Update on RhopressaTM QD (netarsudil ophthalmic solution) 0.02% and RoclatanTM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% Exhibit 99.2

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.

Agenda Overview Rhopressa TM Safety and Efficacy Roclatan TM Regulatory Perspective AGS 2016 Physicians’ Perspective Q & A
++ TIMOPTIC® Package Insert In a study of plasma drug concentration in six subjects, the systemic exposure to timolol was determined following twice daily administration of TIMOPTIC 0.5%. The mean peak plasma concentration following morning dosing was 0.46 ng/mL and following afternoon dosing was 0.35 ng/mL. In these studies, TIMOPTIC was generally well tolerated and produced fewer and less severe side effects than either pilocarpine or epinephrine. A slight reduction of resting heart rate in some patients receiving. TIMOPTIC (mean reduction 2.9 beats/minute standard deviation 10.2) was observed. CONTRAINDICATIONS TIMOPTIC is contraindicated in patients with (1) bronchial asthma; (2) a history of bronchial asthma; (3) severe chronic obstructive pulmonary disease (see WARNINGS); (4) sinus bradycardia; (5) second or third degree atrioventricular block; (6) overt cardiac failure (see WARNINGS); (7) cardiogenic shock; or (8) hypersensitivity to any component of this product. Warnings As with many topically applied ophthalmic drugs, this drug is absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported following systemic or ophthalmic administration of timolol maleate. Systemic Safety Warnings for Timolol Use TIMOPTIC® Package Insert

Additional Timolol Considerations http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6146a2.htm Summary Health Statistics: National Health Interview Survey, 2014 Nelson-1986: Between September 1978 and December 1985, 450 case reports of serious respiratory and cardiovascular events and 32 case reports of death attributed to ophthalmic timolol were received by the United States Food and Drug Administration and the National Registry of Drug-Induced Ocular Side Effects. Based on Centers for Disease Control and Prevention data from 2011 and 2014, an estimated 47% of the population older than 65 years of age has heart disease and chronic obstructive pulmonary disease, all of which are contraindications to timolol.

Update on RhopressaTM QD (netarsudil ophthalmic solution) 0.02%

RhopressaTM FDA Communications FDA Pre-NDA Clinical Meeting (October ‘15) discussions: Minimum of 100 RhopressaTM QD patients with 12 months of safety data needed for NDA filing Detailed 3-month safety data on RhopressaTM QD were shared and discussed during the Pre-NDA meeting No requirement for Risk Management Plan ++Data on File
RhopressaTM QD Safety Profile To Date There were no drug-related serious adverse events (SAEs) No new adverse events observed over the twelve-month period compared to three-month period There was no evidence of treatment-related systemic effects (e.g., clinical laboratory or haematology values, heart rate or blood pressure) No systemic absorption (i.e. below level of detection) The most common adverse event was conjunctival hyperemia ~50% Other ocular AEs AEs occurring in ~5-23% of patients included: corneal deposits, conjunctival hemorrhage, vision blurred and visual acuity reduced ++Data on File Based on Rocket 2 3-Month Safety and 2 Interim 12-month safety
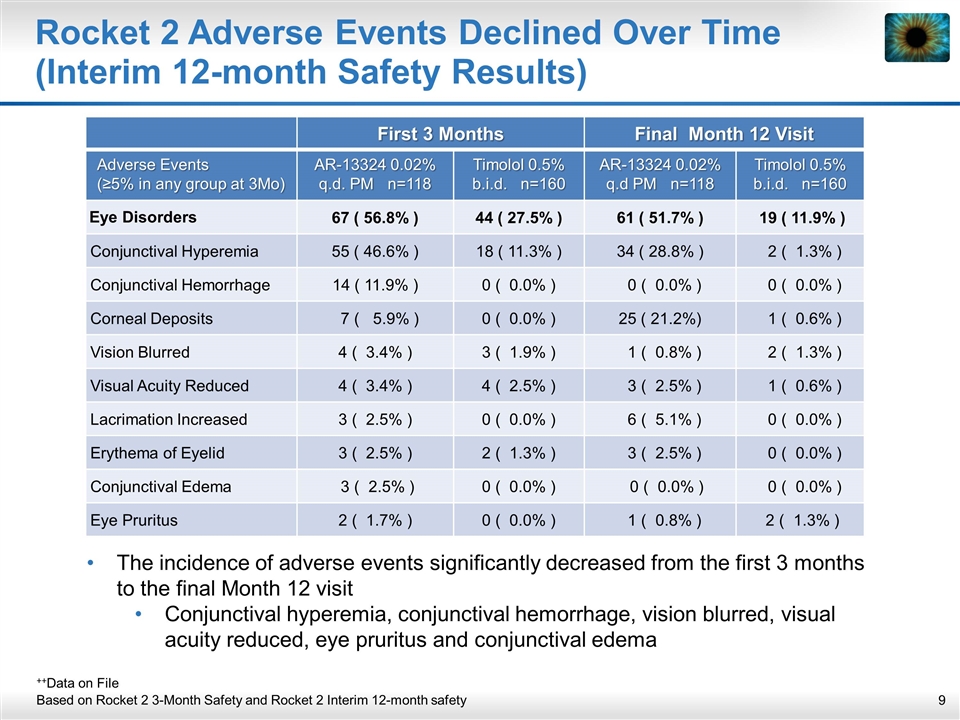
Rocket 2 Adverse Events Declined Over Time (Interim 12-month Safety Results) The incidence of adverse events significantly decreased from the first 3 months to the final Month 12 visit Conjunctival hyperemia, conjunctival hemorrhage, vision blurred, visual acuity reduced, eye pruritus and conjunctival edema ++Data on File Based on Rocket 2 3-Month Safety and Rocket 2 Interim 12-month safety First 3 Months Final Month 12 Visit Adverse Events (≥5% in any group at 3Mo) AR-13324 0.02% q.d. PM n=118 Timolol 0.5% b.i.d. n=160 AR-13324 0.02% q.d PM n=118 Timolol 0.5% b.i.d. n=160 Eye Disorders 67 ( 56.8% ) 44 ( 27.5% ) 61 ( 51.7% ) 19 ( 11.9% ) Conjunctival Hyperemia 55 ( 46.6% ) 18 ( 11.3% ) 34 ( 28.8% ) 2 ( 1.3% ) Conjunctival Hemorrhage 14 ( 11.9% ) 0 ( 0.0% ) 0 ( 0.0% ) 0 ( 0.0% ) Corneal Deposits 7 ( 5.9% ) 0 ( 0.0% ) 25 ( 21.2%) 1 ( 0.6% ) Vision Blurred 4 ( 3.4% ) 3 ( 1.9% ) 1 ( 0.8% ) 2 ( 1.3% ) Visual Acuity Reduced 4 ( 3.4% ) 4 ( 2.5% ) 3 ( 2.5% ) 1 ( 0.6% ) Lacrimation Increased 3 ( 2.5% ) 0 ( 0.0% ) 6 ( 5.1% ) 0 ( 0.0% ) Erythema of Eyelid 3 ( 2.5% ) 2 ( 1.3% ) 3 ( 2.5% ) 0 ( 0.0% ) Conjunctival Edema 3 ( 2.5% ) 0 ( 0.0% ) 0 ( 0.0% ) 0 ( 0.0% ) Eye Pruritus 2 ( 1.7% ) 0 ( 0.0% ) 1 ( 0.8% ) 2 ( 1.3% )
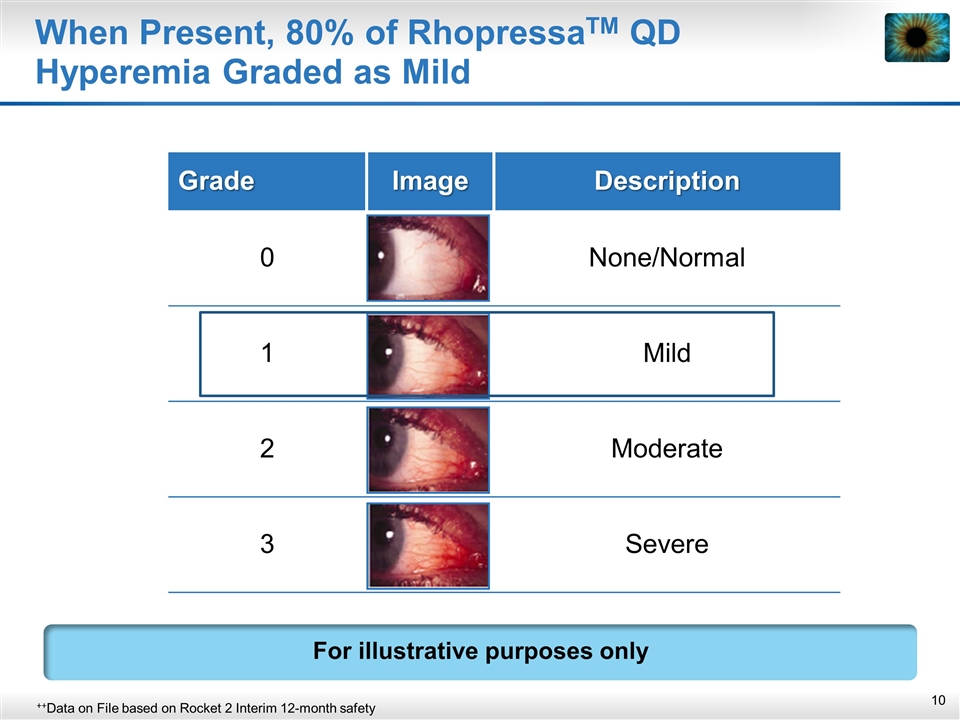
When Present, 80% of RhopressaTM QD Hyperemia Graded as Mild Grade Image Description 0 None/Normal 1 Mild 2 Moderate 3 Severe ++Data on File based on Rocket 2 Interim 12-month safety For illustrative purposes only
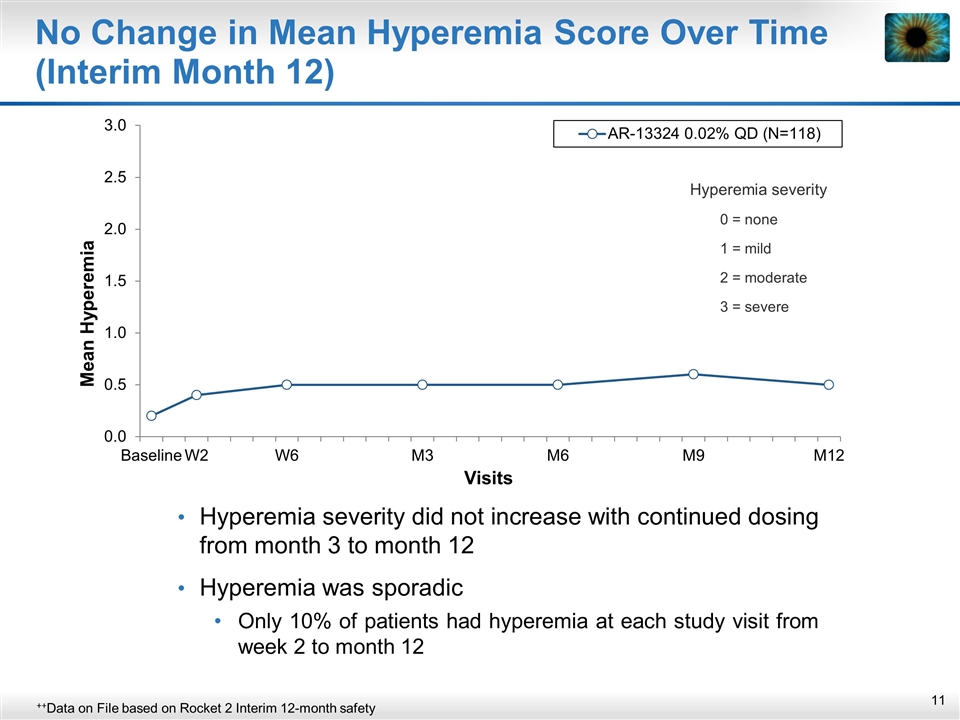
No Change in Mean Hyperemia Score Over Time (Interim Month 12) Hyperemia severity did not increase with continued dosing from month 3 to month 12 Hyperemia was sporadic Only 10% of patients had hyperemia at each study visit from week 2 to month 12 ++Data on File based on Rocket 2 Interim 12-month safety Hyperemia severity 0 = none 1 = mild 2 = moderate 3 = severe
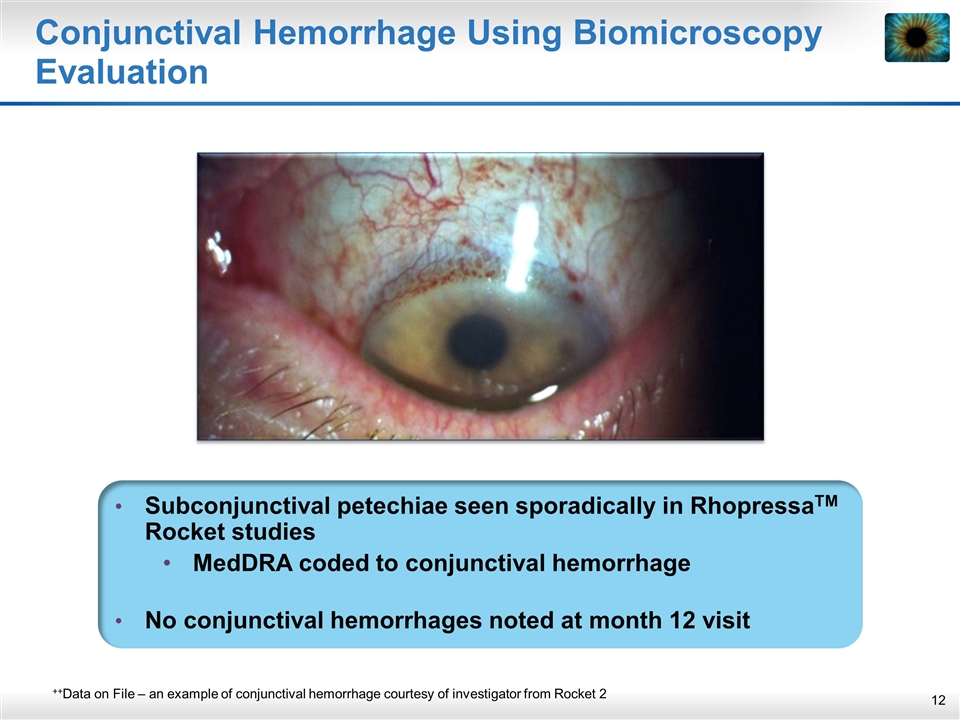
Conjunctival Hemorrhage Using Biomicroscopy Evaluation Subconjunctival petechiae seen sporadically in RhopressaTM Rocket studies MedDRA coded to conjunctival hemorrhage No conjunctival hemorrhages noted at month 12 visit ++Data on File – an example of conjunctival hemorrhage courtesy of investigator from Rocket 2
Corneal Deposits Are Asymptomatic And Did Not Affect Visual Acuity Corneal deposits (lipid microdeposits in the corneal epithelial layer) noted were: Asymptomatic - Did not affect visual acuity Only visible via biomicroscopy evaluation ~75% resolved to date ~30% resolved during continued RhopressaTM dosing Noted in RhopressaTM and timolol subjects RhopressaTM corneal deposits due to phospholipidosis Follow-up program in place Ophthalmologists are familiar with corneal deposits with other drugs such as Amiodarone ++Data on File – an example from Rocket 1 and 2 (courtesy of investigators) Cordarone (Amiodarone) Prescribing Information
Phospholipidosis: Well Understood by FDA ++FDA Phospholipidosis workshop 2010 FDA Phospholipidosis (PLD) workshop in 2010 Adaptive process More than 290 PLD-inducing compounds in FDA database Over 50 marketed products across ~25 drug classes
Other Corneal Evaluations in the Rocket Studies NDA submission with endothelial cell counts for 100 treated patients plus a control group for at least 3 months No significant changes in corneal endothelial cell density or hexagonality (shape) using specular microscopy seen in RhopressaTM No other significant corneal findings (including guttatae-like finding) were reported in RhopressaTM ++Data on File Topline 3-Month and Interim 12-Month Rocket 2
Incidence reduced over time: Topline 3-month: 4.4% for RhopressaTM, 1.6% for timolol Interim 12-month: 2.5% for RhopressaTM, 0.6% for timolol Visual Acuity Reduced Adverse Event: Infrequent, Sporadic and Self-Resolving Topline 3-month: Only 2 (0.8%) RhopressaTM patients had reduced visual acuity at more than 1 visit (with no change in severity) Typically limited to one eye (both eyes are treated) ++Data on File Topline 3-Month and Interim 12-Month Rocket 2
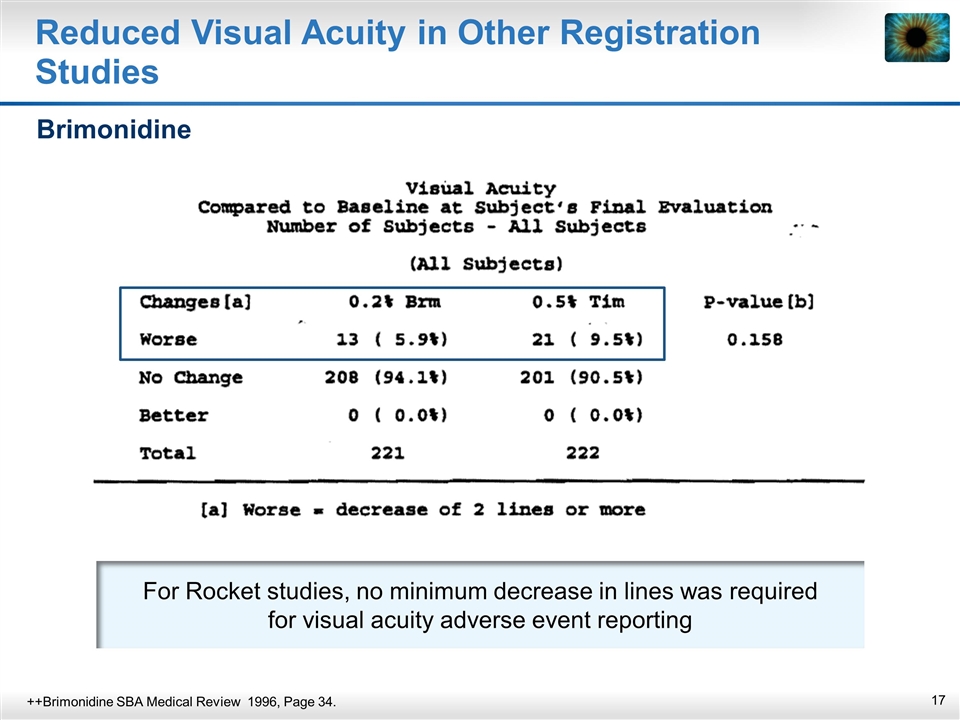
For Rocket studies, no minimum decrease in lines was required for visual acuity adverse event reporting Reduced Visual Acuity in Other Registration Studies Brimonidine ++Brimonidine SBA Medical Review 1996, Page 34.
Vision Blurred Adverse Event: Intermittent and Self-Resolving Topline 3-month: Patient reported adverse event 17 out of 18 RhopressaTM patients reporting blurred vision had no reduced visual acuity at office visit ++Data on File Based on Rocket 2 3-Month Safety and 2 Interim 12-month safety Incidence reduced over time: Topline 3-month: 7.2% for RhopressaTM, 2.8% for timolol Interim 12-month: 0.8% for RhopressaTM, 1.3% for timolol
Systemic Safety: RhopressaTM There was no evidence of treatment-related systemic effects (e.g., clinical laboratory or haematology values, heart rate or blood pressure) No systemic absorption in prior PK study (i.e. below level of detection) ++Data on File Based on Rocket 2 3-Month Safety and 2 Interim 12-month safety
Systemic Safety: Warnings for Timolol Use Systemic AEs Referenced in the timolol Label WARNINGS The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported following systemic or ophthalmic administration of timolol maleate ++Package Insert Because timolol is the comparator in the Rocket studies, patients with known contraindication or hypersensitivity to timolol/Beta-blockers were excluded.
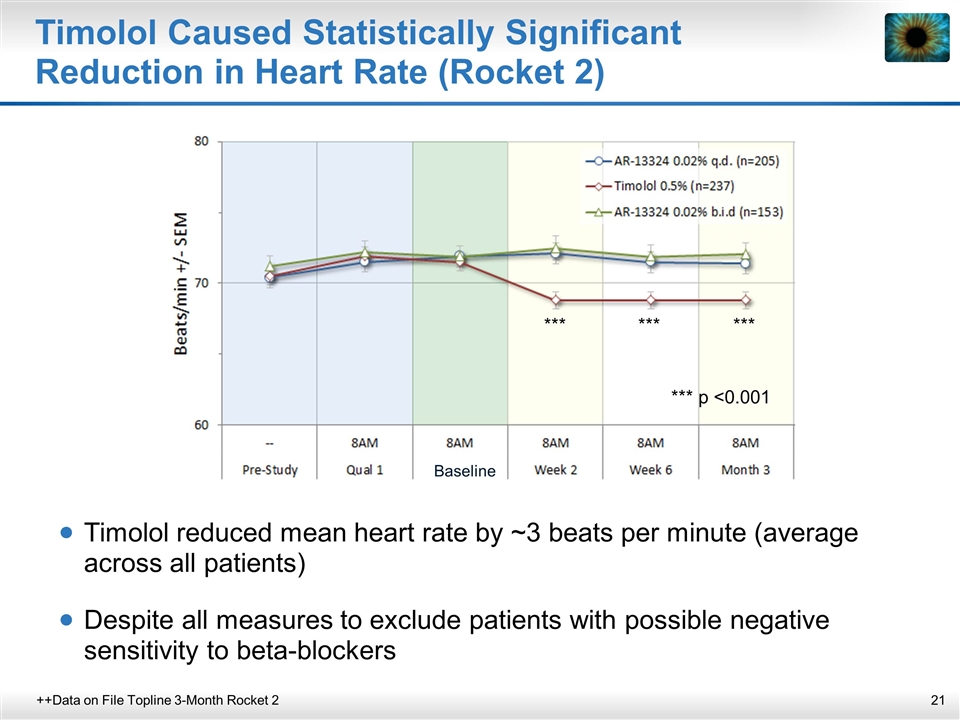
Timolol Caused Statistically Significant Reduction in Heart Rate (Rocket 2) ++Data on File Topline 3-Month Rocket 2 Timolol reduced mean heart rate by ~3 beats per minute (average across all patients) Despite all measures to exclude patients with possible negative sensitivity to beta-blockers *** *** *** *** p <0.001 Baseline
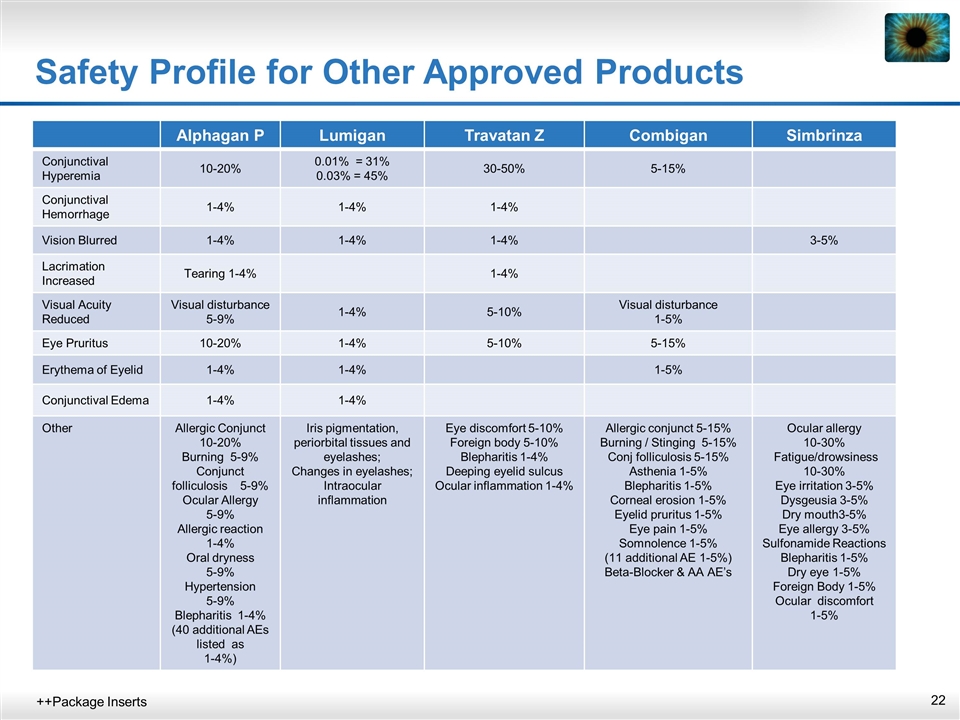
Safety Profile for Other Approved Products ++Package Inserts Alphagan P Lumigan Travatan Z Combigan Simbrinza Conjunctival Hyperemia 10-20% 0.01% = 31% 0.03% = 45% 30-50% 5-15% Conjunctival Hemorrhage 1-4% 1-4% 1-4% Vision Blurred 1-4% 1-4% 1-4% 3-5% Lacrimation Increased Tearing 1-4% 1-4% Visual Acuity Reduced Visual disturbance 5-9% 1-4% 5-10% Visual disturbance 1-5% Eye Pruritus 10-20% 1-4% 5-10% 5-15% Erythema of Eyelid 1-4% 1-4% 1-5% Conjunctival Edema 1-4% 1-4% Other Allergic Conjunct 10-20% Burning 5-9% Conjunct folliculosis 5-9% Ocular Allergy 5-9% Allergic reaction 1-4% Oral dryness 5-9% Hypertension 5-9% Blepharitis 1-4% (40 additional AEs listed as 1-4%) Iris pigmentation, periorbital tissues and eyelashes; Changes in eyelashes; Intraocular inflammation Eye discomfort 5-10% Foreign body 5-10% Blepharitis 1-4% Deeping eyelid sulcus Ocular inflammation 1-4% Allergic conjunct 5-15% Burning / Stinging 5-15% Conj folliculosis 5-15% Asthenia 1-5% Blepharitis 1-5% Corneal erosion 1-5% Eyelid pruritus 1-5% Eye pain 1-5% Somnolence 1-5% (11 additional AE 1-5%) Beta-Blocker & AA AE’s Ocular allergy 10-30% Fatigue/drowsiness 10-30% Eye irritation 3-5% Dysgeusia 3-5% Dry mouth3-5% Eye allergy 3-5% Sulfonamide Reactions Blepharitis 1-5% Dry eye 1-5% Foreign Body 1-5% Ocular discomfort 1-5%
RhopressaTM On Target To File NDA Completed 90-day efficacy and 90-day safety for Rocket 2 and Rocket 1, which was shared with FDA, in October 2015 The first 118 patients on RhopressaTM QD completed the 12-month period Adverse event profile in Month 12 similar to Month 3 Stable efficacy from Month 3 to Month 12 On target to file NDA in Q3 2016 ++Data on File. Based on Rocket 2 3-month and Interim 12-month data

Update on RoclatanTM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005%
RoclatanTM Phase 3 Mercury Studies Were Designed to Replicate the Positive Phase 2b Results RoclatanTM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% Phase 2b Study Demonstrated Significant IOP-Lowering Therapy Achieved primary efficacy outcome of superiority over each of the components on day 29 Mean diurnal IOP reduction on day 29 was approximately 2 mmHg better than latanoprost Achieved statistical superiority over the individual components at all time points More efficacious than latanoprost by 1.6 – 3.2 mmHg More efficacious than AR-13324 by 1.7 – 3.4 mmHg The main adverse event was hyperemia, or eye redness, reported in 40 percent of patients and scored as mild for the large majority of patients No systemic drug-related adverse events Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Lewis RA, et al. Br J Ophthalmol 2016;100:339–344. doi:10.1136/bjophthalmol-2015-306778
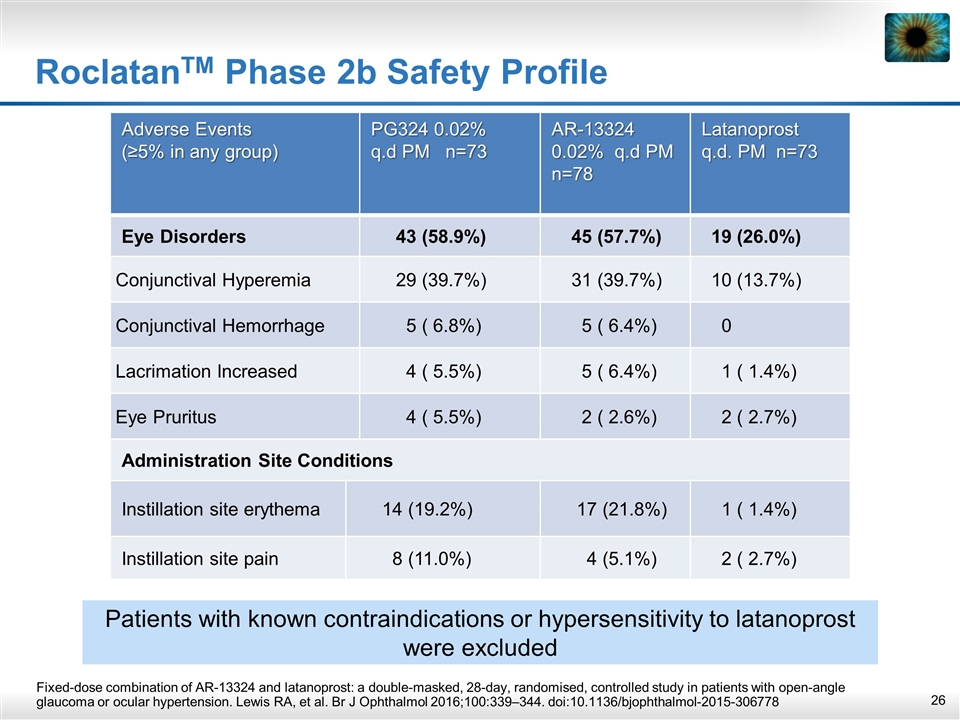
RoclatanTM Phase 2b Safety Profile Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Lewis RA, et al. Br J Ophthalmol 2016;100:339–344. doi:10.1136/bjophthalmol-2015-306778 Adverse Events (≥5% in any group) PG324 0.02% q.d PM n=73 AR-13324 0.02% q.d PM n=78 Latanoprost q.d. PM n=73 Eye Disorders 43 (58.9%) 45 (57.7%) 19 (26.0%) Conjunctival Hyperemia 29 (39.7%) 31 (39.7%) 10 (13.7%) Conjunctival Hemorrhage 5 ( 6.8%) 5 ( 6.4%) 0 Lacrimation Increased 4 ( 5.5%) 5 ( 6.4%) 1 ( 1.4%) Eye Pruritus 4 ( 5.5%) 2 ( 2.6%) 2 ( 2.7%) Administration Site Conditions Instillation site erythema 14 (19.2%) 17 (21.8%) 1 ( 1.4%) Instillation site pain 8 (11.0%) 4 (5.1%) 2 ( 2.7%) Patients with known contraindications or hypersensitivity to latanoprost were excluded
Fixed-Dose Combination ++Data on File. FDA guidance, presentations and sponsor meetings. Trial design follows FDA requirement for fixed-dose combination (FDC) Superiority of combination over each individual component Statistically significant difference at measured time points Higher combo efficacy vs. components of at least ~1–3 mmHg, as previously accepted by FDA for product approval Favorable safety profile
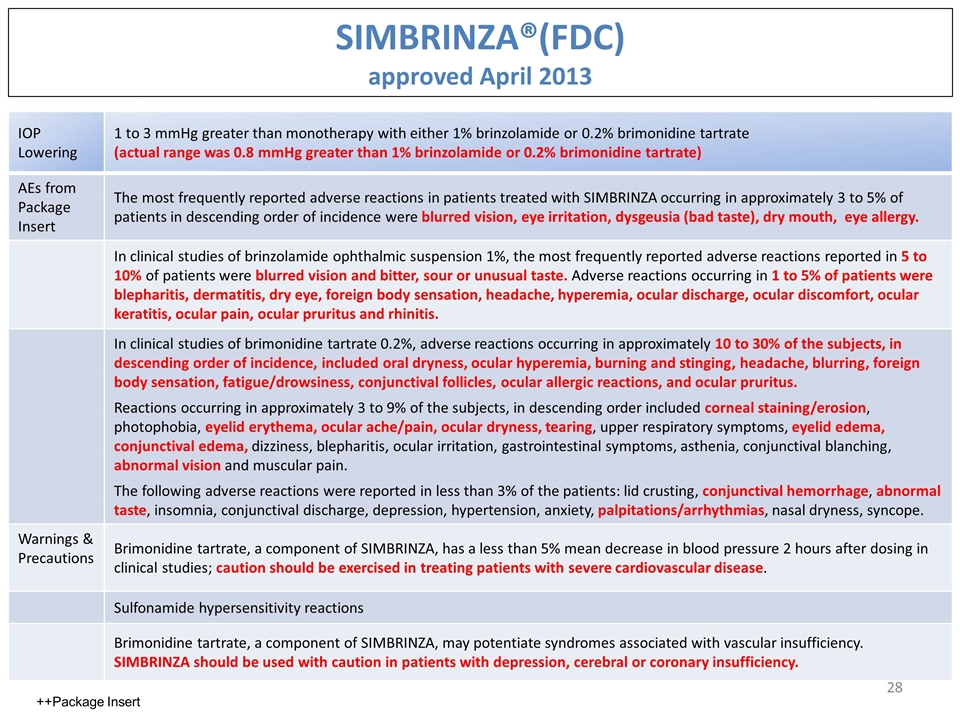
SIMBRINZA®(FDC) approved April 2013 IOP Lowering 1 to 3 mmHg greater than monotherapy with either 1% brinzolamide or 0.2% brimonidine tartrate (actual range was 0.8 mmHg greater than 1% brinzolamide or 0.2% brimonidine tartrate) AEs from Package Insert The most frequently reported adverse reactions in patients treated with SIMBRINZA occurring in approximately 3 to 5% of patients in descending order of incidence were blurred vision, eye irritation, dysgeusia (bad taste), dry mouth, eye allergy. In clinical studies of brinzolamide ophthalmic suspension 1%, the most frequently reported adverse reactions reported in 5 to 10% of patients were blurred vision and bitter, sour or unusual taste. Adverse reactions occurring in 1 to 5% of patients were blepharitis, dermatitis, dry eye, foreign body sensation, headache, hyperemia, ocular discharge, ocular discomfort, ocular keratitis, ocular pain, ocular pruritus and rhinitis. In clinical studies of brimonidine tartrate 0.2%, adverse reactions occurring in approximately 10 to 30% of the subjects, in descending order of incidence, included oral dryness, ocular hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus. Reactions occurring in approximately 3 to 9% of the subjects, in descending order included corneal staining/erosion, photophobia, eyelid erythema, ocular ache/pain, ocular dryness, tearing, upper respiratory symptoms, eyelid edema, conjunctival edema, dizziness, blepharitis, ocular irritation, gastrointestinal symptoms, asthenia, conjunctival blanching, abnormal vision and muscular pain. The following adverse reactions were reported in less than 3% of the patients: lid crusting, conjunctival hemorrhage, abnormal taste, insomnia, conjunctival discharge, depression, hypertension, anxiety, palpitations/arrhythmias, nasal dryness, syncope. Warnings & Precautions Brimonidine tartrate, a component of SIMBRINZA, has a less than 5% mean decrease in blood pressure 2 hours after dosing in clinical studies; caution should be exercised in treating patients with severe cardiovascular disease. Sulfonamide hypersensitivity reactions Brimonidine tartrate, a component of SIMBRINZA, may potentiate syndromes associated with vascular insufficiency. SIMBRINZA should be used with caution in patients with depression, cerebral or coronary insufficiency. ++Package Insert

Physicians’ Perspectives AGS 2016 Market Research Findings

Market Research from the American Glaucoma Society Annual Meeting (March 2016) ++Data on File 30 Glaucoma specialists participated in 90-minute sessions Rhopressa™ pre-clinical and clinical data were presented Rhopressa™ data were compared to all products used as adjunctive therapy to prostaglandins (PGA): MOAs Efficacy Safety Ease of Use

Physicians’ Perspectives on Clinical Data Glaucoma specialists were impressed with Rhopressa™ QD efficacy Rhopressa™ met non-inferiority to timolol at IOPs below 25 Brimonidine and CAIs did not The 12 month interim safety data which showed consistent IOP control from month 3 to month 12 Timolol and brimonidine are known to lose effectiveness through time There was a great deal of enthusiasm for using Rhopressa™ with a PGA Synergy with PGAs from Phase 2b and Phase 3 trials Physicians believe timolol, brimonidine and the CAIs are not very effective as adjuncts to PGAs QD dose & multiple and new MOAs Safety: No Serious Adverse Events (SAE’s) Physicians believe the side effects of Rhopressa™ are manageable and are not serious ++Data on File

Physicians’ Perspectives on Adverse Events Glaucoma specialists felt that the Rhopressa™ absence of systemic or serious AE’s was a significant benefit over timolol, brimonidine and CAIs After reviewing the data, when asked to select words that best describe Rhopressa™, the 3rd most popular choice (of the 15 selections) was “Safe” #1 was Unique and #2 was Effective Additionally: Conjunctival hemorrhages are not an issue when properly characterized as focal and small in size Corneal deposits, although benign, will need to be managed like the ocular side effects of PGAs (pigmentation, orbital fat loss) Hyperemia appears to be the same in incidence and severity as PGAs and can be managed Hyperemia is not as worrisome as brimonidine induced allergic conjunctivitis or timolol induced heart/lung effects ++Data on File
Physicians’ Perspectives: Rhopressa™ Summary ++Data on File Physicians recognized several potential important benefits that Rhopressa™ has over other adjunctive agents: Effectiveness as an additive therapy to PGAs “Rhopressa™ shines as an adjunct to a PGA” QD dosing put Rhopressa™ ahead of all others “The easiest adjunctive regimen” No serious AEs (SAEs) “Takes an unnecessary worry off the table” 93% of participants put Rhopressa™ in 1st position as an adjunct to PGAs
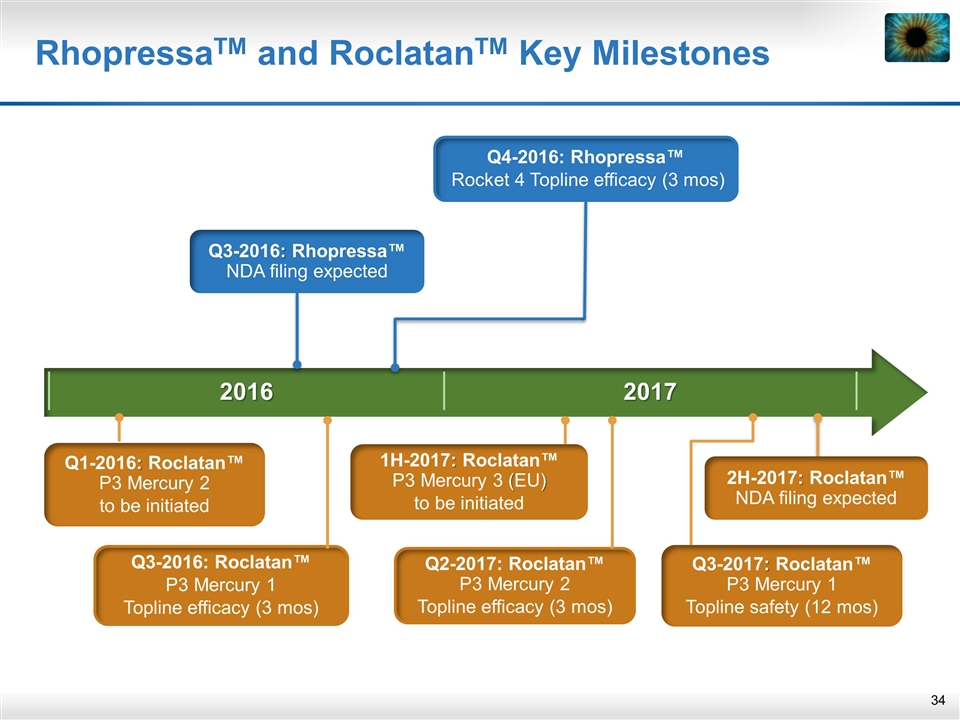
2016 2017 RhopressaTM and RoclatanTM Key Milestones Q3-2017: Roclatan™ P3 Mercury 1 Topline safety (12 mos) 2H-2017: Roclatan™ NDA filing expected 1H-2017: Roclatan™ P3 Mercury 3 (EU) to be initiated Q3-2016: Rhopressa™ NDA filing expected Q4-2016: Rhopressa™ Rocket 4 Topline efficacy (3 mos) Q3-2016: Roclatan™ P3 Mercury 1 Topline efficacy (3 mos) Q2-2017: Roclatan™ P3 Mercury 2 Topline efficacy (3 mos) Q1-2016: Roclatan™ P3 Mercury 2 to be initiated