
Company Overview Investor Presentation April 2017 Exhibit 99.1

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. In addition, any discussion of clinical trial results for RhopressaTM (netarsudil ophthalmic solution) 0.02% relate to the results in its first Phase 3 registration trials named Rocket 1 and Rocket 2, or Rocket 4 which will be used primarily for European regulatory filing purposes, and for RoclatanTM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% relate to the results in its Phase 3 registration trial. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation, including the updated guidance presented herein, are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. Any top line data presented herein is preliminary and based solely on information available to us as of the date of this document and additional information about the results may be disclosed at any time. In addition, the preclinical research discussed in this presentation is preliminary and the outcome of such preclinical studies may not be predictive of the outcome of later trials. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
Aerie Late Stage IOP–Lowering Products Pre-Clinical Research Rhopressa™ Potential for disease modification AR-13154 Significant lesion size reduction in wet AMD Drug Delivery - Front and back of the eye Rhopressa™ (netarsudil ophthalmic solution) 0.02% Inhibits ROCK, NET, lowers EVP, targets diseased tissue NDA resubmitted in February 2017; entering launch mode Roclatan™ (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% Fixed combination of Rhopressa™ and latanoprost Two P3’s in process; first P3 achieved primary efficacy endpoint Aerie – Building a Major Ophthalmic Pharmaceutical Company Data on file For Investor Use
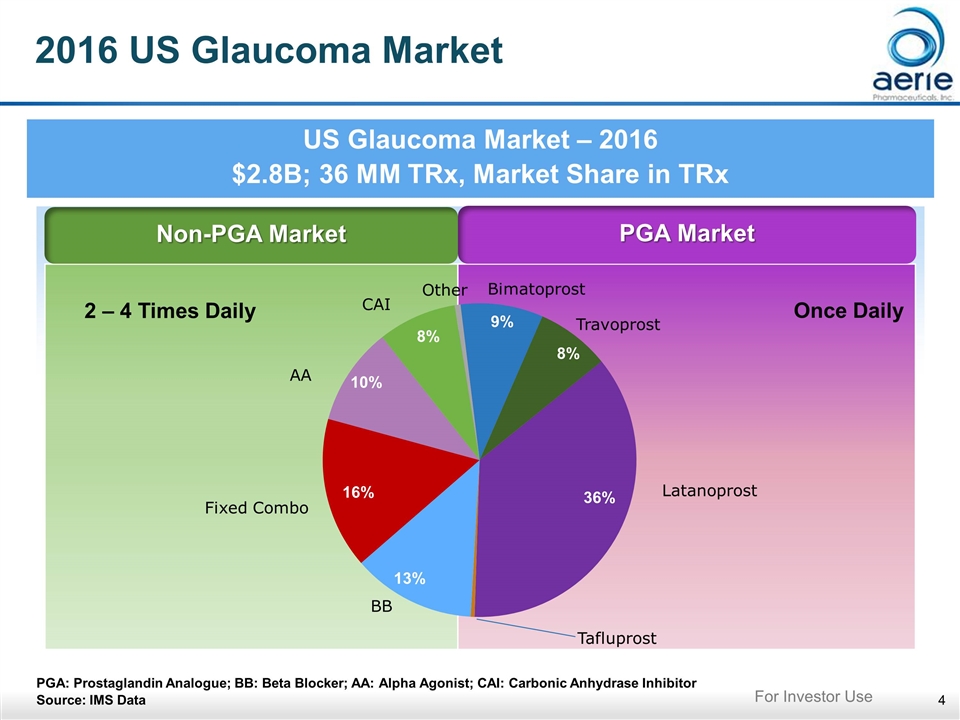
2016 US Glaucoma Market US Glaucoma Market – 2016 $2.8B; 36 MM TRx, Market Share in TRx PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor Source: IMS Data Non-PGA Market PGA Market 16% 13% 10% 8% 36% 9% 8% 2 – 4 Times Daily Once Daily For Investor Use
Potential drug of choice as adjunctive therapy to PGAs when additional IOP lowering is desired Potential drug of choice for patients requiring maximal IOP lowering Initial therapy for PGA non-responders and those with tolerability concerns Patients using two or more glaucoma therapies Normal-tension glaucoma Patients with high / very high IOP Patients at any IOP with significant disease progression Rhopressa™ Positioning Roclatan™ Positioning Rhopressa™ Advantages Efficacy vs. other adjunctive therapies QD PM dose Lack of serious and systemic drug-related AE’s Roclatan™ Advantages Efficacy vs. all other glaucoma therapies QD PM dose Lack of serious and systemic product-related AE’s RhopressaTM and RoclatanTM Potential Product Advantages For Investor Use
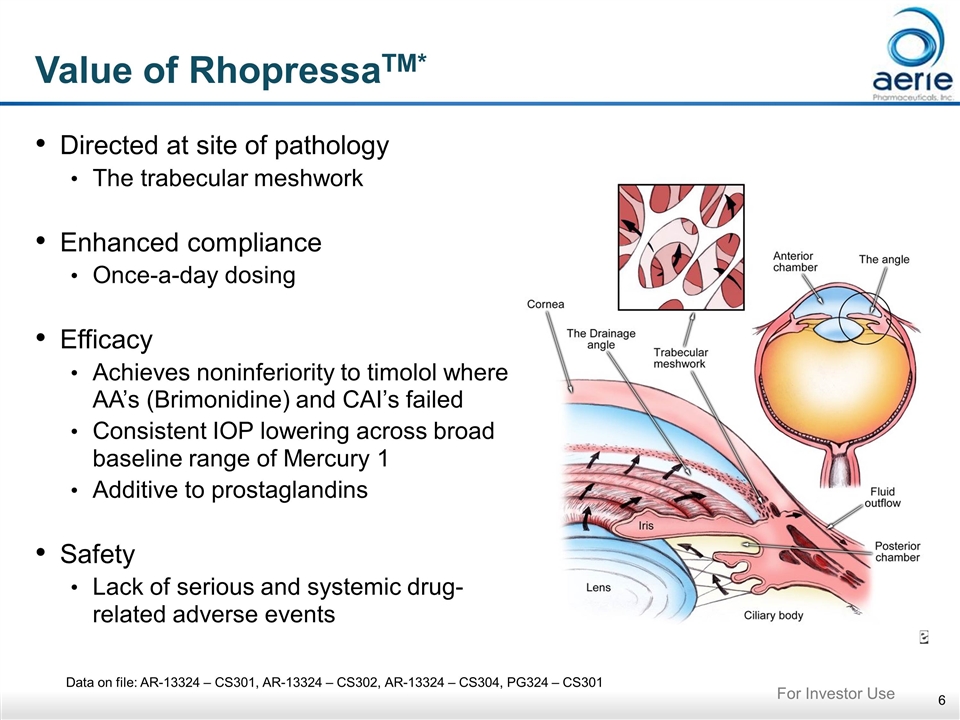
Data on file: AR-13324 – CS301, AR-13324 – CS302, AR-13324 – CS304, PG324 – CS301 Value of RhopressaTM* Directed at site of pathology The trabecular meshwork Enhanced compliance Once-a-day dosing Efficacy Achieves noninferiority to timolol where AA’s (Brimonidine) and CAI’s failed Consistent IOP lowering across broad baseline range of Mercury 1 Additive to prostaglandins Safety Lack of serious and systemic drug-related adverse events For Investor Use
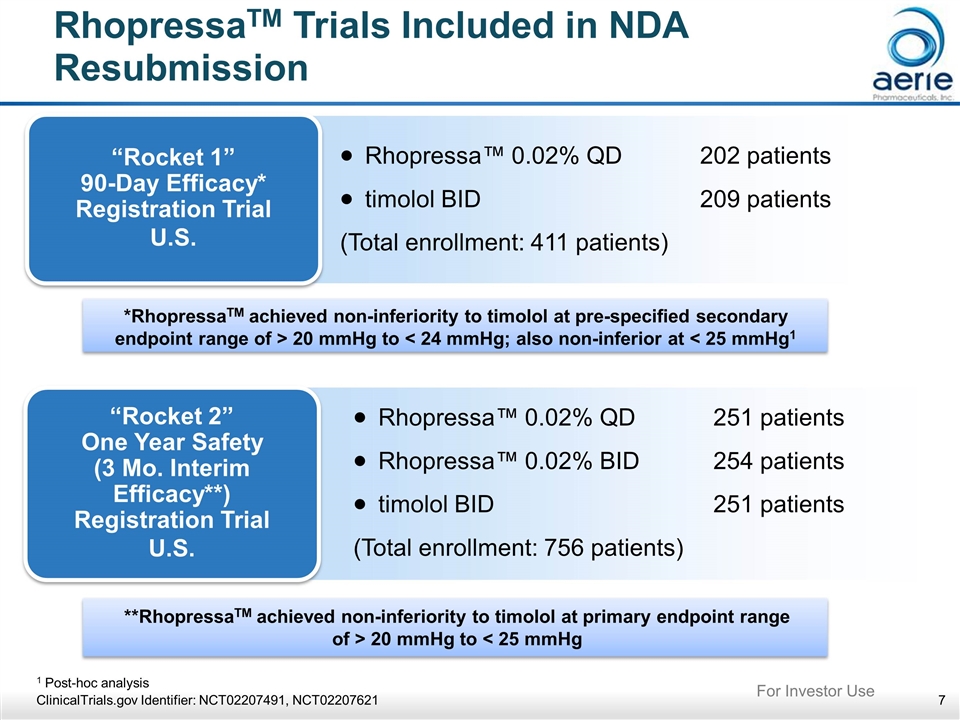
**RhopressaTM achieved non-inferiority to timolol at primary endpoint range of > 20 mmHg to < 25 mmHg *RhopressaTM achieved non-inferiority to timolol at pre-specified secondary endpoint range of > 20 mmHg to < 24 mmHg; also non-inferior at < 25 mmHg1 RhopressaTM Trials Included in NDA Resubmission “Rocket 1” 90-Day Efficacy* Registration Trial U.S. Rhopressa™ 0.02% QD202 patients timolol BID209 patients (Total enrollment: 411 patients) “Rocket 2” One Year Safety (3 Mo. Interim Efficacy**) Registration Trial U.S. Rhopressa™ 0.02% QD251 patients Rhopressa™ 0.02% BID254 patients timolol BID251 patients (Total enrollment: 756 patients) 1 Post-hoc analysis ClinicalTrials.gov Identifier: NCT02207491, NCT02207621 For Investor Use
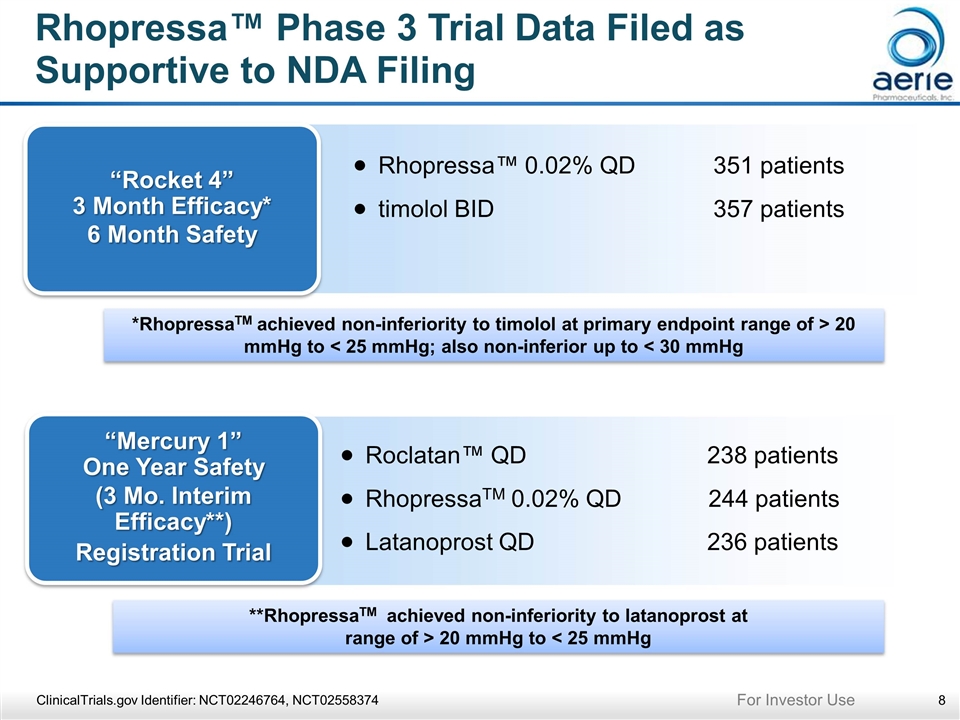
Rhopressa™ Phase 3 Trial Data Filed as Supportive to NDA Filing ClinicalTrials.gov Identifier: NCT02246764, NCT02558374 “Rocket 4” 3 Month Efficacy* 6 Month Safety Rhopressa™ 0.02% QD351 patients timolol BID 357 patients *RhopressaTM achieved non-inferiority to timolol at primary endpoint range of > 20 mmHg to < 25 mmHg; also non-inferior up to < 30 mmHg “Mercury 1” One Year Safety (3 Mo. Interim Efficacy**) Registration Trial Roclatan™ QD 238 patients RhopressaTM 0.02% QD 244 patients Latanoprost QD 236 patients **RhopressaTM achieved non-inferiority to latanoprost at range of > 20 mmHg to < 25 mmHg For Investor Use
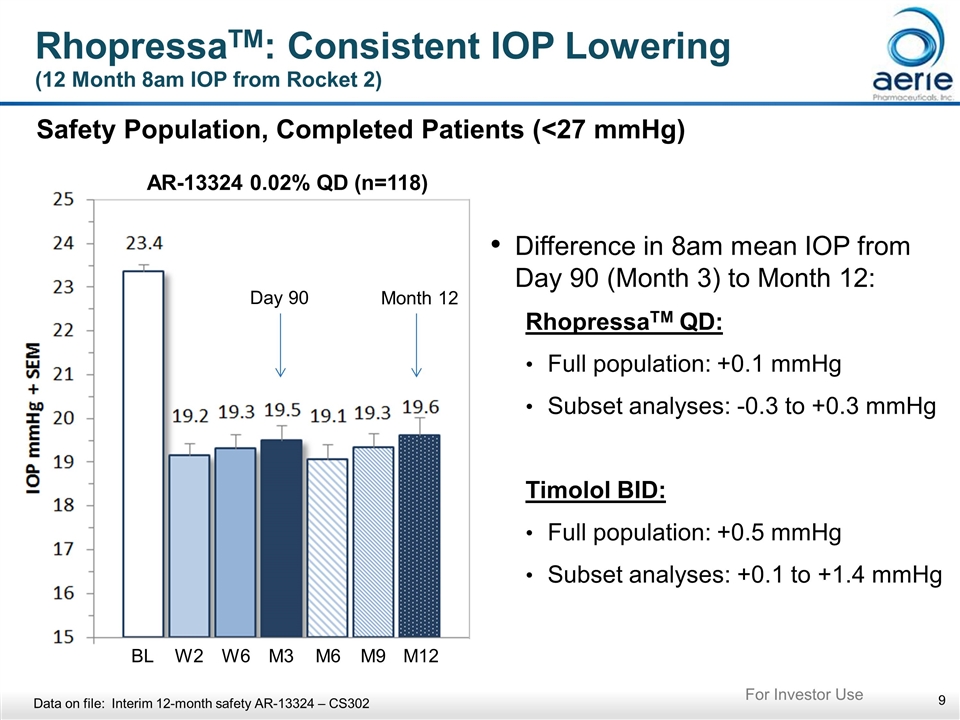
RhopressaTM: Consistent IOP Lowering (12 Month 8am IOP from Rocket 2) Safety Population, Completed Patients (<27 mmHg) Difference in 8am mean IOP from Day 90 (Month 3) to Month 12: RhopressaTM QD: Full population: +0.1 mmHg Subset analyses: -0.3 to +0.3 mmHg Timolol BID: Full population: +0.5 mmHg Subset analyses: +0.1 to +1.4 mmHg Day 90 Month 12 AR-13324 0.02% QD (n=118) BL W2 W6 M3 M6 M9 M12 Data on file: Interim 12-month safety AR-13324 – CS302 For Investor Use
Rocket 2: Safety/Tolerability Overview of RhopressaTM QD (Interim 12-Month) * Incidence of conjunctival hyperemia ~50% including baseline at ~20% There were no drug-related serious adverse events (SAEs) No new adverse events introduced over the twelve-month period The most common adverse event was conjunctival hyperemia with ~50% incidence*, the majority mild Other ocular AEs AEs occurring in ~5-23% of patients included: conjunctival hemorrhage, corneal verticillata, blurry vision and reduced visual acuity For Investor Use
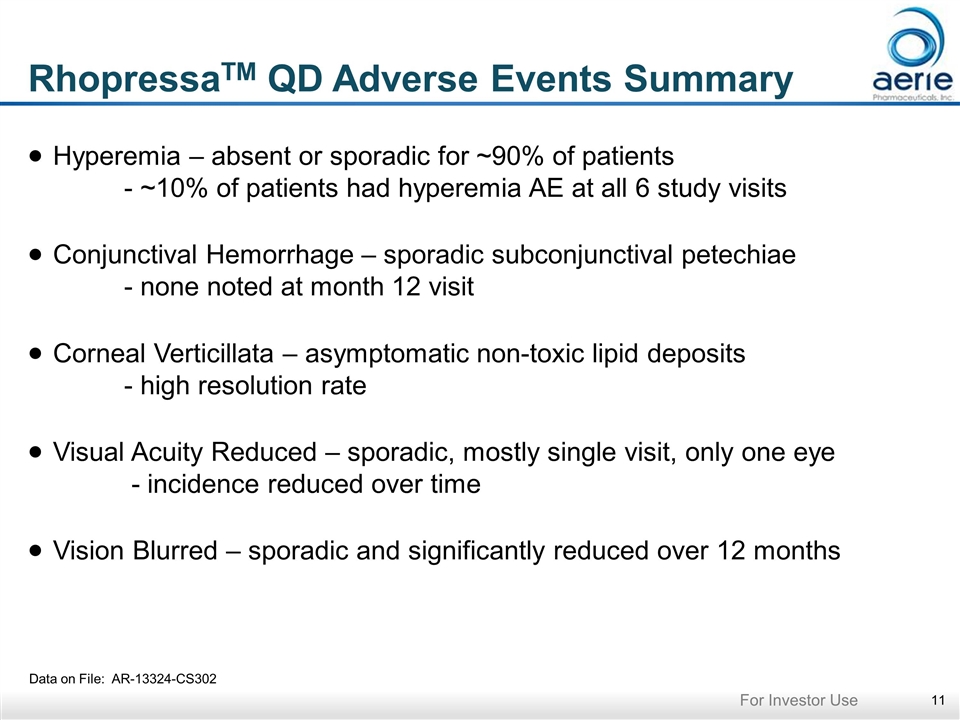
RhopressaTM QD Adverse Events Summary Data on File: AR-13324-CS302 Hyperemia – absent or sporadic for ~90% of patients - ~10% of patients had hyperemia AE at all 6 study visits Conjunctival Hemorrhage – sporadic subconjunctival petechiae - none noted at month 12 visit Corneal Verticillata – asymptomatic non-toxic lipid deposits - high resolution rate Visual Acuity Reduced – sporadic, mostly single visit, only one eye - incidence reduced over time Vision Blurred – sporadic and significantly reduced over 12 months For Investor Use
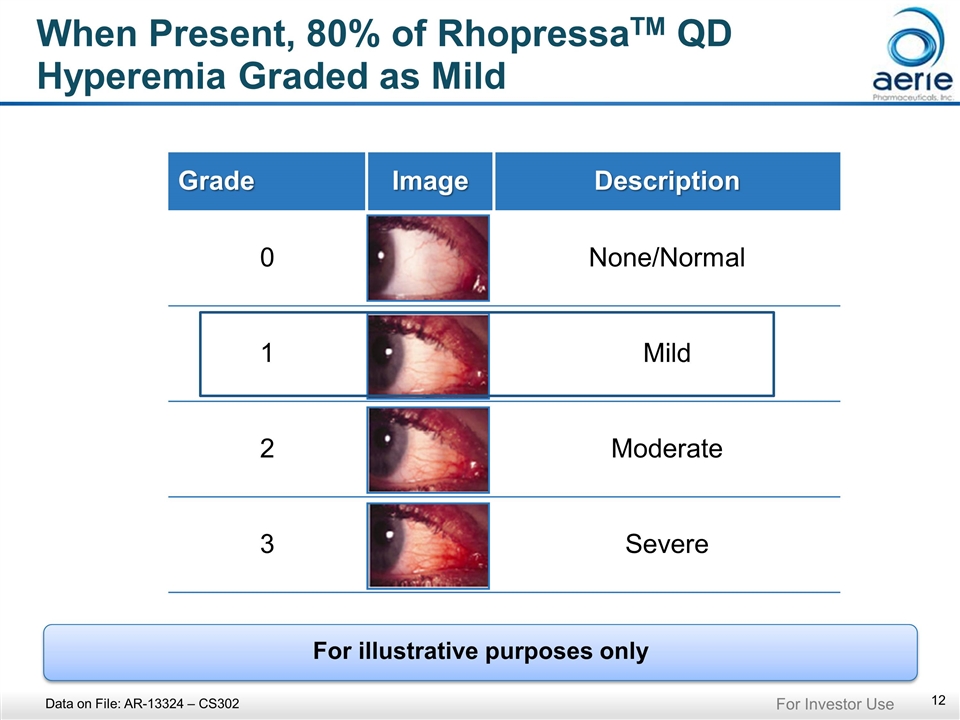
When Present, 80% of RhopressaTM QD Hyperemia Graded as Mild Grade Image Description 0 None/Normal 1 Mild 2 Moderate 3 Severe Data on File: AR-13324 – CS302 For illustrative purposes only For Investor Use
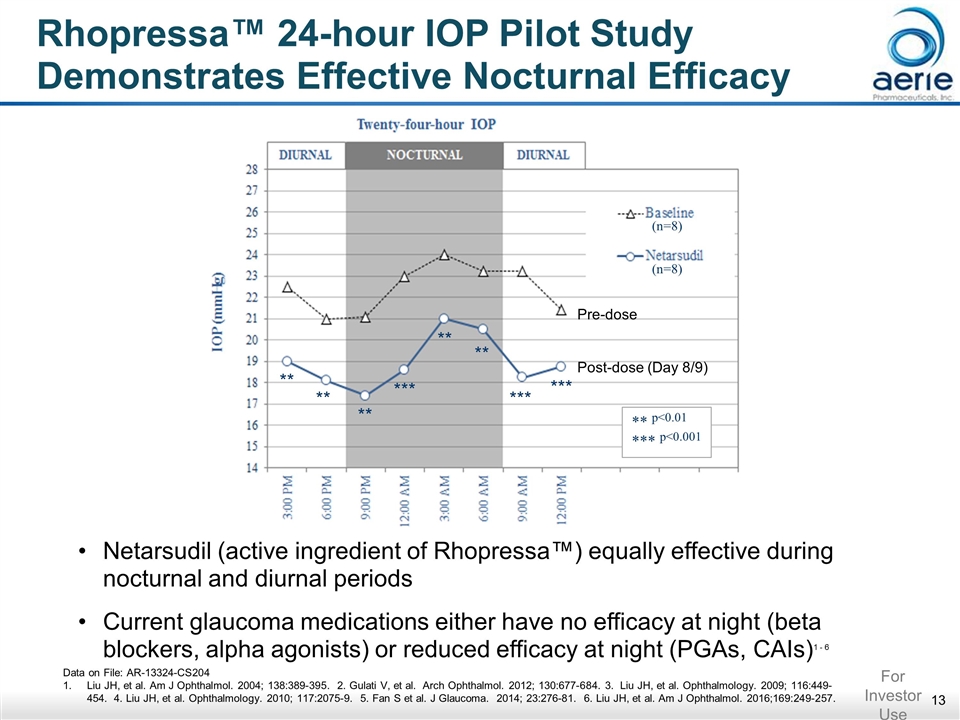
Rhopressa™ 24-hour IOP Pilot Study Demonstrates Effective Nocturnal Efficacy (n=8) (n=8) ** ** ** *** ** ** *** *** ** p<0.01 *** p<0.001 Netarsudil (active ingredient of Rhopressa™) equally effective during nocturnal and diurnal periods Current glaucoma medications either have no efficacy at night (beta blockers, alpha agonists) or reduced efficacy at night (PGAs, CAIs)1 - 6 Data on File: AR-13324-CS204 Liu JH, et al. Am J Ophthalmol. 2004; 138:389-395. 2. Gulati V, et al. Arch Ophthalmol. 2012; 130:677-684. 3. Liu JH, et al. Ophthalmology. 2009; 116:449-454. 4. Liu JH, et al. Ophthalmology. 2010; 117:2075-9. 5. Fan S et al. J Glaucoma. 2014; 23:276-81. 6. Liu JH, et al. Am J Ophthalmol. 2016;169:249-257. For Investor Use Pre-dose Post-dose (Day 8/9)
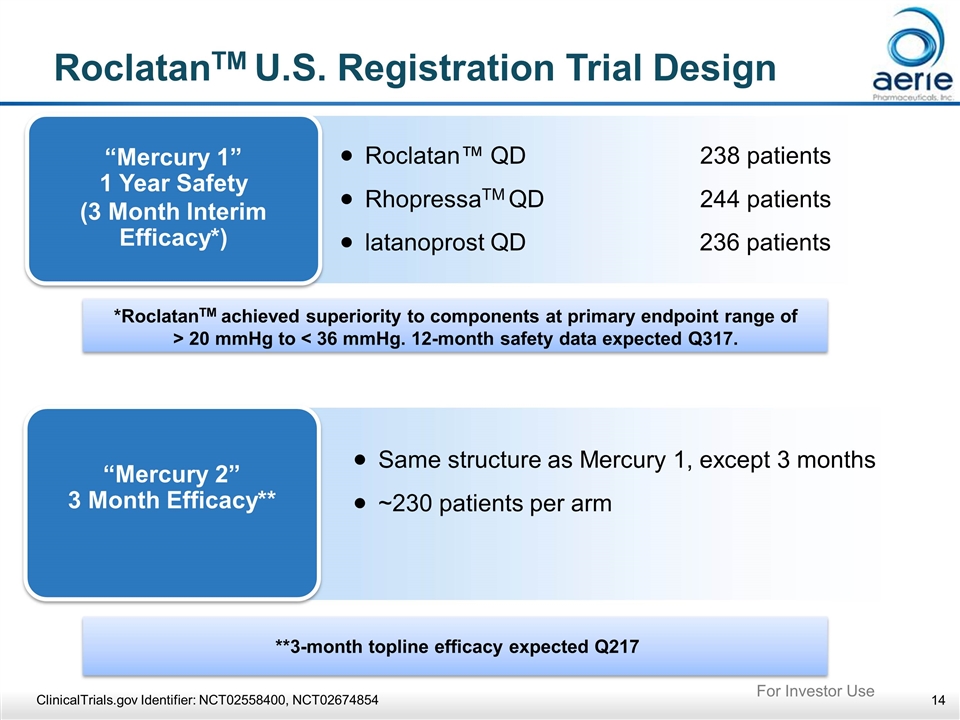
**3-month topline efficacy expected Q217 *RoclatanTM achieved superiority to components at primary endpoint range of > 20 mmHg to < 36 mmHg. 12-month safety data expected Q317. RoclatanTM U.S. Registration Trial Design “Mercury 1” 1 Year Safety (3 Month Interim Efficacy*) Roclatan™ QD 238 patients RhopressaTM QD 244 patients latanoprost QD 236 patients “Mercury 2” 3 Month Efficacy** Same structure as Mercury 1, except 3 months ~230 patients per arm ClinicalTrials.gov Identifier: NCT02558400, NCT02674854 For Investor Use

Roclatan™ met the criteria for demonstrating statistical superiority over both latanoprost and Rhopressa™ for the primary efficacy analysis IOP-lowering effect of Roclatan™ was greater (1-3 mmHg) than monotherapy with either latanoprost or Rhopressa™ throughout the duration of the study RoclatanTM reduced mean diurnal IOPs to 16 mmHg or lower in 61% of patients, a significantly higher percentage than observed in the comparator arms The RhopressaTM arm achieved non-inferiority to latanoprost in the pre-specified range of >20 mmHg to <25 mmHg Mercury 1: RoclatanTM Achieves Primary Clinical Endpoint Data on file and PG324 - CS301 For Investor Use

RoclatanTM Achieved Statistical Superiority Over Individual Components at All 9 Time Points Mean IOP at Each Time Point (ITT) ***p<0.0001 vs Latanoprost and RhopressaTM Data on File For Investor Use
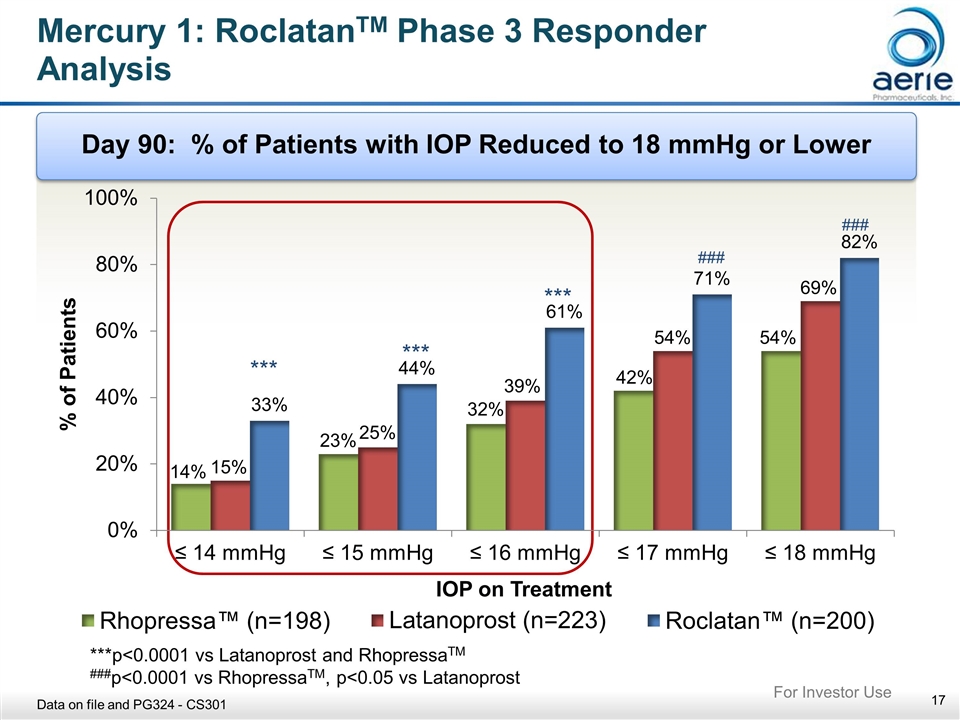
Mercury 1: RoclatanTM Phase 3 Responder Analysis Day 90: % of Patients with IOP Reduced to 18 mmHg or Lower *** *** *** ***p<0.0001 vs Latanoprost and RhopressaTM ###p<0.0001 vs RhopressaTM, p<0.05 vs Latanoprost Data on file and PG324 - CS301 For Investor Use

Mercury 1: Safety/Tolerability Overview of RoclatanTM There were no drug-related serious adverse events (SAEs) There was no evidence of treatment-related systemic effects The most common adverse event was conjunctival hyperemia with ~50% incidence*, ~80% mild on biomicroscopy Other ocular AEs AEs occurring in ~5-11% of subjects receiving RoclatanTM included: conjunctival hemorrhage, eye pruritus, lacrimation increased and cornea verticillata. * Incidence of conjunctival hyperemia ~50% including baseline at ~20% Data on file and PG324 - CS301 For Investor Use

Expanding Aerie Franchise to Europe and Japan Europe Current clinical plan expected to satisfy EU regulatory requirements (including Rocket 4 for RhopressaTM and Mercury 3 for RoclatanTM) Mercury 3: 6-month efficacy and safety registration trial for Europe, comparing RoclatanTM for non-inferiority to a fixed-dose combo in Europe starting mid-17. Approximately 230 patients per arm. Expect to file EU MAA for Rhopressa™ in 2018 Commenced construction of Ireland Plant to support worldwide commercial supply Japan Preparing to advance clinical development on our own Phases 1 and 2 to be conducted in U.S. on Japanese / Japanese Americans Phase 3 trials expected to be conducted in Japan commencing 2H18 For Investor Use
Advancing Earlier-Stage Pipeline Data on file Rhopressa™ Potential for disease modification AR-13154 Significant lesion size reduction in wet AMD Drug Delivery Rhopressa™: Front of the eye AR-13154: Back of the eye For Investor Use
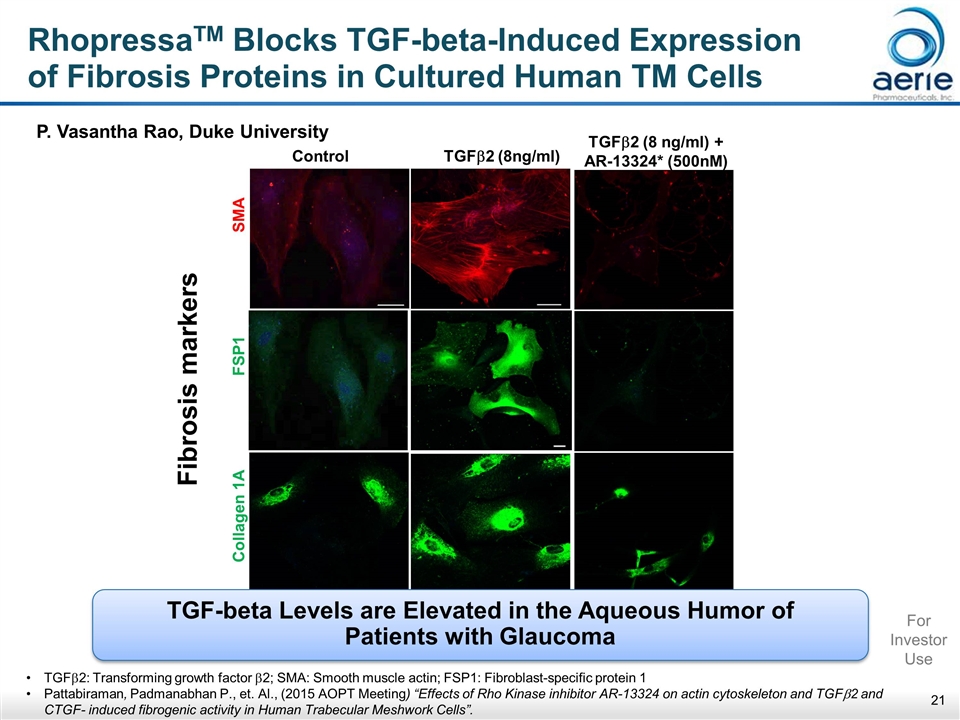
RhopressaTM Blocks TGF-beta-Induced Expression of Fibrosis Proteins in Cultured Human TM Cells Fibrosis markers Control TGFb2 (8ng/ml) Collagen 1A FSP1 SMA TGFb2 (8 ng/ml) + AR-13324* (500nM) P. Vasantha Rao, Duke University TGF-beta Levels are Elevated in the Aqueous Humor of Patients with Glaucoma TGFb2: Transforming growth factor b2; SMA: Smooth muscle actin; FSP1: Fibroblast-specific protein 1 Pattabiraman, Padmanabhan P., et. Al., (2015 AOPT Meeting) “Effects of Rho Kinase inhibitor AR-13324 on actin cytoskeleton and TGFb2 and CTGF- induced fibrogenic activity in Human Trabecular Meshwork Cells”. For Investor Use
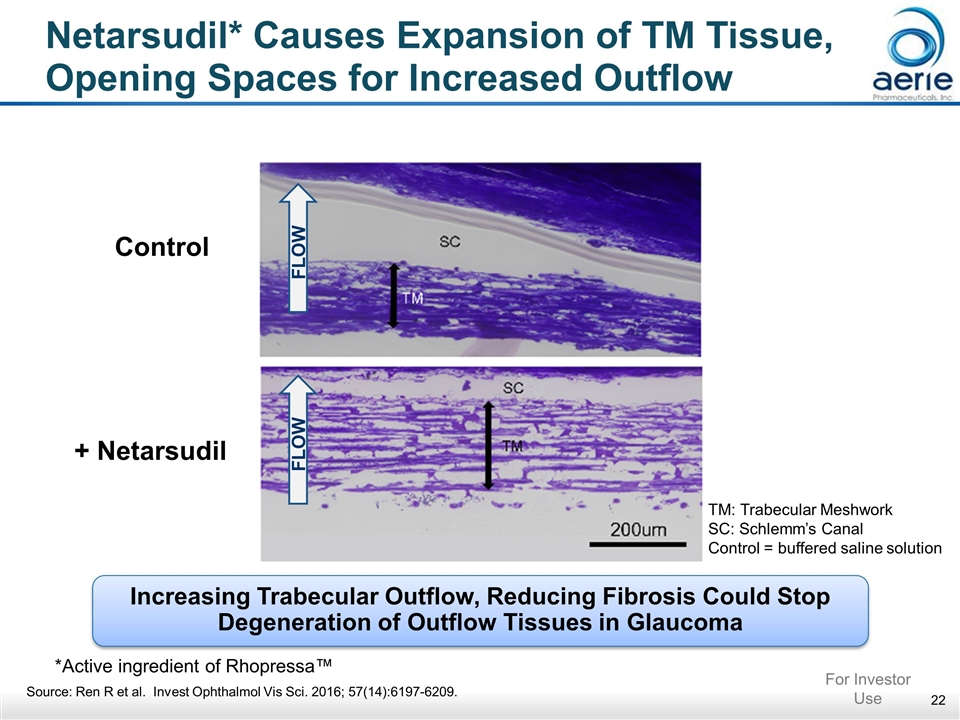
Netarsudil* Causes Expansion of TM Tissue, Opening Spaces for Increased Outflow Control + Netarsudil FLOW FLOW TM: Trabecular Meshwork SC: Schlemm’s Canal Control = buffered saline solution Increasing Trabecular Outflow, Reducing Fibrosis Could Stop Degeneration of Outflow Tissues in Glaucoma *Active ingredient of Rhopressa™ Source: Ren R et al. Invest Ophthalmol Vis Sci. 2016; 57(14):6197-6209. For Investor Use
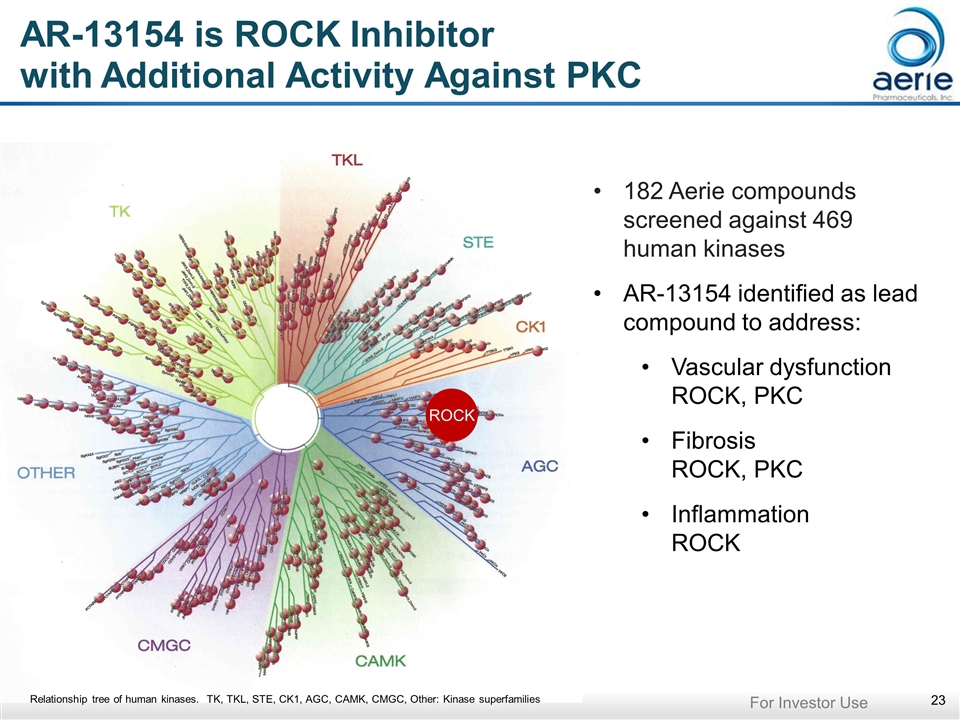
AR-13154 is ROCK Inhibitor with Additional Activity Against PKC ROCK 182 Aerie compounds screened against 469 human kinases AR-13154 identified as lead compound to address: Vascular dysfunction ROCK, PKC Fibrosis ROCK, PKC Inflammation ROCK Relationship tree of human kinases. TK, TKL, STE, CK1, AGC, CAMK, CMGC, Other: Kinase superfamilies For Investor Use
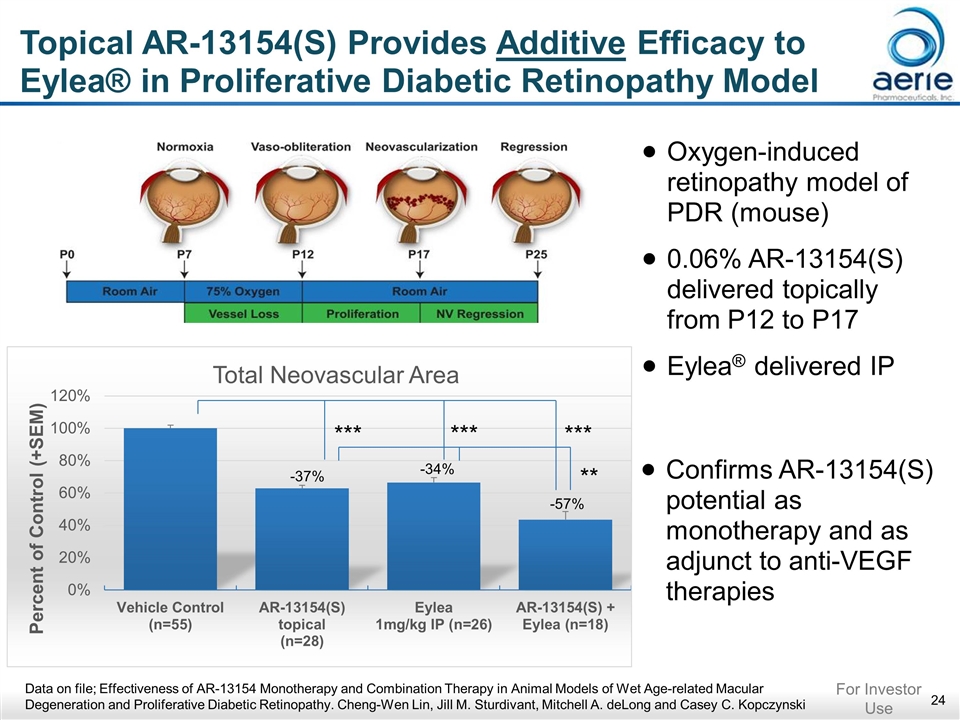
Topical AR-13154(S) Provides Additive Efficacy to Eylea® in Proliferative Diabetic Retinopathy Model -37% -34% -57% ** *** *** *** Oxygen-induced retinopathy model of PDR (mouse) 0.06% AR-13154(S) delivered topically from P12 to P17 Eylea® delivered IP Confirms AR-13154(S) potential as monotherapy and as adjunct to anti-VEGF therapies Data on file; Effectiveness of AR-13154 Monotherapy and Combination Therapy in Animal Models of Wet Age-related Macular Degeneration and Proliferative Diabetic Retinopathy. Cheng-Wen Lin, Jill M. Sturdivant, Mitchell A. deLong and Casey C. Kopczynski For Investor Use
Products in Development: Preclinical Netarsudil* sustained-release implant for Glaucoma Single injection to lower IOP for ~6 months Potential for increased efficacy, reduced ocular side effects, neuroprotection Addresses patient adherence for subset of population AR-13154 implant for AMD, DR/DME ROCK/PKC inhibitor Addresses multiple drivers of disease - fibrosis, inflammation, vessel leakage and neovascularization Efficacy demonstrated as monotherapy and as adjunct to anti-VEGF therapy (preclinical) Potential to improve long-term outcomes * Active ingredient of RhopressaTM For Investor Use

Summary Key Clinical Priorities RhopressaTM: NDA resubmitted in February 2017 Rocket 4 completion RoclatanTM: Mercury 1 and Mercury 2 completion Mercury 3 commencement mid-2017 (EU) Research Initiatives Rhopressa™ disease modification, sustained release AR-13154 potential in wet AMD, etc. Evaluating Aerie’s 3,000+ proprietary molecules Business Development and Expansion Opportunities Drug delivery opportunities for front and back of the eye EU/JP clinical path and commercialization strategy Ireland Manufacturing Facility Well-Financed* * $233.7 million in cash and investments at 12/31/16 For Investor Use
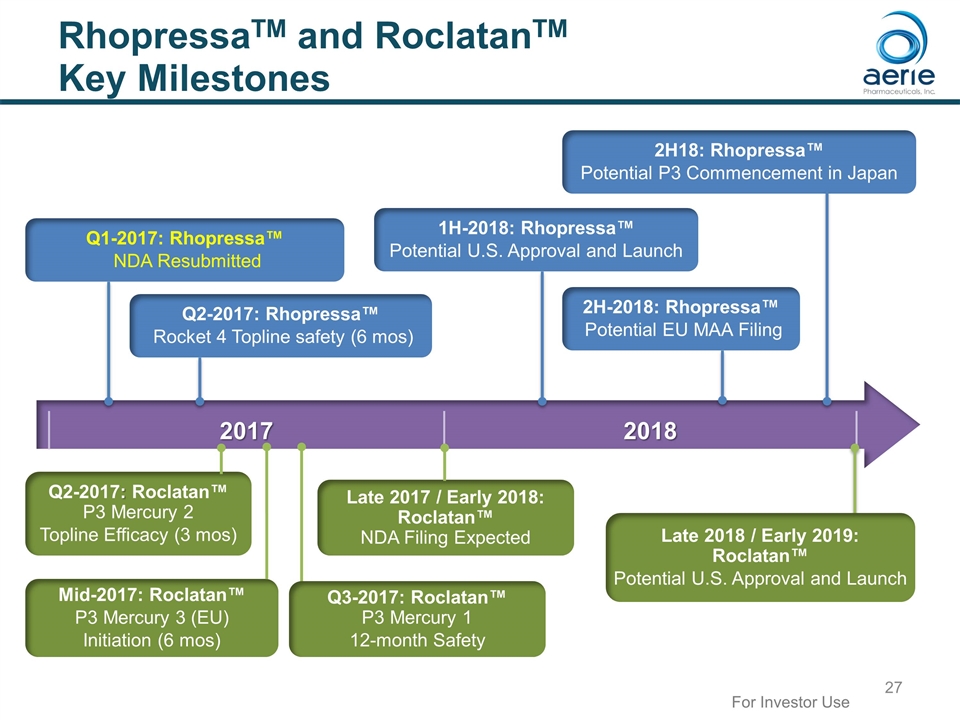
2017 2018 RhopressaTM and RoclatanTM Key Milestones Late 2018 / Early 2019: Roclatan™ Potential U.S. Approval and Launch Late 2017 / Early 2018: Roclatan™ NDA Filing Expected Q1-2017: Rhopressa™ NDA Resubmitted 1H-2018: Rhopressa™ Potential U.S. Approval and Launch Q2-2017: Rhopressa™ Rocket 4 Topline safety (6 mos) Mid-2017: Roclatan™ P3 Mercury 3 (EU) Initiation (6 mos) Q2-2017: Roclatan™ P3 Mercury 2 Topline Efficacy (3 mos) 2H-2018: Rhopressa™ Potential EU MAA Filing For Investor Use 2H18: Rhopressa™ Potential P3 Commencement in Japan Q3-2017: Roclatan™ P3 Mercury 1 12-month Safety