
Aerie Pharmaceuticals, Inc. Investor and Media Day November 13, 2015 Building a Major Ophthalmic Pharmaceutical Company Exhibit 99.1
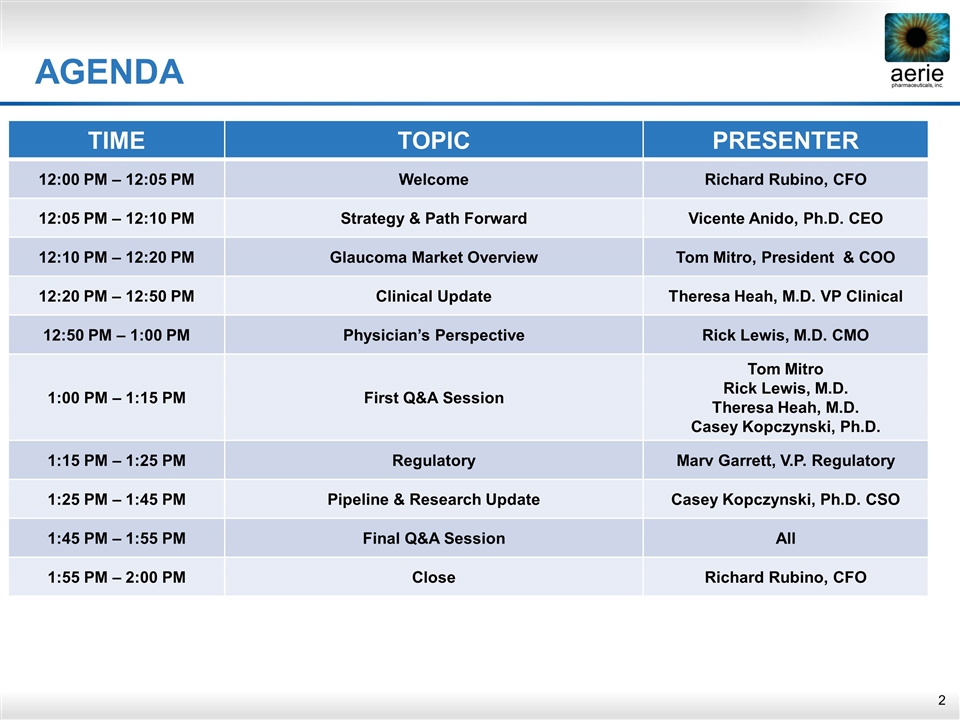
AGENDA TIME TOPIC PRESENTER 12:00 PM – 12:05 PM Welcome Richard Rubino, CFO 12:05 PM – 12:10 PM Strategy & Path Forward Vicente Anido, Ph.D. CEO 12:10 PM – 12:20 PM Glaucoma Market Overview Tom Mitro, President & COO 12:20 PM – 12:50 PM Clinical Update Theresa Heah, M.D. VP Clinical 12:50 PM – 1:00 PM Physician’s Perspective Rick Lewis, M.D. CMO 1:00 PM – 1:15 PM First Q&A Session Tom Mitro Rick Lewis, M.D. Theresa Heah, M.D. Casey Kopczynski, Ph.D. 1:15 PM – 1:25 PM Regulatory Marv Garrett, V.P. Regulatory 1:25 PM – 1:45 PM Pipeline & Research Update Casey Kopczynski, Ph.D. CSO 1:45 PM – 1:55 PM Final Q&A Session All 1:55 PM – 2:00 PM Close Richard Rubino, CFO

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. In addition, any discussion of clinical trial results for RhopressaTM relate to the results in its first Phase 3 registration trials, Rocket 1 and Rocket 2, and for RoclatanTM relate to the results in its Phase 2b clinical trial. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, the preclinical research discussed in this presentation is preliminary and the outcome of such preclinical studies may not be predictive of the outcome of later trials. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.

Our Strategy: Investor Day September 10, 2014 Advance the development of our product candidates to approval Establish internal capabilities to commercialize our product candidates in North America (and potentially Europe) Explore partnerships with leading pharmaceutical and biotechnology companies to maximize the value of our product candidates outside of North America Continue to leverage and strengthen our intellectual property portfolio Expand our product portfolio through internal discovery efforts and possible in-licensing or acquisitions of additional ophthalmic product candidates or products
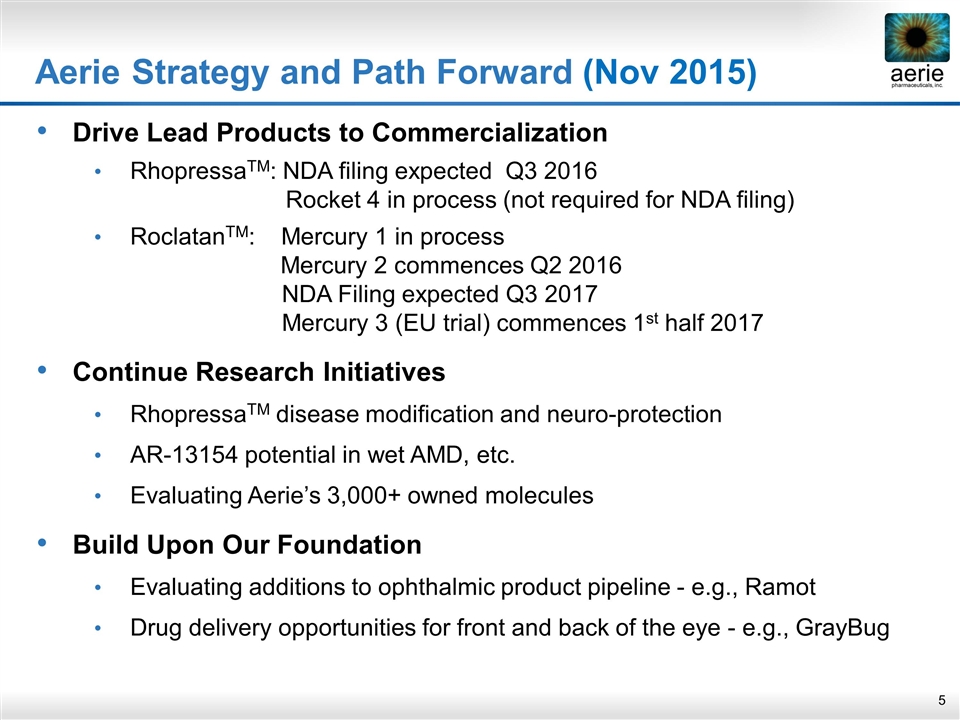
Aerie Strategy and Path Forward (Nov 2015) Drive Lead Products to Commercialization RhopressaTM: NDA filing expected Q3 2016 Rocket 4 in process (not required for NDA filing) RoclatanTM: Mercury 1 in process Mercury 2 commences Q2 2016 NDA Filing expected Q3 2017 Mercury 3 (EU trial) commences 1st half 2017 Continue Research Initiatives RhopressaTM disease modification and neuro-protection AR-13154 potential in wet AMD, etc. Evaluating Aerie’s 3,000+ owned molecules Build Upon Our Foundation Evaluating additions to ophthalmic product pipeline - e.g., Ramot Drug delivery opportunities for front and back of the eye - e.g., GrayBug

Glaucoma Market Overview Tom Mitro President and COO
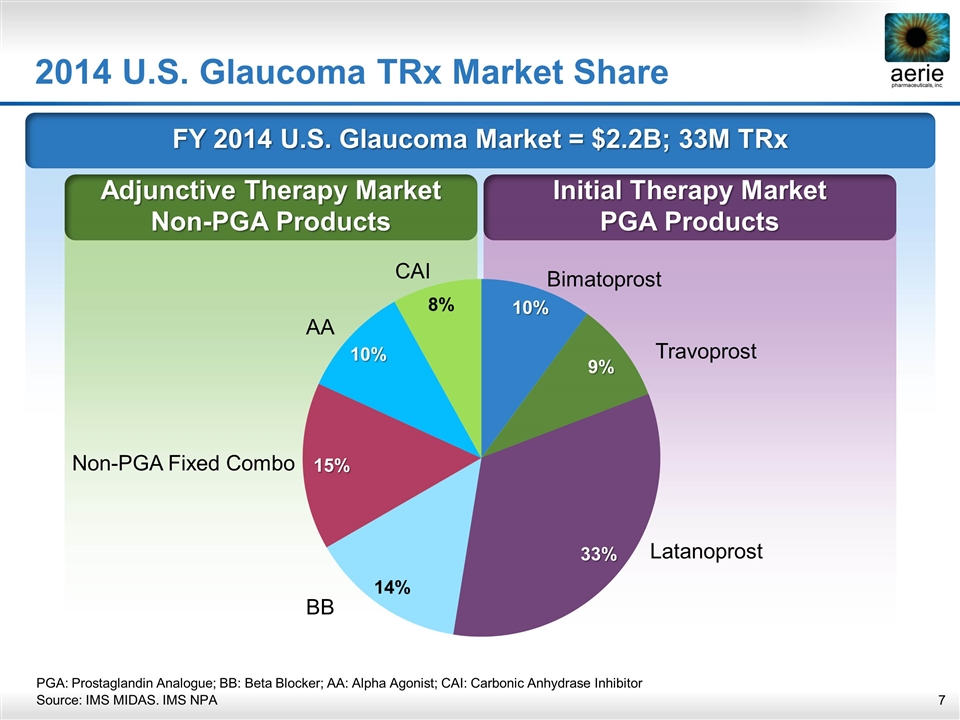
FY 2014 U.S. Glaucoma Market = $2.2B; 33M TRx Initial Therapy Market PGA Products Adjunctive Therapy Market Non-PGA Products 2014 U.S. Glaucoma TRx Market Share Bimatoprost Travoprost Latanoprost BB Non-PGA Fixed Combo AA CAI PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor Source: IMS MIDAS. IMS NPA
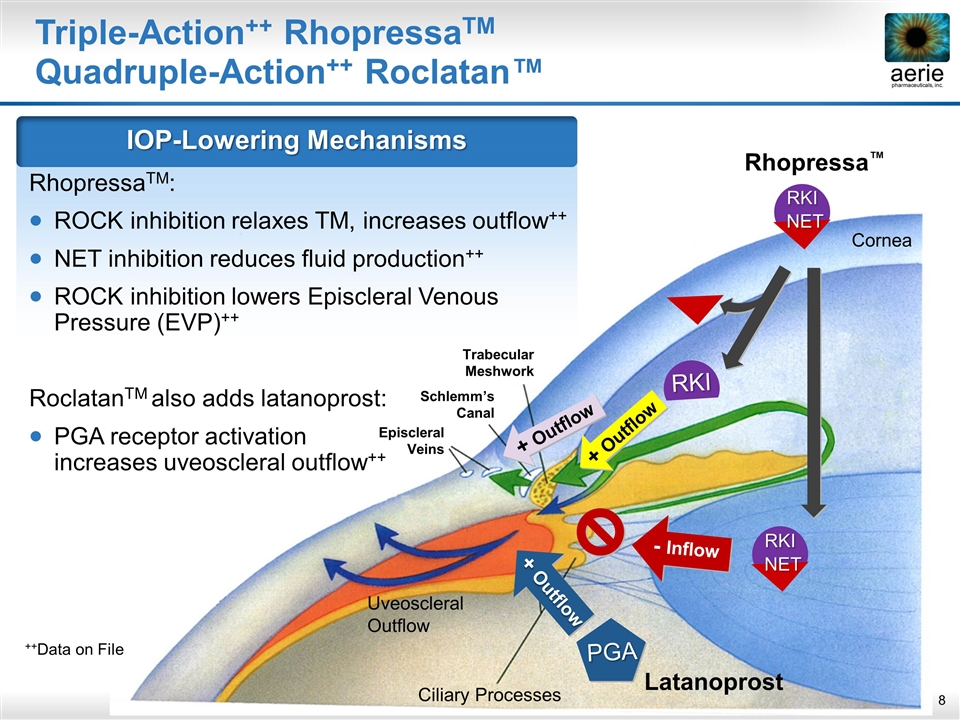
Ciliary Processes Cornea Uveoscleral Outflow + Outflow + Outflow RKI NET RKI NET RKI - Inflow Trabecular Meshwork Episcleral Veins Schlemm’s Canal Latanoprost PGA + Outflow Rhopressa™ Triple-Action++ RhopressaTM Quadruple-Action++ Roclatan™ IOP-Lowering Mechanisms RhopressaTM: ROCK inhibition relaxes TM, increases outflow++ NET inhibition reduces fluid production++ ROCK inhibition lowers Episcleral Venous Pressure (EVP)++ RoclatanTM also adds latanoprost: PGA receptor activation increases uveoscleral outflow++ ++Data on File
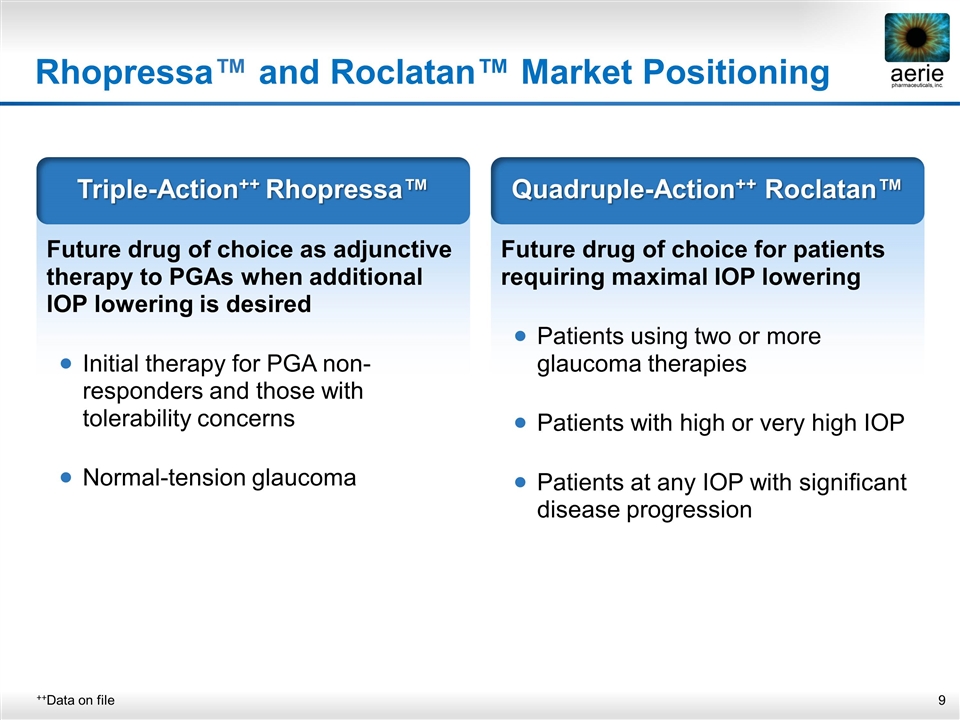
Rhopressa™ and Roclatan™ Market Positioning ++Data on file Future drug of choice as adjunctive therapy to PGAs when additional IOP lowering is desired Initial therapy for PGA non-responders and those with tolerability concerns Normal-tension glaucoma Triple-Action++ Rhopressa™ Future drug of choice for patients requiring maximal IOP lowering Patients using two or more glaucoma therapies Patients with high or very high IOP Patients at any IOP with significant disease progression Quadruple-Action++ Roclatan™
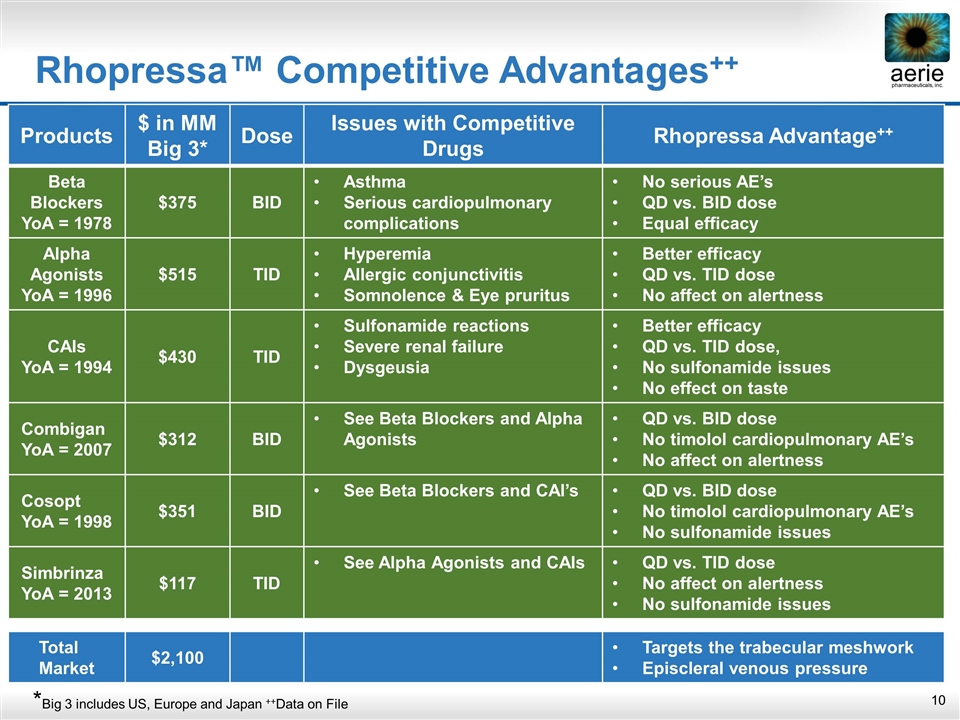
Products $ in MM Big 3* Dose Issues with Competitive Drugs Rhopressa Advantage++ Beta Blockers YoA = 1978 $375 BID Asthma Serious cardiopulmonary complications No serious AE’s QD vs. BID dose Equal efficacy Alpha Agonists YoA = 1996 $515 TID Hyperemia Allergic conjunctivitis Somnolence & Eye pruritus Better efficacy QD vs. TID dose No affect on alertness CAIs YoA = 1994 $430 TID Sulfonamide reactions Severe renal failure Dysgeusia Better efficacy QD vs. TID dose, No sulfonamide issues No effect on taste Combigan YoA = 2007 $312 BID See Beta Blockers and Alpha Agonists QD vs. BID dose No timolol cardiopulmonary AE’s No affect on alertness Cosopt YoA = 1998 $351 BID See Beta Blockers and CAI’s QD vs. BID dose No timolol cardiopulmonary AE’s No sulfonamide issues Simbrinza YoA = 2013 $117 TID See Alpha Agonists and CAIs QD vs. TID dose No affect on alertness No sulfonamide issues Total Market $2,100 Targets the trabecular meshwork Episcleral venous pressure Rhopressa™ Competitive Advantages++ *Big 3 includes US, Europe and Japan ++Data on File

Summary of Rhopressa™ Market Opportunities $2.1 Billion in the “Big 3”* Adjunctive Glaucoma Market Rhopressa™ has a number of unique advantages++ Targeting the Trabecular Meshwork and lowering Episcleral Venous Pressure QD dosing No systemic side effects Better efficacy than Alpha Agonists and CAIs Rhopressa™ is expected to be the only QD adjunctive therapy with no systemic side effects *Big 3 includes US, Europe and Japan ++Data on File
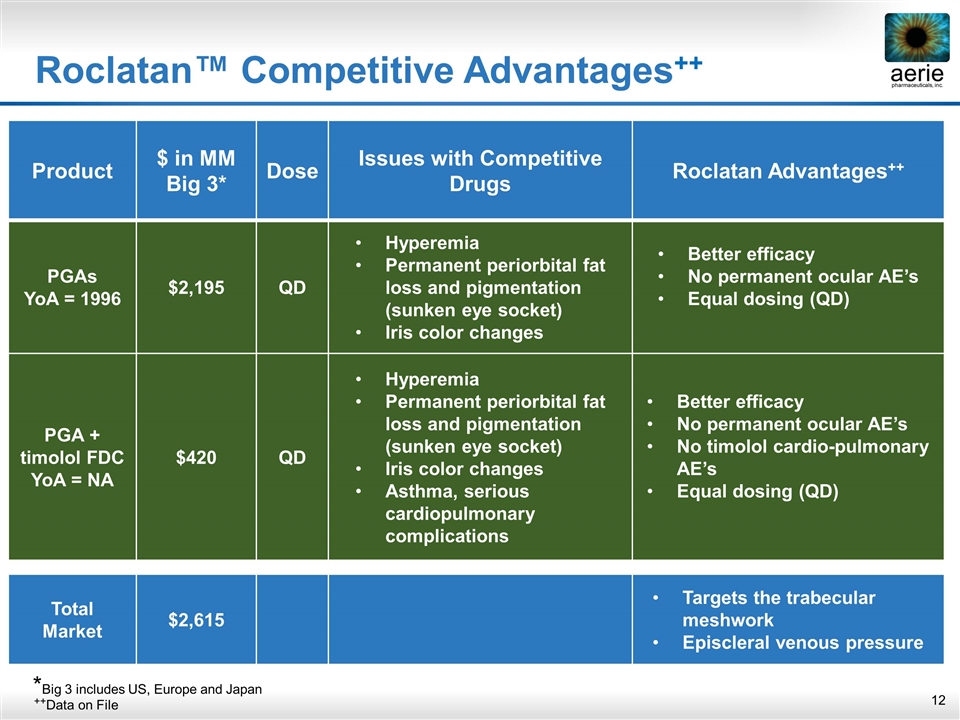
Product $ in MM Big 3* Dose Issues with Competitive Drugs Roclatan Advantages++ PGAs YoA = 1996 $2,195 QD Hyperemia Permanent periorbital fat loss and pigmentation (sunken eye socket) Iris color changes Better efficacy No permanent ocular AE’s Equal dosing (QD) PGA + timolol FDC YoA = NA $420 QD Hyperemia Permanent periorbital fat loss and pigmentation (sunken eye socket) Iris color changes Asthma, serious cardiopulmonary complications Better efficacy No permanent ocular AE’s No timolol cardio-pulmonary AE’s Equal dosing (QD) Total Market $2,615 Targets the trabecular meshwork Episcleral venous pressure Roclatan™ Competitive Advantages++ ++Data on File *Big 3 includes US, Europe and Japan

Glaucoma Market: Key Observations The 150MM TRx and $4.7B Glaucoma Market* is Very Large and is Dominated by Very Old Products Rhopressa™ and Roclatan™ expected to be enthusiastically embraced by prescribers: Rhopressa™ will bring new discussions (restoring TM outflow, EVP, triple-action) into the market Roclatan™ could set new Global standard for IOP lowering In addition, Rhopressa™ & Roclatan™++: are QD have shown no systemic side effects in clinical trials could both offer much more, if the disease modification efforts continue to be researched and proven Glaucoma products are not a focal point with MCO’s: Interviews with 12 MCOs covering 42+ million lives found 100% recommended Tier 2 or 3 for Rhopressa and Roclatan, no “Not Covered” recommendations++ *Includes US, Europe and Japan ++Data on File

Rhopressa™ Commercialization Plan Now in Launch Mode Will begin identifying and hiring additional marketing and sales leadership personnel Podium and press to educate the market Development of launch strategies and plans Educate glaucoma KOLs (U.S., Europe and Japan) Development and testing of key product messages North American sales and marketing organization to support 100 sales representatives calling on 11,000 Ophthalmologists Cost per sales representative: ~$275K

Rhopressa™ / Roclatan™ Clinical Update Theresa Heah, MD VP, Clinical Research and Medical Affairs
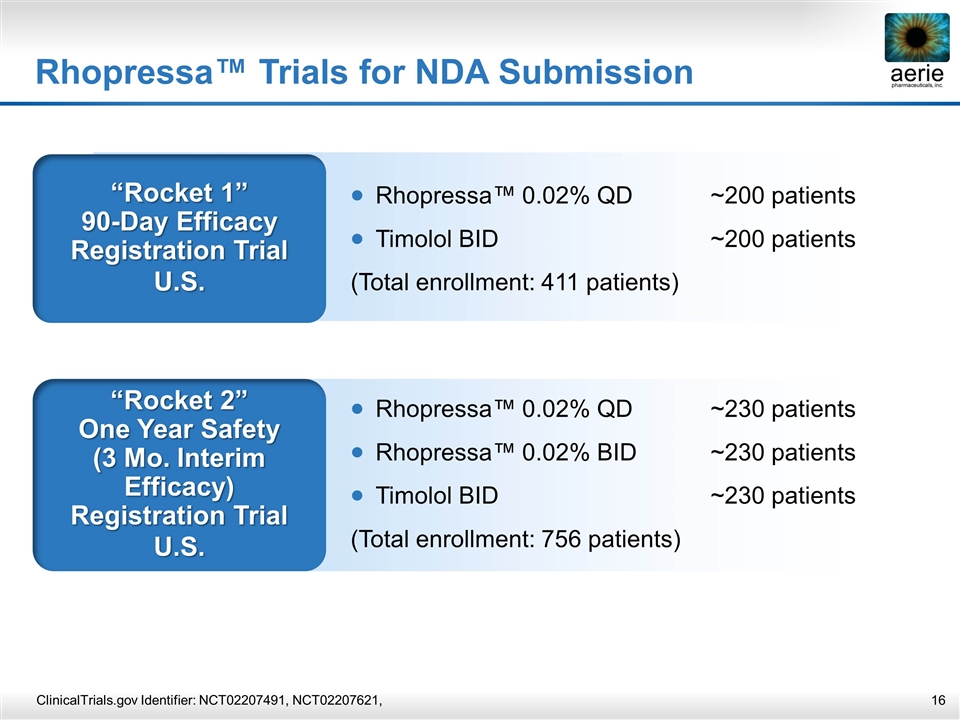
Rhopressa™ Trials for NDA Submission “Rocket 1” 90-Day Efficacy Registration Trial U.S. Rhopressa™ 0.02% QD~200 patients Timolol BID~200 patients (Total enrollment: 411 patients) “Rocket 2” One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. Rhopressa™ 0.02% QD~230 patients Rhopressa™ 0.02% BID~230 patients Timolol BID~230 patients (Total enrollment: 756 patients) ClinicalTrials.gov Identifier: NCT02207491, NCT02207621,
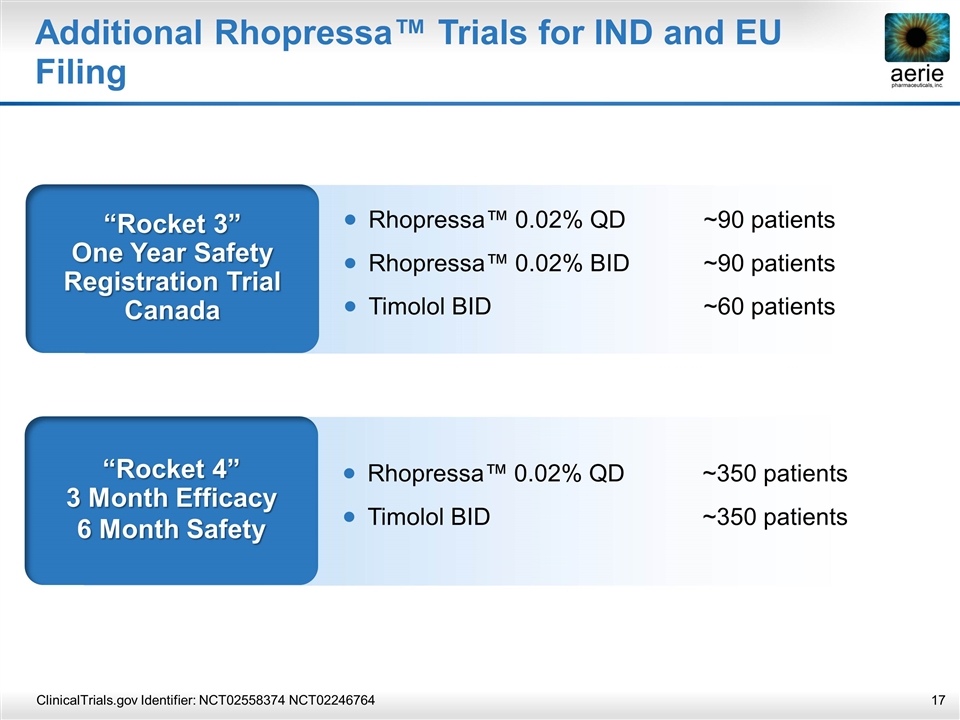
Additional Rhopressa™ Trials for IND and EU Filing ClinicalTrials.gov Identifier: NCT02558374 NCT02246764 “Rocket 4” 3 Month Efficacy 6 Month Safety Rhopressa™ 0.02% QD~350 patients Timolol BID~350 patients “Rocket 3” One Year Safety Registration Trial Canada Rhopressa™ 0.02% QD~90 patients Rhopressa™ 0.02% BID ~90 patients Timolol BID~60 patients

Rhopressa™ Registration Trial Overview AR-13324 CS301 and CS302 study protocols, FDA End of Phase 2 meeting minutes, Pre-NDA meeting minutes Non-inferiority design vs. timolol 95% CI within 1.5 mmHg at all time points, within 1.0 mmHg at a majority of time points Rocket 2 primary efficacy endpoint: mean IOP at all time points through Day 90 (for maximum baseline IOP <25 mmHg) Data from Rocket 1 is supportive FDA discussions on design of Phase 3 studies Minimum of 100 RhopressaTM QD patients with 12 months of safety data needed for NDA filing Should meet efficacy requirements for European filing Rocket 4 adds adequate RhopressaTM patients for 6 months EU safety data
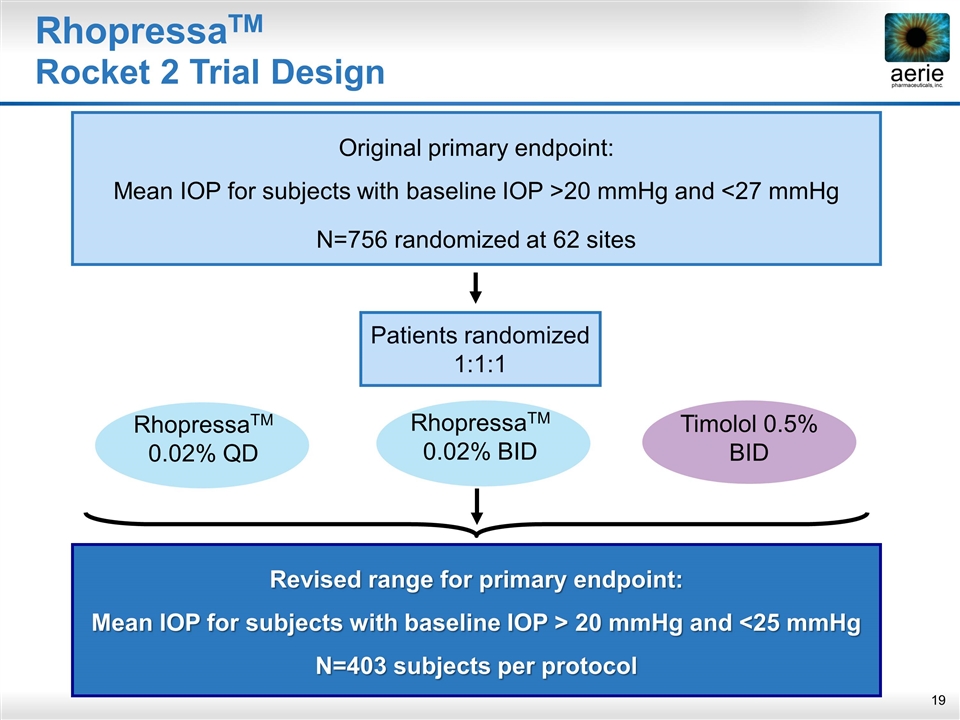
Patients randomized 1:1:1 Revised range for primary endpoint: Mean IOP for subjects with baseline IOP > 20 mmHg and <25 mmHg N=403 subjects per protocol Original primary endpoint: Mean IOP for subjects with baseline IOP >20 mmHg and <27 mmHg N=756 randomized at 62 sites Timolol 0.5% BID RhopressaTM Rocket 2 Trial Design RhopressaTM 0.02% QD RhopressaTM 0.02% BID
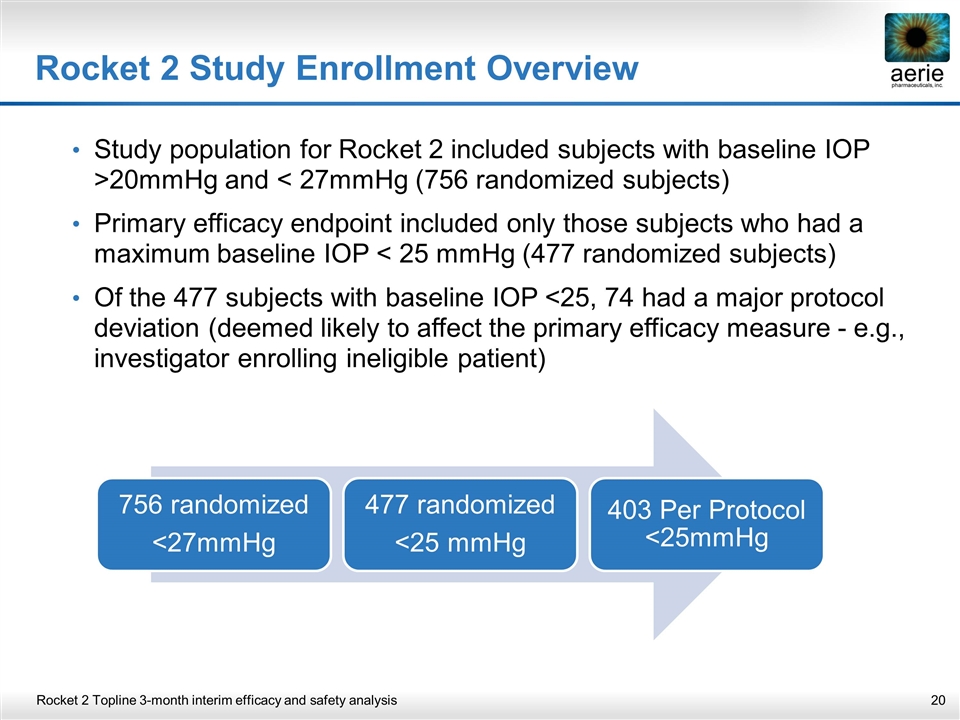
Rocket 2 Study Enrollment Overview Study population for Rocket 2 included subjects with baseline IOP >20mmHg and < 27mmHg (756 randomized subjects) Primary efficacy endpoint included only those subjects who had a maximum baseline IOP < 25 mmHg (477 randomized subjects) Of the 477 subjects with baseline IOP <25, 74 had a major protocol deviation (deemed likely to affect the primary efficacy measure - e.g., investigator enrolling ineligible patient) Rocket 2 Topline 3-month interim efficacy and safety analysis 756 randomized <27mmHg 477 randomized <25 mmHg 403 Per Protocol <25mmHg
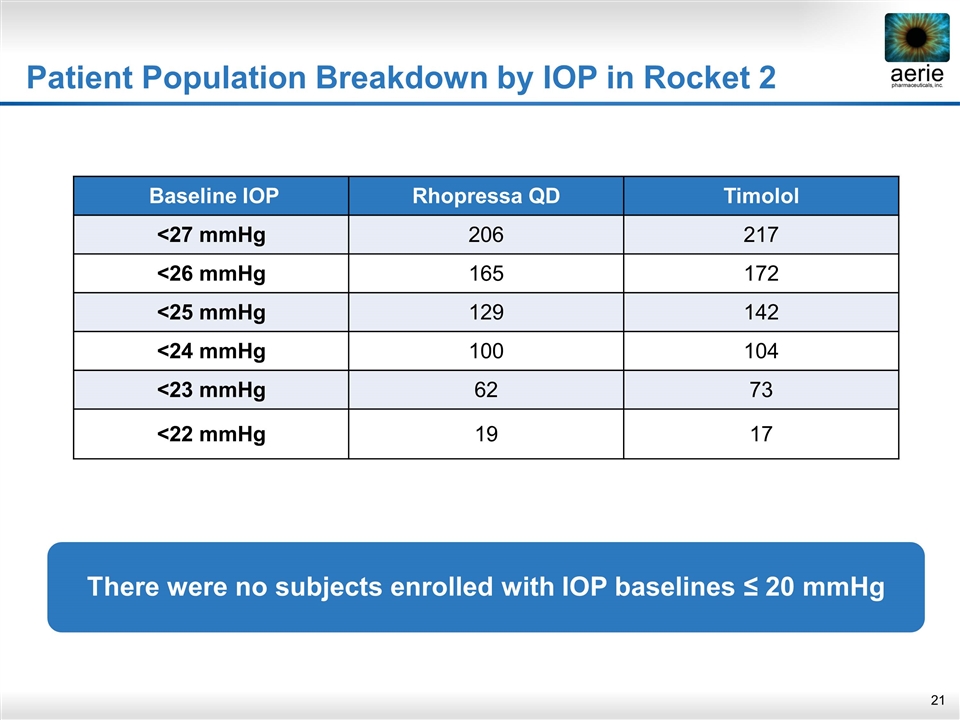
Patient Population Breakdown by IOP in Rocket 2 Baseline IOP Rhopressa QD Timolol <27 mmHg 206 217 <26 mmHg 165 172 <25 mmHg 129 142 <24 mmHg 100 104 <23 mmHg 62 73 <22 mmHg 19 17 There were no subjects enrolled with IOP baselines ≤ 20 mmHg
Study Conduct Of Intraocular Pressure Measurements Measuring Intraocular Pressure (IOP) – Goldmann Applanation Tonometry webeye.ophth.uiowa.edu Goldmann is standard against which others are measured Inter- and intra-observer variability (>30% varied by 2-3 mmHg) Protocol-specified clinical technique results in ~0.5 mmHg variability in IOP measurements
Rocket 2 Statistical Analysis Rocket 2 Statistical Analysis Plan, RhopressaTM End of Phase 2 FDA meeting minutes Intent-to-Treat (ITT) population (477 subjects) Included all randomized subjects who received at least one dose of study medication Per Protocol (PP) population (403 subjects) A subset of the ITT population Was the secondary population for efficacy analyses Used to summarize a subset of efficacy variables Included those subjects (and their visits) who did not have major protocol violations likely to seriously affect the primary outcome of the study Used to summarize all efficacy variables Was the primary population for efficacy analyses Both the PP and ITT efficacy analyses will be submitted in the NDA as agreed with the FDA
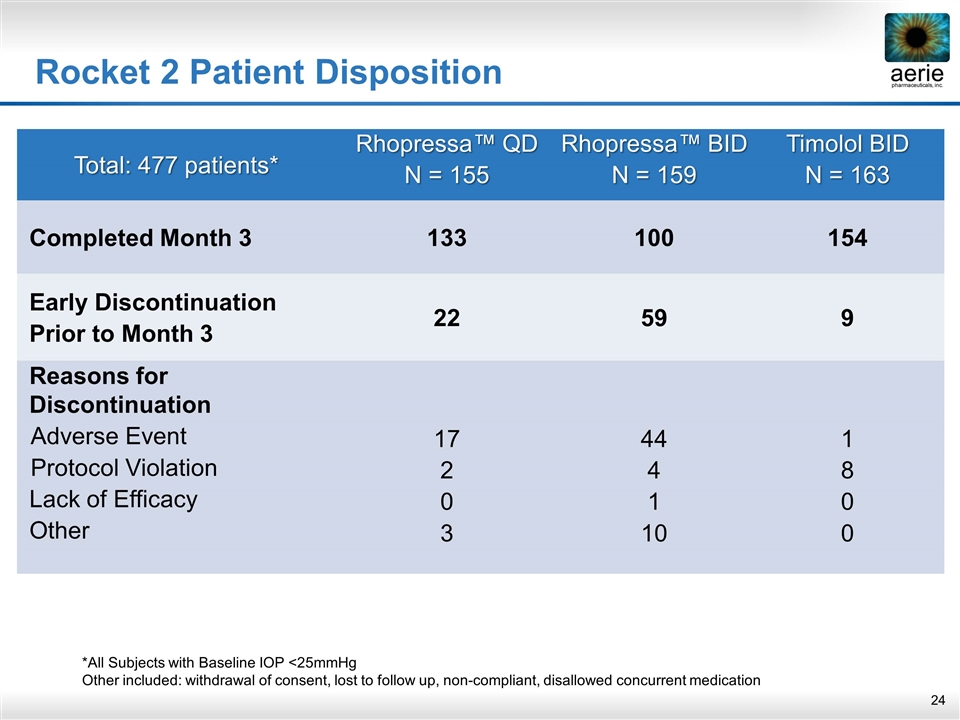
Rocket 2 Patient Disposition Total: 477 patients* Rhopressa™ QD N = 155 Rhopressa™ BID N = 159 Timolol BID N = 163 Completed Month 3 133 100 154 Early Discontinuation Prior to Month 3 22 59 9 Reasons for Discontinuation Adverse Event Protocol Violation Lack of Efficacy Other 17 2 0 3 44 4 1 10 1 8 0 0 *All Subjects with Baseline IOP <25mmHg Other included: withdrawal of consent, lost to follow up, non-compliant, disallowed concurrent medication
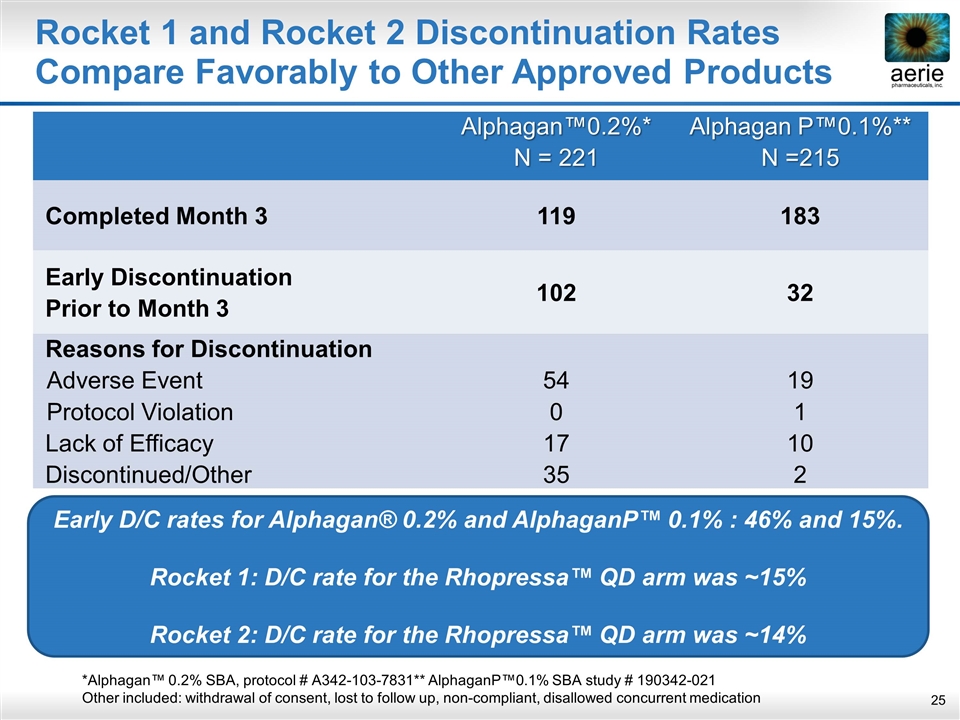
Rocket 1 and Rocket 2 Discontinuation Rates Compare Favorably to Other Approved Products Alphagan™0.2%* N = 221 Alphagan P™0.1%** N =215 Completed Month 3 119 183 Early Discontinuation Prior to Month 3 102 32 Reasons for Discontinuation Adverse Event Protocol Violation Lack of Efficacy Discontinued/Other 54 0 17 35 19 1 10 2 *Alphagan™ 0.2% SBA, protocol # A342-103-7831** AlphaganP™0.1% SBA study # 190342-021 Other included: withdrawal of consent, lost to follow up, non-compliant, disallowed concurrent medication Early D/C rates for Alphagan® 0.2% and AlphaganP™ 0.1% : 46% and 15%. Rocket 1: D/C rate for the Rhopressa™ QD arm was ~15% Rocket 2: D/C rate for the Rhopressa™ QD arm was ~14%
Rocket 2 Achieves Primary Clinical Endpoint RhopressaTM QD and RhopressaTM BID met the criteria for non-inferiority to timolol BID for the primary efficacy analysis (baseline IOP <25 mmHg) RhopressaTM QD showed stable efficacy from Week 2 to Month 3 Consistent efficacy results across multiple statistical analysis imputations (PP/ITT/LOCF) NDA submission for RhopressaTM QD with Rocket 1 and 2 as agreed with the FDA Focus on Rhopressa™ QD vs. Timolol BID only
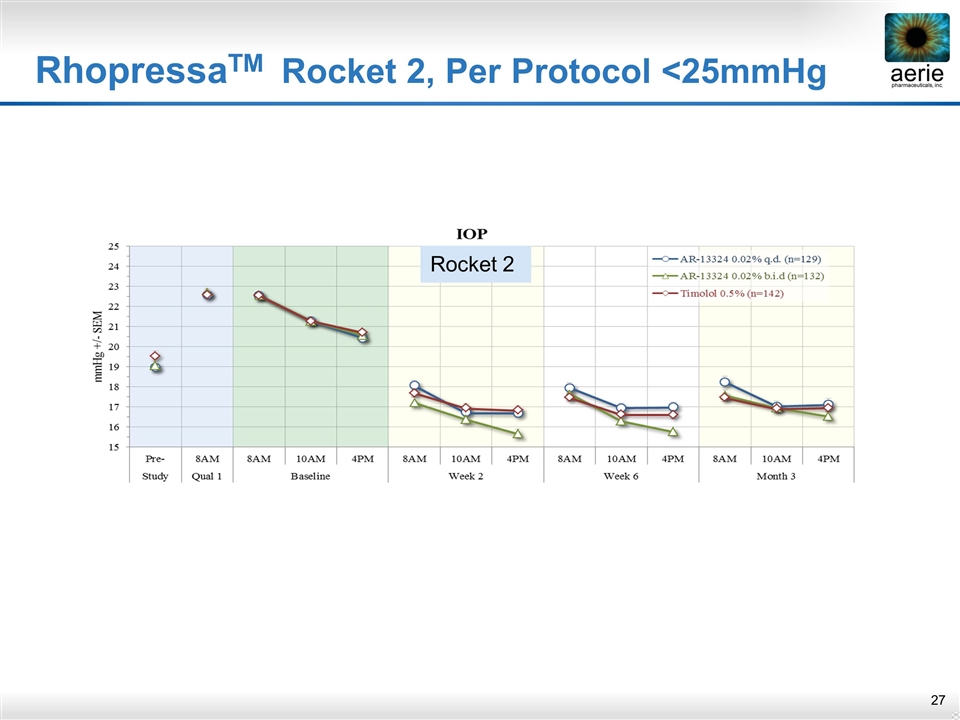
RhopressaTM Rocket 2, Per Protocol <25mmHg IOP Rocket 2
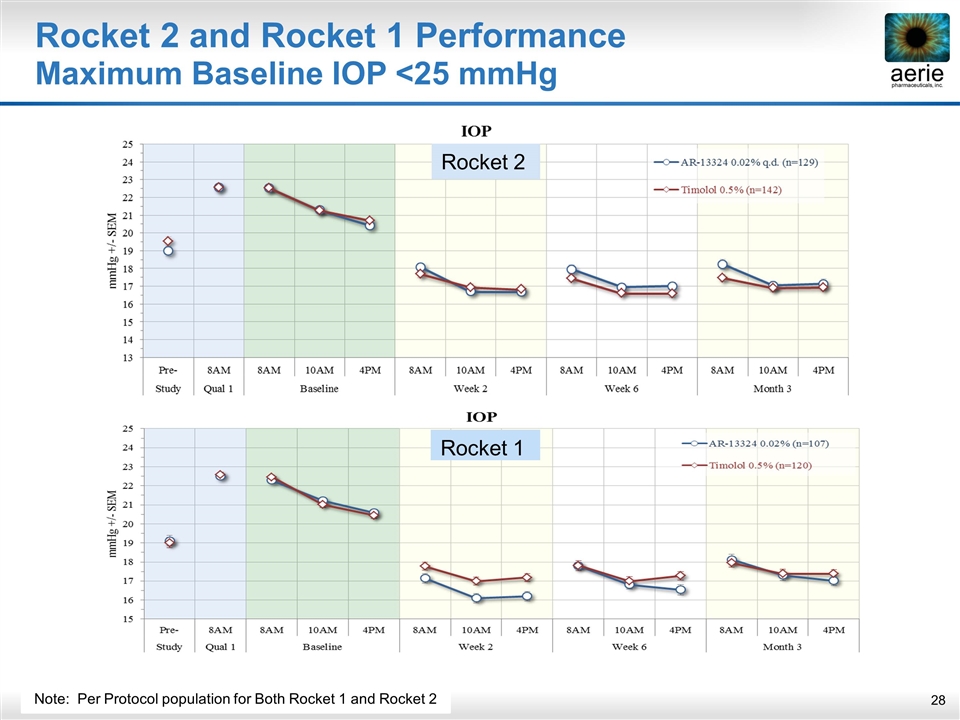
Rocket 2 and Rocket 1 Performance Maximum Baseline IOP <25 mmHg Rocket 1 Note: Per Protocol population for Both Rocket 1 and Rocket 2 Rocket 2
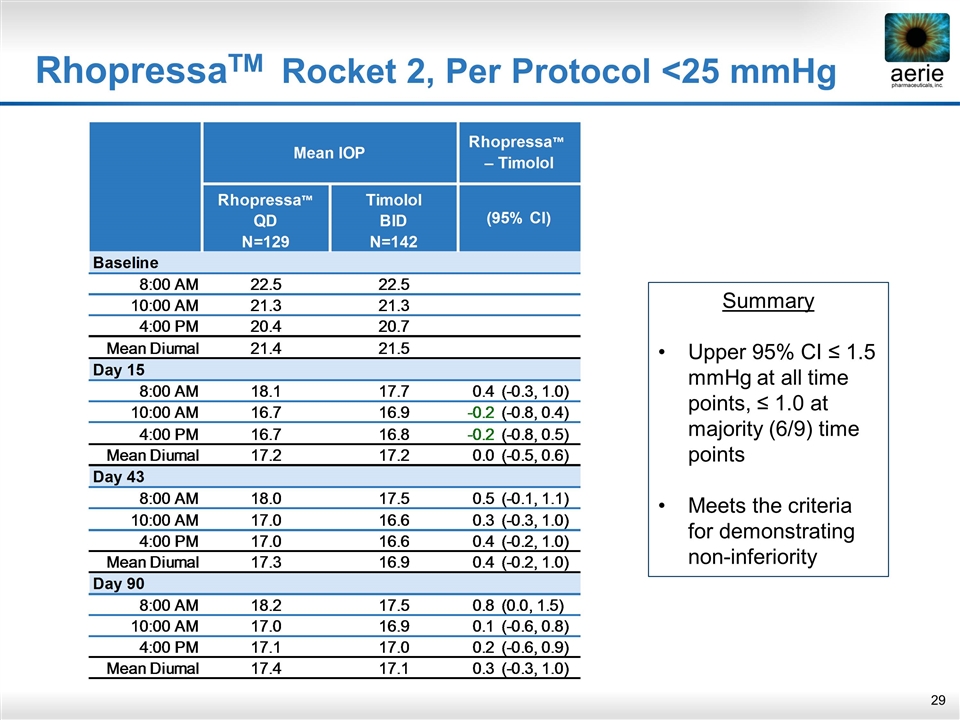
RhopressaTM Rocket 2, Per Protocol <25 mmHg Summary Upper 95% CI ≤ 1.5 mmHg at all time points, ≤ 1.0 at majority (6/9) time points Meets the criteria for demonstrating non-inferiority
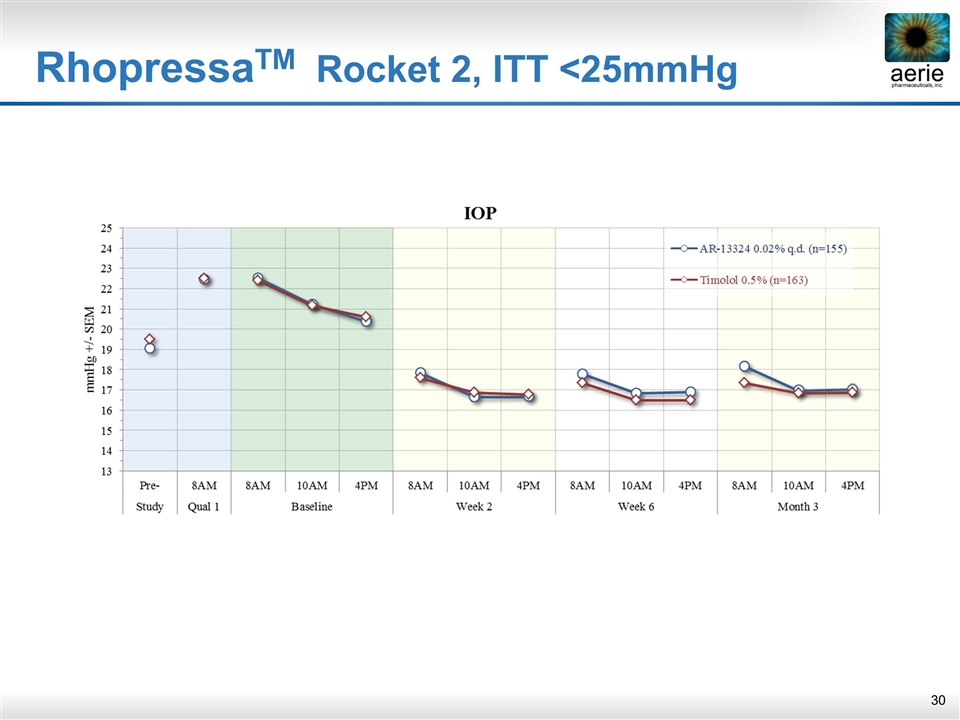
RhopressaTM Rocket 2, ITT <25mmHg

Similar Results with ITT Population RhopressaTM Rocket 2, ITT <25mmHg Summary Upper 95% CI ≤ 1.5 mmHg at all time points, ≤ 1.0 at majority (8/9) time points Meets the criteria for demonstrating non-inferiority
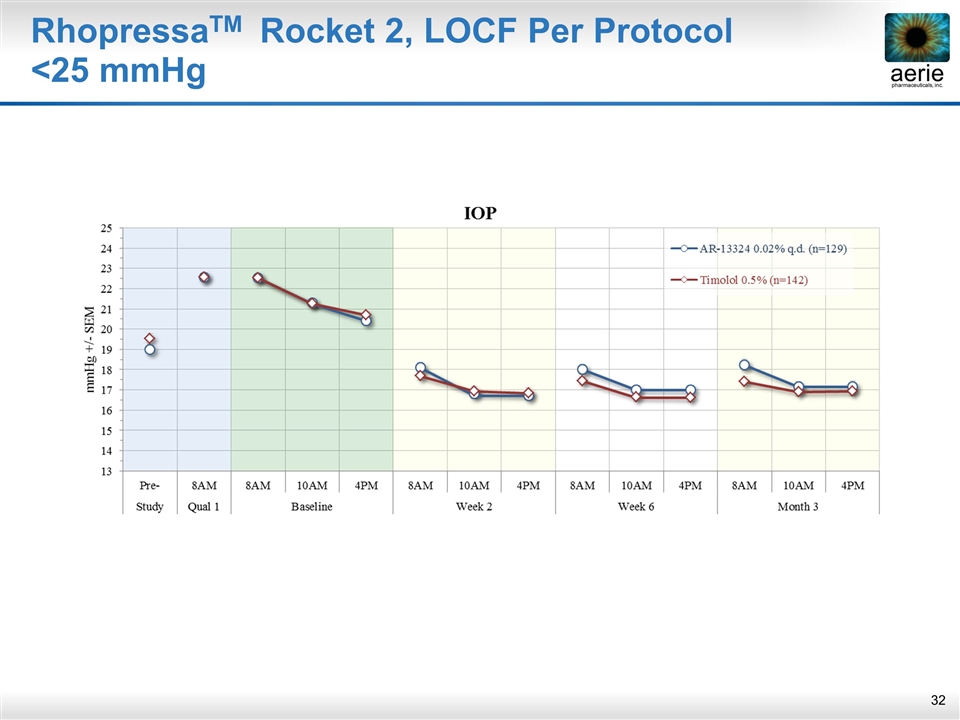
RhopressaTM Rocket 2, LOCF Per Protocol <25 mmHg
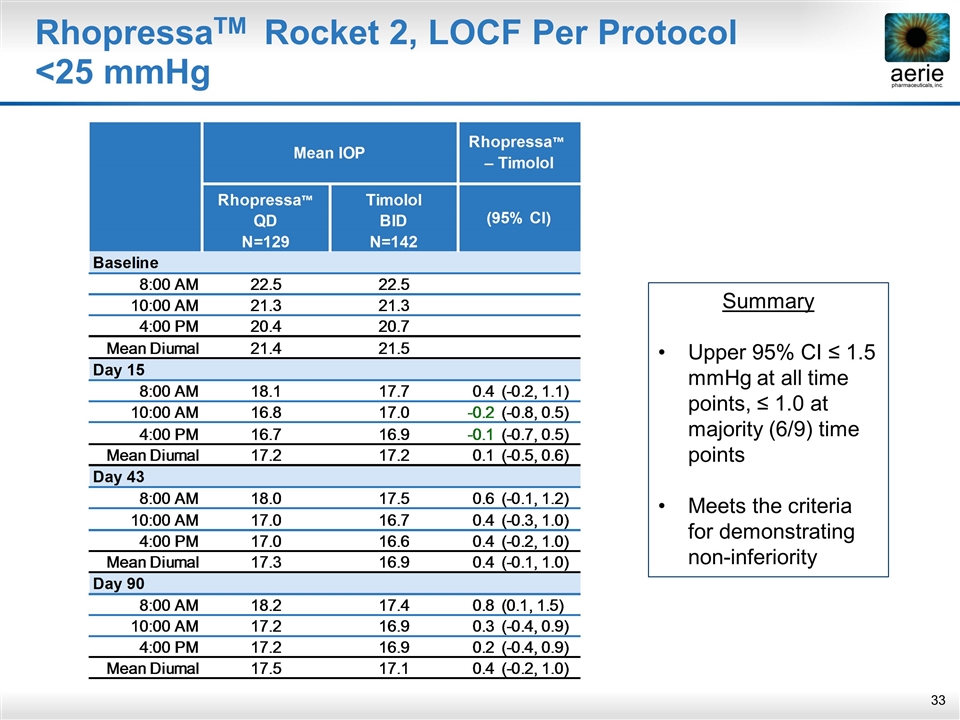
RhopressaTM Rocket 2, LOCF Per Protocol <25 mmHg Summary Upper 95% CI ≤ 1.5 mmHg at all time points, ≤ 1.0 at majority (6/9) time points Meets the criteria for demonstrating non-inferiority
Rocket 2: No Significant Effect of Discontinuations on Efficacy Results The Per Protocol population included data from subjects who discontinued the study early Statistical methods for imputation of missing data (such as LOCF) were used to test the robustness of the data Efficacy results were similar for Per Protocol and imputation analyses
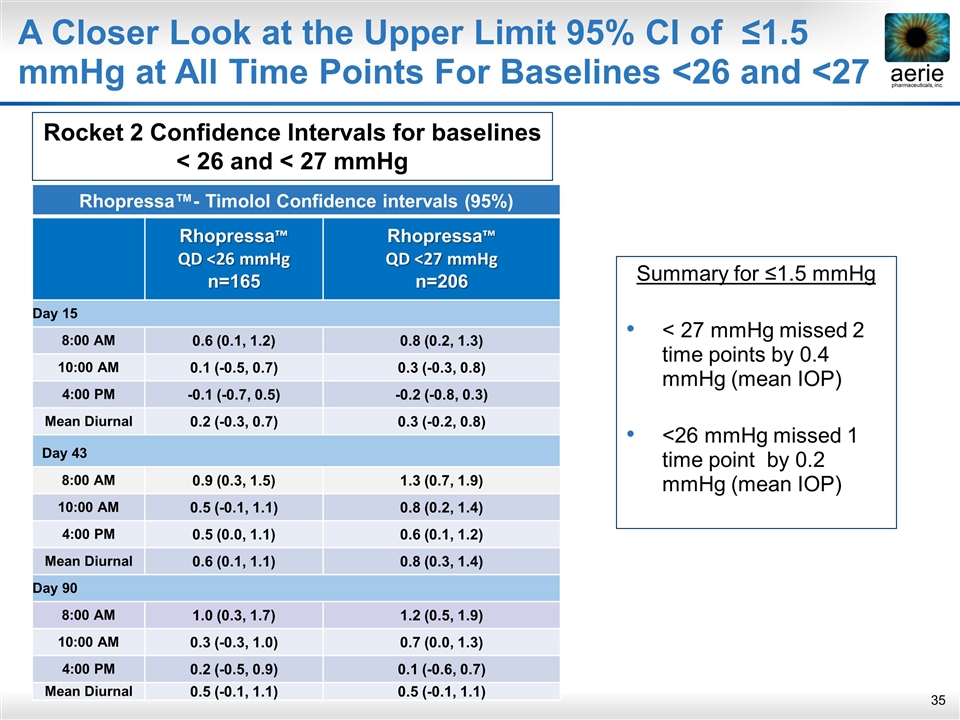
A Closer Look at the Upper Limit 95% CI of ≤1.5 mmHg at All Time Points For Baselines <26 and <27 Summary for ≤1.5 mmHg < 27 mmHg missed 2 time points by 0.4 mmHg (mean IOP) <26 mmHg missed 1 time point by 0.2 mmHg (mean IOP) Rhopressa™- Timolol Confidence intervals (95%) Rhopressa™ QD <26 mmHg n=165 Rhopressa™ QD <27 mmHg n=206 Day 15 8:00 AM 0.6 (0.1, 1.2) 0.8 (0.2, 1.3) 10:00 AM 0.1 (-0.5, 0.7) 0.3 (-0.3, 0.8) 4:00 PM -0.1 (-0.7, 0.5) -0.2 (-0.8, 0.3) Mean Diurnal 0.2 (-0.3, 0.7) 0.3 (-0.2, 0.8) Day 43 8:00 AM 0.9 (0.3, 1.5) 1.3 (0.7, 1.9) 10:00 AM 0.5 (-0.1, 1.1) 0.8 (0.2, 1.4) 4:00 PM 0.5 (0.0, 1.1) 0.6 (0.1, 1.2) Mean Diurnal 0.6 (0.1, 1.1) 0.8 (0.3, 1.4) Day 90 8:00 AM 1.0 (0.3, 1.7) 1.2 (0.5, 1.9) 10:00 AM 0.3 (-0.3, 1.0) 0.7 (0.0, 1.3) 4:00 PM 0.2 (-0.5, 0.9) 0.1 (-0.6, 0.7) Mean Diurnal 0.5 (-0.1, 1.1) 0.5 (-0.1, 1.1) Rocket 2 Confidence Intervals for baselines < 26 and < 27 mmHg
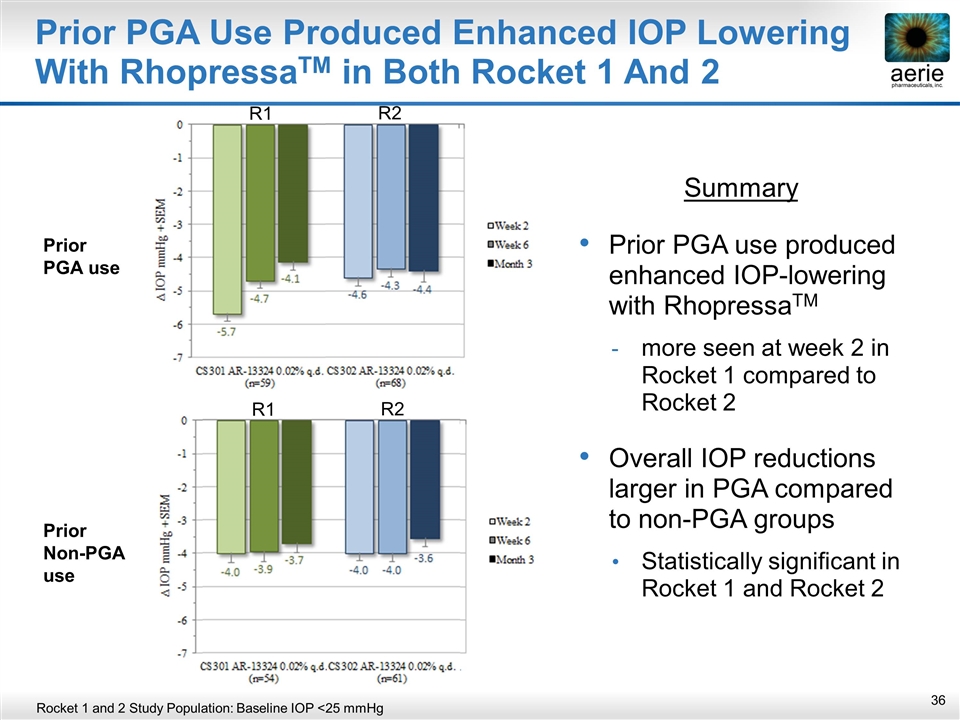
Prior PGA Use Produced Enhanced IOP Lowering With RhopressaTM in Both Rocket 1 And 2 Summary Prior PGA use produced enhanced IOP-lowering with RhopressaTM more seen at week 2 in Rocket 1 compared to Rocket 2 Overall IOP reductions larger in PGA compared to non-PGA groups Statistically significant in Rocket 1 and Rocket 2 Prior PGA use R1 R2 Prior Non-PGA use R1 R2 Rocket 1 and 2 Study Population: Baseline IOP <25 mmHg
Summary of Patients Using PGAs Prior to Rhopressa™ in Clinical Trials There was a synergistic effect with patients previously on a PGA in Rocket 2 that was more pronounced in Rocket 1 Effect was statistically significant in Rocket 1 and Rocket 2 Differences between Rocket 2 and Rocket 1 Rhopressa™ efficacy may have resulted from a more effective washout of PGAs in the Rocket 2 trial Retrospective analysis of Phase 2b trial results for Rhopressa™ and Roclatan™ shows prior PGA use enhanced RhopressaTM IOP-lowering by 1 mmHg (p=0.007) and 1.2 mmHg (p=0.002) at weeks 2 and 4, respectively, relative to subjects not previously on PGA
Rocket 2 QD Safety/Tolerability (Days 15-90) Rocket 2 3-month Topline results There were no drug-related serious adverse events (SAEs) The most common adverse event was conjunctival hyperemia RhopressaTM QD: ~35% increased incidence of which 83% was mild and16% was moderate Other ocular AEs AEs occurring in ~5-15% of subjects receiving RhopressaTM QD included: conjunctival hemorrhage, corneal deposits, and blurry vision
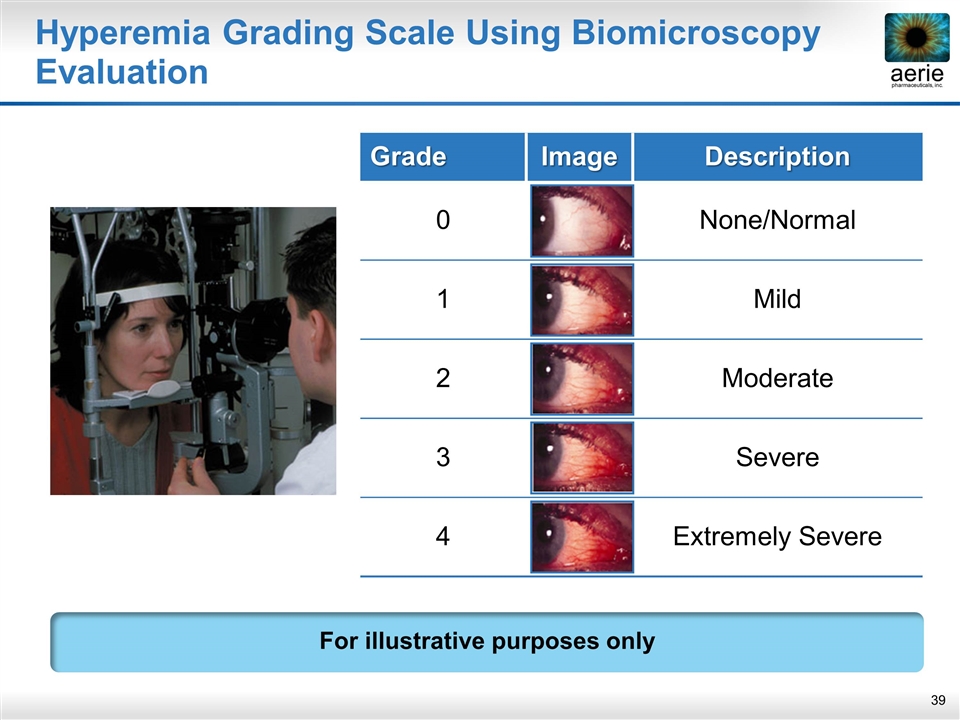
Hyperemia Grading Scale Using Biomicroscopy Evaluation For illustrative purposes only Grade Image Description 0 None/Normal 1 Mild 2 Moderate 3 Severe 4 Extremely Severe
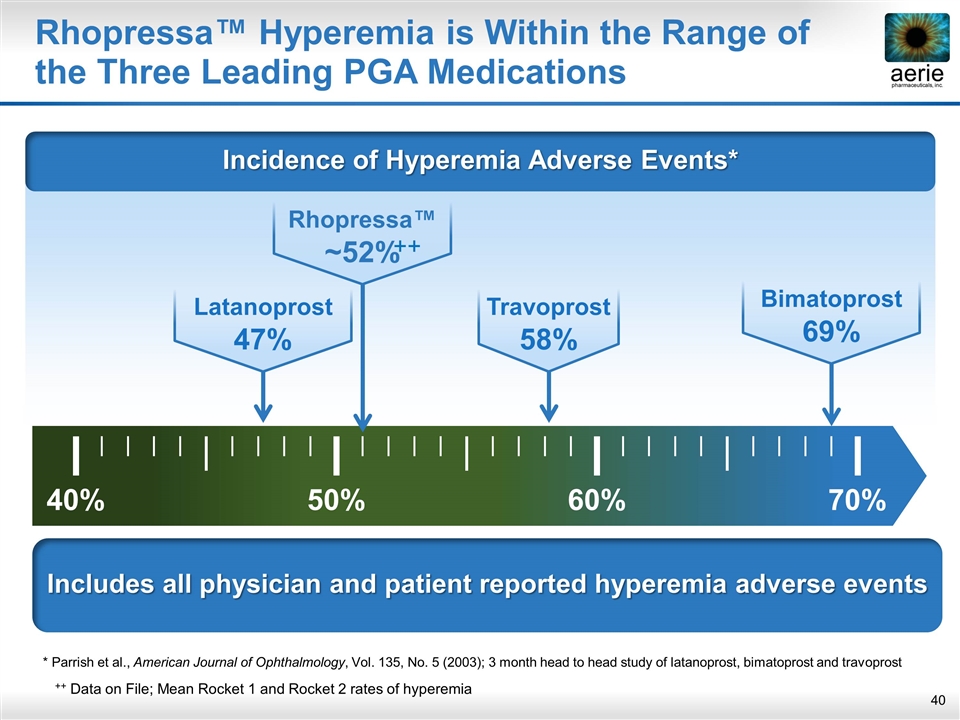
Includes all physician and patient reported hyperemia adverse events Rhopressa™ Hyperemia is Within the Range of the Three Leading PGA Medications * Parrish et al., American Journal of Ophthalmology, Vol. 135, No. 5 (2003); 3 month head to head study of latanoprost, bimatoprost and travoprost Latanoprost 47% 40% 50% 60% 70% Travoprost 58% Bimatoprost 69% Rhopressa™ ~52% ++ ++ Data on File; Mean Rocket 1 and Rocket 2 rates of hyperemia Incidence of Hyperemia Adverse Events*
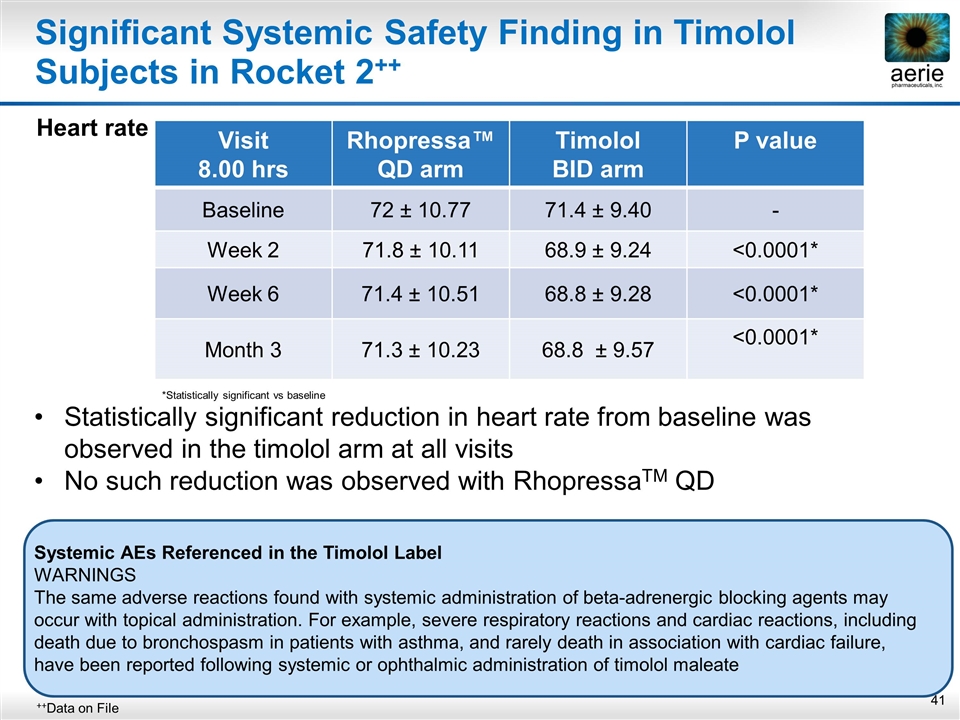
Significant Systemic Safety Finding in Timolol Subjects in Rocket 2++ Heart rate ++Data on File Visit 8.00 hrs Rhopressa™ QD arm Timolol BID arm P value Baseline 72 ± 10.77 71.4 ± 9.40 - Week 2 71.8 ± 10.11 68.9 ± 9.24 <0.0001* Week 6 71.4 ± 10.51 68.8 ± 9.28 <0.0001* Month 3 71.3 ± 10.23 68.8 ± 9.57 <0.0001* *Statistically significant vs baseline Statistically significant reduction in heart rate from baseline was observed in the timolol arm at all visits No such reduction was observed with RhopressaTM QD Systemic AEs Referenced in the Timolol Label WARNINGS The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported following systemic or ophthalmic administration of timolol maleate
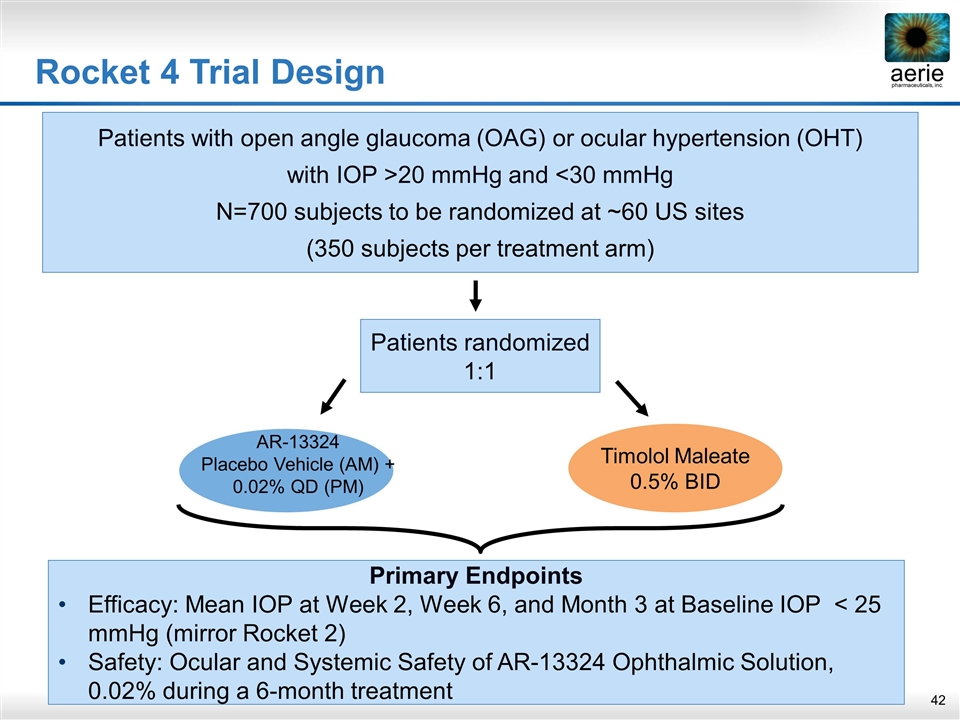
Patients randomized 1:1 Primary Endpoints Efficacy: Mean IOP at Week 2, Week 6, and Month 3 at Baseline IOP < 25 mmHg (mirror Rocket 2) Safety: Ocular and Systemic Safety of AR-13324 Ophthalmic Solution, 0.02% during a 6‑month treatment Patients with open angle glaucoma (OAG) or ocular hypertension (OHT) with IOP >20 mmHg and <30 mmHg N=700 subjects to be randomized at ~60 US sites (350 subjects per treatment arm) Timolol Maleate 0.5% BID AR-13324 Placebo Vehicle (AM) + 0.02% QD (PM) Rocket 4 Trial Design

Roclatan™ Update
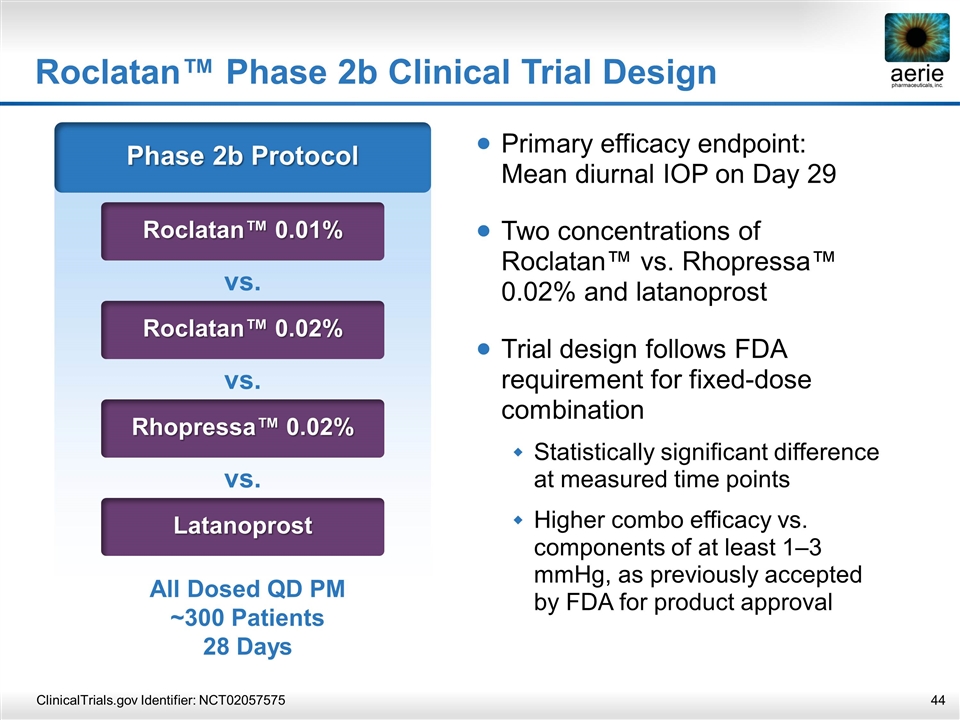
Roclatan™ Phase 2b Clinical Trial Design ClinicalTrials.gov Identifier: NCT02057575 Phase 2b Protocol Roclatan™ 0.01% vs. Roclatan™ 0.02% vs. Rhopressa™ 0.02% vs. Latanoprost All Dosed QD PM ~300 Patients 28 Days Primary efficacy endpoint: Mean diurnal IOP on Day 29 Two concentrations of Roclatan™ vs. Rhopressa™ 0.02% and latanoprost Trial design follows FDA requirement for fixed-dose combination Statistically significant difference at measured time points Higher combo efficacy vs. components of at least 1–3 mmHg, as previously accepted by FDA for product approval
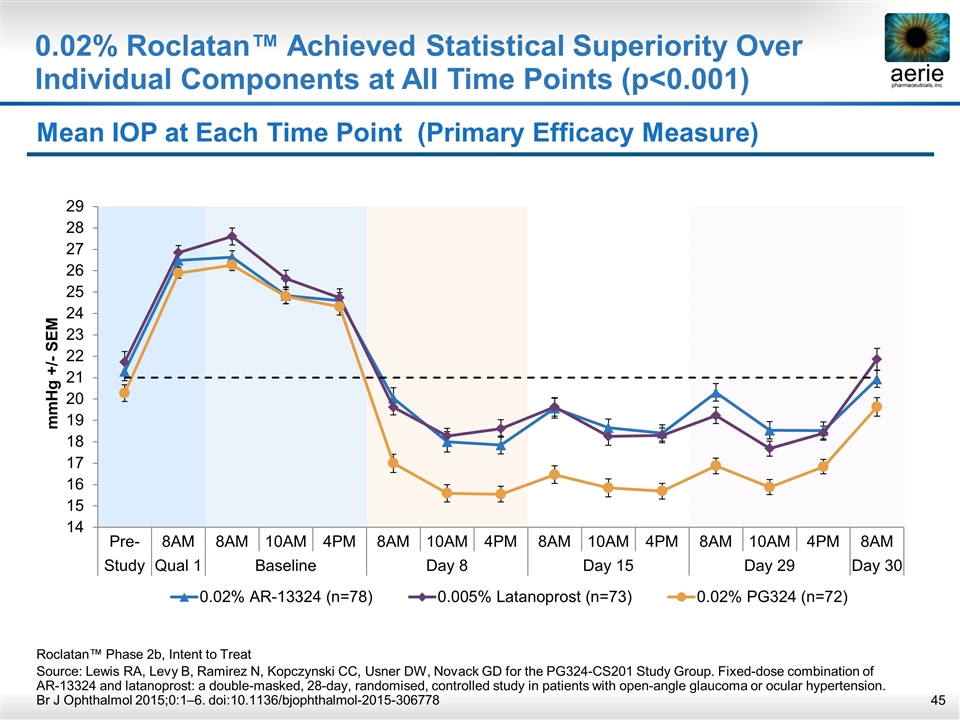
Mean IOP at Each Time Point (Primary Efficacy Measure) Roclatan™ Phase 2b, Intent to Treat Source: Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD for the PG324-CS201 Study Group. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol 2015;0:1–6. doi:10.1136/bjophthalmol-2015-306778 0.02% Roclatan™ Achieved Statistical Superiority Over Individual Components at All Time Points (p<0.001)
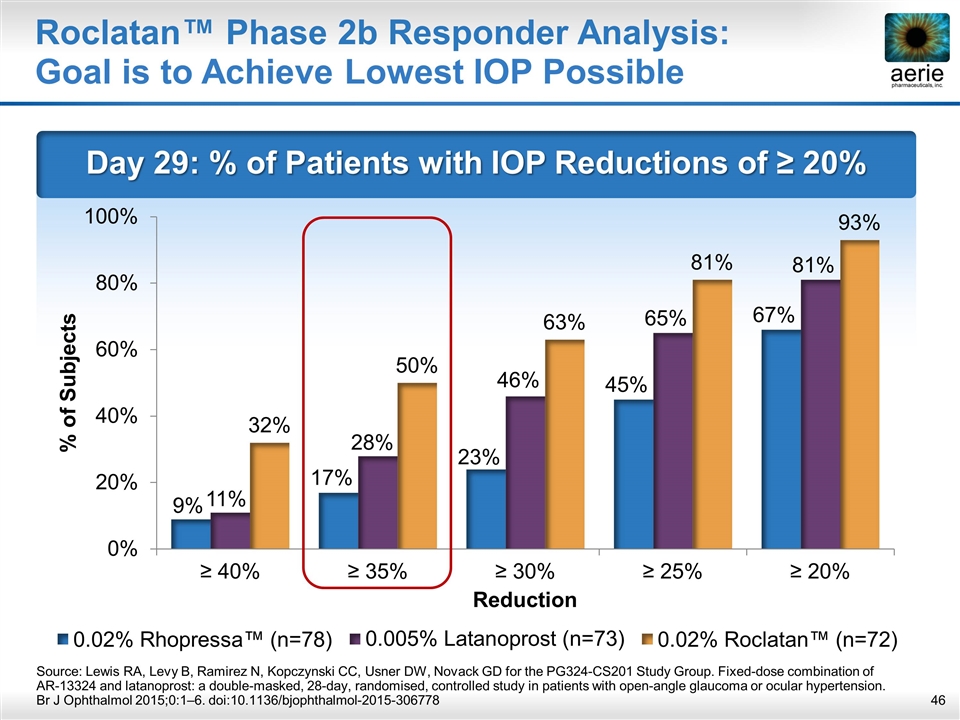
Day 29: % of Patients with IOP Reductions of ≥ 20% Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible Source: Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD for the PG324-CS201 Study Group. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol 2015;0:1–6. doi:10.1136/bjophthalmol-2015-306778
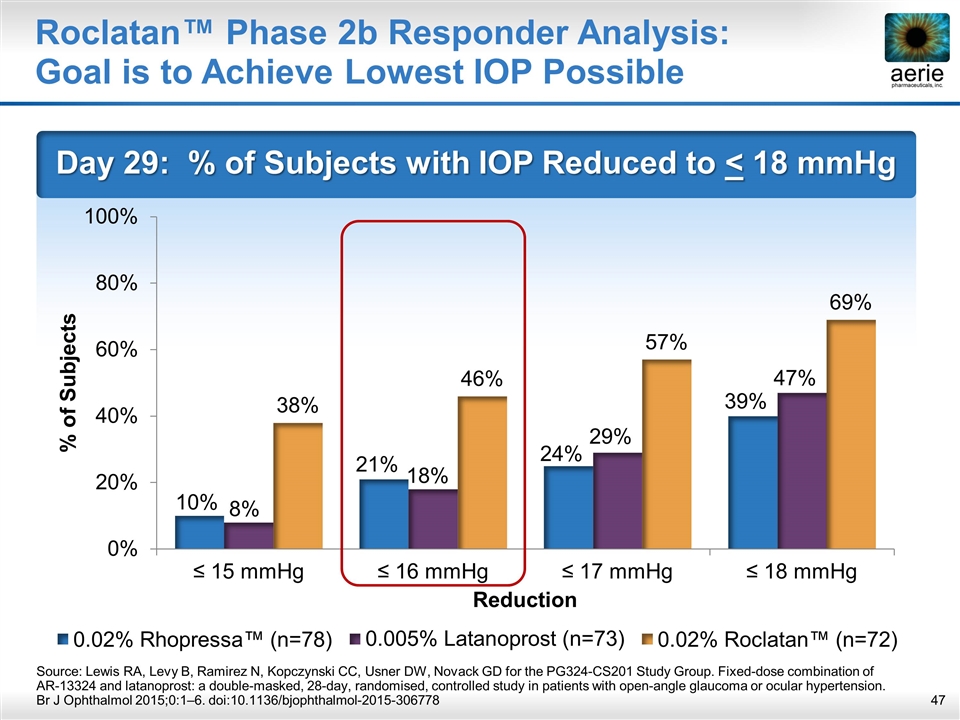
Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible Day 29: % of Subjects with IOP Reduced to < 18 mmHg Source: Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD for the PG324-CS201 Study Group. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol 2015;0:1–6. doi:10.1136/bjophthalmol-2015-306778
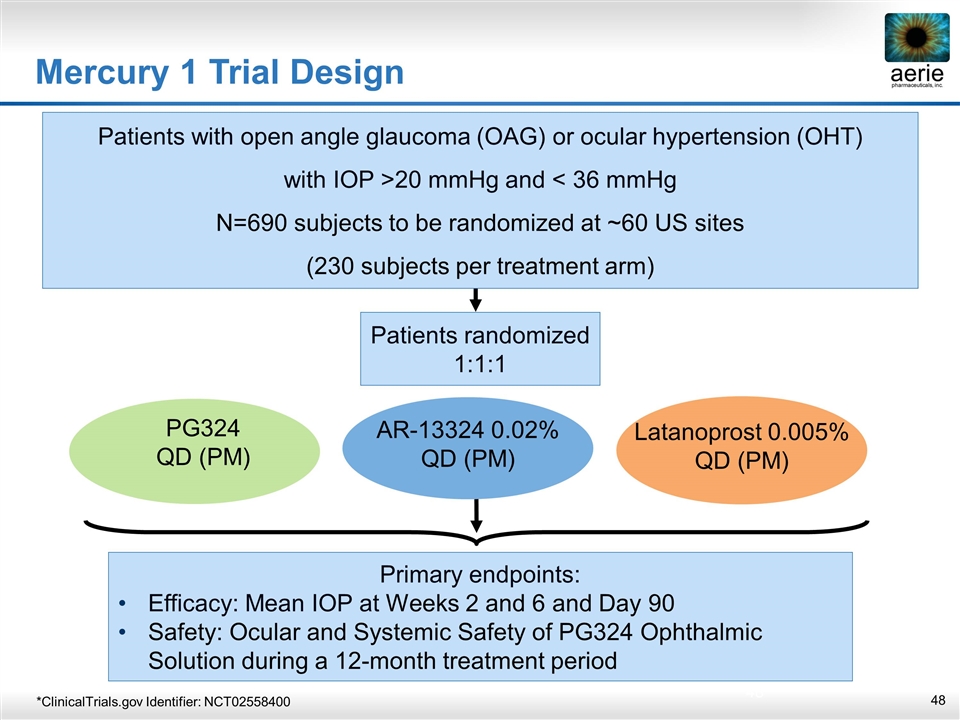
Patients randomized 1:1:1 Primary endpoints: Efficacy: Mean IOP at Weeks 2 and 6 and Day 90 Safety: Ocular and Systemic Safety of PG324 Ophthalmic Solution during a 12‑month treatment period Patients with open angle glaucoma (OAG) or ocular hypertension (OHT) with IOP >20 mmHg and < 36 mmHg N=690 subjects to be randomized at ~60 US sites (230 subjects per treatment arm) Latanoprost 0.005% QD (PM) PG324 QD (PM) AR-13324 0.02% QD (PM) Mercury 1 Trial Design *ClinicalTrials.gov Identifier: NCT02558400
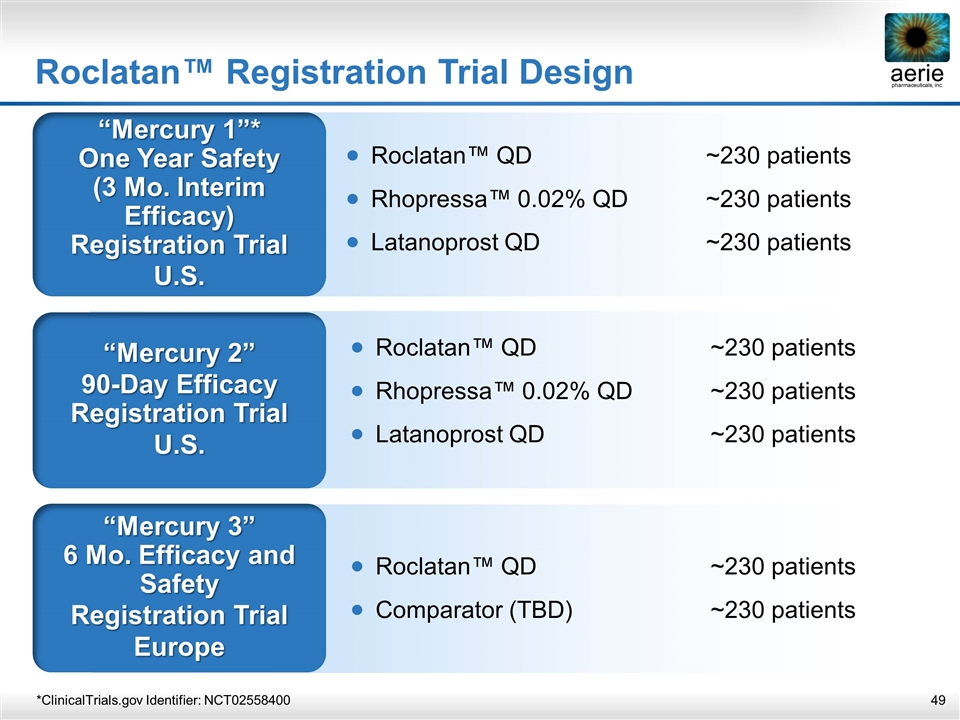
Roclatan™ Registration Trial Design “Mercury 1”* One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. “Mercury 2” 90-Day Efficacy Registration Trial U.S. “Mercury 3” 6 Mo. Efficacy and Safety Registration Trial Europe Roclatan™ QD~230 patients Rhopressa™ 0.02% QD~230 patients Latanoprost QD~230 patients Roclatan™ QD~230 patients Comparator (TBD)~230 patients *ClinicalTrials.gov Identifier: NCT02558400 Roclatan™ QD~230 patients Rhopressa™ 0.02% QD~230 patients Latanoprost QD~230 patients

Physician’s Perspective Richard Lewis, MD Chief Medical Officer

Physician’s Perspective Glaucoma in 2015 Challenges treating this disease Need for new medications Value of RhopressaTM Directed at site of pathology Enhanced compliance Additive to existing medications RoclatanTM will be a paradigm shift in treating glaucoma

Q & A

Regulatory Update Marv Garrett V.P. Regulatory/Quality

Rhopressa™ Update We have continual dialog with FDA Rocket 1 & Rocket 2 (QD) sufficient for NDA filing Rocket 3 results not required for NDA filing Supplemental to Rocket 2 (12 month trial) Rocket 4 results are not required for NDA filing All safety data (including a 4 month update) will be filed to the NDA Rocket 3 & 4 clinical study reports will be filed to the IND (not the NDA) NDA preparation activities Electronic submissions have been set up, tested and accepted by FDA NDA filing planned for Q3 2016
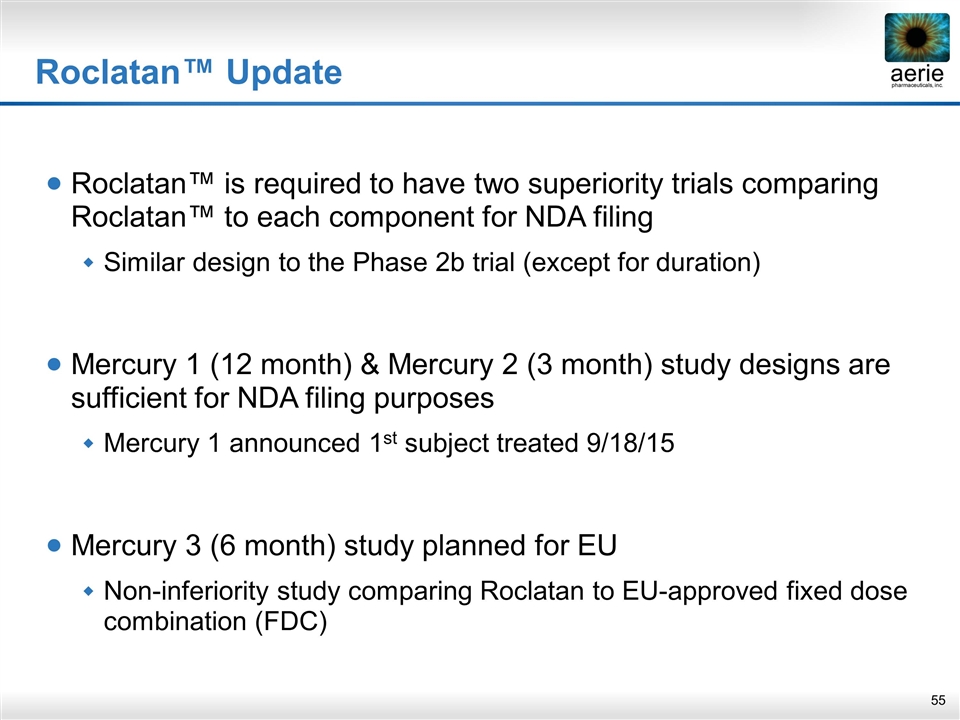
Roclatan™ Update Roclatan™ is required to have two superiority trials comparing Roclatan™ to each component for NDA filing Similar design to the Phase 2b trial (except for duration) Mercury 1 (12 month) & Mercury 2 (3 month) study designs are sufficient for NDA filing purposes Mercury 1 announced 1st subject treated 9/18/15 Mercury 3 (6 month) study planned for EU Non-inferiority study comparing Roclatan to EU-approved fixed dose combination (FDC)
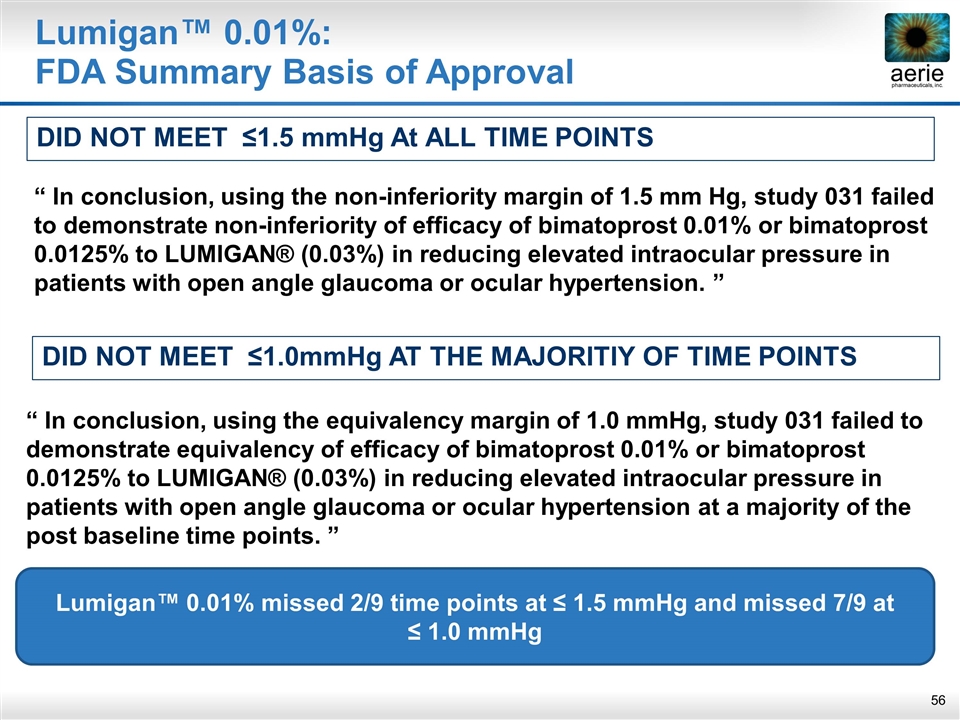
Lumigan™ 0.01%: FDA Summary Basis of Approval DID NOT MEET ≤1.0mmHg AT THE MAJORITIY OF TIME POINTS “ In conclusion, using the non-inferiority margin of 1.5 mm Hg, study 031 failed to demonstrate non-inferiority of efficacy of bimatoprost 0.01% or bimatoprost 0.0125% to LUMIGAN® (0.03%) in reducing elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension. ” “ In conclusion, using the equivalency margin of 1.0 mmHg, study 031 failed to demonstrate equivalency of efficacy of bimatoprost 0.01% or bimatoprost 0.0125% to LUMIGAN® (0.03%) in reducing elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension at a majority of the post baseline time points. ” DID NOT MEET ≤1.5 mmHg At ALL TIME POINTS Lumigan™ 0.01% missed 2/9 time points at ≤ 1.5 mmHg and missed 7/9 at ≤ 1.0 mmHg
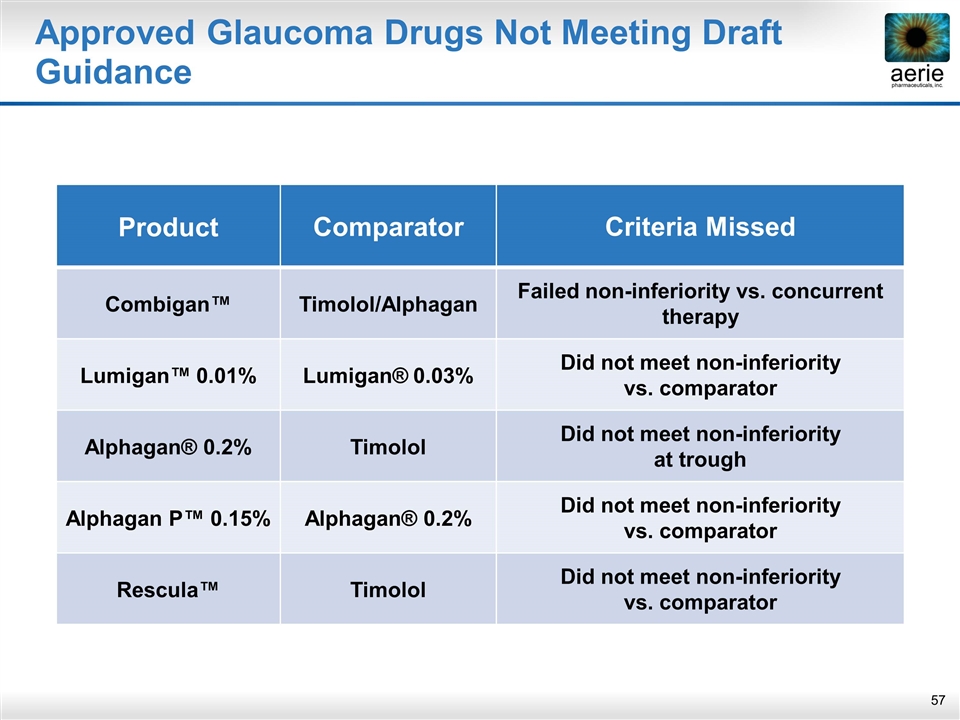
Approved Glaucoma Drugs Not Meeting Draft Guidance Product Comparator Criteria Missed Combigan™ Timolol/Alphagan Failed non-inferiority vs. concurrent therapy Lumigan™ 0.01% Lumigan® 0.03% Did not meet non-inferiority vs. comparator Alphagan® 0.2% Timolol Did not meet non-inferiority at trough Alphagan P™ 0.15% Alphagan® 0.2% Did not meet non-inferiority vs. comparator Rescula™ Timolol Did not meet non-inferiority vs. comparator

Preclinical Research Casey Kopczynski, Ph.D. Chief Scientific Officer
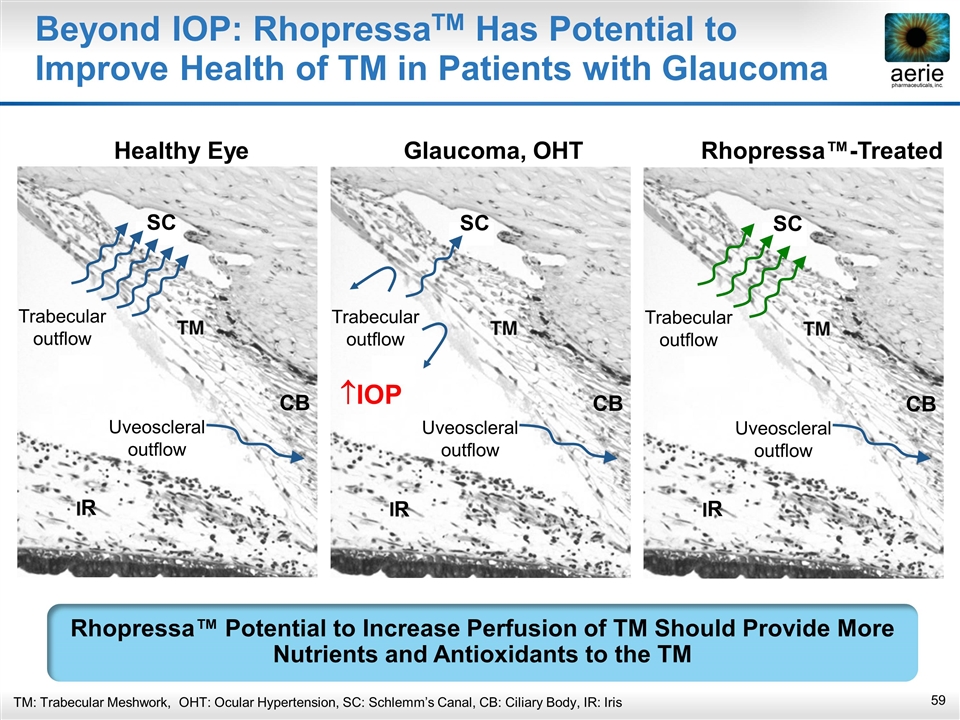
TM: Trabecular Meshwork, OHT: Ocular Hypertension, SC: Schlemm’s Canal, CB: Ciliary Body, IR: Iris Beyond IOP: RhopressaTM Has Potential to Improve Health of TM in Patients with Glaucoma Rhopressa™ Potential to Increase Perfusion of TM Should Provide More Nutrients and Antioxidants to the TM DC SC CB R Trabecular outflow Uveoscleral outflow DC SC CB R Trabecular outflow Uveoscleral outflow Healthy Eye Glaucoma, OHT Rhopressa™-Treated DC SC CB R Trabecular outflow Uveoscleral outflow IOP
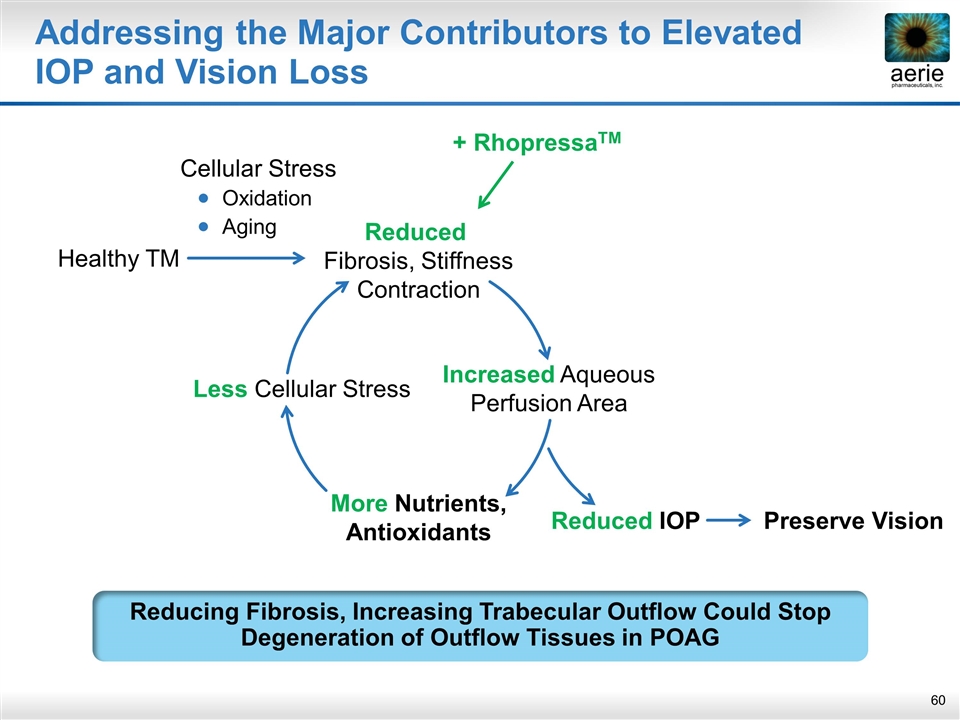
Addressing the Major Contributors to Elevated IOP and Vision Loss Healthy TM More Nutrients, Antioxidants Less Cellular Stress Reduced Fibrosis, Stiffness Contraction Increased Aqueous Perfusion Area Reduced IOP Cellular Stress Oxidation Aging Reducing Fibrosis, Increasing Trabecular Outflow Could Stop Degeneration of Outflow Tissues in POAG + RhopressaTM Preserve Vision
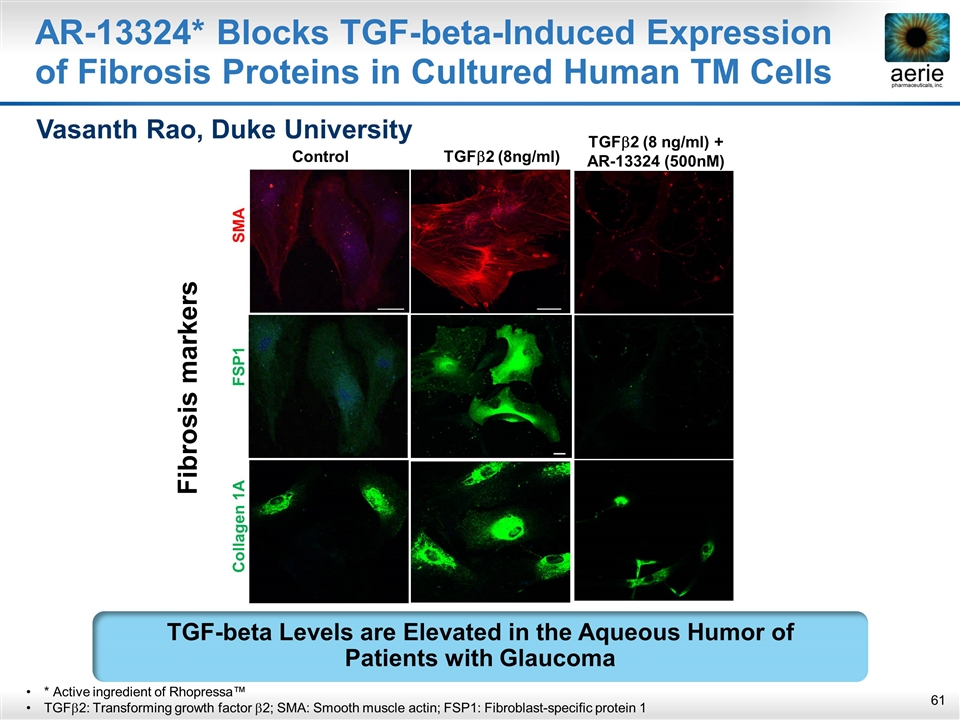
AR-13324* Blocks TGF-beta-Induced Expression of Fibrosis Proteins in Cultured Human TM Cells Fibrosis markers Control TGFb2 (8ng/ml) Collagen 1A FSP1 SMA TGFb2 (8 ng/ml) + AR-13324 (500nM) Vasanth Rao, Duke University * Active ingredient of Rhopressa™ TGFb2: Transforming growth factor b2; SMA: Smooth muscle actin; FSP1: Fibroblast-specific protein 1 TGF-beta Levels are Elevated in the Aqueous Humor of Patients with Glaucoma
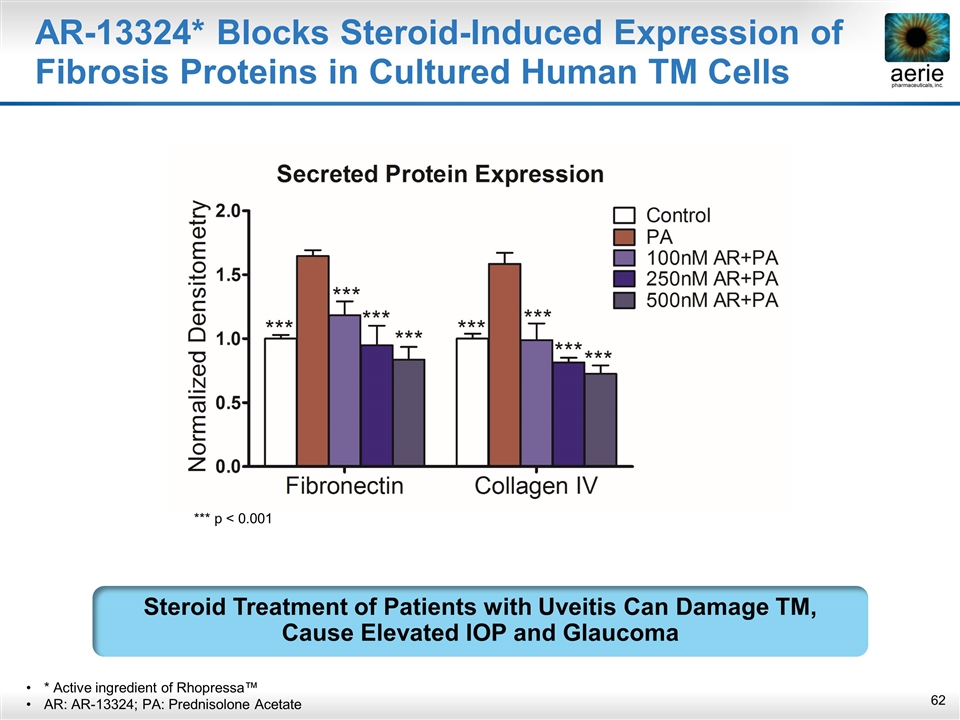
AR-13324* Blocks Steroid-Induced Expression of Fibrosis Proteins in Cultured Human TM Cells * Active ingredient of Rhopressa™ AR: AR-13324; PA: Prednisolone Acetate Steroid Treatment of Patients with Uveitis Can Damage TM, Cause Elevated IOP and Glaucoma *** p < 0.001
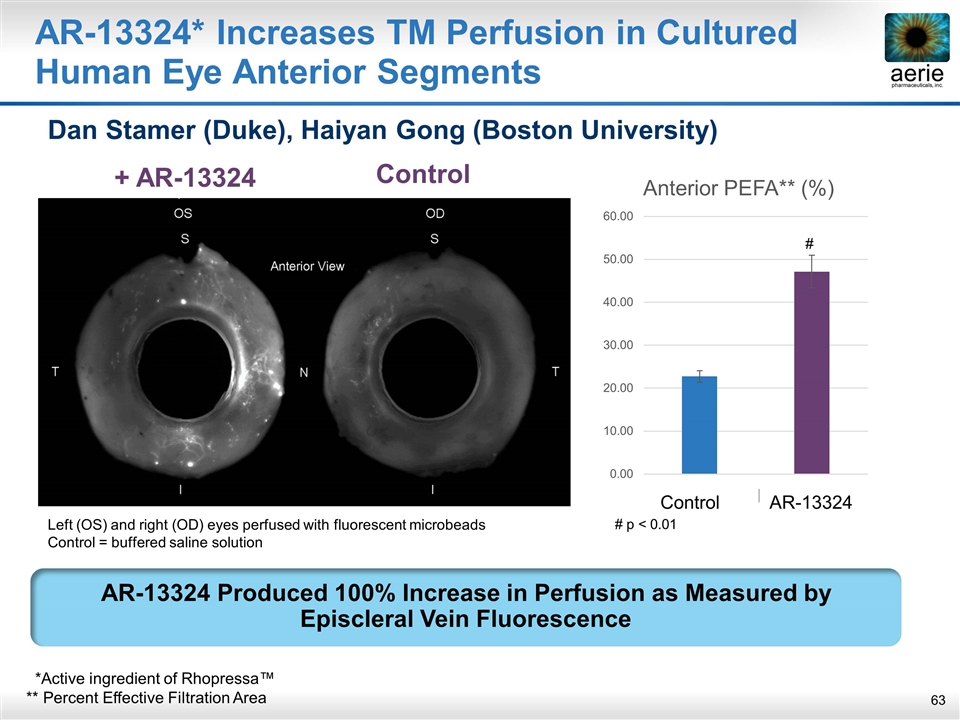
+ AR-13324 Control AR-13324* Increases TM Perfusion in Cultured Human Eye Anterior Segments Dan Stamer (Duke), Haiyan Gong (Boston University) AR-13324 Produced 100% Increase in Perfusion as Measured by Episcleral Vein Fluorescence AR-13324 Control Left (OS) and right (OD) eyes perfused with fluorescent microbeads Control = buffered saline solution *Active ingredient of Rhopressa™ ** Percent Effective Filtration Area # # p < 0.01
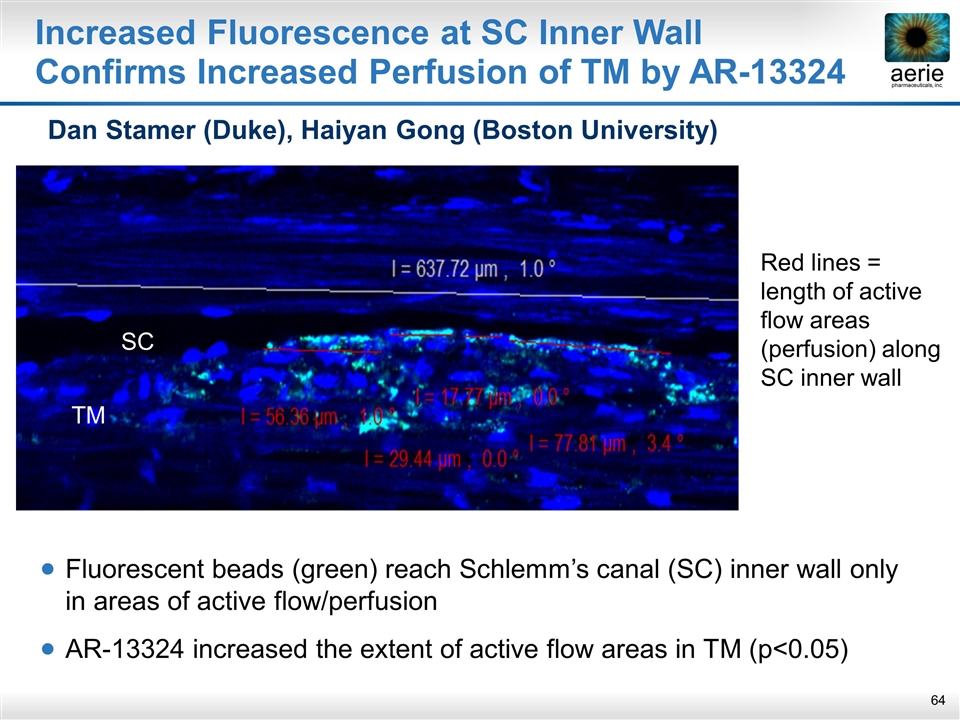
Increased Fluorescence at SC Inner Wall Confirms Increased Perfusion of TM by AR-13324 Fluorescent beads (green) reach Schlemm’s canal (SC) inner wall only in areas of active flow/perfusion AR-13324 increased the extent of active flow areas in TM (p<0.05) Red lines = length of active flow areas (perfusion) along SC inner wall SC TM Dan Stamer (Duke), Haiyan Gong (Boston University)
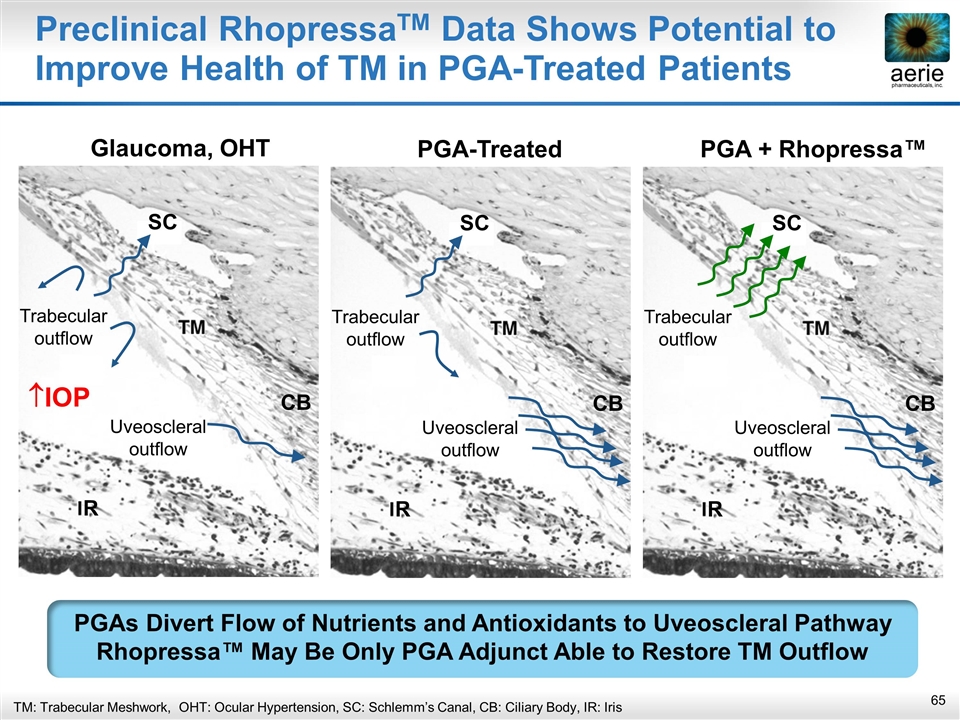
Glaucoma, OHT PGA + Rhopressa™ PGA-Treated Preclinical RhopressaTM Data Shows Potential to Improve Health of TM in PGA-Treated Patients PGAs Divert Flow of Nutrients and Antioxidants to Uveoscleral Pathway Rhopressa™ May Be Only PGA Adjunct Able to Restore TM Outflow DC SC CB R Trabecular outflow Uveoscleral outflow DC SC CB R Trabecular outflow Uveoscleral outflow DC SC CB R Trabecular outflow Uveoscleral outflow IOP TM: Trabecular Meshwork, OHT: Ocular Hypertension, SC: Schlemm’s Canal, CB: Ciliary Body, IR: Iris
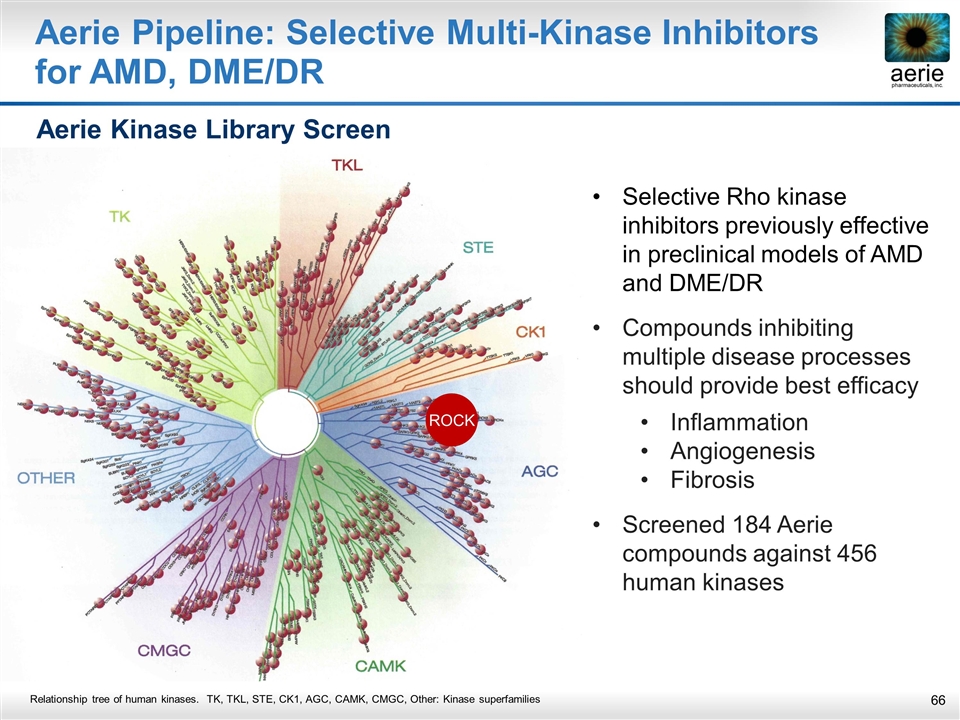
Aerie Pipeline: Selective Multi-Kinase Inhibitors for AMD, DME/DR ROCK Selective Rho kinase inhibitors previously effective in preclinical models of AMD and DME/DR Compounds inhibiting multiple disease processes should provide best efficacy Inflammation Angiogenesis Fibrosis Screened 184 Aerie compounds against 456 human kinases Aerie Kinase Library Screen Relationship tree of human kinases. TK, TKL, STE, CK1, AGC, CAMK, CMGC, Other: Kinase superfamilies
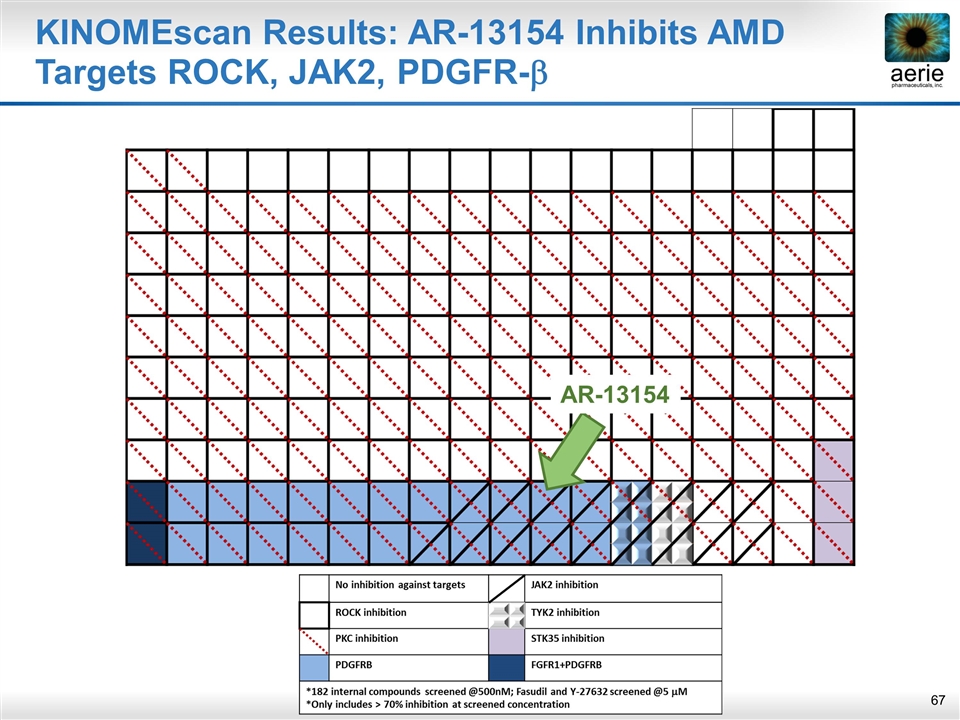
KINOMEscan Results: AR-13154 Inhibits AMD Targets ROCK, JAK2, PDGFR-b AR-13154
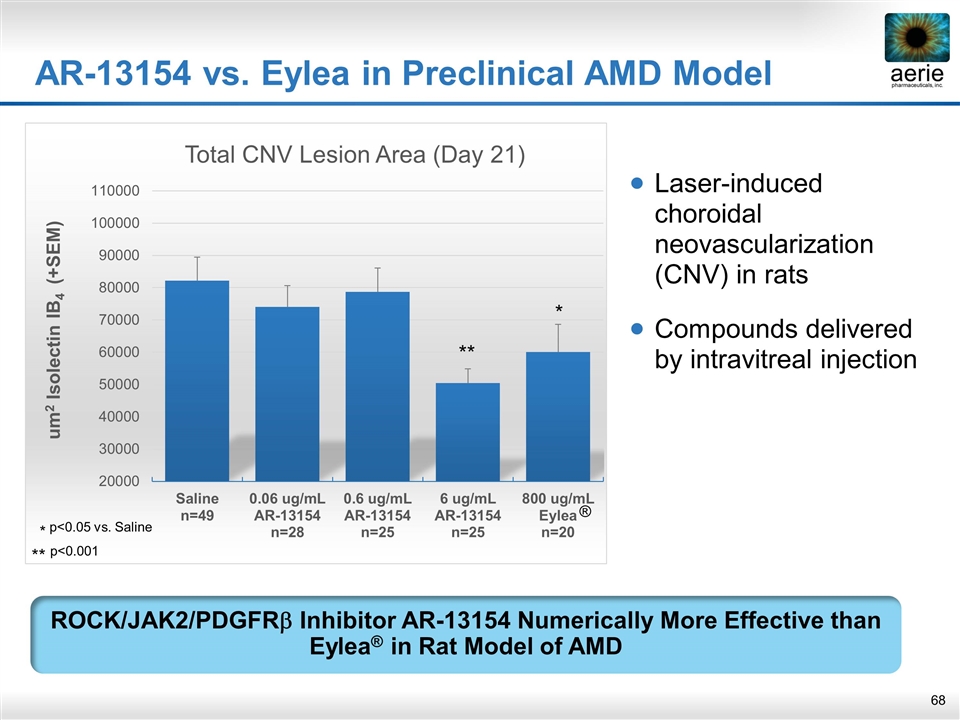
Laser-induced choroidal neovascularization (CNV) in rats Compounds delivered by intravitreal injection AR-13154 vs. Eylea in Preclinical AMD Model ROCK/JAK2/PDGFRb Inhibitor AR-13154 Numerically More Effective than Eylea® in Rat Model of AMD ** * * p<0.05 vs. Saline ** p<0.001 ®
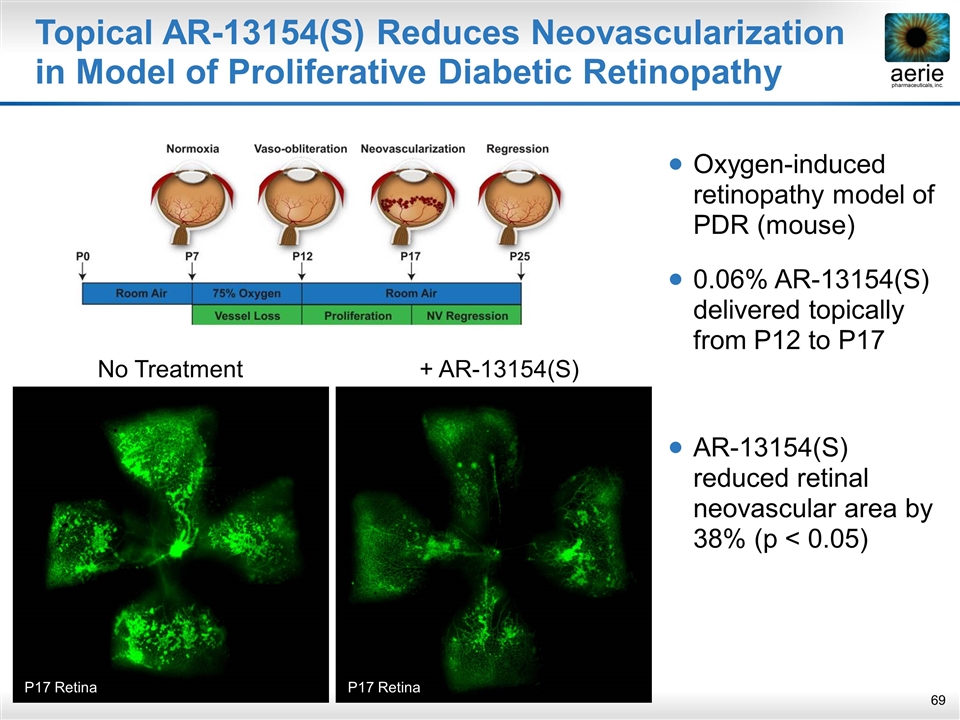
Topical AR-13154(S) Reduces Neovascularization in Model of Proliferative Diabetic Retinopathy Oxygen-induced retinopathy model of PDR (mouse) 0.06% AR-13154(S) delivered topically from P12 to P17 AR-13154(S) reduced retinal neovascular area by 38% (p < 0.05) No Treatment P17 Retina + AR-13154(S) P17 Retina

Aerie Pipeline: New Research Collaborations Neuroprotection/Dry AMD Pre-clinical anti-beta amyloid small molecule product candidate (EG-30) from Ramot at Tel Aviv University Potential for neuroprotection in glaucoma and reduction of geographic atrophy in advanced dry AMD Collaboration and license agreement covering all ophthalmic indications Sustained intraocular drug delivery GrayBug technology, spin-out from Johns Hopkins University Sustained release injectable technology capable of delivering compounds to the front and back of the eye Biodegradable polymer technology with potential for multi-month delivery Efforts initially focused on delivery of AR-13154 to back of the eye

Q & A
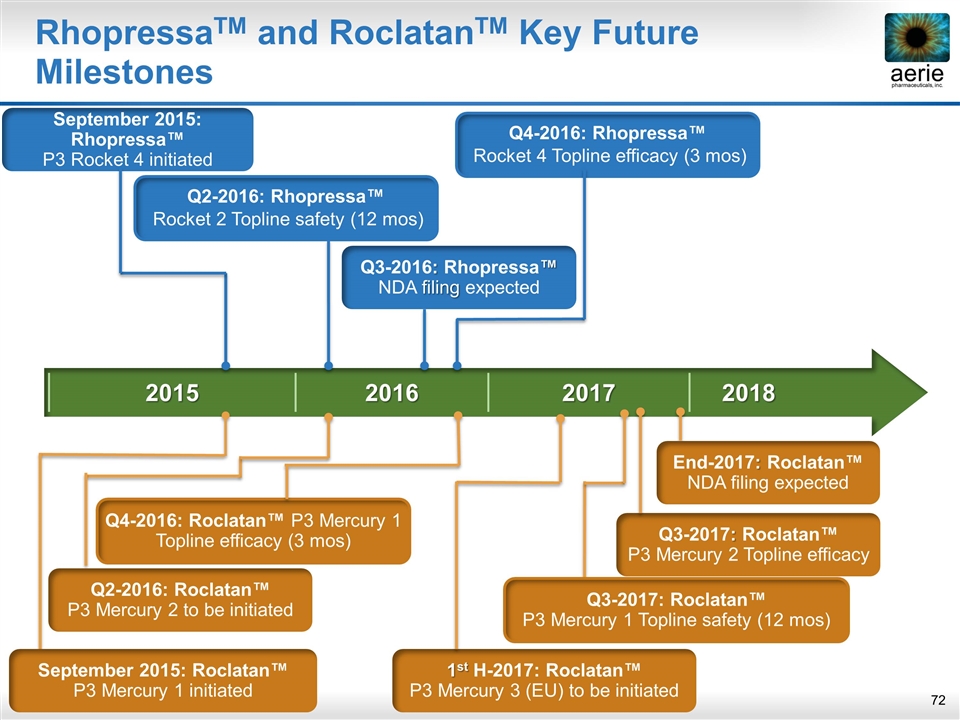
2015 2016 2017 2018 RhopressaTM and RoclatanTM Key Future Milestones Q3-2017: Roclatan™ P3 Mercury 2 Topline efficacy End-2017: Roclatan™ NDA filing expected 1st H-2017: Roclatan™ P3 Mercury 3 (EU) to be initiated September 2015: Rhopressa™ P3 Rocket 4 initiated Q2-2016: Rhopressa™ Rocket 2 Topline safety (12 mos) Q3-2016: Rhopressa™ NDA filing expected Q4-2016: Rhopressa™ Rocket 4 Topline efficacy (3 mos) Q4-2016: Roclatan™ P3 Mercury 1 Topline efficacy (3 mos) Q3-2017: Roclatan™ P3 Mercury 1 Topline safety (12 mos) September 2015: Roclatan™ P3 Mercury 1 initiated Q2-2016: Roclatan™ P3 Mercury 2 to be initiated

Building a Major Ophthalmic Pharmaceutical Company

Speaker Biographies

Speaker Biographies Vicente Anido, Jr., PhD has served as our Chief Executive Officer since July 2013 and as a Chairman and member of our Board since April 2013. Dr. Anido is the former President, Chief Executive Officer and Director of ISTA Pharmaceuticals, Inc., which was acquired by Bausch + Lomb, Inc. in 2012. Prior to joining ISTA Pharmaceuticals, Dr. Anido served as general partner of Windamere Venture Partners from 2000 to 2001. From 1996 to 1999, Dr. Anido served as President and Chief Executive Officer of CombiChem, Inc., a drug discovery company. From 1993 to 1996, Dr. Anido served as President of the Americas Region of Allergan, Inc., where he was responsible for Allergan’s commercial operations for North and South America. Prior to joining Allergan, Dr. Anido spent 17 years at Marion Laboratories and Marion Merrell Dow, Inc., including as Vice President, Business Management of Marion’s U.S. Prescription Products Division. Dr. Anido currently serves as a member of the boards of directors of Depomed, Inc. Dr. Anido holds a BS and a MS from West Virginia University and a PhD from the University of Missouri, Kansas City.

Speaker Biographies Richard J. Rubino has served as our Chief Financial Officer since October 2012. From March 2008 to April 2012, Mr. Rubino served as Senior Vice President, Finance and Chief Financial Officer of Medco Health Solutions, Inc. and from May 1993 to March 2008 served as Controller and Vice President of Planning, with responsibilities for financial, business and strategic planning. Previously, Mr. Rubino held various positions at International Business Machines Corporation from 1983 to May 1993 and PricewaterhouseCoopers LLP (formerly Price Waterhouse & Co.) from 1979 to 1983. Mr. Rubino is a Certified Public Accountant and a member of the American Institute of Certified Public Accountants. Mr. Rubino received a B.S. degree in Accounting from Manhattan College. He has been a director of the Northside Center for Child Development since 2009, the Treasurer since 2012 and also currently serves as a member of the Finance Committee and the Executive Committee.

Speaker Biographies Thomas A. Mitro has served as our President and Chief Operating Officer since August 2013. From November 2012 to August 2013, Mr. Mitro served as Vice President, Sales and Marketing at Omeros Corporation, a clinical-stage biopharmaceutical company. Prior to this, Mr. Mitro was Vice President, Sales and Marketing at ISTA Pharmaceuticals from July 2002 to July 2012. Previously, Mr. Mitro held various positions at Allergan, Inc., including Vice President, Skin Care; Vice President, Business Development; and Vice President, e-Business. Mr. Mitro received a B.S. degree from Miami University. Theresa G.H. Heah, MD, has served as our Vice President, Clinical Research and Medical Affairs since January 2015. From 2011 to 2015, Dr. Heah lead the global medical affairs pre-launch and launch of EYLEA® at Bayer Healthcare as Director, Sr. Global Medical Affairs Physician followed by Global Brand and Strategic Marketing Director position responsible for global brand management and commercial strategy across ophthalmology franchise. Prior to Bayer Healthcare, Dr. Heah has held several Global R&D roles and clinical development leadership positions within Orion Clinical Services, Allergan Inc., and Sanofi-Fovea in addition to clinical experience as an ophthalmologist in the UK. She obtained her medical degree from Guy’s, Kings and St. Thomas School of Medicine, King’s College, University of London, U.K. She was a past-committee member of the British Association of Pharmaceutical Physicians (BrAPP), member of the Royal College of Ophthalmologists, London and the Royal College of Surgeons, Edinburgh.

Speaker Biographies Richard A. Lewis, M.D., is the former director of glaucoma at the University of California, Davis. In addition to his busy clinical practice located in Sacramento, California, Dr. Lewis is actively involved in clinical research in national and international trials in anterior segment disease and glaucoma therapy. He is the past President of the American Society of Cataract and Refractive Surgery (2014-15) and past President of the American Glaucoma Society (2000-02). Dr. Lewis is the medical Editor Emeritus of Glaucoma Today and on the editorial board of the Journal of Cataract and Refractive Surgery, Journal of Glaucoma, Advanced Ocular Care and Ocular Surgery News. He is co-founder of Sacramento’s Capital City Surgery Center (2003).Dr. Lewis attended the University of California, Berkeley as an undergraduate and received his doctorate in medicine from Northwestern University Medical School in Chicago in 1978. His ophthalmology training included a residency at the Department of Ophthalmology at the University of California, Davis, and a fellowship in glaucoma at the University of Iowa Department Of Ophthalmology in Iowa City (1982-83). He is a Diplomat of the American Board of Ophthalmology and the National Board of Medical Examiners. Dr. Lewis has published over ninety articles and book chapters focusing on glaucoma, ophthalmic surgery, and ophthalmic pharmacology. He is co-author of the book, Curbsides in Glaucoma. He teaches and lectures extensively on glaucoma and cataract surgery. He has received the American Academy of Ophthalmology Honor and Senior Honor as well as the Secretariat and Life Achievement Awards for his contributions in teaching and leadership and for initiating the AAO Subspecialty Day meeting.A native of San Francisco, Dr. Lewis lives in Sacramento with his wife; the couple has two grown children.

Speaker Biographies Marvin J. Garrett has served as our Vice President of Regulatory Affairs and Quality since February 2014. In his role at Aerie he works directly with the US Food and Drug Administration and foreign ministries of health and oversees product quality. Mr. Garrett has more than forty years of experience in the fields of regulatory affairs, quality and clinical research. Mr. Garrett previously served as Vice President of Regulatory Affairs, Quality and Compliance at Bausch + Lomb, Inc., following the acquisition of ISTA Pharmaceuticals, Inc., where he held the same position. Previously, Mr. Garrett was Vice President of Regulatory Affairs, Clinical Research, Quality and Compliance at XOMA, Ltd. Prior to this, he served as President and Chief Executive Officer at Coopervision Pharmaceuticals and Corporate Vice President of Regulatory Affairs for Cooper Companies. Previously he served as Vice President of Regulatory Affairs, Clinical Research, Quality and Technical Development for IOLAB Pharmaceuticals/Johnson & Johnson. Earlier in his career held several positions at Allergan Pharmaceuticals attaining the position of Director of Regulatory Affairs and was the designated company liaison with CDER and CDRH. Prior to joining Allergan, Mr. Garrett served in Regulatory and Quality roles for ORTHO/Johnson & Johnson and McGaw Laboratories/American Hospital. Mr. Garrett is a past President and Chairman of the Regulatory Affairs Professional Society (RAPS) and is a current board member of SoCalBio. He received a B.S. degree in Microbiology from California State University Long Beach and studied business at the University of Redlands.

Speaker Biographies Casey C. Kopczynski, Ph.D., has served as our Chief Scientific Officer since co-founding our company in 2005. From 2002 to 2005, Dr. Kopczynski was the Managing Partner at Biotech Initiative, LLC, a consulting practice dedicated to emerging biotech companies. He was also previously the Vice President of Research at Ercole Biotech, Inc. from 2003 to 2004, a company developing drugs for the treatment of cancer, inflammation and orphan genetic diseases. Prior to Ercole Biotech, Inc., Dr. Kopczynski was Director of Research and a founding member of the scientific staff at Exelixis, Inc. from 1996 to 2002. Dr. Kopczynski received a Ph.D. degree in Molecular, Cellular and Developmental Biology from Indiana University and was a Jane Coffin Childs Research Fellow at the University of California, Berkeley.















































































