
Acquisition of Spitfire Pharma Inc. Adding NASH Drug Candidate ALT-801 Exhibit 99.2
Forward-looking statements Safe-Harbor Statement Any statements made in this presentation relating to future financial or business performance, conditions, plans, prospects, trends, or strategies and other financial and business matters, including without limitation, the closing of the Spitfire Pharma acquisition, the timing of key milestones for ALT-801, the filing of the IND for ALT-801 in 2020, the initiation of a Phase 1 clinical study in 2020, cash on hand to fund the development of ALT-801, and the prospects for regulatory approval or commercializing ALT-801, are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In addition, when or if used in this press release, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants, as they relate to Altimmune, Inc. (the “Company”) may identify forward-looking statements. The Company cautions that these forward-looking statements are subject to numerous assumptions, risks, and uncertainties, which change over time. Important factors that may cause actual results to differ materially from the results discussed in the forward looking statements or historical experience include risks and uncertainties, including risks relating to: the Company’s ability to close the Spitfire Pharma acquisition on the timelines anticipated, or at all, the reliability of the results of the studies relating to human safety and possible adverse effects resulting from the administration of ALT-801; the Company may encounter substantial delays in its clinical trials, or its clinical trials may fail to demonstrate the safety and efficacy of our product candidates to the satisfaction of applicable regulatory authorities; the Company’s ability to predict the time and cost of product development; competition from other pharmaceutical and biotechnology companies, which may result in others discovering, developing or commercializing NASH products before, or more successfully, than the Company the Company’s ability to obtain potential regulatory approvals on the timelines anticipated, or at all; the Company’s ability to obtain additional patents or extend existing patents on the timelines anticipated, or at all; the Company’s ability to expand its pipeline of products and the success of future product advancements, including the success of future clinical trials, and the Company’s ability to commercialize its products; third-party claims of intellectual property infringement or misappropriation may prevent or delay the Company’s development and commercialization efforts the Company’s anticipated financial or operational results; the Company’s ability to obtain additional capital resources; unforeseen safety and efficacy issues; the Company’s ability to receive stockholder approval to issue shares of its common stock in satisfaction of milestone payments; and the Company’s ability to continue to satisfy the listing requirements of the NASDAQ Global Market. Further information on the factors and risks that could affect the Company's business, financial conditions and results of operations are contained in the Company’s filings with the U.S. Securities and Exchange Commission, including under the heading “Risk Factors” in the Company’s annual reports on Form 10-K and quarterly reports on Form 10-Q filed with the SEC, which are available at www.sec.gov.

AGENDA Spitfire Pharma, Inc. overview Strategic rationale ALT-801 overview Financial considerations Q&A 1 2 3 4 5 Vipin K. Garg, Ph.D. President and CEO . Will Brown, CPA Chief Financial Officer . Scot Roberts, Ph.D. Chief Scientific Officer John J. Nestor, Jr., Ph.D. Co-Founder Spitfire Pharma, Inc. Sybil Tasker, M.D. Clinical Advisor .
SPITFIRE PHARMA OVERVIEW Founded by Dr. John Nestor and Velocity Pharmaceutical Development, in San Francisco, California Developing a novel dual GLP-1/glucagon receptor agonist for the treatment of NASH Compelling results in established preclinical animal models for NASH Product candidate to be renamed ALT-801 upon consummation of merger Poised to enter clinical development in H2 2020 ALT-801
STRATEGIC RATIONALE NASH is a significant unmet need with no currently approved therapy ALT-801: Potent dual agonist peptide that treats a root cause of NASH – obesity Demonstrated beneficial effects on liver fat, fibrosis and inflammation COMPLEMENTARY HEPTCELL | PEPTIDES Leverages our expertise in liver diseases with HepTcell for chronic hepatitis B Deep knowledge in developing peptide based therapeutics DIFFERENTIATED BALANCED DUAL AGONIST | WEEKLY DOSING GLP-1/ Glucagon dual agonist reverses both metabolic and liver dysfunctions Compelling preclinical effects across multiple NASH endpoints
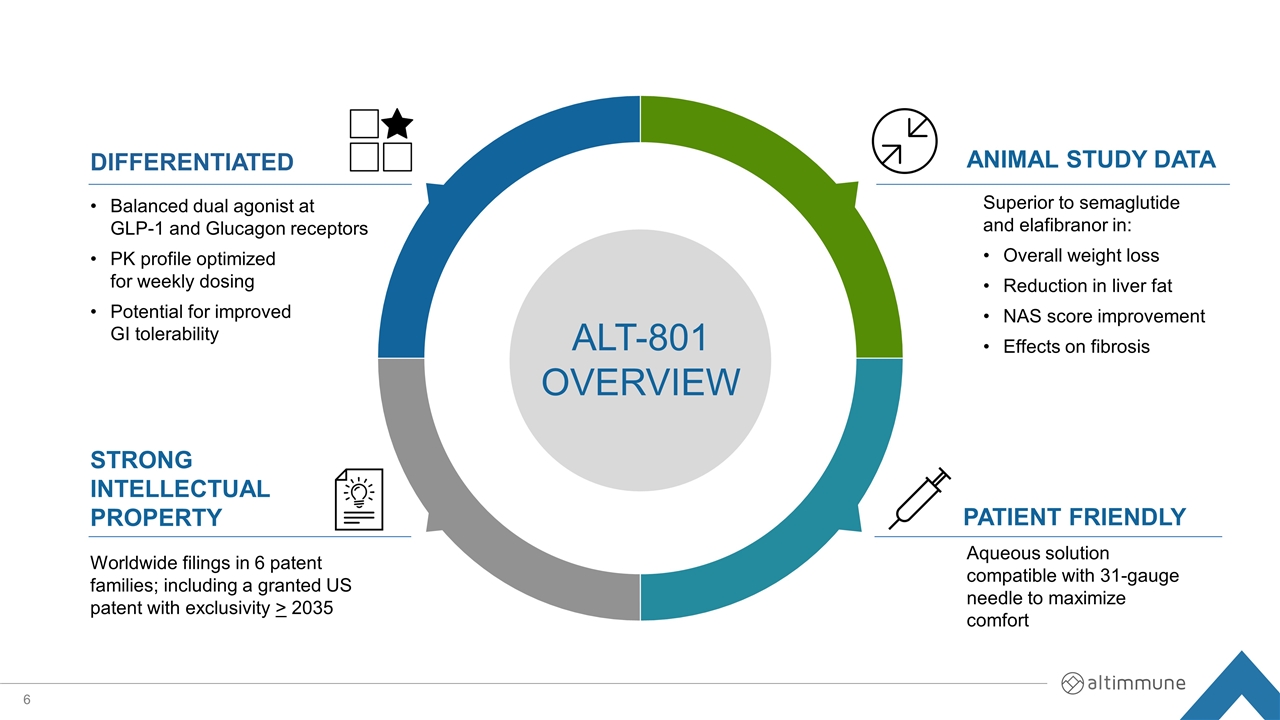
DIFFERENTIATED PATIENT FRIENDLY STRONG INTELLECTUAL PROPERTY Balanced dual agonist at GLP-1 and Glucagon receptors PK profile optimized for weekly dosing Potential for improved GI tolerability Aqueous solution compatible with 31-gauge needle to maximize comfort Superior to semaglutide and elafibranor in: Overall weight loss Reduction in liver fat NAS score improvement Effects on fibrosis Worldwide filings in 6 patent families; including a granted US patent with exclusivity > 2035 ANIMAL STUDY DATA ALT-801 OVERVIEW
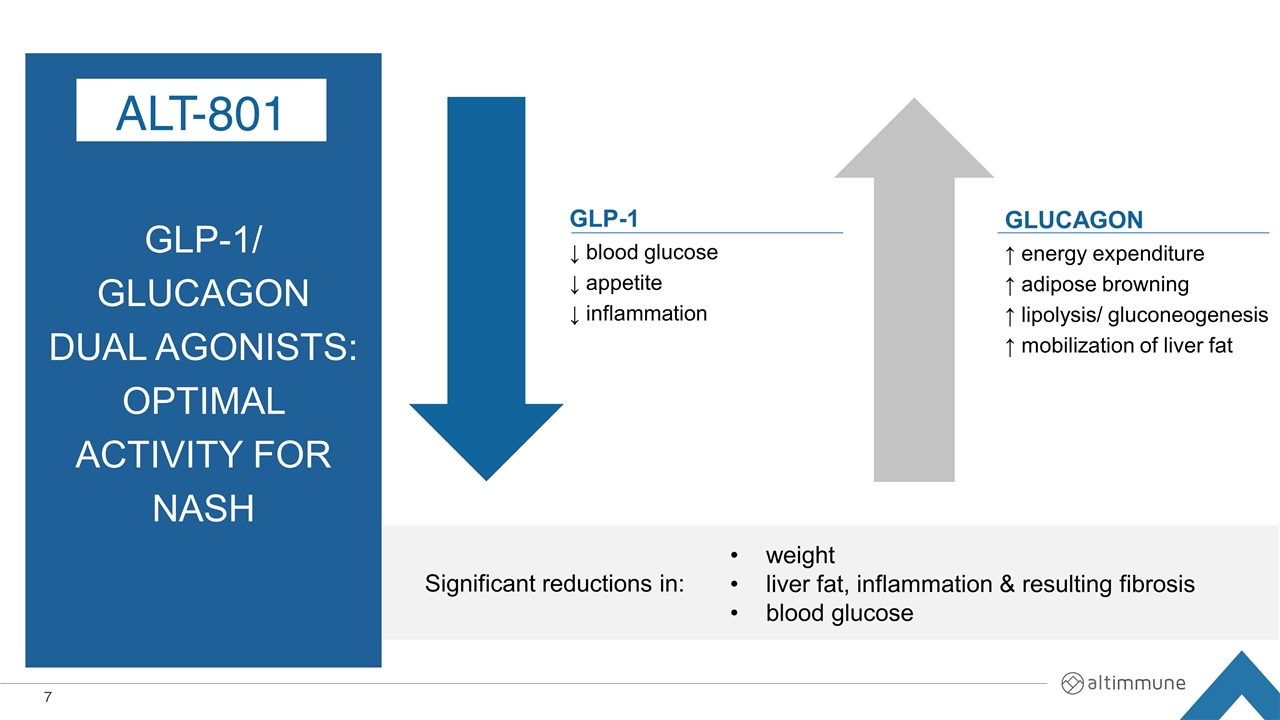
GLP-1/ Glucagon Dual Agonists: Optimal Activity for NASH ALT-801 Significant reductions in: weight liver fat, inflammation & resulting fibrosis blood glucose GLP-1 ↓ blood glucose ↓ appetite ↓ inflammation GLUCAGON ↑ energy expenditure ↑ adipose browning ↑ lipolysis/ gluconeogenesis ↑ mobilization of liver fat
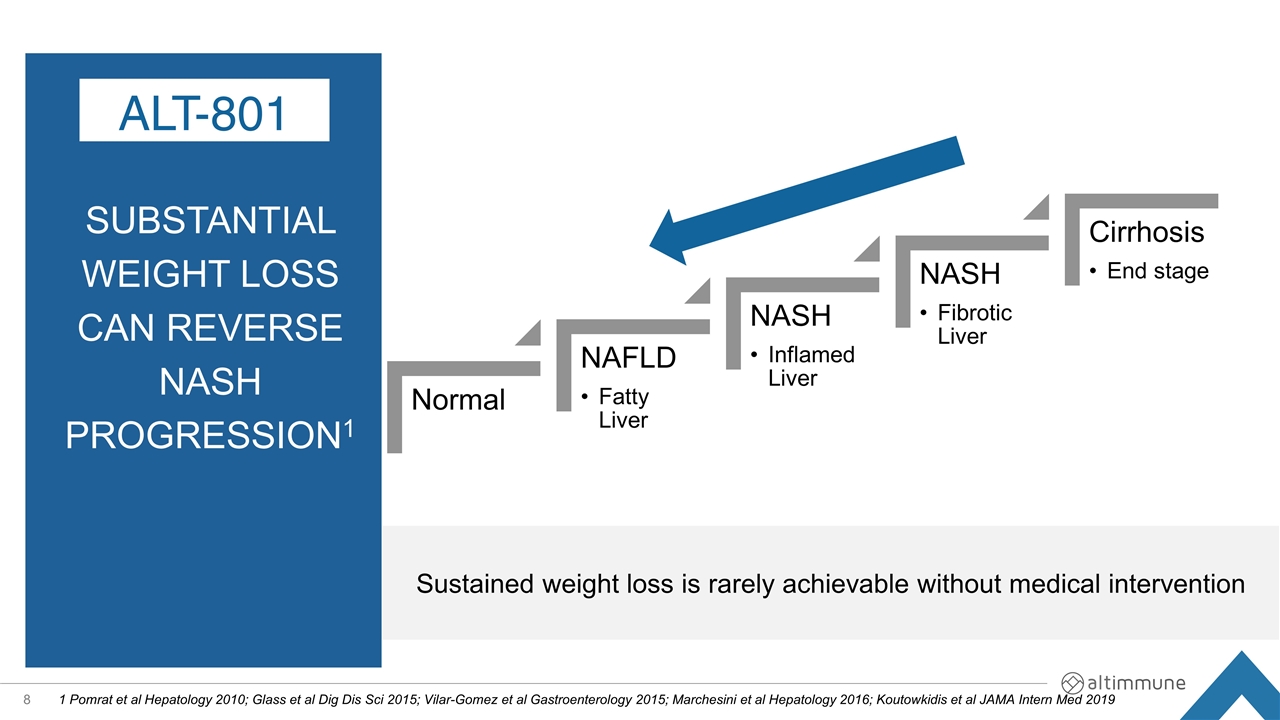
Substantial weight loss can REVERSE nash pROGRESSION1 1 Pomrat et al Hepatology 2010; Glass et al Dig Dis Sci 2015; Vilar-Gomez et al Gastroenterology 2015; Marchesini et al Hepatology 2016; Koutowkidis et al JAMA Intern Med 2019 ALT-801 Sustained weight loss is rarely achievable without medical intervention Normal NAFLD NASH NASH Cirrhosis Fatty Liver Inflamed Liver Fibrotic Liver End stage
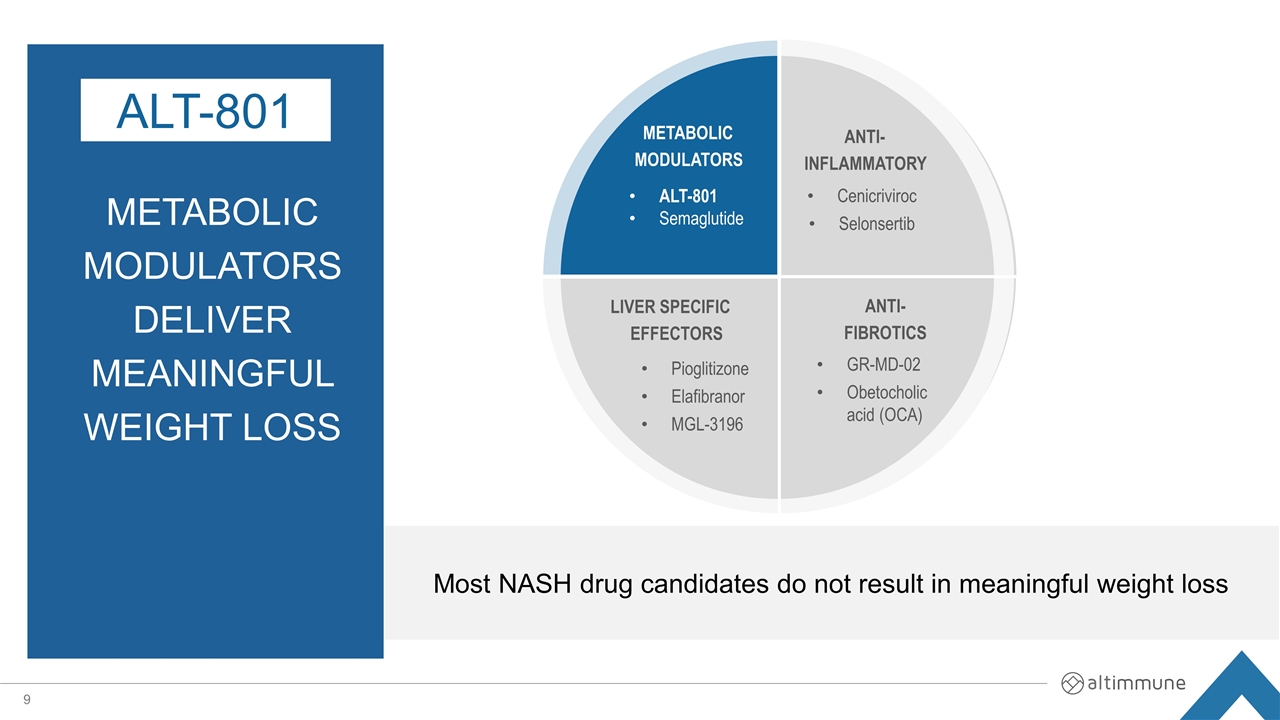
ALT-801 Semaglutide METABOLIC MODULATORS Pioglitizone Elafibranor MGL-3196 LIVER SPECIFIC EFFECTORS ANTI-INFLAMMATORY ANTI- FIBROTICS Cenicriviroc Selonsertib GR-MD-02 Obetocholic acid (OCA) Most NASH drug candidates do not result in meaningful weight loss Metabolic modulatorsdeliver meaningful weight loss ALT-801
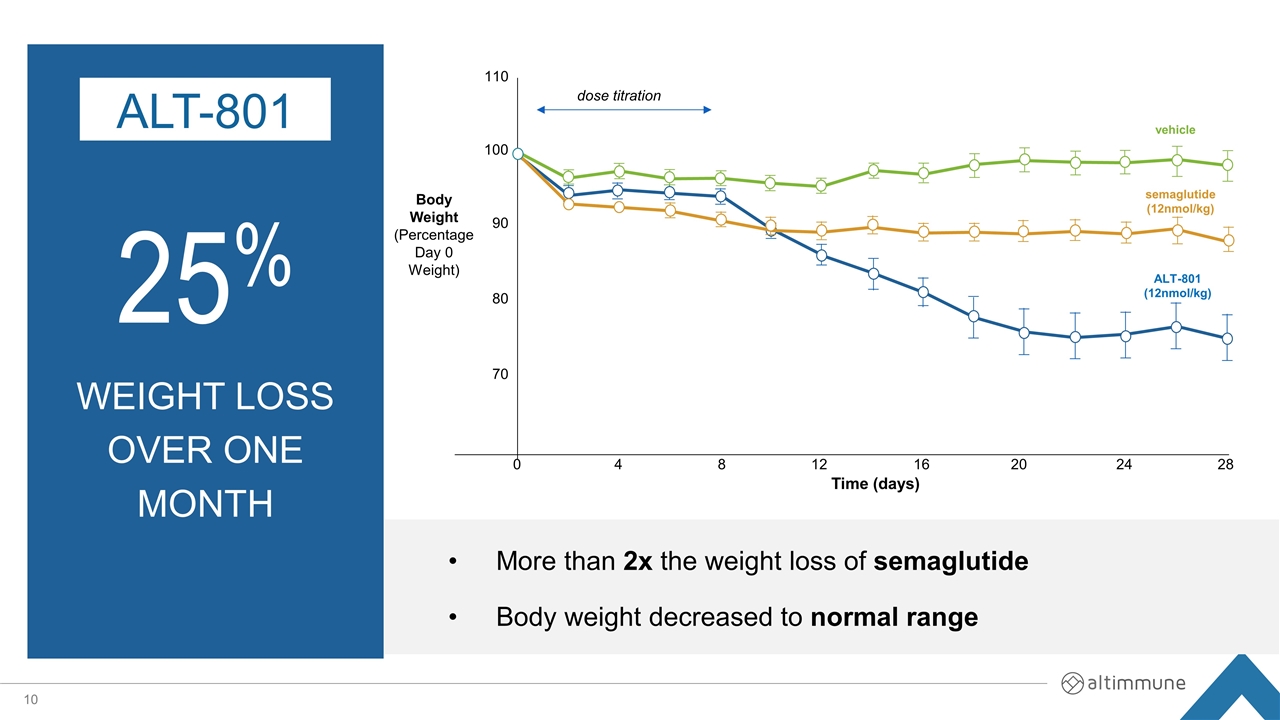
25% weight loss over one month ALT-801 More than 2x the weight loss of semaglutide Body weight decreased to normal range Body Weight (Percentage Day 0 Weight) 110 100 90 80 70 0 4 8 12 16 20 24 28 Time (days) vehicle dose titration semaglutide (12nmol/kg) ALT-801 (12nmol/kg)
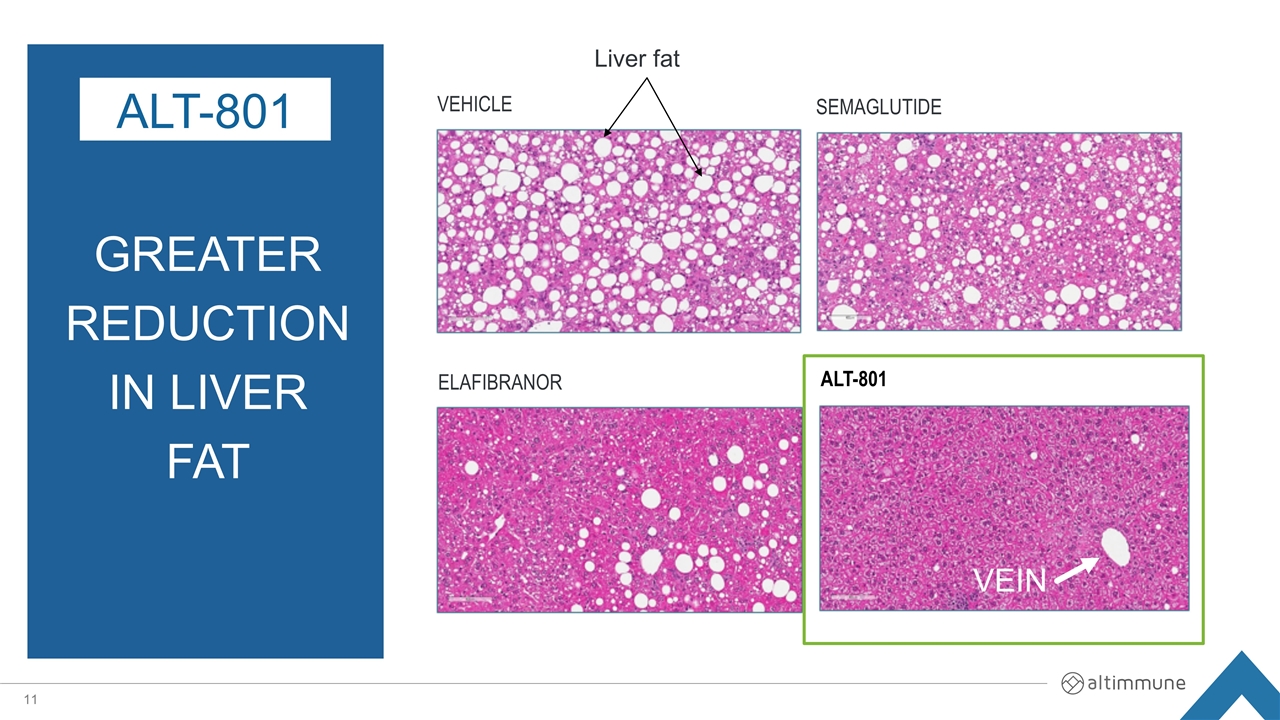
GREATER reduction in Liver Fat ALT-801 VEHICLE SEMAGLUTIDE ELAFIBRANOR ALT-801 Liver fat VEIN
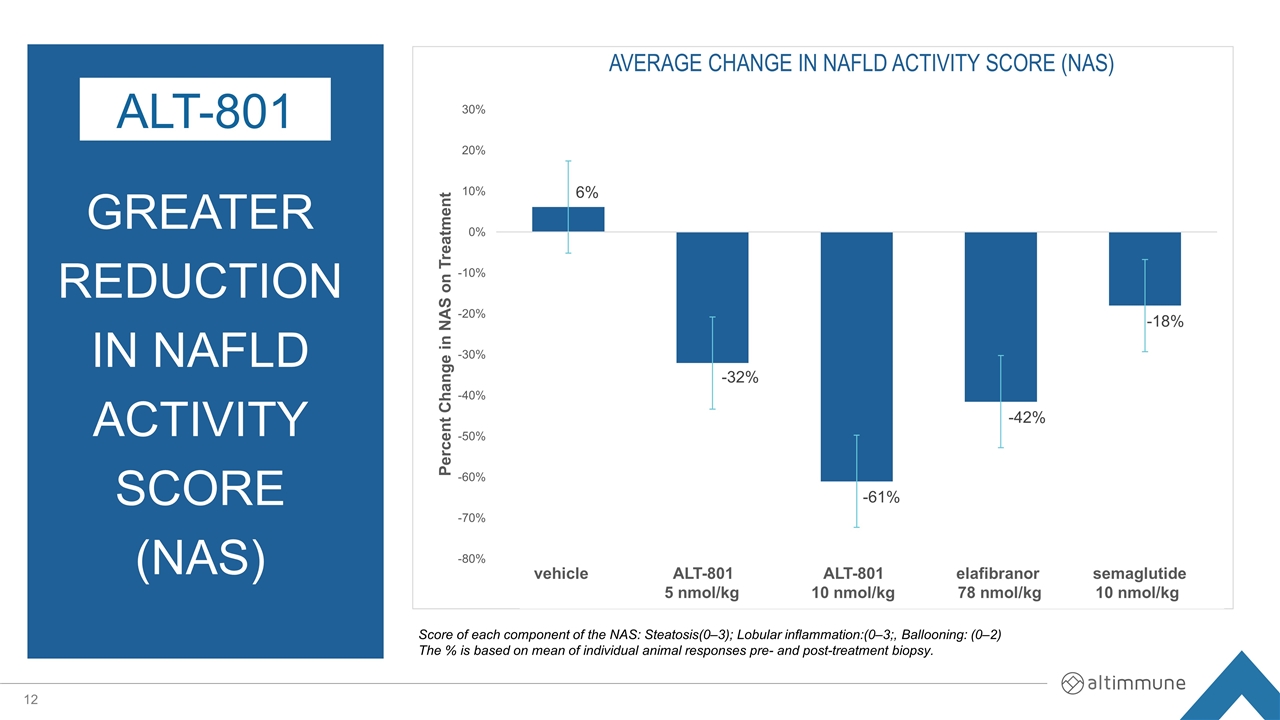
GREATER Reduction in NAFLD Activity Score (NAS) ALT-801 Score of each component of the NAS: Steatosis(0–3); Lobular inflammation:(0–3;, Ballooning: (0–2) The % is based on mean of individual animal responses pre- and post-treatment biopsy. vehicle ALT-801 ALT-801 elafibranor semaglutide 5 nmol/kg 10 nmol/kg 78 nmol/kg 10 nmol/kg
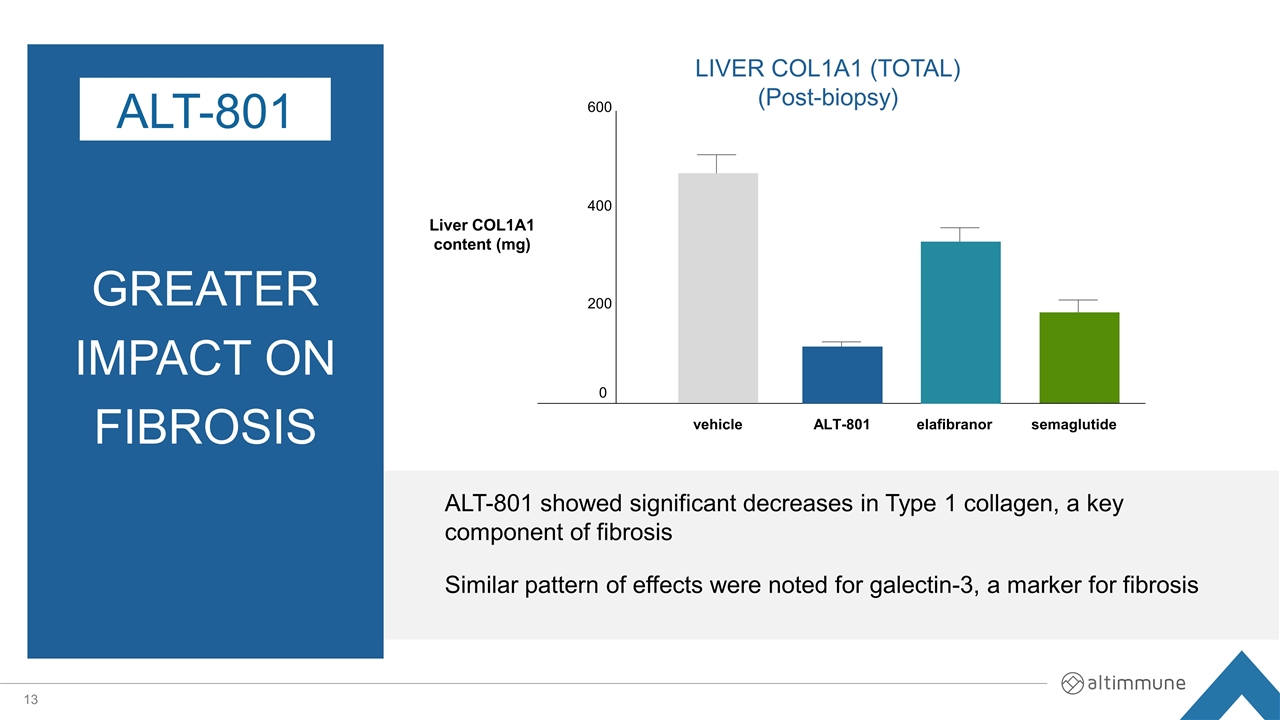
GREATER Impact on Fibrosis ALT-801 LIVER COL1A1 (TOTAL) (Post-biopsy) Liver COL1A1 content (mg) 600 400 200 0 vehicle ALT-801 elafibranor semaglutide ALT-801 showed significant decreases in Type 1 collagen, a key component of fibrosis Similar pattern of effects were noted for galectin-3, a marker for fibrosis

Financial considerations – Deal terms Cash-free and debt-free acquisition of 100% Spitfire Pharma Inc. SALES BASED MILESTONES SINGLE ASSET ENTITY no employees or facilities $5M UPFRONT all stock consideration up to $8M CLINICAL AND REGULATORY MILESTONES payable in cash or stock C
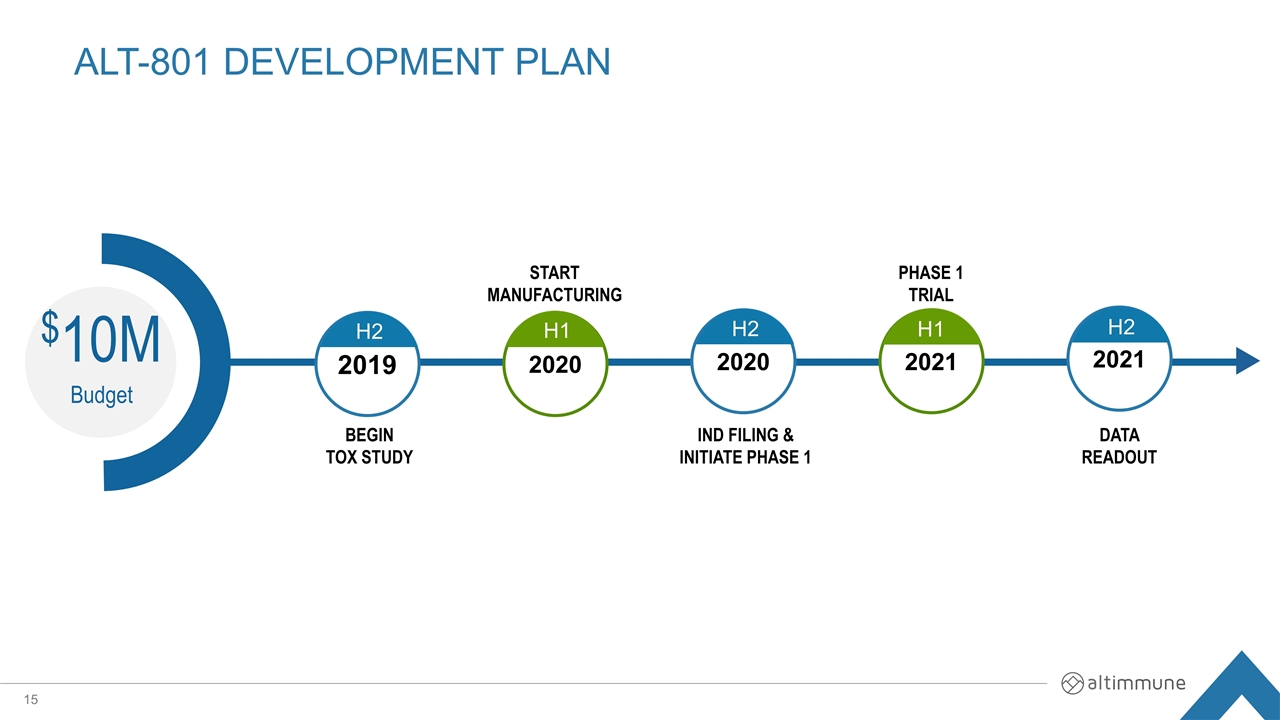
ALT-801 Development plan 2019 2020 2020 2021 2021 $10M Budget H2 H1 H2 H2 H1 BEGIN TOX STUDY IND FILING & INITIATE PHASE 1 START MANUFACTURING PHASE 1 TRIAL DATA READOUT

Spitfire pharma Acquisition: key takeaways Complementary asset addressing a significant unmet need Highly differentiated product candidate that addresses a root cause of NASH Outperforms other NASH candidates in preclinical models All stock deal structure with payment terms that minimize risk Cash on hand to support development through Phase 1 data

Acquisition of Spitfire Pharma