
A Phase 2b Study Evaluating the Safety and Efficacy of AR-15512 Ophthalmic Solution for the Treatment of Dry Eye Disease Study AR-15512-CS201 (COMET-1) September 2021 Exhibit 99.2

Important Information Any discussion of the potential use or expected success of our product candidates is subject to our regulatory approval. The information in this presentation is current only as of its date and may have changed prior to it being read or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. Forward-looking statements in this presentation includes statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our expectations regarding the effectiveness of our product candidates, including AR-15512, and the success, timing and cost of our ongoing and anticipated clinical trials for our product candidates, including AR-15512. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law. For Investor Use
Dry Eye 1Stonecipher et al. Therapeutics and Clinical Risk Management 2013 Over 30 million individuals in the US alone suffer from dry eye, yet less than 10% are treated. Prevalence of dry eye continues to rise as a result of an aging population, and more frequent use of contact lenses, computers, smartphones, and tablets. Dry eye represents one of the most common reasons for patients seeing an ophthalmologist or optometrist. The majority of patients seeking medical attention for dry eye do not receive a pharmaceutical intervention or punctal plugs.1 Dry eye is a symptomatic disease, yet most products (except Lifitegrast) approved for the chronic treatment of dry eye are not indicated for the improvement of dry eye symptoms. We designed the Phase 2b clinical study to fully characterize a novel TRPM8 agonist in order to optimize the Phase 3 program. For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
Executive Summary In this Phase 2b clinical study, two concentrations (0.0014% and 0.003%) of AR-15512 dosed twice daily were evaluated for safety and efficacy vs. vehicle in subjects with dry eye. Statistically significant efficacy was demonstrated across multiple pre-specified symptom and sign endpoints with 0.003% AR-15512. Statistically significant improvements in symptoms of Ocular Discomfort, SANDE*, and Eye Dryness. Statistically significant improvements in signs for tear production, conjunctival redness, and ocular surface staining. Efficacy for both symptoms and signs observed as early as 14 days. Continued improvement in symptoms and signs over the 3-month (84-day) study. The formulations were safe and well-tolerated. Most common AE was instillation site burning or stinging (~95% rated as mild). < 2% of subjects in the 0.003% treatment group discontinued the study due to AEs. No systemic or serious AEs attributed to study medication. 0.003% AR-15512 selected for advancement with planned initiation of Phase 3 pivotal studies in H1 2022. For Investor Use Data on file * Symptom Assessment iN Dry Eye AR-15512 is a development stage product candidate and is not approved by any regulatory agency.

TRPM8 (transient receptor potential melastatin) receptors are cold-sensitive thermoreceptors that play key role in tear film homeostasis.1-2 Located on eyelid and cornea. TRPM8 channels detect drops in corneal temperature associated with tear evaporation on ocular surface. AR-15512 is a potent and highly-selective TRPM8 agonist designed as a potential treatment for DED. AR-15512 shown in vivo to increase tearing in a concentration-dependent manner. AR-15512 produces cooling sensation (via TRPM8 receptor) designed to reduce ocular discomfort and pain. Targeting the TRPM8 receptor offers a new modality for treating symptoms and signs dry eye. Neuro-sensory abnormalities now recognized to play a role in the etiology of dry eye disease.3 1Eguchi et al. Biomed Res Int 2017; 2Yang et al. Pharmaceuticals 2018; 3Craig et al. TFOS DEWSII Report. Ocular Surface 2017. Cold-Sensitive Neuron Response to Temperature AR-15512: A Selective TRPM8 Agonist for Dry Eye For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
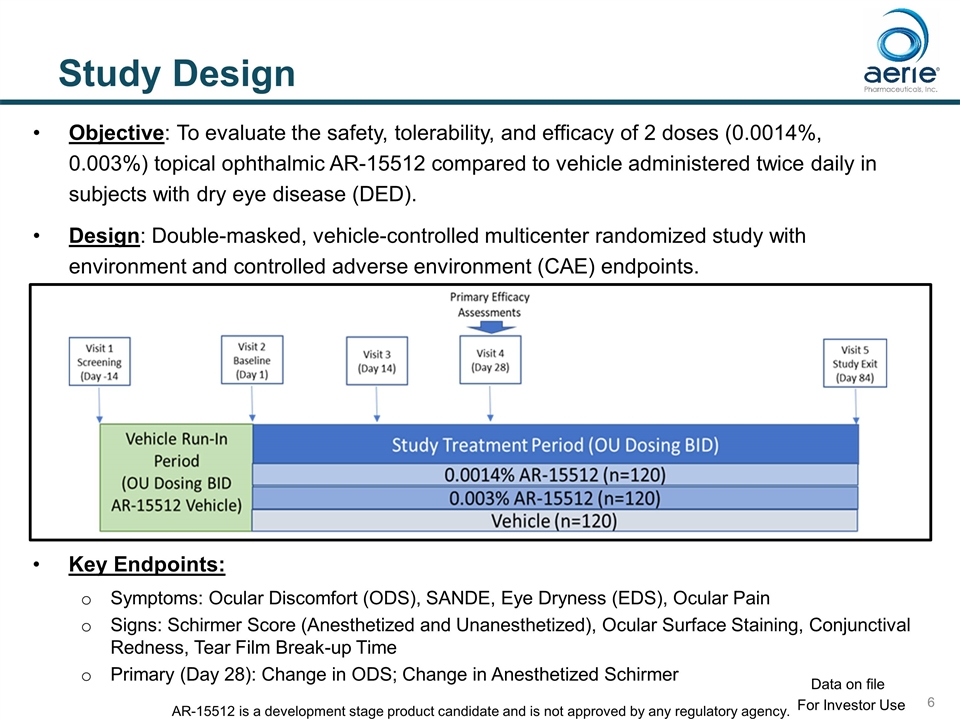
Study Design Objective: To evaluate the safety, tolerability, and efficacy of 2 doses (0.0014%, 0.003%) topical ophthalmic AR-15512 compared to vehicle administered twice daily in subjects with dry eye disease (DED). Design: Double-masked, vehicle-controlled multicenter randomized study with environment and controlled adverse environment (CAE) endpoints. Key Endpoints: Symptoms: Ocular Discomfort (ODS), SANDE, Eye Dryness (EDS), Ocular Pain Signs: Schirmer Score (Anesthetized and Unanesthetized), Ocular Surface Staining, Conjunctival Redness, Tear Film Break-up Time Primary (Day 28): Change in ODS; Change in Anesthetized Schirmer For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
Key Inclusion Criteria Male or female, 30 years of age or older at the Screening visit. Have a history of dry eye disease within the previous 6 months. Have used, and/or desired to use artificial tears for dry eye disease (DED) within 2 months prior to the Screening visit. Symptoms of DED based Ocular Discomfort Score(ODS) – VAS and Global SANDE questionnaires at both the Screening and Baseline visits. Anesthetized Schirmer test score ≥ 2 and 10 mm/5 min at both Screening and Baseline visits. Total corneal fluorescein staining score of ≥ 2 and ≤ 15 based on modified NEI grading scheme (0-20), with no one region scoring >3 at Screening visit. BCVA of 20/200 (0.70 LogMAR) or better in both eyes at both the Screening and Baseline visits. Good general and ocular health, as determined by the investigator using medical history, ophthalmic examination, blood chemistry and hematology, urinalysis and vital signs at the Screening visit. Capable of giving signed informed consent, which includes compliance with the requirements and restrictions listed in the informed consent form (ICF) and in this protocol. Able, as assessed by the investigator, and willing to follow study instructions and likely to complete all required study visits. For Investor Use Data on file

Key Exclusion Criteria History or presence of any ocular disorder or condition (other than DED) in either eye that would, in the opinion of the investigator, likely interfere with the interpretation of the study results or subject safety. History of ocular surgery within 1 year prior to the Screening visit. Punctal or intracanalicular plug present in either eyelid within 1 year prior to the Screening visit or anticipated plug insertion or occlusion at any time during the study. Use of contact lenses in either eye within 7 days prior to Screening visit or planned use during the study. Regular use of lid hygiene within 14 days prior to the Screening visit or any planned use during study. Use of any topical ocular medications for DED, ocular corticosteroid or NSAID, glaucoma medications, eye whitening, topical antibiotics, topical antihistamines, mast cell stabilizers or other OTC or nutritional supplements with exception of ATs within 30 days prior to Screening or anticipated use during study. Use of artificial tears within 2 hours prior to the Screening visit or anticipated use during the study. Use of systemic medications associated with treatment of severe DED and/or Meibomian gland disease (such as oral pilocarpine, oral cevimeline, oral macrolides, oral tetracyclines, oral retinoids within 90 days prior to Baseline Visit or anticipated use during the study. Use of systemic corticosteroids started < 90 days prior to Baseline visit or change in dosage during study. Non-ocular topically applied corticosteroids (including nasal inhalers) will be permitted. Known allergies or sensitivity to the study interventions or study diagnostic agents. Positive pregnancy test at Screening or Baseline, currently breastfeeding or plans to become pregnant during the study. Women of childbearing potential not using a medically acceptable form of birth control. The subject has a condition or is in a situation that, in the investigator’s opinion, may put the subject at significant risk or may confound the study results. For Investor Use Data on file
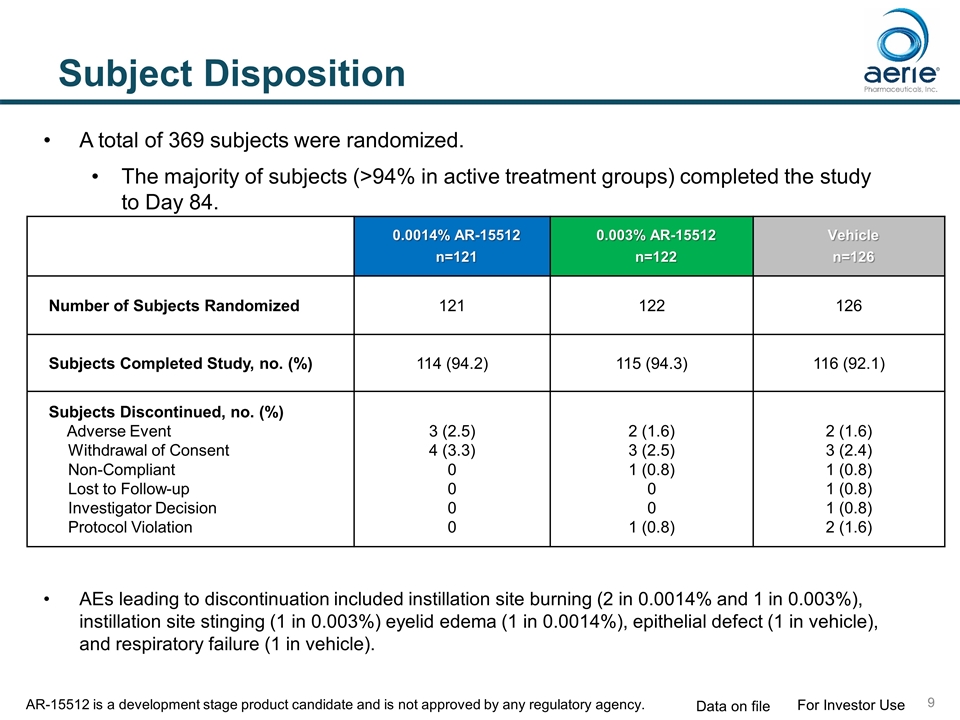
Subject Disposition 0.0014% AR-15512 n=121 0.003% AR-15512 n=122 Vehicle n=126 Number of Subjects Randomized 121 122 126 Subjects Completed Study, no. (%) 114 (94.2) 115 (94.3) 116 (92.1) Subjects Discontinued, no. (%) Adverse Event Withdrawal of Consent Non-Compliant Lost to Follow-up Investigator Decision Protocol Violation 3 (2.5) 4 (3.3) 0 0 0 0 2 (1.6) 3 (2.5) 1 (0.8) 0 0 1 (0.8) 2 (1.6) 3 (2.4) 1 (0.8) 1 (0.8) 1 (0.8) 2 (1.6) A total of 369 subjects were randomized. The majority of subjects (>94% in active treatment groups) completed the study to Day 84. AEs leading to discontinuation included instillation site burning (2 in 0.0014% and 1 in 0.003%), instillation site stinging (1 in 0.003%) eyelid edema (1 in 0.0014%), epithelial defect (1 in vehicle), and respiratory failure (1 in vehicle). For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.

Baseline Demographics 0.0014% AR-15512 n=121 0.003% AR-15512 n=122 Vehicle n=126 Age (years) 39 (32.2%) 30 (24.6%) 34 (27.0%) Mean (SD) 65.5 (10.89) 62.6 (13.01) 63.1 (11.90) Range 31 – 85 30 - 87 30 - 90 Gender Female, no (%) 82 (67.8%) 92 (75.4%) 92 (73.0%) Race, no (%) White/Caucasian 97 (80.2%) 92 (75.4%) 99 (78.6%) Black/African American 15 (12.4%) 18 (14.8%) 18 (14.3%) Asian 8 (6.6%) 11 (9.0%) 7 (5.6%) Other 1 (0.8%) 1 (0.8%) 2 (1.6%) For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
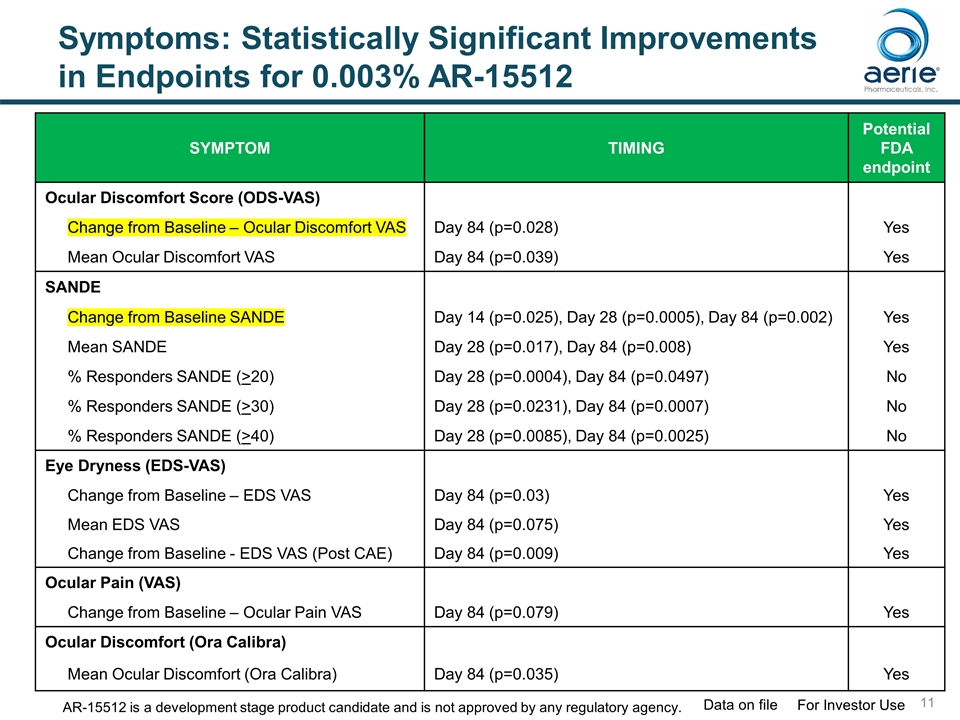
Symptoms: Statistically Significant Improvements in Endpoints for 0.003% AR-15512 SYMPTOM TIMING Potential FDA endpoint Ocular Discomfort Score (ODS-VAS) Change from Baseline – Ocular Discomfort VAS Day 84 (p=0.028) Yes Mean Ocular Discomfort VAS Day 84 (p=0.039) Yes SANDE Change from Baseline SANDE Day 14 (p=0.025), Day 28 (p=0.0005), Day 84 (p=0.002) Yes Mean SANDE Day 28 (p=0.017), Day 84 (p=0.008) Yes % Responders SANDE (>20) Day 28 (p=0.0004), Day 84 (p=0.0497) No % Responders SANDE (>30) Day 28 (p=0.0231), Day 84 (p=0.0007) No % Responders SANDE (>40) Day 28 (p=0.0085), Day 84 (p=0.0025) No Eye Dryness (EDS-VAS) Change from Baseline – EDS VAS Day 84 (p=0.03) Yes Mean EDS VAS Day 84 (p=0.075) Yes Change from Baseline - EDS VAS (Post CAE) Day 84 (p=0.009) Yes Ocular Pain (VAS) Change from Baseline – Ocular Pain VAS Day 84 (p=0.079) Yes Ocular Discomfort (Ora Calibra) Mean Ocular Discomfort (Ora Calibra) Day 84 (p=0.035) Yes For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
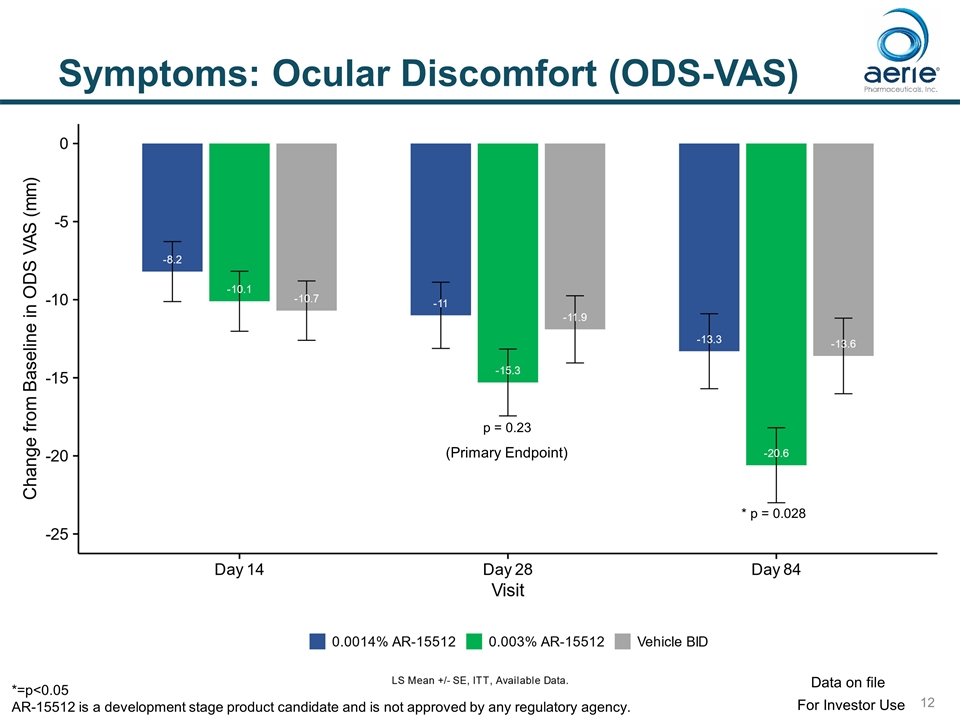
Symptoms: Ocular Discomfort (ODS-VAS) p = 0.23 * p = 0.028 (Primary Endpoint) For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
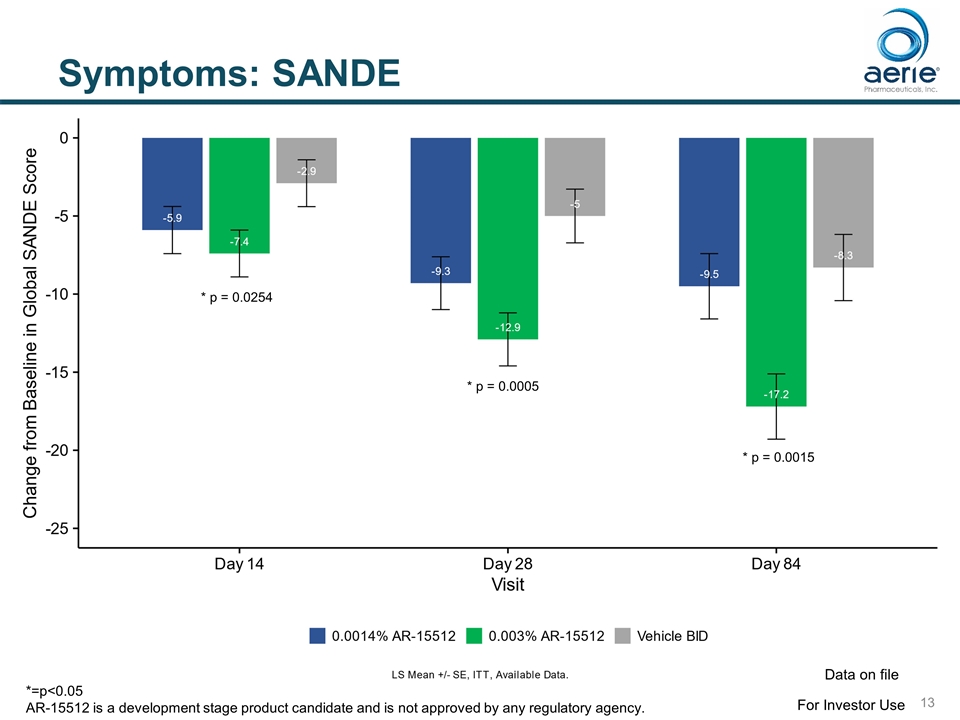
Symptoms: SANDE * p = 0.0015 * p = 0.0005 * p = 0.0254 For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.

Symptoms: Eye Dryness (EDS-VAS) * p = 0.03 For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
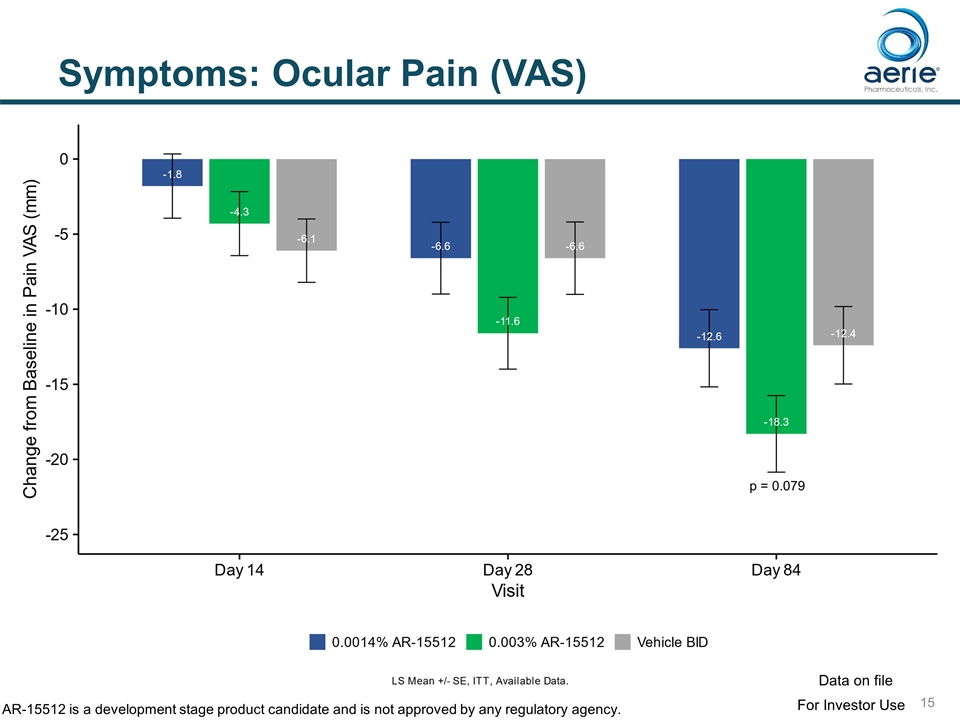
Symptoms: Ocular Pain (VAS) p = 0.079 For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
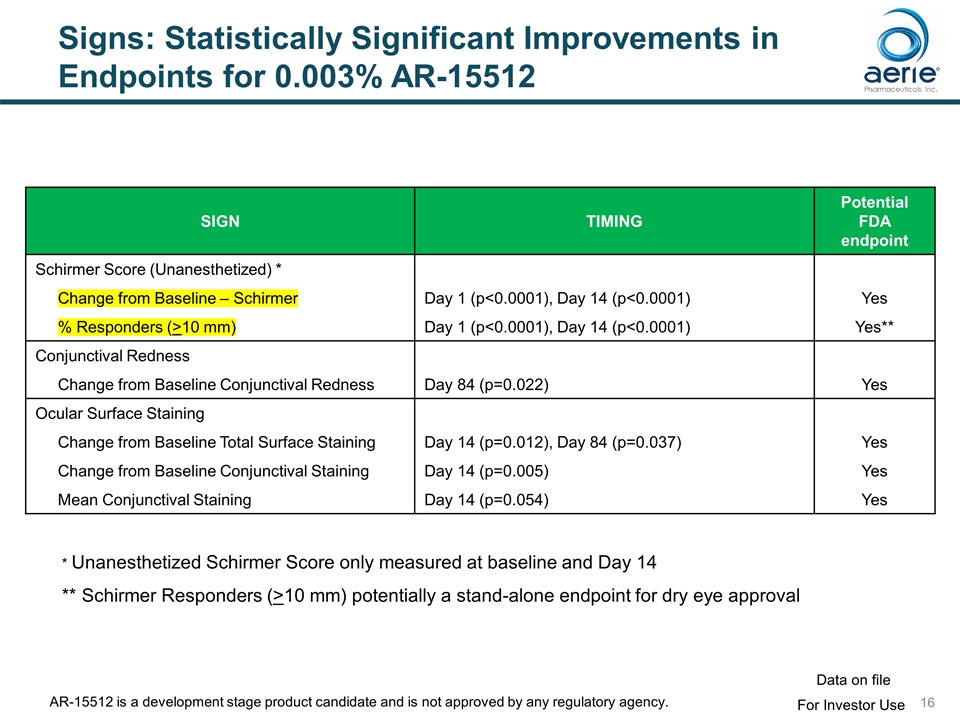
Signs: Statistically Significant Improvements in Endpoints for 0.003% AR-15512 SIGN TIMING Potential FDA endpoint Schirmer Score (Unanesthetized) * Change from Baseline – Schirmer Day 1 (p<0.0001), Day 14 (p<0.0001) Yes % Responders (>10 mm) Day 1 (p<0.0001), Day 14 (p<0.0001) Yes** Conjunctival Redness Change from Baseline Conjunctival Redness Day 84 (p=0.022) Yes Ocular Surface Staining Change from Baseline Total Surface Staining Day 14 (p=0.012), Day 84 (p=0.037) Yes Change from Baseline Conjunctival Staining Day 14 (p=0.005) Yes Mean Conjunctival Staining Day 14 (p=0.054) Yes * Unanesthetized Schirmer Score only measured at baseline and Day 14 ** Schirmer Responders (>10 mm) potentially a stand-alone endpoint for dry eye approval For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
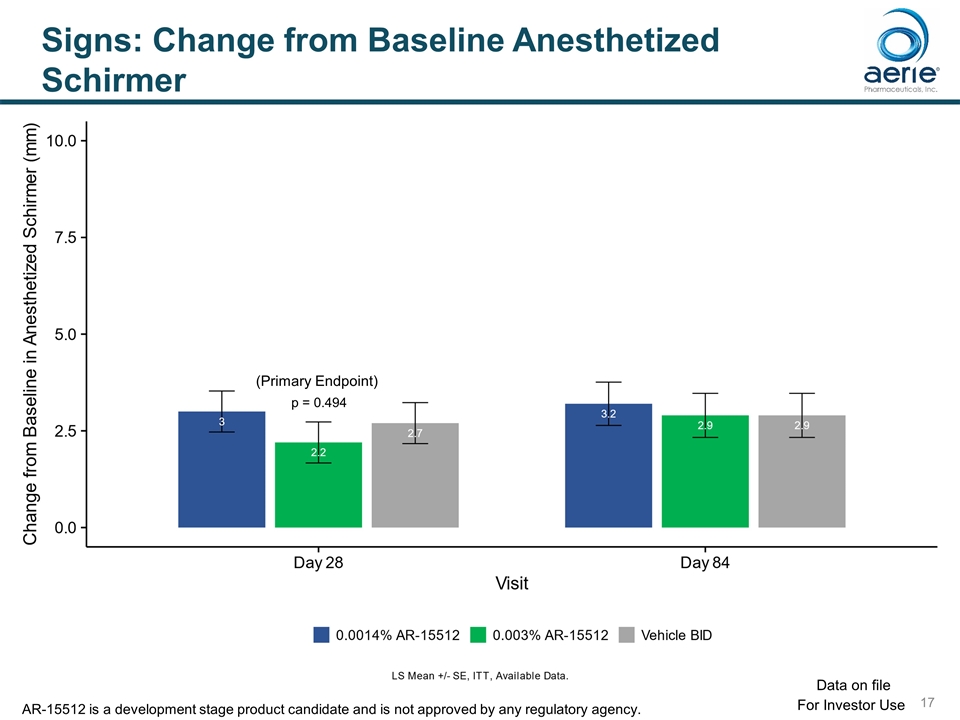
Signs: Change from Baseline Anesthetized Schirmer p = 0.494 (Primary Endpoint) For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
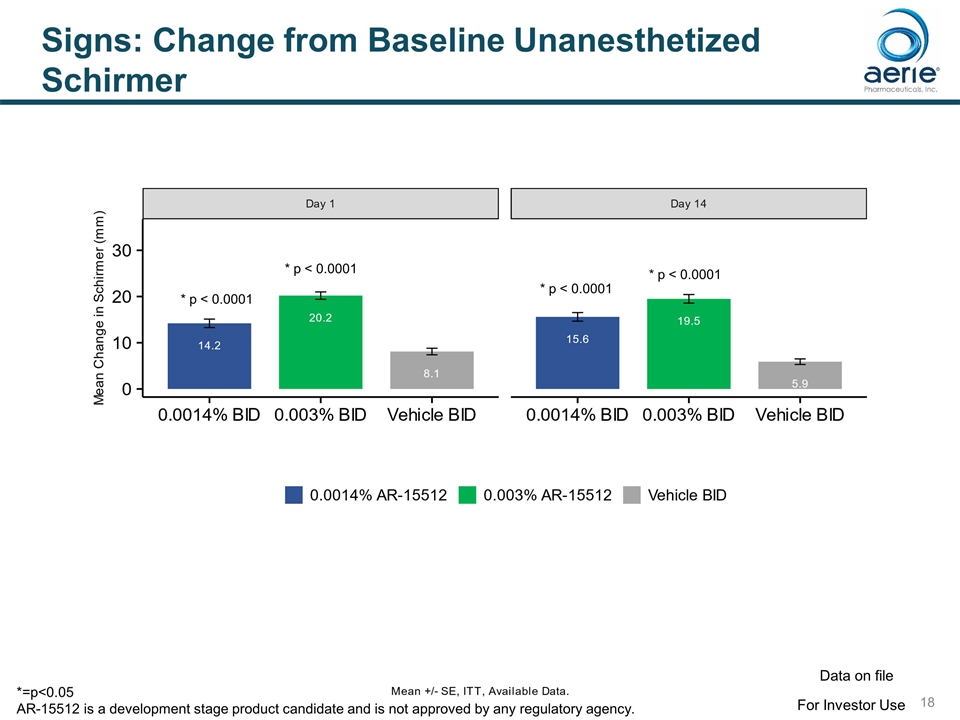
Signs: Change from Baseline Unanesthetized Schirmer * p < 0.0001 * p < 0.0001 * p < 0.0001 * p < 0.0001 For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
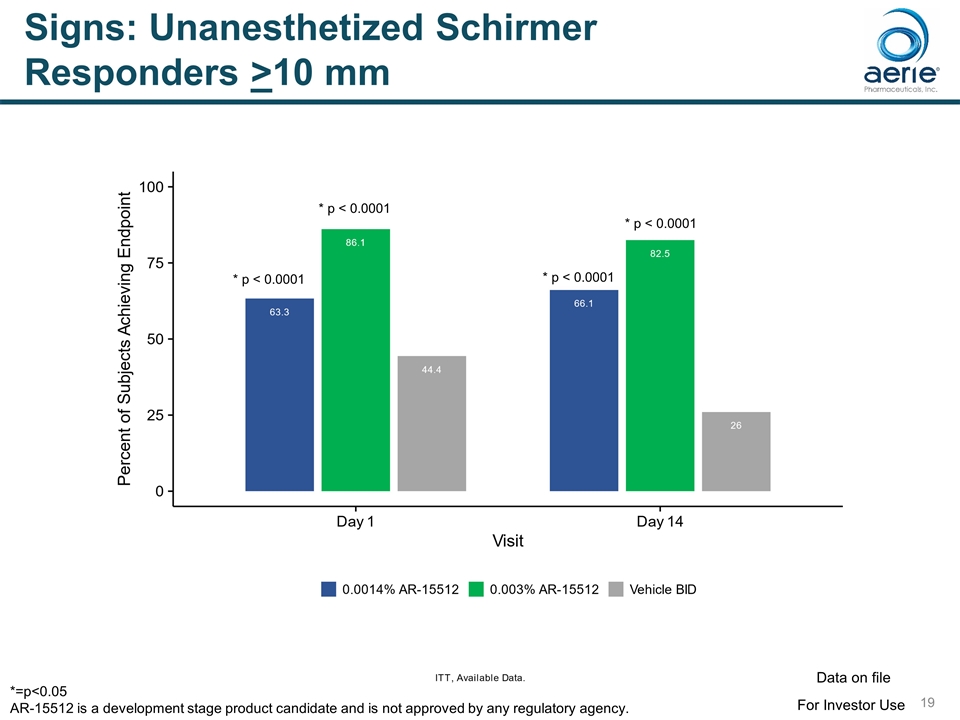
Signs: Unanesthetized Schirmer Responders >10 mm * p < 0.0001 * p < 0.0001 * p < 0.0001 * p < 0.0001 For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
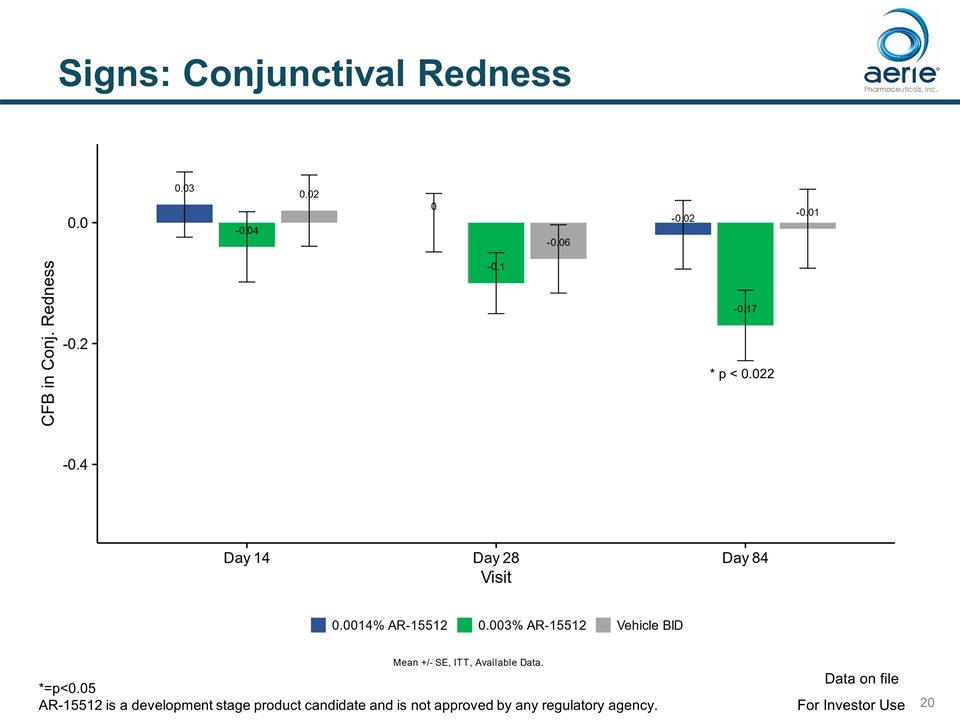
Signs: Conjunctival Redness * p < 0.022 For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
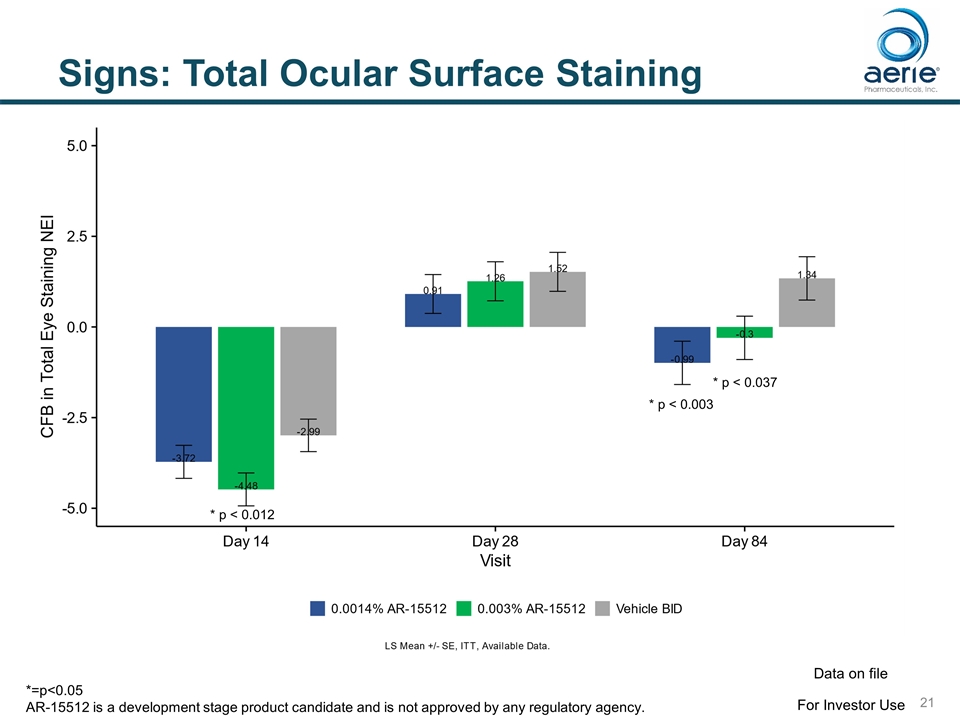
Signs: Total Ocular Surface Staining * p < 0.012 * p < 0.003 * p < 0.037 For Investor Use Data on file *=p<0.05 AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
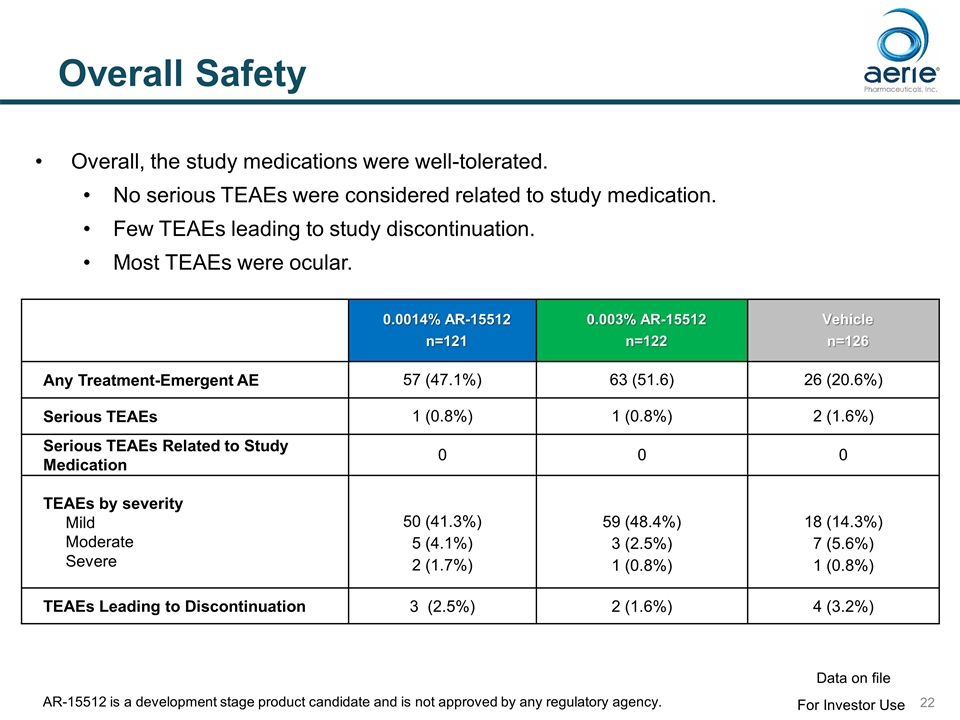
Overall Safety 0.0014% AR-15512 n=121 0.003% AR-15512 n=122 Vehicle n=126 Any Treatment-Emergent AE 57 (47.1%) 63 (51.6) 26 (20.6%) Serious TEAEs 1 (0.8%) 1 (0.8%) 2 (1.6%) Serious TEAEs Related to Study Medication 0 0 0 TEAEs by severity Mild Moderate Severe 50 (41.3%) 5 (4.1%) 2 (1.7%) 59 (48.4%) 3 (2.5%) 1 (0.8%) 18 (14.3%) 7 (5.6%) 1 (0.8%) TEAEs Leading to Discontinuation 3 (2.5%) 2 (1.6%) 4 (3.2%) Overall, the study medications were well-tolerated. No serious TEAEs were considered related to study medication. Few TEAEs leading to study discontinuation. Most TEAEs were ocular. For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
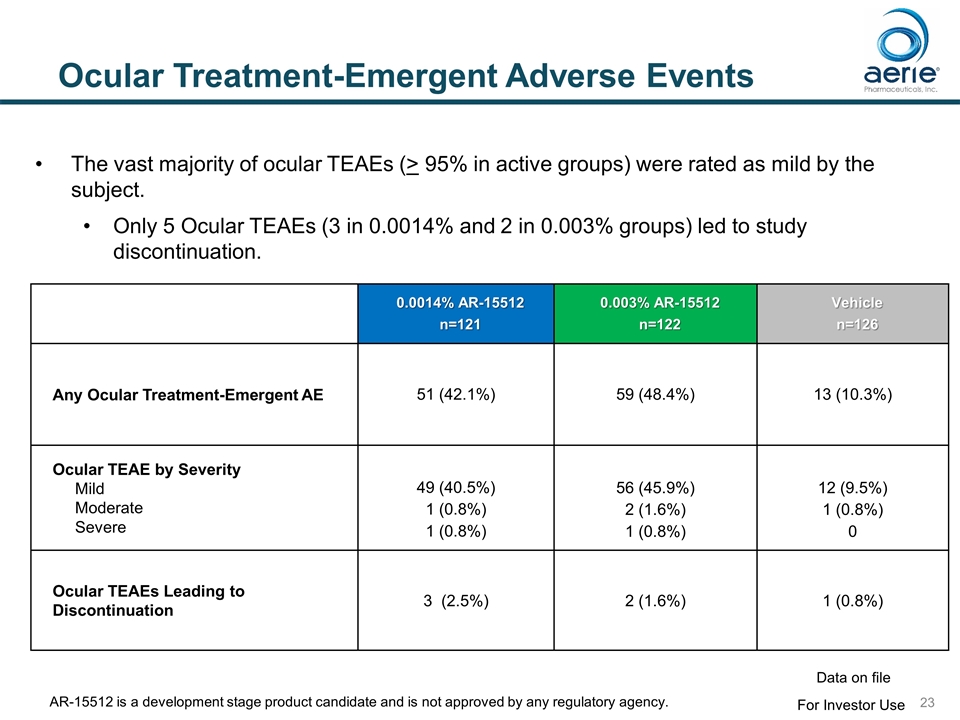
Ocular Treatment-Emergent Adverse Events 0.0014% AR-15512 n=121 0.003% AR-15512 n=122 Vehicle n=126 Any Ocular Treatment-Emergent AE 51 (42.1%) 59 (48.4%) 13 (10.3%) Ocular TEAE by Severity Mild Moderate Severe 49 (40.5%) 1 (0.8%) 1 (0.8%) 56 (45.9%) 2 (1.6%) 1 (0.8%) 12 (9.5%) 1 (0.8%) 0 Ocular TEAEs Leading to Discontinuation 3 (2.5%) 2 (1.6%) 1 (0.8%) The vast majority of ocular TEAEs (> 95% in active groups) were rated as mild by the subject. Only 5 Ocular TEAEs (3 in 0.0014% and 2 in 0.003% groups) led to study discontinuation. For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
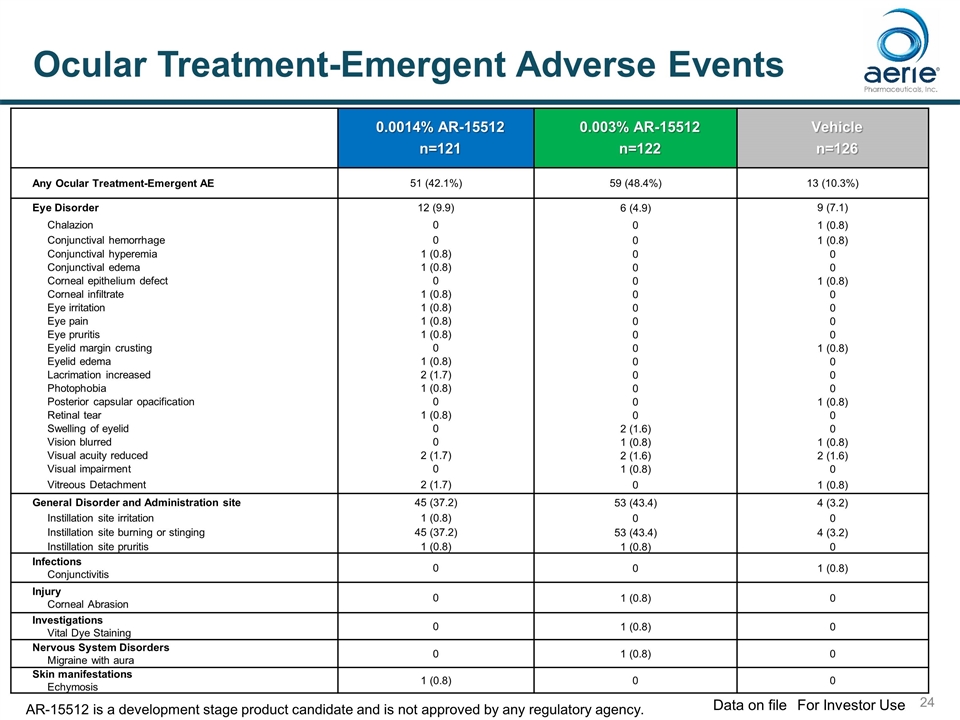
0.0014% AR-15512 n=121 0.003% AR-15512 n=122 Vehicle n=126 Any Ocular Treatment-Emergent AE 51 (42.1%) 59 (48.4%) 13 (10.3%) Eye Disorder 12 (9.9) 6 (4.9) 9 (7.1) Chalazion 0 0 1 (0.8) Conjunctival hemorrhage 0 0 1 (0.8) Conjunctival hyperemia 1 (0.8) 0 0 Conjunctival edema 1 (0.8) 0 0 Corneal epithelium defect 0 0 1 (0.8) Corneal infiltrate 1 (0.8) 0 0 Eye irritation 1 (0.8) 0 0 Eye pain 1 (0.8) 0 0 Eye pruritis 1 (0.8) 0 0 Eyelid margin crusting 0 0 1 (0.8) Eyelid edema 1 (0.8) 0 0 Lacrimation increased 2 (1.7) 0 0 Photophobia 1 (0.8) 0 0 Posterior capsular opacification 0 0 1 (0.8) Retinal tear 1 (0.8) 0 0 Swelling of eyelid 0 2 (1.6) 0 Vision blurred 0 1 (0.8) 1 (0.8) Visual acuity reduced 2 (1.7) 2 (1.6) 2 (1.6) Visual impairment 0 1 (0.8) 0 Vitreous Detachment 2 (1.7) 0 1 (0.8) General Disorder and Administration site 45 (37.2) 53 (43.4) 4 (3.2) Instillation site irritation 1 (0.8) 0 0 Instillation site burning or stinging 45 (37.2) 53 (43.4) 4 (3.2) Instillation site pruritis 1 (0.8) 1 (0.8) 0 Infections Conjunctivitis 0 0 1 (0.8) Injury Corneal Abrasion 0 1 (0.8) 0 Investigations Vital Dye Staining 0 1 (0.8) 0 Nervous System Disorders Migraine with aura 0 1 (0.8) 0 Skin manifestations Echymosis 1 (0.8) 0 0 Ocular Treatment-Emergent Adverse Events For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
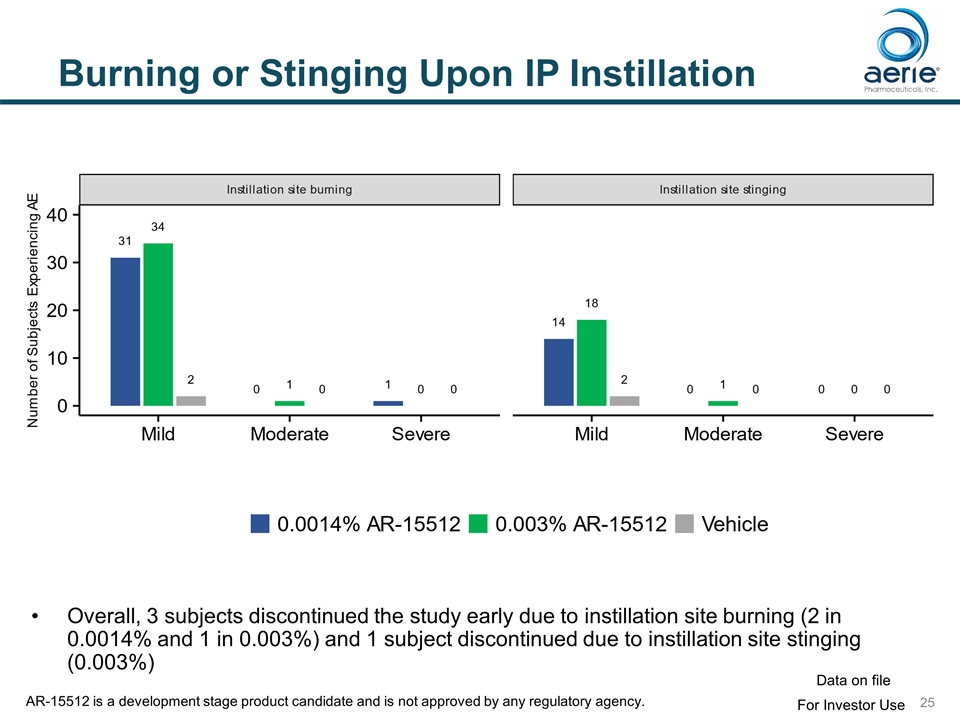
Burning or Stinging Upon IP Instillation Overall, 3 subjects discontinued the study early due to instillation site burning (2 in 0.0014% and 1 in 0.003%) and 1 subject discontinued due to instillation site stinging (0.003%) For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
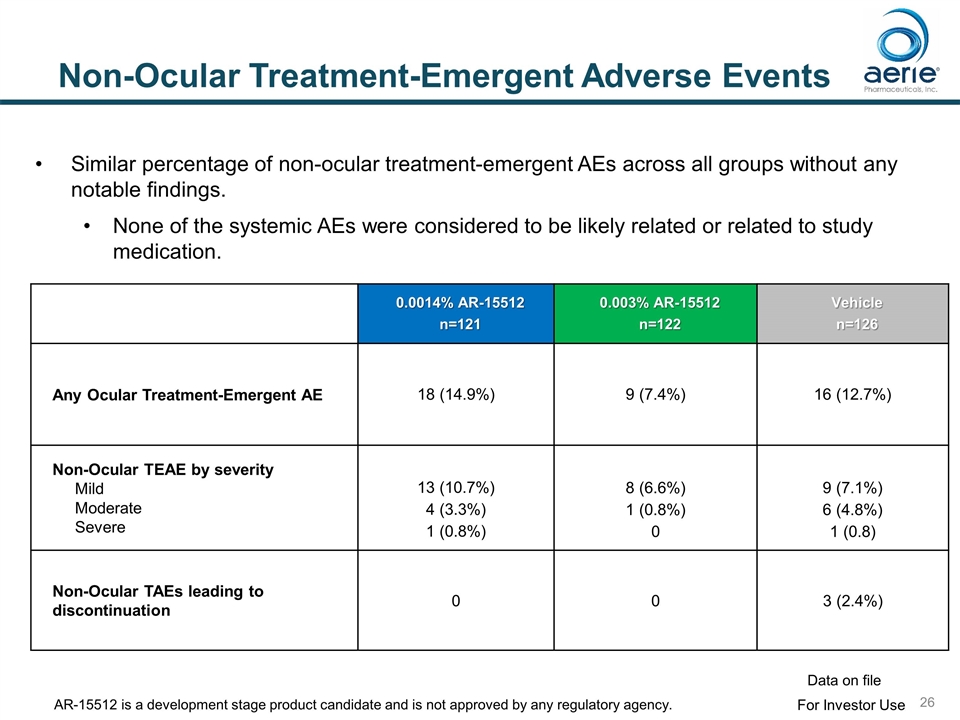
Non-Ocular Treatment-Emergent Adverse Events 0.0014% AR-15512 n=121 0.003% AR-15512 n=122 Vehicle n=126 Any Ocular Treatment-Emergent AE 18 (14.9%) 9 (7.4%) 16 (12.7%) Non-Ocular TEAE by severity Mild Moderate Severe 13 (10.7%) 4 (3.3%) 1 (0.8%) 8 (6.6%) 1 (0.8%) 0 9 (7.1%) 6 (4.8%) 1 (0.8) Non-Ocular TAEs leading to discontinuation 0 0 3 (2.4%) Similar percentage of non-ocular treatment-emergent AEs across all groups without any notable findings. None of the systemic AEs were considered to be likely related or related to study medication. For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
Summary In this Phase 2b clinical study, 369 dry eye subjects were randomized to one of 3 treatment groups (0.0014% AR-15512, 0.003% AR-15512 or vehicle). Statistically significant efficacy was demonstrated across multiple pre-specified symptom and sign endpoints with 0.003% AR-15512. Symptoms: Ocular Discomfort, SANDE, Eye Dryness. Signs: Tear production, conjunctival redness, ocular surface staining. Early onset (within 14 days) of efficacy as well as continued improvement in symptoms and signs demonstrated over 3-months. The formulations were safe and well-tolerated. Vast majority (~95%) of all ocular AEs rated as mild and < 2% of subjects in the 0.003% treatment group discontinued due to AEs. No systemic or serious AEs attributed to study medication. 0.003% AR-15512 selected for advancement to Phase 3 development. For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.

Next Steps: End-of-Phase 2 meeting with FDA in Q1 2022 with Phase 3 initiation in Q2 2022. Plan two 3-month efficacy studies and safety study. Expected NDA submission H1 2024. For Investor Use Data on file AR-15512 is a development stage product candidate and is not approved by any regulatory agency.
Background on Presenters David Hollander, MD, MBA Chief R&D Officer Cornea-trained ophthalmologist Former leadership roles at Allergan (Global VP and Therapeutic Area Head) and Ora, Inc. (Chief Medical Officer) Michelle Senchyna, PhD Vice President, Clinical Development and Medical Affairs Worked on over 20 dry eye studies Former leadership roles at Allergan (Executive Director of Ophthalmology) and Alcon (R&D and Medical Affairs) For Investor Use




























