
Aerie Pharmaceuticals, Inc. April 12, 2016 Building a Major Ophthalmic Pharmaceutical Company Exhibit 99.1

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. In addition, any discussion of clinical trial results for RhopressaTM (netarsudil ophthalmic solution) 0.02% relate to the results in its first Phase 3 registration trials, Rocket 1 and Rocket 2, and for RoclatanTM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% relate to the results in its Phase 2b clinical trial. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, the preclinical research discussed in this presentation is preliminary and the outcome of such preclinical studies may not be predictive of the outcome of later trials. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
Aerie Late Stage IOP–Lowering Products Pre-Clinical Research Rhopressa™ Potential for disease modification and neuroprotection AR-13154 Significant lesion size reduction in wet AMD Drug Delivery - Front and back of the eye Data on File Rhopressa™ (netarsudil ophthalmic solution) 0.02% Inhibits ROCK, NET, lowers EVP, targets diseased tissue NDA filing expected Q3 2016 Roclatan™ (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% Fixed combination of Rhopressa™ and latanoprost Two P3’s in process; topline efficacy expected Q3 2016 Aerie – Building a Major Ophthalmic Pharmaceutical Company
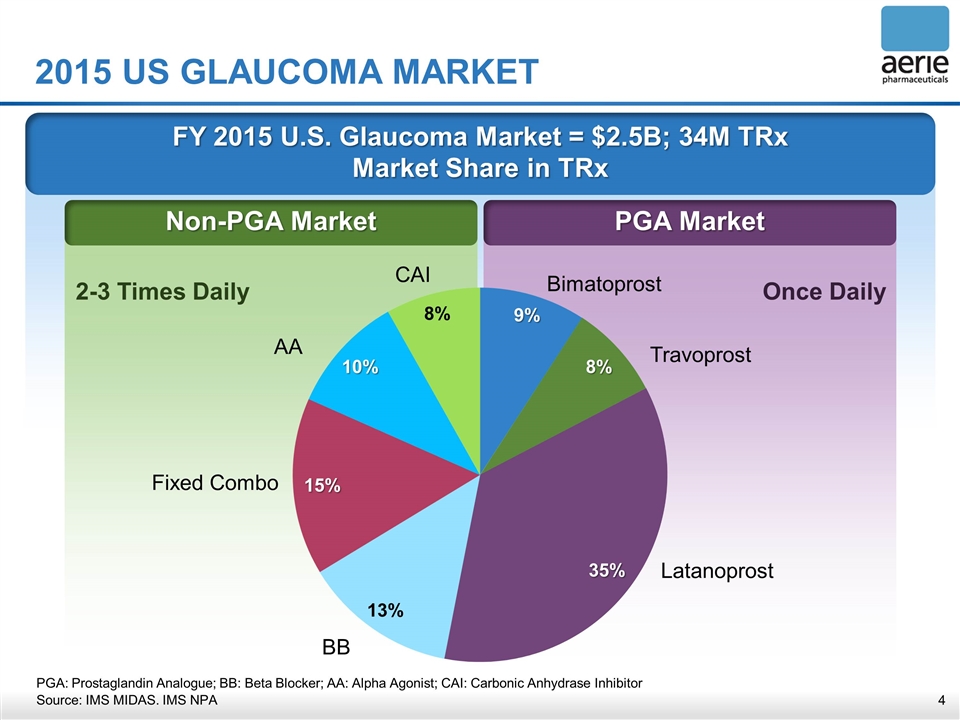
FY 2015 U.S. Glaucoma Market = $2.5B; 34M TRx Market Share in TRx PGA Market Non-PGA Market 2015 US GLAUCOMA MARKET Once Daily 2-3 Times Daily Bimatoprost Travoprost Latanoprost BB Fixed Combo AA CAI PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor Source: IMS MIDAS. IMS NPA
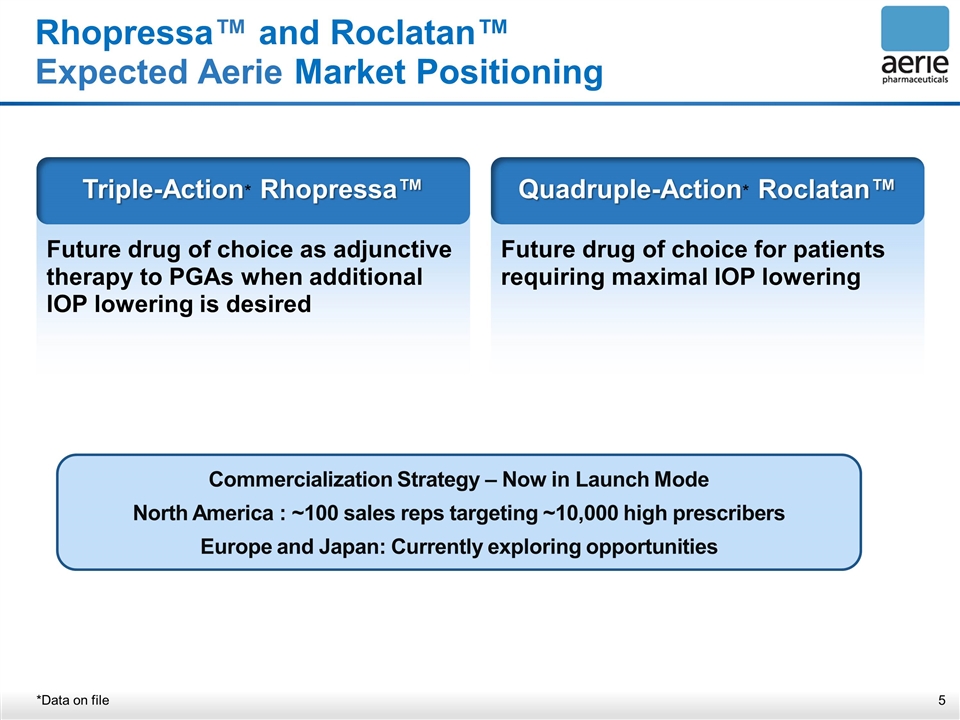
Rhopressa™ and Roclatan™ Expected Aerie Market Positioning *Data on file Future drug of choice as adjunctive therapy to PGAs when additional IOP lowering is desired Triple-Action* Rhopressa™ Future drug of choice for patients requiring maximal IOP lowering Quadruple-Action* Roclatan™ Commercialization Strategy – Now in Launch Mode North America : ~100 sales reps targeting ~10,000 high prescribers Europe and Japan: Currently exploring opportunities
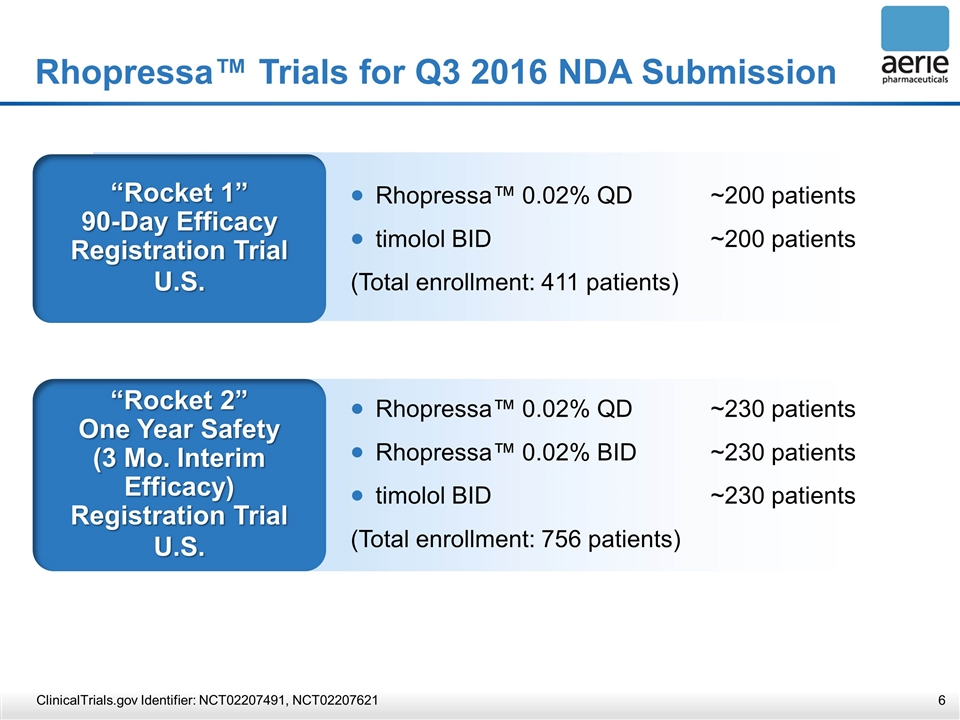
Rhopressa™ Trials for Q3 2016 NDA Submission “Rocket 1” 90-Day Efficacy Registration Trial U.S. Rhopressa™ 0.02% QD~200 patients timolol BID~200 patients (Total enrollment: 411 patients) “Rocket 2” One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. Rhopressa™ 0.02% QD~230 patients Rhopressa™ 0.02% BID~230 patients timolol BID~230 patients (Total enrollment: 756 patients) ClinicalTrials.gov Identifier: NCT02207491, NCT02207621
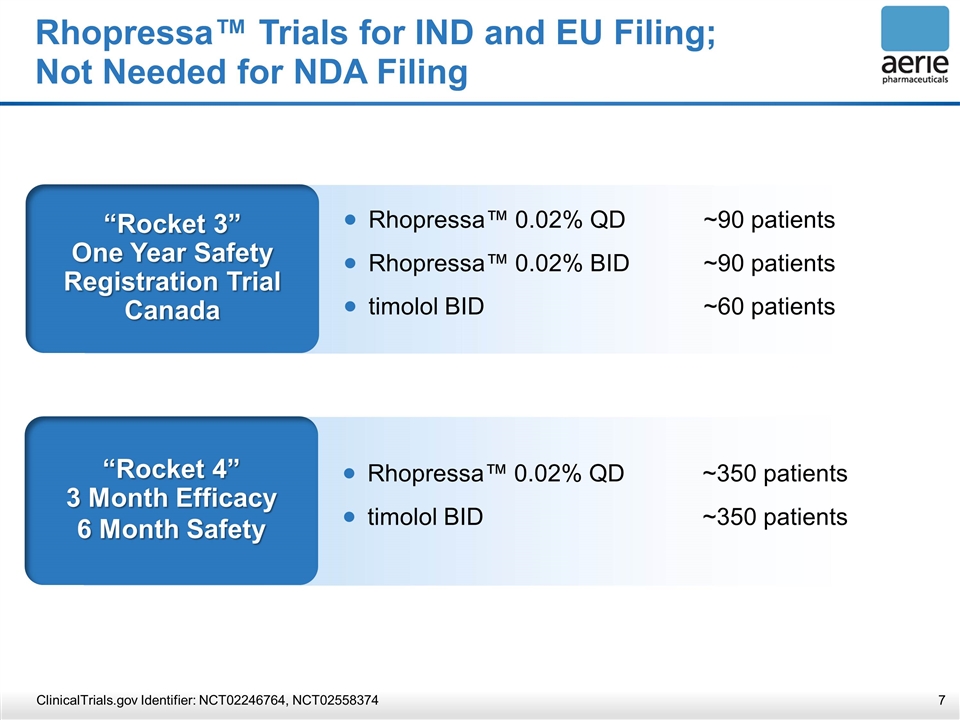
Rhopressa™ Trials for IND and EU Filing; Not Needed for NDA Filing ClinicalTrials.gov Identifier: NCT02246764, NCT02558374 “Rocket 4” 3 Month Efficacy 6 Month Safety Rhopressa™ 0.02% QD~350 patients timolol BID~350 patients “Rocket 3” One Year Safety Registration Trial Canada Rhopressa™ 0.02% QD~90 patients Rhopressa™ 0.02% BID ~90 patients timolol BID~60 patients

Rocket 2 and Rocket 1 Performance Maximum Baseline IOP <25 mmHg *Data on File PLACEHOLDER Rocket 2 Rocket 1
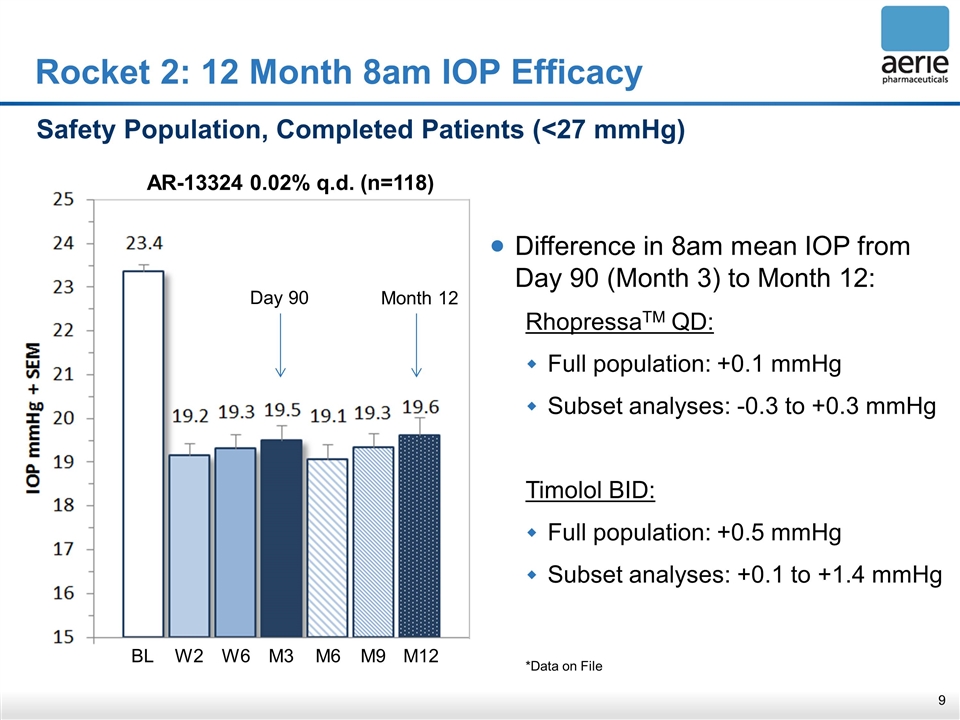
Rocket 2: 12 Month 8am IOP Efficacy Safety Population, Completed Patients (<27 mmHg) Difference in 8am mean IOP from Day 90 (Month 3) to Month 12: RhopressaTM QD: Full population: +0.1 mmHg Subset analyses: -0.3 to +0.3 mmHg Timolol BID: Full population: +0.5 mmHg Subset analyses: +0.1 to +1.4 mmHg *Data on File Day 90 Month 12 AR-13324 0.02% q.d. (n=118) BL W2 W6 M3 M6 M9 M12
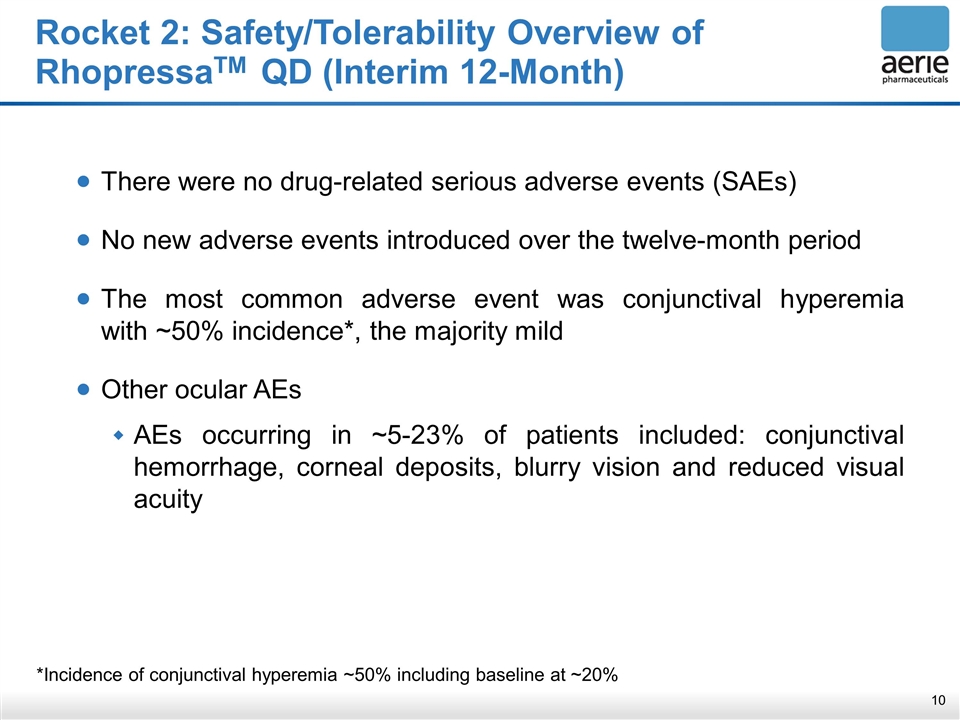
Rocket 2: Safety/Tolerability Overview of RhopressaTM QD (Interim 12-Month) *Incidence of conjunctival hyperemia ~50% including baseline at ~20% There were no drug-related serious adverse events (SAEs) No new adverse events introduced over the twelve-month period The most common adverse event was conjunctival hyperemia with ~50% incidence*, the majority mild Other ocular AEs AEs occurring in ~5-23% of patients included: conjunctival hemorrhage, corneal deposits, blurry vision and reduced visual acuity
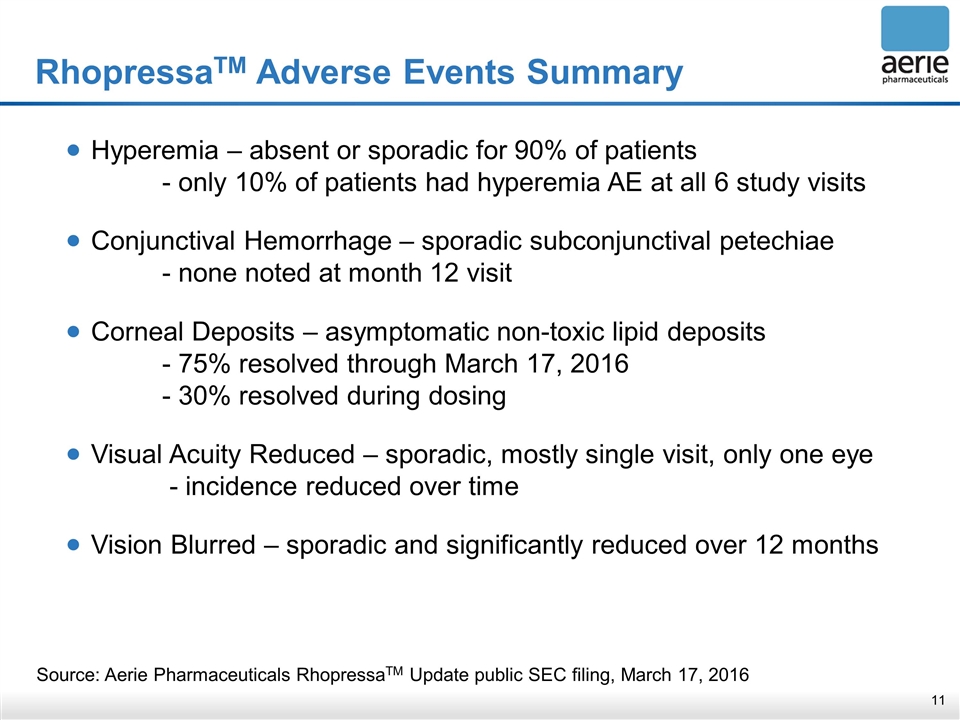
RhopressaTM Adverse Events Summary Source: Aerie Pharmaceuticals RhopressaTM Update public SEC filing, March 17, 2016 Hyperemia – absent or sporadic for 90% of patients - only 10% of patients had hyperemia AE at all 6 study visits Conjunctival Hemorrhage – sporadic subconjunctival petechiae - none noted at month 12 visit Corneal Deposits – asymptomatic non-toxic lipid deposits - 75% resolved through March 17, 2016 - 30% resolved during dosing Visual Acuity Reduced – sporadic, mostly single visit, only one eye - incidence reduced over time Vision Blurred – sporadic and significantly reduced over 12 months
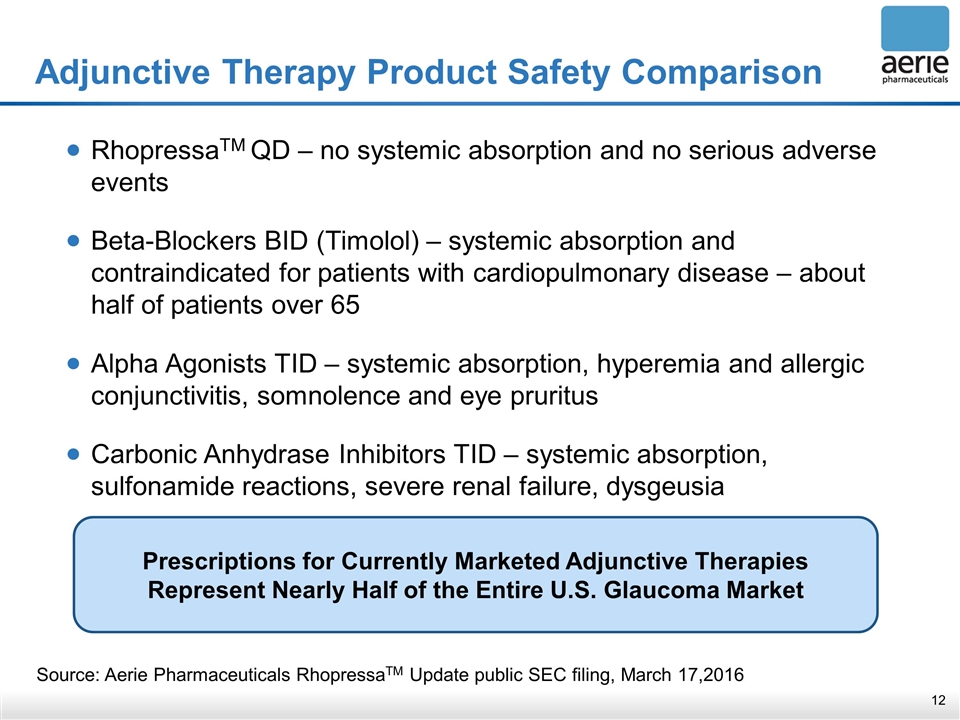
Adjunctive Therapy Product Safety Comparison Source: Aerie Pharmaceuticals RhopressaTM Update public SEC filing, March 17,2016 RhopressaTM QD – no systemic absorption and no serious adverse events Beta-Blockers BID (Timolol) – systemic absorption and contraindicated for patients with cardiopulmonary disease – about half of patients over 65 Alpha Agonists TID – systemic absorption, hyperemia and allergic conjunctivitis, somnolence and eye pruritus Carbonic Anhydrase Inhibitors TID – systemic absorption, sulfonamide reactions, severe renal failure, dysgeusia Prescriptions for Currently Marketed Adjunctive Therapies Represent Nearly Half of the Entire U.S. Glaucoma Market
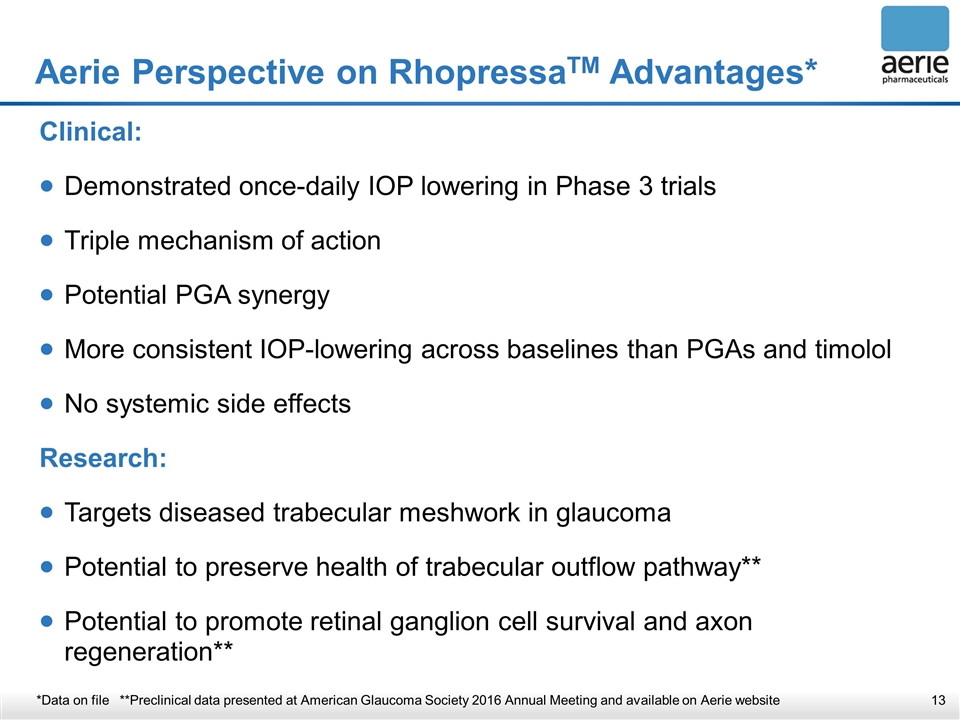
Aerie Perspective on RhopressaTM Advantages* *Data on file **Preclinical data presented at American Glaucoma Society 2016 Annual Meeting and available on Aerie website Clinical: Demonstrated once-daily IOP lowering in Phase 3 trials Triple mechanism of action Potential PGA synergy More consistent IOP-lowering across baselines than PGAs and timolol No systemic side effects Research: Targets diseased trabecular meshwork in glaucoma Potential to preserve health of trabecular outflow pathway** Potential to promote retinal ganglion cell survival and axon regeneration**
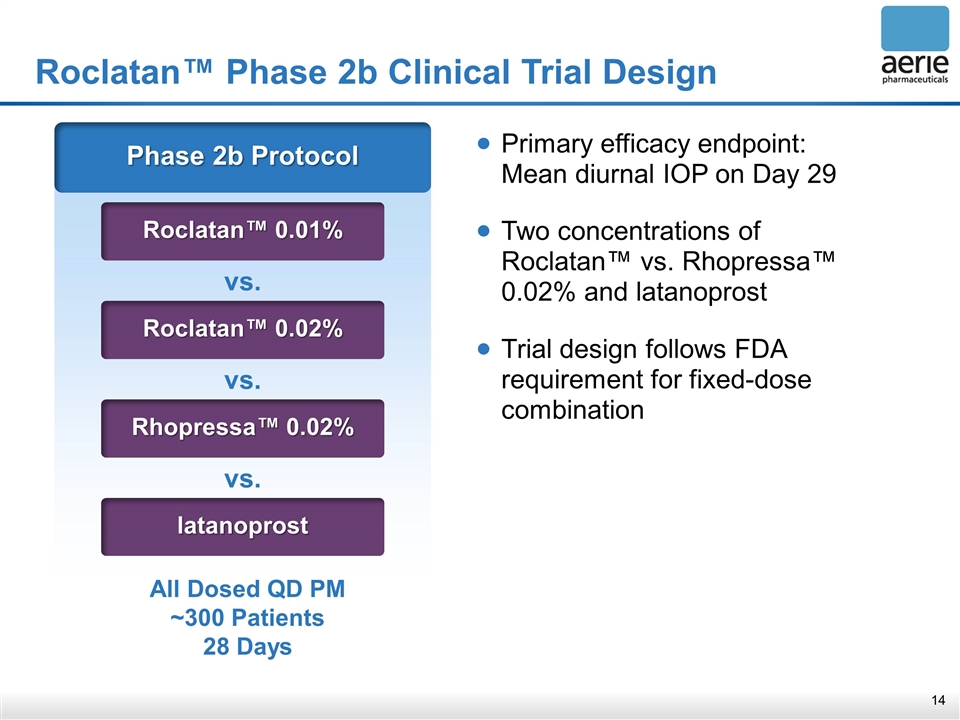
Roclatan™ Phase 2b Clinical Trial Design Phase 2b Protocol Roclatan™ 0.01% vs. Roclatan™ 0.02% vs. Rhopressa™ 0.02% vs. latanoprost All Dosed QD PM ~300 Patients 28 Days Primary efficacy endpoint: Mean diurnal IOP on Day 29 Two concentrations of Roclatan™ vs. Rhopressa™ 0.02% and latanoprost Trial design follows FDA requirement for fixed-dose combination
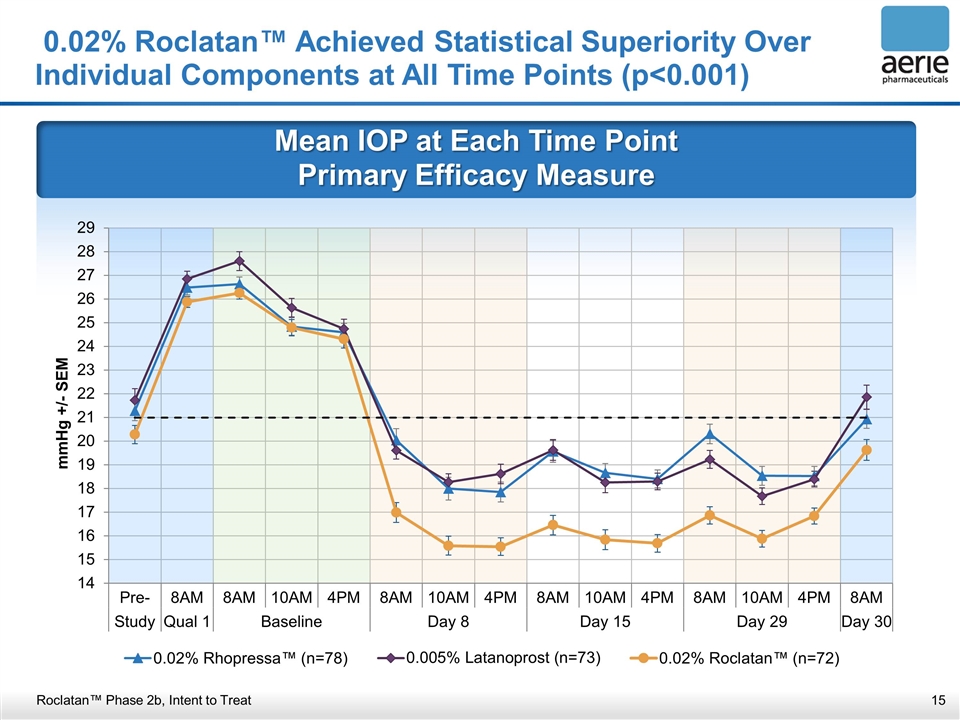
Mean IOP at Each Time Point Primary Efficacy Measure 0.02% Roclatan™ Achieved Statistical Superiority Over Individual Components at All Time Points (p<0.001) Roclatan™ Phase 2b, Intent to Treat
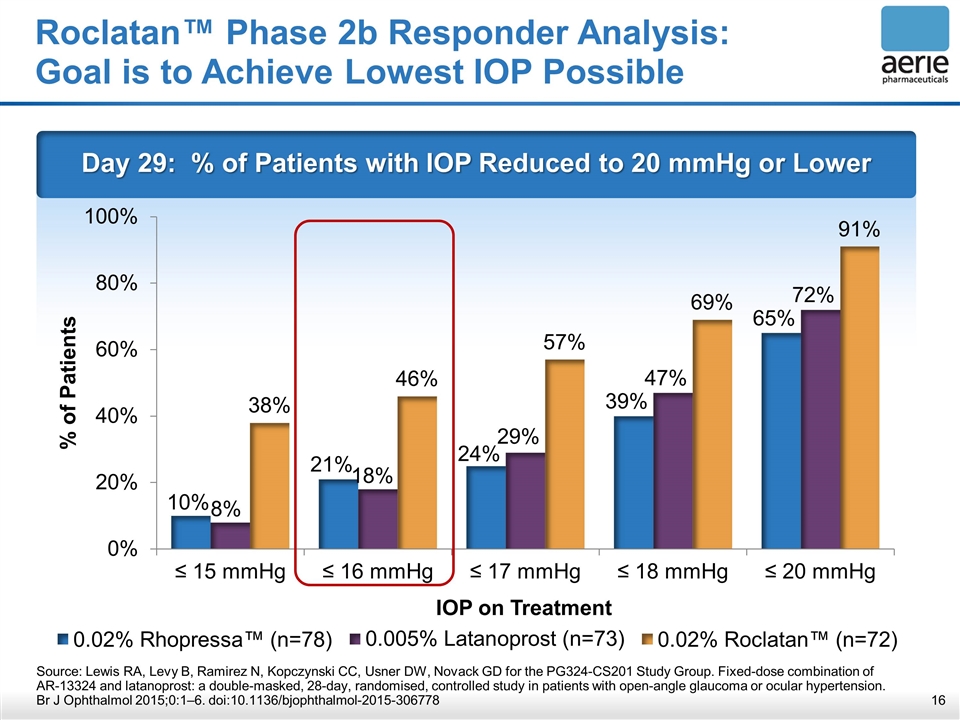
Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible Day 29: % of Patients with IOP Reduced to 20 mmHg or Lower Source: Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD for the PG324-CS201 Study Group. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol 2015;0:1–6. doi:10.1136/bjophthalmol-2015-306778
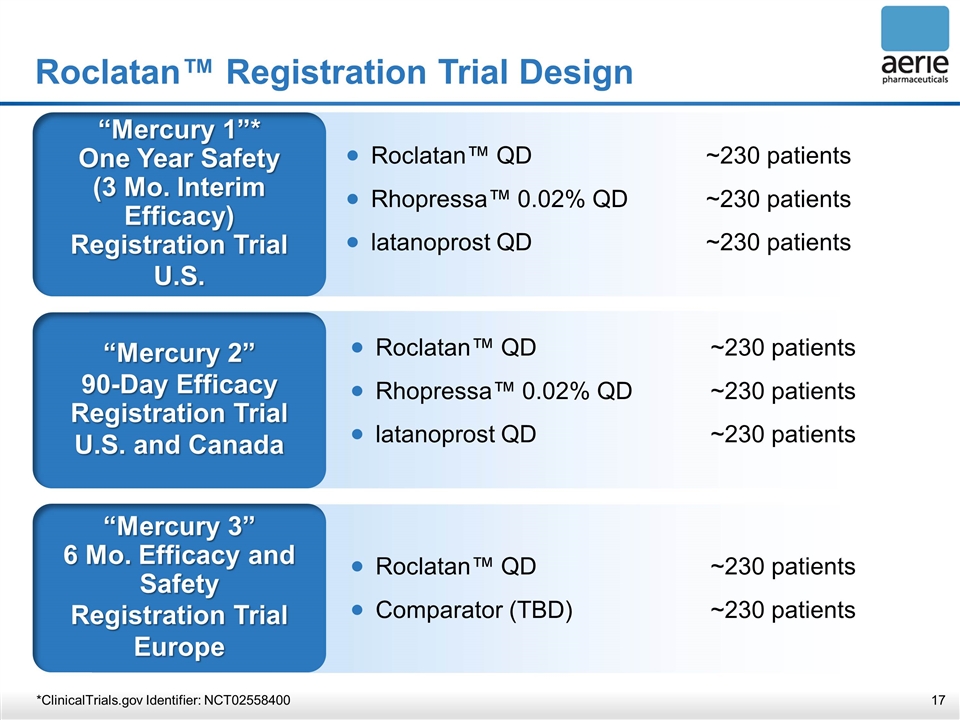
Roclatan™ Registration Trial Design “Mercury 1”* One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. “Mercury 2” 90-Day Efficacy Registration Trial U.S. and Canada “Mercury 3” 6 Mo. Efficacy and Safety Registration Trial Europe Roclatan™ QD~230 patients Rhopressa™ 0.02% QD~230 patients latanoprost QD~230 patients Roclatan™ QD~230 patients Comparator (TBD)~230 patients *ClinicalTrials.gov Identifier: NCT02558400 Roclatan™ QD~230 patients Rhopressa™ 0.02% QD~230 patients latanoprost QD~230 patients
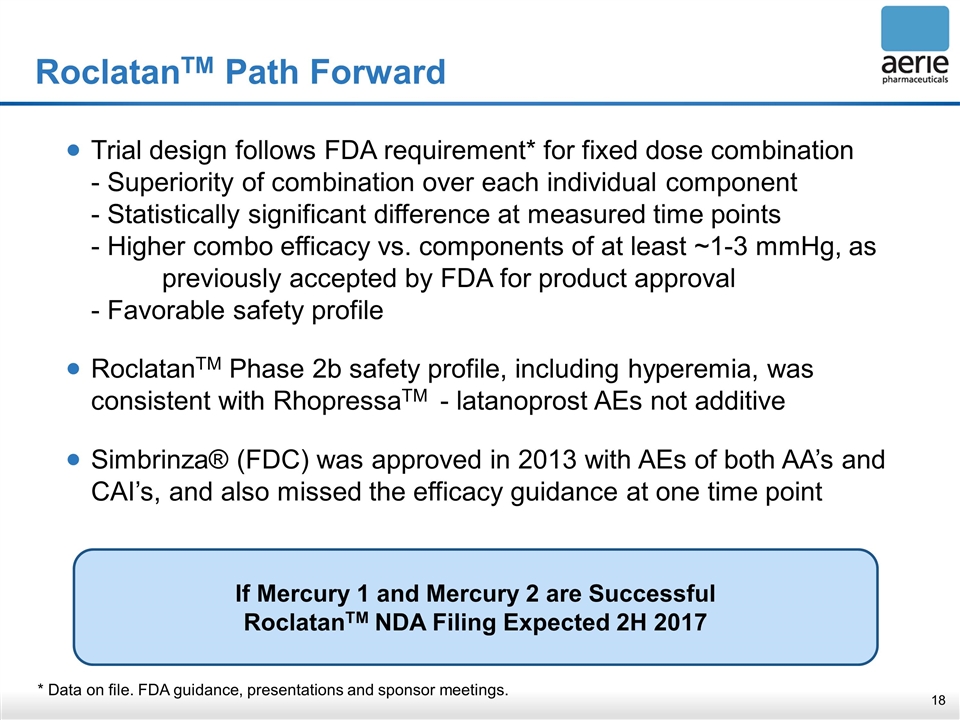
RoclatanTM Path Forward * Data on file. FDA guidance, presentations and sponsor meetings. Trial design follows FDA requirement* for fixed dose combination - Superiority of combination over each individual component - Statistically significant difference at measured time points - Higher combo efficacy vs. components of at least ~1-3 mmHg, as previously accepted by FDA for product approval - Favorable safety profile RoclatanTM Phase 2b safety profile, including hyperemia, was consistent with RhopressaTM - latanoprost AEs not additive Simbrinza® (FDC) was approved in 2013 with AEs of both AA’s and CAI’s, and also missed the efficacy guidance at one time point If Mercury 1 and Mercury 2 are Successful RoclatanTM NDA Filing Expected 2H 2017

Expanding Franchise to Europe and Japan Europe Current clinical plan expected to satisfy EU regulatory requirements (including Rocket 4 for RhopressaTM and Mercury 3 for RoclatanTM) Establishing KOL relationships in top 5 countries Targeting completion of EU commercialization strategy by YE16 Expect to file EU MAA for Rhopressa™ by approximately mid-2017 Japan Discussions regarding potential Rhopressa™ out-licensing are ongoing Also preparing to advance clinical development on our own Conferring with PMDA to confirm path to approval Prepared to secure a CRO relationship in 2016 to conduct trials
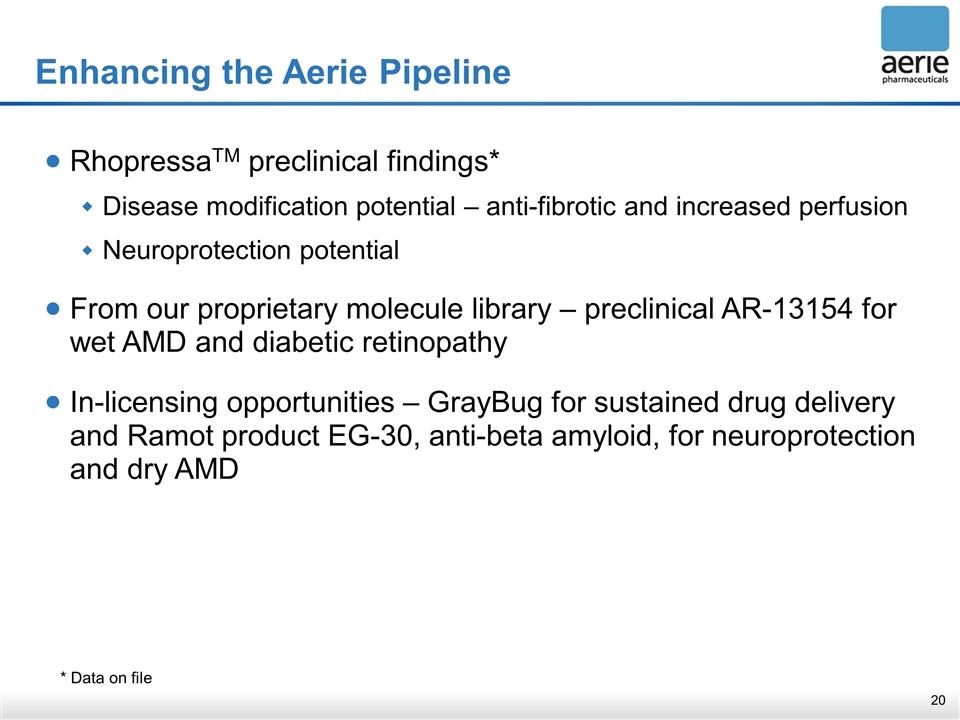
Enhancing the Aerie Pipeline RhopressaTM preclinical findings* Disease modification potential – anti-fibrotic and increased perfusion Neuroprotection potential From our proprietary molecule library – preclinical AR-13154 for wet AMD and diabetic retinopathy In-licensing opportunities – GrayBug for sustained drug delivery and Ramot product EG-30, anti-beta amyloid, for neuroprotection and dry AMD * Data on file
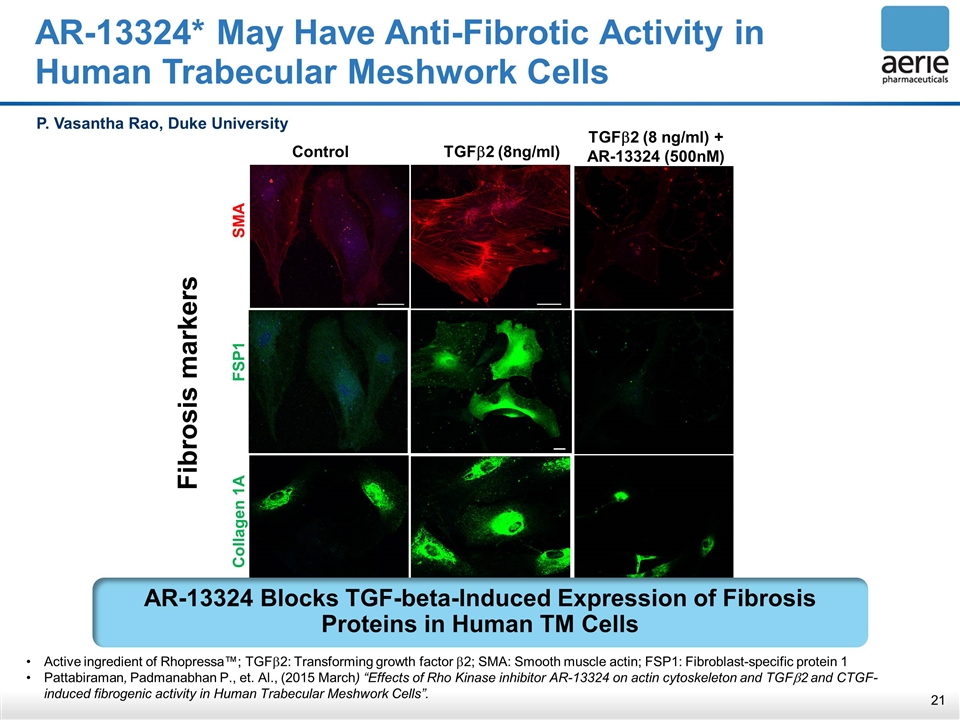
AR-13324* May Have Anti-Fibrotic Activity in Human Trabecular Meshwork Cells Fibrosis markers Control TGFb2 (8ng/ml) Collagen 1A FSP1 SMA TGFb2 (8 ng/ml) + AR-13324 (500nM) P. Vasantha Rao, Duke University Active ingredient of Rhopressa™; TGFb2: Transforming growth factor b2; SMA: Smooth muscle actin; FSP1: Fibroblast-specific protein 1 Pattabiraman, Padmanabhan P., et. Al., (2015 March) “Effects of Rho Kinase inhibitor AR-13324 on actin cytoskeleton and TGFb2 and CTGF- induced fibrogenic activity in Human Trabecular Meshwork Cells”. AR-13324 Blocks TGF-beta-Induced Expression of Fibrosis Proteins in Human TM Cells
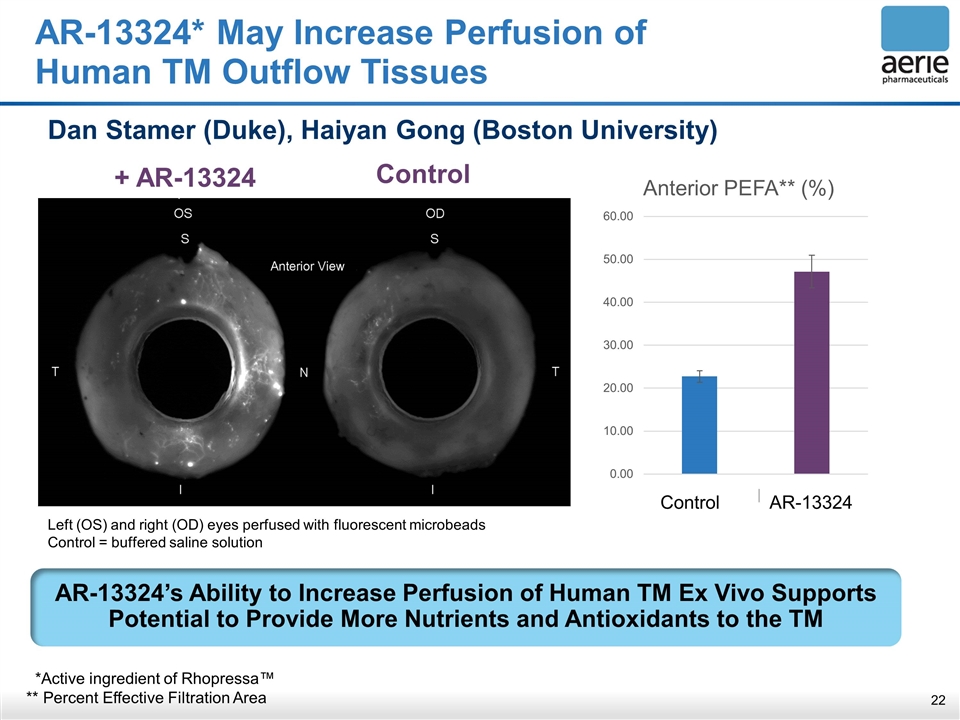
+ AR-13324 Control AR-13324* May Increase Perfusion of Human TM Outflow Tissues Dan Stamer (Duke), Haiyan Gong (Boston University) AR-13324’s Ability to Increase Perfusion of Human TM Ex Vivo Supports Potential to Provide More Nutrients and Antioxidants to the TM AR-13324 Control Left (OS) and right (OD) eyes perfused with fluorescent microbeads Control = buffered saline solution *Active ingredient of Rhopressa™ ** Percent Effective Filtration Area
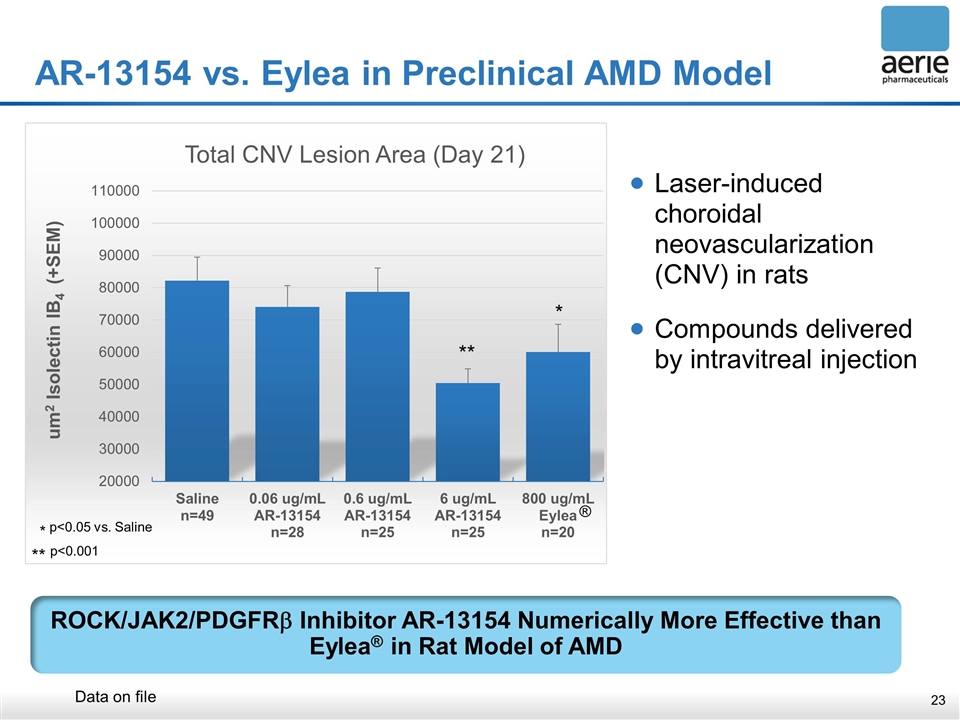
Laser-induced choroidal neovascularization (CNV) in rats Compounds delivered by intravitreal injection AR-13154 vs. Eylea in Preclinical AMD Model ROCK/JAK2/PDGFRb Inhibitor AR-13154 Numerically More Effective than Eylea® in Rat Model of AMD ** * * p<0.05 vs. Saline ** p<0.001 ® Data on file

Glaucoma Delivery Technology - RhopressaTM Evaluating sustained release formulation technologies Bioerodible implant with capability of delivering netarsudil/AR-13324 (the active ingredient in RhopressaTM) over several months Intracameral injection – provides physician greater control over compliance Potential to enhance efficacy, reduce side effects More localized delivery to targeted tissues: trabecular meshwork (outflow) and ciliary body (aqueous humor production) Less drug exposure to conjunctiva, cornea vs. topical dosing Provides physicians and patients with another therapeutic option to consider

Aerie Research Collaborations GrayBug Drug delivery technology spin-out from Johns Hopkins University Sustained release injectable technology with capability of delivering compounds to the front and back of the eye Biodegradable polymer technology with potential for multi-month delivery Efforts initially focused on delivery of AR-13154 to back of the eye Ramot at Tel Aviv University Pre-clinical anti-beta amyloid small molecule product candidate (EG 30) Potential for neuroprotection in glaucoma and reduction of geographic atrophy in advanced dry AMD Collaboration and license agreement covering all ophthalmic indications
Summary Key Clinical Priorities RhopressaTM: NDA filing expected Q3 2016 Rocket 4 in process (not required for NDA filing) RoclatanTM: Mercury 1 and 2 in process Mercury 1 topline efficacy expected Q3 2016 Research Initiatives Rhopressa™ disease modification, neuroprotection, sustained release AR-13154 potential in wet AMD, etc. Evaluating Aerie’s 3,000+ proprietary molecules Business Development Opportunities Evaluating additions to ophthalmic product pipeline - e.g., Ramot Drug delivery opportunities for front and back of the eye - e.g., GrayBug Note: As of December 31, 2015, Aerie had $150 million of cash and marketable securities on the balance sheet
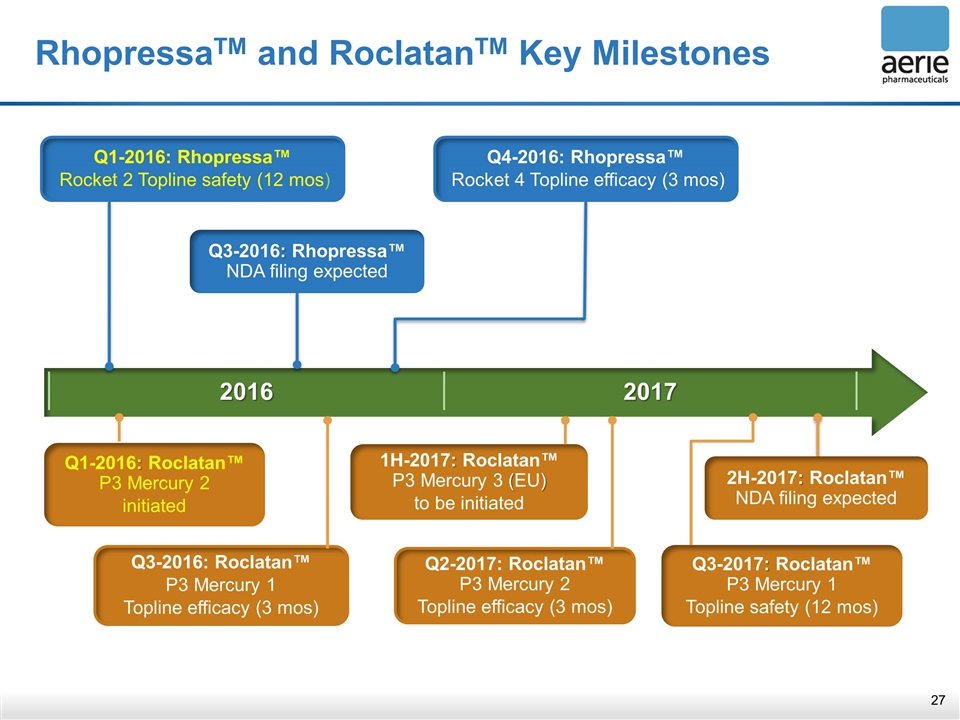
2016 2017 RhopressaTM and RoclatanTM Key Milestones Q3-2017: Roclatan™ P3 Mercury 1 Topline safety (12 mos) 2H-2017: Roclatan™ NDA filing expected 1H-2017: Roclatan™ P3 Mercury 3 (EU) to be initiated Q1-2016: Rhopressa™ Rocket 2 Topline safety (12 mos) Q3-2016: Rhopressa™ NDA filing expected Q4-2016: Rhopressa™ Rocket 4 Topline efficacy (3 mos) Q3-2016: Roclatan™ P3 Mercury 1 Topline efficacy (3 mos) Q2-2017: Roclatan™ P3 Mercury 2 Topline efficacy (3 mos) Q1-2016: Roclatan™ P3 Mercury 2 initiated

Building a Major Ophthalmic Pharmaceutical Company