On December 7, 2020, Lyra Therapeutics, Inc. (the “Company”) reported positive top-line results from its Phase 2 LANTERN clinical trial, a randomized, controlled, patient blinded clinical trial designed to evaluate safety and efficacy in chronic rhinosinusitis (“CRS”) patients both with and without nasal polyps who have failed previous medical management but have not undergone endoscopic sinus surgery. The trial was designed to enroll 99 evaluable patients with the potential to increase to up to 150 patients and was initiated in May 2019 at sites in Australia, Austria, Czech Republic, New Zealand and Poland. In December 2019, the U.S. Food and Drug Administration (the “FDA”) authorized the Company’s investigational new drug application, and, prior to the COVID-19 global pandemic, the Company planned to enroll patients in the United States. However, in light of developments relating to the COVID-19 global pandemic, the Company discontinued enrollment at 67 patients in its Phase 2 LANTERN clinical trial and did not enroll any patients in the United States.
In its readout of top-line results, the Company reported that, at the 7,500 µg dose, LYR-210 achieved statistically significant improvement in the composite four cardinal symptoms score (“4CSS”) in favor of the treatment arm as measured by the change from baseline at week 16 (-1.47) (p=0.021), week 20 (-1.61) (p=0.012) and week 24 (-1.64) (p=0.016) (see Figure 1, below). However, although a strong treatment effect was observed at week 4, LYR-210 did not achieve the primary endpoint of change from baseline in 4CSS at week 4 at either the 7,500 µg dose (-0.36) (p=0.306) or 2,500 µg dose (0.04) (p=0.525). The Company believes this was due primarily to the discontinuation of enrollment related to the COVID-19 global pandemic. As a result of the decrease in the number of patients enrolled from planned (99 evaluable) to actually enrolled (67), a greater magnitude of change from baseline in 4CSS at week 4 and/or a smaller standard deviation associated with the change from baseline was required in order to achieve statistical significance for the primary endpoint at week 4.
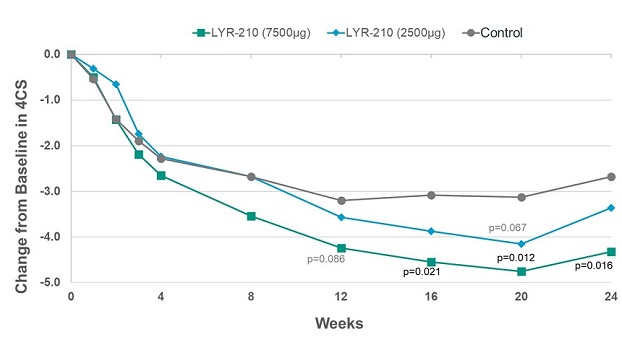
Figure 1. Total Symptom Improvement by 4CSS for Phase 2 LANTERN Clinical Trial.
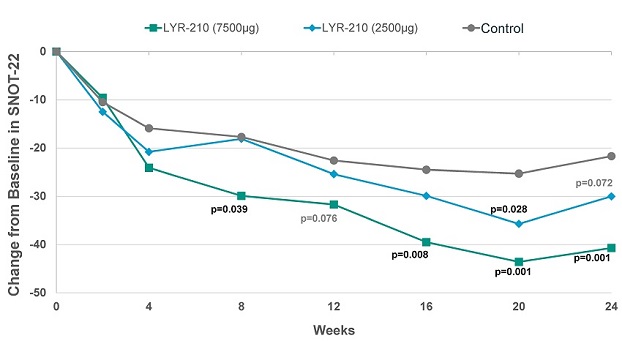
Furthermore, at the 7,500 µg dose, LYR-210 achieved statistically significant improvement in SinoNasal Outcome Test (“SNOT-22”) score in favor of the treatment arm as measured by the change from baseline at week 8 (-12.2) (p=0.039), week 16 (-15.0) (p=0.008), week 20 (-18.4) (p=0.001) and week 24 (-19.0) (p=0.001) (see Figure 2, below). In particular, the improvement of the 7,500 µg dose of LYR-210 at week 24 over the control group (-19.0) was over two times the minimal clinically important difference of -8.9.