
Aerie Pharmaceuticals, Inc. November 16 - 19 2015 Building a Major Ophthalmic Pharmaceutical Company Exhibit 99.1

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. In addition, any discussion of clinical trial results for RhopressaTM relate to the results in its first Phase 3 registration trials, Rocket 1 and Rocket 2, and for RoclatanTM relate to the results in its Phase 2b clinical trial. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, the preclinical research discussed in this presentation is preliminary and the outcome of such preclinical studies may not be predictive of the outcome of later trials. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
Current Aerie Products: Once-Daily IOP-Lowering Eye Drops for Glaucoma Pre-Clinical Research Findings Rhopressa™ shows potential to modify diseased tissue May block fibrotic response, increase perfusion AR-13154 shows potential for the treatment of wet AMD May inhibit ROCK/JAK/PDGFR-β, lesion size reduction exceeds market-leading product In-licensing opportunities GrayBug drug delivery and Ramot small molecule anti-beta amyloid for neuroprotection and dry AMD Rhopressa™ Inhibits ROCK and NET, lowers EVP, targets diseased tissue Successful P3 trials; NDA filing expected Q3 2016 Roclatan™ Fixed combination of Rhopressa™ and latanoprost P2b achieved all clinical endpoints, P3 in process Potentially most efficacious IOP-lowering therapy Aerie – Building a Major Ophthalmic Pharmaceutical Company Aerie Products - Full patent protection through at least 2030; Blockbuster Potential
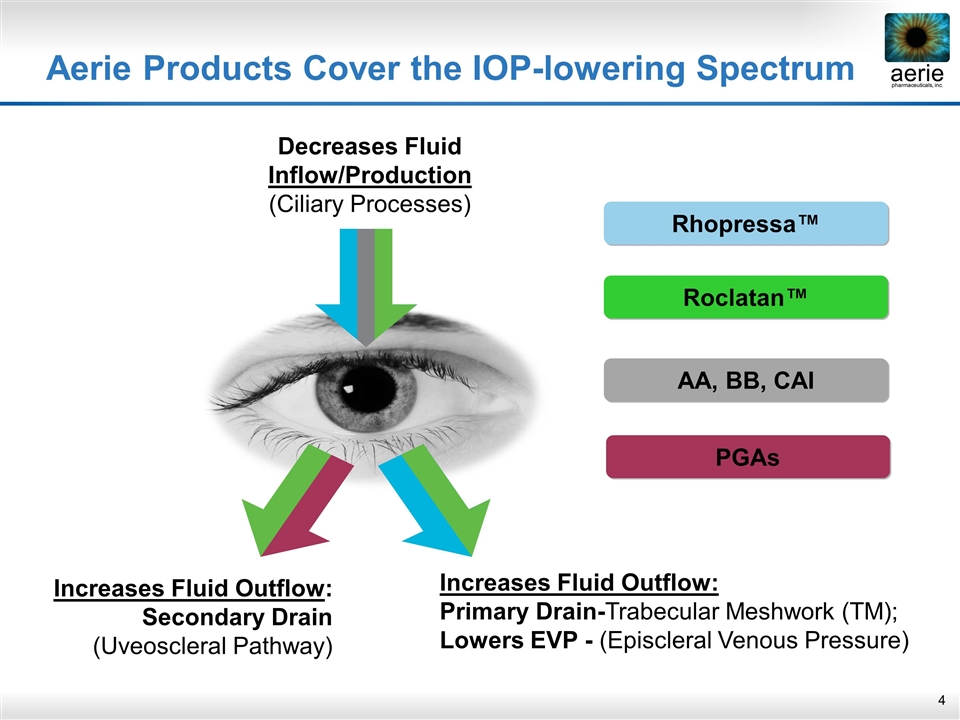
increase Decreases Fluid Inflow/Production (Ciliary Processes) Increases Fluid Outflow: Secondary Drain (Uveoscleral Pathway) Increases Fluid Outflow: Primary Drain-Trabecular Meshwork (TM); Lowers EVP - (Episcleral Venous Pressure) Rhopressa™ Roclatan™ AA, BB, CAI PGAs Aerie Products Cover the IOP-lowering Spectrum
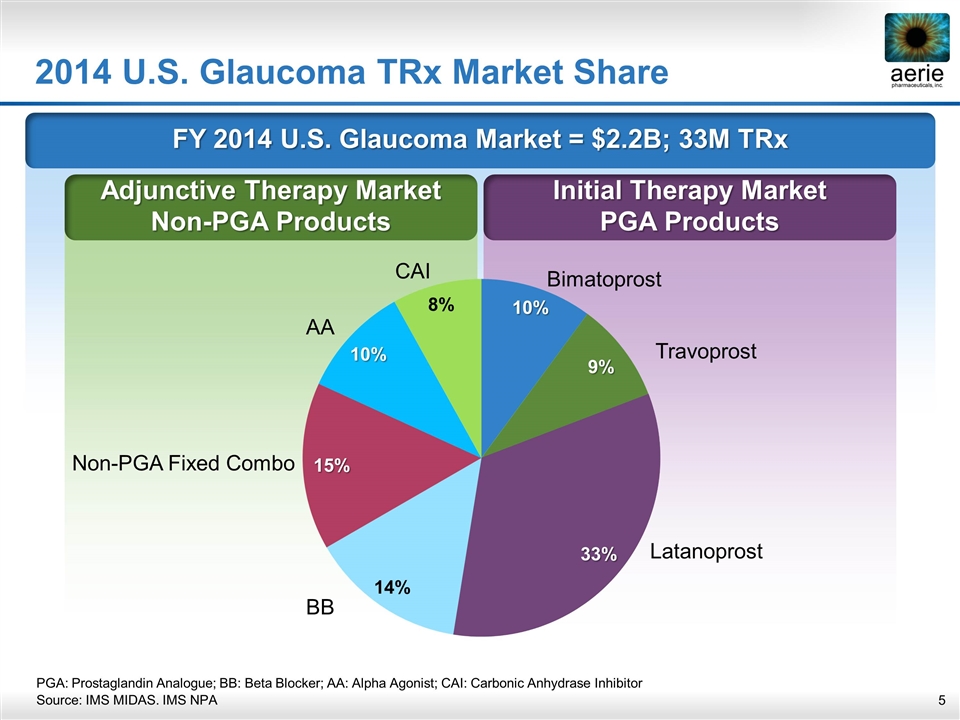
FY 2014 U.S. Glaucoma Market = $2.2B; 33M TRx Initial Therapy Market PGA Products Adjunctive Therapy Market Non-PGA Products 2014 U.S. Glaucoma TRx Market Share Bimatoprost Travoprost Latanoprost BB Non-PGA Fixed Combo AA CAI PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor Source: IMS MIDAS. IMS NPA
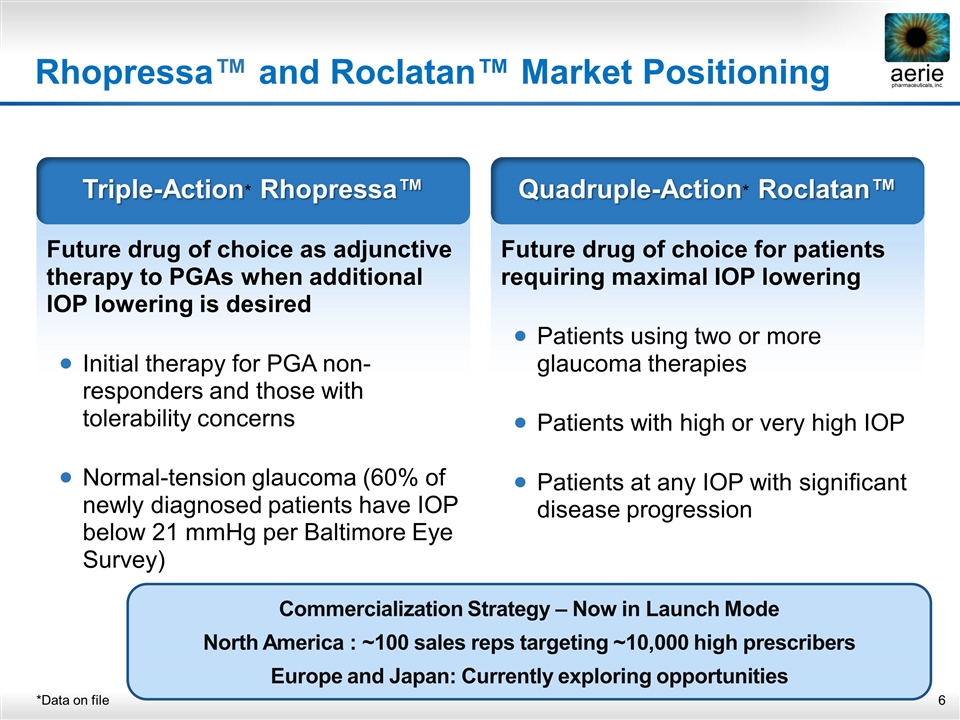
Rhopressa™ and Roclatan™ Market Positioning *Data on file Future drug of choice as adjunctive therapy to PGAs when additional IOP lowering is desired Initial therapy for PGA non-responders and those with tolerability concerns Normal-tension glaucoma (60% of newly diagnosed patients have IOP below 21 mmHg per Baltimore Eye Survey) Triple-Action* Rhopressa™ Future drug of choice for patients requiring maximal IOP lowering Patients using two or more glaucoma therapies Patients with high or very high IOP Patients at any IOP with significant disease progression Quadruple-Action* Roclatan™ Commercialization Strategy – Now in Launch Mode North America : ~100 sales reps targeting ~10,000 high prescribers Europe and Japan: Currently exploring opportunities
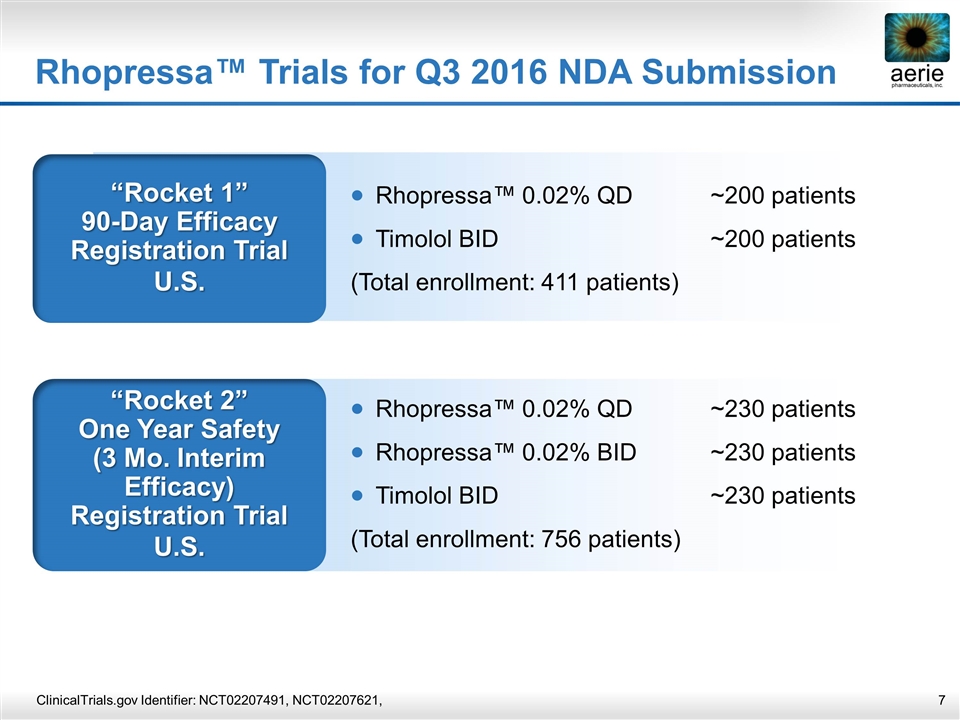
Rhopressa™ Trials for Q3 2016 NDA Submission “Rocket 1” 90-Day Efficacy Registration Trial U.S. Rhopressa™ 0.02% QD~200 patients Timolol BID~200 patients (Total enrollment: 411 patients) “Rocket 2” One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. Rhopressa™ 0.02% QD~230 patients Rhopressa™ 0.02% BID~230 patients Timolol BID~230 patients (Total enrollment: 756 patients) ClinicalTrials.gov Identifier: NCT02207491, NCT02207621,
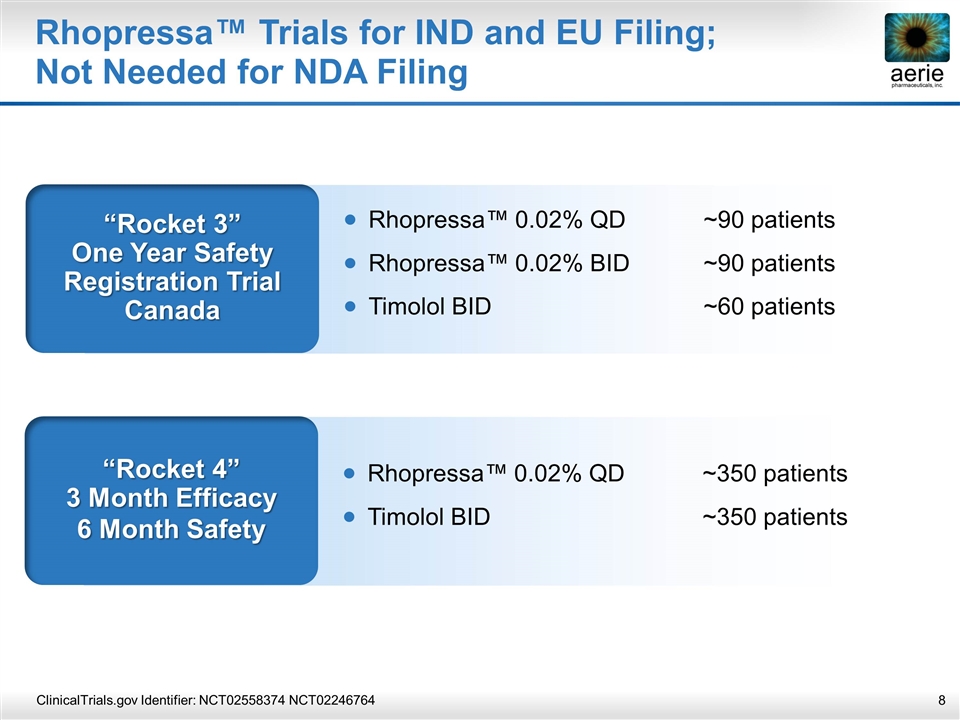
Rhopressa™ Trials for IND and EU Filing; Not Needed for NDA Filing ClinicalTrials.gov Identifier: NCT02558374 NCT02246764 “Rocket 4” 3 Month Efficacy 6 Month Safety Rhopressa™ 0.02% QD~350 patients Timolol BID~350 patients “Rocket 3” One Year Safety Registration Trial Canada Rhopressa™ 0.02% QD~90 patients Rhopressa™ 0.02% BID ~90 patients Timolol BID~60 patients

Rhopressa™ Registration Trial Overview AR-13324 CS301 and CS302 study protocols, FDA End of Phase 2 meeting minutes, Pre-NDA meeting minutes Non-inferiority design vs. timolol 95% CI within 1.5 mmHg at all time points, within 1.0 mmHg at a majority of time points Rocket 2 primary efficacy endpoint: mean IOP at all time points through Day 90 (for maximum baseline IOP <25 mmHg) Data from Rocket 1 is supportive FDA discussions on design of Phase 3 studies Minimum of 100 RhopressaTM QD patients with 12 months of safety data needed for NDA filing Should meet efficacy requirements for European filing Rocket 4 adds adequate RhopressaTM patients for 6 months EU safety data
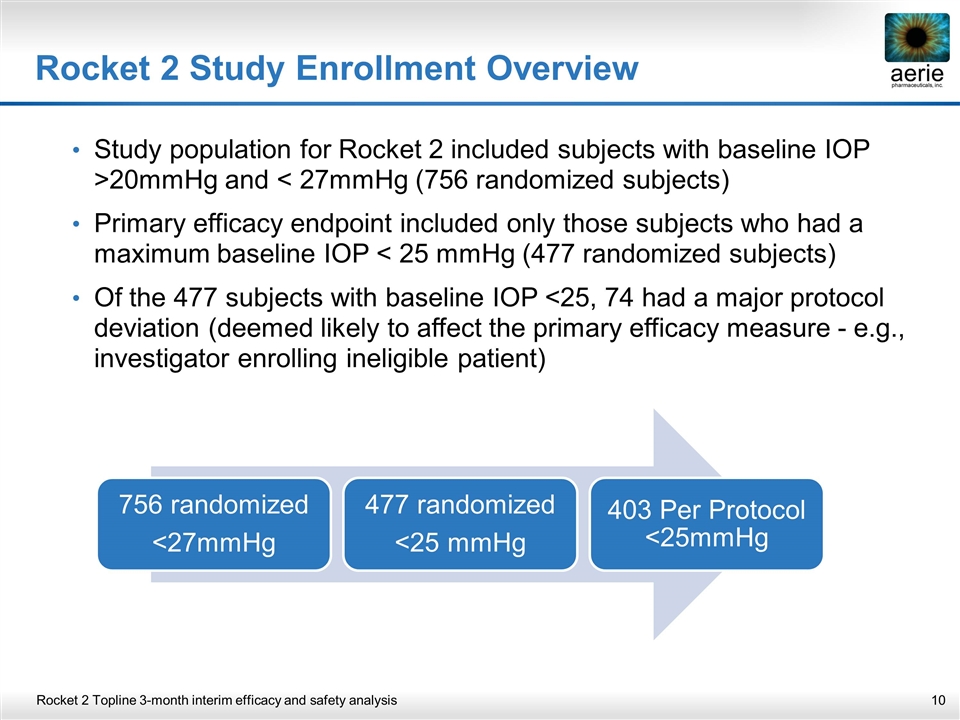
Rocket 2 Study Enrollment Overview Study population for Rocket 2 included subjects with baseline IOP >20mmHg and < 27mmHg (756 randomized subjects) Primary efficacy endpoint included only those subjects who had a maximum baseline IOP < 25 mmHg (477 randomized subjects) Of the 477 subjects with baseline IOP <25, 74 had a major protocol deviation (deemed likely to affect the primary efficacy measure - e.g., investigator enrolling ineligible patient) Rocket 2 Topline 3-month interim efficacy and safety analysis 756 randomized <27mmHg 477 randomized <25 mmHg 403 Per Protocol <25mmHg
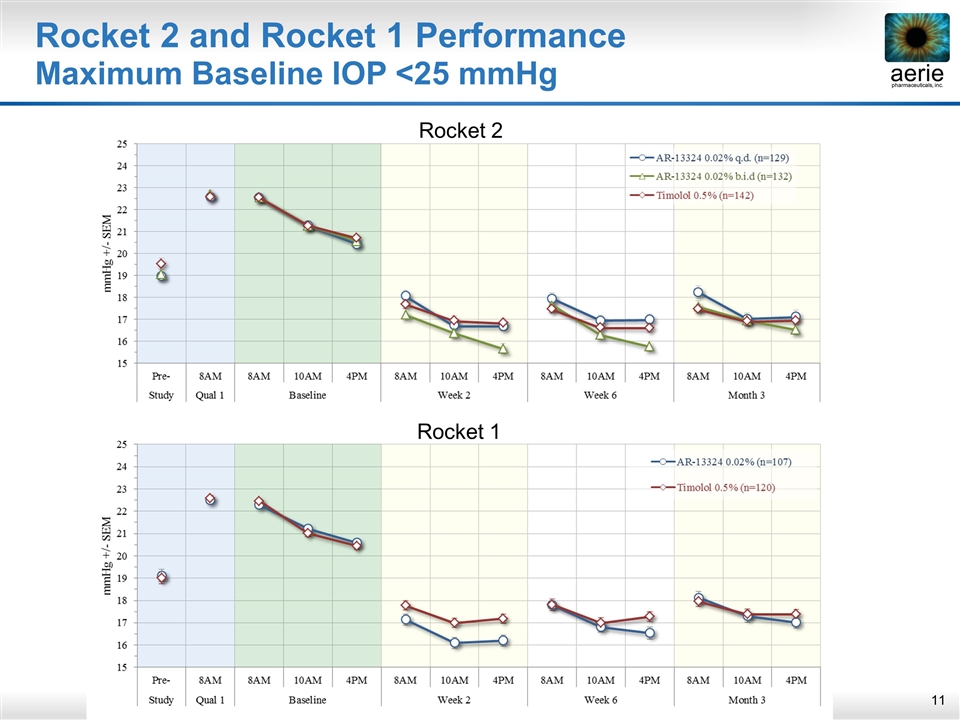
Rocket 2 and Rocket 1 Performance Maximum Baseline IOP <25 mmHg PLACEHOLDER Rocket 2 Rocket 1
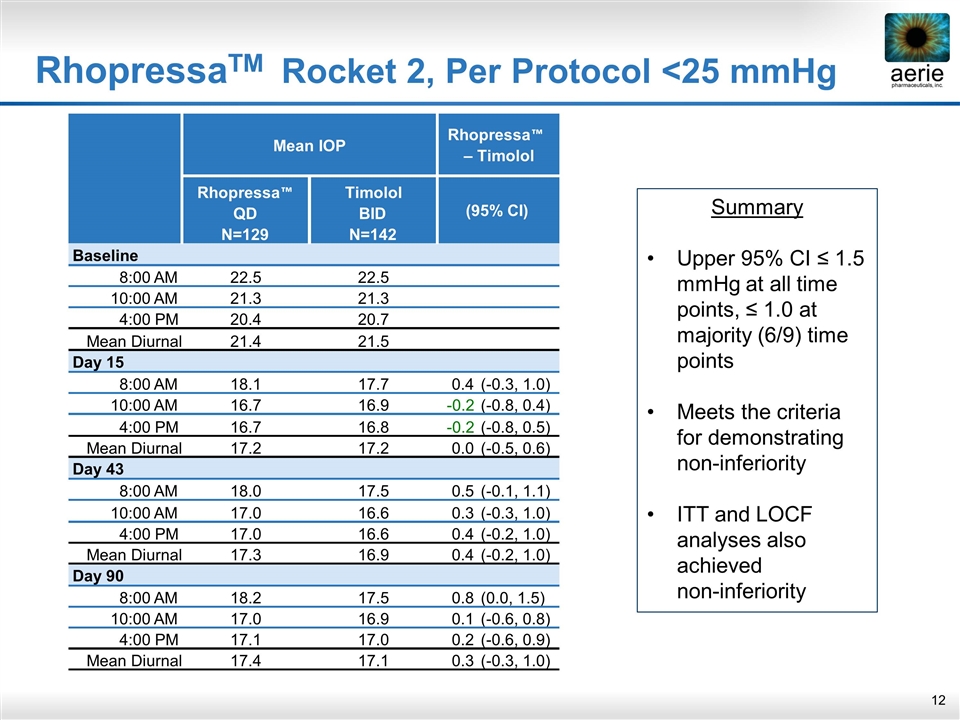
RhopressaTM Rocket 2, Per Protocol <25 mmHg Summary Upper 95% CI ≤ 1.5 mmHg at all time points, ≤ 1.0 at majority (6/9) time points Meets the criteria for demonstrating non-inferiority ITT and LOCF analyses also achieved non-inferiority Rhopressa ™ QD Timolol BID N=129 N=142 Baseline 8:00 AM 22.5 22.5 10:00 AM 21.3 21.3 4:00 PM 20.4 20.7 Mean Diurnal 21.4 21.5 Day 15 8:00 AM 18.1 17.7 0.4 (-0.3, 1.0) 10:00 AM 16.7 16.9 -0.2 (-0.8, 0.4) 4:00 PM 16.7 16.8 -0.2 (-0.8, 0.5) Mean Diurnal 17.2 17.2 0.0 (-0.5, 0.6) Day 43 8:00 AM 18.0 17.5 0.5 (-0.1, 1.1) 10:00 AM 17.0 16.6 0.3 (-0.3, 1.0) 4:00 PM 17.0 16.6 0.4 (-0.2, 1.0) Mean Diurnal 17.3 16.9 0.4 (-0.2, 1.0) Day 90 8:00 AM 18.2 17.5 0.8 (0.0, 1.5) 10:00 AM 17.0 16.9 0.1 (-0.6, 0.8) 4:00 PM 17.1 17.0 0.2 (-0.6, 0.9) Mean Diurnal 17.4 17.1 0.3 (-0.3, 1.0) (95% CI) Mean IOP Rhopressa ™ – Timolol
Summary of Patients Using PGAs Prior to Rhopressa™ in Clinical Trials There was a synergistic effect with patients previously on a PGA in Rocket 2 that was more pronounced in Rocket 1 Differences between Rocket 2 and Rocket 1 Rhopressa™ efficacy may have resulted from a more effective washout of PGAs in the Rocket 2 trial Synergy was statistically significant in both Rocket 1 and Rocket 2 Retrospective analysis of Phase 2 trial results shows prior PGA use enhanced RhopressaTM IOP-lowering by 1 mmHg (p=0.007) and 1.2 mmHg (p=0.002) at weeks 2 and 4, respectively, relative to subjects not previously on PGA Synergistic effect may explain the very high efficacy observed in the RoclatanTM (combo of RhopressaTM with latanoprost) P2b trial
Rocket 2 QD Safety/Tolerability (Days 15-90) Rocket 2 3-month Topline results There were no drug-related serious adverse events (SAEs) The most common adverse event was conjunctival hyperemia RhopressaTM QD: ~35% increased incidence of which 83% was mild and16% was moderate Other ocular AEs AEs occurring in ~5-15% of subjects receiving RhopressaTM QD included: conjunctival hemorrhage, corneal deposits, and blurry vision
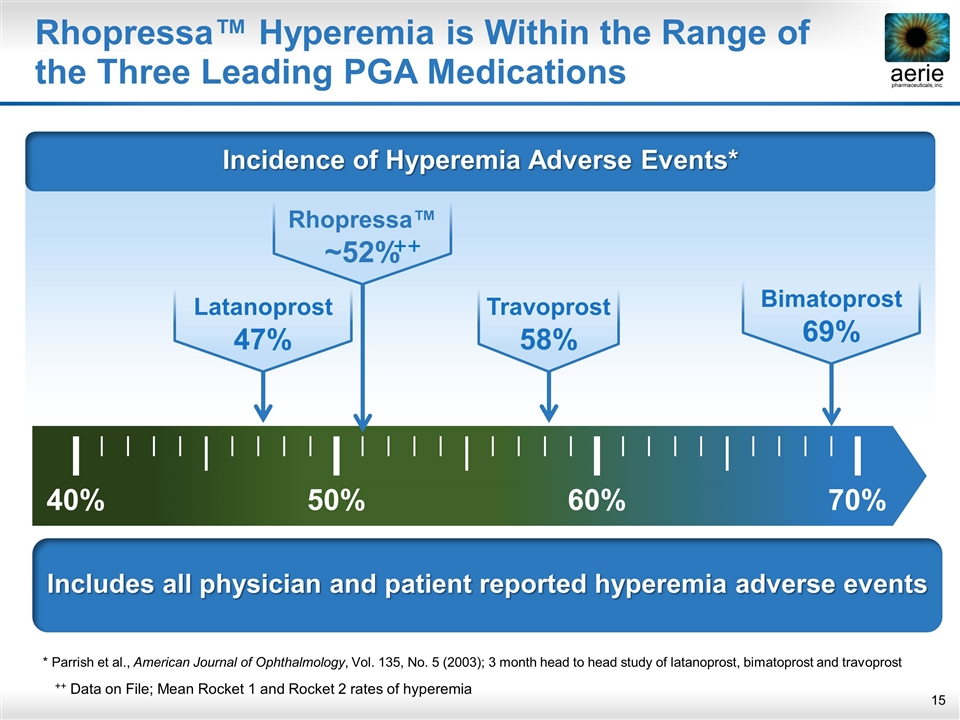
Includes all physician and patient reported hyperemia adverse events Rhopressa™ Hyperemia is Within the Range of the Three Leading PGA Medications * Parrish et al., American Journal of Ophthalmology, Vol. 135, No. 5 (2003); 3 month head to head study of latanoprost, bimatoprost and travoprost Latanoprost 47% 40% 50% 60% 70% Travoprost 58% Bimatoprost 69% Rhopressa™ ~52% ++ ++ Data on File; Mean Rocket 1 and Rocket 2 rates of hyperemia Incidence of Hyperemia Adverse Events*
RhopressaTM Status Preparing to file NDA for RhopressaTM QD in Q3 2016: - Successful Rocket 2 is pivotal – non-inferior to Timolol - Rocket 1 successful at secondary endpoint range – supportive - One year safety and product stability in process Rocket 4 commenced September 2015: - Adds adequate RhopressaTM patients for 6 months EU Safety - Not required for NDA filing - 700 total patients – RhopressaTM QD vs. Timolol BID - Same clinical endpoint ranges as Rocket 2 - 90-day efficacy readout expected in one year
RhopressaTM Advantages *Data on file Demonstrated once-daily IOP lowering in Phase 3 trials Triple mechanism of action* Potential PGA Synergy More consistent IOP-lowering across baselines than PGAs and timolol No systemic side effects Targets diseased trabecular meshwork in glaucoma Potential to preserve health of trabecular outflow pathway Anti-fibrotic effect demonstrated preclinically in human trabecular meshwork cells Increased perfusion demonstrated preclinically in human trabecular meshwork and episcleral tissues
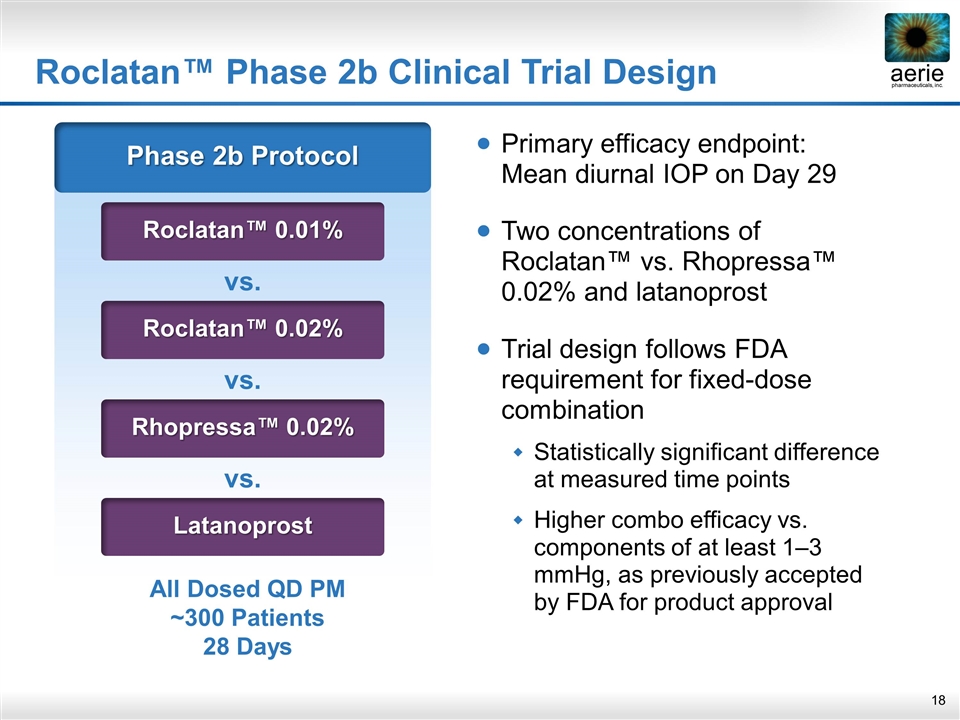
Roclatan™ Phase 2b Clinical Trial Design Phase 2b Protocol Roclatan™ 0.01% vs. Roclatan™ 0.02% vs. Rhopressa™ 0.02% vs. Latanoprost All Dosed QD PM ~300 Patients 28 Days Primary efficacy endpoint: Mean diurnal IOP on Day 29 Two concentrations of Roclatan™ vs. Rhopressa™ 0.02% and latanoprost Trial design follows FDA requirement for fixed-dose combination Statistically significant difference at measured time points Higher combo efficacy vs. components of at least 1–3 mmHg, as previously accepted by FDA for product approval
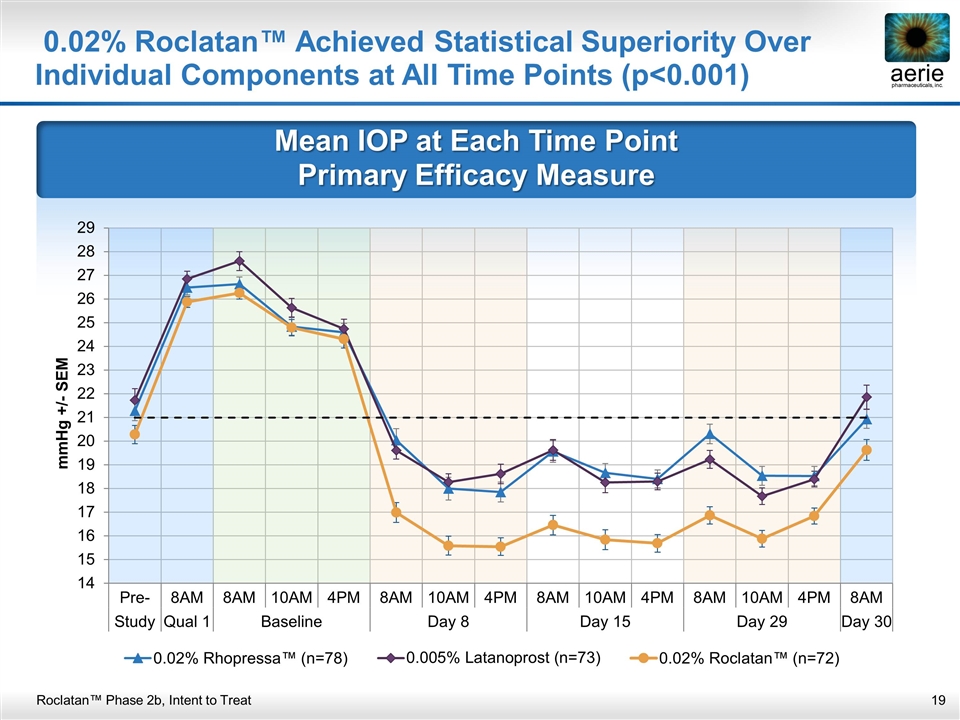
Mean IOP at Each Time Point Primary Efficacy Measure 0.02% Roclatan™ Achieved Statistical Superiority Over Individual Components at All Time Points (p<0.001) Roclatan™ Phase 2b, Intent to Treat
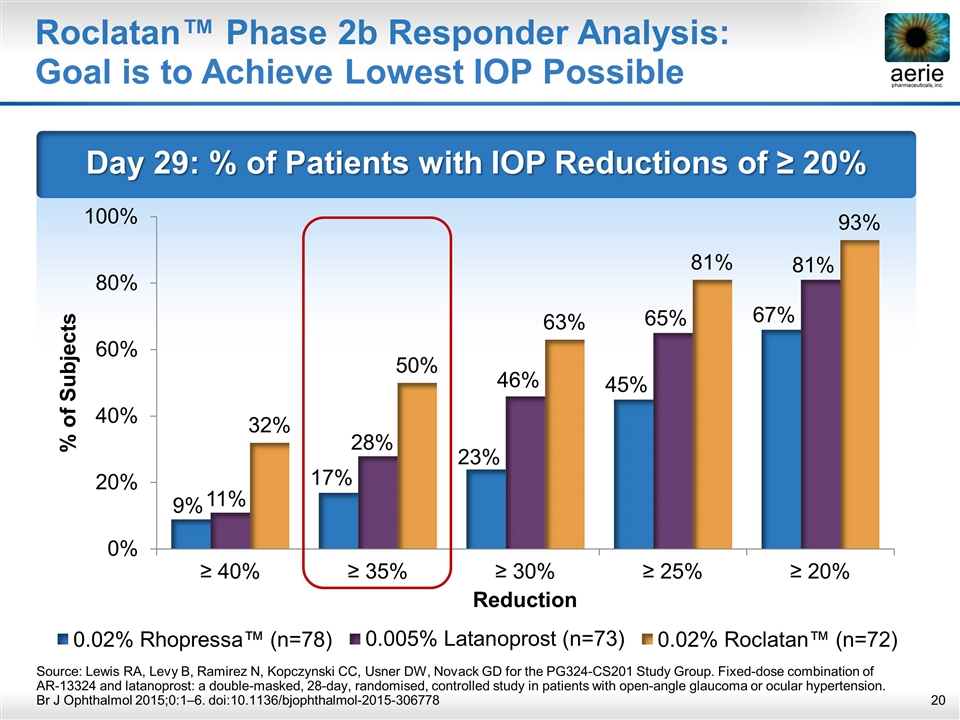
Day 29: % of Patients with IOP Reductions of ≥ 20% Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible Source: Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD for the PG324-CS201 Study Group. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol 2015;0:1–6. doi:10.1136/bjophthalmol-2015-306778
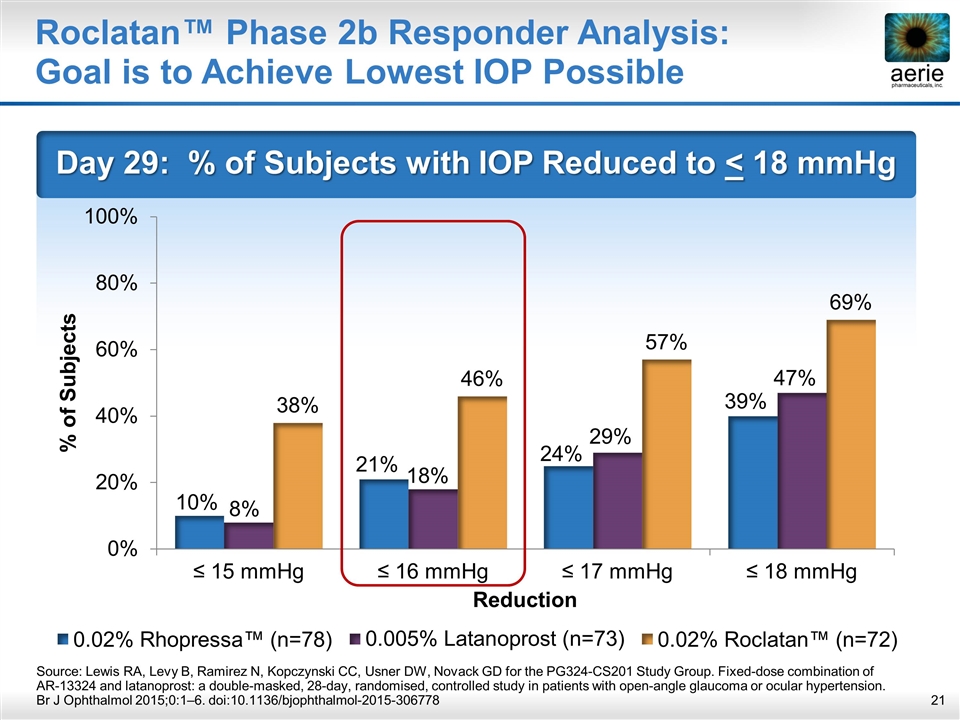
Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible Day 29: % of Subjects with IOP Reduced to < 18 mmHg Source: Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD for the PG324-CS201 Study Group. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol 2015;0:1–6. doi:10.1136/bjophthalmol-2015-306778
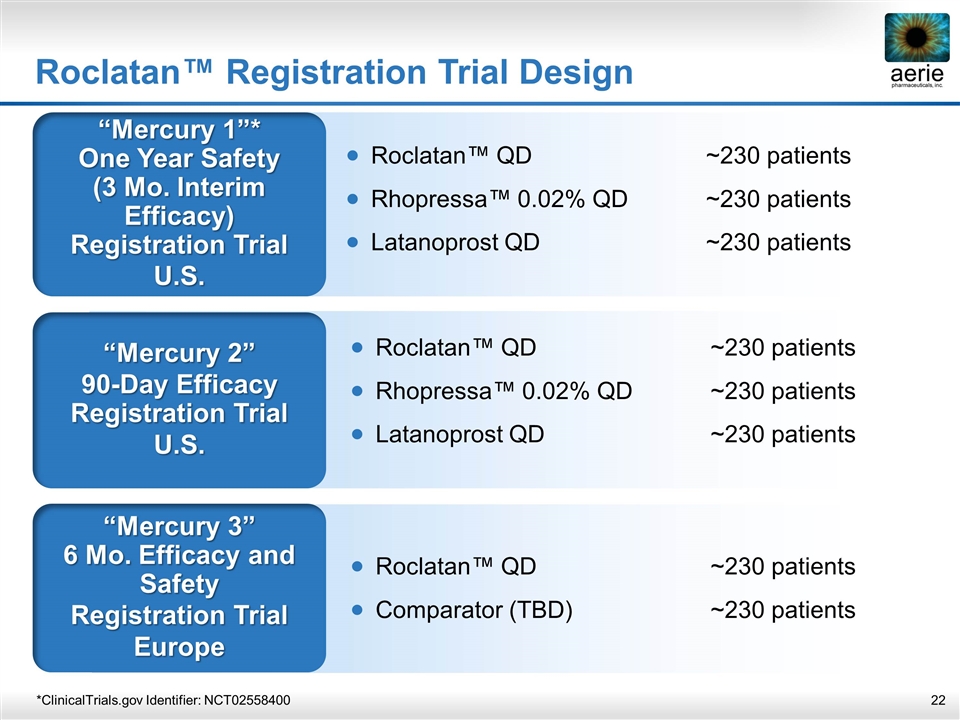
Roclatan™ Registration Trial Design “Mercury 1”* One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. “Mercury 2” 90-Day Efficacy Registration Trial U.S. “Mercury 3” 6 Mo. Efficacy and Safety Registration Trial Europe Roclatan™ QD~230 patients Rhopressa™ 0.02% QD~230 patients Latanoprost QD~230 patients Roclatan™ QD~230 patients Comparator (TBD)~230 patients *ClinicalTrials.gov Identifier: NCT02558400 Roclatan™ QD~230 patients Rhopressa™ 0.02% QD~230 patients Latanoprost QD~230 patients
Enhancing the Aerie Pipeline RhopressaTM preclinical findings Disease modification potential – anti-fibrotic and increased perfusion Neuroprotection potential From our owned molecule library – preclinical AR-13154 for wet AMD and diabetic retinopathy In-licensing opportunities – GrayBug for sustained drug delivery and Ramot product EG-30, anti-beta amyloid, for neuroprotection and dry AMD
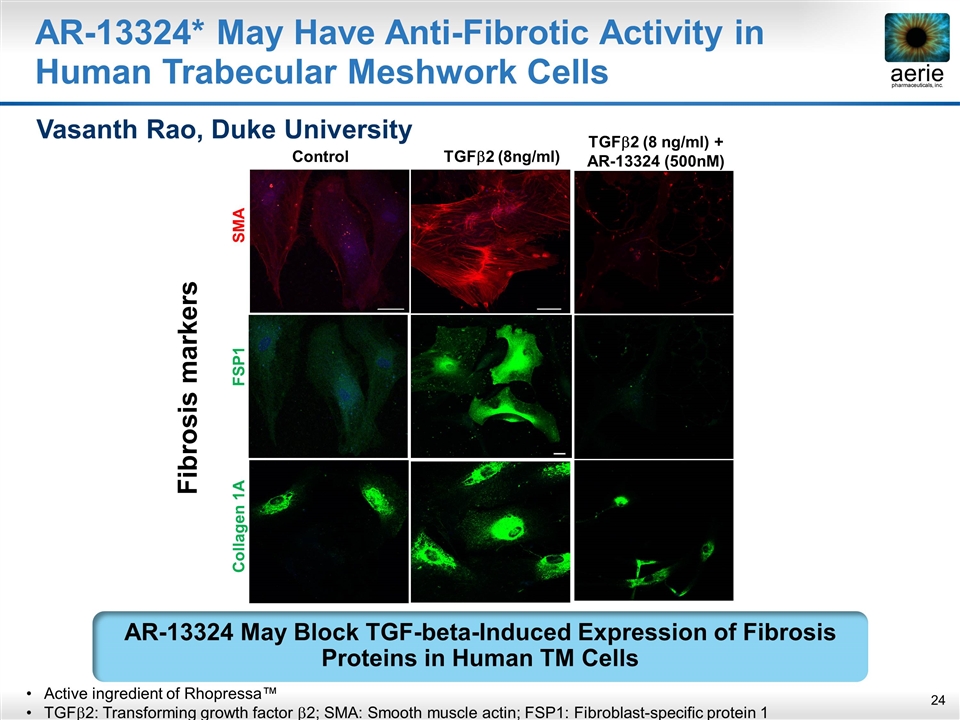
AR-13324* May Have Anti-Fibrotic Activity in Human Trabecular Meshwork Cells Fibrosis markers Control TGFb2 (8ng/ml) Collagen 1A FSP1 SMA TGFb2 (8 ng/ml) + AR-13324 (500nM) Vasanth Rao, Duke University Active ingredient of Rhopressa™ TGFb2: Transforming growth factor b2; SMA: Smooth muscle actin; FSP1: Fibroblast-specific protein 1 AR-13324 May Block TGF-beta-Induced Expression of Fibrosis Proteins in Human TM Cells
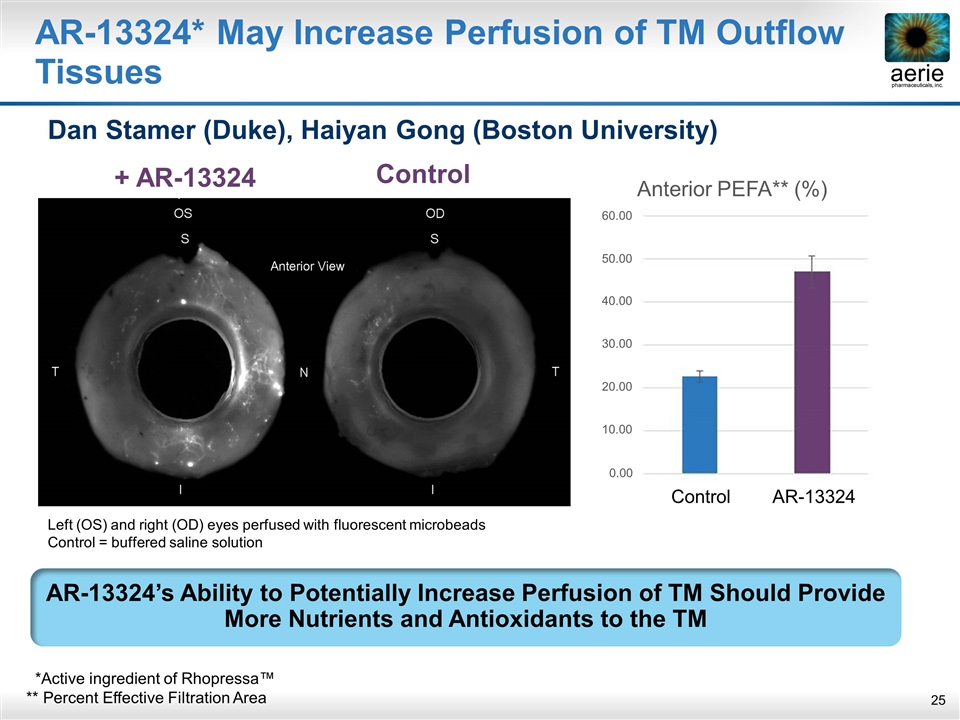
+ AR-13324 Control AR-13324* May Increase Perfusion of TM Outflow Tissues Dan Stamer (Duke), Haiyan Gong (Boston University) AR-13324’s Ability to Potentially Increase Perfusion of TM Should Provide More Nutrients and Antioxidants to the TM Left (OS) and right (OD) eyes perfused with fluorescent microbeads Control = buffered saline solution *Active ingredient of Rhopressa™ ** Percent Effective Filtration Area Control AR-13324 0.00 10.00 20.00 30.00 40.00 50.00 60.00 Anterior PEFA** (%)
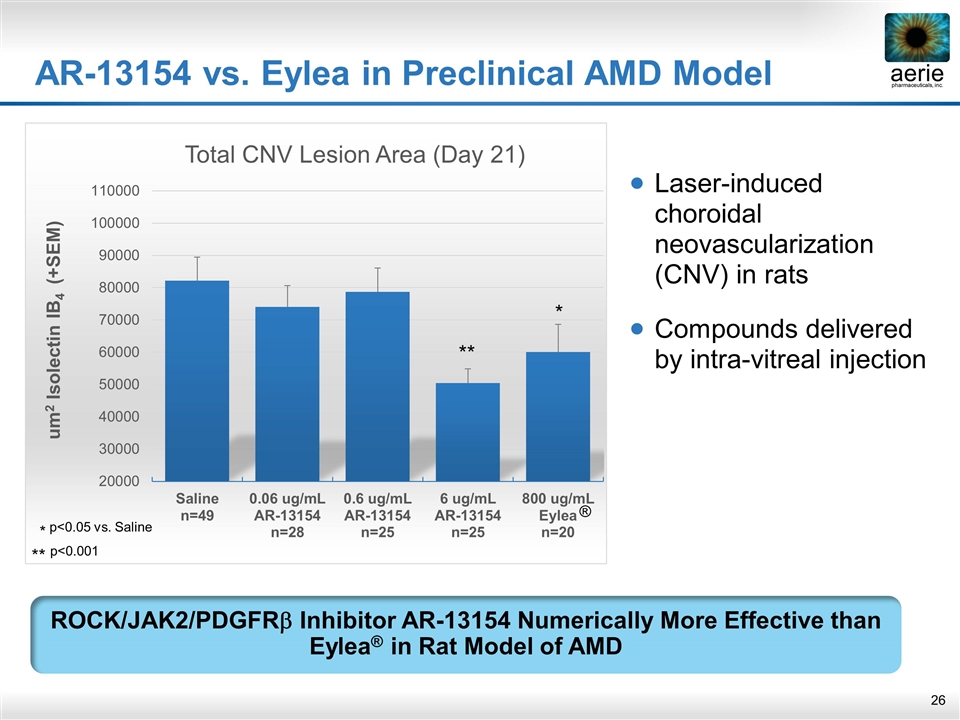
Laser-induced choroidal neovascularization (CNV) in rats Compounds delivered by intra-vitreal injection AR-13154 vs. Eylea in Preclinical AMD Model ROCK/JAK2/PDGFRb Inhibitor AR-13154 Numerically More Effective than Eylea® in Rat Model of AMD ** * * p<0.05 vs. Saline ** p<0.001 ®
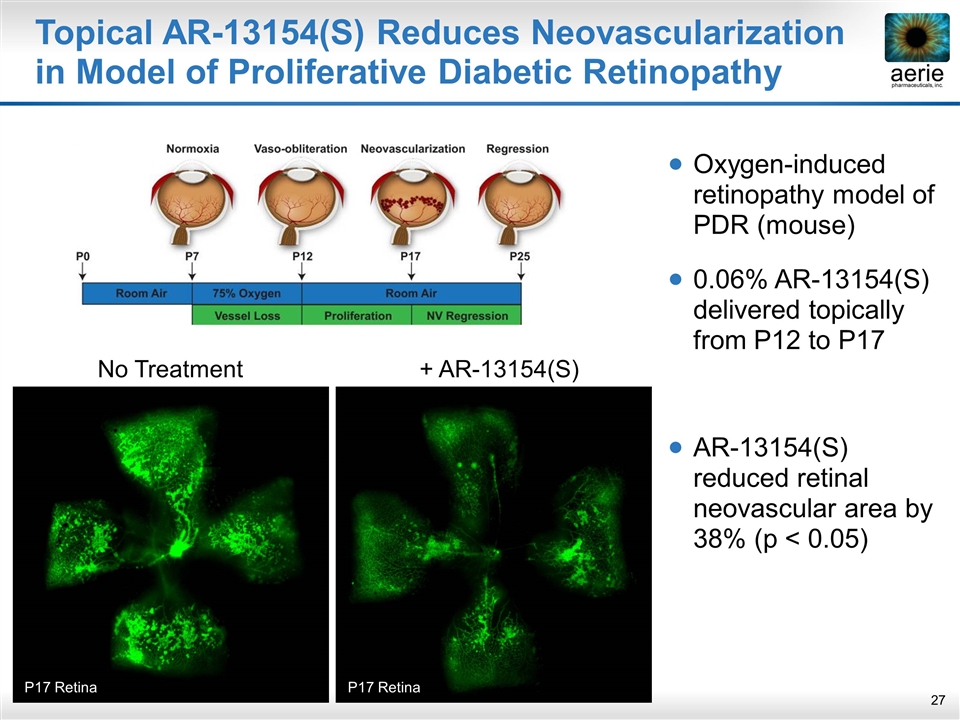
Topical AR-13154(S) Reduces Neovascularization in Model of Proliferative Diabetic Retinopathy Oxygen-induced retinopathy model of PDR (mouse) 0.06% AR-13154(S) delivered topically from P12 to P17 AR-13154(S) reduced retinal neovascular area by 38% (p < 0.05) No Treatment P17 Retina + AR-13154(S) P17 Retina

Aerie Research Collaborations GrayBug Drug delivery technology spin-out from Johns Hopkins University Sustained release injectable technology with capability of delivering compounds to the front and back of the eye Biodegradable polymer technology with potential for multi-month delivery Efforts initially focused on delivery of AR-13154 to back of the eye Ramot at Tel Aviv University Pre-clinical anti-beta amyloid small molecule product candidate (EG 30) Potential for neuroprotection in glaucoma and reduction of geographic atrophy in advanced dry AMD Collaboration and license agreement covering all ophthalmic indications
Summary Key Clinical Priorities RhopressaTM: NDA filing expected Q3 2016 Rocket 4 in process (not required for NDA filing) RoclatanTM: Mercury 1 in process Mercury 2 commences Q2 2016 Research Initiatives RhopressaTM disease modification and neuro-protection AR-13154 potential in wet AMD, etc. Evaluating Aerie’s 3,000+ owned molecules Business Development Opportunities Evaluating additions to ophthalmic product pipeline - e.g., Ramot Drug delivery opportunities for front and back of the eye - e.g., GrayBug Note: As of September 30, 2015, Aerie had $163 million of cash and marketable securities on the balance sheet
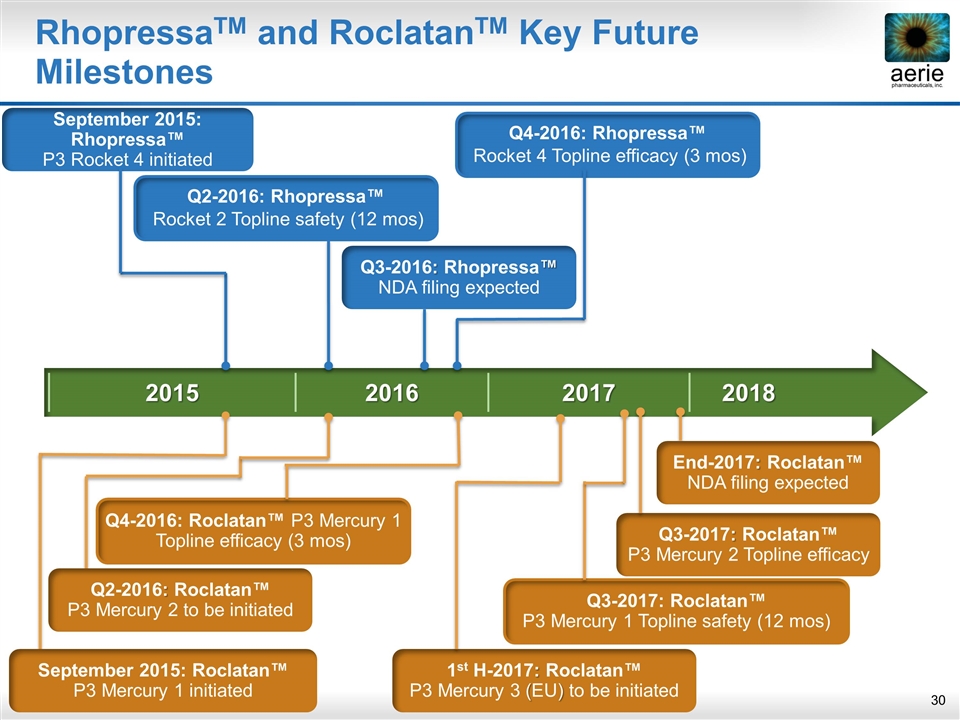
2015 2016 2017 2018 RhopressaTM and RoclatanTM Key Future Milestones Q3-2017: Roclatan™ P3 Mercury 2 Topline efficacy End-2017: Roclatan™ NDA filing expected 1st H-2017: Roclatan™ P3 Mercury 3 (EU) to be initiated September 2015: Rhopressa™ P3 Rocket 4 initiated Q2-2016: Rhopressa™ Rocket 2 Topline safety (12 mos) Q3-2016: Rhopressa™ NDA filing expected Q4-2016: Rhopressa™ Rocket 4 Topline efficacy (3 mos) Q4-2016: Roclatan™ P3 Mercury 1 Topline efficacy (3 mos) Q3-2017: Roclatan™ P3 Mercury 1 Topline safety (12 mos) September 2015: Roclatan™ P3 Mercury 1 initiated Q2-2016: Roclatan™ P3 Mercury 2 to be initiated

Building a Major Ophthalmic Pharmaceutical Company






























