UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
| ¨ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2010
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| ¨ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 000-51440
CHINA MEDICAL TECHNOLOGIES, INC.
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant’s name into English)
Cayman Islands
(Jurisdiction of incorporation or organization)
No. 24 Yong Chang North Road
Beijing Economic-Technological Development Area
Beijing 100176
People’s Republic of China
(Address of principal executive offices)
Takyung (Sam) Tsang
Chief Financial Officer
No. 24 Yong Chang North Road
Beijing Economic-Technological Development Area
Beijing 100176
People’s Republic of China
Telephone: (86) 10 6787 1166
Fax: (86) 10 6788 9588
Email: IR@chinameditech.com
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
| American Depositary Shares, each representing 10 ordinary shares, par value $0.10 per share | | The NASDAQ Stock Market LLC (NASDAQ Global Select Market) |
Securities registered or to be registered pursuant to Section 12(g) of the Act
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report. 322,680,001 Ordinary Shares
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP x International Financial Reporting Standards as issued by the International Accounting Standards Board ¨ Other ¨
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ¨ Item 18 ¨
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ¨ No ¨
TABLE OF CONTENTS
i
INTRODUCTION
Unless otherwise indicated, references in this annual report on Form 20-F to:
| | • | | “ADSs” are to our American depositary shares, each of which represents 10 ordinary shares; |
| | • | | “Articles of association” are to our memorandum and articles of association, as amended and restated from time to time; |
| | • | | “China” and the “PRC” are to the People’s Republic of China, excluding, for the purposes of this annual report on Form 20-F only, Taiwan and the special administrative regions of Hong Kong and Macau; |
| | • | | “RMB” and “Renminbi” are to the legal currency of the PRC; |
| | • | | “SEC” are to the U.S. Securities and Exchange Commission; |
| | • | | “shares” and “ordinary shares” are to our ordinary shares, par value US$0.10 per share; and |
| | • | | “US$” and “U.S. dollars” are to the legal currency of the United States. |
Unless the context indicates otherwise, “we,” “us,” “our Company,” “our,” “China Medical” and “CMED” refer to China Medical Technologies, Inc., its predecessor and all other direct and indirect consolidated subsidiaries of China Medical Technologies, Inc.
In addition, unless otherwise indicated, references in this annual report on Form 20-F to:
| | • | | “2011 Notes” are to our US$150 million aggregate principal amount of 3.5% Convertible Senior Subordinated Notes due November 2011; |
| | • | | “2013 Notes” are to our US$276 million aggregate principal amount of 4.0% Convertible Senior Notes due August 2013; |
| | • | | “BBE” are to Beijing Bio-Ekon Biotechnology Co., Ltd., one of our wholly-owned subsidiaries in the PRC; |
| | • | | “Beijing Chengxuan” are to Beijing Chengxuan Economic and Trade Co., Ltd., a related party; |
| | • | | “Beijing GP” are to Beijing GP Medical Technologies Co., Ltd., one of our wholly-owned subsidiaries in the PRC; |
| | • | | “Beijing Weixiao” are to Beijing Weixiao Biological Technology Development Co., Ltd., a related party; |
| | • | | “Chengxuan” are to Chengxuan International Ltd., a related party; |
| | • | | “Convertible Notes” are collectively the 2011 Notes and the 2013 Notes; and |
| | • | | “YDME Beijing” are to Beijing Yuande Bio-Medical Engineering Co., Ltd., our predecessor and one of our wholly-owned subsidiaries in the PRC. |
The following technical and industry specific terms used in this annual report on Form 20-F have the meanings set forth below:
| | • | | “CCD camera” means charge-couple-device camera equipped with a chip to control the brightness and brilliance of colors of the recorded images; |
| | • | | “DNA” means deoxyribonucleic acid, a nucleic acid molecule that contains the genetic instructions for the development and functioning of all known living organisms; |
| | • | | “DNA chip” means a molecular diagnostic biosensor chip used with SPR system for the diagnosis of infectious diseases, cancers, cardiovascular disorders and immune system disorders; |
1
| | • | | “ECLIA” means enhanced chemiluminescence immunoassay, a diagnostic technology which uses analyzer and reagent kits; |
| | • | | “ECLIA analyzer” means an IVD instrument capable of detecting minute levels of light triggered by combining reagents with body fluid samples to produce quantitative diagnostic results; |
| | • | | “ECLIA reagent kits” means reagent kits designed to be used with the ECLIA analyzers to perform IVD tests; |
| | • | | “ECLIA system” means an integrated luminescence immunoassay IVD system comprised of an analyzer and reagent kits for detecting and quantifying a specific antigen or antibody in a blood or body fluid sample using an immunological reaction; |
| | • | | “FISH business” means the business of manufacturing and selling FISH probes that we acquired in March 2007, and until April 2009, included the sale of fluorescent microscopes manufactured by third parties; |
| | • | | “FISH probes” means a synthetic piece of DNA coupled with a fluorescent indicator, or tag, so that the chromosomes or genes that it binds to can be directly visualized under a fluorescent microscope; |
| | • | | “FISH system” means an integrated molecular diagnostic IVD system comprised of a fluorescent microscope and FISH probes for detecting and localizing the presence of specific DNA sequences in chromosomes for an identification of chromosomal abnormalities; |
| | • | | “Fluorescent in situ hybridization technology” or “FISH technology” means a molecular diagnostic technology that uses a fluorescent-labeled probe to detect and localize the presence or absence of specific DNA sequences on chromosome; |
| | • | | “Fluorescent microscope” means an instrument that enables medical practitioners to visualize and locate DNA sequences in human cells for identifying chromosomal abnormalities using FISH technology; |
| | • | | “HIFU” means high intensity focused ultrasound; |
| | • | | “HIFU therapy system” means an ultrasound-guided ablation system that is used for the non-invasive treatment of solid tumors; |
| | • | | “HIV” means human immunodeficiency virus, a retrovirus that can lead to acquired immunodeficiency syndrome, a condition in humans in which the immune system begins to fail, leading to life-threatening opportunistic infections; |
| | • | | “HPV” means human papillomavirus, a common virus that infects the skin and mucous membranes of humans, causing various kinds of warts as well as cervical cancer; |
| | • | | “HPV-DNA chip” means a label-free DNA chip for the diagnosis of HPV infection and genotyping of HPV; |
| | • | | “Immunodiagnostic system sales” means (i) the sales of ECLIA reagent kits and (ii) the sales of semi-automated ECLIA analyzers until April 2008; |
| | • | | “In-vitro diagnostics” or “IVD” means the detection and monitoring of diseases through laboratory evaluation and the analysis of bodily fluids and tissues from patients; |
| | • | | “IVD systems” consists of the ECLIA analyzer, ECLIA reagent kits, FISH probes, SPR analyzer and HPV-DNA chips; |
| | • | | “Luminescence immunoassay IVD system” means an advanced, light-based IVD system commonly used in the United States and Western Europe; |
| | • | | “Molecular diagnostic system sales” means (i) the sales of FISH probes, (ii) the sales of fluorescent microscopes until April 2009 and (iii) the sales of HPV-DNA chips which commenced in the three month period ended June 30, 2010; |
2
| | • | | “Probes” means a piece of labeled DNA used to detect the presence of a complementary DNA sequence by molecular hybridization; |
| | • | | “Reagent” means a substance used in a chemical reaction to detect, measure and produce other substances; |
| | • | | “Reagent kits” means commercially prepared reagent sets, with accessory devices, containing all major components necessary to perform IVD tests; |
| | • | | “SPR” means surface plasmon resonance, a leading biosensor technology in molecular biology used for the analysis of proteins, nucleic acids and viruses; |
| | • | | “SPR analyzer” means a label-free, fully-automated analysis system that utilizes SPR technology on DNA chips; |
| | • | | “SPR system” means an integrated molecular diagnostic system comprised of a SPR analyzer and DNA chips for detecting and quantifying biomarkers related to infectious diseases, cancers, cardiovascular disorders and immune system disorders; and |
| | • | | “SPR business” means the business of manufacturing and marketing SPR analyzer and DNA chips. |
We completed the initial public offering of 7,360,000 ADSs, each representing 10 ordinary shares in August 2005. On August 10, 2005, we listed our ADSs on the NASDAQ Global Select Market (formerly the NASDAQ National Market) under the symbol “CMED”. On March 27, 2006, we completed a follow-on offering of 5,750,000 ADSs, which were sold by certain shareholders. On November 21, 2006, we completed an offering of the 2011 Notes to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”). Concurrently with the offering of the 2011 Notes, we entered into a prepaid forward repurchase contract with an affiliate of Merrill Lynch & Co. Pursuant to the forward repurchase contract, we repurchased contemporaneously with the sales of the 2011 Notes, an aggregate of US$30 million of ADSs, equivalent to 1,163,392 ADS at US$25.78 per ADS. We filed a registration statement on Form F-3 (333-139777) to register the resale of the 2011 Notes and the ADSs issuable upon conversion of the 2011 Notes in January 2007 and the registration statement was declared effective by the SEC on January 26, 2007. On August 15, 2008, we completed an offering of the 2013 Notes pursuant to a registration statement on Form F-3 (333-152937) which was filed and became effective on August 11, 2008. Concurrently with the offering of the 2013 Notes, we entered into an ADS issuance and repurchase agreement with affiliates of Credit Suisse Securities (USA) LLC and Morgan Stanley & Co. Incorporated, or the Dealers. Pursuant to the ADS issuance and repurchase agreement, we issued 4,700,000 ADSs to the Dealers at a consideration of US$1.00 per ADS. The Dealers will be obligated to deliver to us an equal number of ADSs in the future. On the same day we issued the ADSs to the Dealers, we prepaid a purchase price of US$1.00 per ADS for the 4,700,000 ADSs to be repurchased from the Dealers by us in the future.
On September 18, 2009, we announced a share repurchase program under which we were authorized by our board to repurchase up to US$30 million worth of our outstanding ADSs for a period of one year, commencing on October 1, 2009. We repurchased a total of 500,000 ADSs pursuant to this share repurchase program and the program was terminated in May 2010. During the fiscal year ended March 31, 2010, we also bought back a portion of our 2013 Notes. Pursuant to the ADS issuance and repurchase agreement entered into in connection with the offering of such notes with affiliates of Credit Suisse Securities (USA) LLC and Morgan Stanley & Co. Incorporated, or the Dealers, the Dealers delivered back to us 192,000 ADSs in relation to the portion of the 2013 Notes which we bought back. The ADSs repurchased by us and returned by the Dealers have been retained by us and may be cancelled (in which case the underlying ordinary shares will be cancelled) or used by us for other arrangements such as issuance of ADSs to employees upon the exercise of their share incentive awards.
3
PART I
| ITEM 1. | IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
Not applicable.
| ITEM 2. | OFFER STATISTICS AND EXPECTED TIMETABLE |
Not applicable.
| A. | Selected Financial Data |
The following selected consolidated statement of income data (other than ADS data) for the fiscal years ended March 31, 2008, 2009 and 2010 and the selected consolidated balance sheet data as of March 31, 2009 and 2010 have been derived from our audited consolidated financial statements included elsewhere in this annual report on Form 20-F. The following selected consolidated statement of income data (other than ADS data) for the fiscal years ended March 31, 2006 and 2007 and the selected consolidated balance sheet data as of March 31, 2006, 2007 and 2008 have been derived from our unaudited consolidated financial statements, which are not included in this annual report on Form 20-F. In December 2008, we sold the HIFU business to Chengxuan and discontinued the HIFU business. As a result, the HIFU business was accounted for as a discontinued operation in accordance with U.S. Generally Accepted Accounting Principles (“U.S. GAAP”) in our consolidated financial statements. As required by U.S. GAAP, we reclassified the comparative operating results of the discontinued operation for the fiscal years ended March 31, 2006, 2007, 2008 and 2009 as discontinued operations in our consolidated statements of income for the respective fiscal years. In May 2008, the Financial Accounting Standards Board (FASB) issued new authoritative guidance on accounting for convertible debt instruments that may be settled in cash upon conversion (including partial settlement), which requires that the liability and equity components of the convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) be separately accounted for in a manner that reflects an issuer’s non-convertible debt borrowing rate. The resulting debt discount is amortized over the period that the convertible debt is expected to be outstanding as additional non-cash interest expense. This guidance became effective for us on April 1, 2009 and requires retrospective application and our historical selected financial data has been retrospectively adjusted accordingly. A more in depth discussion of how the adoption of this new guidance impacted our consolidated financial statements can be found in the accompanying notes to our consolidated financial statements. Our consolidated statements of income for the fiscal years ended March 31, 2007 and consolidated balance sheets as of March 31, 2007 and 2008 have been adjusted accordingly and were not audited by our independent registered public accountants.
You should read this information together with the consolidated financial statements and related notes and information under “Item 5. Operating and Financial Review and Prospects” included elsewhere in this annual report on Form 20-F. Our consolidated financial statements are prepared and presented in accordance with U.S. GAAP. Our historical results do not necessarily indicate our results expected for any future periods.
4
| | | | | | | | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2010 | |
| | | RMB | | | RMB | | | RMB | | | RMB | | | RMB | | | US$ | |
| | | | | | (As adjusted) | | | (As adjusted) | | | (As adjusted) | | | | | | | |
| | | (in thousands, except for number of shares, per share and per ADS data) | |
Consolidated Statement of Income Data: | | | | | | | | | | | | | | | | | | |
Revenues | | 124,889 | | | 215,550 | | | 547,421 | | | 829,950 | | | 723,071 | | | 105,932 | |
Gross profit | | 87,244 | | | 157,800 | | | 301,984 | | | 597,379 | | | 485,521 | | | 71,131 | |
Operating income | | 44,033 | | | 91,507 | | | 188,130 | | | 144,228 | | | 125,107 | | | 18,329 | |
Other income (expense), net | | 20,098 | | | 13,905 | | | (51,776 | ) | | (100,793 | ) | | (118,592 | ) | | (17,374 | ) |
Income from continuing operations before income taxes | | 64,131 | | | 105,412 | | | 136,354 | | | 43,435 | | | 6,515 | | | 955 | |
Income tax expense | | (4,490 | ) | | (12,049 | ) | | (51,999 | ) | | (73,042 | ) | | (63,556 | ) | | (9,311 | ) |
Income (loss) from continuing operations | | 59,641 | | | 93,363 | | | 84,355 | | | (29,607 | ) | | (57,041 | ) | | (8,356 | ) |
Income from and gain on sale of discontinued operation, net | | 139,372 | | | 186,043 | | | 212,656 | | | 364,409 | | | — | | | — | |
Net income (loss) | | 199,013 | | | 279,406 | | | 297,011 | | | 334,802 | | | (57,041 | ) | | (8,356 | ) |
Net income (loss) per share—Basic(1): | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | 0.24 | | | 0.35 | | | 0.32 | | | (0.11 | ) | | (0.22 | ) | | (0.03 | ) |
Income from and gain on sale of discontinued operation, net | | 0.56 | | | 0.69 | | | 0.81 | | | 1.39 | | | — | | | — | |
Total | | 0.80 | | | 1.04 | | | 1.13 | | | 1.28 | | | (0.22 | ) | | (0.03 | ) |
Net income (loss) per share—Diluted(1): | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | 0.24 | | | 0.35 | | | 0.32 | | | (0.11 | ) | | (0.22 | ) | | (0.03 | ) |
Income from and gain on sale of discontinued operation, net | | 0.56 | | | 0.69 | | | 0.81 | | | 1.39 | | | — | | | — | |
Total | | 0.80 | | | 1.04 | | | 1.13 | | | 1.28 | | | (0.22 | ) | | (0.03 | ) |
Net income (loss) per ADS—Basic(2): | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | 2.42 | | | 3.47 | | | 3.22 | | | (1.13 | ) | | (2.17 | ) | | (0.32 | ) |
Income from and gain on sale of discontinued operation, net | | 5.65 | | | 6.91 | | | 8.11 | | | 13.87 | | | — | | | — | |
Total | | 8.07 | | | 10.38 | | | 11.33 | | | 12.74 | | | (2.17 | ) | | (0.32 | ) |
Net income (loss) per ADS—Diluted(2): | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | 2.41 | | | 3.46 | | | 3.20 | | | (1.13 | ) | | (2.17 | ) | | (0.32 | ) |
Income from and gain on sale of discontinued operation, net | | 5.64 | | | 6.90 | | | 8.07 | | | 13.87 | | | — | | | — | |
Total | | 8.05 | | | 10.36 | | | 11.27 | | | 12.74 | | | (2.17 | ) | | (0.32 | ) |
Dividend paid per share | | — | | | — | | | 0.28 | | | 0.34 | | | 0.38 | | | 0.06 | |
Dividend paid per ADS | | — | | | — | | | 2.80 | | | 3.42 | | | 3.75 | | | 0.55 | |
Weighted average number of shares outstanding | | | | | | | | | | | | | | | | | | |
Basic | | 246,878,001 | | | 269,232,171 | | | 262,218,999 | | | 262,776,289 | | | 262,546,385 | | | 262,546,385 | |
Diluted | | 247,163,001 | | | 269,508,465 | | | 263,464,624 | | | 262,776,289 | | | 262,546,385 | | | 262,546,385 | |
| (1) | If YDME Beijing and Beijing GP had not had any preferential tax rates or tax holidays, our basic and diluted earnings per share for the following periods would have been reduced as follows: |
| | | | | | | | | | | | |
| | | Year Ended March 31, |
| | | 2006 | | 2007 | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB | | RMB | | RMB | | RMB | | RMB | | US$ |
| | | | | (As adjusted) | | (As adjusted) | | (As adjusted) | | | | |
| | | (in thousands) |
Income from continuing operations per share | | | | | | | | | | | | |
Basic | | 0.01 | | 0.02 | | 0.19 | | 0.41 | | 0.23 | | 0.03 |
Diluted | | 0.01 | | 0.02 | | 0.19 | | 0.41 | | 0.23 | | 0.03 |
| (2) | Each ADS represents 10 ordinary shares. |
5
| | | | | | | | | | | | |
| | | As of March 31, |
| | | 2006 | | 2007 | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB | | RMB | | RMB | | RMB | | RMB | | US$ |
| | | | | (As adjusted) | | (As adjusted) | | (As adjusted) | | | | |
| | | (in thousands) |
Consolidated Balance Sheet Data: | | | | | | | | | | | | |
Cash | | 843,791 | | 1,173,640 | | 682,679 | | 1,456,410 | | 815,453 | | 119,466 |
Working capital(1) | | 906,875 | | 764,090 | | 671,990 | | 1,043,911 | | 1,108,839 | | 162,448 |
Goodwill and intangible assets | | 224,744 | | 1,565,362 | | 1,550,447 | | 3,496,128 | | 3,293,844 | | 482,558 |
Total assets | | 1,375,841 | | 3,186,692 | | 2,927,865 | | 5,780,396 | | 4,873,391 | | 713,966 |
Convertible notes | | — | | 1,000,415 | | 935,905 | | 2,826,348 | | 2,777,086 | | 406,851 |
Payables and deferred credit related to FISH and SPR acquisitions (current and non-current) | | — | | 530,598 | | 65,793 | | 707,205 | | — | | — |
Total liabilities | | 129,307 | | 1,748,424 | | 1,293,148 | | 3,854,126 | | 3,105,373 | | 454,946 |
Total shareholders’ equity | | 1,246,534 | | 1,438,268 | | 1,634,717 | | 1,926,270 | | 1,768,018 | | 259,020 |
| (1) | Working capital equals current assets minus current liabilities. |
Exchange Rate Information
This annual report on Form 20-F contains translations of certain RMB amounts into U.S. dollar amounts at specified rates for the convenience of the reader. Unless otherwise stated, the translations of RMB into U.S. dollars have been made at the noon buying rate in effect on March 31, 2010 as set forth in the H.10 statistical release of the U.S. Federal Reserve Board, which was RMB6.8258 to US$1.00. We make no representation that the RMB or U.S. dollar amounts referred to in this annual report on Form 20-F could have been or could be converted into U.S. dollars or RMB, as the case may be, at any particular rate or at all. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in the PRC— Fluctuation in the value of the Renminbi may have a material adverse effect on your investment” and “—Restrictions on currency exchange may limit our ability to receive and use our revenues effectively” for discussions of the effects of fluctuating exchange rates and currency control on the value of our ADSs. On September 3, 2010, the noon buying rate was RMB6.8014 to US$1.00.
The following table sets forth information concerning exchange rates between the RMB and the U.S. dollar for the periods indicated.
| | | | | | | | |
| | | RMB per U.S. Dollar Exchange Rate(1) |
Period | | Period End(2) | | Average(3) | | Low | | High |
| | | (RMB per US$1.00) |
2005 | | 8.0702 | | 8.1826 | | 8.2765 | | 8.0702 |
2006 | | 7.8041 | | 7.9579 | | 8.0702 | | 7.8041 |
2007 | | 7.2946 | | 7.5806 | | 7.8127 | | 7.2946 |
2008 | | 6.8225 | | 6.9193 | | 7.2946 | | 6.7800 |
2009 | | 6.8259 | | 6.8295 | | 6.8470 | | 6.8176 |
2010 | | | | | | | | |
January | | 6.8268 | | 6.8269 | | 6.8295 | | 6.8258 |
February | | 6.8258 | | 6.8285 | | 6.8330 | | 6.8258 |
March | | 6.8258 | | 6.8262 | | 6.8270 | | 6.8254 |
April | | 6.8247 | | 6.8256 | | 6.8275 | | 6.8229 |
May | | 6.8305 | | 6.8275 | | 6.8310 | | 6.8245 |
June | | 6.7815 | | 6.8184 | | 6.8323 | | 6.7815 |
July | | 6.7735 | | 6.7762 | | 6.7807 | | 6.7709 |
August | | 6.8069 | | 6.7873 | | 6.8069 | | 6.7670 |
September (through September 3) | | 6.8014 | | 6.8062 | | 6.8102 | | 6.8014 |
6
| (1) | For all periods prior to January 1, 2009, the exchange rate refers to the noon buying rate as reported by the Federal Reserve Bank of New York. For periods beginning on or after January 1, 2009, the exchange rate refers to the exchange rate as set forth in the H.10 statistical release of the U.S. Federal Reserve Board. |
| (2) | Represents the exchange rate on the last business day of the applicable period. |
| (3) | Annual averages are calculated from month-end rates. Monthly averages are calculated using the average of the daily rates during the relevant period. |
| B. | Capitalization and Indebtedness |
Not applicable.
| C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
Risks Related to Our Business
We may not succeed in sustaining and expanding the market for our ECLIA business and our free arrangement for ECLIA analyzers, whereby we provide ECLIA analyzers to certain hospitals free of charge, may not be successful in generating additional recurring revenue from sales of our ECLIA reagent kits.
We began marketing and selling our semi-automated ECLIA analyzers and reagent kits in September 2004. Our ECLIA system is referred to as a “closed” system since our ECLIA analyzer can only be used with reagent kits manufactured by us and our reagent kits can only be used with our ECLIA analyzer. Gross profit derived from the ECLIA business was RMB277.4 million, RMB366.2 million and RMB200.0 million (US$29.2 million) for the fiscal years ended March 31, 2008, 2009 and 2010, respectively. Seeking to further expand the installed base of our ECLIA analyzers and derive additional recurring revenue from sales of our reagent kits, we started to provide our semi-automated ECLIA analyzers to small and medium-sized hospitals with certain diagnostic volume free of charge to promote the sales of our ECLIA reagent kits in April 2008. We also expect to deliver our fully-automated ECLIA analyzers free of charge to certain large hospitals in 2011.
Going forward, the long-term success of our ECLIA business depends on several factors, including our ability to:
| | • | | successfully promote product awareness of our ECLIA analyzers and reagent kits in the PRC; |
| | • | | develop new reagent kits to expand the applications of our ECLIA reagent kits; |
| | • | | increase the usage of reagent kits in hospitals; |
| | • | | continue to expand the installed base of our ECLIA analyzers and derive recurring revenue from sales of our reagent kits; |
| | • | | competitively price our ECLIA reagent kits; |
| | • | | select effective distributors, |
| | • | | comply with new or changing regulatory requirements and obtain additional regulatory approvals or clearances for new reagent kits in a timely manner; and |
| | • | | successfully launch our fully-automated ECLIA analyzers and reagent kits in the PRC. |
7
Luminescence immunoassay technology is a well established method of clinical diagnosis. As a result, our ECLIA business is competing in a market in which there are already many established industry players. We cannot assure you that we will be able to successfully market or sell our ECLIA analyzers and reagent kits in the future. In addition, we cannot assure you that our free arrangement for ECLIA analyzers would successfully expand the installed base of our ECLIA analyzers and increase recurring revenue from sales of reagent kits and that there would be no negative impact on our existing customer base or revenue stream. We also cannot assure you that our ECLIA business, or any future enhancements to our ECLIA business, will generate adequate revenue to offset our investments and costs in acquiring, developing or marketing our ECLIA business. If there is insufficient demand for our ECLIA analyzers and reagent kits, our business, financial condition and results of operations may be harmed. In addition, any announcement of new products, services or enhancements by us or our competitors may cause our customers to cancel or postpone purchasing decisions for our existing products in anticipation of these new products, services or enhancements.
Our limited operational experience with FISH probes makes evaluating our FISH business and its prospects difficult.
We only introduced our FISH business to the market in June 2007. We develop and manufacture FISH probes internally. Our FISH probes can be used with fluorescent microscopes purchased directly from the manufacturers. We also sold fluorescent microscopes to customers which were manufactured by third parties until April 2009. Gross profit derived from the FISH business was RMB24.6 million, RMB231.2 million and RMB285.9 million (US$41.9 million) for the fiscal years ended March 31, 2008, 2009 and 2010, respectively.
The success of our FISH business depends on several factors, including our ability to:
| | • | | successfully promote product awareness of FISH probes in the PRC; |
| | • | | expand our direct sales force targeting large hospitals; |
| | • | | maintain our competitive position in the market and set competitive prices for our FISH probes; |
| | • | | develop new FISH probes to expand the applications of diagnosis for new diseases and disorders; and |
| | • | | comply with new or changing regulatory requirements and obtain additional regulatory approvals or clearances for new FISH probes in a timely manner. |
FISH is an established technology for clinical diagnosis of genetic syndromes in the prenatal and postnatal settings and diagnosis of cancers in western countries. As a result, our FISH probes are competing in the Chinese market in which there are international players with extensive experience. The penetration rate of FISH probes in the PRC is low and we cannot assure you that we will be able to successfully market or sell our FISH probes despite our cost advantage and our distribution experience in the PRC. We also cannot assure you that our FISH probes, or any future enhancements to our FISH probes, will generate adequate revenue to offset our investments and costs in acquiring, developing or marketing our FISH probes. If there is insufficient demand for our FISH probes, our business, financial condition and results of operations may be materially and adversely affected. In addition, any announcement of price change, new products, services or enhancements by us or our competitors may cause our customers to cancel or postpone purchasing decisions for our FISH probes.
In addition, our limited operating history with the FISH probes may not provide a meaningful basis for you to evaluate our business, financial performance and prospects in our FISH business. We may not have sufficient experience to address the risks frequently encountered by early-stage companies or companies exploring in new industries, and as a result we may not be able to:
| | • | | maintain our market share and profitability; |
| | • | | continue to effectively commercialize our FISH probes; |
| | • | | acquire and retain customers; |
8
| | • | | attract, train, motivate and retain qualified personnel; |
| | • | | keep up with evolving industry standards and market developments; |
| | • | | increase the market awareness and increase the penetration rate of our FISH probes in the PRC; |
| | • | | respond to competitive market conditions; |
| | • | | maintain adequate control of our expenses; |
| | • | | manage our relationships with our suppliers, distributors and direct-selling hospitals; or |
| | • | | protect our proprietary technologies. |
If we are unsuccessful in addressing any of these risks, our business may be materially and adversely affected.
We may not succeed in developing and expanding a market for our SPR analyzers and HPV-DNA chips.
We acquired our SPR technology and related assets in December 2008 and only began to introduce our SPR analyzers and HPV-DNA chips to certain existing hospital customers in April 2009. Since then, the progress of the placement of our SPR analyzers with hospitals was significantly affected because the attention of our senior management was significantly diverted to the internal investigation which is discussed below under the heading “—Allegations of irregularities or improper conduct of our management, whether true or false, may cause us to undertake internal investigations and may cause government agencies to initiate investigations against us and could have a material adverse effect on our business, prospects and reputation and negatively impact our financial condition and results of operations”. As a result, we only started to deliver our SPR analyzers to our customers free of charge in January 2010 and generate revenue from the sale of HPV-DNA chips following the approval by the PRC State Food and Drug Administration (“SFDA”) which was granted in June 2010. As of June 30, 2010, we delivered 49 SPR analyzers free of charge to large hospitals which are also users of our FISH probes. Our SPR system is referred to as a “closed” system since our SPR analyzer can only be used with DNA chips manufactured by us and our DNA chips can only be used with our SPR analyzer. We provide our SPR analyzers free of charge to large hospitals, in order to expand the installed base of our SPR analyzers and derive recurring revenue from sales of our DNA chips.
The success of our SPR business depends on several factors, including our ability to:
| | • | | devote sufficient management attention and resources to the promotion and development of our SPR business; |
| | • | | successfully promote product awareness of our SPR analyzers and DNA chips in the PRC; |
| | • | | develop new DNA chips to expand the applications for the diagnosis of new diseases and disorders; |
| | • | | continue to expand the installed base of our SPR analyzers and derive recurring revenue from sales of our DNA chips; |
| | • | | competitively price our DNA chips; and |
| | • | | comply with new or changing regulatory requirements and obtain additional regulatory approvals or clearances for new DNA chips in a timely manner. |
We are the first company in the PRC to manufacture SPR analyzers and HPV-DNA chips for clinical diagnosis and we cannot assure you that we will be able to successfully market or sell our DNA chips. In addition, we cannot assure you that our free arrangement for SPR analyzers would successfully expand the installed base of our SPR analyzers and increase recurring revenue from sales of DNA chips. We also cannot assure you that our HPV-DNA chips, or any new DNA chips that we may develop in the future, will generate adequate revenue to offset our investments and costs in acquiring, developing and marketing our SPR analyzers
9
and DNA chips. If there is insufficient demand for our DNA chips, our business, financial condition and results of operations may be adversely affected. In addition, any announcement of price change, new products, services or enhancements by us or our competitors may cause our customers to cancel or postpone purchasing decisions for our DNA chips.
A reduction in, or a failure to increase, the revenues of our ECLIA reagent kits and FISH probes would cause our revenues to decline and could materially harm our business.
We have derived substantially all or all of our revenues from sales of our ECLIA reagent kits and FISH probes. Sales of our ECLIA reagent kits and FISH probes accounted for 70%, 98% and 100% of our total revenues for the fiscal years ended March 31, 2008, 2009 and 2010, respectively. We expect sales of our ECLIA reagent kits and FISH probes to continue to comprise substantially all of our revenues until we generate material revenues from our SPR business, which only commenced generating revenue in June 2010, and sales of such kits and probes will continue to comprise a major portion of our revenues thereafter. Therefore, a reduction in, or our failure to increase, revenues from sales of our ECLIA reagent kits and FISH probes, could materially harm our business, results of operations and prospects.
Our ECLIA customers, mainly distributors, reduced their inventory levels in the first half of the fiscal year ended March 31, 2010 and certain customers ceased purchasing from us in anticipation of price reductions on our ECLIA reagent kits and we reduced the selling price of our ECLIA reagent kits in September 2009. The reduction in orders for our ECLIA reagent kits and their selling prices negatively affected our revenues from the ECLIA business and such negative impact may continue. While we have adopted, and will continue to adopt, measures to increase the sales of our ECLIA reagent kits, we cannot assure you that these measures will be effective or that our revenues from sales of ECLIA reagent kits will not decline further. A similar reduction in orders and prices of our FISH probes could occur at any time, which would cause our revenues from the FISH business to decline. If we fail to successfully address the decline in the sales of our ECLIA reagent kits or experience a decline in the sales of our FISH probes, our revenues may decline significantly and our business and operating results may be materially and adversely affected.
If we are unable to protect our intellectual property rights, our competitors may take advantage of our proprietary technology and know-how and compete directly against us.
Our success depends, in part, on our ability to protect our proprietary technologies. In the PRC, we own 11 invention patents and 13 utility patents relating to our ECLIA technology. We also own two invention patents relating to our SPR technology and we have filed patent applications relating to our FISH technology in the PRC. Invention patents are granted for invention or discovery of any new and useful process or article of manufacture, or any new and useful improvement thereof. Utility patents are granted for invention of a new and practical structure or form, or a combination of both, for an article of manufacture.
We have been granted one patent for ECLIA technology in the United States. In addition, we have 22 patent applications related to our SPR technology in the United States and the European Union. We have also filed patent applications relating to our FISH technology in the United States.
Due to the different regulatory bodies and varying requirements in the United States, we cannot assure you that we will be able to obtain patent protection for all or any aspects of our ECLIA, FISH and SPR technologies in the United States other than those we have already received. The process of seeking patent protection can be lengthy and expensive, and we cannot assure you that our patent applications will result in patents being issued or that our existing or future issued patents will be sufficient to provide us with meaningful protection or commercial advantage. Our patents and patent applications may be challenged, invalidated or circumvented in the future. We cannot assure you that our current or potential competitors, many of which have substantial resources and have made substantial investments in competing technologies, do not have, and will not develop, products that compete directly with our products despite our intellectual property rights.
10
We also rely on trade secrets, proprietary know-how and other non-patentable technologies, which we seek to protect through non-disclosure agreements with employees. We cannot assure you that these non-disclosure agreements will not be breached, that we will have adequate remedies for any breach, or that our trade secrets, proprietary know-how and other non-patentable technologies will not otherwise become known to, or be independently developed by, our competitors.
Implementation and enforcement of the PRC intellectual property-related laws have historically been deficient and ineffective, and are hampered by corruption and local protectionism. Accordingly, intellectual property rights and confidentiality protections in the PRC may not be as effective as in the United States or other countries. Policing unauthorized use of proprietary technology is difficult and expensive, and we might need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. The experience and capabilities of the PRC courts in handling intellectual property litigation varies, and outcomes are unpredictable. Further, such litigation may require significant expenditure of cash and management efforts and could adversely affect our business, financial condition and results of operations. An adverse determination in any such litigation will impair our intellectual property rights and may adversely affect our business, prospects and reputation.
We may be exposed to intellectual property infringement and other claims by third parties, which, if successful, could cause us to pay significant damage awards and incur other costs.
Our success also depends in large part on our ability to use and develop our technology and know-how without infringing the intellectual property rights of third parties. As litigation becomes more common in the PRC in resolving commercial disputes, we face a higher risk of being the subject of intellectual property infringement claims. The validity and scope of claims relating to medical device technology patents involve complex scientific, legal and factual questions and analysis and, therefore, may be highly uncertain. The defense and prosecution of intellectual property suits, patent opposition proceedings and related legal and administrative proceedings can be costly and time consuming, with unpredictable outcomes, and may significantly divert the efforts and resources of our technical and management personnel. Courts in some jurisdictions in the PRC are inexperienced in these types of cases, and may be influenced by local protectionism. An adverse determination in any such litigation or proceedings to which we may become a party could (i) subject us to significant liability, including damage awards, to third parties, (ii) require us to seek licenses from third parties, to pay ongoing royalties, or to redesign our products or (iii) subject us to injunctions preventing the manufacture and sale of our products. Protracted litigation could also result in our customers or potential customers deferring or limiting their purchase or use of our products until resolution of such litigation.
Unauthorized use of our brand name by third parties, and the expenses incurred in developing and preserving the value of our brand name, may adversely affect our business.
Our brand name is critical to our success. Unauthorized use of our brand name by third parties may adversely affect our business and reputation, including the perceived quality and reliability of our products. We rely on trademark law, company brand name protection policies and agreements with our employees, customers, business partners and others to protect the value of our brand name. Despite our precautions, we cannot assure you that those procedures provide effective prevention for unauthorized third-party use of our brand name. Enforceability, scope and validity of protection of trademarks in the PRC are uncertain and still evolving, and we may not be successful in prosecuting unauthorized third-party use. Future litigation could also result in substantial costs and diversion of our resources, and could adversely affect our business, financial condition and results of operations.
Our business strategy to grow through acquisitions of new products or technologies may result in integration costs, failures and dilution to existing shareholders.
An important business strategy of ours is to acquire and commercialize medical technologies and products with significant market potential. We continue to seek attractive opportunities to acquire new products or
11
technologies, particularly those that could assist us in advancing our current products, technologies and market penetration, or in expanding our product offerings or technologies. If we decide to acquire another company or its assets in order to obtain its products or technologies, we would face a number of risks including consummating the acquisition on unfavorable terms and not obtaining adequate financing, which may adversely affect our ability to develop new products and services and to compete in our rapidly changing marketplace. These acquisitions could also require that our management develop expertise in new areas, manage new business relationships and trade models, and attract new customers. Successful management and integration of acquisitions are subject to a number of risks, including difficulties in assimilating acquired operations and managing remote operations, potential loss of key employees, diversion of management’s attention from existing business operations, assumption of contingent liabilities and incurrence of potentially significant write-offs, which may adversely affect our business or results of operations. In addition, if we consummate such an acquisition through an exchange of our securities, our existing shareholders could suffer dilution which could adversely affect the value of their investment in our Company.
Rapid growth and a rapidly changing operating environment could place a strain on our limited resources.
Our growth strategy includes our efforts to build our brand, develop new products, increase the installed base of our semi-automated ECLIA systems by targeting small to medium-sized hospitals, launch our fully-automated ECLIA systems by targeting large hospitals, as well as high volume users among medium-sized hospitals, and accelerate market acceptance of our FISH probes and SPR systems in large hospitals. This growth strategy requires significant capital resources, and we may not generate an adequate return on our investment. As noted above, growth may also involve the acquisition of new products or technologies which could further strain our resources. We may also experience difficulties integrating any acquisition into our existing businesses and operations. The success of our growth strategy also depends in part on our ability to utilize our financial, operational and management resources and to attract, train, motivate and manage an increasing number of employees. The success of our growth strategy depends on a number of internal and external factors, such as:
| | • | | the growth of the market for medical devices and supplies in the PRC; |
| | • | | increased customer awareness and acceptance of our products; |
| | • | | continued enhancement of our research and development capabilities; |
| | • | | development of new ECLIA reagent kits, FISH probes and DNA chips to expand the applications of diagnosis for new diseases and disorders; and |
| | • | | competition from companies that offer IVD products or alternative diagnostic products in the PRC. |
Many of these factors are beyond our control and we may not be able to implement our growth strategy successfully or manage our expansion effectively.
We have a major shareholder that could exert substantial influence over our business, and its interests may not be aligned with the interests of our other shareholders.
Currently, Chengxuan, which is beneficially owned by Mr. Xiaodong Wu, our founder, the chairman of our board of directors and our chief executive officer, beneficially owns approximately 23% of our outstanding ordinary shares. Chengxuan could exert substantial influence over matters such as electing directors and approving material mergers, acquisitions or other business combination transactions. The interests of Chengxuan may differ from the interests of our other shareholders. In addition, the concentration of ownership in Chengxuan may discourage, delay or prevent a change in control of our Company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our Company and might reduce the price of our ADSs. These actions may be taken even if they are opposed by our other shareholders. In cases where the interests of our significant shareholders are aligned and they vote together, these shareholders will also have the power to prevent or cause a change in control.
12
If we grant additional share options, restricted shares or other share-based compensation in the future, our operating results could be materially adversely affected.
We adopted a stock option plan in 2005 and an equity incentive plan in 2009. As of June 30, 2010, we have granted options to purchase 1,550,000 of our ordinary shares and 7,930,000 restricted shares under our 2005 stock option plan, as amended and restated, and 3,250,000 restricted shares under our 2009 equity incentive plan. Our 2005 stock option plan expired on December 31, 2009.
For accounting purposes, we measure the fair value of share options and other share-based compensation based on the fair value of equity-classified awards on the date of the grant, with the related compensation expense recognized generally over the period in which the recipient is required to provide service in exchange for the equity award. If we grant additional options, restricted shares or other equity incentives to our employees in the future, we could incur significant compensation expenses which could materially reduce our net income, and your investment in our ADSs could be significantly diluted.
We are highly dependent on senior management and key research and development personnel.
We are highly dependent on our senior management to manage our businesses and operations and our key research and development personnel for the development of new technologies and applications and the enhancement of our existing products. In particular, we rely substantially on our chairman and chief executive officer, Mr. Xiaodong Wu, to manage our operations. We also depend on our key research personnel. In addition, we also rely on customer service personnel for the installation and support of our products and on marketing and sales personnel, engineers and other personnel with technical and industry knowledge to market, sell, install and service our products. We do not maintain key man life insurance on any of our senior management or key personnel. The loss of any of our senior management or key personnel could have a material adverse effect on our business and operations. Competition for senior management and research and development personnel is intense, and the pool of suitable candidates is limited. We may be unable to locate a suitable replacement for any senior management or key research and development personnel that we lose. In addition, if any member of our senior management or key research and development personnel joins a competitor or forms a competing company, they may compete with us for customers, business partners and other key professionals and staff members of our Company. Although each of our senior management and key research and development personnel has signed a confidentiality and non-competition agreement in connection with his employment with us, we cannot assure you that we will be able to successfully enforce these provisions in the event of a dispute between us and any member of our senior management or key research and development personnel.
We compete for qualified personnel with other medical technology companies, medical device and supplies manufacturers, universities and research institutions. Intense competition for these personnel could cause our compensation costs to increase significantly, which could have a material adverse effect on our results of operations. Our future success and ability to grow our business will depend in part on the continued service of these individuals and our ability to identify, hire and retain additional qualified personnel. If we are unable to attract and retain qualified employees, we may be unable to meet our business and financial goals.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain of our employees and consultants were previously employed at other biotechnology or medical device companies, including our competitors or potential competitors, or at universities or other research institutions. We may be subject to claims that these employees, consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could delay or prevent us from commercializing one or more of our products.
13
Allegations of irregularities or improper conduct of our management, whether true or false, may cause us to undertake internal investigations and may cause government agencies to initiate investigations against us and could have a material adverse effect on our business, prospects and reputation and negatively impact our financial condition and results of operations.
Our audit committee received an anonymous letter, in which allegations of irregularities and improper conduct were made against our senior management in connection with our acquisition of the FISH business and the SPR technology and related assets, our disposal of the HIFU business, our revenues as well as our relationships with stock analysts. Our audit committee, consisting of three independent directors, initiated an independent internal investigation into the allegations and engaged an independent law firm as well as independent forensic accountants to assist the audit committee to carry out the independent internal investigation in April 2009. Our audit committee completed the investigation and reported that the investigation did not identify any evidence to support the allegations made in the anonymous letter. During the course of the investigation, our senior management team and certain management personnel were required to devote significant time and effort to assist with the investigation and therefore had less time to spend on our operations, which we believe had contributed to the decline in our sales and the delay for the launch of our SPR system. The costs of the investigation also increased our general and administrative expenses substantially for the quarter ended June 30, 2009 and the quarter ended September 30, 2009.
In addition, our audit committee noted that our PRC subsidiaries had provided gifts to third parties mainly during festivals in the PRC for the past few years but, after further inquiry, it was determined that the value of the gifts was small and there was no evidence that the gifts were used for an improper purpose. Thereafter, our audit committee proposed measures to enhance our policies and procedures with respect to gifts. These measures were approved by our board of directors and have been implemented by us. We cannot assure you that similar allegations of irregularities or improper conduct of our management or questions regarding the conduct of our employees and management will not occur in the future.
If similar allegations are made or potential impermissible conduct within our Company otherwise comes to our attention, we may need to undertake an internal investigation. In addition, the allegations we have received and any future allegations of similar nature may cause government agencies that have supervisory power or regulatory authority over us to initiate investigations against us. Any of these investigations could be costly and time-consuming and may divert a significant amount of our management attention and resources. Therefore, any allegation of irregularity or improper conduct of our management with evidence could have material adverse effect on our business, prospects and reputation and negatively impact our financial condition and results of operations.
If we fail to expand and maintain an effective sales force or successfully develop and maintain our relationship with distributors, our business, prospects and brand may be materially and adversely affected.
We currently market and sell substantially all of our ECLIA products to third-party distributors, while our FISH probes, SPR analyzers and HPV-DNA chips are promoted, marketed or sold solely through our own direct sales force. As part of our growth plan, we intend to increase the number of distributors we utilize to distribute our ECLIA products and strengthen and grow our direct sales force targeting large hospitals to sell our FISH probes and HPV-DNA chips. We have limited experience in managing a large sales force and distributor network. We cannot assure you that we will be able to maintain an effective sales force or maintain our relationships with third-party distributors. If we fail to do any one of those, our sales could fail to grow or could even decline, and our ability to grow our business could be adversely affected. The expansion of our sales force and distribution network is also likely to require a significant investment of financial resources and management efforts, and the benefits, if any, which we gain from such expansion may not be sufficient to generate an adequate return on our investment.
14
If we fail to effectively manage our distribution network, our business, prospects and brand may be materially affected by actions taken by our distributors.
We have a limited ability to manage the activities of our third-party distributors, who are independent from us. Our distributors could take one or more of the following actions, any of which could have a material adverse effect on our business, prospects and brand:
| | • | | sell products that compete with our products in breach of their non-competition agreements with us; |
| | • | | fail to adequately promote our products; |
| | • | | fail to provide proper service to our end-users; or |
| | • | | violate the anti-corruption laws that apply to us in the PRC or elsewhere. |
Failure to adequately manage our distribution network or the non-compliance of our distributors with their obligations under distribution agreements with us could harm our corporate image among end users of our products and disrupt our sales, resulting in a failure to meet our sales goals. The PRC government has increased its anti-bribery efforts in the healthcare sector to reduce improper payments received by hospital administrators and doctors in connection with the purchase of pharmaceutical products and medical devices. Our distributors may violate these laws or otherwise engage in illegal practices with respect to their sales or marketing of our products which could damage our reputation and materially adversely affect our business, financial condition and results of operations.
A significant interruption in supply could prevent or limit our ability to provide our products to customers on a timely basis.
We purchase all our materials and major components from third-party suppliers and assemble and manufacture our products in-house. Currently, we do not have any material long-term supply contracts with our suppliers. Our purchases are made on a purchase order basis. If the supply of certain materials, components or services is interrupted, our own manufacturing process would be delayed. We may be unable to secure alternative sources of supply in a timely and cost-effective manner. We purchase the components of our ECLIA analyzer and SPR analyzer from various domestic and international suppliers. The raw materials for our ECLIA reagent kits, FISH probes and HPV-DNA chips are also supplied by various domestic and international vendors. The failure of any of these suppliers to provide materials to us, particularly if we are unable to obtain materials and major components from alternative sources on a timely basis or on commercially reasonable terms, could impair our ability to manufacture our products or increase our costs. We also plan to further increase the production and sales of our products in the future. If we are unable to obtain required materials and components that meet our production standards in sufficient quantities or at acceptable cost, we will be unable to increase our production and sales as planned. Failure to obtain adequate supplies of materials, components or services in a timely and cost-effective manner could delay our delivery to our customers. This delay could harm our reputation, cause us to lose sales, and force us to use more expensive sources of supply, which could significantly increase our production costs and harm our operating results. Any of these occurrences could have a material adverse effect on our business, financial condition and results of operations.
Our costs could substantially increase if we experience a significant number of warranty claims or repair requests.
We generally provide 12-month product warranties against technical defects of our ECLIA analyzers, fluorescent microscopes (prior to the cessation of its sales in April 2009) and SPR analyzers, including free analyzers delivered to customers. Our product warranty requires us to repair defects arising from product design and production process and if necessary, replace defective components. Historically, we have received a limited number of warranty claims or repair requests for our ECLIA analyzers and fluorescent microscopes. The costs associated with our warranty claims or repair requests have historically been relatively low. Thus, we generally do not accrue any liability for potential warranty claims at the time of sales, but rather at the time claims are
15
actually made. We also replace ECLIA reagent kits and FISH probes if they are found to be defective. As we only began installing SPR analyzers in hospitals in January 2010 and selling our HPV-DNA chips in June 2010, we currently have no historical data on the warranty claims or repair requests for these products.
If we experience an increase in warranty claims or if our repair and replacement costs associated with warranty claims increase significantly, we will begin to incur liabilities for potential warranty claims at the time of delivery of our products. An increase in the frequency of warranty claims or amount of warranty costs may harm our reputation and could have a material adverse effect on our financial condition and results of operations.
We are subject to product liability exposure and have no relevant insurance coverage.
Our products are medical devices and we are exposed to potential product liability claims in the event that our products fail to function properly or the use of our products causes or is alleged to have caused personal injuries or other adverse effects. A successful product liability claim against us could require us to pay substantial damages. Product liability claims against us, whether or not successful, are costly and time-consuming to defend. Also, in the event that our products prove to be defective, we may be required to recall or redesign such products. We do not have any product liability insurance to cover potential product liability arising from the use of our products. In addition, as the insurance industry in the PRC is still in an early stage of development, product liability insurance available in the PRC offers limited coverage compared to coverage offered in many other countries. We cannot assure you that a product liability claim will not be brought against us in the future. A product liability claim, with or without merit, could result in significant adverse publicity against us, and could have a material adverse effect on the marketability of our products and our reputation, which in turn, could have a material adverse effect on our business, financial condition and results of operations.
Our operations might be interrupted by the occurrence of a natural disaster or other catastrophic events.
Substantially all of our manufacturing and research and development facilities are located in Beijing, PRC. We do not maintain back-up facilities, so we depend on our manufacturing and other facilities for the continued operation of our business. Natural disasters or other catastrophic events, including power interruptions, water shortages, storms, fires, earthquakes, floods, terrorist attacks and wars could disrupt our operations. We might suffer losses as a result of business interruptions, for which we do not have insurance coverage, and our operations and financial results might be materially and adversely affected. Moreover, any such event could delay our research and development programs.
If we are unable to successfully operate and manage our manufacturing operations, we may experience a decrease in revenues and gross margin.
As we ramp up our manufacturing operations to accommodate our planned growth, we may encounter difficulties associated with increasing production scale, including shortages of qualified personnel to operate our equipment, assemble our products or manage manufacturing operations, as well as shortages of key raw materials or components for our products. In addition, we may also experience difficulties in producing sufficient quantities of products or in achieving desired product quality. If we are unable to successfully operate and manage our manufacturing operations to meet our needs, we may not be able to provide our customers with the quantity or quality of products they require in a timely manner. This could cause us to lose customers and result in reduced revenues and gross margin.
Fluctuations in our quarterly operating results could cause our ADS price to decline.
Our revenues and operating results have fluctuated in the past and may continue to fluctuate significantly from quarter to quarter depending upon numerous factors. Factors that may affect the fluctuation of our quarterly operating results include changes in pricing policies by us or our competitors, the length of our sales cycle, the timing and market acceptance of new product introductions and product enhancements by us or our competitors,
16
customer order deferrals in anticipation of new or enhanced products offered by us or our competitors, the loss of key sales personnel or distributors, the commencement of any internal investigation, changes in government policies or regulations and a downturn in general economic conditions in the PRC. Many of these factors are beyond our control, and you should not rely on our results of operations for prior quarters as an indication of our results in any future period. As our revenues vary from quarter to quarter, our business is difficult to predict, and our quarterly results could fall below investor expectations, which could cause our ADS price to decline.
Our future capital needs are uncertain and we may need to raise additional funds in the future.
We may require additional cash resources in the future due to:
| | • | | changed business conditions or other future developments; |
| | • | | the receipt of, and the time and expenses required to obtain and maintain, regulatory clearances and approvals; |
| | • | | the resources we devote to developing, marketing and producing our products; |
| | • | | our ability to identify and our desire or need to pursue acquisitions or other investments; |
| | • | | the extent to which our products generate market acceptance and demand; and |
| | • | | the need to repay our outstanding Convertible Notes or any other indebtedness we may incur from time to time. |
We cannot assure you that our revenues will be sufficient to meet our operational needs and capital requirements in the future. In addition, we cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. Our future capital needs and other business reasons could require us to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity or equity-linked securities could result in additional dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends to our shareholders.
If a poll is not demanded at our shareholder meeting, voting will be by show of hands and shares will not be proportionately represented. Shareholder resolutions may be passed without the presence of the majority of our shareholders in person or by proxy.
Voting at any of our shareholder meetings is by show of hands unless a poll is demanded. A poll may be demanded by the chairman of our board of directors or by any shareholder present in person or by proxy. If a poll is demanded, each shareholder present in person or by proxy will have one vote for each ordinary share registered in his name. If a poll is not demanded, voting will be by show of hands and each shareholder present in person or by proxy will have one vote regardless of the number of shares registered in his name. In the absence of a poll, shares will therefore not be proportionately represented. In addition, the quorum required for our shareholder meetings consists of shareholders who hold at least one-third of our ordinary shares being present at a meeting in person or by proxy. Therefore, subject to the requisite majorities, shareholder resolutions may be passed at our shareholder meetings without the presence of the majority of our shareholders in person or by proxy.
Our earnings could be adversely affected if we recognize impairment losses on goodwill and other intangible assets relating to our acquisitions of the ECLIA, FISH and SPR technologies and BBE business.
As a result of our acquisitions of the ECLIA, FISH and SPR technologies and BBE business in August 2004, March 2007, December 2008 and January 2008, respectively, we have recognized goodwill and other intangible assets. Our intangible assets, other than goodwill, are amortized over their respective estimated useful lives, and are tested for impairment whenever events or changes in circumstances indicate that the carrying
17
amount of an intangible asset may not be recoverable. Goodwill is tested for impairment by us annually as of year end or more frequently if an event or a circumstance indicates that goodwill might be impaired. Examples of such events or circumstances include, but are not limited to, a significant adverse change in legal or business climate, an adverse regulatory action or unanticipated competition. See “Item 5. Operating and Financial Review and Prospects—Critical Accounting Policies”. In the future, we could recognize impairment losses on goodwill and other intangible assets and that impairment could result in a charge to our reported results of operations.
We may fail to achieve and maintain effective internal controls.
We are subject to the reporting obligations under the United States securities laws. We are required by the SEC, as directed by Section 404 of the Sarbanes-Oxley Act, to include a report of management on the effectiveness of our internal control over financial reporting. In addition, an independent registered public accounting firm must attest to and report on the effectiveness of our internal control over financial reporting.
Our management concluded that our internal control over financial reporting was effective as of March 31, 2010, and our independent registered public accounting firm has issued an attestation report, which has concluded that we maintained, in all material respects, effective internal control over financial reporting as of March 31, 2010. See “Item 15. Controls and Procedures”.
Our management may not conclude that our internal controls are effective in the future. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent public registered accounting firm may disagree. If our independent public registered accounting firm is not satisfied with our internal control over financial reporting or the level at which our controls are designed, operated, documented or reviewed, it then may issue an adverse opinion on the effectiveness of our internal control over financial reporting. Any of these possible outcomes could result in the loss of investor confidence in the reliability of our financial statements.
Furthermore, we have expended, and anticipate that we will continue to expend, considerable costs, management time, and other resources, in an effort to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
Risks Related to Our Industry
New product development in the medical device and supply industry is both costly and labor-intensive and has a very low rate of successful commercialization.
Our success will depend in part on our ability to enhance our existing products and technologies and to develop and acquire new products or technologies. The development process for medical technology is complex and uncertain, as well as time-consuming and costly. Product development requires the accurate assessment of technological and market trends as well as precise technological execution. We cannot assure you that:
| | • | | our product or technology development will be successfully completed; |
| | • | | necessary regulatory clearances or approvals will be granted by the SFDA or other regulatory bodies for the commercialization and marketing of any new products or technologies as required on a timely basis or at all; or |
| | • | | any product or technology we develop can be commercialized or will achieve market acceptance. |
In particular, obtaining SFDA approval for new product registration can be time-consuming. In addition to applying for a production permit, SFDA product registration is required for our IVD products. The product registration entails, among other things, on-site manufacturing inspections, pre-clinical and clinical trials and technical evaluation. It has generally taken us 12 months or more to obtain SFDA approval for our products.
18
Also, we may be unable to locate suitable products or technologies to acquire or acquire such products or technologies on commercially reasonable terms. Failure to develop, acquire, obtain necessary regulatory clearances or approvals for the commercialization of new products and technologies, or successfully commercialize or market potential new products or technologies could have a material adverse effect on our business, financial condition, results of operations or prospects.
In order to manufacture and market our products, we are required to obtain various authorizations from governmental regulatory authorities in the PRC and other countries. If we fail to obtain clearances or approvals in a timely fashion, our business may be significantly affected.
The sales and marketing of our medical device products are subject to regulation in the PRC and other countries where we plan to expand our product distribution. We are required to obtain registrations with the SFDA and the regulators administering the approval in countries where we plan to export. The process for obtaining regulatory clearances or approvals can be lengthy and expensive, and the results are unpredictable. In addition, the relevant regulatory authorities may introduce additional requirements or procedures that have the effect of delaying or prolonging the regulatory clearance or approval for our existing or new products. If we are unable to obtain clearances or approvals needed to market existing or new products, or obtain such clearances or approvals in a timely fashion, our business could be significantly disrupted, and sales and profitability could be materially and adversely affected.
We need the SFDA approval for our semi-automated ECLIA analyzer, fully-automated ECLIA analyzer and SPR analyzer before they can be used by the hospitals. The SFDA approvals we have obtained for our semi-automated ECLIA analyzer, fully-automated ECLIA analyzer and SPR analyzer will expire in July 2012, April 2014 and September 2013, respectively, and we will need to renew these approvals once they expire. We are also required to obtain a production permit from the provincial level food and drug administration before commencing the manufacture of our products. Our production permit for the manufacture of our ECLIA analyzer and reagent kits will expire in June 2015 and we will need to renew the production permit once it expires. Our production permit for the manufacture of our FISH probes, SPR analyzers and DNA chips will expire in May 2011, and we will need to renew the production permit once it expires. Renewing our registration certificates and production permit entails submission of various information and the review of that submission by the applicable regulatory authorities. We do not foresee any significant difficulties in obtaining such renewal. But if we fail to obtain such renewal in a timely fashion, our business may be adversely affected. See “Item 4. Information on the Company—B. Business Overview—Regulation”.
In April 2007, the SFDA announced a new regulation that became effective on June 1, 2007. Reagent kits used for IVD testing are divided into three different categories, Class I, Class II and Class III, depending on the degree of risk associated with each reagent kit. Our ECLIA reagent kits, FISH probes and DNA chips are classified as Class III, and they therefore are subject to all regulatory controls governing the Class III category. We are required to obtain a registration certificate for each reagent kit prior to selling that reagent kit for clinical use. A reagent kit that is used for research purposes only, however, is exempted from registration and/or approval. A reagent kit intended for research use only must comply with the labeling requirements that present the statement: “For research use only. Not for use in diagnostic procedures” on the package. Of the 95 ECLIA reagent kits and 46 FISH probes that we sell, 76 ECLIA reagent kits and 15 FISH probes are covered by registration certificates. Of the 19 ECLIA reagent kits we currently sell without registration certificates, 11 are food safety reagent kits which are not subject to SFDA registration and the remaining 8 reagent kits are being sold for research use only. Of the 8 ECLIA reagent kits and 31 FISH probes we currently sell for research use only, we have submitted registration applications for most of them. The registration certificate for our HPV-DNA chip was granted to us in May 2010. We intend to apply for registration certificates for most of our ECLIA reagent kits, FISH probes and DNA chips, but we may not succeed in obtaining registration certificates for these ECLIA reagent kits, FISH probes and DNA chips or for any ECLIA reagent kit, FISH probe or DNA chip that we develop in the future. Before receiving the necessary registration certificate, these ECLIA reagent kits, FISH
19
probes and DNA chips can be sold for research use only. Thus, this may delay or limit growth in sales of our ECLIA reagent kits, FISH probes and DNA chips. See “Item 4. Information on the Company—B. Business Overview—Regulation”.
Competition in the markets in which we operate is expected to increase in the future.
Our ECLIA system competes with IVD systems and reagent kits offered in the PRC market by many established international companies, including Abbott Diagnostics, Siemens Healthcare, Beckman Coulter, PerkinElmer, Johnson & Johnson and Roche Group. There are also a number of China-based manufacturers who also offer luminescence immunoassay systems.
Our FISH probes compete with probes offered by Abbott Diagnostics. We are not aware of any China-based manufacturers who offer FISH probes in the PRC.
Our SPR analyzers and HPV-DNA chips compete with HPV test products offered by Qiagen and certain China-based manufacturers. We are not aware of any China-based manufacturers who offer both SPR analyzers and HPV-DNA chips in the PRC.
A number of our existing and potential competitors have significantly greater financial, research and development, sales and marketing, personnel resources and other resources than we do. Competition will intensify as other companies enter our markets. Competing companies may succeed in developing products that are more effective or less costly than those that we may offer, and these companies may also be more successful in marketing their products. Competing companies may also introduce competitive pricing measures that adversely affect our sales levels and margins. If we do not adequately address our competitive challenges, we could lose sales and market share and fail to grow our business as planned, which would have a material adverse effect on our financial condition, results of operations and future growth.
In addition, we believe that corrupt practices in the healthcare industry in the PRC still occur. In order to increase sales, certain manufacturers or distributors of medical devices may pay kickbacks to hospital personnel who make procurement decisions. We prohibit our employees from engaging in such practices and, to our knowledge, none of our distributors engages in such practices. However, as competition intensifies in the medical device and supplies industry in the PRC, we may lose sales, customers or contracts to competitors to the extent we or our distributors refuse to engage in such practices.
The IVD industry is characterized by constant technological change, and if we fail to respond effectively to technological changes, we could lose our competitive advantage.
The IVD industry in which we currently compete are characterized by:
| | • | | frequent new product introductions and enhancements; |
| | • | | changing customer needs; and |
To develop new products and designs, we must develop or acquire and use leading technologies in a cost-effective and timely manner and continue to expand our technical and design expertise. Failure to do so could cause us to lose our competitive position and may cause a material adverse effect on our revenues in the future.
20
Risks Related to Doing Business in the PRC
Adverse changes in political, economic and other policies of the Chinese government could have a material adverse effect on the overall economic growth of the PRC, which could reduce the demand for our products and materially and adversely affect our competitive position.
Substantially all of our business operations are conducted in the PRC, and all of our sales are made in the PRC. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in the PRC. The Chinese economy differs from the economies of most developed countries in many respects, including:
| | • | | the extent of government involvement; |
| | • | | the level of development; |
| | • | | the control of foreign exchange; |
| | • | | the allocation of resources; |
| | • | | an evolving regulatory system; and |
| | • | | lack of sufficient transparency in the regulatory process. |
While the Chinese economy has experienced significant growth in the past 20 years, growth has been uneven, both geographically and among various sectors of the economy. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us.
The Chinese economy has been transitioning from a planned economy to a more market-oriented economy. Although in recent years the Chinese government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of the productive assets in the PRC is still owned by the Chinese government. The continued control of these assets and other aspects of the national economy by the Chinese government could materially and adversely affect our business. The Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Efforts by the Chinese government to slow the pace of growth of the Chinese economy could result in decreased expenditure by hospitals, which in turn could reduce demand for our products.
Moreover, the political relationship between the United States, Europe, or other Asian nations and the PRC is subject to sudden fluctuation and periodic tension. Changes in political conditions in the PRC and changes in the state of foreign relations are difficult to predict and could adversely affect our operations or cause our services to become less attractive. This could lead to a decline in our profitability.
Any adverse change in the economic conditions or government policies in the PRC could have a material adverse effect on overall economic growth and the level of healthcare investments and expenditures in the PRC, which in turn could lead to a reduction in demand for our products and consequently have a material adverse effect on our businesses.
21
Future changes in laws, regulations or enforcement policies in the PRC could adversely affect our business.
Laws, regulations or enforcement policies in the PRC, including those regulating medical devices and supplies, are evolving and subject to future change. For example, under a regulation enacted by the SFDA in September 2002 and was renewed in April 2007, reagent kits are required to be registered with the SFDA and are subject to regulatory controls. As of August 31, 2010, we have obtained SFDA registrations for 76 ECLIA reagent kits and 15 FISH probes we sell. We are in the process of obtaining the required registrations for most of the 8 ECLIA reagent kits and 31 FISH probes that are currently sold with research labels. The 11 food safety ECLIA reagent kits are not subject to SFDA registration. The registration certificate for our HPV-DNA chip was granted to us in May 2010. In 2007, the SFDA imposed certain quality control system assessment requirements for certain reagent products, covering our ECLIA reagent kits, FISH probes and HPV-DNA chips. We met such requirements for ECLIA reagent kits, FISH probes and HPV-DNA chips, respectively in February 2009, September 2009 and September 2009.
Future changes in laws, regulations or administrative interpretations, or stricter enforcement policies by the Chinese government, could impose more stringent requirements on us, including fines or other penalties. Changes in applicable laws and regulations may also increase our operating costs. Compliance with such requirements could impose substantial additional costs or otherwise have a material adverse effect on our business, financial condition and results of operations. These changes may relax some requirements, which could be beneficial to our competitors or could lower market entry barriers and increase competition. Further, regulatory agencies in the PRC may periodically, and sometimes abruptly, change their enforcement practice. Therefore, prior enforcement activity, or lack of enforcement activity, is not necessarily predictive of future actions. Any enforcement actions against us could have a material and adverse effect on us and the market price of our ADSs. In addition, any litigation or governmental investigation or enforcement proceedings in the PRC may be protracted and may result in substantial cost and diversion of resources and management attention, negative publicity, damage to our reputation and decline in the price of our ADSs.
We may not be able to cause our subsidiaries to distribute sufficient amount of dividends to us to satisfy our cash needs.
We are a holding company and have historically relied on proceeds from equity and debt financings as well as dividends paid by our wholly-owned operating subsidiaries, for our cash needs, including the funds necessary to pay dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. In the event equity and debt financings are not available in amounts or on terms acceptable to us, we may need to cause our wholly-owned subsidiaries to pay dividends to us for our cash needs. The payment of dividends in the PRC is subject to limitations. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in the PRC. YDME Beijing, Beijing GP and BBE are also required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves until the reserves reach 50% of the subsidiaries’ own registered capital. These reserves are not distributable as cash dividends. In addition, if these three PRC operating subsidiaries incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other distributions to us. Therefore, we may not be able to receive sufficient amount of dividends from our subsidiaries to satisfy our cash needs, and if such situation arises, we will not have the funds necessary to pay dividends and other cash distributions to our shareholders, to service our debt or pay our operating expenses. See “—If we receive dividends from our operating subsidiaries located in the PRC, such dividends may be subject to PRC withholding tax.”
Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.
We receive all of our revenues in Renminbi, which currently is not a freely convertible currency. A portion of our revenues may be converted into other currencies to meet our foreign currency obligations, including,
22
among others, payment of dividends declared, if any, in respect of our ordinary shares. Under the PRC’s existing foreign exchange regulations, YDME Beijing, Beijing GP and BBE are able to pay dividends in foreign currencies without prior approval from the State Administration of Foreign Exchange, or the SAFE, by complying with certain procedural requirements. However, we cannot assure you that the PRC government will not take future measures to restrict access to foreign currencies for current account transactions.
All our PRC subsidiaries’ ability to obtain foreign exchange is subject to significant foreign exchange controls and, in the case of amounts under the capital account, requires the approval of and/or registration with the PRC government authorities, including the SAFE. In particular, if any of these subsidiaries borrow foreign currency loans from us or other foreign lenders, they must do so within approved limits that satisfy their approval documentation and PRC debt to equity ratio requirements. Further, such loans must be registered with the SAFE. If we finance any of these subsidiaries by means of additional capital contributions, the amount of these capital contributions must first be approved by the relevant government approval authority. These limitations could affect the ability of these subsidiaries to obtain foreign exchange through debt or equity financing.
Fluctuation in the value of the Renminbi may have a material adverse effect on your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. The conversion of Renminbi into foreign currencies, including U.S. dollars, has historically been set by the People’s Bank of China. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a band against a basket of certain foreign currencies, determined by the People’s Bank of China, against which it can rise or fall by as much as 0.3% each day. On May 18, 2009, the PRC government further widened the daily trading band from 0.3% to 0.5%. On June 19, 2010, the People’s Bank of China decided to proceed further with reform of the exchange rate regime and to enhance the exchange rate flexibility. These changes in the policy resulted in a significant appreciation in the value of the Renminbi against the U.S. dollar between July 21, 2005 and September 3, 2010. Since the adoption of this new policy, the value of Renminbi against the U.S. dollar has fluctuated on a daily basis within narrow ranges, but overall has further strengthened against the U.S. dollar. There remains significant international pressure on the PRC government to further liberalize its currency policy, which could result in a further and more significant appreciation in the value of the Renminbi against the U.S. dollar. However, it is uncertain what additional measures will be taken and what their impact could be on exchange rates in the future. As we import certain materials and supplies for ECLIA reagent kits, FISH probes and HPV-DNA chips from the United States and some European countries, fluctuations in the value of the Renminbi against the currencies of those countries may increase the cost of our ECLIA reagent kits, FISH probes and HPV-DNA chips. For example, to the extent that we need to convert U.S. dollars we received from our initial public offering, the offering of the 2011 Notes and 2013 Notes into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount we receive from the conversion. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or ADSs or for other business purposes, appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount available to us. In addition, appreciation or depreciation in the value of the Renminbi relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations.
The discontinuation of any of the preferential tax treatments or the financial incentives currently available to us in the PRC could adversely affect our financial condition and results of operation.
On March 16, 2007, the PRC Enterprise Income Tax Law, or the EIT Law, was enacted, and, along with its implementing regulations, became effective on January 1, 2008. The EIT Law adopts a uniform tax rate of 25% for all enterprises (including foreign-invested enterprises) and eliminates most of the tax exemptions, reductions and preferential treatments available under the previous tax laws and regulations. Under the EIT Law, enterprises that were established and already enjoyed preferential tax treatments before March 16, 2007 (i) in the case of
23
preferential tax rates, continue to enjoy the tax rates which will be gradually increased to the new tax rates within five years from January 1, 2008 or (ii) in the case of preferential tax exemption or reduction for a specified term, continue to enjoy the preferential tax holiday until the expiration of such term. Specifically, an enterprise that was enjoying tax holidays on January 1, 2008 will continue to enjoy its existing tax holidays, unless such tax holidays did not yet start on January 1, 2008 due to the loss position of the enterprise, in which case such tax holidays commenced on January 1, 2008. In accordance with a notice issued by the State Council in December 2007 on the implementation of transitional preferential tax treatments, or the Transition Implementation Notice, enterprises that were subject to income tax at the rate of 15% prior to 2008 are subject to income tax at the rates of 18%, 20%, 22%, 24% and 25% in the years of 2008, 2009, 2010, 2011 and 2012, respectively.
The EIT Law, however, introduces new tax incentives, subject to various qualification criteria. The EIT Law and its implementing rules permit qualified “high and new technology enterprises” to enjoy a reduced 15% EIT rate. The recently published qualification criteria are significantly higher than those prescribed by the old tax rules under which we had been granted preferential treatment. YDME Beijing and BBE obtained the qualification certificates of “high and new technology enterprises” in 2008 with a validity period of three years starting from 2008 to 2010. However, the continuing qualification of YDME Beijing and BBE as “high and new technology enterprises” for the calendar year of 2010 will be subject to annual evaluation by the relevant government authorities in the PRC. In addition, YDME Beijing and BBE will need to apply for a three-year extension upon the expiration of the current qualification certificates if they want to continue to enjoy the 15% reduced rate. We cannot assure you that YDME Beijing and BBE will continue to qualify as “high and new technology enterprises” under the EIT Law, or that the local tax authorities will not, in the future, change their position and revoke any of our past preferential tax treatments. The discontinuation of any preferential tax treatments could materially increase our tax obligations.
Beijing GP was previously entitled to an EIT rate of 15% and was entitled to an exemption from EIT from January 1, 2008 to December 31, 2009 and a 50% income tax reduction from January 1, 2010 to December 31, 2012. While Beijing GP will be able to enjoy the tax holiday under the EIT Law and the Transition Implementation Notice, its 50% income tax reduction from January 1, 2010 to December 31, 2012 will be partially eliminated. Under the EIT Law and the Transition Implementation Notice, Beijing GP’s EIT rates are 0%, 0%, 11%, 12% and 12.5% for the calendar years from 2008 to 2012.
Any increase in the enterprise income tax rate applicable to us or discontinuation or reduction of any of the preferential tax treatments or financial incentives currently enjoyed by our PRC subsidiaries could adversely affect our business, operating results and financial condition.
If we receive dividends from our operating subsidiaries located in the PRC, such dividends may be subject to PRC withholding tax.
The EIT Law imposes a withholding tax of 10% on dividends distributed by a foreign-invested enterprise to its immediate holding company outside of the PRC, if such immediate holding company is considered as a “non-resident enterprise” without any establishment or place within the PRC or if the received dividends have no connection with the establishment or place of such immediate holding company within the PRC, unless such immediate holding company’s jurisdiction of incorporation has a tax treaty with the PRC that provides for a different withholding arrangement. The Cayman Islands, where we are incorporated, does not have such tax treaty with the PRC. According to the Arrangement Between Mainland China and Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion, dividends paid by a foreign-invested enterprise in the PRC to its direct holding company in Hong Kong will be subject to withholding tax at a rate of no more than 5% (if the foreign investor owns directly at least 25% of the shares of the foreign-invested enterprise). All of our subsidiaries in the PRC are invested and held by Hong Kong registered entities. Thus, dividends paid to us by our subsidiaries in the PRC may be subject to the 5% withholding tax if such Hong Kong registered entities are each considered as a “non-resident enterprise” under the EIT Law.
24
Nevertheless, the PRC State Administration of Taxation promulgated a tax notice on October 27, 2009, or Circular 601, which provides that tax treaty benefits will be denied to “conduit” or shell companies without business substance, and a beneficial ownership analysis will be used based on a “substance-over-form” principle to determine whether or not to grant tax treaty benefits. It is unclear at this early stage whether Circular 601 applies to dividends from our PRC subsidiaries paid to us through our Hong Kong subsidiaries. It is possible, however, that under Circular 601 our Hong Kong subsidiaries would not be considered to be the beneficial owners of any such dividends, and that such dividends would as a result be subject to income tax withholding at the rate of 10% rather than the favorable 5% rate applicable under the tax treaty between mainland China and Hong Kong.
We may be deemed a PRC resident enterprise under the EIT Law and be subject to PRC taxation on our worldwide income.
The EIT Law also provides that enterprises established outside of the PRC whose “de facto management bodies” are located in the PRC are considered “resident enterprises” and are generally subject to the uniform 25% EIT rate as to their worldwide income. Under the implementation regulations for the EIT Law issued by the PRC State Council, “de facto management body” is defined as a body that has material and overall management and control over the manufacturing and business operations, personnel and human resources, finances and treasury, and acquisition and disposition of properties and other assets of an enterprise. On April 22, 2009, the PRC State Administration of Taxation further issued a notice entitled “Notice Regarding Recognizing Offshore-Established Enterprises Controlled by PRC Shareholders as Resident Enterprises Based on Their Place of Effective Management.” Under this notice, a foreign company controlled by a PRC company or a group of PRC companies shall be deemed as a PRC resident enterprise, if (i) the senior management and the core management departments in charge of its daily operations mainly function in the PRC; (ii) its financial decisions and human resource decisions are subject to decisions or approvals of persons or institutions in the PRC; (iii) its major assets, accounting books, company seals, minutes and files of board meetings and shareholders’ meetings are located or kept in the PRC; and (iv) more than half of the directors or senior management personnel with voting rights reside in the PRC. Substantially all of our operational management is currently based in the PRC. If we are treated as a resident enterprise for PRC tax purposes, we will be subject to PRC tax on our worldwide income at the 25% uniform tax rate, which could have an impact on our effective tax rate and an adverse effect on our net income and results of operations, although dividends distributed from our PRC subsidiaries to us could be exempt from PRC dividend withholding tax, since such income is exempted under the EIT Law to a PRC resident recipient.
Interest and dividends payable by us to our foreign investors and gain on the sale of our Convertible Notes, ADSs and ordinary shares may become subject to taxes under PRC tax laws.
Under the EIT Law and implementation regulations issued by the State Council, PRC income tax at the rate of 10% is applicable to interest and dividends payable by resident enterprises to investors that are “non-resident enterprises,” which do not have an establishment or place of business in the PRC, or which have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such interest and dividends have their sources within the PRC. Similarly, any gain realized on the transfer of the notes, ADSs or ordinary shares issued by resident enterprises to such investors is also subject to 10% PRC income tax if such gain is regarded as income derived from sources within the PRC. Therefore, if we are considered a PRC “resident enterprise,” and interest we pay with respect to our Convertible Notes and dividends we pay with respect to ADSs or ordinary shares, gain realized from the transfer of our Convertible Notes, ADSs or ordinary shares are considered as income derived from sources within the PRC, such income may be subject to PRC tax. If we are required under the EIT Law to withhold PRC income tax on interest or dividends payable to our non-PRC investors that are “non-resident enterprises,” our ability to make payments on our Convertible Notes may be affected. In addition, if such non-PRC investors are required to pay PRC income tax on the transfer of our Convertible Notes, ADSs or ordinary shares, the value of an investment in our Convertible Notes, ADSs or ordinary shares may be materially and adversely affected.
25
We may need to pay additional tax in connection with the gain on sale of the HIFU business in December 2008, which could negatively impact our financial condition and results of operations.
The PRC State Administration of Taxation promulgated a tax notice on December 10, 2009, or Circular 698, which provides the administration and tax reporting guidance for any gains generated from indirect offshore transfers of equity interests in PRC enterprises that are not taxable overseas. It is unclear at this early stage whether Circular 698 applies to the gain on sale of our HIFU business in December 2008. It is possible, however, that under Circular 698, we will need to pay additional tax in connection with the gain on sale of our HIFU business generated from indirect offshore transfers of equity interests in PRC enterprises that are not taxable overseas. Any additional tax that we may need to pay in the future will negatively impact our financial condition and results of operations.
PRC regulation of loans and direct investment by offshore holding companies to PRC entities may delay or prevent us from using the proceeds of our equity or debt offerings to make loans or additional capital contributions to our PRC operating subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
In utilizing the proceeds of any equity or debt offerings, as an offshore holding company of our PRC operating subsidiaries, we may make loans to our PRC subsidiaries, or we may make additional capital contributions to our PRC subsidiaries. Any loans to our PRC subsidiaries are subject to PRC regulations and approvals. For example, loans by us to our wholly owned subsidiaries in the PRC, each of which is a foreign-invested enterprise, to finance their activities cannot exceed statutory limits and must be registered with the SAFE or its local counterpart.
We may also decide to finance our wholly owned subsidiaries by means of capital contributions. These capital contributions must be approved by the Ministry of Commerce in the PRC or its local counterpart. We cannot assure you that we will be able to obtain these government registrations or approvals on a timely basis, if at all, with respect to future loans or capital contributions by us to our PRC subsidiaries or any of their respective subsidiaries. If we fail to receive such registrations or approvals, our ability to use the proceeds of any equity or debt offerings and to capitalize our PRC operations may be negatively affected, which could adversely and materially affect our liquidity and our ability to fund and expand our business.
All participants in our equity compensation plans who are PRC citizens may be required to register with SAFE. We may also face regulatory uncertainties that could restrict our ability to adopt additional equity compensation plans for our directors, employees and other parties under PRC law.
In December 2006, PBOC promulgated the Administrative Measures for Individual Foreign Exchange, which set forth the respective requirements for foreign exchange transactions by PRC individuals under either the current account or the capital account. The Implementation Rules of the Administrative Measures for Individual Foreign Exchange, issued in January 2007 by SAFE, specify the approval requirements for PRC citizens who are granted shares or share options by an overseas listed company according to its employee stock ownership plan or stock option plan.
In March 2007, SAFE promulgated the Processing Guidance on Foreign Exchange Administration for Domestic Individuals Participating in Employee Stock Holding Plans or Stock Option Plans of Overseas-Listed Companies, or the Share Option Rule. According to the Share Option Rule, PRC citizens who are granted shares or share options by an overseas listed company according to its employee share option or share incentive plan are required, through the PRC subsidiary of such overseas listed company or other qualified PRC agents, to register with SAFE and complete certain other procedures related to the share option or other share incentive plan. Foreign exchange income from the sale of shares or dividends distributed by the overseas listed company may be remitted into a foreign currency account of such PRC citizen or be exchanged into Renminbi. In addition, the overseas listed company or its PRC subsidiary or other qualified PRC agent is required to appoint an asset manager or administrator, appoint a custodian bank and open dedicated foreign currency accounts to handle
26
transactions relating to the share option scheme or other share incentive plan. We and our PRC citizen employees who have been and will be granted share options or restricted shares, or PRC option holders, are subject to these rules. If we or our PRC option holders fail to comply with these rules, we or our PRC option holders may be subject to fines and legal or administrative sanctions.
We may incur substantial increases in labor cost due to the promulgation of the new labor contract law.
In June 2007, the Standing Committee of the National People’s Congress enacted the Labor Contract Law, which became effective on January 1, 2008. Compared to the Labor Law, the Labor Contract Law establishes more restrictions and increases the cost to employers upon termination of employees, including specific provisions related to fixed-term employment contracts, temporary employment, probation, consultation with the labor union and employee general assembly, employment without a contract, dismissal of employees, compensation upon termination and overtime work, and collective bargaining. According to the Labor Contract Law, an employer is obligated to sign an unlimited term labor contract with an employee if the employer continues to employ the employee after two consecutive fixed term labor contracts. The employer also has to pay compensation to employees if the employer terminates an unlimited term labor contract. Unless an employee refuses to extend an expired labor contract, compensation is also required when the labor contract expires and the employer does not extend the labor contract with the employee under the same terms or better terms than those in the original contract. Further, under the Regulations on Paid Annual Leave for Employees, which became effective on January 1, 2008, employees who have served more than one year with an employer are entitled to a paid vacation ranging from five to 15 days, depending on their length of service. Employees who waive such vacation time at the request of employers shall be compensated at three times their normal salaries for each waived vacation day. As a result of these new protective labor measures, our labor costs may increase. We cannot give assurance that any disputes, work stoppages or strikes will not arise in the future.
We could be subject to labor disruptions that interfere with our operations and adversely affect our business, financial condition and results of operations.
We could be subject to labor disputes and adverse employee relations which could disrupt our operations and adversely affect our business, financial condition and results of operations. A portion of our employees is represented by labor unions, and it is possible that such employees, and our other employees, could attempt to take collective action against us if they are unhappy with their employment conditions. Existing employment agreements with our employees and labor union agreements may not prevent a strike or work stoppage in the future. There can be no assurance that we will not experience labor disputes and/or adverse employee relations in the future.
We face risks related to health epidemics and other outbreaks of contagious diseases, including swine flu, avian flu, severe acute respiratory syndrome, or SARS.
Our business could be adversely affected by the effects of swine flu, avian flu, SARS or another epidemic or outbreak. During April and May 2009, there have been outbreaks of highly pathogenic swine flu, caused by the H1N1A virus, in certain regions of the world, including parts of Asia. In 2007 and early 2008, there were reports of outbreaks of a highly pathogenic avian flu, caused by the H5N1 virus, in certain regions of Asia and Europe. In 2005 and 2006, there were reports on the occurrences of avian flu in various parts of the PRC, including a few confirmed human cases. An outbreak of avian flu in the human population could result in a widespread health crisis that could adversely affect the economies and financial markets of many countries, particularly in Asia. Additionally, any recurrence of SARS, a highly contagious form of atypical pneumonia, similar to the occurrence in 2003 which affected the PRC, Hong Kong, Taiwan, Singapore, Vietnam and certain other countries, would also have similar adverse effects. These outbreaks of contagious diseases, and other adverse public health developments in the PRC, would have a material adverse effect on our business operations. These could include restrictions on our ability to travel or to ship our products within the PRC, as well as cause temporary closure of our manufacturing facilities. Such closures or travel or shipment restrictions would severely disrupt our business
27
operations and adversely affect our financial condition and results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of swine flu, avian flu, SARS or any other epidemic.
Our operations are subject to the uncertainty associated with the legal system in the PRC, which could adversely affect our business, or limit the legal protection available to us or to existing or potential investors.
We conduct our business through our operating subsidiaries in the PRC, which are governed by the PRC law. The PRC is a civil law jurisdiction based on written codes and statutes. Unlike common law jurisdictions, prior court decisions may be cited as persuasive authority but do not have legally binding force. The PRC government has promulgated laws and regulations in relation to economic matters in general, such as foreign investment, corporate organization and governance, commerce, taxation and trade, with a view to establishing a comprehensive legal system conducive to investment activities. However, the implementation, interpretation and enforcement of these laws and regulations may involve greater uncertainty compared to those in the common law jurisdictions due to a relatively short legislative history, limited volume of court cases and their non-binding nature. Furthermore, many laws, regulations and legal requirements have only recently been adopted by the central or local government agencies, and their implementation, interpretation and enforcement may involve uncertainty due to the lack of established practice available for reference. The PRC administrative and court authorities also have significant discretion in interpreting and enforcing statutory and contractual terms. It thus may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection available than in more developed legal systems. These uncertainties may also impede our ability to enforce the contracts we have entered into with our business partners, customers and suppliers. Vis-à-vis our competitors, depending on the government agency or how an application or a case is presented to such agency or other variables, we may receive less favorable application of law. In addition, any litigation or legal proceeding in the PRC may be protracted and result in substantial legal costs and diversion of resources and management attention. We cannot predict the effect of future legal developments in the PRC, including promulgation of new laws, changes to existing laws or the interpretation or enforcement thereof, the preemption of local regulations by national law, the overturn or modification of the lower-level authority’s decisions at the higher level, or the changes in judiciary and administrative practices. As a result, there is substantial uncertainty as to the legal protection available to us or to our shareholders.
Risks Related to Our Ordinary Shares and our ADSs
Activities in connection with hedging transactions may affect the market price of our ADSs.
Activities by investors in our Convertible Notes, affiliates of the underwriters and others in connection with hedging transactions may affect the market price of our ADSs from time to time. For example, in connection with the settlement of a hedging transaction, the underwriters or their affiliates may purchase ADSs and investors in our Convertible Notes may sell ADSs, which could temporarily increase or decrease (or delay an increase or decrease in) the market price of our ADSs. In addition, investors in our Convertible Notes may wish to purchase or sell Convertible Notes or adjust any hedging transactions from time to time, such that the short position created through the concurrent offering of the purchased ADSs may be larger or smaller than needed. As a result, hedge adjustments may result in the buying or selling of ADSs by investors, affiliates of the underwriters or others, which may affect the market price of our ADSs.
Because the purchased ADSs may be considered outstanding for the purpose of computing and reporting our earnings per ADS, the purchased ADSs could decrease our earnings per ADS and potentially the market price of our ADSs.
While the ordinary shares underlying the purchased ADSs will be considered issued and outstanding for purposes of Cayman Islands Companies Law, we believe that under U.S. GAAP currently in effect, the purchased ADSs will not be considered outstanding for the purpose of computing earnings per ADS because,
28
pursuant to the ADS issuance and repurchase agreements, among other things, upon conversion, repurchase or payment at maturity of all of the Convertible Notes, the ADS Purchasers are obligated to deliver to us a number of purchased ADSs, unless earlier delivered pursuant to the ADS issuance and repurchase agreements. If these accounting principles, or our interpretation thereof, were to change in the future, we may become required to treat the purchased ADSs as outstanding for purposes of computing and reporting our earnings per ADS, our earnings per ADS would be reduced and the market price of our ADSs could decrease, possibly significantly.
The market price for our ADSs may be volatile.
The market price for our ADSs has been, and may continue to be, subject to significant fluctuations. Since August 10, 2005, the intraday sales prices of our ADSs on the NASDAQ Global Select Market (then called “the NASDAQ National Market”) have ranged from US$9.40 to US$57.50 per ADS, and the closing sale price on September 3, 2010 was US$11.61 per ADS. The price of our ADSs may fluctuate in response to factors including the following:
| | • | | announcements of technological or competitive developments; |
| | • | | announcements regarding patent litigation or the issuance of patents to us or our competitors; |
| | • | | governmental developments in the PRC, such as changes in fiscal policies, tax policies or developments relating to the regulatory or health care reimbursement environment, affecting us or our competitors; |
| | • | | announcements of studies and reports relating to the effectiveness or safety of our products or those of our competitors; |
| | • | | actual or anticipated fluctuations in our quarterly operating results and changes or revisions of our expected results; |
| | • | | changes in financial estimates by securities research analysts; |
| | • | | changes in the economic performance or market valuations of other medical technology companies; |
| | • | | addition or departure of our senior management and key research and development personnel; |
| | • | | negative publicity about us or our products or allegation of irregularities or improper conduct of our management, whether true or false; |
| | • | | any litigation, governmental investigation or enforcement proceedings brought against us by authorities and industry regulators in the PRC or elsewhere; |
| | • | | release or expiry of lock-up or other transfer restrictions on our outstanding ordinary shares or ADSs; and |
| | • | | sales of additional ordinary shares or ADSs, or the perception that such sales might occur. |
Accordingly, holders of our ADSs will be subject to the risk of volatility and depressed prices of our ADSs.
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also have a material adverse effect on the market price of our ADSs.
In addition, any sales in the public market of ADSs issued upon conversion of the Convertible Notes could adversely affect prevailing market prices of our ADSs.
29
Future sales of our ordinary shares or ADSs in the public market or the issuance of securities senior to our ordinary shares could adversely affect the trading price of our ADSs and our ability to raise funds in new share offerings.
Future sales of substantial amounts of our ordinary shares or ADSs or other equity-related securities in the public market, including sales by any existing shareholders, or the perception that such sales could occur, could adversely affect prevailing trading prices of our ADSs and could impair our ability to raise capital through future offerings of equity or equity-related securities. No prediction can be made as to the effect, if any, that future sales of our ordinary shares or ADSs or the availability of our ordinary shares or ADSs for future sale, will have on the trading price of our ADSs.
Holders of ADSs will have fewer rights than holders of ordinary shares and must act through the depositary to exercise those rights.
As a holder of our ADSs, you will not have the same rights as holders of our ordinary shares, and you may only exercise the voting rights with respect to the underlying ordinary shares in accordance with the provisions of the deposit agreement. Under the deposit agreement, if the vote is by show of hands, the depositary will vote the deposited securities in accordance with the voting instructions received from a majority of holders of ADSs that provided timely voting instructions. If the vote is by poll, the depositary will vote the deposited securities in accordance with the voting instructions it timely receives from ADS holders. In the event of poll voting, deposited securities for which no instructions are received will not be voted. Under our articles of association, the minimum notice period required to convene a general meeting is seven days. When a general meeting is convened, you may not receive sufficient notice of a shareholders’ meeting to permit you to withdraw your ordinary shares to allow you to cast your vote with respect to any specific matter. In addition, the depositary and its agents may not be able to send voting instructions to you or carry out your voting instructions in a timely manner. We will make all reasonable efforts to cause the depositary to extend voting rights to you in a timely manner, but we cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. Furthermore, the depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, you may not be able to exercise your right to vote and you may lack recourse if your ADSs are not voted as you requested. In addition, in your capacity as an ADS holder, you will not be able to call a shareholder meeting.
You may be subject to limitations on transfers of your ADSs.
Although your ADSs will be transferable on the books of the depositary, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
You may not receive cash dividends if it is impractical to make them available.
The depositary of our ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary may, at its discretion, decide that it is inequitable or impractical to make a distribution available to any holders of ADSs. For example, the depositary may determine that it is not practicable to distribute certain property through the mail, or that the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may decide not to distribute such property, in which event you would not receive such distribution.
30
Your right to participate in any future rights offerings may be limited, which may cause dilution to your holdings.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to holders of our ADSs in the United States unless we register the rights and the securities to which the rights relate under the Securities Act of 1933, as amended, or the Securities Act, or an exemption from the registration requirements is available. Also, under the deposit agreement, the depositary will not make rights available to holders of our ADSs unless the distribution to ADS holders of both the rights and any related securities are either registered under the Securities Act, or exempted from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. Accordingly, you may be unable to participate in our rights offerings and may experience dilution in your holdings of ADSs.
Our articles of association contain anti-takeover provisions that could adversely affect the rights of holders of our ordinary shares and ADSs.
Our articles of association contain provisions that could limit the ability of others to acquire control of our Company or cause us to engage in change-of-control transactions. These provisions could have the effect of depriving our holders of our ordinary shares and holders of our ADSs of an opportunity to sell their ordinary shares and ADSs, as applicable, at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of our Company in a tender offer or similar transaction. For example, our board of directors has the authority, without further action by our shareholders, to issue preferred shares in one or more series and to fix their designations, powers, preferences, privileges, and relative participating, optional or special rights and the qualifications, limitations or restrictions, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights associated with our ordinary shares, in the form of ADS or otherwise. Preferred shares could be issued quickly with terms calculated to delay or prevent a change in control of our Company or make removal of management more difficult. If our board of directors issue preferred shares, the price of our ADSs may fall and the voting and other rights of the holders of our ordinary shares and ADSs may be adversely affected.
We are a Cayman Islands company, and because the rights of shareholders under Cayman Islands law differ from those under United States law, you may have difficulty protecting your shareholder rights.
We are an exempted company with limited liability incorporated under Cayman Islands law, and substantially all of our assets are located outside the United States. In addition, a majority of our directors and executive officers reside outside the United States, and a substantial portion of their assets is located outside of the United States. As a result, it may be difficult for you to effect service of process within the United States upon our directors or executive officers, or enforce judgments obtained in the United States courts against our directors or executive officers.
Our corporate affairs are governed by our memorandum and articles of association, the Cayman Islands Companies Law and the common law of the Cayman Islands. The rights of shareholders to take legal action against our directors and us, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, which has persuasive, but not binding, authority on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law may not be as clearly established as they would be under statutes or judicial precedents in the United States. In particular, the Cayman Islands has a less developed body of securities laws as compared to the United States and some states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law. In addition, shareholders in Cayman Islands companies may not have standing to initiate a
31
shareholder derivative action before the federal courts of the United States and will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records.
Walkers, our counsel as to Cayman Islands Law, have advised that there is no statutory mechanism by which a judgment obtained in the United States courts can be recognized or enforced in the Cayman Islands. At common law, a foreign judgment which is in personam (a judgment against a person or other legal entity such as a company) may be recognized if:
| | • | | the judgment debtor was, at the time the foreign proceedings were instituted, present in the foreign country (which could be presence through an agent or representative office); |
| | • | | the judgment debtor was plaintiff, or counter-claimed, in the proceedings in the foreign country; |
| | • | | the judgment debtor, being a defendant in the foreign court, voluntarily submitted to the jurisdiction of that court by participating in the foreign proceedings; or |
| | • | | if the judgment debtor had, before the commencement of the foreign proceedings agreed, in respect of the subject matter of the proceedings, to submit to the jurisdiction of that court or the courts of that country. |
The Cayman Islands court must also be satisfied that the judgment was not obtained by fraud and the proceedings in which the judgment was obtained were not contrary to natural justice and the judgment is final and conclusive on the merits. The usual procedure for enforcement of a foreign judgment is to commence proceedings on the judgment.
Walkers has further advised us that the courts of the Cayman Islands would not entertain original actions brought in the Cayman Islands against us or our directors or officers which only relate to the securities laws of the United States or any state in the United States. An original action brought in the Cayman Islands must give rise to a cause of action in the Cayman Islands (whether pursuant to Cayman Islands statutes or the common law).
In relation to statutory law, an action may only be commenced in a Cayman Islands court in respect of statutes enacted or in force in the Cayman Islands.
There is uncertainty regarding whether Cayman Islands courts would:
| | • | | recognize or enforce against us judgments of courts of the United States based on certain civil liability provisions of United States securities laws; and |
| | • | | impose liability against us, in original actions brought in the Cayman Islands, based on certain civil liability provisions of United States securities laws. |
There is no statutory recognition in the Cayman Islands of judgments obtained in the United States, although the courts of the Cayman Islands will generally recognize and enforce a non-penal judgment of a foreign court of competent jurisdiction of a fixed sum that is not contrary to natural justice or the public policy of the Cayman Islands without retrial on the merit.
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests with respect to actions taken by our management, directors or major shareholders than they would as public shareholders of a United States company.
We may be or become a passive foreign investment company, which could result in adverse U.S. federal income tax consequences to U.S. investors.
We may be classified as a passive foreign investment company, or PFIC, by the U.S. Internal Revenue Service for U.S. federal income tax purposes. Such characterization could result in adverse U.S. federal income
32
tax consequences to you if you are a U.S. investor. For example, U.S. investors who owned our ordinary shares or ADSs during any taxable year in which we were a PFIC generally are subject to increased U.S. tax liabilities and reporting requirements for that taxable year and all succeeding years, regardless of whether we actually continue to be a PFIC, although a shareholder election to terminate such deemed PFIC status may be available in certain circumstances.
The determination of whether or not we are a PFIC is made on an annual basis and depends on the composition of our income and assets, including goodwill, from time to time. Specifically, we will be classified as a PFIC for U.S. tax purposes for a taxable year if either (a) 75.0% or more of our gross income for such taxable year is passive income, or (b) 50.0% or more of the average percentage of our assets during such taxable year either produce passive income or are held for the production of passive income. For such purposes, if we directly or indirectly own 25.0% or more of the shares of another corporation, we generally will be treated as if we (a) held directly a proportionate share of the other corporation’s assets, and (b) received directly a proportionate share of the other corporation’s income.
We do not believe that we are currently a PFIC. However, because the PFIC determination is highly fact intensive and made at the end of each taxable year, there can be no assurance that we will not be a PFIC for the current or any future taxable year or that the IRS will not challenge our determination concerning our PFIC status.
Under recently enacted U.S. legislation and subject to future guidance, if we are a PFIC, U.S. Holders will be required to file, for returns due after March 18, 2010, an annual information return with the IRS relating to their ownership of our common shares or ADSs. Although expected, no guidance has yet been issued about such return, including on the information required to be reported on such return, the form of the return, or the due date for the return.
For further discussion of the adverse U.S. federal income tax consequences of our possible classification as a PFIC, see “Item 10. Additional Information—E. Taxation—United States Federal Income Taxation.”
| ITEM 4. | INFORMATION ON THE COMPANY |
| A. | History and Development of our Company |
Our predecessor company, Beijing Yuande Bio-Medical Engineering Co., Ltd., or YDME Beijing, was incorporated in the PRC and commenced business operations in July 1999. In anticipation of our initial public offering, we incorporated CMED in the Cayman Islands as a listing vehicle in July 2004. CMED is an exempted company with limited liability incorporated under Cayman Islands law. CMED became our ultimate holding company when it issued shares to the existing shareholders of YDME Beijing in January 2005 in exchange for all of the shares that these shareholders held in YDME Beijing. Prior to our acquisition of the FISH business in March 2007, we conducted substantially all of our operations through YDME Beijing. In June 2007, we completed the full integration of the FISH business and launched it to the market through Beijing GP Medical Technologies Co., Ltd., or Beijing GP. In December 2008, we acquired the SPR technology and related assets through CMED Technologies Ltd. In December 2008, we sold the HIFU business to Chengxuan International Ltd. and discontinued the HIFU business. We started to conduct the SPR business through Beijing GP in April 2009.
In March 2007, we established a wholly-owned subsidiary, CMED ECLIA Diagnostic Technology Ltd, for investment holding purposes. CMED (HK) Limited was set up in April 2007 to provide administrative services to our group companies. CMED ECLIA Diagnostic Technology Ltd acquired Beijing Bio-Ekon Biotechnology Co., Ltd., or BBE, in January 2008. BBE develops, manufactures and sells semi-automated ECLIA analyzers and also offers a range of ECLIA reagent kits. In April and May 2010, we established two wholly-owned subsidiaries, CMED International Ltd and CMED South East Asia Ltd, for investment holding purposes. In June 2010, we
33
established a wholly-owned subsidiary, CMT Diagnostics (Singapore) Pte. Ltd. We expect to develop our diagnostics business in Singapore through this newly established subsidiary. We currently conduct substantially all of our operations through YDME Beijing, Beijing GP and BBE.
Our principal executive offices are located at No. 24 Yong Chang North Road, Beijing Economic-Technological Development Area, Beijing 100176, People’s Republic of China. Our telephone number at this address is (8610) 6787-1166 and our fax number is (8610) 6788-9588.
Investor inquiries should be directed to us at the address and telephone number of our principal executive offices set forth above. Our website ishttp://www.chinameditech.com. The information contained on our website is not part of this annual report on Form 20-F. Our agent for service of process in the United States is Corporation Service Company located at 2711 Centerville Road, Wilmington, DE 19808.
We made capital expenditures of RMB664.9 million, RMB1,789.7 million and RMB715.5 million (US$104.8 million) in the fiscal years ended March 31, 2008, 2009 and 2010, respectively. Such capital expenditures principally consisted of expansion of production facilities for ECLIA products, FISH probes, SPR analyzers and HPV-DNA chips and payments in relation to our acquisition of the FISH and BBE businesses as well as SPR technologies and related assets. We expect to spend approximately RMB34.1 million (US$5.0 million) in the year ending March 31, 2011, consisting primarily of the purchase of new manufacturing equipment to enlarge our production capacity and the renovation of our existing facilities.
Overview
We are a leading China-based medical device company that develops, manufactures and markets advanced immunodiagnostic and molecular diagnostic products utilizing ECLIA technology, FISH technology and SPR technology. Immunodiagnostic products include ECLIA analyzers and reagent kits, while molecular diagnostic products include FISH probes, SPR analyzers and HPV-DNA chips (and fluorescent microscopes until April 2009). Our IVD diagnostic products are used for the detection of various cancers and other diseases and disorders and also serve as companion diagnostics tests for targeted cancer drugs.
Our ECLIA business, which consists of ECLIA analyzers and reagent kits, is an IVD system based on ECLIA technology we acquired in August 2004 and January 2008. The ECLIA system is a type of IVD system commonly used in the United States, Western Europe and the PRC. Our ECLIA system is a “closed” system in that only our ECLIA reagent kits can be used with our ECLIA analyzers and our ECLIA analyzers can only be used with our ECLIA reagent kits. In April 2008, in order to further expand the installed base of our semi-automated ECLIA analyzers and increase recurring revenue from sales of reagent kits, we started to provide our semi-automated ECLIA analyzers free of charge to hospitals with certain diagnostic volume to promote the sales of our ECLIA reagent kits. We had over 3,500 units of our semi-automated ECLIA analyzers installed in small and medium-sized hospitals as of March 31, 2010. In April 2010, we received the SFDA approval for our fully-automated ECLIA analyzers, which automatically mix patient samples with reagents allowing for more accurate and higher volume of tests within a period of time in comparison to semi-automated analyzers. The target market for our fully-automated ECLIA analyzers is large hospitals as well as high volume users among medium-sized hospitals. We expect to deliver our fully-automated ECLIA analyzers free of charge to certain large hospitals in 2011. We currently offer 95 types of reagent kits for use with our ECLIA analyzers. These reagent kits are used to detect and monitor various types of diseases and disorders, including anemia, cardiac diseases, diabetes, food safety, growth disorders, infectious diseases, infertility, liver fibrosis, reproductive endocrinology, thyroid disorders and various types of tumors and viruses.
Our FISH business, which consists of FISH probes, utilizes molecular diagnostic reagents based on FISH technology we acquired in March 2007. FISH probes are molecular diagnostic reagents used with a fluorescent
34
microscope for (i) the prenatal and postnatal diagnosis of various genetic diseases, (ii) the early detection and prognosis of various cancers and (iii) the identification of cancer patients who would benefit from targeted drugs in the treatment of various cancers. FISH technology enables medical practitioners to visualize and locate DNA sequences in human cells for identifying chromosomal abnormalities. Although widely used in hospitals in the United States and Western Europe, FISH technology is relatively new to the PRC market. We began marketing and selling FISH probes in June 2007. We currently offer 46 FISH probes that are used to detect various genetic syndromes and cancers including bladder cancer, cervical cancer, prostate cancer, hematological malignancies, as well as companion diagnostic tests for targeted cancer drugs for breast cancer and lung cancer. Our FISH probes can be used with fluorescent microscopes purchased from third party manufacturers. We also sold fluorescent microscopes to customers which were manufactured by third parties until April 2009.
Our SPR business, which consists of SPR analyzers and HPV-DNA chips, is a molecular diagnostic system based on SPR technology we acquired in December 2008. Our SPR system is referred to as a “closed” system since our SPR analyzers can only be used with DNA chips manufactured by us and our DNA chips can only be used with our SPR analyzers. SPR is a leading biosensor technology in molecular biology used for the analysis of proteins, nucleic acids and viruses, including testing for HPV. The HPV-DNA chips, which we currently offer for use with our SPR analyzers, are label-free DNA chips for the diagnosis of HPV infection and genotyping of HPV. This technology is relatively new to the PRC market and offers significant market potential as HPV is responsible for nearly all cases of cervical cancer. We started to deliver our SPR analyzers free of charge to large hospitals in January 2010 and generate revenue from the sale of HPV-DNA chips in June 2010 following approval by the SFDA which was granted in May 2010. As of June 30, 2010, we delivered 49 SPR analyzers free of charge to large hospitals which are also users of our FISH probes.
We have significant experience and demonstrated ability in identifying, acquiring and commercializing medical technologies with significant market potential. We have acquired the ECLIA, FISH and SPR technology platforms, successfully integrated these technologies into our existing operations and developed and marketed products utilizing these technologies.
We sell our ECLIA reagent kits solely through distributors, who mainly target small to medium-sized hospitals. As our ECLIA system is a “closed” system, we believe that a large installed base of our ECLIA analyzers will enable us to derive a steady source of recurring revenue from sales of our reagent kits. In April 2008, in order to further expand the installed base of our ECLIA analyzers and to derive additional recurring revenue from sales of our reagent kits, we started to provide our semi-automated ECLIA analyzers free of charge to hospitals with certain diagnostic volume to promote the sales of our reagent kits. We also expect to deliver our fully-automated ECLIA analyzers free of charge to certain large hospitals in 2011. In April 2008, in order to promote the sales of our FISH probes, we started to recommend hospital customers to purchase fluorescent microscopes directly from microscope manufacturers so that these hospitals could reduce the cost of setting up the FISH test platform and expedite the commencement of providing this diagnostic service to patients. Our FISH probes and HPV-DNA chips are marketed and sold through our own sales force which mainly targets large hospitals. We had 214 sales and marketing employees as of July 31, 2010. We also provide our SPR analyzers free of charge to large hospitals which are also users of our FISH probes to promote the sales of our HPV-DNA chips. Our revenue streams currently come primarily from the recurring sales of ECLIA reagent kits and FISH probes, and we expect the recurring sales of HPV-DNA chips will become an additional main revenue stream in the future.
Our Strengths
We believe that our principal strengths include the following:
| | • | | leading position in the advanced immunoassay and molecular diagnostic market in the PRC; |
| | • | | established direct sales team and network of independent distributors in the PRC; |
| | • | | strong research and development capabilities; |
35
| | • | | expanding product portfolio and recurring base of revenue; |
| | • | | ability to identify, acquire and commercialize advanced medical technologies with significant market potential; and |
| | • | | high quality and low cost manufacturing base. |
Our Strategies
We intend to grow our business through the following strategies:
| | • | | expand and leverage our sales networks and our relationships with large hospitals; |
| | • | | expand product portfolio through strong research and development capabilities; |
| | • | | continue to promote market awareness and clinical benefits of our products; and |
| | • | | selectively acquire, license or distribute complementary products or technologies. |
Our Products and Services
ECLIA Reagent Kits and ECLIA Analyzers
Our ECLIA system, which consists of reagent kits and an ECLIA analyzer, is a closed IVD system based on ECLIA technology we acquired in August 2004 and January 2008.
To perform a test using our ECLIA system, an ECLIA reagent is mixed with a sample of body fluid from the patient in a microplate provided in our reagent kit. The mixture reacts and emits light, which is measured and analyzed by the ECLIA analyzer. The result is compared with the reference ranges for the particular reagent in order to reach a diagnosis. Our ECLIA analyzer is a sensitive, accurate, low cost and simple-to-use IVD device capable of detecting minute levels of light triggered by combining reagents with body fluid samples to produce diagnostic results. We have developed several methods that make our photon counter, a key component of our ECLIA analyzer, more sensitive and accurate. Our ECLIA analyzer uses our proprietary technology to analyze and organize the information produced and contains encryption codes that prevent users from conducting tests with reagent kits produced by other manufacturers.
We derive revenue from recurring sales of ECLIA reagent kits. In April 2008, in order to further expand the installed base of our semi-automated ECLIA analyzers and increase recurring revenue from sales of reagent kits, we started to provide our semi-automated ECLIA analyzers free of charge to hospitals with certain diagnostic volume to promote the sales of our ECLIA reagent kits.
Recently, we have received SFDA approval for our fully-automated ECLIA analyzers. The target market for our fully-automated ECLIA analyzers is large hospitals as well as high volume users among medium-sized hospitals. We expect to deliver our fully-automated ECLIA analyzers free of charge to certain large hospitals in 2011.
36
We currently offer 95 types of reagent kits for detecting and monitoring various types of diseases and disorders, including anemia, cardiac diseases, diabetes, food safety, growth disorders, infectious diseases, infertility, liver fibrosis, reproductive endocrinology, thyroid disorders and various types of tumors and viruses. We are also developing reagent kits for other applications such as infectious diseases, infertility disorders and cardiac diseases. The following table sets out the major clinical applications of our reagent kits:
| | | | |
Type of Test | | Number of
Reagent Kits | | Description |
| Anemia | | 1 | | To evaluate levels of serum ferritin in diagnosing iron deficiency anemia. |
| Cardiac diseases | | 1 | | To test the effectiveness of digoxin, a widely used drug for treatment of heart failure. |
| Food safety | | 11 | | To test for toxic chemicals in food products. Food safety reagents are not subject to SFDA approval. |
| Growth disorders | | 1 | | Early diagnosis, identification and prognosis evaluation of problems relating to growth and development. |
| Hepatitis | | 15 | | Basic tests hospitals perform to test for hepatitis A, B and C. |
| HIV | | 1 | | To test for human immunodeficiency virus (HIV), a virus that can lead to acquired immunodeficiency syndrome (AIDS). |
| Infertility | | 7 | | To test for the sterility caused by the malfunction of immune system. |
| Liver fibrosis | | 4 | | Main tests performed by hospitals for evaluating the presence and degree of liver fibrosis. |
| Metabolic function (including diabetes) | | 4 | | To test insulin and C-Peptide levels, two common indications of diabetes and to test for and monitor diabetes where insulin and C-Peptide levels may not be a reliable indicator. |
| Other viruses | | 12 | | To test for the infection of other viruses including the five viruses that could cause the fetal malformation if infected during pregnancy. |
| Reproductive endocrinology | | 18 | | Main tests performed by hospitals to evaluate the functions of the female and male reproductive organs as well as tests to assess Down syndrome. |
| SARS | | 1 | | To test for SARS, an atypical pneumonia of unknown etiology recognized in February 2003 in the PRC, Taiwan, Hong Kong, Singapore and certain other regions in the world. |
| Thyroid disorders | | 7 | | The basic tests hospitals perform to evaluate the functions of a patient’s thyroid gland. |
| Tumors | | 12 | | To detect the following types of tumors: breast, colon, intestine, kidney, liver, lung, ovary, pancreas, prostate and stomach. |
| Total | | 95 | | |
FISH Probes
Our FISH probes are molecular diagnostic IVD reagents used with fluorescent microscopes for the prenatal diagnosis of various genetic diseases, for the early detection and prognosis of various cancers and for identifying cancer patients who would benefit from targeted drugs in the treatment of various cancers.
To perform a FISH test, a specific FISH probe is mixed with a sample of patient body fluid or tissue so that the probe can hybridize with its complementary target DNA in the patient’s cells. Once hybridized, the fluorescent fragments on the FISH probe will show precisely where the target DNA lies along the patient’s chromosomes. Under the fluorescent microscope, the medical practitioners examine the labeled sample, check the quality of the fluorescent signals, analyze the findings and record the areas for analysis. These fluorescent images are captured and saved on the computer for further analysis by the fluorescent microscope if needed.
37
In April 2008, in order to promote the sales of our FISH probes, we started to recommend hospital customers to purchase fluorescent microscopes directly from microscope manufacturers so that these hospitals could reduce the cost of setting up the FISH test platform and expedite the commencement of providing this diagnostic service to patients. We believe such practice could increase our sales of FISH probes, which are recurring in nature and generate higher gross margin. We also sold fluorescent microscopes to customers which were manufactured by third parties until April 2009.
In addition, some of our FISH probes were chosen to be used in a research project sponsored by the Chinese Ministry of Health focusing on cancer targeted therapy standardization as well as early cancer detection and treatment.
We currently offer 46 types of FISH probes. The following table sets out the major clinical applications of these FISH probes:
| | | | |
Application | | Number
of Probes | | Description |
Bladder cancer | | 4 | | An advanced approach for early detection and monitoring the course of bladder cancer. |
Breast cancer | | 2 | | An effective tool to guide treatment. Mostly used to determine whether a breast cancer patient is suitable for receiving Herceptin therapy which has been recently approved to treat stomach cancer patients. |
Cervical cancer | | 2 | | An effective tool for early diagnosis of cervical cancer and for prediction of the risk of developing cervical cancer. |
Hematological malignancies | | 23 | | For diagnosis and prognosis of various leukemia, lymphoma, multiple myeloma and related blood disorders. |
Lung cancer | | 2 | | For detecting epidermal growth factor receptor (“EGFR”) abnormalities in patients with non-small cell lung cancer to guide treatment. |
Prenatal and postnatal | | 9 | | For detecting Trisomy 21, Trisomy 18, Trisomy 13, Turner syndrome, Trisomy X, XYY syndrome, DiGeorge syndrome, triploidy. |
Prostate cancer | | 4 | | An effective approach for early diagnosis of prostate cancer. |
Total | | 46 | | |
HPV-DNA Chips and SPR Analyzers
Our HPV-DNA chips are molecular diagnostic biosensor chips used with our SPR analyzer for the diagnosis of HPV infection and genotyping of HPV.
To perform a test using our SPR system, a sample of patient cervical cells is obtained. After abstraction, DNA is amplified and then placed on a HPV-DNA chip. HPV genotype specific probes on the chip can hybridize with their complementary target DNA abstracted from the cervical cells. Once hybridized, the SPR system can read the hybridization signals and interpret the results based on control signals. The hybridization and analysis procedures are automatically performed by the SPR analyzers. Medical practitioners examine and record the final results.
We started to provide our SPR analyzers free of charge to large hospitals with a certain level of diagnostic volume in the PRC in January 2010 and generate revenue from the sale of HPV-DNA chips in June 2010. As of June 30, 2010, we had delivered 49 SPR analyzers free of charge to large hospitals which are also users of our FISH probes.
Manufacturing
Our manufacturing strategy combines our internal design expertise and proprietary technology with strategic sourcing. We produce our ECLIA reagent kits, FISH probes and HPV-DNA chips and conduct the final product
38
assembly, testing and packaging of our ECLIA analyzers and SPR analyzers at our facilities in Beijing, PRC. We outsource the production of a majority of our product components to, and purchase raw materials from, third-party suppliers.
We design or configure many key components of our products and outsource their production to qualified manufacturers. Our suppliers undergo quality certification in accordance with the standards developed by us, and we conduct frequent quality inspections of each of our suppliers’ manufacturing facilities. We believe we maintain components and raw materials at an adequate level to ensure a stable supply.
The major components of our ECLIA analyzer are the photon-multiplier, motor drive and various types of other electrical and plastic parts that are generally readily available in sufficient quantities from our local suppliers. The raw materials of our ECLIA reagent kits are antibodies and antigens for the production of coated microplate and conjugator, chemicals for the production of reagent kits, and plastics and packaging such as vacant microplate and bottles. We purchase a portion of our supplies of antibodies and antigens overseas and source the other raw materials from local suppliers. The raw materials of our FISH probes are substances from our DNA clone bank and fluorescent dye. The major components of our SPR analyzer are auto-sampling robot, a high resolution CCD camera and various types of electrical and plastic parts that are generally readily available in sufficient quantities from our local suppliers. The raw materials of our HPV-DNA chips are substances from our DNA clone bank and gold or silver-coated glass slides.
We generally produce our products based on firm orders from our customers and anticipated orders that we are reasonably confident will be obtained. Lead times for raw materials and components vary and depend on the specific supplier and the availability and demand for the raw materials or components. We currently use one or more suppliers for some of our key components for quality control purposes. We believe that we will be able to source most of our key components from other suppliers if necessary. We do not currently have any long-term supply contracts, and our purchases are made on a purchase order basis.
We have streamlined our product assembly process to improve our responsiveness to changes in our business volume by training our employees to assemble multiple components. As our sales fluctuate from month to month, employees with multiple skill sets and multi-tasked teams will allow us to make quicker deliveries while enabling us to more effectively manage our costs. In 2007, the SFDA imposed certain quality control system assessment requirements for certain reagent products, covering our ECLIA reagent kits, FISH probes and DNA chips. We met such requirements for ECLIA reagent kits, FISH probes and DNA chips, respectively, in February 2009, September 2009 and September 2009.
We are required to obtain production permits and to renew these permits six months prior to the expiration of the original permit. Our production permit for the manufacture of our ECLIA analyzer and reagent kits is valid until June 2015 and our production permit for the manufacture of our FISH probes, SPR analyzers and DNA chips is valid until May 2011. In addition, we are required to complete the SFDA registration of our products before we may sell the products for clinical diagnosis purpose. See “—Regulation.”
Quality Control
We have our own independent quality control system and devote significant attention to quality control for the designing, manufacturing and testing of our products. We have established a quality control system in accordance with the SFDA regulations. To indicate our compliance with recognized international standards for quality control, we obtained ISO9001 and ISO13485:2003 certifications for the production of our ECLIA systems and FISH probes in May 2006 and May 2008, respectively. We inspect components prior to assembly, and inspect and test internally manufactured products both during and after the manufacturing process. We also conduct regular quality inspections of each of our major suppliers’ manufacturing facilities.
39
Our quality control team is also responsible for ensuring that we are in compliance with all applicable regulations, standards and internal policies. Our management team is actively involved in setting quality policies and managing internal and external quality performance.
Markets and Customers
We believe that our products have significant market potential in the PRC as there is a strong demand for sensitive and reliable IVD tests. According to the Ministry of Health of the People’s Republic of China, or the MOH, there were approximately 20,291 hospitals in the PRC in 2009. The target market for our semi-automated ECLIA system is small to medium-sized hospitals in the PRC. We began marketing and selling our ECLIA system in September 2004 and had installed over 3,500 units of semi-automated ECLIA analyzers as of March 31, 2010. The target market for our fully-automated ECLIA system is large hospitals as well as high volume users among medium-sized hospitals. We expect to deliver our fully-automated ECLIA system free of charge to certain large hospitals in 2011. The target market for our FISH probes, SPR analyzers and HPV-DNA chips is large hospitals in the PRC. We began marketing and selling our FISH probes and HPV-DNA chips in June 2007 and June 2010, respectively. As of March 31, 2010, about 400 large hospitals were using our FISH probes. We began delivery of our SPR analyzers to some hospitals in January 2010, and we began generating revenues from sales of our HPV-DNA chips in June 2010.
Sales and Marketing
We market and sell our ECLIA reagent kits solely to distributors. We sold ECLIA analyzers until April 2008. For sales of reagent kits, we do not require down payments, and we collect payments three months after acceptance. We started to provide our semi-automated ECLIA analyzers to qualified hospitals free of charge in April 2008. We also expect to deliver our fully-automated ECLIA analyzers free of charge to certain large hospitals in 2011. The costs of the ECLIA analyzers that we provided free of charge to our customers are included in cost of revenues. As our ECLIA analyzers are closed systems, we believe such practice could increase the sales of our ECLIA reagent kits, which are recurring in nature.
We market and sell our FISH probes directly to hospitals. For sales of FISH probes, we do not require down payments, and we generally collect payments four to nine months after acceptance. We sold fluorescent microscopes until April 2009, and for sales of our fluorescent microscopes, we generally required a payment of a portion of the sale price before delivery or after acceptance and the balance within six months of acceptance. In April 2008, in order to promote the sales of our FISH probes, we started to recommend hospital customers to purchase fluorescent microscopes directly from microscope manufacturers so that these hospitals can reduce the cost of setting up the FISH test platform and expedite the commencement of providing this diagnostic service to patients. We believe such practice could increase our sales of FISH probes, which are recurring in nature and generate a higher gross margin.
We market our SPR analyzers and HPV-DNA chips directly to hospitals. Similar to our free arrangement for ECLIA analyzers, we provide our SPR analyzers to qualified hospitals free of charge. The costs of free SPR analyzers are included in cost of revenues after delivery. As our SPR systems are closed systems, we expect such practice to increase the sales of our HPV-DNA chips, which are recurring in nature.
We recognize revenues at the time our products are accepted by either our distributors, the hospitals designated by these distributors or our hospital customers. Customer acceptance is evidenced by signed acceptance documents. Certain of our distributors or hospital customers are required to pay in advance for purchase of products. Our distributors take ownership of our products when either they or the hospitals they designate accept the products.
We select our third-party distributors based on their reputation, market coverage, sales experience and the size of their sales force. We conduct credit assessments of each of our distributors or hospital customers before we sell our products to them. Currently, all our products are being marketed and sold in the PRC.
40
As of July 31, 2010, we had a team of 214 marketing and sales personnel who conduct direct sales of our FISH probes and HPV-DNA chips and perform other sales related tasks. We intend to further increase the size of our sales force as our business grows.
The hospital’s head of the department of diagnostics or pathology typically make the purchasing decision for our products. We believe that hospitals make purchase decisions on diagnostics equipment and regents based on the performance and the range of ECLIA reagent kits, FISH probes or HPV-DNA chips available, the prices of equipment and ECLIA reagent kits, FISH probes or HPV-DNA chips, ease of use and the turnaround time of results.
We have established relationships with many hospital administrators at prominent hospitals and other leading medical institutions. We believe our relationships with these major hospitals and medical institutions raise our profile, enhance awareness of our products in the medical community, medical equipment and supplies industry and among patients, provide us with valuable clinical data to improve our products and keep us abreast of industry trends and developments, all of which in turn helps us market and sell our products. We manage our relationships with ECLIA distributors by pricing our ECLIA system more attractively to distributors than imported alternatives. Our FISH probes address large unmet market in the PRC, primarily the prenatal and cancer diagnosis market. Our direct sales force formed for FISH probes distribution helps us build closer relationships with the end-users which are large hospitals. We believe these relationships also facilitate cross-selling of our ECLIA system, SPR analyzers and HPV-DNA chips.
Customer Support and Service
We maintain a dedicated team to provide customer support and service for our products. Our customer support and service teams are based in Beijing and other cities in the PRC and provide on-site and off-site technical and clinical support to hospitals across the PRC upon customer request. As of July 31, 2010, this team consisted of 250 full-time employees, including physicians, biomedical engineers, computer science engineers, and service and repair technicians. We believe that our ability to provide good customer support and service is an important part of our business.
We provide training to hospitals that purchase our ECLIA products, FISH probes and SPR products. Training sessions are provided directly by us or distributors. Training for our ECLIA system, FISH probes and SPR system generally takes one to two weeks.
Each of our free ECLIA analyzer and SPR analyzer delivered to customers is typically provided with a 12-month warranty against technical defects. We have experienced a very limited number of warranty claims or repair requests in the past for our ECLIA analyzers. As we only began installing SPR analyzers in hospitals in January 2010, we currently have no historical data on the warranty claims or repair requests for this product. The costs associated with our warranty claims or repair requests have historically been insignificant. We do not accrue any liability for potential warranty costs at the time of sale.
Competition
With respect to our ECLIA systems, the majority of small and medium-sized hospitals in the PRC are currently using less sophisticated technologies such as ELISA (Enzyme-Linked Immunosorbent Assay) and radioimmunoassay systems. In contrast, in the United States and Western Europe, the majority of such tests are performed with luminescence immunoassay IVD systems. The supply of luminescence immunoassay IVD systems to large hospitals in the PRC is currently dominated by established overseas-based companies such as Abbott Diagnostics, Siemens Healthcare, Beckman Coulter, PerkinElmer, Johnson & Johnson and Roche Diagnostics, all of which primarily offer automatic luminescence immunoassay systems. There are a number of China-based manufacturers who offer semi-automated luminescence immunoassay systems to small and medium-sized hospitals. The target market for our semi-automated ECLIA system consists of small and medium-sized hospitals in the PRC. We believe that most of these hospitals do not have sufficient financial means to
41
purchase the more expensive imported automatic luminescence immunoassay systems or do not have sufficient patient volume to justify the cost. As a result, we believe we are not in direct competition with overseas-based companies with respect to these hospitals. However, as we are now offering fully-automated ECLIA analyzers to large hospitals, we will face competition with these overseas-based companies.
Our FISH probes compete with probes offered by Abbott Diagnostics. We are not aware of any China-based manufacturers who offer FISH probes in the PRC.
Our SPR analyzers and HPV-DNA chips compete with HPV products offered by Qiagen and certain China-based manufacturers. We are not aware of any China-based manufacturers who offer both SPR analyzers and HPV-DNA chips in the PRC.
Rapid product development, technological advances, intense competition and a strong emphasis on proprietary products characterize the industry in which we compete. Our products could be rendered obsolete or uneconomical by the introduction and market acceptance of competing products or by technological advances of our current or potential competitors. We face direct competition from a number of publicly traded and privately held companies, including other manufacturers of diagnostic products. Many of our existing and potential competitors have substantially greater financial, research and development, sales and marketing, personnel and other resources than we do and may have more experience in developing, manufacturing, marketing and supporting new products. Competition will intensify as more players enter this field. We believe that an important competitive advantage for a medical technology company is its ability to make continuous investments in research and development and to identify commercially viable technologies and successfully bring the technologies into the market.
Insurance
We have property insurance coverage from China Pacific Property Insurance Co., Ltd. to cover certain of our fixed assets. Our insurance coverage, however, may not be sufficient to cover any claims for damages to our fixed assets. We do not maintain any product liability insurance. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Business—We are subject to product liability exposure and have no relevant insurance coverage.”
Regulation
Our ECLIA analyzers and SPR analyzers are medical devices and are subject to regulatory controls governing medical devices. Pursuant to the SFDA regulation effective on June 1, 2007, reagents used for IVD testing are divided into three different categories depending on the degree of risk associated with the reagent. All reagents are subject to regulatory controls governing IVD reagents. As a manufacturer of medical equipment and supplies, we are subject to regulation and oversight by different levels of the SFDA. We are also subject to other government laws and regulations which are applicable to manufacturers in general. The SFDA requirements include obtaining production permits, compliance with clinical testing standards, manufacturing practices, quality standards, applicable industry and adverse reporting, and advertising and packing standards.
Classification of medical devices
In the PRC, medical devices are classified into three different categories, Class I, Class II and Class III, depending on the degree of risk associated with each medical device and the extent of control needed to ensure safety and effectiveness. Classification of a medical device is important because the class to which a medical device is assigned determines, among other things, whether a manufacturer needs to obtain a production permit and the level of regulatory authority involved in obtaining such permit. Classification of a device also determines the types of registration required and the level of regulatory authority involved in effecting the registration.
42
Class I devices are those with low risk to the human body and are subject to “general controls”. Class I devices are regulated by the city level food and drug administration where the manufacturer is located. Class II devices are those with medium risk to the human body and are subject to “special controls”. Class II devices require product certification, usually through a quality system assessment, and are regulated by the provincial level food and drug administration where the manufacturer is located. Class III devices are those with high risk to the human body, such as life-sustaining, life-supporting or implantable devices, and are regulated by the SFDA under the strictest regulatory control.
Our ECLIA analyzer and SPR analyzer are classified as Class II devices.
Production permits for medical devices
A manufacturer must obtain a production permit from the provincial level food and drug administration before commencing the manufacture of Class II or Class III medical devices. A production permit, once obtained, is valid for five years and is renewable upon expiration. Our production permit for the manufacture of our ECLIA analyzer will expire on June 11, 2015. Our production permit for the manufacture of our SPR analyzer will expire on May 14, 2011. To renew a production permit, a manufacturer needs to submit to the provincial level food and drug administration an application to renew the permit, along with required information six months before the expiration date of the permit. We do not believe it will be difficult for us to renew our production permit.
Registration requirements of medical devices
Before a medical device can be manufactured for commercial distribution, a manufacturer must effect medical device registration by proving the safety and effectiveness of the medical device to the satisfaction of respective levels of the food and drug administration. In order to conduct a clinical trial on a Class II or Class III medical device, the SFDA requires manufacturers to apply for and to obtain in advance a favorable inspection result for the device from a third party inspection center recognized by the SFDA. The application to the inspection center must be supported by appropriate data, such as animal and laboratory testing results. If the inspection center approves the application for clinical trial, and the respective levels of the food and drug administration approve the institutions which will conduct the clinical trials, the manufacturer may begin the clinical trial. A registration application for a Class II or Class III device must provide certain pre-clinical and clinical trial data and information about the device and its components regarding, among other things, device design, manufacturing and labeling. The provincial level food and drug administration, within 60 days of receiving an application for the registration of a Class II device, and the SFDA, within 90 days of receiving an application for the registration of a Class III device, will notify the applicant whether the application for registration is approved. If approved, a registration certificate will be issued within 10 days of the notification. If the food and drug administration requires supplemental information, the approval process may take much longer. The registration is valid for four years.
The SFDA occasionally changes its policies, adopts additional regulations, revises existing regulations or tightens enforcement, each of which could block or delay the approval process for a medical device.
The SFDA approval for our ECLIA analyzer and SPR analyzer will expire in July 2012 and September 2013, respectively. We do not believe it will be difficult for us to renew these approvals.
Classification of ECLIA reagent kits, FISH probes and HPV-DNA chips
Reagents used for IVD testing are divided into three different categories, Class I, Class II and Class III, depending on the degree of risk associated with each reagent. Classification of a reagent is important because the class to which a reagent is assigned determines, among other things, the types of registration required and the level of regulatory authority involved in effecting the registration.
43
Class I reagents are those with low risk and are subject to “general controls”. Class I reagents are regulated by the city level food and drug administration where the manufacturer is located. Class II reagents are those with medium risk and are subject to “special controls”. Class II reagents are regulated by the provincial level food and drug administration where the manufacturer is located. Class III devices are those with high risk and are regulated by the SFDA under the strictest regulatory control.
Our ECLIA reagent kits, FISH probes and HPV-DNA chips are classified as Class III reagents, and they therefore are subject to all regulatory controls governing Class III reagents.
Production permits for ECLIA reagent kits, FISH probes and HPV-DNA chips
A manufacturer must obtain a production permit from the food and drug administration at the provincial level. A production permit, once obtained, is valid for five years and is renewable upon expiration. Our production permit for the manufacture of ECLIA reagent kits will expire in June 2015. Our production permit for the manufacture of FISH probes and HPV-DNA chips will expire in May 2011. To renew the production permit, a manufacturer needs to submit to the provincial level food and drug administration an application to renew the permit, along with required information six months before the expiration date of the permit. We do not believe it will be difficult for us to renew our production permit. In addition, our manufacturing operations are also subject to periodic re-inspection and must comply with applicable quality regulations.
Registration requirements of ECLIA reagent kits, FISH probes and HPV-DNA chips
Prior to selling an IVD reagent kit, a manufacturer must obtain the SFDA registration of the product it plans to sell. The registration process starts with submitting a registration dossier to the SFDA. The registration dossier contains primarily detailed information concerning the effectiveness and quality of the reagent kit. It also contains details concerning the manufacturing process and the production facilities. The registration process can take a few months and the timing depends on the nature of the product under review, the quality of the data and the general efficiency of the SFDA. The approval process involves the detailed examination of the testing, effectiveness, labeling, manufacturing and marketing of IVD reagents by the SFDA. The registration is valid for four years.
We currently offer a total of 95 ECLIA reagent kits and 46 FISH probes in the PRC. Of the 95 ECLIA reagent kits, 76 are covered by registration certificates. Of the 46 FISH probes, 15 are covered by registration certificates. Of the 19 ECLIA reagent kits we currently sell without registration certificates, 11 are food safety reagent kits which are not subject to SFDA registration and the remaining 8 reagent kits are being sold for research use only. Of the 8 ECLIA reagent kits and 31 FISH probes we currently sell for research use only, we have submitted registration applications for most of them. The registration certificate for our HPV-DNA chips was granted to us in May 2010.
Thus, although regulations require that the SFDA registration be obtained before a reagent kit is sold, a reagent kit may be exempt from registration if it is used for research purposes only. A reagent kit intended for research use only may not be applied in human clinical diagnostic or prognostic uses. In addition, a reagent kit intended for research use only must comply with the labeling requirements that require the statement: “For research use only. Not for use in diagnostic procedures.” We have sold some of our ECLIA reagent kits, FISH probes and HPV-DNA chips without required registrations. In addition, we believe that some ECLIA reagent kits, FISH probes and HPV-DNA chips sold in the PRC, including some manufactured by our overseas-based competitors, are also sold without required registrations. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Industry—In order to manufacture and market our products, we are required to obtain various authorizations from governmental regulatory authorities in the PRC and other countries. If we fail to obtain clearances or approvals in a timely fashion, our business may be significantly affected.” and “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in the PRC—Future changes in laws, regulations or enforcement policies in the PRC could adversely affect our business.”
44
In 2007, the SFDA imposed certain quality control system assessment requirements for certain reagent products, covering our ECLIA reagent kits, FISH probes and HPV-DNA chips. We met such requirements for ECLIA reagent kits, FISH probes and HPV-DNA chips, respectively in February 2009, September 2009 and September 2009.
Continuing SFDA regulations
We are subject to continuing regulation by the SFDA. In the event of significant modification to an approved medical device, its labeling or its manufacturing process, a new pre-market approval or pre-market approval supplement may be required. Our ECLIA analyzer and ECLIA reagent kits, FISH probes, and SPR analyzers and HPV-DNA chips are subject to, among others, the following regulations:
| | • | | the SFDA’s quality system regulations, which require manufacturers to create, implement and follow certain design, testing, control, documentation and other quality assurance procedures; |
| | • | | medical device reporting regulations, which require that manufacturers report to the SFDA certain types of adverse reactions and other events involving their products; and |
| | • | | the SFDA’s general prohibition against promoting products for unapproved uses. |
Class II and III devices may also be subject to special controls applicable to them, such as supply purchase information, performance standards, quality inspection procedures and product testing, which may not be required for Class I devices. We believe we are in compliance with the applicable SFDA guidelines, but we could be required to change our compliance activities or be subject to other special controls if the SFDA changes or modifies its existing regulations or adopts new requirements.
We are also subject to inspection and market surveillance by the SFDA to determine compliance with regulatory requirements. If the SFDA decides to enforce its regulations and rules, the agency can institute a wide variety of enforcement actions, such as:
| | • | | fines, injunctions and civil penalties; |
| | • | | recall or seizure of our products; |
| | • | | the imposition of operating restrictions, partial suspension or complete shutdown of production; and |
Other national and provincial level laws and regulations
We are subject to changing regulation under many other laws and regulations administered by governmental authorities at the national, provincial and city levels, some of which are, or may be, applicable to our business. Our hospital customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
Laws regulating medical device manufacturers and hospitals cover a broad array of subjects. For example, regulations control the confidentiality of patient medical information and the circumstances under which patient medical information may be released for inclusion in our databases, or released by us to third parties. These laws and regulations governing both the disclosure and the use of confidential patient medical information may become more restrictive in the future.
We must also comply with numerous additional state and local laws relating to matters such as labor contracts, safe working conditions, manufacturing practices, environmental protection and fire hazard control. We believe we are currently in compliance with these laws and regulations; however, we may be required to incur significant costs to comply with these laws and regulations in the future. Unanticipated changes in existing regulatory requirements or adoption of new requirements could therefore have a material adverse effect on our business, results of operations and financial condition.
45
Regulation of foreign currency exchange and dividend distribution
Foreign currency exchange regulation in the PRC is primarily governed by the following rules:
| | • | | Foreign Currency Administration Rules (1996), as amended, or the Exchange Rules; and |
| | • | | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Under the Exchange Rules, the Renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of Renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of the SAFE.
Under the Administration Rules, companies in the PRC with foreign investments, such as YDME Beijing, Beijing GP or BBE, may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from the SAFE. Capital investments by foreign-invested enterprises outside of the PRC are also subject to limitations, which include approvals by the SAFE and other relevant government authorities.
The principal regulations governing distribution of dividends paid by wholly foreign owned enterprises include:
| | • | | Wholly Foreign Owned Enterprise Law (1986), as amended; and |
| | • | | Wholly Foreign Owned Enterprise Law Implementation Rules (1990), as amended. |
Under these regulations, foreign-invested enterprises in the PRC may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, foreign-invested enterprises in the PRC are required to set aside certain amounts out of their accumulated profits each year, if any, to fund certain reserve funds. These reserves are not distributable as cash dividends.
Regulation of foreign exchange in certain onshore and offshore transactions
In January and April 2005, the SAFE issued two rules that require PRC residents to register with and receive approvals from the SAFE in connection with their offshore investment activities. The SAFE has announced that the purpose of these regulations is to achieve the proper balance of foreign exchange and the standardization of the cross-border flow of funds.
On October 21, 2005, the SAFE issued the Notice on Issues Relating to the Administration of Foreign Exchange in Fund-raising and Reverse Investment Activities of Domestic Residents Conducted via Offshore Special Purpose Companies, or Notice 75, which became effective as of November 1, 2005. Notice 75 replaced the two rules issued by the SAFE in January and April 2005 mentioned above.
According to Notice 75:
(i) prior to establishing or assuming control of an offshore company for the purpose of financing that offshore company with assets or equity interests in an onshore enterprise in the PRC, each PRC resident who is an ultimate controller, whether a natural or legal person, must complete the overseas investment foreign exchange registration procedures with the relevant local SAFE branch;
(ii) an amendment to the registration with the local SAFE branch is required to be filed by any PRC resident that directly or indirectly holds interests in that offshore company upon either (a) the injection of equity interests or assets of an onshore enterprise to the offshore company, or (b) the completion of any overseas fund raising by such offshore company; and
46
(iii) an amendment to the registration with the local SAFE branch is also required to be filed by such PRC resident when there is any material change involving a change in the capital of the offshore company, such as (a) an increase or decrease in its capital, (b) a transfer or swap of shares, (c) a merger or division, (d) a long-term equity or debt investment, or (e) the provision of a guarantee to third parties.
Moreover, Notice 75 applies retroactively and to indirect shareholdings. As a result, PRC residents who have established or acquired direct or indirect control of offshore companies that have made onshore investments in the PRC in the past had to complete the relevant overseas investment foreign exchange registration procedures by March 31, 2006. Under the relevant rules, failure to comply with the registration procedures set forth in Notice 75 may result in restrictions being imposed on the foreign exchange activities of the relevant onshore company, including the payment of dividends and other distributions to its offshore parent or affiliate and the capital inflow from the offshore entity, and may also subject relevant PRC residents to penalties under PRC foreign exchange administration regulations.
In order to clarify the issues arising from the implementation and application of Notice 75, on May 29, 2007, SAFE promulgated Implementing Procedures of Notice 75, or Notice 106. However, the complex rules and requirements set forth under Notice 106 need even more interpretation and operating guidance. For example, it is not clear under Notice 106 if our establishment of the three entities in the British Virgin Islands would be subject to the registration requirement of Notice 106.
As a result of the lack of implementing rules and other uncertainties relating to the interpretation and implementation of Notice 75 and Notice 106, we cannot predict how these regulations will affect our business operations or strategies. For example, our present or future PRC subsidiaries’ ability to conduct foreign exchange activities, such as remittance of dividends and foreign-currency-denominated borrowings, may be subject to compliance with such SAFE registration requirements by relevant PRC residents, over whom we have no control. In addition, we cannot assure you that any such PRC residents will complete the necessary approval and registration procedures required by the SAFE regulations.
On March 28, 2007, SAFE promulgated the Operating Procedures on Administration of Foreign Exchange regarding PRC Individuals’ Participation in Employee Share Ownership Plans and Employee Share Option Plans of Overseas Listed Companies, or the Share Option Rules. Under the Share Option Rules, PRC citizens who participate in employment share ownership or share option plans of an overseas listed company are required to appoint a domestic agent to deal with the relevant foreign exchange matters in the PRC. For participants of an employment share ownership plan, an overseas custodian bank should be retained by the domestic agent to hold on trusteeship all overseas assets held by such participants under the employment share ownership plan. In the case of a share option plan, a financial institution with stock brokerage qualification at the place where the overseas listed company is listed or a qualified institution designated by the overseas listed company is required to be retained to handle matters in connection with exercise or sale of stock options for the share option plan participants. For participants who had already participated in an employment share ownership plan or share option plan before the date of the Share Option Rules, the Share Option Rules require their domestic employers or domestic agents to complete the relevant formalities within three months of the date of the Share Option Rules. The failure to comply with the Share Option Rules may subject us as the company offering the plan to penalties under PRC foreign exchange regime.
Company Law
On October 27, 2005, the Standing Committee of the National People’s Congress adopted amendments to the PRC Company Law, which substantially overhauled the PRC company law system and removed a number of legal restrictions and hurdles on the management and operations of limited liability companies and companies limited by shares. It is expected that the PRC Law of Wholly Foreign-owned Enterprises, or the WFOE Law, and its implementing regulations will be amended accordingly in order to align the WFOE Law with the amendments to the PRC Company Law. Each of YDME Beijing, Beijing GP and BBE is governed by both the PRC Company
47
Law and the WFOE Law and their implementing regulations, and we believe that YDME Beijing, Beijing GP and BBE will be able to benefit from a more flexible and business friendly company law regime under the new PRC Company Law. For example, the amended PRC Company Law eliminated a restriction which limited the amount of equity investments a company could make to a maximum of 50% of such company’s net assets. With the removal of this restriction, YDME Beijing, Beijing GP and BBE may have increased flexibility in making equity investments or planning potential acquisitions. In addition, the amended PRC Company Law now permits the establishment of single-shareholder limited liability companies. As a result, YDME Beijing, Beijing GP and BBE may acquire 100% of the equity interest in a PRC limited liability company and become the sole shareholder of such limited liability company.
| C. | Organizational Structure |
The following diagram illustrates our Company’s organizational structure, the place of formation, ownership interest and affiliation of each of our significant subsidiaries as of August 31, 2010.
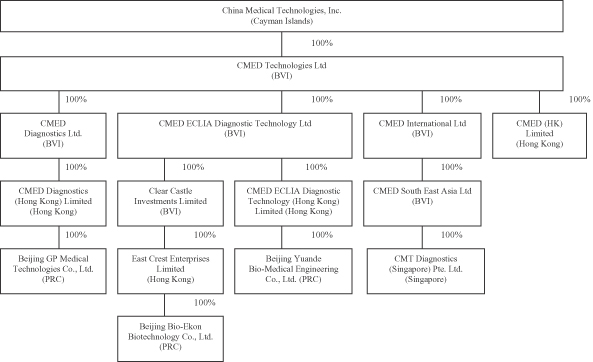
| D. | Property, Plant and Equipment |
Most of our manufacturing facilities are located in the Beijing Economic-Technological Development Area, where we own two buildings with an aggregate of approximately 10,000 square meters of office, research and development and manufacturing spaces.
To further expand our production capacity, we have leased properties of over 10,000 square meters in the Beijing Economic-Technological Development Area for the production of our ECLIA analyzers, FISH probes, SPR analyzers and HPV-DNA chips.
48
Through the acquisition of BBE in January 2008, we obtained an aggregate of approximately 2,000 square meters of research and development and manufacturing spaces in Fengtai district, Beijing, which are owned by BBE. BBE also entered into a one-year lease which was renewed for another one-year term in January 2010 for approximately 583 square meters of office space in Fengtai district, Beijing.
| ITEM 4A. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| ITEM 5. | OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Item 3. Key Information—D. Risk Factors” or in other parts of this annual report on Form 20-F.
Overview
We are a leading China-based medical device company that develops, manufactures and markets advanced IVD products using ECLIA, FISH and SPR technologies to detect and monitor various diseases and disorders. As of March 31, 2010, our sales were generated from two products, our ECLIA system and our FISH probes. We started reporting our financial results in segments along product lines since our acquisition of the ECLIA technology in August 2004. We added the FISH business line for the fiscal year ended March 31, 2008 as the sales and distribution of our FISH probes began in June 2007 and removed the HIFU product line for the fiscal year ended March 31, 2009 as we sold the HIFU business in December 2008. In December 2008, we completed the SPR acquisition for cash consideration of US$346.1 million. After the sale of the HIFU business and the SPR acquisition, we re-classified our reportable operating segments for the fiscal year ended March 31, 2009 into (i) immunodiagnostic systems and (ii) molecular diagnostic systems. Immunodiagnostic systems currently include ECLIA analyzers and reagent kits while molecular diagnostic systems include FISH probes, SPR analyzers and HPV-DNA chips (and fluorescent microscopes until April 2009).
One of our main products, the ECLIA system, is an IVD system that can be used to diagnose and analyze a variety of diseases and conditions, including various thyroid disorders, diabetes, hepatitis, Down syndrome, liver fibrosis, disorders related to reproduction and growth, various types of tumors and food safety. Our FISH probes are molecular diagnostic reagents used with fluorescent microscope for (i) the prenatal and postnatal diagnosis of various genetic diseases, (ii) the early detection and prognosis of various cancers and (iii) the identification of cancer patients who would benefit from targeted drugs in the treatment of various cancers. Our SPR analyzers and HPV-DNA chips utilize a biosensor technology in molecular biology which is currently used for the diagnosis of HPV infection and genotyping of HPV, which can cause cervical cancer and sexually transmitted diseases.
In December 2008, we completed the sale of the HIFU business to Chengxuan for cash consideration of US$53.5 million, of which US$23.5 million has been paid. As a result, the HIFU business has been accounted for as a discontinued operation in accordance with U.S. GAAP. Chengxuan is owned by Mr. Xiaodong Wu, the chairman of our board of directors and our chief executive officer. As we completed the sale of the HIFU business, we have become a pure advanced IVD company. We expect to use the proceeds from the sale of the HIFU business to support further development of the fast growing molecular diagnostic businesses and strive to become the leading molecular diagnostic company in the PRC. See “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions—Transactions with Chengxuan” for more information.
49
Pricing
We believe that our ECLIA system is competitively priced to offer attractive economic returns to our distributors. We reduced the selling price of our ECLIA reagent kits in September 2009 in response to increasing market competition and to stimulate usage of our ECLIA reagent kits. The prices of our ECLIA reagent kits are now significantly lower than those of foreign imports after the price reduction.
We believe that the prices of our FISH probes are lower than those of foreign imports. We are not aware of any China-based manufacturers who currently offer FISH probes in the PRC.
We believe that our HPV-DNA chips are competitively priced in comparison to other comparable HPV diagnostic tests available in the PRC.
Revenues
As of March 31, 2010, we derived revenues from two sources, which were sales of our ECLIA reagent kits and our FISH probes. We began generating revenues from sales of our HPV-DNA chips in June 2010.
Our revenues, growth and results of operations depend on several factors, including the level of acceptance of our products among doctors, hospitals and patients and our ability to maintain prices for our products at levels that provide favorable margins. The level of acceptance among doctors, hospitals and patients is influenced by the performance and pricing of our products, our ability to educate distributors and the medical community about our products, our relationships with hospitals and major distributors, government reimbursement levels as well as other factors.
Our sales have historically been made on the basis of unit-by-unit purchase orders rather than long-term commitments, and we do not have long-term contracts with any of our customers. In the fiscal years ended March 31, 2008, 2009 and 2010, we marketed and sold our ECLIA system solely to distributors. At present, we market and sell our FISH probes and HPV-DNA chips directly to hospitals. We cannot assure you that any customer will continue to purchase our products at the same levels as in prior years or that our relationship with any of them will continue.
For sales of ECLIA reagent kits, we do not require down payments, and collect payments within three months after product acceptance. For sales of FISH probes and HPV-DNA chips, we do not require down payments, and we collect payments four to nine months after acceptance.
For sales of our fluorescent microscopes, prior to our cessation of sales in April 2009, we required a payment of a portion of the sale price before delivery or after acceptance and the balance within six months of acceptance.
To promote the sales of our ECLIA reagent kits, we provide our semi-automated ECLIA analyzers to qualified hospitals free of charge. We also expect to deliver our fully-automated ECLIA analyzers free of charge to certain large hospitals in 2011. As our ECLIA analyzers are “closed” systems, although it eliminates the sales of our ECLIA analyzers, we believe such practice could increase the sales of our ECLIA reagent kits, which are recurring in nature and generate higher gross margin than the ECLIA analyzers. To promote the sales of our FISH probes, we recommend our hospital customers to purchase fluorescent microscopes directly from microscope manufacturers so that these hospitals could reduce the cost of setting up the FISH test platform and expedite the commencement of providing this diagnostic service to patients. We ceased selling fluorescent microscopes in April 2009. We believe such practice could increase our sales of FISH probes, which are recurring in nature and generate high gross margin. We also provide our SPR analyzers to qualified hospitals free of charge to increase the sales of our HPV-DNA chips.
50
Costs
Cost of revenues
Our cost of revenues primarily consists of material and component costs, including the cost of ECLIA analyzers and SPR analyzers that we provided free of charge. Our cost of revenues also includes amortization of intangible assets and direct costs incurred in the assembly, installation and service of our products, such as salaries and related personnel expenses and depreciation costs of plant and equipment used for production purposes. Depreciation of property, plant and equipment for the fiscal years ended March 31, 2008, 2009 and 2010 in the amount of RMB6.1 million, RMB9.6 million and RMB13.1 million (US$1.9 million), respectively, were recorded as cost of revenues. Amortization of intangible assets in the amount of RMB90.2 million, RMB90.1 million and RMB89.7 million (US$13.1 million) were included in cost of revenues for the fiscal years ended March 31, 2008, 2009 and 2010, respectively. The cost of free ECLIA analyzers and SPR analyzers were RMB9.0 million and RMB11.5 million for the fiscal year ended March 31, 2010, respectively. The cost of free ECLIA analyzers was RMB13.2 million for the fiscal year ended March 31, 2009 and there was no such cost for the fiscal year ended March 31, 2008. The cost of free SPR analyzers for the fiscal years ended March 31, 2008 and 2009 was nil. Our in-house production team manufactures the ECLIA reagent kits, FISH probes and HPV-DNA chips, and conducts the final product assembly, testing and packaging of our ECLIA analyzers and SPR analyzers. We source a majority of our components from third-party suppliers.
As we source a significant portion of our components and raw materials in the PRC, we currently have a relatively low cost base compared to medical technology companies in more developed countries. We expect the costs of components and raw materials in the PRC will increase in the future as a result of further economic development in the PRC. In addition, our focus on new generations and applications of our products may require higher cost components and raw materials. We plan to offset increases in our cost of raw materials and components through more efficient product designs and product assembly enhancements as well as through savings due to economies of scale.
Operating expenses
Our operating expenses primarily consist of research and development expenses, acquired in-process research and development, sales and marketing expenses, general and administrative expenses and amortization of SPR intangible assets.
| | • | | Research and development. Research and development expenses primarily consist of costs associated with the design, development, testing and enhancement of our existing products and development of new ECLIA reagent kits, FISH probes and SPR-based DNA chips. These costs consist of expenditures for purchases of supplies, clinical trials, salaries and related personnel expenses, and other relevant costs. Share-based compensation of RMB2.8 million, RMB8.2 million and RMB6.3 million (US$0.9 million) was included in research and development expenses for the fiscal years ended March 31, 2008, 2009 and 2010, respectively. Going forward, we expect to increase our research and development expenses to develop new applications and improve the product designs of our immunodiagnostic systems and molecular diagnostic systems. |
| | • | | Acquired in-process research and development. The value of acquired in-process research and development is determined by estimating the costs to develop the acquired in-process research and development into a commercially viable product, estimating the resulting cash flows from the sale of the products resulting from the completion of the in-process research and development and discounting the net cash flows using an appropriate discount rate, net of returns on contributory assets. For acquisitions prior to April 2009, acquired in-process research and development with no future alternative use was expensed at the acquisition date. |
In-process research and development costs acquired in business combinations and asset acquisitions are expensed as research and development expense upon acquisition and amounted to RMB0.7 million (relating to our BBE acquisition) and RMB244.9 million (relating to our SPR acquisition) for the fiscal
51
years ended March 31, 2008 and 2009, respectively. There were no acquisitions of in-process research and development during the fiscal year ended March 31, 2010.
| | • | | Sales and marketing. Sales and marketing expenses consist primarily of salaries and related expenses for personnel engaged in sales, marketing and customer support functions and costs associated with advertising and other marketing activities. Similar to most China-based manufacturers of medical equipment and supplies, our sales of ECLIA reagent kits are made primarily to distributors. As a result, our sales and marketing expenses for the ECLIA system as a percentage of revenues are significantly lower than manufacturers of medical equipment and supplies that operate their own marketing and distribution networks and sell directly to hospitals. At present, we market and sell our FISH probes directly to hospitals. We recently began selling our HPV-DNA chips directly to hospitals. Going forward, we expect to increase our expenditures on sales and marketing to promote the sales of our FISH probes and HPV-DNA chips directly to hospitals. |
| | • | | General and administrative. General and administrative expenses consist primarily of salaries and benefits and related costs for our administrative personnel and management, fees and expenses of our outside advisers, including legal, audit and valuation expenses, expenses associated with our administrative offices and the depreciation of equipment used for administrative purposes. We expect that our general and administrative expenses will increase as we hire additional personnel and incur costs related to the anticipated growth of our business. Share-based compensation of RMB13.9 million, RMB42.0 million and RMB34.1 million (US$5.0 million) was included in general and administrative expenses for the fiscal years ended March 31, 2008, 2009 and 2010, respectively. We have granted restricted shares and share options to our directors, officers and employees under our stock option plan and equity incentive plan. We record a compensation charge measured at the grant date fair value of the restricted shares and share options. We recognize the compensation expense over the applicable service period of the grantee, which is usually the vesting period. |
| | • | | Amortization of SPR intangible assets. Amortization of SPR intangible assets is recognized as operating expenses instead of cost of revenues until significant revenues are generated from these intangible assets. Due to the commencement of sales of HPV-DNA chips, we expect to begin recognizing the amortization of SPR intangible assets as cost of revenues starting from the quarter ending September 30, 2010. |
Taxation
Cayman Islands
Under the current laws of the Cayman Islands, we are not subject to tax on our income or capital gains. In addition, no Cayman Islands withholding tax will be imposed on payments of dividends by us to our shareholders.
British Virgin Islands (“BVI”)
Under the current laws of BVI, our BVI subsidiaries are not subject to tax on income or capital gains. In addition, payments of dividends by our BVI subsidiaries to their shareholders are not subject to withholding tax in the BVI.
Hong Kong
Under the current Hong Kong Inland Revenue Ordinance, our Hong Kong subsidiaries are subject to 16.5% income tax on its taxable income generated from operations in Hong Kong. In addition, payments of dividends by our Hong Kong subsidiaries to their shareholders are not subject to any Hong Kong withholding tax.
52
PRC
The EIT Law, and the implementation regulations for the EIT Law issued by the PRC State Council, became effective as of January 1, 2008. The EIT Law imposes a uniform tax rate of 25% on all PRC enterprises, including foreign-invested enterprises, and eliminates or modifies most of the tax exemptions, reductions and preferential treatments available under the old EIT law. Under the EIT law, enterprises that were established before March 16, 2007 and already enjoy preferential tax treatments, in accordance with any detailed directives to be issued by the State Council, (i) in the case of preferential tax rates, continue to enjoy the preferential tax rates which will be gradually increased to the new tax rates within five years from January 1, 2008 or (ii) in the case of preferential tax exemption or reduction for a specified term, continue to enjoy the preferential tax holiday until the expiration of such term. The State Council issued the Notice on Implementation of the Transition Period for Preferential Enterprise Income Tax, or the Transition Implementation Notice, on December 26, 2007, which provides detailed rules on how preferential tax rates under previous income tax laws or regulations would transition to the uniform 25% EIT rate. In addition, entities that qualify as “high and new technology enterprises” will enjoy a 15% preferential tax rate under the EIT law. The Ministry of Science and Technology, the Ministry of Finance and the State Administration of Taxation issued the Measures on Qualification of High and New Technology Enterprises, or Circular 172, on April 14, 2008, which provides detailed standards for “high and new technology enterprises”. In addition, according to the Notice on Prepayment of Enterprise Income Tax issued by the State Administration of Taxation, an enterprise that has been certified as a “high and new technology enterprise” should pre-pay its EIT at the rate of 25% temporarily until it is re-certified as a “high and new technology enterprise” under Circular 172.
Under the current PRC laws, YDME Beijing, Beijing GP and BBE are subject to EIT and value added taxes, or VAT. YDME Beijing and BBE obtained the qualification certificates of “high and new technology enterprises” in 2008 with a validity period of three years from 2008 to 2010. However, the continuing qualification of YDME Beijing and BBE as “high and new technology enterprises” for the calendar year of 2010 will be subject to annual evaluation by the relevant government authorities in the PRC. In addition, YDME Beijing and BBE will need to apply for a three-year extension upon the expiration of the current qualification certificates if they want to continue to enjoy the 15% reduced EIT rate after 2010.
Beijing GP was previously entitled to an EIT rate of 15% and was entitled to an exemption from EIT from January 1, 2008 to December 31, 2009 and a 50% income tax reduction from January 1, 2010 to December 31, 2012. While Beijing GP will be able to enjoy the tax holiday under the EIT Law and the Transition Implementation Notice, its 50% income tax reduction from January 1, 2010 to December 31, 2012 will be partially eliminated. Under the EIT Law and the Transition Implementation Notice, Beijing GP’s EIT rates are 0%, 0%, 11%, 12% and 12.5% for the calendar years from 2008 to 2012. We may apply for qualification of Beijing GP as a “high and new technology enterprise” under the EIT Law to enjoy the 15% reduced EIT rate after 2012.
If YDME Beijing and Beijing GP had not had any preferential tax rate or tax holiday, our basic earnings from continuing operations per share for the fiscal years ended March 31, 2008, 2009 and 2010 would have been reduced by RMB0.19, RMB0.41 and RMB0.23 (US$0.03), respectively, and our diluted earnings from continuing operations per share for the fiscal years ended March 31, 2008, 2009 and 2010 would have been reduced by RMB0.19, RMB0.41 and RMB0.23 (US$0.03), respectively.
VAT is charged based on the selling price of our products at a general rate of 17%, or at a reduced rate of 6% for products that we have obtained the appropriate approvals from the relevant tax authorities. Our revenues are recorded net of this VAT.
The EIT Law also provides that enterprises established outside of the PRC whose “de facto management bodies” are located in the PRC are considered “resident enterprises” and are generally subject to the uniform
53
25% EIT rate as to their worldwide income. Under the implementation regulations for the EIT Law issued by the PRC State Council, “de facto management body” is defined as a body that has material and overall management and control over the manufacturing and business operations, personnel and human resources, finances and treasury, and acquisition and disposition of properties and other assets of an enterprise. On April 22, 2009, the PRC State Administration of Taxation further issued a notice entitled “Notice Regarding Recognizing Offshore-Established Enterprises Controlled by PRC Shareholders as Resident Enterprises Based on Their Place of Effective Management”. Under this notice, a foreign company controlled by a PRC company or a group of PRC companies shall be deemed as a PRC resident enterprise, if (i) the senior management and the core management departments in charge of its daily operations mainly function in the PRC; (ii) its financial decisions and human resource decisions are subject to decisions or approvals of persons or institutions in the PRC; (iii) its major assets, accounting books, company seals, minutes and files of board meetings and shareholders’ meetings are located or kept in the PRC; and (iv) more than half of the directors or senior management personnel with voting rights reside in the PRC. Although substantially all of our operational management is currently based in the PRC, it is unclear whether the PRC tax authorities would require (or permit) us to be treated as a PRC resident enterprise. If we are treated as a resident enterprise for PRC tax purposes, we will be subject to PRC tax on our worldwide income at the 25% uniform tax rate, which could have an impact on our effective tax rate and an adverse effect on our net income and results of operations, although dividends distributed from our PRC subsidiaries to us could be exempt from PRC dividend withholding tax, since such income is exempted under the EIT Law to a PRC resident recipient.
The EIT Law imposes a withholding tax of 10% on dividends distributed by a foreign-invested enterprise to its immediate holding company outside of the PRC, if such immediate holding company is considered as a “non-resident enterprise” without any establishment or place within the PRC or if the received dividends have no connection with the establishment or place of such immediate holding company within the PRC, unless such immediate holding company’s jurisdiction of incorporation has a tax treaty with the PRC that provides for a different withholding arrangement. Such withholding income tax was exempted under the previous income tax regulations. The Cayman Islands, where we are incorporated, does not have such tax treaty with the PRC. According to the Arrangement Between Mainland China and Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion, dividends paid by a foreign-invested enterprise in the PRC to its direct holding company in Hong Kong will be subject to withholding tax at a rate of no more than 5% (if the foreign investor owns directly at least 25% of the shares of the foreign-invested enterprise). All of our subsidiaries in the PRC are invested and held by Hong Kong registered entities. Nevertheless, the PRC State Administration of Taxation promulgated a tax notice on October 27, 2009, or Circular 601, which provides that tax treaty benefits will be denied to “conduit” or shell companies without business substance, and a beneficial ownership analysis will be used based on a “substance-over-form” principle to determine whether or not to grant tax treaty benefits. It is unclear at this early stage whether Circular 601 applies to dividends from our PRC subsidiaries paid to us through our Hong Kong subsidiaries. It is possible, however, that under Circular 601 our Hong Kong subsidiaries would not be considered to be the beneficial owners of any such dividends, and that such dividends would as a result be subject to income tax withholding at the rate of 10% rather than the favorable 5% rate applicable under the tax treaty between mainland China and Hong Kong. In accordance with U.S. GAAP, all undistributed earnings of our subsidiaries in the PRC are presumed to be transferred to their respective parent companies and are subject to the withholding tax unless they are going to be permanently reinvested in the PRC or can be distributed in a tax-free manner. Based on the subsequently issued interpretation of the EIT Law, however, dividends on earnings prior to 2008 but distributed after 2008 are not subject to the withholding tax.
As of March 31, 2008, we intended to reinvest all undistributed earnings indefinitely and therefore no deferred tax liability was recognized. After we sold the HIFU business and completed the acquisition of the SPR technology and related assets, in the fiscal year ended March 31, 2009, certain undistributed earnings were no longer required to be reinvested indefinitely and therefore the related deferred tax liability was recognized. Undistributed earnings that we intend to reinvest indefinitely were RMB419 million, RMB249 million and RMB249 million (US$36 million) as of March 31, 2008, 2009 and 2010, respectively. The unrecognized deferred
54
tax liability related to the undistributed earnings subject to withholding tax was RMB42 million, RMB12 million and RMB12 million (US$2 million) as of March 31, 2008, 2009 and 2010, respectively.
Critical Accounting Policies
We prepare our financial statements in accordance with U.S. GAAP, which requires us to make judgments, estimates and assumptions that affect the reported amounts of our assets and liabilities and the disclosure of our contingent assets and liabilities at the end of each fiscal period and the reported amounts of revenues and expenses during each fiscal period. On an on-going basis, we evaluate our judgment and estimate areas, including those related to revenues, provision for allowance for doubtful accounts, share-based compensation, income taxes, inventory valuation, intangible assets and goodwill, estimated useful lives of assets, accounting for convertible notes, foreign currency and contingencies. We continually evaluate these judgments and estimates based on our own historical experience, knowledge and assessment of current business and other conditions, our expectations regarding the future considering available information and assumptions that we believe to be reasonable, which together form our basis for making judgments about matters that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates. Some of our accounting policies involve a greater use of estimates or require a higher degree of judgment than others in their application.
The selection of critical accounting policies, the judgments and other uncertainties affecting application of those policies and the sensitivity of reported results to changes in conditions and assumptions are factors that should be considered when reviewing our consolidated financial statements. We believe the following accounting policies involve the most significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue recognition
We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred and services performed, the sales price is fixed or determinable, and collection is reasonably assured. Written sales agreements or customers purchase orders, which specify price, product specifications, and quantity, are used as evidence of an arrangement. We recognize revenue for reagent kits and probes upon customer acceptance, and the customer acceptance is evidenced by signed acceptance documents. Our equipment sales agreements include the provision of training and installation services. These services are ancillary to the customers’ purchase of medical equipment and are considered by the customers to be an integral part of the acquired equipment. We recognize revenue for the entire equipment sales arrangement upon customer acceptance, which occurs after delivery, installation and training. Customer acceptance is evidenced by signed acceptance documents.
Convertible notes
Effective April 1, 2009, we adopted the new authoritative guidance on accounting for convertible debt instruments that may be settled in cash upon conversion retrospectively, which changes the accounting treatment for convertible securities which the issuer may settle fully or partially in cash. Upon the adoption of this accounting standard, we separate our cash settled securities into debt and equity components. The value assigned to the debt component is the estimated fair value, as of the issuance date, of a similar debt instrument without the conversion feature, and the difference between the proceeds from the issuance and the amount reflected as a debt liability is assigned to equity. As a result, the debt will be recorded at a discount reflecting its below market coupon interest rate. The debt will subsequently be accreted to its par value over its expected life, with the rate of interest that reflects the market rate at issuance. The resulting debt discount is amortized over the period that the convertible debt is expected to be outstanding, as additional non-cash interest expense. The effect of applying this guidance on our Convertible Notes has reduced net income for the fiscal years ended March 31, 2008 and 2009 by approximately RMB28.2 million and RMB27.6 million, respectively.
55
Share-based compensation
We recognize share-based compensation based on grant date fair value of the award. We recognize compensation cost for an award with only service conditions that have a graded vesting schedule on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in-substance, multiple awards.
We use the Black-Scholes option pricing model or the Hull and White Binomial option pricing model to determine the fair value of share options. The determination of the fair value of stock-based compensation awards on the date of grant using an option-pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables, including our expected stock price volatility over the term of the awards, actual and projected employee share option exercise behaviors, risk-free interest rate and expected dividends. If we use different assumptions for estimating share-based compensation expense in future periods or if we decide to use a different valuation model, the change in our share-based compensation expense could materially affect our operating income, net income and net income per share.
Furthermore, we are required to estimate forfeitures at the time of grant and record share-based compensation expense only for those awards that are expected to vest. If actual forfeitures differ materially from our estimated forfeitures, we may need to revise those estimates used in subsequent periods.
Income taxes
Income taxes are accounted for under the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates or change in tax status is recognized in income in the period the change in tax rates or the tax law is enacted. A valuation allowance is provided to reduce the amount of deferred income tax assets if it is considered more likely than not that some portion, or all, of the deferred income tax assets will not be realized.
To assess uncertain tax positions, we apply a more likely than not threshold and a two-step approach for the tax position measurement and financial statement recognition. Under the two-step approach, the first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement.
All undistributed earnings are presumed to be transferred to the parent company and are subject to the withholding taxes. This presumption may be overcome if we have sufficient evidence to demonstrate that the undistributed dividends will be re-invested and the remittance of the dividends will be postponed indefinitely. Accordingly, deferred income tax liabilities are only recognized for the undistributed earnings that we do not intend to reinvest indefinitely.
Discontinued operation
We segregate the revenues and expenses related to the discontinued operation from continuing operations and these are reported as discontinued operation for all periods presented.
Depreciation and amortization of long-lived assets and intangible assets, other than goodwill
We depreciate property, plant and equipment and amortize our intangible assets other than goodwill on a straight-line basis over the respective estimated useful lives of these assets, after taking into account their estimated residual values.
56
The patent we acquired as part of our ECLIA acquisition in August 2004 is amortized over its remaining protected period at time of acquisition of 17 years as allowed by the relevant regulatory authorities.
In determining the estimated useful lives of our unpatented technology in our ECLIA and BBE acquisitions, we consider the following pertinent factors:
| | • | | the expected protective period of similarly patented technologies as allowed by the relevant regulatory authorities; |
| | • | | the planning horizon of our operations taking into account the medical equipment and supply market in the PRC as a whole; and |
| | • | | the economic lives and history of other similar diagnostic technologies currently used by hospitals in the world. |
As a result, we have estimated that the useful life of the unpatented technology we acquired as part of our ECLIA and BBE acquisitions range between 15 and 20 years.
The estimated useful life of the non-compete commitment acquired as part of our ECLIA acquisition in August 2004 is based on the period of time during which the non-compete commitment is expected to be of value to us, which we consider to be the estimated time required for Beijing Weixiao to develop a similar technology and the length of the impact if Beijing Weixiao were to develop the technology and compete with us. As a result, we have estimated the useful life of the non-compete commitment to be nine years.
The customer relationships acquired in the BBE acquisition is amortized over the estimated remaining life for existing customers of 15 years based on historical customer retention rates.
The intangible assets we acquired in our FISH acquisition and SPR acquisition are amortized over their respective estimated useful lives. These intangible assets are amortized over the length of time that they are expected to bring economic value to us.
In determining the estimated useful lives of FISH and SPR related technology and know-how acquired, we consider the following pertinent factors:
| | • | | the useful life of similar technology and technical know-how; |
| | • | | the planning horizon of our operations taking into account the medical equipment and supply market in the PRC as a whole; and |
| | • | | the economic lives and history of other similar diagnostic technologies currently used by hospitals in the world. |
Our estimated useful life for the technical know-how for production of FISH systems and FISH probes are 5 years and 20 years, respectively.
Our estimated useful life for the technical know-how for production for SPR systems and HPV-DNA chips are 5 years and 20 years, respectively.
The estimated useful life of the non-compete commitment acquired as part of our SPR acquisition in December 2008 is based on the period of time during which the non-compete commitment is expected to be of value to us, which we consider to be the estimated time required to develop a similar technology and the length of the impact if Molecular Diagnostic Technologies Limited, the party from whom we acquired the SPR technology and related assets, were to develop the technology and to compete with us. As a result, we have estimated the useful life of the non-compete commitment to be five years.
57
We review the estimated useful lives and estimated residual values of our assets regularly in order to determine the amount of depreciation and amortization expenses to be recorded during any reporting period. We determine the estimated useful lives for property, plant and equipment based on our historical usage experience with similar assets and taking into account anticipated technological changes.
We adjust depreciation and amortization expense for future periods if there are significant changes from previous estimates. For the fiscal years ended March 31, 2008, 2009 and 2010, there were no significant changes in the estimated useful lives or residual values of these long-lived assets.
Acquisitions
We completed the acquisitions of the BBE business and SPR assets during the fiscal years ended March 31, 2008 and 2009, respectively. We made no acquisitions during the fiscal year ended March 31, 2010.
We evaluate whether the net assets acquired in a business combination constitute a business. For an acquired set of activities and assets to qualify for a business, it must contain all of the inputs and processes necessary for it to continue to conduct normal operations after being separated from the seller, which includes the ability to sustain a revenue stream by providing its outputs to customers. As the assets acquired upon the ECLIA and SPR acquisitions lack some of the necessary processes like distribution, strategic management, accounting and human resources to continue to conduct normal operations and do not have access to the customers to sustain their revenue stream, we determined them to be asset acquisition.
We allocate the cost of acquisitions based on the estimated fair value of the net assets acquired on the date of acquisition. This process is commonly referred to as the purchase price allocation. As part of the purchase price allocation, we are required to determine the fair value of any identifiable intangible assets acquired. The determination of the fair value of the intangible assets acquired involves certain judgments and estimates. These judgments can include, but are not limited to, the cash flows that an asset is expected to generate in the future. A change in the amount allocated to identifiable intangible assets would change the amount of amortization expense recognized related to those identifiable intangible assets. When a business combination involves an excess of fair value of the acquired net assets over cost and a contingent consideration agreement that, when resolved, might result in the recognition of an additional element of cost with respect to the acquired entity, we recognize a liability for the lesser of the maximum amount of contingent consideration or the initial amount of negative goodwill. If an amount of negative goodwill remains after we recognize this liability, we first write down the eligible acquired assets, and recognize any remaining unallocated amount as an extraordinary gain. When a business involves an excess of cost over the fair value of net assets acquired, goodwill arises. Goodwill is not amortized, but is instead tested for impairment at least annually.
As part of the purchase price allocation, we allocate the estimated fair value based on risk-adjusted cash flows related to in-process projects that have not yet reached technological feasibility and have no alternative future uses as of the date of the acquisition to in-process research and development. The fair value attributable to these in-process projects was expensed at the time of the acquisition for those acquisitions prior to March 31, 2009. For acquisitions after April 1, 2009, these amounts may be capitalized regardless of whether there is an alternative future use for the in-process research and development as a result of change in relevant accounting guidance. If the projects are not successful or completed in a timely manner, the Group may not realize the financial benefits expected for these projects. The determination of in-process projects involves significant judgment by management, including but not limited to, the assessment of whether the in-process projects reached technological feasibility or have alternative future uses as of the date of the acquisition. The determination of the fair value of the in-process projects acquired also involves judgments and estimates, including but not limited to, the cash flows that a project is expected to generate in the future.
The fair values of our identifiable intangible assets were determined by management with the assistance of independent appraisers using mainly the income approach.
58
For unpatented ECLIA reagent technologies and customer relationships acquired upon the BBE acquisition, the fair values were determined by using the multi-period excess earnings method. The principle of the multi-period excess earnings method implies that the value of an asset is the capitalized amount of incremental profits achieved through use of the intangible assets as compared with the profits of the same business not using it, or that the value of an asset is the capitalized amount of earnings relating to that asset less the returns on all other assets that contribute to that earning stream. For the unpatented ECLIA core technologies, the fair values were determined by using the relief-from-royalty method under the income approach. Under the relief-from-royalty method, a hypothetical construct is used to represent what we would be willing to pay to continue to use the intangible asset in business operations if we no longer had legal ownership of the intangible asset. Since ownership of the asset relieves the business from being required to make these payments, financial results are improved to the extent the upfront payment and royalty payments are avoided. After considering the profit margins of comparable companies, the estimate of the reasonable royalty rates for ECLIA products are from 20% to 25% of sales revenue. They were based on the entire operating income of the business enterprise selling and marketing the ECLIA products in the PRC with the unpatented technologies. For the non-compete agreements in BBE acquisition, we have adopted an income approach that compares the prospective cash flows with and without the subject competition in place.
For unpatented SPR technologies acquired upon the SPR acquisition, the fair values were determined by using the multi-period excess earnings method. The principle of the multi-period excess earnings method implies that the value of an asset is the capitalized amount of incremental profits achieved through use of the intangible assets as compared with the profits of the same business not using it, or that the value of an asset is the capitalized amount of earnings relating to that asset less the returns on all other assets that contribute to that earning stream. For the non-compete agreements in SPR acquisition, we have adopted an income approach that compares the prospective cash flows with and without the subject competition in place. For assembled workforce in SPR acquisition, we have adopted a cost approach that estimates the replacement cost for the experienced employees acquired.
Impairment assessment of long-lived assets
Our long-lived assets consisted of property, plant and equipment, land use rights and intangible assets. We review periodically the carrying amounts of our long-lived assets to be held and used in order to assess whether the recoverable amounts, based on expected undiscounted net cash flows, have declined below the carrying amounts. We test these assets for impairment whenever events or changes in circumstances indicate that their recorded carrying amounts may not be recoverable. When such a decline has occurred, we reduce the carrying amount to the estimated fair value and record the difference as an impairment loss, which is charged against earnings. We determine estimated fair value using expected future cash flows generated by the assets and such future cash flows are discounted to their present value, which requires significant judgment in terms of projection of cash flows for future years and the assumption on the pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. For the fiscal years ended March 31, 2008, 2009 and 2010, we did not recognize any impairment losses in our consolidated financial statements.
Collectibility of our accounts receivable
We evaluate the collectibility of our accounts receivable based on the aging of account balances, collection history, credit quality of the customer and current economic conditions that may affect a customer’s ability to pay.
We have recognized an allowance for doubtful accounts in our consolidated financial statements. We have been dependent on a relatively small number of distributors for a significant portion of our ECLIA revenues. If a major distributor customer’s financial condition deteriorates, or if our customer default rates become higher than historical levels, our estimates of the recoverability of amounts due to us could change, and an additional allowance could be required, which could have a material adverse effect on our results of operations.
59
Inventory valuation
Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out method. Cost of work-in-progress and finished goods comprise direct materials, direct production costs and an allocation of production overheads based on normal operating capacity. We assess the valuation of inventory continuously and periodically. We will write down the value for estimated excess and obsolete inventory based on estimates about future demand. The evaluation may take into consideration historical usage, expected demand, anticipated sales prices, new product development schedules, the effect that new products might have on the sale of existing products, product obsolescence, and other factors. If actual market conditions are less favorable than those projected by management, additional write-downs may be required that could negatively impact our gross margin and operating results. We wrote down inventory in the amount of RMB4.4 million (US$0.6 million) for the fiscal year ended March 31, 2010, and there was no such charge for the fiscal years ended March 31, 2008 and 2009.
Results of Operations
The following table sets forth a summary, for the periods indicated, our consolidated results of operations with each item expressed as a percentage of our total revenues. Our historical results presented below are not necessarily indicative of the results that may be expected for any future period.
| | | | | | | | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008 | | | 2009 | | | 2010 | |
| | | RMB (in thousands) | | | % of
revenues | | | RMB (in thousands) | | | % of
revenues | | | RMB (in thousands) | | | % of
revenues | |
| | | (As adjusted) | | | | | | (As adjusted) | | | | | | | | | | |
Revenues | | 547,421 | | | 100.0 | | | 829,950 | | | 100.0 | | | 723,071 | | | 100.0 | |
Cost of revenues | | (245,437 | ) | | (44.8 | ) | | (232,571 | ) | | (28.0 | ) | | (237,550 | ) | | (32.9 | ) |
| | | | | | | | | | | | | | | | | | |
Gross profit | | 301,984 | | | 55.2 | | | 597,379 | | | 72.0 | | | 485,521 | | | 67.1 | |
Operating expenses: | | | | | | | | | | | | | | | | | | |
Research and development | | (20,231 | ) | | (3.7 | ) | | (31,450 | ) | | (3.8 | ) | | (42,293 | ) | | (5.8 | ) |
Acquired in-process research and development | | (672 | ) | | (0.1 | ) | | (244,872 | ) | | (29.5 | ) | | — | | | — | |
Sales and marketing | | (22,012 | ) | | (4.0 | ) | | (42,722 | ) | | (5.1 | ) | | (64,055 | ) | | (8.9 | ) |
General and administrative | | (70,939 | ) | | (13.0 | ) | | (97,596 | ) | | (11.8 | ) | | (144,671 | ) | | (20.0 | ) |
Amortization of SPR intangible assets | | — | | | — | | | (36,511 | ) | | (4.4 | ) | | (109,395 | ) | | (15.1 | ) |
| | | | | | | | | | | | | | | | | | |
Total operating expenses | | (113,854 | ) | | (20.8 | ) | | (453,151 | ) | | (54.6 | ) | | (360,414 | ) | | (49.8 | ) |
Operating income | | 188,130 | | | 34.4 | | | 144,228 | | | 17.4 | | | 125,107 | | | 17.3 | |
Other expense, net(1) | | (51,776 | ) | | (9.5 | ) | | (100,793 | ) | | (12.1 | ) | | (118,592 | ) | | (16.4 | ) |
| | | | | | | | | | | | | | | | | | |
Income before income taxes | | 136,354 | | | 24.9 | | | 43,435 | | | 5.3 | | | 6,515 | | | 0.9 | |
Income tax expense | | (51,999 | ) | | (9.5 | ) | | (73,042 | ) | | (8.8 | ) | | (63,556 | ) | | (8.8 | ) |
| | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | 84,355 | | | 15.4 | | | (29,607 | ) | | (3.5 | ) | | (57,041 | ) | | (7.9 | ) |
Income from and gain on sale of discontinued operation | | 212,656 | | | 38.8 | | | 364,409 | | | 43.9 | | | — | | | — | |
| | | | | | | | | | | | | | | | | | |
Net income (loss) | | 297,011 | | | 54.2 | | | 334,802 | | | 40.4 | | | (57,041 | ) | | (7.9 | ) |
| | | | | | | | | | | | | | | | | | |
| (1) | Other expense includes “interest expense”, “interest income”, “amortization of Convertible Notes issuance costs” and “other income, net”. |
60
Year Ended March 31, 2010 Compared to Year Ended March 31, 2009
Revenues.Our revenues were derived from two operating segments, immunodiagnostic system sales and molecular diagnostic system sales. Immunodiagnostic system sales include the sales of ECLIA reagent kits. Molecular diagnostic system sales include the sales of FISH probes. Immunodiagnostics system sales and molecular diagnostics system sales accounted for 46.8% and 53.2% of our revenues, respectively, for the fiscal year ended March 31, 2010 and 60.8% and 39.2%, respectively, for the fiscal year ended March 31, 2009.
Our revenues decreased 12.9% to RMB723.1 million (US$105.9 million) for the fiscal year ended March 31, 2010 from RMB830.0 million for the fiscal year ended March 31, 2009. This decrease was due to the decrease in revenues from sales of our immunodiagnostic systems which was partially offset by the increase in revenues from sales of our molecular diagnostic systems.
Revenues from sales of our immunodiagnostic systems decreased 33.0% to RMB338.3 million (US$49.6 million) for the fiscal year ended March 31, 2010 from RMB504.7 million for the fiscal year ended March 31, 2009. This decrease was primarily attributable to the price reduction of ECLIA reagent kits.
Revenues from sales of our molecular diagnostic systems increased 18.3% to RMB384.8 million (US$56.4 million) for the fiscal year ended March 31, 2010 from RMB325.3 million for the fiscal year ended March 31, 2009. The increase was primarily attributable to strong growth in sales of our FISH probes to hospitals as a result of an increase in new hospital customers and increased usage of our FISH probes by existing hospital customers.
Cost of revenues and gross margin. Our cost of revenues increased 2.1% to RMB237.6 million (US$34.8 million) for the fiscal year ended March 31, 2010 from RMB232.6 million for the fiscal year ended March 31, 2009. Our overall gross margin decreased to 67.1% for the fiscal year ended March 31, 2010 as compared to 72.0% for the fiscal year ended March 31, 2009. This decrease in our overall gross margin was primarily due to the price reduction of ECLIA reagent kits. Gross margin for our immunodiagnostic systems decreased to 59.0% for the fiscal year ended March 31, 2010 from 72.6% for the fiscal year ended March 31, 2009 primarily due to the price reduction of ECLIA reagent kits starting from September 2009. In comparison, gross margin for our molecular diagnostic systems increased to 74.3% for the fiscal year ended March 31, 2010 from 71.1% for the fiscal year ended March 31, 2009. The lower gross margin for molecular diagnostic systems in the fiscal year ended March 31, 2009 was mainly due to the sales of fluorescent microscopes in that fiscal year which generated lower gross margin than sales of FISH probes. We discontinued sales of fluorescent microscopes in April 2009.
Operating expenses. Our operating expenses decreased significantly to RMB360.4 million (US$52.8 million) for the fiscal year ended March 31, 2010 from RMB453.2 million for the fiscal year ended March 31, 2009. This decrease was primarily due to the fact that there was no charge of acquired in-process research and development in connection with the SPR acquisition for the fiscal year ended March 31, 2010 as compared to a charge of RMB244.9 million for the fiscal year ended March 31, 2009, which was partially offset by an increase in our general and administrative expenses of RMB47.1 million (US$6.9 million) and an increase in amortization of SPR intangible assets of RMB72.9 million (US$10.7 million). Our operating expenses as a percentage of revenues decreased to 49.8% for the fiscal year ended March 31, 2010 from 54.6% for the fiscal year ended March 31, 2009.
Our research and development expenses increased 34.5% to RMB42.3 million (US$6.2 million) for the fiscal year ended March 31, 2010 from RMB31.5 million for the fiscal year ended March 31, 2009. The increase was primarily due to the development of new FISH probes, SPR-based chips, ECLIA reagent kits and fully-automated ECLIA analyzers. Our research and development expenses as a percentage of revenues increased to 5.8% for the fiscal year ended March 31, 2010 from 3.8% for the fiscal year ended March 31, 2009.
61
Our charge of acquired in-process research and development of RMB244.9 million for the fiscal year ended March 31, 2009 was in connection with the SPR acquisition. There was no such charge for the fiscal year ended March 31, 2010.
Our sales and marketing expenses increased 49.9% to RMB64.1 million (US$9.4 million) for the fiscal year ended March 31, 2010 from RMB42.7 million for the fiscal year ended March 31, 2009. This increase was primarily due to the increase in our direct sales efforts for molecular diagnostic systems. Our sales and marketing expenses as a percentage of revenues increased to 8.9% for the fiscal year ended March 31, 2010 from 5.1% for the fiscal year ended March 31, 2009.
Our general and administrative expenses increased 48.2% to RMB144.7 million (US$21.2 million) for the fiscal year ended March 31, 2010 from RMB97.6 million for the fiscal year ended March 31, 2009. This increase was primarily due to the costs for the independent internal investigation started in April 2009 and provision for allowance for doubtful accounts related to certain ECLIA customers. Our general and administrative expenses as a percentage of revenues increased to 20.0% for the fiscal year ended March 31, 2010 from 11.8% for the fiscal year ended March 31, 2009.
Our amortization of SPR intangible assets related to the SPR acquisition completed in December 2008.
Other expense. We incurred net interest expense of RMB145.1 million (US$21.3 million) for the fiscal year ended March 31, 2010, as compared to RMB97.1 million for the fiscal year ended March 31, 2009. Our interest expense for our Convertible Notes increased significantly to RMB141.1 million (US$20.7 million) in the fiscal year ended March 31, 2010 from RMB111.9 million for the fiscal year ended March 31, 2009. The increase was primarily due to the full year impact of the issuance of the 2013 Notes in August 2008. Also included in the calculation of the net interest expense was the amortization of Convertible Notes issuance costs of RMB17.4 million (US$2.5 million) and RMB13.3 million for the fiscal years ended March 31, 2010 and 2009, respectively. Such amortization of Convertible Notes issuance costs related to the 2011 Notes and 2013 Notes. The issuance costs are amortized over the five-year term of the Convertible Notes. We also realized a gain from the repurchase of our Convertible Notes of RMB25.7 million (US$3.8 million) during the fiscal year ended March 31, 2010. There was no such gain for the fiscal year ended March 31, 2009.
Income tax expense. Income tax expense decreased 13.0% to RMB63.6 million (US$9.3 million) for the fiscal year ended March 31, 2010 from RMB73.0 million for the fiscal year ended March 31, 2009. This decrease was primarily due to the decrease in revenues for the fiscal year ended March 31, 2010. Our income tax expense remained relatively high in the fiscal year ended March 31, 2010 primarily due to the fact that certain of our expenses such as share-based compensation expense, amortization of acquired intangible assets and interest expense associated with Convertible Notes were not deductible for income tax purposes. In addition, we were required to accrue for withholding income tax on distributable earnings generated in the PRC during the year.
Income from and gain on sale of discontinued operation, net. There was no income from and gain on sale of discontinued operation for the fiscal year ended March 31, 2010, as compared to our income from and gain on sale of discontinued operation of RMB364.4 million, resulting from the sale of the HIFU business during the fiscal year ended March 31, 2009.
Net income (loss). Net loss was RMB57.0 million (US$8.4 million) for the fiscal year ended March 31, 2010, as compared to net income of RMB334.8 million for the fiscal year ended March 31, 2009, as a result of the cumulative effect of the above factors.
Year Ended March 31, 2009 Compared to Year Ended March 31, 2008
Revenues.Our revenues were derived from two operating segments, immunodiagnostic system sales and molecular diagnostic system sales. Immunodiagnostic system sales include the sales of ECLIA reagent kits.
62
Molecular diagnostic system sales include the sales of FISH probes and, to a lesser extent, the sales of fluorescent microscopes. Immunodiagnostics system sales and molecular diagnostics system sales accounted for 60.8% and 39.2% of our revenues, respectively, for the fiscal year ended March 31, 2009 and 69.5% and 30.5%, respectively, for the fiscal year ended March 31, 2008.
Our revenues increased 51.6% to RMB830.0 million for the fiscal year ended March 31, 2009, from RMB547.4 million for the fiscal year ended March 31, 2008. This increase was primarily due to the significant increase in the ECLIA reagent kits sales and FISH probes sales.
Revenues from sales of our immunodiagnostic system increased 32.6% to RMB504.7 million for the fiscal year ended March 31, 2009 from RMB380.5 million for the fiscal year ended March 31, 2008. This increase was attributable to the increased utilization of ECLIA analyzers by hospitals and the expanded installed base of the analyzers, both of which increased the demand for our reagent kits and our revenues from sale of reagent kits.
Revenues from sales of our molecular diagnostic system increased 94.9% to RMB325.3 million for the fiscal year ended March 31, 2009 from RMB166.9 million for the fiscal year ended March 31, 2008. The significant increase was attributable to strong growth in sales of our FISH probes to hospitals as a result of increase in new hospital customers and increased usage of our FISH probes by existing hospital customers.
Cost of revenues and gross margin. Our cost of revenues decreased 5.2% to RMB232.6 million for the fiscal year ended March 31, 2009 from RMB245.4 million for the fiscal year ended March 31, 2008. Our overall gross margin increased to 72.0% for the fiscal year ended March 31, 2009 as compared to 55.2% for the fiscal year ended March 31, 2008. This increase in our overall gross margin was primarily due to the change in revenue mix where almost all revenues were derived from recurring sales of higher margin ECLIA reagent kits and FISH probes for the fiscal year ended March 31, 2009. Gross margin for our immunodiagnostic systems decreased to 72.6% for the fiscal year ended March 31, 2009 from 72.9% for the fiscal year ended March 31, 2008 primarily due to the costs of ECLIA analyzers provided free of charge to customers starting from April 2008. In comparison, lower gross margin for our molecular diagnostic systems in the fiscal year ended March 31, 2008 was mainly due to the amortization of FISH intangible assets and the sale of fluorescent microscopes which generated lower gross margin than the sale of FISH probes.
Operating expenses. Our operating expenses increased significantly to RMB453.2 million for the fiscal year ended March 31, 2009 from RMB113.9 million for the fiscal year ended March 31, 2008. This increase was primarily due to the charge of acquired in-process research and development and additional amortization of acquired intangible assets in connection with the SPR acquisition. Our operating expenses as a percentage of revenues increased to 54.6% for the fiscal year ended March 31, 2009 from 20.8% for the fiscal year ended March 31, 2008.
Our research and development expenses increased 55.5% to RMB31.5 million for the fiscal year ended March 31, 2009 from RMB20.2 million for the fiscal year ended March 31, 2008. The increase was primarily due to the development of new FISH probes and fully-automated ECLIA analyzer. Our research and development expenses as a percentage of revenues slightly increased to 3.8% for the fiscal year ended March 31, 2009 from 3.7% for the fiscal year ended March 31, 2008.
Our charge of acquired in-process research and development of RMB244.9 million for the fiscal year ended March 31, 2009 was in connection with the SPR acquisition. The charge of acquired in-process research and development of RMB0.7 million for the fiscal year ended March 31, 2008 was in connection with the BBE acquisition. Our charge of acquired in-process research and development as a percentage of revenues increased to 29.5% for the fiscal year ended March 31, 2009 from 0.1% for the fiscal year ended March 31, 2008.
Our sales and marketing expenses increased 94.1% to RMB42.7 million for the fiscal year ended March 31, 2009 from RMB22.0 million for the fiscal year ended March 31, 2008. This increase was primarily due to the
63
expansion of our direct sales force for the FISH business and increased product promotional activities. Our sales and marketing expenses as a percentage of revenues increased to 5.1% for the fiscal year ended March 31, 2009 from 4.0% for the fiscal year ended March 31, 2008.
Our general and administrative expenses increased 37.6% to RMB97.6 million for the fiscal year ended March 31, 2009 from RMB70.9 million for the fiscal year ended March 31, 2008. This increase was primarily due to an increase in employee expenses as a result of the increased headcount associated with the expansion of our operation and an increase in share-based compensation expenses. Our general and administrative expenses as a percentage of revenues decreased to 11.8% for the fiscal year ended March 31, 2009 from 13.0% for the fiscal year ended March 31, 2008.
Our amortization of SPR intangible assets related to the SPR acquisition completed in December 2008.
Other expense. We incurred net interest expense of RMB97.1 million for the fiscal year ended March 31, 2009, as compared to RMB51.9 million for the fiscal year ended March 31, 2008. Our interest expense for the Convertible Notes increased significantly to RMB111.9 million in the fiscal year ended March 31, 2009 from RMB68.5 million for the fiscal year ended March 31, 2008. The increase was primarily due to the issuance of the 2013 Notes in August 2008. Also included in the calculation of the net interest expense was the amortization of Convertible Notes issuance costs of RMB13.3 million and RMB6.8 million for the fiscal years ended March 31, 2009 and 2008, respectively. Such amortization of Convertible Notes issuance costs were related to the 2011 Notes and 2013 Notes. The issuance costs are amortized over the five-year term of the Convertible Notes. Due to the adoption of new authoritative guidance on accounting for convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) on April 1, 2009, we recorded additional non-cash interest expense for the 2011 Notes. We also made an adjustment related to the 2011 Notes for the fiscal year ended March 31, 2008 to adopt this new guidance retrospectively in accordance with U.S. GAAP. This new guidance does not apply to the 2013 Notes.
Income tax expense. Income tax expense increased 40.5% to RMB73.0 million for the fiscal year ended March 31, 2009 from RMB52.0 million for the fiscal year ended March 31, 2008. Our effective tax rate for the fiscal year ended March 31, 2009 was 168.2%, as compared to 38.1% for the fiscal year ended March 31, 2008. The effective tax rate in the fiscal year ended March 31, 2009 increased significantly primarily due to the fact that the charge for acquired in-process research and development was not deductible for income tax purposes. In addition, certain of our expenses such as share-based compensation expense, amortization of acquired intangible assets and interest expense of Convertible Notes were not deductible for income tax purposes. In addition, we were required to accrue for withholding income tax on distributable earnings generated in the PRC during the year.
Income from and gain on sale of discontinued operation, net. Our income from and gain on sale of discontinued operation increased to RMB364.4 million for the fiscal year ended March 31, 2009 from RMB212.7 million for the fiscal year ended March 31, 2008. This increase was primarily due to a gain from the sale of the HIFU business of RMB243.3 million. However, in September 2009, we received a request for compensation from Chengxuan relating to the sale of the HIFU business and we reduced the gain on the sale of the HIFU business by deferring approximately US$15.5 million of the gain. We then received another letter from Chengxuan in September 2009 which informed us that Chengxuan decided to withdraw its request for compensation. As such, we considered that the deferral was no longer necessary and recognized the full gain on the sale of the HIFU business in the amount of RMB243.3 million. See “Item 7. Major Shareholders and Related Party Translations—B. Related Party Transactions—Transactions with Chengxuan” for more information.
Net income. Net income increased 12.7% to RMB334.8 million for the fiscal year ended March 31, 2009 from RMB297.0 million for the fiscal year ended March 31, 2008, as a result of the cumulative effect of the above factors.
64
| B. | Liquidity and Capital Resources |
Liquidity and Capital Resources
The following table sets forth a summary of our net cash flows for the periods indicated:
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008 | | | 2009 | | | 2010 | | | 2010 | |
| | | RMB | | | RMB | | | RMB | | | US$ | |
| | | (in thousands) | |
Net cash provided by operating activities | | 463,334 | | | 490,758 | | | 279,877 | | | 41,003 | |
Net cash used in investing activities | | (831,551 | ) | | (1,467,195 | ) | | (715,522 | ) | | (104,826 | ) |
Net cash (used in) provided by financing activities | | (86,149 | ) | | 1,751,297 | | | (205,061 | ) | | (30,042 | ) |
Net (decrease) increase in cash and cash equivalents | | (490,961 | ) | | 773,731 | | | (640,957 | ) | | (93,902 | ) |
Cash and cash equivalents at beginning of year | | 1,173,640 | | | 682,679 | | | 1,456,410 | | | 213,368 | |
Cash and cash equivalents at end of year | | 682,679 | | | 1,456,410 | | | 815,453 | | | 119,466 | |
As of March 31, 2010, we had working capital of RMB1,108.8 million (US$162.4 million). We believe that our current cash and cash flow from operations will be sufficient to meet our anticipated cash needs, including our cash needs for working capital, research and development expenditures and capital expenditures for the foreseeable future, although we may need to consider additional financing activities to refinance our Convertible Notes in the future. We may require additional cash resources due to changing business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our existing cash resources are insufficient to meet our requirements, we may seek to sell additional equity securities, debt securities or borrow from banks. We cannot assure you that financing will be available in the amounts we need or on terms acceptable to us, if at all. The sale of additional equity securities, including convertible debt securities, would result in additional dilution to our shareholders. The incurrence of indebtedness would result in debt service obligations and could result in operating and financial covenants that restrict our operations and our ability to pay dividends to our shareholders. If we are unable to obtain additional equity or debt financing as required, our business, operations and prospects may suffer.
In the fiscal years ended March 31, 2008, 2009 and 2010, we have financed our operations primarily through cash generated by our operating activities and our issuance of Convertible Notes in November 2006 and August 2008. We received net proceeds of approximately US$144.7 million and US$267.7 million from our issuance of Convertible Notes in November 2006 and August 2008, respectively.
Our principal uses of cash during the fiscal year ended March 31, 2010 were for our working capital requirements, payment of RMB706.9 million (US$103.6 million) in relation to our SPR acquisition, payment of cash dividends in the aggregate amount of RMB99.7 million (US$14.6 million) to our shareholders, payment of RMB49.4 million (US$7.2 million) in relation to repurchase of our Convertible Notes, payment of RMB43.8 million (US$6.4 million) in relation to repurchase of our own ADSs, prepayment of RMB14.3 million (US$2.1 million) in relation to the repurchase of our Convertible Notes, and payment of RMB8.6 million (US$1.3 million) in relation to the expansion of our production facility and purchase of other property, plant and equipment.
As of March 31, 2010, we had RMB815.5 million (US$119.5 million) in cash. Our cash primarily consists of cash on hand and bank balances which are mainly held in RMB denominated accounts with banks in the PRC and U.S. dollar denominated accounts with banks in Hong Kong and the United States.
In September 2009, our board of directors approved a share repurchase program with a view to demonstrate our commitment to maximize shareholder value. Under the terms of the program, we were authorized by our board to repurchase up to US$30 million worth of our outstanding ADSs for a period of one year, commencing on October 1, 2009. We repurchased a total of 500,000 ADSs at a cost of approximately US$6.4 million (including transaction costs). We funded such repurchases from our available cash balance. We terminated this share repurchase program in May 2010.
65
Starting in January 2010, we repurchased RMB76.8 million (US$11.2 million) of our Convertible Notes up to March 31, 2010 and continued to repurchase our Convertible Notes subsequently. Following these repurchases, we had Convertible Notes with outstanding principal amount of RMB2,831 million (US$415 million) as of March 31, 2010. We funded the repurchase of our Convertible Notes from our available cash balance. We intend to continue to reduce our leverage to an appropriate level by accumulating cash generated from our operations.
Operating Activities
Net cash provided by operating activities decreased to RMB279.9 million (US$41.0 million) for the fiscal year ended March 31, 2010 from RMB490.8 million for the fiscal year ended March 31, 2009. This decrease was mainly attributable to the decrease in revenues and increase in operating expenses and interest expenses in the fiscal year ended March 31, 2010.
Net cash provided by operating activities increased to RMB490.8 million for the fiscal year ended March 31, 2009 from RMB463.3 million for the fiscal year ended March 31, 2008. This increase was mainly attributable to the increase in revenues in the fiscal year ended March 31, 2009.
Investing Activities
Net cash used in investing activities decreased to RMB715.5 million (US$104.8 million) for the fiscal year ended March 31, 2010 from RMB1,467.2 million for the fiscal year ended March 31, 2009. Net cash used in investing activities for the fiscal year ended March 31, 2010 consisted of the payment of RMB706.9 million (US$103.6 million) in relation to our SPR acquisition and payment of RMB8.6 million (US$1.3 million) in relation to the expansion of our production facility and purchase of other property, plant and equipment. As of March 31, 2010, all payments in relation to our SPR acquisition were made.
Net cash used in investing activities increased to RMB1,467.2 million for the fiscal year ended March 31, 2009 from RMB831.6 million for the fiscal year ended March 31, 2008. Net cash used in investing activities for the fiscal year ended March 31, 2009 consisted primarily of the payment of RMB1,514.0 million in relation to our SPR acquisition, payment of RMB68.3 million in relation to our acquisition of the FISH business, payment of RMB26.0 million in relation to the expansion of our production facility and purchase of other property, plant and equipment, and payment of RMB14.8 million in relation to our BBE acquisition.
Financing Activities
Net cash used in financing activities was RMB205.1 million (US$30.0 million) for the fiscal year ended March 31, 2010, as compared to net cash provided by financing activities of RMB1,751.3 million for the fiscal year ended March 31, 2009. Net cash used in financing activities for the fiscal year ended March 31, 2010 consisted primarily of the payment of cash dividends of RMB99.7 million (US$14.6 million) to our shareholders in October 2009, payment of RMB49.4 million (US$7.2 million) in relation to repurchase of our Convertible Notes, payment of RMB43.8 million (US$6.4 million) in relation to repurchase of our own ADSs and prepayment of RMB14.3 million (US$2.1 million) in relation to repurchase of our Convertible Notes.
Net cash used in financing activities was RMB1,751.3 million for the fiscal year ended March 31, 2009, as compared to net cash used in financing activities of RMB86.1 million for the fiscal year ended March 31, 2008. Net cash provided by financing activities for the fiscal year ended March 31, 2009 consisted primarily of proceeds of RMB1,837.9 million raised from our issuance of the 2013 Notes in August 2008, partially offset by cash dividends in the aggregate amount of RMB86.6 million paid to our shareholders in August 2008.
We are a holding company and have historically relied on proceeds from our equity and debt financings as well as dividends paid by our wholly owned subsidiaries for our cash needs, including the funds necessary to pay
66
dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. In the event equity and debt financings are not available in amounts or on terms acceptable to us, we may need to cause our wholly owned subsidiaries to pay dividends to us for our cash needs. The payment of dividends in the PRC is subject to limitations. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in the PRC. YDME Beijing, Beijing GP and BBE are also required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to fund certain reserve funds until the reserves reach 50% of the subsidiaries own registered capital. These reserves can be used to recoup previous years’ losses, if any, and, subject to the approval of the relevant government authority, may be converted into share capital in proportion to their existing shareholdings, or by increasing the par value of the shares currently held by them. Such reserves, however, are not distributable as cash dividends. In addition, if YDME Beijing, Beijing GP and BBE incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other distributions to us.
The ability of our subsidiaries, YDME Beijing, Beijing GP and BBE to convert Renminbi into U.S. dollars and make payments to us is subject to PRC foreign exchange regulations. Under these regulations, the Renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of Renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of the SAFE.
Our working capital requirements vary from period to period depending on manufacturing volumes, the timing of deliveries, our payment cycles to our suppliers and the payment cycles of our customers.
Capital Expenditures
We made capital expenditures of RMB664.9 million, RMB1,789.7 million and RMB715.5 (US$104.8 million) in the fiscal years ended March 31, 2008, 2009 and 2010, respectively. We expect to spend approximately RMB34.1 million (US$5.0 million) in the fiscal year ending March 31, 2011, consisting primarily of the purchase of new manufacturing equipment to expand our production capacity and the renovation of our existing facilities. We expect to finance such capital expenditures primarily through our existing cash balances carried forward and cash generated by our operating activities. Our past capital expenditures principally consisted of expansion of production facility and payments in relation to our acquisitions of the FISH business, BBE business and the SPR technology and related assets.
Recent Accounting Pronouncements
Effective July 2009, the Financial Accounting Standards Board (FASB) codified accounting literature into a single source of authoritative accounting principles. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative GAAP for SEC registrants. Since the codification did not alter existing GAAP, it did not have an impact on our consolidated financial statements and related disclosures. All references to pre-codified GAAP have been removed in our consolidated financial statements and related disclosures.
In June 2009, the FASB issued an amendment to the accounting for transfers of financial assets. The amendment requires additional information disclosures on the transfer of financial assets, including securitization transactions, and where an entity has continuing exposure to risks related to transferred financial assets. The amendment eliminates the concept of a “qualifying special-purpose entity,” changes the requirements for derecognizing financial assets and requires additional disclosures. This amendment is effective at the start of a reporting entity’s first fiscal year beginning after November 15, 2009. We do not believe the adoption of this guidance will have a material effect on our consolidated financial statements and related disclosures.
67
In October 2009, the FASB issued new guidance on revenue recognition for arrangements with multiple deliverables and certain revenue arrangements that include software elements. The new guidance establishes a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. This guidance eliminates the residual method of allocation and requires that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. The relative selling price method allocates any discount in the arrangement proportionally to each deliverable on the basis of each deliverable’s selling price. In addition, this guidance significantly expands required disclosures related to a vendor’s multiple-deliverable revenue arrangements. The new guidance is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. We do not believe the adoption of this guidance will have a material effect on our consolidated financial statements and related disclosures.
In October 2009, the FASB issued new guidance on accounting for own-share lending arrangements in contemplation of convertible debt issuance, which is effective for financial statements issued for fiscal years beginning on or after December 15, 2009. The guidance applies to an equity-classified share lending arrangement on an entity’s own shares when executed in contemplation of a convertible debt offering or other financing. The share lending arrangement is required to be measured at fair value and recognized as an issuance cost associated with the convertible debt offering or other financing. If counterparty default is probable, the share lender is required to recognize an expense equal to the then fair value of the unreturned shares, net of the fair value of probable recoveries. In addition, the loaned shares are excluded from basic and diluted earnings per share unless default of the share-lending arrangement occurs, at which time the loaned shares would be included in the common and diluted earnings-per-share calculation. If dividends on the loaned shares are not reimbursed to the entity, any amounts, including contractual (accumulated) dividends and participation rights in undistributed earnings, attributable to the loaned shares shall be deducted in computing income available to common shareholders, consistent with the “two-class” method. We have evaluated the impact of implementing this new guidance in the fiscal year beginning April 1, 2010 on our financial position, results of operations and cash flows and determined that we will record additional debt issuance costs at issuance of RMB55.4 million (US$8.1 million) related to our loaned shares. Such costs will be amortized over the life of the Convertible Notes and will result in an adjustment of RMB19.4 million (US$2.8 million) to beginning retained earnings on April 1, 2010. The effect of applying this guidance on our Convertible Notes will reduce net income for the fiscal year ended March 31, 2009 by approximately RMB6.9 million and increased net loss for the fiscal year ended March 31, 2010 by approximately RMB12.5 million (US$1.8 million).
| C. | Research and Development and Intellectual Property |
Research and Development
As of July 31, 2010, our research and development team consisted of 138 research personnel and medical professionals, which accounted for 14.2% of our employees. Many of the key research and development personnel who developed the ECLIA technologies at the Peking University People’s Hospital are currently employed by us. The key research and development personnel of the FISH technology and the SPR technology that we acquired in March 2007 and December 2008, respectively, were also transferred to us and are employed by us. Our research and development staff consists of medical professionals, biomedical engineers, electrical engineers and computer science engineers, many of whom have experience in the healthcare and medical device research fields, including experience working with hospitals who are customers of our products. Most of our research and development staff hold bachelor or higher degrees.
Our research and development focuses primarily on developing new applications and improving the product designs of our ECLIA analyzer and reagent kits, our FISH probes and our SPR analyzers and DNA chips.
| | • | | ECLIA analyzer and reagent kits research and development. Our ECLIA technology research and development focuses on expanding the range of reagent kits for applications that are commonly used in |
68
| | the PRC. We plan to continue to add new reagent kits to increase the competitiveness of our ECLIA system. The reagent kits under development include tests for infectious diseases, infertility disorders and cardiac diseases. We also developed fully-automated ECLIA analyzer and are working to improve both semi-automated and fully-automated ECLIA analyzers. |
| | • | | FISH probes research and development. Our FISH technology research and development focuses on expanding the range of probes for applications that address large unmet demand in the PRC. The probes we currently offer are used in these applications: prenatal and postnatal disorders, early detection and prognosis of bladder cancer, cervical cancer, hematological malignancies and prostate cancer, and identify cancer patients who would benefit from targeted drugs in the treatment of breast cancer and lung cancer. FISH probes under development include applications in lymphoma, soft tissue sarcoma, tuberculosis and infertility. We are also working on enhancing the sensitivity and specificity of our probes. |
| | • | | SPR analyzers and DNA chips research and development. Our SPR technology research and development focuses on expanding the range of DNA chips for applications that address large unmet demand in the PRC. We currently offer one type of DNA chip for HPV detection and genotyping. We plan to continue to add new DNA chips to our portfolio. DNA chips under development include applications in low risk HPV genotyping for sexually transmitted diseases, bacterial infection and risk prediction of coagulation related disorders. We are also working on improving the response time and efficiency of our SPR analyzers. |
We actively involve scientists and physicians during our research and development process to produce products that provide clinical and economic benefits to hospitals and their patients. In recent years, we have increased our investment in research and development to accelerate the commercialization of new technologies. Over the past years, we initiated various long term collaborations with leading institutions such as Biomed-X Center of Peking University to strengthen our research capabilities, further enhance the functionality of existing products and expedite the development of new products. We expect to continue devoting significant resources to research and development.
Our research and development expenditures were RMB20.2 million, RMB31.5 million and RMB42.3 million (US$6.2 million) in the fiscal years ended March 31, 2008, 2009 and 2010, respectively.
Intellectual Property
We are committed to developing and protecting our intellectual property portfolio. We own and have applied for patents to protect the technologies, inventions and improvements that we believe are significant to our business. As of August 31, 2010, we have obtained 24 patents in the PRC, including 13 utility patents and 11 invention patents relating to our ECLIA technology. We also own two invention patents relating to our SPR technology. The validity periods for our utility patents and invention patents are 10 years and 20 years, respectively, from the date the application was filed. We have filed patent applications relating to our FISH and SPR technologies in the PRC. Generally, invention patents are viewed as offering more commercial protection than utility patents. As with patent rights in most other jurisdictions, a patent holder in the PRC enjoys the exclusive right to exclude others from using, licensing and otherwise exploiting the patent within the PRC. To protect some of the most important proprietary technologies internationally, we have been granted one patent for ECLIA technology in the United States. In addition, we have 22 patent applications related to our SPR technology in the United States and the European Union. We have also filed patent applications relating to our FISH technology in the United States.
69
We have developed, among others, the following key technologies for our ECLIA system, FISH probes and SPR system:
| | • | | Enhanced chemiluminescence substrate technology—this technology improves the chemical reaction of reagents with the patient’s body fluid sample to produce a long lasting and linear signal and enables our reagent kits to be more sensitive and accurate. We have a pending patent application directed toward this technology in the PRC. |
| | • | | Substance storage technology—this technology can improve the shelf life of our reagent kits from six months to two years, thus reducing the risk of inventory obsolescence. We have obtained a patent directed toward this technology in the PRC. |
| | • | | FISH methodology—this methodology discloses an innovative method to deplete repetitive sequences from human DNA. The approach has consistently demonstrated that our present subtraction method is efficient in removing DNA hybridized complexes, thus leading to a significant depletion of repetitive sequences from a source DNA. We have filed a patent application directed toward this methodology in the United States. |
| | • | | SPR methodology—this leading biosensor technology in molecular biology is used for the analysis of proteins, nucleic acids and viruses. The HPV-DNA chips, which we currently offer for use with our SPR analyzers, are label-free DNA chips for the diagnosis of HPV infection and genotyping of HPV. Once the HPV genotype specific probes on the HPV-DNA chip hybridize with their complementary target DNA abstracted from the cervical cells, the SPR analyzers can read the hybridization signals and interpret the results based on control signals. We have filed patent applications relating to our SPR technology in the PRC, the United States and the European Union. |
We require our employees, consultants and advisers to execute confidentiality agreements in connection with their employment, consulting and advisory relationships with us. We also require our employees, consultants and advisers who are privy to confidential information to agree to disclose and assign to us all inventions conceived during their terms of employment or contract. Despite any measures we take to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or our proprietary technology or to obtain and use information that we regard as proprietary.
We, like other medical device manufacturers in the PRC and elsewhere, are subject to a number of uncertainties regarding the commercial value of our patent portfolio. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Business—If we are unable to protect our intellectual property rights, our competitors may take advantage of our proprietary technology and know-how and compete directly against us.”
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the period from April 1, 2008 to March 31, 2010 that are reasonably likely to have a material adverse effect on our revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
| E. | Off-Balance Sheet Arrangements |
We do not, and did not, have any interest in variable interest entities or any other off-balance sheet arrangements that require disclosure.
70
| F. | Tabular Disclosure of Contractual Obligations |
The following table sets forth our contractual obligations as of March 31, 2010:
| | | | | | | | | | |
| | | Payment due by period |
Contractual obligations | | Less than
1 year | | 1-3
Years | | 3-5
years | | More than
5 years | | Total |
| | | (RMB in thousands) |
Convertible notes—principal | | — | | 1,023,870 | | 1,807,131 | | — | | 2,831,001 |
Convertible notes—interest | | 140,756 | | 188,333 | | 33,131 | | — | | 362,220 |
Operating lease obligations | | 6,669 | | 8,861 | | 1,448 | | — | | 16,978 |
Purchase commitments | | 7,433 | | 13,417 | | — | | — | | 20,850 |
| | | | | | | | | | |
Total | | 154,858 | | 1,234,481 | | 1,841,710 | | — | | 3,231,049 |
| | | | | | | | | | |
Other than the contractual obligations and commercial commitments set forth above, we did not have any other long-term debt obligations, operating lease obligations, purchase commitments or other long-term liabilities as of March 31, 2010.
Inflation
Inflation in the PRC has not had a material impact on our results of operations in recent years. According to the National Bureau of Statistics of China, the change in the Consumer Price Index in the PRC was 4.8%, 5.9% and (0.7)% in 2007, 2008 and 2009, respectively.
This annual report on Form 20-F contains forward-looking statements that relate to future events, including our future operating results and conditions, our prospects and our future financial performance and condition. The forward-looking statements are contained principally in the sections entitled “Item 3. Key Information—D. Risk Factors,” “Item 4. Information on the Company” and “Item 5. Operating and Financial Review and Prospects.” These statements involve known and unknown risks, uncertainties and other factors, which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
Forward-looking statements typically are identified by words or phrases such as “may,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “potential,” “continue,” “is/are likely to” or other similar expressions or the negative of these words or expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. We cannot guarantee the accuracy of the forward-looking statements, and you should be aware that our actual results could differ materially from those contained in the forward-looking statements due to a number of factors, including:
| | • | | our anticipated growth strategies; |
| | • | | our future business development, results of operations and financial condition; |
| | • | | our ability to develop and market future applications of our ECLIA system, FISH probes and SPR system; |
| | • | | market acceptance of fully-automated ECLIA analyzers, FISH probes, SPR analyzers and DNA chips; |
| | • | | the expected market growth for medical devices and supplies in the PRC; |
| | • | | market acceptance of our technology and products; |
| | • | | our ability to expand our production, sales and distribution network and other aspects of our operations; |
71
| | • | | our ability to effectively build and manage a direct sales force for the distribution of FISH probes, SPR analyzers and DNA chips; |
| | • | | our ability to diversify our product range and stay abreast of technological changes; |
| | • | | competition from other companies that offer IVD systems in the PRC; |
| | • | | our ability to effectively protect our intellectual property and not infringe on the intellectual property of others; |
| | • | | our ability to identify and acquire new medical technologies and products; |
| | • | | changes in the healthcare industry in the PRC, including changes in the healthcare policies and regulations of the PRC government and changes in the healthcare insurance sector in the PRC; and |
| | • | | fluctuations in general economic and business conditions in the PRC. |
This annual report contains market information related to the medical devices and supplies industry, healthcare insurance, hospitals and IVD markets in the PRC. Unlike in the United States, there is limited authoritative data on the healthcare industry in the PRC, particularly on a nationwide basis. For example, there is limited data concerning health and patient trends or the medical devices and supplies industry. In addition, any data that is available may not be current. This annual report contains projections that are based on a number of assumptions. For example, our business and marketing plan for the ECLIA system assumes that small and medium-sized hospitals in the PRC will be interested in buying an ECLIA system such as ours. We have also projected a certain penetration rate for our FISH probes and SPR system in large hospitals in the PRC. Any or all of our assumptions may turn out to be incorrect. If demand for our ECLIA system, FISH probes and SPR system does not grow at the projected rates, our business and the market price of our ADSs would suffer. In addition, the complex and changing nature of broad macroeconomic factors subject any projections or estimates relating to the growth prospects or future conditions of our market to significant uncertainties.
The forward-looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this annual report and the documents that we reference in this annual report and have filed as exhibits to the registration statement, of which this annual report is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
| ITEM 6. | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
| A. | Directors and Senior Management |
Directors and Executive Officers
The following table sets forth information regarding our directors and executive officers as of June 30, 2010.
| | | | |
Name | | Age | | Position/title |
Xiaodong Wu | | 54 | | Chairman of the board of directors, chief executive officer |
Iain Ferguson Bruce(1)(2)(3) | | 70 | | Independent director |
Lawrence A. Crum(1)(2)(3) | | 69 | | Independent director |
Yuedong Li(1)(3) | | 61 | | Independent director |
Guoming Qi(2) | | 64 | | Independent director |
Takyung (Sam) Tsang | | 39 | | Director, chief financial officer |
Zhong Chen | | 45 | | Chief technology officer |
Feng (Charles) Zhu | | 41 | | Senior vice president—operations |
72
| (1) | Member of the audit committee. |
| (2) | Member of the compensation committee. |
| (3) | Member of the nomination committee. |
Mr. Xiaodong Wu, our founder, currently serves as our chairman of the board of directors and chief executive officer. Mr. Wu has served on our board of directors since July 2004 and as the chairman of our board of directors since February 2005. Mr. Wu is also a director and the chief executive officer of YDME Beijing, positions he has served since the inception of YDME Beijing in July 1999. Mr. Wu serves on the board of directors of Chengxuan, Beijing Chengxuan, an investment holding company, Beijing Weixiao, a medical technology company, and Beijing Wan De Jin Da Industry and Trade Co., Ltd., a property management company. Mr. Wu worked in the foreign investment department of the Jiangxi provincial government from 1990 to 1996. From 1980 to 1989, Mr. Wu worked in Jiangxi Municipal Science and Technology Commission and accumulated extensive experience in assessing and managing hi-tech projects. Mr. Wu is an advisor to Beijing Medical University for technology development projects. Mr. Wu received his bachelor’s degree in Physics from Jiangxi University.
Mr. Iain Ferguson Bruce has served as a member of our board of directors since February 2005. Mr. Bruce is a member of the audit, compensation and nomination committees. Mr. Bruce joined KPMG in Hong Kong in 1964 and was elected to its partnership in 1971. He was the Senior Partner of KPMG from 1991 until his retirement in 1996 and also concurrently served as Chairman of KPMG Asia Pacific from 1993 to 1997. Since 1964, Mr. Bruce has been a member of the Chartered Accountants of Scotland and is a fellow of the Hong Kong Institute of Certified Public Accountants with over 40 years’ experience in the accounting profession. Mr. Bruce is currently an independent non-executive director of Paul Y Engineering Group Limited, a construction and engineering company, Vitasoy International Holdings Ltd., a beverage manufacturing company, Wing On Company International Ltd., a department store operating and real property investment company, Tencent Holdings Limited, a provider of Internet services and mobile value-added services, and Sands China Ltd., an operator of integrated resorts and casinos. All of these companies are listed companies on the Hong Kong Stock Exchange. Mr. Bruce is also a non-executive director of Noble Group Limited, a commodity trading company that is listed on the Singapore Stock Exchange, and a non-executive director of Yingli Green Energy Holding Company Limited, a photovoltaic product manufacturing company that is listed on the New York Stock Exchange.
Dr. Lawrence A. Crum has served as a member of our board of directors since February 2005 and serves as a member of the audit, compensation and nomination committees. Dr. Crum is the director of the Center for Industrial and Medical Ultrasound and a research professor in electrical engineering and bioengineering at the University of Washington in Seattle in the United States. He holds a Ph.D. degree in physics from the University of Ohio, has served as the president of the Acoustical Society of America and the International Commission for Acoustics and holds an honorary doctorate degree from the University of Brussels. He was recently elected as a member of the Danish Academy of Natural Sciences and as President of the International Society for Therapeutic Ultrasound.
Dr. Yuedong Li has served as a member of our board of directors since October 2007 and serves as a member of the audit and nomination committees. He is currently the chief secretary of the China Hospital Association and the deputy director of Center of Organ Transplant at Beijing University from October 2006. Prior to that, he was the vice-president of Peking University People’s Hospital from 1986 to 2006. Dr. Li also holds various positions in a number of medical associations in the PRC and he obtained a bachelor’s degree from Beijing Medical University in 1976.
Dr. Guoming Qi has served as a member of our board of directors since November 2007. He is also currently the vice president of the Chinese Medical Association, the largest and long established medical association in the PRC. From 1997 to 2006, he was the general director of the Department of Medical Science,
73
Technology and Education of the Ministry of Health in the PRC. From 1992 to 1997, he was the vice president of the Chinese Center for Disease Control and Prevention (formerly known as the Chinese Academy of Preventive Medicine). Dr. Qi obtained his bachelor’s degree from Beijing Medical University in 1970 and a master’s degree from Chinese Academy of Medical Sciences in 1981.
Mr. Takyung (Sam) Tsang has served as our chief financial officer since January 2005 and as a member of our board of director since June 2007. Mr. Tsang was an advisor to our Company for the areas of accounting and finance from February 2004 to December 2004. Prior to joining us, Mr. Tsang worked as a manager of KPMG Hong Kong and Ernst & Young China. He is a Certified Public Accountant in the United States and Hong Kong. He received his bachelor’s degree in business administration from the Chinese University of Hong Kong.
Dr. Zhong Chen has served as our chief technology officer since November 2007. He was the medical director of cytogenetics program at the Associated Regional and University Pathologists Laboratories in Utah and a professor of Pediatrics and Pathology at the University of Utah. Prior to that, he served as the director of cytogenetics laboratories at Genetrix Inc. from 1993 to 1996 and of Genzyme Genetics from 1996 to 1998, two leading biotechnology companies in the United States. Dr. Chen obtained his bachelor’s degree in Medicine in 1986 and a M.D. degree in 1989 from Tongji Medical University. From 1990 to 1993, he also completed a fellowship in Medical Genetics/Cancer Genetics at Southwest Biomedical Research Institute in conjunction with the University of Arizona Genetics Program in Phoenix, Arizona, U.S. He is certificated by the American Board of Medical Genetics.
Mr. Feng (Charles) Zhu joined our Company as our vice president for business development and investor relations in January 2005 and became our senior vice president of operations in October 2009. Prior to joining us, Mr. Zhu worked as a business development manager for GE Healthcare in the PRC from February 2002 to December 2004, responsible for merger and acquisition projects. From May 2000 to February 2002, Mr. Zhu also worked as a business analyst for a Siemens joint venture in the PRC, advising clients on fund raising projects. Mr. Zhu received his bachelor’s degree in English from Foreign Affairs College in the PRC and his master’s degree in business administration from Texas Christian University.
The business address of our directors and executive officers is China Medical Technologies, Inc. at No. 24 Yong Chang North Road, Beijing Economic-Technological Development Area, Beijing 100176, People’s Republic of China.
Compensation of Directors and Executive Officers
For the fiscal year ended March 31, 2010, the aggregate cash compensation to our directors and executive officers was approximately RMB14.9 million (US$2.2 million). The aggregate amount set aside or accrued to provide retirement, pension or other similar benefits for our executive officers was approximately RMB100,000 (US$15,000) for the fiscal year ended March 31, 2010. If we terminate an executive officer without cause, we are required to pay compensation equal to nine months of the executive officer’s salary. Except for directors who are also executive officers, none of our directors receive benefits upon termination.
2005 Stock Option Plan
Our board of directors adopted the 2005 stock option plan on February 2, 2005 and amended and restated the 2005 stock option plan on November 12, 2006. The 2005 stock option plan, as amended and restated, provides for the grant of share options as well as restricted stock, referred to as “awards”. The purpose of the plan is to attract and retain the best available personnel for positions of substantial responsibility, provide additional incentive to employees, directors and consultants and promote the success of our business. Our board of directors believes that our Company’s long-term success is dependent upon our ability to attract and retain superior individuals who, by virtue of their ability, experience and qualifications, make important contributions to our business.
74
We have reserved an aggregate of 30,000,000 of our ordinary shares for issuance under our 2005 stock option plan, as amended and restated. As of June 30, 2010, 6,360,000 restricted shares and share options to purchase an aggregate of 600,000 of our ordinary shares were issued and outstanding under the 2005 stock option plan.
Termination of Awards. Options and restricted shares shall have the specified termination set forth in an award agreement. Where the option agreement permits the exercise of the options granted for a certain period of time following the recipient’s termination of services with us, or the recipient’s disability or death, the options will terminate to the extent any is not exercised or purchased on the last day of the specified period or the last day of the original term of the options, whichever occurs first.
Administration. Our 2005 stock option plan is administered by an administrative committee designated by our board of directors. The administrative committee will determine the provisions, terms and conditions of each award grant, including, but not limited to, the option vesting schedule, repurchase provisions, rights of first refusal, forfeiture provisions, form of payment upon settlement of the award, payment contingencies and satisfaction of any performance criteria.
Vesting Schedule. Options and restricted shares granted under our 2005 stock option plan vest over a period of two to three years after vesting commencement date. The vesting schedule is subject to the optionee continuing to be a service provider of our Company over the vesting period.
Option Agreement. Options granted under our stock option plan are evidenced by an option agreement that contains, among other things, provisions concerning exercisability and forfeiture upon termination of employment or consulting arrangements by reason of death, disability or otherwise, as determined by our board. In addition, the option agreement also provides that options granted under each plan are subject to a 180-day lock-up period following the effective date of a registration statement filed by us under the Securities Act, if so requested by us or any representative of the underwriters in connection with any registration of the offering of any of our securities.
Option Exercise. The term of options granted under the 2005 stock option plan, as amended and restated, may not exceed five years from the date of grant. The consideration to be paid for our ordinary shares upon exercise of an option or purchase of shares underlying the option will be determined by the administrative committee and may include cash, check, ordinary shares, a promissory note, consideration received by us under a cashless exercise program implemented by us in connection with our stock option plan, or any combination of the foregoing methods of payment.
Third-party Acquisition. If a third-party acquires us through the purchase of all or substantially all of our assets, a merger or other business combination, all outstanding share options or share purchase rights will be assumed or equivalent share options or share purchase rights will be substituted by the successor corporation or parent or subsidiary of successor corporation. In the event that the successor corporation refuses to assume or substitute for the stock options or share purchase rights, all share options or share purchase rights will become fully vested and exercisable immediately prior to such transaction and all unexercised options will terminate unless, in either case, the options are assumed by the successor corporation or its parent.
Termination of Plan. Our board of directors has the authority to amend or terminate our stock option plan subject to shareholder approval to the extent necessary to comply with applicable law and regulations. However, no such action may (i) impair the rights of any optionee unless agreed by the optionee and the stock option plan administrative committee, or (ii) affect the stock option plan administrative committee’s ability to exercise the powers granted to it under our stock option plan. The 2005 stock option plan, as amended and restated, expired on December 31, 2009, which means that no new awards may be made under this plan but it will continue to govern any awards previously granted that remain outstanding.
75
The following table summarizes the share options and restricted shares that we granted to our directors and management personnel under our 2005 stock option plan, as amended and restated, as of June 30, 2010:
| | | | | | | | | | |
Name | | Type of awards | | Number of shares
to be issued upon
exercise of
options and/or
number of
restricted shares
granted | | Per share exercise
price/per share
purchase price
(in US$) | | Date of grant | | Date of expiration |
Xiaodong Wu | | Restricted shares | | * | | US$0.10 | | June 6, 2008 November 16, 2009 | | June 5, 2013 November 15, 2014 |
Iain Ferguson Bruce | | Restricted shares | | * | | US$0.10 | | June 11, 2007 November 16, 2009 | | May 31, 2012 November 15, 2014 |
Lawrence A. Crum | | Restricted shares | | * | | US$0.10 | | June 11, 2007 November 16, 2009 | | May 31, 2012 November 15, 2014 |
Takyung (Sam) Tsang | | Restricted shares | | * | | US$0.10 | | June 11, 2007 June 6, 2008 November 16, 2009 | | May 31, 2012 June 5, 2013 November 15, 2014 |
Yuedong Li | | Restricted shares | | * | | US$0.10 | | October 1, 2007 November 16, 2009 | | September 30, 2012 November 15, 2014 |
Guoming Qi | | Restricted shares | | * | | US$0.10 | | November 16, 2007 November 16, 2009 | | November 15, 2012 November 15, 2014 |
Other management personnel as a group | | Share option Restricted shares | | * | | US$2.53 US$0.10 | | June 11, 2007 June 11, 2007 June 6, 2008 November 16, 2009 | | May 31, 2012 May 31, 2012 June 5, 2013 November 15, 2014 |
Total | | Options to purchase 1,550,000 ordinary shares** and 7,930,000 restricted shares*** | | | | | | |
| * | Upon exercise of all options and vesting of all restricted shares granted, would beneficially own less than 1% of our outstanding ordinary shares. |
| ** | Including options that have been exercised and forfeited after they were granted. |
| *** | Including restricted shares that were subsequently forfeited after they were granted. |
These options and restricted shares vest over a period of two to three years after the grant date.
2009 Equity Incentive Plan
Our 2009 equity incentive plan was adopted by our board of directors in August 2009 and approved by a meeting of our shareholders in November 2009. The 2009 equity incentive plan provides for the grant of share options, stock appreciation rights and other stock-based awards including restricted shares, which collectively referred to as “awards”. The purpose of the plan is to aid our Company and our affiliates in recruiting and retaining key employees, directors or consultants of outstanding ability and to motivate such employees, directors or consultants to exert their best efforts on behalf of our Company and affiliates by providing incentives through the granting of awards. We expect that we will benefit from the added interest which such key employees, directors or consultants will have in the welfare of our Company as a result of their proprietary interest in our Company's success.
We have reserved an aggregate of 20,000,000 of our ordinary shares for issuance under our 2009 equity incentive plan. As of June 30, 2010, 3,250,000 restricted shares were issued and outstanding under the 2009 equity incentive plan.
76
Termination of Awards. Awards shall have the specified termination set forth in an award agreement. The agreement permits the exercise of the options granted for a certain period of time following the recipient’s termination of services with us, or the recipient’s disability or death, the options will terminate to the extent any is not exercised or purchased on the last day of the specified period or the last day of the original term of the options, whichever occurs first.
Administration. Our 2009 equity incentive plan is administered by our compensation committee or administrative committee. Our compensation committee or administrative committee will determine the provisions, terms and conditions of each award grant, including, but not limited to, the option vesting schedule, repurchase provisions, rights of first refusal, forfeiture provisions, form of payment upon settlement of the award, payment contingencies and satisfaction of any performance criteria.
Vesting Schedule. Awards granted under our 2009 equity incentive plan vest over a period of two to three years after vesting commencement date. The vesting schedule is subject to the grantee continuing to be a service provider of our Company over the vesting period.
Option Agreement. Options granted under our 2009 equity incentive plan shall be non-qualified or incentive stock options for U.S. federal income tax purposes and are evidenced by an option agreement that contains, among other things, provisions concerning exercisability and forfeiture upon termination of employment or consulting arrangements by reason of death, disability or otherwise, as determined by our board.
Option Exercise. The term of options granted under the 2009 equity incentive plan may not exceed five years from the date of grant. The consideration to be paid for our ordinary shares upon exercise of an option or purchase of shares underlying the option will be determined by our compensation committee or administrative committee and may include cash, check, ordinary shares or proceeds received by us through the delivery of irrevocable instructions to a broker to sell ordinary shares upon the exercise of the options.
Stock Appreciation Rights Agreement. Stock appreciation rights may be granted independent of an option or in connection with an option or a portion thereof and are evidenced by a stock appreciation right agreement.
Other Stock-Based Awards Agreement. Our compensation committee or administrative committee may grant or sell awards of shares, awards of restricted shares and awards that are valued in whole or in part by reference to, or are otherwise based on the fair market value of, shares, and shall determine to whom and when such awards will be granted, as well as the terms and conditions of such awards.
Change in Control. In the event of a change in control, if determined by our compensation committee or administrative committee in the applicable award agreement or otherwise, any outstanding awards which are unexercisable, unvested or subject to lapse restrictions will automatically be deemed exercisable, vested or no longer subject to lapse restrictions, as the case may be, immediately prior to such change in control. In addition, our compensation committee or administrative committee may, but is not obligated to, cancel such awards for fair value (as determined by the compensation committee or administrative committee), provide for the issuance of substitute awards that substantially preserve the terms of the previously granted awards or provide that for a period of at least 15 days prior to the change in control, options will be exercisable as to all shares subject to the options and upon the change in control, the options will terminate. For purposes of the 2009 equity incentive plan, a change in control includes the sale or disposition of all or substantially all of our assets, the acquisition by any person or group of more than 50% of the total voting power of our voting stock or a change in our board of directors such that, during any period of two consecutive years, individuals who at the beginning of the period constituted our board cease for any reason to constitute a majority of our board.
Termination of Plan. Unless terminated earlier, our 2009 equity incentive plan will expire in 2014. Our board of directors has the authority to amend or terminate our 2009 equity incentive plan, subject to applicable laws and regulations, but no amendment or termination shall be made (i) without the approval of our
77
shareholders, if such action would increase the total number of shares reserved for the purposes of the 2009 equity incentive plan or change the maximum number of shares for which awards may be granted to any grantee or (ii) without the consent of a grantee, if such action would diminish any of the rights of the grantee under any award granted to such grantee under the 2009 equity incentive plan.
The following table summarizes the restricted shares that we granted to our directors and management personnel under our 2009 equity incentive plan as of June 30, 2010:
| | | | | | | | | | |
Name | | Type of awards | | Number of
restricted shares
granted | | Per share
purchase price
(in US$) | | Date of grant | | Date of expiration |
Xiaodong Wu | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Iain Ferguson Bruce | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Lawrence A. Crum | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Takyung (Sam) Tsang | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Yuedong Li | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Guoming Qi | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Other management
personnel as a group | | Restricted shares | | * | | US$0.10 | | May 21, 2010 | | May 20, 2015 |
Total | | 3,250,000 restricted shares | | | | | | | | |
| * | Upon vesting of all restricted shares granted, would beneficially own less than 1% of our outstanding ordinary shares. |
These restricted shares vest over a period of three years after the grant date.
Committees of the Board of Directors
We believe that our corporate governance practices comply with those required by domestic companies under NASDAQ Listing Rules and do not differ in any significant ways.
Audit Committee
Our audit committee consists of Mr. Iain Ferguson Bruce, Dr. Lawrence A. Crum and Dr. Yuedong Li, and is chaired by Mr. Bruce. All of the three directors satisfy the “independence” requirements of the NASDAQ Listing Rules and meet the criteria for independence set forth in Section 10A(m)(3)(B)(i) of the Exchange Act. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our Company. The audit committee is responsible for, among other things:
| | • | | selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors; |
| | • | | reviewing with our independent auditors any audit problems or difficulties and management’s response; |
| | • | | reviewing and approving all proposed related-party transactions, as defined in Item 404 of Regulation S-K under the Securities Act; |
| | • | | discussing our annual audited consolidated financial statements with management and our independent auditors; |
| | • | | reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies; |
| | • | | annually reviewing and reassessing the adequacy of our audit committee charter; |
78
| | • | | such other matters that are specifically delegated to our audit committee by our board of directors from time to time; |
| | • | | meeting separately and periodically with management and our internal and independent auditors; and |
| | • | | reporting regularly to the full board of directors. |
Compensation Committee
Our compensation committee consists of Mr. Iain Ferguson Bruce, Dr. Lawrence A. Crum and Dr. Guoming Qi, all of them satisfy the “independence” requirements of the NASDAQ Listing Rules and meet the criteria for independence set forth in Section 10A(m)(3)(B)(i) of the Exchange Act. The committee is chaired by Mr. Bruce. Our compensation committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Members of the compensation committee are not prohibited from direct involvement in determining their own compensation. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
| | • | | approving and overseeing the compensation package for our executive officers; |
| | • | | reviewing and making recommendations to the board with respect to the compensation of our directors; |
| | • | | reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, evaluating the performance of our chief executive officer in light of those goals and objectives, and setting the compensation level of our chief executive officer based on this evaluation; and |
| | • | | reviewing periodically and making recommendations to the board regarding any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans. |
Nomination Committee
Our nomination committee consists of Mr. Iain Ferguson Bruce, Dr. Lawrence A. Crum and Dr. Yuedong Li, and is chaired by Mr. Bruce. All of the three directors satisfy the “independence” requirements of the NASDAQ Listing Rules and meet the criteria for independence set forth in Section 10A(m)(3)(B)(i) of the Exchange Act. The nomination committee assists the board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The nomination committee is responsible for, among other things:
| | • | | identifying and recommending to the board nominees for election or re-election to the board, or for appointment to fill any vacancy; and |
| | • | | reviewing annually with the board the current composition of the board in light of the characteristics of independence, qualification, experience and availability of service to us. |
Duties of Directors
Under Cayman Islands law, our directors have a fiduciary duty to act honestly, in good faith and with a view to our best interests. Our directors also have a duty to exercise the skill they actually possess with such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association, as amended and restated from time to time. A shareholder has the right to seek damages if a duty owed by our directors is breached.
The functions and powers of our board of directors include, among others:
| | • | | convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings; |
79
| | • | | declaring dividends and distributions; |
| | • | | appointing officers and determining the term of office of officers; |
| | • | | exercising the borrowing powers of our Company and mortgaging the property of our Company; and |
| | • | | approving the transfer of shares of our Company, including the registering of such shares in our share register. |
Terms of Directors and Executive Officers
All directors hold office until their successors have been duly elected and qualified. Officers are elected by and serve at the discretion of the board of directors. Currently, directors are not subject to a term of office and hold office until such time as they are removed from office by special resolution or the unanimous written resolution of all shareholders. If a director has been appointed by Chengxuan, upon written notice to us, such director may be removed and replaced by his or her nominator, at any time for any reason.
A director will be removed from office automatically if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors; or (ii) dies or is found by our Company to be or becomes of unsound mind.
Employees
We had 708, 733 and 879 employees as of March 31, 2008, 2009 and 2010, respectively. As of July 31, 2010, we had 974 employees. The following table sets forth the number of our employees categorized by function as of July 31, 2010.
| | | | |
| | | As of July 31,
2010 | | Percentage of
Total (%) |
Manufacturing and services | | 264 | | 27.1 |
Research and development | | 138 | | 14.2 |
General and administration | | 108 | | 11.0 |
Sales and marketing | | 214 | | 22.0 |
Customer support and service | | 250 | | 25.7 |
| | | | |
Total | | 974 | | 100.0 |
| | | | |
From time to time, we also employ independent contractors to support our marketing and sales and clinical support and research. We plan to hire additional employees for sales and marketing, customer support and service, research and development and manufacturing as we grow our business. A number of our PRC employees are represented by a labor union. We consider our relationship with our employees to be good.
In accordance with applicable regulations in the PRC, we participate in a pension contribution plan, a medical insurance plan, an unemployment insurance plan and a personal injury insurance plan for our employees. We have made adequate provisions in accordance with applicable regulations, which require us to contribute amounts equal to 20%, 10%, 1% and 0.3-0.5%, respectively, of our employees’ aggregate base salaries to these statutory plans.
Also, in accordance with PRC regulations, we contribute amounts equal to 12% and 0.8% of our employees’ aggregate base salaries towards a housing fund and a maternity fund, respectively. We also contribute RMB150 per employee per year for a supplemental medical insurance fund.
80
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of June 30, 2010, the latest practicable date, by:
| | • | | each of our directors and executive officers; and |
| | • | | each person known to us to own beneficially more than 5.0% of our ordinary shares. |
| | | | |
| | | Ordinary shares
beneficially owned(1) |
| | | Number | | % |
Directors and executive officers: | | | | |
Xiaodong Wu(2)(4) | | 74,220,001 | | 23.0 |
Iain Ferguson Bruce | | * | | * |
Lawrence A. Crum | | * | | * |
Guoming Qi | | * | | * |
Yuedong Li | | * | | * |
Takyung (Sam) Tsang | | * | | * |
Zhong Chen | | * | | * |
Feng (Charles) Zhu | | * | | * |
All directors and executive officers as a group(3) | | 77,050,001 | | 23.9 |
Principal and 5% shareholders: | | | | |
Chengxuan International Ltd.(4) | | 73,420,001 | | 22.8 |
Keywise Capital Management Limited(5) | | 22,701,100 | | 7.1 |
Putnam Investment Management, LLC (6) | | 19,922,850 | | 6.2 |
Wellington Management Company, LLP(7) | | 18,284,150 | | 5.7 |
| (1) | Beneficial ownership is determined in accordance with Rule 13d-3 of the General Rules and Regulations under the Securities Exchange Act of 1934, as amended, and includes shares as to which the indicated person holds voting or investment power with respect to the securities. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. |
| (2) | Includes 71,820,001 ordinary shares and 200,000 ADSs (equivalent to underlying 2,000,000 ordinary shares) owned by Chengxuan International Ltd., which is beneficially owned by Mr. Xiaodong Wu, and 400,000 restricted shares granted to Mr. Xiaodong Wu which already vested. |
| (3) | Includes ordinary shares issuable upon exercise of options and restricted shares beneficially held by all of our directors and executive officers as a group. |
| (4) | Includes 71,420,001 ordinary shares and 200,000 ADSs (equivalent to underlying 2,000,000 ordinary shares) owned by Chengxuan International Ltd., a British Virgin Islands company beneficially owned by Mr. Xiaodong Wu. The address for Chengxuan is The Mill Mall, PO Box 92, Road Town, Tortola, British Virgin Islands. |
| (5) | Based solely upon a Form 13F filed by Keywise Capital Management Limited with the SEC on August 16, 2010. The address of the principal business office of Keywise Capital Management Limited is Walker House, 87 Mary Street, George Town, Grand Cayman, KY1-9005, Cayman Islands. |
| (6) | Based solely upon a Form 13F filed by Putnam Investment Management, LLC with the SEC on August 13, 2010. The address of Putnam Investment Management, LLC is One Post Office Square, Boston, MA 02109. |
| (7) | Based solely upon a Form 13F filed by Wellington Management Company, LLP with the SEC on August 16, 2010, or the Form 13F. According to the Form 13F, Wellington Management Company, LLP has sole investment discretion with respect to 1,574,165 of our ADSs and shared investment discretion with respect to 254,250 of our ADSs. As such, Wellington Management Company, LLP may be deemed to have beneficial ownership of the 18,284,150 ordinary shares underlying the ADSs. The address of Wellington Management Company, LLP is 75 State Street, Boston, MA 02109. |
81
As of June 30, 2010, other than the 77.4% of our outstanding ordinary shares underlying the outstanding ADSs which were held by our custodian, Citibank, N.A., Hong Kong Branch, on behalf of Citibank, N.A., the depositary, none of our ordinary shares were held in the United States. Our ordinary shares underlying the ADSs quoted on the NASDAQ Global Select Market are held in Hong Kong by the custodian, Citibank, N.A., Hong Kong Branch, on behalf of Citibank, N.A., the depositary.
None of our shareholders has different voting rights from other shareholders. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
| ITEM 7. | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
Please refer to “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
| B. | Related Party Transactions |
After the completion of our initial public offering on August 15, 2005, we adopted an audit committee charter, which requires that the audit committee review all related party transactions on an ongoing basis and all such transactions be approved by the committee. Set forth below is a description of all of our related party transactions for the fiscal years ended March 31, 2008, 2009 and 2010.
Transactions with Chengxuan
Chengxuan International Ltd., or Chengxuan, is one of our shareholders and controlled by Mr. Xiaodong Wu. In December 2008, we completed the sale of the HIFU business to Chengxuan for US$53.5 million in cash. Under the agreement, Chengxuan paid US$23.5 million upon the closing of the sale and will make two other payments during the year after the closing.
In June 2009, we received a letter (“First Letter”) from Chengxuan in connection with a notice from the State Food and Drug Administration, or the SFDA, in April 2009. The notice from the SFDA required the submission of new clinical trial data for the renewal application of the registration certificate for the HIFU system and did not permit the sale of the HIFU system starting from April 2009 until approval of the renewal application. In August 2009, we received another letter (“Second Letter”) from Chengxuan which updated us their ongoing discussion with the SFDA about the requirements for the new clinical trial data for the HIFU system, Chengxuan’s loss of revenues due to the prohibition on selling the HIFU system since April 2009 and their preliminary indication of seeking maximum compensation of approximately US$15.5 million us. We established a special committee comprising two independent directors to evaluate and handle the related matters with Chengxuan and the special committee has engaged legal counsel to advise on Chengxuan’s request for compensation. In September 2009, we received another letter (“Third Letter”) from Chengxuan in which they decided to withdraw the compensation request in the Second Letter. While Chengxuan may make a claim of compensation to us again in the future, we do not believe, based on our current knowledge, that it is likely to happen. Following Chengxuan’s withdrawal of the compensation request, Chengxuan asked us for an extension of the due date for the remaining US$30.0 million consideration payable to us with respect to the sale of the HIFU business. After evaluating this request, the special committee approved an extension of the payment date from December 31, 2009 to December 31, 2010, and in connection with this approval, Chengxuan agreed to pay interest on the unpaid amount at a market rate on a quarterly basis. To date, we have received all quarterly interest payments from Chengxuan.
Shareholders’ Agreement
In June 2005, we and Chengxuan, Golden Meditech (BVI) Company Ltd., or Golden Meditech, and General Electric Company, or GE, entered into a shareholders’ agreement. If any of Chengxuan’s, Golden Meditech’s or GE’s ownership interest in our Company falls below 5% of our outstanding shares, calculated on a fully diluted basis, such shareholder will cease to be a party to the June 2005 shareholders’ agreement and will no longer have any rights or obligations under that agreement. In 2006, both GE and Golden Meditech’s ownership interest in our Company fell below 5% of our outstanding shares and ceased to be parties to the shareholders’ agreement. In July and October 2006, GE and Golden Meditech ceased to be our shareholders.
82
Certain provisions of the June 2005 shareholders’ agreement are still applicable to Chengxuan. Under these provisions, all transactions between Chengxuan and us, including any changes to the material terms thereof, should be approved by our audit committee and unanimously approved by our board of directors.
Equity Incentive Plan
See “Item 6. Directors, Senior Management and Employees—B. Compensation—2005 Stock Option Plan” and “—2009 Equity Incentive Plan”.
| C. | Interests of Experts and Counsel |
Not applicable.
| ITEM 8. | FINANCIAL INFORMATION |
| A. | Consolidated Statements and Other Financial Information |
We have appended consolidated financial statements filed as part of this annual report.
Legal and Administrative Proceedings
Neither we nor any of our directors and executive officers are a party to any material legal or administrative proceedings, and we are not aware of threatened material legal or administrative proceedings against us. We may from time to time become a party to various legal or administrative proceedings arising in the ordinary course of our business.
Dividend Policy
Our board of directors declared a cash dividend on our ordinary shares of US$0.04 per share, equivalent to US$0.40 per ADS based on our net income for the fiscal year ended March 31, 2007 and the cash dividend was paid in August 2007. Our board of directors declared a cash dividend on our ordinary shares of US$0.05 per share, equivalent to US$0.50 per ADS based on our net income for the fiscal year ended March 31, 2008 and the cash dividend was paid in August 2008. Our board of directors declared a cash dividend on our ordinary shares of US$0.055 per share, equivalent to US$0.55 per ADS based on our net income for the fiscal year ended March 31, 2009 and the cash dividend was paid in October 2009. Our board of directors has determined that no dividends will be declared on our ordinary shares for the fiscal year ended March 31, 2010. Cash dividends, if any, will be at the discretion of our board of directors and will depend upon our future operations and earnings, capital requirements and surplus, general financial conditions, shareholders’ interests, contractual restrictions and other factors as our board of directors may deem relevant. We can pay dividends only out of profits or other distributable reserves.
We have historically relied on proceeds from equity and debt offerings as well as dividends paid to us by our operating subsidiaries in the PRC, for our cash requirements, including the funds necessary to pay dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. In the event equity and debt financings are not available in amounts or on terms acceptable to us, we may need to cause our subsidiaries in the PRC to pay dividends to us for our cash needs. In the PRC, the payment of dividends is subject to limitations. PRC regulations currently permit payment of dividends only out of accumulated profits as determined in accordance with PRC accounting standards and regulations. Under current PRC laws, regulations and accounting standards, our PRC operating subsidiaries are required to allocate at least 10% of their after-tax profits to their general reserves. Allocation to these reserves is not required after these reserves have reached 50% of the registered capital of these subsidiaries. In addition, at the discretion of its board of directors, these operating subsidiaries may allocate a portion of their after-tax profits to their enterprise expansion funds and staff welfare and bonus funds. The general reserves, the enterprise expansion funds and staff welfare and bonus funds may not be distributed to equity owners.
83
Our board of directors has complete discretion as to whether we will distribute dividends in the future. Even if our board of directors decides to distribute dividends, the form, frequency and amount of our dividends will depend upon our future operations and earnings, capital requirements and surplus, financial condition, contractual restrictions and other factors that our board of directors may deem relevant. Any dividend we declare will be paid to the holders of ADSs, subject to the terms of the deposit agreement and applicable laws, to the same extent as holders of our ordinary shares, less the fees and expenses payable under the deposit agreement. Any dividend we declare will be distributed by the depositary bank to the holders of our ADSs. Cash dividends on our ordinary shares, if any, will be paid in U.S. dollars.
We have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
| ITEM 9. | THE OFFER AND LISTING |
| A. | Offering and Listing Details |
Our ADSs, each representing 10 of our ordinary shares, have been listed on the NASDAQ Global Select Market (formerly the NASDAQ National Market) since August 10, 2005 under the symbol “CMED”. The following table provides, for the periods indicated, the high and low market prices for our ADSs.
| | | | | | |
| | | Sales Price |
| | | High | | Low |
| Annual High and Low | | | | | | |
2005 (from August 10) | | US$ | 42.60 | | US$ | 14.95 |
2006 | | US$ | 44.93 | | US$ | 18.30 |
2007 | | US$ | 48.25 | | US$ | 21.65 |
2008 | | US$ | 57.50 | | US$ | 16.70 |
2009 | | US$ | 26.07 | | US$ | 11.41 |
| | |
| Quarterly High and Low | | | | | | |
First Quarter 2008 | | US$ | 57.50 | | US$ | 35.61 |
Second Quarter 2008 | | US$ | 49.82 | | US$ | 32.52 |
Third Quarter 2008 | | US$ | 55.24 | | US$ | 32.04 |
Fourth Quarter 2008 | | US$ | 36.65 | | US$ | 16.70 |
First Quarter 2009 | | US$ | 21.74 | | US$ | 11.41 |
Second Quarter 2009 | | US$ | 26.07 | | US$ | 13.26 |
Third Quarter 2009 | | US$ | 20.72 | | US$ | 11.43 |
Fourth Quarter 2009 | | US$ | 18.10 | | US$ | 12.30 |
First Quarter 2010 | | US$ | 15.75 | | US$ | 12.27 |
Second Quarter 2010 | | US$ | 14.25 | | US$ | 9.40 |
| | |
| Monthly Highs and Lows | | | | | | |
January 2010 | | US$ | 15.75 | | US$ | 13.03 |
February 2010 | | US$ | 14.42 | | US$ | 12.27 |
March 2010 | | US$ | 14.75 | | US$ | 12.81 |
April 2010 | | US$ | 14.25 | | US$ | 13.30 |
May 2010 | | US$ | 14.00 | | US$ | 10.05 |
June 2010 | | US$ | 12.45 | | US$ | 9.40 |
July 2010 | | US$ | 10.89 | | US$ | 9.62 |
August 2010 | | US$ | 11.90 | | US$ | 9.90 |
September 2010 (through September 3) | | US$ | 11.64 | | US$ | 10.82 |
84
Not applicable.
Our ADSs, each representing 10 of our ordinary shares, have been listed on the NASDAQ Global Select Market (formerly the NASDAQ National Market) since August 10, 2005 under the symbol “CMED”.
Not applicable.
Not applicable.
Not applicable.
| ITEM 10. | ADDITIONAL INFORMATION |
Not applicable.
| B. | Memorandum and Articles of Association |
We incorporate by reference into this annual report the description of our amended and restated memorandum of association contained in our F-1 registration statement (File No. 333-132214), as amended, initially filed with the SEC on July 15, 2005. Our shareholders adopted our amended and restated memorandum and articles of association by unanimous resolutions in June 2004.
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report on Form 20-F.
Foreign Currency Exchange
Foreign currency exchange regulation in the PRC is primarily governed by the following rules:
| | • | | Foreign Currency Administration Rules (1996), as amended, or the Exchange Rules; and |
| | • | | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Under the Exchange Rules, the Renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of Renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of the SAFE.
Under the Administration Rules, companies in the PRC with foreign investments, such as YDME Beijing, Beijing GP or BBE, may only buy, sell and/or remit foreign currencies at those banks authorized to conduct
85
foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from the SAFE. Capital investments by foreign-invested enterprises outside of the PRC are also subject to limitations, which include approvals by the SAFE and other relevant government authorities.
Cayman Islands Taxation
The Cayman Islands currently levy no taxes on individuals or corporations based upon profits, income, gains or appreciation, and there is no taxation in the nature of inheritance tax or estate duty. No Cayman Islands stamp duty will be payable unless an instrument is executed in, brought to, or produced before a court of the Cayman Islands. The Cayman Islands are not parties to any double tax treaties. There are no exchange control regulations or currency restrictions in the Cayman Islands.
People’s Republic of China Taxation
The PRC Enterprise Income Tax Law, or the EIT Law, and the implementation rules for the EIT Law issued by the PRC State Council, became effective as of January 1, 2008. The EIT Law provides that enterprises established outside of the PRC whose “de facto management bodies” are located in the PRC are considered “resident enterprises” and are generally subject to the uniform 25% enterprise income tax rate as to their worldwide income. Under the implementation rules for the EIT Law issued by the PRC State Council, “de facto management body” is defined as a body that has material and overall management and control over the manufacturing and business operations, personnel and human resources, finances and treasury, and acquisition and disposition of properties and other assets of an enterprise. On April 22, 2009, the PRC State Administration of Taxation further issued a notice entitled “Notice Regarding Recognizing Offshore-Established Enterprises Controlled by PRC Shareholders as Resident Enterprises Based on Their Place of Effective Management.” Under this notice, a foreign company controlled by a PRC company or a group of PRC companies shall be deemed as a PRC resident enterprise, if (i) the senior management and the core management departments in charge of its daily operations mainly function in the PRC; (ii) its financial decisions and human resource decisions are subject to decisions or approvals of persons or institutions in the PRC; (iii) its major assets, accounting books, company seals, minutes and files of board meetings and shareholders’ meetings are located or kept in the PRC; and (iv) more than half of the directors or senior management personnel with voting rights reside in the PRC. Although we are not controlled by any PRC company or company group, we cannot assure you that we will not be deemed to be a PRC resident enterprise under the EIT Law, its implementation rules and the above notice. Substantially all of our operational management is currently based in the PRC. If we are treated as a resident enterprise for PRC tax purposes, we will be subject to PRC tax on our worldwide income at the 25% uniform tax rate, which could have an impact on our effective tax rate and an adverse effect on our net income and results of operations, although dividends distributed from our PRC subsidiaries to us could be exempt from PRC dividend withholding tax, since such income is exempted under the EIT Law to a PRC resident recipient.
Under the EIT Law and implementation rules issued by the State Council, PRC income tax at the rate of 10% is applicable to dividends payable to investors that are “non-resident enterprises,” which do not have an establishment or place of business in the PRC, or which have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends driven from sources within the PRC. Similarly, any gain realized on the transfer of ADSs or shares by such investors is also subject to 10% PRC income tax if such gain is regarded as income derived from sources within the PRC. If we are considered a PRC “resident enterprise,” it is unclear whether dividends we pay with respect to our ordinary shares or ADSs, or the gain you may realize from the transfer of our ordinary shares or ADSs, would be treated as income derived from sources within the PRC and be subject to PRC tax. It is also unclear whether, if we are considered a PRC “resident enterprise,” holders of our ordinary shares or ADSs might be able to claim the benefit of income tax treaties entered into between the PRC and other countries.
86
United States Federal Income Taxation
The following discussion is a summary of certain United States federal income tax consequences applicable to the ownership and disposition of shares or ADSs by a U.S. Holder (as defined below), but does not purport to be a complete analysis of all potential United States federal income tax effects. This summary is based on the Internal Revenue Code of 1986, as amended, or the Code, United States Treasury regulations promulgated thereunder, Internal Revenue Service, or IRS, rulings and judicial decisions in effect on the date hereof. All of these are subject to change, possibly with retroactive effect, or different interpretations.
This summary does not address all aspects of United States federal income taxation that may be relevant to particular U.S. Holders in light of their specific circumstances (for example, U.S. Holders subject to the alternative minimum tax provisions of the Code) or to holders that may be subject to special rules under United States federal income tax law, including:
| | • | | dealers in stocks, securities or currencies; |
| | • | | securities traders that use a mark-to-market accounting method; |
| | • | | banks and financial institutions; |
| | • | | regulated investment companies; |
| | • | | real estate investment trusts; |
| | • | | tax-exempt organizations; |
| | • | | persons holding shares or ADSs as part of a hedging or conversion transaction or a straddle; |
| | • | | persons deemed to sell shares or ADSs under the constructive sale provisions of the Code; |
| | • | | persons who or that are, or may become, subject to the expatriation provisions of the Code; |
| | • | | persons whose functional currency is not the United States dollar; and |
| | • | | direct, indirect or constructive owners of 10% or more of the total combined voting power of all classes of our voting stock. |
This summary also does not discuss any aspect of state, local or foreign law, or United States federal estate or gift tax law as applicable to U.S. Holders. In addition, this discussion is limited to U.S. Holders holding shares or ADSs as capital assets. Prospective purchasers are urged to consult their tax advisers about the United States federal, state and local tax consequences to them of the purchase, ownership and disposition of shares or ADSs.
For purposes of this summary, “U.S. Holder” means a beneficial holder of shares or ADSs who or that for United States federal income tax purposes is:
| | • | | an individual citizen or resident of the United States; |
| | • | | a corporation or other entity classified as a corporation for United States federal income tax purposes created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| | • | | an estate, the income of which is subject to United States federal income taxation regardless of its source; or |
| | • | | a trust, if a court within the United States is able to exercise primary supervision over the administration of such trust and one or more “U.S. persons” (within the meaning of the Code) have the authority to control all substantial decisions of the trust, or if a valid election is in effect to be treated as a U.S. person. |
87
If a partnership or other entity or arrangement classified as a partnership for United States federal income tax purposes holds shares or ADSs, the United States federal income tax treatment of a partner generally will depend on the status of the partner and the activities of the partnership. This summary does not address the tax consequences of any such partner. If you are a partner of a partnership holding shares or ADSs, you should consult your tax advisers.
The discussion below is written on the basis that the representations contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement will be performed in accordance with the terms. If you hold ADSs, you generally will be treated as the owner of the underlying ordinary shares represented by those ADSs for United States federal income tax purposes. Accordingly, deposits or withdrawal of shares for ADSs will not be subject to United States federal income tax.
U.S. Holders
Taxation of Dividends and Other Distributions on the Shares or ADSs
Subject to the passive foreign investment company, or PFIC, rules discussed below, distributions paid by the company out of current or accumulated earnings and profits (as determined for United States federal income tax purposes) generally will be taxable to a U.S. Holder as foreign source dividend income, and will not be eligible for the dividends received deduction generally allowed to corporations. Distributions in excess of current and accumulated earnings and profits will be treated as a non-taxable return of capital to the extent of the U.S. Holder’s adjusted tax basis in the shares or ADSs and thereafter as capital gain. However, the company does not maintain calculations of its earnings and profits in accordance with United States federal income tax accounting principles. U.S. Holders should therefore assume that any distribution by the company with respect to the shares or ADSs will constitute dividend income. U.S. Holders should consult their own tax advisors with respect to the appropriate United States federal income tax treatment of any distribution received from the company.
For taxable years beginning before January 1, 2011, dividends paid by the company should be taxable to a non-corporate U.S. Holder at the special reduced rate normally applicable to long term capital gains, provided that certain conditions are satisfied. A U.S. Holder will not be able to claim the reduced rate for any year in which the company is treated as a PFIC. See “Passive Foreign Investment Company Considerations,” below. Dividends may be taxed at the lower applicable capital gains rate provided that (1) the ADS or ordinary shares, as applicable, are readily tradable on an established securities market in the United States, (2) the company is not a passive foreign investment company (as discussed below) for either our taxable year in which the dividend was paid or the preceding taxable year, and (3) certain holding period requirements are met. Since our ADSs are listed on the NASDAQ Global Select Market, they are considered for purposes of clause (1) above to be readily tradable on an established securities market in the United States.
In the event that dividends from the company are subject to withholding by the PRC, a U.S. Holder may be eligible, subject to a number of complex limitations, to claim a foreign tax credit in respect of any foreign withholding taxes imposed on dividends received on the shares or ADSs. A U.S. Holder who does not elect to claim a foreign tax credit for foreign income tax withheld, may instead claim a deduction, for United States federal income tax purposes, in respect of such withholdings, but only for a year in which such holder elects to do so for all creditable foreign income taxes. Dividends will constitute foreign source income for United States foreign tax credit purposes.
Taxation of Disposition of Shares or ADSs
Subject to the PFIC rules discussed below, you will recognize taxable gain or loss on any sale or exchange of a share or ADS in an amount equal to the difference between the amount realized (in U.S. dollars) for the share or ADS and your tax basis (in U.S. dollars) in the share or ADS. The gain or loss will generally be capital gain or loss and will be long-term capital gain or loss if you have held the share or ADS for more than one year.
88
Long-term capital gains of non-corporate U.S. Holders currently are eligible for reduced rates of taxation. The deductibility of a capital loss may be subject to limitations. Any gain or loss that you recognize generally will be treated as United States source gain or loss for United States foreign tax credit purposes.
Passive Foreign Investment Company Considerations
A foreign corporation will be classified as a PFIC for any taxable year in which, after taking into account the income and assets of the corporation and certain subsidiaries pursuant to applicable “look-through rules,” either (i) at least 75 percent of its gross income is “passive income” or (ii) at least 50 percent of the average value of its assets is attributable to assets which produce passive income or are held for the production of passive income.
For this purpose, cash and investment securities are categorized as passive assets and our company’s unbooked intangibles are taken into account. We will be treated as owning our proportionate share of the assets and earning our proportionate share of the income of any other corporation in which we own, directly or indirectly, more than 25% (by value) of the stock.
We do not believe that we are currently a PFIC for United States federal income tax purposes and do not expect to become a PFIC in the future. However, the determination of whether we will be classified as a PFIC is made annually and may involve facts that are not within our control. In particular, the fair market value of the company’s assets may be determined in large part by the market price of the shares, which is likely to fluctuate. In addition, the composition of the company’s income and assets will be affected by how, and how quickly, the company spends any cash that is raised. Thus, no assurance can be provided that the company would not be classified as a PFIC for the current or any future taxable year. Furthermore, while we believe our valuation approach is reasonable, it is possible that the IRS could challenge our determination concerning our PFIC status.
If the company is classified as a PFIC for any taxable year during which a U.S. Holder owns shares or ADSs, the U.S. Holder, absent certain elections (including a mark-to-market election), will generally be subject to adverse rules (regardless of whether the company continues to be classified as a PFIC) with respect to (i) any “excess distributions” (generally, any distributions received by the U.S. Holder on the shares or ADSs in a taxable year that are greater than 125 percent of the average annual distributions received by the U.S. Holder in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for the shares or ADSs) and (ii) any gain realized on the sale or other disposition of shares or ADSs.
Under these adverse rules (a) the excess distribution or gain will be allocated rateably over the U.S. Holder’s holding period, (b) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which the company is classified as a PFIC will be taxed as ordinary income, and (c) the amount allocated to each of the other taxable years during which the company was classified as a PFIC will be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year and an interest charge will be imposed with respect to the resulting tax attributable to each such other taxable year.
Alternatively, a U.S. Holder may be eligible to make a mark-to-market election. A U.S. Holder that makes a mark-to-market election must include in ordinary income, rather than capital gain, for each year an amount equal to the excess, if any, of the fair market value of the shares or ADSs, as applicable, at the close of the taxable year over the U.S. Holder’s adjusted basis in the shares or ADSs. Additional complex rules apply and the election cannot be revoked without the consent of the IRS unless the shares or ADSs cease to be marketable.
Under recently enacted U.S. legislation and subject to future guidance, if we are a PFIC, U.S. Holders will be required to file, for returns due after March 18, 2010, an annual information return with the IRS relating to their ownership of our common shares or ADSs. Although expected, no guidance has yet been issued about such return, including on the information required to be reported on such return, the form of the return, or the due date for the return.
89
U.S. Holders should consult their tax advisors regarding the potential application of the PFIC regime, including eligibility for and the manner and advisability of making a mark-to-market election.
Information Reporting and Backup Withholding
The proceeds of a sale or other disposition, as well as dividends paid with respect to shares or ADSs by a United States payor (including any payments received from a U.S. financial intermediary), generally will be reported to the IRS and to the U.S. Holder as required under applicable regulations. Backup withholding tax may apply to these payments if the U.S. Holder fails to timely provide an accurate taxpayer identification number or otherwise fails to comply with, or establish an exemption from, such backup withholding tax requirements. Certain U.S. Holders (including, among others, corporations) are not subject to the information reporting or backup withholding tax requirements described herein. U.S. Holders should consult their tax advisors as to their qualification for exemption from backup withholding tax and the procedure for obtaining an exemption.
| F. | Dividends and Paying Agents |
Not applicable.
Not applicable.
We have filed this annual report on Form 20-F, including exhibits, with the SEC. As allowed by the SEC, in Item 19 of this annual report, we incorporate by reference certain information we filed with the SEC. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this annual report.
You may read and copy this annual report, including the exhibits incorporated by reference in this annual report, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 and at the SEC’s regional offices in New York, New York and Chicago, Illinois. You can also request copies of this annual report, including the exhibits incorporated by reference in this annual report, upon payment of a duplicating fee, by writing information on the operation of the SEC’s Public Reference Room.
The SEC also maintains a website at www.sec.gov that contains reports, proxy statements and other information regarding registrants that file electronically with the SEC. Our annual report and some of the other information submitted by us to the SEC may be accessed through this web site.
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
Our financial statements have been prepared in accordance with U.S. GAAP.
We will furnish our shareholders with annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with U.S. GAAP.
In accordance with NASDAQ Listing Rule 5250(d)(1), we will post this annual report on Form 20-F on our Company website athttp://ir.chinameditech.com. In addition, we will provide hard copies of our annual report free of charge to shareholders and ADS holders upon request.
90
Not applicable.
| ITEM 11. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Foreign Exchange Risk
Our revenues, costs and expenses are currently denominated mainly in Renminbi, but the Renminbi prices of some of the materials and supplies for reagent kits that are imported from companies in the United States and European Union may be affected by fluctuations in the value of Renminbi against the currencies of those countries. We do not believe that we currently have any significant direct foreign currency exchange rate risk and have not hedged exposures denominated in foreign currencies or any other derivative financial instruments. Although our revenues and cost of revenues are denominated in Renminbi, fluctuations in the value of the Renminbi may affect the price competitiveness of our products if we commence international sales of our products in the future. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi will be permitted to fluctuate within a band against a basket of certain foreign currencies. This change in policy resulted initially in an approximately 2.0% appreciation in the value of the Renminbi against the U.S. dollar. Since the adoption of this new policy, the value of Renminbi against the U.S. dollar has fluctuated on a daily basis within narrow ranges but overall has further strengthened against the U.S. dollar. On May 18, 2009, the PRC government further widened the daily trading band from 0.3% to 0.5%. On June 19, 2010, the People’s Bank of China has announced that it will proceed further with reform of the RMB exchange rate regime and to enhance the RMB exchange rate flexibility. However, it is uncertain what additional measures will be taken and what their impact could be on exchange rates in the future. There remains significant international pressure on the PRC government to further liberalize its currency policy, which could result in a further and more significant appreciation in the value of the Renminbi against the U.S. dollar. Furthermore, a decline in the value of Renminbi against the U.S. dollar could reduce the U.S. dollar equivalent amounts of our financial results, the value of your investment in our Company and the dividends we may pay in the future, if any, all of which may have a material adverse effect on the prices of our ADSs.
Net assets of China Medical Technologies, Inc. and another non-PRC group entity, whose functional currency is the U.S. dollar accounted for 2% of our consolidated net assets as of March 31, 2010, which results in our exposure to foreign currency exchange risk. Primarily as a result of the appreciation of the Renminbi against the U.S. dollar, the translation of the net assets of China Medical Technologies, Inc. and another non-PRC group entity to Renminbi during consolidation resulted in accumulative translation loss of RMB70.3 million (US$10.3 million) which was recognized as a component of comprehensive loss as of March 31, 2010. If the Renminbi against U.S. dollar as of March 31, 2010 had further appreciated by 10% from 6.8258 to 6.1432 as of March 31, 2010, the translation loss would have increased by RMB3.4 million (US$0.5 million).
Interest Rate Risk
Our risk exposure from changes in interest rates relates primarily to the interest expenses associated with our historical bank borrowings, as well as the interest income generated by excess cash invested in demand and time deposits. We recognized interest income of RMB8.3 million (US$1.2 million) for the fiscal year ended March 31, 2010. A one percent decrease in the average applicable interest rate would have decreased our interest income by RMB8.3 million (US$1.2 million) for the fiscal year. On November 21, 2006, we completed an offering of US$150 million principal amount of the 2011 Notes. On August 15, 2008, we completed an offering of the 2013 Notes. As the Convertible Notes carry a fixed rate of interest, historical changes in market interest rates have not exposed us to material interest rate risks.
91
As of March 31, 2010, the book value and fair value of our Convertible Notes were US$407 million and US$285 million, respectively. We believe the fair value of our Convertible Notes is subject to changes, primarily based on the trading price of our ADSs and our credit risk, and to a lesser extent changes in market interest rates. Changes in the fair value of our Convertible Notes do not have any impact on our financial position or results of operations. To the extent that we may need to raise debt financing in the future, increases in market interest rates will increase the cost of new debt.
We have not historically used, and do not expect to use in the future, any derivative financial instruments to manage our interest risk exposure. Such interest-earning instruments and borrowings carry a degree of interest rate risk. We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates.
| ITEM 12. | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
Not applicable.
Not applicable.
Not applicable.
| D. | American Depositary Shares |
Fee and charges our ADS holders may have to pay
Citibank, N.A. is our depositary. The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deducting from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide services for which fees are payable, until such fees are paid.
92
The table below sets forth all fees and charges, which may change from time to time, that a holder of our ADSs may have to pay to the depositary bank of our ADS program, either directly or indirectly:
| | |
| |
| Persons depositing or withdrawing shares must pay: | | For: |
| Up to US$5.00 per 100 ADSs (or portion thereof) | | Issuance of ADSs upon deposit of shares Delivery of deposited securities against surrender of ADSs Distribution of ADSs pursuant to (i) stock dividends or other free stock distributions or (ii) exercise of rights to purchase additional ADSs Distributions of securities other than ADSs or rights to purchase additional ADSs |
| |
| Up to US$2.00 per 100 ADSs (or portion thereof) | | Distributions of cash dividends or other cash distributions Annual depositary services |
| |
| US$1.50 per certificate presented for transfer | | Transfer of American depositary receipts, or ADRs |
| |
| Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or ordinary shares underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes | | As necessary |
| |
| Any charges, fees or expenses incurred by the depositary or its agents for servicing the deposited securities | | As necessary |
Fee and other payments made by the depositary to us
Our depositary has agreed to reimburse us for certain expenses we incur that are related to the administration and maintenance of the ADS program. There are limits on the amount of expenses for which the depositary will reimburse us, but the amount of reimbursement available to us is not related to the amounts of fees the depositary collects from investors. The depositary reimbursed us US$143,000 for expenses related to the administration and maintenance of the facility for the period from January 1, 2009 to March 31, 2010.
93
PART II
| ITEM 13. | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
None of these events occurred in any of the fiscal years ended March 31, 2008, 2009 and 2010.
| ITEM 14. | MATERIAL MODIFICATIONS TO THE RIGHTS TO SECURITY HOLDERS AND USE OF PROCEEDS |
There are no material modification to, or qualifications of, the rights of securities holders that are required to be disclosed.
On August 15, 2008, we closed an offering of the 2013 Notes pursuant to a registration statement on Form F-3 (333-152937) which was filed and became effective on August 11, 2008. The aggregate principal amount of notes sold reflects the exercise in full by the underwriters of their option to purchase up to an additional US$36 million aggregate principal amount of the notes to cover over-allotments. We received net proceeds of approximately US$267.7 million from our issuance of the 2013 Notes. Credit Suisse Securities (USA) LLC and Morgan Stanley & Co. Incorporated were the underwriters for the offering of the 2013 Notes. Concurrently with the offering of 2013 Notes, we entered into an ADS issuance and repurchase agreement with affiliates of Credit Suisse Securities (USA) LLC and Morgan Stanley & Co. Incorporated, or the Dealers, pursuant to which we issued 4,700,000 ADSs to the Dealers at a consideration of US$1.00 per ADS. On the same day we issued the ADSs to the Dealers, we prepaid to the Dealers a US$1.00 per ADS repurchase price for the 4,700,000 ADSs to be repurchased from the Dealers by us in the future. The Dealers will be obligated to deliver to us an equal number of ADSs in the future.
In the fiscal year ended March 31, 2010, we had used all the net proceeds of US$267.7 million received from the 2013 Notes offering for the SPR acquisition.
As of March 31, 2010, our cash resources amounted to RMB815.5 million (US$119.5 million), comprising of cash on hand and demand deposits.
| ITEM 15. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this annual report, an evaluation has been carried out under the supervision and with the participation of our management, including our chief executive officer and our chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as such term is defined under Rules 13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended. Based on that evaluation, our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures are effective in ensuring that material information required to be disclosed in this annual report is recorded, processed, summarized and reported to them for assessment, and required disclosure is made within the time period specified in the rules and forms of the SEC.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a–15(f) under the Securities Exchange Act of 1934, as amended, for our Company. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with generally accepted accounting principles and includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions
94
and dispositions of a company’s assets, (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that a company’s receipts and expenditures are being made only in accordance with authorizations of a company’s management and directors, and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of a company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance with respect to consolidated financial statement preparation and presentation and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As required by Section 404 of the Sarbanes-Oxley Act of 2002 and related rules as promulgated by the SEC, management assessed the effectiveness of the our internal control over financial reporting as of March 31, 2010 using criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on this assessment, management concluded that our internal control over financial reporting was effective as of March 31, 2010 based on the criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Attestation Report of the Registered Public Accounting Firm
PricewaterhouseCoopers Zhong Tian CPAs Limited Company, or PwC, our independent registered public accounting firm, has audited the effectiveness of our internal control over financial reporting as of March 31, 2010, as stated in its report, which appears on page F-2 of this annual report on Form 20-F.
Changes in Internal Controls
There were no changes in our internal control over financial reporting that occurred during the year ended March 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 16A. | AUDIT COMMITTEE FINANCIAL EXPERT |
Our board of directors has determined that Mr. Iain Ferguson Bruce qualifies as an “audit committee financial expert” as defined in Item 16A of Form 20-F. Each of the members of the audit committee is an “independent director” as defined in the NASDAQ Listing Rules.
Our board of directors has adopted a code of ethics that applies to our directors, officers, employees and advisors, including certain provisions that specifically apply to our chief executive officer, chief financial officer, vice presidents and any other persons who perform similar functions for us. We previously filed our code of business conduct and ethics with the annual report on Form 20-F on September 29, 2006. We hereby undertake to provide to any person without charge, a copy of our code of business conduct and ethics within 10 working days after we receive such person’s written request.
95
| ITEM 16C. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The following table sets forth the aggregate fees by the categories specified below in connection with certain professional services rendered by KPMG, independent registered public accounting firm for the year ended March 31, 2008, and PwC, independent registered public accounting firm for the years ended March 31, 2009 and 2010. We did not pay any other fees to our auditors during the periods indicated below.
| | | | | | |
| | | For the Year Ended March 31, |
| | | 2008 | | 2009 | | 2010 |
| | | US$ | | US$ | | US$ |
| | | (In thousands) |
KPMG | | | | | | |
Audit fees(1) | | 799 | | 556 | | 41 |
Audit-related fees(2) | | — | | 42 | | — |
Tax fees(3) | | — | | — | | — |
Other fees(4) | | — | | — | | — |
| | | |
PwC | | | | | | |
Audit fees(1) | | — | | 659 | | 857 |
Audit-related fees(2) | | — | | — | | — |
Tax fees(3) | | — | | — | | — |
Other fees(4) | | — | | 82 | | — |
| (1) | “Audit fees” represent the aggregate fees billed for professional services rendered by our principal auditors for the audit of our annual financial statements for each of the three fiscal years ended March 31, 2008, 2009 and 2010. |
| (2) | “Audit-related fees” represent the aggregate fees billed by our principal auditors for services rendered that are reasonably related to the performance of the audit or review of our financial statements and are not reported under “Audit Fees”. The fees for the fiscal year ended March 31, 2009 paid to KPMG related to providing advice in connection with a SEC comment letter we received. |
| (3) | “Tax fees” represent the aggregate fees billed for professional services rendered by our principal auditors for tax compliance, tax advice and tax planning. |
| (4) | “Other fees” represent the aggregate fees billed by our principal auditors for services rendered other than services reported under “Audit fees”, “Audit-related fees” and “Tax fees”. “Other fees” for the fiscal year ended March 31, 2009 paid to PwC related primarily to the accounting advisory services provided by PwC before they were engaged to be our principal auditors in August 2009. |
The policy of our audit committee is to pre-approve all audit and non-audit services provided by KPMG and PwC, including audit services, audit-related services, tax services and other services as described above, other than those for de minimus services which are approved by the audit committee prior to the completion of the audit.
| ITEM 16D. | EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
Not applicable.
96
| ITEM 16E. | PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
In September 2009, our board of directors approved a share repurchase program with a view to demonstrate our commitment to maximize shareholder value. Under the terms of the program, we were authorized by our board to repurchase up to US$30 million worth of our outstanding ADSs for a period of one year, commencing on October 1, 2009. Repurchases could be made from time to time in the open market at prevailing market prices or in block trades, in accordance with the “Safe Harbor” requirements of Rule 10b-18 under the Exchange Act. The repurchases could also be effected pursuant to a 10b5-1 plan (which allows us to repurchase our ADSs during periods in which we may be in possession of material non-public information) or otherwise. We repurchased a total of 500,000 ADSs at a cost of approximately US$6.4 million (including transaction costs). We funded such repurchases from our available cash balance. We terminated this share repurchase program in May 2010.
| | | | | | | | |
Period | | Total
Number of
ADSs
Purchased(1) | | Average
Price
Paid
Per ADS | | Total Number
of ADSs
Purchased
as Part of
Publicly
Announced
Plans
or Programs | | Approximate
Dollar Value
of ADSs that
May Yet Be
Purchased
Under the Plans
or Programs |
| | | | | US$ | | | | US$ million |
October 1 through October 30, 2009 | | — | | — | | — | | 30.0 |
November 1 through November 30, 2009 | | 388,562 | | 12.79 | | 388,562 | | 25.0 |
December 1 through December 31, 2009 | | 761 | | 13.05 | | 761 | | 25.0 |
January 1 through January 31, 2010 | | — | | — | | — | | 25.0 |
February 1 through February 28, 2010 | | 110,677 | | 13.00 | | 110,677 | | 23.6 |
March 1 through March 31, 2010 | | — | | — | | — | | 23.6 |
Total | | 500,000 | | 12.84 | | 500,000 | | 23.6 |
| (1) | Our ADS to ordinary share ratio is one ADS for every 10 ordinary shares. |
| ITEM 16F. | CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
As previously reported in our annual report on Form 20-F for the fiscal year ended March 31, 2009 filed with the SEC on September 30, 2009, our audit committee and board of directors approved in August 2009 (i) the engagement of PwC to serve as our independent registered public accounting firm in connection with the audit of our consolidated financial statements for the fiscal year ended March 31, 2009 and (ii) the removal of KPMG, our previous independent registered public accounting firm.
| ITEM 16G. | CORPORATE GOVERNANCE |
None.
97
PART III
| ITEM 17. | FINANCIAL STATEMENTS |
Not applicable.
| ITEM 18. | FINANCIAL STATEMENTS |
The following financial statements are filed as part of this Annual Report on Form 20-F, together with the reports of the independent registered public accounting firms:
INDEX TO THE FINANCIAL STATEMENTS
| | |
Exhibit
Number | | Description of Document |
| 1.1† | | Second Amended and Restated Memorandum and Articles of Association of the Registrant |
| |
| 2.1† | | Specimen Certificate for Ordinary Shares of the Registrant |
| |
| 2.2 | | Specimen American Depositary Receipt of the Registrant (included in Exhibit 2.3) |
| |
| 2.3† | | Deposit Agreement, dated as of August 9, 2005, among the Registrant, the depositary and holders and beneficial owners of the American Depositary Shares issued thereunder, as amended by the letter agreement, dated as of November 21, 2006, between the Registrant and the depositary |
| |
| 2.4†††† | | Indenture, dated as of November 21, 2006, among the Registrant, Wilmington Trust Company, as the trustee, and Citibank, N.A., as the initial agent |
| |
| 2.5 | | Form of China Medical Technologies, Inc. 3.5% Convertible Senior Subordinated Notes due 2011 (included in Exhibit 2.4) |
| |
| 2.6†††† | | Registration Rights Agreement, dated as of November 21, 2006, between the Registrant and Merrill Lynch, Pierce, Fenner & Smith Incorporated |
| |
| 2.7††††† | | Form of First Supplemental Indenture, dated as of August 15, 2008, between the Registrant and Wilmington Trust Company, as the trustee |
| |
| 2.8 | | Form of China Medical Technologies, Inc. 4.00% Convertible Senior Notes due 2013 (included in Exhibit 2.7) |
| |
| 2.9††††† | | Form of ADS Issuance and Repurchase Agreement, dated as of August 12, 2008, between the Registrant and Credit Suisse International |
| |
| 2.10††††† | | Form of ADS Issuance and Repurchase Agreement, dated as of August 12, 2008, between the Registrant and Morgan Stanley & Co. International PLC. |
98
| | |
Exhibit
Number | | Description of Document |
| 4.1††† | | Business Acquisition Agreement, dated as of February 6, 2007, among the Registrant, CMED Technologies Ltd., Supreme Well Investments Limited and Molecular Diagnostic Technologies Limited |
| |
| 4.2†††††† | | Asset Acquisition Agreement, dated as of October 5, 2008, among the Registrant, CMED Technologies Ltd., Molecular Diagnostic Technologies Limited and Supreme Well Investments Limited |
| |
| 4.3†††††† | | Share Purchase Agreement, dated as of December 17, 2008, between CMED ECLIA Diagnostic Technology Ltd. and Chengxuan International Ltd. |
| |
| 4.4††††††† | | Amended and Restated 2005 Stock Option Plan |
| |
| 4.5†††††††† | | 2009 Equity Incentive Plan |
| |
| 8.1* | | List of Subsidiaries |
| |
| 11.1†† | | Code of Business Conduct and Ethics |
| |
| 12.1* | | CEO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 12.2* | | CFO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.1* | | CEO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.2* | | CFO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 15.1* | | Consent of KPMG, Independent Registered Public Accounting Firm |
| |
| 15.2* | | Consent of PricewaterhouseCoopers Zhong Tian CPAs Limited Company, Independent Registered Public Accounting Firm |
| † | Previously filed with the Registrant’s registration statement on Form F-1 (File No. 333-126630), as amended, initially filed with the SEC on July 15, 2005, and incorporated herein by reference. |
| †† | Previously filed with the Registrant’s annual report on Form 20-F on September 29, 2006, and incorporated herein by reference. |
| ††† | Previously filed with the Registrant’s annual report on Form 20-F on September 28, 2007, and incorporated herein by reference. |
| †††† | Previous filed with the Registrant’s registration statement on Form F-3 (File No. 333-139777), as amended, initially filed with the SEC on January 3, 2007, and incorporated herein by reference. |
| ††††† | Previous filed with the Registrant’s report on Form 6-K on August 15, 2008, and incorporated herein by reference. |
| †††††† | Previously filed with the Registrant’s annual report on Form 20-F on September 30, 2009, and incorporated herein by reference. |
| ††††††† | Previous filed with the Registrant’s registration statement on Form S-8 (File No. 333-138954) on November 27, 2006, and incorporated herein by reference. |
| †††††††† | Previous filed with the Registrant’s report on Form 6-K on November 17, 2009, and incorporated herein by reference. |
| * | Filed with this Annual Report on Form 20-F. |
99
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing its annual report on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | |
| CHINA MEDICAL TECHNOLOGIES, INC. |
| |
| By | | /S/ XIAODONG WU |
| Name: | | Mr. Xiaodong Wu |
| Title: | | Chief Executive Officer |
Date: September 8, 2010
100
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
China Medical Technologies, Inc.:
We have audited the accompanying consolidated statements of income, shareholders’ equity and comprehensive income and cash flows of China Medical Technologies, Inc. and subsidiaries for the year ended March 31, 2008. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the results of operations and cash flows of China Medical Technologies, Inc. and subsidiaries for the year ended March 31, 2008, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 2(y) to the consolidated financial statements, in 2009 the Company retrospectively changed its method of accounting for convertible notes due to the adoption of a new accounting pronouncement.
/s/ KPMG
Hong Kong, China
June 27, 2008, except for
Note 9 and Note 21, which
are as of September 30, 2009,
and Note 2(y), which is as of
September 8, 2010
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of China Medical Technologies, Inc.:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, of shareholders’ equity and comprehensive income and of cash flows present fairly, in all material respects, the financial position of China Medical Technologies, Inc. and its subsidiaries at March 31, 2010 and March 31, 2009, and the results of their operations and their cash flows for each of the two years in the period ended March 31, 2010 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 15 of the accompanying Form 20-F. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinion.
As disclosed in Note 2(y) to the consolidated financial statements, in this fiscal year the Company changed the manner in which it accounts for convertible debt instruments due to adopting new accounting pronouncement.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers Zhong Tian CPAs Limited Company
Beijing, the People’s Republic of China
September 8, 2010
F-2
CHINA MEDICAL TECHNOLOGIES, INC.
Consolidated Balance Sheets
(RMB and US$ expressed in thousands, except share data)
| | | | | | | | | | | |
| | | | | March 31, | |
| | | Note | | 2009
(As adjusted)
(Note 2(y)) | | | 2010 | | | 2010 | |
| | | | | RMB | | | RMB | | | US$ | |
| | | | |
Assets | | | | | | | | | | | |
Current assets: | | | | | | | | | | | |
Cash and cash equivalents | | | | 1,456,410 | | | 815,453 | | | 119,466 | |
Accounts receivable, net | | 2(e) | | 343,037 | | | 303,368 | | | 44,444 | |
Prepayments and other assets | | | | 20,425 | | | 21,508 | | | 3,151 | |
Inventories | | 4 | | 16,932 | | | 24,889 | | | 3,646 | |
Due from a related party | | 13 | | 204,987 | | | 204,774 | | | 30,000 | |
| | | | | | | | | | | |
Total current assets | | | | 2,041,791 | | | 1,369,992 | | | 200,707 | |
| | | | | | | | | | | |
Property, plant and equipment, net | | 5 | | 169,422 | | | 155,825 | | | 22,829 | |
Land use rights | | | | 7,239 | | | 7,049 | | | 1,033 | |
Intangible assets, net | | 6 | | 3,487,474 | | | 3,285,190 | | | 481,290 | |
Goodwill | | 3 | | 8,654 | | | 8,654 | | | 1,268 | |
Convertible notes issuance costs | | | | 65,816 | | | 46,681 | | | 6,839 | |
| | | | | | | | | | | |
Total assets | | | | 5,780,396 | | | 4,873,391 | | | 713,966 | |
| | | | | | | | | | | |
Liabilities and Shareholders’ Equity | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | |
Accounts payable | | | | 27,863 | | | 20,126 | | | 2,948 | |
Accrued expenses and other liabilities | | 7 | | 185,700 | | | 183,498 | | | 26,883 | |
Payables related to SPR acquisition | | 3 | | 707,205 | | | — | | | — | |
Income taxes payable | | | | 77,112 | | | 57,529 | | | 8,428 | |
| | | | | | | | | | | |
Total current liabilities | | | | 997,880 | | | 261,153 | | | 38,259 | |
| | | | | | | | | | | |
Convertible notes | | 8 | | 2,826,348 | | | 2,777,086 | | | 406,851 | |
Deferred income taxes | | 12 | | 29,898 | | | 67,134 | | | 9,836 | |
| | | | | | | | | | | |
Total liabilities | | | | 3,854,126 | | | 3,105,373 | | | 454,946 | |
| | | | | | | | | | | |
Commitments and contingencies | | 18 | | — | | | — | | | — | |
| | | | | | | | | | | |
| | | | |
Shareholders’ equity: | | | | | | | | | | | |
Ordinary shares: | | | | | | | | | | | |
Par value: US$0.10 | | | | | | | | | | | |
Authorized: 500,000,000 shares
Issued and outstanding:
321,066,661 and 322,680,001 shares as of March 31, 2009 and 2010, respectively | | | | 257,738 | | | 258,840 | | | 37,921 | |
Additional paid-in capital | | | | 709,949 | | | 752,862 | | | 110,297 | |
Treasury stock (Nil and 6,946,370 shares as of March 31, 2009 and 2010, respectively) | | 10(c), 19 | | — | | | (45,143 | ) | | (6,614 | ) |
Accumulated other comprehensive loss | | | | (69,957 | ) | | (70,316 | ) | | (10,302 | ) |
Retained earnings | | | | 1,028,540 | | | 871,775 | | | 127,718 | |
| | | | | | | | | | | |
Total shareholders’ equity | | | | 1,926,270 | | | 1,768,018 | | | 259,020 | |
| | | | | | | | | | | |
Total liabilities and shareholders’ equity | | | | 5,780,396 | | | 4,873,391 | | | 713,966 | |
| | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
CHINA MEDICAL TECHNOLOGIES, INC.
Consolidated Statements of Income
(RMB and US$ expressed in thousands, except per share data)
| | | | | | | | | | | | | | |
| | | | | Year ended March 31, | |
| | | Note | | 2008
(As adjusted)
(Note 2(y)) | | | 2009
(As adjusted)
(Note 2(y)) | | | 2010 | | | 2010 | |
| | | | | RMB | | | RMB | | | RMB | | | US$ | |
| | | | | |
Revenues | | | | 547,421 | | | 829,950 | | | 723,071 | | | 105,932 | |
Cost of revenues | | | | (245,437 | ) | | (232,571 | ) | | (237,550 | ) | | (34,801 | ) |
| | | | | | | | | | | | | | |
Gross profit | | | | 301,984 | | | 597,379 | | | 485,521 | | | 71,131 | |
| | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | |
Research and development | | | | (20,231 | ) | | (31,450 | ) | | (42,293 | ) | | (6,196 | ) |
Acquired in-process research and development | | | | (672 | ) | | (244,872 | ) | | — | | | — | |
Sales and marketing | | | | (22,012 | ) | | (42,722 | ) | | (64,055 | ) | | (9,384 | ) |
General and administrative | | | | (70,939 | ) | | (97,596 | ) | | (144,671 | ) | | (21,195 | ) |
Amortization of SPR intangible assets | | | | — | | | (36,511 | ) | | (109,395 | ) | | (16,027 | ) |
| | | | | | | | | | | | | | |
Total operating expenses | | | | (113,854 | ) | | (453,151 | ) | | (360,414 | ) | | (52,802 | ) |
| | | | | | | | | | | | | | |
Operating income | | | | 188,130 | | | 144,228 | | | 125,107 | | | 18,329 | |
Other income (expense): | | | | | | | | | | | | | | |
Interest income | | | | 28,650 | | | 32,354 | | | 13,456 | | | 1,971 | |
Interest expense | | | | (73,745 | ) | | (116,092 | ) | | (141,123 | ) | | (20,675 | ) |
Amortization of convertible notes issuance costs | | 8 | | (6,781 | ) | | (13,323 | ) | | (17,402 | ) | | (2,549 | ) |
Other income (expense) | | 8 | | 100 | | | (3,732 | ) | | 26,477 | | | 3,879 | |
| | | | | | | | | | | | | | |
Income before income taxes | | | | 136,354 | | | 43,435 | | | 6,515 | | | 955 | |
Income tax expense | | 12 | | (51,999 | ) | | (73,042 | ) | | (63,556 | ) | | (9,311 | ) |
| | | | | | | | | | | | | | |
Income (loss) from continuing operations | | | | 84,355 | | | (29,607 | ) | | (57,041 | ) | | (8,356 | ) |
Income from and gain on sale of discontinued operation, net | | 9 | | 212,656 | | | 364,409 | | | — | | | — | |
| | | | | | | | | | | | | | |
Net income (loss) | | | | 297,011 | | | 334,802 | | | (57,041 | ) | | (8,356 | ) |
| | | | | | | | | | | | | | |
Net income (loss) per share—Basic: | | | | | | | | | | | | | | |
| | | | | |
Income (loss) from continuing operations | | 11 | | 0.32 | | | (0.11 | ) | | (0.22 | ) | | (0.03 | ) |
Income from and gain on sale of discontinued operation, net | | 11 | | 0.81 | | | 1.39 | | | — | | | — | |
| | | | | | | | | | | | | | |
Net income (loss) | | | | 1.13 | | | 1.28 | | | (0.22 | ) | | (0.03 | ) |
| | | | | | | | | | | | | | |
Net income (loss) per share—Diluted: | | | | | | | | | | | | | | |
| | | | | |
Income (loss) from continuing operations | | 11 | | 0.32 | | | (0.11 | ) | | (0.22 | ) | | (0.03 | ) |
Income from and gain on sale of discontinued operation, net | | 11 | | 0.81 | | | 1.39 | | | — | | | — | |
| | | | | | | | | | | | | | |
Net income (loss) | | | | 1.13 | | | 1.28 | | | (0.22 | ) | | (0.03 | ) |
| | | | | | | | | | | | | | |
Weighted average shares used in computing net income per share (‘000): | | | | | | | | | | | | | | |
| | | | | |
Basic | | | | 262,219 | | | 262,776 | | | 262,546 | | | 262,546 | |
Diluted | | | | 263,465 | | | 262,776 | | | 262,546 | | | 262,546 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
CHINA MEDICAL TECHNOLOGIES, INC.
Consolidated Statements of Shareholders’ Equity and Comprehensive Income
(RMB and US$ expressed in thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Note | | Ordinary Shares | | Additional
Paid-in
Capital | | | Treasury
Stock | | | Accumulated
Other
Comprehensive
Loss | | | Retained
Earnings | | | Total
Shareholders’
Equity | | | Comprehensive
income | |
| | | | | Number | | RMB | | RMB | | | RMB | | | RMB | | | RMB | | | RMB | | | RMB | |
Balance as of April 1,
2007 | | | | 273,600,001 | | 225,125 | | 504,795 | | | — | | | (21,335 | ) | | 577,154 | | | 1,285,739 | | | | |
Adjustment to initially apply the new guidance on accounting for convertible debt instruments | | 2(y) | | — | | — | | 165,771 | | | — | | | (2,930 | ) | | (10,312 | ) | | 152,529 | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of April 1, 2007 (As adjusted) | | | | 273,600,001 | | 225,125 | | 670,566 | | | — | | | (24,265 | ) | | 566,842 | | | 1,438,268 | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | — | | — | | — | | | — | | | — | | | 297,011 | | | 297,011 | | | 297,011 | |
Foreign currency translation adjustment, net of nil tax | | | | — | | — | | — | | | — | | | (39,072 | ) | | — | | | (39,072 | ) | | (39,072 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | 257,939 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of shares upon exercise of options | | 17 | | 466,660 | | 348 | | 4,809 | | | — | | | — | | | — | | | 5,157 | | | | |
Share-based compensation | | 17 | | — | | — | | 16,660 | | | — | | | — | | | — | | | 16,660 | | | | |
Dividends declared and paid | | 10(d) | | — | | — | | — | | | — | | | — | | | (83,306 | ) | | (83,306 | ) | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of March 31, 2008 (As adjusted) | | | | 274,066,661 | | 225,473 | | 692,035 | | | — | | | (63,337 | ) | | 780,547 | | | 1,634,718 | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | — | | — | | — | | | — | | | — | | | 334,802 | | | 334,802 | | | 334,802 | |
Foreign currency translation adjustment, net of nil tax | | | | — | | — | | — | | | — | | | (6,620 | ) | | — | | | (6,620 | ) | | (6,620 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | 328,182 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Issuance and repurchase of shares under share issuance and repurchase agreement | | 10(c) | | 47,000,000 | | 32,265 | | (32,265 | ) | | — | | | — | | | — | | | — | | | | |
Share-based compensation | | 17 | | — | | — | | 50,179 | | | — | | | — | | | — | | | 50,179 | | | | |
Dividends declared and paid | | 10(d) | | — | | — | | — | | | — | | | — | | | (86,809 | ) | | (86,809 | ) | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of March 31, 2009 (As adjusted) | | | | 321,066,661 | | 257,738 | | 709,949 | | | — | | | (69,957 | ) | | 1,028,540 | | | 1,926,270 | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | | — | | — | | — | | | — | | | — | | | (57,041 | ) | | (57,041 | ) | | (57,041 | ) |
Foreign currency translation adjustment, net of nil tax | | | | — | | — | | — | | | — | | | (359 | ) | | — | | | (359 | ) | | (359 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | — | | — | | — | | | — | | | — | | | — | | | — | | | (57,400 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of shares upon exercise of options and restricted shares | | | | 1,613,340 | | 1,102 | | 1,147 | | | — | | | — | | | — | | | 2,249 | | | | |
Return of shares under share issuance and repurchase agreement | | 10(c) | | — | | — | | 1,329 | | | (1,329 | ) | | — | | | — | | | — | | | | |
Repurchase of shares | | 19 | | — | | — | | — | | | (43,814 | ) | | — | | | — | | | (43,814 | ) | | | |
Share-based compensation | | 17 | | — | | — | | 40,437 | | | — | | | — | | | — | | | 40,437 | | | | |
Dividends declared and paid | | 10(d) | | — | | — | | — | | | — | | | — | | | (99,724 | ) | | (99,724 | ) | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of March 31, 2010 | | | | 322,680,001 | | 258,840 | | 752,862 | | | (45,143 | ) | | (70,316 | ) | | 871,775 | | | 1,768,018 | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of March 31, 2010 (US$) | | | | | | 37,921 | | 110,297 | | | (6,614 | ) | | (10,302 | ) | | 127,718 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
CHINA MEDICAL TECHNOLOGIES, INC.
Consolidated Statements of Cash Flows
(RMB and US$ expressed in thousands)
| | | | | | | | | | | | | | | |
| | | | | | Year ended March 31, | |
| | | Note | | | 2008
(As adjusted)
(Note 2(y)) | | | 2009
(As adjusted)
(Note 2(y)) | | | 2010 | | | 2010 | |
| | | | | | RMB | | | RMB | | | RMB | | | US$ | |
Cash flow from operating activities: | | | | | | | | | | | | | | | |
Net income (loss) | | | | | 297,011 | | | 334,802 | | | (57,041 | ) | | (8,356 | ) |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | | | | | | | |
Depreciation and amortization of property, plant and equipment | | | | | 16,067 | | | 20,150 | | | 22,137 | | | 3,243 | |
Amortization of intangible assets | | 6 | | | 90,233 | | | 126,588 | | | 199,115 | | | 29,171 | |
Acquired in-process research and development | | | | | 672 | | | 244,872 | | | — | | | — | |
Non-cash interest expense on convertible notes | | 2 | (y) | | 29,367 | | | 28,614 | | | 30,477 | | | 4,465 | |
Amortization of convertible notes issuance costs | | | | | 6,781 | | | 13,323 | | | 17,402 | | | 2,549 | |
Accrued interest on FISH hold-back payment | | | | | 5,087 | | | 4,240 | | | — | | | — | |
Share-based compensation | | | | | 16,660 | | | 50,179 | | | 40,437 | | | 5,924 | |
Charges of excess and obsolete inventory | | 4 | | | — | | | — | | | 4,406 | | | 645 | |
Land use rights expense | | | | | 189 | | | 191 | | | 190 | | | 28 | |
Loss on disposal of property, plant and equipment | | | | | 179 | | | 188 | | | 75 | | | 11 | |
Deferred income taxes | | | | | 1,666 | | | 28,774 | | | 37,236 | | | 5,455 | |
Gain on repurchase of convertible notes | | 8 | | | — | | | — | | | (25,693 | ) | | (3,764 | ) |
Gain on sale of HIFU Business, net | | | | | — | | | (243,329 | ) | | — | | | — | |
Provision for allowance for doubtful accounts | | | | | — | | | 3,373 | | | 15,143 | | | 2,218 | |
Changes in operating assets and liabilities net of effects of acquisitions and discontinued operation: | | | | | | | | | | | | | | | |
Accounts receivable | | | | | (80,326 | ) | | (184,459 | ) | | 24,526 | | | 3,594 | |
Prepayments and other assets | | | | | 15,260 | | | (1,305 | ) | | 13,263 | | | 1,943 | |
Inventories | | | | | 9,928 | | | 5,171 | | | (12,363 | ) | | (1,811 | ) |
Accounts payable | | | | | (3,201 | ) | | 18,959 | | | (7,737 | ) | | (1,133 | ) |
Accrued expenses and other liabilities | | | | | 26,729 | | | 36,337 | | | (2,113 | ) | | (310 | ) |
Income taxes payable | | | | | 31,032 | | | 4,090 | | | (19,583 | ) | | (2,869 | ) |
| | | | | | | | | | | | | | | |
Net cash provided by operating activities | | | | | 463,334 | | �� | 490,758 | | | 279,877 | | | 41,003 | |
| | | | | | | | | | | | | | | |
Cash flow from investing activities: | | | | | | | | | | | | | | | |
Purchase of property, plant and equipment | | | | | (29,323 | ) | | (25,972 | ) | | (8,616 | ) | | (1,262 | ) |
Payments for FISH acquisition | | | | | (439,926 | ) | | (68,329 | ) | | — | | | — | |
Payments for BBE acquisition | | | | | (195,637 | ) | | (14,788 | ) | | — | | | — | |
Deposit and payments for SPR acquisition | | 3 | (c) | | (166,665 | ) | | (1,513,964 | ) | | (706,906 | ) | | (103,564 | ) |
Proceeds from sale of HIFU Business | | | | | — | | | 155,858 | | | — | | | — | |
| | | | | | | | | | | | | | | |
Net cash used in investing activities | | | | | (831,551 | ) | | (1,467,195 | ) | | (715,522 | ) | | (104,826 | ) |
| | | | | | | | | | | | | | | |
Cash flow from financing activities: | | | | | | | | | | | | | | | |
Dividends paid | | | | | (83,306 | ) | | (86,574 | ) | | (99,724 | ) | | (14,610 | ) |
Principal payments on bank loan | | | | | (8,000 | ) | | — | | | — | | | — | |
Proceeds from exercise of stock options and restricted shares | | | | | 5,157 | | | — | | | 2,249 | | | 330 | |
Net proceeds from issuance of convertible notes | | | | | — | | | 1,837,871 | | | — | | | — | |
Payment for repurchase of convertible notes | | 8 | | | — | | | — | | | (49,426 | ) | | (7,241 | ) |
Prepayment for repurchase of convertible notes | | | | | — | | | — | | | (14,346 | ) | | (2,102 | ) |
Repurchase of shares under share repurchase program | | 19 | | | — | | | — | | | (43,814 | ) | | (6,419 | ) |
Proceeds from issuance of shares under share issuance and repurchase agreement | | 10 | (c) | | — | | | 32,265 | | | — | | | — | |
Payment for repurchase of shares under share issuance and repurchase agreement | | 10 | (c) | | — | | | (32,265 | ) | | — | | | — | |
| | | | | | | | | | | | | | | |
Net cash (used in) provided by financing activities | | | | | (86,149 | ) | | 1,751,297 | | | (205,061 | ) | | (30,042 | ) |
| | | | | | | | | | | | | | | |
Effect of foreign currency exchange rate change on cash and cash equivalents | | | | | (36,595 | ) | | (1,129 | ) | | (251 | ) | | (37 | ) |
| | | | | | | | | | | | | | | |
Net (decrease) increase in cash and cash equivalents | | | | | (490,961 | ) | | 773,731 | | | (640,957 | ) | | (93,902 | ) |
Cash and cash equivalents at beginning of year | | | | | 1,173,640 | | | 682,679 | | | 1,456,410 | | | 213,368 | |
| | | | | | | | | | | | | | | |
Cash and cash equivalents at end of year | | | | | 682,679 | | | 1,456,410 | | | 815,453 | | | 119,466 | |
| | | | | | | | | | | | | | | |
Supplemental disclosures of cash flow information: | | | | | | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | | | | | | |
Income taxes | | | | | 45,499 | | | 79,028 | | | 45,903 | | | 6,725 | |
Interest expenses | | | | | 39,104 | | | 74,024 | | | 109,988 | | | 16,113 | |
Non-cash financing activities during the year: | | | | | | | | | | | | | | | |
Return of shares under share issuance and repurchase agreement | | 10 | (c) | | — | | | — | | | 1,329 | | | 195 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements
For the years ended March 31, 2008, 2009 and 2010
1 Principal Activities and Organization
China Medical Technologies, Inc. (the “Company”) was incorporated in the Cayman Islands on July 6, 2004 as part of a reorganization of entities under common control and in connection with its initial public offering. China Medical Technologies, Inc. and its subsidiaries (collectively the “Group”) are principally engaged in the commercialization, manufacture and sale of medical devices and supplies to customers primarily in the People’s Republic of China (the “PRC”).
On August 10, 2005, the Company was listed on the Nasdaq Global Select Market (formerly the Nasdaq National Market) when the Company issued 6,400,000 American Depositary Shares (“ADSs”), representing 64,000,000 ordinary shares, at an initial public offering price of US$15.00 per ADS. On August 29, 2005, an additional 960,000 ADS, representing 9,600,000 ordinary shares, were sold by the Company at the initial public offering price of US$15.00 per ADS. In connection with this initial public offering, the Company received proceeds of RMB799,067,000, net of related offering expenses paid.
On March 27, 2006, the Company completed a secondary public offering of 5,750,000 ADSs, representing 57,500,000 ordinary shares held by certain shareholders. The selling shareholders received the entire net proceeds from the sale of shares in this secondary offering.
On November 21, 2006, the Company completed an offering of US$150 million principal amount of 3.5% Convertible Senior Subordinated Notes due November 2011 to qualified institutional buyers.
On August 15, 2008, the Company completed an offering of US$276 million principal amount of 4.0% Convertible Senior Notes due August 2013 to qualified buyers.
2 Summary of Significant Accounting Policies
(a) Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. Generally Accepted Accounting Principles (“U.S. GAAP”).
(b) Principles of consolidation
The accompanying consolidated financial statements include the financial statements of China Medical Technologies, Inc. and its subsidiaries. All significant inter-company balances and transactions have been eliminated upon consolidation.
(c) Fair value of financial instruments
All financial assets and liabilities are recognized or disclosed at fair value in the consolidated financial statements.
Accounting guidance defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Group considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
F-7
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Accounting guidance establishes three levels of inputs that may be used to measure fair value:
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included within Level 1 that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical asset or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The carrying amount of cash and cash equivalents, accounts receivable, other receivables, accounts payable and other current payables, approximates their fair value because of the short-term maturity of these instruments.
(d) Cash and cash equivalents
Cash and cash equivalents consist of cash on hand and highly liquid short-term deposits which are unrestricted as to withdrawal and use, and which have original maturities less than three months.
(e) Accounts receivable
Accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is management’s best estimate of the amount of probable credit losses in the Group’s existing accounts receivable. Management determines the allowance for doubtful accounts based on historical write-off experience, customer specific facts and economic conditions. Accounts receivable balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered unlikely. The allowance for doubtful accounts were RMB3,373,000 and RMB18,516,000 (US$2,713,000) as of March 31, 2009 and 2010, respectively.
(f) Inventories
Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out method. Cost of work-in-progress and finished goods comprise direct materials, direct production costs and an allocation of production overheads based on normal operating capacity. The Group assesses the valuation of inventory continuously and periodically. The Group will write down the value for estimated excess and obsolete inventory based on estimates about future demand. The evaluation may take into consideration historical usage, expected demand, anticipated sales prices, new product development schedules, the effect of new products might have on the sale of existing products, product obsolescence, and other factors. If actual market conditions are less favorable than those projected by management, additional write-downs may be required that could negatively impact our gross margin and operating results. The Group wrote down inventory in the amount of RMB4,406,000 (US$645,000) for the year ended March 31, 2010 and there was no such charges for the years ended March 31, 2008 and 2009.
F-8
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
(g) Property, plant and equipment
Property, plant and equipment are stated at cost. Depreciation is provided on the straight-line method (after taking into consideration any estimated residual value), over the following estimated useful lives:
| | |
Buildings | | 10-30 years |
Machinery and equipment | | 5-10 years |
Furniture, fixtures and equipment | | 3-5 years |
Motor vehicles | | 5 years |
Leasehold improvements are amortized using the straight-line method over 2 to 5 years which represents the shorter of the remaining lease term or estimated useful life of the leasehold improvement.
Depreciation and amortization of property, plant and equipment attributable to manufacturing activities is capitalized as part of inventories, and recognized as cost of revenues when the inventory is sold. Cost incurred in the construction of property, plant and equipment, including progress payments and deposits, are initially capitalized as construction-in-progress and transferred into their respective asset categories when the assets are ready for their intended use, at which time depreciation commences.
When items are retired or otherwise disposed of, income is charged or credited for the difference between net book value and the proceeds received thereon. Ordinary maintenance and repairs are charged to expense as incurred, and replacements and betterments are capitalized and amortized over the remaining useful life.
(h) Goodwill and other intangible assets
Goodwill represents the excess of the cost of an acquisition over the fair value of net assets acquired. Goodwill is not amortized, but is instead tested for impairment at least annually as of year-end or when a triggering event occurs.
Intangible assets are amortized on a straight-line basis over their respective estimated useful lives, which range from 3 to 20 years. The Group has no intangible assets with indefinite useful lives.
(i) Land use rights
Land use rights represent the exclusive right to occupy and use a piece of land in the PRC for a specified contractual term. Land use rights are carried at cost and charged to expense on a straight-line basis over the contractual term of the rights, which range from 44 to 47 years. The current portion of land use rights of RMB192,000 and RMB192,000 (US$28,000) as of March 31, 2009 and 2010, respectively, has been included in prepayments and other assets in the accompanying consolidated balance sheets.
(j) Impairment of goodwill and other long-lived assets
Long-lived assets, such as property, plant, and equipment and intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized for the amount by which the carrying amount of the asset exceeds its fair value. Assets to be disposed of are separately presented in the consolidated balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and are no longer depreciated.
F-9
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Goodwill is tested annually for impairment, and is tested for impairment more frequently if events and circumstances indicate that goodwill might be impaired. The determination of goodwill impairment is made at the reporting unit level and is a two-step test. In the first step, management determines the fair value of the reporting unit (determined using a discounted cash flow analysis) and compares it to its carrying value (including goodwill). Second, if the carrying amount of the reporting unit exceeds its fair value, an impairment loss is recognized for any excess of the carrying amount of the reporting unit’s goodwill over the implied fair value of that goodwill. The implied fair value of goodwill is determined by allocating the fair value of the reporting unit in a manner similar to a purchase price allocation. The residual fair value after this allocation is the implied fair value of the reporting unit goodwill.
There was no impairment of goodwill or other long-lived assets as of March 31, 2009 and 2010.
(k) Revenue recognition
The Group derived revenues from immunodiagnostic system sales and molecular diagnostic system sales for the fiscal year ended March 31, 2010. Immunodiagnostic system sales include the sales of the Enhanced Chemiluminescence Immunoassay (“ECLIA”) reagent kits. Molecular diagnostic system sales include the sales of the Fluorescent In Situ Hybridization (“FISH”) probes. For the fiscal year ended March 31, 2008, the Group’s immunodiagnostic system sales also included sales of ECLIA analyzers. No revenues were generated from the sales of ECLIA analyzers for the fiscal years ended March 31, 2009 and 2010 as the Company provided its ECLIA analyzers to qualified potential customers free of charge starting from April 2008 to promote its sales of ECLIA reagent kits. For the fiscal years ended March 31, 2008 and 2009, the Group’s Molecular diagnostic systems sales also included the sales of fluorescent microscopes. No revenues were generated from the sales of fluorescent microscopes for the fiscal year ended March 31, 2010 as the Company started to recommend to its hospital customers to purchase fluorescent microscopes directly from the microscope manufacturers so that these hospitals could reduce the cost of setting up the FISH test platform and expedite the commencement of providing this diagnostic service to patients. The Group sells its immunodiagnostic systems primarily through distributors and sells its molecular diagnostic systems directly to hospitals.
The Group recognizes revenues when persuasive evidence of an arrangement exists, delivery has occurred and services have been performed, the sales price is fixed or determinable, and collection is reasonably assured. Written sales agreements or customers purchase orders, which specify price, product specifications, and quantity, are used as evidence of an arrangement.
The Group recognizes revenue for ECLIA reagent kits and FISH probes upon customer acceptance and the customer acceptance is evidenced by signed acceptance documents.
The Group’s fluorescent microscope and ECLIA analyzers sales agreements included the provision of training and installation services. These services are ancillary to the customers’ purchase of medical equipment and are considered by the customers to be an integral part of the acquired equipment. The Group recognized revenue for the entire equipment sales arrangement upon customer acceptance, which occurred after delivery, installation and training. Customer acceptance was evidenced by signed acceptance documents.
All of the Group’s products sold through distributors are non-exchangeable, non-refundable, non-returnable and are without any rights of price protection or stock rotation. Accordingly, the Group considers all the distributors as end-customers.
F-10
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
In the PRC, value added tax (“VAT”) of 6% or 17% on invoiced amount is collected on behalf of tax authorities. Revenue is recorded net of VAT. VAT collected from customers, net of VAT paid for purchases, is recorded as a liability and included in “accrued expenses and other liabilities” in the consolidated balance sheets until paid.
Pursuant to the laws and regulations of the PRC, Beijing Yuande Bio-Medical Engineering Co., Ltd. (“Beijing Yuande”), a subsidiary of the Company, is entitled to a refund of VAT on sales of self-developed software embedded in the medical equipment. The VAT refund represents the amount of VAT collected from customers and paid to tax authorities in excess of 3% of relevant sales. As the refund relates directly to sales of ECLIA analyzers, the Group recognizes the VAT refund when the corresponding product revenue is recognized. VAT refunds included in revenue for the years ended March 31, 2008, 2009 and 2010 were RMB4,443,000, RMB13,000 and RMB nil, respectively.
(l) Government grants
Government grants, of RMB100,000, RMB nil and RMB1,248,000 (US$183,000) for the years ended March 31, 2008, 2009 and 2010, respectively, which do not have specific terms of usage and are not refundable, are recognized as other income when received.
(m) Convertible notes issuance costs
Cost incurred by the Company that are directly attributable to the issuance of convertible notes, are deferred and are charged to the consolidated statements of income using the straight line method over the term of the convertible notes, the results of which approximate the effective interest rate method.
(n) Income taxes
Income taxes are accounted for under the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates or change in tax status is recognized in income in the period the change in tax rates or the tax law is enacted. A valuation allowance is provided to reduce the amount of deferred income tax assets if it is considered more likely than not that some portion, or all, of the deferred income tax assets will not be realized.
To assess uncertain tax positions, the Group applies a more likely than not threshold and a two-step approach for the tax position measurement and financial statement recognition. Under the two-step approach, the first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement.
All undistributed earnings of the Group’s subsidiaries are presumed to be transferred to the company and are subject to withholding taxes. The presumption may be overcome if the Group has sufficient evidence to demonstrate that the undistributed dividends will be re-invested and the remittance of the dividends will be postponed indefinitely or that the earnings can be distributed in a tax-free manner. Accordingly, deferred income tax liabilities are only recognized for the portion of undistributed earnings that the Group does not intend to reinvest indefinitely.
F-11
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
(o) Share-based compensation
The Group recognizes share-based compensation based on the grant date fair value of the award. The Group recognizes compensation cost for an award with only service conditions that have a graded vesting schedule on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in-substance, multiple awards.
Forfeitures are estimated at the time of grant and revised in subsequent periods if actual forfeitures differ from those estimates. The Group uses historical data to estimate pre-vesting option and restricted share forfeitures and records share-based compensation expense only for those awards that are expected to vest.
(p) Research and development, and advertising costs
Research and development and advertising costs are expensed as incurred.
The value of acquired in-process research and development is determined by estimating the costs to develop the purchased in-process research and development into a commercially viable product, estimating the resulting cash flows from the sale of the products resulting from the completion of the in-process research and development and discounting the net cash flows using an appropriate discount rate, net of returns on contributory assets. For acquisitions prior to April 2009, acquired in-process research and development with no future alternative use was expensed at the acquisition date. For subsequent acquisitions, all acquired in-process research and development even those with no future alternative use will be capitalized in accordance with the new guidance on purchase accounting.
In-process research and development costs acquired in business combinations and asset acquisitions prior to April 2009 are expensed as research and development expense upon acquisition and amounted to RMB672,000, RMB244,872,000 for the years ended March 31, 2008 and 2009, respectively. There were no acquisitions of in-process research and development during the year ended March 31, 2010.
Advertising costs, which are included in sales and marketing expense, amounted to RMB1,145,000, RMB1,625,000 and RMB1,155,000 (US$169,000) for the years ended March 31, 2008, 2009 and 2010, respectively.
(q) Cost of free ECLIA analyzers and SPR analyzers
To acquire new customers for its ECLIA reagent kits, the Group started to provide free ECLIA analyzers to its potential customers from April 2008. Similarly, the Group started the Surface Plasmon Resonance (“SPR”) business in January 2010 by providing free SPR analyzers to potential customers to acquire more new customers for its Human Papillomavirus (“HPV”)-DNA chips in a shorter period of time. As the ECLIA analyzer and SPR analyzer can only be used with ECLIA reagent kits and HPV-DNA chips, respectively, manufactured by the Group and the successful rate of obtaining customers within a short period of time after providing free ECLIA analyzers and SPR analyzers is high, the costs of the free ECLIA analyzers and SPR analyzer are included in cost of revenues in the period the ECLIA analyzers and SPR analyzers are delivered to the customers. The costs of ECLIA analyzers given to customers were RMB13,234,000 and RMB8,951,000 (US$1,311,000) for the years ended March 31, 2009 and 2010, respectively, and there was no such cost for the year ended March 31, 2008. The costs of SPR analyzers given to customers were RMB11,465,000 (US$1,680,000) for the year ended March 31, 2010 and there was no such cost for the years ended March 31, 2008 and 2009.
F-12
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
(r) Retirement and other postretirement benefits
Pursuant to the relevant PRC regulations, the Company’s PRC subsidiaries are required to make contributions at approximately 20% on a standard salary base as determined by the Beijing Social Security Bureau to a defined contribution retirement plan organized by the Beijing Social Security Bureau. The Chinese government is responsible for the pension liability to be paid to these employees and the Group’s obligations are limited to the amounts contributed. Contributions to the defined contribution plans are charged to the consolidated statements of income as the related employee service is provided. For the years ended March 31, 2008, 2009 and 2010, contributions to the defined contribution plans amounted to RMB2,177,000, RMB3,888,000 and RMB6,221,000 (US$911,000), respectively.
(s) Foreign currency transactions and translation
The Company’s reporting currency is the Renminbi (“RMB”). The functional currency of the Company is the U.S. dollar, whereas the functional currency of the Company’s subsidiaries located in the PRC is the RMB. Since the RMB is not freely convertible into foreign currencies, all foreign exchange transactions involving RMB must take place either through the People’s Bank of China (the “PBOC”) or other institutions authorized to buy and sell foreign exchange. The exchange rate adopted for the foreign exchange transactions are the rates of exchange quoted by the PBOC.
Transactions denominated in currencies other than the functional currency are converted into the functional currency at the exchange rates prevailing at the date of transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are converted into the functional currency using the applicable exchange rates at each balance sheet date. The resulting exchange differences are recorded as a component of other income, net in the consolidated statements of income.
Assets and liabilities of the Company and other non-PRC group entities with functional currencies other than the RMB, are translated into RMB using the applicable exchange rate at each balance sheet date. Revenues, if any, and expenses are translated at average rates prevailing during the year. The gains and losses from such translations are recorded as a separate component of accumulated other comprehensive loss within shareholders’ equity.
For the United States dollar convenience translation amounts included in the accompanying financial statements, the Renminbi equivalents were translated into United States dollar (“US$”) at the rate of US$1.00=RMB6.8258 on March 31, 2010, representing the noon buying rate in the City of New York for cable transfers of Renminbi, as set forth in the H.10 weekly statistical release of the Federal Reserve Bank of New York. No representation is made that the Renminbi amounts could have been, or could be, converted into United States dollars at that rate or at any other certain rate on March 31, 2010 or at any other date.
(t) Warranty costs
The Group provides a warranty that its products will meet certain functionality standards. The term of the product warranty is generally twelve months. Based on the limited number of actual warranty claims and the historically low cost of such repairs, the Group has not recognized a liability for warranty claims. Warranty expenses were insignificant for the years ended March 31, 2008, 2009 and 2010.
F-13
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
(u) Income (loss) from continuing operations per share
Income (loss) from continuing operations per share is computed by dividing income (loss) from continuing operations by the weighted average number of ordinary shares outstanding, excluding the ordinary shares to be purchased under a forward share repurchase contract and the ordinary shares issued and to be repurchased under a share issuance and repurchase agreement (note 10(b) and note 10(c)), during the year. Diluted income (loss) from continuing operations per share is calculated by dividing income (loss) from continuing operations by the weighted average number of ordinary and dilutive ordinary equivalent shares outstanding, excluding the ordinary shares repurchased pursuant to the forward share repurchase contract and ordinary shares issued and to be repurchased under a share issuance and repurchase agreement, during the year. Ordinary equivalent shares consist of (a) ordinary shares issuable upon the conversion of convertible notes (using the if-converted method) and (b) ordinary shares issuable upon the exercise of outstanding share options and restricted shares (using the treasury share method). Potential dilutive securities are not included in the calculation of dilutive income (loss) from continuing operations per share if the impact is anti-dilutive.
(v) Use of estimates
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires management of the Group to make a number of estimates and assumptions relating to the reported amount of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the period. Significant items subject to such estimates and assumptions include the recoverability of the carrying amount and the estimated useful lives of long-lived assets, allowance for doubtful accounts, valuation for inventories, share-based compensation, accounting for convertible notes, accounting for income taxes and fair value of assets and liabilities acquired in business combinations. Actual results could differ from those estimates.
(w) Segment reporting
The Group uses the “management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Group’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Group’s reportable segments. The Group’s chief operating decision maker is the chief executive officer of the Group. The Group started reporting financial results in segments along product lines since acquisition of the ECLIA technology in August 2004 and added the FISH product line for the fiscal year ended March 31, 2008 as the sales and distribution of our FISH products began in June 2007 and removed the High Intensity Focused Ultrasound (“HIFU”) product line for the fiscal year ended March 31, 2009 as the Group sold HIFU Business in December 2008. In December 2008, the Group completed the acquisition of the SPR technology related assets. After the sale of the HIFU Business and the acquisition of SPR technology related assets, the Group re-classified the reportable operating segments for the fiscal year ended March 31, 2009 into (i) immunodiagnostic systems and (ii) molecular diagnostic systems, and this change was applied retrospectively for all the periods presented for comparability. Immunodiagnostic systems currently include ECLIA analyzers and reagent kits while molecular diagnostic systems include FISH probes, SPR analyzers and HPV-DNA chips (and fluorescent microscopes until April 2009).
The Group generates substantially all revenues from customers in the PRC and the Group’s tangible long-lived assets are substantially located in the PRC. Consequently, no geographic information is presented.
F-14
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
(x) Contingencies
In the normal course of business, the Group is subject to loss contingencies, such as legal proceedings and claims arising out of its businesses, which cover a wide range of matters, including among others, product liability. The Group records accruals for such loss contingencies when it is probable that a liability has been incurred and the amount of loss can be reasonably estimated.
(y) Adoption of new authoritative guidance
In May 2008, the FASB issued new authoritative guidance on accounting for convertible debt instruments that may be settled in cash upon conversion which requires that the liability and equity components of convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) be separately accounted for in a manner that reflects an issuer’s nonconvertible debt borrowing rate. The resulting debt discount is amortized over the period that the convertible debt is expected to be outstanding, as additional non-cash interest expense. This guidance became effective for the Group on April 1, 2009 and requires retrospective application and has changed the accounting treatment for the Company’s convertible notes (Note 8). The effect of applying this guidance on the Company’s convertible notes has reduced net income for the years ended March 31, 2008 and 2009 by approximately RMB28.2 million and RMB27.6 million, respectively. The following financial statement line items were affected as of March 31, 2009 and for the years ended March 31, 2008 and 2009 as a result of the retrospective adoption of the new guidance on April 1, 2009.
Consolidated Balance Sheet
| | | | | | | | | |
| | | As originally
reported | | | Effect of
adoption of
new guidance | | | As
adjusted | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
March 31, 2009 | | | | | | | | | |
Assets | | | | | | | | | |
Convertible notes issuance costs | | 68,596 | | | (2,780 | ) | | 65,816 | |
Liabilities | | | | | | | | | |
Convertible notes | | 2,910,815 | | | (84,467 | ) | | 2,826,348 | |
Shareholders’ equity | | | | | | | | | |
Additional paid-in capital | | 544,178 | | | 165,771 | | | 709,949 | |
Accumulated other comprehensive loss | | (51,946 | ) | | (18,011 | ) | | (69,957 | ) |
Retained earnings | | 1,094,613 | | | (66,073 | ) | | 1,028,540 | |
F-15
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Consolidated Statements of Income
| | | | | | | | | |
| | | Year ended March 31, 2009 | |
| | | As originally
reported | | | Effect of
adoption of
new guidance | | | As adjusted | |
| | | (RMB’000, except for per share data) | |
Interest expense | | (87,478 | ) | | (28,614 | ) | | (116,092 | ) |
Amortization of convertible notes issuance costs | | (14,387 | ) | | 1,064 | | | (13,323 | ) |
Income before income taxes | | 70,985 | | | (27,550 | ) | | 43,435 | |
Loss from continuing operations | | (2,057 | ) | | (27,550 | ) | | (29,607 | ) |
Income from and gain on sale of discontinued operation, net | | 364,409 | | | — | | | 364,409 | |
Net income | | 362,352 | | | (27,550 | ) | | 334,802 | |
| | | |
Net income (loss) per share—Basic | | | | | | | | | |
Loss from continuing operations | | (0.01 | ) | | (0.10 | ) | | (0.11 | ) |
Income from and gain on sale of discontinued operation, net | | 1.39 | | | — | | | 1.39 | |
| | | | | | | | | |
Total | | 1.38 | | | (0.10 | ) | | 1.28 | |
| | | | | | | | | |
Net income (loss) per share—Diluted | | | | | | | | | |
Loss from continuing operations | | (0.01 | ) | | (0.10 | ) | | (0.11 | ) |
Income from and gain on sale of discontinued operation, net | | 1.39 | | | — | | | 1.39 | |
| | | | | | | | | |
Total | | 1.38 | | | (0.10 | ) | | 1.28 | |
| | | | | | | | | |
| | | | | | | | | |
| | | Year ended March 31, 2008 | |
| | | As originally
reported | | | Effect of
adoption of
new guidance | | | As adjusted | |
| | | (RMB’000, except for per share data) | |
Interest expense | | (44,378 | ) | | (29,367 | ) | | (73,745 | ) |
Amortization of convertible notes issuance costs | | (7,937 | ) | | 1,156 | | | (6,781 | ) |
Income before income taxes | | 164,565 | | | (28,211 | ) | | 136,354 | |
Income from continuing operations | | 112,566 | | | (28,211 | ) | | 84,355 | |
Income from and gain on sale of discontinued operation, net | | 212,656 | | | — | | | 212,656 | |
Net income | | 325,222 | | | (28,211 | ) | | 297,011 | |
| | | |
Net income per share—Basic | | | | | | | | | |
Income from continuing operations | | 0.43 | | | (0.11 | ) | | 0.32 | |
Income from and gain on sale of discontinued operation, net | | 0.81 | | | — | | | 0.81 | |
| | | | | | | | | |
Total | | 1.24 | | | (0.11 | ) | | 1.13 | |
| | | | | | | | | |
Net income per share—Diluted | | | | | | | | | |
Income from continuing operations | | 0.43 | | | (0.11 | ) | | 0.32 | |
Income from and gain on sale of discontinued operation, net | | 0.81 | | | — | | | 0.81 | |
| | | | | | | | | |
Total | | 1.24 | | | (0.11 | ) | | 1.13 | |
| | | | | | | | | |
F-16
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Consolidated Statements of Cash Flows
| | | | | | | |
| | | Year ended March 31, 2009 |
| | | As originally
reported | | Effect of
adoption of
new guidance | | | As
adjusted |
| | | RMB’000 | | RMB’000 | | | RMB’000 |
Net income | | 362,352 | | (27,550 | ) | | 334,802 |
Adjustments to reconcile net income to net cash provided by operating activities | | | | | | | |
Non-cash interest expense on convertible notes | | — | | 28,614 | | | 28,614 |
Amortization of convertible notes issuance costs | | 14,387 | | (1,064 | ) | | 13,323 |
Net cash provided by operating activities | | 490,758 | | — | | | 490,758 |
| | | | | | | |
| | | Year ended March 31, 2008 |
| | | As originally
reported | | Effect of
adoption of
new guidance | | | As
adjusted |
| | | RMB’000 | | RMB’000 | | | RMB’000 |
Net income | | 325,222 | | (28,211 | ) | | 297,011 |
Adjustments to reconcile net income to net cash provided by operating activities | | | | | | | |
Non-cash interest expense on convertible notes | | — | | 29,367 | | | 29,367 |
Amortization of convertible notes issuance costs | | 7,937 | | (1,156 | ) | | 6,781 |
Net cash provided by operating activities | | 463,334 | | — | | | 463,334 |
As a result of the accounting change, retained earnings as of April 1, 2007, decreased from RMB577,154,000 as originally reported, to RMB566,842,000. Additional paid-in capital and accumulated other comprehensive loss as of April 1, 2007, increased from RMB504,795,000 and RMB21,335,000 as originally reported, to RMB670,566,000 and RMB24,265,000, respectively. The foreign currency translation adjustment, net of nil tax for the year ended March 31, 2008, increased from RMB26,711,000 as originally reported, to RMB39,072,000. The comprehensive income for the year ended March 31, 2008 decreased from RMB298,511,000 as originally reported to RMB257,939,000.
(z) Recently issued accounting pronouncements
Effective July 2009, the Financial Accounting Standards Board (FASB) codified accounting literature into a single source of authoritative accounting principles. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative GAAP for SEC registrants. Since the codification did not alter existing GAAP, it did not have an impact on the Company’s consolidated financial statements and related disclosures. All references to pre-codified GAAP have been removed in the Company’s consolidated financial statements and related disclosures.
In June 2009, the FASB issued an amendment to the accounting for transfers of financial assets. The amendment requires additional information disclosures on the transfer of financial assets, including securitization transactions, and where an entity has continuing exposure to risks related to transferred financial assets. The amendment eliminates the concept of a “qualifying special-purpose entity,” changes the requirements for derecognizing financial assets and requires additional disclosures. This amendment is effective at the start of a reporting entity’s first fiscal year beginning after November 15, 2009 and will therefore be applicable to the Group for its fiscal year beginning April 1, 2010. The Group does not believe the adoption of this guidance will have a material effect on its consolidated financial statements and related disclosures.
F-17
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
In October 2009, the FASB issued new guidance on revenue recognition for arrangements with multiple deliverables and certain revenue arrangements that include software elements. The new guidance establishes a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. This guidance eliminates the residual method of allocation and requires that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. The relative selling price method allocates any discount in the arrangement proportionally to each deliverable on the basis of each deliverable’s selling price. In addition, this guidance significantly expands required disclosures related to a vendor’s multiple-deliverable revenue arrangements. The new guidance is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. The Group does not believe the adoption of this guidance will have a material effect on its consolidated financial statements and related disclosures.
In October 2009, the FASB issued new guidance on accounting for own-share lending arrangements in contemplation of convertible debt issuance, which is effective for financial statements issued for fiscal years beginning on or after December 15, 2009. The guidance applies to an equity-classified share lending arrangement on an entity’s own shares when executed in contemplation of a convertible debt offering or other financing. The share lending arrangement is required to be measured at fair value and recognized as an issuance costs associated with the convertible debt offering or other financing. If counterparty default is probable, the share lender is required to recognize an expense equal to the then fair value of the unreturned shares, net of the fair value of probable recoveries. In addition, the loaned shares are excluded from basic and diluted earnings per share unless default of the share-lending arrangement occurs, at which time the loaned shares would be included in the common and diluted earnings-per-share calculation. If dividends on the loaned shares are not reimbursed to the entity, any amounts, including contractual (accumulated) dividends and participation rights in undistributed earnings, attributable to the loaned shares shall be deducted in computing income available to common shareholders, consistent with the “two-class” method. In August 2008, the Company entered into a share lending arrangement in contemplation of its 2013 convertible notes (Note 10(c)), which will be impacted by this new guidance. This guidance became effective for the Group on April 1, 2010 and requires retrospective application and will change the accounting treatment for the Company’s convertible notes in its fiscal year beginning April 1, 2010.
The Group has evaluated the impact of retrospectively implementing this new guidance in its fiscal year beginning April 1, 2010 on its financial position, results of operations and cash flows and determined that it will record additional debt issuance costs at issuance of RMB55.4 million (US$8.1 million) related to its loaned shares. Such costs will be amortized over the life of the convertible notes and will result in an adjustment of RMB19.4 million (US$2.8 million) to beginning retained earnings on April 1, 2010. The effect of applying this guidance on the Company’s convertible notes will reduce net income for the year ended March 31, 2009 by approximately RMB6.9 million and will increase net loss for the year ended March 31, 2010 by approximately RMB12.5 million (US$1.8 million). The following financial statement line items will be affected as of April 1, 2010 and for the years ended March 31, 2009 and 2010 as a result of the retrospective adoption of the new guidance on April 1, 2010.
F-18
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Consolidated Balance Sheet
| | | | | | | | | |
| | | As reported on
March 31, 2010 | | | Effect of
adoption of
guidance on
own-share
lending
arrangements | | | As adjusted
upon
adoption on
April 1,
2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
March 31, 2010 | | | | | | | | | |
Assets | | | | | | | | | |
Share lending costs | | — | | | 35,678 | | | 35,678 | |
Shareholders’ equity | | | | | | | | | |
Additional paid-in capital | | 752,862 | | | 55,359 | | | 808,221 | |
Accumulated other comprehensive loss | | (70,316 | ) | | (240 | ) | | (70,556 | ) |
Retained earnings | | 871,775 | | | (19,441 | ) | | 852,334 | |
| | | | | | | | | |
| | | As reported on
March 31, 2010 | | | Effect of
adoption of
guidance on
own-share
lending
arrangements | | | As adjusted
upon
adoption on
April 1,
2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
March 31, 2009 | | | | | | | | | |
Assets | | | | | | | | | |
Share lending costs | | — | | | 48,263 | | | 48,263 | |
Shareholders’ equity | | | | | | | | | |
Additional paid-in capital | | 709,949 | | | 55,359 | | | 765,308 | |
Accumulated other comprehensive loss | | (69,957 | ) | | (196 | ) | | (70,153 | ) |
Retained earnings | | 1,028,540 | | | (6,900 | ) | | 1,021,640 | |
Consolidated Statements of Income
| | | | | | | | | |
| | | Year ended March 31, 2010 | |
| | | As reported on
March 31,
2010 | | | Effect of
adoption of
guidance on
own-share
lending
arrangements | | | As adjusted
upon
adoption on
April 1,
2010 | |
| | | (RMB’000, except for per share data) | |
Amortization of share lending costs | | — | | | (10,912 | ) | | (10,912 | ) |
Other income | | 26,477 | | | (1,629 | ) | | 24,848 | |
Income (loss) before income taxes | | 6,515 | | | (12,541 | ) | | (6,026 | ) |
Loss from continuing operations | | (57,041 | ) | | (12,541 | ) | | (69,582 | ) |
Net loss | | (57,041 | ) | | (12,541 | ) | | (69,582 | ) |
| | | |
Net income (loss) per share—Basic | | | | | | | | | |
Loss from continuing operations | | (0.22 | ) | | (0.05 | ) | | (0.27 | ) |
Income from and gain on sale of discontinued operation, net | | N/A | | | N/A | | | N/A | |
| | | | | | | | | |
Total | | (0.22 | ) | | (0.05 | ) | | (0.27 | ) |
| | | | | | | | | |
Net income (loss) per share—Diluted | | | | | | | | | |
Loss from continuing operations | | (0.22 | ) | | (0.05 | ) | | (0.27 | ) |
Income from and gain on sale of discontinued operation, net | | N/A | | | N/A | | | N/A | |
| | | | | | | | | |
Total | | (0.22 | ) | | (0.05 | ) | | (0.27 | ) |
| | | | | | | | | |
F-19
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
| | | | | | | | | |
| | | Year ended March 31, 2009 | |
| | | As reported on
March 31,
2010 | | | Effect of
adoption of
guidance on
own-share
lending
arrangements | | | As adjusted
upon
adoption on
April 1,
2010 | |
| | | (RMB’000, except for per share data) | |
| | | |
Amortization of share lending costs | | — | | | (6,900 | ) | | (6,900 | ) |
Income before income taxes | | 43,435 | | | (6,900 | ) | | 36,535 | |
Loss from continuing operations | | (29,607 | ) | | (6,900 | ) | | (36,507 | ) |
Net income | | 334,802 | | | (6,900 | ) | | 327,902 | |
| | | |
Net income (loss) per share—Basic | | | | | | | | | |
Loss from continuing operations | | (0.11 | ) | | (0.03 | ) | | (0.14 | ) |
Income from and gain on sale of discontinued operation, net | | 1.39 | | | — | | | 1.39 | |
| | | | | | | | | |
Total | | 1.28 | | | (0.03 | ) | | 1.25 | |
| | | | | | | | | |
Net income (loss) per share—Diluted | | | | | | | | | |
Loss from continuing operations | | (0.11 | ) | | (0.03 | ) | | (0.14 | ) |
Income from and gain on sale of discontinued operation, net | | 1.39 | | | — | | | 1.39 | |
| | | | | | | | | |
Total | | 1.28 | | | (0.03 | ) | | 1.25 | |
| | | | | | | | | |
Consolidated Statements of Cash Flows
| | | | | | | | | |
| | | Year ended March 31, 2010 | |
| | | As reported on
March 31,
2010 | | | Effect of
adoption of
guidance of
own-share
lending
arrangements | | | As adjusted
upon
adoption on
April 1,
2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Net loss | | (57,041 | ) | | (12,541 | ) | | (69,582 | ) |
Adjustments to reconcile net income to net cash provided by operating activities | | | | | | | | | |
Amortization of share lending costs | | — | | | 10,912 | | | 10,912 | |
Gain on repurchase of convertible notes | | (25,693 | ) | | 1,629 | | | (24,064 | ) |
Net cash provided by operating activities | | 279,877 | | | — | | | 279,877 | |
| | | | | | | |
| | | Year ended March 31, 2009 |
| | | As reported on
March 31,
2010 | | Effect of
adoption of
guidance of
own-share
lending
arrangements | | | As adjusted
upon
adoption on
April 1,
2010 |
| | | RMB’000 | | RMB’000 | | | RMB’000 |
Net income | | 334,802 | | (6,900 | ) | | 327,902 |
Adjustments to reconcile net income to net cash provided by operating activities | | | | | | | |
Amortization of share lending costs | | — | | 6,900 | | | 6,900 |
Net cash provided by operating activities | | 490,758 | | — | | | 490,758 |
F-20
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
3 Acquisitions
(a) FISH Acquisition
On March 1, 2007, the Group completed the acquisition of the FISH business of Supreme Well Investments Limited and its subsidiaries (the “FISH acquisition”). The Group has accounted for the FISH acquisition using the purchase method. The sum of the fair values of the assets acquired less liabilities assumed exceeded the acquisition cost, excluding contingent consideration, by RMB571,517,000, resulting in negative goodwill. Since the contingent consideration could result in the recognition of additional purchase price in a future period, the Group recognized, as if it were a liability, an amount equal to the lesser of the maximum amount of the contingent consideration or the total amount of the negative goodwill. Accordingly, the maximum amount of the contingent earnout payments of RMB308,928,000 (US$40,000,000) has been recognized as if it were a liability at the date of acquisition. The remaining negative goodwill of RMB262,589,000 was allocated to reduce the amounts assigned to certain long-lived assets.
The results of FISH operation have been included in the Group’s consolidated statements of income from March 1, 2007. The total purchase consideration, including the contingent earnout payments, is as follows:
| | |
| | | RMB’000 |
Cash consideration (note (i)) | | 902,204 |
Transaction costs | | 23,200 |
Holdback payments (note (i)) | | 144,438 |
| | |
Total purchase consideration | | 1,069,842 |
Contingent consideration (note (ii)) | | 308,928 |
| | |
Total purchase consideration, including contingent earnout payments | | 1,378,770 |
| | |
Notes:
| | (i) | Cash consideration includes US$10,000,000 which was paid by the Group in June 2007. Further, pursuant to the acquisition agreement US$20,000,000 was held back by the Group to settle any claims that arise if the sellers acted fraudulently or made misrepresentation to the Group. US$10,000,000 was due by and paid in March 2008 and the remaining US$10,000,000 was paid in March, 2009. The US$10,000,000 holdback payment due by March 1, 2009 was recorded at its present value as of the acquisition date of March 1, 2007 or US$8,702,000 using a discount rate of 7.2%, representing the borrowing rate for debt with similar terms. All amounts related to the FISH acquisition had been paid as of March 31, 2009. |
| | (ii) | Pursuant to acquisition agreement, the sellers are entitled to additional consideration of US$20,000,000 if the Group receives bona fide, irrevocable, binding purchase orders of an amount equal to or in excess of US$20,000,000 during the 12-month period ending March 1, 2008. In addition, the sellers are entitled to an additional consideration of US$20,000,000, if the Group recognizes revenue of US$20,000,000 as reported under U.S. GAAP during the 12-month period ending March 1, 2008. During the 12-month period ended March 1, 2008, the purchase order and revenue targets were achieved and the Group paid the contingent payments in cash in February and March 2008. |
F-21
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The Group has accounted for the FISH acquisition using the purchase method. The following table summarizes the allocation of the purchase consideration to the fair value of the assets acquired and liabilities assumed on March 1, 2007:
| | | | |
| | | Amortization
period | | RMB’000 |
Net tangible assets acquired | | | | 19,222 |
Identifiable intangible assets: | | | | |
—Unpatented technology—FISH probes | | 20 | | 1,323,960 |
—Unpatented technology—FISH imaging analysis system | | 5 | | 18,767 |
—Non-compete agreement | | 5 | | 16,821 |
| | | | |
Purchase price allocated | | | | 1,378,770 |
| | | | |
(b) BBE Acquisition
On January 8, 2008, the Group acquired 100% interest in Beijing Bio-Ekon Biotechnology Co., Ltd. (“BBE”) from Finnea International Limited (the “BBE acquisition”) for cash consideration of RMB212,230,000, including transaction costs of RMB1,709,000.
BBE is a medical device company engaged in the commercialization, manufacture and sale of medical devices using ECLIA technology. The acquisition is expected to strengthen the Group’s position in the advanced in-vitro diagnostics market in China. The Group paid a premium (i.e. goodwill) over the fair value of the net tangible and identified intangible assets mainly due to expected cost savings and synergies resulting from selling similar products to similar customers.
The Group has accounted for the BBE acquisition using the purchase method. The results of BBE have been included in the Group’s consolidated statements of income from January 8, 2008. The following table summarizes the allocation of the purchase consideration to the fair value of the assets acquired and liabilities assumed on January 8, 2008:
| | | | | | | | |
| | | Amortization
period (years) | | RMB’000 | | | US$’000 | |
Cash | | | | 1,650 | | | 235 | |
Net current liabilities | | | | (1,521 | ) | | (216 | ) |
Property and equipment | | | | 15,630 | | | 2,229 | |
Identifiable intangible assets: | | | | | | | | |
—Unpatented technology—ECLIA core technology | | 20 | | 174,347 | | | 24,864 | |
—Unpatented technology—ECLIA reagent technology | | 15 | | 7,828 | | | 1,116 | |
—Customer relationships | | 15 | | 2,019 | | | 288 | |
—Non-compete agreement | | 5 | | 2,951 | | | 421 | |
In-process research and development charged to the statement of income | | | | 672 | | | 96 | |
Goodwill | | | | 8,654 | | | 1,234 | |
| | | | | | | | |
Purchase price allocated | | | | 212,230 | | | 30,267 | |
| | | | | | | | |
The acquired identifiable intangible assets, all of which are being amortized, have a weighted average life of approximately 20 years. RMB8,654,000 of goodwill, which has been allocated to the Group’s immunodiagnostic system segment, is not deductible for tax purposes.
F-22
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The following unaudited pro forma financial information presents the results of the operations of the Group as if the BBE acquisition had occurred on April 1, 2007. Amortization expense of acquired identifiable intangible assets in the amount of RMB9,964,000 was included in the unaudited pro forma financial information for the years ended March 31, 2008. The unaudited pro forma financial information is not necessarily indicative of what the Group’s consolidated results of operations actually would have been had it completed the BBE acquisition on April 1, 2007. In addition, the unaudited pro forma financial information does not attempt to project the future results of operations of the combined entity.
| | | | | | | |
| | | Year ended March 31, 2008 |
| | | Actual | | Proforma | | | Unaudited
proforma
results |
| | | RMB’000 | | RMB’000 | | | RMB’000 |
| | | except per share data (unaudited) |
Revenues | | 567,369 | | — | | | 567,369 |
Income from continuing operations | | 93,131 | | (28,211 | ) | | 64,920 |
Net income | | 305,787 | | (28,211 | ) | | 277,576 |
Income per share from continuing operations | | | | | | | |
Basic | | 0.36 | | (0.11 | ) | | 0.25 |
Diluted | | 0.35 | | (0.11 | ) | | 0.24 |
(c) HPV-DNA Chip and SPR System Asset Acquisition
On December 4, 2008, the Group acquired HPV-DNA Biosensor Chip (“HPV-DNA Chip”) and SPR-based Analysis System (“SPR System”) from Molecular Diagnostic Technologies Limited for cash consideration of RMB2,380,064,000 (US$346,071,000), including transaction costs of RMB7,370,000 (US$1,071,000). As of March 31, 2009, the unpaid consideration was RMB707,205,000 (US$103,500,000), which has been paid in two equal installments in July and December 2009.
As the acquired net assets does not constitute a business, the Group accounted for this acquisition as an asset acquisition and allocated the entire purchase price to the individual assets acquired and liabilities assumed based on their relative fair values, with no goodwill recorded.
The following table summarizes the allocation of the purchase consideration to the fair value of the assets acquired on December 4, 2008:
| | | | | | |
| | | Amortization
period (years) | | RMB’000 | | US$’000 |
Equipment | | | | 12,387 | | 1,800 |
Inventories | | | | 6,194 | | 900 |
Identifiable intangible assets: | | | | | | |
—Unpatented technology—HPV | | 20 | | 2,088,396 | | 303,471 |
—Unpatented technology—Life Sciences | | 5 | | 24,774 | | 3,600 |
—Non-compete agreement and assembled workforce | | 3-5 | | 3,441 | | 500 |
In-process research and development charged to the statement of income | | | | 244,872 | | 35,800 |
| | | | | | |
Purchase price allocated | | | | 2,380,064 | | 346,071 |
| | | | | | |
F-23
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The acquired identifiable intangible assets, all of which are being amortized, have a weighted average life of approximately 20 years.
Acquired in-process research and development
As part of the purchase price allocation, RMB244,872,000 (US$35,800,000) of the purchase price was allocated to acquired in-process research and development projects. The amount allocated to acquired in-process research and development represents the estimated fair value based on risk-adjusted cash flows related to in-process projects that have not yet reached technological feasibility and have no alternative future use as of the date of the acquisition. The fair value attributable to these in-process projects was expensed at the time of the acquisition. If the projects are not successful or completed in a timely manner, the Group may not realize the financial benefits expected for these projects.
4 Inventories
Inventories by major class consist of the following:
| | | | | | |
| | | March 31, |
| | | 2009 | | 2010 | | 2010 |
| | | RMB’000 | | RMB’000 | | US$’000 |
Raw materials | | 10,293 | | 10,817 | | 1,585 |
Work-in-progress | | 3,969 | | 10,608 | | 1,554 |
Finished goods | | 2,670 | | 3,464 | | 507 |
| | | | | | |
Total inventories | | 16,932 | | 24,889 | | 3,646 |
| | | | | | |
The related charges of excess and obsolete inventory in the amount of RMB 4,406,000 (US$645,000) was recorded in cost of revenues for the year ended March 31, 2010 and there was no such charge for the years ended March 31, 2008 and 2009.
5 Property, Plant and Equipment, net
Property, plant and equipment consist of the following:
| | | | | | | | | |
| | | March 31, | |
| | | 2009 | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | US$’000 | |
Buildings | | 134,394 | | | 134,394 | | | 19,689 | |
Leasehold improvements | | 14,288 | | | 14,781 | | | 2,166 | |
Machinery and equipment | | 49,794 | | | 55,125 | | | 8,076 | |
Furniture, fixtures and equipment | | 10,232 | | | 11,649 | | | 1,707 | |
Motor vehicles | | 6,151 | | | 7,293 | | | 1,068 | |
| | | | | | | | | |
Total property, plant and equipment | | 214,859 | | | 223,242 | | | 32,706 | |
Accumulated depreciation and amortization | | (45,437 | ) | | (67,417 | ) | | (9,877 | ) |
| | | | | | | | | |
Total property, plant and equipment, net | | 169,422 | | | 155,825 | | | 22,829 | |
| | | | | | | | | |
F-24
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Depreciation and amortization of property, plant and equipment was allocated to the following expense items:
| | | | | | | | |
| | | Year ended March 31, |
| | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB’000 | | RMB’000 | | RMB’000 | | US$’000 |
Cost of revenues | | 6,141 | | 9,601 | | 13,081 | | 1,916 |
Research and development | | 673 | | 1,204 | | 3,833 | | 562 |
Sales and marketing | | 171 | | 139 | | 255 | | 37 |
General and administrative | | 3,888 | | 5,253 | | 4,968 | | 728 |
Income from discontinued operation | | 5,194 | | 3,953 | | — | | — |
| | | | | | | | |
Total depreciation and amortization expense | | 16,067 | | 20,150 | | 22,137 | | 3,243 |
| | | | | | | | |
6 Intangible Assets, net
The following table summarizes the Group’s intangible assets as of March 31, 2009 and 2010:
| | | | | | | | | |
| | | March 31, 2009 |
| | | Gross
carrying
amount | | Accumulated
amortization | | | Net | | Weighted
average
amortization
period |
| | | RMB’000 | | RMB’000 | | | RMB’000 | | Years |
Unpatented technology | | 3,657,989 | | (220,153 | ) | | 3,437,836 | | 20 |
Non-compete agreements | | 68,018 | | (29,137 | ) | | 38,881 | | 9 |
Patented technology | | 10,530 | | (2,839 | ) | | 7,691 | | 17 |
Customer relationships | | 2,019 | | (168 | ) | | 1,851 | | 15 |
Assembled workforce | | 1,367 | | (152 | ) | | 1,215 | | 3 |
| | | | | | | | | |
Total intangible assets | | 3,739,923 | | (252,449 | ) | | 3,487,474 | | |
| | | | | | | | | |
| | | | | | | | | |
| | | March 31, 2010 |
| | | Gross
carrying
amount | | Accumulated
amortization | | | Net | | Weighted
average
amortization
period |
| | | RMB’000 | | RMB’000 | | | RMB’000 | | Years |
Unpatented technology | | 3,654,575 | | (409,014 | ) | | 3,245,561 | | 20 |
Non-compete agreements | | 68,001 | | (37,917 | ) | | 30,084 | | 9 |
Patented technology | | 10,530 | | (3,459 | ) | | 7,071 | | 17 |
Customer relationships | | 2,019 | | (303 | ) | | 1,716 | | 15 |
Assembled workforce | | 1,365 | | (607 | ) | | 758 | | 3 |
| | | | | | | | | |
Total intangible assets | | 3,736,490 | | (451,300 | ) | | 3,285,190 | | |
| | | | | | | | | |
F-25
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Amortization of intangible assets was allocated to the following expense items:
| | | | | | | | |
| | | Year ended March 31, |
| | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB’000 | | RMB’000 | | RMB’000 | | US$’000 |
Cost of revenues | | 90,233 | | 90,077 | | 89,720 | | 13,144 |
Operating expenses | | — | | 36,511 | | 109,395 | | 16,027 |
| | | | | | | | |
Total amortization expense | | 90,233 | | 126,588 | | 199,115 | | 29,171 |
| | | | | | | | |
The amortization of acquired intangible assets in connection with SPR acquisitions is classified as operating expenses until significant revenues are generated from these intangible assets.
Estimated annual amortization expense for each of the next five years is approximately RMB192,630,000 each year.
7 Accrued Expenses and Other Liabilities
Accrued expenses and other liabilities consist of the following:
| | | | | | |
| | | March 31, |
| | | 2009 | | 2010 | | 2010 |
| | | RMB’000 | | RMB’000 | | US$’000 |
VAT payables | | 90,416 | | 70,054 | | 10,263 |
Accrued salaries and welfare | | 33,311 | | 51,936 | | 7,609 |
Convertible notes interest payable | | 23,480 | | 23,027 | | 3,374 |
Accrued professional fees | | 9,810 | | 11,112 | | 1,628 |
Receipts in advance | | 6,339 | | 5,581 | | 818 |
Other payables | | 22,344 | | 21,788 | | 3,191 |
| | | | | | |
Total accrued expenses and other liabilities | | 185,700 | | 183,498 | | 26,883 |
| | | | | | |
8 Convertible Notes
3.5% Convertible Senior Subordinated Notes due November 2011
On November 21, 2006, the Company received an aggregate US$150 million from the issuance of 3.5% convertible senior subordinated notes due on November 15, 2011 (the “2011 Convertible Notes”) to Merrill Lynch & Co. and Merrill Lynch, Pierce, Fenner & Smith Incorporated. The net proceeds from the offering, after deducting the offering expenses, were approximately US$144.7 million. The 2011 Convertible Notes bear interest at a rate of 3.5% per year, payable semiannually in arrears on May 15 and November 15 of each year beginning on May 15, 2007.
The 2011 Convertible Notes are convertible, at the discretion of the holders of the 2011 Convertible Notes (the “2011 Convertible Notes Holders”) at any time prior to November 15, 2011, the maturity date, at an initial conversion rate of 31.0318 ADSs per US$1,000 principal amount of 2011 Convertible Notes (equivalent to a conversion price of approximately US$32.23 per ADS), subject to dilution protection adjustment for the issuance of shares and dividends, as defined in the agreement.
F-26
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Upon conversion of the 2011 Convertible Notes, in lieu of delivery of ADSs, the Company may elect to deliver cash or a combination of cash and ADSs based on the conversion price then in effect, the average trading price of the ADSs (over a 20 day period as defined in the agreement) and the portion the Company elects to settle with cash.
4.0% Convertible Senior Notes due August 2013
On August 15, 2008, the Company received an aggregate US$276 million from the issuance of 4.0% convertible senior subordinated notes due on August 15, 2013 (the “2013 Convertible Notes”) to Credit Suisse Securities (USA) LLC and Morgan Stanley & Co. Incorporated. The net proceeds from the offering, after deducting the offering expenses, were approximately US$267.7 million. The 2013 Convertible Notes bear interest at a rate of 4.0% per year, payable semiannually in arrears on February 15 and August 15 of each year beginning on February 15, 2009.
The 2013 Convertible Notes are convertible, at the discretion of the holders of the 2013 Convertible Notes (the “2013 Convertible Notes Holders”) at any time prior to August 15, 2013, the maturity date, at an initial conversion rate of 17.0068 ADSs per US$1,000 principal amount of 2013 Convertible Notes (equivalent to a conversion price of approximately US$58.80 per ADS), subject to dilution protection adjustment for the issuance of shares and dividends, as defined in the agreement.
Upon conversion of the 2013 Convertible Notes, the Company can only deliver ADSs.
If a change of control or a termination of trading, as defined in the agreements, occurs, both the 2011 Convertible Notes Holders and the 2013 Convertible Notes Holders may be entitled to an increase in the conversion rate and may require the Company to repurchase all or a portion of their convertible notes for cash at a repurchase price equal to 100% of the principal amount plus accrued and unpaid interest, if any.
The 2013 Convertible Notes are senior unsecured obligations and will rank equally in right of payment with the Company’s future senior indebtedness. The 2013 Convertible Notes are senior in right of payment to the Company’s existing, and future subordinated indebtedness, including the 2011 Convertible Notes. In addition, both the 2011 Convertible Notes and the 2013 Convertible Notes are effectively junior to any secured indebtedness to the extent of the value of the related collateral, and structurally subordinated to the existing and future indebtedness and other liabilities, including trade payables, of the subsidiaries.
The Company has determined that the conversion feature embedded in the 2013 Convertible Notes is not required to be bifurcated and accounted for as a derivative, since the embedded conversion feature of the 2013 Convertible Notes is indexed to the Company’s own shares and would be classified in shareholders’ equity if they were free-standing instruments. Further, since the effective conversion price of the 2013 Convertible Notes exceeded the market price of the Company’s ordinary shares on the date of issuance of respective convertible notes, no portion of the proceeds from the issuance was accounted for as a beneficial conversion feature.
The Company has accounted for the conversion feature embedded in the 2011 Convertible Notes in accordance with new authoritative guidance on accounting for convertible debt instruments that may be settled in cash upon conversion, which was effective for the Company from April 1, 2009. The effect of applying this new guidance on the Company’s 2011 Convertible Notes is illustrated under Note 2(y)— “Adoption of new authoritative guidance”.
F-27
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
As a result of the declarations of dividends by the Company, the conversion rate of the 2011 Convertible Notes was adjusted from 31.0318 ADSs to 31.3953 ADSs, from 31.3953 ADSs to 31.7378 ADSs and from 31.7378 ADSs to 32.8322 ADSs per US$1,000 principal amount of 2011 Convertible Notes (equivalent to changing the conversion price from US$32.23 to US$31.85, from US$31.85 to US$31.51 and from US$31.51 to US$30.46) in July 2007, July 2008 and September 2009, respectively. As the adjusted conversion price of US$31.85, US$31.51 and US$30.46 per ADS exceeded the market price of the Company’s ordinary shares on the date of issuance of 2011 Convertible Notes, there was no beneficial conversion feature recognized.
As a result of the declarations of dividends by the Company, the conversion rate of the 2013 Convertible Notes was adjusted from 17.0068 ADSs to 17.5899 ADSs per US$1,000 principal amount of 2013 Convertible Notes (equivalent to changing the conversion price from US$58.80 to US$56.85) in September 2009. As the adjusted conversion price of US$56.85 per ADS exceeded the market price of the Company’s ordinary shares on the date of issuance of 2013 Convertible Notes, there was no beneficial conversion feature recognized.
The Company recognized the amortization of convertible notes issuance costs of RMB6,781,000 (as adjusted, note 2(y)), RMB13,323,000 (as adjusted, note 2(y)) and RMB17,402,000 (US$2,549,000) for the years ended March 31, 2008, 2009 and 2010.
The effective interest rate is 7.0% for 2011 Convertible Notes for the years ended March 31, 2008, 2009 and 2010. The Company recognized RMB68,517,000, RMB64,670,000 and RMB66,326,000 (US$9,717,000) interest expenses including non-cash interest expense of RMB29,367,000, RMB28,614,000 and RMB30,477,000 (US$4,465,000) for 2011 Convertible Notes for the years ended March 31, 2008, 2009 and 2010, respectively.
During the year ended March 31, 2010, the Company bought back a portion of the 2013 Convertible Notes with a carrying value which included unamortized issuance costs related to the portion of the 2013 Convertible Notes repurchased of RMB75.1 million (US$11.0 million) for a total consideration which included the transaction cost and excluded the accrued interest up to the date of purchase of RMB49.4 million (US$7.2 million). The gain from convertible notes repurchase in the amount of RMB25.7 million (US$3.8 million) was recognized as other income. As of March 31, 2010, the outstanding amount of the 2013 Convertible Notes excluding unamortized issuance costs was RMB1,807.1 million (US$264.8 million). There was no convertible notes repurchase for the years ended March 31, 2008 and 2009.
9 Discontinued Operation
On December 17, 2008, the Company decided to sell its High Intensity Focused Ultrasound tumor therapy system business (the “HIFU Business”) to Chengxuan International Ltd. (“Chengxuan”), a major shareholder of the Company and beneficially owned by Mr. Xiaodong Wu, the Company’s founder, chairman of the board of directors and chief executive officer. On December 30, 2008, the Group completed the sale for cash consideration of RMB365,378,000 (US$53,500,000). The HIFU Business was previously presented in HIFU System segment.
F-28
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The consolidated balance sheet at March 31, 2009 does not reflect the assets held for sale due to the fact that the sale was completed prior to March 31, 2009. As there was no continuing cash flow from the disposed HIFU Business expected to be generated by the on going entity and the Group will not have significant continuing involvement in the operations of the disposed HIFU Business after December 30, 2008, the revenues and expenses related to the operations of HIFU Business have been segregated from continuing operations and reported as discontinued operation for all periods presented. Following are revenues, income from discontinued operation and gain on sale of discontinued operation:
| | | | | | |
| | | Year ended March 31, | |
| | | 2008 | | | 2009 | |
| | | RMB’000 | | | RMB’000 | |
Revenues | | 368,317 | | | 246,588 | |
| | | | | | |
Income before income taxes | | 238,854 | | | 159,930 | |
Income tax expense | | (26,198 | ) | | (38,850 | ) |
| | | | | | |
Income from discontinued operation, net of tax | | 212,656 | | | 121,080 | |
| | | | | | |
Gain on sale of discontinued operation before income taxes | | — | | | 263,947 | |
Income tax expense | | — | | | (20,618 | ) |
| | | | | | |
Gain on sale of discontinued operation, net of tax | | — | | | 243,329 | |
| | | | | | |
Income from and gain on sale of discontinued operation, net of tax | | 212,656 | | | 364,409 | |
| | | | | | |
HIFU Business claim
In June 2009, the Company received a letter (“First Letter”) from Chengxuan International Ltd. (“Chengxuan”), the buyer of the HIFU Business and a major shareholder of the Company in connection with a notice from the State Food and Drug Administration, P.R.China (“the SFDA”) in April 2009. The notice from the SFDA required the submission of new clinical trial data for the renewal application of the registration certificate for the HIFU system and did not permit sales of the HIFU system starting from April 2009 until approval of the renewal application. In August, the Company received another letter (“Second Letter”) from Chengxuan which provided updated information on their ongoing discussions with the SFDA about the requirements for the new clinical trial data for the HIFU system, Chengxuan’s loss of revenues due to the prohibition on selling the HIFU system since April 2009 and their preliminary indication of seeking maximum compensation of approximately US$15.5 million from the Company. The Company established a special committee comprising two independent directors to evaluate and handle the related matters with Chengxuan and the special committee engaged legal counsel to advise on Chengxuan’s request for compensation. In September, 2009, the Company received another letter (“Third Letter”) from Chengxuan in which Chengxuan indicated that it had decided to withdraw the compensation request contained in the Second Letter. While Chengxuan may make a claim for compensation to the Company again in the future, the Company does not believe, based on current knowledge, that this is likely to happen.
10 Shareholders’ Equity
(a) Statutory reserves
In accordance with the relevant laws and regulations of the PRC, the Company’s PRC subsidiaries are required to transfer 10% of their respective after tax profit, as determined in accordance with PRC accounting standards and regulations to a general reserve fund until the balance of the fund reaches 50% of
F-29
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
the registered capital of the respective subsidiary. The transfer to this general reserve fund must be made before distribution of dividends can be made. As of March 31, 2009 and 2010, the PRC subsidiaries had appropriated RMB120,849,000 and RMB171,412,000 (US$25,112,000), respectively to the general reserve funds, which are restricted from being distributed to its shareholders. General reserve funds are restricted for set off against losses, expansion of production and operation or increase in registered capital of the respective company. These reserves are therefore not available for distribution except in liquidation.
Further, the PRC subsidiaries may, at the discretion of the enterprise, make appropriations to a staff welfare and bonus reserve in accordance with the relevant laws and regulations in the PRC. The appropriation to staff welfare and bonus reserve is recognized as an expense as it is a current liability to the employees. The PRC subsidiaries made appropriations to the staff welfare and bonus reserve of RMB4,575,000 for the year ended March 31, 2008 and there was no such appropriation made for the years ended March 31, 2009 and 2010.
(b) Forward share repurchase contract
In connection with the issuance of the 2011 Convertible Notes, the Company entered into a prepaid forward repurchase contract (the “Forward Repurchase Contract”) on November 15, 2006 with an affiliate of Merrill Lynch & Co. (the “Dealer”). Pursuant to the Forward Repurchase Contract, the Company paid, an aggregate of US$30 million (1,163,692 ADSs at US$25.78 per ADS) for the Forward Repurchase Contract. The Dealer may deliver the shares pursuant to the Forward Repurchase Contract at its discretion, in full or in part, over a period of five years from the date of the contract. As of March 31, 2010, the Dealer had not delivered any shares. Since the Forward Repurchase Contract requires physical settlement of a fixed number of ADSs at a fixed price per ADS, the shares to be repurchased pursuant to the Forward Repurchase Contract are treated as retired for purposes of the Company’s basic and diluted earnings per share calculations. The Company has accounted for the prepaid forward repurchase of the shares as a reduction of shareholders’ equity in the consolidated financial statements. As a result of the Forward Repurchase Contract, the ADSs to be repurchased do not effectively have any voting or dividend rights.
(c) Share issuance and repurchase agreement
In connection with the issuance of the 2013 Convertible Notes, the Company entered into a share issuance and repurchase agreement (the “Share Issuance and Repurchase Agreement”) on August 15, 2008 with affiliates of Credit Suisse Securities (USA) LLC and Morgan Stanley & Co. Incorporated (the “Dealers”). Pursuant to the Share Issuance and Repurchase Agreement, the Company issued 4,700,000 ADSs to the Dealers at a consideration of US$1.00 per ADS. On the same day the Company issued the ADSs to the Dealers, the Company prepaid to the Dealers a US$1.00 per ADS repurchase price for the 4,700,000 ADSs to be repurchased from the Dealers by the Company in August 2013. The Dealers will be obligated to deliver to the Company an equal number of ADSs. Since the Share Issuance and Repurchase Agreement requires physical settlement of a fixed number of ADSs at a fixed price per ADS, the shares to be repurchased pursuant to the Share Issuance and Repurchase Agreement are treated as retired for purposes of the Company’s basic and diluted earnings per share calculations. The Company has accounted for the issuance and repurchase of the shares as one connected transaction in shareholders’ equity in the consolidated financial statements. As a result of the Share Issuance and Repurchase Agreement, the issued ADSs do not effectively have any voting or dividend rights. During the year ended March 31, 2010, the Company bought back a portion of the 2013 Convertible Notes (Note 8) with carrying value of RMB76.8 million (US$11.2 million) excluding unamortized issuance costs. Pursuant to the Share Issuance and Repurchase Agreement, the Dealers have delivered back to the Company 192,000 ADSs in relation to the portion of the 2013 Convertible Notes buyback. Shares returned by the Dealers became part of the
F-30
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Company’s treasury stock and may be retired or used by the Company for other arrangements such as issuance of shares to employees upon the exercise of their share incentive awards.
(d) Declaration of dividend
On June 18, 2007, the Company declared a cash dividend on its ordinary shares of US$0.04 per share, equivalent to US$0.40 per ADS to the shareholders of record as of July 18, 2007. The dividend was paid in August 2007.
On June 18, 2008, the Company declared a cash dividend on its ordinary shares of US$0.05 per share, equivalent to US$0.50 per ADS to the shareholders of record as of July 25, 2008. The dividend was paid in August 2008.
On September 1, 2009, the Company declared a cash dividend on its ordinary shares of US$0.055 per share, equivalent to US$0.55 per ADS to the shareholders of record as of September 30, 2009. The dividend was paid in October 2009.
11 Net Income (Loss) Per Share
The following table sets forth the computation of basic and diluted net income (loss) per share for the years indicated:
| | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008
(As adjusted)
(Note 2(y)) | | | 2009
(As adjusted)
(Note 2(y)) | | | 2010 | |
| | | RMB | | | RMB | | | RMB | |
| | | (in thousands, except per share data) | |
Income (loss) from continuing operations | | 84,355 | | | (29,607 | ) | | (57,041 | ) |
Income from and gain on sale of discontinued operation | | 212,656 | | | 364,409 | | | — | |
| | | | | | | | | |
Net income (loss) | | 297,011 | | | 334,802 | | | (57,041 | ) |
| | | | | | | | | |
Shares issued and outstanding at the beginning of the year | | 273,600 | | | 274,067 | | | 321,067 | |
Add: weighted average shares issued upon exercise of options and restricted shares | | 256 | | | 346 | | | 1,681 | |
Add: weighted average shares issued under share issuance and repurchase agreement | | — | | | 29,616 | | | — | |
Less: weighted average shares to be purchased under the prepaid forward contract and share issuance and repurchase agreement | | (11,637 | ) | | (41,253 | ) | | (58,638 | ) |
Less: weighted average shares purchased under share repurchase program | | — | | | — | | | (1,564 | ) |
| | | | | | | | | |
Weighted average ordinary shares outstanding—Basic | | 262,219 | | | 262,776 | | | 262,546 | |
Effect of dilutive ordinary equivalent shares | | 1,246 | | | — | | | — | |
| | | | | | | | | |
Weighted average ordinary shares outstanding—Diluted | | 263,465 | | | 262,776 | | | 262,546 | |
| | | | | | | | | |
Basic net income (loss) per share: | | | | | | | | | |
Income (loss) from continuing operations | | 0.32 | | | (0.11 | ) | | (0.22 | ) |
Income from and gain on sale of discontinued operation, net | | 0.81 | | | 1.39 | | | — | |
| | | | | | | | | |
| | 1.13 | | | 1.28 | | | (0.22 | ) |
| | | | | | | | | |
Diluted net income (loss) per share: | | | | | | | | | |
Income (loss) from continuing operations | | 0.32 | | | (0.11 | ) | | (0.22 | ) |
Income from and gain on sale of discontinued operation, net | | 0.81 | | | 1.39 | | | — | |
| | | | | | | | | |
| | 1.13 | | | 1.28 | | | (0.22 | ) |
| | | | | | | | | |
F-31
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The Company’s ordinary equivalent shares for the years ended March 31, 2008, 2009 and 2010 consist of 1,245,625, 1,876,181 and 682,586, respectively, ordinary shares issuable upon exercise of outstanding share options and restricted shares (using the treasury stock method); and 46,932,057, 94,383,602 and 95,675,608 ordinary shares issuable upon conversion of convertible notes (using the if-converted method). The Company has 46,932,057, 96,259,783 and 96,358,194 potential ordinary equivalent shares that were anti-dilutive for the years ended March 31, 2008, 2009 and 2010.
12 Income Taxes
The Company is incorporated in the Cayman Islands, and is not subject to tax on income or capital gain under the current laws of the Cayman Islands. In addition, upon payment of dividends by the Company to its shareholders, no Cayman Islands withholding tax is imposed. The Company’s other subsidiaries are subject to income tax as described below.
British Virgin Islands (“BVI”)
Under the current laws of BVI, our BVI subsidiaries are not subject to tax on income or capital gain. In addition, payments of dividends by our BVI subsidiaries to their shareholders are not subject to withholding tax in the BVI.
Hong Kong
Under the current Hong Kong Inland Revenue Ordinance, our Hong Kong subsidiaries are subject to 16.5% income tax on its taxable income generated from operations in Hong Kong. In addition, payments of dividends by our Hong Kong subsidiaries to their shareholders are not subject to any Hong Kong withholding tax.
PRC
Prior to January 1, 2008, the Group was governed by the previous Income Tax Law (the “Previous Tax Law”) of China. Under the Previous Tax Law, the Company’s subsidiaries, including Beijing Yuande Bio-Medical Engineering Co., Ltd. (“Beijing Yuande”), Beijing GP Medical Technologies, Ltd. (“Beijing GP”) and BBE, were entitled to various preferential tax treatments.
On February 8, 2006, Beijing Yuande, being a “foreign-invested advanced technology enterprise” accredited by Beijing Commerce Bureau, was granted a preferential rate of 10% for income tax for the tax period from January 1, 2005 to December 31, 2007.
Beijing GP is located in an approved economic-technological development area in the PRC, and was entitled to a PRC income tax rate of 15%. Further, Beijing GP, which was deemed a production-oriented foreign investment enterprise, was entitled to an exemption from PRC income tax from January 1, 2008 to December 31, 2009 and a 50% income tax reduction from January 1, 2010 to December 31, 2012.
BBE, an entity qualified as a “High and New Technology Enterprises” under the Previous Tax Law was entitled to a PRC income tax rate of 15%.
On March 16, 2007, the National People’s Congress passed the new Enterprise Income Tax law (the “new EIT law”) which imposes a single income tax rate of 25% for most domestic enterprises and foreign investment
F-32
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
enterprises. The new EIT law was effective as of January 1, 2008. The new EIT law provides a five-year transition period from its effective date for those enterprises which were established before March 16, 2007 and which were entitled to a preferential lower tax rate under the then effective tax laws or regulations, as well as grandfathering tax holidays. Further, according to the new EIT law, entities that qualify as “High and New Technology Enterprises” are entitled to the preferential EIT rate of 15%. Beijing Yuande and BBE have received approval for the status as a “High and New Technology Enterprises”. The status is valid for three years starting from January 2008 and will be renewed after evaluation by relevant government authorities every three years. Further, on December 26, 2007, the PRC government passed the detailed implementing rules which allow enterprises to continue to enjoy their unexpired tax holiday under the previous income tax laws and rules. As a result, under the new EIT law, Beijing Yuande’s tax rate and BBE’s tax rate are 15% for the calendar years from 2008 to 2010 and subject to renewal of the status of “High and New Technology Enterprises” after calendar year 2010; and Beijing GP’s tax rates are 0%, 0%, 11%, 12%, 12.5% for the calendar years from 2008 to 2012 and 25% thereafter. Beijing GP can apply for the status of “High and New Technology Enterprises”. Upon approval, Beijing GP will be entitled to the preferential EIT rate of 15%.
The new EIT Law also provides that an enterprise established under the laws of foreign countries or regions but whose “de facto management body” is located in the PRC be treated as a resident enterprise for PRC tax purposes and consequently be subject to the PRC income tax at the rate of 25% for its worldwide income. The Implementing Rules of the new EIT Law merely defines the location of the “de facto management body” as “the place where the exercising, in substance, of the overall management and control of the production and business operation, personnel, accounting, properties, etc., of a non-PRC company is located.” On April 22, 2009, the PRC State Administration of Taxation further issued a notice entitled “Notice Regarding Recognizing Offshore-Established Enterprises Controlled by PRC Shareholders as Resident Enterprises Based on Their Place of Effective Management.” Under this notice, a foreign company controlled by a PRC company or a group of PRC companies shall be deemed as a PRC resident enterprise, if (i) the senior management and the core management departments in charge of its daily operations mainly function in the PRC; (ii) its financial decisions and human resource decisions are subject to decisions or approvals of persons or institutions in the PRC; (iii) its major assets, accounting books, company seals, minutes and files of board meetings and shareholders’ meetings are located or kept in the PRC; and (iv) more than half of the directors or senior management personnel with voting rights reside in the PRC. Based on a review of surrounding facts and circumstances, the Group does not believe that it is likely that its operations outside of the PRC should be considered a resident enterprise for PRC tax purposes. However, due to limited guidance and implementation history of the new EIT Law, should the Company be treated as a resident enterprise for PRC tax purposes, the Company will be subject to PRC tax on worldwide income at a uniform tax rate of 25% retroactive to January 1, 2008.
The new EIT Law also imposes a withholding income tax of 10% on dividends distributed by a foreign invested enterprise to its immediate holding company outside of China, if such immediate holding company is considered as a non-resident enterprise without any establishment or place within China or if the received dividends have no connection with the establishment or place of such immediate holding company within China, unless such immediate holding company’s jurisdiction of incorporation has a tax treaty with China that provides for a different withholding arrangement. Such withholding income tax was exempted under the previous income tax regulations. The Cayman Islands, where the Company is incorporated, does not have such tax treaty with China. According to the arrangement between Mainland China and Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion in August 2006, dividends paid by a foreign-invested enterprise in China to its direct holding company incorporated in Hong Kong will be subject to withholding tax at a rate of no more than 5% (if the foreign investor owns directly at least 25% of the shares of the FIE). All of the Company’s subsidiaries in China are invested and held by Hong Kong registered entities and the Company recognizes deferred tax liabilities related to withholding income tax at the rate of 5%. Nevertheless,
F-33
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
the PRC State Administration of Taxation promulgated a tax notice on October 27, 2009, or Circular 601, which provides that tax treaty benefits will be denied to “conduit” or shell companies without business substance, and a beneficial ownership analysis will be used based on a “substance-over-form” principle to determine whether or not to grant tax treaty benefits. It is unclear at this early stage whether Circular 601 applies to dividends from our PRC subsidiaries paid to us through our Hong Kong subsidiaries. It is possible, however, that under Circular 601 our Hong Kong subsidiaries would not be considered to be the beneficial owners of any such dividends, and that such dividends would as a result be subject to income tax withholding at the rate of 10% rather than the favorable 5% rate applicable under the tax treaty between mainland China and Hong Kong. Based on the subsequently issued interpretation of the new EIT, Article 4 of Cai Shui [2008] Circular No. 1, dividends on earnings earned prior to January 1, 2008 but distributed after 2008 are not subject to withholding income tax.
Undistributed earnings that the Group intends to reinvest indefinitely, and for which no deferred tax liability was recognized, were RMB249 million and RMB249 million (US$36 million) at March 31, 2009 and 2010 respectively. The unrecognized deferred tax liability related to the undistributed earnings subject to withholding tax was RMB12 million and RMB12 million (US$2 million) as of March 31, 2009 and 2010, respectively.
The PRC State Administration of Taxation promulgated a tax notice on December 10, 2009, or Circular 698, which provides the administration and tax reporting guidance for any gains generated from indirect offshore transfers of equity interests in PRC enterprises that are not taxable overseas. It is unclear at this early stage whether Circular 698 applies to the gain on sale of the HIFU Business in December 2008. It is possible, however, that under Circular 698, the Company will need to pay additional tax in connection with the gain on sale of the HIFU Business generated from indirect offshore transfers of equity interests in PRC enterprises that are not taxable overseas. Any additional tax that the Company may need to pay in the future will negatively impact its financial condition and results of operations.
The components of income (loss) before income taxes are as follows:
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008
(As adjusted)
(Note 2(y)) | | | 2009
(As adjusted)
(Note 2(y)) | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | | | US$’000 | |
PRC | | 316,444 | | | 592,013 | | | 396,451 | | | 58,081 | |
Other countries | | (180,090 | ) | | (548,578 | ) | | (389,936 | ) | | (57,126 | ) |
| | | | | | | | | | | | |
Total income before income taxes | | 136,354 | | | 43,435 | | | 6,515 | | | 955 | |
| | | | | | | | | | | | |
Income tax expense, which is all incurred in the PRC, consists of:
| | | | | | | | |
| | | Year ended March 31, |
| | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB’000 | | RMB’000 | | RMB’000 | | US$’000 |
Current income tax expense | | 50,333 | | 47,673 | | 42,820 | | 6,273 |
Deferred income tax expense | | 1,666 | | 25,369 | | 20,736 | | 3,038 |
| | | | | | | | |
Total income tax expense | | 51,999 | | 73,042 | | 63,556 | | 9,311 |
| | | | | | | | |
F-34
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Basic and diluted earnings from continuing operations per share effects of the tax holiday and preferential tax rates for the years ended March 31, 2008, 2009 and 2010 are as follows:
| | | | | | | | |
| | | Year ended March 31, |
| | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB | | RMB | | RMB | | US$ |
Basic net income from continuing operations per share | | 0.19 | | 0.41 | | 0.23 | | 0.03 |
| | | | | | | | |
Diluted net income from continuing operations per share | | 0.19 | | 0.41 | | 0.23 | | 0.03 |
| | | | | | | | |
Income tax expense reported in the consolidated statements of income differs from the amount computed by applying the PRC income tax rate of 25% for the years ended March 31, 2008, 2009 and 2010 for the following reasons:
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008
(As adjusted)
(Note 2(y)) | | | 2009
(As adjusted)
(Note 2(y)) | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | | | US$’000 | |
Income before income taxes | | 136,354 | | | 43,435 | | | 6,515 | | | 955 | |
| | | | | | | | | | | | |
Computed “expected” tax expense | | 34,089 | | | 10,859 | | | 1,629 | | | 239 | |
Non-deductible expenses | | 1,479 | | | 369 | | | 463 | | | 68 | |
Non-taxable income | | (1,139 | ) | | (45 | ) | | (3 | ) | | (1 | ) |
Tax benefit resulting from tax holiday and preferential tax rates | | (49,983 | ) | | (107,907 | ) | | (59,353 | ) | | (8,695 | ) |
Change in enacted tax rate | | 23,065 | | | — | | | — | | | — | |
Change in valuation allowance | | — | | | 2,921 | | | 6,513 | | | 954 | |
Tax effect related to foreign entities not subject to income taxes | | 44,743 | | | 136,817 | | | 97,025 | | | 14,214 | |
Withholding tax | | — | | | 22,889 | | | 18,257 | | | 2,675 | |
Restructuring tax | | — | | | 4,895 | | | — | | | — | |
Change in the tax status of prior year non-taxable income | | — | | | 1,642 | | | — | | | — | |
Others | | (255 | ) | | 602 | | | (975 | ) | | (143 | ) |
| | | | | | | | | | | | |
Total income tax expense | | 51,999 | | | 73,042 | | | 63,556 | | | 9,311 | |
| | | | | | | | | | | | |
F-35
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The tax effect of temporary differences that gives rise to significant portions of the deferred income tax assets and deferred income tax liabilities are presented below:
| | | | | | | | | |
| | | March 31, | |
| | | 2009 | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | US$’000 | |
Deferred tax assets: | | | | | | | | | |
Operating loss carryforwards | | 4,699 | | | 7,598 | | | 1,113 | |
Allowances for doubtful accounts | | 707 | | | 4,321 | | | 633 | |
Accrued expenses and other liabilities | | 1,000 | | �� | 1,000 | | | 147 | |
| | | | | | | | | |
Total gross deferred tax assets | | 6,406 | | | 12,919 | | | 1,893 | |
Less: valuation allowance | | (6,406 | ) | | (12,919 | ) | | (1,893 | ) |
| | | | | | | | | |
Net deferred tax assets | | — | | | — | | | — | |
| | | | | | | | | |
Deferred tax liabilities: | | | | | | | | | |
Intangible assets | | (3,604 | ) | | (6,083 | ) | | (892 | ) |
Withholding tax | | (26,294 | ) | | (61,051 | ) | | (8,944 | ) |
| | | | | | | | | |
Total deferred tax liabilities | | (29,898 | ) | | (67,134 | ) | | (9,836 | ) |
| | | | | | | | | |
Net deferred tax liabilities | | (29,898 | ) | | (67,134 | ) | | (9,836 | ) |
| | | | | | | | | |
Tax operating loss carryforwards of the Group, as approved by the PRC tax authorities, amounted to RMB16,658,000 as of March 31, 2010, including RMB9,941,000 which will expire during the year ending March 31, 2012, RMB1,497,000 which will expire during the year ending March 31, 2013 and RMB5,220,000 which will expire during the year ending March 31, 2014.
In assessing the realizability of deferred income tax assets, management considers whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. As of March 31, 2009 and 2010, deferred income tax assets represented the acquired tax operating loss carryforwards of BBE, other deferred tax assets identified in the BBE acquisition and provision of allowance for doubtful accounts in Beijing Yuande. BBE incurred tax operating losses through March 2010. Management believes that BBE’s cumulative losses arising from recurring business in recent years constituted significant negative evidence demonstrating that deferred tax assets would not be realizable and this evidence outweighed the Group’s expectations that BBE would generate future taxable income. Therefore, a full valuation allowance has been provided against BBE’s deferred income tax assets as of the date of acquisition of January 8, 2008, March 31, 2009 and March 31, 2010. The provision of allowance for doubtful accounts was provided for the accounts receivable of Beijing Yuande. The amount of the deferred tax assets considered unrealizable, however, could change in the near term if estimates of future taxable income during the carry forward period also change.
As of March 31, 2009 and March 31, 2010, the Group did not have any unrecognized tax benefits or liabilities and thus, no interest and penalties related to unrecognized tax liabilities were recorded.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of taxes is due to computational errors made by the taxpayer or the withholding agent. The statute of limitations is extended to five years under special circumstances, which are not clearly defined. In the case of
F-36
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
transfer pricing issues, the statute of limitation is ten years. There is no statute of limitation in the case of tax evasion. The income tax returns of the PRC subsidiaries for the tax calendar years 2005 to 2009 are subject to examination by the relevant tax authorities.
13 Related Party Transactions
On December 30, 2008, the Group completed the sale of its HIFU Business to Chengxuan (Note 9), a major shareholder of the Company. The receivables of RMB204,774,000 (US$30,000,000) from the sale of the HIFU Business, bear interest at 5% per annum, is unsecured and repayable on December 31, 2010. The Group recognized interest income related to the receivables in the amount of RMB5,120,000 (US$750,000) for the year ended March 31, 2010 and there was no such interest income for the years ended March 31, 2008 and 2009.
14 Segment Information
The Group’s principal operating segments coincide with the types of products to be sold. The products from which revenues are derived are consistent with the reporting structure of the Group’s internal organization. The Group’s reportable segments for the year ended March 31, 2010 were immunodiagnostic systems and molecular diagnostic systems.
The Group’s chief operating decision-maker has been identified as the chief executive officer. During the year ended March 31, 2009, the Group changed its measure of reported segment profit or loss to be gross profit (i.e., revenues less cost of revenues) in order to align the segment disclosures with a change in the way the chief executive officer assesses performance of the segments. Periods prior to March 31, 2009 were revised to be presented consistently with this change. The change had no impact on previously reported gross profit by segment. There are no inter-segment revenue transactions and, therefore, revenues are only to external customers. The Group does not allocate any specific assets or expenditures for long lived assets to the operating segments, as the chief executive officer does not use the information to measure the performance of the reportable segments.
The accounting policies of the segments are the same as those used by the Group.
As the Group primarily generates its revenues from customers in the PRC, no geographical segments are presented.
| | | | | | | | | |
| | | Immunodiagnostic
Systems | | | Molecular
Diagnostic
Systems | | | Total | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Year ended March 31, 2010 | | | | | | | | | |
Revenues | | 338,309 | | | 384,762 | | | 723,071 | |
Cost of revenues | | (138,666 | ) | | (98,884 | ) | | (237,550 | ) |
| | | | | | | | | |
Segment gross profit | | 199,643 | | | 285,878 | | | 485,521 | |
Depreciation and amortization included in cost of revenues | | (31,889 | ) | | (70,912 | ) | | (102,801 | ) |
| | | | | | | | | |
F-37
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
| | | | | | | | | |
| | | Immunodiagnostic
Systems | | | Molecular
Diagnostic
Systems | | | Total | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Year ended March 31, 2009 | | | | | | | | | |
Revenues | | 504,656 | | | 325,294 | | | 829,950 | |
Cost of revenues | | (138,467 | ) | | (94,104 | ) | | (232,571 | ) |
| | | | | | | | | |
Segment gross profit | | 366,189 | | | 231,190 | | | 597,379 | |
Depreciation and amortization included in cost of revenues | | (32,154 | ) | | (67,524 | ) | | (99,678 | ) |
| | | | | | | | | |
| | | | | | | | | |
| | | |
| | | Immunodiagnostic
Systems | | | Molecular
Diagnostic
Systems | | | Total | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Year ended March 31, 2008 | | | | | | | | | |
Revenues | | 380,520 | | | 166,901 | | | 547,421 | |
Cost of revenues | | (103,143 | ) | | (142,294 | ) | | (245,437 | ) |
| | | | | | | | | |
Segment gross profit | | 277,377 | | | 24,607 | | | 301,984 | |
Depreciation and amortization included in cost of revenues | | (22,726 | ) | | (73,648 | ) | | (96,374 | ) |
| | | | | | | | | |
The reconciliation of depreciation and amortization included in cost of revenues to total depreciation and amortization included in the statements of income is as follows:
| | | | | | | | |
| | | Year ended March 31, |
| | | 2008 | | 2009 | | 2010 | | 2010 |
| | | RMB’000 | | RMB’000 | | RMB’000 | | US$’000 |
Depreciation and amortization included in | | | | | | | | |
Cost of revenues | | 96,374 | | 99,678 | | 102,801 | | 15,060 |
Research and development | | 673 | | 1,204 | | 3,833 | | 562 |
Sales and marketing | | 171 | | 139 | | 255 | | 37 |
General and administrative | | 3,888 | | 5,253 | | 4,968 | | 728 |
Amortization of SPR intangible assets | | — | | 36,511 | | 109,395 | | 16,027 |
Income from discontinued operation | | 5,194 | | 3,953 | | — | | — |
| | | | | | | | |
Total depreciation and amortization expense | | 106,300 | | 146,738 | | 221,252 | | 32,414 |
| | | | | | | | |
F-38
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
15 Fair Value of Financial Instruments
As of March 31, 2009 and 2010, the cash equivalents consisted of time deposits placed with banks with original maturities equal to three months. The Group value time deposits using quoted prices for securities with similar characteristics and other observable inputs, and accordingly, the Group classify the valuation techniques that use these inputs as Level 2.
| | | | | | | | |
| | | Fair value measurement at March 31, 2010 |
| | | Total | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) |
| | | RMB’000 | | RMB’000 | | RMB’000 | | RMB’000 |
Assets | | | | — | | — | | — |
Cash equivalents: | | | | | | | | |
Time deposits | | 400,944 | | — | | 400,944 | | — |
| | | | | | | | |
Total assets | | 400,944 | | — | | 400,944 | | — |
| | | | | | | | |
| | | | | | | | |
| | | Fair value measurement at March 31, 2009 |
| | | Total | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) |
| | | RMB | | RMB | | RMB | | RMB |
Assets | | | | | | | | |
Cash equivalents: | | | | | | | | |
Time deposits | | 343,560 | | — | | 343,560 | | — |
| | | | | | | | |
Total assets | | 343,560 | | — | | 343,560 | | — |
| | | | | | | | |
As of March 31, 2009 and 2010, the fair value of the 2011 Convertible Notes, determined based on quoted market value, was approximately US$95,390,000 (RMB651,790,000) and US$119,063,000 (RMB812,700,000), respectively. As of March 31, 2009 and 2010, the fair value of the 2013 Convertible Notes, determined based on quoted market value, was approximately US$132,480,000 (RMB905,223,000) and US$166,131,000 (RMB1,133,977,000).
16 Business and Credit Concentrations
All of the Group’s customers are located in the PRC. The Group had no customer that individually comprised 10% or more of revenue for the years ended March 31, 2008, 2009 and 2010.
The Group has no customer that individually comprised 10% or more of the outstanding accounts receivable balance as of March 31, 2009 and 2010.
As of March 31, 2009 and 2010, the Group’s cash balance was held at major financial institutions located in the PRC and other countries. Further, as of March 31, 2009 and 2010, the Group’s cash balance included U.S. dollar denominated bank deposits of US$134,273,000 and US$43,846,000 (equivalent to RMB917,474,000 and RMB299,284,000), respectively. Management believes that these major financial institutions are of high credit quality.
F-39
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The Group was dependent on single-source suppliers for certain major materials and components used to manufacture its HIFU products. Purchases (net of VAT) made from the two local suppliers for the ultrasound imaging systems and treatment beds for the year ended March 31, 2008 were RMB73,215,000. After the sale of the HIFU Business (Note 9), the Group is not dependent on single-source suppliers.
The Group’s business is affected by competition in the market for the products that many of the Group’s major customers sell, and any decline in their businesses could reduce purchase orders from these customers. The loss of sales to any of these customers could have a material adverse effect on the Group’s business and results of operations. Furthermore, these customers have sought, from time to time, to prospectively renegotiate the pricing terms of their current agreements with the Group or obtain more favorable terms upon renewal of the contracts. Any adverse revisions to the material terms of the Group’s agreements with its key customers could have a material adverse effect on its business and results of operations.
17 Share-Based Compensation
On February 2, 2005, the Company adopted the 2005 stock option plan (the “Plan 2005”) pursuant to which the Company’s board of directors may grant stock options to directors and employees. The Company has reserved an aggregate of 30,000,000 of its ordinary shares for issuance under the Plan 2005. The Plan 2005 will remain in effect for 5 years starting from the date of adoption.
On November 12, 2006, the Company replaced the Plan 2005 by adopting the Amended and Restated 2005 Stock Option Plan (the “Restated Plan 2005”) pursuant to which the Company’s board of directors may grant stock options or restricted stock to employees, directors and consultants. The Restated Plan 2005 is administered by a compensation committee and an administrative committee. The per share exercise price of the option for the ordinary shares shall be such price, determined by the compensation committee and the administrative committee. The Restated Plan 2005 will remain in effect for 5 years starting from the date of adoption of the Plan 2005. As of March 31, 2010, there were 20,520,000 unissued shares under the Restated Plan 2005. The Restated Plan 2005 expired as at December 31, 2009.
On November 16, 2009, the Company adopted the 2009 equity incentive plan (the “Plan 2009”) pursuant to which the Company’s board of directors may grant stock options as well as restricted stock to employees, directors and consultants. The Company has reserved an aggregate of 20,000,000 of its ordinary shares for issuance under the Plan 2009. The Plan 2009 will remain in effect for 5 years starting from the date of adoption. The Plan 2009 is administered by a compensation committee and an administrative committee. The per share exercise price of the option for the ordinary shares shall be such price, determined by the compensation committee and the administrative committee. As of March 31, 2010, there were 20,000,000 unissued shares under the Plan 2009.
Restricted shares
Prior to March 31, 2007, no restricted shares were granted. On June 11, 2007, the Company granted 1,800,000 restricted shares, equivalent to 180,000 ADS to certain directors and management personnel. The restricted shares vest over a period of three years commencing from the date of grant.
On October 1, 2007 and November 16, 2007, the Company granted a total of 120,000 restricted shares, equivalent to 12,000 ADS to new independent directors. The restricted shares vest over a period of three years commencing from the date of grant.
F-40
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
On June 6, 2008, the Company granted 3,560,000 restricted shares, equivalent to 356,000 ADS to certain directors and management personnel. The restricted shares vest over a period of three years commencing from the date of grant.
On November 16, 2009, the Company granted 2,450,000 restricted shares, equivalent to 245,000 ADS to certain directors and management personnel. The restricted shares vest over a period of three years commencing from the date of grant.
Share-based compensation expense with respect to the restricted shares was measured based on the fair value of the Company’s ordinary shares at the date of grant and is recognized on a straight-line basis over the requisite service period for each separately vesting portion of the restricted shares. The grant-date fair value of restricted shares is based on the market price of the Company’s ADS on the grant date.
The amount of compensation cost recognized for restricted shares was RMB14,225,000, RMB49,152,000 and RMB39,618,000 (US$5,804,000) for the years ended March 31, 2008, 2009 and 2010, respectively, of which RMB1,336,000, RMB7,429,000 and RMB5,721,000 (US$838,000) respectively was recorded as research and development expenses and RMB12,889,000, RMB41,723,000 and RMB33,897,000 (US$4,966,000) respectively was recorded as general and administrative expenses. No income tax benefit was recognized in the income statement for these restricted shares as such compensation expenses were recorded and incurred by the Company, a Cayman Islands incorporated entity which is not subject to income tax under Cayman Islands laws and regulations.
Restricted share activity during the periods indicated is as follows:
| | | | | | |
| | | Number of
Restricted
Shares | | | Grant Date
Weighted
Average
Fair value | |
| | | | | | US$ | |
Balance as of April 1, 2007 | | — | | | — | |
Granted | | 1,920,000 | | | 5,064,000 | |
Issued | | — | | | — | |
Forfeited | | (90,000 | ) | | (227,700 | ) |
| | | | | | |
Balance as of April 1, 2008 | | 1,830,000 | | | 4,836,300 | |
Granted | | 3,560,000 | | | 12,132,480 | |
Issued | | — | | | — | |
Forfeited | | — | | | — | |
| | | | | | |
Balance as of March 31, 2009 | | 5,390,000 | | | 16,968,780 | |
Granted | | 2,450,000 | | | 3,724,000 | |
Issued | | (1,480,000 | ) | | (4,402,900 | ) |
Forfeited | | — | | | — | |
| | | | | | |
Balance as of March 31, 2010 | | 6,360,000 | | | 16,289,880 | |
| | | | | | |
Exercisable as of March 31, 2010 | | 460,000 | | | 1,441,880 | |
Exercisable as of March 31, 2009 | | 450,000 | | | 1,207,300 | |
Stock options
On August 8, 2005, the Company granted options to purchase an aggregate of 400,000 ordinary shares, equivalent to 40,000 ADS, at an exercise price of US$1.50 per share (“August 2005 Option”) to two directors. The share options vested over a period of two years and expired on December 31, 2009.
F-41
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
On June 11, 2007, the Company granted 750,000 share options, equivalent to 75,000 ADS to certain management personnel at an exercise price of US$2.53 per share (“June 2007 Option”). The share options vest over a period of three years and expire on May 31, 2012.
The amount of compensation cost recognized for share options was RMB2,435,000, RMB1,027,000 and RMB819,000 (US$120,000) for the years ended March 31, 2008, 2009 and 2010, respectively, of which RMB1,461,000, RMB761,000 and RMB614,000 (US$90,000) respectively was recorded as research and development expenses, and RMB974,000, RMB266,000 and RMB205,000 (US$30,000) respectively was recorded as general and administrative expenses. No income tax benefit was recognized in the income statement for these stock options as such compensation expenses were recorded and incurred by the Company, a Cayman Islands incorporated entity which is not subject to income tax under Cayman Islands laws and regulations.
Stock option activity during the periods indicated is as follows:
| | | | | | | | | |
| | | Number of
options | | | Weighted
average
exercise
price | | Weighted
average
remaining
contractual
life | | Aggregate
intrinsic
value |
| | | | | | US$ | | Years | | US$ |
Balance as of March 31, 2007 | | 800,000 | | | 1.43 | | | | |
Granted | | 750,000 | | | 2.53 | | | | |
Exercised | | (466,660 | ) | | 1.48 | | | | |
| | | | | | | | | |
Balance as of March 31, 2008 | | 1,083,340 | | | 2.17 | | | | |
Forfeited | | (150,000 | ) | | 2.53 | | | | |
| | | | | | | | | |
Balance as of March 31, 2009 | | 933,340 | | | 2.11 | | | | |
Expired | | (200,000 | ) | | 1.36 | | | | |
Exercised | | (133,340 | ) | | 1.36 | | | | |
| | | | | | | | | |
Balance as of March 31, 2010 | | 600,000 | | | 2.53 | | 2.17 | | — |
| | | | | | | | | |
Exercisable as of March 31, 2010 | | 400,000 | | | 2.53 | | 2.17 | | — |
Vested and expected to vest as of March 31, 2010 | | 600,000 | | | 2.53 | | 2.17 | | — |
The total intrinsic value of options exercised during the years ended March 31, 2008, 2009 and 2010 was US$954,634, US$ nil and US$5,583, respectively. The intrinsic value is calculated as the difference between the market value on the date of exercise and the exercise price of the shares. Cash received from the exercises of stock option was RMB5,157,000, RMB nil and RMB1,238,000 (US$181,000) for the years ended March 31, 2008, 2009 and 2010.
The intrinsic value of options outstanding, options exercisable and options vested and expected to vest is calculated as the difference between the market value as of the last day of the fiscal year and the exercise price of the shares.
Share-based compensation expense with respect to the stock options was measured based on the fair value of the options at the date of grant and is recognized on a straight-line basis over requisite service period for each separately vesting portion of the stock options.
F-42
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The weighted average option fair value of US$0.39 (RMB3.16) per share or an aggregate of RMB1,265,000 (US$156,000) for the options granted on August 8, 2005 was estimated using the Black-Scholes option-pricing model on the grant date. The weighted average option fair value of US$0.90 (RMB6.92) per share or an aggregate of RMB5,189,000 (US$677,000) for the options granted on June 11, 2007 was estimated using the Hull and White Binomial option-pricing model on the grant date. The following assumptions were used to estimate the fair value of the options:
| | | | | | |
| | | August 2005
Option | | June 2007
Option |
Option granted | | | 400,000 | | | 750,000 |
Average risk-free interest rate of return | | | 4.216% | | | 5.07% |
Early Exercise Multiple | | | — | | | 2.0x |
Expected Exit Rate (Post-vesting) | | | — | | | 5.0% |
Weighted average expected option life | | | 3 years | | | — |
Volatility rate | | | 30.00% | | | 40.00% |
Dividend yield | | | 0.0% | | | 1.1% |
Share price | | US$ | 1.50 | | US$ | 2.53 |
As of March 31, 2010, there was RMB38,395,000 (US$5,625,000) of total unrecognized compensation cost related to stock options and restricted shares. This cost is expected to be recognized over a weighed average period of 1.48 years.
18 Commitments and Contingencies
(a) Operating lease obligation
Rental expense under operating leases was RMB5,801,000, RMB5,258,000 and RMB5,734,000 (US$840,000), for the years ended March 31, 2008, 2009 and 2010, respectively. As of March 31, 2010, total future minimum lease payments under non-cancellable operating leases were as follows:
| | |
| | | RMB’000 |
Year ending March 31, | | |
2011 | | 6,669 |
2012 | | 4,458 |
2013 to 2015 | | 5,852 |
| | |
| | 16,979 |
| | |
(b) Purchase commitment
As of March 31, 2010, the Group had outstanding purchase commitments totaling RMB20,850,000 (US$3,055,000), which mainly related to research collaborations for various applications of our FISH probes and our SPR System over the next three years.
19 Share Repurchase Program
On September 18, 2009, the Company announced the authorization of a share repurchase program under which the Company was entitled to repurchase up to US$30 million worth of its outstanding ADSs. Pursuant to this program, the Company was entitled, from time to time for a period of one year commencing on October 1, 2009, depending on market conditions, share price and other factors, to make one or more purchases, on the open
F-43
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
market at prevailing market prices or in block trades, of up to US$30 million worth of its outstanding ADSs. As of March 31, 2010, the Company has repurchased 500,000 ADSs at a total cost of US$6,418,000 pursuant to this repurchase program. Any share repurchased by the Company became part of its treasury stock and may be retired or used by the Company for other arrangements such as issuance of shares to employees upon the exercise of their share incentive awards.
20 Subsequent Events
Convertible Notes
From April to June 2010, the Company purchased about US$15.0 million 2011 Convertible Notes and US$16.8 million 2013 Convertible Notes for a total cash consideration of US$23.3 million.
Share Incentive Awards
On May 21, 2010, the Company granted 3,250,000 restricted shares, equivalent to 325,000 ADSs to certain directors and employees. The restricted shares will vest over a period of three years.
Termination of Share Repurchase Program
On May 21, 2010, the Company’s Board of directors approved the termination of the US$30 million share repurchase program (Note 19). The Company had purchased a total of 500,000 ADSs at a cost of US$6,418,000 pursuant to this repurchase program.
21 China Medical Technologies, Inc. (the Company only)
Under PRC regulations, foreign-invested enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, the Company’s PRC subsidiaries are required to set aside at least 10% of their respective accumulated profits each year, if any, to fund general reserve funds unless such reserve funds have reached 50% of its respective registered capital. These reserves are not distributable in the form of cash dividends to the Company.
As of March 31, 2010, the amount of restricted net assets of the PRC subsidiaries which may not be transferred to the Company in the form of loans, advances or cash dividends without the consent of PRC government authorities, was more than 25% of the Group’s consolidated net assets.
F-44
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The following presents condensed financial information of the Company only.
Condensed Balance Sheets
| | | | | | | | | |
| | | March 31, | |
| | | 2009
(As adjusted
below) | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | US$’000 | |
Assets | | | | | | | | | |
Current assets | | | | | | | | | |
Cash | | 102,145 | | | 57,182 | | | 8,377 | |
Prepayments and other assets | | 422 | | | 14,749 | | | 2,161 | |
| | | | | | | | | |
Total current assets | | 102,567 | | | 71,931 | | | 10,538 | |
Investments in subsidiaries | | 978,897 | | | 1,127,413 | | | 165,169 | |
Amounts due from subsidiaries | | 4,077,444 | | | 4,198,158 | | | 615,043 | |
Convertible notes issuance costs | | 65,816 | | | 46,681 | | | 6,839 | |
| | | | | | | | | |
Total assets | | 5,224,724 | | | 5,444,183 | | | 797,589 | |
| | | | | | | | | |
Liabilities | | | | | | | | | |
Current liabilities | | | | | | | | | |
Accrued expenses and other liabilities | | 39,909 | | | 44,686 | | | 6,547 | |
Amounts due to subsidiaries | | 432,197 | | | 854,393 | | | 125,171 | |
| | | |
Non-current liabilities | | | | | | | | | |
Convertible notes | | 2,826,348 | | | 2,777,086 | | | 406,851 | |
| | | | | | | | | |
Total liabilities | | 3,298,454 | | | 3,676,165 | | | 538,569 | |
| | | | | | | | | |
Shareholders’ equity | | | | | | | | | |
Ordinary shares | | 257,738 | | | 258,840 | | | 37,921 | |
Additional paid-in capital | | 709,949 | | | 752,862 | | | 110,297 | |
Treasury stock | | — | | | (45,143 | ) | | (6,614 | ) |
Accumulated other comprehensive loss | | (69,957 | ) | | (70,316 | ) | | (10,302 | ) |
Retained earnings | | 1,028,540 | | | 871,775 | | | 127,718 | |
| | | | | | | | | |
Total shareholders’ equity | | 1,926,270 | | | 1,768,018 | | | 259,020 | |
| | | | | | | | | |
Total liabilities and shareholders’ equity | | 5,224,724 | | | 5,444,183 | | | 797,589 | |
| | | | | | | | | |
Save for the Convertible Notes, the Company had no other contingencies, long-term obligations and guarantee as of March 31, 2009 and 2010.
F-45
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Condensed Statements of Income
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008
(As adjusted
below) | | | 2009
(As adjusted
below) | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | | | USD’000 | |
Interest income | | 18,546 | | | 12,625 | | | 10 | | | 1 | |
Interest expenses | | (75,297 | ) | | (125,175 | ) | | (158,526 | ) | | (23,224 | ) |
Other income | | — | | | — | | | 25,693 | | | 3,764 | |
Share of net income of subsidiaries | | 395,268 | | | 518,767 | | | 147,980 | | | 21,680 | |
General and administrative expenses | | (41,506 | ) | | (71,415 | ) | | (72,198 | ) | | (10,577 | ) |
| | | | | | | | | | | | |
Income (loss) before income taxes | | 297,011 | | | 334,802 | | | (57,041 | ) | | (8,356 | ) |
Income tax expense | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | |
Net income (loss) | | 297,011 | | | 334,802 | | | (57,041 | ) | | (8,356 | ) |
| | | | | | | | | | | | |
F-46
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
Condensed Statements of Cash Flows
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2008
(As adjusted
below) | | | 2009
(As adjusted
below) | | | 2010 | | | 2010 | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | | | US$’000 | |
Cash flow from operating activities | | | | | | | | | | | | |
Net income (loss) | | 297,011 | | | 334,802 | | | (57,041 | ) | | (8,356 | ) |
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
Share-based compensation | | 16,660 | | | 50,179 | | | 40,437 | | | 5,924 | |
Share of net income of subsidiaries | | (395,268 | ) | | (518,767 | ) | | (147,980 | ) | | (21,680 | ) |
Non-cash interest expense on convertible notes | | 29,367 | | | 28,614 | | | 30,477 | | | 4,465 | |
Amortization of convertible notes issuance costs | | 6,781 | | | 13,323 | | | 17,402 | | | 2,549 | |
Gain on repurchase of convertible notes | | — | | | — | | | (25,693 | ) | | (3,764 | ) |
Changes in assets and liabilities: | | | | | | | | | | | | |
Prepayments and other assets | | 248 | | | 239 | | | 19 | | | 3 | |
Amounts due from subsidiaries | | (2,843 | ) | | — | | | — | | | — | |
Accrued expenses and other liabilities | | (290 | ) | | 11,235 | | | 4,777 | | | 700 | |
Amounts due to subsidiaries | | — | | | 78,817 | | | 422,196 | | | 61,853 | |
| | | | | | | | | | | | |
Net cash (used in) provided by operating activities | | (48,334 | ) | | (1,558 | ) | | 284,594 | | | 41,694 | |
| | | | | | | | | | | | |
Cash flow from investing activities | | | | | | | | | | | | |
Dividends received | | 200,000 | | | 431,066 | | | — | | | — | |
Advance to subsidiaries | | (447,447 | ) | | (2,209,357 | ) | | (120,714 | ) | | (17,685 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | (247,447 | ) | | (1,778,291 | ) | | (120,714 | ) | | (17,685 | ) |
| | | | | | | | | | | | |
Cash flow from financing activities | | | | | | | | | | | | |
Dividends paid | | (83,306 | ) | | (86,574 | ) | | (99,724 | ) | | (14,610 | ) |
Proceeds from exercise of stock options and restricted shares | | 5,157 | | | — | | | 2,249 | | | 330 | |
Net proceeds from issuance of convertible notes | | — | | | 1,837,871 | | | — | | | — | |
Payment for repurchase of convertible notes | | — | | | — | | | (49,426 | ) | | (7,241 | ) |
Prepayment for repurchase of convertible notes | | — | | | — | | | (14,346 | ) | | (2,102 | ) |
Repurchase of shares under share repurchase program | | — | | | — | | | (43,814 | ) | | (6,419 | ) |
Proceeds from issuance of shares under share issuance and repurchase agreement | | — | | | 32,265 | | | — | | | — | |
Payment for repurchase of shares under share issuance and repurchase agreement | | — | | | (32,265 | ) | | — | | | — | |
| | | | | | | | | | | | |
Net cash (used in) provided by financing activities | | (78,149 | ) | | 1,751,297 | | | (205,061 | ) | | (30,042 | ) |
| | | | | | | | | | | | |
Effect of foreign currency exchange rate changes on cash and cash equivalents | | (33,692 | ) | | (39,154 | ) | | (3,782 | ) | | (555 | ) |
| | | | | | | | | | | | |
Net decrease in cash and cash equivalents | | (407,622 | ) | | (67,706 | ) | | (44,963 | ) | | (6,588 | ) |
| | | | |
Cash and cash equivalents: | | | | | | | | | | | | |
At beginning of year | | 577,473 | | | 169,851 | | | 102,145 | | | 14,965 | |
| | | | | | | | | | | | |
At end of year | | 169,851 | | | 102,145 | | | 57,182 | | | 8,377 | |
| | | | | | | | | | | | |
F-47
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
The following condensed financial statement line items were affected as of March 31, 2009 and for the years ended March 31, 2008 and 2009 reflect the retrospective adoption of new authoritative guidance on accounting for convertible debt instruments that may be settled in cash upon conversion (Note 2(y)).
Condensed Balance Sheet of the Company
| | | | | | | | | |
| | | As originally
reported | | | Effect of adoption
of new guidance
(Note 2(y)) | | | As adjusted | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
March 31, 2009 | | | | | | | | | |
Assets | | | | | | | | | |
Convertible notes issuance costs | | 68,596 | | | (2,780 | ) | | 65,816 | |
Liabilities | | | | | | | | | |
Convertible notes | | 2,910,815 | | | (84,467 | ) | | 2,826,348 | |
Shareholders’ equity | | | | | | | | | |
Additional paid-in capital | | 544,178 | | | 165,771 | | | 709,949 | |
Accumulated other comprehensive loss | | (51,946 | ) | | (18,011 | ) | | (69,957 | ) |
Retained earnings | | 1,094,613 | | | (66,073 | ) | | 1,028,540 | |
Condensed Statements of Income of the Company
| | | | | | | | | |
| | | Year ended March 31, 2009 | |
| | | As originally
reported | | | Effect of adoption
of new guidance
(Note 2(y)) | | | As adjusted | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Interest expense | | (97,625 | ) | | (27,550 | ) | | (125,175 | ) |
Income before income taxes | | 362,352 | | | (27,550 | ) | | 334,802 | |
| | | | | | | | | |
Net income | | 362,352 | | | (27,550 | ) | | 334,802 | |
| | | | | | | | | |
| | | | | | | | | |
| | | Year ended March 31, 2008 | |
| | | As originally
reported | | | Effect of adoption
of new guidance
(Note 2(y)) | | | As adjusted | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Interest expense | | (47,086 | ) | | (28,211 | ) | | (75,297 | ) |
Income before income taxes | | 325,222 | | | (28,211 | ) | | 297,011 | |
| | | | | | | | | |
Net income | | 325,222 | | | (28,211 | ) | | 297,011 | |
| | | | | | | | | |
Condensed Statements of Cash Flows of the Company
| | | | | | | | | |
| | | Year ended March 31, 2009 | |
| | | As originally
reported | | | Effect of adoption
of new guidance
(Note 2(y)) | | | As adjusted | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Net income | | 362,352 | | | (27,550 | ) | | 334,802 | |
Adjustments to reconcile net income to net cash provided by (used in) operating activities | | | | | | | | | |
Non-cash interest expense on convertible notes | | — | | | 28,614 | | | 28,614 | |
Amortization of convertible notes issuance costs | | 14,387 | | | (1,064 | ) | | 13,323 | |
Net cash used in operating activities | | (1,558 | ) | | — | | | (1,558 | ) |
F-48
CHINA MEDICAL TECHNOLOGIES, INC.
Notes to the Consolidated Financial Statements (Continued)
For the years ended March 31, 2008, 2009 and 2010
| | | | | | | | | |
| | | Year ended March 31, 2008 | |
| | | As originally
reported | | | Effect of adoption
of new guidance
(Note 2(y)) | | | As adjusted | |
| | | RMB’000 | | | RMB’000 | | | RMB’000 | |
Net income | | 325,222 | | | (28,211 | ) | | 297,011 | |
Adjustments to reconcile net income to net cash provided by (used in) operating activities | | | | | | | | | |
Non-cash interest expense on convertible notes | | — | | | 29,367 | | | 29,367 | |
Amortization of convertible notes issuance costs | | 7,937 | | | (1,156 | ) | | 6,781 | |
Net cash used in operating activities | | (48,334 | ) | | — | | | (48,334 | ) |
F-49
