Exhibit 99.1

Building a Major Ophthalmic Pharmaceutical Company
Aerie Pharmaceuticals, Inc.
Investor Day September 10, 2014

Important Information
aerie pharmaceuticals, inc.
Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. In addition, any discussion of clinical data results for our Rhopressa™ and Roclatan™ product candidates relate to the results in our Phase 2 clinical trials.
The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete.
Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws, including beliefs, expectations, estimates, projections and statements relating to our business plans, prospects and objectives, and the assumptions upon which those statements are based. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operation.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
2

Our Strategy
aerie pharmaceuticals, inc.
Advance the development of our product candidates to approval
Establish internal capabilities to commercialize our product candidates in North America (and potentially Europe)
Explore partnerships with leading pharmaceutical and biotechnology companies to maximize the value of our product candidates outside of North America
Continue to leverage and strengthen our intellectual property portfolio
Expand our product portfolio through internal discovery efforts and possible in-licensing or acquisitions of additional ophthalmic product candidates or products
3

Accomplishments Since October 2013
aerie pharmaceuticals, inc.
Completed IPO – raised $68M
EVP findings in 1Q 2014
Reported successful Roclatan™ Phase 2b results in June 2014
Initiated Rhopressa™ Phase 3 efficacy and safety studies in 3Q 2014
Added key senior executives in 2014 for Commercial, R&D, and Business Development
Recently announced additional $125M financing
4

Introductions
aerie pharmaceuticals, inc.
Vicente Anido, Jr., Ph.D., Chief Executive Officer
Thomas Mitro, Chief Operating Officer
Richard Parrish, M.D. – Professor Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami
Casey Kopczynski, Ph.D., Chief Scientific Officer
Brian Levy, O.D., M.Sc., Chief Medical Officer
Richard Rubino, Chief Financial Officer
Marvin Garrett, V.P. Regulatory Affairs
Craig Skenes, V.P. Business Development
Michael McCleerey, V.P. Marketing
5
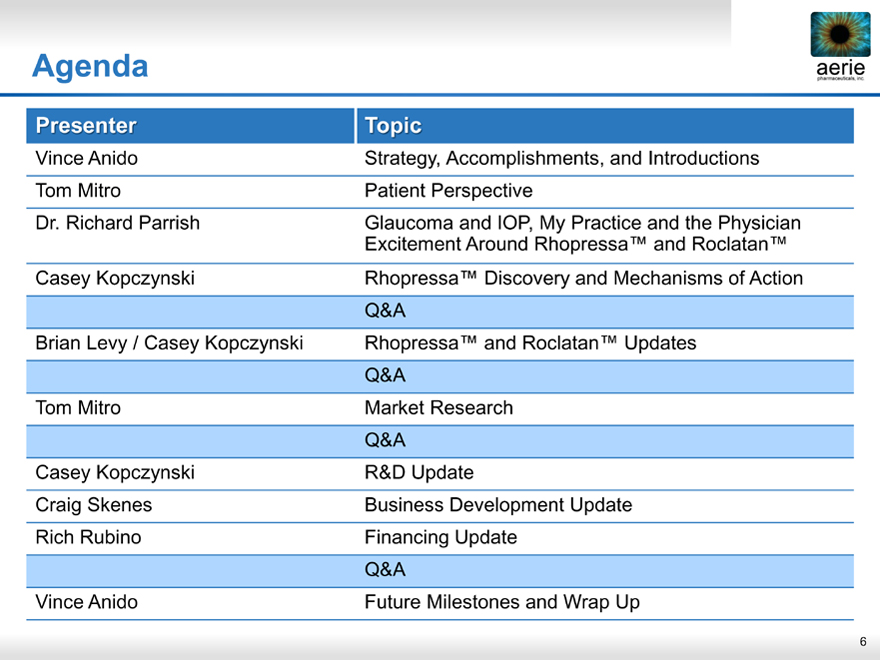
Agenda
aerie pharmaceuticals, inc.
Presenter Topic
Vince Anido Strategy, Accomplishments, and Introductions
Tom Mitro Patient Perspective
Dr. Richard Parrish Glaucoma and IOP, My Practice and the Physician
Excitement Around Rhopressa™ and Roclatan™
Casey Kopczynski Rhopressa™ Discovery and Mechanisms of Action
Q&A
Brian Levy / Casey Kopczynski Rhopressa™ and Roclatan™ Updates
Q&A
Tom Mitro Market Research
Q&A
Casey Kopczynski R&D Update
Craig Skenes Business Development Update
Rich Rubino Financing Update
Q&A
Vince Anido Future Milestones and Wrap Up
6

A Patient’s Perspective
aerie pharmaceuticals, inc.
Glaucoma Patien Background
Glaucoma patient for 35+ years; lost partial vision; both parents had glaucoma
3 failed trabeculectomy surgeries
Now taking 3 glaucoma medications (1 QD, 1 BID, 1 TID)
Quotes from this Patient
“A major consideration for prescribing physicians is compliance. Given the age and sometimes diminished mental state of glaucoma patients… once daily dosing advantage can not be stressed enough” “Your products represent a unique approach to the treatment of glaucoma” “Companies like yours help to make physicians better able to treat their patients”
“The vast majority of existing and undiagnosed glaucoma patients desperately need a drug that provides improved levels of performance and outcomes”
7

Richard Parrish, M.D.:
aerie pharmaceuticals, inc.
Associate Dean for Graduate Medical Education, University of Miami School of Medicine Professor of Ophthalmology
Glaucoma and IOP
About my practice
Why my colleagues and I are excited about Rhopressa™ and Roclatan™
New MOAs
Trabecular Meshwork EVP
20 years
Low tension glaucoma
Where we will use these products
Hyperemia and glaucoma treatment
8
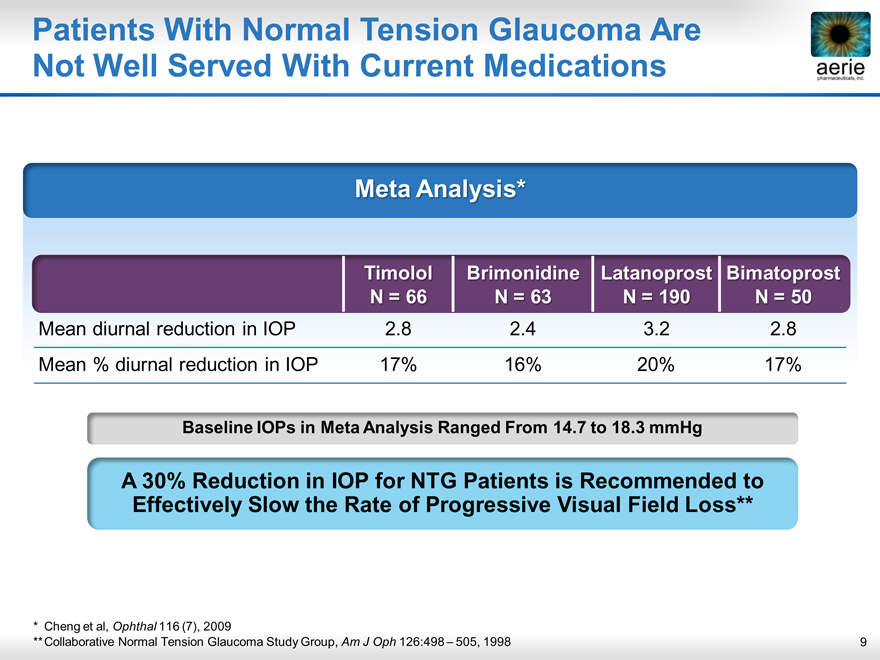
Patients With Normal Tension Glaucoma Are
aerie pharmaceuticals, inc.
Not Well Served With Current Medications
aerie
pharmaceuticals, inc.
Meta Analysis*
Timolol
N = 66 Brimonidine N = 63 Latanoprost N = 190 Bimatoprost
N = 50
Mean diurnal reduction in IOP 2.8 2.4 3.2 2.8
Mean % diurnal reduction in IOP 17% 16% 20% 17%
Baseline IOPs in Meta Analysis Ranged From 14.7 to 18.3 mmHg
A 30% Reduction in IOP for NTG Patients is Recommended to Effectively Slow the Rate of Progressive Visual Field Loss**
* Cheng et al, Ophthal 116 (7), 2009
** Collaborative Normal Tension Glaucoma Study Group, Am J Oph 126:498 – 505, 1998
9

Rhopressa™ Discovery and Mechanisms of Action
Casey Kopczynski
10

The Aerie Rho Kinase R&D Success Story
aerie pharmaceuticals, inc.
Ophthalmic Efficacy by Design
Optimal ocular delivery, formulation properties built into Aerie lead compounds
Rapid cycle time from medicinal chemistry to ophthalmic efficacy, tolerability testing
– >1,500 Rho kinase inhibitors designed, screened and characterized to date
Discovered new ROCK-selective inhibitors and the novel ROCK/NET inhibitor drug class
Large patent portfolio, key patents provide protection through 2030
ROCK/NET inhibitor Rhopressa™ advanced to Phase 3 trials
“Prodrug” design provides strong efficacy, good tolerability with once-daily dosing in clinic
Lowers IOP through 3 different mechanisms of action
Achieves consistent IOP lowering regardless of baseline IOP
11
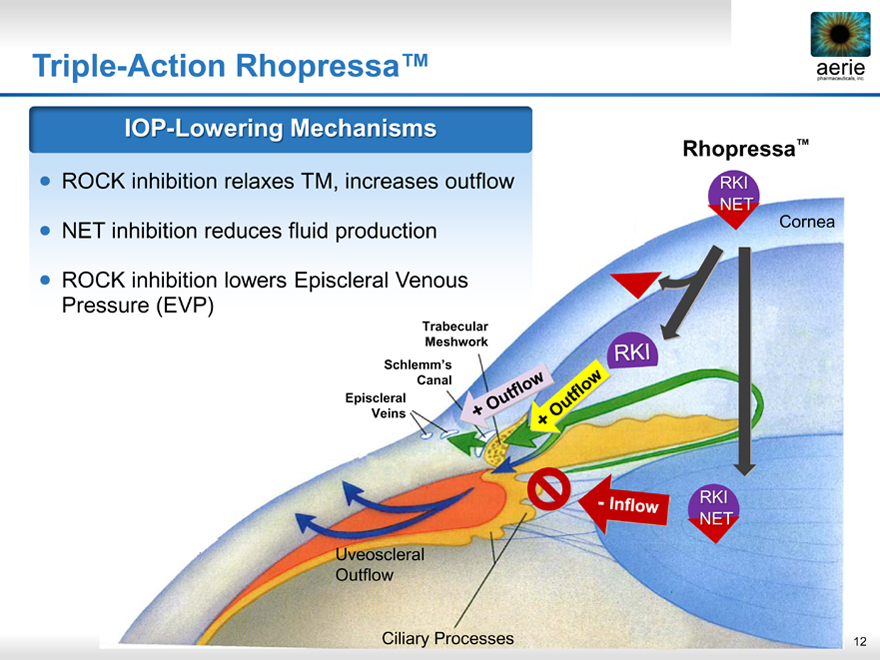
Triple-Action Rhopressa™
aerie pharmaceuticals, inc.
IOP-Lowering Mechanisms
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers Episcleral Venous Pressure (EVP)
Rhopressa™
RKI NET
Cornea
Trabecular Meshwork Schlemm’s Canal Episcleral Veins
RKI
Uveoscleral Outflow
Ciliary Processes 12
-Inflow
RKI NET
+ Outflow
+ Outflow
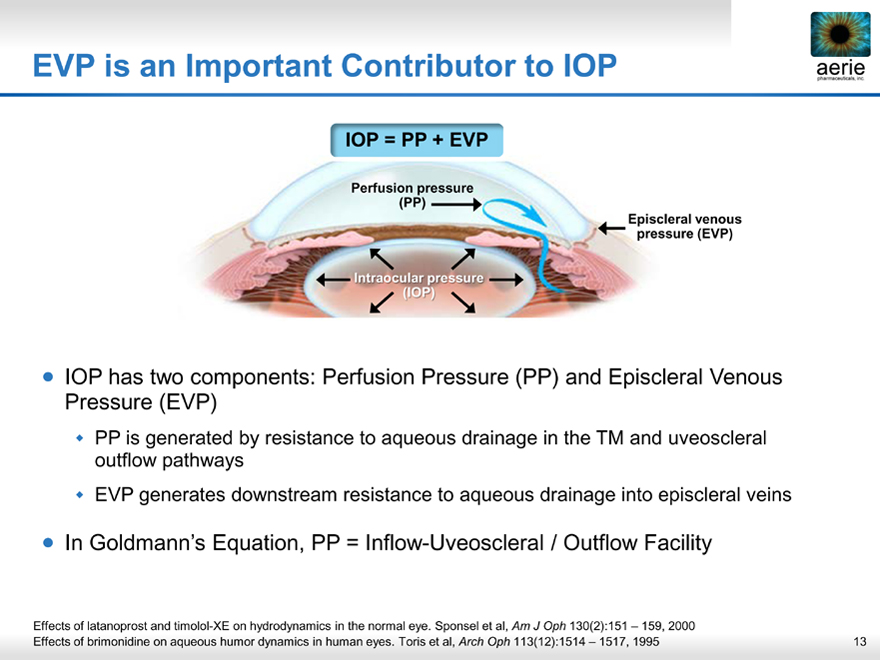
EVP is an Important Contributor to IOP
IOP = PP + EVP
Perfusion pressure (PP)
Episcleral venous pressure (EVP)
aerie pharmaceuticals, inc.
Intraocular pressure (IOP)
• |
| IOP has two components: Perfusion Pressure (PP) and Episcleral Venous Pressure (EVP) |
PP is generated by resistance to aqueous drainage in the TM and uveoscleral outflow pathways EVP generates downstream resistance to aqueous drainage into episcleral veins
• |
| In Goldmann’s Equation, PP = Inflow-Uveoscleral / Outflow Facility |
Effects of latanoprost and timolol-XE on hydrodynamics in the normal eye. Sponsel et al, Am J Oph 130(2):151 – 159, 2000
Effects of brimonidine on aqueous humor dynamics in human eyes. Toris et al, Arch Oph 113(12):1514 – 1517, 1995
13
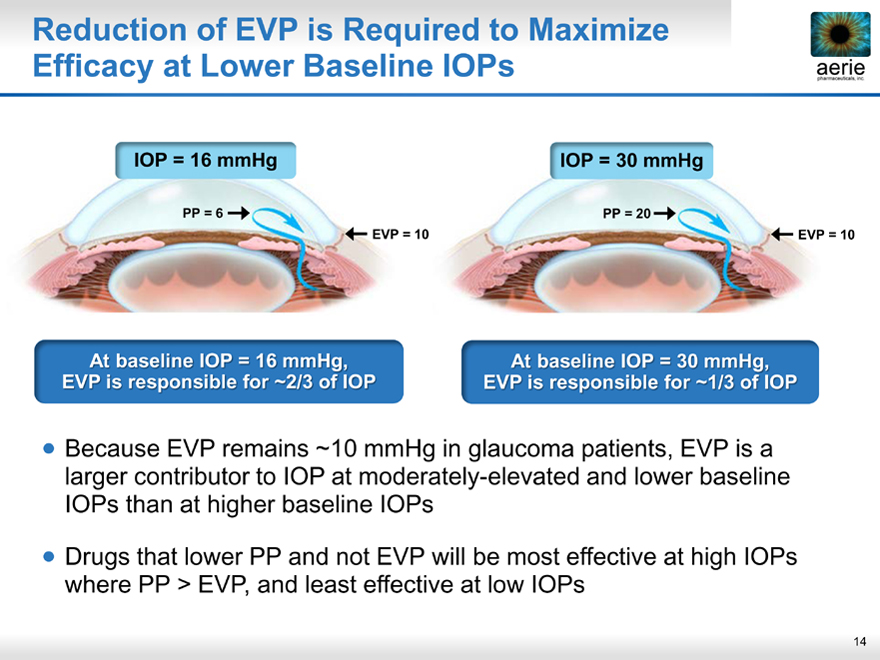
Reduction of EVP is Required to Maximize Efficacy at Lower Baseline IOPs
aerie pharmaceuticals, inc.
IOP = 16 mmHg IOP = 30 mmHg
PP = 6 PP = 20
EVP = 10 EVP = 10
At baseline IOP = 16 mmHg, EVP is responsible for ~2/3 of IOP
At baseline IOP = 30 mmHg, EVP is responsible for ~1/3 of IOP
Because EVP remains ~10 mmHg in glaucoma patients, EVP is a larger contributor to IOP at moderately-elevated and lower baseline IOPs than at higher baseline IOPs
Drugs that lower PP and not EVP will be most effective at high IOPs where PP > EVP, and least effective at low IOPs
14
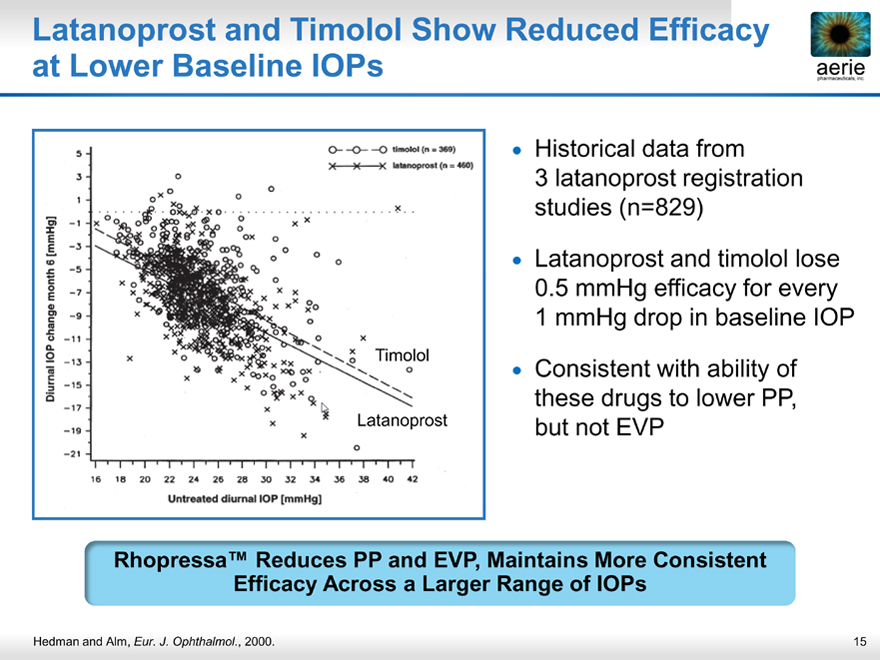
Latanoprost and Timolol Show Reduced Efficacy at Lower Baseline IOPs
aerie pharmaceuticals, inc.
Timolol
Diurnal IOP change month 6 [mmHg]
Untreated diurnal IOP [mmHg]
timolol (n = 369)
latanoprost (n = 460)
Latanoprost
Historical data from 3 latanoprost registration studies (n=829)
Latanoprost and timolol lose 0.5 mmHg efficacy for every 1 mmHg drop in baseline IOP
Consistent with ability of these drugs to lower PP, but not EVP
Rhopressa™ Reduces PP and EVP, Maintains More Consistent Efficacy Across a Larger Range of IOPs
Hedman and Alm, Eur. J. Ophthalmol., 2000.
15
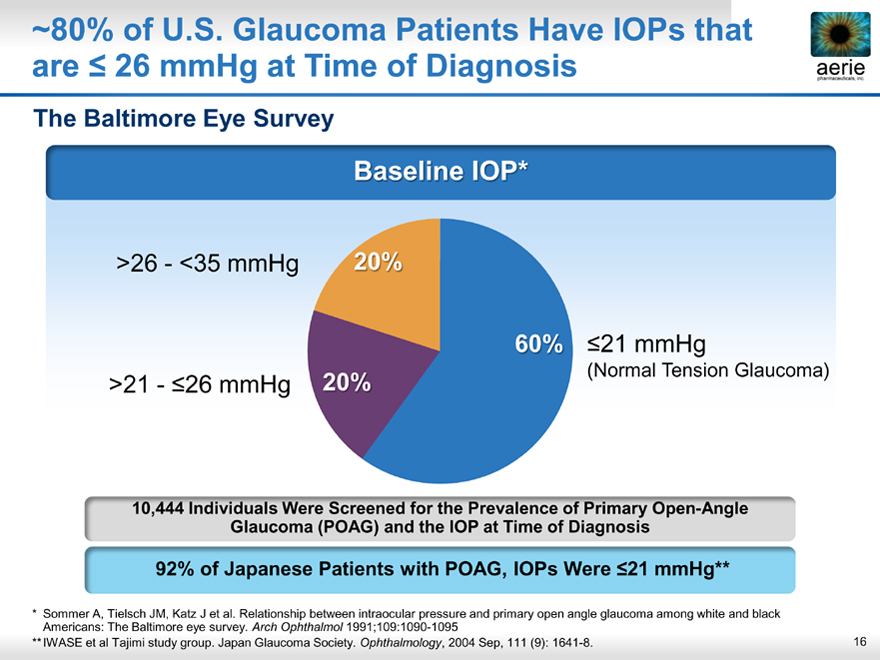
~80% of U.S. Glaucoma Patients Have IOPs that are <26 mmHg at Time of Diagnosis
The Baltimore Eye Survey
Baseline IOP*
>26 - <35 mmHg 20%
60%< 21 mmHg
20% (Normal Tension Glaucoma)
>21 - <26 mmHg
10,444 Individuals Were Screened for the Prevalence of Primary Open-Angle Glaucoma (POAG) and the IOP at Time of Diagnosis
92% of Japanese Patients with POAG, IOPs Were <21 mmHg**
* Sommer A, Tielsch JM, Katz J et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: The Baltimore eye survey. Arch Ophthalmol 1991;109:1090-1095 **IWASE et al Tajimi study group. Japan Glaucoma Society. Ophthalmology, 2004 Sep, 111 (9): 1641-8.
16
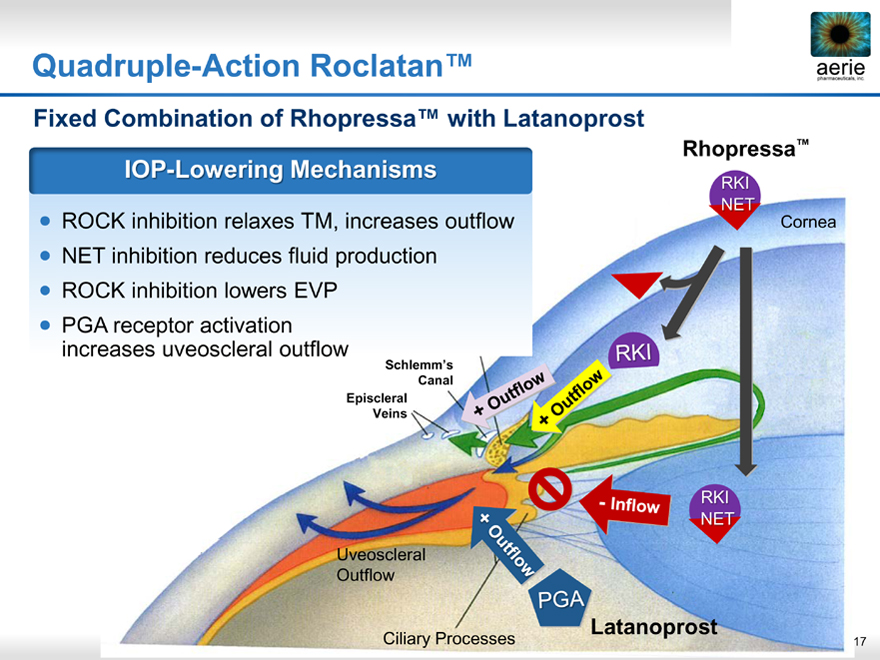
Quadruple-Action Roclatan™
aerie pharmaceuticals, inc.
Fixed Combination of Rhopressa™ with Latanoprost
IOP-Lowering Mechanisms
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers EVP
• PGA receptor activation increases uveoscleral outflow
Rhopressa™
RKI NET
Cornea
Trabecular Meshwork Schlemm’s Canal Episcleral Veins
RKI
Uveoscleral
Outflow
Latanoprost
Ciliary Processes 17
- Inflow
PGA

Q&A
18

Rhopressa™ / Roclatan™ Clinical Updates
Brian Levy
19
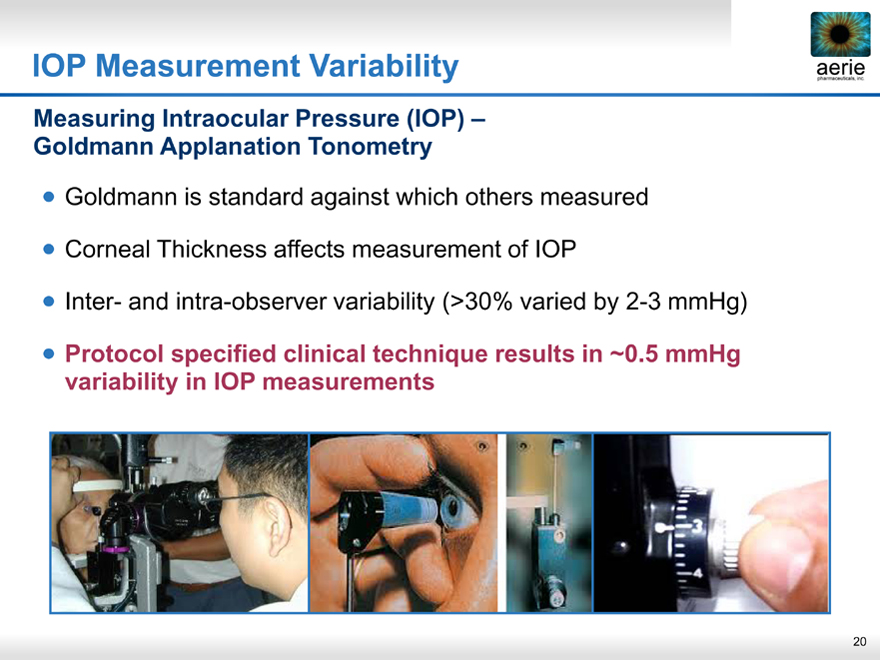
IOP Measurement Variability
aerie pharmaceuticals, inc.
Measuring Intraocular Pressure (IOP) – Goldmann Applanation Tonometry
Goldmann is standard against which others measured
Corneal Thickness affects measurement of IOP
Inter- and intra-observer variability (>30% varied by 2-3 mmHg)
• Protocol specified clinical technique results in ~0.5 mmHg variability in IOP measurements
20
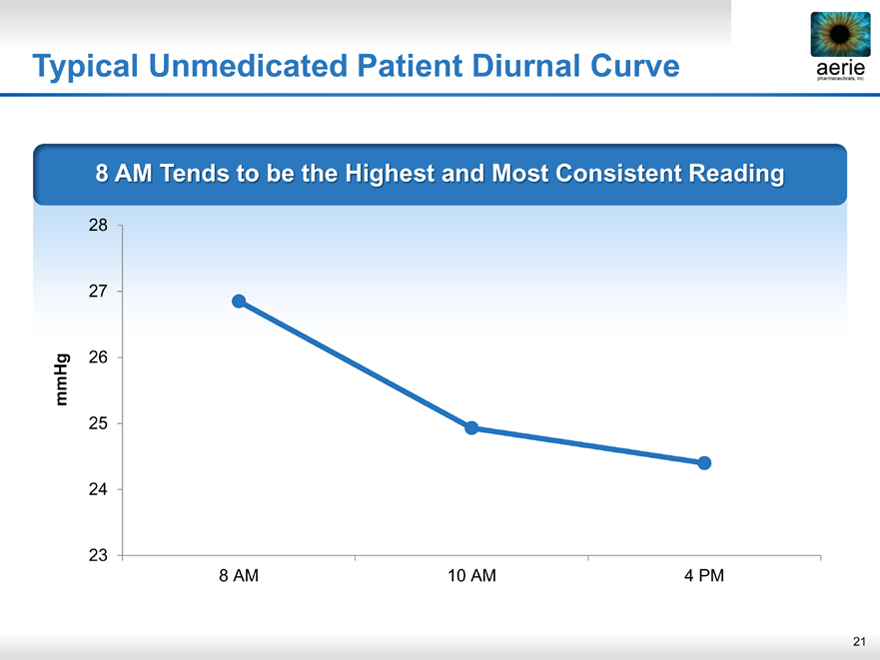
Typical Unmedicated Patient Diurnal Curve
8 AM Tends to be the Highest and Most Consistent Reading
28
27
mmHg 26 25
24
23
8 AM 10 AM 4 PM
21
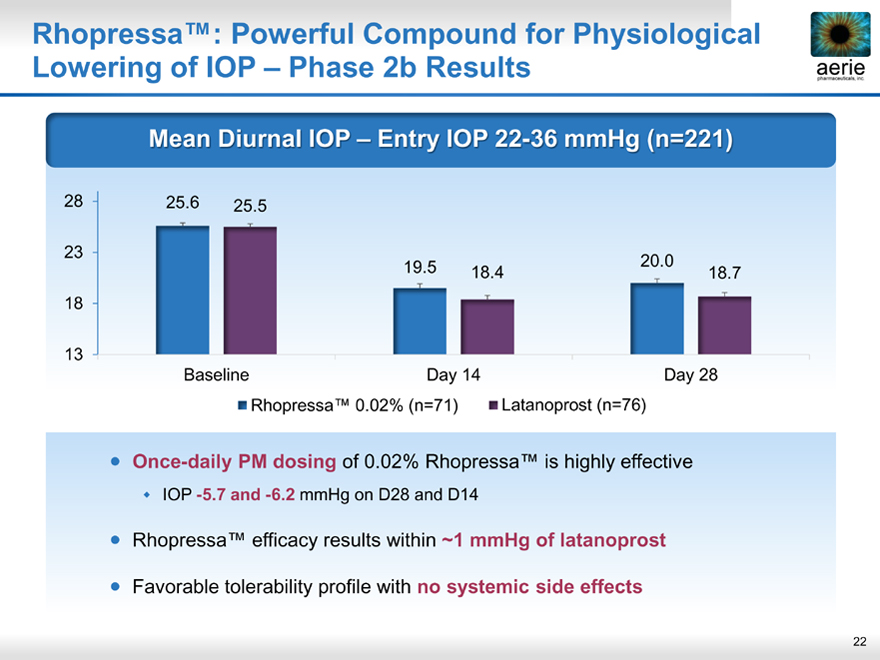
Rhopressa™: Powerful Compound for Physiological Lowering of IOP – Phase 2b Results
aerie pharmaceuticals, inc.
Mean Diurnal IOP – Entry IOP 22-36 mmHg (n=221)
28 25.6 25.5
23
19.5 20.0 18.4 18.7 18
13
Baseline Day 14 Day 28
• Rhopressa™ 0.02% (n=71) • Latanoprost (n=76)
• Once-daily PM dosing of 0.02% Rhopressa™ is highly effective
• IOP -5.7 and -6.2 mmHg on D28 and D14
• Rhopressa™ efficacy results within ~1 mmHg of latanoprost
• Favorable tolerability profile with no systemic side effects
22
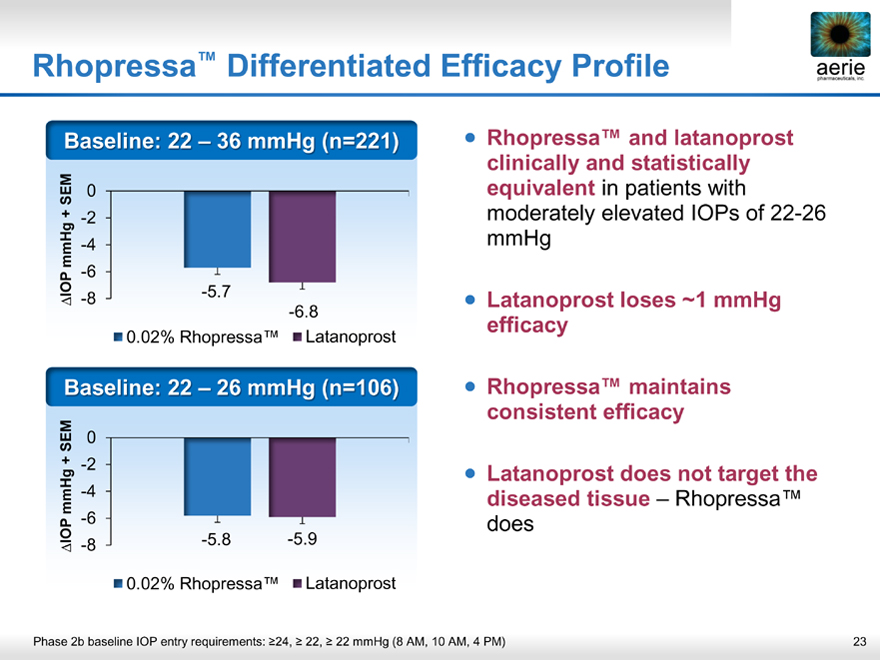
Rhopressa™ Differentiated Efficacy Profile
aerie pharmaceuticals, inc.
Baseline: 22 – 36 mmHg (n=221)
IOP mmHg + SEM
-8 -6 -4 -2 0
-5.7
-6.8
• 0.02% Rhopressa™ • Latanoprost
Baseline: 22 – 26 mmHg (n=106)
IOP mmHg + SEM
-8 -6 -4 -2 0
-5.8 -5.9
• 0.02% Rhopressa™ • Latanoprost
• Rhopressa™ and latanoprost clinically and statistically equivalent in patients with moderately elevated IOPs of 22-26 mmHg
• Latanoprost loses ~1 mmHg efficacy
• Rhopressa™ maintains consistent efficacy
• Latanoprost does not target the diseased tissue – Rhopressa™ does
Phase 2b baseline IOP entry requirements: >24, >22, >22 mmHg (8 AM, 10 AM, 4 PM)
23
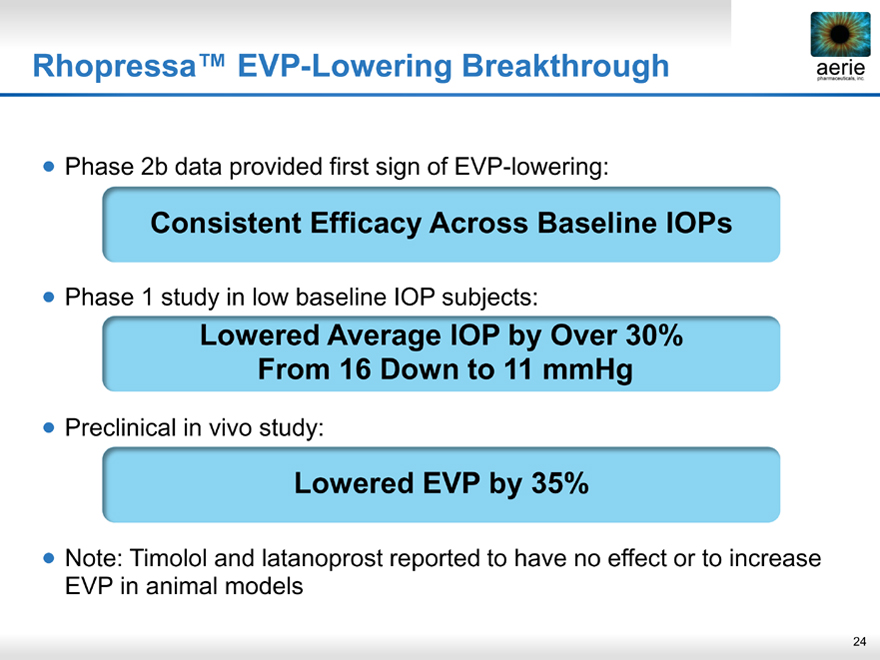
Rhopressa™ EVP-Lowering Breakthrough
aerie pharmaceuticals, inc.
• Phase 2b data provided first sign of EVP-lowering:
Consistent Efficacy Across Baseline IOPs
• Phase 1 study in low baseline IOP subjects:
Lowered Average IOP by Over 30% From 16 Down to 11 mmHg
• Preclinical in vivo study:
Lowered EVP by 35%
• Note: Timolol and latanoprost reported to have no effect or to increase EVP in animal models
24
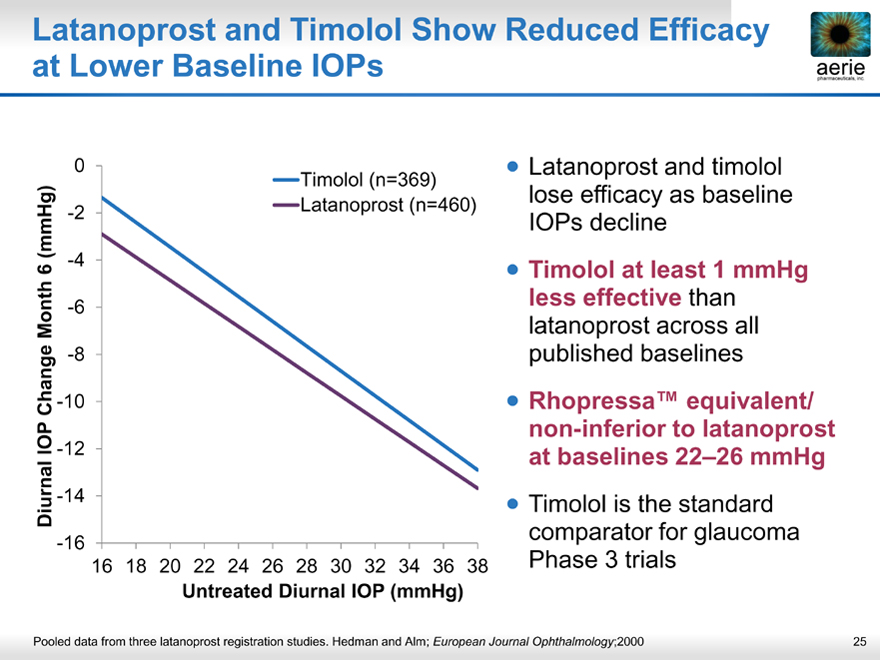
Latanoprost and Timolol Show Reduced Efficacy at Lower Baseline IOPs
aerie pharmaceuticals, inc.
Diurnal IOP Change Month 6 (mmHg)
-16 -14 -12 -10 -8 -6 -4 -2 0
16 18 20 22 24 26 28 30 32 34 36 38
Untreated Diurnal IOP (mmHg)
Timolol (n=369) Latanoprost (n=460)
• Latanoprost and timolol lose efficacy as baseline IOPs decline
• Timolol at least 1 mmHg less effective than latanoprost across all published baselines
• Rhopressa™ equivalent/ non-inferior to latanoprost at baselines 22–26 mmHg
• Timolol is the standard comparator for glaucoma Phase 3 trials
Pooled data from three latanoprost registration studies. Hedman and Alm; European Journal Ophthalmology;2000
25

Rhopressa™ Phase 3 De-risking / Confidence Summary
aerie pharmaceuticals, inc.
• Rhopressa™ Phase 3 entry IOP is >20 mmHg and <27 mmHg
• Rhopressa™ QD non-inferior to latanoprost at entry IOPs of 22-26 mmHg
• Timolol is comparator for Phase 3 trials (standard comparator)
• Timolol BID at least 1 mmHg less effective than latanoprost across all published baselines • FDA agreed to these entry IOPs with no expected impact on label
• Rhopressa™ well-tolerated with hyperemia rates within range of current PGA drugs
• No drug-related systemic safety issues in Phase1/Phase 2 trials
• Approximately 250 patients exposed to 0.02% Rhopressa™ to date
26
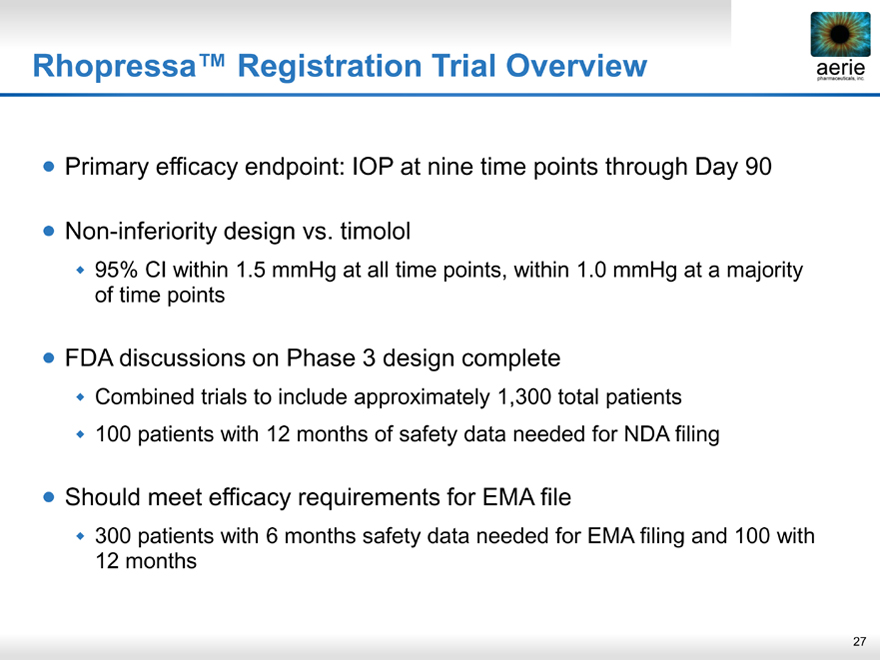
Rhopressa™ Registration Trial Overview
aerie pharmaceuticals, inc.
Š Primary efficacy endpoint: IOP at nine time points through Day 90
Š Non-inferiority design vs. timolol
‰ 95% Cl within 1.5 mmHg at all time points, within 1.0 mmHg at a majority of time points
FDA discussions on Phase 3 design complete
‰ Combined trials to include approximately 1,300 total patients
‰ 100 patients with 12 months of safety data needed for NDA filing
Š Should meet efficacy requirements for EMA file
‰ 300 patients with 6 months safety data needed for EMA filing and 100 with 12 months
27
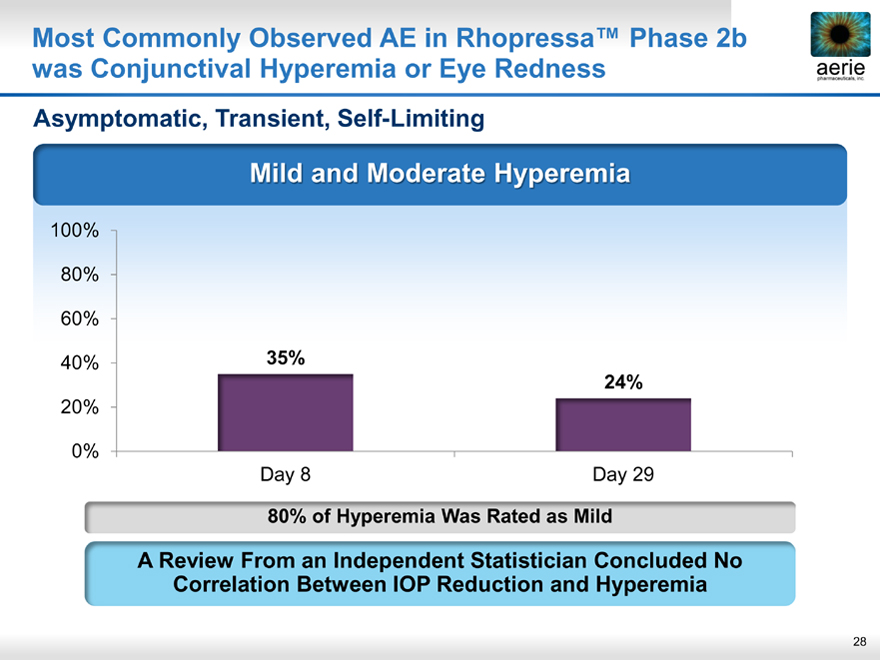
Most Commonly Observed AE in Rhopressa™ Phase 2b was Conjunctival Hyperemia or Eye Rednessaerie pharmaceuticals, inc.
Asymptomatic, Transient, Self-Limiting
Mild and Moderate Hyperemia
100%
80%
60%
40% 35%
24%
20%
0%
Day 8 Day 29
80% of Hyperemia Was Rated as Mild
A Review From an Independent Statistician Concluded No Correlation Between IOP Reduction and Hyperemia
28
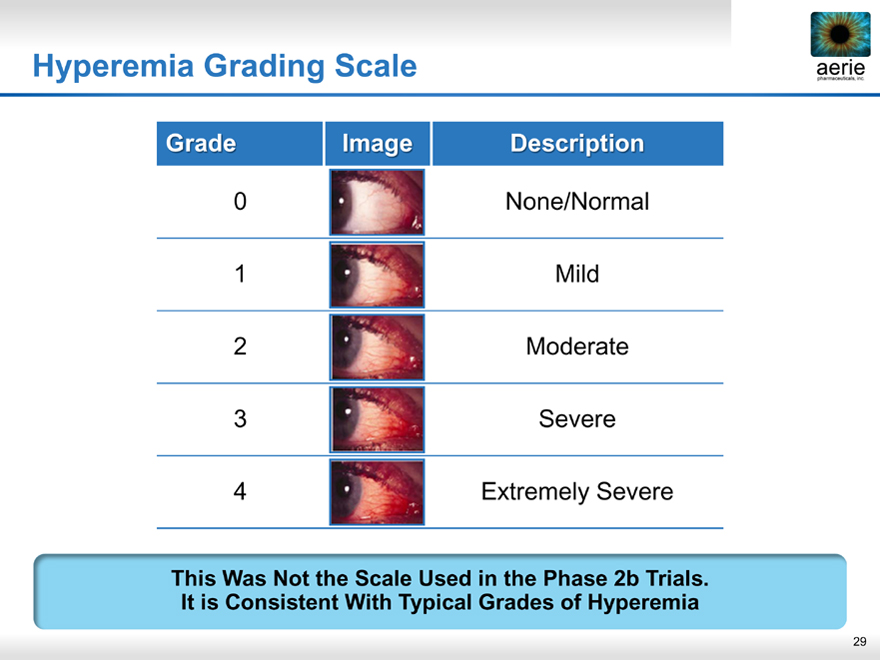
Hyperemia Grading Scale
aerie pharmaceuticals, inc.
Grade Image Description
0 None/Normal
1 Mild
2 Moderate
3 Severe
4 Extremely Severe
This Was Not the Scale Used in the Phase 2b Trials. It is Consistent With Typical Grades of Hyperemia
29
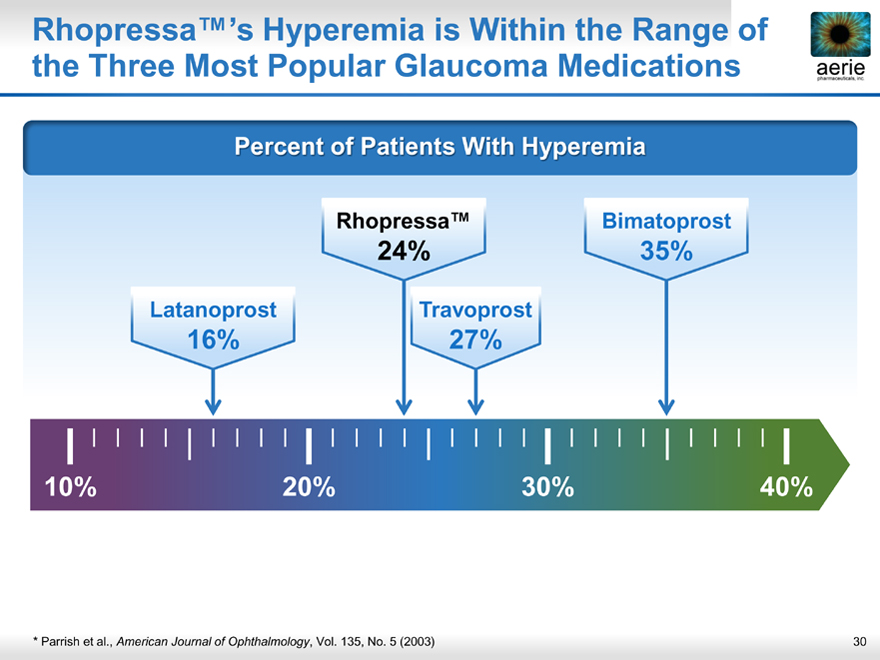
Rhopressa™’s Hyperemia is Within the Range of the Three Most Popular Glaucoma Medications
aerie pharmaceuticals, inc.
Percent of Patients With Hyperemia
Rhopressa™ Bimatoprost
24% 35%
Latanoprost Travoprost
16% 27%
10% 20% 30% 40%
* Parrish et al., American Journal of Ophthalmology, Vol. 135, No. 5 (2003)
30
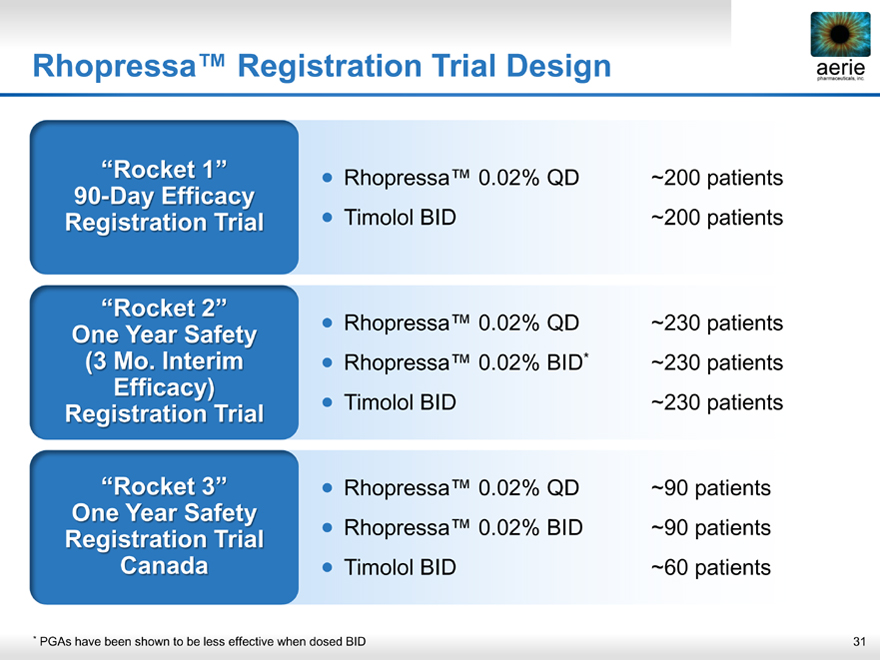
Rhopressa™ Registration Trial Design
aerie pharmaceuticals, inc.
“Rocket 1” • Rhopressa™ 0.02% QD
~200patients
90-Day Efficacy
Registration Trial • Timolol BID
~200 patients
“Rocket 2” • Rhopressa™ 0.02% QD
~230 patients
One Year Safety
(3 Mo Interim • Rhopressa™ 0.02% BID*
~230 patients
Efficacy)
Registration Trial • Timolol BID
~230 patients
“Rocket 3” • Rhopressa™ 0.02% QD
~90 patients
One Year Safety
Registration Trial • Rhopressa™ 0.02% BID
~90 patients
Canada • Timolol BID
~60 patients
* PGAs have been shown to be less effective when dosed BID
31
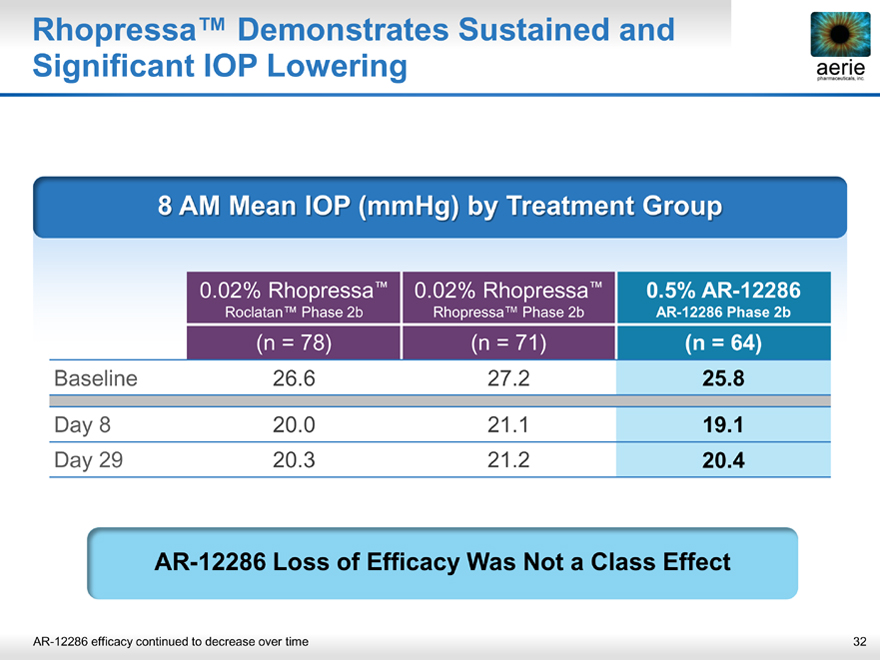
Rhopressa™ Demonstrates Sustained and Significant IOP Lowering aerie
aerie pharmaceuticals, inc.
8 AM Mean IOP (mmHg) by Treatment Group
0.02% Rhopressa™ 0.02% Rhopressa™ 0.5% AR-12286
Roclatan™ Phase 2b Rhopressa™ Phase 2b AR-12286 Phase 2b
(n = 78)(n = 71)(n = 64)
Baseline 26.6 27.2 25.8
Day 8 20.0 21.1 19.1
Day 29 20.3 21.2 20.4
AR-12286 Loss of Efficacy Was Not a Class Effect
AR-12286 efficacy continued to decrease over time
32
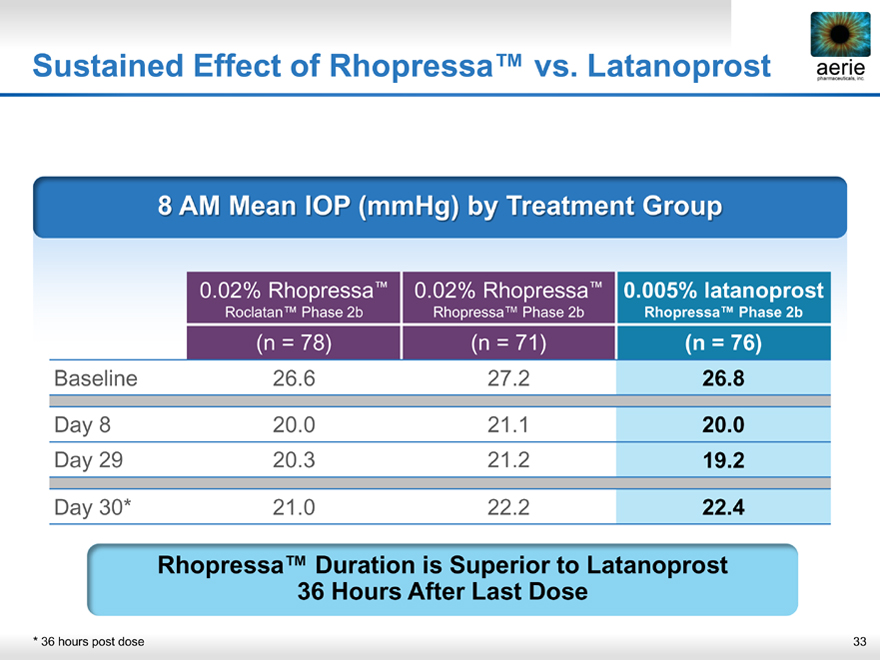
Sustained Effect of Rhopressa™ vs. Latanoprost
aerie pharmaceuticals, inc.
8 AM Mean IOP (mmHg) by Treatment Group
0.02% Rhopressa™ 0.02% Rhopressa™ 0.005% latanoprost
Roclatan™ Phase 2b Rhopressa™ Phase 2b Rhopressa™ Phase 2b
(n = 78) (n = 71) (n = 76)
Baseline 26.6 27.2 26.8
Day 8 20.0 21.1 20.0
Day 29 20.3 21.2 19.2
Day 30* 21.0 22.2 22.4
Rhopressa™ Duration is Superior to Latanoprost
36 Hours After Last Dose
* 36 hours post dose
33
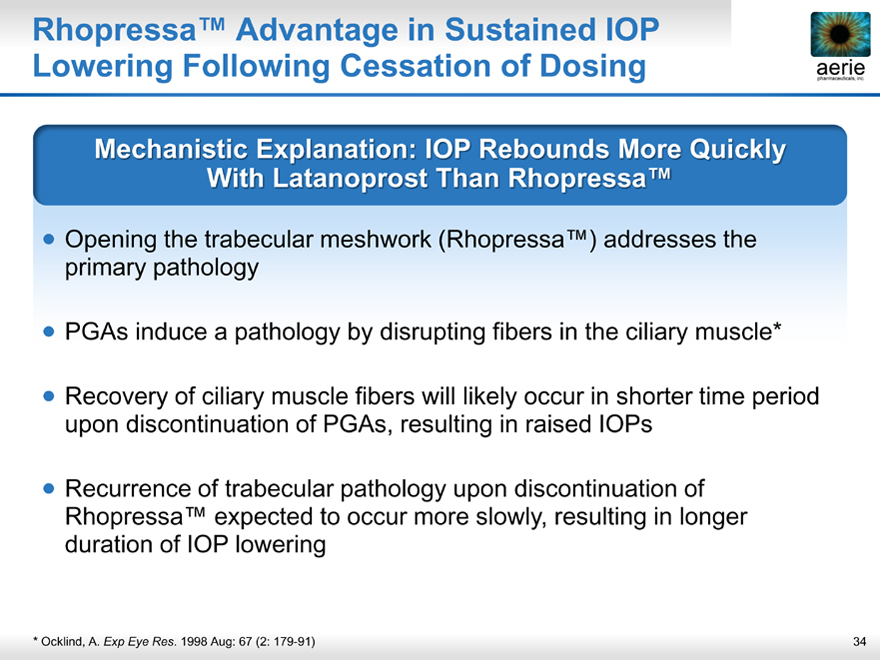
Rhopressa™ Advantage in Sustained IOP
aerie pharmaceuticals, inc. Lowering Following Cessation of Dosing aerie
Mechanistic Explanation: IOP Rebounds More Quickly With Latanoprost Than Rhopressa™
Opening the trabecular meshwork (Rhopressa™) addresses the primary pathology
PGAs induce a pathology by disrupting fibers in the ciliary muscle*
Recovery of ciliary muscle fibers will likely occur in shorter time period upon discontinuation of PGAs, resulting in raised IOPs
Recurrence of trabecular pathology upon discontinuation of Rhopressa™ expected to occur more slowly, resulting in longer duration of IOP lowering
* Ocklind, A. Exp Eye Res. 1998 Aug: 67 (2: 179-91)
34
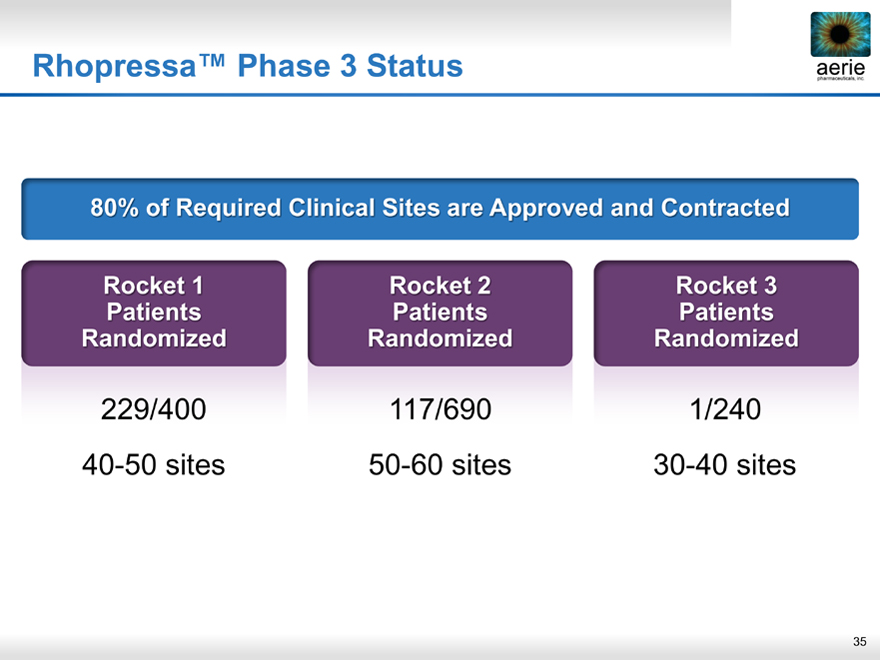
Rhopressa™ Phase 3 Status aerie
aerie pharmaceuticals, inc.
80% of Required Clinical Sites are Approved and Contracted
Rocket 1 Rocket 2 Rocket 3
Patients Patients Patients
Randomized Randomized Randomized
229/400 117/690 1/240
40-50 sites 50-60 sites 30-40 sites
35

Roclatan™ Discussion
36
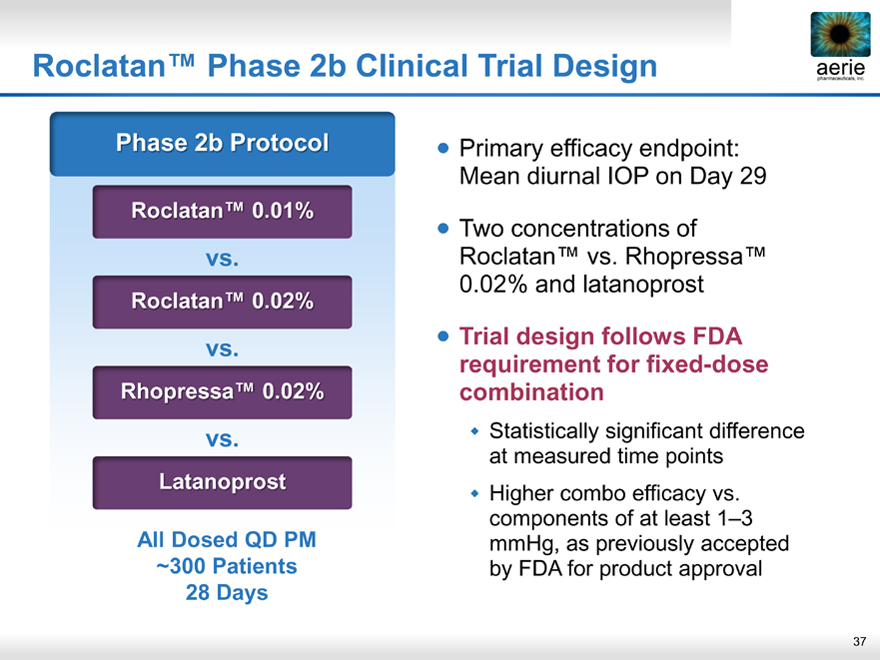
Roclatan™ Phase 2b Clinical Trial Design aerie
aerie pharmaceuticals, inc.
Phase 2b Protocol
Roclatan ™ 0.01%
vs.
Roclatan ™ 0.02%
vs.
Rhopressa™ 0.02%
vs.
Latanoprost
All Dosed QD PM ~300 Patients
28 Days
Primary efficacy endpoint: Mean diurnal IOP on Day 29
Two concentrations of Roclatan™ vs. Rhopressa™ 0.02% and latanoprost
Trial design follows FDA requirement for fixed-dose combination
Statistically significant difference at measured time points
Higher combo efficacy vs. components of at least 1–3 mmHg, as previously accepted by FDA for product approval
37
Roclatan™ Phase 2b Clinical Trial Performance aerie
aerie pharmaceuticals, inc.
Potential to be Highest Efficacy IOP-Lowering Therapy
Achieved primary efficacy measure of superiority over each of the components on day 29
• Mean diurnal IOP reduction on day 29 was approximately 2 mmHg superior to latanoprost
• Achieved statistical superiority over the individual components at all time points
More efficacious than latanoprost by 1.6 – 3.2 mmHg More efficacious than Rhopressa™ by 1.7 – 3.4 mmHg
• The main adverse event was hyperemia, or eye redness, reported in 40 percent of patients and scored as mild for the large majority of patients
No systemic drug-related adverse events
38
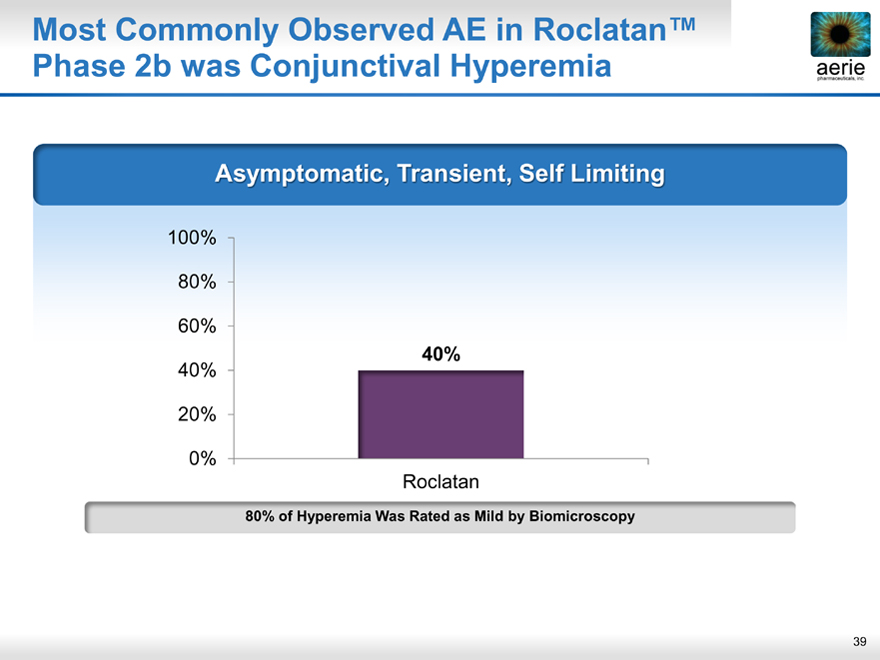
Most Commonly Observed AE in Roclatan™ Phase 2b was Conjunctival Hyperemia
aerie pharmaceuticals, inc.
Asymptomati , Transient, Self Limiting
100%
80%
60%
40%
40%
20%
0%
Roclatan
80% of Hyperemia Was Rated as Mild by Biomicroscopy
39
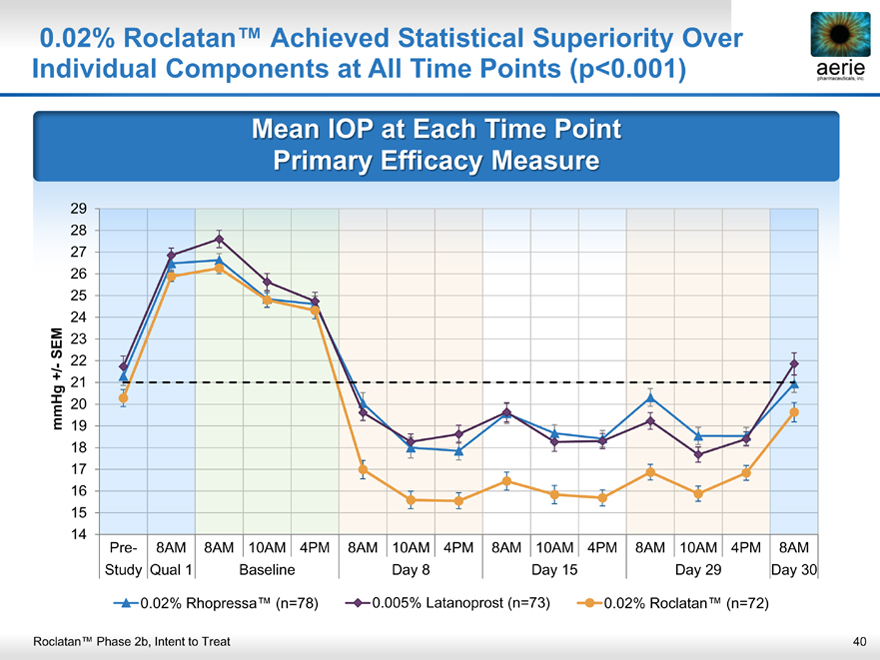
0.02% Roclatan™ Achieved Statistical Superiority Over Individual Components at All Time Points (p<0.001)
aerie pharmaceuticals, inc.
Mean IOP at Each Time Point Primary Efficacy Measure
29
28
27
26
25
24
SEM 23
- 22
+/ 21
mmHg 20
19
18
17
16
15
14
Pre- 8AM 8AM 10AM 4PM 8AM 10AM 4PM 8AM 10AM 4PM 8AM 10AM 4PM 8AM Study Qual 1 Baseline Day 8 Day 15 Day 29 Day 30
0.02% Rhopressa™ (n=78) 0.005% Latanoprost (n=73) 0.02% Roclatan™ (n=72)
Roclatan™ Phase 2b, Intent to Treat
40
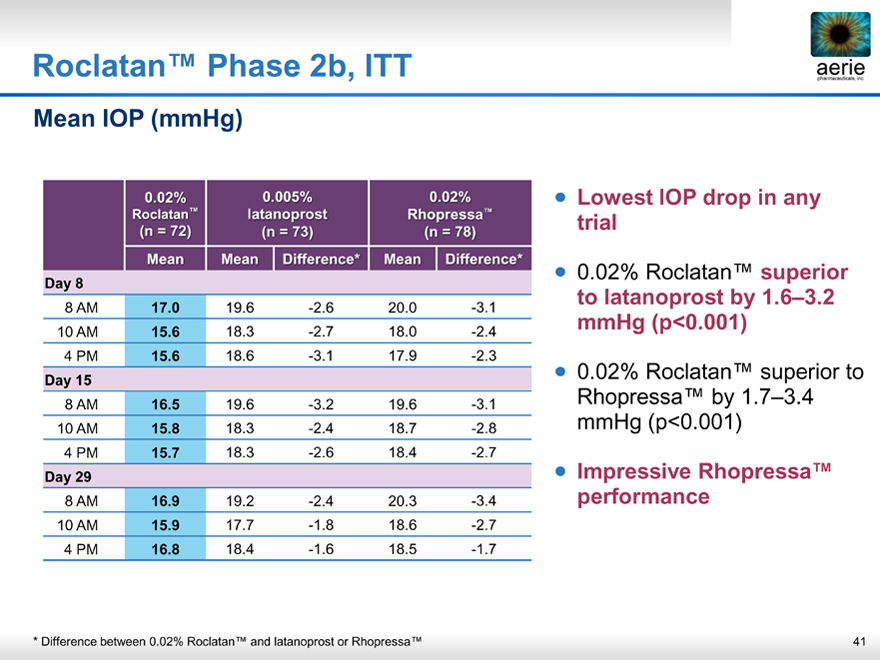
Roclatan™ Phase 2b, ITT
aeria pharmaceuticals, inc.
Mean IOP (mmHg)
0.02% 0.005% 0.02%
Roclatan™ latanoprost Rhopress ™
(n = 72)(n = 73)(n = 78)
Mean Mean Difference* Mean Difference*
Day 8
8 AM 17.0 19.6 -2.6 20.0 -3.1
10 AM 15.6 18.3 -2.7 18.0 -2.4
4 PM 15.6 18.6 -3.1 17.9 -2.3
Day 15
8 AM 16.5 19.6 -3.2 19.6 -3.1
10 AM 15.8 18.3 -2.4 18.7 -2.8
4 PM 15.7 18.3 -2.6 18.4 -2.7
Day 29
8 AM 16.9 19.2 -2.4 20.3 -3.4
10 AM 15.9 17.7 -1.8 18.6 -2.7
4 PM 16.8 18.4 -1.6 18.5 -1.7
Lowest IOP drop in any trial
0.02% Roclatan™ superior to latanoprost by 1.6–3.2 mmHg (p<0.001)
0.02% Roclatan™ superior to Rhopressa™ by 1.7–3.4 mmHg (p<0.001)
Impressive Rhopressa™ performance
* Difference between 0.02% Roclatan™ and latanoprost or Rhopressa™
41
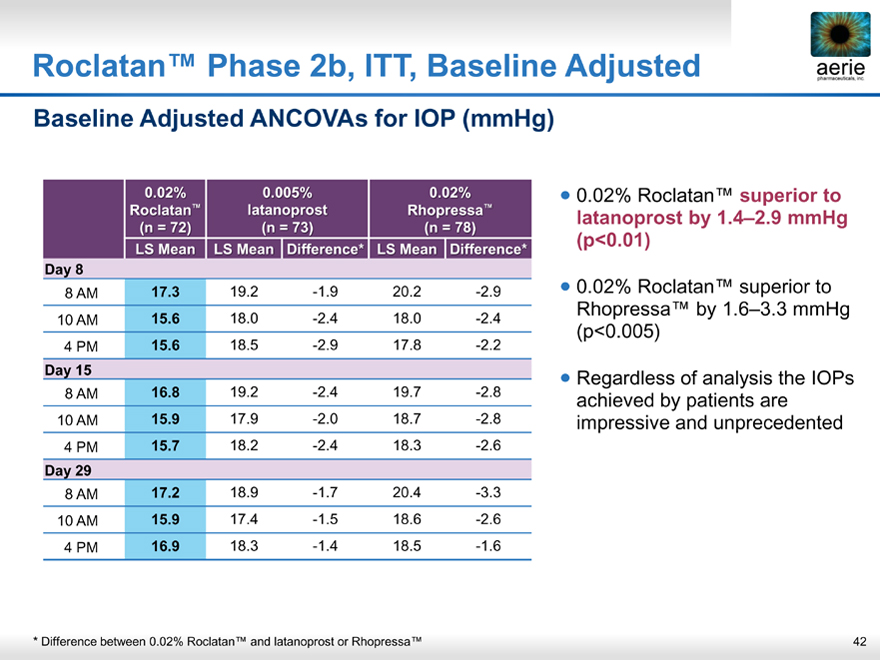
Roclatan™ Phase 2b, ITT, Baseline Adjusted
aeria pharmaceuticals, inc.
Baseline Adjusted ANCOVAs for IOP (mmHg)
0.02% 0.005% 0.02%
Roclatan™ latanoprost Rhopress ™
(n = 72)(n = 73)(n = 78)
LS Mean LS Mean Difference* LS Mean Difference*
Day 8
8 AM 17.3 19.2 -1.9 20.2 -2.9
10 AM 15.6 18.0 -2.4 18.0 -2.4
4 PM 15.6 18.5 -2.9 17.8 -2.2
Day 15
8 AM 16.8 19.2 -2.4 19.7 -2.8
10 AM 15.9 17.9 -2.0 18.7 -2.8
4 PM 15.7 18.2 -2.4 18.3 -2.6
Day 29
8 AM 17.2 18.9 -1.7 20.4 -3.3
10 AM 15.9 17.4 -1.5 18.6 -2.6
4 PM 16.9 18.3 -1.4 18.5 -1.6
0.02% Roclatan™ superior to latanoprost by 1.4–2.9 mmHg (p<0.01)
0.02% Roclatan™ superior to Rhopressa™ by 1.6–3.3 mmHg (p<0.005)
Regardless of analysis the IOPs achieved by patients are impressive and unprecedented
* Difference between 0.02% Roclatan™ and latanoprost or Rhopressa™
42
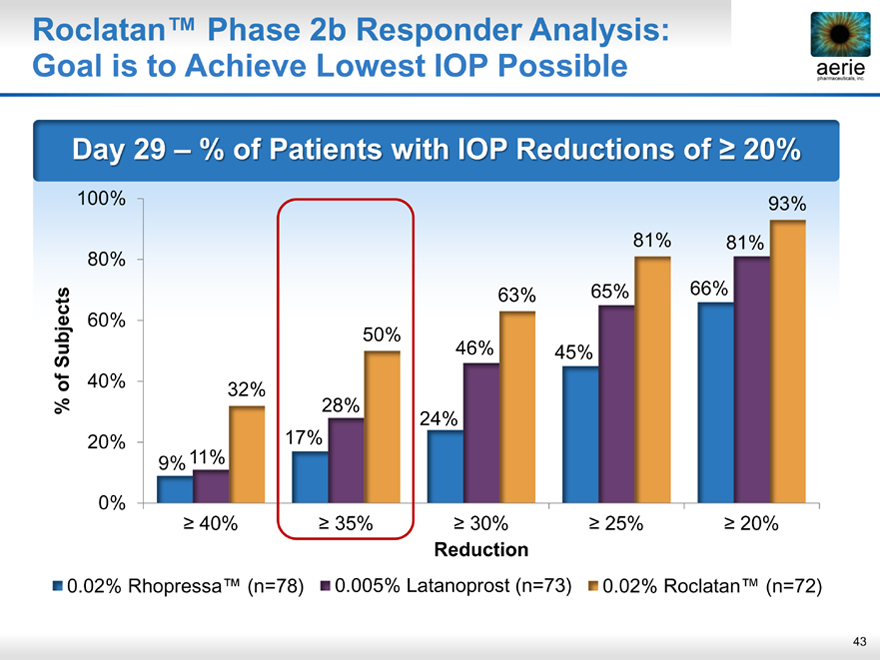
Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible
aeria pharmaceuticals, inc.
Day 29 % of Patients with IOP Reductions of > 20%
100%
80%
Subjects 60% of 40% %
20%
0%
93% 81% 81% 63% 65% 66% 50% 46% 45% 32% 28% 24% 11% 17% 9%
> 40% > 35% > 30% > 25% > 20%
Reduction
0.02% Rhopressa™ (n=78) 0.005% Latanoprost (n=73) 0.02% Roclatan™ (n=72)
43
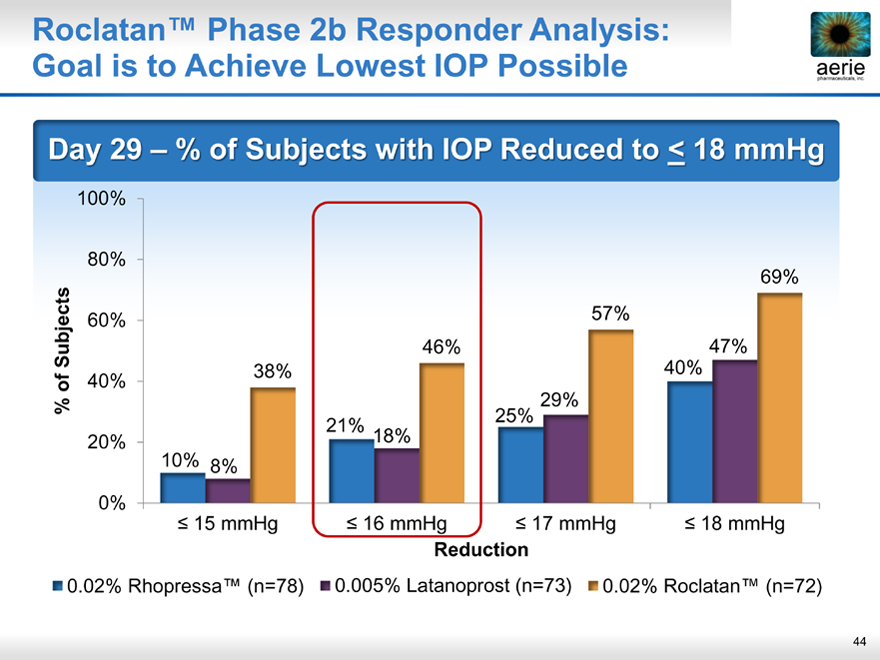
Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible
aeria pharmaceuticals, inc.
Day 29 % of Subjects with IOP Reduced to < 18 mmHg
100%
80%
Subjects 60% of 40% %
20%
0%
69%
57%
46% 47%
38% 40%
29%
21% 18% 25%
10% 8%
< 15 mmHg < 16 mmHg < 17 mmHg < 18 mmHg
Reduction
0.02% Rhopressa™ (n=78) 0.005% Latanoprost (n=73) 0.02% Roclatan™ (n=72)
44
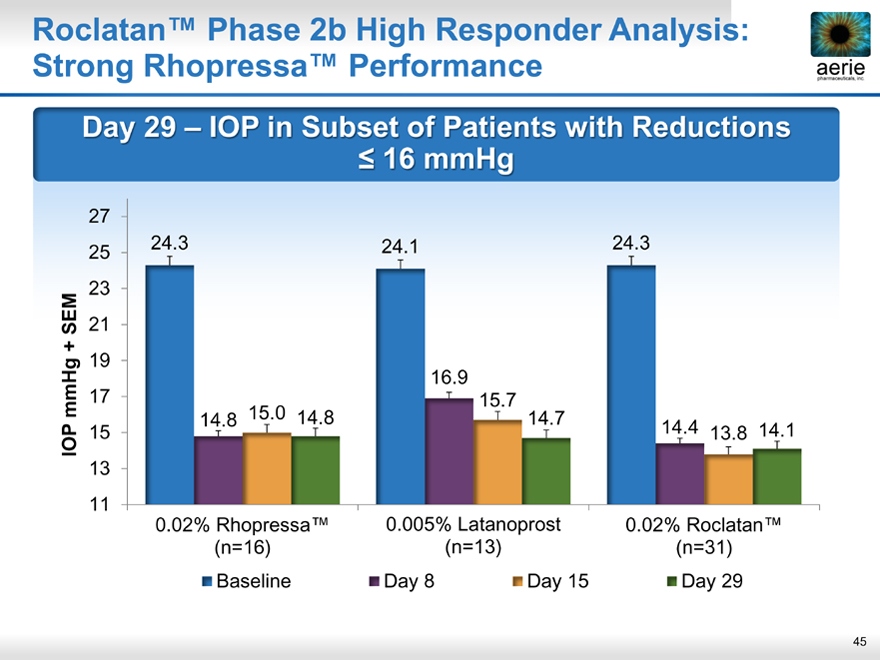
aeria pharmaceuticals, inc.
Roclatan™ Phase 2b High Responder Analysis: Strong Rhopressa™ Performance
Day 29 – IOP in Subset of Patients with Reductions
< 16 mmHg
27
25 24.3 24.1 24.3 23
SEM 21 + 19
16.9 mmHg 17 15.7 15.0
IOP 15 14.8 14.8 14.7 14.4 13.8 14.1 13 11
0.02% Rhopressa™ 0.005% Latanoprost 0.02% Roclatan™ (n=16) (n=13) (n=31)
Baseline Day 8 Day 15 Day 29
45
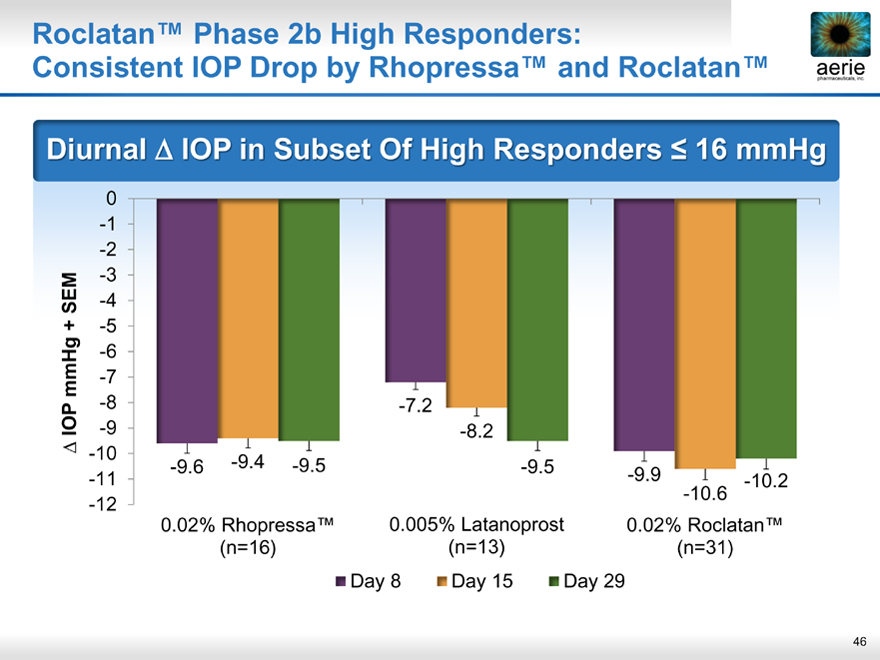
Roclatan™ Phase 2b High Responders:
aeria pharmaceuticals, inc.
Consistent IOP Drop by Rhopressa™ and Roclatan™
Diurnal IOP in Subset Of High Responders < 16 mmHg
0 -1 -2 -3
SEM -4
+ -5 -6 mmHg -7
-8
IOP -9
-10 -11 -12
-7.2 -8.2
-9.6 -9.4 -9.5 -9.5
-9.9 -10.6 -10.2
0.02% Rhopressa™ 0.005% Latanoprost 0.02% Roclatan™
(n=16) (n=13) (n=31)
• Day 8 • Day 15 • Day 29
46
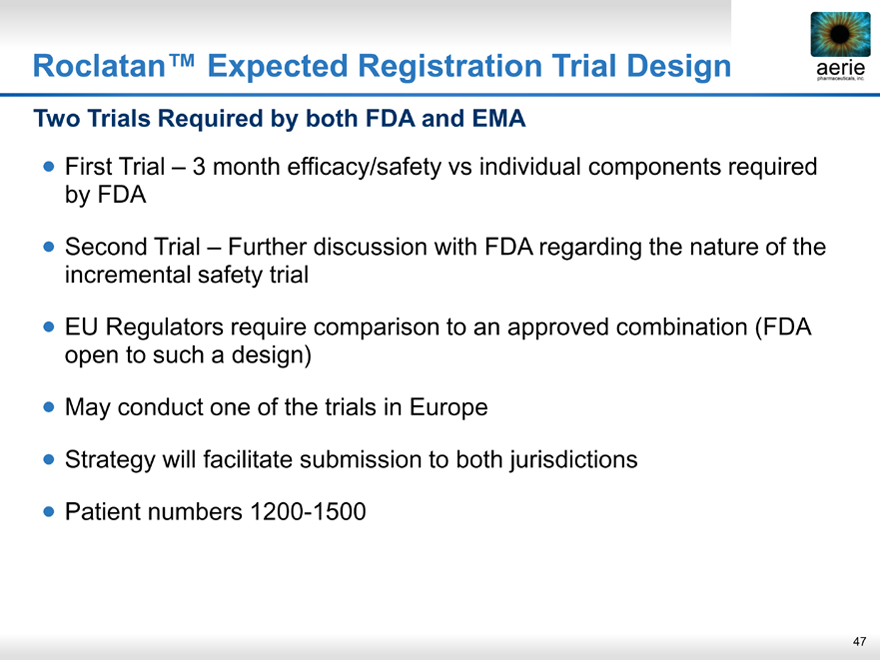
Roclatan™ Expected Registration Trial Design
aeria pharmaceuticals, inc.
Two Trials Required by both FDA and EMA
• First Trial – 3 month efficacy/safety vs individual components required by FDA
• Second Trial – Further discussion with FDA regarding the nature of the incremental safety trial
• EU Regulators require comparison to an approved combination (FDA open to such a design)
• May conduct one of the trials in Europe
• Strategy will facilitate submission to both jurisdictions
• Patient numbers 1200-1500
47
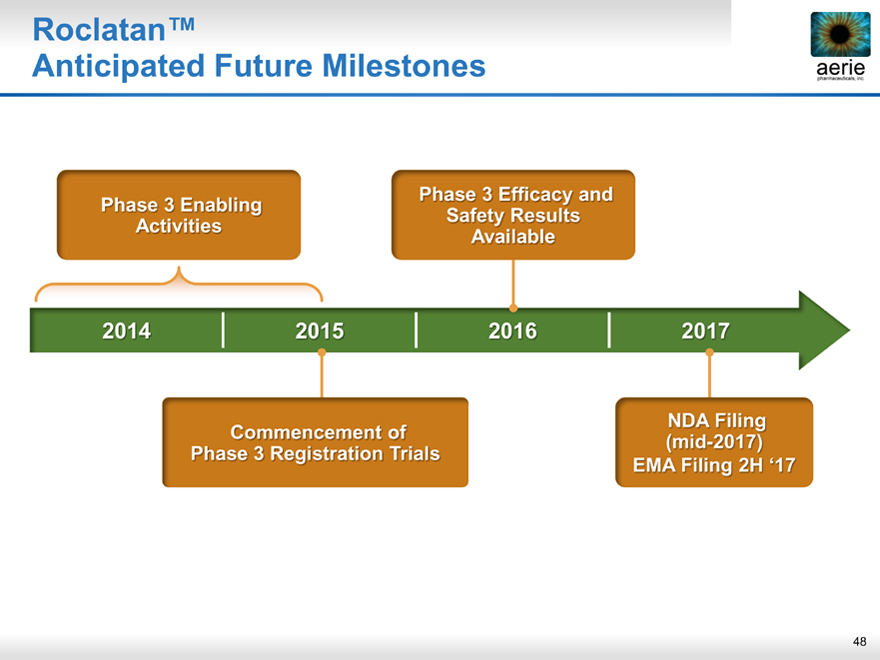
Roclatan™
aeria pharmaceuticals, inc.
Anticipated Future Milestones
Phase 3 Enabling Activities Phase 3 Efficacy and Safety Results Available
2014 2015 2016 2017
Commencement of Phase 3 Registration Trials NDA Filing (mid-2017) EMA Filing 2H ‘17
48
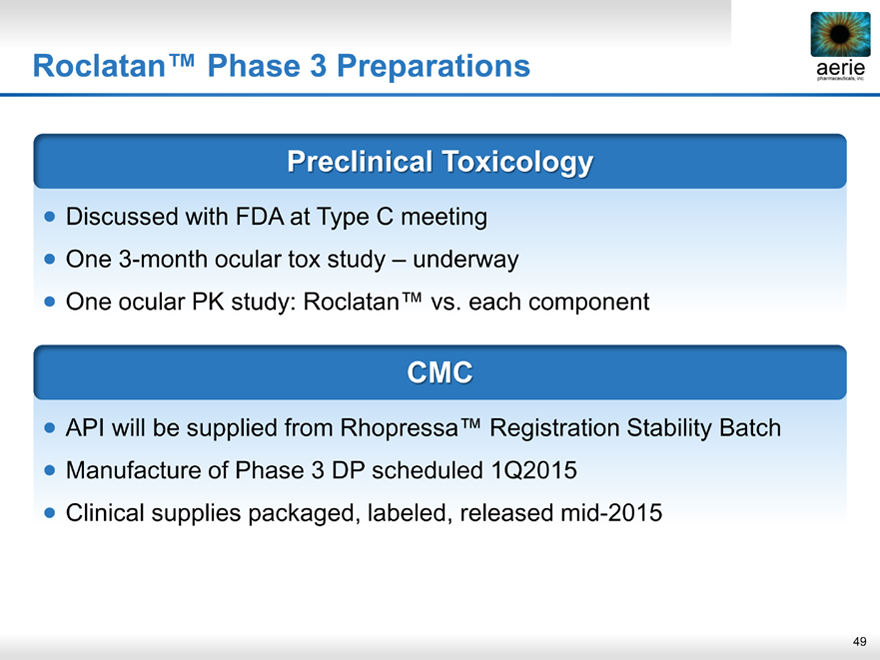
Roclatan™ Phase 3 Preparations
aeria pharmaceuticals, inc.
Preclinical Toxicology
• Discussed with FDA at Type C meeting
• One 3-month ocular tox study – underway
• One ocular PK study: Roclatan™ vs. each component
CMC
• API will be supplied from Rhopressa™ Registration Stability Batch
• Manufacture of Phase 3 DP scheduled 1Q2015
• Clinical supplies packaged, labeled, released mid-2015
49

Q&A
50

Marketing Research
Thomas Mitro
51
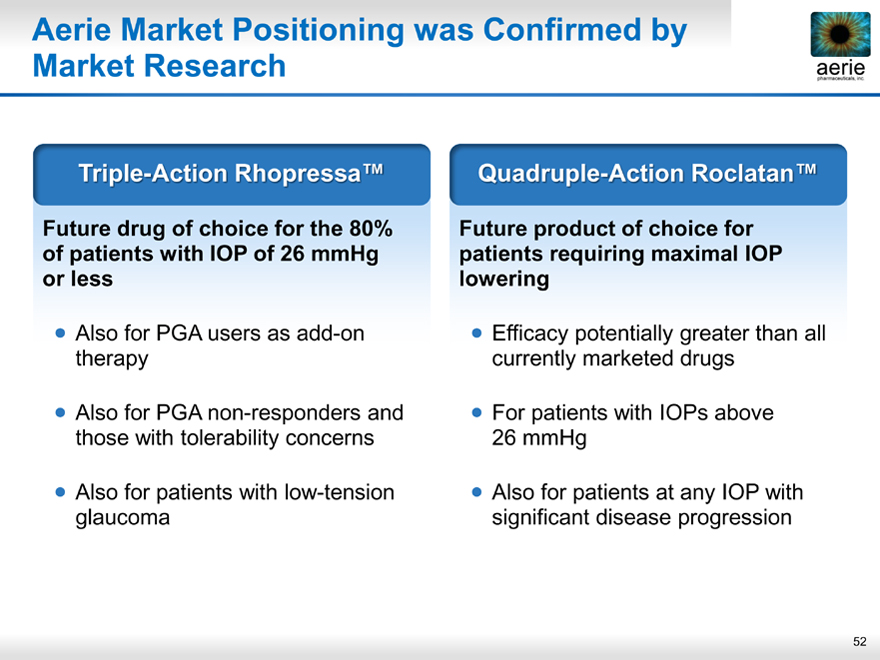
Aerie Market Positioning was Confirmed by Market Research
aeria pharmaceuticals, inc.
Triple-Action Rhopressa™
Future drug of choice for the 80% of patients with IOP of 26 mmHg or less
Also for PGA users as add-on therapy
Also for PGA non-responders and those with tolerability concerns
Also for patients with low-tension glaucoma
Quadruple-Action Roclatan™
Future product of choice for patients requiring maximal IOP lowering
Efficacy potentially greater than all currently marketed drugs
For patients with IOPs above 26 mmHg
Also for patients at any IOP with significant disease progression
52
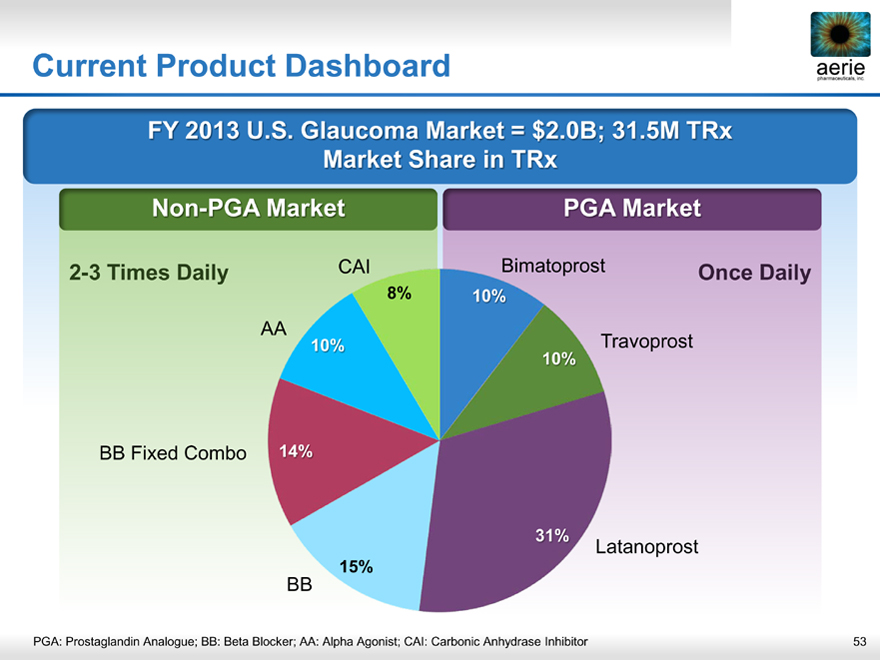
Current Product Dashboard
aeria pharmaceuticals, inc.
FY 2013 U.S. Glaucoma Market = $2.0B; 31.5M TRx Market Share in TRx
Non- PGA Market PGA Market
2-3 Times Daily CAI Bimatoprost Once Daily
8% 10%
AA
10% 10% Travoprost
BB Fixed Combo 14%
31%
Latanoprost
15%
BB
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor 53
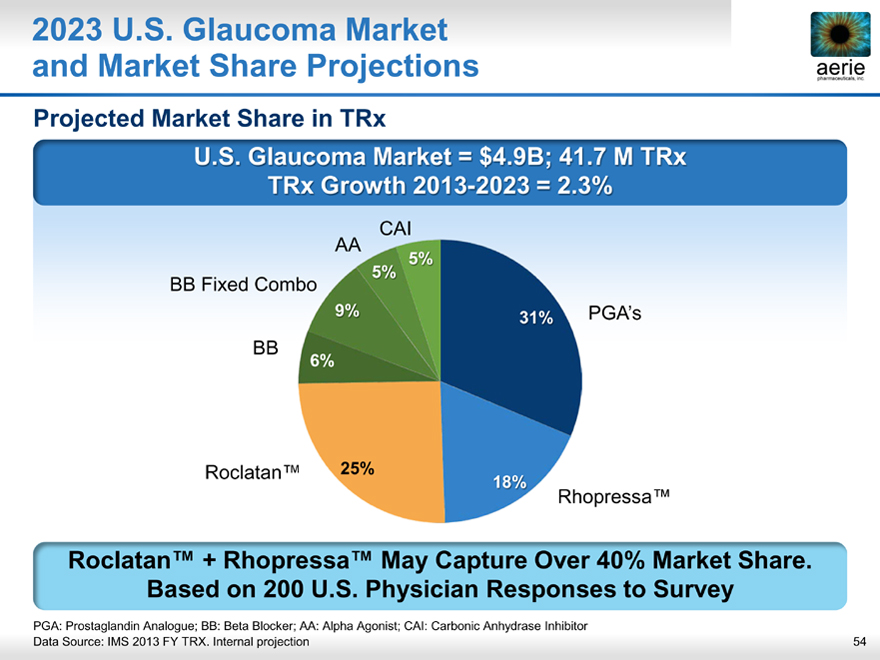
2023 U.S. Glaucoma Market and Market Share Projections
aeria pharmaceuticals, inc.
Projected Market Share in TRx
U.S. Glaucoma Market = $4.9B; 41.7 M TRx TRx Growth 2013- 2023 = 2.3%
CAI AA
5% 5%
BB Fixed Combo
9% 31% PGA’s BB
6%
Roclatan™ 25%
18%
Rhopressa™
Roclatan™ + Rhopressa™ May Capture Over 40% Market Share. Based on 200 U.S. Physician Responses to Survey
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Data Source: IMS 2013 FY TRX. Internal projection
54
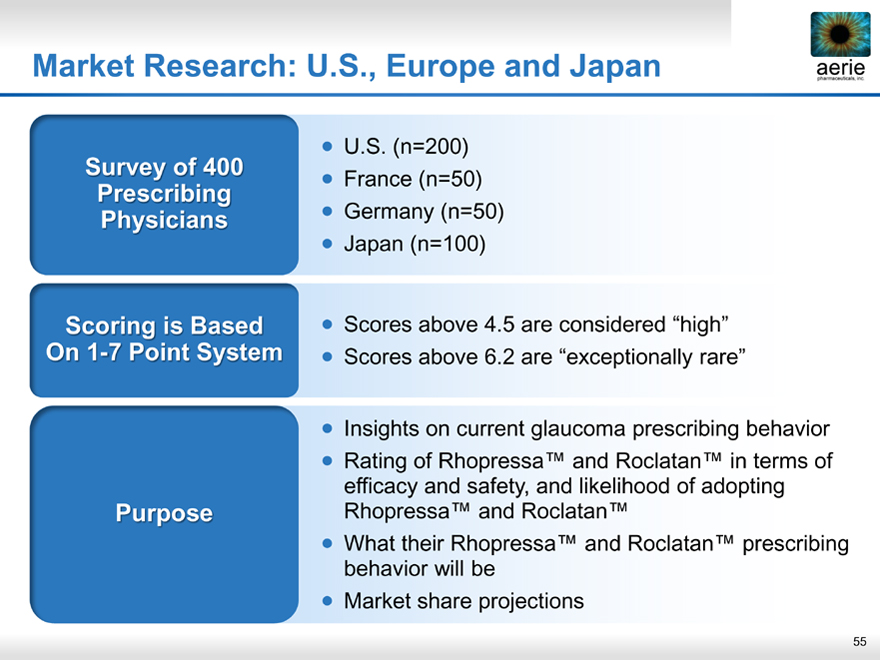
Market Research: U.S., Europe and Japan aerie
aeria pharmaceuticals, inc.
Survey of 400 Prescribing Physicians
Scoring is Based On1- 7 Point System
Purpose
U.S. (n=200)
France (n=50)
Germany (n=50)
Japan (n=100)
Scores above 4.5 are considered “high”
Scores above 6.2 are “exceptionally rare”
Insights on current glaucoma prescribing behavior
Rating of Rhopressa™ and Roclatan™ in terms of efficacy and safety, and likelihood of adopting Rhopressa™ and Roclatan™
What their Rhopressa™ and Roclatan™ prescribing behavior will be
Market share projections
55

Most Important Attributes in a New Topical Glaucoma Medication
aeria pharmaceuticals, inc.
Key Product Attributes
1. Greater IOP lowering
2. Once daily dosing regimen
3. Greater efficacy as an add-on to PGA
4. Lack of contraindications/AE’s
5. Better tolerated
6. Ability to work in low tension Glaucoma
7. As a fixed combo with another drug
Desired Key Product Attributes Align with the Benefits Offered by Rhopressa™ and Roclatan™
56

Efficacy, Safety, and Tolerability of Rhopressa™ and Roclatan™
aeria pharmaceuticals, inc.
Physician Ratings for Rhopressa™ and Roclatan™ Efficacy Were High From All Geographies
Rhopressa™ score ranges: 5.5 to 5.8
Roclatan™ score ranges: 5.5 to 6.2
Physicians Felt That the Safety and Tolerability of Rhopressa™ and Roclatan™ Was Excellent With High Scores From All Countries
Score ranges: 5.3 to 5.7
Market Research Company States Ratings Above 6.2 Are Extremely Rare
Respondents ranked products on a scale of 1-7.
57
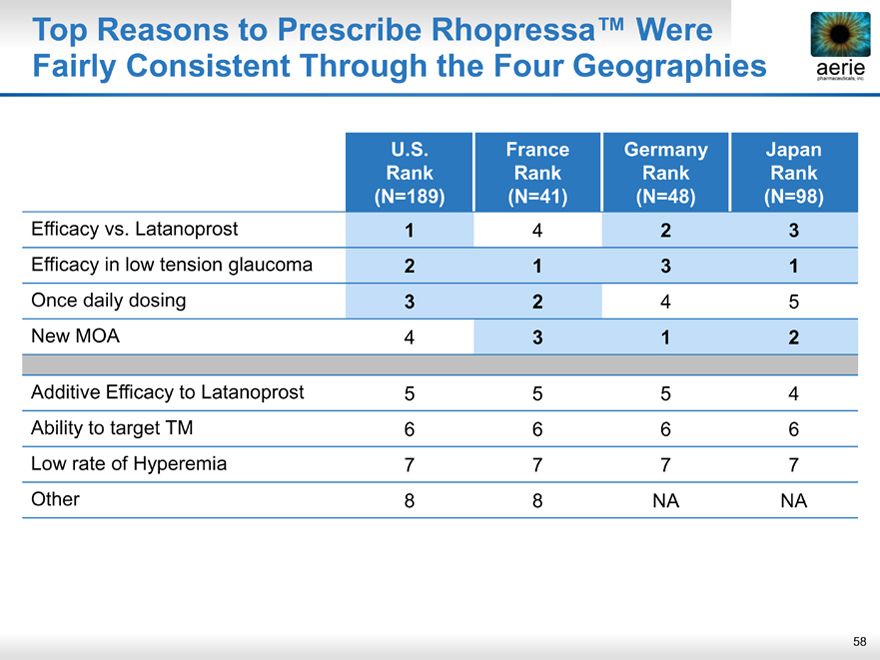
Top Reasons to Prescribe Rhopressa™ Were Fairly Consistent Through the Four Geographies
aeria pharmaceuticals, inc.
U.S. France Germany Japan
Rank Rank Rank Rank
(N=189)(N=41)(N=48)(N=98)
Efficacy vs. Latanoprost 1 4 2 3
Efficacy in low tension glaucoma 2 1 3 1
Once daily dosing 3 2 4 5
New MOA 4 3 1 2
Additive Efficacy to Latanoprost 5 5 5 4
Ability to target TM 6 6 6 6
Low rate of Hyperemia 7 7 7 7
Other 8 8 NA NA
58
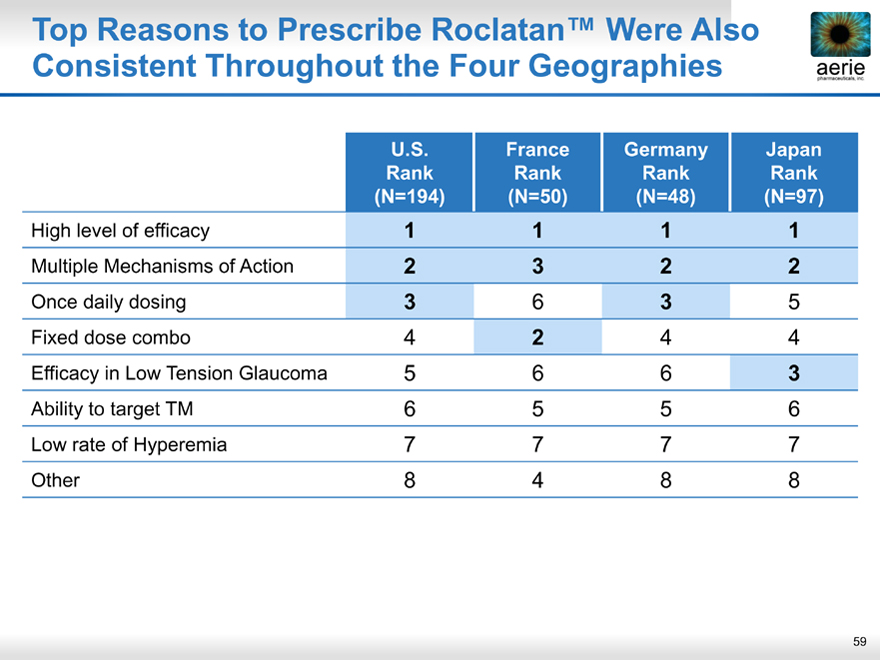
Top Reasons to Prescribe Roclatan™ Were Also Consistent Throughout the Four Geographies
aeria pharmaceuticals, inc.
U.S. France Germany Japan
Rank Rank Rank Rank
(N=194)(N=50)(N=48)(N=97)
High level of efficacy 1 1 1 1
Multiple Mechanisms of Action 2 3 2 2
Once daily dosing 3 6 3 5
Fixed dose combo 4 2 4 4
Efficacy in Low Tension Glaucoma 5 6 6 3
Ability to target TM 6 5 5 6
Low rate of Hyperemia 7 7 7 7
Other 8 4 8 8
59

Likelihood to use Rhopressa™ and Roclatan™ Rated “High”
aeria pharmaceuticals, inc.
Likelihood to Use Rhopressa™ and Roclatan™ Were Rated High for All Countries
Rhopressa™ score ranges: 5.5 to 5.8
Roclatan™ score ranges: 5.5 to 6.0
Respondents ranked products on a scale of 1-7.
60
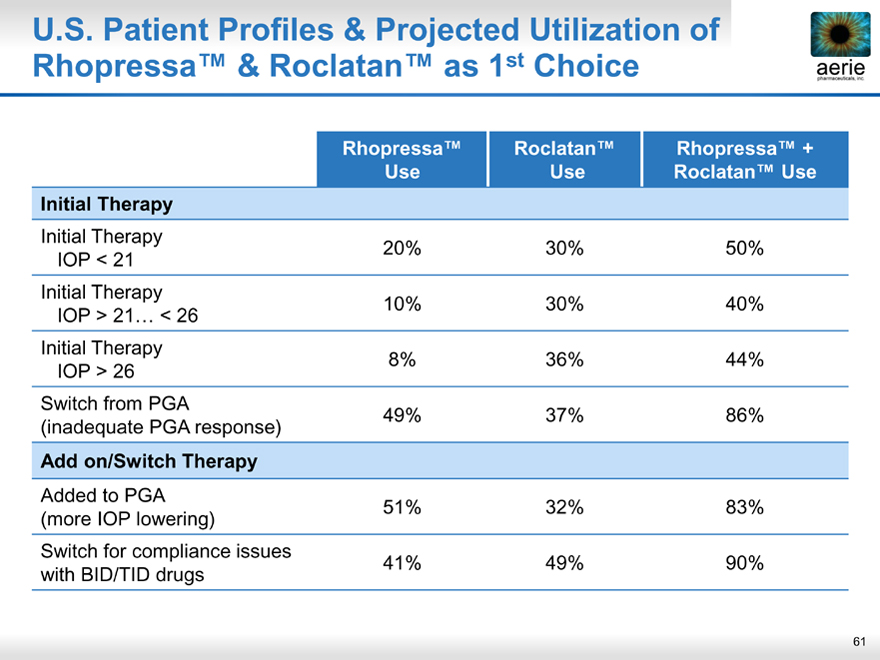
U.S. Patient Profiles & Projected Utilization of Rhopressa™ & Roclatan™ as 1st Choice
aeria pharmaceuticals, inc.
Rhopressa™ Roclatan™ Rhopressa™ +
Use Use Roclatan™ Use
Initial Therapy
Initial Therapy 20% 30% 50%
IOP < 21
Initial Therapy 10% 30% 40%
IOP > 21… < 26
Initial Therapy 8% 36% 44%
IOP > 26
Switch from PGA 49% 37% 86%
(inadequate PGA response)
Add on/Switch Therapy
Added to PGA 51% 32% 83%
(more IOP lowering)
Switch for compliance issues 41% 49% 90%
with BID/TID drugs
61
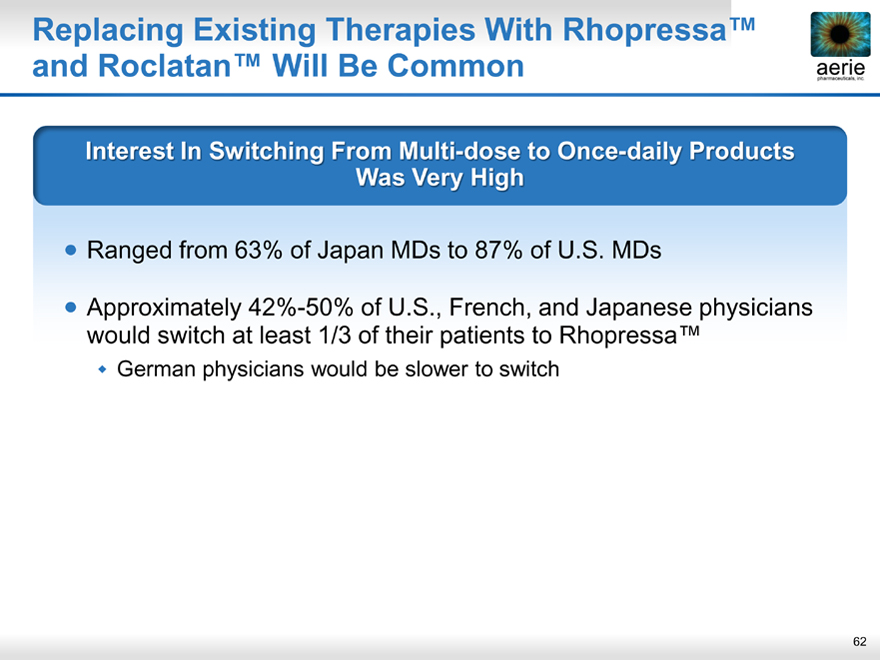
Replacing Existing Therapies With Rhopressa™ and Roclatan™ Will Be Common
aeria pharmaceuticals, inc.
Interest In Switching From Multi-dose to Once-daily Products Was Very High
Ranged from 63% of Japan MDs to 87% of U.S. MDs
Approximately 42%-50% of U.S., French, and Japanese physicians would switch at least 1/3 of their patients to Rhopressa™
German physicians would be slower to switch
62
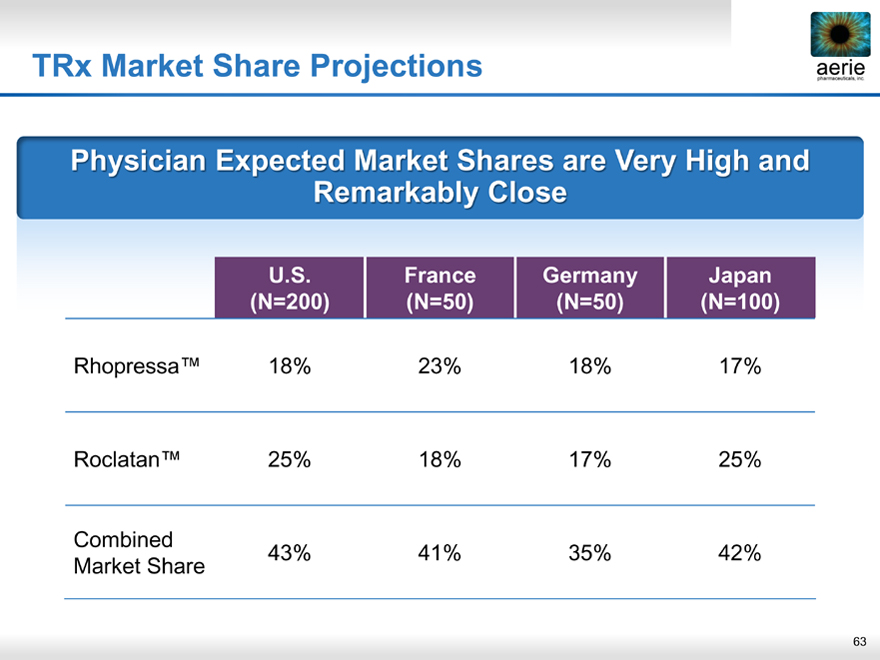
TRx Market Share Projections
aeria pharmaceuticals, inc.
Physician Expected Market Shares are Very High and Remarkably Close
U.S. France Germany Japan
(N=200)(N=50)(N=50)(N=100)
Rhopressa™ 18% 23% 18% 17%
Roclatan™ 25% 18% 17% 25%
Combined 43% 41% 35% 42%
Market Share
63
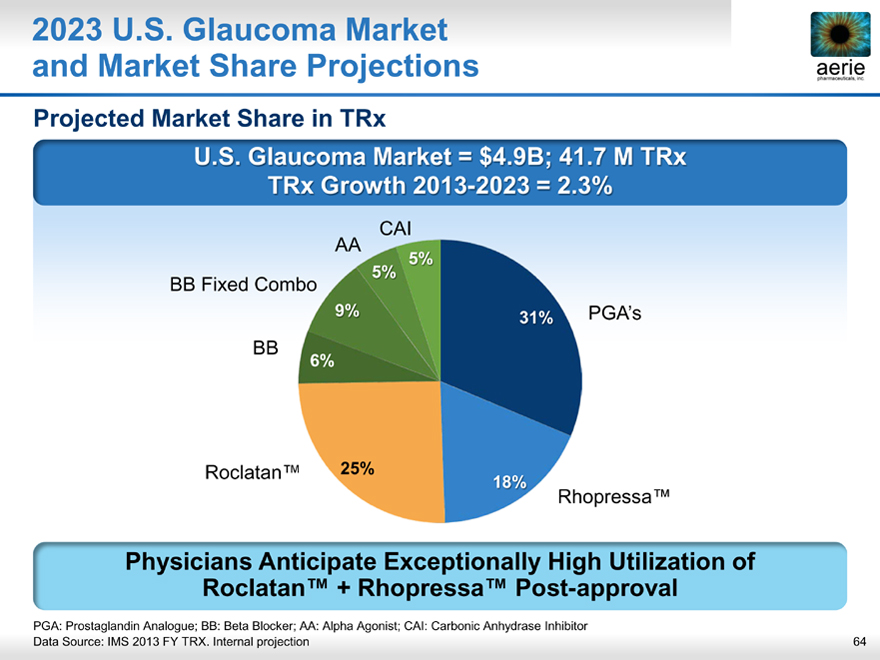
2023 U.S. Glaucoma Market and Market Share Projections
aeria pharmaceuticals, inc.
Projected Market Share in TRx
U.S. Glaucoma Market = $4.9B; 41.7 M TRx TRx Growth 2013-2023 = 2.3%
CAI
AA
5%
5%
BB Fixed Combo
9% 31% PGA’s
BB
6%
Roclatan™ 25%
18%
Rhopressa™
Physicians Anticipate Exceptionally High Utilization of Roclatan™ + Rhopressa™ Post-approval
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Data Source: IMS 2013 FY TRX. Internal projection
64

Q&A
65

R&D Update
Casey Kopczynski
66
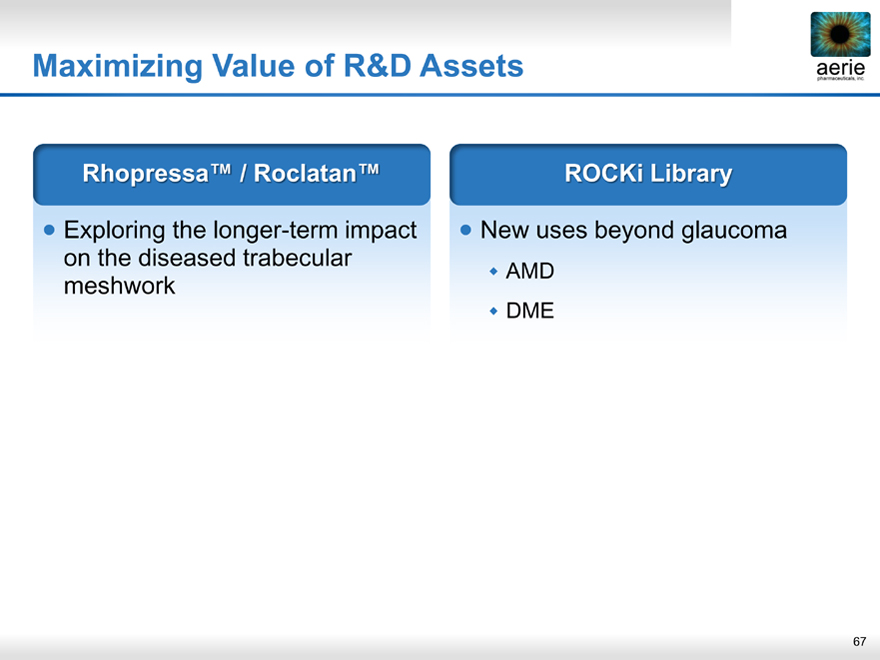
Maximizing Value of R&D Assets
aerie pharmaceuticals, inc.
Rhopressa™ / Roclatan™
Exploring the longer-term impact on the diseased trabecular meshwork
ROCKi Library
New uses beyond glaucoma
• AMD
• DME
67
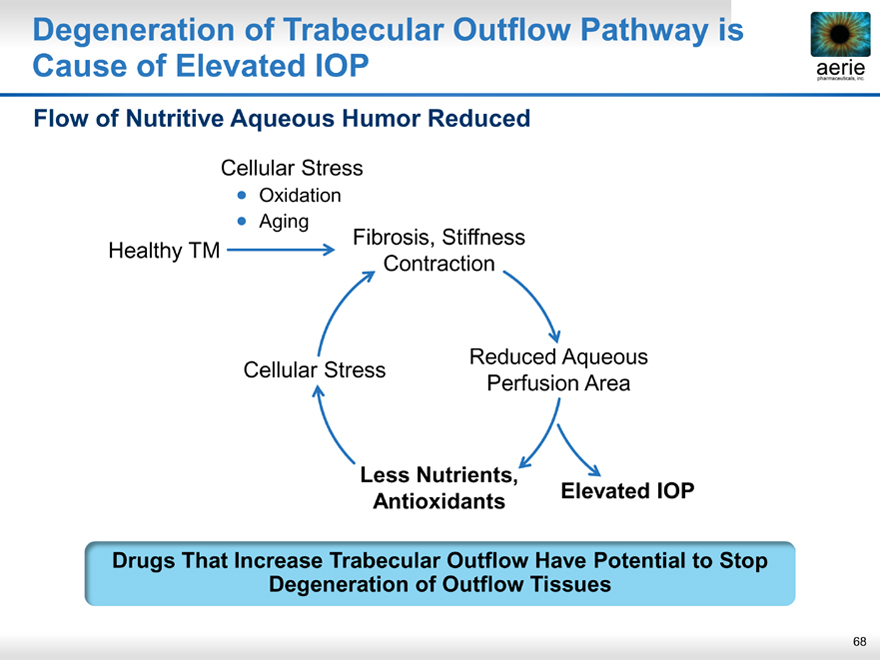
Degeneration of Trabecular Outflow Pathway is Cause of Elevated IOP
aeria pharmaceuticals, inc.
Flow of Nutritive Aqueous Humor Reduced
Cellular Stress
• Oxidation
• Aging
Fibrosis, Stiffness Healthy TM
Contraction
Reduced Aqueous Cellular Stress Perfusion Area
Less Nutrients,
Antioxidants Elevated IOP
Drugs That Increase Trabecular Outflow Have Potential to Stop Degeneration of Outflow Tissues
68

Can Rhopressa™ Preserve TM Health?
aeria pharmaceuticals, inc.
Tissues of the trabecular outflow pathway are avascular – they rely on aqueous humor to deliver nutrients and antioxidants
Research collaborations underway to explore whether Rhopressa™ may be able to prevent, or even reverse, damage to the TM pathway
Can Rhopressa™ reduce fibrosis in TM outflow tissues?
Can Rhopressa™ increase the effective filtration area of the TM, increasing aqueous humor perfusion into TM outflow tissues?
ROCK inhibitors have previously been shown to:
Reduce fibrosis in kidney, liver
Increase the effective filtration area of the TM
69
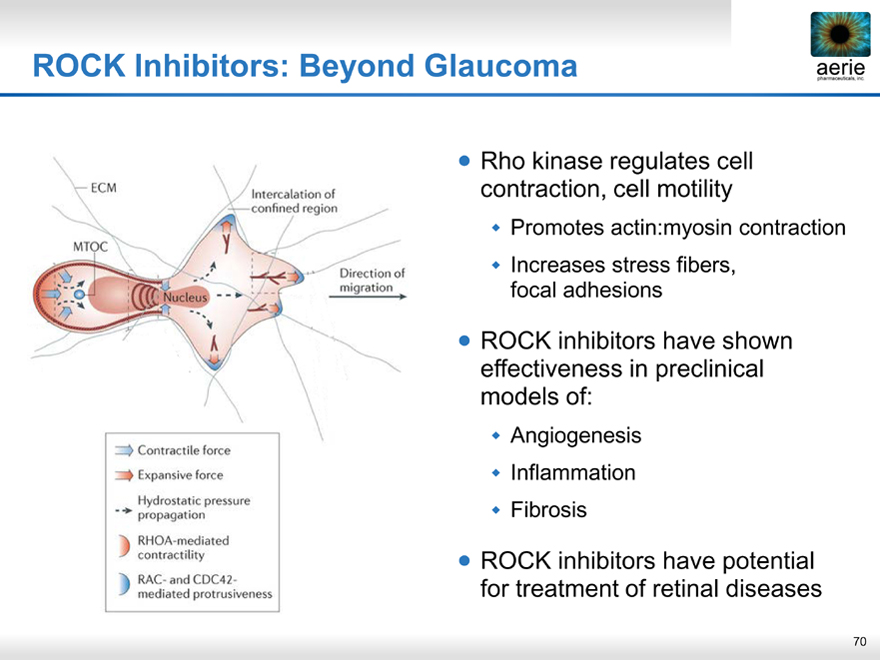
ROCK Inhibitors: Beyond Glaucoma
aeria pharmaceuticals, inc.
ECM
MTOC
Intercalation of confined region
Direction of migration
Contractile force
Expansive force
Hydrostatic pressure propagation
RHOA-mediated contractility
RAC- and CDC42- mediated protrusiveness
Rho kinase regulates cell contraction, cell motility
Promotes actin:myosin contraction
Increases stress fibers, focal adhesions
ROCK inhibitors have shown effectiveness in preclinical models of:
Angiogenesis
Inflammation
Fibrosis
ROCK inhibitors have potential for treatment of retinal diseases
70
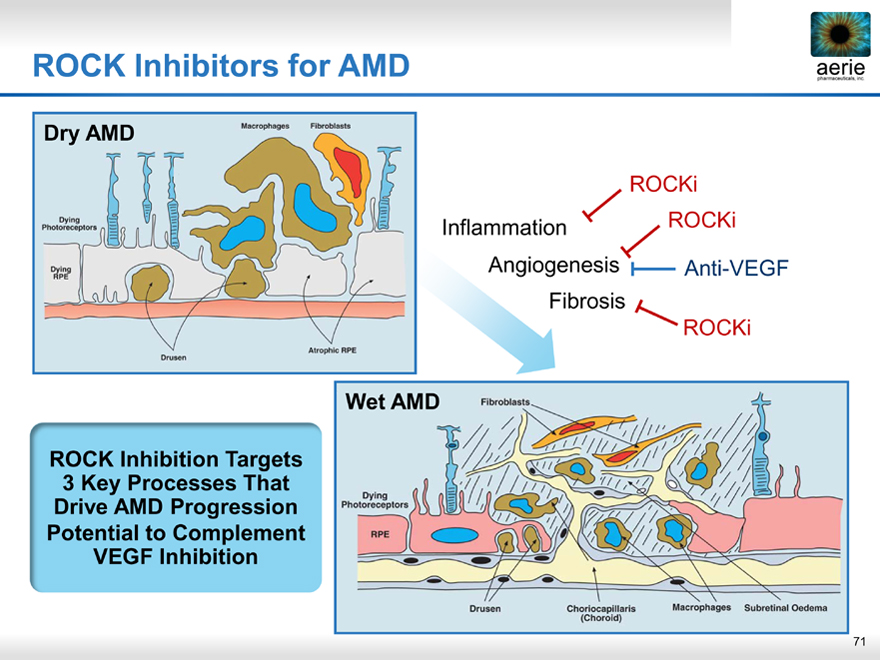
ROCK Inhibitors for AMD
aeria pharmaceuticals, inc.
Dry AMD
Macrophages
Fibroblasts
Dying
Photoreceptors
Dying RPE
Drusen
Atophic RPE
ROCKi Inflammation ROCKi Angiogenesis Anti-VEGF
Fibrosis
ROCKi
ROCK Inhibition Targets
3 Key Processes That Drive AMD Progression Potential to Complement VEGF Inhibition
Wet AMD
Fibroblasts
Dying Photoreceptors
Drusen
Choriocapillaris
(Choroid)
Macrophages
Subretinal Oedema
71
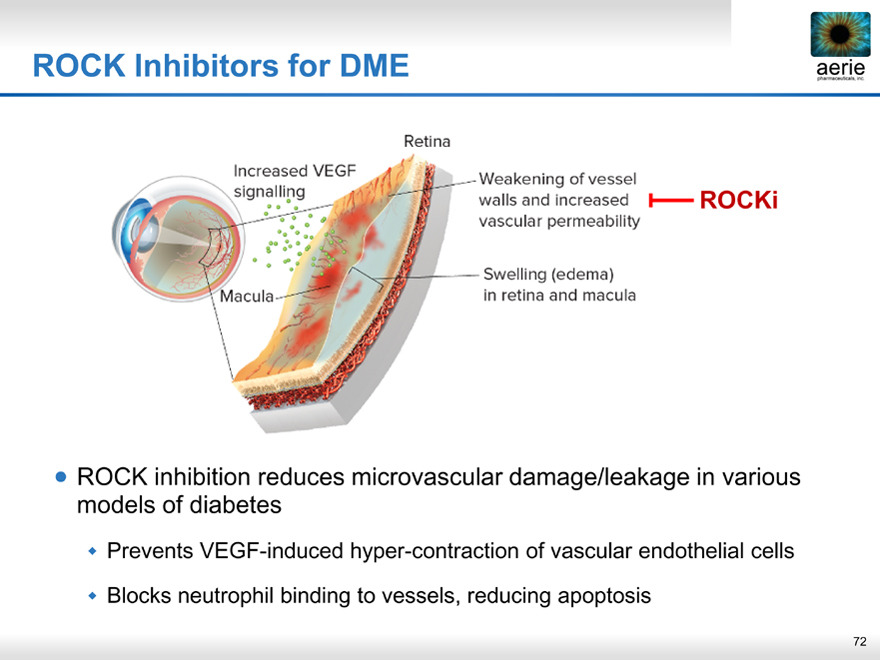
ROCK Inhibitors for DME aerie pharmaceuticals, inc.
aeria pharmaceuticals, inc.
Retina
Increased VEGF signalling
Macula
Weakening of vessel walls and increased vascular permeability
ROCKi
Swelling (edema) in retina and macula
ROCK inhibition reduces microvascular damage/leakage in various models of diabetes
Prevents VEGF-induced hyper-contraction of vascular endothelial cells
Blocks neutrophil binding to vessels, reducing apoptosis
72
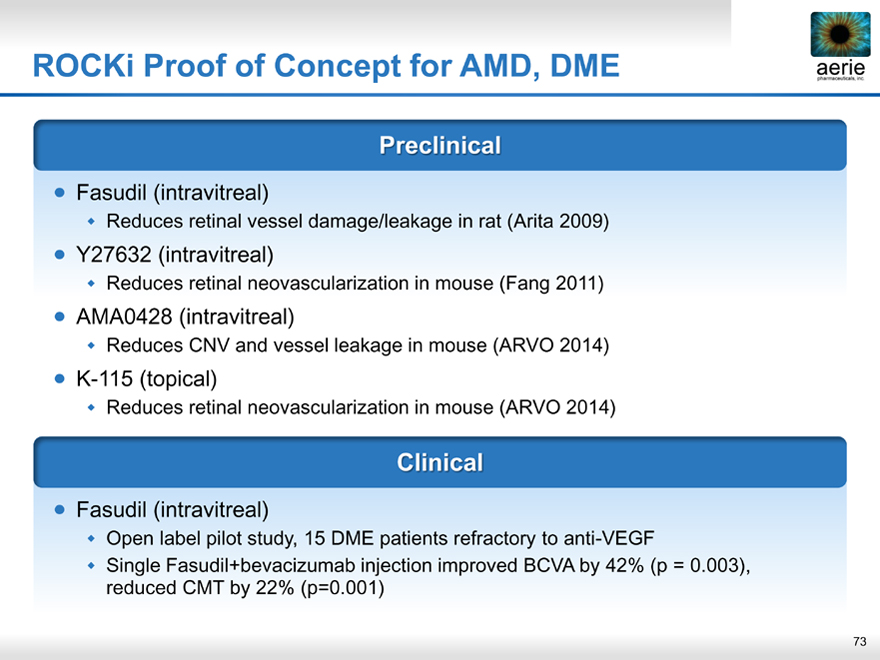
ROCKi Proof of Concept for AMD, DME aerie pharmaceuticals, inc.
aeria pharmaceuticals, inc.
Preclinical
Fasudil (intravitreal)
Reduces retinal vessel damage/leakage in rat (Arita 2009)
Y27632 (intravitreal)
Reduces retinal neovascularization in mouse (Fang 2011)
AMA0428 (intravitreal)
Reduces CNV and vessel leakage in mouse (ARVO 2014)
K-115 (topical)
Reduces retinal neovascularization in mouse (ARVO 2014)
Clinical
Fasudil (intravitreal)
Open label pilot study, 15 DME patients refractory to anti-VEGF
Single Fasudil+bevacizumab injection improved BCVA by 42% (p = 0.003), reduced CMT by 22% (p=0.001)
73
Aerie R&D – Back of the Eye Explorations
aeria pharmaceuticals, inc.
Leverage large collection of proprietary ROCK inhibitors
Diverse physicochemical properties
Wide range of kinase inhibition profiles (e.g., ROCK+PKC)
Test select compounds in laser-induced CNV model
Widely accepted model for neovascular AMD
Compare compounds with different kinase selectivity profiles
Test intravitreal injection, topical delivery
Test as adjunct to anti-VEGF treatment
• Repeat above for established models of DME
74

Business Development
Craig Skenes
75
Rhopressa™ / Roclatan™ Partnering Strategy
aeria pharmaceuticals, inc.
Advance development of Rhopressa™ and Roclatan™ to approval in North America and potentially Europe
Establish our own commercial infrastructure in North America
Exploring Aerie’s more direct involvement of development and commercial objectives for Europe
Seeking collaboration for territories outside North America with focus on Japan and Europe
Japan prioritized over Europe at this point
Importance of finding partner with interest in developing and commercializing both products
– Ensure global development is aligned between parties
76

Partnering Update
aeria pharmaceuticals, inc.
Initiated outreach in Q3 upon conclusion of Roclatan™ Phase 2b study
Connected with broad spectrum of partnering candidates
Experiencing significant global interest
Discussions are in progress with approximately 20 companies
Range of regional to global players, inside and outside ophthalmology
Prospective partners are progressing in their evaluations
Dataroom is available and dialogue is ongoing
No requirement or timeline for completing a transaction
Ensure that value and structures are aligned with our strategy and recognize the extent we have de-risked these assets
77

Building the Enterprise
aeria pharmaceuticals, inc.
We have demonstrated capabilities in drug discovery, research, development and commercialization
Initiating process of evaluating opportunities for in-licensing or acquisition within ophthalmology
Seek opportunities with market potential consistent with Aerie’s other portfolio products
Focus on remaining unmet needs in ophthalmology
First-in-class or differentiable to existing therapeutics
Spin-outs, divestitures or discontinued programs from other ophthalmic players
We Are Driving to Make Aerie a Major Ophthalmic Pharmaceutical Company
78

Financing Update
Richard Rubino
79
New Deerfield Financing
aeria pharmaceuticals, inc.
$125 Million Senior Secured Convertible Debt Facility
Proceeds expected to fully finance Aerie through product commercialization
Facility also provides further operational and strategic flexibility
Summary of Key Terms
Interest: 1.75% annual coupon paid quarterly
Term: 7 year maturity
Conversion: Convertible into Aerie common stock at 30% premium to Aerie closing price on September 8, 2014, resulting in a conversion price of $24.80 per share.
80

Q&A
81

Our Strategy
aeria pharmaceuticals, inc.
Advance the development of our product candidates to approval
Establish internal capabilities to commercialize our product candidates in North America (and potentially Europe)
Explore partnerships with leading pharmaceutical and biotechnology companies to maximize the value of our product candidates outside of North America
Continue to leverage and strengthen our intellectual property portfolio
Expand our product portfolio through internal discovery efforts and possible in-licensing or acquisitions of additional ophthalmic product candidates or products
82
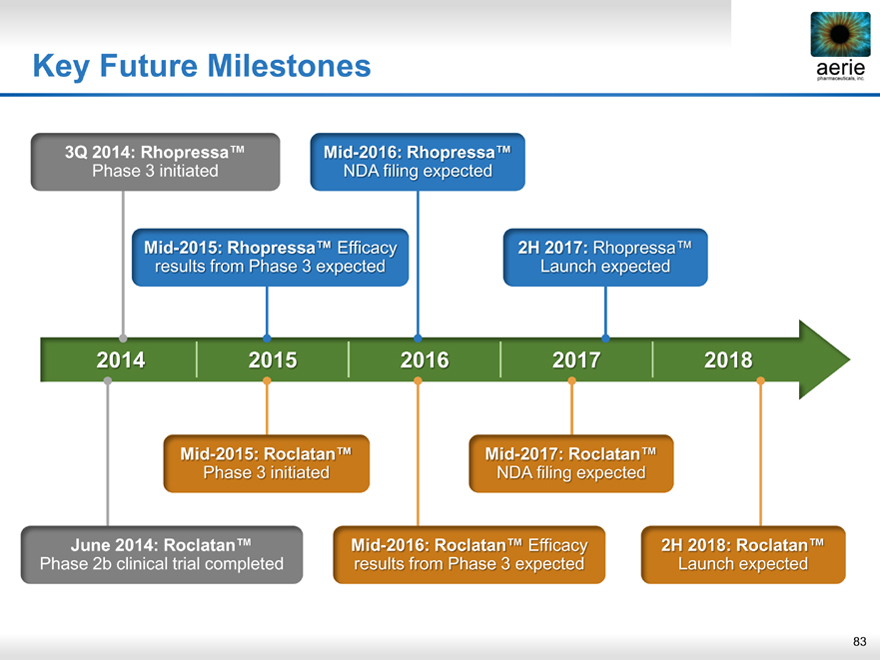
Key Future Milestones
aeria pharmaceuticals, inc.
3Q 2014: Rhopressa™
Phase 3 initiated
Mid-2016: Rhopressa™
NDA filing expected
Mid-2015: Rhopressa™ Efficacy results from Phase 3 expected
2H 2017: Rhopressa™ Launch expected
2014 2015 2016 2017 2018
Mid-2015: Roclatan™
Phase 3 initiated
Mid-2017: Roclatan™
NDA filing expected
June 2014: Roclatan™
Phase 2b clinical trial completed
Mid-2016: Roclatan™ Efficacy results from Phase 3 expected
2H 2018: Roclatan™
Launch expected
83

Building a Major Ophthalmic Pharmaceutical Company
Aerie Pharmaceuticals, Inc.
Investor Day September 10, 2014