RhopressaTM (netarsudil ophthalmic solution) 0.02% Rocket 2 Phase 3 Interim 12-Month Safety Results Exhibit 99.2

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
RhopressaTM Achieves Positive Interim 12-month Safety Results FDA requires at least 100 patients on RhopressaTM QD completing twelve-month period for NDA The first 118 patients on RhopressaTM QD completed the twelve-month period Adverse event profile in Month 12 similar to Month 3 Stable efficacy from Month 3 to Month 12 On target to file NDA in Q3 2016
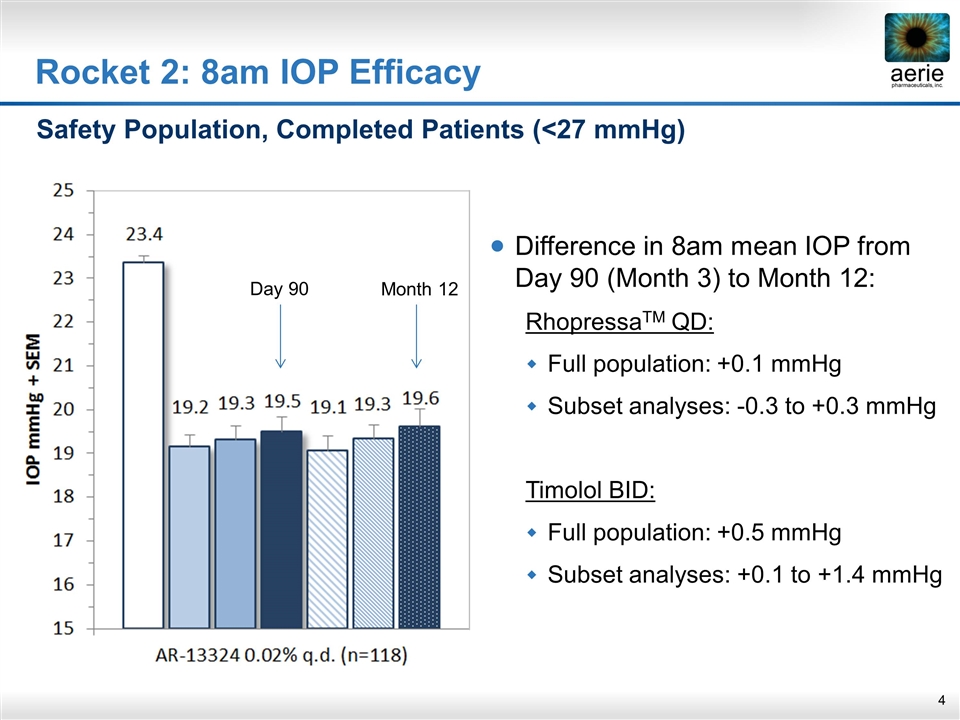
Rocket 2: 8am IOP Efficacy Safety Population, Completed Patients (<27 mmHg) Difference in 8am mean IOP from Day 90 (Month 3) to Month 12: RhopressaTM QD: Full population: +0.1 mmHg Subset analyses: -0.3 to +0.3 mmHg Timolol BID: Full population: +0.5 mmHg Subset analyses: +0.1 to +1.4 mmHg Day 90 Month 12
Safety/Tolerability Overview of RhopressaTM QD (Interim 12-Month) *Incidence of conjunctival hyperemia ~50% (baseline 20%) There were no drug-related serious adverse events (SAEs) No new adverse events introduced over the twelve-month period The most common adverse event was conjunctival hyperemia Increased conjunctival hyperemia measured by biomicroscopy at 8 am was ~30%* of which 76% was mild at month 12 Hyperemia was sporadic – 70% of patients with prior conjunctival hyperemia had no hyperemia at month 12 Other ocular AEs AEs occurring in ~5-23% of patients included: conjunctival hemorrhage, corneal deposits, blurry vision and reduced visual acuity
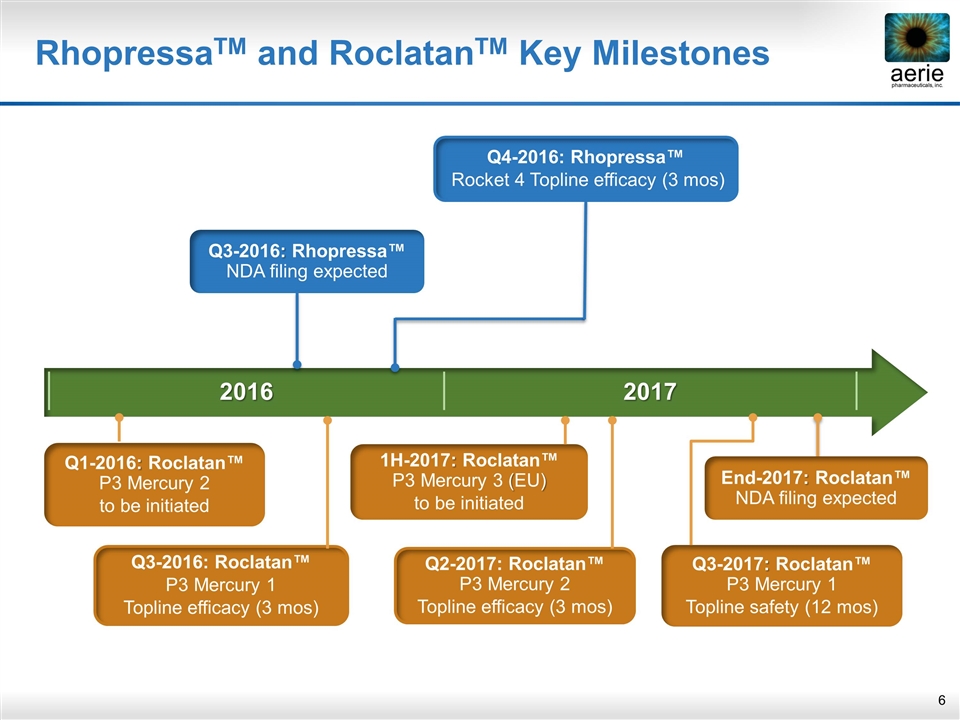
2016 2017 RhopressaTM and RoclatanTM Key Milestones Q3-2017: Roclatan™ P3 Mercury 1 Topline safety (12 mos) End-2017: Roclatan™ NDA filing expected 1H-2017: Roclatan™ P3 Mercury 3 (EU) to be initiated Q3-2016: Rhopressa™ NDA filing expected Q4-2016: Rhopressa™ Rocket 4 Topline efficacy (3 mos) Q3-2016: Roclatan™ P3 Mercury 1 Topline efficacy (3 mos) Q2-2017: Roclatan™ P3 Mercury 2 Topline efficacy (3 mos) Q1-2016: Roclatan™ P3 Mercury 2 to be initiated





