
TRPM8: A New Target for the Treatment of Dry Eye David A. Hollander, MD, MBA Chief R&D Officer March 11, 2021 Exhibit 99.1

Important Information The information in this presentation does not contain all of the information that a potential investor should review before investing in Aerie shares. The descriptions of Aerie Pharmaceuticals, Inc. (the “Company” or “Aerie”) in this presentation are qualified in their entirety by reference to reports filed with the SEC. Certain information in this presentation has been obtained from outside sources or is anecdotal in nature. While such information is believed to be reliable for the purposes used herein, no representations are made as to the accuracy or completeness thereof and we take no responsibility for such information. Any discussion in this presentation of the potential efficacy or viability of AR-15512 as a treatment for dry eye is subject to regulatory approval. Aerie’s commercial or regulatory experience with Rhopressa, Rocklatan or any product candidate is in no way predictive of the potential success of AR-15512. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. This presentation shall not constitute an offer to sell, nor a solicitation of an offer to buy, any of Aerie’s securities. Certain statements in this presentation, including any guidance or timelines presented herein, are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. For example, uncertainties around the duration and severity of the current global COVID-19 pandemic including its possible impact on our clinical and commercial operations and our global supply chain could cause our actual results to be materially different than those expressed in our forward-looking statements. Any top line data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may be disclosed at any time. In addition, any discussion in this presentation about preclinical activities or opportunities associated with our products or discussions involving the potential for our dry eye or retinal product candidates are preliminary and the outcome of any studies may not be predictive of the outcome of later trials and ultimate regulatory approval. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. Any statements regarding Aerie’s future liquidity, cash balances or financing transactions also constitute forward-looking statements as are discussions of the possibility of, or possible results of, any commercial transactions or collaborations. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law.
Majority of Dry Eye Patients Are Untreated 1Stonecipher et al. Therapeutics and Clinical Risk Management 2013 Over 30 million individuals in the US alone suffer from dry eye, yet less than 10% are treated. Dry eye has significant impact on reading, driving at night, workplace productivity and overall quality of life. Prevalence of dry eye continues to rise as a result of aging population, more frequent use of contact lenses, computers, smartphones, and tablets. Dry eye represents one of the most common reasons for patients seeing an ophthalmologist or optometrist. The majority of patients seeking medical attention for dry eye do not receive a pharmaceutical intervention or punctal plugs.1 Dry eye is a symptomatic disease, yet most products (except Lifitegrast) approved for the chronic treatment of dry eye are not indicated for the improvement of dry eye symptoms.

Unmet Need for a Novel MOA that Addresses Symptoms and Signs of Dry Eye Pharmaceutical treatments approved today for dry eye are anti-inflammatories. These anti-inflammatory therapies are often associated with poor compliance secondary to delayed onset or poor tolerability. With the evolving understanding of dry eye, it is now recognized that etiologies beyond inflammation play a central role in dry eye disease (DED). Novel modalities are needed that address other etiologies of this multifactorial disease. 2017 DEWSII Dry Eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neuro-sensory abnormalities play etiological roles. 1 Neuro-sensory abnormalities now recognized to play a role in the underlying disease and therefore offer new targets for treatment. Despite considerable progress, there remains a significant unmet need for a novel MOA that addresses underlying etiology and relieves symptoms in addition to treating signs of dry eye. 1Craig et al. TFOS DEWSII Report. Ocular Surface 2017.
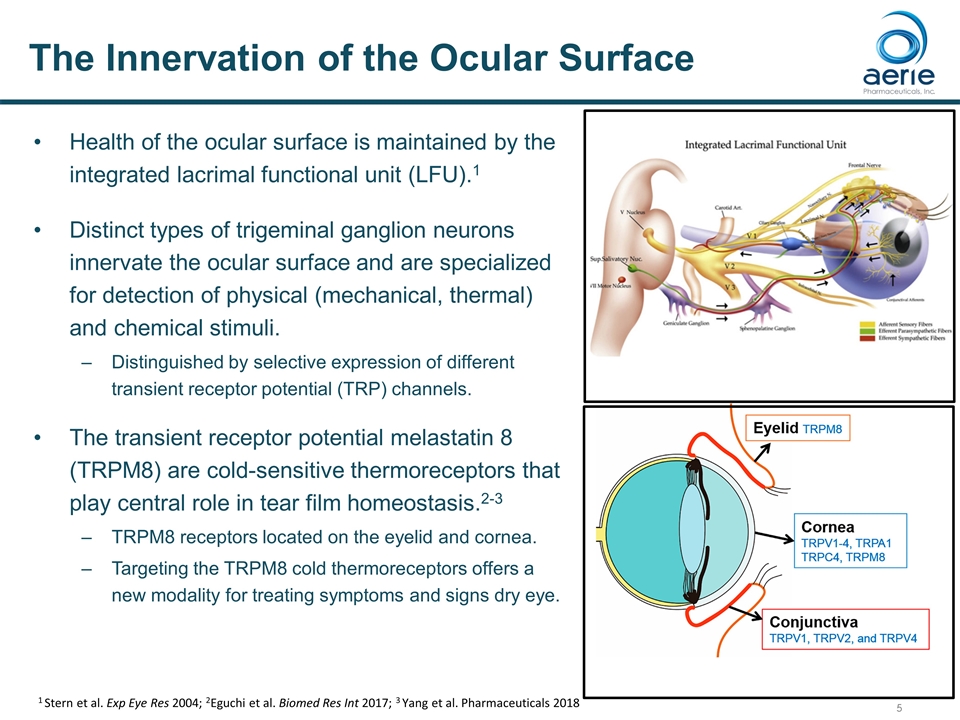
The Innervation of the Ocular Surface Health of the ocular surface is maintained by the integrated lacrimal functional unit (LFU).1 Distinct types of trigeminal ganglion neurons innervate the ocular surface and are specialized for detection of physical (mechanical, thermal) and chemical stimuli. Distinguished by selective expression of different transient receptor potential (TRP) channels. The transient receptor potential melastatin 8 (TRPM8) are cold-sensitive thermoreceptors that play central role in tear film homeostasis.2-3 TRPM8 receptors located on the eyelid and cornea. Targeting the TRPM8 cold thermoreceptors offers a new modality for treating symptoms and signs dry eye. 1 Stern et al. Exp Eye Res 2004; 2Eguchi et al. Biomed Res Int 2017; 3 Yang et al. Pharmaceuticals 2018
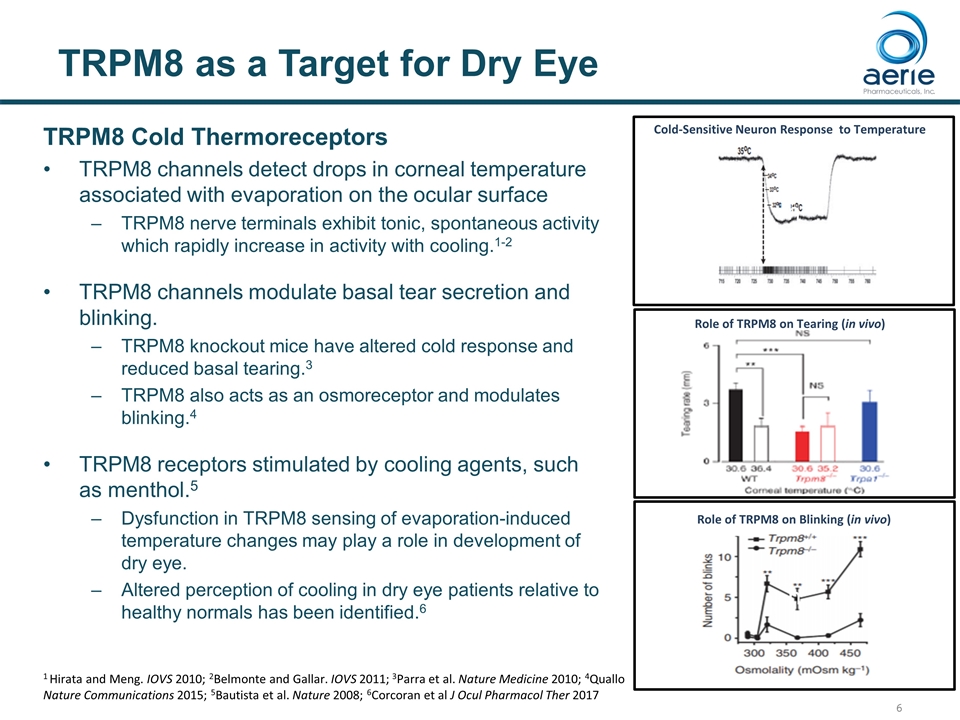
TRPM8 as a Target for Dry Eye TRPM8 Cold Thermoreceptors TRPM8 channels detect drops in corneal temperature associated with evaporation on the ocular surface TRPM8 nerve terminals exhibit tonic, spontaneous activity which rapidly increase in activity with cooling.1-2 TRPM8 channels modulate basal tear secretion and blinking. TRPM8 knockout mice have altered cold response and reduced basal tearing.3 TRPM8 also acts as an osmoreceptor and modulates blinking.4 TRPM8 receptors stimulated by cooling agents, such as menthol.5 Dysfunction in TRPM8 sensing of evaporation-induced temperature changes may play a role in development of dry eye. Altered perception of cooling in dry eye patients relative to healthy normals has been identified.6 Cold-Sensitive Neuron Response to Temperature h Role of TRPM8 on Tearing (in vivo) Role of TRPM8 on Blinking (in vivo) 1 Hirata and Meng. IOVS 2010; 2Belmonte and Gallar. IOVS 2011; 3Parra et al. Nature Medicine 2010; 4Quallo Nature Communications 2015; 5Bautista et al. Nature 2008; 6Corcoran et al J Ocul Pharmacol Ther 2017 h h
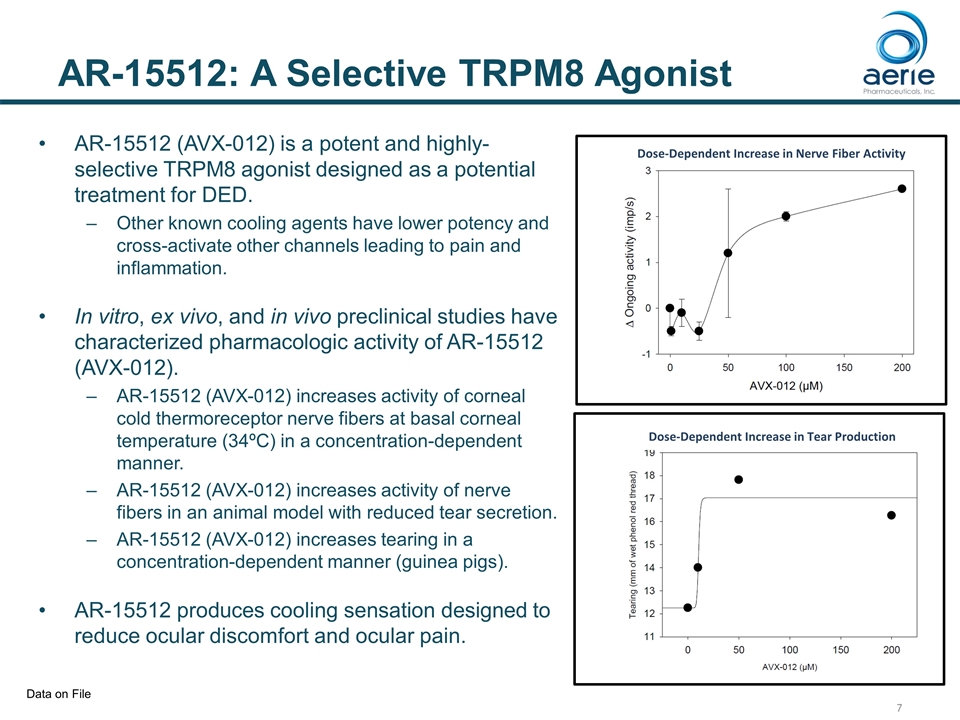
AR-15512: A Selective TRPM8 Agonist AR-15512 (AVX-012) is a potent and highly-selective TRPM8 agonist designed as a potential treatment for DED. Other known cooling agents have lower potency and cross-activate other channels leading to pain and inflammation. In vitro, ex vivo, and in vivo preclinical studies have characterized pharmacologic activity of AR-15512 (AVX-012). AR-15512 (AVX-012) increases activity of corneal cold thermoreceptor nerve fibers at basal corneal temperature (34ºC) in a concentration-dependent manner. AR-15512 (AVX-012) increases activity of nerve fibers in an animal model with reduced tear secretion. AR-15512 (AVX-012) increases tearing in a concentration-dependent manner (guinea pigs). AR-15512 produces cooling sensation designed to reduce ocular discomfort and ocular pain. Dose-Dependent Increase in Nerve Fiber Activity Dose-Dependent Increase in Tear Production Data on File
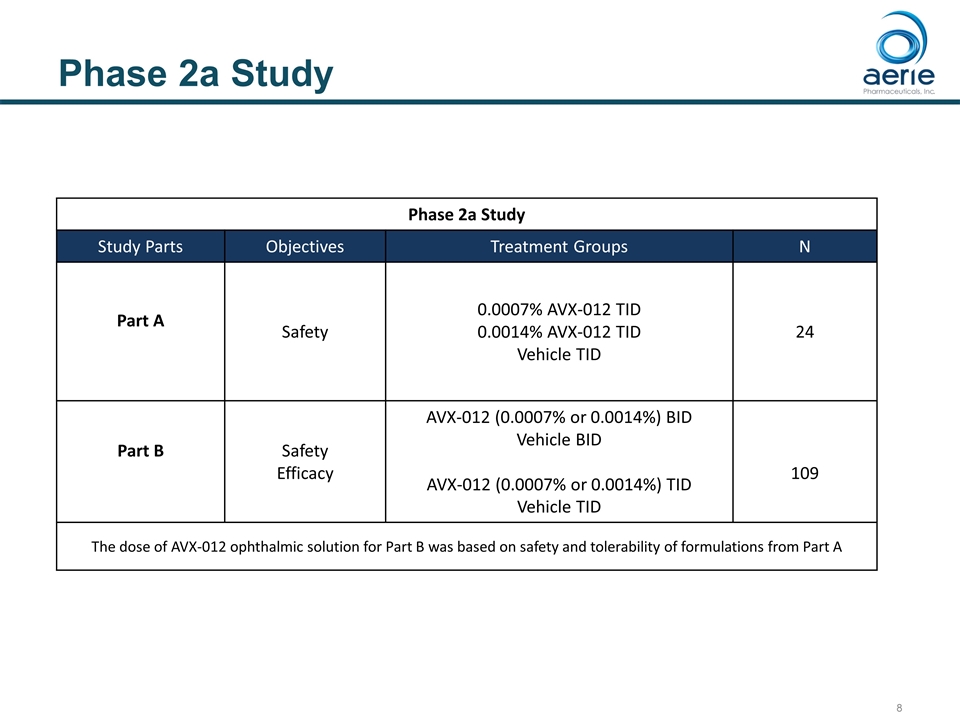
Phase 2a Study Phase 2a Study Study Parts Objectives Treatment Groups N Part A Safety 0.0007% AVX-012 TID 0.0014% AVX-012 TID Vehicle TID 24 Part B Safety Efficacy AVX-012 (0.0007% or 0.0014%) BID Vehicle BID AVX-012 (0.0007% or 0.0014%) TID Vehicle TID 109 The dose of AVX-012 ophthalmic solution for Part B was based on safety and tolerability of formulations from Part A
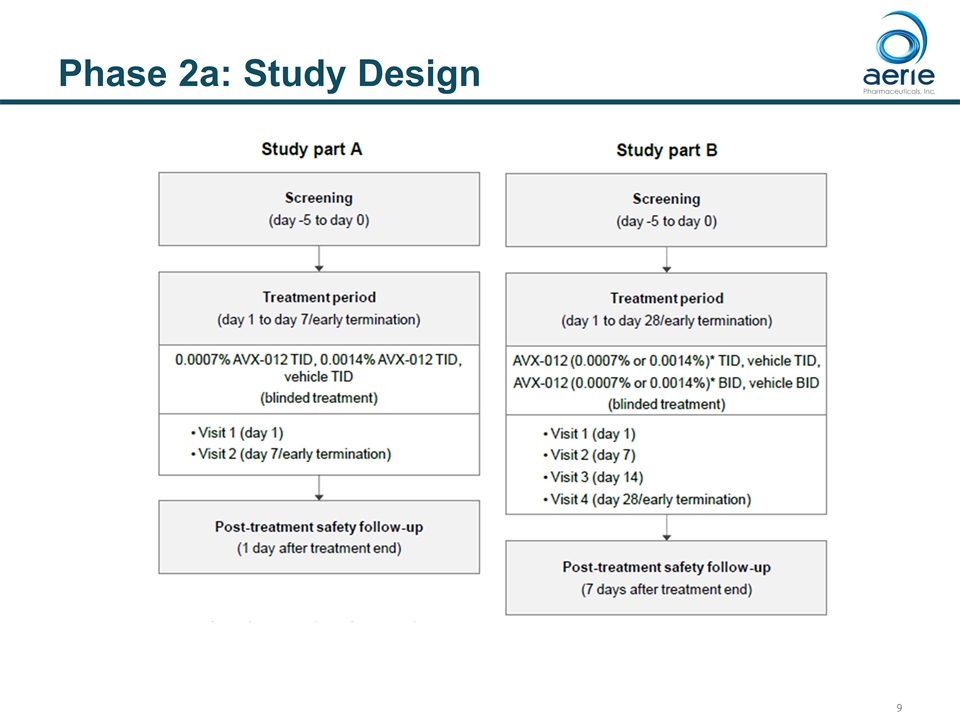
Phase 2a: Study Design
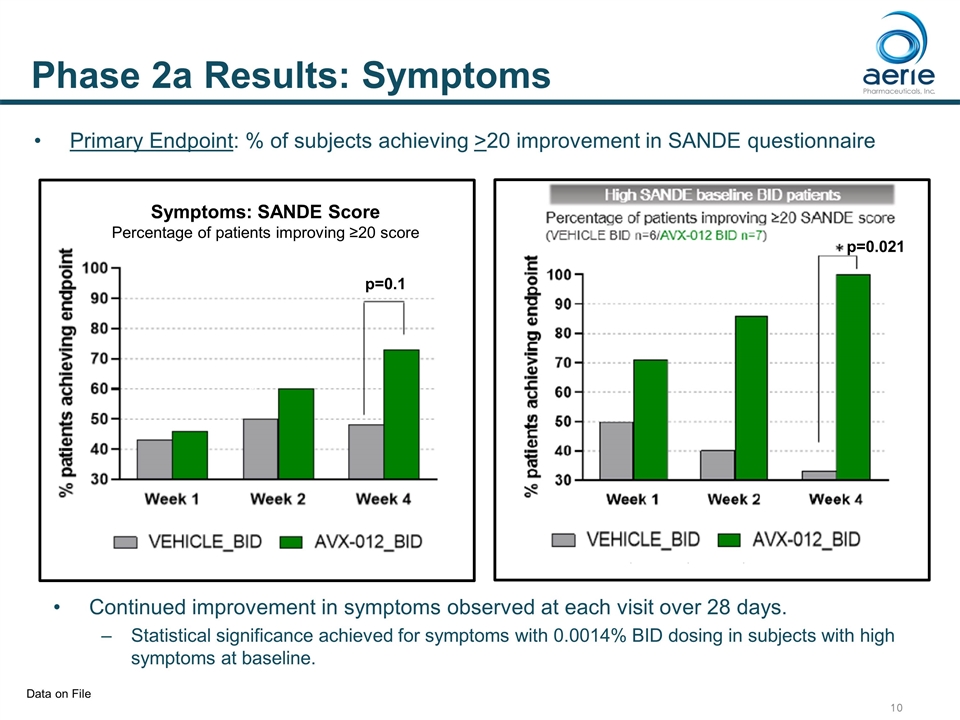
Phase 2a Results: Symptoms p=0.021 h Symptoms: SANDE Score Percentage of patients improving ≥20 score h Primary Endpoint: % of subjects achieving >20 improvement in SANDE questionnaire Continued improvement in symptoms observed at each visit over 28 days. Statistical significance achieved for symptoms with 0.0014% BID dosing in subjects with high symptoms at baseline. Data on File p=0.1
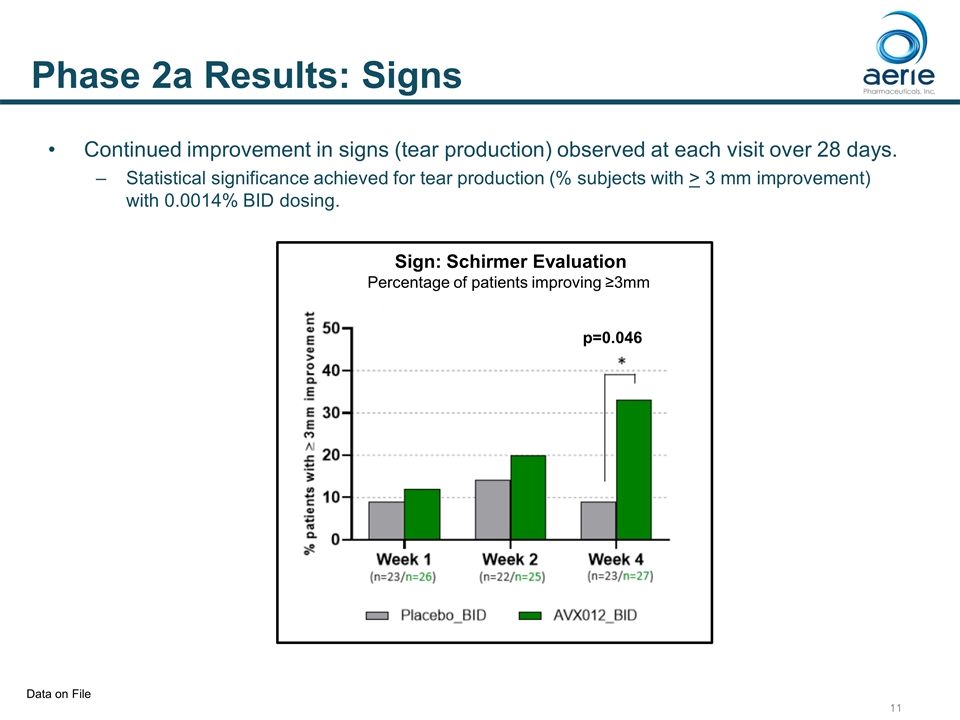
Data on File Phase 2a Results: Signs Continued improvement in signs (tear production) observed at each visit over 28 days. Statistical significance achieved for tear production (% subjects with > 3 mm improvement) with 0.0014% BID dosing. Sign: Schirmer Evaluation Percentage of patients improving ≥3mm h p=0.046
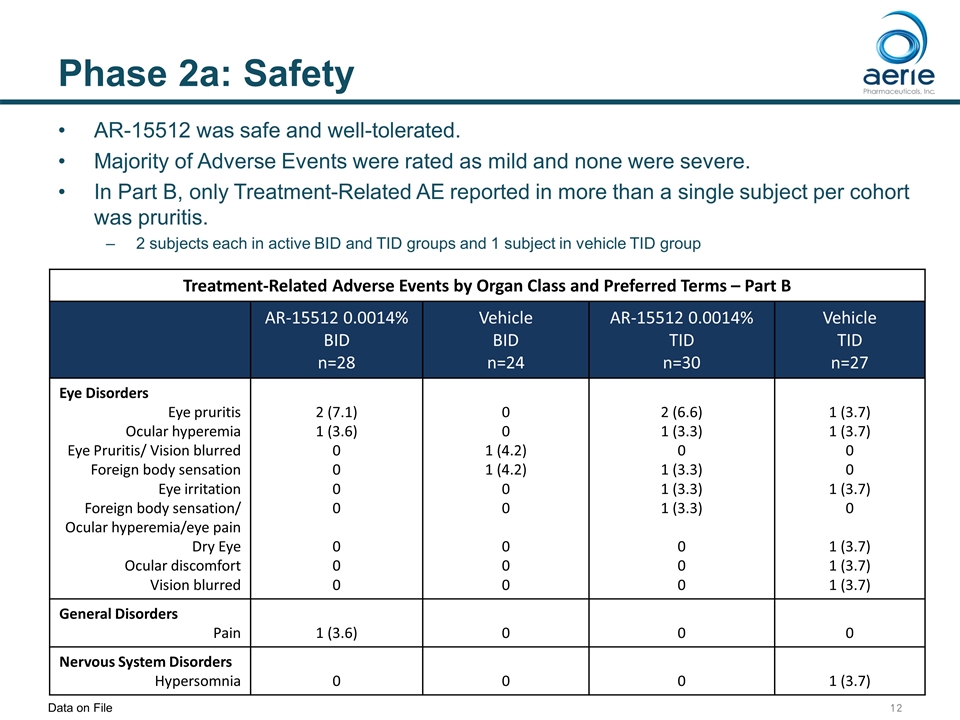
Phase 2a: Safety Treatment-Related Adverse Events by Organ Class and Preferred Terms – Part B AR-15512 0.0014% BID n=28 Vehicle BID n=24 AR-15512 0.0014% TID n=30 Vehicle TID n=27 Eye Disorders Eye pruritis Ocular hyperemia Eye Pruritis/ Vision blurred Foreign body sensation Eye irritation Foreign body sensation/ Ocular hyperemia/eye pain Dry Eye Ocular discomfort Vision blurred 2 (7.1) 1 (3.6) 0 0 0 0 0 0 0 0 0 1 (4.2) 1 (4.2) 0 0 0 0 0 2 (6.6) 1 (3.3) 0 1 (3.3) 1 (3.3) 1 (3.3) 0 0 0 1 (3.7) 1 (3.7) 0 0 1 (3.7) 0 1 (3.7) 1 (3.7) 1 (3.7) General Disorders Pain 1 (3.6) 0 0 0 Nervous System Disorders Hypersomnia 0 0 0 1 (3.7) AR-15512 was safe and well-tolerated. Majority of Adverse Events were rated as mild and none were severe. In Part B, only Treatment-Related AE reported in more than a single subject per cohort was pruritis. 2 subjects each in active BID and TID groups and 1 subject in vehicle TID group Data on File
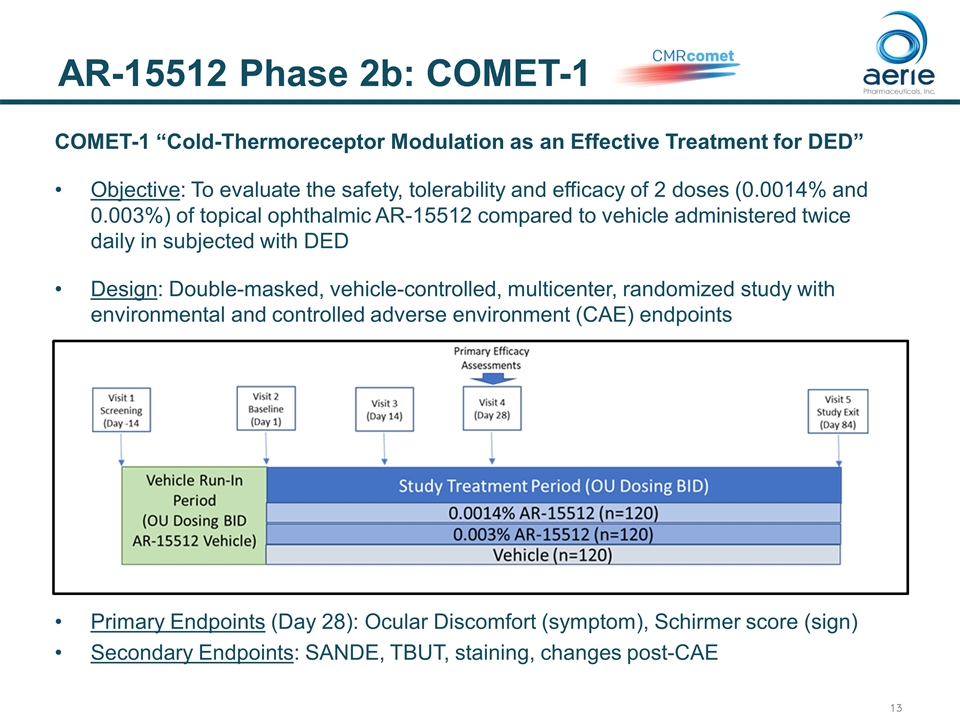
AR-15512 Phase 2b: COMET-1 COMET-1 “Cold-Thermoreceptor Modulation as an Effective Treatment for DED” Objective: To evaluate the safety, tolerability and efficacy of 2 doses (0.0014% and 0.003%) of topical ophthalmic AR-15512 compared to vehicle administered twice daily in subjected with DED Design: Double-masked, vehicle-controlled, multicenter, randomized study with environmental and controlled adverse environment (CAE) endpoints Primary Endpoints (Day 28): Ocular Discomfort (symptom), Schirmer score (sign) Secondary Endpoints: SANDE, TBUT, staining, changes post-CAE
AR-15512: A TRPM8 Agonist for the Treatment of Dry Eye AR-15512 is a selective agonist of the TRPM8 cold thermoreceptor with potential to improve both the symptoms and signs of DED. A novel MOA (different from currently approved anti-inflammatories) designed to produce a cooling sensation to relieve ocular discomfort as well as increase tear production. Impacts natural basal tear production and not an acute reflex tear TRPM8 stimulation is independent of pain-evoked reflex tearing. Phase 2b study (powered as a Phase 3) ongoing with topline expected Q3 2021. Supported by preclinical, toxicology, and Phase 2a clinical studies. AR-15512 has been shown to be safe and well-tolerated. IP protection for composition of matter and method of use through 2031. The MOA supports the use of AR-15512 as both monotherapy as well as in conjunction with other approved dry eye treatments.













