Exhibit 99.1

An effective Solution for the Prevention of Bacterial Load and CAUTI, Through the Application of Surface Acoustic Waves on Indwelling Catheters March 11, 2019 NASDAQ : NAOV

This presentation contains forward - looking statements. Forward - looking statements are based on current expectations and projections about future events and are subject to risks, uncertainties and assumptions about our Company, economic and market sectors and the industry in which we do business, among other things. These statements are not guarantees of future performance, and except as required by law, we undertake no obligation to publicly update any forward - looking statements whether as a result of new information, future events or otherwise. Actual events and results may differ from those expressed in any forward - looking statements due to a number of factors. Factors that could cause our actual performance, future results and actions to differ materially from any forward - looking statements include, but are not limited to, those discussed in risk factors within our Annual Report on Form 10 - K for the fiscal year ended December 31, 2017, and our Quarterly Report on Form 10 - Q for the quarterly period ended Secpetember 30, 2018, as filed with the Securities and Exchange Commission Disclaimer
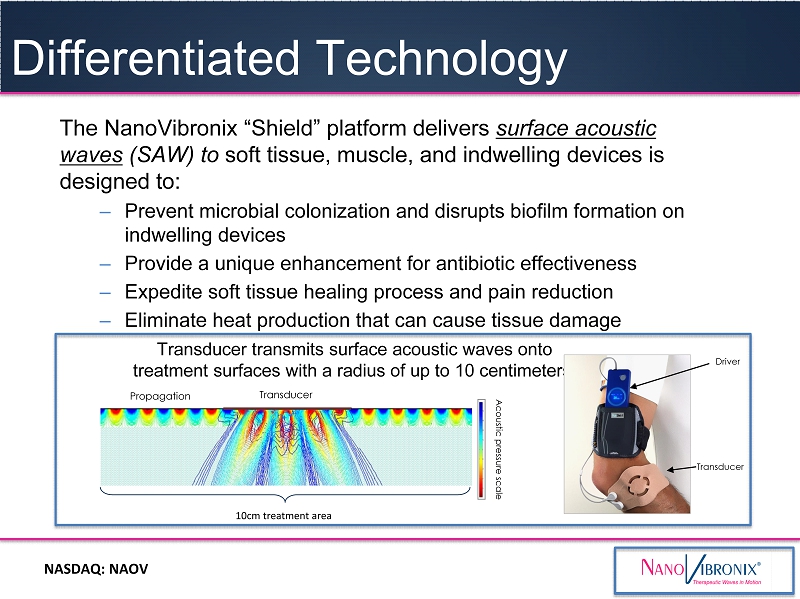
NASDAQ: NAOV The NanoVibronix “Shield” platform delivers surface acoustic waves (SAW) to soft tissue , muscle , and indwelling devices is designed to : – Prevent microbial colonization and disrupts biofilm formation on indwelling devices – Provide a unique enhancement for antibiotic effectiveness – Expedite soft tissue healing process and pain reduction – Eliminate heat production that can cause tissue damage Differentiated Technology Acoustic pressure scale Transducer Propagation Transducer Driver Transducer transmits surface acoustic waves onto treatment surfaces with a radius of up to 10 centimeters 10cm treatment area

NASDAQ: NAOV • A therapeutic surface acoustic wave device, consisting of a reusable driver unit and a clamp - on disposable, which contains a proprietary therapeutic transducer • D esigned to prevent bacterial docking, colonization and biofilm on indwelling urinary catheters, decrease UT and urine infections, increas e antibiotic efficacy , and decrease pain and discomfort associated with urinary catheter use . UroShield Regulatory approval in the EU , Canada, India & Israel ; actively pursuing marketing clearance in the U.S
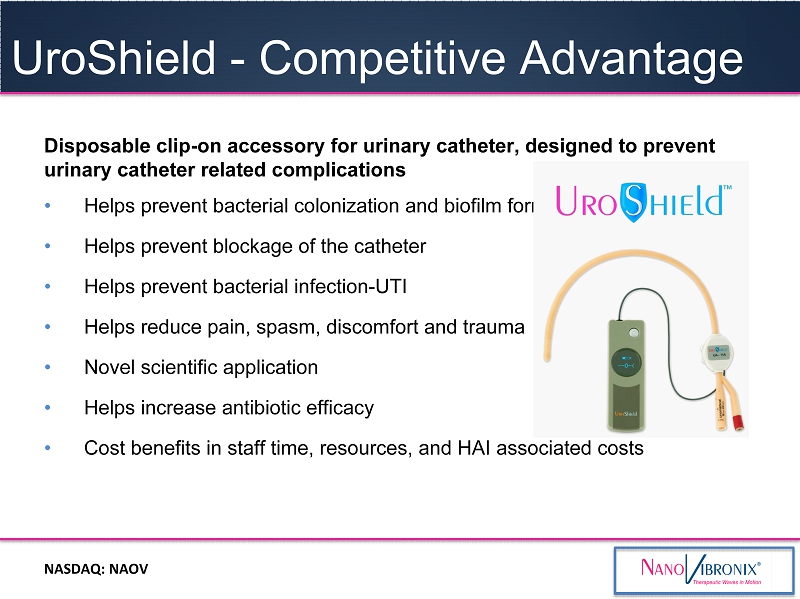
NASDAQ : NAOV Disposable clip - on accessory for urinary catheter, designed to prevent urinary catheter related complications • Helps p revent bacterial colonization and biofilm formation • Helps prevent blockage of the catheter • Helps prevent bacterial infection - UTI • Helps r educe pain, spasm, discomfort and trauma • Novel scientific application • Helps i ncrease antibiotic efficacy • Cost benefits in staff time, resources, and HAI associated costs UroShield - Competit i ve Advantage
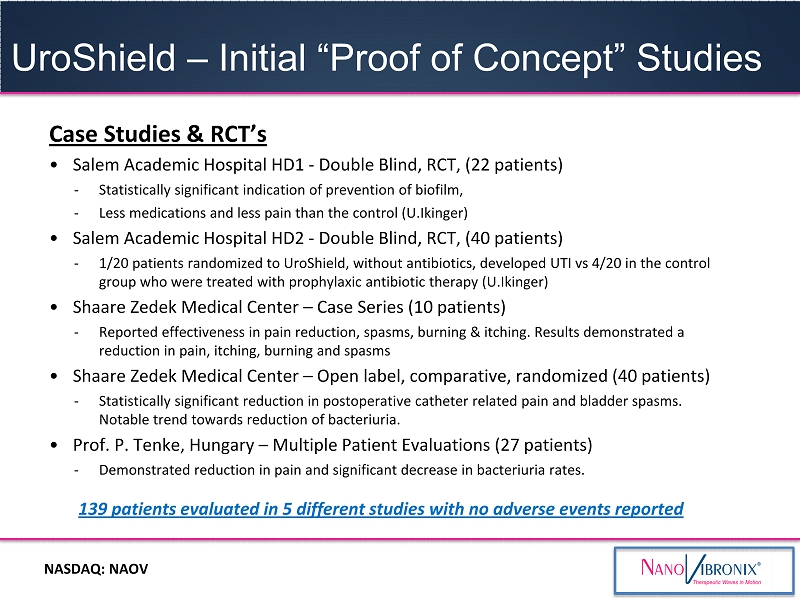
UroShield – Initial “Proof of Concept” Studies NASDAQ: NAOV Case Studies & RCT’s • Salem Academic Hospital HD1 - Double Blind, RCT, (22 patients) - Statistically significant indication of prevention of biofilm, - Less medications and less pain than the control (U.Ikinger) • Salem Academic Hospital HD2 - Double Blind, RCT, (40 patients) - 1/20 patients randomized to UroShield, without antibiotics, developed UTI vs 4/20 in the control group who were treated with prophylaxic antibiotic therapy (U.Ikinger) • Shaare Zedek Medical Center – Case Series (10 patients) - Reported effectiveness in pain reduction, spasms, burning & itching. Results demonstrated a reduction in pain, itching, burning and spasms • Shaare Zedek Medical Center – Open label, comparative, randomized (40 patients) - Statistically significant reduction in postoperative catheter related pain and bladder spasms. Notable trend towards reduction of bacteriuria. • Prof. P. Tenke, Hungary – Multiple Patient Evaluations (27 patients) - Demonstrated reduction in pain and significant decrease in bacteriuria rates. 139 patients evaluated in 5 different studies with no adverse events reported
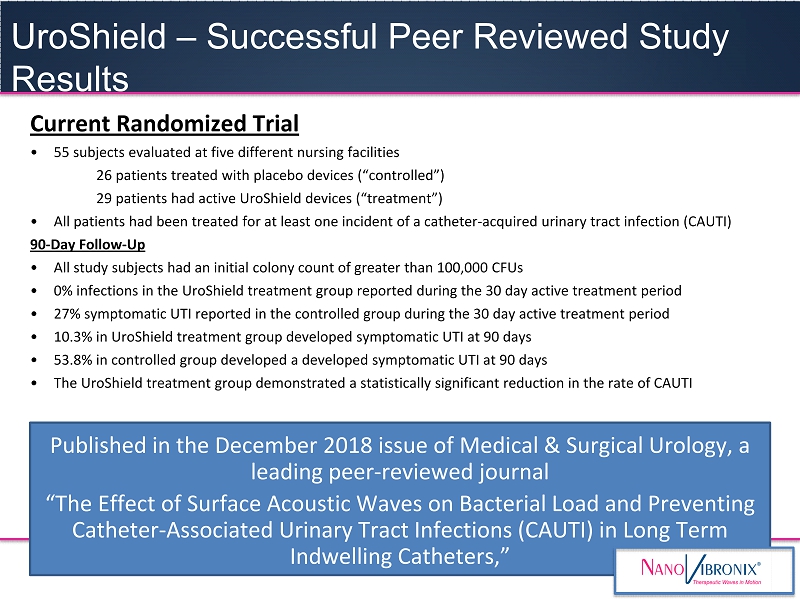
NASDAQ: NAOV Current Randomized Trial • 55 subjects evaluated at five different nursing facilities 26 patients treated with placebo devices (“controlled”) 29 patients had active UroShield devices (“treatment”) • All patients had been treated for at least one incident of a catheter - acquired urinary tract infection (CAUTI) 90 - Day Follow - Up • All study subjects had an initial colony count of greater than 100,000 CFUs • 0% infections in the UroShield treatment group reported during the 30 day active treatment period • 27% symptomatic UTI reported in the controlled group during the 30 day active treatment period • 10.3% in UroShield treatment group developed symptomatic UTI at 90 days • 53.8% in controlled group developed a developed symptomatic UTI at 90 days • The UroShield treatment group demonstrated a statistically significant reduction in the rate of CAUTI Published in the December 2018 issue of Medical & Surgical Urology, a leading peer - reviewed journal “The Effect of Surface Acoustic Waves on Bacterial Load and Preventing Catheter - Associated Urinary Tract Infections (CAUTI) in Long Term Indwelling Catheters,” UroShield – Successful Peer Reviewed Study Results
“Understanding and controlling urinary catheter - associated biofilms: a complex problem” Sandra A. Wilks 1,2 1 School of Health Sciences, 2 School of Biological Sciences, Faculty of Environmental & Life Sciences Contact: S.A.Wilks@soton.ac.uk Lecturer in Medical Microbiology Presented at Knowlex IPC Conference 27 February 2019 UNIVERSITY OF Southampton Summary Results of In Vitro Study Presented at Knowlex Infection Prevention and Control Conference 2019
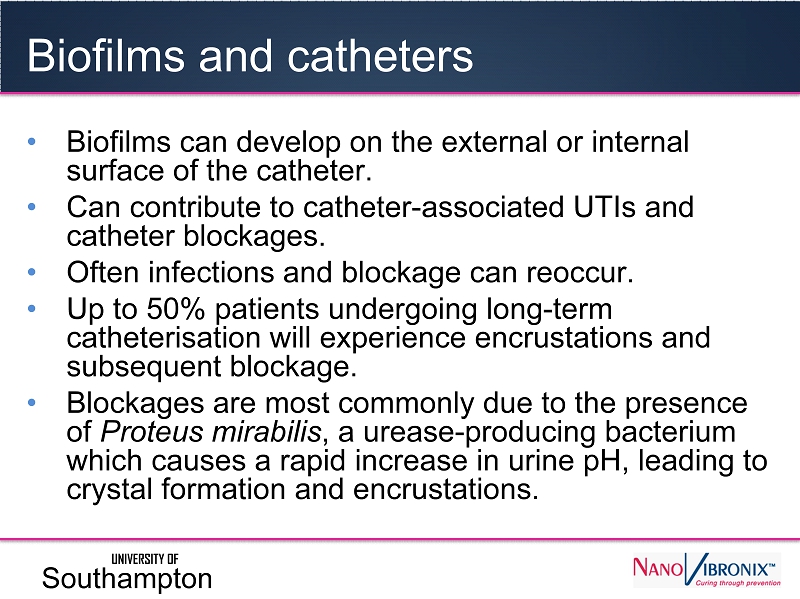
Biofilms and catheters • Biofilms can develop on the external or internal surface of the catheter. • Can contribute to catheter - associated UTIs and catheter blockages. • Often infections and blockage can reoccur. • Up to 50% patients undergoing long - term catheterisation will experience encrustations and subsequent blockage. • Blockages are most commonly due to the presence of Proteus mirabilis , a urease - producing bacterium which causes a rapid increase in urine pH, leading to crystal formation and encrustations. UNIVERSITY OF Southampton

Scheme of artificial bladder model assembly: 1, aspirator with artificial urine; 2, peristaltic pump; 3, glass artificial bladder; 4, heating jacket; 5, water bath; 6, drainage bag; 7, artificial urethra. (Kazmierska et al ., 2010). Images taken from Maierl et al., 2015. This is the most widely accepted setup for in - vitro testing Controlled laboratory modelling: UNIVERSITY OF Southampton
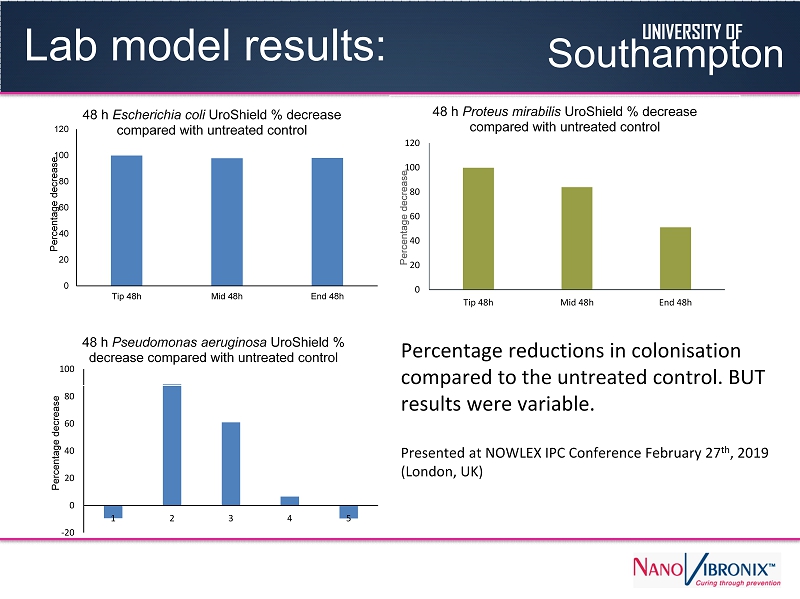
Lab model results: 0 20 40 60 80 100 120 Tip 48h Mid 48h End 48h Percentage decrease 48 h Escherichia coli UroShield % decrease compared with untreated control 0 20 40 60 80 100 120 Tip 48h Mid 48h End 48h Percentage decrease 48 h Proteus mirabilis UroShield % decrease compared with untreated control -20 0 20 40 60 80 100 1 2 3 4 5 Percentage decrease 48 h Pseudomonas aeruginosa UroShield % decrease compared with untreated control Percentage reductions in colonisation compared to the untreated control. BUT results were variable. Presented at NOWLEX IPC Conference February 27 th , 2019 (London, UK) UNIVERSITY OF Southampton
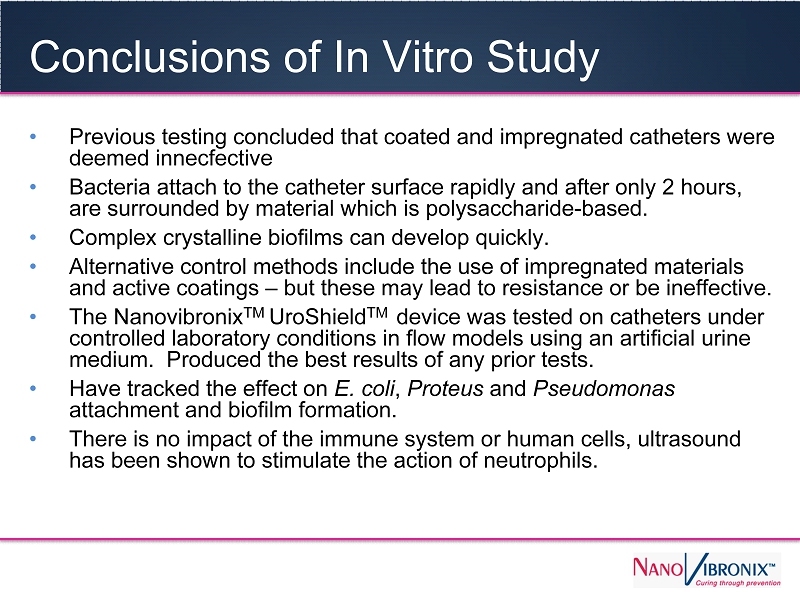
Conclusions of In Vitro Study • Previous testing concluded that coated and impregnated catheters were deemed innecfective • Bacteria attach to the catheter surface rapidly and after only 2 hours, are surrounded by material which is polysaccharide - based. • Complex crystalline biofilms can develop quickly. • Alternative control methods include the use of impregnated materials and active coatings – but these may lead to resistance or be ineffective. • The Nanovibronix TM UroShield TM device was tested on catheters under controlled laboratory conditions in flow models using an artificial urine medium. Produced the best results of any prior tests. • Have tracked the effect on E. coli , Proteus and Pseudomonas attachment and biofilm formation. • There is no impact of the immune system or human cells, ultrasound has been shown to stimulate the action of neutrophils.

UroShield Shows Great Promise in the Effort to Reduce CAUTI!!! Summary Conclusion

Brian Murphy, CEO, Contact Info : bmurphy@nanovibronix.com 630 - 338 - 5022 Company Contact: NanoVibronix Inc. Email: info@nanovibronix.com Phone: 914 - 233 - 3004 Thank You













