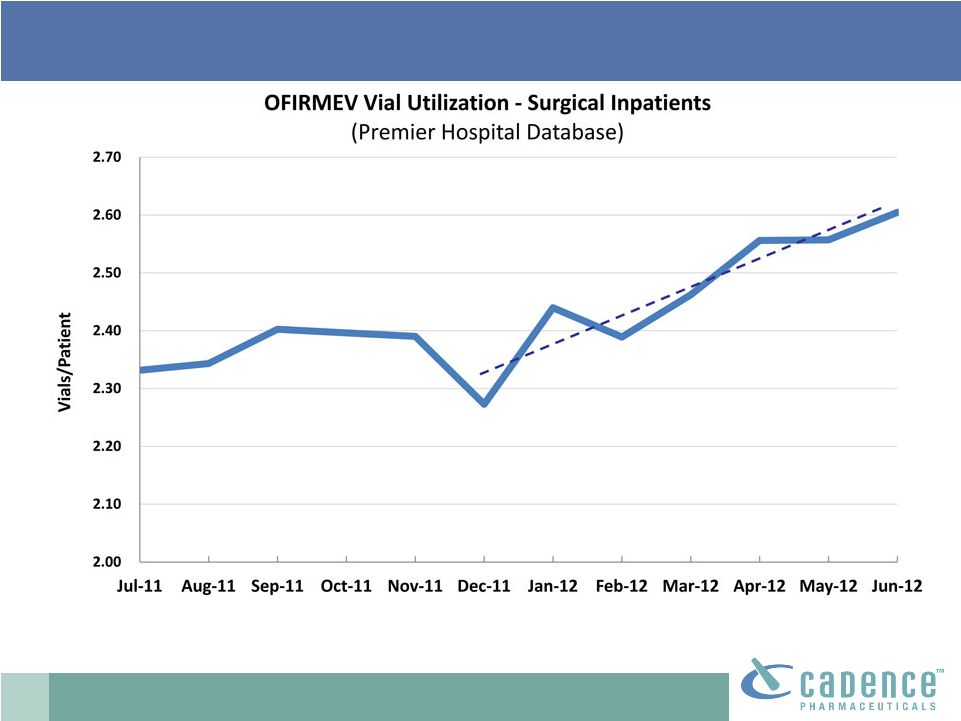
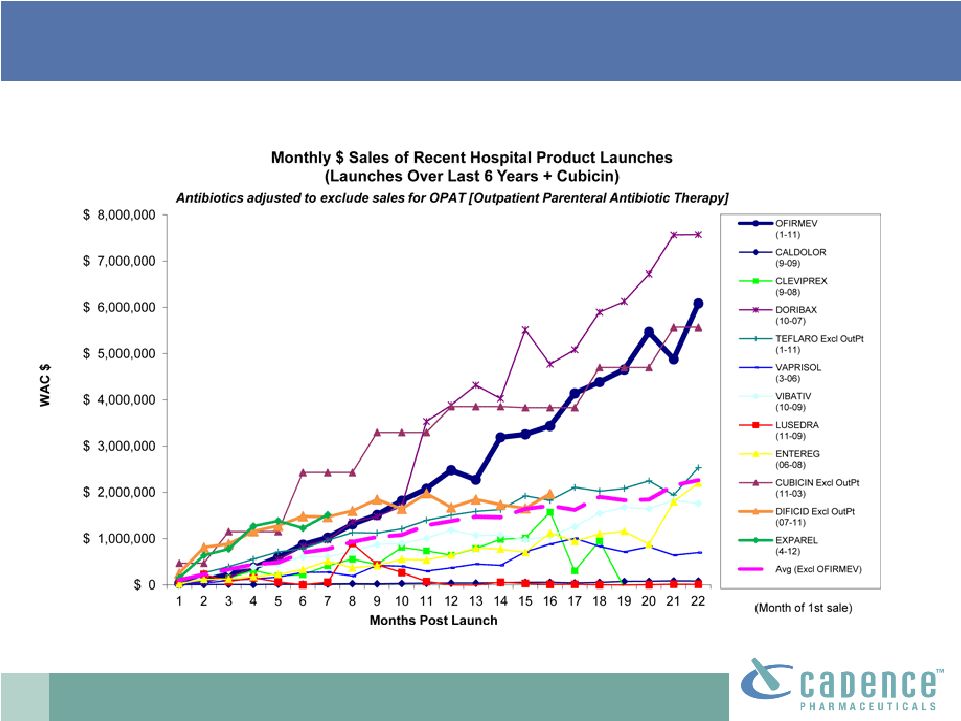
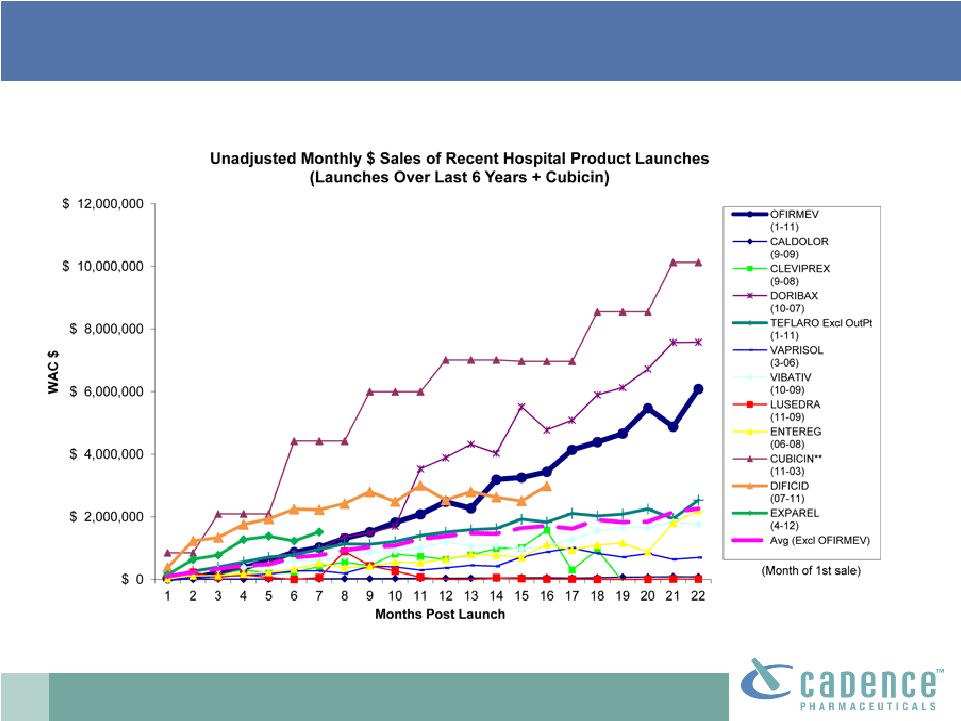
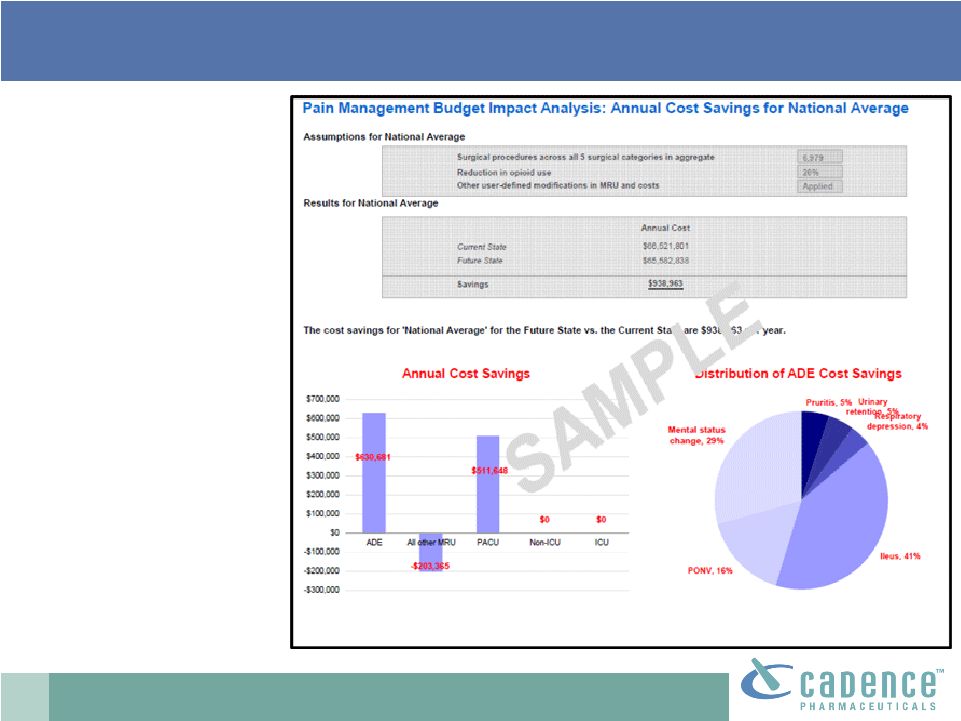
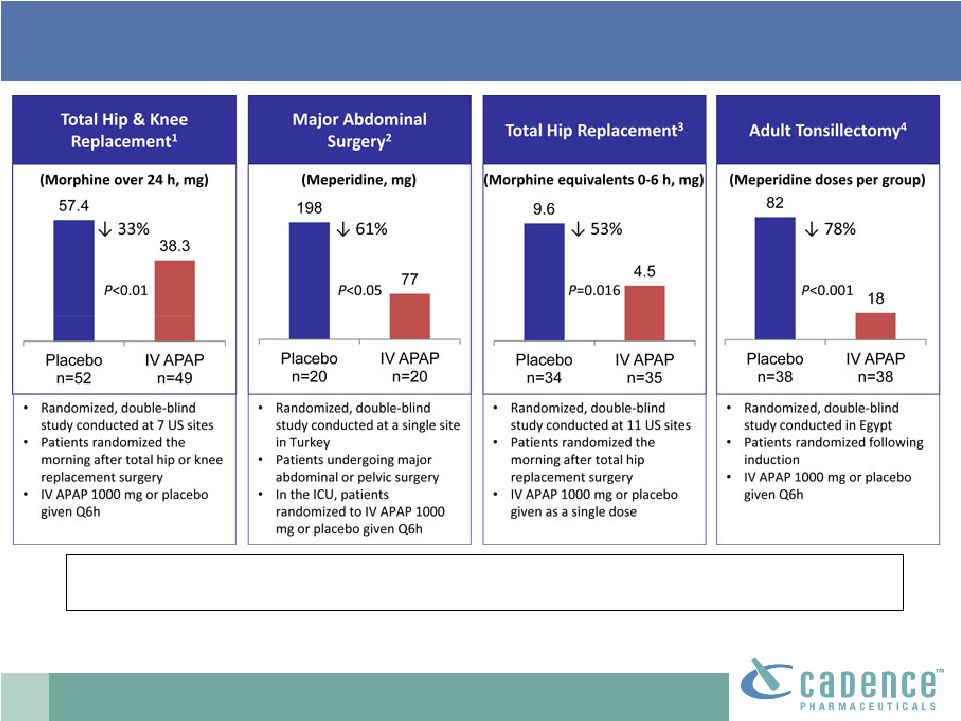
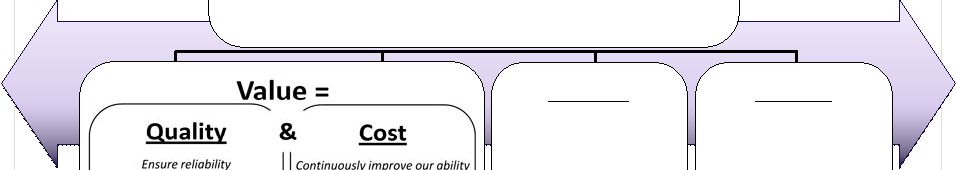
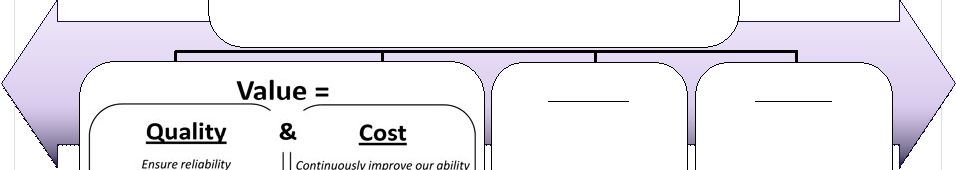
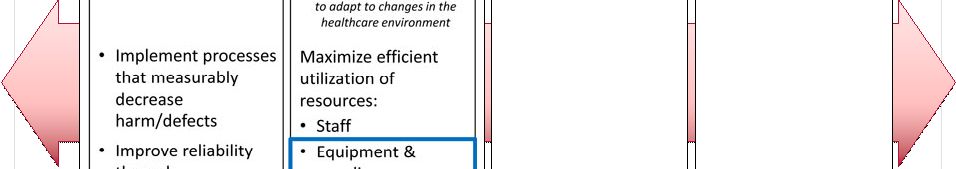
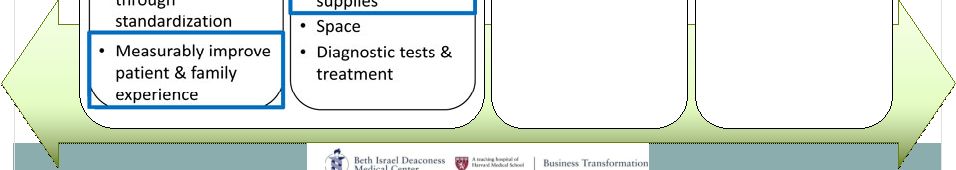
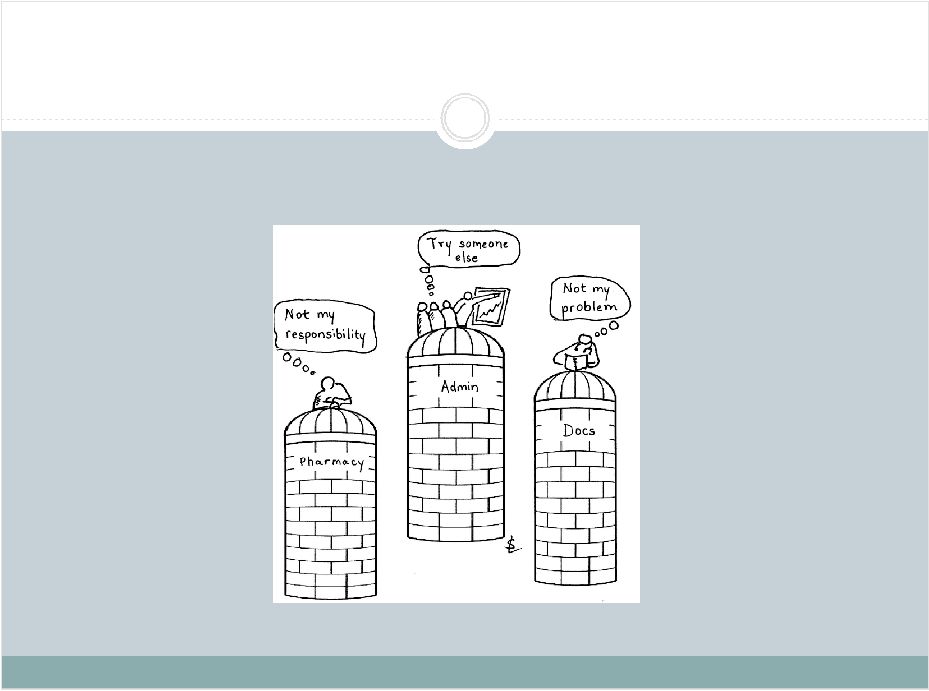
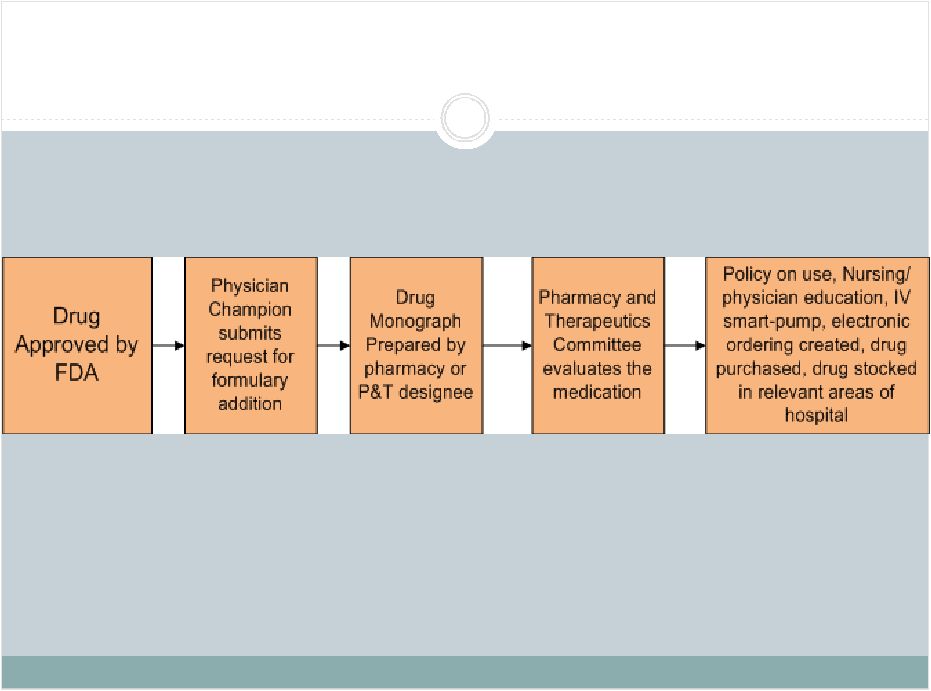
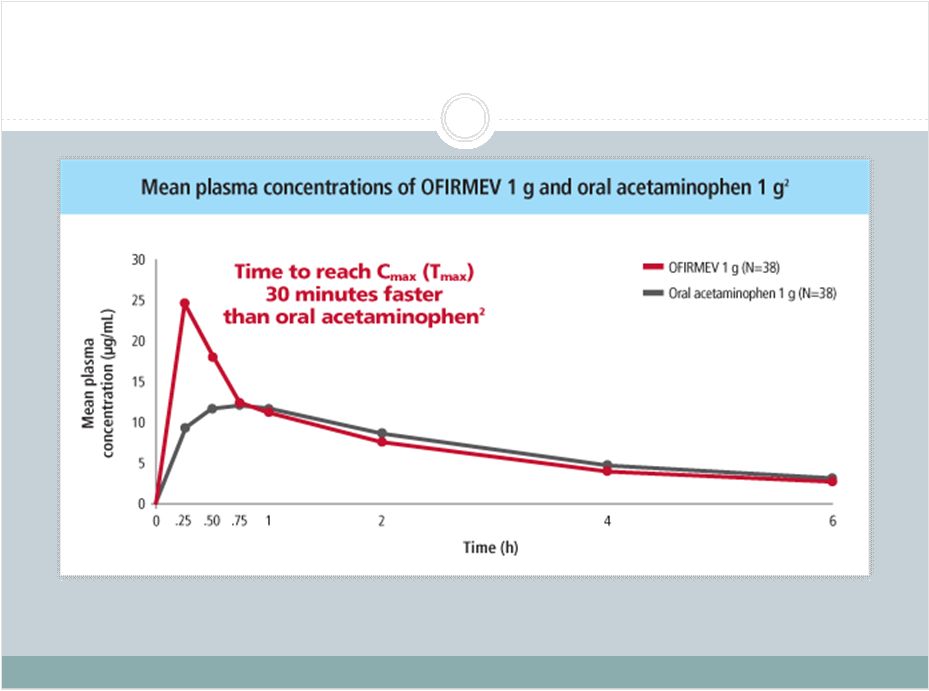
2 This presentation includes forward-looking statements, which are based on our current beliefs and expectations. Such statements include, without limitation, statements regarding: the anticipated U.S. market opportunity for OFIRMEV; our financial estimates and projections; our expectations regarding growth in customer base, market base, order rates and market penetration, and their ability to drive revenue growth for OFIRMEV; physician projections regarding OFIRMEV utilization in surgical inpatients and the average number of doses to be administered to patients; statements regarding the prospects for approval by the FTC and DOJ of our settlement with Perrigo Company, and for ultimately receiving any payments under that settlement from Perrigo; our confidence in the strength of the patents covering OFIRMEV and ability to prevail in the ongoing intellectual property litigation against Exela PharmaSci and its affiliates; the sustainability of our core business; the sufficiency of our capital resources to fund our operations; and our ability to execute our strategies for acquiring, in- licensing, developing and commercializing proprietary products principally for use in the hospital setting. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Our actual future results may differ materially from our current expectations due to the risks and uncertainties inherent in our business. In addition, past results and trends may not be indicative or a guarantee of future results or trends. These risks include, but are not limited to: our dependence on the successful commercialization of OFIRMEV, which is our only product; our ability to achieve broad market acceptance and generate revenues from sales of OFIRMEV; our dependence on our contract manufacturers and our ability to ensure an adequate and continued supply of OFIRMEV to meet market demand; our ability to successfully enforce our marketing exclusivities and intellectual property rights, and to defend the patents covering OFIRMEV, including our current patent litigation; the potential that we may be required to continue patent litigation for substantial lengths of time, file additional lawsuits to defend our patent rights from challenges by Exela or other companies that may submit ANDAs for generic versions of OFIRMEV, and the substantial costs associated with such lawsuits; potential product liability exposure; the risk that we may not be able to raise sufficient capital when needed, or at all; and other risks detailed under “Risk Factors” and elsewhere in our Annual Report on Form 10-K for the period ended December 31, 2011, and our other filings made with the Securities and Exchange Commission from time to time. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and we undertake no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Caution on forward-looking statements CADENCE ® , OFIRMEV ® and the Cadence and OFIRMEV logos are trademarks of Cadence Pharmaceuticals, Inc. © 2012 Cadence Pharmaceuticals, Inc. All rights reserved. |