Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C., 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2007
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 000-51902
INFUSYSTEM HOLDINGS, INC.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 20-3341405 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
1551 East Lincoln Avenue, Suite 200
Madison Heights, Michigan 48071
(Address of Principal Executive Offices) (Zip Code)
Registrant’s Telephone Number, including Area Code:
(248) 546-7047
Securities Registered Pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Each Exchange on which Registered | |
| None | None |
Securities Registered Pursuant to 12(g) of the Act
Common Stock, par value $0.0001 per share
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ¨ NO x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ¨ NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter periods as the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. YES x NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act. (check one)
| Large accelerated filer | ¨ | Accelerated filer x | ||
| Non-accelerated filer | ¨ | Smaller reporting company ¨ | ||
| (Do not check if smaller reporting company.) | ||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). YES ¨ NO x
The aggregate market value of the registrant’s voting equity held by non-affiliates of the registrant, computed by reference to the closing sales price for the registrant’s common stock on June 29, 2007, as reported on the OTC Bulletin Board, was approximately $97,707,703. In determining the market value of the voting equity held by non-affiliates, securities of the registrant beneficially owned by directors and officers of the registrant have been excluded. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of shares of the registrant’s common stock outstanding as of March 13, 2008 was16,824,295.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of this registrant’s definitive proxy statement for its 2008 Annual Meeting of Stockholders to be filed with the SEC no later than 120 days after the end of the registrant’s fiscal year are incorporated herein by reference in Part III of this Annual Report on Form 10-K.
Table of Contents
i
Table of Contents
Cautionary Statement about Forward-Looking Statements
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical facts contained in this Annual Report on Form 10-K, including statements regarding the future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on current expectations and projections about future events and financial trends that we believe may affect financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, without limitation, those described in “Risk Factors” and elsewhere in this Annual Report on Form 10-K, including, among other things:
| • | dependence on our Medicare Supplier Number; |
| • | changes in third-party reimbursement rates; |
| • | availability of chemotherapy drugs used in our infusion pump systems; |
| • | physician’s acceptance of infusion pump therapy over oral medications; |
| • | growth strategy, involving entry into new fields of infusion-based therapy; |
| • | industry competition; |
| • | dependence upon our suppliers; and |
| • | compliance with U.S. GAAP. |
These risks are not exhaustive. Other sections of this Annual Report on Form 10-K include additional factors which could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for us to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
You should not rely upon forward looking statements as predictions of future events. We cannot assure you that the events and circumstances reflected in the forward looking statements will be achieved or occur. Although we believe that the expectations reflected in the forward looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
Table of Contents
References in this Annual Report on Form 10-K to “we,” “us,” or the “Company” are to InfuSystem Holdings, Inc. and its subsidiary. From September 28, 2006 through October 25, 2007, the subsidiary was Iceland Acquisition Subsidiary, Inc. (“Iceland Acquisition Subsidiary”). As of October 25, 2007, the subsidiary was InfuSystem, Inc. (“InfuSystem”).
| Item 1. | Business. |
Background
We were formed as a Delaware blank check company in 2005 for the purpose of acquiring through a merger, capital stock exchange, asset acquisition or other similar business combination, one or more operating businesses in the healthcare sector. We completed our initial public offering on April 18, 2006. On September 29, 2006, we entered into a Stock Purchase Agreement (as, amended the “Stock Purchase Agreement”) with I-Flow Corporation (“I-Flow”), Iceland Acquisition Subsidiary, our wholly-owned subsidiary, and InfuSystem, a wholly-owned subsidiary of I-Flow Corporation. Upon the closing of the transactions contemplated by the Stock Purchase Agreement on October 25, 2007, Iceland Acquisition Subsidiary purchased all of the issued and outstanding capital stock of InfuSystem from I-Flow and concurrently merged with and into InfuSystem. As a result of the merger, Iceland Acquisition Subsidiary ceased to exist as an independent entity and InfuSystem, as the corporation surviving the merger, became our wholly-owned subsidiary. Effective October 25, 2007, we changed our corporate name from “HAPC, INC.” to InfuSystem Holdings, Inc.
InfuSystem was incorporated under the laws of the State of California in December 1997 under the name I-Flow Subsidiary, Inc., as a wholly owned subsidiary of I-Flow. In February 1998, I-Flow Subsidiary, Inc. acquired Venture Medical, Inc. and InfuSystem II, Inc. in a merger transaction pursuant to which I-Flow Subsidiary, Inc. as the surviving corporation changed its name to InfuSystem, Inc.
Business Concept and Strategy
We are a provider of ambulatory infusion pump management services for oncologists in the United States. Ambulatory infusion pumps are small, lightweight electronic pumps designed to be worn by patients in their homes which allow patients the freedom to move about while receiving chemotherapy treatments. The pumps are battery powered and attached to intravenous administration tubing, which is in turn attached to a bag or plastic cassette that contains the chemotherapy drug.
Our business model is currently highly focused on oncology chemotherapy infusion. To our knowledge, we are the only national ambulatory infusion pump service provider focused on oncology.
We supply electronic ambulatory infusion pumps and associated disposable supply kits to physicians’ offices, infusion clinics and hospital outpatient chemotherapy clinics to be utilized by patients who receive continuous chemotherapy infusions. We obtain an assignment of insurance benefits from the patient, bill the insurance company or patient accordingly, and collect payment. We provide pump management services for the pumps and associated disposable supply kits to approximately 1,550 oncology practices in the United States. We retain title to the pumps during this process. In addition, we sell safety devices for cytotoxic drug transfer and administration and we rent pole-mounted or ambulatory infusion pumps for use within the oncology practice.
We purchase electronic ambulatory infusion pumps from a variety of suppliers on a non-exclusive basis. Such pumps are generic in nature and are available to our competitors. The pumps are currently used primarily for continuous infusion of chemotherapy drugs for patients with colorectal cancer.
One aspect of our business strategy over the next one to three years is to expand into treatment of other cancers such as head, neck and gastric. In addition to treatments for colorectal cancer, there are a number of other drugs currently approved by the U.S. Food and Drug Administration (the “FDA”), as well as agents in the pharmaceutical development pipeline, which we believe could potentially be used with continuous infusion
2
Table of Contents
protocols for the treatment of other diseases. These approved drugs and drugs in the pharmaceutical development pipeline include the following: Gemcitabine, Idarubicin and Topotecan. We currently generate approximately 15% of our revenue from treatments for disease states other than colorectal cancer. Drugs or protocols currently in clinical trials may also obtain regulatory approval over the next several years. If these new drugs obtain regulatory approval for use with continuous infusion protocols, we expect the pharmaceutical companies to focus their sales and marketing forces on promoting the new drugs and protocols to physicians.
Another aspect of our business strategy over the next one to three years is to actively pursue opportunities for expansion through acquisitions, joint ventures and strategic alliances. We believe there are numerous opportunities to acquire smaller, regional competitors that perform similar services to us, but do not have the national presence, network of managed care contracts or economies of scale that we currently enjoy. We believe that the successful integration of these businesses into our operations will potentially enable us to achieve higher profit margins from them than they could achieve operating independently.
We face risks that other competitors can provide the same services as us. Those risks are currently mitigated by our existing managed care contracts and economies of scale, which allow for less costly purchase and management of the pumps. Additionally, we have already established a long standing relationship as a provider of pumps to approximately 1,550 oncology practices in the United States. We believe that there are competitive barriers to entry against other suppliers with respect to these oncology practices because we have an established national presence and managed care contracts in place covering over 125,000,000 managed care lives, (i.e. persons enrolled in various managed care plans or commercial insurance carriers such as health maintenance organizations and preferred provider organizations) increasing the likelihood that we participate in the insurance networks of patients to whom physicians wish to refer to an ambulatory infusion pump provider. Moreover, we have an available inventory of approximately 17,000 active ambulatory infusion pumps, which may allow us to be more responsive to the needs of physicians and patients than a new market entrant. We do not perform any research and development.
Continuous Infusion Therapy
Continuous infusion of chemotherapy involves the gradual administration of a drug via a small, lightweight, portable electronic infusion pump over a prolonged period of time, defined as greater than 8 hours, and up to 24 hours daily. A cancer patient can receive his or her medicine on average 5 to 30 days a month depending on the chemotherapy regimen that is most appropriate to that individual’s health status and disease process. This may be followed by periods of rest and then repeated cycles with treatment goals of progression free disease survival. This drug administration method has replaced “bolus” administration in specific circumstances. The advantages of slow continuous low doses of specific drugs are well documented. Clinical studies support use of continuous infusion chemotherapy for decreased toxicity without loss of anti-tumor efficacy while emerging evidence shows improved drug activity. The 2007/2008 National Comprehensive Cancer Network Guidelines recommend the use of continuous infusion for treatment of numerous cancer diagnoses. We believe that the growth of continuous infusion therapy is driven by three factors: superior clinical outcomes; enhanced patient response and convenience; and recent changes to physician reimbursement.
| • | In the past decade, significant progress has been made in the treatment of colorectal cancer due to advances in surgery, radiotherapy and chemotherapy. The survival benefit of 5-Fluorouracil based chemotherapy for patients with Stage III colorectal cancer has been established during this time. In the late 1990s, overwhelming evidence revealed that the delivery method of the drug (or schedule) was key to drug availability, efficacy and tolerability. Schedule dependant anti-tumor activity and toxicity has resulted in continuous infusion 5-Fluorouracil being adopted as standard of care. In 2000, the FDA approved Camptosar (the trade name for the generic chemotherapy drug Irinotecan), a drug developed by Pfizer, for first-line therapy in combination with 5-Fluorouracil for the treatment of colorectal cancer. In 2002, the FDA approved Eloxatin (the trade name for the generic chemotherapy drug Oxaliplatin), a drug developed by Sanofi-Aventis, for use in combination with continuous infusion 5-Fluorouracil for the treatment of colorectal cancer. FOLFIRI, the chemotherapy protocol which includes Camptosar in combination with continuous infusion 5-Fluorouracil and the drug Leucovorin, and FOLFOX, the chemotherapy protocol which includes Eloxatin, in combination with continuous infusion 5-Fluorouracil and the Leucovorin, have resulted in significantly improved overall |
3
Table of Contents
survival rates for colorectal cancer patients at various stages of the disease state. Sanofi-Aventis and Pfizer are each dedicating significant resources to educate physicians and promote the use of FOLFOX and FOLFIRI. Oncologists have responded and the adoption of continuous infusion treatments has steadily grown for colorectal cancer. |
| • | The use of continuous infusion has been demonstrated to decrease or alter the toxicity of a number of cytotoxic, or cell killing agents. Higher doses of drugs can be infused over longer periods of time, leading to improved tolerance and decreased toxicity. For example, the cardiotoxicity (heart muscle damage) of the chemotherapy drug Doxorubicin is decreased by schedules of administration (The Chemotherapy Source Book, Perry, M.C., 2008). Nausea, vomiting, diarrhea and decreased white blood cell and platelet counts are all affected by duration of delivery. Continuous infusion can lead to improved tolerance and patient comfort while enhancing the patient’s ability to remain on the chemotherapy regimen. |
| • | Continuous infusion offers convenience to the cancer patient. Fatigue, a prevalent side-effect of cancer treatment, is compounded by repeated day-long treatments at the physician’s office. Ambulatory pump use for drug therapy allows the patient to complete treatment while attending to activities of daily living or relaxing in his or her own home. Physician administration of the pump provides for continuity of care. The partnering of physician management and patient autonomy provide for the highest quality of care with the greatest patient satisfaction. |
| • | The Medicare Modernization Act of 2003 reduced levels of Medicare reimbursement for oncology drugs administered in the physician office setting. To offset this reduction, Medicare increased the service fees paid to oncologists. We believe that this has resulted in doctors shifting to treatments that provide superior efficacy and patient satisfaction while optimizing their potential to earn service fees. |
Products and Services
Our core service is to provide oncology offices, infusion clinics and hospital out-patient chemotherapy clinics with ambulatory infusion pumps in addition to related supplies for patient use, and then directly bill and collect payment from payors and patients for the use of these pumps. We own approximately 17,000 pumps. At any given time, it is estimated that approximately 60% of the pumps are in the possession of patients. The remainder of the pumps is in transport for cleaning, calibration or as back-ups.
After a doctor determines that a patient is eligible for ambulatory infusion pump therapy, the doctor arranges for the patient to receive an infusion pump and provides the necessary chemotherapy drugs. The oncologist and nursing staff train the patient in the use of the pump and initiate service. The physician bills insurers, Medicare, Medicaid, managed care companies or patients (collectively, “payors”) for the physician’s professional services associated with initiating and supervising the infusion pump administration, as well as the supply of drugs. We directly bill payors for the use of the pump and related disposable supplies. We have contracts with more than 100 payors that cover more than 125 million managed care lives. Billing to payors requires coordination with patients and physicians who initiate the service, as physicians’ offices must provide us with appropriate paperwork (patient’s insurance information, certificate of medical necessity and an acknowledgement of benefits that shows receipt of equipment by the patient) in order for us to bill the payors.
In addition to providing high quality and convenient care, we believe that our pump management program offers significant economic benefits for patients, providers and payors.
| • | We benefit patients by providing high quality, reliable pumps and accessories as well as 24-hour service and support. We employ oncology and intravenous certified registered nurses trained on ambulatory infusion pump equipment who staff our 24-hour hotline to address questions that patients may have about their pump treatment, the infusion pumps or other medical or technical questions related to the pumps. |
4
Table of Contents
| • | Physicians benefit from our service in several ways. For those physicians wishing to provide pumps to their patients, by utilizing our model, we can relieve such physicians of the capital commitment, pump service, maintenance and billing and administrative burdens associated with pump ownership. Rather than referring patients to home care, our service allows the doctor to continue a direct relationship with the patient and to receive professional service fees for setting up treatment and administering drugs. We provide physicians the pumps and related administration sets and accessories while retaining title to the pumps. We directly bill the patients’ insurance companies for the use of the pumps. As there is no purchase on the part of the physician, there is no capital commitment. We bill insurance providers for the technical component (pump usage and related supplies) and the physician bills insurance providers for the related professional services. |
| • | Payors support us because our service is generally less expensive than hospitalization or home care. |
Relationships with Physician Offices
Through our direct sales force, we maintain relationships with clinical oncologists in more than 1,550 practices. Though this represents a substantial portion of the oncologists in the United States, we believe we can continue to expand our network to further penetrate the oncology market. Over the past three years, we have added approximately 425 new accounts to our services. We believe our relationships with physician offices are strong, as evidenced by our significant retention rate (98.5% of the physician offices serviced during fiscal year 2006 remained customers during fiscal year 2007).
We believe that, in general, we do not compete directly with hospitals and physician offices to treat patients. Rather, by providing products and services to hospitals and physician offices and other care facilities and providers, we believe that we can help providers keep up with increasing patient demand and manage institutional constraints on capital and manpower due to the nature of limited resources in hospitals and physician offices.
Billing Collection Services
Prior to our acquisition of InfuSystem, InfuSystem had been providing billing and collection services to I-Flow for its ON-Q® product. On October 25, 2007, InfuSystem and I-Flow entered into an Amended and Restated Services Agreement (the “Services Agreement”) pursuant to which InfuSystem agreed to continue to provide I-Flow with these services. The initial term of the Services Agreement is three years. The Services Agreement will automatically be renewed for succeeding one year terms unless terminated pursuant to certain cancellation provisions. I-Flow has agreed to pay InfuSystem a monthly service fee equal to the greater of (i) the monthly expenses for those InfuSystem employees devoted to the billing and collection and management services provided to I-Flow which expenses shall consist of (a) salaries and wages, (b) payroll taxes and (c) group insurance, in addition to an amount equal to 40% of the sums of items (a) through (c) or (ii) a performance-based fee equal to 25% of the total actual net cash collections (net of adjustments) received during such month on behalf of I-Flow. InfuSystem currently maintains a staff of 9 people to provide billing and collection services to I-Flow.
In order to facilitate the continued business relationship between InfuSystem and I-Flow under the Services Agreement, described above, InfuSystem and I-Flow entered into a license agreement on October 25, 2007 (the “License Agreement”) pursuant to which InfuSystem granted I-Flow a license to use InfuSystem’s intellectual property related to the third-party billing and collection services and management services provided by InfuSystem to I-Flow. Specifically, InfuSystem granted I-Flow (i) an unrestricted, perpetual, irrevocable, worldwide, assignable, royalty-free and exclusive license to use and/or sublicense InfuSystem’s intellectual property with respect to acute post-operative pain management treatments, and (ii) an unrestricted, perpetual, irrevocable, worldwide, assignable, royalty-free and non-exclusive license to use and/or sublicense InfuSystem’s intellectual property with respect to all fields other than post-operative pain management treatments, including, without limitation, the fields of wound site management and post-operative surgical treatments.
5
Table of Contents
The term of the License Agreement is perpetual, but may be terminated by I-Flow following the third anniversary of the effective date of the License Agreement. Upon the later of the third anniversary of the effective date or the termination of the Services Agreement for any reason, the exclusive license will be deemed amended to become a non-exclusive license. InfuSystem may not terminate the License Agreement.
On November 8, 2007, I-Flow informed InfuSystem that it was terminating the Services Agreement effective May 10, 2008. I-Flow’s license to use and/or sublicense InfuSystem’s intellectual property with respect to acute post-operative pain management treatments will thereafter become non-exclusive. During 2007, we recorded revenues of $103,000 from this arrangement, which are included in net revenues.
Employees
As of December 31, 2007, we had 107 employees, including 100 full-time employees and 7 part-time employees. None of our employees are unionized.
Material Suppliers
We supply a wide variety of pumps and associated equipment, as well as disposables and ancillary supplies. The majority of our pumps are electronic ambulatory pumps purchased from the following manufacturers, each of which is material and supplies more than 10% of the pumps purchased by us: Smiths Medical, Inc.; Hospira Worldwide, Inc.; and McKinley Medical, LLC. There are no supply agreements in place with any of the suppliers. All purchases are handled pursuant to pricing agreements, which contain no material terms other than prices that are subject to change by the manufacturer. As of December 31, 2007, we owned approximately 17,000 active pumps.
Seasonality
We do not believe that there is significant seasonality of our business.
Environmental Laws
We are required to comply with applicable environmental laws regulating the disposal of cleaning agents used in the process of cleaning our ambulatory infusion pumps, as well as the disposal of sharps and blood products used in connection with the pumps. We do not believe that compliance with such laws has a material effect on our business.
Significant Customers
We have sought to establish contracts with as many managed care organizations as commercially practicable, in an effort to ensure that reimbursement is not a significant obstacle for providers who recommend continuous infusion therapy and wish to utilize our services. A managed care organization is a health care payor (or a group of medical services payors) that contracts to provide a wide variety of healthcare services to enrolled members through participating providers (such as us). A payor is any entity that pays on behalf of a member patient.
We currently have contracts with more than 100 managed care plans that cover approximately 125 million lives. Material terms of contracts with managed care organizations are typically a set fee or rate, or discount from billed charges for equipment provided. These contracts generally provide for a term of one year, with automatic one-year renewals, unless we or the contracted payor do not wish to renew. Contracted payors include managed care organizations, Medicare, commercial Medicare replacement plans, Blue Cross/Blue Shield plans, self-insured plans and numerous other commercial insurance carriers.
Through December 31, 2007, we experienced increased collection delays from non-contracted Blue Cross/Blue Shield affiliates. This was primarily due to the fact that all of our non-contracted billings were submitted to Blue Cross of Michigan (“BCBSM”) through the national BlueCard program. BCBSM processes our hard copy claims and generates electronic files to out of state Blue Cross plans under which the patients’ policies are carried. This results in a very slow payment process and a high volume of claim rejections requiring additional information. We are working with BCBSM personnel to rectify this issue.
6
Table of Contents
Competitors
We believe that our competition is primarily composed of regional providers, hospital-owned durable medical equipment (“DME”) providers, physician providers and home care infusion providers. An estimate of the number of competitors is not known or reasonably available, due to the wide variety in type and size of the market participants described below. We are not aware of any industry reports with respect to the competitive market described below. The description of market segments and business activities within those market segments is based on our experiences in the industry.
| • | Regional Providers: Regional DME providers act as distributors for a variety of medical products. We believe regional DME provider sales forces generally consist of a relatively small number of salespeople, usually covering one or two states in total. Regional DME providers tend to carry a limited selection of infusion pumps and their salespeople generally have limited resources. Regional DME providers usually do not have 24-hour nursing service. InfuSystem believes that regional DME providers have relatively few managed care contracts, which may prevent these providers from being paid at acceptable levels and may also result in higher out-of-pocket costs for patients. |
| • | Hospital—Owned DME Providers: Many hospitals have in-house DME providers to supply basic equipment. In general, however, these providers have limited capital and tend to stock a small inventory of infusion pumps. As a result, InfuSystem believes that hospital-owned providers have limited ability to grow because of restricted patient populations. Growth from outside of the hospital may pose a challenge because hospitals typically will not provide referrals to competitors, instead preferring to offer patients a choice of non-hospital-affiliated DME providers. |
| • | Physician Providers: A limited number of physicians maintain an inventory of their own infusion pumps and collect both the professional and technical fees. However, we believe that pump utilization in this area tends to be low and the costs associated with ongoing supplies, preventative maintenance and repairs can be relatively high. Moreover, we believe that a high percentage of DME claims are rejected by payors upon first submission, requiring a provider’s staff to spend significant time and effort to resubmit claims and receive payment for treatment. The numerous service and technical questions from patients may present another significant cost to a physician provider’s staff. |
| • | Home Care Infusion Providers: Home care infusion providers provide chemotherapy drugs and services to allow for in-home patient treatment. Although the doctor is still responsible for overseeing the treatment and assuming the liability for the patient’s treatment and outcome, we believe that the physician is often not reimbursed for this ongoing responsibility. Moreover, we believe that home care infusion treatment can be very costly and that many patients do not carry this type of insurance coverage, resulting in larger out-of-pocket costs. Because home care treatments may take as long as six months, these costs can be high and can result in higher patient co-payments. We believe that home care providers may also be reluctant to offer 24-hour coverage or additional patient visits, due to capped fees. |
Regulation of Our Business
Our business is subject to certain regulations. Specifically, as a Medicare supplier of DME and related supplies, we must comply with the rules (the “DMEPOS Supplier Standards”) established by the Health Care Financing Administration regulating Medicare suppliers of DME and prosthetics, orthotics and supplies (“DMEPOS”). The DMEPOS Supplier Standards consist of 21 requirements that must be met in order for a DMEPOS supplier to be eligible to receive payment for a Medicare-covered item. The most significant DMEPOS Supplier Standards require us to (i) advise Medicare beneficiaries of their option to purchase certain equipment, (ii) honor all warranties under state law and not charge Medicare beneficiaries for the repair or replacement of equipment or for services covered under warranty, (iii) permit agents of the Centers for Medicare and Medicaid Services to conduct on-site inspections to ascertain compliance with the DMEPOS Supplier Standards, (iv) maintain
7
Table of Contents
liability insurance, (v) refrain from contacting Medicare beneficiaries by telephone, except in certain limited circumstances, (vi) answer questions and respond to complaints of beneficiaries regarding the supplied equipment, (vii) disclose the DMEPOS Supplier Standards to each Medicare beneficiary to whom it supplies equipment and (viii) maintain a complaint resolution procedure and record certain information regarding each complaint.
We are also subject to the provisions of the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) designed to protect the security and confidentiality of certain patient health information. Under HIPAA, we must provide patients with access to certain records and must notify patients of our use of personal medical information and patient privacy rights. Moreover, HIPAA sets limits on how we may use individually identifiable health information and prohibits the use of patient information for marketing purposes.
Available Information
Our Internet address iswww.infusystem.com. On this Web site, we post the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the U.S. Securities and Exchange Commission (the “SEC”): our Annual Report on Form 10-K; our Quarterly Reports on Form 10-Q; our Current Reports on Form 8-K; our proxy statement related to our annual stockholders’ meeting; and any amendments to those reports or statements. All such filings are available on our Web site free of charge. The content on our Web site is not incorporated by reference into this Annual Report on Form 10-K unless expressly noted.
| Item 1A. | Risk Factors. |
An investment in our securities involves a high degree of risk. You should consider carefully all of the material risks described below, together with the other information contained in this Annual Report on Form 10-K. If any of the following events occur, our business, financial conditions and results of operations may be materially adversely affected.
RISK FACTORS RELATING TO OUR BUSINESS AND THE INDUSTRY IN WHICH WE OPERATE.
We are dependent on our Medicare Supplier Number.
We have obtained a Medicare Supplier Number and are required to comply with Medicare Supplier Standards in order to maintain such number. If we are unable to comply with the relevant standards, we could lose our Medicare Supplier Number, which is the primary identification number used with our various third-party payors. The loss of such identification number for any reason would prevent us from billing Medicare for patients who rely on Medicare to pay their medical expenses and, as a result, we would experience a decrease in our revenues. Furthermore, all managed care and Medicaid contracts require us to have a Medicare Supplier Number. Without such a number, we would be unable to continue our managed care and Medicaid contracts and would experience a material decrease in revenues as a result. The majority of our revenue is dependent upon our Medicare Supplier Number.
Changes in third-party reimbursement rates may adversely impact our revenues.
We depend primarily on third-party reimbursement for the collection of our revenues. We are paid directly by private insurers and governmental agencies, often on a fixed fee basis, for infusion equipment and related disposable supplies provided to patients. If the average fees allowable by private insurers or governmental agencies were reduced, the negative impact on revenues could have a material adverse effect on our financial condition, results of operations and cash flows. Also, if collection amounts owed to us by patients and insurers is reduced, we may be required to increase our bad debt expense and/or decrease our revenues.
8
Table of Contents
Our customers frequently receive reimbursement from private insurers and governmental agencies. Any change in the overall reimbursement system may adversely impact our business. The health care reimbursement system is in a constant state of change.
Changes often create financial incentives and disincentives that encourage or discourage the use of a particular type of product, therapy or clinical procedure. Market acceptance of infusion therapy may be adversely affected by changes or trends within the reimbursement system. Changes to the health care system that favor technologies or treatment regimens other than InfuSystem’s or that reduce reimbursements to providers or treatment facilities that use InfuSystem’s products may adversely affect InfuSystem’s ability to market its products profitably.
Our customers are heavily dependent on payment for their services by private insurers and governmental agencies. Changes in the reimbursement system could adversely affect our participation in the industry. We believe that the current trend in the insurance industry (both private and governmental) has been to eliminate cost-based reimbursement and to move towards fixed or limited fees for service, thereby encouraging health care providers to use the lowest cost method of delivering medications. Furthermore, certain payors may transition to competitive bidding programs to lower costs even more. These trends may discourage the use of our products, create downward pressure on our average prices, and, ultimately, negatively affect our revenues and profit margins.
Our success is impacted by the availability of the chemotherapy drugs that are used in our infusion pump systems.
We primarily derive our revenue from the rental of ambulatory infusion pump systems to oncology patients through physicians’ offices and chemotherapy clinics. A shortage in the availability of chemotherapy drugs that are used in the infusion pump systems, including the commonly-used chemotherapy drug known as 5-Fluorouracil, could have a material adverse effect on InfuSystem’s financial condition, results of operations and cash flows. For instance, we believe that a shortage of 5-Fluorouracil in the fourth quarter of 2005 resulted in an unfavorable revenue impact in excess of $1,000,000. The 5-Fluorouracil shortage continued into the first quarter of 2006 and, although availability of 5-Fluorouracil returned to normal at the end of the first quarter of 2006, revenue was affected during the second quarter of 2006 due to a decline in the number of patients in the pipeline who had to turn to other medications. Future shortages of 5-Fluorouracil or other commonly-used chemotherapy drugs could negatively impact InfuSystem’s revenue, financial condition and cash flows.
Our revenues are heavily dependent on physicians’ acceptance of infusion therapy as a preferred therapy since a significant percentage of patients are treated with oral medications. If new oral medications are introduced or future clinical studies demonstrate that oral medications are as effective or more effective than infusion therapy, our business could be adversely affected.
Continuous infusion therapy is currently preferred by many physicians over oral medication treatment despite the more cumbersome aspects of maintaining a continuous infusion regimen. The reasons for these physicians’ preference are varied, including a belief that infusion therapy involves fewer adverse side effects, reduces compliance issues and may provide greater therapeutic benefits. Numerous clinical trials are currently ongoing, evaluating and comparing the therapeutic benefits of current infusion-based regimens with various oral medication regimens. If these clinical trials demonstrate that oral medications provide equal or greater therapeutic benefits and/or demonstrate reduced side effects from prior oral medication regimens, our revenues and overall business could be materially and adversely affected. Additionally, if new oral medications are introduced to the market that are superior to existing oral therapies, physicians’ willingness to prescribe infusion-based regimens could decline, which would adversely affect our financial condition, results of operations and cash flows.
We operate our business in all 50 states of the United States and we are subject to each state’s licensure laws as a DME supplier. State licensure laws for DME suppliers are subject to change and if we fail to comply with any state’s laws, we will be unable to operate as a DME supplier in such state and our business operations will be adversely affected.
As a DME supplier operating in all 50 states of the United States, we are subject to each state’s licensure laws regulating DME suppliers. State licensure laws for DME suppliers are subject to change and we must ensure that we are continually in compliance with the laws of all 50 states. In the event that we fail to comply with any
9
Table of Contents
state’s laws governing the licensing of DME suppliers, we will be unable to operate as a DME supplier in such state until we regain compliance. We may also be subject to certain fines and/or penalties and our business operations will be adversely affected.
Our growth strategy includes expansion into infusion treatment for cancers other than the colorectal type. There can be no assurance that infusion-based regimens for these other cancers will become accepted or that we will be successful in penetrating these different markets.
An aspect of our growth strategy is to expand into the treatment of other cancers, such as head, neck and gastric. Currently, however, there is not widespread acceptance of infusion-based therapies in the treatment of these other cancers and future acceptance of continuous infusion therapies depends on new protocols for existing drugs and approval of new drugs currently in clinical trials. No assurances can be given that these new drugs will be approved or will prove superior to oral medication or other treatment alternatives. In addition, no assurances can be given that we will be able to successfully penetrate any new markets that may develop in the future or manage the growth in additional resources that would be required.
The industry in which we operate is intensely competitive and changes rapidly. If we are unable to successfully compete with our competitors, our business operations may suffer.
The drug infusion industry is highly competitive. We compete in this industry based primarily on price, service and performance. Some of our competitors and potential competitors have significantly greater resources than we do for research and development, marketing and sales. As a result, they may be better able to compete for market share, even in areas in which our services may be superior. The industry is subject to technological changes and such changes may put our current fleet of pumps at a competitive disadvantage. If we are unable to effectively compete in our market, our financial condition, results of operations and cash flows may materially suffer.
We rely on independent suppliers for our products. Any delay or disruption in the supply of products, particularly our supply of electronic ambulatory pumps, may negatively impact our operations.
Our infusion pumps are obtained from outside vendors. The majority of our pumps are electronic ambulatory pumps which are supplied to us by three major suppliers: Smiths Medical, Inc.; Hospira Worldwide, Inc.; and McKinley Medical, LLC. The loss or breakdown of our relationships with even one of these outside vendors could subject us to substantial delays in the delivery of our products to customers. Significant delays in the delivery of products could result in possible cancellation of orders and the loss of customers. Our inability to provide products to meet delivery schedules could have a material adverse effect on our reputation in the industry, as well as our financial condition, results of operations and cash flows.
Although we do not manufacture the products we distribute, if one of the products distributed by us proves to be defective or is misused by a health care practitioner or patient, we may be subject to liability that could adversely affect our financial condition and results of operations.
Although we do not manufacture the products that we distribute, a defect in the design or manufacture of one of the products distributed by us, or a failure of products distributed by us to perform for the use specified, could have a material adverse effect on our reputation in the industry and subject us to claims of liability for injuries and otherwise. Misuse of products distributed by us by a practitioner or patient that results in injury could similarly subject us to liability. Any substantial underinsured loss could have a material adverse effect on our financial condition, results of operations and cash flows. Furthermore, any impairment of our reputation could have a material adverse effect on our revenues and prospects for future business.
The preparation of our financial statements in accordance with accounting principles generally accepted in the United States requires us to make estimates, judgments, and assumptions that may ultimately prove to be incorrect.
The accounting estimates and judgments that management must make in the ordinary course of business affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts
10
Table of Contents
of revenue and expenses during the periods presented. If the underlying estimates are ultimately proven to be incorrect, subsequent adjustments resulting from errors could have a material adverse effect on our operating results for the period or periods in which the change is identified. Additionally, subsequent adjustments from errors could require us to restate our financial statements. Restating financial statements could result in a material decline in the price of our stock.
RISK FACTORS RELATING SPECIFICALLY TO OUR COMMON STOCK AND WARRANTS
The market price of our common stock and warrants has been, and is likely to remain volatile and may decline in value.
The market price of our common stock and warrants has been and is likely to continue to be volatile. Market prices for securities of medical device companies, including ours, have historically been volatile, and the market has from time to time experienced significant price and volume fluctuations that appear unrelated to the operating performance of particular companies. The following factors, among others, can have a significant effect on the market price of our securities:
| • | announcements of technological innovations, new products, or clinical studies by others; |
| • | government regulation; |
| • | changes in the coverage or reimbursement rates of private insurers and governmental agencies; |
| • | developments in patent or other proprietary rights; |
| • | changes in economic conditions in the United States or globally; and |
| • | comments by securities analysts and general market conditions. |
The realization of any risks described in these “Risk Factors” could also have a negative effect on the market price of our common stock and warrants.
We do not pay dividends and this may negatively affect the price of our stock.
We have not paid dividends on our common stock and do not anticipate paying dividends on our common stock in the foreseeable future. The future price of our common stock may be adversely impacted because we do not pay dividends.
Holders of our warrants may not be able to exercise the warrants if we do not maintain an effective registration statement and current prospectus covering the shares of common stock underlying the warrants.
If we do not maintain an effective registration statement and a current prospectus covering the shares of common stock issuable upon exercise of our outstanding warrants or comply with applicable state securities laws, holders of our warrants may not be able to exercise the warrants issued by us. In order for holders of our warrants to be able to exercise the warrants, the shares of common stock underlying the warrants must be covered by an effective registration statement and a current prospectus or be qualified for sale or exempt from qualification under the applicable securities laws of the state in which the warrant holder resides. Although we cannot assure holders of our warrants that we will actually be able to do so, we will use our best efforts to maintain an effective registration statement and a current prospectus covering the shares of our common stock underlying the outstanding warrants at all times when the market price of the common stock exceeds the exercise price of the warrants until the expiration of the warrants.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our
11
Table of Contents
common stock in the public market, or the perception that these sales could occur. In addition to the shares of our common stock currently available for sale in the public market, shares of our common stock sold in past private placements (which include shares held by certain members of our board of directors) and the shares of common stock underlying our outstanding warrants are subject to registration rights. If the holders of these securities choose to exercise their registration rights, this would result in an increase in the number of shares our common stock available for resale in the public market, which in turn could lead to a decrease in our stock price and a dilution of stockholders’ ownership interests. These factors could also make it more difficult for us to raise funds through future equity offerings. As of March 13, 2008 we had 16,824,295 shares of our common stock outstanding not including the 35,108,219 shares of common stock underlying our outstanding warrants.
| Item 1B. | Unresolved Staff Comments. |
Not applicable.
| Item 2. | Properties. |
We do not own any real property. We lease office and warehouse space at 1551 E. Lincoln Avenue, Madison Heights, Michigan. We believe that such office and warehouse space is suitable and adequate for our business.
| Item 3. | Legal Proceedings. |
We are involved in legal proceedings arising out of the ordinary course and conduct of our business, the outcomes of which are not determinable at this time. We have insurance policies covering such potential losses where such coverage is cost effective. In our opinion, any liability that might be incurred by us upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on our financial condition or results of operations.
| Item 4. | Submission of Matters to a Vote of Security Holders. |
We held our Special Annual Meeting of Stockholders on October 24, 2007. Each of the proposals set forth below was approved by our stockholders at the Special Annual Meeting.
Our acquisition of all of the issued and outstanding capital stock of InfuSystem pursuant to the Stock Purchase Agreement dated as of September 29, 2006, by and among us, Iceland Acquisition Subsidiary, I-Flow and InfuSystem was approved by the vote set forth below.
| Votes | ||
For | 13,945,464 | |
Against | 3,181,118 | |
Abstain | 666,667 |
The amendment of our Amended and Restated Certificate of Incorporation to change our name from “HAPC, INC.” to “InfuSystem Holdings, Inc.” was approved by the vote set forth below.
| Votes | ||
For | 15,282,735 | |
Against | 931,747 | |
Abstain | 1,895,000 |
The adoption of our 2007 Stock Incentive Plan was approved by the vote set forth below.
| Votes | ||
For | 12,924,094 | |
Against | 2,906,436 | |
Abstain | 1,895,000 |
12
Table of Contents
The following nominees were elected to serve as members of our board of directors, each to hold office until his successor is duly elected and qualified, by the vote set forth below:
Nominee | For | Withhold Authority | ||
Sean McDevitt | 15,409,463 | 2,701,016 | ||
John Voris | 15,411,828 | 2,698,651 | ||
Pat LaVecchia | 15,411,828 | 2,698,651 | ||
Wayne Yetter | 15,411,828 | 2,698,651 | ||
Jean Pierre Millon | 15,411,828 | 2,698,651 |
Each of the foregoing individuals served as members of our board of directors immediately prior to the Special Annual Meeting of Stockholders.
The ratification of the appointment of Deloitte & Touche LLP as our registered public accounting firm for the fiscal year ended December 31, 2007 was approved by the vote set forth below.
| Votes | ||
For | 15,289,011 | |
Against | 886,968 | |
Abstain | 1,934,500 |
13
Table of Contents
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Our common stock, warrants and units currently traded on the OTC Bulletin Board under the symbols INHI.OB, INHIW.OB and INHIU.OB, respectively. Prior to January 14, 2008, our common stock, warrants and units were traded on the OTC Bulletin Board under the symbols HAPN.OB, HAPNW.OB and HANPU.OB, respectively.
Each warrant entitles the holder to purchase from us one share of our common stock at an exercise price of $5.00. Our warrants will expire at 5:00 p.m., New York City time, on April 11, 2011, or earlier upon redemption.
The following tables set forth, for the calendar quarter indicated, the quarterly high and low bid information of our units, common stock and warrants, respectively, as reported on the OTC Bulletin Board. The quotations listed below reflect interdealer prices, without retail markup, markdown or commission and may not necessarily represent actual transactions.
Units
Quarter ended | High | Low | ||||
December 31, 2007 | $ | 6.44 | $ | 3.65 | ||
September 30, 2007 | $ | 6.40 | $ | 6.00 | ||
June 30, 2007 | $ | 6.55 | $ | 6.05 | ||
March 30, 2007 | $ | 6.25 | $ | 6.00 | ||
December 31, 2006 | $ | 6.15 | $ | 5.76 | ||
September 30, 2006 | $ | 6.08 | $ | 5.90 | ||
June 30, 2006 (1) | $ | 6.25 | $ | 5.90 | ||
| (1) | Represents the high and low bid information for our shares of units from June 15, 2006, the date that our Units first became separately tradable, through June 30, 2006. |
Common Stock
Quarter ended | High | Low | ||||
December 31, 2007 | $ | 5.84 | $ | 3.10 | ||
September 30, 2007 | $ | 5.86 | $ | 5.59 | ||
June 30, 2007 | $ | 5.86 | $ | 5.65 | ||
March 30, 2007 | $ | 5.67 | $ | 5.56 | ||
December 31, 2006 | $ | 5.59 | $ | 5.45 | ||
September 30, 2006 | $ | 5.50 | $ | 5.35 | ||
June 30, 2006 (1) | $ | 5.37 | $ | 5.35 | ||
| (1) | Represents the high and low bid information for our shares of common stock from June 15, 2006, the date that our common stock first became separately tradable, through June 30, 2006. |
Warrants
Quarter ended | High | Low | ||||
December 31, 2007 | $ | .495 | $ | .165 | ||
September 30, 2007 | $ | .355 | $ | .215 | ||
June 30, 2007 | $ | .395 | $ | .192 | ||
March 30, 2007 | $ | .34 | $ | .20 | ||
December 31, 2006 | $ | .32 | $ | .16 | ||
September 30, 2006 | $ | .31 | $ | .27 | ||
June 30, 2006 (1) | $ | .30 | $ | .28 | ||
| (1) | Represents the high and low bid information for our warrants from June 15, 2006, the date that our warrants first became separately tradable, through June 30, 2006. |
14
Table of Contents
Holders of Common Equity
As of March 13, 2008, we had approximately 7 stockholders of record of our common stock. This does not include beneficial owners of our common stock, including Cede & Co., nominee of the Depository Trust Company.
Dividends
We have not paid any dividends on our common stock to date. The payment of dividends in the future will be contingent upon our revenues and earnings, if any, capital requirements and general financial condition. It is the present intention of our board of directors to retain all earnings, if any, for use in our business operations and, accordingly, our board of directors does not anticipate declaring any dividends in the foreseeable future. There are no restrictions on our ability to pay dividends.
Equity Compensation Plan Information
The following table provides information as of December 31, 2007 with respect to compensation plans (including individual compensation arrangements) under which our equity securities are authorized for issuance.
Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||
| (a) | (b) | (c) | ||||
Equity compensation plans approved by security holders | None | Not Applicable | 2,000,000 | |||
Equity compensation plans not approved by security holders | Not Applicable | Not Applicable | Not Applicable | |||
Total | None | Not Applicable | 2,000,000 |
15
Table of Contents
Stock Performance Graph
The graph set forth below compares the change in the our cumulative total stockholder return on our common stock between June 15, 2006, (the date that our common stock became separately tradeable from our units) and December 31, 2007 with the cumulative total return of the NASDAQ Composite Index and the NASDAQ Biotechnology Index during the same period. This graph assumes the investment of $100 on June 15, 2006 in our common stock and each of the comparison groups and assumes reinvestment of dividends, if any. We have not paid any dividends on our common stock, and no dividends are included in the report of our performance. This graph is not “soliciting material,” is not deemed filed with the SEC and is not to be incorporated by reference in of our filings under the Securities Act or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
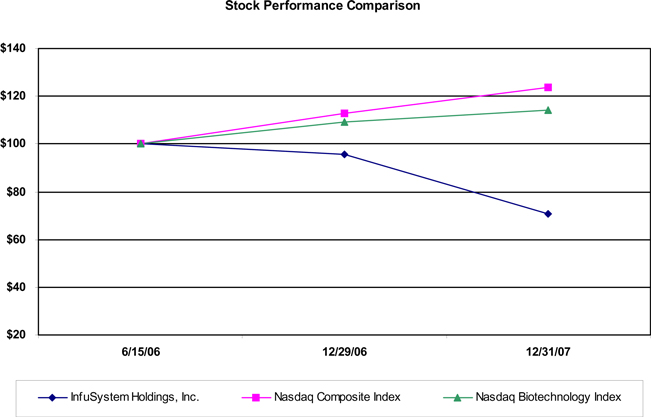
| 6/15/06 | 12/29/06 | 12/31/07 | |||||||
InfuSystem Holdings, Inc. Common Stock | $ | 100 | $ | 95.56 | $ | 70.94 | |||
Nasdaq Composite Index | $ | 100 | $ | 112.65 | $ | 123.70 | |||
Nasdaq Biotechnology Index | $ | 100 | $ | 109.14 | $ | 114.14 | |||
16
Table of Contents
Recent Sales of Unregistered Securities
As described in our Current Reports on Form 8-K filed on January 3, 2007, May 4, 2007 and September 12, 2007, during 2006 and 2007, we sold an aggregate of 1,357,717 warrants to purchase our common stock to members of our board of directors and our former Chief Financial Officer in private placement transactions made in reliance upon the exemption from securities registration afforded by Section 4(2) under the Securities Act and Regulation D thereunder.
Use of Proceeds from Registered Offering
On April 11, 2006, we commenced our initial public offering of 16,666,667 units, each consisting of one share of our common stock, par value $0.0001 per share, and two warrants, each exercisable for one share of our common stock, pursuant to a registration statement on Form S-1 (File No. 333-129035), which was declared effective on April 11, 2006. FTN Midwest Securities Corp. (“FTN Midwest”) served as underwriter for our initial public offering, which closed on April 18, 2006. The net proceeds from our initial public offering were $96,903,000, after deducting offering expenses of $497,000 and underwriting discounts of $2,600,000, including $1,000,000 evidencing the underwriters’ non-accountable expense allowance of 1% of the gross proceeds of the initial public offering. As of April 18, 2006, $95,000,000 of this amount had been placed in trust (including $5,400,000 payable to FTN Midwest for deferred underwriting discounts and commissions) for purposes of consummating a business combination, with $1,903,000 remaining.
On May 18, 2006, FTN Midwest exercised its over-allotment option in connection with our initial public offering and purchased 208,584 additional units. The net proceeds from the exercise of the over-allotment option were $1,232,000, after deducting underwriting discounts of $20,000. $1,215,000 of the net proceeds from the exercise of the over-allotment option was placed in trust (including $68,000 in deferred underwriters discounts and commissions) with $17,000 remaining.
As of December 31, 2006, we had expended all of the proceeds of our initial public offering held outside the trust account.
On October 25, 2007, we completed our acquisition of InfuSystem from I-Flow and disbursed the $101,257,000 balance in the trust account as set forth below.
Description | Amount | |||
Payment to I-Flow in connection with closing of acquisition of InfuSystem | $ | 73,829,000 | (1) | |
Conversion of shares of common stock | 16,899,000 | (2) | ||
Legal and other fees payable in connection with closing of acquisition of InfuSystem | 2,400,000 | (3) | ||
Transferred to operating account | 8,129,000 | |||
Total | $ | 101,257,000 | ||
| (1) | Represents $67,297,000 cash portion of the $100,000,000 purchase price paid for InfuSystem plus an additional $6,532,000, which consisted of a $3,000,000 termination fee, financing fees of approximately $2,002,000, a ticking fee of $693,000 and other acquisition related reimbursements of approximately $837,000. The $32,703,000 remainder of the purchase price was paid in the form of a loan from I-Flow. |
| (2) | Represents cash consideration paid to stockholders who held 2,816,488 shares in the aggregate, and who voted against our acquisition of InfuSystem and elected to convert their shares of common stock into their pro rata portion of the proceeds of the trust account, or $6.00 per share. As a result of 90,000 less shares ultimately converted by stockholders voting against the acquisition, we received a refund of $6.00 per share or $540,000. |
| (3) | Includes payments of $200,000 to each of Sean McDevitt and Philip B. Harris pursuant to the Guarantee Fee and Reimbursement Agreement, dated as of September 29, 2006, by and among Sean McDevitt, Philip B. Harris, Pat LaVecchia and the Company in connection with their guaranty of the break up fee payable to I-Flow upon a termination of the Stock Purchase Agreement. |
17
Table of Contents
On October 31, 2007, we paid a balance of $6,405,000 owed to FTN Midwest out of our operating account. The balance owed consisted of the items set forth below.
Deferred underwriting fee | $ | 4,555,000 | |
M&A advisory fees and expenses | 1,850,000 |
As a result of 90,000 less shares ultimately converted by shareholders voting against our acquisition of InfuSystem, we paid an additional underwriting fee of $29,000 to FTN Midwest.
The remaining cash of approximately $2,200,000 has and will continue to be used for general corporate purposes.
Repurchases of Equity Securities
None.
| Item 6. | Selected Financial Data. |
InfuSystem Holdings, Inc. and Subsidiary
The following historical information was derived from the audited consolidated financial statements of InfuSystem Holdings, Inc. for the fiscal years ended December 31, 2007 and 2006 and for the period from August 15, 2005 (inception) to December 31, 2005 and the related notes and schedules thereto, which are included in this Annual Report on Form 10-K. The information for InfuSystem Holdings, Inc. for the fiscal year ended December 31, 2007 includes operations for InfuSystem from October 26, 2007 through December 31, 2007.
Statement of Operations Data (1)
| (in thousands, except per share data) | Year Ended December 31, 2007 | Year Ended December 31, 2006 | Period from August 15, 2005 (inception) to December 31, 2005 | |||||||||
Net revenues | $ | 6,582 | $ | — | $ | — | ||||||
Total operating expenses | 8,079 | 20,824 | 24 | |||||||||
Total other (expense) income | (189 | ) | 14,003 | (1 | ) | |||||||
Income tax expense | (1,110 | ) | (1,038 | ) | — | |||||||
Net loss | (2,796 | ) | (7,859 | ) | (25 | ) | ||||||
Net loss per share – basic and diluted | $ | (0.15 | ) | $ | (0.58 | ) | $ | (0.01 | ) | |||
Balance Sheet Data (at period end) (1)
|
| |||||||||||
| (in thousands) | December 31, 2007 | December 31, 2006 | December 31, 2005 | |||||||||
Total assets | $ | 116,426 | $ | 100,298 | $ | 177 | ||||||
Long-term debt, including current maturities | 32,294 | — | — | |||||||||
Stockholders’ equity | 68,759 | 65,146 | — | |||||||||
| (1) | On October 25, 2007, we completed our acquisition of 100% of the issued and outstanding capital stock of InfuSystem from I-Flow pursuant to the terms of the Stock Purchase Agreement. InfuSystem’s results of operations are included in our Consolidated Statements of Operations from the date of the acquisition. For more information, see Note 3 “Acquisitions” to our Consolidated Financial Statements which are included in this Annual Report on Form 10-K. |
18
Table of Contents
Predecessor InfuSystem
The following historical information was derived from the audited financial statements of Predecessor InfuSystem for the period commencing after December 31, 2006 to October 25, 2007, the fiscal years ended December 31, 2006, 2005, 2004 and 2003 and the related notes and schedules thereto (“Predecessor InfuSystem”), which are included in this Annual Report on Form 10-K.
Statement of Operations Data
| January 1, 2007 to October 25, 2007 | Year Ended December 31, 2006 | Year Ended December 31, 2005 | Year Ended December 31, 2004 | Year Ended December 31, 2003 | |||||||||||
Net revenues | $ | 25,001 | $ | 31,716 | $ | 28,525 | $ | 19,349 | $ | 13,022 | |||||
Cost of revenues | 6,702 | 8,455 | 7,735 | 5,555 | 3,993 | ||||||||||
Total operating expenses | 15,673 | 15,091 | 12,709 | 9,142 | 7,130 | ||||||||||
Income tax expense | 1,086 | 3,094 | 2,938 | 1,699 | 720 | ||||||||||
Net income | 1,777 | 4,963 | 5,093 | 2,924 | 1,147 | ||||||||||
Balance Sheet Data (at period end)
| December 31, 2006 | December 31, 2005 | December 31, 2004 | December 31, 2003 | |||||||||
Total assets | $ | 27,628 | $ | 27,831 | $ | 17,665 | $ | 11,647 | ||||
Stockholders’ equity | 22,008 | 22,455 | 12,823 | 9,215 | ||||||||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operation. |
Executive Overview
Included in this Annual Report on Form 10-K are the results of operations of both the Company and Predecessor InfuSystem. The Company’s results of operations are presented for the fiscal years ended December 31, 2007 and 2006 and for the period from August 15, 2005 (inception) to December 31, 2005. The Company’s results of operations for the fiscal year ended December 31, 2007 include the operations of InfuSystem for a sixty seven day period from October 26, 2007 to December 31, 2007. The results of operations of Predecessor InfuSystem are presented for the fiscal years ended December 31, 2006 and 2005 and for the period commencing after the end of the fiscal year ended December 31, 2006 to October 25, 2007, the date of consummation of the acquisition. Also included in this Annual Report on Form 10-K is a discussion and analysis of the Company’s management with respect to Predecessor InfuSystem’s fiscal years ended December 31, 2006 and 2005 and for the period commencing after the end of the fiscal year ended December 31, 2006 to October 25, 2007, the date of consummation of the acquisition. Management’s Discussion and Analysis has been prepared by the Company based upon its understanding of and in reliance upon information communicated to the Company by former InfuSystem management regarding the reasons for the changes in Predecessor InfuSystem’s operations.
The financial statements and supplementary data of Predecessor InfuSystem presented for the period prior to October 26, 2007 are not those of the Company and were prepared by the former management of Predecessor InfuSystem and audited by Predecessor InfuSystem’s independent registered public accounting firm. The financial statements and supplementary data of Predecessor InfuSystem for the period prior to October 26, 2007 may be particularly unrepresentative of the operations of the Company going forward for the following reasons, among others:
| • | Both the Company’s financials and Predecessor InfuSystem’s financials contain items which require management to make considerable judgments and estimates. There can be no assurance that the judgments and estimates made by the Company’s management will be identical or even similar to the historical judgments and estimates made by Predecessor InfuSystem’s former management. |
19
Table of Contents
| • | The financials of Predecessor InfuSystem contain allocations of certain general and administrative expenses specific to I-Flow. |
| • | The Company’s financials are prepared utilizing a different basis of accounting than Predecessor InfuSystem’s financials. Specific differences include, but are not limited to, the Company’s accounting for net property, intangible assets and goodwill at fair value on the date of acquisition in accordance with SFAS No. 141,Business Combinations. Predecessor InfuSystem accounted for these items on a historical cost basis. |
InfuSystem Holdings, Inc. Overview
We were formed as a Delaware blank check company in 2005 for the purpose of acquiring through a merger, capital stock exchange, asset acquisition or other similar business combination, one or more operating businesses in the healthcare sector. On September 29, 2006, we entered into a Stock Purchase Agreement with I-Flow, Iceland Acquisition Subsidiary and InfuSystem. Upon the closing of the transactions contemplated by the Stock Purchase Agreement on October 25, 2007, Iceland Acquisition Subsidiary purchased all of the issued and outstanding capital stock of InfuSystem from I-Flow and concurrently merged with and into InfuSystem. As a result of the merger, Iceland Acquisition Subsidiary ceased to exist as in independent entity and InfuSystem, as the corporation surviving the merger, became our wholly-owned subsidiary. Effective October 25, 2007, we changed our corporate name from “HAPC, INC.” to InfuSystem Holdings, Inc. Upon our acquisition of InfuSystem, we ceased to be a development stage company and became an operating company.
Summary of Revenue and Expenses
The following table provides a summary of our revenues and expenses for the fiscal years ended December 31, 2007, 2006 and 2005 to supplement the more detailed discussions below:
| Years ended December 31, | ||||||||||||
(in thousands) | 2007 | 2006 | 2005 | |||||||||
Net revenues | $ | 6,582 | $ | — | $ | — | ||||||
Cost of revenues | ||||||||||||
Product and supply costs | $ | (923 | ) | $ | — | $ | — | |||||
Pump depreciation | (697 | ) | — | — | ||||||||
Total cost of revenues | $ | (1,620 | ) | $ | — | $ | — | |||||
Provision for doubtful accounts | $ | (584 | ) | $ | — | $ | — | |||||
Amortization of intangibles | $ | (335 | ) | $ | — | $ | — | |||||
Selling and marketing expenses | ||||||||||||
Sales salaries, commissions, fringes and payroll-related | $ | (524 | ) | $ | — | $ | — | |||||
Travel and entertainment | (88 | ) | — | — | ||||||||
Marketing | (27 | ) | — | — | ||||||||
Other | (10 | ) | — | — | ||||||||
Total selling and marketing expenses | $ | (649 | ) | $ | — | $ | — | |||||
General and administrative expenses | ||||||||||||
Share-based compensation expense | $ | (1,750 | ) | $ | (19,710 | ) | $ | (24 | ) | |||
Admin. salaries, fringes and payroll-related | (1,168 | ) | — | — | ||||||||
Ticking fee | (630 | ) | (95 | ) | ||||||||
Insurance | (307 | ) | — | — | ||||||||
Other | (1,036 | ) | (1,019 | ) | — | |||||||
Total general and administrative expenses | $ | (4,891 | ) | $ | (20,824 | ) | $ | (24 | ) | |||
(Loss) gain on derivatives | ||||||||||||
(Loss) gain on warrants | $ | (3,037 | ) | $ | 10,800 | $ | — | |||||
Loss on interest rate swap | (257 | ) | — | — | ||||||||
Total (loss) gain on derivatives | $ | (3,294 | ) | $ | 10,800 | $ | — | |||||
Interest income | $ | 3,879 | $ | 3,204 | $ | — | ||||||
Interest expense | ||||||||||||
Interest on term loan | $ | (637 | ) | $ | — | $ | — | |||||
Amortization of deferred debt issuance costs | (134 | ) | — | — | ||||||||
Swap income | 4 | — | — | |||||||||
Other | (7 | ) | (1 | ) | (1 | ) | ||||||
Total interest expense | $ | (774 | ) | $ | (1 | ) | $ | (1 | ) | |||
Income tax expense | $ | (1,110 | ) | $ | (1,038 | ) | $ | — | ||||
20
Table of Contents
InfuSystem Holdings, Inc. Results of Operations for the Fiscal Year ended December 31, 2007
Our results of operations for the year ended December 31, 2007 are not comparable with the prior periods presented. Effective October 25, 2007, upon our acquisition of InfuSystem, we ceased to be a development stage company and became an operating company.Substantially all activity through October 25, 2007 relates to our formation, initial public offering (the “IPO”) and efforts related to the acquisition of InfuSystem.
Our revenue consists predominantly of rental revenue derived from our rental of ambulatory infusion pumps which are primarily used for continuous infusion of chemotherapy drugs for patients with colorectal cancer. Our revenue for the fiscal year ended December 31, 2007 was $6,582,000, which includes InfuSystem revenue only for the sixty seven day period from October 26, 2007 to December 31, 2007. Management anticipates that new revenue growth will come from the expansion of the existing use of our ambulatory infusion pumps for the treatment of colorectal cancer as well as the potential future use of our ambulatory infusion pumps for continuous infusion of chemotherapy drugs for the treatment of head, neck and gastric cancer. Another aspect of our business strategy over the next one to three years is to actively pursue opportunities for the expansion of our business through acquisitions, joint ventures and strategic alliances.
Cost of revenues for the fiscal year ended December 31, 2007 was $1,620,000. This consisted of product and supply costs, including freight costs for the transport of pumps and supplies to and from oncology practices, and depreciation on our infusion pumps.
Provision for doubtful accounts for the fiscal year ended December 31, 2007 was $584,000.
21
Table of Contents
Amortization of our intangible assets for the fiscal year ended December 31, 2007 was $335,000.
During the fiscal year ended December 31, 2007, our selling and marketing expenses were $649,000. Selling and marketing expenses during this period consisted of sales salaries, commissions and associated fringe benefit and payroll-related items, travel and entertainment, marketing and other miscellaneous expenses.
During the fiscal year ended December 31, 2007, our general and administrative expenses were $4,891,000. General and administrative expenses during this period consist primarily of share-based compensation expense, administrative personnel (including management and officers) salaries, fringes and payroll-related items, the ticking fee we paid to I-Flow on the date of the acquisition of InfuSystem, insurance (including directors’ and officers’ insurance) and other miscellaneous expenses.
During the fiscal year ended December 31, 2007, we recorded a loss on derivatives of $3,294,000. This amount represents an unrealized loss which resulted from the change in the fair value of our warrants and the change in the fair value of our single interest rate swap. For more information, please refer to the discussion under “Summary of Significant Accounting Policies—Warrants and Derivative Financial Instruments” included in Note 2 and “Warrants and Derivative Financial Instruments” included in Note 7 to our Consolidated Financial Statements included in this Annual Report on Form 10-K.
During the fiscal year ended December 31, 2007, we recorded interest income of $3,879,000. This amount consists primarily of interest earned on the trust account (in which a substantial portion of the proceeds of our initial public offering were deposited) through the date of the acquisition. Interest income is not expected to be significant going forward.
During the fiscal year ended December 31, 2007, we recorded interest expense of $774,000. This amount consists of interest paid to I-Flow on our term loan, the amortization of deferred debt issuance costs incurred in conjunction with the loan, income on the interest rate swap and other interest expense.
During the fiscal year ended December 31, 2007, we recorded income tax expense of $1,110,000. This amount consists of federal, state and local tax provisions.
Management believes that there has been no material effect on our operations or financial condition as a result of inflation or changing prices of our ambulatory infusion pumps during the period from August 15, 2005 (inception) through December 31, 2007.
InfuSystem Holdings, Inc. Results of Operations for the Fiscal Year ended December 31, 2006
During the fiscal year ended December 31, 2006, we had not yet consummated our acquisition of InfuSystem, however, we completed our initial public offering, signed a Material Definitive Agreement (see Note 3 to our Consolidated Financial Statements included in this Annual Report on Form 10-K) and incurred non-cash expenses of $6,660,000 and $13,050,000, which represented the amortization of stock based compensation and the value of 2,416,666 shares granted to two members of our board of directors, respectively. The balance of expenses was offset by interest income of $3,204,000 and an unrealized gain on warrant liabilities of $10,800,000, resulting in a net loss of $7,859,000 for the fiscal year ended December 31, 2006.
InfuSystem Holdings, Inc. Results of Operations from August 15, 2005 (inception) to December 31, 2005
For the period from August 15, 2005 (date of inception) to December 31, 2005, we had substantially no operations.
Predecessor InfuSystem Overview
The financial statements and supplementary data of Predecessor InfuSystem presented for the period prior to October 26, 2007 are not those of the Company and were prepared by the former management of Predecessor InfuSystem and audited by Predecessor InfuSystem’s independent registered public accounting firm. The financial statements and supplementary data of Predecessor InfuSystem for the period prior to October 26, 2007 may be particularly unrepresentative of the operations of the Company going forward for the following reasons, among others:
| • | Both the Company’s financials and Predecessor InfuSystem’s financials contain items which require management to make considerable judgments and estimates. There can be no assurance that the judgments and estimates made by the Company’s management will be identical or even similar to the historical judgments and estimates made by Predecessor InfuSystem’s former management. |
22
Table of Contents
| • | The financials of Predecessor InfuSystem contain allocations of certain general and administrative expenses specific to I-Flow. |
| • | The Company’s financials are prepared utilizing a different basis of accounting than Predecessor InfuSystem’s financials. Specific differences include, but are not limited to, the Company’s accounting for net property, intangible assets and goodwill on a fair value basis. Predecessor InfuSystem accounted for those items on a historical cost basis. |
Predecessor InfuSystem Results of Operations from January 1, 2007 to October 25, 2007 Compared to the Year Ended December 31, 2006
Revenues
Revenues for the period from January 1, 2007 to October 25, 2007 were $25,001,000, compared to $31,716,000 for the year ended December 31, 2006. Revenues for the comparable January 1 to October 25 periods were approximately even year-over-year. This was primarily the result of increased gross billings, offset by a decrease in revenue recognition by Predecessor InfuSystem effective January 1, 2007, as well as management focus during 2007 on completing the acquisition.
Cost of Revenues
Cost of revenues, which includes depreciation on infusion pumps, product and supply costs and freight costs, were $6,702,000 for the period from January 1, 2007 to October 25, 2007, compared to $8,455,000 for the year ended December 31, 2006. Cost of revenues as a percentage of revenues for the period from January 1, 2007 to October 25, 2007 was equal to that of the year ended December 31, 2006.
Selling and Marketing Expenses
Selling and marketing expenses for the period from January 1, 2007 to October 25, 2007 were $3,390,000, compared to $3,803,000 for the year ended December 31, 2006. Predecessor InfuSystem recognized stock-based compensation costs related to selling and marketing expenses of approximately $116,000 and $254,000 for the period from January 1, 2007 to October 25, 2007 and the year ended December 31, 2006, respectively.
As a percentage of net revenues, selling and marketing expenses increased by approximately two percentage points for the period from January 1, 2007 to October 25, 2007 compared to the year ended December 31, 2006, primarily related to transaction-related bonuses paid to salespersons.
General and Administrative Expenses
General and administrative expenses for the period from January 1, 2007 to October 25, 2007 were $12,283,000, compared to $11,288,000 for the year ended December 31, 2006. As a percentage of net revenues, general and administrative expenses increased by approximately thirteen percentage points for the period from January 1, 2007 to October 25, 2007 compared to the year ended December 31, 2006. The increase was primarily attributable to increases in transaction-related management bonuses (increased from 0% to 4% of revenues), bad debt expense (increased from 13% to 20% of revenues) and stock-based compensation expense (increased from 1% to 3% of revenues). The increase in bad debt expense for the period from January 1, 2007 to October 25, 2007 compared to the year ended December 31, 2006 resulted primarily from the continued delay in collections from a specific large third party insurer. The delays resulted from procedural and processing changes within the insurer’s affiliated group
23
Table of Contents
of companies. Predecessor InfuSystem recognized stock-based compensation costs related to general and administrative expenses of approximately $630,000 and $142,000 for the period from January 1, 2007 to October 25, 2007 and the year ended December 31, 2006, respectively. The increase in stock-based compensation expense related to the immediate vesting of restricted stock units and stock options of management in connection with the closing of the acquisition.
Predecessor InfuSystem management estimates that unreimbursed processing costs borne by Predecessor InfuSystem for I-Flow’s ON-Q® billings and reflected in its financial statements were approximately $1,101,000 and $1,457,000 for the period from January 1, 2007 to October 25, 2007 and the year ended December 31, 2006, respectively. Predecessor InfuSystem continued to bear these processing costs until the closing of the acquisition. Pursuant to the terms of the Services Agreement between Predecessor InfuSystem and I-Flow, I-Flow compensates InfuSystem for its processing costs related to I-Flow’s ON-Q® billings and provides InfuSystem with an incentive-based reimbursement arrangement. Pursuant to the terms of the Services Agreement, InfuSystem will continue to provide to I-Flow, from and after the closing, the billing and collection services and management services Predecessor InfuSystem has been providing prior to the date of the closing of the acquisition. Fees paid by I-Flow under the Services Agreement are set at the higher of a cost-plus or percentage of collections. On November 8, 2007, I-Flow informed InfuSystem that it was terminating the Services Agreement effective May 10, 2008.
In connection with the sale of Predecessor InfuSystem, I-Flow incurred certain expenses related to the divestiture, including legal and professional fees that resulted directly from the transaction. Divestiture expenses incurred by I-Flow for the period January 1, 2007 to October 25, 2007 and the year ended December 31, 2006, were approximately $2,329,000 and $2,090,000, respectively. At the close of the sale of Predecessor InfuSystem, we reimbursed I-Flow approximately $946,000 in certain divestiture expenses incurred in 2006 and 2007. The divestiture expenses incurred were not reimbursed by Predecessor InfuSystem to I-Flow and, accordingly, the amounts, including the reimbursement by us for divestiture expenses, are not included in Predecessor InfuSystem’s financial statements.
Interest Income / Expense
Interest income for the period from January 1, 2007 to October 25, 2007 was $237,000, compared to interest expense of $113,000 for the year ended December 31, 2006. The improvement during 2007 was due primarily to the reversal of $267,000 of interest expense as explained below.
In August 2005, the State of Michigan Department of Treasury issued a decision and order of determination which provided that InfuSystem was liable for use taxes on its purchases of infusion pumps. As a result, Predecessor InfuSystem recorded through March 31, 2007 a cumulative net increase to net fixed assets of $700,000, a tax liability of $1,466,000 and total expense of $1,033,000, consisting of $766,000 cost of sales and $267,000 accrued interest expense. InfuSystem appealed the decision. InfuSystem believes that portable infusion pumps which allow cancer patients to be ambulatory and lead a reasonably normal life, qualify for an exemption from use tax under Michigan law. On April 24, 2007, the Michigan Tax Tribunal granted a Motion for Summary Disposition in favor of InfuSystem, which was not appealed by the State of Michigan Department of Treasury. The review period for the ruling by the Michigan Tax Tribunal has ended which effectively forecloses further appeal of the August 2005 decision and order. As a result of the favorable ruling, Predecessor InfuSystem reversed in the second quarter of 2007 the cumulative effects of the liability and expense recorded to date. Predecessor InfuSystem’s balance sheet reflects the decrease of $1,466,000 and $267,000 of accrued tax liability and accrued interest expense, respectively, and a decrease of $700,000 in cumulative net fixed assets. Predecessor InfuSystem’s statement of income has been impacted by a reversal of $1,033,000 in total expense, consisting of $766,000 of cost of sales and $267,000 of interest expense.
24
Table of Contents
Income Tax Provision
The income tax provision for the period from January 1, 2007 to October 25, 2007 was $1,086,000, compared to $3,094,000 for the year ended December 31, 2006. The decrease was primarily attributable to a decrease in pretax income. InfuSystem’s effective tax expense rates for the period from January 1, 2007 to October 25, 2007 and the year ended December 31, 2006 were 37.9% and 38.4%, respectively.
Net Income
Net income for the period from January 1, 2007 to October 25, 2007 was $1,777,000, compared to $4,963,000 in the year ended December 31, 2006. The decrease was primarily due to less than a full year of revenues, the payment of transaction-related management bonuses, the acceleration of stock-based compensation, and an increase in bad debt expense.
Predecessor InfuSystem Results of Operations for the Year Ended December 31, 2006 Compared to the Year Ended December 31, 2005
Revenues
Revenues increased 11% or $3,191,000, to $31,716,000 for the year ended December 31, 2006 from $28,525,000 for the year ended December 31, 2005. The increase in revenues for the year ended December 31, 2006 compared to the prior year was primarily due to an increased usage of new drugs and clinical protocols that require the use of ambulatory infusion electronic pumps. Net revenues for the year ended December 31, 2006 increased despite the unfavorable impact of shortages of 5-Fluorouracil, a commonly used chemotherapy drug, that began in the fourth quarter of 2005 and continued into the first quarter of 2006. Availability of 5-Fluorouracil returned to normal at the end of the first quarter of 2006. However, revenue is believed to have been adversely affected during the second, and possibly third, quarters of 2006 due to a decline in the number of patients utilizing the ambulatory infusion pumps. Predecessor InfuSystem believes that these patients were forced to turn to other medications or treatments during this time.
Cost of Revenues
Cost of revenues increased 9%, or $720,000, to $8,455,000 for the year ended December 31, 2006 from $7,735,000 for the year ended December 31, 2005. Cost of revenues increased for the year ended December 31, 2006 compared to the prior year primarily due to the increase in revenues. Cost of revenues as a percentage of revenues for the year ended December 31, 2006 was equal to that of the prior year.
Selling and Marketing Expenses
Selling and marketing expenses decreased 12%, or $512,000, to $3,803,000 for the year ended December 31, 2006 from $4,315,000 for the year ended December 31, 2005. The decrease in expenses was primarily attributable to a decrease in commissions ($700,000), which was due to the unfavorable impact of the 5-Fluorouracil shortage during the first quarter of 2006, resulting in less compensation expense recorded for the achievement of sales goals, offset in part by an increase in advertising expense ($100,000). Predecessor InfuSystem recognized stock-based compensation costs related to selling and marketing expenses of approximately $254,000 and $266,000 for the years ended December 31, 2006 and 2005, respectively. The adoption of SFAS 123R in fiscal 2006 did not have a significant impact on stock-based compensation expense for selling and marketing expenses because Predecessor InfuSystem was required to recognize such expenses under the prior accounting guidance for the stock awards issued to the sales force, which were generally granted with an exercise price below fair market value.
As a percentage of net revenues, selling and marketing expenses decreased by approximately three percentage points for the year ended December 31, 2006 compared to the prior year, primarily because of the decrease in selling and marketing expenses described above.
General and Administrative Expenses
General and administrative expenses increased 34%, or $2,894,000, to $11,288,000 for the year ended December 31, 2006 from $8,394,000 for the year ended December 31, 2005. The increase was primarily attributable to increases in bad debt expense ($2,780,000) and salaries, wages and fringe benefits related expenses ($581,000),
25
Table of Contents
offset in part by a decrease in non-cash compensation expense related to the amortization of deferred compensation ($678,000). The increase in bad debt expense for the year ended December 31, 2006 compared to the prior year resulted primarily from a delay in collections from a specific large third party insurer. The delays resulted from procedural and processing changes within the insurer’s affiliated group of companies. Increases in salaries, wages and fringe benefits and related expenses for the year ended December 31, 2006 compared to the prior year were primarily due to increased staffing to support the growth of Predecessor InfuSystem. The decrease in non-cash compensation expense related to the amortization of deferred compensation was primarily due to the upward repricing and acceleration of the out-of-the-money stock options on November 9, 2005, offset in part by the adoption of SFAS 123R in fiscal 2006. Predecessor InfuSystem recognized stock-based compensation costs related to general and administrative expenses of approximately $142,000 and $819,000 during the year ended December 31, 2006 and 2005, respectively.
Predecessor InfuSystem management estimates that unreimbursed processing costs borne by Predecessor InfuSystem for I-Flow’s ON-Q® billings and reflected in its financial statements were approximately $1,457,000 and $1,165,000 for the years ended December 31, 2006 and 2005, respectively. Predecessor InfuSystem continued to bear these processing costs until the closing of the acquisition. Pursuant to the terms of the Services Agreement between Predecessor InfuSystem and I-Flow, I-Flow compensates InfuSystem for its processing costs related to I-Flow’s ON-Q® billings and provides InfuSystem with an incentive-based reimbursement arrangement. Pursuant to the terms of the Services Agreement, InfuSystem will continue to provide to I-Flow, from and after the closing, the billing and collection services and management services Predecessor InfuSystem has been providing prior to the date of the closing of the acquisition. Fees paid by I-Flow under the Services Agreement are set at the higher of a cost-plus or percentage of collections. On November 8, 2007, I-Flow informed InfuSystem that it was terminating the Services Agreement effective May 10, 2008.
I-Flow incurred certain expenses related to the divestiture of Predecessor InfuSystem, including legal and professional fees that resulted directly from the transaction. Divestiture expenses for the year ended December 31, 2006 were $2,090,000, which were not reimbursed to I-Flow by Predecessor InfuSystem and are not reflected in Predecessor InfuSystem’s financial statements.
Interest Expense
Interest expense increased 126%, or $63,000, to $113,000 for the year ended December 31, 2006 from $50,000 for the year ended December 31, 2005. The increase for the year ended December 31, 2006 was primarily due to an increase in the accrual of interest expense in connection with a dispute with the State of Michigan Department of Treasury related to use taxes on purchases of infusion pumps.
In August 2005, the State of Michigan Department of Treasury issued a decision and order of determination which provided that InfuSystem was liable for use taxes on its purchases of infusion pumps. As a result, Predecessor InfuSystem recorded through December 31, 2006 a cumulative net increase to gross fixed assets of $1,276,000, a tax liability of $1,392,000, and total expense of $943,000 (of which $302,000 and $236,000 were recorded during the years ended December 31, 2006 and 2005, respectively). The $943,000 total expense recorded through December 31, 2006 consists of $706,000 cost of sales (of which $209,000 and $187,000 were recorded during the years ended December 31, 2006 and 2005, respectively) and $237,000 accrued interest expense (of which $93,000 and $49,000 were recorded during the years ended December 31, 2006 and 2005, respectively). Predecessor InfuSystem appealed the decision. Predecessor InfuSystem believes that portable infusion pumps which allow cancer patients to be ambulatory and lead a reasonably normal life, qualify for an exemption from use tax under Michigan law. On April 24, 2007, the Michigan Tax Tribunal granted a Motion for Summary Disposition in favor of InfuSystem, which was not appealed by the State of Michigan Department of Treasury. The review period for the ruling by the Michigan Tax Tribunal has ended which effectively forecloses further appeal of the August 2005 decision and order. As a result of the favorable ruling, Predecessor InfuSystem reversed in the second quarter of 2007 the cumulative effects of the liability and expense recorded to date.
26
Table of Contents
Income Tax Provision
The income tax provision increased 5%, or $156,000, to $3,094,000 for the year ended December 31, 2006 from $2,938,000 for the year ended December 31, 2005. The increase was primarily attributable to an increase in pretax income. InfuSystem’s effective tax expense rates for the years ended December 31, 2006 and 2005 were 38.4% and 36.6%, respectively.
Net Income
Net income for the year ended December 31, 2006 was $4,963,000, compared to $5,093,000 in the prior year, a decrease of 2%. The decrease was primarily due to an increase of $2,893,000 in general and administrative expenses, partially offset by an increase in gross profit of $2,471,000 and a decrease in selling and marketing expense of $511,000.
Liquidity and Capital Resources
As of December 31, 2007 we had cash resources of $3,960,000 compared to $427,000 at December 31, 2006. The increase in cash available to us was associated with our closing of the acquisition of InfuSystem on October 25, 2007 and the liquidation of the trust account in which a substantial portion of the net proceeds from our initial public offering were held. See Item 5 – “Use of Proceeds from a Registered Offering” for a detailed description of the disbursements made from the trust account in connection with the closing of the acquisition.
Cash provided by operating activities for the fiscal year ended December 31, 2007 was $4,377,000, compared to cash used in operating activities of $912,000 for the fiscal year ended December 31, 2006. The increase for the fiscal year ended December 31, 2007 was due primarily to the acquisition of InfuSystem on October 25, 2007, the inclusion of its operating results and cash flows from that date through the end of the period and the withdrawal of interest earned on the investments held in trust.
Cash provided by investing activities for the fiscal year ended December 31, 2007 was $22,018,000, compared to cash used in investing activities of $96,956,000 for the fiscal year ended December 31, 2006. The increase for the fiscal year ended December 31, 2007 is primarily due to consummation of the acquisition on October 25, 2007 and the associated $96,215,000 withdrawal from the trust account, as well as $2,343,000 of cash acquired from I-Flow as part of the acquisition. Partial offsets to this increase included $67,297,000 paid to I-Flow representing the cash portion of the purchase price, $8,690,000 of deferred acquisition costs paid and capital expenditures of $553,000.
Cash used in financing activities for the fiscal year ended December 31, 2007 was $22,862,000, compared to cash provided by financing activities of $98,282,000 for the fiscal year ended December 31, 2006. The decrease for the fiscal year ended December 31, 2007 reflects the $16,359,000 paid to shareholders who voted against the acquisition and converted their shares, payment to FTN Midwest of $4,555,000 in deferred underwriting fees, $2,052,000 of capitalized debt issuance costs associated with the term loan from I-Flow and $409,000 in principal payments on the term loan. The decrease was partially offset by $513,000 received from the issuance of warrants. During the fiscal year ended December 31, 2006, we received significant proceeds from our initial public offering.
As of December 31, 2007, we had cash and cash equivalents of $3,960,000, net accounts receivable of $6,304,000 and net working capital (excluding derivative liabilities) of $6,889,000. Management believes the current funds, together with expected cash flows from ongoing operations, are sufficient to fund our operations for at least the next 12 months. We intend to further supplement liquidity by securing a line of credit during the fiscal year ended December 31, 2008.
As of December 31, 2007, we did not have a line of credit in place. We have, however, initiated discussions with several financial institutions with the intent to enter into a line of credit. Any line of credit is
27
Table of Contents
expected to be collateralized by certain of our assets (as permitted under our Credit and Guaranty Agreement, dated as of October 25, 2007, with I-Flow), and will likely require us to comply with certain covenants relating to profitability and liquidity measures. Other than customary operating expenditures and payments of principal and interest on our loan from I-Flow, cash requirements for the fiscal year ended December 31, 2008 are expected to consist primarily of capital expenditures for new infusion pumps. Management expects capital expenditures to be in the range of $4,000,000 to $5,000,000 during 2008. We intend to pursue alternative financing options for some or all of these expenditures.
Contractual Obligations
As of December 31, 2007, future payments related to contractual obligations are as follows:
| Payment Due by Period(1)(2) | |||||||||||||||
| Less than 1 Year | 1 to 3 Years | 3 to 5 Years | More than 5 Years | Total | |||||||||||
| (Amounts in Thousands) | |||||||||||||||
Debt obligations | $ | 2,044 | $ | 30,250 | $ | — | $ | — | $ | 32,294 | |||||
Operating Lease Obligations | 116 | — | — | — | 116 | ||||||||||
Total | $ | 2,160 | $ | 30,250 | $ | — | $ | — | $ | 32,410 | |||||
| (1) | The table above does not include any potential payout to I-Flow associated with the earn-out provision. For more information, please refer to the discussion under “Acquisitions” included in Note 3 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. |
| (2) | The table above does not include any interest payments associated with our variable rate term debt. For more information, please refer to the discussion under “Debt” included in Note 8 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. |
The operating lease obligations represent future minimum lease payments as of December 31, 2007 under a non-cancelable operating lease for our Madison Heights, Michigan office and a non-cancelable operating lease for our warehouse facility at the same location. Both leases expire on June 30, 2008. The Company is presently considering its options upon the expiry of the leases, including the possibility of remaining in the same location with new lease agreements.
Contingent Liabilities
We do not have any contingent liabilities.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Critical Accounting Policies and Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires the appropriate application of certain accounting policies, many of which require management to make estimates and assumptions about future events and their impact on amounts reported in our consolidated financial statements and related notes. Since future events and their impact cannot be determined with certainty, actual results may differ from management’s estimates. Such differences may be material to our consolidated financial statements.
Management believes its application of accounting policies, and the estimates inherently required therein, are reasonable. These accounting policies and estimates are periodically reevaluated, and adjustments are made when facts and circumstances dictate a change.
Our accounting policies are more fully described under the heading “Summary of Significant Accounting Policies” in Note 2 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. We believe the following critical accounting estimates are the most significant to the presentation of our financial statements and require the most difficult, subjective and complex judgments:
28
Table of Contents
Revenue Recognition
Our strategic focus is rental revenue in the oncology market. Revenues are recognized predominantly under fee for service arrangements through equipment we rent to patients. We recognize revenue only when all of the following criteria are met: persuasive evidence of an arrangement exists; services have been rendered; the price to the customer is fixed or determinable; and collectability is reasonably assured. Persuasive evidence of an arrangement is determined to exist, and collectability is reasonably assured, when we receive a physician’s order (or certificate of medical necessity) and assignment of benefits, signed by the physician and patient, respectively, and we have verified actual pump usage and insurance coverage. We recognize rental revenue from electronic infusion pumps as earned, normally on a month-to-month basis. We bill pump rentals at established rates, which often differ from contractually allowable rates provided by third-party payors such as Medicare, Medicaid and commercial insurance carriers. All billings to third party payors are recorded net of provision for contractual adjustments to arrive at net revenues.
Due to the nature of the industry and the reimbursement environment in which we operate, certain estimates are required to record net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Due to continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on results of operations and cash flows.
Accounts Receivable and Allowance for Doubtful Accounts
We have agreements with third-party payors which provide for payments at amounts different from established rates. Patient accounts receivable and net revenue are reported at the estimated net realizable amounts from patients, third-party payors and others for service rendered. We perform periodic analyses to assess the accounts receivable balances. We record an allowance for doubtful accounts based on the estimated collectability of the accounts such that the recorded amounts reflect estimated net realizable value. Upon determination that an account is uncollectible, the account is written-off and charged to the allowance.
Substantially all of our receivables are related to providing healthcare services to patients. Accounts receivable are reduced by an allowance for amounts that could become uncollectible in the future. Our estimate for allowance for doubtful accounts is based upon management’s assessment of historical and expected net collections by payor. Due to the continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on our financial position, results of operations, and cash flows.
Warrants and Derivative Instruments
On April 18, 2006, we consummated our IPO of 16,666,667 units. Each unit consists of one share of common stock and two redeemable common stock purchase warrants. Each warrant entitles the holder to purchase from us one share of common stock at an exercise price of $5.00. On May 18, 2006, we sold an additional 208,584 units to FTN Midwest, the underwriter of its IPO, pursuant to a partial exercise by FTN Midwest of its overallotment option. We are required by the Warrant Agreement to register the shares underlying the warrants in the absence of our ability to deliver registered shares to the warrant holders upon warrant exercise.
In September 2000, the Emerging Issues Task Force issued EITF 00-19,Accounting for Derivative Financial Instruments Indexed to and Potentially Settled in, a Company’s Own Stock, (“EITF 00-19”) which requires freestanding derivative contracts that are settled in a company’s own stock, including common stock warrants, to be designated as equity instruments, assets or liabilities. Under the provisions of EITF 00-19, a contract designated as an asset or a liability must be carried at its fair value on a company’s balance sheet, with any changes in fair value recorded in the company’s results of operations. A contract designated as an equity instrument must be included within equity, and no fair value adjustments are required from period to period.
29
Table of Contents
In accordance with EITF 00-19, the 33,750,502 warrants issued in connection with the IPO and overallotment option must be settled in registered shares and are separately accounted for as liabilities as discussed in Note 7 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. The fair value of these warrants is reflected on our balance sheet and the unrealized changes in the value of these warrants are reflected in our statement of operations as “Gain (loss) on warrant liabilities.” These warrants are freely traded on the “Over The Counter Bulletin Board.” Consequently, the fair value of these warrants is estimated as the market price of the warrant at each period end. To the extent the market price increases or decreases, our warrant liabilities will also increase or decrease with a corresponding impact on our results of operations.
Sales of warrants that may be settled in unregistered shares of common stock are treated as equity and included in additional paid in capital as discussed in Note 11 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. The total number of warrants issued to date that may be settled in unregistered shares of common stock is 1,357,717 at an issue price of $.70 per warrant or a total issue price of $950,000.
SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities, requires that an entity recognize all derivatives as either assets or liabilities in the statement of financial position and measure those instruments at fair value.
In December 2007, we entered into an interest rate swap to hedge the exposure associated with our floating rate debt. We have elected not to designate the swap as a cash flow hedge, in accordance with SFAS No. 133. The fair value of the swap is therefore reflected on our balance sheet and the unrealized changes in the value of the swap are reflected in our statement of operations within “Gain (loss) on derivatives”.
Income Taxes
We account for income taxes in accordance with SFAS No. 109,Accounting for Income Taxes, which requires that we recognize deferred tax liabilities and assets based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities, using enacted tax rates in effect in the years the differences are expected to reverse. Deferred income tax benefit (expense) results from the change in net deferred tax assets or deferred tax liabilities. A valuation allowance is recorded when, in the opinion of management, it is more likely than not that some or all of any deferred tax assets will not be realized. For more information, please refer to the “Income Taxes” discussion included in Note 9 to the Consolidated Financial Statements.
Goodwill Valuation
Goodwill arising from business combinations represents the excess of the purchase price over the estimated fair value of the net assets of the businesses acquired. In accordance with the provisions of SFAS No. 142,Goodwill and Other Intangible Assets,goodwill is tested annually for impairment or more frequently if circumstances indicate the possibility of impairment. We have selected October 31 to perform our annual impairment test. Management does not believe impairment of our goodwill existed at December 31, 2007. The goodwill amount for the October 25, 2007 acquisition of InfuSystem, totaling $56,544,000, is based upon preliminary estimates that are subject to change in 2008 upon completion of the final valuation analysis. Final determination of these estimates could result in an adjustment to the purchase price allocation with an offsetting adjustment to goodwill.
Recent Accounting Pronouncements
On January 1, 2007, we adopted FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes(FIN 48). FIN 48 clarifies the accounting for income taxes by prescribing a minimum probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is defined in FIN 48 as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. FIN 48 must be applied to all existing tax positions upon initial adoption.
30
Table of Contents
The cumulative effect of applying FIN 48 at adoption, if any, is to be reported as an adjustment to opening retained earnings for the year of adoption. The adoption of FIN 48 did not have a material effect on our consolidated financial position or results of operations.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements, which relates to the definition of fair value, the methods used to measure fair value and the expanded disclosures about fair value measurements. The provisions of SFAS No. 157 are effective for financial statements issued for fiscal years beginning after November 15, 2007. We have not yet completed our analysis of the impact of adopting SFAS No. 157 on the consolidated financial position, results of operations or cash flows.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB Statement No. 115, which permits entities to choose to measure many financial instruments and certain other items at fair value that are not currently required to be measured at fair value. The objective is to improve financial reporting by providing entities with the opportunity to mitigate volatility in reported earnings caused by measuring related assets and liabilities differently without having to apply complex hedge accounting provisions. SFAS No. 159 is expected to expand the use of fair value measurement, which is consistent with the Board’s long-term measurement objectives for accounting for financial instruments. SFAS No. 159 also establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. SFAS No. 159 does not affect any existing accounting literature that requires certain assets and liabilities to be carried at fair value. SFAS No. 159 does not establish requirements for recognizing and measuring dividend income, interest income, or interest expense. SFAS No. 159 does not eliminate disclosure requirements included in other accounting standards, including requirements for disclosures about fair value measurements, included in SFAS No. 157,Fair Value Measurements, and SFAS No. 107,Disclosures about Fair Value of Financial Instruments. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. We have not yet completed our assessment of the impact upon adoption of SFAS No. 159 on the consolidated financial position, results of operations or cash flows.
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations. This statement retains the fundamental requirements of the original pronouncement requiring that the acquisition method of accounting, or purchase method, be used for all business combinations. SFAS No. 141(R) defines the acquirer as the entity that obtains control of one or more businesses in the business combination, establishes the acquisition date as the date that the acquirer achieves control and requires the acquirer to recognize the assets acquired, liabilities assumed and any noncontrolling interest at their fair values as of the acquisition date. In addition, SFAS No. 141(R) requires, among other things, expensing of acquisition related and restructuring related costs, measurement of pre-acquisition contingencies at fair value, measurement of equity securities issued for purchase at the date of close of the transaction and capitalization of in process research and development, all of which represent modifications to current accounting for business combinations. SFAS No. 141(R) is effective for fiscal years beginning after December 15, 2008. Adoption is prospective and early adoption is not permitted. Adoption of SFAS No. 141(R) will not impact our accounting for business combinations closed prior to its adoption, but given the nature of the changes noted above, we expect our accounting for business combinations occurring subsequent to adoption will be significantly different than that applied following current accounting literature.
| Item 7A. | Quantitative and Qualitative Disclosure About Market Risk. |
We are exposed to interest rate fluctuations on our underlying variable rate long-term debt. We utilize an interest rate swap agreement to moderate the majority of such exposure. We do not use derivative financial instruments for trading or other speculative purposes.
At December 31, 2007, the principal plus accrued interest on our term loan with I-Flow was $32,294,000. The term loan bears interest at either LIBOR plus 5.5% or Prime plus 4.5%, at our option. The loan is a variable rate loan and therefore fair value approximates book value. Please see the heading “Debt” under Note 8 to our Consolidated Financial Statements included in this Annual Report on Form 10-K for a further discussion of our term loan with I-Flow.
31
Table of Contents
At December 31, 2007, we had one interest rate swap agreement in effect to fix our LIBOR-based variable rate debt. The interest rate swap agreement, which expires in December 2010, had a notional amount of $19,750,000 on December 31, 2007 and a fixed rate of 4.29%.
Based on the term loan outstanding and the swap agreement in place at December 31, 2007, a 100 basis point change in the applicable interest rates would increase or decrease our 2007 cash flow and pretax earnings by approximately $55,000.
We have classified certain warrants as derivative liabilities, which resulted in a liability of $12,150,000 at December 31, 2007. We classified the warrants as derivative liabilities because there is a possibility that we may be required to settle the warrants in registered shares of common stock. We are required to compare the fair market value of these instruments from the date of the initial recording to their fair market value as of the end of each reporting period and to reflect the change in fair market value in our Consolidated Statements of Operations as a gain or loss for the applicable period.
32
Table of Contents
| Item 8. | Financial Statements and Supplementary Data. |
33
Table of Contents

Deloitte & Touche LLP |
600 Renaissance Center |
Suite 900 |
Detroit, MI 48243-1895 |
USA |
Tel: +1 313 396 3000 |
Fax: +1 313 396 3678 www.deloitte.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
InfuSystem Holdings, Inc.:
We have audited the accompanying consolidated balance sheets of InfuSystem Holdings, Inc. and Subsidiary (formerly HAPC, Inc.) (the “Company”) as of December 31, 2007 and 2006, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2007. Our audits also included the financial statement schedule listed in the Index at Item 15. These consolidated financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on the consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of InfuSystem Holdings, Inc. and Subsidiary as of December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2007, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly in all material respects the information set forth therein.
As discussed in Note 1 to the consolidated financial statements, prior to October 25, 2007, the Company was in the development stage.
/S/ DELOITTE & TOUCHE LLP
March 17, 2008
Member of Deloitte Touche Tohmatsu |
34
Table of Contents
 | 750 Lexington Avenue | |||
| New York, NY 10022-1200 | ||||
Certified Public Accountants | 212 750 9100 | phone | ||
| 212 750 2727 | fax | |||
| mec@millerellin.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Infusystem Holdings, Inc.
(formerly HAPC, Inc.)
We have audited the accompanying statements of operations, stockholders’ deficit, and cash flows of Infusystem Holdings, Inc. (formerly HAPC, Inc.) for the period from August 15, 2005 (date of inception) through December 31, 2005. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the results of operations and cash flows of Infusystem Holdings, Inc. (formerly HAPC, Inc.) for the period from August 15, 2005 (date of inception) to December 31, 2005, in conformity with United States generally accepted accounting principles.
/s/ MILLER, ELLIN & COMPANY, LLP CERTIFIED PUBLIC ACCOUNTANTS |
New York, New York
April 6, 2006
35
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
CONSOLIDATED BALANCE SHEETS
(in thousands, except share data) | December 31, 2007 | December 31, 2006 | ||||||
ASSETS | ||||||||
Current Assets: | ||||||||
Cash and cash equivalents | $ | 3,960 | $ | 427 | ||||
Investments held in trust | — | 98,151 | ||||||
Accounts receivable, less allowance for doubtful accounts of $1,638 and $0 at December 31, 2007 and 2006, respectively; December 31, 2007 includes $103 due from I-Flow | 6,304 | — | ||||||
Inventory supplies | 364 | — | ||||||
Prepaid expenses and other current assets | 1,263 | 446 | ||||||
Deferred income taxes | 4 | — | ||||||
Deferred acquisition costs | — | 1,274 | ||||||
Total Current Assets | 11,895 | 100,298 | ||||||
Property & equipment, net | 13,504 | — | ||||||
Deferred debt issuance costs, net | 1,918 | — | ||||||
Goodwill | 56,544 | — | ||||||
Intangible assets, net | 32,565 | — | ||||||
Total Assets | $ | 116,426 | $ | 100,298 | ||||
LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
Current Liabilities: | ||||||||
Accounts payable | $ | 1,076 | $ | 353 | ||||
Other current liabilities | 700 | 598 | ||||||
Income taxes payable | 1,186 | — | ||||||
Deferred underwriting fees | — | 5,468 | ||||||
Derivative liabilities | 12,407 | 9,113 | ||||||
Current portion of long-term debt payable to I-Flow | 2,044 | — | ||||||
Total Current Liabilities | 17,413 | 15,532 | ||||||
Long-term debt payable to I-Flow, net of current portion | 30,250 | — | ||||||
Deferred income taxes | 4 | — | ||||||
Total Liabilities | $ | 47,667 | $ | 15,532 | ||||
Commitments and Contingencies: | ||||||||
Common stock subject to possible conversion | — | 19,620 | ||||||
Stockholders' Equity | ||||||||
Preferred stock, $.0001 par value: authorized 1,000,000 | — | — | ||||||
shares; none issued | ||||||||
Common stock, $.0001 par value; authorized 200,000,000 shares; issued 18,315,430 and 21,041,918, respectively; outstanding 16,824,295 and 18,625,252, respectively | 2 | 2 | ||||||
Additional paid-in capital | 79,437 | 73,028 | ||||||
Retained deficit | (10,680 | ) | (7,884 | ) | ||||
Total Stockholders' Equity | 68,759 | 65,146 | ||||||
Total Liabilities and Stockholders' Equity | $ | 116,426 | $ | 100,298 | ||||
See accompanying notes to consolidated financial statements.
36
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data) | Year Ended December 31, 2007 | Year Ended December 31, 2006 | Period from August 15, 2005 (inception) to December 31, 2005 | |||||||||
Net revenues | $ | 6,582 | $ | — | $ | — | ||||||
Operating expenses: | ||||||||||||
Cost of revenues – Product and supply costs | 923 | — | — | |||||||||
Cost of revenues – Pump depreciation | 697 | — | — | |||||||||
Provision for doubtful accounts | 584 | — | — | |||||||||
Amortization of intangibles | 335 | — | — | |||||||||
Selling and marketing | 649 | — | — | |||||||||
General and administrative | 4,891 | 20,824 | 24 | |||||||||
Total Operating Expenses | 8,079 | 20,824 | 24 | |||||||||
Other (expense) income: | ||||||||||||
(Loss) gain on derivatives | (3,294 | ) | 10,800 | — | ||||||||
Interest income | 3,879 | 3,204 | — | |||||||||
Interest expense | (774 | ) | (1 | ) | (1 | ) | ||||||
Total other (expense) income | (189 | ) | 14,003 | (1 | ) | |||||||
Loss before income taxes | (1,686 | ) | (6,821 | ) | (25 | ) | ||||||
Income tax expense | (1,110 | ) | (1,038 | ) | — | |||||||
Net loss | $ | (2,796 | ) | $ | (7,859 | ) | $ | (25 | ) | |||
Loss per share: | ||||||||||||
basic and diluted | $ | (0.15 | ) | $ | (0.58 | ) | $ | (0.01 | ) | |||
Weighted average shares outstanding: | ||||||||||||
basic and diluted | 18,299,600 | 13,661,117 | 3,250,000 | |||||||||
See accompanying notes to consolidated financial statements.
37
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
CONSOLIDATED STATEMENTS OF
STOCKHOLDERS’ EQUITY
| Common Stock | Treasury Stock | ||||||||||||||||||||||||
| Shares | Par Value $0.0001 Amount | Paid in Capital in Excess of Par | Retained Deficit | Shares | Amount | Total Stockholders’ Equity | |||||||||||||||||||
Balances at August 15, 2005 | — | $ | — | — | $ | — | — | $ | — | $ | — | ||||||||||||||
Common stock issues September 13, 2005 | 4,167 | — | 25 | — | — | — | 25 | ||||||||||||||||||
Treasury stock purchased | — | — | — | — | (4,167 | ) | (25 | ) | (25 | ) | |||||||||||||||
Issuance of treasury shares for services | — | — | (25 | ) | — | 1,750 | 25 | — | |||||||||||||||||
Amortization of stock based compensation expense | — | — | 24 | — | — | — | 24 | ||||||||||||||||||
Net loss | — | — | — | (25 | ) | — | — | (25 | ) | ||||||||||||||||
Balances at December 31, 2005 | 4,167 | — | 24 | (25 | ) | (2,417 | ) | — | (1 | ) | |||||||||||||||
Issuance of common stock and warrants | 16,875 | 2 | 101,250 | — | — | — | 101,252 | ||||||||||||||||||
Reclassifications of proceeds allocated to warrants-derivative liabilities | — | — | (19,913 | ) | — | — | — | (19,913 | ) | ||||||||||||||||
Non-cash compensation | — | — | 13,050 | — | — | — | 13,050 | ||||||||||||||||||
Expenses of offering | — | — | (10,827 | ) | — | — | — | (10,827 | ) | ||||||||||||||||
Non-cash charge related to sale of option | — | — | 1,967 | — | — | — | 1,967 | ||||||||||||||||||
Amortization of stock based compensation expense | — | — | 6,660 | — | — | — | 6,660 | ||||||||||||||||||
Proceeds subject to possible conversion of 3,373,363 shares | — | — | (19,620 | ) | — | — | — | (19,620 | ) | ||||||||||||||||
Issuance of warrants | — | — | 437 | — | — | — | 437 | ||||||||||||||||||
Net loss | — | — | — | (7,859 | ) | — | — | (7,859 | ) | ||||||||||||||||
Balances at December 31, 2006 | 21,042 | 2 | $ | 73,028 | $ | (7,884 | ) | (2,417 | ) | $ | — | $ | 65,146 | ||||||||||||
Net loss | — | — | — | (2,796 | ) | — | — | (2,796 | ) | ||||||||||||||||
Adjustment to reflect actual conversion of 2,726,488 shares | (2,726 | ) | — | 3,262 | — | — | — | 3,262 | |||||||||||||||||
Issuance of option to FTN Midwest to purchase 833,333 units | — | — | — | — | — | — | — | ||||||||||||||||||
Issuance of warrants | — | — | 513 | — | — | — | 513 | ||||||||||||||||||
Amortization of stock-based compensation expense | — | — | 1,750 | — | — | — | 1,750 | ||||||||||||||||||
To adjust deferred underwriting fee paid to FTN Midwest to reflect actual share redemption | — | — | 884 | — | — | — | 884 | ||||||||||||||||||
Issuance of shares of treasury stock for services | — | — | — | — | 926 | — | — | ||||||||||||||||||
Balances at December 31, 2007 | 18,316 | 2 | $ | 79,437 | $ | (10,680 | ) | (1,491 | ) | $ | — | $ | 68,759 | ||||||||||||
See accompanying notes to consolidated financial statements.
38
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands) | Year Ended December 31, 2007 | Year Ended December 31, 2006 | Period from August 15, 2005 (inception) to December 31, 2005 | |||||||||
OPERATING ACTIVITIES | ||||||||||||
Net Loss | $ | (2,796 | ) | $ | (7,859 | ) | $ | (25 | ) | |||
Items included in net loss not requiring cash: | ||||||||||||
Loss (gain) on derivative liabilities | 3,294 | (10,800 | ) | — | ||||||||
Provision for doubtful accounts | 584 | |||||||||||
Depreciation | 728 | — | — | |||||||||
Amortization of intangible assets | 335 | |||||||||||
Amortization of deferred debt issuance costs | 134 | |||||||||||
Loss on disposal of assets | 107 | — | — | |||||||||
Interest Income on investments held in trust | (3,879 | ) | (3,184 | ) | — | |||||||
Withdrawal of interest earned on investments held in trust | 5,815 | 1,247 | — | |||||||||
Amortization of stock-based compensation expense | 1,750 | 6,660 | 24 | |||||||||
Non-cash compensation satisfied by grant of stock | — | 13,050 | — | |||||||||
Changes in current assets and liabilities, exclusive of effects of acquisitions: | ||||||||||||
Decrease (increase) in accounts receivable | (698 | ) | ||||||||||
Decrease (increase) in prepaid expenses and other current assets | (431 | ) | (445 | ) | — | |||||||
Increase (decrease) in income taxes payable | 1,186 | — | — | |||||||||
Increase (decrease) in accounts payable and other current liabilities | (1,752 | ) | 419 | — | ||||||||
NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | 4,377 | (912 | ) | (1 | ) | |||||||
INVESTING ACTIVITIES | ||||||||||||
Purchase of investments held in trust | (96,215 | ) | — | |||||||||
Cash from trust, excluding interest earned | 96,215 | |||||||||||
Cash paid for acquisition, net of cash acquired | (64,954 | ) | — | — | ||||||||
Payment of deferred acquisition costs | (8,690 | ) | (741 | ) | ||||||||
Capital expenditures | (553 | ) | ||||||||||
NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | 22,018 | (96,956 | ) | — | ||||||||
FINANCING ACTIVITIES | ||||||||||||
Share conversion payments to stockholders | (16,359 | ) | ||||||||||
Capitalized debt issuance costs | (2,052 | ) | ||||||||||
Payments on term loan | (409 | ) | ||||||||||
Payment to FTN Midwest of deferred underwriting fees | (4,555 | ) | — | — | ||||||||
Proceeds from (payment of) notes payable | — | (85 | ) | 85 | ||||||||
Proceeds from issuance of warrants | 513 | 437 | — | |||||||||
Payment of offering costs | — | (3,321 | ) | (71 | ) | |||||||
Proceeds from public offering | — | 81,631 | — | |||||||||
Proceeds from issuance of shares of stock subject to possible conversion | — | 19,620 | — | |||||||||
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES | (22,862 | ) | 98,282 | 14 | ||||||||
Net change in cash and cash equivalents | 3,533 | 414 | 13 | |||||||||
Cash and cash equivalents, beginning of period | 427 | 13 | — | |||||||||
Cash and cash equivalents, end of period | $ | 3,960 | $ | 427 | $ | 13 | ||||||
39
Table of Contents
The following table presents certain supplementary cash flow information for the years ended December 31, 2007 and 2006 and the period from August 15, 2005 (inception) to December 31, 2005:
(in thousands) | Year Ended December 31, 2007 | Year Ended December 31, 2006 | Period from August 15, 2005 (inception) to December 31, 2005 | ||||||||
Cash paid for interest (net of swap proceeds, and excluding capitalized interest) | $ | 640 | $ | 1 | $ | — | |||||
Cash paid for income taxes | $ | 431 | $ | 1,254 | $ | — | |||||
Supplementary non-cash activities: | |||||||||||
Origination of long term debt | $ | 32,703 | $ | — | $ | — | |||||
Additions to property (a) | $ | 356 | $ | — | $ | — | |||||
Current assets assumed in acquisition (b) | $ | 8,499 | $ | — | $ | — | |||||
Current liabilities assumed in acquisition (b) | $ | (3,206 | ) | $ | — | $ | — | ||||
Treasury stock transactions | 926 | — | (2,417 | ) | |||||||
| (a) | Amounts consist of current liabilities for net property that have not been included in investing activities. These amounts have not been paid for as of December 31, 2007, but will be included as a cash outflow from investing activities for capital expenditures when paid. |
| (b) | See Note 3 – "Acquisitions" |
See accompanying notes to consolidated financial statements.
40
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Basis of Presentation and Nature of Operations |
The information in this Annual Report on Form 10-K includes the financial position of InfuSystem Holdings, Inc. (formerly HAPC, INC.) and its consolidated subsidiary, InfuSystem, Inc. (“InfuSystem,” together with InfuSystem Holdings, Inc., the “Company”) as of December 31, 2007 and 2006, and the results of operations and cash flows for the years ended December 31, 2007, 2006 and the period from August 15, 2005 (inception) to December 31, 2005, and stockholders’ equity (deficit) from August 15, 2005 (inception) to December 31, 2007.
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The Company was incorporated in Delaware on August 15, 2005 as a blank check company whose objective was to acquire through a merger, capital stock exchange, asset acquisition or other similar business combination, one or more operating businesses in the healthcare sector.
Substantially all activity through October 25, 2007 relates to the Company’s formation, its initial public offering (the “IPO”) and efforts related to the acquisition of InfuSystem described below. The Company has selected December 31 as its fiscal year end. The Company completed its initial public offering (“IPO”) on April 18, 2006 and received gross proceeds of $100,000,000. Substantially all of the net proceeds of the IPO were used to acquire InfuSystem. On September 29, 2006, the Company entered into a Stock Purchase Agreement (as amended, the “Stock Purchase Agreement”) with I-Flow Corporation (“I-Flow”), Iceland Acquisition Subsidiary, the Company’s wholly-owned subsidiary (“Acquisition Subsidiary”), and InfuSystem, a wholly-owned subsidiary of I-Flow. Upon the closing of the transactions contemplated by the Stock Purchase Agreement on October 25, 2007, Acquisition Subsidiary purchased all of the issued and outstanding capital stock of InfuSystem from I-Flow and concurrently merged with and into InfuSystem. As a result of the merger, Acquisition Subsidiary ceased to exist as an independent entity and InfuSystem, as the corporation surviving the merger, became the Company’s wholly-owned subsidiary. Effective October 25, 2007, the Company changed its corporate name from “HAPC, INC.” to InfuSystem Holdings, Inc., and the Company ceased its existence as a development stage company. Prior to October 25, 2007, the Company was in the development stage. For accounting purposes, the acquisition has been treated as a purchase business combination. The results of InfuSystem are included in the consolidated financial statements subsequent to the acquisition date.
The Company is a provider of ambulatory infusion pump management services for oncologists in the United States. Ambulatory infusion pumps are small, lightweight electronic pumps designed to be worn by patients in their homes which allow patients the freedom to move about while receiving chemotherapy treatments. The pumps are battery powered and attached to intravenous administration tubing, which is in turn attached to a bag or plastic cassette that contains the chemotherapy drug.
The Company’s business model is currently highly focused on oncology chemotherapy infusion. To the Company’s knowledge, it is the only national ambulatory infusion pump service provider focused on oncology.
The Company supplies electronic ambulatory infusion pumps and associated disposable supply kits to physicians’ offices, infusion clinics and hospital outpatient chemotherapy clinics to be utilized by patients who receive continuous chemotherapy infusions. The Company obtains an assignment of insurance benefits from the patient, bills the insurance company or patient accordingly and collects payment. The Company provides billing and collection services for the pumps and associated disposable supply kits to approximately 1,550 physician practices in the United States. The Company retains title to the pumps during this process.
41
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Basis of Presentation and Nature of Operations (Continued) |
The Company purchases electronic ambulatory infusion pumps from a variety of suppliers on a non-exclusive basis. Such pumps are generic in nature and are available to the Company’s competitors. The pumps are currently used primarily for continuous infusion of chemotherapy drugs for patients with colorectal cancer.
The Company has one operating segment, which consists solely of InfuSystem, and represents the only reportable segment in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 131,Disclosures about Segments of an Enterprise and Related Information.
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and all wholly owned, majority owned or controlled organizations. All intercompany transactions and account balances have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates, assumptions and judgments that affect the amounts reported in the financial statements, including the notes thereto. The Company considers critical accounting policies to be those that require more significant judgments and estimates in the preparation of its consolidated financial statements, including the following: revenue recognition, which includes contractual allowances; accounts receivable and allowance for doubtful accounts; income taxes; and goodwill valuation. Management relies on historical experience and other assumptions believed to be reasonable in making its judgment and estimates. Actual results could differ materially from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. The Company maintains its cash and cash equivalents primarily with a single financial institution, which potentially subjects the Company to concentrations of credit risk related to temporary cash investments that are in excess of the federally insured amounts of $100,000 per account. The Company has not experienced any losses to date as a result of this policy, and management believes there is little risk of loss.
Investments Held in Trust
The Company previously invested amounts held in trust in a United States Treasury money market account. Under the terms of the trust agreement, these amounts were restricted to the funding of an acquisition and to the payment of income taxes on the trust’s taxable income. At December 31, 2007, the Company has $0 of investments held in trust, as the funds were used to consummate the acquisition of InfuSystem.
Accounts Receivable and Allowance for Doubtful Accounts
The Company has agreements with third-party payors which provide for payments at amounts different from established rates. Patient accounts receivable and net revenue are reported at the estimated net realizable amounts from patients, third-party payors and others for service rendered. The Company performs periodic analyses to assess the accounts receivable balances. It records an allowance for doubtful accounts based on the estimated collectability of the accounts such that the recorded amounts reflect estimated net realizable value. Upon determination that an account is uncollectible, the account is written-off and charged to the allowance.
42
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2. | Summary of Significant Accounting Policies (Continued) |
Substantially all of the Company’s receivables are related to providing healthcare services to patients. Accounts receivable are reduced by an allowance for amounts that could become uncollectible in the future. The Company’s estimate for its allowance for doubtful accounts is based upon management’s assessment of historical and expected net collections by payor. Due to the continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on its financial position, results of operations, and cash flows.
Inventory Supplies
Inventory supplies are stated at the lower of cost (determined on a first in, first out basis) or market. The Company records a period expense for inventory supplies obsolescence when incurred.
Property and Equipment
Property and equipment is stated at acquired cost and depreciated using the straight-line method over the estimated useful lives of the related assets, ranging from three to seven years. Rental equipment, consisting of ambulatory infusion pumps that the Company acquires from third-party manufacturers, is depreciated over five years. Leasehold improvements are amortized using the straight-line method over the life of the asset or the remaining term of the lease, whichever is shorter. Maintenance and minor repairs are charged to operations as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is recorded in the current period.
During the fiscal year ended December 31, 2007, the Company made purchases from two suppliers, each of which supplies more than 10% of the pumps purchased by the Company. There are no supply agreements in place with either of the suppliers. All purchases are handled pursuant to pricing agreements, which contain no material terms other than prices that are subject to change by the manufacturer.
Long-Lived Assets
The Company accounts for the impairment and disposition of long-lived assets in accordance with SFAS No. 144,Accounting for Impairment or Disposal of Long-Lived Assets. SFAS No. 144 addresses financial accounting and reporting for the impairment of long-lived assets and for the disposal of long-lived assets. In accordance with SFAS No. 144, long-lived assets to be held are reviewed for events or changes in circumstances, which indicate that their carrying value may not be recoverable. If an impairment indicator exists, the Company assesses the asset (or asset group) for recoverability. Recoverability of these assets is determined based upon the expected undiscounted future net cash flows from the operations to which the assets relate, utilizing management’s best estimates, appropriate assumptions and projections at the time. If the carrying value is determined not to be recoverable from future operating cash flows, the asset is deemed impaired and an impairment loss would be recognized to the extent the carrying value exceeded the estimated fair market value of the asset. The Company periodically reviews the carrying value of long-lived assets to determine whether impairment to such value has occurred. The Company has determined that no impairment existed as of December 31, 2007.
43
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2. | Summary of Significant Accounting Policies (Continued) |
Goodwill Valuation
Goodwill arising from business combinations represents the excess of the purchase price over the estimated fair value of the net assets of the businesses acquired. In accordance with the provisions of SFAS No. 142,Goodwill and Other Intangible Assets,goodwill is tested annually for impairment or more frequently if circumstances indicate the possibility of impairment. The Company has selected October 31 to perform its annual impairment test. Management does not believe impairment of its goodwill existed at December 31, 2007. The goodwill amount for the October 25, 2007 acquisition of InfuSystem, totaling $56,544,000, is based upon preliminary estimates that are subject to change in 2008 upon completion of the final valuation analysis. Final determination of these estimates could result in an adjustment to the purchase price allocation with an offsetting adjustment to goodwill.
Intangible Assets
Intangible assets consist of trade names and physician relationships, both of which arose from the acquisition of InfuSystem. The Company amortizes the value assigned to the physician relationships on a straight-line basis over the period of expected benefit. Management tests intangible assets for impairment in accordance with SFAS No. 142. The intangible assets resulting from the October 25, 2007 acquisition of InfuSystem are based upon preliminary estimates which are subject to change during the fiscal year ended December 31, 2008 upon completion of final valuation analysis.
Revenue Recognition
The Company’s strategic focus is rental revenue in the oncology market. Revenues are recognized predominantly under fee for service arrangements through equipment that the Company rents to patients. The Company recognizes revenue only when all of the following criteria are met: persuasive evidence of an arrangement exists; services have been rendered; the price to the customer is fixed or determinable; and collectability is reasonably assured. Persuasive evidence of an arrangement is determined to exist, and collectability is reasonably assured, when the Company receives a physician’s order (or certificate of medical necessity) and assignment of benefits, signed by the physician and patient, respectively, and the Company has verified actual pump usage and insurance coverage. The Company recognizes rental revenue from electronic infusion pumps as earned, normally on a month-to-month basis. Pump rentals are billed at the Company’s established rates, which often differ from contractually allowable rates provided by third-party payors such as Medicare, Medicaid and commercial insurance carriers. All billings to third party payors are recorded net of provision for contractual adjustments to arrive at net revenues.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Due to continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on results of operations and cash flows.
Our largest contracted payor is Medicare, which accounted for approximately 31% of our gross billings for the fiscal year ended December 31, 2007. We contract with various individual Blue Cross/Blue Shield affiliates which in the aggregate accounted for approximately 22% of our gross billings for the fiscal year ended December 31, 2007. No individual payor (other than Medicare and the Blue Cross/Blue Shield entities) accounts for greater than approximately 7% of our gross billings.
44
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2. | Summary of Significant Accounting Policies (Continued) |
Income Taxes
The Company accounts for income taxes in accordance with SFAS No. 109,Accounting for Income Taxes, which requires that the Company recognize deferred tax liabilities and assets based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities, using enacted tax rates in effect in the years the differences are expected to reverse. Deferred income tax benefit (expense) results from the change in net deferred tax assets or deferred tax liabilities. A valuation allowance is recorded when, in the opinion of management, it is more likely than not that some or all of any deferred tax assets will not be realized. For more information, please refer to the “Income Taxes” discussion included in Note 9.
Share Based Payment
SFAS No. 123 (R),Share-Based Payment, requires all entities to recognize compensation expense in an amount equal to the fair value of share based payments made to employees, among other requirements. Under the fair value based method, compensation cost is measured at the grant date based on the fair value of the award and is recognized on a straight-line basis over the award vesting period.
Accordingly, share based payments issued to officers, directors and vendors are measured at fair value and recognized as expense over the related vesting periods.
Share based compensation expense recognized for the years ended December 31, 2007 and 2006 was $1,750,000 and $19,710,000, respectively. The share based compensation expense recognized for the period from August 15, 2005 (inception) to December 31, 2007 was $21,484,000. All such compensation expense relates to the Company’s operations prior to the acquisition of InfuSystem; no such expenses were incurred by the Company from the date of the acquisition of InfuSystem through December 31, 2007.
Warrants and Derivative Financial Instruments
On April 18, 2006, the Company consummated its IPO of 16,666,667 units. Each unit consists of one share of common stock and two redeemable common stock purchase warrants. Each warrant entitles the holder to purchase from the Company one share of its common stock at an exercise price of $5.00. On May 18, 2006, the Company sold an additional 208,584 units to FTN Midwest Securities Corp., the underwriter of its IPO (“FTN Midwest”), pursuant to a partial exercise by FTN Midwest of its overallotment option. The Warrant Agreement provides for the Company to register the shares underlying the warrants in the absence of the Company’s ability to deliver registered shares to the warrant holders upon warrant exercise.
In September 2000, the Emerging Issues Task Force issued EITF 00-19,Accounting for Derivative Financial Instruments Indexed to and Potentially Settled in, a Company’s Own Stock, (“EITF 00-19”) which requires freestanding derivative contracts that are settled in a company’s own stock, including common stock warrants, to be designated as equity instruments, assets or liabilities. Under the provisions of EITF 00-19, a contract designated as an asset or a liability must be carried at its fair value on a company’s balance sheet, with any changes in fair value recorded in the company’s results of operations. A contract designated as an equity instrument must be included within equity, and no fair value adjustments are required from period to period.
45
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2. | Summary of Significant Accounting Policies (Continued) |
In accordance with EITF 00-19, the 33,750,502 warrants issued in connection with the IPO and overallotment to purchase stock must be settled in registered shares and are separately accounted for as liabilities as discussed in Note 7. The fair value of these warrants is shown on the Company’s balance sheet and the unrealized changes in the value of these warrants are shown in the Company’s statement of operations as “(Loss) gain on derivatives.” These warrants are freely traded on the “Over The Counter Bulletin Board.” Consequently, the fair value of these warrants is estimated as the market price of the warrant at each period end. To the extent the market price increases or decreases, the Company’s warrant liabilities will also increase or decrease with a corresponding impact on the Company’s results of operations.
Sales of warrants that can be settled in unregistered shares of common stock are treated as equity and included in additional paid in capital as discussed in Note 11. The total warrants issued to date that can be settled in unregistered shares of common stock are 1,357,717 at an issue price of $.70 per warrant or a total issue price of $950,000.
SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities, requires that an entity recognize all derivatives as either assets or liabilities in the statement of financial position and measure those instruments at fair value.
In December 2007, the Company entered into an interest rate swap to hedge the exposure associated with its floating rate debt. The Company has elected not to designate the swap as a cash flow hedge, in accordance with SFAS No. 133. The fair value of the swap is therefore shown on the Company’s balance sheet and the unrealized changes in the value of the swap are shown in the Company’s statement of operations within “Loss (gain) on derivatives”.
Deferred Debt Issuance Costs
Capitalized debt issuance costs include those associated with the Company’s term loan with I-Flow. The Company classifies the costs as non-current assets and is amortizing the costs using the interest method through the maturity date of October 2011. For a further discussion of the Company’s deferred debt issuance costs, please see Note 8.
Earnings Per Share
Basic earnings (loss) per share is computed by dividing net loss by the weighted average number of common shares outstanding during the period. Diluted earnings per share assumes the issuance of potentially dilutive shares of common stock during the periods related to the warrants. The following table reconciles the numerators and denominators of the basic and diluted loss per share computations for net loss for the following periods:
| (in thousands, except share and per share data) | Year Ended December 31, 2007 | Year Ended December 31, 2006 | For the Period From August 15, 2005 (inception) to December 31, 2005 | |||||||||
Numerator: | ||||||||||||
Net loss | $ | (2,796 | ) | $ | (7,859 | ) | $ | (25 | ) | |||
Denominator: | ||||||||||||
Weighted average common shares outstanding – basic and diluted | 18,299,600 | 13,661,117 | 3,250,000 | |||||||||
Net loss per share – basic and diluted | $ | (0.15 | ) | $ | (0.58 | ) | $ | (0.01 | ) | |||
46
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2. | Summary of Significant Accounting Policies (Continued) |
Potential shares of common stock related to our 33,750,502 outstanding warrants issued in connection with our IPO and the 1,357,717 warrants issued privately were not included as the impact would be anti-dilutive.
Recently Issued Accounting Pronouncements
On January 1, 2007, the Company adopted FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes(FIN 48). FIN 48 clarifies the accounting for income taxes by prescribing a minimum probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is defined in FIN 48 as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. FIN 48 must be applied to all existing tax positions upon initial adoption. The cumulative effect of applying FIN 48 at adoption, if any, is to be reported as an adjustment to opening retained earnings for the year of adoption. The adoption of FIN 48 did not have a material effect on the Company’s consolidated financial position or results of operations.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements, which relates to the definition of fair value, the methods used to measure fair value and the expanded disclosures about fair value measurements. The provisions of SFAS No. 157 are effective for financial statements issued for fiscal years beginning after November 15, 2007. The Company has not yet completed its analysis of the impact of adopting SFAS No. 157 on the consolidated financial position, results of operations or cash flows.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB Statement No. 115, which permits entities to choose to measure many financial instruments and certain other items at fair value that are not currently required to be measured at fair value. The objective is to improve financial reporting by providing entities with the opportunity to mitigate volatility in reported earnings caused by measuring related assets and liabilities differently without having to apply complex hedge accounting provisions. SFAS No. 159 is expected to expand the use of fair value measurement, which is consistent with the Board’s long-term measurement objectives for accounting for financial instruments. SFAS No. 159 also establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. SFAS No. 159 does not affect any existing accounting literature that requires certain assets and liabilities to be carried at fair value. SFAS No. 159 does not establish requirements for recognizing and measuring dividend income, interest income, or interest expense. SFAS No. 159 does not eliminate disclosure requirements included in other accounting standards, including requirements for disclosures about fair value measurements, included in SFAS No. 157,Fair Value Measurements, and SFAS No. 107,Disclosures about Fair Value of Financial Instruments. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The Company has not yet completed its assessment of the impact upon adoption of SFAS No. 159 on the consolidated financial position, results of operations or cash flows.
47
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2. | Summary of Significant Accounting Policies (Continued) |
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations. This statement retains the fundamental requirements of the original pronouncement requiring that the acquisition method of accounting, or purchase method, be used for all business combinations. SFAS No. 141(R) defines the acquirer as the entity that obtains control of one or more businesses in the business combination, establishes the acquisition date as the date that the acquirer achieves control and requires the acquirer to recognize the assets acquired, liabilities assumed and any noncontrolling interest at their fair values as of the acquisition date. In addition, SFAS No. 141(R) requires, among other things, expensing of acquisition related and restructuring related costs, measurement of pre–acquisition contingencies at fair value, measurement of equity securities issued for purchase at the date of close of the transaction and capitalization of in process research and development, all of which represent modifications to current accounting for business combinations. SFAS No. 141(R) is effective for fiscal years beginning after December 15, 2008. Adoption is prospective and early adoption is not permitted. Adoption of SFAS No. 141(R) will not impact the Company’s accounting for business combinations closed prior to its adoption, but given the nature of the changes noted above, the Company expects that its accounting for business combinations occurring subsequent to adoption will be significantly different than that applied following current accounting literature.
| 3. | Acquisitions |
Entry into a Material Definitive Agreement
On September 29, 2006, the Company entered into a Stock Purchase Agreement with I-Flow Corporation, a Delaware corporation (“I-Flow”), Iceland Acquisition Subsidiary, Inc., a Delaware corporation and wholly-owned subsidiary of the Company (“Acquisition Sub”) and InfuSystem, Inc. (“InfuSystem”), a California corporation and wholly-owned subsidiary of I-Flow. Pursuant to the terms of the Stock Purchase Agreement, Acquisition Sub purchased all of the issued and outstanding capital stock of InfuSystem. Concurrently with the acquisition, Acquisition Sub merged with and into InfuSystem. After the merger, Acquisition Sub ceased to exist as an independent entity and InfuSystem, as the surviving corporation, continued its corporate existence under the laws of the State of California. The name of the surviving corporation is InfuSystem, Inc. The Stock Purchase Agreement originally provided that in the event the acquisition of InfuSystem was not consummated by April 30, 2007, the Stock Purchase Agreement was terminable by the Company or I-Flow. On April 30, 2007, the Company, I-Flow, InfuSystem and Acquisition Sub entered into Amendment No. 1 to the Stock Purchase Agreement extending the termination date from April 30, 2007 to June 29, 2007. On June 29, 2007, the Company, I-Flow, InfuSystem and Acquisition Sub entered into Amendment No. 2 to the Stock Purchase Agreement extending the termination date from June 29, 2007 to July 31, 2007. On July 31, 2007, the Company, I-Flow, InfuSystem and Acquisition Sub entered into Amendment No. 3 to the Stock Purchase Agreement extending the termination date from July 31, 2007 to October 1, 2007. On September 18, 2007, the Company, I-Flow, InfuSystem and Acquisition Sub entered into Amendment No. 4 to the Stock Purchase Agreement extending the termination date from October 1, 2007 to October 22, 2007. Amendment No. 4 to the Stock Purchase Agreement also amended the Stock Purchase Agreement to provide for, among other things, a reduction in the purchase price for InfuSystem from $140,000,000 to $100,000,000 and an earn-out provision.
48
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 3. | Acquisitions (Continued) |
The earn-out provision provides for a potential additional payment of up to $12,000,000 to be paid to I-Flow in 2011, provided that certain consolidated net revenue growth targets related to the Company’s future operations are met. The contingent consideration is based upon the compound annual growth rate or “CAGR” of the Company’s consolidated net revenues over the three-year period ended December 31, 2010 as compared to InfuSystem’s 2007 net revenues, excluding certain revenues not part of InfuSystem’s core business. The additional payment would be paid in 2011. No additional payment will be made unless the Company achieves a consolidated net revenue CAGR of at least 40% over the three-year period. The additional payment will range from $3,000,000 to $12,000,000 depending upon the extent to which consolidated net revenue CAGR for the three year period exceeds 40%. The maximum potential amount of the contingent consideration is $12,000,000 and would be payable to I-Flow if the Company achieves a consolidated net revenue CAGR of 50% over the three-year period.
On October 17, 2007, the Company, I-Flow, InfuSystem and Acquisition Sub entered into a Further Agreement Regarding Project Iceland (the Company’s acquisition of InfuSystem from I-Flow) pursuant to which the termination date was extended to November 1, 2007, the Company agreed to pay the termination fee of $3,000,000 unconditionally and I-Flow was able to purchase shares of the Company’s stock at its discretion from the third parties in privately negotiated or market transactions in order to increase the likelihood of shareholder approval.
Purchase Price
In consideration for the acquisition of all of the issued and outstanding shares of capital stock of InfuSystem, the Company paid I-Flow a purchase price of $100,000,000, subject to certain working capital adjustments as set forth in the Stock Purchase Agreement. The purchase price was paid in the combination of (i) a secured promissory note (the “Promissory Note”) payable to I-Flow and (ii) cash. In connection with I-Flow’s commitment to accept the Promissory Note, a $100,000 delivery fee was paid by the Company to I-Flow on October 4, 2006 and a “Ticking Fee” (between 0.50% and 1.0% per annum of the Maximum Amount) was payable from September 29, 2006 until the earlier of the closing under the Stock Purchase Agreement, termination of the Stock Purchase Agreement or the Company’s notice that, because alternative financing has been secured, the Promissory Note to I-Flow will no longer be required. On October 4, 2006, the Company paid $100,000 to I-Flow representing the delivery fee.
Consummation of Transaction
On October 25, 2007, the Company completed its acquisition of 100% of the issued and outstanding equity of InfuSystem from I-Flow pursuant to the Stock Purchase Agreement. InfuSystem’s results of operations are included in the Company’s consolidated statements of operations from the acquisition date. The total purchase price for InfuSystem, Inc. was $108,717,000 and is comprised of the following (in thousands):
Cash payment to I-Flow | $ | 67,297 | ||
Working Capital Adjustment | (784 | ) | ||
Loan from I-Flow | 32,703 | |||
Acquisition-related transaction costs | 9,501 | |||
Total purchase price | $ | 108,717 | ||
49
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 3. | Acquisitions (Continued) |
The following payments were made pursuant to the Stock Purchase Agreement:
Cash payment to I-Flow: Pursuant to the terms of the Stock Purchase Agreement, the Company paid I-Flow $67,297,000 on the transaction date, which was subject to customary working capital adjustments. The final calculated working capital resulted in a downward adjustment to the purchase price of $784,000, which I-Flow subsequently paid to the Company in 2008, with interest from the date of the transaction.
Loan from I-Flow: Pursuant to the terms of the Stock Purchase Agreement, the secured promissory note payable to I-Flow was to be for a principal amount equal to the $100,000,000 purchase price, less the cash portion of the purchase price paid at closing. This resulted in a principal amount of $32,703,000, subject to the terms and conditions of the Credit and Guaranty Agreement dated as of October 25, 2007.
Acquisition Related Transaction Costs
Acquisition related transaction costs include legal and accounting fees and other external costs directly related to the acquisition.
Additional Contingent Payment
The Stock Purchase Agreement also provides for a potential additional payment of up to $12,000,000, or the earn-out, to I-Flow in 2011, provided that certain consolidated net revenue growth targets related to the Company's future operations are met. Any amounts ultimately paid out in 2011 per the earn-out will increase Goodwill at the time of payment.
Cash Payment to Shareholders
We paid $16,359,000 in cash to shareholders, who held 2,726,488 shares in the aggregate, and who voted against our acquisition of InfuSystem and elected to convert their shares of common stock into their pro rata portion of the proceeds of the trust account, of $6.00 per share.
Purchase Price Allocation
Pursuant to SFAS No. 141,Business Combinations, the purchase price has been allocated to the assets acquired and liabilities assumed based upon their estimated fair values as of the acquisition date. The purchase price allocation was primarily based upon a valuation using income and cost approaches, and management’s estimates and assumptions. The excess of the purchase price over the net tangible and identifiable intangible assets was recorded as goodwill. For tax purposes, goodwill consists of both identifiable intangible assets (trade name and physician relationships from the table below) and unidentifiable intangible assets (goodwill from the table below). Goodwill of $89,444,000 is expected to be deductible for tax purposes. The purchase price allocation, while substantially complete, is subject to further adjustments. The allocation of the purchase price to the fair values of the assets acquired and liabilities assumed as of the transaction date is presented below (in thousands):
Current assets | $ | 8,499 | ||
Property and equipment | 13,980 | |||
Goodwill | 56,544 | |||
Trade Name | 5,500 | |||
Physician Relationships | 27,400 | |||
Current liabilities | (3,206 | ) | ||
Total purchase price | $ | 108,717 | ||
50
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 3. | Acquisitions (Continued) |
Trade Name
All of InfuSystem’s services are sold under the InfuSystem trade name. The use of the InfuSystem trade name is considered to be paramount to the continued success of the Company and provides for a seamless transition of ownership and continuity in the minds of patients, physicians, insurance companies and employees. This indefinite-lived trade name is not subject to amortization, as there are no plans to retire the trade name and management expects it to generate cash flows indefinitely.
Physician Relationships
The acquired physician relationship base represents a valuable asset of InfuSystem due to the expectation of future business opportunities to be leveraged from the existing relationship with each physician. InfuSystem has long-standing relationships with numerous oncology clinics and physicians. These relationships are expected, on average, to have a 15 year useful life, based on minimal attrition experienced to date by the Company and expectations of continued minimal attrition.
Pro Forma Financial Information
The pro forma financial information in the table below summarizes the combined results of operations of the Company and InfuSystem as though the companies had been combined as of the beginning of each period presented. The pro forma financial information is presented for informational purposes only and is not indicative of the results of operations that would have been achieved if the acquisition had taken place at the beginning of each period presented or that may result in the future. The pro forma financial information for all periods presented also includes the pro forma depreciation and amortization charges from acquired tangible and intangible assets, interest expense and related tax effects for the years ended December, 31:
| (in thousands, except per share data) | 2007 | 2006 | |||||
Net revenues | $ | 31,583 | $ | 31,716 | |||
(Loss) income before income taxes | (4,760 | ) | 12,618 | ||||
Net income | (4,036 | ) | 11,854 | ||||
Earnings per share - basic | (0.22 | ) | 0.87 | ||||
Earnings per share - diluted | (0.22 | ) | 0.72 | ||||
| 4. | Initial Public Offering |
On April 18, 2006, the Company sold 16,666,667 units in connection with its IPO to the public at a price of $6.00 per unit. Each unit consists of one share of the Company’s common stock, $.0001 par value, and two redeemable common stock purchase warrants. Each warrant entitles the holder to purchase from the Company one share of common stock at an exercise price of $5.00 commencing on the later of the completion of a business combination or one year from the effective date of the IPO and expiring five years from the effective date of the IPO. The Company may call the warrants for redemption in whole and not in part at a price of $.01 per warrant at any time after the warrants become exercisable. They cannot be redeemed unless the warrant holders receive written notice not less than 30 days prior to the redemption; and, if, and only if, the reported last sale price of the common stock equals or exceeds $8.50 per share for any 20 trading days within a 30 trading day period ending on the third business day prior to the notice of redemption to warrant holders. In connection with the IPO, the Company paid to FTN Midwest an underwriting discount of 7% of the IPO price and a non–accountable expense allowance of 1% of the IPO price.
51
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 4. | Initial Public Offering (Continued) |
In addition, on April 18, 2006, the Company issued to FTN Midwest, for $100, an option to purchase up to a total of 833,333 units. The units issuable upon exercise of this option are identical to those offered in the IPO, except that each of the warrants underlying this option entitles the holder to purchase one share of the Company’s common stock at a price of $6.25. This option is exercisable at $7.50 per unit commencing on the later of the consummation of a business combination or one year from the date of the IPO prospectus dated April 11, 2006 and expiring five years from the date of the IPO prospectus dated April 11, 2006. The option may only be exercised or converted by the option holder. The Company received payment of $100 for this option in the first quarter of 2007.
The sale of the option was accounted for as an equity transaction. Accordingly, there was no net impact on the Company’s financial position or results of operations, except for the recording of the $100 proceeds from the sale. The Company determined that the fair value of the option on the date of sale was $2.36 per unit, or $1,966,666 total, using an expected life of five years, volatility of 47% and a risk-free interest rate of 3.98%. Accordingly, this amount was recorded as an expense of the offering resulting in a charge directly to stockholders’ equity during 2006.
The volatility calculation of 47% was based on the 180 day average volatility of a representative sample of 41 healthcare industry companies (the “Sample Companies”) with market capitalization under $200 million. Because it does not have a long trading history, the Company needed to estimate the potential volatility of its common stock price. The Company referred to the 180 day average volatility of the Sample Companies because Management believes that the average volatility of such companies was a reasonable benchmark to use in estimating the expected volatility of the Company’s common stock post-business combination.
On May 18, 2006, the Company sold 208,584 units (the “Overallotment Units”) to FTN Midwest pursuant to a partial exercise by FTN Midwest of its overallotment option. The Overallotment Units were sold at the offering price of $6.00 per unit, less FTN Midwest’s 7% underwriting discount.
| 5. | Property and Equipment |
Property and equipment consisted of the following as of December 31 (amounts in thousands):
| 2007 | 2006 | ||||||
Pump equipment | $ | 13,816 | $ | 0 | |||
Furniture, fixtures, and equipment | 413 | 0 | |||||
Accumulated depreciation | (725 | ) | 0 | ||||
Total | $ | 13,504 | $ | 0 | |||
Depreciation expense for 2007 was $728,000, which was recorded in cost of revenues and general and administrative expenses, for pump equipment and other fixed assets, respectively.
52
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 6. | Identifiable Intangible Assets |
The carrying amount and accumulated amortization of identifiable intangible assets as of December 31, 2007 were as follows (in thousands):
Nonamortizable intangible assets: | ||||
Trade names | $ | 5,500 | ||
Amortizable intangible assets: | ||||
Physician Relationships | 27,400 | |||
Total nonamortizable and amortizable intangible assets | 32,900 | |||
Less accumulated amortization | (335 | ) | ||
Total identifiable intangible assets | $ | 32,565 | ||
Amortization expense for intangible assets for 2007 was $335,000, which was recorded in operating expenses. Expected annual amortization expense for intangible assets recorded as of December 31, 2007 is as follows (in thousands):
| (in thousands) | 2008 | 2009 | 2010 | 2011 | 2012 | ||||||||||
Amortization expense | $ | 1,827 | $ | 1,827 | $ | 1,827 | $ | 1,827 | $ | 1,827 | |||||
| 7. | Warrants and Derivative Financial Instruments |
The warrants will be exercisable only if at the time of exercise (i) a registration statement under the Securities Act of 1933, as amended (the “Securities Act”), with respect to the common stock underlying the warrants issuable upon exercise of the option is effective, or (ii) in the opinion of counsel to the Company or counsel to the option holder reasonably satisfactory to the Company, the exercise of the warrants is exempt from the registration requirements of the Securities Act and such securities are qualified for sale or exempt from qualification under applicable securities laws of the states or other jurisdictions in which the registered holders reside. The warrants may not be exercised by, or securities issued to, any registered holder in any state in which such exercise or issuance would be unlawful. The option holder is not entitled to receive a net cash settlement or other settlement in lieu of physical settlement if the common stock underlying the warrants, or securities underlying the option, as applicable, are not covered by an effective registration statement.
The Company has determined that the warrants issued in connection with the IPO including the Overallotment Units issued on May 18, 2006 are classified as liabilities in accordance with EITF 00-19. Therefore, the fair value of each instrument must be recorded as a liability on the Company’s balance sheet. Changes in the fair values of these instruments will result in adjustments to the amount of the recorded liabilities, and the corresponding gain or loss will be recorded in the Company’s statement of operations. At the date of the conversion of each warrant or portion thereof (or exercise of the warrants or portion thereof, as the case may be), the corresponding liability will be reclassified as equity.
The fair value of the Company’s 33,750,502 warrants issued in connection with the IPO outstanding at December 31, 2007 and December 31, 2006 were liabilities of $12,150,000 or $0.36 per warrant and $9,113,000 or $0.27 per warrant, respectively.
At December 31, 2007, the Company had one interest rate swap agreement in effect to fix its LIBOR-based variable rate debt. The interest rate swap agreement, which expires in December 2010, had a notional amount of $19,750,000 on December 31, 2007 and a fixed rate of 4.29%. The fair value of the Company’s interest rate swap outstanding at December 31, 2007 and December 31, 2006 was a liability of $257,000 and $0, respectively.
53
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 7. | Warrants and Derivative Financial Instruments (Continued) |
Total derivative liabilities are as follows (in thousands):
| December 31, 2007 | December 31, 2006 | |||||
Warrant Liability | $ | 12,150 | $ | 9,113 | ||
Interest Rate Swap Liability | 257 | — | ||||
Total Derivative Liabilities | 12,407 | 9,113 | ||||
| 8. | Debt |
The Company entered into a $32,703,000 term loan from I-Flow, subject to the Credit and Guaranty Agreement, dated as of October 25, 2007, by and among the Company, Iceland Acquisition Subsidiary, Inc. and I-Flow (the “Credit and Guaranty Agreement”). The loan expires on October 25, 2011. The loan bears interest at either LIBOR plus 5.5%, or Prime plus 4.5%, at the Company’s option. The loan is a variable rate loan and therefore fair value approximates book value. At December 31, 2007, the rate in effect was 10.7%. The Company paid $637,000 in cash interest payments to I-Flow during 2007.
Maturities on the loan are as follows:
Year Ending December 31, | (in thousands) | ||
2008 | $ | 2,044 | |
2009 | 3,270 | ||
2010 | 3,679 | ||
2011 | 23,301 | ||
2012 | — | ||
Thereafter | — | ||
| $ | 32,294 | ||
The loan is collateralized by substantially all of the Company’s assets and requires the Company to comply with covenants principally relating to satisfaction of a Fixed Charge Coverage Ratio, a Leverage Ratio, and Minimum Earnings Before Interest, Taxes, Depreciation and Amortization (“EBITDA”).
In conjunction with the Credit and Guaranty Agreement, the Company incurred deferred debt issuance costs of $2,052,000. These costs will be recognized in income using the interest method through the maturity date of October 2011. Amortization of these costs for 2007 was $134,000, which was recorded in interest expense.
54
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 9. | Income Taxes |
The components of consolidated provision for income taxes for the years ended December 31, 2007, 2006 and 2005 are as follows:
| 2007 | 2006 | 2005 | |||||||
Provision for Federal income taxes – | |||||||||
Current | $ | 886 | $ | 642 | $ | — | |||
Deferred | — | — | — | ||||||
Total provision for Federal income taxes | 886 | 642 | — | ||||||
Provision for state and local income taxes – | |||||||||
Current | 224 | 396 | — | ||||||
Deferred | — | — | — | ||||||
Total provision for state and local income taxes | 224 | 396 | — | ||||||
Consolidated provision for income taxes | $ | 1,110 | $ | 1,038 | $ | — | |||
The significant components of net deferred income taxes as of December 31, 2007 and 2006 are as follows:
| 2007 | 2006 | |||||||
Deferred Federal income tax assets – | ||||||||
Bad debt reserves | $ | 23 | $ | — | ||||
Depreciation and asset basis differences | 11 | — | ||||||
Stock based compensation | 4,503 | 4,437 | ||||||
Other | 88 | 66 | ||||||
Valuation Allowance | (4,401 | ) | (4,503 | ) | ||||
Total deferred Federal income tax assets | 224 | — | ||||||
Deferred income tax liabilities – | ||||||||
Amortization | (224 | ) | — | |||||
Total deferred income tax liabilities | (224 | ) | — | |||||
Net deferred income taxes | $ | — | $ | — | ||||
The classification of net deferred income taxes as of December 31, 2006 is summarized as follows:
| Current | Long-term | Total | |||||||
Deferred tax assets | $ | — | $ | — | $ | — | |||
Deferred tax liabilities | — | — | — | ||||||
Net deferred income taxes | $ | — | $ | — | $ | — | |||
55
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 9. | Income Taxes (Continued) |
The classification of net deferred income taxes as of December 31, 2007 is summarized as follows:
| Current | Long-term | Total | |||||||||
Deferred tax assets | $ | 4 | $ | 250 | $ | 254 | |||||
Deferred tax liabilities | — | (254 | ) | (254 | ) | ||||||
Net deferred income taxes | $ | 4 | $ | (4 | ) | $ | — | ||||
The differences between the United States Federal statutory income tax provision (using the statutory rate of 34%) and the Company’s consolidated provision for income taxes for the years ended December 31, 2007 and December 31, 2006 are summarized as follows:
| 2007 | 2006 | |||||||
Federal income tax provision based on the statutory rate | $ | (573 | ) | $ | (2,319 | ) | ||
State and local income taxes, net of related Federal income taxes | 224 | 263 | ||||||
Non-deductible Expenses | 3 | — | ||||||
Non-deductible stock based compensation | 595 | 2,263 | ||||||
Non-deductible (gain) loss on warrant liability | 1,033 | (3,672 | ) | |||||
Valuation allowance | (102 | ) | 4,503 | |||||
Prior year adjustments | (70 | ) | — | |||||
Income tax expense | $ | 1,110 | $ | 1,038 | ||||
The Company’s realization of its deferred tax assets is dependent upon many factors, including, but not limited to, the Company’s ability to generate sufficient taxable income. At December 31, 2007 and 2006, valuation allowances of $4,401,000 and $4,503,000 respectively, were applied against the deferred tax assets because it was uncertain whether the benefit of the deferred tax assets would be fully utilized.
The Company and its subsidiary will file a consolidated federal and certain combined state tax returns as of October 25, 2007, the date of acquisition. Through these filings, income generated by the Company is offset by losses in the consolidated group.
| 10. | Related Party Transactions |
Two of the members of the Company’s board of directors are former Managing Directors of FTN Midwest, the underwriter of the Company’s IPO. Sean McDevitt resigned from his position as Managing Director of FTN Midwest effective January 19, 2007 and Pat LaVecchia resigned from his position as Managing Director of FTN Midwest effective February 2, 2007. FTN Midwest received an underwriting discount of 7%, a non-accountable expense allowance of 1% and an option to purchase 833,333 shares for a fee of $100. The Company reserved in its treasury 2,000,000 shares of common stock for issuance to Sean McDevitt and 416,666 shares of common stock for issuance to Pat LaVecchia. The consummation of the transaction resulted in 925,531 of these shares being issued at October 25, 2007. The remaining 1,491,135 shares will be issued six months after the consummation of the transaction.
56
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 10. | Related Party Transactions (Continued) |
Prior to its acquisition of InfuSystem, the Company utilized certain administrative, technological and secretarial services, as well as certain limited office space provided by FTN Midwest. The Company agreed to pay $1 per year for such services commencing on the effective date of the IPO and continuing monthly thereafter.
As discussed in Note 8 “Debt”, the Company entered into a $32,703,000 term loan from I-Flow, subject to the Credit and Guaranty Agreement, dated as of October 25, 2007, by and among the Company, Iceland Acquisition Subsidiary, Inc. and I-Flow (the “Credit and Guaranty Agreement”).
Prior to the Company’s acquisition of InfuSystem, InfuSystem had been providing billing and collection services to I-Flow for its ON-Q® product. On October 25, 2007, InfuSystem and I-Flow entered into an Amended and Restated Services Agreement (the “Services Agreement”) pursuant to which InfuSystem agreed to continue to provide I-Flow with these services, and I-Flow agreed to pay InfuSystem a monthly service fee. During 2007, the Company recorded revenues of $103,000 from this arrangement, which included net revenues. The entire $103,000 was an outstanding receivable at December 31, 2007. On November 8, 2007, I-Flow informed InfuSystem that it was terminating the Services Agreement effective May 10, 2008.
Steven Watkins, the Chief Executive Officer of the Company, owns 5% of Tu-Effs Limited Partnership, which owns the Madison Heights, Michigan office and warehouse facility currently leased by the Company. Rent expense for the leased premises was $42,000 for the fiscal year ended December 31, 2007, which was recorded in general and administrative expenses. Both the office and warehouse leases expire on June 30, 2008. As of December 31, 2007, the future minimum lease payments related to the lease obligations were $116,000.
| 11. | Commitments and Contingencies |
Certain of the Company’s directors committed to purchase up to $1,000,000 of the Company’s warrants from the Company in a private placement at a price of $.70 per warrant subsequent to the filing of the preliminary proxy statement seeking stockholder approval of the acquisition of InfuSystem. Such officers and directors agreed not to sell or transfer the warrants until after the Company has consummated a business combination. On December 28, 2006, the Company issued 624,286 warrants to purchase common stock to Sean McDevitt, Chairman of the Board of Directors of the Company, at a purchase price of $0.70 per warrant for an aggregate purchase price of $437,000. The warrants have an exercise price of $5.00 per share of common stock and became exercisable commencing on the acquisition date and expire April 11, 2011 or earlier upon redemption by the Company. The Company may call the warrants for redemption in whole and not in part at a price of $0.01 per warrant at anytime after the warrant becomes exercisable. The warrants cannot be redeemed unless the holder receives written notice not less than 30 days prior to the redemption and if and only if, the reported last price of the common stock equals or exceeds $8.50 per share for any 20 trading days within a 30 day period ending on the third day of business prior to the notice of redemption to warrant holder. The Company has fully reserved these shares as authorized but not issued. The Company issued to Sean McDevitt an additional 447,143 warrants to purchase common stock at a purchase price of $0.70 per warrant for an aggregate purchase price of $313,000 on April 12, 2007. In addition, on September 12, 2007, the Company issued 286,288 warrants to purchase common stock to Sean McDevitt, John Voris, Wayne Yetter, Erin Enright and Jean Pierre Millon at a purchase price of $0.70 per warrant for an aggregate purchase price of $200,000. The warrants issued to Sean McDevitt on April 12, 2007 and the warrants issued to Sean McDevitt, John Voris, Wayne Yetter, Erin Enright and Jean Pierre Millon on September 12, 2007, are subject to the same terms and conditions as the warrants issued to Sean McDevitt in December 2006. Sean. McDevitt and the other warrant purchasers agreed not to sell or transfer their warrant purchases until after the Company had consummated a business combination. The warrants issued and sold in December 2006, April 2007 and September 2007 were not registered under the Securities Act. As a result, the warrants and the common stock issuable upon exercise of the warrants may not be sold unless they have been registered pursuant to a registration statement filed under the Securities Act or pursuant to an available exemption from the registration requirements of the Securities Act as evidenced by an opinion of counsel reasonably satisfactory to the Company.
57
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 11. | Commitments and Contingencies (Continued) |
The initial stockholders, who received shares prior to the IPO, are entitled to demand that the Company register the resale of their shares of common stock at any time six months following the consummation of the acquisition, pursuant to the terms of their respective lock-up agreements.
The Company has agreed to reimburse its initial stockholders for (a) any income tax liability incurred by the Company’s initial stockholders as a result of the award of their shares and/or the vesting of such shares (other than tax liability due as a result of their sale of such shares) and (b) all reasonable out-of-pocket expenses incurred by the initial stockholders in connection with their activities on the Company’s behalf.
The Company and FTN Midwest executed an engagement letter dated April 27, 2007 pursuant to which FTN Midwest agreed to advisory services to the Company for a fee of $1,000,000. FTN Midwest provided the Company with financial advice and assistance in connection with acquisition of InfuSystem. The services included performing valuation analysis, assisting the Company in performing its due diligence, coordinating visits with InfuSystem and assisting the Company in negotiating financial terms. Pursuant to the terms of the engagement letter, the $1,000,000 fee was not payable until the Company consummated a business combination. The Company paid the $1,000,000 fee on October 31, 2007, after the October 25, 2007 closing.
We are involved in legal proceedings arising out of the ordinary course and conduct of our business, the outcomes of which are not determinable at this time. We have insurance policies covering such potential losses where such coverage is cost effective. In our opinion, any liability that might be incurred by us upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on our financial condition or results of operations.
The Company enters into contracts with payors that require the Company to indemnify the payors against any claims by third parties arising from the Company’s actions in connection with the contracts. Such contracts typically provide that the payor will also indemnify the Company against any claims by third parties arising from the payor’s actions in connection with the contact. A maximum obligation arising out of these types of agreements in not explicitly stated and, therefore, the overall maximum amount of these obligations cannot be reasonably estimated. Historically, the Company has not been obligated to make significant payments for these obligations and thus, no liabilities have been recorded for these obligations on its balance sheet as of December 31, 2006 and December 31, 2007.
| 12. | Share-based Compensation |
Effective December 30, 2005, Healthcare Acquisition Partners Holdings, LLC sold the 4,166,667 shares of common stock that it had received upon formation of the Company back to the Company. The shares were purchased for a $25,000 note payable. Simultaneously, the Company transferred 1,750,001 of these shares to certain members of its management team resulting in aggregate compensation of $8,435,000 to them, computed at $4.82 per share, which has been charged to expense ratably over the forfeiture period. Of this amount, $1,750,000 and $6,660,000 were charged to expense for the years ended December 31, 2007 and 2006, respectively. The amount charged to expense for the period from August 15, 2005 (inception) to December 31, 2007 was $8,435,000, representing full amortization of the total compensation expense, as the forfeiture period expired on the date of the acquisition. These expenses were recorded in general and administrative expenses.
The remaining 2,416,666 shares of the Company’s common stock transferred back to the Company and not transferred to members of the Company’s management team on December 30, 2005 were being held as treasury shares and reserved for transfer by the Company’s board of directors to present or future officers, directors or employees.
On July 24, 2006, the Company reserved for grant to two of the Company’s directors 2,416,666 shares of the Company’s common stock. These shares were originally held as treasury shares and reserved for transfer to present or future officers, directors or employees. The consummation of the transaction resulted in 925,531 of these shares being issued at October 25, 2007. The remaining 1,491,135 shares will be issued six months after the consummation of the transaction.
As a result of the above, the Company recorded compensation expense of $13,050,000 in its quarter ended September 30, 2006 which is based upon the number of shares reserved of 2,416,666 at the July 24, 2006 closing stock price of $5.40 per share.
The Company is authorized to issue 1,000,000 shares of preferred stock with such designations, voting and other rights and preferences as may be determined from time to time by the board of directors.
58
Table of Contents
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARY
(formerly HAPC, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 12. | Share-based Compensation (Continued) |
The Company has adopted the 2007 Stock Incentive Plan providing for the issuance of a maximum of 2,000,000 shares of common stock in connection with the grant of options and/or other stock-based or stock-denominated awards. As of December 31, 2007, no such awards have been granted.
| 13. | Employee Benefit Plans |
At December 31, 2007, the Company did not have a 401(k) defined contribution plan in place. The Company has enacted a 401(k) Plan effective February 1, 2008. Employees of the Company working more than 1,040 hours annually may participate in the 401(k) Plan. The Company plans to contribute $0.33 for each dollar of employee contribution up to a maximum contribution by the Company of 1.32% of each participant’s annual salary. The maximum contribution by the Company of 1.32% corresponds to an employee contribution of 4% of annual salary. Participants will vest in the Company’s contribution ratably over five years. Such contributions totaled $0 for the fiscal year ended December 31, 2007. The Company does not provide post-retirement or post-employment benefits to its employees.
| 14. | Unaudited Quarterly Information |
| Quarter Ended | ||||||||||||||||
| (in thousands, except per share data) | March 31, 2007 | June 30, 2007 | September 30, 2007 | December 31, 2007(1) | ||||||||||||
Net revenues | $ | — | $ | — | $ | — | $ | 6,582 | ||||||||
Gross margin | — | — | — | 4,962 | ||||||||||||
Total operating expenses | 1,018 | 936 | 507 | 5,618 | ||||||||||||
Total other income (expense) | 3,013 | (1,053 | ) | 326 | (2,475 | ) | ||||||||||
Income (loss) before income taxes | 1,995 | (1,989 | ) | (182 | ) | (1,510 | ) | |||||||||
Net income (loss) | 1,775 | (2,198 | ) | (505 | ) | (1,868 | ) | |||||||||
Earnings (loss) per share - basic | 0.10 | (0.12 | ) | (0.03 | ) | (0.11 | ) | |||||||||
Earnings (loss) per share - diluted | 0.08 | (0.12 | ) | (0.03 | ) | (0.11 | ) | |||||||||
| Quarter Ended | ||||||||||||||||
| (in thousands, except per share data) | March 31, 2006 | June 30, 2006 | September 30, 2006 | December 31, 2006 | ||||||||||||
Net revenues | $ | — | $ | — | $ | — | $ | — | ||||||||
Gross margin | — | — | — | — | ||||||||||||
Total operating expenses | 2,197 | 2,363 | 14,336 | 1,928 | ||||||||||||
Total other income (expense) | (1 | ) | 10,644 | 837 | 2,523 | |||||||||||
Income (loss) before income taxes | (2,198 | ) | 8,282 | (13,499 | ) | 594 | ||||||||||
Net income (loss) | (2,199 | ) | 8,091 | (13,920 | ) | 169 | ||||||||||
Earnings (loss) per share - basic | (1.26 | ) | 0.53 | (0.75 | ) | 0.01 | ||||||||||
Earnings (loss) per share - diluted | (1.26 | ) | 0.46 | (0.75 | ) | 0.01 | ||||||||||
| (1) | The quarter ended December 31, 2007 is not comparable to the other quarters presented in the table above. As discussed in Note 3 “Acquisitions”, on October 25, 2007, the Company completed its acquisition of 100% of the issued and outstanding equity of InfuSystem from I-Flow pursuant to the Stock Purchase Agreement. InfuSystem’s results of operations are included in the Company’s consolidated statements of operations from the acquisition date. Prior to October 25, 2007, the Company was in the development stage. |
59
Table of Contents
[LETTERHEAD OF DELOITTE & TOUCHE LLP]
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholder of
InfuSystem, Inc.:
We have audited the accompanying balance sheet of InfuSystem, Inc. (a California corporation) (the “Company”) (a wholly owned subsidiary of I-Flow Corporation (the “Parent”)) as of December 31, 2006, and the related statements of income, stockholder’s equity, and cash flows for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005. Our audits also included the information of InfuSystem, Inc. included in the financial statement schedule listed in the Index at Item 15. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of InfuSystem, Inc. as of December 31, 2006, and the results of its operations and its cash flows for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such information of InfuSystem, Inc. included in the financial statement schedule, when considered in relation to the basic financial statements taken as a whole, present fairly in all material respects the information set forth therein.
As discussed in Note 1 to the financial statements, the Company adopted the provisions of Financial Accounting Standards Board (FASB) Interpretation (FIN) No. 48,Accounting for Uncertainty in Income Taxes, in 2007. Also, as discussed in Note 1 to the financial statements, the Company changed its method of accounting for share-based compensation in 2006 as a result of adopting FASB Statement No. 123(R),Share-Based Payment.
As further discussed in Note 1 to the financial statements, on October 25, 2007, the Company was sold by the Parent to InfuSystem Holdings, Inc. (formerly known as HAPC, Inc.).
As further described in Note 1, the accompanying financial statements have been prepared from the separate records maintained by the Company and may not necessarily be indicative of the conditions that would have existed or the results of operations if the Company had been operated as an unaffiliated company.
/s/ DELOITTE & TOUCHE LLP
Costa Mesa, CA
March 20, 2008
Member of
Deloitte Touche Tohmatsu
60
Table of Contents
INFUSYSTEM, INC.
(A Wholly Owned Subsidiary of I-Flow Corporation)
BALANCE SHEET
AS OF DECEMBER 31, 2006
(Amounts in thousands)
| December 31, 2006 | |||
ASSETS | |||
CURRENT ASSETS: | |||
Cash | $ | 1,956 | |
Accounts receivable, less allowance for doubtful accounts of $1,668 | 9,630 | ||
Inventory supplies | 252 | ||
Prepaid expenses and other current assets | 155 | ||
Deferred taxes | 689 | ||
Total current assets | 12,682 | ||
PROPERTY — Net | 12,307 | ||
GOODWILL | 2,639 | ||
TOTAL | $ | 27,628 | |
LIABILITIES AND STOCKHOLDER’S EQUITY | |||
CURRENT LIABILITIES: | |||
Accounts payable | $ | 1,884 | |
Accrued payroll and related expenses | 1,055 | ||
Accrued use taxes payable | 1,392 | ||
Other current liabilities | 4 | ||
Total current liabilities | 4,335 | ||
DEFERRED TAXES | 1,285 | ||
COMMITMENTS AND CONTINGENCIES (Note 6) | |||
STOCKHOLDER’S EQUITY: | |||
Common stock, $0.01 par value — 100 shares authorized, issued and outstanding at October 25, 2007 and December 31, 2006 | |||
Additional paid-in capital | 8,544 | ||
Retained earnings | 13,464 | ||
Total stockholder’s equity | 22,008 | ||
TOTAL | $ | 27,628 | |
See notes to financial statements.
61
Table of Contents
INFUSYSTEM, INC.
(A Wholly Owned Subsidiary of I-Flow Corporation)
STATEMENTS OF INCOME
FOR THE PERIOD FROM JANUARY 1, 2007 TO OCTOBER 25, 2007, AND THE
YEARS ENDED DECEMBER 31, 2006 AND 2005
(Amounts in thousands)
| For the Period From January 1, 2007 to October 25, 2007 | Fiscal Year Ended December 31, 2006 | Fiscal Year Ended December 31, 2005 | |||||||||
NET REVENUES | $ | 25,001 | $ | 31,716 | $ | 28,525 | |||||
COST OF REVENUES | 6,702 | 8,455 | 7,735 | ||||||||
GROSS PROFIT | 18,299 | 23,261 | 20,790 | ||||||||
OPERATING EXPENSES: | |||||||||||
Selling and marketing | 3,390 | 3,803 | 4,315 | ||||||||
General and administrative | 12,283 | 11,288 | 8,394 | ||||||||
Total operating expenses | 15,673 | 15,091 | 12,709 | ||||||||
OPERATING INCOME | 2,626 | 8,170 | 8,081 | ||||||||
INTEREST INCOME (EXPENSE) | 237 | (113 | ) | (50 | ) | ||||||
INCOME BEFORE INCOME TAXES | 2,863 | 8,057 | 8,031 | ||||||||
INCOME TAX EXPENSE | 1,086 | 3,094 | 2,938 | ||||||||
NET INCOME | $ | 1,777 | $ | 4,963 | $ | 5,093 | |||||
See notes to financial statements.
62
Table of Contents
INFUSYSTEM, INC.
(A Wholly Owned Subsidiary of I-Flow Corporation)
STATEMENTS OF STOCKHOLDER’S EQUITY
FOR THE PERIOD FROM JANUARY 1, 2007 TO OCTOBER 25, 2007, AND THE
YEARS ENDED DECEMBER 31, 2006 AND 2005
(Amounts in thousands)
| Common Stock | Additional Paid-In Capital | Retained Earnings | Total | |||||||||||||
| Shares | Amount | |||||||||||||||
BALANCE — January 1, 2005 | 100 | $ | — | $ | 4,005 | $ | 8,818 | $ | 12,823 | |||||||
Net contributions from Parent | 4,539 | 4,539 | ||||||||||||||
Net income | 5,093 | 5,093 | ||||||||||||||
BALANCE — December 31, 2005 | 100 | — | 8,544 | 13,911 | 22,455 | |||||||||||
Net dividends to Parent | (5,410 | ) | (5,410 | ) | ||||||||||||
Net income | 4,963 | 4,963 | ||||||||||||||
BALANCE — December 31, 2006 | 100 | — | 8,544 | 13,464 | 22,008 | |||||||||||
Cumulative effect of adjustments from adoption of FIN No. 48 (Note 5) | (821 | ) | (821 | ) | ||||||||||||
BALANCE — January 1, 2007 | 100 | — | 8,544 | 12,643 | 21,187 | |||||||||||
Net dividends to Parent | (3,593 | ) | (3,593 | ) | ||||||||||||
Net income | 1,777 | 1,777 | ||||||||||||||
BALANCE — October 25, 2007 | 100 | $ | — | $ | 8,544 | $ | 10,827 | $ | 19,371 | |||||||
See notes to financial statements.
63
Table of Contents
INFUSYSTEM, INC.
(A Wholly Owned Subsidiary of I-Flow Corporation)
STATEMENTS OF CASH FLOWS
FOR THE PERIOD FROM JANUARY 1, 2007 TO OCTOBER 25, 2007, AND THE
YEARS ENDED DECEMBER 31, 2006 AND 2005
(Amounts in thousands)
| For the Period January 1, 2007 to October 25, 2007 | Fiscal Year Ended December 31, 2006 | Fiscal Year Ended December 31, 2005 | ||||||||||
CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
Net income | $ | 1,777 | $ | 4,963 | $ | 5,093 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation and amortization | 2,713 | 3,706 | 3,259 | |||||||||
Provision for doubtful accounts receivable | 4,971 | 4,041 | 1,260 | |||||||||
Deferred taxes | (1,732 | ) | (187 | ) | (351 | ) | ||||||
Loss on disposal of property | 255 | 302 | 370 | |||||||||
Stock-based compensation | 746 | 396 | 1,085 | |||||||||
Write-off of obsolete inventory supplies | 1 | 12 | ||||||||||
Changes in operating assets and liabilities: | ||||||||||||
Accounts receivable | (965 | ) | (4,511 | ) | (5,500 | ) | ||||||
Inventory supplies | (158 | ) | (67 | ) | 27 | |||||||
Prepaid expenses and other current assets | (455 | ) | 80 | (120 | ) | |||||||
Accounts payable | (1,131 | ) | (131 | ) | (565 | ) | ||||||
Accrued payroll and related expenses | 716 | (608 | ) | 366 | ||||||||
State income taxes payable | (173 | ) | 78 | |||||||||
Other current liabilities | 670 | (8 | ) | 12 | ||||||||
Net cash provided by operating activities | 7,407 | 7,804 | 5,026 | |||||||||
CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
Capital expenditures | (2,912 | ) | (2,506 | ) | (6,878 | ) | ||||||
Proceeds from sale of property | 231 | 1 | 1 | |||||||||
Net cash used in investing activities | (2,681 | ) | (2,505 | ) | (6,877 | ) | ||||||
CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
Net capital contributions from parent | 3,454 | |||||||||||
Net capital distributions to parent | (4,339 | ) | (5,806 | ) | ||||||||
Net cash (used in) provided by financing activities | (4,339 | ) | (5,806 | ) | 3,454 | |||||||
NET INCREASE (DECREASE) IN CASH | 387 | (507 | ) | 1,603 | ||||||||
CASH — Beginning of year | 1,956 | 2,463 | 860 | |||||||||
CASH — End of period | $ | 2,343 | $ | 1,956 | $ | 2,463 | ||||||
SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||||||
Interest paid | $ | — | $ | 20 | $ | 1 | ||||||
State income tax payments | $ | 241 | $ | 266 | $ | 138 | ||||||
SUPPLEMENTAL SCHEDULE OF NONCASH INVESTING ACTIVITIES — Property acquisitions | $ | 550 | $ | 1,126 | $ | 1,135 | ||||||
See notes to financial statements.
64
Table of Contents
INFUSYSTEM, INC.
(A Wholly Owned Subsidiary of I-Flow Corporation)
NOTES TO FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2006, AND FOR THE PERIOD FROM JANUARY 1, 2007 TO
OCTOBER 25, 2007, AND THE YEARS DECEMBER 31, 2006 AND 2005
| 1. | GENERAL |
InfuSystem, Inc. (“InfuSystem” or the “Company”) is a leading provider of ambulatory infusion pump management services and is based in Madison Heights, MI. It is primarily engaged in the rental of ambulatory electronic infusion pumps on a month-to-month basis for the administration of chemotherapy drugs for the treatment of cancer. The Company was incorporated in California on December 18, 1997, and was a wholly owned subsidiary of I-Flow Corporation (“I-Flow”).
On September 29, 2006, I-Flow signed a definitive agreement to sell the Company to InfuSystem Holdings, Inc., (formerly known as HAPC, Inc., (“InfuSystem Holdings”) for $140,000,000 in the form of cash and a secured note, subject to certain purchase price adjustments based on the level of working capital. On September 18, 2007, due to the prevailing conditions within the financial markets, I-Flow amended the definitive agreement resulting in a new purchase price of $100,000,000 (subject to working capital adjustments in the definitive agreement), plus a contingent payment right to I-Flow of up to a maximum of $12,000,000 (the “Earn-Out”). The amended purchase price is payable in the form of cash or a combination of cash equal to $85,000,000 less the amount paid to InfuSystem Holdings’ shareholders who chose to convert their InfuSystem Holdings shares into cash and a secured promissory note.
On October 19, 2007, I-Flow purchased approximately 2.8 million shares of common stock of InfuSystem Holdings at $5.97 per share through private transactions with third parties totaling approximately $16,651,000. With the shares purchased, I-Flow owns approximately 15% of the issued and outstanding InfuSystem Holdings common stock and disclosed its intentions to vote such shares in favor of the acquisition.
On October 24, 2007, the shareholders of InfuSystem Holdings approved the acquisition of the Company. The sale was completed on October 25, 2007, and the I-Flow received the $100,000,000 purchase price at the closing in a combination of (i) cash equal to $67,297,000 and (ii) a secured promissory note with a principal amount equal to $32,703,000.
On October 25, 2007, in connection with the closing of the sale, the Company and I-Flow entered into a services agreement that will result in I-Flow compensating the Company for its processing costs related to I-Flow’s ON-Q® billings and providing the Company with an incentive-based reimbursement arrangement. Pursuant to the terms of the services agreement, the Company will continue to provide to I-Flow, from and after the closing, the billing and collection services and management services the Company has been providing prior to the date of the closing. The term of the services agreement will be three years, but it may be terminated earlier, after 18 months. Fees paid by I-Flow under the services agreement will be set at the higher of a cost-plus or percentage of collections.
Pursuant to the 2007 Management Incentive Plan (“2007 MIP”) that was established and adopted by I-Flow’s executive management prior to the sale of the Company, the Company’s executive officers are entitled to share a bonus pool of $150,000 upon the closing of the sale of the Company. In addition to the 2007 MIP, the Company entered into change in control agreements with certain of its executive
65
Table of Contents
officers. The change in control agreements provide for success bonuses to be paid to the executives equal to twelve months of the nonsales executive’s current base salary or W-2 Medicare wages or six months of the sales executive’s base salary, plus six times the monthly average of commissions and overrides earned during the twelve months immediately preceding the closing date. The change in control agreements also provided for the immediate vesting of all restricted stock units and unvested stock options issued by I-Flow. All restrictions with respect to the restricted stock units would lapse and all vested options would remain exercisable for 30 days after the closing date of the transaction. Upon the closing of the sale of the Company on October 25, 2007, the Company paid a total of $150,000 under the 2007 MIP bonus and approximately $815,000 of success bonuses to the executives. The Company also recorded approximately $583,000 of stock-based compensation expense related to the immediate vesting of the restricted stock units and stock options of the executives in connection with the close of the sale.
In connection with the sale of the Company to InfuSystem Holdings, I-Flow incurred certain expenses related to the divestiture, including legal and professional fees that resulted directly from the sale transaction. Divestiture expenses incurred by I-Flow for the period from January 1, 2007 to October 25, 2007, and the year ended December 31, 2006, were approximately $2,329,000 and $2,090,000, respectively. At the close of the sale of the Company, I-Flow received reimbursement of approximately $946,000 by InfuSystem Holdings of certain divestiture expenses incurred in 2006 and 2007. The divestiture expenses incurred were not reimbursed by the Company to I-Flow, and accordingly, the amounts, including the reimbursement by InfuSystem Holdings for divestiture expenses, are not included in the Company’s financial statements.
The Company provided certain administrative services to I-Flow for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, related to the I-Flow’s ON-Q PainBuster® Pain Management System (“ON-Q”). Specifically, the Company provided customer service, billings to third-party insurance, collections, and solicitation of managed care contracts with insurance companies on behalf of I-Flow and its ON-Q product line, primarily in ambulatory surgery centers (ASC’s). The Company was not reimbursed from I-Flow for these services.
The Company estimates that processing costs borne by the Company for ON-Q billings and reflected in its financial statements for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, respectively, were approximately $1,101,000, $1,457,000, and $1,165,000. The estimated expense for the period from January 1, 2007 to October 25, 2007, consisted of approximately $459,000 in direct payroll expenses and $642,000 of indirect or allocated expenses. The estimated expense for the year ended December 31, 2006, consisted of approximately $745,000 in direct payroll expenses and $712,000 of indirect or allocated expenses. The estimated expense for the year ended December 31, 2005, consisted of approximately $452,000 in direct payroll expenses and $713,000 of indirect or allocated expenses.
66
Table of Contents
I-Flow also provided certain administrative services to the Company during the same time periods. Costs incurred by I-Flow on behalf of the Company that were clearly applicable to the Company were charged to the Company and included the following administrative services:
| • | I-Flow provided workers’ compensation insurance to employees of the Company. The Company’s financial statements for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, include workers’ compensation insurance expense of approximately $21,000, $28,000, and $29,000, respectively, which was recorded in general and administrative expenses. |
| • | Many of the Company’s employees received stock options and other stock-based awards relating to the stock of I-Flow. I-Flow determined the expense based on the outstanding stock-based awards granted specifically to employees of the Company. Stock-based compensation expense reflected in the Company’s financial statements for the period from January 1, 2007 to October 25, 2007, which includes the expense related to the immediate vesting of the restricted stock units and stock options of the executives in connection with the close of the sale, and the expense for the years ended December 31, 2006 and 2005, were as follows: |
| For the Period January 1, 2007 to October 25, 2007 | Fiscal Year Ended December 31, 2006 | Fiscal Year Ended December 31, 2005 | |||||||
Selling and marketing expenses | $ | 116,000 | $ | 254,000 | $ | 266,000 | |||
General and administrative expenses | 630,000 | 142,000 | 819,000 | ||||||
Total stock-based compensation expense | $ | 746,000 | $ | 396,000 | $ | 1,085,000 | |||
| • | Although the Company files a consolidated and certain combined state tax returns with its Parent, the Company recorded income taxes payable as a result of preparing its financial statements on a separate tax return basis. Income taxes payable and excess stock option income tax benefits totaling $2,297,000, $2,870,000, and $2,993,000 for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, respectively, were recognized as contributions from I-Flow. |
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation — The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Cash — As of October 25, 2007 and December 31, 2006, the Company maintains its cash primarily with a single financial institution.
Accounts Receivable — The Company performs periodic analyses to evaluate its accounts receivable balances. It records an allowance for doubtful accounts based on the estimated collectibility of the accounts such that the recorded amounts reflect estimated net realizable value. Upon determination that an account is uncollectible, the account is written-off and charged to the allowance.
In determining its accounts receivable balances and allowance for doubtful accounts, management considers historical realization data, accounts receivable aging trends, operating trends, and other relevant business conditions, such as governmental and managed care payor claims processing
67
Table of Contents
procedures and system changes. The Company’s analysis includes the application of specified percentages to the accounts receivable agings to estimate the amount that will ultimately be uncollectible and therefore should be reserved. The percentages are increased as the accounts age. Due to the continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on its financial position, results of operations, and cash flows.
Inventory Supplies — Inventory supplies are stated at the lower of cost (determined on a first in, first out basis) or market. The Company records a period expense for inventory supplies obsolescence when incurred.
Long-Lived Assets — The Company accounts for the impairment and disposition of long-lived assets in accordance with Financial Accounting Standards Board (FASB) Statement No. 144,Accounting for Impairment or Disposal of Long-Lived Assets. FASB Statement No. 144 addresses financial accounting and reporting for the impairment of long-lived assets and for the disposal of long-lived assets. In accordance with FASB Statement No. 144, long-lived assets to be held are reviewed for events or changes in circumstances, which indicate that their carrying value may not be recoverable. Recoverability of these assets is determined based upon the expected undiscounted future net cash flows from the operations to which the assets relate, utilizing management’s best estimates, appropriate assumptions and projections at the time. If the carrying value is determined not to be recoverable from future operating cash flows, the asset is deemed impaired and an impairment loss would be recognized to the extent the carrying value exceeded the estimated fair market value of the asset. The Company periodically reviews the carrying value of long-lived assets to determine whether an impairment to such value has occurred. The Company has determined that there was no impairment as of October 25, 2007 or December 31, 2006.
Property — Property is stated at cost and depreciated using the straight-line method over the estimated useful lives of the related assets, ranging from three to seven years. Rental equipment, consisting of ambulatory infusion pumps that the Company acquires from third-party manufacturers, is depreciated over five years. Leasehold improvements are amortized using the straight-line method over the life of the asset or the remaining term of the lease, whichever is shorter. Maintenance and minor repairs are charged to operations as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is recorded in the current period. Property as of October 25, 2007 and December 31, 2006, consisted of the following:
| October 25, 2007 | December 31, 2006 | |||||||
Pump equipment | $ | 23,240,000 | $ | 23,010,000 | ||||
Furniture, fixtures, and equipment | 1,337,000 | 1,279,000 | ||||||
Accumulated depreciation and amortization | (13,282,000 | ) | (11,982,000 | ) | ||||
Total | $ | 11,295,000 | $ | 12,307,000 | ||||
During the period from January 1, 2007 to October 25, 2007, the Company made purchases from two suppliers, each of which supplies more than 10% of the pumps purchased by the Company. During the year ended December 31, 2006, the Company made purchases from three suppliers, each of which supplies more than 10% of the pumps purchased by the Company. There are no supply agreements in place with any of the three suppliers. All purchases are handled pursuant to pricing agreements, which contain no material terms other than prices that are subject to change by the manufacturer. The products offered by all three suppliers are fungible, such that no one supplier is material to the Company’s business.
68
Table of Contents
Goodwill — The Company recognizes goodwill in accordance with FASB Statement No. 142,Goodwill and Other Intangible Assets. Under FASB Statement No. 142, goodwill is recorded at its carrying value and is tested for impairment at least annually. The Company reviews the recoverability of the carrying value of goodwill on an annual basis or more frequently if an event occurs or circumstances change to indicate that an impairment of goodwill has possibly occurred. The Company compares the fair value of its reporting unit to the carrying value, as well as other factors, to determine whether or not any potential impairment of goodwill exists. If a potential impairment exists, an impairment loss is recognized to the extent the carrying value of goodwill exceeds the difference between the fair value of the reporting unit and the fair value of its other assets and liabilities. No goodwill impairment existed as of October 25, 2007 and December 31, 2006.
Revenue Recognition — Rental revenue in the oncology market is the Company’s strategic focus. The Company does not recognize revenue until all of the following criteria are met: persuasive evidence of an arrangement exists, services have been rendered, the price to the customer is fixed or determinable, and collectibility is reasonably assured. Persuasive evidence of an arrangement is determined to exist, and collectibility is reasonably assured, at the point in which a certificate of medical necessity and assignment of benefits, signed by the physician and patient, respectively, have been received by the Company, and the Company has verified actual pump usage and insurance coverage. Rental revenue from electronic infusion pumps is recognized as earned over the term of the related rental agreements, normally on a month-to-month basis. Pump rentals are billed at the Company’s established rates, which often differ from contractually allowable rates provided by third-party payors, such as Medicare, Medicaid, and commercial insurance carriers. Provision is made currently to reduce revenue to the estimated allowable amount per such contractual rates. For the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, revenue from Medicare accounted for 32%, 33%, and 35% of total revenue, respectively. For the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, revenue from Blue Cross Blue Shield accounted for 21%, 21%, and 22% of total revenue, respectively. For the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, revenue from Medicaid accounted for 5%, 3%, and 3% of total revenue, respectively.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Because of continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on operations and cash flows.
Income Taxes — The Company accounts for income taxes in accordance with FASB Statement No. 109,Accounting for Income Taxes, which requires that the Company recognize deferred tax liabilities and assets based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities, using enacted tax rates in effect in the years the differences are expected to reverse. Deferred income tax benefit (expense) results from the change in net deferred tax assets or deferred tax liabilities. A valuation allowance is recorded when it is more likely than not that some or all of any deferred tax assets will not be realized. The Company’s income taxes as presented in the financial statements have been prepared on a separate return basis.
Accounting for Stock-Based Compensation — I-Flow, the former parent company, historically granted stock-based awards to certain officers and employees of the Company. Total stock-based compensation expense incurred by the Company related to stock options and stock grants by I-Flow to
69
Table of Contents
the Company’s employees for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, were $746,000, $396,000, and $1,085,000, respectively. The stock-based compensation expense recorded for the period from January 1, 2007 to October 25, 2007, includes a) approximately $583,000 of expense related to the restricted stock units and stock options granted to the Company’s executives that immediately vested upon the sale of the Company on October 25, 2007, pursuant to the change in control agreements in effect and b) approximately $142,000 of expense reversal related to the true up of actual forfeitures on stock options and stock grants that were cancelled for all other employees by I-Flow due to the sale of the Company.
Effective January 1, 2006, the Company adopted FASB Statement No. 123-revised 2004,Share-Based Payment, which requires the measurement and recognition of compensation expense based on estimated fair values for all equity-based compensation made to employees and directors. FASB Statement No. 123R replaces the guidance in FASB Statement No. 123,Accounting for Stock-Based Compensation, and supersedes Accounting Principles Board (“APB”) Opinion No. 25,Accounting for Stock Issued to Employees.
The Company adopted FASB Statement No. 123R using the modified prospective application transition method. In accordance with the modified prospective application transition method, the Company’s financial statements for prior periods do not have to be restated to reflect, and do not include, the impact of FASB Statement No. 123R. Prior to the adoption of FASB Statement No. 123R, the Company accounted for stock-based awards to officers and employees using the intrinsic value method in accordance with APB 25 and adopted the disclosure-only alternative of FASB Statement No. 123. Because the Company had adopted the disclosure-only provisions of FASB Statement No. 123, no compensation cost was recognized in 2005 for stock option grants to employees and officers with exercise prices at least equal to the fair market value of the underlying shares at the grant date.
FASB Statement No. 123R requires companies to estimate the fair value of equity awards on the date of grant using an option-pricing model. The Company uses the Black-Scholes option-pricing model, which it had previously used for valuation of option-based awards for its pro forma information required under FASB Statement No. 123 for periods prior to fiscal 2006. The determination of the fair value of option-based awards using the Black-Scholes model incorporates various assumptions including volatility, expected life of awards, risk-free interest rates, and expected dividends. The expected volatility is based on the historical volatility of the price of I-Flow’s common stock over the most recent period commensurate with the estimated expected life of the Company’s stock options and adjusted for the impact of unusual fluctuations not reasonably expected to recur. The expected life of an award is based on historical experience and on the terms and conditions of the stock awards granted to employees and nonemployee directors. The fair value of each option grant is estimated on the date of grant using the Black-Scholes option-pricing model. No stock options were granted to employees of the Company for the period from January 1, 2007 to October 25, 2007, and the year ended December 31, 2006. During the year ended December 31, 2005, 101,000 stock options were granted to employees of the Company with the following weighted-average assumptions used for grants: no dividend yield; expected volatility of 86%; risk-free interest rate of 4.17%; and estimated life of five years.
Stock-based compensation expense was recognized for all new and unvested equity awards that are expected to vest as the requisite service is rendered beginning on January 1, 2006. Stock-based compensation for awards granted prior to January 1, 2006, was based on the grant date fair value as determined under the pro forma provisions of FASB Statement No. 123. In conjunction with the adoption of FASB Statement No. 123R, the Company changed its method of attributing the value of stock-based compensation expense from the accelerated multiple-option approach to the straight-line single-option method. Compensation expense for all unvested equity awards granted on or prior to December 31, 2005, continued to be recognized using the accelerated multiple-option approach. Compensation expense for
70
Table of Contents
all equity awards granted subsequent to December 31, 2005, was recognized using the straight-line single-option method. In accordance with FASB Statement No. 123R, the Company has factored in forfeitures in its recognition of stock-based compensation. FASB Statement No. 123R requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company accounted for forfeitures as they occurred in the pro forma information required under FASB Statement No. 123 for periods prior to fiscal 2006.
FASB Statement No. 123R requires that cash flows resulting from tax deductions in excess of the cumulative compensation cost recognized for options exercised (excess tax benefits) be classified as cash inflows from financing activities and cash outflows from operating activities. Excess tax benefits that were attributed to the share-based compensation expense are currently reflected in contributions from Parent as a result of the Company preparing its financial statements on a separate tax return basis (see Notes 4 and 5).
On November 10, 2005, the FASB issued FASB Staff Position No. FAS 123R-3,Transition Election Related to Accounting for Tax Effects of Share-Based Payment Awards. The Company has elected to adopt the alternative transition method provided in the FASB Staff Position for calculating the tax effects of share-based compensation pursuant to FASB Statement No. 123R. The alternative transition method includes simplified methods to establish the beginning balance of the additional paid-in capital pool (“APIC Pool”) related to the tax effects of employee share-based compensation and to determine the subsequent impact on the APIC Pool and Statements of Cash Flows of the tax effects of employee share-based awards that were outstanding upon adoption of FASB Statement No. 123R.
From and after May 26, 2005, stock-based awards granted to officers and employees of the Company are granted from I-Flow active equity incentive plans that were approved by I-Flow’s stockholders. All future grants of stock options (including incentive stock options or nonqualified stock options), restricted stock, restricted stock units, or other forms of equity-based compensation to officers and employees of the Company are expected to be made under the I-Flow Corporation 2001 Equity Incentive Plan (the “2001 Plan”), which was approved by I-Flow’s stockholders in May 2001. The maximum number of shares of common stock that may be issued pursuant to awards under the 2001 Plan is currently 7,750,000, subject to adjustments for stock splits or other adjustments as defined in the 2001 Plan.
Stock Options — Options granted under the 2001 Plan became exercisable at such times as determined by the I-Flow compensation committee of the board of directors or the I-Flow board of directors itself. Options granted to officers and employees of the Company generally have an exercise price equal to the market price of the I-Flow’s stock at the date of the grant, with vesting and contractual terms of five years. Options generally provide for accelerated vesting if there is a change in control for I-Flow (as defined in the 2001 Plan or, as applicable, the officers’ employment and change in control agreements). I-Flow issues new shares upon the exercise of stock options. Pursuant to the change in control agreements entered into with I-Flow and the Company’s executives, all unvested stock options would immediately vest upon the sale of the Company and would remain exercisable for 30 days after the closing date of the transaction. All unvested stock options that were granted to nonexecutive employees were cancelled by I-Flow as of October 25, 2007. All vested but unexercised stock options would remain exercisable for 30 days after the closing date of the transaction for nonexecutive employees. A summary of all the Company’s outstanding options as of October 25, 2007, and of changes in options outstanding for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, is as follows.
71
Table of Contents
| Number of Shares | Weighted- Average Exercise Price per Share | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | ||||||||
Options outstanding — January 1, 2005 | 317,273 | $ | 5.06 | ||||||||
Options granted | 101,000 | 17.54 | |||||||||
Options exercised | (80,949 | ) | 1.39 | ||||||||
Options forfeited or expired | |||||||||||
Options outstanding — December 31, 2005 | 337,324 | 9.68 | |||||||||
Options granted | |||||||||||
Options exercised | (103,634 | ) | 3.57 | ||||||||
Options forfeited or expired | (3,304 | ) | 10.45 | ||||||||
Options outstanding — December 31, 2006 | 230,386 | 12.42 | |||||||||
Options granted | |||||||||||
Options exercised | (102,203 | ) | 11.39 | ||||||||
Options forfeited or expired | (979 | ) | 6.38 | ||||||||
Options outstanding — October 25, 2007 | 127,204 | 13.29 | 0.15 | $ | 707,605 | ||||||
Options vested and exercisable — October 25, 2007 | 127,204 | 13.29 | 0.15 | $ | 707,605 | ||||||
The above table excludes equity awards granted to sales representatives and sales management with exercise prices below fair market value. Such awards have been included in the restricted stock units table below.
No options were granted for the period from January 1, 2007 to October 25, 2007, and the year ended December 31, 2006. The weighted-average fair value of options granted during the year ended December 31, 2005, estimated as of the grant date using the Black-Scholes option valuation model was $12.23 per option. The total intrinsic value of options exercised for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, was $761,000, $1,161,000 and $1,159,000, respectively.
72
Table of Contents
The following table summarizes information concerning outstanding and exercisable options for employees of the Company for the purchase of I-Flow’s common stock for all plans and grants as of October 25, 2007:
| Options Outstanding | Options Exercisable | |||||||||||
Range of Exercise Prices | Number of Shares Outstanding | Weighted-Average Remaining Contractual Life in Years | Average Weighted- Exercise Price | Number of Shares Exercisable | Weighted- Average Exercise Price | |||||||
$ 1.33–$ 1.33 | 4,000 | 0.08 | $ | 1.33 | 4,000 | $ | 1.33 | |||||
$ 1.66–$ 1.66 | 13,055 | 0.12 | 1.66 | 13,055 | 1.66 | |||||||
$ 4.40–$ 4.40 | 151 | 0.08 | 4.40 | 151 | 4.40 | |||||||
$11.41–$11.41 | 661 | 0.08 | 11.41 | 661 | 11.41 | |||||||
$11.52–$11.52 | 4,337 | 0.11 | 11.52 | 4,337 | 11.52 | |||||||
$13.52–$13.52 | 1,000 | 0.08 | 13.52 | 1,000 | 13.52 | |||||||
$13.55–$13.55 | 57,000 | 0.16 | 13.55 | 57,000 | 13.55 | |||||||
$14.03–$14.03 | 2,000 | 0.08 | 14.03 | 2,000 | 14.03 | |||||||
$17.58–$17.58 | 45,000 | 0.15 | 17.58 | 45,000 | 17.58 | |||||||
Total | 127,204 | 0.15 | 13.29 | 127,204 | 13.29 | |||||||
On November 9, 2005, the board of directors of I-Flow approved the amendment of stock options that were previously granted to employees and officers with exercise prices at a discount to the fair market value. The amendments increased the exercise price to the fair market value on the date the options were granted and accelerated the vesting of approximately 150,000 unvested, “out-of-the-money” stock options previously awarded to officers and employees of the Company. A total of 175,000 stock options granted to officers and employees of the Company with original exercise prices of $11.52 and $14.94 per share were increased to $13.55 and $17.58 per share, respectively, effective November 9, 2005. In 2005, I-Flow compensated the officers and employees of the Company for the increased exercise prices by granting approximately 23,000 shares of I-Flow’s common stock such that the value of the shares granted (based on the closing price of I-Flow’s common stock on November 9, 2005, of $11.91) equaled the value of the lost discount in exercise price, net of shares withheld to pay withholding taxes. With respect to the acceleration of vesting, options with an exercise price greater than $11.91 per share (giving effect to the increased exercise price) were deemed “out-of-the-money.” The accelerated options granted to officers and employees of the Company, which are considered fully vested as of November 9, 2005, have exercise prices ranging from $13.52 to $17.58 per share and a weighted-average exercise price of $16.24 per share. Among the primary purposes of the amended exercise price and acceleration were to comply with new deferred compensation tax laws, to promote employee motivation, retention and the perception of option value, and to avoid recognizing future compensation expense associated with “out-of-the-money” stock options upon adoption of FASB Statement No. 123R, which replaces FASB Statement No. 123, and supersedes APB Opinion No. 25 in fiscal 2006.
Effective January 1, 2006, under FASB Statement No. 123R, approximately 81,500 stock options with exercise prices of $1.33 or $2.47 per share were increased to $1.66 and $2.91 per share, respectively. In January 2006, I-Flow compensated the eight affected option holders for the increased exercise price by granting approximately 1,500 shares of the I-Flow’s common stock for a total incremental compensation cost of $14,000 for the year ended December 31, 2006.
73
Table of Contents
For stock options granted prior to the adoption of FASB Statement No. 123R, the following table illustrates the pro forma effect on net income as if the Company had applied the fair value recognition provisions of FASB Statement No. 123 in determining stock-based compensation for options during the year ended December 31, 2005:
Net income — as reported | $ | 5,093,000 | ||
Stock-based compensation expense included in net loss — net of tax | 688,000 | |||
Total stock-based compensation expense determined under fair-value-based method for all awards — net of tax | (1,471,000 | ) | ||
Net income — pro forma | $ | 4,310,000 | ||
Restricted Stock Units — Restricted stock units were granted pursuant to the 2001 Plan and as determined by the I-Flow compensation committee of the I-Flow board of directors or the board of directors itself. Restricted stock units granted to officers and employees of the Company generally have vesting periods of five years from the date of grant. Restricted stock units granted to sales representatives and sales management have a maximum vesting term of three years from the date of grant. The Company issues new shares upon the vesting of restricted stock units. In accordance with FASB Statement No. 123R, the fair value of restricted stock units was estimated based on the closing market value stock price on the date of grant and the expense is recognized straight-lined over the requisite period. The total number of shares of restricted stock units expected to vest is adjusted by estimated forfeiture rates. Pursuant to the change in control agreements entered into with I-Flow and the Company’s executives, all restrictions with respect to the restricted stock units would lapse upon the sale of the Company. All unvested restricted stock units that were granted to nonexecutive employees were cancelled by I-Flow as of October 25, 2007.
74
Table of Contents
The following table provides a summary of the Company’s restricted stock units as of October 25, 2007, and of changes in restricted stock units outstanding under the 2001 Plan for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005:
| Number of Shares | Weighted-Average Grant Date Fair Value Per Share | |||||
Nonvested shares outstanding — January 1, 2005 | — | $ | — | |||
Shares issued | 16,000 | 14.12 | ||||
Shares vested or released | 16,000 | 16.01 | ||||
Shares forfeited | (3,000 | ) | 14.12 | |||
Nonvested shares outstanding — December 31, 2005 | 29,000 | 15.16 | ||||
Shares issued | 85,140 | 13.73 | ||||
Shares vested or released | (27,000 | ) | 15.20 | |||
Shares forfeited | (2,000 | ) | 13.22 | |||
Nonvested shares outstanding — December 31, 2006 | 85,140 | 13.76 | ||||
Shares issued | 10,000 | 16.43 | ||||
Shares vested or released | (69,475 | ) | 14.05 | |||
Shares forfeited | (25,665 | ) | 14.01 | |||
Nonvested shares outstanding — October 25, 2007 | — | |||||
The total value of shares vested during the period from January 1, 2007 and October 25, 2007, and the year ended December 31, 2006, was approximately $976,000 and $410,000, respectively.
Use of Estimates — The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America necessarily requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from these estimates.
New Accounting Pronouncements — In July 2006, the FASB issued FASB Interpretation (FIN) No. 48,Accounting for Uncertainty in Income Taxes— an interpretation of FASB Statement No. 109, which prescribes a recognition threshold and measurement process for recording in the financial statements uncertain tax positions taken or expected to be taken in a tax return. Additionally, FIN No. 48 provides guidance on the derecognition, classification, interest and penalties, accounting in interim periods, and disclosure requirements for uncertain tax positions. The accounting provisions of FIN No. 48 are effective for reporting periods beginning after December 15, 2006. The Company adopted FIN No. 48 effective January 1, 2007. See Note 5 on Income Taxes for additional information, including the effects of the adoption on the Company’s financial statements.
In September 2006, the FASB issued FASB Statement No. 157,Fair Value Measurements, which defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. The provisions of FASB Statement No. 157 are effective as of the beginning of the Company’s 2008 fiscal year. The Company is currently assessing the impact of the adoption of FASB Statement No. 157 and its impact on the Company’s financial statements.
In February 2007, the FASB issued FASB Statement No. 159,The Fair Value Option for Financial Assets and Financial Liabilities — Including an amendment of FASB Statement No. 115. FASB Statement No. 159
75
Table of Contents
permits companies to measure many financial instruments and certain other items at fair value at specified election dates. FASB Statement No. 159 will be effective beginning January 1, 2008. The Company is currently assessing the impact of the adoption of FASB Statement No. 159 and its impact on the Company’s financial statements.
| 3. | LINES OF CREDIT |
The Company had a loan agreement containing a revolving line of credit with a bank under which it could borrow up to the lesser of $3.5 million or 80% of eligible accounts receivable, as defined, at the bank’s prime rate less 0.25%. Under a separate loan agreement with the same bank, the Company also had a loan facility under which it could borrow up to $2,500,000 for the purchase of equipment. The line of credit and loan facility expired on June 30, 2006, and were not renewed.
| 4. | SHAREHOLDER’S EQUITY |
At inception, the Company received capital contributions of approximately $3,321,000 from I-Flow, which were used primarily in the acquisition of the Company’s operations. Net contributions from and distributions to I-Flow for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, were as follows:
| For the Period January 1, 2007 to October 25, 2007 | Fiscal Year Ended December 31, 2006 | Fiscal Year Ended December 31, 2005 | |||||||||
Stock-based compensation expense charges | $ | 746,000 | $ | 396,000 | $ | 1,085,000 | |||||
Workers’ compensation insurance charges | 21,000 | 28,000 | 29,000 | ||||||||
Excess stock option income tax benefit | 522,000 | 531,000 | 196,000 | ||||||||
Income tax liability forgiveness by Parent | 1,775,000 | 2,339,000 | 2,797,000 | ||||||||
Net cash transfers (to) from the Parent | (6,657,000 | ) | (8,704,000 | ) | 432,000 | ||||||
Net contributions from (dividends to) Parent | $ | (3,593,000 | ) | $ | (5,410,000 | ) | $ | 4,539,000 | |||
| 5. | INCOME TAXES |
The Company accounts for income taxes under the provisions of FASB Statement No. 109. Under this method, deferred tax assets and liabilities are established for temporary differences between the financial reporting basis and the tax basis of the Company’s assets and liabilities at tax rates expected to be in effect when such assets or liabilities are realized or settled.
The Company files a consolidated federal and certain combined state tax returns with its Parent. Through these filings, income generated by the Company is offset by losses in the consolidated group.
76
Table of Contents
The income tax provision for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, is summarized as follows:
| For the Period January 1, 2007 to October 25, 2007 | Fiscal Year Ended December 31, 2006 | Fiscal Year Ended December 31, 2005 | ||||||||||
Current: | ||||||||||||
Federal | $ | 2,278,000 | $ | 2,916,000 | $ | 2,994,000 | ||||||
State | 161,000 | 365,000 | 295,000 | |||||||||
Subtotal | 2,439,000 | 3,281,000 | 3,289,000 | |||||||||
Deferred: | ||||||||||||
Federal | (1,323,000 | ) | (188,000 | ) | (336,000 | ) | ||||||
State | (30,000 | ) | 1,000 | (15,000 | ) | |||||||
Subtotal | (1,353,000 | ) | (187,000 | ) | (351,000 | ) | ||||||
Total income tax provision | $ | 1,086,000 | $ | 3,094,000 | $ | 2,938,000 | ||||||
| The reconciliations of the effective income tax rate to the federal statutory rate are as follows: | ||||||||||||
| For the Period January 1, 2007 to October 25, 2007 | Fiscal Year Ended December 31, 2006 | Fiscal Year Ended December 31, 2005 | ||||||||||
Tax at federal statutory rate | 34.0 | % | 34.0 | % | 34.0 | % | ||||||
State income taxes — net of federal benefit | 3.9 | 3.3 | 2.3 | |||||||||
Nondeductible charges | 1.1 | 0.3 | ||||||||||
Total | 37.9 | % | 38.4 | % | 36.6 | % | ||||||
| As of December 31, 2006 and 2005, the Company had a net deferred tax liability comprised of the following: | ||||||||||||
| December 31, 2006 | December 31, 2005 | |||||||||||
Reserves not currently deductible |
| $ | 689,000 | $ | 687,000 | |||||||
Share-based compensation |
| 114,000 | ||||||||||
State taxes |
| (9,000 | ) | (10,000 | ) | |||||||
Depreciation |
| (1,390,000 | ) | (1,460,000 | ) | |||||||
Net deferred tax liability |
| $ | (596,000 | ) | $ | (783,000 | ) | |||||
Although the Company files a consolidated and certain combined state tax returns with its Parent, the Company recorded income taxes payable as a result of preparing its financial statements on a separate tax return basis. Income taxes payable and excess stock option income tax benefits totaling $2,297,000, $2,870,000, and $2,993,000 for the years ended October 25, 2007, and December 31, 2006 and 2005, respectively, were recognized as contributions from the Parent.
77
Table of Contents
In July 2006, the FASB issued FIN No. 48. FIN No. 48 is an interpretation of FASB Statement No. 109, and it seeks to reduce the diversity in practice associated with certain aspects of measurement and recognition in accounting for income taxes. In addition, FIN No. 48 provides guidance on derecognition, classification, interest and penalties, and accounting in interim periods and requires expanded disclosure with respect to the uncertainty in income taxes. FIN No. 48 is effective as of the beginning of the Company’s 2007 fiscal year. The cumulative effect, if any, of applying FIN No. 48 is to be reported as an adjustment to the opening balance of retained earnings in the year of adoption.
As a result of the implementation of FIN No. 48, the Company recognized a $1,198,000 increase to its FIN No. 48 liability for uncertain tax positions. Of this increase, approximately $821,000 is the cumulative affect adjustment to the opening balance of retained earnings.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2006, the Company has approximately $62,000 of accrued interest expense related to uncertain tax positions and approximately $133,000 of penalties. As of October 25, 2007, the Company has approximately $100,000 of additional accrued interest and approximately $82,000 of penalties related to uncertain tax positions related to the FIN No. 48 liability balance as of December 31, 2006. An additional $98,000 relating to additional state tax liabilities and $25,000 related to penalties was identified in the current year ended October 25, 2007. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
Unrecognized tax benefits balance at December 31, 2006 | $ | 1,198,000 | |
Gross increases for tax positions of prior years | 157,000 | ||
Gross increases for tax positions of current year | 123,000 | ||
Unrecognized tax benefits balance at October 25, 2007 | $ | 1,478,000 | |
The Company had $1,478,000 of gross unrecognized tax benefits as of October 25, 2007. The total amount of net unrecognized tax benefits that, if recognized, would affect the effective tax rate was $1,031,000 as of October 25, 2007.
The Company is subject to U.S. federal income tax as well as income tax of multiple state jurisdictions. The tax years 2003 and forward remain open to examination by the major state taxing jurisdictions to which the Company is subject depending on the state taxing authority. In addition, there are a number of state taxing jurisdictions in which the Company is not filing tax returns that may consider the Company to have taxable income. The tax years 2004 and forward remain open to examination by the Internal Revenue Service.
| 6. | COMMITMENTS AND CONTINGENCIES |
Concentrations of Credit Risk — The Company maintains cash deposits within a single depository institution in excess of federally insured limits.
Leases — The Company entered into leases in July 2002 for approximately 14,000 square feet of general office space and approximately 4,000 square feet of warehouse space in Madison Heights, MI. Both leases have a term of five years. In July 2007, both leases were renewed and extended for one additional year. Future minimum lease payments under these leases total $115,000 in 2008. Rent expense for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, was $187,000, $221,000, and $177,000, respectively, which were recorded in general and administrative expenses.
78
Table of Contents
Michigan Use Taxes — In accordance with a decision and order of determination received from the State of Michigan Department of Treasury in August 2005, the Company was subject to sales and use taxes in the state of Michigan. As a result, the Company recorded use taxes on its purchases of ambulatory infusion pumps as an increase in fixed assets. As of March 31, 2007, the Company recorded a cumulative net increase to net fixed assets of $700,000, a tax liability of $1,466,000, and total expense of $1,033,000, consisting of $766,000 cost of sales and $267,000 accrued interest expense. The Company appealed the decision. The Company believes that portable infusion pumps qualify for an exemption from tax under Michigan law. On April 24, 2007, the Michigan Tax Tribunal granted a Motion for Summary Disposition in favor of the Company, which was not appealed by the State of Michigan Department of Treasury. The review period for the ruling by the Michigan Tax Tribunal has ended which effectively forecloses further appeal of the August 2005 decision and order. As such, the Company reversed in the second quarter of 2007 the cumulative effects of the liability and expense recorded to date. The Company’s balance sheet reflected the decrease of $1,466,000 and $267,000 of accrued tax liability and accrued interest expense, respectively, and a decrease of $700,000 in cumulative net fixed assets. On its statement of income the Company recorded a reversal of $1,033,000 in total expense, consisting of $766,000 of cost of sales and $267,000 of interest expense.
Private Insurers and Government Reimbursement — The Company is paid directly by private insurers and governmental agencies, often on a fixed fee bases, for infusion pump management services provided by the Company to patients. The health care reimbursement system is in a constant state of change. Changes to the health care system that favor technologies other than the Company’s or that reduce the average fees allowable by private insurers or governmental agencies could have a material adverse effect on the Company’s financial position, results of operations and cash flows.
Guarantees and Indemnities — The Company enters into certain types of contracts from time to time that contingently require the Company to indemnify parties against third party claims. These contracts primarily relate to Company license, consulting, distribution and purchase agreements with its customers and other parties, under which the Company may be required to indemnify such parties for intellectual property infringement claims, product liability claims, and other claims arising from the Company’s provision of products or services to such parties.
The terms of the foregoing types of obligations vary. A maximum obligation arising out of these types of agreements is not explicitly stated and, therefore, the overall maximum amount of these obligations cannot be reasonably estimated. Historically, the Company has not been obligated to make significant payments for these obligations and, thus, no liabilities have been recorded for these obligations on its balance sheet as of October 25, 2007 and December 31, 2006.
Other Litigation — The Company is involved in litigation arising from the normal course of operations. In the opinion of management, the ultimate impact of such litigation will not have a material adverse effect on the Company’s financial position and results of operations.
79
Table of Contents
| 7. | EMPLOYEE BENEFIT PLAN |
Employees of the Company working more than 1,040 hours annually were able to participate in I-Flow’s 401(k) retirement plan. The Company contributes $0.33 for each dollar of employee contribution up to a maximum contribution by the Company of 1.32% of each participant’s annual salary. The maximum contribution by the Company of 1.32% corresponds to an employee contribution of 4% of annual salary. Participants vest in the Company’s contribution ratably over five years. Such contributions totaled $72,000, $75,000, and $66,000 for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, respectively, and were included in general and administrative expenses in the accompanying financial statements. The Company does not provide postretirement or postemployment benefits to its employees.
| 8. | RELATED-PARTY TRANSACTIONS |
Steven Watkins, President of the Company, owns 5% of Tu-Effs Limited Partnership, which owns the premises currently leased by the Company. Rent expense for the leased premises was $187,000, $221,000, and $177,000 for the period from January 1, 2007 to October 25, 2007, and the years ended December 31, 2006 and 2005, respectively, which was recorded in general and administrative expenses.
******
80
Table of Contents
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosures. |
None.
| Item 9A. | Controls and Procedures. |
Disclosure Controls and Procedures
We maintain disclosure controls and procedures, as defined by Rule 15d-15(e) that are designed to ensure that information required to be disclosed in our reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal accounting and financial officer), as appropriate, to allow timely decisions regarding required financial disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that a control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, with a company have been detected.
As of the end of the period covered by this Annual Report on Form 10-K, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2007. Based upon that evaluation, the principal executive officer and principal accounting and financial officer each concluded that our disclosure controls and procedures are effective as of the end of the period covered by this Annual Report on Form 10-K, as of December 31, 2007.
Our former Chief Executive Officer and former Chief Financial Officer previously concluded that as of September 30, 2007, our disclosure controls and procedures were not effective at the reasonable assurance level due to a material weakness. A material weakness is a deficiency or combination of deficiencies in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the registrant’s annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, at September 30, 2007, we did not have sufficient resources with the appropriate expertise to adequately evaluate complex transactions entered into by us and determine the appropriate application of generally accepted accounting principles (“GAAP”). This material weakness resulted in the error in accounting for the effects of our warrants issued to purchase common stock. Management now believes this material weakness has been remediated.
Internal Control Over Financial Reporting
This Annual Report on Form 10-K does not include a report of management’s assessment regarding internal control over financial reporting or a report of our independent registered public accounting firm regarding internal control over financial reporting. We were previously a development stage company formed with the objective to acquire one or more operating businesses in the healthcare sector. On October 25, 2007, we acquired InfuSystem and changed our name from “HAPC, INC.” to “InfuSystem Holdings, Inc.” Upon the acquisition, our legacy internal controls were entirely supplanted by those of InfuSystem and the processes and systems that were previously part of us were replaced entirely by controls relevant to us combined with InfuSystem. While our management is responsible for establishing and maintaining adequate internal control over financial reporting, due to our acquisition of InfuSystem in late 2007 and the complete replacement of our internal controls with those of InfuSystem, management has excluded the assessment of all of our internal control over financial reporting and has not conducted, and is not required to conduct, an evaluation or assessment of the effectiveness of our internal control over financial reporting as of December 31, 2007. Management considers the acquisition of InfuSystem to be material and significant to our consolidated financial statements and internal control over financial reporting process.
81
Table of Contents
Changes In Internal Control Over Financial Reporting
The following changes were made to our internal control over financial reporting during the fiscal quarter ended December 31, 2007. Such changes have materially affected our internal control over financial reporting, including remediation of the material weakness noted above:
| • | Upon our acquisition of InfuSystem, certain members of our management team, including our Chief Executive Officer and Chief Financial Officer, in addition to our four previously existing financial reporting processes, which consisted of management of cash investments, management of cash disbursements, management of period end closing and management of external reporting, were all replaced by Predecessor InfuSystem internal controls; |
| • | Various functions, controls and financial reporting processes of Predecessor InfuSystem were adopted as our functions, controls and financial processes, which include entity level controls, computer controls and significant business processes addressing financial reporting, accounting close, revenue and receivables, purchasing and payables, fixed assets, treasury, inventory, payroll, employee benefits and tax accounting; |
| • | We hired a Chief Financial Officer (principal accounting and financial officer) who is a Certified Public Accountant with substantial prior public company and public accounting experience; he specifically adds to the Company’s finance resources and possesses the appropriate expertise to adequately evaluate complex transactions entered into by us and determine the appropriate application of generally accepted accounting principles (“GAAP”); and |
| • | We retained all key Predecessor InfuSystem management, including the Chief Executive Officer (principal executive officer, and former President of Predecessor InfuSystem), Vice President of Operations, Controller, and Vice President of Sales |
| Item 9B. | Other Information. |
None.
| Item 10. | Directors, Executive Officers and Corporate Governance. |
The information required by Part III, Item 10 is incorporated herein by reference to our definitive proxy statement relating to the 2008 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Form 10-K.
| Item 11. | Executive Compensation. |
The information required by Part III, Item 11 is incorporated herein by reference to our definitive proxy statement relating to the 2008 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Form 10-K.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The information required by Part III, Item 12 is incorporated herein by reference to our definitive proxy statement relating to the 2008 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Form 10-K.
82
Table of Contents
| Item 13. | Certain Relationships and Related Transactions and Director Independence. |
The information required by Part III, Item 13 is incorporated herein by reference to our definitive proxy statement relating to the 2008 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Form 10-K.
| Item 14. | Principal Accounting Fees and Services. |
The information required by Part III, Item 14 is incorporated herein by reference to our definitive proxy statement relating to the 2008 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Form 10-K.
| Item 15. | Exhibits and Financial Statement Schedules. |
| (a) | 1. | Financial Statements | ||
| Reference is made to the Index to Financial Statements under Item 8, Part II hereof. | ||||
| 2. | Financial Statement Schedules | |||
| Schedule II – Valuation and Qualifying Accounts | ||||
| 3. | Exhibits | |||
| The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Form 10-K. | ||||
| (b) | See Item 15(a)(3) | |||
| (c) | See Item 15(a)(3) | |||
83
Table of Contents
Exhibit Index
Exhibit Number | Description of Document | |
| 3.1 | Amended and Restated Certificate of Incorporation (1) | |
| 3.2 | Certificate of Amendment of Amended and Restated Certificate of Incorporation (7) | |
| 3.3 | By-Laws (1) | |
| 3.4 | Amended and Restated By-Laws (2) | |
| 3.5 | Certificate of Amendment of Amended and Restated Certificate of Incorporation (20) | |
| 4.1 | Specimen Unit Certificate (4) | |
| 4.2 | Specimen Common Stock Certificate (4) | |
| 4.3 | Specimen Warrant Certificate (4) | |
| 4.4 | Form of Warrant Agreement between Registrant and Mellon Investor Services LLC (2) | |
| 4.5 | Form of Purchase Option granted to FTN Midwest Securities Corp. (4) | |
| 4.6 | Form of Warrant issued to Sean McDevitt (10) | |
| 4.7 | Unit Purchase Option Clarification Agreement, dated as of February 9, 2007, by and between Registrant and FTN Midwest Securities Corp. (11) | |
| 4.8 | Warrant, dated as of April 12, 2007, issued to Sean McDevitt (13) | |
| 4.9 | Form of Warrant between Registrant and each of Sean McDevitt, John Voris, Wayne Yetter, Jean Pierre Millon and Erin Enright. (16) | |
| 10.1 | Form of Letter Agreement entered into by and between Registrant and each of its initial stockholders (4) | |
| 10.2 | Form of Registration Rights Agreement (4) | |
| 10.3 | Form of Trust Account Agreement by and between Registrant and JPMorgan Chase Bank, N.A. (5) | |
| 10.4 | Amendment to Trust Account Agreement by and between Registrant and JPMorgan Chase Bank, N.A. (12) | |
| 10.5 | Form of Stock Transfer Agency Agreement (2) | |
| 10.6 | Form of Lock-up Agreements by and between FTN Midwest Securities Corp., Registrant and its initial stockholders (6) | |
| 10.7 | Form of Letter Agreement to be entered into by and between Registrant and members of management other than initial stockholders (6) | |
| 10.8 | Compensation Agreements by and between Registrant and its officers and directors (4) | |
| 10.9 | Form of Warrant Purchase Agreement by and among each of Registrant’s officers and directors and FTN Midwest Securities Corp. (5) | |
| 10.10 | Stock Purchase Agreement, dated as of September 29, 2006, by and among Registrant, Iceland Acquisition Subsidiary, Inc., InfuSystem, Inc. and I-Flow Corporation (8) | |
| 10.11 | Amendment No. 1 to Stock Purchase Agreement, dated as of April 30, 2007, by and among Registrant, Iceland Acquisition Subsidiary, Inc., InfuSystem, Inc. and I-Flow Corporation (13) | |
| 10.12 | Amendment No. 2 to Stock Purchase Agreement, dated as of June 29, 2007, by and among Registrant, Iceland Acquisition Subsidiary, Inc., InfuSystem, Inc. and I-Flow Corporation (14) | |
| 10.13 | Amendment No. 3 to Stock Purchase Agreement, dated as of July 31, 2007, by and among Registrant, Iceland Acquisition Subsidiary, Inc., InfuSystem, Inc. and I-Flow Corporation (15) | |
| 10.14 | Amendment No. 4 to Stock Purchase Agreement, dated as of September 18, 2007, by and among Registrant, Iceland Acquisition Subsidiary, Inc., InfuSystem, Inc. and I-Flow Corporation (18) | |
| 10.15 | Continuing Guaranty, dated as of September 29, 2006, by and among Sean McDevitt, Pat LaVecchia, Philip B. Harris and I-Flow Corporation (8) | |
| 10.16 | Guarantee Fee and Reimbursement Agreement, dated as of September 29, 2006, by and among Registrant, Sean McDevitt, Pat LaVecchia and Philip B. Harris (8) | |
| 10.17 | Subscription Agreement, dated as of December 28, 2006, between FTN Midwest Securities Corp. and Sean McDevitt (10) | |
| 10.18 | Subscription Agreement, dated as of April 12, 2007, between Registrant and Sean McDevitt (13) | |
| 10.19 | Form of Subscription Agreement between Registrant and each of Sean McDevitt, John Voris, Wayne Yetter, Jean Pierre Millon and Erin Enright (16) | |
| 10.20 | Memorandum of Intent, dated as of September 12, 2007, by and among Registrant, I-Flow Corporation, InfuSystem, Inc. and Iceland Acquisition Subsidiary, Inc. (17) | |
84
Table of Contents
Exhibit Number | Description of Document | |
| 10.21 | Further Agreement Regarding Project Iceland, dated as of October 17, 2007, by and among Registrant, I-Flow Corporation, InfuSystem, Inc. and Iceland Acquisition Subsidiary, Inc. (19) | |
| 10.22 | Acknowledgment and Agreement, dated as of October 8, 2007, by and among Registrant, I-Flow Corporation, InfuSystem, Inc. and Iceland Acquisition Subsidiary, Inc. (19) | |
| 10.23 | Credit and Guaranty Agreement, dated as of October 25, 2007, by and among Registrant, Iceland Acquisition Subsidiary, Inc. and I-Flow Corporation (20) | |
| 10.24 | Security Agreement, dated as of October 25, 2007, by and among Registrant, Iceland Acquisition Subsidiary, Inc. and I-Flow Corporation (20) | |
| 10.25 | Employment Agreement, dated as of November 12, 2007, by and between Registrant and Steven Watkins (21) | |
| 10.26 | Employment Agreement, dated as of November 12, 2007, by and between Registrant and Sean Whelan (21) | |
| 10.27 | Employment Agreement, dated as of November 12, 2007, by and between Registrant and Janet Skonieczny (21) | |
| 14.1 | Code of Ethics (3) | |
| 16.1 | Letter, dated October 27, 2006, from Miller Ellin and Company, LLP to the U.S. Securities and Exchange Commission (9) | |
| 21.1 | Subsidiaries of Registrant* | |
| 31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14 of the Securities Exchange Act of 1934, as amended * | |
| 31.2 | Certification of Principal Accounting Officer pursuant to Rule 13a-14 of the Securities Exchange Act of 1934, as amended * | |
| 32.1 | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| 32.2 | Certification of Principal Accounting Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| * | Filed herewith |
| (1) | Incorporated by reference to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on October 14, 2005. |
| (2) | Incorporated by reference to Amendment No. 1 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on December 8, 2005. |
| (3) | Incorporated by reference to Amendment No. 2 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on December 17, 2005. |
| (4) | Incorporated by reference to Amendment No. 3 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on March 3, 2006. |
| (5) | Incorporated by reference to Amendment No. 4 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on March 24, 2006. |
| (6) | Incorporated by reference to Amendment No. 5 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on April 6, 2006. |
| (7) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on April 24, 2006. |
| (8) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 4, 2006. |
| (9) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 27, 2006. |
| (10) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on January 3, 2007. |
85
Table of Contents
| (11) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on February 14, 2007. |
| (12) | Incorporated by reference to Registrant’s Annual Report on Form 10-K filed on March 30, 2007. |
| (13) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on May 4, 2007. |
| (14) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on July 5, 2007. |
| (15) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on August 1, 2007. |
| (16) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on September 12, 2007. |
| (17) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on September 13, 2007. |
| (18) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on September 21, 2007. |
| (19) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 22, 2007. |
| (20) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 31, 2007. |
| (21) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on November 16, 2007. |
86
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| INFUSYSTEM HOLDINGS, INC. | ||||
| Date: March 24, 2008 | By: | /s/ Steven Watkins | ||
| Steven Watkins | ||||
| Chief Executive Officer | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacity and on the dates indicated.
| Date: March 24, 2008 | /s/ Steven Watkins | |
| Steven Watkins | ||
| Chief Executive Officer and Director | ||
| (Principal Executive Officer) | ||
| Date: March 24, 2008 | /s/ Sean Whelan | |
| Sean Whelan | ||
| Chief Financial Officer | ||
| (Principal Accounting and Financial Officer) | ||
| Date: March 24, 2008 | /s/ Sean McDevitt | |
| Sean McDevitt | ||
| Chairman of the Board | ||
| Date: March 24, 2008 | /s/ John Voris | |
| John Voris | ||
| Director | ||
| Date: March 24, 2008 | /s/ Pat LaVecchia | |
| Pat LaVecchia | ||
| Director | ||
| Date: March 24, 2008 | /s/ Wayne Yetter | |
| Wayne Yetter | ||
| Director | ||
| Date: March 24, 2008 | /s/ Jean-Pierre Millon | |
| Jean-Pierre Millon | ||
| Director | ||
87
Table of Contents
INFUSYSTEM HOLDINGS, INC.
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
Following is an analysis of the allowance for doubtful accounts for InfuSystem Holdings, Inc.
for the year ended December 31, 2007 ($000’s) (1):
| Balance at beginning of Period | Acquired in acquisition | Charged to costs and expenses | Deductions (2) | Balance at end of Period | ||||||||||||
Allowance for doubtful accounts — 2007 | $ | — | $ | 1,673 | $ | 584 | $ | (619 | ) | $ | 1,638 | |||||
| (1) | The Company had no such allowance in the years ended December 31, 2006 and 2005. |
| (2) | Deductions represent the write-off of uncollectible account receivable balances. |
Following is an analysis of the allowance for doubtful accounts for Predecessor InfuSystem
for the years ended December 31, 2006 and 2005 and period ended October 25, 2007:
| Balance at beginning of Period | Charged to costs and expenses | Deductions (1) | Balance at end of Period | ||||||||||
Allowance for doubtful accounts — 2007 | $ | 1,668 | $ | 4,971 | $ | (4,777 | ) | $ | 1,862 | ||||
Allowance for doubtful accounts — 2006 | $ | 1,723 | $ | 4,041 | $ | (4,096 | ) | $ | 1,668 | ||||
Allowance for doubtful accounts — 2005 | $ | 757 | $ | 1,260 | $ | (294 | ) | $ | 1,723 | ||||
| (1) | Deductions represent the write-off of uncollectible account receivable balances. |
88