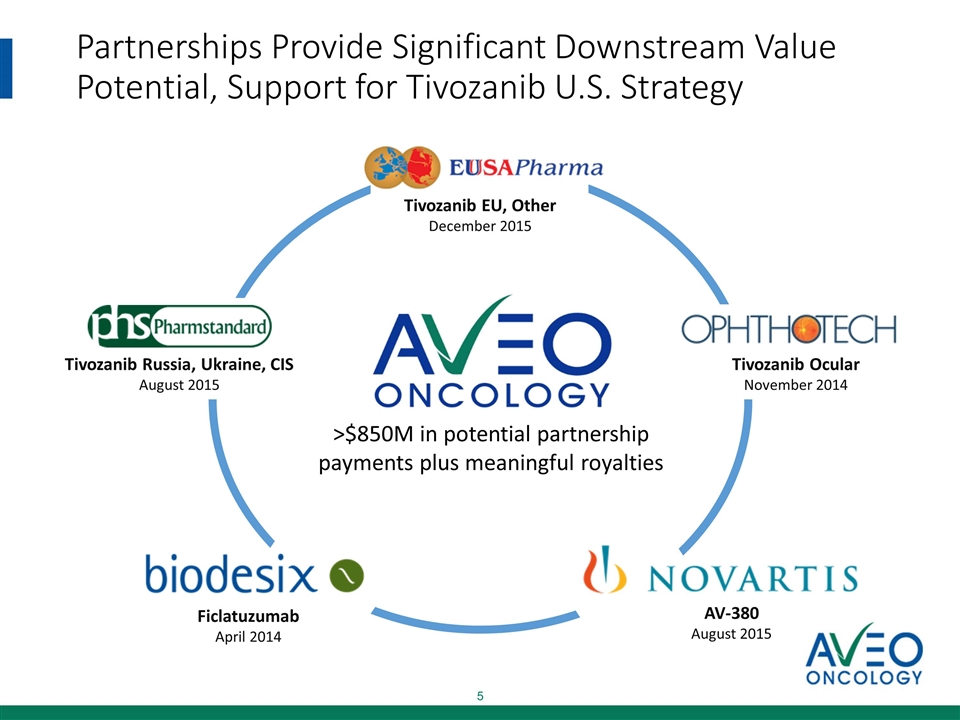
Partnerships Provide Significant Downstream Value Potential, Support for Tivozanib U.S. Strategy Tivozanib EU, Other December 2015 Tivozanib Ocular November 2014 Tivozanib Russia, Ukraine, CIS August 2015 AV-380 August 2015 Ficlatuzumab April 2014 >$850M in potential partnership payments plus meaningful royalties

Tivozanib A potent, selective, long half-life inhibitor of vascular endothelial growth factor (VEGF) 1, 2 and 3 receptors
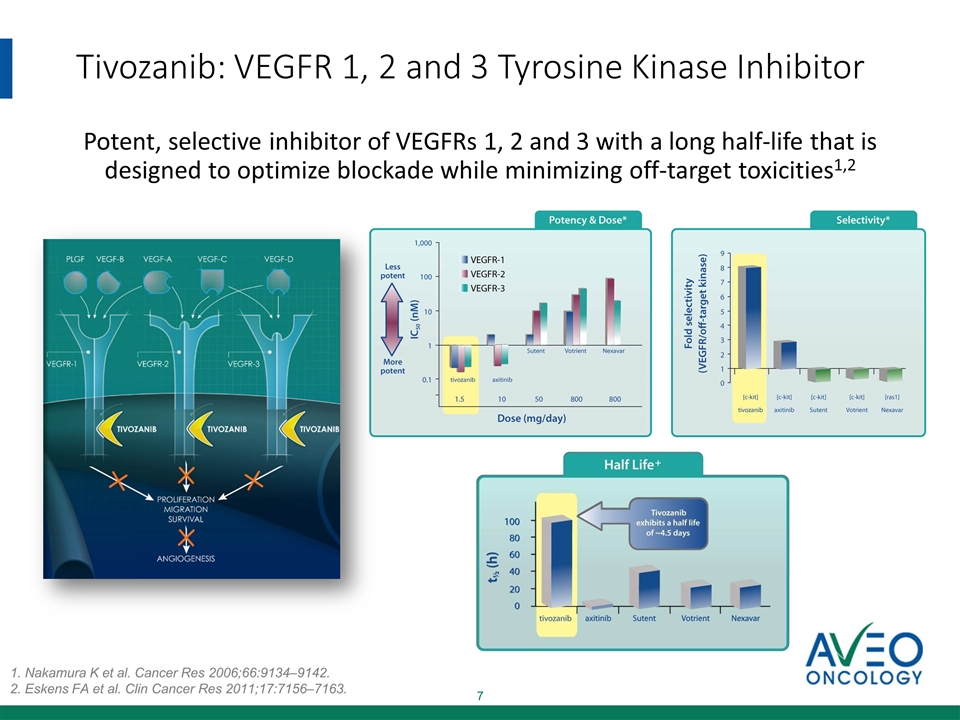
Tivozanib: VEGFR 1, 2 and 3 Tyrosine Kinase Inhibitor Potent, selective inhibitor of VEGFRs 1, 2 and 3 with a long half-life that is designed to optimize blockade while minimizing off-target toxicities1,2 1. Nakamura K et al. Cancer Res 2006;66:9134–9142. 2. Eskens FA et al. Clin Cancer Res 2011;17:7156–7163.
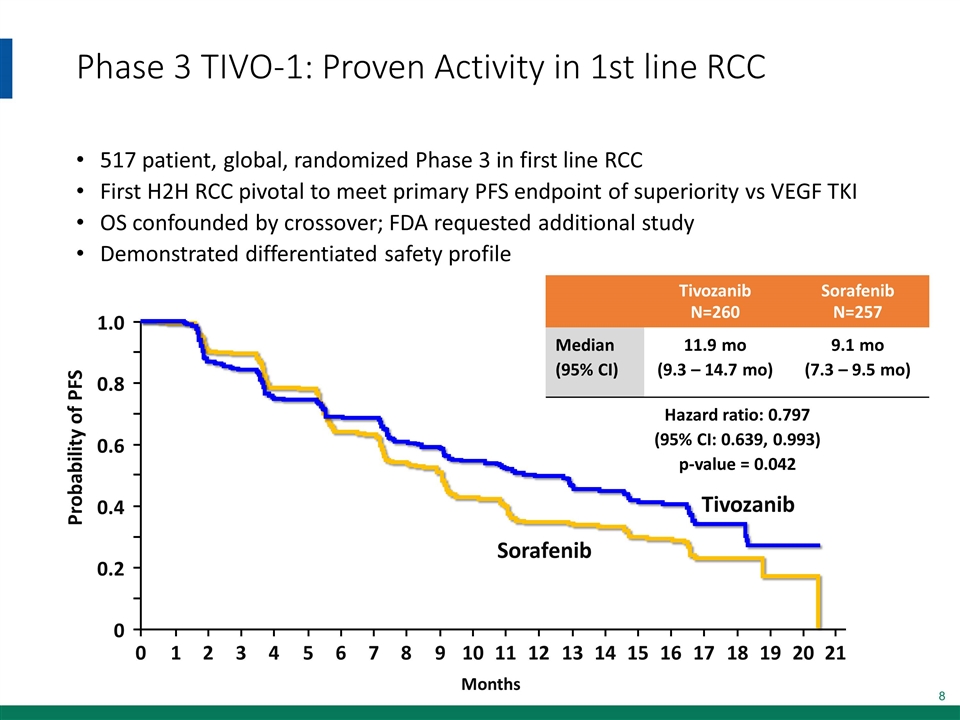
Phase 3 TIVO-1: Proven Activity in 1st line RCC 517 patient, global, randomized Phase 3 in first line RCC First H2H RCC pivotal to meet primary PFS endpoint of superiority vs VEGF TKI OS confounded by crossover; FDA requested additional study Demonstrated differentiated safety profile Months Probability of PFS 0 Sorafenib 1.0 0.8 0.6 0.4 0.2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Tivozanib Tivozanib N=260 Sorafenib N=257 Median (95% CI) 11.9 mo (9.3 – 14.7 mo) 9.1 mo (7.3 – 9.5 mo) Hazard ratio: 0.797 (95% CI: 0.639, 0.993) p-value = 0.042
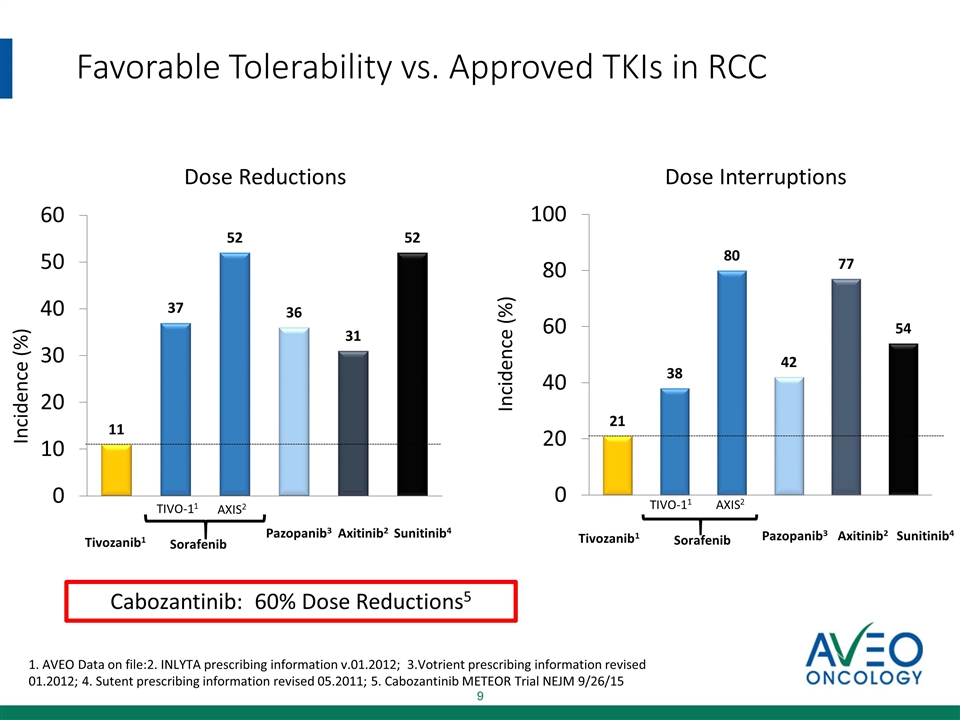
Favorable Tolerability vs. Approved TKIs in RCC Incidence (%) Incidence (%) Tivozanib1 Sorafenib Pazopanib3 Axitinib2 TIVO-11 AXIS2 Sunitinib4 Dose Reductions Dose Interruptions Tivozanib1 Sorafenib Pazopanib3 Axitinib2 TIVO-11 AXIS2 Sunitinib4 1. AVEO Data on file:2. INLYTA prescribing information v.01.2012; 3.Votrient prescribing information revised 01.2012; 4. Sutent prescribing information revised 05.2011; 5. Cabozantinib METEOR Trial NEJM 9/26/15 Cabozantinib: 60% Dose Reductions5
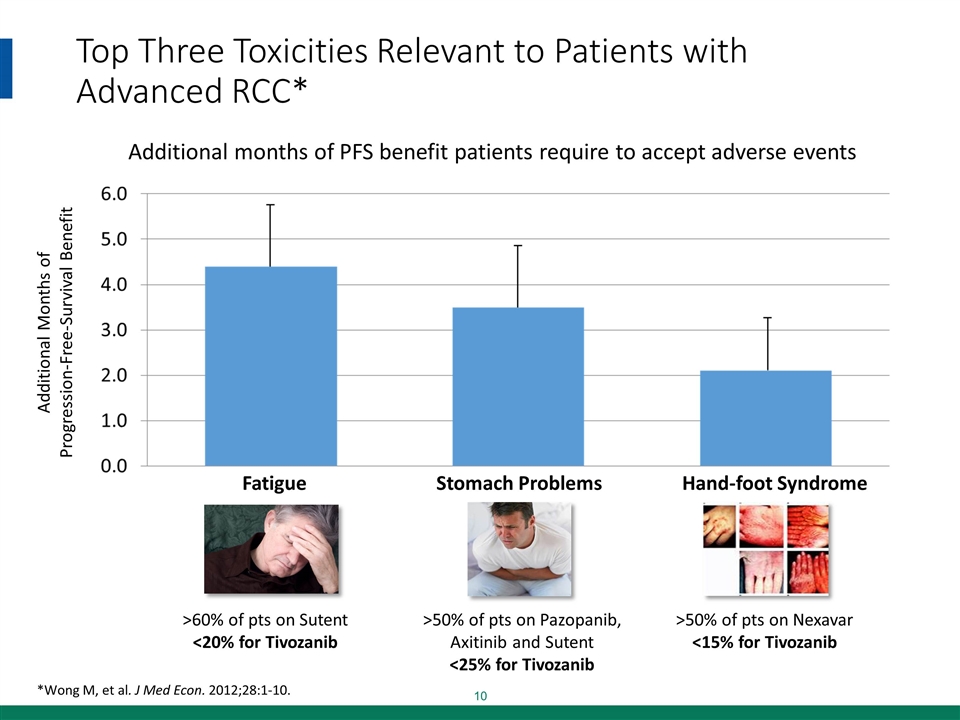
Top Three Toxicities Relevant to Patients with Advanced RCC* *Wong M, et al. J Med Econ. 2012;28:1-10. Additional months of PFS benefit patients require to accept adverse events >50% of pts on Nexavar <15% for Tivozanib >60% of pts on Sutent <20% for Tivozanib >50% of pts on Pazopanib, Axitinib and Sutent <25% for Tivozanib Additional Months of Progression-Free-Survival Benefit Fatigue Stomach Problems Hand-foot Syndrome
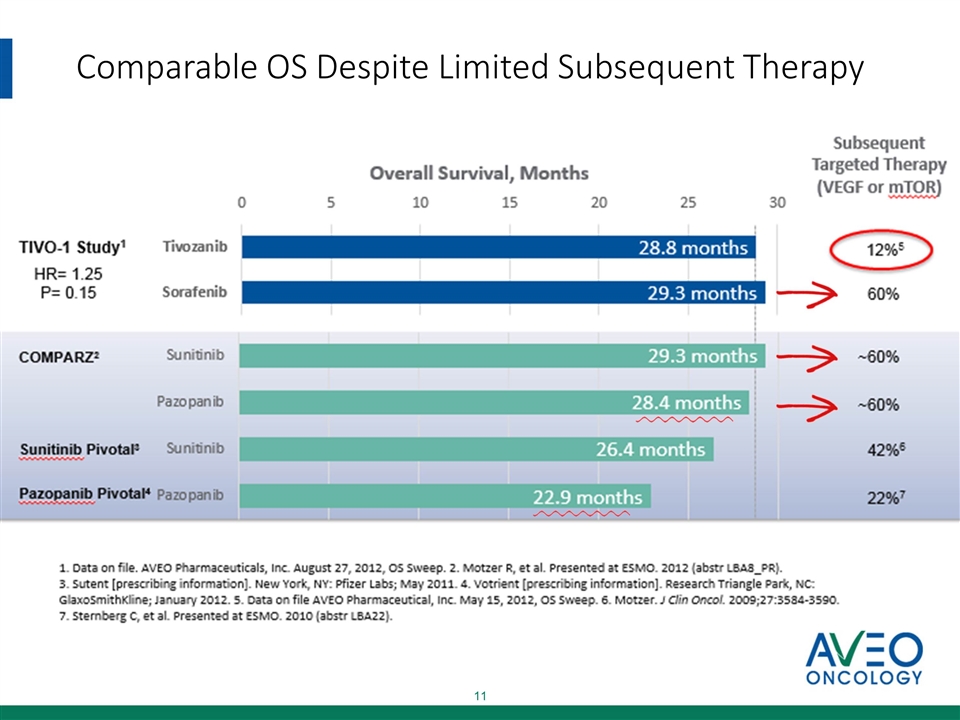
Comparable OS Despite Limited Subsequent Therapy

Tivozanib North American Registration Strategy in RCC
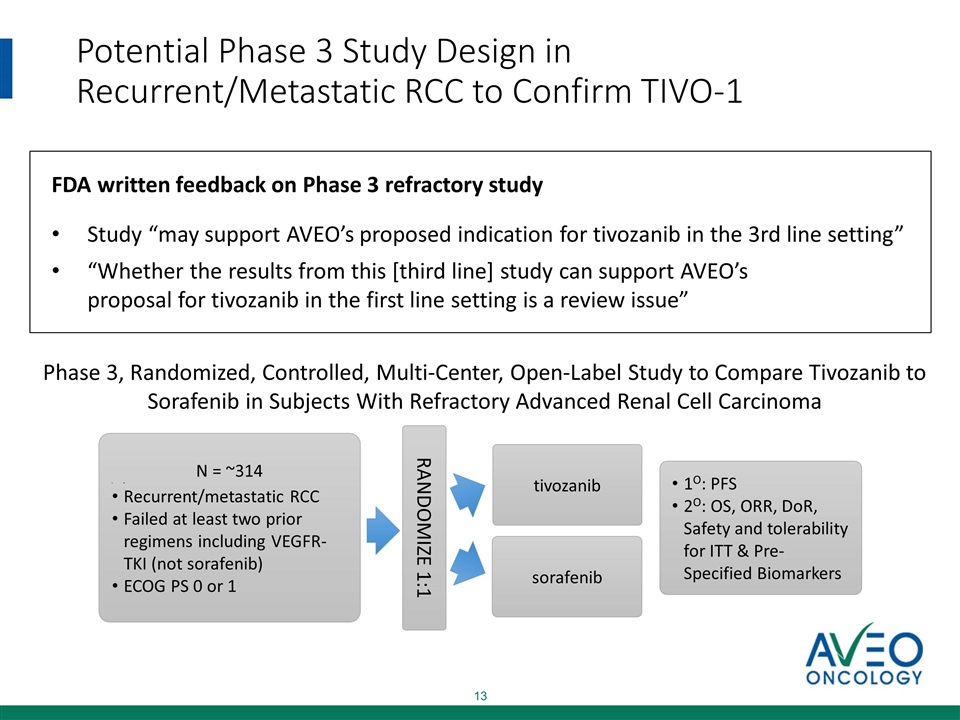
Potential Phase 3 Study Design in Recurrent/Metastatic RCC to Confirm TIVO-1 N = ~314 F Recurrent/metastatic RCC Failed at least two prior regimens including VEGFR-TKI (not sorafenib) ECOG PS 0 or 1 RANDOMIZE 1:1 tivozanib sorafenib 1O: PFS 2O: OS, ORR, DoR, Safety and tolerability for ITT & Pre-Specified Biomarkers Phase 3, Randomized, Controlled, Multi-Center, Open-Label Study to Compare Tivozanib to Sorafenib in Subjects With Refractory Advanced Renal Cell Carcinoma FDA written feedback on Phase 3 refractory study Study “may support AVEO’s proposed indication for tivozanib in the 3rd line setting” “Whether the results from this [third line] study can support AVEO’s proposal for tivozanib in the first line setting is a review issue”
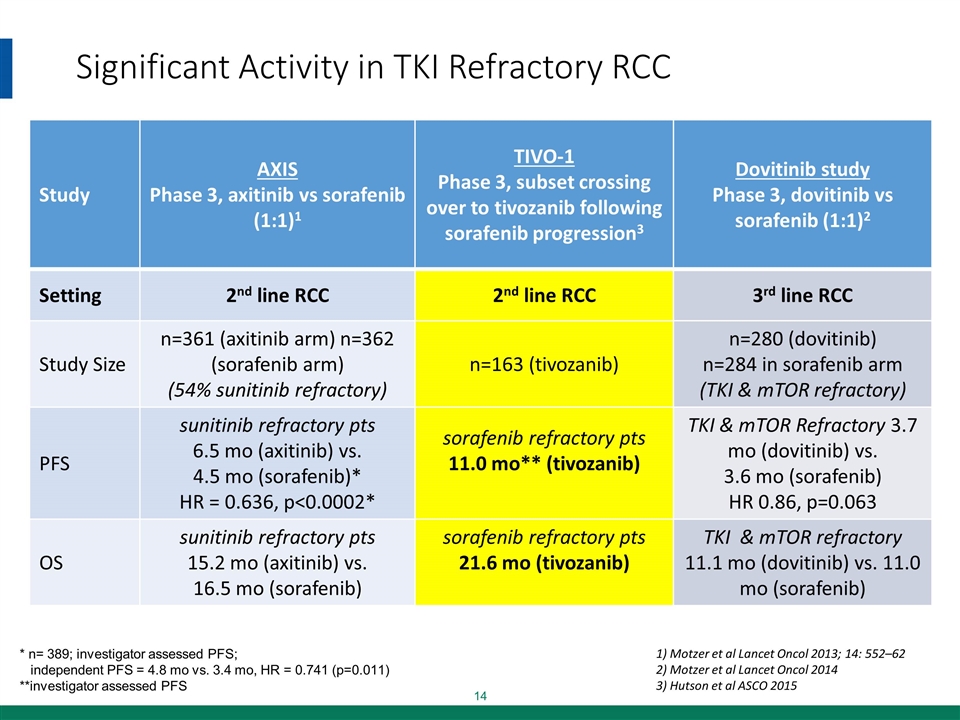
Significant Activity in TKI Refractory RCC Study AXIS Phase 3, axitinib vs sorafenib (1:1)1 TIVO-1 Phase 3, subset crossing over to tivozanib following sorafenib progression3 Dovitinib study Phase 3, dovitinib vs sorafenib (1:1)2 Setting 2nd line RCC 2nd line RCC 3rd line RCC Study Size n=361 (axitinib arm) n=362 (sorafenib arm) (54% sunitinib refractory) n=163 (tivozanib) n=280 (dovitinib) n=284 in sorafenib arm (TKI & mTOR refractory) PFS sunitinib refractory pts 6.5 mo (axitinib) vs. 4.5 mo (sorafenib)* HR = 0.636, p<0.0002* sorafenib refractory pts 11.0 mo** (tivozanib) TKI & mTOR Refractory 3.7 mo (dovitinib) vs. 3.6 mo (sorafenib) HR 0.86, p=0.063 OS sunitinib refractory pts 15.2 mo (axitinib) vs. 16.5 mo (sorafenib) sorafenib refractory pts 21.6 mo (tivozanib) TKI & mTOR refractory 11.1 mo (dovitinib) vs. 11.0 mo (sorafenib) * n= 389; investigator assessed PFS; independent PFS = 4.8 mo vs. 3.4 mo, HR = 0.741 (p=0.011) **investigator assessed PFS 1) Motzer et al Lancet Oncol 2013; 14: 552–62 2) Motzer et al Lancet Oncol 2014 3) Hutson et al ASCO 2015
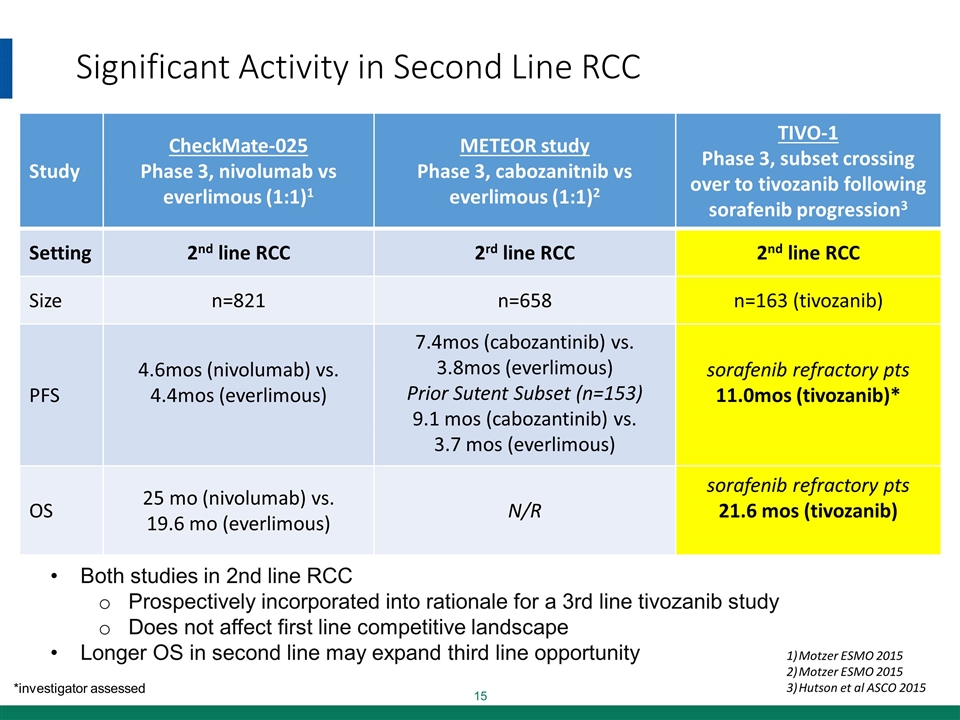
Significant Activity in Second Line RCC Study CheckMate-025 Phase 3, nivolumab vs everlimous (1:1)1 METEOR study Phase 3, cabozanitnib vs everlimous (1:1)2 TIVO-1 Phase 3, subset crossing over to tivozanib following sorafenib progression3 Setting 2nd line RCC 2rd line RCC 2nd line RCC Size n=821 n=658 n=163 (tivozanib) PFS 4.6mos (nivolumab) vs. 4.4mos (everlimous) 7.4mos (cabozantinib) vs. 3.8mos (everlimous) Prior Sutent Subset (n=153) 9.1 mos (cabozantinib) vs. 3.7 mos (everlimous) sorafenib refractory pts 11.0mos (tivozanib)* OS 25 mo (nivolumab) vs. 19.6 mo (everlimous) N/R sorafenib refractory pts 21.6 mos (tivozanib) *investigator assessed Motzer ESMO 2015 Motzer ESMO 2015 Hutson et al ASCO 2015 Both studies in 2nd line RCC Prospectively incorporated into rationale for a 3rd line tivozanib study Does not affect first line competitive landscape Longer OS in second line may expand third line opportunity
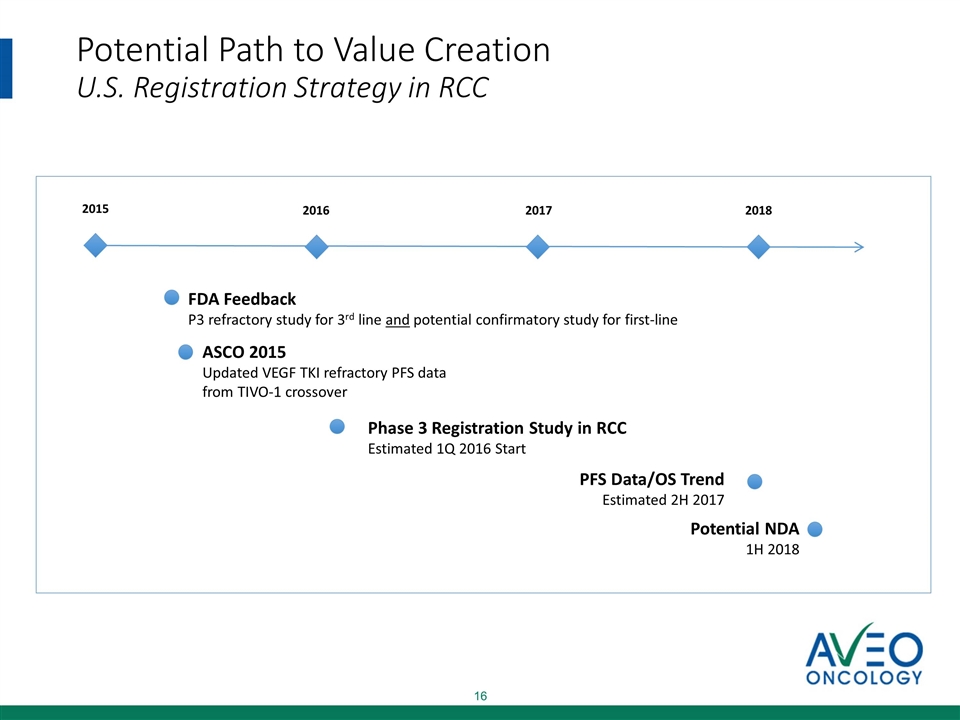
Potential Path to Value Creation U.S. Registration Strategy in RCC 2015 2016 ASCO 2015 Updated VEGF TKI refractory PFS data from TIVO-1 crossover FDA Feedback P3 refractory study for 3rd line and potential confirmatory study for first-line Phase 3 Registration Study in RCC Estimated 1Q 2016 Start 2017 Potential NDA 1H 2018 2018 PFS Data/OS Trend Estimated 2H 2017

Tivozanib EU Registration Opportunity for 1st Line RCC
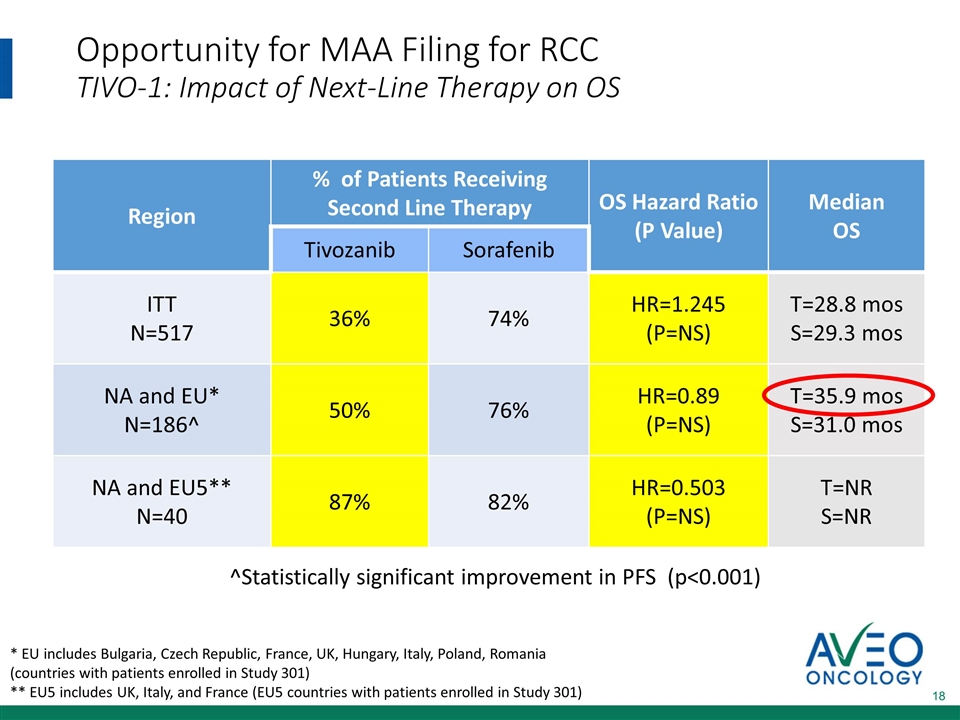
Opportunity for MAA Filing for RCC TIVO-1: Impact of Next-Line Therapy on OS * EU includes Bulgaria, Czech Republic, France, UK, Hungary, Italy, Poland, Romania (countries with patients enrolled in Study 301) ** EU5 includes UK, Italy, and France (EU5 countries with patients enrolled in Study 301) Region % of Patients Receiving Second Line Therapy OS Hazard Ratio (P Value) Median OS Tivozanib Sorafenib ITT N=517 36% 74% HR=1.245 (P=NS) T=28.8 mos S=29.3 mos NA and EU* N=186^ 50% 76% HR=0.89 (P=NS) T=35.9 mos S=31.0 mos NA and EU5** N=40 87% 82% HR=0.503 (P=NS) T=NR S=NR ^Statistically significant improvement in PFS (p<0.001)

Exclusive Licensing Agreement with EUSA: Dec. 2015 Opportunity to realize value for tivozanib outside of North America Agreement Exclusive License Territory EU and other* Upfront (R&D) $2.5M Clinical and Regulatory Milestones** $37M R&D Reimbursement $22M Sales Milestones $335M Royalties Tiered royalties on product sales ranging from low double digits to mid twenties R&D reimbursement, milestone and royalty payments subject to the successful development or commercialization of the product A portion of payments received by AVEO subject to sublicense revenue obligation to Kyowa Hakko Kirin, excluding R&D payments * Excludes North America, Asia, Russian, Ukraine and CIS ** Includes $2.0M per reimbursement approval in each EU5 country EU filing by EUSA expected in 1Q 2016

EUSA Pharma Formed through acquisition from Jazz Pharmaceuticals (2015) Five approved specialty hospital products Several named-patient specialty hospital products Multi-national commercial infrastructure EUSA Pharma brand name Headquartered in the UK, with wholly-owned subs in the US, Germany, France and Holland Experienced management team with a record of building successful specialty pharmaceutical companies Funded by leading life science investor Essex Woodlands

Exclusive Licensing Agreement with Pharmstandard in Russia, Ukraine and CIS: Aug. 2015 Agreement Exclusive license agreement Territory Russia, Ukraine and the Commonwealth of Independent States Upfront $1.5M* Marketing Authorization Milestone $7.5Mᶧ Additional Indication Milestones $3.0M Royalties High single-digit royalty on net sales Opportunity to realize value for tivozanib outside of North America Milestone and royalty payments subject to the successful development or commercialization of the product All payments received by AVEO subject to sublicense revenue obligation to Kyowa Hakko Kirin * $0.5M due upon registration with Rospatent ᶧ $3.0M if further study is needed prior to approval Russian Filing by Pharmstandard 12/28/15

Tivozanib Additional Development Opportunities
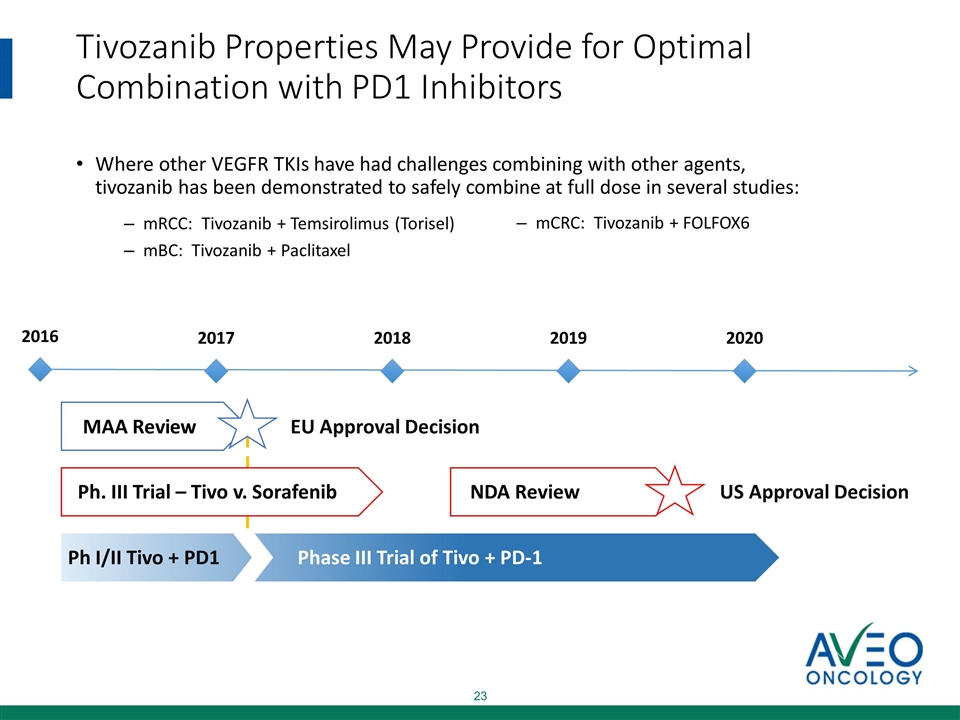
Tivozanib Properties May Provide for Optimal Combination with PD1 Inhibitors Where other VEGFR TKIs have had challenges combining with other agents, tivozanib has been demonstrated to safely combine at full dose in several studies: mRCC: Tivozanib + Temsirolimus (Torisel) mBC: Tivozanib + Paclitaxel mCRC: Tivozanib + FOLFOX6 MAA Review Ph. III Trial – Tivo v. Sorafenib US Approval Decision EU Approval Decision NDA Review 2016 2017 2018 2019 2020 Ph I/II Tivo + PD1 Phase III Trial of Tivo + PD-1
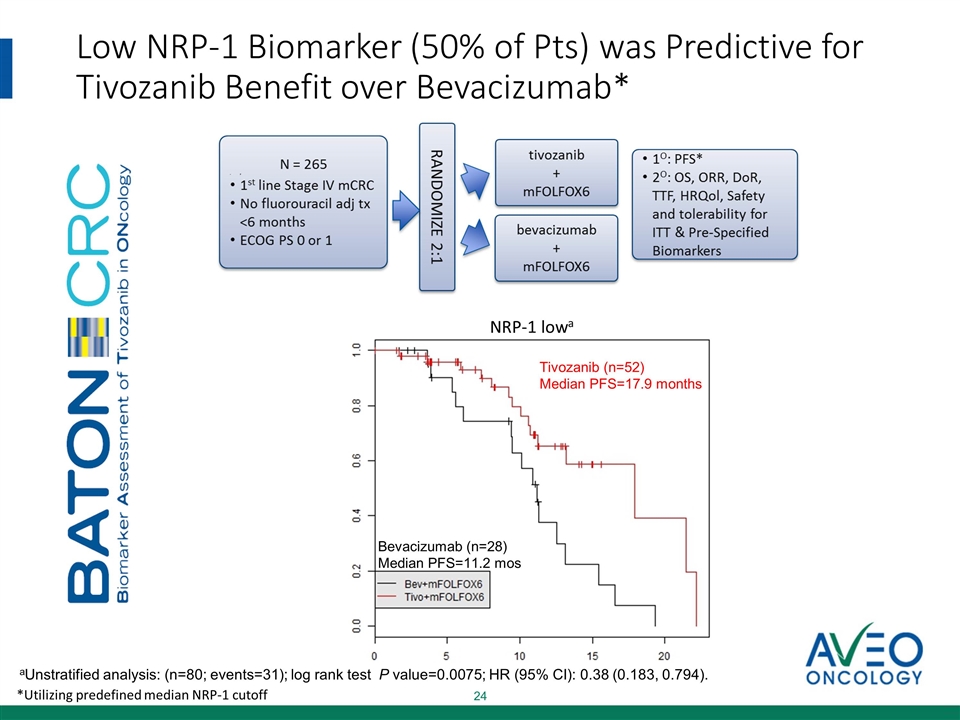
Low NRP-1 Biomarker (50% of Pts) was Predictive for Tivozanib Benefit over Bevacizumab* Bevacizumab (n=28) Median PFS=11.2 mos Tivozanib (n=52) Median PFS=17.9 months NRP-1 lowa aUnstratified analysis: (n=80; events=31); log rank test P value=0.0075; HR (95% CI): 0.38 (0.183, 0.794). *Utilizing predefined median NRP-1 cutoff

Robust Pipeline Development Funded by Partnerships
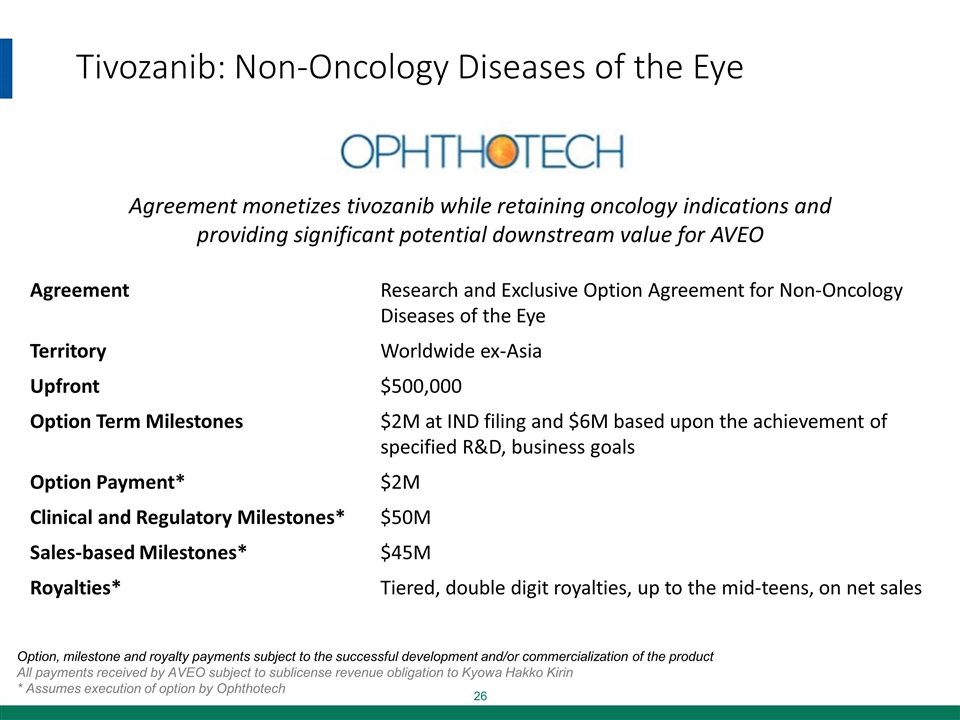
Tivozanib: Non-Oncology Diseases of the Eye Agreement Research and Exclusive Option Agreement for Non-Oncology Diseases of the Eye Territory Worldwide ex-Asia Upfront $500,000 Option Term Milestones $2M at IND filing and $6M based upon the achievement of specified R&D, business goals Option Payment* $2M Clinical and Regulatory Milestones* $50M Sales-based Milestones* $45M Royalties* Tiered, double digit royalties, up to the mid-teens, on net sales Agreement monetizes tivozanib while retaining oncology indications and providing significant potential downstream value for AVEO Option, milestone and royalty payments subject to the successful development and/or commercialization of the product All payments received by AVEO subject to sublicense revenue obligation to Kyowa Hakko Kirin * Assumes execution of option by Ophthotech
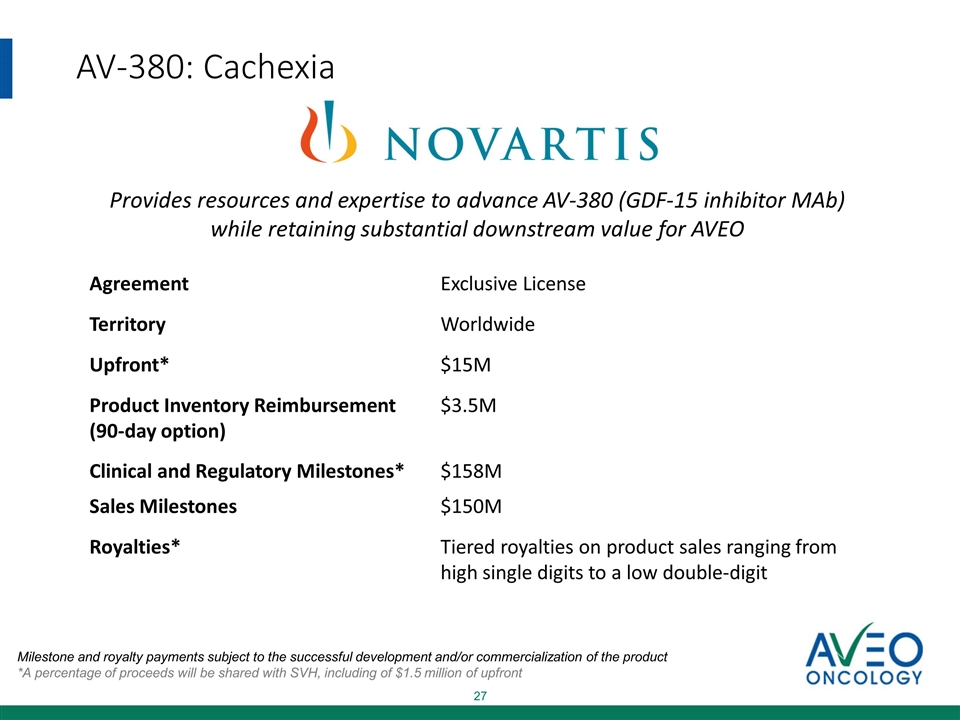
AV-380: Cachexia Agreement Exclusive License Territory Worldwide Upfront* $15M Product Inventory Reimbursement (90-day option) $3.5M Clinical and Regulatory Milestones* $158M Sales Milestones $150M Royalties* Tiered royalties on product sales ranging from high single digits to a low double-digit Provides resources and expertise to advance AV-380 (GDF-15 inhibitor MAb) while retaining substantial downstream value for AVEO Milestone and royalty payments subject to the successful development and/or commercialization of the product *A percentage of proceeds will be shared with SVH, including of $1.5 million of upfront
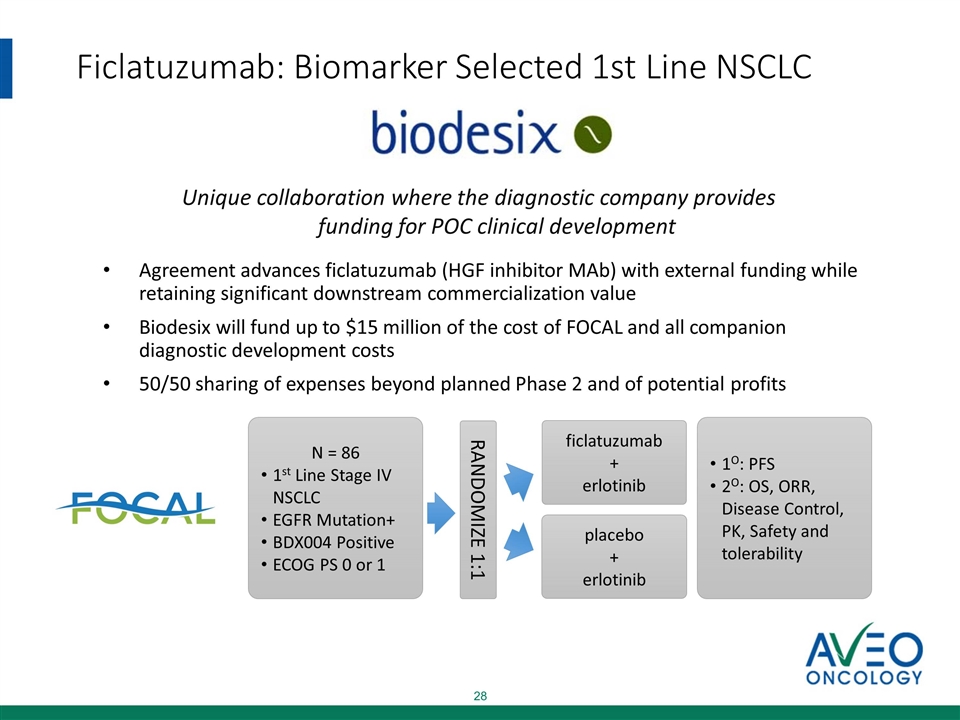
Ficlatuzumab: Biomarker Selected 1st Line NSCLC Agreement advances ficlatuzumab (HGF inhibitor MAb) with external funding while retaining significant downstream commercialization value Biodesix will fund up to $15 million of the cost of FOCAL and all companion diagnostic development costs 50/50 sharing of expenses beyond planned Phase 2 and of potential profits N = 86 1st Line Stage IV NSCLC EGFR Mutation+ BDX004 Positive ECOG PS 0 or 1 RANDOMIZE 1:1 ficlatuzumab + erlotinib placebo + erlotinib 1O: PFS 2O: OS, ORR, Disease Control, PK, Safety and tolerability Unique collaboration where the diagnostic company provides funding for POC clinical development
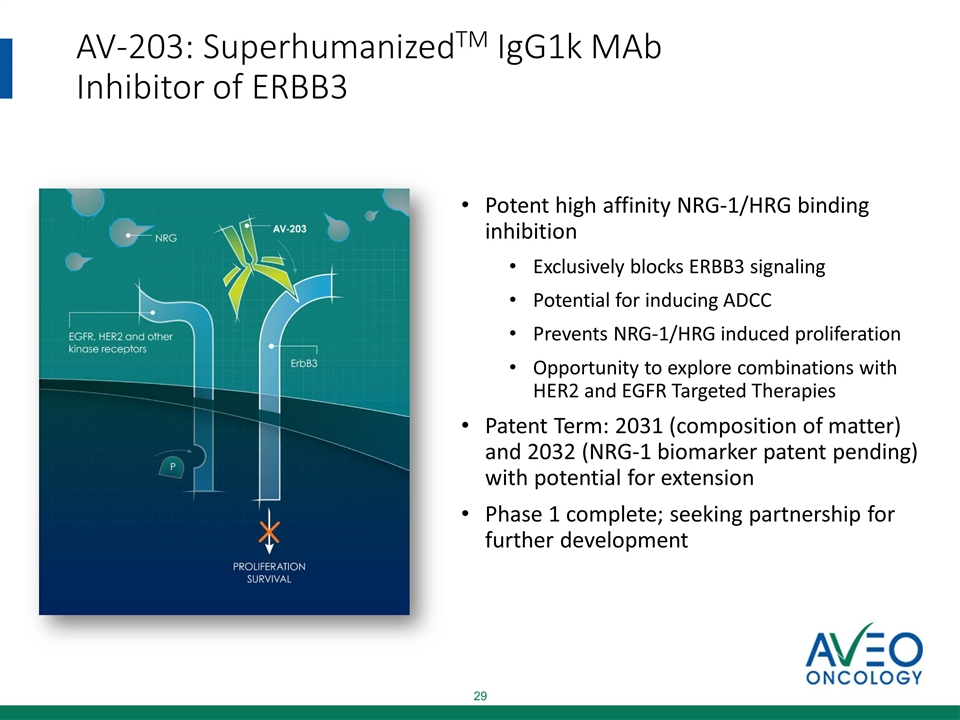
AV-203: SuperhumanizedTM IgG1k MAb Inhibitor of ERBB3 Potent high affinity NRG-1/HRG binding inhibition Exclusively blocks ERBB3 signaling Potential for inducing ADCC Prevents NRG-1/HRG induced proliferation Opportunity to explore combinations with HER2 and EGFR Targeted Therapies Patent Term: 2031 (composition of matter) and 2032 (NRG-1 biomarker patent pending) with potential for extension Phase 1 complete; seeking partnership for further development

Financials & Summary
Financial Highlights Estimated $34M in cash and securities as of year end 2015 Excludes $3.5M due from Novartis Sufficient to fund operations at least through the fourth quarter of 2017* >$40M in potential payments in next 18 months Ophthotech option exercise and development milestones Pharmstandard regulatory milestone EUSA R&D funding, as well as regulatory and reimbursement milestones Novartis development milestones Streamlined operations with a headcount of 20 Shares outstanding as of 11/2/15: ~58M *Does not include potential development expense, SEC settlement or milestone payments associated with partnerships
Significant Progress During 2015 Tivozanib Presentation of encouraging CRC data FDA feedback RCC registration plan ASCO presentation of refractory RCC data Rapporteur feedback for European filing Partnership in Russia (Pharmstandard) Partnership in EU (EUSA) Filing in Russia Pipeline AV-380 partnership and option exercise (Novartis) Corporate Streamlined operations – facilities & staffing

Potential 2016/2017 Milestones Tivozanib MAA filing (EUSA) Initiation of Phase 3 third-line confirmatory RCC study Initiation of Phase 1 PD-1 combination study Proof of concept in AMD (Ophthotech) Initiation of Phase 2/Phase 3 CRC study (with CDx identification) Regulatory decision in Russia/Ukraine/CIS (Pharmstandard) Regulatory decision in EU (EUSA) Data readout of third line RCC Phase 3 study Pipeline Development of AV-380 (Novartis) AV-203 partnership Data readout of FOCAL (ficlatuzumab) study

AVEO Oncology Highlights Lead Asset: Tivozanib Core focus: retain North American tivozanib rights >$1Bn U.S. RCC market Partner Ex-N.A. (EUSA, Pharmstandard) and for ocular (Ophthotech) in 2014/15 Phase 3 TIVO-1 study demonstrated improved PFS in advanced RCC Study may support registration in Europe/Russia OS confounded by crossover; FDA requested additional study Phase 3 refractory RCC study may support 1st and 3rd line indications in U.S. Exploring immunotherapy combination in RCC and CRC biomarker Pipeline to be funded through partnerships Novartis with AV-380; Biodesix with ficlatuzumab; seeking partner for AV-203 Estimated $34M in cash and securities at year end 2015 Excludes $3.5M due from Novartis Potential for >$40M in additional partnership payments in next 18 months Total of >$850M in potential partnership payments Lean organization, experienced management team and Board

January 2016 Corporate Overview


































