- NAOV Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
FWP Filing
NanoVibronix (NAOV) FWPFree writing prospectus
Filed: 27 Sep 17, 12:00am

Investor Presentation September 2017 OTC QB : NAOV Issuer Free Writing Prospectus Filed Pursuant to Rule 433 of the Securities Act of 1933, as amended Registration Statement No. 333 - 218871

OTC QB : NAOV 2 Safe Harbor This presentation may contain “forward - looking statements” that are made pursuant to the “safe harbor” provisions as defined within the Private Securities Litigation Reform Act of 1995 . Forward - looking statements may be identified by words including “anticipates,” “believes,” “intends,” “estimates,” and similar expressions . These statements are based upon management’s current expectations as of the date of this presentation . Such forward - looking statements may include statements regarding the Company’s future financial performance or results of operations, including expected revenue growth, cash flow growth, future expenses and other future or expected performances . The Company cautions readers there may be events in the future that the Company is not able to accurately predict or control and the information contained in the forward - looking statements is inherently uncertain and subject to a number of risks that could cause actual results to differ materially from those indicated in the forward - looking statements . Further information on these and other potential factors that could affect the Company’s financial results is included in the Company’s filings with the SEC under the “Risk Factors” sections and elsewhere in those filings .

This presentation highlights basic information about NanoVibronix , Inc. and the offering. NanoVibronix , Inc. has filed a registration statement on Form S - 1 (Registration No. 333 - 218871) (including a prospectus) with the U.S. Securities and Exchange Commission (the “SEC”) for the offering to which this presentation relates. The registration statement is not yet effective. Before you invest, you should read the prospectus in that registration statement (including, among other things, risk factors described therein) and other documents the issuer has filed with the SEC for more complete information about NanoVibronix , Inc. and this offering. The preliminary prospectus dated June 21, 2017, and subsequent amendments are available at the SEC website. You may get these documents for free by visiting EDGAR on the SEC website at www.sec.gov. Alternatively, NanoVibronix , Inc. or any underwriter or any dealer participating in the offering will arrange to send you the prospectus if you request it by contacting Dawson James Securities, Inc., Attention: Prospectus Department, 1 North Federal Highway, 5 th Floor, Boca Raton, FL 33432, mmaclaren@dawsonjames.com or toll free at 866.928.0928. Free Writing Prospectus OTC QB : NAOV 3
OTC QB : NAOV 4 Key Highlights x Addresses multi - billion $ markets - catheter infections, pain relief & wound healing x New senior management team; previously introduced medical devices generating $ billions in sales; previously Kinetics Concepts (KCI) sold for $6B in 2011 x NanoVibronix was selected by the Israeli government as one of the eight innovation leading companies to present at their United Nations conference in 2016 x Potential breakthrough medical platform and devices – now entering commercial stage x Extensive existing and growing clinical evidence and regulatory approvals x In discussions with potential strategic partners x Scalable, high - margin recurring revenue business model x Extensive IP, 8 issued patents, covers the technology and unique applications Inflection Point: New Management Team Leading to Commercialization

OTC QB : NAOV Brian Murphy, CEO – Led the initiative for V.A.C. therapy into the homecare market from $0 - $400 million in 6 years at Kinetics Concepts (KCI) USA – Experience includes 30+ years in medical device and biologics industry – Leadership positions with MiMedx , KCI/ Acelity , ConvaTec , O2 Insights, Kodak Chris Fashek , Chairman of the Board – Former Vice Chairman and President of Kinetics Concepts (KCI) USA . Led the team that introduced Negative Pressure Wound Therapy technology in 1995; KCI was acquired for $6 billion in 2011 – Former Chairman and President of Spiracur Inc. and Chairman of Systagenix Ltd. – CEO of Atteris Healthcare, wound and skin care start - up Dr. Harold Jacob, MD - CMO – Board Certified in Internal Medicine and Gastroenterology – Former Director of Medical Affairs at Given Imaging ( Nasdaq:GIVN ) – Personally holds multiple medical device patents and licensing agreements Steve Brown, CPA – CFO – Former CFO of IDT Corporation (NYSE: IDT) 5 New Experienced Management Team Previous Success at Kinetics Concepts (KCI) USA

OTC QB: NAOV Surface Acoustic Wave (SAW) Technology : therapeutic ultrasound – Delivered through miniature transducers that transmit low - frequency, low - intensity ultrasound to induce repair of soft tissue and prevent bacterial colonization – Indicated for indwelling catheter infection, resolution of pain and wound healing with a strong pipeline of additional healthcare modalities – Can be administered at home, without the assistance of medical professionals Lead Products : – UroShield ® ● CE Mark, Canadian clearance ● FDA 510K filing targeted in Q1 2018 – PainShield ® ● FDA clearance, Canadian clearance , and CE Mark ● Preparing market plans for lead, large market indications – WoundShield ® ● CE Mark, Canadian clearance 6 What We Do Transitioning from Development into Commercialization

OTC QB: NAOV The NanoVibronix “Shield” platform delivers surface acoustic waves (SAW) to soft tissue , muscle , and indwelling devices is designed to : – Prevent microbial colonization and disrupts biofilm formation on indwelling devices – Provide a unique enhancement for antibiotic effectiveness – Expedite soft tissue healing process and pain reduction – Eliminate heat production that can cause tissue damage 7 Differentiated Technology Acoustic pressure scale Transducer Propagation Transducer Driver Transducer transmits surface acoustic waves onto treatment surfaces with a radius of up to 10 centimeters 10cm treatment area
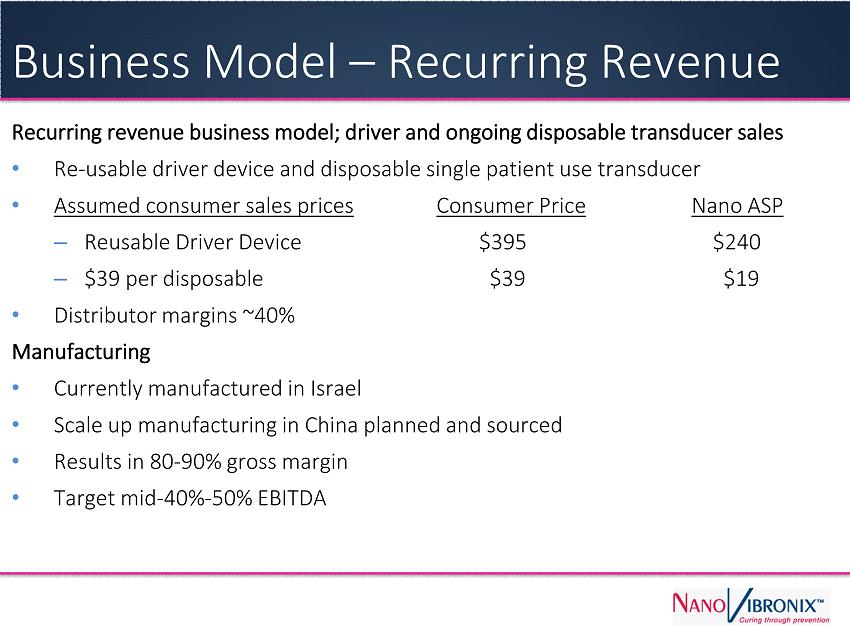
Recurring revenue business model; driver and ongoing disposable transducer sales ● Re - usable driver device and disposable single patient use transducer ● Assumed consumer sales prices Consumer Price Nano ASP – Reusable Driver Device $395 $240 – $39 per disposable $39 $19 ● Distributor margins ~40% Manufacturing ● Currently manufactured in Israel ● Scale up manufacturing in China planned and sourced ● Results in 80 - 90% gross margin ● Target mid - 40% - 50% EBITDA Business Model – Recurring Revenue

OTC QB: NAOV ● A therapeutic surface acoustic wave device, consisting of a reusable driver unit and a clamp - on disposable, which contains a proprietary therapeutic transducer ● D esigned to prevent bacterial docking, colonization and biofilm on indwelling urinary catheters, decrease UT and urine infections, increas e antibiotic efficacy , and decrease pain and discomfort associated with urinary catheter use . 9 UroShield
OTC QB : NAOV – The Global Catheter market : $26. 6 B in 201 5; growing at an estimated 9.7% through 202 1; – 100 million foley catheters sold worldwide annually, with 25 million in the U.S.; represents a multi - billion dollar opportunity ● Urinary Tract Infections (UTI) account for more than 40% of the Hospital Acquired Infections (HAI) ● Catheter Acquired UTIs are the source for 20% of Healthcare Acquired Bacteremia in acute care and 50% in long term care facilities – Applicable on any current catheter and expect to expand to other indications for additional catheters 10 UroShield – Market Opportunity We Believe UroShield Offers the Most Effective Option for Prevention of Catheter Acquired Urinary Infections
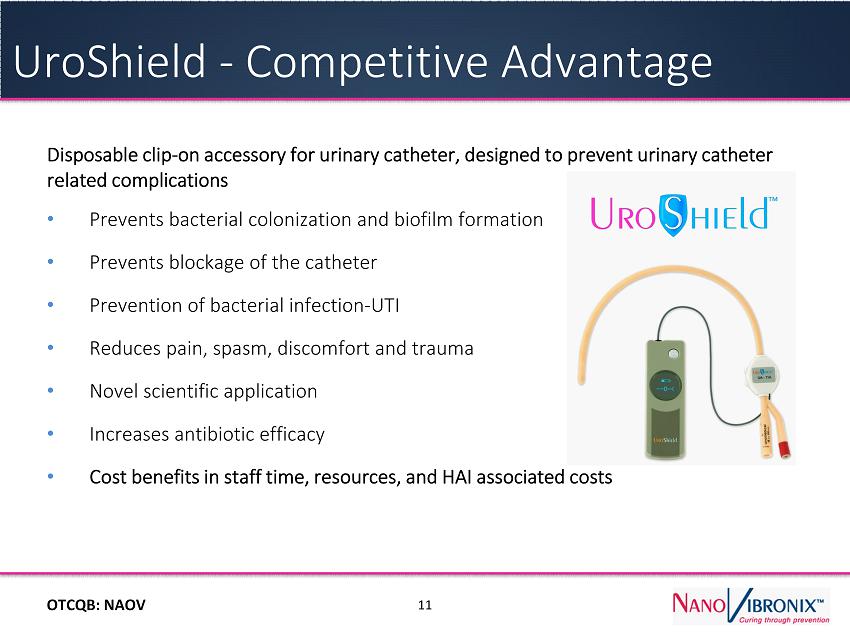
OTC QB : NAOV Disposable clip - on accessory for urinary catheter, designed to prevent urinary catheter related complications ● Prevents bacterial colonization and biofilm formation ● Prevents blockage of the catheter ● Prevention of bacterial infection - UTI ● Reduces pain, spasm, discomfort and trauma ● Novel scientific application ● Increases antibiotic efficacy ● Cost benefits in staff time, resources, and HAI associated costs 11 UroShield - Competit i ve Advantage
OTC QB: NAOV In Vivo Urinary Catheter Studies (Rabbits) ● Foundational, initial evaluation utilizing low level ultrasound, comparing the result to standard catheter without low level ultrasound. ● Evaluated both arms for biofilm formation, urinary tract infections & bacteriuria ● Non - vibrated catheter results: – Grossly pathological urinary tracts, infected urine, and heavily biofilm coated – High incidence of UT and urine infections – Heavy colonialization and biofilm coating on catheters ● Nano - Vibrated catheter results: – Urinary tracts remained normal – Urine free of infection – Catheters almost free of biofilm – Without the risk of tissue damage! …Human studies further the validation 12 UroShield - Initial Foundational Validation
UroShield - Clinical Validation OTC QB: NAOV 13 Case Studies & RCT’s ● Salem Academic Hospital HD1 - Double Blind, RCT, (22 patients) - statistically significant proof of prevention of biofilm, - less medications and less pain than the control ( U.Ikinger ) ● Salem Academic Hospital HD2 - Double Blind, RCT, (40 patients) - 1/20 patients randomized to UroShield , without antibiotics, developed UTI vs 4/20 in the control group who were treated with prophylaxic antibiotic therapy ( U.Ikinger ) ● Shaare Zedek Medical Center – Case Series (10 patients) - effectiveness in pain reduction, spasms, burning & itching. Results demonstrated a reduction in pain, itching, burning and spasms ● Shaare Zedek Medical Center – Open label, comparative, randomized (40 patients) - statistically significant reduction in postoperative catheter related pain and bladder spasms. Notable trend towards reduction of bacteriuria. ● Prof. P. Tenke , Hungary – Multiple Patient Evaluations (27 patients) - demonstrated reduction in pain and significant decrease in bacteriuria rates. 139 patients evaluated in 5 different clinical studies with no adverse events reported
UroShield – Successful Interim Trial Results OTC QB: NAOV 14 Current Randomized Trial (60 patients) ● 22 subjects evaluated at two skilled nursing facilities near Buffalo, NY 11 patients treated with placebo devices (“controlled”) 11 patients had active UroShield devices (“treatment”) ● All exhibited significant colonization of multiple microorganisms in both the catheter device as well as retained urine from the bladder Most counted at greater than 100,000 colony forming unit (CFUs) 30 & 60 Day evaluation results ● All 11 subjects in controlled group still exhibited greater than 100,000 CFUs ● All 11 subjects in UroShield treatment group exhibited reductions in CFUs Most measured less than 10,000 CFUs Many measured less than 1,000 CFUs; a greater than 90% reduction ● None of the subjects in UroShield treatment group developed a clinical infection 90 - Day Follow - Up - Pending ● UroShield and Control clinical infection incidence to be determined Study will be use to support FDA submission and attainment of clearances with desired indications for use

OTC QB : NAOV 15 Market Preparation and Strategy Market Strategy US Clinical and Regulatory ● Target completion of randomized trial in 1Q 2018 ● Expect trial results compiled and 510(K) Class 2 filing to FDA in 2Q 2018 ● Target FDA approval of 510K in 3Q 2018 ● Projected target US launch in 3 - 4Q 2018 ● Non - US Areas: Utilize region specific distributors to begin selling immediately under CE and Canadian approval – Relationships completed in United Kingdom, Austria, Italy, India, and Canada – Agreements include minimum sales requirements and in some cases, future commitment – Expect results from the pending completion of US randomized trial to augment market demand ● US: Target completion of partnership with leading catheter product companies for US sales/marketing – ACTIVE discussions with qualified distributors and domestic license partners Manufacturing ● Current capacity sufficient for targeted launch ● Expanded capacity and higher margin manufacturer expected to be in place in Q3 2018

OTC QB : NAOV A patch - based therapeutic Surface Acoustic Wave reusable driver unit and a disposable transducer patch T reats pain in nerves, muscles, joints and soft tissue through delivery of a localized ultrasound , and induce s soft tissue healing in targeted area s. 16 PainShield FDA Cleared, CE, and Canadian License approvals
OTC QB : NAOV Trigeminal Neuralgia – A chronic pain condition of the most widely distributed 5 th cranial nerve in the head – Potentially as many as 280,000 sufferers in the U.S. & 7,000,000 worldwide. 15% growth estimated between 2012 and 2022 – Current treatments include opioid and surgical interventions. Both have a high rate of recurrence The General Pain Market – HHS estimates that 54% of the adult population experience musculoskeletal pain – Internal Analgesics are $ 4.1 B in the U.S. – Worldwide opioid prevention initiative Other Treatments: – Pelvic Pain (Exclusive distributor recently signed) – Sports injuries, tennis elbow and joint pain (National distributor recently signed - Henry Schein) – Chronic Neuropathic Pain – Fibromyalgia – Equine (Exclusive distributor negotiations are close to being finalized) 17 PainShield – Market Opportunity We Believe PainShield is Differentiated and Unique Due to SAW Technology

OTC QB: NAOV 18 PainShield – Targeted Marketing Strategy ● Treats the source of the pain ● Alleviate pain Pain Management: - Chiropractic - Physical therapy - Sports medicine ● 73% of pts. achieving complete or near complete relief ● Definitive study planned Trigeminal Neuralgia ● Pain ● RX abuse & over prescribing ● Narcotic RX / dependency Organizations: - Hospitals & clinics - Long - term care facilities - VA / DOD ● performance ● down time Veterinary Market - Trainers - Equine Therapists Target Benefit Sales Channel Independent Sales Reps Private Label Distributors Direct Sales: - Social Media - Pain oriented journals - Neurology Intermediary - Does not require FSS - Internal sales resources - <$3,500 PO - No oversight or scrutiny Specialized Sales Force National Distributors
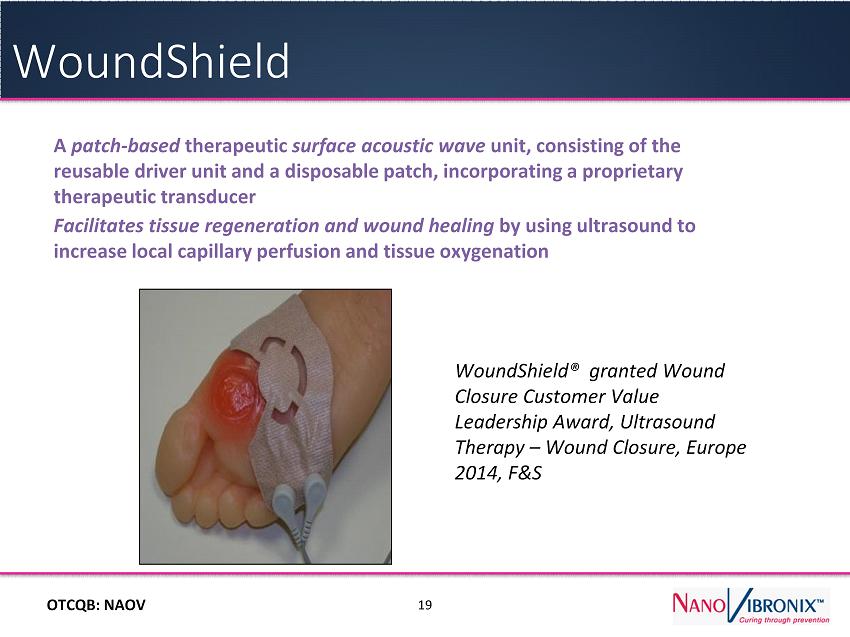
OTC QB : NAOV A patch - based therapeutic surface acoustic wave unit, consisting of the reusable driver unit and a disposable patch, incorporating a proprietary therapeutic transducer F acilitates tissue regeneration and wound healing by using ultrasound to increase local capillary perfusion and tissue oxygenation 19 WoundShield WoundShield ® granted Wound Closure Customer Value Leadership Award, Ultrasound Therapy – Wound Closure, Europe 2014, F&S
OTC QB: NAOV Disposable, patch based therapeutic ultrasound device ● Induces perfusion in soft tissue for wound healing ● Encourages epithelial tissue growth and migration ● Accelerates healing of chronic and acute wounds ● Hands - free, portable and easy to use ● For use in both clinical and home settings 20 WoundShield – Status & Strategy We Believe WoundShield is Differentiated and Unique Due to SAW Technology Two published clinical studies done to - date suggest: ● Improves localized blood flow and oxygenation ● Improves topical oxygen saturation Cultivating Strategic Partnership opportunities: ● Multiple licensing opportunities currently being considered
Product Pipeline OTC QB : NAOV 21 ● LungShield ™ , SAW ultrasound device to manage biofilm adhesion and infection in endotracheal tubes. LungShield is currently being studied in a hospital setting to determine its efficacy for reducing infection and pain. Infection in endotracheal tubes is a common problem in hospitals. ● RenooSkin ™ , is a SAW ultrasound facial skin rejuvenation device utilizing bilateral transducers placed near the ears on the face. Based on clinical preliminary research, NanoVibronix’s SAW ultrasound technology enhances human skin regeneration. Na noVibronix SAW ultrasound technology increases cytokeratin 14, collagen III & glicoseaminoglycan (gag) expression in human skin In Strategic Discussions with a Global Cosmetics Company
OTC QB : NAOV ● Ticker: NAOV ● Exchange: OTC Q B ● Share Price ( 9 / 8 / 17 ): $ 5.80 ● Common Shares Outstanding: 2.6 M ● Preferred Shares: 1.9 M ● Fiscal year - end: December 3 1 22 Key Statistics

OTC QB : NAOV 23 Investment Summary x Potential breakthrough medical platform and devices – now entering commercial stage x Extensive existing and growing clinical evidence and regulatory approvals x Addresses multi - billion $ markets - catheter infections, pain relief & wound healing x New senior management team; previously introduced medical devices generating $ billions in sales; previously Kinetics Concepts (KCI) sold for $6B in 2011 x Advanced discussions with potential strategic partners x Scalable, high - margin recurring revenue business model Inflection Point: New Management Team Leading to Commercialization

Investor Relations Contact s : Crescendo Communications / David Waldman NAOV@crescendo - ir.com / 212 - 671 - 102 1 Hayden IR / Brett Maas brett@haydenir.com / 646 - 536 - 7331 Company Contact: NanoVibronix Inc. Email: info@nanovibronix.com Phone: 914 - 233 - 3004 Thank You