
AR-13324-CS208 Japan Phase 2 Study Topline Results November 6, 2019 Exhibit 99.1

Important Information The information in this presentation is current only as of its date and may have changed or may change in the future. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. The results generated by the studies discussed herein are not predictors of future results or outcomes of any ongoing or future studies conducted by the Company pertaining to any of our products or product candidates. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In particular, the topline AR-13324-CS208 data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may be disclosed at any time. Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law. For Investor Use
Japan Phase 2 Executive Summary 28-day prospective, double-masked, placebo-controlled, dose-ranging study of netarsudil efficacy and safety in Japanese subjects with open-angle glaucoma (OAG) or ocular hypertension (OHT) Netarsudil 0.01%, 0.02% and 0.04% were efficacious and met primary endpoint of superiority to placebo in mean diurnal IOP at Week 41 Baseline mean diurnal IOPs 20-21 mmHg across study arms2 (Japanese IOPs ~3 – 4 mmHg lower than in Caucasians) Week 4 mean diurnal IOP was 16.3 (-4.1), 15.4 (-4.8), 16.2 (-4.8) and 19.3 (-1.7) mmHg in the netarsudil 0.01%, 0.02%, 0.04%, and placebo groups, respectively2 Netarsudil 0.01%, 0.02% and 0.04% were safe and generally well tolerated in Japanese subjects No serious adverse events Netarsudil 0.02% provided optimal efficacy and safety profile Most common AEs were Conjunctival Hyperemia (37.0%) and Eye Irritation (9.3%) Discontinuations rate was 1.9% (1/54 subjects) Hyperemia and discontinuation rates lower than in US trials3-5 1. ANCOVA with MCMC imputation. 2. Observed data. 3. Bacharach J, et al. Ophthalmology. 2015 Feb;122(2):302-7. 4. Serle JB, et al. Am J Ophthalmol. 2018 Feb;186:116-127. 5. Khouri AS, et al. Am J Ophthalmol. 2019 Aug;204:97-104. For Investor Use
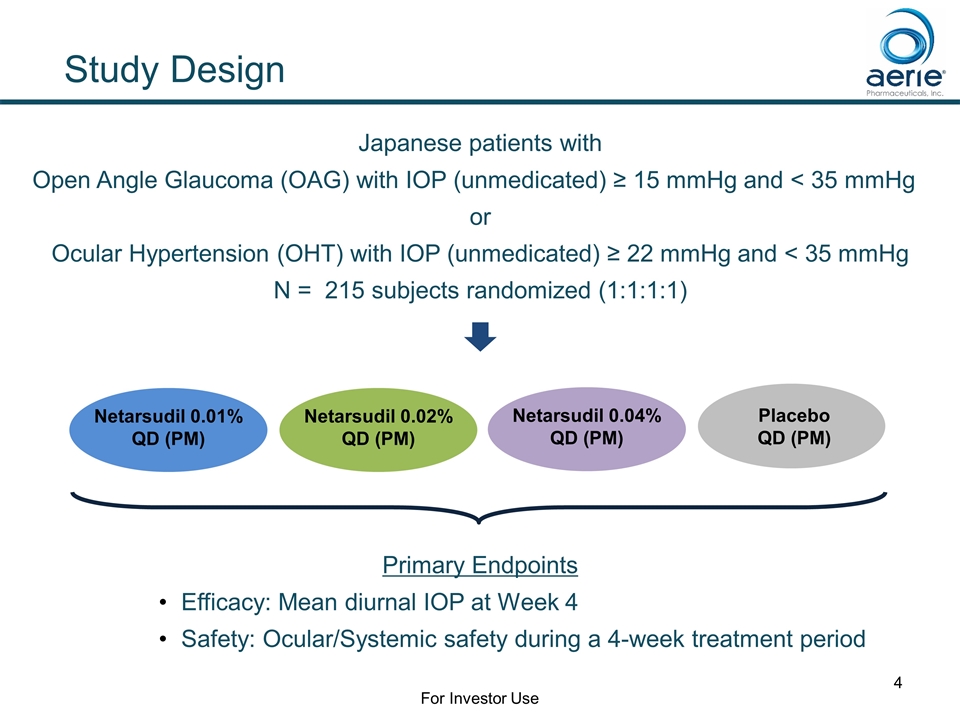
Primary Endpoints Efficacy: Mean diurnal IOP at Week 4 Safety: Ocular/Systemic safety during a 4-week treatment period Japanese patients with Open Angle Glaucoma (OAG) with IOP (unmedicated) ≥ 15 mmHg and < 35 mmHg or Ocular Hypertension (OHT) with IOP (unmedicated) ≥ 22 mmHg and < 35 mmHg N = 215 subjects randomized (1:1:1:1) Netarsudil 0.02% QD (PM) Placebo QD (PM) Netarsudil 0.04% QD (PM) Study Design Netarsudil 0.01% QD (PM) For Investor Use
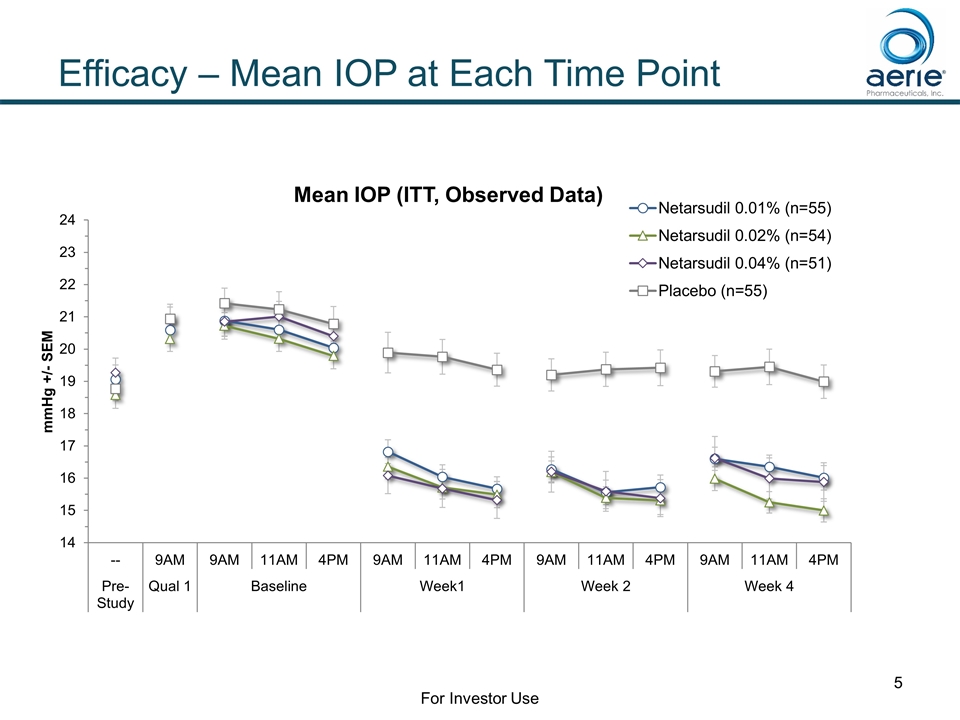
Efficacy – Mean IOP at Each Time Point For Investor Use
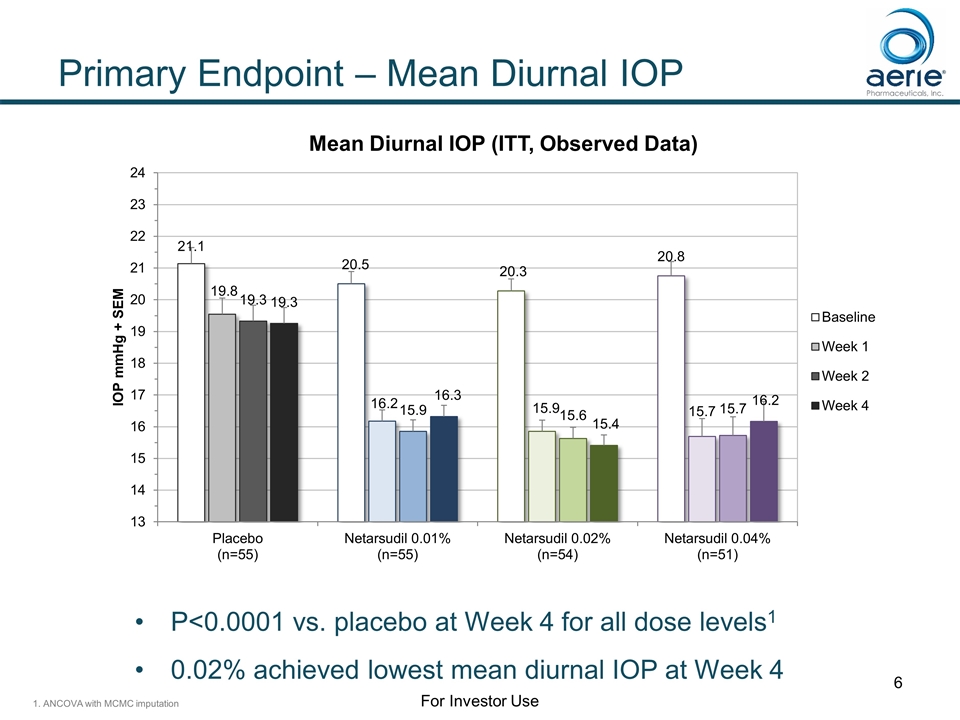
Primary Endpoint – Mean Diurnal IOP P<0.0001 vs. placebo at Week 4 for all dose levels1 0.02% achieved lowest mean diurnal IOP at Week 4 1. ANCOVA with MCMC imputation For Investor Use
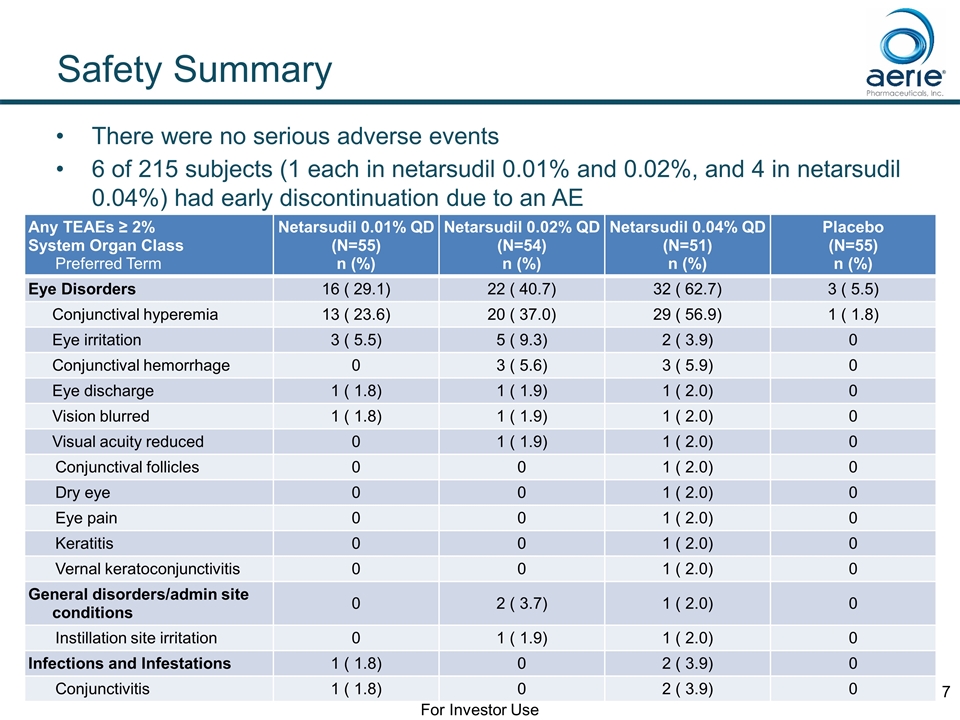
Any TEAEs ≥ 2% System Organ Class Preferred Term Netarsudil 0.01% QD (N=55) n (%) Netarsudil 0.02% QD (N=54) n (%) Netarsudil 0.04% QD (N=51) n (%) Placebo (N=55) n (%) Eye Disorders 16 ( 29.1) 22 ( 40.7) 32 ( 62.7) 3 ( 5.5) Conjunctival hyperemia 13 ( 23.6) 20 ( 37.0) 29 ( 56.9) 1 ( 1.8) Eye irritation 3 ( 5.5) 5 ( 9.3) 2 ( 3.9) 0 Conjunctival hemorrhage 0 3 ( 5.6) 3 ( 5.9) 0 Eye discharge 1 ( 1.8) 1 ( 1.9) 1 ( 2.0) 0 Vision blurred 1 ( 1.8) 1 ( 1.9) 1 ( 2.0) 0 Visual acuity reduced 0 1 ( 1.9) 1 ( 2.0) 0 Conjunctival follicles 0 0 1 ( 2.0) 0 Dry eye 0 0 1 ( 2.0) 0 Eye pain 0 0 1 ( 2.0) 0 Keratitis 0 0 1 ( 2.0) 0 Vernal keratoconjunctivitis 0 0 1 ( 2.0) 0 General disorders/admin site conditions 0 2 ( 3.7) 1 ( 2.0) 0 Instillation site irritation 0 1 ( 1.9) 1 ( 2.0) 0 Infections and Infestations 1 ( 1.8) 0 2 ( 3.9) 0 Conjunctivitis 1 ( 1.8) 0 2 ( 3.9) 0 Safety Summary There were no serious adverse events 6 of 215 subjects (1 each in netarsudil 0.01% and 0.02%, and 4 in netarsudil 0.04%) had early discontinuation due to an AE For Investor Use
Japan Phase 2 Executive Summary Netarsudil 0.01%, 0.02% and 0.04% were efficacious and met primary endpoint of superiority to placebo in mean diurnal IOP1 Baseline mean diurnal IOPs were 20 - 21 mmHg across study arms2 Week 4 mean diurnal IOP was 16.3 (-4.1), 15.4 (-4.8), 16.2 (-4.8) and 19.3 (-1.7) mmHg in the netarsudil 0.01%, 0.02%, 0.04%, and placebo groups, respectively2 Netarsudil 0.01%, 0.02% and 0.04% were safe and generally well tolerated in Japanese subjects Netarsudil 0.02% provided optimal efficacy and safety profile Most common AEs were Conjunctival Hyperemia (37.0%) and Eye Irritation (9.3%) Discontinuations rate was 1.9% (1/54 subjects) Hyperemia and discontinuation rates lower than in US trials3-5 Efficacy at low baseline IOPs predicts efficacy in Normal Tension Glaucoma Meeting with PMDA in 1H’20 and expect start of P3 trials in 2H ‘20 1. ANCOVA with MCMC imputation. 2. Observed data. 3. Bacharach J, et al. Ophthalmology. 2015 Feb;122(2):302-7. 4. Serle JB, et al. Am J Ophthalmol. 2018 Feb;186:116-127. 5. Khouri AS, et al. Am J Ophthalmol. 2019 Aug;204:97-104. For Investor Use







