Exhibit 99.2
Exhibit 99.2
RhopressaTM
Rocket 2 Phase 3 Topline Results
Important Information
Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities.
The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete.
Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
RhopressaTM Achieves Primary Clinical Endpoint
RhopressaTM QD and RhopressaTM BID met the criteria for non-
inferiority to Timolol BID for the primary efficacy analysis (baseline
IOP <25 mmHg)
RhopressaTM QD showed stable efficacy from Week 2 to Month 3
RhopressaTM QD adverse event profile in Rocket 2 similar to
Rocket 1
RhopressaTM BID slightly more effective than QD, but had higher
incidence of adverse events which led to greater early terminations
On target to file RhopressaTM NDA in mid-2016
RhopressaTM
Rocket 2 Trial Design
Original primary endpoint:
Mean IOP for subjects with baseline IOP >20 mmHg and <27 mmHg N=756 randomized at 62 sites
Patients randomized 1:1:1
RhopressaTM 0.02% QD
RhopressaTM
Timolol 0.5%
0.02% BID
BID
Revised range for primary endpoint:
Mean IOP for subjects with baseline IOP > 20 mmHg and <25 mmHg N=403 subjects per protocol
Study Endpoints
Efficacy
Primary efficacy endpoint was the mean IOP for subjects with baseline
IOP >20 mmHg and <25 mmHg in the study eye at nine time points:
08:00, 10:00, and 16:00 at Week 2, Week 6, and Month 3
Multiple secondary endpoints including baseline IOP <27 mmHg
Safety
Ocular and systemic safety measures at each visit through 12 months
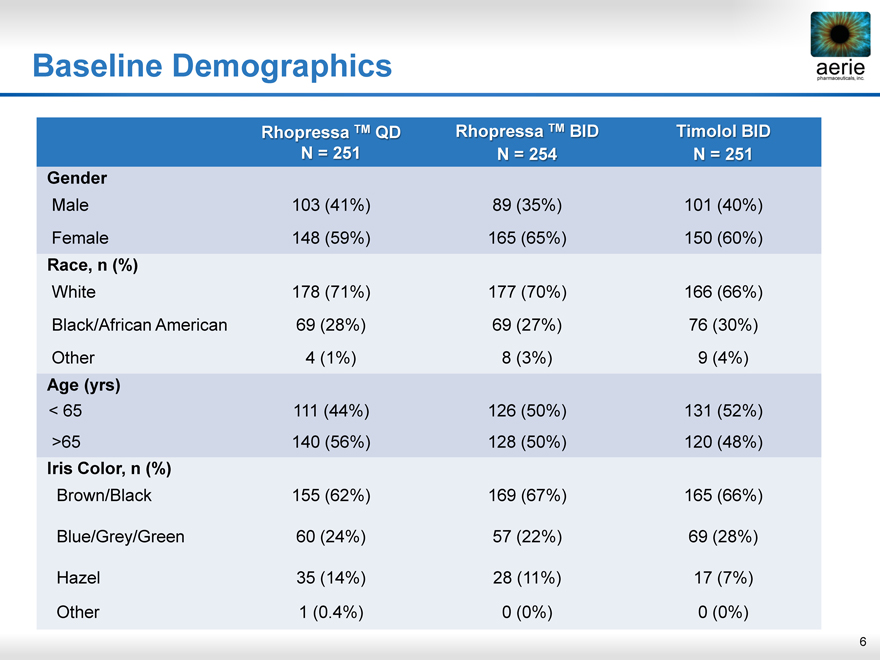
Baseline Demographics
Rhopressa TM QD Rhopressa TM BID Timolol BID
N = 251 N = 254 N = 251
Gender
Male 103 (41%) 89 (35%) 101 (40%)
Female 148 (59%) 165 (65%) 150 (60%)
Race, n (%)
White 178 (71%) 177 (70%) 166 (66%)
Black/African American 69 (28%) 69 (27%) 76 (30%)
Other 4 (1%) 8 (3%) 9 (4%)
Age (yrs)
< 65 111 (44%) 126 (50%) 131 (52%)
>65 140 (56%) 128 (50%) 120 (48%)
Iris Color, n (%)
Brown/Black 155 (62%) 169 (67%) 165 (66%)
Blue/Grey/Green 60 (24%) 57 (22%) 69 (28%)
Hazel 35 (14%) 28 (11%) 17 (7%)
Other 1 (0.4%) 0 (0%) 0 (0%)
Early Termination Summary
Early termination rates comparable to Rocket 1
RhopressaTM QD: 18%
RhopressaTM BID: 40% (Rocket 2 only)
Timolol BID: 6%
Early termination rates for conjunctival hyperemia
RhopressaTM QD: 9%
RhopressaTM BID: 17%
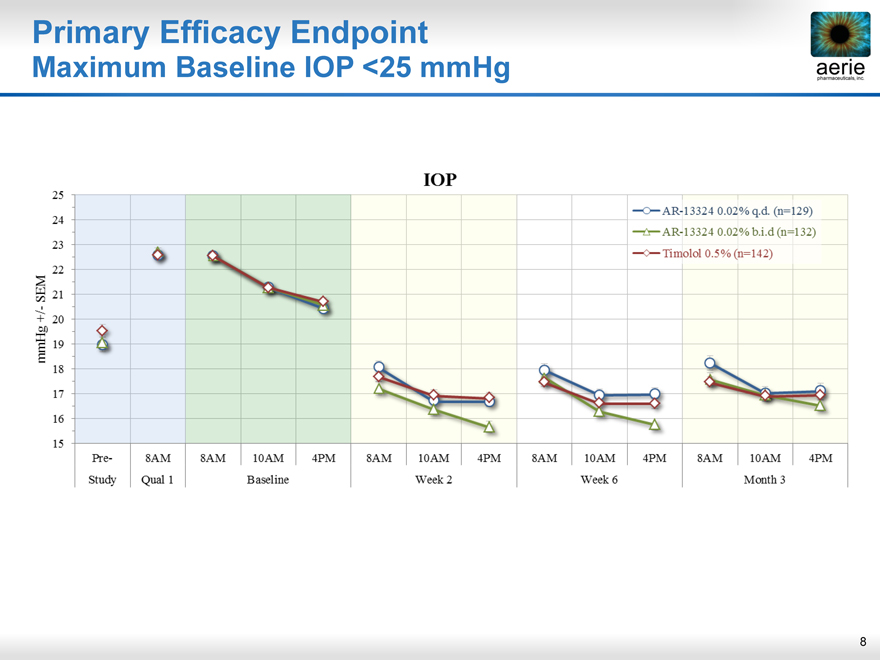
Primary Efficacy Endpoint
Maximum Baseline IOP <25 mmHg
mmHg +/- SEM
pre- 8AM 10AM 4PM
Study Qual 1 Baseline Week 2 Week 6 Month 3
25 24 23 22 21 20 19 18 17 16 15
AR-13324 0.02% q.d. (n=129)
AR-13324 0.02% b.i.d. (n=132)
Timolol 0.5% (n=142) IOP
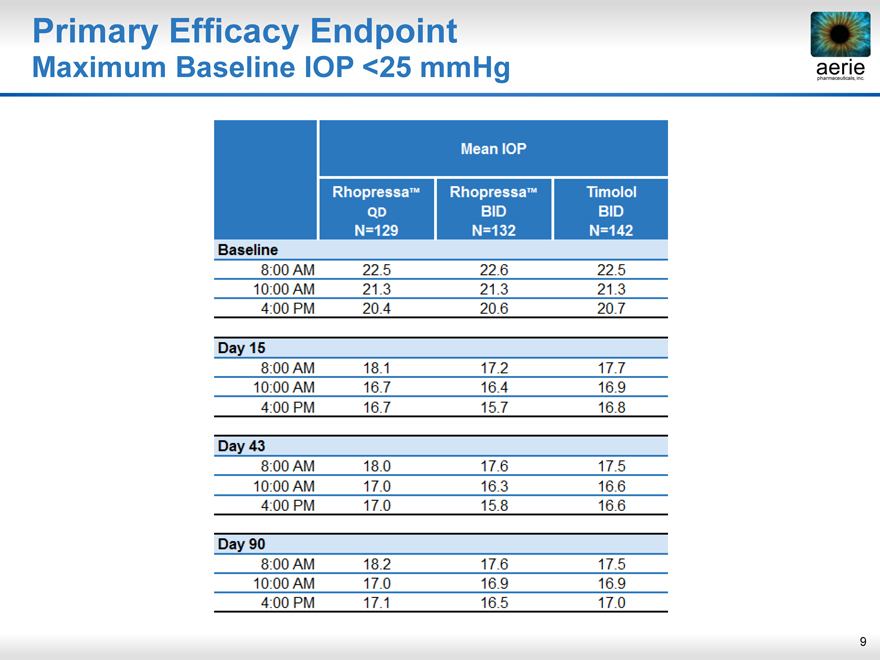
Primary Efficacy Endpoint
Maximum Baseline IOP <25 mmHg
Mean IOP
RhopressaTM qd N=129
RhopressaTM BID N=132
Timolol BID N=142
Baseline
8:00 AM 22.5 22.6 22.5
10:00 AM 21.3 21.3 21.3
4:00 PM 20.4 20.6 20.7
Day 15
8:00 AM 18.1 17.2 17.7
10:00 AM 16.7 16.4 16.9
4:00 PM 16.7 15.7 16.8
Day 43
8:00 AM 18.0 17.6 17.5
10:00 AM 17.0 16.3 16.6
4:00 PM 17.0 15.8 16.6
Day 90
8:00 AM 18.2 17.6 17.5
10:00 AM 17.0 16.9 16.9
4:00 PM 17.1 16.5 17.0
9
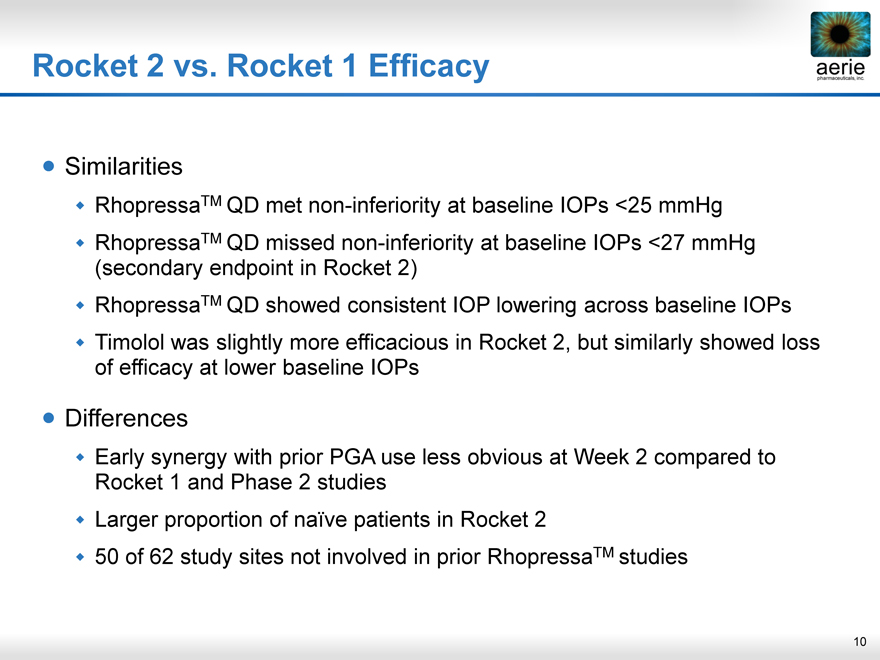
Rocket 2 vs. Rocket 1 Efficacy
Similarities
RhopressaTM QD met non-inferiority at baseline IOPs <25 mmHg
RhopressaTM QD missed non-inferiority at baseline IOPs <27 mmHg
(secondary endpoint in Rocket 2)
RhopressaTM QD showed consistent IOP lowering across baseline IOPs
Timolol was slightly more efficacious in Rocket 2, but similarly showed loss
of efficacy at lower baseline IOPs
Differences
Early synergy with prior PGA use less obvious at Week 2 compared to
Rocket 1 and Phase 2 studies
Larger proportion of naïve patients in Rocket 2
50 of 62 study sites not involved in prior RhopressaTM studies
10
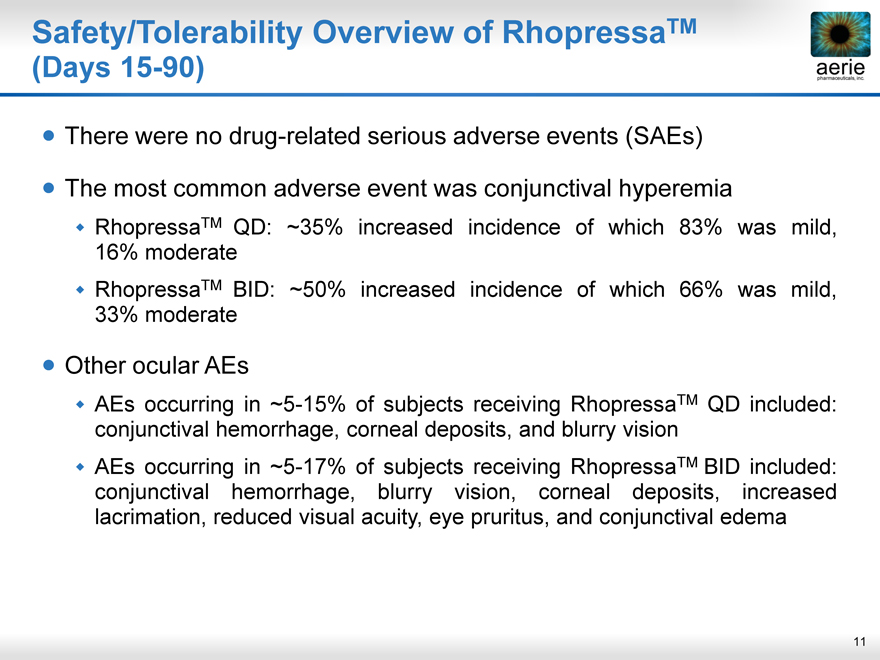
Safety/Tolerability Overview of RhopressaTM
(Days 15-90)
There were no drug-related serious adverse events (SAEs)
The most common adverse event was conjunctival hyperemia
RhopressaTM QD: ~35% increased incidence of which 83% was mild,
16% moderate
RhopressaTM BID: ~50% increased incidence of which 66% was mild,
33% moderate
Other ocular AEs
AEs occurring in ~5-15% of subjects receiving RhopressaTM QD included:
conjunctival hemorrhage, corneal deposits, and blurry vision
AEs occurring in ~5-17% of subjects receiving RhopressaTM BID included:
conjunctival hemorrhage, blurry vision, corneal deposits, increased
lacrimation, reduced visual acuity, eye pruritus, and conjunctival edema
11
RhopressaTM and RoclatanTM Next Steps
RhopressaTM
Rocket 2: First 100 patients on RhopressaTM 12-month safety results at
the end of 2015 or early 2016
On track to file our RhopressaTM NDA in mid-2016
Rocket 4: A fourth Phase 3 trial expected to commence by the end of
September 2015
RoclatanTM
Mercury 1: First Phase 3 12-month study expected to commence by the end of September 2015 Mercury 2: Phase 3 3-month study expected to commence in 2016 Mercury 3 (Europe): Efficacy study, comparing to a leading combo product marketed in Europe, expected to commence in H2 2016
12
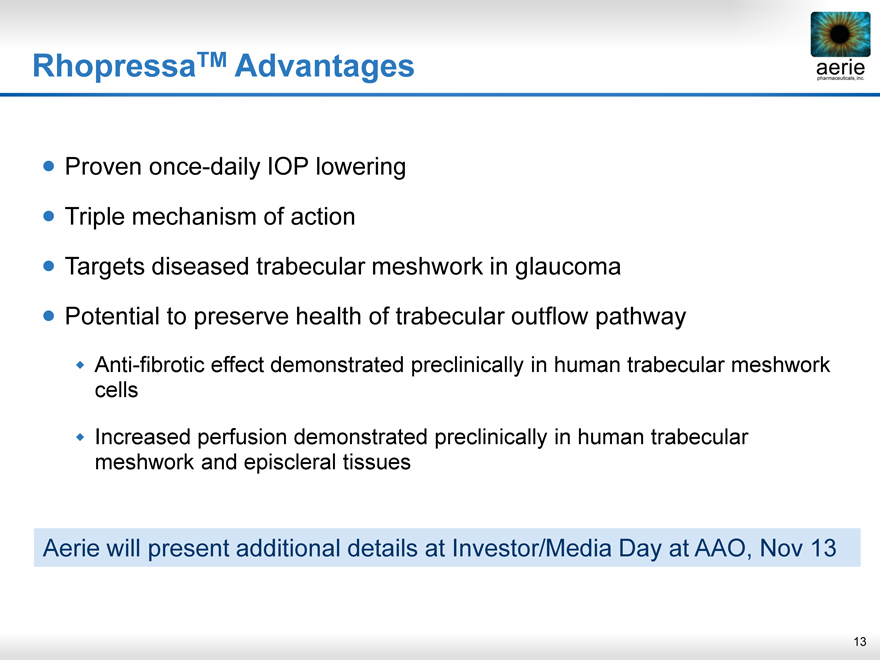
RhopressaTM Advantages
Proven once-daily IOP lowering Triple mechanism of action
Targets diseased trabecular meshwork in glaucoma Potential to preserve health of trabecular outflow pathway
Anti-fibrotic effect demonstrated preclinically in human trabecular meshwork cells
Increased perfusion demonstrated preclinically in human trabecular meshwork and episcleral tissues
Aerie will present additional details at Investor/Media Day at AAO, Nov 13
aerie pharamaceuticals, Inc.
13