Exhibit 99.1

1 NASDAQ:CPXX | celatorpharma.com Transforming the Science of Combination Therapy to Improve Patient Outcomes in Cancer July 16, 2015 Celator Pharmaceuticals May 28, 2015 © Copyright 2015 Celator Pharmaceuticals. All rights reserved.

Welcome and Introductions Scott Jackson Chief Executive Officer

Option 2 with people Celator Photography Celator Pharmaceuticals 3 Celator Corporate Overview Our mission is to improve and extend the lives of patients with cancer by transforming the science of combination therapy

Safe Harbor Celator Pharmaceuticals 4 To the extent that statements contained in this presentation are not descriptions of historical facts regarding Celator, they are forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward - looking statements. Forward - looking statements in this presentation involve substantial risks and uncertainties that could cause our pre - clinical and clinical research and development programs, future results, working capital requirements, performance or achievements to differ significantly from those expressed or implied by the forward - looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the initiation and conduct of clinical studies and other research programs, enrollment in clinical studies, availability of data from ongoing clinical studies, the outcome of other research programs, the potential efficacy and therapeutic and commercial potential of our drug candidates, our prospects for long - term growth, our ability to raise capital, whether final results of clinical studies will be supportive of regulatory approvals required to market licensed products and other matters that could affect the availability or commercial potential of our drug candidates. Celator undertakes no obligation to update or revise any forward - looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward - looking statements, as well as risks relating to the business of the company in general, see Celator's Form 10 - K for the year ended December 31, 2014 and other filings by the company with the U.S. Securities and Exchange Commission.

Agenda Celator Pharmaceuticals 5 9:00am - 9:15am Welcome and Introductions Scott Jackson Chief E xecutive Officer 9:15am - 9:30am Overview and Update on CPX - 351 Studies Arthur Louie, MD Chief Medical Officer 9:30am - 9:50am AML Therapy: A GIANT Unmet Need Gail Roboz, MD Cornell University – Weill Medical College 9:50am - 10:10am Developing CPX - 351 beyond the initial indication Bruno C. Medeiros, MD Stanford University School of Medicine 10:10am - 10:35am Fireside Chat followed by Question and Answer Session Participants : Dr. Roboz, Dr. Medeiros and Dr. Louie Moderator: Derek Miller 10:35am - 10:50am Paving the Way to CPX - 351 Commercialization Derek Miller Chief Business Officer

Agenda Celator Pharmaceuticals 6 11:00am - 11:20am Challenges and Opportunities for Developing Combinations of Molecularly Targeted Agents Anthony Tolcher , MD South Texas Accelerated Research Therapeutics 11:20am - 11:40am CombiPlex Technology Platform: Application to Molecularly Targeted Agent Combinations Lawrence Mayer, PhD Founder, President, and Chief Scientific Officer 11:40am - 11:50am Question and Answer Session - Technology Platform – Dr. Tolcher and Dr. Mayer - General – Management Team 11:50am - 12:00pm Closing Remarks Scott Jackson Chief Executive Officer
Key Value Drivers • CPX - 351: Late - stage, lead CombiPlex ® - based product • First available data, induction response rate, from a Phase 3 study produced a 43% relative improvement in favor of CPX - 351 over the current standard of care, the 7+3 regimen • Primary endpoint, overall survival, is expected in 1Q2016 • Potential to become the new foundation of care in AML • Orphan Drug Status – US and EU • Fast Track Designation – US • CombiPlex ® - A platform for optimizing development of drug combinations including: • Conventional chemotherapeutics • Novel molecularly targeted agents • Epigenetic modulating agents • Growing proprietary oncology pipeline based on CombiPlex ® Technology • Rapid, broadly - applicable, rational design of drug combinations, optimized in the lab before clinical testing • Financial resources into 2H2016, which deliver key, near - term inflection points, including a NDA submission for CPX - 351 Celator Pharmaceuticals 7

Reaction to Induction Response Rate • Thought - leader feedback has been very positive: • “Impressive CR rate…you beat 7+3 again” • “Response rate is the best surrogate available for clinical benefit” • “Results are favorable…I’ll take it anytime” • “…optimism is justified based on this secondary endpoint, response rate. Nicely done.” • “Exciting results. Congratulations !” • “That is great news! Congratulations!” • “Third time you’ve demonstrated a better response rate.” • Overall survival benefit for CPX - 351 remains likely Celator Pharmaceuticals 8

Exciting Future for Celator Establishing CPX - 351 as a new foundation of care for AML patients • Phase 3 study - going head - to - head against the 7+3 regimen • Preparing for a NDA submission in 3Q2016 • Commercialization activities are underway • Expanding clinical development to demonstrate broader opportunity Technology Platform is transforming the science of combination therapies • Novel combination data packages by the end of 3Q2015 • Pursuing R&D collaborations • Identify a product candidate to advance toward clinical development Celator Pharmaceuticals 9

Track Record of Delivering on Milestones: Since Analyst and Investor Day 2014 10 Celator Pharmaceuticals CPX - 351 Milestone Projected Achieved Phase 3 Study Achieve Target Enrollment 4Q2014 October 2014 DSMB review of first 225 patients randomized 4Q2014 December 2014 Fast Track Designation 1Q2015 January 2015 Induction Response Rate analysis 2Q2015 June 2015 Phase 2 Study PK/PD Study Initiated and Completed Enrollment 2Q2015 June 2015 Investigator - Initiated Studies Expansion of cohort in MDS or AML patients at High - risk of Treatment - Related Mortality Cohort expansion Est. Publication June 2015 2Q2016 Enrollment commences in AML patients at high - risk of treatment - related mortality 3Q2014 Est. Publication July 2015 1Q2017

11 Celator Pharmaceuticals CPX - 351 Milestone Projected Achieved Publications/Presentations Ongoing Acute Lymphoblastic Leukemia (ALL) preclinical data published in Pediatric Blood & Cancer September 2014 Presented at the American Association of Pharmaceutical Scientists October 2014 Presented CPX - 351 preclinical and clinical data at the American Society of Hematology December 2014 Phase 2 first relapse AML data published in Cancer January 2015 Presented CPX - 351 preclinical data at the American Association for Cancer Research April 2015 Track Record of Delivering on Milestones: Since Analyst and Investor Day 2014

12 Celator Pharmaceuticals General Milestone Achieved Financing Raised capital to now fund activities to NDA submission October 2014 Announced NJEDA program resulted in >$1.9M January 2015 LLS amended agreement and increased milestone payment for induction response rate to $900K June 2015 Intellectual Property EU patent allowance on nanoparticle delivery technology December 2015 Track Record of Delivering on Milestones: Since Analyst and Investor Day 2014

Upcoming Milestones • CPX - 351 milestones on path to approval and commercialization: • 1Q2016 - Phase 3 – Overall Survival • 3Q2016 - NDA submission • 1Q2017 - MAA submission • 2Q2017 - FDA Approval • 1Q2018 - EMA Approval • Technology Platform milestones: • 3Q2015 - Data packages on novel combinations 13 Celator Pharmaceuticals

Welcome and Introductions Arthur Louie, MD Chief Medical Officer

CPX - 351: Clinical Development Celator Pharmaceuticals 15 Phase 1 • Safety and Dose - finding • MTD 100 units/m 2 • Myelosuppression > other Aes • Very encouraging activity Phase 2 • Newly Diagnosed AML 60 - 75y “ 204 Study” • Randomized, controlled study • High - risk – strong signal • Safety • First Relapse AML 18 - 65y “ 205 Study” • Randomized, controlled study • Poor - risk – strong signal Phase 3 • Previously Untreated High - risk AML • Randomized, controlled study • DSMB • Independent Hematopathologist • Hierarchical analysis • Based on observations from Phase 2
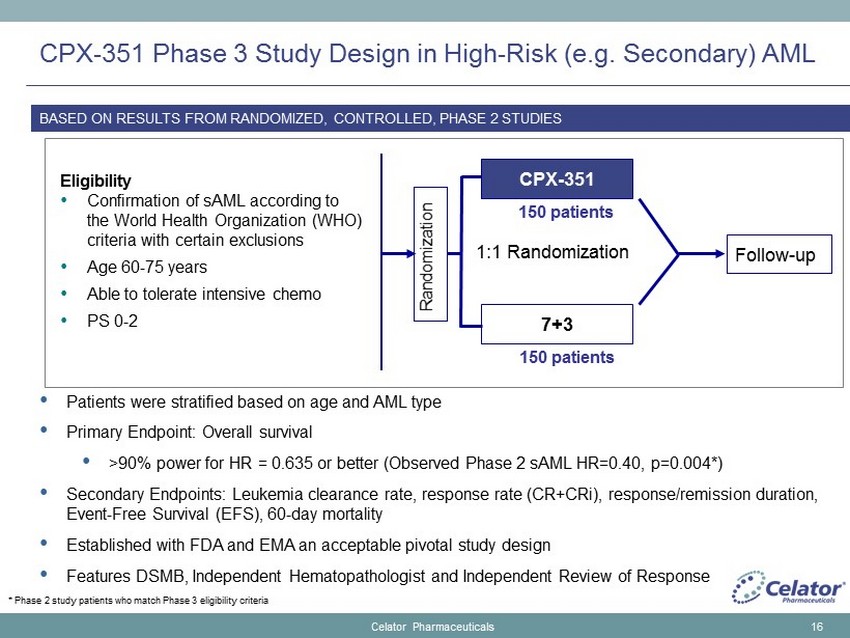
CPX - 351 Phase 3 Study Design in High - Risk (e.g. Secondary) AML • Patients were stratified based on age and AML type • Primary Endpoint: Overall survival • > 90% power for HR = 0.635 or better (Observed Phase 2 sAML HR=0.40, p=0.004*) • Secondary Endpoints: Leukemia clearance rate, response rate (CR+CRi), response/remission duration, Event - Free Survival (EFS), 60 - day mortality • Established with FDA and EMA an acceptable pivotal study design • Features DSMB, Independent Hematopathologist and Independent Review of Response 16 Eligibility • Confirmation of sAML according to the World Health Organization (WHO) criteria with certain exclusions • Age 60 - 75 years • Able to tolerate intensive chemo • PS 0 - 2 Randomization CPX - 351 7+3 1:1 Randomization Follow - up 150 patients 150 patients BASED ON RESULTS FROM RANDOMIZED, CONTROLLED, PHASE 2 STUDIES Celator Pharmaceuticals * Phase 2 study patients who match Phase 3 eligibility criteria

What Patients Are Considered High - Risk for the Phase 3 Study? Celator Pharmaceuticals 17 The Phase 3 study included the following types of patients: • Treatment – related AML • AML with documented history of MDS with prior treatment with hypomethylating agents • AML with documented history of MDS without prior treatment with hypomethylating agents • de novo AML with karyotype characteristic of MDS • AML with documented history of CMMoL

CPX - 351 Phase 3 Study Update Celator Pharmaceuticals 18 Induction Response Rate • Final analysis of Induction Response Rate ( CR+CRi ), a secondary endpoint in the study • B reakout of CR vs. CRi will be available at the end of the study • Discussed with FDA for impact • Prospectively described in the protocol and SAP • Statistical comparison will be available at the end of the study • Conducted after all patients have completed both induction and consolidation treatment • Patients are only being followed for relapse and/or survival • Rigorous independent review of all patient responses by a hematopathologist • Time - defined assessment for both peripheral blood count recovery and transfusion independence

CPX - 351 Phase 3 study is w ell b alanced b ased on patient d emographics and AML t ype Celator Pharmaceuticals 19 CPX - 351 n=153 (%) 7+3 n=156 (%) Total n=309 (%) Gender Male 94 (60.3) 96 (61.5) 190 (61.5) Race Caucasian 128 (83.7) 139 (89.1) 267 (86.4) Age Group ≥70 years 57 (37.3) 54 (34.6) 91 (29.4) median 68 68 68 ECOG 0 - 1 138 (90.2) 132 (84.6) 189 (61.2) 2 15 (9.8) 22 (14.1) 37 (12.0) missing 0 (0) 2 (1.3) 2 (0.6) Strata: CPX - 351 n=153 (%) 7+3 n=156 (%) Total n=309 (%) Therapy - related 30 (19.6) 33 (21.2) 63 (20.4) MDS with Prior HMA 51 (33.3) 55 (35.6) 106 (34.3) MDS without Prior HMA 21 (13.7) 20 (12.8) 41 (13.3) CMML 11 (7.2) 11 (7.1) 22 (7.1) De novo with MDS Karyotype 40 (26.1) 37 (23.7) 77 (24.9)

Third randomized, controlled trial to show a response improvement for CPX - 351 over a cytarabine & anthracycline - based control Celator Pharmaceuticals 20 • Impressive 43% relative improvement in induction response rate • Response is the best available surrogate for overall survival and clinical benefit

Response Correlation with Overall Survival Celator Pharmaceuticals 21 • Response is the best available surrogate for overall survival and clinical benefit • It is not the only factor as overall survival is impacted by early mortality, duration of response, etc. • Walter et al (2010) - MD Anderson Cancer Center database of >2,200 AML patients showed statistically significant difference in OS for those achieving induction response (CR > CRp ) vs . those who did not (p≤0.001)

Response Correlates with Overall Survival: CPX - 351 Phase 2 Study in Newly Diagnosed AML Patients Celator Pharmaceuticals 22 Lancet et al, ASH 2012 Overall Survival Variable n/N (%) HR (95% CI) P - value Univariate Previous AHD 49/126 (39%) 1.65 (1.01, 2.70) 0.044 High overall risk 84/122 (69%) 1.98 (1.11, 3.53) 0.022 Secondary AML 52/126 (41%) 1.71 (1.05, 2.79) 0.031 ≥2 Risk Factors 41/118 (35%) 1.86 (1.12, 3.10) 0.017 Adverse Cytogenetic Risk 36/117 (31%) 1.85 (1.10, 3.11) 0.019 Response 0.51 (0.30, 0.86) 0.012 Multivariate ≥2 Risk Factors 40/117 (34%) 1.94 (1.15, 3.25) 0.013 Response 0.47 (0.27, 0.82) 0.008

Response Assessment Used in Phase 2 Compared to Phase 3 Phase 2 Principal Investigator Based on Cheson , et. al. 2003 Phase 3 Independent Reviewer Based on Dohner , et al. 2010 Bone Marrow Assessment • <5% blasts • Absence of Auer rods Same Peripheral blood • ANC ≥1000/mL • Platelets ≥100,000/mL • Variable / Subjective time frame Peripheral blood • ANC ≥1000/mL (same) • Platelets ≥100,000/mL (same) • 14 - day window around biopsy Transfusions • Variable / Subjective timing • “ independent of transfusions” Transfusions • 14 day transfusion free period from 7 days before biopsy to 21 days after biopsy • “i ndependence from red cell transfusions” Celator Pharmaceuticals 23

Applying Phase 3 Criteria to Phase 2 Population *Phase 2 patient response using the RBC transfusion rules and the 14 - day window requirement for peripheral blood counts. Celator Pharmaceuticals 24 CPX - 351 n=39 7+3 n=19 Relative Improvement Phase 2 criteria Remission Rate (CR+CRi) 73.7% 42.1% 75.1% Phase 3 criteria* Remission Rate ( CR+CRi ) 63.2% 42.1% 50.0% Phase 2 Population matched for Phase 3 Overall Survival HR=0.40 P value= 0.004 • Phase 2 data was not reviewed by an independent hematopathologist which may preclude the declaration of a CR or CRi in patients. • Applying the time period criteria, the results were:

Summary • Terrific progress in Phase 3 Study • Impressive 43% relative improvement in Induction Response Rate • Overall survival, the primary endpoint of the study, is expected in 1Q2016 • Other required studies for NDA are on track • NDA projected in 3Q2016 • Broad development incorporating Investigator - Initiated Studies, Cooperative Group Studies and Company Sponsored Studies to support CPX - 351 utilization Celator Pharmaceuticals 25

Clinical Development Strategy is Designed to Explore Important Areas of Unmet Need in AML and other Hematologic Malignancies Celator Pharmaceuticals 26 RESEARCH PRECLINICAL PHASE 1 PHASE 2 PHASE 3 APPROVAL High Risk (e.g. secondary) Acute Myeloid Leukemia age 60 and ≤75 years (enrolled) Registration Study Randomized, Controlled Study Pre - conditioning prior to Cord Blood HSCT (Ongoing) Unfit AML due to co - morbidities ( Ongoing - Expanded) HR - MDS and AML after HMA therapy (Ongoing) Unfit AML due to poor risk (Ongoing) Pediatric Pilot (Ongoing) Investigator - initiated Studies Planned Sponsored Studies Estimated Data 2Q16 2Q16 3Q16 1Q17 Lead into COG Induction failure AML patients age ≥18 years ( Planned ) High - risk AML patients age <60 years (Planned) Elderly AML (≥65 years) (Planned) 2Q17 Cooperative Group Studies Children with Relapsed AML ( Planned ) Newly Diagnosed AML or high - risk MDS Aged 18 - 60 ( Planned ) 2Q21 4Q17

Gail J. Roboz, M.D. Director, Leukemia Program Professor of Medicine AML Therapy: A GIANT Unmet Need

Acute Myeloid Leukemia • Most common acute leukemia in adults • 2015 ≈ 20,800 cases with 10,460 deaths • Median age at diagnosis ≈ 70 • Associated with chemo +/ - radiation exposure, antecedent hematologic disorders, environmental factors • Dangerous and difficult - to - treat disease, progress is lagging behind other hematologic malignancies, most young patients not cured, almost no older patients cured Available at: http://seer.cancer.gov/statfacts/html/amyl.html. Accessed July 5, 2015. Yamamoto JF, et al. Cancer Causes Control. 2008;19:379 - 390; Juliusson G, et al. Blood . 2009;113:4179 - 4187.

What Happens in AML? • Bone marrow is the factory that makes blood cells • You need a working bone marrow to live • Abnormal white blood cells (blasts) take over marrow • May have no symptoms, abnormal blood counts picked up on screening • More commonly signs/symptoms of bone marrow failure: infection, bleeding, shortness of breath, fatigue • Patients often extremely sick, many organs affected • Need transfusions, antibiotics, intensive supportive care, potential for life - threatening complications • Prolonged and multiple hospitalizations common

Treatment Options for Older Patients with AML • Intensive chemotherapy ( “7+3”) • Low - intensity chemotherapy (low - dose cytarabine, decitabine, azacitidine) • Clinical trials (the best option) • Supportive care • No treatment/hospice

CR, complete response. Kantarjian et al, 2006. AML in Older Adults: Prognostic Model Prognostic Factor CR Rate 8 - wk Mortality 1 - y Survival Age ≥75 y ■ ■ ■ Poor performance status ■ ■ ■ Unfavorable karyotype ■ ■ ■ Anemia ■ Leukocytosis ■ Antecedent hematologic disease ■ ■ ■ Creatinine >1.3 mg/dL ■ ■ ■ Elevated lactate dehydrogenase ■ Treated in laminar flow room ■ ■ ■

Secondary AML • Therapy related AML: history of prior cytotoxic therapy or ionizing radiotherapy for an unrelated disease • AML with a history of myelodysplasia or CMMoL • Different disease biology (characteristic molecular signature) • Associated with unfavorable prognosis and needs allo transplant for long - term survival

What does it mean to be “ fit ” for chemo?

Fit for chemo • 75 y/o math professor

? Fit for chemo • 75 y/o homeless man

Separated by 5 minutes

AML has a dismal prognosis in patients >60 years. (All lectures, manuscripts, grants and FDA submissions involving elderly AML begin and/or end with this statement.)
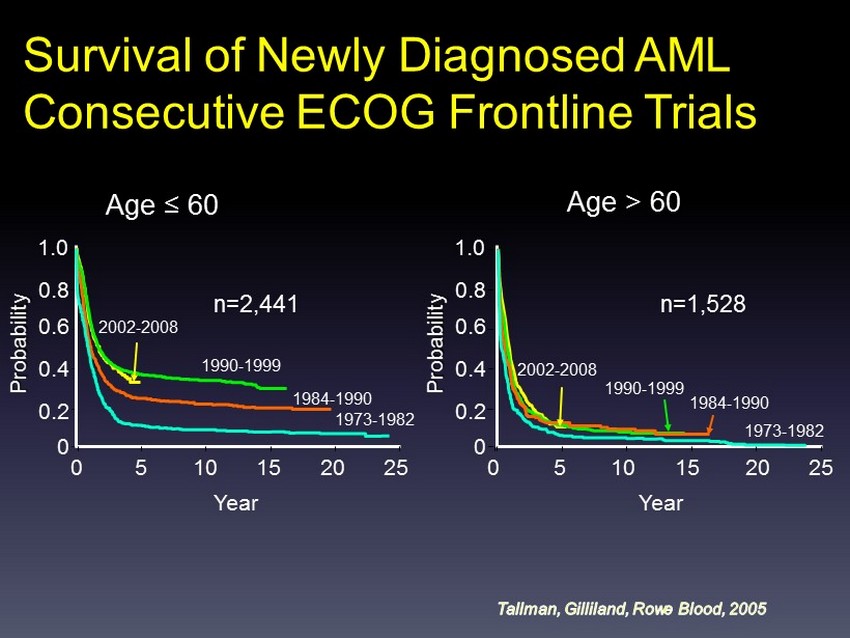
Age ≤ 60 1.0 Year Probability 25 1973 - 1982 1984 - 1990 1990 - 1999 2002 - 2008 n=2,441 0.8 0.6 0.4 0.2 0 20 15 10 5 0 Age > 60 n=1,528 1.0 Probability 0.8 0.6 0.4 0.2 0 0 Year 25 20 15 10 5 1973 - 1982 1984 - 1990 1990 - 1999 2002 - 2008 Survival of Newly Diagnosed AML Consecutive ECOG Frontline Trials

1.0 0.8 0.6 0.4 0.2 0.0 0 1 2 3 4 5 6 7 8 Proportion Surviving Years MDACC: AML Survival by Decade Patients > 60 ERA N Deaths 1960s 39 39 1970s 166 164 1980s 263 259 1990s 495 461 2000s 486 350 Kantarjian H, O’Brien S . Cancer . 2010;116(21):4896 - 901.

Overall Survival of AML Patients on MRC Trials: 1970 to 2004 Burnett, 2012. N = 3,541 N = 7,704 Age 15 - 59 y Age 60+ y

Overall Survival: WCMC N=298 patients, 253 deaths Median OS = 6.5 months (95% CI = 5.1, 8.0)

Survival of Older AML Patients by Therapy NA, not applicable. Menzin et al, 2006. Median survival ( mo ) 1 - y survival (%) Overall 4.4 NA Chemotherapy 6.1 ; 30 No therapy 1.7 ; 1 0 o 3,317 elderly patients with AML aged ≥65 years o 1,193 (36%) received chemotherapy (younger, fewer comorbidities) o 888 patients matched in both cohorts

YATES et al: 1973 (!!!!) Pilot of infusional cytarabine and daunorubicin “7&3 DNR45”

Treatment of acute myelocytic leukemia: a study by Cancer and Leukemia Group B, Rai et al., Blood 1981 • N = 352, 27 centers, 13 months (1974 - 75): 47 pts < 20 yrs, 105 >60 yrs • Daunorubicin 45 mg/m 2 , ara - C 100 mg/m 2 infusion IVB or CI, 5 or 7 days • 3+7 infusional best regimen • 56% CR Year 0 1 2 3 4 5 6 CR 152 105 67 53 44 36 11 PR 38 21 7 5 1 0 0 NR 162 12 4 2 1 1 0

Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: Cancer and Leukemia Group B Study 9720 Baer et al. Leukemia May 2011; 25: 800

Burnett et al. Cancer , 2007; 109: 1114 - 1124. UK NCRI AML 14 Trial (Non - Intensive) Poor - risk karyotype Response Ara - C (N=102) HU (N=99) Induction Death 26% 26% CR 18% 1% Favorable/intermediate - risk karyotype

Intensive vs. Lower - intensity Rx Intensive i) Myelosuppression ii) Extramedullary toxicity iii) Significant induction mortality iv) Prototype: 7&3 What you get for your trouble: Highest response rates Lower - intensity i) Less myelosuppression ii) Less extramedullary toxicity iii) Less induction mortality iv) Prototype: low - dose ara - C What you get for your trouble: - some CRs, approaching intensive in certain subgroups ?? Survival ?? Quality of life

Response Assessment in AML • Improved complete remission, improved survival • Improved complete remission, no improved survival • No improvement in complete remission, but improved survival

Is there a role for chemotherapy in the era of “targeted” and “personalized” medicine?
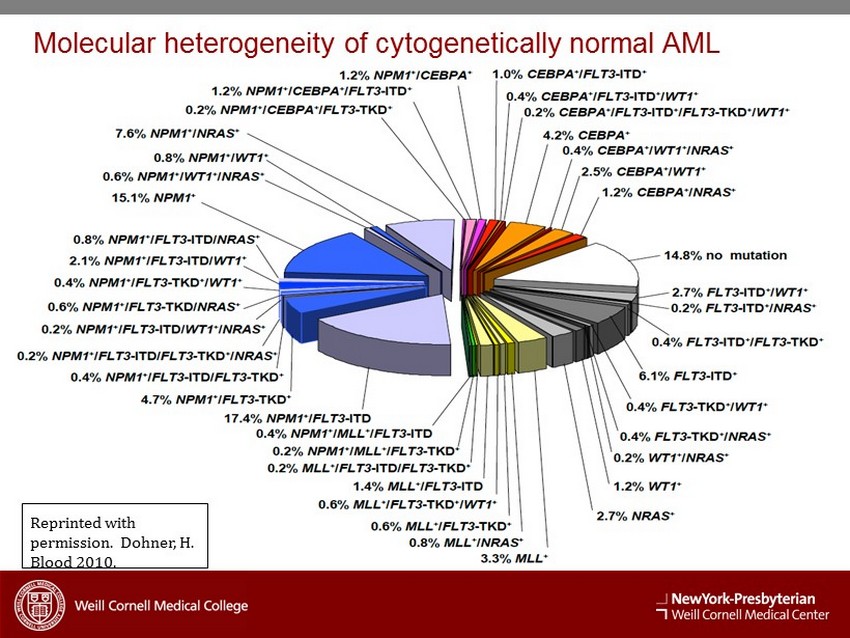
Reprinted with permission. Dohner, H. Blood 2010. Molecular heterogeneity of cytogenetically normal AML
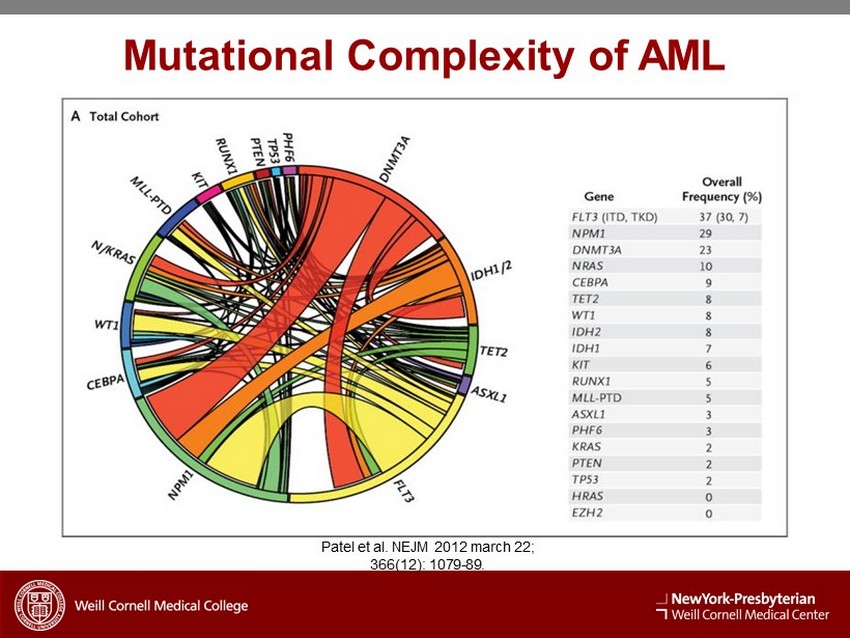
Mutational Complexity of AML Patel et al. NEJM 2012 march 22; 366(12): 1079 - 89.

L S D 1 ? ? ? ? I D H 1 T A R G E T P R A C T I C E

D EVELOPING CPX - 351 BEYOND THE INITIAL INDICATION Bruno Medeiros, MD New York, NY July 16, 2015
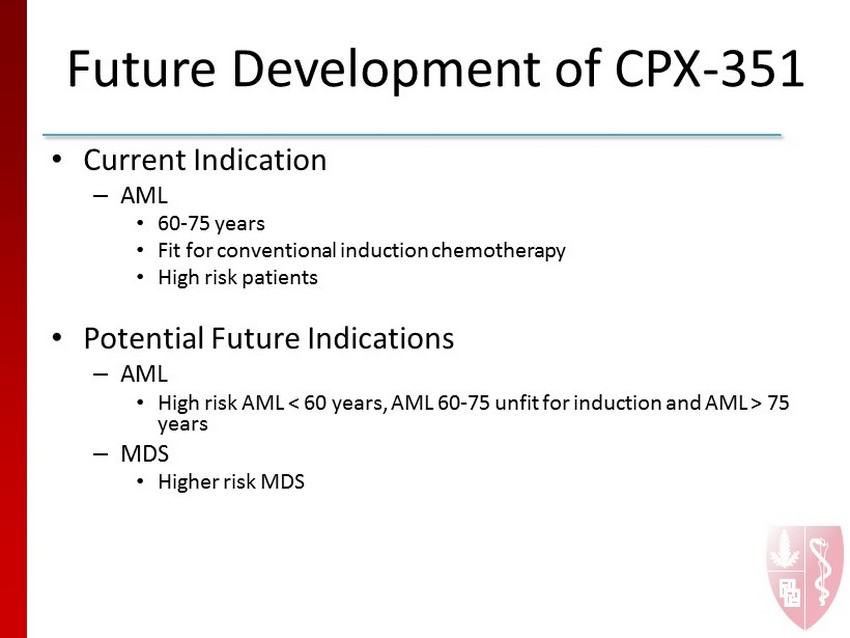
Future Development of CPX - 351 • Current Indication – AML • 60 - 75 years • Fit for conventional induction chemotherapy • High risk patients • Potential Future Indications – AML • High risk AML < 60 years, AML 60 - 75 unfit for induction and AML > 75 years – MDS • Higher risk MDS

High Risk AML < 60 years – Why? Multifactorial MDR Activity in AML • Delivery – Liposomal encapsulation • Molar ratio – 5:1 cytarabine / dauno • Distribution – Long half life in BM environment

High Risk AML < 60 years – Volume? Swedish Acute Leukemia Database Danish Acute Leukemia Database 2000 - 2013 – AML < 60 years – Secondary AML – 17% – Adverse Karyotype – 15% Gunnar J et al. Blood 2012;119:3890 - 3899 Secondary AML – 22% Adverse Karyotype - ?? Østgård L, Medeiros BC et al. JCO, 2015. Accepted for publication

AML Treatment Algorithm
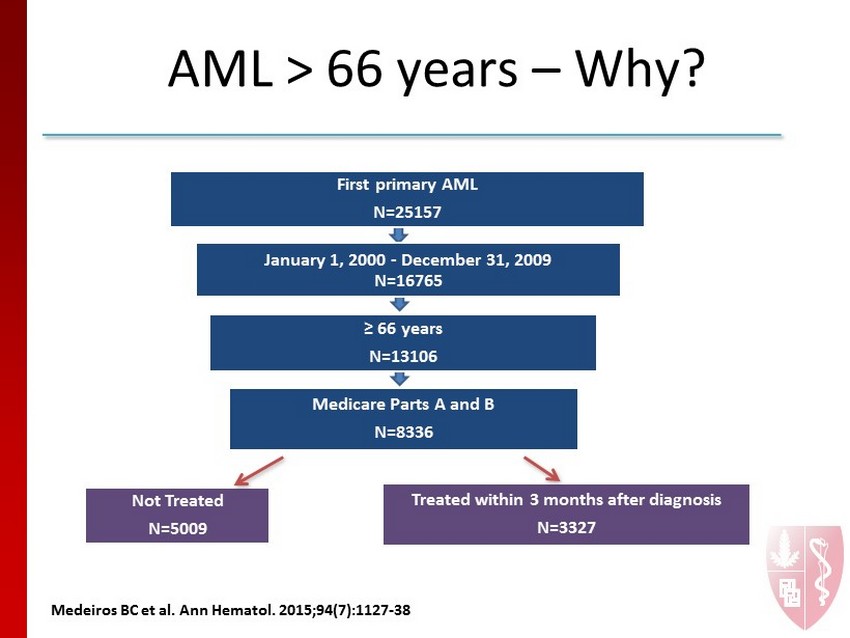
First primary AML N=25157 January 1, 2000 - December 31, 2009 N=16765 ≥ 66 years N=13106 Medicare Parts A and B N=8336 Treated within 3 months after diagnosis N=3327 Not Treated N=5009 AML > 66 years – Why? M e deiros BC et al. Ann Hematol. 2015;94 (7):1127 - 38

AML > 66 years – Why? 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 2000 (N=868) 2001 (N=845) 2002 (N=959) 2003 (N=880) 2004 (N=848) 2005 (N=824) 2006 (N= 827) 2007 (N= 833) 2008 (N= 820) 2009 (N= 732) Proportion of Patients Year of Diagnosis Not Treated Treated M e deiros BC et al. Ann Hematol. 2015;94 (7):1127 - 38
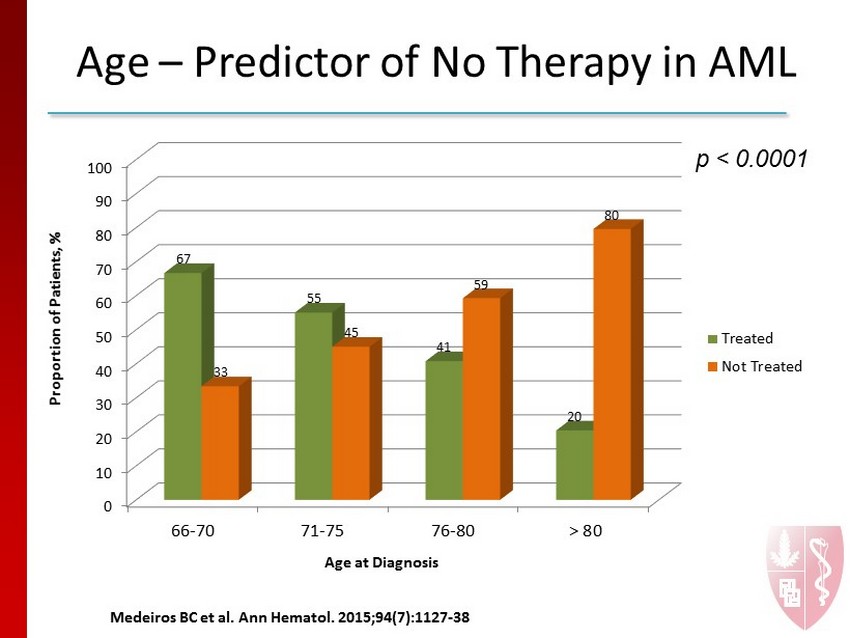
Age – Predictor of No Therapy in AML p < 0.0001 0 10 20 30 40 50 60 70 80 90 100 66-70 71-75 76-80 > 80 67 55 41 20 33 45 59 80 Proportion of Patients, % Age at Diagnosis Treated Not Treated M e deiros BC et al. Ann Hematol. 2015;94 (7):1127 - 38
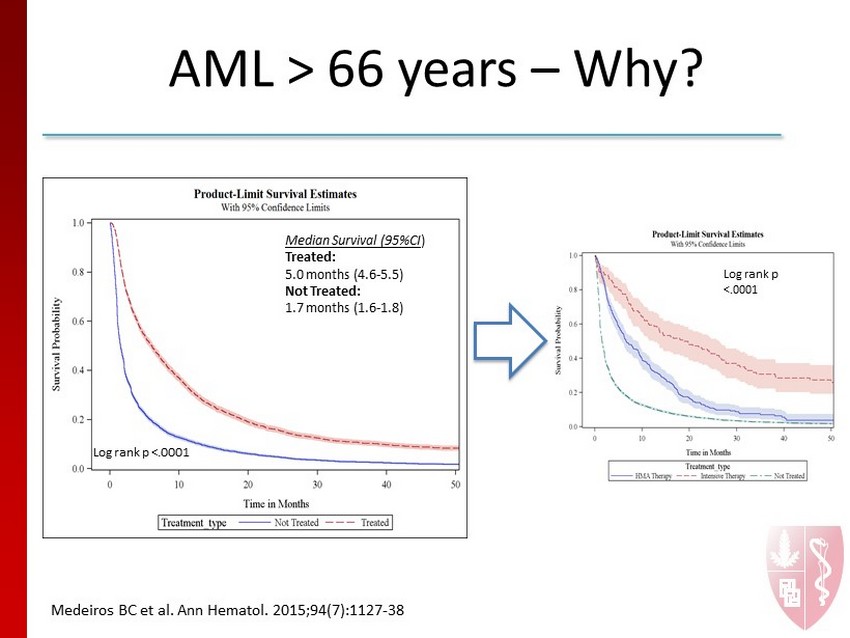
AML > 66 years – Why? Log rank p <.0001 Median Survival (95%CI ) Treated: 5.0 months (4.6 - 5.5) Not Treated: 1.7 months (1.6 - 1.8) M e deiros BC et al. Ann Hematol. 2015;94 (7):1127 - 38 Log rank p <.0001

AML Therapy for Unfit Patients J Clin Oncol 2012; 30:2670 - 2677 Decitabine

AML Therapy for Unfit Patients Dombret H et al. EHA, 2014

Myelodysplastic Syndromes

Azacitidine in Higher Risk MDS 0 5 10 15 20 25 30 35 40 Months From Randomization 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Proportion Surviving CCR VIDAZA Log - rank P =0.0001 HR=0.58 (95% CI: 0.43 - 0.77) 24.5 months 15 months

HMA Failures Thomas Prébet et al. JCO 2011;29:3322 - 3327
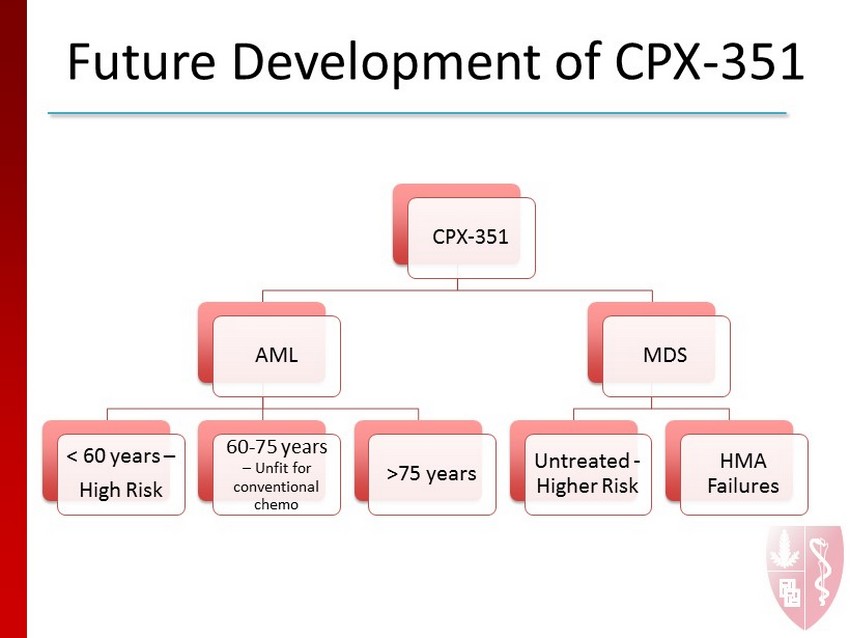
Future Development of CPX - 351 CPX - 351 AML < 60 years – High Risk 60 - 75 years – Unfit for conventional chemo >75 years MDS Untreated - Higher Risk HMA Failures

Potential CPX - 351 Combinations – Flt3 inhibitors • FLT3 - ITD positive patients – In combination with hypomethylating agents • DNMT3a mutations? • All patients – IDH1 or IDH2 inhibitors – In combination with conjugated MAB • Gemtuzumab • SGN - 33a

Fireside Chat Participants: Dr. Gail Roboz , Dr. Bruno Medeiros & Dr. Arthur Louie Moderator: Derek Miller

Paving the Way to CPX - 351 Commercialization Derek Miller Chief Business Officer

CPX - 351 is Well - Positioned to become the Foundation of Care in AML, which provides an Attractive Commercial Opportunity • No significant improvements to 7+3 in more than 40 years • OS improvement can address large unmet need for AML Patients • Compelling Product Profile (OS, HECON) can Deliver Meaningful Value Proposition to key Stakeholders • Focused Market: Nearly half of top 50 AML centers involved in Phase 3 • Lean Commercial Infrastructure (~30 Reps) • On - going Clinical Development Plan can lead to Substantial global product opportunity beyond primary indication 71 Celator Pharmaceuticals

0 10 20 30 40 50 60 70 80 90 0% 20% 40% 60% 80% 100% Source: SEER 18 areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose - Monterey , Los Angeles, Alaska Native Registry, Rural Georgia, California excluding SF/SJM/LA, Kentucky, Louisiana, New Jersey and Georgia excluding ATL/RG). Median age and incidence counts include c ase s diagnosed in 2006 - 2010. Relative survival rates include cases diagnosed in 2003 - 2009. Annual US incidence for all heme malignancies ~ 160,000 cases; ~ 50,000 deaths per year; > 1 million survivors in US. 5 - Year Relative Survival Rate CLL CML Median Age at Diagnosis AML MDS Courtesy of, and adapted from, the Leukemia and Lymphoma Society ® Significant Unmet Need in AML: Highest Incidence, Lowest 5 - year Survival of a ll Leukemias and MDS A LL U.S. incidence and mortality in 2015: • 20,830 new AML cases • 10,460 deaths from AML Patients who can tolerate intensive chemotherapy receive the 7+3 regimen which is cytarabine plus an anthracycline (i.e. daunorubicin) • The 7+3 regimen has not been displaced after more than 40 years Celator Pharmaceuticals 72

AML Induction Therapy Consistent Across Risk Categories with Exception of Unfit Patient Population 73 Celator Pharmaceuticals Source: Results from Hematologist/Oncologist interviews conducted in US and EU (May/June 2014). Induction Therapy Used by Region 100% 100% 100% 100% 20% 20% 6% 11% 18% 27% 29% 13% 47% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% US Europe US Europe US Europe % Frequency of Mentions 7+3 Decitabine Azacitidine LD Ara-C Palliative Care Unfit for intensive Tx Poor/ Int Risk Good Risk ~28,000 Patients ~ 15,000 Patients ~9,000 Patients

AML Patients Initially Considered CPX - 351 - Eligible based on Risk and “Fitness” 74 Celator Pharmaceuticals 60% >60y 40% <60y ~75% Intermediate/High Risk 50% >60y 85% <60y Age “FIT” for Intensive Tx Age based on SEER Incidence for AML. Risk and Fitness based on primary market research CPX - eligible

Current and Planned Clinical Activities are Designed to Address Intermediate and High Risk Populations with Greatest Unmet Needs 7800 8100 3700 3400 15000 5700 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 >60 1st Line Fit <60 1st Line Fit Relapsed AML Refractory AML Unfit AML High Risk MDS Celator Pharmaceuticals 75 Estimated Patients in US, EU, and JPN >43,000* Ph 3 CPXX IIS/Co - op Ongoing/Planned Studies

Global Market Opportunities are Sufficient to Support Life Cycle Plans 76 Celator Pharmaceuticals AML 1L, Int/HR, >60 "Fit" , $450 AML 1L, Int/HR, <60 "Fit" , $300 AML Relapse , $300 AML Refractory , $200 AML 1L all risk, >60 "Unfit" , $400 AML 1L all risk, <60 "Unit" , $100 HR MDS (HMA failure) , $300 Estimated Market Potential ($Ms) CPX - 351 1 st Indication: $200 - 250M

CPX - 351 Potential Positioning* • For Acute Myeloid Leukemia patients who are deemed appropriate to receive intensive myeloablative therapy, CPX - 351 is the foundational treatment that prolongs survival by improving response and reducing treatment - related mortality 77 Celator Pharmaceuticals * For Planning Purposes only and pending FDA approval

CPX - 351 is Poised to Deliver Meaningful Value Proposition to All Key Stakeholders • Prolonged overall survival • Significant reduction in 60 - day early mortality • Higher remission rates could result in more transplant candidates • More convenient and less resource - intensive dosing schedule • 3 x 90 minute infusions vs 7 - day continuous infusion coupled with an IV push on days 1 - 3 during induction • Fixed, co - formulation of 2 drugs eliminates need to mix or identify optimal ratio • Alopecia rarely observed* • Potential for outpatient treatment in consolidation phase* Celator Pharmaceuticals 78 * As reported by investigators anecdotally Patients HCPs Health Systems Payers
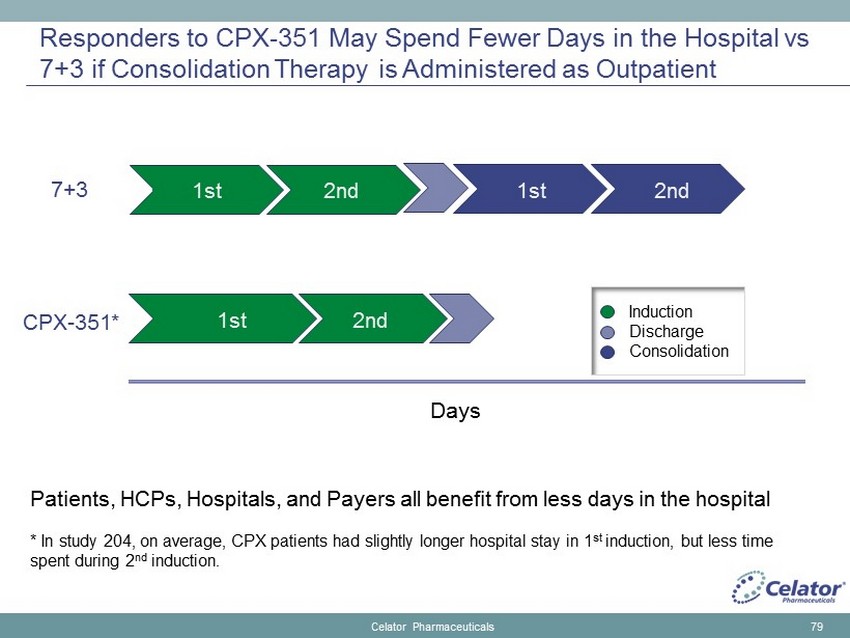
Responders to CPX - 351 May Spend Fewer Days in the Hospital vs 7+3 if Consolidation Therapy is Administered as Outpatient Celator Pharmaceuticals 79 7+3 CPX - 351* Patients, HCPs, Hospitals, and Payers all benefit from less days in the hospital * In study 204, on average, CPX patients had slightly longer hospital stay in 1 st induction, but less time spent during 2 nd induction. 1st 1st 2nd 1st 2nd Days 2nd Induction Discharge Consolidation

US Market Can be Served Effectively and Efficiently with Lean Infrastructure 80 Celator Pharmaceuticals ~ 30 Sales Reps and Managers 50 Centers Performed >70% of AML SCTs States with centers performing >20 AML SCTs in 2014

Important Investments Have Been Made to Prepare for Commercialization x KOL and Investigator Engagement • International Advisory Board and ongoing investigator dialogue x Core Scientific Messages x Publication Planning x Developed and Tested Target Product Profile • US and EU (West, Central, East) x Initial pricing and reimbursement research • US and EU x Branding • Name and logo generation, testing, legal search, and filing □ Landscape Assessment - INITIATED □ Value Proposition - INITIATED □ Pricing/Access/Reimbursement Research – pending completion of initiated efforts Celator Pharmaceuticals 81

Critical Success Factors to Optimize Launch of CPX - 351 • Expand Global Awareness and Experience through Clinical Trials and HCP interactions • Identify Strategic Commercialization Partner • Establish Compelling Value Proposition and Key Supporting Messages • Statistical improvement in Overall Survival and other key endpoints • Develop Support for Effective Coverage and Reimbursement • Refine Pricing and Contracting Strategy • Develop Access Strategy, including Channel and Distribution Plan • Continue Partnership with the Leukemia and Lymphoma Society • Attract High C aliber T alent to Lead various aspects of the Launch Celator Pharmaceuticals 82

Throughout 2015 - 2016 Celator w ill Transform from a Late - Stage Development Company to a Development and Commercial Company Commercial Partnership Discussions Phase 3 Induction Remission Analysis 2015 2016 Commercial Launch Prep and Positioning CPX - 351 Begin building Commercial Infrastrucure Phase 3 Overall Survival NDA Submission 2015 2016 2 Q 3Q 4Q 1Q 2Q 3Q 4Q Establish R&D Collaborations for Technology Platforms First Corporate Presence at ASH 2Q17 US Launch 83 Celator Pharmaceuticals

CPX - 351 is Well - Positioned to become the Foundation of Care in AML, which provides an Attractive Commercial Opportunity • No significant improvements to 7+3 in more than 40 years • OS improvement can address large unmet need for AML Patients • Compelling Product Profile (OS, HECON) can Deliver Meaningful Value Proposition to key Stakeholders • Focused Market: Nearly half of top 50 AML centers involved in Ph 3 • Lean Commercial Infrastructure (~30 Reps) • On - going Clinical Development Plan can lead to Substantial global product opportunity beyond primary indication 84 Celator Pharmaceuticals

vyxeos cytarabine and duanorubicin liposome for injection celator pharmaceauticals 85

Developing A New Drug or A New Therapy: There is a Difference Anthony W. Tolcher MD FRCPC FACP Director of Clinical Research START San Antonio

Magnitude of Benefit For single agents, improvement in patient outcomes meeting FDA criteria for approval: • Are often modest survival improvements, PFS, or response • Are rarely, if ever Complete Response or cure Yet, for most patients with cancer, meaningful improvements are likely to come with improved therapies — combinations of active agents

Curative Therapy with Came with the Advent of Cytotoxic Combinations Hodgkin’s Disease • Individually many chemotherapy agents shrunk disease but not curative until MOPP & ABVD Non - Hodgkin’s Lymphoma • CHOP and then R - RCHOP Testicular Cancer • BEP and EP

Combinations with Molecularly Targeted Agents Have Proven Difficult • Sorafenib , Sunitinib , Pazopanib combinations; very difficult to develop – Too toxic to GI, skin – Dose reductions to very low doses of each agent • mTOR inhibitors – Only combined with endocrine therapy – Too toxic, GI, Skin, metabolic

High Value Combinations • High propensity for BRAF, KRAS mutations existing with PIK3Ca, AKT, PTEN mutations • Resistance to BRAFi / MEKi pre - clinically and clinically for PTEN/AKT/PIK3Ca mutations • Preclinical synergy for combining MEKi to mTOR , PI3Ki, AKTi agents


Unable to Deliver Combinations at Full Doses Toxicity • Stomatitis • skin Activity • HRAS ACC • Few others Dose Reductions Trametinib & GSK 2141795 Toxicity • Skin (Rash) • GI (NVDS) Activity • Melanoma • Breast Dose Reductions Trametinib & Everolimus Tolcher et al CCR 2015 Kurzrock et al ASCO 2011 Toxicity • Skin (Rash) • GI (NVDS) Activity • KRAS NSCLC • Others Dose Reductions AZD 6244 & MK2206 Tolcher et al ASCO 2011

Toxicity • Skin (Rash) • GI (NVDS) Activity • KRAS NSCLC • Others Trametinib Afursetinib Tolcher et al 2015 Can Chemo Pharm Toxicity • Skin (Rash) • GI (NVDS) Activity • Endometrial • Ovary • Breast Cobimetinib & Ipatasertib Bendell et al AACR 2014 Unable to Deliver Combinations at Full Doses


Despite Synergy In Preclinical Models • Synergistic normal tissue toxicity also occurs • Cannot give both drugs at, or near full doses • Antitumor activity has been modest even with biomarker - driven patient selection

To Combine the Two Drugs We Reduce Doses… Do We Compromise Each Components Drug Dose & Inhibition of Molecular Target Effectiveness?

Is Sub - Maximal I nhibition of MEK+AKT Better than Maximal I nhibition of E ither MEK or AKT Inhibitors Alone? Mutations 50 MEK + 50 AKT Vs 100% MEK (number of cell lines) 75 MEK + 75 AKT Vs 100% MEK (number of cell lines) 50 MEK + 50 AKT Vs 100% AKT (number of cell lines) 75 MEK + 75 AKT Vs 100% AKT (number of cell lines) BRAF M 0/5 0/5 4/5 4/5 PIK3CA M 1/5 4/5 0/5 0/5 KRAS M 1/5 2/5 1/5 2/5 BRAF WT / PIK3CA WT / KRAS WT 1/5 2/5 0/5 0/5 Udai Banerji et al: TAT 2014

Is Sub - Maximal I nhibition of MEK+AKT Better than Maximal I nhibition of E ither MEK or AKT Inhibitors Alone? Conclusions In BRAF mutant cell lines, reducing dose of MEK inhibitor to add AKT is inferior to full dose MEK If PIK3CA mutant, reducing dose of AKT inhibitor, to add MEK is inferior to full dose AKT Udai Banerji et al: TAT 2014 These results suggest that optimizing the degree of combination target inhibition (drug ratio) is important to obtain the best efficacy outcome

Many More Challenges to Come CDK4/6 inhibitors (e.g. Palbociclib ) • Tolerable with endocrine therapy but diarrhea and neutropenia mean combinations will be difficult FGFR inhibitors (many) • GI, liver function, and phosphorous alterations BET Inhibitors (many)

If we compromise the dose(s), we compromise the benefit T herapeutic index of combinations is low: • Prevents full dose of both agents • Diminishes therapeutic benefit, leading to increased risk of clinical trial failure • How can we ensure coordinated inhibition of combined targets without causing toxicities that block the full therapeutic potential?

How Do We Improve Therapeutic Index? Combination of non - cross - toxic agents • Basis of cytotoxic combinations, e.g vincristine with alkylator Preferential Delivery to tumor vs normal tissue • Basis of Antibody Drug Conjugate platform; utility for coordinated combination therapies unknown • Celator’s CombiPlex technology is well poised to address many of the problems experienced to date

Conclusions • Developing combinations of potentially synergistic combinations must continue to achieve clinically meaningful therapies • If synergistic toxicities occur, we must explore new delivery systems • Preferential delivery to tumor over normal tissue is necessary for many of the high value combinations that are currently unfeasible and require coordinated tumor drug exposure

CombiPlex Technology Platform Application to Molecularly Targeted Agent Combinations

Celator Pharmaceuticals Rationale for CombiPlex ® in the Development of Targeted Agent Combinations • Single molecularly targeted agents often have modest or transient antitumor activity • Individual targets rarely dictate tumor cell survival independently • Inter - pathway communication and feedback loops • Many combinations will require simultaneous target inhibition and drug ratio control due to drug ratio - dependent synergy • Need for coordinated PK and tumor cell exposure • Conventional dosing formats are unable to coordinate exposure • Must saturate the body with both agents in order to inhibit both targets and this often causes excessive toxicity • CombiPlex is uniquely positioned to address these challenges 103

Traditional Approach Fails to Optimize Combination Regimens Many drug combinations require simultaneous exposure, however: • Combining drugs at ratios based on MTD may be antagonistic and/or more toxic • Even if a synergistic ratio is identified , it will be lost without a delivery vehicle due to PK/PD differences • Optimizing combination regimens in the clinic is very time consuming and costly Celator Pharmaceuticals 104

Celator Pharmaceuticals Unwanted Exposure to Healthy Tissue and Changing Drug Ratios are Typical for Conventional Combinations • Significant drug exposure to healthy tissues leads to toxicity problems • Drug elimination from body occurs rapidly after administration stops and varies between drugs • Very difficult to coordinate timing and ratio of drug combination exposure to tumors Treatment Hours after Administration [ 14 C]Cytarabine 1 24 Non-Liposomal Treatment Hours after Administration [ 14 C]Daunorubicin 1 48 Non-Liposomal Free Cytarabine Free Daunorubicin ASH 2014 Abst . 3740

The CombiPlex ® Solution: True Combination Products Celator Pharmaceuticals 106 • Identify molar synergy in vitro • Lock optimal ratio in a delivery complex • Nano - scale liposomes and nanoparticles engineered to: • Stably encapsulate drug ratios • Harmonize PK through controlled release to maintain optimal ratio in vivo • Provide elevated, prolonged and selective exposure of synergistic drug ratios to tumor cells

Celator Pharmaceuticals CombiPlex ® Solves the Coordinated Exposure Problem for Drug Combinations • Markedly reduced drug exposure to healthy tissues reduces toxicity problems and increases exposure to tumor tissue • Drug elimination occurs slowly and at the same rate for both drugs • CombiPlex gets the right ratio of drugs to the right site for the right amount of time Treatment Hours after Administration [ 14 C]Cytarabine 1 24 CPX-351 Treatment Hours after Administration [ 14 C]Daunorubicin 1 48 CPX-351 CPX - 351 Cytarabine CPX - 351 Daunorubicin ASH 2014 Abst . 3740

Celator Pharmaceuticals 108 Molecularly Targeted Agent Combinations Selected for CombiPlex ® Application Combinations of epigenetic modulators • Histone deacetylase inhibitors ( vorinostat ); plus • Hypomethylating agents (e.g. decitabine ) Combinations targeting major signaling pathways • Inhibitors of PI3K/AKT/ mTOR pathway (e.g. Ipatasertib , Genentech); plus • Inhibitors of Ras / Raf /MEK/ERK pathway (e.g. Selumetinib , Astrazeneca ) Combinations of existing chemotherapeutics with molecularly targeted potentiating agents • Widely utilized and active cytotoxics (e.g. docetaxel ); plus • Modulators that impact apoptosis (e.g. HSP90i AUY922 , Vernalis )

Celator Pharmaceuticals Nanoparticle - Based CombiPlex ® Systems: Hydrophobic Prodrug Nanoparticles (HPN) • Used to control the PK of hydrophobic drugs; most targeted agents are hydrophobic • Anchor and linker chemistry of prodrugs controls release of parent drug • Iterative process of structure - performance evaluations via preclinical PK testing • Goal: Maintain encapsulated drug ratio for up to 24h after in vivo administration Cleavable Links Cross - linker Drug Hydrophobic Anchor Prodrug 1 Prodrug 2 Cross - linker Drug Hydrophobic Anchor Prodrug 1 Prodrug 2 Stabilizer Co - lipid 109

HPN Formulation Development Flowchart Excipient and Solvent Evaluation • Selection of well - characterized diblock polymers such as PEG - PLA, PEG - PCL, PEG - PS, PEG - PLGA for different degrees of hydrophobicity and crystallinity • Optimization of copolymer block lengths to achieve good drug loading • Selection of compatible organic solvents for the drugs and excipients • Optimization of organic to aqueous solvent ratios and rates of mixing Physical and Chemical Stability Assessment • Incorporation of stabilizing agents such as Pluronics ®, polysorbates , PVAs, and phosphatidylcholines to prevent aggregation • Stability evaluation on the size and drug contents of nanoparticles at different storage conditions Optimization of drug to drug and drug to polymer ratios • Optimization of drug to drug and drug to polymer ratios based on the results of physical and chemical stability and pharmacokinetic performance Celator Pharmaceuticals 110 Drug Anchor and cross - linker optimization Stability re - evaluation # HPN formulations generated: 80 66 # HPN formulations evaluated for PK: 19 36 Docetaxel/AUY922 Selumetinib / Ipatasertib

Issues CombiPlex ® Optimizing Combinations of Molecularly Targeted Agents: Docetaxel + HSP90 inhibitor (AUY922) 111 Docetaxel plus HSP90 inhibitors PI3K/AKT/ mTOR plus Ras / Raf /MEK/ERK HDAC inhibitors plus Hypomethylating agents Concurrent and prolonged exposure required for optimal efficacy Pathway cross - talk requires concurrent inhibition for optimal efficacy Hypomethylators exhibit rapid degradation and elimination HSP90i associated with GI and ocular toxicities Toxicities of the combination limit dose of current formulations Inter - patient PK variability plus encephalopathy with combination Enhance PK and Toxicity Profile: • Extend plasma elimination time • Minimize normal tissue exposure • Reduce GI and ocular toxicity • Uncouple GI tox from efficacy I ncrease therapeutic index: • Shift exposure away from healthy tissues to tumor • Ensure simultaneous target inhibition at tumor site Enhance Toxicity and Tumor Exposure Profile: • Potential to r educe CNS toxicity • Elevate and prolong exposure of optimal drug ratio Celator Pharmaceuticals

Celator Pharmaceuticals 112 HPN Formulation of Docetaxel:AUY922 Dramatically Increases Plasma Drug Concentrations and Coordinates PK Compared to Free Drugs Plasma concentrations of AUY922 and docetaxel in nanoparticle formulation are more than 2 - 4 orders of magnitude greater than conventional IV formulation Hours After Administration 0 5 10 15 20 25 Percent Injected Dose 0.0001 0.001 0.01 0.1 1 10 100 1000 Docetaxel prodrug AUY922 prodrug Free AUY922 Free Docetaxel

Celator Pharmaceuticals 113 Docetaxel:AUY - 922 Free Drug Combination Must Reduce Dose of Individual Agents Markedly Compared to CombiPlex ® Formulation % Free Drug MTD 0 20 40 60 80 100 Docetaxel MTD AUY922 MTD Free Drug Combination Nanoparticle Combination Maximum tolerated dose (MTD*) of Docetaxel and AUY922 administered as a combination was compared to the MTD of free drugs administered individually * MTD defined as < 15% loss of body wt. and < 10% mortality

Efficacy of Docetaxel:AUY922 in the HCT15 Tumor Model ( Taxane Resistant Model) Celator Pharmaceuticals 114 Nanoparticle formulation of Docetaxel and AUY922 is more efficacious than the free drug combination in a taxane resistant colorectal cancer model Days After Treatment 0 10 20 30 40 50 Tumor Volume (mm3) 0 500 1000 1500 2000 Control Free Drug MTD (10:25 mg/kg) Nanoparticle MTD (20:40 mg/kg) Treatments administered IV weekly x 3

Days After Administration 0 5 10 15 20 25 30 35 Cubic Millimeters 0 200 400 600 8001000 1200 1400 1600 1800 Saline Control Nanoparticle DOC:AUY (10:20 mg/kg) Nanoparticle DOC:AUY (20:40 mg/kg) Efficacy of Docetaxel:AUY922in the ST996 PDX Tumor Model ( Taxane Resistant Model) Celator Pharmaceuticals 115 Nanoparticle formulation of Docetaxel and AUY922 exhibits significant growth inhibition of a taxane resistant PDX breast cancer model • Note: lowest free drug combination dose tested using 10 mg/kg docetaxel in this expt. was excessively toxic (>25% body wt loss)

Days After Administration -5 0 5 10 15 20 25 30 Tumor Size (cubic millimeters) 0 1000 2000 3000 4000 Saline Control Nanoparticle DOC:AUY (20:40 mg/kg) Efficacy of Docetaxel:AUY922 in the HCC1569 Tumor Model ( Taxane Sensitive Model) Celator Pharmaceuticals 116 Nanoparticle formulation of Docetaxel and AUY922 exhibits significant tumor regression in the HCC1569 human breast cancer xenograft model • Note: lowest free drug combination dose tested using 5 mg/kg docetaxel in this expt. was excessively toxic (>20% body wt loss)

Issues CombiPlex ® Optimizing Combinations of Molecularly Targeted Agents: MEK inhibitor ( S elumetinib ) + Akt inhibitor ( Ipatasertib ) 117 Docetaxel plus HSP90 inhibitors PI3K/AKT/mTOR plus Ras/Raf/MEK/ERK HDAC inhibitors plus Hypomethylating agents Concurrent and prolonged exposure required for optimal efficacy Pathway cross - talk requires concurrent inhibition for optimal efficacy Hypomethylators exhibit rapid degradation and elimination HSP90i associated with GI and/or ocular toxicities Toxicities (GI and sever rash) of the combination limit dose of current formulations Inter - patient PK variability plus encephalopathy with combination Enhance PK and Toxicity Profile: • Extend plasma elimination time • Minimize normal tissue exposure • Reduce GI and/or ocular toxicity I ncrease therapeutic index: • Shift exposure away from healthy tissues to tumor • Ensure simultaneous target inhibition at tumor site • Uncouple severe rash toxicity from efficacy Enhance Toxicity and Tumor Exposure Profile: • Potential to r educe CNS toxicity • Elevate and prolong exposure of optimal drug ratio Celator Pharmaceuticals

Celator Pharmaceuticals 118 HPN Formulation of Selumetinib:Ipatasertib Dramatically Increases Plasma Drug Concentrations and Coordinates PK Compared to Free Drug Hours After Injection 0 5 10 15 20 25 Percent Injected Dose 0.01 0.1 1 10 100 1000 Nano Selumetinib Nano Ipatasertib Oral Selumetinib Extended circulation lifetimes and coordinated PK are observed for Selumetinib and Ipatasertib when co - formulated in polymer nanoparticles

Celator Pharmaceuticals 119 Improved Tolerability of CombiPlex Formulation of Selumetinib:Ipatasertib Compared to the Free Drug Combination Maximum tolerated dose ( MTD) of Ipatasertib administered as a combination was compared to the MTD of free drug administered individually* *Note that bioavailability of oral dosage form is only approximately 25% % Free Drug MTD 0 20 40 60 80 100 120 Free Drug Combination Nanoparticle Combination

Celator Pharmaceuticals 120 Ongoing/Future Studies with Combinations Utilizing Molecularly Targeted Agents • Numerous efficacy studies with nanoparticle formulations ongoing • Drug ratio optimization in nanoparticle • Efficacy vs free drug combinations • Individual vs combination agents • Characterize reduction of key target organ toxicities • GI toxicity • Rash (MEK/ Akt combination); model development • Pharmaceutical stability • Apply CombiPlex technology to additional combinations • Incorporate new agents (e.g. CDK 4/6 inhibitor, FGFR inhibitor) • Examine “modular” nature of hydrophobic prodrug nanoparticle platform (e.g. docetaxel + selumetinib , AUY922 + selumetinib )

Hours After Administration 0 5 10 15 20 25 Percent Injected Dose 1 10 100 Nano Selumetinib Nano Docetaxel Celator Pharmaceuticals 121 HPN Formulation of Selumetinib and Docetaxel Exhibits Coordinated PK: Demonstrates “Modular” Properties of HPN Platform Plasma concentrations of Selumetinib and Docetaxel co - formulated in Hydrophobic prodrug nanoparticles

Issues CombiPlex ® Optimizing Combinations of Molecularly Targeted Agents: Hypomethylator ( Decitabiine ) + HDAC inhibitor ( Vorinostat ) 122 Docetaxel plus HSP90 inhibitors PI3K/AKT/ mTOR plus Ras / Raf /MEK/ERK HDAC inhibitors plus Hypomethylating agents Concurrent and prolonged exposure required for optimal efficacy Pathway cross - talk requires concurrent inhibition for optimal efficacy Hypomethylators exhibit rapid degradation and elimination HSP90i associated with GI and/or ocular toxicities Toxicities of the combination limit dose of current formulations Inter - patient PK variability plus encephalopathy with combination Enhance PK and Toxicity Profile: • Extend plasma elimination time • Minimize normal tissue exposure • Reduce GI and/or ocular toxicity I ncrease therapeutic index: • Shift exposure away from healthy tissues to tumor • Ensure simultaneous target inhibition at tumor site Enhance Toxicity and Tumor Exposure Profile: • Potential to r educe CNS toxicity • Elevate and prolong exposure of optimal drug ratio Celator Pharmaceuticals

Celator Pharmaceuticals 123 CombiPlex ® Formulation Development of Decitabine:Vorinostat • Decitabine degrades under conditions required for liposome encapsulation • Hydrophobic prodrugs of decitabine and vorinostat were synthesized and incorporated into nanoparticles • Numerous formulations prepared under a variety of conditions and compositions • Pharmacokinetic analyses performed on multiple formulations revealed low drug levels in the plasma soon after IV administration • Further investigation revealed that decitabine is unstable even as a prodrug within the hydrophobic core of the nanoparticles. • This problem may be surmountable, however, a decision was made to move on to new combinations rather than allocate additional resources to this combination.

Celator Pharmaceuticals 124 CombiPlex ® Platform R&D Summary • CombiPlex technology can be applied to a range of molecularly targeted agents • Hydrophobic prodrug nanoparticle (HPN) platform is well suited for optimizing and coordinating PK of targeted agent combinations • Nanoparticle delivery markedly reduces toxicities of targeted agent combinations studied to date • Promising evidence of improved efficacy observed with nanoparticle formulations of targeted agent combinations • HPN platform provides versatile and modular capabilities to generate a wide range of novel targeted combinations.

Celator Pharmaceuticals 125 Upcoming Activities and Milestones of Note • Full data packages available end of 3Q 2015 • Formulation and pharmaceutical stability • Pharmacokinetics • Tolerability + selected target organ toxicity testing • Efficacy vs free drug in multiple tumor models + drug ratio dependency • Abstract submission and data presentation at EORTC/NCI/AACR conference on Molecularly Targeted Therapeutics; November 5 - 9, 2015, Boston, MA • Outreach to Pharma for potential R&D collaborations applying CombiPlex ® technology to their compounds • Previous outreach generated significant interest but required data • Data packages will be used to leverage previous interactions and generate new opportunities • Assess opportunity to advance one CombiPlex ® combination into formal development towards clinical testing

Question and Answer Session - Technology Platform - General

Closing Remarks Scott Jackson Chief Executive Officer

Exciting Future for Celator Establishing CPX - 351 as a new foundation of care for AML patients • Phase 3 study - going head - to - head against the 7+3 regimen • Preparing for a NDA submission in 3Q2016 • Commercialization activities are underway • Expanding clinical development to demonstrate broader opportunity Technology Platform is transforming the science of combination therapies • Novel combination data packages by the end of 3Q2015 • Pursuing R&D collaborations • Identify a product candidate to advance toward clinical development Celator Pharmaceuticals 128 Creating Shareholder Value

Fast Track Designation 2015 - 2016 Selected News Flow and Expected Milestones Potential NDA Filing in 3Q2016 Phase 3 Induction Remission Rate First Relapse Phase 2 Publication 2015 2016 AACR abstracts ASH abstracts AACR abstracts ASCO abstracts ASH abstracts Technology Platform Programs – data packages Phase 3 Overall Survival NDA Sumission Data Presentations / Clinical Updates Phase 3 Milestones Technology Platform Milestones 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q Celator Pharmaceuticals 129 NJEDA Announcement JP Bizzari L Mayer Study 206 IIS expansion Company News EHA abstracts New IIS study LLS Amendment

Throughout 2015 - 2016 Celator w ill Transform from a Late - Stage Development Company to a Development and Commercial Company Commercial Partnership Discussions Phase 3 Induction Remission Analysis 2015 2016 Commercial Launch Prep and Positioning CPX - 351 Begin building Commercial Infrastrucure Phase 3 Overall Survival NDA Submission 2015 2016 2 Q 3Q 4Q 1Q 2Q 3Q 4Q Establish R&D Collaborations for Technology Platforms First Corporate Presence at ASH 2Q17 US Launch 130 Celator Pharmaceuticals

131 NASDAQ:CPXX | celatorpharma.com Thank You ! © Copyright 2015 Celator Pharmaceuticals. All rights reserved.



































































































































